User login
Things We Do for No Reason™: Tumor Markers CA125, CA19-9, and CEA in the Initial Diagnosis of Malignancy
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old woman presents to the emergency department with a 2-week history of abdominal pain associated with nausea and an episode of nonbilious, nonbloody emesis. Her last bowel movement was 2 days prior to her presentation. The patient has tachycardia to 105 beats per minute but otherwise normal vital signs. Findings on her physical examination include dry mucous membranes and increased bowel sounds. A review of systems reveals an unintentional weight loss of 15 kg over the past 4 months and increased fatigue. Computed tomography scan of the abdomen and pelvis with contrast reveals multiple areas of attenuation in the liver and small bowel obstruction. The hospitalist admits the patient to the medicine service for supportive treatment and workup for underlying malignancy. Her admitting team orders serum tumor biomarkers on admission to expedite the diagnosis.
BACKGROUND
When patients present with unexplained weight loss or with metastasis from an unknown primary location, the initial workup often includes imaging and a tumor biomarker panel (eg, cancer antigen 125 [CA125], carbohydrate antigen 19-9 [CA19-9], carcinoembryonic antigen [CEA]). The CA125, CA19-9, and CEA biomarkers are traditionally associated with ovarian, pancreatic, and colorectal cancer, respectively.1 While clinicians initially used these serum biomarkers to monitor for cancer recurrence or treatment response, they have since become widely used in multiple clinical stages of oncological evaluation.
WHY YOU MIGHT THINK CA125, CA19-9, AND CEA ARE HELPFUL IN THE DIAGNOSIS OF CANCER
Hospitalists routinely order biomarkers as part of the malignancy workup. More than a dozen oncology biomarkers are used in the clinical setting to risk stratify, plan treatment, and monitor for recurrence. For example, studies associate elevated preoperative levels of CEA and CA19-9 with metastatic invasion of colorectal2 and gastric3 cancers and with poor prognosis of intrahepatic cholangiocarcinoma. Similarly, CA125 has demonstrated utility in monitoring response to ovarian cancer treatment.4 Specific biomarkers, such as alpha-fetoprotein, improve diagnosis of liver and nonseminomatous testicular tumors.5 Clinicians often apply the same paradigm to other biomarkers due to their widespread availability, noninvasiveness, reproducibility, and ease of use, particularly in acute settings wherein any new information is perceived to be potentially helpful.
WHY YOU SHOULD NOT USE CA125, CA19-9, AND CEA TO DIAGNOSE CANCER
Utilizing these serum biomarkers to diagnose cancer has the potential for diagnostic error and can result in unnecessary patient anxiety and follow-up testing. Since tissue sampling is necessary and remains the gold standard in most cancer diagnoses, obtaining these tumor biomarkers in the early diagnostic stage does not change management and may even lead to harm. Furthermore, due to their poor sensitivity and specificity, these biomarkers cannot rule in or rule out cancer. Elevated CA125, CA19-9, and CEA biomarkers occur in a variety of malignancies, including gastric, gallbladder, hepatocellular, bladder, and breast cancers.1,3,6 In addition, these biomarkers have a very limited role in the workup of cancer of unknown primary origin.7
Even in the setting of a known pelvic mass, the use of CA125 alone has poor sensitivity at a cut-off level of 35 U/mL as a biomarker for the diagnosis of early ovarian cancer.8
Serum CA19-9 is not a useful diagnostic biomarker as elevated CA19-9 can occur in benign conditions, including cirrhosis, chronic pancreatitis, and cholangitis. In a systematic review of patients with histologic confirmation of pancreatic malignancy, the median positive predictive value of CA19-9 was 72% (interquartile range, 41%-95%).9 Additionally, patients with Lewis-null blood type, which is present in 5% to 10% of the Caucasian population, do not produce CA19-9.10 Therefore, CA19-9 will be 0% specific for tumors in this population.
The use of CEA in the diagnosis of colorectal cancer is also questionable. In stage I colorectal cancer, CEA was only 38.1% sensitive at a cut-off level of 2.41 ng/mL; it was 78.3% sensitive in stage IV disease.11 The specificity of CEA is limited since elevated CEA occurs in benign conditions, such as inflammatory bowel disease, smoking, hypothyroidism, pancreatitis, biliary obstruction, peptic ulcers, and cirrhosis—though CEA levels in these conditions are rarely >10 ng/mL.11 Regardless of the results of biomarker testing, definitive diagnosis requires tissue biopsy; therefore, biomarker findings are of little utility in the initial workup.
In addition to variable diagnostic utility, overreliance on these biomarkers has the potential for serious patient harm. In a study examining patients with established rectal cancer, combination CEA and CA19-9 testing alone was insufficient to predict the pathologic stage of disease correctly.2 A cancer misdiagnosis not only traumatizes patients but also erodes their trust in clinicians and creates anxiety during future clinical encounters. Overutilization of these tumor biomarkers is also costly and contributes to waste in the US healthcare system.
WHEN YOU SHOULD USE CA125, CA19-9, AND CEA
There is a role for tumor biomarker testing in specific cancers after the primary source of malignancy has been determined. When evaluating a known pelvic mass, CA125 testing is performed in conjunction with transvaginal ultrasound and assessment of menopausal status in the risk of ovarian malignancy algorithm for prognostication of disease prior to surgery.12 This algorithm takes into account levels of CA125 in addition to levels of human epididymis protein 4 and patient age, yielding an area under the curve as high as 0.93 for ovarian cancer risk classification.8 Beyond the prognostication process, oncologists follow CA125 to monitor response to first-line ovarian cancer treatment. However, CA125 has a less defined role in surveillance for ovarian cancer recurrence.
CA19-9 has demonstrated utility for pancreatic cancer and cholangiocarcinoma survival estimates. A national cohort analysis of patients with established intrahepatic cholangiocarcinoma found that CA19-9 independently predicted increased mortality. Patients with elevated CA19-9 also had significantly more nodal metastases and positive-margin resections.6 A study of 353 patients with pancreatic ductal adenocarcinoma undergoing radical resection further demonstrated the utility of CA19-9. In this study, patients with postoperative CA19-9 normalization had improved survival by almost 12 months when compared to those with consistently elevated CA19-9.13
Last, the literature describes CEA biomarker testing in the surveillance of patients after curative treatment of colon and rectal cancer. The American Society of Colon and Rectal Surgeons recommends regularly tracking this biomarker following curative resection, in conjunction with colonoscopy and chest and liver imaging studies.14 A prospective randomized controlled study that followed this monitoring protocol in cured asymptomatic patients on a bimonthly basis found that early diagnosis of recurrent colorectal cancer improved survival.15 The use of CEA testing as a monitoring tool should therefore be a point of discussion between providers and patients, as its utility varies based on patient comorbidities, their ability to tolerate surgery or chemotherapy, risk factors for recurrence, performance status, compliance, age, and preference.14
WHAT YOU SHOULD DO INSTEAD
The use of CA125, CA19-9, and CEA testing alone as initial diagnostic tools for malignancy are problematic due to their poor sensitivities and/or positive predictive value. Multiple studies have demonstrated their utility as markers of metastasis or malignancy progression rather than as clinically useful markers for the detection of any one type of cancer.1,3,6 In an undiagnosed symptomatic patient with unexplained weight loss or symptoms of a tumor mass, elevated CA125, CA19-9, and CEA add no new information as metastatic pancreatic, colorectal, ovarian, gastric, gallbladder, hepatocellular, bladder, ovarian, and breast cancers all remain in the differential diagnosis. Clinicians should approach the initial diagnosis of cancer in such patients with appropriate imaging studies, a thorough physical examination, and prompt biopsy of abnormal findings, as long as these are consistent with the patient’s goals of care. After establishing a tissue diagnosis, some tumor biomarkers have valid prognostic, staging, and monitoring roles.6,13,14
RECOMMENDATIONS
- Do not routinely order CA125, CA19-9, and CEA tests for the initial diagnostic workup of visceral malignancy of unknown origin regardless of whether imaging studies have been obtained.
