User login
Update on Noninvasive Body Contouring Techniques
In today’s society there is a ubiquitous pressure to lose weight, reduce fat, and rejuvenate the skin that stems not only from images of idealized bodies in the media but also from our growing knowledge of the detrimental effects of obesity. Along with diet and exercise, it has become popular to use noninvasive devices to attain these goals by means of body contouring—the optimization of the definition, smoothness, and shape of the human physique.1 In fact, body contouring currently is the fastest-growing area of cosmetic dermatology.2
Previously, body contouring primarily involved invasive procedures (eg, liposuction) that are associated with various adverse effects, financial costs, and lengthy downtime.3 More recently, a growing demand for safer and less painful procedures for adipose tissue reduction and skin tightening have led to the development of several novel modalities for noninvasive body contouring. Although the results achieved using these new technologies may be less dramatic than invasive techniques and are not immediate, they do not carry the risks and adverse effects that are associated with surgical procedures and therefore are increasingly requested by cosmetic patients.4,5 New noninvasive techniques primarily target the physical properties of fat, resulting in an efflux of triglycerides from fat cells, causing either reduced size, necrosis, or apoptosis of adipocytes.3,6 Of these modalities, cold-induced adipocyte apoptosis has been commercially available the longest and has been the most researched; however, other noninvasive body contouring techniques have been increasingly explored by researchers since the first reports of human adipose tissue explants exhibiting features of apoptosis after heat injury became available.7,8
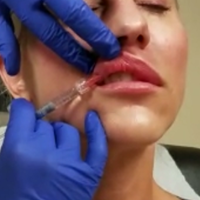
There currently are 4 leading modalities used for noninvasive body contouring: cryolipolysis, radiofrequency (RF), high-intensity focused ultrasound (HIFU), and laser therapy (Table). Although no procedure has yet been accepted as the gold standard, investigators are working to determine which technique is the most effective.9 In this article, we provide an overview of these techniques to help dermatologists choose appropriate modalities for their cosmetic patients.
Cryolipolysis
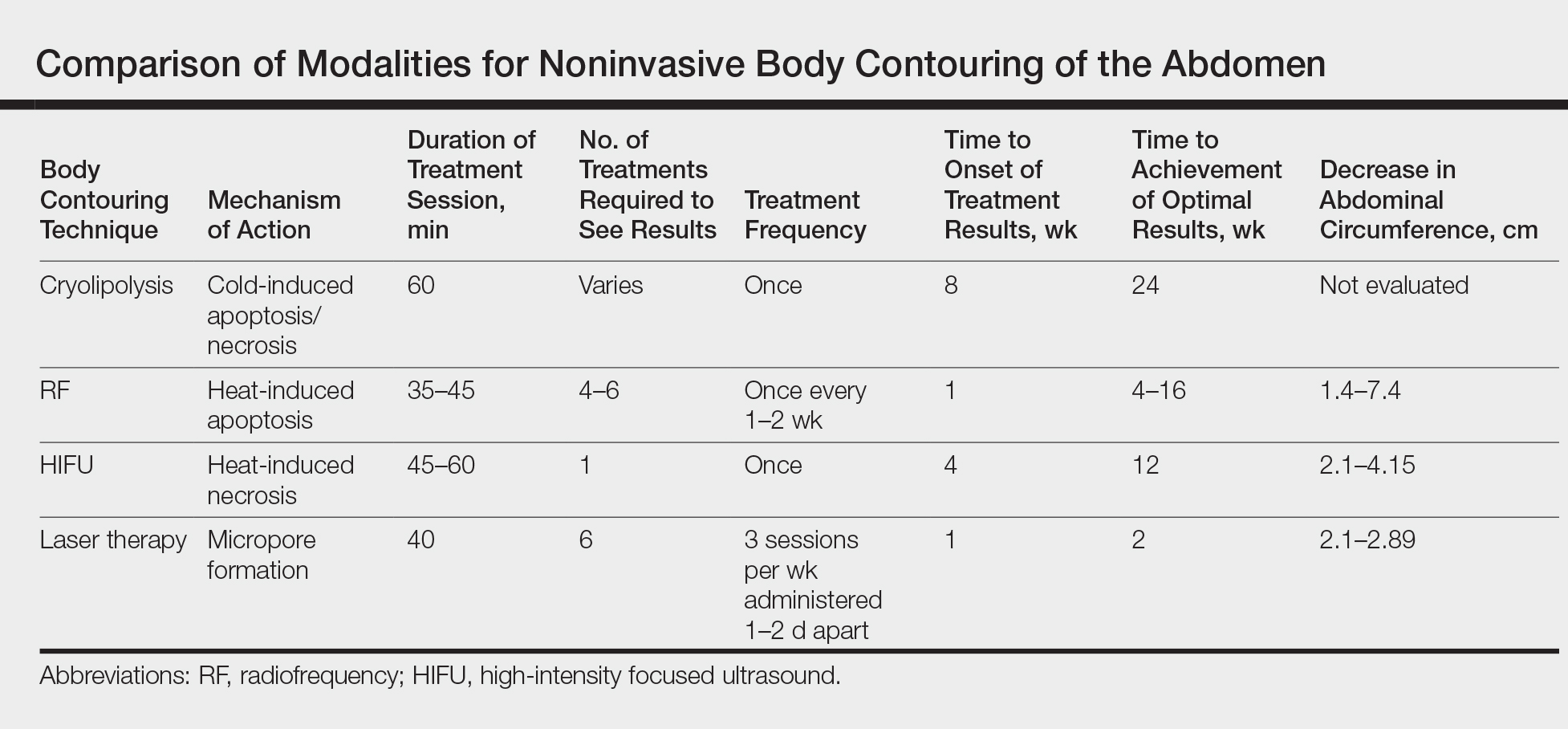
Cryolipolysis is unique in that it employs the principle that lipid-rich adipocytes are more susceptible to freezing than surrounding water-rich cells, allowing selective apoptosis while preserving the adjacent structures. As macrophages digest the apoptotic adipocytes, patients experience a decrease in subcutaneous fat volume over the subsequent 2 to 3 months.10-13 Cryolipolysis has been gaining popularity since 2010, when it was first approved by the US Food and Drug Administration (FDA) for fat reduction in the flank areas; it was later approved for the abdomen in 2012, thighs in 2014, and submental area in 2015.14 Most recently, cryolipolysis was approved for fat reduction in the arms, back, and buttocks in 2016.
The most popular cryolipolysis device applies suction to the treatment area and vacuums the tissue between 2 cooling panels for 30 to 60 minutes.9 Clinical studies investigating the safety and efficacy of cryolipolysis have reported a high degree of patient satisfaction with the procedure and only minimal side effects.4,6,15,16 Common complications of cryolipolysis include erythema, swelling, and sensitivity at the treatment site followed by a lesser incidence of pain, tingling, and bruising, all of which generally resolve within a few weeks of treatment.6 With the removal of adipocytes, there has been concern regarding elevations in blood lipid levels and liver enzymes; however, these laboratory values have been reported to remain within normal limits during and after cryolipolysis.17,18 Of note, patients should be advised of the risk of paradoxical adipose hyperplasia, a rare side effect of cryolipolysis in which a large, demarcated, tender fat mass develops at the treatment site 2 to 3 months after treatment, with an estimated incidence of 1 in 20,000.19 However, the incidence of paradoxical adipose hyperplasia may be underestimated, as a single practice reported an incidence of 0.47% in 422 cryolipolysis treatments.20 This complication has not been associated with any of the heat-induced fat reduction modalities.
Cryolipolysis has been found to be safe for all skin types with no reported pigmentary changes.16 It should not be performed in patients with cold-induced conditions (eg, cryoglobulinemia, cold urticaria) or in those with severe varicose veins or atopic dermatitis.21,22 Patients benefitting most from this procedure are those who require only small or moderate amounts of adipose tissue and cellulite removal with separate fat bulges.12,17 Interestingly, cryolipolysis also has been used off label to treat pseudogynecomastia in male patients.23
Radiofrequency
Radiofrequency has become an important and frequently used modality in cosmetic dermatology.24 This modality differs from cryolipolysis in that it relies on exploiting the difference in water content and impedance between tissues: the skin has low impedance, whereas fat tissue has high impedance. Radiofrequency induces thermal injury to targeted tissue layers, rather than the cold-induced damage seen in cryolipolysis, through devices that focus thermal energy on tissues with high impedance, inducing apoptosis of cells in the subcutaneous adipose tissue with minimal risk of damaging the epidermis, dermis, and muscle.9,25 Ultimately, thermal exposure to 43°C to 45°C over several minutes results in a delayed adipocyte death response.4 In addition to adipocyte death, RF has been shown to cause denaturation of collagen fibrils, leading to subsequent remodeling, neocollagenesis, and skin tightening.26
Radiofrequency devices can be broadly classified as monopolar or bipolar.24,27 Bipolar devices generally require more frequent treatments, whereas monopolar devices tend to require fewer treatment sessions with superior circumference and fat reduction.28
Overall, RF devices have a favorable side effect profile. The most common side effects are erythema and edema at the treatment site lasting less than 24 hours after the procedure.25 The absence of complications such as abdominal discomfort, erythema, and burning during treatment have been reported,27 with the exception of 1 case of hyperesthesia on the abdomen that lasted for 3 days after a treatment session.5 Although RF has beneficial effects on circumference reduction in the abdomen and thighs and can improve the appearance of cellulite, an increase in body weight may occur during treatment. When a localized area of fat such as the thigh is targeted for treatment but the remaining fat cells in the body are not affected, the remaining cells can continue to grow and expand; for instance, although fat cells destroyed with RF will not continue to expand, fat cells in untreated areas may continue to grow due to continued weight gain (eg, from excessive eating), leading to overall weight gain. Thus, patients must understand that weight gain is not an indication of treatment failure after RF or any other method of irreversible fat destruction.5
High-intensity Focused Ultrasound
High-intensity focused ultrasound recently was introduced as a new treatment modality for body contouring, specifically for skin tightening and rejuvenation.5 The mechanism of HIFU is similar to that of RF in that it also relies on heat to cause adipocyte apoptosis; however, it utilizes acoustic energy rather than electric energy. High-intensity focused ultrasound devices can deliver energy to the deep dermis, subdermal connective tissue, and fibromuscular layers in precise microcoagulation zones without damage to the epidermis. The focused energy induces a high temperature (>65°C) within 1 to 3 seconds, causing cell protein coagulation in the targeted area. In addition to its thermal effects, HIFU induces a mechanical effect that disrupts cell membranes immediately, which contributes to the coagulation necrosis process, further promoting necrosis and apoptosis. The effects of these devices can be visualized, as there always is a sharp demarcation between the targeted and untargeted tissue.29 Additionally, microcoagulation is thought to cause gradual skin tightening through collagen contraction and remodeling.30
High-intensity focused ultrasound first received FDA approval for eyebrow lifting and has been used safely and effectively to treat facial and neck skin in a variety of skin types as well as to improve the clinical appearance of the abdomen and thighs.31 This technique is best suited for patients with mild to moderate laxity of the skin or soft tissue who have a body mass index less than 30 kg/m2 and are seeking mild body contouring.32 The ideal patient is young with normal wound healing, since the clinical response to treatment is partly dependent on new collagen synthesis.33 Older patients with extensive photoaging or severe skin laxity are not good candidates for HIFU.
There are a variety of available HIFU devices,34 which utilize special transducers that direct ultrasound energy to a small focal point in the subcutaneous tissues that harmlessly passes through the skin.35 By using newly developed transducers with different energy outputs and focal depths, dermatologists can tailor HIFU treatment to meet the unique physical characteristics of each patient.31
Adverse effects of HIFU are limited to transient pain in most patients and occasional erythema and ecchymosis in some cases.31 In general, most adverse effects resolve spontaneously within 4 weeks and all by 12 weeks posttreatment. Studies also have reported hard subcutaneous nodules, discomfort, burning sensation, mild blisters, and one case of purpuric lesions, all at the treatment site.36-39 There is no evidence that HIFU can cause abnormalities in serum lipids or liver function tests.
Lasers
Laser technology is a rapidly growing modality in noninvasive body contouring. A novel device recently emerged as the first and only FDA-cleared hyperthermic laser for fat reduction and noninvasive body contouring of the abdomen, flanks, back, inner and outer thighs, and submental area.40,41 The device is a 1060-nm diode laser that uses thermal energy to destroy adipose tissue, leading to permanent reduction in stubborn fat without surgery or downtime through the use of a flat, nonsuction applicator that is designed for consistent, natural-looking results. The device includes a contact cooling system that helps to limit thermal discomfort and prevent damage to the surface of the skin during the procedure. Initial improvement can be seen as quickly as 6 weeks posttreatment, and optimal results usually occur in as few as 12 weeks. This device was found to have an excellent safety profile and was well tolerated among patients, with only mild pain reported.42,43
Prior to the development of this new 1060-nm diode laser, the initial application of lasers for noninvasive body contouring involved low-level laser therapy (LLLT), also known as cold laser therapy.40 One device has 5 rotating diode laser heads that work at a wavelength of 635 nm. Treatment sessions last up to 30 minutes, and 6 to 8 sessions are required to obtain optimal results. Low-level laser therapy is a unique modality that is not based on thermal tissue damage, but rather on producing transient microscopic pores in adipocytes that allow lipids to leak out, leading to fat reduction.34 Because LLLT causes immediate emptying of targeted adipocytes, results are noticeable as soon as treatment is completed; however, there is no necrosis or apoptosis of adipocytes, so the recurrence of fat deposition is believed to be greater when compared to the other modalities. Because the results are temporary, long-term or permanent results should not be expected with LLLT. Depending on the patient’s goals, the temporary nature of the results can be either an advantage or disadvantage: some may prefer immediate results despite gradual diminishment over subsequent months, whereas others may prefer results that progressively increase over time and are more permanent, as seen with cryolipolysis, HIFU, and RF.3
Complications of LLLT generally are fewer and more mild than with all other body contouring procedures, with several studies reporting no adverse effects.44-48 Others reported swelling or erythema at the treatment area, pain or tingling during treatment, and increased urination, all of which were temporary and resolved spontaneously.49 Additionally, although the lipids released from treatment are cleared through the lymphatic system, LLLT has not been shown to increase serum lipid levels.50
Conclusion
The field of noninvasive body contouring is undoubtedly growing and will likely continue to rise in popularity as the efficacy and safety of these treatments improve. Although the available technologies vary by mechanism and side effect profiles, several devices have been revealed to be safe and effective in reducing subcutaneous fat tissue and improving skin laxity.1 However, additional studies are needed to evaluate these devices in a standardized manner, especially considering the high costs associated with treatment.32 Current studies investigating these devices vary in treatment protocol, treatment area, number and timing of follow-up sessions, and outcome measures, making it challenging to compare the results objectively.3 Dermatologists offering body contouring treatments need to be intimately familiar with the available devices and determine which treatment is appropriate for each patient in order to provide the highest quality care. Most importantly, patients and physicians must discuss individual goals when choosing a body-contouring method in order to maximize patient satisfaction.
- Jalian HR, Avram MM. Body contouring: the skinny on noninvasive fat removal. Semin Cutan Med Surg. 2012;31:121-125.
- Ho D, Jagdeo J. A systematic review of paradoxical adipose hyperplasia (PAH) post-cryolipolysis. J Drugs Dermatol. 2017;16:62-67.
- Kennedy J, Verne S, Griffith R, et al. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol. 2015;29:1679-1688.
- Krueger N, Mai SV, Luebberding S, et al. Cryolipolysis for noninvasive body contouring: clinical efficacy and patient satisfaction. Clin Cosmet Investig Dermatol. 2014;7:201-205.
- Suh DH, Kim CM, Lee SJ, et al. Safety and efficacy of a non-contact radiofrequency device for body contouring in Asians. J Cosmet Laser Ther. 2017;19:89-92.
- Ingargiola MJ, Motakef S, Chung MT, et al. Cryolipolysis for fat reduction and body contouring: safety and efficacy of current treatment paradigms. Plast Reconstr Surg. 2015;135:1581-1590.
- Prins JB, Walker NI, Winterford CM, et al. Apoptosis of human adipocytes in vitro. Biochem Biophys Res Commun. 1994;201:500-507.
- Sorisky A, Magun R, Gagnon AM. Adipose cell apoptosis: death in the energy depot. Int J Obes Relat Metab Disord. 2000;24(suppl 4):S3-S7.
- Chilukuri S, Mueller G. “Hands-free” noninvasive body contouring devices: review of effectiveness and patient satisfaction. J Drugs Dermatol. 2016;15:1402-1406.
- Manstein D, Laubach H, Watanabe K, et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595-604.
- Zelickson B, Egbert BM, Preciado J, et al. Cryolipolysis for noninvasive fat cell destruction: initial results from a pig model. Dermatol Surg. 2009;35:1462-1470.
- Nelson AA, Wasserman D, Avram MM. Cryolipolysis for reduction of excess adipose tissue. Semin Cutan Med Surg. 2009;28:244-249.
- Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med. 2009;41:703-708.
- Klein KB, Bachelor EP, Becker EV, et al. Multiple same day cryolipolysis treatments for the reduction of subcutaneous fat are safe and do not affect serum lipid levels or liver function tests. Lasers Surg Med. 2017;49:640-644.
- Dierickx CC, Mazer JM, Sand M, et al. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg. 2013;39:1209-1216.
- Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J. 2013;33:835-846.
- Ferraro GA, De Francesco F, Cataldo C, et al. Synergistic effects of cryolipolysis and shock waves for noninvasive body contouring. Aesthetic Plast Surg. 2012;36:666-679.
- Lee KR. Clinical efficacy of fat reduction on the thigh of Korean women through cryolipolysis. J Obes Weight Loss Ther. 2013;3:203.
- Jalian HR, Avram MM, Garibyan L, et al. Paradoxical adipose hyperplasia after cryolipolysis. JAMA Dermatol. 2014;150:317-319.
- Singh SM, Geddes ER, Boutrous SG, et al. Paradoxical adipose hyperplasia secondary to cryolipolysis: an underreported entity? Lasers Surg Med. 2015;47:476-478.
- Pinto H, Arredondo E, Ricart-Jane D. Evaluation of adipocytic changes after a simil-lipocryolysis stimulus. Cryo Letters. 2013;34:100-105.
- Pinto HR, Garcia-Cruz E, Melamed GE. A study to evaluate the action of lipocryolysis. Cryo Letters. 2012;33:177-181.
- Singh B, Keaney T, Rossi AM. Male body contouring. J Drugs Dermatol. 2015;14:1052-1059.
- Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32:79-90.
- Weiss R, Weiss M, Beasley K, et al. Operator independent focused high frequency ISM band for fat reduction: porcine model. Lasers Surg Med. 2013;45:235-239.
- Hantash BM, Ubeid AA, Chang H, et al. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009;41:1-9.
- Harth Y. Painless, safe, and efficacious noninvasive skin tightening, body contouring, and cellulite reduction using multisource 3DEEP radiofrequency. J Cosmet Dermatol. 2015;14:70-75.
- Nassab R. The evidence behind noninvasive body contouring devices. Aesthet Surg J. 2015;35:279-293.
- Luo W, Zhou X, Gong X, et al. Study of sequential histopathologic changes, apoptosis, and cell proliferation in rabbit livers after high-intensity focused ultrasound ablation. J Ultrasound Med. 2007;26:477-485.
- Minkis K, Alam M. Ultrasound skin tightening. Dermatol Clin. 2014;32:71-77.
- Ko EJ, Hong JY, Kwon TR, et al. Efficacy and safety of non-invasive body tightening with high-intensity focused ultrasound (HIFU). Skin Res Technol. 2017;23:558-562.
- Sklar LR, El Tal AK, Kerwin LY. Use of transcutaneous ultrasound for lipolysis and skin tightening: a review. Aesthetic Plast Surg. 2014;38:429-441.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 2013;32:18-25.
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14:E36727 .
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Fatemi A. High-intensity focused ultrasound effectively reduces adipose tissue. Semin Cutan Med Surg. 2009;28:257-262.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety and efficacy of the Contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Hotta TA. Nonsurgical body contouring with focused ultrasound. Plast Surg Nurs. 2010;30:77-82; quiz 83-84.
- Fatemi A, Kane MA. High-intensity focused ultrasound effectively reduces waist circumference by ablating adipose tissue from the abdomen and flanks: a retrospective case series. Aesthetic Plast Surg. 2010;34:577-582.
- Schilling L, Saedi N, Weiss R. 1060 nm diode hyperthermic laser lipolysis: the latest in non-invasive body contouring. J Drugs Dermatol. 2017;16:48-52.
- Body contouring. CynoSure website. https://www.cynosure.com/treatment/body-contouring/SculpSure. Accessed March 28, 2018.
- Decorato JW, Chen B, Sierra R. Subcutaneous adipose tissue response to a non-invasive hyperthermic treatment using a 1,060 nm laser. Lasers Surg Med. 2017;49:480-489.
- Weiss R, McDaniel D, Doherty S. Clinical evaluation of fat reduction treatment of the flanks and abdomen with a non-invasive 1060 nm diode laser: a multicenter study. Paper presented at: 2016 Annual American Society for Laser Medicine and Surgery Conference; March 30–April 3, 2016; Boston, MA.
- Caruso-Davis MK, Guillot TS, Podichetty VK, et al. Efficacy of low-level laser therapy for body contouring and spot fat reduction. Obes Surg. 2011;21:722-729.
- McRae E, Boris J. Independent evaluation of low-level laser therapy at 635 nm for non-invasive body contouring of the waist, hips, and thighs. Lasers Surg Med. 2013;45:1-7.
- Nestor MS, Newburger J, Zarraga MB. Body contouring using 635-nm low level laser therapy. Semin Cutan Med Surg. 2013;32:35-40.
- Jackson RF, Stern FA, Neira R, et al. Application of low-level laser therapy for noninvasive body contouring. Lasers Surg Med. 2012;44:211-217.
- Jackson RF, Dedo DD, Roche GC, et al. Low-level laser therapy as a non-invasive approach for body contouring: a randomized, controlled study. Lasers Surg Med. 2009;41:799-809.
- Gold MH, Khatri KA, Hails K, et al. Reduction in thigh circumference and improvement in the appearance of cellulite with dual-wavelength, low-level laser energy and massage. J Cosmet Laser Ther. 2011;13:13-20.
- Avci P, Nyame TT, Gupta GK, et al. Low-level laser therapy for fat layer reduction: a comprehensive review. Lasers Surg Med. 2013;45:349-357.
In today’s society there is a ubiquitous pressure to lose weight, reduce fat, and rejuvenate the skin that stems not only from images of idealized bodies in the media but also from our growing knowledge of the detrimental effects of obesity. Along with diet and exercise, it has become popular to use noninvasive devices to attain these goals by means of body contouring—the optimization of the definition, smoothness, and shape of the human physique.1 In fact, body contouring currently is the fastest-growing area of cosmetic dermatology.2
Previously, body contouring primarily involved invasive procedures (eg, liposuction) that are associated with various adverse effects, financial costs, and lengthy downtime.3 More recently, a growing demand for safer and less painful procedures for adipose tissue reduction and skin tightening have led to the development of several novel modalities for noninvasive body contouring. Although the results achieved using these new technologies may be less dramatic than invasive techniques and are not immediate, they do not carry the risks and adverse effects that are associated with surgical procedures and therefore are increasingly requested by cosmetic patients.4,5 New noninvasive techniques primarily target the physical properties of fat, resulting in an efflux of triglycerides from fat cells, causing either reduced size, necrosis, or apoptosis of adipocytes.3,6 Of these modalities, cold-induced adipocyte apoptosis has been commercially available the longest and has been the most researched; however, other noninvasive body contouring techniques have been increasingly explored by researchers since the first reports of human adipose tissue explants exhibiting features of apoptosis after heat injury became available.7,8
There currently are 4 leading modalities used for noninvasive body contouring: cryolipolysis, radiofrequency (RF), high-intensity focused ultrasound (HIFU), and laser therapy (Table). Although no procedure has yet been accepted as the gold standard, investigators are working to determine which technique is the most effective.9 In this article, we provide an overview of these techniques to help dermatologists choose appropriate modalities for their cosmetic patients.
Cryolipolysis
Cryolipolysis is unique in that it employs the principle that lipid-rich adipocytes are more susceptible to freezing than surrounding water-rich cells, allowing selective apoptosis while preserving the adjacent structures. As macrophages digest the apoptotic adipocytes, patients experience a decrease in subcutaneous fat volume over the subsequent 2 to 3 months.10-13 Cryolipolysis has been gaining popularity since 2010, when it was first approved by the US Food and Drug Administration (FDA) for fat reduction in the flank areas; it was later approved for the abdomen in 2012, thighs in 2014, and submental area in 2015.14 Most recently, cryolipolysis was approved for fat reduction in the arms, back, and buttocks in 2016.
The most popular cryolipolysis device applies suction to the treatment area and vacuums the tissue between 2 cooling panels for 30 to 60 minutes.9 Clinical studies investigating the safety and efficacy of cryolipolysis have reported a high degree of patient satisfaction with the procedure and only minimal side effects.4,6,15,16 Common complications of cryolipolysis include erythema, swelling, and sensitivity at the treatment site followed by a lesser incidence of pain, tingling, and bruising, all of which generally resolve within a few weeks of treatment.6 With the removal of adipocytes, there has been concern regarding elevations in blood lipid levels and liver enzymes; however, these laboratory values have been reported to remain within normal limits during and after cryolipolysis.17,18 Of note, patients should be advised of the risk of paradoxical adipose hyperplasia, a rare side effect of cryolipolysis in which a large, demarcated, tender fat mass develops at the treatment site 2 to 3 months after treatment, with an estimated incidence of 1 in 20,000.19 However, the incidence of paradoxical adipose hyperplasia may be underestimated, as a single practice reported an incidence of 0.47% in 422 cryolipolysis treatments.20 This complication has not been associated with any of the heat-induced fat reduction modalities.
Cryolipolysis has been found to be safe for all skin types with no reported pigmentary changes.16 It should not be performed in patients with cold-induced conditions (eg, cryoglobulinemia, cold urticaria) or in those with severe varicose veins or atopic dermatitis.21,22 Patients benefitting most from this procedure are those who require only small or moderate amounts of adipose tissue and cellulite removal with separate fat bulges.12,17 Interestingly, cryolipolysis also has been used off label to treat pseudogynecomastia in male patients.23
Radiofrequency
Radiofrequency has become an important and frequently used modality in cosmetic dermatology.24 This modality differs from cryolipolysis in that it relies on exploiting the difference in water content and impedance between tissues: the skin has low impedance, whereas fat tissue has high impedance. Radiofrequency induces thermal injury to targeted tissue layers, rather than the cold-induced damage seen in cryolipolysis, through devices that focus thermal energy on tissues with high impedance, inducing apoptosis of cells in the subcutaneous adipose tissue with minimal risk of damaging the epidermis, dermis, and muscle.9,25 Ultimately, thermal exposure to 43°C to 45°C over several minutes results in a delayed adipocyte death response.4 In addition to adipocyte death, RF has been shown to cause denaturation of collagen fibrils, leading to subsequent remodeling, neocollagenesis, and skin tightening.26
Radiofrequency devices can be broadly classified as monopolar or bipolar.24,27 Bipolar devices generally require more frequent treatments, whereas monopolar devices tend to require fewer treatment sessions with superior circumference and fat reduction.28
Overall, RF devices have a favorable side effect profile. The most common side effects are erythema and edema at the treatment site lasting less than 24 hours after the procedure.25 The absence of complications such as abdominal discomfort, erythema, and burning during treatment have been reported,27 with the exception of 1 case of hyperesthesia on the abdomen that lasted for 3 days after a treatment session.5 Although RF has beneficial effects on circumference reduction in the abdomen and thighs and can improve the appearance of cellulite, an increase in body weight may occur during treatment. When a localized area of fat such as the thigh is targeted for treatment but the remaining fat cells in the body are not affected, the remaining cells can continue to grow and expand; for instance, although fat cells destroyed with RF will not continue to expand, fat cells in untreated areas may continue to grow due to continued weight gain (eg, from excessive eating), leading to overall weight gain. Thus, patients must understand that weight gain is not an indication of treatment failure after RF or any other method of irreversible fat destruction.5
High-intensity Focused Ultrasound
High-intensity focused ultrasound recently was introduced as a new treatment modality for body contouring, specifically for skin tightening and rejuvenation.5 The mechanism of HIFU is similar to that of RF in that it also relies on heat to cause adipocyte apoptosis; however, it utilizes acoustic energy rather than electric energy. High-intensity focused ultrasound devices can deliver energy to the deep dermis, subdermal connective tissue, and fibromuscular layers in precise microcoagulation zones without damage to the epidermis. The focused energy induces a high temperature (>65°C) within 1 to 3 seconds, causing cell protein coagulation in the targeted area. In addition to its thermal effects, HIFU induces a mechanical effect that disrupts cell membranes immediately, which contributes to the coagulation necrosis process, further promoting necrosis and apoptosis. The effects of these devices can be visualized, as there always is a sharp demarcation between the targeted and untargeted tissue.29 Additionally, microcoagulation is thought to cause gradual skin tightening through collagen contraction and remodeling.30
High-intensity focused ultrasound first received FDA approval for eyebrow lifting and has been used safely and effectively to treat facial and neck skin in a variety of skin types as well as to improve the clinical appearance of the abdomen and thighs.31 This technique is best suited for patients with mild to moderate laxity of the skin or soft tissue who have a body mass index less than 30 kg/m2 and are seeking mild body contouring.32 The ideal patient is young with normal wound healing, since the clinical response to treatment is partly dependent on new collagen synthesis.33 Older patients with extensive photoaging or severe skin laxity are not good candidates for HIFU.
There are a variety of available HIFU devices,34 which utilize special transducers that direct ultrasound energy to a small focal point in the subcutaneous tissues that harmlessly passes through the skin.35 By using newly developed transducers with different energy outputs and focal depths, dermatologists can tailor HIFU treatment to meet the unique physical characteristics of each patient.31
Adverse effects of HIFU are limited to transient pain in most patients and occasional erythema and ecchymosis in some cases.31 In general, most adverse effects resolve spontaneously within 4 weeks and all by 12 weeks posttreatment. Studies also have reported hard subcutaneous nodules, discomfort, burning sensation, mild blisters, and one case of purpuric lesions, all at the treatment site.36-39 There is no evidence that HIFU can cause abnormalities in serum lipids or liver function tests.
Lasers
Laser technology is a rapidly growing modality in noninvasive body contouring. A novel device recently emerged as the first and only FDA-cleared hyperthermic laser for fat reduction and noninvasive body contouring of the abdomen, flanks, back, inner and outer thighs, and submental area.40,41 The device is a 1060-nm diode laser that uses thermal energy to destroy adipose tissue, leading to permanent reduction in stubborn fat without surgery or downtime through the use of a flat, nonsuction applicator that is designed for consistent, natural-looking results. The device includes a contact cooling system that helps to limit thermal discomfort and prevent damage to the surface of the skin during the procedure. Initial improvement can be seen as quickly as 6 weeks posttreatment, and optimal results usually occur in as few as 12 weeks. This device was found to have an excellent safety profile and was well tolerated among patients, with only mild pain reported.42,43
