User login
What Is the Best E&M of Heparin-Induced Thrombocytopenia?
Case
A 52-year-old white woman presents to the ED after a motor vehicle accident with a fractured left femur. After surgical repair of the fracture, she is treated with enoxaparin 40 mg daily for VTE prophylaxis. Upon admission to the hospital, her platelet count is 180x109/L. On postoperative day three, it is 140x109/L; on postoperative day six, it is 78x109/L. Because of persistent swelling of the left leg, a venous ultrasound is obtained; results are negative for DVT. Is the decrease in the platelet count concerning for heparin-induced thrombocytopenia?
Overview
Approximately one-third of hospitalized patients are exposed to heparin each year.1 A well-described, life-threatening adverse effect of heparin use is thrombocytopenia, also called heparin-induced thrombocytopenia (HIT). Studies suggest that the frequency of HIT in the U.S. is as high as 1% to 5% in patients exposed to unfractionated heparin.1,2
There are two types of HIT. Type 2 HIT is more serious, with risk for life- or limb-threatening complications. Type 1 HIT is a nonimmune disorder caused by the direct effect of heparin on platelet activation, which is characterized by a drop in thrombocyte count within the first 48 hours of heparin exposure. The platelet count is expected to normalize with continued heparin exposure in Type 1 HIT. Type 2 HIT is an immune-mediated disorder in which heparin-dependent IgG recognizes complexes of heparin and platelet factor 4 (PF4), which subsequently induce platelet activation via the platelet Fc gammaRIIa receptor. A positive feedback loop occurs, causing further release of PF4 and platelet activation, which can lead to devastating prothrombotic complications.
Individuals affected by Type 2 HIT have a 20% to 50% risk of developing new thrombotic events, and also have a 10% rate of major morbidity, including limb ischemia requiring amputation, cerebrovascular events, myocardial infarction, DVT, or pulmonary embolus.1,2
Until recently, the mortality rate in HIT has been reported as high as 20%; however, earlier diagnosis and treatment have resulted in a better prognosis, with mortality and major morbidity of 6% to 10%.2 Low-molecular-weight heparin (LMWH) carries a lower risk for development of HIT; as such, one measure to reduce the risk of HIT is to use LMWH in place of unfractionated heparin.3
Review of the Data
When to suspect HIT. HIT should be considered as a potential diagnosis anytime there is a drop in platelet count, either during or shortly following heparin exposure. The differential diagnosis for thrombocytopenia during heparin exposure is broad and includes:
- Disseminated intravascular coagulation;
- Drug-induced thrombocytopenia;
- Hemolytic-uremic syndrome;
- Immune thrombocytopenic purpura;
- Post-transfusion thrombocytopenia;
- Systemic lupus erythematosus; and
- Thrombotic thrombocytopenic purpura.
The 2009 Clinical Practice Guideline on Evaluation and Management of HIT provided by the American Society of Hematology recommends the use of Warkentin’s 4Ts clinical probability scoring system as a guide in determining the probability of HIT in patients with thrombocytopenia who are exposed to heparin.4 The 4Ts scoring system is detailed in Table 1.
In patients with intermediate to high clinical probability of HIT (4-5 points and 6-8 points, respectively, on the 4Ts scoring system), immunologic and functional assays could further guide management. In patients with a low probability of HIT (4Ts score <3), the diagnosis is unlikely and an alternative diagnoses should be considered. Immunologic and functional assays are not recommended for these patients, and heparin can be continued.
