User login
Chest Tube Management
A pneumothorax is a collection of air in the space outside the lungs that is trapped within the thorax. This abnormality can occur spontaneously or as the result of trauma. Traumatic pneumothoraces include those resulting from medical interventions such as a transthoracic and transbronchial needle biopsy, central line placement, and positive‐pressure mechanical ventilation. This group is most accurately described as iatrogenic pneumothorax (IP).[1]
IP can be an expected complication of many routine thoracic procedures, but it can also occur accidentally during procedures near the lung or thoracic cavity. Some IPs may be asymptomatic and go undiagnosed, or their diagnosis may be delayed.[2] The majority of iatrogenic pneumothoraces will resolve without complications, and patients will not require medical attention. A small percentage can, however, expand and have the potential to develop into a tension pneumothorax causing severe respiratory distress and mediastinal shift.[3, 4]
The incidence of IP ranges from 0.11% with mechanical ventilation to 2.68% with thoracentesis, according to an analysis of 7.5 million uniform hospital discharge abstracts from 2000.5 A 2010 systematic review of 24 studies that included 6605 patients suggested a 6.0% incidence of pneumothorax following thoracentesis.[3] The highest risk of IP is seen with computed tomography (CT)‐guided lung biopsy, with 1 series of 1098 biopsies showing a 42% incidence; chest tube evacuation was required in 12% of these cases.[4] A Veterans Administration study of patient safety indicators from 2001 to 2004 found that risk‐adjusted rates of IP were increasing over time.[6] It is unclear whether this increase is due to increasing numbers of interventional procedures or to better rates of detection. IP poses a considerable cost to the medical system, with safety studies finding that patients with IP will stay in the hospital approximately 4 days longer and incur an additional $17,000 in charges.[7]
In addition to this financial burden, the lack of consistency in training and guidelines for management of pneumothorax is thought to add to chest tube‐related complications.[8] In 2001, the American College of Chest Physicians (ACCP) published guidelines for the management of spontaneous pneumothorax that do not specifically address IP.[9] In 2010, the British Thoracic Society (BTS) updated their guidelines and included a brief statement on IP that described a higher incidence for it than for spontaneous pneumothorax and noted its relative ease of management.[10] Despite the lack of specific guidelines dedicated to IPs, common clinical practice is to manage iatrogenic defects in a manner similar to that for spontaneous ones. However, studies have shown that the management of pneumothorax remains diverse and that the adherence to these published guidelines is suboptimal.[10, 11] The BTS guidelines favor needle aspiration as the first‐line treatment,[10] whereas the ACCP recommends drainage with catheters over aspiration.[9]
The possibility of this complication, along with the rising rate of invasive interventions being performed, has led to expanded surveillance criteria for IP. Surveillance imaging, clinical observation, or a combination of the 2 may be required, depending on the institution, the risk of the procedure, and the preference of the treating clinician. The algorithms presented here were designed in alignment with both major society guidelines and with the intention of simplifying the treatment regimen for the ease of adoption by hospitalists.
ETIOLOGY AND RISK FACTORS
The etiology and risk factors for IP are multiple, with the most common being interventional‐based procedures. In 535 Veterans Administration patients, the most common precursor procedures were transthoracic needle biopsy (24%), subclavian vein catheterization (22%), thoracentesis (20%), transbronchial biopsy (10%), pleural biopsy (8%), and positive pressure ventilation.[12] IP can also be a rare complication of pacemaker manipulations,[5] and less commonly, bronchoscopy.[13] Patient factors that increase the risk of pneumothorax in the setting of an intervention include age, chronic obstructive lung disease, primary lung cancer, malignant and parapneumonic pleural effusions, empyema, and chronic corticosteroid use.[4] As might be expected, patients with structural lung disease (eg, emphysema with bullae) and poor healing ability (eg, corticosteroid dependent), tend to have IPs more often and to require more complicated interventions for resolution.[14, 15] In some studies, operator experience seems to be inversely related to the rate of IP, and the use of ultrasound is correlated with lower rates of this complication.[1, 3]
PATIENT PRESENTATIONS AND DIAGNOSIS
Clinical signs and symptoms of a significant pneumothorax vary in severity but most often include dyspnea, tachypnea, chest pain, and pleurisy (see Box 1). Post procedure signs or symptoms require further evaluation with imaging, usually a plain chest radiograph. CT can be useful for further evaluation. Small anterior pneumothoraces may be difficult to detect without lateral radiographic imaging or computed tomogram. Ultrasound is being used more frequently at the bedside to make this diagnosis, and various studies of trauma patients have found that it has good sensitivity and specificity.[16, 17] These results have been validated by a recent meta‐analysis comparing ultrasound to chest radiographs for the detection of pneumothorax among trauma, critically ill, and postprocedural patients.[18] This study demonstrated superior sensitivity and similar specificity for ultrasound versus chest radiographs for detection of pneumothorax. More ominous signs, such as tachycardia or hypotension, can be indicative of tension pneumothorax, which requires emergent evacuation.
MANAGEMENT
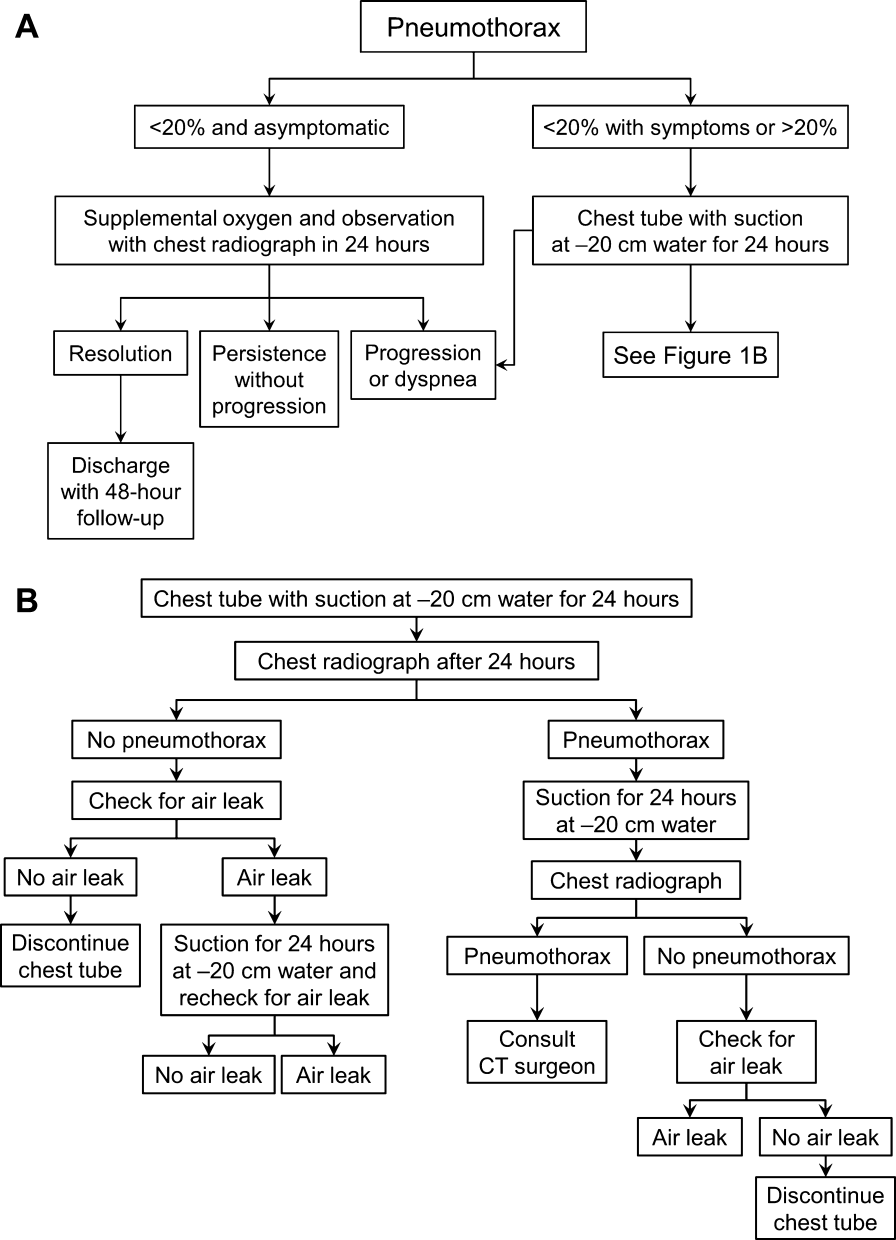
Once the diagnosis of pneumothorax has been established, treatment options should be guided by defect size and clinical assessment following a defined treatment algorithm (Figure 1). As emphasized by the BTS and ACCP guidelines, we advocate considering the use of symptoms along with defect size to determine the best management course.
Observation
Defects that involve<20% of the hemithorax in a patient who is clinically asymptomatic and hemodynamically stable can be safely managed by oxygen supplementation and hospital observation. Repeat imaging can be obtained after 12 to 24 hours of defect detection or with symptom change. Patients who display resolution may be discharged home.
Patients who show persistence without progression but are asymptomatic may also be discharged safely, with follow‐up imaging and clinical evaluation 48 hours later.[9, 10] This was demonstrated by Kelly and colleagues,[19] who described the outcomes of 154 patients in a retrospective cohort study. Of the 91 patients treated with outpatient observation, 82 resolved without additional interventions. A recent review article by the same author cites conservative management of small pneumothoraces as being widely accepted.[20] If reimaging shows progression of defect or if the patient becomes more symptomatic, the pneumothorax should be evacuated by 1 of the methods described below.
Aspiration
Aspiration is defined by the ACCP Delphi consensus statement as the removal of pleural air via needle or cannula followed by immediate removal of needle or cannula.[9] This option mandates careful patient selection. It should be considered for small pneumothoraces that cause only mild dyspnea in patients who have no known parenchymal disease. These patients should be observed overnight in the hospital and reimaged 24 hours after aspiration of the pneumothorax. Several authors have reported success with aspiration alone. Yamagami et al.[21] noted the efficacy of manual aspiration immediately after CT‐guided biopsy, with a success rate exceeding 90%. They also noted that evacuated volumes >543 mL correlated with the need for further intervention with a chest tube. This technique is advocated for small pneumothoraces that are recognized shortly after the procedure.
Similarly, Delius and colleagues[22] managed 131 pneumothoraces with aspiration as an alternative to chest tube placement. Of these, 79 were iatrogenic. Aspiration achieved a 75% success rate for all IPs. Small defects defined as <20% of volume had an even higher resolution rate of 87%. Similar findings were demonstrated by Talbot‐Stern et al.[23] in their prospective study of 76 pneumothoraces. Among those that were iatrogenic, 82% resolved after simple aspiration. Faruqi et al.[24] also showed that aspiration is a viable option for IPs. Of the 57 patients with pneumothorax included in their study, 35 were treated with aspiration alone. Iatrogenesis was the culprit in 12 of the 35 manually aspirated cases. Aspiration achieved a success rate of 91.7% in IP. A recent Cochrane database systematic review compared simple aspiration with intercostal tube drainage for primary spontaneous pneumothorax.[25] The authors reported no difference between these methods in terms of success rate, early failure rate, duration of hospital stay, 1‐year success rate, or number of patients who required pleurodesis at 1‐year follow‐up.
Because the algorithms presented in this article were specifically designed for the use by hospitalists, we intentionally omitted aspiration from the decision trees. Most hospitalists would not be expected to evacuate IPs. However, knowledge regarding this option and appropriate follow‐up are valuable to internists, because many interventionalists admit patients to the hospital service for overnight observation. An asymptomatic postaspiration patient, who on subsequent imaging demonstrates resolution or persistence without progression of pneumothorax, may be discharged with 48‐hour follow‐up.
Placement of Catheter or Chest Tube Drainage
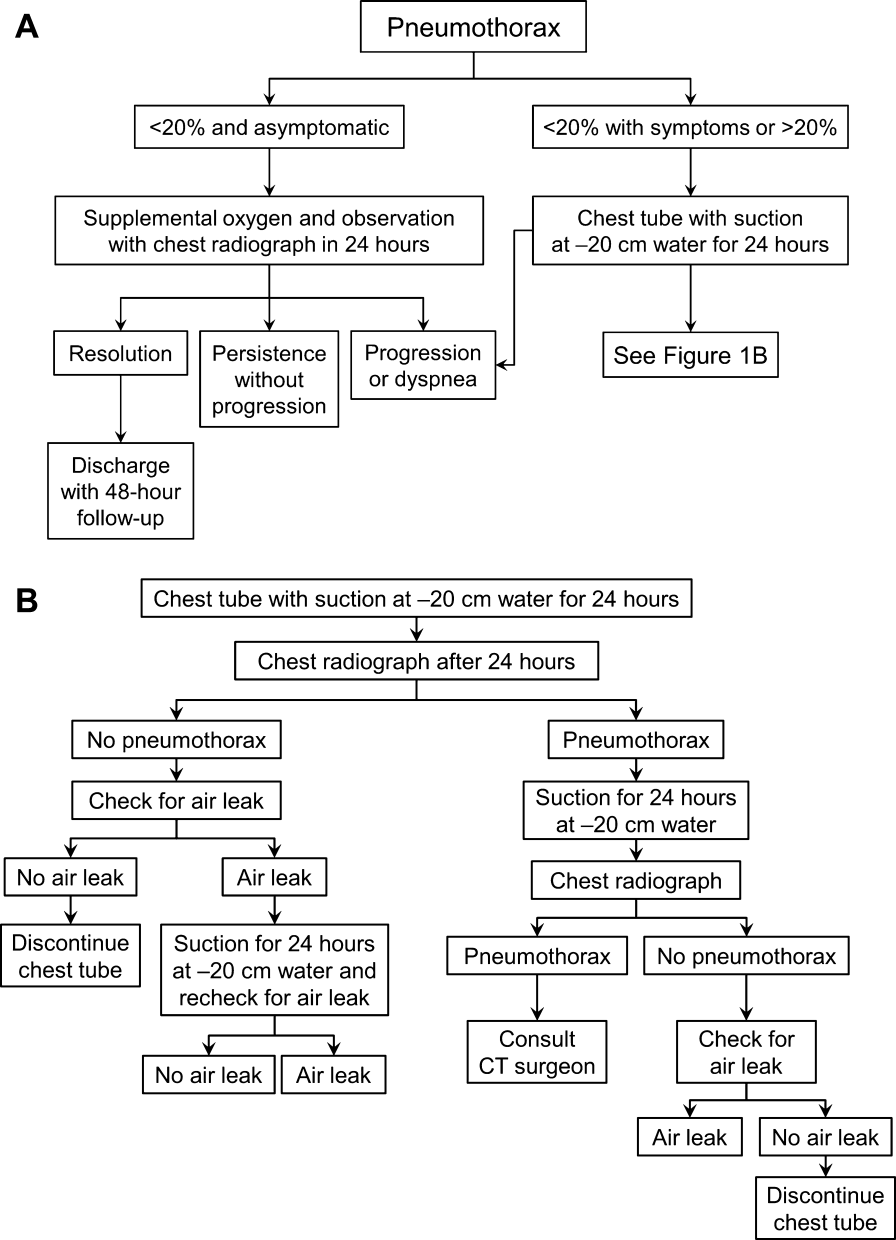
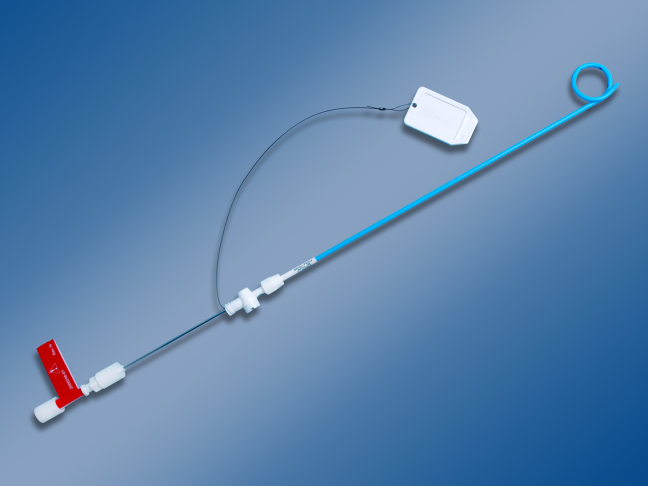
Most patients with a clinically significant pneumothorax will require evacuation of the air. Pneumothoraces larger than 20% or that produce symptoms warrant chest tube management and inpatient observation (Figure 1B). Traditionally, large tubes with 20 cm of water on continuous suction are used and have been studied the most widely. Several authors have shown that smaller tubes can effectively drain a pneumothorax.[26, 27, 28, 29] Small‐bore catheters (8F14F), which can be inserted percutaneously, have been shown to provide effective lung re‐expansion with minimal morbidity[8] and may be better tolerated by patients with uncomplicated pneumothoraces (Figure 2). Terzi and colleagues[30] have shown that smaller tubes cause less discomfort to patients at rest, with cough, and at the time of tube removal.
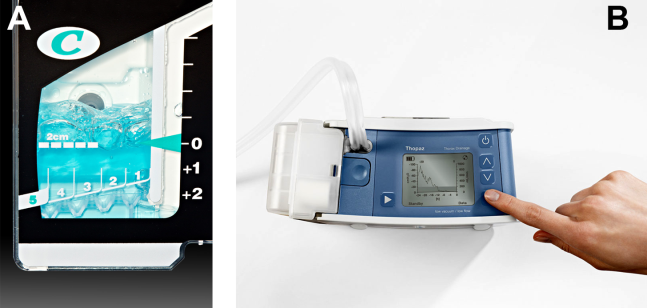
At most US institutions, catheters and chest tubes are connected to all‐purpose drainage systems. Although commercially available through a variety of manufacturers, they share similar design principles because they replicate the 3‐bottle system described in detail elsewhere in the literature.[31] We have limited our discussion to 3 pleural evacuation systems because it is our intention to familiarize hospitalists with the units that they are most likely to encounter. The first 2 systems have been studied and described by Baumann and colleagues[32] as being commonplace and reasonably reliable. These include the Oasis (Atrium Medical Corp., Hudson, NH) (Figure 3A) and the Pleur‐evac (Teleflex Inc., Limerick, PA). The third unit is the Thopaz digital thoracic drainage system (Medela Inc., McHenry, IL) (Figure 3B). The Thopaz is unique in its inclusion of a suction source and digital capability. Although it utilizes the same principles of all pleural evacuation devices, its setup and information output require that one be familiar with its digital format.

