User login
Elective Total Hip Arthroplasty: Which Surgical Approach Is Optimal?
Total hip arthroplasty (THA) is one of the most successful orthopedic interventions performed today in terms of pain relief, cost effectiveness, and clinical outcomes.1 As a definitive treatment for end-stage arthritis of the hip, more than 330,000 procedures are performed in the Unites States each year. The number performed is growing by > 5% per year and is predicted to double by 2030, partly due to patients living longer, older individuals seeking a higher level of functionality than did previous generations, and better access to health care.2,3
The THA procedure also has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early-onset secondary arthritis, such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoroacetabular impingement.4 Younger patients are more likely to need a revision. According to a study by Evans and colleagues using available arthroplasty registry data, about three-quarters of hip replacements last 15 to 20 years, and 58% of hip replacements last 25 years in patients with osteoarthritis.5
For decades, the THA procedure of choice has been a standard posterior approach (PA). The PA was used because it allowed excellent intraoperative exposure and was applicable to a wide range of hip problems.6 In the past several years, modified muscle-sparing surgical approaches have been introduced. Two performed frequently are the mini PA (MPA) and the direct anterior approach (DAA).
The MPA is a modification of the PA. Surgeons perform the THA through a small incision without cutting the abductor muscles that are critical to hip stability and gait. A study published in 2010 concluded that the MPA was associated with less pain, shorter hospital length of stay (LOS) (therefore, an economic saving), and an earlier return to walking postoperatively.7
The DAA has been around since the early days of THA. Carl Hueter first described the anterior approach to the hip in 1881 (referred to as the Hueter approach). Smith-Peterson is frequently credited with popularizing the DAA technique during his career after publishing his first description of the approach in 1917.8 About 10 years ago, the DAA showed a resurgence as another muscle-sparing alternative for THAs. The DAA is considered to be a true intermuscular approach that preserves the soft tissues around the hip joint, thereby preserving the stability of the joint.9-11 The optimal surgical approach is still the subject of debate.
We present a male with right hip end-stage degenerative joint disease (DJD) and review some medical literature. Although other approaches to THA can be used (lateral, anterolateral), the discussion focuses on 2 muscle-sparing approaches performed frequently, the MPA and the DAA, and can be of value to primary care practitioners in their discussion with patients.
Case Presentation
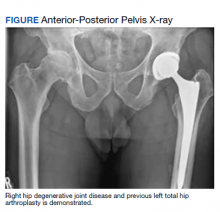
A 61-year-old male patient presented with progressive right hip pain. At age 37, he had a left THA via a PA due to hip dysplasia and a revision on the same hip at age 55 (the polyethylene liner was replaced and the cobalt chromium head was changed to ceramic), again through a PA. An orthopedic clinical evaluation and X-rays confirmed end-stage DJD of the right hip (Figure). He was informed to return to plan an elective THA when the “bad days were significantly greater than the good days” and/or when his functionality or quality of life was unacceptable. The orthopedic surgeon favored an MPA but offered a hand-off to colleagues who preferred the DAA. The patient was given information to review.
Discussion
No matter which approach is used, one study concluded that surgeons who perform > 50 hip replacements each year have better overall outcomes.12
The MPA emerged in the past decade as a muscle-sparing modification of the PA. The incision length (< 10 cm) is the simplest way of categorizing the surgery as an MPA. However, the amount of deep surgical dissection is a more important consideration for sparing muscle (for improved postoperative functionality, recovery, and joint stability) due to the gluteus maximus insertion, the quadratus femoris, and the piriformis tendons being left intact.13-16
Multiple studies have directly compared the MPA and PA, with variable results. One study concluded that the MPA was associated with lower surgical blood loss, lower pain at rest, and a faster recovery compared with that of the PA. Still, the study found no significant difference in postoperative laboratory values of possible markers of increased tissue damage and surgical invasiveness, such as creatinine phosphokinase (CPK) levels.15 Another randomized controlled trial (RCT) of 100 patients concluded that there was a trend for improved walking times and patient satisfaction at 6 weeks post-MPA vs PA.16 Other studies have found that the MPA and PA were essentially equivalent to each other regarding operative time, early postoperative outcomes, transfusion rate, hospital LOS, and postoperative complications.14 However, a recent meta-analysis found positive trends in favor of the MPA. The MPA was associated with a slight decrease in operating time, blood loss, hospital LOS, and earlier improvement in Harris hip scores. The meta-analysis found no significant decrease in the rate of dislocation or femoral fracture.13 Studies are still needed to evaluate long-term implant survival and outcomes for MPA and PA.
The DAA has received renewed attention as surgeons seek minimally invasive techniques and more rapid recoveries.6 The DAA involves a 3- to 4-inch incision on the front of the hip and enters the hip joint through the intermuscular interval between the tensor fasciae latae and gluteus medius muscles laterally and the sartorius muscle and rectus fascia medially.9 The DAA is considered a true intermuscular approach that preserves the soft tissues around the hip joint (including the posterior capsule), thereby presumably preserving the stability of the joint.9 The popularity for this approach has been attributed primarily to claims of improved recovery times, lower pain levels, improved patient satisfaction, as well as improved accuracy on both implant placement/alignment and leg length restoration.17 Orthopedic surgeons are increasingly being trained in the DAA during their residency and fellowship training.
There are many potential disadvantages to DAA. For example, DAA may present intraoperative radiation exposure for patients and surgeons during a fluoroscopy-assisted procedure. In addition, neuropraxia, particularly to the lateral femoral cutaneous nerve, can cause transient or permanent meralgia paresthetica. Wound healing may also present problems for female and obese patients, particularly those with a body mass index > 39 who are at increased risk of wound complications. DAA also increases time under anesthesia. Patients may experience proximal femoral fractures and dislocations and complex/challenging femoral exposure and bone preparation. Finally, sagittal malalignment of the stem could lead to loosening and an increased need for revision surgery.18
Another disadvantage of the DAA compared with the PA and MPA is the steep learning curve. Most studies find that the complication rate decreases only when the surgeon performs a significant number of DAA procedures. DeSteiger and colleagues noted a learning curve of 50 to 100 cases needed, and Masonis and colleagues concluded that at least 100 cases needed to be done to decrease operating and fluoroscopy times.19,20 Many orthopedic surgeons perform < 25 THA procedures a year.21
With the recent surge in popularity of the DAA, several studies have evaluated the DAA vs the MPA. A prospective RCT of 54 patients comparing the 2 approaches found that DAA patients walked without assistive devices sooner than did MPA patients: 22 days for DAA and 28 days for MPA.22 Improved cup position and a faster return of functionality were found in another study. DAA patients transitioned to a cane at 12 days vs 15.5 days for MPA patients and had a negative Trendelenburg sign at 16.7 days vs 24.8 days for MPA patients.23
Comparing DAA and MPA for inflammatory markers (serum CPK, C-reactive protein, interleukin-6, interleukin-1 β and tumor necrosis factor-α), the level of CPK postoperatively was 5.5 times higher in MPA patients, consistent with significantly more muscle damage. However, the overall physiologic burden as demonstrated by the measurement of all inflammatory markers was similar between the MPA and the DAA. This suggests that the inflammatory cascade associated with THA may be influenced more by the osteotomy and prosthesis implantation than by the surgical approach.24
Of note, some surgeons who perform the DAA recommend fewer postoperative precautions and suggest that physical therapy may not be necessary after discharge.25,26 Nevertheless, physiotherapeutic rehabilitation after all THA surgery is recommended as the standard treatment to minimize postoperative complications, such as hip dislocation, wound infection, deep venous thrombosis, and pulmonary embolism, and to maximize the patient’s functionality.27-29 RCTs are needed to look at long-term data on clinical outcomes between the MPA and DAA. Dislocation is a risk regardless of the approach used. Nevertheless, rates of dislocation, in general, are now very low, given the use of larger femoral head implants for all approaches.
Conclusions
THA is one of the most successful surgical procedures performed today. Patients desire hip pain relief and a return to function with as little interruption in their life as possible. Additionally, health care systems and insurers require THA procedures to be as efficient and cost-effective as possible. The debate regarding the most effective or preferable approach for THA continues. Although some prospective RCTs found that patients who underwent the DAA had objectively faster recovery than patients who had the MPA, it is also acknowledged that the results were dependent on surgeons who are very skilled in performing DAAs. The hope of both approaches is to get the individual moving as quickly and safely as possible to avoid a cascade of deterioration in the postoperative period. Factors other than the surgical approach, including patient selection, surgical volume and experience, careful preoperative assessments, attentive pain management, and rapid rehabilitation protocols, may be just as important as to which procedure is performed.30 The final decision should still be dependent on the patient-surgeon relationship and informed decision making.
In this case, the patient reviewed all the information he was given and independently researched the 2 procedures over many months. Ultimately, he decided to undergo a right THA via the DAA.
1. Elmallah RK, Chughtai M, Khlopas A. et al. Determining cost-effectiveness of total hip and knee arthroplasty using the Short Form-6D utility measure. J Arthroplasty. 2017;32(2):351-354. doi:10.1016/j.arth.2016.08.006
2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
3. Kurtz, S, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
4. Sheahan WT, Parvataneni HK. Asymptomatic but time for a hip revision. Fed Pract. 2016;33(2):39-43.
5. Evans, JT, Evans JP, Walker RW, et al. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647-654. doi:10.1016/S0140-6736(18)31665-9
6. Yang X, Huang H-F, Sun L , Yang Z, Deng C-Y, Tian XB. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis of randomized controlled studies. Orthop Surg. 2020;12:1065-1073. doi:10.1111/os.12669
7. Varela Egocheaga JR, Suárez-Suárez MA, Fernández-Villán M, González-Sastre V, Varela-Gómez JR, Murcia-Mazón A. Minimally invasive posterior approach in total hip arthroplasty. Prospective randomized trial. An Sist Sanit Navar. 2010:33(2):133-143. doi:10.4321/s1137-66272010000300002
8. Raxhbauer F, Kain MS, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320. doi:10.1016/j.ocl.2009.02.007
9. Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int. 2019;29(6):584-596. doi:10.1177/1120700018820652
10. Kennon RE Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A(suppl 4):39-48. doi:10.2106/00004623-200300004-00005
11. Bal BS, Vallurupalli S. Minimally invasive total hip arthroplasty with the anterior approach. Indian J Orthop. 2008;42(3):301-308. doi:10.4103/0019-5413.41853
12. Katz JN, Losina E, Barrett E. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629. doi:10.2106/00004623-200111000-00002
13. Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision approach to a total hip arthroplasty. J Arthroplasty. 2014;29(10):1970-1982. doi:10.1016/j.arth.2014.05.021
14. Chimento GF, Pavone V, Sharrock S, Kahn K, Cahill J, Sculco TP. Minimally invasive total hip arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20(2):139-144. doi:10.1016/j.arth.2004.09.061
15. Fink B, Mittelstaedt A, Schulz MS, Sebena P, Sing J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty. A prospective and comparative study. J Orthop Surg Res. 2010;5:46. doi:10.1186/1749-799X-5-46
16. Khan RJ, Maor D, Hofmann M, Haebich S. A comparison of a less invasive piriformis-sparing approach versus the standard approach to the hip: a randomized controlled trial. J Bone Joint Surg Br. 2012;94:43-50. doi:10.1302/0301-620X.94B1.27001
17. Galakatos GR. Direct anterior total hip arthroplasty. Missouri Med. 2018;115(6):537-541.
18. Flevas, DA, Tsantes AG, Mavrogenis, AE. Direct anterior approach total hip arthroplasty revisited. JBJS Rev. 2020;8(4):e0144. doi:10.2106/JBJS.RVW.19.00144
19. DeSteiger RN, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res. 2015;473(12):3860-3866. doi:10.1007/s11999-015-4565-6
20. Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12)(suppl 2).
21. Bal BS. Clinical faceoff: anterior total hip versus mini-posterior: Which one is better? Clin Orthop Relat Res. 2015;473(4):1192-1196. doi:10.1007/s11999-014-3684-9
22. Taunton MJ, Mason JB, Odum SM, Bryan D, Springer BD. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty. 2014;29;(suppl 9):169-172. doi:10.1016/j.arth.2014.03.05
23. Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698-704. doi:10.1016/j.arth.2008.04.012
24. Bergin PF, Doppelt JD, Kephart CJ. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. Bone Joint Surg Am. 2011; 93(15):1392-1398. doi:10.2106/JBJS.J.00557
25. Carli AV, Poitras S, Clohisy JC, Beaule PE. Variation in use of postoperative precautions and equipment following total hip arthroplasty: a survey of the AAHKS and CAS membership. J Arthroplasty. 2018;33(10):3201-3205. doi:10.1016/j.arth.2018.05.043
26. Kavcˇicˇ G, Mirt PK, Tumpej J, Bedenčič. The direct anterior approach for total hip arthroplasty without specific table: surgical approach and our seven years of experience. Published June 14, 2019. Accessed March 4, 2022. https://crimsonăpublishers.com/rabs/fulltext/RABS.000520.php27. American Academy of Orthopedic Surgeons. Total hip replacement exercise guide. Published 2017. Updated February 2022. Accessed March 4, 2022. https://orthoinfo.aaos.org/en/recovery/total-hip-replacement-exercise-guide
28. Medical Advisory Secretariat. Physiotherapy rehabilitation after total knee or hip replacement: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(8):1-91.
29. Pa˘unescu F, Didilescu A, Antonescu DM. Factors that may influence the functional outcome after primary total hip arthroplasty. Clujul Med. 2013;86(2):121-127.
30. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631. doi:10.1007/s11999-014-3827-z
Total hip arthroplasty (THA) is one of the most successful orthopedic interventions performed today in terms of pain relief, cost effectiveness, and clinical outcomes.1 As a definitive treatment for end-stage arthritis of the hip, more than 330,000 procedures are performed in the Unites States each year. The number performed is growing by > 5% per year and is predicted to double by 2030, partly due to patients living longer, older individuals seeking a higher level of functionality than did previous generations, and better access to health care.2,3
The THA procedure also has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early-onset secondary arthritis, such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoroacetabular impingement.4 Younger patients are more likely to need a revision. According to a study by Evans and colleagues using available arthroplasty registry data, about three-quarters of hip replacements last 15 to 20 years, and 58% of hip replacements last 25 years in patients with osteoarthritis.5
For decades, the THA procedure of choice has been a standard posterior approach (PA). The PA was used because it allowed excellent intraoperative exposure and was applicable to a wide range of hip problems.6 In the past several years, modified muscle-sparing surgical approaches have been introduced. Two performed frequently are the mini PA (MPA) and the direct anterior approach (DAA).
The MPA is a modification of the PA. Surgeons perform the THA through a small incision without cutting the abductor muscles that are critical to hip stability and gait. A study published in 2010 concluded that the MPA was associated with less pain, shorter hospital length of stay (LOS) (therefore, an economic saving), and an earlier return to walking postoperatively.7
The DAA has been around since the early days of THA. Carl Hueter first described the anterior approach to the hip in 1881 (referred to as the Hueter approach). Smith-Peterson is frequently credited with popularizing the DAA technique during his career after publishing his first description of the approach in 1917.8 About 10 years ago, the DAA showed a resurgence as another muscle-sparing alternative for THAs. The DAA is considered to be a true intermuscular approach that preserves the soft tissues around the hip joint, thereby preserving the stability of the joint.9-11 The optimal surgical approach is still the subject of debate.
We present a male with right hip end-stage degenerative joint disease (DJD) and review some medical literature. Although other approaches to THA can be used (lateral, anterolateral), the discussion focuses on 2 muscle-sparing approaches performed frequently, the MPA and the DAA, and can be of value to primary care practitioners in their discussion with patients.
Case Presentation
A 61-year-old male patient presented with progressive right hip pain. At age 37, he had a left THA via a PA due to hip dysplasia and a revision on the same hip at age 55 (the polyethylene liner was replaced and the cobalt chromium head was changed to ceramic), again through a PA. An orthopedic clinical evaluation and X-rays confirmed end-stage DJD of the right hip (Figure). He was informed to return to plan an elective THA when the “bad days were significantly greater than the good days” and/or when his functionality or quality of life was unacceptable. The orthopedic surgeon favored an MPA but offered a hand-off to colleagues who preferred the DAA. The patient was given information to review.
Discussion
No matter which approach is used, one study concluded that surgeons who perform > 50 hip replacements each year have better overall outcomes.12
The MPA emerged in the past decade as a muscle-sparing modification of the PA. The incision length (< 10 cm) is the simplest way of categorizing the surgery as an MPA. However, the amount of deep surgical dissection is a more important consideration for sparing muscle (for improved postoperative functionality, recovery, and joint stability) due to the gluteus maximus insertion, the quadratus femoris, and the piriformis tendons being left intact.13-16
Multiple studies have directly compared the MPA and PA, with variable results. One study concluded that the MPA was associated with lower surgical blood loss, lower pain at rest, and a faster recovery compared with that of the PA. Still, the study found no significant difference in postoperative laboratory values of possible markers of increased tissue damage and surgical invasiveness, such as creatinine phosphokinase (CPK) levels.15 Another randomized controlled trial (RCT) of 100 patients concluded that there was a trend for improved walking times and patient satisfaction at 6 weeks post-MPA vs PA.16 Other studies have found that the MPA and PA were essentially equivalent to each other regarding operative time, early postoperative outcomes, transfusion rate, hospital LOS, and postoperative complications.14 However, a recent meta-analysis found positive trends in favor of the MPA. The MPA was associated with a slight decrease in operating time, blood loss, hospital LOS, and earlier improvement in Harris hip scores. The meta-analysis found no significant decrease in the rate of dislocation or femoral fracture.13 Studies are still needed to evaluate long-term implant survival and outcomes for MPA and PA.
The DAA has received renewed attention as surgeons seek minimally invasive techniques and more rapid recoveries.6 The DAA involves a 3- to 4-inch incision on the front of the hip and enters the hip joint through the intermuscular interval between the tensor fasciae latae and gluteus medius muscles laterally and the sartorius muscle and rectus fascia medially.9 The DAA is considered a true intermuscular approach that preserves the soft tissues around the hip joint (including the posterior capsule), thereby presumably preserving the stability of the joint.9 The popularity for this approach has been attributed primarily to claims of improved recovery times, lower pain levels, improved patient satisfaction, as well as improved accuracy on both implant placement/alignment and leg length restoration.17 Orthopedic surgeons are increasingly being trained in the DAA during their residency and fellowship training.
