User login
Balancing needs and risks as the opioid pendulum swings
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
Meaningful improvement for patients like Tante Ilse
Last year, after a long delay due to COVID, my father’s ashes were finally laid to rest at Arlington National Cemetery. Among the loved ones who came was my favorite aunt, Tante Ilse, who was suffering from dementia. While she wasn’t “following” everything that was going on, she did perk up when she heard my father’s name and would comment on how she liked him and how wonderful he had been to her.
After the ceremony, our family of about 30 gathered at a restaurant where we shared stories and old pictures. Tante Ilse seemed to relish the photos and the time with family. She was doing so well that when we went back to my mom’s home after the reception, my cousins decided to bring Tante Ilse there, too. She had a great time, as evidenced by her famous total-body laugh. In the months before her death, we all commented about that day and how happy she seemed.
My aunt’s decline comes to mind as I reflect on media reports of 2 Alzheimer drugs— aducanumab and lecanemab—that have been billed by some as “gamechangers.” These new drugs are monoclonal antibodies directed at amyloid, one of several agents thought to cause Alzheimer disease. The details of aducanumab’s approval by the US Food and Drug Administration (FDA) generated a great deal of criticism—with good reason.
Two manufacturer-sponsored studies of aducanumab were halted due to futility of finding a benefit.1 The FDA’s scientific advisory panel recommended against approval due to a lack of evidence that it did anything more than remove amyloid plaque from the brain. And yet aducanumab received accelerated approval from the FDA. (This author collaborated on an additional analysis using data presented to the FDA, after its approval, which also reported no clinically meaningful effects.2) The other agent, lecanemab, also reduces markers of amyloid and was shown to be only moderately better than placebo in decreasing the rate of decline on various measures of cognition.3 Quite notably, both aducanumab and lecanemab, which are administered parenterally, cost more than $25,000 per year4,5 and cause amyloid-related imaging abnormalities (brain edema or hemorrhage).
Expensive agents without meaningful benefit. So far, neither of these agents has shown a reduction in things that are truly important to our patients and their families/caregivers: a reduction in caregiver burden and a reduction in the need for placement in long-term care facilities.
This is in contrast to cholinesterase inhibitors, which also slow the rate of cognitive decline.6 Among the differences that exist between these agents: Cholinesterase inhibitors are taken orally and are available as generics, which cost less than a thousand dollars per year.7 Limited data also suggest that they are associated with a lower risk for nursing home placement.8,9 (A February 2023 search of clinicaltrials.gov did not reveal any completed or planned head-to-head comparisons of monoclonal antibodies and anticholinergic agents.)
Our patients, their families, and caregivers hold out hope for something that will improve the patient’s cognition and extend the meaningful time they have with their loved ones. So far, the best we have to offer falls far short of these goals. I certainly would have hoped for something better than merely clearing amyloid for my aunt.
It’s time that the FDA adopt more rigorous standards requiring new drugs to, among other things, demonstrate meaningful clinical benefits, provide real cost savings, and be safer than currently available therapies. Other nations seem to be able to do this.10,11 It is bad enough to provide “hope in a bottle”; it is worse when what is offered is false hope.
1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197-210. doi: 10.14283/jpad.2022.30
2. Ebell MH, Barry HC. Why physicians should not prescribe aducanumab for Alzheimer disease. Am Fam Physician. 2022;105:353-354.
3. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948
4. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023; 613:227-228. doi: 10.1038/d41586-023-00030-3
5. Biogen announces reduced price for Aduhelm to improve access for patients with early Alzheimer’s disease. December 20, 2021. Accessed February 20, 2023. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-reduced-price-aduhelmr-improve-access-patients
6. Takramah WK, Asem L. The efficacy of pharmacological interventions to improve cognitive and behavior symptoms in people with dementia: A systematic review and meta-analysis. Health Sci Rep. 2022;5:e913. doi: 10.1002/hsr2.913
7. GoodRx. Donepezil generic Aricept. Accessed February 20, 2023. www.goodrx.com/donepezil
8. Howard R, McShane R, Lindesay J, et al. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer’s disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171-1181. doi: 10.1016/S1474-4422(15)00258-6
9. Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937-944. doi: 10.1046/j.1365-2389.2003.51306.x
10. Pham C, Le K, Draves M, et al. Assessment of FDA-approved drugs not recommended for use or reimbursement in other countries, 2017-2020. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6787
11. Johnston JL, Ross JS, Ramachandran R. US Food and Drug Administration approval of drugs not meeting pivotal trial primary end points, 2018-2021. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6444
Last year, after a long delay due to COVID, my father’s ashes were finally laid to rest at Arlington National Cemetery. Among the loved ones who came was my favorite aunt, Tante Ilse, who was suffering from dementia. While she wasn’t “following” everything that was going on, she did perk up when she heard my father’s name and would comment on how she liked him and how wonderful he had been to her.
After the ceremony, our family of about 30 gathered at a restaurant where we shared stories and old pictures. Tante Ilse seemed to relish the photos and the time with family. She was doing so well that when we went back to my mom’s home after the reception, my cousins decided to bring Tante Ilse there, too. She had a great time, as evidenced by her famous total-body laugh. In the months before her death, we all commented about that day and how happy she seemed.
My aunt’s decline comes to mind as I reflect on media reports of 2 Alzheimer drugs— aducanumab and lecanemab—that have been billed by some as “gamechangers.” These new drugs are monoclonal antibodies directed at amyloid, one of several agents thought to cause Alzheimer disease. The details of aducanumab’s approval by the US Food and Drug Administration (FDA) generated a great deal of criticism—with good reason.
Two manufacturer-sponsored studies of aducanumab were halted due to futility of finding a benefit.1 The FDA’s scientific advisory panel recommended against approval due to a lack of evidence that it did anything more than remove amyloid plaque from the brain. And yet aducanumab received accelerated approval from the FDA. (This author collaborated on an additional analysis using data presented to the FDA, after its approval, which also reported no clinically meaningful effects.2) The other agent, lecanemab, also reduces markers of amyloid and was shown to be only moderately better than placebo in decreasing the rate of decline on various measures of cognition.3 Quite notably, both aducanumab and lecanemab, which are administered parenterally, cost more than $25,000 per year4,5 and cause amyloid-related imaging abnormalities (brain edema or hemorrhage).
Expensive agents without meaningful benefit. So far, neither of these agents has shown a reduction in things that are truly important to our patients and their families/caregivers: a reduction in caregiver burden and a reduction in the need for placement in long-term care facilities.
This is in contrast to cholinesterase inhibitors, which also slow the rate of cognitive decline.6 Among the differences that exist between these agents: Cholinesterase inhibitors are taken orally and are available as generics, which cost less than a thousand dollars per year.7 Limited data also suggest that they are associated with a lower risk for nursing home placement.8,9 (A February 2023 search of clinicaltrials.gov did not reveal any completed or planned head-to-head comparisons of monoclonal antibodies and anticholinergic agents.)
Our patients, their families, and caregivers hold out hope for something that will improve the patient’s cognition and extend the meaningful time they have with their loved ones. So far, the best we have to offer falls far short of these goals. I certainly would have hoped for something better than merely clearing amyloid for my aunt.
It’s time that the FDA adopt more rigorous standards requiring new drugs to, among other things, demonstrate meaningful clinical benefits, provide real cost savings, and be safer than currently available therapies. Other nations seem to be able to do this.10,11 It is bad enough to provide “hope in a bottle”; it is worse when what is offered is false hope.
Last year, after a long delay due to COVID, my father’s ashes were finally laid to rest at Arlington National Cemetery. Among the loved ones who came was my favorite aunt, Tante Ilse, who was suffering from dementia. While she wasn’t “following” everything that was going on, she did perk up when she heard my father’s name and would comment on how she liked him and how wonderful he had been to her.
After the ceremony, our family of about 30 gathered at a restaurant where we shared stories and old pictures. Tante Ilse seemed to relish the photos and the time with family. She was doing so well that when we went back to my mom’s home after the reception, my cousins decided to bring Tante Ilse there, too. She had a great time, as evidenced by her famous total-body laugh. In the months before her death, we all commented about that day and how happy she seemed.
My aunt’s decline comes to mind as I reflect on media reports of 2 Alzheimer drugs— aducanumab and lecanemab—that have been billed by some as “gamechangers.” These new drugs are monoclonal antibodies directed at amyloid, one of several agents thought to cause Alzheimer disease. The details of aducanumab’s approval by the US Food and Drug Administration (FDA) generated a great deal of criticism—with good reason.
Two manufacturer-sponsored studies of aducanumab were halted due to futility of finding a benefit.1 The FDA’s scientific advisory panel recommended against approval due to a lack of evidence that it did anything more than remove amyloid plaque from the brain. And yet aducanumab received accelerated approval from the FDA. (This author collaborated on an additional analysis using data presented to the FDA, after its approval, which also reported no clinically meaningful effects.2) The other agent, lecanemab, also reduces markers of amyloid and was shown to be only moderately better than placebo in decreasing the rate of decline on various measures of cognition.3 Quite notably, both aducanumab and lecanemab, which are administered parenterally, cost more than $25,000 per year4,5 and cause amyloid-related imaging abnormalities (brain edema or hemorrhage).
Expensive agents without meaningful benefit. So far, neither of these agents has shown a reduction in things that are truly important to our patients and their families/caregivers: a reduction in caregiver burden and a reduction in the need for placement in long-term care facilities.
This is in contrast to cholinesterase inhibitors, which also slow the rate of cognitive decline.6 Among the differences that exist between these agents: Cholinesterase inhibitors are taken orally and are available as generics, which cost less than a thousand dollars per year.7 Limited data also suggest that they are associated with a lower risk for nursing home placement.8,9 (A February 2023 search of clinicaltrials.gov did not reveal any completed or planned head-to-head comparisons of monoclonal antibodies and anticholinergic agents.)
Our patients, their families, and caregivers hold out hope for something that will improve the patient’s cognition and extend the meaningful time they have with their loved ones. So far, the best we have to offer falls far short of these goals. I certainly would have hoped for something better than merely clearing amyloid for my aunt.
It’s time that the FDA adopt more rigorous standards requiring new drugs to, among other things, demonstrate meaningful clinical benefits, provide real cost savings, and be safer than currently available therapies. Other nations seem to be able to do this.10,11 It is bad enough to provide “hope in a bottle”; it is worse when what is offered is false hope.
1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197-210. doi: 10.14283/jpad.2022.30
2. Ebell MH, Barry HC. Why physicians should not prescribe aducanumab for Alzheimer disease. Am Fam Physician. 2022;105:353-354.
3. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948
4. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023; 613:227-228. doi: 10.1038/d41586-023-00030-3
5. Biogen announces reduced price for Aduhelm to improve access for patients with early Alzheimer’s disease. December 20, 2021. Accessed February 20, 2023. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-reduced-price-aduhelmr-improve-access-patients
6. Takramah WK, Asem L. The efficacy of pharmacological interventions to improve cognitive and behavior symptoms in people with dementia: A systematic review and meta-analysis. Health Sci Rep. 2022;5:e913. doi: 10.1002/hsr2.913
7. GoodRx. Donepezil generic Aricept. Accessed February 20, 2023. www.goodrx.com/donepezil
8. Howard R, McShane R, Lindesay J, et al. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer’s disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171-1181. doi: 10.1016/S1474-4422(15)00258-6
9. Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937-944. doi: 10.1046/j.1365-2389.2003.51306.x
10. Pham C, Le K, Draves M, et al. Assessment of FDA-approved drugs not recommended for use or reimbursement in other countries, 2017-2020. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6787
11. Johnston JL, Ross JS, Ramachandran R. US Food and Drug Administration approval of drugs not meeting pivotal trial primary end points, 2018-2021. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6444
1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197-210. doi: 10.14283/jpad.2022.30
2. Ebell MH, Barry HC. Why physicians should not prescribe aducanumab for Alzheimer disease. Am Fam Physician. 2022;105:353-354.
3. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948
4. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023; 613:227-228. doi: 10.1038/d41586-023-00030-3
5. Biogen announces reduced price for Aduhelm to improve access for patients with early Alzheimer’s disease. December 20, 2021. Accessed February 20, 2023. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-reduced-price-aduhelmr-improve-access-patients
6. Takramah WK, Asem L. The efficacy of pharmacological interventions to improve cognitive and behavior symptoms in people with dementia: A systematic review and meta-analysis. Health Sci Rep. 2022;5:e913. doi: 10.1002/hsr2.913
7. GoodRx. Donepezil generic Aricept. Accessed February 20, 2023. www.goodrx.com/donepezil
8. Howard R, McShane R, Lindesay J, et al. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer’s disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171-1181. doi: 10.1016/S1474-4422(15)00258-6
9. Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937-944. doi: 10.1046/j.1365-2389.2003.51306.x
10. Pham C, Le K, Draves M, et al. Assessment of FDA-approved drugs not recommended for use or reimbursement in other countries, 2017-2020. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6787
11. Johnston JL, Ross JS, Ramachandran R. US Food and Drug Administration approval of drugs not meeting pivotal trial primary end points, 2018-2021. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6444
A reason for hope in the face of long COVID
In this issue, Mayo and colleagues1 summarize what we know about patients with long COVID. The report made me pause and realize that it has been 3 years since we heard the very first reports of patients infected with SARS-CoV-2, which would eventually cause the COVID-19 pandemic. I suspect that I am not alone in having been fascinated by the rapid communication of information (of variable quality and veracity) via peer-reviewed papers, pre-print servers, the media, and social media.
The early studies were largely descriptive, focusing on symptom constellations and outbreak data. Much of what we had by way of treatment was supportive and “let’s try anything”—whether reasonable or, in some cases, not. In relatively short order, though, we developed effective vaccines to help protect people from getting seriously ill, being hospitalized, and dying; we also identified targeted therapies for those who became ill.2 But variants continued—or rather, continue—to emerge, and we remain committed to meeting the demands of the day.
The Centers for Disease Control and Prevention reports that more than 98 million Americans have contracted COVID, and more than 1 million have died.3 Besides the astonishingly high total mortality, the ravages of COVID-19 include new-onset respiratory, cardiovascular, neurologic, and psychiatric illnesses.4,5 As many as half of adults hospitalized for COVID report having persistent symptoms.6
As described in this issue, what we know about long COVID appears to be following the early course of its parent illness. As was true then, we are learning about the symptoms, etiology, and best ways to manage our patients. As in the early days of the pandemic, treatment is supportive, and we await definitive therapies.
I am optimistic, though. Why? Because shortly after the first reports of COVID-19, the virus’ DNA sequence was shared online. Based on that information, diagnostic assays were developed. Within 2 years of the outbreak, we had effective vaccines and specific therapies.
Another call to action. If 5% of patients contracting COVID (a very low estimate) develop long COVID, that would translate to 4.9 million people with long COVID in the United States. That is an astounding burden of suffering that I have no doubt will motivate innovation.
Innovation is a strength of the US health care system. I believe we will rise to the next challenge that COVID-19 has put before us. We have reason to be hopeful.
1. Mayo NL, Ellenbogen RL, Mendoza MD, et al. The family physician’s role in long COVID management. J Fam Pract. 2022;71:426-431. doi: 10.12788/jfp.0517
2. Kulshreshtha A, Sizemore S, Barry HC. COVID-19 therapy: What works? What doesn’t? And what’s on the horizon? J Fam Pract. 2022;71:E3-E16. doi: 10.12788/jfp.0474
3. CDC. COVID data tracker. Accessed December 5, 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
4. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. doi: 10.1016/s2215-0366(21) 00084-5
5. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693
6. Writing Committee for the Comebac Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525-1534. doi: 10.1001/jama.2021.3331
In this issue, Mayo and colleagues1 summarize what we know about patients with long COVID. The report made me pause and realize that it has been 3 years since we heard the very first reports of patients infected with SARS-CoV-2, which would eventually cause the COVID-19 pandemic. I suspect that I am not alone in having been fascinated by the rapid communication of information (of variable quality and veracity) via peer-reviewed papers, pre-print servers, the media, and social media.
The early studies were largely descriptive, focusing on symptom constellations and outbreak data. Much of what we had by way of treatment was supportive and “let’s try anything”—whether reasonable or, in some cases, not. In relatively short order, though, we developed effective vaccines to help protect people from getting seriously ill, being hospitalized, and dying; we also identified targeted therapies for those who became ill.2 But variants continued—or rather, continue—to emerge, and we remain committed to meeting the demands of the day.
The Centers for Disease Control and Prevention reports that more than 98 million Americans have contracted COVID, and more than 1 million have died.3 Besides the astonishingly high total mortality, the ravages of COVID-19 include new-onset respiratory, cardiovascular, neurologic, and psychiatric illnesses.4,5 As many as half of adults hospitalized for COVID report having persistent symptoms.6
As described in this issue, what we know about long COVID appears to be following the early course of its parent illness. As was true then, we are learning about the symptoms, etiology, and best ways to manage our patients. As in the early days of the pandemic, treatment is supportive, and we await definitive therapies.
I am optimistic, though. Why? Because shortly after the first reports of COVID-19, the virus’ DNA sequence was shared online. Based on that information, diagnostic assays were developed. Within 2 years of the outbreak, we had effective vaccines and specific therapies.
Another call to action. If 5% of patients contracting COVID (a very low estimate) develop long COVID, that would translate to 4.9 million people with long COVID in the United States. That is an astounding burden of suffering that I have no doubt will motivate innovation.
Innovation is a strength of the US health care system. I believe we will rise to the next challenge that COVID-19 has put before us. We have reason to be hopeful.
In this issue, Mayo and colleagues1 summarize what we know about patients with long COVID. The report made me pause and realize that it has been 3 years since we heard the very first reports of patients infected with SARS-CoV-2, which would eventually cause the COVID-19 pandemic. I suspect that I am not alone in having been fascinated by the rapid communication of information (of variable quality and veracity) via peer-reviewed papers, pre-print servers, the media, and social media.
