User login
Hospitalists in Internal Medicine Residency
By the year 2010, more than 20,000 hospitalists will be in practice, compared to 5000 rheumatologists and 8000 pulmonologists.13 The growth of this career option has been driven by an industry need to reduce healthcare costs, increase the emphasis on quality improvement of healthcare services, and improve the efficiency and delivery of care between that provided in the hospital and that provided by the primary care physician.49
While hospitalists' roots can be traced back to community hospitals in the late 1980s and early 1990s, this career option is now flourishing in academic centers, with the rise of hospitalist faculty and hospitalist faculty tracks.10, 11 One potential advantage of having faculty who are hospitalists is the availability and expertise of physicians who specialize in the care of hospitalized patients.8, 12 Additionally, hospitalist faculty have been reported to achieve high resident satisfaction scores, increase understanding of cost‐effective measures, and improve the supervision of hospital procedures.12 To our knowledge, the medical literature does not provide an estimate of the percentage of internal medicine residency programs utilizing hospitalist faculty.
Critics of hospitalist faculty point to the potential loss of teaching opportunities from shorter hospital stays, bemoan the decreased physician‐patient continuity between inpatient and outpatient arenas, and fear that the hospitalists' presence may decrease resident autonomy and decrease subspecialty consultations by fellows.5, 13, 14 Hospitalist faculty need development, education, and training to match the teaching activities they are expected to fulfill. The challenge is for hospitalist societies and national residency organizations to define, plan for, and meet their faculty development needs.
Goals
The goals of this study were to describe the current involvement of hospitalists in internal medicine residencies. More specifically, we wanted to determine: (1) the percentage of programs with hospitalists as faculty, (2) the teaching activities of hospitalists, (3) regional differences in academic hospitalist activity, and (4) the number of programs with hospitalist training tracks.
Materials and Methods
Questionnaire Development
The Survey Committee of the Association of Program Directors in Internal Medicine (APDIM) is charged with developing questionnaires to track the baseline characteristics of the 391 internal medicine residencies in the United States as well as to address current issues facing residencies and residency directors. The Survey Committee's goal is to create a longitudinal data warehouse to: (1) track changes over time, (2) create valid outcome measures, and (3) facilitate educational studies and interventions. Two of the authors (B.B. and F.M.) were members of this committee. This work contains results from 2 successive questionnaires.
The first questionnaire, completed in 2005, and its administration have been described in our previous reports.1517 The second, completed in 2007, repeated many of the baseline characteristic questions, and introduced new questions regarding current residency issues, in particular hospitalism in residencies. Whereas the 2005 questionnaire was sent as an e‐mail attachment to the residency programs, the 2007 questionnaire used a web‐based format for completion and data collection. We e‐mailed a notification of the questionnaire with a link to the website in November 2006 to each member program of APDIM (total = 381 programs in 2006), representing 97% of the training programs in internal medicine. The directions and glossary for the questionnaire provided definitions and explained that the first section about the baseline characteristics could be completed by a program administrator or an associate program director. In both surveys, we defined the term faculty as any physician who serves as an attending or preceptor, provides lectures, noon conferences, physical diagnosis rounds, etc., or attends educational conferences (eg, morning report) on a regular basis. The survey assumed that program directors identified hospitalists as physicians whose primary professional focus is the general medical care of hospitalized patients. We asked that the program director review and approve the first section, and complete the remaining questions on his or her own. We sent subsequent request e‐mails in December 2006 and January 2007. The survey was confidential with respondents tracked by numerical codes.
Data Analysis
We used SPSS for Windows 15.0.0 (SPSS, Inc., Chicago, IL) statistics program for all analyses. Each program was categorized by setting (university‐based, community‐based, military, Veterans Administration, multispecialty group), number of residents, and the state in which the program was located. Respondents were assigned a region based upon the categorization used by the U.S. Census Bureau.18 We combined response categories for variables when we found sparsely selected responses. We examined continuous variables for evidence of skewness, outliers, and nonnormality.
In order to avoid misinterpretation of the results of multiple comparisons, we are reporting only bivariate associations that are significant at the P < 0.01 level. We used Spearman's rho to find correlations with the number of hospitalists and continuous variables. Chi‐square analyses were used to compare nominal variables. Fisher's exact test was used to compare the increase in the prevalence of hospitalists at primary teaching hospitals over time. For all the analyses we used 2‐sided tests.
Results
A total of 272 (response rate 70%) programs completed the 2005 survey, and 236 (response rate 62%) completed the 2007 survey. A total of 171 programs completed both surveys. In 2007, 15 (6%) program directors reported that they were hospitalists while 118 (50%) claimed to be traditional general internists.
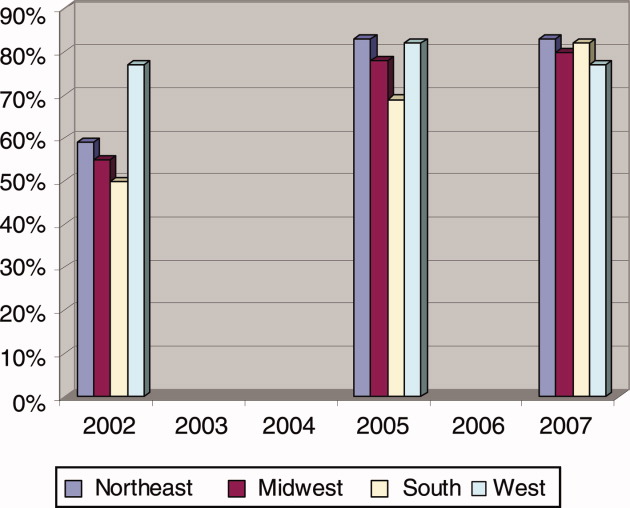
For the program directors who answered both surveys, 57% indicated that their primary teaching hospital employed hospitalists before the residency work‐hour limits were implemented (before June 2002). At the time of the survey in 2005, 77% said that hospitalists were employed, a 20% increase in 3 years. When we surveyed these same programs again in 2007, the proportion had risen to 81% (Fisher's exact P = 0.02 compared to before work‐hour limits; Figure 1).
Hospitalist Data from 2007
Eighty‐three percent of program directors identified using hospitalists as a part of their residency faculty. There was no significant difference between community‐based and university‐based residency programs (Table 1). There was an expected positive correlation (Spearman rho = 0.39, P < 0.001) between the total number of hospital beds and the number of hospitalists. The number and proportion of hospitalists had no correlation with residency program size, Residency Review Committee (RRC) cycle length (P = 0.99), or the American Board of Internal Medicine (ABIM) board exam pass rate (P = 0.60).
| Community‐Based (n = 130) | University‐Based (n = 63) | Northeast Region (n = 76) | Midwest Region (n = 53) | Southern Region (n = 59) | Western Region (n = 30) | |
|---|---|---|---|---|---|---|
| ||||||
| Does your primary teaching hospital employ hospitalists now?* | 98 (75) | 54 (86) | 61 (80) | 41 (77) | 48 (81) | 22 (73) |
| Are the hospitalists involved in teaching residents?* | 90 (91) | 47 (87) | 60 (98) | 35 (85) | 38 (79) | 22 (100) |
| If Yes, what teaching activities?* | ||||||
| Hospitalists serve as attending on resident service | 81 (92) | 43 (91) | 57 (95) | 32 (91) | 33 (87) | 20 (91) |
| Hospitalists conduct teaching rounds | 71 (79) | 39 (83) | 55 (92) | 30 (86) | 24 (63) | 18 (82) |
| Hospitalists perform direct observation of inpatient clinical skills | 60 (67) | 32 (68) | 45 (75) | 26 (74) | 20 (53) | 17 (77) |
| Hospitalists provide lectures | 56 (67) | 35 (74) | 43 (72) | 26 (74) | 19 (50) | 18 (82) |
| Hospitalists attend morning report | 43 (49) | 27 (59) | 35 (58) | 19 (54) | 19 (50) | 13 (57) |
| Hospitalists teach physical diagnosis | 41 (46) | 23 (49) | 28 (47) | 21 (60) | 14 (37) | 13 (59) |
| Hospitalists conduct interdisciplinary education rounds | 25 (28) | 18 (38) | 24 (40) | 10 (29) | 11 (29) | 7 (32) |
| Do you have a hospitalist track/focus? | 13 (11) | 6 (10) | 6 (8) | 5 (9) | 7 (12) | 5 (17) |
Teaching hospitals of the university‐based residencies employed hospitalists more often than those of community‐based programs (87% vs. 76%, P = 0.07; Table 1). And, while programs across the United States employed hospitalists at near the same proportions, programs in the Northeast (92%) and the West coast (92%) trended toward involving them in teaching residents more often (vs. Midwest 78% and South 76%, P = 0.04). Compared to programs in the Northeast, those in the Southern region generally utilize hospitalists less for common teaching activities, and in particular, significantly less (63%, P = 0.001) for conducting teaching rounds. Eleven percent of residencies had a hospitalist track or a hospitalist training focus, and this did not vary between community‐based and university‐based programs, but the Western region trended toward a higher percentage (17%, P = 0.18, compared to the Northeast 8%).
Of those programs that utilize hospitalists, program directors indicated that hospitalists contributed the following teaching activities to their residency programs (Table 1): serve as attending on resident service (92%), conduct teaching rounds (81%), perform direct observation of inpatient clinical skills (67%), provide lectures (68%), attend morning report (52%), teach physical diagnosis (48%), and conduct interdisciplinary education rounds (31%). Notable comments provided by program directors about other teaching activities of hospitalists included: accept and review night float patients, residents do inpatient consultations with hospitalists, serve as one of the associate program directors, and write curriculum updates and develop evaluation methods (ie, oral exams, multiple choice questions, etc.).
Discussion
This is the first study to document the national rise of hospitalist faculty in internal medicine residency programs. Program directors noted a 20% increase in teaching hospitals that employed hospitalists after the work‐hour regulations went into effecta trend that continued to rise. The tendency was seen first on the coasts, where managed care has higher penetration, and particularly in the Northeast, where New York's resident work‐hour reforms occurred by state mandate prior to the residency accreditation action that affected the rest of the country. Not only have hospitalists picked up the burden of service at these hospitals,19, 20 but the vast majority of programs (>80%) have utilized hospitalists as teachers in important areas of their residency. The magnitude of hospitalist involvement in residency training may have important implications.
Beyond the financial significance of hospitalists at academic teaching hospitals,21 only a few studies have addressed their impact on resident education. On the monthly evaluations at the University of California, San Francisco (San Francisco, CA), residents' satisfaction with their attendings was significantly higher when the physician was a hospitalist rather than a traditional faculty member.22 Residents believed hospitalists were more effective teachers, and provided more effective feedback. At Emory University (Atlanta, GA), a methodologically more rigorous study of postrotation assessment of faculty demonstrated that ratings of hospitalists were not different from traditional general internists; both scored higher than subspecialists.23 The hospitalists as a group had completed training more recently, which also was associated with higher scores.
Even community hospitals that sponsor residency programs have benefited from hospitalist faculty. At Norwalk Hospital (Norwalk, CT), the program had used resident teams led by a group of community physicians and a small group of employed internists. But time pressures and reimbursement concerns created tension between the workload and education balance. After hiring 2 hospitalist clinician‐educators, the length of stay and cost per case were substantially reduced, while resident evaluations indicated improved teaching rounds, conferences, and bedside teaching.24
The results of our study fit with the role of hospitalists as well as what individual programs have reported about hospitalist faculty in the past. Hospitalist faculty serve by and large (92%) as attendings on the hospital ward services. Theoretically, who better to have round with residents in the hospital than the specialists of hospital medicine. For a pulmonary curricular experience, residents work with pulmonologists. But beyond serving as attendings in the hospital, they perform the traditional functions of hospital attendings: providing teaching rounds (>80%), evaluating clinical skills (67%), and even lecturing to residents (>65%). The Southern region trends toward slower adoption of hospitalists as faculty, particularly compared with the Northeast. Overall, what is striking is how much hospitalist faculty already are filling the roles expected of all academic faculty.
We found that only 11% of programs have a hospitalist track through which internal medicine residents may develop the specialized skills and knowledge needed to function optimally in a hospitalist career.25 But given the rapid growth of this specialty, we might expect to see a similar rise in programs providing such specialized training.
