User login
A new way to classify endometrial cancer
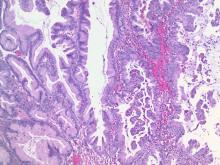
We classify endometrial cancer so that we can communicate and define each patient’s disease status, the potential for harm, and the likelihood that adjuvant therapies might provide help. Traditional forms of classification have clearly fallen short in achieving this aim, as we all know of patients with apparent low-risk disease (such as stage IA grade 1 endometrioid carcinoma) who have had recurrences and died from their disease, and we know that many patients have been subjected to overtreatment for their cancer and have acquired lifelong toxicities of therapy. This column will explore the newer, more sophisticated molecular-based classifications that are being validated for endometrial cancer, and the ways in which this promises to personalize the treatment of endometrial cancer.
Breast cancer and melanoma are examples of the inclusion of molecular data such as hormone receptor status, HER2/neu status, or BRAF positivity resulting in advancements in personalizing therapeutics. We are now moving toward this for endometrial cancer.
What is the Cancer Genome Atlas?
In 2006 the National Institutes of Health announced an initiative to coordinate work between the National Cancer Institute and the National Human Genome Research Institute taking information about the human genome and analyzing it for key genomic alterations found in 33 common cancers. These data were combined with clinical information (such as survival) to classify the behaviors of those cancers with respect to their individual genomic alternations, in order to look for patterns in mutations and behaviors. The goal of this analysis was to shift the paradigm of cancer classification from being centered around primary organ site toward tumors’ shared genomic patterns.
In 2013 the Cancer Genome Atlas published their results of complete gene sequencing in endometrial cancer.3 The authors identified four discrete subgroups of endometrial cancer with distinct molecular mutational profiles and distinct clinical outcomes: polymerase epsilon (POLE, pronounced “pole-ee”) ultramutated, microsatellite instability (MSI) high, copy number high, and copy number low.
POLE ultramutated
An important subgroup identified in the Cancer Genome Atlas was a group of patients with a POLE ultramutated state. POLE encodes for a subunit of DNA polymerase, the enzyme responsible for replicating the leading DNA strand. Nonfunctioning POLE results in proofreading errors and a subsequent ultramutated cellular state with a predominance of single nucleotide variants. POLE proofreading domain mutations in endometrial cancer and colon cancer are associated with excellent prognosis, likely secondary to the immune response that is elicited by this ultramutated state from creation of “antigenic neoepitopes” that stimulate T-cell response. Effectively, the very mutated cell is seen as “more foreign” to the body’s immune system.
Approximately 10% of patients with endometrial cancer have a POLE ultramutated state, and, as stated above, prognosis is excellent, even if coexisting with a histologic cell type (such as serous) that is normally associated with adverse outcomes. These women tend to be younger, with a lower body mass index, higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and low stage.
MSI high
MSI (microsatellite instability) is a result of epigenetic/hypermethylations or loss of expression in mismatch repair genes (such as MLH1, MSH2, MSH6, PMS2). These genes code for proteins critical in the repair of mismatches in short repeated sequences of DNA. Loss of their function results in an accumulation of errors in these sequences: MSI. It is a feature of the Lynch syndrome inherited state, but is also found sporadically in endometrial tumors. These tumors accumulate a number of mutations during cell replication that, as in POLE hypermutated tumors, are associated with eliciting an immune response.
These tumors tend to be associated with a higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and an advanced stage. Patients with tumors that have been described as MSI high are candidates for “immune therapy” with the PDL1 inhibitor pembrolizumab because of their proinflammatory state and observed favorable responses in clinical trials.4
Copy number high/low
Copy number (CN) high and low refers to the results of microarrays in which hierarchical clustering was applied to identify reoccurring amplification or deletion regions. The CN-high group was associated with the poorest outcomes (recurrence and survival). There is significant overlap with mutations in TP53. Most serous carcinomas were CN high; however, 25% of patients with high-grade endometrioid cell type shared the CN-high classification. These tumors shared great molecular similarity to high-grade serous ovarian cancers and basal-like breast cancer.
Those patients who did not possess mutations that classified them as POLE hypermutated, MSI high, or CN high were classified as CN low. This group included predominantly grades 1 and 2 endometrioid adenocarcinomas of an early stage and had a favorable prognostic profile, though less favorable than those with a POLE ultramutated state, which appears to be somewhat protective.
Molecular/metabolic interactions
While molecular data are clearly important in driving a cancer cell’s behavior, other clinical and metabolic factors influence cancer behavior. For example, body mass index, adiposity, glucose, and lipid metabolism have been shown to be important drivers of cellular behavior and responsiveness to targeted therapies.5,6 Additionally age, race, and other metabolic states contribute to oncologic behavior. Future classifications of endometrial cancer are unlikely to use molecular profiles in isolation but will need to incorporate these additional patient-specific data to better predict and prognosticate outcomes.
Clinical applications
If researchers can better define and describe a patient’s endometrial cancer from the time of their biopsy, important clinical decisions might be able to be tackled. For example, in a premenopausal patient with an endometrial cancer who is considering fertility-sparing treatments, preoperative knowledge of a POLE ultramutated state (and therefore an anticipated good prognosis) might favor fertility preservation or avoid comprehensive staging which may be of limited value. Similarly, if an MSI-high profile is identified leading to a Lynch syndrome diagnosis, she may be more inclined to undergo a hysterectomy with bilateral salpingo-oophorectomy and staging as she is at known increased risk for a more advanced endometrial cancer, as well as the potential for ovarian cancer.
Postoperative incorporation of molecular data promises to be particularly helpful in guiding adjuvant therapies and sparing some women from unnecessary treatments. For example, women with high-grade endometrioid tumors who are CN high were historically treated with radiotherapy but might do better treated with systemic adjuvant therapies traditionally reserved for nonendometrioid carcinomas. Costly therapies such as immunotherapy can be directed toward those with MSI-high tumors, and the rare patient with a POLE ultramutated state who has a recurrence or advanced disease. Clinical trials will be able to cluster enrollment of patients with CN-high, serouslike cancers with those with serous cancers, rather than combining them with patients whose cancers predictably behave much differently.
Much work is still needed to validate this molecular profiling in endometrial cancer and define the algorithms associated with treatment decisions; however, it is likely that the way we describe endometrial cancer in the near future will be quite different.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no disclosures.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-7.
2. Clarke BA et al. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol. 2010;63(5):410-5.
3. Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
4. Ott PA et al. Pembrolizumab in advanced endometrial cancer: Preliminary results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2016;34(suppl):Abstract 5581.
5. Roque DR et al. Association between differential gene expression and body mass index among endometrial cancers from the Cancer Genome Atlas Project. Gynecol Oncol. 2016;142(2):317-22.
6. Talhouk A et al. New classification of endometrial cancers: The development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract. 2016 Dec;3:14.
We classify endometrial cancer so that we can communicate and define each patient’s disease status, the potential for harm, and the likelihood that adjuvant therapies might provide help. Traditional forms of classification have clearly fallen short in achieving this aim, as we all know of patients with apparent low-risk disease (such as stage IA grade 1 endometrioid carcinoma) who have had recurrences and died from their disease, and we know that many patients have been subjected to overtreatment for their cancer and have acquired lifelong toxicities of therapy. This column will explore the newer, more sophisticated molecular-based classifications that are being validated for endometrial cancer, and the ways in which this promises to personalize the treatment of endometrial cancer.
Breast cancer and melanoma are examples of the inclusion of molecular data such as hormone receptor status, HER2/neu status, or BRAF positivity resulting in advancements in personalizing therapeutics. We are now moving toward this for endometrial cancer.
What is the Cancer Genome Atlas?
In 2006 the National Institutes of Health announced an initiative to coordinate work between the National Cancer Institute and the National Human Genome Research Institute taking information about the human genome and analyzing it for key genomic alterations found in 33 common cancers. These data were combined with clinical information (such as survival) to classify the behaviors of those cancers with respect to their individual genomic alternations, in order to look for patterns in mutations and behaviors. The goal of this analysis was to shift the paradigm of cancer classification from being centered around primary organ site toward tumors’ shared genomic patterns.
In 2013 the Cancer Genome Atlas published their results of complete gene sequencing in endometrial cancer.3 The authors identified four discrete subgroups of endometrial cancer with distinct molecular mutational profiles and distinct clinical outcomes: polymerase epsilon (POLE, pronounced “pole-ee”) ultramutated, microsatellite instability (MSI) high, copy number high, and copy number low.
POLE ultramutated
An important subgroup identified in the Cancer Genome Atlas was a group of patients with a POLE ultramutated state. POLE encodes for a subunit of DNA polymerase, the enzyme responsible for replicating the leading DNA strand. Nonfunctioning POLE results in proofreading errors and a subsequent ultramutated cellular state with a predominance of single nucleotide variants. POLE proofreading domain mutations in endometrial cancer and colon cancer are associated with excellent prognosis, likely secondary to the immune response that is elicited by this ultramutated state from creation of “antigenic neoepitopes” that stimulate T-cell response. Effectively, the very mutated cell is seen as “more foreign” to the body’s immune system.
Approximately 10% of patients with endometrial cancer have a POLE ultramutated state, and, as stated above, prognosis is excellent, even if coexisting with a histologic cell type (such as serous) that is normally associated with adverse outcomes. These women tend to be younger, with a lower body mass index, higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and low stage.
MSI high
MSI (microsatellite instability) is a result of epigenetic/hypermethylations or loss of expression in mismatch repair genes (such as MLH1, MSH2, MSH6, PMS2). These genes code for proteins critical in the repair of mismatches in short repeated sequences of DNA. Loss of their function results in an accumulation of errors in these sequences: MSI. It is a feature of the Lynch syndrome inherited state, but is also found sporadically in endometrial tumors. These tumors accumulate a number of mutations during cell replication that, as in POLE hypermutated tumors, are associated with eliciting an immune response.
These tumors tend to be associated with a higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and an advanced stage. Patients with tumors that have been described as MSI high are candidates for “immune therapy” with the PDL1 inhibitor pembrolizumab because of their proinflammatory state and observed favorable responses in clinical trials.4
Copy number high/low
Copy number (CN) high and low refers to the results of microarrays in which hierarchical clustering was applied to identify reoccurring amplification or deletion regions. The CN-high group was associated with the poorest outcomes (recurrence and survival). There is significant overlap with mutations in TP53. Most serous carcinomas were CN high; however, 25% of patients with high-grade endometrioid cell type shared the CN-high classification. These tumors shared great molecular similarity to high-grade serous ovarian cancers and basal-like breast cancer.
Those patients who did not possess mutations that classified them as POLE hypermutated, MSI high, or CN high were classified as CN low. This group included predominantly grades 1 and 2 endometrioid adenocarcinomas of an early stage and had a favorable prognostic profile, though less favorable than those with a POLE ultramutated state, which appears to be somewhat protective.
Molecular/metabolic interactions
While molecular data are clearly important in driving a cancer cell’s behavior, other clinical and metabolic factors influence cancer behavior. For example, body mass index, adiposity, glucose, and lipid metabolism have been shown to be important drivers of cellular behavior and responsiveness to targeted therapies.5,6 Additionally age, race, and other metabolic states contribute to oncologic behavior. Future classifications of endometrial cancer are unlikely to use molecular profiles in isolation but will need to incorporate these additional patient-specific data to better predict and prognosticate outcomes.
Clinical applications
If researchers can better define and describe a patient’s endometrial cancer from the time of their biopsy, important clinical decisions might be able to be tackled. For example, in a premenopausal patient with an endometrial cancer who is considering fertility-sparing treatments, preoperative knowledge of a POLE ultramutated state (and therefore an anticipated good prognosis) might favor fertility preservation or avoid comprehensive staging which may be of limited value. Similarly, if an MSI-high profile is identified leading to a Lynch syndrome diagnosis, she may be more inclined to undergo a hysterectomy with bilateral salpingo-oophorectomy and staging as she is at known increased risk for a more advanced endometrial cancer, as well as the potential for ovarian cancer.
Postoperative incorporation of molecular data promises to be particularly helpful in guiding adjuvant therapies and sparing some women from unnecessary treatments. For example, women with high-grade endometrioid tumors who are CN high were historically treated with radiotherapy but might do better treated with systemic adjuvant therapies traditionally reserved for nonendometrioid carcinomas. Costly therapies such as immunotherapy can be directed toward those with MSI-high tumors, and the rare patient with a POLE ultramutated state who has a recurrence or advanced disease. Clinical trials will be able to cluster enrollment of patients with CN-high, serouslike cancers with those with serous cancers, rather than combining them with patients whose cancers predictably behave much differently.
Much work is still needed to validate this molecular profiling in endometrial cancer and define the algorithms associated with treatment decisions; however, it is likely that the way we describe endometrial cancer in the near future will be quite different.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no disclosures.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-7.
2. Clarke BA et al. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol. 2010;63(5):410-5.
3. Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
4. Ott PA et al. Pembrolizumab in advanced endometrial cancer: Preliminary results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2016;34(suppl):Abstract 5581.
5. Roque DR et al. Association between differential gene expression and body mass index among endometrial cancers from the Cancer Genome Atlas Project. Gynecol Oncol. 2016;142(2):317-22.
6. Talhouk A et al. New classification of endometrial cancers: The development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract. 2016 Dec;3:14.
We classify endometrial cancer so that we can communicate and define each patient’s disease status, the potential for harm, and the likelihood that adjuvant therapies might provide help. Traditional forms of classification have clearly fallen short in achieving this aim, as we all know of patients with apparent low-risk disease (such as stage IA grade 1 endometrioid carcinoma) who have had recurrences and died from their disease, and we know that many patients have been subjected to overtreatment for their cancer and have acquired lifelong toxicities of therapy. This column will explore the newer, more sophisticated molecular-based classifications that are being validated for endometrial cancer, and the ways in which this promises to personalize the treatment of endometrial cancer.
Breast cancer and melanoma are examples of the inclusion of molecular data such as hormone receptor status, HER2/neu status, or BRAF positivity resulting in advancements in personalizing therapeutics. We are now moving toward this for endometrial cancer.
What is the Cancer Genome Atlas?
In 2006 the National Institutes of Health announced an initiative to coordinate work between the National Cancer Institute and the National Human Genome Research Institute taking information about the human genome and analyzing it for key genomic alterations found in 33 common cancers. These data were combined with clinical information (such as survival) to classify the behaviors of those cancers with respect to their individual genomic alternations, in order to look for patterns in mutations and behaviors. The goal of this analysis was to shift the paradigm of cancer classification from being centered around primary organ site toward tumors’ shared genomic patterns.
In 2013 the Cancer Genome Atlas published their results of complete gene sequencing in endometrial cancer.3 The authors identified four discrete subgroups of endometrial cancer with distinct molecular mutational profiles and distinct clinical outcomes: polymerase epsilon (POLE, pronounced “pole-ee”) ultramutated, microsatellite instability (MSI) high, copy number high, and copy number low.
POLE ultramutated
An important subgroup identified in the Cancer Genome Atlas was a group of patients with a POLE ultramutated state. POLE encodes for a subunit of DNA polymerase, the enzyme responsible for replicating the leading DNA strand. Nonfunctioning POLE results in proofreading errors and a subsequent ultramutated cellular state with a predominance of single nucleotide variants. POLE proofreading domain mutations in endometrial cancer and colon cancer are associated with excellent prognosis, likely secondary to the immune response that is elicited by this ultramutated state from creation of “antigenic neoepitopes” that stimulate T-cell response. Effectively, the very mutated cell is seen as “more foreign” to the body’s immune system.
Approximately 10% of patients with endometrial cancer have a POLE ultramutated state, and, as stated above, prognosis is excellent, even if coexisting with a histologic cell type (such as serous) that is normally associated with adverse outcomes. These women tend to be younger, with a lower body mass index, higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and low stage.
MSI high
MSI (microsatellite instability) is a result of epigenetic/hypermethylations or loss of expression in mismatch repair genes (such as MLH1, MSH2, MSH6, PMS2). These genes code for proteins critical in the repair of mismatches in short repeated sequences of DNA. Loss of their function results in an accumulation of errors in these sequences: MSI. It is a feature of the Lynch syndrome inherited state, but is also found sporadically in endometrial tumors. These tumors accumulate a number of mutations during cell replication that, as in POLE hypermutated tumors, are associated with eliciting an immune response.
These tumors tend to be associated with a higher-grade endometrioid cell type, the presence of lymphovascular space invasion, and an advanced stage. Patients with tumors that have been described as MSI high are candidates for “immune therapy” with the PDL1 inhibitor pembrolizumab because of their proinflammatory state and observed favorable responses in clinical trials.4
Copy number high/low
Copy number (CN) high and low refers to the results of microarrays in which hierarchical clustering was applied to identify reoccurring amplification or deletion regions. The CN-high group was associated with the poorest outcomes (recurrence and survival). There is significant overlap with mutations in TP53. Most serous carcinomas were CN high; however, 25% of patients with high-grade endometrioid cell type shared the CN-high classification. These tumors shared great molecular similarity to high-grade serous ovarian cancers and basal-like breast cancer.
Those patients who did not possess mutations that classified them as POLE hypermutated, MSI high, or CN high were classified as CN low. This group included predominantly grades 1 and 2 endometrioid adenocarcinomas of an early stage and had a favorable prognostic profile, though less favorable than those with a POLE ultramutated state, which appears to be somewhat protective.