The early studies were largely descriptive, focusing on symptom constellations and outbreak data. Much of what we had by way of treatment was supportive and “let’s try anything”—whether reasonable or, in some cases, not. In relatively short order, though, we developed effective vaccines to help protect people from getting seriously ill, being hospitalized, and dying; we also identified targeted therapies for those who became ill.2 But variants continued—or rather, continue—to emerge, and we remain committed to meeting the demands of the day.
The Centers for Disease Control and Prevention reports that more than 98 million Americans have contracted COVID, and more than 1 million have died.3 Besides the astonishingly high total mortality, the ravages of COVID-19 include new-onset respiratory, cardiovascular, neurologic, and psychiatric illnesses.4,5 As many as half of adults hospitalized for COVID report having persistent symptoms.6
As described in this issue, what we know about long COVID appears to be following the early course of its parent illness. As was true then, we are learning about the symptoms, etiology, and best ways to manage our patients. As in the early days of the pandemic, treatment is supportive, and we await definitive therapies.
I am optimistic, though. Why? Because shortly after the first reports of COVID-19, the virus’ DNA sequence was shared online. Based on that information, diagnostic assays were developed. Within 2 years of the outbreak, we had effective vaccines and specific therapies.
Another call to action. If 5% of patients contracting COVID (a very low estimate) develop long COVID, that would translate to 4.9 million people with long COVID in the United States. That is an astounding burden of suffering that I have no doubt will motivate innovation.
Innovation is a strength of the US health care system. I believe we will rise to the next challenge that COVID-19 has put before us. We have reason to be hopeful.
1. Mayo NL, Ellenbogen RL, Mendoza MD, et al. The family physician’s role in long COVID management. J Fam Pract. 2022;71:426-431. doi: 10.12788/jfp.0517
2. Kulshreshtha A, Sizemore S, Barry HC. COVID-19 therapy: What works? What doesn’t? And what’s on the horizon? J Fam Pract. 2022;71:E3-E16. doi: 10.12788/jfp.0474
3. CDC. COVID data tracker. Accessed December 5, 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
4. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. doi: 10.1016/s2215-0366(21) 00084-5
5. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693
6. Writing Committee for the Comebac Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525-1534. doi: 10.1001/jama.2021.3331
1. Mayo NL, Ellenbogen RL, Mendoza MD, et al. The family physician’s role in long COVID management. J Fam Pract. 2022;71:426-431. doi: 10.12788/jfp.0517
2. Kulshreshtha A, Sizemore S, Barry HC. COVID-19 therapy: What works? What doesn’t? And what’s on the horizon? J Fam Pract. 2022;71:E3-E16. doi: 10.12788/jfp.0474
3. CDC. COVID data tracker. Accessed December 5, 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
4. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416-427. doi: 10.1016/s2215-0366(21) 00084-5
5. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693
6. Writing Committee for the Comebac Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525-1534. doi: 10.1001/jama.2021.3331
Going the distance with our patients
Many years ago, I had a patient I’ll call “Hannah,” who was well into her 80s and always came into the office with her daughter. She was a heavy smoker and had hypertension and type 2 diabetes.
At each visit, I would ask her if she still smoked and if she was interested in talking about quitting. At every visit, she would say that she was still smoking and didn’t want to quit. My response was always something along the lines of: “When you’re ready, we can talk more. But I think it is the most important thing you can do to improve your health.” From there, we would discuss any concerns she or her daughter had.
A few years shy of her 100th birthday, Hannah told me she had quit smoking. I was amazed and asked her why, after all these years, she’d done it.
“I quit,” she said, “because I was tired of you nagging me, sonny!” And we both had a good laugh about that.
Hannah’s story reminds me that, as family physicians, we often have an impact on our patients in ways we don’t see in the short term. It is our longitudinal relationships with patients that allow us to plant seeds and reap the benefits over time.
It is these relationships that we can draw upon when counseling our patients with type 2 diabetes to address lifestyle issues such as exercise and a healthy diet. In this issue, McMullan et al1 provide us with a rather hopeful review of the evidence in support of lifestyle changes. For our patients with type 2 diabetes, lifestyle changes can decrease A1C levels by 0.5% (with environmental changes related to diet)2 and 0.7% (with moderate aerobic exercise).3 This is comparable to what is reported for the starting doses of most medications.4 In fact, a meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients.5 (Caveat: The result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used.)
And yet, we often focus more on the various medications we can prescribe, with professional guidelines pointing the way.
Continue to: The National Institute for Health and Care Excellence
The National Institute for Health and Care Excellence,6 American Diabetes Association,7 American College of Physicians,8 and American Academy of Family Physicians8 have followed the accumulating evidence that various medications improve outcomes—especially in patients at high risk or with established atherosclerotic cardiovascular disease. They have endorsed a stepwise pharmacologic approach beginning with metformin and recommend assessing each patient’s comorbidities to guide whether to add a sodium glucose co-transporter 2 (SGLT2) inhibitor or another agent. Where the groups diverge is what that second agent should be (glucagon-like peptide 1 receptor agonist, SGLT2 inhibitor, or dipeptidyl peptidase-4 inhibitor).
But what about lifestyle? Each organization’s guidelines address lifestyle changes as a foundation for managing patients with type 2 diabetes. But is that call loud enough? Do we heed it well enough?
Implementing lifestyle changes in office practice can be time consuming. Many clinicians lack adequate training or experience to gain any traction with it. Also, there is skepticism about success and sustainability.
I believe change starts when we recognize that while we have a priority list for each patient encounter, so do our patients. But they may not share that list with us unless we open the door by asking questions, such as:
- Of all the things you have heard about caring for your diabetes, what would you like to work on?
- What are you currently doing and what prevents you from meeting your goals?
- How would you like me to help you?
From there, we can start small and build on successes over time. We can go the distance with our patients. In the case of Hannah, I had the honor of caring for her until she died at age 104.
1. McMullan S, Smith DK, Kimsey J. Maximizing lifestyle changes to manage type 2 diabetes. J Fam Pract. 2022;71;342-348. doi: 10.12788/jfp.0482
2. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
3. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. doi: 10.1186/s12933-017-0518-6
Many years ago, I had a patient I’ll call “Hannah,” who was well into her 80s and always came into the office with her daughter. She was a heavy smoker and had hypertension and type 2 diabetes.
At each visit, I would ask her if she still smoked and if she was interested in talking about quitting. At every visit, she would say that she was still smoking and didn’t want to quit. My response was always something along the lines of: “When you’re ready, we can talk more. But I think it is the most important thing you can do to improve your health.” From there, we would discuss any concerns she or her daughter had.
A few years shy of her 100th birthday, Hannah told me she had quit smoking. I was amazed and asked her why, after all these years, she’d done it.
“I quit,” she said, “because I was tired of you nagging me, sonny!” And we both had a good laugh about that.
Hannah’s story reminds me that, as family physicians, we often have an impact on our patients in ways we don’t see in the short term. It is our longitudinal relationships with patients that allow us to plant seeds and reap the benefits over time.
It is these relationships that we can draw upon when counseling our patients with type 2 diabetes to address lifestyle issues such as exercise and a healthy diet. In this issue, McMullan et al1 provide us with a rather hopeful review of the evidence in support of lifestyle changes. For our patients with type 2 diabetes, lifestyle changes can decrease A1C levels by 0.5% (with environmental changes related to diet)2 and 0.7% (with moderate aerobic exercise).3 This is comparable to what is reported for the starting doses of most medications.4 In fact, a meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients.5 (Caveat: The result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used.)
And yet, we often focus more on the various medications we can prescribe, with professional guidelines pointing the way.
Continue to: The National Institute for Health and Care Excellence
The National Institute for Health and Care Excellence,6 American Diabetes Association,7 American College of Physicians,8 and American Academy of Family Physicians8 have followed the accumulating evidence that various medications improve outcomes—especially in patients at high risk or with established atherosclerotic cardiovascular disease. They have endorsed a stepwise pharmacologic approach beginning with metformin and recommend assessing each patient’s comorbidities to guide whether to add a sodium glucose co-transporter 2 (SGLT2) inhibitor or another agent. Where the groups diverge is what that second agent should be (glucagon-like peptide 1 receptor agonist, SGLT2 inhibitor, or dipeptidyl peptidase-4 inhibitor).
But what about lifestyle? Each organization’s guidelines address lifestyle changes as a foundation for managing patients with type 2 diabetes. But is that call loud enough? Do we heed it well enough?
Implementing lifestyle changes in office practice can be time consuming. Many clinicians lack adequate training or experience to gain any traction with it. Also, there is skepticism about success and sustainability.
I believe change starts when we recognize that while we have a priority list for each patient encounter, so do our patients. But they may not share that list with us unless we open the door by asking questions, such as:
- Of all the things you have heard about caring for your diabetes, what would you like to work on?
- What are you currently doing and what prevents you from meeting your goals?
- How would you like me to help you?
From there, we can start small and build on successes over time. We can go the distance with our patients. In the case of Hannah, I had the honor of caring for her until she died at age 104.
Many years ago, I had a patient I’ll call “Hannah,” who was well into her 80s and always came into the office with her daughter. She was a heavy smoker and had hypertension and type 2 diabetes.
At each visit, I would ask her if she still smoked and if she was interested in talking about quitting. At every visit, she would say that she was still smoking and didn’t want to quit. My response was always something along the lines of: “When you’re ready, we can talk more. But I think it is the most important thing you can do to improve your health.” From there, we would discuss any concerns she or her daughter had.
A few years shy of her 100th birthday, Hannah told me she had quit smoking. I was amazed and asked her why, after all these years, she’d done it.
“I quit,” she said, “because I was tired of you nagging me, sonny!” And we both had a good laugh about that.
Hannah’s story reminds me that, as family physicians, we often have an impact on our patients in ways we don’t see in the short term. It is our longitudinal relationships with patients that allow us to plant seeds and reap the benefits over time.
It is these relationships that we can draw upon when counseling our patients with type 2 diabetes to address lifestyle issues such as exercise and a healthy diet. In this issue, McMullan et al1 provide us with a rather hopeful review of the evidence in support of lifestyle changes. For our patients with type 2 diabetes, lifestyle changes can decrease A1C levels by 0.5% (with environmental changes related to diet)2 and 0.7% (with moderate aerobic exercise).3 This is comparable to what is reported for the starting doses of most medications.4 In fact, a meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients.5 (Caveat: The result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used.)
And yet, we often focus more on the various medications we can prescribe, with professional guidelines pointing the way.
Continue to: The National Institute for Health and Care Excellence
The National Institute for Health and Care Excellence,6 American Diabetes Association,7 American College of Physicians,8 and American Academy of Family Physicians8 have followed the accumulating evidence that various medications improve outcomes—especially in patients at high risk or with established atherosclerotic cardiovascular disease. They have endorsed a stepwise pharmacologic approach beginning with metformin and recommend assessing each patient’s comorbidities to guide whether to add a sodium glucose co-transporter 2 (SGLT2) inhibitor or another agent. Where the groups diverge is what that second agent should be (glucagon-like peptide 1 receptor agonist, SGLT2 inhibitor, or dipeptidyl peptidase-4 inhibitor).
But what about lifestyle? Each organization’s guidelines address lifestyle changes as a foundation for managing patients with type 2 diabetes. But is that call loud enough? Do we heed it well enough?
Implementing lifestyle changes in office practice can be time consuming. Many clinicians lack adequate training or experience to gain any traction with it. Also, there is skepticism about success and sustainability.
I believe change starts when we recognize that while we have a priority list for each patient encounter, so do our patients. But they may not share that list with us unless we open the door by asking questions, such as:
- Of all the things you have heard about caring for your diabetes, what would you like to work on?
- What are you currently doing and what prevents you from meeting your goals?
- How would you like me to help you?
From there, we can start small and build on successes over time. We can go the distance with our patients. In the case of Hannah, I had the honor of caring for her until she died at age 104.
1. McMullan S, Smith DK, Kimsey J. Maximizing lifestyle changes to manage type 2 diabetes. J Fam Pract. 2022;71;342-348. doi: 10.12788/jfp.0482
2. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
3. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. doi: 10.1186/s12933-017-0518-6
1. McMullan S, Smith DK, Kimsey J. Maximizing lifestyle changes to manage type 2 diabetes. J Fam Pract. 2022;71;342-348. doi: 10.12788/jfp.0482
2. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
3. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. doi: 10.1186/s12933-017-0518-6
COVID-19 therapy: What works? What doesn’t? And what’s on the horizon?
The ongoing COVID-19 pandemic has caused more than 1 million deaths in the United States and continues to be a major public health challenge. Cases can be asymptomatic, or symptoms can range from a mild respiratory tract infection to acute respiratory distress and multiorgan failure.
Three strategies can successfully contain the pandemic and its consequences:
- Public health measures, such as masking and social distancing
- Prophylactic vaccines to reduce transmission
- Safe and effective drugs for reducing morbidity and mortality among infected patients.
Optimal treatment strategies for patients in ambulatory and hospital settings continue to evolve as new studies are reported and new strains of the virus arise. Many medical and scientific organizations, including the National Institutes of Health (NIH) COVID-19 treatment panel,1 Infectious Diseases Society of America (IDSA),2 World Health Organization (WHO),3 and Centers for Disease Control and Prevention,4 provide recommendations for managing patients with COVID-19. Their guidance is based on the strongest research available and is updated intermittently; nevertheless, a plethora of new data emerges weekly and controversies surround several treatments.
In this article, we
We encourage clinicians, in planning treatment, to consider:
- The availability of medications (ie, use the COVID-19 Public Therapeutic Locatora)
- The local COVID-19 situation
- Patient factors and preferences
- Evolving evidence regarding new and existing treatments.
Most evidence about the treatment of COVID-19 comes from studies conducted when the Omicron variant of SARS-CoV-2 was not the dominant variant, as it is today in the United States. As such, drugs authorized or approved by the US Food and Drug Administration (FDA) to treat COVID-19 or used off-label for that purpose might not be as efficacious today as they were almost a year ago. Furthermore, many trials of potential therapies against new viral variants are ongoing; if your patient is interested in enrolling in a clinical trial of an investigational COVID-19 treatment, refer them to www.clinicaltrials.gov.
General managementof COVID-19
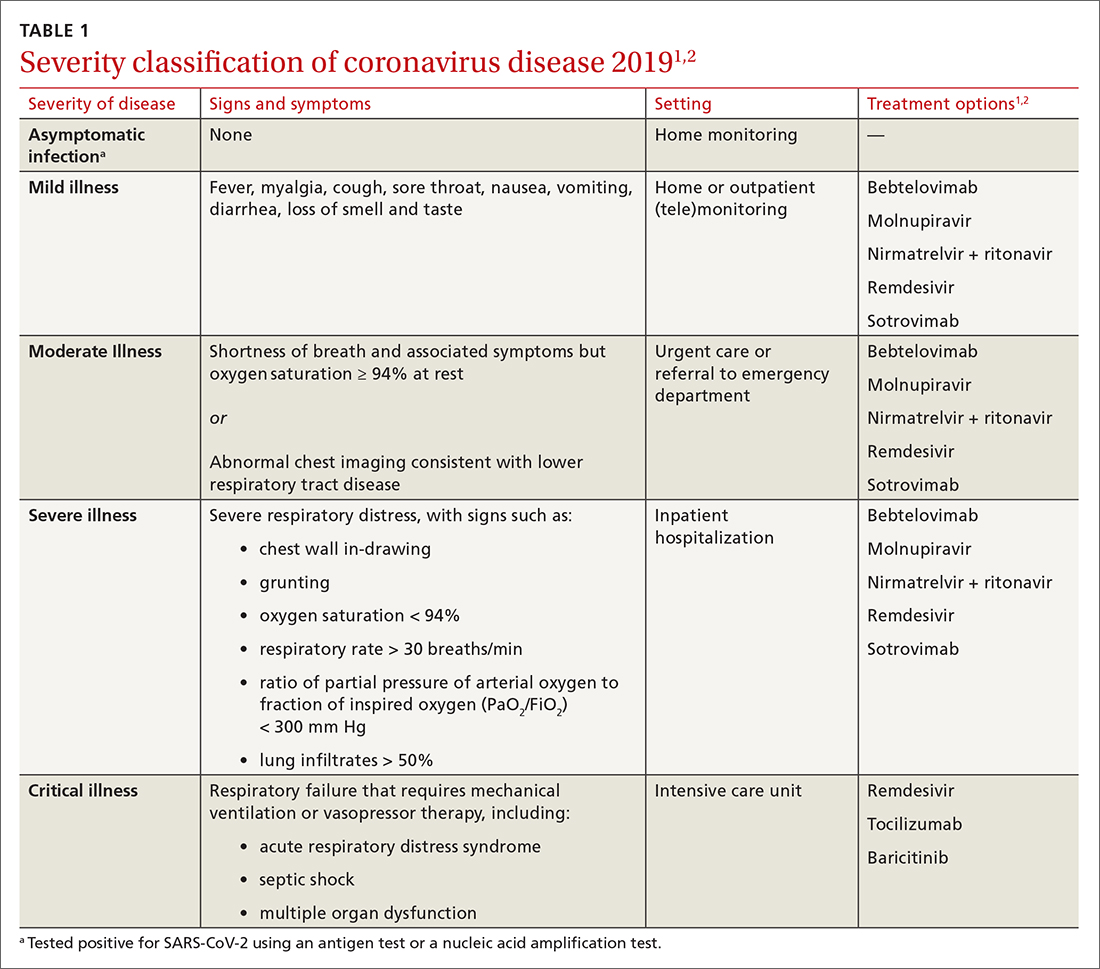
Patients with COVID-19 experience a range of illness severity—from asymptomatic to mild symptoms, such as fever and myalgia, to critical illness requiring intensive care (TABLE 11,2). Patients with COVID-19 should therefore be monitored for progression, remotely or in person, until full recovery is achieved. Key concepts of general management include:
Assess and monitor patients’ oxygenation status by pulse oximetry; identify those with low or declining oxygen saturation before further clinical deterioration.
Continue to: Consider the patient's age and general health
Consider the patient’s age and general health. Patients are at higher risk of severe disease if they are > 65 years or have an underlying comorbidity.4
Emphasize self-isolation and supportive care, including rest, hydration, and over-the-counter medications to relieve cough, reduce fever, and alleviate other symptoms.
Drugs: Few approved, some under study
The antiviral remdesivir is the only drug fully approved for clinical use by the FDA to treat COVID-19 in patients > 12 years.5,6
In addition, the FDA has issued an emergency use authorization (EUA) for several monoclonal antibodies as prophylaxis and treatment: tixagevimab packaged with cilgavimab (Evusheld) is the first antibody combination for pre-exposure prophylaxis (PrEP) against COVID-19; the separately packaged injectables are recommended for patients who have a history of severe allergy that prevents them from being vaccinated or those with moderate or severe immune-compromising disorders.7
In the pipeline. Several treatments are being tested in clinical trials to evaluate their effectiveness and safety in combating COVID-19, including:
- Antivirals, which prevent viruses from multiplying
- Immunomodulators, which reduce the body’s immune reaction to the virus
- Antibody therapies, which are manufactured antibodies against the virus
- Anti-inflammatory drugs, which reduce systemic inflammation and prevent organ dysfunction
- Cell therapies and gene therapies, which alter the expression of cells and genes.
Continue to: Outpatient treatment
Outpatient treatment
Several assessment tools that take into account patients’ age, respiratory status, and comorbidities are available for triage of patients infected with COVID-19.8
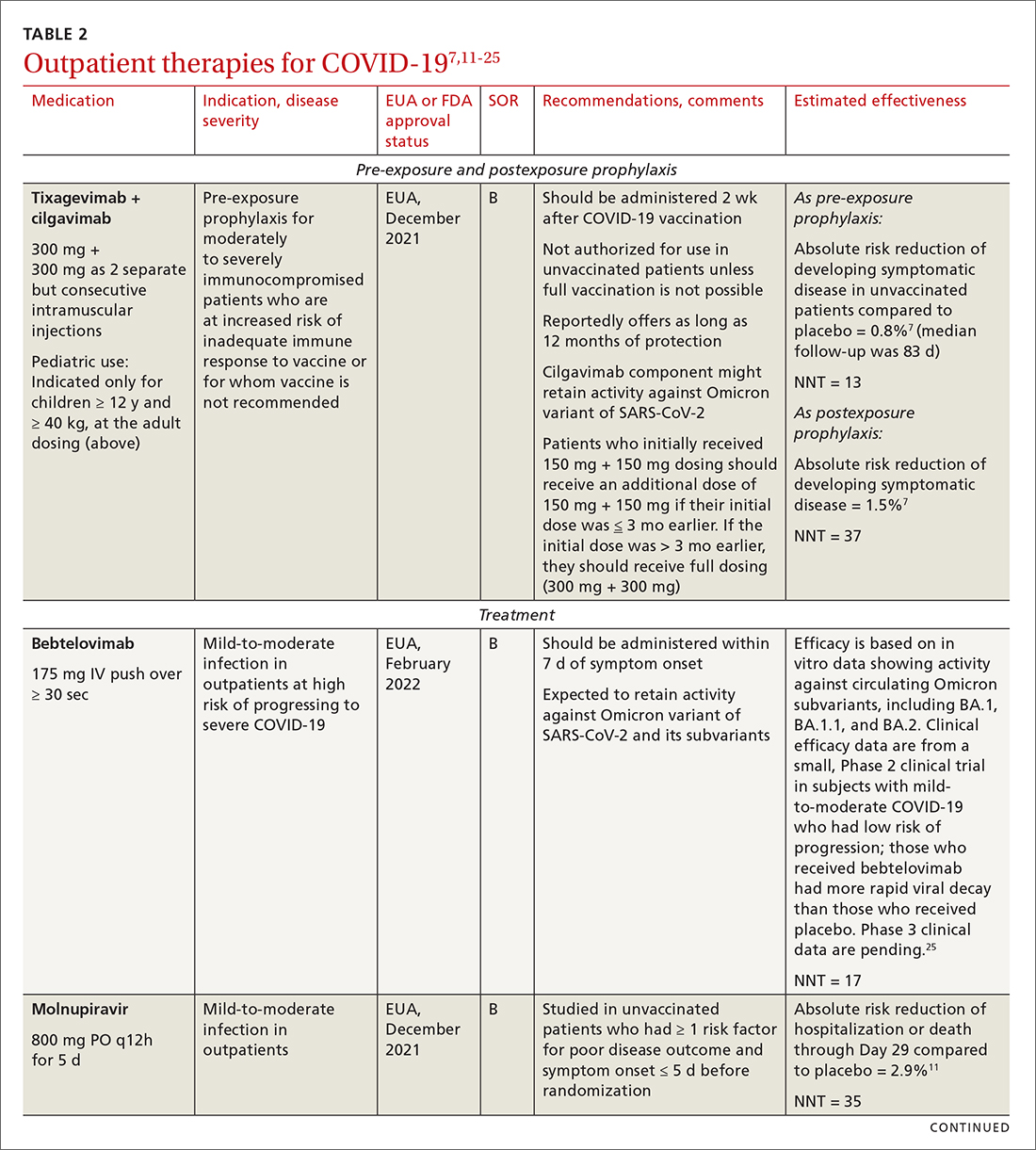
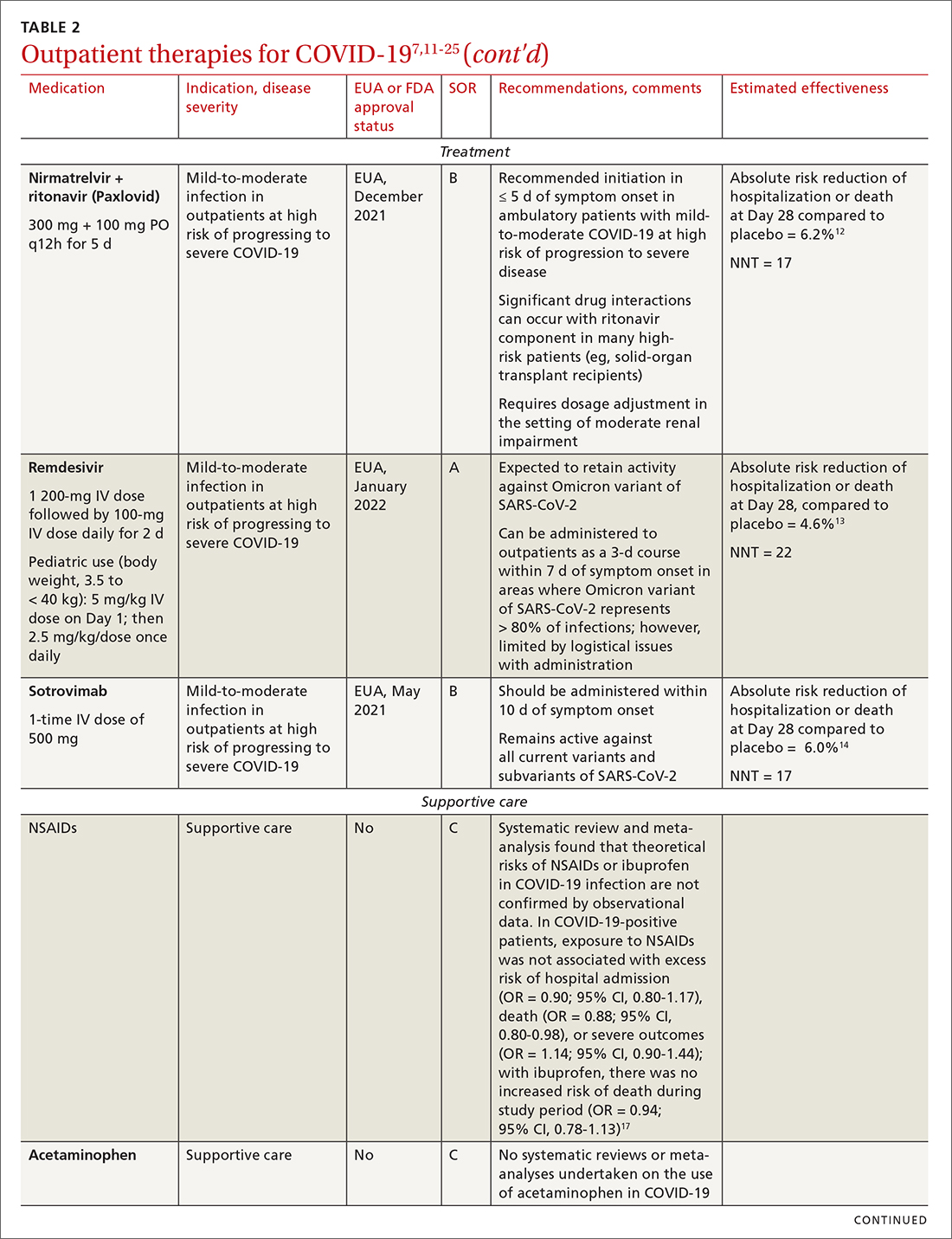
Most (> 80%) patients with COVID-19 have mild infection and are safely managed as outpatients or at home.9,10 For patients at high risk of severe disease, a few options are recommended for patients who do not require hospitalization or supplemental oxygen; guidelines on treatment of COVID-19 in outpatient settings that have been developed by various organizations are summarized in TABLE 2.7,11-25
Antiviral drugs target different stages of the SARS-CoV-2 replication cycle. They should be used early in the course of infection, particularly in patients at high risk of severe disease.
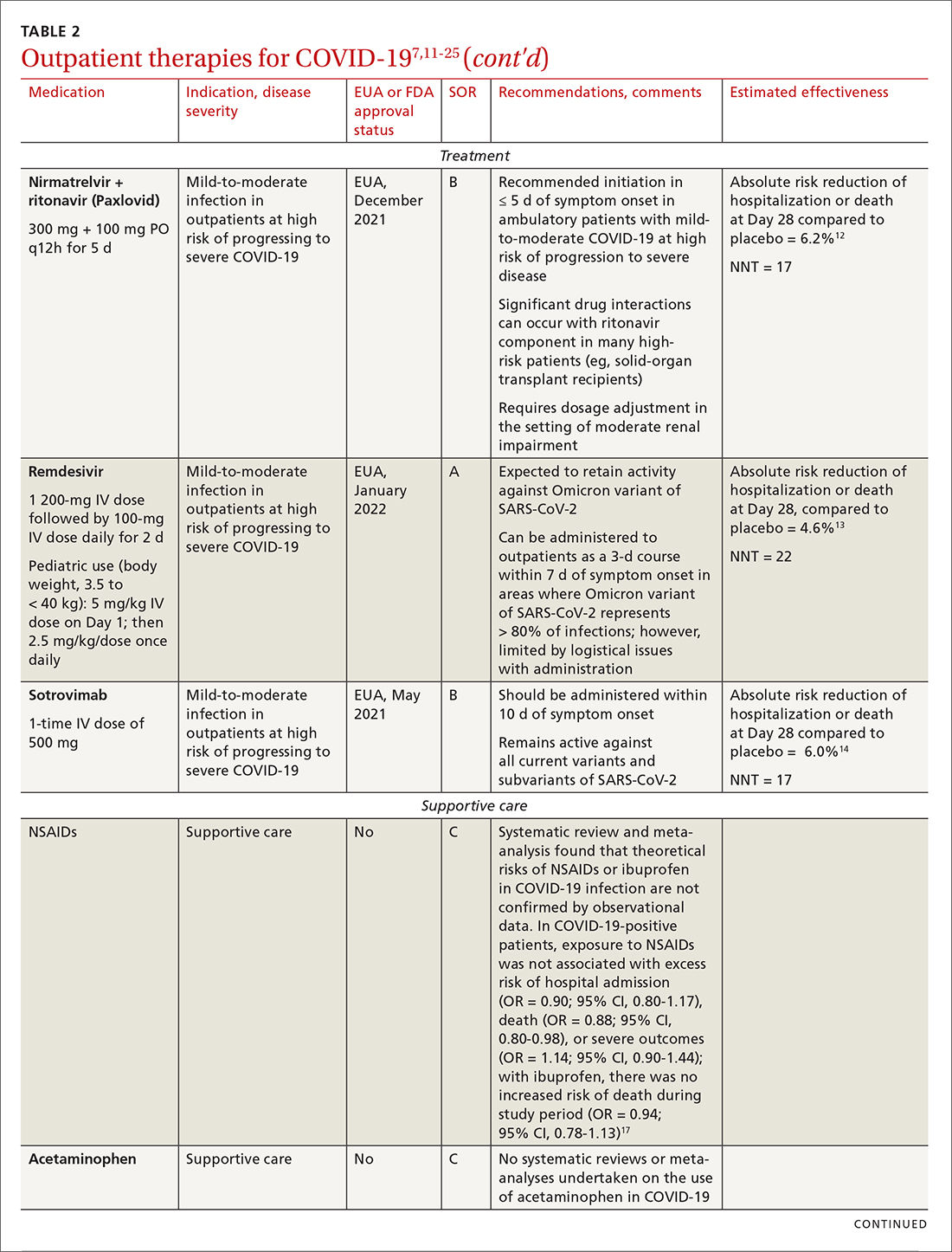
IDSA recommends antiviral therapy with molnupiravir, nirmatrelvir + ritonavir packaged together (Paxlovid), or remdesivir.11,12,26,27 Remdesivir requires intravenous (IV) infusion on 3 consecutive days, which can be difficult in some clinic settings.13,28 Nirmatrelvir + ritonavir should be initiated within 5 days after symptom onset. Overall, for most patients, nirmatrelvir + ritonavir is preferred because of oral dosing and higher efficacy in comparison to other antivirals. With nirmatrelvir + ritonavir, carefully consider drug–drug interactions and the need to adjust dosing in the presence of renal disease.28,29 There are no data on the efficacy of any combination treatments with these agents (other than co-packaged Paxlovid).
Monoclonal antibodies for COVID-19 are given primarily intravenously. They bind to the viral spike protein, thus preventing SARS-CoV-2 from attaching to and entering cells. Bamlanivimab + etesevimab and bebtelovimab are available under an EUA for outpatient treatment.14b Treatment should be initiated as early as possible in the course of infection—ideally, within 7 to 10 days after onset of symptoms.
Continue to: Bebtelovimab was recently given...