Prior to the development of this new 1060-nm diode laser, the initial application of lasers for noninvasive body contouring involved low-level laser therapy (LLLT), also known as cold laser therapy.40 One device has 5 rotating diode laser heads that work at a wavelength of 635 nm. Treatment sessions last up to 30 minutes, and 6 to 8 sessions are required to obtain optimal results. Low-level laser therapy is a unique modality that is not based on thermal tissue damage, but rather on producing transient microscopic pores in adipocytes that allow lipids to leak out, leading to fat reduction.34 Because LLLT causes immediate emptying of targeted adipocytes, results are noticeable as soon as treatment is completed; however, there is no necrosis or apoptosis of adipocytes, so the recurrence of fat deposition is believed to be greater when compared to the other modalities. Because the results are temporary, long-term or permanent results should not be expected with LLLT. Depending on the patient’s goals, the temporary nature of the results can be either an advantage or disadvantage: some may prefer immediate results despite gradual diminishment over subsequent months, whereas others may prefer results that progressively increase over time and are more permanent, as seen with cryolipolysis, HIFU, and RF.3
Complications of LLLT generally are fewer and more mild than with all other body contouring procedures, with several studies reporting no adverse effects.44-48 Others reported swelling or erythema at the treatment area, pain or tingling during treatment, and increased urination, all of which were temporary and resolved spontaneously.49 Additionally, although the lipids released from treatment are cleared through the lymphatic system, LLLT has not been shown to increase serum lipid levels.50
Conclusion
The field of noninvasive body contouring is undoubtedly growing and will likely continue to rise in popularity as the efficacy and safety of these treatments improve. Although the available technologies vary by mechanism and side effect profiles, several devices have been revealed to be safe and effective in reducing subcutaneous fat tissue and improving skin laxity.1 However, additional studies are needed to evaluate these devices in a standardized manner, especially considering the high costs associated with treatment.32 Current studies investigating these devices vary in treatment protocol, treatment area, number and timing of follow-up sessions, and outcome measures, making it challenging to compare the results objectively.3 Dermatologists offering body contouring treatments need to be intimately familiar with the available devices and determine which treatment is appropriate for each patient in order to provide the highest quality care. Most importantly, patients and physicians must discuss individual goals when choosing a body-contouring method in order to maximize patient satisfaction.
In today’s society there is a ubiquitous pressure to lose weight, reduce fat, and rejuvenate the skin that stems not only from images of idealized bodies in the media but also from our growing knowledge of the detrimental effects of obesity. Along with diet and exercise, it has become popular to use noninvasive devices to attain these goals by means of body contouring—the optimization of the definition, smoothness, and shape of the human physique.1 In fact, body contouring currently is the fastest-growing area of cosmetic dermatology.2
Previously, body contouring primarily involved invasive procedures (eg, liposuction) that are associated with various adverse effects, financial costs, and lengthy downtime.3 More recently, a growing demand for safer and less painful procedures for adipose tissue reduction and skin tightening have led to the development of several novel modalities for noninvasive body contouring. Although the results achieved using these new technologies may be less dramatic than invasive techniques and are not immediate, they do not carry the risks and adverse effects that are associated with surgical procedures and therefore are increasingly requested by cosmetic patients.4,5 New noninvasive techniques primarily target the physical properties of fat, resulting in an efflux of triglycerides from fat cells, causing either reduced size, necrosis, or apoptosis of adipocytes.3,6 Of these modalities, cold-induced adipocyte apoptosis has been commercially available the longest and has been the most researched; however, other noninvasive body contouring techniques have been increasingly explored by researchers since the first reports of human adipose tissue explants exhibiting features of apoptosis after heat injury became available.7,8
There currently are 4 leading modalities used for noninvasive body contouring: cryolipolysis, radiofrequency (RF), high-intensity focused ultrasound (HIFU), and laser therapy (Table). Although no procedure has yet been accepted as the gold standard, investigators are working to determine which technique is the most effective.9 In this article, we provide an overview of these techniques to help dermatologists choose appropriate modalities for their cosmetic patients.
Cryolipolysis
Cryolipolysis is unique in that it employs the principle that lipid-rich adipocytes are more susceptible to freezing than surrounding water-rich cells, allowing selective apoptosis while preserving the adjacent structures. As macrophages digest the apoptotic adipocytes, patients experience a decrease in subcutaneous fat volume over the subsequent 2 to 3 months.10-13 Cryolipolysis has been gaining popularity since 2010, when it was first approved by the US Food and Drug Administration (FDA) for fat reduction in the flank areas; it was later approved for the abdomen in 2012, thighs in 2014, and submental area in 2015.14 Most recently, cryolipolysis was approved for fat reduction in the arms, back, and buttocks in 2016.
The most popular cryolipolysis device applies suction to the treatment area and vacuums the tissue between 2 cooling panels for 30 to 60 minutes.9 Clinical studies investigating the safety and efficacy of cryolipolysis have reported a high degree of patient satisfaction with the procedure and only minimal side effects.4,6,15,16 Common complications of cryolipolysis include erythema, swelling, and sensitivity at the treatment site followed by a lesser incidence of pain, tingling, and bruising, all of which generally resolve within a few weeks of treatment.6 With the removal of adipocytes, there has been concern regarding elevations in blood lipid levels and liver enzymes; however, these laboratory values have been reported to remain within normal limits during and after cryolipolysis.17,18 Of note, patients should be advised of the risk of paradoxical adipose hyperplasia, a rare side effect of cryolipolysis in which a large, demarcated, tender fat mass develops at the treatment site 2 to 3 months after treatment, with an estimated incidence of 1 in 20,000.19 However, the incidence of paradoxical adipose hyperplasia may be underestimated, as a single practice reported an incidence of 0.47% in 422 cryolipolysis treatments.20 This complication has not been associated with any of the heat-induced fat reduction modalities.
Cryolipolysis has been found to be safe for all skin types with no reported pigmentary changes.16 It should not be performed in patients with cold-induced conditions (eg, cryoglobulinemia, cold urticaria) or in those with severe varicose veins or atopic dermatitis.21,22 Patients benefitting most from this procedure are those who require only small or moderate amounts of adipose tissue and cellulite removal with separate fat bulges.12,17 Interestingly, cryolipolysis also has been used off label to treat pseudogynecomastia in male patients.23
Radiofrequency
Radiofrequency has become an important and frequently used modality in cosmetic dermatology.24 This modality differs from cryolipolysis in that it relies on exploiting the difference in water content and impedance between tissues: the skin has low impedance, whereas fat tissue has high impedance. Radiofrequency induces thermal injury to targeted tissue layers, rather than the cold-induced damage seen in cryolipolysis, through devices that focus thermal energy on tissues with high impedance, inducing apoptosis of cells in the subcutaneous adipose tissue with minimal risk of damaging the epidermis, dermis, and muscle.9,25 Ultimately, thermal exposure to 43°C to 45°C over several minutes results in a delayed adipocyte death response.4 In addition to adipocyte death, RF has been shown to cause denaturation of collagen fibrils, leading to subsequent remodeling, neocollagenesis, and skin tightening.26
Radiofrequency devices can be broadly classified as monopolar or bipolar.24,27 Bipolar devices generally require more frequent treatments, whereas monopolar devices tend to require fewer treatment sessions with superior circumference and fat reduction.28
Overall, RF devices have a favorable side effect profile. The most common side effects are erythema and edema at the treatment site lasting less than 24 hours after the procedure.25 The absence of complications such as abdominal discomfort, erythema, and burning during treatment have been reported,27 with the exception of 1 case of hyperesthesia on the abdomen that lasted for 3 days after a treatment session.5 Although RF has beneficial effects on circumference reduction in the abdomen and thighs and can improve the appearance of cellulite, an increase in body weight may occur during treatment. When a localized area of fat such as the thigh is targeted for treatment but the remaining fat cells in the body are not affected, the remaining cells can continue to grow and expand; for instance, although fat cells destroyed with RF will not continue to expand, fat cells in untreated areas may continue to grow due to continued weight gain (eg, from excessive eating), leading to overall weight gain. Thus, patients must understand that weight gain is not an indication of treatment failure after RF or any other method of irreversible fat destruction.5
High-intensity Focused Ultrasound
High-intensity focused ultrasound recently was introduced as a new treatment modality for body contouring, specifically for skin tightening and rejuvenation.5 The mechanism of HIFU is similar to that of RF in that it also relies on heat to cause adipocyte apoptosis; however, it utilizes acoustic energy rather than electric energy. High-intensity focused ultrasound devices can deliver energy to the deep dermis, subdermal connective tissue, and fibromuscular layers in precise microcoagulation zones without damage to the epidermis. The focused energy induces a high temperature (>65°C) within 1 to 3 seconds, causing cell protein coagulation in the targeted area. In addition to its thermal effects, HIFU induces a mechanical effect that disrupts cell membranes immediately, which contributes to the coagulation necrosis process, further promoting necrosis and apoptosis. The effects of these devices can be visualized, as there always is a sharp demarcation between the targeted and untargeted tissue.29 Additionally, microcoagulation is thought to cause gradual skin tightening through collagen contraction and remodeling.30
High-intensity focused ultrasound first received FDA approval for eyebrow lifting and has been used safely and effectively to treat facial and neck skin in a variety of skin types as well as to improve the clinical appearance of the abdomen and thighs.31 This technique is best suited for patients with mild to moderate laxity of the skin or soft tissue who have a body mass index less than 30 kg/m2 and are seeking mild body contouring.32 The ideal patient is young with normal wound healing, since the clinical response to treatment is partly dependent on new collagen synthesis.33 Older patients with extensive photoaging or severe skin laxity are not good candidates for HIFU.
There are a variety of available HIFU devices,34 which utilize special transducers that direct ultrasound energy to a small focal point in the subcutaneous tissues that harmlessly passes through the skin.35 By using newly developed transducers with different energy outputs and focal depths, dermatologists can tailor HIFU treatment to meet the unique physical characteristics of each patient.31
Adverse effects of HIFU are limited to transient pain in most patients and occasional erythema and ecchymosis in some cases.31 In general, most adverse effects resolve spontaneously within 4 weeks and all by 12 weeks posttreatment. Studies also have reported hard subcutaneous nodules, discomfort, burning sensation, mild blisters, and one case of purpuric lesions, all at the treatment site.36-39 There is no evidence that HIFU can cause abnormalities in serum lipids or liver function tests.
Lasers
Laser technology is a rapidly growing modality in noninvasive body contouring. A novel device recently emerged as the first and only FDA-cleared hyperthermic laser for fat reduction and noninvasive body contouring of the abdomen, flanks, back, inner and outer thighs, and submental area.40,41 The device is a 1060-nm diode laser that uses thermal energy to destroy adipose tissue, leading to permanent reduction in stubborn fat without surgery or downtime through the use of a flat, nonsuction applicator that is designed for consistent, natural-looking results. The device includes a contact cooling system that helps to limit thermal discomfort and prevent damage to the surface of the skin during the procedure. Initial improvement can be seen as quickly as 6 weeks posttreatment, and optimal results usually occur in as few as 12 weeks. This device was found to have an excellent safety profile and was well tolerated among patients, with only mild pain reported.42,43
Prior to the development of this new 1060-nm diode laser, the initial application of lasers for noninvasive body contouring involved low-level laser therapy (LLLT), also known as cold laser therapy.40 One device has 5 rotating diode laser heads that work at a wavelength of 635 nm. Treatment sessions last up to 30 minutes, and 6 to 8 sessions are required to obtain optimal results. Low-level laser therapy is a unique modality that is not based on thermal tissue damage, but rather on producing transient microscopic pores in adipocytes that allow lipids to leak out, leading to fat reduction.34 Because LLLT causes immediate emptying of targeted adipocytes, results are noticeable as soon as treatment is completed; however, there is no necrosis or apoptosis of adipocytes, so the recurrence of fat deposition is believed to be greater when compared to the other modalities. Because the results are temporary, long-term or permanent results should not be expected with LLLT. Depending on the patient’s goals, the temporary nature of the results can be either an advantage or disadvantage: some may prefer immediate results despite gradual diminishment over subsequent months, whereas others may prefer results that progressively increase over time and are more permanent, as seen with cryolipolysis, HIFU, and RF.3
Complications of LLLT generally are fewer and more mild than with all other body contouring procedures, with several studies reporting no adverse effects.44-48 Others reported swelling or erythema at the treatment area, pain or tingling during treatment, and increased urination, all of which were temporary and resolved spontaneously.49 Additionally, although the lipids released from treatment are cleared through the lymphatic system, LLLT has not been shown to increase serum lipid levels.50
Conclusion
The field of noninvasive body contouring is undoubtedly growing and will likely continue to rise in popularity as the efficacy and safety of these treatments improve. Although the available technologies vary by mechanism and side effect profiles, several devices have been revealed to be safe and effective in reducing subcutaneous fat tissue and improving skin laxity.1 However, additional studies are needed to evaluate these devices in a standardized manner, especially considering the high costs associated with treatment.32 Current studies investigating these devices vary in treatment protocol, treatment area, number and timing of follow-up sessions, and outcome measures, making it challenging to compare the results objectively.3 Dermatologists offering body contouring treatments need to be intimately familiar with the available devices and determine which treatment is appropriate for each patient in order to provide the highest quality care. Most importantly, patients and physicians must discuss individual goals when choosing a body-contouring method in order to maximize patient satisfaction.
- Jalian HR, Avram MM. Body contouring: the skinny on noninvasive fat removal. Semin Cutan Med Surg. 2012;31:121-125.
- Ho D, Jagdeo J. A systematic review of paradoxical adipose hyperplasia (PAH) post-cryolipolysis. J Drugs Dermatol. 2017;16:62-67.
- Kennedy J, Verne S, Griffith R, et al. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol. 2015;29:1679-1688.
- Krueger N, Mai SV, Luebberding S, et al. Cryolipolysis for noninvasive body contouring: clinical efficacy and patient satisfaction. Clin Cosmet Investig Dermatol. 2014;7:201-205.
- Suh DH, Kim CM, Lee SJ, et al. Safety and efficacy of a non-contact radiofrequency device for body contouring in Asians. J Cosmet Laser Ther. 2017;19:89-92.
- Ingargiola MJ, Motakef S, Chung MT, et al. Cryolipolysis for fat reduction and body contouring: safety and efficacy of current treatment paradigms. Plast Reconstr Surg. 2015;135:1581-1590.
- Prins JB, Walker NI, Winterford CM, et al. Apoptosis of human adipocytes in vitro. Biochem Biophys Res Commun. 1994;201:500-507.
- Sorisky A, Magun R, Gagnon AM. Adipose cell apoptosis: death in the energy depot. Int J Obes Relat Metab Disord. 2000;24(suppl 4):S3-S7.
- Chilukuri S, Mueller G. “Hands-free” noninvasive body contouring devices: review of effectiveness and patient satisfaction. J Drugs Dermatol. 2016;15:1402-1406.
- Manstein D, Laubach H, Watanabe K, et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595-604.
- Zelickson B, Egbert BM, Preciado J, et al. Cryolipolysis for noninvasive fat cell destruction: initial results from a pig model. Dermatol Surg. 2009;35:1462-1470.
- Nelson AA, Wasserman D, Avram MM. Cryolipolysis for reduction of excess adipose tissue. Semin Cutan Med Surg. 2009;28:244-249.
- Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med. 2009;41:703-708.
- Klein KB, Bachelor EP, Becker EV, et al. Multiple same day cryolipolysis treatments for the reduction of subcutaneous fat are safe and do not affect serum lipid levels or liver function tests. Lasers Surg Med. 2017;49:640-644.
- Dierickx CC, Mazer JM, Sand M, et al. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg. 2013;39:1209-1216.
- Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J. 2013;33:835-846.
- Ferraro GA, De Francesco F, Cataldo C, et al. Synergistic effects of cryolipolysis and shock waves for noninvasive body contouring. Aesthetic Plast Surg. 2012;36:666-679.
- Lee KR. Clinical efficacy of fat reduction on the thigh of Korean women through cryolipolysis. J Obes Weight Loss Ther. 2013;3:203.
- Jalian HR, Avram MM, Garibyan L, et al. Paradoxical adipose hyperplasia after cryolipolysis. JAMA Dermatol. 2014;150:317-319.
- Singh SM, Geddes ER, Boutrous SG, et al. Paradoxical adipose hyperplasia secondary to cryolipolysis: an underreported entity? Lasers Surg Med. 2015;47:476-478.
- Pinto H, Arredondo E, Ricart-Jane D. Evaluation of adipocytic changes after a simil-lipocryolysis stimulus. Cryo Letters. 2013;34:100-105.
- Pinto HR, Garcia-Cruz E, Melamed GE. A study to evaluate the action of lipocryolysis. Cryo Letters. 2012;33:177-181.
- Singh B, Keaney T, Rossi AM. Male body contouring. J Drugs Dermatol. 2015;14:1052-1059.
- Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32:79-90.
- Weiss R, Weiss M, Beasley K, et al. Operator independent focused high frequency ISM band for fat reduction: porcine model. Lasers Surg Med. 2013;45:235-239.
- Hantash BM, Ubeid AA, Chang H, et al. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009;41:1-9.
- Harth Y. Painless, safe, and efficacious noninvasive skin tightening, body contouring, and cellulite reduction using multisource 3DEEP radiofrequency. J Cosmet Dermatol. 2015;14:70-75.
- Nassab R. The evidence behind noninvasive body contouring devices. Aesthet Surg J. 2015;35:279-293.
- Luo W, Zhou X, Gong X, et al. Study of sequential histopathologic changes, apoptosis, and cell proliferation in rabbit livers after high-intensity focused ultrasound ablation. J Ultrasound Med. 2007;26:477-485.
- Minkis K, Alam M. Ultrasound skin tightening. Dermatol Clin. 2014;32:71-77.
- Ko EJ, Hong JY, Kwon TR, et al. Efficacy and safety of non-invasive body tightening with high-intensity focused ultrasound (HIFU). Skin Res Technol. 2017;23:558-562.
- Sklar LR, El Tal AK, Kerwin LY. Use of transcutaneous ultrasound for lipolysis and skin tightening: a review. Aesthetic Plast Surg. 2014;38:429-441.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 2013;32:18-25.
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14:E36727 .
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Fatemi A. High-intensity focused ultrasound effectively reduces adipose tissue. Semin Cutan Med Surg. 2009;28:257-262.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety and efficacy of the Contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Hotta TA. Nonsurgical body contouring with focused ultrasound. Plast Surg Nurs. 2010;30:77-82; quiz 83-84.
- Fatemi A, Kane MA. High-intensity focused ultrasound effectively reduces waist circumference by ablating adipose tissue from the abdomen and flanks: a retrospective case series. Aesthetic Plast Surg. 2010;34:577-582.
- Schilling L, Saedi N, Weiss R. 1060 nm diode hyperthermic laser lipolysis: the latest in non-invasive body contouring. J Drugs Dermatol. 2017;16:48-52.
- Body contouring. CynoSure website. https://www.cynosure.com/treatment/body-contouring/SculpSure. Accessed March 28, 2018.
- Decorato JW, Chen B, Sierra R. Subcutaneous adipose tissue response to a non-invasive hyperthermic treatment using a 1,060 nm laser. Lasers Surg Med. 2017;49:480-489.
- Weiss R, McDaniel D, Doherty S. Clinical evaluation of fat reduction treatment of the flanks and abdomen with a non-invasive 1060 nm diode laser: a multicenter study. Paper presented at: 2016 Annual American Society for Laser Medicine and Surgery Conference; March 30–April 3, 2016; Boston, MA.
- Caruso-Davis MK, Guillot TS, Podichetty VK, et al. Efficacy of low-level laser therapy for body contouring and spot fat reduction. Obes Surg. 2011;21:722-729.
- McRae E, Boris J. Independent evaluation of low-level laser therapy at 635 nm for non-invasive body contouring of the waist, hips, and thighs. Lasers Surg Med. 2013;45:1-7.
- Nestor MS, Newburger J, Zarraga MB. Body contouring using 635-nm low level laser therapy. Semin Cutan Med Surg. 2013;32:35-40.
- Jackson RF, Stern FA, Neira R, et al. Application of low-level laser therapy for noninvasive body contouring. Lasers Surg Med. 2012;44:211-217.
- Jackson RF, Dedo DD, Roche GC, et al. Low-level laser therapy as a non-invasive approach for body contouring: a randomized, controlled study. Lasers Surg Med. 2009;41:799-809.
- Gold MH, Khatri KA, Hails K, et al. Reduction in thigh circumference and improvement in the appearance of cellulite with dual-wavelength, low-level laser energy and massage. J Cosmet Laser Ther. 2011;13:13-20.
- Avci P, Nyame TT, Gupta GK, et al. Low-level laser therapy for fat layer reduction: a comprehensive review. Lasers Surg Med. 2013;45:349-357.
- Jalian HR, Avram MM. Body contouring: the skinny on noninvasive fat removal. Semin Cutan Med Surg. 2012;31:121-125.
- Ho D, Jagdeo J. A systematic review of paradoxical adipose hyperplasia (PAH) post-cryolipolysis. J Drugs Dermatol. 2017;16:62-67.
- Kennedy J, Verne S, Griffith R, et al. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol. 2015;29:1679-1688.
- Krueger N, Mai SV, Luebberding S, et al. Cryolipolysis for noninvasive body contouring: clinical efficacy and patient satisfaction. Clin Cosmet Investig Dermatol. 2014;7:201-205.
- Suh DH, Kim CM, Lee SJ, et al. Safety and efficacy of a non-contact radiofrequency device for body contouring in Asians. J Cosmet Laser Ther. 2017;19:89-92.
- Ingargiola MJ, Motakef S, Chung MT, et al. Cryolipolysis for fat reduction and body contouring: safety and efficacy of current treatment paradigms. Plast Reconstr Surg. 2015;135:1581-1590.
- Prins JB, Walker NI, Winterford CM, et al. Apoptosis of human adipocytes in vitro. Biochem Biophys Res Commun. 1994;201:500-507.
- Sorisky A, Magun R, Gagnon AM. Adipose cell apoptosis: death in the energy depot. Int J Obes Relat Metab Disord. 2000;24(suppl 4):S3-S7.
- Chilukuri S, Mueller G. “Hands-free” noninvasive body contouring devices: review of effectiveness and patient satisfaction. J Drugs Dermatol. 2016;15:1402-1406.
- Manstein D, Laubach H, Watanabe K, et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595-604.
- Zelickson B, Egbert BM, Preciado J, et al. Cryolipolysis for noninvasive fat cell destruction: initial results from a pig model. Dermatol Surg. 2009;35:1462-1470.
- Nelson AA, Wasserman D, Avram MM. Cryolipolysis for reduction of excess adipose tissue. Semin Cutan Med Surg. 2009;28:244-249.
- Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med. 2009;41:703-708.
- Klein KB, Bachelor EP, Becker EV, et al. Multiple same day cryolipolysis treatments for the reduction of subcutaneous fat are safe and do not affect serum lipid levels or liver function tests. Lasers Surg Med. 2017;49:640-644.
- Dierickx CC, Mazer JM, Sand M, et al. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg. 2013;39:1209-1216.
- Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J. 2013;33:835-846.
- Ferraro GA, De Francesco F, Cataldo C, et al. Synergistic effects of cryolipolysis and shock waves for noninvasive body contouring. Aesthetic Plast Surg. 2012;36:666-679.
- Lee KR. Clinical efficacy of fat reduction on the thigh of Korean women through cryolipolysis. J Obes Weight Loss Ther. 2013;3:203.
- Jalian HR, Avram MM, Garibyan L, et al. Paradoxical adipose hyperplasia after cryolipolysis. JAMA Dermatol. 2014;150:317-319.
- Singh SM, Geddes ER, Boutrous SG, et al. Paradoxical adipose hyperplasia secondary to cryolipolysis: an underreported entity? Lasers Surg Med. 2015;47:476-478.
- Pinto H, Arredondo E, Ricart-Jane D. Evaluation of adipocytic changes after a simil-lipocryolysis stimulus. Cryo Letters. 2013;34:100-105.
- Pinto HR, Garcia-Cruz E, Melamed GE. A study to evaluate the action of lipocryolysis. Cryo Letters. 2012;33:177-181.
- Singh B, Keaney T, Rossi AM. Male body contouring. J Drugs Dermatol. 2015;14:1052-1059.
- Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32:79-90.
- Weiss R, Weiss M, Beasley K, et al. Operator independent focused high frequency ISM band for fat reduction: porcine model. Lasers Surg Med. 2013;45:235-239.
- Hantash BM, Ubeid AA, Chang H, et al. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009;41:1-9.
- Harth Y. Painless, safe, and efficacious noninvasive skin tightening, body contouring, and cellulite reduction using multisource 3DEEP radiofrequency. J Cosmet Dermatol. 2015;14:70-75.
- Nassab R. The evidence behind noninvasive body contouring devices. Aesthet Surg J. 2015;35:279-293.
- Luo W, Zhou X, Gong X, et al. Study of sequential histopathologic changes, apoptosis, and cell proliferation in rabbit livers after high-intensity focused ultrasound ablation. J Ultrasound Med. 2007;26:477-485.
- Minkis K, Alam M. Ultrasound skin tightening. Dermatol Clin. 2014;32:71-77.
- Ko EJ, Hong JY, Kwon TR, et al. Efficacy and safety of non-invasive body tightening with high-intensity focused ultrasound (HIFU). Skin Res Technol. 2017;23:558-562.
- Sklar LR, El Tal AK, Kerwin LY. Use of transcutaneous ultrasound for lipolysis and skin tightening: a review. Aesthetic Plast Surg. 2014;38:429-441.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 2013;32:18-25.
- Alizadeh Z, Halabchi F, Mazaheri R, et al. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int J Endocrinol Metab. 2016;14:E36727 .
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Fatemi A. High-intensity focused ultrasound effectively reduces adipose tissue. Semin Cutan Med Surg. 2009;28:257-262.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety and efficacy of the Contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Hotta TA. Nonsurgical body contouring with focused ultrasound. Plast Surg Nurs. 2010;30:77-82; quiz 83-84.
- Fatemi A, Kane MA. High-intensity focused ultrasound effectively reduces waist circumference by ablating adipose tissue from the abdomen and flanks: a retrospective case series. Aesthetic Plast Surg. 2010;34:577-582.
- Schilling L, Saedi N, Weiss R. 1060 nm diode hyperthermic laser lipolysis: the latest in non-invasive body contouring. J Drugs Dermatol. 2017;16:48-52.
- Body contouring. CynoSure website. https://www.cynosure.com/treatment/body-contouring/SculpSure. Accessed March 28, 2018.
- Decorato JW, Chen B, Sierra R. Subcutaneous adipose tissue response to a non-invasive hyperthermic treatment using a 1,060 nm laser. Lasers Surg Med. 2017;49:480-489.
- Weiss R, McDaniel D, Doherty S. Clinical evaluation of fat reduction treatment of the flanks and abdomen with a non-invasive 1060 nm diode laser: a multicenter study. Paper presented at: 2016 Annual American Society for Laser Medicine and Surgery Conference; March 30–April 3, 2016; Boston, MA.
- Caruso-Davis MK, Guillot TS, Podichetty VK, et al. Efficacy of low-level laser therapy for body contouring and spot fat reduction. Obes Surg. 2011;21:722-729.
- McRae E, Boris J. Independent evaluation of low-level laser therapy at 635 nm for non-invasive body contouring of the waist, hips, and thighs. Lasers Surg Med. 2013;45:1-7.
- Nestor MS, Newburger J, Zarraga MB. Body contouring using 635-nm low level laser therapy. Semin Cutan Med Surg. 2013;32:35-40.
- Jackson RF, Stern FA, Neira R, et al. Application of low-level laser therapy for noninvasive body contouring. Lasers Surg Med. 2012;44:211-217.
- Jackson RF, Dedo DD, Roche GC, et al. Low-level laser therapy as a non-invasive approach for body contouring: a randomized, controlled study. Lasers Surg Med. 2009;41:799-809.
- Gold MH, Khatri KA, Hails K, et al. Reduction in thigh circumference and improvement in the appearance of cellulite with dual-wavelength, low-level laser energy and massage. J Cosmet Laser Ther. 2011;13:13-20.
- Avci P, Nyame TT, Gupta GK, et al. Low-level laser therapy for fat layer reduction: a comprehensive review. Lasers Surg Med. 2013;45:349-357.
Practice Points
- There currently are 4 leading modalities used for noninvasive body contouring: cryolipolysis, radiofrequency, high-intensity focused ultrasound, and laser therapy.
- Devices utilizing these 4 modalities have been found to be safe and effective in reducing subcutaneous fat tissue and improving skin laxity.
- Dermatologists utilizing body contouring treatments need to be familiar with available devices to determine which treatment is appropriate for each patient.
Hyaluronic Acid for Lip Rejuvenation



