User login
Cancer Screening for Dermatomyositis: A Survey of Indirect Costs, Burden, and Patient Willingness to Pay
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
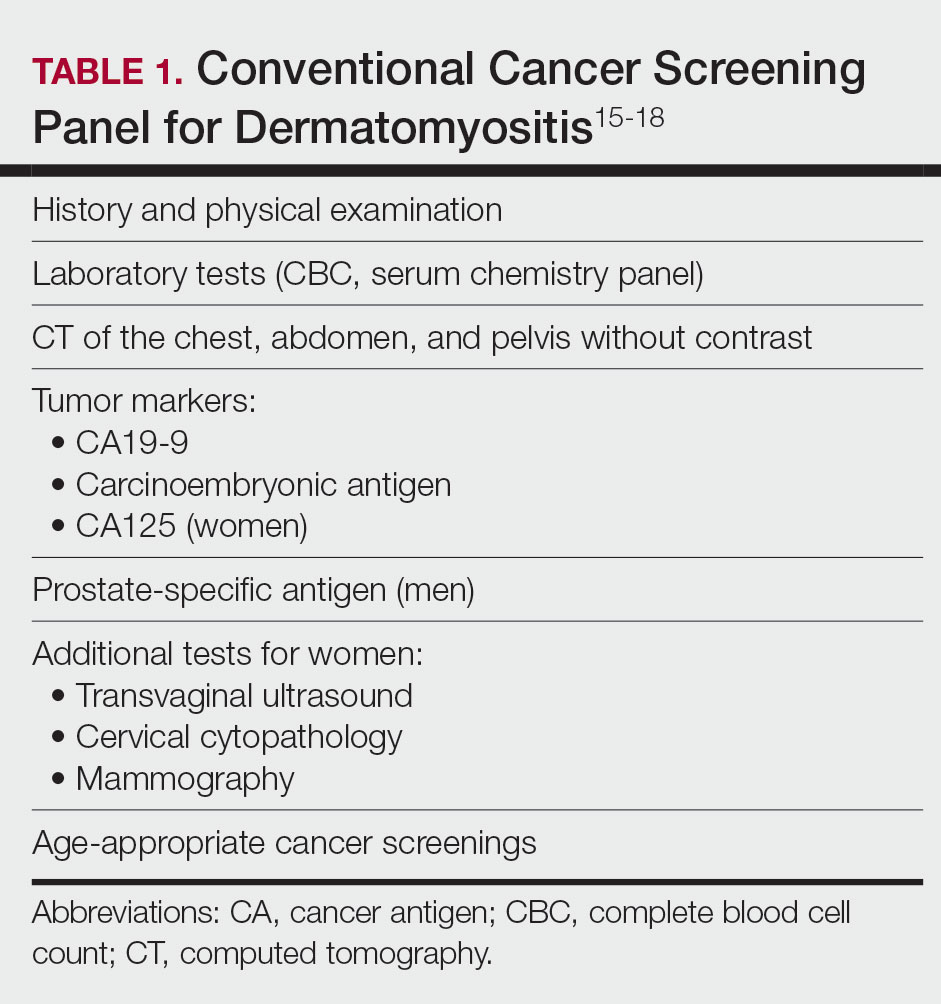
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18
The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).
Results
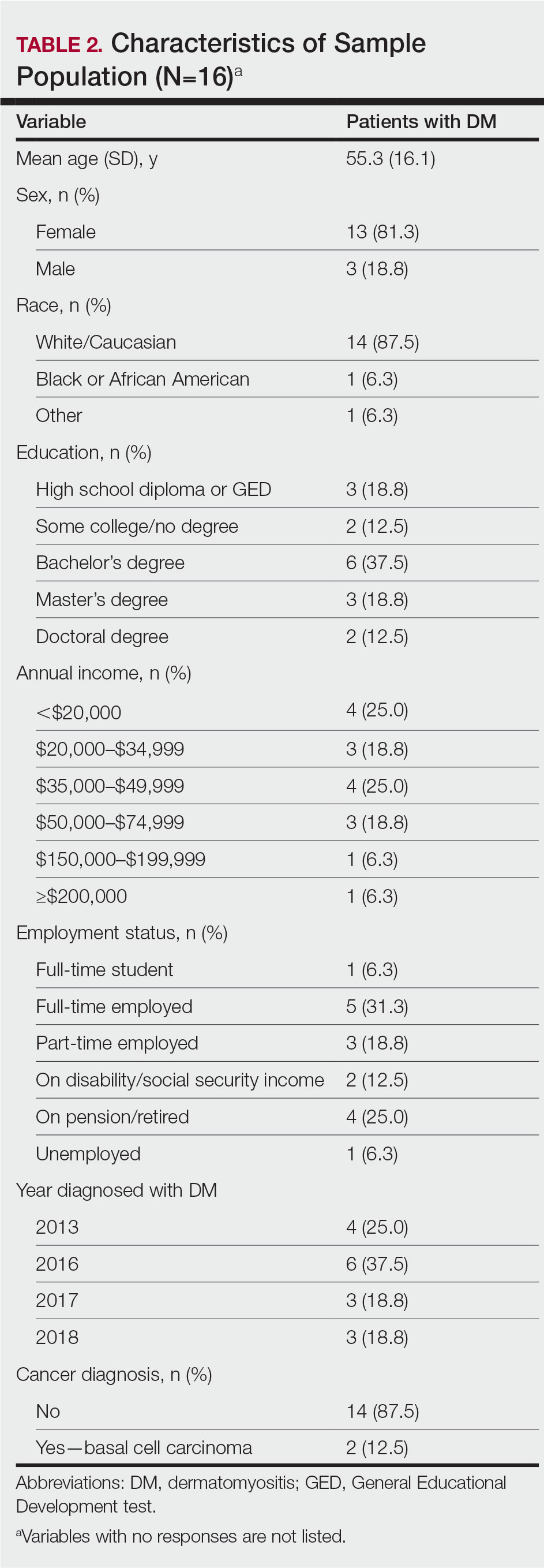
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.
