User login
Impact of a Connected Care model on 30-day readmission rates from skilled nursing facilities
Approximately 20% of hospitalized Medicare beneficiaries in the U.S. are discharged to skilled nursing facilities (SNFs) for post-acute care,1,2 and 23.5% of these patients are readmitted within 30 days.3 Because hospital readmissions are costly and associated with worse outcomes,4,5 30-day readmission rates are considered a quality indicator,6 and there are financial penalties for hospitals with higher than expected rates.7 As a result, hospitals invest substantial resources in programs to reduce readmissions.8-10 The SNFs represent an attractive target for readmission reduction efforts, since SNFs contribute a disproportionate share of readmissions.3,4 Because SNF patients are in a monitored environment with high medication adherence, risk factors for readmission likely differ between patients discharged to SNFs and those sent home. For example, 1 study showed that among heart failure patients with cognitive impairment, those discharged to SNFs had lower readmissions during the first 20 days, likely due to better medication adherence.11 Patients discharged to SNFs generally have more complex illnesses, lower functional status, and higher 1-year mortality than patients discharged to the community.12,13 Despite this, SNF patients might have infrequent contact with physicians. Federal regulations require only that patients discharged to SNFs need to be seen within 30 days and then at least once every 30 days thereafter.14 According to the 2014 Office of Inspector General report, one-third of Medicare beneficiaries in SNFs experience adverse events from substandard treatment, inadequate resident monitoring and failure or delay of necessary care, most of which are thought to be preventable.15
To address this issue, the Cleveland Clinic developed a program called “Connected Care SNF,” in which hospital-employed physicians and advanced practice professionals visit patients in selected SNFs 4 to 5 times per week, for the purpose of reducing preventable readmissions. The aim of this study was to assess whether the program reduced 30-day readmissions, and to identify which patients benefited most from the program.
METHODS
Setting and Intervention
The Cleveland Clinic main campus is a tertiary academic medical center with 1400 beds and approximately 50,000 admissions per year. In late 2012, the Cleveland Clinic implemented the Connected Care SNF program, wherein Cleveland Clinic physicians regularly visited patients who were discharged from the Cleveland Clinic main campus to 7 regional SNFs. Beginning in December 2012, these 7 high-volume referral SNFs that were not part of the Cleveland Clinic Health System (CCHS) agreed to participate in the program, which focused on reducing avoidable hospital readmissions and delivering quality care (Table 1). The Connected Care team, comprised of 2 geriatricians (1 of whom was also a palliative medicine specialist), 1 internist, 1 family physician, and 5 advanced practice professionals (nurse practitioners and physician assistants), provided medical services at the participating SNFs. These providers aimed to see patients 4 to 5 times per week, were available on site during working hours, and provided telephone coverage at nights and on weekends. All providers had access to hospital electronic medical records and could communicate with the discharging physician and with specialists familiar with the patient as needed. Prior to the admission, providers were informed about patient arrival and, at the time of admission to the SNF, providers reviewed medications and discussed goals of care with patients and their families. In the SNF, providers worked closely with staff members to deliver medications and timely treatment. They also met monthly with multidisciplinary teams for continuous quality improvement and to review outcomes. Patients at Connected Care SNFs who had their own physicians, including most long-stay and some short-stay residents, did not receive the Connected Care intervention. They constituted less than 10% of the patients discharged from Cleveland Clinic main campus.
Study Design and Population
We reviewed administrative and clinical data from a retrospective cohort of patients discharged to SNF from the Cleveland Clinic main campus from January 1, 2011 to December 31, 2014. We included all patients who were discharged to an SNF during the study period. Our main outcome measure was 30-day all-cause readmissions to any hospital in the Cleveland Clinic Health System (CCHS), including the main campus and 8 regional community hospitals. Study patients were followed until January 30, 2015 to capture 30-day readmissions. According to 2012 Medicare data, of CCHS patients who were readmitted within 30 days, 83% of pneumonia, 81% of major joint replacement, 72% of heart failure and 57% of acute myocardial infarction patients were readmitted to a CCHS facility. As the Cleveland Clinic main campus attracts cardiac patients from a 100+-mile radius, they may be more likely to seek care readmission near home and are not reflective of CCHS patients overall. Because we did not have access to readmissions data from non-CCHS hospitals, we excluded patients who were discharged to SNFs beyond a 25-mile radius from the main campus, where they may be more likely to utilize non-CCHS hospitals for acute hospitalization. We also excluded patients discharged to non-CCHS hospital-based SNFs, which may refer readmissions to their own hospital system. Because the Connected Care program began in December 2012, the years 2011-2012 served as the baseline period. The intervention was conducted at 7 SNFs. All other SNFs within the 25-mile radius were included as controls, except for 3 hospital-based SNFs that would be unlikely to admit patients to CCHS. We compared the change in all-cause 30-day readmission rates after implementation of Connected Care, using all patients discharged to SNFs within 25 miles to control for temporal changes in local readmission rates. Discharge to specific SNFs was determined solely by patient choice.
Data Collection
For each patient, we collected the following data that has been shown to be associated with readmissions:16-18 demographics (age, race, sex, ZIP code), lab values on discharge (hemoglobin and sodium); hemodialysis status; medicine or surgical service; elective surgery or nonelective surgery; details of the index admission index (diagnosis-related group [DRG], Medicare severity-diagnosis-related groups [MS-DRG] weight, primary diagnosis code; principal procedure code; admission date; discharge date, length of stay, and post-acute care provider); and common comorbidities, as listed in Table 2. We also calculated each patient’s HOSPITAL19,20 score. The HOSPITAL score was developed to predict risk of preventable 30-day readmissions,19 but it has also been validated to predict 30-day all-cause readmission rates for patients discharged to SNF.21 The model contains 7 elements (hemoglobin, oncology service, sodium, procedure, index type, admissions within the last year, length of stay) (supplemental Table).Patients with a high score (7 or higher) have a 41% chance of readmission, while those with a low score (4 or lower) have only a 15% chance. 21 We assessed all cause 30-day readmission status from CCHS administrative data. Observation patients and outpatient same-day surgeries were not considered to be admissions. For patients with multiple admissions, each admission was counted as a separate index hospitalization. Cleveland Clinic’s Institutional Review Board approved the study.
Statistical Analysis
For the 7 intervention SNFs, patient characteristics were summarized as means and standard deviations or frequencies and percentages for the periods of 2011-2012 and 2013-2014, respectively, and the 2 periods were compared using the Student t test or χ2 test as appropriate.
Mixed-effects logistic regression models were used to model 30-day readmission rates. Since the intervention was implemented in the last quarter of 2012, we examined the difference in readmission rates before and after that time point. The model included the following fixed effects: SNF type (intervention or usual care), time points (quarters of 2011-2014), whether the time is pre- or postintervention (binary), and the 3-way interaction between SNF type, pre- or postintervention and time points, and patient characteristics. The model also contained a Gaussian random effect at the SNF level to account for possible correlations among the outcomes of patients from the same SNF. For each quarter, the mean adjusted readmission rates of 2 types of SNFs were calculated from the fitted mixed models and plotted over time. Furthermore, we compared the mean readmission rates of the 2 groups in the pre- and postintervention periods. Subgroup analyses were performed for medical and surgical patients, and for patients in the low, intermediate and high HOSPITAL score groups.
All analyses were performed using RStudio (Boston, Massachusetts). Statistical significance was established with 2-sided P values less than 0.05.
RESULTS
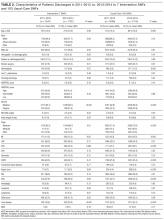
We identified 119 SNFs within a 25-mile radius of the hospital. Of these, 6 did not receive any referrals. Three non-CCHS hospital-based SNFs were excluded, leaving a total of 110 SNFs in the study sample: 7 intervention SNFs and 103 usual-care SNFs. Between January 2011 and December 2014, there were 23,408 SNF discharges from Cleveland Clinic main campus, including 13,544 who were discharged to study SNFs (Supplemental Figure). Of these, 3334 were discharged to 7 intervention SNFs and 10,210 were discharged to usual care SNFs. Characteristics of patients in both periods appear in Table 2. At baseline, patients in the intervention and control SNFs varied in a number of ways. Patients at intervention SNFs were older (75.6 vs. 70.2 years; P < 0.001), more likely to be African American (45.5% vs. 35.9%; P < 0.001), female (61% vs. 55.4%; P < 0.001) and to be insured by Medicare (85.2% vs. 71.4%; P < 0.001). Both groups had similar proportions of patients with high, intermediate, and low readmission risk as measured by HOSPITAL score. Compared to the 2011-2012 pre-intervention period, during the 2013-2014 intervention period, there were more surgeries (34.3% vs. 41.9%; P < 0.001), more elective surgeries (21.8% vs. 25.5%; P = 0.01), fewer medical patients (65.7% vs. 58.1%; P < 0.001), and an increase in comorbidities, including myocardial infarction, peripheral vascular disease, and liver disease (Table 2).
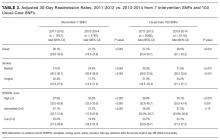
Table 3 shows adjusted 30-day readmissions rates, before and during the intervention period at the intervention and usual care SNFs. Compared to the pre-intervention period, 30-day all-cause adjusted readmission rates declined in the intervention SNFs (28.1% to 21.7%, P < 0.001), while it increased slightly at control sites (27.1% to 28.5%, P < 0.001). The Figure shows the adjusted 30-day readmission rates by quarter throughout the study period.
Declines in 30-day readmission rates were greater for medical patients (31.0% to 24.6%, P < 0.001) than surgical patients (22.4% to 17.7%, P < 0.001). Patients with high HOSPITAL scores had the greatest decline, while those with low HOSPITAL scores had smaller declines.
DISCUSSION
In this retrospective study of 4 years of discharges to 110 SNFs, we report on the impact of a Connected Care program, in which a physician visited patients on admission to the SNF and 4 to 5 times per week during their stay. Introduction of the program was followed by a 6.8% absolute reduction in all-cause 30-day readmission rates compared to usual care. The absolute reductions ranged from 4.6% for patients at low risk for readmission to 9.1% for patients at high risk, and medical patients benefited more than surgical patients.
Most studies of interventions to reduce hospital readmissions have focused on patients discharged to the community setting.7-9 Interventions have centered on discharge planning, medication reconciliation, and close follow-up to assess for medication adherence and early signs of deterioration. Because patients in SNFs have their medications administered by staff and are under frequent surveillance, such interventions are unlikely to be helpful in this population. We found no studies that focus on short-stay or skilled patients discharged to SNF. Two studies have demonstrated that interventions can reduce hospitalization from nursing homes.22,23 Neither study included readmissions. The Evercare model consisted of nurse practitioners providing active primary care services within the nursing home, as well as offering incentive payments to nursing homes for not hospitalizing patients.22 During a 2-year period, long term residents who enrolled in Evercare had an almost 50% reduction in incident hospitalizations compared to those who did not.22 INTERACT II was a quality improvement intervention that provided tools, education, and strategies to help identify and manage acute conditions proactively.23 In 25 nursing homes employing INTERACT II, there was a 17% reduction in self-reported hospital admissions during the 6-month project, with higher rates of reduction among nursing homes rated as more engaged in the process.23 Although nursing homes may serve some short-stay or skilled patients, they generally serve long-term populations, and studies have shown that short-stay patients are at higher risk for 30-day readmissions.24
There are a number of reasons that short-term SNF patients are at higher risk for readmission. Although prior to admission, they were considered hospital level of care and received a physician visit daily, on transfer to the SNF, relatively little medical care is available. Current federal regulations regarding physician services at a SNF require the resident to be seen by a physician at least once every 30 days for the first 90 days after admission, and at least once every 60 days thereafter.25
The Connected Care program physicians provided a smooth transition of care from hospital to SNF as well as frequent reassessment. Physicians were alerted prior to hospital discharge and performed an initial comprehensive visit generally on the day of admission to the SNF and always within 48 hours. The initial evaluation is important because miscommunication during the handoff from hospital to SNF may result in incorrect medication regimens or inaccurate assessments. By performing prompt medication reconciliation and periodic reassessments of a patient’s medical condition, the Connected Care providers recreate some of the essential elements of successful outpatient readmissions prevention programs.
They also worked together with each SNF’s interdisciplinary team to deliver quality care. There were monthly meetings at each participating Connected Care SNF. Physicians reviewed monthly 30-day readmissions and performed root-cause analysis. When they discovered challenges to timely medication and treatment delivery during daily rounds, they provided in-services to SNF nurses.
In addition, Connected Care providers discussed goals of care—something that is often overlooked on admission to a SNF. This is particularly important because patients with chronic illnesses who are discharged to SNF often have poor prognoses. For example, Medicare patients with heart failure who are discharged to SNFs have 1-year mortality in excess of 50%.13 By implementing a plan of care consistent with patient and family goals, inappropriate readmissions for terminal patients may be avoided.
Reducing readmissions is important for hospitals because under the Hospital Readmissions Reduction Program, hospitals now face substantial penalties for higher than expected readmissions rates. Hospitals involved in bundled payments or other total cost-of-care arrangements have additional incentive to avoid readmissions. Beginning in 2019, SNFs will also receive incentive payments based on their 30-day all-cause hospital readmissions as part of the Skilled Nursing Facility Value-Based Purchasing program.25 The Connected Care model offers 1 means of achieving this goal through partnership between hospitals and SNFs.
