User login
Caught in the Hotbox
A 19-year-old woman presented to the emergency department (ED) with a 14-day history of progressive fevers, night sweats, abdominal pain, nonbloody and nonbilious vomiting, diarrhea, cough, and myalgia. The fever occurred daily with no noted temporal pattern, and she had no significant weight loss. The abdominal pain was diffuse and exacerbated by eating. She experienced multiple sporadic episodes of vomiting and diarrhea daily. She denied any rash or arthralgia.
She had no known medical history and took no medications. Family history was negative for autoinflammatory and autoimmune conditions. She had emigrated from Kenya to the United States 28 days ago. Her immunization status was unknown.
This patient has prolonged fevers and evidence of multisystem involvement. The most likely etiologic categories are infectious, inflammatory, rheumatologic, and neoplastic. For febrile patients who have recently emigrated to or travelled outside of the United States, it is important to consider common infections, as well as those endemic to the nation of exposure, which in this case includes malaria, typhoid fever, tuberculosis, cholera, acute viral hepatitis, chikungunya fever, dengue fever, yellow fever, and rickettsial disease. All of these, other than tuberculosis, commonly present with fever, vomiting, diarrhea, and myalgia. She may also have bacterial pneumonia or influenza given her fever and cough, although the chronicity and persistence of symptoms would be atypical. Acute infectious gastroenteritis is a common cause of fever, vomiting, and diarrhea. Most cases resolve in 7 to 10 days, so the duration raises suspicion for a nonviral etiology or an immunocompromised state.
Inflammatory causes could include the first presentation of inflammatory bowel disease (IBD), particularly if the patient develops weight loss or eye, skin, or joint manifestations. The lack of rash or arthralgia makes rheumatologic conditions less likely. Prolonged fevers and night sweats could indicate malignancy such as intra-abdominal lymphoma, although infectious etiologies should be ruled out first.
Previously, on day 9 of symptoms, the patient presented to an ED at another institution. Laboratory evaluation at that time demonstrated an elevated aspartate aminotransferase (AST) level of 229 IU/L (reference, 0-40 IU/L) and alanine aminotransferase (ALT) level of 60 IU/L (reference, 0-32 IU/L) with normal alkaline phosphatase and bilirubin levels, proteinuria to 3+ (normal, negative/trace), ketonuria to 2+ (normal, negative), and hematuria to 2+ (normal, negative). Complete blood count and electrolytes were normal. Computed tomography (CT) scans of the chest, abdomen, and pelvis with intravenous contrast were normal and without evidence of organomegaly.
AST and ALT elevations often indicate hepatocellular damage, although the normal bilirubin levels suggest normal hepatic function. Because CT may miss extrahepatic biliary pathology, a right upper quadrant ultrasound should be obtained to better evaluate patency of the biliary system and hepatic echotexture. Coagulation studies and viral hepatitis serology should be obtained. The disproportionate elevation of AST versus ALT can suggest alcohol use or nonhepatic etiologies such as myositis. Acute viral hepatitis is less likely given there is only mild to moderate elevation in aminotransferase levels. However, the remaining infectious etiologies can have this level of elevation and should still be considered.
Enteritis and IBD are still considerations despite the normal CT results. Transient asymptomatic hematuria or proteinuria can be seen in multiple conditions, particularly proteinuria with febrile illnesses. Urine microscopy to evaluate for casts could indicate a glomerular origin of the hematuria. First morning urine protein-to-creatinine ratio would help quantify the degree of proteinuria. Serum creatinine level should be measured to determine whether there is any renal dysfunction.
While early imaging can be falsely negative, the unremarkable chest CT makes pneumonia and active pulmonary tuberculosis less likely.
Vital signs during this presentation were: temperature, 39.7 °C; heart rate, 126 beats per minute; blood pressure, 109/64 mm Hg; respiratory rate, 20 breaths per minute; and oxygen saturation, 98% on room air. She was ill-appearing, with diffuse abdominal tenderness without peritoneal signs. Other than her tachycardia, findings from her cardiopulmonary, neurologic, and skin exams were normal.
Laboratory testing revealed a white blood cell count of 4,300/µL (reference range, 4,500-13,000/µL), a hemoglobin level of 10.9 g/dL (reference range, 11.7-15.7 g/dL) with a mean corpuscular volume of 77 fL (reference range, 80-96 fL) and reticulocyte percentage of 0.5% (reference range, 0.5%-1.5%), and a platelet count of 59,000/µL (reference range, 135,000-466,000/µL). Her prothrombin time was 13.5 seconds (reference range, 9.6-11.6 seconds) with an international normalized ratio of 1.3 (reference range, 0.8-1.1), erythrocyte sedimentation rate of 46 mm/h (reference range, 0-20 mm/h), C-reactive protein level of 7.49 mg/dL (reference range, <0.3 mg/dL), and AST level of 194 units/L (reference range, 9-35 units/L). ALT, total and direct bilirubin, lipase, electrolytes, and creatinine levels were normal. An abdominal x-ray showed scattered air-fluid levels in a nonobstructed pattern.
Her mildly elevated prothrombin time and international normalized ratio suggest a coagulopathy involving either her extrinsic or common coagulation pathway, with disseminated intravascular coagulation (DIC) being most likely given her new thrombocytopenia and anemia. Hemolytic uremic syndrome and thrombotic thrombocytopenic purpura should be considered but would not cause coagulopathy. A peripheral smear to evaluate for schistocytes associated with microangiopathic hemolysis and serum fibrinogen to distinguish between DIC (low) and thrombocytopenic purpura or hemolytic uremic syndrome (normal or elevated) should be obtained. A thick and thin smear for malaria should also be performed.
Her new pancytopenia suggests bone marrow suppression or infiltration with or without a concomitant consumptive process such as sepsis with resulting DIC. Given her clinical picture, marrow infiltrative processes might include tuberculosis or malignancy, and marrow suppression may be caused by HIV or other viral infection. If she is found to have HIV, disseminated fungal or mycobacterial infections would become more likely. She now has an isolated elevated AST level, which could be secondary to hemolysis rather than hepatocyte damage. Findings from her abdominal exam are nonfocal; this is consistent with her x-ray findings, which reflect possible enteritis or colitis.
The most likely diagnosis currently is an infectious enteritis with resulting hematologic and hepatic abnormalities. Given her recent emigration from Kenya, typhoid fever and cholera are both possible, although cholera typically does not present with prolonged fever or severe abdominal pain. The severity and duration of her illness, and the abnormalities of her laboratory findings, warrant empiric therapy with ceftriaxone to treat possible severe Salmonella enterica infection while awaiting blood and stool cultures.
The patient was admitted to the hospital and her symptoms continued. Results of serum HIV 1 and 2 polymerase chain reactions, herpes simplex virus 1 and 2 polymerase chain reactions, three malaria smears, human T-lymphotropic virus serologies, Toxoplasma serology, Bartonella serology, a stool culture, and multiple large volume blood cultures were negative. Serologic testing for hepatitis A, B, and C, Epstein-Barr virus, cytomegalovirus, and dengue virus were negative for acute infection. Results of an interferon-gamma release assay were indeterminate; results of purified protein derivative (PPD) and Candida antigen control testing were both negative. Ceruloplasmin and α1-antitrypsin levels were normal. An abdominal ultrasound showed central intrahepatic biliary duct dilatation, splenomegaly, and sluggish portal venous flow.
While central intrahepatic biliary ductal dilation could be caused by an obstructive lesion, none were seen on CT or ultrasound. Her normal alkaline phosphatase and bilirbuin levels also suggest functional patency of the biliary system. The thrombocytopenia, splenomegaly, and sluggish portal venous flow suggest possible portal hypertension, though no cirrhotic changes were noted on the ultrasound or abdominal CT. Her negative PPD and Candida antigen control results may suggest underlying immune dysregulation or suppression, though anergy could be secondary to sepsis.
Given her negative initial infectious evaluation, other etiologies such as atypical infections, rheumatologic disorders, and malignancies warrant consideration. She has no murmur; however, subacute bacterial endocarditis with a fastidious organism is possible, which could be investigated with a transthoracic echocardiogram. Other tests to consider include blood cultures for fungi and atypical mycobacterial species, and serology for Coxiella burnetii, chikungunya virus, and yellow fever. Rheumatologic conditions such as systemic lupus erythematosus, hemophagocytic lymphohistiocytosis (HLH), or adult Still’s disease should be considered. Complement levels and an antinuclear antibody panel, including those for dsDNA and Smith antigen, should be performed to evaluate for systemic lupus erythematosus. Serum ferritin, fibrinogen, and triglyceride levels should be measured to evaluate for HLH. Hematologic malignancy is also a consideration, particularly given her pancytopenia. Multicentric Castleman disease can cause prolonged fevers, pancytopenia, and elevated inflammatory markers, but is less likely without lymphadenopathy. A peripheral blood smear should be sent, and a bone marrow biopsy may be needed.
Empiric ciprofloxacin was initiated; however, the patient continued to have fevers up to 39.9 °C, abdominal pain, and myalgia. Ferritin level was over 3,000 ng/mL (reference range, 8-255 ng/mL), and a soluble interleukin-2 (IL-2) receptor level was 1,188 units/mL (reference range, 45-1,105 units/mL). Triglycerides were normal.
The elevated ferritin and soluble IL-2 levels raise concern for HLH. Hyperferritinemia is relatively nonspecific because extremely elevated ferritin can be seen with other conditions, such as renal failure, hepatocellular injury, infection, rheumatologic conditions, and hematologic malignancy. Soluble IL-2 receptor elevation is more specific for HLH than ferritin or triglycerides, but alone does not make the diagnosis because it can be elevated in other rheumatologic disorders and malignancy. The HLH-2004 criteria are commonly used and require either molecular diagnostic testing or meeting at least five out of eight clinical and lab criteria to make the diagnosis. Our patient currently meets three criteria (fever, splenomegaly, and elevated ferritin). Elevated soluble IL-2 is part of the HLH-2004 criteria, but her level of elevation does not meet the required threshold (≥2,400 units/mL). Her cytopenias have also not quite met the HLH-2004 thresholds (two of the following three: hemoglobin <9 g/dL, platelets <100,000/µL, and/or absolute neutrophil count <1,000/µL). Elevated aminotransferase levels and DIC are not part of the HLH-2004 criteria but are often seen with HLH.
Evaluation for an underlying infectious, rheumatologic, or malignant trigger should continue as previously discussed. If this patient does have HLH, it is most likely secondary to an infection, autoimmune disease, or malignancy rather than genetic HLH. HLH has a high mortality rate, but before beginning treatment with immunosuppressive agents, a peripheral smear and a bone marrow biopsy should be performed to evaluate for hematologic malignancy or signs of hemophagocytosis.
Empiric ciprofloxacin covers most bacterial etiologies of diarrhea, including those previously mentioned such as cholera and most strains of S enterica. Her symptoms and laboratory findings (including cytopenias, elevated aminotransferases, and coagulopathy) could suggest enteric fever due to S enterica serovar Typhi, which is endemic in Kenya. Results of blood and stool cultures, though negative, are relatively insensitive for this organism, particularly this far into the illness course. A bone marrow biopsy may also help with diagnosis of occult typhoid fever because marrow culture can be more sensitive than blood or stool culture.
A bone marrow aspiration revealed hemophagocytic histiocytes, no malignant cells, and negative bacterial (including anaerobic), fungal, and acid-fast bacilli cultures. Considering the high mortality rate of untreated HLH/macrophage activation syndrome (MAS), empiric glucocorticoid administration was considered. However, this was withheld due to concern for ongoing undetected infection given her persistent fever and systemic symptoms.
There should still be high suspicion for HLH. Further evaluation for other laboratory manifestations of HLH such as fibrinogen and natural killer cell activity should be considered, as well as repeating her complete blood count to see if her cytopenias have progressed. Her marrow shows no evidence of hematologic malignancy, so other triggers of possible HLH should be sought out by continuing the workup. Consulting specialists from rheumatology and infectious disease may help clarify possible underlying diagnoses and the best management plan. If she continues to have organ damage or clinically worsens, it may be prudent to empirically broaden her antibiotic coverage and begin antifungal agents while starting glucocorticoid therapy for suspected HLH.
A stool molecular screen from admission was returned positive for S enterica serovar Typhi. Ciprofloxacin was discontinued and ceftriaxone was started out of concern for antibiotic resistance. On hospital day 14, the patient’s brother presented to the ED with fever. His blood and stool cultures were positive for S enterica serovar Typhi with intermediate sensitivity to ciprofloxacin and sensitivity to ceftriaxone. With continued treatment with ceftriaxone, the patient improved significantly. Following discharge, she remained afebrile and asymptomatic. During outpatient follow up, a repeat PPD was positive and she was diagnosed with and treated for latent tuberculosis.
COMMENTARY
The evaluation of a patient who has recently emigrated from a foreign nation requires a broad differential diagnosis and a keen awareness of the clinical conditions present in the patient’s country of origin. This often involves knowledge of diseases infrequently encountered in daily practice, as well as awareness of the nuances of rare presentations and possible complications. When the presentation is not classic for a relevant infectious disease and clinical conditions from other diagnostic classes are considered, the evaluation and management of the patient is particularly challenging.
Typhoid fever is a severe systemic illness caused by the organism S enterica serovar Typhi. The organism is ingested, penetrates the small intestinal epithelium, enters the lymphoid tissue, and disseminates via the lymphatic and hematogenous routes. Onset of symptoms typically occurs 5 to 21 days after ingestion of contaminated food or water. Clinical features include fever, chills, relative bradycardia (pulse-temperature dissociation), abdominal pain, rose spots (salmon-colored macules) on the trunk and abdomen, and hepatosplenomegaly. Diarrhea is not a typical symptom of patients with typhoid fever, which can lead to a delayed or missed diagnosis. Life-threatening complications can be seen, including gastrointestinal bleeding, intestinal perforation, and meningitis.1 Typhoid fever is most prevalent in impoverished areas with poor access to sanitation. Regions with the highest incidence include south-central Asia, southeast Asia, and southern Africa.2-4 Approximately 200 to 300 cases are reported in the United States each year.5
Classically, the diagnosis is made by means of clinical symptoms and a positive culture from a sterile site. A recent study of 529 patients found that 61% had positive blood cultures and 96% had positive bone marrow cultures.6 Our patient’s diagnosis was significantly delayed by multiple negative cultures and failure to improve on first-line antibiotics, which initially suggested that the S enterica serovar Typhi stool molecular screen likely represented carriage caused by colonization. Chronic S enterica serovar Typhi carriage is defined as excretion of the organism in stool or urine 1 year or longer after acute infection. Rates of carriage range from 1% to 6%, and up to 25% of carriers have no history of typhoid fever.1,7,8 Carriage is more common in females and in those with biliary tract abnormalities.9,10
Once a presumptive diagnosis is made, antibiotic choice remains a challenge. Resistance to fluoroquinolones, the preferred drug for multidrug-resistant typhoid fever, is growing but remains rare, at approximately 5%.11,12 Ceftriaxone and azithromycin have been used successfully in areas with high resistance.13 Given the patient’s slow response to therapy even after transitioning from ciprofloxacin to ceftriaxone, her brother’s presentation and obtaining the antibiotic sensitivities for his organism were critical to confirming that our diagnosis and management decisions were correct.
One strongly considered diagnosis was HLH/MAS. MAS is an aggressive syndrome of excessive inflammation and tissue destruction caused by inappropriate immune system activation. It belongs to a group of histiocytic disorders collectively known as HLH. Aside from primary (genetic) forms, secondary forms exist that can be triggered by malignancy, infection, or rheumatologic disorders. In infection-associated HLH/MAS, viral infections are a common trigger, with Epstein-Barr virus being the most common. Association with bacterial infections, including tuberculosis and typhoid fever, has also been reported.14 Prompt therapy, often with immunosuppressive agents such as glucocorticoids, is essential for survival because there is a reported mortality rate of up to 50% when untreated.15 When infection-induced HLH/MAS occurs, treatment of the underlying infection is critical.14,15 The greatest barrier to a favorable outcome from HLH/MAS is often a delay in diagnosis because the rarity of this disease, the variable clinical presentation, and the lack of specificity of the clinical and laboratory findings make a conclusive diagnosis challenging.
In the presented case, diagnostic uncertainty challenged the decision to administer systemic glucocorticoids. Glucocorticoids conferred a risk of harm for multiple diagnoses that remained on the differential, including malignancy and infection. Her diagnostic evaluation made malignancy less likely, but because testing was unable to rule out tuberculosis as either the underlying cause or coinfection, the team opted to defer initiating glucocorticoids and instead closely monitor for a worsening inflammatory response. Following appropriate treatment of her systemic infection, her PPD was repeated and became positive. The negative PPD and Candida control obtained during her hospitalization were, therefore, likely caused by anergy in the setting of overwhelming systemic illness. Initiation of glucocorticoids prematurely in this case could have led to further harm because immunosuppression may have led to reactivation of latent tuberculosis or exacerbation of illness from an alternative but then undiagnosed infection.
The patient’s ultimate unifying diagnosis was typhoid fever; however, there are mixed expert opinions as to whether the systemic immune activation was significant enough to merit the diagnosis of infection-induced secondary HLH/MAS. Despite the high morbidity and mortality that can accompany HLH/MAS, it has been reported that a significant proportion of cases of secondary HLH/MAS can be managed effectively with treatment of the underlying etiology; this may have been the case for our patient.14,15 The clinicians in this case were caught between diagnoses, unable to safely reach either one—much like a baseball player stranded between bases. Fortunately for this patient, the diagnosis ultimately emerged after a careful and thorough workup, assisted by the more straightforward diagnosis of her brother with the same disease.
KEY TEACHING POINTS
- Salmonella enterica serovar Typhi has a high false-negative rate in blood and stool cultures; therefore, clinical suspicion should remain high in the setting of a high pre-test probability.
- Fluoroquinolones are traditionally first-line therapy for typhoid fever, but the use of ceftriaxone and azithromycin is increasing because of rising fluoroquinolone resistance.
- Hemophagocytic lymphohistiocytosis/macrophage activation syndrome is characterized by excessive inflammation and tissue destruction caused by inappropriate immune system activation. This syndrome can be fatal without appropriate immunosuppressive therapy; however, glucocorticoid administration must be pursued with caution when infection and malignancy are on the differential diagnosis.
1. Parry CM, Hien TT, Dougan G, et al. Typhoid fever. N Engl J Med. 2002;347(22):1770-1782. https://doi.org/10.1056/nejmra020201
2. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82(5):346-353.
3. Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2(1):010401. https://doi.org/10.7189/jogh.02.010401
4. Mogasale V, Maskery B, Ochiai RL, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2(10):e570-e580. https://doi.org/10.1016/s2214-109x(14)70301-8
5. Lynch MF, Blanton EM, Bulens S, et al. Typhoid fever in the United States, 1999-2006. JAMA. 2009;302(8):859-865. https://doi.org/10.1001/jama.2009.1229
6. Mogasale V, Ramani E, Mogasale VV, Park J. What proportion of Salmonella typhi cases are detected by blood culture? a systematic literature review. Ann Clin Microbiol Antimicrob. 2016;15(1):32. https://doi.org/10.1186/s12941-016-0147-z
7. Merselis JG Jr, Kaye D, Connolly CS, Hook EW. Quantitative bacteriology of the Typhoid carrier state. Am J Trop Med Hyg. 1964;13:425-429. https://doi.org/10.4269/ajtmh.1964.13.425
8. Lanata CF, Levine MM, Ristori C, et al. Vi serology in detection of chronic Salmonella typhi carriers in an endemic area. Lancet. 1983;2(8347):441-443. https://doi.org/10.1016/s0140-6736(83)90401-4
9. Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. Common bile duct stones: a cause of chronic salmonellosis. Am J Gastroenterol. 1992;87(9):1198-1199.
