User login
Chest Tube Management
A pneumothorax is a collection of air in the space outside the lungs that is trapped within the thorax. This abnormality can occur spontaneously or as the result of trauma. Traumatic pneumothoraces include those resulting from medical interventions such as a transthoracic and transbronchial needle biopsy, central line placement, and positive‐pressure mechanical ventilation. This group is most accurately described as iatrogenic pneumothorax (IP).[1]
IP can be an expected complication of many routine thoracic procedures, but it can also occur accidentally during procedures near the lung or thoracic cavity. Some IPs may be asymptomatic and go undiagnosed, or their diagnosis may be delayed.[2] The majority of iatrogenic pneumothoraces will resolve without complications, and patients will not require medical attention. A small percentage can, however, expand and have the potential to develop into a tension pneumothorax causing severe respiratory distress and mediastinal shift.[3, 4]
The incidence of IP ranges from 0.11% with mechanical ventilation to 2.68% with thoracentesis, according to an analysis of 7.5 million uniform hospital discharge abstracts from 2000.5 A 2010 systematic review of 24 studies that included 6605 patients suggested a 6.0% incidence of pneumothorax following thoracentesis.[3] The highest risk of IP is seen with computed tomography (CT)‐guided lung biopsy, with 1 series of 1098 biopsies showing a 42% incidence; chest tube evacuation was required in 12% of these cases.[4] A Veterans Administration study of patient safety indicators from 2001 to 2004 found that risk‐adjusted rates of IP were increasing over time.[6] It is unclear whether this increase is due to increasing numbers of interventional procedures or to better rates of detection. IP poses a considerable cost to the medical system, with safety studies finding that patients with IP will stay in the hospital approximately 4 days longer and incur an additional $17,000 in charges.[7]
In addition to this financial burden, the lack of consistency in training and guidelines for management of pneumothorax is thought to add to chest tube‐related complications.[8] In 2001, the American College of Chest Physicians (ACCP) published guidelines for the management of spontaneous pneumothorax that do not specifically address IP.[9] In 2010, the British Thoracic Society (BTS) updated their guidelines and included a brief statement on IP that described a higher incidence for it than for spontaneous pneumothorax and noted its relative ease of management.[10] Despite the lack of specific guidelines dedicated to IPs, common clinical practice is to manage iatrogenic defects in a manner similar to that for spontaneous ones. However, studies have shown that the management of pneumothorax remains diverse and that the adherence to these published guidelines is suboptimal.[10, 11] The BTS guidelines favor needle aspiration as the first‐line treatment,[10] whereas the ACCP recommends drainage with catheters over aspiration.[9]
The possibility of this complication, along with the rising rate of invasive interventions being performed, has led to expanded surveillance criteria for IP. Surveillance imaging, clinical observation, or a combination of the 2 may be required, depending on the institution, the risk of the procedure, and the preference of the treating clinician. The algorithms presented here were designed in alignment with both major society guidelines and with the intention of simplifying the treatment regimen for the ease of adoption by hospitalists.
ETIOLOGY AND RISK FACTORS
The etiology and risk factors for IP are multiple, with the most common being interventional‐based procedures. In 535 Veterans Administration patients, the most common precursor procedures were transthoracic needle biopsy (24%), subclavian vein catheterization (22%), thoracentesis (20%), transbronchial biopsy (10%), pleural biopsy (8%), and positive pressure ventilation.[12] IP can also be a rare complication of pacemaker manipulations,[5] and less commonly, bronchoscopy.[13] Patient factors that increase the risk of pneumothorax in the setting of an intervention include age, chronic obstructive lung disease, primary lung cancer, malignant and parapneumonic pleural effusions, empyema, and chronic corticosteroid use.[4] As might be expected, patients with structural lung disease (eg, emphysema with bullae) and poor healing ability (eg, corticosteroid dependent), tend to have IPs more often and to require more complicated interventions for resolution.[14, 15] In some studies, operator experience seems to be inversely related to the rate of IP, and the use of ultrasound is correlated with lower rates of this complication.[1, 3]
PATIENT PRESENTATIONS AND DIAGNOSIS
Clinical signs and symptoms of a significant pneumothorax vary in severity but most often include dyspnea, tachypnea, chest pain, and pleurisy (see Box 1). Post procedure signs or symptoms require further evaluation with imaging, usually a plain chest radiograph. CT can be useful for further evaluation. Small anterior pneumothoraces may be difficult to detect without lateral radiographic imaging or computed tomogram. Ultrasound is being used more frequently at the bedside to make this diagnosis, and various studies of trauma patients have found that it has good sensitivity and specificity.[16, 17] These results have been validated by a recent meta‐analysis comparing ultrasound to chest radiographs for the detection of pneumothorax among trauma, critically ill, and postprocedural patients.[18] This study demonstrated superior sensitivity and similar specificity for ultrasound versus chest radiographs for detection of pneumothorax. More ominous signs, such as tachycardia or hypotension, can be indicative of tension pneumothorax, which requires emergent evacuation.
MANAGEMENT
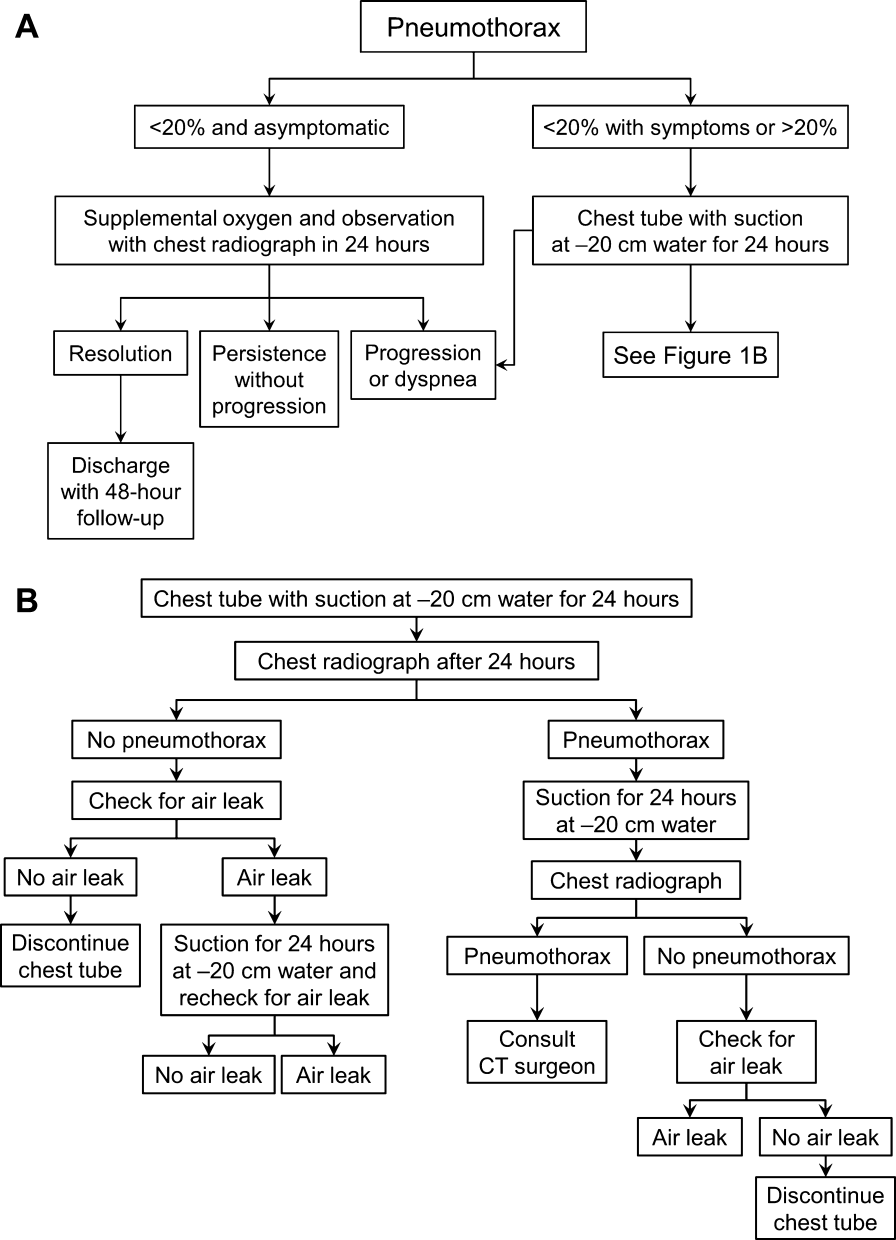
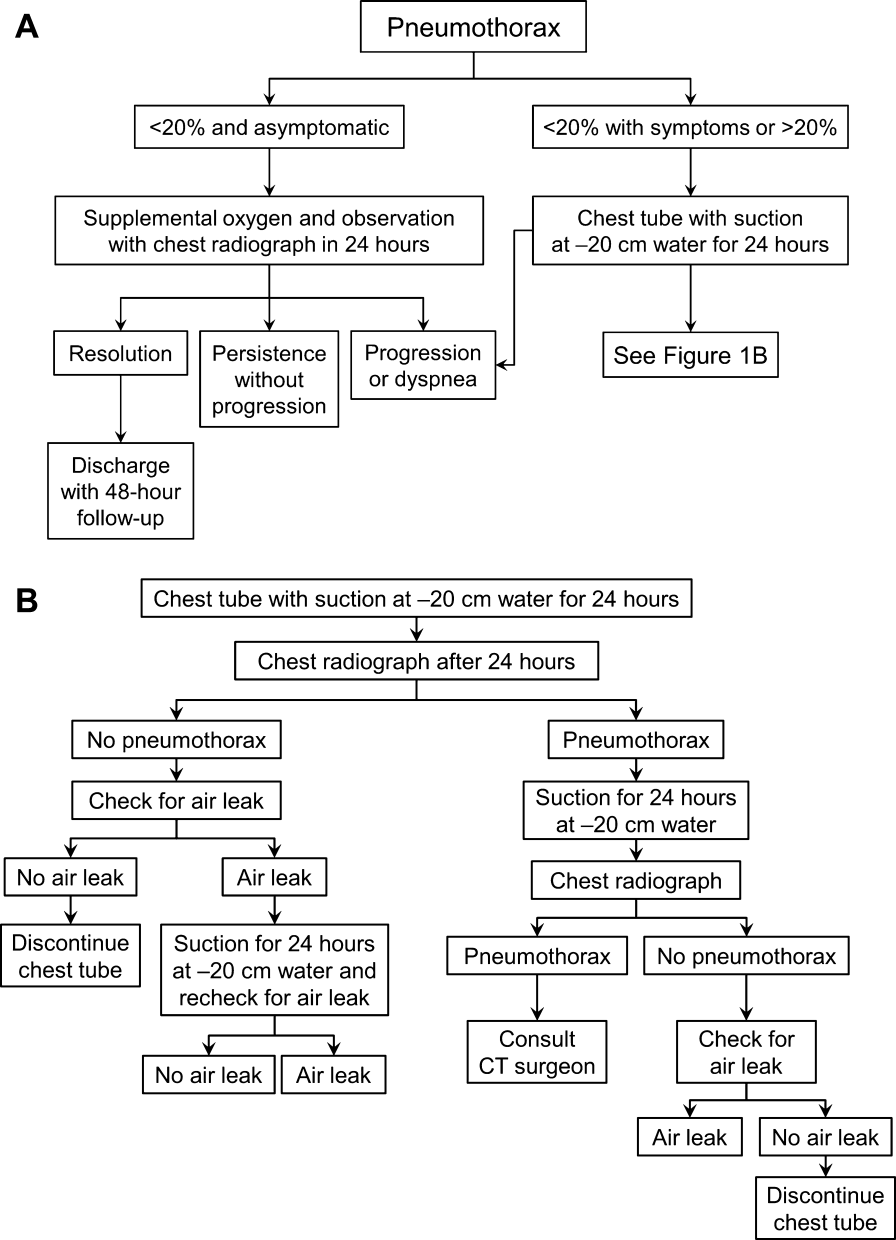
Once the diagnosis of pneumothorax has been established, treatment options should be guided by defect size and clinical assessment following a defined treatment algorithm (Figure 1). As emphasized by the BTS and ACCP guidelines, we advocate considering the use of symptoms along with defect size to determine the best management course.
Observation
Defects that involve<20% of the hemithorax in a patient who is clinically asymptomatic and hemodynamically stable can be safely managed by oxygen supplementation and hospital observation. Repeat imaging can be obtained after 12 to 24 hours of defect detection or with symptom change. Patients who display resolution may be discharged home.
Patients who show persistence without progression but are asymptomatic may also be discharged safely, with follow‐up imaging and clinical evaluation 48 hours later.[9, 10] This was demonstrated by Kelly and colleagues,[19] who described the outcomes of 154 patients in a retrospective cohort study. Of the 91 patients treated with outpatient observation, 82 resolved without additional interventions. A recent review article by the same author cites conservative management of small pneumothoraces as being widely accepted.[20] If reimaging shows progression of defect or if the patient becomes more symptomatic, the pneumothorax should be evacuated by 1 of the methods described below.
Aspiration
Aspiration is defined by the ACCP Delphi consensus statement as the removal of pleural air via needle or cannula followed by immediate removal of needle or cannula.[9] This option mandates careful patient selection. It should be considered for small pneumothoraces that cause only mild dyspnea in patients who have no known parenchymal disease. These patients should be observed overnight in the hospital and reimaged 24 hours after aspiration of the pneumothorax. Several authors have reported success with aspiration alone. Yamagami et al.[21] noted the efficacy of manual aspiration immediately after CT‐guided biopsy, with a success rate exceeding 90%. They also noted that evacuated volumes >543 mL correlated with the need for further intervention with a chest tube. This technique is advocated for small pneumothoraces that are recognized shortly after the procedure.
Similarly, Delius and colleagues[22] managed 131 pneumothoraces with aspiration as an alternative to chest tube placement. Of these, 79 were iatrogenic. Aspiration achieved a 75% success rate for all IPs. Small defects defined as <20% of volume had an even higher resolution rate of 87%. Similar findings were demonstrated by Talbot‐Stern et al.[23] in their prospective study of 76 pneumothoraces. Among those that were iatrogenic, 82% resolved after simple aspiration. Faruqi et al.[24] also showed that aspiration is a viable option for IPs. Of the 57 patients with pneumothorax included in their study, 35 were treated with aspiration alone. Iatrogenesis was the culprit in 12 of the 35 manually aspirated cases. Aspiration achieved a success rate of 91.7% in IP. A recent Cochrane database systematic review compared simple aspiration with intercostal tube drainage for primary spontaneous pneumothorax.[25] The authors reported no difference between these methods in terms of success rate, early failure rate, duration of hospital stay, 1‐year success rate, or number of patients who required pleurodesis at 1‐year follow‐up.
Because the algorithms presented in this article were specifically designed for the use by hospitalists, we intentionally omitted aspiration from the decision trees. Most hospitalists would not be expected to evacuate IPs. However, knowledge regarding this option and appropriate follow‐up are valuable to internists, because many interventionalists admit patients to the hospital service for overnight observation. An asymptomatic postaspiration patient, who on subsequent imaging demonstrates resolution or persistence without progression of pneumothorax, may be discharged with 48‐hour follow‐up.
Placement of Catheter or Chest Tube Drainage
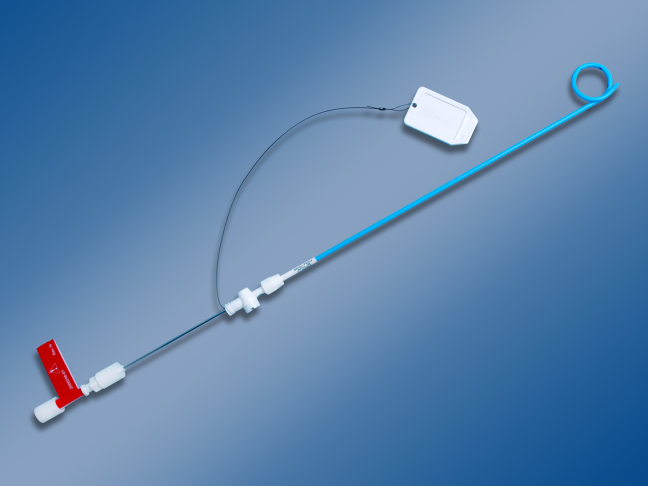
Most patients with a clinically significant pneumothorax will require evacuation of the air. Pneumothoraces larger than 20% or that produce symptoms warrant chest tube management and inpatient observation (Figure 1B). Traditionally, large tubes with 20 cm of water on continuous suction are used and have been studied the most widely. Several authors have shown that smaller tubes can effectively drain a pneumothorax.[26, 27, 28, 29] Small‐bore catheters (8F14F), which can be inserted percutaneously, have been shown to provide effective lung re‐expansion with minimal morbidity[8] and may be better tolerated by patients with uncomplicated pneumothoraces (Figure 2). Terzi and colleagues[30] have shown that smaller tubes cause less discomfort to patients at rest, with cough, and at the time of tube removal.
At most US institutions, catheters and chest tubes are connected to all‐purpose drainage systems. Although commercially available through a variety of manufacturers, they share similar design principles because they replicate the 3‐bottle system described in detail elsewhere in the literature.[31] We have limited our discussion to 3 pleural evacuation systems because it is our intention to familiarize hospitalists with the units that they are most likely to encounter. The first 2 systems have been studied and described by Baumann and colleagues[32] as being commonplace and reasonably reliable. These include the Oasis (Atrium Medical Corp., Hudson, NH) (Figure 3A) and the Pleur‐evac (Teleflex Inc., Limerick, PA). The third unit is the Thopaz digital thoracic drainage system (Medela Inc., McHenry, IL) (Figure 3B). The Thopaz is unique in its inclusion of a suction source and digital capability. Although it utilizes the same principles of all pleural evacuation devices, its setup and information output require that one be familiar with its digital format.