Regenerative Medicine in Cosmetic Dermatology
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
Practice Points
- Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, and hair follicle bulge show promise in tissue regeneration for various dermatologic conditions and aesthetic applications.
- Induced pluripotent stem cells, progenitor cells that result from the dedifferentiation of specialized adult cells, have potential for collagen generation.
Ideals of Facial Beauty
Several concepts of ideal aesthetic measurements can be traced back to ancient Greek and European Renaissance art. In examining canons of beauty, these classical ideals often are compared to modern-day standards, allowing clinicians to delineate the parameters of an attractive facial appearance and facilitate the planning of cosmetic procedures.
Given the growing number of available cosmetic interventions, dermatologists have a powerful ability to modify facial proportions; however, changes to individual structures should be made with a mindful approach to improving overall facial harmony. This article reviews the established parameters of facial beauty to assist the clinician in enhancing cosmetic outcomes.
Canons of Facial Aesthetics
Horizontal Thirds
In his writings on human anatomy, Leonardo da Vinci described dividing the face into equal thirds (Figure 1). The upper third measures from the trichion (the midline point of the normal hairline) to the glabella (the smooth prominence between the eyebrows). The middle third measures from the glabella to the subnasale (the midline point where the nasal septum meets the upper lip). The lower third measures from the subnasale to the menton (the most inferior point of the chin).1
Although the validity of the canon is intended to apply across race and gender, these proportions may vary by ethnicity (Table). In white individuals, the middle third of the face tends to be shorter than the upper and lower thirds.2 This same relationship has been observed in black males.3 In Chinese females, the upper third commonly is shorter than the middle and lower thirds, correlating with a less prominent forehead. In contrast, black females tend to have a relatively longer upper third.4
The relationship between modern perceptions of attractiveness and the neoclassical norm of equal thirds remains a topic of interest. Milutinovic et al1 examined facial thirds in white female celebrities from beauty and fashion magazines and compared them to a group of anonymous white females from the general population. The group of anonymous females showed statistically significant (P<.05) differences between the sizes of the 3 facial segments, whereas the group of celebrity faces demonstrated uniformity between the facial thirds.1
The lower face can itself be divided into thirds, with the upper third measured from the subnasale to the stomion (the midline point of the oral fissure when the lips are closed), and the lower two-thirds measured from the stomion to the menton (Figure 1). Mommaerts and Moerenhout5 examined photographs of 105 attractive celebrity faces and compared their proportions to those of classical sculptures of gods and goddesses (antique faces). The authors identified an upper one-third to lower two-thirds ratio of 69.8% in celebrity females and 69.1% in celebrity males; these ratios were not significantly different from the 72.4% seen in antique females and 73.1% in antique males. The authors concluded that a 30% upper lip to 70% lower lip-chin proportion may be the most appropriate to describe contemporary standards.5
Vertical Fifths
In the vertical dimension, the neoclassical canon of facial proportions divides the face into equal fifths (Figure 2).6 The 2 most lateral fifths are measured from the lateral helix of each ear to the exocanthus of each eye. The eye fissure lengths (measured between the endocanthion and exocanthion of each eye) represent one-fifth. The middle fifth is measured between the medial canthi of both eyes (endocanthion to endocanthion). This distance is equal to the width of the nose, as measured between both alae. Finally, the width of the mouth represents 1.5-times the width of the nose. These ratios of the vertical fifths apply to both males and females.6
Anthropometric studies have examined deviations from the neoclassical canon according to ethnicity. Wang et al7 compared the measurements of North American white and Han Chinese patients to these standards. White patients demonstrated a greater ratio of mouth width to nose width relative to the canon. In contrast, Han Chinese patients demonstrated a relatively wider nose and narrower mouth.7
In black individuals, it has been observed that the dimensions of most facial segments correspond to the neoclassical standards; however, nose width is relatively wider in black individuals relative to the canon as well as relative to white individuals.8
Milutinovic et al1 also compared vertical fifths between white celebrities and anonymous females. In the anonymous female group, statistically significant (P<.05) variations were found between the sizes of the different facial components. In contrast, the celebrity female group showed balance between the widths of vertical fifths.1
Lips
In the lower facial third, the lips represent a key element of attractiveness. Recently, lip augmentation, aimed at creating fuller and plumper lips, has dominated the popular culture and social media landscape.9 Although the aesthetic ideal of lips continues to evolve over time, recent studies have aimed at quantifying modern notions of attractive lip appearance.
Popenko et al10 examined lip measurements using computer-generated images of white women with different variations of lip sizes and lower face proportions. Computer-generated faces were graded on attractiveness by more than 400 individuals from focus groups. An upper lip to lower lip ratio of 1:2 was judged to be the most attractive, while a ratio of 2:1 was judged to be the least attractive. Results also showed that the surface area of the most attractive lips comprised roughly 10% of the lower third of the face.10
Penna et al11 analyzed various parameters of the lips and lower facial third using photographs of 176 white males and females that were judged on attractiveness by 250 volunteer evaluators. Faces were graded on a scale from 1 (absolutely attractive) to 7 (absolutely unattractive). Attractive males and females (grades 1 and 2) both demonstrated an average ratio of upper vermilion height to nose-mouth distance (measured from the subnasalae to the lower edge of the upper vermilion border) of 0.28, which was significantly greater than the average ratio observed in less attractive individuals (grades 6 or 7)(P<.05). In addition, attractive males and females demonstrated a ratio of upper vermilion height to nose-chin distance (measured from the subnasalae to the menton) of 0.09, which again was larger than the average ratio seen in less attractive individuals. Figure 3 demonstrates an aesthetic ideal of the lips derived from these 2 studies, though consideration should be given to the fact that these studies were based in white populations.
Golden Ratio
The golden ratio, also known as Phi, can be observed in nature, art, and architecture. Approximately equal to 1.618, the golden ratio also has been identified as a possible marker of beauty in the human face and has garnered attention in the lay press. The ratio has been applied to several proportions and structures in the face, such as the ratio of mouth width to nose width or the ratio of tooth height to tooth width, with investigation providing varying levels of validation about whether these ratios truly correlate with perceptions of beauty.12 Swift and Remington13 advocated for application of the golden ratio toward a comprehensive set of facial proportions. Marquardt14 used the golden ratio to create a 3-dimensional representation of an idealized face, known as the golden decagon mask. Although the golden ratio and the golden decagon mask have been proposed as analytic tools, their utility in clinical practice may be limited. Firstly, due to its popularity in the lay press, the golden ratio has been inconsistently applied to a wide range of facial ratios, which may undermine confidence in its representation as truth rather than coincidence. Secondly, although some authors have found validity of the golden decagon mask in representing unified ratios of attractiveness, others have asserted that it characterizes a masculinized white female and fails to account for ethnic differences.15-19
Age-Related Changes
In addition to the facial proportions guided by genetics, several changes occur with increased age. Over the course of a lifetime, predictable patterns emerge in the dimensions of the skin, soft tissue, and bone. These alterations in structural proportions may ultimately lead to an unevenness in facial aesthetics.
In skeletal structure, gradual bone resorption and expansion causes a reduction in facial height as well as an increase in facial width and depth.20 Fat atrophy and hypertrophy affect soft tissue proportions, visualized as hollowing at the temples, cheeks, and around the eyes, along with fullness in the submental region and jowls.21 Finally, decreases in skin elasticity and collagen exacerbate the appearance of rhytides and sagging. In older patients who desire a more youthful appearance, various applications of dermal fillers, fat grafting, liposuction, and skin tightening techniques can help to mitigate these changes.
Conclusion
Improving facial aesthetics relies on an understanding of the norms of facial proportions. Although cosmetic interventions commonly are advertised or described based on a single anatomical unit, it is important to appreciate the relationships between facial structures. Most notably, clinicians should be mindful of facial ratios when considering the introduction of filler materials or implants. Augmentation procedures at the temples, zygomatic arch, jaw, chin, and lips all have the possibility to alter facial ratios. Changes should therefore be considered in the context of improving overall facial harmony, with the clinician remaining cognizant of the ideal vertical and horizontal divisions of the face. Understanding such concepts and communicating them to patients can help in appropriately addressing all target areas, thereby leading to greater patient satisfaction.
- Milutinovic J, Zelic K, Nedeljkovic N. Evaluation of facial beauty using anthropometric proportions. ScientificWorldJournal. 2014;2014:428250. doi:10.1155/2014/428250.
- Farkas LG, Hreczko TA, Kolar JC, et al. Vertical and horizontal proportions of the face in young-adult North-American Caucasians: revision of neoclassical canons. Plast Reconstr Surg. 1985;75:328-338.
- Porter JP. The average African American male face: an anthropometric analysis. Arch Facial Plast Surg. 2004;6:78-81.
- Porter JP, Olson KL. Anthropometric facial analysis of the African American woman. Arch Facial Plast Surg. 2001;3:191-197.
- Mommaerts MY, Moerenhout BA. Ideal proportions in full face front view, contemporary versus antique. J Craniomaxillofac Surg. 2011;39:107-110.
- Vegter F, Hage JJ. Clinical anthropometry and canons of the face in historical perspective. Plast Reconstr Surg. 2000;106:1090-1096.
- Wang D, Qian G, Zhang M, et al. Differences in horizontal, neoclassical facial canons in Chinese (Han) and North American Caucasian populations. Aesthetic Plast Surg. 1997;21:265-269.
- Farkas LG, Forrest CR, Litsas L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast Surg. 2000;24:179-184.
- Coleman GG, Lindauer SJ, Tüfekçi E, et al. Influence of chin prominence on esthetic lip profile preferences. Am J Orthod Dentofacial Orthop. 2007;132:36-42.
- Popenko NA, Tripathi PB, Devcic Z, et al. A quantitative approach to determining the ideal female lip aesthetic and its effect on facial attractiveness. JAMA Facial Plast Surg. 2017;19:261-267.
- Penna V, Fricke A, Iblher N, et al. The attractive lip: a photomorphometric analysis. J Plast Reconstr Aesthet Surg. 2015;68:920-929.
- Prokopakis EP, Vlastos IM, Picavet VA, et al. The golden ratio in facial symmetry. Rhinology. 2013;51:18-21.
- Swift A, Remington K. BeautiPHIcationTM: a global approach to facial beauty. Clin Plast Surg. 2011;38:247-277.
- Marquardt SR. Dr. Stephen R. Marquardt on the Golden Decagon and human facial beauty. interview by Dr. Gottlieb. J Clin Orthod. 2002;36:339-347.
- Veerala G, Gandikota CS, Yadagiri PK, et al. Marquardt’s facial Golden Decagon mask and its fitness with South Indian facial traits. J Clin Diagn Res. 2016;10:ZC49-ZC52.
- Holland E. Marquardt’s Phi mask: pitfalls of relying on fashion models and the golden ratio to describe a beautiful face. Aesthetic Plast Surg. 2008;32:200-208.
- Alam MK, Mohd Noor NF, Basri R, et al. Multiracial facial golden ratio and evaluation of facial appearance. PLoS One. 2015;10:e0142914.
- Kim YH. Easy facial analysis using the facial golden mask. J Craniofac Surg. 2007;18:643-649.
- Bashour M. An objective system for measuring facial attractiveness. Plast Reconstr Surg. 2006;118:757-774; discussion 775-776.
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592-600.
- Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg. 2000;26:1107-1112.
Several concepts of ideal aesthetic measurements can be traced back to ancient Greek and European Renaissance art. In examining canons of beauty, these classical ideals often are compared to modern-day standards, allowing clinicians to delineate the parameters of an attractive facial appearance and facilitate the planning of cosmetic procedures.
Given the growing number of available cosmetic interventions, dermatologists have a powerful ability to modify facial proportions; however, changes to individual structures should be made with a mindful approach to improving overall facial harmony. This article reviews the established parameters of facial beauty to assist the clinician in enhancing cosmetic outcomes.
Canons of Facial Aesthetics
Horizontal Thirds
In his writings on human anatomy, Leonardo da Vinci described dividing the face into equal thirds (Figure 1). The upper third measures from the trichion (the midline point of the normal hairline) to the glabella (the smooth prominence between the eyebrows). The middle third measures from the glabella to the subnasale (the midline point where the nasal septum meets the upper lip). The lower third measures from the subnasale to the menton (the most inferior point of the chin).1
Although the validity of the canon is intended to apply across race and gender, these proportions may vary by ethnicity (Table). In white individuals, the middle third of the face tends to be shorter than the upper and lower thirds.2 This same relationship has been observed in black males.3 In Chinese females, the upper third commonly is shorter than the middle and lower thirds, correlating with a less prominent forehead. In contrast, black females tend to have a relatively longer upper third.4
The relationship between modern perceptions of attractiveness and the neoclassical norm of equal thirds remains a topic of interest. Milutinovic et al1 examined facial thirds in white female celebrities from beauty and fashion magazines and compared them to a group of anonymous white females from the general population. The group of anonymous females showed statistically significant (P<.05) differences between the sizes of the 3 facial segments, whereas the group of celebrity faces demonstrated uniformity between the facial thirds.1
The lower face can itself be divided into thirds, with the upper third measured from the subnasale to the stomion (the midline point of the oral fissure when the lips are closed), and the lower two-thirds measured from the stomion to the menton (Figure 1). Mommaerts and Moerenhout5 examined photographs of 105 attractive celebrity faces and compared their proportions to those of classical sculptures of gods and goddesses (antique faces). The authors identified an upper one-third to lower two-thirds ratio of 69.8% in celebrity females and 69.1% in celebrity males; these ratios were not significantly different from the 72.4% seen in antique females and 73.1% in antique males. The authors concluded that a 30% upper lip to 70% lower lip-chin proportion may be the most appropriate to describe contemporary standards.5