- Use appropriate imaging, perform a thorough physical examination, and obtain tissue biopsy in the initial diagnostic workup of a visceral malignancy of unknown origin.
CONCLUSION
Clinicians should use serum biomarkers, like any other diagnostic test, to maximize benefit while preventing patient harm. In general, CA125, CA19-9, and CEA do not have a role in cancer diagnosis. The patient described in our clinical scenario would not benefit from a serum tumor biomarker panel at the time of admission. Regardless of findings from these tests, a tissue sample is required to make a definitive diagnosis of underlying malignancy in this patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Yotsukura S, Mamitsuka H. Evaluation of serum-based cancer biomarkers: a brief review from a clinical and computational viewpoint. Crit Rev Oncol Hematol. 2015;93(2):103-115. https://doi.org/10.1016/j.critrevonc.2014.10.002
2. Zhang B, Sun Z, Song M, et al. Ultrasound/CT combined with serum CEA/CA19.9 in the diagnosis and prognosis of rectal cancer. J Buon. 2018;23(3):592-597.
3. Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16(7):2685-2691. https://doi.org/10.7314/apjcp.2015.16.7.2685
4. Karam AK, Karlan BY. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol. 2010;7(6):335-339. https://doi.org/10.1038/nrclinonc.2010.44
5. Gilligan TD, Seidenfeld J, Basch EM, et al; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404. https://doi.org/10.1200/jco.2009.26.4481
6. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: a national cohort analysis. J Surg Oncol. 2016;114(4):475-482. https://doi.org/10.1002/jso.24381
7. Milovic M, Popov I, Jelic S. Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit. 2002;8(2):MT25-MT30.
8. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/10.1186/s13048-019-0503-7
9. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266-270. https://doi.org/10.1016/j.ejso.2006.10.004
10. Loosen SH, Neumann UP, Trautwein C, Roderburg C, Luedde T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017;39(6):1010428317692231. https://doi.org/10.1177/1010428317692231
11. Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1-e7. https://doi.org/10.3747/co.21.1711
12. Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43-51. https://doi.org/10.1097/igc.0000000000000586
13. Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161(2):373-384. https://doi.org/10.1016/j.surg.2016.08.005
14. Steele SR, Chang GJ, Hendren S, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713-725. https://doi.org/10.1097/dcr.0000000000000410
15. Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized “CEAwatch” trial. Eur J Surg Oncol. 2015;41(9):1188-1196. https://doi.org/10.1016/j.ejso.2015.06.008
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old woman presents to the emergency department with a 2-week history of abdominal pain associated with nausea and an episode of nonbilious, nonbloody emesis. Her last bowel movement was 2 days prior to her presentation. The patient has tachycardia to 105 beats per minute but otherwise normal vital signs. Findings on her physical examination include dry mucous membranes and increased bowel sounds. A review of systems reveals an unintentional weight loss of 15 kg over the past 4 months and increased fatigue. Computed tomography scan of the abdomen and pelvis with contrast reveals multiple areas of attenuation in the liver and small bowel obstruction. The hospitalist admits the patient to the medicine service for supportive treatment and workup for underlying malignancy. Her admitting team orders serum tumor biomarkers on admission to expedite the diagnosis.
BACKGROUND
When patients present with unexplained weight loss or with metastasis from an unknown primary location, the initial workup often includes imaging and a tumor biomarker panel (eg, cancer antigen 125 [CA125], carbohydrate antigen 19-9 [CA19-9], carcinoembryonic antigen [CEA]). The CA125, CA19-9, and CEA biomarkers are traditionally associated with ovarian, pancreatic, and colorectal cancer, respectively.1 While clinicians initially used these serum biomarkers to monitor for cancer recurrence or treatment response, they have since become widely used in multiple clinical stages of oncological evaluation.
WHY YOU MIGHT THINK CA125, CA19-9, AND CEA ARE HELPFUL IN THE DIAGNOSIS OF CANCER
Hospitalists routinely order biomarkers as part of the malignancy workup. More than a dozen oncology biomarkers are used in the clinical setting to risk stratify, plan treatment, and monitor for recurrence. For example, studies associate elevated preoperative levels of CEA and CA19-9 with metastatic invasion of colorectal2 and gastric3 cancers and with poor prognosis of intrahepatic cholangiocarcinoma. Similarly, CA125 has demonstrated utility in monitoring response to ovarian cancer treatment.4 Specific biomarkers, such as alpha-fetoprotein, improve diagnosis of liver and nonseminomatous testicular tumors.5 Clinicians often apply the same paradigm to other biomarkers due to their widespread availability, noninvasiveness, reproducibility, and ease of use, particularly in acute settings wherein any new information is perceived to be potentially helpful.
WHY YOU SHOULD NOT USE CA125, CA19-9, AND CEA TO DIAGNOSE CANCER
Utilizing these serum biomarkers to diagnose cancer has the potential for diagnostic error and can result in unnecessary patient anxiety and follow-up testing. Since tissue sampling is necessary and remains the gold standard in most cancer diagnoses, obtaining these tumor biomarkers in the early diagnostic stage does not change management and may even lead to harm. Furthermore, due to their poor sensitivity and specificity, these biomarkers cannot rule in or rule out cancer. Elevated CA125, CA19-9, and CEA biomarkers occur in a variety of malignancies, including gastric, gallbladder, hepatocellular, bladder, and breast cancers.1,3,6 In addition, these biomarkers have a very limited role in the workup of cancer of unknown primary origin.7
Even in the setting of a known pelvic mass, the use of CA125 alone has poor sensitivity at a cut-off level of 35 U/mL as a biomarker for the diagnosis of early ovarian cancer.8
Serum CA19-9 is not a useful diagnostic biomarker as elevated CA19-9 can occur in benign conditions, including cirrhosis, chronic pancreatitis, and cholangitis. In a systematic review of patients with histologic confirmation of pancreatic malignancy, the median positive predictive value of CA19-9 was 72% (interquartile range, 41%-95%).9 Additionally, patients with Lewis-null blood type, which is present in 5% to 10% of the Caucasian population, do not produce CA19-9.10 Therefore, CA19-9 will be 0% specific for tumors in this population.