Molecular/metabolic interactions
While molecular data are clearly important in driving a cancer cell’s behavior, other clinical and metabolic factors influence cancer behavior. For example, body mass index, adiposity, glucose, and lipid metabolism have been shown to be important drivers of cellular behavior and responsiveness to targeted therapies.5,6 Additionally age, race, and other metabolic states contribute to oncologic behavior. Future classifications of endometrial cancer are unlikely to use molecular profiles in isolation but will need to incorporate these additional patient-specific data to better predict and prognosticate outcomes.
Clinical applications
If researchers can better define and describe a patient’s endometrial cancer from the time of their biopsy, important clinical decisions might be able to be tackled. For example, in a premenopausal patient with an endometrial cancer who is considering fertility-sparing treatments, preoperative knowledge of a POLE ultramutated state (and therefore an anticipated good prognosis) might favor fertility preservation or avoid comprehensive staging which may be of limited value. Similarly, if an MSI-high profile is identified leading to a Lynch syndrome diagnosis, she may be more inclined to undergo a hysterectomy with bilateral salpingo-oophorectomy and staging as she is at known increased risk for a more advanced endometrial cancer, as well as the potential for ovarian cancer.
Postoperative incorporation of molecular data promises to be particularly helpful in guiding adjuvant therapies and sparing some women from unnecessary treatments. For example, women with high-grade endometrioid tumors who are CN high were historically treated with radiotherapy but might do better treated with systemic adjuvant therapies traditionally reserved for nonendometrioid carcinomas. Costly therapies such as immunotherapy can be directed toward those with MSI-high tumors, and the rare patient with a POLE ultramutated state who has a recurrence or advanced disease. Clinical trials will be able to cluster enrollment of patients with CN-high, serouslike cancers with those with serous cancers, rather than combining them with patients whose cancers predictably behave much differently.
Much work is still needed to validate this molecular profiling in endometrial cancer and define the algorithms associated with treatment decisions; however, it is likely that the way we describe endometrial cancer in the near future will be quite different.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no disclosures.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-7.
2. Clarke BA et al. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol. 2010;63(5):410-5.
3. Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
4. Ott PA et al. Pembrolizumab in advanced endometrial cancer: Preliminary results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2016;34(suppl):Abstract 5581.
5. Roque DR et al. Association between differential gene expression and body mass index among endometrial cancers from the Cancer Genome Atlas Project. Gynecol Oncol. 2016;142(2):317-22.
6. Talhouk A et al. New classification of endometrial cancers: The development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract. 2016 Dec;3:14.
What is HIPEC?
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Same-day discharge for hysterectomy
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
Telling her she has cancer: A patient-centered approach to breaking bad news
Hearing a diagnosis of cancer is one of the most significant moments of a patient’s life and informing a patient of her diagnosis is an emotionally and technically challenging task for an obstetrician gynecologist who is frequently on the front line of making this diagnosis. In this column, we will explore some patient-centered strategies to perform this difficult task well so that patients come away informed but with the highest chance for positive emotional adjustment.
Fewer than 10% of physicians report receiving formal training in techniques of breaking bad news. For the majority of clinicians concerns are centered on being honest and not taking away hope, and in responding to a patient’s emotions.1 The SPIKES approach was developed to arm physicians with strategies to discuss a cancer diagnosis with their patients. This approach includes six key elements to incorporate during the encounter. These strategies are not meant to be formulaic but rather consistent principles that can be adjusted for individual patient needs.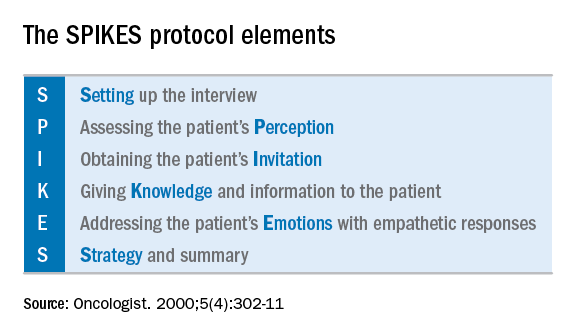
Setting up the discussion
Breaking bad news should not be a one-size-fits-all approach. Age, educational level, culture, religion, race and ethnicity, and socioeconomic opportunities each affects what and how patients may want to have this kind of information communicated to them. So how do you know how best to deliver a patient-centered approach for your patient? I recommend this simple strategy: Ask her. When ordering a test or performing a biopsy, let the patient know then why you are ordering the test and inform her of the possibility that the results may show cancer. Ask her how she would like for you to communicate that result. Would she like to be called by phone, the benefit of which is quick dissemination of information? Or would she like to receive the information face to face in the office? Research supports that most patients prefer to learn the result in the office.2 If so, I recommend scheduling a follow-up appointment in advance to prevent delays. Ask her if she would like a family member or a supportive friend to be present for the conveying of results so that she will have time to make these arrangements. Ask her if she would prefer for an alternate person to be provided with the results on her behalf.
When preparing to speak with the patient, it is valuable to mentally rehearse the words that you’ll use. Arrange for privacy and manage time constraints and interruptions (silence pagers and phones, ensure there is adequate time allocated in the schedule). Sit down to deliver the news and make a connection with eye contact and, if appropriate, touch.
Assessing the patient’s perception. Before you tell, ask. For example, “what is your understanding about why we did the biopsy?” This will guide you in where her head and heart are and can ensure you meet her wherever she is.
Obtaining the patient’s invitation. Ask the patient what she would like to be told and how much information. What would she like you to focus on? What does she not want to hear?
Giving knowledge and information to the patient. Especially now, it is important to avoid jargon and use nontechnical terms. However, do not shy away from using specific words like “cancer” by substituting them for more vague and confusing terms such as “malignancy” or “tumor.” It is important to find the balance between expressing information without being overly emotive, while avoiding excessive bluntness. Word choice is critical. Communication styles in the breaking of bad news can be separated broadly into three styles: disease centered, emotion centered, and patient-centered.3 The patient-centered approach is achieved by balancing emotional connection, information sharing, nondominance, and conveying hope. (For example, “I have some disappointing news to share. Shall we talk about the next steps in treatment? I understand this is that this is difficult for you.”) In general, this approach is most valued by patients and is associated with better information recall.
Addressing the patient’s emotions with empathetic responses. It is important that physicians take a moment to pause after communicating the test result. Even if prepared, most patients will still have a moment of shock, and their minds will likely spin through a multitude of thoughts preventing them from being able to “hear” and focus on the subsequent information. This is a moment to reflect on her reactions, her body language, and nonverbal communications to guide you on how to approach the rest of the encounter. Offer her your comfort and condolence in whichever way feels appropriate for you and her.
Beware of your own inclinations to “soften the blow.” It is a natural, compassionate instinct to follow-up giving a bad piece of information by balancing a good piece of information. For example, after just telling a woman that she has endometrial cancer, following with a statement such as “but it’s just stage 1 and is curable with surgery.” While this certainly may have immediate comforting effects, it has a couple of unintended consequences. First, it can result in difficulties later adjusting to a change in diagnosis when more information comes in (for example, upstaging after surgery or imaging). It is better to be honest and tell patients only what you know for sure in these immediate first moments of diagnosis when complete information is lacking. A more general statement such as “for most women, this is found at an early stage and is highly treatable” may be more appropriate and still provide some comfort. Second, attempts to soften the blow with a qualifying statement of positivity, such as “this is a good kind of cancer to have” might be interpreted by some patients as failing to acknowledge their devastation. She may feel that you are minimizing her condition and not allowing her to grieve or be distressed.
Strategy and summary. Patients who leave the encounter with some kind of plan for the future feel less distressed and anxious. The direction at this point of the encounter should be led by the patient. What are her greatest concerns (such as mortality, loss of fertility, time off work for treatment), and what does she want to know right now? Most patients express a desire to know more about treatment or prognosis.2,4 Unfortunately, it often is not possible to furnish this yet, particularly if this falls into the realm of a subspecialist, and prognostication typically requires more information than a provider has at initial diagnosis. However, leaving these questions unanswered is likely to result in a patient feeling helpless. For example, if an ob.gyn. discovers an apparent advanced ovarian cancer on a CT scan, tell her that, despite its apparent advanced case, it is usually treatable and that a gynecologic oncologist will discuss those best treatment options with her. Assure her that you will expeditiously refer her to a specialist who will provide her with those specifics.
The aftermath
That interval between initial diagnosis and specialist consultation is extraordinarily difficult and a high anxiety time. It is not unreasonable, in such cases, to recommend the patient to reputable online information sources, such as the Society of Gynecologic Oncology or American Cancer Society websites so that she and her family can do some research prior to that visit in order to prepare them better and give them a sense of understanding in their disease.
It is a particularly compassionate touch to reach out to the patient in the days following her cancer diagnosis, even if she has moved on to a specialist. Patients often tell me that they felt enormous reassurance and appreciation when their ob.gyn. reached out to them to “check on how they are doing.” This can usually reasonably be done by phone. This second contact serves another critical purpose: it allows for repetition of the diagnosis and initial plan, and the ability to fill in the blanks of what the patient may have missed during the prior visit, if her mind was, naturally, elsewhere. It also, quite simply, shows that you care.
Ultimately, none of us can break bad news perfectly every time. We all need to be insightful with each of these encounters as to what we did well, what we did not, and how we can adjust in the future. With respect to the SPIKES approach, patients report that physicians struggle most with the “perception,” “invitation,” and “strategy and summary” components.5 Our objective should be keeping the patient’s needs in mind, rather than our own, to maximize the chance of doing a good job. If this task is done well, not only are patients more likely to have positive emotional adjustments to their diagnosis but also more adherence with future therapies.4 In the end, it is the patient who has the final say on whether it was done well or not.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reports no relevant financial disclosures.
References
1. Baile WF et al. Oncologist. 2000;5(4):302-11.
2. Girgis A et al. Behav Med. 1999 Summer;25(2):69-77.
3. Schmid MM et al. Patient Educ Couns. 2005 Sep;58(3):244-51.
4. Girgis A et al. J Clin Oncol. 1995 Sep;13(9):2449-56.
5. Marscholiek P et al. J Cancer Educ. 2018 Feb 5. doi: 10.1007/s13187-017-1315-3.
Hearing a diagnosis of cancer is one of the most significant moments of a patient’s life and informing a patient of her diagnosis is an emotionally and technically challenging task for an obstetrician gynecologist who is frequently on the front line of making this diagnosis. In this column, we will explore some patient-centered strategies to perform this difficult task well so that patients come away informed but with the highest chance for positive emotional adjustment.
Fewer than 10% of physicians report receiving formal training in techniques of breaking bad news. For the majority of clinicians concerns are centered on being honest and not taking away hope, and in responding to a patient’s emotions.1 The SPIKES approach was developed to arm physicians with strategies to discuss a cancer diagnosis with their patients. This approach includes six key elements to incorporate during the encounter. These strategies are not meant to be formulaic but rather consistent principles that can be adjusted for individual patient needs.
Setting up the discussion
Breaking bad news should not be a one-size-fits-all approach. Age, educational level, culture, religion, race and ethnicity, and socioeconomic opportunities each affects what and how patients may want to have this kind of information communicated to them. So how do you know how best to deliver a patient-centered approach for your patient? I recommend this simple strategy: Ask her. When ordering a test or performing a biopsy, let the patient know then why you are ordering the test and inform her of the possibility that the results may show cancer. Ask her how she would like for you to communicate that result. Would she like to be called by phone, the benefit of which is quick dissemination of information? Or would she like to receive the information face to face in the office? Research supports that most patients prefer to learn the result in the office.2 If so, I recommend scheduling a follow-up appointment in advance to prevent delays. Ask her if she would like a family member or a supportive friend to be present for the conveying of results so that she will have time to make these arrangements. Ask her if she would prefer for an alternate person to be provided with the results on her behalf.
When preparing to speak with the patient, it is valuable to mentally rehearse the words that you’ll use. Arrange for privacy and manage time constraints and interruptions (silence pagers and phones, ensure there is adequate time allocated in the schedule). Sit down to deliver the news and make a connection with eye contact and, if appropriate, touch.
Assessing the patient’s perception. Before you tell, ask. For example, “what is your understanding about why we did the biopsy?” This will guide you in where her head and heart are and can ensure you meet her wherever she is.
Obtaining the patient’s invitation. Ask the patient what she would like to be told and how much information. What would she like you to focus on? What does she not want to hear?
Giving knowledge and information to the patient. Especially now, it is important to avoid jargon and use nontechnical terms. However, do not shy away from using specific words like “cancer” by substituting them for more vague and confusing terms such as “malignancy” or “tumor.” It is important to find the balance between expressing information without being overly emotive, while avoiding excessive bluntness. Word choice is critical. Communication styles in the breaking of bad news can be separated broadly into three styles: disease centered, emotion centered, and patient-centered.3 The patient-centered approach is achieved by balancing emotional connection, information sharing, nondominance, and conveying hope. (For example, “I have some disappointing news to share. Shall we talk about the next steps in treatment? I understand this is that this is difficult for you.”) In general, this approach is most valued by patients and is associated with better information recall.
Addressing the patient’s emotions with empathetic responses. It is important that physicians take a moment to pause after communicating the test result. Even if prepared, most patients will still have a moment of shock, and their minds will likely spin through a multitude of thoughts preventing them from being able to “hear” and focus on the subsequent information. This is a moment to reflect on her reactions, her body language, and nonverbal communications to guide you on how to approach the rest of the encounter. Offer her your comfort and condolence in whichever way feels appropriate for you and her.
Beware of your own inclinations to “soften the blow.” It is a natural, compassionate instinct to follow-up giving a bad piece of information by balancing a good piece of information. For example, after just telling a woman that she has endometrial cancer, following with a statement such as “but it’s just stage 1 and is curable with surgery.” While this certainly may have immediate comforting effects, it has a couple of unintended consequences. First, it can result in difficulties later adjusting to a change in diagnosis when more information comes in (for example, upstaging after surgery or imaging). It is better to be honest and tell patients only what you know for sure in these immediate first moments of diagnosis when complete information is lacking. A more general statement such as “for most women, this is found at an early stage and is highly treatable” may be more appropriate and still provide some comfort. Second, attempts to soften the blow with a qualifying statement of positivity, such as “this is a good kind of cancer to have” might be interpreted by some patients as failing to acknowledge their devastation. She may feel that you are minimizing her condition and not allowing her to grieve or be distressed.
Strategy and summary. Patients who leave the encounter with some kind of plan for the future feel less distressed and anxious. The direction at this point of the encounter should be led by the patient. What are her greatest concerns (such as mortality, loss of fertility, time off work for treatment), and what does she want to know right now? Most patients express a desire to know more about treatment or prognosis.2,4 Unfortunately, it often is not possible to furnish this yet, particularly if this falls into the realm of a subspecialist, and prognostication typically requires more information than a provider has at initial diagnosis. However, leaving these questions unanswered is likely to result in a patient feeling helpless. For example, if an ob.gyn. discovers an apparent advanced ovarian cancer on a CT scan, tell her that, despite its apparent advanced case, it is usually treatable and that a gynecologic oncologist will discuss those best treatment options with her. Assure her that you will expeditiously refer her to a specialist who will provide her with those specifics.
The aftermath
That interval between initial diagnosis and specialist consultation is extraordinarily difficult and a high anxiety time. It is not unreasonable, in such cases, to recommend the patient to reputable online information sources, such as the Society of Gynecologic Oncology or American Cancer Society websites so that she and her family can do some research prior to that visit in order to prepare them better and give them a sense of understanding in their disease.
It is a particularly compassionate touch to reach out to the patient in the days following her cancer diagnosis, even if she has moved on to a specialist. Patients often tell me that they felt enormous reassurance and appreciation when their ob.gyn. reached out to them to “check on how they are doing.” This can usually reasonably be done by phone. This second contact serves another critical purpose: it allows for repetition of the diagnosis and initial plan, and the ability to fill in the blanks of what the patient may have missed during the prior visit, if her mind was, naturally, elsewhere. It also, quite simply, shows that you care.
Ultimately, none of us can break bad news perfectly every time. We all need to be insightful with each of these encounters as to what we did well, what we did not, and how we can adjust in the future. With respect to the SPIKES approach, patients report that physicians struggle most with the “perception,” “invitation,” and “strategy and summary” components.5 Our objective should be keeping the patient’s needs in mind, rather than our own, to maximize the chance of doing a good job. If this task is done well, not only are patients more likely to have positive emotional adjustments to their diagnosis but also more adherence with future therapies.4 In the end, it is the patient who has the final say on whether it was done well or not.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reports no relevant financial disclosures.
References
1. Baile WF et al. Oncologist. 2000;5(4):302-11.
2. Girgis A et al. Behav Med. 1999 Summer;25(2):69-77.
3. Schmid MM et al. Patient Educ Couns. 2005 Sep;58(3):244-51.
4. Girgis A et al. J Clin Oncol. 1995 Sep;13(9):2449-56.
5. Marscholiek P et al. J Cancer Educ. 2018 Feb 5. doi: 10.1007/s13187-017-1315-3.
Hearing a diagnosis of cancer is one of the most significant moments of a patient’s life and informing a patient of her diagnosis is an emotionally and technically challenging task for an obstetrician gynecologist who is frequently on the front line of making this diagnosis. In this column, we will explore some patient-centered strategies to perform this difficult task well so that patients come away informed but with the highest chance for positive emotional adjustment.
