User login
Asymptomatic but Time for a Hip Revision
Total hip arthroplasty (THA) is considered to be one of the most successful orthopedic interventions of its generation.1 In 2010, 332,000 THAs were performed in the U.S.2 Although used to correct advanced joint diseases in the elderly, the THA procedure has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early onset secondary arthritis such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoro-acetabular impingement.
Current hip replacements are expected to function at least 10 to 20 years in 90% of patients.3 As increasing numbers of young patients have these procedures and as seniors continue to live longer, patients will outlast their implants. Younger and more active patients have a higher rate of revision, because the longevity of the prosthesis is usually a function of usage.3 The number of revision THAs is projected to increase 137% by 2030.4
Hip resurfacing has been developed as a bone preserving surgical alternative to THA. The first system for use in the U.S. received FDA approval in 2006, but concerns about the metal on metal bearing surfaces, high failure and revision rates, and early catastrophic modes of failure compared with THAs has resulted in the recall of many of these devices. Hip resurfacing may offer some advantages compared with those of a THA in a carefully selected population, but its use will not be further discussed in this case study.5 Periprosthetic osteolysis and aseptic loosening are 2 of the long-term consequences of THA.6 Bone loss is felt to be secondary to a biologic reaction to particulate debris from implants.6 Some patients, especially those with loosening, complete wear, or fracture, will be symptomatic with pain. However, wear and osteolysis is a silent disease unless there is mechanical failure. Other patients may not experience discomfort. Radiographic studies may reveal significant changes, which warrant the recommendation for a hip revision.
Hip revision surgery has 3 major purposes: relieving pain in the affected joint, restoring the patient’s mobility, and removing a loose or damaged prosthesis before irreversible harm is done to the joint. It’s anticipated that most primary care providers (PCPs) will encounter patients who seek advice on the need for a revision hip arthroplasty.
This case will present an asymptomatic patient who underwent a THA in 1997 at age 37, to address developmental dysplasia of the hip (DDH) and was advised to undergo a revision hip arthroplasty due to abnormal radiographic findings at age 55 years. A discussion will follow that includes a brief review of the history of THA, the materials and bearings commonly used, the presenting symptoms or radiographic changes that signal the need for a revision, and the current options available for a patient such as this.
Case Report
A man aged 55 years presented to a new orthopedic surgeon for his first orthopedic appointment in 10 years. The patient had a left metal-on-polyethylene (M-on-PE) THA 18 years prior due to early onset secondary degenerative joint disease from DDH. The patient’s M-on-PE THA was a titanium acetabular socket and femoral stem with a cobalt-chromium alloy femoral head and a polyethylene liner. The patient remained physically active with an exercise routine consisting of walking, swimming, and weight training.
The patient’s orthopedic history was notable for a right knee arthroscopy for intervention due to a torn medial and lateral meniscus, and birth history was noteworthy for a breech presentation. The physical exam was unremarkable except for a slight leg length discrepancy, but the patient did not exhibit a Trendelenburg gait.
Plain X-rays and a computed tomography (CT) scan showed eccentric PE wear and superior migration of the femoral head, which was indicative of significant PE liner wear. No significant osteolysis or periprosthetic loosening was observed on the X-rays or CT scan. He was advised that a hip revision procedure would need to be done, optimally, within the next 6 months to a year.
Discussion
Hip dysplasia represents a broad group of disorders and generally means abnormal development of the hip joint. The term is most commonly used to refer to DDH with inadequate coverage of the femoral head. In one study, 25% of hip replacements performed in patients aged ≤ 40 years were due to underlying hip dysplasia.7
Developmental dysplasia of the hip occurs more often in children who present in the breech position.8 One theory argues that packaging issues in utero may account for the increased incidence of DDH.9 The earliest recorded attempts at hip replacement occurred in Germany, in 1891, when ivory was used to replace the femoral heads of patients whose hip joints had been destroyed by tuberculosis.1
The orthopedic surgeon Sir John Charnley, who worked at the Manchester Royal Infirmary, is considered the father of the modern THA.1 His low friction arthroplasty, designed in the early 1960s is identical, in principle, to the M-on-PE prosthesis used today.1 The PE liner used was ultrahigh molecular weight polyethylene (UHMWPE).1
Due to the early success of the Charnley prosthesis, the M-on-PE prosthesis became the most widely used. Although PE is the most studied and understood of all acetabular liner materials, it will eventually wear and shed debris. Acetabular cup wear is the most frequent reason for mid-to-long-term revisions, especially in young and active patients.10 More active patients shed more debris.3 The PE debris instigates the release of inflammatory mediators, which results in chronic inflammation and tissue damage that erodes the supporting bone and can lead to implant loosening or fracture.6 Ongoing studies seek to optimize and improve properties of the UHMWPE and to develop alternative bearings. After FDA approval in 1999, highly cross-linked polyethylene liners (HXLPE) rapidly became the standard of care for THAs, at least in the U.S.11 Highly cross-linked polyethylene liners are created from UHMWPE through a process of cross-linking by exposure to gamma radiation, and subsequent heat treatment to neutralize free radicals and limit oxidative degradation.12
In one study, the 5-year annual linear wear rate for a HXLPE liner was only 45% of that seen with the UHMWPE liner, although the qualitative wear pattern was the same.13 In a study that followed patients for 7 years postoperatively, the mean steady-state wear rate of the HXLPE was 0.005 mm/y compared with 0.037 mm/y for UHMWPE.14 In a long-term study (a minimum follow-up of 10 years) of 50 patients who were aged < 50 years and underwent THA using HXLPE liners, there was no radiographic evidence of osteolysis or component loosening, and liner wear was 0.020 ± 0.0047 mm/y.12 In 2005, second-generation HXLPE liners were introduced clinically and have been shown to further reduce wear in vitro compared with both UHMWPE and first-generation HXLPE liners. Callary and colleagues calculated that the wear rates between 1 year and 5 years were all < 0.001 mm/y.15
The use of ceramic for THAs began in 1970, and ceramic heads on polyethylene (C-on-PE) liners and ceramic-on-ceramic (C-on-C) bearings have been in continual use for > 30 years in Europe. Premarket FDA approval based on European data was granted in 1983; however, the manufacturer voluntarily removed it from the market because of a high incidence of stem loosening (> 30% within 3 years in some series).16 FDA approvals came much later for C-on-PE (1989) and C-on-C (2003) bearings.