The use of CEA in the diagnosis of colorectal cancer is also questionable. In stage I colorectal cancer, CEA was only 38.1% sensitive at a cut-off level of 2.41 ng/mL; it was 78.3% sensitive in stage IV disease.11 The specificity of CEA is limited since elevated CEA occurs in benign conditions, such as inflammatory bowel disease, smoking, hypothyroidism, pancreatitis, biliary obstruction, peptic ulcers, and cirrhosis—though CEA levels in these conditions are rarely >10 ng/mL.11 Regardless of the results of biomarker testing, definitive diagnosis requires tissue biopsy; therefore, biomarker findings are of little utility in the initial workup.
In addition to variable diagnostic utility, overreliance on these biomarkers has the potential for serious patient harm. In a study examining patients with established rectal cancer, combination CEA and CA19-9 testing alone was insufficient to predict the pathologic stage of disease correctly.2 A cancer misdiagnosis not only traumatizes patients but also erodes their trust in clinicians and creates anxiety during future clinical encounters. Overutilization of these tumor biomarkers is also costly and contributes to waste in the US healthcare system.
WHEN YOU SHOULD USE CA125, CA19-9, AND CEA
There is a role for tumor biomarker testing in specific cancers after the primary source of malignancy has been determined. When evaluating a known pelvic mass, CA125 testing is performed in conjunction with transvaginal ultrasound and assessment of menopausal status in the risk of ovarian malignancy algorithm for prognostication of disease prior to surgery.12 This algorithm takes into account levels of CA125 in addition to levels of human epididymis protein 4 and patient age, yielding an area under the curve as high as 0.93 for ovarian cancer risk classification.8 Beyond the prognostication process, oncologists follow CA125 to monitor response to first-line ovarian cancer treatment. However, CA125 has a less defined role in surveillance for ovarian cancer recurrence.
CA19-9 has demonstrated utility for pancreatic cancer and cholangiocarcinoma survival estimates. A national cohort analysis of patients with established intrahepatic cholangiocarcinoma found that CA19-9 independently predicted increased mortality. Patients with elevated CA19-9 also had significantly more nodal metastases and positive-margin resections.6 A study of 353 patients with pancreatic ductal adenocarcinoma undergoing radical resection further demonstrated the utility of CA19-9. In this study, patients with postoperative CA19-9 normalization had improved survival by almost 12 months when compared to those with consistently elevated CA19-9.13
Last, the literature describes CEA biomarker testing in the surveillance of patients after curative treatment of colon and rectal cancer. The American Society of Colon and Rectal Surgeons recommends regularly tracking this biomarker following curative resection, in conjunction with colonoscopy and chest and liver imaging studies.14 A prospective randomized controlled study that followed this monitoring protocol in cured asymptomatic patients on a bimonthly basis found that early diagnosis of recurrent colorectal cancer improved survival.15 The use of CEA testing as a monitoring tool should therefore be a point of discussion between providers and patients, as its utility varies based on patient comorbidities, their ability to tolerate surgery or chemotherapy, risk factors for recurrence, performance status, compliance, age, and preference.14
WHAT YOU SHOULD DO INSTEAD
The use of CA125, CA19-9, and CEA testing alone as initial diagnostic tools for malignancy are problematic due to their poor sensitivities and/or positive predictive value. Multiple studies have demonstrated their utility as markers of metastasis or malignancy progression rather than as clinically useful markers for the detection of any one type of cancer.1,3,6 In an undiagnosed symptomatic patient with unexplained weight loss or symptoms of a tumor mass, elevated CA125, CA19-9, and CEA add no new information as metastatic pancreatic, colorectal, ovarian, gastric, gallbladder, hepatocellular, bladder, ovarian, and breast cancers all remain in the differential diagnosis. Clinicians should approach the initial diagnosis of cancer in such patients with appropriate imaging studies, a thorough physical examination, and prompt biopsy of abnormal findings, as long as these are consistent with the patient’s goals of care. After establishing a tissue diagnosis, some tumor biomarkers have valid prognostic, staging, and monitoring roles.6,13,14
RECOMMENDATIONS
- Do not routinely order CA125, CA19-9, and CEA tests for the initial diagnostic workup of visceral malignancy of unknown origin regardless of whether imaging studies have been obtained.
- Use appropriate imaging, perform a thorough physical examination, and obtain tissue biopsy in the initial diagnostic workup of a visceral malignancy of unknown origin.
CONCLUSION
Clinicians should use serum biomarkers, like any other diagnostic test, to maximize benefit while preventing patient harm. In general, CA125, CA19-9, and CEA do not have a role in cancer diagnosis. The patient described in our clinical scenario would not benefit from a serum tumor biomarker panel at the time of admission. Regardless of findings from these tests, a tissue sample is required to make a definitive diagnosis of underlying malignancy in this patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old woman presents to the emergency department with a 2-week history of abdominal pain associated with nausea and an episode of nonbilious, nonbloody emesis. Her last bowel movement was 2 days prior to her presentation. The patient has tachycardia to 105 beats per minute but otherwise normal vital signs. Findings on her physical examination include dry mucous membranes and increased bowel sounds. A review of systems reveals an unintentional weight loss of 15 kg over the past 4 months and increased fatigue. Computed tomography scan of the abdomen and pelvis with contrast reveals multiple areas of attenuation in the liver and small bowel obstruction. The hospitalist admits the patient to the medicine service for supportive treatment and workup for underlying malignancy. Her admitting team orders serum tumor biomarkers on admission to expedite the diagnosis.
BACKGROUND
When patients present with unexplained weight loss or with metastasis from an unknown primary location, the initial workup often includes imaging and a tumor biomarker panel (eg, cancer antigen 125 [CA125], carbohydrate antigen 19-9 [CA19-9], carcinoembryonic antigen [CEA]). The CA125, CA19-9, and CEA biomarkers are traditionally associated with ovarian, pancreatic, and colorectal cancer, respectively.1 While clinicians initially used these serum biomarkers to monitor for cancer recurrence or treatment response, they have since become widely used in multiple clinical stages of oncological evaluation.
WHY YOU MIGHT THINK CA125, CA19-9, AND CEA ARE HELPFUL IN THE DIAGNOSIS OF CANCER
Hospitalists routinely order biomarkers as part of the malignancy workup. More than a dozen oncology biomarkers are used in the clinical setting to risk stratify, plan treatment, and monitor for recurrence. For example, studies associate elevated preoperative levels of CEA and CA19-9 with metastatic invasion of colorectal2 and gastric3 cancers and with poor prognosis of intrahepatic cholangiocarcinoma. Similarly, CA125 has demonstrated utility in monitoring response to ovarian cancer treatment.4 Specific biomarkers, such as alpha-fetoprotein, improve diagnosis of liver and nonseminomatous testicular tumors.5 Clinicians often apply the same paradigm to other biomarkers due to their widespread availability, noninvasiveness, reproducibility, and ease of use, particularly in acute settings wherein any new information is perceived to be potentially helpful.