Fewer than 10% of physicians report receiving formal training in techniques of breaking bad news. For the majority of clinicians concerns are centered on being honest and not taking away hope, and in responding to a patient’s emotions.1 The SPIKES approach was developed to arm physicians with strategies to discuss a cancer diagnosis with their patients. This approach includes six key elements to incorporate during the encounter. These strategies are not meant to be formulaic but rather consistent principles that can be adjusted for individual patient needs.
Setting up the discussion
Breaking bad news should not be a one-size-fits-all approach. Age, educational level, culture, religion, race and ethnicity, and socioeconomic opportunities each affects what and how patients may want to have this kind of information communicated to them. So how do you know how best to deliver a patient-centered approach for your patient? I recommend this simple strategy: Ask her. When ordering a test or performing a biopsy, let the patient know then why you are ordering the test and inform her of the possibility that the results may show cancer. Ask her how she would like for you to communicate that result. Would she like to be called by phone, the benefit of which is quick dissemination of information? Or would she like to receive the information face to face in the office? Research supports that most patients prefer to learn the result in the office.2 If so, I recommend scheduling a follow-up appointment in advance to prevent delays. Ask her if she would like a family member or a supportive friend to be present for the conveying of results so that she will have time to make these arrangements. Ask her if she would prefer for an alternate person to be provided with the results on her behalf.
When preparing to speak with the patient, it is valuable to mentally rehearse the words that you’ll use. Arrange for privacy and manage time constraints and interruptions (silence pagers and phones, ensure there is adequate time allocated in the schedule). Sit down to deliver the news and make a connection with eye contact and, if appropriate, touch.
Assessing the patient’s perception. Before you tell, ask. For example, “what is your understanding about why we did the biopsy?” This will guide you in where her head and heart are and can ensure you meet her wherever she is.
Obtaining the patient’s invitation. Ask the patient what she would like to be told and how much information. What would she like you to focus on? What does she not want to hear?
Giving knowledge and information to the patient. Especially now, it is important to avoid jargon and use nontechnical terms. However, do not shy away from using specific words like “cancer” by substituting them for more vague and confusing terms such as “malignancy” or “tumor.” It is important to find the balance between expressing information without being overly emotive, while avoiding excessive bluntness. Word choice is critical. Communication styles in the breaking of bad news can be separated broadly into three styles: disease centered, emotion centered, and patient-centered.3 The patient-centered approach is achieved by balancing emotional connection, information sharing, nondominance, and conveying hope. (For example, “I have some disappointing news to share. Shall we talk about the next steps in treatment? I understand this is that this is difficult for you.”) In general, this approach is most valued by patients and is associated with better information recall.
Addressing the patient’s emotions with empathetic responses. It is important that physicians take a moment to pause after communicating the test result. Even if prepared, most patients will still have a moment of shock, and their minds will likely spin through a multitude of thoughts preventing them from being able to “hear” and focus on the subsequent information. This is a moment to reflect on her reactions, her body language, and nonverbal communications to guide you on how to approach the rest of the encounter. Offer her your comfort and condolence in whichever way feels appropriate for you and her.
Beware of your own inclinations to “soften the blow.” It is a natural, compassionate instinct to follow-up giving a bad piece of information by balancing a good piece of information. For example, after just telling a woman that she has endometrial cancer, following with a statement such as “but it’s just stage 1 and is curable with surgery.” While this certainly may have immediate comforting effects, it has a couple of unintended consequences. First, it can result in difficulties later adjusting to a change in diagnosis when more information comes in (for example, upstaging after surgery or imaging). It is better to be honest and tell patients only what you know for sure in these immediate first moments of diagnosis when complete information is lacking. A more general statement such as “for most women, this is found at an early stage and is highly treatable” may be more appropriate and still provide some comfort. Second, attempts to soften the blow with a qualifying statement of positivity, such as “this is a good kind of cancer to have” might be interpreted by some patients as failing to acknowledge their devastation. She may feel that you are minimizing her condition and not allowing her to grieve or be distressed.
Strategy and summary. Patients who leave the encounter with some kind of plan for the future feel less distressed and anxious. The direction at this point of the encounter should be led by the patient. What are her greatest concerns (such as mortality, loss of fertility, time off work for treatment), and what does she want to know right now? Most patients express a desire to know more about treatment or prognosis.2,4 Unfortunately, it often is not possible to furnish this yet, particularly if this falls into the realm of a subspecialist, and prognostication typically requires more information than a provider has at initial diagnosis. However, leaving these questions unanswered is likely to result in a patient feeling helpless. For example, if an ob.gyn. discovers an apparent advanced ovarian cancer on a CT scan, tell her that, despite its apparent advanced case, it is usually treatable and that a gynecologic oncologist will discuss those best treatment options with her. Assure her that you will expeditiously refer her to a specialist who will provide her with those specifics.
The aftermath
That interval between initial diagnosis and specialist consultation is extraordinarily difficult and a high anxiety time. It is not unreasonable, in such cases, to recommend the patient to reputable online information sources, such as the Society of Gynecologic Oncology or American Cancer Society websites so that she and her family can do some research prior to that visit in order to prepare them better and give them a sense of understanding in their disease.
It is a particularly compassionate touch to reach out to the patient in the days following her cancer diagnosis, even if she has moved on to a specialist. Patients often tell me that they felt enormous reassurance and appreciation when their ob.gyn. reached out to them to “check on how they are doing.” This can usually reasonably be done by phone. This second contact serves another critical purpose: it allows for repetition of the diagnosis and initial plan, and the ability to fill in the blanks of what the patient may have missed during the prior visit, if her mind was, naturally, elsewhere. It also, quite simply, shows that you care.
Ultimately, none of us can break bad news perfectly every time. We all need to be insightful with each of these encounters as to what we did well, what we did not, and how we can adjust in the future. With respect to the SPIKES approach, patients report that physicians struggle most with the “perception,” “invitation,” and “strategy and summary” components.5 Our objective should be keeping the patient’s needs in mind, rather than our own, to maximize the chance of doing a good job. If this task is done well, not only are patients more likely to have positive emotional adjustments to their diagnosis but also more adherence with future therapies.4 In the end, it is the patient who has the final say on whether it was done well or not.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reports no relevant financial disclosures.
References
1. Baile WF et al. Oncologist. 2000;5(4):302-11.
2. Girgis A et al. Behav Med. 1999 Summer;25(2):69-77.
3. Schmid MM et al. Patient Educ Couns. 2005 Sep;58(3):244-51.
4. Girgis A et al. J Clin Oncol. 1995 Sep;13(9):2449-56.
5. Marscholiek P et al. J Cancer Educ. 2018 Feb 5. doi: 10.1007/s13187-017-1315-3.
Complex atypical hyperplasia: When is it appropriate to refer?
Complex atypical hyperplasia (CAH) of the endometrium is considered the precursor for endometrioid endometrial cancer, the most common gynecologic cancer in the United States. This disease is most frequently diagnosed by gynecologists who are evaluating symptoms of abnormal uterine bleeding in premenopausal women or in postmenopausal women who experience new bleeding. Medical therapies, typically progestin-based treatments, can be employed, particularly when fertility preservation is desired or among patients who are poor surgical candidates. However, the most definitive therapy remains surgery with total hysterectomy for two reasons: CAH is associated with a 28% risk for the development of invasive cancer, and occult invasive cancer frequently coexists with CAH.1,2 This raises a question for gynecologists: Given the risk for occult endometrial cancer, should patients be referred to a gynecologic oncologist for their surgery?
What is the risk for cancer?
What is the significance of occult malignancy with CAH?
If surgeons are aware of endometrial cancer preoperatively or intraoperatively, decisions can be made about staging, particularly the need for lymphadenectomy. The virtues of staging in endometrial cancer is a controversial and frequently debated topic. No survival (therapeutic) benefit from lymphadenectomy has been observed in prospective trials when the information from staging results is not used to guide adjuvant therapy.4 However, the administration of adjuvant chemotherapy is associated with improved survival for patients with lymph node metastases.5 Therefore, if there is a benefit to staging with lymphadenectomy, it is its ability to identify patients who most need this life-saving systemic therapy.
Not all patients with endometrial cancer are at equal risk for harboring lymph node metastases and the majority may not benefit from lymphadenectomy. Patients with tumors that are deeply invasive, moderate or high grade, larger than 2 cm, or that have lymphovascular space invasion are at higher risk for lymph node metastases. Women with low grade, minimally invasive tumors that are smaller than 2 cm have extremely low risk for metastases.6 These criteria are commonly employed to stratify women at lowest risk and minimize unnecessary lymphadenectomy procedures. It should be noted that all three of these low risk features must be present to convey that negligible risk profile. The finding of a grade 1 invasive tumor alone is not enough to exclude potential lymph node metastases, particularly in the case of large or deeply invasive cancers.
How can the diagnosis be made preoperatively or intraoperatively?
The gold standard for discriminating between CAH and endometrial cancer is definitive surgical pathology. However, if surgeons wait until these results are available, they have lost the opportunity to stage the patient without subjecting them to a second surgery. The preoperative discovery of cancer may be increased by performing diagnostic curettage rather than relying on office endometrial biopsy sampling.7 This is likely due to the increased volume of tissue removed with dilation and curettage, and a reduction in the risk for sampling error. The addition of hysteroscopy to curettage does not improve upon the detection of cancer. Preoperative MRI to evaluate for depth of myometrial invasion has been described in cases of known endometrial cancer; however, its role in discriminating between CAH and invasive cancer is not well studied.
Intraoperative frozen section is commonly employed to evaluate the hysterectomy specimen for cancer in order to triage patients to staging during that same surgery. However, the accuracy of frozen section with definitive pathology is only approximately 50%.8 This means that at least half of women with CAH will have a false negative frozen section result and will have lost the opportunity for staging at the same procedure. The inaccuracy of frozen section is often overlooked by surgeons who may feel that it is a very straightforward diagnostic procedure. In reality, the characterization of CAH and invasive cancer is technically challenging and relies on multiple sectioning and significant experience in gynecologic pathology.9
Should all patients with CAH be referred and staged?
An alternative to relying on the frozen section process and its inherent inaccuracies would be to routinely stage all women with CAH, knowing that approximately 40% of them have occult cancer, and more than a third of those will have high risk features for lymph node metastases. However, due to the risks associated with lymphadenectomy, particularly lymphedema, most gynecologic oncologists do not routinely stage patients with preoperative CAH with complete lymphadenectomy.
An alternative to the all (complete lymphadenectomy) or none (hysterectomy alone) approach is to perform sentinel lymph node (SLN) biopsy for patients with CAH. SLN biopsy involves removing scant, but high yield lymphatic tissue, and has been shown to be extremely sensitive in detecting metastatic disease.10 This approach is commonly employed by surgeons in the treatment of ductal carcinoma in situ of the breast which, like CAH, is a stage 0 cancer that can be associated with invasive carcinoma on final pathology. In the case of ductal carcinoma in situ, the risk for upstaging is actually substantially lower (25%) than what is observed in CAH.11 Therefore, it would seem even more compelling to apply this approach for endometrial pathologies. The ability to apply the SLN technique is lost after hysterectomy is performed, as there is no longer the target organ into which tracer can be injected; therefore, if SLN biopsy is to be offered to these patients, it needs to be performed using only the preoperative diagnosis of CAH. In this approach, there will be overtreatment of approximately two-thirds of patients, albeit with a less radical and morbid staging procedure.
Making the decision to refer
Ultimately, decisions to refer or not are guided by comprehensive discussions between patient and provider that outline the potential risks and benefits of various approaches. Patients frequently have strong relationships with confidence in their gynecologists who may have cared for them for many years, and may be motivated to have them perform their surgery. For others, the uncertainty and possibility of an unstaged cancer and the potential of a second surgery drives their decision to seek an oncology consultation. Clinicians should discuss the inherent uncertainties in the diagnosis of CAH and the potential for underlying cancer and lymph node metastases, and help patients determine the balance of their underlying competing concerns regarding the risk for inadequate surgery versus the risk of unnecessary surgical procedures.
Summary of recommendations
Invasive endometrial cancer will be identified in the hysterectomy specimens of approximately 40% of women with a preoperative diagnosis of complex endometrial hyperplasia. Preoperative dilation and curettage may reduce the potential for missed occult cancer. Frozen section is an option for determining which patients might benefit from staging but is associated with significant inaccuracies. Failure to diagnose malignancy pre- or intraoperatively handicaps postoperative decision making regarding the necessity of adjuvant chemotherapy, and prevents the ability to offer patients potentially less morbid staging techniques such as SLN biopsy. When gynecologists without oncology training perform these hysterectomies, they should discuss these scenarios to patients and consider referral to gynecologic oncology for patients who desire the potential for comprehensive staging if necessary.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. She reports no relevant financial disclosures.
References
1. J Clin Oncol. 2010 Feb;28:788-92.
2. Cancer. 2006 Feb;106:812-9.
3. Int J Gynecol Cancer. 2005 Jan-Feb;15:127-31.
4. Lancet. 2009 Jan;373(9658):125-36.
5. J Clin Oncol. 2006 Jan;24:36-44.
6. Gynecol Oncol 2008 Apr;109:11-8.
7. Am J Obstet Gynecol. 2010 Oct;203(4):349. e1-6.
8. Am J Obstet Gynecol. 2007 May;196(5):e40-2.
9. Obstet Gynecol. 2012 Nov;120(5):1160-75.
10. Lancet Oncol. 2017 Mar;18(3):384-92.
11. Radiology. 2011 Jul;260:119-28.
Complex atypical hyperplasia (CAH) of the endometrium is considered the precursor for endometrioid endometrial cancer, the most common gynecologic cancer in the United States. This disease is most frequently diagnosed by gynecologists who are evaluating symptoms of abnormal uterine bleeding in premenopausal women or in postmenopausal women who experience new bleeding. Medical therapies, typically progestin-based treatments, can be employed, particularly when fertility preservation is desired or among patients who are poor surgical candidates. However, the most definitive therapy remains surgery with total hysterectomy for two reasons: CAH is associated with a 28% risk for the development of invasive cancer, and occult invasive cancer frequently coexists with CAH.1,2 This raises a question for gynecologists: Given the risk for occult endometrial cancer, should patients be referred to a gynecologic oncologist for their surgery?
What is the risk for cancer?
What is the significance of occult malignancy with CAH?
If surgeons are aware of endometrial cancer preoperatively or intraoperatively, decisions can be made about staging, particularly the need for lymphadenectomy. The virtues of staging in endometrial cancer is a controversial and frequently debated topic. No survival (therapeutic) benefit from lymphadenectomy has been observed in prospective trials when the information from staging results is not used to guide adjuvant therapy.4 However, the administration of adjuvant chemotherapy is associated with improved survival for patients with lymph node metastases.5 Therefore, if there is a benefit to staging with lymphadenectomy, it is its ability to identify patients who most need this life-saving systemic therapy.
Not all patients with endometrial cancer are at equal risk for harboring lymph node metastases and the majority may not benefit from lymphadenectomy. Patients with tumors that are deeply invasive, moderate or high grade, larger than 2 cm, or that have lymphovascular space invasion are at higher risk for lymph node metastases. Women with low grade, minimally invasive tumors that are smaller than 2 cm have extremely low risk for metastases.6 These criteria are commonly employed to stratify women at lowest risk and minimize unnecessary lymphadenectomy procedures. It should be noted that all three of these low risk features must be present to convey that negligible risk profile. The finding of a grade 1 invasive tumor alone is not enough to exclude potential lymph node metastases, particularly in the case of large or deeply invasive cancers.
How can the diagnosis be made preoperatively or intraoperatively?
The gold standard for discriminating between CAH and endometrial cancer is definitive surgical pathology. However, if surgeons wait until these results are available, they have lost the opportunity to stage the patient without subjecting them to a second surgery. The preoperative discovery of cancer may be increased by performing diagnostic curettage rather than relying on office endometrial biopsy sampling.7 This is likely due to the increased volume of tissue removed with dilation and curettage, and a reduction in the risk for sampling error. The addition of hysteroscopy to curettage does not improve upon the detection of cancer. Preoperative MRI to evaluate for depth of myometrial invasion has been described in cases of known endometrial cancer; however, its role in discriminating between CAH and invasive cancer is not well studied.
Intraoperative frozen section is commonly employed to evaluate the hysterectomy specimen for cancer in order to triage patients to staging during that same surgery. However, the accuracy of frozen section with definitive pathology is only approximately 50%.8 This means that at least half of women with CAH will have a false negative frozen section result and will have lost the opportunity for staging at the same procedure. The inaccuracy of frozen section is often overlooked by surgeons who may feel that it is a very straightforward diagnostic procedure. In reality, the characterization of CAH and invasive cancer is technically challenging and relies on multiple sectioning and significant experience in gynecologic pathology.9
Should all patients with CAH be referred and staged?