Suction Versus Water Seal
The chest tube should be placed initially to a suction pressure level of 20 cm of water for 24 hours to maximize lung expansion and evacuate all extrapulmonary air. Suction pressure is set on the Pleur‐evac and Atrium drainage systems by a manual dial that reads to a water pressure of 0 to 40 cm. The default setting from the manufacturer is 20 cm of water. This level of suction is present only when the drainage system is connected to a wall or a portable suction device. The only confirmation of suction presence in the Atrium system is the deployment of the orange bellows (located under the dial) to the level of the arrow tip (Figure 3A). The Pleur‐evac system has a red stripe along the circular edge of the dial that appears at the set level of suction when negative pressure is being applied. It is important to be aware that when patients are disconnected from the wall or the portable suction apparatus, they are on water seal or gravity. These terms are synonymous with no suction. On the Thopaz, a digital menu directs operation, and levels of suction can be selected from water seal (no suction) up to 40 cm of water. We recommend using suction to 20 cm of water given the scarce evidence supporting higher levels of negative pressure. Some clinicians prefer placing patients on water seal for some time before moving toward tube discontinuance, but this is a matter of preference, and no substantial evidence exists to show that any 1 method is superior.[8, 33]
Assessing for Air Leak
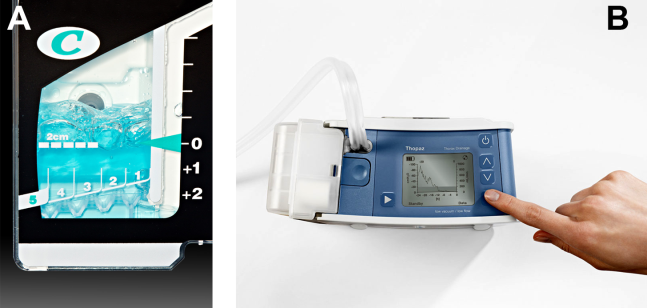
If there is improvement or resolution of the pneumothorax after 24 hours, the presence of an air leak should be assessed; if no leak is present, the chest tube can be safely removed. In the context of chest tubes, the term air leak refers to residual air between the lung and the chest wall. It is possible to see resolution of a pneumothorax on chest radiographs and still have an air leak. This situation is created by a perfect balance between the pleural air evacuation by the catheter and the flow of air exiting from the lung puncture. This would result in reaccumulation of the pneumothorax if the chest tube is removed prematurely. It should also be kept in mind that chest radiographs may miss a small pneumothorax given their relatively low sensitivity.[18] Therefore, the absence of an air leak needs to be documented before the chest tube is discontinued. Depending on the type of drainage system (Atrium, Pleur‐evac, or Thopaz), this assessment can be done in several ways. All systems can be assessed for air leak by clamping the actual chest tube for 2 to 4 hours and then repeating the chest radiograph. Clamping a chest tube simulates the condition of not having a chest tube. Chest tubes should never be clamped without supervision and only with the knowledge of nursing personnel. The onset of chest pain or dyspnea in a patient with a clamped tube mandates immediate removal of the clamp and a return to suction. A repeat chest radiograph showing reaccumulation or expansion of the pneumothorax after clamping indicates that the air leak has not resolved and the chest tube must remain in place and returned to suction. Simpler and more time‐efficient methods of detecting air leaks are available with both cardiothoracic drainage systems.
For the Atrium and Pleur‐evac models, there is a graded panel through which one can visualize air leaks being funneled through water (Figure 4A). Having the patient cough several times or perform a Valsalva maneuver should release any air trapped within the chest into this chamber, where bubbles can be visualized as they travel through the water. The presence of bubbles indicates the presence of residual air in the chest, pointing to a possible leak. In contrast, the Thopaz system offers a graphical display of the air flowing into the system that can be reviewed over the 24‐hour period. When the graph line reaches a 0 flatline graph, no airflow is being detected and no air leakage is suspected (Figure 4B). If no air leaks are detected, the chest tube may be discontinued. Those patients with a failed air leak test should have their chest tubes continued under suction for another 24 hours, with the above tests then repeated. The same holds true for those patients with persistent pneumothorax at 24 hours.
Removal of chest tubes is a simple process that requires the tube to be pulled out of the patient without allowing air to enter the site where the tube was present and where it entered the thorax. Most interventionalists will discontinue the tubes that they have placed. Some small catheters have an internal string that has to be released so the catheter will straighten and pull out easily. Knowledge of the type of catheter or tube that was placed is critical before removal to prevent complications and patient discomfort. Standard chest tubes are straight, smooth plastic and pull out easily but require rapid occlusion of the larger puncture site in the chest wall with an occlusive dressing that often includes petroleum or water‐soluble gel. Some physicians will leave a suture tie when placing the chest tube so that it can be tied down to occlude the site instead of using a dressing.
Consultation of the Cardiothoracic Surgeon or Interventional Pulmonologist
We recommend the involvement of cardiothoracic surgery or interventional pulmonology for patients with nonresolving pneumothorax lasting longer than 48 hours because additional procedures may be necessary. One of the rare but serious complications of a persistent pneumothorax is the formation of a bronchopleural fistula. This communication between the bronchial tree and the pleural space can lead to significant morbidity and mortality. The treatment of a bronchopleural fistula includes medical and surgical options that are beyond the scope of this article but require the expertise of a cardiothoracic surgeon or interventional pulmonologist.[34] Most patients who will not require additional procedures will heal within 48 hours.[11, 35] Decisions regarding more invasive treatment measures can then be made as necessary.[26]
PRACTICAL TIPS
Hospitalists caring for patients with chest tubes are often asked to troubleshoot at the bedside. Scenarios that may be encountered include nonfunctioning tubes, catheter migration, and tube discomfort. Ensuring patency of the tube entails visualizing the tube from the point of entry into the chest wall to the collection chamber and inspecting for kinks or debris clogging the tube. Smaller catheters can be easily kinked during patient positioning and can become clogged. Respiratory variation, which is the movement of the column of fluid in the collection chamber or in the tubing with inspiration and expiration, suggests that the chest tube is patent. This should be part of the daily examination in a patient with a chest tube, and it should also be the first step in assessing sudden dyspnea, hypoxia, pain, or hemodynamic instability. Clogged tubes should be referred to the interventionalists or other physicians who placed them. Chest tubes are typically sutured at the site of entry and securely bandaged to avoid migration but occasionally can be dislodged. This should prompt placement of another tube by an experienced operator. Last, chest tubes can be uncomfortable for patients who may require systemic analgesics. Additionally, tube positioning may ease some of the discomfort. Chest tubes are commonly placed along the midaxillary line and the posterior thorax, leading to discomfort in the recumbent position. Directing the tube anteriorly helps ease some of the discomfort. This can be done using all‐purpose sponges to build a barrier between the skin and the chest tube as it is directed anteriorly. Additional sponges are placed above the tube for extra protection. The gentle curve accomplished by padding the underside of the tube also keeps the tube patent by avoiding sharp kinks as the catheter exits the thorax.
FUTURE TRENDS
Future trends in the management of IP may include shorter duration of tube management (14 hours of suction with air leak evaluation and removal) and the use of even smaller catheters. Outpatient management of IP with small pigtail catheters or small‐caliber tubes in addition to 1‐way valves is also being investigated. The benefits of these practices may include greater patient comfort and lower cost. These approaches will need larger‐scale replication and careful patient selection before they become standard practice.[28, 36]
Ultrasound can be used to assess the presence and size of pneumothoraces that are difficult to visualize by standard chest radiographs. Several studies have established ultrasonography as an effective method of diagnosing pneumothorax and have shown it to have superior sensitivity compared with chest radiography.[1, 18] In the future, the use of ultrasound will likely be more widespread given its performance, portability, ease of use, and relatively low cost.
SUMMARY
IP is a known and costly complication of many medical procedures. The aforementioned algorithms help simplify the management of chest tubes for hospitalists caring for patients with this common complication. This stepwise approach may not only help curtail added expenses related to IPs by decreasing the length of inpatient stays but may also improve patient satisfaction.
Acknowledgments
Disclosure: Mayo does not endorse the products mentioned in this article. The authors report no conflicts of interest.
Box
Clinical Signs and Symptoms of Pneumothorax
Dyspnea
Pleuritic chest pain
Tachypnea
Hypoxia
Decreased breath sounds on affected side
Hyper‐resonant percussion on affected side
Subcutaneous emphysema
- , . Management of pneumothorax. Semin Respir Crit Care Med. 2010;31(6):769–780.
- , . Pneumothorax. Respirology. 2004;9(2):157–164.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy‐guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9‐year period. AJR Am J Roentgenol. 2010;194(3):809–814.
- , , . Accidental iatrogenic pneumothorax in hospitalized patients. Med Care. 2006;44(2):182–186.
- , , , et al. Tracking rates of patient safety indicators over time: lessons from the Veterans Administration. Med Care. 2006;44(9):850–861.
- , . Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290(14):1868–1874.
- , , , , . Preliminary report of a prospective, randomized trial of underwater seal for spontaneous and iatrogenic pneumothorax. J Am Coll Surg. 2007;204(1):84–90.
- , , , et al.; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119(2):590–602.
- , , ; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii18–ii31.
- , . The clinician's perspective on pneumothorax management. Chest. 1997;112(3):822–828.
- , , , . Iatrogenic pneumothorax: etiology and morbidity: results of a Department of Veterans Affairs Cooperative Study. Respiration. 1992;59(4):215–220.
- , , , , , . Severe complications of bronchoscopy. Respiration. 2008;76(4):429–433.
- , , , et al. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest. 2004;126(5):1516–1521.
- , , , , , . Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15(5):479–483.
- , . Ultrasound detection of pneumothorax compared with chest X‐ray and computed tomography scan. Am Surg. 2011;77(4):480–484.
- , . Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010;17(1):11–17.
- , , , , . Diagnosis of pneumothorax by radiography and ultrasonography: a meta‐analysis. Chest. 2011;140(4):859–866.
- , , . Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest. 2008;134(5):1033–1036.
- . Review of management of primary spontaneous pneumothorax: is the best evidence clearer 15 years on? Emerg Med Australas. 2007;19(4):303–308.
- , , , , , . Efficacy of manual aspiration immediately after complicated pneumothorax in CT‐guided lung biopsy. J Vasc Interv Radiol. 2005;16(4):477–483.
- , , , , , . Catheter aspiration for simple pneumothorax: experience with 114 patients. Arch Surg. 1998;124(7):833–836.
- , , , , . Catheter aspiration for simple pneumothorax. J Emerg Med. 1986;4(6):437–442.
- , , , . Role of simple needle aspiration in the management of pneumothorax. Indian J Chest Dis Allied Sci. 2004;46(3):183–190.
- , , . Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev. 2007;(1):CD004479.
- , , , . Evaluation of conventional chest tube therapy for iatrogenic pneumothorax. Chest. 1993;104(6):1770–1772.
- , , , . Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology. 1997;205(1):249–252.
- , , , , , . Outpatient management of postbiopsy pneumothorax with small‐caliber chest tubes: factors affecting the need for prolonged drainage and additional interventions. Cardiovasc Intervent Radiol. 2008;31(2):342–348.
- , . Management of primary and secondary pneumothorax using a small‐bore thoracic catheter. Interact Cardiovasc Thorac Surg. 2010;11(2):146–149.
- , , , et al. The use of flexible spiral drains after non‐cardiac thoracic surgery: a clinical study. Eur J Cardiothorac Surg. 2005;27(1):134–137.
- . Pleural Diseases. 5th ed. Philadelphia: PA: Lippincott Williams 2007.
- , , , . Comparison of function of commercially available pleural drainage units and catheters. Chest. 2003;123(6):1878–1886.
- , . Catheter drainage of spontaneous pneumothorax: suction or no suction, early or late removal? Thorax. 1982;37(1):46–48.
- , . Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128(6):3955–3965.
- , , , . Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg. 1991;126(6):764–766.
- , , , , . A treatment algorithm for pneumothoraces complicating central venous catheter insertion. Am J Surg. 2000;180(6):523–526.
A pneumothorax is a collection of air in the space outside the lungs that is trapped within the thorax. This abnormality can occur spontaneously or as the result of trauma. Traumatic pneumothoraces include those resulting from medical interventions such as a transthoracic and transbronchial needle biopsy, central line placement, and positive‐pressure mechanical ventilation. This group is most accurately described as iatrogenic pneumothorax (IP).[1]
IP can be an expected complication of many routine thoracic procedures, but it can also occur accidentally during procedures near the lung or thoracic cavity. Some IPs may be asymptomatic and go undiagnosed, or their diagnosis may be delayed.[2] The majority of iatrogenic pneumothoraces will resolve without complications, and patients will not require medical attention. A small percentage can, however, expand and have the potential to develop into a tension pneumothorax causing severe respiratory distress and mediastinal shift.[3, 4]
The incidence of IP ranges from 0.11% with mechanical ventilation to 2.68% with thoracentesis, according to an analysis of 7.5 million uniform hospital discharge abstracts from 2000.5 A 2010 systematic review of 24 studies that included 6605 patients suggested a 6.0% incidence of pneumothorax following thoracentesis.[3] The highest risk of IP is seen with computed tomography (CT)‐guided lung biopsy, with 1 series of 1098 biopsies showing a 42% incidence; chest tube evacuation was required in 12% of these cases.[4] A Veterans Administration study of patient safety indicators from 2001 to 2004 found that risk‐adjusted rates of IP were increasing over time.[6] It is unclear whether this increase is due to increasing numbers of interventional procedures or to better rates of detection. IP poses a considerable cost to the medical system, with safety studies finding that patients with IP will stay in the hospital approximately 4 days longer and incur an additional $17,000 in charges.[7]
In addition to this financial burden, the lack of consistency in training and guidelines for management of pneumothorax is thought to add to chest tube‐related complications.[8] In 2001, the American College of Chest Physicians (ACCP) published guidelines for the management of spontaneous pneumothorax that do not specifically address IP.[9] In 2010, the British Thoracic Society (BTS) updated their guidelines and included a brief statement on IP that described a higher incidence for it than for spontaneous pneumothorax and noted its relative ease of management.[10] Despite the lack of specific guidelines dedicated to IPs, common clinical practice is to manage iatrogenic defects in a manner similar to that for spontaneous ones. However, studies have shown that the management of pneumothorax remains diverse and that the adherence to these published guidelines is suboptimal.[10, 11] The BTS guidelines favor needle aspiration as the first‐line treatment,[10] whereas the ACCP recommends drainage with catheters over aspiration.[9]
The possibility of this complication, along with the rising rate of invasive interventions being performed, has led to expanded surveillance criteria for IP. Surveillance imaging, clinical observation, or a combination of the 2 may be required, depending on the institution, the risk of the procedure, and the preference of the treating clinician. The algorithms presented here were designed in alignment with both major society guidelines and with the intention of simplifying the treatment regimen for the ease of adoption by hospitalists.
ETIOLOGY AND RISK FACTORS
The etiology and risk factors for IP are multiple, with the most common being interventional‐based procedures. In 535 Veterans Administration patients, the most common precursor procedures were transthoracic needle biopsy (24%), subclavian vein catheterization (22%), thoracentesis (20%), transbronchial biopsy (10%), pleural biopsy (8%), and positive pressure ventilation.[12] IP can also be a rare complication of pacemaker manipulations,[5] and less commonly, bronchoscopy.[13] Patient factors that increase the risk of pneumothorax in the setting of an intervention include age, chronic obstructive lung disease, primary lung cancer, malignant and parapneumonic pleural effusions, empyema, and chronic corticosteroid use.[4] As might be expected, patients with structural lung disease (eg, emphysema with bullae) and poor healing ability (eg, corticosteroid dependent), tend to have IPs more often and to require more complicated interventions for resolution.[14, 15] In some studies, operator experience seems to be inversely related to the rate of IP, and the use of ultrasound is correlated with lower rates of this complication.[1, 3]
PATIENT PRESENTATIONS AND DIAGNOSIS
Clinical signs and symptoms of a significant pneumothorax vary in severity but most often include dyspnea, tachypnea, chest pain, and pleurisy (see Box 1). Post procedure signs or symptoms require further evaluation with imaging, usually a plain chest radiograph. CT can be useful for further evaluation. Small anterior pneumothoraces may be difficult to detect without lateral radiographic imaging or computed tomogram. Ultrasound is being used more frequently at the bedside to make this diagnosis, and various studies of trauma patients have found that it has good sensitivity and specificity.[16, 17] These results have been validated by a recent meta‐analysis comparing ultrasound to chest radiographs for the detection of pneumothorax among trauma, critically ill, and postprocedural patients.[18] This study demonstrated superior sensitivity and similar specificity for ultrasound versus chest radiographs for detection of pneumothorax. More ominous signs, such as tachycardia or hypotension, can be indicative of tension pneumothorax, which requires emergent evacuation.
MANAGEMENT
Once the diagnosis of pneumothorax has been established, treatment options should be guided by defect size and clinical assessment following a defined treatment algorithm (Figure 1). As emphasized by the BTS and ACCP guidelines, we advocate considering the use of symptoms along with defect size to determine the best management course.