Laboratory and diagnostic workups. Immunologic assays (polyspecific ELISA, IgG-specific ELISA, and particle gel immunoassay) detect antibodies against the PF4 heparin complexes regardless of their capacity to activate platelets. These tests are highly sensitive but less specific for HIT because they also detect PF4-heparin antibodies in patients who do not have HIT; therefore, immunoassays have a lower positive predictive value but a high negative predictive value (>95%).5
Functional assays (serotonin release assay, heparin-induced platelet activation assay, and platelet aggregation test) detect antibodies that induce heparin-dependent platelet activation. These assays are highly sensitive and specific but are not available at many medical centers. The positive predictive value of these assays is higher (89% to 100%).5
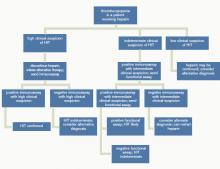
Figure 1 provides a diagnostic and initial treatment algorithm for suspected HIT. Immunoassays to detect PF4-heparin antibodies are recommended when clinical probability of HIT is intermediate to high. In these patients, a negative result on serologic testing has a high negative predictive value and suggests that an alternative diagnosis is more likely. In patients with a positive serologic test and intermediate probability of HIT, a functional assay might be beneficial, as a positive result increases the probability of HIT. For patients with high probability of HIT and a positive immunologic assay, functional assays might not be indicated as the diagnosis is likely.
Treatment. If the probability of HIT is intermediate to high based on the 4Ts scoring system, all heparin products, including heparin flushes, should be immediately discontinued and a laboratory investigation for HIT antibodies should be undertaken. An investigation for lower-limb DVT also should be pursued in patients with high probability of HIT, as the risk of thrombosis is more than 30-fold higher than controls, and studies show that approximately 25% of patients with HIT present with both thrombocytopenia and thrombosis.5 In addition, the presence of thrombosis might influence duration of anticoagulation.
Avoid platelet transfusions, as this might propagate thrombosis.
Anticoagulation. With a significant risk of thrombosis associated with this disorder, treatment with an alternative anticoagulant should be started. Vitamin K antagonists, such as warfarin, cannot be given in acute HIT because of the high risk of inducing skin necrosis and venous limb gangrene. Such anticoagulation should not be used until the platelet count increases to greater than 150x109/L. If warfarin already has been given, reversal with vitamin K is indicated.
Consequently, an alternative anticoagulant bridge to warfarin therapy must be used. Usually, the bridging agent will be one of two intravenous direct thrombin inhibitors (argatroban and lepirudin) approved for this purpose.6 Both are associated with a higher risk of bleeding. Argatroban is hepatically cleared; lepirudin is renally cleared. Table 2 summarizes dosing information for these agents. A third direct thrombin inhibitor, bivalirudin, is approved for treatment of HIT, but only during percutaneous coronary intervention.6
Finally, the recently FDA-approved oral direct thrombin inhibitor dabigatrin has not been studied in or approved for HIT.
Other rational therapies include the factor Xa inhibitors danaparoid and fondaparinux. However, only danaparoid is FDA-approved for use in the treatment of HIT. It can, in cases of low or moderate suspicion of HIT, be given in prophylactic doses, lowering the risk of major bleeding.
Duration of treatment. Whichever bridging anticoagulant is chosen, it should be continued until the platelet count has fully recovered. Further, prior to discontinuation, warfarin therapy should be administered for at least five days and the international normalized ratio (INR) should be therapeutic for approximately 48 hours.
The subsequent length of warfarin therapy is dependent upon the presence or absence of an associated thrombosis. With the presence of a thrombus, the duration should be as defined for other provoked thromboses (three to six months). With no thrombus, the duration should be at least 30 days.
Future anticoagulation in patients with a prior diagnosis of HIT. A history of HIT does not appear to be a risk factor for a higher frequency of forming heparin antibodies upon re-exposure to heparin.7 Therefore, in patients with an important indication for heparin (i.e. cardiac or vascular surgery) and a remote history of HIT (>100 days), heparin can be used. In patients with a subacute history of HIT in whom surgery cannot be delayed, heparin products should be avoided and laboratory investigation should be pursued.
If the immunoassay is positive but the functional assay is negative, it is reasonable to use heparin. If both the immunologic and the functional assays are positive, the patient should be considered as having acute HIT, and bivalirudin is recommended.4
Back to the Case
Our patient has acute thrombocytopenia with a fall in platelets greater than 50% from baseline. The decrease is within the appropriate time frame for HIT. No thrombosis is found, but no alternate explanation for the thrombocytopenia is apparent. The 4Ts score of 6 indicates high risk for HIT. Heparin was discontinued, and argatroban at a rate of 2 mcg/kg/min was initiated. The immunoassay was positive.