An alternative to relying on the frozen section process and its inherent inaccuracies would be to routinely stage all women with CAH, knowing that approximately 40% of them have occult cancer, and more than a third of those will have high risk features for lymph node metastases. However, due to the risks associated with lymphadenectomy, particularly lymphedema, most gynecologic oncologists do not routinely stage patients with preoperative CAH with complete lymphadenectomy.
An alternative to the all (complete lymphadenectomy) or none (hysterectomy alone) approach is to perform sentinel lymph node (SLN) biopsy for patients with CAH. SLN biopsy involves removing scant, but high yield lymphatic tissue, and has been shown to be extremely sensitive in detecting metastatic disease.10 This approach is commonly employed by surgeons in the treatment of ductal carcinoma in situ of the breast which, like CAH, is a stage 0 cancer that can be associated with invasive carcinoma on final pathology. In the case of ductal carcinoma in situ, the risk for upstaging is actually substantially lower (25%) than what is observed in CAH.11 Therefore, it would seem even more compelling to apply this approach for endometrial pathologies. The ability to apply the SLN technique is lost after hysterectomy is performed, as there is no longer the target organ into which tracer can be injected; therefore, if SLN biopsy is to be offered to these patients, it needs to be performed using only the preoperative diagnosis of CAH. In this approach, there will be overtreatment of approximately two-thirds of patients, albeit with a less radical and morbid staging procedure.
Making the decision to refer
Ultimately, decisions to refer or not are guided by comprehensive discussions between patient and provider that outline the potential risks and benefits of various approaches. Patients frequently have strong relationships with confidence in their gynecologists who may have cared for them for many years, and may be motivated to have them perform their surgery. For others, the uncertainty and possibility of an unstaged cancer and the potential of a second surgery drives their decision to seek an oncology consultation. Clinicians should discuss the inherent uncertainties in the diagnosis of CAH and the potential for underlying cancer and lymph node metastases, and help patients determine the balance of their underlying competing concerns regarding the risk for inadequate surgery versus the risk of unnecessary surgical procedures.
Summary of recommendations
Invasive endometrial cancer will be identified in the hysterectomy specimens of approximately 40% of women with a preoperative diagnosis of complex endometrial hyperplasia. Preoperative dilation and curettage may reduce the potential for missed occult cancer. Frozen section is an option for determining which patients might benefit from staging but is associated with significant inaccuracies. Failure to diagnose malignancy pre- or intraoperatively handicaps postoperative decision making regarding the necessity of adjuvant chemotherapy, and prevents the ability to offer patients potentially less morbid staging techniques such as SLN biopsy. When gynecologists without oncology training perform these hysterectomies, they should discuss these scenarios to patients and consider referral to gynecologic oncology for patients who desire the potential for comprehensive staging if necessary.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. She reports no relevant financial disclosures.
References
1. J Clin Oncol. 2010 Feb;28:788-92.
2. Cancer. 2006 Feb;106:812-9.
3. Int J Gynecol Cancer. 2005 Jan-Feb;15:127-31.
4. Lancet. 2009 Jan;373(9658):125-36.
5. J Clin Oncol. 2006 Jan;24:36-44.
6. Gynecol Oncol 2008 Apr;109:11-8.
7. Am J Obstet Gynecol. 2010 Oct;203(4):349. e1-6.
8. Am J Obstet Gynecol. 2007 May;196(5):e40-2.
9. Obstet Gynecol. 2012 Nov;120(5):1160-75.
10. Lancet Oncol. 2017 Mar;18(3):384-92.
11. Radiology. 2011 Jul;260:119-28.
Complex atypical hyperplasia (CAH) of the endometrium is considered the precursor for endometrioid endometrial cancer, the most common gynecologic cancer in the United States. This disease is most frequently diagnosed by gynecologists who are evaluating symptoms of abnormal uterine bleeding in premenopausal women or in postmenopausal women who experience new bleeding. Medical therapies, typically progestin-based treatments, can be employed, particularly when fertility preservation is desired or among patients who are poor surgical candidates. However, the most definitive therapy remains surgery with total hysterectomy for two reasons: CAH is associated with a 28% risk for the development of invasive cancer, and occult invasive cancer frequently coexists with CAH.1,2 This raises a question for gynecologists: Given the risk for occult endometrial cancer, should patients be referred to a gynecologic oncologist for their surgery?
What is the risk for cancer?
What is the significance of occult malignancy with CAH?
If surgeons are aware of endometrial cancer preoperatively or intraoperatively, decisions can be made about staging, particularly the need for lymphadenectomy. The virtues of staging in endometrial cancer is a controversial and frequently debated topic. No survival (therapeutic) benefit from lymphadenectomy has been observed in prospective trials when the information from staging results is not used to guide adjuvant therapy.4 However, the administration of adjuvant chemotherapy is associated with improved survival for patients with lymph node metastases.5 Therefore, if there is a benefit to staging with lymphadenectomy, it is its ability to identify patients who most need this life-saving systemic therapy.
Not all patients with endometrial cancer are at equal risk for harboring lymph node metastases and the majority may not benefit from lymphadenectomy. Patients with tumors that are deeply invasive, moderate or high grade, larger than 2 cm, or that have lymphovascular space invasion are at higher risk for lymph node metastases. Women with low grade, minimally invasive tumors that are smaller than 2 cm have extremely low risk for metastases.6 These criteria are commonly employed to stratify women at lowest risk and minimize unnecessary lymphadenectomy procedures. It should be noted that all three of these low risk features must be present to convey that negligible risk profile. The finding of a grade 1 invasive tumor alone is not enough to exclude potential lymph node metastases, particularly in the case of large or deeply invasive cancers.
How can the diagnosis be made preoperatively or intraoperatively?
The gold standard for discriminating between CAH and endometrial cancer is definitive surgical pathology. However, if surgeons wait until these results are available, they have lost the opportunity to stage the patient without subjecting them to a second surgery. The preoperative discovery of cancer may be increased by performing diagnostic curettage rather than relying on office endometrial biopsy sampling.7 This is likely due to the increased volume of tissue removed with dilation and curettage, and a reduction in the risk for sampling error. The addition of hysteroscopy to curettage does not improve upon the detection of cancer. Preoperative MRI to evaluate for depth of myometrial invasion has been described in cases of known endometrial cancer; however, its role in discriminating between CAH and invasive cancer is not well studied.
Intraoperative frozen section is commonly employed to evaluate the hysterectomy specimen for cancer in order to triage patients to staging during that same surgery. However, the accuracy of frozen section with definitive pathology is only approximately 50%.8 This means that at least half of women with CAH will have a false negative frozen section result and will have lost the opportunity for staging at the same procedure. The inaccuracy of frozen section is often overlooked by surgeons who may feel that it is a very straightforward diagnostic procedure. In reality, the characterization of CAH and invasive cancer is technically challenging and relies on multiple sectioning and significant experience in gynecologic pathology.9
Should all patients with CAH be referred and staged?
An alternative to relying on the frozen section process and its inherent inaccuracies would be to routinely stage all women with CAH, knowing that approximately 40% of them have occult cancer, and more than a third of those will have high risk features for lymph node metastases. However, due to the risks associated with lymphadenectomy, particularly lymphedema, most gynecologic oncologists do not routinely stage patients with preoperative CAH with complete lymphadenectomy.
An alternative to the all (complete lymphadenectomy) or none (hysterectomy alone) approach is to perform sentinel lymph node (SLN) biopsy for patients with CAH. SLN biopsy involves removing scant, but high yield lymphatic tissue, and has been shown to be extremely sensitive in detecting metastatic disease.10 This approach is commonly employed by surgeons in the treatment of ductal carcinoma in situ of the breast which, like CAH, is a stage 0 cancer that can be associated with invasive carcinoma on final pathology. In the case of ductal carcinoma in situ, the risk for upstaging is actually substantially lower (25%) than what is observed in CAH.11 Therefore, it would seem even more compelling to apply this approach for endometrial pathologies. The ability to apply the SLN technique is lost after hysterectomy is performed, as there is no longer the target organ into which tracer can be injected; therefore, if SLN biopsy is to be offered to these patients, it needs to be performed using only the preoperative diagnosis of CAH. In this approach, there will be overtreatment of approximately two-thirds of patients, albeit with a less radical and morbid staging procedure.
Making the decision to refer
Ultimately, decisions to refer or not are guided by comprehensive discussions between patient and provider that outline the potential risks and benefits of various approaches. Patients frequently have strong relationships with confidence in their gynecologists who may have cared for them for many years, and may be motivated to have them perform their surgery. For others, the uncertainty and possibility of an unstaged cancer and the potential of a second surgery drives their decision to seek an oncology consultation. Clinicians should discuss the inherent uncertainties in the diagnosis of CAH and the potential for underlying cancer and lymph node metastases, and help patients determine the balance of their underlying competing concerns regarding the risk for inadequate surgery versus the risk of unnecessary surgical procedures.
Summary of recommendations
Invasive endometrial cancer will be identified in the hysterectomy specimens of approximately 40% of women with a preoperative diagnosis of complex endometrial hyperplasia. Preoperative dilation and curettage may reduce the potential for missed occult cancer. Frozen section is an option for determining which patients might benefit from staging but is associated with significant inaccuracies. Failure to diagnose malignancy pre- or intraoperatively handicaps postoperative decision making regarding the necessity of adjuvant chemotherapy, and prevents the ability to offer patients potentially less morbid staging techniques such as SLN biopsy. When gynecologists without oncology training perform these hysterectomies, they should discuss these scenarios to patients and consider referral to gynecologic oncology for patients who desire the potential for comprehensive staging if necessary.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. She reports no relevant financial disclosures.
References
1. J Clin Oncol. 2010 Feb;28:788-92.
2. Cancer. 2006 Feb;106:812-9.
3. Int J Gynecol Cancer. 2005 Jan-Feb;15:127-31.
4. Lancet. 2009 Jan;373(9658):125-36.
5. J Clin Oncol. 2006 Jan;24:36-44.
6. Gynecol Oncol 2008 Apr;109:11-8.
7. Am J Obstet Gynecol. 2010 Oct;203(4):349. e1-6.
8. Am J Obstet Gynecol. 2007 May;196(5):e40-2.
9. Obstet Gynecol. 2012 Nov;120(5):1160-75.
10. Lancet Oncol. 2017 Mar;18(3):384-92.
11. Radiology. 2011 Jul;260:119-28.
Strategies to evaluate postmenopausal bleeding
Postmenopausal bleeding is a symptom that can announce the presence of a gynecologic malignancy. In this column, we will discuss the important considerations to make in the work-up of this symptom.
Roughly 10% of women will present for evaluation of postmenopausal bleeding.1 More than a third of these women will have benign pathology, with the incidence of endometrial cancer in this group at only about 5%.2 Other gynecologic malignancies should be considered as well, including cervical, vaginal, vulvar, and more rarely, those of the fallopian tubes or ovaries.
Use of ultrasound
Ultrasound is a commonly performed initial approach to work-up because of its noninvasive nature. Transvaginal ultrasound has a high negative predictive value of 99.4%-100% in ruling out malignancy.3 Among women with postmenopausal bleeding, the risk of cancer is 7.3% if their endometrial lining is 5 mm or greater and less than 0.07% risk if their lining is 4 mm or less. Therefore, this cutoff dimension is typically used to triage patients to additional sampling.
If ultrasound is performed on postmenopausal women who are asymptomatic (no bleeding), then an endometrial stripe of greater than 11 mm is considered justification for further work-up and is associated with a 6.7% risk of endometrial cancer.4 If the ultrasound reveals intracavitary lesions, a sonohysterogram would be preferred to characterize intrauterine pathology. In fact, sonohysterography is superior to transvaginal ultrasound (with a sensitivity of 80% vs. 49%, respectively) in detecting endometrial polypoid lesions.5 Preoperative identification of an intracavitary lesion may assist in selecting the best sampling technique (blind vs. hysteroscopy-guided approach).
Endometrial sampling
If an ultrasound reveals a thickened or unevaluable endometrial stripe or if the clinician chooses to proceed directly with diagnostic confirmation, several options for endometrial sampling exist, including office-based or operative procedures, as well as blind or visually guided ones. Endometrial pipelle biopsy, D&C without hysteroscopy, endometrial lavage, and endometrial brush biopsy all constitute “blind” sampling techniques. Targeted biopsy techniques include hysteroscopy D&C and saline infusion sonohysterography–guided biopsy.
Blind D&C
Although D&C may be considered the gold standard of diagnostic sampling techniques, it should be noted that 60% of these procedures sample less than half of the endometrium.6 When used in conjunction with hysteroscopy, the sensitivity in detecting cancer is high at 97% with a specificity of 93%-100%.7
While some patients are candidates for office-based procedures, D&C often requires regional or general anesthesia and is frequently performed in a hospital-based environment or surgical center. This may be most appropriate for patients who have had failed office attempts at sampling, have multiple medical comorbidities that limit the feasibility of office-based procedures (such as morbid obesity), or have severe cervical stenosis. D&C is associated with an increased risk for uterine perforation, compared with outpatient sampling procedures.
The need to go to the operating room rather than to an ambulatory setting also may increase the costs borne by the patient. The advantages of D&C include the potential for large-volume sampling and the potentially therapeutic nature of the procedure in cases of benign pathology.
Office-based procedures
Office-based sampling techniques include those using a pipelle, those employing an endometrial brush, and those guided by saline infusion sonohysterography. If performed in the office, they require minimal or no cervical dilation, are associated with a lower risk of perforation or adverse reaction to anesthesia, and usually have lower costs for patients.
Endometrial pipelle biopsies are a very effective diagnostic tool when there is global, endometrial pathology; they have a sensitivity of 83% in confirming cancer.8 It is an inexpensive and technically straightforward technique that can be easily performed in an office setting.
However, when the endometrial lining is atrophied, alternative tools may provide superior results. Endometrial brushes have been shown to be 33% more successful in collecting adequate samples,compared with pipelles, because they sample a larger endometrial surface area.9
There is ongoing development of sampling techniques, such as endometrial lavage or the combination of saline infusion sonohysterography and endometrial biopsy.10 However, future studies regarding accuracy, cost, and patient acceptability are needed before these techniques are translated to the clinical setting.
Targeted endometrial sampling
Targeted or visually guided sampling, such as hysteroscopy, has been shown to be very accurate in identifying benign pathology, although the sensitivity of hysteroscopic diagnosis of cancer is significantly lower at approximately 50%.11 Therefore, the benefit of hysteroscopy is in complementing the blind nature of D&C by guiding sampling of intracavitary lesions, should they exist.
Hysteroscopy is safe in endometrial cancer and is not associated with upstaging the cancer from transtubal extirpation of malignant cells.12
The addition of hysteroscopy contributes some cost and equipment to the blind D&C procedure; therefore, it might be best applied in cases where there is known intracavitary pathology or inadequate prior sampling. In well-selected patients, hysteroscopy often can be used in an office setting, which improves the practicality of the procedure. Smaller and, in some cases, disposable equipment aids in the feasibility of adding visual guidance to office sampling.
Optimizing sampling
Postmenopausal women have a higher risk for sampling failure, compared with younger women. Obesity also is a risk for failed sampling.13 Cervical ripening with misoprostol may increase access to the endometrial cavity, and ultrasound guidance may decrease the risk of uterine perforation in a stenotic cervix.
Clinicians should ensure that histology results are concordant with clinical data. Discordant results should be reevaluated. For example, if an ultrasound demonstrates a thickened endometrial stripe, but the sampling reveals “scant atrophic tissue,” then there is unexplained pathology to address. Further work-up, such as more comprehensive sampling with hysteroscopy, should be considered in such cases. Additionally, persistent postmenopausal bleeding, despite a benign endometrial biopsy, should be reevaluated over time to rule out occult disease missed during prior sampling.
Clinicians are now equipped with multiple ways of obtaining clinical data, and patients have options that may decrease barriers to their care. Hysteroscopy does not improve upon D&C in the diagnosis of endometrial cancer, although it may be helpful in distinguishing and treating nonmalignant lesions.
Dr. Cotangco is a resident in the department of obstetrics and gynecology at the University of Illinois, Chicago. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Acta Obstet Gynecol Scand. 2004 Feb;83(2):203-7.
2. Menopause Int. 2010 Mar;16(1):5-8.
3. Obstet Gynecol. 2009 Aug;114(2 Pt 1):409-11.
4. Ultrasound Obstet Gynecol. 2004 Oct;24(5):558-65.
5. Ultrasound Obstet Gynecol. 2001 Aug;18(2):157-62.
6. Am J Obstet Gynecol. 2009 Jul;201(1):5-11.
7. Obstet Gynecol Clin North Am. 2000 Jun;27(2):235-44.
8. J Reprod Med. 1995 Aug;40(8):553-5.
9. BJOG. 2008 Jul;115(8):1028-36.
10. PLoS Med. 2016 Dec. doi: 10.1371/journal.pmed.1002206.
11. Arch Gynecol Obstet. 2012 Mar;285(3):839-43.
12. Am J Obstet Gynecol. 2012 Jul;207(1):71.e1-5.
13. Gynecol Oncol. 2017 Feb;144(2):324-8.
Postmenopausal bleeding is a symptom that can announce the presence of a gynecologic malignancy. In this column, we will discuss the important considerations to make in the work-up of this symptom.