There are many potential disadvantages to DAA. For example, DAA may present intraoperative radiation exposure for patients and surgeons during a fluoroscopy-assisted procedure. In addition, neuropraxia, particularly to the lateral femoral cutaneous nerve, can cause transient or permanent meralgia paresthetica. Wound healing may also present problems for female and obese patients, particularly those with a body mass index > 39 who are at increased risk of wound complications. DAA also increases time under anesthesia. Patients may experience proximal femoral fractures and dislocations and complex/challenging femoral exposure and bone preparation. Finally, sagittal malalignment of the stem could lead to loosening and an increased need for revision surgery.18
Another disadvantage of the DAA compared with the PA and MPA is the steep learning curve. Most studies find that the complication rate decreases only when the surgeon performs a significant number of DAA procedures. DeSteiger and colleagues noted a learning curve of 50 to 100 cases needed, and Masonis and colleagues concluded that at least 100 cases needed to be done to decrease operating and fluoroscopy times.19,20 Many orthopedic surgeons perform < 25 THA procedures a year.21
With the recent surge in popularity of the DAA, several studies have evaluated the DAA vs the MPA. A prospective RCT of 54 patients comparing the 2 approaches found that DAA patients walked without assistive devices sooner than did MPA patients: 22 days for DAA and 28 days for MPA.22 Improved cup position and a faster return of functionality were found in another study. DAA patients transitioned to a cane at 12 days vs 15.5 days for MPA patients and had a negative Trendelenburg sign at 16.7 days vs 24.8 days for MPA patients.23
Comparing DAA and MPA for inflammatory markers (serum CPK, C-reactive protein, interleukin-6, interleukin-1 β and tumor necrosis factor-α), the level of CPK postoperatively was 5.5 times higher in MPA patients, consistent with significantly more muscle damage. However, the overall physiologic burden as demonstrated by the measurement of all inflammatory markers was similar between the MPA and the DAA. This suggests that the inflammatory cascade associated with THA may be influenced more by the osteotomy and prosthesis implantation than by the surgical approach.24
Of note, some surgeons who perform the DAA recommend fewer postoperative precautions and suggest that physical therapy may not be necessary after discharge.25,26 Nevertheless, physiotherapeutic rehabilitation after all THA surgery is recommended as the standard treatment to minimize postoperative complications, such as hip dislocation, wound infection, deep venous thrombosis, and pulmonary embolism, and to maximize the patient’s functionality.27-29 RCTs are needed to look at long-term data on clinical outcomes between the MPA and DAA. Dislocation is a risk regardless of the approach used. Nevertheless, rates of dislocation, in general, are now very low, given the use of larger femoral head implants for all approaches.
Conclusions
THA is one of the most successful surgical procedures performed today. Patients desire hip pain relief and a return to function with as little interruption in their life as possible. Additionally, health care systems and insurers require THA procedures to be as efficient and cost-effective as possible. The debate regarding the most effective or preferable approach for THA continues. Although some prospective RCTs found that patients who underwent the DAA had objectively faster recovery than patients who had the MPA, it is also acknowledged that the results were dependent on surgeons who are very skilled in performing DAAs. The hope of both approaches is to get the individual moving as quickly and safely as possible to avoid a cascade of deterioration in the postoperative period. Factors other than the surgical approach, including patient selection, surgical volume and experience, careful preoperative assessments, attentive pain management, and rapid rehabilitation protocols, may be just as important as to which procedure is performed.30 The final decision should still be dependent on the patient-surgeon relationship and informed decision making.
In this case, the patient reviewed all the information he was given and independently researched the 2 procedures over many months. Ultimately, he decided to undergo a right THA via the DAA.
Total hip arthroplasty (THA) is one of the most successful orthopedic interventions performed today in terms of pain relief, cost effectiveness, and clinical outcomes.1 As a definitive treatment for end-stage arthritis of the hip, more than 330,000 procedures are performed in the Unites States each year. The number performed is growing by > 5% per year and is predicted to double by 2030, partly due to patients living longer, older individuals seeking a higher level of functionality than did previous generations, and better access to health care.2,3
The THA procedure also has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early-onset secondary arthritis, such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoroacetabular impingement.4 Younger patients are more likely to need a revision. According to a study by Evans and colleagues using available arthroplasty registry data, about three-quarters of hip replacements last 15 to 20 years, and 58% of hip replacements last 25 years in patients with osteoarthritis.5
For decades, the THA procedure of choice has been a standard posterior approach (PA). The PA was used because it allowed excellent intraoperative exposure and was applicable to a wide range of hip problems.6 In the past several years, modified muscle-sparing surgical approaches have been introduced. Two performed frequently are the mini PA (MPA) and the direct anterior approach (DAA).
The MPA is a modification of the PA. Surgeons perform the THA through a small incision without cutting the abductor muscles that are critical to hip stability and gait. A study published in 2010 concluded that the MPA was associated with less pain, shorter hospital length of stay (LOS) (therefore, an economic saving), and an earlier return to walking postoperatively.7
The DAA has been around since the early days of THA. Carl Hueter first described the anterior approach to the hip in 1881 (referred to as the Hueter approach). Smith-Peterson is frequently credited with popularizing the DAA technique during his career after publishing his first description of the approach in 1917.8 About 10 years ago, the DAA showed a resurgence as another muscle-sparing alternative for THAs. The DAA is considered to be a true intermuscular approach that preserves the soft tissues around the hip joint, thereby preserving the stability of the joint.9-11 The optimal surgical approach is still the subject of debate.
We present a male with right hip end-stage degenerative joint disease (DJD) and review some medical literature. Although other approaches to THA can be used (lateral, anterolateral), the discussion focuses on 2 muscle-sparing approaches performed frequently, the MPA and the DAA, and can be of value to primary care practitioners in their discussion with patients.
Case Presentation
A 61-year-old male patient presented with progressive right hip pain. At age 37, he had a left THA via a PA due to hip dysplasia and a revision on the same hip at age 55 (the polyethylene liner was replaced and the cobalt chromium head was changed to ceramic), again through a PA. An orthopedic clinical evaluation and X-rays confirmed end-stage DJD of the right hip (Figure). He was informed to return to plan an elective THA when the “bad days were significantly greater than the good days” and/or when his functionality or quality of life was unacceptable. The orthopedic surgeon favored an MPA but offered a hand-off to colleagues who preferred the DAA. The patient was given information to review.
Discussion
No matter which approach is used, one study concluded that surgeons who perform > 50 hip replacements each year have better overall outcomes.12
The MPA emerged in the past decade as a muscle-sparing modification of the PA. The incision length (< 10 cm) is the simplest way of categorizing the surgery as an MPA. However, the amount of deep surgical dissection is a more important consideration for sparing muscle (for improved postoperative functionality, recovery, and joint stability) due to the gluteus maximus insertion, the quadratus femoris, and the piriformis tendons being left intact.13-16
Multiple studies have directly compared the MPA and PA, with variable results. One study concluded that the MPA was associated with lower surgical blood loss, lower pain at rest, and a faster recovery compared with that of the PA. Still, the study found no significant difference in postoperative laboratory values of possible markers of increased tissue damage and surgical invasiveness, such as creatinine phosphokinase (CPK) levels.15 Another randomized controlled trial (RCT) of 100 patients concluded that there was a trend for improved walking times and patient satisfaction at 6 weeks post-MPA vs PA.16 Other studies have found that the MPA and PA were essentially equivalent to each other regarding operative time, early postoperative outcomes, transfusion rate, hospital LOS, and postoperative complications.14 However, a recent meta-analysis found positive trends in favor of the MPA. The MPA was associated with a slight decrease in operating time, blood loss, hospital LOS, and earlier improvement in Harris hip scores. The meta-analysis found no significant decrease in the rate of dislocation or femoral fracture.13 Studies are still needed to evaluate long-term implant survival and outcomes for MPA and PA.
The DAA has received renewed attention as surgeons seek minimally invasive techniques and more rapid recoveries.6 The DAA involves a 3- to 4-inch incision on the front of the hip and enters the hip joint through the intermuscular interval between the tensor fasciae latae and gluteus medius muscles laterally and the sartorius muscle and rectus fascia medially.9 The DAA is considered a true intermuscular approach that preserves the soft tissues around the hip joint (including the posterior capsule), thereby presumably preserving the stability of the joint.9 The popularity for this approach has been attributed primarily to claims of improved recovery times, lower pain levels, improved patient satisfaction, as well as improved accuracy on both implant placement/alignment and leg length restoration.17 Orthopedic surgeons are increasingly being trained in the DAA during their residency and fellowship training.
There are many potential disadvantages to DAA. For example, DAA may present intraoperative radiation exposure for patients and surgeons during a fluoroscopy-assisted procedure. In addition, neuropraxia, particularly to the lateral femoral cutaneous nerve, can cause transient or permanent meralgia paresthetica. Wound healing may also present problems for female and obese patients, particularly those with a body mass index > 39 who are at increased risk of wound complications. DAA also increases time under anesthesia. Patients may experience proximal femoral fractures and dislocations and complex/challenging femoral exposure and bone preparation. Finally, sagittal malalignment of the stem could lead to loosening and an increased need for revision surgery.18
Another disadvantage of the DAA compared with the PA and MPA is the steep learning curve. Most studies find that the complication rate decreases only when the surgeon performs a significant number of DAA procedures. DeSteiger and colleagues noted a learning curve of 50 to 100 cases needed, and Masonis and colleagues concluded that at least 100 cases needed to be done to decrease operating and fluoroscopy times.19,20 Many orthopedic surgeons perform < 25 THA procedures a year.21
With the recent surge in popularity of the DAA, several studies have evaluated the DAA vs the MPA. A prospective RCT of 54 patients comparing the 2 approaches found that DAA patients walked without assistive devices sooner than did MPA patients: 22 days for DAA and 28 days for MPA.22 Improved cup position and a faster return of functionality were found in another study. DAA patients transitioned to a cane at 12 days vs 15.5 days for MPA patients and had a negative Trendelenburg sign at 16.7 days vs 24.8 days for MPA patients.23
Comparing DAA and MPA for inflammatory markers (serum CPK, C-reactive protein, interleukin-6, interleukin-1 β and tumor necrosis factor-α), the level of CPK postoperatively was 5.5 times higher in MPA patients, consistent with significantly more muscle damage. However, the overall physiologic burden as demonstrated by the measurement of all inflammatory markers was similar between the MPA and the DAA. This suggests that the inflammatory cascade associated with THA may be influenced more by the osteotomy and prosthesis implantation than by the surgical approach.24
Of note, some surgeons who perform the DAA recommend fewer postoperative precautions and suggest that physical therapy may not be necessary after discharge.25,26 Nevertheless, physiotherapeutic rehabilitation after all THA surgery is recommended as the standard treatment to minimize postoperative complications, such as hip dislocation, wound infection, deep venous thrombosis, and pulmonary embolism, and to maximize the patient’s functionality.27-29 RCTs are needed to look at long-term data on clinical outcomes between the MPA and DAA. Dislocation is a risk regardless of the approach used. Nevertheless, rates of dislocation, in general, are now very low, given the use of larger femoral head implants for all approaches.
Conclusions
THA is one of the most successful surgical procedures performed today. Patients desire hip pain relief and a return to function with as little interruption in their life as possible. Additionally, health care systems and insurers require THA procedures to be as efficient and cost-effective as possible. The debate regarding the most effective or preferable approach for THA continues. Although some prospective RCTs found that patients who underwent the DAA had objectively faster recovery than patients who had the MPA, it is also acknowledged that the results were dependent on surgeons who are very skilled in performing DAAs. The hope of both approaches is to get the individual moving as quickly and safely as possible to avoid a cascade of deterioration in the postoperative period. Factors other than the surgical approach, including patient selection, surgical volume and experience, careful preoperative assessments, attentive pain management, and rapid rehabilitation protocols, may be just as important as to which procedure is performed.30 The final decision should still be dependent on the patient-surgeon relationship and informed decision making.
In this case, the patient reviewed all the information he was given and independently researched the 2 procedures over many months. Ultimately, he decided to undergo a right THA via the DAA.
1. Elmallah RK, Chughtai M, Khlopas A. et al. Determining cost-effectiveness of total hip and knee arthroplasty using the Short Form-6D utility measure. J Arthroplasty. 2017;32(2):351-354. doi:10.1016/j.arth.2016.08.006
2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
3. Kurtz, S, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
4. Sheahan WT, Parvataneni HK. Asymptomatic but time for a hip revision. Fed Pract. 2016;33(2):39-43.
5. Evans, JT, Evans JP, Walker RW, et al. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647-654. doi:10.1016/S0140-6736(18)31665-9
6. Yang X, Huang H-F, Sun L , Yang Z, Deng C-Y, Tian XB. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis of randomized controlled studies. Orthop Surg. 2020;12:1065-1073. doi:10.1111/os.12669
7. Varela Egocheaga JR, Suárez-Suárez MA, Fernández-Villán M, González-Sastre V, Varela-Gómez JR, Murcia-Mazón A. Minimally invasive posterior approach in total hip arthroplasty. Prospective randomized trial. An Sist Sanit Navar. 2010:33(2):133-143. doi:10.4321/s1137-66272010000300002
8. Raxhbauer F, Kain MS, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320. doi:10.1016/j.ocl.2009.02.007
9. Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int. 2019;29(6):584-596. doi:10.1177/1120700018820652
10. Kennon RE Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A(suppl 4):39-48. doi:10.2106/00004623-200300004-00005
11. Bal BS, Vallurupalli S. Minimally invasive total hip arthroplasty with the anterior approach. Indian J Orthop. 2008;42(3):301-308. doi:10.4103/0019-5413.41853
12. Katz JN, Losina E, Barrett E. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629. doi:10.2106/00004623-200111000-00002
13. Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision approach to a total hip arthroplasty. J Arthroplasty. 2014;29(10):1970-1982. doi:10.1016/j.arth.2014.05.021
14. Chimento GF, Pavone V, Sharrock S, Kahn K, Cahill J, Sculco TP. Minimally invasive total hip arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20(2):139-144. doi:10.1016/j.arth.2004.09.061
15. Fink B, Mittelstaedt A, Schulz MS, Sebena P, Sing J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty. A prospective and comparative study. J Orthop Surg Res. 2010;5:46. doi:10.1186/1749-799X-5-46
16. Khan RJ, Maor D, Hofmann M, Haebich S. A comparison of a less invasive piriformis-sparing approach versus the standard approach to the hip: a randomized controlled trial. J Bone Joint Surg Br. 2012;94:43-50. doi:10.1302/0301-620X.94B1.27001
17. Galakatos GR. Direct anterior total hip arthroplasty. Missouri Med. 2018;115(6):537-541.
18. Flevas, DA, Tsantes AG, Mavrogenis, AE. Direct anterior approach total hip arthroplasty revisited. JBJS Rev. 2020;8(4):e0144. doi:10.2106/JBJS.RVW.19.00144
19. DeSteiger RN, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res. 2015;473(12):3860-3866. doi:10.1007/s11999-015-4565-6
20. Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12)(suppl 2).
21. Bal BS. Clinical faceoff: anterior total hip versus mini-posterior: Which one is better? Clin Orthop Relat Res. 2015;473(4):1192-1196. doi:10.1007/s11999-014-3684-9
22. Taunton MJ, Mason JB, Odum SM, Bryan D, Springer BD. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty. 2014;29;(suppl 9):169-172. doi:10.1016/j.arth.2014.03.05
23. Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698-704. doi:10.1016/j.arth.2008.04.012
24. Bergin PF, Doppelt JD, Kephart CJ. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. Bone Joint Surg Am. 2011; 93(15):1392-1398. doi:10.2106/JBJS.J.00557
25. Carli AV, Poitras S, Clohisy JC, Beaule PE. Variation in use of postoperative precautions and equipment following total hip arthroplasty: a survey of the AAHKS and CAS membership. J Arthroplasty. 2018;33(10):3201-3205. doi:10.1016/j.arth.2018.05.043
26. Kavcˇicˇ G, Mirt PK, Tumpej J, Bedenčič. The direct anterior approach for total hip arthroplasty without specific table: surgical approach and our seven years of experience. Published June 14, 2019. Accessed March 4, 2022. https://crimsonăpublishers.com/rabs/fulltext/RABS.000520.php27. American Academy of Orthopedic Surgeons. Total hip replacement exercise guide. Published 2017. Updated February 2022. Accessed March 4, 2022. https://orthoinfo.aaos.org/en/recovery/total-hip-replacement-exercise-guide
28. Medical Advisory Secretariat. Physiotherapy rehabilitation after total knee or hip replacement: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(8):1-91.
29. Pa˘unescu F, Didilescu A, Antonescu DM. Factors that may influence the functional outcome after primary total hip arthroplasty. Clujul Med. 2013;86(2):121-127.
30. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631. doi:10.1007/s11999-014-3827-z
1. Elmallah RK, Chughtai M, Khlopas A. et al. Determining cost-effectiveness of total hip and knee arthroplasty using the Short Form-6D utility measure. J Arthroplasty. 2017;32(2):351-354. doi:10.1016/j.arth.2016.08.006
2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
3. Kurtz, S, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
4. Sheahan WT, Parvataneni HK. Asymptomatic but time for a hip revision. Fed Pract. 2016;33(2):39-43.
5. Evans, JT, Evans JP, Walker RW, et al. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647-654. doi:10.1016/S0140-6736(18)31665-9
6. Yang X, Huang H-F, Sun L , Yang Z, Deng C-Y, Tian XB. Direct anterior approach versus posterolateral approach in total hip arthroplasty: a systematic review and meta-analysis of randomized controlled studies. Orthop Surg. 2020;12:1065-1073. doi:10.1111/os.12669
7. Varela Egocheaga JR, Suárez-Suárez MA, Fernández-Villán M, González-Sastre V, Varela-Gómez JR, Murcia-Mazón A. Minimally invasive posterior approach in total hip arthroplasty. Prospective randomized trial. An Sist Sanit Navar. 2010:33(2):133-143. doi:10.4321/s1137-66272010000300002
8. Raxhbauer F, Kain MS, Leunig M. The history of the anterior approach to the hip. Orthop Clin North Am. 2009;40(3):311-320. doi:10.1016/j.ocl.2009.02.007
9. Jia F, Guo B, Xu F, Hou Y, Tang X, Huang L. A comparison of clinical, radiographic and surgical outcomes of total hip arthroplasty between direct anterior and posterior approaches: a systematic review and meta-analysis. Hip Int. 2019;29(6):584-596. doi:10.1177/1120700018820652
10. Kennon RE Keggi JM, Wetmore RS, Zatorski LE, Huo MH, Keggi KJ. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A(suppl 4):39-48. doi:10.2106/00004623-200300004-00005
11. Bal BS, Vallurupalli S. Minimally invasive total hip arthroplasty with the anterior approach. Indian J Orthop. 2008;42(3):301-308. doi:10.4103/0019-5413.41853
12. Katz JN, Losina E, Barrett E. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83(11):1622-1629. doi:10.2106/00004623-200111000-00002
13. Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision approach to a total hip arthroplasty. J Arthroplasty. 2014;29(10):1970-1982. doi:10.1016/j.arth.2014.05.021
14. Chimento GF, Pavone V, Sharrock S, Kahn K, Cahill J, Sculco TP. Minimally invasive total hip arthroplasty: a prospective randomized study. J Arthroplasty. 2005;20(2):139-144. doi:10.1016/j.arth.2004.09.061
15. Fink B, Mittelstaedt A, Schulz MS, Sebena P, Sing J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty. A prospective and comparative study. J Orthop Surg Res. 2010;5:46. doi:10.1186/1749-799X-5-46
16. Khan RJ, Maor D, Hofmann M, Haebich S. A comparison of a less invasive piriformis-sparing approach versus the standard approach to the hip: a randomized controlled trial. J Bone Joint Surg Br. 2012;94:43-50. doi:10.1302/0301-620X.94B1.27001
17. Galakatos GR. Direct anterior total hip arthroplasty. Missouri Med. 2018;115(6):537-541.
18. Flevas, DA, Tsantes AG, Mavrogenis, AE. Direct anterior approach total hip arthroplasty revisited. JBJS Rev. 2020;8(4):e0144. doi:10.2106/JBJS.RVW.19.00144
19. DeSteiger RN, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res. 2015;473(12):3860-3866. doi:10.1007/s11999-015-4565-6
20. Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12)(suppl 2).
21. Bal BS. Clinical faceoff: anterior total hip versus mini-posterior: Which one is better? Clin Orthop Relat Res. 2015;473(4):1192-1196. doi:10.1007/s11999-014-3684-9
22. Taunton MJ, Mason JB, Odum SM, Bryan D, Springer BD. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty. 2014;29;(suppl 9):169-172. doi:10.1016/j.arth.2014.03.05
23. Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698-704. doi:10.1016/j.arth.2008.04.012
24. Bergin PF, Doppelt JD, Kephart CJ. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. Bone Joint Surg Am. 2011; 93(15):1392-1398. doi:10.2106/JBJS.J.00557
25. Carli AV, Poitras S, Clohisy JC, Beaule PE. Variation in use of postoperative precautions and equipment following total hip arthroplasty: a survey of the AAHKS and CAS membership. J Arthroplasty. 2018;33(10):3201-3205. doi:10.1016/j.arth.2018.05.043
26. Kavcˇicˇ G, Mirt PK, Tumpej J, Bedenčič. The direct anterior approach for total hip arthroplasty without specific table: surgical approach and our seven years of experience. Published June 14, 2019. Accessed March 4, 2022. https://crimsonăpublishers.com/rabs/fulltext/RABS.000520.php27. American Academy of Orthopedic Surgeons. Total hip replacement exercise guide. Published 2017. Updated February 2022. Accessed March 4, 2022. https://orthoinfo.aaos.org/en/recovery/total-hip-replacement-exercise-guide
28. Medical Advisory Secretariat. Physiotherapy rehabilitation after total knee or hip replacement: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(8):1-91.
29. Pa˘unescu F, Didilescu A, Antonescu DM. Factors that may influence the functional outcome after primary total hip arthroplasty. Clujul Med. 2013;86(2):121-127.
30. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631. doi:10.1007/s11999-014-3827-z
Asymptomatic but Time for a Hip Revision
Total hip arthroplasty (THA) is considered to be one of the most successful orthopedic interventions of its generation.1 In 2010, 332,000 THAs were performed in the U.S.2 Although used to correct advanced joint diseases in the elderly, the THA procedure has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early onset secondary arthritis such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoro-acetabular impingement.
Current hip replacements are expected to function at least 10 to 20 years in 90% of patients.3 As increasing numbers of young patients have these procedures and as seniors continue to live longer, patients will outlast their implants. Younger and more active patients have a higher rate of revision, because the longevity of the prosthesis is usually a function of usage.3 The number of revision THAs is projected to increase 137% by 2030.4
Hip resurfacing has been developed as a bone preserving surgical alternative to THA. The first system for use in the U.S. received FDA approval in 2006, but concerns about the metal on metal bearing surfaces, high failure and revision rates, and early catastrophic modes of failure compared with THAs has resulted in the recall of many of these devices. Hip resurfacing may offer some advantages compared with those of a THA in a carefully selected population, but its use will not be further discussed in this case study.5 Periprosthetic osteolysis and aseptic loosening are 2 of the long-term consequences of THA.6 Bone loss is felt to be secondary to a biologic reaction to particulate debris from implants.6 Some patients, especially those with loosening, complete wear, or fracture, will be symptomatic with pain. However, wear and osteolysis is a silent disease unless there is mechanical failure. Other patients may not experience discomfort. Radiographic studies may reveal significant changes, which warrant the recommendation for a hip revision.
Hip revision surgery has 3 major purposes: relieving pain in the affected joint, restoring the patient’s mobility, and removing a loose or damaged prosthesis before irreversible harm is done to the joint. It’s anticipated that most primary care providers (PCPs) will encounter patients who seek advice on the need for a revision hip arthroplasty.
This case will present an asymptomatic patient who underwent a THA in 1997 at age 37, to address developmental dysplasia of the hip (DDH) and was advised to undergo a revision hip arthroplasty due to abnormal radiographic findings at age 55 years. A discussion will follow that includes a brief review of the history of THA, the materials and bearings commonly used, the presenting symptoms or radiographic changes that signal the need for a revision, and the current options available for a patient such as this.
Case Report
A man aged 55 years presented to a new orthopedic surgeon for his first orthopedic appointment in 10 years. The patient had a left metal-on-polyethylene (M-on-PE) THA 18 years prior due to early onset secondary degenerative joint disease from DDH. The patient’s M-on-PE THA was a titanium acetabular socket and femoral stem with a cobalt-chromium alloy femoral head and a polyethylene liner. The patient remained physically active with an exercise routine consisting of walking, swimming, and weight training.
The patient’s orthopedic history was notable for a right knee arthroscopy for intervention due to a torn medial and lateral meniscus, and birth history was noteworthy for a breech presentation. The physical exam was unremarkable except for a slight leg length discrepancy, but the patient did not exhibit a Trendelenburg gait.
Plain X-rays and a computed tomography (CT) scan showed eccentric PE wear and superior migration of the femoral head, which was indicative of significant PE liner wear. No significant osteolysis or periprosthetic loosening was observed on the X-rays or CT scan. He was advised that a hip revision procedure would need to be done, optimally, within the next 6 months to a year.
Discussion
Hip dysplasia represents a broad group of disorders and generally means abnormal development of the hip joint. The term is most commonly used to refer to DDH with inadequate coverage of the femoral head. In one study, 25% of hip replacements performed in patients aged ≤ 40 years were due to underlying hip dysplasia.7
Developmental dysplasia of the hip occurs more often in children who present in the breech position.8 One theory argues that packaging issues in utero may account for the increased incidence of DDH.9 The earliest recorded attempts at hip replacement occurred in Germany, in 1891, when ivory was used to replace the femoral heads of patients whose hip joints had been destroyed by tuberculosis.1
The orthopedic surgeon Sir John Charnley, who worked at the Manchester Royal Infirmary, is considered the father of the modern THA.1 His low friction arthroplasty, designed in the early 1960s is identical, in principle, to the M-on-PE prosthesis used today.1 The PE liner used was ultrahigh molecular weight polyethylene (UHMWPE).1
Due to the early success of the Charnley prosthesis, the M-on-PE prosthesis became the most widely used. Although PE is the most studied and understood of all acetabular liner materials, it will eventually wear and shed debris. Acetabular cup wear is the most frequent reason for mid-to-long-term revisions, especially in young and active patients.10 More active patients shed more debris.3 The PE debris instigates the release of inflammatory mediators, which results in chronic inflammation and tissue damage that erodes the supporting bone and can lead to implant loosening or fracture.6 Ongoing studies seek to optimize and improve properties of the UHMWPE and to develop alternative bearings. After FDA approval in 1999, highly cross-linked polyethylene liners (HXLPE) rapidly became the standard of care for THAs, at least in the U.S.11 Highly cross-linked polyethylene liners are created from UHMWPE through a process of cross-linking by exposure to gamma radiation, and subsequent heat treatment to neutralize free radicals and limit oxidative degradation.12
In one study, the 5-year annual linear wear rate for a HXLPE liner was only 45% of that seen with the UHMWPE liner, although the qualitative wear pattern was the same.13 In a study that followed patients for 7 years postoperatively, the mean steady-state wear rate of the HXLPE was 0.005 mm/y compared with 0.037 mm/y for UHMWPE.14 In a long-term study (a minimum follow-up of 10 years) of 50 patients who were aged < 50 years and underwent THA using HXLPE liners, there was no radiographic evidence of osteolysis or component loosening, and liner wear was 0.020 ± 0.0047 mm/y.12 In 2005, second-generation HXLPE liners were introduced clinically and have been shown to further reduce wear in vitro compared with both UHMWPE and first-generation HXLPE liners. Callary and colleagues calculated that the wear rates between 1 year and 5 years were all < 0.001 mm/y.15
The use of ceramic for THAs began in 1970, and ceramic heads on polyethylene (C-on-PE) liners and ceramic-on-ceramic (C-on-C) bearings have been in continual use for > 30 years in Europe. Premarket FDA approval based on European data was granted in 1983; however, the manufacturer voluntarily removed it from the market because of a high incidence of stem loosening (> 30% within 3 years in some series).16 FDA approvals came much later for C-on-PE (1989) and C-on-C (2003) bearings.
Ceramic is the hardest implant material used, and it can be concluded from many clinical and laboratory reports that C-on-PE and C-on-C combinations confer a potentially significant reduction in wear on THA bearings.16 Ceramic hips initially had 2 concerns: catastrophic shattering and squeaking. Current ceramic hips have been substantially improved, and some experts feel shattering has been essentially eliminated.16 Other experts note that ceramic brittleness remains a major concern.17 Squeaking remains a problem for some, but it usually abates over time. No study has correlated squeaking with impending failure or increased pain or disability.
While C-on-C bearings are now felt to be a good implant for young active patients, these bearings have generally not resulted in significantly lower wear rates and fewer revisions.18 High rates of wear and osteolysis have been sporadically documented over the 35-year history of ceramic implants.16 The FDA approved the first ceramic-on-metal total hip replacement system on June 13, 2011.
Metal-on-metal (M-on-M) implants have been used by some for decades, although they were not approved by the FDA until the late 1990s. However, some device recalls have brought negative attention to M-on-M implants.19 It was felt that they would generate less wear debris than PE, but reports of pseudotumors (from inflammatory mediators) and metallosis have significantly tempered enthusiasm for these products.20,21 The wear rates are very low, estimated to be only 0.01 mm/y, but concerns about the carcinogenetic potential of systemically increased metal ions remains a possible and much debated concern.19,22,23 In January 2013, FDA issued a safety communication on M-on-M implants.
Many experts feel that modern ceramic or metal on second-generation HXLPE represents the gold standard and the most predictable bearing choice for young, active patients.18 Others feel that the optimal choice of bearing surfaces in THA, particularly in the younger and more active patient, remains controversial.24
Follow-Up
Intermittent orthopedic monitoring is recommended for all patients who have undergone a THA. The frequency of hip X-rays on follow-up appointments is left to the orthopedic surgeon. After the initial recovery, serial images every 2 to 5 years can identify progressive failure, and annual X-rays may be used for closer follow-up in high-risk patients.
Patients who experience dislocations, fractures, infections, or pain usually maintain close orthopedic follow-up. Significant wear of the prosthesis damages the socket; osteolysis can cause irreversible bone loss, fracture, and loosening. Massive acetabular bone loss is very difficult to reverse and creates major reconstruction challenges.
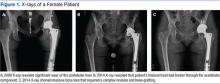
Figure 1A is a 2009 X-ray of a woman aged 44 years who underwent a THA after a motor vehicle accident in 1997 and who was advised to have a revision THA when seen in 2009.
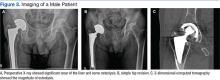
Figure 3A is an X-ray of a man aged 71 years who had undergone THA 21 years earlier and had complied with routine follow-up. When his X-rays showed significant wear of the liner and some osteolysis, he was able to undergo a simple revision (Figure 3B).
Three-dimensional CT is useful for quantifying the presence and severity of osteolytic lesions, because plain radiographs may underestimate the amount of bone loss that is present.25 The CT in Figure 3C shows the magnitude of osteolysis that was underestimated by the preoperative plain X-rays (Figure 3A). Computed tomography scans are crucial for surgical planning in the setting of severe acetabular bone loss.
There is a wide spectrum of signs and symptoms that can occur in the setting of acetabular component failure. Pain is a common presenting symptom. Groin pain can represent acetabular failure; thigh pain may be correlated to femoral component failure.25 The clinical patient presentation ultimately depends on the underlying cause: an infection, polyethylene wear, instability, or aseptic loosening.25 Leg-length discrepancy, joint deformity, location of prior incisions, functional status, and baseline neurologic status should be evaluated and documented during the preoperative evaluation as well.25
Case Study Revision Options
The X-rays and CT scans for this case study patient showed that he was a possible candidate for the simplest revision surgery; an isolated liner exchange and replacement of the femoral head. When the original surgery was performed (1997), the only FDA approved PE liner was UHMWPE. To justify isolated liner exchange, the modular acetabular metallic shell also should be well-fixed and appropriately oriented.26 This is evaluated both preoperatively and intraoperatively.
If found to be well fixed with an appropriate orientation and locking mechanism, the UHMWPE liner could be replaced with a HXLPE liner and a larger metal femoral head for improved wear and stability. Acetabular revision is indicted for an asymptomatic patient who has progressive osteolysis, severe wear, or bone loss that would compromise future reconstruction.
Conclusions
Over the past several decades, THA has become recognized as an effective treatment option for the reduction of pain and disability associated with hip joint disease and is associated with successful clinical outcomes. The most frequently noted recommendations for trying to increase the life expectancy of an artificial hip replacement include maintaining a normal weight, keeping leg muscles strong, and avoiding repetitive squatting and kneeling.
As the number of primary THAs has increased and the average age of those undergoing a primary THA has decreased, the need for revisions has risen. Reviews have demonstrated that the most common causes for early total hip revision, regardless of component, included infection, instability/dislocation, and fracture, whereas wear is the most common reason for mid to late revisions.
The wear of all materials used has been shown to be greatest in the most active patients.
Studies continue to identify ways to potentially prevent or reverse osteolysis from wear debris. Alendronate therapy has been shown to prevent and treat PE debris-induced periprosthetic bone loss in rats.27 It also was successfully used in a case report of an asymptomatic woman aged 39 years who had rapid PE wear and aggressive periprosthetic osteolysis within just 2 years of a bilateral THA.28 Other areas of research on decreasing osteolysis in THA recipients include trials with mesenchymal stem cells, bone morphogenic proteins, and gene therapy.6
In the U.S., 46,000 revisions were performed in 2004 and this number is expected to more than double by 2030.4 Primary care providers are sure to encounter patients who will be in need of a hip revision procedure. It’s important for them to make sure that their patients who have undergone a THA are periodically seen for orthopedic follow-up. Despite the long history of primary THAs, there is still not a single technique and material to suit all patient characteristics.1 Unfortunately, the same currently applies to hip revision procedures.
1. Knight SR, Aujla R, Biswas SP. Total hip arthroplasty--over 100 years of operative history. Orthop Rev (Pavia). 2011;3(2):e16.
2. Centers for Disease Control and Prevention. FastStats: inpatient surgery. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed January 18, 2016.
3. Joint Revision Surgery-When do I need it? American Academy of Orthopedic Surgeons Website. http://www.tlhoc.com/uploads/documents/when_do_I_need_it.pdf. Accessed January 18, 2016.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467(1):56-65.
6. Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312-316.
7. Engesæter IØ, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesæter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82(2):149-154.
8. Salter RB. Etiology, pathogenesis and possible prevention of congenital dislocation of the hip. Can Med Assoc J. 1968;98(20):933-945.
9. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
10. Pace TB, Keith KC, Alvarez E, Snider RG, Tanner, SL, Desjardins JD. Comparison of conventional polyethylene wear and signs of cup failure in two similar total hip designs. Adv Orthop. 2013;2013:710621.
11. Kurtz SM. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement. Academic Press: London; 2014.
12. Babovic N, Trousdale RT. Total hip athroplasty using highly cross-linked polyethylene in patients younger than 50 years with minimum 10-year follow-up. J Arthroplasty. 2013;29(5):815-817.
13. Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasal highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816-1821.
14. Thomas G, Simpson D, Mehmmod S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
15. Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study. Clin Orthop Relat Res. 2013;471(11):3596-3600.
16. Tateiwa T, Clarke IC, Williams PA, et al. Ceramic total hip arthroplasty in the United States: safety and risk issues revisited. Am J Orthop (Belle Mead NJ). 2008;37(2):E26-E31.
17. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. BioMed Res Int. 2013;2013:157247.
18. Haidukewych GJ, Petrie J. Bearing surface considerations for total hip arthroplasty in young patients. Orthop Clin N Am. 2012;43(3):395-402.
19. Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410.
20. Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321-2327.
21. Pritchett JW. Adverse reaction to metal debris: metallosis of the resurfaced hip. Curr Orthop Pract. 2012;23(1):50-58.