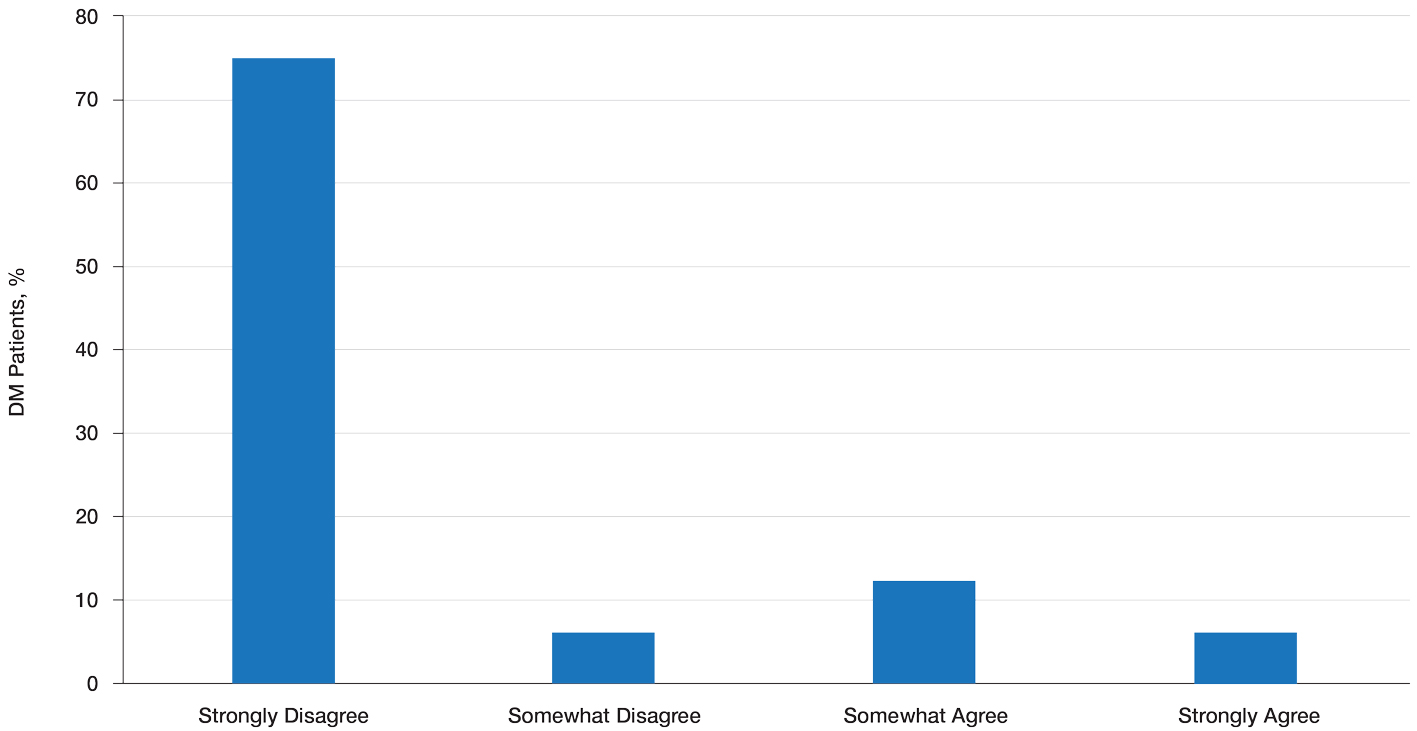
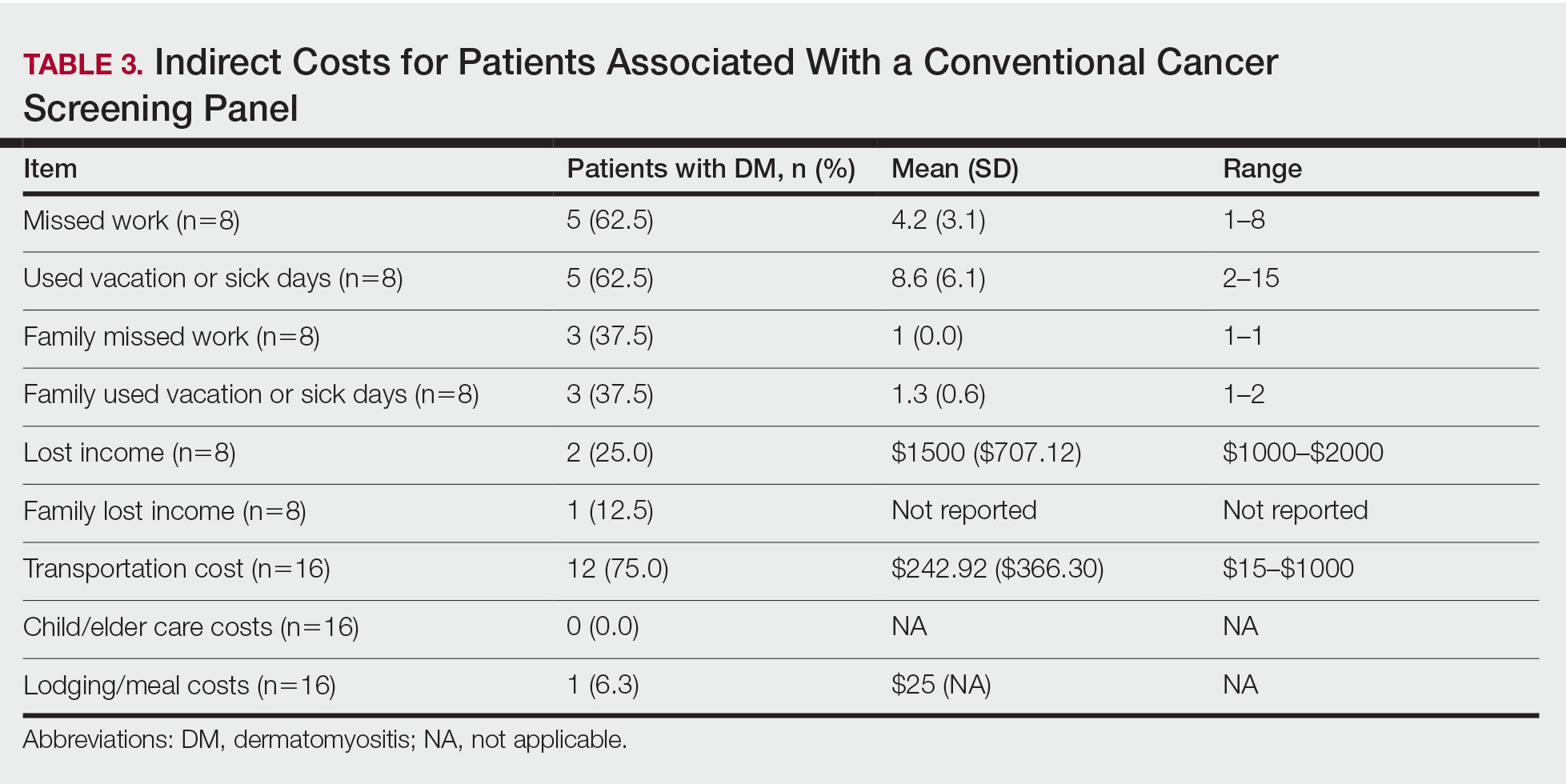
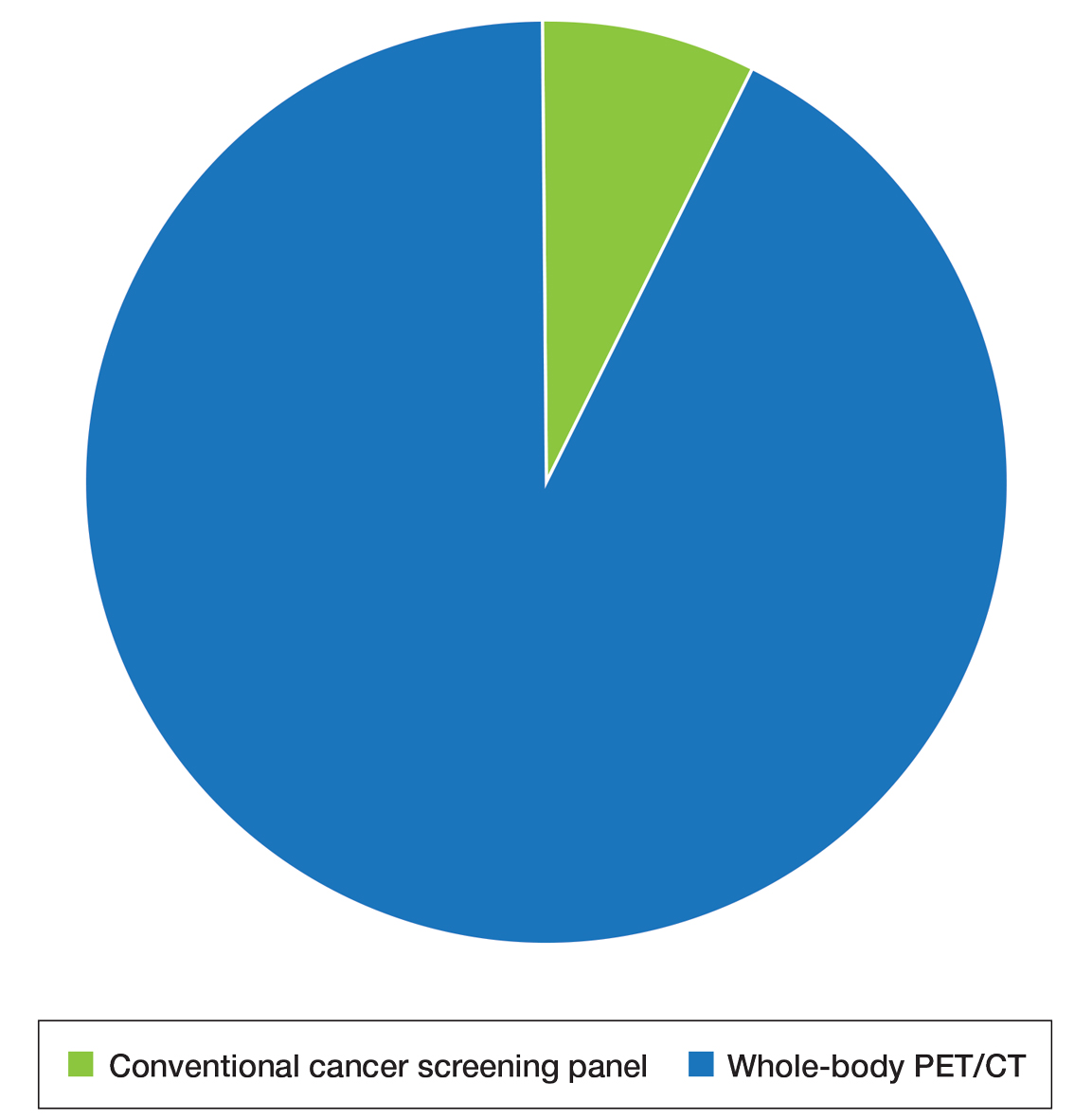
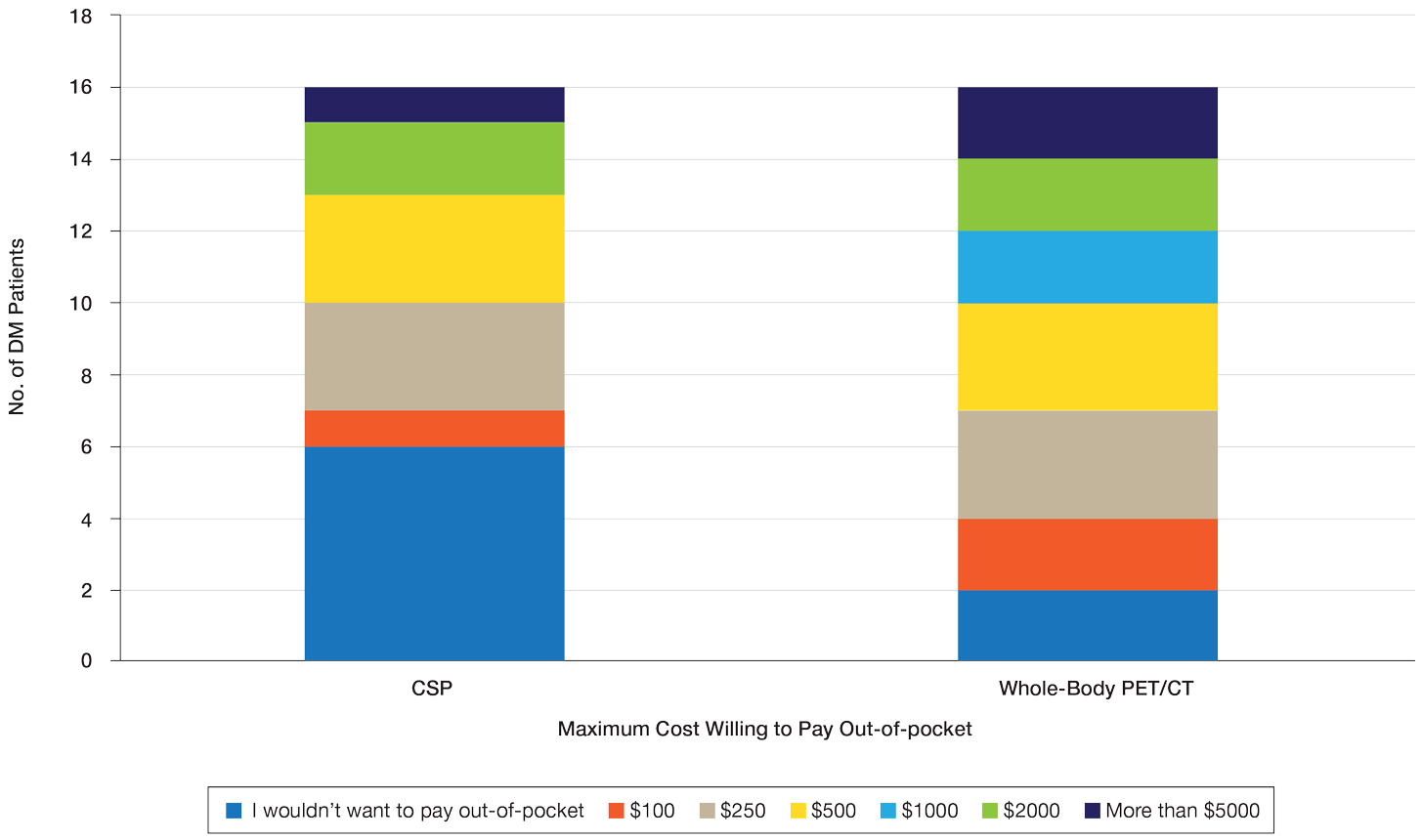
Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.
Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17
The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)
“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18