Suction Versus Water Seal
The chest tube should be placed initially to a suction pressure level of 20 cm of water for 24 hours to maximize lung expansion and evacuate all extrapulmonary air. Suction pressure is set on the Pleur‐evac and Atrium drainage systems by a manual dial that reads to a water pressure of 0 to 40 cm. The default setting from the manufacturer is 20 cm of water. This level of suction is present only when the drainage system is connected to a wall or a portable suction device. The only confirmation of suction presence in the Atrium system is the deployment of the orange bellows (located under the dial) to the level of the arrow tip (Figure 3A). The Pleur‐evac system has a red stripe along the circular edge of the dial that appears at the set level of suction when negative pressure is being applied. It is important to be aware that when patients are disconnected from the wall or the portable suction apparatus, they are on water seal or gravity. These terms are synonymous with no suction. On the Thopaz, a digital menu directs operation, and levels of suction can be selected from water seal (no suction) up to 40 cm of water. We recommend using suction to 20 cm of water given the scarce evidence supporting higher levels of negative pressure. Some clinicians prefer placing patients on water seal for some time before moving toward tube discontinuance, but this is a matter of preference, and no substantial evidence exists to show that any 1 method is superior.[8, 33]
Assessing for Air Leak
If there is improvement or resolution of the pneumothorax after 24 hours, the presence of an air leak should be assessed; if no leak is present, the chest tube can be safely removed. In the context of chest tubes, the term air leak refers to residual air between the lung and the chest wall. It is possible to see resolution of a pneumothorax on chest radiographs and still have an air leak. This situation is created by a perfect balance between the pleural air evacuation by the catheter and the flow of air exiting from the lung puncture. This would result in reaccumulation of the pneumothorax if the chest tube is removed prematurely. It should also be kept in mind that chest radiographs may miss a small pneumothorax given their relatively low sensitivity.[18] Therefore, the absence of an air leak needs to be documented before the chest tube is discontinued. Depending on the type of drainage system (Atrium, Pleur‐evac, or Thopaz), this assessment can be done in several ways. All systems can be assessed for air leak by clamping the actual chest tube for 2 to 4 hours and then repeating the chest radiograph. Clamping a chest tube simulates the condition of not having a chest tube. Chest tubes should never be clamped without supervision and only with the knowledge of nursing personnel. The onset of chest pain or dyspnea in a patient with a clamped tube mandates immediate removal of the clamp and a return to suction. A repeat chest radiograph showing reaccumulation or expansion of the pneumothorax after clamping indicates that the air leak has not resolved and the chest tube must remain in place and returned to suction. Simpler and more time‐efficient methods of detecting air leaks are available with both cardiothoracic drainage systems.
For the Atrium and Pleur‐evac models, there is a graded panel through which one can visualize air leaks being funneled through water (Figure 4A). Having the patient cough several times or perform a Valsalva maneuver should release any air trapped within the chest into this chamber, where bubbles can be visualized as they travel through the water. The presence of bubbles indicates the presence of residual air in the chest, pointing to a possible leak. In contrast, the Thopaz system offers a graphical display of the air flowing into the system that can be reviewed over the 24‐hour period. When the graph line reaches a 0 flatline graph, no airflow is being detected and no air leakage is suspected (Figure 4B). If no air leaks are detected, the chest tube may be discontinued. Those patients with a failed air leak test should have their chest tubes continued under suction for another 24 hours, with the above tests then repeated. The same holds true for those patients with persistent pneumothorax at 24 hours.
Removal of chest tubes is a simple process that requires the tube to be pulled out of the patient without allowing air to enter the site where the tube was present and where it entered the thorax. Most interventionalists will discontinue the tubes that they have placed. Some small catheters have an internal string that has to be released so the catheter will straighten and pull out easily. Knowledge of the type of catheter or tube that was placed is critical before removal to prevent complications and patient discomfort. Standard chest tubes are straight, smooth plastic and pull out easily but require rapid occlusion of the larger puncture site in the chest wall with an occlusive dressing that often includes petroleum or water‐soluble gel. Some physicians will leave a suture tie when placing the chest tube so that it can be tied down to occlude the site instead of using a dressing.
Consultation of the Cardiothoracic Surgeon or Interventional Pulmonologist
We recommend the involvement of cardiothoracic surgery or interventional pulmonology for patients with nonresolving pneumothorax lasting longer than 48 hours because additional procedures may be necessary. One of the rare but serious complications of a persistent pneumothorax is the formation of a bronchopleural fistula. This communication between the bronchial tree and the pleural space can lead to significant morbidity and mortality. The treatment of a bronchopleural fistula includes medical and surgical options that are beyond the scope of this article but require the expertise of a cardiothoracic surgeon or interventional pulmonologist.[34] Most patients who will not require additional procedures will heal within 48 hours.[11, 35] Decisions regarding more invasive treatment measures can then be made as necessary.[26]
PRACTICAL TIPS
Hospitalists caring for patients with chest tubes are often asked to troubleshoot at the bedside. Scenarios that may be encountered include nonfunctioning tubes, catheter migration, and tube discomfort. Ensuring patency of the tube entails visualizing the tube from the point of entry into the chest wall to the collection chamber and inspecting for kinks or debris clogging the tube. Smaller catheters can be easily kinked during patient positioning and can become clogged. Respiratory variation, which is the movement of the column of fluid in the collection chamber or in the tubing with inspiration and expiration, suggests that the chest tube is patent. This should be part of the daily examination in a patient with a chest tube, and it should also be the first step in assessing sudden dyspnea, hypoxia, pain, or hemodynamic instability. Clogged tubes should be referred to the interventionalists or other physicians who placed them. Chest tubes are typically sutured at the site of entry and securely bandaged to avoid migration but occasionally can be dislodged. This should prompt placement of another tube by an experienced operator. Last, chest tubes can be uncomfortable for patients who may require systemic analgesics. Additionally, tube positioning may ease some of the discomfort. Chest tubes are commonly placed along the midaxillary line and the posterior thorax, leading to discomfort in the recumbent position. Directing the tube anteriorly helps ease some of the discomfort. This can be done using all‐purpose sponges to build a barrier between the skin and the chest tube as it is directed anteriorly. Additional sponges are placed above the tube for extra protection. The gentle curve accomplished by padding the underside of the tube also keeps the tube patent by avoiding sharp kinks as the catheter exits the thorax.
FUTURE TRENDS
Future trends in the management of IP may include shorter duration of tube management (14 hours of suction with air leak evaluation and removal) and the use of even smaller catheters. Outpatient management of IP with small pigtail catheters or small‐caliber tubes in addition to 1‐way valves is also being investigated. The benefits of these practices may include greater patient comfort and lower cost. These approaches will need larger‐scale replication and careful patient selection before they become standard practice.[28, 36]
Ultrasound can be used to assess the presence and size of pneumothoraces that are difficult to visualize by standard chest radiographs. Several studies have established ultrasonography as an effective method of diagnosing pneumothorax and have shown it to have superior sensitivity compared with chest radiography.[1, 18] In the future, the use of ultrasound will likely be more widespread given its performance, portability, ease of use, and relatively low cost.
SUMMARY
IP is a known and costly complication of many medical procedures. The aforementioned algorithms help simplify the management of chest tubes for hospitalists caring for patients with this common complication. This stepwise approach may not only help curtail added expenses related to IPs by decreasing the length of inpatient stays but may also improve patient satisfaction.
Acknowledgments
Disclosure: Mayo does not endorse the products mentioned in this article. The authors report no conflicts of interest.
Box
Clinical Signs and Symptoms of Pneumothorax
Dyspnea
Pleuritic chest pain
Tachypnea
Hypoxia
Decreased breath sounds on affected side
Hyper‐resonant percussion on affected side
Subcutaneous emphysema
- , . Management of pneumothorax. Semin Respir Crit Care Med. 2010;31(6):769–780.
- , . Pneumothorax. Respirology. 2004;9(2):157–164.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy‐guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9‐year period. AJR Am J Roentgenol. 2010;194(3):809–814.
- , , . Accidental iatrogenic pneumothorax in hospitalized patients. Med Care. 2006;44(2):182–186.
- , , , et al. Tracking rates of patient safety indicators over time: lessons from the Veterans Administration. Med Care. 2006;44(9):850–861.
- , . Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290(14):1868–1874.
- , , , , . Preliminary report of a prospective, randomized trial of underwater seal for spontaneous and iatrogenic pneumothorax. J Am Coll Surg. 2007;204(1):84–90.
- , , , et al.; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119(2):590–602.
- , , ; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii18–ii31.
- , . The clinician's perspective on pneumothorax management. Chest. 1997;112(3):822–828.
- , , , . Iatrogenic pneumothorax: etiology and morbidity: results of a Department of Veterans Affairs Cooperative Study. Respiration. 1992;59(4):215–220.
- , , , , , . Severe complications of bronchoscopy. Respiration. 2008;76(4):429–433.
- , , , et al. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest. 2004;126(5):1516–1521.
- , , , , , . Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15(5):479–483.
- , . Ultrasound detection of pneumothorax compared with chest X‐ray and computed tomography scan. Am Surg. 2011;77(4):480–484.
- , . Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010;17(1):11–17.
- , , , , . Diagnosis of pneumothorax by radiography and ultrasonography: a meta‐analysis. Chest. 2011;140(4):859–866.
- , , . Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest. 2008;134(5):1033–1036.
- . Review of management of primary spontaneous pneumothorax: is the best evidence clearer 15 years on? Emerg Med Australas. 2007;19(4):303–308.
- , , , , , . Efficacy of manual aspiration immediately after complicated pneumothorax in CT‐guided lung biopsy. J Vasc Interv Radiol. 2005;16(4):477–483.
- , , , , , . Catheter aspiration for simple pneumothorax: experience with 114 patients. Arch Surg. 1998;124(7):833–836.
- , , , , . Catheter aspiration for simple pneumothorax. J Emerg Med. 1986;4(6):437–442.
- , , , . Role of simple needle aspiration in the management of pneumothorax. Indian J Chest Dis Allied Sci. 2004;46(3):183–190.
- , , . Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev. 2007;(1):CD004479.
- , , , . Evaluation of conventional chest tube therapy for iatrogenic pneumothorax. Chest. 1993;104(6):1770–1772.
- , , , . Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology. 1997;205(1):249–252.
- , , , , , . Outpatient management of postbiopsy pneumothorax with small‐caliber chest tubes: factors affecting the need for prolonged drainage and additional interventions. Cardiovasc Intervent Radiol. 2008;31(2):342–348.
- , . Management of primary and secondary pneumothorax using a small‐bore thoracic catheter. Interact Cardiovasc Thorac Surg. 2010;11(2):146–149.
- , , , et al. The use of flexible spiral drains after non‐cardiac thoracic surgery: a clinical study. Eur J Cardiothorac Surg. 2005;27(1):134–137.
- . Pleural Diseases. 5th ed. Philadelphia: PA: Lippincott Williams 2007.
- , , , . Comparison of function of commercially available pleural drainage units and catheters. Chest. 2003;123(6):1878–1886.
- , . Catheter drainage of spontaneous pneumothorax: suction or no suction, early or late removal? Thorax. 1982;37(1):46–48.
- , . Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128(6):3955–3965.
- , , , . Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg. 1991;126(6):764–766.
- , , , , . A treatment algorithm for pneumothoraces complicating central venous catheter insertion. Am J Surg. 2000;180(6):523–526.
A pneumothorax is a collection of air in the space outside the lungs that is trapped within the thorax. This abnormality can occur spontaneously or as the result of trauma. Traumatic pneumothoraces include those resulting from medical interventions such as a transthoracic and transbronchial needle biopsy, central line placement, and positive‐pressure mechanical ventilation. This group is most accurately described as iatrogenic pneumothorax (IP).[1]
IP can be an expected complication of many routine thoracic procedures, but it can also occur accidentally during procedures near the lung or thoracic cavity. Some IPs may be asymptomatic and go undiagnosed, or their diagnosis may be delayed.[2] The majority of iatrogenic pneumothoraces will resolve without complications, and patients will not require medical attention. A small percentage can, however, expand and have the potential to develop into a tension pneumothorax causing severe respiratory distress and mediastinal shift.[3, 4]
The incidence of IP ranges from 0.11% with mechanical ventilation to 2.68% with thoracentesis, according to an analysis of 7.5 million uniform hospital discharge abstracts from 2000.5 A 2010 systematic review of 24 studies that included 6605 patients suggested a 6.0% incidence of pneumothorax following thoracentesis.[3] The highest risk of IP is seen with computed tomography (CT)‐guided lung biopsy, with 1 series of 1098 biopsies showing a 42% incidence; chest tube evacuation was required in 12% of these cases.[4] A Veterans Administration study of patient safety indicators from 2001 to 2004 found that risk‐adjusted rates of IP were increasing over time.[6] It is unclear whether this increase is due to increasing numbers of interventional procedures or to better rates of detection. IP poses a considerable cost to the medical system, with safety studies finding that patients with IP will stay in the hospital approximately 4 days longer and incur an additional $17,000 in charges.[7]
In addition to this financial burden, the lack of consistency in training and guidelines for management of pneumothorax is thought to add to chest tube‐related complications.[8] In 2001, the American College of Chest Physicians (ACCP) published guidelines for the management of spontaneous pneumothorax that do not specifically address IP.[9] In 2010, the British Thoracic Society (BTS) updated their guidelines and included a brief statement on IP that described a higher incidence for it than for spontaneous pneumothorax and noted its relative ease of management.[10] Despite the lack of specific guidelines dedicated to IPs, common clinical practice is to manage iatrogenic defects in a manner similar to that for spontaneous ones. However, studies have shown that the management of pneumothorax remains diverse and that the adherence to these published guidelines is suboptimal.[10, 11] The BTS guidelines favor needle aspiration as the first‐line treatment,[10] whereas the ACCP recommends drainage with catheters over aspiration.[9]
The possibility of this complication, along with the rising rate of invasive interventions being performed, has led to expanded surveillance criteria for IP. Surveillance imaging, clinical observation, or a combination of the 2 may be required, depending on the institution, the risk of the procedure, and the preference of the treating clinician. The algorithms presented here were designed in alignment with both major society guidelines and with the intention of simplifying the treatment regimen for the ease of adoption by hospitalists.
ETIOLOGY AND RISK FACTORS
The etiology and risk factors for IP are multiple, with the most common being interventional‐based procedures. In 535 Veterans Administration patients, the most common precursor procedures were transthoracic needle biopsy (24%), subclavian vein catheterization (22%), thoracentesis (20%), transbronchial biopsy (10%), pleural biopsy (8%), and positive pressure ventilation.[12] IP can also be a rare complication of pacemaker manipulations,[5] and less commonly, bronchoscopy.[13] Patient factors that increase the risk of pneumothorax in the setting of an intervention include age, chronic obstructive lung disease, primary lung cancer, malignant and parapneumonic pleural effusions, empyema, and chronic corticosteroid use.[4] As might be expected, patients with structural lung disease (eg, emphysema with bullae) and poor healing ability (eg, corticosteroid dependent), tend to have IPs more often and to require more complicated interventions for resolution.[14, 15] In some studies, operator experience seems to be inversely related to the rate of IP, and the use of ultrasound is correlated with lower rates of this complication.[1, 3]
PATIENT PRESENTATIONS AND DIAGNOSIS
Clinical signs and symptoms of a significant pneumothorax vary in severity but most often include dyspnea, tachypnea, chest pain, and pleurisy (see Box 1). Post procedure signs or symptoms require further evaluation with imaging, usually a plain chest radiograph. CT can be useful for further evaluation. Small anterior pneumothoraces may be difficult to detect without lateral radiographic imaging or computed tomogram. Ultrasound is being used more frequently at the bedside to make this diagnosis, and various studies of trauma patients have found that it has good sensitivity and specificity.[16, 17] These results have been validated by a recent meta‐analysis comparing ultrasound to chest radiographs for the detection of pneumothorax among trauma, critically ill, and postprocedural patients.[18] This study demonstrated superior sensitivity and similar specificity for ultrasound versus chest radiographs for detection of pneumothorax. More ominous signs, such as tachycardia or hypotension, can be indicative of tension pneumothorax, which requires emergent evacuation.
MANAGEMENT
Once the diagnosis of pneumothorax has been established, treatment options should be guided by defect size and clinical assessment following a defined treatment algorithm (Figure 1). As emphasized by the BTS and ACCP guidelines, we advocate considering the use of symptoms along with defect size to determine the best management course.