Our study has several limitations. First, our study was observational in nature, so the observed reduction in readmissions could have been due to temporal trends unrelated to the intervention. However, no significant reduction was noted during the same time period in other area SNFs. There was also little change in the characteristics of patients admitted to the intervention SNFs. Importantly, the HOSPITAL score, which can predict 30-day readmission rates,20 did not change throughout the study period. Second, the results reflect patients discharged from a single hospital and may not be generalizable to other geographic areas. However, because the program included 7 SNFs, we believe it could be reproduced in other settings. Third, our readmissions measure included only those patients who returned to a CCHS facility. Although we may have missed some readmissions to other hospital systems, such leakage is uncommon—more than 80% of CCHS patients are readmitted to CCHS facilities—and would be unlikely to differ across the short duration of the study. Finally, at the intervention SNFs, most long-stay and some short-stay residents did not receive the Connected Care intervention because they were cared for by their own physicians who did not participate in Connected Care. Had these patients’ readmissions been excluded from our results, the intervention might appear even more effective.
CONCLUSION
A Connected Care intervention reduced 30-day readmission rates among patients discharged to SNFs from a tertiary academic center. While all subgroups had substantial reductions in readmissions following the implementation of the intervention, patients who are at the highest risk of readmission benefited the most. Further study is necessary to know whether Connected Care can be reproduced in other health care systems and whether it reduces overall costs.
Acknowledgments
The authors would like to thank Michael Felver, MD, and teams for their clinical care of patients; Michael Felver, MD, William Zafirau, MD, Dan Blechschmid, MHA, and Kathy Brezine, and Seth Vilensky, MBA, for their administrative support; and Brad Souder, MPT, for assistance with data collection.
Disclosure
Nothing to report.
1. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Chapter 8. Skilled Nursing Facility Services. March 2013. http://www.medpac.gov/docs/default-source/reports/mar13_entirereport.pdf?sfvrsn=0. Accessed March 1, 2017.
2. Kim DG, Messinger-Rapport BJ. Clarion call for a dedicated clinical and research approach to post-acute care. J Am Med Dir Assoc. 2014;15(8):607. e1-e3. PubMed
3. Mor V, Intrator O, Feng Z, Grabowski D. The revolving door of rehospitalization from skilled nursing facilities. Health Aff. 2010;29(1):57-64. PubMed
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. PubMed
5. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med 1993;118(3):219-223. PubMed
6. Van Walraven C, Bennett C, Jennings A, Austin PC, Forester AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391-E402. PubMed
7. Brenson RA, Paulus RA, Kalman NS. Medicare’s readmissions-reduction program – a positive alternative. N Engl J Med 2012;366(15):1364-1366. PubMed
8. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. PubMed
9. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613-620. PubMed
10. Coleman EA, Parry C, Chalmers S, Min SJ. The care transition intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
11. Patel A, Parikh R, Howell EH, Hsich E, Landers SH, Gorodeski EZ. Mini-cog performance: novel marker of post discharge risk among patients hospitalized for heart failure. Circ Heart Fail. 2015;8(1):8-16. PubMed
12. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987-2994. PubMed
13. Allen LA, Hernandez AF, Peterson ED, et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4(3):293-300. PubMed
14. 42 CFR 483.40 – Physician services. US government Publishing Office. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec483-40. Published October 1, 2011. Accessed August 31, 2016.
15. Office of Inspector General. Adverse Events in Skilled Nursing Facilities: National Incidence among Medicare Beneficiaries. OEI-06-11-00370. February 2014. http://oig.hhs.gov/oei/reports/oei-06-11-00370.pdf. Accessed March 22, 2016.
16. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. PubMed
17. Boult C, Dowd B, McCaffrey D, Boult L, Hernandez R, Krulewitch H. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811-817. PubMed
18. Silverstein MD, Qin H, Mercer SQ, Fong J, Haydar Z. Risk factors for 30-day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21(4):363-372. PubMed
19. Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. PubMed
20. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. PubMed
21. Kim LD, Kou L, Messinger-Rapport BJ, Rothberg MB. Validation of the HOSPITAL score for 30-day all-cause readmissions of patients discharged to skilled nursing facilities. J Am Med Dir Assoc. 2016;17(9):e15-e18. PubMed
22. Kane RL, Keckhafer G, Flood S, Bershardsky B, Siadaty MS. The effect of Evercare on hospital use. J Am Geriatr Soc. 2003;51(10):1427-1434. PubMed
23. Ouslander JG, Lamb G, Tappen R, et al. Interventions to reduce hospitalizations from nursing homes: Evaluation of the INTERACT II collaboration quality improvement project. J Am Geriatr Soc. 2011;59(4):745-753. PubMed
24. Cost drivers for dually eligible beneficiaries: Potentially avoidable hospitalizations from nursing facility, skilled nursing facility, and home and community based service waiver programs. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/costdriverstask2.pdf. Accessed August 31, 2016.
25. H.R. 4302 (113th), Section 215, Protecting Access to Medicare Act of 2014 (PAMA). April 2, 2014. https://www.govtrack.us/congress/bills/113/hr4302/text. Accessed August 31, 2016.
Approximately 20% of hospitalized Medicare beneficiaries in the U.S. are discharged to skilled nursing facilities (SNFs) for post-acute care,1,2 and 23.5% of these patients are readmitted within 30 days.3 Because hospital readmissions are costly and associated with worse outcomes,4,5 30-day readmission rates are considered a quality indicator,6 and there are financial penalties for hospitals with higher than expected rates.7 As a result, hospitals invest substantial resources in programs to reduce readmissions.8-10 The SNFs represent an attractive target for readmission reduction efforts, since SNFs contribute a disproportionate share of readmissions.3,4 Because SNF patients are in a monitored environment with high medication adherence, risk factors for readmission likely differ between patients discharged to SNFs and those sent home. For example, 1 study showed that among heart failure patients with cognitive impairment, those discharged to SNFs had lower readmissions during the first 20 days, likely due to better medication adherence.11 Patients discharged to SNFs generally have more complex illnesses, lower functional status, and higher 1-year mortality than patients discharged to the community.12,13 Despite this, SNF patients might have infrequent contact with physicians. Federal regulations require only that patients discharged to SNFs need to be seen within 30 days and then at least once every 30 days thereafter.14 According to the 2014 Office of Inspector General report, one-third of Medicare beneficiaries in SNFs experience adverse events from substandard treatment, inadequate resident monitoring and failure or delay of necessary care, most of which are thought to be preventable.15
To address this issue, the Cleveland Clinic developed a program called “Connected Care SNF,” in which hospital-employed physicians and advanced practice professionals visit patients in selected SNFs 4 to 5 times per week, for the purpose of reducing preventable readmissions. The aim of this study was to assess whether the program reduced 30-day readmissions, and to identify which patients benefited most from the program.
METHODS
Setting and Intervention
The Cleveland Clinic main campus is a tertiary academic medical center with 1400 beds and approximately 50,000 admissions per year. In late 2012, the Cleveland Clinic implemented the Connected Care SNF program, wherein Cleveland Clinic physicians regularly visited patients who were discharged from the Cleveland Clinic main campus to 7 regional SNFs. Beginning in December 2012, these 7 high-volume referral SNFs that were not part of the Cleveland Clinic Health System (CCHS) agreed to participate in the program, which focused on reducing avoidable hospital readmissions and delivering quality care (Table 1). The Connected Care team, comprised of 2 geriatricians (1 of whom was also a palliative medicine specialist), 1 internist, 1 family physician, and 5 advanced practice professionals (nurse practitioners and physician assistants), provided medical services at the participating SNFs. These providers aimed to see patients 4 to 5 times per week, were available on site during working hours, and provided telephone coverage at nights and on weekends. All providers had access to hospital electronic medical records and could communicate with the discharging physician and with specialists familiar with the patient as needed. Prior to the admission, providers were informed about patient arrival and, at the time of admission to the SNF, providers reviewed medications and discussed goals of care with patients and their families. In the SNF, providers worked closely with staff members to deliver medications and timely treatment. They also met monthly with multidisciplinary teams for continuous quality improvement and to review outcomes. Patients at Connected Care SNFs who had their own physicians, including most long-stay and some short-stay residents, did not receive the Connected Care intervention. They constituted less than 10% of the patients discharged from Cleveland Clinic main campus.
Study Design and Population
We reviewed administrative and clinical data from a retrospective cohort of patients discharged to SNF from the Cleveland Clinic main campus from January 1, 2011 to December 31, 2014. We included all patients who were discharged to an SNF during the study period. Our main outcome measure was 30-day all-cause readmissions to any hospital in the Cleveland Clinic Health System (CCHS), including the main campus and 8 regional community hospitals. Study patients were followed until January 30, 2015 to capture 30-day readmissions. According to 2012 Medicare data, of CCHS patients who were readmitted within 30 days, 83% of pneumonia, 81% of major joint replacement, 72% of heart failure and 57% of acute myocardial infarction patients were readmitted to a CCHS facility. As the Cleveland Clinic main campus attracts cardiac patients from a 100+-mile radius, they may be more likely to seek care readmission near home and are not reflective of CCHS patients overall. Because we did not have access to readmissions data from non-CCHS hospitals, we excluded patients who were discharged to SNFs beyond a 25-mile radius from the main campus, where they may be more likely to utilize non-CCHS hospitals for acute hospitalization. We also excluded patients discharged to non-CCHS hospital-based SNFs, which may refer readmissions to their own hospital system. Because the Connected Care program began in December 2012, the years 2011-2012 served as the baseline period. The intervention was conducted at 7 SNFs. All other SNFs within the 25-mile radius were included as controls, except for 3 hospital-based SNFs that would be unlikely to admit patients to CCHS. We compared the change in all-cause 30-day readmission rates after implementation of Connected Care, using all patients discharged to SNFs within 25 miles to control for temporal changes in local readmission rates. Discharge to specific SNFs was determined solely by patient choice.
Data Collection
For each patient, we collected the following data that has been shown to be associated with readmissions:16-18 demographics (age, race, sex, ZIP code), lab values on discharge (hemoglobin and sodium); hemodialysis status; medicine or surgical service; elective surgery or nonelective surgery; details of the index admission index (diagnosis-related group [DRG], Medicare severity-diagnosis-related groups [MS-DRG] weight, primary diagnosis code; principal procedure code; admission date; discharge date, length of stay, and post-acute care provider); and common comorbidities, as listed in Table 2. We also calculated each patient’s HOSPITAL19,20 score. The HOSPITAL score was developed to predict risk of preventable 30-day readmissions,19 but it has also been validated to predict 30-day all-cause readmission rates for patients discharged to SNF.21 The model contains 7 elements (hemoglobin, oncology service, sodium, procedure, index type, admissions within the last year, length of stay) (supplemental Table).Patients with a high score (7 or higher) have a 41% chance of readmission, while those with a low score (4 or lower) have only a 15% chance. 21 We assessed all cause 30-day readmission status from CCHS administrative data. Observation patients and outpatient same-day surgeries were not considered to be admissions. For patients with multiple admissions, each admission was counted as a separate index hospitalization. Cleveland Clinic’s Institutional Review Board approved the study.
Statistical Analysis
For the 7 intervention SNFs, patient characteristics were summarized as means and standard deviations or frequencies and percentages for the periods of 2011-2012 and 2013-2014, respectively, and the 2 periods were compared using the Student t test or χ2 test as appropriate.
Mixed-effects logistic regression models were used to model 30-day readmission rates. Since the intervention was implemented in the last quarter of 2012, we examined the difference in readmission rates before and after that time point. The model included the following fixed effects: SNF type (intervention or usual care), time points (quarters of 2011-2014), whether the time is pre- or postintervention (binary), and the 3-way interaction between SNF type, pre- or postintervention and time points, and patient characteristics. The model also contained a Gaussian random effect at the SNF level to account for possible correlations among the outcomes of patients from the same SNF. For each quarter, the mean adjusted readmission rates of 2 types of SNFs were calculated from the fitted mixed models and plotted over time. Furthermore, we compared the mean readmission rates of the 2 groups in the pre- and postintervention periods. Subgroup analyses were performed for medical and surgical patients, and for patients in the low, intermediate and high HOSPITAL score groups.
All analyses were performed using RStudio (Boston, Massachusetts). Statistical significance was established with 2-sided P values less than 0.05.
RESULTS
We identified 119 SNFs within a 25-mile radius of the hospital. Of these, 6 did not receive any referrals. Three non-CCHS hospital-based SNFs were excluded, leaving a total of 110 SNFs in the study sample: 7 intervention SNFs and 103 usual-care SNFs. Between January 2011 and December 2014, there were 23,408 SNF discharges from Cleveland Clinic main campus, including 13,544 who were discharged to study SNFs (Supplemental Figure). Of these, 3334 were discharged to 7 intervention SNFs and 10,210 were discharged to usual care SNFs. Characteristics of patients in both periods appear in Table 2. At baseline, patients in the intervention and control SNFs varied in a number of ways. Patients at intervention SNFs were older (75.6 vs. 70.2 years; P < 0.001), more likely to be African American (45.5% vs. 35.9%; P < 0.001), female (61% vs. 55.4%; P < 0.001) and to be insured by Medicare (85.2% vs. 71.4%; P < 0.001). Both groups had similar proportions of patients with high, intermediate, and low readmission risk as measured by HOSPITAL score. Compared to the 2011-2012 pre-intervention period, during the 2013-2014 intervention period, there were more surgeries (34.3% vs. 41.9%; P < 0.001), more elective surgeries (21.8% vs. 25.5%; P = 0.01), fewer medical patients (65.7% vs. 58.1%; P < 0.001), and an increase in comorbidities, including myocardial infarction, peripheral vascular disease, and liver disease (Table 2).