22. Smith AJ, Dieppe P, Porter M, Blom AW; National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint registry of England and Wales and hospital episode statistics. BMJ. 2012;344:e2383.
23. Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531-1536.
24. Cash, D, Khanduja V. The case for ceramics-on-polyethylene as the preferred bearing for a young adult hip replacement. Hip Int. 2014;24(5):421-427.
25. Taylor ED, Browne JA. Reconstruction options for acetabular revision. World J Orthop. 2012;3(7):95-100.
26. Lombardi AV, Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg. 2008;16(5):243-248.
27. Millet PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A(2):236-249.
28. O'Hara LJ, Nivbrant B, Rohrl S.Cross-linked polyethylene and bisphosphonate therapy for osteolysis in total hip athroplasty: a case report. J Orthop Surg (Hong Kong). 2004;12(1):114-121.
Total hip arthroplasty (THA) is considered to be one of the most successful orthopedic interventions of its generation.1 In 2010, 332,000 THAs were performed in the U.S.2 Although used to correct advanced joint diseases in the elderly, the THA procedure has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early onset secondary arthritis such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoro-acetabular impingement.
Current hip replacements are expected to function at least 10 to 20 years in 90% of patients.3 As increasing numbers of young patients have these procedures and as seniors continue to live longer, patients will outlast their implants. Younger and more active patients have a higher rate of revision, because the longevity of the prosthesis is usually a function of usage.3 The number of revision THAs is projected to increase 137% by 2030.4
Hip resurfacing has been developed as a bone preserving surgical alternative to THA. The first system for use in the U.S. received FDA approval in 2006, but concerns about the metal on metal bearing surfaces, high failure and revision rates, and early catastrophic modes of failure compared with THAs has resulted in the recall of many of these devices. Hip resurfacing may offer some advantages compared with those of a THA in a carefully selected population, but its use will not be further discussed in this case study.5 Periprosthetic osteolysis and aseptic loosening are 2 of the long-term consequences of THA.6 Bone loss is felt to be secondary to a biologic reaction to particulate debris from implants.6 Some patients, especially those with loosening, complete wear, or fracture, will be symptomatic with pain. However, wear and osteolysis is a silent disease unless there is mechanical failure. Other patients may not experience discomfort. Radiographic studies may reveal significant changes, which warrant the recommendation for a hip revision.
Hip revision surgery has 3 major purposes: relieving pain in the affected joint, restoring the patient’s mobility, and removing a loose or damaged prosthesis before irreversible harm is done to the joint. It’s anticipated that most primary care providers (PCPs) will encounter patients who seek advice on the need for a revision hip arthroplasty.
This case will present an asymptomatic patient who underwent a THA in 1997 at age 37, to address developmental dysplasia of the hip (DDH) and was advised to undergo a revision hip arthroplasty due to abnormal radiographic findings at age 55 years. A discussion will follow that includes a brief review of the history of THA, the materials and bearings commonly used, the presenting symptoms or radiographic changes that signal the need for a revision, and the current options available for a patient such as this.
Case Report
A man aged 55 years presented to a new orthopedic surgeon for his first orthopedic appointment in 10 years. The patient had a left metal-on-polyethylene (M-on-PE) THA 18 years prior due to early onset secondary degenerative joint disease from DDH. The patient’s M-on-PE THA was a titanium acetabular socket and femoral stem with a cobalt-chromium alloy femoral head and a polyethylene liner. The patient remained physically active with an exercise routine consisting of walking, swimming, and weight training.
The patient’s orthopedic history was notable for a right knee arthroscopy for intervention due to a torn medial and lateral meniscus, and birth history was noteworthy for a breech presentation. The physical exam was unremarkable except for a slight leg length discrepancy, but the patient did not exhibit a Trendelenburg gait.
Plain X-rays and a computed tomography (CT) scan showed eccentric PE wear and superior migration of the femoral head, which was indicative of significant PE liner wear. No significant osteolysis or periprosthetic loosening was observed on the X-rays or CT scan. He was advised that a hip revision procedure would need to be done, optimally, within the next 6 months to a year.
Discussion
Hip dysplasia represents a broad group of disorders and generally means abnormal development of the hip joint. The term is most commonly used to refer to DDH with inadequate coverage of the femoral head. In one study, 25% of hip replacements performed in patients aged ≤ 40 years were due to underlying hip dysplasia.7
Developmental dysplasia of the hip occurs more often in children who present in the breech position.8 One theory argues that packaging issues in utero may account for the increased incidence of DDH.9 The earliest recorded attempts at hip replacement occurred in Germany, in 1891, when ivory was used to replace the femoral heads of patients whose hip joints had been destroyed by tuberculosis.1
The orthopedic surgeon Sir John Charnley, who worked at the Manchester Royal Infirmary, is considered the father of the modern THA.1 His low friction arthroplasty, designed in the early 1960s is identical, in principle, to the M-on-PE prosthesis used today.1 The PE liner used was ultrahigh molecular weight polyethylene (UHMWPE).1
Due to the early success of the Charnley prosthesis, the M-on-PE prosthesis became the most widely used. Although PE is the most studied and understood of all acetabular liner materials, it will eventually wear and shed debris. Acetabular cup wear is the most frequent reason for mid-to-long-term revisions, especially in young and active patients.10 More active patients shed more debris.3 The PE debris instigates the release of inflammatory mediators, which results in chronic inflammation and tissue damage that erodes the supporting bone and can lead to implant loosening or fracture.6 Ongoing studies seek to optimize and improve properties of the UHMWPE and to develop alternative bearings. After FDA approval in 1999, highly cross-linked polyethylene liners (HXLPE) rapidly became the standard of care for THAs, at least in the U.S.11 Highly cross-linked polyethylene liners are created from UHMWPE through a process of cross-linking by exposure to gamma radiation, and subsequent heat treatment to neutralize free radicals and limit oxidative degradation.12
In one study, the 5-year annual linear wear rate for a HXLPE liner was only 45% of that seen with the UHMWPE liner, although the qualitative wear pattern was the same.13 In a study that followed patients for 7 years postoperatively, the mean steady-state wear rate of the HXLPE was 0.005 mm/y compared with 0.037 mm/y for UHMWPE.14 In a long-term study (a minimum follow-up of 10 years) of 50 patients who were aged < 50 years and underwent THA using HXLPE liners, there was no radiographic evidence of osteolysis or component loosening, and liner wear was 0.020 ± 0.0047 mm/y.12 In 2005, second-generation HXLPE liners were introduced clinically and have been shown to further reduce wear in vitro compared with both UHMWPE and first-generation HXLPE liners. Callary and colleagues calculated that the wear rates between 1 year and 5 years were all < 0.001 mm/y.15
The use of ceramic for THAs began in 1970, and ceramic heads on polyethylene (C-on-PE) liners and ceramic-on-ceramic (C-on-C) bearings have been in continual use for > 30 years in Europe. Premarket FDA approval based on European data was granted in 1983; however, the manufacturer voluntarily removed it from the market because of a high incidence of stem loosening (> 30% within 3 years in some series).16 FDA approvals came much later for C-on-PE (1989) and C-on-C (2003) bearings.
Ceramic is the hardest implant material used, and it can be concluded from many clinical and laboratory reports that C-on-PE and C-on-C combinations confer a potentially significant reduction in wear on THA bearings.16 Ceramic hips initially had 2 concerns: catastrophic shattering and squeaking. Current ceramic hips have been substantially improved, and some experts feel shattering has been essentially eliminated.16 Other experts note that ceramic brittleness remains a major concern.17 Squeaking remains a problem for some, but it usually abates over time. No study has correlated squeaking with impending failure or increased pain or disability.
While C-on-C bearings are now felt to be a good implant for young active patients, these bearings have generally not resulted in significantly lower wear rates and fewer revisions.18 High rates of wear and osteolysis have been sporadically documented over the 35-year history of ceramic implants.16 The FDA approved the first ceramic-on-metal total hip replacement system on June 13, 2011.
Metal-on-metal (M-on-M) implants have been used by some for decades, although they were not approved by the FDA until the late 1990s. However, some device recalls have brought negative attention to M-on-M implants.19 It was felt that they would generate less wear debris than PE, but reports of pseudotumors (from inflammatory mediators) and metallosis have significantly tempered enthusiasm for these products.20,21 The wear rates are very low, estimated to be only 0.01 mm/y, but concerns about the carcinogenetic potential of systemically increased metal ions remains a possible and much debated concern.19,22,23 In January 2013, FDA issued a safety communication on M-on-M implants.
Many experts feel that modern ceramic or metal on second-generation HXLPE represents the gold standard and the most predictable bearing choice for young, active patients.18 Others feel that the optimal choice of bearing surfaces in THA, particularly in the younger and more active patient, remains controversial.24
Follow-Up
Intermittent orthopedic monitoring is recommended for all patients who have undergone a THA. The frequency of hip X-rays on follow-up appointments is left to the orthopedic surgeon. After the initial recovery, serial images every 2 to 5 years can identify progressive failure, and annual X-rays may be used for closer follow-up in high-risk patients.
Patients who experience dislocations, fractures, infections, or pain usually maintain close orthopedic follow-up. Significant wear of the prosthesis damages the socket; osteolysis can cause irreversible bone loss, fracture, and loosening. Massive acetabular bone loss is very difficult to reverse and creates major reconstruction challenges.
Figure 1A is a 2009 X-ray of a woman aged 44 years who underwent a THA after a motor vehicle accident in 1997 and who was advised to have a revision THA when seen in 2009.
Figure 3A is an X-ray of a man aged 71 years who had undergone THA 21 years earlier and had complied with routine follow-up. When his X-rays showed significant wear of the liner and some osteolysis, he was able to undergo a simple revision (Figure 3B).
Three-dimensional CT is useful for quantifying the presence and severity of osteolytic lesions, because plain radiographs may underestimate the amount of bone loss that is present.25 The CT in Figure 3C shows the magnitude of osteolysis that was underestimated by the preoperative plain X-rays (Figure 3A). Computed tomography scans are crucial for surgical planning in the setting of severe acetabular bone loss.
There is a wide spectrum of signs and symptoms that can occur in the setting of acetabular component failure. Pain is a common presenting symptom. Groin pain can represent acetabular failure; thigh pain may be correlated to femoral component failure.25 The clinical patient presentation ultimately depends on the underlying cause: an infection, polyethylene wear, instability, or aseptic loosening.25 Leg-length discrepancy, joint deformity, location of prior incisions, functional status, and baseline neurologic status should be evaluated and documented during the preoperative evaluation as well.25
Case Study Revision Options
The X-rays and CT scans for this case study patient showed that he was a possible candidate for the simplest revision surgery; an isolated liner exchange and replacement of the femoral head. When the original surgery was performed (1997), the only FDA approved PE liner was UHMWPE. To justify isolated liner exchange, the modular acetabular metallic shell also should be well-fixed and appropriately oriented.26 This is evaluated both preoperatively and intraoperatively.
If found to be well fixed with an appropriate orientation and locking mechanism, the UHMWPE liner could be replaced with a HXLPE liner and a larger metal femoral head for improved wear and stability. Acetabular revision is indicted for an asymptomatic patient who has progressive osteolysis, severe wear, or bone loss that would compromise future reconstruction.
Conclusions
Over the past several decades, THA has become recognized as an effective treatment option for the reduction of pain and disability associated with hip joint disease and is associated with successful clinical outcomes. The most frequently noted recommendations for trying to increase the life expectancy of an artificial hip replacement include maintaining a normal weight, keeping leg muscles strong, and avoiding repetitive squatting and kneeling.
As the number of primary THAs has increased and the average age of those undergoing a primary THA has decreased, the need for revisions has risen. Reviews have demonstrated that the most common causes for early total hip revision, regardless of component, included infection, instability/dislocation, and fracture, whereas wear is the most common reason for mid to late revisions.
The wear of all materials used has been shown to be greatest in the most active patients.
Studies continue to identify ways to potentially prevent or reverse osteolysis from wear debris. Alendronate therapy has been shown to prevent and treat PE debris-induced periprosthetic bone loss in rats.27 It also was successfully used in a case report of an asymptomatic woman aged 39 years who had rapid PE wear and aggressive periprosthetic osteolysis within just 2 years of a bilateral THA.28 Other areas of research on decreasing osteolysis in THA recipients include trials with mesenchymal stem cells, bone morphogenic proteins, and gene therapy.6
In the U.S., 46,000 revisions were performed in 2004 and this number is expected to more than double by 2030.4 Primary care providers are sure to encounter patients who will be in need of a hip revision procedure. It’s important for them to make sure that their patients who have undergone a THA are periodically seen for orthopedic follow-up. Despite the long history of primary THAs, there is still not a single technique and material to suit all patient characteristics.1 Unfortunately, the same currently applies to hip revision procedures.
Total hip arthroplasty (THA) is considered to be one of the most successful orthopedic interventions of its generation.1 In 2010, 332,000 THAs were performed in the U.S.2 Although used to correct advanced joint diseases in the elderly, the THA procedure has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early onset secondary arthritis such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoro-acetabular impingement.
Current hip replacements are expected to function at least 10 to 20 years in 90% of patients.3 As increasing numbers of young patients have these procedures and as seniors continue to live longer, patients will outlast their implants. Younger and more active patients have a higher rate of revision, because the longevity of the prosthesis is usually a function of usage.3 The number of revision THAs is projected to increase 137% by 2030.4
Hip resurfacing has been developed as a bone preserving surgical alternative to THA. The first system for use in the U.S. received FDA approval in 2006, but concerns about the metal on metal bearing surfaces, high failure and revision rates, and early catastrophic modes of failure compared with THAs has resulted in the recall of many of these devices. Hip resurfacing may offer some advantages compared with those of a THA in a carefully selected population, but its use will not be further discussed in this case study.5 Periprosthetic osteolysis and aseptic loosening are 2 of the long-term consequences of THA.6 Bone loss is felt to be secondary to a biologic reaction to particulate debris from implants.6 Some patients, especially those with loosening, complete wear, or fracture, will be symptomatic with pain. However, wear and osteolysis is a silent disease unless there is mechanical failure. Other patients may not experience discomfort. Radiographic studies may reveal significant changes, which warrant the recommendation for a hip revision.
Hip revision surgery has 3 major purposes: relieving pain in the affected joint, restoring the patient’s mobility, and removing a loose or damaged prosthesis before irreversible harm is done to the joint. It’s anticipated that most primary care providers (PCPs) will encounter patients who seek advice on the need for a revision hip arthroplasty.
This case will present an asymptomatic patient who underwent a THA in 1997 at age 37, to address developmental dysplasia of the hip (DDH) and was advised to undergo a revision hip arthroplasty due to abnormal radiographic findings at age 55 years. A discussion will follow that includes a brief review of the history of THA, the materials and bearings commonly used, the presenting symptoms or radiographic changes that signal the need for a revision, and the current options available for a patient such as this.
Case Report
A man aged 55 years presented to a new orthopedic surgeon for his first orthopedic appointment in 10 years. The patient had a left metal-on-polyethylene (M-on-PE) THA 18 years prior due to early onset secondary degenerative joint disease from DDH. The patient’s M-on-PE THA was a titanium acetabular socket and femoral stem with a cobalt-chromium alloy femoral head and a polyethylene liner. The patient remained physically active with an exercise routine consisting of walking, swimming, and weight training.
The patient’s orthopedic history was notable for a right knee arthroscopy for intervention due to a torn medial and lateral meniscus, and birth history was noteworthy for a breech presentation. The physical exam was unremarkable except for a slight leg length discrepancy, but the patient did not exhibit a Trendelenburg gait.
Plain X-rays and a computed tomography (CT) scan showed eccentric PE wear and superior migration of the femoral head, which was indicative of significant PE liner wear. No significant osteolysis or periprosthetic loosening was observed on the X-rays or CT scan. He was advised that a hip revision procedure would need to be done, optimally, within the next 6 months to a year.
Discussion
Hip dysplasia represents a broad group of disorders and generally means abnormal development of the hip joint. The term is most commonly used to refer to DDH with inadequate coverage of the femoral head. In one study, 25% of hip replacements performed in patients aged ≤ 40 years were due to underlying hip dysplasia.7
Developmental dysplasia of the hip occurs more often in children who present in the breech position.8 One theory argues that packaging issues in utero may account for the increased incidence of DDH.9 The earliest recorded attempts at hip replacement occurred in Germany, in 1891, when ivory was used to replace the femoral heads of patients whose hip joints had been destroyed by tuberculosis.1
The orthopedic surgeon Sir John Charnley, who worked at the Manchester Royal Infirmary, is considered the father of the modern THA.1 His low friction arthroplasty, designed in the early 1960s is identical, in principle, to the M-on-PE prosthesis used today.1 The PE liner used was ultrahigh molecular weight polyethylene (UHMWPE).1
Due to the early success of the Charnley prosthesis, the M-on-PE prosthesis became the most widely used. Although PE is the most studied and understood of all acetabular liner materials, it will eventually wear and shed debris. Acetabular cup wear is the most frequent reason for mid-to-long-term revisions, especially in young and active patients.10 More active patients shed more debris.3 The PE debris instigates the release of inflammatory mediators, which results in chronic inflammation and tissue damage that erodes the supporting bone and can lead to implant loosening or fracture.6 Ongoing studies seek to optimize and improve properties of the UHMWPE and to develop alternative bearings. After FDA approval in 1999, highly cross-linked polyethylene liners (HXLPE) rapidly became the standard of care for THAs, at least in the U.S.11 Highly cross-linked polyethylene liners are created from UHMWPE through a process of cross-linking by exposure to gamma radiation, and subsequent heat treatment to neutralize free radicals and limit oxidative degradation.12
In one study, the 5-year annual linear wear rate for a HXLPE liner was only 45% of that seen with the UHMWPE liner, although the qualitative wear pattern was the same.13 In a study that followed patients for 7 years postoperatively, the mean steady-state wear rate of the HXLPE was 0.005 mm/y compared with 0.037 mm/y for UHMWPE.14 In a long-term study (a minimum follow-up of 10 years) of 50 patients who were aged < 50 years and underwent THA using HXLPE liners, there was no radiographic evidence of osteolysis or component loosening, and liner wear was 0.020 ± 0.0047 mm/y.12 In 2005, second-generation HXLPE liners were introduced clinically and have been shown to further reduce wear in vitro compared with both UHMWPE and first-generation HXLPE liners. Callary and colleagues calculated that the wear rates between 1 year and 5 years were all < 0.001 mm/y.15
The use of ceramic for THAs began in 1970, and ceramic heads on polyethylene (C-on-PE) liners and ceramic-on-ceramic (C-on-C) bearings have been in continual use for > 30 years in Europe. Premarket FDA approval based on European data was granted in 1983; however, the manufacturer voluntarily removed it from the market because of a high incidence of stem loosening (> 30% within 3 years in some series).16 FDA approvals came much later for C-on-PE (1989) and C-on-C (2003) bearings.