Bebtelovimab was recently given an EUA. It is a next-generation antibody that neutralizes all currently known variants and is the most potent monoclonal antibody against the Omicron variant, including its BA.2 subvariant.31 However, data about its activity against the BA.2 subvariant are based on laboratory testing and have not been confirmed in clinical trials. Clinical data were similar for this agent alone and for its use in combination with other monoclonal antibodies, but those trials were conducted before the emergence of Omicron.
In your decision-making about the most appropriate therapy, consider (1) the requirement that monoclonal antibodies be administered parenterally and (2) the susceptibility of the locally predominating viral variant.
Other monoclonal antibody agents are in the investigative pipeline; however, data about them have been largely presented through press releases or selectively reported in applications to the FDA for EUA. For example, preliminary reports show cilgavimab coverage against the Omicron variant14; so far, cilgavimab is not approved for treatment but is used in combination with tixagevimab for PreP—reportedly providing as long as 12 months of protection for patients who are less likely to respond to a vaccine.32
Corticosteroids. Guidelines recommend against dexamethasone and other systemic corticosteroids in outpatient settings. For patients with moderate-to-severe symptoms but for whom hospitalization is not possible (eg, beds are unavailable), the NIH panel recommends dexamethasone, 6 mg/d, for the duration of supplemental oxygen, not to exceed 10 days of treatment.1
Patients who were recently discharged after COVID-19 hospitalization should not continue remdesivir, dexamethasone, or baricitinib at home, even if they still require supplemental oxygen.
Continue to: Some treatments should not be in your COVID-19 toolbox
Some treatments should not be in your COVID-19 toolbox
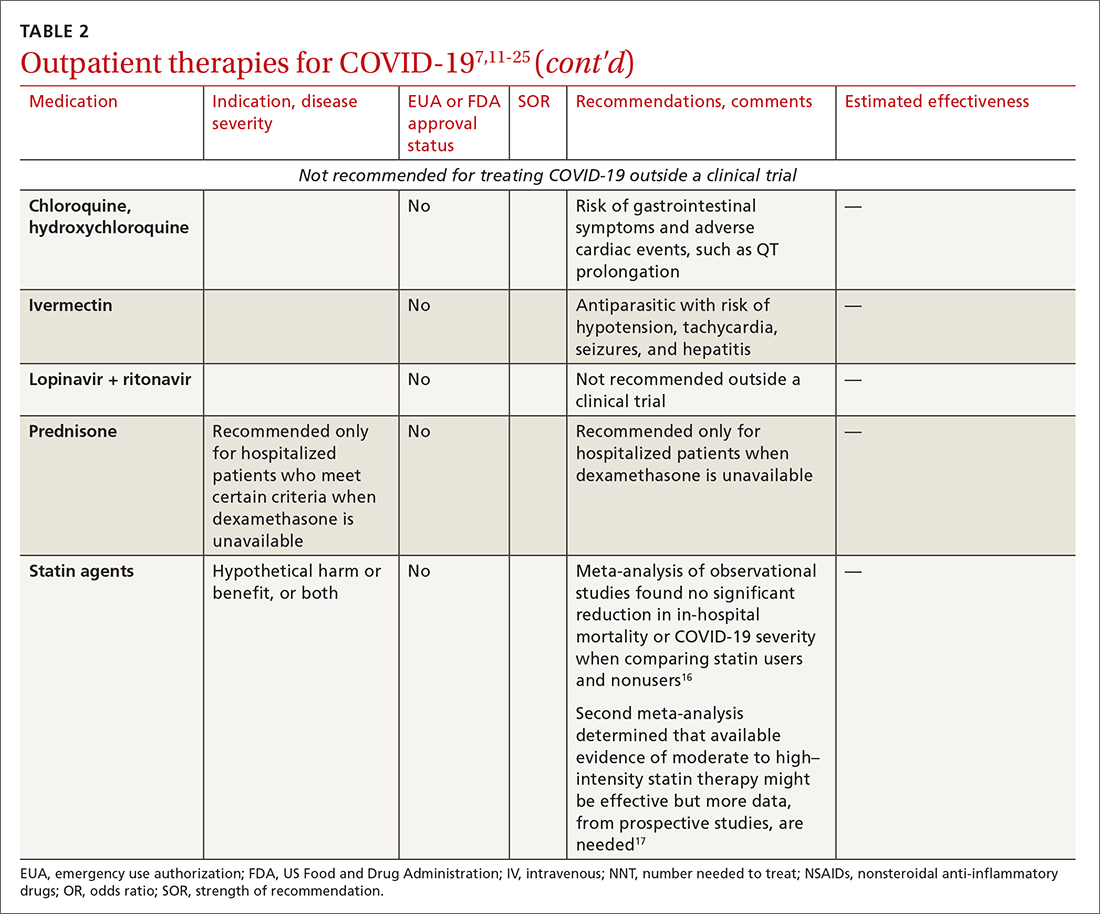
High-quality studies are lacking for several other potential COVID-19 treatments. Some of these drugs are under investigation, with unclear benefit and with the potential risk of toxicity—and therefore should not be prescribed or used outside a clinical trial. See “Treatments not recommended for COVID-19,” page E14. 1-4,15-19,33-41
SIDEBAR
Treatments not recommended for COVID-191-4,15-19,33-41
Fluvoxamine. A few studies suggest that the selective serotonin reuptake inhibitor fluvoxamine reduces progression to severe disease; however, those studies have methodologic challenges.33 The drug is not FDA approved for treating COVID.33
Convalescent plasma, given to high-risk outpatients early in the course of disease, can reduce progression to severe disease,34,35 but it remains investigational for COVID-19 because trials have yielded mixed results.34-36
Ivermectin. The effect of ivermectin in patients with COVID-19 is unclear because high-quality studies do not exist and cases of ivermectin toxicity have occurred with incorrect administration.39
Hydroxychloroquine showed potential in a few observational studies, but randomized clinical trials have not shown any benefit.15
Azithromycin likewise showed potential in a few observational studies; randomized clinical trials have not shown any benefit, however.15
Statins. A few meta-analyses, based on observational studies, reported benefit from statins, but recent studies have shown that this class of drugs does not provide clinical benefit in alleviating COVID-19 symptoms.16,17,37
Inhaled corticosteroids. A systematic review reported no benefit or harm from using an inhaled corticosteroid.18 More recent studies show that the inhaled corticosteroid budesonide used in early COVID-19 might reduce the need for urgent care38 and, in patients who are at higher risk of COVID-19-related complications, shorten time to recovery.19
Vitamins and minerals. Limited observational studies suggest an association between vitamin and mineral deficiency (eg, vitamin C, zinc, and vitamin D) and risk of severe disease, but high-quality data about this finding do not exist.40,41
Casirivimab + imdevimab [REGEN-COV2]. This unapproved investigational combination treatment was granted an EUA in 2020 for postexposure prophylaxis. The EUA was withdrawn in January 2022 because of the limited efficacy of casirivimab + imdevimab against the Omicron variant of SARS-CoV-2.
Postexposure prophylaxis. National guidelines1-4 recommend against postexposure prophylaxis with hydroxychloroquine, colchicine, inhaled corticosteroids, or azithromycin.
TABLE 27,11-25 and TABLE 326,42-46 provide additional information on treatments not recommended outside trials, or not recommended at all, for COVID-19.
Treatment during hospitalization
The NIH COVID-19 treatment panel recommends hospitalization for patients who have any of the following findings1:
- Oxygen saturation < 94% while breathing room air
- Respiratory rate > 30 breaths/min
- A ratio of partial pressure of arterial O2 to fraction of inspired O2 (PaO2/FiO2) < 300 mm Hg
- Lung infiltrates > 50%.
General guidance for the care of hospitalized patients:
- Treatments that target the virus have the greatest efficacy when given early in the course of disease.
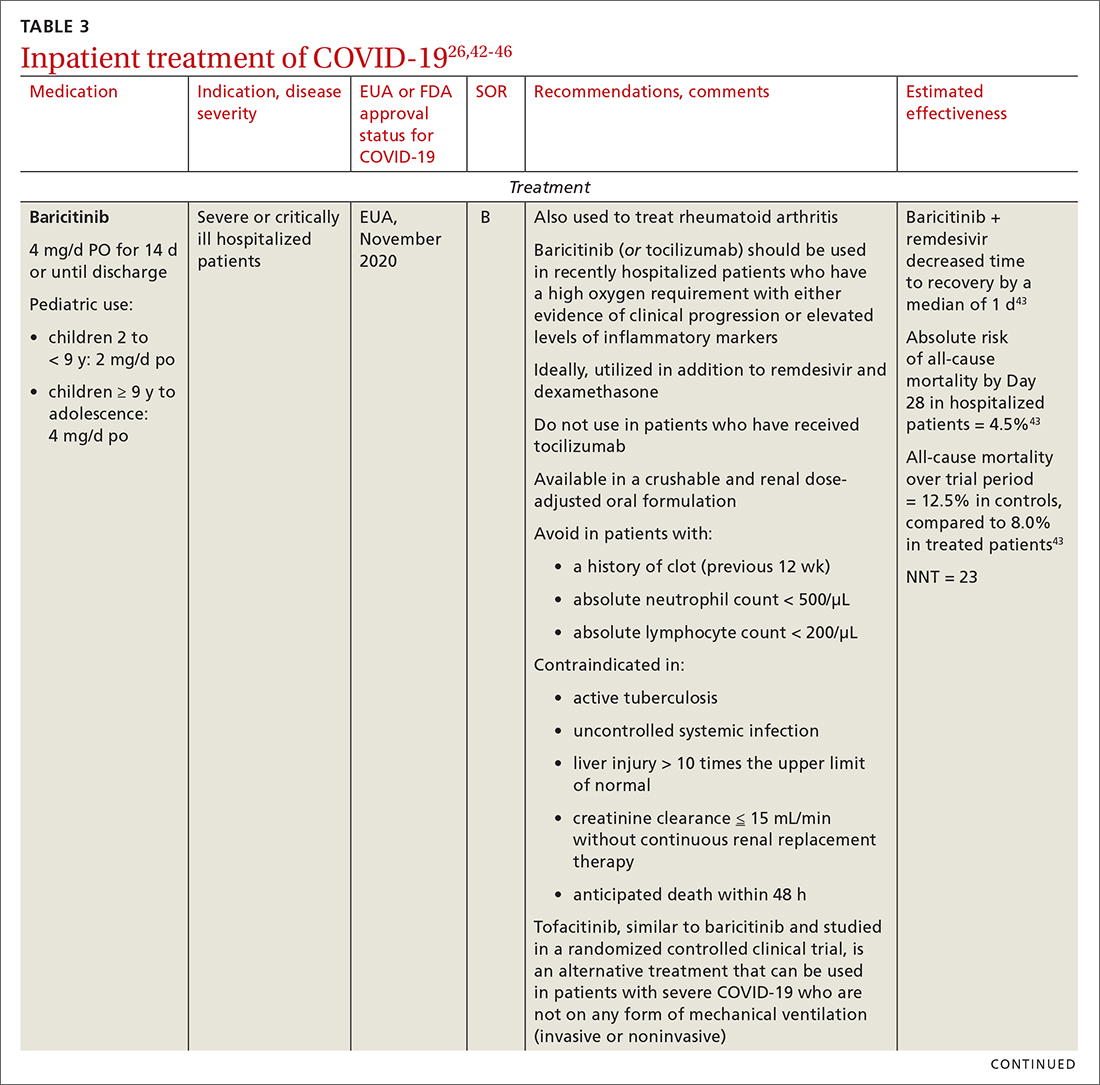
- Anti-inflammatory and immunosuppressive agents help prevent tissue damage from a dysregulated immune system. (See TABLE 326,42-46)
- The NIH panel,1 IDSA,2 and WHO3 recommend against dexamethasone and other corticosteroids for hospitalized patients who do not require supplemental oxygen.
- Prone positioning distributes oxygen more evenly in the lungs and improves overall oxygenation, thus reducing the need for mechanical ventilation.
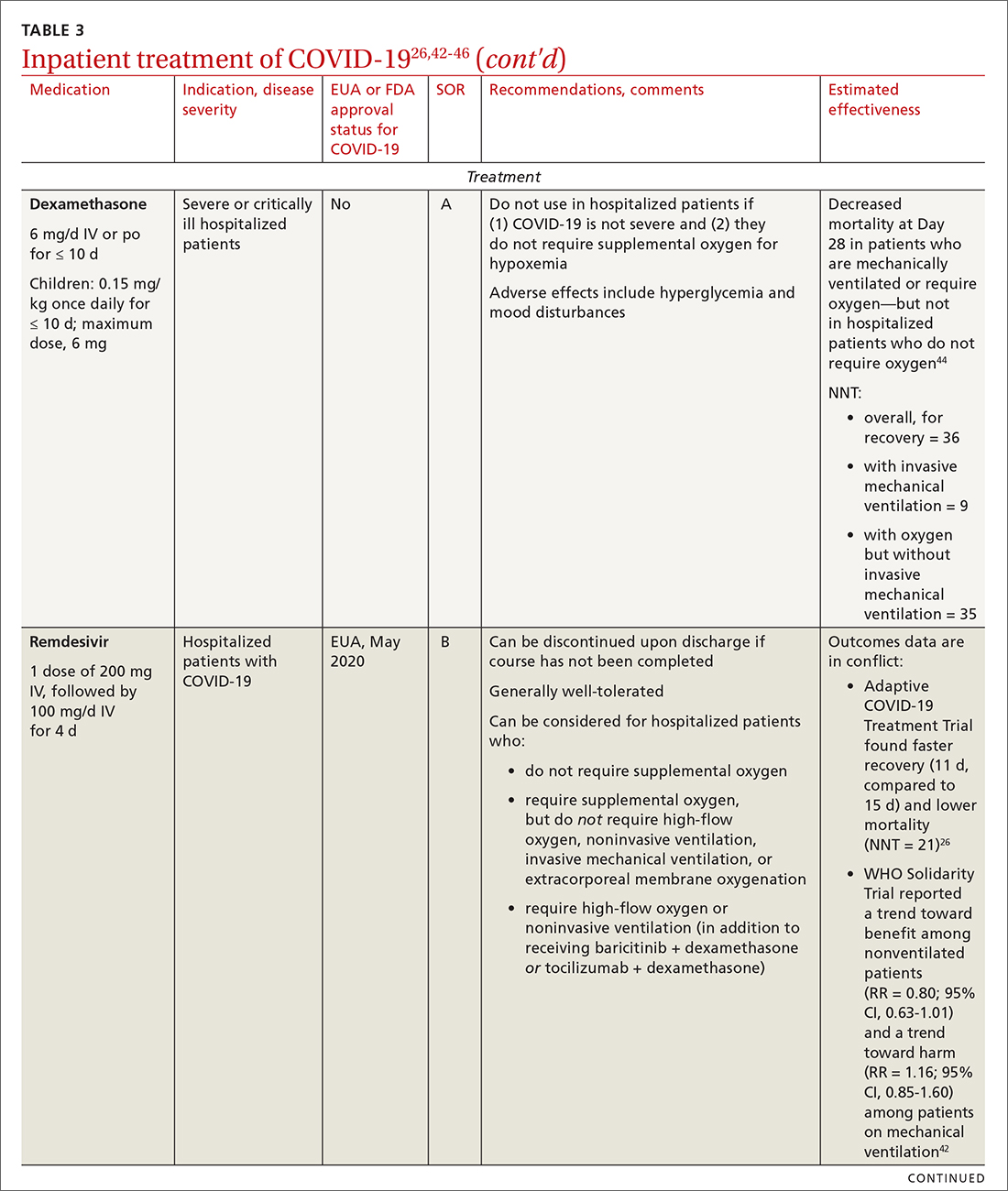
Remdesivir. Once a hospitalized patient does require supplemental oxygen, the NIH panel,1 IDSA,2 and WHO3 recommend remdesivir; however, remdesivir is not recommended in many other countries because WHO has noted its limited efficacy.42 Dexamethasone is recommended alone, or in combination with remdesivir for patients who require increasing supplemental oxygen and those on mechanical ventilation.
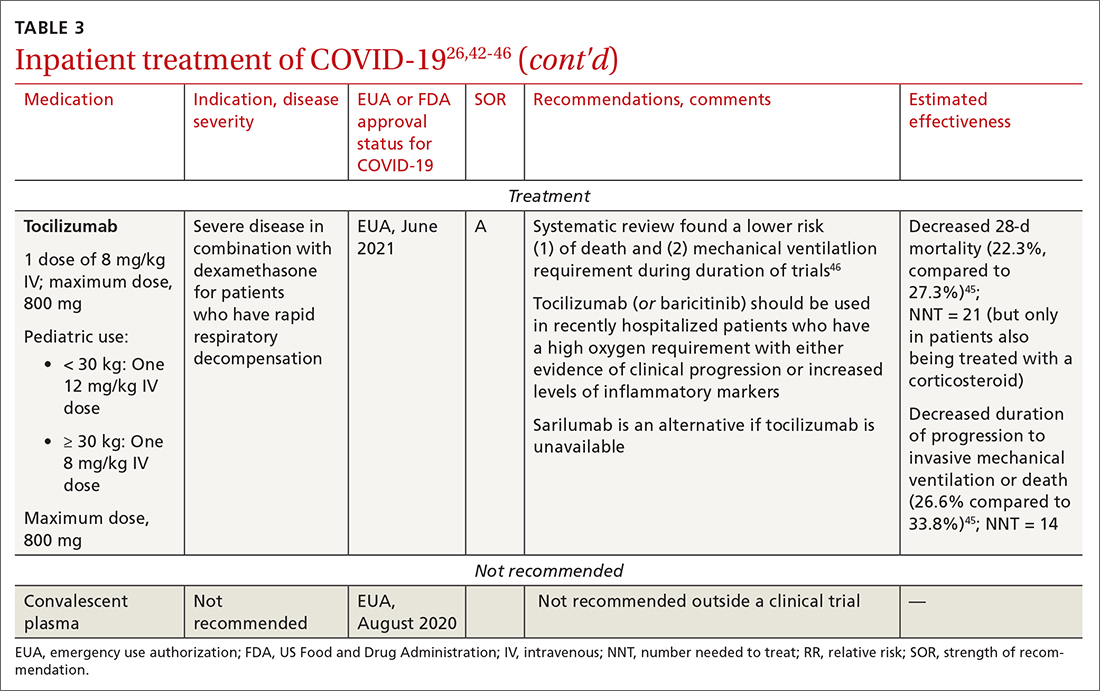
Baricitinib. For patients with rapidly increasing oxygen requirements, invasive mechanical ventilation, and systemic inflammation, baricitinib, a Janus kinase inhibitor, can be administered, in addition to dexamethasone, with or without remdesivir.47
Continue to: Tocilizumab
Tocilizumab. A monoclonal antibody and interleukin (IL)-6 inhibitor, tocilizumab is also recommended in addition to dexamethasone, with or without remdesivir.48 Tocilizumab should be given only in combination with dexamethasone.49 Patients should receive baricitinib or tocilizumab—not both. IDSA recommends tofacitinib, with a prophylactic dose of an anticoagulant, for patients who are hospitalized with severe COVID-19 but who are not on any form of ventilation.50
Care of special populations
Special patient populations often seek primary care. Although many questions remain regarding the appropriate care of these populations, it is useful to summarize existing evidence and recommendations from current guidelines.
Children. COVID-19 is generally milder in children than in adults; many infected children are asymptomatic. However, infants and children who have an underlying medical condition are at risk of severe disease, including multisystem inflammatory syndrome.51
The NIH panel recommends supportive care alone for most children with mild-to-moderate disease.1 Remdesivir is recommended for hospitalized children ≥ 12 years who weigh ≥ 40 kg, have risk factors for severe disease, and have an emergent or increasing need for supplemental oxygen. Dexamethasone is recommended for hospitalized children requiring high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation. Molnupiravir is not authorized for patients < 18 years because it can impede bone and cartilage growth.
There is insufficient evidence for or against the use of monoclonal antibody products for children with COVID-19 in an ambulatory setting. For hospitalized children, there is insufficient evidence for or against use of baricitinib and tocilizumab.
Continue to: Patients who are pregnant
Patients who are pregnant are at increased risk of severe COVID-19.52,53 The NIH states that, in general, treatment and vaccination of pregnant patients with COVID-19 should be the same as for nonpregnant patients.1
Pregnant subjects were excluded from several trials of COVID-19 treatments.54 Because Janus kinase inhibitors, such as baricitinib, are associated with an increased risk of thromboembolism, they are not recommended in pregnant patients who are already at risk of thromboembolic complications. Molnupiravir is not recommended for pregnant patients because of its potential for teratogenic effects.
The Society for Maternal-Fetal Medicine states that there are no absolute contraindications to the use of monoclonal antibodies in appropriate pregnant patients with COVID-19.55 Remdesivir has no known fetal toxicity and is recommended as a treatment that can be offered to pregnant patients. Dexamethasone can also be administered to pregnant patients who require oxygen; however, if dexamethasone is also being used to accelerate fetal lung maturity, more frequent initial dosing is needed.
Older people. COVID-19 treatments for older patients are the same as for the general adult population. However, because older people are more likely to have impaired renal function, renal function should be monitored when an older patient is being treated with COVID-19 medications that are eliminated renally (eg, remdesivir, baricitinib). Furthermore, drug–drug interactions have been reported in older patients treated with nirmatrelvir + ritonavir, primarily because of the effects of ritonavir. Review all of a patient’s medications, including over-the-counter drugs and herbal supplements, when prescribing treatment for COVID-19, and adjust the dosage by following guidance in FDA-approved prescribing information—ideally, in consultation with a pharmacist.
Immunocompromised patients. The combination product tixagevimab + cilgavimab [Evusheld] is FDA approved for COVID-19 PrEP, under an EUA, in patients who are not infected with SARS-CoV-2 who have an immune-compromising condition, who are unlikely to mount an adequate immune response to the COVID-19 vaccine, or those in whom vaccination is not recommended because of their history of a severe adverse reaction to a COVID-19 vaccine or one of its components.7
Continue to: Summing up
Summing up
With a growing need for effective and readily available COVID-19 treatments, there are an unprecedented number of clinical trials in process. Besides antivirals, immunomodulators, and antibody therapies, some novel mechanisms being tested include Janus kinase inhibitors, IL-6-receptor blockers, and drugs that target adult respiratory distress syndrome and cytokine release.
Once larger trials are completed, we can expect stronger evidence of potential treatment options and of safety and efficacy in children, pregnant women, and vulnerable populations. During the pandemic, the FDA’s EUA program has brought emerging treatments rapidly to clinicians; nevertheless, high-quality evidence, with thorough peer review, remains critical to inform COVID-19 treatment guidelines.
ahttps://healthdata.gov/Health/COVID-19-PublicTherapeutic-Locator/rxn6-qnx8/data
b Sotrovimab was effective against the Omicron variant of SARS-CoV-2—the dominant variant in early 2022— but is currently not FDA authorized in any region of the United States because of the prevalence of the Omicron BA.2 subvariant.30
CORRESPONDENCE
Ambar Kulshreshtha, MD, PhD, Department of Epidemiology, Emory Rollins School of Public Health, 4500 North Shallowford Road, Suite 134, Atlanta, GA 30338; akulshr@emory.edu
1. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. July 19, 2022. Accessed July 21, 2022. www.covid19treatmentguidelines.nih.gov
2. IDSA guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America. Updated June 29, 2022. Accessed July 21, 2022. www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-23
3. Therapeutics and COVID-19: living guideline. World Health Organization. July 14, 2022. Accessed July 21, 2022. https://apps.who.int/iris/rest/bitstreams/1449398/retrieve
4. Centers for Disease Control and Prevention. Clinical care considerations. Updated May 27, 2022. Accessed July 21, 2022. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
5. Coronavirus (COVID-19) update: FDA approves first COVID-19 treatment for young children. Press release. US Food and Drug Administration. April 25, 2022. Accessed August 11, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-approves-first-covid-19-treatment-young-children
6. Know your treatment options for COVID-19. US Food and Drug Administration. Updated August 15, 2022. Accessed July 21, 2022. www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19
7. Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA. 2022;327:384-385. doi: 10.1001/jama.2021.24931
8. Judson TJ, Odisho AY, Neinstein AB, et al. Rapid design and implementation of an integrated patient self-triage and self-scheduling tool for COVID-19. J Am Med Inform Assoc. 2020;27:860-866. doi: 10.1093/jamia/ocaa051
9. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757-1766. doi: 10.1056/NEJMcp2009249
10. Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. BMJ. 2020;368:m1182. doi: 10.1136/bmj.m1182
11. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al; . Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509-520. doi: 10.1056/NEJMoa2116044
12. Hammond J, Leister-Tebbe H, Gardner A, et al; . Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397-1408. doi: 10.1056/NEJMoa2118542
13. Gottlieb RL, Vaca CE, Paredes R, et al; . Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305-315. doi: 10.1056/NEJMoa2116846
14. Gupta A, Gonzalez-Rojas Y, Juarez E, et al; . Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941-1950. doi: 10.1056/NEJMoa2107934
15. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623-631. doi: 10.7326/M20-4207
16. Scheen AJ. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2021;47:101220. doi: 10.1016/j.diabet.2020.101220
17. Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153-155. doi: 10.1016/j.amjcard.2020.08.004
18. Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55:2001009. doi: 10.1183/13993003.01009-2020
19. Yu L-M, Bafadhel M, Dorward J, et al; . Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843-855. doi: 10.1016/S0140-6736(21)01744-X
20. Siemieniuk RA, Bartoszko JJ, JP, et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. doi: 10.1136/bmj.n2231
21. Siemieniuk RA, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980
22. Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379
23. Goldstein KM, Ghadimi K, Mystakelis H, et al. Risk of transmitting coronavirus disease 2019 during nebulizer treatment: a systematic review. J Aerosol Med Pulm Drug Deliv. 2021;34:155-170. doi: 10.1089/jamp.2020.1659
24. Schultze A, Walker AJ, MacKenna B, et al; . Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106-1120. doi: 10.1016/S2213-2600(20)30415-X
25. What are the safety and efficacy results of bebtelovimab from BLAZE-4? Lilly USA. January 12, 2022. Accessed August 17, 2022. www.lillymedical.com/en-us/answers/what-are-the-safety-and-efficacy-results-of-bebtelovimab-from-blaze-4-159290
26. Beigel JH, Tomashek KM, Dodd LE, et al; . Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi: 10.1056/NEJMoa2007764
27. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787-1799. doi: 10.1056/NEJMoa2001282
28. Gandhi RT, Malani PN, Del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA. 2022;327:617-618. doi: 10.1001/jama.2022.0335
29. Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54:516-523. doi: 10.1080/07853890.2022.2034936
30. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022:602:671-675. doi: 10.1038/s41586-021-04389-z
31. Emergency use authorization (EUA) for bebtelovimab (LY-CoV1404): Center for Drug Evaluation and Research (CDER) review. US Food and Drug Administration. Updated February 11, 2022. Accessed July 21, 2022. www.fda.gov/media/156396/download
32. Bartoszko JJ, Siemieniuk RAC, Kum E, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373:n949. doi: 10.1136/bmj.n949
33. Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al; TOGETHER Investigators. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42-e51. doi: 10.1016/S2214-109X(21)00448-4
34. RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049-2059. doi: 10.1016/S0140-6736(21)00897-7
35. Simonovich VA, Burgos Pratx LD, Scibona P, et al; . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619-629. doi: 10.1056/NEJMoa2031304
36. Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384:1015-1027. doi: 10.1056/NEJMoa2031893
37. Ayeh SK, Abbey EJ, Khalifa BAA, et al. Statins use and COVID-19 outcomes in hospitalized patients. PLoS One. 2021;16:e0256899. doi: 10.1371/journal.pone.0256899
38. Ramakrishnan S, Nicolau DV Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763-772. doi: 10.1016/S2213-2600(21)00160-0
39. Temple C, Hoang R, Hendrickson RG. Toxic effects from ivermectin use associated with prevention and treatment of Covid-19. N Engl J Med. 2021;385:2197-2198. doi: 10.1056/NEJMc2114907
40. Thomas S, Patel D, Bittel B, et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4:e210369. doi: 10.1001/jamanetworkopen.2021.0369
41. Adams KK, Baker WL, Sobieraj DM. Myth busters: dietary supplements and COVID-19. Ann Pharmacother. 2020;54:820-826. doi: 10.1177/1060028020928052
42. Pan H, Peto R, Henao-Restrepo A-M, et al. Repurposed antiviral drugs for Covid-19—interim WHO Solidarity trial results. N Engl J Med. 2021;384:497-511. doi: 10.1056/NEJMoa2023184
43. Kalil AC, Patterson TF, Mehta AK, et al; . Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795-807. doi: 10.1056/NEJMoa2031994
44. ; Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330-1341. doi: 10.1001/jama.2020.17023
45. ; Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499-518. doi: 10.1001/jama.2021.11330
46. Wei QW, Lin H, Wei R-G, et al. Tocilizumab treatment for COVID-19 patients: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10:71. doi: 10.1186/s40249-021-00857-w
47. Zhang X, Shang L, Fan G, et al. The efficacy and safety of Janus kinase inhibitors for patients with COVID-19: a living systematic review and meta-analysis. Front Med (Lausanne). 2021;8:800492. doi: 10.3389/fmed.2021.800492
48. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637-1645. doi: 10.1016/S0140-6736(21)00676-0
49. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491-1502. doi: 10.1056/NEJMoa2100433
50. Guimaraes PO, Quirk D, Furtado RH, et al; . Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406-415. doi: 10.1056/NEJMoa2101643
51. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-346. doi: 10.1056/NEJMoa2021680
52. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320
53. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817-826. doi: 10.1001/jamapediatrics.2021.1050
54. Jorgensen SCJ, Davis MR, Lapinsky SE. A review of remdesivir for COVID-19 in pregnancy and lactation. J Antimicrob Chemother. 2021;77:24-30. doi: 10.1093/jac/dkab311
55. Management considerations for pregnant patients with COVID-19. Society for Maternal-Fetal Medicine. Accessed July 21, 2022. https://s3.amazonaws.com/cdn.smfm.org/media/2336/SMFM_COVID_Management_of_COVID_pos_preg_patients_4-30-20_final.pdf
The ongoing COVID-19 pandemic has caused more than 1 million deaths in the United States and continues to be a major public health challenge. Cases can be asymptomatic, or symptoms can range from a mild respiratory tract infection to acute respiratory distress and multiorgan failure.
Three strategies can successfully contain the pandemic and its consequences:
- Public health measures, such as masking and social distancing
- Prophylactic vaccines to reduce transmission
- Safe and effective drugs for reducing morbidity and mortality among infected patients.
Optimal treatment strategies for patients in ambulatory and hospital settings continue to evolve as new studies are reported and new strains of the virus arise. Many medical and scientific organizations, including the National Institutes of Health (NIH) COVID-19 treatment panel,1 Infectious Diseases Society of America (IDSA),2 World Health Organization (WHO),3 and Centers for Disease Control and Prevention,4 provide recommendations for managing patients with COVID-19. Their guidance is based on the strongest research available and is updated intermittently; nevertheless, a plethora of new data emerges weekly and controversies surround several treatments.
In this article, we
We encourage clinicians, in planning treatment, to consider:
- The availability of medications (ie, use the COVID-19 Public Therapeutic Locatora)
- The local COVID-19 situation
- Patient factors and preferences
- Evolving evidence regarding new and existing treatments.
Most evidence about the treatment of COVID-19 comes from studies conducted when the Omicron variant of SARS-CoV-2 was not the dominant variant, as it is today in the United States. As such, drugs authorized or approved by the US Food and Drug Administration (FDA) to treat COVID-19 or used off-label for that purpose might not be as efficacious today as they were almost a year ago. Furthermore, many trials of potential therapies against new viral variants are ongoing; if your patient is interested in enrolling in a clinical trial of an investigational COVID-19 treatment, refer them to www.clinicaltrials.gov.
General managementof COVID-19
Patients with COVID-19 experience a range of illness severity—from asymptomatic to mild symptoms, such as fever and myalgia, to critical illness requiring intensive care (TABLE 11,2). Patients with COVID-19 should therefore be monitored for progression, remotely or in person, until full recovery is achieved. Key concepts of general management include:
Assess and monitor patients’ oxygenation status by pulse oximetry; identify those with low or declining oxygen saturation before further clinical deterioration.