Table 3 shows adjusted 30-day readmissions rates, before and during the intervention period at the intervention and usual care SNFs. Compared to the pre-intervention period, 30-day all-cause adjusted readmission rates declined in the intervention SNFs (28.1% to 21.7%, P < 0.001), while it increased slightly at control sites (27.1% to 28.5%, P < 0.001). The Figure shows the adjusted 30-day readmission rates by quarter throughout the study period.
Declines in 30-day readmission rates were greater for medical patients (31.0% to 24.6%, P < 0.001) than surgical patients (22.4% to 17.7%, P < 0.001). Patients with high HOSPITAL scores had the greatest decline, while those with low HOSPITAL scores had smaller declines.
DISCUSSION
In this retrospective study of 4 years of discharges to 110 SNFs, we report on the impact of a Connected Care program, in which a physician visited patients on admission to the SNF and 4 to 5 times per week during their stay. Introduction of the program was followed by a 6.8% absolute reduction in all-cause 30-day readmission rates compared to usual care. The absolute reductions ranged from 4.6% for patients at low risk for readmission to 9.1% for patients at high risk, and medical patients benefited more than surgical patients.
Most studies of interventions to reduce hospital readmissions have focused on patients discharged to the community setting.7-9 Interventions have centered on discharge planning, medication reconciliation, and close follow-up to assess for medication adherence and early signs of deterioration. Because patients in SNFs have their medications administered by staff and are under frequent surveillance, such interventions are unlikely to be helpful in this population. We found no studies that focus on short-stay or skilled patients discharged to SNF. Two studies have demonstrated that interventions can reduce hospitalization from nursing homes.22,23 Neither study included readmissions. The Evercare model consisted of nurse practitioners providing active primary care services within the nursing home, as well as offering incentive payments to nursing homes for not hospitalizing patients.22 During a 2-year period, long term residents who enrolled in Evercare had an almost 50% reduction in incident hospitalizations compared to those who did not.22 INTERACT II was a quality improvement intervention that provided tools, education, and strategies to help identify and manage acute conditions proactively.23 In 25 nursing homes employing INTERACT II, there was a 17% reduction in self-reported hospital admissions during the 6-month project, with higher rates of reduction among nursing homes rated as more engaged in the process.23 Although nursing homes may serve some short-stay or skilled patients, they generally serve long-term populations, and studies have shown that short-stay patients are at higher risk for 30-day readmissions.24
There are a number of reasons that short-term SNF patients are at higher risk for readmission. Although prior to admission, they were considered hospital level of care and received a physician visit daily, on transfer to the SNF, relatively little medical care is available. Current federal regulations regarding physician services at a SNF require the resident to be seen by a physician at least once every 30 days for the first 90 days after admission, and at least once every 60 days thereafter.25
The Connected Care program physicians provided a smooth transition of care from hospital to SNF as well as frequent reassessment. Physicians were alerted prior to hospital discharge and performed an initial comprehensive visit generally on the day of admission to the SNF and always within 48 hours. The initial evaluation is important because miscommunication during the handoff from hospital to SNF may result in incorrect medication regimens or inaccurate assessments. By performing prompt medication reconciliation and periodic reassessments of a patient’s medical condition, the Connected Care providers recreate some of the essential elements of successful outpatient readmissions prevention programs.
They also worked together with each SNF’s interdisciplinary team to deliver quality care. There were monthly meetings at each participating Connected Care SNF. Physicians reviewed monthly 30-day readmissions and performed root-cause analysis. When they discovered challenges to timely medication and treatment delivery during daily rounds, they provided in-services to SNF nurses.
In addition, Connected Care providers discussed goals of care—something that is often overlooked on admission to a SNF. This is particularly important because patients with chronic illnesses who are discharged to SNF often have poor prognoses. For example, Medicare patients with heart failure who are discharged to SNFs have 1-year mortality in excess of 50%.13 By implementing a plan of care consistent with patient and family goals, inappropriate readmissions for terminal patients may be avoided.
Reducing readmissions is important for hospitals because under the Hospital Readmissions Reduction Program, hospitals now face substantial penalties for higher than expected readmissions rates. Hospitals involved in bundled payments or other total cost-of-care arrangements have additional incentive to avoid readmissions. Beginning in 2019, SNFs will also receive incentive payments based on their 30-day all-cause hospital readmissions as part of the Skilled Nursing Facility Value-Based Purchasing program.25 The Connected Care model offers 1 means of achieving this goal through partnership between hospitals and SNFs.
Our study has several limitations. First, our study was observational in nature, so the observed reduction in readmissions could have been due to temporal trends unrelated to the intervention. However, no significant reduction was noted during the same time period in other area SNFs. There was also little change in the characteristics of patients admitted to the intervention SNFs. Importantly, the HOSPITAL score, which can predict 30-day readmission rates,20 did not change throughout the study period. Second, the results reflect patients discharged from a single hospital and may not be generalizable to other geographic areas. However, because the program included 7 SNFs, we believe it could be reproduced in other settings. Third, our readmissions measure included only those patients who returned to a CCHS facility. Although we may have missed some readmissions to other hospital systems, such leakage is uncommon—more than 80% of CCHS patients are readmitted to CCHS facilities—and would be unlikely to differ across the short duration of the study. Finally, at the intervention SNFs, most long-stay and some short-stay residents did not receive the Connected Care intervention because they were cared for by their own physicians who did not participate in Connected Care. Had these patients’ readmissions been excluded from our results, the intervention might appear even more effective.
CONCLUSION
A Connected Care intervention reduced 30-day readmission rates among patients discharged to SNFs from a tertiary academic center. While all subgroups had substantial reductions in readmissions following the implementation of the intervention, patients who are at the highest risk of readmission benefited the most. Further study is necessary to know whether Connected Care can be reproduced in other health care systems and whether it reduces overall costs.
Acknowledgments
The authors would like to thank Michael Felver, MD, and teams for their clinical care of patients; Michael Felver, MD, William Zafirau, MD, Dan Blechschmid, MHA, and Kathy Brezine, and Seth Vilensky, MBA, for their administrative support; and Brad Souder, MPT, for assistance with data collection.
Disclosure
Nothing to report.
Approximately 20% of hospitalized Medicare beneficiaries in the U.S. are discharged to skilled nursing facilities (SNFs) for post-acute care,1,2 and 23.5% of these patients are readmitted within 30 days.3 Because hospital readmissions are costly and associated with worse outcomes,4,5 30-day readmission rates are considered a quality indicator,6 and there are financial penalties for hospitals with higher than expected rates.7 As a result, hospitals invest substantial resources in programs to reduce readmissions.8-10 The SNFs represent an attractive target for readmission reduction efforts, since SNFs contribute a disproportionate share of readmissions.3,4 Because SNF patients are in a monitored environment with high medication adherence, risk factors for readmission likely differ between patients discharged to SNFs and those sent home. For example, 1 study showed that among heart failure patients with cognitive impairment, those discharged to SNFs had lower readmissions during the first 20 days, likely due to better medication adherence.11 Patients discharged to SNFs generally have more complex illnesses, lower functional status, and higher 1-year mortality than patients discharged to the community.12,13 Despite this, SNF patients might have infrequent contact with physicians. Federal regulations require only that patients discharged to SNFs need to be seen within 30 days and then at least once every 30 days thereafter.14 According to the 2014 Office of Inspector General report, one-third of Medicare beneficiaries in SNFs experience adverse events from substandard treatment, inadequate resident monitoring and failure or delay of necessary care, most of which are thought to be preventable.15
To address this issue, the Cleveland Clinic developed a program called “Connected Care SNF,” in which hospital-employed physicians and advanced practice professionals visit patients in selected SNFs 4 to 5 times per week, for the purpose of reducing preventable readmissions. The aim of this study was to assess whether the program reduced 30-day readmissions, and to identify which patients benefited most from the program.
METHODS
Setting and Intervention
The Cleveland Clinic main campus is a tertiary academic medical center with 1400 beds and approximately 50,000 admissions per year. In late 2012, the Cleveland Clinic implemented the Connected Care SNF program, wherein Cleveland Clinic physicians regularly visited patients who were discharged from the Cleveland Clinic main campus to 7 regional SNFs. Beginning in December 2012, these 7 high-volume referral SNFs that were not part of the Cleveland Clinic Health System (CCHS) agreed to participate in the program, which focused on reducing avoidable hospital readmissions and delivering quality care (Table 1). The Connected Care team, comprised of 2 geriatricians (1 of whom was also a palliative medicine specialist), 1 internist, 1 family physician, and 5 advanced practice professionals (nurse practitioners and physician assistants), provided medical services at the participating SNFs. These providers aimed to see patients 4 to 5 times per week, were available on site during working hours, and provided telephone coverage at nights and on weekends. All providers had access to hospital electronic medical records and could communicate with the discharging physician and with specialists familiar with the patient as needed. Prior to the admission, providers were informed about patient arrival and, at the time of admission to the SNF, providers reviewed medications and discussed goals of care with patients and their families. In the SNF, providers worked closely with staff members to deliver medications and timely treatment. They also met monthly with multidisciplinary teams for continuous quality improvement and to review outcomes. Patients at Connected Care SNFs who had their own physicians, including most long-stay and some short-stay residents, did not receive the Connected Care intervention. They constituted less than 10% of the patients discharged from Cleveland Clinic main campus.
Study Design and Population
We reviewed administrative and clinical data from a retrospective cohort of patients discharged to SNF from the Cleveland Clinic main campus from January 1, 2011 to December 31, 2014. We included all patients who were discharged to an SNF during the study period. Our main outcome measure was 30-day all-cause readmissions to any hospital in the Cleveland Clinic Health System (CCHS), including the main campus and 8 regional community hospitals. Study patients were followed until January 30, 2015 to capture 30-day readmissions. According to 2012 Medicare data, of CCHS patients who were readmitted within 30 days, 83% of pneumonia, 81% of major joint replacement, 72% of heart failure and 57% of acute myocardial infarction patients were readmitted to a CCHS facility. As the Cleveland Clinic main campus attracts cardiac patients from a 100+-mile radius, they may be more likely to seek care readmission near home and are not reflective of CCHS patients overall. Because we did not have access to readmissions data from non-CCHS hospitals, we excluded patients who were discharged to SNFs beyond a 25-mile radius from the main campus, where they may be more likely to utilize non-CCHS hospitals for acute hospitalization. We also excluded patients discharged to non-CCHS hospital-based SNFs, which may refer readmissions to their own hospital system. Because the Connected Care program began in December 2012, the years 2011-2012 served as the baseline period. The intervention was conducted at 7 SNFs. All other SNFs within the 25-mile radius were included as controls, except for 3 hospital-based SNFs that would be unlikely to admit patients to CCHS. We compared the change in all-cause 30-day readmission rates after implementation of Connected Care, using all patients discharged to SNFs within 25 miles to control for temporal changes in local readmission rates. Discharge to specific SNFs was determined solely by patient choice.
Data Collection
For each patient, we collected the following data that has been shown to be associated with readmissions:16-18 demographics (age, race, sex, ZIP code), lab values on discharge (hemoglobin and sodium); hemodialysis status; medicine or surgical service; elective surgery or nonelective surgery; details of the index admission index (diagnosis-related group [DRG], Medicare severity-diagnosis-related groups [MS-DRG] weight, primary diagnosis code; principal procedure code; admission date; discharge date, length of stay, and post-acute care provider); and common comorbidities, as listed in Table 2. We also calculated each patient’s HOSPITAL19,20 score. The HOSPITAL score was developed to predict risk of preventable 30-day readmissions,19 but it has also been validated to predict 30-day all-cause readmission rates for patients discharged to SNF.21 The model contains 7 elements (hemoglobin, oncology service, sodium, procedure, index type, admissions within the last year, length of stay) (supplemental Table).Patients with a high score (7 or higher) have a 41% chance of readmission, while those with a low score (4 or lower) have only a 15% chance. 21 We assessed all cause 30-day readmission status from CCHS administrative data. Observation patients and outpatient same-day surgeries were not considered to be admissions. For patients with multiple admissions, each admission was counted as a separate index hospitalization. Cleveland Clinic’s Institutional Review Board approved the study.
Statistical Analysis
For the 7 intervention SNFs, patient characteristics were summarized as means and standard deviations or frequencies and percentages for the periods of 2011-2012 and 2013-2014, respectively, and the 2 periods were compared using the Student t test or χ2 test as appropriate.
