User login
The Effects of Care Team Roles on Situation Awareness in the Pediatric Intensive Care Unit: A Prospective Cross-Sectional Study
Reduction in serious pediatric medical errors has been achieved through sharing of best practices and structured collaboration.1 However, limited progress has been made in reducing complex, multifactorial events such as unrecognized and undertreated patient deterioration events.2 To address this critical gap, interventions to improve clinician situation awareness (SA) have increasingly been applied.3
SA is the ability to recognize and monitor cues regarding what is happening, create a comprehensive picture with available information, and extrapolate whether it indicates adverse developments either immediately or in the near future.4 Methods such as care team huddling5-8 and using standardized patient acuity scoring instruments9 increase SA shared across care team roles. Shared SA is the degree to which each team member possesses a common understanding of what is going on. A team is considered to have shared SA when all the individuals agree on both what is happening (accurate perception and comprehension) and what is going to happen in the future (correct projection). Shared SA for high-risk patients in the pediatric intensive care unit (PICU) has not previously been described and may be an opportunity to improve interprofessional team communication for the sickest patients. Shared SA for high-risk patient status is only one aspect of SA, but it facilitates team-based mitigation planning and is an important starting place for understanding opportunities to improve SA. The primary objective of this study was to measure and compare SA among care team roles regarding patients with high-risk status in the PICU.
METHODS
We conducted a prospective, cross-sectional study from March 2018 to July 2019 examining the individual and shared SA of patient care team trios: the nurse, respiratory therapist (RT), and pediatric resident. The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) determined this study to be non–human-subjects research.
Setting
Research was conducted in the 35-bed PICU of CCHMC, a 500-bed academic free-standing quaternary care children’s hospital.
Participants
We conducted independent surveys of the nurse, RT, and pediatric resident (care team trio) caring for each patient regarding the patient’s clinical deterioration risk status. No patients or care team trios were excluded.
Reference Standard
In 2016, a local panel of experts derived clinical criteria to determine high-risk status for PICU patients, the definition of which, as well as other study terms, appears in Table 1. A PICU attending or fellow identifies a patient as “high risk” when these clinical criteria are met. A plan for prevention and mitigation is formulated and documented for high-risk patients by the PICU attending or fellow at two preexisting daily SA huddles. This plan includes prevention measures to take immediately, specific vital sign thresholds for early identification of deterioration, and guidance on which emergency medication order sets should be utilized to expedite treatment in the event of clinical decline. Dissemination of the care team’s plan is the responsibility of the PICU fellow with additional follow-up by the charge nurse to improve reliability. Identification of high-risk status and development of the prevention and mitigation plan, as completed by the PICU fellow or attending, served as the reference standard for this study.

Survey Instrument Development
The locally developed survey tool was modeled after a validated handoff communication instrument.10 The tool covered the patient’s risk status, which high-risk clinical criteria were met, the presence and content of a mitigation plan, and planned patient interventions (Appendix).
Data Collection
Care team trios were sampled weekly on weekdays during day and night shifts within 4 to 6 hours of the SA huddle by a core group of three research assistants. Care team trios for one group of five to nine patients within a small geographically isolated pod were surveyed each time. The care team trio was surveyed individually regarding the patient’s risk status, the high-risk clinical criteria met, the presence and content of a mitigation plan, and planned patient interventions. The responses were compared for accuracy against the reference standard, which was defined as identification of high-risk patient status and development of the prevention and mitigation plan as completed by the PICU fellow or attending.
Data Analysis
Rates of agreement between the reference standard and individual members of the care team trio were evaluated via a calculation of proportions by care team role. The agreement between each care team trio member and the reference standard was compared with the nurse role performance using chi-square tests. Rates of concordance within the members of the care team trio were calculated via Light’s kappa for determination of high-risk status.11 Assuming a correct assessment of high-risk status of 62%,12 with a difference between groups of 10%, a sample size of 400 bedside provider trios gives a power of 85% at the P < .05 significance level for a two-sided chi-square test.
RESULTS
Between March 1, 2018, and July 11, 2019, 400 care team trios were surveyed. Seventy-three trios cared for patients designated high risk (Table 2 for N and proportions). Among all surveyed trios, 94% of nurses (reference), 95% of RTs (P = .4), and 87% of residents (P = .002) identified patient’s risk status correctly. Care trio member concordance for high-risk status was moderate agreement as assessed by a kappa of 0.57 (95% CI, 0.25-0.90).

Of the 73 high-risk patients, nurses correctly identified risk status for 82% (reference), RTs 85% (P = .7), and residents 67% (P = .04). For high-risk patients, nurses identified the presence of a mitigation plan for 98% of patients (reference), RTs 90% (P = .06), and residents 88% (P = .03). Among the care team members who correctly identified the presence of a mitigation plan, nurses were able to specify the correct plan for 83% of patients (reference), RTs for 68% (P = .09), and residents for 70% (P = .11; Figure).
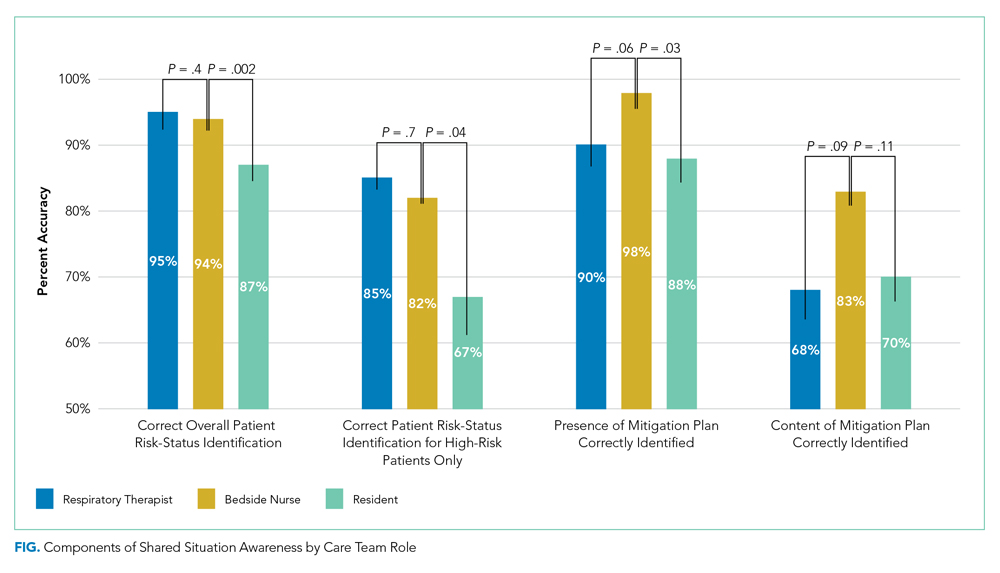
When shared SA for high-risk patients was examined more closely, all three care team roles correctly identified the clinical reason for high-risk status for 32% of patients, with only one or two clinicians being correct for 53%. All three care team clinicians were incorrect for 15% of high-risk patients. Among trios with partial accuracy in which two of three care team members correctly identified a patient as high risk, we examined which care-member was most likely to be incorrect. Nurses incorrectly identified risk for 17% of patients (reference), RTs 19% (P = .8), and residents 64% (P < .0001).
DISCUSSION
Examining 400 care team trios, we found lower individual SA for residents, compared with nurses, regarding high-risk status, the reason for this status, and the presence of a mitigation plan. In all reported measures except for the content of mitigation plans, residents were significantly less correct than the bedside nurses while RTs performed similarly to bedside nurses throughout. In addition, there was only moderate agreement between care team roles, which shows further opportunities for improvement in shared SA. The disparities between care team roles are consistent with studies that suggest certain factors grounded in institutional culture and interpersonal dynamics, such as poor communication, can lead to breakdowns in shared knowledge.13,14 Communication issues demonstrate differences across care team roles14 and may provide insight into barriers to individual and shared SA throughout the care team.