Are there risks to having hospitalists teaching residents? One concern is the potential to model fragmented medical care to trainees when hospitals and ambulatory health systems neglect to ensure quality handoffs.26 In an era that heralds the demise of the primary care general internist,27 the impact of hospitalist faculty on general internal medicine nationally, the gravitation of residents toward or away from hospitalist and ambulatory careers, and the role of the traditional general internist in residency training programs in the future remain to be seen. These were not addressed by our data, but are ripe areas for study.
This study has several limitations. It relies on self‐reported data from program directors that, while knowing the intimate details of their educational program, may not have exact knowledge of the number of hospitalists employed by their hospitals. There is also the potential for recall bias by asking the group to remember the number of hospitalists before duty‐hour implementation. Both points in time of our 2 surveys (2005 and 2007) were after the incident growth of hospital medicine as evidenced by the high prevalence of hospitalists in both surveys. Yet, most program directors know that the service needs of the hospitals were acutely increased when the duty‐hours policies went into affect, and probably were fairly involved in hospital decisions to utilize hospitalist physicians to meet these needs. Finally, our study does not address hospitalism within the family medicine and pediatric specialties, both of which have a significant stake in hospital medicine.
In conclusion, our study documents the recent growth and current prevalence of hospitalists' activities in the teaching hospitals of internal medicine residencies in the United States, the duties they perform in resident education, and the magnitude of their penetration in the geographic regions of the country, both in community‐based and university‐based programs. The high degree of involvement of hospitalists in resident education may have important implications for the future of internal medicine as a discipline both with regard to the need for academic faculty development of this important sector of the education community as well as for the education and career development of the residents whom they train.
Acknowledgements
The authors thank the Mayo Clinic Survey Research Center for their assistance with survey design and data collection.
- , , , et al.Implementation of a voluntary hospitalist service at a community teaching hospital; improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- , , , et al.The United States rheumatology workforce: supply and demand, 2005–2025.Arthritis Rheum.2007;56(3):722–729.
- , , , , ;Committee on Manpower for Pulmonary and Critical Care Societies.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284(21):2762–2770.
- , .The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- , , , , .Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- , , .The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- , .The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- , , , , .What effect does inpatient physician specialty and experience have on clinical outcomes and resource utilization on a general medical service?J Gen Intern Med.2004;19:395–401.
- , , , et al.The presence of hospitalists in medical education.Acad Med.2000;75(suppl):S34–S36.
- , , , et al.Assessing the value of hospitalists to academic health centers: Brigham and Women's Hospital and Harvard Medical School.Am J Med.1999;106:134–137.
- , .Role of hospitalists in medical education.Am J Med.1999;107(4):305–309.
- , .The hospitalists: new boon for internal medicine or retreat from primary care?Ann Intern Med.1999;130:382–387.
- .The hospitalist movement: caution lights flashing at the crossroads.Am J Med.1999;107:409–413.
- , , .The state of competency evaluation in internal medicine residency.J Gen Intern Med.2008;23(7):1010–1015.
- , , .Sources of satisfaction for residency program directors: a second administration of the PD‐Sat.Am J Med.2009;122(2):196–201.
- , , .What predicts residency accreditation cycle length?Acad Med.2009;84(3):356–361.
- U.S. Census Bureau. Census Regions and Divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed May 2009.
- , , , et al.Complying with ACGME resident duty hours restrictions: restructuring the 80‐hour workweek to enhance education and patient safety at Texas A81(12):1026–1031.
- Association of Program Directors in Internal Medicine;, , , , .Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144(12):920–926.
- , , , et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–874.
- , , , , .Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations.Arch Intern Med.2004;164:1866–1871.
- , , , et al.Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- , , , et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19(4):293–301.
- , , , , .How to use The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1:48–56.
- .The hospitalist model: perspectives of the patient, the internist, and internal medicine.Ann Intern Med.1999;130:368–372.
- American College of Physicians. The impending collapse of primary care medicine and its implications for the state of the nation's health care: a report from the American College of Physicians. January 30,2006. Available at: http://www.acponline.org/advocacy/events/state_of_healthcare/statehc06_1.pdf. Accessed May 2009.
By the year 2010, more than 20,000 hospitalists will be in practice, compared to 5000 rheumatologists and 8000 pulmonologists.13 The growth of this career option has been driven by an industry need to reduce healthcare costs, increase the emphasis on quality improvement of healthcare services, and improve the efficiency and delivery of care between that provided in the hospital and that provided by the primary care physician.49
While hospitalists' roots can be traced back to community hospitals in the late 1980s and early 1990s, this career option is now flourishing in academic centers, with the rise of hospitalist faculty and hospitalist faculty tracks.10, 11 One potential advantage of having faculty who are hospitalists is the availability and expertise of physicians who specialize in the care of hospitalized patients.8, 12 Additionally, hospitalist faculty have been reported to achieve high resident satisfaction scores, increase understanding of cost‐effective measures, and improve the supervision of hospital procedures.12 To our knowledge, the medical literature does not provide an estimate of the percentage of internal medicine residency programs utilizing hospitalist faculty.
Critics of hospitalist faculty point to the potential loss of teaching opportunities from shorter hospital stays, bemoan the decreased physician‐patient continuity between inpatient and outpatient arenas, and fear that the hospitalists' presence may decrease resident autonomy and decrease subspecialty consultations by fellows.5, 13, 14 Hospitalist faculty need development, education, and training to match the teaching activities they are expected to fulfill. The challenge is for hospitalist societies and national residency organizations to define, plan for, and meet their faculty development needs.
Goals
The goals of this study were to describe the current involvement of hospitalists in internal medicine residencies. More specifically, we wanted to determine: (1) the percentage of programs with hospitalists as faculty, (2) the teaching activities of hospitalists, (3) regional differences in academic hospitalist activity, and (4) the number of programs with hospitalist training tracks.
Materials and Methods
Questionnaire Development
The Survey Committee of the Association of Program Directors in Internal Medicine (APDIM) is charged with developing questionnaires to track the baseline characteristics of the 391 internal medicine residencies in the United States as well as to address current issues facing residencies and residency directors. The Survey Committee's goal is to create a longitudinal data warehouse to: (1) track changes over time, (2) create valid outcome measures, and (3) facilitate educational studies and interventions. Two of the authors (B.B. and F.M.) were members of this committee. This work contains results from 2 successive questionnaires.
The first questionnaire, completed in 2005, and its administration have been described in our previous reports.1517 The second, completed in 2007, repeated many of the baseline characteristic questions, and introduced new questions regarding current residency issues, in particular hospitalism in residencies. Whereas the 2005 questionnaire was sent as an e‐mail attachment to the residency programs, the 2007 questionnaire used a web‐based format for completion and data collection. We e‐mailed a notification of the questionnaire with a link to the website in November 2006 to each member program of APDIM (total = 381 programs in 2006), representing 97% of the training programs in internal medicine. The directions and glossary for the questionnaire provided definitions and explained that the first section about the baseline characteristics could be completed by a program administrator or an associate program director. In both surveys, we defined the term faculty as any physician who serves as an attending or preceptor, provides lectures, noon conferences, physical diagnosis rounds, etc., or attends educational conferences (eg, morning report) on a regular basis. The survey assumed that program directors identified hospitalists as physicians whose primary professional focus is the general medical care of hospitalized patients. We asked that the program director review and approve the first section, and complete the remaining questions on his or her own. We sent subsequent request e‐mails in December 2006 and January 2007. The survey was confidential with respondents tracked by numerical codes.
Data Analysis
We used SPSS for Windows 15.0.0 (SPSS, Inc., Chicago, IL) statistics program for all analyses. Each program was categorized by setting (university‐based, community‐based, military, Veterans Administration, multispecialty group), number of residents, and the state in which the program was located. Respondents were assigned a region based upon the categorization used by the U.S. Census Bureau.18 We combined response categories for variables when we found sparsely selected responses. We examined continuous variables for evidence of skewness, outliers, and nonnormality.
In order to avoid misinterpretation of the results of multiple comparisons, we are reporting only bivariate associations that are significant at the P < 0.01 level. We used Spearman's rho to find correlations with the number of hospitalists and continuous variables. Chi‐square analyses were used to compare nominal variables. Fisher's exact test was used to compare the increase in the prevalence of hospitalists at primary teaching hospitals over time. For all the analyses we used 2‐sided tests.
Results
A total of 272 (response rate 70%) programs completed the 2005 survey, and 236 (response rate 62%) completed the 2007 survey. A total of 171 programs completed both surveys. In 2007, 15 (6%) program directors reported that they were hospitalists while 118 (50%) claimed to be traditional general internists.
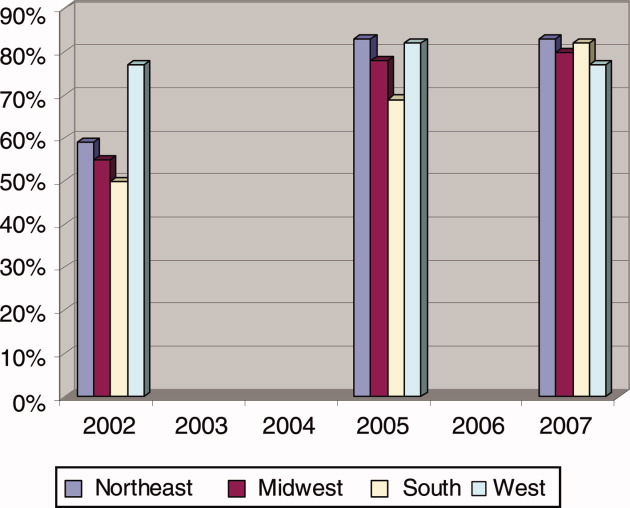
For the program directors who answered both surveys, 57% indicated that their primary teaching hospital employed hospitalists before the residency work‐hour limits were implemented (before June 2002). At the time of the survey in 2005, 77% said that hospitalists were employed, a 20% increase in 3 years. When we surveyed these same programs again in 2007, the proportion had risen to 81% (Fisher's exact P = 0.02 compared to before work‐hour limits; Figure 1).

Hospitalist Data from 2007
Eighty‐three percent of program directors identified using hospitalists as a part of their residency faculty. There was no significant difference between community‐based and university‐based residency programs (Table 1). There was an expected positive correlation (Spearman rho = 0.39, P < 0.001) between the total number of hospital beds and the number of hospitalists. The number and proportion of hospitalists had no correlation with residency program size, Residency Review Committee (RRC) cycle length (P = 0.99), or the American Board of Internal Medicine (ABIM) board exam pass rate (P = 0.60).
| Community‐Based (n = 130) | University‐Based (n = 63) | Northeast Region (n = 76) | Midwest Region (n = 53) | Southern Region (n = 59) | Western Region (n = 30) | |
|---|---|---|---|---|---|---|
| ||||||
| Does your primary teaching hospital employ hospitalists now?* | 98 (75) | 54 (86) | 61 (80) | 41 (77) | 48 (81) | 22 (73) |
| Are the hospitalists involved in teaching residents?* | 90 (91) | 47 (87) | 60 (98) | 35 (85) | 38 (79) | 22 (100) |
| If Yes, what teaching activities?* | ||||||
| Hospitalists serve as attending on resident service | 81 (92) | 43 (91) | 57 (95) | 32 (91) | 33 (87) | 20 (91) |
| Hospitalists conduct teaching rounds | 71 (79) | 39 (83) | 55 (92) | 30 (86) | 24 (63) | 18 (82) |
| Hospitalists perform direct observation of inpatient clinical skills | 60 (67) | 32 (68) | 45 (75) | 26 (74) | 20 (53) | 17 (77) |
| Hospitalists provide lectures | 56 (67) | 35 (74) | 43 (72) | 26 (74) | 19 (50) | 18 (82) |
| Hospitalists attend morning report | 43 (49) | 27 (59) | 35 (58) | 19 (54) | 19 (50) | 13 (57) |
| Hospitalists teach physical diagnosis | 41 (46) | 23 (49) | 28 (47) | 21 (60) | 14 (37) | 13 (59) |
| Hospitalists conduct interdisciplinary education rounds | 25 (28) | 18 (38) | 24 (40) | 10 (29) | 11 (29) | 7 (32) |
| Do you have a hospitalist track/focus? | 13 (11) | 6 (10) | 6 (8) | 5 (9) | 7 (12) | 5 (17) |
Teaching hospitals of the university‐based residencies employed hospitalists more often than those of community‐based programs (87% vs. 76%, P = 0.07; Table 1). And, while programs across the United States employed hospitalists at near the same proportions, programs in the Northeast (92%) and the West coast (92%) trended toward involving them in teaching residents more often (vs. Midwest 78% and South 76%, P = 0.04). Compared to programs in the Northeast, those in the Southern region generally utilize hospitalists less for common teaching activities, and in particular, significantly less (63%, P = 0.001) for conducting teaching rounds. Eleven percent of residencies had a hospitalist track or a hospitalist training focus, and this did not vary between community‐based and university‐based programs, but the Western region trended toward a higher percentage (17%, P = 0.18, compared to the Northeast 8%).