WHY YOU SHOULD NOT USE CA125, CA19-9, AND CEA TO DIAGNOSE CANCER
Utilizing these serum biomarkers to diagnose cancer has the potential for diagnostic error and can result in unnecessary patient anxiety and follow-up testing. Since tissue sampling is necessary and remains the gold standard in most cancer diagnoses, obtaining these tumor biomarkers in the early diagnostic stage does not change management and may even lead to harm. Furthermore, due to their poor sensitivity and specificity, these biomarkers cannot rule in or rule out cancer. Elevated CA125, CA19-9, and CEA biomarkers occur in a variety of malignancies, including gastric, gallbladder, hepatocellular, bladder, and breast cancers.1,3,6 In addition, these biomarkers have a very limited role in the workup of cancer of unknown primary origin.7
Even in the setting of a known pelvic mass, the use of CA125 alone has poor sensitivity at a cut-off level of 35 U/mL as a biomarker for the diagnosis of early ovarian cancer.8
Serum CA19-9 is not a useful diagnostic biomarker as elevated CA19-9 can occur in benign conditions, including cirrhosis, chronic pancreatitis, and cholangitis. In a systematic review of patients with histologic confirmation of pancreatic malignancy, the median positive predictive value of CA19-9 was 72% (interquartile range, 41%-95%).9 Additionally, patients with Lewis-null blood type, which is present in 5% to 10% of the Caucasian population, do not produce CA19-9.10 Therefore, CA19-9 will be 0% specific for tumors in this population.
The use of CEA in the diagnosis of colorectal cancer is also questionable. In stage I colorectal cancer, CEA was only 38.1% sensitive at a cut-off level of 2.41 ng/mL; it was 78.3% sensitive in stage IV disease.11 The specificity of CEA is limited since elevated CEA occurs in benign conditions, such as inflammatory bowel disease, smoking, hypothyroidism, pancreatitis, biliary obstruction, peptic ulcers, and cirrhosis—though CEA levels in these conditions are rarely >10 ng/mL.11 Regardless of the results of biomarker testing, definitive diagnosis requires tissue biopsy; therefore, biomarker findings are of little utility in the initial workup.
In addition to variable diagnostic utility, overreliance on these biomarkers has the potential for serious patient harm. In a study examining patients with established rectal cancer, combination CEA and CA19-9 testing alone was insufficient to predict the pathologic stage of disease correctly.2 A cancer misdiagnosis not only traumatizes patients but also erodes their trust in clinicians and creates anxiety during future clinical encounters. Overutilization of these tumor biomarkers is also costly and contributes to waste in the US healthcare system.
WHEN YOU SHOULD USE CA125, CA19-9, AND CEA
There is a role for tumor biomarker testing in specific cancers after the primary source of malignancy has been determined. When evaluating a known pelvic mass, CA125 testing is performed in conjunction with transvaginal ultrasound and assessment of menopausal status in the risk of ovarian malignancy algorithm for prognostication of disease prior to surgery.12 This algorithm takes into account levels of CA125 in addition to levels of human epididymis protein 4 and patient age, yielding an area under the curve as high as 0.93 for ovarian cancer risk classification.8 Beyond the prognostication process, oncologists follow CA125 to monitor response to first-line ovarian cancer treatment. However, CA125 has a less defined role in surveillance for ovarian cancer recurrence.
CA19-9 has demonstrated utility for pancreatic cancer and cholangiocarcinoma survival estimates. A national cohort analysis of patients with established intrahepatic cholangiocarcinoma found that CA19-9 independently predicted increased mortality. Patients with elevated CA19-9 also had significantly more nodal metastases and positive-margin resections.6 A study of 353 patients with pancreatic ductal adenocarcinoma undergoing radical resection further demonstrated the utility of CA19-9. In this study, patients with postoperative CA19-9 normalization had improved survival by almost 12 months when compared to those with consistently elevated CA19-9.13
Last, the literature describes CEA biomarker testing in the surveillance of patients after curative treatment of colon and rectal cancer. The American Society of Colon and Rectal Surgeons recommends regularly tracking this biomarker following curative resection, in conjunction with colonoscopy and chest and liver imaging studies.14 A prospective randomized controlled study that followed this monitoring protocol in cured asymptomatic patients on a bimonthly basis found that early diagnosis of recurrent colorectal cancer improved survival.15 The use of CEA testing as a monitoring tool should therefore be a point of discussion between providers and patients, as its utility varies based on patient comorbidities, their ability to tolerate surgery or chemotherapy, risk factors for recurrence, performance status, compliance, age, and preference.14
WHAT YOU SHOULD DO INSTEAD
The use of CA125, CA19-9, and CEA testing alone as initial diagnostic tools for malignancy are problematic due to their poor sensitivities and/or positive predictive value. Multiple studies have demonstrated their utility as markers of metastasis or malignancy progression rather than as clinically useful markers for the detection of any one type of cancer.1,3,6 In an undiagnosed symptomatic patient with unexplained weight loss or symptoms of a tumor mass, elevated CA125, CA19-9, and CEA add no new information as metastatic pancreatic, colorectal, ovarian, gastric, gallbladder, hepatocellular, bladder, ovarian, and breast cancers all remain in the differential diagnosis. Clinicians should approach the initial diagnosis of cancer in such patients with appropriate imaging studies, a thorough physical examination, and prompt biopsy of abnormal findings, as long as these are consistent with the patient’s goals of care. After establishing a tissue diagnosis, some tumor biomarkers have valid prognostic, staging, and monitoring roles.6,13,14
RECOMMENDATIONS
- Do not routinely order CA125, CA19-9, and CEA tests for the initial diagnostic workup of visceral malignancy of unknown origin regardless of whether imaging studies have been obtained.
- Use appropriate imaging, perform a thorough physical examination, and obtain tissue biopsy in the initial diagnostic workup of a visceral malignancy of unknown origin.
CONCLUSION
Clinicians should use serum biomarkers, like any other diagnostic test, to maximize benefit while preventing patient harm. In general, CA125, CA19-9, and CEA do not have a role in cancer diagnosis. The patient described in our clinical scenario would not benefit from a serum tumor biomarker panel at the time of admission. Regardless of findings from these tests, a tissue sample is required to make a definitive diagnosis of underlying malignancy in this patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Yotsukura S, Mamitsuka H. Evaluation of serum-based cancer biomarkers: a brief review from a clinical and computational viewpoint. Crit Rev Oncol Hematol. 2015;93(2):103-115. https://doi.org/10.1016/j.critrevonc.2014.10.002
2. Zhang B, Sun Z, Song M, et al. Ultrasound/CT combined with serum CEA/CA19.9 in the diagnosis and prognosis of rectal cancer. J Buon. 2018;23(3):592-597.
3. Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16(7):2685-2691. https://doi.org/10.7314/apjcp.2015.16.7.2685
4. Karam AK, Karlan BY. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol. 2010;7(6):335-339. https://doi.org/10.1038/nrclinonc.2010.44
5. Gilligan TD, Seidenfeld J, Basch EM, et al; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404. https://doi.org/10.1200/jco.2009.26.4481
6. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: a national cohort analysis. J Surg Oncol. 2016;114(4):475-482. https://doi.org/10.1002/jso.24381
7. Milovic M, Popov I, Jelic S. Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit. 2002;8(2):MT25-MT30.
8. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/10.1186/s13048-019-0503-7
9. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266-270. https://doi.org/10.1016/j.ejso.2006.10.004
10. Loosen SH, Neumann UP, Trautwein C, Roderburg C, Luedde T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017;39(6):1010428317692231. https://doi.org/10.1177/1010428317692231
11. Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1-e7. https://doi.org/10.3747/co.21.1711
12. Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43-51. https://doi.org/10.1097/igc.0000000000000586
13. Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161(2):373-384. https://doi.org/10.1016/j.surg.2016.08.005
14. Steele SR, Chang GJ, Hendren S, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713-725. https://doi.org/10.1097/dcr.0000000000000410
15. Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized “CEAwatch” trial. Eur J Surg Oncol. 2015;41(9):1188-1196. https://doi.org/10.1016/j.ejso.2015.06.008
1. Yotsukura S, Mamitsuka H. Evaluation of serum-based cancer biomarkers: a brief review from a clinical and computational viewpoint. Crit Rev Oncol Hematol. 2015;93(2):103-115. https://doi.org/10.1016/j.critrevonc.2014.10.002
2. Zhang B, Sun Z, Song M, et al. Ultrasound/CT combined with serum CEA/CA19.9 in the diagnosis and prognosis of rectal cancer. J Buon. 2018;23(3):592-597.
3. Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16(7):2685-2691. https://doi.org/10.7314/apjcp.2015.16.7.2685
4. Karam AK, Karlan BY. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol. 2010;7(6):335-339. https://doi.org/10.1038/nrclinonc.2010.44
5. Gilligan TD, Seidenfeld J, Basch EM, et al; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404. https://doi.org/10.1200/jco.2009.26.4481
6. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: a national cohort analysis. J Surg Oncol. 2016;114(4):475-482. https://doi.org/10.1002/jso.24381
7. Milovic M, Popov I, Jelic S. Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit. 2002;8(2):MT25-MT30.
8. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/10.1186/s13048-019-0503-7
9. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266-270. https://doi.org/10.1016/j.ejso.2006.10.004
10. Loosen SH, Neumann UP, Trautwein C, Roderburg C, Luedde T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017;39(6):1010428317692231. https://doi.org/10.1177/1010428317692231
11. Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1-e7. https://doi.org/10.3747/co.21.1711
12. Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43-51. https://doi.org/10.1097/igc.0000000000000586
13. Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161(2):373-384. https://doi.org/10.1016/j.surg.2016.08.005
14. Steele SR, Chang GJ, Hendren S, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713-725. https://doi.org/10.1097/dcr.0000000000000410
15. Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized “CEAwatch” trial. Eur J Surg Oncol. 2015;41(9):1188-1196. https://doi.org/10.1016/j.ejso.2015.06.008
© 2021 Society of Hospital Medicine
Reducing Overuse of Proton Pump Inhibitors for Stress Ulcer Prophylaxis and Nonvariceal Gastrointestinal Bleeding in the Hospital: A Narrative Review and Implementation Guide
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).
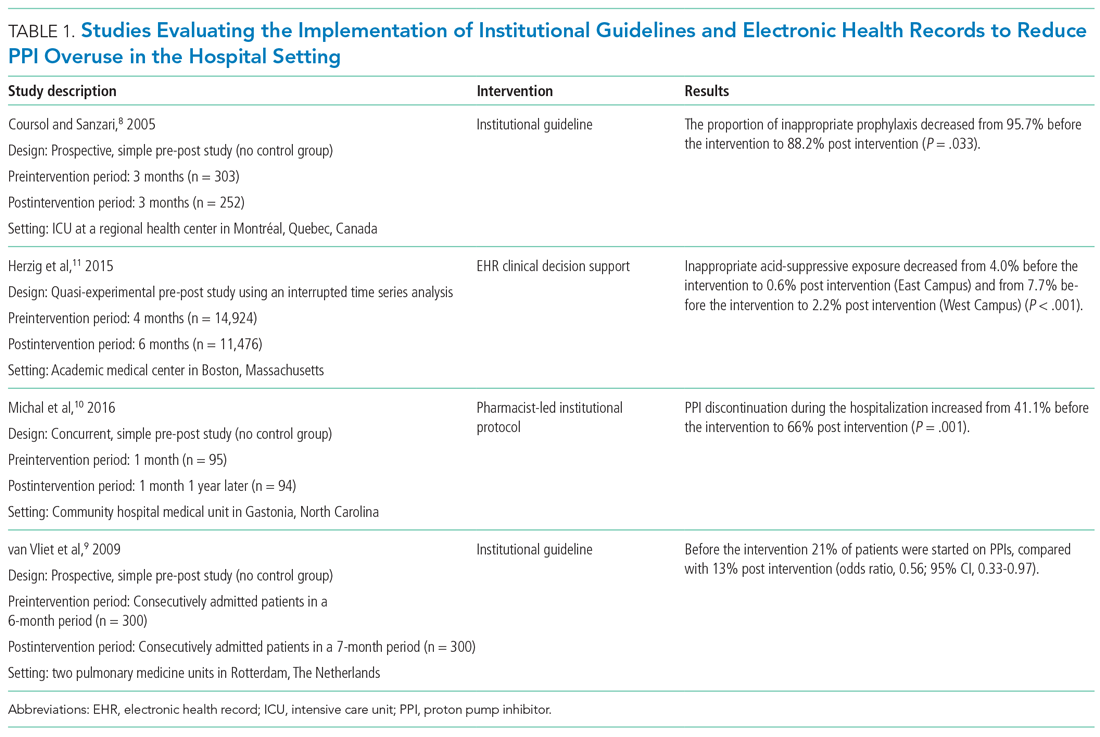
Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.
