Quiz

Erythematous Nodular Plaque Encircling the Lower Leg
- Author:
- Fnu Nutan, MD
- Chandralekha Banerjee, MD
- Terina S. Chen, MD
A 66-year-old woman presented with red to violaceous, rapidly growing nodules on the skin. Her medical history was remarkable for diabetes...
Quiz
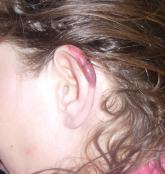
Cutaneous Manifestations of Cocaine Use
- Author:
- Fnu Nutan, MD
- Barry Ladizinski, MD
- Kachiu C. Lee, MD, MPH
A 43-year-old woman presented to the emergency department with painful skin lesions of 1 day’s duration. Physical examination revealed tender...