Vertical Fifths
In the vertical dimension, the neoclassical canon of facial proportions divides the face into equal fifths (Figure 2).6 The 2 most lateral fifths are measured from the lateral helix of each ear to the exocanthus of each eye. The eye fissure lengths (measured between the endocanthion and exocanthion of each eye) represent one-fifth. The middle fifth is measured between the medial canthi of both eyes (endocanthion to endocanthion). This distance is equal to the width of the nose, as measured between both alae. Finally, the width of the mouth represents 1.5-times the width of the nose. These ratios of the vertical fifths apply to both males and females.6

Anthropometric studies have examined deviations from the neoclassical canon according to ethnicity. Wang et al7 compared the measurements of North American white and Han Chinese patients to these standards. White patients demonstrated a greater ratio of mouth width to nose width relative to the canon. In contrast, Han Chinese patients demonstrated a relatively wider nose and narrower mouth.7
In black individuals, it has been observed that the dimensions of most facial segments correspond to the neoclassical standards; however, nose width is relatively wider in black individuals relative to the canon as well as relative to white individuals.8
Milutinovic et al1 also compared vertical fifths between white celebrities and anonymous females. In the anonymous female group, statistically significant (P<.05) variations were found between the sizes of the different facial components. In contrast, the celebrity female group showed balance between the widths of vertical fifths.1
Lips
In the lower facial third, the lips represent a key element of attractiveness. Recently, lip augmentation, aimed at creating fuller and plumper lips, has dominated the popular culture and social media landscape.9 Although the aesthetic ideal of lips continues to evolve over time, recent studies have aimed at quantifying modern notions of attractive lip appearance.
Popenko et al10 examined lip measurements using computer-generated images of white women with different variations of lip sizes and lower face proportions. Computer-generated faces were graded on attractiveness by more than 400 individuals from focus groups. An upper lip to lower lip ratio of 1:2 was judged to be the most attractive, while a ratio of 2:1 was judged to be the least attractive. Results also showed that the surface area of the most attractive lips comprised roughly 10% of the lower third of the face.10
Penna et al11 analyzed various parameters of the lips and lower facial third using photographs of 176 white males and females that were judged on attractiveness by 250 volunteer evaluators. Faces were graded on a scale from 1 (absolutely attractive) to 7 (absolutely unattractive). Attractive males and females (grades 1 and 2) both demonstrated an average ratio of upper vermilion height to nose-mouth distance (measured from the subnasalae to the lower edge of the upper vermilion border) of 0.28, which was significantly greater than the average ratio observed in less attractive individuals (grades 6 or 7)(P<.05). In addition, attractive males and females demonstrated a ratio of upper vermilion height to nose-chin distance (measured from the subnasalae to the menton) of 0.09, which again was larger than the average ratio seen in less attractive individuals. Figure 3 demonstrates an aesthetic ideal of the lips derived from these 2 studies, though consideration should be given to the fact that these studies were based in white populations.

Golden Ratio
The golden ratio, also known as Phi, can be observed in nature, art, and architecture. Approximately equal to 1.618, the golden ratio also has been identified as a possible marker of beauty in the human face and has garnered attention in the lay press. The ratio has been applied to several proportions and structures in the face, such as the ratio of mouth width to nose width or the ratio of tooth height to tooth width, with investigation providing varying levels of validation about whether these ratios truly correlate with perceptions of beauty.12 Swift and Remington13 advocated for application of the golden ratio toward a comprehensive set of facial proportions. Marquardt14 used the golden ratio to create a 3-dimensional representation of an idealized face, known as the golden decagon mask. Although the golden ratio and the golden decagon mask have been proposed as analytic tools, their utility in clinical practice may be limited. Firstly, due to its popularity in the lay press, the golden ratio has been inconsistently applied to a wide range of facial ratios, which may undermine confidence in its representation as truth rather than coincidence. Secondly, although some authors have found validity of the golden decagon mask in representing unified ratios of attractiveness, others have asserted that it characterizes a masculinized white female and fails to account for ethnic differences.15-19
Age-Related Changes
In addition to the facial proportions guided by genetics, several changes occur with increased age. Over the course of a lifetime, predictable patterns emerge in the dimensions of the skin, soft tissue, and bone. These alterations in structural proportions may ultimately lead to an unevenness in facial aesthetics.
In skeletal structure, gradual bone resorption and expansion causes a reduction in facial height as well as an increase in facial width and depth.20 Fat atrophy and hypertrophy affect soft tissue proportions, visualized as hollowing at the temples, cheeks, and around the eyes, along with fullness in the submental region and jowls.21 Finally, decreases in skin elasticity and collagen exacerbate the appearance of rhytides and sagging. In older patients who desire a more youthful appearance, various applications of dermal fillers, fat grafting, liposuction, and skin tightening techniques can help to mitigate these changes.
Conclusion
Improving facial aesthetics relies on an understanding of the norms of facial proportions. Although cosmetic interventions commonly are advertised or described based on a single anatomical unit, it is important to appreciate the relationships between facial structures. Most notably, clinicians should be mindful of facial ratios when considering the introduction of filler materials or implants. Augmentation procedures at the temples, zygomatic arch, jaw, chin, and lips all have the possibility to alter facial ratios. Changes should therefore be considered in the context of improving overall facial harmony, with the clinician remaining cognizant of the ideal vertical and horizontal divisions of the face. Understanding such concepts and communicating them to patients can help in appropriately addressing all target areas, thereby leading to greater patient satisfaction.
Several concepts of ideal aesthetic measurements can be traced back to ancient Greek and European Renaissance art. In examining canons of beauty, these classical ideals often are compared to modern-day standards, allowing clinicians to delineate the parameters of an attractive facial appearance and facilitate the planning of cosmetic procedures.
Given the growing number of available cosmetic interventions, dermatologists have a powerful ability to modify facial proportions; however, changes to individual structures should be made with a mindful approach to improving overall facial harmony. This article reviews the established parameters of facial beauty to assist the clinician in enhancing cosmetic outcomes.
Canons of Facial Aesthetics
Horizontal Thirds
In his writings on human anatomy, Leonardo da Vinci described dividing the face into equal thirds (Figure 1). The upper third measures from the trichion (the midline point of the normal hairline) to the glabella (the smooth prominence between the eyebrows). The middle third measures from the glabella to the subnasale (the midline point where the nasal septum meets the upper lip). The lower third measures from the subnasale to the menton (the most inferior point of the chin).1
Although the validity of the canon is intended to apply across race and gender, these proportions may vary by ethnicity (Table). In white individuals, the middle third of the face tends to be shorter than the upper and lower thirds.2 This same relationship has been observed in black males.3 In Chinese females, the upper third commonly is shorter than the middle and lower thirds, correlating with a less prominent forehead. In contrast, black females tend to have a relatively longer upper third.4
The relationship between modern perceptions of attractiveness and the neoclassical norm of equal thirds remains a topic of interest. Milutinovic et al1 examined facial thirds in white female celebrities from beauty and fashion magazines and compared them to a group of anonymous white females from the general population. The group of anonymous females showed statistically significant (P<.05) differences between the sizes of the 3 facial segments, whereas the group of celebrity faces demonstrated uniformity between the facial thirds.1
The lower face can itself be divided into thirds, with the upper third measured from the subnasale to the stomion (the midline point of the oral fissure when the lips are closed), and the lower two-thirds measured from the stomion to the menton (Figure 1). Mommaerts and Moerenhout5 examined photographs of 105 attractive celebrity faces and compared their proportions to those of classical sculptures of gods and goddesses (antique faces). The authors identified an upper one-third to lower two-thirds ratio of 69.8% in celebrity females and 69.1% in celebrity males; these ratios were not significantly different from the 72.4% seen in antique females and 73.1% in antique males. The authors concluded that a 30% upper lip to 70% lower lip-chin proportion may be the most appropriate to describe contemporary standards.5

Vertical Fifths
In the vertical dimension, the neoclassical canon of facial proportions divides the face into equal fifths (Figure 2).6 The 2 most lateral fifths are measured from the lateral helix of each ear to the exocanthus of each eye. The eye fissure lengths (measured between the endocanthion and exocanthion of each eye) represent one-fifth. The middle fifth is measured between the medial canthi of both eyes (endocanthion to endocanthion). This distance is equal to the width of the nose, as measured between both alae. Finally, the width of the mouth represents 1.5-times the width of the nose. These ratios of the vertical fifths apply to both males and females.6

Anthropometric studies have examined deviations from the neoclassical canon according to ethnicity. Wang et al7 compared the measurements of North American white and Han Chinese patients to these standards. White patients demonstrated a greater ratio of mouth width to nose width relative to the canon. In contrast, Han Chinese patients demonstrated a relatively wider nose and narrower mouth.7
In black individuals, it has been observed that the dimensions of most facial segments correspond to the neoclassical standards; however, nose width is relatively wider in black individuals relative to the canon as well as relative to white individuals.8
Milutinovic et al1 also compared vertical fifths between white celebrities and anonymous females. In the anonymous female group, statistically significant (P<.05) variations were found between the sizes of the different facial components. In contrast, the celebrity female group showed balance between the widths of vertical fifths.1
Lips
In the lower facial third, the lips represent a key element of attractiveness. Recently, lip augmentation, aimed at creating fuller and plumper lips, has dominated the popular culture and social media landscape.9 Although the aesthetic ideal of lips continues to evolve over time, recent studies have aimed at quantifying modern notions of attractive lip appearance.
Popenko et al10 examined lip measurements using computer-generated images of white women with different variations of lip sizes and lower face proportions. Computer-generated faces were graded on attractiveness by more than 400 individuals from focus groups. An upper lip to lower lip ratio of 1:2 was judged to be the most attractive, while a ratio of 2:1 was judged to be the least attractive. Results also showed that the surface area of the most attractive lips comprised roughly 10% of the lower third of the face.10
Penna et al11 analyzed various parameters of the lips and lower facial third using photographs of 176 white males and females that were judged on attractiveness by 250 volunteer evaluators. Faces were graded on a scale from 1 (absolutely attractive) to 7 (absolutely unattractive). Attractive males and females (grades 1 and 2) both demonstrated an average ratio of upper vermilion height to nose-mouth distance (measured from the subnasalae to the lower edge of the upper vermilion border) of 0.28, which was significantly greater than the average ratio observed in less attractive individuals (grades 6 or 7)(P<.05). In addition, attractive males and females demonstrated a ratio of upper vermilion height to nose-chin distance (measured from the subnasalae to the menton) of 0.09, which again was larger than the average ratio seen in less attractive individuals. Figure 3 demonstrates an aesthetic ideal of the lips derived from these 2 studies, though consideration should be given to the fact that these studies were based in white populations.