Continue to: Consider the patient's age and general health
Consider the patient’s age and general health. Patients are at higher risk of severe disease if they are > 65 years or have an underlying comorbidity.4
Emphasize self-isolation and supportive care, including rest, hydration, and over-the-counter medications to relieve cough, reduce fever, and alleviate other symptoms.
Drugs: Few approved, some under study
The antiviral remdesivir is the only drug fully approved for clinical use by the FDA to treat COVID-19 in patients > 12 years.5,6
In addition, the FDA has issued an emergency use authorization (EUA) for several monoclonal antibodies as prophylaxis and treatment: tixagevimab packaged with cilgavimab (Evusheld) is the first antibody combination for pre-exposure prophylaxis (PrEP) against COVID-19; the separately packaged injectables are recommended for patients who have a history of severe allergy that prevents them from being vaccinated or those with moderate or severe immune-compromising disorders.7
In the pipeline. Several treatments are being tested in clinical trials to evaluate their effectiveness and safety in combating COVID-19, including:
- Antivirals, which prevent viruses from multiplying
- Immunomodulators, which reduce the body’s immune reaction to the virus
- Antibody therapies, which are manufactured antibodies against the virus
- Anti-inflammatory drugs, which reduce systemic inflammation and prevent organ dysfunction
- Cell therapies and gene therapies, which alter the expression of cells and genes.
Continue to: Outpatient treatment
Outpatient treatment
Several assessment tools that take into account patients’ age, respiratory status, and comorbidities are available for triage of patients infected with COVID-19.8
Most (> 80%) patients with COVID-19 have mild infection and are safely managed as outpatients or at home.9,10 For patients at high risk of severe disease, a few options are recommended for patients who do not require hospitalization or supplemental oxygen; guidelines on treatment of COVID-19 in outpatient settings that have been developed by various organizations are summarized in TABLE 2.7,11-25