10. Hofmann E, Chianale J, Rollán A, Pereira J, Ferrecio C, Sotomayor V. Blood group antigen secretion and gallstone disease in the Salmonella typhi chronic carrier state. J Infect Dis. 1993;167(4):993-994. https://doi.org/10.1093/infdis/167.4.993
11. Steel AD, Hay Burgess DC, Diaz Z, Carey ME, Zaidi AKM. Challenges and opportunities for typhoid fever control: a call for coordinated action. Clin Infect Dis. 2016;62 (Suppl 1):S4-S8. https://doi.org/10.1093/cid/civ976
12. Hendriksen RS, Leekitcharoenphon P, Lukjancenko O, et al. Genomic signature of multidrug resistant Salmonella enterica serovar Typhi isolates related to a massive outbreak in Zambia between 2010 and 2012. J Clin Microbiol. 2015;53(1):262-272. https://doi.org/10.1128/jcm.02026-14
13. Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of Salmonella infections. Clin Microbiol Rev. 2015;28(4):901-936. https://doi.org/10.1128/cmr.00002-15
14. Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7(12):814-822. https://doi.org/10.1016/s1473-3099(07)70290-6
15. Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000;6(6):601-608. https://doi.org/10.3201/eid0606.000608
A 19-year-old woman presented to the emergency department (ED) with a 14-day history of progressive fevers, night sweats, abdominal pain, nonbloody and nonbilious vomiting, diarrhea, cough, and myalgia. The fever occurred daily with no noted temporal pattern, and she had no significant weight loss. The abdominal pain was diffuse and exacerbated by eating. She experienced multiple sporadic episodes of vomiting and diarrhea daily. She denied any rash or arthralgia.
She had no known medical history and took no medications. Family history was negative for autoinflammatory and autoimmune conditions. She had emigrated from Kenya to the United States 28 days ago. Her immunization status was unknown.
This patient has prolonged fevers and evidence of multisystem involvement. The most likely etiologic categories are infectious, inflammatory, rheumatologic, and neoplastic. For febrile patients who have recently emigrated to or travelled outside of the United States, it is important to consider common infections, as well as those endemic to the nation of exposure, which in this case includes malaria, typhoid fever, tuberculosis, cholera, acute viral hepatitis, chikungunya fever, dengue fever, yellow fever, and rickettsial disease. All of these, other than tuberculosis, commonly present with fever, vomiting, diarrhea, and myalgia. She may also have bacterial pneumonia or influenza given her fever and cough, although the chronicity and persistence of symptoms would be atypical. Acute infectious gastroenteritis is a common cause of fever, vomiting, and diarrhea. Most cases resolve in 7 to 10 days, so the duration raises suspicion for a nonviral etiology or an immunocompromised state.
Inflammatory causes could include the first presentation of inflammatory bowel disease (IBD), particularly if the patient develops weight loss or eye, skin, or joint manifestations. The lack of rash or arthralgia makes rheumatologic conditions less likely. Prolonged fevers and night sweats could indicate malignancy such as intra-abdominal lymphoma, although infectious etiologies should be ruled out first.
Previously, on day 9 of symptoms, the patient presented to an ED at another institution. Laboratory evaluation at that time demonstrated an elevated aspartate aminotransferase (AST) level of 229 IU/L (reference, 0-40 IU/L) and alanine aminotransferase (ALT) level of 60 IU/L (reference, 0-32 IU/L) with normal alkaline phosphatase and bilirubin levels, proteinuria to 3+ (normal, negative/trace), ketonuria to 2+ (normal, negative), and hematuria to 2+ (normal, negative). Complete blood count and electrolytes were normal. Computed tomography (CT) scans of the chest, abdomen, and pelvis with intravenous contrast were normal and without evidence of organomegaly.
AST and ALT elevations often indicate hepatocellular damage, although the normal bilirubin levels suggest normal hepatic function. Because CT may miss extrahepatic biliary pathology, a right upper quadrant ultrasound should be obtained to better evaluate patency of the biliary system and hepatic echotexture. Coagulation studies and viral hepatitis serology should be obtained. The disproportionate elevation of AST versus ALT can suggest alcohol use or nonhepatic etiologies such as myositis. Acute viral hepatitis is less likely given there is only mild to moderate elevation in aminotransferase levels. However, the remaining infectious etiologies can have this level of elevation and should still be considered.
Enteritis and IBD are still considerations despite the normal CT results. Transient asymptomatic hematuria or proteinuria can be seen in multiple conditions, particularly proteinuria with febrile illnesses. Urine microscopy to evaluate for casts could indicate a glomerular origin of the hematuria. First morning urine protein-to-creatinine ratio would help quantify the degree of proteinuria. Serum creatinine level should be measured to determine whether there is any renal dysfunction.
While early imaging can be falsely negative, the unremarkable chest CT makes pneumonia and active pulmonary tuberculosis less likely.
Vital signs during this presentation were: temperature, 39.7 °C; heart rate, 126 beats per minute; blood pressure, 109/64 mm Hg; respiratory rate, 20 breaths per minute; and oxygen saturation, 98% on room air. She was ill-appearing, with diffuse abdominal tenderness without peritoneal signs. Other than her tachycardia, findings from her cardiopulmonary, neurologic, and skin exams were normal.
Laboratory testing revealed a white blood cell count of 4,300/µL (reference range, 4,500-13,000/µL), a hemoglobin level of 10.9 g/dL (reference range, 11.7-15.7 g/dL) with a mean corpuscular volume of 77 fL (reference range, 80-96 fL) and reticulocyte percentage of 0.5% (reference range, 0.5%-1.5%), and a platelet count of 59,000/µL (reference range, 135,000-466,000/µL). Her prothrombin time was 13.5 seconds (reference range, 9.6-11.6 seconds) with an international normalized ratio of 1.3 (reference range, 0.8-1.1), erythrocyte sedimentation rate of 46 mm/h (reference range, 0-20 mm/h), C-reactive protein level of 7.49 mg/dL (reference range, <0.3 mg/dL), and AST level of 194 units/L (reference range, 9-35 units/L). ALT, total and direct bilirubin, lipase, electrolytes, and creatinine levels were normal. An abdominal x-ray showed scattered air-fluid levels in a nonobstructed pattern.
Her mildly elevated prothrombin time and international normalized ratio suggest a coagulopathy involving either her extrinsic or common coagulation pathway, with disseminated intravascular coagulation (DIC) being most likely given her new thrombocytopenia and anemia. Hemolytic uremic syndrome and thrombotic thrombocytopenic purpura should be considered but would not cause coagulopathy. A peripheral smear to evaluate for schistocytes associated with microangiopathic hemolysis and serum fibrinogen to distinguish between DIC (low) and thrombocytopenic purpura or hemolytic uremic syndrome (normal or elevated) should be obtained. A thick and thin smear for malaria should also be performed.
Her new pancytopenia suggests bone marrow suppression or infiltration with or without a concomitant consumptive process such as sepsis with resulting DIC. Given her clinical picture, marrow infiltrative processes might include tuberculosis or malignancy, and marrow suppression may be caused by HIV or other viral infection. If she is found to have HIV, disseminated fungal or mycobacterial infections would become more likely. She now has an isolated elevated AST level, which could be secondary to hemolysis rather than hepatocyte damage. Findings from her abdominal exam are nonfocal; this is consistent with her x-ray findings, which reflect possible enteritis or colitis.
The most likely diagnosis currently is an infectious enteritis with resulting hematologic and hepatic abnormalities. Given her recent emigration from Kenya, typhoid fever and cholera are both possible, although cholera typically does not present with prolonged fever or severe abdominal pain. The severity and duration of her illness, and the abnormalities of her laboratory findings, warrant empiric therapy with ceftriaxone to treat possible severe Salmonella enterica infection while awaiting blood and stool cultures.
The patient was admitted to the hospital and her symptoms continued. Results of serum HIV 1 and 2 polymerase chain reactions, herpes simplex virus 1 and 2 polymerase chain reactions, three malaria smears, human T-lymphotropic virus serologies, Toxoplasma serology, Bartonella serology, a stool culture, and multiple large volume blood cultures were negative. Serologic testing for hepatitis A, B, and C, Epstein-Barr virus, cytomegalovirus, and dengue virus were negative for acute infection. Results of an interferon-gamma release assay were indeterminate; results of purified protein derivative (PPD) and Candida antigen control testing were both negative. Ceruloplasmin and α1-antitrypsin levels were normal. An abdominal ultrasound showed central intrahepatic biliary duct dilatation, splenomegaly, and sluggish portal venous flow.
While central intrahepatic biliary ductal dilation could be caused by an obstructive lesion, none were seen on CT or ultrasound. Her normal alkaline phosphatase and bilirbuin levels also suggest functional patency of the biliary system. The thrombocytopenia, splenomegaly, and sluggish portal venous flow suggest possible portal hypertension, though no cirrhotic changes were noted on the ultrasound or abdominal CT. Her negative PPD and Candida antigen control results may suggest underlying immune dysregulation or suppression, though anergy could be secondary to sepsis.
Given her negative initial infectious evaluation, other etiologies such as atypical infections, rheumatologic disorders, and malignancies warrant consideration. She has no murmur; however, subacute bacterial endocarditis with a fastidious organism is possible, which could be investigated with a transthoracic echocardiogram. Other tests to consider include blood cultures for fungi and atypical mycobacterial species, and serology for Coxiella burnetii, chikungunya virus, and yellow fever. Rheumatologic conditions such as systemic lupus erythematosus, hemophagocytic lymphohistiocytosis (HLH), or adult Still’s disease should be considered. Complement levels and an antinuclear antibody panel, including those for dsDNA and Smith antigen, should be performed to evaluate for systemic lupus erythematosus. Serum ferritin, fibrinogen, and triglyceride levels should be measured to evaluate for HLH. Hematologic malignancy is also a consideration, particularly given her pancytopenia. Multicentric Castleman disease can cause prolonged fevers, pancytopenia, and elevated inflammatory markers, but is less likely without lymphadenopathy. A peripheral blood smear should be sent, and a bone marrow biopsy may be needed.
Empiric ciprofloxacin was initiated; however, the patient continued to have fevers up to 39.9 °C, abdominal pain, and myalgia. Ferritin level was over 3,000 ng/mL (reference range, 8-255 ng/mL), and a soluble interleukin-2 (IL-2) receptor level was 1,188 units/mL (reference range, 45-1,105 units/mL). Triglycerides were normal.
The elevated ferritin and soluble IL-2 levels raise concern for HLH. Hyperferritinemia is relatively nonspecific because extremely elevated ferritin can be seen with other conditions, such as renal failure, hepatocellular injury, infection, rheumatologic conditions, and hematologic malignancy. Soluble IL-2 receptor elevation is more specific for HLH than ferritin or triglycerides, but alone does not make the diagnosis because it can be elevated in other rheumatologic disorders and malignancy. The HLH-2004 criteria are commonly used and require either molecular diagnostic testing or meeting at least five out of eight clinical and lab criteria to make the diagnosis. Our patient currently meets three criteria (fever, splenomegaly, and elevated ferritin). Elevated soluble IL-2 is part of the HLH-2004 criteria, but her level of elevation does not meet the required threshold (≥2,400 units/mL). Her cytopenias have also not quite met the HLH-2004 thresholds (two of the following three: hemoglobin <9 g/dL, platelets <100,000/µL, and/or absolute neutrophil count <1,000/µL). Elevated aminotransferase levels and DIC are not part of the HLH-2004 criteria but are often seen with HLH.
Evaluation for an underlying infectious, rheumatologic, or malignant trigger should continue as previously discussed. If this patient does have HLH, it is most likely secondary to an infection, autoimmune disease, or malignancy rather than genetic HLH. HLH has a high mortality rate, but before beginning treatment with immunosuppressive agents, a peripheral smear and a bone marrow biopsy should be performed to evaluate for hematologic malignancy or signs of hemophagocytosis.
Empiric ciprofloxacin covers most bacterial etiologies of diarrhea, including those previously mentioned such as cholera and most strains of S enterica. Her symptoms and laboratory findings (including cytopenias, elevated aminotransferases, and coagulopathy) could suggest enteric fever due to S enterica serovar Typhi, which is endemic in Kenya. Results of blood and stool cultures, though negative, are relatively insensitive for this organism, particularly this far into the illness course. A bone marrow biopsy may also help with diagnosis of occult typhoid fever because marrow culture can be more sensitive than blood or stool culture.
A bone marrow aspiration revealed hemophagocytic histiocytes, no malignant cells, and negative bacterial (including anaerobic), fungal, and acid-fast bacilli cultures. Considering the high mortality rate of untreated HLH/macrophage activation syndrome (MAS), empiric glucocorticoid administration was considered. However, this was withheld due to concern for ongoing undetected infection given her persistent fever and systemic symptoms.
There should still be high suspicion for HLH. Further evaluation for other laboratory manifestations of HLH such as fibrinogen and natural killer cell activity should be considered, as well as repeating her complete blood count to see if her cytopenias have progressed. Her marrow shows no evidence of hematologic malignancy, so other triggers of possible HLH should be sought out by continuing the workup. Consulting specialists from rheumatology and infectious disease may help clarify possible underlying diagnoses and the best management plan. If she continues to have organ damage or clinically worsens, it may be prudent to empirically broaden her antibiotic coverage and begin antifungal agents while starting glucocorticoid therapy for suspected HLH.
A stool molecular screen from admission was returned positive for S enterica serovar Typhi. Ciprofloxacin was discontinued and ceftriaxone was started out of concern for antibiotic resistance. On hospital day 14, the patient’s brother presented to the ED with fever. His blood and stool cultures were positive for S enterica serovar Typhi with intermediate sensitivity to ciprofloxacin and sensitivity to ceftriaxone. With continued treatment with ceftriaxone, the patient improved significantly. Following discharge, she remained afebrile and asymptomatic. During outpatient follow up, a repeat PPD was positive and she was diagnosed with and treated for latent tuberculosis.
COMMENTARY
The evaluation of a patient who has recently emigrated from a foreign nation requires a broad differential diagnosis and a keen awareness of the clinical conditions present in the patient’s country of origin. This often involves knowledge of diseases infrequently encountered in daily practice, as well as awareness of the nuances of rare presentations and possible complications. When the presentation is not classic for a relevant infectious disease and clinical conditions from other diagnostic classes are considered, the evaluation and management of the patient is particularly challenging.
Typhoid fever is a severe systemic illness caused by the organism S enterica serovar Typhi. The organism is ingested, penetrates the small intestinal epithelium, enters the lymphoid tissue, and disseminates via the lymphatic and hematogenous routes. Onset of symptoms typically occurs 5 to 21 days after ingestion of contaminated food or water. Clinical features include fever, chills, relative bradycardia (pulse-temperature dissociation), abdominal pain, rose spots (salmon-colored macules) on the trunk and abdomen, and hepatosplenomegaly. Diarrhea is not a typical symptom of patients with typhoid fever, which can lead to a delayed or missed diagnosis. Life-threatening complications can be seen, including gastrointestinal bleeding, intestinal perforation, and meningitis.1 Typhoid fever is most prevalent in impoverished areas with poor access to sanitation. Regions with the highest incidence include south-central Asia, southeast Asia, and southern Africa.2-4 Approximately 200 to 300 cases are reported in the United States each year.5
Classically, the diagnosis is made by means of clinical symptoms and a positive culture from a sterile site. A recent study of 529 patients found that 61% had positive blood cultures and 96% had positive bone marrow cultures.6 Our patient’s diagnosis was significantly delayed by multiple negative cultures and failure to improve on first-line antibiotics, which initially suggested that the S enterica serovar Typhi stool molecular screen likely represented carriage caused by colonization. Chronic S enterica serovar Typhi carriage is defined as excretion of the organism in stool or urine 1 year or longer after acute infection. Rates of carriage range from 1% to 6%, and up to 25% of carriers have no history of typhoid fever.1,7,8 Carriage is more common in females and in those with biliary tract abnormalities.9,10
Once a presumptive diagnosis is made, antibiotic choice remains a challenge. Resistance to fluoroquinolones, the preferred drug for multidrug-resistant typhoid fever, is growing but remains rare, at approximately 5%.11,12 Ceftriaxone and azithromycin have been used successfully in areas with high resistance.13 Given the patient’s slow response to therapy even after transitioning from ciprofloxacin to ceftriaxone, her brother’s presentation and obtaining the antibiotic sensitivities for his organism were critical to confirming that our diagnosis and management decisions were correct.
One strongly considered diagnosis was HLH/MAS. MAS is an aggressive syndrome of excessive inflammation and tissue destruction caused by inappropriate immune system activation. It belongs to a group of histiocytic disorders collectively known as HLH. Aside from primary (genetic) forms, secondary forms exist that can be triggered by malignancy, infection, or rheumatologic disorders. In infection-associated HLH/MAS, viral infections are a common trigger, with Epstein-Barr virus being the most common. Association with bacterial infections, including tuberculosis and typhoid fever, has also been reported.14 Prompt therapy, often with immunosuppressive agents such as glucocorticoids, is essential for survival because there is a reported mortality rate of up to 50% when untreated.15 When infection-induced HLH/MAS occurs, treatment of the underlying infection is critical.14,15 The greatest barrier to a favorable outcome from HLH/MAS is often a delay in diagnosis because the rarity of this disease, the variable clinical presentation, and the lack of specificity of the clinical and laboratory findings make a conclusive diagnosis challenging.
In the presented case, diagnostic uncertainty challenged the decision to administer systemic glucocorticoids. Glucocorticoids conferred a risk of harm for multiple diagnoses that remained on the differential, including malignancy and infection. Her diagnostic evaluation made malignancy less likely, but because testing was unable to rule out tuberculosis as either the underlying cause or coinfection, the team opted to defer initiating glucocorticoids and instead closely monitor for a worsening inflammatory response. Following appropriate treatment of her systemic infection, her PPD was repeated and became positive. The negative PPD and Candida control obtained during her hospitalization were, therefore, likely caused by anergy in the setting of overwhelming systemic illness. Initiation of glucocorticoids prematurely in this case could have led to further harm because immunosuppression may have led to reactivation of latent tuberculosis or exacerbation of illness from an alternative but then undiagnosed infection.
The patient’s ultimate unifying diagnosis was typhoid fever; however, there are mixed expert opinions as to whether the systemic immune activation was significant enough to merit the diagnosis of infection-induced secondary HLH/MAS. Despite the high morbidity and mortality that can accompany HLH/MAS, it has been reported that a significant proportion of cases of secondary HLH/MAS can be managed effectively with treatment of the underlying etiology; this may have been the case for our patient.14,15 The clinicians in this case were caught between diagnoses, unable to safely reach either one—much like a baseball player stranded between bases. Fortunately for this patient, the diagnosis ultimately emerged after a careful and thorough workup, assisted by the more straightforward diagnosis of her brother with the same disease.