Observation
Defects that involve<20% of the hemithorax in a patient who is clinically asymptomatic and hemodynamically stable can be safely managed by oxygen supplementation and hospital observation. Repeat imaging can be obtained after 12 to 24 hours of defect detection or with symptom change. Patients who display resolution may be discharged home.
Patients who show persistence without progression but are asymptomatic may also be discharged safely, with follow‐up imaging and clinical evaluation 48 hours later.[9, 10] This was demonstrated by Kelly and colleagues,[19] who described the outcomes of 154 patients in a retrospective cohort study. Of the 91 patients treated with outpatient observation, 82 resolved without additional interventions. A recent review article by the same author cites conservative management of small pneumothoraces as being widely accepted.[20] If reimaging shows progression of defect or if the patient becomes more symptomatic, the pneumothorax should be evacuated by 1 of the methods described below.
Aspiration
Aspiration is defined by the ACCP Delphi consensus statement as the removal of pleural air via needle or cannula followed by immediate removal of needle or cannula.[9] This option mandates careful patient selection. It should be considered for small pneumothoraces that cause only mild dyspnea in patients who have no known parenchymal disease. These patients should be observed overnight in the hospital and reimaged 24 hours after aspiration of the pneumothorax. Several authors have reported success with aspiration alone. Yamagami et al.[21] noted the efficacy of manual aspiration immediately after CT‐guided biopsy, with a success rate exceeding 90%. They also noted that evacuated volumes >543 mL correlated with the need for further intervention with a chest tube. This technique is advocated for small pneumothoraces that are recognized shortly after the procedure.
Similarly, Delius and colleagues[22] managed 131 pneumothoraces with aspiration as an alternative to chest tube placement. Of these, 79 were iatrogenic. Aspiration achieved a 75% success rate for all IPs. Small defects defined as <20% of volume had an even higher resolution rate of 87%. Similar findings were demonstrated by Talbot‐Stern et al.[23] in their prospective study of 76 pneumothoraces. Among those that were iatrogenic, 82% resolved after simple aspiration. Faruqi et al.[24] also showed that aspiration is a viable option for IPs. Of the 57 patients with pneumothorax included in their study, 35 were treated with aspiration alone. Iatrogenesis was the culprit in 12 of the 35 manually aspirated cases. Aspiration achieved a success rate of 91.7% in IP. A recent Cochrane database systematic review compared simple aspiration with intercostal tube drainage for primary spontaneous pneumothorax.[25] The authors reported no difference between these methods in terms of success rate, early failure rate, duration of hospital stay, 1‐year success rate, or number of patients who required pleurodesis at 1‐year follow‐up.
Because the algorithms presented in this article were specifically designed for the use by hospitalists, we intentionally omitted aspiration from the decision trees. Most hospitalists would not be expected to evacuate IPs. However, knowledge regarding this option and appropriate follow‐up are valuable to internists, because many interventionalists admit patients to the hospital service for overnight observation. An asymptomatic postaspiration patient, who on subsequent imaging demonstrates resolution or persistence without progression of pneumothorax, may be discharged with 48‐hour follow‐up.
Placement of Catheter or Chest Tube Drainage
Most patients with a clinically significant pneumothorax will require evacuation of the air. Pneumothoraces larger than 20% or that produce symptoms warrant chest tube management and inpatient observation (Figure 1B). Traditionally, large tubes with 20 cm of water on continuous suction are used and have been studied the most widely. Several authors have shown that smaller tubes can effectively drain a pneumothorax.[26, 27, 28, 29] Small‐bore catheters (8F14F), which can be inserted percutaneously, have been shown to provide effective lung re‐expansion with minimal morbidity[8] and may be better tolerated by patients with uncomplicated pneumothoraces (Figure 2). Terzi and colleagues[30] have shown that smaller tubes cause less discomfort to patients at rest, with cough, and at the time of tube removal.

At most US institutions, catheters and chest tubes are connected to all‐purpose drainage systems. Although commercially available through a variety of manufacturers, they share similar design principles because they replicate the 3‐bottle system described in detail elsewhere in the literature.[31] We have limited our discussion to 3 pleural evacuation systems because it is our intention to familiarize hospitalists with the units that they are most likely to encounter. The first 2 systems have been studied and described by Baumann and colleagues[32] as being commonplace and reasonably reliable. These include the Oasis (Atrium Medical Corp., Hudson, NH) (Figure 3A) and the Pleur‐evac (Teleflex Inc., Limerick, PA). The third unit is the Thopaz digital thoracic drainage system (Medela Inc., McHenry, IL) (Figure 3B). The Thopaz is unique in its inclusion of a suction source and digital capability. Although it utilizes the same principles of all pleural evacuation devices, its setup and information output require that one be familiar with its digital format.

Suction Versus Water Seal
The chest tube should be placed initially to a suction pressure level of 20 cm of water for 24 hours to maximize lung expansion and evacuate all extrapulmonary air. Suction pressure is set on the Pleur‐evac and Atrium drainage systems by a manual dial that reads to a water pressure of 0 to 40 cm. The default setting from the manufacturer is 20 cm of water. This level of suction is present only when the drainage system is connected to a wall or a portable suction device. The only confirmation of suction presence in the Atrium system is the deployment of the orange bellows (located under the dial) to the level of the arrow tip (Figure 3A). The Pleur‐evac system has a red stripe along the circular edge of the dial that appears at the set level of suction when negative pressure is being applied. It is important to be aware that when patients are disconnected from the wall or the portable suction apparatus, they are on water seal or gravity. These terms are synonymous with no suction. On the Thopaz, a digital menu directs operation, and levels of suction can be selected from water seal (no suction) up to 40 cm of water. We recommend using suction to 20 cm of water given the scarce evidence supporting higher levels of negative pressure. Some clinicians prefer placing patients on water seal for some time before moving toward tube discontinuance, but this is a matter of preference, and no substantial evidence exists to show that any 1 method is superior.[8, 33]
Assessing for Air Leak
If there is improvement or resolution of the pneumothorax after 24 hours, the presence of an air leak should be assessed; if no leak is present, the chest tube can be safely removed. In the context of chest tubes, the term air leak refers to residual air between the lung and the chest wall. It is possible to see resolution of a pneumothorax on chest radiographs and still have an air leak. This situation is created by a perfect balance between the pleural air evacuation by the catheter and the flow of air exiting from the lung puncture. This would result in reaccumulation of the pneumothorax if the chest tube is removed prematurely. It should also be kept in mind that chest radiographs may miss a small pneumothorax given their relatively low sensitivity.[18] Therefore, the absence of an air leak needs to be documented before the chest tube is discontinued. Depending on the type of drainage system (Atrium, Pleur‐evac, or Thopaz), this assessment can be done in several ways. All systems can be assessed for air leak by clamping the actual chest tube for 2 to 4 hours and then repeating the chest radiograph. Clamping a chest tube simulates the condition of not having a chest tube. Chest tubes should never be clamped without supervision and only with the knowledge of nursing personnel. The onset of chest pain or dyspnea in a patient with a clamped tube mandates immediate removal of the clamp and a return to suction. A repeat chest radiograph showing reaccumulation or expansion of the pneumothorax after clamping indicates that the air leak has not resolved and the chest tube must remain in place and returned to suction. Simpler and more time‐efficient methods of detecting air leaks are available with both cardiothoracic drainage systems.
For the Atrium and Pleur‐evac models, there is a graded panel through which one can visualize air leaks being funneled through water (Figure 4A). Having the patient cough several times or perform a Valsalva maneuver should release any air trapped within the chest into this chamber, where bubbles can be visualized as they travel through the water. The presence of bubbles indicates the presence of residual air in the chest, pointing to a possible leak. In contrast, the Thopaz system offers a graphical display of the air flowing into the system that can be reviewed over the 24‐hour period. When the graph line reaches a 0 flatline graph, no airflow is being detected and no air leakage is suspected (Figure 4B). If no air leaks are detected, the chest tube may be discontinued. Those patients with a failed air leak test should have their chest tubes continued under suction for another 24 hours, with the above tests then repeated. The same holds true for those patients with persistent pneumothorax at 24 hours.