Antiviral drugs target different stages of the SARS-CoV-2 replication cycle. They should be used early in the course of infection, particularly in patients at high risk of severe disease.

IDSA recommends antiviral therapy with molnupiravir, nirmatrelvir + ritonavir packaged together (Paxlovid), or remdesivir.11,12,26,27 Remdesivir requires intravenous (IV) infusion on 3 consecutive days, which can be difficult in some clinic settings.13,28 Nirmatrelvir + ritonavir should be initiated within 5 days after symptom onset. Overall, for most patients, nirmatrelvir + ritonavir is preferred because of oral dosing and higher efficacy in comparison to other antivirals. With nirmatrelvir + ritonavir, carefully consider drug–drug interactions and the need to adjust dosing in the presence of renal disease.28,29 There are no data on the efficacy of any combination treatments with these agents (other than co-packaged Paxlovid).

Monoclonal antibodies for COVID-19 are given primarily intravenously. They bind to the viral spike protein, thus preventing SARS-CoV-2 from attaching to and entering cells. Bamlanivimab + etesevimab and bebtelovimab are available under an EUA for outpatient treatment.14b Treatment should be initiated as early as possible in the course of infection—ideally, within 7 to 10 days after onset of symptoms.

Continue to: Bebtelovimab was recently given...
Bebtelovimab was recently given an EUA. It is a next-generation antibody that neutralizes all currently known variants and is the most potent monoclonal antibody against the Omicron variant, including its BA.2 subvariant.31 However, data about its activity against the BA.2 subvariant are based on laboratory testing and have not been confirmed in clinical trials. Clinical data were similar for this agent alone and for its use in combination with other monoclonal antibodies, but those trials were conducted before the emergence of Omicron.
In your decision-making about the most appropriate therapy, consider (1) the requirement that monoclonal antibodies be administered parenterally and (2) the susceptibility of the locally predominating viral variant.
Other monoclonal antibody agents are in the investigative pipeline; however, data about them have been largely presented through press releases or selectively reported in applications to the FDA for EUA. For example, preliminary reports show cilgavimab coverage against the Omicron variant14; so far, cilgavimab is not approved for treatment but is used in combination with tixagevimab for PreP—reportedly providing as long as 12 months of protection for patients who are less likely to respond to a vaccine.32
Corticosteroids. Guidelines recommend against dexamethasone and other systemic corticosteroids in outpatient settings. For patients with moderate-to-severe symptoms but for whom hospitalization is not possible (eg, beds are unavailable), the NIH panel recommends dexamethasone, 6 mg/d, for the duration of supplemental oxygen, not to exceed 10 days of treatment.1
Patients who were recently discharged after COVID-19 hospitalization should not continue remdesivir, dexamethasone, or baricitinib at home, even if they still require supplemental oxygen.
Continue to: Some treatments should not be in your COVID-19 toolbox
Some treatments should not be in your COVID-19 toolbox
High-quality studies are lacking for several other potential COVID-19 treatments. Some of these drugs are under investigation, with unclear benefit and with the potential risk of toxicity—and therefore should not be prescribed or used outside a clinical trial. See “Treatments not recommended for COVID-19,” page E14. 1-4,15-19,33-41
SIDEBAR
Treatments not recommended for COVID-191-4,15-19,33-41
Fluvoxamine. A few studies suggest that the selective serotonin reuptake inhibitor fluvoxamine reduces progression to severe disease; however, those studies have methodologic challenges.33 The drug is not FDA approved for treating COVID.33
Convalescent plasma, given to high-risk outpatients early in the course of disease, can reduce progression to severe disease,34,35 but it remains investigational for COVID-19 because trials have yielded mixed results.34-36
Ivermectin. The effect of ivermectin in patients with COVID-19 is unclear because high-quality studies do not exist and cases of ivermectin toxicity have occurred with incorrect administration.39
Hydroxychloroquine showed potential in a few observational studies, but randomized clinical trials have not shown any benefit.15
Azithromycin likewise showed potential in a few observational studies; randomized clinical trials have not shown any benefit, however.15
Statins. A few meta-analyses, based on observational studies, reported benefit from statins, but recent studies have shown that this class of drugs does not provide clinical benefit in alleviating COVID-19 symptoms.16,17,37
Inhaled corticosteroids. A systematic review reported no benefit or harm from using an inhaled corticosteroid.18 More recent studies show that the inhaled corticosteroid budesonide used in early COVID-19 might reduce the need for urgent care38 and, in patients who are at higher risk of COVID-19-related complications, shorten time to recovery.19
Vitamins and minerals. Limited observational studies suggest an association between vitamin and mineral deficiency (eg, vitamin C, zinc, and vitamin D) and risk of severe disease, but high-quality data about this finding do not exist.40,41
Casirivimab + imdevimab [REGEN-COV2]. This unapproved investigational combination treatment was granted an EUA in 2020 for postexposure prophylaxis. The EUA was withdrawn in January 2022 because of the limited efficacy of casirivimab + imdevimab against the Omicron variant of SARS-CoV-2.
Postexposure prophylaxis. National guidelines1-4 recommend against postexposure prophylaxis with hydroxychloroquine, colchicine, inhaled corticosteroids, or azithromycin.
TABLE 27,11-25 and TABLE 326,42-46 provide additional information on treatments not recommended outside trials, or not recommended at all, for COVID-19.
Treatment during hospitalization
The NIH COVID-19 treatment panel recommends hospitalization for patients who have any of the following findings1:
- Oxygen saturation < 94% while breathing room air
- Respiratory rate > 30 breaths/min
- A ratio of partial pressure of arterial O2 to fraction of inspired O2 (PaO2/FiO2) < 300 mm Hg
- Lung infiltrates > 50%.