Mixed-effects logistic regression models were used to model 30-day readmission rates. Since the intervention was implemented in the last quarter of 2012, we examined the difference in readmission rates before and after that time point. The model included the following fixed effects: SNF type (intervention or usual care), time points (quarters of 2011-2014), whether the time is pre- or postintervention (binary), and the 3-way interaction between SNF type, pre- or postintervention and time points, and patient characteristics. The model also contained a Gaussian random effect at the SNF level to account for possible correlations among the outcomes of patients from the same SNF. For each quarter, the mean adjusted readmission rates of 2 types of SNFs were calculated from the fitted mixed models and plotted over time. Furthermore, we compared the mean readmission rates of the 2 groups in the pre- and postintervention periods. Subgroup analyses were performed for medical and surgical patients, and for patients in the low, intermediate and high HOSPITAL score groups.
All analyses were performed using RStudio (Boston, Massachusetts). Statistical significance was established with 2-sided P values less than 0.05.
RESULTS
We identified 119 SNFs within a 25-mile radius of the hospital. Of these, 6 did not receive any referrals. Three non-CCHS hospital-based SNFs were excluded, leaving a total of 110 SNFs in the study sample: 7 intervention SNFs and 103 usual-care SNFs. Between January 2011 and December 2014, there were 23,408 SNF discharges from Cleveland Clinic main campus, including 13,544 who were discharged to study SNFs (Supplemental Figure). Of these, 3334 were discharged to 7 intervention SNFs and 10,210 were discharged to usual care SNFs. Characteristics of patients in both periods appear in Table 2. At baseline, patients in the intervention and control SNFs varied in a number of ways. Patients at intervention SNFs were older (75.6 vs. 70.2 years; P < 0.001), more likely to be African American (45.5% vs. 35.9%; P < 0.001), female (61% vs. 55.4%; P < 0.001) and to be insured by Medicare (85.2% vs. 71.4%; P < 0.001). Both groups had similar proportions of patients with high, intermediate, and low readmission risk as measured by HOSPITAL score. Compared to the 2011-2012 pre-intervention period, during the 2013-2014 intervention period, there were more surgeries (34.3% vs. 41.9%; P < 0.001), more elective surgeries (21.8% vs. 25.5%; P = 0.01), fewer medical patients (65.7% vs. 58.1%; P < 0.001), and an increase in comorbidities, including myocardial infarction, peripheral vascular disease, and liver disease (Table 2).
Table 3 shows adjusted 30-day readmissions rates, before and during the intervention period at the intervention and usual care SNFs. Compared to the pre-intervention period, 30-day all-cause adjusted readmission rates declined in the intervention SNFs (28.1% to 21.7%, P < 0.001), while it increased slightly at control sites (27.1% to 28.5%, P < 0.001). The Figure shows the adjusted 30-day readmission rates by quarter throughout the study period.
Declines in 30-day readmission rates were greater for medical patients (31.0% to 24.6%, P < 0.001) than surgical patients (22.4% to 17.7%, P < 0.001). Patients with high HOSPITAL scores had the greatest decline, while those with low HOSPITAL scores had smaller declines.
DISCUSSION
In this retrospective study of 4 years of discharges to 110 SNFs, we report on the impact of a Connected Care program, in which a physician visited patients on admission to the SNF and 4 to 5 times per week during their stay. Introduction of the program was followed by a 6.8% absolute reduction in all-cause 30-day readmission rates compared to usual care. The absolute reductions ranged from 4.6% for patients at low risk for readmission to 9.1% for patients at high risk, and medical patients benefited more than surgical patients.
Most studies of interventions to reduce hospital readmissions have focused on patients discharged to the community setting.7-9 Interventions have centered on discharge planning, medication reconciliation, and close follow-up to assess for medication adherence and early signs of deterioration. Because patients in SNFs have their medications administered by staff and are under frequent surveillance, such interventions are unlikely to be helpful in this population. We found no studies that focus on short-stay or skilled patients discharged to SNF. Two studies have demonstrated that interventions can reduce hospitalization from nursing homes.22,23 Neither study included readmissions. The Evercare model consisted of nurse practitioners providing active primary care services within the nursing home, as well as offering incentive payments to nursing homes for not hospitalizing patients.22 During a 2-year period, long term residents who enrolled in Evercare had an almost 50% reduction in incident hospitalizations compared to those who did not.22 INTERACT II was a quality improvement intervention that provided tools, education, and strategies to help identify and manage acute conditions proactively.23 In 25 nursing homes employing INTERACT II, there was a 17% reduction in self-reported hospital admissions during the 6-month project, with higher rates of reduction among nursing homes rated as more engaged in the process.23 Although nursing homes may serve some short-stay or skilled patients, they generally serve long-term populations, and studies have shown that short-stay patients are at higher risk for 30-day readmissions.24
There are a number of reasons that short-term SNF patients are at higher risk for readmission. Although prior to admission, they were considered hospital level of care and received a physician visit daily, on transfer to the SNF, relatively little medical care is available. Current federal regulations regarding physician services at a SNF require the resident to be seen by a physician at least once every 30 days for the first 90 days after admission, and at least once every 60 days thereafter.25
The Connected Care program physicians provided a smooth transition of care from hospital to SNF as well as frequent reassessment. Physicians were alerted prior to hospital discharge and performed an initial comprehensive visit generally on the day of admission to the SNF and always within 48 hours. The initial evaluation is important because miscommunication during the handoff from hospital to SNF may result in incorrect medication regimens or inaccurate assessments. By performing prompt medication reconciliation and periodic reassessments of a patient’s medical condition, the Connected Care providers recreate some of the essential elements of successful outpatient readmissions prevention programs.
They also worked together with each SNF’s interdisciplinary team to deliver quality care. There were monthly meetings at each participating Connected Care SNF. Physicians reviewed monthly 30-day readmissions and performed root-cause analysis. When they discovered challenges to timely medication and treatment delivery during daily rounds, they provided in-services to SNF nurses.
In addition, Connected Care providers discussed goals of care—something that is often overlooked on admission to a SNF. This is particularly important because patients with chronic illnesses who are discharged to SNF often have poor prognoses. For example, Medicare patients with heart failure who are discharged to SNFs have 1-year mortality in excess of 50%.13 By implementing a plan of care consistent with patient and family goals, inappropriate readmissions for terminal patients may be avoided.
Reducing readmissions is important for hospitals because under the Hospital Readmissions Reduction Program, hospitals now face substantial penalties for higher than expected readmissions rates. Hospitals involved in bundled payments or other total cost-of-care arrangements have additional incentive to avoid readmissions. Beginning in 2019, SNFs will also receive incentive payments based on their 30-day all-cause hospital readmissions as part of the Skilled Nursing Facility Value-Based Purchasing program.25 The Connected Care model offers 1 means of achieving this goal through partnership between hospitals and SNFs.
Our study has several limitations. First, our study was observational in nature, so the observed reduction in readmissions could have been due to temporal trends unrelated to the intervention. However, no significant reduction was noted during the same time period in other area SNFs. There was also little change in the characteristics of patients admitted to the intervention SNFs. Importantly, the HOSPITAL score, which can predict 30-day readmission rates,20 did not change throughout the study period. Second, the results reflect patients discharged from a single hospital and may not be generalizable to other geographic areas. However, because the program included 7 SNFs, we believe it could be reproduced in other settings. Third, our readmissions measure included only those patients who returned to a CCHS facility. Although we may have missed some readmissions to other hospital systems, such leakage is uncommon—more than 80% of CCHS patients are readmitted to CCHS facilities—and would be unlikely to differ across the short duration of the study. Finally, at the intervention SNFs, most long-stay and some short-stay residents did not receive the Connected Care intervention because they were cared for by their own physicians who did not participate in Connected Care. Had these patients’ readmissions been excluded from our results, the intervention might appear even more effective.
CONCLUSION
A Connected Care intervention reduced 30-day readmission rates among patients discharged to SNFs from a tertiary academic center. While all subgroups had substantial reductions in readmissions following the implementation of the intervention, patients who are at the highest risk of readmission benefited the most. Further study is necessary to know whether Connected Care can be reproduced in other health care systems and whether it reduces overall costs.
Acknowledgments
The authors would like to thank Michael Felver, MD, and teams for their clinical care of patients; Michael Felver, MD, William Zafirau, MD, Dan Blechschmid, MHA, and Kathy Brezine, and Seth Vilensky, MBA, for their administrative support; and Brad Souder, MPT, for assistance with data collection.
Disclosure
Nothing to report.
1. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Chapter 8. Skilled Nursing Facility Services. March 2013. http://www.medpac.gov/docs/default-source/reports/mar13_entirereport.pdf?sfvrsn=0. Accessed March 1, 2017.
2. Kim DG, Messinger-Rapport BJ. Clarion call for a dedicated clinical and research approach to post-acute care. J Am Med Dir Assoc. 2014;15(8):607. e1-e3. PubMed
3. Mor V, Intrator O, Feng Z, Grabowski D. The revolving door of rehospitalization from skilled nursing facilities. Health Aff. 2010;29(1):57-64. PubMed
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. PubMed
5. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med 1993;118(3):219-223. PubMed
6. Van Walraven C, Bennett C, Jennings A, Austin PC, Forester AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391-E402. PubMed
7. Brenson RA, Paulus RA, Kalman NS. Medicare’s readmissions-reduction program – a positive alternative. N Engl J Med 2012;366(15):1364-1366. PubMed
8. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. PubMed
9. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613-620. PubMed
10. Coleman EA, Parry C, Chalmers S, Min SJ. The care transition intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
11. Patel A, Parikh R, Howell EH, Hsich E, Landers SH, Gorodeski EZ. Mini-cog performance: novel marker of post discharge risk among patients hospitalized for heart failure. Circ Heart Fail. 2015;8(1):8-16. PubMed
12. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987-2994. PubMed
13. Allen LA, Hernandez AF, Peterson ED, et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4(3):293-300. PubMed
14. 42 CFR 483.40 – Physician services. US government Publishing Office. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec483-40. Published October 1, 2011. Accessed August 31, 2016.
15. Office of Inspector General. Adverse Events in Skilled Nursing Facilities: National Incidence among Medicare Beneficiaries. OEI-06-11-00370. February 2014. http://oig.hhs.gov/oei/reports/oei-06-11-00370.pdf. Accessed March 22, 2016.
16. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. PubMed
17. Boult C, Dowd B, McCaffrey D, Boult L, Hernandez R, Krulewitch H. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811-817. PubMed
18. Silverstein MD, Qin H, Mercer SQ, Fong J, Haydar Z. Risk factors for 30-day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21(4):363-372. PubMed
19. Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. PubMed
20. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. PubMed
21. Kim LD, Kou L, Messinger-Rapport BJ, Rothberg MB. Validation of the HOSPITAL score for 30-day all-cause readmissions of patients discharged to skilled nursing facilities. J Am Med Dir Assoc. 2016;17(9):e15-e18. PubMed
22. Kane RL, Keckhafer G, Flood S, Bershardsky B, Siadaty MS. The effect of Evercare on hospital use. J Am Geriatr Soc. 2003;51(10):1427-1434. PubMed
23. Ouslander JG, Lamb G, Tappen R, et al. Interventions to reduce hospitalizations from nursing homes: Evaluation of the INTERACT II collaboration quality improvement project. J Am Geriatr Soc. 2011;59(4):745-753. PubMed
24. Cost drivers for dually eligible beneficiaries: Potentially avoidable hospitalizations from nursing facility, skilled nursing facility, and home and community based service waiver programs. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/costdriverstask2.pdf. Accessed August 31, 2016.
25. H.R. 4302 (113th), Section 215, Protecting Access to Medicare Act of 2014 (PAMA). April 2, 2014. https://www.govtrack.us/congress/bills/113/hr4302/text. Accessed August 31, 2016.
1. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Chapter 8. Skilled Nursing Facility Services. March 2013. http://www.medpac.gov/docs/default-source/reports/mar13_entirereport.pdf?sfvrsn=0. Accessed March 1, 2017.
2. Kim DG, Messinger-Rapport BJ. Clarion call for a dedicated clinical and research approach to post-acute care. J Am Med Dir Assoc. 2014;15(8):607. e1-e3. PubMed
3. Mor V, Intrator O, Feng Z, Grabowski D. The revolving door of rehospitalization from skilled nursing facilities. Health Aff. 2010;29(1):57-64. PubMed
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. PubMed
5. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med 1993;118(3):219-223. PubMed
6. Van Walraven C, Bennett C, Jennings A, Austin PC, Forester AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391-E402. PubMed
7. Brenson RA, Paulus RA, Kalman NS. Medicare’s readmissions-reduction program – a positive alternative. N Engl J Med 2012;366(15):1364-1366. PubMed
8. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. PubMed
9. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613-620. PubMed
10. Coleman EA, Parry C, Chalmers S, Min SJ. The care transition intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
11. Patel A, Parikh R, Howell EH, Hsich E, Landers SH, Gorodeski EZ. Mini-cog performance: novel marker of post discharge risk among patients hospitalized for heart failure. Circ Heart Fail. 2015;8(1):8-16. PubMed
12. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987-2994. PubMed
13. Allen LA, Hernandez AF, Peterson ED, et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4(3):293-300. PubMed
14. 42 CFR 483.40 – Physician services. US government Publishing Office. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec483-40. Published October 1, 2011. Accessed August 31, 2016.