Ceramic is the hardest implant material used, and it can be concluded from many clinical and laboratory reports that C-on-PE and C-on-C combinations confer a potentially significant reduction in wear on THA bearings.16 Ceramic hips initially had 2 concerns: catastrophic shattering and squeaking. Current ceramic hips have been substantially improved, and some experts feel shattering has been essentially eliminated.16 Other experts note that ceramic brittleness remains a major concern.17 Squeaking remains a problem for some, but it usually abates over time. No study has correlated squeaking with impending failure or increased pain or disability.
While C-on-C bearings are now felt to be a good implant for young active patients, these bearings have generally not resulted in significantly lower wear rates and fewer revisions.18 High rates of wear and osteolysis have been sporadically documented over the 35-year history of ceramic implants.16 The FDA approved the first ceramic-on-metal total hip replacement system on June 13, 2011.
Metal-on-metal (M-on-M) implants have been used by some for decades, although they were not approved by the FDA until the late 1990s. However, some device recalls have brought negative attention to M-on-M implants.19 It was felt that they would generate less wear debris than PE, but reports of pseudotumors (from inflammatory mediators) and metallosis have significantly tempered enthusiasm for these products.20,21 The wear rates are very low, estimated to be only 0.01 mm/y, but concerns about the carcinogenetic potential of systemically increased metal ions remains a possible and much debated concern.19,22,23 In January 2013, FDA issued a safety communication on M-on-M implants.
Many experts feel that modern ceramic or metal on second-generation HXLPE represents the gold standard and the most predictable bearing choice for young, active patients.18 Others feel that the optimal choice of bearing surfaces in THA, particularly in the younger and more active patient, remains controversial.24
Follow-Up
Intermittent orthopedic monitoring is recommended for all patients who have undergone a THA. The frequency of hip X-rays on follow-up appointments is left to the orthopedic surgeon. After the initial recovery, serial images every 2 to 5 years can identify progressive failure, and annual X-rays may be used for closer follow-up in high-risk patients.
Patients who experience dislocations, fractures, infections, or pain usually maintain close orthopedic follow-up. Significant wear of the prosthesis damages the socket; osteolysis can cause irreversible bone loss, fracture, and loosening. Massive acetabular bone loss is very difficult to reverse and creates major reconstruction challenges.
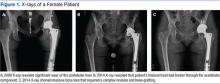
Figure 1A is a 2009 X-ray of a woman aged 44 years who underwent a THA after a motor vehicle accident in 1997 and who was advised to have a revision THA when seen in 2009.
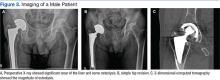
Figure 3A is an X-ray of a man aged 71 years who had undergone THA 21 years earlier and had complied with routine follow-up. When his X-rays showed significant wear of the liner and some osteolysis, he was able to undergo a simple revision (Figure 3B).
Three-dimensional CT is useful for quantifying the presence and severity of osteolytic lesions, because plain radiographs may underestimate the amount of bone loss that is present.25 The CT in Figure 3C shows the magnitude of osteolysis that was underestimated by the preoperative plain X-rays (Figure 3A). Computed tomography scans are crucial for surgical planning in the setting of severe acetabular bone loss.
There is a wide spectrum of signs and symptoms that can occur in the setting of acetabular component failure. Pain is a common presenting symptom. Groin pain can represent acetabular failure; thigh pain may be correlated to femoral component failure.25 The clinical patient presentation ultimately depends on the underlying cause: an infection, polyethylene wear, instability, or aseptic loosening.25 Leg-length discrepancy, joint deformity, location of prior incisions, functional status, and baseline neurologic status should be evaluated and documented during the preoperative evaluation as well.25
Case Study Revision Options
The X-rays and CT scans for this case study patient showed that he was a possible candidate for the simplest revision surgery; an isolated liner exchange and replacement of the femoral head. When the original surgery was performed (1997), the only FDA approved PE liner was UHMWPE. To justify isolated liner exchange, the modular acetabular metallic shell also should be well-fixed and appropriately oriented.26 This is evaluated both preoperatively and intraoperatively.
If found to be well fixed with an appropriate orientation and locking mechanism, the UHMWPE liner could be replaced with a HXLPE liner and a larger metal femoral head for improved wear and stability. Acetabular revision is indicted for an asymptomatic patient who has progressive osteolysis, severe wear, or bone loss that would compromise future reconstruction.
Conclusions
Over the past several decades, THA has become recognized as an effective treatment option for the reduction of pain and disability associated with hip joint disease and is associated with successful clinical outcomes. The most frequently noted recommendations for trying to increase the life expectancy of an artificial hip replacement include maintaining a normal weight, keeping leg muscles strong, and avoiding repetitive squatting and kneeling.
As the number of primary THAs has increased and the average age of those undergoing a primary THA has decreased, the need for revisions has risen. Reviews have demonstrated that the most common causes for early total hip revision, regardless of component, included infection, instability/dislocation, and fracture, whereas wear is the most common reason for mid to late revisions.