Ceramic is the hardest implant material used, and it can be concluded from many clinical and laboratory reports that C-on-PE and C-on-C combinations confer a potentially significant reduction in wear on THA bearings.16 Ceramic hips initially had 2 concerns: catastrophic shattering and squeaking. Current ceramic hips have been substantially improved, and some experts feel shattering has been essentially eliminated.16 Other experts note that ceramic brittleness remains a major concern.17 Squeaking remains a problem for some, but it usually abates over time. No study has correlated squeaking with impending failure or increased pain or disability.
While C-on-C bearings are now felt to be a good implant for young active patients, these bearings have generally not resulted in significantly lower wear rates and fewer revisions.18 High rates of wear and osteolysis have been sporadically documented over the 35-year history of ceramic implants.16 The FDA approved the first ceramic-on-metal total hip replacement system on June 13, 2011.
Metal-on-metal (M-on-M) implants have been used by some for decades, although they were not approved by the FDA until the late 1990s. However, some device recalls have brought negative attention to M-on-M implants.19 It was felt that they would generate less wear debris than PE, but reports of pseudotumors (from inflammatory mediators) and metallosis have significantly tempered enthusiasm for these products.20,21 The wear rates are very low, estimated to be only 0.01 mm/y, but concerns about the carcinogenetic potential of systemically increased metal ions remains a possible and much debated concern.19,22,23 In January 2013, FDA issued a safety communication on M-on-M implants.
Many experts feel that modern ceramic or metal on second-generation HXLPE represents the gold standard and the most predictable bearing choice for young, active patients.18 Others feel that the optimal choice of bearing surfaces in THA, particularly in the younger and more active patient, remains controversial.24
Follow-Up
Intermittent orthopedic monitoring is recommended for all patients who have undergone a THA. The frequency of hip X-rays on follow-up appointments is left to the orthopedic surgeon. After the initial recovery, serial images every 2 to 5 years can identify progressive failure, and annual X-rays may be used for closer follow-up in high-risk patients.
Patients who experience dislocations, fractures, infections, or pain usually maintain close orthopedic follow-up. Significant wear of the prosthesis damages the socket; osteolysis can cause irreversible bone loss, fracture, and loosening. Massive acetabular bone loss is very difficult to reverse and creates major reconstruction challenges.
Figure 1A is a 2009 X-ray of a woman aged 44 years who underwent a THA after a motor vehicle accident in 1997 and who was advised to have a revision THA when seen in 2009.
Figure 3A is an X-ray of a man aged 71 years who had undergone THA 21 years earlier and had complied with routine follow-up. When his X-rays showed significant wear of the liner and some osteolysis, he was able to undergo a simple revision (Figure 3B).
Three-dimensional CT is useful for quantifying the presence and severity of osteolytic lesions, because plain radiographs may underestimate the amount of bone loss that is present.25 The CT in Figure 3C shows the magnitude of osteolysis that was underestimated by the preoperative plain X-rays (Figure 3A). Computed tomography scans are crucial for surgical planning in the setting of severe acetabular bone loss.
There is a wide spectrum of signs and symptoms that can occur in the setting of acetabular component failure. Pain is a common presenting symptom. Groin pain can represent acetabular failure; thigh pain may be correlated to femoral component failure.25 The clinical patient presentation ultimately depends on the underlying cause: an infection, polyethylene wear, instability, or aseptic loosening.25 Leg-length discrepancy, joint deformity, location of prior incisions, functional status, and baseline neurologic status should be evaluated and documented during the preoperative evaluation as well.25
Case Study Revision Options
The X-rays and CT scans for this case study patient showed that he was a possible candidate for the simplest revision surgery; an isolated liner exchange and replacement of the femoral head. When the original surgery was performed (1997), the only FDA approved PE liner was UHMWPE. To justify isolated liner exchange, the modular acetabular metallic shell also should be well-fixed and appropriately oriented.26 This is evaluated both preoperatively and intraoperatively.
If found to be well fixed with an appropriate orientation and locking mechanism, the UHMWPE liner could be replaced with a HXLPE liner and a larger metal femoral head for improved wear and stability. Acetabular revision is indicted for an asymptomatic patient who has progressive osteolysis, severe wear, or bone loss that would compromise future reconstruction.
Conclusions
Over the past several decades, THA has become recognized as an effective treatment option for the reduction of pain and disability associated with hip joint disease and is associated with successful clinical outcomes. The most frequently noted recommendations for trying to increase the life expectancy of an artificial hip replacement include maintaining a normal weight, keeping leg muscles strong, and avoiding repetitive squatting and kneeling.
As the number of primary THAs has increased and the average age of those undergoing a primary THA has decreased, the need for revisions has risen. Reviews have demonstrated that the most common causes for early total hip revision, regardless of component, included infection, instability/dislocation, and fracture, whereas wear is the most common reason for mid to late revisions.
The wear of all materials used has been shown to be greatest in the most active patients.
Studies continue to identify ways to potentially prevent or reverse osteolysis from wear debris. Alendronate therapy has been shown to prevent and treat PE debris-induced periprosthetic bone loss in rats.27 It also was successfully used in a case report of an asymptomatic woman aged 39 years who had rapid PE wear and aggressive periprosthetic osteolysis within just 2 years of a bilateral THA.28 Other areas of research on decreasing osteolysis in THA recipients include trials with mesenchymal stem cells, bone morphogenic proteins, and gene therapy.6
In the U.S., 46,000 revisions were performed in 2004 and this number is expected to more than double by 2030.4 Primary care providers are sure to encounter patients who will be in need of a hip revision procedure. It’s important for them to make sure that their patients who have undergone a THA are periodically seen for orthopedic follow-up. Despite the long history of primary THAs, there is still not a single technique and material to suit all patient characteristics.1 Unfortunately, the same currently applies to hip revision procedures.
1. Knight SR, Aujla R, Biswas SP. Total hip arthroplasty--over 100 years of operative history. Orthop Rev (Pavia). 2011;3(2):e16.
2. Centers for Disease Control and Prevention. FastStats: inpatient surgery. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed January 18, 2016.
3. Joint Revision Surgery-When do I need it? American Academy of Orthopedic Surgeons Website. http://www.tlhoc.com/uploads/documents/when_do_I_need_it.pdf. Accessed January 18, 2016.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467(1):56-65.
6. Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312-316.
7. Engesæter IØ, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesæter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82(2):149-154.
8. Salter RB. Etiology, pathogenesis and possible prevention of congenital dislocation of the hip. Can Med Assoc J. 1968;98(20):933-945.
9. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
10. Pace TB, Keith KC, Alvarez E, Snider RG, Tanner, SL, Desjardins JD. Comparison of conventional polyethylene wear and signs of cup failure in two similar total hip designs. Adv Orthop. 2013;2013:710621.
11. Kurtz SM. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement. Academic Press: London; 2014.
12. Babovic N, Trousdale RT. Total hip athroplasty using highly cross-linked polyethylene in patients younger than 50 years with minimum 10-year follow-up. J Arthroplasty. 2013;29(5):815-817.
13. Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasal highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816-1821.
14. Thomas G, Simpson D, Mehmmod S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
15. Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study. Clin Orthop Relat Res. 2013;471(11):3596-3600.
16. Tateiwa T, Clarke IC, Williams PA, et al. Ceramic total hip arthroplasty in the United States: safety and risk issues revisited. Am J Orthop (Belle Mead NJ). 2008;37(2):E26-E31.
17. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. BioMed Res Int. 2013;2013:157247.
18. Haidukewych GJ, Petrie J. Bearing surface considerations for total hip arthroplasty in young patients. Orthop Clin N Am. 2012;43(3):395-402.
19. Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410.
20. Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321-2327.
21. Pritchett JW. Adverse reaction to metal debris: metallosis of the resurfaced hip. Curr Orthop Pract. 2012;23(1):50-58.
22. Smith AJ, Dieppe P, Porter M, Blom AW; National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint registry of England and Wales and hospital episode statistics. BMJ. 2012;344:e2383.
23. Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531-1536.
24. Cash, D, Khanduja V. The case for ceramics-on-polyethylene as the preferred bearing for a young adult hip replacement. Hip Int. 2014;24(5):421-427.
25. Taylor ED, Browne JA. Reconstruction options for acetabular revision. World J Orthop. 2012;3(7):95-100.
26. Lombardi AV, Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg. 2008;16(5):243-248.
27. Millet PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A(2):236-249.
28. O'Hara LJ, Nivbrant B, Rohrl S.Cross-linked polyethylene and bisphosphonate therapy for osteolysis in total hip athroplasty: a case report. J Orthop Surg (Hong Kong). 2004;12(1):114-121.
1. Knight SR, Aujla R, Biswas SP. Total hip arthroplasty--over 100 years of operative history. Orthop Rev (Pavia). 2011;3(2):e16.
2. Centers for Disease Control and Prevention. FastStats: inpatient surgery. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed January 18, 2016.
3. Joint Revision Surgery-When do I need it? American Academy of Orthopedic Surgeons Website. http://www.tlhoc.com/uploads/documents/when_do_I_need_it.pdf. Accessed January 18, 2016.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467(1):56-65.
6. Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312-316.
7. Engesæter IØ, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesæter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82(2):149-154.
8. Salter RB. Etiology, pathogenesis and possible prevention of congenital dislocation of the hip. Can Med Assoc J. 1968;98(20):933-945.
9. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
10. Pace TB, Keith KC, Alvarez E, Snider RG, Tanner, SL, Desjardins JD. Comparison of conventional polyethylene wear and signs of cup failure in two similar total hip designs. Adv Orthop. 2013;2013:710621.
11. Kurtz SM. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement. Academic Press: London; 2014.
12. Babovic N, Trousdale RT. Total hip athroplasty using highly cross-linked polyethylene in patients younger than 50 years with minimum 10-year follow-up. J Arthroplasty. 2013;29(5):815-817.
13. Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasal highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816-1821.
14. Thomas G, Simpson D, Mehmmod S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
15. Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study. Clin Orthop Relat Res. 2013;471(11):3596-3600.
16. Tateiwa T, Clarke IC, Williams PA, et al. Ceramic total hip arthroplasty in the United States: safety and risk issues revisited. Am J Orthop (Belle Mead NJ). 2008;37(2):E26-E31.
17. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. BioMed Res Int. 2013;2013:157247.
18. Haidukewych GJ, Petrie J. Bearing surface considerations for total hip arthroplasty in young patients. Orthop Clin N Am. 2012;43(3):395-402.
19. Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410.
20. Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321-2327.
21. Pritchett JW. Adverse reaction to metal debris: metallosis of the resurfaced hip. Curr Orthop Pract. 2012;23(1):50-58.
22. Smith AJ, Dieppe P, Porter M, Blom AW; National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint registry of England and Wales and hospital episode statistics. BMJ. 2012;344:e2383.
23. Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531-1536.
24. Cash, D, Khanduja V. The case for ceramics-on-polyethylene as the preferred bearing for a young adult hip replacement. Hip Int. 2014;24(5):421-427.
25. Taylor ED, Browne JA. Reconstruction options for acetabular revision. World J Orthop. 2012;3(7):95-100.
26. Lombardi AV, Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg. 2008;16(5):243-248.
27. Millet PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A(2):236-249.
28. O'Hara LJ, Nivbrant B, Rohrl S.Cross-linked polyethylene and bisphosphonate therapy for osteolysis in total hip athroplasty: a case report. J Orthop Surg (Hong Kong). 2004;12(1):114-121.
Testosterone Replacement Therapy: Playing Catch-up With Patients
The objective of this article is to help primary care providers (PCPs) council patients regarding testosterone replacement therapy (TRT). This case will present a patient who initiated TRT at a community-based alternative medicine clinic. The case will be followed by a discussion regarding the standard diagnosis of hypogonadism, the potential benefits and risks of TRT, and a review of the current clinical guideline recommendations. Examples of information being disseminated to the general public by the complementary and alternative medicine (CAM) providers will be briefly reviewed for an increased awareness of the questions patients may pose regarding TRT.
Background
From 2000 to 2011, total testosterone sales increased 12-fold globally.1 Possible causes for the increase involved the aging population, newer options for TRT administration, and increased direct-to-consumer advertising. A low testosterone level (sometimes referred to as low T in consumer marketing materials) is associated with a variety of medical conditions (ie, low mood, increased body fat, declining athletic performance, and decreased sexual performance) that have become increasingly prevalent among middle aged and older men.2 It has also received attention as an intervention to reverse frailty and sarcopenia.3
Testosterone replacement therapy options include injectable solutions, transdermal gels and patches, pellet implants, or buccal tablets. The ease of administration of transdermal testosterone comes at a relatively high cost. Injectable testosterone preparations are generally the least expensive option, and many patients choose injections for this reason.
Related: Keeping an Open Mind on HRT
Testosterone prescriptions were most frequently written by PCPs with 36% coming from family practitioners and 20.1% from internal medicine practices, according to a Kaiser Permanente study.4 Endocrinologists (13.5%) and urologists (6.6%) were less likely to have written the prescriptions for patients.
Due, in part, to direct-to-consumer advertising and to the availability of online medical information, many men now present to their PCP questioning whether they might have low T. Others may have already started therapy at a CAM, integrative medicine, or anti-aging clinic.
Confusing the issue further, some CAM providers promote a variety of off-label medications and nutritional supplements for the treatment of low T, which seems to have struck a chord in the baby boomer generation. No other age group in history has tried to work so intensely on its physical condition and appearance.5 Much of the information marketed to consumers emphasizes that many traditionally trained physicians are not educated in the treatment of low T.
Case Report
Mr. C. is a 65-year-old man who was seen in the primary care clinic for the first time. He was accompanied by his much younger fiancée. She reported that Mr. C.’s energy and sexual interest were declining, and the patient reported his “get up and go had gotten up and left.” They sought medical advice from a CAM provider who ordered blood work and then explained that the symptoms were due to low testosterone. For the past 6 months he had been visiting the clinic weekly for testosterone injections.
Mr. C. reported feeling as good as a “40 year old.” He also reported that he started working with a personal trainer and had given up most junk food and alcohol. He had no symptoms of chest pain, erectile dysfunction, or significant urinary urgency, frequency, or nocturia.
Related: Will Testosterone Therapy Kill Your Patient?
The visits to a CAM provider had been an out-of-pocket expense, and he was hoping to transfer his treatment to the VA so the costs could be covered. Mr. C. failed to bring medical records from the other provider but remembered being told that all his tests were “fine” except for the low testosterone level.
His past history was notable for controlled type 2 diabetes mellitus for 8 years, hypertension, hyperlipidemia, and spinal stenosis. He had no history of benign prostatic hyperplasia or prostate cancer.
In addition to the testosterone (100 mg intramuscular injection weekly), his medication regimen included metoprolol 25 mg twice daily, atorvastatin 20 mg daily, acetaminophen 650 mg 3 times daily as needed, aspirin 81 mg daily, metformin 500 mg twice daily, vitamin D 2,000 IU daily, vitamin B12 1,000 mg daily, and Co-Q10 200 mg daily.
On physical examination, Mr. C.’s vitals were stable and his body mass index was in the overweight range at 29.8 kg/m2. His cardiopulmonary examination was normal. There was increased central obesity without palpable organomegaly. There was no gynecomastia, and he had normal amounts of axillary and pubic hair. There was no peripheral edema; his genitourinary examination included normal-sized testicles, and the prostate was smooth without nodules.
The PCP informed Mr. C. that he was familiar with the evaluation and management of testosterone therapy. He was advised that additional evaluation would be needed before determining whether the clinical benefit of TRT outweighed the potential risks.
Andropause
Testosterone levels in men are known to decline at a rate of 1% per year after aged 30 years.6 About 20% of men aged ≥ 60 years and 50% of men aged ≥ 80 years have low (hypogonadal) total testosterone levels.7 The clinical diagnosis of hypogonadism, however, is made on the basis of signs and symptoms consistent with androgen deficiency and a low serum morning testosterone level measured on serum on multiple occasions.8
Specific clinical signs and symptoms (“A” list) consistent with androgen deficiency include low libido and sexual activity; diminished spontaneous erections; gynecomastia; reduced facial, axillary, or pubic hair; small (≤ 5 mL) testes; inability to father children; loss of height, fractures, or other signs of bone loss; and hot flashes and night sweats.9
Less specific signs and symptoms (“B” list) of androgen deficiency include a decrease in energy or motivation, feelings of sadness or depression, poor concentration or memory, trouble sleeping, increased sleepiness, mild anemia, reduced muscle bulk or strength, increased body fat, and diminished physical performance.9
Making the clinical diagnosis of hypogonadism is challenging, because the clinical symptoms have a high prevalence in the older male population and overlap with many nonendocrine diseases. Testosterone replacement therapy has been associated weakly, but consistently, with improved sexual function,10-12 bone mineral density,13,14 fat free mass,13,14 strength,15,16 lipid profiles,17,18 insulin resistance,17,18 and with an increased time to ST segment depression during stress testing.19,20
Laboratory Evaluation
Serum total testosterone circulates in 3 forms: free testosterone, sex hormone-binding globulin (SHBG)-bound testosterone, and albumin-bound testosterone. Free testosterone is the most bio-available testosterone but represents only 2% to 3% of total testosterone.21 Whether total testosterone or free testosterone measurements most closely correlate with symptomatic androgen deficiency is a matter of debate.21 A total testosterone level is an appropriate screening test in young, healthy, and lean men for whom SHBG levels are presumably normal. However, a free or bioavailable testosterone level should be considered for men when there is a high likelihood of conditions that can affect SHBG levels.