The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).

Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.

Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.

Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17

The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)

“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18

The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).

Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.

Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.

Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17

The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)

“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
Practice Points
- Dermatomyositis (DM) is associated with an increased risk for malignancy. Patient perspective needs to be considered in developing cancer screening guidelines for patients with DM, particularly given the similar efficacy of available screening modalities.
- Current modalities for cancer screening in DM include whole-body positron emission tomography/computed tomography (PET/CT) and a conventional cancer screening panel (CSP), which includes a battery of tests typically requiring multiple visits. Patients may find the simplicity of PET/CT more preferrable than the more complex CSP.
- Indirect costs of cancer screening include missed work, travel and childcare expenses, and lost wages. Conventional cancer screening has greater indirect costs than PET/CT.
Long-standing Dermatitis Treated With Dupilumab With Subsequent Progression to Cutaneous T-cell Lymphoma
Dupilumab is a novel medication that is approved by the US Food and Drug Administration to treat moderate to severe atopic dermatitis (AD) in patients 6 years and older. Dupilumab is an injectable fully human monoclonal antibody. It provides a giant leap toward a better quality of life for patients with AD. Dupilumab works by binding to the shared α subunit of the IL-4 receptor (IL-4R), thus inhibiting IL-4 and IL-13 from using that signaling pathway. The documented side-effect profile includes injection-site reaction, keratitis, nasopharyngitis, and headache.1
We initiated off-label treatment with dupilumab in 3 adult patients who had a history of long-standing adult-onset dermatitis confirmed by histopathology. The 3 patients received a loading dose of 600 mg subcutaneously, followed by 300 mg every other week. Following treatment, the patients had expansion of their disease, with features consistent with cutaneous T-cell lymphoma (CTCL) on subsequent biopsies. These 3 cases demonstrate the well-known adage that the diagnosis of CTCL often requires multiple biopsies performed over time. Although dupilumab has proved efficacious and safe for treating AD, dermatologists should be cautious before starting this medication in an adult who has new-onset dermatitis and no history of atopy.
Case Reports
Patient 1
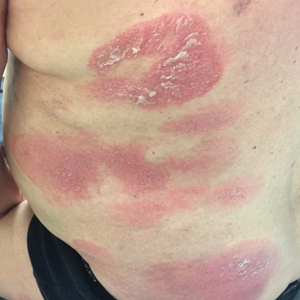
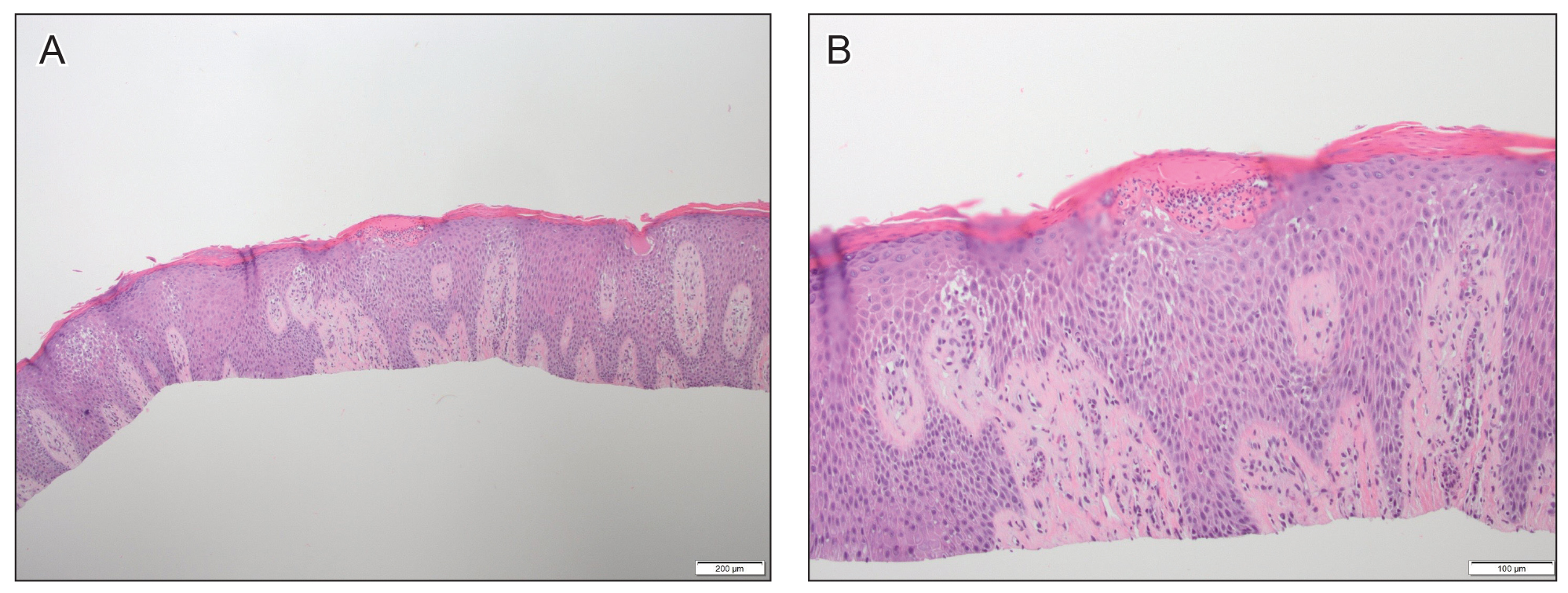
A 61-year-old man presented to dermatology after being lost to follow-up for several years and was started on dupilumab for long-standing nonspecific eczematous dermatitis based on histopathology. He had a pruritic rash of 10 years’ duration that had been biopsied multiple times and was found to be consistent with dermatitis and lichen simplex chronicus (Figure 1). He had been treated with triamcinolone ointment 0.1% and narrowband UVB as often as 3 times weekly over many years. The patient also had a history of idiopathic CD4 lymphopenia with consistently negative tests for human immunodeficiency virus.
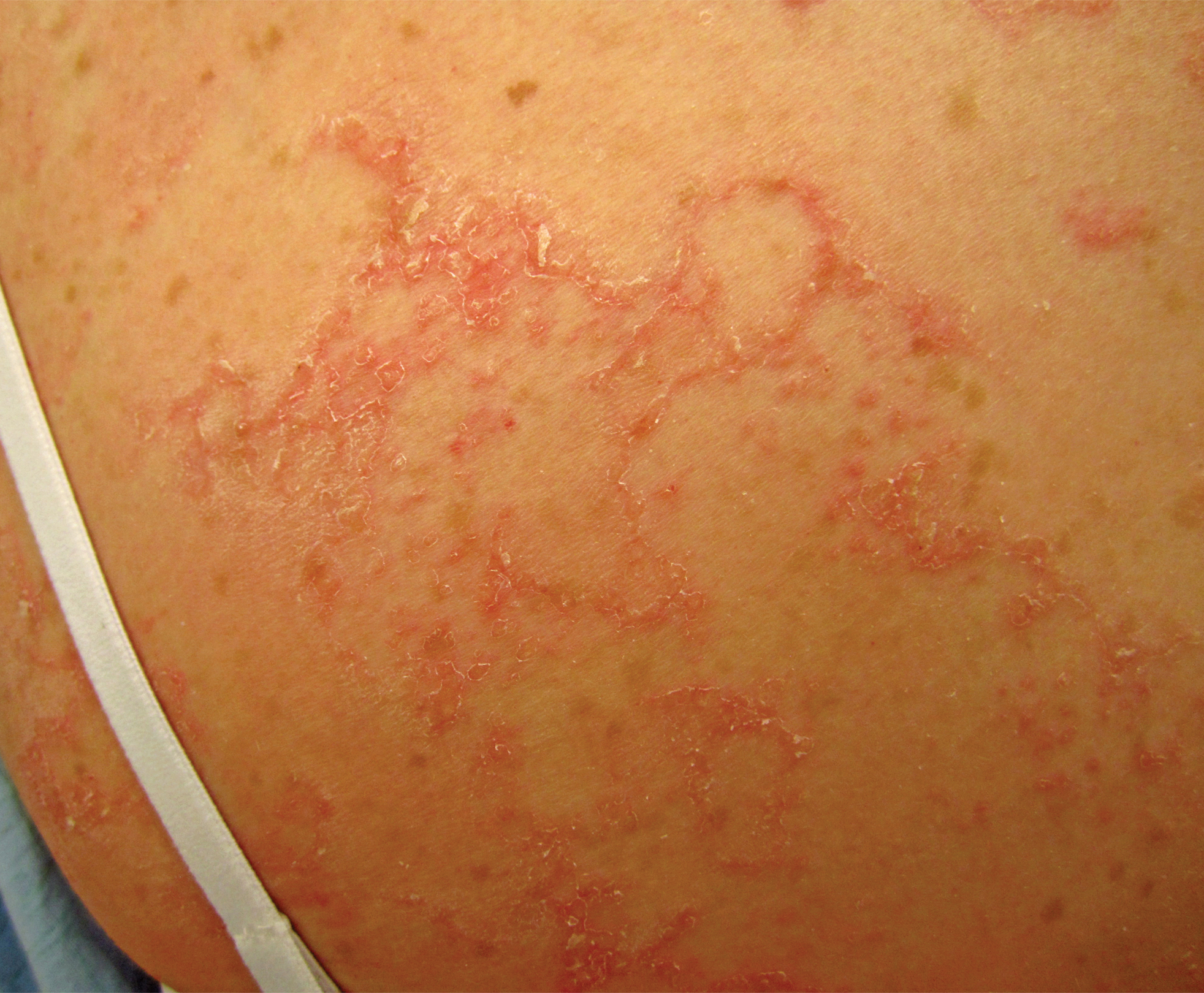
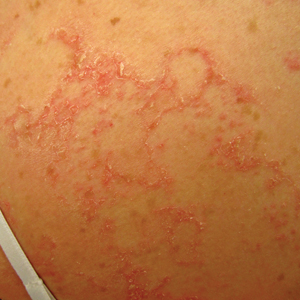
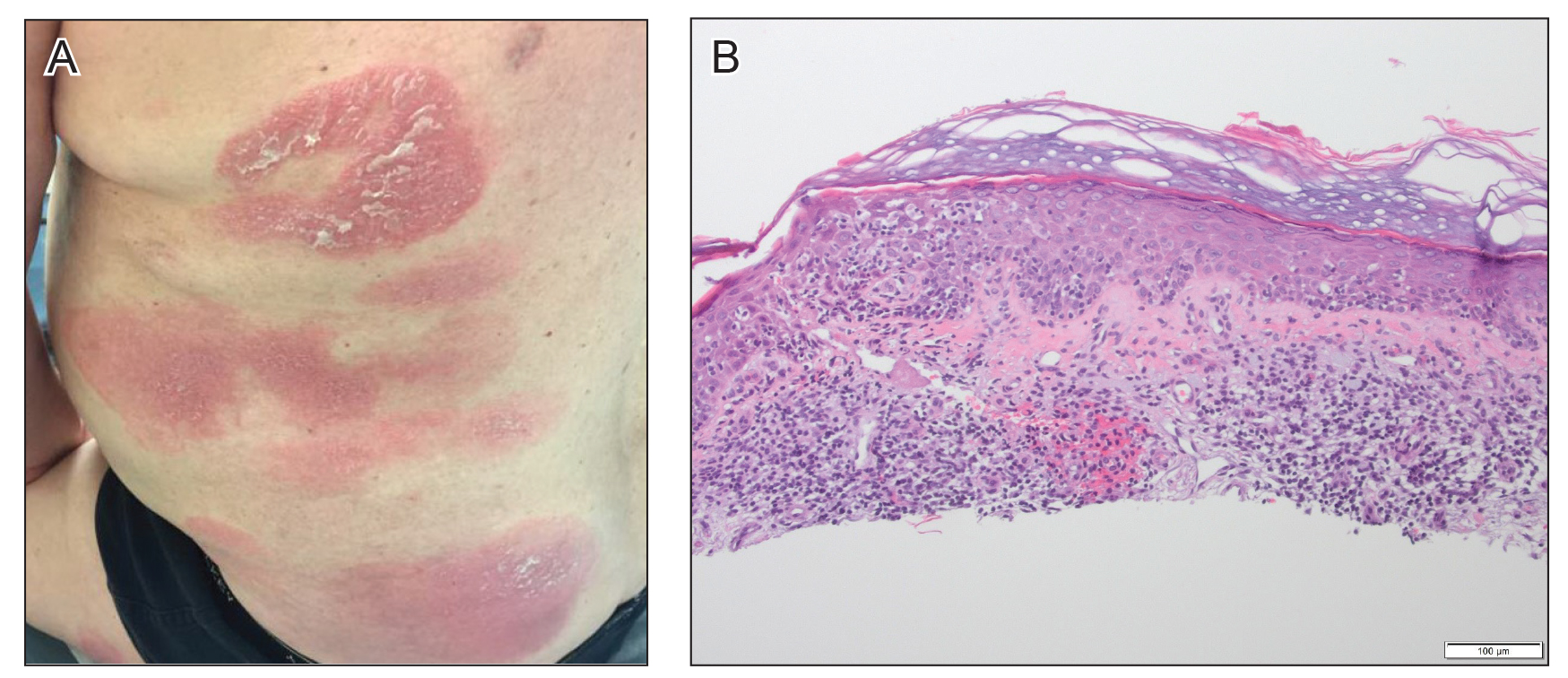
At approximately the same time as dupilumab was initiated, he was started on 60 mg daily of prednisone by his pulmonologist because of a history of restrictive lung disease of unknown cause. While taking prednisone, he experienced notable improvement in his skin condition; however, as he was slowly tapered off prednisone, he noted remarkable worsening of the dermatitis. Dupilumab was discontinued. Two more biopsies were performed; findings on both were consistent with mycosis fungoides (MF)(Figure 2).
Patient 2
A 52-year-old man presented with indurated, red, scaly plaques on the legs and arms. Initial biopsy was consistent with psoriasiform dermatitis that was thought to be due to a primarily eczematous process. Because of the clinical suspicion of psoriasis, the patient was at first treated with topical betamethasone and eventually was transitioned to multiple injectable biologics without improvement. There was no response to multiple psoriasis treatments, and the original pathology report was re-reviewed. The report noted a substantial eczematous component; therefore, a decision was made to transition him to dupilumab. He also was at first provided with a prednisone taper due to the severity of the cutaneous disease.
Initially, the patient noted 15% to 20% improvement; however, after 6 injections, dupilumab appeared to lose efficacy. Due to a lack of response to multiple biologic medications as well as dupilumab, another biopsy was performed. Findings were consistent with MF.
Patient 3
A 60-year-old woman with diffuse, pruritic, and erythematous dermatitis of 3 years’ duration was referred from an outside dermatology group. Prior biopsies were consistent with eczematous dermatitis. However, because 1 isolated plaque demonstrated findings consistent with psoriasis, she was started on guselkumab, which was discontinued after 12 weeks of therapy for lack of efficacy. The patient also had been treated with a short course of narrowband UVB and topical corticosteroids without benefit.
Upon initial evaluation in our clinic, there was concern for Sézary syndrome; however, peripheral blood studies were normal, and there was no monoclonal spike or irregularity in the patient’s Sézary flow cytometry panel. A biopsy demonstrated lichenoid dermatitis, possibly consistent with drug eruption. All supplements and likely medication culprits were discontinued without improvement.
Prior to follow-up in our clinic, the patient was again evaluated by an outside dermatologist and started on dupilumab. After 3 doses, she discontinued the medication because there was no improvement in the cutaneous symptoms. Findings on repeat biopsy following dupilumab treatment were consistent with MF.
Comment
Mycosis fungoides is a rare chronic T-cell lymphoma that can smolder for decades as nonspecific dermatitis before declaring itself fully on skin biopsy.2 In many cases, MF masquerades as eczema, psoriasis, contact dermatitis, or other dermatitides, and it often responds to the same medications, making diagnosis even more challenging. Treatment options include topical steroids, narrowband UVB, topical nitrogen mustard, topical carmustine, and bexarotene gel for early-stage disease.3 Although it cannot be determined which patients will progress, some do, and therapies must then be upgraded.