Removal of chest tubes is a simple process that requires the tube to be pulled out of the patient without allowing air to enter the site where the tube was present and where it entered the thorax. Most interventionalists will discontinue the tubes that they have placed. Some small catheters have an internal string that has to be released so the catheter will straighten and pull out easily. Knowledge of the type of catheter or tube that was placed is critical before removal to prevent complications and patient discomfort. Standard chest tubes are straight, smooth plastic and pull out easily but require rapid occlusion of the larger puncture site in the chest wall with an occlusive dressing that often includes petroleum or water‐soluble gel. Some physicians will leave a suture tie when placing the chest tube so that it can be tied down to occlude the site instead of using a dressing.
Consultation of the Cardiothoracic Surgeon or Interventional Pulmonologist
We recommend the involvement of cardiothoracic surgery or interventional pulmonology for patients with nonresolving pneumothorax lasting longer than 48 hours because additional procedures may be necessary. One of the rare but serious complications of a persistent pneumothorax is the formation of a bronchopleural fistula. This communication between the bronchial tree and the pleural space can lead to significant morbidity and mortality. The treatment of a bronchopleural fistula includes medical and surgical options that are beyond the scope of this article but require the expertise of a cardiothoracic surgeon or interventional pulmonologist.[34] Most patients who will not require additional procedures will heal within 48 hours.[11, 35] Decisions regarding more invasive treatment measures can then be made as necessary.[26]
PRACTICAL TIPS
Hospitalists caring for patients with chest tubes are often asked to troubleshoot at the bedside. Scenarios that may be encountered include nonfunctioning tubes, catheter migration, and tube discomfort. Ensuring patency of the tube entails visualizing the tube from the point of entry into the chest wall to the collection chamber and inspecting for kinks or debris clogging the tube. Smaller catheters can be easily kinked during patient positioning and can become clogged. Respiratory variation, which is the movement of the column of fluid in the collection chamber or in the tubing with inspiration and expiration, suggests that the chest tube is patent. This should be part of the daily examination in a patient with a chest tube, and it should also be the first step in assessing sudden dyspnea, hypoxia, pain, or hemodynamic instability. Clogged tubes should be referred to the interventionalists or other physicians who placed them. Chest tubes are typically sutured at the site of entry and securely bandaged to avoid migration but occasionally can be dislodged. This should prompt placement of another tube by an experienced operator. Last, chest tubes can be uncomfortable for patients who may require systemic analgesics. Additionally, tube positioning may ease some of the discomfort. Chest tubes are commonly placed along the midaxillary line and the posterior thorax, leading to discomfort in the recumbent position. Directing the tube anteriorly helps ease some of the discomfort. This can be done using all‐purpose sponges to build a barrier between the skin and the chest tube as it is directed anteriorly. Additional sponges are placed above the tube for extra protection. The gentle curve accomplished by padding the underside of the tube also keeps the tube patent by avoiding sharp kinks as the catheter exits the thorax.
FUTURE TRENDS
Future trends in the management of IP may include shorter duration of tube management (14 hours of suction with air leak evaluation and removal) and the use of even smaller catheters. Outpatient management of IP with small pigtail catheters or small‐caliber tubes in addition to 1‐way valves is also being investigated. The benefits of these practices may include greater patient comfort and lower cost. These approaches will need larger‐scale replication and careful patient selection before they become standard practice.[28, 36]
Ultrasound can be used to assess the presence and size of pneumothoraces that are difficult to visualize by standard chest radiographs. Several studies have established ultrasonography as an effective method of diagnosing pneumothorax and have shown it to have superior sensitivity compared with chest radiography.[1, 18] In the future, the use of ultrasound will likely be more widespread given its performance, portability, ease of use, and relatively low cost.
SUMMARY
IP is a known and costly complication of many medical procedures. The aforementioned algorithms help simplify the management of chest tubes for hospitalists caring for patients with this common complication. This stepwise approach may not only help curtail added expenses related to IPs by decreasing the length of inpatient stays but may also improve patient satisfaction.
Acknowledgments
Disclosure: Mayo does not endorse the products mentioned in this article. The authors report no conflicts of interest.
Box
Clinical Signs and Symptoms of Pneumothorax
Dyspnea
Pleuritic chest pain
Tachypnea
Hypoxia
Decreased breath sounds on affected side
Hyper‐resonant percussion on affected side
Subcutaneous emphysema
A pneumothorax is a collection of air in the space outside the lungs that is trapped within the thorax. This abnormality can occur spontaneously or as the result of trauma. Traumatic pneumothoraces include those resulting from medical interventions such as a transthoracic and transbronchial needle biopsy, central line placement, and positive‐pressure mechanical ventilation. This group is most accurately described as iatrogenic pneumothorax (IP).[1]
IP can be an expected complication of many routine thoracic procedures, but it can also occur accidentally during procedures near the lung or thoracic cavity. Some IPs may be asymptomatic and go undiagnosed, or their diagnosis may be delayed.[2] The majority of iatrogenic pneumothoraces will resolve without complications, and patients will not require medical attention. A small percentage can, however, expand and have the potential to develop into a tension pneumothorax causing severe respiratory distress and mediastinal shift.[3, 4]
The incidence of IP ranges from 0.11% with mechanical ventilation to 2.68% with thoracentesis, according to an analysis of 7.5 million uniform hospital discharge abstracts from 2000.5 A 2010 systematic review of 24 studies that included 6605 patients suggested a 6.0% incidence of pneumothorax following thoracentesis.[3] The highest risk of IP is seen with computed tomography (CT)‐guided lung biopsy, with 1 series of 1098 biopsies showing a 42% incidence; chest tube evacuation was required in 12% of these cases.[4] A Veterans Administration study of patient safety indicators from 2001 to 2004 found that risk‐adjusted rates of IP were increasing over time.[6] It is unclear whether this increase is due to increasing numbers of interventional procedures or to better rates of detection. IP poses a considerable cost to the medical system, with safety studies finding that patients with IP will stay in the hospital approximately 4 days longer and incur an additional $17,000 in charges.[7]
In addition to this financial burden, the lack of consistency in training and guidelines for management of pneumothorax is thought to add to chest tube‐related complications.[8] In 2001, the American College of Chest Physicians (ACCP) published guidelines for the management of spontaneous pneumothorax that do not specifically address IP.[9] In 2010, the British Thoracic Society (BTS) updated their guidelines and included a brief statement on IP that described a higher incidence for it than for spontaneous pneumothorax and noted its relative ease of management.[10] Despite the lack of specific guidelines dedicated to IPs, common clinical practice is to manage iatrogenic defects in a manner similar to that for spontaneous ones. However, studies have shown that the management of pneumothorax remains diverse and that the adherence to these published guidelines is suboptimal.[10, 11] The BTS guidelines favor needle aspiration as the first‐line treatment,[10] whereas the ACCP recommends drainage with catheters over aspiration.[9]
The possibility of this complication, along with the rising rate of invasive interventions being performed, has led to expanded surveillance criteria for IP. Surveillance imaging, clinical observation, or a combination of the 2 may be required, depending on the institution, the risk of the procedure, and the preference of the treating clinician. The algorithms presented here were designed in alignment with both major society guidelines and with the intention of simplifying the treatment regimen for the ease of adoption by hospitalists.
ETIOLOGY AND RISK FACTORS
The etiology and risk factors for IP are multiple, with the most common being interventional‐based procedures. In 535 Veterans Administration patients, the most common precursor procedures were transthoracic needle biopsy (24%), subclavian vein catheterization (22%), thoracentesis (20%), transbronchial biopsy (10%), pleural biopsy (8%), and positive pressure ventilation.[12] IP can also be a rare complication of pacemaker manipulations,[5] and less commonly, bronchoscopy.[13] Patient factors that increase the risk of pneumothorax in the setting of an intervention include age, chronic obstructive lung disease, primary lung cancer, malignant and parapneumonic pleural effusions, empyema, and chronic corticosteroid use.[4] As might be expected, patients with structural lung disease (eg, emphysema with bullae) and poor healing ability (eg, corticosteroid dependent), tend to have IPs more often and to require more complicated interventions for resolution.[14, 15] In some studies, operator experience seems to be inversely related to the rate of IP, and the use of ultrasound is correlated with lower rates of this complication.[1, 3]
PATIENT PRESENTATIONS AND DIAGNOSIS
Clinical signs and symptoms of a significant pneumothorax vary in severity but most often include dyspnea, tachypnea, chest pain, and pleurisy (see Box 1). Post procedure signs or symptoms require further evaluation with imaging, usually a plain chest radiograph. CT can be useful for further evaluation. Small anterior pneumothoraces may be difficult to detect without lateral radiographic imaging or computed tomogram. Ultrasound is being used more frequently at the bedside to make this diagnosis, and various studies of trauma patients have found that it has good sensitivity and specificity.[16, 17] These results have been validated by a recent meta‐analysis comparing ultrasound to chest radiographs for the detection of pneumothorax among trauma, critically ill, and postprocedural patients.[18] This study demonstrated superior sensitivity and similar specificity for ultrasound versus chest radiographs for detection of pneumothorax. More ominous signs, such as tachycardia or hypotension, can be indicative of tension pneumothorax, which requires emergent evacuation.
MANAGEMENT
Once the diagnosis of pneumothorax has been established, treatment options should be guided by defect size and clinical assessment following a defined treatment algorithm (Figure 1). As emphasized by the BTS and ACCP guidelines, we advocate considering the use of symptoms along with defect size to determine the best management course.

Observation
Defects that involve<20% of the hemithorax in a patient who is clinically asymptomatic and hemodynamically stable can be safely managed by oxygen supplementation and hospital observation. Repeat imaging can be obtained after 12 to 24 hours of defect detection or with symptom change. Patients who display resolution may be discharged home.
Patients who show persistence without progression but are asymptomatic may also be discharged safely, with follow‐up imaging and clinical evaluation 48 hours later.[9, 10] This was demonstrated by Kelly and colleagues,[19] who described the outcomes of 154 patients in a retrospective cohort study. Of the 91 patients treated with outpatient observation, 82 resolved without additional interventions. A recent review article by the same author cites conservative management of small pneumothoraces as being widely accepted.[20] If reimaging shows progression of defect or if the patient becomes more symptomatic, the pneumothorax should be evacuated by 1 of the methods described below.
Aspiration
Aspiration is defined by the ACCP Delphi consensus statement as the removal of pleural air via needle or cannula followed by immediate removal of needle or cannula.[9] This option mandates careful patient selection. It should be considered for small pneumothoraces that cause only mild dyspnea in patients who have no known parenchymal disease. These patients should be observed overnight in the hospital and reimaged 24 hours after aspiration of the pneumothorax. Several authors have reported success with aspiration alone. Yamagami et al.[21] noted the efficacy of manual aspiration immediately after CT‐guided biopsy, with a success rate exceeding 90%. They also noted that evacuated volumes >543 mL correlated with the need for further intervention with a chest tube. This technique is advocated for small pneumothoraces that are recognized shortly after the procedure.
Similarly, Delius and colleagues[22] managed 131 pneumothoraces with aspiration as an alternative to chest tube placement. Of these, 79 were iatrogenic. Aspiration achieved a 75% success rate for all IPs. Small defects defined as <20% of volume had an even higher resolution rate of 87%. Similar findings were demonstrated by Talbot‐Stern et al.[23] in their prospective study of 76 pneumothoraces. Among those that were iatrogenic, 82% resolved after simple aspiration. Faruqi et al.[24] also showed that aspiration is a viable option for IPs. Of the 57 patients with pneumothorax included in their study, 35 were treated with aspiration alone. Iatrogenesis was the culprit in 12 of the 35 manually aspirated cases. Aspiration achieved a success rate of 91.7% in IP. A recent Cochrane database systematic review compared simple aspiration with intercostal tube drainage for primary spontaneous pneumothorax.[25] The authors reported no difference between these methods in terms of success rate, early failure rate, duration of hospital stay, 1‐year success rate, or number of patients who required pleurodesis at 1‐year follow‐up.
Because the algorithms presented in this article were specifically designed for the use by hospitalists, we intentionally omitted aspiration from the decision trees. Most hospitalists would not be expected to evacuate IPs. However, knowledge regarding this option and appropriate follow‐up are valuable to internists, because many interventionalists admit patients to the hospital service for overnight observation. An asymptomatic postaspiration patient, who on subsequent imaging demonstrates resolution or persistence without progression of pneumothorax, may be discharged with 48‐hour follow‐up.
Placement of Catheter or Chest Tube Drainage
Most patients with a clinically significant pneumothorax will require evacuation of the air. Pneumothoraces larger than 20% or that produce symptoms warrant chest tube management and inpatient observation (Figure 1B). Traditionally, large tubes with 20 cm of water on continuous suction are used and have been studied the most widely. Several authors have shown that smaller tubes can effectively drain a pneumothorax.[26, 27, 28, 29] Small‐bore catheters (8F14F), which can be inserted percutaneously, have been shown to provide effective lung re‐expansion with minimal morbidity[8] and may be better tolerated by patients with uncomplicated pneumothoraces (Figure 2). Terzi and colleagues[30] have shown that smaller tubes cause less discomfort to patients at rest, with cough, and at the time of tube removal.

At most US institutions, catheters and chest tubes are connected to all‐purpose drainage systems. Although commercially available through a variety of manufacturers, they share similar design principles because they replicate the 3‐bottle system described in detail elsewhere in the literature.[31] We have limited our discussion to 3 pleural evacuation systems because it is our intention to familiarize hospitalists with the units that they are most likely to encounter. The first 2 systems have been studied and described by Baumann and colleagues[32] as being commonplace and reasonably reliable. These include the Oasis (Atrium Medical Corp., Hudson, NH) (Figure 3A) and the Pleur‐evac (Teleflex Inc., Limerick, PA). The third unit is the Thopaz digital thoracic drainage system (Medela Inc., McHenry, IL) (Figure 3B). The Thopaz is unique in its inclusion of a suction source and digital capability. Although it utilizes the same principles of all pleural evacuation devices, its setup and information output require that one be familiar with its digital format.

Suction Versus Water Seal
The chest tube should be placed initially to a suction pressure level of 20 cm of water for 24 hours to maximize lung expansion and evacuate all extrapulmonary air. Suction pressure is set on the Pleur‐evac and Atrium drainage systems by a manual dial that reads to a water pressure of 0 to 40 cm. The default setting from the manufacturer is 20 cm of water. This level of suction is present only when the drainage system is connected to a wall or a portable suction device. The only confirmation of suction presence in the Atrium system is the deployment of the orange bellows (located under the dial) to the level of the arrow tip (Figure 3A). The Pleur‐evac system has a red stripe along the circular edge of the dial that appears at the set level of suction when negative pressure is being applied. It is important to be aware that when patients are disconnected from the wall or the portable suction apparatus, they are on water seal or gravity. These terms are synonymous with no suction. On the Thopaz, a digital menu directs operation, and levels of suction can be selected from water seal (no suction) up to 40 cm of water. We recommend using suction to 20 cm of water given the scarce evidence supporting higher levels of negative pressure. Some clinicians prefer placing patients on water seal for some time before moving toward tube discontinuance, but this is a matter of preference, and no substantial evidence exists to show that any 1 method is superior.[8, 33]
Assessing for Air Leak
If there is improvement or resolution of the pneumothorax after 24 hours, the presence of an air leak should be assessed; if no leak is present, the chest tube can be safely removed. In the context of chest tubes, the term air leak refers to residual air between the lung and the chest wall. It is possible to see resolution of a pneumothorax on chest radiographs and still have an air leak. This situation is created by a perfect balance between the pleural air evacuation by the catheter and the flow of air exiting from the lung puncture. This would result in reaccumulation of the pneumothorax if the chest tube is removed prematurely. It should also be kept in mind that chest radiographs may miss a small pneumothorax given their relatively low sensitivity.[18] Therefore, the absence of an air leak needs to be documented before the chest tube is discontinued. Depending on the type of drainage system (Atrium, Pleur‐evac, or Thopaz), this assessment can be done in several ways. All systems can be assessed for air leak by clamping the actual chest tube for 2 to 4 hours and then repeating the chest radiograph. Clamping a chest tube simulates the condition of not having a chest tube. Chest tubes should never be clamped without supervision and only with the knowledge of nursing personnel. The onset of chest pain or dyspnea in a patient with a clamped tube mandates immediate removal of the clamp and a return to suction. A repeat chest radiograph showing reaccumulation or expansion of the pneumothorax after clamping indicates that the air leak has not resolved and the chest tube must remain in place and returned to suction. Simpler and more time‐efficient methods of detecting air leaks are available with both cardiothoracic drainage systems.
For the Atrium and Pleur‐evac models, there is a graded panel through which one can visualize air leaks being funneled through water (Figure 4A). Having the patient cough several times or perform a Valsalva maneuver should release any air trapped within the chest into this chamber, where bubbles can be visualized as they travel through the water. The presence of bubbles indicates the presence of residual air in the chest, pointing to a possible leak. In contrast, the Thopaz system offers a graphical display of the air flowing into the system that can be reviewed over the 24‐hour period. When the graph line reaches a 0 flatline graph, no airflow is being detected and no air leakage is suspected (Figure 4B). If no air leaks are detected, the chest tube may be discontinued. Those patients with a failed air leak test should have their chest tubes continued under suction for another 24 hours, with the above tests then repeated. The same holds true for those patients with persistent pneumothorax at 24 hours.