Observation
Defects that involve<20% of the hemithorax in a patient who is clinically asymptomatic and hemodynamically stable can be safely managed by oxygen supplementation and hospital observation. Repeat imaging can be obtained after 12 to 24 hours of defect detection or with symptom change. Patients who display resolution may be discharged home.
Patients who show persistence without progression but are asymptomatic may also be discharged safely, with follow‐up imaging and clinical evaluation 48 hours later.[9, 10] This was demonstrated by Kelly and colleagues,[19] who described the outcomes of 154 patients in a retrospective cohort study. Of the 91 patients treated with outpatient observation, 82 resolved without additional interventions. A recent review article by the same author cites conservative management of small pneumothoraces as being widely accepted.[20] If reimaging shows progression of defect or if the patient becomes more symptomatic, the pneumothorax should be evacuated by 1 of the methods described below.
Aspiration
Aspiration is defined by the ACCP Delphi consensus statement as the removal of pleural air via needle or cannula followed by immediate removal of needle or cannula.[9] This option mandates careful patient selection. It should be considered for small pneumothoraces that cause only mild dyspnea in patients who have no known parenchymal disease. These patients should be observed overnight in the hospital and reimaged 24 hours after aspiration of the pneumothorax. Several authors have reported success with aspiration alone. Yamagami et al.[21] noted the efficacy of manual aspiration immediately after CT‐guided biopsy, with a success rate exceeding 90%. They also noted that evacuated volumes >543 mL correlated with the need for further intervention with a chest tube. This technique is advocated for small pneumothoraces that are recognized shortly after the procedure.
Similarly, Delius and colleagues[22] managed 131 pneumothoraces with aspiration as an alternative to chest tube placement. Of these, 79 were iatrogenic. Aspiration achieved a 75% success rate for all IPs. Small defects defined as <20% of volume had an even higher resolution rate of 87%. Similar findings were demonstrated by Talbot‐Stern et al.[23] in their prospective study of 76 pneumothoraces. Among those that were iatrogenic, 82% resolved after simple aspiration. Faruqi et al.[24] also showed that aspiration is a viable option for IPs. Of the 57 patients with pneumothorax included in their study, 35 were treated with aspiration alone. Iatrogenesis was the culprit in 12 of the 35 manually aspirated cases. Aspiration achieved a success rate of 91.7% in IP. A recent Cochrane database systematic review compared simple aspiration with intercostal tube drainage for primary spontaneous pneumothorax.[25] The authors reported no difference between these methods in terms of success rate, early failure rate, duration of hospital stay, 1‐year success rate, or number of patients who required pleurodesis at 1‐year follow‐up.
Because the algorithms presented in this article were specifically designed for the use by hospitalists, we intentionally omitted aspiration from the decision trees. Most hospitalists would not be expected to evacuate IPs. However, knowledge regarding this option and appropriate follow‐up are valuable to internists, because many interventionalists admit patients to the hospital service for overnight observation. An asymptomatic postaspiration patient, who on subsequent imaging demonstrates resolution or persistence without progression of pneumothorax, may be discharged with 48‐hour follow‐up.
Placement of Catheter or Chest Tube Drainage
Most patients with a clinically significant pneumothorax will require evacuation of the air. Pneumothoraces larger than 20% or that produce symptoms warrant chest tube management and inpatient observation (Figure 1B). Traditionally, large tubes with 20 cm of water on continuous suction are used and have been studied the most widely. Several authors have shown that smaller tubes can effectively drain a pneumothorax.[26, 27, 28, 29] Small‐bore catheters (8F14F), which can be inserted percutaneously, have been shown to provide effective lung re‐expansion with minimal morbidity[8] and may be better tolerated by patients with uncomplicated pneumothoraces (Figure 2). Terzi and colleagues[30] have shown that smaller tubes cause less discomfort to patients at rest, with cough, and at the time of tube removal.

At most US institutions, catheters and chest tubes are connected to all‐purpose drainage systems. Although commercially available through a variety of manufacturers, they share similar design principles because they replicate the 3‐bottle system described in detail elsewhere in the literature.[31] We have limited our discussion to 3 pleural evacuation systems because it is our intention to familiarize hospitalists with the units that they are most likely to encounter. The first 2 systems have been studied and described by Baumann and colleagues[32] as being commonplace and reasonably reliable. These include the Oasis (Atrium Medical Corp., Hudson, NH) (Figure 3A) and the Pleur‐evac (Teleflex Inc., Limerick, PA). The third unit is the Thopaz digital thoracic drainage system (Medela Inc., McHenry, IL) (Figure 3B). The Thopaz is unique in its inclusion of a suction source and digital capability. Although it utilizes the same principles of all pleural evacuation devices, its setup and information output require that one be familiar with its digital format.

Suction Versus Water Seal
The chest tube should be placed initially to a suction pressure level of 20 cm of water for 24 hours to maximize lung expansion and evacuate all extrapulmonary air. Suction pressure is set on the Pleur‐evac and Atrium drainage systems by a manual dial that reads to a water pressure of 0 to 40 cm. The default setting from the manufacturer is 20 cm of water. This level of suction is present only when the drainage system is connected to a wall or a portable suction device. The only confirmation of suction presence in the Atrium system is the deployment of the orange bellows (located under the dial) to the level of the arrow tip (Figure 3A). The Pleur‐evac system has a red stripe along the circular edge of the dial that appears at the set level of suction when negative pressure is being applied. It is important to be aware that when patients are disconnected from the wall or the portable suction apparatus, they are on water seal or gravity. These terms are synonymous with no suction. On the Thopaz, a digital menu directs operation, and levels of suction can be selected from water seal (no suction) up to 40 cm of water. We recommend using suction to 20 cm of water given the scarce evidence supporting higher levels of negative pressure. Some clinicians prefer placing patients on water seal for some time before moving toward tube discontinuance, but this is a matter of preference, and no substantial evidence exists to show that any 1 method is superior.[8, 33]
Assessing for Air Leak
If there is improvement or resolution of the pneumothorax after 24 hours, the presence of an air leak should be assessed; if no leak is present, the chest tube can be safely removed. In the context of chest tubes, the term air leak refers to residual air between the lung and the chest wall. It is possible to see resolution of a pneumothorax on chest radiographs and still have an air leak. This situation is created by a perfect balance between the pleural air evacuation by the catheter and the flow of air exiting from the lung puncture. This would result in reaccumulation of the pneumothorax if the chest tube is removed prematurely. It should also be kept in mind that chest radiographs may miss a small pneumothorax given their relatively low sensitivity.[18] Therefore, the absence of an air leak needs to be documented before the chest tube is discontinued. Depending on the type of drainage system (Atrium, Pleur‐evac, or Thopaz), this assessment can be done in several ways. All systems can be assessed for air leak by clamping the actual chest tube for 2 to 4 hours and then repeating the chest radiograph. Clamping a chest tube simulates the condition of not having a chest tube. Chest tubes should never be clamped without supervision and only with the knowledge of nursing personnel. The onset of chest pain or dyspnea in a patient with a clamped tube mandates immediate removal of the clamp and a return to suction. A repeat chest radiograph showing reaccumulation or expansion of the pneumothorax after clamping indicates that the air leak has not resolved and the chest tube must remain in place and returned to suction. Simpler and more time‐efficient methods of detecting air leaks are available with both cardiothoracic drainage systems.
For the Atrium and Pleur‐evac models, there is a graded panel through which one can visualize air leaks being funneled through water (Figure 4A). Having the patient cough several times or perform a Valsalva maneuver should release any air trapped within the chest into this chamber, where bubbles can be visualized as they travel through the water. The presence of bubbles indicates the presence of residual air in the chest, pointing to a possible leak. In contrast, the Thopaz system offers a graphical display of the air flowing into the system that can be reviewed over the 24‐hour period. When the graph line reaches a 0 flatline graph, no airflow is being detected and no air leakage is suspected (Figure 4B). If no air leaks are detected, the chest tube may be discontinued. Those patients with a failed air leak test should have their chest tubes continued under suction for another 24 hours, with the above tests then repeated. The same holds true for those patients with persistent pneumothorax at 24 hours.