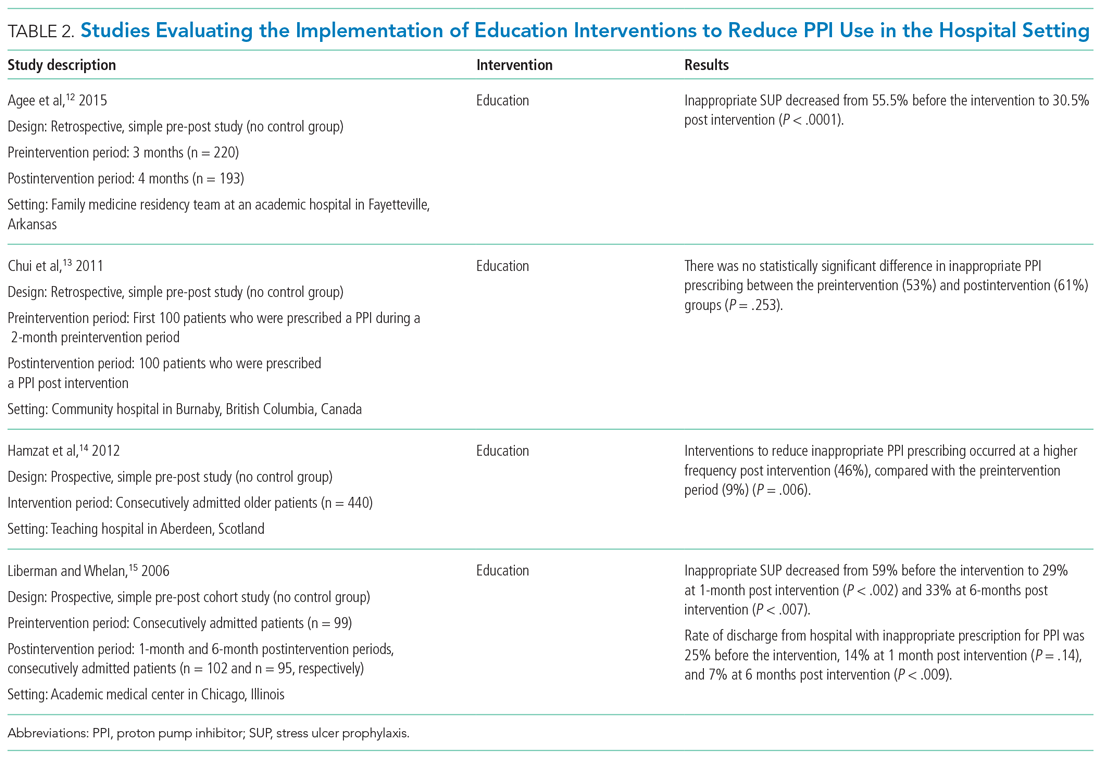
Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.
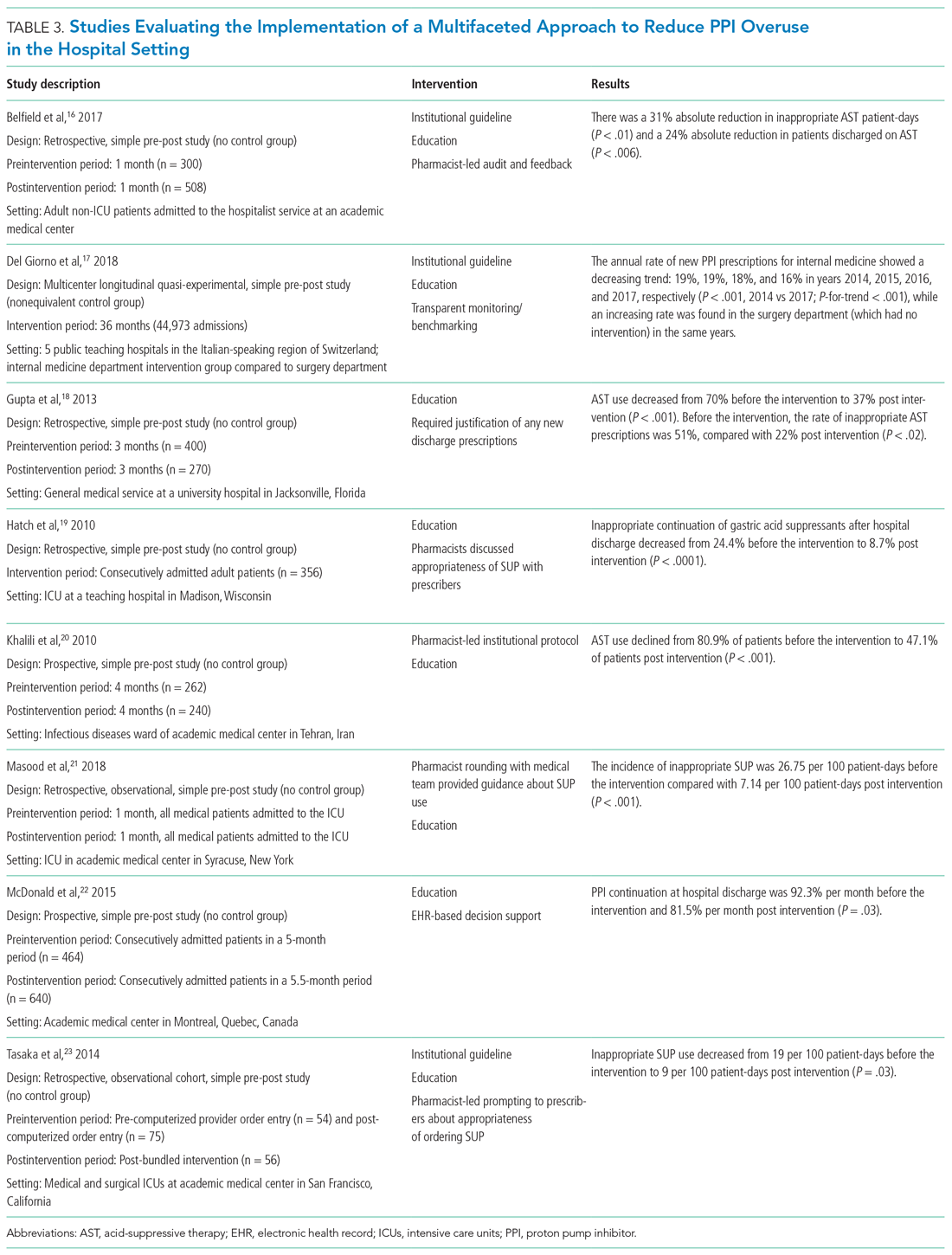
Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).

Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.

Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.

Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).

Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.

Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.

Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
© 2021 Society of Hospital Medicine
Choosing Wisely in the COVID-19 Era: Preventing Harm to Healthcare Workers
With more than 3 million people diagnosed and more than 200,000 deaths worldwide at the time this article was written, coronavirus disease of 2019 (COVID-19) poses an unprecedented challenge to the public and to our healthcare system.1 The United States has surpassed every other country in the total number of COVID-19 cases. Hospitals in hotspots are operating beyond capacity, while others prepare for a predicted surge of patients suffering from COVID-19. Now more than ever, clinicians need to prioritize limited time and resources wisely in this rapidly changing environment. Our most precious limited resource, healthcare workers (HCWs), bravely care for patients while trying to avoid acquiring the infection. With each test and treatment, clinicians must carefully consider harms and benefits, including exposing themselves and other HCWs to SARS-CoV-2, the virus causing this disease.