Argatroban was continued until the platelet count reached 150x109/L, at which point warfarin therapy, 5 mg daily, was started. After four days, the INR was 2.2. After another 24 hours, argatroban was discontinued. She was instructed to continue warfarin for another 30 days.
Bottom Line
Evaluation for HIT combines clinical judgment, summarized in the 4Ts, with laboratory evaluation including an immunoassay and possibly a functional assay. Treatment requires immediate discontinuation of heparin, early initiation of a direct thrombin inhibitor, and bridging to warfarin to continue treatment for at least 30 days. TH
Drs. Smith and Rice are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn.
References
- Heparin-Induced Thrombocytopenia. MedScape Reference website. Available at: http://emedicine.medscape.com/article/1357846. Accessed Aug. 31, 2010.
- Heparin-Induced Thrombocytopenia. Orpha.net website. Available at: http://www.orpha.net/data/patho/GB/uk-HIT.pdf. Accessed Aug. 31, 2010.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332(20):1330-1335.
- American Society of Hematology Guidelines: Immune Thrombocytopenia (HIT). American Society of Hematology website. Available at: www.hematology.org/Practice/Guidelines/2934.aspx. Accessed Jan. 28, 2011.
- Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. Annu Rev Med. 2010;61:77-90.
- Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:340S-380S.
- Warkentin TE. Agents for the treatment of heparin-induced thrombocytopenia. Hematol Oncol Clin N Am. 2010;24:755-775.
Case
A 52-year-old white woman presents to the ED after a motor vehicle accident with a fractured left femur. After surgical repair of the fracture, she is treated with enoxaparin 40 mg daily for VTE prophylaxis. Upon admission to the hospital, her platelet count is 180x109/L. On postoperative day three, it is 140x109/L; on postoperative day six, it is 78x109/L. Because of persistent swelling of the left leg, a venous ultrasound is obtained; results are negative for DVT. Is the decrease in the platelet count concerning for heparin-induced thrombocytopenia?
Overview
Approximately one-third of hospitalized patients are exposed to heparin each year.1 A well-described, life-threatening adverse effect of heparin use is thrombocytopenia, also called heparin-induced thrombocytopenia (HIT). Studies suggest that the frequency of HIT in the U.S. is as high as 1% to 5% in patients exposed to unfractionated heparin.1,2
There are two types of HIT. Type 2 HIT is more serious, with risk for life- or limb-threatening complications. Type 1 HIT is a nonimmune disorder caused by the direct effect of heparin on platelet activation, which is characterized by a drop in thrombocyte count within the first 48 hours of heparin exposure. The platelet count is expected to normalize with continued heparin exposure in Type 1 HIT. Type 2 HIT is an immune-mediated disorder in which heparin-dependent IgG recognizes complexes of heparin and platelet factor 4 (PF4), which subsequently induce platelet activation via the platelet Fc gammaRIIa receptor. A positive feedback loop occurs, causing further release of PF4 and platelet activation, which can lead to devastating prothrombotic complications.
Individuals affected by Type 2 HIT have a 20% to 50% risk of developing new thrombotic events, and also have a 10% rate of major morbidity, including limb ischemia requiring amputation, cerebrovascular events, myocardial infarction, DVT, or pulmonary embolus.1,2
Until recently, the mortality rate in HIT has been reported as high as 20%; however, earlier diagnosis and treatment have resulted in a better prognosis, with mortality and major morbidity of 6% to 10%.2 Low-molecular-weight heparin (LMWH) carries a lower risk for development of HIT; as such, one measure to reduce the risk of HIT is to use LMWH in place of unfractionated heparin.3
Review of the Data
When to suspect HIT. HIT should be considered as a potential diagnosis anytime there is a drop in platelet count, either during or shortly following heparin exposure. The differential diagnosis for thrombocytopenia during heparin exposure is broad and includes:
- Disseminated intravascular coagulation;
- Drug-induced thrombocytopenia;
- Hemolytic-uremic syndrome;
- Immune thrombocytopenic purpura;
- Post-transfusion thrombocytopenia;
- Systemic lupus erythematosus; and
- Thrombotic thrombocytopenic purpura.