Removal of chest tubes is a simple process that requires the tube to be pulled out of the patient without allowing air to enter the site where the tube was present and where it entered the thorax. Most interventionalists will discontinue the tubes that they have placed. Some small catheters have an internal string that has to be released so the catheter will straighten and pull out easily. Knowledge of the type of catheter or tube that was placed is critical before removal to prevent complications and patient discomfort. Standard chest tubes are straight, smooth plastic and pull out easily but require rapid occlusion of the larger puncture site in the chest wall with an occlusive dressing that often includes petroleum or water‐soluble gel. Some physicians will leave a suture tie when placing the chest tube so that it can be tied down to occlude the site instead of using a dressing.
Consultation of the Cardiothoracic Surgeon or Interventional Pulmonologist
We recommend the involvement of cardiothoracic surgery or interventional pulmonology for patients with nonresolving pneumothorax lasting longer than 48 hours because additional procedures may be necessary. One of the rare but serious complications of a persistent pneumothorax is the formation of a bronchopleural fistula. This communication between the bronchial tree and the pleural space can lead to significant morbidity and mortality. The treatment of a bronchopleural fistula includes medical and surgical options that are beyond the scope of this article but require the expertise of a cardiothoracic surgeon or interventional pulmonologist.[34] Most patients who will not require additional procedures will heal within 48 hours.[11, 35] Decisions regarding more invasive treatment measures can then be made as necessary.[26]
PRACTICAL TIPS
Hospitalists caring for patients with chest tubes are often asked to troubleshoot at the bedside. Scenarios that may be encountered include nonfunctioning tubes, catheter migration, and tube discomfort. Ensuring patency of the tube entails visualizing the tube from the point of entry into the chest wall to the collection chamber and inspecting for kinks or debris clogging the tube. Smaller catheters can be easily kinked during patient positioning and can become clogged. Respiratory variation, which is the movement of the column of fluid in the collection chamber or in the tubing with inspiration and expiration, suggests that the chest tube is patent. This should be part of the daily examination in a patient with a chest tube, and it should also be the first step in assessing sudden dyspnea, hypoxia, pain, or hemodynamic instability. Clogged tubes should be referred to the interventionalists or other physicians who placed them. Chest tubes are typically sutured at the site of entry and securely bandaged to avoid migration but occasionally can be dislodged. This should prompt placement of another tube by an experienced operator. Last, chest tubes can be uncomfortable for patients who may require systemic analgesics. Additionally, tube positioning may ease some of the discomfort. Chest tubes are commonly placed along the midaxillary line and the posterior thorax, leading to discomfort in the recumbent position. Directing the tube anteriorly helps ease some of the discomfort. This can be done using all‐purpose sponges to build a barrier between the skin and the chest tube as it is directed anteriorly. Additional sponges are placed above the tube for extra protection. The gentle curve accomplished by padding the underside of the tube also keeps the tube patent by avoiding sharp kinks as the catheter exits the thorax.
FUTURE TRENDS
Future trends in the management of IP may include shorter duration of tube management (14 hours of suction with air leak evaluation and removal) and the use of even smaller catheters. Outpatient management of IP with small pigtail catheters or small‐caliber tubes in addition to 1‐way valves is also being investigated. The benefits of these practices may include greater patient comfort and lower cost. These approaches will need larger‐scale replication and careful patient selection before they become standard practice.[28, 36]
Ultrasound can be used to assess the presence and size of pneumothoraces that are difficult to visualize by standard chest radiographs. Several studies have established ultrasonography as an effective method of diagnosing pneumothorax and have shown it to have superior sensitivity compared with chest radiography.[1, 18] In the future, the use of ultrasound will likely be more widespread given its performance, portability, ease of use, and relatively low cost.
SUMMARY
IP is a known and costly complication of many medical procedures. The aforementioned algorithms help simplify the management of chest tubes for hospitalists caring for patients with this common complication. This stepwise approach may not only help curtail added expenses related to IPs by decreasing the length of inpatient stays but may also improve patient satisfaction.
Acknowledgments
Disclosure: Mayo does not endorse the products mentioned in this article. The authors report no conflicts of interest.
Box
Clinical Signs and Symptoms of Pneumothorax
Dyspnea
Pleuritic chest pain
Tachypnea
Hypoxia
Decreased breath sounds on affected side
Hyper‐resonant percussion on affected side
Subcutaneous emphysema
A pneumothorax is a collection of air in the space outside the lungs that is trapped within the thorax. This abnormality can occur spontaneously or as the result of trauma. Traumatic pneumothoraces include those resulting from medical interventions such as a transthoracic and transbronchial needle biopsy, central line placement, and positive‐pressure mechanical ventilation. This group is most accurately described as iatrogenic pneumothorax (IP).[1]
IP can be an expected complication of many routine thoracic procedures, but it can also occur accidentally during procedures near the lung or thoracic cavity. Some IPs may be asymptomatic and go undiagnosed, or their diagnosis may be delayed.[2] The majority of iatrogenic pneumothoraces will resolve without complications, and patients will not require medical attention. A small percentage can, however, expand and have the potential to develop into a tension pneumothorax causing severe respiratory distress and mediastinal shift.[3, 4]
The incidence of IP ranges from 0.11% with mechanical ventilation to 2.68% with thoracentesis, according to an analysis of 7.5 million uniform hospital discharge abstracts from 2000.5 A 2010 systematic review of 24 studies that included 6605 patients suggested a 6.0% incidence of pneumothorax following thoracentesis.[3] The highest risk of IP is seen with computed tomography (CT)‐guided lung biopsy, with 1 series of 1098 biopsies showing a 42% incidence; chest tube evacuation was required in 12% of these cases.[4] A Veterans Administration study of patient safety indicators from 2001 to 2004 found that risk‐adjusted rates of IP were increasing over time.[6] It is unclear whether this increase is due to increasing numbers of interventional procedures or to better rates of detection. IP poses a considerable cost to the medical system, with safety studies finding that patients with IP will stay in the hospital approximately 4 days longer and incur an additional $17,000 in charges.[7]
In addition to this financial burden, the lack of consistency in training and guidelines for management of pneumothorax is thought to add to chest tube‐related complications.[8] In 2001, the American College of Chest Physicians (ACCP) published guidelines for the management of spontaneous pneumothorax that do not specifically address IP.[9] In 2010, the British Thoracic Society (BTS) updated their guidelines and included a brief statement on IP that described a higher incidence for it than for spontaneous pneumothorax and noted its relative ease of management.[10] Despite the lack of specific guidelines dedicated to IPs, common clinical practice is to manage iatrogenic defects in a manner similar to that for spontaneous ones. However, studies have shown that the management of pneumothorax remains diverse and that the adherence to these published guidelines is suboptimal.[10, 11] The BTS guidelines favor needle aspiration as the first‐line treatment,[10] whereas the ACCP recommends drainage with catheters over aspiration.[9]
The possibility of this complication, along with the rising rate of invasive interventions being performed, has led to expanded surveillance criteria for IP. Surveillance imaging, clinical observation, or a combination of the 2 may be required, depending on the institution, the risk of the procedure, and the preference of the treating clinician. The algorithms presented here were designed in alignment with both major society guidelines and with the intention of simplifying the treatment regimen for the ease of adoption by hospitalists.
ETIOLOGY AND RISK FACTORS
The etiology and risk factors for IP are multiple, with the most common being interventional‐based procedures. In 535 Veterans Administration patients, the most common precursor procedures were transthoracic needle biopsy (24%), subclavian vein catheterization (22%), thoracentesis (20%), transbronchial biopsy (10%), pleural biopsy (8%), and positive pressure ventilation.[12] IP can also be a rare complication of pacemaker manipulations,[5] and less commonly, bronchoscopy.[13] Patient factors that increase the risk of pneumothorax in the setting of an intervention include age, chronic obstructive lung disease, primary lung cancer, malignant and parapneumonic pleural effusions, empyema, and chronic corticosteroid use.[4] As might be expected, patients with structural lung disease (eg, emphysema with bullae) and poor healing ability (eg, corticosteroid dependent), tend to have IPs more often and to require more complicated interventions for resolution.[14, 15] In some studies, operator experience seems to be inversely related to the rate of IP, and the use of ultrasound is correlated with lower rates of this complication.[1, 3]
PATIENT PRESENTATIONS AND DIAGNOSIS
Clinical signs and symptoms of a significant pneumothorax vary in severity but most often include dyspnea, tachypnea, chest pain, and pleurisy (see Box 1). Post procedure signs or symptoms require further evaluation with imaging, usually a plain chest radiograph. CT can be useful for further evaluation. Small anterior pneumothoraces may be difficult to detect without lateral radiographic imaging or computed tomogram. Ultrasound is being used more frequently at the bedside to make this diagnosis, and various studies of trauma patients have found that it has good sensitivity and specificity.[16, 17] These results have been validated by a recent meta‐analysis comparing ultrasound to chest radiographs for the detection of pneumothorax among trauma, critically ill, and postprocedural patients.[18] This study demonstrated superior sensitivity and similar specificity for ultrasound versus chest radiographs for detection of pneumothorax. More ominous signs, such as tachycardia or hypotension, can be indicative of tension pneumothorax, which requires emergent evacuation.
MANAGEMENT
Once the diagnosis of pneumothorax has been established, treatment options should be guided by defect size and clinical assessment following a defined treatment algorithm (Figure 1). As emphasized by the BTS and ACCP guidelines, we advocate considering the use of symptoms along with defect size to determine the best management course.

Observation
Defects that involve<20% of the hemithorax in a patient who is clinically asymptomatic and hemodynamically stable can be safely managed by oxygen supplementation and hospital observation. Repeat imaging can be obtained after 12 to 24 hours of defect detection or with symptom change. Patients who display resolution may be discharged home.
Patients who show persistence without progression but are asymptomatic may also be discharged safely, with follow‐up imaging and clinical evaluation 48 hours later.[9, 10] This was demonstrated by Kelly and colleagues,[19] who described the outcomes of 154 patients in a retrospective cohort study. Of the 91 patients treated with outpatient observation, 82 resolved without additional interventions. A recent review article by the same author cites conservative management of small pneumothoraces as being widely accepted.[20] If reimaging shows progression of defect or if the patient becomes more symptomatic, the pneumothorax should be evacuated by 1 of the methods described below.
Aspiration
Aspiration is defined by the ACCP Delphi consensus statement as the removal of pleural air via needle or cannula followed by immediate removal of needle or cannula.[9] This option mandates careful patient selection. It should be considered for small pneumothoraces that cause only mild dyspnea in patients who have no known parenchymal disease. These patients should be observed overnight in the hospital and reimaged 24 hours after aspiration of the pneumothorax. Several authors have reported success with aspiration alone. Yamagami et al.[21] noted the efficacy of manual aspiration immediately after CT‐guided biopsy, with a success rate exceeding 90%. They also noted that evacuated volumes >543 mL correlated with the need for further intervention with a chest tube. This technique is advocated for small pneumothoraces that are recognized shortly after the procedure.
Similarly, Delius and colleagues[22] managed 131 pneumothoraces with aspiration as an alternative to chest tube placement. Of these, 79 were iatrogenic. Aspiration achieved a 75% success rate for all IPs. Small defects defined as <20% of volume had an even higher resolution rate of 87%. Similar findings were demonstrated by Talbot‐Stern et al.[23] in their prospective study of 76 pneumothoraces. Among those that were iatrogenic, 82% resolved after simple aspiration. Faruqi et al.[24] also showed that aspiration is a viable option for IPs. Of the 57 patients with pneumothorax included in their study, 35 were treated with aspiration alone. Iatrogenesis was the culprit in 12 of the 35 manually aspirated cases. Aspiration achieved a success rate of 91.7% in IP. A recent Cochrane database systematic review compared simple aspiration with intercostal tube drainage for primary spontaneous pneumothorax.[25] The authors reported no difference between these methods in terms of success rate, early failure rate, duration of hospital stay, 1‐year success rate, or number of patients who required pleurodesis at 1‐year follow‐up.
Because the algorithms presented in this article were specifically designed for the use by hospitalists, we intentionally omitted aspiration from the decision trees. Most hospitalists would not be expected to evacuate IPs. However, knowledge regarding this option and appropriate follow‐up are valuable to internists, because many interventionalists admit patients to the hospital service for overnight observation. An asymptomatic postaspiration patient, who on subsequent imaging demonstrates resolution or persistence without progression of pneumothorax, may be discharged with 48‐hour follow‐up.
Placement of Catheter or Chest Tube Drainage
Most patients with a clinically significant pneumothorax will require evacuation of the air. Pneumothoraces larger than 20% or that produce symptoms warrant chest tube management and inpatient observation (Figure 1B). Traditionally, large tubes with 20 cm of water on continuous suction are used and have been studied the most widely. Several authors have shown that smaller tubes can effectively drain a pneumothorax.[26, 27, 28, 29] Small‐bore catheters (8F14F), which can be inserted percutaneously, have been shown to provide effective lung re‐expansion with minimal morbidity[8] and may be better tolerated by patients with uncomplicated pneumothoraces (Figure 2). Terzi and colleagues[30] have shown that smaller tubes cause less discomfort to patients at rest, with cough, and at the time of tube removal.

At most US institutions, catheters and chest tubes are connected to all‐purpose drainage systems. Although commercially available through a variety of manufacturers, they share similar design principles because they replicate the 3‐bottle system described in detail elsewhere in the literature.[31] We have limited our discussion to 3 pleural evacuation systems because it is our intention to familiarize hospitalists with the units that they are most likely to encounter. The first 2 systems have been studied and described by Baumann and colleagues[32] as being commonplace and reasonably reliable. These include the Oasis (Atrium Medical Corp., Hudson, NH) (Figure 3A) and the Pleur‐evac (Teleflex Inc., Limerick, PA). The third unit is the Thopaz digital thoracic drainage system (Medela Inc., McHenry, IL) (Figure 3B). The Thopaz is unique in its inclusion of a suction source and digital capability. Although it utilizes the same principles of all pleural evacuation devices, its setup and information output require that one be familiar with its digital format.