KEY TEACHING POINTS
- Salmonella enterica serovar Typhi has a high false-negative rate in blood and stool cultures; therefore, clinical suspicion should remain high in the setting of a high pre-test probability.
- Fluoroquinolones are traditionally first-line therapy for typhoid fever, but the use of ceftriaxone and azithromycin is increasing because of rising fluoroquinolone resistance.
- Hemophagocytic lymphohistiocytosis/macrophage activation syndrome is characterized by excessive inflammation and tissue destruction caused by inappropriate immune system activation. This syndrome can be fatal without appropriate immunosuppressive therapy; however, glucocorticoid administration must be pursued with caution when infection and malignancy are on the differential diagnosis.
A 19-year-old woman presented to the emergency department (ED) with a 14-day history of progressive fevers, night sweats, abdominal pain, nonbloody and nonbilious vomiting, diarrhea, cough, and myalgia. The fever occurred daily with no noted temporal pattern, and she had no significant weight loss. The abdominal pain was diffuse and exacerbated by eating. She experienced multiple sporadic episodes of vomiting and diarrhea daily. She denied any rash or arthralgia.
She had no known medical history and took no medications. Family history was negative for autoinflammatory and autoimmune conditions. She had emigrated from Kenya to the United States 28 days ago. Her immunization status was unknown.
This patient has prolonged fevers and evidence of multisystem involvement. The most likely etiologic categories are infectious, inflammatory, rheumatologic, and neoplastic. For febrile patients who have recently emigrated to or travelled outside of the United States, it is important to consider common infections, as well as those endemic to the nation of exposure, which in this case includes malaria, typhoid fever, tuberculosis, cholera, acute viral hepatitis, chikungunya fever, dengue fever, yellow fever, and rickettsial disease. All of these, other than tuberculosis, commonly present with fever, vomiting, diarrhea, and myalgia. She may also have bacterial pneumonia or influenza given her fever and cough, although the chronicity and persistence of symptoms would be atypical. Acute infectious gastroenteritis is a common cause of fever, vomiting, and diarrhea. Most cases resolve in 7 to 10 days, so the duration raises suspicion for a nonviral etiology or an immunocompromised state.
Inflammatory causes could include the first presentation of inflammatory bowel disease (IBD), particularly if the patient develops weight loss or eye, skin, or joint manifestations. The lack of rash or arthralgia makes rheumatologic conditions less likely. Prolonged fevers and night sweats could indicate malignancy such as intra-abdominal lymphoma, although infectious etiologies should be ruled out first.
Previously, on day 9 of symptoms, the patient presented to an ED at another institution. Laboratory evaluation at that time demonstrated an elevated aspartate aminotransferase (AST) level of 229 IU/L (reference, 0-40 IU/L) and alanine aminotransferase (ALT) level of 60 IU/L (reference, 0-32 IU/L) with normal alkaline phosphatase and bilirubin levels, proteinuria to 3+ (normal, negative/trace), ketonuria to 2+ (normal, negative), and hematuria to 2+ (normal, negative). Complete blood count and electrolytes were normal. Computed tomography (CT) scans of the chest, abdomen, and pelvis with intravenous contrast were normal and without evidence of organomegaly.
AST and ALT elevations often indicate hepatocellular damage, although the normal bilirubin levels suggest normal hepatic function. Because CT may miss extrahepatic biliary pathology, a right upper quadrant ultrasound should be obtained to better evaluate patency of the biliary system and hepatic echotexture. Coagulation studies and viral hepatitis serology should be obtained. The disproportionate elevation of AST versus ALT can suggest alcohol use or nonhepatic etiologies such as myositis. Acute viral hepatitis is less likely given there is only mild to moderate elevation in aminotransferase levels. However, the remaining infectious etiologies can have this level of elevation and should still be considered.
Enteritis and IBD are still considerations despite the normal CT results. Transient asymptomatic hematuria or proteinuria can be seen in multiple conditions, particularly proteinuria with febrile illnesses. Urine microscopy to evaluate for casts could indicate a glomerular origin of the hematuria. First morning urine protein-to-creatinine ratio would help quantify the degree of proteinuria. Serum creatinine level should be measured to determine whether there is any renal dysfunction.
While early imaging can be falsely negative, the unremarkable chest CT makes pneumonia and active pulmonary tuberculosis less likely.
Vital signs during this presentation were: temperature, 39.7 °C; heart rate, 126 beats per minute; blood pressure, 109/64 mm Hg; respiratory rate, 20 breaths per minute; and oxygen saturation, 98% on room air. She was ill-appearing, with diffuse abdominal tenderness without peritoneal signs. Other than her tachycardia, findings from her cardiopulmonary, neurologic, and skin exams were normal.
Laboratory testing revealed a white blood cell count of 4,300/µL (reference range, 4,500-13,000/µL), a hemoglobin level of 10.9 g/dL (reference range, 11.7-15.7 g/dL) with a mean corpuscular volume of 77 fL (reference range, 80-96 fL) and reticulocyte percentage of 0.5% (reference range, 0.5%-1.5%), and a platelet count of 59,000/µL (reference range, 135,000-466,000/µL). Her prothrombin time was 13.5 seconds (reference range, 9.6-11.6 seconds) with an international normalized ratio of 1.3 (reference range, 0.8-1.1), erythrocyte sedimentation rate of 46 mm/h (reference range, 0-20 mm/h), C-reactive protein level of 7.49 mg/dL (reference range, <0.3 mg/dL), and AST level of 194 units/L (reference range, 9-35 units/L). ALT, total and direct bilirubin, lipase, electrolytes, and creatinine levels were normal. An abdominal x-ray showed scattered air-fluid levels in a nonobstructed pattern.
Her mildly elevated prothrombin time and international normalized ratio suggest a coagulopathy involving either her extrinsic or common coagulation pathway, with disseminated intravascular coagulation (DIC) being most likely given her new thrombocytopenia and anemia. Hemolytic uremic syndrome and thrombotic thrombocytopenic purpura should be considered but would not cause coagulopathy. A peripheral smear to evaluate for schistocytes associated with microangiopathic hemolysis and serum fibrinogen to distinguish between DIC (low) and thrombocytopenic purpura or hemolytic uremic syndrome (normal or elevated) should be obtained. A thick and thin smear for malaria should also be performed.
Her new pancytopenia suggests bone marrow suppression or infiltration with or without a concomitant consumptive process such as sepsis with resulting DIC. Given her clinical picture, marrow infiltrative processes might include tuberculosis or malignancy, and marrow suppression may be caused by HIV or other viral infection. If she is found to have HIV, disseminated fungal or mycobacterial infections would become more likely. She now has an isolated elevated AST level, which could be secondary to hemolysis rather than hepatocyte damage. Findings from her abdominal exam are nonfocal; this is consistent with her x-ray findings, which reflect possible enteritis or colitis.
The most likely diagnosis currently is an infectious enteritis with resulting hematologic and hepatic abnormalities. Given her recent emigration from Kenya, typhoid fever and cholera are both possible, although cholera typically does not present with prolonged fever or severe abdominal pain. The severity and duration of her illness, and the abnormalities of her laboratory findings, warrant empiric therapy with ceftriaxone to treat possible severe Salmonella enterica infection while awaiting blood and stool cultures.
The patient was admitted to the hospital and her symptoms continued. Results of serum HIV 1 and 2 polymerase chain reactions, herpes simplex virus 1 and 2 polymerase chain reactions, three malaria smears, human T-lymphotropic virus serologies, Toxoplasma serology, Bartonella serology, a stool culture, and multiple large volume blood cultures were negative. Serologic testing for hepatitis A, B, and C, Epstein-Barr virus, cytomegalovirus, and dengue virus were negative for acute infection. Results of an interferon-gamma release assay were indeterminate; results of purified protein derivative (PPD) and Candida antigen control testing were both negative. Ceruloplasmin and α1-antitrypsin levels were normal. An abdominal ultrasound showed central intrahepatic biliary duct dilatation, splenomegaly, and sluggish portal venous flow.
While central intrahepatic biliary ductal dilation could be caused by an obstructive lesion, none were seen on CT or ultrasound. Her normal alkaline phosphatase and bilirbuin levels also suggest functional patency of the biliary system. The thrombocytopenia, splenomegaly, and sluggish portal venous flow suggest possible portal hypertension, though no cirrhotic changes were noted on the ultrasound or abdominal CT. Her negative PPD and Candida antigen control results may suggest underlying immune dysregulation or suppression, though anergy could be secondary to sepsis.
Given her negative initial infectious evaluation, other etiologies such as atypical infections, rheumatologic disorders, and malignancies warrant consideration. She has no murmur; however, subacute bacterial endocarditis with a fastidious organism is possible, which could be investigated with a transthoracic echocardiogram. Other tests to consider include blood cultures for fungi and atypical mycobacterial species, and serology for Coxiella burnetii, chikungunya virus, and yellow fever. Rheumatologic conditions such as systemic lupus erythematosus, hemophagocytic lymphohistiocytosis (HLH), or adult Still’s disease should be considered. Complement levels and an antinuclear antibody panel, including those for dsDNA and Smith antigen, should be performed to evaluate for systemic lupus erythematosus. Serum ferritin, fibrinogen, and triglyceride levels should be measured to evaluate for HLH. Hematologic malignancy is also a consideration, particularly given her pancytopenia. Multicentric Castleman disease can cause prolonged fevers, pancytopenia, and elevated inflammatory markers, but is less likely without lymphadenopathy. A peripheral blood smear should be sent, and a bone marrow biopsy may be needed.
Empiric ciprofloxacin was initiated; however, the patient continued to have fevers up to 39.9 °C, abdominal pain, and myalgia. Ferritin level was over 3,000 ng/mL (reference range, 8-255 ng/mL), and a soluble interleukin-2 (IL-2) receptor level was 1,188 units/mL (reference range, 45-1,105 units/mL). Triglycerides were normal.
The elevated ferritin and soluble IL-2 levels raise concern for HLH. Hyperferritinemia is relatively nonspecific because extremely elevated ferritin can be seen with other conditions, such as renal failure, hepatocellular injury, infection, rheumatologic conditions, and hematologic malignancy. Soluble IL-2 receptor elevation is more specific for HLH than ferritin or triglycerides, but alone does not make the diagnosis because it can be elevated in other rheumatologic disorders and malignancy. The HLH-2004 criteria are commonly used and require either molecular diagnostic testing or meeting at least five out of eight clinical and lab criteria to make the diagnosis. Our patient currently meets three criteria (fever, splenomegaly, and elevated ferritin). Elevated soluble IL-2 is part of the HLH-2004 criteria, but her level of elevation does not meet the required threshold (≥2,400 units/mL). Her cytopenias have also not quite met the HLH-2004 thresholds (two of the following three: hemoglobin <9 g/dL, platelets <100,000/µL, and/or absolute neutrophil count <1,000/µL). Elevated aminotransferase levels and DIC are not part of the HLH-2004 criteria but are often seen with HLH.
Evaluation for an underlying infectious, rheumatologic, or malignant trigger should continue as previously discussed. If this patient does have HLH, it is most likely secondary to an infection, autoimmune disease, or malignancy rather than genetic HLH. HLH has a high mortality rate, but before beginning treatment with immunosuppressive agents, a peripheral smear and a bone marrow biopsy should be performed to evaluate for hematologic malignancy or signs of hemophagocytosis.
Empiric ciprofloxacin covers most bacterial etiologies of diarrhea, including those previously mentioned such as cholera and most strains of S enterica. Her symptoms and laboratory findings (including cytopenias, elevated aminotransferases, and coagulopathy) could suggest enteric fever due to S enterica serovar Typhi, which is endemic in Kenya. Results of blood and stool cultures, though negative, are relatively insensitive for this organism, particularly this far into the illness course. A bone marrow biopsy may also help with diagnosis of occult typhoid fever because marrow culture can be more sensitive than blood or stool culture.
A bone marrow aspiration revealed hemophagocytic histiocytes, no malignant cells, and negative bacterial (including anaerobic), fungal, and acid-fast bacilli cultures. Considering the high mortality rate of untreated HLH/macrophage activation syndrome (MAS), empiric glucocorticoid administration was considered. However, this was withheld due to concern for ongoing undetected infection given her persistent fever and systemic symptoms.
There should still be high suspicion for HLH. Further evaluation for other laboratory manifestations of HLH such as fibrinogen and natural killer cell activity should be considered, as well as repeating her complete blood count to see if her cytopenias have progressed. Her marrow shows no evidence of hematologic malignancy, so other triggers of possible HLH should be sought out by continuing the workup. Consulting specialists from rheumatology and infectious disease may help clarify possible underlying diagnoses and the best management plan. If she continues to have organ damage or clinically worsens, it may be prudent to empirically broaden her antibiotic coverage and begin antifungal agents while starting glucocorticoid therapy for suspected HLH.
A stool molecular screen from admission was returned positive for S enterica serovar Typhi. Ciprofloxacin was discontinued and ceftriaxone was started out of concern for antibiotic resistance. On hospital day 14, the patient’s brother presented to the ED with fever. His blood and stool cultures were positive for S enterica serovar Typhi with intermediate sensitivity to ciprofloxacin and sensitivity to ceftriaxone. With continued treatment with ceftriaxone, the patient improved significantly. Following discharge, she remained afebrile and asymptomatic. During outpatient follow up, a repeat PPD was positive and she was diagnosed with and treated for latent tuberculosis.
COMMENTARY
The evaluation of a patient who has recently emigrated from a foreign nation requires a broad differential diagnosis and a keen awareness of the clinical conditions present in the patient’s country of origin. This often involves knowledge of diseases infrequently encountered in daily practice, as well as awareness of the nuances of rare presentations and possible complications. When the presentation is not classic for a relevant infectious disease and clinical conditions from other diagnostic classes are considered, the evaluation and management of the patient is particularly challenging.
Typhoid fever is a severe systemic illness caused by the organism S enterica serovar Typhi. The organism is ingested, penetrates the small intestinal epithelium, enters the lymphoid tissue, and disseminates via the lymphatic and hematogenous routes. Onset of symptoms typically occurs 5 to 21 days after ingestion of contaminated food or water. Clinical features include fever, chills, relative bradycardia (pulse-temperature dissociation), abdominal pain, rose spots (salmon-colored macules) on the trunk and abdomen, and hepatosplenomegaly. Diarrhea is not a typical symptom of patients with typhoid fever, which can lead to a delayed or missed diagnosis. Life-threatening complications can be seen, including gastrointestinal bleeding, intestinal perforation, and meningitis.1 Typhoid fever is most prevalent in impoverished areas with poor access to sanitation. Regions with the highest incidence include south-central Asia, southeast Asia, and southern Africa.2-4 Approximately 200 to 300 cases are reported in the United States each year.5
Classically, the diagnosis is made by means of clinical symptoms and a positive culture from a sterile site. A recent study of 529 patients found that 61% had positive blood cultures and 96% had positive bone marrow cultures.6 Our patient’s diagnosis was significantly delayed by multiple negative cultures and failure to improve on first-line antibiotics, which initially suggested that the S enterica serovar Typhi stool molecular screen likely represented carriage caused by colonization. Chronic S enterica serovar Typhi carriage is defined as excretion of the organism in stool or urine 1 year or longer after acute infection. Rates of carriage range from 1% to 6%, and up to 25% of carriers have no history of typhoid fever.1,7,8 Carriage is more common in females and in those with biliary tract abnormalities.9,10
Once a presumptive diagnosis is made, antibiotic choice remains a challenge. Resistance to fluoroquinolones, the preferred drug for multidrug-resistant typhoid fever, is growing but remains rare, at approximately 5%.11,12 Ceftriaxone and azithromycin have been used successfully in areas with high resistance.13 Given the patient’s slow response to therapy even after transitioning from ciprofloxacin to ceftriaxone, her brother’s presentation and obtaining the antibiotic sensitivities for his organism were critical to confirming that our diagnosis and management decisions were correct.
One strongly considered diagnosis was HLH/MAS. MAS is an aggressive syndrome of excessive inflammation and tissue destruction caused by inappropriate immune system activation. It belongs to a group of histiocytic disorders collectively known as HLH. Aside from primary (genetic) forms, secondary forms exist that can be triggered by malignancy, infection, or rheumatologic disorders. In infection-associated HLH/MAS, viral infections are a common trigger, with Epstein-Barr virus being the most common. Association with bacterial infections, including tuberculosis and typhoid fever, has also been reported.14 Prompt therapy, often with immunosuppressive agents such as glucocorticoids, is essential for survival because there is a reported mortality rate of up to 50% when untreated.15 When infection-induced HLH/MAS occurs, treatment of the underlying infection is critical.14,15 The greatest barrier to a favorable outcome from HLH/MAS is often a delay in diagnosis because the rarity of this disease, the variable clinical presentation, and the lack of specificity of the clinical and laboratory findings make a conclusive diagnosis challenging.
In the presented case, diagnostic uncertainty challenged the decision to administer systemic glucocorticoids. Glucocorticoids conferred a risk of harm for multiple diagnoses that remained on the differential, including malignancy and infection. Her diagnostic evaluation made malignancy less likely, but because testing was unable to rule out tuberculosis as either the underlying cause or coinfection, the team opted to defer initiating glucocorticoids and instead closely monitor for a worsening inflammatory response. Following appropriate treatment of her systemic infection, her PPD was repeated and became positive. The negative PPD and Candida control obtained during her hospitalization were, therefore, likely caused by anergy in the setting of overwhelming systemic illness. Initiation of glucocorticoids prematurely in this case could have led to further harm because immunosuppression may have led to reactivation of latent tuberculosis or exacerbation of illness from an alternative but then undiagnosed infection.
The patient’s ultimate unifying diagnosis was typhoid fever; however, there are mixed expert opinions as to whether the systemic immune activation was significant enough to merit the diagnosis of infection-induced secondary HLH/MAS. Despite the high morbidity and mortality that can accompany HLH/MAS, it has been reported that a significant proportion of cases of secondary HLH/MAS can be managed effectively with treatment of the underlying etiology; this may have been the case for our patient.14,15 The clinicians in this case were caught between diagnoses, unable to safely reach either one—much like a baseball player stranded between bases. Fortunately for this patient, the diagnosis ultimately emerged after a careful and thorough workup, assisted by the more straightforward diagnosis of her brother with the same disease.
KEY TEACHING POINTS
- Salmonella enterica serovar Typhi has a high false-negative rate in blood and stool cultures; therefore, clinical suspicion should remain high in the setting of a high pre-test probability.
- Fluoroquinolones are traditionally first-line therapy for typhoid fever, but the use of ceftriaxone and azithromycin is increasing because of rising fluoroquinolone resistance.
- Hemophagocytic lymphohistiocytosis/macrophage activation syndrome is characterized by excessive inflammation and tissue destruction caused by inappropriate immune system activation. This syndrome can be fatal without appropriate immunosuppressive therapy; however, glucocorticoid administration must be pursued with caution when infection and malignancy are on the differential diagnosis.
1. Parry CM, Hien TT, Dougan G, et al. Typhoid fever. N Engl J Med. 2002;347(22):1770-1782. https://doi.org/10.1056/nejmra020201
2. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82(5):346-353.
3. Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2(1):010401. https://doi.org/10.7189/jogh.02.010401
4. Mogasale V, Maskery B, Ochiai RL, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2(10):e570-e580. https://doi.org/10.1016/s2214-109x(14)70301-8
5. Lynch MF, Blanton EM, Bulens S, et al. Typhoid fever in the United States, 1999-2006. JAMA. 2009;302(8):859-865. https://doi.org/10.1001/jama.2009.1229
6. Mogasale V, Ramani E, Mogasale VV, Park J. What proportion of Salmonella typhi cases are detected by blood culture? a systematic literature review. Ann Clin Microbiol Antimicrob. 2016;15(1):32. https://doi.org/10.1186/s12941-016-0147-z
7. Merselis JG Jr, Kaye D, Connolly CS, Hook EW. Quantitative bacteriology of the Typhoid carrier state. Am J Trop Med Hyg. 1964;13:425-429. https://doi.org/10.4269/ajtmh.1964.13.425
8. Lanata CF, Levine MM, Ristori C, et al. Vi serology in detection of chronic Salmonella typhi carriers in an endemic area. Lancet. 1983;2(8347):441-443. https://doi.org/10.1016/s0140-6736(83)90401-4
9. Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. Common bile duct stones: a cause of chronic salmonellosis. Am J Gastroenterol. 1992;87(9):1198-1199.
10. Hofmann E, Chianale J, Rollán A, Pereira J, Ferrecio C, Sotomayor V. Blood group antigen secretion and gallstone disease in the Salmonella typhi chronic carrier state. J Infect Dis. 1993;167(4):993-994. https://doi.org/10.1093/infdis/167.4.993
11. Steel AD, Hay Burgess DC, Diaz Z, Carey ME, Zaidi AKM. Challenges and opportunities for typhoid fever control: a call for coordinated action. Clin Infect Dis. 2016;62 (Suppl 1):S4-S8. https://doi.org/10.1093/cid/civ976
12. Hendriksen RS, Leekitcharoenphon P, Lukjancenko O, et al. Genomic signature of multidrug resistant Salmonella enterica serovar Typhi isolates related to a massive outbreak in Zambia between 2010 and 2012. J Clin Microbiol. 2015;53(1):262-272. https://doi.org/10.1128/jcm.02026-14
13. Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of Salmonella infections. Clin Microbiol Rev. 2015;28(4):901-936. https://doi.org/10.1128/cmr.00002-15
14. Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7(12):814-822. https://doi.org/10.1016/s1473-3099(07)70290-6
15. Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000;6(6):601-608. https://doi.org/10.3201/eid0606.000608
1. Parry CM, Hien TT, Dougan G, et al. Typhoid fever. N Engl J Med. 2002;347(22):1770-1782. https://doi.org/10.1056/nejmra020201
2. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82(5):346-353.
3. Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2(1):010401. https://doi.org/10.7189/jogh.02.010401
4. Mogasale V, Maskery B, Ochiai RL, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2(10):e570-e580. https://doi.org/10.1016/s2214-109x(14)70301-8
5. Lynch MF, Blanton EM, Bulens S, et al. Typhoid fever in the United States, 1999-2006. JAMA. 2009;302(8):859-865. https://doi.org/10.1001/jama.2009.1229
6. Mogasale V, Ramani E, Mogasale VV, Park J. What proportion of Salmonella typhi cases are detected by blood culture? a systematic literature review. Ann Clin Microbiol Antimicrob. 2016;15(1):32. https://doi.org/10.1186/s12941-016-0147-z
7. Merselis JG Jr, Kaye D, Connolly CS, Hook EW. Quantitative bacteriology of the Typhoid carrier state. Am J Trop Med Hyg. 1964;13:425-429. https://doi.org/10.4269/ajtmh.1964.13.425
8. Lanata CF, Levine MM, Ristori C, et al. Vi serology in detection of chronic Salmonella typhi carriers in an endemic area. Lancet. 1983;2(8347):441-443. https://doi.org/10.1016/s0140-6736(83)90401-4
9. Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. Common bile duct stones: a cause of chronic salmonellosis. Am J Gastroenterol. 1992;87(9):1198-1199.
10. Hofmann E, Chianale J, Rollán A, Pereira J, Ferrecio C, Sotomayor V. Blood group antigen secretion and gallstone disease in the Salmonella typhi chronic carrier state. J Infect Dis. 1993;167(4):993-994. https://doi.org/10.1093/infdis/167.4.993
11. Steel AD, Hay Burgess DC, Diaz Z, Carey ME, Zaidi AKM. Challenges and opportunities for typhoid fever control: a call for coordinated action. Clin Infect Dis. 2016;62 (Suppl 1):S4-S8. https://doi.org/10.1093/cid/civ976
12. Hendriksen RS, Leekitcharoenphon P, Lukjancenko O, et al. Genomic signature of multidrug resistant Salmonella enterica serovar Typhi isolates related to a massive outbreak in Zambia between 2010 and 2012. J Clin Microbiol. 2015;53(1):262-272. https://doi.org/10.1128/jcm.02026-14
13. Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of Salmonella infections. Clin Microbiol Rev. 2015;28(4):901-936. https://doi.org/10.1128/cmr.00002-15
14. Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7(12):814-822. https://doi.org/10.1016/s1473-3099(07)70290-6
15. Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000;6(6):601-608. https://doi.org/10.3201/eid0606.000608
© 2021 Society of Hospital Medicine
Decreasing Hospital Observation Time for Febrile Infants
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
© 2021 Society of Hospital Medicine
Comparing Two Proximal Measures of Unrecognized Clinical Deterioration in Children
Unrecognized in-hospital clinical deterioration can lead to substantial morbidity and mortality.1 As a result, hospitals have implemented systems to identify and mitigate this form of potentially preventable harm.2-4 Cardiopulmonary arrest rates are useful metrics to evaluate the effectiveness of systems designed to identify and respond to deteriorating adult patients.5 Pediatric arrests outside of the intensive care unit (ICU) are rare; therefore, the identification of valid and more frequent proximal measures of deterioration is critical to the assessment of current systems and to guide future improvement efforts.6
Bonafide et al developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had an over 13-fold increased risk of in-hospital mortality.7 Implementation of a rapid response system was subsequently associated with a decrease in the trajectory of CDEs.2 At Cincinnati Children’s Hospital Medical Center (CCHMC), an additional proximal outcome measure was developed for unrecognized clinical deterioration: emergency transfers (ETs).8,9 An event meets criteria for an ET when the patient undergoes intubation, inotropic support, or three or more fluid boluses in the first hour after arrival or prior to ICU transfer.9 Recently, ETs were associated with an increased in-hospital mortality, ICU length of stay, and post-transfer hospital length of stay when compared with nonemergent transfers.10,11
While both CDEs and ETs were associated with adverse outcomes in children and may be modifiable through better rapid response systems, researchers have not previously compared the extent to which CDEs and ETs capture similar versus distinct events. Furthermore, the ability of focused situation awareness interventions to identify high-risk patients has not previously been assessed. Situation awareness is defined as the perception of elements in the environment, the comprehension of their meaning, and the projection of their status in the near future.12 Clinically, improved situation awareness can lead to earlier recognition of deterioration and a reduction in failure to rescue events.9 The objectives of this study were to (1) describe CDEs and ETs and assess for similarities, differences, and trends, and (2) evaluate the utility of situation awareness interventions to detect patients who experience these events.
METHODS
Setting and Inclusion Criteria
We conducted a retrospective cross-sectional study at CCHMC, a free-standing tertiary care children’s hospital. We included all patients cared for outside of the ICU during their hospitalization from January 2016 to July 2018. Transfer to the ICU included the pediatric and the cardiac ICUs.
Study Definitions
CDEs were events in which a patient received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of ICU transfer (Figure).7 ETs were events in which a patient underwent intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer (Figure).9 We examined two distinct situation awareness interventions: watcher identification and the pediatric early warning score (PEWS). A watcher is a situation awareness concern based on clinician perception, or “gut feeling,” that the patient is at high risk for deterioration.9,13 When clinicians designate a patient as a watcher in the electronic medical record, they establish an action plan, reassessment timeline, and objective criteria for activation of the rapid response team to assess the patient. Watcher patients are discussed at institution-wide safety huddles three times daily. The PEWS is a reproducible assessment of the patient’s status based on physiologic parameters, including behavior, cardiovascular, and respiratory assessments.3,4 At CCHMC, a Monaghan PEWS score is calculated with each assessment of vital signs.14 The bedside nurse calls the physician or advanced practice provider to assess the patient for a score of 4 or greater.
Event Identification and Classification
Two trained research nurses (C.F. and D.H.) manually reviewed all ICU transfers during the study period to determine if CDE criteria were met. Events meeting CDE criteria were classified as respiratory (requiring noninvasive or invasive ventilation), cardiac (requiring inotropes), or cardiopulmonary resuscitation (CPR) in which cardiac and respiratory interventions were initiated simultaneously. Additional information obtained included the time the patient met CDE criteria relative to the time of ICU transfer, watcher identification prior to the event, and the highest PEWS documented within 12 hours of the event. A physician (T.S.) performed manual chart review of each CDE as an additional validation step. ETs during the study period were obtained from an existing institutional database. ICU transfers meeting ET criteria are entered into this database in nearly real time by the inpatient nurse manager; this nurse attends all rapid response team calls and is aware of the disposition for each event. A physician (T.S.) performed manual chart review of each ET to determine event classification by intervention type, watcher identification, and the highest PEWS documented within 12 hours of the event. All CDEs and ETs were cross-referenced to determine overlap.
Outcome Measures and Statistical Analysis
The primary outcomes were CDEs and ETs, calculated as absolute counts and number of events per 10,000 non-ICU patient days. Events were classified by (1) category of intervention, (2) watcher identification prior to the event, and (3) PEWS of 4 or greater documented in the 12 hours prior to the event.
RESULTS
Incidence and Overlap of CDEs and ETs
There were 1,828 ICU transfers during the study period, of which 365 (20%) met criteria for a CDE, ET, or both. Among events captured, 359 (98.4%) met criteria for a CDE, occurring at a rate of 16.7 per 10,000 non-ICU patient days, and 88 (24.1%) met criteria for an ET, occurring at a rate of 4.1 per 10,000 non-ICU patient days (Table). Of the 88 ETs, 82 also met criteria for a CDE.
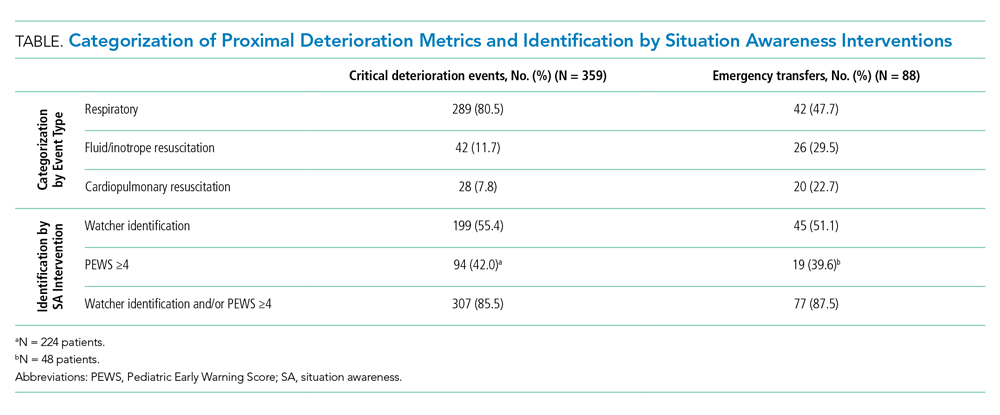
Timing and Categorization of CDEs and ETs
Despite the 12-hour time horizon, most CDEs (62.1%) met criteria within 1 hour of ICU transfer, and 79.9% met criteria within 3 hours (Figure). Respiratory events were most common for both CDEs (80.5%) and ETs (47.7%) (Table). Of respiratory CDEs, 67.4% required noninvasive ventilation, and 32.5% required invasive ventilation. Fluid or inotrope support were responsible for 11.7% of CDEs and nearly one-third of ETs; of note, the CDE definition does not include fluid boluses. Less than 10% of CDEs were characterized by CPR, whereas this accounted for 22.7% of ETs.

Identification of Events by Situation Awareness Interventions
The Table depicts the identification of events by watcher status and PEWS. All events were included for watcher identification, and events with a documented score in the 12 hours prior to transfer were included for PEWS. While half or less of the events were captured by watcher or PEWS separately, over 85% of events were captured by either one or both of the situation awareness interventions. The situation awareness interventions identified CDEs and ETs similarly.
DISCUSSION
This study is the first to classify and compare two proximal measures of clinical deterioration in children. Given that children with escalating respiratory symptoms are often treated successfully outside of the ICU, the findings that most events are respiratory in nature and occur within 1 hour of transfer are not unexpected. The analysis of situation awareness interventions suggests that neither watcher identification nor PEWS is independently sufficient to predict future deterioration. These findings support the necessity of both a clinician “gut feeling” and objective vital sign and physical exam findings to indicate a patient’s clinical status.9 Initiatives to improve the early recognition and mitigation of patient deterioration should focus on both tools to initiate an escalation of care, and work to understand gaps in these identification systems, which currently miss approximately 15% of acutely deteriorating patients. Although most patients had watcher identification or elevated PEWS prior to the event, they still required emergent life-sustaining care, which suggests that opportunities exist to improve mitigation and escalation pathways as a critical prevention effort.7,10
It is likely that CDEs and ETs are important outcome metrics in the evaluation of pediatric escalation systems, including rapid response systems.15 ETs are less common and more specific for unrecognized deterioration, which makes them a more feasible early metric for assessment. CDEs, which are likely more sensitive, may be useful in settings in which deterioration is rare or a more common outcome enhances power to detect the effect of interventions.10
This study has limitations and lends itself to future work. While CDEs and ETs are more common than cardiopulmonary arrest, they remain relatively uncommon. This was a single-site study at a large, tertiary care, free-standing children’s hospital, so generalizability to centers with different characteristics and patient populations may be limited. Future work should focus on comparing patient-level outcomes of CDEs and ETs, including length of stay and mortality. The determination of specific diagnoses and conditions associated with CDEs and ETs may inform targeted preventive improvement science interventions.
CONCLUSION
CDEs were roughly fourfold more common than ETs, with most CDEs occurring within 1 hour of ICU transfer. Most patients were identified by either watcher status or elevated PEWS, suggesting that these tools, when utilized as complementary situation awareness interventions, are important for identifying patients at risk for deterioration. Opportunities exist for improved escalation plans for patients identified as high-risk to prevent the need for emergent life-sustaining intervention.
1. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
2. Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of rapid response system implementation on critical deterioration events in children. JAMA Pediatr. 2014;168(1):25-33. https://doi.org/10.1001/jamapediatrics.2013.3266
3. Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271-278. https://doi.org/10.1016/j.jcrc.2006.06.007
4. Sefton G, McGrath C, Tume L, Lane S, Lisboa PJ, Carrol ED. What impact did a Paediatric Early Warning system have on emergency admissions to the paediatric intensive care unit? an observational cohort study. Intensive Crit Care Nurs. 2015;31(2):91-99. https://doi.org/10.1016/j.iccn.2014.01.001
5. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388
6. Feudtner C, Berry JG, Parry G, et al. Statistical uncertainty of mortality rates and rankings for children’s hospitals. Pediatrics. 2011;128(4):e966-e972. https://doi.org/10.1542/peds.2010-3074
7. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784
8. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747
9. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364
10. Hussain FS, Sosa T, Ambroggio L, Gallagher R, Brady PW. Emergency transfers: an important predictor of adverse outcomes in hospitalized children. J Hosp Med. 2019;14(8):482-485. https://doi.org/10.12788/jhm.3219
11. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2019;55(2):213-215. https://doi.org/10.1111/jpc.14185
12. Endsley MR. Toward a theory of situation awareness in dynamic systems. Human Factors. 1995;37(1):32-64. https://doi.org/10.1518/001872095779049543
13. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042
14. Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32-35. https://doi.org/10.7748/paed2005.02.17.1.32.c964
15. Subbe CP, Bannard-Smith J, Bunch J, et al. Quality metrics for the evaluation of Rapid Response Systems: proceedings from the third international consensus conference on Rapid Response Systems. Resuscitation. 2019;141:1-12. https://doi.org/10.1016/j.resuscitation.2019.05.012
Unrecognized in-hospital clinical deterioration can lead to substantial morbidity and mortality.1 As a result, hospitals have implemented systems to identify and mitigate this form of potentially preventable harm.2-4 Cardiopulmonary arrest rates are useful metrics to evaluate the effectiveness of systems designed to identify and respond to deteriorating adult patients.5 Pediatric arrests outside of the intensive care unit (ICU) are rare; therefore, the identification of valid and more frequent proximal measures of deterioration is critical to the assessment of current systems and to guide future improvement efforts.6
Bonafide et al developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had an over 13-fold increased risk of in-hospital mortality.7 Implementation of a rapid response system was subsequently associated with a decrease in the trajectory of CDEs.2 At Cincinnati Children’s Hospital Medical Center (CCHMC), an additional proximal outcome measure was developed for unrecognized clinical deterioration: emergency transfers (ETs).8,9 An event meets criteria for an ET when the patient undergoes intubation, inotropic support, or three or more fluid boluses in the first hour after arrival or prior to ICU transfer.9 Recently, ETs were associated with an increased in-hospital mortality, ICU length of stay, and post-transfer hospital length of stay when compared with nonemergent transfers.10,11
While both CDEs and ETs were associated with adverse outcomes in children and may be modifiable through better rapid response systems, researchers have not previously compared the extent to which CDEs and ETs capture similar versus distinct events. Furthermore, the ability of focused situation awareness interventions to identify high-risk patients has not previously been assessed. Situation awareness is defined as the perception of elements in the environment, the comprehension of their meaning, and the projection of their status in the near future.12 Clinically, improved situation awareness can lead to earlier recognition of deterioration and a reduction in failure to rescue events.9 The objectives of this study were to (1) describe CDEs and ETs and assess for similarities, differences, and trends, and (2) evaluate the utility of situation awareness interventions to detect patients who experience these events.
METHODS
Setting and Inclusion Criteria
We conducted a retrospective cross-sectional study at CCHMC, a free-standing tertiary care children’s hospital. We included all patients cared for outside of the ICU during their hospitalization from January 2016 to July 2018. Transfer to the ICU included the pediatric and the cardiac ICUs.
Study Definitions
CDEs were events in which a patient received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of ICU transfer (Figure).7 ETs were events in which a patient underwent intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer (Figure).9 We examined two distinct situation awareness interventions: watcher identification and the pediatric early warning score (PEWS). A watcher is a situation awareness concern based on clinician perception, or “gut feeling,” that the patient is at high risk for deterioration.9,13 When clinicians designate a patient as a watcher in the electronic medical record, they establish an action plan, reassessment timeline, and objective criteria for activation of the rapid response team to assess the patient. Watcher patients are discussed at institution-wide safety huddles three times daily. The PEWS is a reproducible assessment of the patient’s status based on physiologic parameters, including behavior, cardiovascular, and respiratory assessments.3,4 At CCHMC, a Monaghan PEWS score is calculated with each assessment of vital signs.14 The bedside nurse calls the physician or advanced practice provider to assess the patient for a score of 4 or greater.