The wear of all materials used has been shown to be greatest in the most active patients.
Studies continue to identify ways to potentially prevent or reverse osteolysis from wear debris. Alendronate therapy has been shown to prevent and treat PE debris-induced periprosthetic bone loss in rats.27 It also was successfully used in a case report of an asymptomatic woman aged 39 years who had rapid PE wear and aggressive periprosthetic osteolysis within just 2 years of a bilateral THA.28 Other areas of research on decreasing osteolysis in THA recipients include trials with mesenchymal stem cells, bone morphogenic proteins, and gene therapy.6
In the U.S., 46,000 revisions were performed in 2004 and this number is expected to more than double by 2030.4 Primary care providers are sure to encounter patients who will be in need of a hip revision procedure. It’s important for them to make sure that their patients who have undergone a THA are periodically seen for orthopedic follow-up. Despite the long history of primary THAs, there is still not a single technique and material to suit all patient characteristics.1 Unfortunately, the same currently applies to hip revision procedures.
1. Knight SR, Aujla R, Biswas SP. Total hip arthroplasty--over 100 years of operative history. Orthop Rev (Pavia). 2011;3(2):e16.
2. Centers for Disease Control and Prevention. FastStats: inpatient surgery. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed January 18, 2016.
3. Joint Revision Surgery-When do I need it? American Academy of Orthopedic Surgeons Website. http://www.tlhoc.com/uploads/documents/when_do_I_need_it.pdf. Accessed January 18, 2016.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467(1):56-65.
6. Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312-316.
7. Engesæter IØ, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesæter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82(2):149-154.
8. Salter RB. Etiology, pathogenesis and possible prevention of congenital dislocation of the hip. Can Med Assoc J. 1968;98(20):933-945.
9. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
10. Pace TB, Keith KC, Alvarez E, Snider RG, Tanner, SL, Desjardins JD. Comparison of conventional polyethylene wear and signs of cup failure in two similar total hip designs. Adv Orthop. 2013;2013:710621.
11. Kurtz SM. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement. Academic Press: London; 2014.
12. Babovic N, Trousdale RT. Total hip athroplasty using highly cross-linked polyethylene in patients younger than 50 years with minimum 10-year follow-up. J Arthroplasty. 2013;29(5):815-817.
13. Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasal highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816-1821.
14. Thomas G, Simpson D, Mehmmod S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
15. Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study. Clin Orthop Relat Res. 2013;471(11):3596-3600.
16. Tateiwa T, Clarke IC, Williams PA, et al. Ceramic total hip arthroplasty in the United States: safety and risk issues revisited. Am J Orthop (Belle Mead NJ). 2008;37(2):E26-E31.
17. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. BioMed Res Int. 2013;2013:157247.
18. Haidukewych GJ, Petrie J. Bearing surface considerations for total hip arthroplasty in young patients. Orthop Clin N Am. 2012;43(3):395-402.
19. Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410.
20. Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321-2327.
21. Pritchett JW. Adverse reaction to metal debris: metallosis of the resurfaced hip. Curr Orthop Pract. 2012;23(1):50-58.
22. Smith AJ, Dieppe P, Porter M, Blom AW; National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint registry of England and Wales and hospital episode statistics. BMJ. 2012;344:e2383.
23. Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531-1536.
24. Cash, D, Khanduja V. The case for ceramics-on-polyethylene as the preferred bearing for a young adult hip replacement. Hip Int. 2014;24(5):421-427.
25. Taylor ED, Browne JA. Reconstruction options for acetabular revision. World J Orthop. 2012;3(7):95-100.
26. Lombardi AV, Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg. 2008;16(5):243-248.
27. Millet PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A(2):236-249.
28. O'Hara LJ, Nivbrant B, Rohrl S.Cross-linked polyethylene and bisphosphonate therapy for osteolysis in total hip athroplasty: a case report. J Orthop Surg (Hong Kong). 2004;12(1):114-121.
Total hip arthroplasty (THA) is considered to be one of the most successful orthopedic interventions of its generation.1 In 2010, 332,000 THAs were performed in the U.S.2 Although used to correct advanced joint diseases in the elderly, the THA procedure has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early onset secondary arthritis such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoro-acetabular impingement.
Current hip replacements are expected to function at least 10 to 20 years in 90% of patients.3 As increasing numbers of young patients have these procedures and as seniors continue to live longer, patients will outlast their implants. Younger and more active patients have a higher rate of revision, because the longevity of the prosthesis is usually a function of usage.3 The number of revision THAs is projected to increase 137% by 2030.4
Hip resurfacing has been developed as a bone preserving surgical alternative to THA. The first system for use in the U.S. received FDA approval in 2006, but concerns about the metal on metal bearing surfaces, high failure and revision rates, and early catastrophic modes of failure compared with THAs has resulted in the recall of many of these devices. Hip resurfacing may offer some advantages compared with those of a THA in a carefully selected population, but its use will not be further discussed in this case study.5 Periprosthetic osteolysis and aseptic loosening are 2 of the long-term consequences of THA.6 Bone loss is felt to be secondary to a biologic reaction to particulate debris from implants.6 Some patients, especially those with loosening, complete wear, or fracture, will be symptomatic with pain. However, wear and osteolysis is a silent disease unless there is mechanical failure. Other patients may not experience discomfort. Radiographic studies may reveal significant changes, which warrant the recommendation for a hip revision.
Hip revision surgery has 3 major purposes: relieving pain in the affected joint, restoring the patient’s mobility, and removing a loose or damaged prosthesis before irreversible harm is done to the joint. It’s anticipated that most primary care providers (PCPs) will encounter patients who seek advice on the need for a revision hip arthroplasty.