Roughly 10% of women will present for evaluation of postmenopausal bleeding.1 More than a third of these women will have benign pathology, with the incidence of endometrial cancer in this group at only about 5%.2 Other gynecologic malignancies should be considered as well, including cervical, vaginal, vulvar, and more rarely, those of the fallopian tubes or ovaries.
Use of ultrasound
Ultrasound is a commonly performed initial approach to work-up because of its noninvasive nature. Transvaginal ultrasound has a high negative predictive value of 99.4%-100% in ruling out malignancy.3 Among women with postmenopausal bleeding, the risk of cancer is 7.3% if their endometrial lining is 5 mm or greater and less than 0.07% risk if their lining is 4 mm or less. Therefore, this cutoff dimension is typically used to triage patients to additional sampling.
If ultrasound is performed on postmenopausal women who are asymptomatic (no bleeding), then an endometrial stripe of greater than 11 mm is considered justification for further work-up and is associated with a 6.7% risk of endometrial cancer.4 If the ultrasound reveals intracavitary lesions, a sonohysterogram would be preferred to characterize intrauterine pathology. In fact, sonohysterography is superior to transvaginal ultrasound (with a sensitivity of 80% vs. 49%, respectively) in detecting endometrial polypoid lesions.5 Preoperative identification of an intracavitary lesion may assist in selecting the best sampling technique (blind vs. hysteroscopy-guided approach).
Endometrial sampling
If an ultrasound reveals a thickened or unevaluable endometrial stripe or if the clinician chooses to proceed directly with diagnostic confirmation, several options for endometrial sampling exist, including office-based or operative procedures, as well as blind or visually guided ones. Endometrial pipelle biopsy, D&C without hysteroscopy, endometrial lavage, and endometrial brush biopsy all constitute “blind” sampling techniques. Targeted biopsy techniques include hysteroscopy D&C and saline infusion sonohysterography–guided biopsy.
Blind D&C
Although D&C may be considered the gold standard of diagnostic sampling techniques, it should be noted that 60% of these procedures sample less than half of the endometrium.6 When used in conjunction with hysteroscopy, the sensitivity in detecting cancer is high at 97% with a specificity of 93%-100%.7
While some patients are candidates for office-based procedures, D&C often requires regional or general anesthesia and is frequently performed in a hospital-based environment or surgical center. This may be most appropriate for patients who have had failed office attempts at sampling, have multiple medical comorbidities that limit the feasibility of office-based procedures (such as morbid obesity), or have severe cervical stenosis. D&C is associated with an increased risk for uterine perforation, compared with outpatient sampling procedures.
The need to go to the operating room rather than to an ambulatory setting also may increase the costs borne by the patient. The advantages of D&C include the potential for large-volume sampling and the potentially therapeutic nature of the procedure in cases of benign pathology.
Office-based procedures
Office-based sampling techniques include those using a pipelle, those employing an endometrial brush, and those guided by saline infusion sonohysterography. If performed in the office, they require minimal or no cervical dilation, are associated with a lower risk of perforation or adverse reaction to anesthesia, and usually have lower costs for patients.
Endometrial pipelle biopsies are a very effective diagnostic tool when there is global, endometrial pathology; they have a sensitivity of 83% in confirming cancer.8 It is an inexpensive and technically straightforward technique that can be easily performed in an office setting.
However, when the endometrial lining is atrophied, alternative tools may provide superior results. Endometrial brushes have been shown to be 33% more successful in collecting adequate samples,compared with pipelles, because they sample a larger endometrial surface area.9
There is ongoing development of sampling techniques, such as endometrial lavage or the combination of saline infusion sonohysterography and endometrial biopsy.10 However, future studies regarding accuracy, cost, and patient acceptability are needed before these techniques are translated to the clinical setting.
Targeted endometrial sampling
Targeted or visually guided sampling, such as hysteroscopy, has been shown to be very accurate in identifying benign pathology, although the sensitivity of hysteroscopic diagnosis of cancer is significantly lower at approximately 50%.11 Therefore, the benefit of hysteroscopy is in complementing the blind nature of D&C by guiding sampling of intracavitary lesions, should they exist.
Hysteroscopy is safe in endometrial cancer and is not associated with upstaging the cancer from transtubal extirpation of malignant cells.12
The addition of hysteroscopy contributes some cost and equipment to the blind D&C procedure; therefore, it might be best applied in cases where there is known intracavitary pathology or inadequate prior sampling. In well-selected patients, hysteroscopy often can be used in an office setting, which improves the practicality of the procedure. Smaller and, in some cases, disposable equipment aids in the feasibility of adding visual guidance to office sampling.
Optimizing sampling
Postmenopausal women have a higher risk for sampling failure, compared with younger women. Obesity also is a risk for failed sampling.13 Cervical ripening with misoprostol may increase access to the endometrial cavity, and ultrasound guidance may decrease the risk of uterine perforation in a stenotic cervix.
Clinicians should ensure that histology results are concordant with clinical data. Discordant results should be reevaluated. For example, if an ultrasound demonstrates a thickened endometrial stripe, but the sampling reveals “scant atrophic tissue,” then there is unexplained pathology to address. Further work-up, such as more comprehensive sampling with hysteroscopy, should be considered in such cases. Additionally, persistent postmenopausal bleeding, despite a benign endometrial biopsy, should be reevaluated over time to rule out occult disease missed during prior sampling.
Clinicians are now equipped with multiple ways of obtaining clinical data, and patients have options that may decrease barriers to their care. Hysteroscopy does not improve upon D&C in the diagnosis of endometrial cancer, although it may be helpful in distinguishing and treating nonmalignant lesions.
Dr. Cotangco is a resident in the department of obstetrics and gynecology at the University of Illinois, Chicago. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Acta Obstet Gynecol Scand. 2004 Feb;83(2):203-7.
2. Menopause Int. 2010 Mar;16(1):5-8.
3. Obstet Gynecol. 2009 Aug;114(2 Pt 1):409-11.
4. Ultrasound Obstet Gynecol. 2004 Oct;24(5):558-65.
5. Ultrasound Obstet Gynecol. 2001 Aug;18(2):157-62.
6. Am J Obstet Gynecol. 2009 Jul;201(1):5-11.
7. Obstet Gynecol Clin North Am. 2000 Jun;27(2):235-44.
8. J Reprod Med. 1995 Aug;40(8):553-5.
9. BJOG. 2008 Jul;115(8):1028-36.
10. PLoS Med. 2016 Dec. doi: 10.1371/journal.pmed.1002206.
11. Arch Gynecol Obstet. 2012 Mar;285(3):839-43.
12. Am J Obstet Gynecol. 2012 Jul;207(1):71.e1-5.
13. Gynecol Oncol. 2017 Feb;144(2):324-8.
Postmenopausal bleeding is a symptom that can announce the presence of a gynecologic malignancy. In this column, we will discuss the important considerations to make in the work-up of this symptom.
Roughly 10% of women will present for evaluation of postmenopausal bleeding.1 More than a third of these women will have benign pathology, with the incidence of endometrial cancer in this group at only about 5%.2 Other gynecologic malignancies should be considered as well, including cervical, vaginal, vulvar, and more rarely, those of the fallopian tubes or ovaries.
Use of ultrasound
Ultrasound is a commonly performed initial approach to work-up because of its noninvasive nature. Transvaginal ultrasound has a high negative predictive value of 99.4%-100% in ruling out malignancy.3 Among women with postmenopausal bleeding, the risk of cancer is 7.3% if their endometrial lining is 5 mm or greater and less than 0.07% risk if their lining is 4 mm or less. Therefore, this cutoff dimension is typically used to triage patients to additional sampling.
If ultrasound is performed on postmenopausal women who are asymptomatic (no bleeding), then an endometrial stripe of greater than 11 mm is considered justification for further work-up and is associated with a 6.7% risk of endometrial cancer.4 If the ultrasound reveals intracavitary lesions, a sonohysterogram would be preferred to characterize intrauterine pathology. In fact, sonohysterography is superior to transvaginal ultrasound (with a sensitivity of 80% vs. 49%, respectively) in detecting endometrial polypoid lesions.5 Preoperative identification of an intracavitary lesion may assist in selecting the best sampling technique (blind vs. hysteroscopy-guided approach).
Endometrial sampling
If an ultrasound reveals a thickened or unevaluable endometrial stripe or if the clinician chooses to proceed directly with diagnostic confirmation, several options for endometrial sampling exist, including office-based or operative procedures, as well as blind or visually guided ones. Endometrial pipelle biopsy, D&C without hysteroscopy, endometrial lavage, and endometrial brush biopsy all constitute “blind” sampling techniques. Targeted biopsy techniques include hysteroscopy D&C and saline infusion sonohysterography–guided biopsy.
Blind D&C
Although D&C may be considered the gold standard of diagnostic sampling techniques, it should be noted that 60% of these procedures sample less than half of the endometrium.6 When used in conjunction with hysteroscopy, the sensitivity in detecting cancer is high at 97% with a specificity of 93%-100%.7
While some patients are candidates for office-based procedures, D&C often requires regional or general anesthesia and is frequently performed in a hospital-based environment or surgical center. This may be most appropriate for patients who have had failed office attempts at sampling, have multiple medical comorbidities that limit the feasibility of office-based procedures (such as morbid obesity), or have severe cervical stenosis. D&C is associated with an increased risk for uterine perforation, compared with outpatient sampling procedures.
The need to go to the operating room rather than to an ambulatory setting also may increase the costs borne by the patient. The advantages of D&C include the potential for large-volume sampling and the potentially therapeutic nature of the procedure in cases of benign pathology.
Office-based procedures
Office-based sampling techniques include those using a pipelle, those employing an endometrial brush, and those guided by saline infusion sonohysterography. If performed in the office, they require minimal or no cervical dilation, are associated with a lower risk of perforation or adverse reaction to anesthesia, and usually have lower costs for patients.
Endometrial pipelle biopsies are a very effective diagnostic tool when there is global, endometrial pathology; they have a sensitivity of 83% in confirming cancer.8 It is an inexpensive and technically straightforward technique that can be easily performed in an office setting.
However, when the endometrial lining is atrophied, alternative tools may provide superior results. Endometrial brushes have been shown to be 33% more successful in collecting adequate samples,compared with pipelles, because they sample a larger endometrial surface area.9
There is ongoing development of sampling techniques, such as endometrial lavage or the combination of saline infusion sonohysterography and endometrial biopsy.10 However, future studies regarding accuracy, cost, and patient acceptability are needed before these techniques are translated to the clinical setting.
Targeted endometrial sampling
Targeted or visually guided sampling, such as hysteroscopy, has been shown to be very accurate in identifying benign pathology, although the sensitivity of hysteroscopic diagnosis of cancer is significantly lower at approximately 50%.11 Therefore, the benefit of hysteroscopy is in complementing the blind nature of D&C by guiding sampling of intracavitary lesions, should they exist.
Hysteroscopy is safe in endometrial cancer and is not associated with upstaging the cancer from transtubal extirpation of malignant cells.12
The addition of hysteroscopy contributes some cost and equipment to the blind D&C procedure; therefore, it might be best applied in cases where there is known intracavitary pathology or inadequate prior sampling. In well-selected patients, hysteroscopy often can be used in an office setting, which improves the practicality of the procedure. Smaller and, in some cases, disposable equipment aids in the feasibility of adding visual guidance to office sampling.
Optimizing sampling
Postmenopausal women have a higher risk for sampling failure, compared with younger women. Obesity also is a risk for failed sampling.13 Cervical ripening with misoprostol may increase access to the endometrial cavity, and ultrasound guidance may decrease the risk of uterine perforation in a stenotic cervix.
Clinicians should ensure that histology results are concordant with clinical data. Discordant results should be reevaluated. For example, if an ultrasound demonstrates a thickened endometrial stripe, but the sampling reveals “scant atrophic tissue,” then there is unexplained pathology to address. Further work-up, such as more comprehensive sampling with hysteroscopy, should be considered in such cases. Additionally, persistent postmenopausal bleeding, despite a benign endometrial biopsy, should be reevaluated over time to rule out occult disease missed during prior sampling.
Clinicians are now equipped with multiple ways of obtaining clinical data, and patients have options that may decrease barriers to their care. Hysteroscopy does not improve upon D&C in the diagnosis of endometrial cancer, although it may be helpful in distinguishing and treating nonmalignant lesions.
Dr. Cotangco is a resident in the department of obstetrics and gynecology at the University of Illinois, Chicago. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Acta Obstet Gynecol Scand. 2004 Feb;83(2):203-7.
2. Menopause Int. 2010 Mar;16(1):5-8.
3. Obstet Gynecol. 2009 Aug;114(2 Pt 1):409-11.
4. Ultrasound Obstet Gynecol. 2004 Oct;24(5):558-65.
5. Ultrasound Obstet Gynecol. 2001 Aug;18(2):157-62.
6. Am J Obstet Gynecol. 2009 Jul;201(1):5-11.
7. Obstet Gynecol Clin North Am. 2000 Jun;27(2):235-44.
8. J Reprod Med. 1995 Aug;40(8):553-5.
9. BJOG. 2008 Jul;115(8):1028-36.
10. PLoS Med. 2016 Dec. doi: 10.1371/journal.pmed.1002206.
11. Arch Gynecol Obstet. 2012 Mar;285(3):839-43.
12. Am J Obstet Gynecol. 2012 Jul;207(1):71.e1-5.
13. Gynecol Oncol. 2017 Feb;144(2):324-8.
Approaching intraoperative bowel injury
Enterotomy can be a serious complication in abdominopelvic surgery, particularly if it is not immediately recognized and treated. Risk of visceral injury increases when complex dissection is required for treatment of cancer, resection of endometriosis, and extensive lysis of adhesions.
In a retrospective review from 1984 to 2003, investigators assessed intestinal injuries at the time of gynecologic operations. Of the 110 cases reported, about 37% occurred during the opening of the peritoneal cavity, 38% during adhesiolysis and pelvic dissection, 9% during laparoscopy, 9% during vaginal surgery, and 8% during dilation and curettage. Of the bowel injuries, more than 75% were minor.1 Mortality from unrecognized bowel injury is significant, and as such, appropriate recognition and management of these injuries is critical.2
Some basic principles are critical when surgeons face a bowel injury:
1. Recognize the extent of the injury, including the size of the breach, the depth (full or partial thickness), and the nature of the injury (thermal or cold).
2. Assess the integrity of the bowel, including adequacy of blood supply, prior bowel damage from radiation, and absence of downstream obstruction.
3. Ensure no other occult injuries exist in other segments.
4. Obtain adequate exposure and mobilization of the bowel beyond the site of injury, including the adjacent bowel. This involves releasing other adhesions so that adequate bowel length is available for a tension-free repair.
Methods of repair
The decision to employ each is influenced by multiple factors. Primary closure is best suited to small lesions (1 cm or less) that are a result of cold or sharp injury. However, thermal injury sustained via electrosurgical devices induces delayed tissue damage beyond the visible edges of the immediate defect, and surgeons should consider a resection of bowel to at least 1 cm beyond the immediately apparent injury site. Additionally, resection and re-anastamosis should also be considered if the damaged segment of bowel has poor blood supply, integrity, or the repair would result in tension along the suture/staple line or luminal narrowing.
Simple small bowel closures
Serosal abrasions need not be repaired; however, small tears of the serosa and muscularis can be managed with a single layer of interrupted 3-0 absorbable or permanent silk suture on a tapered needle. The suture line should be perpendicular to the longitudinal axis of the bowel at 2-mm to 3-mm intervals in order to prevent narrowing of the lumen. The suture should pass through serosal and muscular layers in an imbricating (Lembert) stitch. For smaller defects of less than 6 mm, a single layer closure is typically adequate.
Small bowel resection
Some larger defects, thermal injuries, and segments with multiple enterotomies may be best repaired with resection and re-anastamosis technique. A segment of resectable bowel is chosen such that the afferent and efferent limbs to be re-anastamosed can be reapproximated in a tension-free fashion. A mesenterotomy is made at the proximal and distal portions of the involved bowel. A gastrointestinal anastomotic stapler is then inserted perpendicularly across the bowel. The remaining wedge of connected mesentery can then be efficiently excised with an electrothermal bipolar coagulator device ensuring that maximal mesentery and blood supply are preserved to the remaining limbs of intestine. The proximal and distal segments are then aligned at the antimesenteric sides.
Large bowel repair
Defects in the serosa and small lacerations can be managed with a primary closure, similar to the small intestine. For more extensive injuries that may require resection, diversion, or complicated repair, consultation with a gynecologic oncologist or general or colorectal surgeon may be indicated as colotomy repairs are associated with higher rates of breakdown and fistula. If fecal contamination is present, copious irrigation should be performed and placement of a peritoneal drain to reduce the likelihood of abscess formation should be considered. If appropriate antibiotic prophylaxis for colonic surgery has not been given prior to skin incision, it should be administered once the colotomy is identified.
Standard prophylaxis for hysterectomy (such as a first-generation cephalosporin like cefazolin) is not adequate for large bowel surgery, and either metronidazole should be added or a second-generation cephalosporin such as cefoxitin should be given. For patients with penicillin allergy, clindamycin or vancomycin with either gentamicin or a fluoroquinolone should be administered.6
Postoperative management
The potential for postoperative morbidity must be understood for appropriate management following bowel surgery. Ileus is common and the clinician should understand how to diagnose and manage it. Additionally, intra-abdominal abscess, anastomotic leak, fistula formation, and mechanical obstruction are complications that may require surgical intervention and must be vigilantly managed.