Golden Ratio
The golden ratio, also known as Phi, can be observed in nature, art, and architecture. Approximately equal to 1.618, the golden ratio also has been identified as a possible marker of beauty in the human face and has garnered attention in the lay press. The ratio has been applied to several proportions and structures in the face, such as the ratio of mouth width to nose width or the ratio of tooth height to tooth width, with investigation providing varying levels of validation about whether these ratios truly correlate with perceptions of beauty.12 Swift and Remington13 advocated for application of the golden ratio toward a comprehensive set of facial proportions. Marquardt14 used the golden ratio to create a 3-dimensional representation of an idealized face, known as the golden decagon mask. Although the golden ratio and the golden decagon mask have been proposed as analytic tools, their utility in clinical practice may be limited. Firstly, due to its popularity in the lay press, the golden ratio has been inconsistently applied to a wide range of facial ratios, which may undermine confidence in its representation as truth rather than coincidence. Secondly, although some authors have found validity of the golden decagon mask in representing unified ratios of attractiveness, others have asserted that it characterizes a masculinized white female and fails to account for ethnic differences.15-19
Age-Related Changes
In addition to the facial proportions guided by genetics, several changes occur with increased age. Over the course of a lifetime, predictable patterns emerge in the dimensions of the skin, soft tissue, and bone. These alterations in structural proportions may ultimately lead to an unevenness in facial aesthetics.
In skeletal structure, gradual bone resorption and expansion causes a reduction in facial height as well as an increase in facial width and depth.20 Fat atrophy and hypertrophy affect soft tissue proportions, visualized as hollowing at the temples, cheeks, and around the eyes, along with fullness in the submental region and jowls.21 Finally, decreases in skin elasticity and collagen exacerbate the appearance of rhytides and sagging. In older patients who desire a more youthful appearance, various applications of dermal fillers, fat grafting, liposuction, and skin tightening techniques can help to mitigate these changes.
Conclusion
Improving facial aesthetics relies on an understanding of the norms of facial proportions. Although cosmetic interventions commonly are advertised or described based on a single anatomical unit, it is important to appreciate the relationships between facial structures. Most notably, clinicians should be mindful of facial ratios when considering the introduction of filler materials or implants. Augmentation procedures at the temples, zygomatic arch, jaw, chin, and lips all have the possibility to alter facial ratios. Changes should therefore be considered in the context of improving overall facial harmony, with the clinician remaining cognizant of the ideal vertical and horizontal divisions of the face. Understanding such concepts and communicating them to patients can help in appropriately addressing all target areas, thereby leading to greater patient satisfaction.
- Milutinovic J, Zelic K, Nedeljkovic N. Evaluation of facial beauty using anthropometric proportions. ScientificWorldJournal. 2014;2014:428250. doi:10.1155/2014/428250.
- Farkas LG, Hreczko TA, Kolar JC, et al. Vertical and horizontal proportions of the face in young-adult North-American Caucasians: revision of neoclassical canons. Plast Reconstr Surg. 1985;75:328-338.
- Porter JP. The average African American male face: an anthropometric analysis. Arch Facial Plast Surg. 2004;6:78-81.
- Porter JP, Olson KL. Anthropometric facial analysis of the African American woman. Arch Facial Plast Surg. 2001;3:191-197.
- Mommaerts MY, Moerenhout BA. Ideal proportions in full face front view, contemporary versus antique. J Craniomaxillofac Surg. 2011;39:107-110.
- Vegter F, Hage JJ. Clinical anthropometry and canons of the face in historical perspective. Plast Reconstr Surg. 2000;106:1090-1096.
- Wang D, Qian G, Zhang M, et al. Differences in horizontal, neoclassical facial canons in Chinese (Han) and North American Caucasian populations. Aesthetic Plast Surg. 1997;21:265-269.
- Farkas LG, Forrest CR, Litsas L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast Surg. 2000;24:179-184.
- Coleman GG, Lindauer SJ, Tüfekçi E, et al. Influence of chin prominence on esthetic lip profile preferences. Am J Orthod Dentofacial Orthop. 2007;132:36-42.
- Popenko NA, Tripathi PB, Devcic Z, et al. A quantitative approach to determining the ideal female lip aesthetic and its effect on facial attractiveness. JAMA Facial Plast Surg. 2017;19:261-267.
- Penna V, Fricke A, Iblher N, et al. The attractive lip: a photomorphometric analysis. J Plast Reconstr Aesthet Surg. 2015;68:920-929.
- Prokopakis EP, Vlastos IM, Picavet VA, et al. The golden ratio in facial symmetry. Rhinology. 2013;51:18-21.
- Swift A, Remington K. BeautiPHIcationTM: a global approach to facial beauty. Clin Plast Surg. 2011;38:247-277.
- Marquardt SR. Dr. Stephen R. Marquardt on the Golden Decagon and human facial beauty. interview by Dr. Gottlieb. J Clin Orthod. 2002;36:339-347.
- Veerala G, Gandikota CS, Yadagiri PK, et al. Marquardt’s facial Golden Decagon mask and its fitness with South Indian facial traits. J Clin Diagn Res. 2016;10:ZC49-ZC52.
- Holland E. Marquardt’s Phi mask: pitfalls of relying on fashion models and the golden ratio to describe a beautiful face. Aesthetic Plast Surg. 2008;32:200-208.
- Alam MK, Mohd Noor NF, Basri R, et al. Multiracial facial golden ratio and evaluation of facial appearance. PLoS One. 2015;10:e0142914.
- Kim YH. Easy facial analysis using the facial golden mask. J Craniofac Surg. 2007;18:643-649.
- Bashour M. An objective system for measuring facial attractiveness. Plast Reconstr Surg. 2006;118:757-774; discussion 775-776.
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592-600.
- Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg. 2000;26:1107-1112.
- Milutinovic J, Zelic K, Nedeljkovic N. Evaluation of facial beauty using anthropometric proportions. ScientificWorldJournal. 2014;2014:428250. doi:10.1155/2014/428250.
- Farkas LG, Hreczko TA, Kolar JC, et al. Vertical and horizontal proportions of the face in young-adult North-American Caucasians: revision of neoclassical canons. Plast Reconstr Surg. 1985;75:328-338.
- Porter JP. The average African American male face: an anthropometric analysis. Arch Facial Plast Surg. 2004;6:78-81.
- Porter JP, Olson KL. Anthropometric facial analysis of the African American woman. Arch Facial Plast Surg. 2001;3:191-197.
- Mommaerts MY, Moerenhout BA. Ideal proportions in full face front view, contemporary versus antique. J Craniomaxillofac Surg. 2011;39:107-110.
- Vegter F, Hage JJ. Clinical anthropometry and canons of the face in historical perspective. Plast Reconstr Surg. 2000;106:1090-1096.
- Wang D, Qian G, Zhang M, et al. Differences in horizontal, neoclassical facial canons in Chinese (Han) and North American Caucasian populations. Aesthetic Plast Surg. 1997;21:265-269.
- Farkas LG, Forrest CR, Litsas L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast Surg. 2000;24:179-184.
- Coleman GG, Lindauer SJ, Tüfekçi E, et al. Influence of chin prominence on esthetic lip profile preferences. Am J Orthod Dentofacial Orthop. 2007;132:36-42.
- Popenko NA, Tripathi PB, Devcic Z, et al. A quantitative approach to determining the ideal female lip aesthetic and its effect on facial attractiveness. JAMA Facial Plast Surg. 2017;19:261-267.
- Penna V, Fricke A, Iblher N, et al. The attractive lip: a photomorphometric analysis. J Plast Reconstr Aesthet Surg. 2015;68:920-929.
- Prokopakis EP, Vlastos IM, Picavet VA, et al. The golden ratio in facial symmetry. Rhinology. 2013;51:18-21.
- Swift A, Remington K. BeautiPHIcationTM: a global approach to facial beauty. Clin Plast Surg. 2011;38:247-277.
- Marquardt SR. Dr. Stephen R. Marquardt on the Golden Decagon and human facial beauty. interview by Dr. Gottlieb. J Clin Orthod. 2002;36:339-347.
- Veerala G, Gandikota CS, Yadagiri PK, et al. Marquardt’s facial Golden Decagon mask and its fitness with South Indian facial traits. J Clin Diagn Res. 2016;10:ZC49-ZC52.
- Holland E. Marquardt’s Phi mask: pitfalls of relying on fashion models and the golden ratio to describe a beautiful face. Aesthetic Plast Surg. 2008;32:200-208.
- Alam MK, Mohd Noor NF, Basri R, et al. Multiracial facial golden ratio and evaluation of facial appearance. PLoS One. 2015;10:e0142914.
- Kim YH. Easy facial analysis using the facial golden mask. J Craniofac Surg. 2007;18:643-649.
- Bashour M. An objective system for measuring facial attractiveness. Plast Reconstr Surg. 2006;118:757-774; discussion 775-776.
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592-600.
- Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg. 2000;26:1107-1112.
Practice Points
- Canons of ideal facial dimensions have existed since antiquity and remain relevant in modern times.
- Horizontal and vertical anatomical ratios can provide a useful framework for cosmetic interventions.
- To maximize aesthetic results, alterations to individual cosmetic units should be made with thoughtful consideration of overall facial harmony.
Microneedling With Stem Cells
Psoriasis Oral Therapy Update: Report From the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dermatopathology Pearls: Report From the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Topical Cannabinoids in Dermatology
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.
Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
Practice Points
- Topical cannabinoids are advertised by companies as treatment options for numerous dermatologic conditions.
- Despite promising data in rodent models, there have been no rigorous studies to date confirming efficacy or safety in humans.
- Dermatologists should therefore inquire with patients about the use of any topical cannabinoid products, especially around the time of planned procedures, as they may affect treatment outcomes.
Local Anesthetics in Cosmetic Dermatology
Local anesthesia is a central component of successful interventions in cosmetic dermatology. The number of anesthetic medications and administration techniques has grown in recent years as outpatient cosmetic procedures continue to expand. Pain is a common barrier to cosmetic procedures, and alleviating the fear of painful interventions is critical to patient satisfaction and future visits. To accommodate a multitude of cosmetic interventions, it is important for clinicians to be well versed in applications of topical and regional anesthesia. In this article, we review pain management strategies for use in cosmetic practice.
Local Anesthetics
The sensation of pain is carried to the central nervous system by unmyelinated C nerve fibers. Local anesthetics (LAs) act by blocking fast voltage-gated sodium channels in the cell membrane of the nerve, thereby inhibiting downstream propagation of an action potential and the transmission of painful stimuli.1 The chemical structure of LAs is fundamental to their mechanism of action and metabolism. Local anesthetics contain a lipophilic aromatic group, an intermediate chain, and a hydrophilic amine group. Broadly, agents are classified as amides or esters depending on the chemical group attached to the intermediate chain.2 Amides (eg, lidocaine, bupivacaine, articaine, mepivacaine, prilocaine, levobupivacaine) are metabolized by the hepatic system; esters (eg, procaine, proparacaine, benzocaine, chlorprocaine, tetracaine, cocaine) are metabolized by plasma cholinesterase, which produces para-aminobenzoic acid, a potentially dangerous metabolite that has been implicated in allergic reactions.3
Lidocaine is the most prevalent LA used in dermatology practices. Importantly, lidocaine is a class IB antiarrhythmic agent used in cardiology to treat ventricular arrhythmias.4 As an anesthetic, a maximum dose of 4.5 mg/kg can be administered, increasing to 7.0 mg/kg when mixed with epinephrine; with higher doses, there is a risk for central nervous system and cardiovascular toxicity.5 Initial symptoms of lidocaine toxicity include dizziness, tinnitus, circumoral paresthesia, blurred vision, and a metallic taste in the mouth.6 Systemic absorption of topical anesthetics is heightened across mucosal membranes, and care should be taken when applying over large surface areas.
Allergic reactions to LAs may be local or less frequently systemic. It is important to note that LAs tend to show cross-reactivity within their class rather than across different classes.7 Reactions can be classified as type I or type IV. Type I (IgE-mediated) reactions evolve in minutes to hours, affecting the skin and possibly leading to respiratory and circulatory collapse. Delayed reactions to LAs have increased in recent years, with type IV contact allergy most frequently found in connection with benzocaine and lidocaine.8
Topical Anesthesia
Topical anesthetics are effective and easy to use and are particularly valuable in patients with needle phobia. In certain cases, these medications may be applied by the patient prior to arrival, thereby reducing visit time. Topical agents act on nerve fibers running through the dermis; therefore, efficacy is dependent on successful penetration through the stratum corneum and viable epidermis. To enhance absorption, agents may be applied under an occlusive dressing.
Topical anesthetics are most commonly used for injectable fillers, ablative and nonablative laser resurfacing, laser hair removal, and tattoo removal. The eutectic mixture of 2.5% lidocaine and 2.5% prilocaine as well as topical 4% or 5% lidocaine are the most commonly used US Food and Drug Administration–approved products for topical anesthesia. In addition, several compounded pharmacy products are available.
After 60 minutes of application of the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine, a 3-mm depth of analgesia is reached, and after 120 minutes, a 4.5-mm depth is reached.9 It elicits a biphasic vascular response of vasoconstriction and blanching followed by vasodilation and erythema.10 Most adverse events are mild and transient, but allergic contact dermatitis and contact urticaria have been reported.11-13 In older children and adults, the maximum application area is 200 cm2, with a maximum dose of 20 g used for no longer than 4 hours.
The 4% or 5% lidocaine cream uses a liposomal delivery system, which is designed to improve cutaneous penetration and has been shown to provide longer durations of anesthesia than nonliposomal lidocaine preparations.14 Application should be performed 30 to 60 minutes prior to a procedure. In a study comparing the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine versus lidocaine cream 5% for pain control during laser hair removal with a 1064-nm Nd:YAG laser, no significant differences were found.15 The maximum application area is 100 cm2 in children weighing less than 20 kg. A study of healthy adults demonstrated safety with the use of 30 to 60 g of occluded liposomal lidocaine cream 4%.16
In addition to US Food and Drug Administration–approved products, several compounded pharmacy products are available for topical anesthesia. These formulations include benzocaine-lidocaine-tetracaine gel, tetracaine-adrenaline-cocaine solution, and lidocaine-epinephrine-tetracaine solution. A triple-anesthetic gel, benzocaine-lidocaine-tetracaine is widely used in cosmetic practice. The product has been shown to provide adequate anesthesia for laser resurfacing after 20 minutes without occlusion.17 Of note, compounded anesthetics lack standardization, and different pharmacies may follow their own individual protocols.
Regional Anesthesia
Regional nerve blockade is a useful option for more widespread or complex interventions. Using regional nerve blockade, effective analgesia can be delivered to a target area while avoiding the toxicity and pain associated with numerous anesthetic infiltrations. In addition, there is no distortion of the tissue architecture, allowing for improved visual evaluation during the procedure. Recently, hyaluronic acid fillers have been compounded with lidocaine as a means of reducing procedural pain.
Blocks for Dermal Fillers
Forehead
For dermal filler injections of the glabellar and frontalis lines, anesthesia of the forehead may be desired. The supraorbital and supratrochlear nerves supply this area. The supraorbital nerve can be injected at the supraorbital notch, which is measured roughly 2.7 cm from the glabella. The orbital rim should be palpated with the nondominant hand, and 1 to 2 mL of anesthetic should be injected just below the rim (Figure 1). The supratrochlear nerve is located roughly 1.7 cm from the midline and can be similarly injected under the orbital rim with 1 to 2 mL of anesthetic (Figure 1).
Lateral Temple Region
Anesthesia of the zygomaticotemporal nerve can be used to reduce pain from dermal filler injections of the lateral canthal and temporal areas. The nerve is identified by first palpating the zygomaticofrontal suture. A long needle is then inserted posteriorly, immediately behind the concave surface of the lateral orbital rim, and 1 to 2 mL of anesthetic is injected (Figure 1).
Malar Region
Blockade of the zygomaticofacial nerve is commonly performed in conjunction with the zygomaticotemporal nerve and provides anesthesia to the malar region for cheek augmentation procedures. To identify the target area, the junction of the lateral and inferior orbital rim should be palpated. With the needle placed just lateral to this point, 1 to 2 mL of anesthetic is injected (Figure 1).
Blocks for Perioral Fillers
Upper Lips/Nasolabial Folds
Bilateral blockade of the infraorbital nerves provides anesthesia to the upper lip and nasolabial folds prior to filler injections. The infraorbital nerve can be targeted via an intraoral route where it exits the maxilla at the infraorbital foramen. The nerve is anesthetized by palpating the infraorbital ridge and injecting 3 to 5 mL of anesthetic roughly 1 cm below this point on the vertical axis of the midpupillary line (Figure 1). The external nasal nerve, thought to be a branch of cranial nerve V, also may be targeted if there is inadequate anesthesia from the infraorbital block. This nerve is reached by injecting at the osseocartilaginous junction of the nasal bones (Figure 1).
Lower Lips
Blockade of the mental nerve provides anesthesia to the lower lips for augmentation procedures. The mental nerve can be targeted on each side at the mental foramen, which is located below the root of the lower second premolar. Aiming roughly 1 cm below the gumline, 3 to 5 mL of anesthetic is injected intraorally (Figure 1). A transcutaneous approach toward the same target also is possible, though this technique risks visible bruising. Alternatively, the upper or lower lips can be anesthetized using 4 to 5 submucosal injections at evenly spaced intervals between the canine teeth.18
Blocks for Palmoplantar Hyperhidrosis
The treatment of palmoplantar hyperhidrosis benefits from regional blocks. Botulinum toxin has been well established as an effective therapy for the condition.19-21 Given the sensitivity of palmoplantar sites, it is valuable to achieve effective analgesia of the region prior to dermal injections of botulinum toxin.
Wrists
Sensory innervation of the palm is provided by the median, ulnar, and radial nerves (Figure 2A).
The ulnar nerve is anesthetized between the ulnar artery and the flexor carpi ulnaris muscle. The artery is identified by palpation, and special care should be taken to avoid intra-arterial injection. The needle is directed toward the radial styloid, and 3 to 5 mL of anesthetic is injected roughly 1 cm proximal to the wrist crease (Figure 2B).
Anesthesia of the radial nerve can be considered a field block given the numerous small branches that supply the hand. These branches are reached by injecting anesthetic roughly 2 to 3 cm proximal to the radial styloid with the needle aimed medially and extending the injection dorsally (Figure 2B). A total of 4 to 6 mL of anesthetic is used.
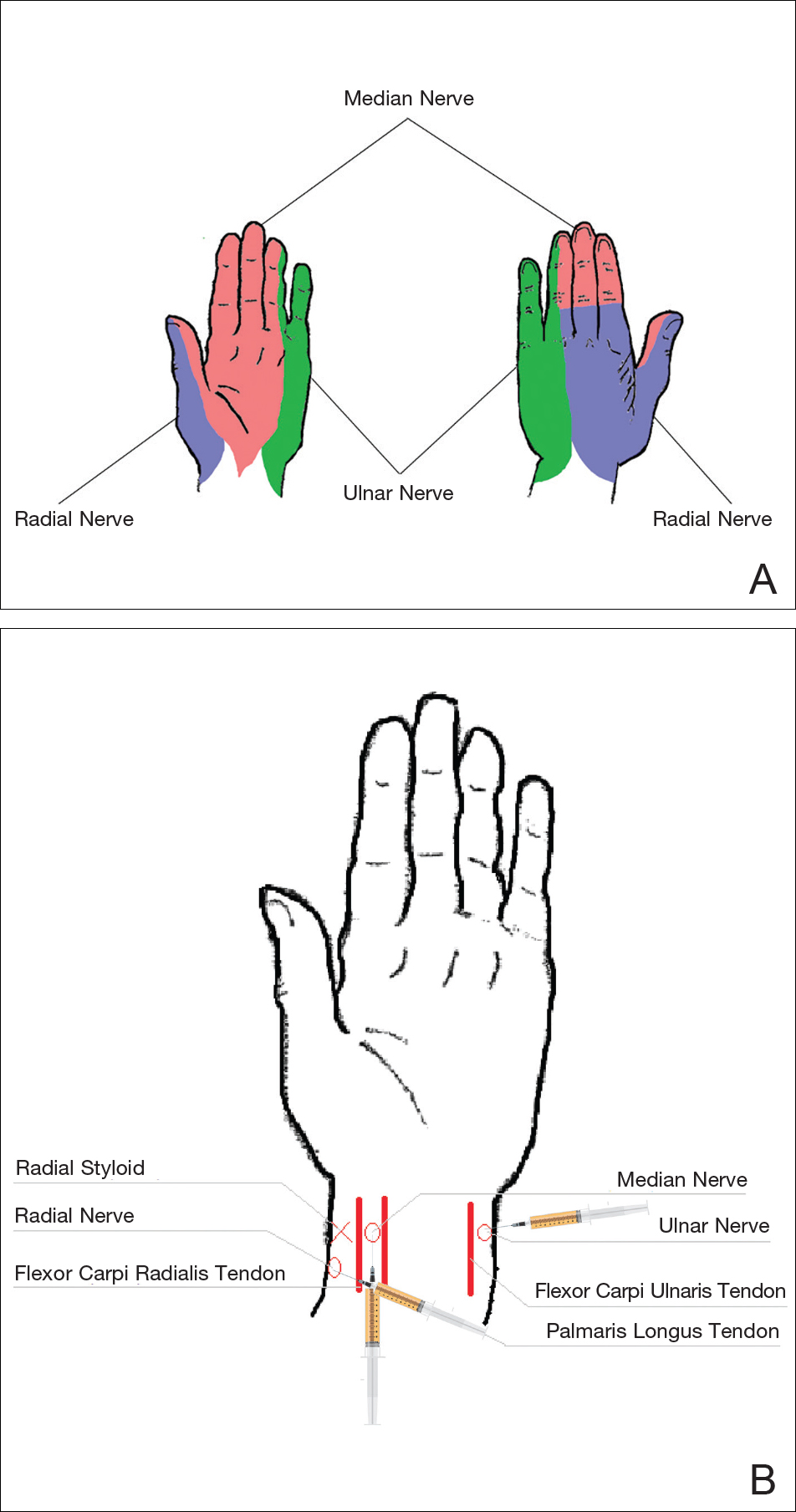
Ankles
An ankle block provides anesthesia to the dorsal and plantar surfaces of the foot.22 The region is supplied by the superficial peroneal nerve, deep peroneal nerve, sural nerve, saphenous nerve, and branches of the posterior tibial nerve (Figure 3A).
To anesthetize the deep peroneal nerve, the extensor hallucis longus tendon is first identified on the anterior surface of the ankle through dorsiflexion of the toes; the dorsalis pedis artery runs in close proximity. The injection should be placed lateral to the tendon and artery (Figure 3B). The needle should be inserted until bone is reached, withdrawn slightly, and then 3 to 5 mL of anesthetic should be injected. To block the saphenous nerve, the needle can then be directed superficially toward the medial malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C). To block the superficial peroneal nerve, the needle should then be directed toward the lateral malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C).
The posterior tibial nerve is located posterior to the medial malleolus. The dorsalis pedis artery can be palpated near this location. The needle should be inserted posterior to the artery, extending until bone is reached (Figure 3C). The needle is then withdrawn slightly, and 3 to 5 mL of anesthetic is injected. Finally, the sural nerve is anesthetized between the Achilles tendon and the lateral malleolus, using 5 mL of anesthetic to raise a subcutaneous wheal (Figure 3C).
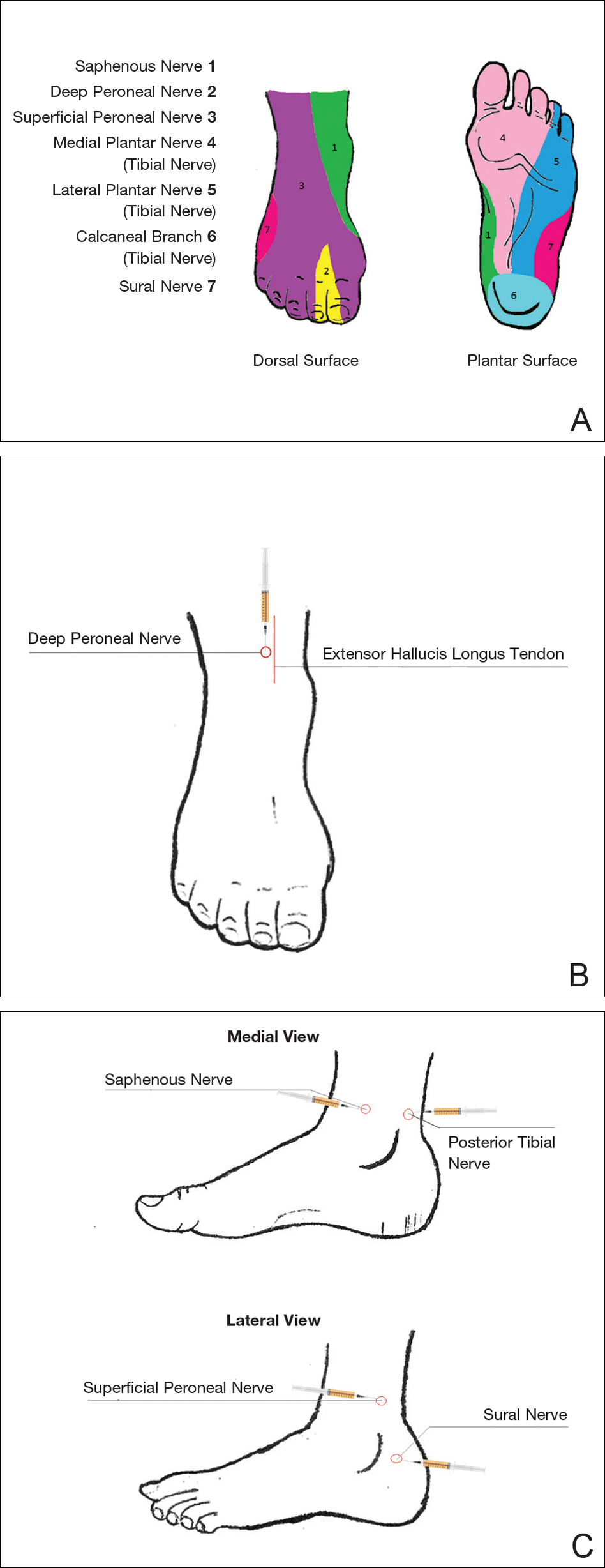
Conclusion
Proper pain management is integral to ensuring a positive experience for cosmetic patients. Enhanced knowledge of local anesthetic techniques allows the clinician to provide for a variety of procedural indications and patient preferences. As anesthetic strategies are continually evolving, it is important for practitioners to remain informed of these developments.
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52-61.
- Auletta MJ. Local anesthesia for dermatologic surgery. Semin Dermatol. 1994;13:35-42.
- Park KK, Sharon VR. A review of local anesthetics: minimizing risk and side effects in cutaneous surgery. Dermatol Surg. 2017;43:173-187.
- Reiz S, Nath S. Cardiotoxicity of local anaesthetic agents. Br J Anaesth. 1986;58:736-746.
- Klein JA, Kassarjdian N. Lidocaine toxicity with tumescent liposuction. a case report of probable drug interactions. Dermatol Surg. 1997;23:1169-1174.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Morais-Almeida M, Gaspar A, Marinho S, et al. Allergy to local anesthetics of the amide group with tolerance to procaine. Allergy. 2003;58:827-828.
- To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372.
- Wahlgren CF, Quiding H. Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream). J Am Acad Dermatol. 2000;42:584-588.
- Bjerring P, Andersen PH, Arendt-Nielsen L. Vascular response of human skin after analgesia with EMLA cream. Br J Anaesth. 1989;63:655-660.
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111.
- Thakur BK, Murali MR. EMLA cream-induced allergic contact dermatitis: a role for prilocaine as an immunogen. J Allergy Clin Immunol. 1995;95:776-778.
- Waton J, Boulanger A, Trechot PH, et al. Contact urticaria from EMLA cream. Contact Dermatitis. 2004;51:284-287.
- Bucalo BD, Mirikitani EJ, Moy RL. Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol Surg. 1998;24:537-541.
- Guardiano RA, Norwood CW. Direct comparison of EMLA versus lidocaine for pain control in Nd:YAG 1,064 nm laser hair removal. Dermatol Surg. 2005;31:396-398.
- Nestor MS. Safety of occluded 4% liposomal lidocaine cream. J Drugs Dermatol. 2006;5:618-620.
- Oni G, Rasko Y, Kenkel J. Topical lidocaine enhanced by laser pretreatment: a safe and effective method of analgesia for facial rejuvenation. Aesthet Surg J. 2013;33:854-861.
- Niamtu J 3rd. Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatol Surg. 2005;31:1330-1332.
- Naumann M, Flachenecker P, Brocker EB, et al. Botulinum toxin for palmar hyperhidrosis. Lancet. 1997;349:252.
- Naumann M, Hofmann U, Bergmann I, et al. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301-304.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38(2, pt 1):227-229.
- Davies T, Karanovic S, Shergill B. Essential regional nerve blocks for the dermatologist: part 2. Clin Exp Dermatol. 2014;39:861-867.
Local anesthesia is a central component of successful interventions in cosmetic dermatology. The number of anesthetic medications and administration techniques has grown in recent years as outpatient cosmetic procedures continue to expand. Pain is a common barrier to cosmetic procedures, and alleviating the fear of painful interventions is critical to patient satisfaction and future visits. To accommodate a multitude of cosmetic interventions, it is important for clinicians to be well versed in applications of topical and regional anesthesia. In this article, we review pain management strategies for use in cosmetic practice.
Local Anesthetics
The sensation of pain is carried to the central nervous system by unmyelinated C nerve fibers. Local anesthetics (LAs) act by blocking fast voltage-gated sodium channels in the cell membrane of the nerve, thereby inhibiting downstream propagation of an action potential and the transmission of painful stimuli.1 The chemical structure of LAs is fundamental to their mechanism of action and metabolism. Local anesthetics contain a lipophilic aromatic group, an intermediate chain, and a hydrophilic amine group. Broadly, agents are classified as amides or esters depending on the chemical group attached to the intermediate chain.2 Amides (eg, lidocaine, bupivacaine, articaine, mepivacaine, prilocaine, levobupivacaine) are metabolized by the hepatic system; esters (eg, procaine, proparacaine, benzocaine, chlorprocaine, tetracaine, cocaine) are metabolized by plasma cholinesterase, which produces para-aminobenzoic acid, a potentially dangerous metabolite that has been implicated in allergic reactions.3
Lidocaine is the most prevalent LA used in dermatology practices. Importantly, lidocaine is a class IB antiarrhythmic agent used in cardiology to treat ventricular arrhythmias.4 As an anesthetic, a maximum dose of 4.5 mg/kg can be administered, increasing to 7.0 mg/kg when mixed with epinephrine; with higher doses, there is a risk for central nervous system and cardiovascular toxicity.5 Initial symptoms of lidocaine toxicity include dizziness, tinnitus, circumoral paresthesia, blurred vision, and a metallic taste in the mouth.6 Systemic absorption of topical anesthetics is heightened across mucosal membranes, and care should be taken when applying over large surface areas.
Allergic reactions to LAs may be local or less frequently systemic. It is important to note that LAs tend to show cross-reactivity within their class rather than across different classes.7 Reactions can be classified as type I or type IV. Type I (IgE-mediated) reactions evolve in minutes to hours, affecting the skin and possibly leading to respiratory and circulatory collapse. Delayed reactions to LAs have increased in recent years, with type IV contact allergy most frequently found in connection with benzocaine and lidocaine.8
Topical Anesthesia
Topical anesthetics are effective and easy to use and are particularly valuable in patients with needle phobia. In certain cases, these medications may be applied by the patient prior to arrival, thereby reducing visit time. Topical agents act on nerve fibers running through the dermis; therefore, efficacy is dependent on successful penetration through the stratum corneum and viable epidermis. To enhance absorption, agents may be applied under an occlusive dressing.
Topical anesthetics are most commonly used for injectable fillers, ablative and nonablative laser resurfacing, laser hair removal, and tattoo removal. The eutectic mixture of 2.5% lidocaine and 2.5% prilocaine as well as topical 4% or 5% lidocaine are the most commonly used US Food and Drug Administration–approved products for topical anesthesia. In addition, several compounded pharmacy products are available.
After 60 minutes of application of the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine, a 3-mm depth of analgesia is reached, and after 120 minutes, a 4.5-mm depth is reached.9 It elicits a biphasic vascular response of vasoconstriction and blanching followed by vasodilation and erythema.10 Most adverse events are mild and transient, but allergic contact dermatitis and contact urticaria have been reported.11-13 In older children and adults, the maximum application area is 200 cm2, with a maximum dose of 20 g used for no longer than 4 hours.
The 4% or 5% lidocaine cream uses a liposomal delivery system, which is designed to improve cutaneous penetration and has been shown to provide longer durations of anesthesia than nonliposomal lidocaine preparations.14 Application should be performed 30 to 60 minutes prior to a procedure. In a study comparing the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine versus lidocaine cream 5% for pain control during laser hair removal with a 1064-nm Nd:YAG laser, no significant differences were found.15 The maximum application area is 100 cm2 in children weighing less than 20 kg. A study of healthy adults demonstrated safety with the use of 30 to 60 g of occluded liposomal lidocaine cream 4%.16
In addition to US Food and Drug Administration–approved products, several compounded pharmacy products are available for topical anesthesia. These formulations include benzocaine-lidocaine-tetracaine gel, tetracaine-adrenaline-cocaine solution, and lidocaine-epinephrine-tetracaine solution. A triple-anesthetic gel, benzocaine-lidocaine-tetracaine is widely used in cosmetic practice. The product has been shown to provide adequate anesthesia for laser resurfacing after 20 minutes without occlusion.17 Of note, compounded anesthetics lack standardization, and different pharmacies may follow their own individual protocols.
Regional Anesthesia
Regional nerve blockade is a useful option for more widespread or complex interventions. Using regional nerve blockade, effective analgesia can be delivered to a target area while avoiding the toxicity and pain associated with numerous anesthetic infiltrations. In addition, there is no distortion of the tissue architecture, allowing for improved visual evaluation during the procedure. Recently, hyaluronic acid fillers have been compounded with lidocaine as a means of reducing procedural pain.
Blocks for Dermal Fillers
Forehead
For dermal filler injections of the glabellar and frontalis lines, anesthesia of the forehead may be desired. The supraorbital and supratrochlear nerves supply this area. The supraorbital nerve can be injected at the supraorbital notch, which is measured roughly 2.7 cm from the glabella. The orbital rim should be palpated with the nondominant hand, and 1 to 2 mL of anesthetic should be injected just below the rim (Figure 1). The supratrochlear nerve is located roughly 1.7 cm from the midline and can be similarly injected under the orbital rim with 1 to 2 mL of anesthetic (Figure 1).
Lateral Temple Region
Anesthesia of the zygomaticotemporal nerve can be used to reduce pain from dermal filler injections of the lateral canthal and temporal areas. The nerve is identified by first palpating the zygomaticofrontal suture. A long needle is then inserted posteriorly, immediately behind the concave surface of the lateral orbital rim, and 1 to 2 mL of anesthetic is injected (Figure 1).
Malar Region
Blockade of the zygomaticofacial nerve is commonly performed in conjunction with the zygomaticotemporal nerve and provides anesthesia to the malar region for cheek augmentation procedures. To identify the target area, the junction of the lateral and inferior orbital rim should be palpated. With the needle placed just lateral to this point, 1 to 2 mL of anesthetic is injected (Figure 1).