This case will present an asymptomatic patient who underwent a THA in 1997 at age 37, to address developmental dysplasia of the hip (DDH) and was advised to undergo a revision hip arthroplasty due to abnormal radiographic findings at age 55 years. A discussion will follow that includes a brief review of the history of THA, the materials and bearings commonly used, the presenting symptoms or radiographic changes that signal the need for a revision, and the current options available for a patient such as this.
Case Report
A man aged 55 years presented to a new orthopedic surgeon for his first orthopedic appointment in 10 years. The patient had a left metal-on-polyethylene (M-on-PE) THA 18 years prior due to early onset secondary degenerative joint disease from DDH. The patient’s M-on-PE THA was a titanium acetabular socket and femoral stem with a cobalt-chromium alloy femoral head and a polyethylene liner. The patient remained physically active with an exercise routine consisting of walking, swimming, and weight training.
The patient’s orthopedic history was notable for a right knee arthroscopy for intervention due to a torn medial and lateral meniscus, and birth history was noteworthy for a breech presentation. The physical exam was unremarkable except for a slight leg length discrepancy, but the patient did not exhibit a Trendelenburg gait.
Plain X-rays and a computed tomography (CT) scan showed eccentric PE wear and superior migration of the femoral head, which was indicative of significant PE liner wear. No significant osteolysis or periprosthetic loosening was observed on the X-rays or CT scan. He was advised that a hip revision procedure would need to be done, optimally, within the next 6 months to a year.
Discussion
Hip dysplasia represents a broad group of disorders and generally means abnormal development of the hip joint. The term is most commonly used to refer to DDH with inadequate coverage of the femoral head. In one study, 25% of hip replacements performed in patients aged ≤ 40 years were due to underlying hip dysplasia.7
Developmental dysplasia of the hip occurs more often in children who present in the breech position.8 One theory argues that packaging issues in utero may account for the increased incidence of DDH.9 The earliest recorded attempts at hip replacement occurred in Germany, in 1891, when ivory was used to replace the femoral heads of patients whose hip joints had been destroyed by tuberculosis.1
The orthopedic surgeon Sir John Charnley, who worked at the Manchester Royal Infirmary, is considered the father of the modern THA.1 His low friction arthroplasty, designed in the early 1960s is identical, in principle, to the M-on-PE prosthesis used today.1 The PE liner used was ultrahigh molecular weight polyethylene (UHMWPE).1
Due to the early success of the Charnley prosthesis, the M-on-PE prosthesis became the most widely used. Although PE is the most studied and understood of all acetabular liner materials, it will eventually wear and shed debris. Acetabular cup wear is the most frequent reason for mid-to-long-term revisions, especially in young and active patients.10 More active patients shed more debris.3 The PE debris instigates the release of inflammatory mediators, which results in chronic inflammation and tissue damage that erodes the supporting bone and can lead to implant loosening or fracture.6 Ongoing studies seek to optimize and improve properties of the UHMWPE and to develop alternative bearings. After FDA approval in 1999, highly cross-linked polyethylene liners (HXLPE) rapidly became the standard of care for THAs, at least in the U.S.11 Highly cross-linked polyethylene liners are created from UHMWPE through a process of cross-linking by exposure to gamma radiation, and subsequent heat treatment to neutralize free radicals and limit oxidative degradation.12
In one study, the 5-year annual linear wear rate for a HXLPE liner was only 45% of that seen with the UHMWPE liner, although the qualitative wear pattern was the same.13 In a study that followed patients for 7 years postoperatively, the mean steady-state wear rate of the HXLPE was 0.005 mm/y compared with 0.037 mm/y for UHMWPE.14 In a long-term study (a minimum follow-up of 10 years) of 50 patients who were aged < 50 years and underwent THA using HXLPE liners, there was no radiographic evidence of osteolysis or component loosening, and liner wear was 0.020 ± 0.0047 mm/y.12 In 2005, second-generation HXLPE liners were introduced clinically and have been shown to further reduce wear in vitro compared with both UHMWPE and first-generation HXLPE liners. Callary and colleagues calculated that the wear rates between 1 year and 5 years were all < 0.001 mm/y.15
The use of ceramic for THAs began in 1970, and ceramic heads on polyethylene (C-on-PE) liners and ceramic-on-ceramic (C-on-C) bearings have been in continual use for > 30 years in Europe. Premarket FDA approval based on European data was granted in 1983; however, the manufacturer voluntarily removed it from the market because of a high incidence of stem loosening (> 30% within 3 years in some series).16 FDA approvals came much later for C-on-PE (1989) and C-on-C (2003) bearings.
Ceramic is the hardest implant material used, and it can be concluded from many clinical and laboratory reports that C-on-PE and C-on-C combinations confer a potentially significant reduction in wear on THA bearings.16 Ceramic hips initially had 2 concerns: catastrophic shattering and squeaking. Current ceramic hips have been substantially improved, and some experts feel shattering has been essentially eliminated.16 Other experts note that ceramic brittleness remains a major concern.17 Squeaking remains a problem for some, but it usually abates over time. No study has correlated squeaking with impending failure or increased pain or disability.
While C-on-C bearings are now felt to be a good implant for young active patients, these bearings have generally not resulted in significantly lower wear rates and fewer revisions.18 High rates of wear and osteolysis have been sporadically documented over the 35-year history of ceramic implants.16 The FDA approved the first ceramic-on-metal total hip replacement system on June 13, 2011.