The routine use of postoperative nasogastric tube (NGT) does not hasten return of bowel function or prevent leak from sites of gastrointestinal repair. In fact, early feeding has been associated with reduced perioperative complications and earlier return of bowel function has been observed without the use of NGT.7 In general, for small and large intestinal injuries, early feeding is considered acceptable.8
Prolonged antibiotic prophylaxis, beyond 24 hours, is not recommended.6
Avoiding injury
Gynecologic surgeons should adhere to surgical principles with sharp dissection for adhesions, gentle tissue handling, adequate exposure, and light retraction to prevent bowel injury or minimize their extent. Laparoscopic entry sites should be chosen based on the likelihood of abdominal adhesions. When the patient’s history predicts a high likelihood of intraperitoneal adhesions, the left upper quadrant site should be strongly considered as the entry site. The likelihood of gastrointestinal injury is not influenced by open versus closed laparoscopic entry and surgeons should use the technique with which they have the greatest experience and skill.9 However, in patients who have had prior laparotomies, there is an increased risk of periumbilical adhesions, and consideration should be made for a nonumbilical entry site.10 Methodical sharp dissection and sparing use of thermal energy should be used with adhesiolysis. When injury occurs, prompt recognition, preparation, and methodical management can mitigate the impact.
Dr. Staley is a gynecologic oncology fellow at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
References
1. Int Surg. 2006 Nov-Dec;91(6):336-40.
2. J Am Coll Surg. 2001 Jun;192(6):677-83.
3. Doherty, G. Current Diagnosis and Treatment: Surgery. Thirteenth Edition. New York: McGraw Hill, 2010.
4. Hoffman B. Williams Gynecology. Third Edition. New York: McGraw Hill, 2016.
5. Berek J, Hacker N. Berek & Hacker’s Gynecologic Oncology. Sixth Edition. Philadelphia: Wolters Kluwer, 2015.
6. Surg Infect (Larchmt). 2013 Feb;14(1):73-156.
7. Br J Surg. 2005 Jun;92(6):673-80.
8. Am J Obstet Gynecol. 2001 Jul;185(1):1-4.
9. Cochrane Database Syst Rev. 2015 Aug 31;8:CD006583.
10. Br J Obstet Gynaecol. 1997 May;104(5):595-600.
Enterotomy can be a serious complication in abdominopelvic surgery, particularly if it is not immediately recognized and treated. Risk of visceral injury increases when complex dissection is required for treatment of cancer, resection of endometriosis, and extensive lysis of adhesions.
In a retrospective review from 1984 to 2003, investigators assessed intestinal injuries at the time of gynecologic operations. Of the 110 cases reported, about 37% occurred during the opening of the peritoneal cavity, 38% during adhesiolysis and pelvic dissection, 9% during laparoscopy, 9% during vaginal surgery, and 8% during dilation and curettage. Of the bowel injuries, more than 75% were minor.1 Mortality from unrecognized bowel injury is significant, and as such, appropriate recognition and management of these injuries is critical.2
Some basic principles are critical when surgeons face a bowel injury:
1. Recognize the extent of the injury, including the size of the breach, the depth (full or partial thickness), and the nature of the injury (thermal or cold).
2. Assess the integrity of the bowel, including adequacy of blood supply, prior bowel damage from radiation, and absence of downstream obstruction.
3. Ensure no other occult injuries exist in other segments.
4. Obtain adequate exposure and mobilization of the bowel beyond the site of injury, including the adjacent bowel. This involves releasing other adhesions so that adequate bowel length is available for a tension-free repair.
Methods of repair
The decision to employ each is influenced by multiple factors. Primary closure is best suited to small lesions (1 cm or less) that are a result of cold or sharp injury. However, thermal injury sustained via electrosurgical devices induces delayed tissue damage beyond the visible edges of the immediate defect, and surgeons should consider a resection of bowel to at least 1 cm beyond the immediately apparent injury site. Additionally, resection and re-anastamosis should also be considered if the damaged segment of bowel has poor blood supply, integrity, or the repair would result in tension along the suture/staple line or luminal narrowing.
Simple small bowel closures
Serosal abrasions need not be repaired; however, small tears of the serosa and muscularis can be managed with a single layer of interrupted 3-0 absorbable or permanent silk suture on a tapered needle. The suture line should be perpendicular to the longitudinal axis of the bowel at 2-mm to 3-mm intervals in order to prevent narrowing of the lumen. The suture should pass through serosal and muscular layers in an imbricating (Lembert) stitch. For smaller defects of less than 6 mm, a single layer closure is typically adequate.
Small bowel resection
Some larger defects, thermal injuries, and segments with multiple enterotomies may be best repaired with resection and re-anastamosis technique. A segment of resectable bowel is chosen such that the afferent and efferent limbs to be re-anastamosed can be reapproximated in a tension-free fashion. A mesenterotomy is made at the proximal and distal portions of the involved bowel. A gastrointestinal anastomotic stapler is then inserted perpendicularly across the bowel. The remaining wedge of connected mesentery can then be efficiently excised with an electrothermal bipolar coagulator device ensuring that maximal mesentery and blood supply are preserved to the remaining limbs of intestine. The proximal and distal segments are then aligned at the antimesenteric sides.
Large bowel repair
Defects in the serosa and small lacerations can be managed with a primary closure, similar to the small intestine. For more extensive injuries that may require resection, diversion, or complicated repair, consultation with a gynecologic oncologist or general or colorectal surgeon may be indicated as colotomy repairs are associated with higher rates of breakdown and fistula. If fecal contamination is present, copious irrigation should be performed and placement of a peritoneal drain to reduce the likelihood of abscess formation should be considered. If appropriate antibiotic prophylaxis for colonic surgery has not been given prior to skin incision, it should be administered once the colotomy is identified.
Standard prophylaxis for hysterectomy (such as a first-generation cephalosporin like cefazolin) is not adequate for large bowel surgery, and either metronidazole should be added or a second-generation cephalosporin such as cefoxitin should be given. For patients with penicillin allergy, clindamycin or vancomycin with either gentamicin or a fluoroquinolone should be administered.6
Postoperative management
The potential for postoperative morbidity must be understood for appropriate management following bowel surgery. Ileus is common and the clinician should understand how to diagnose and manage it. Additionally, intra-abdominal abscess, anastomotic leak, fistula formation, and mechanical obstruction are complications that may require surgical intervention and must be vigilantly managed.
The routine use of postoperative nasogastric tube (NGT) does not hasten return of bowel function or prevent leak from sites of gastrointestinal repair. In fact, early feeding has been associated with reduced perioperative complications and earlier return of bowel function has been observed without the use of NGT.7 In general, for small and large intestinal injuries, early feeding is considered acceptable.8
Prolonged antibiotic prophylaxis, beyond 24 hours, is not recommended.6
Avoiding injury
Gynecologic surgeons should adhere to surgical principles with sharp dissection for adhesions, gentle tissue handling, adequate exposure, and light retraction to prevent bowel injury or minimize their extent. Laparoscopic entry sites should be chosen based on the likelihood of abdominal adhesions. When the patient’s history predicts a high likelihood of intraperitoneal adhesions, the left upper quadrant site should be strongly considered as the entry site. The likelihood of gastrointestinal injury is not influenced by open versus closed laparoscopic entry and surgeons should use the technique with which they have the greatest experience and skill.9 However, in patients who have had prior laparotomies, there is an increased risk of periumbilical adhesions, and consideration should be made for a nonumbilical entry site.10 Methodical sharp dissection and sparing use of thermal energy should be used with adhesiolysis. When injury occurs, prompt recognition, preparation, and methodical management can mitigate the impact.
Dr. Staley is a gynecologic oncology fellow at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
References
1. Int Surg. 2006 Nov-Dec;91(6):336-40.
2. J Am Coll Surg. 2001 Jun;192(6):677-83.
3. Doherty, G. Current Diagnosis and Treatment: Surgery. Thirteenth Edition. New York: McGraw Hill, 2010.
4. Hoffman B. Williams Gynecology. Third Edition. New York: McGraw Hill, 2016.
5. Berek J, Hacker N. Berek & Hacker’s Gynecologic Oncology. Sixth Edition. Philadelphia: Wolters Kluwer, 2015.
6. Surg Infect (Larchmt). 2013 Feb;14(1):73-156.
7. Br J Surg. 2005 Jun;92(6):673-80.
8. Am J Obstet Gynecol. 2001 Jul;185(1):1-4.
9. Cochrane Database Syst Rev. 2015 Aug 31;8:CD006583.
10. Br J Obstet Gynaecol. 1997 May;104(5):595-600.
Enterotomy can be a serious complication in abdominopelvic surgery, particularly if it is not immediately recognized and treated. Risk of visceral injury increases when complex dissection is required for treatment of cancer, resection of endometriosis, and extensive lysis of adhesions.
In a retrospective review from 1984 to 2003, investigators assessed intestinal injuries at the time of gynecologic operations. Of the 110 cases reported, about 37% occurred during the opening of the peritoneal cavity, 38% during adhesiolysis and pelvic dissection, 9% during laparoscopy, 9% during vaginal surgery, and 8% during dilation and curettage. Of the bowel injuries, more than 75% were minor.1 Mortality from unrecognized bowel injury is significant, and as such, appropriate recognition and management of these injuries is critical.2
Some basic principles are critical when surgeons face a bowel injury:
1. Recognize the extent of the injury, including the size of the breach, the depth (full or partial thickness), and the nature of the injury (thermal or cold).
2. Assess the integrity of the bowel, including adequacy of blood supply, prior bowel damage from radiation, and absence of downstream obstruction.
3. Ensure no other occult injuries exist in other segments.
4. Obtain adequate exposure and mobilization of the bowel beyond the site of injury, including the adjacent bowel. This involves releasing other adhesions so that adequate bowel length is available for a tension-free repair.
Methods of repair
The decision to employ each is influenced by multiple factors. Primary closure is best suited to small lesions (1 cm or less) that are a result of cold or sharp injury. However, thermal injury sustained via electrosurgical devices induces delayed tissue damage beyond the visible edges of the immediate defect, and surgeons should consider a resection of bowel to at least 1 cm beyond the immediately apparent injury site. Additionally, resection and re-anastamosis should also be considered if the damaged segment of bowel has poor blood supply, integrity, or the repair would result in tension along the suture/staple line or luminal narrowing.
Simple small bowel closures
Serosal abrasions need not be repaired; however, small tears of the serosa and muscularis can be managed with a single layer of interrupted 3-0 absorbable or permanent silk suture on a tapered needle. The suture line should be perpendicular to the longitudinal axis of the bowel at 2-mm to 3-mm intervals in order to prevent narrowing of the lumen. The suture should pass through serosal and muscular layers in an imbricating (Lembert) stitch. For smaller defects of less than 6 mm, a single layer closure is typically adequate.
Small bowel resection
Some larger defects, thermal injuries, and segments with multiple enterotomies may be best repaired with resection and re-anastamosis technique. A segment of resectable bowel is chosen such that the afferent and efferent limbs to be re-anastamosed can be reapproximated in a tension-free fashion. A mesenterotomy is made at the proximal and distal portions of the involved bowel. A gastrointestinal anastomotic stapler is then inserted perpendicularly across the bowel. The remaining wedge of connected mesentery can then be efficiently excised with an electrothermal bipolar coagulator device ensuring that maximal mesentery and blood supply are preserved to the remaining limbs of intestine. The proximal and distal segments are then aligned at the antimesenteric sides.
Large bowel repair
Defects in the serosa and small lacerations can be managed with a primary closure, similar to the small intestine. For more extensive injuries that may require resection, diversion, or complicated repair, consultation with a gynecologic oncologist or general or colorectal surgeon may be indicated as colotomy repairs are associated with higher rates of breakdown and fistula. If fecal contamination is present, copious irrigation should be performed and placement of a peritoneal drain to reduce the likelihood of abscess formation should be considered. If appropriate antibiotic prophylaxis for colonic surgery has not been given prior to skin incision, it should be administered once the colotomy is identified.
Standard prophylaxis for hysterectomy (such as a first-generation cephalosporin like cefazolin) is not adequate for large bowel surgery, and either metronidazole should be added or a second-generation cephalosporin such as cefoxitin should be given. For patients with penicillin allergy, clindamycin or vancomycin with either gentamicin or a fluoroquinolone should be administered.6
Postoperative management
The potential for postoperative morbidity must be understood for appropriate management following bowel surgery. Ileus is common and the clinician should understand how to diagnose and manage it. Additionally, intra-abdominal abscess, anastomotic leak, fistula formation, and mechanical obstruction are complications that may require surgical intervention and must be vigilantly managed.
The routine use of postoperative nasogastric tube (NGT) does not hasten return of bowel function or prevent leak from sites of gastrointestinal repair. In fact, early feeding has been associated with reduced perioperative complications and earlier return of bowel function has been observed without the use of NGT.7 In general, for small and large intestinal injuries, early feeding is considered acceptable.8
Prolonged antibiotic prophylaxis, beyond 24 hours, is not recommended.6
Avoiding injury
Gynecologic surgeons should adhere to surgical principles with sharp dissection for adhesions, gentle tissue handling, adequate exposure, and light retraction to prevent bowel injury or minimize their extent. Laparoscopic entry sites should be chosen based on the likelihood of abdominal adhesions. When the patient’s history predicts a high likelihood of intraperitoneal adhesions, the left upper quadrant site should be strongly considered as the entry site. The likelihood of gastrointestinal injury is not influenced by open versus closed laparoscopic entry and surgeons should use the technique with which they have the greatest experience and skill.9 However, in patients who have had prior laparotomies, there is an increased risk of periumbilical adhesions, and consideration should be made for a nonumbilical entry site.10 Methodical sharp dissection and sparing use of thermal energy should be used with adhesiolysis. When injury occurs, prompt recognition, preparation, and methodical management can mitigate the impact.
Dr. Staley is a gynecologic oncology fellow at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
References
1. Int Surg. 2006 Nov-Dec;91(6):336-40.
2. J Am Coll Surg. 2001 Jun;192(6):677-83.
3. Doherty, G. Current Diagnosis and Treatment: Surgery. Thirteenth Edition. New York: McGraw Hill, 2010.
4. Hoffman B. Williams Gynecology. Third Edition. New York: McGraw Hill, 2016.
5. Berek J, Hacker N. Berek & Hacker’s Gynecologic Oncology. Sixth Edition. Philadelphia: Wolters Kluwer, 2015.
6. Surg Infect (Larchmt). 2013 Feb;14(1):73-156.
7. Br J Surg. 2005 Jun;92(6):673-80.
8. Am J Obstet Gynecol. 2001 Jul;185(1):1-4.
9. Cochrane Database Syst Rev. 2015 Aug 31;8:CD006583.
10. Br J Obstet Gynaecol. 1997 May;104(5):595-600.
Low malignant potential tumors of the ovary: A review
Low malignant potential tumors of the ovary – otherwise known as borderline tumors – include ovarian tumors with atypical cellularity, which lack stromal invasion that differentiates them from low grade and high grade invasive carcinomas. They can coexist with extraovarian disease, however, in the setting of borderline tumors these foci are referred to as “implants” rather than metastases. As discussed below, these implants can exhibit the presence of invasion or not.
Classification
The two most common histologic categories of borderline tumors are serous and mucinous cell types. Rarer histologic types such as endometrioid, clear cell, and Brenner also exist. However, these are so infrequent that they will not be covered in this discussion as there are very limited data to make generalizations about these histologies.
Serous borderline tumors contain cellularity similar to that of fallopian tubal epithelium. Approximately 25% of all serous ovarian tumors exhibit borderline features. Compared with mucinous tumors, they are more commonly bilateral and smaller in size (mean size of 12 cm) at the time of diagnosis and they are more likely to be associated with extraovarian implants (typically peritoneal). In fact, up to 25% of serous borderline tumors have concomitant extraovarian implants. Cancer antigen (CA) 125 is commonly a tumor marker for these tumors (elevated in 45% of early stage disease and 80% of advanced stage disease).1
Incidence
The incidence of borderline ovarian tumors is 2.5 per 100,000 woman years in the United States. About 70% are diagnosed at stage I.3 They arise in a younger population compared with invasive ovarian carcinomas. Risk factors for development of borderline tumors are similar to those of invasive ovarian carcinomas (such as nulliparity) but there may be a stronger association between the development of borderline ovarian tumors and infertility, as well as prior use of infertility treatment.4
Diagnosis
The diagnosis of borderline tumors of the ovaries occurs almost exclusively at the time of surgical pathology (either frozen section or definitive pathology).
Preoperative assessments with imaging and tumor markers – usually CA 125 and carcinoembryonic antigen (CEA) – are nonspecific for this tumor type. Preoperative imaging will typically reveal complex ovarian cysts with papillations and vascularity. However, in the case of mucinous borderline tumors, unilocular cysts are common.1 The presence of ascites and peritoneal implants can be observed on preoperative imaging of serous borderline tumors with extraovarian disease. However, it is not possible for this imaging to accurately differentiate borderline tumors with implants from low grade and high grade carcinomas with metastases.