Of those programs that utilize hospitalists, program directors indicated that hospitalists contributed the following teaching activities to their residency programs (Table 1): serve as attending on resident service (92%), conduct teaching rounds (81%), perform direct observation of inpatient clinical skills (67%), provide lectures (68%), attend morning report (52%), teach physical diagnosis (48%), and conduct interdisciplinary education rounds (31%). Notable comments provided by program directors about other teaching activities of hospitalists included: accept and review night float patients, residents do inpatient consultations with hospitalists, serve as one of the associate program directors, and write curriculum updates and develop evaluation methods (ie, oral exams, multiple choice questions, etc.).
Discussion
This is the first study to document the national rise of hospitalist faculty in internal medicine residency programs. Program directors noted a 20% increase in teaching hospitals that employed hospitalists after the work‐hour regulations went into effecta trend that continued to rise. The tendency was seen first on the coasts, where managed care has higher penetration, and particularly in the Northeast, where New York's resident work‐hour reforms occurred by state mandate prior to the residency accreditation action that affected the rest of the country. Not only have hospitalists picked up the burden of service at these hospitals,19, 20 but the vast majority of programs (>80%) have utilized hospitalists as teachers in important areas of their residency. The magnitude of hospitalist involvement in residency training may have important implications.
Beyond the financial significance of hospitalists at academic teaching hospitals,21 only a few studies have addressed their impact on resident education. On the monthly evaluations at the University of California, San Francisco (San Francisco, CA), residents' satisfaction with their attendings was significantly higher when the physician was a hospitalist rather than a traditional faculty member.22 Residents believed hospitalists were more effective teachers, and provided more effective feedback. At Emory University (Atlanta, GA), a methodologically more rigorous study of postrotation assessment of faculty demonstrated that ratings of hospitalists were not different from traditional general internists; both scored higher than subspecialists.23 The hospitalists as a group had completed training more recently, which also was associated with higher scores.
Even community hospitals that sponsor residency programs have benefited from hospitalist faculty. At Norwalk Hospital (Norwalk, CT), the program had used resident teams led by a group of community physicians and a small group of employed internists. But time pressures and reimbursement concerns created tension between the workload and education balance. After hiring 2 hospitalist clinician‐educators, the length of stay and cost per case were substantially reduced, while resident evaluations indicated improved teaching rounds, conferences, and bedside teaching.24
The results of our study fit with the role of hospitalists as well as what individual programs have reported about hospitalist faculty in the past. Hospitalist faculty serve by and large (92%) as attendings on the hospital ward services. Theoretically, who better to have round with residents in the hospital than the specialists of hospital medicine. For a pulmonary curricular experience, residents work with pulmonologists. But beyond serving as attendings in the hospital, they perform the traditional functions of hospital attendings: providing teaching rounds (>80%), evaluating clinical skills (67%), and even lecturing to residents (>65%). The Southern region trends toward slower adoption of hospitalists as faculty, particularly compared with the Northeast. Overall, what is striking is how much hospitalist faculty already are filling the roles expected of all academic faculty.
We found that only 11% of programs have a hospitalist track through which internal medicine residents may develop the specialized skills and knowledge needed to function optimally in a hospitalist career.25 But given the rapid growth of this specialty, we might expect to see a similar rise in programs providing such specialized training.
Are there risks to having hospitalists teaching residents? One concern is the potential to model fragmented medical care to trainees when hospitals and ambulatory health systems neglect to ensure quality handoffs.26 In an era that heralds the demise of the primary care general internist,27 the impact of hospitalist faculty on general internal medicine nationally, the gravitation of residents toward or away from hospitalist and ambulatory careers, and the role of the traditional general internist in residency training programs in the future remain to be seen. These were not addressed by our data, but are ripe areas for study.
This study has several limitations. It relies on self‐reported data from program directors that, while knowing the intimate details of their educational program, may not have exact knowledge of the number of hospitalists employed by their hospitals. There is also the potential for recall bias by asking the group to remember the number of hospitalists before duty‐hour implementation. Both points in time of our 2 surveys (2005 and 2007) were after the incident growth of hospital medicine as evidenced by the high prevalence of hospitalists in both surveys. Yet, most program directors know that the service needs of the hospitals were acutely increased when the duty‐hours policies went into affect, and probably were fairly involved in hospital decisions to utilize hospitalist physicians to meet these needs. Finally, our study does not address hospitalism within the family medicine and pediatric specialties, both of which have a significant stake in hospital medicine.
In conclusion, our study documents the recent growth and current prevalence of hospitalists' activities in the teaching hospitals of internal medicine residencies in the United States, the duties they perform in resident education, and the magnitude of their penetration in the geographic regions of the country, both in community‐based and university‐based programs. The high degree of involvement of hospitalists in resident education may have important implications for the future of internal medicine as a discipline both with regard to the need for academic faculty development of this important sector of the education community as well as for the education and career development of the residents whom they train.
Acknowledgements
The authors thank the Mayo Clinic Survey Research Center for their assistance with survey design and data collection.
By the year 2010, more than 20,000 hospitalists will be in practice, compared to 5000 rheumatologists and 8000 pulmonologists.13 The growth of this career option has been driven by an industry need to reduce healthcare costs, increase the emphasis on quality improvement of healthcare services, and improve the efficiency and delivery of care between that provided in the hospital and that provided by the primary care physician.49
While hospitalists' roots can be traced back to community hospitals in the late 1980s and early 1990s, this career option is now flourishing in academic centers, with the rise of hospitalist faculty and hospitalist faculty tracks.10, 11 One potential advantage of having faculty who are hospitalists is the availability and expertise of physicians who specialize in the care of hospitalized patients.8, 12 Additionally, hospitalist faculty have been reported to achieve high resident satisfaction scores, increase understanding of cost‐effective measures, and improve the supervision of hospital procedures.12 To our knowledge, the medical literature does not provide an estimate of the percentage of internal medicine residency programs utilizing hospitalist faculty.
Critics of hospitalist faculty point to the potential loss of teaching opportunities from shorter hospital stays, bemoan the decreased physician‐patient continuity between inpatient and outpatient arenas, and fear that the hospitalists' presence may decrease resident autonomy and decrease subspecialty consultations by fellows.5, 13, 14 Hospitalist faculty need development, education, and training to match the teaching activities they are expected to fulfill. The challenge is for hospitalist societies and national residency organizations to define, plan for, and meet their faculty development needs.
Goals
The goals of this study were to describe the current involvement of hospitalists in internal medicine residencies. More specifically, we wanted to determine: (1) the percentage of programs with hospitalists as faculty, (2) the teaching activities of hospitalists, (3) regional differences in academic hospitalist activity, and (4) the number of programs with hospitalist training tracks.
Materials and Methods
Questionnaire Development
The Survey Committee of the Association of Program Directors in Internal Medicine (APDIM) is charged with developing questionnaires to track the baseline characteristics of the 391 internal medicine residencies in the United States as well as to address current issues facing residencies and residency directors. The Survey Committee's goal is to create a longitudinal data warehouse to: (1) track changes over time, (2) create valid outcome measures, and (3) facilitate educational studies and interventions. Two of the authors (B.B. and F.M.) were members of this committee. This work contains results from 2 successive questionnaires.
The first questionnaire, completed in 2005, and its administration have been described in our previous reports.1517 The second, completed in 2007, repeated many of the baseline characteristic questions, and introduced new questions regarding current residency issues, in particular hospitalism in residencies. Whereas the 2005 questionnaire was sent as an e‐mail attachment to the residency programs, the 2007 questionnaire used a web‐based format for completion and data collection. We e‐mailed a notification of the questionnaire with a link to the website in November 2006 to each member program of APDIM (total = 381 programs in 2006), representing 97% of the training programs in internal medicine. The directions and glossary for the questionnaire provided definitions and explained that the first section about the baseline characteristics could be completed by a program administrator or an associate program director. In both surveys, we defined the term faculty as any physician who serves as an attending or preceptor, provides lectures, noon conferences, physical diagnosis rounds, etc., or attends educational conferences (eg, morning report) on a regular basis. The survey assumed that program directors identified hospitalists as physicians whose primary professional focus is the general medical care of hospitalized patients. We asked that the program director review and approve the first section, and complete the remaining questions on his or her own. We sent subsequent request e‐mails in December 2006 and January 2007. The survey was confidential with respondents tracked by numerical codes.
Data Analysis
We used SPSS for Windows 15.0.0 (SPSS, Inc., Chicago, IL) statistics program for all analyses. Each program was categorized by setting (university‐based, community‐based, military, Veterans Administration, multispecialty group), number of residents, and the state in which the program was located. Respondents were assigned a region based upon the categorization used by the U.S. Census Bureau.18 We combined response categories for variables when we found sparsely selected responses. We examined continuous variables for evidence of skewness, outliers, and nonnormality.
In order to avoid misinterpretation of the results of multiple comparisons, we are reporting only bivariate associations that are significant at the P < 0.01 level. We used Spearman's rho to find correlations with the number of hospitalists and continuous variables. Chi‐square analyses were used to compare nominal variables. Fisher's exact test was used to compare the increase in the prevalence of hospitalists at primary teaching hospitals over time. For all the analyses we used 2‐sided tests.
Results
A total of 272 (response rate 70%) programs completed the 2005 survey, and 236 (response rate 62%) completed the 2007 survey. A total of 171 programs completed both surveys. In 2007, 15 (6%) program directors reported that they were hospitalists while 118 (50%) claimed to be traditional general internists.
For the program directors who answered both surveys, 57% indicated that their primary teaching hospital employed hospitalists before the residency work‐hour limits were implemented (before June 2002). At the time of the survey in 2005, 77% said that hospitalists were employed, a 20% increase in 3 years. When we surveyed these same programs again in 2007, the proportion had risen to 81% (Fisher's exact P = 0.02 compared to before work‐hour limits; Figure 1).

Hospitalist Data from 2007
Eighty‐three percent of program directors identified using hospitalists as a part of their residency faculty. There was no significant difference between community‐based and university‐based residency programs (Table 1). There was an expected positive correlation (Spearman rho = 0.39, P < 0.001) between the total number of hospital beds and the number of hospitalists. The number and proportion of hospitalists had no correlation with residency program size, Residency Review Committee (RRC) cycle length (P = 0.99), or the American Board of Internal Medicine (ABIM) board exam pass rate (P = 0.60).
| Community‐Based (n = 130) | University‐Based (n = 63) | Northeast Region (n = 76) | Midwest Region (n = 53) | Southern Region (n = 59) | Western Region (n = 30) | |
|---|---|---|---|---|---|---|
| ||||||
| Does your primary teaching hospital employ hospitalists now?* | 98 (75) | 54 (86) | 61 (80) | 41 (77) | 48 (81) | 22 (73) |
| Are the hospitalists involved in teaching residents?* | 90 (91) | 47 (87) | 60 (98) | 35 (85) | 38 (79) | 22 (100) |
| If Yes, what teaching activities?* | ||||||
| Hospitalists serve as attending on resident service | 81 (92) | 43 (91) | 57 (95) | 32 (91) | 33 (87) | 20 (91) |
| Hospitalists conduct teaching rounds | 71 (79) | 39 (83) | 55 (92) | 30 (86) | 24 (63) | 18 (82) |
| Hospitalists perform direct observation of inpatient clinical skills | 60 (67) | 32 (68) | 45 (75) | 26 (74) | 20 (53) | 17 (77) |
| Hospitalists provide lectures | 56 (67) | 35 (74) | 43 (72) | 26 (74) | 19 (50) | 18 (82) |
| Hospitalists attend morning report | 43 (49) | 27 (59) | 35 (58) | 19 (54) | 19 (50) | 13 (57) |
| Hospitalists teach physical diagnosis | 41 (46) | 23 (49) | 28 (47) | 21 (60) | 14 (37) | 13 (59) |
| Hospitalists conduct interdisciplinary education rounds | 25 (28) | 18 (38) | 24 (40) | 10 (29) | 11 (29) | 7 (32) |
| Do you have a hospitalist track/focus? | 13 (11) | 6 (10) | 6 (8) | 5 (9) | 7 (12) | 5 (17) |
Teaching hospitals of the university‐based residencies employed hospitalists more often than those of community‐based programs (87% vs. 76%, P = 0.07; Table 1). And, while programs across the United States employed hospitalists at near the same proportions, programs in the Northeast (92%) and the West coast (92%) trended toward involving them in teaching residents more often (vs. Midwest 78% and South 76%, P = 0.04). Compared to programs in the Northeast, those in the Southern region generally utilize hospitalists less for common teaching activities, and in particular, significantly less (63%, P = 0.001) for conducting teaching rounds. Eleven percent of residencies had a hospitalist track or a hospitalist training focus, and this did not vary between community‐based and university‐based programs, but the Western region trended toward a higher percentage (17%, P = 0.18, compared to the Northeast 8%).