Removal of chest tubes is a simple process that requires the tube to be pulled out of the patient without allowing air to enter the site where the tube was present and where it entered the thorax. Most interventionalists will discontinue the tubes that they have placed. Some small catheters have an internal string that has to be released so the catheter will straighten and pull out easily. Knowledge of the type of catheter or tube that was placed is critical before removal to prevent complications and patient discomfort. Standard chest tubes are straight, smooth plastic and pull out easily but require rapid occlusion of the larger puncture site in the chest wall with an occlusive dressing that often includes petroleum or water‐soluble gel. Some physicians will leave a suture tie when placing the chest tube so that it can be tied down to occlude the site instead of using a dressing.
Consultation of the Cardiothoracic Surgeon or Interventional Pulmonologist
We recommend the involvement of cardiothoracic surgery or interventional pulmonology for patients with nonresolving pneumothorax lasting longer than 48 hours because additional procedures may be necessary. One of the rare but serious complications of a persistent pneumothorax is the formation of a bronchopleural fistula. This communication between the bronchial tree and the pleural space can lead to significant morbidity and mortality. The treatment of a bronchopleural fistula includes medical and surgical options that are beyond the scope of this article but require the expertise of a cardiothoracic surgeon or interventional pulmonologist.[34] Most patients who will not require additional procedures will heal within 48 hours.[11, 35] Decisions regarding more invasive treatment measures can then be made as necessary.[26]
PRACTICAL TIPS
Hospitalists caring for patients with chest tubes are often asked to troubleshoot at the bedside. Scenarios that may be encountered include nonfunctioning tubes, catheter migration, and tube discomfort. Ensuring patency of the tube entails visualizing the tube from the point of entry into the chest wall to the collection chamber and inspecting for kinks or debris clogging the tube. Smaller catheters can be easily kinked during patient positioning and can become clogged. Respiratory variation, which is the movement of the column of fluid in the collection chamber or in the tubing with inspiration and expiration, suggests that the chest tube is patent. This should be part of the daily examination in a patient with a chest tube, and it should also be the first step in assessing sudden dyspnea, hypoxia, pain, or hemodynamic instability. Clogged tubes should be referred to the interventionalists or other physicians who placed them. Chest tubes are typically sutured at the site of entry and securely bandaged to avoid migration but occasionally can be dislodged. This should prompt placement of another tube by an experienced operator. Last, chest tubes can be uncomfortable for patients who may require systemic analgesics. Additionally, tube positioning may ease some of the discomfort. Chest tubes are commonly placed along the midaxillary line and the posterior thorax, leading to discomfort in the recumbent position. Directing the tube anteriorly helps ease some of the discomfort. This can be done using all‐purpose sponges to build a barrier between the skin and the chest tube as it is directed anteriorly. Additional sponges are placed above the tube for extra protection. The gentle curve accomplished by padding the underside of the tube also keeps the tube patent by avoiding sharp kinks as the catheter exits the thorax.
FUTURE TRENDS
Future trends in the management of IP may include shorter duration of tube management (14 hours of suction with air leak evaluation and removal) and the use of even smaller catheters. Outpatient management of IP with small pigtail catheters or small‐caliber tubes in addition to 1‐way valves is also being investigated. The benefits of these practices may include greater patient comfort and lower cost. These approaches will need larger‐scale replication and careful patient selection before they become standard practice.[28, 36]
Ultrasound can be used to assess the presence and size of pneumothoraces that are difficult to visualize by standard chest radiographs. Several studies have established ultrasonography as an effective method of diagnosing pneumothorax and have shown it to have superior sensitivity compared with chest radiography.[1, 18] In the future, the use of ultrasound will likely be more widespread given its performance, portability, ease of use, and relatively low cost.
SUMMARY
IP is a known and costly complication of many medical procedures. The aforementioned algorithms help simplify the management of chest tubes for hospitalists caring for patients with this common complication. This stepwise approach may not only help curtail added expenses related to IPs by decreasing the length of inpatient stays but may also improve patient satisfaction.
Acknowledgments
Disclosure: Mayo does not endorse the products mentioned in this article. The authors report no conflicts of interest.
Box
Clinical Signs and Symptoms of Pneumothorax
Dyspnea
Pleuritic chest pain
Tachypnea
Hypoxia
Decreased breath sounds on affected side
Hyper‐resonant percussion on affected side
Subcutaneous emphysema
- , . Management of pneumothorax. Semin Respir Crit Care Med. 2010;31(6):769–780.
- , . Pneumothorax. Respirology. 2004;9(2):157–164.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy‐guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9‐year period. AJR Am J Roentgenol. 2010;194(3):809–814.
- , , . Accidental iatrogenic pneumothorax in hospitalized patients. Med Care. 2006;44(2):182–186.
- , , , et al. Tracking rates of patient safety indicators over time: lessons from the Veterans Administration. Med Care. 2006;44(9):850–861.
- , . Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290(14):1868–1874.
- , , , , . Preliminary report of a prospective, randomized trial of underwater seal for spontaneous and iatrogenic pneumothorax. J Am Coll Surg. 2007;204(1):84–90.
- , , , et al.; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119(2):590–602.
- , , ; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii18–ii31.
- , . The clinician's perspective on pneumothorax management. Chest. 1997;112(3):822–828.
- , , , . Iatrogenic pneumothorax: etiology and morbidity: results of a Department of Veterans Affairs Cooperative Study. Respiration. 1992;59(4):215–220.
- , , , , , . Severe complications of bronchoscopy. Respiration. 2008;76(4):429–433.
- , , , et al. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest. 2004;126(5):1516–1521.
- , , , , , . Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15(5):479–483.
- , . Ultrasound detection of pneumothorax compared with chest X‐ray and computed tomography scan. Am Surg. 2011;77(4):480–484.
- , . Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010;17(1):11–17.
- , , , , . Diagnosis of pneumothorax by radiography and ultrasonography: a meta‐analysis. Chest. 2011;140(4):859–866.
- , , . Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest. 2008;134(5):1033–1036.
- . Review of management of primary spontaneous pneumothorax: is the best evidence clearer 15 years on? Emerg Med Australas. 2007;19(4):303–308.
- , , , , , . Efficacy of manual aspiration immediately after complicated pneumothorax in CT‐guided lung biopsy. J Vasc Interv Radiol. 2005;16(4):477–483.
- , , , , , . Catheter aspiration for simple pneumothorax: experience with 114 patients. Arch Surg. 1998;124(7):833–836.
- , , , , . Catheter aspiration for simple pneumothorax. J Emerg Med. 1986;4(6):437–442.
- , , , . Role of simple needle aspiration in the management of pneumothorax. Indian J Chest Dis Allied Sci. 2004;46(3):183–190.
- , , . Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev. 2007;(1):CD004479.
- , , , . Evaluation of conventional chest tube therapy for iatrogenic pneumothorax. Chest. 1993;104(6):1770–1772.
- , , , . Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology. 1997;205(1):249–252.
- , , , , , . Outpatient management of postbiopsy pneumothorax with small‐caliber chest tubes: factors affecting the need for prolonged drainage and additional interventions. Cardiovasc Intervent Radiol. 2008;31(2):342–348.
- , . Management of primary and secondary pneumothorax using a small‐bore thoracic catheter. Interact Cardiovasc Thorac Surg. 2010;11(2):146–149.
- , , , et al. The use of flexible spiral drains after non‐cardiac thoracic surgery: a clinical study. Eur J Cardiothorac Surg. 2005;27(1):134–137.
- . Pleural Diseases. 5th ed. Philadelphia: PA: Lippincott Williams 2007.
- , , , . Comparison of function of commercially available pleural drainage units and catheters. Chest. 2003;123(6):1878–1886.
- , . Catheter drainage of spontaneous pneumothorax: suction or no suction, early or late removal? Thorax. 1982;37(1):46–48.
- , . Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128(6):3955–3965.
- , , , . Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg. 1991;126(6):764–766.
- , , , , . A treatment algorithm for pneumothoraces complicating central venous catheter insertion. Am J Surg. 2000;180(6):523–526.
- , . Management of pneumothorax. Semin Respir Crit Care Med. 2010;31(6):769–780.
- , . Pneumothorax. Respirology. 2004;9(2):157–164.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy‐guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9‐year period. AJR Am J Roentgenol. 2010;194(3):809–814.
- , , . Accidental iatrogenic pneumothorax in hospitalized patients. Med Care. 2006;44(2):182–186.
- , , , et al. Tracking rates of patient safety indicators over time: lessons from the Veterans Administration. Med Care. 2006;44(9):850–861.
- , . Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290(14):1868–1874.
- , , , , . Preliminary report of a prospective, randomized trial of underwater seal for spontaneous and iatrogenic pneumothorax. J Am Coll Surg. 2007;204(1):84–90.
- , , , et al.; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119(2):590–602.
- , , ; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii18–ii31.
- , . The clinician's perspective on pneumothorax management. Chest. 1997;112(3):822–828.
- , , , . Iatrogenic pneumothorax: etiology and morbidity: results of a Department of Veterans Affairs Cooperative Study. Respiration. 1992;59(4):215–220.
- , , , , , . Severe complications of bronchoscopy. Respiration. 2008;76(4):429–433.
- , , , et al. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest. 2004;126(5):1516–1521.
- , , , , , . Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15(5):479–483.
- , . Ultrasound detection of pneumothorax compared with chest X‐ray and computed tomography scan. Am Surg. 2011;77(4):480–484.
- , . Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010;17(1):11–17.
- , , , , . Diagnosis of pneumothorax by radiography and ultrasonography: a meta‐analysis. Chest. 2011;140(4):859–866.
- , , . Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest. 2008;134(5):1033–1036.
- . Review of management of primary spontaneous pneumothorax: is the best evidence clearer 15 years on? Emerg Med Australas. 2007;19(4):303–308.
- , , , , , . Efficacy of manual aspiration immediately after complicated pneumothorax in CT‐guided lung biopsy. J Vasc Interv Radiol. 2005;16(4):477–483.
- , , , , , . Catheter aspiration for simple pneumothorax: experience with 114 patients. Arch Surg. 1998;124(7):833–836.
- , , , , . Catheter aspiration for simple pneumothorax. J Emerg Med. 1986;4(6):437–442.
- , , , . Role of simple needle aspiration in the management of pneumothorax. Indian J Chest Dis Allied Sci. 2004;46(3):183–190.
- , , . Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev. 2007;(1):CD004479.
- , , , . Evaluation of conventional chest tube therapy for iatrogenic pneumothorax. Chest. 1993;104(6):1770–1772.
- , , , . Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology. 1997;205(1):249–252.
- , , , , , . Outpatient management of postbiopsy pneumothorax with small‐caliber chest tubes: factors affecting the need for prolonged drainage and additional interventions. Cardiovasc Intervent Radiol. 2008;31(2):342–348.
- , . Management of primary and secondary pneumothorax using a small‐bore thoracic catheter. Interact Cardiovasc Thorac Surg. 2010;11(2):146–149.
- , , , et al. The use of flexible spiral drains after non‐cardiac thoracic surgery: a clinical study. Eur J Cardiothorac Surg. 2005;27(1):134–137.
- . Pleural Diseases. 5th ed. Philadelphia: PA: Lippincott Williams 2007.
- , , , . Comparison of function of commercially available pleural drainage units and catheters. Chest. 2003;123(6):1878–1886.
- , . Catheter drainage of spontaneous pneumothorax: suction or no suction, early or late removal? Thorax. 1982;37(1):46–48.
- , . Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128(6):3955–3965.
- , , , . Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg. 1991;126(6):764–766.
- , , , , . A treatment algorithm for pneumothoraces complicating central venous catheter insertion. Am J Surg. 2000;180(6):523–526.
Esophageal Perforation, Complication of EGD
Esophagogastroduodenoscopy (EGD) carries a small but serious risk of esophageal perforation.13 With its potential for sepsis and fatal mediastinitis, prompt recognition and treatment are essential for favorable outcomes. The risk of perforation with diagnostic flexible EGD is 0.03%, which is an improvement from the 0.1%0.4% risk associated with rigid endoscopy.4 However, the risk of perforation can dramatically increase to 17% depending on the methods of therapeutic intervention and underlying risk factors (Table 1).1, 57
| Level of operator experience |
| Underlying esophageal disease |
| Zenker's diverticulum |
| Eosinophilic esophagitis |
| Esophageal or mediastinal irradiation |
| Esophageal malignancy |
| Esophageal strictures |
| Systemic disease |
| Anterior cervical osteophytes |
| Advanced liver cirrhosis |
| Diabetes mellitus |
| Scleroderma |
| Complexity of intervention |
| Esophageal stent placement |
| Pneumatic dilation |
| Other |
| Heavy sedation |
| Advanced age |
It is estimated that 33%75% of all esophageal perforations are iatrogenic.8 Of those caused by EGD, therapeutic interventions portend an increased risk compared with the risk of diagnostic endoscopy alone (Table 2).4 With the expanding role of flexible EGD and the increasing number of procedures performed, this modest risk per procedure still translates into a sizable number of perforations with their ensuing complications.4, 7 Mortality rates following esophageal perforation may approach 25%.9
| Endoscopic procedure | Esophageal perforation risk |
|---|---|
| Diagnostic | 0.03% |
| Dilation | 0.25% (normal esophagus)4%7% (achalasia)*7% (gastric outlet obstruction)*17% (strictures due to caustic agent) |
| Thermal method (treatment of malignancy) | 10% |
| Endoprosthesis | 3% |
| Variceal sclerotherapy | 1%5% (acute perforation)2%5% (delayed perforation) |
| Band ligation | 0.7% (perforation) |
| Nonvariceal hemostasis (use of sclerosant or cautery) | 0%2% (first hemostasis)4% (hemostasis repeated within 2448 hours) |
ANATOMY AND PATHOPHYSIOLOGY
The most common site of perforation is at the level of the cricopharyngeus, as it is a narrow introitus leading to the esophagus. The risk of perforation at this location is further increased with the presence of a Zenker's diverticulum or cervical osteophytes. The second most common site is proximal to the lower esophageal sphincter because of the angulation of the hiatus and the high frequency of esophageal webs, rings, reflux strictures, and hiatal hernias. The relatively straight middle esophagus is an uncommon site for perforations.
Cervical perforations are less commonly caused by organic lesions of the esophagus. Often, they are the result of technique and manipulation of the endoscope, or of certain conditions associated with the jaw, neck, or spinal column that are unfavorable for endoscopy. The risk of cervical perforation increases with the presence of bony spurs, as the upper esophagus is compressed over the underlying spinal column. Thoracic perforations, however, are more commonly seen with organic esophageal obstruction. These obstructions may be caused by an underlying inflammatory process, benign stricture, or neoplasm. In these cases, the risk of thoracic perforation is increased with blind procedures. Thoracic perforations carry a worse prognosis if diagnosis is delayed, or if the underlying obstruction cannot be removed.10
Esophageal perforation leads to periesophageal tissues being contaminated by food, secretions, air, or gastric contents and may be followed by chemical tissue injury and infection. The nature and extent of infection depend on the site of esophageal perforation. Cervical esophageal perforation can cause retropharyngeal space infection, which has the potential to extend directly into the posterior mediastinum via the danger space, which is between the retropharyngeal and prevertebral spaces and extends from the base of the skull descending freely throughout the entire length of the posterior mediastinum. With thoracic perforations, esophageal contents can enter the pleural space by negative intrathoracic pressure with subsequent pleural contamination and empyema.8, 1113
Pathogens responsible for infections after esophageal perforation vary based on several factors including site of perforation, clinical status of patient when perforation occurs (hospitalized versus not hospitalized, critically ill versus healthy), receipt of enteral nutrition, gastric acid suppression with H2‐receptor antagonists or proton‐pump inhibitors, immunosuppression, and recent (or current) receipt of antimicrobials. In nonintubated, healthy adults not on antimicrobial therapy, organisms in the upper esophagus are essentially identical to those in the oropharynx and include viridans streptococci, Haemophilus species, and anaerobes. During critical illness and following antibiotic therapy, the normal oral flora is rapidly replaced by aerobic Gram‐negative bacilli, Staphylococcus aureus, and yeast.14 The stomach, which is normally devoid of bacteria, can likewise be colonized with pathogenic organisms in the setting of gastric acid suppression and enteral nutrition.15, 16
SIGNS AND SYMPTOMS
Esophageal perforation should be considered after EGD, dilation, sclerotherapy, variceal banding, and esophageal stenting. However, perforation can also result from other invasive procedures such as insertion of feeding and nasogastric tubes, rapid sequence intubation, and transesophageal echocardiography.
The clinical triad of esophageal perforation includes pain, fever, and subcutaneous air.17 In a study by Wychulis et al., among 33 patients with esophageal perforation, 75% demonstrated all 3 findings.10 Pain is the most sensitive finding and occurs in nearly all patients identified with esophageal perforation. Crepitation, which results from air dissecting along soft tissue planes of the mediastinum and into the neck, occurs in up to 70% with cervical perforation and 30% with thoracic perforation.8, 10, 18
Clinical presentation and outcomes vary depending on the location of the perforation (Table 3).8 Cervical perforation is usually associated with anterior neck pain, located at the anterior border of the sternocleidomastoid muscle. Movement of the neck and palpation typically aggravate the pain. Thoracic perforation typically presents as substernal chest pain, often with a component of pleurisy. Pleural effusions are present in 50% of thoracic perforations, and mediastinitis is more likely to occur.19 Hamman's sign, a finding characterized by an audible crunch with chest auscultation, is suggestive of mediastinal emphysema. Perforation of the intra‐abdominal esophagus can result in epigastric pain and signs of acute abdomen.10, 17 Subcutaneous emphysema occurs more frequently with cervical perforation but can be present regardless of location.10 Secondary infections following esophageal perforation can manifest with an accelerated clinical course leading to sepsis and shock.
| Location of perforation | Symptom | Sign* |
|---|---|---|
| ||
| Cervical esophagus | Muscle spasm Dysphonia Hoarseness Dysphagia | Anterior neck tendernessTenderness on cervical motionSubcutaneous emphysema |
| Thoracic esophagus | Substernal chest pain Dysphagia Odynophagia | Cyanosis, Dyspnea Hamman's sign Pleural effusion Subcutaneous emphysema |
| Intraabdominal esophagus | Epigastric pain | Acute abdomenSubcutaneous emphysema |
DIAGNOSIS
Clinical suspicion of esophageal perforation should prompt necessary radiographic studies to establish the diagnosis.18, 20 Contrast‐enhanced computed tomography (CT) scans of the neck and chest are preferable because of their increased sensitivity in localizing the site and showing the extent of perforation and abscess. CT scans may reveal subcutaneous or mediastinal air, abscess cavities adjacent to the esophagus, and fistulas between the esophagus and mediastinum (Figs. 1 and 2).2022 Results of contrast studies may be negative and warrant repeating within several hours.19