Delivering any healthcare service in which the potential harm exceeds benefit represents one form of overuse. In the era of COVID-19, the harmful consequences of overuse go beyond the patient to the healthcare team. For example, unnecessary chest computed tomography (CT) to help diagnose COVID-19 comes with the usual risks to the patient including radiation, but it may also reveal a suspicious nodule. That incidental finding can lead to downstream consequences, such as more imaging, blood work, and biopsy. In the current pandemic, however, that CT comes with more than just the usual risk. The initial unnecessary chest CT can risk exposing the transporter, the staff in the hallways and elevator en route, the radiology staff operating the CT scanner, and the maintenance staff who must clean the room and scanner afterward. Potential downstream harms to staff include exposure of the pulmonary and interventional radiology consultants, as well as the staff who perform repeat imaging after the biopsy. Evaluation of the nodule potentially prolongs the patient’s stay and exposes more staff. Clinicians must weigh the benefits and harms of each test and treatment carefully with consideration of both the patient and the staff involved. Moreover, it may turn out that the patient and staff without symptoms of COVID-19 may pose the most risk to one another.
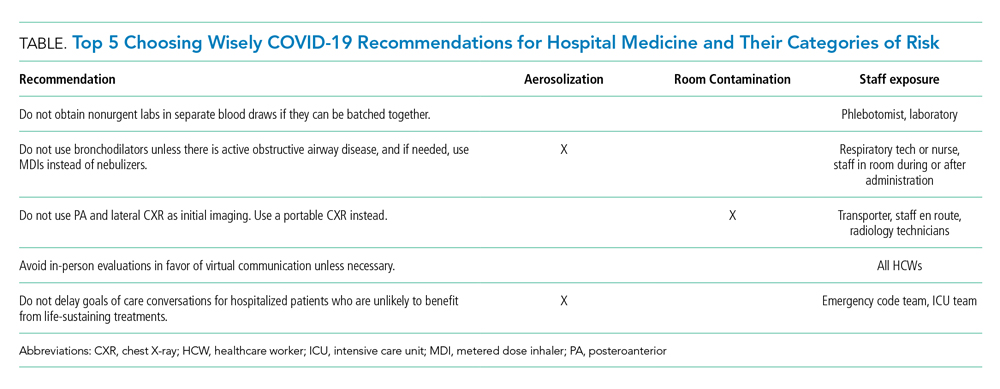
RECOMMENDATIONS
Choosing Wisely® partnered with patients and clinician societies to develop a Top 5 recommendations list for eliminating unnecessary testing and treatment. Our multi-institutional group from the High Value Practice Academic Alliance proposed this Top 5 list of overuse practices in hospital medicine that can lead to harm of both patients and HCWs in the COVID-19 era (Table). The following recommendations apply to all patients with unsuspected, suspected, or confirmed SARS-CoV-2 infection in the hospital setting.
- Do not obtain nonurgent labs in separate blood draws if they can be batched together.
This recommendation expands on the original Society of Hospital Medicine Choosing Wisely recommendation: Don’t perform repetitive complete blood count and chemistry testing in the face of clinical and lab stability.2 Aside from patient harms such as pain and hospital-acquired anemia, the risk of exposure to HCWs who perform phlebotomy (phlebotomists, nurses, and other clinicians), as well as staff who transport, handle, and process the bloodwork in the lab, must be minimized. Most prior interventions to eliminate unnecessary bloodwork focused on the number of lab tests,3 but some also aimed to batch nonurgent labs together to effectively reduce unnecessary needlesticks (“think twice, stick once”).4 This concept can be brought into this pandemic to provide safe and appropriate care for both patients and HCWs.
- Do not use bronchodilators unless there is active obstructive airway disease, and if needed, use metered dose inhalers instead of nebulizers.
We do not recommend using bronchodilators to treat COVID-19 symptoms unless patients develop acute bronchospastic symptoms of their underlying obstructive airway disease.5 When needed, use metered dose inhalers (MDIs),6 if available, instead of nebulizers because the latter potentiates aerosolization that could lead to higher risk of spreading the infection. The risk extends to respiratory technicians and nurses who administer the nebulizer, as well as other HCWs who enter the room during or after administration. The Centers for Disease Control and Prevention (CDC) considers nebulized bronchodilator therapy a “high-risk” exposure for HCWs not wearing the proper personal protectvie equipment.7 Moreover, MDI therapy produces equivalent outcomes to nebulized treatments for patients who are not critically ill.6 Unfortunately, the supply of MDIs during this crisis has not kept up with the increased demand.8
There are no clear guidelines for reuse of MDIs in COVID-19; however, options include labeling patients’ MDIs to use for hospitalization and discharge or labeling an MDI for use during hospitalization and then disinfecting for reuse. For safety reasons, MDIs of COVID-19 patients should be reused only for other patients with COVID-19.8
- Do not use posteroanterior and lateral chest X-ray as initial imaging. Use a portable chest X-ray instead.
The CDC does not currently recommend diagnosing COVID-19 by chest X-ray (CXR).7 When used appropriately, CXR can provide information to support a COVID-19 diagnosis and rule out other etiologies that cause respiratory symptoms.9 Posteroanterior (PA) and lateral CXR are more sensitive than portable CXR for detecting pleural effusions, and lateral CXR is needed to examine structures along the axis of the body. Portable CXR also may cause the heart to appear magnified and the mediastinum widened, the diaphragm to appear higher, and vascular shadows to be obscured.10 The improved ability to detect these subtle differences should be weighed against the increased risk to HCWs required to perform PA and lateral CXR. A portable CXR exposes a relatively smaller number of staff who come to the bedside versus the larger number of people exposed in transporting the patient out of the room and into the hallway, elevator, and the radiology suite for a PA and lateral CXR.
- Avoid in-person evaluations in favor of virtual communication unless necessary.
To minimize HCW exposure to COVID-19 and optimize infection control, the CDC recommends the use of telemedicine when possible.7 Telemedicine refers to the use of technology to support clinical care across some distance, which includes video visits and remote clinical monitoring. At the time of writing, the Centers for Medicare & Medicaid Services had waived the rural site of care requirement for Medicare beneficiaries, granted 49 Medicaid waivers to states to enhance flexibility, and (at least temporarily) added inpatient care to the list of reimbursed telemedicine services.11 Funding for expanded coverage under Medicare is included in the recent Coronavirus Preparedness and Response Supplemental Appropriations Act.12 These federal changes open the door for commercial payers and state Medicaid programs to further boost telemedicine through reimbursement parity to in-person visits and other coverage policies. Hospitalists can ride this momentum and learn from ambulatory colleagues to harness the power of telemedicine and minimize unnecessary face-to-face interactions with patients who are suspected or confirmed to have COVID-19.13 Even if providers have to enter the patient’s room, telemedicine may still allow for large virtual family meetings despite strict visitor restrictions and physical distance with loved ones. If in-person visits are necessary, only one designated person should enter the patient’s room instead of the entire team.
- Do not delay goals of care conversations for hospitalized patients who are unlikely to benefit from life-sustaining treatments.