Metal-on-metal (M-on-M) implants have been used by some for decades, although they were not approved by the FDA until the late 1990s. However, some device recalls have brought negative attention to M-on-M implants.19 It was felt that they would generate less wear debris than PE, but reports of pseudotumors (from inflammatory mediators) and metallosis have significantly tempered enthusiasm for these products.20,21 The wear rates are very low, estimated to be only 0.01 mm/y, but concerns about the carcinogenetic potential of systemically increased metal ions remains a possible and much debated concern.19,22,23 In January 2013, FDA issued a safety communication on M-on-M implants.
Many experts feel that modern ceramic or metal on second-generation HXLPE represents the gold standard and the most predictable bearing choice for young, active patients.18 Others feel that the optimal choice of bearing surfaces in THA, particularly in the younger and more active patient, remains controversial.24
Follow-Up
Intermittent orthopedic monitoring is recommended for all patients who have undergone a THA. The frequency of hip X-rays on follow-up appointments is left to the orthopedic surgeon. After the initial recovery, serial images every 2 to 5 years can identify progressive failure, and annual X-rays may be used for closer follow-up in high-risk patients.
Patients who experience dislocations, fractures, infections, or pain usually maintain close orthopedic follow-up. Significant wear of the prosthesis damages the socket; osteolysis can cause irreversible bone loss, fracture, and loosening. Massive acetabular bone loss is very difficult to reverse and creates major reconstruction challenges.
Figure 1A is a 2009 X-ray of a woman aged 44 years who underwent a THA after a motor vehicle accident in 1997 and who was advised to have a revision THA when seen in 2009.
Figure 3A is an X-ray of a man aged 71 years who had undergone THA 21 years earlier and had complied with routine follow-up. When his X-rays showed significant wear of the liner and some osteolysis, he was able to undergo a simple revision (Figure 3B).
Three-dimensional CT is useful for quantifying the presence and severity of osteolytic lesions, because plain radiographs may underestimate the amount of bone loss that is present.25 The CT in Figure 3C shows the magnitude of osteolysis that was underestimated by the preoperative plain X-rays (Figure 3A). Computed tomography scans are crucial for surgical planning in the setting of severe acetabular bone loss.
There is a wide spectrum of signs and symptoms that can occur in the setting of acetabular component failure. Pain is a common presenting symptom. Groin pain can represent acetabular failure; thigh pain may be correlated to femoral component failure.25 The clinical patient presentation ultimately depends on the underlying cause: an infection, polyethylene wear, instability, or aseptic loosening.25 Leg-length discrepancy, joint deformity, location of prior incisions, functional status, and baseline neurologic status should be evaluated and documented during the preoperative evaluation as well.25
Case Study Revision Options
The X-rays and CT scans for this case study patient showed that he was a possible candidate for the simplest revision surgery; an isolated liner exchange and replacement of the femoral head. When the original surgery was performed (1997), the only FDA approved PE liner was UHMWPE. To justify isolated liner exchange, the modular acetabular metallic shell also should be well-fixed and appropriately oriented.26 This is evaluated both preoperatively and intraoperatively.
If found to be well fixed with an appropriate orientation and locking mechanism, the UHMWPE liner could be replaced with a HXLPE liner and a larger metal femoral head for improved wear and stability. Acetabular revision is indicted for an asymptomatic patient who has progressive osteolysis, severe wear, or bone loss that would compromise future reconstruction.
Conclusions
Over the past several decades, THA has become recognized as an effective treatment option for the reduction of pain and disability associated with hip joint disease and is associated with successful clinical outcomes. The most frequently noted recommendations for trying to increase the life expectancy of an artificial hip replacement include maintaining a normal weight, keeping leg muscles strong, and avoiding repetitive squatting and kneeling.
As the number of primary THAs has increased and the average age of those undergoing a primary THA has decreased, the need for revisions has risen. Reviews have demonstrated that the most common causes for early total hip revision, regardless of component, included infection, instability/dislocation, and fracture, whereas wear is the most common reason for mid to late revisions.
The wear of all materials used has been shown to be greatest in the most active patients.
Studies continue to identify ways to potentially prevent or reverse osteolysis from wear debris. Alendronate therapy has been shown to prevent and treat PE debris-induced periprosthetic bone loss in rats.27 It also was successfully used in a case report of an asymptomatic woman aged 39 years who had rapid PE wear and aggressive periprosthetic osteolysis within just 2 years of a bilateral THA.28 Other areas of research on decreasing osteolysis in THA recipients include trials with mesenchymal stem cells, bone morphogenic proteins, and gene therapy.6
In the U.S., 46,000 revisions were performed in 2004 and this number is expected to more than double by 2030.4 Primary care providers are sure to encounter patients who will be in need of a hip revision procedure. It’s important for them to make sure that their patients who have undergone a THA are periodically seen for orthopedic follow-up. Despite the long history of primary THAs, there is still not a single technique and material to suit all patient characteristics.1 Unfortunately, the same currently applies to hip revision procedures.
Total hip arthroplasty (THA) is considered to be one of the most successful orthopedic interventions of its generation.1 In 2010, 332,000 THAs were performed in the U.S.2 Although used to correct advanced joint diseases in the elderly, the THA procedure has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early onset secondary arthritis such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoro-acetabular impingement.
Current hip replacements are expected to function at least 10 to 20 years in 90% of patients.3 As increasing numbers of young patients have these procedures and as seniors continue to live longer, patients will outlast their implants. Younger and more active patients have a higher rate of revision, because the longevity of the prosthesis is usually a function of usage.3 The number of revision THAs is projected to increase 137% by 2030.4
Hip resurfacing has been developed as a bone preserving surgical alternative to THA. The first system for use in the U.S. received FDA approval in 2006, but concerns about the metal on metal bearing surfaces, high failure and revision rates, and early catastrophic modes of failure compared with THAs has resulted in the recall of many of these devices. Hip resurfacing may offer some advantages compared with those of a THA in a carefully selected population, but its use will not be further discussed in this case study.5 Periprosthetic osteolysis and aseptic loosening are 2 of the long-term consequences of THA.6 Bone loss is felt to be secondary to a biologic reaction to particulate debris from implants.6 Some patients, especially those with loosening, complete wear, or fracture, will be symptomatic with pain. However, wear and osteolysis is a silent disease unless there is mechanical failure. Other patients may not experience discomfort. Radiographic studies may reveal significant changes, which warrant the recommendation for a hip revision.