Conditions that can decrease SHBG (and may result in a low total testosterone reading even when the free fraction may be normal) include obesity, metabolic syndrome, type 2 diabetes mellitus, hypothyroidism, nephrotic syndrome, chronic glucocorticoid use, and the use of progestins and anabolic steroids.21 Conditions that can increase SHBG (and may result in a normal total testosterone level in patients with hypogonadism, as they have low levels of free testosterone) include aging, cirrhosis, anticonvulsant use, hyperthyroidism, catabolic conditions, and HIV.21
Related: Effect of Statins on Total Testosterone Levels in Male Veterans
Serum testosterone levels generally peak in the early morning, followed by a progressive decline over the course of the day until they reach a nadir in the evening.21 Although it has been debated that morning testosterone levels are not necessary in older men due to a blunting of the circadian rhythm, many men aged 65 to 80 years who have low T in the afternoon will have normal testosterone levels when retested in the morning.22,23 Readings below a reference range of 280 ng/dL to 300 ng/dL on at least 2 different occasions support a diagnosis of hypogonadism.9
Follicle stimulating hormone (FSH) and luteinizing hormone (LH) laboratory tests may be ordered following confirmation of a low testosterone level. Prolactin levels and iron saturation can help evaluate for the presence of hyperprolactinemia and hemochromatosis, respectively. Primary hypogonadism due to testicular failure is diagnosed with high FSH, high LH, and low testosterone levels. Secondary hypogonadism due to hypothalamic or pituitary failure is diagnosed with low FSH, low LH, and low testosterone levels.
Hypothalamic or pituitary suppression from a nonendocrine condition may result in functional hypogonadotropic hypogonadism (FHH), which can be identified with low (or normal) FSH; low (or normal) LH; and low testosterone levels. Hypogonadotropic hypogonadism has been associated with depression, obesity, stress, and physical exertion; and FHH may also be associated with the use of multiple drugs and drug classes (spironolactone, anabolic and corticosteroids, ketoconazole, ethanol, anticonvulsants, immunosuppressants, tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotics, and opioids).24,25 Even statin therapy has been associated with FHH.26,27 Testosterone levels will often recover if or when modifiable factors for FHH are corrected.28
Although there is no consensus on an absolute number that defines a low testosterone level, concern exists that there are economic incentives to raise the bar for normal and thereby increase the potential market for testosterone-raising products.29 Many commercial avenues for the treatment of low T do not follow the standards of the established medical community. Some websites suggest screening for low T with total and free testosterone levels for all men aged > 40 years. Others advise men to consider TRT if they have a total testosterone level of < 500 ng/dL or a free testosterone level that is not in the upper one-third range for men aged 21 to 49 years.30 Of even greater concern, Baillargeon and colleagues reported that 25% of all new androgen users had not had their testosterone levels measured in the 12 months before starting treatment.31 In another study, 40% of men who initiated TRT did not have a baseline measurement.32
Treatments
Before considering TRT, physicians need to emphasize lifestyle modifications as first-line treatment for hypogonadism. The most important modifications include weight loss, tobacco cessation, and moderation in alcohol use.
Patients need to be advised of possible adverse events (AEs) of TRT, which may include gynecomastia, polycythemia, sleep apnea, decreased high-density lipoprotein cholesterol, benign prostatic hypertrophy, infertility, testicular atrophy, and abnormal liver function tests. More recently, several studies have shown an association between TRT and an increase in cardiovascular complications, such as stroke, heart attacks, and death.
Prior to considering TRT, a careful history and physical examination, including a clinical prostate examination, should be performed. Minimum additional tests should include hematocrit, fasting lipid profile (FLP), complete metabolic profile (CMP), and prostate-specific antigen (PSA). Initiation of TRT is not recommended for patients with metastatic prostate cancer; breast cancer; an unevaluated prostate nodule; a PSA > 4 ng/mL (or > 3 ng/mL in African Americans or men with a first-degree relative with prostate cancer); hematocrit > 50%; untreated severe obstructive sleep apnea; uncontrolled or poorly controlled congestive heart failure; or an International Prostate Symptoms Score (IPSS) > 19.9
A past history of prostate cancer had previously been a contraindication for the use of TRT. However, more recent studies have shown that TRT can be used in those who have no evidence of active or metastatic disease and who are under the close supervision of a physician.33-35
Widespread screening is not recommended, and population-based surveys can be unreliable. Fifteen percent of healthy young men, for example, will have a low serum testosterone level in a given 24-hour period.9 Thirty percent of men with an initial testosterone level in the mildly hypogonadal range will have a normal testosterone level when retested; moreover the threshold below which AEs occur remains unknown.9
The goal of TRT is to achieve a total testosterone level in the 400 ng/mL to 700 ng/mL range with improved clinical signs and symptoms.9 Laboratory tests should be conducted at 3 months, 6 months, and then annually. These tests include hematocrit, PSA, and a testosterone level.32 Testing for CMP and FLP should also be considered. If, during therapy, the hematocrit is > 54%, the patient should be assessed for hypoxia and sleep apnea, and treatment should resume at a lower dose only when the hematocrit returns to baseline.9 A digital examination of the prostate is recommended for men with a PSA of > 0.6 ng/mL. A urologic consultation should be obtained for an increase in the PSA of > 1.4 ng/mL over 12 months, a PSA velocity of > 0.4 ng/mL per year (using the PSA after 6 months as a reference), or for an IPPS of > 19.9
Emerging Cardiovascular Concerns
The Testosterone for Older Men study, a randomized, placebo- controlled clinical trial of testosterone therapy in men with a high prevalence of cardiovascular disease, showed significantly greater improvements in leg-press, chest-press, and stair-climbing exercises while carrying a load compared with that in the placebo group.36 However, the study was stopped early due to an increased risk of cardiovascular AEs in those who received testosterone gel.
Vigen and colleagues examined a cohort of veterans who underwent coronary angiography and had a low serum testosterone level.37 The use of TRT in this cohort was also associated with an increased risk of adverse cardiovascular outcomes. This study generated several letters and a recent article in response that vigorously questioned the validity of the methods used and the conclusions reached.38-44 Prior clinical studies of TRT had not detected cardiac AEs, but these trials were generally of short duration and not powered for clinical endpoints.37
A FDA Safety Announcement as well as a VA National Pharmacy Benefits Management bulletin were based on the results of these studies.45 The FDA did not conclude that TRT increased the risk of stroke, heart attack, or death, but health care providers were asked to consider whether the benefits of TRT are likely to exceed the potential risk of treatment.
Direct-to-Consumer Marketing
Some direct-to-consumer marketing promotes the use of aromatase inhibitors, such as anastrozole. This class of medications prevents the conversion of endogenous and exogenous testosterone to estrogen by the aromatase enzyme, which is found predominately in abdominal adipose tissue. There is no evidence that naturally occurring elevations in estrogen cause low testosterone or that treatment of elevated estrogen with an aromatase inhibitor during TRT has any significant clinical benefit in terms of male sexuality.46 Nevertheless, some CAM providers now hypothesize that the increase in cardiovascular AEs with TRT noted in the recent studies may have been due to the increase in estrogen that is associated with TRT.46
The off-label use of clomiphene citrate to block the negative feedback of estrogen on the production of LH has been promoted as another potential treatment to increase testosterone levels. Luteinizing hormone is the pituitary analog of human chorionic gonadotropin (HCG). Many CAM providers also prescribe HCG to increase the testicles’ testosterone production.
Some consumer-focused media insist that the use of either clomiphene citrate or HCG will increase testosterone production and does not cause testicular atrophy, a known TRT- associated AE. This seems to increase the motivation of many men to try these off-label medications.
Some sources even posit a “conspiracy theory” that the FDA and pharmaceutical companies conspire to keep the price of transdermal TRT options high. Men are told that testosterone creams made at compounding pharmacies are much less expensive than are the transdermal pharmaceuticals, and they are urged to see a CAM provider to obtain a prescription for the compounded testosterone. In some cases, a sample prescription is included.47
Many supplements are available that claim to boost testosterone or suppress estrogen. Chrysin, for example, is a bioflavonoid that is marketed as having the potential to act as a natural aromatase inhibitor. Although studies have suggested the potential for chrysin to work in such a manner, the effectiveness may be attenuated by its low bioavailability in supplements.48 Long-term studies have not been conducted.49 Nettle root is a plant-derived compound that is stated to increase free testosterone levels by binding to SHBG, in place of testosterone, and by inhibiting the enzyme that converts testosterone to dihydrotestosterone. The clinical evidence of effectiveness is based on many open studies, and the significance and magnitude of the effect still needs more rigorous evaluation.50
Conclusions
Patients today are barraged with medical information through television, print advertising, radio, and the Internet. A recent study of online sources of herbal product information found that only 10.5% recommended a consultation with a health care professional and < 3% cited scientific literature to accompany their claims.51 Many patients present to their PCP with questions about TRT or have already started an intervention for low T. Complementary and alternative medicine providers of TRT have been able to capture a segment of the population that often has the motivation and disposable income to pursue nontraditional therapies.
All nutritional supplements contain a standard warning from the FDA: “The above statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” Providers should remind patients of the statement and point out the contradictions between the statement and the benefits touted by the supplement marketing literature.
Finally, despite the well- established role of testosterone in enhancing libido, its definitive role in erectile function had been controversial until evidence substantiated a key function for this hormone.52 Testosterone may facilitate erection by acting as a vasodilator of the penile arterioles and cavernous sinusoids and may ameliorate the response to the phosphodiesterase-5 inhibitors in hypogonadal men.53 Testosterone replacement alone in hypogonadal men can restore erectile dysfunction.51 However, hypogonadism is not a common finding in those with erectile dysfunction, only occurring in about 5% of cases.53
Allopathic providers are concerned about the vitality and sexual health of their aging male patients, but their enthusiasm for anti-aging treatments is often tempered by evidence-based studies that have shown a lack of efficacy or potentially serious health care risks. Unfortunately, many patients remain unaware of the controversies regarding TRT. For those patients who receive treatment through CAM providers and are convinced of the efficacy of their low-T treatment regimen, it is important to keep lines of communication open.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Handelsman D. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
2. Hackett G. Testosterone and the heart. Int J Clin Pract. 2012;66(7):648-655.
3. Morley JE. Hypogonadism, testosterone, and nursing home residents. J Am Med Dir Assoc. 2013;14(6):381-383.
4. An J, Cheetham TC, Van Den Eeden S. PS3-36: testosterone replacement therapy patterns for aging males in a managed care setting. Clin Med Res. 2013;11(3):141.
5. Moschis G, Lee E, Marthur A, Strautman J. The Maturing Marketplace: Buying Habits of Baby Boomers and Their Parents. Westport, CT: Quorum Books; 2000.
6. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413.
7. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731.
8. Basaria S. Male hypogonadism. Lancet. 2014;383 (9924):1250-1263.
9. Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
10. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839-2853.
11. Bolona ER, Uranga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
12. Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005;63(4):381-394.
13. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280-293.
14. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670-2677.
15. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661-1667.
16. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090-1099.
17. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome. Diabetes Care. 2011;34(4):828-837.
18. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318-327.
19. Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443-449.
20. Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871-876.
21. Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. White Paper: The Laboratory Diagnosis of Testosterone Deficiency. http://www.auanet.org/common/pdf/education/clinical -guidance/Testosterone-Deficiency-WhitePaper.pdf. Published 2013. Accessed April 9, 2015.
22. Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509-513.
23. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67(6):853-862.
24. Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: symptoms and treatment. J Adv Pharm Technol Res. 2010;1(3):297-301.
25. Montgomery K. Sexual desire disorders. Psychiatry (Edgmont). 2008;5(6):50-55.
26. Corona G, Boddi V, Balercia G, et al. The effect of statin therapy on testosterone levels in subjects consulting for erectile dysfunction. J Sex Med. 2010; 7(4 pt 1):1547-1556.
27. Schooling CM, Yeung SLA, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
28. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
29. Schwartz LM, Woloshin S. Low “T” as in “template”: how to sell disease. JAMA Intern Med. 2013;173(15):1460-1462.
30. Male Hormone Modulation Therapy, Part 2. Life Extension Vitamins Website. http://www.lifeextension vitamins.com/mahomothpa2.html. Accessed April 9, 2015.
31. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465-1466.
32. Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99(3):835-842.
33. Ramasamy R, Fisher ES, Schlegel PN. Testosterone replacement and prostate cancer. Indian J Urol. 2012;28(2):123-128.
34. Marks, LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2351-2361.
35. Coward RM, Simhan J, Carson CC 3rd. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103(9):1179-1183.
36. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
37. Vigen R, O’Donnell Cl, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
38. Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):961-962.
39. Jones TH, Channer K. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):962-963.
40. Katz J, Nadelberg R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963.
41. Riche D, Baker WL, Koch CA. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963-964.
42. Dhindsa S, Batra M, Dandona P. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):964.
43. Ho PM, Barón AE, Wierman M. Deaths and cardiovascular events in men receiving testosterone—reply. JAMA. 2014;311(9):964-965.
44. Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11(3):624-629.
45. U.S. Department of Veterans Affairs, Veterans Health Administration (VHA), Pharmacy Benefit Management Services (PBM), Medical Advisory Panel (MAP), and Center for Medication Safety (VA Medsafe. National PBM Bulletin. Testosterone products and cardiovascular safety. http://www.pbm.va.gov/PBM/vacenterformedicationsafety/nationalpbmbulletin/Testosterone_Products_and_Cardiovascular_Safety_NATIONAL_PBM_BULLETIN_02.pdf. Published February 7, 2014. Accessed April 9, 2015.
46. Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681-1696.
47. Faloon W. Vindication. Life Extension Magazine Website. http://www.lef.org/magazine /mag2008/dec2008_Harvard-Experts-Recommend -Testosterone-Replacement_02.htm. Published December 2008. Accessed April 9,2015.
48. Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51(2):143-146.
49. Jana K, Yin X, Schiffer, et al. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197(2):315-323.
50. Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7-8):568-579.
51. Owens C, Baergen R, Puckett D. Online sources of herbal product information. Am J Med. 2014;127(2):109-115.
52. Blute W, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108-122.
53. Mikhail N. Does testosterone have a role in erectile dysfunction? Am J Med. 2006;119(5):373-382.
The objective of this article is to help primary care providers (PCPs) council patients regarding testosterone replacement therapy (TRT). This case will present a patient who initiated TRT at a community-based alternative medicine clinic. The case will be followed by a discussion regarding the standard diagnosis of hypogonadism, the potential benefits and risks of TRT, and a review of the current clinical guideline recommendations. Examples of information being disseminated to the general public by the complementary and alternative medicine (CAM) providers will be briefly reviewed for an increased awareness of the questions patients may pose regarding TRT.
Background
From 2000 to 2011, total testosterone sales increased 12-fold globally.1 Possible causes for the increase involved the aging population, newer options for TRT administration, and increased direct-to-consumer advertising. A low testosterone level (sometimes referred to as low T in consumer marketing materials) is associated with a variety of medical conditions (ie, low mood, increased body fat, declining athletic performance, and decreased sexual performance) that have become increasingly prevalent among middle aged and older men.2 It has also received attention as an intervention to reverse frailty and sarcopenia.3
Testosterone replacement therapy options include injectable solutions, transdermal gels and patches, pellet implants, or buccal tablets. The ease of administration of transdermal testosterone comes at a relatively high cost. Injectable testosterone preparations are generally the least expensive option, and many patients choose injections for this reason.
Related: Keeping an Open Mind on HRT
Testosterone prescriptions were most frequently written by PCPs with 36% coming from family practitioners and 20.1% from internal medicine practices, according to a Kaiser Permanente study.4 Endocrinologists (13.5%) and urologists (6.6%) were less likely to have written the prescriptions for patients.
Due, in part, to direct-to-consumer advertising and to the availability of online medical information, many men now present to their PCP questioning whether they might have low T. Others may have already started therapy at a CAM, integrative medicine, or anti-aging clinic.
Confusing the issue further, some CAM providers promote a variety of off-label medications and nutritional supplements for the treatment of low T, which seems to have struck a chord in the baby boomer generation. No other age group in history has tried to work so intensely on its physical condition and appearance.5 Much of the information marketed to consumers emphasizes that many traditionally trained physicians are not educated in the treatment of low T.
Case Report
Mr. C. is a 65-year-old man who was seen in the primary care clinic for the first time. He was accompanied by his much younger fiancée. She reported that Mr. C.’s energy and sexual interest were declining, and the patient reported his “get up and go had gotten up and left.” They sought medical advice from a CAM provider who ordered blood work and then explained that the symptoms were due to low testosterone. For the past 6 months he had been visiting the clinic weekly for testosterone injections.
Mr. C. reported feeling as good as a “40 year old.” He also reported that he started working with a personal trainer and had given up most junk food and alcohol. He had no symptoms of chest pain, erectile dysfunction, or significant urinary urgency, frequency, or nocturia.
Related: Will Testosterone Therapy Kill Your Patient?
The visits to a CAM provider had been an out-of-pocket expense, and he was hoping to transfer his treatment to the VA so the costs could be covered. Mr. C. failed to bring medical records from the other provider but remembered being told that all his tests were “fine” except for the low testosterone level.
His past history was notable for controlled type 2 diabetes mellitus for 8 years, hypertension, hyperlipidemia, and spinal stenosis. He had no history of benign prostatic hyperplasia or prostate cancer.
In addition to the testosterone (100 mg intramuscular injection weekly), his medication regimen included metoprolol 25 mg twice daily, atorvastatin 20 mg daily, acetaminophen 650 mg 3 times daily as needed, aspirin 81 mg daily, metformin 500 mg twice daily, vitamin D 2,000 IU daily, vitamin B12 1,000 mg daily, and Co-Q10 200 mg daily.
On physical examination, Mr. C.’s vitals were stable and his body mass index was in the overweight range at 29.8 kg/m2. His cardiopulmonary examination was normal. There was increased central obesity without palpable organomegaly. There was no gynecomastia, and he had normal amounts of axillary and pubic hair. There was no peripheral edema; his genitourinary examination included normal-sized testicles, and the prostate was smooth without nodules.
The PCP informed Mr. C. that he was familiar with the evaluation and management of testosterone therapy. He was advised that additional evaluation would be needed before determining whether the clinical benefit of TRT outweighed the potential risks.