15. Office of Inspector General. Adverse Events in Skilled Nursing Facilities: National Incidence among Medicare Beneficiaries. OEI-06-11-00370. February 2014. http://oig.hhs.gov/oei/reports/oei-06-11-00370.pdf. Accessed March 22, 2016.
16. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. PubMed
17. Boult C, Dowd B, McCaffrey D, Boult L, Hernandez R, Krulewitch H. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811-817. PubMed
18. Silverstein MD, Qin H, Mercer SQ, Fong J, Haydar Z. Risk factors for 30-day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21(4):363-372. PubMed
19. Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632-638. PubMed
20. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. PubMed
21. Kim LD, Kou L, Messinger-Rapport BJ, Rothberg MB. Validation of the HOSPITAL score for 30-day all-cause readmissions of patients discharged to skilled nursing facilities. J Am Med Dir Assoc. 2016;17(9):e15-e18. PubMed
22. Kane RL, Keckhafer G, Flood S, Bershardsky B, Siadaty MS. The effect of Evercare on hospital use. J Am Geriatr Soc. 2003;51(10):1427-1434. PubMed
23. Ouslander JG, Lamb G, Tappen R, et al. Interventions to reduce hospitalizations from nursing homes: Evaluation of the INTERACT II collaboration quality improvement project. J Am Geriatr Soc. 2011;59(4):745-753. PubMed
24. Cost drivers for dually eligible beneficiaries: Potentially avoidable hospitalizations from nursing facility, skilled nursing facility, and home and community based service waiver programs. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/costdriverstask2.pdf. Accessed August 31, 2016.
25. H.R. 4302 (113th), Section 215, Protecting Access to Medicare Act of 2014 (PAMA). April 2, 2014. https://www.govtrack.us/congress/bills/113/hr4302/text. Accessed August 31, 2016.
© 2017 Society of Hospital Medicine
Home-based care for heart failure: Cleveland Clinic’s “Heart Care at Home” transitional care program
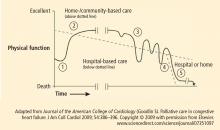
The home is the most important context of care for individuals with chronic heart failure and yet it is the least accessible to caregivers. Patients often struggle to manage a complex regimen of medications, follow an unfamiliar diet, monitor weight and vital signs, and work to coordinate care among various providers who, in some cases, fail to communicate effectively. Heart failure patients do all this while making difficult decisions about their livelihoods, social condition, and future direction. With progression of the disease and comorbidity, these patients often experience a downward cycle of repeat hospitalization and worsening functional capacity (Figure 1). Each subsequent transition from acute care to home becomes incrementally more difficult to manage.
According to the latest American College of Cardiology/American Heart Association Guidelines for the Diagnosis and Management of Heart Failure in Adults, appropriate care for patients with heart failure should include:
- Intensive patient education
- Encouragement of patients to be more aggressive participants in their care
- Close monitoring of patients through telephone follow-up or home nursing
- Careful review of medications to improve adherence to evidence-based guidelines
- Multidisciplinary care with nurse case management directed by a physician1
Beyond these general suggestions, recommendations about specific approaches and models of care in the home are lacking.
Contemporary research suggests that postacute, home-based care of heart failure patients may yield outcomes similar to those of clinic-based outpatient care. Results of the Which Heart Failure Intervention is Most Cost-Effective & Consumer-Friendly in Reducing Hospital Care (WHICH?) trial support this hypothesis. This multicenter, randomized clinical trial (n = 280) compared home- with clinic-based multidisciplinary management for postacute heart failure patients.2 Investigators compared outcomes in patients managed at a heart failure clinic with those managed at home. They found that postdischarge home visits by heart failure nurses did not significantly alter the primary composite end point of death or unplanned rehospitalization from any cause over 18 months (hazard ratio [HR] 0.97, 95% confidence interval [CI] 0.73–1.30, P = .8621). The rate of unplanned and total hospitalization was also similar in the two groups. However, the average length of hospital stay was significantly lower in the home care group (4 days) than in the clinic-based group (6 days); P = .004. A cost-effectiveness analysis is planned but has not yet been presented.
HEART CARE AT HOME
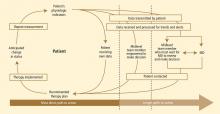
At Cleveland Clinic, our group of physicians (geriatrics and cardiology), nurses, nurse practitioners, and hospital administrators founded a primarily home-based postacute transitional care program in 2010 called “Heart Care at Home.” The design of our program was influenced by Coleman et al’s care transitions interventions program,3 Naylor et al’s transitional care intervention,4 and the contemporary remote monitoring literature.5 The program focuses primarily on older adults hospitalized for heart failure who are transitioning from hospital to home. In our model:
- Inpatient care advocates identify candidates during the index inpatient stay, introduce a model of care, and begin a coaching intervention.
- After discharge, home liaisons visit the patient at home, continue coaching intervention, and teach the patient to use the newly installed remote monitoring equipment.
- For 30 to 40 days after discharge, a team of telehealth nurses monitors the patient, makes contact with him or her weekly in order to reinforce coaching intervention, coordinates care, and tracks outcomes.
- Nurse practitioners experienced both in home care and heart failure provide clinical oversight and leadership and visit the highest-acuity patients at home.
To date, the program has provided care in more than 2,100 patient encounters, with approximately 50 to 80 patients actively enrolled at any time. We identified potential program candidates using a digital list tool embedded in Cleveland Clinic’s electronic medical record (EMR) system. This tool was developed by our team together with an internal business intelligence team. We have been approximately 65% successful in identifying eligible inpatients. Patients enrolled in our transitional care program tend to be older, have longer hospital stays, and have more comorbidities than other older adults hospitalized at Cleveland Clinic for similar reasons.
Following index hospital discharge, our home liaisons have been able to make an initial home visit after a median of 2 days (25th to 75th percentile: 1 to 3 days). Patients thought to be at higher risk for hospital readmissions have been seen at home by our nurse practitioners within the first week of discharge. The most common challenge that our at-home team members have faced relates to patients’ medications (for example, unfilled prescriptions and errors in utilization). On many occasions our at-home team has succeeded in transitioning patients not benefiting from care at home to nonhospital venues (skilled nursing facilities, chronic care facilities, inpatient hospice) or to higher levels of at-home care (at-home physician visits, home-care nursing and therapy, at-home hospice).
To date, patients have been enrolled in our program for a median of 30 days (25th to 75th percentile: 20 to 35 days). We have observed an increased level of patient satisfaction. Among heart failure patients enrolled in our program for the first time, we have observed a lower readmission rate compared with publicly reported Cleveland Clinic rates (24.5% vs 28.2%). However, there are several ongoing challenges in the care of heart failure patients in the home environment. These relate to longitudinal care across venues, cross training of providers, and home monitoring.
Longitudinal care across venues
Our program aims to address the lack of integrated care over time and between care venues. This problem lies at the intersection of health care reimbursement policy and clinical practice. Currently, the hospital reimbursement system does not encourage care coordination across settings. The system has, in fact, evolved into a string of disconnected care providers who act as “toll booths” providing services for a fee in isolation from other providers. Coleman and colleagues have documented the complexity of the transitions among these care providers for older patients with chronic disease, noting the implications for patient safety and cost.6
Hospitals receive a fixed payment for an inpatient admission, which increases the financial incentive to discharge patients faster to other venues of care. The study by Bueno et al of a Medicare population treated between 1993 and 2006 confirms that such a trend exists for heart failure patients.7 The authors found a steady decrease in the mean length of hospital stay from 8.81 days to 6.33 days over the study period (28% relative reduction, P < .001). During this same period, the 30-day all-cause readmission rate increased from 17.2% to 20.1% (a 17% relative increase, P < .001) with an associated 10% relative reduction in the proportion of patients discharged to home.7 Experience in other populations with heart failure, such as patients in the Veterans Affairs health care system, has shown similar trends in length of hospital stay and readmissions.8
During these transitions, information is often lost in the handoff from the discharging hospital to the next venue of care. Medication management is the most common problem area with the potential for patient noncompliance with prescriptions,9,10 which can have serious deleterious effects on quality and safety. Forster et al found that 66% of untoward outcomes in discharged patients were due to adverse drug events.11 Similarly, Gray et al identified adverse drug events in 20% of patients discharged from hospital to home with home health care services.12
In the Cleveland Clinic Health System, we are coupling our “Heart Care at Home” transitional care program with an aggressive plan to develop a more comprehensive cross-venue EMR. Connecting the hospital EMR with our health system–owned home health agency will enable a consistent medication record and communication system for patients transitioning from our hospitals to Cleveland Clinic home care services (nearly 20,000 patients per year).
Despite these issues, several care transition interventions have shown promising clinical and economic results. Coleman and colleagues conducted a randomized, controlled trial of a transition coaching model in which patients and caregivers were encouraged to take a more active role in care transitions. Results of this trial showed a significant decrease in 30- and 90-day rehospitalizations (the 90-day read-mission rate in the treatment group was 16.7 vs 22.5 in the control group, P = .04) with associated cost savings.3 Voss et al showed similar results in reduction of readmissions in a nonintegrated delivery system.13 Additionally, telephone-based chronic disease management programs have been shown to be cost-effective in chronically ill Medicare patients.14
When will the clinical evidence behind care transitions and financial incentives converge to create an atmosphere conducive to more optimal care coordination? Today, this question remains unanswered. Health care reform, with the passage of the Patient Protection and Affordable Care Act (PPACA) (http://housedocs.house.gov/energycommerce/ppacacon.pdf), may spur the creation of programs to increase incentives for care coordination. These include a move to episodic reimbursement that would bundle payments for acute and postacute care, thus creating more incentives for coordinating care across settings. The “Bundled Payments for Care Improvement” project run by the Center for Medicare & Medicaid Innovation will test different models and approaches to bundled payments (http://innovations.cms.gov/initiatives/bundled-payments). Additionally, beginning in fiscal year 2013, Medicare will penalize hospitals that have high readmission rates for heart failure, acute myocardial infarction, and pneumonia with a financial risk of up to 3% of total hospital Medicare payments by year 3 of the program.
The PPACA will have a significant effect on home-based care for older adults with chronic conditions. The PPACA reforms will likely lead to more patients being treated at home (the lower-cost care setting), ideally under the care of highly skilled teams. Payment reforms will also create new incentives for providers to better coordinate care, keep patients healthy at home, and avoid the “toll-booth” description entirely, enabling providers to focus on patient care. However, more research and experimentation are required to streamline the elements on the transitions spectrum in order to create the most value for specific patient populations. New infrastructure, use of technology, changing culture, and dedicated clinical teams will be necessary to deliver on the hopes of more integrated longitudinal care across venues.
Cross training of providers
Older community-dwelling adults with heart failure exhibit more health instability; take more medications; have more comorbidities; and receive more nursing, homemaking, and meal services than do other home care clients.15 Nurses thus have a unique opportunity to improve outcomes for home-based heart failure patients,16,17 but are often insufficiently trained to do so. Delaney et al administered a validated 20-item heart failure knowledge questionnaire to 94 home care nurses from four different home care agencies.18 The investigators found a 79% knowledge level in overall heart failure education principles, with lowest scores related to issues of asymptomatic hypotension (25% answered correctly), daily weight monitoring (27%), and transient dizziness (31%). Nurses with poorer heart failure–related knowledge may partially explain worse process and outcome measures among this patient population.19
The home-based nursing workforce of the future, and specifically nurses who care for heart failure patients at home, will need to be better trained and specialized in issues relating both to home-based nursing and medical heart failure. These “hybrid nurses” should be allowed a central clinical leadership role among their peers, as they will need to be empowered to make medical and care coordination decisions.
At our center, hybrid-trained home care/heart failure nurse practitioners make home visits for higher-acuity home-based patients and provide clinical leadership and support for other home care nurses. These nurse practitioners have been instrumental in identifying and correcting heart failure medication–related problems, as well as effectively coordinating care. Examples include: independently prescribing and coordinating administration of intravenous diuretics at home for patients who have difficulty managing volume overload, avoiding hospital readmissions by transitioning ill patients to a skilled nursing facility or an at-home hospice, and effectively educating patients and families about appropriate heart failure self-care.
Home monitoring
Home monitoring of selected physiologic parameters and patient-reported health status measures among heart failure patients may facilitate early detection of clinical deterioration and direct timely intervention to prevent adverse outcomes.20 Desai and Stevenson have previously proposed the “circle from home to heart-failure disease management,” a concept illustrating how home monitoring can be embedded in a comprehensive heart failure management approach (Figure 2).20 This concept emphasizes the following:
- Home monitoring should facilitate early detection of clinical deterioration.20
- Home monitoring data will most directly lead to action if the data can be used by the patient to improve self-care.
- In the setting of multidisciplinary care, data should be remotely transmitted to a midlevel team, preferably one empowered to make therapeutic decisions.
- Further engagement of physicians or other clinical providers may be beneficial but will delay the clinical response.
The most commonly monitored physiologic parameter of heart failure patients is daily weight. While nearly universally used, this parameter is in fact a poor surrogate for subclinical hemodynamic congestion and has poor diagnostic performance for clinical decompensation. Results are conflicting from studies evaluating the utility of daily body weight measurements in patients with heart failure who are being cared for in the home environment.