If CT scans cannot be performed, neck (soft‐tissue) and chest x‐rays may be useful. Although plain films have limited value in evaluating the retropharyngeal space, they can reveal soft‐tissue emphysema, a widened mediastinum, pulmonary infiltrates or effusions, neck abscess, and mediastinal air‐fluid levels. In cervical perforation, a lateral film of the neck can show air in deep cervical tissue before clinical signs are apparent.
Swallow studies with Gastrografin (meglumine diatrizoate) are useful in defining the exact location of the perforation (Fig. 3). However, the false‐negative rate of swallow studies can exceed 10%, especially if the patient is upright during the study. When the contrast propagates past the site of perforation too quickly, it may not extravasate.23 Although barium may provide slightly greater contrast, it may add to the problem of foreign body reaction in the area of perforation.18 An additional complication of barium is that once it has extravasated, it is not readily absorbed. The persistence of extravasated barium makes it difficult to assess the resolution of an esophageal tear on subsequent fluoroscopic or CT exams. Hence, our institution avoids using barium to evaluate esophageal perforation, unless Gastrografin swallow has excluded any major esophageal perforation. Barium swallow may then be used to exclude small mural tears. Some medical centers elect to routinely screen their high‐risk patients with swallow evaluations after an EGD, although this is not common practice.8, 24
If the above workup is negative, the use of EGD may be considered for establishing the diagnosis if a high index of suspicion remains. However, the risks of EGD in this situation include extension of the perforation, further extravasation of esophageal contents, and difficulty with subsequent radiographic studies to visualize the perforation.19
MANAGEMENT
Once the diagnosis of esophageal perforation has been established, treatment options are individualized based on the clinical scenario. Currently, there are no established guidelines, and large randomized clinical trials comparing outcomes of operative versus nonoperative management have not been conducted (Fig. 4).25, 26 Outcomes associated with esophageal perforations depend on preoperative clinical condition, comorbidities, location and size of the perforation, nature of underlying esophageal disease (if any), and time to establish the diagnosis and initiate therapy.10 Delay in patient presentation or diagnosis beyond 24 hours following esophageal perforation has been associated with adverse outcomes.18, 27, 28
A conservative approach is appropriate when clinically stable patients with minimal symptoms have well‐contained, nontransmural tears. Management entails broad‐spectrum antibiotics, nothing by mouth, nasogastric suction, and parenteral nutrition.24 Early surgical consultation is recommended in all cases. Serial CT scanning is useful for following the resolution of fistulas and tears. An oral diet can be resumed when contrast or swallow studies show no extravasation of dye. Cervical perforations typically fare well with this approach.26, 29
Surgical therapy is recommended for patients with large or uncontained esophageal perforations, mediastinal abscesses, and/or sepsis.25, 27 Surgical options include esophageal diversion, esophagectomy, or drainage with or without primary repair. Drainage with primary repair is considered the treatment of choice, regardless of time to presentation. Esophagectomy is considered in cases of delayed or neglected perforations, extensive transmural necrosis or underlying cancer.30 Operative mortality is 0%30% when treated within 24 hours. This rate increases to 26%64% when treatment is delayed beyond 24 hours, reaffirming the importance of making a prompt diagnosis.8
Endoscopic intervention is gaining recognition for its role in the management of esophageal perforations, especially when the risks make surgery prohibitive. Therapeutic options include stenting and clipping a perforation, as well as debriding and draining an abscess. Endoscopists can successfully treat traumatic nonmalignant esophageal perforations smaller than 50% to 70% of the circumference with self‐expanding plastic stents.26 Another option is to use metallic clipping devices to treat small esophageal perforations (<1 cm).3133 Combined with medical management and appropriate patient selection, the benefits of an endoscopic approach may potentially outweigh the risks of surgery.26, 29, 33, 34
Regardless of treatment approach, the appropriate and timely selection of empiric antibiotic therapy improves outcomes. Empiric antimicrobial therapy for esophageal perforation will depend on several host factors as well as the site of perforation. In healthy nonhospitalized adults, ampicillin‐sulbactam, clindamycin, and penicillin G plus metronidazole are good choices because of their excellent activity against oral microflora. In patients who are critically ill, are hospitalized, are immunosuppressed, or have gastric acid suppression, initial broad‐spectrum antimicrobials such as piperacillin‐tazobactam, imipenem, meropenem, or a third‐generation cephalosporin plus metronidazole (or clindamycin) should be initiated. Additional therapy against methicillin‐resistant Staphylococcus aureus or Candida sp. should be considered if the patient is critically ill or is known to be colonized with these organisms. Initial empiric therapy should be modified as necessary based on culture results. Total duration of therapy will vary based on location and magnitude of the infection, adjunctive surgical debridement, and pathogens involved.
SUMMARY
Despite being an extremely safe procedure, EGD carries a known serious risk of esophageal perforation. Mortality after esophageal perforation can approach 25%. Although diagnostic endoscopy has a perforation rate of less than 0.03%, the risk can approach 17% with therapeutic interventions such as stent placement and esophageal dilation. Factors influencing the risks of perforation include procedural complexity, operator experience, and underlying esophageal and systemic diseases. Furthermore, perforations complicated by infection can lead to fatal mediastinitis and sepsis. The clinical triad of esophageal perforation is fever, neck pain, and crepitus. The optimal diagnostic study is CT scan of the neck and thorax with water‐soluble oral contrast. Treatment options range from conservative management with broad‐spectrum antibiotics to surgery. Diagnosis of esophageal perforation within 24 hours is essential for favorable outcomes.
- .Complications of endoscopic gastrointestinal dilation techniques.Gastrointest Endosc Clin N Am.1996;6:323–341.
- .Complications of upper gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.1996;6:287–303.
- ,.Complications of upper gastrointestinal endoscopy and their management.Gastrointest Endosc Clin N Am.1994;4:551–570.
- ,,,,.Treatment of endoscopic esophageal perforation.Surg Endosc.1999;13:962–966.
- ,,,.Unsedated small‐caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study [see comment].Gastroenterology.1999;117:1301–1307.
- ,,,,.Complications associated with esophagogastroduodenoscopy and with esophageal dilation.Gastrointest Endosc.1976;23(1):16–19.
- ,,.Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures.Gastrointest Endosc.2000;51(4 Pt 1):460–462.
- ,.Esophageal emergencies: things that will wake you from a sound sleep.Gastroenterol Clin N Am.2003;32:1035–1052.
- ,,, et al.Complications of upper GI endoscopy.Gastrointest Endosc.2002;55:784–793.
- ,,.Instrumental perforations of the esophagus.Dis Chest.1969;55(3):184–189.
- ,Complications of esophageal dilation and guidelines for their prevention.Gastrointest Endosc.1981;27:229–234.
- .Infectious complications associated with gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.2000;10:215–232.
- ,,,The occurrence of bacteremia after esophageal dilation.Gastrointestinal Endoscopy1975;22(2):86–87.
- ,,,,,.The pathogenesis of ventilator‐associated pneumonia: its relevance to developing effective strategies for prevention.Respir Care.2005;50:725–739; discussion39–41.
- ,,, et al.Stomach as a source of colonization of the respiratory tract during mechanical ventilation: association with ventilator‐associated pneumonia.Eur Respir J.1996;9:1729–1735.
- ,,, et al.Gastric microbiota in elderly patients fed via nasogastric tubes for prolonged periods.J Hosp Infect.2006;63(1):79–83.
- ,.Retroesophageal abscess twenty‐five days after esophagoscopy. An unusual complication of endoscopy.Gastrointest Endosc.1972;18:167–168.
- ,,.The radiologist in prevention and diagnosis of instrumental perforation of the esophagus.South Med J.1974;67:830–836.
- ,,.Short‐ and long‐term outcome of esophageal perforation.Gastrointest Endosc.1995;41(2):130–134.
- ,,,.The diagnosis and treatment of esophageal perforations resulting from nonmalignant causes.Surg Today.1997;27:793–800.
- ,.Radiology of the retropharyngeal space.Clin Radiol.2000;55:740–748.
- ,,.Diagnosis and management decisions in infections of the deep fascial spaces of the head and neck utilizing computerized tomography.Laryngoscope.1982;92(6, Pt. 1):630–633.
- .Perforation of the esophagus.Ann Thorac Surg.1986;42:231–232.
- ,,, et al.Successfully treated case of cervical abscess and mediastinitis due to esophageal perforation after gastrointestinal endoscopy.Dis Esophagus.2002;15:250–252.
- ,.The spectrum of spontaneous and iatrogenic esophageal injury: perforations, Mallory‐Weiss tears, and hematomas.J Clin Gastroenterol.1999;29:306–317.
- .Treatment of esophageal perforations and anastomotic leaks: the endoscopist is stepping into the arena.Gastrointest Endosc.2005;61:897–900.
- ,,.Operative and nonoperative management of esophageal perforations.Ann Surg.1981;194(1):57–63.
- ,,.Current results of therapy for esophageal perforation.Am J Surg.1995;169:615–617.
- ,,.Successful endoscopic management of a cervical pharyngeal perforation and mediastinal abscess.Gastrointest Endosc.2005;61(1):158–160.
- ,,,,.Thoracic esophageal perforations: a decade of experience.[see comment].Ann Thorac Surg.2003;75:1071–1074.
- ,.Perforation: part and parcel of endoscopic resection? [comment].Gastrointest Endosc.2006;63:602–605.
- ,,,.Endoscopic clip application as an adjunct to closure of mature esophageal perforation with fistulae.Clin Gastroenterol Hepatol.2003;1(1):44–50.
- ,,.Endoscopic clipping of esophageal perforation after pneumatic dilation for achalasia.Endoscopy.1995;27:608–611.
- ,,, et al.Esophageal dilation.Gastrointest Endosc.2006;63:755–760.
Esophagogastroduodenoscopy (EGD) carries a small but serious risk of esophageal perforation.13 With its potential for sepsis and fatal mediastinitis, prompt recognition and treatment are essential for favorable outcomes. The risk of perforation with diagnostic flexible EGD is 0.03%, which is an improvement from the 0.1%0.4% risk associated with rigid endoscopy.4 However, the risk of perforation can dramatically increase to 17% depending on the methods of therapeutic intervention and underlying risk factors (Table 1).1, 57
| Level of operator experience |
| Underlying esophageal disease |
| Zenker's diverticulum |
| Eosinophilic esophagitis |
| Esophageal or mediastinal irradiation |
| Esophageal malignancy |
| Esophageal strictures |
| Systemic disease |
| Anterior cervical osteophytes |
| Advanced liver cirrhosis |
| Diabetes mellitus |
| Scleroderma |
| Complexity of intervention |
| Esophageal stent placement |
| Pneumatic dilation |
| Other |
| Heavy sedation |
| Advanced age |
It is estimated that 33%75% of all esophageal perforations are iatrogenic.8 Of those caused by EGD, therapeutic interventions portend an increased risk compared with the risk of diagnostic endoscopy alone (Table 2).4 With the expanding role of flexible EGD and the increasing number of procedures performed, this modest risk per procedure still translates into a sizable number of perforations with their ensuing complications.4, 7 Mortality rates following esophageal perforation may approach 25%.9
| Endoscopic procedure | Esophageal perforation risk |
|---|---|
| Diagnostic | 0.03% |
| Dilation | 0.25% (normal esophagus)4%7% (achalasia)*7% (gastric outlet obstruction)*17% (strictures due to caustic agent) |
| Thermal method (treatment of malignancy) | 10% |
| Endoprosthesis | 3% |
| Variceal sclerotherapy | 1%5% (acute perforation)2%5% (delayed perforation) |
| Band ligation | 0.7% (perforation) |
| Nonvariceal hemostasis (use of sclerosant or cautery) | 0%2% (first hemostasis)4% (hemostasis repeated within 2448 hours) |
ANATOMY AND PATHOPHYSIOLOGY
The most common site of perforation is at the level of the cricopharyngeus, as it is a narrow introitus leading to the esophagus. The risk of perforation at this location is further increased with the presence of a Zenker's diverticulum or cervical osteophytes. The second most common site is proximal to the lower esophageal sphincter because of the angulation of the hiatus and the high frequency of esophageal webs, rings, reflux strictures, and hiatal hernias. The relatively straight middle esophagus is an uncommon site for perforations.
Cervical perforations are less commonly caused by organic lesions of the esophagus. Often, they are the result of technique and manipulation of the endoscope, or of certain conditions associated with the jaw, neck, or spinal column that are unfavorable for endoscopy. The risk of cervical perforation increases with the presence of bony spurs, as the upper esophagus is compressed over the underlying spinal column. Thoracic perforations, however, are more commonly seen with organic esophageal obstruction. These obstructions may be caused by an underlying inflammatory process, benign stricture, or neoplasm. In these cases, the risk of thoracic perforation is increased with blind procedures. Thoracic perforations carry a worse prognosis if diagnosis is delayed, or if the underlying obstruction cannot be removed.10
Esophageal perforation leads to periesophageal tissues being contaminated by food, secretions, air, or gastric contents and may be followed by chemical tissue injury and infection. The nature and extent of infection depend on the site of esophageal perforation. Cervical esophageal perforation can cause retropharyngeal space infection, which has the potential to extend directly into the posterior mediastinum via the danger space, which is between the retropharyngeal and prevertebral spaces and extends from the base of the skull descending freely throughout the entire length of the posterior mediastinum. With thoracic perforations, esophageal contents can enter the pleural space by negative intrathoracic pressure with subsequent pleural contamination and empyema.8, 1113
Pathogens responsible for infections after esophageal perforation vary based on several factors including site of perforation, clinical status of patient when perforation occurs (hospitalized versus not hospitalized, critically ill versus healthy), receipt of enteral nutrition, gastric acid suppression with H2‐receptor antagonists or proton‐pump inhibitors, immunosuppression, and recent (or current) receipt of antimicrobials. In nonintubated, healthy adults not on antimicrobial therapy, organisms in the upper esophagus are essentially identical to those in the oropharynx and include viridans streptococci, Haemophilus species, and anaerobes. During critical illness and following antibiotic therapy, the normal oral flora is rapidly replaced by aerobic Gram‐negative bacilli, Staphylococcus aureus, and yeast.14 The stomach, which is normally devoid of bacteria, can likewise be colonized with pathogenic organisms in the setting of gastric acid suppression and enteral nutrition.15, 16
SIGNS AND SYMPTOMS
Esophageal perforation should be considered after EGD, dilation, sclerotherapy, variceal banding, and esophageal stenting. However, perforation can also result from other invasive procedures such as insertion of feeding and nasogastric tubes, rapid sequence intubation, and transesophageal echocardiography.
The clinical triad of esophageal perforation includes pain, fever, and subcutaneous air.17 In a study by Wychulis et al., among 33 patients with esophageal perforation, 75% demonstrated all 3 findings.10 Pain is the most sensitive finding and occurs in nearly all patients identified with esophageal perforation. Crepitation, which results from air dissecting along soft tissue planes of the mediastinum and into the neck, occurs in up to 70% with cervical perforation and 30% with thoracic perforation.8, 10, 18
Clinical presentation and outcomes vary depending on the location of the perforation (Table 3).8 Cervical perforation is usually associated with anterior neck pain, located at the anterior border of the sternocleidomastoid muscle. Movement of the neck and palpation typically aggravate the pain. Thoracic perforation typically presents as substernal chest pain, often with a component of pleurisy. Pleural effusions are present in 50% of thoracic perforations, and mediastinitis is more likely to occur.19 Hamman's sign, a finding characterized by an audible crunch with chest auscultation, is suggestive of mediastinal emphysema. Perforation of the intra‐abdominal esophagus can result in epigastric pain and signs of acute abdomen.10, 17 Subcutaneous emphysema occurs more frequently with cervical perforation but can be present regardless of location.10 Secondary infections following esophageal perforation can manifest with an accelerated clinical course leading to sepsis and shock.
| Location of perforation | Symptom | Sign* |
|---|---|---|
| ||
| Cervical esophagus | Muscle spasm Dysphonia Hoarseness Dysphagia | Anterior neck tendernessTenderness on cervical motionSubcutaneous emphysema |
| Thoracic esophagus | Substernal chest pain Dysphagia Odynophagia | Cyanosis, Dyspnea Hamman's sign Pleural effusion Subcutaneous emphysema |
| Intraabdominal esophagus | Epigastric pain | Acute abdomenSubcutaneous emphysema |
DIAGNOSIS
Clinical suspicion of esophageal perforation should prompt necessary radiographic studies to establish the diagnosis.18, 20 Contrast‐enhanced computed tomography (CT) scans of the neck and chest are preferable because of their increased sensitivity in localizing the site and showing the extent of perforation and abscess. CT scans may reveal subcutaneous or mediastinal air, abscess cavities adjacent to the esophagus, and fistulas between the esophagus and mediastinum (Figs. 1 and 2).2022 Results of contrast studies may be negative and warrant repeating within several hours.19