Of those programs that utilize hospitalists, program directors indicated that hospitalists contributed the following teaching activities to their residency programs (Table 1): serve as attending on resident service (92%), conduct teaching rounds (81%), perform direct observation of inpatient clinical skills (67%), provide lectures (68%), attend morning report (52%), teach physical diagnosis (48%), and conduct interdisciplinary education rounds (31%). Notable comments provided by program directors about other teaching activities of hospitalists included: accept and review night float patients, residents do inpatient consultations with hospitalists, serve as one of the associate program directors, and write curriculum updates and develop evaluation methods (ie, oral exams, multiple choice questions, etc.).
Discussion
This is the first study to document the national rise of hospitalist faculty in internal medicine residency programs. Program directors noted a 20% increase in teaching hospitals that employed hospitalists after the work‐hour regulations went into effecta trend that continued to rise. The tendency was seen first on the coasts, where managed care has higher penetration, and particularly in the Northeast, where New York's resident work‐hour reforms occurred by state mandate prior to the residency accreditation action that affected the rest of the country. Not only have hospitalists picked up the burden of service at these hospitals,19, 20 but the vast majority of programs (>80%) have utilized hospitalists as teachers in important areas of their residency. The magnitude of hospitalist involvement in residency training may have important implications.
Beyond the financial significance of hospitalists at academic teaching hospitals,21 only a few studies have addressed their impact on resident education. On the monthly evaluations at the University of California, San Francisco (San Francisco, CA), residents' satisfaction with their attendings was significantly higher when the physician was a hospitalist rather than a traditional faculty member.22 Residents believed hospitalists were more effective teachers, and provided more effective feedback. At Emory University (Atlanta, GA), a methodologically more rigorous study of postrotation assessment of faculty demonstrated that ratings of hospitalists were not different from traditional general internists; both scored higher than subspecialists.23 The hospitalists as a group had completed training more recently, which also was associated with higher scores.
Even community hospitals that sponsor residency programs have benefited from hospitalist faculty. At Norwalk Hospital (Norwalk, CT), the program had used resident teams led by a group of community physicians and a small group of employed internists. But time pressures and reimbursement concerns created tension between the workload and education balance. After hiring 2 hospitalist clinician‐educators, the length of stay and cost per case were substantially reduced, while resident evaluations indicated improved teaching rounds, conferences, and bedside teaching.24
The results of our study fit with the role of hospitalists as well as what individual programs have reported about hospitalist faculty in the past. Hospitalist faculty serve by and large (92%) as attendings on the hospital ward services. Theoretically, who better to have round with residents in the hospital than the specialists of hospital medicine. For a pulmonary curricular experience, residents work with pulmonologists. But beyond serving as attendings in the hospital, they perform the traditional functions of hospital attendings: providing teaching rounds (>80%), evaluating clinical skills (67%), and even lecturing to residents (>65%). The Southern region trends toward slower adoption of hospitalists as faculty, particularly compared with the Northeast. Overall, what is striking is how much hospitalist faculty already are filling the roles expected of all academic faculty.
We found that only 11% of programs have a hospitalist track through which internal medicine residents may develop the specialized skills and knowledge needed to function optimally in a hospitalist career.25 But given the rapid growth of this specialty, we might expect to see a similar rise in programs providing such specialized training.
Are there risks to having hospitalists teaching residents? One concern is the potential to model fragmented medical care to trainees when hospitals and ambulatory health systems neglect to ensure quality handoffs.26 In an era that heralds the demise of the primary care general internist,27 the impact of hospitalist faculty on general internal medicine nationally, the gravitation of residents toward or away from hospitalist and ambulatory careers, and the role of the traditional general internist in residency training programs in the future remain to be seen. These were not addressed by our data, but are ripe areas for study.
This study has several limitations. It relies on self‐reported data from program directors that, while knowing the intimate details of their educational program, may not have exact knowledge of the number of hospitalists employed by their hospitals. There is also the potential for recall bias by asking the group to remember the number of hospitalists before duty‐hour implementation. Both points in time of our 2 surveys (2005 and 2007) were after the incident growth of hospital medicine as evidenced by the high prevalence of hospitalists in both surveys. Yet, most program directors know that the service needs of the hospitals were acutely increased when the duty‐hours policies went into affect, and probably were fairly involved in hospital decisions to utilize hospitalist physicians to meet these needs. Finally, our study does not address hospitalism within the family medicine and pediatric specialties, both of which have a significant stake in hospital medicine.
In conclusion, our study documents the recent growth and current prevalence of hospitalists' activities in the teaching hospitals of internal medicine residencies in the United States, the duties they perform in resident education, and the magnitude of their penetration in the geographic regions of the country, both in community‐based and university‐based programs. The high degree of involvement of hospitalists in resident education may have important implications for the future of internal medicine as a discipline both with regard to the need for academic faculty development of this important sector of the education community as well as for the education and career development of the residents whom they train.
Acknowledgements
The authors thank the Mayo Clinic Survey Research Center for their assistance with survey design and data collection.
- , , , et al.Implementation of a voluntary hospitalist service at a community teaching hospital; improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- , , , et al.The United States rheumatology workforce: supply and demand, 2005–2025.Arthritis Rheum.2007;56(3):722–729.
- , , , , ;Committee on Manpower for Pulmonary and Critical Care Societies.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284(21):2762–2770.
- , .The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- , , , , .Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- , , .The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- , .The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- , , , , .What effect does inpatient physician specialty and experience have on clinical outcomes and resource utilization on a general medical service?J Gen Intern Med.2004;19:395–401.
- , , , et al.The presence of hospitalists in medical education.Acad Med.2000;75(suppl):S34–S36.
- , , , et al.Assessing the value of hospitalists to academic health centers: Brigham and Women's Hospital and Harvard Medical School.Am J Med.1999;106:134–137.
- , .Role of hospitalists in medical education.Am J Med.1999;107(4):305–309.
- , .The hospitalists: new boon for internal medicine or retreat from primary care?Ann Intern Med.1999;130:382–387.
- .The hospitalist movement: caution lights flashing at the crossroads.Am J Med.1999;107:409–413.
- , , .The state of competency evaluation in internal medicine residency.J Gen Intern Med.2008;23(7):1010–1015.
- , , .Sources of satisfaction for residency program directors: a second administration of the PD‐Sat.Am J Med.2009;122(2):196–201.
- , , .What predicts residency accreditation cycle length?Acad Med.2009;84(3):356–361.
- U.S. Census Bureau. Census Regions and Divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed May 2009.
- , , , et al.Complying with ACGME resident duty hours restrictions: restructuring the 80‐hour workweek to enhance education and patient safety at Texas A81(12):1026–1031.
- Association of Program Directors in Internal Medicine;, , , , .Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144(12):920–926.
- , , , et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–874.
- , , , , .Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations.Arch Intern Med.2004;164:1866–1871.
- , , , et al.Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- , , , et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19(4):293–301.
- , , , , .How to use The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1:48–56.
- .The hospitalist model: perspectives of the patient, the internist, and internal medicine.Ann Intern Med.1999;130:368–372.
- American College of Physicians. The impending collapse of primary care medicine and its implications for the state of the nation's health care: a report from the American College of Physicians. January 30,2006. Available at: http://www.acponline.org/advocacy/events/state_of_healthcare/statehc06_1.pdf. Accessed May 2009.
- , , , et al.Implementation of a voluntary hospitalist service at a community teaching hospital; improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- , , , et al.The United States rheumatology workforce: supply and demand, 2005–2025.Arthritis Rheum.2007;56(3):722–729.
- , , , , ;Committee on Manpower for Pulmonary and Critical Care Societies.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284(21):2762–2770.
- , .The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- , , , , .Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- , , .The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- , .The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- , , , , .What effect does inpatient physician specialty and experience have on clinical outcomes and resource utilization on a general medical service?J Gen Intern Med.2004;19:395–401.
- , , , et al.The presence of hospitalists in medical education.Acad Med.2000;75(suppl):S34–S36.
- , , , et al.Assessing the value of hospitalists to academic health centers: Brigham and Women's Hospital and Harvard Medical School.Am J Med.1999;106:134–137.
- , .Role of hospitalists in medical education.Am J Med.1999;107(4):305–309.
- , .The hospitalists: new boon for internal medicine or retreat from primary care?Ann Intern Med.1999;130:382–387.
- .The hospitalist movement: caution lights flashing at the crossroads.Am J Med.1999;107:409–413.
- , , .The state of competency evaluation in internal medicine residency.J Gen Intern Med.2008;23(7):1010–1015.
- , , .Sources of satisfaction for residency program directors: a second administration of the PD‐Sat.Am J Med.2009;122(2):196–201.
- , , .What predicts residency accreditation cycle length?Acad Med.2009;84(3):356–361.
- U.S. Census Bureau. Census Regions and Divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed May 2009.
- , , , et al.Complying with ACGME resident duty hours restrictions: restructuring the 80‐hour workweek to enhance education and patient safety at Texas A81(12):1026–1031.
- Association of Program Directors in Internal Medicine;, , , , .Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144(12):920–926.
- , , , et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–874.
- , , , , .Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations.Arch Intern Med.2004;164:1866–1871.
- , , , et al.Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- , , , et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19(4):293–301.
- , , , , .How to use The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1:48–56.
- .The hospitalist model: perspectives of the patient, the internist, and internal medicine.Ann Intern Med.1999;130:368–372.
- American College of Physicians. The impending collapse of primary care medicine and its implications for the state of the nation's health care: a report from the American College of Physicians. January 30,2006. Available at: http://www.acponline.org/advocacy/events/state_of_healthcare/statehc06_1.pdf. Accessed May 2009.
Copyright © 2009 Society of Hospital Medicine
Should ACEIs/ARAs Be Continued Before Surgery?
Clinicians commonly use renin‐angiotensin‐aldosterone‐system (RAAS) antagonists such as angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor subtype 1 antagonists (ARAs) to treat hypertension, congestive heart failure, and diabetic nephropathy. Hospitalists and other clinicians involved in the preoperative care of patients treated chronically with these agents are faced with the uncertainty of whether to continue these medications immediately prior to surgery.
The concern among those who recommend holding therapy is that pharmacologic suppression of the RAAS in patients undergoing general anesthesia may lead to severe or refractory (to intravenous fluid support) hypotension requiring vasopressors. On the other hand, if complications are no more likely when continuing one of these agents up to the day of surgery, withholding it could represent an unnecessary and potentially harmful intervention (eg, when a clinician caring postoperatively for a patient forgets to restart it). Although several studies have attempted to address this dilemma, a systematic and comprehensive summary of the pertinent evidence has not been published.
In this systematic review and meta‐analysis, we sought to summarize the best available evidence about the relative incidence of patient‐important outcomes1 in patients who do or do not receive ACEI/ARA therapy on the day of their nonemergent surgery.
METHODS
We report this protocol‐driven review in accordance with the Quality of Reporting of Meta‐analyses (QUOROM) standards for reporting systematic reviews of randomized trials.2
Search Strategy
In collaboration with an expert reference librarian (P.J.E.), we designed a search strategy that included the electronic databases MEDLINE, EMBASE, CINAHL, Web of Science, Current Contents, CENTRAL, DARE, and SCOPUS from 1981 (when captopril, the first ACEI, was approved by the FDA) until March 2006. We also reviewed the reference lists of included articles, retrieved articles from our personal files, and consulted with anesthesiologists and hospitalists with an interest in perioperative care in order to identify unpublished studies or studies missed by our strategy.