We reported 3 patients with adult-onset dermatitis and multiple biopsies demonstrating nondiagnostic findings, which, in retrospect, likely represented early smoldering CTCL. Each of these patients was treated with dupilumab because multiple biopsies demonstrated findings consistent with nondiagnostic dermatitis, along with a lack of response to standard therapies. In all 3 cases, however, the patients had no history of eczema or atopy. After starting dupilumab, each patient had an acute exacerbation of dermatitis; immediately thereafter, biopsies were consistent with CTCL.
These patients most likely had smoldering CTCL that expressed itself fully after dupilumab was started. Biologic medications and their effects on the immune system have been shown to have multiple unanticipated effects on the skin.4-6 We are not insinuating that dupilumab was the cause of our patients having developed CTCL, but we do propose that the underlying interplay of dupilumab with the immune system might have accelerated progression of underlying CTCL, resulting in the lymphoma presenting itself clinically and histopathologically. We also must mention that all 3 cases could represent a “true, true, and unrelated” phenomenon.
A proposed mechanism for how dupilumab might hasten progression of CTCL is based on a functional increase of IL-13 available for binding at the IL-13 receptor (IL-13R) α2 site following blockade of the IL-13Rα1 site by dupilumab (Figure 3). The pathway that is blocked by dupilumab provides improvement in AD by blocking the α subunit of the IL-4R, making it a receptor antagonist for both IL-4 and IL-13. The IL-4R forms a heterodimer with both γ c and separately with IL-13Rα1. As a result, IL-4 and IL-13 cannot bind to their respective targets; thus, downstream signaling that is required for AD is halted.7 IL-13, in addition to IL-4R, also binds to an IL-13Rα2. IL-13 and both of its receptors are upregulated in CTCL, particularly IL-13Rα2.8
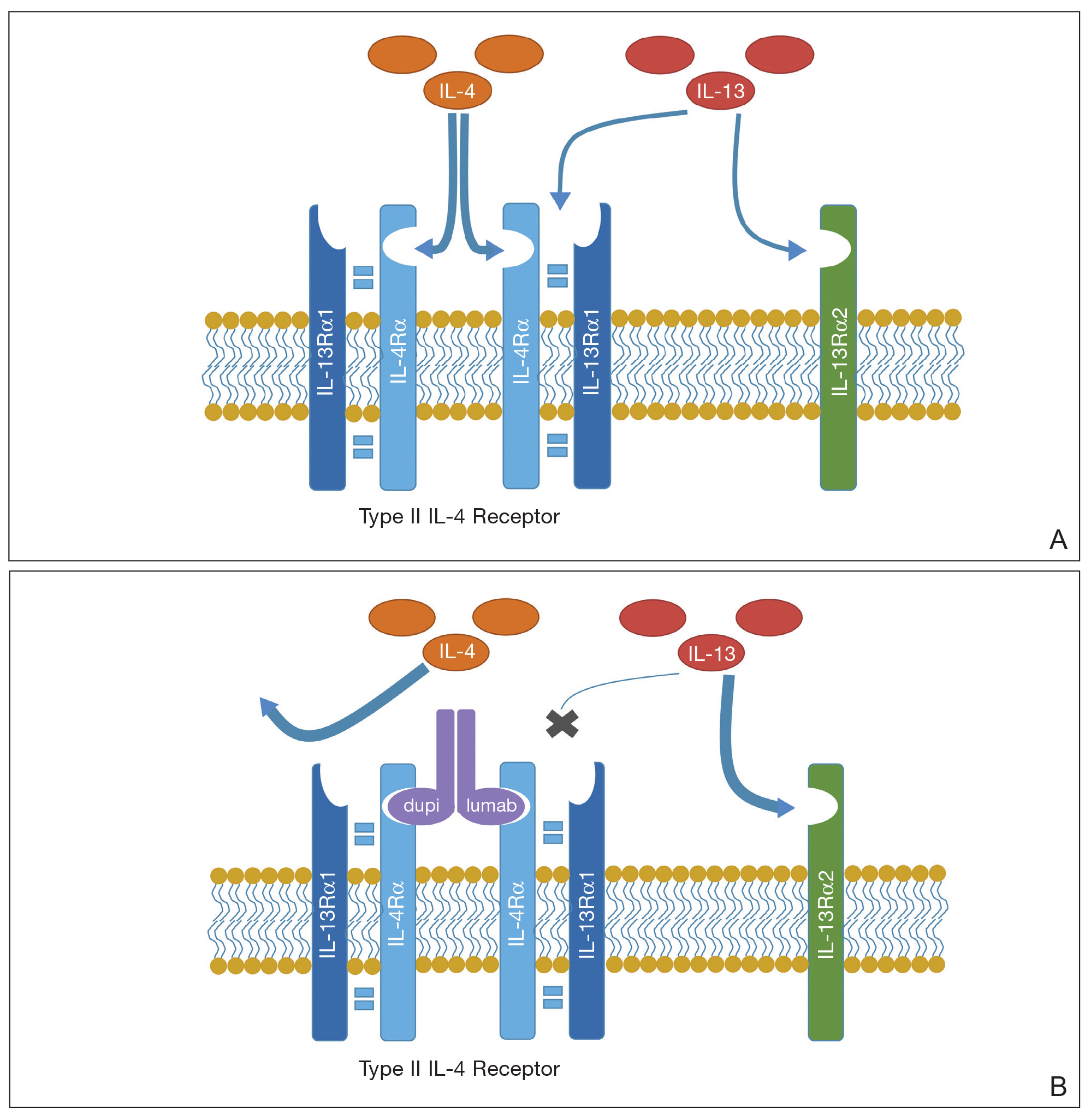
One of the principal ways that CTCL survives is through autocrine signaling, inducing more IL-13 and more IL-13Rα2, which is not seen in normal skin.8 Autocrine signaling plays a critical role in cancer activation and in providing self-sustaining growth signals to tumors.9 In addition, it has been documented that IL-13Rα2 has a higher affinity for IL-13 than the affinity of IL-13Rα1.10 As such, when the dupilumab receptor is blocked, our proposed mechanism of acceleration of CTCL is based on a functional increase in IL-13 available for binding at the IL-13Rα2 site, following indirect blockade of the α1 receptor with dupilumab, which effectively increases available IL-13 to be shunted down the tumorigenic pathway.
We recognize that this proposed mechanism is a theory; additionally, it should be noted that dupilumab is approved only for the treatment of AD and asthma. In our 3 cases, we used dupilumab off label in patients who did not have a clear case of AD or a childhood history of the disease.
When screening patients for the use of dupilumab, it is important to treat only those who have a classic history of moderate to severe AD, including itch, family history, and rash in the classic atopic distribution. We propose that these cases represent potential exacerbation of extant CTCL following exposure to dupilumab.
The manufacturer of dupilumab has reported 1 case of stage IV MF in a 57-year-old man 48 days after the first dose of dupilumab, leading to permanent discontinuation. The patient had ongoing disease at the time of the report, and the manufacturer stated that use of dupilumab was unrelated to disease.11 Studies are needed to explore any potential immunologic link between dupilumab and progression of CTCL.
- Raedler LA. Dupixent (dupilumab) first biologic drug approved for patients with moderate-to-severe atopic dermatitis. Am Health Drug Benefits. 2018;11:58-60.
- Skov AG, Gniadecki R. Delay in the histopathologic diagnosis of mycosis fungoides. Acta Derm Venereol. 2015;95:472-475.
- Ramsay DL, Meller JA, Zackheim HS. Topical treatment of early cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9:1031-1056.
- Mazloom SE, Yan D, Hu JZ, et al. TNF-α inhibitor-induced psoriasis: a decade of experience at the Cleveland Clinic [published online December 18, 2018]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2018.12.018.
- Tierney E, Kirthi S, Ramsay B, et al. Ustekinumab-induced subacute cutaneous lupus. JAAD Case Rep. 2019;5:271-273.
- Orrell KA, Murphrey M, Kelm RC, et al. Inflammatory bowel disease events after exposure to interleukin 17 inhibitors secukinumab and ixekizumab: postmarketing analysis from the RADAR (“Research on Adverse Drug events And Reports”) program. J Am Acad Dermatol. 2018;79:777-778.
- Sastre J, Dávila I. Dupilumab: a new paradigm for the treatment of allergic diseases. J Investig Allergol Clin Immunol. 2018;28:139-150.
- Geskin LJ, Viragova S, Stolz DB, et al. Interleukin-13 is over-expressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood. 2015;125:2798-2805.
- Barderas R, Bartolomé RA, Fernandez-Aceñero MJ, et al. High expression of IL-13 receptor α2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72:2780-2790.
- Andrews A-L, Holloway JW, Puddicombe SM, et al. Kinetic analysis of the interleukin-13 receptor complex. J Biol Chem. 2002;277:46073-46078.
- Data on file. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
Dupilumab is a novel medication that is approved by the US Food and Drug Administration to treat moderate to severe atopic dermatitis (AD) in patients 6 years and older. Dupilumab is an injectable fully human monoclonal antibody. It provides a giant leap toward a better quality of life for patients with AD. Dupilumab works by binding to the shared α subunit of the IL-4 receptor (IL-4R), thus inhibiting IL-4 and IL-13 from using that signaling pathway. The documented side-effect profile includes injection-site reaction, keratitis, nasopharyngitis, and headache.1
We initiated off-label treatment with dupilumab in 3 adult patients who had a history of long-standing adult-onset dermatitis confirmed by histopathology. The 3 patients received a loading dose of 600 mg subcutaneously, followed by 300 mg every other week. Following treatment, the patients had expansion of their disease, with features consistent with cutaneous T-cell lymphoma (CTCL) on subsequent biopsies. These 3 cases demonstrate the well-known adage that the diagnosis of CTCL often requires multiple biopsies performed over time. Although dupilumab has proved efficacious and safe for treating AD, dermatologists should be cautious before starting this medication in an adult who has new-onset dermatitis and no history of atopy.
Case Reports
Patient 1
A 61-year-old man presented to dermatology after being lost to follow-up for several years and was started on dupilumab for long-standing nonspecific eczematous dermatitis based on histopathology. He had a pruritic rash of 10 years’ duration that had been biopsied multiple times and was found to be consistent with dermatitis and lichen simplex chronicus (Figure 1). He had been treated with triamcinolone ointment 0.1% and narrowband UVB as often as 3 times weekly over many years. The patient also had a history of idiopathic CD4 lymphopenia with consistently negative tests for human immunodeficiency virus.