In addition, the effects of patient load on SA needs further study. While our PICU nurses are commonly assigned to 1 to 2 patients, RTs care for 7 to 11 patients, and an on-call resident may be covering 15 to 20 patients during a high-census season. The increased patient load cannot serve as an excuse for the knowledge gap regarding high-risk status and mitigation plan, but may provide an opportunity to support residents and other medical providers through the use of clinical decision-support tools that indicate high-risk status and represent mitigation plans.12
This study has multiple limitations. First, while we based our survey tool on a communication assessment tool with prior validity evidence,10,12 our tool has not been used prior to this study. The adapted tool contained relevant categorizations of patient information, including explicit statement of patient status and planned treatment consistent with study definitions of SA, and has been used in the critical care setting previously.11 The survey tool used to measure SA in this study was locally designed and implemented only within the study unit, which could lead to decreased reliability and generalizability of the results to other units and institutions at large. Second, while the sample size for the primary measure (N = 400) was adequately powered because our baseline SA was higher than estimated, we had insufficient power for some subgroup analyses that can lead to type II errors. Third, care team trios may have been surveyed repeatedly on the same patient without adjustment in the results for repeated measures. However, as we surveyed on average only once a week and alternated areas of the PICU surveyed, it is unlikely that it affected results given that the most lengths of stay within the PICU range from 3 to 4 days. Finally, individual characteristics of patients were not collected for this work, and therefore, no adjustments or further analysis can be made on the effect of the patient characteristic on the care team role SA.
CONCLUSION
This study is the first to assess differences in individual and shared SA within a PICU by care team role. Efforts to expand on these findings should include investigation into the causes for the disparities in SA among care team roles for individual patients and among the care teams of high-risk and normal-risk patients. Given the association between increased SA and improved patient outcomes,4 future efforts should be structured to address care team role–specific gaps in SA because these may advance the quality of care in the pediatric inpatient setting.
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://doi.org/10.1542/peds.2016-3494
2. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
3. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-308. https://doi.org/10.1542/peds.2012-1364
4. Endsley MR. Theoretical underpinnings of situation awareness: a critical review. In: Endsley MR, Garland DJ, eds. Situation Awareness Analysis and Measurement. Lawrence Erlbaum Associates; 2000.
5. Dewan M, Wolfe H, Lin R, et al. Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patients. J Hosp Med. 2017;12(8):652‐657. https://doi.org/10.12788/jhm.2782
6. Bonafide CP, Localio AR, Stemler S, et al. Safety huddle intervention for reducing physiologic monitor alarms: a hybrid effectiveness-implementation cluster randomized trial. J Hosp Med. 2018;13(9):609‐615. https://doi.org/10.12788/jhm.2956
7. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. https://doi.org/10.1097/HMR.0000000000000009
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE, Anderson JM. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. https://doi.org/10.1136/bmjqs-2012-001467
9. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. https://doi.org/10.1002/jhm.886
10. Shahian DM, McEachern K, Rossi L, Chisari RG, Mort E. Large-scale implementation of the I-PASS handover system at an academic medical centre. BMJ Qual Saf. 2017;26(9):760-770. https://doi.org/10.1136/bmjqs-2016-006195
11. Gamer M, Lemon J, Fellows I, Singh P. Various Coefficients of Interrater Reliability and Agreement. January 26, 2019. Accessed January 24, 2020. http://cran.r-project.org/web/packages/irr/irr.pdf
12. Shelov E, Muthu N, Wolfe H, et al. Design and implementation of a pediatric ICU acuity scoring tool as clinical decision support. Appl Clin Inf. 2018;09(3):576-587. https://doi.org/10.1055/s-0038-1667122
13. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019
14. Sexton B, Thomas E, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320(7237):745-749. doi:10.1136/bmj.320.7237.745
Reduction in serious pediatric medical errors has been achieved through sharing of best practices and structured collaboration.1 However, limited progress has been made in reducing complex, multifactorial events such as unrecognized and undertreated patient deterioration events.2 To address this critical gap, interventions to improve clinician situation awareness (SA) have increasingly been applied.3
SA is the ability to recognize and monitor cues regarding what is happening, create a comprehensive picture with available information, and extrapolate whether it indicates adverse developments either immediately or in the near future.4 Methods such as care team huddling5-8 and using standardized patient acuity scoring instruments9 increase SA shared across care team roles. Shared SA is the degree to which each team member possesses a common understanding of what is going on. A team is considered to have shared SA when all the individuals agree on both what is happening (accurate perception and comprehension) and what is going to happen in the future (correct projection). Shared SA for high-risk patients in the pediatric intensive care unit (PICU) has not previously been described and may be an opportunity to improve interprofessional team communication for the sickest patients. Shared SA for high-risk patient status is only one aspect of SA, but it facilitates team-based mitigation planning and is an important starting place for understanding opportunities to improve SA. The primary objective of this study was to measure and compare SA among care team roles regarding patients with high-risk status in the PICU.
METHODS
We conducted a prospective, cross-sectional study from March 2018 to July 2019 examining the individual and shared SA of patient care team trios: the nurse, respiratory therapist (RT), and pediatric resident. The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) determined this study to be non–human-subjects research.
Setting
Research was conducted in the 35-bed PICU of CCHMC, a 500-bed academic free-standing quaternary care children’s hospital.
Participants
We conducted independent surveys of the nurse, RT, and pediatric resident (care team trio) caring for each patient regarding the patient’s clinical deterioration risk status. No patients or care team trios were excluded.
Reference Standard
In 2016, a local panel of experts derived clinical criteria to determine high-risk status for PICU patients, the definition of which, as well as other study terms, appears in Table 1. A PICU attending or fellow identifies a patient as “high risk” when these clinical criteria are met. A plan for prevention and mitigation is formulated and documented for high-risk patients by the PICU attending or fellow at two preexisting daily SA huddles. This plan includes prevention measures to take immediately, specific vital sign thresholds for early identification of deterioration, and guidance on which emergency medication order sets should be utilized to expedite treatment in the event of clinical decline. Dissemination of the care team’s plan is the responsibility of the PICU fellow with additional follow-up by the charge nurse to improve reliability. Identification of high-risk status and development of the prevention and mitigation plan, as completed by the PICU fellow or attending, served as the reference standard for this study.

Survey Instrument Development
The locally developed survey tool was modeled after a validated handoff communication instrument.10 The tool covered the patient’s risk status, which high-risk clinical criteria were met, the presence and content of a mitigation plan, and planned patient interventions (Appendix).
Data Collection
Care team trios were sampled weekly on weekdays during day and night shifts within 4 to 6 hours of the SA huddle by a core group of three research assistants. Care team trios for one group of five to nine patients within a small geographically isolated pod were surveyed each time. The care team trio was surveyed individually regarding the patient’s risk status, the high-risk clinical criteria met, the presence and content of a mitigation plan, and planned patient interventions. The responses were compared for accuracy against the reference standard, which was defined as identification of high-risk patient status and development of the prevention and mitigation plan as completed by the PICU fellow or attending.
Data Analysis
Rates of agreement between the reference standard and individual members of the care team trio were evaluated via a calculation of proportions by care team role. The agreement between each care team trio member and the reference standard was compared with the nurse role performance using chi-square tests. Rates of concordance within the members of the care team trio were calculated via Light’s kappa for determination of high-risk status.11 Assuming a correct assessment of high-risk status of 62%,12 with a difference between groups of 10%, a sample size of 400 bedside provider trios gives a power of 85% at the P < .05 significance level for a two-sided chi-square test.