Suction Versus Water Seal
The chest tube should be placed initially to a suction pressure level of 20 cm of water for 24 hours to maximize lung expansion and evacuate all extrapulmonary air. Suction pressure is set on the Pleur‐evac and Atrium drainage systems by a manual dial that reads to a water pressure of 0 to 40 cm. The default setting from the manufacturer is 20 cm of water. This level of suction is present only when the drainage system is connected to a wall or a portable suction device. The only confirmation of suction presence in the Atrium system is the deployment of the orange bellows (located under the dial) to the level of the arrow tip (Figure 3A). The Pleur‐evac system has a red stripe along the circular edge of the dial that appears at the set level of suction when negative pressure is being applied. It is important to be aware that when patients are disconnected from the wall or the portable suction apparatus, they are on water seal or gravity. These terms are synonymous with no suction. On the Thopaz, a digital menu directs operation, and levels of suction can be selected from water seal (no suction) up to 40 cm of water. We recommend using suction to 20 cm of water given the scarce evidence supporting higher levels of negative pressure. Some clinicians prefer placing patients on water seal for some time before moving toward tube discontinuance, but this is a matter of preference, and no substantial evidence exists to show that any 1 method is superior.[8, 33]
Assessing for Air Leak
If there is improvement or resolution of the pneumothorax after 24 hours, the presence of an air leak should be assessed; if no leak is present, the chest tube can be safely removed. In the context of chest tubes, the term air leak refers to residual air between the lung and the chest wall. It is possible to see resolution of a pneumothorax on chest radiographs and still have an air leak. This situation is created by a perfect balance between the pleural air evacuation by the catheter and the flow of air exiting from the lung puncture. This would result in reaccumulation of the pneumothorax if the chest tube is removed prematurely. It should also be kept in mind that chest radiographs may miss a small pneumothorax given their relatively low sensitivity.[18] Therefore, the absence of an air leak needs to be documented before the chest tube is discontinued. Depending on the type of drainage system (Atrium, Pleur‐evac, or Thopaz), this assessment can be done in several ways. All systems can be assessed for air leak by clamping the actual chest tube for 2 to 4 hours and then repeating the chest radiograph. Clamping a chest tube simulates the condition of not having a chest tube. Chest tubes should never be clamped without supervision and only with the knowledge of nursing personnel. The onset of chest pain or dyspnea in a patient with a clamped tube mandates immediate removal of the clamp and a return to suction. A repeat chest radiograph showing reaccumulation or expansion of the pneumothorax after clamping indicates that the air leak has not resolved and the chest tube must remain in place and returned to suction. Simpler and more time‐efficient methods of detecting air leaks are available with both cardiothoracic drainage systems.
For the Atrium and Pleur‐evac models, there is a graded panel through which one can visualize air leaks being funneled through water (Figure 4A). Having the patient cough several times or perform a Valsalva maneuver should release any air trapped within the chest into this chamber, where bubbles can be visualized as they travel through the water. The presence of bubbles indicates the presence of residual air in the chest, pointing to a possible leak. In contrast, the Thopaz system offers a graphical display of the air flowing into the system that can be reviewed over the 24‐hour period. When the graph line reaches a 0 flatline graph, no airflow is being detected and no air leakage is suspected (Figure 4B). If no air leaks are detected, the chest tube may be discontinued. Those patients with a failed air leak test should have their chest tubes continued under suction for another 24 hours, with the above tests then repeated. The same holds true for those patients with persistent pneumothorax at 24 hours.

Removal of chest tubes is a simple process that requires the tube to be pulled out of the patient without allowing air to enter the site where the tube was present and where it entered the thorax. Most interventionalists will discontinue the tubes that they have placed. Some small catheters have an internal string that has to be released so the catheter will straighten and pull out easily. Knowledge of the type of catheter or tube that was placed is critical before removal to prevent complications and patient discomfort. Standard chest tubes are straight, smooth plastic and pull out easily but require rapid occlusion of the larger puncture site in the chest wall with an occlusive dressing that often includes petroleum or water‐soluble gel. Some physicians will leave a suture tie when placing the chest tube so that it can be tied down to occlude the site instead of using a dressing.
Consultation of the Cardiothoracic Surgeon or Interventional Pulmonologist
We recommend the involvement of cardiothoracic surgery or interventional pulmonology for patients with nonresolving pneumothorax lasting longer than 48 hours because additional procedures may be necessary. One of the rare but serious complications of a persistent pneumothorax is the formation of a bronchopleural fistula. This communication between the bronchial tree and the pleural space can lead to significant morbidity and mortality. The treatment of a bronchopleural fistula includes medical and surgical options that are beyond the scope of this article but require the expertise of a cardiothoracic surgeon or interventional pulmonologist.[34] Most patients who will not require additional procedures will heal within 48 hours.[11, 35] Decisions regarding more invasive treatment measures can then be made as necessary.[26]
PRACTICAL TIPS
Hospitalists caring for patients with chest tubes are often asked to troubleshoot at the bedside. Scenarios that may be encountered include nonfunctioning tubes, catheter migration, and tube discomfort. Ensuring patency of the tube entails visualizing the tube from the point of entry into the chest wall to the collection chamber and inspecting for kinks or debris clogging the tube. Smaller catheters can be easily kinked during patient positioning and can become clogged. Respiratory variation, which is the movement of the column of fluid in the collection chamber or in the tubing with inspiration and expiration, suggests that the chest tube is patent. This should be part of the daily examination in a patient with a chest tube, and it should also be the first step in assessing sudden dyspnea, hypoxia, pain, or hemodynamic instability. Clogged tubes should be referred to the interventionalists or other physicians who placed them. Chest tubes are typically sutured at the site of entry and securely bandaged to avoid migration but occasionally can be dislodged. This should prompt placement of another tube by an experienced operator. Last, chest tubes can be uncomfortable for patients who may require systemic analgesics. Additionally, tube positioning may ease some of the discomfort. Chest tubes are commonly placed along the midaxillary line and the posterior thorax, leading to discomfort in the recumbent position. Directing the tube anteriorly helps ease some of the discomfort. This can be done using all‐purpose sponges to build a barrier between the skin and the chest tube as it is directed anteriorly. Additional sponges are placed above the tube for extra protection. The gentle curve accomplished by padding the underside of the tube also keeps the tube patent by avoiding sharp kinks as the catheter exits the thorax.
FUTURE TRENDS
Future trends in the management of IP may include shorter duration of tube management (14 hours of suction with air leak evaluation and removal) and the use of even smaller catheters. Outpatient management of IP with small pigtail catheters or small‐caliber tubes in addition to 1‐way valves is also being investigated. The benefits of these practices may include greater patient comfort and lower cost. These approaches will need larger‐scale replication and careful patient selection before they become standard practice.[28, 36]
Ultrasound can be used to assess the presence and size of pneumothoraces that are difficult to visualize by standard chest radiographs. Several studies have established ultrasonography as an effective method of diagnosing pneumothorax and have shown it to have superior sensitivity compared with chest radiography.[1, 18] In the future, the use of ultrasound will likely be more widespread given its performance, portability, ease of use, and relatively low cost.
SUMMARY
IP is a known and costly complication of many medical procedures. The aforementioned algorithms help simplify the management of chest tubes for hospitalists caring for patients with this common complication. This stepwise approach may not only help curtail added expenses related to IPs by decreasing the length of inpatient stays but may also improve patient satisfaction.
Acknowledgments
Disclosure: Mayo does not endorse the products mentioned in this article. The authors report no conflicts of interest.
Box
Clinical Signs and Symptoms of Pneumothorax
Dyspnea
Pleuritic chest pain
Tachypnea
Hypoxia
Decreased breath sounds on affected side
Hyper‐resonant percussion on affected side
Subcutaneous emphysema
- , . Management of pneumothorax. Semin Respir Crit Care Med. 2010;31(6):769–780.
- , . Pneumothorax. Respirology. 2004;9(2):157–164.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy‐guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9‐year period. AJR Am J Roentgenol. 2010;194(3):809–814.
- , , . Accidental iatrogenic pneumothorax in hospitalized patients. Med Care. 2006;44(2):182–186.
- , , , et al. Tracking rates of patient safety indicators over time: lessons from the Veterans Administration. Med Care. 2006;44(9):850–861.
- , . Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290(14):1868–1874.
- , , , , . Preliminary report of a prospective, randomized trial of underwater seal for spontaneous and iatrogenic pneumothorax. J Am Coll Surg. 2007;204(1):84–90.
- , , , et al.; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119(2):590–602.
- , , ; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii18–ii31.
- , . The clinician's perspective on pneumothorax management. Chest. 1997;112(3):822–828.
- , , , . Iatrogenic pneumothorax: etiology and morbidity: results of a Department of Veterans Affairs Cooperative Study. Respiration. 1992;59(4):215–220.
- , , , , , . Severe complications of bronchoscopy. Respiration. 2008;76(4):429–433.
- , , , et al. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest. 2004;126(5):1516–1521.
- , , , , , . Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15(5):479–483.
- , . Ultrasound detection of pneumothorax compared with chest X‐ray and computed tomography scan. Am Surg. 2011;77(4):480–484.
- , . Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010;17(1):11–17.
- , , , , . Diagnosis of pneumothorax by radiography and ultrasonography: a meta‐analysis. Chest. 2011;140(4):859–866.
- , , . Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest. 2008;134(5):1033–1036.
- . Review of management of primary spontaneous pneumothorax: is the best evidence clearer 15 years on? Emerg Med Australas. 2007;19(4):303–308.
- , , , , , . Efficacy of manual aspiration immediately after complicated pneumothorax in CT‐guided lung biopsy. J Vasc Interv Radiol. 2005;16(4):477–483.
- , , , , , . Catheter aspiration for simple pneumothorax: experience with 114 patients. Arch Surg. 1998;124(7):833–836.
- , , , , . Catheter aspiration for simple pneumothorax. J Emerg Med. 1986;4(6):437–442.
- , , , . Role of simple needle aspiration in the management of pneumothorax. Indian J Chest Dis Allied Sci. 2004;46(3):183–190.
- , , . Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev. 2007;(1):CD004479.
- , , , . Evaluation of conventional chest tube therapy for iatrogenic pneumothorax. Chest. 1993;104(6):1770–1772.
- , , , . Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology. 1997;205(1):249–252.
- , , , , , . Outpatient management of postbiopsy pneumothorax with small‐caliber chest tubes: factors affecting the need for prolonged drainage and additional interventions. Cardiovasc Intervent Radiol. 2008;31(2):342–348.
- , . Management of primary and secondary pneumothorax using a small‐bore thoracic catheter. Interact Cardiovasc Thorac Surg. 2010;11(2):146–149.
- , , , et al. The use of flexible spiral drains after non‐cardiac thoracic surgery: a clinical study. Eur J Cardiothorac Surg. 2005;27(1):134–137.
- . Pleural Diseases. 5th ed. Philadelphia: PA: Lippincott Williams 2007.
- , , , . Comparison of function of commercially available pleural drainage units and catheters. Chest. 2003;123(6):1878–1886.
- , . Catheter drainage of spontaneous pneumothorax: suction or no suction, early or late removal? Thorax. 1982;37(1):46–48.
- , . Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128(6):3955–3965.
- , , , . Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg. 1991;126(6):764–766.
- , , , , . A treatment algorithm for pneumothoraces complicating central venous catheter insertion. Am J Surg. 2000;180(6):523–526.
- , . Management of pneumothorax. Semin Respir Crit Care Med. 2010;31(6):769–780.
- , . Pneumothorax. Respirology. 2004;9(2):157–164.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy‐guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9‐year period. AJR Am J Roentgenol. 2010;194(3):809–814.
- , , . Accidental iatrogenic pneumothorax in hospitalized patients. Med Care. 2006;44(2):182–186.
- , , , et al. Tracking rates of patient safety indicators over time: lessons from the Veterans Administration. Med Care. 2006;44(9):850–861.
- , . Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290(14):1868–1874.
- , , , , . Preliminary report of a prospective, randomized trial of underwater seal for spontaneous and iatrogenic pneumothorax. J Am Coll Surg. 2007;204(1):84–90.
- , , , et al.; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119(2):590–602.
- , , ; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii18–ii31.
- , . The clinician's perspective on pneumothorax management. Chest. 1997;112(3):822–828.
- , , , . Iatrogenic pneumothorax: etiology and morbidity: results of a Department of Veterans Affairs Cooperative Study. Respiration. 1992;59(4):215–220.
- , , , , , . Severe complications of bronchoscopy. Respiration. 2008;76(4):429–433.
- , , , et al. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest. 2004;126(5):1516–1521.
- , , , , , . Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15(5):479–483.
- , . Ultrasound detection of pneumothorax compared with chest X‐ray and computed tomography scan. Am Surg. 2011;77(4):480–484.
- , . Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010;17(1):11–17.
- , , , , . Diagnosis of pneumothorax by radiography and ultrasonography: a meta‐analysis. Chest. 2011;140(4):859–866.
- , , . Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest. 2008;134(5):1033–1036.
- . Review of management of primary spontaneous pneumothorax: is the best evidence clearer 15 years on? Emerg Med Australas. 2007;19(4):303–308.
- , , , , , . Efficacy of manual aspiration immediately after complicated pneumothorax in CT‐guided lung biopsy. J Vasc Interv Radiol. 2005;16(4):477–483.
- , , , , , . Catheter aspiration for simple pneumothorax: experience with 114 patients. Arch Surg. 1998;124(7):833–836.
- , , , , . Catheter aspiration for simple pneumothorax. J Emerg Med. 1986;4(6):437–442.
- , , , . Role of simple needle aspiration in the management of pneumothorax. Indian J Chest Dis Allied Sci. 2004;46(3):183–190.
- , , . Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev. 2007;(1):CD004479.
- , , , . Evaluation of conventional chest tube therapy for iatrogenic pneumothorax. Chest. 1993;104(6):1770–1772.
- , , , . Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology. 1997;205(1):249–252.
- , , , , , . Outpatient management of postbiopsy pneumothorax with small‐caliber chest tubes: factors affecting the need for prolonged drainage and additional interventions. Cardiovasc Intervent Radiol. 2008;31(2):342–348.
- , . Management of primary and secondary pneumothorax using a small‐bore thoracic catheter. Interact Cardiovasc Thorac Surg. 2010;11(2):146–149.
- , , , et al. The use of flexible spiral drains after non‐cardiac thoracic surgery: a clinical study. Eur J Cardiothorac Surg. 2005;27(1):134–137.
- . Pleural Diseases. 5th ed. Philadelphia: PA: Lippincott Williams 2007.
- , , , . Comparison of function of commercially available pleural drainage units and catheters. Chest. 2003;123(6):1878–1886.
- , . Catheter drainage of spontaneous pneumothorax: suction or no suction, early or late removal? Thorax. 1982;37(1):46–48.
- , . Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128(6):3955–3965.
- , , , . Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg. 1991;126(6):764–766.
- , , , , . A treatment algorithm for pneumothoraces complicating central venous catheter insertion. Am J Surg. 2000;180(6):523–526.
Hospitalist Postgraduate PA Training Program
In recent years, the demand for hospitalists has outstripped the supply, creating a national shortage.1, 2 A recent Society of Hospital Medicine (SHM) survey found that in the last 2 years there has been a 31% mean growth increase in the number of hospitalist groups.3 As hospitalists are becoming more difficult to recruit, many practices are utilizing physician assistants (PAs) and nurse practitioners (NPs), collectively referred to as nonphysician providers (NPPs) to help offset the workload.4 The SHM survey also noted that the number of hospitalist groups utilizing NPPs increased from 29% to 38%.3 The exact number of NPPs working for hospitalist groups is unknown.
Hospitalist NPPs are in demand for reasons other than just physician shortages. NPPs have been utilized to fill the gap in many institutions where the workforce was impacted by the 2002 Accreditation Council for Graduate Medical Education (ACGME) ruling to restrict resident work hours. Several studies have documented NPPs' ability to assist with the compliance of ACCGME resident work‐hour restrictions while maintaining patient continuity of care, improving length of stays, and reducing health care costs on various hospital services.59 Dresselhaus et al.10 found that 56% of medical resident's time on service was delegated to tasks not related to direct patient care. They proposed that these tasks can be delegated to the NPPs, leaving more time for the residents to focus on direct patient care. In a recent study performed at a Pennsylvania hospital, patients presenting to the emergency department with low‐risk chest pain (based upon thrombolysis in myocardial infarction [TIMI] risk score) were admitted to a nonteaching service staffed with NPPs and attending physicians. Simultaneously, a similar group of low‐risk chest pain patients were admitted to a traditional internal medicine resident service. The results demonstrated lower median length of stay and hospital charges on the nonteaching service. This study suggested that NPPs can offset the workload volume for medical residents, allowing them to focus on patients with higher acuity and greater learning value.11
Barriers to Finding Experienced NPPs in Hospital Medicine
Although many hospitalist groups are interested in hiring NPPs, there can be significant obstacles to recruitment. For example, most experienced PAs and NPs have clinical backgrounds in either surgical or medical subspecialties and therefore typically need extensive on‐the‐job training in hospital medicine, which can often take at least 6 to 12 months to acquire the basic skill set.12 Hiring new graduates may require even longer training periods.
The inexperience of new graduates has become an even more pertinent issue due to recent changes in PA education. Traditionally, PA programs attracted older students with prior healthcare experience, who wished to return to school for additional training. However, in 2005 a major shift occurred in PA education: programs began transitioning from graduating trainees with a bachelor's degree to now requiring a master's level degree for completion of the PA program.13 The acquisition of more advanced degrees has changed the demographics of the students matriculating into PA programs, attracting younger students, straight from undergraduate institutions, with less prior healthcare experience.14 As a result, not only are new PA graduates less experienced overall, but they are particularly lacking in exposure to hospital medicine. After PA students complete their first 12 months of PA school in the basic sciences and didactic coursework, they embark on 12 to 15 months of clinical rotations, which are largely rooted in primary care. In fact, many PA programs find it difficult to offer hospital‐based rotations while fulfilling the required rotations in primary care. These factors have resulted in the need for more extensive on‐the‐job training particularly for those new graduates interested in hospital medicine. In light of these challenges, our institution created a 12‐month postgraduate PA fellowship program in Hospital Medicine.
Postgraduate PA Training Programs
Postgraduate PA fellowships, interchangeably called residencies, are voluntary 1‐year training programs that provide both didactic instruction and clinical experience in a medical or surgical subspecialty, thereby lessening the need for on‐the‐job training. These programs are recognized by the Association of Postgraduate Physician Assistant Programs.15 Currently, there are 44 postgraduate training programs in the United States, in a wide range of medical and surgical specialties. At the end of these 1‐year postgraduate PA programs, most graduates receive a certificate of completion. Until now, the only postgraduate education option for PAs interested in Hospital Medicine was a master's completion program only available to PAs who were already employed by a hospitalist group.15 This work reviews the first reported postgraduate hospitalist training program for PAs. Specifically, the program's background, curriculum, anticipated program outcomes, and future plans are discussed.
Background for A Hospitalist Postgraduate PA Fellowship
Mayo Clinic Arizona is a multispecialty private group comprised of both outpatient services and a tertiary care hospital medical center, located in the metropolitan Phoenix, AZ, area. The Mayo Clinic Hospital is a 7‐story facility with 244 licensed beds, 18 operating rooms, and a Level II emergency department. The Mayo Hospitalist group is composed of 15 full time hospitalists and 6 part‐time hospitalists, all of whom are salaried Mayo employees. The group provides 24‐hour in‐house staffing, covering both resident services (teams composed of interns and residents supervised by a staff hospitalist) and nonresident services (staff hospitalists). Over the years there has been steady growth in the number of nonresident services, in part due to resident work‐hour restrictions. To support the physicians working on these nonresident services, the first PA was hired in 2001. Since then, the number of NPPs in our Hospitalist group has increased to 9.35 full‐time equivalents (FTEs), including 1 nurse practitioner. However, one of the greatest challenges in expanding the NPP service was the difficulty finding candidates with experience in hospital internal medicine. This need inspired the creation of a PA fellowship in Hospital Medicine. At the time, there were 2 other postgraduate PA training programs at the Mayo Clinic Arizona in Hepatology and Otolaryngology/Ear, Nose, and Throat (ENT) Surgery.
Program Description
The Mayo Clinic Arizona PA fellowship in Hospital Medicine began in October 2007 and currently accepts 1 fellow per year. Applicants must be graduates of an Accreditation Review Commission in Education for the Physician Assistant (ARC‐PA)‐accredited PA program and be certified through the National Commission on Certification of Physician Assistants (NCCPA). Furthermore, they must be licensed to work as a PA in the state of Arizona. The program is 12 months in duration, and is comprised of both didactic and clinical components. Upon graduation, the fellow earns a certificate of completion from the Mayo Clinic College of Medicine. The program has received recognition with the Association of Postgraduate Physician Assistant Programs (APPAP).
Two physician assistants act as co‐program directors of the PA fellowship in hospital medicine. They are given 0.10 full‐time equivalent (FTE) for management of the program, which includes day‐to‐day operations, curriculum development, and candidate selection. The program also has 2 volunteer physician medical directors, both of whom have previous medical residency experience. The physicians and NPPs in our hospitalist group volunteer their time to serve as faculty for the program, assisting with much of the didactic and clinical education. The program receives a budget of $99,500 per year, which is funded by the organization's foundation through the department of education. This includes the fellow stipend of $44,000 per 12 months and institutional malpractice insurance coverage. The fellow also receives health and dental insurance, 2 weeks of paid vacation, and $500 stipend toward attendance of a continuing medical education (CME) conference.
CURRICULUM
The PA fellowship curriculum is designed in a diverse unique format that strives to accommodate all types of learners. It includes clinical rotations in various medicine/surgical subspecialties, didactic instruction, and teaching modules (Figure 1). The curriculum is based upon the SHM Core Competencies.15
Clinical Rotations
The PA fellow completes 12 to 14 general hospital medicine and medical specialty rotations, each 2 to 4 weeks in duration. The rotation calendar for the current fellow is given in Figure 2. These rotations are all inpatient‐based and are supervised by either the hospitalist or the respective inpatient subspecialists. The PA fellow's specific clinical responsibilities vary from rotation to rotation, and are designed to maximize the fellow's exposure to that particular specialty. Each rotation has specific written objectives created by the program directors and reviewed by the rotation's preceptor(s) (Figure 2). During the clinical rotations, complementary didactic lectures, coursework, and readings are provided to ensure the PA fellow receives a strong foundation. Didactic instruction is designed by the program directors, physician preceptors and staff NPPs, and is coordinated with the clinical rotation specialty. At the end of each rotation the fellow is evaluated by the preceptor and given direct feedback on their performance.