Hip revision surgery has 3 major purposes: relieving pain in the affected joint, restoring the patient’s mobility, and removing a loose or damaged prosthesis before irreversible harm is done to the joint. It’s anticipated that most primary care providers (PCPs) will encounter patients who seek advice on the need for a revision hip arthroplasty.
This case will present an asymptomatic patient who underwent a THA in 1997 at age 37, to address developmental dysplasia of the hip (DDH) and was advised to undergo a revision hip arthroplasty due to abnormal radiographic findings at age 55 years. A discussion will follow that includes a brief review of the history of THA, the materials and bearings commonly used, the presenting symptoms or radiographic changes that signal the need for a revision, and the current options available for a patient such as this.
Case Report
A man aged 55 years presented to a new orthopedic surgeon for his first orthopedic appointment in 10 years. The patient had a left metal-on-polyethylene (M-on-PE) THA 18 years prior due to early onset secondary degenerative joint disease from DDH. The patient’s M-on-PE THA was a titanium acetabular socket and femoral stem with a cobalt-chromium alloy femoral head and a polyethylene liner. The patient remained physically active with an exercise routine consisting of walking, swimming, and weight training.
The patient’s orthopedic history was notable for a right knee arthroscopy for intervention due to a torn medial and lateral meniscus, and birth history was noteworthy for a breech presentation. The physical exam was unremarkable except for a slight leg length discrepancy, but the patient did not exhibit a Trendelenburg gait.
Plain X-rays and a computed tomography (CT) scan showed eccentric PE wear and superior migration of the femoral head, which was indicative of significant PE liner wear. No significant osteolysis or periprosthetic loosening was observed on the X-rays or CT scan. He was advised that a hip revision procedure would need to be done, optimally, within the next 6 months to a year.
Discussion
Hip dysplasia represents a broad group of disorders and generally means abnormal development of the hip joint. The term is most commonly used to refer to DDH with inadequate coverage of the femoral head. In one study, 25% of hip replacements performed in patients aged ≤ 40 years were due to underlying hip dysplasia.7
Developmental dysplasia of the hip occurs more often in children who present in the breech position.8 One theory argues that packaging issues in utero may account for the increased incidence of DDH.9 The earliest recorded attempts at hip replacement occurred in Germany, in 1891, when ivory was used to replace the femoral heads of patients whose hip joints had been destroyed by tuberculosis.1
The orthopedic surgeon Sir John Charnley, who worked at the Manchester Royal Infirmary, is considered the father of the modern THA.1 His low friction arthroplasty, designed in the early 1960s is identical, in principle, to the M-on-PE prosthesis used today.1 The PE liner used was ultrahigh molecular weight polyethylene (UHMWPE).1
Due to the early success of the Charnley prosthesis, the M-on-PE prosthesis became the most widely used. Although PE is the most studied and understood of all acetabular liner materials, it will eventually wear and shed debris. Acetabular cup wear is the most frequent reason for mid-to-long-term revisions, especially in young and active patients.10 More active patients shed more debris.3 The PE debris instigates the release of inflammatory mediators, which results in chronic inflammation and tissue damage that erodes the supporting bone and can lead to implant loosening or fracture.6 Ongoing studies seek to optimize and improve properties of the UHMWPE and to develop alternative bearings. After FDA approval in 1999, highly cross-linked polyethylene liners (HXLPE) rapidly became the standard of care for THAs, at least in the U.S.11 Highly cross-linked polyethylene liners are created from UHMWPE through a process of cross-linking by exposure to gamma radiation, and subsequent heat treatment to neutralize free radicals and limit oxidative degradation.12
In one study, the 5-year annual linear wear rate for a HXLPE liner was only 45% of that seen with the UHMWPE liner, although the qualitative wear pattern was the same.13 In a study that followed patients for 7 years postoperatively, the mean steady-state wear rate of the HXLPE was 0.005 mm/y compared with 0.037 mm/y for UHMWPE.14 In a long-term study (a minimum follow-up of 10 years) of 50 patients who were aged < 50 years and underwent THA using HXLPE liners, there was no radiographic evidence of osteolysis or component loosening, and liner wear was 0.020 ± 0.0047 mm/y.12 In 2005, second-generation HXLPE liners were introduced clinically and have been shown to further reduce wear in vitro compared with both UHMWPE and first-generation HXLPE liners. Callary and colleagues calculated that the wear rates between 1 year and 5 years were all < 0.001 mm/y.15
The use of ceramic for THAs began in 1970, and ceramic heads on polyethylene (C-on-PE) liners and ceramic-on-ceramic (C-on-C) bearings have been in continual use for > 30 years in Europe. Premarket FDA approval based on European data was granted in 1983; however, the manufacturer voluntarily removed it from the market because of a high incidence of stem loosening (> 30% within 3 years in some series).16 FDA approvals came much later for C-on-PE (1989) and C-on-C (2003) bearings.
Ceramic is the hardest implant material used, and it can be concluded from many clinical and laboratory reports that C-on-PE and C-on-C combinations confer a potentially significant reduction in wear on THA bearings.16 Ceramic hips initially had 2 concerns: catastrophic shattering and squeaking. Current ceramic hips have been substantially improved, and some experts feel shattering has been essentially eliminated.16 Other experts note that ceramic brittleness remains a major concern.17 Squeaking remains a problem for some, but it usually abates over time. No study has correlated squeaking with impending failure or increased pain or disability.