General guidance for the care of hospitalized patients:
- Treatments that target the virus have the greatest efficacy when given early in the course of disease.
- Anti-inflammatory and immunosuppressive agents help prevent tissue damage from a dysregulated immune system. (See TABLE 326,42-46)
- The NIH panel,1 IDSA,2 and WHO3 recommend against dexamethasone and other corticosteroids for hospitalized patients who do not require supplemental oxygen.
- Prone positioning distributes oxygen more evenly in the lungs and improves overall oxygenation, thus reducing the need for mechanical ventilation.

Remdesivir. Once a hospitalized patient does require supplemental oxygen, the NIH panel,1 IDSA,2 and WHO3 recommend remdesivir; however, remdesivir is not recommended in many other countries because WHO has noted its limited efficacy.42 Dexamethasone is recommended alone, or in combination with remdesivir for patients who require increasing supplemental oxygen and those on mechanical ventilation.

Baricitinib. For patients with rapidly increasing oxygen requirements, invasive mechanical ventilation, and systemic inflammation, baricitinib, a Janus kinase inhibitor, can be administered, in addition to dexamethasone, with or without remdesivir.47
Continue to: Tocilizumab
Tocilizumab. A monoclonal antibody and interleukin (IL)-6 inhibitor, tocilizumab is also recommended in addition to dexamethasone, with or without remdesivir.48 Tocilizumab should be given only in combination with dexamethasone.49 Patients should receive baricitinib or tocilizumab—not both. IDSA recommends tofacitinib, with a prophylactic dose of an anticoagulant, for patients who are hospitalized with severe COVID-19 but who are not on any form of ventilation.50
Care of special populations
Special patient populations often seek primary care. Although many questions remain regarding the appropriate care of these populations, it is useful to summarize existing evidence and recommendations from current guidelines.
Children. COVID-19 is generally milder in children than in adults; many infected children are asymptomatic. However, infants and children who have an underlying medical condition are at risk of severe disease, including multisystem inflammatory syndrome.51
The NIH panel recommends supportive care alone for most children with mild-to-moderate disease.1 Remdesivir is recommended for hospitalized children ≥ 12 years who weigh ≥ 40 kg, have risk factors for severe disease, and have an emergent or increasing need for supplemental oxygen. Dexamethasone is recommended for hospitalized children requiring high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation. Molnupiravir is not authorized for patients < 18 years because it can impede bone and cartilage growth.
There is insufficient evidence for or against the use of monoclonal antibody products for children with COVID-19 in an ambulatory setting. For hospitalized children, there is insufficient evidence for or against use of baricitinib and tocilizumab.
Continue to: Patients who are pregnant
Patients who are pregnant are at increased risk of severe COVID-19.52,53 The NIH states that, in general, treatment and vaccination of pregnant patients with COVID-19 should be the same as for nonpregnant patients.1
Pregnant subjects were excluded from several trials of COVID-19 treatments.54 Because Janus kinase inhibitors, such as baricitinib, are associated with an increased risk of thromboembolism, they are not recommended in pregnant patients who are already at risk of thromboembolic complications. Molnupiravir is not recommended for pregnant patients because of its potential for teratogenic effects.
The Society for Maternal-Fetal Medicine states that there are no absolute contraindications to the use of monoclonal antibodies in appropriate pregnant patients with COVID-19.55 Remdesivir has no known fetal toxicity and is recommended as a treatment that can be offered to pregnant patients. Dexamethasone can also be administered to pregnant patients who require oxygen; however, if dexamethasone is also being used to accelerate fetal lung maturity, more frequent initial dosing is needed.
Older people. COVID-19 treatments for older patients are the same as for the general adult population. However, because older people are more likely to have impaired renal function, renal function should be monitored when an older patient is being treated with COVID-19 medications that are eliminated renally (eg, remdesivir, baricitinib). Furthermore, drug–drug interactions have been reported in older patients treated with nirmatrelvir + ritonavir, primarily because of the effects of ritonavir. Review all of a patient’s medications, including over-the-counter drugs and herbal supplements, when prescribing treatment for COVID-19, and adjust the dosage by following guidance in FDA-approved prescribing information—ideally, in consultation with a pharmacist.
Immunocompromised patients. The combination product tixagevimab + cilgavimab [Evusheld] is FDA approved for COVID-19 PrEP, under an EUA, in patients who are not infected with SARS-CoV-2 who have an immune-compromising condition, who are unlikely to mount an adequate immune response to the COVID-19 vaccine, or those in whom vaccination is not recommended because of their history of a severe adverse reaction to a COVID-19 vaccine or one of its components.7
Continue to: Summing up
Summing up
With a growing need for effective and readily available COVID-19 treatments, there are an unprecedented number of clinical trials in process. Besides antivirals, immunomodulators, and antibody therapies, some novel mechanisms being tested include Janus kinase inhibitors, IL-6-receptor blockers, and drugs that target adult respiratory distress syndrome and cytokine release.
Once larger trials are completed, we can expect stronger evidence of potential treatment options and of safety and efficacy in children, pregnant women, and vulnerable populations. During the pandemic, the FDA’s EUA program has brought emerging treatments rapidly to clinicians; nevertheless, high-quality evidence, with thorough peer review, remains critical to inform COVID-19 treatment guidelines.
ahttps://healthdata.gov/Health/COVID-19-PublicTherapeutic-Locator/rxn6-qnx8/data
b Sotrovimab was effective against the Omicron variant of SARS-CoV-2—the dominant variant in early 2022— but is currently not FDA authorized in any region of the United States because of the prevalence of the Omicron BA.2 subvariant.30
CORRESPONDENCE
Ambar Kulshreshtha, MD, PhD, Department of Epidemiology, Emory Rollins School of Public Health, 4500 North Shallowford Road, Suite 134, Atlanta, GA 30338; akulshr@emory.edu
The ongoing COVID-19 pandemic has caused more than 1 million deaths in the United States and continues to be a major public health challenge. Cases can be asymptomatic, or symptoms can range from a mild respiratory tract infection to acute respiratory distress and multiorgan failure.
Three strategies can successfully contain the pandemic and its consequences:
- Public health measures, such as masking and social distancing
- Prophylactic vaccines to reduce transmission
- Safe and effective drugs for reducing morbidity and mortality among infected patients.
Optimal treatment strategies for patients in ambulatory and hospital settings continue to evolve as new studies are reported and new strains of the virus arise. Many medical and scientific organizations, including the National Institutes of Health (NIH) COVID-19 treatment panel,1 Infectious Diseases Society of America (IDSA),2 World Health Organization (WHO),3 and Centers for Disease Control and Prevention,4 provide recommendations for managing patients with COVID-19. Their guidance is based on the strongest research available and is updated intermittently; nevertheless, a plethora of new data emerges weekly and controversies surround several treatments.
In this article, we
We encourage clinicians, in planning treatment, to consider:
- The availability of medications (ie, use the COVID-19 Public Therapeutic Locatora)
- The local COVID-19 situation
- Patient factors and preferences
- Evolving evidence regarding new and existing treatments.
Most evidence about the treatment of COVID-19 comes from studies conducted when the Omicron variant of SARS-CoV-2 was not the dominant variant, as it is today in the United States. As such, drugs authorized or approved by the US Food and Drug Administration (FDA) to treat COVID-19 or used off-label for that purpose might not be as efficacious today as they were almost a year ago. Furthermore, many trials of potential therapies against new viral variants are ongoing; if your patient is interested in enrolling in a clinical trial of an investigational COVID-19 treatment, refer them to www.clinicaltrials.gov.
General managementof COVID-19
Patients with COVID-19 experience a range of illness severity—from asymptomatic to mild symptoms, such as fever and myalgia, to critical illness requiring intensive care (TABLE 11,2). Patients with COVID-19 should therefore be monitored for progression, remotely or in person, until full recovery is achieved. Key concepts of general management include:
Assess and monitor patients’ oxygenation status by pulse oximetry; identify those with low or declining oxygen saturation before further clinical deterioration.

Continue to: Consider the patient's age and general health
Consider the patient’s age and general health. Patients are at higher risk of severe disease if they are > 65 years or have an underlying comorbidity.4
Emphasize self-isolation and supportive care, including rest, hydration, and over-the-counter medications to relieve cough, reduce fever, and alleviate other symptoms.
Drugs: Few approved, some under study
The antiviral remdesivir is the only drug fully approved for clinical use by the FDA to treat COVID-19 in patients > 12 years.5,6
In addition, the FDA has issued an emergency use authorization (EUA) for several monoclonal antibodies as prophylaxis and treatment: tixagevimab packaged with cilgavimab (Evusheld) is the first antibody combination for pre-exposure prophylaxis (PrEP) against COVID-19; the separately packaged injectables are recommended for patients who have a history of severe allergy that prevents them from being vaccinated or those with moderate or severe immune-compromising disorders.7
In the pipeline. Several treatments are being tested in clinical trials to evaluate their effectiveness and safety in combating COVID-19, including:
- Antivirals, which prevent viruses from multiplying
- Immunomodulators, which reduce the body’s immune reaction to the virus
- Antibody therapies, which are manufactured antibodies against the virus
- Anti-inflammatory drugs, which reduce systemic inflammation and prevent organ dysfunction
- Cell therapies and gene therapies, which alter the expression of cells and genes.
Continue to: Outpatient treatment
Outpatient treatment
Several assessment tools that take into account patients’ age, respiratory status, and comorbidities are available for triage of patients infected with COVID-19.8
Most (> 80%) patients with COVID-19 have mild infection and are safely managed as outpatients or at home.9,10 For patients at high risk of severe disease, a few options are recommended for patients who do not require hospitalization or supplemental oxygen; guidelines on treatment of COVID-19 in outpatient settings that have been developed by various organizations are summarized in TABLE 2.7,11-25

Antiviral drugs target different stages of the SARS-CoV-2 replication cycle. They should be used early in the course of infection, particularly in patients at high risk of severe disease.

IDSA recommends antiviral therapy with molnupiravir, nirmatrelvir + ritonavir packaged together (Paxlovid), or remdesivir.11,12,26,27 Remdesivir requires intravenous (IV) infusion on 3 consecutive days, which can be difficult in some clinic settings.13,28 Nirmatrelvir + ritonavir should be initiated within 5 days after symptom onset. Overall, for most patients, nirmatrelvir + ritonavir is preferred because of oral dosing and higher efficacy in comparison to other antivirals. With nirmatrelvir + ritonavir, carefully consider drug–drug interactions and the need to adjust dosing in the presence of renal disease.28,29 There are no data on the efficacy of any combination treatments with these agents (other than co-packaged Paxlovid).

Monoclonal antibodies for COVID-19 are given primarily intravenously. They bind to the viral spike protein, thus preventing SARS-CoV-2 from attaching to and entering cells. Bamlanivimab + etesevimab and bebtelovimab are available under an EUA for outpatient treatment.14b Treatment should be initiated as early as possible in the course of infection—ideally, within 7 to 10 days after onset of symptoms.

Continue to: Bebtelovimab was recently given...
Bebtelovimab was recently given an EUA. It is a next-generation antibody that neutralizes all currently known variants and is the most potent monoclonal antibody against the Omicron variant, including its BA.2 subvariant.31 However, data about its activity against the BA.2 subvariant are based on laboratory testing and have not been confirmed in clinical trials. Clinical data were similar for this agent alone and for its use in combination with other monoclonal antibodies, but those trials were conducted before the emergence of Omicron.
In your decision-making about the most appropriate therapy, consider (1) the requirement that monoclonal antibodies be administered parenterally and (2) the susceptibility of the locally predominating viral variant.
Other monoclonal antibody agents are in the investigative pipeline; however, data about them have been largely presented through press releases or selectively reported in applications to the FDA for EUA. For example, preliminary reports show cilgavimab coverage against the Omicron variant14; so far, cilgavimab is not approved for treatment but is used in combination with tixagevimab for PreP—reportedly providing as long as 12 months of protection for patients who are less likely to respond to a vaccine.32
Corticosteroids. Guidelines recommend against dexamethasone and other systemic corticosteroids in outpatient settings. For patients with moderate-to-severe symptoms but for whom hospitalization is not possible (eg, beds are unavailable), the NIH panel recommends dexamethasone, 6 mg/d, for the duration of supplemental oxygen, not to exceed 10 days of treatment.1
Patients who were recently discharged after COVID-19 hospitalization should not continue remdesivir, dexamethasone, or baricitinib at home, even if they still require supplemental oxygen.
Continue to: Some treatments should not be in your COVID-19 toolbox
Some treatments should not be in your COVID-19 toolbox
High-quality studies are lacking for several other potential COVID-19 treatments. Some of these drugs are under investigation, with unclear benefit and with the potential risk of toxicity—and therefore should not be prescribed or used outside a clinical trial. See “Treatments not recommended for COVID-19,” page E14. 1-4,15-19,33-41
SIDEBAR
Treatments not recommended for COVID-191-4,15-19,33-41
Fluvoxamine. A few studies suggest that the selective serotonin reuptake inhibitor fluvoxamine reduces progression to severe disease; however, those studies have methodologic challenges.33 The drug is not FDA approved for treating COVID.33
Convalescent plasma, given to high-risk outpatients early in the course of disease, can reduce progression to severe disease,34,35 but it remains investigational for COVID-19 because trials have yielded mixed results.34-36
Ivermectin. The effect of ivermectin in patients with COVID-19 is unclear because high-quality studies do not exist and cases of ivermectin toxicity have occurred with incorrect administration.39
Hydroxychloroquine showed potential in a few observational studies, but randomized clinical trials have not shown any benefit.15
Azithromycin likewise showed potential in a few observational studies; randomized clinical trials have not shown any benefit, however.15
Statins. A few meta-analyses, based on observational studies, reported benefit from statins, but recent studies have shown that this class of drugs does not provide clinical benefit in alleviating COVID-19 symptoms.16,17,37
Inhaled corticosteroids. A systematic review reported no benefit or harm from using an inhaled corticosteroid.18 More recent studies show that the inhaled corticosteroid budesonide used in early COVID-19 might reduce the need for urgent care38 and, in patients who are at higher risk of COVID-19-related complications, shorten time to recovery.19
Vitamins and minerals. Limited observational studies suggest an association between vitamin and mineral deficiency (eg, vitamin C, zinc, and vitamin D) and risk of severe disease, but high-quality data about this finding do not exist.40,41
Casirivimab + imdevimab [REGEN-COV2]. This unapproved investigational combination treatment was granted an EUA in 2020 for postexposure prophylaxis. The EUA was withdrawn in January 2022 because of the limited efficacy of casirivimab + imdevimab against the Omicron variant of SARS-CoV-2.
Postexposure prophylaxis. National guidelines1-4 recommend against postexposure prophylaxis with hydroxychloroquine, colchicine, inhaled corticosteroids, or azithromycin.
TABLE 27,11-25 and TABLE 326,42-46 provide additional information on treatments not recommended outside trials, or not recommended at all, for COVID-19.
Treatment during hospitalization
The NIH COVID-19 treatment panel recommends hospitalization for patients who have any of the following findings1:
- Oxygen saturation < 94% while breathing room air
- Respiratory rate > 30 breaths/min
- A ratio of partial pressure of arterial O2 to fraction of inspired O2 (PaO2/FiO2) < 300 mm Hg
- Lung infiltrates > 50%.

General guidance for the care of hospitalized patients:
- Treatments that target the virus have the greatest efficacy when given early in the course of disease.
- Anti-inflammatory and immunosuppressive agents help prevent tissue damage from a dysregulated immune system. (See TABLE 326,42-46)
- The NIH panel,1 IDSA,2 and WHO3 recommend against dexamethasone and other corticosteroids for hospitalized patients who do not require supplemental oxygen.
- Prone positioning distributes oxygen more evenly in the lungs and improves overall oxygenation, thus reducing the need for mechanical ventilation.

Remdesivir. Once a hospitalized patient does require supplemental oxygen, the NIH panel,1 IDSA,2 and WHO3 recommend remdesivir; however, remdesivir is not recommended in many other countries because WHO has noted its limited efficacy.42 Dexamethasone is recommended alone, or in combination with remdesivir for patients who require increasing supplemental oxygen and those on mechanical ventilation.