The 2009 Clinical Practice Guideline on Evaluation and Management of HIT provided by the American Society of Hematology recommends the use of Warkentin’s 4Ts clinical probability scoring system as a guide in determining the probability of HIT in patients with thrombocytopenia who are exposed to heparin.4 The 4Ts scoring system is detailed in Table 1.
In patients with intermediate to high clinical probability of HIT (4-5 points and 6-8 points, respectively, on the 4Ts scoring system), immunologic and functional assays could further guide management. In patients with a low probability of HIT (4Ts score <3), the diagnosis is unlikely and an alternative diagnoses should be considered. Immunologic and functional assays are not recommended for these patients, and heparin can be continued.
Laboratory and diagnostic workups. Immunologic assays (polyspecific ELISA, IgG-specific ELISA, and particle gel immunoassay) detect antibodies against the PF4 heparin complexes regardless of their capacity to activate platelets. These tests are highly sensitive but less specific for HIT because they also detect PF4-heparin antibodies in patients who do not have HIT; therefore, immunoassays have a lower positive predictive value but a high negative predictive value (>95%).5
Functional assays (serotonin release assay, heparin-induced platelet activation assay, and platelet aggregation test) detect antibodies that induce heparin-dependent platelet activation. These assays are highly sensitive and specific but are not available at many medical centers. The positive predictive value of these assays is higher (89% to 100%).5
Figure 1 provides a diagnostic and initial treatment algorithm for suspected HIT. Immunoassays to detect PF4-heparin antibodies are recommended when clinical probability of HIT is intermediate to high. In these patients, a negative result on serologic testing has a high negative predictive value and suggests that an alternative diagnosis is more likely. In patients with a positive serologic test and intermediate probability of HIT, a functional assay might be beneficial, as a positive result increases the probability of HIT. For patients with high probability of HIT and a positive immunologic assay, functional assays might not be indicated as the diagnosis is likely.
Treatment. If the probability of HIT is intermediate to high based on the 4Ts scoring system, all heparin products, including heparin flushes, should be immediately discontinued and a laboratory investigation for HIT antibodies should be undertaken. An investigation for lower-limb DVT also should be pursued in patients with high probability of HIT, as the risk of thrombosis is more than 30-fold higher than controls, and studies show that approximately 25% of patients with HIT present with both thrombocytopenia and thrombosis.5 In addition, the presence of thrombosis might influence duration of anticoagulation.
Avoid platelet transfusions, as this might propagate thrombosis.
Anticoagulation. With a significant risk of thrombosis associated with this disorder, treatment with an alternative anticoagulant should be started. Vitamin K antagonists, such as warfarin, cannot be given in acute HIT because of the high risk of inducing skin necrosis and venous limb gangrene. Such anticoagulation should not be used until the platelet count increases to greater than 150x109/L. If warfarin already has been given, reversal with vitamin K is indicated.
Consequently, an alternative anticoagulant bridge to warfarin therapy must be used. Usually, the bridging agent will be one of two intravenous direct thrombin inhibitors (argatroban and lepirudin) approved for this purpose.6 Both are associated with a higher risk of bleeding. Argatroban is hepatically cleared; lepirudin is renally cleared. Table 2 summarizes dosing information for these agents. A third direct thrombin inhibitor, bivalirudin, is approved for treatment of HIT, but only during percutaneous coronary intervention.6
Finally, the recently FDA-approved oral direct thrombin inhibitor dabigatrin has not been studied in or approved for HIT.
Other rational therapies include the factor Xa inhibitors danaparoid and fondaparinux. However, only danaparoid is FDA-approved for use in the treatment of HIT. It can, in cases of low or moderate suspicion of HIT, be given in prophylactic doses, lowering the risk of major bleeding.
Duration of treatment. Whichever bridging anticoagulant is chosen, it should be continued until the platelet count has fully recovered. Further, prior to discontinuation, warfarin therapy should be administered for at least five days and the international normalized ratio (INR) should be therapeutic for approximately 48 hours.