Event Identification and Classification
Two trained research nurses (C.F. and D.H.) manually reviewed all ICU transfers during the study period to determine if CDE criteria were met. Events meeting CDE criteria were classified as respiratory (requiring noninvasive or invasive ventilation), cardiac (requiring inotropes), or cardiopulmonary resuscitation (CPR) in which cardiac and respiratory interventions were initiated simultaneously. Additional information obtained included the time the patient met CDE criteria relative to the time of ICU transfer, watcher identification prior to the event, and the highest PEWS documented within 12 hours of the event. A physician (T.S.) performed manual chart review of each CDE as an additional validation step. ETs during the study period were obtained from an existing institutional database. ICU transfers meeting ET criteria are entered into this database in nearly real time by the inpatient nurse manager; this nurse attends all rapid response team calls and is aware of the disposition for each event. A physician (T.S.) performed manual chart review of each ET to determine event classification by intervention type, watcher identification, and the highest PEWS documented within 12 hours of the event. All CDEs and ETs were cross-referenced to determine overlap.
Outcome Measures and Statistical Analysis
The primary outcomes were CDEs and ETs, calculated as absolute counts and number of events per 10,000 non-ICU patient days. Events were classified by (1) category of intervention, (2) watcher identification prior to the event, and (3) PEWS of 4 or greater documented in the 12 hours prior to the event.
RESULTS
Incidence and Overlap of CDEs and ETs
There were 1,828 ICU transfers during the study period, of which 365 (20%) met criteria for a CDE, ET, or both. Among events captured, 359 (98.4%) met criteria for a CDE, occurring at a rate of 16.7 per 10,000 non-ICU patient days, and 88 (24.1%) met criteria for an ET, occurring at a rate of 4.1 per 10,000 non-ICU patient days (Table). Of the 88 ETs, 82 also met criteria for a CDE.

Timing and Categorization of CDEs and ETs
Despite the 12-hour time horizon, most CDEs (62.1%) met criteria within 1 hour of ICU transfer, and 79.9% met criteria within 3 hours (Figure). Respiratory events were most common for both CDEs (80.5%) and ETs (47.7%) (Table). Of respiratory CDEs, 67.4% required noninvasive ventilation, and 32.5% required invasive ventilation. Fluid or inotrope support were responsible for 11.7% of CDEs and nearly one-third of ETs; of note, the CDE definition does not include fluid boluses. Less than 10% of CDEs were characterized by CPR, whereas this accounted for 22.7% of ETs.

Identification of Events by Situation Awareness Interventions
The Table depicts the identification of events by watcher status and PEWS. All events were included for watcher identification, and events with a documented score in the 12 hours prior to transfer were included for PEWS. While half or less of the events were captured by watcher or PEWS separately, over 85% of events were captured by either one or both of the situation awareness interventions. The situation awareness interventions identified CDEs and ETs similarly.
DISCUSSION
This study is the first to classify and compare two proximal measures of clinical deterioration in children. Given that children with escalating respiratory symptoms are often treated successfully outside of the ICU, the findings that most events are respiratory in nature and occur within 1 hour of transfer are not unexpected. The analysis of situation awareness interventions suggests that neither watcher identification nor PEWS is independently sufficient to predict future deterioration. These findings support the necessity of both a clinician “gut feeling” and objective vital sign and physical exam findings to indicate a patient’s clinical status.9 Initiatives to improve the early recognition and mitigation of patient deterioration should focus on both tools to initiate an escalation of care, and work to understand gaps in these identification systems, which currently miss approximately 15% of acutely deteriorating patients. Although most patients had watcher identification or elevated PEWS prior to the event, they still required emergent life-sustaining care, which suggests that opportunities exist to improve mitigation and escalation pathways as a critical prevention effort.7,10
It is likely that CDEs and ETs are important outcome metrics in the evaluation of pediatric escalation systems, including rapid response systems.15 ETs are less common and more specific for unrecognized deterioration, which makes them a more feasible early metric for assessment. CDEs, which are likely more sensitive, may be useful in settings in which deterioration is rare or a more common outcome enhances power to detect the effect of interventions.10
This study has limitations and lends itself to future work. While CDEs and ETs are more common than cardiopulmonary arrest, they remain relatively uncommon. This was a single-site study at a large, tertiary care, free-standing children’s hospital, so generalizability to centers with different characteristics and patient populations may be limited. Future work should focus on comparing patient-level outcomes of CDEs and ETs, including length of stay and mortality. The determination of specific diagnoses and conditions associated with CDEs and ETs may inform targeted preventive improvement science interventions.
CONCLUSION
CDEs were roughly fourfold more common than ETs, with most CDEs occurring within 1 hour of ICU transfer. Most patients were identified by either watcher status or elevated PEWS, suggesting that these tools, when utilized as complementary situation awareness interventions, are important for identifying patients at risk for deterioration. Opportunities exist for improved escalation plans for patients identified as high-risk to prevent the need for emergent life-sustaining intervention.
Unrecognized in-hospital clinical deterioration can lead to substantial morbidity and mortality.1 As a result, hospitals have implemented systems to identify and mitigate this form of potentially preventable harm.2-4 Cardiopulmonary arrest rates are useful metrics to evaluate the effectiveness of systems designed to identify and respond to deteriorating adult patients.5 Pediatric arrests outside of the intensive care unit (ICU) are rare; therefore, the identification of valid and more frequent proximal measures of deterioration is critical to the assessment of current systems and to guide future improvement efforts.6
Bonafide et al developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had an over 13-fold increased risk of in-hospital mortality.7 Implementation of a rapid response system was subsequently associated with a decrease in the trajectory of CDEs.2 At Cincinnati Children’s Hospital Medical Center (CCHMC), an additional proximal outcome measure was developed for unrecognized clinical deterioration: emergency transfers (ETs).8,9 An event meets criteria for an ET when the patient undergoes intubation, inotropic support, or three or more fluid boluses in the first hour after arrival or prior to ICU transfer.9 Recently, ETs were associated with an increased in-hospital mortality, ICU length of stay, and post-transfer hospital length of stay when compared with nonemergent transfers.10,11
While both CDEs and ETs were associated with adverse outcomes in children and may be modifiable through better rapid response systems, researchers have not previously compared the extent to which CDEs and ETs capture similar versus distinct events. Furthermore, the ability of focused situation awareness interventions to identify high-risk patients has not previously been assessed. Situation awareness is defined as the perception of elements in the environment, the comprehension of their meaning, and the projection of their status in the near future.12 Clinically, improved situation awareness can lead to earlier recognition of deterioration and a reduction in failure to rescue events.9 The objectives of this study were to (1) describe CDEs and ETs and assess for similarities, differences, and trends, and (2) evaluate the utility of situation awareness interventions to detect patients who experience these events.
METHODS
Setting and Inclusion Criteria
We conducted a retrospective cross-sectional study at CCHMC, a free-standing tertiary care children’s hospital. We included all patients cared for outside of the ICU during their hospitalization from January 2016 to July 2018. Transfer to the ICU included the pediatric and the cardiac ICUs.
Study Definitions
CDEs were events in which a patient received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of ICU transfer (Figure).7 ETs were events in which a patient underwent intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer (Figure).9 We examined two distinct situation awareness interventions: watcher identification and the pediatric early warning score (PEWS). A watcher is a situation awareness concern based on clinician perception, or “gut feeling,” that the patient is at high risk for deterioration.9,13 When clinicians designate a patient as a watcher in the electronic medical record, they establish an action plan, reassessment timeline, and objective criteria for activation of the rapid response team to assess the patient. Watcher patients are discussed at institution-wide safety huddles three times daily. The PEWS is a reproducible assessment of the patient’s status based on physiologic parameters, including behavior, cardiovascular, and respiratory assessments.3,4 At CCHMC, a Monaghan PEWS score is calculated with each assessment of vital signs.14 The bedside nurse calls the physician or advanced practice provider to assess the patient for a score of 4 or greater.
Event Identification and Classification
Two trained research nurses (C.F. and D.H.) manually reviewed all ICU transfers during the study period to determine if CDE criteria were met. Events meeting CDE criteria were classified as respiratory (requiring noninvasive or invasive ventilation), cardiac (requiring inotropes), or cardiopulmonary resuscitation (CPR) in which cardiac and respiratory interventions were initiated simultaneously. Additional information obtained included the time the patient met CDE criteria relative to the time of ICU transfer, watcher identification prior to the event, and the highest PEWS documented within 12 hours of the event. A physician (T.S.) performed manual chart review of each CDE as an additional validation step. ETs during the study period were obtained from an existing institutional database. ICU transfers meeting ET criteria are entered into this database in nearly real time by the inpatient nurse manager; this nurse attends all rapid response team calls and is aware of the disposition for each event. A physician (T.S.) performed manual chart review of each ET to determine event classification by intervention type, watcher identification, and the highest PEWS documented within 12 hours of the event. All CDEs and ETs were cross-referenced to determine overlap.
Outcome Measures and Statistical Analysis
The primary outcomes were CDEs and ETs, calculated as absolute counts and number of events per 10,000 non-ICU patient days. Events were classified by (1) category of intervention, (2) watcher identification prior to the event, and (3) PEWS of 4 or greater documented in the 12 hours prior to the event.
RESULTS
Incidence and Overlap of CDEs and ETs
There were 1,828 ICU transfers during the study period, of which 365 (20%) met criteria for a CDE, ET, or both. Among events captured, 359 (98.4%) met criteria for a CDE, occurring at a rate of 16.7 per 10,000 non-ICU patient days, and 88 (24.1%) met criteria for an ET, occurring at a rate of 4.1 per 10,000 non-ICU patient days (Table). Of the 88 ETs, 82 also met criteria for a CDE.

Timing and Categorization of CDEs and ETs
Despite the 12-hour time horizon, most CDEs (62.1%) met criteria within 1 hour of ICU transfer, and 79.9% met criteria within 3 hours (Figure). Respiratory events were most common for both CDEs (80.5%) and ETs (47.7%) (Table). Of respiratory CDEs, 67.4% required noninvasive ventilation, and 32.5% required invasive ventilation. Fluid or inotrope support were responsible for 11.7% of CDEs and nearly one-third of ETs; of note, the CDE definition does not include fluid boluses. Less than 10% of CDEs were characterized by CPR, whereas this accounted for 22.7% of ETs.

Identification of Events by Situation Awareness Interventions
The Table depicts the identification of events by watcher status and PEWS. All events were included for watcher identification, and events with a documented score in the 12 hours prior to transfer were included for PEWS. While half or less of the events were captured by watcher or PEWS separately, over 85% of events were captured by either one or both of the situation awareness interventions. The situation awareness interventions identified CDEs and ETs similarly.
DISCUSSION
This study is the first to classify and compare two proximal measures of clinical deterioration in children. Given that children with escalating respiratory symptoms are often treated successfully outside of the ICU, the findings that most events are respiratory in nature and occur within 1 hour of transfer are not unexpected. The analysis of situation awareness interventions suggests that neither watcher identification nor PEWS is independently sufficient to predict future deterioration. These findings support the necessity of both a clinician “gut feeling” and objective vital sign and physical exam findings to indicate a patient’s clinical status.9 Initiatives to improve the early recognition and mitigation of patient deterioration should focus on both tools to initiate an escalation of care, and work to understand gaps in these identification systems, which currently miss approximately 15% of acutely deteriorating patients. Although most patients had watcher identification or elevated PEWS prior to the event, they still required emergent life-sustaining care, which suggests that opportunities exist to improve mitigation and escalation pathways as a critical prevention effort.7,10
It is likely that CDEs and ETs are important outcome metrics in the evaluation of pediatric escalation systems, including rapid response systems.15 ETs are less common and more specific for unrecognized deterioration, which makes them a more feasible early metric for assessment. CDEs, which are likely more sensitive, may be useful in settings in which deterioration is rare or a more common outcome enhances power to detect the effect of interventions.10
This study has limitations and lends itself to future work. While CDEs and ETs are more common than cardiopulmonary arrest, they remain relatively uncommon. This was a single-site study at a large, tertiary care, free-standing children’s hospital, so generalizability to centers with different characteristics and patient populations may be limited. Future work should focus on comparing patient-level outcomes of CDEs and ETs, including length of stay and mortality. The determination of specific diagnoses and conditions associated with CDEs and ETs may inform targeted preventive improvement science interventions.
CONCLUSION
CDEs were roughly fourfold more common than ETs, with most CDEs occurring within 1 hour of ICU transfer. Most patients were identified by either watcher status or elevated PEWS, suggesting that these tools, when utilized as complementary situation awareness interventions, are important for identifying patients at risk for deterioration. Opportunities exist for improved escalation plans for patients identified as high-risk to prevent the need for emergent life-sustaining intervention.
1. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
2. Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of rapid response system implementation on critical deterioration events in children. JAMA Pediatr. 2014;168(1):25-33. https://doi.org/10.1001/jamapediatrics.2013.3266
3. Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271-278. https://doi.org/10.1016/j.jcrc.2006.06.007
4. Sefton G, McGrath C, Tume L, Lane S, Lisboa PJ, Carrol ED. What impact did a Paediatric Early Warning system have on emergency admissions to the paediatric intensive care unit? an observational cohort study. Intensive Crit Care Nurs. 2015;31(2):91-99. https://doi.org/10.1016/j.iccn.2014.01.001
5. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388
6. Feudtner C, Berry JG, Parry G, et al. Statistical uncertainty of mortality rates and rankings for children’s hospitals. Pediatrics. 2011;128(4):e966-e972. https://doi.org/10.1542/peds.2010-3074
7. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784
8. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747
9. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364
10. Hussain FS, Sosa T, Ambroggio L, Gallagher R, Brady PW. Emergency transfers: an important predictor of adverse outcomes in hospitalized children. J Hosp Med. 2019;14(8):482-485. https://doi.org/10.12788/jhm.3219
11. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2019;55(2):213-215. https://doi.org/10.1111/jpc.14185
12. Endsley MR. Toward a theory of situation awareness in dynamic systems. Human Factors. 1995;37(1):32-64. https://doi.org/10.1518/001872095779049543
13. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042
14. Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32-35. https://doi.org/10.7748/paed2005.02.17.1.32.c964
15. Subbe CP, Bannard-Smith J, Bunch J, et al. Quality metrics for the evaluation of Rapid Response Systems: proceedings from the third international consensus conference on Rapid Response Systems. Resuscitation. 2019;141:1-12. https://doi.org/10.1016/j.resuscitation.2019.05.012
1. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
2. Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of rapid response system implementation on critical deterioration events in children. JAMA Pediatr. 2014;168(1):25-33. https://doi.org/10.1001/jamapediatrics.2013.3266
3. Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271-278. https://doi.org/10.1016/j.jcrc.2006.06.007
4. Sefton G, McGrath C, Tume L, Lane S, Lisboa PJ, Carrol ED. What impact did a Paediatric Early Warning system have on emergency admissions to the paediatric intensive care unit? an observational cohort study. Intensive Crit Care Nurs. 2015;31(2):91-99. https://doi.org/10.1016/j.iccn.2014.01.001
5. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388
6. Feudtner C, Berry JG, Parry G, et al. Statistical uncertainty of mortality rates and rankings for children’s hospitals. Pediatrics. 2011;128(4):e966-e972. https://doi.org/10.1542/peds.2010-3074
7. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784
8. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747
9. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364
10. Hussain FS, Sosa T, Ambroggio L, Gallagher R, Brady PW. Emergency transfers: an important predictor of adverse outcomes in hospitalized children. J Hosp Med. 2019;14(8):482-485. https://doi.org/10.12788/jhm.3219
11. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2019;55(2):213-215. https://doi.org/10.1111/jpc.14185
12. Endsley MR. Toward a theory of situation awareness in dynamic systems. Human Factors. 1995;37(1):32-64. https://doi.org/10.1518/001872095779049543
13. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042
14. Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32-35. https://doi.org/10.7748/paed2005.02.17.1.32.c964
15. Subbe CP, Bannard-Smith J, Bunch J, et al. Quality metrics for the evaluation of Rapid Response Systems: proceedings from the third international consensus conference on Rapid Response Systems. Resuscitation. 2019;141:1-12. https://doi.org/10.1016/j.resuscitation.2019.05.012
© 2020 Society of Hospital Medicine
The Effects of Care Team Roles on Situation Awareness in the Pediatric Intensive Care Unit: A Prospective Cross-Sectional Study
Reduction in serious pediatric medical errors has been achieved through sharing of best practices and structured collaboration.1 However, limited progress has been made in reducing complex, multifactorial events such as unrecognized and undertreated patient deterioration events.2 To address this critical gap, interventions to improve clinician situation awareness (SA) have increasingly been applied.3
SA is the ability to recognize and monitor cues regarding what is happening, create a comprehensive picture with available information, and extrapolate whether it indicates adverse developments either immediately or in the near future.4 Methods such as care team huddling5-8 and using standardized patient acuity scoring instruments9 increase SA shared across care team roles. Shared SA is the degree to which each team member possesses a common understanding of what is going on. A team is considered to have shared SA when all the individuals agree on both what is happening (accurate perception and comprehension) and what is going to happen in the future (correct projection). Shared SA for high-risk patients in the pediatric intensive care unit (PICU) has not previously been described and may be an opportunity to improve interprofessional team communication for the sickest patients. Shared SA for high-risk patient status is only one aspect of SA, but it facilitates team-based mitigation planning and is an important starting place for understanding opportunities to improve SA. The primary objective of this study was to measure and compare SA among care team roles regarding patients with high-risk status in the PICU.
METHODS
We conducted a prospective, cross-sectional study from March 2018 to July 2019 examining the individual and shared SA of patient care team trios: the nurse, respiratory therapist (RT), and pediatric resident. The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) determined this study to be non–human-subjects research.
Setting
Research was conducted in the 35-bed PICU of CCHMC, a 500-bed academic free-standing quaternary care children’s hospital.
Participants
We conducted independent surveys of the nurse, RT, and pediatric resident (care team trio) caring for each patient regarding the patient’s clinical deterioration risk status. No patients or care team trios were excluded.
Reference Standard
In 2016, a local panel of experts derived clinical criteria to determine high-risk status for PICU patients, the definition of which, as well as other study terms, appears in Table 1. A PICU attending or fellow identifies a patient as “high risk” when these clinical criteria are met. A plan for prevention and mitigation is formulated and documented for high-risk patients by the PICU attending or fellow at two preexisting daily SA huddles. This plan includes prevention measures to take immediately, specific vital sign thresholds for early identification of deterioration, and guidance on which emergency medication order sets should be utilized to expedite treatment in the event of clinical decline. Dissemination of the care team’s plan is the responsibility of the PICU fellow with additional follow-up by the charge nurse to improve reliability. Identification of high-risk status and development of the prevention and mitigation plan, as completed by the PICU fellow or attending, served as the reference standard for this study.

Survey Instrument Development
The locally developed survey tool was modeled after a validated handoff communication instrument.10 The tool covered the patient’s risk status, which high-risk clinical criteria were met, the presence and content of a mitigation plan, and planned patient interventions (Appendix).