If CT scans cannot be performed, neck (soft‐tissue) and chest x‐rays may be useful. Although plain films have limited value in evaluating the retropharyngeal space, they can reveal soft‐tissue emphysema, a widened mediastinum, pulmonary infiltrates or effusions, neck abscess, and mediastinal air‐fluid levels. In cervical perforation, a lateral film of the neck can show air in deep cervical tissue before clinical signs are apparent.
Swallow studies with Gastrografin (meglumine diatrizoate) are useful in defining the exact location of the perforation (Fig. 3). However, the false‐negative rate of swallow studies can exceed 10%, especially if the patient is upright during the study. When the contrast propagates past the site of perforation too quickly, it may not extravasate.23 Although barium may provide slightly greater contrast, it may add to the problem of foreign body reaction in the area of perforation.18 An additional complication of barium is that once it has extravasated, it is not readily absorbed. The persistence of extravasated barium makes it difficult to assess the resolution of an esophageal tear on subsequent fluoroscopic or CT exams. Hence, our institution avoids using barium to evaluate esophageal perforation, unless Gastrografin swallow has excluded any major esophageal perforation. Barium swallow may then be used to exclude small mural tears. Some medical centers elect to routinely screen their high‐risk patients with swallow evaluations after an EGD, although this is not common practice.8, 24

If the above workup is negative, the use of EGD may be considered for establishing the diagnosis if a high index of suspicion remains. However, the risks of EGD in this situation include extension of the perforation, further extravasation of esophageal contents, and difficulty with subsequent radiographic studies to visualize the perforation.19
MANAGEMENT
Once the diagnosis of esophageal perforation has been established, treatment options are individualized based on the clinical scenario. Currently, there are no established guidelines, and large randomized clinical trials comparing outcomes of operative versus nonoperative management have not been conducted (Fig. 4).25, 26 Outcomes associated with esophageal perforations depend on preoperative clinical condition, comorbidities, location and size of the perforation, nature of underlying esophageal disease (if any), and time to establish the diagnosis and initiate therapy.10 Delay in patient presentation or diagnosis beyond 24 hours following esophageal perforation has been associated with adverse outcomes.18, 27, 28

A conservative approach is appropriate when clinically stable patients with minimal symptoms have well‐contained, nontransmural tears. Management entails broad‐spectrum antibiotics, nothing by mouth, nasogastric suction, and parenteral nutrition.24 Early surgical consultation is recommended in all cases. Serial CT scanning is useful for following the resolution of fistulas and tears. An oral diet can be resumed when contrast or swallow studies show no extravasation of dye. Cervical perforations typically fare well with this approach.26, 29
Surgical therapy is recommended for patients with large or uncontained esophageal perforations, mediastinal abscesses, and/or sepsis.25, 27 Surgical options include esophageal diversion, esophagectomy, or drainage with or without primary repair. Drainage with primary repair is considered the treatment of choice, regardless of time to presentation. Esophagectomy is considered in cases of delayed or neglected perforations, extensive transmural necrosis or underlying cancer.30 Operative mortality is 0%30% when treated within 24 hours. This rate increases to 26%64% when treatment is delayed beyond 24 hours, reaffirming the importance of making a prompt diagnosis.8
Endoscopic intervention is gaining recognition for its role in the management of esophageal perforations, especially when the risks make surgery prohibitive. Therapeutic options include stenting and clipping a perforation, as well as debriding and draining an abscess. Endoscopists can successfully treat traumatic nonmalignant esophageal perforations smaller than 50% to 70% of the circumference with self‐expanding plastic stents.26 Another option is to use metallic clipping devices to treat small esophageal perforations (<1 cm).3133 Combined with medical management and appropriate patient selection, the benefits of an endoscopic approach may potentially outweigh the risks of surgery.26, 29, 33, 34
Regardless of treatment approach, the appropriate and timely selection of empiric antibiotic therapy improves outcomes. Empiric antimicrobial therapy for esophageal perforation will depend on several host factors as well as the site of perforation. In healthy nonhospitalized adults, ampicillin‐sulbactam, clindamycin, and penicillin G plus metronidazole are good choices because of their excellent activity against oral microflora. In patients who are critically ill, are hospitalized, are immunosuppressed, or have gastric acid suppression, initial broad‐spectrum antimicrobials such as piperacillin‐tazobactam, imipenem, meropenem, or a third‐generation cephalosporin plus metronidazole (or clindamycin) should be initiated. Additional therapy against methicillin‐resistant Staphylococcus aureus or Candida sp. should be considered if the patient is critically ill or is known to be colonized with these organisms. Initial empiric therapy should be modified as necessary based on culture results. Total duration of therapy will vary based on location and magnitude of the infection, adjunctive surgical debridement, and pathogens involved.
SUMMARY
Despite being an extremely safe procedure, EGD carries a known serious risk of esophageal perforation. Mortality after esophageal perforation can approach 25%. Although diagnostic endoscopy has a perforation rate of less than 0.03%, the risk can approach 17% with therapeutic interventions such as stent placement and esophageal dilation. Factors influencing the risks of perforation include procedural complexity, operator experience, and underlying esophageal and systemic diseases. Furthermore, perforations complicated by infection can lead to fatal mediastinitis and sepsis. The clinical triad of esophageal perforation is fever, neck pain, and crepitus. The optimal diagnostic study is CT scan of the neck and thorax with water‐soluble oral contrast. Treatment options range from conservative management with broad‐spectrum antibiotics to surgery. Diagnosis of esophageal perforation within 24 hours is essential for favorable outcomes.
Esophagogastroduodenoscopy (EGD) carries a small but serious risk of esophageal perforation.13 With its potential for sepsis and fatal mediastinitis, prompt recognition and treatment are essential for favorable outcomes. The risk of perforation with diagnostic flexible EGD is 0.03%, which is an improvement from the 0.1%0.4% risk associated with rigid endoscopy.4 However, the risk of perforation can dramatically increase to 17% depending on the methods of therapeutic intervention and underlying risk factors (Table 1).1, 57
| Level of operator experience |
| Underlying esophageal disease |
| Zenker's diverticulum |
| Eosinophilic esophagitis |
| Esophageal or mediastinal irradiation |
| Esophageal malignancy |
| Esophageal strictures |
| Systemic disease |
| Anterior cervical osteophytes |
| Advanced liver cirrhosis |
| Diabetes mellitus |
| Scleroderma |
| Complexity of intervention |
| Esophageal stent placement |
| Pneumatic dilation |
| Other |
| Heavy sedation |
| Advanced age |
It is estimated that 33%75% of all esophageal perforations are iatrogenic.8 Of those caused by EGD, therapeutic interventions portend an increased risk compared with the risk of diagnostic endoscopy alone (Table 2).4 With the expanding role of flexible EGD and the increasing number of procedures performed, this modest risk per procedure still translates into a sizable number of perforations with their ensuing complications.4, 7 Mortality rates following esophageal perforation may approach 25%.9
| Endoscopic procedure | Esophageal perforation risk |
|---|---|
| Diagnostic | 0.03% |
| Dilation | 0.25% (normal esophagus)4%7% (achalasia)*7% (gastric outlet obstruction)*17% (strictures due to caustic agent) |
| Thermal method (treatment of malignancy) | 10% |
| Endoprosthesis | 3% |
| Variceal sclerotherapy | 1%5% (acute perforation)2%5% (delayed perforation) |
| Band ligation | 0.7% (perforation) |
| Nonvariceal hemostasis (use of sclerosant or cautery) | 0%2% (first hemostasis)4% (hemostasis repeated within 2448 hours) |
ANATOMY AND PATHOPHYSIOLOGY
The most common site of perforation is at the level of the cricopharyngeus, as it is a narrow introitus leading to the esophagus. The risk of perforation at this location is further increased with the presence of a Zenker's diverticulum or cervical osteophytes. The second most common site is proximal to the lower esophageal sphincter because of the angulation of the hiatus and the high frequency of esophageal webs, rings, reflux strictures, and hiatal hernias. The relatively straight middle esophagus is an uncommon site for perforations.
Cervical perforations are less commonly caused by organic lesions of the esophagus. Often, they are the result of technique and manipulation of the endoscope, or of certain conditions associated with the jaw, neck, or spinal column that are unfavorable for endoscopy. The risk of cervical perforation increases with the presence of bony spurs, as the upper esophagus is compressed over the underlying spinal column. Thoracic perforations, however, are more commonly seen with organic esophageal obstruction. These obstructions may be caused by an underlying inflammatory process, benign stricture, or neoplasm. In these cases, the risk of thoracic perforation is increased with blind procedures. Thoracic perforations carry a worse prognosis if diagnosis is delayed, or if the underlying obstruction cannot be removed.10
Esophageal perforation leads to periesophageal tissues being contaminated by food, secretions, air, or gastric contents and may be followed by chemical tissue injury and infection. The nature and extent of infection depend on the site of esophageal perforation. Cervical esophageal perforation can cause retropharyngeal space infection, which has the potential to extend directly into the posterior mediastinum via the danger space, which is between the retropharyngeal and prevertebral spaces and extends from the base of the skull descending freely throughout the entire length of the posterior mediastinum. With thoracic perforations, esophageal contents can enter the pleural space by negative intrathoracic pressure with subsequent pleural contamination and empyema.8, 1113
Pathogens responsible for infections after esophageal perforation vary based on several factors including site of perforation, clinical status of patient when perforation occurs (hospitalized versus not hospitalized, critically ill versus healthy), receipt of enteral nutrition, gastric acid suppression with H2‐receptor antagonists or proton‐pump inhibitors, immunosuppression, and recent (or current) receipt of antimicrobials. In nonintubated, healthy adults not on antimicrobial therapy, organisms in the upper esophagus are essentially identical to those in the oropharynx and include viridans streptococci, Haemophilus species, and anaerobes. During critical illness and following antibiotic therapy, the normal oral flora is rapidly replaced by aerobic Gram‐negative bacilli, Staphylococcus aureus, and yeast.14 The stomach, which is normally devoid of bacteria, can likewise be colonized with pathogenic organisms in the setting of gastric acid suppression and enteral nutrition.15, 16
SIGNS AND SYMPTOMS
Esophageal perforation should be considered after EGD, dilation, sclerotherapy, variceal banding, and esophageal stenting. However, perforation can also result from other invasive procedures such as insertion of feeding and nasogastric tubes, rapid sequence intubation, and transesophageal echocardiography.
The clinical triad of esophageal perforation includes pain, fever, and subcutaneous air.17 In a study by Wychulis et al., among 33 patients with esophageal perforation, 75% demonstrated all 3 findings.10 Pain is the most sensitive finding and occurs in nearly all patients identified with esophageal perforation. Crepitation, which results from air dissecting along soft tissue planes of the mediastinum and into the neck, occurs in up to 70% with cervical perforation and 30% with thoracic perforation.8, 10, 18
Clinical presentation and outcomes vary depending on the location of the perforation (Table 3).8 Cervical perforation is usually associated with anterior neck pain, located at the anterior border of the sternocleidomastoid muscle. Movement of the neck and palpation typically aggravate the pain. Thoracic perforation typically presents as substernal chest pain, often with a component of pleurisy. Pleural effusions are present in 50% of thoracic perforations, and mediastinitis is more likely to occur.19 Hamman's sign, a finding characterized by an audible crunch with chest auscultation, is suggestive of mediastinal emphysema. Perforation of the intra‐abdominal esophagus can result in epigastric pain and signs of acute abdomen.10, 17 Subcutaneous emphysema occurs more frequently with cervical perforation but can be present regardless of location.10 Secondary infections following esophageal perforation can manifest with an accelerated clinical course leading to sepsis and shock.
| Location of perforation | Symptom | Sign* |
|---|---|---|
| ||
| Cervical esophagus | Muscle spasm Dysphonia Hoarseness Dysphagia | Anterior neck tendernessTenderness on cervical motionSubcutaneous emphysema |
| Thoracic esophagus | Substernal chest pain Dysphagia Odynophagia | Cyanosis, Dyspnea Hamman's sign Pleural effusion Subcutaneous emphysema |
| Intraabdominal esophagus | Epigastric pain | Acute abdomenSubcutaneous emphysema |
DIAGNOSIS
Clinical suspicion of esophageal perforation should prompt necessary radiographic studies to establish the diagnosis.18, 20 Contrast‐enhanced computed tomography (CT) scans of the neck and chest are preferable because of their increased sensitivity in localizing the site and showing the extent of perforation and abscess. CT scans may reveal subcutaneous or mediastinal air, abscess cavities adjacent to the esophagus, and fistulas between the esophagus and mediastinum (Figs. 1 and 2).2022 Results of contrast studies may be negative and warrant repeating within several hours.19