RESULTS
Between March 1, 2018, and July 11, 2019, 400 care team trios were surveyed. Seventy-three trios cared for patients designated high risk (Table 2 for N and proportions). Among all surveyed trios, 94% of nurses (reference), 95% of RTs (P = .4), and 87% of residents (P = .002) identified patient’s risk status correctly. Care trio member concordance for high-risk status was moderate agreement as assessed by a kappa of 0.57 (95% CI, 0.25-0.90).

Of the 73 high-risk patients, nurses correctly identified risk status for 82% (reference), RTs 85% (P = .7), and residents 67% (P = .04). For high-risk patients, nurses identified the presence of a mitigation plan for 98% of patients (reference), RTs 90% (P = .06), and residents 88% (P = .03). Among the care team members who correctly identified the presence of a mitigation plan, nurses were able to specify the correct plan for 83% of patients (reference), RTs for 68% (P = .09), and residents for 70% (P = .11; Figure).

When shared SA for high-risk patients was examined more closely, all three care team roles correctly identified the clinical reason for high-risk status for 32% of patients, with only one or two clinicians being correct for 53%. All three care team clinicians were incorrect for 15% of high-risk patients. Among trios with partial accuracy in which two of three care team members correctly identified a patient as high risk, we examined which care-member was most likely to be incorrect. Nurses incorrectly identified risk for 17% of patients (reference), RTs 19% (P = .8), and residents 64% (P < .0001).
DISCUSSION
Examining 400 care team trios, we found lower individual SA for residents, compared with nurses, regarding high-risk status, the reason for this status, and the presence of a mitigation plan. In all reported measures except for the content of mitigation plans, residents were significantly less correct than the bedside nurses while RTs performed similarly to bedside nurses throughout. In addition, there was only moderate agreement between care team roles, which shows further opportunities for improvement in shared SA. The disparities between care team roles are consistent with studies that suggest certain factors grounded in institutional culture and interpersonal dynamics, such as poor communication, can lead to breakdowns in shared knowledge.13,14 Communication issues demonstrate differences across care team roles14 and may provide insight into barriers to individual and shared SA throughout the care team.
In addition, the effects of patient load on SA needs further study. While our PICU nurses are commonly assigned to 1 to 2 patients, RTs care for 7 to 11 patients, and an on-call resident may be covering 15 to 20 patients during a high-census season. The increased patient load cannot serve as an excuse for the knowledge gap regarding high-risk status and mitigation plan, but may provide an opportunity to support residents and other medical providers through the use of clinical decision-support tools that indicate high-risk status and represent mitigation plans.12
This study has multiple limitations. First, while we based our survey tool on a communication assessment tool with prior validity evidence,10,12 our tool has not been used prior to this study. The adapted tool contained relevant categorizations of patient information, including explicit statement of patient status and planned treatment consistent with study definitions of SA, and has been used in the critical care setting previously.11 The survey tool used to measure SA in this study was locally designed and implemented only within the study unit, which could lead to decreased reliability and generalizability of the results to other units and institutions at large. Second, while the sample size for the primary measure (N = 400) was adequately powered because our baseline SA was higher than estimated, we had insufficient power for some subgroup analyses that can lead to type II errors. Third, care team trios may have been surveyed repeatedly on the same patient without adjustment in the results for repeated measures. However, as we surveyed on average only once a week and alternated areas of the PICU surveyed, it is unlikely that it affected results given that the most lengths of stay within the PICU range from 3 to 4 days. Finally, individual characteristics of patients were not collected for this work, and therefore, no adjustments or further analysis can be made on the effect of the patient characteristic on the care team role SA.
CONCLUSION
This study is the first to assess differences in individual and shared SA within a PICU by care team role. Efforts to expand on these findings should include investigation into the causes for the disparities in SA among care team roles for individual patients and among the care teams of high-risk and normal-risk patients. Given the association between increased SA and improved patient outcomes,4 future efforts should be structured to address care team role–specific gaps in SA because these may advance the quality of care in the pediatric inpatient setting.
Reduction in serious pediatric medical errors has been achieved through sharing of best practices and structured collaboration.1 However, limited progress has been made in reducing complex, multifactorial events such as unrecognized and undertreated patient deterioration events.2 To address this critical gap, interventions to improve clinician situation awareness (SA) have increasingly been applied.3
SA is the ability to recognize and monitor cues regarding what is happening, create a comprehensive picture with available information, and extrapolate whether it indicates adverse developments either immediately or in the near future.4 Methods such as care team huddling5-8 and using standardized patient acuity scoring instruments9 increase SA shared across care team roles. Shared SA is the degree to which each team member possesses a common understanding of what is going on. A team is considered to have shared SA when all the individuals agree on both what is happening (accurate perception and comprehension) and what is going to happen in the future (correct projection). Shared SA for high-risk patients in the pediatric intensive care unit (PICU) has not previously been described and may be an opportunity to improve interprofessional team communication for the sickest patients. Shared SA for high-risk patient status is only one aspect of SA, but it facilitates team-based mitigation planning and is an important starting place for understanding opportunities to improve SA. The primary objective of this study was to measure and compare SA among care team roles regarding patients with high-risk status in the PICU.
METHODS
We conducted a prospective, cross-sectional study from March 2018 to July 2019 examining the individual and shared SA of patient care team trios: the nurse, respiratory therapist (RT), and pediatric resident. The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) determined this study to be non–human-subjects research.
Setting
Research was conducted in the 35-bed PICU of CCHMC, a 500-bed academic free-standing quaternary care children’s hospital.
Participants
We conducted independent surveys of the nurse, RT, and pediatric resident (care team trio) caring for each patient regarding the patient’s clinical deterioration risk status. No patients or care team trios were excluded.
Reference Standard
In 2016, a local panel of experts derived clinical criteria to determine high-risk status for PICU patients, the definition of which, as well as other study terms, appears in Table 1. A PICU attending or fellow identifies a patient as “high risk” when these clinical criteria are met. A plan for prevention and mitigation is formulated and documented for high-risk patients by the PICU attending or fellow at two preexisting daily SA huddles. This plan includes prevention measures to take immediately, specific vital sign thresholds for early identification of deterioration, and guidance on which emergency medication order sets should be utilized to expedite treatment in the event of clinical decline. Dissemination of the care team’s plan is the responsibility of the PICU fellow with additional follow-up by the charge nurse to improve reliability. Identification of high-risk status and development of the prevention and mitigation plan, as completed by the PICU fellow or attending, served as the reference standard for this study.

Survey Instrument Development
The locally developed survey tool was modeled after a validated handoff communication instrument.10 The tool covered the patient’s risk status, which high-risk clinical criteria were met, the presence and content of a mitigation plan, and planned patient interventions (Appendix).
Data Collection
Care team trios were sampled weekly on weekdays during day and night shifts within 4 to 6 hours of the SA huddle by a core group of three research assistants. Care team trios for one group of five to nine patients within a small geographically isolated pod were surveyed each time. The care team trio was surveyed individually regarding the patient’s risk status, the high-risk clinical criteria met, the presence and content of a mitigation plan, and planned patient interventions. The responses were compared for accuracy against the reference standard, which was defined as identification of high-risk patient status and development of the prevention and mitigation plan as completed by the PICU fellow or attending.