Study Selection
Eligible studies were prospective cohort studies or randomized controlled trials enrolling adult patients (ie, most patients > 18 years) undergoing nonemergent surgery and using ACEI or ARA chronically and assessing the effect of withdrawing or continuing these agents up to the morning of surgery. Eligible studies measured and reported either events of great patient importance (death, myocardial infarction, transient ischemic attack or stroke, and hepatic or renal failure) or of potentially less importance such as unplanned admission to the intensive care unit or treatment‐requiring hypotension, arrhythmias, or hyperkalemia.
Study Selection
Two reviewers (D.J.R. and F.S.M.) independently screened the titles and abstracts for potential inclusion and retrieved potentially eligible articles for full‐text evaluation. Two reviewers (D.J.R. and M.L.B.) working in duplicate independently selected studies for inclusion. The reviewers were in agreement for full text inclusion 100% of the time.
Data Extraction
Two hospitalists with experience in perioperative care and trained in clinical research (D.J.R. and F.S.M.) working independently and in duplicate extracted data from each eligible article using a standardized structured data extraction form. We extracted information about the study authors and publication, the patients (numbers in each group, indications for chronic ACEI/ARA therapy, type of surgery, agents used for anesthesia), event rates of surgical and perioperative complications (death, stroke, myocardial infarction, unplanned admission to the intensive care unit, treatment‐requiring hypotension, arrhythmias, or hyperkalemia), and relevant periods (e.g., between last dose of ACEI/ARA and surgery, between surgery and clinical end points, total follow‐up). When key information was not available in the published report, we contacted authors by electronic mail. We made 2 attempts to contact authors who failed to respond. Three of the 4 authors contacted responded with the requested information.
Quality Assessment
For randomized trials, we noted whether authors reported adequate allocation concealment, blinding of patients, clinicians, data collectors, data analysts, outcome assessors, and loss to follow‐up. The same reviewers (D.J.R. and F.S.M.) assessed study quality and were in agreement for each article and each domain of quality (kappa statistic in each case was 1.0). For cohort studies we noted details of cohort selection and comparability according to the Newcastle‐Ottawa approach.3
Statistical Analysis
We used a DerSimonian and Laird random effects method4 to conduct meta‐analyses across eligible outcomes. Random effects meta‐analysis incorporates both within‐study and between‐study variability. We chose a random effects approach because of the important degree of clinical heterogeneity expected between the included studies. For rare events we followed the approach by Sweeting et al. for the choice of a continuity correction factor.5 We report the pooled relative risk and the associated 95% confidence interval.
Inconsistency and Subgroup Analyses
To ascertain the magnitude of inconsistency across trials, we measured the I2 statistic, an estimate of the proportion of the overall between‐study variability that is not a result of random error or chance but of true clinical heterogeneity.6 When possible, we explored subgroup analyses to explain heterogeneity, including subgroups defined by type of surgery (cardiovascular versus noncardiovascular), timing of measurement of outcomes (in relation to anesthesia induction postoperatively), and type of agent (ACEI or ARA). We estimated the difference in treatment effects between subgroups by testing the hypothesis of treatmentsubgroup interaction with a nominal significance level of 5%.7
RESULTS
Search Results
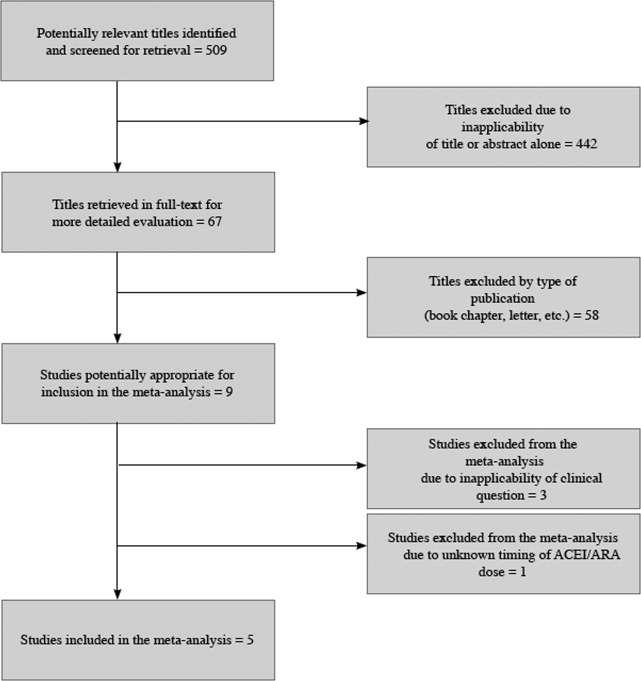
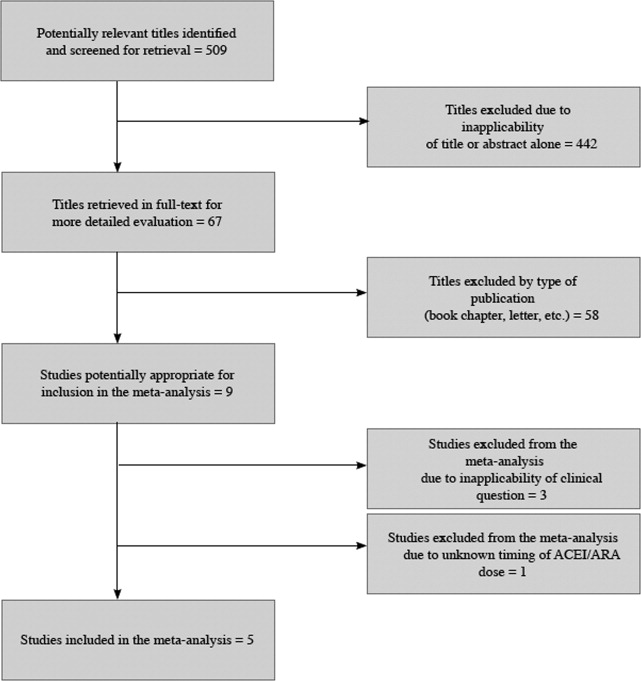
The 509 titles reviewed included 410 titles produced by electronic searches and an additional 99 titles from other sources (Fig. 1).
Study Characteristics
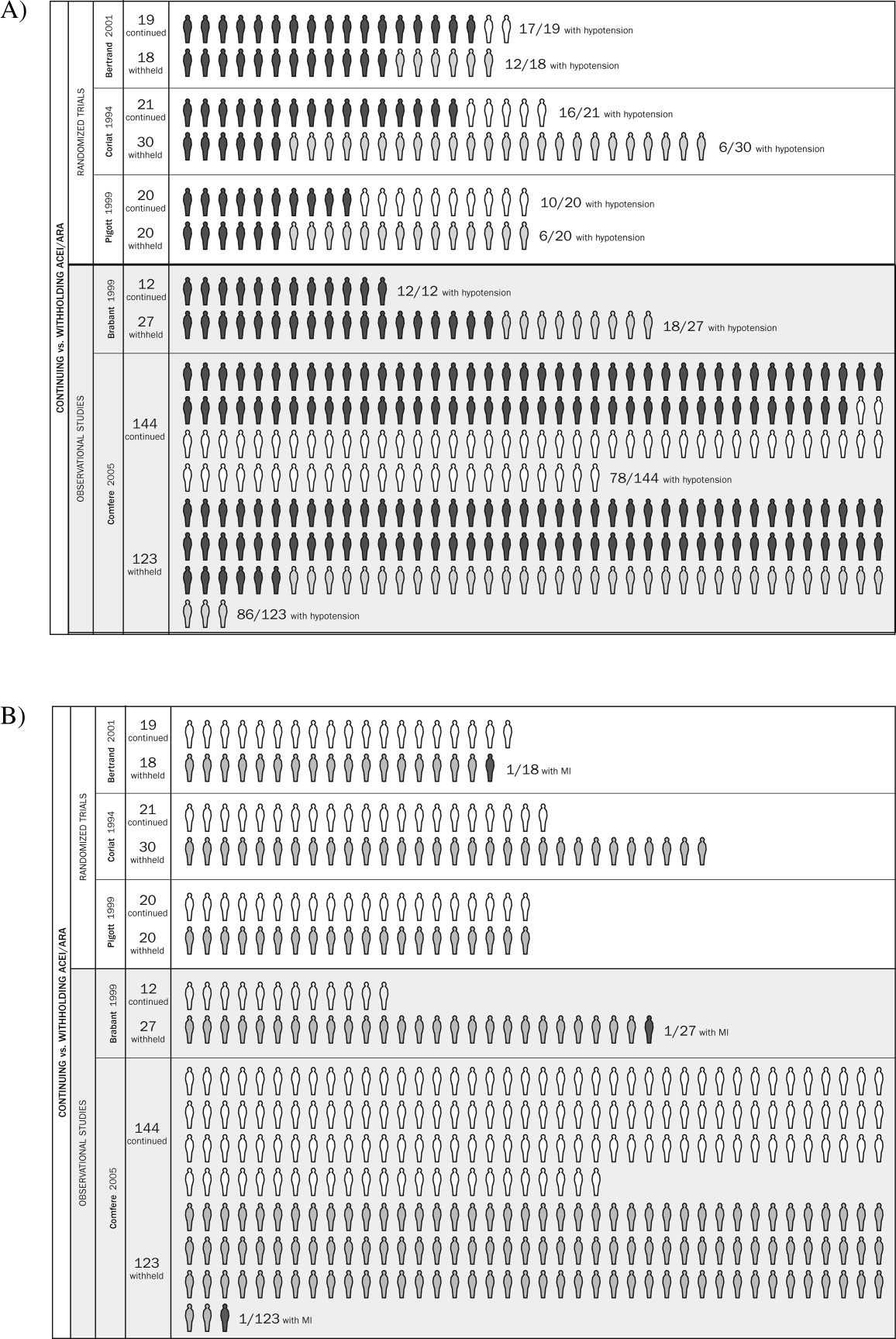
Table 1 summarizes the characteristics of the 5 included studies (n = 434 patients). Myocardial infarction was an end point in 3 studies (Brabant, Bertrand, and Comfere); 1 event was reported in the withheld arm of each of these studies (none in the continuing arms). Hypotension requiring vasopressors was reported in all 5 studies. The other end points of interest were reported sparsely. There was considerable heterogeneity across studies regarding follow‐up period, which ranged from ending at incision to ending at dismissal from the hospital.
| Author/Year | Patients (n) | Indication for ACEI/ARA | Type of surgery | End points measured |
|---|---|---|---|---|
| ||||
| Randomized trials | ||||
| Bertrand, 200111 | 19 continued 18 withheld | Hypertension | Elective major vascular | Hypotension, need for vasoactive drugs (at or shortly after induction) |
| Coriat, 19948 | 21 continued 30 withheld | Hypertension | Peripheral vascular (>2 hours) | Systolic blood pressure (at or shortly after induction), plasma ACEI and catecholamine levels |
| Pigott, 199917 | 20 continued 20 withheld | Hypertension (n = 17); previous myocardial infarction (n = 23) | Coronary artery bypass graft | Arterial pressure (at or shortly after induction), cardiac index, systemic vascular resistance, use of vasoactive drugs |
| Observational studies | ||||
| Brabant, 199910 | 12 continued 27 withheld | Previous myocardial infarction (n = 6); diabetes mellitus (n = 6; n with diabetic nephropathy unknown); hypertension (n = unknown) | Elective vascular surgery | Blood pressure (at or shortly after induction) |
| Comfere, 20059 | 144 continued 123 withheld | Hypertension | Noncardiovascular | Blood pressure (at or shortly after induction), unplanned ICU admissions, hemodynamic instability in the PACU (ABP or HR out of range), acute renal impairment, TIA, stroke, myocardial ischemia/emnfarction, and death |
Methodological Quality of Included Studies
Table 2 describes the methodological quality, as reported, of the included studies. Allocation concealment was unclear in 2 of the 3 randomized trials. Details of blinding either were not reported or otherwise were unclear in 2 of these 3 studies. Only 1 study specified the extent of loss to follow‐up.8 In 1 of the observational studies,9 details of cohort selection were generally appropriate. The 12 patients examined in another study10 had been scheduled consecutively for surgery. Both studies controlled for a variety of demographic and other key variables. Duration of follow‐up ranged from 3 days after surgery (for ECG)10 to as long as duration of hospitalization.9
| Randomized trials | |||
|---|---|---|---|
| Allocation concealment | Blinding | Loss to follow‐up | |
| Bertrand, 200111 | Unclear | Unclear | Not reported |
| Coriat, 19948 | Unclear | None | 19% |
| Pigott, 199917 | Adequate | Investigator, cardiac anesthetists, perfusionists, and recovery staff were blinded to allocation. Blinding not reported for other data collectors, assessors of outcome, or data analysts | Not reported |
| Observational studies | |||
| Details of cohort selection | Comparability of cohorts | ||
| Brabant, 199910 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery. The unexposed cohort was drawn from the same community as the exposed cohort | Similar with 2 exceptions: compared with the ACEI‐withheld group, the ARA‐given group contained more than twice the proportion of patients with previous myocardial revascularization Compared with the ARA‐given group, the ACEI‐withheld group contained more than twice the proportion of patients with diabetes mellitus | |
| Comfere, 20059 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery (referral center population may not truly represent overall population). The unexposed cohort was drawn from the same community as the exposed cohort. Data were extracted from a secure record | Adequate This study controls for a variety of demographic and other variables | |
Meta‐analyses
Pooled results suggested that patients receiving the immediate preoperative ACEI/ARA dose were more likely (RR 1.51, 95% CI 1.14‐2.01) to develop hypotension requiring vasopressors at or shortly after induction of anesthesia (Fig. 2A). There was important inconsistency between studies (I2 = 59%). The pooled effect derived from randomized trials (RR = 2.26, 95% CI 0.84‐6.12) seemed greater than that derived from the 2 observational studies (RR = 1.33, 95% CI 1.02‐1.73), but the treatment‐study design interaction was not significant (P = .3). Similarly, other subgroup explorations were not contributory.