Baricitinib. For patients with rapidly increasing oxygen requirements, invasive mechanical ventilation, and systemic inflammation, baricitinib, a Janus kinase inhibitor, can be administered, in addition to dexamethasone, with or without remdesivir.47
Continue to: Tocilizumab
Tocilizumab. A monoclonal antibody and interleukin (IL)-6 inhibitor, tocilizumab is also recommended in addition to dexamethasone, with or without remdesivir.48 Tocilizumab should be given only in combination with dexamethasone.49 Patients should receive baricitinib or tocilizumab—not both. IDSA recommends tofacitinib, with a prophylactic dose of an anticoagulant, for patients who are hospitalized with severe COVID-19 but who are not on any form of ventilation.50
Care of special populations
Special patient populations often seek primary care. Although many questions remain regarding the appropriate care of these populations, it is useful to summarize existing evidence and recommendations from current guidelines.
Children. COVID-19 is generally milder in children than in adults; many infected children are asymptomatic. However, infants and children who have an underlying medical condition are at risk of severe disease, including multisystem inflammatory syndrome.51
The NIH panel recommends supportive care alone for most children with mild-to-moderate disease.1 Remdesivir is recommended for hospitalized children ≥ 12 years who weigh ≥ 40 kg, have risk factors for severe disease, and have an emergent or increasing need for supplemental oxygen. Dexamethasone is recommended for hospitalized children requiring high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation. Molnupiravir is not authorized for patients < 18 years because it can impede bone and cartilage growth.
There is insufficient evidence for or against the use of monoclonal antibody products for children with COVID-19 in an ambulatory setting. For hospitalized children, there is insufficient evidence for or against use of baricitinib and tocilizumab.
Continue to: Patients who are pregnant
Patients who are pregnant are at increased risk of severe COVID-19.52,53 The NIH states that, in general, treatment and vaccination of pregnant patients with COVID-19 should be the same as for nonpregnant patients.1
Pregnant subjects were excluded from several trials of COVID-19 treatments.54 Because Janus kinase inhibitors, such as baricitinib, are associated with an increased risk of thromboembolism, they are not recommended in pregnant patients who are already at risk of thromboembolic complications. Molnupiravir is not recommended for pregnant patients because of its potential for teratogenic effects.
The Society for Maternal-Fetal Medicine states that there are no absolute contraindications to the use of monoclonal antibodies in appropriate pregnant patients with COVID-19.55 Remdesivir has no known fetal toxicity and is recommended as a treatment that can be offered to pregnant patients. Dexamethasone can also be administered to pregnant patients who require oxygen; however, if dexamethasone is also being used to accelerate fetal lung maturity, more frequent initial dosing is needed.
Older people. COVID-19 treatments for older patients are the same as for the general adult population. However, because older people are more likely to have impaired renal function, renal function should be monitored when an older patient is being treated with COVID-19 medications that are eliminated renally (eg, remdesivir, baricitinib). Furthermore, drug–drug interactions have been reported in older patients treated with nirmatrelvir + ritonavir, primarily because of the effects of ritonavir. Review all of a patient’s medications, including over-the-counter drugs and herbal supplements, when prescribing treatment for COVID-19, and adjust the dosage by following guidance in FDA-approved prescribing information—ideally, in consultation with a pharmacist.
Immunocompromised patients. The combination product tixagevimab + cilgavimab [Evusheld] is FDA approved for COVID-19 PrEP, under an EUA, in patients who are not infected with SARS-CoV-2 who have an immune-compromising condition, who are unlikely to mount an adequate immune response to the COVID-19 vaccine, or those in whom vaccination is not recommended because of their history of a severe adverse reaction to a COVID-19 vaccine or one of its components.7
Continue to: Summing up
Summing up
With a growing need for effective and readily available COVID-19 treatments, there are an unprecedented number of clinical trials in process. Besides antivirals, immunomodulators, and antibody therapies, some novel mechanisms being tested include Janus kinase inhibitors, IL-6-receptor blockers, and drugs that target adult respiratory distress syndrome and cytokine release.
Once larger trials are completed, we can expect stronger evidence of potential treatment options and of safety and efficacy in children, pregnant women, and vulnerable populations. During the pandemic, the FDA’s EUA program has brought emerging treatments rapidly to clinicians; nevertheless, high-quality evidence, with thorough peer review, remains critical to inform COVID-19 treatment guidelines.
ahttps://healthdata.gov/Health/COVID-19-PublicTherapeutic-Locator/rxn6-qnx8/data
b Sotrovimab was effective against the Omicron variant of SARS-CoV-2—the dominant variant in early 2022— but is currently not FDA authorized in any region of the United States because of the prevalence of the Omicron BA.2 subvariant.30
CORRESPONDENCE
Ambar Kulshreshtha, MD, PhD, Department of Epidemiology, Emory Rollins School of Public Health, 4500 North Shallowford Road, Suite 134, Atlanta, GA 30338; akulshr@emory.edu
1. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. July 19, 2022. Accessed July 21, 2022. www.covid19treatmentguidelines.nih.gov
2. IDSA guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America. Updated June 29, 2022. Accessed July 21, 2022. www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-23
3. Therapeutics and COVID-19: living guideline. World Health Organization. July 14, 2022. Accessed July 21, 2022. https://apps.who.int/iris/rest/bitstreams/1449398/retrieve
4. Centers for Disease Control and Prevention. Clinical care considerations. Updated May 27, 2022. Accessed July 21, 2022. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
5. Coronavirus (COVID-19) update: FDA approves first COVID-19 treatment for young children. Press release. US Food and Drug Administration. April 25, 2022. Accessed August 11, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-approves-first-covid-19-treatment-young-children
6. Know your treatment options for COVID-19. US Food and Drug Administration. Updated August 15, 2022. Accessed July 21, 2022. www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19
7. Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA. 2022;327:384-385. doi: 10.1001/jama.2021.24931
8. Judson TJ, Odisho AY, Neinstein AB, et al. Rapid design and implementation of an integrated patient self-triage and self-scheduling tool for COVID-19. J Am Med Inform Assoc. 2020;27:860-866. doi: 10.1093/jamia/ocaa051
9. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757-1766. doi: 10.1056/NEJMcp2009249
10. Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. BMJ. 2020;368:m1182. doi: 10.1136/bmj.m1182
11. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al; . Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509-520. doi: 10.1056/NEJMoa2116044
12. Hammond J, Leister-Tebbe H, Gardner A, et al; . Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397-1408. doi: 10.1056/NEJMoa2118542
13. Gottlieb RL, Vaca CE, Paredes R, et al; . Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305-315. doi: 10.1056/NEJMoa2116846
14. Gupta A, Gonzalez-Rojas Y, Juarez E, et al; . Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941-1950. doi: 10.1056/NEJMoa2107934
15. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623-631. doi: 10.7326/M20-4207
16. Scheen AJ. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2021;47:101220. doi: 10.1016/j.diabet.2020.101220
17. Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153-155. doi: 10.1016/j.amjcard.2020.08.004
18. Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55:2001009. doi: 10.1183/13993003.01009-2020
19. Yu L-M, Bafadhel M, Dorward J, et al; . Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843-855. doi: 10.1016/S0140-6736(21)01744-X
20. Siemieniuk RA, Bartoszko JJ, JP, et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. doi: 10.1136/bmj.n2231
21. Siemieniuk RA, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980
22. Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379
23. Goldstein KM, Ghadimi K, Mystakelis H, et al. Risk of transmitting coronavirus disease 2019 during nebulizer treatment: a systematic review. J Aerosol Med Pulm Drug Deliv. 2021;34:155-170. doi: 10.1089/jamp.2020.1659
24. Schultze A, Walker AJ, MacKenna B, et al; . Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106-1120. doi: 10.1016/S2213-2600(20)30415-X
25. What are the safety and efficacy results of bebtelovimab from BLAZE-4? Lilly USA. January 12, 2022. Accessed August 17, 2022. www.lillymedical.com/en-us/answers/what-are-the-safety-and-efficacy-results-of-bebtelovimab-from-blaze-4-159290
26. Beigel JH, Tomashek KM, Dodd LE, et al; . Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi: 10.1056/NEJMoa2007764
27. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787-1799. doi: 10.1056/NEJMoa2001282
28. Gandhi RT, Malani PN, Del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA. 2022;327:617-618. doi: 10.1001/jama.2022.0335
29. Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54:516-523. doi: 10.1080/07853890.2022.2034936
30. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022:602:671-675. doi: 10.1038/s41586-021-04389-z
31. Emergency use authorization (EUA) for bebtelovimab (LY-CoV1404): Center for Drug Evaluation and Research (CDER) review. US Food and Drug Administration. Updated February 11, 2022. Accessed July 21, 2022. www.fda.gov/media/156396/download
32. Bartoszko JJ, Siemieniuk RAC, Kum E, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373:n949. doi: 10.1136/bmj.n949
33. Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al; TOGETHER Investigators. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42-e51. doi: 10.1016/S2214-109X(21)00448-4
34. RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049-2059. doi: 10.1016/S0140-6736(21)00897-7
35. Simonovich VA, Burgos Pratx LD, Scibona P, et al; . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619-629. doi: 10.1056/NEJMoa2031304
36. Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384:1015-1027. doi: 10.1056/NEJMoa2031893
37. Ayeh SK, Abbey EJ, Khalifa BAA, et al. Statins use and COVID-19 outcomes in hospitalized patients. PLoS One. 2021;16:e0256899. doi: 10.1371/journal.pone.0256899
38. Ramakrishnan S, Nicolau DV Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763-772. doi: 10.1016/S2213-2600(21)00160-0
39. Temple C, Hoang R, Hendrickson RG. Toxic effects from ivermectin use associated with prevention and treatment of Covid-19. N Engl J Med. 2021;385:2197-2198. doi: 10.1056/NEJMc2114907
40. Thomas S, Patel D, Bittel B, et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4:e210369. doi: 10.1001/jamanetworkopen.2021.0369
41. Adams KK, Baker WL, Sobieraj DM. Myth busters: dietary supplements and COVID-19. Ann Pharmacother. 2020;54:820-826. doi: 10.1177/1060028020928052
42. Pan H, Peto R, Henao-Restrepo A-M, et al. Repurposed antiviral drugs for Covid-19—interim WHO Solidarity trial results. N Engl J Med. 2021;384:497-511. doi: 10.1056/NEJMoa2023184
43. Kalil AC, Patterson TF, Mehta AK, et al; . Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795-807. doi: 10.1056/NEJMoa2031994
44. ; Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330-1341. doi: 10.1001/jama.2020.17023
45. ; Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499-518. doi: 10.1001/jama.2021.11330
46. Wei QW, Lin H, Wei R-G, et al. Tocilizumab treatment for COVID-19 patients: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10:71. doi: 10.1186/s40249-021-00857-w
47. Zhang X, Shang L, Fan G, et al. The efficacy and safety of Janus kinase inhibitors for patients with COVID-19: a living systematic review and meta-analysis. Front Med (Lausanne). 2021;8:800492. doi: 10.3389/fmed.2021.800492
48. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637-1645. doi: 10.1016/S0140-6736(21)00676-0
49. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491-1502. doi: 10.1056/NEJMoa2100433
50. Guimaraes PO, Quirk D, Furtado RH, et al; . Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406-415. doi: 10.1056/NEJMoa2101643
51. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-346. doi: 10.1056/NEJMoa2021680
52. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320
53. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817-826. doi: 10.1001/jamapediatrics.2021.1050
54. Jorgensen SCJ, Davis MR, Lapinsky SE. A review of remdesivir for COVID-19 in pregnancy and lactation. J Antimicrob Chemother. 2021;77:24-30. doi: 10.1093/jac/dkab311
55. Management considerations for pregnant patients with COVID-19. Society for Maternal-Fetal Medicine. Accessed July 21, 2022. https://s3.amazonaws.com/cdn.smfm.org/media/2336/SMFM_COVID_Management_of_COVID_pos_preg_patients_4-30-20_final.pdf
1. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. July 19, 2022. Accessed July 21, 2022. www.covid19treatmentguidelines.nih.gov
2. IDSA guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America. Updated June 29, 2022. Accessed July 21, 2022. www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-23
3. Therapeutics and COVID-19: living guideline. World Health Organization. July 14, 2022. Accessed July 21, 2022. https://apps.who.int/iris/rest/bitstreams/1449398/retrieve
4. Centers for Disease Control and Prevention. Clinical care considerations. Updated May 27, 2022. Accessed July 21, 2022. www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
5. Coronavirus (COVID-19) update: FDA approves first COVID-19 treatment for young children. Press release. US Food and Drug Administration. April 25, 2022. Accessed August 11, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-approves-first-covid-19-treatment-young-children
6. Know your treatment options for COVID-19. US Food and Drug Administration. Updated August 15, 2022. Accessed July 21, 2022. www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19
7. Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA. 2022;327:384-385. doi: 10.1001/jama.2021.24931
8. Judson TJ, Odisho AY, Neinstein AB, et al. Rapid design and implementation of an integrated patient self-triage and self-scheduling tool for COVID-19. J Am Med Inform Assoc. 2020;27:860-866. doi: 10.1093/jamia/ocaa051
9. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757-1766. doi: 10.1056/NEJMcp2009249
10. Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. BMJ. 2020;368:m1182. doi: 10.1136/bmj.m1182
11. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al; . Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509-520. doi: 10.1056/NEJMoa2116044
12. Hammond J, Leister-Tebbe H, Gardner A, et al; . Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397-1408. doi: 10.1056/NEJMoa2118542
13. Gottlieb RL, Vaca CE, Paredes R, et al; . Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305-315. doi: 10.1056/NEJMoa2116846
14. Gupta A, Gonzalez-Rojas Y, Juarez E, et al; . Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941-1950. doi: 10.1056/NEJMoa2107934
15. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623-631. doi: 10.7326/M20-4207
16. Scheen AJ. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2021;47:101220. doi: 10.1016/j.diabet.2020.101220
17. Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153-155. doi: 10.1016/j.amjcard.2020.08.004
18. Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55:2001009. doi: 10.1183/13993003.01009-2020
19. Yu L-M, Bafadhel M, Dorward J, et al; . Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843-855. doi: 10.1016/S0140-6736(21)01744-X
20. Siemieniuk RA, Bartoszko JJ, JP, et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. doi: 10.1136/bmj.n2231
21. Siemieniuk RA, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980
22. Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379
23. Goldstein KM, Ghadimi K, Mystakelis H, et al. Risk of transmitting coronavirus disease 2019 during nebulizer treatment: a systematic review. J Aerosol Med Pulm Drug Deliv. 2021;34:155-170. doi: 10.1089/jamp.2020.1659
24. Schultze A, Walker AJ, MacKenna B, et al; . Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106-1120. doi: 10.1016/S2213-2600(20)30415-X
25. What are the safety and efficacy results of bebtelovimab from BLAZE-4? Lilly USA. January 12, 2022. Accessed August 17, 2022. www.lillymedical.com/en-us/answers/what-are-the-safety-and-efficacy-results-of-bebtelovimab-from-blaze-4-159290
26. Beigel JH, Tomashek KM, Dodd LE, et al; . Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi: 10.1056/NEJMoa2007764
27. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787-1799. doi: 10.1056/NEJMoa2001282
28. Gandhi RT, Malani PN, Del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA. 2022;327:617-618. doi: 10.1001/jama.2022.0335
29. Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54:516-523. doi: 10.1080/07853890.2022.2034936
30. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022:602:671-675. doi: 10.1038/s41586-021-04389-z
31. Emergency use authorization (EUA) for bebtelovimab (LY-CoV1404): Center for Drug Evaluation and Research (CDER) review. US Food and Drug Administration. Updated February 11, 2022. Accessed July 21, 2022. www.fda.gov/media/156396/download
32. Bartoszko JJ, Siemieniuk RAC, Kum E, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373:n949. doi: 10.1136/bmj.n949
33. Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al; TOGETHER Investigators. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42-e51. doi: 10.1016/S2214-109X(21)00448-4
34. RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049-2059. doi: 10.1016/S0140-6736(21)00897-7
35. Simonovich VA, Burgos Pratx LD, Scibona P, et al; . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619-629. doi: 10.1056/NEJMoa2031304
36. Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384:1015-1027. doi: 10.1056/NEJMoa2031893
37. Ayeh SK, Abbey EJ, Khalifa BAA, et al. Statins use and COVID-19 outcomes in hospitalized patients. PLoS One. 2021;16:e0256899. doi: 10.1371/journal.pone.0256899
38. Ramakrishnan S, Nicolau DV Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763-772. doi: 10.1016/S2213-2600(21)00160-0
39. Temple C, Hoang R, Hendrickson RG. Toxic effects from ivermectin use associated with prevention and treatment of Covid-19. N Engl J Med. 2021;385:2197-2198. doi: 10.1056/NEJMc2114907
40. Thomas S, Patel D, Bittel B, et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4:e210369. doi: 10.1001/jamanetworkopen.2021.0369
41. Adams KK, Baker WL, Sobieraj DM. Myth busters: dietary supplements and COVID-19. Ann Pharmacother. 2020;54:820-826. doi: 10.1177/1060028020928052
42. Pan H, Peto R, Henao-Restrepo A-M, et al. Repurposed antiviral drugs for Covid-19—interim WHO Solidarity trial results. N Engl J Med. 2021;384:497-511. doi: 10.1056/NEJMoa2023184
43. Kalil AC, Patterson TF, Mehta AK, et al; . Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795-807. doi: 10.1056/NEJMoa2031994
44. ; Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330-1341. doi: 10.1001/jama.2020.17023
45. ; Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499-518. doi: 10.1001/jama.2021.11330
46. Wei QW, Lin H, Wei R-G, et al. Tocilizumab treatment for COVID-19 patients: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10:71. doi: 10.1186/s40249-021-00857-w
47. Zhang X, Shang L, Fan G, et al. The efficacy and safety of Janus kinase inhibitors for patients with COVID-19: a living systematic review and meta-analysis. Front Med (Lausanne). 2021;8:800492. doi: 10.3389/fmed.2021.800492
48. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637-1645. doi: 10.1016/S0140-6736(21)00676-0
49. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491-1502. doi: 10.1056/NEJMoa2100433
50. Guimaraes PO, Quirk D, Furtado RH, et al; . Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406-415. doi: 10.1056/NEJMoa2101643
51. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-346. doi: 10.1056/NEJMoa2021680
52. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320
53. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817-826. doi: 10.1001/jamapediatrics.2021.1050
54. Jorgensen SCJ, Davis MR, Lapinsky SE. A review of remdesivir for COVID-19 in pregnancy and lactation. J Antimicrob Chemother. 2021;77:24-30. doi: 10.1093/jac/dkab311
55. Management considerations for pregnant patients with COVID-19. Society for Maternal-Fetal Medicine. Accessed July 21, 2022. https://s3.amazonaws.com/cdn.smfm.org/media/2336/SMFM_COVID_Management_of_COVID_pos_preg_patients_4-30-20_final.pdf
PRACTICE RECOMMENDATIONS
› Use antivirals (eg, molnupiravir, nirmatrelvir packaged with ritonavir [Paxlovid], and remdesivir) and monoclonal antibody agents (eg, bebtelovimab) effective against the circulating Omicron variant, to treat symptoms of mild-to-moderate COVID-19 illness. C
› Treat severely ill hospitalized COVID-19 patients who require supplemental oxygen with dexamethasone, alone or in combination with remdesivir, to produce better outcomes. B
› Consider administering baricitinib or tocilizumab, in addition to dexamethasone with or without remdesivir, to COVID-19 patients with rapidly increasing oxygen requirements. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A Randomized Controlled Trial of Telephone Management of Suspected Urinary Tract Infections in Women
STUDY DESIGN: We randomly assigned women calling their usual provider with a suspected UTI to receive care over the telephone (n=36) or usual office-based care (n=36). All women had urinalyses and urine cultures. All were treated with 7 days of antibiotics. We compared symptom scores at baseline and at day 3 and day 10 after therapy. We also compared patient satisfaction at the end of the study. The settings were family practices in Michigan.
POPULATION: We included healthy nonpregnant women older than 18 years.
RESULTS: A total of 201 women with suspected UTIs called their physician. Of these, 99 were ineligible, and 30 declined to participate. The women were young (mean age=36.6 years) and predominantly white (86%). Sixty-four percent of the urine cultures had significant growth of a single organism. We observed no difference in symptom scores or satisfaction. Overall, satisfaction was high.
CONCLUSIONS: Short-term outcomes of managing suspected UTIs by telephone appear to be comparable with usual office care.
Millions of women with acute dysuria show up at offices, urgent care centers, and emergency departments for suspected urinary tract infections (UTIs), accounting for more than $1 billion in direct costs.1 Since most UTIs are uncomplicated, numerous strategies have been proposed for managing them in more efficient and less costly ways. Berg2 found 82 separate management strategies among 137 family physicians, with costs ranging from negligible to $250.
In a previous study3 we used a cost-utility analysis to demonstrate that among office-based approaches, empiric therapy of suspected UTIs was most cost-effective. This was robust over a wide range of assumptions, including prevalence, test characteristics, costs, complication rates, and outcomes. These findings were recently confirmed by Fenwick and colleagues4 in a British analysis. Among the approaches commonly used, telephone management has the potential for reducing cost, increasing convenience for patients, and reducing barriers to care. Saint and coworkers5 demonstrated that a clinical practice guideline using telephone-based management of presumed UTIs reduced the use of urine tests and increased the use of guideline-specified antibiotics. Although telephone management is common, we were unable to find any studies directly comparing that approach with office-based care.
We report on the first trial in which women with suspected UTIs were randomly assigned to telephone management or office-based management. The purpose of our study was to identify the prevalence of UTIs in women presenting with suspected UTIs, to determine if telephone management was comparable in outcomes with those of office-based care, and to determine if women whose infections were managed by telephone were satisfied with their care.
Methods
Setting
We enrolled patients at 6 primary care offices (including a walk-in clinic) of the Upper Peninsula Research Network (UPRNet) and the Department of Family Practice at Michigan State University in East Lansing. UPRNet is a primary care research network in the Upper Peninsula of Michigan.
Subjects
Nonpregnant women 18 years or older completed an interview to confirm eligibility when they called their primary care physicians with a suspected uncomplicated UTI. We defined a suspected uncomplicated UTI as a complaint of dysuria, pain on urination, complaint of urinary urgency and frequency, or the patient’s saying, “I think I have a bladder infection.” Patients with symptoms compatible with pyelonephritis (fever, chills, sweats, back or flank pain, or vomiting), vaginitis, or cervicitis (presence of a new or changed vaginal discharge) were excluded from our study. We also excluded women with diabetes, a previous history of pyelonephritis or other complicated UTI, a UTI in the preceding month, symptoms lasting longer than 14 days, and known kidney disease, anatomic abnormalities, or previous renal surgery. In addition, we excluded women receiving chemotherapy and those who had received antibiotics in the preceding month. Informed consent was obtained. Enrollment occurred between October of 1997 and March of 1999. All enrolled patients received $25 for participating in the study. The Michigan State University Committee on Research Involving Human Subjects approved our study.
Procedures and Measures
We stratified each practice, and using a central computer-generated random number, we blindly allocated patients to either treatment by telephone (telephone group) or office-based care (control group) by using an opaque envelope containing the group assignment. The envelope also provided instructions appropriate for the assigned group. We asked the telephone strategy patients to come to the clinic to leave a urine sample and pick up a prescription for an antibiotic; the control (usual care) patients were given a same-day appointment for a regular clinic visit.
We asked patients enrolled in both groups to rate the severity of urinary dysuria, urgency, frequency, function, and how they generally felt about their symptoms. Each was rated on a 10-point scale (10 was most severe). The telephone management patients were given a prescription for sulfamethoxazole/trimethoprim (800 mg/160 mg) twice daily for 7 days. If the patient was allergic to sulfa, she received a prescription for nitrofurantoin 100 mg twice daily for 7 days. Patients were required to have a urinalysis and urine culture before receiving the prescription. We asked the health care providers of the control patients to use their usual management strategies. The control group patients were also required to have a urinalysis and urine culture.
A nurse telephoned all women in both groups for follow-up 3 and 10 days after the time of enrollment. During these telephone calls we assessed patient status (including symptom scores and patient satisfaction) and whether the patient sought care with any practitioner since the initial time of enrollment. If UTI symptoms were still evident at either the 3- or 10-day follow-up, the patient was asked to make an appointment to see her practitioner. We recorded start and stop times for all interviews to assist in estimating and comparing costs of care for the 2 groups.
Primary Outcomes
The primary outcomes for our study were the UTI score and overall evaluation rating (OER)6 of the treatment experience. The UTI score is the sum of the ratings of severity of dysuria, urgency, frequency, function, and general symptoms (range=0-50). Since the UTI score has not been previously used, we pilot tested it on 20 women. These same women were reevaluated 2 to 3 days later. Test-retest reliability (measured by the overall correlation between the same items asked on 2 separate occasions with the order of questions changed) was 0.98. Validity (measured by the correlation coefficient between specific questions and a global question for the episode) was 0.52 for burning, 0.89 for frequency, 0.95 for urgency, 0.86 for interference with activities of daily living, and -0.76 for the actual interval between urination (smaller interval associated with greater overall episode severity).
The OER consisted of 2 questions about the overall quality of care and the outcome of care. Each question—answered as poor, fair, good, very good, or excellent—was scored from 1 to 5, respectively. The OER, a validated score,6 is a simple sum of the scores for these items (range 2-10, not normally distributed). Also, we asked the women about their overall satisfaction on a 5-point Likert scale.
Secondary Outcomes
We also evaluated urine culture results. We defined a negative culture as one with either no growth or less than 1000 colony-forming units. A positive culture had any growth of a single organism. A contaminated specimen (mixed flora) was rated as a negative culture, since this is how these are usually handled clinically.
Statistical Analysis
The data were entered into a database, and all entries were double-checked by one of the investigators for transcription errors. We analyzed the data using SAS software (version 7, SAS Institute, Cary, NC). Continuous variables (age, time variables, UTI score) were compared by treatment group using unpaired Student t tests. We used the Shapiro-Wilk test and the Kolmogorov-Smirnov test to confirm that the UTI score and changes in that score between assessments were normally distributed. We compared categorical variables (resolution of symptoms, culture results) by treatment group using a chi-square. We used Wilcoxon rank sum tests to compare ordinal data (OER) between the treatment groups.
For all sample size estimations we wanted to achieve 80% power with two-sided a of 0.05. To detect a 5-point difference in the UTI score, with an estimated a priori standard deviation (SD) of 5, we calculated that 16 subjects in each group are needed (21 for 90% power). With an SD of 7.5, 36 subjects in each group are needed. For patient satisfaction (OER), we estimated a priori that we would need 15 subjects in each group (19 for 90% power) to detect a 1-point difference with an estimated mean of 3.9 and a SD of 0.95.
Results
We identified 201 women with suspected UTIs of whom 99 were not eligible. Of the 102 eligible women, 30 declined to participate. We randomized 36 women to office care (control group) and 36 to telephone management. The subjects were predominantly white (n=62, 86%) and young (mean age=36.6 years, SD=12.3). Five patients had no culture results. Of the 67 remaining cultures, 4 (6%) were contaminated specimens, 20 (29.8%) had negative cultures, and 43 (64.2%) had positive cultures. Of the positive cultures, 34 (79.1%) grew Escherichia coli. Twenty-three of 34 (67.6%) cultures in the control group were positive compared with 20 of 33 (60.6%) in the telephone group (chi-square=0.3611; P=.55).
The groups were similar at baseline Table 1. On day 3 and day 10 there were no significant differences in the change in symptom scores or overall UTI score from baseline. We also found no difference in the change in urinary intervals from baseline. Table 2 shows these data. There was no significant difference in the overall evaluation rating. We also found no difference in satisfaction with care (median response was “very good” in the control group and “excellent” in the telephone group). These are shown in Table 3
On the third day after therapy was inititated, 20 of 33 (60.6%) of the control subjects had persistent urinary symptoms compared with 19 of 34 (55.8%, chi-square=0.1536; P=.70) in the telephone group. By day 10, 6 of 35 control patients (17.1%) had persistent symptoms, compared with 12 of 35 (34.3%) in the telephone group (chi-square=2.6923; P=0.1). Among the patients still symptomatic on the third day, culture results were available for 35, 11 (31%) of which were negative. Among those still symptomatic on the 10th day, 18 had available cultures, 9 (50%) of which were negative.
To evaluate the patients with persistent symptoms at the conclusion of the study we looked at the baseline and final UTI scores and baseline culture results. Six patients in the control group reported persistent symptoms. Three of these patients had final UTI scores less than 10, and 3 had UTI scores greater than 20. Two of these patients also had negative cultures. Twelve patients in the telephone group reported persistent symptoms; all but 1 had final UTI scores less than 10, and only 1 had a final UTI score greater than 20. Seven of the 12 patients had negative cultures at baseline.
We attempted to determine how office care differed from telephone care. Three patients in the control group received no antibiotics. Two of these had negative cultures, and no culture result was available for the third patient. All patients in the telephone group were prescribed antibiotics. Five control group patients who ultimately had positive cultures took antibiotics for less than 7 days, compared with only 3 in the telephone group. Among those receiving antibiotics, 30 of the control group patients received either sulfamethoxazole/trimethoprim or nitrofurantoin, and 3 received second-line agents. Because of allergies, 1 patient in the telephone group did not receive the planned therapy and received cephalexin instead.
We also evaluated the nursing time to administer various elements of the protocol. It took 2.5 minutes (SD=1.3) to determine eligibility to participate in the study and 5.3 minutes (SD=2.1) to enroll the subjects into the study. The nursing time for the day 3 follow-up took on average 5.6 minutes (SD=2.9) and 5.2 minutes (SD=2.0) on day 10.
Discussion
Although managing uncomplicated UTIs by telephone is a common practice in ambulatory primary care settings, we had no previous empiric evidence of its effectiveness compared with seeing patients in the office. In this randomized trial of office management versus management by telephone, two thirds of the women enrolled had culture-confirmed UTIs. The rate was similar in each group and mirrors that reported in the literature.7 We found no difference in improvement in symptom scores from baseline and no significant difference in overall satisfaction with the care provided or the outcome.
Gallagher and colleagues8 reported that when acute medical problems are triaged by nurses, patients are generally satisfied with care. However, UTIs represented only 5% of the telephone encounters. Delichatsios and coworkers9 similarly reported that patients calling to speak with the physician were generally satisfied with the advice given on the telephone, but they did not report outcomes related to specific conditions or therapies. Although 2 independent economic evaluations3,4 have found empiric therapy to be cost-effective, neither included a strategy that avoids an office visit.
The direct cost of telephone management of uncomplicated UTIs is relatively low. It took only 2.5 minutes of nurse time to identify symptomatic women with risk factors for complicated UTIs who were good candidates for telephone management. This may cause a dilemma. Physicians practicing in predominantly fee-for-service settings will lose income by managing UTIs by telephone. In managed care settings, the financial incentives to reduce utilization make this practice inexpensive while simultaneously maintaining high patient satisfaction. Many physicians, however, complain about the complexity of the patients they now see, and having an occasional uncomplicated UTI might provide some breathing space on hectic days.
Limitations
We did not ask the practitioners who provided office-based care to alter their usual approach. By patient report, only 3 control patients received no antibiotics. This may reflect a knee-jerk response in which antibiotics are prescribed for all women with a suspected UTI. It may also reflect a very appropriate therapeutic threshold where physicians have a gestalt about the probability of a UTI that exceeds any diagnostic uncertainty. Although this has been described explicitly,10 we believe that seasoned clinicians do this implicitly. We did not attempt to open the “black box” to further understand this process.
Approximately half the women calling for appointments were not eligible to participate in our study because of the presence of 1 or more complicating factor. The most common reason was the presence of back pain, a complaint that commonly accompanies uncomplicated as well as complicated UTIs. Although the prevalence of acute pyelonephritis is very low, our protocol conservatively put women with this isolated complaint in a potentially high-risk group that required an office visit. It is quite likely that using a constellation of symptoms (such as back or flank pain plus fever or chills or nausea and vomiting, and so forth) would have allowed more women to be eligible. The participation rate was high among eligible women, improving the generalizability of the data. Although we enrolled predominantly white women (reflecting the ethnic mix of the participating practices), we believe the biologic responses in our study are not race dependent. We are not confident, however, that patient satisfaction data will extrapolate to other groups, since women in groups that have traditionally been underserved by the health care system may see telephone management as a way to shortchange them.
Our study was planned to have 80% power to detect important differences in the primary outcome variables but lacks sufficient sample size to determine if patients in either group were more likely to experience pyelonephritis or other complications. Since the specific therapy was similar in each group, one would suspect a similar rate of complications.
Twice as many patients in the telephone group were still symptomatic after 10 days, compared with those seen in the office. The small numbers in our study raise the possibility that clinically meaningful differences did not reach statistical significance. However, a closer look at the UTI scores suggests that only 1 of the 12 patients in the telephone group who reported persistent symptoms had a high score compared with 3 of the 6 control patients. This suggests that the severity of the persistent symptoms was quite low. Also, it raises the possibility that symptoms such as low back pain that were not captured by the UTI score or possibly not related to UTI were unimproved. We also believe that many of these women may have had other conditions causing their persistent symptoms. Finally, it is possible that these findings reflect a significant degree of statistical “noise” due to the wide confidence intervals associated with small studies. This is an area for further study.
This study used 7 days of antibiotic therapy. Currently, 3 days of therapy are increasingly used. Interestingly, more than half the women were still symptomatic on day 3. At the conclusion of the study, though, 75% of all women reported resolution of their symptoms. Although this discussion places this observation in a different context, it may raise potential concerns about whether 3-day therapy (while effective in delivering laboratory “cures”) may not provide enough relief to patients to be worth the tradeoffs.
Conclusions
This study demonstrates that managing uncomplicated UTIs in otherwise healthy women over the telephone has comparable outcomes and patient satisfaction with managing these women with an office visit. Whether symptom resolution is the same is not adequately answered by our study. More research on the optimal use of triage protocols for common acute conditions is needed in the primary care setting.
Acknowledgments
Our research was funded by the Blue Cross Blue Shield of Michigan Foundation grant # 231-II: a randomized clinical trial comparing telephone and usual care strategies for the management of suspected UTI in otherwise healthy adult women. We thank the following practices that participated in our study: Michigan State University Department of Family Practice, East Lansing; Order of St. Francis Medical Group, Escanaba; and Doctors Park Family Physicians, Escanaba. We are especially appreciative of the efforts of the office nurses and physician’s assistants who recruited and provided the telephone follow-up of the patients: Barb Bedient, LPN; Lisa Sweet, LPN; Debi Besson, RN; Grace Borkadi, PAC; Gloria Johnson, LPN; and Mary Baron, RN. Conflict of interest statement: Dr Ebell is editor of The Journal of Family Practice, Dr Hickner is an associate editor, and Dr Barry is an assistant editor. Therefore, the peer review process, including selection of reviewers, editorial review, editing, and the decision to accept or reject the manuscript was performed by Dr Bernard Ewigman, MD, MSPH, Associate Editor of JFP.
1. Johnson JR, Stamm WE. Diagnosis and treatment of acute urinary tract infections [published erratum appears in Infect Dis Clin North Am 1990; 4:following xii.]. Infect Dis Clin North Am 1987;1:773-91.
2. Berg AO. Variations among family physicians’ management strategies for lower urinary tract infection in women: a report from the Washington Family Physicians Collaborative Research Network. J Am Board Fam Pract 1991;4:327-30.
3. Barry H, Ebell M, Hickner J. Evaluation of suspected UTI in ambulatory women: a cost-utility analysis of office-based strategies. J Fam Pract 1997;44:49-60.
4. Fenwick E, Briggs A, Hawke C. Management of urinary tract infection in general practice: a cost-effectiveness analysis. Br J Gen Pract 2000;50:635-39.
5. Saint S, Scholes D, Fihn SD, Farrell RG, Stamm WE. The effectiveness of a clinical practice guideline for the management of presumed uncomplicated urinary tract infection in women. Am J Med 1999;106:636-41.
6. Ross CK, Steward CA, Sinacore JM. A comparative study of seven measures of patient satisfaction. MedCare 1995;33:392-406.
7. Stamm WE, Wagner KF, Amsel R, et al. Causes of the acute urethral syndrome in women. N Engl J Med 1980;303:409-15.