Data Analysis
Rates of agreement between the reference standard and individual members of the care team trio were evaluated via a calculation of proportions by care team role. The agreement between each care team trio member and the reference standard was compared with the nurse role performance using chi-square tests. Rates of concordance within the members of the care team trio were calculated via Light’s kappa for determination of high-risk status.11 Assuming a correct assessment of high-risk status of 62%,12 with a difference between groups of 10%, a sample size of 400 bedside provider trios gives a power of 85% at the P < .05 significance level for a two-sided chi-square test.
RESULTS
Between March 1, 2018, and July 11, 2019, 400 care team trios were surveyed. Seventy-three trios cared for patients designated high risk (Table 2 for N and proportions). Among all surveyed trios, 94% of nurses (reference), 95% of RTs (P = .4), and 87% of residents (P = .002) identified patient’s risk status correctly. Care trio member concordance for high-risk status was moderate agreement as assessed by a kappa of 0.57 (95% CI, 0.25-0.90).

Of the 73 high-risk patients, nurses correctly identified risk status for 82% (reference), RTs 85% (P = .7), and residents 67% (P = .04). For high-risk patients, nurses identified the presence of a mitigation plan for 98% of patients (reference), RTs 90% (P = .06), and residents 88% (P = .03). Among the care team members who correctly identified the presence of a mitigation plan, nurses were able to specify the correct plan for 83% of patients (reference), RTs for 68% (P = .09), and residents for 70% (P = .11; Figure).

When shared SA for high-risk patients was examined more closely, all three care team roles correctly identified the clinical reason for high-risk status for 32% of patients, with only one or two clinicians being correct for 53%. All three care team clinicians were incorrect for 15% of high-risk patients. Among trios with partial accuracy in which two of three care team members correctly identified a patient as high risk, we examined which care-member was most likely to be incorrect. Nurses incorrectly identified risk for 17% of patients (reference), RTs 19% (P = .8), and residents 64% (P < .0001).
DISCUSSION
Examining 400 care team trios, we found lower individual SA for residents, compared with nurses, regarding high-risk status, the reason for this status, and the presence of a mitigation plan. In all reported measures except for the content of mitigation plans, residents were significantly less correct than the bedside nurses while RTs performed similarly to bedside nurses throughout. In addition, there was only moderate agreement between care team roles, which shows further opportunities for improvement in shared SA. The disparities between care team roles are consistent with studies that suggest certain factors grounded in institutional culture and interpersonal dynamics, such as poor communication, can lead to breakdowns in shared knowledge.13,14 Communication issues demonstrate differences across care team roles14 and may provide insight into barriers to individual and shared SA throughout the care team.
In addition, the effects of patient load on SA needs further study. While our PICU nurses are commonly assigned to 1 to 2 patients, RTs care for 7 to 11 patients, and an on-call resident may be covering 15 to 20 patients during a high-census season. The increased patient load cannot serve as an excuse for the knowledge gap regarding high-risk status and mitigation plan, but may provide an opportunity to support residents and other medical providers through the use of clinical decision-support tools that indicate high-risk status and represent mitigation plans.12
This study has multiple limitations. First, while we based our survey tool on a communication assessment tool with prior validity evidence,10,12 our tool has not been used prior to this study. The adapted tool contained relevant categorizations of patient information, including explicit statement of patient status and planned treatment consistent with study definitions of SA, and has been used in the critical care setting previously.11 The survey tool used to measure SA in this study was locally designed and implemented only within the study unit, which could lead to decreased reliability and generalizability of the results to other units and institutions at large. Second, while the sample size for the primary measure (N = 400) was adequately powered because our baseline SA was higher than estimated, we had insufficient power for some subgroup analyses that can lead to type II errors. Third, care team trios may have been surveyed repeatedly on the same patient without adjustment in the results for repeated measures. However, as we surveyed on average only once a week and alternated areas of the PICU surveyed, it is unlikely that it affected results given that the most lengths of stay within the PICU range from 3 to 4 days. Finally, individual characteristics of patients were not collected for this work, and therefore, no adjustments or further analysis can be made on the effect of the patient characteristic on the care team role SA.
CONCLUSION
This study is the first to assess differences in individual and shared SA within a PICU by care team role. Efforts to expand on these findings should include investigation into the causes for the disparities in SA among care team roles for individual patients and among the care teams of high-risk and normal-risk patients. Given the association between increased SA and improved patient outcomes,4 future efforts should be structured to address care team role–specific gaps in SA because these may advance the quality of care in the pediatric inpatient setting.
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://doi.org/10.1542/peds.2016-3494
2. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
3. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-308. https://doi.org/10.1542/peds.2012-1364
4. Endsley MR. Theoretical underpinnings of situation awareness: a critical review. In: Endsley MR, Garland DJ, eds. Situation Awareness Analysis and Measurement. Lawrence Erlbaum Associates; 2000.
5. Dewan M, Wolfe H, Lin R, et al. Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patients. J Hosp Med. 2017;12(8):652‐657. https://doi.org/10.12788/jhm.2782
6. Bonafide CP, Localio AR, Stemler S, et al. Safety huddle intervention for reducing physiologic monitor alarms: a hybrid effectiveness-implementation cluster randomized trial. J Hosp Med. 2018;13(9):609‐615. https://doi.org/10.12788/jhm.2956
7. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. https://doi.org/10.1097/HMR.0000000000000009
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE, Anderson JM. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. https://doi.org/10.1136/bmjqs-2012-001467
9. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. https://doi.org/10.1002/jhm.886
10. Shahian DM, McEachern K, Rossi L, Chisari RG, Mort E. Large-scale implementation of the I-PASS handover system at an academic medical centre. BMJ Qual Saf. 2017;26(9):760-770. https://doi.org/10.1136/bmjqs-2016-006195
11. Gamer M, Lemon J, Fellows I, Singh P. Various Coefficients of Interrater Reliability and Agreement. January 26, 2019. Accessed January 24, 2020. http://cran.r-project.org/web/packages/irr/irr.pdf
12. Shelov E, Muthu N, Wolfe H, et al. Design and implementation of a pediatric ICU acuity scoring tool as clinical decision support. Appl Clin Inf. 2018;09(3):576-587. https://doi.org/10.1055/s-0038-1667122
13. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019
14. Sexton B, Thomas E, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320(7237):745-749. doi:10.1136/bmj.320.7237.745
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://doi.org/10.1542/peds.2016-3494
2. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
3. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-308. https://doi.org/10.1542/peds.2012-1364
4. Endsley MR. Theoretical underpinnings of situation awareness: a critical review. In: Endsley MR, Garland DJ, eds. Situation Awareness Analysis and Measurement. Lawrence Erlbaum Associates; 2000.
5. Dewan M, Wolfe H, Lin R, et al. Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patients. J Hosp Med. 2017;12(8):652‐657. https://doi.org/10.12788/jhm.2782
6. Bonafide CP, Localio AR, Stemler S, et al. Safety huddle intervention for reducing physiologic monitor alarms: a hybrid effectiveness-implementation cluster randomized trial. J Hosp Med. 2018;13(9):609‐615. https://doi.org/10.12788/jhm.2956
7. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. https://doi.org/10.1097/HMR.0000000000000009
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE, Anderson JM. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. https://doi.org/10.1136/bmjqs-2012-001467
9. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. https://doi.org/10.1002/jhm.886
10. Shahian DM, McEachern K, Rossi L, Chisari RG, Mort E. Large-scale implementation of the I-PASS handover system at an academic medical centre. BMJ Qual Saf. 2017;26(9):760-770. https://doi.org/10.1136/bmjqs-2016-006195
11. Gamer M, Lemon J, Fellows I, Singh P. Various Coefficients of Interrater Reliability and Agreement. January 26, 2019. Accessed January 24, 2020. http://cran.r-project.org/web/packages/irr/irr.pdf
12. Shelov E, Muthu N, Wolfe H, et al. Design and implementation of a pediatric ICU acuity scoring tool as clinical decision support. Appl Clin Inf. 2018;09(3):576-587. https://doi.org/10.1055/s-0038-1667122
13. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019
14. Sexton B, Thomas E, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320(7237):745-749. doi:10.1136/bmj.320.7237.745
© 2020 Society of Hospital Medicine
Clinical Progress Note: High Flow Nasal Cannula Therapy for Bronchiolitis Outside the ICU in Infants
Viral bronchiolitis is the most common indication for infant hospitalization in the United States.1 The treatment mainstay remains supportive care, including supplemental oxygen when indicated.1 High flow nasal cannula (HFNC) therapy delivers humidified, heated air blended with oxygen, allowing much higher flow rates than standard nasal cannula therapy and is being used more frequently in inpatient settings.