The subsequent length of warfarin therapy is dependent upon the presence or absence of an associated thrombosis. With the presence of a thrombus, the duration should be as defined for other provoked thromboses (three to six months). With no thrombus, the duration should be at least 30 days.
Future anticoagulation in patients with a prior diagnosis of HIT. A history of HIT does not appear to be a risk factor for a higher frequency of forming heparin antibodies upon re-exposure to heparin.7 Therefore, in patients with an important indication for heparin (i.e. cardiac or vascular surgery) and a remote history of HIT (>100 days), heparin can be used. In patients with a subacute history of HIT in whom surgery cannot be delayed, heparin products should be avoided and laboratory investigation should be pursued.
If the immunoassay is positive but the functional assay is negative, it is reasonable to use heparin. If both the immunologic and the functional assays are positive, the patient should be considered as having acute HIT, and bivalirudin is recommended.4
Back to the Case
Our patient has acute thrombocytopenia with a fall in platelets greater than 50% from baseline. The decrease is within the appropriate time frame for HIT. No thrombosis is found, but no alternate explanation for the thrombocytopenia is apparent. The 4Ts score of 6 indicates high risk for HIT. Heparin was discontinued, and argatroban at a rate of 2 mcg/kg/min was initiated. The immunoassay was positive.
Argatroban was continued until the platelet count reached 150x109/L, at which point warfarin therapy, 5 mg daily, was started. After four days, the INR was 2.2. After another 24 hours, argatroban was discontinued. She was instructed to continue warfarin for another 30 days.
Bottom Line
Evaluation for HIT combines clinical judgment, summarized in the 4Ts, with laboratory evaluation including an immunoassay and possibly a functional assay. Treatment requires immediate discontinuation of heparin, early initiation of a direct thrombin inhibitor, and bridging to warfarin to continue treatment for at least 30 days. TH
Drs. Smith and Rice are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn.
References
- Heparin-Induced Thrombocytopenia. MedScape Reference website. Available at: http://emedicine.medscape.com/article/1357846. Accessed Aug. 31, 2010.
- Heparin-Induced Thrombocytopenia. Orpha.net website. Available at: http://www.orpha.net/data/patho/GB/uk-HIT.pdf. Accessed Aug. 31, 2010.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332(20):1330-1335.
- American Society of Hematology Guidelines: Immune Thrombocytopenia (HIT). American Society of Hematology website. Available at: www.hematology.org/Practice/Guidelines/2934.aspx. Accessed Jan. 28, 2011.
- Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. Annu Rev Med. 2010;61:77-90.
- Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:340S-380S.
- Warkentin TE. Agents for the treatment of heparin-induced thrombocytopenia. Hematol Oncol Clin N Am. 2010;24:755-775.
Case
A 52-year-old white woman presents to the ED after a motor vehicle accident with a fractured left femur. After surgical repair of the fracture, she is treated with enoxaparin 40 mg daily for VTE prophylaxis. Upon admission to the hospital, her platelet count is 180x109/L. On postoperative day three, it is 140x109/L; on postoperative day six, it is 78x109/L. Because of persistent swelling of the left leg, a venous ultrasound is obtained; results are negative for DVT. Is the decrease in the platelet count concerning for heparin-induced thrombocytopenia?
Overview
Approximately one-third of hospitalized patients are exposed to heparin each year.1 A well-described, life-threatening adverse effect of heparin use is thrombocytopenia, also called heparin-induced thrombocytopenia (HIT). Studies suggest that the frequency of HIT in the U.S. is as high as 1% to 5% in patients exposed to unfractionated heparin.1,2
There are two types of HIT. Type 2 HIT is more serious, with risk for life- or limb-threatening complications. Type 1 HIT is a nonimmune disorder caused by the direct effect of heparin on platelet activation, which is characterized by a drop in thrombocyte count within the first 48 hours of heparin exposure. The platelet count is expected to normalize with continued heparin exposure in Type 1 HIT. Type 2 HIT is an immune-mediated disorder in which heparin-dependent IgG recognizes complexes of heparin and platelet factor 4 (PF4), which subsequently induce platelet activation via the platelet Fc gammaRIIa receptor. A positive feedback loop occurs, causing further release of PF4 and platelet activation, which can lead to devastating prothrombotic complications.