Surgical management
Borderline tumors are commonly diagnosed in women of reproductive age and decisions need to be made regarding fertility sparing surgery, ovarian sparing surgery, and whether staging is performed. The recommended surgery for women who have completed child bearing is complete hysterectomy with bilateral salpingo-oophorectomy. However, cystectomy or unilateral salpingo-oophorectomy can be considered for women who desire fertility preservation. Conservative fertility preserving surgery is associated with an increased risk of recurrence, but with no negative impact on survival.1
Staging – with at least omentectomy and comprehensive evaluation of the peritoneal cavity, with or without peritoneal biopsies – can be considered, though staging is not associated with improved survival. Lymphadenectomy is also not associated with improved oncologic outcomes and routine lymphadenectomy is not recommended for borderline tumors.1 However, about a quarter of patients with gross evidence of extraovarian disease have implants within lymph nodes. Bulky lymph nodes should be removed, particularly in this group of patients.
Complete removal of extraovarian implants is the surgical intervention that is most important for survival and recurrence.1 This requires that surgeons thoroughly evaluate the peritoneal cavity and retroperitoneum, and possess the capability to completely resect all sites of disease.
Historically appendectomy was part of surgical staging of mucinous borderline tumors in order to identify a primary appendiceal lesion, but only 1% of patients with a grossly normal appearing appendix have significant pathology identified. This is no longer recommended.2
Treatment
The primary treatment for borderline tumors of the ovary is surgery. A minimally invasive approach is appropriate when feasible, though it may be associated with an increased risk of cyst rupture, particularly if cystectomy is attempted. Outcomes are best when extraovarian implants are completely resected. Adjuvant chemotherapy is not associated with improved survival and is not routinely recommended, though the guidelines from the National Comprehensive Cancer Network include this as an option for patients with advanced stage disease that is either completely or incompletely resected.5
Prognosis
In general, prognosis is excellent for borderline tumors with 5- and 10-year survival of 99% and 97%, 98% and 90%, and 96% and 88% for stages I, II and III tumors, respectively.1 However, several pathologic, molecular, and anatomic features are important in predicting who is at highest risk for recurrence.
Serous borderline tumors with invasive implants (as opposed to desmoplastic implants) and incompletely resected extraovarian implants are associated with increased recurrence and poor prognosis.Micropapillary features and stromal invasion are histologic features that have historically been associated with worse prognosis, but it is unclear if these are independent risk factors, or instead associated with invasive implants. For mucinous borderline tumors, intraepithelial carcinoma has been inconclusively associated with poor prognosis.1,6
Surveillance
Recurrences do occur in patients with a history of borderline tumors of the ovary, however these typically occur late. For this reason, surveillance is important and should continue for many years after diagnosis. Most recurrences are within the peritoneal cavity and are treated with surgical excision and patients should be counseled regarding symptoms of recurrence that include gastrointestinal symptoms, bloating, and pain.
In accordance with guidelines from the Society of Gynecologic Oncology, surveillance examinations can take place annually as there is no evidence that more frequent evaluations improve outcomes. These visits should include physical examinations (with pelvic examinations), symptom assessment, and, if elevated preoperatively, assessment of relevant tumor markers (typically CA 125 and/or CEA).7 Surveillance should continue for at least 10 years postoperatively.
Routine imaging is not recommended for all patients in surveillance. However, for patients who have had fertility-sparing surgery, imaging with pelvic ultrasound is recommended, particularly for women with a history of cystectomy or serous borderline tumor (who are at increased risk for bilateral tumors).
Prognosis is most closely associated with the presence of invasive implants and residual disease following surgery. Surgeons who manage these tumors can safely consider fertility-sparing procedures but should be equipped to completely resect all gross disease.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Lancet Oncol. 2012 Mar;13(3):e103-15.
2. Arch Gynecol Obstet. 2016 Nov;294(6):1283-9.
3. Cancer. 2002 Dec 1;95(11):2380-9.
4. Am J Epidemiol. 2002 Feb 1;155(3):217-24.
5. J Natl Compr Canc Netw. 2016 Sep;14(9):1134-63.
6. BJOG. 2016 Mar;123(4):498-508.
7. Gynecol Oncol. 2017 Jul;146(1):3-10.
Low malignant potential tumors of the ovary – otherwise known as borderline tumors – include ovarian tumors with atypical cellularity, which lack stromal invasion that differentiates them from low grade and high grade invasive carcinomas. They can coexist with extraovarian disease, however, in the setting of borderline tumors these foci are referred to as “implants” rather than metastases. As discussed below, these implants can exhibit the presence of invasion or not.
Classification
The two most common histologic categories of borderline tumors are serous and mucinous cell types. Rarer histologic types such as endometrioid, clear cell, and Brenner also exist. However, these are so infrequent that they will not be covered in this discussion as there are very limited data to make generalizations about these histologies.
Serous borderline tumors contain cellularity similar to that of fallopian tubal epithelium. Approximately 25% of all serous ovarian tumors exhibit borderline features. Compared with mucinous tumors, they are more commonly bilateral and smaller in size (mean size of 12 cm) at the time of diagnosis and they are more likely to be associated with extraovarian implants (typically peritoneal). In fact, up to 25% of serous borderline tumors have concomitant extraovarian implants. Cancer antigen (CA) 125 is commonly a tumor marker for these tumors (elevated in 45% of early stage disease and 80% of advanced stage disease).1
Incidence
The incidence of borderline ovarian tumors is 2.5 per 100,000 woman years in the United States. About 70% are diagnosed at stage I.3 They arise in a younger population compared with invasive ovarian carcinomas. Risk factors for development of borderline tumors are similar to those of invasive ovarian carcinomas (such as nulliparity) but there may be a stronger association between the development of borderline ovarian tumors and infertility, as well as prior use of infertility treatment.4
Diagnosis
The diagnosis of borderline tumors of the ovaries occurs almost exclusively at the time of surgical pathology (either frozen section or definitive pathology).
Preoperative assessments with imaging and tumor markers – usually CA 125 and carcinoembryonic antigen (CEA) – are nonspecific for this tumor type. Preoperative imaging will typically reveal complex ovarian cysts with papillations and vascularity. However, in the case of mucinous borderline tumors, unilocular cysts are common.1 The presence of ascites and peritoneal implants can be observed on preoperative imaging of serous borderline tumors with extraovarian disease. However, it is not possible for this imaging to accurately differentiate borderline tumors with implants from low grade and high grade carcinomas with metastases.
Surgical management
Borderline tumors are commonly diagnosed in women of reproductive age and decisions need to be made regarding fertility sparing surgery, ovarian sparing surgery, and whether staging is performed. The recommended surgery for women who have completed child bearing is complete hysterectomy with bilateral salpingo-oophorectomy. However, cystectomy or unilateral salpingo-oophorectomy can be considered for women who desire fertility preservation. Conservative fertility preserving surgery is associated with an increased risk of recurrence, but with no negative impact on survival.1
Staging – with at least omentectomy and comprehensive evaluation of the peritoneal cavity, with or without peritoneal biopsies – can be considered, though staging is not associated with improved survival. Lymphadenectomy is also not associated with improved oncologic outcomes and routine lymphadenectomy is not recommended for borderline tumors.1 However, about a quarter of patients with gross evidence of extraovarian disease have implants within lymph nodes. Bulky lymph nodes should be removed, particularly in this group of patients.
Complete removal of extraovarian implants is the surgical intervention that is most important for survival and recurrence.1 This requires that surgeons thoroughly evaluate the peritoneal cavity and retroperitoneum, and possess the capability to completely resect all sites of disease.
Historically appendectomy was part of surgical staging of mucinous borderline tumors in order to identify a primary appendiceal lesion, but only 1% of patients with a grossly normal appearing appendix have significant pathology identified. This is no longer recommended.2
Treatment
The primary treatment for borderline tumors of the ovary is surgery. A minimally invasive approach is appropriate when feasible, though it may be associated with an increased risk of cyst rupture, particularly if cystectomy is attempted. Outcomes are best when extraovarian implants are completely resected. Adjuvant chemotherapy is not associated with improved survival and is not routinely recommended, though the guidelines from the National Comprehensive Cancer Network include this as an option for patients with advanced stage disease that is either completely or incompletely resected.5
Prognosis
In general, prognosis is excellent for borderline tumors with 5- and 10-year survival of 99% and 97%, 98% and 90%, and 96% and 88% for stages I, II and III tumors, respectively.1 However, several pathologic, molecular, and anatomic features are important in predicting who is at highest risk for recurrence.
Serous borderline tumors with invasive implants (as opposed to desmoplastic implants) and incompletely resected extraovarian implants are associated with increased recurrence and poor prognosis.Micropapillary features and stromal invasion are histologic features that have historically been associated with worse prognosis, but it is unclear if these are independent risk factors, or instead associated with invasive implants. For mucinous borderline tumors, intraepithelial carcinoma has been inconclusively associated with poor prognosis.1,6
Surveillance
Recurrences do occur in patients with a history of borderline tumors of the ovary, however these typically occur late. For this reason, surveillance is important and should continue for many years after diagnosis. Most recurrences are within the peritoneal cavity and are treated with surgical excision and patients should be counseled regarding symptoms of recurrence that include gastrointestinal symptoms, bloating, and pain.
In accordance with guidelines from the Society of Gynecologic Oncology, surveillance examinations can take place annually as there is no evidence that more frequent evaluations improve outcomes. These visits should include physical examinations (with pelvic examinations), symptom assessment, and, if elevated preoperatively, assessment of relevant tumor markers (typically CA 125 and/or CEA).7 Surveillance should continue for at least 10 years postoperatively.
Routine imaging is not recommended for all patients in surveillance. However, for patients who have had fertility-sparing surgery, imaging with pelvic ultrasound is recommended, particularly for women with a history of cystectomy or serous borderline tumor (who are at increased risk for bilateral tumors).
Prognosis is most closely associated with the presence of invasive implants and residual disease following surgery. Surgeons who manage these tumors can safely consider fertility-sparing procedures but should be equipped to completely resect all gross disease.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Lancet Oncol. 2012 Mar;13(3):e103-15.
2. Arch Gynecol Obstet. 2016 Nov;294(6):1283-9.
3. Cancer. 2002 Dec 1;95(11):2380-9.
4. Am J Epidemiol. 2002 Feb 1;155(3):217-24.
5. J Natl Compr Canc Netw. 2016 Sep;14(9):1134-63.
6. BJOG. 2016 Mar;123(4):498-508.
7. Gynecol Oncol. 2017 Jul;146(1):3-10.
Low malignant potential tumors of the ovary – otherwise known as borderline tumors – include ovarian tumors with atypical cellularity, which lack stromal invasion that differentiates them from low grade and high grade invasive carcinomas. They can coexist with extraovarian disease, however, in the setting of borderline tumors these foci are referred to as “implants” rather than metastases. As discussed below, these implants can exhibit the presence of invasion or not.
Classification
The two most common histologic categories of borderline tumors are serous and mucinous cell types. Rarer histologic types such as endometrioid, clear cell, and Brenner also exist. However, these are so infrequent that they will not be covered in this discussion as there are very limited data to make generalizations about these histologies.
Serous borderline tumors contain cellularity similar to that of fallopian tubal epithelium. Approximately 25% of all serous ovarian tumors exhibit borderline features. Compared with mucinous tumors, they are more commonly bilateral and smaller in size (mean size of 12 cm) at the time of diagnosis and they are more likely to be associated with extraovarian implants (typically peritoneal). In fact, up to 25% of serous borderline tumors have concomitant extraovarian implants. Cancer antigen (CA) 125 is commonly a tumor marker for these tumors (elevated in 45% of early stage disease and 80% of advanced stage disease).1
Incidence
The incidence of borderline ovarian tumors is 2.5 per 100,000 woman years in the United States. About 70% are diagnosed at stage I.3 They arise in a younger population compared with invasive ovarian carcinomas. Risk factors for development of borderline tumors are similar to those of invasive ovarian carcinomas (such as nulliparity) but there may be a stronger association between the development of borderline ovarian tumors and infertility, as well as prior use of infertility treatment.4
Diagnosis
The diagnosis of borderline tumors of the ovaries occurs almost exclusively at the time of surgical pathology (either frozen section or definitive pathology).
Preoperative assessments with imaging and tumor markers – usually CA 125 and carcinoembryonic antigen (CEA) – are nonspecific for this tumor type. Preoperative imaging will typically reveal complex ovarian cysts with papillations and vascularity. However, in the case of mucinous borderline tumors, unilocular cysts are common.1 The presence of ascites and peritoneal implants can be observed on preoperative imaging of serous borderline tumors with extraovarian disease. However, it is not possible for this imaging to accurately differentiate borderline tumors with implants from low grade and high grade carcinomas with metastases.
Surgical management
Borderline tumors are commonly diagnosed in women of reproductive age and decisions need to be made regarding fertility sparing surgery, ovarian sparing surgery, and whether staging is performed. The recommended surgery for women who have completed child bearing is complete hysterectomy with bilateral salpingo-oophorectomy. However, cystectomy or unilateral salpingo-oophorectomy can be considered for women who desire fertility preservation. Conservative fertility preserving surgery is associated with an increased risk of recurrence, but with no negative impact on survival.1
Staging – with at least omentectomy and comprehensive evaluation of the peritoneal cavity, with or without peritoneal biopsies – can be considered, though staging is not associated with improved survival. Lymphadenectomy is also not associated with improved oncologic outcomes and routine lymphadenectomy is not recommended for borderline tumors.1 However, about a quarter of patients with gross evidence of extraovarian disease have implants within lymph nodes. Bulky lymph nodes should be removed, particularly in this group of patients.
Complete removal of extraovarian implants is the surgical intervention that is most important for survival and recurrence.1 This requires that surgeons thoroughly evaluate the peritoneal cavity and retroperitoneum, and possess the capability to completely resect all sites of disease.
Historically appendectomy was part of surgical staging of mucinous borderline tumors in order to identify a primary appendiceal lesion, but only 1% of patients with a grossly normal appearing appendix have significant pathology identified. This is no longer recommended.2
Treatment
The primary treatment for borderline tumors of the ovary is surgery. A minimally invasive approach is appropriate when feasible, though it may be associated with an increased risk of cyst rupture, particularly if cystectomy is attempted. Outcomes are best when extraovarian implants are completely resected. Adjuvant chemotherapy is not associated with improved survival and is not routinely recommended, though the guidelines from the National Comprehensive Cancer Network include this as an option for patients with advanced stage disease that is either completely or incompletely resected.5
Prognosis
In general, prognosis is excellent for borderline tumors with 5- and 10-year survival of 99% and 97%, 98% and 90%, and 96% and 88% for stages I, II and III tumors, respectively.1 However, several pathologic, molecular, and anatomic features are important in predicting who is at highest risk for recurrence.
Serous borderline tumors with invasive implants (as opposed to desmoplastic implants) and incompletely resected extraovarian implants are associated with increased recurrence and poor prognosis.Micropapillary features and stromal invasion are histologic features that have historically been associated with worse prognosis, but it is unclear if these are independent risk factors, or instead associated with invasive implants. For mucinous borderline tumors, intraepithelial carcinoma has been inconclusively associated with poor prognosis.1,6
Surveillance
Recurrences do occur in patients with a history of borderline tumors of the ovary, however these typically occur late. For this reason, surveillance is important and should continue for many years after diagnosis. Most recurrences are within the peritoneal cavity and are treated with surgical excision and patients should be counseled regarding symptoms of recurrence that include gastrointestinal symptoms, bloating, and pain.
In accordance with guidelines from the Society of Gynecologic Oncology, surveillance examinations can take place annually as there is no evidence that more frequent evaluations improve outcomes. These visits should include physical examinations (with pelvic examinations), symptom assessment, and, if elevated preoperatively, assessment of relevant tumor markers (typically CA 125 and/or CEA).7 Surveillance should continue for at least 10 years postoperatively.
Routine imaging is not recommended for all patients in surveillance. However, for patients who have had fertility-sparing surgery, imaging with pelvic ultrasound is recommended, particularly for women with a history of cystectomy or serous borderline tumor (who are at increased risk for bilateral tumors).
Prognosis is most closely associated with the presence of invasive implants and residual disease following surgery. Surgeons who manage these tumors can safely consider fertility-sparing procedures but should be equipped to completely resect all gross disease.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Lancet Oncol. 2012 Mar;13(3):e103-15.
2. Arch Gynecol Obstet. 2016 Nov;294(6):1283-9.
3. Cancer. 2002 Dec 1;95(11):2380-9.
4. Am J Epidemiol. 2002 Feb 1;155(3):217-24.
5. J Natl Compr Canc Netw. 2016 Sep;14(9):1134-63.
6. BJOG. 2016 Mar;123(4):498-508.
7. Gynecol Oncol. 2017 Jul;146(1):3-10.
Optimizing HPV vaccination
Human papillomavirus (HPV) is the most common sexually transmitted infection. Exposure is widespread and most individuals clear the infection without symptoms or development of disease. However, a subset of individuals experience persistent infection, a state which can lead to carcinogenesis of lower genital tract malignancies, particularly cervical cancer.1
Vaccine coverage
Persistent infection with high-risk (oncogenic) HPV is well known to be the cause of cervical cancer. There are two HPV vaccines manufactured for the purposes of cervical cancer, anal cancer, and genital wart prevention (Cervarix and Gardasil). The Cervarix vaccine covers high-risk HPV subtypes 16 and 18 and the Gardasil vaccine prevents both low-risk HPV subtypes 6 and 11, which can cause genital warts, and high-risk HPV subtypes 16, 18, 31, 33, 45, 52 and 58, which cause cervical dysplasia and cancer.