At approximately the same time as dupilumab was initiated, he was started on 60 mg daily of prednisone by his pulmonologist because of a history of restrictive lung disease of unknown cause. While taking prednisone, he experienced notable improvement in his skin condition; however, as he was slowly tapered off prednisone, he noted remarkable worsening of the dermatitis. Dupilumab was discontinued. Two more biopsies were performed; findings on both were consistent with mycosis fungoides (MF)(Figure 2).

Patient 2
A 52-year-old man presented with indurated, red, scaly plaques on the legs and arms. Initial biopsy was consistent with psoriasiform dermatitis that was thought to be due to a primarily eczematous process. Because of the clinical suspicion of psoriasis, the patient was at first treated with topical betamethasone and eventually was transitioned to multiple injectable biologics without improvement. There was no response to multiple psoriasis treatments, and the original pathology report was re-reviewed. The report noted a substantial eczematous component; therefore, a decision was made to transition him to dupilumab. He also was at first provided with a prednisone taper due to the severity of the cutaneous disease.
Initially, the patient noted 15% to 20% improvement; however, after 6 injections, dupilumab appeared to lose efficacy. Due to a lack of response to multiple biologic medications as well as dupilumab, another biopsy was performed. Findings were consistent with MF.
Patient 3
A 60-year-old woman with diffuse, pruritic, and erythematous dermatitis of 3 years’ duration was referred from an outside dermatology group. Prior biopsies were consistent with eczematous dermatitis. However, because 1 isolated plaque demonstrated findings consistent with psoriasis, she was started on guselkumab, which was discontinued after 12 weeks of therapy for lack of efficacy. The patient also had been treated with a short course of narrowband UVB and topical corticosteroids without benefit.
Upon initial evaluation in our clinic, there was concern for Sézary syndrome; however, peripheral blood studies were normal, and there was no monoclonal spike or irregularity in the patient’s Sézary flow cytometry panel. A biopsy demonstrated lichenoid dermatitis, possibly consistent with drug eruption. All supplements and likely medication culprits were discontinued without improvement.
Prior to follow-up in our clinic, the patient was again evaluated by an outside dermatologist and started on dupilumab. After 3 doses, she discontinued the medication because there was no improvement in the cutaneous symptoms. Findings on repeat biopsy following dupilumab treatment were consistent with MF.
Comment
Mycosis fungoides is a rare chronic T-cell lymphoma that can smolder for decades as nonspecific dermatitis before declaring itself fully on skin biopsy.2 In many cases, MF masquerades as eczema, psoriasis, contact dermatitis, or other dermatitides, and it often responds to the same medications, making diagnosis even more challenging. Treatment options include topical steroids, narrowband UVB, topical nitrogen mustard, topical carmustine, and bexarotene gel for early-stage disease.3 Although it cannot be determined which patients will progress, some do, and therapies must then be upgraded.
We reported 3 patients with adult-onset dermatitis and multiple biopsies demonstrating nondiagnostic findings, which, in retrospect, likely represented early smoldering CTCL. Each of these patients was treated with dupilumab because multiple biopsies demonstrated findings consistent with nondiagnostic dermatitis, along with a lack of response to standard therapies. In all 3 cases, however, the patients had no history of eczema or atopy. After starting dupilumab, each patient had an acute exacerbation of dermatitis; immediately thereafter, biopsies were consistent with CTCL.
These patients most likely had smoldering CTCL that expressed itself fully after dupilumab was started. Biologic medications and their effects on the immune system have been shown to have multiple unanticipated effects on the skin.4-6 We are not insinuating that dupilumab was the cause of our patients having developed CTCL, but we do propose that the underlying interplay of dupilumab with the immune system might have accelerated progression of underlying CTCL, resulting in the lymphoma presenting itself clinically and histopathologically. We also must mention that all 3 cases could represent a “true, true, and unrelated” phenomenon.
A proposed mechanism for how dupilumab might hasten progression of CTCL is based on a functional increase of IL-13 available for binding at the IL-13 receptor (IL-13R) α2 site following blockade of the IL-13Rα1 site by dupilumab (Figure 3). The pathway that is blocked by dupilumab provides improvement in AD by blocking the α subunit of the IL-4R, making it a receptor antagonist for both IL-4 and IL-13. The IL-4R forms a heterodimer with both γ c and separately with IL-13Rα1. As a result, IL-4 and IL-13 cannot bind to their respective targets; thus, downstream signaling that is required for AD is halted.7 IL-13, in addition to IL-4R, also binds to an IL-13Rα2. IL-13 and both of its receptors are upregulated in CTCL, particularly IL-13Rα2.8