Individuals affected by Type 2 HIT have a 20% to 50% risk of developing new thrombotic events, and also have a 10% rate of major morbidity, including limb ischemia requiring amputation, cerebrovascular events, myocardial infarction, DVT, or pulmonary embolus.1,2
Until recently, the mortality rate in HIT has been reported as high as 20%; however, earlier diagnosis and treatment have resulted in a better prognosis, with mortality and major morbidity of 6% to 10%.2 Low-molecular-weight heparin (LMWH) carries a lower risk for development of HIT; as such, one measure to reduce the risk of HIT is to use LMWH in place of unfractionated heparin.3
Review of the Data
When to suspect HIT. HIT should be considered as a potential diagnosis anytime there is a drop in platelet count, either during or shortly following heparin exposure. The differential diagnosis for thrombocytopenia during heparin exposure is broad and includes:
- Disseminated intravascular coagulation;
- Drug-induced thrombocytopenia;
- Hemolytic-uremic syndrome;
- Immune thrombocytopenic purpura;
- Post-transfusion thrombocytopenia;
- Systemic lupus erythematosus; and
- Thrombotic thrombocytopenic purpura.
The 2009 Clinical Practice Guideline on Evaluation and Management of HIT provided by the American Society of Hematology recommends the use of Warkentin’s 4Ts clinical probability scoring system as a guide in determining the probability of HIT in patients with thrombocytopenia who are exposed to heparin.4 The 4Ts scoring system is detailed in Table 1.
In patients with intermediate to high clinical probability of HIT (4-5 points and 6-8 points, respectively, on the 4Ts scoring system), immunologic and functional assays could further guide management. In patients with a low probability of HIT (4Ts score <3), the diagnosis is unlikely and an alternative diagnoses should be considered. Immunologic and functional assays are not recommended for these patients, and heparin can be continued.
Laboratory and diagnostic workups. Immunologic assays (polyspecific ELISA, IgG-specific ELISA, and particle gel immunoassay) detect antibodies against the PF4 heparin complexes regardless of their capacity to activate platelets. These tests are highly sensitive but less specific for HIT because they also detect PF4-heparin antibodies in patients who do not have HIT; therefore, immunoassays have a lower positive predictive value but a high negative predictive value (>95%).5
Functional assays (serotonin release assay, heparin-induced platelet activation assay, and platelet aggregation test) detect antibodies that induce heparin-dependent platelet activation. These assays are highly sensitive and specific but are not available at many medical centers. The positive predictive value of these assays is higher (89% to 100%).5
Figure 1 provides a diagnostic and initial treatment algorithm for suspected HIT. Immunoassays to detect PF4-heparin antibodies are recommended when clinical probability of HIT is intermediate to high. In these patients, a negative result on serologic testing has a high negative predictive value and suggests that an alternative diagnosis is more likely. In patients with a positive serologic test and intermediate probability of HIT, a functional assay might be beneficial, as a positive result increases the probability of HIT. For patients with high probability of HIT and a positive immunologic assay, functional assays might not be indicated as the diagnosis is likely.
Treatment. If the probability of HIT is intermediate to high based on the 4Ts scoring system, all heparin products, including heparin flushes, should be immediately discontinued and a laboratory investigation for HIT antibodies should be undertaken. An investigation for lower-limb DVT also should be pursued in patients with high probability of HIT, as the risk of thrombosis is more than 30-fold higher than controls, and studies show that approximately 25% of patients with HIT present with both thrombocytopenia and thrombosis.5 In addition, the presence of thrombosis might influence duration of anticoagulation.
Avoid platelet transfusions, as this might propagate thrombosis.
Anticoagulation. With a significant risk of thrombosis associated with this disorder, treatment with an alternative anticoagulant should be started. Vitamin K antagonists, such as warfarin, cannot be given in acute HIT because of the high risk of inducing skin necrosis and venous limb gangrene. Such anticoagulation should not be used until the platelet count increases to greater than 150x109/L. If warfarin already has been given, reversal with vitamin K is indicated.