While C-on-C bearings are now felt to be a good implant for young active patients, these bearings have generally not resulted in significantly lower wear rates and fewer revisions.18 High rates of wear and osteolysis have been sporadically documented over the 35-year history of ceramic implants.16 The FDA approved the first ceramic-on-metal total hip replacement system on June 13, 2011.
Metal-on-metal (M-on-M) implants have been used by some for decades, although they were not approved by the FDA until the late 1990s. However, some device recalls have brought negative attention to M-on-M implants.19 It was felt that they would generate less wear debris than PE, but reports of pseudotumors (from inflammatory mediators) and metallosis have significantly tempered enthusiasm for these products.20,21 The wear rates are very low, estimated to be only 0.01 mm/y, but concerns about the carcinogenetic potential of systemically increased metal ions remains a possible and much debated concern.19,22,23 In January 2013, FDA issued a safety communication on M-on-M implants.
Many experts feel that modern ceramic or metal on second-generation HXLPE represents the gold standard and the most predictable bearing choice for young, active patients.18 Others feel that the optimal choice of bearing surfaces in THA, particularly in the younger and more active patient, remains controversial.24
Follow-Up
Intermittent orthopedic monitoring is recommended for all patients who have undergone a THA. The frequency of hip X-rays on follow-up appointments is left to the orthopedic surgeon. After the initial recovery, serial images every 2 to 5 years can identify progressive failure, and annual X-rays may be used for closer follow-up in high-risk patients.
Patients who experience dislocations, fractures, infections, or pain usually maintain close orthopedic follow-up. Significant wear of the prosthesis damages the socket; osteolysis can cause irreversible bone loss, fracture, and loosening. Massive acetabular bone loss is very difficult to reverse and creates major reconstruction challenges.
Figure 1A is a 2009 X-ray of a woman aged 44 years who underwent a THA after a motor vehicle accident in 1997 and who was advised to have a revision THA when seen in 2009.
Figure 3A is an X-ray of a man aged 71 years who had undergone THA 21 years earlier and had complied with routine follow-up. When his X-rays showed significant wear of the liner and some osteolysis, he was able to undergo a simple revision (Figure 3B).
Three-dimensional CT is useful for quantifying the presence and severity of osteolytic lesions, because plain radiographs may underestimate the amount of bone loss that is present.25 The CT in Figure 3C shows the magnitude of osteolysis that was underestimated by the preoperative plain X-rays (Figure 3A). Computed tomography scans are crucial for surgical planning in the setting of severe acetabular bone loss.
There is a wide spectrum of signs and symptoms that can occur in the setting of acetabular component failure. Pain is a common presenting symptom. Groin pain can represent acetabular failure; thigh pain may be correlated to femoral component failure.25 The clinical patient presentation ultimately depends on the underlying cause: an infection, polyethylene wear, instability, or aseptic loosening.25 Leg-length discrepancy, joint deformity, location of prior incisions, functional status, and baseline neurologic status should be evaluated and documented during the preoperative evaluation as well.25
Case Study Revision Options
The X-rays and CT scans for this case study patient showed that he was a possible candidate for the simplest revision surgery; an isolated liner exchange and replacement of the femoral head. When the original surgery was performed (1997), the only FDA approved PE liner was UHMWPE. To justify isolated liner exchange, the modular acetabular metallic shell also should be well-fixed and appropriately oriented.26 This is evaluated both preoperatively and intraoperatively.
If found to be well fixed with an appropriate orientation and locking mechanism, the UHMWPE liner could be replaced with a HXLPE liner and a larger metal femoral head for improved wear and stability. Acetabular revision is indicted for an asymptomatic patient who has progressive osteolysis, severe wear, or bone loss that would compromise future reconstruction.
Conclusions
Over the past several decades, THA has become recognized as an effective treatment option for the reduction of pain and disability associated with hip joint disease and is associated with successful clinical outcomes. The most frequently noted recommendations for trying to increase the life expectancy of an artificial hip replacement include maintaining a normal weight, keeping leg muscles strong, and avoiding repetitive squatting and kneeling.
As the number of primary THAs has increased and the average age of those undergoing a primary THA has decreased, the need for revisions has risen. Reviews have demonstrated that the most common causes for early total hip revision, regardless of component, included infection, instability/dislocation, and fracture, whereas wear is the most common reason for mid to late revisions.
The wear of all materials used has been shown to be greatest in the most active patients.
Studies continue to identify ways to potentially prevent or reverse osteolysis from wear debris. Alendronate therapy has been shown to prevent and treat PE debris-induced periprosthetic bone loss in rats.27 It also was successfully used in a case report of an asymptomatic woman aged 39 years who had rapid PE wear and aggressive periprosthetic osteolysis within just 2 years of a bilateral THA.28 Other areas of research on decreasing osteolysis in THA recipients include trials with mesenchymal stem cells, bone morphogenic proteins, and gene therapy.6
In the U.S., 46,000 revisions were performed in 2004 and this number is expected to more than double by 2030.4 Primary care providers are sure to encounter patients who will be in need of a hip revision procedure. It’s important for them to make sure that their patients who have undergone a THA are periodically seen for orthopedic follow-up. Despite the long history of primary THAs, there is still not a single technique and material to suit all patient characteristics.1 Unfortunately, the same currently applies to hip revision procedures.
1. Knight SR, Aujla R, Biswas SP. Total hip arthroplasty--over 100 years of operative history. Orthop Rev (Pavia). 2011;3(2):e16.