OVERVIEW AND CLINICAL QUESTION
Infants and toddlers with bronchiolitis develop increased work of breathing to preserve oxygenation and ventilation in the setting of altered airway resistance and lung compliance.2,3 In addition to oxygen supplementation, HFNC is used to reduce work of breathing through several mechanisms:2-6 (1) Nasopharyngeal dead space washout clears oxygen-depleted gas at the end of expiration, facilitating alveolar ventilation (ie, carbon dioxide retention improves); (2) High flow rates match increased inspiratory flow demands of acutely ill patients, reducing nasopharyngeal inspiratory resistance and optimizing dead space washout, thus decreasing work of breathing; (3) Adequate flow rates generate distending pressure, which prevents pharyngeal collapse, supports lung recruitment, and reduces respiratory effort (demonstrated in younger infants); and (4) HFNC systems heat and humidify the breathing gas, reducing the metabolic work required to condition cool, dry gas and improving conductance and pulmonary compliance.2-5
HFNC therapy is used more commonly in acute care units despite limited literature on its effectiveness outside the intensive care unit (ICU).7,8 We asked the question, “Does use of HFNC therapy for infants with bronchiolitis hospitalized in acute care units result in improved outcomes when compared with standard nasal cannula oxygen therapy, including length of stay (LOS), oxygen therapy duration, and preventing escalations of care such as ICU transfer, positive pressure ventilation, and intubation?” Also, do published studies provide guidance for the initiation and management of HFNC? We focused our search on studies published in the last five years that included patients with bronchiolitis treated with HFNC outside the ICU; here, we review those studies most relevant to pediatric hospitalists.
RECENT LITERATURE REVIEW
No guideline exists for initiating flow or fraction of inspired oxygen (FiO2). HFNC may be initiated for hypoxia, increased work of breathing, or both in patients with bronchiolitis. To achieve optimal dead space washout, inspiratory flow, and distending pressure, initial flow rates should be 1.5 to 2 L/kg/min, particularly for infants and young children.2-5 Weiler et al.3 evaluated the breathing effort of ICU patients at 0.5, 1, 1.5, and 2 L/kg/min and found optimal flow rates for improved work of breathing were 1.5-2 L/kg/min. The smallest patients, ≤8 kg, saw the greatest benefit, a finding likely explained by larger anatomic dead space in infants/small children compared with older children.3 For older/larger children (>20 kg), an initial flow closer to 1 L/kg/min is often appropriate.5 When used for hypoxia, initiating flow without supplemental FiO2 may improve oxygenation by flushing nasopharyngeal dead space. FiO2 should be titrated to achieve the goal set by the treatment team, often ≥90%. Improvement in heart rate and peripheral oxygen saturation (SpO2) can be observed within 60 minutes of initiating HFNC in patients responsive to therapy.6
HFNC therapy is safe when used correctly.6,9,10Potential adverse effects include pneumothorax, pressure injury, mucosal injury/bleeding, and delayed escalation to invasive ventilation. While difficult to quantify, recent studies report low rates or no serious HFNC complications. For example, only 2 of 1,127 patients supported with HFNC developed a pneumothorax and neither required evacuation.2,9-12
Inclusion criteria and HFNC protocols vary among published studies. Most HFNC protocols reviewed may not have optimally supported all of the patients in their HFNC groups, often by limiting flow to <2 L/kg/min.6-9,11,12 These variables may explain the disparate results, with some studies demonstrating apparent benefits and others no difference.7,9,10,12
Two studies of infants with bronchiolitis showed HFNC therapy may prevent ICU transfer, but this benefit may be limited to rescue when standard oxygen therapy fails, rather than as a superior initial support modality.7,9 Kepreotes et al.9 reported a single-center, randomized controlled trial comparing HFNC with standard oxygen therapy with 101 patients in each treatment arm. The primary outcome, median time to wean off oxygen, was not significantly different between the two groups: 24 hours (95% CI: 18-28) in the HFNC group versus 20 hours in the standard therapy group (95% CI: 17-34). The HFNC group had fewer treatment failures (abnormal heart rate, respiratory rate, SpO2 <90%, or severe respiratory distress score while on maximum therapy) than the standard therapy group, and 20 (63%) of the 33 patients who failed standard therapy were rescued with HFNC, avoiding transfer to the ICU. Fourteen patients from the HFNC group and 12 from the standard oxygen group required transfer to the ICU for support escalation. Although this study did not show a significant difference in oxygen weaning time between groups, it appears to support HFNC use as a rescue modality to reduce or prevent ICU transfer.9 Franklin et al.10 conducted a multicenter, randomized, controlled trial to compare standard nasal cannula oxygen therapy with HFNC (2 L/kg/min) in 1,472 patients. Patients receiving HFNC had lower care escalation rates due to treatment failure, defined as the presence of at least three of four clinical criteria and the clinician determining escalation was indicated. Oxygen therapy duration, ICU admission rates, and LOS were not significantly different between groups. Similar to the previous study, a large portion of the standard therapy patients who failed treatment (102 of 167) crossed over to the HFNC arm in an attempt to avoid ICU transfer. Twelve patients required intubation: 8 (1%) receiving HFNC and 4 (0.5%) receiving the standard therapy.10
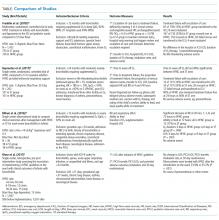
Two additional studies, both with study design limitations, did not demonstrate differences in ICU transfer rates and had variable differences in outcomes. Riese et al.7 retrospectively assessed HFNC use outside the ICU at one institution and included 936 patients admitted before and 1,001 patients admitted after HFNC guideline implementation on the wards. Flow rates were based on age and not weight. They found no difference in LOS, ICU transfer rate, ICU LOS, intubation rates, or 30-day readmission rates, though HFNC use increased over time. The HFNC guideline is a potentially significant limitation as it may not have provided optimal flow rates to all subjects given it was based on age rather than weight. Milani et al.12 performed a single-center observational study of 36 infants aged <12 months, treated for bronchiolitis on the ward, who were informally assigned to HFNC or standard therapy based upon HFNC device availability. HFNC flow rate was determined by the equation: L/min = 8 mL/kg × respiratory rate × 0.3. Using mean weight and respiratory rate for patients in this group, it appears patients in the HFNC group were treated with flow rates less than the 1.5-2 L/kg/min recommended to be effective.2,3,12 Despite this, clinical improvement was faster in the HFNC group, including respiratory rate and effort, ability to feed, days on oxygen supplementation, and hospital LOS. ICU admission was not different between the two groups.12 The Table compares the four studies discussed above.