Data Collection
Care team trios were sampled weekly on weekdays during day and night shifts within 4 to 6 hours of the SA huddle by a core group of three research assistants. Care team trios for one group of five to nine patients within a small geographically isolated pod were surveyed each time. The care team trio was surveyed individually regarding the patient’s risk status, the high-risk clinical criteria met, the presence and content of a mitigation plan, and planned patient interventions. The responses were compared for accuracy against the reference standard, which was defined as identification of high-risk patient status and development of the prevention and mitigation plan as completed by the PICU fellow or attending.
Data Analysis
Rates of agreement between the reference standard and individual members of the care team trio were evaluated via a calculation of proportions by care team role. The agreement between each care team trio member and the reference standard was compared with the nurse role performance using chi-square tests. Rates of concordance within the members of the care team trio were calculated via Light’s kappa for determination of high-risk status.11 Assuming a correct assessment of high-risk status of 62%,12 with a difference between groups of 10%, a sample size of 400 bedside provider trios gives a power of 85% at the P < .05 significance level for a two-sided chi-square test.
RESULTS
Between March 1, 2018, and July 11, 2019, 400 care team trios were surveyed. Seventy-three trios cared for patients designated high risk (Table 2 for N and proportions). Among all surveyed trios, 94% of nurses (reference), 95% of RTs (P = .4), and 87% of residents (P = .002) identified patient’s risk status correctly. Care trio member concordance for high-risk status was moderate agreement as assessed by a kappa of 0.57 (95% CI, 0.25-0.90).

Of the 73 high-risk patients, nurses correctly identified risk status for 82% (reference), RTs 85% (P = .7), and residents 67% (P = .04). For high-risk patients, nurses identified the presence of a mitigation plan for 98% of patients (reference), RTs 90% (P = .06), and residents 88% (P = .03). Among the care team members who correctly identified the presence of a mitigation plan, nurses were able to specify the correct plan for 83% of patients (reference), RTs for 68% (P = .09), and residents for 70% (P = .11; Figure).
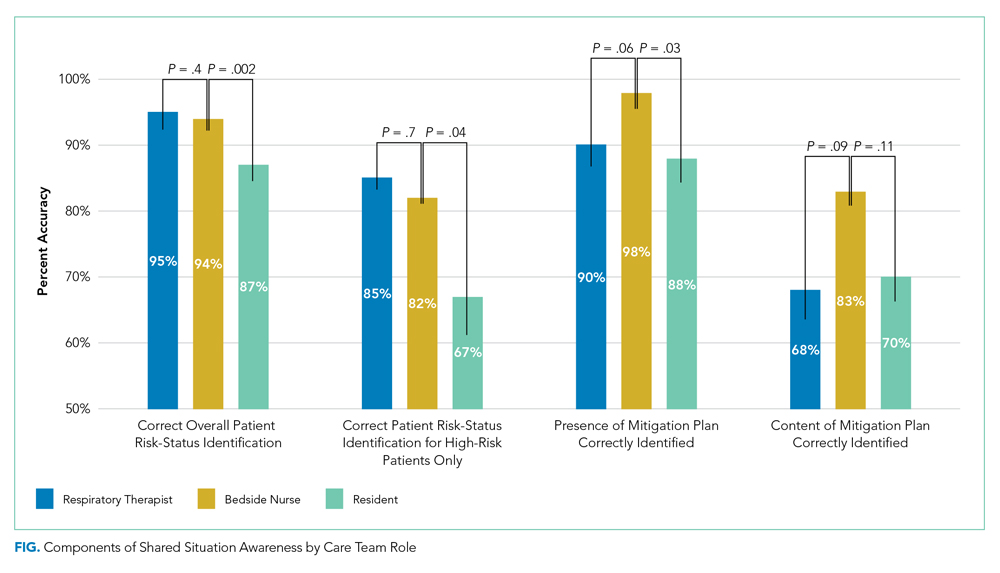
When shared SA for high-risk patients was examined more closely, all three care team roles correctly identified the clinical reason for high-risk status for 32% of patients, with only one or two clinicians being correct for 53%. All three care team clinicians were incorrect for 15% of high-risk patients. Among trios with partial accuracy in which two of three care team members correctly identified a patient as high risk, we examined which care-member was most likely to be incorrect. Nurses incorrectly identified risk for 17% of patients (reference), RTs 19% (P = .8), and residents 64% (P < .0001).
DISCUSSION
Examining 400 care team trios, we found lower individual SA for residents, compared with nurses, regarding high-risk status, the reason for this status, and the presence of a mitigation plan. In all reported measures except for the content of mitigation plans, residents were significantly less correct than the bedside nurses while RTs performed similarly to bedside nurses throughout. In addition, there was only moderate agreement between care team roles, which shows further opportunities for improvement in shared SA. The disparities between care team roles are consistent with studies that suggest certain factors grounded in institutional culture and interpersonal dynamics, such as poor communication, can lead to breakdowns in shared knowledge.13,14 Communication issues demonstrate differences across care team roles14 and may provide insight into barriers to individual and shared SA throughout the care team.
In addition, the effects of patient load on SA needs further study. While our PICU nurses are commonly assigned to 1 to 2 patients, RTs care for 7 to 11 patients, and an on-call resident may be covering 15 to 20 patients during a high-census season. The increased patient load cannot serve as an excuse for the knowledge gap regarding high-risk status and mitigation plan, but may provide an opportunity to support residents and other medical providers through the use of clinical decision-support tools that indicate high-risk status and represent mitigation plans.12
This study has multiple limitations. First, while we based our survey tool on a communication assessment tool with prior validity evidence,10,12 our tool has not been used prior to this study. The adapted tool contained relevant categorizations of patient information, including explicit statement of patient status and planned treatment consistent with study definitions of SA, and has been used in the critical care setting previously.11 The survey tool used to measure SA in this study was locally designed and implemented only within the study unit, which could lead to decreased reliability and generalizability of the results to other units and institutions at large. Second, while the sample size for the primary measure (N = 400) was adequately powered because our baseline SA was higher than estimated, we had insufficient power for some subgroup analyses that can lead to type II errors. Third, care team trios may have been surveyed repeatedly on the same patient without adjustment in the results for repeated measures. However, as we surveyed on average only once a week and alternated areas of the PICU surveyed, it is unlikely that it affected results given that the most lengths of stay within the PICU range from 3 to 4 days. Finally, individual characteristics of patients were not collected for this work, and therefore, no adjustments or further analysis can be made on the effect of the patient characteristic on the care team role SA.
CONCLUSION
This study is the first to assess differences in individual and shared SA within a PICU by care team role. Efforts to expand on these findings should include investigation into the causes for the disparities in SA among care team roles for individual patients and among the care teams of high-risk and normal-risk patients. Given the association between increased SA and improved patient outcomes,4 future efforts should be structured to address care team role–specific gaps in SA because these may advance the quality of care in the pediatric inpatient setting.
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://doi.org/10.1542/peds.2016-3494
2. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
3. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-308. https://doi.org/10.1542/peds.2012-1364
4. Endsley MR. Theoretical underpinnings of situation awareness: a critical review. In: Endsley MR, Garland DJ, eds. Situation Awareness Analysis and Measurement. Lawrence Erlbaum Associates; 2000.
5. Dewan M, Wolfe H, Lin R, et al. Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patients. J Hosp Med. 2017;12(8):652‐657. https://doi.org/10.12788/jhm.2782
6. Bonafide CP, Localio AR, Stemler S, et al. Safety huddle intervention for reducing physiologic monitor alarms: a hybrid effectiveness-implementation cluster randomized trial. J Hosp Med. 2018;13(9):609‐615. https://doi.org/10.12788/jhm.2956
7. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. https://doi.org/10.1097/HMR.0000000000000009
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE, Anderson JM. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. https://doi.org/10.1136/bmjqs-2012-001467
9. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. https://doi.org/10.1002/jhm.886
10. Shahian DM, McEachern K, Rossi L, Chisari RG, Mort E. Large-scale implementation of the I-PASS handover system at an academic medical centre. BMJ Qual Saf. 2017;26(9):760-770. https://doi.org/10.1136/bmjqs-2016-006195
11. Gamer M, Lemon J, Fellows I, Singh P. Various Coefficients of Interrater Reliability and Agreement. January 26, 2019. Accessed January 24, 2020. http://cran.r-project.org/web/packages/irr/irr.pdf
12. Shelov E, Muthu N, Wolfe H, et al. Design and implementation of a pediatric ICU acuity scoring tool as clinical decision support. Appl Clin Inf. 2018;09(3):576-587. https://doi.org/10.1055/s-0038-1667122
13. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019
14. Sexton B, Thomas E, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320(7237):745-749. doi:10.1136/bmj.320.7237.745
Reduction in serious pediatric medical errors has been achieved through sharing of best practices and structured collaboration.1 However, limited progress has been made in reducing complex, multifactorial events such as unrecognized and undertreated patient deterioration events.2 To address this critical gap, interventions to improve clinician situation awareness (SA) have increasingly been applied.3
SA is the ability to recognize and monitor cues regarding what is happening, create a comprehensive picture with available information, and extrapolate whether it indicates adverse developments either immediately or in the near future.4 Methods such as care team huddling5-8 and using standardized patient acuity scoring instruments9 increase SA shared across care team roles. Shared SA is the degree to which each team member possesses a common understanding of what is going on. A team is considered to have shared SA when all the individuals agree on both what is happening (accurate perception and comprehension) and what is going to happen in the future (correct projection). Shared SA for high-risk patients in the pediatric intensive care unit (PICU) has not previously been described and may be an opportunity to improve interprofessional team communication for the sickest patients. Shared SA for high-risk patient status is only one aspect of SA, but it facilitates team-based mitigation planning and is an important starting place for understanding opportunities to improve SA. The primary objective of this study was to measure and compare SA among care team roles regarding patients with high-risk status in the PICU.
METHODS
We conducted a prospective, cross-sectional study from March 2018 to July 2019 examining the individual and shared SA of patient care team trios: the nurse, respiratory therapist (RT), and pediatric resident. The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) determined this study to be non–human-subjects research.
Setting
Research was conducted in the 35-bed PICU of CCHMC, a 500-bed academic free-standing quaternary care children’s hospital.
Participants
We conducted independent surveys of the nurse, RT, and pediatric resident (care team trio) caring for each patient regarding the patient’s clinical deterioration risk status. No patients or care team trios were excluded.
Reference Standard
In 2016, a local panel of experts derived clinical criteria to determine high-risk status for PICU patients, the definition of which, as well as other study terms, appears in Table 1. A PICU attending or fellow identifies a patient as “high risk” when these clinical criteria are met. A plan for prevention and mitigation is formulated and documented for high-risk patients by the PICU attending or fellow at two preexisting daily SA huddles. This plan includes prevention measures to take immediately, specific vital sign thresholds for early identification of deterioration, and guidance on which emergency medication order sets should be utilized to expedite treatment in the event of clinical decline. Dissemination of the care team’s plan is the responsibility of the PICU fellow with additional follow-up by the charge nurse to improve reliability. Identification of high-risk status and development of the prevention and mitigation plan, as completed by the PICU fellow or attending, served as the reference standard for this study.

Survey Instrument Development
The locally developed survey tool was modeled after a validated handoff communication instrument.10 The tool covered the patient’s risk status, which high-risk clinical criteria were met, the presence and content of a mitigation plan, and planned patient interventions (Appendix).
Data Collection
Care team trios were sampled weekly on weekdays during day and night shifts within 4 to 6 hours of the SA huddle by a core group of three research assistants. Care team trios for one group of five to nine patients within a small geographically isolated pod were surveyed each time. The care team trio was surveyed individually regarding the patient’s risk status, the high-risk clinical criteria met, the presence and content of a mitigation plan, and planned patient interventions. The responses were compared for accuracy against the reference standard, which was defined as identification of high-risk patient status and development of the prevention and mitigation plan as completed by the PICU fellow or attending.
Data Analysis
Rates of agreement between the reference standard and individual members of the care team trio were evaluated via a calculation of proportions by care team role. The agreement between each care team trio member and the reference standard was compared with the nurse role performance using chi-square tests. Rates of concordance within the members of the care team trio were calculated via Light’s kappa for determination of high-risk status.11 Assuming a correct assessment of high-risk status of 62%,12 with a difference between groups of 10%, a sample size of 400 bedside provider trios gives a power of 85% at the P < .05 significance level for a two-sided chi-square test.
RESULTS
Between March 1, 2018, and July 11, 2019, 400 care team trios were surveyed. Seventy-three trios cared for patients designated high risk (Table 2 for N and proportions). Among all surveyed trios, 94% of nurses (reference), 95% of RTs (P = .4), and 87% of residents (P = .002) identified patient’s risk status correctly. Care trio member concordance for high-risk status was moderate agreement as assessed by a kappa of 0.57 (95% CI, 0.25-0.90).

Of the 73 high-risk patients, nurses correctly identified risk status for 82% (reference), RTs 85% (P = .7), and residents 67% (P = .04). For high-risk patients, nurses identified the presence of a mitigation plan for 98% of patients (reference), RTs 90% (P = .06), and residents 88% (P = .03). Among the care team members who correctly identified the presence of a mitigation plan, nurses were able to specify the correct plan for 83% of patients (reference), RTs for 68% (P = .09), and residents for 70% (P = .11; Figure).

When shared SA for high-risk patients was examined more closely, all three care team roles correctly identified the clinical reason for high-risk status for 32% of patients, with only one or two clinicians being correct for 53%. All three care team clinicians were incorrect for 15% of high-risk patients. Among trios with partial accuracy in which two of three care team members correctly identified a patient as high risk, we examined which care-member was most likely to be incorrect. Nurses incorrectly identified risk for 17% of patients (reference), RTs 19% (P = .8), and residents 64% (P < .0001).
DISCUSSION
Examining 400 care team trios, we found lower individual SA for residents, compared with nurses, regarding high-risk status, the reason for this status, and the presence of a mitigation plan. In all reported measures except for the content of mitigation plans, residents were significantly less correct than the bedside nurses while RTs performed similarly to bedside nurses throughout. In addition, there was only moderate agreement between care team roles, which shows further opportunities for improvement in shared SA. The disparities between care team roles are consistent with studies that suggest certain factors grounded in institutional culture and interpersonal dynamics, such as poor communication, can lead to breakdowns in shared knowledge.13,14 Communication issues demonstrate differences across care team roles14 and may provide insight into barriers to individual and shared SA throughout the care team.
In addition, the effects of patient load on SA needs further study. While our PICU nurses are commonly assigned to 1 to 2 patients, RTs care for 7 to 11 patients, and an on-call resident may be covering 15 to 20 patients during a high-census season. The increased patient load cannot serve as an excuse for the knowledge gap regarding high-risk status and mitigation plan, but may provide an opportunity to support residents and other medical providers through the use of clinical decision-support tools that indicate high-risk status and represent mitigation plans.12
This study has multiple limitations. First, while we based our survey tool on a communication assessment tool with prior validity evidence,10,12 our tool has not been used prior to this study. The adapted tool contained relevant categorizations of patient information, including explicit statement of patient status and planned treatment consistent with study definitions of SA, and has been used in the critical care setting previously.11 The survey tool used to measure SA in this study was locally designed and implemented only within the study unit, which could lead to decreased reliability and generalizability of the results to other units and institutions at large. Second, while the sample size for the primary measure (N = 400) was adequately powered because our baseline SA was higher than estimated, we had insufficient power for some subgroup analyses that can lead to type II errors. Third, care team trios may have been surveyed repeatedly on the same patient without adjustment in the results for repeated measures. However, as we surveyed on average only once a week and alternated areas of the PICU surveyed, it is unlikely that it affected results given that the most lengths of stay within the PICU range from 3 to 4 days. Finally, individual characteristics of patients were not collected for this work, and therefore, no adjustments or further analysis can be made on the effect of the patient characteristic on the care team role SA.
CONCLUSION
This study is the first to assess differences in individual and shared SA within a PICU by care team role. Efforts to expand on these findings should include investigation into the causes for the disparities in SA among care team roles for individual patients and among the care teams of high-risk and normal-risk patients. Given the association between increased SA and improved patient outcomes,4 future efforts should be structured to address care team role–specific gaps in SA because these may advance the quality of care in the pediatric inpatient setting.
Reduction in serious pediatric medical errors has been achieved through sharing of best practices and structured collaboration.1 However, limited progress has been made in reducing complex, multifactorial events such as unrecognized and undertreated patient deterioration events.2 To address this critical gap, interventions to improve clinician situation awareness (SA) have increasingly been applied.3
SA is the ability to recognize and monitor cues regarding what is happening, create a comprehensive picture with available information, and extrapolate whether it indicates adverse developments either immediately or in the near future.4 Methods such as care team huddling5-8 and using standardized patient acuity scoring instruments9 increase SA shared across care team roles. Shared SA is the degree to which each team member possesses a common understanding of what is going on. A team is considered to have shared SA when all the individuals agree on both what is happening (accurate perception and comprehension) and what is going to happen in the future (correct projection). Shared SA for high-risk patients in the pediatric intensive care unit (PICU) has not previously been described and may be an opportunity to improve interprofessional team communication for the sickest patients. Shared SA for high-risk patient status is only one aspect of SA, but it facilitates team-based mitigation planning and is an important starting place for understanding opportunities to improve SA. The primary objective of this study was to measure and compare SA among care team roles regarding patients with high-risk status in the PICU.
METHODS
We conducted a prospective, cross-sectional study from March 2018 to July 2019 examining the individual and shared SA of patient care team trios: the nurse, respiratory therapist (RT), and pediatric resident. The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) determined this study to be non–human-subjects research.
Setting
Research was conducted in the 35-bed PICU of CCHMC, a 500-bed academic free-standing quaternary care children’s hospital.
Participants
We conducted independent surveys of the nurse, RT, and pediatric resident (care team trio) caring for each patient regarding the patient’s clinical deterioration risk status. No patients or care team trios were excluded.
Reference Standard
In 2016, a local panel of experts derived clinical criteria to determine high-risk status for PICU patients, the definition of which, as well as other study terms, appears in Table 1. A PICU attending or fellow identifies a patient as “high risk” when these clinical criteria are met. A plan for prevention and mitigation is formulated and documented for high-risk patients by the PICU attending or fellow at two preexisting daily SA huddles. This plan includes prevention measures to take immediately, specific vital sign thresholds for early identification of deterioration, and guidance on which emergency medication order sets should be utilized to expedite treatment in the event of clinical decline. Dissemination of the care team’s plan is the responsibility of the PICU fellow with additional follow-up by the charge nurse to improve reliability. Identification of high-risk status and development of the prevention and mitigation plan, as completed by the PICU fellow or attending, served as the reference standard for this study.

Survey Instrument Development
The locally developed survey tool was modeled after a validated handoff communication instrument.10 The tool covered the patient’s risk status, which high-risk clinical criteria were met, the presence and content of a mitigation plan, and planned patient interventions (Appendix).
Data Collection
Care team trios were sampled weekly on weekdays during day and night shifts within 4 to 6 hours of the SA huddle by a core group of three research assistants. Care team trios for one group of five to nine patients within a small geographically isolated pod were surveyed each time. The care team trio was surveyed individually regarding the patient’s risk status, the high-risk clinical criteria met, the presence and content of a mitigation plan, and planned patient interventions. The responses were compared for accuracy against the reference standard, which was defined as identification of high-risk patient status and development of the prevention and mitigation plan as completed by the PICU fellow or attending.