If CT scans cannot be performed, neck (soft‐tissue) and chest x‐rays may be useful. Although plain films have limited value in evaluating the retropharyngeal space, they can reveal soft‐tissue emphysema, a widened mediastinum, pulmonary infiltrates or effusions, neck abscess, and mediastinal air‐fluid levels. In cervical perforation, a lateral film of the neck can show air in deep cervical tissue before clinical signs are apparent.
Swallow studies with Gastrografin (meglumine diatrizoate) are useful in defining the exact location of the perforation (Fig. 3). However, the false‐negative rate of swallow studies can exceed 10%, especially if the patient is upright during the study. When the contrast propagates past the site of perforation too quickly, it may not extravasate.23 Although barium may provide slightly greater contrast, it may add to the problem of foreign body reaction in the area of perforation.18 An additional complication of barium is that once it has extravasated, it is not readily absorbed. The persistence of extravasated barium makes it difficult to assess the resolution of an esophageal tear on subsequent fluoroscopic or CT exams. Hence, our institution avoids using barium to evaluate esophageal perforation, unless Gastrografin swallow has excluded any major esophageal perforation. Barium swallow may then be used to exclude small mural tears. Some medical centers elect to routinely screen their high‐risk patients with swallow evaluations after an EGD, although this is not common practice.8, 24

If the above workup is negative, the use of EGD may be considered for establishing the diagnosis if a high index of suspicion remains. However, the risks of EGD in this situation include extension of the perforation, further extravasation of esophageal contents, and difficulty with subsequent radiographic studies to visualize the perforation.19
MANAGEMENT
Once the diagnosis of esophageal perforation has been established, treatment options are individualized based on the clinical scenario. Currently, there are no established guidelines, and large randomized clinical trials comparing outcomes of operative versus nonoperative management have not been conducted (Fig. 4).25, 26 Outcomes associated with esophageal perforations depend on preoperative clinical condition, comorbidities, location and size of the perforation, nature of underlying esophageal disease (if any), and time to establish the diagnosis and initiate therapy.10 Delay in patient presentation or diagnosis beyond 24 hours following esophageal perforation has been associated with adverse outcomes.18, 27, 28

A conservative approach is appropriate when clinically stable patients with minimal symptoms have well‐contained, nontransmural tears. Management entails broad‐spectrum antibiotics, nothing by mouth, nasogastric suction, and parenteral nutrition.24 Early surgical consultation is recommended in all cases. Serial CT scanning is useful for following the resolution of fistulas and tears. An oral diet can be resumed when contrast or swallow studies show no extravasation of dye. Cervical perforations typically fare well with this approach.26, 29
Surgical therapy is recommended for patients with large or uncontained esophageal perforations, mediastinal abscesses, and/or sepsis.25, 27 Surgical options include esophageal diversion, esophagectomy, or drainage with or without primary repair. Drainage with primary repair is considered the treatment of choice, regardless of time to presentation. Esophagectomy is considered in cases of delayed or neglected perforations, extensive transmural necrosis or underlying cancer.30 Operative mortality is 0%30% when treated within 24 hours. This rate increases to 26%64% when treatment is delayed beyond 24 hours, reaffirming the importance of making a prompt diagnosis.8
Endoscopic intervention is gaining recognition for its role in the management of esophageal perforations, especially when the risks make surgery prohibitive. Therapeutic options include stenting and clipping a perforation, as well as debriding and draining an abscess. Endoscopists can successfully treat traumatic nonmalignant esophageal perforations smaller than 50% to 70% of the circumference with self‐expanding plastic stents.26 Another option is to use metallic clipping devices to treat small esophageal perforations (<1 cm).3133 Combined with medical management and appropriate patient selection, the benefits of an endoscopic approach may potentially outweigh the risks of surgery.26, 29, 33, 34
Regardless of treatment approach, the appropriate and timely selection of empiric antibiotic therapy improves outcomes. Empiric antimicrobial therapy for esophageal perforation will depend on several host factors as well as the site of perforation. In healthy nonhospitalized adults, ampicillin‐sulbactam, clindamycin, and penicillin G plus metronidazole are good choices because of their excellent activity against oral microflora. In patients who are critically ill, are hospitalized, are immunosuppressed, or have gastric acid suppression, initial broad‐spectrum antimicrobials such as piperacillin‐tazobactam, imipenem, meropenem, or a third‐generation cephalosporin plus metronidazole (or clindamycin) should be initiated. Additional therapy against methicillin‐resistant Staphylococcus aureus or Candida sp. should be considered if the patient is critically ill or is known to be colonized with these organisms. Initial empiric therapy should be modified as necessary based on culture results. Total duration of therapy will vary based on location and magnitude of the infection, adjunctive surgical debridement, and pathogens involved.
SUMMARY
Despite being an extremely safe procedure, EGD carries a known serious risk of esophageal perforation. Mortality after esophageal perforation can approach 25%. Although diagnostic endoscopy has a perforation rate of less than 0.03%, the risk can approach 17% with therapeutic interventions such as stent placement and esophageal dilation. Factors influencing the risks of perforation include procedural complexity, operator experience, and underlying esophageal and systemic diseases. Furthermore, perforations complicated by infection can lead to fatal mediastinitis and sepsis. The clinical triad of esophageal perforation is fever, neck pain, and crepitus. The optimal diagnostic study is CT scan of the neck and thorax with water‐soluble oral contrast. Treatment options range from conservative management with broad‐spectrum antibiotics to surgery. Diagnosis of esophageal perforation within 24 hours is essential for favorable outcomes.
- .Complications of endoscopic gastrointestinal dilation techniques.Gastrointest Endosc Clin N Am.1996;6:323–341.
- .Complications of upper gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.1996;6:287–303.
- ,.Complications of upper gastrointestinal endoscopy and their management.Gastrointest Endosc Clin N Am.1994;4:551–570.
- ,,,,.Treatment of endoscopic esophageal perforation.Surg Endosc.1999;13:962–966.
- ,,,.Unsedated small‐caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study [see comment].Gastroenterology.1999;117:1301–1307.
- ,,,,.Complications associated with esophagogastroduodenoscopy and with esophageal dilation.Gastrointest Endosc.1976;23(1):16–19.
- ,,.Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures.Gastrointest Endosc.2000;51(4 Pt 1):460–462.
- ,.Esophageal emergencies: things that will wake you from a sound sleep.Gastroenterol Clin N Am.2003;32:1035–1052.
- ,,, et al.Complications of upper GI endoscopy.Gastrointest Endosc.2002;55:784–793.
- ,,.Instrumental perforations of the esophagus.Dis Chest.1969;55(3):184–189.
- ,Complications of esophageal dilation and guidelines for their prevention.Gastrointest Endosc.1981;27:229–234.
- .Infectious complications associated with gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.2000;10:215–232.
- ,,,The occurrence of bacteremia after esophageal dilation.Gastrointestinal Endoscopy1975;22(2):86–87.
- ,,,,,.The pathogenesis of ventilator‐associated pneumonia: its relevance to developing effective strategies for prevention.Respir Care.2005;50:725–739; discussion39–41.
- ,,, et al.Stomach as a source of colonization of the respiratory tract during mechanical ventilation: association with ventilator‐associated pneumonia.Eur Respir J.1996;9:1729–1735.
- ,,, et al.Gastric microbiota in elderly patients fed via nasogastric tubes for prolonged periods.J Hosp Infect.2006;63(1):79–83.
- ,.Retroesophageal abscess twenty‐five days after esophagoscopy. An unusual complication of endoscopy.Gastrointest Endosc.1972;18:167–168.
- ,,.The radiologist in prevention and diagnosis of instrumental perforation of the esophagus.South Med J.1974;67:830–836.
- ,,.Short‐ and long‐term outcome of esophageal perforation.Gastrointest Endosc.1995;41(2):130–134.
- ,,,.The diagnosis and treatment of esophageal perforations resulting from nonmalignant causes.Surg Today.1997;27:793–800.
- ,.Radiology of the retropharyngeal space.Clin Radiol.2000;55:740–748.
- ,,.Diagnosis and management decisions in infections of the deep fascial spaces of the head and neck utilizing computerized tomography.Laryngoscope.1982;92(6, Pt. 1):630–633.
- .Perforation of the esophagus.Ann Thorac Surg.1986;42:231–232.
- ,,, et al.Successfully treated case of cervical abscess and mediastinitis due to esophageal perforation after gastrointestinal endoscopy.Dis Esophagus.2002;15:250–252.
- ,.The spectrum of spontaneous and iatrogenic esophageal injury: perforations, Mallory‐Weiss tears, and hematomas.J Clin Gastroenterol.1999;29:306–317.
- .Treatment of esophageal perforations and anastomotic leaks: the endoscopist is stepping into the arena.Gastrointest Endosc.2005;61:897–900.
- ,,.Operative and nonoperative management of esophageal perforations.Ann Surg.1981;194(1):57–63.
- ,,.Current results of therapy for esophageal perforation.Am J Surg.1995;169:615–617.
- ,,.Successful endoscopic management of a cervical pharyngeal perforation and mediastinal abscess.Gastrointest Endosc.2005;61(1):158–160.
- ,,,,.Thoracic esophageal perforations: a decade of experience.[see comment].Ann Thorac Surg.2003;75:1071–1074.
- ,.Perforation: part and parcel of endoscopic resection? [comment].Gastrointest Endosc.2006;63:602–605.
- ,,,.Endoscopic clip application as an adjunct to closure of mature esophageal perforation with fistulae.Clin Gastroenterol Hepatol.2003;1(1):44–50.
- ,,.Endoscopic clipping of esophageal perforation after pneumatic dilation for achalasia.Endoscopy.1995;27:608–611.
- ,,, et al.Esophageal dilation.Gastrointest Endosc.2006;63:755–760.
- .Complications of endoscopic gastrointestinal dilation techniques.Gastrointest Endosc Clin N Am.1996;6:323–341.
- .Complications of upper gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.1996;6:287–303.
- ,.Complications of upper gastrointestinal endoscopy and their management.Gastrointest Endosc Clin N Am.1994;4:551–570.
- ,,,,.Treatment of endoscopic esophageal perforation.Surg Endosc.1999;13:962–966.
- ,,,.Unsedated small‐caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study [see comment].Gastroenterology.1999;117:1301–1307.
- ,,,,.Complications associated with esophagogastroduodenoscopy and with esophageal dilation.Gastrointest Endosc.1976;23(1):16–19.
- ,,.Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures.Gastrointest Endosc.2000;51(4 Pt 1):460–462.
- ,.Esophageal emergencies: things that will wake you from a sound sleep.Gastroenterol Clin N Am.2003;32:1035–1052.
- ,,, et al.Complications of upper GI endoscopy.Gastrointest Endosc.2002;55:784–793.
- ,,.Instrumental perforations of the esophagus.Dis Chest.1969;55(3):184–189.
- ,Complications of esophageal dilation and guidelines for their prevention.Gastrointest Endosc.1981;27:229–234.
- .Infectious complications associated with gastrointestinal endoscopy.Gastrointest Endosc Clin N Am.2000;10:215–232.
- ,,,The occurrence of bacteremia after esophageal dilation.Gastrointestinal Endoscopy1975;22(2):86–87.
- ,,,,,.The pathogenesis of ventilator‐associated pneumonia: its relevance to developing effective strategies for prevention.Respir Care.2005;50:725–739; discussion39–41.
- ,,, et al.Stomach as a source of colonization of the respiratory tract during mechanical ventilation: association with ventilator‐associated pneumonia.Eur Respir J.1996;9:1729–1735.
- ,,, et al.Gastric microbiota in elderly patients fed via nasogastric tubes for prolonged periods.J Hosp Infect.2006;63(1):79–83.
- ,.Retroesophageal abscess twenty‐five days after esophagoscopy. An unusual complication of endoscopy.Gastrointest Endosc.1972;18:167–168.
- ,,.The radiologist in prevention and diagnosis of instrumental perforation of the esophagus.South Med J.1974;67:830–836.
- ,,.Short‐ and long‐term outcome of esophageal perforation.Gastrointest Endosc.1995;41(2):130–134.
- ,,,.The diagnosis and treatment of esophageal perforations resulting from nonmalignant causes.Surg Today.1997;27:793–800.
- ,.Radiology of the retropharyngeal space.Clin Radiol.2000;55:740–748.
- ,,.Diagnosis and management decisions in infections of the deep fascial spaces of the head and neck utilizing computerized tomography.Laryngoscope.1982;92(6, Pt. 1):630–633.
- .Perforation of the esophagus.Ann Thorac Surg.1986;42:231–232.
- ,,, et al.Successfully treated case of cervical abscess and mediastinitis due to esophageal perforation after gastrointestinal endoscopy.Dis Esophagus.2002;15:250–252.
- ,.The spectrum of spontaneous and iatrogenic esophageal injury: perforations, Mallory‐Weiss tears, and hematomas.J Clin Gastroenterol.1999;29:306–317.
- .Treatment of esophageal perforations and anastomotic leaks: the endoscopist is stepping into the arena.Gastrointest Endosc.2005;61:897–900.
- ,,.Operative and nonoperative management of esophageal perforations.Ann Surg.1981;194(1):57–63.
- ,,.Current results of therapy for esophageal perforation.Am J Surg.1995;169:615–617.
- ,,.Successful endoscopic management of a cervical pharyngeal perforation and mediastinal abscess.Gastrointest Endosc.2005;61(1):158–160.
- ,,,,.Thoracic esophageal perforations: a decade of experience.[see comment].Ann Thorac Surg.2003;75:1071–1074.
- ,.Perforation: part and parcel of endoscopic resection? [comment].Gastrointest Endosc.2006;63:602–605.
- ,,,.Endoscopic clip application as an adjunct to closure of mature esophageal perforation with fistulae.Clin Gastroenterol Hepatol.2003;1(1):44–50.
- ,,.Endoscopic clipping of esophageal perforation after pneumatic dilation for achalasia.Endoscopy.1995;27:608–611.
- ,,, et al.Esophageal dilation.Gastrointest Endosc.2006;63:755–760.