One of the principal ways that CTCL survives is through autocrine signaling, inducing more IL-13 and more IL-13Rα2, which is not seen in normal skin.8 Autocrine signaling plays a critical role in cancer activation and in providing self-sustaining growth signals to tumors.9 In addition, it has been documented that IL-13Rα2 has a higher affinity for IL-13 than the affinity of IL-13Rα1.10 As such, when the dupilumab receptor is blocked, our proposed mechanism of acceleration of CTCL is based on a functional increase in IL-13 available for binding at the IL-13Rα2 site, following indirect blockade of the α1 receptor with dupilumab, which effectively increases available IL-13 to be shunted down the tumorigenic pathway.
We recognize that this proposed mechanism is a theory; additionally, it should be noted that dupilumab is approved only for the treatment of AD and asthma. In our 3 cases, we used dupilumab off label in patients who did not have a clear case of AD or a childhood history of the disease.
When screening patients for the use of dupilumab, it is important to treat only those who have a classic history of moderate to severe AD, including itch, family history, and rash in the classic atopic distribution. We propose that these cases represent potential exacerbation of extant CTCL following exposure to dupilumab.
The manufacturer of dupilumab has reported 1 case of stage IV MF in a 57-year-old man 48 days after the first dose of dupilumab, leading to permanent discontinuation. The patient had ongoing disease at the time of the report, and the manufacturer stated that use of dupilumab was unrelated to disease.11 Studies are needed to explore any potential immunologic link between dupilumab and progression of CTCL.
Dupilumab is a novel medication that is approved by the US Food and Drug Administration to treat moderate to severe atopic dermatitis (AD) in patients 6 years and older. Dupilumab is an injectable fully human monoclonal antibody. It provides a giant leap toward a better quality of life for patients with AD. Dupilumab works by binding to the shared α subunit of the IL-4 receptor (IL-4R), thus inhibiting IL-4 and IL-13 from using that signaling pathway. The documented side-effect profile includes injection-site reaction, keratitis, nasopharyngitis, and headache.1
We initiated off-label treatment with dupilumab in 3 adult patients who had a history of long-standing adult-onset dermatitis confirmed by histopathology. The 3 patients received a loading dose of 600 mg subcutaneously, followed by 300 mg every other week. Following treatment, the patients had expansion of their disease, with features consistent with cutaneous T-cell lymphoma (CTCL) on subsequent biopsies. These 3 cases demonstrate the well-known adage that the diagnosis of CTCL often requires multiple biopsies performed over time. Although dupilumab has proved efficacious and safe for treating AD, dermatologists should be cautious before starting this medication in an adult who has new-onset dermatitis and no history of atopy.
Case Reports
Patient 1
A 61-year-old man presented to dermatology after being lost to follow-up for several years and was started on dupilumab for long-standing nonspecific eczematous dermatitis based on histopathology. He had a pruritic rash of 10 years’ duration that had been biopsied multiple times and was found to be consistent with dermatitis and lichen simplex chronicus (Figure 1). He had been treated with triamcinolone ointment 0.1% and narrowband UVB as often as 3 times weekly over many years. The patient also had a history of idiopathic CD4 lymphopenia with consistently negative tests for human immunodeficiency virus.

At approximately the same time as dupilumab was initiated, he was started on 60 mg daily of prednisone by his pulmonologist because of a history of restrictive lung disease of unknown cause. While taking prednisone, he experienced notable improvement in his skin condition; however, as he was slowly tapered off prednisone, he noted remarkable worsening of the dermatitis. Dupilumab was discontinued. Two more biopsies were performed; findings on both were consistent with mycosis fungoides (MF)(Figure 2).

Patient 2
A 52-year-old man presented with indurated, red, scaly plaques on the legs and arms. Initial biopsy was consistent with psoriasiform dermatitis that was thought to be due to a primarily eczematous process. Because of the clinical suspicion of psoriasis, the patient was at first treated with topical betamethasone and eventually was transitioned to multiple injectable biologics without improvement. There was no response to multiple psoriasis treatments, and the original pathology report was re-reviewed. The report noted a substantial eczematous component; therefore, a decision was made to transition him to dupilumab. He also was at first provided with a prednisone taper due to the severity of the cutaneous disease.
Initially, the patient noted 15% to 20% improvement; however, after 6 injections, dupilumab appeared to lose efficacy. Due to a lack of response to multiple biologic medications as well as dupilumab, another biopsy was performed. Findings were consistent with MF.
Patient 3
A 60-year-old woman with diffuse, pruritic, and erythematous dermatitis of 3 years’ duration was referred from an outside dermatology group. Prior biopsies were consistent with eczematous dermatitis. However, because 1 isolated plaque demonstrated findings consistent with psoriasis, she was started on guselkumab, which was discontinued after 12 weeks of therapy for lack of efficacy. The patient also had been treated with a short course of narrowband UVB and topical corticosteroids without benefit.
Upon initial evaluation in our clinic, there was concern for Sézary syndrome; however, peripheral blood studies were normal, and there was no monoclonal spike or irregularity in the patient’s Sézary flow cytometry panel. A biopsy demonstrated lichenoid dermatitis, possibly consistent with drug eruption. All supplements and likely medication culprits were discontinued without improvement.
Prior to follow-up in our clinic, the patient was again evaluated by an outside dermatologist and started on dupilumab. After 3 doses, she discontinued the medication because there was no improvement in the cutaneous symptoms. Findings on repeat biopsy following dupilumab treatment were consistent with MF.
Comment
Mycosis fungoides is a rare chronic T-cell lymphoma that can smolder for decades as nonspecific dermatitis before declaring itself fully on skin biopsy.2 In many cases, MF masquerades as eczema, psoriasis, contact dermatitis, or other dermatitides, and it often responds to the same medications, making diagnosis even more challenging. Treatment options include topical steroids, narrowband UVB, topical nitrogen mustard, topical carmustine, and bexarotene gel for early-stage disease.3 Although it cannot be determined which patients will progress, some do, and therapies must then be upgraded.
We reported 3 patients with adult-onset dermatitis and multiple biopsies demonstrating nondiagnostic findings, which, in retrospect, likely represented early smoldering CTCL. Each of these patients was treated with dupilumab because multiple biopsies demonstrated findings consistent with nondiagnostic dermatitis, along with a lack of response to standard therapies. In all 3 cases, however, the patients had no history of eczema or atopy. After starting dupilumab, each patient had an acute exacerbation of dermatitis; immediately thereafter, biopsies were consistent with CTCL.
These patients most likely had smoldering CTCL that expressed itself fully after dupilumab was started. Biologic medications and their effects on the immune system have been shown to have multiple unanticipated effects on the skin.4-6 We are not insinuating that dupilumab was the cause of our patients having developed CTCL, but we do propose that the underlying interplay of dupilumab with the immune system might have accelerated progression of underlying CTCL, resulting in the lymphoma presenting itself clinically and histopathologically. We also must mention that all 3 cases could represent a “true, true, and unrelated” phenomenon.
A proposed mechanism for how dupilumab might hasten progression of CTCL is based on a functional increase of IL-13 available for binding at the IL-13 receptor (IL-13R) α2 site following blockade of the IL-13Rα1 site by dupilumab (Figure 3). The pathway that is blocked by dupilumab provides improvement in AD by blocking the α subunit of the IL-4R, making it a receptor antagonist for both IL-4 and IL-13. The IL-4R forms a heterodimer with both γ c and separately with IL-13Rα1. As a result, IL-4 and IL-13 cannot bind to their respective targets; thus, downstream signaling that is required for AD is halted.7 IL-13, in addition to IL-4R, also binds to an IL-13Rα2. IL-13 and both of its receptors are upregulated in CTCL, particularly IL-13Rα2.8