Blocks for Perioral Fillers
Upper Lips/Nasolabial Folds
Bilateral blockade of the infraorbital nerves provides anesthesia to the upper lip and nasolabial folds prior to filler injections. The infraorbital nerve can be targeted via an intraoral route where it exits the maxilla at the infraorbital foramen. The nerve is anesthetized by palpating the infraorbital ridge and injecting 3 to 5 mL of anesthetic roughly 1 cm below this point on the vertical axis of the midpupillary line (Figure 1). The external nasal nerve, thought to be a branch of cranial nerve V, also may be targeted if there is inadequate anesthesia from the infraorbital block. This nerve is reached by injecting at the osseocartilaginous junction of the nasal bones (Figure 1).
Lower Lips
Blockade of the mental nerve provides anesthesia to the lower lips for augmentation procedures. The mental nerve can be targeted on each side at the mental foramen, which is located below the root of the lower second premolar. Aiming roughly 1 cm below the gumline, 3 to 5 mL of anesthetic is injected intraorally (Figure 1). A transcutaneous approach toward the same target also is possible, though this technique risks visible bruising. Alternatively, the upper or lower lips can be anesthetized using 4 to 5 submucosal injections at evenly spaced intervals between the canine teeth.18
Blocks for Palmoplantar Hyperhidrosis
The treatment of palmoplantar hyperhidrosis benefits from regional blocks. Botulinum toxin has been well established as an effective therapy for the condition.19-21 Given the sensitivity of palmoplantar sites, it is valuable to achieve effective analgesia of the region prior to dermal injections of botulinum toxin.
Wrists
Sensory innervation of the palm is provided by the median, ulnar, and radial nerves (Figure 2A).
The ulnar nerve is anesthetized between the ulnar artery and the flexor carpi ulnaris muscle. The artery is identified by palpation, and special care should be taken to avoid intra-arterial injection. The needle is directed toward the radial styloid, and 3 to 5 mL of anesthetic is injected roughly 1 cm proximal to the wrist crease (Figure 2B).
Anesthesia of the radial nerve can be considered a field block given the numerous small branches that supply the hand. These branches are reached by injecting anesthetic roughly 2 to 3 cm proximal to the radial styloid with the needle aimed medially and extending the injection dorsally (Figure 2B). A total of 4 to 6 mL of anesthetic is used.