Data Analysis
Rates of agreement between the reference standard and individual members of the care team trio were evaluated via a calculation of proportions by care team role. The agreement between each care team trio member and the reference standard was compared with the nurse role performance using chi-square tests. Rates of concordance within the members of the care team trio were calculated via Light’s kappa for determination of high-risk status.11 Assuming a correct assessment of high-risk status of 62%,12 with a difference between groups of 10%, a sample size of 400 bedside provider trios gives a power of 85% at the P < .05 significance level for a two-sided chi-square test.
RESULTS
Between March 1, 2018, and July 11, 2019, 400 care team trios were surveyed. Seventy-three trios cared for patients designated high risk (Table 2 for N and proportions). Among all surveyed trios, 94% of nurses (reference), 95% of RTs (P = .4), and 87% of residents (P = .002) identified patient’s risk status correctly. Care trio member concordance for high-risk status was moderate agreement as assessed by a kappa of 0.57 (95% CI, 0.25-0.90).

Of the 73 high-risk patients, nurses correctly identified risk status for 82% (reference), RTs 85% (P = .7), and residents 67% (P = .04). For high-risk patients, nurses identified the presence of a mitigation plan for 98% of patients (reference), RTs 90% (P = .06), and residents 88% (P = .03). Among the care team members who correctly identified the presence of a mitigation plan, nurses were able to specify the correct plan for 83% of patients (reference), RTs for 68% (P = .09), and residents for 70% (P = .11; Figure).

When shared SA for high-risk patients was examined more closely, all three care team roles correctly identified the clinical reason for high-risk status for 32% of patients, with only one or two clinicians being correct for 53%. All three care team clinicians were incorrect for 15% of high-risk patients. Among trios with partial accuracy in which two of three care team members correctly identified a patient as high risk, we examined which care-member was most likely to be incorrect. Nurses incorrectly identified risk for 17% of patients (reference), RTs 19% (P = .8), and residents 64% (P < .0001).
DISCUSSION
Examining 400 care team trios, we found lower individual SA for residents, compared with nurses, regarding high-risk status, the reason for this status, and the presence of a mitigation plan. In all reported measures except for the content of mitigation plans, residents were significantly less correct than the bedside nurses while RTs performed similarly to bedside nurses throughout. In addition, there was only moderate agreement between care team roles, which shows further opportunities for improvement in shared SA. The disparities between care team roles are consistent with studies that suggest certain factors grounded in institutional culture and interpersonal dynamics, such as poor communication, can lead to breakdowns in shared knowledge.13,14 Communication issues demonstrate differences across care team roles14 and may provide insight into barriers to individual and shared SA throughout the care team.
In addition, the effects of patient load on SA needs further study. While our PICU nurses are commonly assigned to 1 to 2 patients, RTs care for 7 to 11 patients, and an on-call resident may be covering 15 to 20 patients during a high-census season. The increased patient load cannot serve as an excuse for the knowledge gap regarding high-risk status and mitigation plan, but may provide an opportunity to support residents and other medical providers through the use of clinical decision-support tools that indicate high-risk status and represent mitigation plans.12
This study has multiple limitations. First, while we based our survey tool on a communication assessment tool with prior validity evidence,10,12 our tool has not been used prior to this study. The adapted tool contained relevant categorizations of patient information, including explicit statement of patient status and planned treatment consistent with study definitions of SA, and has been used in the critical care setting previously.11 The survey tool used to measure SA in this study was locally designed and implemented only within the study unit, which could lead to decreased reliability and generalizability of the results to other units and institutions at large. Second, while the sample size for the primary measure (N = 400) was adequately powered because our baseline SA was higher than estimated, we had insufficient power for some subgroup analyses that can lead to type II errors. Third, care team trios may have been surveyed repeatedly on the same patient without adjustment in the results for repeated measures. However, as we surveyed on average only once a week and alternated areas of the PICU surveyed, it is unlikely that it affected results given that the most lengths of stay within the PICU range from 3 to 4 days. Finally, individual characteristics of patients were not collected for this work, and therefore, no adjustments or further analysis can be made on the effect of the patient characteristic on the care team role SA.
CONCLUSION
This study is the first to assess differences in individual and shared SA within a PICU by care team role. Efforts to expand on these findings should include investigation into the causes for the disparities in SA among care team roles for individual patients and among the care teams of high-risk and normal-risk patients. Given the association between increased SA and improved patient outcomes,4 future efforts should be structured to address care team role–specific gaps in SA because these may advance the quality of care in the pediatric inpatient setting.
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://doi.org/10.1542/peds.2016-3494
2. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
3. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-308. https://doi.org/10.1542/peds.2012-1364
4. Endsley MR. Theoretical underpinnings of situation awareness: a critical review. In: Endsley MR, Garland DJ, eds. Situation Awareness Analysis and Measurement. Lawrence Erlbaum Associates; 2000.
5. Dewan M, Wolfe H, Lin R, et al. Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patients. J Hosp Med. 2017;12(8):652‐657. https://doi.org/10.12788/jhm.2782
6. Bonafide CP, Localio AR, Stemler S, et al. Safety huddle intervention for reducing physiologic monitor alarms: a hybrid effectiveness-implementation cluster randomized trial. J Hosp Med. 2018;13(9):609‐615. https://doi.org/10.12788/jhm.2956
7. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. https://doi.org/10.1097/HMR.0000000000000009
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE, Anderson JM. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. https://doi.org/10.1136/bmjqs-2012-001467
9. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. https://doi.org/10.1002/jhm.886
10. Shahian DM, McEachern K, Rossi L, Chisari RG, Mort E. Large-scale implementation of the I-PASS handover system at an academic medical centre. BMJ Qual Saf. 2017;26(9):760-770. https://doi.org/10.1136/bmjqs-2016-006195
11. Gamer M, Lemon J, Fellows I, Singh P. Various Coefficients of Interrater Reliability and Agreement. January 26, 2019. Accessed January 24, 2020. http://cran.r-project.org/web/packages/irr/irr.pdf
12. Shelov E, Muthu N, Wolfe H, et al. Design and implementation of a pediatric ICU acuity scoring tool as clinical decision support. Appl Clin Inf. 2018;09(3):576-587. https://doi.org/10.1055/s-0038-1667122
13. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019
14. Sexton B, Thomas E, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320(7237):745-749. doi:10.1136/bmj.320.7237.745
1. Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140(3):e20163494. https://doi.org/10.1542/peds.2016-3494
2. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
3. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-308. https://doi.org/10.1542/peds.2012-1364
4. Endsley MR. Theoretical underpinnings of situation awareness: a critical review. In: Endsley MR, Garland DJ, eds. Situation Awareness Analysis and Measurement. Lawrence Erlbaum Associates; 2000.
5. Dewan M, Wolfe H, Lin R, et al. Impact of a safety huddle-based intervention on monitor alarm rates in low-acuity pediatric intensive care unit patients. J Hosp Med. 2017;12(8):652‐657. https://doi.org/10.12788/jhm.2782
6. Bonafide CP, Localio AR, Stemler S, et al. Safety huddle intervention for reducing physiologic monitor alarms: a hybrid effectiveness-implementation cluster randomized trial. J Hosp Med. 2018;13(9):609‐615. https://doi.org/10.12788/jhm.2956
7. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. https://doi.org/10.1097/HMR.0000000000000009
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE, Anderson JM. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. https://doi.org/10.1136/bmjqs-2012-001467
9. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. https://doi.org/10.1002/jhm.886
10. Shahian DM, McEachern K, Rossi L, Chisari RG, Mort E. Large-scale implementation of the I-PASS handover system at an academic medical centre. BMJ Qual Saf. 2017;26(9):760-770. https://doi.org/10.1136/bmjqs-2016-006195
11. Gamer M, Lemon J, Fellows I, Singh P. Various Coefficients of Interrater Reliability and Agreement. January 26, 2019. Accessed January 24, 2020. http://cran.r-project.org/web/packages/irr/irr.pdf
12. Shelov E, Muthu N, Wolfe H, et al. Design and implementation of a pediatric ICU acuity scoring tool as clinical decision support. Appl Clin Inf. 2018;09(3):576-587. https://doi.org/10.1055/s-0038-1667122
13. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019
14. Sexton B, Thomas E, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320(7237):745-749. doi:10.1136/bmj.320.7237.745
© 2020 Society of Hospital Medicine
Emergency Transfers: An Important Predictor of Adverse Outcomes in Hospitalized Children
Unrecognized in-hospital deterioration can result in tragic consequences for pediatric patients. The majority of deterioration events have antecedents such as increasingly abnormal vital signs and new concerns from nurses.1 Recent meta-analyses have shown that rapid response systems (RRSs), which include trigger mechanisms such as a pediatric early warning score (PEWS), are associated with a reduced rate of arrests and in-hospital mortality.2,3 Cardiopulmonary arrest rates are useful metrics to judge the effectiveness of the system to identify and respond to deteriorating adult patients; however, there are important challenges to their use as an outcome measure in pediatrics. Arrests, which have been relatively uncommon in pediatric patients, are now even less frequent since the adoption of a RRS in the majority of children’s hospitals.4,5 Several innovations in these systems will be context-dependent and hence best first evaluated in a single center, where arrests outside of the intensive care unit (ICU) may occur rarely. Identification of valid, more frequent proximal measures to arrests may better identify the risk factors for deterioration. This could potentially inform quality improvement efforts to mitigate clinical deterioration.
Bonafide et al. at the Children’s Hospital of Philadelphia developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had a >13-fold increased risk of in-hospital mortality.6 At Cincinnati Children’s Hospital Medical Center, an additional proximal outcome measure was developed for unrecognized clinical deterioration, now termed emergency transfers (ETs).7-9 An ET is defined as any patient transferred to the ICU where the patient received intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer.9 Improvement science work that aimed at increasing clinician situation awareness was associated with a reduction in ETs,8 but the association of ETs with mortality or other healthcare utilization outcomes is unknown. The objective of this study was to determine the predictive validity of an ET on in-hospital mortality, ICU length of stay (LOS), and overall hospital LOS.
METHODS
We conducted a case–control study at Cincinnati Children’s Hospital, a free-standing tertiary care children’s hospital. Our center has had an ICU-based RRS in place since 2005. In 2009, we eliminated the ICU consult such that each floor-to-ICU transfer is evaluated by the RRS. Nurses calculate a Monaghan PEWS every four hours on the majority of nursing units.
Patients of all ages cared for outside of the ICU at any point in their hospitalization from January 1, 2013, to July 31, 2017, were eligible for inclusion. There were no other exclusion criteria. The ICU included both the pediatric ICU and the cardiac ICU.
Cases
We identified all ET cases from an existing situation awareness database in which each RRS call is entered by the hospital nursing supervisor, whose role includes responding to each RRS activation. If the patient transfer meets the ET criteria, the nurse indicates this in the database. Each ET entry is later confirmed for assurance purposes by the nurse leader of the RRS committee (RG). For the purposes of this study, all records were again reviewed and validated using manual chart review in the electronic health record (Epic Systems, Verona, Wisconsin).
Controls
We identified nonemergent ICU transfers to serve as controls and matched those to ET in cases to limit the impact of confounders that may increase the likelihood of both an ET and a negative outcome such as ICU mortality. We identified up to three controls for each case from our database and matched in terms of age group (within five years of age), hospital unit before transfer, and time of year (within three months of ET). These variables were chosen to adjust for the impact of age, diversity of disease (as hospital units are generally organized by organ system of illness), and seasonality on outcomes.
Outcome Measures
Posttransfer LOS in the ICU, posttransfer hospital LOS, and in-hospital mortality were the primary outcome measures. Patient demographics, specific diagnoses, and number of medical conditions were a priori defined as covariates of interest. Data for each case and control were entered into a secure, web-based Research Electronic Data Capture (REDCap) database.
Analysis
Descriptive data were summarized using counts and percentages for categorical variables and medians and ranges for continuous variables due to nonnormal distributions. Chi-square test was used to compare in-hospital mortality between the ETs and the controls. The Wilcoxon rank-sum test was used to compare LOS between ETs and controls. All data analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
A total of 45 ETs were identified, and 110 controls were matched. Patient demographics were similar among all cases and controls (P > .05). Patients with ETs had a median age of seven years (interquartile range: 3-18 years), and 51% of them were males. The majority of patients among our examined cases were white (68%) and non-Hispanic (93%). There was no statistical difference in insurance between the ETs and the controls. When evaluating the hospital unit before the transfer, ETs occurred most commonly in the Cardiology (22%), Hematology/Oncology (22%), and Neuroscience (16%) units.
ETs stayed longer in the ICU than non-ETs [median of 4.9 days vs 2.2 days, P = .001; Figure (A)]. Similarly, ET cases had a significantly longer posttransfer hospital LOS [median of 35 days vs 21 days, P = .001; Figure (B)]. ETs had a 22% in-hospital mortality rate, compared with 9% in-hospital mortality in the matched controls (P = .02; Table).
DISCUSSION
Children who experienced an ET had a significantly longer ICU LOS, a longer posttransfer LOS, and a higher in-hospital mortality than the matched controls who were also transferred to the ICU. Researchers and improvement science teams at multiple hospitals have demonstrated that interventions targeting improved situation awareness can reduce ETs; we have demonstrated that reducing ETs may reduce subsequent adverse outcomes.8,10
These findings provide additional support for the use of the ET metric in children’s hospitals as a proximal measure for significant clinical deterioration. We found mortality rates that were overall high for a children’s hospital (22% in ET cases and 9% among controls) compared with a national average mortality rate of 2.3% in pediatric ICUs.11 This is likely due to the study sample containing a significant proportion of children with medical complexity.
Aoki et al. recently demonstrated that ETs, compared with non-ETs, were associated with longer LOS and higher mortality in a bivariate analysis.12 In our study, we found similar results with the important addition that these findings were robust when ETs were compared with matched controls who were likely at a higher risk of poor outcomes than ICU transfers in general. In addition, we demonstrated that ETs were associated with adverse outcomes in a United States children’s hospital with a mature, long-standing RRS process. As ETs are considerably more common than cardiac and respiratory arrests, use of the ET metric in children’s hospitals may enable more rapid learning and systems improvement implementations. We also found that most of the children with ETs present from units that care for children with substantial medical complexity, including Cardiology, Hematology/Oncology, and Neurosciences. Future work should continue to examine the relationship between medical complexity and ET risk.
The ET metric is complementary to the CDE measure developed by Bonafide et al. Both metrics capture potential events of unrecognized clinical deterioration, and both offer researchers the opportunity to better understand and improve their RRSs. Both ETs and CDEs are more common than arrests, and CDEs are more common than ETs. ETs, which by definition occur in the first hour of ICU care, are likely a more specific measure of unrecognized clinical deterioration. CDEs will capture therapies that may have been started up to 12 hours after transfer and thus are possibly more sensitive to identify unrecognized clinical deterioration. However, CDEs also may encompass some patients who arrived at the ICU after prompt recognition and then had a subacute deterioration in the ICU.
The maturity of the RRS and the bandwidth of teams to collect data may inform which metric(s) are best for individual centers. As ETs are less common and likely more specific to unrecognized clinical deterioration, they might be the first tracked as a center improves its RRS through QI methods. Alternatively, CDEs may be a useful metric for centers where unrecognized clinical deterioration is less common or in research studies where this more common outcome would lead to more power to detect the effect of interventions to improve care.
Our study had several limitations. Data collection was confined to one tertiary care children’s hospital with a high burden of complex cardiac and oncology care. The results may not generalize well to children hospitalized in smaller or community hospitals or in hospitals without a mature RRS. There is also the possibility of misclassification of covariates and outcomes, but any misclassification would likely be nondifferential and bias toward the null. Matching was not possible based on exact diagnosis, and the unit is a good but imperfect proxy for diagnosis grouping. At our center, overflow of patients into the Cardiology and Hematology/Oncology units is uncommon, mitigating this partially, although residual confounding may remain. The finding that ETs are associated with adverse outcomes does not necessarily mean that these events were preventable; however, it is important and encouraging that the rate of ETs has been reduced at two centers using improvement science interventions.8,10
CONCLUSION
Patients who experienced an ET had a significantly higher likelihood of in-hospital mortality, spent more time in the ICU, and had a longer hospital LOS posttransfer than matched controls. The use of the ET metric in children’s hospitals would allow for further analysis of such patients in hopes of identifying clinical characteristics that serve as predictors of deterioration. This may facilitate better risk stratification in the clinical system as well as enable more rapid learning and systems improvements targeted toward preventing unrecognized clinical deterioration.
Disclosures
Dr. Hussain, Dr. Sosa, Dr. Ambroggio, and Mrs. Gallagher have nothing to disclose. Patrick Brady reports grants from the Agency for Healthcare Research and Quality, outside the submitted work. The authors certify that this submission is not under review by any other publication. The author team has no conflicts of interest to disclose.
Funding
Ms. Hussain was supported by the Society of Hospital Medicine’s Student Hospitalist Scholar Grant Program in 2017. Dr. Brady receives career development support from AHRQ K08-HS023827. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the SHM, AHRQ, or NIH.
1. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388.
2. Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015;19:254. https://doi.org/10.1186/s13054-015-0973-y.
3. Bonafide CP, Roland D, Brady PW. Rapid response systems 20 years later: new approaches, old challenges. JAMA Pediatrics. 2016;170(8):729-730. https://doi.org/10.1001/jamapediatrics.2016.0398.
4. Hayes LW, Dobyns EL, DiGiovine B, et al. A multicenter collaborative approach to reducing pediatric codes outside the ICU. Pediatrics. 2012;129(3):e785-e791. https://doi.org/10.1542/peds.2011-0227.
5. Raymond TT, Bonafide CP, Praestgaard A, et al. Pediatric medical emergency team events and outcomes: a report of 3647 events from the American Heart Association’s get with the guidelines-resuscitation registry. Hosp Pediatr. 2016;6(2):57-64. https://doi.org/10.1542/hpeds.2015-0132.
6. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784.
7. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747.
8. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364.
9. Brady PW, Wheeler DS, Muething SE, Kotagal UR. Situation awareness: a new model for predicting and preventing patient deterioration. Hosp Pediatr. 2014;4(3):143-146. https://doi.org/10.1542/hpeds.2013-0119.
10. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042.
11. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med. 2017;18(10):915-923. https://doi.org/10.1097/PCC.0000000000001268.
12. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2018;55(2):213-215. https://doi.org/10.1111/jpc.14185.
Unrecognized in-hospital deterioration can result in tragic consequences for pediatric patients. The majority of deterioration events have antecedents such as increasingly abnormal vital signs and new concerns from nurses.1 Recent meta-analyses have shown that rapid response systems (RRSs), which include trigger mechanisms such as a pediatric early warning score (PEWS), are associated with a reduced rate of arrests and in-hospital mortality.2,3 Cardiopulmonary arrest rates are useful metrics to judge the effectiveness of the system to identify and respond to deteriorating adult patients; however, there are important challenges to their use as an outcome measure in pediatrics. Arrests, which have been relatively uncommon in pediatric patients, are now even less frequent since the adoption of a RRS in the majority of children’s hospitals.4,5 Several innovations in these systems will be context-dependent and hence best first evaluated in a single center, where arrests outside of the intensive care unit (ICU) may occur rarely. Identification of valid, more frequent proximal measures to arrests may better identify the risk factors for deterioration. This could potentially inform quality improvement efforts to mitigate clinical deterioration.