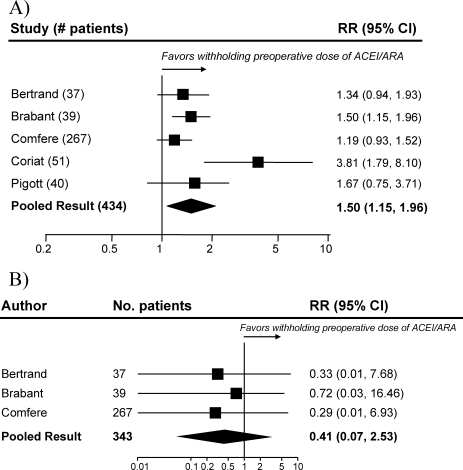
The incidence of perioperative myocardial infarction was not significantly different between continuing and withheld groups (Fig. 2B); the results were consistent across trials (I2 = 0%) but were imprecise (RR = 0.41, 95% CI 0.07‐2.53). Data were insufficient for subgroup analyses.
DISCUSSION
Statement of Principle Findings
Our systematic review identified 3 randomized trials and 2 observational studies examining the clinical consequences of continuing versus deliberately withholding the immediate preoperative dose of a renin‐angiotensin‐aldosterone system antagonist in patients treated chronically with these agents and scheduled to undergo nonemergent surgery.
Results from pooled estimates suggest that continuing chronic therapy up until surgery may increase the risk of perioperative hypotension requiring vasopressors (Fig. 3). Otherwise, this systematic review did not identify any clinically significant consequences associated either with preoperatively withholding or continuing RAAS antagonists. We do note that all 3 of the myocardial infarctions reported occurred in patients from whom the immediate preoperative ACEI/ARA dose was withheld, although no meaningful conclusion can be inferred from so few data points.
Strengths and Weaknesses of This Review
We observed considerable variation in design quality from study to study. With the exception of hypotension, other end points were not examined uniformly in the studies comprising this review. This was due either to study design (retrospective) or to the belief that the outcomes were not likely. With 1 exception,11 patient‐important end points such as myocardial infarction were noted if they occurred but not explicitly sought. Without active surveillance (serial electrocardiographic and biomarker testing), events such as myocardial infarction may remain undetected. Pain from myocardial ischemia, for example, may be masked by postoperative analgesia. Creatine kinase with muscle and brain subunits (CK‐MB) may be elevated in response to extracardiac injury. Postoperative ECG findings often are nonspecific.12 Furthermore, these studies examined the immediate and short‐term postoperative periods, possibly missing late‐manifesting hypotension‐induced or other end‐organ damage. Thus, truly reliable conclusions regarding the frequency of myocardial infarction, cerebrovascular events, and other patient‐important outcomes cannot be reached. Because this review includes small studies, it is particularly vulnerable to the effects of publication bias. The overall quality of the evidence we summarized makes it likely that larger rigorous trials may fail to confirm our findings.1315 Notably, this is to our knowledge the first systematic review addressing the clinical consequences of continuing or withholding the immediate preoperative dose of ACEI/ARAs.
Meaning of the Study
Evidence exists that perioperative ACEI/ARA therapy can impair the body's already anesthesia‐ suppressed blood pressure regulation system. Patients scheduled to undergo cardiovascular surgery may be at increased risk for the development of perioperative hypotension requiring vasopressors if the immediate preoperative ACEI/ARA dose is given. The results of this reviewa review of studies that were relatively small and generally not powered to observe clinically significant consequencesdo not provide sufficient evidence to support the systematic withholding or the systematic continuation of RAAS antagonists. Patients will be served best by hospitalists and other clinicians involved in perioperative care who take into account situation‐specific details in making this decision. A patient at particularly high risk for the complications of a blood pressure extreme (either hyper‐ or hypotension) represents such an example.
For patients who receive the immediate preoperative ACEI/ARA dose and do develop perioperative hypotension, there is inadequate evidence to determine whether that hypotension leads to patient‐important adverse outcomes. In fact, data from literature presently available are insufficient to reach any conclusion about long‐term clinical consequences of continuing or not continuing chronic ACEI/ARA therapy. The available studies were relatively small, reported few if any events, and were not designed to measure accurately the incidence of patient‐important end points.
Unanswered Questions and Future Research
Large and rigorous randomized trials could help to clarify the relationships suggested in this meta‐analysis and provide valid data about the consequences of continuing versus withholding preoperative ACEI/ARA therapy. Such trials are required before strong evidence‐based recommendations can be formulated.
Acknowledgements
The authors are indebted to James M. Naessens, ScD, David R. Danielson, MD, and David O. Warner, MD, for their advice during the conduct of this study. We also gratefully acknowledge Amanda Ebright, MD, for asking the original question that led to this review and Mr. Matthew Maleska for his design of summary Figure 3.
- ,,,,.Patients at the center: in our practice, and in our use of language.ACP J Club.2004;140:A11–A12.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement.Lancet.1999;354:1896–1900.
- ,,, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analysis. Ottawa Health Research Institute, University of Ottawa, Ontario, Canada. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed: March 21,2006.
- ,.Meta‐analysis in clinical trials.Control Clin Trials. Sep1986;7:177–188.
- .,..What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data.Stat Med.2004;23:1351–1375.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21:1539–1558.
- ,.Statistics notes: Interaction revisited: the difference between two estimatesBMJ.2003;326:219
- ,,, et al.Influence of chronic angiotensin‐converting enzyme inhibition on anesthetic induction.Anesthesiology.1994;81:299–307.
- ,,, et al.Angiotensin system inhibitors in a general surgical population.Anesth Analg.2005;100:636–644.
- ,,,,.The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists.Anesth Analg.1999;89:1388–1392.
- ,,,,,.Should the angiotensin II antagonists be discontinued before surgery? [see comment].Anesth Analg.2001;92:26–30.
- Zipes.Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine.7th ed.New York:Saunders, an imprint of Elsevier;2005.
- ,.Summarizing the Evidence: Publication Bias.Chicago:AMA Press;2002.
- ,,, et al.Large trials vsmeta‐analysis of smaller trials: how do their results compare? [see comment].JAMA.1996;276:1332–1338.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315:629–634.
- .Preoperative evaluation of the patient with hypertension.JAMA.2002;287:2043–2046.
- ,,,,.Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements.Br J Anaesth.1999;83:715–720.
Clinicians commonly use renin‐angiotensin‐aldosterone‐system (RAAS) antagonists such as angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor subtype 1 antagonists (ARAs) to treat hypertension, congestive heart failure, and diabetic nephropathy. Hospitalists and other clinicians involved in the preoperative care of patients treated chronically with these agents are faced with the uncertainty of whether to continue these medications immediately prior to surgery.
The concern among those who recommend holding therapy is that pharmacologic suppression of the RAAS in patients undergoing general anesthesia may lead to severe or refractory (to intravenous fluid support) hypotension requiring vasopressors. On the other hand, if complications are no more likely when continuing one of these agents up to the day of surgery, withholding it could represent an unnecessary and potentially harmful intervention (eg, when a clinician caring postoperatively for a patient forgets to restart it). Although several studies have attempted to address this dilemma, a systematic and comprehensive summary of the pertinent evidence has not been published.
In this systematic review and meta‐analysis, we sought to summarize the best available evidence about the relative incidence of patient‐important outcomes1 in patients who do or do not receive ACEI/ARA therapy on the day of their nonemergent surgery.
METHODS
We report this protocol‐driven review in accordance with the Quality of Reporting of Meta‐analyses (QUOROM) standards for reporting systematic reviews of randomized trials.2
Search Strategy
In collaboration with an expert reference librarian (P.J.E.), we designed a search strategy that included the electronic databases MEDLINE, EMBASE, CINAHL, Web of Science, Current Contents, CENTRAL, DARE, and SCOPUS from 1981 (when captopril, the first ACEI, was approved by the FDA) until March 2006. We also reviewed the reference lists of included articles, retrieved articles from our personal files, and consulted with anesthesiologists and hospitalists with an interest in perioperative care in order to identify unpublished studies or studies missed by our strategy.
Study Selection
Eligible studies were prospective cohort studies or randomized controlled trials enrolling adult patients (ie, most patients > 18 years) undergoing nonemergent surgery and using ACEI or ARA chronically and assessing the effect of withdrawing or continuing these agents up to the morning of surgery. Eligible studies measured and reported either events of great patient importance (death, myocardial infarction, transient ischemic attack or stroke, and hepatic or renal failure) or of potentially less importance such as unplanned admission to the intensive care unit or treatment‐requiring hypotension, arrhythmias, or hyperkalemia.
Study Selection
Two reviewers (D.J.R. and F.S.M.) independently screened the titles and abstracts for potential inclusion and retrieved potentially eligible articles for full‐text evaluation. Two reviewers (D.J.R. and M.L.B.) working in duplicate independently selected studies for inclusion. The reviewers were in agreement for full text inclusion 100% of the time.
Data Extraction
Two hospitalists with experience in perioperative care and trained in clinical research (D.J.R. and F.S.M.) working independently and in duplicate extracted data from each eligible article using a standardized structured data extraction form. We extracted information about the study authors and publication, the patients (numbers in each group, indications for chronic ACEI/ARA therapy, type of surgery, agents used for anesthesia), event rates of surgical and perioperative complications (death, stroke, myocardial infarction, unplanned admission to the intensive care unit, treatment‐requiring hypotension, arrhythmias, or hyperkalemia), and relevant periods (e.g., between last dose of ACEI/ARA and surgery, between surgery and clinical end points, total follow‐up). When key information was not available in the published report, we contacted authors by electronic mail. We made 2 attempts to contact authors who failed to respond. Three of the 4 authors contacted responded with the requested information.
Quality Assessment
For randomized trials, we noted whether authors reported adequate allocation concealment, blinding of patients, clinicians, data collectors, data analysts, outcome assessors, and loss to follow‐up. The same reviewers (D.J.R. and F.S.M.) assessed study quality and were in agreement for each article and each domain of quality (kappa statistic in each case was 1.0). For cohort studies we noted details of cohort selection and comparability according to the Newcastle‐Ottawa approach.3
Statistical Analysis
We used a DerSimonian and Laird random effects method4 to conduct meta‐analyses across eligible outcomes. Random effects meta‐analysis incorporates both within‐study and between‐study variability. We chose a random effects approach because of the important degree of clinical heterogeneity expected between the included studies. For rare events we followed the approach by Sweeting et al. for the choice of a continuity correction factor.5 We report the pooled relative risk and the associated 95% confidence interval.
Inconsistency and Subgroup Analyses
To ascertain the magnitude of inconsistency across trials, we measured the I2 statistic, an estimate of the proportion of the overall between‐study variability that is not a result of random error or chance but of true clinical heterogeneity.6 When possible, we explored subgroup analyses to explain heterogeneity, including subgroups defined by type of surgery (cardiovascular versus noncardiovascular), timing of measurement of outcomes (in relation to anesthesia induction postoperatively), and type of agent (ACEI or ARA). We estimated the difference in treatment effects between subgroups by testing the hypothesis of treatmentsubgroup interaction with a nominal significance level of 5%.7
RESULTS
Search Results
The 509 titles reviewed included 410 titles produced by electronic searches and an additional 99 titles from other sources (Fig. 1).