Ankles
An ankle block provides anesthesia to the dorsal and plantar surfaces of the foot.22 The region is supplied by the superficial peroneal nerve, deep peroneal nerve, sural nerve, saphenous nerve, and branches of the posterior tibial nerve (Figure 3A).
To anesthetize the deep peroneal nerve, the extensor hallucis longus tendon is first identified on the anterior surface of the ankle through dorsiflexion of the toes; the dorsalis pedis artery runs in close proximity. The injection should be placed lateral to the tendon and artery (Figure 3B). The needle should be inserted until bone is reached, withdrawn slightly, and then 3 to 5 mL of anesthetic should be injected. To block the saphenous nerve, the needle can then be directed superficially toward the medial malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C). To block the superficial peroneal nerve, the needle should then be directed toward the lateral malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C).
The posterior tibial nerve is located posterior to the medial malleolus. The dorsalis pedis artery can be palpated near this location. The needle should be inserted posterior to the artery, extending until bone is reached (Figure 3C). The needle is then withdrawn slightly, and 3 to 5 mL of anesthetic is injected. Finally, the sural nerve is anesthetized between the Achilles tendon and the lateral malleolus, using 5 mL of anesthetic to raise a subcutaneous wheal (Figure 3C).

Conclusion
Proper pain management is integral to ensuring a positive experience for cosmetic patients. Enhanced knowledge of local anesthetic techniques allows the clinician to provide for a variety of procedural indications and patient preferences. As anesthetic strategies are continually evolving, it is important for practitioners to remain informed of these developments.
Local anesthesia is a central component of successful interventions in cosmetic dermatology. The number of anesthetic medications and administration techniques has grown in recent years as outpatient cosmetic procedures continue to expand. Pain is a common barrier to cosmetic procedures, and alleviating the fear of painful interventions is critical to patient satisfaction and future visits. To accommodate a multitude of cosmetic interventions, it is important for clinicians to be well versed in applications of topical and regional anesthesia. In this article, we review pain management strategies for use in cosmetic practice.
Local Anesthetics
The sensation of pain is carried to the central nervous system by unmyelinated C nerve fibers. Local anesthetics (LAs) act by blocking fast voltage-gated sodium channels in the cell membrane of the nerve, thereby inhibiting downstream propagation of an action potential and the transmission of painful stimuli.1 The chemical structure of LAs is fundamental to their mechanism of action and metabolism. Local anesthetics contain a lipophilic aromatic group, an intermediate chain, and a hydrophilic amine group. Broadly, agents are classified as amides or esters depending on the chemical group attached to the intermediate chain.2 Amides (eg, lidocaine, bupivacaine, articaine, mepivacaine, prilocaine, levobupivacaine) are metabolized by the hepatic system; esters (eg, procaine, proparacaine, benzocaine, chlorprocaine, tetracaine, cocaine) are metabolized by plasma cholinesterase, which produces para-aminobenzoic acid, a potentially dangerous metabolite that has been implicated in allergic reactions.3
Lidocaine is the most prevalent LA used in dermatology practices. Importantly, lidocaine is a class IB antiarrhythmic agent used in cardiology to treat ventricular arrhythmias.4 As an anesthetic, a maximum dose of 4.5 mg/kg can be administered, increasing to 7.0 mg/kg when mixed with epinephrine; with higher doses, there is a risk for central nervous system and cardiovascular toxicity.5 Initial symptoms of lidocaine toxicity include dizziness, tinnitus, circumoral paresthesia, blurred vision, and a metallic taste in the mouth.6 Systemic absorption of topical anesthetics is heightened across mucosal membranes, and care should be taken when applying over large surface areas.
Allergic reactions to LAs may be local or less frequently systemic. It is important to note that LAs tend to show cross-reactivity within their class rather than across different classes.7 Reactions can be classified as type I or type IV. Type I (IgE-mediated) reactions evolve in minutes to hours, affecting the skin and possibly leading to respiratory and circulatory collapse. Delayed reactions to LAs have increased in recent years, with type IV contact allergy most frequently found in connection with benzocaine and lidocaine.8
Topical Anesthesia
Topical anesthetics are effective and easy to use and are particularly valuable in patients with needle phobia. In certain cases, these medications may be applied by the patient prior to arrival, thereby reducing visit time. Topical agents act on nerve fibers running through the dermis; therefore, efficacy is dependent on successful penetration through the stratum corneum and viable epidermis. To enhance absorption, agents may be applied under an occlusive dressing.
Topical anesthetics are most commonly used for injectable fillers, ablative and nonablative laser resurfacing, laser hair removal, and tattoo removal. The eutectic mixture of 2.5% lidocaine and 2.5% prilocaine as well as topical 4% or 5% lidocaine are the most commonly used US Food and Drug Administration–approved products for topical anesthesia. In addition, several compounded pharmacy products are available.
After 60 minutes of application of the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine, a 3-mm depth of analgesia is reached, and after 120 minutes, a 4.5-mm depth is reached.9 It elicits a biphasic vascular response of vasoconstriction and blanching followed by vasodilation and erythema.10 Most adverse events are mild and transient, but allergic contact dermatitis and contact urticaria have been reported.11-13 In older children and adults, the maximum application area is 200 cm2, with a maximum dose of 20 g used for no longer than 4 hours.
The 4% or 5% lidocaine cream uses a liposomal delivery system, which is designed to improve cutaneous penetration and has been shown to provide longer durations of anesthesia than nonliposomal lidocaine preparations.14 Application should be performed 30 to 60 minutes prior to a procedure. In a study comparing the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine versus lidocaine cream 5% for pain control during laser hair removal with a 1064-nm Nd:YAG laser, no significant differences were found.15 The maximum application area is 100 cm2 in children weighing less than 20 kg. A study of healthy adults demonstrated safety with the use of 30 to 60 g of occluded liposomal lidocaine cream 4%.16
In addition to US Food and Drug Administration–approved products, several compounded pharmacy products are available for topical anesthesia. These formulations include benzocaine-lidocaine-tetracaine gel, tetracaine-adrenaline-cocaine solution, and lidocaine-epinephrine-tetracaine solution. A triple-anesthetic gel, benzocaine-lidocaine-tetracaine is widely used in cosmetic practice. The product has been shown to provide adequate anesthesia for laser resurfacing after 20 minutes without occlusion.17 Of note, compounded anesthetics lack standardization, and different pharmacies may follow their own individual protocols.
Regional Anesthesia
Regional nerve blockade is a useful option for more widespread or complex interventions. Using regional nerve blockade, effective analgesia can be delivered to a target area while avoiding the toxicity and pain associated with numerous anesthetic infiltrations. In addition, there is no distortion of the tissue architecture, allowing for improved visual evaluation during the procedure. Recently, hyaluronic acid fillers have been compounded with lidocaine as a means of reducing procedural pain.
Blocks for Dermal Fillers
Forehead
For dermal filler injections of the glabellar and frontalis lines, anesthesia of the forehead may be desired. The supraorbital and supratrochlear nerves supply this area. The supraorbital nerve can be injected at the supraorbital notch, which is measured roughly 2.7 cm from the glabella. The orbital rim should be palpated with the nondominant hand, and 1 to 2 mL of anesthetic should be injected just below the rim (Figure 1). The supratrochlear nerve is located roughly 1.7 cm from the midline and can be similarly injected under the orbital rim with 1 to 2 mL of anesthetic (Figure 1).
Lateral Temple Region
Anesthesia of the zygomaticotemporal nerve can be used to reduce pain from dermal filler injections of the lateral canthal and temporal areas. The nerve is identified by first palpating the zygomaticofrontal suture. A long needle is then inserted posteriorly, immediately behind the concave surface of the lateral orbital rim, and 1 to 2 mL of anesthetic is injected (Figure 1).
Malar Region
Blockade of the zygomaticofacial nerve is commonly performed in conjunction with the zygomaticotemporal nerve and provides anesthesia to the malar region for cheek augmentation procedures. To identify the target area, the junction of the lateral and inferior orbital rim should be palpated. With the needle placed just lateral to this point, 1 to 2 mL of anesthetic is injected (Figure 1).

Blocks for Perioral Fillers
Upper Lips/Nasolabial Folds
Bilateral blockade of the infraorbital nerves provides anesthesia to the upper lip and nasolabial folds prior to filler injections. The infraorbital nerve can be targeted via an intraoral route where it exits the maxilla at the infraorbital foramen. The nerve is anesthetized by palpating the infraorbital ridge and injecting 3 to 5 mL of anesthetic roughly 1 cm below this point on the vertical axis of the midpupillary line (Figure 1). The external nasal nerve, thought to be a branch of cranial nerve V, also may be targeted if there is inadequate anesthesia from the infraorbital block. This nerve is reached by injecting at the osseocartilaginous junction of the nasal bones (Figure 1).
Lower Lips
Blockade of the mental nerve provides anesthesia to the lower lips for augmentation procedures. The mental nerve can be targeted on each side at the mental foramen, which is located below the root of the lower second premolar. Aiming roughly 1 cm below the gumline, 3 to 5 mL of anesthetic is injected intraorally (Figure 1). A transcutaneous approach toward the same target also is possible, though this technique risks visible bruising. Alternatively, the upper or lower lips can be anesthetized using 4 to 5 submucosal injections at evenly spaced intervals between the canine teeth.18
Blocks for Palmoplantar Hyperhidrosis
The treatment of palmoplantar hyperhidrosis benefits from regional blocks. Botulinum toxin has been well established as an effective therapy for the condition.19-21 Given the sensitivity of palmoplantar sites, it is valuable to achieve effective analgesia of the region prior to dermal injections of botulinum toxin.
Wrists
Sensory innervation of the palm is provided by the median, ulnar, and radial nerves (Figure 2A).
The ulnar nerve is anesthetized between the ulnar artery and the flexor carpi ulnaris muscle. The artery is identified by palpation, and special care should be taken to avoid intra-arterial injection. The needle is directed toward the radial styloid, and 3 to 5 mL of anesthetic is injected roughly 1 cm proximal to the wrist crease (Figure 2B).
Anesthesia of the radial nerve can be considered a field block given the numerous small branches that supply the hand. These branches are reached by injecting anesthetic roughly 2 to 3 cm proximal to the radial styloid with the needle aimed medially and extending the injection dorsally (Figure 2B). A total of 4 to 6 mL of anesthetic is used.

Ankles
An ankle block provides anesthesia to the dorsal and plantar surfaces of the foot.22 The region is supplied by the superficial peroneal nerve, deep peroneal nerve, sural nerve, saphenous nerve, and branches of the posterior tibial nerve (Figure 3A).
To anesthetize the deep peroneal nerve, the extensor hallucis longus tendon is first identified on the anterior surface of the ankle through dorsiflexion of the toes; the dorsalis pedis artery runs in close proximity. The injection should be placed lateral to the tendon and artery (Figure 3B). The needle should be inserted until bone is reached, withdrawn slightly, and then 3 to 5 mL of anesthetic should be injected. To block the saphenous nerve, the needle can then be directed superficially toward the medial malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C). To block the superficial peroneal nerve, the needle should then be directed toward the lateral malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C).
The posterior tibial nerve is located posterior to the medial malleolus. The dorsalis pedis artery can be palpated near this location. The needle should be inserted posterior to the artery, extending until bone is reached (Figure 3C). The needle is then withdrawn slightly, and 3 to 5 mL of anesthetic is injected. Finally, the sural nerve is anesthetized between the Achilles tendon and the lateral malleolus, using 5 mL of anesthetic to raise a subcutaneous wheal (Figure 3C).

Conclusion
Proper pain management is integral to ensuring a positive experience for cosmetic patients. Enhanced knowledge of local anesthetic techniques allows the clinician to provide for a variety of procedural indications and patient preferences. As anesthetic strategies are continually evolving, it is important for practitioners to remain informed of these developments.
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52-61.
- Auletta MJ. Local anesthesia for dermatologic surgery. Semin Dermatol. 1994;13:35-42.
- Park KK, Sharon VR. A review of local anesthetics: minimizing risk and side effects in cutaneous surgery. Dermatol Surg. 2017;43:173-187.
- Reiz S, Nath S. Cardiotoxicity of local anaesthetic agents. Br J Anaesth. 1986;58:736-746.
- Klein JA, Kassarjdian N. Lidocaine toxicity with tumescent liposuction. a case report of probable drug interactions. Dermatol Surg. 1997;23:1169-1174.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Morais-Almeida M, Gaspar A, Marinho S, et al. Allergy to local anesthetics of the amide group with tolerance to procaine. Allergy. 2003;58:827-828.
- To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372.
- Wahlgren CF, Quiding H. Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream). J Am Acad Dermatol. 2000;42:584-588.
- Bjerring P, Andersen PH, Arendt-Nielsen L. Vascular response of human skin after analgesia with EMLA cream. Br J Anaesth. 1989;63:655-660.
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111.
- Thakur BK, Murali MR. EMLA cream-induced allergic contact dermatitis: a role for prilocaine as an immunogen. J Allergy Clin Immunol. 1995;95:776-778.
- Waton J, Boulanger A, Trechot PH, et al. Contact urticaria from EMLA cream. Contact Dermatitis. 2004;51:284-287.
- Bucalo BD, Mirikitani EJ, Moy RL. Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol Surg. 1998;24:537-541.
- Guardiano RA, Norwood CW. Direct comparison of EMLA versus lidocaine for pain control in Nd:YAG 1,064 nm laser hair removal. Dermatol Surg. 2005;31:396-398.
- Nestor MS. Safety of occluded 4% liposomal lidocaine cream. J Drugs Dermatol. 2006;5:618-620.
- Oni G, Rasko Y, Kenkel J. Topical lidocaine enhanced by laser pretreatment: a safe and effective method of analgesia for facial rejuvenation. Aesthet Surg J. 2013;33:854-861.
- Niamtu J 3rd. Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatol Surg. 2005;31:1330-1332.
- Naumann M, Flachenecker P, Brocker EB, et al. Botulinum toxin for palmar hyperhidrosis. Lancet. 1997;349:252.
- Naumann M, Hofmann U, Bergmann I, et al. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301-304.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38(2, pt 1):227-229.
- Davies T, Karanovic S, Shergill B. Essential regional nerve blocks for the dermatologist: part 2. Clin Exp Dermatol. 2014;39:861-867.
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52-61.
- Auletta MJ. Local anesthesia for dermatologic surgery. Semin Dermatol. 1994;13:35-42.
- Park KK, Sharon VR. A review of local anesthetics: minimizing risk and side effects in cutaneous surgery. Dermatol Surg. 2017;43:173-187.
- Reiz S, Nath S. Cardiotoxicity of local anaesthetic agents. Br J Anaesth. 1986;58:736-746.
- Klein JA, Kassarjdian N. Lidocaine toxicity with tumescent liposuction. a case report of probable drug interactions. Dermatol Surg. 1997;23:1169-1174.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Morais-Almeida M, Gaspar A, Marinho S, et al. Allergy to local anesthetics of the amide group with tolerance to procaine. Allergy. 2003;58:827-828.
- To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372.
- Wahlgren CF, Quiding H. Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream). J Am Acad Dermatol. 2000;42:584-588.
- Bjerring P, Andersen PH, Arendt-Nielsen L. Vascular response of human skin after analgesia with EMLA cream. Br J Anaesth. 1989;63:655-660.
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111.
- Thakur BK, Murali MR. EMLA cream-induced allergic contact dermatitis: a role for prilocaine as an immunogen. J Allergy Clin Immunol. 1995;95:776-778.
- Waton J, Boulanger A, Trechot PH, et al. Contact urticaria from EMLA cream. Contact Dermatitis. 2004;51:284-287.
- Bucalo BD, Mirikitani EJ, Moy RL. Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol Surg. 1998;24:537-541.
- Guardiano RA, Norwood CW. Direct comparison of EMLA versus lidocaine for pain control in Nd:YAG 1,064 nm laser hair removal. Dermatol Surg. 2005;31:396-398.
- Nestor MS. Safety of occluded 4% liposomal lidocaine cream. J Drugs Dermatol. 2006;5:618-620.
- Oni G, Rasko Y, Kenkel J. Topical lidocaine enhanced by laser pretreatment: a safe and effective method of analgesia for facial rejuvenation. Aesthet Surg J. 2013;33:854-861.
- Niamtu J 3rd. Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatol Surg. 2005;31:1330-1332.
- Naumann M, Flachenecker P, Brocker EB, et al. Botulinum toxin for palmar hyperhidrosis. Lancet. 1997;349:252.
- Naumann M, Hofmann U, Bergmann I, et al. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301-304.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38(2, pt 1):227-229.
- Davies T, Karanovic S, Shergill B. Essential regional nerve blocks for the dermatologist: part 2. Clin Exp Dermatol. 2014;39:861-867.
Practice Points
- The proper delivery of local anesthesia is integral to successful cosmetic interventions.
- Regional nerve blocks can provide effective analgesia while reducing the number of injections and preserving the architecture of the cosmetic field.
Microneedling Therapy With and Without Platelet-Rich Plasma
Microneedling therapy, also known as collagen induction therapy or percutaneous collagen induction, is an increasingly popular treatment modality for skin rejuvenation. The approach employs small needles to puncture the skin and stimulate local collagen production in a minimally invasive manner. Recently, clinicians have incorporated the use of platelet-rich plasma (PRP) with the aim of augmenting cosmetic outcomes. In this article, we examine the utility of this approach by reviewing comparison studies of microneedling therapy with and without the application of PRP.
Dr. Gary Goldenberg demonstrates microneedling with platelet-rich plasma in a procedural video available here.
Microneedling Therapy
The use of microneedling first gained attention in the 1990s. Initially, Camirand and Doucet1 described tattooing without pigment for the treatment of achromatic and hypertrophic scars. Fernandes2 evolved this concept and developed a drum-shaped device with fine protruding needles to puncture the skin. Microneedling devices have expanded in recent years and now include both cord- and battery-powered pens and rollers, with needles ranging in length from 0.25 to 3.0 mm.
Treatment with microneedling promotes skin rejuvenation by creating small puncture wounds in the epidermis and dermis. This injury triggers the wound healing cascade and alters the modulation of growth factors to promote regenerative effects.3,4 Following microneedling therapy, increases occur in elastic fiber formation, collagen deposition, and dermal thickness (Figure).5 Of interesting histologic note, collagen is deposited in the normal lattice pattern following this treatment rather than in the parallel bundles typical of scars.6 Microneedling preserves the overall integrity of the epidermal layers and basement membrane, allowing the epidermis to heal without abnormality, verified on histology by a normal stratum corneum, enhanced stratum granulosum, and normal rete ridges.7
Microneedling has demonstrated several uses beyond general skin rejuvenation. In patients with atrophic acne scars, therapy can lead to improved scar appearance, skin texture, and patient satisfaction.8,9 Hypertrophic and dyspigmented burn scars on the body, face, arms, and legs have shown to be receptive to repeated treatments.10 Microneedling also has shown promise in treating androgenic alopecia, increasing hair regrowth in patients who previously showed poor response to conventional therapy with minoxidil and finasteride.11,12
Platelet-Rich Plasma
Platelet-rich plasma is developed by enriching blood with an autologous concentration of platelets. The preparation of PRP begins with whole blood, commonly obtained peripherally by venipuncture. Samples undergo centrifugation to allow separation of the blood into 3 layers: platelet-poor plasma, PRP, and erythrocytes.13 The typical platelet count of whole blood is approximately 200,000/µL; PRP aims to prepare a platelet count of at least 1,000,000/µL in a 5-mL volume.14
An attractive component of PRP is its high concentration of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and epithelial growth factor.15 Because of the regenerative effects of these proteins, PRP has been investigated as a modality to augment wound healing in a variety of clinical areas, such as maxillofacial surgery, orthopedics, cardiovascular surgery, and treatment of soft tissue ulcers.16
Combination Use of Microneedling and PRP
Several studies have compared the effects of microneedling with and without the application of PRP (Table).17-20 In an animal model, Akcal et al17 examined the effects of microneedling and PRP on skin flap survival. Eight rats were randomly divided into 5 groups: sham, control, microneedling alone, microneedling plus PRP, and microneedling plus platelet-poor plasma. Treatments were applied to skin flaps after 4 hours of induced ischemia. The surviving flap area was measured, with results demonstrating significantly higher viable areas in the microneedling plus PRP group relative to all other groups (P<.01). On histologic examination, the microneedling plus PRP group showed well-organized epidermal layers and a dermal integrity that matched the dermis of the sham group.17
Asif et al18 performed a split-face comparison study of 50 patients with atrophic acne scars. On the right side, microneedling was performed followed by intradermal injections and topical application of PRP. On the left side, microneedling was performed followed by intradermal injections of distilled water. The study included 3 treatment sessions with 1 month between each session. Scars were assessed using the Goodman and Baron scale,21 which is designed to grade the morphology of postacne scarring. Scars on the right side improved by 62.2% and scars on the left side improved by 45.8%; prior to treatment, both sides demonstrated similar severity scores, but final severity scores were significantly reduced in the microneedling plus PRP group relative to the microneedling plus distilled water group (P<.00001). No residual side effects from treatment were reported.18
Examining the degree of improvement more carefully, microneedling plus PRP yielded excellent improvement in 40% (20/50) of patients and good improvement in 60% (30/50).18 Microneedling plus distilled water led to excellent improvement in 10% (5/50) and good improvement in 84% (42/50). Given that microneedling plus distilled water still provided good to excellent results in 94% of patients, the addition of PRP was helpful though not necessary in achieving meaningful benefit.18
In another split-face study, Fabbrocini et al19 evaluated 12 adult patients with acne scars. The right side of the face received microneedling plus PRP, while the left side received microneedling alone. Two treatments were performed 8 weeks apart. Severity scores (0=no lesions; 10=maximum severity) were used to assess patient outcomes throughout the study. Acne scars improved on both sides of the face following the treatment period, but the reduction in scar severity with microneedling plus PRP (3.5 points) was significantly greater than with microneedling alone (2.6 points)(P<.05). Patients tended to experience2 to 3 days of mild swelling and erythema after treatment regardless of PRP addition. With only 12 patients, the study was limited by a small sample size. The 10-point grading system differed from the Goodman and Baron scale in that it lacked corresponding qualitative markers, likely decreasing reproducibility.19
Chawla20 compared the effectiveness of combination therapy with microneedling plus PRP versus microneedling and vitamin C application. In a split-face study of 30 patients with atrophic acne scars, the right side of the face was treated with microneedling plus PRP and the left side was treated with microneedling plus vitamin C. Four sessions were performed with an interval of 1 month in between treatments. The Goodman and Baron Scale was used to assess treatment efficacy. Overall, both treatments led to improved outcomes, but in categorizing patients who demonstrated poor responses, a significantly larger percentage existed in the microneedling plus vitamin C group (37% [10/27]) versus the microneedling plus PRP group (22% [6/27])(P=.021). Additionally, aggregate patient satisfaction scores were higher with microneedling plus PRP relative to microneedling plus vitamin C (P=.01). Of note, assessments of improvement were performed by the treating physician and patient satisfaction reports were completed with knowledge of the therapies and cost factor, which may have influenced results.20
Conclusion
Microneedling therapy continues to evolve with a range of applications now emerging in dermatology. As PRP has gained popularity, there has been increased interest in its utilization to amplify the regenerative effects of microneedling. Although the number of direct comparisons examining microneedling with and without PRP is limited, the available evidence indicates that the addition of PRP may improve cosmetic outcomes. These results have been demonstrated primarily in the management of acne scars, but favorable effects may extend to other indications. Continued study is warranted to further quantify the degree of these benefits and to elucidate optimal treatment schedules.
In addition, it is important to consider a cost-benefit analysis of PRP. The price of PRP varies depending on the clinical site but in certain cases may double the cost of a microneedling treatment session. Although studies have demonstrated a statistically significant benefit to PRP, the clinical significance of this supplementary treatment must be weighed against the increased expense. A discussion should take place with the consideration that microneedling alone can provide a satisfactory result for some patients.
- Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast Surg. 1997;21:48-51.
- Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthet Surg J. 2002;22:307-309.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration [published online February 7, 2014]. Burns. 2014;40:966-973.
- Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the Dermaroller device. Plast Reconstr Surg. 2011;127:E146-E148.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26:192-199.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Leheta T, El Tawdy A, Abdel Hay R, et al. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207-216.
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228.
- Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2 suppl 1):3S-22S.
- Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043-1054.
- Akcal A, Savas SA, Gorgulu T, et al. The effect of platelete rich plasma combined with microneedling on full venous outflow compromise in a rat skin flap model. Plast Reconstr Surg. 2015;136(4 suppl):71-72.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study [published online January 8, 2016]. J Cosmet Dermatol. 2016;15:434-443.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
Microneedling therapy, also known as collagen induction therapy or percutaneous collagen induction, is an increasingly popular treatment modality for skin rejuvenation. The approach employs small needles to puncture the skin and stimulate local collagen production in a minimally invasive manner. Recently, clinicians have incorporated the use of platelet-rich plasma (PRP) with the aim of augmenting cosmetic outcomes. In this article, we examine the utility of this approach by reviewing comparison studies of microneedling therapy with and without the application of PRP.
Dr. Gary Goldenberg demonstrates microneedling with platelet-rich plasma in a procedural video available here.
Microneedling Therapy
The use of microneedling first gained attention in the 1990s. Initially, Camirand and Doucet1 described tattooing without pigment for the treatment of achromatic and hypertrophic scars. Fernandes2 evolved this concept and developed a drum-shaped device with fine protruding needles to puncture the skin. Microneedling devices have expanded in recent years and now include both cord- and battery-powered pens and rollers, with needles ranging in length from 0.25 to 3.0 mm.
Treatment with microneedling promotes skin rejuvenation by creating small puncture wounds in the epidermis and dermis. This injury triggers the wound healing cascade and alters the modulation of growth factors to promote regenerative effects.3,4 Following microneedling therapy, increases occur in elastic fiber formation, collagen deposition, and dermal thickness (Figure).5 Of interesting histologic note, collagen is deposited in the normal lattice pattern following this treatment rather than in the parallel bundles typical of scars.6 Microneedling preserves the overall integrity of the epidermal layers and basement membrane, allowing the epidermis to heal without abnormality, verified on histology by a normal stratum corneum, enhanced stratum granulosum, and normal rete ridges.7