Consequently, an alternative anticoagulant bridge to warfarin therapy must be used. Usually, the bridging agent will be one of two intravenous direct thrombin inhibitors (argatroban and lepirudin) approved for this purpose.6 Both are associated with a higher risk of bleeding. Argatroban is hepatically cleared; lepirudin is renally cleared. Table 2 summarizes dosing information for these agents. A third direct thrombin inhibitor, bivalirudin, is approved for treatment of HIT, but only during percutaneous coronary intervention.6
Finally, the recently FDA-approved oral direct thrombin inhibitor dabigatrin has not been studied in or approved for HIT.
Other rational therapies include the factor Xa inhibitors danaparoid and fondaparinux. However, only danaparoid is FDA-approved for use in the treatment of HIT. It can, in cases of low or moderate suspicion of HIT, be given in prophylactic doses, lowering the risk of major bleeding.
Duration of treatment. Whichever bridging anticoagulant is chosen, it should be continued until the platelet count has fully recovered. Further, prior to discontinuation, warfarin therapy should be administered for at least five days and the international normalized ratio (INR) should be therapeutic for approximately 48 hours.
The subsequent length of warfarin therapy is dependent upon the presence or absence of an associated thrombosis. With the presence of a thrombus, the duration should be as defined for other provoked thromboses (three to six months). With no thrombus, the duration should be at least 30 days.
Future anticoagulation in patients with a prior diagnosis of HIT. A history of HIT does not appear to be a risk factor for a higher frequency of forming heparin antibodies upon re-exposure to heparin.7 Therefore, in patients with an important indication for heparin (i.e. cardiac or vascular surgery) and a remote history of HIT (>100 days), heparin can be used. In patients with a subacute history of HIT in whom surgery cannot be delayed, heparin products should be avoided and laboratory investigation should be pursued.
If the immunoassay is positive but the functional assay is negative, it is reasonable to use heparin. If both the immunologic and the functional assays are positive, the patient should be considered as having acute HIT, and bivalirudin is recommended.4
Back to the Case
Our patient has acute thrombocytopenia with a fall in platelets greater than 50% from baseline. The decrease is within the appropriate time frame for HIT. No thrombosis is found, but no alternate explanation for the thrombocytopenia is apparent. The 4Ts score of 6 indicates high risk for HIT. Heparin was discontinued, and argatroban at a rate of 2 mcg/kg/min was initiated. The immunoassay was positive.
Argatroban was continued until the platelet count reached 150x109/L, at which point warfarin therapy, 5 mg daily, was started. After four days, the INR was 2.2. After another 24 hours, argatroban was discontinued. She was instructed to continue warfarin for another 30 days.
Bottom Line
Evaluation for HIT combines clinical judgment, summarized in the 4Ts, with laboratory evaluation including an immunoassay and possibly a functional assay. Treatment requires immediate discontinuation of heparin, early initiation of a direct thrombin inhibitor, and bridging to warfarin to continue treatment for at least 30 days. TH
Drs. Smith and Rice are members of the Section of Hospital Medicine at Vanderbilt University in Nashville, Tenn.
References
- Heparin-Induced Thrombocytopenia. MedScape Reference website. Available at: http://emedicine.medscape.com/article/1357846. Accessed Aug. 31, 2010.
- Heparin-Induced Thrombocytopenia. Orpha.net website. Available at: http://www.orpha.net/data/patho/GB/uk-HIT.pdf. Accessed Aug. 31, 2010.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332(20):1330-1335.
- American Society of Hematology Guidelines: Immune Thrombocytopenia (HIT). American Society of Hematology website. Available at: www.hematology.org/Practice/Guidelines/2934.aspx. Accessed Jan. 28, 2011.
- Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. Annu Rev Med. 2010;61:77-90.
- Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:340S-380S.
- Warkentin TE. Agents for the treatment of heparin-induced thrombocytopenia. Hematol Oncol Clin N Am. 2010;24:755-775.