Study Characteristics
Table 1 summarizes the characteristics of the 5 included studies (n = 434 patients). Myocardial infarction was an end point in 3 studies (Brabant, Bertrand, and Comfere); 1 event was reported in the withheld arm of each of these studies (none in the continuing arms). Hypotension requiring vasopressors was reported in all 5 studies. The other end points of interest were reported sparsely. There was considerable heterogeneity across studies regarding follow‐up period, which ranged from ending at incision to ending at dismissal from the hospital.
| Author/Year | Patients (n) | Indication for ACEI/ARA | Type of surgery | End points measured |
|---|---|---|---|---|
| ||||
| Randomized trials | ||||
| Bertrand, 200111 | 19 continued 18 withheld | Hypertension | Elective major vascular | Hypotension, need for vasoactive drugs (at or shortly after induction) |
| Coriat, 19948 | 21 continued 30 withheld | Hypertension | Peripheral vascular (>2 hours) | Systolic blood pressure (at or shortly after induction), plasma ACEI and catecholamine levels |
| Pigott, 199917 | 20 continued 20 withheld | Hypertension (n = 17); previous myocardial infarction (n = 23) | Coronary artery bypass graft | Arterial pressure (at or shortly after induction), cardiac index, systemic vascular resistance, use of vasoactive drugs |
| Observational studies | ||||
| Brabant, 199910 | 12 continued 27 withheld | Previous myocardial infarction (n = 6); diabetes mellitus (n = 6; n with diabetic nephropathy unknown); hypertension (n = unknown) | Elective vascular surgery | Blood pressure (at or shortly after induction) |
| Comfere, 20059 | 144 continued 123 withheld | Hypertension | Noncardiovascular | Blood pressure (at or shortly after induction), unplanned ICU admissions, hemodynamic instability in the PACU (ABP or HR out of range), acute renal impairment, TIA, stroke, myocardial ischemia/emnfarction, and death |
Methodological Quality of Included Studies
Table 2 describes the methodological quality, as reported, of the included studies. Allocation concealment was unclear in 2 of the 3 randomized trials. Details of blinding either were not reported or otherwise were unclear in 2 of these 3 studies. Only 1 study specified the extent of loss to follow‐up.8 In 1 of the observational studies,9 details of cohort selection were generally appropriate. The 12 patients examined in another study10 had been scheduled consecutively for surgery. Both studies controlled for a variety of demographic and other key variables. Duration of follow‐up ranged from 3 days after surgery (for ECG)10 to as long as duration of hospitalization.9
| Randomized trials | |||
|---|---|---|---|
| Allocation concealment | Blinding | Loss to follow‐up | |
| Bertrand, 200111 | Unclear | Unclear | Not reported |
| Coriat, 19948 | Unclear | None | 19% |
| Pigott, 199917 | Adequate | Investigator, cardiac anesthetists, perfusionists, and recovery staff were blinded to allocation. Blinding not reported for other data collectors, assessors of outcome, or data analysts | Not reported |
| Observational studies | |||
| Details of cohort selection | Comparability of cohorts | ||
| Brabant, 199910 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery. The unexposed cohort was drawn from the same community as the exposed cohort | Similar with 2 exceptions: compared with the ACEI‐withheld group, the ARA‐given group contained more than twice the proportion of patients with previous myocardial revascularization Compared with the ARA‐given group, the ACEI‐withheld group contained more than twice the proportion of patients with diabetes mellitus | |
| Comfere, 20059 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery (referral center population may not truly represent overall population). The unexposed cohort was drawn from the same community as the exposed cohort. Data were extracted from a secure record | Adequate This study controls for a variety of demographic and other variables | |
Meta‐analyses
Pooled results suggested that patients receiving the immediate preoperative ACEI/ARA dose were more likely (RR 1.51, 95% CI 1.14‐2.01) to develop hypotension requiring vasopressors at or shortly after induction of anesthesia (Fig. 2A). There was important inconsistency between studies (I2 = 59%). The pooled effect derived from randomized trials (RR = 2.26, 95% CI 0.84‐6.12) seemed greater than that derived from the 2 observational studies (RR = 1.33, 95% CI 1.02‐1.73), but the treatment‐study design interaction was not significant (P = .3). Similarly, other subgroup explorations were not contributory.

The incidence of perioperative myocardial infarction was not significantly different between continuing and withheld groups (Fig. 2B); the results were consistent across trials (I2 = 0%) but were imprecise (RR = 0.41, 95% CI 0.07‐2.53). Data were insufficient for subgroup analyses.
DISCUSSION
Statement of Principle Findings
Our systematic review identified 3 randomized trials and 2 observational studies examining the clinical consequences of continuing versus deliberately withholding the immediate preoperative dose of a renin‐angiotensin‐aldosterone system antagonist in patients treated chronically with these agents and scheduled to undergo nonemergent surgery.
Results from pooled estimates suggest that continuing chronic therapy up until surgery may increase the risk of perioperative hypotension requiring vasopressors (Fig. 3). Otherwise, this systematic review did not identify any clinically significant consequences associated either with preoperatively withholding or continuing RAAS antagonists. We do note that all 3 of the myocardial infarctions reported occurred in patients from whom the immediate preoperative ACEI/ARA dose was withheld, although no meaningful conclusion can be inferred from so few data points.

Strengths and Weaknesses of This Review
We observed considerable variation in design quality from study to study. With the exception of hypotension, other end points were not examined uniformly in the studies comprising this review. This was due either to study design (retrospective) or to the belief that the outcomes were not likely. With 1 exception,11 patient‐important end points such as myocardial infarction were noted if they occurred but not explicitly sought. Without active surveillance (serial electrocardiographic and biomarker testing), events such as myocardial infarction may remain undetected. Pain from myocardial ischemia, for example, may be masked by postoperative analgesia. Creatine kinase with muscle and brain subunits (CK‐MB) may be elevated in response to extracardiac injury. Postoperative ECG findings often are nonspecific.12 Furthermore, these studies examined the immediate and short‐term postoperative periods, possibly missing late‐manifesting hypotension‐induced or other end‐organ damage. Thus, truly reliable conclusions regarding the frequency of myocardial infarction, cerebrovascular events, and other patient‐important outcomes cannot be reached. Because this review includes small studies, it is particularly vulnerable to the effects of publication bias. The overall quality of the evidence we summarized makes it likely that larger rigorous trials may fail to confirm our findings.1315 Notably, this is to our knowledge the first systematic review addressing the clinical consequences of continuing or withholding the immediate preoperative dose of ACEI/ARAs.
Meaning of the Study
Evidence exists that perioperative ACEI/ARA therapy can impair the body's already anesthesia‐ suppressed blood pressure regulation system. Patients scheduled to undergo cardiovascular surgery may be at increased risk for the development of perioperative hypotension requiring vasopressors if the immediate preoperative ACEI/ARA dose is given. The results of this reviewa review of studies that were relatively small and generally not powered to observe clinically significant consequencesdo not provide sufficient evidence to support the systematic withholding or the systematic continuation of RAAS antagonists. Patients will be served best by hospitalists and other clinicians involved in perioperative care who take into account situation‐specific details in making this decision. A patient at particularly high risk for the complications of a blood pressure extreme (either hyper‐ or hypotension) represents such an example.
For patients who receive the immediate preoperative ACEI/ARA dose and do develop perioperative hypotension, there is inadequate evidence to determine whether that hypotension leads to patient‐important adverse outcomes. In fact, data from literature presently available are insufficient to reach any conclusion about long‐term clinical consequences of continuing or not continuing chronic ACEI/ARA therapy. The available studies were relatively small, reported few if any events, and were not designed to measure accurately the incidence of patient‐important end points.
Unanswered Questions and Future Research
Large and rigorous randomized trials could help to clarify the relationships suggested in this meta‐analysis and provide valid data about the consequences of continuing versus withholding preoperative ACEI/ARA therapy. Such trials are required before strong evidence‐based recommendations can be formulated.
Acknowledgements
The authors are indebted to James M. Naessens, ScD, David R. Danielson, MD, and David O. Warner, MD, for their advice during the conduct of this study. We also gratefully acknowledge Amanda Ebright, MD, for asking the original question that led to this review and Mr. Matthew Maleska for his design of summary Figure 3.
Clinicians commonly use renin‐angiotensin‐aldosterone‐system (RAAS) antagonists such as angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor subtype 1 antagonists (ARAs) to treat hypertension, congestive heart failure, and diabetic nephropathy. Hospitalists and other clinicians involved in the preoperative care of patients treated chronically with these agents are faced with the uncertainty of whether to continue these medications immediately prior to surgery.
The concern among those who recommend holding therapy is that pharmacologic suppression of the RAAS in patients undergoing general anesthesia may lead to severe or refractory (to intravenous fluid support) hypotension requiring vasopressors. On the other hand, if complications are no more likely when continuing one of these agents up to the day of surgery, withholding it could represent an unnecessary and potentially harmful intervention (eg, when a clinician caring postoperatively for a patient forgets to restart it). Although several studies have attempted to address this dilemma, a systematic and comprehensive summary of the pertinent evidence has not been published.
In this systematic review and meta‐analysis, we sought to summarize the best available evidence about the relative incidence of patient‐important outcomes1 in patients who do or do not receive ACEI/ARA therapy on the day of their nonemergent surgery.
METHODS
We report this protocol‐driven review in accordance with the Quality of Reporting of Meta‐analyses (QUOROM) standards for reporting systematic reviews of randomized trials.2
Search Strategy
In collaboration with an expert reference librarian (P.J.E.), we designed a search strategy that included the electronic databases MEDLINE, EMBASE, CINAHL, Web of Science, Current Contents, CENTRAL, DARE, and SCOPUS from 1981 (when captopril, the first ACEI, was approved by the FDA) until March 2006. We also reviewed the reference lists of included articles, retrieved articles from our personal files, and consulted with anesthesiologists and hospitalists with an interest in perioperative care in order to identify unpublished studies or studies missed by our strategy.
Study Selection
Eligible studies were prospective cohort studies or randomized controlled trials enrolling adult patients (ie, most patients > 18 years) undergoing nonemergent surgery and using ACEI or ARA chronically and assessing the effect of withdrawing or continuing these agents up to the morning of surgery. Eligible studies measured and reported either events of great patient importance (death, myocardial infarction, transient ischemic attack or stroke, and hepatic or renal failure) or of potentially less importance such as unplanned admission to the intensive care unit or treatment‐requiring hypotension, arrhythmias, or hyperkalemia.
Study Selection
Two reviewers (D.J.R. and F.S.M.) independently screened the titles and abstracts for potential inclusion and retrieved potentially eligible articles for full‐text evaluation. Two reviewers (D.J.R. and M.L.B.) working in duplicate independently selected studies for inclusion. The reviewers were in agreement for full text inclusion 100% of the time.
Data Extraction
Two hospitalists with experience in perioperative care and trained in clinical research (D.J.R. and F.S.M.) working independently and in duplicate extracted data from each eligible article using a standardized structured data extraction form. We extracted information about the study authors and publication, the patients (numbers in each group, indications for chronic ACEI/ARA therapy, type of surgery, agents used for anesthesia), event rates of surgical and perioperative complications (death, stroke, myocardial infarction, unplanned admission to the intensive care unit, treatment‐requiring hypotension, arrhythmias, or hyperkalemia), and relevant periods (e.g., between last dose of ACEI/ARA and surgery, between surgery and clinical end points, total follow‐up). When key information was not available in the published report, we contacted authors by electronic mail. We made 2 attempts to contact authors who failed to respond. Three of the 4 authors contacted responded with the requested information.
Quality Assessment
For randomized trials, we noted whether authors reported adequate allocation concealment, blinding of patients, clinicians, data collectors, data analysts, outcome assessors, and loss to follow‐up. The same reviewers (D.J.R. and F.S.M.) assessed study quality and were in agreement for each article and each domain of quality (kappa statistic in each case was 1.0). For cohort studies we noted details of cohort selection and comparability according to the Newcastle‐Ottawa approach.3
Statistical Analysis
We used a DerSimonian and Laird random effects method4 to conduct meta‐analyses across eligible outcomes. Random effects meta‐analysis incorporates both within‐study and between‐study variability. We chose a random effects approach because of the important degree of clinical heterogeneity expected between the included studies. For rare events we followed the approach by Sweeting et al. for the choice of a continuity correction factor.5 We report the pooled relative risk and the associated 95% confidence interval.