2. Centers for Disease Control and Prevention. FastStats: inpatient surgery. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed January 18, 2016.
3. Joint Revision Surgery-When do I need it? American Academy of Orthopedic Surgeons Website. http://www.tlhoc.com/uploads/documents/when_do_I_need_it.pdf. Accessed January 18, 2016.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467(1):56-65.
6. Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312-316.
7. Engesæter IØ, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesæter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82(2):149-154.
8. Salter RB. Etiology, pathogenesis and possible prevention of congenital dislocation of the hip. Can Med Assoc J. 1968;98(20):933-945.
9. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
10. Pace TB, Keith KC, Alvarez E, Snider RG, Tanner, SL, Desjardins JD. Comparison of conventional polyethylene wear and signs of cup failure in two similar total hip designs. Adv Orthop. 2013;2013:710621.
11. Kurtz SM. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement. Academic Press: London; 2014.
12. Babovic N, Trousdale RT. Total hip athroplasty using highly cross-linked polyethylene in patients younger than 50 years with minimum 10-year follow-up. J Arthroplasty. 2013;29(5):815-817.
13. Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasal highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816-1821.
14. Thomas G, Simpson D, Mehmmod S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
15. Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study. Clin Orthop Relat Res. 2013;471(11):3596-3600.
16. Tateiwa T, Clarke IC, Williams PA, et al. Ceramic total hip arthroplasty in the United States: safety and risk issues revisited. Am J Orthop (Belle Mead NJ). 2008;37(2):E26-E31.
17. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. BioMed Res Int. 2013;2013:157247.
18. Haidukewych GJ, Petrie J. Bearing surface considerations for total hip arthroplasty in young patients. Orthop Clin N Am. 2012;43(3):395-402.
19. Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410.
20. Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321-2327.
21. Pritchett JW. Adverse reaction to metal debris: metallosis of the resurfaced hip. Curr Orthop Pract. 2012;23(1):50-58.
22. Smith AJ, Dieppe P, Porter M, Blom AW; National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint registry of England and Wales and hospital episode statistics. BMJ. 2012;344:e2383.
23. Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531-1536.
24. Cash, D, Khanduja V. The case for ceramics-on-polyethylene as the preferred bearing for a young adult hip replacement. Hip Int. 2014;24(5):421-427.
25. Taylor ED, Browne JA. Reconstruction options for acetabular revision. World J Orthop. 2012;3(7):95-100.
26. Lombardi AV, Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg. 2008;16(5):243-248.
27. Millet PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A(2):236-249.
28. O'Hara LJ, Nivbrant B, Rohrl S.Cross-linked polyethylene and bisphosphonate therapy for osteolysis in total hip athroplasty: a case report. J Orthop Surg (Hong Kong). 2004;12(1):114-121.
1. Knight SR, Aujla R, Biswas SP. Total hip arthroplasty--over 100 years of operative history. Orthop Rev (Pavia). 2011;3(2):e16.
2. Centers for Disease Control and Prevention. FastStats: inpatient surgery. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed January 18, 2016.
3. Joint Revision Surgery-When do I need it? American Academy of Orthopedic Surgeons Website. http://www.tlhoc.com/uploads/documents/when_do_I_need_it.pdf. Accessed January 18, 2016.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467(1):56-65.
6. Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312-316.
7. Engesæter IØ, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesæter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82(2):149-154.
8. Salter RB. Etiology, pathogenesis and possible prevention of congenital dislocation of the hip. Can Med Assoc J. 1968;98(20):933-945.
9. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
10. Pace TB, Keith KC, Alvarez E, Snider RG, Tanner, SL, Desjardins JD. Comparison of conventional polyethylene wear and signs of cup failure in two similar total hip designs. Adv Orthop. 2013;2013:710621.
11. Kurtz SM. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement. Academic Press: London; 2014.
12. Babovic N, Trousdale RT. Total hip athroplasty using highly cross-linked polyethylene in patients younger than 50 years with minimum 10-year follow-up. J Arthroplasty. 2013;29(5):815-817.
13. Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasal highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816-1821.
14. Thomas G, Simpson D, Mehmmod S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
15. Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study. Clin Orthop Relat Res. 2013;471(11):3596-3600.
16. Tateiwa T, Clarke IC, Williams PA, et al. Ceramic total hip arthroplasty in the United States: safety and risk issues revisited. Am J Orthop (Belle Mead NJ). 2008;37(2):E26-E31.
17. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. BioMed Res Int. 2013;2013:157247.
18. Haidukewych GJ, Petrie J. Bearing surface considerations for total hip arthroplasty in young patients. Orthop Clin N Am. 2012;43(3):395-402.
19. Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410.
20. Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321-2327.
21. Pritchett JW. Adverse reaction to metal debris: metallosis of the resurfaced hip. Curr Orthop Pract. 2012;23(1):50-58.
22. Smith AJ, Dieppe P, Porter M, Blom AW; National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint registry of England and Wales and hospital episode statistics. BMJ. 2012;344:e2383.
23. Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531-1536.
24. Cash, D, Khanduja V. The case for ceramics-on-polyethylene as the preferred bearing for a young adult hip replacement. Hip Int. 2014;24(5):421-427.
25. Taylor ED, Browne JA. Reconstruction options for acetabular revision. World J Orthop. 2012;3(7):95-100.
26. Lombardi AV, Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg. 2008;16(5):243-248.
27. Millet PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A(2):236-249.
28. O'Hara LJ, Nivbrant B, Rohrl S.Cross-linked polyethylene and bisphosphonate therapy for osteolysis in total hip athroplasty: a case report. J Orthop Surg (Hong Kong). 2004;12(1):114-121.