Didactic Instruction
The didactic instruction is organized in a system‐based manner and occurs on a weekly basis during the Hospital Internal Medicine service and Medicine Consults rotations. Hospitalist NPPs and physician faculty are responsible for most of the teaching. This formal didactic instruction is supplemented by journal club presentations given by the PA fellow to faculty in the division of hospital internal medicine. The fellow is also required to attend daily medical resident lunchtime educational lectures, weekly medical grand rounds, and any lectures provided by the medicine subspecialties while the PA is on that particular rotation.
Teaching Modules
One component of the Hospital Medicine PA fellowship curriculum that may be unique is the concept of teaching modules. While receiving regular didactic instruction and completing their clinical rotations, the PA is also expected to complete self‐directed teaching module assignments. These modules serve to educate the PA fellow on the hospital as a systemthe true essence of hospital medicine. The modules cover a variety of topics not directly addressed during their rotations. These topics are outlined in Figure 3. Each teaching module consists of a didactic component, clinical application, and assessment (Figure 4) and has its own specific objectives and goals. Teaching modules are often taught by the local expert in the hospital in that particular area. For example, for the infectious control teaching module, the PA fellow will rotate with the infection control nursing staff learning about the isolation and infection control policies of the institution.

Assessment Tools
There are several tools utilized to assess both the PA fellow and the fellowship program itself (Figure 5). The assessment tools used include both ongoing and summative assessments. To fulfill the ongoing assessment, each rotation and teaching module contains assessment tools provided by the preceptor, which are reviewed by the program directors. Additionally, during the clinical rotations, skills are assessed using competency checklists that require the preceptor to directly observe the PA fellow perform a specific task or skill‐set and sign off on its successful completion (Supplementary Figures 6, 7).