Inconsistency and Subgroup Analyses
To ascertain the magnitude of inconsistency across trials, we measured the I2 statistic, an estimate of the proportion of the overall between‐study variability that is not a result of random error or chance but of true clinical heterogeneity.6 When possible, we explored subgroup analyses to explain heterogeneity, including subgroups defined by type of surgery (cardiovascular versus noncardiovascular), timing of measurement of outcomes (in relation to anesthesia induction postoperatively), and type of agent (ACEI or ARA). We estimated the difference in treatment effects between subgroups by testing the hypothesis of treatmentsubgroup interaction with a nominal significance level of 5%.7
RESULTS
Search Results
The 509 titles reviewed included 410 titles produced by electronic searches and an additional 99 titles from other sources (Fig. 1).

Study Characteristics
Table 1 summarizes the characteristics of the 5 included studies (n = 434 patients). Myocardial infarction was an end point in 3 studies (Brabant, Bertrand, and Comfere); 1 event was reported in the withheld arm of each of these studies (none in the continuing arms). Hypotension requiring vasopressors was reported in all 5 studies. The other end points of interest were reported sparsely. There was considerable heterogeneity across studies regarding follow‐up period, which ranged from ending at incision to ending at dismissal from the hospital.
| Author/Year | Patients (n) | Indication for ACEI/ARA | Type of surgery | End points measured |
|---|---|---|---|---|
| ||||
| Randomized trials | ||||
| Bertrand, 200111 | 19 continued 18 withheld | Hypertension | Elective major vascular | Hypotension, need for vasoactive drugs (at or shortly after induction) |
| Coriat, 19948 | 21 continued 30 withheld | Hypertension | Peripheral vascular (>2 hours) | Systolic blood pressure (at or shortly after induction), plasma ACEI and catecholamine levels |
| Pigott, 199917 | 20 continued 20 withheld | Hypertension (n = 17); previous myocardial infarction (n = 23) | Coronary artery bypass graft | Arterial pressure (at or shortly after induction), cardiac index, systemic vascular resistance, use of vasoactive drugs |
| Observational studies | ||||
| Brabant, 199910 | 12 continued 27 withheld | Previous myocardial infarction (n = 6); diabetes mellitus (n = 6; n with diabetic nephropathy unknown); hypertension (n = unknown) | Elective vascular surgery | Blood pressure (at or shortly after induction) |
| Comfere, 20059 | 144 continued 123 withheld | Hypertension | Noncardiovascular | Blood pressure (at or shortly after induction), unplanned ICU admissions, hemodynamic instability in the PACU (ABP or HR out of range), acute renal impairment, TIA, stroke, myocardial ischemia/emnfarction, and death |
Methodological Quality of Included Studies
Table 2 describes the methodological quality, as reported, of the included studies. Allocation concealment was unclear in 2 of the 3 randomized trials. Details of blinding either were not reported or otherwise were unclear in 2 of these 3 studies. Only 1 study specified the extent of loss to follow‐up.8 In 1 of the observational studies,9 details of cohort selection were generally appropriate. The 12 patients examined in another study10 had been scheduled consecutively for surgery. Both studies controlled for a variety of demographic and other key variables. Duration of follow‐up ranged from 3 days after surgery (for ECG)10 to as long as duration of hospitalization.9
| Randomized trials | |||
|---|---|---|---|
| Allocation concealment | Blinding | Loss to follow‐up | |
| Bertrand, 200111 | Unclear | Unclear | Not reported |
| Coriat, 19948 | Unclear | None | 19% |
| Pigott, 199917 | Adequate | Investigator, cardiac anesthetists, perfusionists, and recovery staff were blinded to allocation. Blinding not reported for other data collectors, assessors of outcome, or data analysts | Not reported |
| Observational studies | |||
| Details of cohort selection | Comparability of cohorts | ||
| Brabant, 199910 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery. The unexposed cohort was drawn from the same community as the exposed cohort | Similar with 2 exceptions: compared with the ACEI‐withheld group, the ARA‐given group contained more than twice the proportion of patients with previous myocardial revascularization Compared with the ARA‐given group, the ACEI‐withheld group contained more than twice the proportion of patients with diabetes mellitus | |
| Comfere, 20059 | Appropriate Cohort somewhat representative of the adult population undergoing nonemergent surgery (referral center population may not truly represent overall population). The unexposed cohort was drawn from the same community as the exposed cohort. Data were extracted from a secure record | Adequate This study controls for a variety of demographic and other variables | |
Meta‐analyses
Pooled results suggested that patients receiving the immediate preoperative ACEI/ARA dose were more likely (RR 1.51, 95% CI 1.14‐2.01) to develop hypotension requiring vasopressors at or shortly after induction of anesthesia (Fig. 2A). There was important inconsistency between studies (I2 = 59%). The pooled effect derived from randomized trials (RR = 2.26, 95% CI 0.84‐6.12) seemed greater than that derived from the 2 observational studies (RR = 1.33, 95% CI 1.02‐1.73), but the treatment‐study design interaction was not significant (P = .3). Similarly, other subgroup explorations were not contributory.

The incidence of perioperative myocardial infarction was not significantly different between continuing and withheld groups (Fig. 2B); the results were consistent across trials (I2 = 0%) but were imprecise (RR = 0.41, 95% CI 0.07‐2.53). Data were insufficient for subgroup analyses.
DISCUSSION
Statement of Principle Findings
Our systematic review identified 3 randomized trials and 2 observational studies examining the clinical consequences of continuing versus deliberately withholding the immediate preoperative dose of a renin‐angiotensin‐aldosterone system antagonist in patients treated chronically with these agents and scheduled to undergo nonemergent surgery.
Results from pooled estimates suggest that continuing chronic therapy up until surgery may increase the risk of perioperative hypotension requiring vasopressors (Fig. 3). Otherwise, this systematic review did not identify any clinically significant consequences associated either with preoperatively withholding or continuing RAAS antagonists. We do note that all 3 of the myocardial infarctions reported occurred in patients from whom the immediate preoperative ACEI/ARA dose was withheld, although no meaningful conclusion can be inferred from so few data points.

Strengths and Weaknesses of This Review
We observed considerable variation in design quality from study to study. With the exception of hypotension, other end points were not examined uniformly in the studies comprising this review. This was due either to study design (retrospective) or to the belief that the outcomes were not likely. With 1 exception,11 patient‐important end points such as myocardial infarction were noted if they occurred but not explicitly sought. Without active surveillance (serial electrocardiographic and biomarker testing), events such as myocardial infarction may remain undetected. Pain from myocardial ischemia, for example, may be masked by postoperative analgesia. Creatine kinase with muscle and brain subunits (CK‐MB) may be elevated in response to extracardiac injury. Postoperative ECG findings often are nonspecific.12 Furthermore, these studies examined the immediate and short‐term postoperative periods, possibly missing late‐manifesting hypotension‐induced or other end‐organ damage. Thus, truly reliable conclusions regarding the frequency of myocardial infarction, cerebrovascular events, and other patient‐important outcomes cannot be reached. Because this review includes small studies, it is particularly vulnerable to the effects of publication bias. The overall quality of the evidence we summarized makes it likely that larger rigorous trials may fail to confirm our findings.1315 Notably, this is to our knowledge the first systematic review addressing the clinical consequences of continuing or withholding the immediate preoperative dose of ACEI/ARAs.
Meaning of the Study
Evidence exists that perioperative ACEI/ARA therapy can impair the body's already anesthesia‐ suppressed blood pressure regulation system. Patients scheduled to undergo cardiovascular surgery may be at increased risk for the development of perioperative hypotension requiring vasopressors if the immediate preoperative ACEI/ARA dose is given. The results of this reviewa review of studies that were relatively small and generally not powered to observe clinically significant consequencesdo not provide sufficient evidence to support the systematic withholding or the systematic continuation of RAAS antagonists. Patients will be served best by hospitalists and other clinicians involved in perioperative care who take into account situation‐specific details in making this decision. A patient at particularly high risk for the complications of a blood pressure extreme (either hyper‐ or hypotension) represents such an example.
For patients who receive the immediate preoperative ACEI/ARA dose and do develop perioperative hypotension, there is inadequate evidence to determine whether that hypotension leads to patient‐important adverse outcomes. In fact, data from literature presently available are insufficient to reach any conclusion about long‐term clinical consequences of continuing or not continuing chronic ACEI/ARA therapy. The available studies were relatively small, reported few if any events, and were not designed to measure accurately the incidence of patient‐important end points.
Unanswered Questions and Future Research
Large and rigorous randomized trials could help to clarify the relationships suggested in this meta‐analysis and provide valid data about the consequences of continuing versus withholding preoperative ACEI/ARA therapy. Such trials are required before strong evidence‐based recommendations can be formulated.
Acknowledgements
The authors are indebted to James M. Naessens, ScD, David R. Danielson, MD, and David O. Warner, MD, for their advice during the conduct of this study. We also gratefully acknowledge Amanda Ebright, MD, for asking the original question that led to this review and Mr. Matthew Maleska for his design of summary Figure 3.
- ,,,,.Patients at the center: in our practice, and in our use of language.ACP J Club.2004;140:A11–A12.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement.Lancet.1999;354:1896–1900.
- ,,, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analysis. Ottawa Health Research Institute, University of Ottawa, Ontario, Canada. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed: March 21,2006.
- ,.Meta‐analysis in clinical trials.Control Clin Trials. Sep1986;7:177–188.
- .,..What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data.Stat Med.2004;23:1351–1375.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21:1539–1558.
- ,.Statistics notes: Interaction revisited: the difference between two estimatesBMJ.2003;326:219
- ,,, et al.Influence of chronic angiotensin‐converting enzyme inhibition on anesthetic induction.Anesthesiology.1994;81:299–307.
- ,,, et al.Angiotensin system inhibitors in a general surgical population.Anesth Analg.2005;100:636–644.
- ,,,,.The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists.Anesth Analg.1999;89:1388–1392.
- ,,,,,.Should the angiotensin II antagonists be discontinued before surgery? [see comment].Anesth Analg.2001;92:26–30.
- Zipes.Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine.7th ed.New York:Saunders, an imprint of Elsevier;2005.
- ,.Summarizing the Evidence: Publication Bias.Chicago:AMA Press;2002.
- ,,, et al.Large trials vsmeta‐analysis of smaller trials: how do their results compare? [see comment].JAMA.1996;276:1332–1338.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315:629–634.
- .Preoperative evaluation of the patient with hypertension.JAMA.2002;287:2043–2046.
- ,,,,.Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements.Br J Anaesth.1999;83:715–720.
- ,,,,.Patients at the center: in our practice, and in our use of language.ACP J Club.2004;140:A11–A12.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement.Lancet.1999;354:1896–1900.
- ,,, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analysis. Ottawa Health Research Institute, University of Ottawa, Ontario, Canada. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed: March 21,2006.
- ,.Meta‐analysis in clinical trials.Control Clin Trials. Sep1986;7:177–188.
- .,..What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data.Stat Med.2004;23:1351–1375.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21:1539–1558.
- ,.Statistics notes: Interaction revisited: the difference between two estimatesBMJ.2003;326:219
- ,,, et al.Influence of chronic angiotensin‐converting enzyme inhibition on anesthetic induction.Anesthesiology.1994;81:299–307.
- ,,, et al.Angiotensin system inhibitors in a general surgical population.Anesth Analg.2005;100:636–644.
- ,,,,.The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists.Anesth Analg.1999;89:1388–1392.
- ,,,,,.Should the angiotensin II antagonists be discontinued before surgery? [see comment].Anesth Analg.2001;92:26–30.
- Zipes.Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine.7th ed.New York:Saunders, an imprint of Elsevier;2005.
- ,.Summarizing the Evidence: Publication Bias.Chicago:AMA Press;2002.
- ,,, et al.Large trials vsmeta‐analysis of smaller trials: how do their results compare? [see comment].JAMA.1996;276:1332–1338.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315:629–634.
- .Preoperative evaluation of the patient with hypertension.JAMA.2002;287:2043–2046.
- ,,,,.Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements.Br J Anaesth.1999;83:715–720.