Microneedling has demonstrated several uses beyond general skin rejuvenation. In patients with atrophic acne scars, therapy can lead to improved scar appearance, skin texture, and patient satisfaction.8,9 Hypertrophic and dyspigmented burn scars on the body, face, arms, and legs have shown to be receptive to repeated treatments.10 Microneedling also has shown promise in treating androgenic alopecia, increasing hair regrowth in patients who previously showed poor response to conventional therapy with minoxidil and finasteride.11,12
Platelet-Rich Plasma
Platelet-rich plasma is developed by enriching blood with an autologous concentration of platelets. The preparation of PRP begins with whole blood, commonly obtained peripherally by venipuncture. Samples undergo centrifugation to allow separation of the blood into 3 layers: platelet-poor plasma, PRP, and erythrocytes.13 The typical platelet count of whole blood is approximately 200,000/µL; PRP aims to prepare a platelet count of at least 1,000,000/µL in a 5-mL volume.14
An attractive component of PRP is its high concentration of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and epithelial growth factor.15 Because of the regenerative effects of these proteins, PRP has been investigated as a modality to augment wound healing in a variety of clinical areas, such as maxillofacial surgery, orthopedics, cardiovascular surgery, and treatment of soft tissue ulcers.16
Combination Use of Microneedling and PRP
Several studies have compared the effects of microneedling with and without the application of PRP (Table).17-20 In an animal model, Akcal et al17 examined the effects of microneedling and PRP on skin flap survival. Eight rats were randomly divided into 5 groups: sham, control, microneedling alone, microneedling plus PRP, and microneedling plus platelet-poor plasma. Treatments were applied to skin flaps after 4 hours of induced ischemia. The surviving flap area was measured, with results demonstrating significantly higher viable areas in the microneedling plus PRP group relative to all other groups (P<.01). On histologic examination, the microneedling plus PRP group showed well-organized epidermal layers and a dermal integrity that matched the dermis of the sham group.17
Asif et al18 performed a split-face comparison study of 50 patients with atrophic acne scars. On the right side, microneedling was performed followed by intradermal injections and topical application of PRP. On the left side, microneedling was performed followed by intradermal injections of distilled water. The study included 3 treatment sessions with 1 month between each session. Scars were assessed using the Goodman and Baron scale,21 which is designed to grade the morphology of postacne scarring. Scars on the right side improved by 62.2% and scars on the left side improved by 45.8%; prior to treatment, both sides demonstrated similar severity scores, but final severity scores were significantly reduced in the microneedling plus PRP group relative to the microneedling plus distilled water group (P<.00001). No residual side effects from treatment were reported.18
Examining the degree of improvement more carefully, microneedling plus PRP yielded excellent improvement in 40% (20/50) of patients and good improvement in 60% (30/50).18 Microneedling plus distilled water led to excellent improvement in 10% (5/50) and good improvement in 84% (42/50). Given that microneedling plus distilled water still provided good to excellent results in 94% of patients, the addition of PRP was helpful though not necessary in achieving meaningful benefit.18
In another split-face study, Fabbrocini et al19 evaluated 12 adult patients with acne scars. The right side of the face received microneedling plus PRP, while the left side received microneedling alone. Two treatments were performed 8 weeks apart. Severity scores (0=no lesions; 10=maximum severity) were used to assess patient outcomes throughout the study. Acne scars improved on both sides of the face following the treatment period, but the reduction in scar severity with microneedling plus PRP (3.5 points) was significantly greater than with microneedling alone (2.6 points)(P<.05). Patients tended to experience2 to 3 days of mild swelling and erythema after treatment regardless of PRP addition. With only 12 patients, the study was limited by a small sample size. The 10-point grading system differed from the Goodman and Baron scale in that it lacked corresponding qualitative markers, likely decreasing reproducibility.19
Chawla20 compared the effectiveness of combination therapy with microneedling plus PRP versus microneedling and vitamin C application. In a split-face study of 30 patients with atrophic acne scars, the right side of the face was treated with microneedling plus PRP and the left side was treated with microneedling plus vitamin C. Four sessions were performed with an interval of 1 month in between treatments. The Goodman and Baron Scale was used to assess treatment efficacy. Overall, both treatments led to improved outcomes, but in categorizing patients who demonstrated poor responses, a significantly larger percentage existed in the microneedling plus vitamin C group (37% [10/27]) versus the microneedling plus PRP group (22% [6/27])(P=.021). Additionally, aggregate patient satisfaction scores were higher with microneedling plus PRP relative to microneedling plus vitamin C (P=.01). Of note, assessments of improvement were performed by the treating physician and patient satisfaction reports were completed with knowledge of the therapies and cost factor, which may have influenced results.20
Conclusion
Microneedling therapy continues to evolve with a range of applications now emerging in dermatology. As PRP has gained popularity, there has been increased interest in its utilization to amplify the regenerative effects of microneedling. Although the number of direct comparisons examining microneedling with and without PRP is limited, the available evidence indicates that the addition of PRP may improve cosmetic outcomes. These results have been demonstrated primarily in the management of acne scars, but favorable effects may extend to other indications. Continued study is warranted to further quantify the degree of these benefits and to elucidate optimal treatment schedules.
In addition, it is important to consider a cost-benefit analysis of PRP. The price of PRP varies depending on the clinical site but in certain cases may double the cost of a microneedling treatment session. Although studies have demonstrated a statistically significant benefit to PRP, the clinical significance of this supplementary treatment must be weighed against the increased expense. A discussion should take place with the consideration that microneedling alone can provide a satisfactory result for some patients.
Microneedling therapy, also known as collagen induction therapy or percutaneous collagen induction, is an increasingly popular treatment modality for skin rejuvenation. The approach employs small needles to puncture the skin and stimulate local collagen production in a minimally invasive manner. Recently, clinicians have incorporated the use of platelet-rich plasma (PRP) with the aim of augmenting cosmetic outcomes. In this article, we examine the utility of this approach by reviewing comparison studies of microneedling therapy with and without the application of PRP.
Dr. Gary Goldenberg demonstrates microneedling with platelet-rich plasma in a procedural video available here.
Microneedling Therapy
The use of microneedling first gained attention in the 1990s. Initially, Camirand and Doucet1 described tattooing without pigment for the treatment of achromatic and hypertrophic scars. Fernandes2 evolved this concept and developed a drum-shaped device with fine protruding needles to puncture the skin. Microneedling devices have expanded in recent years and now include both cord- and battery-powered pens and rollers, with needles ranging in length from 0.25 to 3.0 mm.
Treatment with microneedling promotes skin rejuvenation by creating small puncture wounds in the epidermis and dermis. This injury triggers the wound healing cascade and alters the modulation of growth factors to promote regenerative effects.3,4 Following microneedling therapy, increases occur in elastic fiber formation, collagen deposition, and dermal thickness (Figure).5 Of interesting histologic note, collagen is deposited in the normal lattice pattern following this treatment rather than in the parallel bundles typical of scars.6 Microneedling preserves the overall integrity of the epidermal layers and basement membrane, allowing the epidermis to heal without abnormality, verified on histology by a normal stratum corneum, enhanced stratum granulosum, and normal rete ridges.7

Microneedling has demonstrated several uses beyond general skin rejuvenation. In patients with atrophic acne scars, therapy can lead to improved scar appearance, skin texture, and patient satisfaction.8,9 Hypertrophic and dyspigmented burn scars on the body, face, arms, and legs have shown to be receptive to repeated treatments.10 Microneedling also has shown promise in treating androgenic alopecia, increasing hair regrowth in patients who previously showed poor response to conventional therapy with minoxidil and finasteride.11,12
Platelet-Rich Plasma
Platelet-rich plasma is developed by enriching blood with an autologous concentration of platelets. The preparation of PRP begins with whole blood, commonly obtained peripherally by venipuncture. Samples undergo centrifugation to allow separation of the blood into 3 layers: platelet-poor plasma, PRP, and erythrocytes.13 The typical platelet count of whole blood is approximately 200,000/µL; PRP aims to prepare a platelet count of at least 1,000,000/µL in a 5-mL volume.14
An attractive component of PRP is its high concentration of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and epithelial growth factor.15 Because of the regenerative effects of these proteins, PRP has been investigated as a modality to augment wound healing in a variety of clinical areas, such as maxillofacial surgery, orthopedics, cardiovascular surgery, and treatment of soft tissue ulcers.16
Combination Use of Microneedling and PRP
Several studies have compared the effects of microneedling with and without the application of PRP (Table).17-20 In an animal model, Akcal et al17 examined the effects of microneedling and PRP on skin flap survival. Eight rats were randomly divided into 5 groups: sham, control, microneedling alone, microneedling plus PRP, and microneedling plus platelet-poor plasma. Treatments were applied to skin flaps after 4 hours of induced ischemia. The surviving flap area was measured, with results demonstrating significantly higher viable areas in the microneedling plus PRP group relative to all other groups (P<.01). On histologic examination, the microneedling plus PRP group showed well-organized epidermal layers and a dermal integrity that matched the dermis of the sham group.17
Asif et al18 performed a split-face comparison study of 50 patients with atrophic acne scars. On the right side, microneedling was performed followed by intradermal injections and topical application of PRP. On the left side, microneedling was performed followed by intradermal injections of distilled water. The study included 3 treatment sessions with 1 month between each session. Scars were assessed using the Goodman and Baron scale,21 which is designed to grade the morphology of postacne scarring. Scars on the right side improved by 62.2% and scars on the left side improved by 45.8%; prior to treatment, both sides demonstrated similar severity scores, but final severity scores were significantly reduced in the microneedling plus PRP group relative to the microneedling plus distilled water group (P<.00001). No residual side effects from treatment were reported.18
Examining the degree of improvement more carefully, microneedling plus PRP yielded excellent improvement in 40% (20/50) of patients and good improvement in 60% (30/50).18 Microneedling plus distilled water led to excellent improvement in 10% (5/50) and good improvement in 84% (42/50). Given that microneedling plus distilled water still provided good to excellent results in 94% of patients, the addition of PRP was helpful though not necessary in achieving meaningful benefit.18
In another split-face study, Fabbrocini et al19 evaluated 12 adult patients with acne scars. The right side of the face received microneedling plus PRP, while the left side received microneedling alone. Two treatments were performed 8 weeks apart. Severity scores (0=no lesions; 10=maximum severity) were used to assess patient outcomes throughout the study. Acne scars improved on both sides of the face following the treatment period, but the reduction in scar severity with microneedling plus PRP (3.5 points) was significantly greater than with microneedling alone (2.6 points)(P<.05). Patients tended to experience2 to 3 days of mild swelling and erythema after treatment regardless of PRP addition. With only 12 patients, the study was limited by a small sample size. The 10-point grading system differed from the Goodman and Baron scale in that it lacked corresponding qualitative markers, likely decreasing reproducibility.19
Chawla20 compared the effectiveness of combination therapy with microneedling plus PRP versus microneedling and vitamin C application. In a split-face study of 30 patients with atrophic acne scars, the right side of the face was treated with microneedling plus PRP and the left side was treated with microneedling plus vitamin C. Four sessions were performed with an interval of 1 month in between treatments. The Goodman and Baron Scale was used to assess treatment efficacy. Overall, both treatments led to improved outcomes, but in categorizing patients who demonstrated poor responses, a significantly larger percentage existed in the microneedling plus vitamin C group (37% [10/27]) versus the microneedling plus PRP group (22% [6/27])(P=.021). Additionally, aggregate patient satisfaction scores were higher with microneedling plus PRP relative to microneedling plus vitamin C (P=.01). Of note, assessments of improvement were performed by the treating physician and patient satisfaction reports were completed with knowledge of the therapies and cost factor, which may have influenced results.20
Conclusion
Microneedling therapy continues to evolve with a range of applications now emerging in dermatology. As PRP has gained popularity, there has been increased interest in its utilization to amplify the regenerative effects of microneedling. Although the number of direct comparisons examining microneedling with and without PRP is limited, the available evidence indicates that the addition of PRP may improve cosmetic outcomes. These results have been demonstrated primarily in the management of acne scars, but favorable effects may extend to other indications. Continued study is warranted to further quantify the degree of these benefits and to elucidate optimal treatment schedules.
In addition, it is important to consider a cost-benefit analysis of PRP. The price of PRP varies depending on the clinical site but in certain cases may double the cost of a microneedling treatment session. Although studies have demonstrated a statistically significant benefit to PRP, the clinical significance of this supplementary treatment must be weighed against the increased expense. A discussion should take place with the consideration that microneedling alone can provide a satisfactory result for some patients.
- Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast Surg. 1997;21:48-51.
- Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthet Surg J. 2002;22:307-309.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration [published online February 7, 2014]. Burns. 2014;40:966-973.
- Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the Dermaroller device. Plast Reconstr Surg. 2011;127:E146-E148.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26:192-199.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Leheta T, El Tawdy A, Abdel Hay R, et al. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207-216.
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228.
- Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2 suppl 1):3S-22S.
- Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043-1054.
- Akcal A, Savas SA, Gorgulu T, et al. The effect of platelete rich plasma combined with microneedling on full venous outflow compromise in a rat skin flap model. Plast Reconstr Surg. 2015;136(4 suppl):71-72.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study [published online January 8, 2016]. J Cosmet Dermatol. 2016;15:434-443.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- Camirand A, Doucet J. Needle dermabrasion. Aesthetic Plast Surg. 1997;21:48-51.
- Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthet Surg J. 2002;22:307-309.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Zeitter S, Sikora Z, Jahn S, et al. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration [published online February 7, 2014]. Burns. 2014;40:966-973.
- Schwarz M, Laaff H. A prospective controlled assessment of microneedling with the Dermaroller device. Plast Reconstr Surg. 2011;127:E146-E148.
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26:192-199.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Leheta T, El Tawdy A, Abdel Hay R, et al. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207-216.
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228.
- Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(2 suppl 1):3S-22S.
- Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043-1054.
- Akcal A, Savas SA, Gorgulu T, et al. The effect of platelete rich plasma combined with microneedling on full venous outflow compromise in a rat skin flap model. Plast Reconstr Surg. 2015;136(4 suppl):71-72.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study [published online January 8, 2016]. J Cosmet Dermatol. 2016;15:434-443.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
Practice Points
- Microneedling is an effective therapy for skin rejuvenation.
- Preliminary evidence indicates that the addition of platelet-rich plasma to microneedling improves cosmetic outcomes.