High-risk HPV is also associated with head and neck, vulvar, vaginal, and penile cancers, though the vaccines are not approved by the Food and Drug Administration for prevention of these diseases.2
Vaccination indications
Since vaccination prevents multiple subtypes of HPV, an individual who has already been exposed will still benefit from protection from other subtypes of HPV through vaccination. HPV vaccination is not approved during pregnancy but can be initiated in the postpartum period when women are engaged in their health care and receiving other vaccinations, such as varicella or the MMR vaccine.
Recommended schedule
Until October 2016, the vaccination schedule was based on a three-dose series (0, 2, and 6 months). Currently, the CDC recommends that children aged under 15 years at the time of first dose may opt for a two-dose series (0 and 6-12 months). For those aged 15-26 years, the three-dose schedule remains the recommended course.
The benefits of two-dose schedule are convenience, cost, and increased likelihood of completion. Data presented at the 2017 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer showed that rates of cervical dysplasia were equivalent for women who completed a two-dose schedule versus a three-dose schedule.4
Efficacy
A recent meta-analysis of clinical trials of the HPV vaccines describe efficacy of 95%-97% in prevention of CIN 1-3.5 While its greatest efficacy is in its ability to prevent primary HPV infection, there still is some benefit for individuals who already were exposed to HPV prior to vaccination. As stated previously, women with a history of prior HPV vaccination have lower rates of recurrence of cervical dysplasia after treatment. Additionally, recent research has shown that women who received HPV vaccinations after a LEEP procedure for CIN 2 or 3 experience significantly lower recurrence rates, compared with women who did not receive vaccinations after LEEP (2.5% vs. 8.5%).6 This raises the possibility of a therapeutic role for HPV vaccination in women infected with HPV. Prospective studies are currently evaluating this question.
Myths
The most common side effects of the HPV vaccine are pain, redness, or swelling at the injection site. Other known side effects include fever, headache or malaise, nausea, syncope, or muscle/joint pain – similar to other vaccinations. Anaphylaxis is a rare complication.
Some parents and pediatricians report concerns that vaccination could lead to earlier sexual activity. Multiple studies have shown that girls who receive HPV vaccination are no more likely to become pregnant or get a sexually transmitted infection (proxies for intercourse) than are girls who were not vaccinated.7,8
Maximizing vaccination rates
HPV vaccination rates in the United States lag significantly behind rates in countries with national vaccine programs, such as Australia and Denmark.9 Early data from Australia already have shown a decrease in genital warts and CIN 2+ incidence within the 10 years of starting its school-based vaccine program, with approximately 73% of 12- to 15-year-olds having completed the vaccine series.2 In contrast, just 40% and 22% of 13- to 17-year-old girls and boys in the United States, respectively, had completed the vaccine series in 2014, according to the CDC.10
Vaccination gaps between girls and boys are narrowing, and more teens will be able to complete the series with the new two-dose recommendation for those younger than 15 years. However, our current rates of vaccination are significantly lower for HPV than for other routinely recommended adolescent vaccines (such as Tdap and meningococcal) and more must be done to encourage vaccination.
Studies have shown that parents are more likely to vaccinate their children if providers recommend the vaccine.11 As women’s health care providers, we do not always see children during the time period that is ideal for vaccination. However, we take care of many women who are presenting for routine gynecologic care, pregnancy, or with abnormal Pap smear screenings. These are ideal opportunities to educate and offer HPV vaccination to women in the approved age groups, as well as to encourage parents to vaccinate their children.
As with other vaccines, the recommendation should be clear and focused on the cancer prevention benefit. Using methods in which the recommendation is “announced” in a brief statement assuming parents/patients are ready to vaccinate versus open-ended conversations, has been studied as a potentially successful method to increase uptake of HPV vaccination.12 Additionally, documentation of HPV vaccination status should be built into electronic medical record templates to prompt clinicians to ask and offer HPV vaccination at visits, including postpartum visits.
Dr. Rahangdale is an associate professor of ob.gyn. at the University of North Carolina, Chapel Hill, and is director of the North Carolina Women’s Hospital Cervical Dysplasia Clinic. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Epidemiol. 2008 Jul 15;168(2):123-37.
2. Int J Cancer. 2012 Nov 1;131(9):1969-82.
3. BMJ. 2012 Mar 27;344:e1401. doi: 10.1136/bmj.e1401.
4. Gynecol Oncol. 2017 Jun. doi. org/10.1016/j.ygyno.2017.03.031.
5. Int J Prev Med. 2017 Jun 1;8:44. doi: 10.4103/ijpvm.IJPVM_413_16.
6. Gynecol Oncol. 2013 Aug;130(2):264-8.
7. Pediatrics. 2012 Nov;130(5):798-805.
8. JAMA Intern Med. 2015 Apr;175(4):617-23.
9. Clin Pediatr (Phila). 2016 Sep;55(10):904-14.
10. MMWR Morb Mortal Wkly Rep. 2015 Jul 31;64(29):784-92.
11. Vaccine. 2016 Feb 24;34(9):1187-92.
12. Pediatrics. 2017 Jan;139(1). pii:e20161764. doi: 10.1542/peds.2016-1764.
Human papillomavirus (HPV) is the most common sexually transmitted infection. Exposure is widespread and most individuals clear the infection without symptoms or development of disease. However, a subset of individuals experience persistent infection, a state which can lead to carcinogenesis of lower genital tract malignancies, particularly cervical cancer.1
Vaccine coverage
Persistent infection with high-risk (oncogenic) HPV is well known to be the cause of cervical cancer. There are two HPV vaccines manufactured for the purposes of cervical cancer, anal cancer, and genital wart prevention (Cervarix and Gardasil). The Cervarix vaccine covers high-risk HPV subtypes 16 and 18 and the Gardasil vaccine prevents both low-risk HPV subtypes 6 and 11, which can cause genital warts, and high-risk HPV subtypes 16, 18, 31, 33, 45, 52 and 58, which cause cervical dysplasia and cancer.
High-risk HPV is also associated with head and neck, vulvar, vaginal, and penile cancers, though the vaccines are not approved by the Food and Drug Administration for prevention of these diseases.2
Vaccination indications
Since vaccination prevents multiple subtypes of HPV, an individual who has already been exposed will still benefit from protection from other subtypes of HPV through vaccination. HPV vaccination is not approved during pregnancy but can be initiated in the postpartum period when women are engaged in their health care and receiving other vaccinations, such as varicella or the MMR vaccine.
Recommended schedule
Until October 2016, the vaccination schedule was based on a three-dose series (0, 2, and 6 months). Currently, the CDC recommends that children aged under 15 years at the time of first dose may opt for a two-dose series (0 and 6-12 months). For those aged 15-26 years, the three-dose schedule remains the recommended course.
The benefits of two-dose schedule are convenience, cost, and increased likelihood of completion. Data presented at the 2017 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer showed that rates of cervical dysplasia were equivalent for women who completed a two-dose schedule versus a three-dose schedule.4
Efficacy
A recent meta-analysis of clinical trials of the HPV vaccines describe efficacy of 95%-97% in prevention of CIN 1-3.5 While its greatest efficacy is in its ability to prevent primary HPV infection, there still is some benefit for individuals who already were exposed to HPV prior to vaccination. As stated previously, women with a history of prior HPV vaccination have lower rates of recurrence of cervical dysplasia after treatment. Additionally, recent research has shown that women who received HPV vaccinations after a LEEP procedure for CIN 2 or 3 experience significantly lower recurrence rates, compared with women who did not receive vaccinations after LEEP (2.5% vs. 8.5%).6 This raises the possibility of a therapeutic role for HPV vaccination in women infected with HPV. Prospective studies are currently evaluating this question.
Myths
The most common side effects of the HPV vaccine are pain, redness, or swelling at the injection site. Other known side effects include fever, headache or malaise, nausea, syncope, or muscle/joint pain – similar to other vaccinations. Anaphylaxis is a rare complication.
Some parents and pediatricians report concerns that vaccination could lead to earlier sexual activity. Multiple studies have shown that girls who receive HPV vaccination are no more likely to become pregnant or get a sexually transmitted infection (proxies for intercourse) than are girls who were not vaccinated.7,8
Maximizing vaccination rates
HPV vaccination rates in the United States lag significantly behind rates in countries with national vaccine programs, such as Australia and Denmark.9 Early data from Australia already have shown a decrease in genital warts and CIN 2+ incidence within the 10 years of starting its school-based vaccine program, with approximately 73% of 12- to 15-year-olds having completed the vaccine series.2 In contrast, just 40% and 22% of 13- to 17-year-old girls and boys in the United States, respectively, had completed the vaccine series in 2014, according to the CDC.10
Vaccination gaps between girls and boys are narrowing, and more teens will be able to complete the series with the new two-dose recommendation for those younger than 15 years. However, our current rates of vaccination are significantly lower for HPV than for other routinely recommended adolescent vaccines (such as Tdap and meningococcal) and more must be done to encourage vaccination.
Studies have shown that parents are more likely to vaccinate their children if providers recommend the vaccine.11 As women’s health care providers, we do not always see children during the time period that is ideal for vaccination. However, we take care of many women who are presenting for routine gynecologic care, pregnancy, or with abnormal Pap smear screenings. These are ideal opportunities to educate and offer HPV vaccination to women in the approved age groups, as well as to encourage parents to vaccinate their children.
As with other vaccines, the recommendation should be clear and focused on the cancer prevention benefit. Using methods in which the recommendation is “announced” in a brief statement assuming parents/patients are ready to vaccinate versus open-ended conversations, has been studied as a potentially successful method to increase uptake of HPV vaccination.12 Additionally, documentation of HPV vaccination status should be built into electronic medical record templates to prompt clinicians to ask and offer HPV vaccination at visits, including postpartum visits.
Dr. Rahangdale is an associate professor of ob.gyn. at the University of North Carolina, Chapel Hill, and is director of the North Carolina Women’s Hospital Cervical Dysplasia Clinic. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Epidemiol. 2008 Jul 15;168(2):123-37.
2. Int J Cancer. 2012 Nov 1;131(9):1969-82.
3. BMJ. 2012 Mar 27;344:e1401. doi: 10.1136/bmj.e1401.
4. Gynecol Oncol. 2017 Jun. doi. org/10.1016/j.ygyno.2017.03.031.
5. Int J Prev Med. 2017 Jun 1;8:44. doi: 10.4103/ijpvm.IJPVM_413_16.
6. Gynecol Oncol. 2013 Aug;130(2):264-8.
7. Pediatrics. 2012 Nov;130(5):798-805.
8. JAMA Intern Med. 2015 Apr;175(4):617-23.
9. Clin Pediatr (Phila). 2016 Sep;55(10):904-14.
10. MMWR Morb Mortal Wkly Rep. 2015 Jul 31;64(29):784-92.
11. Vaccine. 2016 Feb 24;34(9):1187-92.
12. Pediatrics. 2017 Jan;139(1). pii:e20161764. doi: 10.1542/peds.2016-1764.
Human papillomavirus (HPV) is the most common sexually transmitted infection. Exposure is widespread and most individuals clear the infection without symptoms or development of disease. However, a subset of individuals experience persistent infection, a state which can lead to carcinogenesis of lower genital tract malignancies, particularly cervical cancer.1
Vaccine coverage
Persistent infection with high-risk (oncogenic) HPV is well known to be the cause of cervical cancer. There are two HPV vaccines manufactured for the purposes of cervical cancer, anal cancer, and genital wart prevention (Cervarix and Gardasil). The Cervarix vaccine covers high-risk HPV subtypes 16 and 18 and the Gardasil vaccine prevents both low-risk HPV subtypes 6 and 11, which can cause genital warts, and high-risk HPV subtypes 16, 18, 31, 33, 45, 52 and 58, which cause cervical dysplasia and cancer.
High-risk HPV is also associated with head and neck, vulvar, vaginal, and penile cancers, though the vaccines are not approved by the Food and Drug Administration for prevention of these diseases.2
Vaccination indications
Since vaccination prevents multiple subtypes of HPV, an individual who has already been exposed will still benefit from protection from other subtypes of HPV through vaccination. HPV vaccination is not approved during pregnancy but can be initiated in the postpartum period when women are engaged in their health care and receiving other vaccinations, such as varicella or the MMR vaccine.
Recommended schedule
Until October 2016, the vaccination schedule was based on a three-dose series (0, 2, and 6 months). Currently, the CDC recommends that children aged under 15 years at the time of first dose may opt for a two-dose series (0 and 6-12 months). For those aged 15-26 years, the three-dose schedule remains the recommended course.
The benefits of two-dose schedule are convenience, cost, and increased likelihood of completion. Data presented at the 2017 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer showed that rates of cervical dysplasia were equivalent for women who completed a two-dose schedule versus a three-dose schedule.4
Efficacy
A recent meta-analysis of clinical trials of the HPV vaccines describe efficacy of 95%-97% in prevention of CIN 1-3.5 While its greatest efficacy is in its ability to prevent primary HPV infection, there still is some benefit for individuals who already were exposed to HPV prior to vaccination. As stated previously, women with a history of prior HPV vaccination have lower rates of recurrence of cervical dysplasia after treatment. Additionally, recent research has shown that women who received HPV vaccinations after a LEEP procedure for CIN 2 or 3 experience significantly lower recurrence rates, compared with women who did not receive vaccinations after LEEP (2.5% vs. 8.5%).6 This raises the possibility of a therapeutic role for HPV vaccination in women infected with HPV. Prospective studies are currently evaluating this question.
Myths
The most common side effects of the HPV vaccine are pain, redness, or swelling at the injection site. Other known side effects include fever, headache or malaise, nausea, syncope, or muscle/joint pain – similar to other vaccinations. Anaphylaxis is a rare complication.
Some parents and pediatricians report concerns that vaccination could lead to earlier sexual activity. Multiple studies have shown that girls who receive HPV vaccination are no more likely to become pregnant or get a sexually transmitted infection (proxies for intercourse) than are girls who were not vaccinated.7,8
Maximizing vaccination rates
HPV vaccination rates in the United States lag significantly behind rates in countries with national vaccine programs, such as Australia and Denmark.9 Early data from Australia already have shown a decrease in genital warts and CIN 2+ incidence within the 10 years of starting its school-based vaccine program, with approximately 73% of 12- to 15-year-olds having completed the vaccine series.2 In contrast, just 40% and 22% of 13- to 17-year-old girls and boys in the United States, respectively, had completed the vaccine series in 2014, according to the CDC.10
Vaccination gaps between girls and boys are narrowing, and more teens will be able to complete the series with the new two-dose recommendation for those younger than 15 years. However, our current rates of vaccination are significantly lower for HPV than for other routinely recommended adolescent vaccines (such as Tdap and meningococcal) and more must be done to encourage vaccination.
Studies have shown that parents are more likely to vaccinate their children if providers recommend the vaccine.11 As women’s health care providers, we do not always see children during the time period that is ideal for vaccination. However, we take care of many women who are presenting for routine gynecologic care, pregnancy, or with abnormal Pap smear screenings. These are ideal opportunities to educate and offer HPV vaccination to women in the approved age groups, as well as to encourage parents to vaccinate their children.
As with other vaccines, the recommendation should be clear and focused on the cancer prevention benefit. Using methods in which the recommendation is “announced” in a brief statement assuming parents/patients are ready to vaccinate versus open-ended conversations, has been studied as a potentially successful method to increase uptake of HPV vaccination.12 Additionally, documentation of HPV vaccination status should be built into electronic medical record templates to prompt clinicians to ask and offer HPV vaccination at visits, including postpartum visits.
Dr. Rahangdale is an associate professor of ob.gyn. at the University of North Carolina, Chapel Hill, and is director of the North Carolina Women’s Hospital Cervical Dysplasia Clinic. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Epidemiol. 2008 Jul 15;168(2):123-37.
2. Int J Cancer. 2012 Nov 1;131(9):1969-82.
3. BMJ. 2012 Mar 27;344:e1401. doi: 10.1136/bmj.e1401.
4. Gynecol Oncol. 2017 Jun. doi. org/10.1016/j.ygyno.2017.03.031.
5. Int J Prev Med. 2017 Jun 1;8:44. doi: 10.4103/ijpvm.IJPVM_413_16.
6. Gynecol Oncol. 2013 Aug;130(2):264-8.
7. Pediatrics. 2012 Nov;130(5):798-805.
8. JAMA Intern Med. 2015 Apr;175(4):617-23.
9. Clin Pediatr (Phila). 2016 Sep;55(10):904-14.
10. MMWR Morb Mortal Wkly Rep. 2015 Jul 31;64(29):784-92.
11. Vaccine. 2016 Feb 24;34(9):1187-92.
12. Pediatrics. 2017 Jan;139(1). pii:e20161764. doi: 10.1542/peds.2016-1764.
Understanding the human papillomavirus
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.