One of the principal ways that CTCL survives is through autocrine signaling, inducing more IL-13 and more IL-13Rα2, which is not seen in normal skin.8 Autocrine signaling plays a critical role in cancer activation and in providing self-sustaining growth signals to tumors.9 In addition, it has been documented that IL-13Rα2 has a higher affinity for IL-13 than the affinity of IL-13Rα1.10 As such, when the dupilumab receptor is blocked, our proposed mechanism of acceleration of CTCL is based on a functional increase in IL-13 available for binding at the IL-13Rα2 site, following indirect blockade of the α1 receptor with dupilumab, which effectively increases available IL-13 to be shunted down the tumorigenic pathway.
We recognize that this proposed mechanism is a theory; additionally, it should be noted that dupilumab is approved only for the treatment of AD and asthma. In our 3 cases, we used dupilumab off label in patients who did not have a clear case of AD or a childhood history of the disease.
When screening patients for the use of dupilumab, it is important to treat only those who have a classic history of moderate to severe AD, including itch, family history, and rash in the classic atopic distribution. We propose that these cases represent potential exacerbation of extant CTCL following exposure to dupilumab.
The manufacturer of dupilumab has reported 1 case of stage IV MF in a 57-year-old man 48 days after the first dose of dupilumab, leading to permanent discontinuation. The patient had ongoing disease at the time of the report, and the manufacturer stated that use of dupilumab was unrelated to disease.11 Studies are needed to explore any potential immunologic link between dupilumab and progression of CTCL.
- Raedler LA. Dupixent (dupilumab) first biologic drug approved for patients with moderate-to-severe atopic dermatitis. Am Health Drug Benefits. 2018;11:58-60.
- Skov AG, Gniadecki R. Delay in the histopathologic diagnosis of mycosis fungoides. Acta Derm Venereol. 2015;95:472-475.
- Ramsay DL, Meller JA, Zackheim HS. Topical treatment of early cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9:1031-1056.
- Mazloom SE, Yan D, Hu JZ, et al. TNF-α inhibitor-induced psoriasis: a decade of experience at the Cleveland Clinic [published online December 18, 2018]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2018.12.018.
- Tierney E, Kirthi S, Ramsay B, et al. Ustekinumab-induced subacute cutaneous lupus. JAAD Case Rep. 2019;5:271-273.
- Orrell KA, Murphrey M, Kelm RC, et al. Inflammatory bowel disease events after exposure to interleukin 17 inhibitors secukinumab and ixekizumab: postmarketing analysis from the RADAR (“Research on Adverse Drug events And Reports”) program. J Am Acad Dermatol. 2018;79:777-778.
- Sastre J, Dávila I. Dupilumab: a new paradigm for the treatment of allergic diseases. J Investig Allergol Clin Immunol. 2018;28:139-150.
- Geskin LJ, Viragova S, Stolz DB, et al. Interleukin-13 is over-expressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood. 2015;125:2798-2805.
- Barderas R, Bartolomé RA, Fernandez-Aceñero MJ, et al. High expression of IL-13 receptor α2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72:2780-2790.
- Andrews A-L, Holloway JW, Puddicombe SM, et al. Kinetic analysis of the interleukin-13 receptor complex. J Biol Chem. 2002;277:46073-46078.
- Data on file. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
- Raedler LA. Dupixent (dupilumab) first biologic drug approved for patients with moderate-to-severe atopic dermatitis. Am Health Drug Benefits. 2018;11:58-60.
- Skov AG, Gniadecki R. Delay in the histopathologic diagnosis of mycosis fungoides. Acta Derm Venereol. 2015;95:472-475.
- Ramsay DL, Meller JA, Zackheim HS. Topical treatment of early cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9:1031-1056.
- Mazloom SE, Yan D, Hu JZ, et al. TNF-α inhibitor-induced psoriasis: a decade of experience at the Cleveland Clinic [published online December 18, 2018]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2018.12.018.
- Tierney E, Kirthi S, Ramsay B, et al. Ustekinumab-induced subacute cutaneous lupus. JAAD Case Rep. 2019;5:271-273.
- Orrell KA, Murphrey M, Kelm RC, et al. Inflammatory bowel disease events after exposure to interleukin 17 inhibitors secukinumab and ixekizumab: postmarketing analysis from the RADAR (“Research on Adverse Drug events And Reports”) program. J Am Acad Dermatol. 2018;79:777-778.
- Sastre J, Dávila I. Dupilumab: a new paradigm for the treatment of allergic diseases. J Investig Allergol Clin Immunol. 2018;28:139-150.
- Geskin LJ, Viragova S, Stolz DB, et al. Interleukin-13 is over-expressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood. 2015;125:2798-2805.
- Barderas R, Bartolomé RA, Fernandez-Aceñero MJ, et al. High expression of IL-13 receptor α2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72:2780-2790.
- Andrews A-L, Holloway JW, Puddicombe SM, et al. Kinetic analysis of the interleukin-13 receptor complex. J Biol Chem. 2002;277:46073-46078.
- Data on file. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
Practice Points
- Dupilumab is a safe and effective treatment for atopic dermatitis (AD) in both children and adults.
- Prior to starting treatment for presumed adult-onset AD, consider smoldering cutaneous T-cell lymphoma (CTCL).
- Dupilumab may interact with the cutaneous immune system, leading to an expedited presentation of CTCL in patients with chronic adult-onset AD.
Chronic Diarrhea in an Adolescent Girl With a Genetic Skin Condition
The Diagnosis: Netherton Syndrome
Netherton syndrome (NS) is a rare autosomal-recessive disorder characterized by a clinical triad of ichthyosis linearis circumflexa; atopic diathesis; and hair shaft abnormalities, most classically trichorrhexis invaginata.1 Netherton syndrome is caused by a loss-of-function mutation in the serine peptidase inhibitor Kazal-type gene, SPINK5, which encodes LEKTI proteins and is found in all stratified epithelia as well as the thymus.2 A lack of functional LEKTI leads to the activation of a cascade of allergy and inflammation as well as uncontrolled proteolytic activity in the stratum corneum, which causes increased desquamation.1
Netherton syndrome presents with serpiginous or circinate scaling plaques with double-edged scale referred to as ichthyosis linearis circumflexa (quiz image). Skin plaques are intensely pruritic and migratory with fluctuating severity. Alternately, patients may have a generalized scaling erythroderma. Infants are at an especially high risk for recurrent infections, sepsis, hypernatremic dehydration, and failure to thrive.2
Netherton syndrome often gradually improves over time, though adults with NS usually have intensely pruritic, localized patches of redness, scaling, or ichthyosis linearis circumflexa. Lichenification and eczematous plaques of the popliteal and antecubital fossae also are common.1 Therapeutic options for NS include emollients, topical steroids, phototherapy, and intravenous immunoglobulin for severe cases.3 Because there is skin barrier dysfunction in NS, supratherapeutic serum levels of tacrolimus following topical application have been reported.4 Topical pimecrolimus has been demonstrated as an effective and safer application.5 Trichorrhexis invaginata (also known as bamboo hair) of the hair and eyebrows is a pathognomonic finding in NS, involving invagination of the distal hair shaft into the proximal shaft on light microscopy examination.1
Histopathology is variable and nonspecific with psoriasiform hyperplasia as the most frequent finding. Other histologic findings include incomplete keratinization of the epidermis, incomplete cornification with a severely reduced granular layer, and mild to moderate inflammatory dermal infiltrate.6 LEKTI immunostaining is confirmatory and shows the reduction or complete absence of LEKTI in the granular layer and inner root sheath of follicles.1 Patchy LEKTI staining would be suggestive of atopic dermatitis and psoriasis instead of NS.2
Atopic manifestations include angioedema, urticaria, and anaphylaxis, as well as chronic diarrhea or vomiting due to food allergies.1 Elevated IgE levels for staple foods (eg, milk, wheat), elevated total serum IgE, and eosinophilia frequently are seen.7 Biopsy of the esophagus and colon likely would show mucosal eosinophilia.7,8 Elimination of major food triggers through specific serum IgE testing and oral allergen desensitization can lead to the reduction of digestive symptoms.9 Cisapride and omeprazole are effective treatments for gastroesophageal reflux and poor feeding.8 Biopsy of the intestines in this patient likely would not have shown total villous atrophy, which is rare and primarily reported in infants with NS who have failure to thrive.10 There is a limited association between NS and intestinal metaplasia, intraepithelial lymphocytes, and bacterial overgrowth.
The primary morphology of dyskeratosis follicularis includes keratotic papules developing in sebaceous areas of the skin rather than scaly serpiginous plaques as seen in NS. Elastosis perforans serpiginosa is a perforating disorder seen in the context of several genetic conditions. It has a serpiginous appearance but, unlike NS, tends to be localized and features keratotic papules rather than patches with scale. Erythema marginatum is an uncommon feature of rheumatic fever and appears as pink annular macules and tends not to be pruritic. Subacute cutaneous lupus does feature scaly annular and serpiginous plaques but features trailing scale without the double-edge appearance of NS and is acquired rather than genetic.
- Hovnanian A. Netherton syndrome: skin inflammation and allergy by loss of protease inhibition. Cell Tissue Res. 2013;351:289-300.
- Bitoun E, Micheloni A, Lamant L, et al. LEKTI proteolytic processing in human primary keratinocytes, tissue distribution and defective expression in Netherton syndrome. Human Mol Genet. 2003;12:2417-2430.
- Yan AC, Honig PJ, Ming ME, et al. The safety and efficacy of pimecrolimus, 1%, cream for the treatment of Netherton syndrome: results from an exploratory study. Arch Dermatol. 2010;146:57-62.
- Shah KN, Yan AC. Low but detectable serum levels of tacrolimus seen with the use of very dilute, extemporaneously compounded formulations of tacrolimus ointment in the treatment of patients with Netherton syndrome. Arch Dermatol. 2006;142:1362-1363.
- Yan AC, Honig PJ, Ming ME, et al. The safety and efficacy of pimecrolimus, 1%, cream for the treatment of Netherton syndrome: results from an exploratory study. Arch Dermatol. 2010;146:57-62.
- Leclerc-Mercier S, Bodemer C, Furio L, et al. Skin biopsy in Netherton syndrome: a histological review of a large series and new findings. Am J Dermatopathol. 2016;38:83-91.
- Pauluel-Marmont C, Bellon N, Barbet P, et al. Eosinophilic esophagitis and colonic mucosal eosinophilia in Netherton syndrome. J Allergy Clin Immunol. 2017;139:2003-2005.e1.
- Hannula-Jouppi K, Laasanen SL, Heikkila H, et al. IgE allergen component-based profiling and atopic manifestations in patients with Netherton syndrome. J Allergy Clin Immunol. 2014;134:985-988.
- Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097-1102.
- Judge MR, Morgan G , Harper JI. A clinical and immunological study of Netherton's syndrome. Br J Dermatol. 1994;131:615-21.
The Diagnosis: Netherton Syndrome
Netherton syndrome (NS) is a rare autosomal-recessive disorder characterized by a clinical triad of ichthyosis linearis circumflexa; atopic diathesis; and hair shaft abnormalities, most classically trichorrhexis invaginata.1 Netherton syndrome is caused by a loss-of-function mutation in the serine peptidase inhibitor Kazal-type gene, SPINK5, which encodes LEKTI proteins and is found in all stratified epithelia as well as the thymus.2 A lack of functional LEKTI leads to the activation of a cascade of allergy and inflammation as well as uncontrolled proteolytic activity in the stratum corneum, which causes increased desquamation.1