In one study, an increase in body weight of > 2 kg over 24 to 72 hours had a 9% sensitivity for detecting clinical deterioration.21 In another study, Chaudhry et al performed a nested case-control trial in 134 patients with heart failure and 134 matched controls referred to a home monitoring system by managed care organizations. The researchers found that increases in body weight were associated with hospitalization for heart failure and that the increases began at least 1 week before admission.22 However, they did not investigate whether the use of this information by clinicians altered outcomes. In a prior randomized clinical trial of symptom monitoring versus transtelephonic body weight monitoring in patients with symptomatic heart failure, the Weight Monitoring in Heart Failure trial (n = 280), weight monitoring did not result in improvement in the primary outcome of hospitalizations for heart failure over a 6-month period.23
The ideal monitoring parameters in heart failure patients may include direct hemodynamic measurements from the right ventricular outflow tract,24 pulmonary artery,25 or left atrium,26 using implantable devices. For example, the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients) trial (n = 550) was a randomized, single-blind, industry-sponsored trial of heart failure management guided by physiologic hemodynamic data derived from a percutaneously inserted pulmonary artery hemodynamic monitor (Champion HF Monitoring System; CardioMEMS, Atlanta, Georgia). The researchers found that monitoring these parameters was associated with a 28% reduction in heart failure–related hospitalizations during the first 6 months (rate 0.32 vs 0.44, HR 0.72, 95% CI 0.60–0.85, P = .0002) compared with usual care.25 At 6 months, the freedom from device- or system-related complications was 98.6%.
Despite success in the trial, the US Food and Drug Administration Circulatory System Devices Panel voted against approving the device. The panel was concerned that the e-mail–alert and care systems built into the intervention arm of the trial created bias in favor of the device, and that in a real-world situation it may not be as effective. This demonstrates the ongoing challenges and barriers to adoption of invasive hemodynamic monitoring.
At our center, we are conducting an institutional review board–approved investigation of an entirely noninvasive under-the-mattress piezoelectric monitor in a cohort of postacute heart failure patients. Piezoelectricity is the charge that accumulates in certain solid materials in response to mechanical stress. Common applications of piezoelectricity include microphones, push-start propane barbecues, and cigarette lighters. The device under investigation (EverOn; EarlySense, Ramat-Gan, Israel) detects heart rate, respiratory rate, and movement rate through vibrations of the mattress. Case examples are shown in Figure 3. Whether such monitoring technology will play a future role in the home environment remains to be seen.
SUMMARY
At the time of this writing, the Supreme Court of the United States has reaffirmed the constitutionality of the PPACA, clearing the way for implementation of significant changes in the US health care delivery system. The implications for in-home care for older adults with chronic conditions, including heart failure, are significant. The home will become an increasingly common venue of postacute care. Today is the time to investigate beneficial models of care and optimal uses of technology, and to develop a specialized mobile workforce that will confidently care for individuals with heart failure at home, responsibly and at lower cost.
- Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009; 119:e391–e479.
- Stewart S, Carrington MJ, Marwick TH, et al. Impact of home versus clinic-based management of chronic heart failure: the WHICH? (Which Heart Failure Intervention Is Most Cost-Effective and Consumer Friendly in Reducing Hospital Care) multicenter, randomized trial. J Am Coll Cardiol 2012; 60:1239–1248.
- Coleman EA, Parry C, Chalmers S, Min S-J. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006; 166:1822–1828.
- Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc 2004; 52:675–684.
- Klersy C, De Silvestri A, Gabutti G, Regoli F, Auricchio A. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol 2009; 54:1683–1694.
- Coleman EA, Min S-J, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res 2004; 39:1449–1465.
- Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA 2010; 303:2141–2147.
- Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol 2010; 56:362–368.
- Moore C, Wisnivesky J, Williams S, McGinn T. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 2003; 18:646–651.
- Coleman EA, Smith JD, Raha D, Min S-J. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005; 165:1842–1847.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003; 138:161–167.
- Gray SL, Mahoney JE, Blough DK. Adverse drug events in elderly patients receiving home health services following hospital discharge. Ann Pharmacother 1999; 33:1147–1153.
- Voss R, Gardner R, Baier R, Butterfeld K, Lehrman S, Gravenstein S. The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med 2011; 171:1232–1237.
- Baker LC, Johnson SJ, Macaulay D, Birnbaum H. Integrated telehealth and care management program for Medicare benefciaries with chronic disease linked to savings. Health Aff (Millwood) 2011; 30:1689–1697.
- Foebel AD, Hirdes JP, Heckman GA, Tyas SL, Tjam EY. A profile of older community-dwelling home care clients with heart failure in Ontario. Chronic Dis Can 2011; 31:49–57.
- Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol 2002; 39:83–89.
- González B, Lupón J, Herreros J, et al. Patient’s education by nurse: what we really do achieve? Eur J Cardiovasc Nurs 2005; 4:107–111.
- Delaney C, Apostolidis B, Lachapelle L, Fortinsky R. Home care nurses’ knowledge of evidence-based education topics for management of heart failure. Heart Lung 2011; 40:285–292.
- Foebel AD, Heckman GA, Hirdes JP, et al. Clinical, demographic and functional characteristics associated with pharmacotherapy for heart failure in older home care clients: a retrospective, population-level, cross-sectional study. Drugs Aging 2011; 28:561–573.
- Desai AS, Stevenson LW. Connecting the circle from home to heart-failure disease management. N Engl J Med 2010; 363:2364–2367.
- Lewin J, Ledwidge M, O’Loughlin C, McNally C, McDonald K. Clinical deterioration in established heart failure: what is the value of BNP and weight gain in aiding diagnosis? Eur J Heart Fail 2005; 7:953–957.
- Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation 2007; 116:1549–1554.
- Goldberg LR, Piette JD, Walsh MN, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J 2003; 146:705–712.
- Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the Compass-HF study. J Am Coll Cardiol 2008; 51:1073–1079.
- Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation 2010; 121:1086–1095.
The home is the most important context of care for individuals with chronic heart failure and yet it is the least accessible to caregivers. Patients often struggle to manage a complex regimen of medications, follow an unfamiliar diet, monitor weight and vital signs, and work to coordinate care among various providers who, in some cases, fail to communicate effectively. Heart failure patients do all this while making difficult decisions about their livelihoods, social condition, and future direction. With progression of the disease and comorbidity, these patients often experience a downward cycle of repeat hospitalization and worsening functional capacity (Figure 1). Each subsequent transition from acute care to home becomes incrementally more difficult to manage.
According to the latest American College of Cardiology/American Heart Association Guidelines for the Diagnosis and Management of Heart Failure in Adults, appropriate care for patients with heart failure should include:
- Intensive patient education
- Encouragement of patients to be more aggressive participants in their care
- Close monitoring of patients through telephone follow-up or home nursing
- Careful review of medications to improve adherence to evidence-based guidelines
- Multidisciplinary care with nurse case management directed by a physician1
Beyond these general suggestions, recommendations about specific approaches and models of care in the home are lacking.
Contemporary research suggests that postacute, home-based care of heart failure patients may yield outcomes similar to those of clinic-based outpatient care. Results of the Which Heart Failure Intervention is Most Cost-Effective & Consumer-Friendly in Reducing Hospital Care (WHICH?) trial support this hypothesis. This multicenter, randomized clinical trial (n = 280) compared home- with clinic-based multidisciplinary management for postacute heart failure patients.2 Investigators compared outcomes in patients managed at a heart failure clinic with those managed at home. They found that postdischarge home visits by heart failure nurses did not significantly alter the primary composite end point of death or unplanned rehospitalization from any cause over 18 months (hazard ratio [HR] 0.97, 95% confidence interval [CI] 0.73–1.30, P = .8621). The rate of unplanned and total hospitalization was also similar in the two groups. However, the average length of hospital stay was significantly lower in the home care group (4 days) than in the clinic-based group (6 days); P = .004. A cost-effectiveness analysis is planned but has not yet been presented.
HEART CARE AT HOME
At Cleveland Clinic, our group of physicians (geriatrics and cardiology), nurses, nurse practitioners, and hospital administrators founded a primarily home-based postacute transitional care program in 2010 called “Heart Care at Home.” The design of our program was influenced by Coleman et al’s care transitions interventions program,3 Naylor et al’s transitional care intervention,4 and the contemporary remote monitoring literature.5 The program focuses primarily on older adults hospitalized for heart failure who are transitioning from hospital to home. In our model:
- Inpatient care advocates identify candidates during the index inpatient stay, introduce a model of care, and begin a coaching intervention.
- After discharge, home liaisons visit the patient at home, continue coaching intervention, and teach the patient to use the newly installed remote monitoring equipment.
- For 30 to 40 days after discharge, a team of telehealth nurses monitors the patient, makes contact with him or her weekly in order to reinforce coaching intervention, coordinates care, and tracks outcomes.
- Nurse practitioners experienced both in home care and heart failure provide clinical oversight and leadership and visit the highest-acuity patients at home.
To date, the program has provided care in more than 2,100 patient encounters, with approximately 50 to 80 patients actively enrolled at any time. We identified potential program candidates using a digital list tool embedded in Cleveland Clinic’s electronic medical record (EMR) system. This tool was developed by our team together with an internal business intelligence team. We have been approximately 65% successful in identifying eligible inpatients. Patients enrolled in our transitional care program tend to be older, have longer hospital stays, and have more comorbidities than other older adults hospitalized at Cleveland Clinic for similar reasons.
Following index hospital discharge, our home liaisons have been able to make an initial home visit after a median of 2 days (25th to 75th percentile: 1 to 3 days). Patients thought to be at higher risk for hospital readmissions have been seen at home by our nurse practitioners within the first week of discharge. The most common challenge that our at-home team members have faced relates to patients’ medications (for example, unfilled prescriptions and errors in utilization). On many occasions our at-home team has succeeded in transitioning patients not benefiting from care at home to nonhospital venues (skilled nursing facilities, chronic care facilities, inpatient hospice) or to higher levels of at-home care (at-home physician visits, home-care nursing and therapy, at-home hospice).
To date, patients have been enrolled in our program for a median of 30 days (25th to 75th percentile: 20 to 35 days). We have observed an increased level of patient satisfaction. Among heart failure patients enrolled in our program for the first time, we have observed a lower readmission rate compared with publicly reported Cleveland Clinic rates (24.5% vs 28.2%). However, there are several ongoing challenges in the care of heart failure patients in the home environment. These relate to longitudinal care across venues, cross training of providers, and home monitoring.
Longitudinal care across venues
Our program aims to address the lack of integrated care over time and between care venues. This problem lies at the intersection of health care reimbursement policy and clinical practice. Currently, the hospital reimbursement system does not encourage care coordination across settings. The system has, in fact, evolved into a string of disconnected care providers who act as “toll booths” providing services for a fee in isolation from other providers. Coleman and colleagues have documented the complexity of the transitions among these care providers for older patients with chronic disease, noting the implications for patient safety and cost.6
Hospitals receive a fixed payment for an inpatient admission, which increases the financial incentive to discharge patients faster to other venues of care. The study by Bueno et al of a Medicare population treated between 1993 and 2006 confirms that such a trend exists for heart failure patients.7 The authors found a steady decrease in the mean length of hospital stay from 8.81 days to 6.33 days over the study period (28% relative reduction, P < .001). During this same period, the 30-day all-cause readmission rate increased from 17.2% to 20.1% (a 17% relative increase, P < .001) with an associated 10% relative reduction in the proportion of patients discharged to home.7 Experience in other populations with heart failure, such as patients in the Veterans Affairs health care system, has shown similar trends in length of hospital stay and readmissions.8
During these transitions, information is often lost in the handoff from the discharging hospital to the next venue of care. Medication management is the most common problem area with the potential for patient noncompliance with prescriptions,9,10 which can have serious deleterious effects on quality and safety. Forster et al found that 66% of untoward outcomes in discharged patients were due to adverse drug events.11 Similarly, Gray et al identified adverse drug events in 20% of patients discharged from hospital to home with home health care services.12
In the Cleveland Clinic Health System, we are coupling our “Heart Care at Home” transitional care program with an aggressive plan to develop a more comprehensive cross-venue EMR. Connecting the hospital EMR with our health system–owned home health agency will enable a consistent medication record and communication system for patients transitioning from our hospitals to Cleveland Clinic home care services (nearly 20,000 patients per year).
Despite these issues, several care transition interventions have shown promising clinical and economic results. Coleman and colleagues conducted a randomized, controlled trial of a transition coaching model in which patients and caregivers were encouraged to take a more active role in care transitions. Results of this trial showed a significant decrease in 30- and 90-day rehospitalizations (the 90-day read-mission rate in the treatment group was 16.7 vs 22.5 in the control group, P = .04) with associated cost savings.3 Voss et al showed similar results in reduction of readmissions in a nonintegrated delivery system.13 Additionally, telephone-based chronic disease management programs have been shown to be cost-effective in chronically ill Medicare patients.14
When will the clinical evidence behind care transitions and financial incentives converge to create an atmosphere conducive to more optimal care coordination? Today, this question remains unanswered. Health care reform, with the passage of the Patient Protection and Affordable Care Act (PPACA) (http://housedocs.house.gov/energycommerce/ppacacon.pdf), may spur the creation of programs to increase incentives for care coordination. These include a move to episodic reimbursement that would bundle payments for acute and postacute care, thus creating more incentives for coordinating care across settings. The “Bundled Payments for Care Improvement” project run by the Center for Medicare & Medicaid Innovation will test different models and approaches to bundled payments (http://innovations.cms.gov/initiatives/bundled-payments). Additionally, beginning in fiscal year 2013, Medicare will penalize hospitals that have high readmission rates for heart failure, acute myocardial infarction, and pneumonia with a financial risk of up to 3% of total hospital Medicare payments by year 3 of the program.