Given increasing use of HFNC outside the ICU, institutions risk overuse and increased healthcare costs.13 Limited data on HFNC overuse exist, but several studies report increased use after implementation on the wards without robust evidence indicating it improves outcomes.7,14 Overuse of HFNC is a concern that should be considered as institutions develop HFNC protocols. Another important consideration is safe feeding. One study examined 132 children ages one month to two years with bronchiolitis who were receiving HFNC and enteral nutrition.15 Only one patient had aspiration respiratory failure, and 12 had nutrition interruptions, demonstrating oral nutrition is generally well tolerated15 and should be considered in patients with stable respiratory status on HFNC.
CONCLUSIONS
Many children’s hospitals have extended the use of HFNC outside the ICU for children with bronchiolitis despite the paucity of evidence demonstrating its benefit over standard flow oxygen. Given variation in protocols, study designs, outcomes, and number of patients studied, it is difficult to assess its efficacy outside the ICU. However, based on the studies reviewed herein, HFNC therapy does not appear to decrease LOS, time on oxygen, or escalations of care, such as ICU transfers, positive pressure ventilation, or intubation, when used as a primary therapy.7,9,11,12 Future research will ideally use optimal flow rates to determine the effectiveness of HFNC on acute care units. Although not addressed in the above studies, additional benefits to be considered in future studies include: (1) increased critical care capacity by allowing patients to be supported on the floor and (2) the ability for patients to remain closer to home when HFNC is used in the community hospital setting.
In each of the large, randomized studies reviewed, most (66%-75%) patients treated with standard low flow oxygen were supported successfully and did not require escalation to HFNC.9,10 Hospitalists should continue to use standard low flow oxygen as first-line respiratory support for patients with bronchiolitis.1 No evidence supports the use of HFNC therapy early in a child’s inpatient course; rather, it should be used when standard oxygen therapy fails. Future research should focus on better elucidating which patients will benefit most from HFNC to prevent overuse.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-1502. https://doi.org/10.1542/peds.2014-2742.
2. Milesi C, Baleine J, Matecki S, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013;39(6):1088-1094. https://doi.org/10.1007/s00134-013-2879-y.
3. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71. https://doi.org/10.1016/j.jpeds.2017.06.006.
4. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400-1405. https://doi.org/10.1016/j.rmed.2009.04.007.
5. Milesi C, Boubal M, Jacquot A, et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann Intensive Care. 2014;4(1):29. https://doi.org/10.1186/s13613-014-0029-5.
6. Heikkila P, Sokuri P, Mecklin M, et al. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018;107(11):1971-1976. https://doi.org/10.1111/apa.14421.
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
8. Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. 2017;52(6):806-812. https://doi.org/10.1002/ppul.23626.
9. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
10. Franklin D, Babl FE, Schibler A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2446-2447. https://doi.org/10.1056/NEJMc1805312.
11. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
12. Milani GP, Plebani AM, Arturi E, et al. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr. 2016;105(8):e368-e372. https://doi.org/10.1111/apa.13444.
13. Modesto i Alapont V, Garcia Cusco M, Medina A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2444. https://doi.org/10.1056/NEJMc1805312.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
Viral bronchiolitis is the most common indication for infant hospitalization in the United States.1 The treatment mainstay remains supportive care, including supplemental oxygen when indicated.1 High flow nasal cannula (HFNC) therapy delivers humidified, heated air blended with oxygen, allowing much higher flow rates than standard nasal cannula therapy and is being used more frequently in inpatient settings.
OVERVIEW AND CLINICAL QUESTION
Infants and toddlers with bronchiolitis develop increased work of breathing to preserve oxygenation and ventilation in the setting of altered airway resistance and lung compliance.2,3 In addition to oxygen supplementation, HFNC is used to reduce work of breathing through several mechanisms:2-6 (1) Nasopharyngeal dead space washout clears oxygen-depleted gas at the end of expiration, facilitating alveolar ventilation (ie, carbon dioxide retention improves); (2) High flow rates match increased inspiratory flow demands of acutely ill patients, reducing nasopharyngeal inspiratory resistance and optimizing dead space washout, thus decreasing work of breathing; (3) Adequate flow rates generate distending pressure, which prevents pharyngeal collapse, supports lung recruitment, and reduces respiratory effort (demonstrated in younger infants); and (4) HFNC systems heat and humidify the breathing gas, reducing the metabolic work required to condition cool, dry gas and improving conductance and pulmonary compliance.2-5
HFNC therapy is used more commonly in acute care units despite limited literature on its effectiveness outside the intensive care unit (ICU).7,8 We asked the question, “Does use of HFNC therapy for infants with bronchiolitis hospitalized in acute care units result in improved outcomes when compared with standard nasal cannula oxygen therapy, including length of stay (LOS), oxygen therapy duration, and preventing escalations of care such as ICU transfer, positive pressure ventilation, and intubation?” Also, do published studies provide guidance for the initiation and management of HFNC? We focused our search on studies published in the last five years that included patients with bronchiolitis treated with HFNC outside the ICU; here, we review those studies most relevant to pediatric hospitalists.
RECENT LITERATURE REVIEW
No guideline exists for initiating flow or fraction of inspired oxygen (FiO2). HFNC may be initiated for hypoxia, increased work of breathing, or both in patients with bronchiolitis. To achieve optimal dead space washout, inspiratory flow, and distending pressure, initial flow rates should be 1.5 to 2 L/kg/min, particularly for infants and young children.2-5 Weiler et al.3 evaluated the breathing effort of ICU patients at 0.5, 1, 1.5, and 2 L/kg/min and found optimal flow rates for improved work of breathing were 1.5-2 L/kg/min. The smallest patients, ≤8 kg, saw the greatest benefit, a finding likely explained by larger anatomic dead space in infants/small children compared with older children.3 For older/larger children (>20 kg), an initial flow closer to 1 L/kg/min is often appropriate.5 When used for hypoxia, initiating flow without supplemental FiO2 may improve oxygenation by flushing nasopharyngeal dead space. FiO2 should be titrated to achieve the goal set by the treatment team, often ≥90%. Improvement in heart rate and peripheral oxygen saturation (SpO2) can be observed within 60 minutes of initiating HFNC in patients responsive to therapy.6
HFNC therapy is safe when used correctly.6,9,10Potential adverse effects include pneumothorax, pressure injury, mucosal injury/bleeding, and delayed escalation to invasive ventilation. While difficult to quantify, recent studies report low rates or no serious HFNC complications. For example, only 2 of 1,127 patients supported with HFNC developed a pneumothorax and neither required evacuation.2,9-12
Inclusion criteria and HFNC protocols vary among published studies. Most HFNC protocols reviewed may not have optimally supported all of the patients in their HFNC groups, often by limiting flow to <2 L/kg/min.6-9,11,12 These variables may explain the disparate results, with some studies demonstrating apparent benefits and others no difference.7,9,10,12
Two studies of infants with bronchiolitis showed HFNC therapy may prevent ICU transfer, but this benefit may be limited to rescue when standard oxygen therapy fails, rather than as a superior initial support modality.7,9 Kepreotes et al.9 reported a single-center, randomized controlled trial comparing HFNC with standard oxygen therapy with 101 patients in each treatment arm. The primary outcome, median time to wean off oxygen, was not significantly different between the two groups: 24 hours (95% CI: 18-28) in the HFNC group versus 20 hours in the standard therapy group (95% CI: 17-34). The HFNC group had fewer treatment failures (abnormal heart rate, respiratory rate, SpO2 <90%, or severe respiratory distress score while on maximum therapy) than the standard therapy group, and 20 (63%) of the 33 patients who failed standard therapy were rescued with HFNC, avoiding transfer to the ICU. Fourteen patients from the HFNC group and 12 from the standard oxygen group required transfer to the ICU for support escalation. Although this study did not show a significant difference in oxygen weaning time between groups, it appears to support HFNC use as a rescue modality to reduce or prevent ICU transfer.9 Franklin et al.10 conducted a multicenter, randomized, controlled trial to compare standard nasal cannula oxygen therapy with HFNC (2 L/kg/min) in 1,472 patients. Patients receiving HFNC had lower care escalation rates due to treatment failure, defined as the presence of at least three of four clinical criteria and the clinician determining escalation was indicated. Oxygen therapy duration, ICU admission rates, and LOS were not significantly different between groups. Similar to the previous study, a large portion of the standard therapy patients who failed treatment (102 of 167) crossed over to the HFNC arm in an attempt to avoid ICU transfer. Twelve patients required intubation: 8 (1%) receiving HFNC and 4 (0.5%) receiving the standard therapy.10
Two additional studies, both with study design limitations, did not demonstrate differences in ICU transfer rates and had variable differences in outcomes. Riese et al.7 retrospectively assessed HFNC use outside the ICU at one institution and included 936 patients admitted before and 1,001 patients admitted after HFNC guideline implementation on the wards. Flow rates were based on age and not weight. They found no difference in LOS, ICU transfer rate, ICU LOS, intubation rates, or 30-day readmission rates, though HFNC use increased over time. The HFNC guideline is a potentially significant limitation as it may not have provided optimal flow rates to all subjects given it was based on age rather than weight. Milani et al.12 performed a single-center observational study of 36 infants aged <12 months, treated for bronchiolitis on the ward, who were informally assigned to HFNC or standard therapy based upon HFNC device availability. HFNC flow rate was determined by the equation: L/min = 8 mL/kg × respiratory rate × 0.3. Using mean weight and respiratory rate for patients in this group, it appears patients in the HFNC group were treated with flow rates less than the 1.5-2 L/kg/min recommended to be effective.2,3,12 Despite this, clinical improvement was faster in the HFNC group, including respiratory rate and effort, ability to feed, days on oxygen supplementation, and hospital LOS. ICU admission was not different between the two groups.12 The Table compares the four studies discussed above.