8. Gallagher M, Huddart T, Henderson B. Telephone triage of acute illness by a practice nurse in general practice: outcomes of care. Br J Gen Pract 1998;48:1141-45.
9. Delichatsios H, Callahan M, Charlson M. Outcomes of telephone medical care. J Gen Intern Med 1998;13:579-85.
10. Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med 1980;302:1109-17.
STUDY DESIGN: We randomly assigned women calling their usual provider with a suspected UTI to receive care over the telephone (n=36) or usual office-based care (n=36). All women had urinalyses and urine cultures. All were treated with 7 days of antibiotics. We compared symptom scores at baseline and at day 3 and day 10 after therapy. We also compared patient satisfaction at the end of the study. The settings were family practices in Michigan.
POPULATION: We included healthy nonpregnant women older than 18 years.
RESULTS: A total of 201 women with suspected UTIs called their physician. Of these, 99 were ineligible, and 30 declined to participate. The women were young (mean age=36.6 years) and predominantly white (86%). Sixty-four percent of the urine cultures had significant growth of a single organism. We observed no difference in symptom scores or satisfaction. Overall, satisfaction was high.
CONCLUSIONS: Short-term outcomes of managing suspected UTIs by telephone appear to be comparable with usual office care.
Millions of women with acute dysuria show up at offices, urgent care centers, and emergency departments for suspected urinary tract infections (UTIs), accounting for more than $1 billion in direct costs.1 Since most UTIs are uncomplicated, numerous strategies have been proposed for managing them in more efficient and less costly ways. Berg2 found 82 separate management strategies among 137 family physicians, with costs ranging from negligible to $250.
In a previous study3 we used a cost-utility analysis to demonstrate that among office-based approaches, empiric therapy of suspected UTIs was most cost-effective. This was robust over a wide range of assumptions, including prevalence, test characteristics, costs, complication rates, and outcomes. These findings were recently confirmed by Fenwick and colleagues4 in a British analysis. Among the approaches commonly used, telephone management has the potential for reducing cost, increasing convenience for patients, and reducing barriers to care. Saint and coworkers5 demonstrated that a clinical practice guideline using telephone-based management of presumed UTIs reduced the use of urine tests and increased the use of guideline-specified antibiotics. Although telephone management is common, we were unable to find any studies directly comparing that approach with office-based care.
We report on the first trial in which women with suspected UTIs were randomly assigned to telephone management or office-based management. The purpose of our study was to identify the prevalence of UTIs in women presenting with suspected UTIs, to determine if telephone management was comparable in outcomes with those of office-based care, and to determine if women whose infections were managed by telephone were satisfied with their care.
Methods
Setting
We enrolled patients at 6 primary care offices (including a walk-in clinic) of the Upper Peninsula Research Network (UPRNet) and the Department of Family Practice at Michigan State University in East Lansing. UPRNet is a primary care research network in the Upper Peninsula of Michigan.
Subjects
Nonpregnant women 18 years or older completed an interview to confirm eligibility when they called their primary care physicians with a suspected uncomplicated UTI. We defined a suspected uncomplicated UTI as a complaint of dysuria, pain on urination, complaint of urinary urgency and frequency, or the patient’s saying, “I think I have a bladder infection.” Patients with symptoms compatible with pyelonephritis (fever, chills, sweats, back or flank pain, or vomiting), vaginitis, or cervicitis (presence of a new or changed vaginal discharge) were excluded from our study. We also excluded women with diabetes, a previous history of pyelonephritis or other complicated UTI, a UTI in the preceding month, symptoms lasting longer than 14 days, and known kidney disease, anatomic abnormalities, or previous renal surgery. In addition, we excluded women receiving chemotherapy and those who had received antibiotics in the preceding month. Informed consent was obtained. Enrollment occurred between October of 1997 and March of 1999. All enrolled patients received $25 for participating in the study. The Michigan State University Committee on Research Involving Human Subjects approved our study.
Procedures and Measures
We stratified each practice, and using a central computer-generated random number, we blindly allocated patients to either treatment by telephone (telephone group) or office-based care (control group) by using an opaque envelope containing the group assignment. The envelope also provided instructions appropriate for the assigned group. We asked the telephone strategy patients to come to the clinic to leave a urine sample and pick up a prescription for an antibiotic; the control (usual care) patients were given a same-day appointment for a regular clinic visit.
We asked patients enrolled in both groups to rate the severity of urinary dysuria, urgency, frequency, function, and how they generally felt about their symptoms. Each was rated on a 10-point scale (10 was most severe). The telephone management patients were given a prescription for sulfamethoxazole/trimethoprim (800 mg/160 mg) twice daily for 7 days. If the patient was allergic to sulfa, she received a prescription for nitrofurantoin 100 mg twice daily for 7 days. Patients were required to have a urinalysis and urine culture before receiving the prescription. We asked the health care providers of the control patients to use their usual management strategies. The control group patients were also required to have a urinalysis and urine culture.
A nurse telephoned all women in both groups for follow-up 3 and 10 days after the time of enrollment. During these telephone calls we assessed patient status (including symptom scores and patient satisfaction) and whether the patient sought care with any practitioner since the initial time of enrollment. If UTI symptoms were still evident at either the 3- or 10-day follow-up, the patient was asked to make an appointment to see her practitioner. We recorded start and stop times for all interviews to assist in estimating and comparing costs of care for the 2 groups.
Primary Outcomes
The primary outcomes for our study were the UTI score and overall evaluation rating (OER)6 of the treatment experience. The UTI score is the sum of the ratings of severity of dysuria, urgency, frequency, function, and general symptoms (range=0-50). Since the UTI score has not been previously used, we pilot tested it on 20 women. These same women were reevaluated 2 to 3 days later. Test-retest reliability (measured by the overall correlation between the same items asked on 2 separate occasions with the order of questions changed) was 0.98. Validity (measured by the correlation coefficient between specific questions and a global question for the episode) was 0.52 for burning, 0.89 for frequency, 0.95 for urgency, 0.86 for interference with activities of daily living, and -0.76 for the actual interval between urination (smaller interval associated with greater overall episode severity).
The OER consisted of 2 questions about the overall quality of care and the outcome of care. Each question—answered as poor, fair, good, very good, or excellent—was scored from 1 to 5, respectively. The OER, a validated score,6 is a simple sum of the scores for these items (range 2-10, not normally distributed). Also, we asked the women about their overall satisfaction on a 5-point Likert scale.
Secondary Outcomes
We also evaluated urine culture results. We defined a negative culture as one with either no growth or less than 1000 colony-forming units. A positive culture had any growth of a single organism. A contaminated specimen (mixed flora) was rated as a negative culture, since this is how these are usually handled clinically.
Statistical Analysis
The data were entered into a database, and all entries were double-checked by one of the investigators for transcription errors. We analyzed the data using SAS software (version 7, SAS Institute, Cary, NC). Continuous variables (age, time variables, UTI score) were compared by treatment group using unpaired Student t tests. We used the Shapiro-Wilk test and the Kolmogorov-Smirnov test to confirm that the UTI score and changes in that score between assessments were normally distributed. We compared categorical variables (resolution of symptoms, culture results) by treatment group using a chi-square. We used Wilcoxon rank sum tests to compare ordinal data (OER) between the treatment groups.
For all sample size estimations we wanted to achieve 80% power with two-sided a of 0.05. To detect a 5-point difference in the UTI score, with an estimated a priori standard deviation (SD) of 5, we calculated that 16 subjects in each group are needed (21 for 90% power). With an SD of 7.5, 36 subjects in each group are needed. For patient satisfaction (OER), we estimated a priori that we would need 15 subjects in each group (19 for 90% power) to detect a 1-point difference with an estimated mean of 3.9 and a SD of 0.95.
Results
We identified 201 women with suspected UTIs of whom 99 were not eligible. Of the 102 eligible women, 30 declined to participate. We randomized 36 women to office care (control group) and 36 to telephone management. The subjects were predominantly white (n=62, 86%) and young (mean age=36.6 years, SD=12.3). Five patients had no culture results. Of the 67 remaining cultures, 4 (6%) were contaminated specimens, 20 (29.8%) had negative cultures, and 43 (64.2%) had positive cultures. Of the positive cultures, 34 (79.1%) grew Escherichia coli. Twenty-three of 34 (67.6%) cultures in the control group were positive compared with 20 of 33 (60.6%) in the telephone group (chi-square=0.3611; P=.55).
The groups were similar at baseline Table 1. On day 3 and day 10 there were no significant differences in the change in symptom scores or overall UTI score from baseline. We also found no difference in the change in urinary intervals from baseline. Table 2 shows these data. There was no significant difference in the overall evaluation rating. We also found no difference in satisfaction with care (median response was “very good” in the control group and “excellent” in the telephone group). These are shown in Table 3
On the third day after therapy was inititated, 20 of 33 (60.6%) of the control subjects had persistent urinary symptoms compared with 19 of 34 (55.8%, chi-square=0.1536; P=.70) in the telephone group. By day 10, 6 of 35 control patients (17.1%) had persistent symptoms, compared with 12 of 35 (34.3%) in the telephone group (chi-square=2.6923; P=0.1). Among the patients still symptomatic on the third day, culture results were available for 35, 11 (31%) of which were negative. Among those still symptomatic on the 10th day, 18 had available cultures, 9 (50%) of which were negative.
To evaluate the patients with persistent symptoms at the conclusion of the study we looked at the baseline and final UTI scores and baseline culture results. Six patients in the control group reported persistent symptoms. Three of these patients had final UTI scores less than 10, and 3 had UTI scores greater than 20. Two of these patients also had negative cultures. Twelve patients in the telephone group reported persistent symptoms; all but 1 had final UTI scores less than 10, and only 1 had a final UTI score greater than 20. Seven of the 12 patients had negative cultures at baseline.
We attempted to determine how office care differed from telephone care. Three patients in the control group received no antibiotics. Two of these had negative cultures, and no culture result was available for the third patient. All patients in the telephone group were prescribed antibiotics. Five control group patients who ultimately had positive cultures took antibiotics for less than 7 days, compared with only 3 in the telephone group. Among those receiving antibiotics, 30 of the control group patients received either sulfamethoxazole/trimethoprim or nitrofurantoin, and 3 received second-line agents. Because of allergies, 1 patient in the telephone group did not receive the planned therapy and received cephalexin instead.
We also evaluated the nursing time to administer various elements of the protocol. It took 2.5 minutes (SD=1.3) to determine eligibility to participate in the study and 5.3 minutes (SD=2.1) to enroll the subjects into the study. The nursing time for the day 3 follow-up took on average 5.6 minutes (SD=2.9) and 5.2 minutes (SD=2.0) on day 10.
Discussion
Although managing uncomplicated UTIs by telephone is a common practice in ambulatory primary care settings, we had no previous empiric evidence of its effectiveness compared with seeing patients in the office. In this randomized trial of office management versus management by telephone, two thirds of the women enrolled had culture-confirmed UTIs. The rate was similar in each group and mirrors that reported in the literature.7 We found no difference in improvement in symptom scores from baseline and no significant difference in overall satisfaction with the care provided or the outcome.
Gallagher and colleagues8 reported that when acute medical problems are triaged by nurses, patients are generally satisfied with care. However, UTIs represented only 5% of the telephone encounters. Delichatsios and coworkers9 similarly reported that patients calling to speak with the physician were generally satisfied with the advice given on the telephone, but they did not report outcomes related to specific conditions or therapies. Although 2 independent economic evaluations3,4 have found empiric therapy to be cost-effective, neither included a strategy that avoids an office visit.
The direct cost of telephone management of uncomplicated UTIs is relatively low. It took only 2.5 minutes of nurse time to identify symptomatic women with risk factors for complicated UTIs who were good candidates for telephone management. This may cause a dilemma. Physicians practicing in predominantly fee-for-service settings will lose income by managing UTIs by telephone. In managed care settings, the financial incentives to reduce utilization make this practice inexpensive while simultaneously maintaining high patient satisfaction. Many physicians, however, complain about the complexity of the patients they now see, and having an occasional uncomplicated UTI might provide some breathing space on hectic days.
Limitations
We did not ask the practitioners who provided office-based care to alter their usual approach. By patient report, only 3 control patients received no antibiotics. This may reflect a knee-jerk response in which antibiotics are prescribed for all women with a suspected UTI. It may also reflect a very appropriate therapeutic threshold where physicians have a gestalt about the probability of a UTI that exceeds any diagnostic uncertainty. Although this has been described explicitly,10 we believe that seasoned clinicians do this implicitly. We did not attempt to open the “black box” to further understand this process.
Approximately half the women calling for appointments were not eligible to participate in our study because of the presence of 1 or more complicating factor. The most common reason was the presence of back pain, a complaint that commonly accompanies uncomplicated as well as complicated UTIs. Although the prevalence of acute pyelonephritis is very low, our protocol conservatively put women with this isolated complaint in a potentially high-risk group that required an office visit. It is quite likely that using a constellation of symptoms (such as back or flank pain plus fever or chills or nausea and vomiting, and so forth) would have allowed more women to be eligible. The participation rate was high among eligible women, improving the generalizability of the data. Although we enrolled predominantly white women (reflecting the ethnic mix of the participating practices), we believe the biologic responses in our study are not race dependent. We are not confident, however, that patient satisfaction data will extrapolate to other groups, since women in groups that have traditionally been underserved by the health care system may see telephone management as a way to shortchange them.
Our study was planned to have 80% power to detect important differences in the primary outcome variables but lacks sufficient sample size to determine if patients in either group were more likely to experience pyelonephritis or other complications. Since the specific therapy was similar in each group, one would suspect a similar rate of complications.
Twice as many patients in the telephone group were still symptomatic after 10 days, compared with those seen in the office. The small numbers in our study raise the possibility that clinically meaningful differences did not reach statistical significance. However, a closer look at the UTI scores suggests that only 1 of the 12 patients in the telephone group who reported persistent symptoms had a high score compared with 3 of the 6 control patients. This suggests that the severity of the persistent symptoms was quite low. Also, it raises the possibility that symptoms such as low back pain that were not captured by the UTI score or possibly not related to UTI were unimproved. We also believe that many of these women may have had other conditions causing their persistent symptoms. Finally, it is possible that these findings reflect a significant degree of statistical “noise” due to the wide confidence intervals associated with small studies. This is an area for further study.
This study used 7 days of antibiotic therapy. Currently, 3 days of therapy are increasingly used. Interestingly, more than half the women were still symptomatic on day 3. At the conclusion of the study, though, 75% of all women reported resolution of their symptoms. Although this discussion places this observation in a different context, it may raise potential concerns about whether 3-day therapy (while effective in delivering laboratory “cures”) may not provide enough relief to patients to be worth the tradeoffs.
Conclusions
This study demonstrates that managing uncomplicated UTIs in otherwise healthy women over the telephone has comparable outcomes and patient satisfaction with managing these women with an office visit. Whether symptom resolution is the same is not adequately answered by our study. More research on the optimal use of triage protocols for common acute conditions is needed in the primary care setting.
Acknowledgments
Our research was funded by the Blue Cross Blue Shield of Michigan Foundation grant # 231-II: a randomized clinical trial comparing telephone and usual care strategies for the management of suspected UTI in otherwise healthy adult women. We thank the following practices that participated in our study: Michigan State University Department of Family Practice, East Lansing; Order of St. Francis Medical Group, Escanaba; and Doctors Park Family Physicians, Escanaba. We are especially appreciative of the efforts of the office nurses and physician’s assistants who recruited and provided the telephone follow-up of the patients: Barb Bedient, LPN; Lisa Sweet, LPN; Debi Besson, RN; Grace Borkadi, PAC; Gloria Johnson, LPN; and Mary Baron, RN. Conflict of interest statement: Dr Ebell is editor of The Journal of Family Practice, Dr Hickner is an associate editor, and Dr Barry is an assistant editor. Therefore, the peer review process, including selection of reviewers, editorial review, editing, and the decision to accept or reject the manuscript was performed by Dr Bernard Ewigman, MD, MSPH, Associate Editor of JFP.
STUDY DESIGN: We randomly assigned women calling their usual provider with a suspected UTI to receive care over the telephone (n=36) or usual office-based care (n=36). All women had urinalyses and urine cultures. All were treated with 7 days of antibiotics. We compared symptom scores at baseline and at day 3 and day 10 after therapy. We also compared patient satisfaction at the end of the study. The settings were family practices in Michigan.
POPULATION: We included healthy nonpregnant women older than 18 years.
RESULTS: A total of 201 women with suspected UTIs called their physician. Of these, 99 were ineligible, and 30 declined to participate. The women were young (mean age=36.6 years) and predominantly white (86%). Sixty-four percent of the urine cultures had significant growth of a single organism. We observed no difference in symptom scores or satisfaction. Overall, satisfaction was high.
CONCLUSIONS: Short-term outcomes of managing suspected UTIs by telephone appear to be comparable with usual office care.
Millions of women with acute dysuria show up at offices, urgent care centers, and emergency departments for suspected urinary tract infections (UTIs), accounting for more than $1 billion in direct costs.1 Since most UTIs are uncomplicated, numerous strategies have been proposed for managing them in more efficient and less costly ways. Berg2 found 82 separate management strategies among 137 family physicians, with costs ranging from negligible to $250.
In a previous study3 we used a cost-utility analysis to demonstrate that among office-based approaches, empiric therapy of suspected UTIs was most cost-effective. This was robust over a wide range of assumptions, including prevalence, test characteristics, costs, complication rates, and outcomes. These findings were recently confirmed by Fenwick and colleagues4 in a British analysis. Among the approaches commonly used, telephone management has the potential for reducing cost, increasing convenience for patients, and reducing barriers to care. Saint and coworkers5 demonstrated that a clinical practice guideline using telephone-based management of presumed UTIs reduced the use of urine tests and increased the use of guideline-specified antibiotics. Although telephone management is common, we were unable to find any studies directly comparing that approach with office-based care.
We report on the first trial in which women with suspected UTIs were randomly assigned to telephone management or office-based management. The purpose of our study was to identify the prevalence of UTIs in women presenting with suspected UTIs, to determine if telephone management was comparable in outcomes with those of office-based care, and to determine if women whose infections were managed by telephone were satisfied with their care.
Methods
Setting
We enrolled patients at 6 primary care offices (including a walk-in clinic) of the Upper Peninsula Research Network (UPRNet) and the Department of Family Practice at Michigan State University in East Lansing. UPRNet is a primary care research network in the Upper Peninsula of Michigan.
Subjects
Nonpregnant women 18 years or older completed an interview to confirm eligibility when they called their primary care physicians with a suspected uncomplicated UTI. We defined a suspected uncomplicated UTI as a complaint of dysuria, pain on urination, complaint of urinary urgency and frequency, or the patient’s saying, “I think I have a bladder infection.” Patients with symptoms compatible with pyelonephritis (fever, chills, sweats, back or flank pain, or vomiting), vaginitis, or cervicitis (presence of a new or changed vaginal discharge) were excluded from our study. We also excluded women with diabetes, a previous history of pyelonephritis or other complicated UTI, a UTI in the preceding month, symptoms lasting longer than 14 days, and known kidney disease, anatomic abnormalities, or previous renal surgery. In addition, we excluded women receiving chemotherapy and those who had received antibiotics in the preceding month. Informed consent was obtained. Enrollment occurred between October of 1997 and March of 1999. All enrolled patients received $25 for participating in the study. The Michigan State University Committee on Research Involving Human Subjects approved our study.
Procedures and Measures
We stratified each practice, and using a central computer-generated random number, we blindly allocated patients to either treatment by telephone (telephone group) or office-based care (control group) by using an opaque envelope containing the group assignment. The envelope also provided instructions appropriate for the assigned group. We asked the telephone strategy patients to come to the clinic to leave a urine sample and pick up a prescription for an antibiotic; the control (usual care) patients were given a same-day appointment for a regular clinic visit.
We asked patients enrolled in both groups to rate the severity of urinary dysuria, urgency, frequency, function, and how they generally felt about their symptoms. Each was rated on a 10-point scale (10 was most severe). The telephone management patients were given a prescription for sulfamethoxazole/trimethoprim (800 mg/160 mg) twice daily for 7 days. If the patient was allergic to sulfa, she received a prescription for nitrofurantoin 100 mg twice daily for 7 days. Patients were required to have a urinalysis and urine culture before receiving the prescription. We asked the health care providers of the control patients to use their usual management strategies. The control group patients were also required to have a urinalysis and urine culture.
A nurse telephoned all women in both groups for follow-up 3 and 10 days after the time of enrollment. During these telephone calls we assessed patient status (including symptom scores and patient satisfaction) and whether the patient sought care with any practitioner since the initial time of enrollment. If UTI symptoms were still evident at either the 3- or 10-day follow-up, the patient was asked to make an appointment to see her practitioner. We recorded start and stop times for all interviews to assist in estimating and comparing costs of care for the 2 groups.
Primary Outcomes
The primary outcomes for our study were the UTI score and overall evaluation rating (OER)6 of the treatment experience. The UTI score is the sum of the ratings of severity of dysuria, urgency, frequency, function, and general symptoms (range=0-50). Since the UTI score has not been previously used, we pilot tested it on 20 women. These same women were reevaluated 2 to 3 days later. Test-retest reliability (measured by the overall correlation between the same items asked on 2 separate occasions with the order of questions changed) was 0.98. Validity (measured by the correlation coefficient between specific questions and a global question for the episode) was 0.52 for burning, 0.89 for frequency, 0.95 for urgency, 0.86 for interference with activities of daily living, and -0.76 for the actual interval between urination (smaller interval associated with greater overall episode severity).
The OER consisted of 2 questions about the overall quality of care and the outcome of care. Each question—answered as poor, fair, good, very good, or excellent—was scored from 1 to 5, respectively. The OER, a validated score,6 is a simple sum of the scores for these items (range 2-10, not normally distributed). Also, we asked the women about their overall satisfaction on a 5-point Likert scale.
Secondary Outcomes
We also evaluated urine culture results. We defined a negative culture as one with either no growth or less than 1000 colony-forming units. A positive culture had any growth of a single organism. A contaminated specimen (mixed flora) was rated as a negative culture, since this is how these are usually handled clinically.
Statistical Analysis
The data were entered into a database, and all entries were double-checked by one of the investigators for transcription errors. We analyzed the data using SAS software (version 7, SAS Institute, Cary, NC). Continuous variables (age, time variables, UTI score) were compared by treatment group using unpaired Student t tests. We used the Shapiro-Wilk test and the Kolmogorov-Smirnov test to confirm that the UTI score and changes in that score between assessments were normally distributed. We compared categorical variables (resolution of symptoms, culture results) by treatment group using a chi-square. We used Wilcoxon rank sum tests to compare ordinal data (OER) between the treatment groups.
For all sample size estimations we wanted to achieve 80% power with two-sided a of 0.05. To detect a 5-point difference in the UTI score, with an estimated a priori standard deviation (SD) of 5, we calculated that 16 subjects in each group are needed (21 for 90% power). With an SD of 7.5, 36 subjects in each group are needed. For patient satisfaction (OER), we estimated a priori that we would need 15 subjects in each group (19 for 90% power) to detect a 1-point difference with an estimated mean of 3.9 and a SD of 0.95.
Results
We identified 201 women with suspected UTIs of whom 99 were not eligible. Of the 102 eligible women, 30 declined to participate. We randomized 36 women to office care (control group) and 36 to telephone management. The subjects were predominantly white (n=62, 86%) and young (mean age=36.6 years, SD=12.3). Five patients had no culture results. Of the 67 remaining cultures, 4 (6%) were contaminated specimens, 20 (29.8%) had negative cultures, and 43 (64.2%) had positive cultures. Of the positive cultures, 34 (79.1%) grew Escherichia coli. Twenty-three of 34 (67.6%) cultures in the control group were positive compared with 20 of 33 (60.6%) in the telephone group (chi-square=0.3611; P=.55).
The groups were similar at baseline Table 1. On day 3 and day 10 there were no significant differences in the change in symptom scores or overall UTI score from baseline. We also found no difference in the change in urinary intervals from baseline. Table 2 shows these data. There was no significant difference in the overall evaluation rating. We also found no difference in satisfaction with care (median response was “very good” in the control group and “excellent” in the telephone group). These are shown in Table 3
On the third day after therapy was inititated, 20 of 33 (60.6%) of the control subjects had persistent urinary symptoms compared with 19 of 34 (55.8%, chi-square=0.1536; P=.70) in the telephone group. By day 10, 6 of 35 control patients (17.1%) had persistent symptoms, compared with 12 of 35 (34.3%) in the telephone group (chi-square=2.6923; P=0.1). Among the patients still symptomatic on the third day, culture results were available for 35, 11 (31%) of which were negative. Among those still symptomatic on the 10th day, 18 had available cultures, 9 (50%) of which were negative.
To evaluate the patients with persistent symptoms at the conclusion of the study we looked at the baseline and final UTI scores and baseline culture results. Six patients in the control group reported persistent symptoms. Three of these patients had final UTI scores less than 10, and 3 had UTI scores greater than 20. Two of these patients also had negative cultures. Twelve patients in the telephone group reported persistent symptoms; all but 1 had final UTI scores less than 10, and only 1 had a final UTI score greater than 20. Seven of the 12 patients had negative cultures at baseline.
We attempted to determine how office care differed from telephone care. Three patients in the control group received no antibiotics. Two of these had negative cultures, and no culture result was available for the third patient. All patients in the telephone group were prescribed antibiotics. Five control group patients who ultimately had positive cultures took antibiotics for less than 7 days, compared with only 3 in the telephone group. Among those receiving antibiotics, 30 of the control group patients received either sulfamethoxazole/trimethoprim or nitrofurantoin, and 3 received second-line agents. Because of allergies, 1 patient in the telephone group did not receive the planned therapy and received cephalexin instead.
We also evaluated the nursing time to administer various elements of the protocol. It took 2.5 minutes (SD=1.3) to determine eligibility to participate in the study and 5.3 minutes (SD=2.1) to enroll the subjects into the study. The nursing time for the day 3 follow-up took on average 5.6 minutes (SD=2.9) and 5.2 minutes (SD=2.0) on day 10.
Discussion
Although managing uncomplicated UTIs by telephone is a common practice in ambulatory primary care settings, we had no previous empiric evidence of its effectiveness compared with seeing patients in the office. In this randomized trial of office management versus management by telephone, two thirds of the women enrolled had culture-confirmed UTIs. The rate was similar in each group and mirrors that reported in the literature.7 We found no difference in improvement in symptom scores from baseline and no significant difference in overall satisfaction with the care provided or the outcome.
Gallagher and colleagues8 reported that when acute medical problems are triaged by nurses, patients are generally satisfied with care. However, UTIs represented only 5% of the telephone encounters. Delichatsios and coworkers9 similarly reported that patients calling to speak with the physician were generally satisfied with the advice given on the telephone, but they did not report outcomes related to specific conditions or therapies. Although 2 independent economic evaluations3,4 have found empiric therapy to be cost-effective, neither included a strategy that avoids an office visit.
The direct cost of telephone management of uncomplicated UTIs is relatively low. It took only 2.5 minutes of nurse time to identify symptomatic women with risk factors for complicated UTIs who were good candidates for telephone management. This may cause a dilemma. Physicians practicing in predominantly fee-for-service settings will lose income by managing UTIs by telephone. In managed care settings, the financial incentives to reduce utilization make this practice inexpensive while simultaneously maintaining high patient satisfaction. Many physicians, however, complain about the complexity of the patients they now see, and having an occasional uncomplicated UTI might provide some breathing space on hectic days.
Limitations
We did not ask the practitioners who provided office-based care to alter their usual approach. By patient report, only 3 control patients received no antibiotics. This may reflect a knee-jerk response in which antibiotics are prescribed for all women with a suspected UTI. It may also reflect a very appropriate therapeutic threshold where physicians have a gestalt about the probability of a UTI that exceeds any diagnostic uncertainty. Although this has been described explicitly,10 we believe that seasoned clinicians do this implicitly. We did not attempt to open the “black box” to further understand this process.
Approximately half the women calling for appointments were not eligible to participate in our study because of the presence of 1 or more complicating factor. The most common reason was the presence of back pain, a complaint that commonly accompanies uncomplicated as well as complicated UTIs. Although the prevalence of acute pyelonephritis is very low, our protocol conservatively put women with this isolated complaint in a potentially high-risk group that required an office visit. It is quite likely that using a constellation of symptoms (such as back or flank pain plus fever or chills or nausea and vomiting, and so forth) would have allowed more women to be eligible. The participation rate was high among eligible women, improving the generalizability of the data. Although we enrolled predominantly white women (reflecting the ethnic mix of the participating practices), we believe the biologic responses in our study are not race dependent. We are not confident, however, that patient satisfaction data will extrapolate to other groups, since women in groups that have traditionally been underserved by the health care system may see telephone management as a way to shortchange them.
Our study was planned to have 80% power to detect important differences in the primary outcome variables but lacks sufficient sample size to determine if patients in either group were more likely to experience pyelonephritis or other complications. Since the specific therapy was similar in each group, one would suspect a similar rate of complications.
Twice as many patients in the telephone group were still symptomatic after 10 days, compared with those seen in the office. The small numbers in our study raise the possibility that clinically meaningful differences did not reach statistical significance. However, a closer look at the UTI scores suggests that only 1 of the 12 patients in the telephone group who reported persistent symptoms had a high score compared with 3 of the 6 control patients. This suggests that the severity of the persistent symptoms was quite low. Also, it raises the possibility that symptoms such as low back pain that were not captured by the UTI score or possibly not related to UTI were unimproved. We also believe that many of these women may have had other conditions causing their persistent symptoms. Finally, it is possible that these findings reflect a significant degree of statistical “noise” due to the wide confidence intervals associated with small studies. This is an area for further study.
This study used 7 days of antibiotic therapy. Currently, 3 days of therapy are increasingly used. Interestingly, more than half the women were still symptomatic on day 3. At the conclusion of the study, though, 75% of all women reported resolution of their symptoms. Although this discussion places this observation in a different context, it may raise potential concerns about whether 3-day therapy (while effective in delivering laboratory “cures”) may not provide enough relief to patients to be worth the tradeoffs.
Conclusions
This study demonstrates that managing uncomplicated UTIs in otherwise healthy women over the telephone has comparable outcomes and patient satisfaction with managing these women with an office visit. Whether symptom resolution is the same is not adequately answered by our study. More research on the optimal use of triage protocols for common acute conditions is needed in the primary care setting.
Acknowledgments
Our research was funded by the Blue Cross Blue Shield of Michigan Foundation grant # 231-II: a randomized clinical trial comparing telephone and usual care strategies for the management of suspected UTI in otherwise healthy adult women. We thank the following practices that participated in our study: Michigan State University Department of Family Practice, East Lansing; Order of St. Francis Medical Group, Escanaba; and Doctors Park Family Physicians, Escanaba. We are especially appreciative of the efforts of the office nurses and physician’s assistants who recruited and provided the telephone follow-up of the patients: Barb Bedient, LPN; Lisa Sweet, LPN; Debi Besson, RN; Grace Borkadi, PAC; Gloria Johnson, LPN; and Mary Baron, RN. Conflict of interest statement: Dr Ebell is editor of The Journal of Family Practice, Dr Hickner is an associate editor, and Dr Barry is an assistant editor. Therefore, the peer review process, including selection of reviewers, editorial review, editing, and the decision to accept or reject the manuscript was performed by Dr Bernard Ewigman, MD, MSPH, Associate Editor of JFP.
1. Johnson JR, Stamm WE. Diagnosis and treatment of acute urinary tract infections [published erratum appears in Infect Dis Clin North Am 1990; 4:following xii.]. Infect Dis Clin North Am 1987;1:773-91.
2. Berg AO. Variations among family physicians’ management strategies for lower urinary tract infection in women: a report from the Washington Family Physicians Collaborative Research Network. J Am Board Fam Pract 1991;4:327-30.
3. Barry H, Ebell M, Hickner J. Evaluation of suspected UTI in ambulatory women: a cost-utility analysis of office-based strategies. J Fam Pract 1997;44:49-60.
4. Fenwick E, Briggs A, Hawke C. Management of urinary tract infection in general practice: a cost-effectiveness analysis. Br J Gen Pract 2000;50:635-39.
5. Saint S, Scholes D, Fihn SD, Farrell RG, Stamm WE. The effectiveness of a clinical practice guideline for the management of presumed uncomplicated urinary tract infection in women. Am J Med 1999;106:636-41.
6. Ross CK, Steward CA, Sinacore JM. A comparative study of seven measures of patient satisfaction. MedCare 1995;33:392-406.
7. Stamm WE, Wagner KF, Amsel R, et al. Causes of the acute urethral syndrome in women. N Engl J Med 1980;303:409-15.
8. Gallagher M, Huddart T, Henderson B. Telephone triage of acute illness by a practice nurse in general practice: outcomes of care. Br J Gen Pract 1998;48:1141-45.
9. Delichatsios H, Callahan M, Charlson M. Outcomes of telephone medical care. J Gen Intern Med 1998;13:579-85.
10. Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med 1980;302:1109-17.
1. Johnson JR, Stamm WE. Diagnosis and treatment of acute urinary tract infections [published erratum appears in Infect Dis Clin North Am 1990; 4:following xii.]. Infect Dis Clin North Am 1987;1:773-91.
2. Berg AO. Variations among family physicians’ management strategies for lower urinary tract infection in women: a report from the Washington Family Physicians Collaborative Research Network. J Am Board Fam Pract 1991;4:327-30.
3. Barry H, Ebell M, Hickner J. Evaluation of suspected UTI in ambulatory women: a cost-utility analysis of office-based strategies. J Fam Pract 1997;44:49-60.
4. Fenwick E, Briggs A, Hawke C. Management of urinary tract infection in general practice: a cost-effectiveness analysis. Br J Gen Pract 2000;50:635-39.
5. Saint S, Scholes D, Fihn SD, Farrell RG, Stamm WE. The effectiveness of a clinical practice guideline for the management of presumed uncomplicated urinary tract infection in women. Am J Med 1999;106:636-41.
6. Ross CK, Steward CA, Sinacore JM. A comparative study of seven measures of patient satisfaction. MedCare 1995;33:392-406.
7. Stamm WE, Wagner KF, Amsel R, et al. Causes of the acute urethral syndrome in women. N Engl J Med 1980;303:409-15.
8. Gallagher M, Huddart T, Henderson B. Telephone triage of acute illness by a practice nurse in general practice: outcomes of care. Br J Gen Pract 1998;48:1141-45.
9. Delichatsios H, Callahan M, Charlson M. Outcomes of telephone medical care. J Gen Intern Med 1998;13:579-85.
10. Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med 1980;302:1109-17.