Andropause
Testosterone levels in men are known to decline at a rate of 1% per year after aged 30 years.6 About 20% of men aged ≥ 60 years and 50% of men aged ≥ 80 years have low (hypogonadal) total testosterone levels.7 The clinical diagnosis of hypogonadism, however, is made on the basis of signs and symptoms consistent with androgen deficiency and a low serum morning testosterone level measured on serum on multiple occasions.8
Specific clinical signs and symptoms (“A” list) consistent with androgen deficiency include low libido and sexual activity; diminished spontaneous erections; gynecomastia; reduced facial, axillary, or pubic hair; small (≤ 5 mL) testes; inability to father children; loss of height, fractures, or other signs of bone loss; and hot flashes and night sweats.9
Less specific signs and symptoms (“B” list) of androgen deficiency include a decrease in energy or motivation, feelings of sadness or depression, poor concentration or memory, trouble sleeping, increased sleepiness, mild anemia, reduced muscle bulk or strength, increased body fat, and diminished physical performance.9
Making the clinical diagnosis of hypogonadism is challenging, because the clinical symptoms have a high prevalence in the older male population and overlap with many nonendocrine diseases. Testosterone replacement therapy has been associated weakly, but consistently, with improved sexual function,10-12 bone mineral density,13,14 fat free mass,13,14 strength,15,16 lipid profiles,17,18 insulin resistance,17,18 and with an increased time to ST segment depression during stress testing.19,20
Laboratory Evaluation
Serum total testosterone circulates in 3 forms: free testosterone, sex hormone-binding globulin (SHBG)-bound testosterone, and albumin-bound testosterone. Free testosterone is the most bio-available testosterone but represents only 2% to 3% of total testosterone.21 Whether total testosterone or free testosterone measurements most closely correlate with symptomatic androgen deficiency is a matter of debate.21 A total testosterone level is an appropriate screening test in young, healthy, and lean men for whom SHBG levels are presumably normal. However, a free or bioavailable testosterone level should be considered for men when there is a high likelihood of conditions that can affect SHBG levels.
Conditions that can decrease SHBG (and may result in a low total testosterone reading even when the free fraction may be normal) include obesity, metabolic syndrome, type 2 diabetes mellitus, hypothyroidism, nephrotic syndrome, chronic glucocorticoid use, and the use of progestins and anabolic steroids.21 Conditions that can increase SHBG (and may result in a normal total testosterone level in patients with hypogonadism, as they have low levels of free testosterone) include aging, cirrhosis, anticonvulsant use, hyperthyroidism, catabolic conditions, and HIV.21
Related: Effect of Statins on Total Testosterone Levels in Male Veterans
Serum testosterone levels generally peak in the early morning, followed by a progressive decline over the course of the day until they reach a nadir in the evening.21 Although it has been debated that morning testosterone levels are not necessary in older men due to a blunting of the circadian rhythm, many men aged 65 to 80 years who have low T in the afternoon will have normal testosterone levels when retested in the morning.22,23 Readings below a reference range of 280 ng/dL to 300 ng/dL on at least 2 different occasions support a diagnosis of hypogonadism.9
Follicle stimulating hormone (FSH) and luteinizing hormone (LH) laboratory tests may be ordered following confirmation of a low testosterone level. Prolactin levels and iron saturation can help evaluate for the presence of hyperprolactinemia and hemochromatosis, respectively. Primary hypogonadism due to testicular failure is diagnosed with high FSH, high LH, and low testosterone levels. Secondary hypogonadism due to hypothalamic or pituitary failure is diagnosed with low FSH, low LH, and low testosterone levels.
Hypothalamic or pituitary suppression from a nonendocrine condition may result in functional hypogonadotropic hypogonadism (FHH), which can be identified with low (or normal) FSH; low (or normal) LH; and low testosterone levels. Hypogonadotropic hypogonadism has been associated with depression, obesity, stress, and physical exertion; and FHH may also be associated with the use of multiple drugs and drug classes (spironolactone, anabolic and corticosteroids, ketoconazole, ethanol, anticonvulsants, immunosuppressants, tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotics, and opioids).24,25 Even statin therapy has been associated with FHH.26,27 Testosterone levels will often recover if or when modifiable factors for FHH are corrected.28
Although there is no consensus on an absolute number that defines a low testosterone level, concern exists that there are economic incentives to raise the bar for normal and thereby increase the potential market for testosterone-raising products.29 Many commercial avenues for the treatment of low T do not follow the standards of the established medical community. Some websites suggest screening for low T with total and free testosterone levels for all men aged > 40 years. Others advise men to consider TRT if they have a total testosterone level of < 500 ng/dL or a free testosterone level that is not in the upper one-third range for men aged 21 to 49 years.30 Of even greater concern, Baillargeon and colleagues reported that 25% of all new androgen users had not had their testosterone levels measured in the 12 months before starting treatment.31 In another study, 40% of men who initiated TRT did not have a baseline measurement.32
Treatments
Before considering TRT, physicians need to emphasize lifestyle modifications as first-line treatment for hypogonadism. The most important modifications include weight loss, tobacco cessation, and moderation in alcohol use.
Patients need to be advised of possible adverse events (AEs) of TRT, which may include gynecomastia, polycythemia, sleep apnea, decreased high-density lipoprotein cholesterol, benign prostatic hypertrophy, infertility, testicular atrophy, and abnormal liver function tests. More recently, several studies have shown an association between TRT and an increase in cardiovascular complications, such as stroke, heart attacks, and death.
Prior to considering TRT, a careful history and physical examination, including a clinical prostate examination, should be performed. Minimum additional tests should include hematocrit, fasting lipid profile (FLP), complete metabolic profile (CMP), and prostate-specific antigen (PSA). Initiation of TRT is not recommended for patients with metastatic prostate cancer; breast cancer; an unevaluated prostate nodule; a PSA > 4 ng/mL (or > 3 ng/mL in African Americans or men with a first-degree relative with prostate cancer); hematocrit > 50%; untreated severe obstructive sleep apnea; uncontrolled or poorly controlled congestive heart failure; or an International Prostate Symptoms Score (IPSS) > 19.9
A past history of prostate cancer had previously been a contraindication for the use of TRT. However, more recent studies have shown that TRT can be used in those who have no evidence of active or metastatic disease and who are under the close supervision of a physician.33-35
Widespread screening is not recommended, and population-based surveys can be unreliable. Fifteen percent of healthy young men, for example, will have a low serum testosterone level in a given 24-hour period.9 Thirty percent of men with an initial testosterone level in the mildly hypogonadal range will have a normal testosterone level when retested; moreover the threshold below which AEs occur remains unknown.9
The goal of TRT is to achieve a total testosterone level in the 400 ng/mL to 700 ng/mL range with improved clinical signs and symptoms.9 Laboratory tests should be conducted at 3 months, 6 months, and then annually. These tests include hematocrit, PSA, and a testosterone level.32 Testing for CMP and FLP should also be considered. If, during therapy, the hematocrit is > 54%, the patient should be assessed for hypoxia and sleep apnea, and treatment should resume at a lower dose only when the hematocrit returns to baseline.9 A digital examination of the prostate is recommended for men with a PSA of > 0.6 ng/mL. A urologic consultation should be obtained for an increase in the PSA of > 1.4 ng/mL over 12 months, a PSA velocity of > 0.4 ng/mL per year (using the PSA after 6 months as a reference), or for an IPPS of > 19.9
Emerging Cardiovascular Concerns
The Testosterone for Older Men study, a randomized, placebo- controlled clinical trial of testosterone therapy in men with a high prevalence of cardiovascular disease, showed significantly greater improvements in leg-press, chest-press, and stair-climbing exercises while carrying a load compared with that in the placebo group.36 However, the study was stopped early due to an increased risk of cardiovascular AEs in those who received testosterone gel.
Vigen and colleagues examined a cohort of veterans who underwent coronary angiography and had a low serum testosterone level.37 The use of TRT in this cohort was also associated with an increased risk of adverse cardiovascular outcomes. This study generated several letters and a recent article in response that vigorously questioned the validity of the methods used and the conclusions reached.38-44 Prior clinical studies of TRT had not detected cardiac AEs, but these trials were generally of short duration and not powered for clinical endpoints.37
A FDA Safety Announcement as well as a VA National Pharmacy Benefits Management bulletin were based on the results of these studies.45 The FDA did not conclude that TRT increased the risk of stroke, heart attack, or death, but health care providers were asked to consider whether the benefits of TRT are likely to exceed the potential risk of treatment.
Direct-to-Consumer Marketing
Some direct-to-consumer marketing promotes the use of aromatase inhibitors, such as anastrozole. This class of medications prevents the conversion of endogenous and exogenous testosterone to estrogen by the aromatase enzyme, which is found predominately in abdominal adipose tissue. There is no evidence that naturally occurring elevations in estrogen cause low testosterone or that treatment of elevated estrogen with an aromatase inhibitor during TRT has any significant clinical benefit in terms of male sexuality.46 Nevertheless, some CAM providers now hypothesize that the increase in cardiovascular AEs with TRT noted in the recent studies may have been due to the increase in estrogen that is associated with TRT.46
The off-label use of clomiphene citrate to block the negative feedback of estrogen on the production of LH has been promoted as another potential treatment to increase testosterone levels. Luteinizing hormone is the pituitary analog of human chorionic gonadotropin (HCG). Many CAM providers also prescribe HCG to increase the testicles’ testosterone production.
Some consumer-focused media insist that the use of either clomiphene citrate or HCG will increase testosterone production and does not cause testicular atrophy, a known TRT- associated AE. This seems to increase the motivation of many men to try these off-label medications.
Some sources even posit a “conspiracy theory” that the FDA and pharmaceutical companies conspire to keep the price of transdermal TRT options high. Men are told that testosterone creams made at compounding pharmacies are much less expensive than are the transdermal pharmaceuticals, and they are urged to see a CAM provider to obtain a prescription for the compounded testosterone. In some cases, a sample prescription is included.47
Many supplements are available that claim to boost testosterone or suppress estrogen. Chrysin, for example, is a bioflavonoid that is marketed as having the potential to act as a natural aromatase inhibitor. Although studies have suggested the potential for chrysin to work in such a manner, the effectiveness may be attenuated by its low bioavailability in supplements.48 Long-term studies have not been conducted.49 Nettle root is a plant-derived compound that is stated to increase free testosterone levels by binding to SHBG, in place of testosterone, and by inhibiting the enzyme that converts testosterone to dihydrotestosterone. The clinical evidence of effectiveness is based on many open studies, and the significance and magnitude of the effect still needs more rigorous evaluation.50
Conclusions
Patients today are barraged with medical information through television, print advertising, radio, and the Internet. A recent study of online sources of herbal product information found that only 10.5% recommended a consultation with a health care professional and < 3% cited scientific literature to accompany their claims.51 Many patients present to their PCP with questions about TRT or have already started an intervention for low T. Complementary and alternative medicine providers of TRT have been able to capture a segment of the population that often has the motivation and disposable income to pursue nontraditional therapies.
All nutritional supplements contain a standard warning from the FDA: “The above statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” Providers should remind patients of the statement and point out the contradictions between the statement and the benefits touted by the supplement marketing literature.
Finally, despite the well- established role of testosterone in enhancing libido, its definitive role in erectile function had been controversial until evidence substantiated a key function for this hormone.52 Testosterone may facilitate erection by acting as a vasodilator of the penile arterioles and cavernous sinusoids and may ameliorate the response to the phosphodiesterase-5 inhibitors in hypogonadal men.53 Testosterone replacement alone in hypogonadal men can restore erectile dysfunction.51 However, hypogonadism is not a common finding in those with erectile dysfunction, only occurring in about 5% of cases.53
Allopathic providers are concerned about the vitality and sexual health of their aging male patients, but their enthusiasm for anti-aging treatments is often tempered by evidence-based studies that have shown a lack of efficacy or potentially serious health care risks. Unfortunately, many patients remain unaware of the controversies regarding TRT. For those patients who receive treatment through CAM providers and are convinced of the efficacy of their low-T treatment regimen, it is important to keep lines of communication open.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The objective of this article is to help primary care providers (PCPs) council patients regarding testosterone replacement therapy (TRT). This case will present a patient who initiated TRT at a community-based alternative medicine clinic. The case will be followed by a discussion regarding the standard diagnosis of hypogonadism, the potential benefits and risks of TRT, and a review of the current clinical guideline recommendations. Examples of information being disseminated to the general public by the complementary and alternative medicine (CAM) providers will be briefly reviewed for an increased awareness of the questions patients may pose regarding TRT.
Background
From 2000 to 2011, total testosterone sales increased 12-fold globally.1 Possible causes for the increase involved the aging population, newer options for TRT administration, and increased direct-to-consumer advertising. A low testosterone level (sometimes referred to as low T in consumer marketing materials) is associated with a variety of medical conditions (ie, low mood, increased body fat, declining athletic performance, and decreased sexual performance) that have become increasingly prevalent among middle aged and older men.2 It has also received attention as an intervention to reverse frailty and sarcopenia.3
Testosterone replacement therapy options include injectable solutions, transdermal gels and patches, pellet implants, or buccal tablets. The ease of administration of transdermal testosterone comes at a relatively high cost. Injectable testosterone preparations are generally the least expensive option, and many patients choose injections for this reason.
Related: Keeping an Open Mind on HRT
Testosterone prescriptions were most frequently written by PCPs with 36% coming from family practitioners and 20.1% from internal medicine practices, according to a Kaiser Permanente study.4 Endocrinologists (13.5%) and urologists (6.6%) were less likely to have written the prescriptions for patients.
Due, in part, to direct-to-consumer advertising and to the availability of online medical information, many men now present to their PCP questioning whether they might have low T. Others may have already started therapy at a CAM, integrative medicine, or anti-aging clinic.
Confusing the issue further, some CAM providers promote a variety of off-label medications and nutritional supplements for the treatment of low T, which seems to have struck a chord in the baby boomer generation. No other age group in history has tried to work so intensely on its physical condition and appearance.5 Much of the information marketed to consumers emphasizes that many traditionally trained physicians are not educated in the treatment of low T.
Case Report
Mr. C. is a 65-year-old man who was seen in the primary care clinic for the first time. He was accompanied by his much younger fiancée. She reported that Mr. C.’s energy and sexual interest were declining, and the patient reported his “get up and go had gotten up and left.” They sought medical advice from a CAM provider who ordered blood work and then explained that the symptoms were due to low testosterone. For the past 6 months he had been visiting the clinic weekly for testosterone injections.
Mr. C. reported feeling as good as a “40 year old.” He also reported that he started working with a personal trainer and had given up most junk food and alcohol. He had no symptoms of chest pain, erectile dysfunction, or significant urinary urgency, frequency, or nocturia.
Related: Will Testosterone Therapy Kill Your Patient?
The visits to a CAM provider had been an out-of-pocket expense, and he was hoping to transfer his treatment to the VA so the costs could be covered. Mr. C. failed to bring medical records from the other provider but remembered being told that all his tests were “fine” except for the low testosterone level.
His past history was notable for controlled type 2 diabetes mellitus for 8 years, hypertension, hyperlipidemia, and spinal stenosis. He had no history of benign prostatic hyperplasia or prostate cancer.
In addition to the testosterone (100 mg intramuscular injection weekly), his medication regimen included metoprolol 25 mg twice daily, atorvastatin 20 mg daily, acetaminophen 650 mg 3 times daily as needed, aspirin 81 mg daily, metformin 500 mg twice daily, vitamin D 2,000 IU daily, vitamin B12 1,000 mg daily, and Co-Q10 200 mg daily.
On physical examination, Mr. C.’s vitals were stable and his body mass index was in the overweight range at 29.8 kg/m2. His cardiopulmonary examination was normal. There was increased central obesity without palpable organomegaly. There was no gynecomastia, and he had normal amounts of axillary and pubic hair. There was no peripheral edema; his genitourinary examination included normal-sized testicles, and the prostate was smooth without nodules.
The PCP informed Mr. C. that he was familiar with the evaluation and management of testosterone therapy. He was advised that additional evaluation would be needed before determining whether the clinical benefit of TRT outweighed the potential risks.
Andropause
Testosterone levels in men are known to decline at a rate of 1% per year after aged 30 years.6 About 20% of men aged ≥ 60 years and 50% of men aged ≥ 80 years have low (hypogonadal) total testosterone levels.7 The clinical diagnosis of hypogonadism, however, is made on the basis of signs and symptoms consistent with androgen deficiency and a low serum morning testosterone level measured on serum on multiple occasions.8
Specific clinical signs and symptoms (“A” list) consistent with androgen deficiency include low libido and sexual activity; diminished spontaneous erections; gynecomastia; reduced facial, axillary, or pubic hair; small (≤ 5 mL) testes; inability to father children; loss of height, fractures, or other signs of bone loss; and hot flashes and night sweats.9
Less specific signs and symptoms (“B” list) of androgen deficiency include a decrease in energy or motivation, feelings of sadness or depression, poor concentration or memory, trouble sleeping, increased sleepiness, mild anemia, reduced muscle bulk or strength, increased body fat, and diminished physical performance.9
Making the clinical diagnosis of hypogonadism is challenging, because the clinical symptoms have a high prevalence in the older male population and overlap with many nonendocrine diseases. Testosterone replacement therapy has been associated weakly, but consistently, with improved sexual function,10-12 bone mineral density,13,14 fat free mass,13,14 strength,15,16 lipid profiles,17,18 insulin resistance,17,18 and with an increased time to ST segment depression during stress testing.19,20
Laboratory Evaluation
Serum total testosterone circulates in 3 forms: free testosterone, sex hormone-binding globulin (SHBG)-bound testosterone, and albumin-bound testosterone. Free testosterone is the most bio-available testosterone but represents only 2% to 3% of total testosterone.21 Whether total testosterone or free testosterone measurements most closely correlate with symptomatic androgen deficiency is a matter of debate.21 A total testosterone level is an appropriate screening test in young, healthy, and lean men for whom SHBG levels are presumably normal. However, a free or bioavailable testosterone level should be considered for men when there is a high likelihood of conditions that can affect SHBG levels.