The PPACA will have a significant effect on home-based care for older adults with chronic conditions. The PPACA reforms will likely lead to more patients being treated at home (the lower-cost care setting), ideally under the care of highly skilled teams. Payment reforms will also create new incentives for providers to better coordinate care, keep patients healthy at home, and avoid the “toll-booth” description entirely, enabling providers to focus on patient care. However, more research and experimentation are required to streamline the elements on the transitions spectrum in order to create the most value for specific patient populations. New infrastructure, use of technology, changing culture, and dedicated clinical teams will be necessary to deliver on the hopes of more integrated longitudinal care across venues.
Cross training of providers
Older community-dwelling adults with heart failure exhibit more health instability; take more medications; have more comorbidities; and receive more nursing, homemaking, and meal services than do other home care clients.15 Nurses thus have a unique opportunity to improve outcomes for home-based heart failure patients,16,17 but are often insufficiently trained to do so. Delaney et al administered a validated 20-item heart failure knowledge questionnaire to 94 home care nurses from four different home care agencies.18 The investigators found a 79% knowledge level in overall heart failure education principles, with lowest scores related to issues of asymptomatic hypotension (25% answered correctly), daily weight monitoring (27%), and transient dizziness (31%). Nurses with poorer heart failure–related knowledge may partially explain worse process and outcome measures among this patient population.19
The home-based nursing workforce of the future, and specifically nurses who care for heart failure patients at home, will need to be better trained and specialized in issues relating both to home-based nursing and medical heart failure. These “hybrid nurses” should be allowed a central clinical leadership role among their peers, as they will need to be empowered to make medical and care coordination decisions.
At our center, hybrid-trained home care/heart failure nurse practitioners make home visits for higher-acuity home-based patients and provide clinical leadership and support for other home care nurses. These nurse practitioners have been instrumental in identifying and correcting heart failure medication–related problems, as well as effectively coordinating care. Examples include: independently prescribing and coordinating administration of intravenous diuretics at home for patients who have difficulty managing volume overload, avoiding hospital readmissions by transitioning ill patients to a skilled nursing facility or an at-home hospice, and effectively educating patients and families about appropriate heart failure self-care.
Home monitoring
Home monitoring of selected physiologic parameters and patient-reported health status measures among heart failure patients may facilitate early detection of clinical deterioration and direct timely intervention to prevent adverse outcomes.20 Desai and Stevenson have previously proposed the “circle from home to heart-failure disease management,” a concept illustrating how home monitoring can be embedded in a comprehensive heart failure management approach (Figure 2).20 This concept emphasizes the following:
- Home monitoring should facilitate early detection of clinical deterioration.20
- Home monitoring data will most directly lead to action if the data can be used by the patient to improve self-care.
- In the setting of multidisciplinary care, data should be remotely transmitted to a midlevel team, preferably one empowered to make therapeutic decisions.
- Further engagement of physicians or other clinical providers may be beneficial but will delay the clinical response.
The most commonly monitored physiologic parameter of heart failure patients is daily weight. While nearly universally used, this parameter is in fact a poor surrogate for subclinical hemodynamic congestion and has poor diagnostic performance for clinical decompensation. Results are conflicting from studies evaluating the utility of daily body weight measurements in patients with heart failure who are being cared for in the home environment.
In one study, an increase in body weight of > 2 kg over 24 to 72 hours had a 9% sensitivity for detecting clinical deterioration.21 In another study, Chaudhry et al performed a nested case-control trial in 134 patients with heart failure and 134 matched controls referred to a home monitoring system by managed care organizations. The researchers found that increases in body weight were associated with hospitalization for heart failure and that the increases began at least 1 week before admission.22 However, they did not investigate whether the use of this information by clinicians altered outcomes. In a prior randomized clinical trial of symptom monitoring versus transtelephonic body weight monitoring in patients with symptomatic heart failure, the Weight Monitoring in Heart Failure trial (n = 280), weight monitoring did not result in improvement in the primary outcome of hospitalizations for heart failure over a 6-month period.23
The ideal monitoring parameters in heart failure patients may include direct hemodynamic measurements from the right ventricular outflow tract,24 pulmonary artery,25 or left atrium,26 using implantable devices. For example, the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients) trial (n = 550) was a randomized, single-blind, industry-sponsored trial of heart failure management guided by physiologic hemodynamic data derived from a percutaneously inserted pulmonary artery hemodynamic monitor (Champion HF Monitoring System; CardioMEMS, Atlanta, Georgia). The researchers found that monitoring these parameters was associated with a 28% reduction in heart failure–related hospitalizations during the first 6 months (rate 0.32 vs 0.44, HR 0.72, 95% CI 0.60–0.85, P = .0002) compared with usual care.25 At 6 months, the freedom from device- or system-related complications was 98.6%.
Despite success in the trial, the US Food and Drug Administration Circulatory System Devices Panel voted against approving the device. The panel was concerned that the e-mail–alert and care systems built into the intervention arm of the trial created bias in favor of the device, and that in a real-world situation it may not be as effective. This demonstrates the ongoing challenges and barriers to adoption of invasive hemodynamic monitoring.
At our center, we are conducting an institutional review board–approved investigation of an entirely noninvasive under-the-mattress piezoelectric monitor in a cohort of postacute heart failure patients. Piezoelectricity is the charge that accumulates in certain solid materials in response to mechanical stress. Common applications of piezoelectricity include microphones, push-start propane barbecues, and cigarette lighters. The device under investigation (EverOn; EarlySense, Ramat-Gan, Israel) detects heart rate, respiratory rate, and movement rate through vibrations of the mattress. Case examples are shown in Figure 3. Whether such monitoring technology will play a future role in the home environment remains to be seen.
SUMMARY
At the time of this writing, the Supreme Court of the United States has reaffirmed the constitutionality of the PPACA, clearing the way for implementation of significant changes in the US health care delivery system. The implications for in-home care for older adults with chronic conditions, including heart failure, are significant. The home will become an increasingly common venue of postacute care. Today is the time to investigate beneficial models of care and optimal uses of technology, and to develop a specialized mobile workforce that will confidently care for individuals with heart failure at home, responsibly and at lower cost.
The home is the most important context of care for individuals with chronic heart failure and yet it is the least accessible to caregivers. Patients often struggle to manage a complex regimen of medications, follow an unfamiliar diet, monitor weight and vital signs, and work to coordinate care among various providers who, in some cases, fail to communicate effectively. Heart failure patients do all this while making difficult decisions about their livelihoods, social condition, and future direction. With progression of the disease and comorbidity, these patients often experience a downward cycle of repeat hospitalization and worsening functional capacity (Figure 1). Each subsequent transition from acute care to home becomes incrementally more difficult to manage.
According to the latest American College of Cardiology/American Heart Association Guidelines for the Diagnosis and Management of Heart Failure in Adults, appropriate care for patients with heart failure should include:
- Intensive patient education
- Encouragement of patients to be more aggressive participants in their care
- Close monitoring of patients through telephone follow-up or home nursing
- Careful review of medications to improve adherence to evidence-based guidelines
- Multidisciplinary care with nurse case management directed by a physician1
Beyond these general suggestions, recommendations about specific approaches and models of care in the home are lacking.
Contemporary research suggests that postacute, home-based care of heart failure patients may yield outcomes similar to those of clinic-based outpatient care. Results of the Which Heart Failure Intervention is Most Cost-Effective & Consumer-Friendly in Reducing Hospital Care (WHICH?) trial support this hypothesis. This multicenter, randomized clinical trial (n = 280) compared home- with clinic-based multidisciplinary management for postacute heart failure patients.2 Investigators compared outcomes in patients managed at a heart failure clinic with those managed at home. They found that postdischarge home visits by heart failure nurses did not significantly alter the primary composite end point of death or unplanned rehospitalization from any cause over 18 months (hazard ratio [HR] 0.97, 95% confidence interval [CI] 0.73–1.30, P = .8621). The rate of unplanned and total hospitalization was also similar in the two groups. However, the average length of hospital stay was significantly lower in the home care group (4 days) than in the clinic-based group (6 days); P = .004. A cost-effectiveness analysis is planned but has not yet been presented.
HEART CARE AT HOME
At Cleveland Clinic, our group of physicians (geriatrics and cardiology), nurses, nurse practitioners, and hospital administrators founded a primarily home-based postacute transitional care program in 2010 called “Heart Care at Home.” The design of our program was influenced by Coleman et al’s care transitions interventions program,3 Naylor et al’s transitional care intervention,4 and the contemporary remote monitoring literature.5 The program focuses primarily on older adults hospitalized for heart failure who are transitioning from hospital to home. In our model:
- Inpatient care advocates identify candidates during the index inpatient stay, introduce a model of care, and begin a coaching intervention.
- After discharge, home liaisons visit the patient at home, continue coaching intervention, and teach the patient to use the newly installed remote monitoring equipment.
- For 30 to 40 days after discharge, a team of telehealth nurses monitors the patient, makes contact with him or her weekly in order to reinforce coaching intervention, coordinates care, and tracks outcomes.
- Nurse practitioners experienced both in home care and heart failure provide clinical oversight and leadership and visit the highest-acuity patients at home.
To date, the program has provided care in more than 2,100 patient encounters, with approximately 50 to 80 patients actively enrolled at any time. We identified potential program candidates using a digital list tool embedded in Cleveland Clinic’s electronic medical record (EMR) system. This tool was developed by our team together with an internal business intelligence team. We have been approximately 65% successful in identifying eligible inpatients. Patients enrolled in our transitional care program tend to be older, have longer hospital stays, and have more comorbidities than other older adults hospitalized at Cleveland Clinic for similar reasons.
Following index hospital discharge, our home liaisons have been able to make an initial home visit after a median of 2 days (25th to 75th percentile: 1 to 3 days). Patients thought to be at higher risk for hospital readmissions have been seen at home by our nurse practitioners within the first week of discharge. The most common challenge that our at-home team members have faced relates to patients’ medications (for example, unfilled prescriptions and errors in utilization). On many occasions our at-home team has succeeded in transitioning patients not benefiting from care at home to nonhospital venues (skilled nursing facilities, chronic care facilities, inpatient hospice) or to higher levels of at-home care (at-home physician visits, home-care nursing and therapy, at-home hospice).
To date, patients have been enrolled in our program for a median of 30 days (25th to 75th percentile: 20 to 35 days). We have observed an increased level of patient satisfaction. Among heart failure patients enrolled in our program for the first time, we have observed a lower readmission rate compared with publicly reported Cleveland Clinic rates (24.5% vs 28.2%). However, there are several ongoing challenges in the care of heart failure patients in the home environment. These relate to longitudinal care across venues, cross training of providers, and home monitoring.
Longitudinal care across venues
Our program aims to address the lack of integrated care over time and between care venues. This problem lies at the intersection of health care reimbursement policy and clinical practice. Currently, the hospital reimbursement system does not encourage care coordination across settings. The system has, in fact, evolved into a string of disconnected care providers who act as “toll booths” providing services for a fee in isolation from other providers. Coleman and colleagues have documented the complexity of the transitions among these care providers for older patients with chronic disease, noting the implications for patient safety and cost.6
Hospitals receive a fixed payment for an inpatient admission, which increases the financial incentive to discharge patients faster to other venues of care. The study by Bueno et al of a Medicare population treated between 1993 and 2006 confirms that such a trend exists for heart failure patients.7 The authors found a steady decrease in the mean length of hospital stay from 8.81 days to 6.33 days over the study period (28% relative reduction, P < .001). During this same period, the 30-day all-cause readmission rate increased from 17.2% to 20.1% (a 17% relative increase, P < .001) with an associated 10% relative reduction in the proportion of patients discharged to home.7 Experience in other populations with heart failure, such as patients in the Veterans Affairs health care system, has shown similar trends in length of hospital stay and readmissions.8
During these transitions, information is often lost in the handoff from the discharging hospital to the next venue of care. Medication management is the most common problem area with the potential for patient noncompliance with prescriptions,9,10 which can have serious deleterious effects on quality and safety. Forster et al found that 66% of untoward outcomes in discharged patients were due to adverse drug events.11 Similarly, Gray et al identified adverse drug events in 20% of patients discharged from hospital to home with home health care services.12
In the Cleveland Clinic Health System, we are coupling our “Heart Care at Home” transitional care program with an aggressive plan to develop a more comprehensive cross-venue EMR. Connecting the hospital EMR with our health system–owned home health agency will enable a consistent medication record and communication system for patients transitioning from our hospitals to Cleveland Clinic home care services (nearly 20,000 patients per year).