There are 2 forms of summative assessment for the PA fellow. First, to assess the PA fellow's knowledge, comprehensive mid‐year and end‐year examinations are utilized. These multiple‐choice examinations are comprised of questions which align with the didactic lectures/objectives provided by the Hospital Medicine faculty throughout the year. The second form of summative evaluation of the fellow is project‐based and divided into 2 parts. First, the fellow is expected to write a publication‐quality manuscript on a hospital medicine topic by the end of the year. Second, the PA fellow is expected to create a professional portfolio, which is comprised of a collection of all of the rotation/module assessments, the formal program assessments, and documentation of all of the skills obtained by the fellow throughout year (competency checklists). This portfolio can be used by the graduate to demonstrate to future employers what skills they possess and provide documentation of knowledge gained during the fellowship.
The program itself is evaluated by several measures. First, the fellow provides formal feedback during the mid‐year and end‐of‐the‐year assessments, which are used to enhance the experience of future fellows. Second, there is ongoing review by both the division of Hospital Medicine and the institution's Allied Health Education Committee, which ensures that the program maintains the appropriate standards and goals.
Future Goals for the PA Fellowship
The program graduated its first fellow at the end of October 2008 and has enjoyed early success. Integrating the PA fellow onto the hospitalist services augmented the present mid‐level and physician teams. There has been excellent institutional support for the program with extremely positive feedback from the rotation preceptors. There are several futures plans for the program. Our first goal is to seek accreditation from the Accreditation Review Commission for Physician Assistants (ARC‐PA), the organization that accredits entry level PA programs and which began formal, voluntary accreditation of postgraduate programs in early 2008. We plan to begin this process within the next academic year.
Our second long‐term goal for the program is to include NPs in the training program. Because of the desire to seek accreditation, the program directors felt temporarily limiting the fellowship to PAs would aide in the rigorous accreditation process, which can take approximately 1 year to complete. There is an NP on our faculty and the program has received interest from NPs. Once we obtain accreditation, expand the program enrollment, and develop an NP curriculum, we plan to open the fellowship to either PA or NP applicants.
Our third goal is to substantiate our PA Fellowship validity with outcome measures and ultimately publishable data. Thus far, the success of the PA fellowship is qualitative, and with small numbers of graduates it is difficult to quantify. After graduation of many subsequent PA fellows, our goal is to obtain quantifiable data that can be used to improve the quality of the PA fellowship and demonstrate the value of postgraduate training for physician assistants.
Perhaps the most important goal of the program is to eventually accept additional PA/NP fellows per year. While 1 program does not meet the demands of a national shortage of hospitalist providers, it may serve as a model that other institutions can adapt to their own needs. Since the program is based upon the SHM Core Competencies, the curriculum can be applied to a variety of hospitalist programs, and its relatively low operating cost makes it feasible for both academic‐based and community‐based institutions. Importantly, since recruitment and retention of employees is such a challenge for most hospitalist groups, this PA fellowship program may serve as a vehicle for recruitment and long‐term retention of well‐trained employees. This precedent has been set, as our division has hired our first PA fellow, whose transition from PA fellow to PA staff was seamless.
In conclusion, our PA fellowship in Hospital Medicine represents the first reported postgraduate PA program of this kind in the United States offering a certificate of completion. As the need for hospitalists increase so will the need for NPPs, particularly those with additional training in hospital medicine. This program serves as an example of 1 type of training tool for physician assistants looking to work in hospital medicine.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2004;20:101–107.
- .Innovations in the management of hospitalized patients. Nurse Pract Spring2006 (suppl):2–3.
- .Hospitalist pay up, productivity steady in SHM's latest survey.Hospitalist.2008;12(5):7,16.
- .Physician assistants: filling the gap in patient care in academic hospitals.Perspect Physician Assist Educ.2003;14(3):158–167.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,, et al.Physician extenders impact trauma systems.J Trauma.2005;58(5):917–920.
- ,,.The role of physician assistants in critical care units.Chest.1991;99:89–91.
- .Alliances: invaluable assistants.Hospitalist.2006;April:32–33.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–42.
- ,,,,,.Analyzing the time and value of house staff inpatient work.J Intern Med.1998;13:534–540.
- ,,, et al.Improving resource utilization in a teaching hospital: Development of a nonteaching service for chest pain admissions.Acad Med.2006;81(5):432–435.
- .Midlevels make a rocky entrance into hospital medicine.Todays Hospitalist.2007;5(1):28–32.
- Accreditation Review Commission for Physician Assistant Education.3rd ed. 2005. Available at: http://www.arc‐pa.org/Standards/standards.html. Accessed September2009.
- 22nd Annual Report on Physician Assistant Education in the U.S., 2005–2006. Available at: http://www.paeaonline.org. Accessed September2009.
- Association of Postgraduate Physician Assistant Programs. Available at: http://www.appap.org. Accessed September2009.
- ,,,,.The core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
In recent years, the demand for hospitalists has outstripped the supply, creating a national shortage.1, 2 A recent Society of Hospital Medicine (SHM) survey found that in the last 2 years there has been a 31% mean growth increase in the number of hospitalist groups.3 As hospitalists are becoming more difficult to recruit, many practices are utilizing physician assistants (PAs) and nurse practitioners (NPs), collectively referred to as nonphysician providers (NPPs) to help offset the workload.4 The SHM survey also noted that the number of hospitalist groups utilizing NPPs increased from 29% to 38%.3 The exact number of NPPs working for hospitalist groups is unknown.
Hospitalist NPPs are in demand for reasons other than just physician shortages. NPPs have been utilized to fill the gap in many institutions where the workforce was impacted by the 2002 Accreditation Council for Graduate Medical Education (ACGME) ruling to restrict resident work hours. Several studies have documented NPPs' ability to assist with the compliance of ACCGME resident work‐hour restrictions while maintaining patient continuity of care, improving length of stays, and reducing health care costs on various hospital services.59 Dresselhaus et al.10 found that 56% of medical resident's time on service was delegated to tasks not related to direct patient care. They proposed that these tasks can be delegated to the NPPs, leaving more time for the residents to focus on direct patient care. In a recent study performed at a Pennsylvania hospital, patients presenting to the emergency department with low‐risk chest pain (based upon thrombolysis in myocardial infarction [TIMI] risk score) were admitted to a nonteaching service staffed with NPPs and attending physicians. Simultaneously, a similar group of low‐risk chest pain patients were admitted to a traditional internal medicine resident service. The results demonstrated lower median length of stay and hospital charges on the nonteaching service. This study suggested that NPPs can offset the workload volume for medical residents, allowing them to focus on patients with higher acuity and greater learning value.11
Barriers to Finding Experienced NPPs in Hospital Medicine
Although many hospitalist groups are interested in hiring NPPs, there can be significant obstacles to recruitment. For example, most experienced PAs and NPs have clinical backgrounds in either surgical or medical subspecialties and therefore typically need extensive on‐the‐job training in hospital medicine, which can often take at least 6 to 12 months to acquire the basic skill set.12 Hiring new graduates may require even longer training periods.
The inexperience of new graduates has become an even more pertinent issue due to recent changes in PA education. Traditionally, PA programs attracted older students with prior healthcare experience, who wished to return to school for additional training. However, in 2005 a major shift occurred in PA education: programs began transitioning from graduating trainees with a bachelor's degree to now requiring a master's level degree for completion of the PA program.13 The acquisition of more advanced degrees has changed the demographics of the students matriculating into PA programs, attracting younger students, straight from undergraduate institutions, with less prior healthcare experience.14 As a result, not only are new PA graduates less experienced overall, but they are particularly lacking in exposure to hospital medicine. After PA students complete their first 12 months of PA school in the basic sciences and didactic coursework, they embark on 12 to 15 months of clinical rotations, which are largely rooted in primary care. In fact, many PA programs find it difficult to offer hospital‐based rotations while fulfilling the required rotations in primary care. These factors have resulted in the need for more extensive on‐the‐job training particularly for those new graduates interested in hospital medicine. In light of these challenges, our institution created a 12‐month postgraduate PA fellowship program in Hospital Medicine.
Postgraduate PA Training Programs
Postgraduate PA fellowships, interchangeably called residencies, are voluntary 1‐year training programs that provide both didactic instruction and clinical experience in a medical or surgical subspecialty, thereby lessening the need for on‐the‐job training. These programs are recognized by the Association of Postgraduate Physician Assistant Programs.15 Currently, there are 44 postgraduate training programs in the United States, in a wide range of medical and surgical specialties. At the end of these 1‐year postgraduate PA programs, most graduates receive a certificate of completion. Until now, the only postgraduate education option for PAs interested in Hospital Medicine was a master's completion program only available to PAs who were already employed by a hospitalist group.15 This work reviews the first reported postgraduate hospitalist training program for PAs. Specifically, the program's background, curriculum, anticipated program outcomes, and future plans are discussed.
Background for A Hospitalist Postgraduate PA Fellowship
Mayo Clinic Arizona is a multispecialty private group comprised of both outpatient services and a tertiary care hospital medical center, located in the metropolitan Phoenix, AZ, area. The Mayo Clinic Hospital is a 7‐story facility with 244 licensed beds, 18 operating rooms, and a Level II emergency department. The Mayo Hospitalist group is composed of 15 full time hospitalists and 6 part‐time hospitalists, all of whom are salaried Mayo employees. The group provides 24‐hour in‐house staffing, covering both resident services (teams composed of interns and residents supervised by a staff hospitalist) and nonresident services (staff hospitalists). Over the years there has been steady growth in the number of nonresident services, in part due to resident work‐hour restrictions. To support the physicians working on these nonresident services, the first PA was hired in 2001. Since then, the number of NPPs in our Hospitalist group has increased to 9.35 full‐time equivalents (FTEs), including 1 nurse practitioner. However, one of the greatest challenges in expanding the NPP service was the difficulty finding candidates with experience in hospital internal medicine. This need inspired the creation of a PA fellowship in Hospital Medicine. At the time, there were 2 other postgraduate PA training programs at the Mayo Clinic Arizona in Hepatology and Otolaryngology/Ear, Nose, and Throat (ENT) Surgery.
Program Description
The Mayo Clinic Arizona PA fellowship in Hospital Medicine began in October 2007 and currently accepts 1 fellow per year. Applicants must be graduates of an Accreditation Review Commission in Education for the Physician Assistant (ARC‐PA)‐accredited PA program and be certified through the National Commission on Certification of Physician Assistants (NCCPA). Furthermore, they must be licensed to work as a PA in the state of Arizona. The program is 12 months in duration, and is comprised of both didactic and clinical components. Upon graduation, the fellow earns a certificate of completion from the Mayo Clinic College of Medicine. The program has received recognition with the Association of Postgraduate Physician Assistant Programs (APPAP).
Two physician assistants act as co‐program directors of the PA fellowship in hospital medicine. They are given 0.10 full‐time equivalent (FTE) for management of the program, which includes day‐to‐day operations, curriculum development, and candidate selection. The program also has 2 volunteer physician medical directors, both of whom have previous medical residency experience. The physicians and NPPs in our hospitalist group volunteer their time to serve as faculty for the program, assisting with much of the didactic and clinical education. The program receives a budget of $99,500 per year, which is funded by the organization's foundation through the department of education. This includes the fellow stipend of $44,000 per 12 months and institutional malpractice insurance coverage. The fellow also receives health and dental insurance, 2 weeks of paid vacation, and $500 stipend toward attendance of a continuing medical education (CME) conference.
CURRICULUM
The PA fellowship curriculum is designed in a diverse unique format that strives to accommodate all types of learners. It includes clinical rotations in various medicine/surgical subspecialties, didactic instruction, and teaching modules (Figure 1). The curriculum is based upon the SHM Core Competencies.15

Clinical Rotations
The PA fellow completes 12 to 14 general hospital medicine and medical specialty rotations, each 2 to 4 weeks in duration. The rotation calendar for the current fellow is given in Figure 2. These rotations are all inpatient‐based and are supervised by either the hospitalist or the respective inpatient subspecialists. The PA fellow's specific clinical responsibilities vary from rotation to rotation, and are designed to maximize the fellow's exposure to that particular specialty. Each rotation has specific written objectives created by the program directors and reviewed by the rotation's preceptor(s) (Figure 2). During the clinical rotations, complementary didactic lectures, coursework, and readings are provided to ensure the PA fellow receives a strong foundation. Didactic instruction is designed by the program directors, physician preceptors and staff NPPs, and is coordinated with the clinical rotation specialty. At the end of each rotation the fellow is evaluated by the preceptor and given direct feedback on their performance.

Didactic Instruction
The didactic instruction is organized in a system‐based manner and occurs on a weekly basis during the Hospital Internal Medicine service and Medicine Consults rotations. Hospitalist NPPs and physician faculty are responsible for most of the teaching. This formal didactic instruction is supplemented by journal club presentations given by the PA fellow to faculty in the division of hospital internal medicine. The fellow is also required to attend daily medical resident lunchtime educational lectures, weekly medical grand rounds, and any lectures provided by the medicine subspecialties while the PA is on that particular rotation.
Teaching Modules
One component of the Hospital Medicine PA fellowship curriculum that may be unique is the concept of teaching modules. While receiving regular didactic instruction and completing their clinical rotations, the PA is also expected to complete self‐directed teaching module assignments. These modules serve to educate the PA fellow on the hospital as a systemthe true essence of hospital medicine. The modules cover a variety of topics not directly addressed during their rotations. These topics are outlined in Figure 3. Each teaching module consists of a didactic component, clinical application, and assessment (Figure 4) and has its own specific objectives and goals. Teaching modules are often taught by the local expert in the hospital in that particular area. For example, for the infectious control teaching module, the PA fellow will rotate with the infection control nursing staff learning about the isolation and infection control policies of the institution.


Assessment Tools
There are several tools utilized to assess both the PA fellow and the fellowship program itself (Figure 5). The assessment tools used include both ongoing and summative assessments. To fulfill the ongoing assessment, each rotation and teaching module contains assessment tools provided by the preceptor, which are reviewed by the program directors. Additionally, during the clinical rotations, skills are assessed using competency checklists that require the preceptor to directly observe the PA fellow perform a specific task or skill‐set and sign off on its successful completion (Supplementary Figures 6, 7).