Netherton syndrome presents with serpiginous or circinate scaling plaques with double-edged scale referred to as ichthyosis linearis circumflexa (quiz image). Skin plaques are intensely pruritic and migratory with fluctuating severity. Alternately, patients may have a generalized scaling erythroderma. Infants are at an especially high risk for recurrent infections, sepsis, hypernatremic dehydration, and failure to thrive.2
Netherton syndrome often gradually improves over time, though adults with NS usually have intensely pruritic, localized patches of redness, scaling, or ichthyosis linearis circumflexa. Lichenification and eczematous plaques of the popliteal and antecubital fossae also are common.1 Therapeutic options for NS include emollients, topical steroids, phototherapy, and intravenous immunoglobulin for severe cases.3 Because there is skin barrier dysfunction in NS, supratherapeutic serum levels of tacrolimus following topical application have been reported.4 Topical pimecrolimus has been demonstrated as an effective and safer application.5 Trichorrhexis invaginata (also known as bamboo hair) of the hair and eyebrows is a pathognomonic finding in NS, involving invagination of the distal hair shaft into the proximal shaft on light microscopy examination.1
Histopathology is variable and nonspecific with psoriasiform hyperplasia as the most frequent finding. Other histologic findings include incomplete keratinization of the epidermis, incomplete cornification with a severely reduced granular layer, and mild to moderate inflammatory dermal infiltrate.6 LEKTI immunostaining is confirmatory and shows the reduction or complete absence of LEKTI in the granular layer and inner root sheath of follicles.1 Patchy LEKTI staining would be suggestive of atopic dermatitis and psoriasis instead of NS.2
Atopic manifestations include angioedema, urticaria, and anaphylaxis, as well as chronic diarrhea or vomiting due to food allergies.1 Elevated IgE levels for staple foods (eg, milk, wheat), elevated total serum IgE, and eosinophilia frequently are seen.7 Biopsy of the esophagus and colon likely would show mucosal eosinophilia.7,8 Elimination of major food triggers through specific serum IgE testing and oral allergen desensitization can lead to the reduction of digestive symptoms.9 Cisapride and omeprazole are effective treatments for gastroesophageal reflux and poor feeding.8 Biopsy of the intestines in this patient likely would not have shown total villous atrophy, which is rare and primarily reported in infants with NS who have failure to thrive.10 There is a limited association between NS and intestinal metaplasia, intraepithelial lymphocytes, and bacterial overgrowth.
The primary morphology of dyskeratosis follicularis includes keratotic papules developing in sebaceous areas of the skin rather than scaly serpiginous plaques as seen in NS. Elastosis perforans serpiginosa is a perforating disorder seen in the context of several genetic conditions. It has a serpiginous appearance but, unlike NS, tends to be localized and features keratotic papules rather than patches with scale. Erythema marginatum is an uncommon feature of rheumatic fever and appears as pink annular macules and tends not to be pruritic. Subacute cutaneous lupus does feature scaly annular and serpiginous plaques but features trailing scale without the double-edge appearance of NS and is acquired rather than genetic.
The Diagnosis: Netherton Syndrome
Netherton syndrome (NS) is a rare autosomal-recessive disorder characterized by a clinical triad of ichthyosis linearis circumflexa; atopic diathesis; and hair shaft abnormalities, most classically trichorrhexis invaginata.1 Netherton syndrome is caused by a loss-of-function mutation in the serine peptidase inhibitor Kazal-type gene, SPINK5, which encodes LEKTI proteins and is found in all stratified epithelia as well as the thymus.2 A lack of functional LEKTI leads to the activation of a cascade of allergy and inflammation as well as uncontrolled proteolytic activity in the stratum corneum, which causes increased desquamation.1
Netherton syndrome presents with serpiginous or circinate scaling plaques with double-edged scale referred to as ichthyosis linearis circumflexa (quiz image). Skin plaques are intensely pruritic and migratory with fluctuating severity. Alternately, patients may have a generalized scaling erythroderma. Infants are at an especially high risk for recurrent infections, sepsis, hypernatremic dehydration, and failure to thrive.2
Netherton syndrome often gradually improves over time, though adults with NS usually have intensely pruritic, localized patches of redness, scaling, or ichthyosis linearis circumflexa. Lichenification and eczematous plaques of the popliteal and antecubital fossae also are common.1 Therapeutic options for NS include emollients, topical steroids, phototherapy, and intravenous immunoglobulin for severe cases.3 Because there is skin barrier dysfunction in NS, supratherapeutic serum levels of tacrolimus following topical application have been reported.4 Topical pimecrolimus has been demonstrated as an effective and safer application.5 Trichorrhexis invaginata (also known as bamboo hair) of the hair and eyebrows is a pathognomonic finding in NS, involving invagination of the distal hair shaft into the proximal shaft on light microscopy examination.1
Histopathology is variable and nonspecific with psoriasiform hyperplasia as the most frequent finding. Other histologic findings include incomplete keratinization of the epidermis, incomplete cornification with a severely reduced granular layer, and mild to moderate inflammatory dermal infiltrate.6 LEKTI immunostaining is confirmatory and shows the reduction or complete absence of LEKTI in the granular layer and inner root sheath of follicles.1 Patchy LEKTI staining would be suggestive of atopic dermatitis and psoriasis instead of NS.2
Atopic manifestations include angioedema, urticaria, and anaphylaxis, as well as chronic diarrhea or vomiting due to food allergies.1 Elevated IgE levels for staple foods (eg, milk, wheat), elevated total serum IgE, and eosinophilia frequently are seen.7 Biopsy of the esophagus and colon likely would show mucosal eosinophilia.7,8 Elimination of major food triggers through specific serum IgE testing and oral allergen desensitization can lead to the reduction of digestive symptoms.9 Cisapride and omeprazole are effective treatments for gastroesophageal reflux and poor feeding.8 Biopsy of the intestines in this patient likely would not have shown total villous atrophy, which is rare and primarily reported in infants with NS who have failure to thrive.10 There is a limited association between NS and intestinal metaplasia, intraepithelial lymphocytes, and bacterial overgrowth.
The primary morphology of dyskeratosis follicularis includes keratotic papules developing in sebaceous areas of the skin rather than scaly serpiginous plaques as seen in NS. Elastosis perforans serpiginosa is a perforating disorder seen in the context of several genetic conditions. It has a serpiginous appearance but, unlike NS, tends to be localized and features keratotic papules rather than patches with scale. Erythema marginatum is an uncommon feature of rheumatic fever and appears as pink annular macules and tends not to be pruritic. Subacute cutaneous lupus does feature scaly annular and serpiginous plaques but features trailing scale without the double-edge appearance of NS and is acquired rather than genetic.
- Hovnanian A. Netherton syndrome: skin inflammation and allergy by loss of protease inhibition. Cell Tissue Res. 2013;351:289-300.
- Bitoun E, Micheloni A, Lamant L, et al. LEKTI proteolytic processing in human primary keratinocytes, tissue distribution and defective expression in Netherton syndrome. Human Mol Genet. 2003;12:2417-2430.
- Yan AC, Honig PJ, Ming ME, et al. The safety and efficacy of pimecrolimus, 1%, cream for the treatment of Netherton syndrome: results from an exploratory study. Arch Dermatol. 2010;146:57-62.
- Shah KN, Yan AC. Low but detectable serum levels of tacrolimus seen with the use of very dilute, extemporaneously compounded formulations of tacrolimus ointment in the treatment of patients with Netherton syndrome. Arch Dermatol. 2006;142:1362-1363.
- Yan AC, Honig PJ, Ming ME, et al. The safety and efficacy of pimecrolimus, 1%, cream for the treatment of Netherton syndrome: results from an exploratory study. Arch Dermatol. 2010;146:57-62.
- Leclerc-Mercier S, Bodemer C, Furio L, et al. Skin biopsy in Netherton syndrome: a histological review of a large series and new findings. Am J Dermatopathol. 2016;38:83-91.
- Pauluel-Marmont C, Bellon N, Barbet P, et al. Eosinophilic esophagitis and colonic mucosal eosinophilia in Netherton syndrome. J Allergy Clin Immunol. 2017;139:2003-2005.e1.
- Hannula-Jouppi K, Laasanen SL, Heikkila H, et al. IgE allergen component-based profiling and atopic manifestations in patients with Netherton syndrome. J Allergy Clin Immunol. 2014;134:985-988.
- Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097-1102.
- Judge MR, Morgan G , Harper JI. A clinical and immunological study of Netherton's syndrome. Br J Dermatol. 1994;131:615-21.
- Hovnanian A. Netherton syndrome: skin inflammation and allergy by loss of protease inhibition. Cell Tissue Res. 2013;351:289-300.
- Bitoun E, Micheloni A, Lamant L, et al. LEKTI proteolytic processing in human primary keratinocytes, tissue distribution and defective expression in Netherton syndrome. Human Mol Genet. 2003;12:2417-2430.
- Yan AC, Honig PJ, Ming ME, et al. The safety and efficacy of pimecrolimus, 1%, cream for the treatment of Netherton syndrome: results from an exploratory study. Arch Dermatol. 2010;146:57-62.
- Shah KN, Yan AC. Low but detectable serum levels of tacrolimus seen with the use of very dilute, extemporaneously compounded formulations of tacrolimus ointment in the treatment of patients with Netherton syndrome. Arch Dermatol. 2006;142:1362-1363.
- Yan AC, Honig PJ, Ming ME, et al. The safety and efficacy of pimecrolimus, 1%, cream for the treatment of Netherton syndrome: results from an exploratory study. Arch Dermatol. 2010;146:57-62.
- Leclerc-Mercier S, Bodemer C, Furio L, et al. Skin biopsy in Netherton syndrome: a histological review of a large series and new findings. Am J Dermatopathol. 2016;38:83-91.
- Pauluel-Marmont C, Bellon N, Barbet P, et al. Eosinophilic esophagitis and colonic mucosal eosinophilia in Netherton syndrome. J Allergy Clin Immunol. 2017;139:2003-2005.e1.
- Hannula-Jouppi K, Laasanen SL, Heikkila H, et al. IgE allergen component-based profiling and atopic manifestations in patients with Netherton syndrome. J Allergy Clin Immunol. 2014;134:985-988.
- Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097-1102.
- Judge MR, Morgan G , Harper JI. A clinical and immunological study of Netherton's syndrome. Br J Dermatol. 1994;131:615-21.
A 17-year-old adolescent girl visited our clinic to establish care for her genetic skin condition. She exhibited red scaly plaques and patches over much of the body surface area consistent with atopic dermatitis but also had areas on the trunk with serpiginous red plaques with scale on the leading and trailing edges. She also noted fragile hair with sparse eyebrows. The patient reported that she had experienced chronic diarrhea and abdominal pain since childhood. She asked if it could be related to her genetic condition.