Despite these issues, several care transition interventions have shown promising clinical and economic results. Coleman and colleagues conducted a randomized, controlled trial of a transition coaching model in which patients and caregivers were encouraged to take a more active role in care transitions. Results of this trial showed a significant decrease in 30- and 90-day rehospitalizations (the 90-day read-mission rate in the treatment group was 16.7 vs 22.5 in the control group, P = .04) with associated cost savings.3 Voss et al showed similar results in reduction of readmissions in a nonintegrated delivery system.13 Additionally, telephone-based chronic disease management programs have been shown to be cost-effective in chronically ill Medicare patients.14
When will the clinical evidence behind care transitions and financial incentives converge to create an atmosphere conducive to more optimal care coordination? Today, this question remains unanswered. Health care reform, with the passage of the Patient Protection and Affordable Care Act (PPACA) (http://housedocs.house.gov/energycommerce/ppacacon.pdf), may spur the creation of programs to increase incentives for care coordination. These include a move to episodic reimbursement that would bundle payments for acute and postacute care, thus creating more incentives for coordinating care across settings. The “Bundled Payments for Care Improvement” project run by the Center for Medicare & Medicaid Innovation will test different models and approaches to bundled payments (http://innovations.cms.gov/initiatives/bundled-payments). Additionally, beginning in fiscal year 2013, Medicare will penalize hospitals that have high readmission rates for heart failure, acute myocardial infarction, and pneumonia with a financial risk of up to 3% of total hospital Medicare payments by year 3 of the program.
The PPACA will have a significant effect on home-based care for older adults with chronic conditions. The PPACA reforms will likely lead to more patients being treated at home (the lower-cost care setting), ideally under the care of highly skilled teams. Payment reforms will also create new incentives for providers to better coordinate care, keep patients healthy at home, and avoid the “toll-booth” description entirely, enabling providers to focus on patient care. However, more research and experimentation are required to streamline the elements on the transitions spectrum in order to create the most value for specific patient populations. New infrastructure, use of technology, changing culture, and dedicated clinical teams will be necessary to deliver on the hopes of more integrated longitudinal care across venues.
Cross training of providers
Older community-dwelling adults with heart failure exhibit more health instability; take more medications; have more comorbidities; and receive more nursing, homemaking, and meal services than do other home care clients.15 Nurses thus have a unique opportunity to improve outcomes for home-based heart failure patients,16,17 but are often insufficiently trained to do so. Delaney et al administered a validated 20-item heart failure knowledge questionnaire to 94 home care nurses from four different home care agencies.18 The investigators found a 79% knowledge level in overall heart failure education principles, with lowest scores related to issues of asymptomatic hypotension (25% answered correctly), daily weight monitoring (27%), and transient dizziness (31%). Nurses with poorer heart failure–related knowledge may partially explain worse process and outcome measures among this patient population.19
The home-based nursing workforce of the future, and specifically nurses who care for heart failure patients at home, will need to be better trained and specialized in issues relating both to home-based nursing and medical heart failure. These “hybrid nurses” should be allowed a central clinical leadership role among their peers, as they will need to be empowered to make medical and care coordination decisions.
At our center, hybrid-trained home care/heart failure nurse practitioners make home visits for higher-acuity home-based patients and provide clinical leadership and support for other home care nurses. These nurse practitioners have been instrumental in identifying and correcting heart failure medication–related problems, as well as effectively coordinating care. Examples include: independently prescribing and coordinating administration of intravenous diuretics at home for patients who have difficulty managing volume overload, avoiding hospital readmissions by transitioning ill patients to a skilled nursing facility or an at-home hospice, and effectively educating patients and families about appropriate heart failure self-care.
Home monitoring
Home monitoring of selected physiologic parameters and patient-reported health status measures among heart failure patients may facilitate early detection of clinical deterioration and direct timely intervention to prevent adverse outcomes.20 Desai and Stevenson have previously proposed the “circle from home to heart-failure disease management,” a concept illustrating how home monitoring can be embedded in a comprehensive heart failure management approach (Figure 2).20 This concept emphasizes the following:
- Home monitoring should facilitate early detection of clinical deterioration.20
- Home monitoring data will most directly lead to action if the data can be used by the patient to improve self-care.
- In the setting of multidisciplinary care, data should be remotely transmitted to a midlevel team, preferably one empowered to make therapeutic decisions.
- Further engagement of physicians or other clinical providers may be beneficial but will delay the clinical response.
The most commonly monitored physiologic parameter of heart failure patients is daily weight. While nearly universally used, this parameter is in fact a poor surrogate for subclinical hemodynamic congestion and has poor diagnostic performance for clinical decompensation. Results are conflicting from studies evaluating the utility of daily body weight measurements in patients with heart failure who are being cared for in the home environment.
In one study, an increase in body weight of > 2 kg over 24 to 72 hours had a 9% sensitivity for detecting clinical deterioration.21 In another study, Chaudhry et al performed a nested case-control trial in 134 patients with heart failure and 134 matched controls referred to a home monitoring system by managed care organizations. The researchers found that increases in body weight were associated with hospitalization for heart failure and that the increases began at least 1 week before admission.22 However, they did not investigate whether the use of this information by clinicians altered outcomes. In a prior randomized clinical trial of symptom monitoring versus transtelephonic body weight monitoring in patients with symptomatic heart failure, the Weight Monitoring in Heart Failure trial (n = 280), weight monitoring did not result in improvement in the primary outcome of hospitalizations for heart failure over a 6-month period.23
The ideal monitoring parameters in heart failure patients may include direct hemodynamic measurements from the right ventricular outflow tract,24 pulmonary artery,25 or left atrium,26 using implantable devices. For example, the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients) trial (n = 550) was a randomized, single-blind, industry-sponsored trial of heart failure management guided by physiologic hemodynamic data derived from a percutaneously inserted pulmonary artery hemodynamic monitor (Champion HF Monitoring System; CardioMEMS, Atlanta, Georgia). The researchers found that monitoring these parameters was associated with a 28% reduction in heart failure–related hospitalizations during the first 6 months (rate 0.32 vs 0.44, HR 0.72, 95% CI 0.60–0.85, P = .0002) compared with usual care.25 At 6 months, the freedom from device- or system-related complications was 98.6%.
Despite success in the trial, the US Food and Drug Administration Circulatory System Devices Panel voted against approving the device. The panel was concerned that the e-mail–alert and care systems built into the intervention arm of the trial created bias in favor of the device, and that in a real-world situation it may not be as effective. This demonstrates the ongoing challenges and barriers to adoption of invasive hemodynamic monitoring.
At our center, we are conducting an institutional review board–approved investigation of an entirely noninvasive under-the-mattress piezoelectric monitor in a cohort of postacute heart failure patients. Piezoelectricity is the charge that accumulates in certain solid materials in response to mechanical stress. Common applications of piezoelectricity include microphones, push-start propane barbecues, and cigarette lighters. The device under investigation (EverOn; EarlySense, Ramat-Gan, Israel) detects heart rate, respiratory rate, and movement rate through vibrations of the mattress. Case examples are shown in Figure 3. Whether such monitoring technology will play a future role in the home environment remains to be seen.
SUMMARY
At the time of this writing, the Supreme Court of the United States has reaffirmed the constitutionality of the PPACA, clearing the way for implementation of significant changes in the US health care delivery system. The implications for in-home care for older adults with chronic conditions, including heart failure, are significant. The home will become an increasingly common venue of postacute care. Today is the time to investigate beneficial models of care and optimal uses of technology, and to develop a specialized mobile workforce that will confidently care for individuals with heart failure at home, responsibly and at lower cost.
- Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009; 119:e391–e479.
- Stewart S, Carrington MJ, Marwick TH, et al. Impact of home versus clinic-based management of chronic heart failure: the WHICH? (Which Heart Failure Intervention Is Most Cost-Effective and Consumer Friendly in Reducing Hospital Care) multicenter, randomized trial. J Am Coll Cardiol 2012; 60:1239–1248.
- Coleman EA, Parry C, Chalmers S, Min S-J. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006; 166:1822–1828.
- Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc 2004; 52:675–684.
- Klersy C, De Silvestri A, Gabutti G, Regoli F, Auricchio A. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol 2009; 54:1683–1694.
- Coleman EA, Min S-J, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res 2004; 39:1449–1465.
- Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA 2010; 303:2141–2147.
- Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol 2010; 56:362–368.
- Moore C, Wisnivesky J, Williams S, McGinn T. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 2003; 18:646–651.
- Coleman EA, Smith JD, Raha D, Min S-J. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005; 165:1842–1847.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003; 138:161–167.
- Gray SL, Mahoney JE, Blough DK. Adverse drug events in elderly patients receiving home health services following hospital discharge. Ann Pharmacother 1999; 33:1147–1153.
- Voss R, Gardner R, Baier R, Butterfeld K, Lehrman S, Gravenstein S. The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med 2011; 171:1232–1237.
- Baker LC, Johnson SJ, Macaulay D, Birnbaum H. Integrated telehealth and care management program for Medicare benefciaries with chronic disease linked to savings. Health Aff (Millwood) 2011; 30:1689–1697.
- Foebel AD, Hirdes JP, Heckman GA, Tyas SL, Tjam EY. A profile of older community-dwelling home care clients with heart failure in Ontario. Chronic Dis Can 2011; 31:49–57.
- Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol 2002; 39:83–89.
- González B, Lupón J, Herreros J, et al. Patient’s education by nurse: what we really do achieve? Eur J Cardiovasc Nurs 2005; 4:107–111.
- Delaney C, Apostolidis B, Lachapelle L, Fortinsky R. Home care nurses’ knowledge of evidence-based education topics for management of heart failure. Heart Lung 2011; 40:285–292.
- Foebel AD, Heckman GA, Hirdes JP, et al. Clinical, demographic and functional characteristics associated with pharmacotherapy for heart failure in older home care clients: a retrospective, population-level, cross-sectional study. Drugs Aging 2011; 28:561–573.
- Desai AS, Stevenson LW. Connecting the circle from home to heart-failure disease management. N Engl J Med 2010; 363:2364–2367.
- Lewin J, Ledwidge M, O’Loughlin C, McNally C, McDonald K. Clinical deterioration in established heart failure: what is the value of BNP and weight gain in aiding diagnosis? Eur J Heart Fail 2005; 7:953–957.
- Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation 2007; 116:1549–1554.
- Goldberg LR, Piette JD, Walsh MN, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J 2003; 146:705–712.
- Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the Compass-HF study. J Am Coll Cardiol 2008; 51:1073–1079.
- Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation 2010; 121:1086–1095.
- Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009; 119:e391–e479.
- Stewart S, Carrington MJ, Marwick TH, et al. Impact of home versus clinic-based management of chronic heart failure: the WHICH? (Which Heart Failure Intervention Is Most Cost-Effective and Consumer Friendly in Reducing Hospital Care) multicenter, randomized trial. J Am Coll Cardiol 2012; 60:1239–1248.
- Coleman EA, Parry C, Chalmers S, Min S-J. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006; 166:1822–1828.
- Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc 2004; 52:675–684.
- Klersy C, De Silvestri A, Gabutti G, Regoli F, Auricchio A. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol 2009; 54:1683–1694.
- Coleman EA, Min S-J, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res 2004; 39:1449–1465.
- Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA 2010; 303:2141–2147.
- Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol 2010; 56:362–368.
- Moore C, Wisnivesky J, Williams S, McGinn T. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 2003; 18:646–651.
- Coleman EA, Smith JD, Raha D, Min S-J. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005; 165:1842–1847.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003; 138:161–167.
- Gray SL, Mahoney JE, Blough DK. Adverse drug events in elderly patients receiving home health services following hospital discharge. Ann Pharmacother 1999; 33:1147–1153.
- Voss R, Gardner R, Baier R, Butterfeld K, Lehrman S, Gravenstein S. The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med 2011; 171:1232–1237.
- Baker LC, Johnson SJ, Macaulay D, Birnbaum H. Integrated telehealth and care management program for Medicare benefciaries with chronic disease linked to savings. Health Aff (Millwood) 2011; 30:1689–1697.
- Foebel AD, Hirdes JP, Heckman GA, Tyas SL, Tjam EY. A profile of older community-dwelling home care clients with heart failure in Ontario. Chronic Dis Can 2011; 31:49–57.
- Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol 2002; 39:83–89.
- González B, Lupón J, Herreros J, et al. Patient’s education by nurse: what we really do achieve? Eur J Cardiovasc Nurs 2005; 4:107–111.
- Delaney C, Apostolidis B, Lachapelle L, Fortinsky R. Home care nurses’ knowledge of evidence-based education topics for management of heart failure. Heart Lung 2011; 40:285–292.
- Foebel AD, Heckman GA, Hirdes JP, et al. Clinical, demographic and functional characteristics associated with pharmacotherapy for heart failure in older home care clients: a retrospective, population-level, cross-sectional study. Drugs Aging 2011; 28:561–573.
- Desai AS, Stevenson LW. Connecting the circle from home to heart-failure disease management. N Engl J Med 2010; 363:2364–2367.
- Lewin J, Ledwidge M, O’Loughlin C, McNally C, McDonald K. Clinical deterioration in established heart failure: what is the value of BNP and weight gain in aiding diagnosis? Eur J Heart Fail 2005; 7:953–957.
- Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation 2007; 116:1549–1554.
- Goldberg LR, Piette JD, Walsh MN, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J 2003; 146:705–712.
- Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the Compass-HF study. J Am Coll Cardiol 2008; 51:1073–1079.
- Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation 2010; 121:1086–1095.