There are 2 forms of summative assessment for the PA fellow. First, to assess the PA fellow's knowledge, comprehensive mid‐year and end‐year examinations are utilized. These multiple‐choice examinations are comprised of questions which align with the didactic lectures/objectives provided by the Hospital Medicine faculty throughout the year. The second form of summative evaluation of the fellow is project‐based and divided into 2 parts. First, the fellow is expected to write a publication‐quality manuscript on a hospital medicine topic by the end of the year. Second, the PA fellow is expected to create a professional portfolio, which is comprised of a collection of all of the rotation/module assessments, the formal program assessments, and documentation of all of the skills obtained by the fellow throughout year (competency checklists). This portfolio can be used by the graduate to demonstrate to future employers what skills they possess and provide documentation of knowledge gained during the fellowship.
The program itself is evaluated by several measures. First, the fellow provides formal feedback during the mid‐year and end‐of‐the‐year assessments, which are used to enhance the experience of future fellows. Second, there is ongoing review by both the division of Hospital Medicine and the institution's Allied Health Education Committee, which ensures that the program maintains the appropriate standards and goals.
Future Goals for the PA Fellowship
The program graduated its first fellow at the end of October 2008 and has enjoyed early success. Integrating the PA fellow onto the hospitalist services augmented the present mid‐level and physician teams. There has been excellent institutional support for the program with extremely positive feedback from the rotation preceptors. There are several futures plans for the program. Our first goal is to seek accreditation from the Accreditation Review Commission for Physician Assistants (ARC‐PA), the organization that accredits entry level PA programs and which began formal, voluntary accreditation of postgraduate programs in early 2008. We plan to begin this process within the next academic year.
Our second long‐term goal for the program is to include NPs in the training program. Because of the desire to seek accreditation, the program directors felt temporarily limiting the fellowship to PAs would aide in the rigorous accreditation process, which can take approximately 1 year to complete. There is an NP on our faculty and the program has received interest from NPs. Once we obtain accreditation, expand the program enrollment, and develop an NP curriculum, we plan to open the fellowship to either PA or NP applicants.
Our third goal is to substantiate our PA Fellowship validity with outcome measures and ultimately publishable data. Thus far, the success of the PA fellowship is qualitative, and with small numbers of graduates it is difficult to quantify. After graduation of many subsequent PA fellows, our goal is to obtain quantifiable data that can be used to improve the quality of the PA fellowship and demonstrate the value of postgraduate training for physician assistants.
Perhaps the most important goal of the program is to eventually accept additional PA/NP fellows per year. While 1 program does not meet the demands of a national shortage of hospitalist providers, it may serve as a model that other institutions can adapt to their own needs. Since the program is based upon the SHM Core Competencies, the curriculum can be applied to a variety of hospitalist programs, and its relatively low operating cost makes it feasible for both academic‐based and community‐based institutions. Importantly, since recruitment and retention of employees is such a challenge for most hospitalist groups, this PA fellowship program may serve as a vehicle for recruitment and long‐term retention of well‐trained employees. This precedent has been set, as our division has hired our first PA fellow, whose transition from PA fellow to PA staff was seamless.
In conclusion, our PA fellowship in Hospital Medicine represents the first reported postgraduate PA program of this kind in the United States offering a certificate of completion. As the need for hospitalists increase so will the need for NPPs, particularly those with additional training in hospital medicine. This program serves as an example of 1 type of training tool for physician assistants looking to work in hospital medicine.
In recent years, the demand for hospitalists has outstripped the supply, creating a national shortage.1, 2 A recent Society of Hospital Medicine (SHM) survey found that in the last 2 years there has been a 31% mean growth increase in the number of hospitalist groups.3 As hospitalists are becoming more difficult to recruit, many practices are utilizing physician assistants (PAs) and nurse practitioners (NPs), collectively referred to as nonphysician providers (NPPs) to help offset the workload.4 The SHM survey also noted that the number of hospitalist groups utilizing NPPs increased from 29% to 38%.3 The exact number of NPPs working for hospitalist groups is unknown.
Hospitalist NPPs are in demand for reasons other than just physician shortages. NPPs have been utilized to fill the gap in many institutions where the workforce was impacted by the 2002 Accreditation Council for Graduate Medical Education (ACGME) ruling to restrict resident work hours. Several studies have documented NPPs' ability to assist with the compliance of ACCGME resident work‐hour restrictions while maintaining patient continuity of care, improving length of stays, and reducing health care costs on various hospital services.59 Dresselhaus et al.10 found that 56% of medical resident's time on service was delegated to tasks not related to direct patient care. They proposed that these tasks can be delegated to the NPPs, leaving more time for the residents to focus on direct patient care. In a recent study performed at a Pennsylvania hospital, patients presenting to the emergency department with low‐risk chest pain (based upon thrombolysis in myocardial infarction [TIMI] risk score) were admitted to a nonteaching service staffed with NPPs and attending physicians. Simultaneously, a similar group of low‐risk chest pain patients were admitted to a traditional internal medicine resident service. The results demonstrated lower median length of stay and hospital charges on the nonteaching service. This study suggested that NPPs can offset the workload volume for medical residents, allowing them to focus on patients with higher acuity and greater learning value.11
Barriers to Finding Experienced NPPs in Hospital Medicine
Although many hospitalist groups are interested in hiring NPPs, there can be significant obstacles to recruitment. For example, most experienced PAs and NPs have clinical backgrounds in either surgical or medical subspecialties and therefore typically need extensive on‐the‐job training in hospital medicine, which can often take at least 6 to 12 months to acquire the basic skill set.12 Hiring new graduates may require even longer training periods.
The inexperience of new graduates has become an even more pertinent issue due to recent changes in PA education. Traditionally, PA programs attracted older students with prior healthcare experience, who wished to return to school for additional training. However, in 2005 a major shift occurred in PA education: programs began transitioning from graduating trainees with a bachelor's degree to now requiring a master's level degree for completion of the PA program.13 The acquisition of more advanced degrees has changed the demographics of the students matriculating into PA programs, attracting younger students, straight from undergraduate institutions, with less prior healthcare experience.14 As a result, not only are new PA graduates less experienced overall, but they are particularly lacking in exposure to hospital medicine. After PA students complete their first 12 months of PA school in the basic sciences and didactic coursework, they embark on 12 to 15 months of clinical rotations, which are largely rooted in primary care. In fact, many PA programs find it difficult to offer hospital‐based rotations while fulfilling the required rotations in primary care. These factors have resulted in the need for more extensive on‐the‐job training particularly for those new graduates interested in hospital medicine. In light of these challenges, our institution created a 12‐month postgraduate PA fellowship program in Hospital Medicine.
Postgraduate PA Training Programs
Postgraduate PA fellowships, interchangeably called residencies, are voluntary 1‐year training programs that provide both didactic instruction and clinical experience in a medical or surgical subspecialty, thereby lessening the need for on‐the‐job training. These programs are recognized by the Association of Postgraduate Physician Assistant Programs.15 Currently, there are 44 postgraduate training programs in the United States, in a wide range of medical and surgical specialties. At the end of these 1‐year postgraduate PA programs, most graduates receive a certificate of completion. Until now, the only postgraduate education option for PAs interested in Hospital Medicine was a master's completion program only available to PAs who were already employed by a hospitalist group.15 This work reviews the first reported postgraduate hospitalist training program for PAs. Specifically, the program's background, curriculum, anticipated program outcomes, and future plans are discussed.
Background for A Hospitalist Postgraduate PA Fellowship
Mayo Clinic Arizona is a multispecialty private group comprised of both outpatient services and a tertiary care hospital medical center, located in the metropolitan Phoenix, AZ, area. The Mayo Clinic Hospital is a 7‐story facility with 244 licensed beds, 18 operating rooms, and a Level II emergency department. The Mayo Hospitalist group is composed of 15 full time hospitalists and 6 part‐time hospitalists, all of whom are salaried Mayo employees. The group provides 24‐hour in‐house staffing, covering both resident services (teams composed of interns and residents supervised by a staff hospitalist) and nonresident services (staff hospitalists). Over the years there has been steady growth in the number of nonresident services, in part due to resident work‐hour restrictions. To support the physicians working on these nonresident services, the first PA was hired in 2001. Since then, the number of NPPs in our Hospitalist group has increased to 9.35 full‐time equivalents (FTEs), including 1 nurse practitioner. However, one of the greatest challenges in expanding the NPP service was the difficulty finding candidates with experience in hospital internal medicine. This need inspired the creation of a PA fellowship in Hospital Medicine. At the time, there were 2 other postgraduate PA training programs at the Mayo Clinic Arizona in Hepatology and Otolaryngology/Ear, Nose, and Throat (ENT) Surgery.
Program Description
The Mayo Clinic Arizona PA fellowship in Hospital Medicine began in October 2007 and currently accepts 1 fellow per year. Applicants must be graduates of an Accreditation Review Commission in Education for the Physician Assistant (ARC‐PA)‐accredited PA program and be certified through the National Commission on Certification of Physician Assistants (NCCPA). Furthermore, they must be licensed to work as a PA in the state of Arizona. The program is 12 months in duration, and is comprised of both didactic and clinical components. Upon graduation, the fellow earns a certificate of completion from the Mayo Clinic College of Medicine. The program has received recognition with the Association of Postgraduate Physician Assistant Programs (APPAP).
Two physician assistants act as co‐program directors of the PA fellowship in hospital medicine. They are given 0.10 full‐time equivalent (FTE) for management of the program, which includes day‐to‐day operations, curriculum development, and candidate selection. The program also has 2 volunteer physician medical directors, both of whom have previous medical residency experience. The physicians and NPPs in our hospitalist group volunteer their time to serve as faculty for the program, assisting with much of the didactic and clinical education. The program receives a budget of $99,500 per year, which is funded by the organization's foundation through the department of education. This includes the fellow stipend of $44,000 per 12 months and institutional malpractice insurance coverage. The fellow also receives health and dental insurance, 2 weeks of paid vacation, and $500 stipend toward attendance of a continuing medical education (CME) conference.
CURRICULUM
The PA fellowship curriculum is designed in a diverse unique format that strives to accommodate all types of learners. It includes clinical rotations in various medicine/surgical subspecialties, didactic instruction, and teaching modules (Figure 1). The curriculum is based upon the SHM Core Competencies.15

Clinical Rotations
The PA fellow completes 12 to 14 general hospital medicine and medical specialty rotations, each 2 to 4 weeks in duration. The rotation calendar for the current fellow is given in Figure 2. These rotations are all inpatient‐based and are supervised by either the hospitalist or the respective inpatient subspecialists. The PA fellow's specific clinical responsibilities vary from rotation to rotation, and are designed to maximize the fellow's exposure to that particular specialty. Each rotation has specific written objectives created by the program directors and reviewed by the rotation's preceptor(s) (Figure 2). During the clinical rotations, complementary didactic lectures, coursework, and readings are provided to ensure the PA fellow receives a strong foundation. Didactic instruction is designed by the program directors, physician preceptors and staff NPPs, and is coordinated with the clinical rotation specialty. At the end of each rotation the fellow is evaluated by the preceptor and given direct feedback on their performance.

Didactic Instruction
The didactic instruction is organized in a system‐based manner and occurs on a weekly basis during the Hospital Internal Medicine service and Medicine Consults rotations. Hospitalist NPPs and physician faculty are responsible for most of the teaching. This formal didactic instruction is supplemented by journal club presentations given by the PA fellow to faculty in the division of hospital internal medicine. The fellow is also required to attend daily medical resident lunchtime educational lectures, weekly medical grand rounds, and any lectures provided by the medicine subspecialties while the PA is on that particular rotation.
Teaching Modules
One component of the Hospital Medicine PA fellowship curriculum that may be unique is the concept of teaching modules. While receiving regular didactic instruction and completing their clinical rotations, the PA is also expected to complete self‐directed teaching module assignments. These modules serve to educate the PA fellow on the hospital as a systemthe true essence of hospital medicine. The modules cover a variety of topics not directly addressed during their rotations. These topics are outlined in Figure 3. Each teaching module consists of a didactic component, clinical application, and assessment (Figure 4) and has its own specific objectives and goals. Teaching modules are often taught by the local expert in the hospital in that particular area. For example, for the infectious control teaching module, the PA fellow will rotate with the infection control nursing staff learning about the isolation and infection control policies of the institution.


Assessment Tools
There are several tools utilized to assess both the PA fellow and the fellowship program itself (Figure 5). The assessment tools used include both ongoing and summative assessments. To fulfill the ongoing assessment, each rotation and teaching module contains assessment tools provided by the preceptor, which are reviewed by the program directors. Additionally, during the clinical rotations, skills are assessed using competency checklists that require the preceptor to directly observe the PA fellow perform a specific task or skill‐set and sign off on its successful completion (Supplementary Figures 6, 7).

There are 2 forms of summative assessment for the PA fellow. First, to assess the PA fellow's knowledge, comprehensive mid‐year and end‐year examinations are utilized. These multiple‐choice examinations are comprised of questions which align with the didactic lectures/objectives provided by the Hospital Medicine faculty throughout the year. The second form of summative evaluation of the fellow is project‐based and divided into 2 parts. First, the fellow is expected to write a publication‐quality manuscript on a hospital medicine topic by the end of the year. Second, the PA fellow is expected to create a professional portfolio, which is comprised of a collection of all of the rotation/module assessments, the formal program assessments, and documentation of all of the skills obtained by the fellow throughout year (competency checklists). This portfolio can be used by the graduate to demonstrate to future employers what skills they possess and provide documentation of knowledge gained during the fellowship.
The program itself is evaluated by several measures. First, the fellow provides formal feedback during the mid‐year and end‐of‐the‐year assessments, which are used to enhance the experience of future fellows. Second, there is ongoing review by both the division of Hospital Medicine and the institution's Allied Health Education Committee, which ensures that the program maintains the appropriate standards and goals.
Future Goals for the PA Fellowship
The program graduated its first fellow at the end of October 2008 and has enjoyed early success. Integrating the PA fellow onto the hospitalist services augmented the present mid‐level and physician teams. There has been excellent institutional support for the program with extremely positive feedback from the rotation preceptors. There are several futures plans for the program. Our first goal is to seek accreditation from the Accreditation Review Commission for Physician Assistants (ARC‐PA), the organization that accredits entry level PA programs and which began formal, voluntary accreditation of postgraduate programs in early 2008. We plan to begin this process within the next academic year.
Our second long‐term goal for the program is to include NPs in the training program. Because of the desire to seek accreditation, the program directors felt temporarily limiting the fellowship to PAs would aide in the rigorous accreditation process, which can take approximately 1 year to complete. There is an NP on our faculty and the program has received interest from NPs. Once we obtain accreditation, expand the program enrollment, and develop an NP curriculum, we plan to open the fellowship to either PA or NP applicants.
Our third goal is to substantiate our PA Fellowship validity with outcome measures and ultimately publishable data. Thus far, the success of the PA fellowship is qualitative, and with small numbers of graduates it is difficult to quantify. After graduation of many subsequent PA fellows, our goal is to obtain quantifiable data that can be used to improve the quality of the PA fellowship and demonstrate the value of postgraduate training for physician assistants.
Perhaps the most important goal of the program is to eventually accept additional PA/NP fellows per year. While 1 program does not meet the demands of a national shortage of hospitalist providers, it may serve as a model that other institutions can adapt to their own needs. Since the program is based upon the SHM Core Competencies, the curriculum can be applied to a variety of hospitalist programs, and its relatively low operating cost makes it feasible for both academic‐based and community‐based institutions. Importantly, since recruitment and retention of employees is such a challenge for most hospitalist groups, this PA fellowship program may serve as a vehicle for recruitment and long‐term retention of well‐trained employees. This precedent has been set, as our division has hired our first PA fellow, whose transition from PA fellow to PA staff was seamless.
In conclusion, our PA fellowship in Hospital Medicine represents the first reported postgraduate PA program of this kind in the United States offering a certificate of completion. As the need for hospitalists increase so will the need for NPPs, particularly those with additional training in hospital medicine. This program serves as an example of 1 type of training tool for physician assistants looking to work in hospital medicine.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2004;20:101–107.
- .Innovations in the management of hospitalized patients. Nurse Pract Spring2006 (suppl):2–3.
- .Hospitalist pay up, productivity steady in SHM's latest survey.Hospitalist.2008;12(5):7,16.
- .Physician assistants: filling the gap in patient care in academic hospitals.Perspect Physician Assist Educ.2003;14(3):158–167.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,, et al.Physician extenders impact trauma systems.J Trauma.2005;58(5):917–920.
- ,,.The role of physician assistants in critical care units.Chest.1991;99:89–91.
- .Alliances: invaluable assistants.Hospitalist.2006;April:32–33.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–42.
- ,,,,,.Analyzing the time and value of house staff inpatient work.J Intern Med.1998;13:534–540.
- ,,, et al.Improving resource utilization in a teaching hospital: Development of a nonteaching service for chest pain admissions.Acad Med.2006;81(5):432–435.
- .Midlevels make a rocky entrance into hospital medicine.Todays Hospitalist.2007;5(1):28–32.
- Accreditation Review Commission for Physician Assistant Education.3rd ed. 2005. Available at: http://www.arc‐pa.org/Standards/standards.html. Accessed September2009.
- 22nd Annual Report on Physician Assistant Education in the U.S., 2005–2006. Available at: http://www.paeaonline.org. Accessed September2009.
- Association of Postgraduate Physician Assistant Programs. Available at: http://www.appap.org. Accessed September2009.
- ,,,,.The core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2004;20:101–107.
- .Innovations in the management of hospitalized patients. Nurse Pract Spring2006 (suppl):2–3.
- .Hospitalist pay up, productivity steady in SHM's latest survey.Hospitalist.2008;12(5):7,16.
- .Physician assistants: filling the gap in patient care in academic hospitals.Perspect Physician Assist Educ.2003;14(3):158–167.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,, et al.Physician extenders impact trauma systems.J Trauma.2005;58(5):917–920.
- ,,.The role of physician assistants in critical care units.Chest.1991;99:89–91.
- .Alliances: invaluable assistants.Hospitalist.2006;April:32–33.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15:33–42.
- ,,,,,.Analyzing the time and value of house staff inpatient work.J Intern Med.1998;13:534–540.
- ,,, et al.Improving resource utilization in a teaching hospital: Development of a nonteaching service for chest pain admissions.Acad Med.2006;81(5):432–435.
- .Midlevels make a rocky entrance into hospital medicine.Todays Hospitalist.2007;5(1):28–32.
- Accreditation Review Commission for Physician Assistant Education.3rd ed. 2005. Available at: http://www.arc‐pa.org/Standards/standards.html. Accessed September2009.
- 22nd Annual Report on Physician Assistant Education in the U.S., 2005–2006. Available at: http://www.paeaonline.org. Accessed September2009.
- Association of Postgraduate Physician Assistant Programs. Available at: http://www.appap.org. Accessed September2009.
- ,,,,.The core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
Copyright © 2010 Society of Hospital Medicine