Given increasing use of HFNC outside the ICU, institutions risk overuse and increased healthcare costs.13 Limited data on HFNC overuse exist, but several studies report increased use after implementation on the wards without robust evidence indicating it improves outcomes.7,14 Overuse of HFNC is a concern that should be considered as institutions develop HFNC protocols. Another important consideration is safe feeding. One study examined 132 children ages one month to two years with bronchiolitis who were receiving HFNC and enteral nutrition.15 Only one patient had aspiration respiratory failure, and 12 had nutrition interruptions, demonstrating oral nutrition is generally well tolerated15 and should be considered in patients with stable respiratory status on HFNC.
CONCLUSIONS
Many children’s hospitals have extended the use of HFNC outside the ICU for children with bronchiolitis despite the paucity of evidence demonstrating its benefit over standard flow oxygen. Given variation in protocols, study designs, outcomes, and number of patients studied, it is difficult to assess its efficacy outside the ICU. However, based on the studies reviewed herein, HFNC therapy does not appear to decrease LOS, time on oxygen, or escalations of care, such as ICU transfers, positive pressure ventilation, or intubation, when used as a primary therapy.7,9,11,12 Future research will ideally use optimal flow rates to determine the effectiveness of HFNC on acute care units. Although not addressed in the above studies, additional benefits to be considered in future studies include: (1) increased critical care capacity by allowing patients to be supported on the floor and (2) the ability for patients to remain closer to home when HFNC is used in the community hospital setting.
In each of the large, randomized studies reviewed, most (66%-75%) patients treated with standard low flow oxygen were supported successfully and did not require escalation to HFNC.9,10 Hospitalists should continue to use standard low flow oxygen as first-line respiratory support for patients with bronchiolitis.1 No evidence supports the use of HFNC therapy early in a child’s inpatient course; rather, it should be used when standard oxygen therapy fails. Future research should focus on better elucidating which patients will benefit most from HFNC to prevent overuse.
Viral bronchiolitis is the most common indication for infant hospitalization in the United States.1 The treatment mainstay remains supportive care, including supplemental oxygen when indicated.1 High flow nasal cannula (HFNC) therapy delivers humidified, heated air blended with oxygen, allowing much higher flow rates than standard nasal cannula therapy and is being used more frequently in inpatient settings.
OVERVIEW AND CLINICAL QUESTION
Infants and toddlers with bronchiolitis develop increased work of breathing to preserve oxygenation and ventilation in the setting of altered airway resistance and lung compliance.2,3 In addition to oxygen supplementation, HFNC is used to reduce work of breathing through several mechanisms:2-6 (1) Nasopharyngeal dead space washout clears oxygen-depleted gas at the end of expiration, facilitating alveolar ventilation (ie, carbon dioxide retention improves); (2) High flow rates match increased inspiratory flow demands of acutely ill patients, reducing nasopharyngeal inspiratory resistance and optimizing dead space washout, thus decreasing work of breathing; (3) Adequate flow rates generate distending pressure, which prevents pharyngeal collapse, supports lung recruitment, and reduces respiratory effort (demonstrated in younger infants); and (4) HFNC systems heat and humidify the breathing gas, reducing the metabolic work required to condition cool, dry gas and improving conductance and pulmonary compliance.2-5
HFNC therapy is used more commonly in acute care units despite limited literature on its effectiveness outside the intensive care unit (ICU).7,8 We asked the question, “Does use of HFNC therapy for infants with bronchiolitis hospitalized in acute care units result in improved outcomes when compared with standard nasal cannula oxygen therapy, including length of stay (LOS), oxygen therapy duration, and preventing escalations of care such as ICU transfer, positive pressure ventilation, and intubation?” Also, do published studies provide guidance for the initiation and management of HFNC? We focused our search on studies published in the last five years that included patients with bronchiolitis treated with HFNC outside the ICU; here, we review those studies most relevant to pediatric hospitalists.