Bonafide et al. at the Children’s Hospital of Philadelphia developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had a >13-fold increased risk of in-hospital mortality.6 At Cincinnati Children’s Hospital Medical Center, an additional proximal outcome measure was developed for unrecognized clinical deterioration, now termed emergency transfers (ETs).7-9 An ET is defined as any patient transferred to the ICU where the patient received intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer.9 Improvement science work that aimed at increasing clinician situation awareness was associated with a reduction in ETs,8 but the association of ETs with mortality or other healthcare utilization outcomes is unknown. The objective of this study was to determine the predictive validity of an ET on in-hospital mortality, ICU length of stay (LOS), and overall hospital LOS.
METHODS
We conducted a case–control study at Cincinnati Children’s Hospital, a free-standing tertiary care children’s hospital. Our center has had an ICU-based RRS in place since 2005. In 2009, we eliminated the ICU consult such that each floor-to-ICU transfer is evaluated by the RRS. Nurses calculate a Monaghan PEWS every four hours on the majority of nursing units.
Patients of all ages cared for outside of the ICU at any point in their hospitalization from January 1, 2013, to July 31, 2017, were eligible for inclusion. There were no other exclusion criteria. The ICU included both the pediatric ICU and the cardiac ICU.
Cases
We identified all ET cases from an existing situation awareness database in which each RRS call is entered by the hospital nursing supervisor, whose role includes responding to each RRS activation. If the patient transfer meets the ET criteria, the nurse indicates this in the database. Each ET entry is later confirmed for assurance purposes by the nurse leader of the RRS committee (RG). For the purposes of this study, all records were again reviewed and validated using manual chart review in the electronic health record (Epic Systems, Verona, Wisconsin).
Controls
We identified nonemergent ICU transfers to serve as controls and matched those to ET in cases to limit the impact of confounders that may increase the likelihood of both an ET and a negative outcome such as ICU mortality. We identified up to three controls for each case from our database and matched in terms of age group (within five years of age), hospital unit before transfer, and time of year (within three months of ET). These variables were chosen to adjust for the impact of age, diversity of disease (as hospital units are generally organized by organ system of illness), and seasonality on outcomes.
Outcome Measures
Posttransfer LOS in the ICU, posttransfer hospital LOS, and in-hospital mortality were the primary outcome measures. Patient demographics, specific diagnoses, and number of medical conditions were a priori defined as covariates of interest. Data for each case and control were entered into a secure, web-based Research Electronic Data Capture (REDCap) database.
Analysis
Descriptive data were summarized using counts and percentages for categorical variables and medians and ranges for continuous variables due to nonnormal distributions. Chi-square test was used to compare in-hospital mortality between the ETs and the controls. The Wilcoxon rank-sum test was used to compare LOS between ETs and controls. All data analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
A total of 45 ETs were identified, and 110 controls were matched. Patient demographics were similar among all cases and controls (P > .05). Patients with ETs had a median age of seven years (interquartile range: 3-18 years), and 51% of them were males. The majority of patients among our examined cases were white (68%) and non-Hispanic (93%). There was no statistical difference in insurance between the ETs and the controls. When evaluating the hospital unit before the transfer, ETs occurred most commonly in the Cardiology (22%), Hematology/Oncology (22%), and Neuroscience (16%) units.
ETs stayed longer in the ICU than non-ETs [median of 4.9 days vs 2.2 days, P = .001; Figure (A)]. Similarly, ET cases had a significantly longer posttransfer hospital LOS [median of 35 days vs 21 days, P = .001; Figure (B)]. ETs had a 22% in-hospital mortality rate, compared with 9% in-hospital mortality in the matched controls (P = .02; Table).
DISCUSSION
Children who experienced an ET had a significantly longer ICU LOS, a longer posttransfer LOS, and a higher in-hospital mortality than the matched controls who were also transferred to the ICU. Researchers and improvement science teams at multiple hospitals have demonstrated that interventions targeting improved situation awareness can reduce ETs; we have demonstrated that reducing ETs may reduce subsequent adverse outcomes.8,10
These findings provide additional support for the use of the ET metric in children’s hospitals as a proximal measure for significant clinical deterioration. We found mortality rates that were overall high for a children’s hospital (22% in ET cases and 9% among controls) compared with a national average mortality rate of 2.3% in pediatric ICUs.11 This is likely due to the study sample containing a significant proportion of children with medical complexity.
Aoki et al. recently demonstrated that ETs, compared with non-ETs, were associated with longer LOS and higher mortality in a bivariate analysis.12 In our study, we found similar results with the important addition that these findings were robust when ETs were compared with matched controls who were likely at a higher risk of poor outcomes than ICU transfers in general. In addition, we demonstrated that ETs were associated with adverse outcomes in a United States children’s hospital with a mature, long-standing RRS process. As ETs are considerably more common than cardiac and respiratory arrests, use of the ET metric in children’s hospitals may enable more rapid learning and systems improvement implementations. We also found that most of the children with ETs present from units that care for children with substantial medical complexity, including Cardiology, Hematology/Oncology, and Neurosciences. Future work should continue to examine the relationship between medical complexity and ET risk.
The ET metric is complementary to the CDE measure developed by Bonafide et al. Both metrics capture potential events of unrecognized clinical deterioration, and both offer researchers the opportunity to better understand and improve their RRSs. Both ETs and CDEs are more common than arrests, and CDEs are more common than ETs. ETs, which by definition occur in the first hour of ICU care, are likely a more specific measure of unrecognized clinical deterioration. CDEs will capture therapies that may have been started up to 12 hours after transfer and thus are possibly more sensitive to identify unrecognized clinical deterioration. However, CDEs also may encompass some patients who arrived at the ICU after prompt recognition and then had a subacute deterioration in the ICU.
The maturity of the RRS and the bandwidth of teams to collect data may inform which metric(s) are best for individual centers. As ETs are less common and likely more specific to unrecognized clinical deterioration, they might be the first tracked as a center improves its RRS through QI methods. Alternatively, CDEs may be a useful metric for centers where unrecognized clinical deterioration is less common or in research studies where this more common outcome would lead to more power to detect the effect of interventions to improve care.
Our study had several limitations. Data collection was confined to one tertiary care children’s hospital with a high burden of complex cardiac and oncology care. The results may not generalize well to children hospitalized in smaller or community hospitals or in hospitals without a mature RRS. There is also the possibility of misclassification of covariates and outcomes, but any misclassification would likely be nondifferential and bias toward the null. Matching was not possible based on exact diagnosis, and the unit is a good but imperfect proxy for diagnosis grouping. At our center, overflow of patients into the Cardiology and Hematology/Oncology units is uncommon, mitigating this partially, although residual confounding may remain. The finding that ETs are associated with adverse outcomes does not necessarily mean that these events were preventable; however, it is important and encouraging that the rate of ETs has been reduced at two centers using improvement science interventions.8,10
CONCLUSION
Patients who experienced an ET had a significantly higher likelihood of in-hospital mortality, spent more time in the ICU, and had a longer hospital LOS posttransfer than matched controls. The use of the ET metric in children’s hospitals would allow for further analysis of such patients in hopes of identifying clinical characteristics that serve as predictors of deterioration. This may facilitate better risk stratification in the clinical system as well as enable more rapid learning and systems improvements targeted toward preventing unrecognized clinical deterioration.
Disclosures
Dr. Hussain, Dr. Sosa, Dr. Ambroggio, and Mrs. Gallagher have nothing to disclose. Patrick Brady reports grants from the Agency for Healthcare Research and Quality, outside the submitted work. The authors certify that this submission is not under review by any other publication. The author team has no conflicts of interest to disclose.
Funding
Ms. Hussain was supported by the Society of Hospital Medicine’s Student Hospitalist Scholar Grant Program in 2017. Dr. Brady receives career development support from AHRQ K08-HS023827. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the SHM, AHRQ, or NIH.
Unrecognized in-hospital deterioration can result in tragic consequences for pediatric patients. The majority of deterioration events have antecedents such as increasingly abnormal vital signs and new concerns from nurses.1 Recent meta-analyses have shown that rapid response systems (RRSs), which include trigger mechanisms such as a pediatric early warning score (PEWS), are associated with a reduced rate of arrests and in-hospital mortality.2,3 Cardiopulmonary arrest rates are useful metrics to judge the effectiveness of the system to identify and respond to deteriorating adult patients; however, there are important challenges to their use as an outcome measure in pediatrics. Arrests, which have been relatively uncommon in pediatric patients, are now even less frequent since the adoption of a RRS in the majority of children’s hospitals.4,5 Several innovations in these systems will be context-dependent and hence best first evaluated in a single center, where arrests outside of the intensive care unit (ICU) may occur rarely. Identification of valid, more frequent proximal measures to arrests may better identify the risk factors for deterioration. This could potentially inform quality improvement efforts to mitigate clinical deterioration.
Bonafide et al. at the Children’s Hospital of Philadelphia developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had a >13-fold increased risk of in-hospital mortality.6 At Cincinnati Children’s Hospital Medical Center, an additional proximal outcome measure was developed for unrecognized clinical deterioration, now termed emergency transfers (ETs).7-9 An ET is defined as any patient transferred to the ICU where the patient received intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer.9 Improvement science work that aimed at increasing clinician situation awareness was associated with a reduction in ETs,8 but the association of ETs with mortality or other healthcare utilization outcomes is unknown. The objective of this study was to determine the predictive validity of an ET on in-hospital mortality, ICU length of stay (LOS), and overall hospital LOS.
METHODS
We conducted a case–control study at Cincinnati Children’s Hospital, a free-standing tertiary care children’s hospital. Our center has had an ICU-based RRS in place since 2005. In 2009, we eliminated the ICU consult such that each floor-to-ICU transfer is evaluated by the RRS. Nurses calculate a Monaghan PEWS every four hours on the majority of nursing units.
Patients of all ages cared for outside of the ICU at any point in their hospitalization from January 1, 2013, to July 31, 2017, were eligible for inclusion. There were no other exclusion criteria. The ICU included both the pediatric ICU and the cardiac ICU.
Cases
We identified all ET cases from an existing situation awareness database in which each RRS call is entered by the hospital nursing supervisor, whose role includes responding to each RRS activation. If the patient transfer meets the ET criteria, the nurse indicates this in the database. Each ET entry is later confirmed for assurance purposes by the nurse leader of the RRS committee (RG). For the purposes of this study, all records were again reviewed and validated using manual chart review in the electronic health record (Epic Systems, Verona, Wisconsin).
Controls
We identified nonemergent ICU transfers to serve as controls and matched those to ET in cases to limit the impact of confounders that may increase the likelihood of both an ET and a negative outcome such as ICU mortality. We identified up to three controls for each case from our database and matched in terms of age group (within five years of age), hospital unit before transfer, and time of year (within three months of ET). These variables were chosen to adjust for the impact of age, diversity of disease (as hospital units are generally organized by organ system of illness), and seasonality on outcomes.
Outcome Measures
Posttransfer LOS in the ICU, posttransfer hospital LOS, and in-hospital mortality were the primary outcome measures. Patient demographics, specific diagnoses, and number of medical conditions were a priori defined as covariates of interest. Data for each case and control were entered into a secure, web-based Research Electronic Data Capture (REDCap) database.
Analysis
Descriptive data were summarized using counts and percentages for categorical variables and medians and ranges for continuous variables due to nonnormal distributions. Chi-square test was used to compare in-hospital mortality between the ETs and the controls. The Wilcoxon rank-sum test was used to compare LOS between ETs and controls. All data analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
A total of 45 ETs were identified, and 110 controls were matched. Patient demographics were similar among all cases and controls (P > .05). Patients with ETs had a median age of seven years (interquartile range: 3-18 years), and 51% of them were males. The majority of patients among our examined cases were white (68%) and non-Hispanic (93%). There was no statistical difference in insurance between the ETs and the controls. When evaluating the hospital unit before the transfer, ETs occurred most commonly in the Cardiology (22%), Hematology/Oncology (22%), and Neuroscience (16%) units.
ETs stayed longer in the ICU than non-ETs [median of 4.9 days vs 2.2 days, P = .001; Figure (A)]. Similarly, ET cases had a significantly longer posttransfer hospital LOS [median of 35 days vs 21 days, P = .001; Figure (B)]. ETs had a 22% in-hospital mortality rate, compared with 9% in-hospital mortality in the matched controls (P = .02; Table).
DISCUSSION
Children who experienced an ET had a significantly longer ICU LOS, a longer posttransfer LOS, and a higher in-hospital mortality than the matched controls who were also transferred to the ICU. Researchers and improvement science teams at multiple hospitals have demonstrated that interventions targeting improved situation awareness can reduce ETs; we have demonstrated that reducing ETs may reduce subsequent adverse outcomes.8,10
These findings provide additional support for the use of the ET metric in children’s hospitals as a proximal measure for significant clinical deterioration. We found mortality rates that were overall high for a children’s hospital (22% in ET cases and 9% among controls) compared with a national average mortality rate of 2.3% in pediatric ICUs.11 This is likely due to the study sample containing a significant proportion of children with medical complexity.
Aoki et al. recently demonstrated that ETs, compared with non-ETs, were associated with longer LOS and higher mortality in a bivariate analysis.12 In our study, we found similar results with the important addition that these findings were robust when ETs were compared with matched controls who were likely at a higher risk of poor outcomes than ICU transfers in general. In addition, we demonstrated that ETs were associated with adverse outcomes in a United States children’s hospital with a mature, long-standing RRS process. As ETs are considerably more common than cardiac and respiratory arrests, use of the ET metric in children’s hospitals may enable more rapid learning and systems improvement implementations. We also found that most of the children with ETs present from units that care for children with substantial medical complexity, including Cardiology, Hematology/Oncology, and Neurosciences. Future work should continue to examine the relationship between medical complexity and ET risk.
The ET metric is complementary to the CDE measure developed by Bonafide et al. Both metrics capture potential events of unrecognized clinical deterioration, and both offer researchers the opportunity to better understand and improve their RRSs. Both ETs and CDEs are more common than arrests, and CDEs are more common than ETs. ETs, which by definition occur in the first hour of ICU care, are likely a more specific measure of unrecognized clinical deterioration. CDEs will capture therapies that may have been started up to 12 hours after transfer and thus are possibly more sensitive to identify unrecognized clinical deterioration. However, CDEs also may encompass some patients who arrived at the ICU after prompt recognition and then had a subacute deterioration in the ICU.
The maturity of the RRS and the bandwidth of teams to collect data may inform which metric(s) are best for individual centers. As ETs are less common and likely more specific to unrecognized clinical deterioration, they might be the first tracked as a center improves its RRS through QI methods. Alternatively, CDEs may be a useful metric for centers where unrecognized clinical deterioration is less common or in research studies where this more common outcome would lead to more power to detect the effect of interventions to improve care.
Our study had several limitations. Data collection was confined to one tertiary care children’s hospital with a high burden of complex cardiac and oncology care. The results may not generalize well to children hospitalized in smaller or community hospitals or in hospitals without a mature RRS. There is also the possibility of misclassification of covariates and outcomes, but any misclassification would likely be nondifferential and bias toward the null. Matching was not possible based on exact diagnosis, and the unit is a good but imperfect proxy for diagnosis grouping. At our center, overflow of patients into the Cardiology and Hematology/Oncology units is uncommon, mitigating this partially, although residual confounding may remain. The finding that ETs are associated with adverse outcomes does not necessarily mean that these events were preventable; however, it is important and encouraging that the rate of ETs has been reduced at two centers using improvement science interventions.8,10
CONCLUSION
Patients who experienced an ET had a significantly higher likelihood of in-hospital mortality, spent more time in the ICU, and had a longer hospital LOS posttransfer than matched controls. The use of the ET metric in children’s hospitals would allow for further analysis of such patients in hopes of identifying clinical characteristics that serve as predictors of deterioration. This may facilitate better risk stratification in the clinical system as well as enable more rapid learning and systems improvements targeted toward preventing unrecognized clinical deterioration.
Disclosures
Dr. Hussain, Dr. Sosa, Dr. Ambroggio, and Mrs. Gallagher have nothing to disclose. Patrick Brady reports grants from the Agency for Healthcare Research and Quality, outside the submitted work. The authors certify that this submission is not under review by any other publication. The author team has no conflicts of interest to disclose.
Funding
Ms. Hussain was supported by the Society of Hospital Medicine’s Student Hospitalist Scholar Grant Program in 2017. Dr. Brady receives career development support from AHRQ K08-HS023827. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the SHM, AHRQ, or NIH.
1. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388.
2. Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015;19:254. https://doi.org/10.1186/s13054-015-0973-y.
3. Bonafide CP, Roland D, Brady PW. Rapid response systems 20 years later: new approaches, old challenges. JAMA Pediatrics. 2016;170(8):729-730. https://doi.org/10.1001/jamapediatrics.2016.0398.
4. Hayes LW, Dobyns EL, DiGiovine B, et al. A multicenter collaborative approach to reducing pediatric codes outside the ICU. Pediatrics. 2012;129(3):e785-e791. https://doi.org/10.1542/peds.2011-0227.
5. Raymond TT, Bonafide CP, Praestgaard A, et al. Pediatric medical emergency team events and outcomes: a report of 3647 events from the American Heart Association’s get with the guidelines-resuscitation registry. Hosp Pediatr. 2016;6(2):57-64. https://doi.org/10.1542/hpeds.2015-0132.
6. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784.
7. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747.
8. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364.
9. Brady PW, Wheeler DS, Muething SE, Kotagal UR. Situation awareness: a new model for predicting and preventing patient deterioration. Hosp Pediatr. 2014;4(3):143-146. https://doi.org/10.1542/hpeds.2013-0119.
10. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042.
11. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med. 2017;18(10):915-923. https://doi.org/10.1097/PCC.0000000000001268.
12. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2018;55(2):213-215. https://doi.org/10.1111/jpc.14185.
1. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388.
2. Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015;19:254. https://doi.org/10.1186/s13054-015-0973-y.
3. Bonafide CP, Roland D, Brady PW. Rapid response systems 20 years later: new approaches, old challenges. JAMA Pediatrics. 2016;170(8):729-730. https://doi.org/10.1001/jamapediatrics.2016.0398.
4. Hayes LW, Dobyns EL, DiGiovine B, et al. A multicenter collaborative approach to reducing pediatric codes outside the ICU. Pediatrics. 2012;129(3):e785-e791. https://doi.org/10.1542/peds.2011-0227.
5. Raymond TT, Bonafide CP, Praestgaard A, et al. Pediatric medical emergency team events and outcomes: a report of 3647 events from the American Heart Association’s get with the guidelines-resuscitation registry. Hosp Pediatr. 2016;6(2):57-64. https://doi.org/10.1542/hpeds.2015-0132.
6. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784.
7. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747.
8. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364.
9. Brady PW, Wheeler DS, Muething SE, Kotagal UR. Situation awareness: a new model for predicting and preventing patient deterioration. Hosp Pediatr. 2014;4(3):143-146. https://doi.org/10.1542/hpeds.2013-0119.
10. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042.
11. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med. 2017;18(10):915-923. https://doi.org/10.1097/PCC.0000000000001268.
12. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2018;55(2):213-215. https://doi.org/10.1111/jpc.14185.
© 2019 Society of Hospital Medicine