Conditions that can decrease SHBG (and may result in a low total testosterone reading even when the free fraction may be normal) include obesity, metabolic syndrome, type 2 diabetes mellitus, hypothyroidism, nephrotic syndrome, chronic glucocorticoid use, and the use of progestins and anabolic steroids.21 Conditions that can increase SHBG (and may result in a normal total testosterone level in patients with hypogonadism, as they have low levels of free testosterone) include aging, cirrhosis, anticonvulsant use, hyperthyroidism, catabolic conditions, and HIV.21
Related: Effect of Statins on Total Testosterone Levels in Male Veterans
Serum testosterone levels generally peak in the early morning, followed by a progressive decline over the course of the day until they reach a nadir in the evening.21 Although it has been debated that morning testosterone levels are not necessary in older men due to a blunting of the circadian rhythm, many men aged 65 to 80 years who have low T in the afternoon will have normal testosterone levels when retested in the morning.22,23 Readings below a reference range of 280 ng/dL to 300 ng/dL on at least 2 different occasions support a diagnosis of hypogonadism.9
Follicle stimulating hormone (FSH) and luteinizing hormone (LH) laboratory tests may be ordered following confirmation of a low testosterone level. Prolactin levels and iron saturation can help evaluate for the presence of hyperprolactinemia and hemochromatosis, respectively. Primary hypogonadism due to testicular failure is diagnosed with high FSH, high LH, and low testosterone levels. Secondary hypogonadism due to hypothalamic or pituitary failure is diagnosed with low FSH, low LH, and low testosterone levels.
Hypothalamic or pituitary suppression from a nonendocrine condition may result in functional hypogonadotropic hypogonadism (FHH), which can be identified with low (or normal) FSH; low (or normal) LH; and low testosterone levels. Hypogonadotropic hypogonadism has been associated with depression, obesity, stress, and physical exertion; and FHH may also be associated with the use of multiple drugs and drug classes (spironolactone, anabolic and corticosteroids, ketoconazole, ethanol, anticonvulsants, immunosuppressants, tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotics, and opioids).24,25 Even statin therapy has been associated with FHH.26,27 Testosterone levels will often recover if or when modifiable factors for FHH are corrected.28
Although there is no consensus on an absolute number that defines a low testosterone level, concern exists that there are economic incentives to raise the bar for normal and thereby increase the potential market for testosterone-raising products.29 Many commercial avenues for the treatment of low T do not follow the standards of the established medical community. Some websites suggest screening for low T with total and free testosterone levels for all men aged > 40 years. Others advise men to consider TRT if they have a total testosterone level of < 500 ng/dL or a free testosterone level that is not in the upper one-third range for men aged 21 to 49 years.30 Of even greater concern, Baillargeon and colleagues reported that 25% of all new androgen users had not had their testosterone levels measured in the 12 months before starting treatment.31 In another study, 40% of men who initiated TRT did not have a baseline measurement.32
Treatments
Before considering TRT, physicians need to emphasize lifestyle modifications as first-line treatment for hypogonadism. The most important modifications include weight loss, tobacco cessation, and moderation in alcohol use.
Patients need to be advised of possible adverse events (AEs) of TRT, which may include gynecomastia, polycythemia, sleep apnea, decreased high-density lipoprotein cholesterol, benign prostatic hypertrophy, infertility, testicular atrophy, and abnormal liver function tests. More recently, several studies have shown an association between TRT and an increase in cardiovascular complications, such as stroke, heart attacks, and death.
Prior to considering TRT, a careful history and physical examination, including a clinical prostate examination, should be performed. Minimum additional tests should include hematocrit, fasting lipid profile (FLP), complete metabolic profile (CMP), and prostate-specific antigen (PSA). Initiation of TRT is not recommended for patients with metastatic prostate cancer; breast cancer; an unevaluated prostate nodule; a PSA > 4 ng/mL (or > 3 ng/mL in African Americans or men with a first-degree relative with prostate cancer); hematocrit > 50%; untreated severe obstructive sleep apnea; uncontrolled or poorly controlled congestive heart failure; or an International Prostate Symptoms Score (IPSS) > 19.9
A past history of prostate cancer had previously been a contraindication for the use of TRT. However, more recent studies have shown that TRT can be used in those who have no evidence of active or metastatic disease and who are under the close supervision of a physician.33-35
Widespread screening is not recommended, and population-based surveys can be unreliable. Fifteen percent of healthy young men, for example, will have a low serum testosterone level in a given 24-hour period.9 Thirty percent of men with an initial testosterone level in the mildly hypogonadal range will have a normal testosterone level when retested; moreover the threshold below which AEs occur remains unknown.9
The goal of TRT is to achieve a total testosterone level in the 400 ng/mL to 700 ng/mL range with improved clinical signs and symptoms.9 Laboratory tests should be conducted at 3 months, 6 months, and then annually. These tests include hematocrit, PSA, and a testosterone level.32 Testing for CMP and FLP should also be considered. If, during therapy, the hematocrit is > 54%, the patient should be assessed for hypoxia and sleep apnea, and treatment should resume at a lower dose only when the hematocrit returns to baseline.9 A digital examination of the prostate is recommended for men with a PSA of > 0.6 ng/mL. A urologic consultation should be obtained for an increase in the PSA of > 1.4 ng/mL over 12 months, a PSA velocity of > 0.4 ng/mL per year (using the PSA after 6 months as a reference), or for an IPPS of > 19.9
Emerging Cardiovascular Concerns
The Testosterone for Older Men study, a randomized, placebo- controlled clinical trial of testosterone therapy in men with a high prevalence of cardiovascular disease, showed significantly greater improvements in leg-press, chest-press, and stair-climbing exercises while carrying a load compared with that in the placebo group.36 However, the study was stopped early due to an increased risk of cardiovascular AEs in those who received testosterone gel.
Vigen and colleagues examined a cohort of veterans who underwent coronary angiography and had a low serum testosterone level.37 The use of TRT in this cohort was also associated with an increased risk of adverse cardiovascular outcomes. This study generated several letters and a recent article in response that vigorously questioned the validity of the methods used and the conclusions reached.38-44 Prior clinical studies of TRT had not detected cardiac AEs, but these trials were generally of short duration and not powered for clinical endpoints.37
A FDA Safety Announcement as well as a VA National Pharmacy Benefits Management bulletin were based on the results of these studies.45 The FDA did not conclude that TRT increased the risk of stroke, heart attack, or death, but health care providers were asked to consider whether the benefits of TRT are likely to exceed the potential risk of treatment.
Direct-to-Consumer Marketing
Some direct-to-consumer marketing promotes the use of aromatase inhibitors, such as anastrozole. This class of medications prevents the conversion of endogenous and exogenous testosterone to estrogen by the aromatase enzyme, which is found predominately in abdominal adipose tissue. There is no evidence that naturally occurring elevations in estrogen cause low testosterone or that treatment of elevated estrogen with an aromatase inhibitor during TRT has any significant clinical benefit in terms of male sexuality.46 Nevertheless, some CAM providers now hypothesize that the increase in cardiovascular AEs with TRT noted in the recent studies may have been due to the increase in estrogen that is associated with TRT.46
The off-label use of clomiphene citrate to block the negative feedback of estrogen on the production of LH has been promoted as another potential treatment to increase testosterone levels. Luteinizing hormone is the pituitary analog of human chorionic gonadotropin (HCG). Many CAM providers also prescribe HCG to increase the testicles’ testosterone production.
Some consumer-focused media insist that the use of either clomiphene citrate or HCG will increase testosterone production and does not cause testicular atrophy, a known TRT- associated AE. This seems to increase the motivation of many men to try these off-label medications.
Some sources even posit a “conspiracy theory” that the FDA and pharmaceutical companies conspire to keep the price of transdermal TRT options high. Men are told that testosterone creams made at compounding pharmacies are much less expensive than are the transdermal pharmaceuticals, and they are urged to see a CAM provider to obtain a prescription for the compounded testosterone. In some cases, a sample prescription is included.47
Many supplements are available that claim to boost testosterone or suppress estrogen. Chrysin, for example, is a bioflavonoid that is marketed as having the potential to act as a natural aromatase inhibitor. Although studies have suggested the potential for chrysin to work in such a manner, the effectiveness may be attenuated by its low bioavailability in supplements.48 Long-term studies have not been conducted.49 Nettle root is a plant-derived compound that is stated to increase free testosterone levels by binding to SHBG, in place of testosterone, and by inhibiting the enzyme that converts testosterone to dihydrotestosterone. The clinical evidence of effectiveness is based on many open studies, and the significance and magnitude of the effect still needs more rigorous evaluation.50
Conclusions
Patients today are barraged with medical information through television, print advertising, radio, and the Internet. A recent study of online sources of herbal product information found that only 10.5% recommended a consultation with a health care professional and < 3% cited scientific literature to accompany their claims.51 Many patients present to their PCP with questions about TRT or have already started an intervention for low T. Complementary and alternative medicine providers of TRT have been able to capture a segment of the population that often has the motivation and disposable income to pursue nontraditional therapies.
All nutritional supplements contain a standard warning from the FDA: “The above statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” Providers should remind patients of the statement and point out the contradictions between the statement and the benefits touted by the supplement marketing literature.
Finally, despite the well- established role of testosterone in enhancing libido, its definitive role in erectile function had been controversial until evidence substantiated a key function for this hormone.52 Testosterone may facilitate erection by acting as a vasodilator of the penile arterioles and cavernous sinusoids and may ameliorate the response to the phosphodiesterase-5 inhibitors in hypogonadal men.53 Testosterone replacement alone in hypogonadal men can restore erectile dysfunction.51 However, hypogonadism is not a common finding in those with erectile dysfunction, only occurring in about 5% of cases.53
Allopathic providers are concerned about the vitality and sexual health of their aging male patients, but their enthusiasm for anti-aging treatments is often tempered by evidence-based studies that have shown a lack of efficacy or potentially serious health care risks. Unfortunately, many patients remain unaware of the controversies regarding TRT. For those patients who receive treatment through CAM providers and are convinced of the efficacy of their low-T treatment regimen, it is important to keep lines of communication open.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Handelsman D. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
2. Hackett G. Testosterone and the heart. Int J Clin Pract. 2012;66(7):648-655.
3. Morley JE. Hypogonadism, testosterone, and nursing home residents. J Am Med Dir Assoc. 2013;14(6):381-383.
4. An J, Cheetham TC, Van Den Eeden S. PS3-36: testosterone replacement therapy patterns for aging males in a managed care setting. Clin Med Res. 2013;11(3):141.
5. Moschis G, Lee E, Marthur A, Strautman J. The Maturing Marketplace: Buying Habits of Baby Boomers and Their Parents. Westport, CT: Quorum Books; 2000.
6. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413.
7. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731.
8. Basaria S. Male hypogonadism. Lancet. 2014;383 (9924):1250-1263.
9. Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
10. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839-2853.
11. Bolona ER, Uranga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
12. Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005;63(4):381-394.
13. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280-293.
14. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670-2677.
15. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661-1667.
16. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090-1099.
17. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome. Diabetes Care. 2011;34(4):828-837.
18. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318-327.
19. Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443-449.
20. Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871-876.
21. Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. White Paper: The Laboratory Diagnosis of Testosterone Deficiency. http://www.auanet.org/common/pdf/education/clinical -guidance/Testosterone-Deficiency-WhitePaper.pdf. Published 2013. Accessed April 9, 2015.
22. Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509-513.
23. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67(6):853-862.
24. Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: symptoms and treatment. J Adv Pharm Technol Res. 2010;1(3):297-301.
25. Montgomery K. Sexual desire disorders. Psychiatry (Edgmont). 2008;5(6):50-55.
26. Corona G, Boddi V, Balercia G, et al. The effect of statin therapy on testosterone levels in subjects consulting for erectile dysfunction. J Sex Med. 2010; 7(4 pt 1):1547-1556.
27. Schooling CM, Yeung SLA, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
28. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
29. Schwartz LM, Woloshin S. Low “T” as in “template”: how to sell disease. JAMA Intern Med. 2013;173(15):1460-1462.
30. Male Hormone Modulation Therapy, Part 2. Life Extension Vitamins Website. http://www.lifeextension vitamins.com/mahomothpa2.html. Accessed April 9, 2015.
31. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465-1466.
32. Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99(3):835-842.
33. Ramasamy R, Fisher ES, Schlegel PN. Testosterone replacement and prostate cancer. Indian J Urol. 2012;28(2):123-128.
34. Marks, LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2351-2361.
35. Coward RM, Simhan J, Carson CC 3rd. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103(9):1179-1183.
36. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
37. Vigen R, O’Donnell Cl, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
38. Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):961-962.
39. Jones TH, Channer K. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):962-963.
40. Katz J, Nadelberg R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963.
41. Riche D, Baker WL, Koch CA. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963-964.
42. Dhindsa S, Batra M, Dandona P. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):964.
43. Ho PM, Barón AE, Wierman M. Deaths and cardiovascular events in men receiving testosterone—reply. JAMA. 2014;311(9):964-965.
44. Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11(3):624-629.
45. U.S. Department of Veterans Affairs, Veterans Health Administration (VHA), Pharmacy Benefit Management Services (PBM), Medical Advisory Panel (MAP), and Center for Medication Safety (VA Medsafe. National PBM Bulletin. Testosterone products and cardiovascular safety. http://www.pbm.va.gov/PBM/vacenterformedicationsafety/nationalpbmbulletin/Testosterone_Products_and_Cardiovascular_Safety_NATIONAL_PBM_BULLETIN_02.pdf. Published February 7, 2014. Accessed April 9, 2015.
46. Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681-1696.
47. Faloon W. Vindication. Life Extension Magazine Website. http://www.lef.org/magazine /mag2008/dec2008_Harvard-Experts-Recommend -Testosterone-Replacement_02.htm. Published December 2008. Accessed April 9,2015.
48. Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51(2):143-146.
49. Jana K, Yin X, Schiffer, et al. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197(2):315-323.
50. Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7-8):568-579.
51. Owens C, Baergen R, Puckett D. Online sources of herbal product information. Am J Med. 2014;127(2):109-115.
52. Blute W, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108-122.
53. Mikhail N. Does testosterone have a role in erectile dysfunction? Am J Med. 2006;119(5):373-382.
1. Handelsman D. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
2. Hackett G. Testosterone and the heart. Int J Clin Pract. 2012;66(7):648-655.
3. Morley JE. Hypogonadism, testosterone, and nursing home residents. J Am Med Dir Assoc. 2013;14(6):381-383.
4. An J, Cheetham TC, Van Den Eeden S. PS3-36: testosterone replacement therapy patterns for aging males in a managed care setting. Clin Med Res. 2013;11(3):141.
5. Moschis G, Lee E, Marthur A, Strautman J. The Maturing Marketplace: Buying Habits of Baby Boomers and Their Parents. Westport, CT: Quorum Books; 2000.
6. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413.
7. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731.
8. Basaria S. Male hypogonadism. Lancet. 2014;383 (9924):1250-1263.
9. Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
10. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839-2853.
11. Bolona ER, Uranga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
12. Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005;63(4):381-394.
13. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280-293.
14. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670-2677.
15. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661-1667.
16. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090-1099.
17. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome. Diabetes Care. 2011;34(4):828-837.
18. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318-327.
19. Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443-449.
20. Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871-876.
21. Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. White Paper: The Laboratory Diagnosis of Testosterone Deficiency. http://www.auanet.org/common/pdf/education/clinical -guidance/Testosterone-Deficiency-WhitePaper.pdf. Published 2013. Accessed April 9, 2015.
22. Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509-513.
23. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67(6):853-862.
24. Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: symptoms and treatment. J Adv Pharm Technol Res. 2010;1(3):297-301.
25. Montgomery K. Sexual desire disorders. Psychiatry (Edgmont). 2008;5(6):50-55.
26. Corona G, Boddi V, Balercia G, et al. The effect of statin therapy on testosterone levels in subjects consulting for erectile dysfunction. J Sex Med. 2010; 7(4 pt 1):1547-1556.
27. Schooling CM, Yeung SLA, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
28. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
29. Schwartz LM, Woloshin S. Low “T” as in “template”: how to sell disease. JAMA Intern Med. 2013;173(15):1460-1462.
30. Male Hormone Modulation Therapy, Part 2. Life Extension Vitamins Website. http://www.lifeextension vitamins.com/mahomothpa2.html. Accessed April 9, 2015.
31. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465-1466.
32. Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99(3):835-842.
33. Ramasamy R, Fisher ES, Schlegel PN. Testosterone replacement and prostate cancer. Indian J Urol. 2012;28(2):123-128.
34. Marks, LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2351-2361.
35. Coward RM, Simhan J, Carson CC 3rd. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103(9):1179-1183.
36. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
37. Vigen R, O’Donnell Cl, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
38. Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):961-962.
39. Jones TH, Channer K. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):962-963.
40. Katz J, Nadelberg R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963.
41. Riche D, Baker WL, Koch CA. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963-964.
42. Dhindsa S, Batra M, Dandona P. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):964.
43. Ho PM, Barón AE, Wierman M. Deaths and cardiovascular events in men receiving testosterone—reply. JAMA. 2014;311(9):964-965.
44. Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11(3):624-629.
45. U.S. Department of Veterans Affairs, Veterans Health Administration (VHA), Pharmacy Benefit Management Services (PBM), Medical Advisory Panel (MAP), and Center for Medication Safety (VA Medsafe. National PBM Bulletin. Testosterone products and cardiovascular safety. http://www.pbm.va.gov/PBM/vacenterformedicationsafety/nationalpbmbulletin/Testosterone_Products_and_Cardiovascular_Safety_NATIONAL_PBM_BULLETIN_02.pdf. Published February 7, 2014. Accessed April 9, 2015.
46. Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681-1696.
47. Faloon W. Vindication. Life Extension Magazine Website. http://www.lef.org/magazine /mag2008/dec2008_Harvard-Experts-Recommend -Testosterone-Replacement_02.htm. Published December 2008. Accessed April 9,2015.
48. Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51(2):143-146.
49. Jana K, Yin X, Schiffer, et al. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197(2):315-323.
50. Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7-8):568-579.
51. Owens C, Baergen R, Puckett D. Online sources of herbal product information. Am J Med. 2014;127(2):109-115.
52. Blute W, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108-122.
53. Mikhail N. Does testosterone have a role in erectile dysfunction? Am J Med. 2006;119(5):373-382.