RECENT LITERATURE REVIEW
No guideline exists for initiating flow or fraction of inspired oxygen (FiO2). HFNC may be initiated for hypoxia, increased work of breathing, or both in patients with bronchiolitis. To achieve optimal dead space washout, inspiratory flow, and distending pressure, initial flow rates should be 1.5 to 2 L/kg/min, particularly for infants and young children.2-5 Weiler et al.3 evaluated the breathing effort of ICU patients at 0.5, 1, 1.5, and 2 L/kg/min and found optimal flow rates for improved work of breathing were 1.5-2 L/kg/min. The smallest patients, ≤8 kg, saw the greatest benefit, a finding likely explained by larger anatomic dead space in infants/small children compared with older children.3 For older/larger children (>20 kg), an initial flow closer to 1 L/kg/min is often appropriate.5 When used for hypoxia, initiating flow without supplemental FiO2 may improve oxygenation by flushing nasopharyngeal dead space. FiO2 should be titrated to achieve the goal set by the treatment team, often ≥90%. Improvement in heart rate and peripheral oxygen saturation (SpO2) can be observed within 60 minutes of initiating HFNC in patients responsive to therapy.6
HFNC therapy is safe when used correctly.6,9,10Potential adverse effects include pneumothorax, pressure injury, mucosal injury/bleeding, and delayed escalation to invasive ventilation. While difficult to quantify, recent studies report low rates or no serious HFNC complications. For example, only 2 of 1,127 patients supported with HFNC developed a pneumothorax and neither required evacuation.2,9-12
Inclusion criteria and HFNC protocols vary among published studies. Most HFNC protocols reviewed may not have optimally supported all of the patients in their HFNC groups, often by limiting flow to <2 L/kg/min.6-9,11,12 These variables may explain the disparate results, with some studies demonstrating apparent benefits and others no difference.7,9,10,12
Two studies of infants with bronchiolitis showed HFNC therapy may prevent ICU transfer, but this benefit may be limited to rescue when standard oxygen therapy fails, rather than as a superior initial support modality.7,9 Kepreotes et al.9 reported a single-center, randomized controlled trial comparing HFNC with standard oxygen therapy with 101 patients in each treatment arm. The primary outcome, median time to wean off oxygen, was not significantly different between the two groups: 24 hours (95% CI: 18-28) in the HFNC group versus 20 hours in the standard therapy group (95% CI: 17-34). The HFNC group had fewer treatment failures (abnormal heart rate, respiratory rate, SpO2 <90%, or severe respiratory distress score while on maximum therapy) than the standard therapy group, and 20 (63%) of the 33 patients who failed standard therapy were rescued with HFNC, avoiding transfer to the ICU. Fourteen patients from the HFNC group and 12 from the standard oxygen group required transfer to the ICU for support escalation. Although this study did not show a significant difference in oxygen weaning time between groups, it appears to support HFNC use as a rescue modality to reduce or prevent ICU transfer.9 Franklin et al.10 conducted a multicenter, randomized, controlled trial to compare standard nasal cannula oxygen therapy with HFNC (2 L/kg/min) in 1,472 patients. Patients receiving HFNC had lower care escalation rates due to treatment failure, defined as the presence of at least three of four clinical criteria and the clinician determining escalation was indicated. Oxygen therapy duration, ICU admission rates, and LOS were not significantly different between groups. Similar to the previous study, a large portion of the standard therapy patients who failed treatment (102 of 167) crossed over to the HFNC arm in an attempt to avoid ICU transfer. Twelve patients required intubation: 8 (1%) receiving HFNC and 4 (0.5%) receiving the standard therapy.10
Two additional studies, both with study design limitations, did not demonstrate differences in ICU transfer rates and had variable differences in outcomes. Riese et al.7 retrospectively assessed HFNC use outside the ICU at one institution and included 936 patients admitted before and 1,001 patients admitted after HFNC guideline implementation on the wards. Flow rates were based on age and not weight. They found no difference in LOS, ICU transfer rate, ICU LOS, intubation rates, or 30-day readmission rates, though HFNC use increased over time. The HFNC guideline is a potentially significant limitation as it may not have provided optimal flow rates to all subjects given it was based on age rather than weight. Milani et al.12 performed a single-center observational study of 36 infants aged <12 months, treated for bronchiolitis on the ward, who were informally assigned to HFNC or standard therapy based upon HFNC device availability. HFNC flow rate was determined by the equation: L/min = 8 mL/kg × respiratory rate × 0.3. Using mean weight and respiratory rate for patients in this group, it appears patients in the HFNC group were treated with flow rates less than the 1.5-2 L/kg/min recommended to be effective.2,3,12 Despite this, clinical improvement was faster in the HFNC group, including respiratory rate and effort, ability to feed, days on oxygen supplementation, and hospital LOS. ICU admission was not different between the two groups.12 The Table compares the four studies discussed above.
Given increasing use of HFNC outside the ICU, institutions risk overuse and increased healthcare costs.13 Limited data on HFNC overuse exist, but several studies report increased use after implementation on the wards without robust evidence indicating it improves outcomes.7,14 Overuse of HFNC is a concern that should be considered as institutions develop HFNC protocols. Another important consideration is safe feeding. One study examined 132 children ages one month to two years with bronchiolitis who were receiving HFNC and enteral nutrition.15 Only one patient had aspiration respiratory failure, and 12 had nutrition interruptions, demonstrating oral nutrition is generally well tolerated15 and should be considered in patients with stable respiratory status on HFNC.
CONCLUSIONS
Many children’s hospitals have extended the use of HFNC outside the ICU for children with bronchiolitis despite the paucity of evidence demonstrating its benefit over standard flow oxygen. Given variation in protocols, study designs, outcomes, and number of patients studied, it is difficult to assess its efficacy outside the ICU. However, based on the studies reviewed herein, HFNC therapy does not appear to decrease LOS, time on oxygen, or escalations of care, such as ICU transfers, positive pressure ventilation, or intubation, when used as a primary therapy.7,9,11,12 Future research will ideally use optimal flow rates to determine the effectiveness of HFNC on acute care units. Although not addressed in the above studies, additional benefits to be considered in future studies include: (1) increased critical care capacity by allowing patients to be supported on the floor and (2) the ability for patients to remain closer to home when HFNC is used in the community hospital setting.
In each of the large, randomized studies reviewed, most (66%-75%) patients treated with standard low flow oxygen were supported successfully and did not require escalation to HFNC.9,10 Hospitalists should continue to use standard low flow oxygen as first-line respiratory support for patients with bronchiolitis.1 No evidence supports the use of HFNC therapy early in a child’s inpatient course; rather, it should be used when standard oxygen therapy fails. Future research should focus on better elucidating which patients will benefit most from HFNC to prevent overuse.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-1502. https://doi.org/10.1542/peds.2014-2742.
2. Milesi C, Baleine J, Matecki S, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013;39(6):1088-1094. https://doi.org/10.1007/s00134-013-2879-y.
3. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71. https://doi.org/10.1016/j.jpeds.2017.06.006.
4. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400-1405. https://doi.org/10.1016/j.rmed.2009.04.007.
5. Milesi C, Boubal M, Jacquot A, et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann Intensive Care. 2014;4(1):29. https://doi.org/10.1186/s13613-014-0029-5.
6. Heikkila P, Sokuri P, Mecklin M, et al. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018;107(11):1971-1976. https://doi.org/10.1111/apa.14421.
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
8. Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. 2017;52(6):806-812. https://doi.org/10.1002/ppul.23626.
9. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
10. Franklin D, Babl FE, Schibler A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2446-2447. https://doi.org/10.1056/NEJMc1805312.
11. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
12. Milani GP, Plebani AM, Arturi E, et al. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr. 2016;105(8):e368-e372. https://doi.org/10.1111/apa.13444.
13. Modesto i Alapont V, Garcia Cusco M, Medina A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2444. https://doi.org/10.1056/NEJMc1805312.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-1502. https://doi.org/10.1542/peds.2014-2742.
2. Milesi C, Baleine J, Matecki S, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013;39(6):1088-1094. https://doi.org/10.1007/s00134-013-2879-y.
3. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71. https://doi.org/10.1016/j.jpeds.2017.06.006.
4. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400-1405. https://doi.org/10.1016/j.rmed.2009.04.007.
5. Milesi C, Boubal M, Jacquot A, et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann Intensive Care. 2014;4(1):29. https://doi.org/10.1186/s13613-014-0029-5.
6. Heikkila P, Sokuri P, Mecklin M, et al. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018;107(11):1971-1976. https://doi.org/10.1111/apa.14421.
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
8. Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. 2017;52(6):806-812. https://doi.org/10.1002/ppul.23626.
9. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
10. Franklin D, Babl FE, Schibler A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2446-2447. https://doi.org/10.1056/NEJMc1805312.
11. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
12. Milani GP, Plebani AM, Arturi E, et al. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr. 2016;105(8):e368-e372. https://doi.org/10.1111/apa.13444.
13. Modesto i Alapont V, Garcia Cusco M, Medina A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2444. https://doi.org/10.1056/NEJMc1805312.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
© 2020 Society of Hospital Medicine