The COVID-19 pandemic amplifies the need for early goals of care discussions. Mortality rates range higher with acute respiratory distress syndrome from COVID-19, compared with other etiologies, and is associated with extended intensive care unit stays.14 The harms extend beyond the patient and families to our HCWs through psychological distress and heightened exposure from aerosolization during resuscitation. Advance care planning should center on the values and preferences of the patient. Rather than asking if the patient or family would want certain treatments, it is crucial for clinicians to be direct in making do-not-resuscitate recommendations if deemed futile care.15 This practice is well within legal confines and is distinct from withdrawal or withholding of life-sustaining resources.15
CONCLUSION
HCWs providing inpatient care during this pandemic remain among the highest risk for contracting the infection. As of April 9, 2020, nearly 9,300 HCWs in the United States have contracted COVID-19.16 One thing remains clear: If we want to protect our patients, we must start by protecting our HCWs. We must think critically to evaluate the potential harms to our extended healthcare teams and strive further to eliminate overuse from our care.
Acknowledgment
The authors represent members of the High Value Practice Academic Alliance. The High Value Practice Academic Alliance is a consortium of academic medical centers in the United States and Canada working to advance high-value healthcare through collaborative quality improvement, research, and education. Additional information is available at http://www.hvpaa.org.
1. World Health Organization. Coronavirus disease (COVID-19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed May 3, 2020.
2. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063.
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. https://doi.org/10.1001/jamainternmed.2017.5152.
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549.
5. Respiratory care committee of Chinese Thoracic Society. [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;17(0):E020. https://doi.org/10.3760/cma.j.issn.1001-0939.2020.0020.
6. Moriates C, Feldman L. Nebulized bronchodilators instead of metered-dose inhalers for obstructive pulmonary symptoms. J Hosp Med. 2015;10(10):691-693. https://doi.org/10.1002/jhm.2386.
7. Centers for Disease Control and Prevention. Interim US Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19). April 15, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed May 3, 2020.
8. Institute for Safe Medication Practices. Revisiting the Need for MDI Common Canister Protocols During the COVID-19 Pandemic. March 26, 2020. https://ismp.org/resources/revisiting-need-mdi-common-canister-protocols-during-covid-19-pandemic. Accessed May 3, 2020.
9. American College of Radiology. ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. March 11, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed May 3, 2020.
10. Bell DJ, Jones J, et al. https://radiopaedia.org/articles/chest-radiograph?lang=us. Accessed April 4, 2020.
11. Centers for Medicare & Medicaid Services. List of Telehealth Services. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes. Accessed April 17, 2020.
12. Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020, HR 6074, 116th Cong (2020). Accessed May 3, 2020. https://congress.gov/bill/116th-congress/house-bill/6074/.
13. Doshi A, Platt Y, Dressen JR, Mathews Benji, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(5):302-304. https://doi.org/10.12788/jhm.3419.
14. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. https://doi.org/10.1001/jama.2020.5394.
15. Curtis JR, Kross EK, Stapleton RD. The importance of addressing advance care planning and decisions about do-not-resuscitate orders during novel coronavirus 2019 (COVID-19) [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.4894.
16. CDC COVID-19 Response Team. Characteristics of health care personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-481.
With more than 3 million people diagnosed and more than 200,000 deaths worldwide at the time this article was written, coronavirus disease of 2019 (COVID-19) poses an unprecedented challenge to the public and to our healthcare system.1 The United States has surpassed every other country in the total number of COVID-19 cases. Hospitals in hotspots are operating beyond capacity, while others prepare for a predicted surge of patients suffering from COVID-19. Now more than ever, clinicians need to prioritize limited time and resources wisely in this rapidly changing environment. Our most precious limited resource, healthcare workers (HCWs), bravely care for patients while trying to avoid acquiring the infection. With each test and treatment, clinicians must carefully consider harms and benefits, including exposing themselves and other HCWs to SARS-CoV-2, the virus causing this disease.
Delivering any healthcare service in which the potential harm exceeds benefit represents one form of overuse. In the era of COVID-19, the harmful consequences of overuse go beyond the patient to the healthcare team. For example, unnecessary chest computed tomography (CT) to help diagnose COVID-19 comes with the usual risks to the patient including radiation, but it may also reveal a suspicious nodule. That incidental finding can lead to downstream consequences, such as more imaging, blood work, and biopsy. In the current pandemic, however, that CT comes with more than just the usual risk. The initial unnecessary chest CT can risk exposing the transporter, the staff in the hallways and elevator en route, the radiology staff operating the CT scanner, and the maintenance staff who must clean the room and scanner afterward. Potential downstream harms to staff include exposure of the pulmonary and interventional radiology consultants, as well as the staff who perform repeat imaging after the biopsy. Evaluation of the nodule potentially prolongs the patient’s stay and exposes more staff. Clinicians must weigh the benefits and harms of each test and treatment carefully with consideration of both the patient and the staff involved. Moreover, it may turn out that the patient and staff without symptoms of COVID-19 may pose the most risk to one another.

RECOMMENDATIONS
Choosing Wisely® partnered with patients and clinician societies to develop a Top 5 recommendations list for eliminating unnecessary testing and treatment. Our multi-institutional group from the High Value Practice Academic Alliance proposed this Top 5 list of overuse practices in hospital medicine that can lead to harm of both patients and HCWs in the COVID-19 era (Table). The following recommendations apply to all patients with unsuspected, suspected, or confirmed SARS-CoV-2 infection in the hospital setting.
- Do not obtain nonurgent labs in separate blood draws if they can be batched together.
This recommendation expands on the original Society of Hospital Medicine Choosing Wisely recommendation: Don’t perform repetitive complete blood count and chemistry testing in the face of clinical and lab stability.2 Aside from patient harms such as pain and hospital-acquired anemia, the risk of exposure to HCWs who perform phlebotomy (phlebotomists, nurses, and other clinicians), as well as staff who transport, handle, and process the bloodwork in the lab, must be minimized. Most prior interventions to eliminate unnecessary bloodwork focused on the number of lab tests,3 but some also aimed to batch nonurgent labs together to effectively reduce unnecessary needlesticks (“think twice, stick once”).4 This concept can be brought into this pandemic to provide safe and appropriate care for both patients and HCWs.
- Do not use bronchodilators unless there is active obstructive airway disease, and if needed, use metered dose inhalers instead of nebulizers.
We do not recommend using bronchodilators to treat COVID-19 symptoms unless patients develop acute bronchospastic symptoms of their underlying obstructive airway disease.5 When needed, use metered dose inhalers (MDIs),6 if available, instead of nebulizers because the latter potentiates aerosolization that could lead to higher risk of spreading the infection. The risk extends to respiratory technicians and nurses who administer the nebulizer, as well as other HCWs who enter the room during or after administration. The Centers for Disease Control and Prevention (CDC) considers nebulized bronchodilator therapy a “high-risk” exposure for HCWs not wearing the proper personal protectvie equipment.7 Moreover, MDI therapy produces equivalent outcomes to nebulized treatments for patients who are not critically ill.6 Unfortunately, the supply of MDIs during this crisis has not kept up with the increased demand.8
There are no clear guidelines for reuse of MDIs in COVID-19; however, options include labeling patients’ MDIs to use for hospitalization and discharge or labeling an MDI for use during hospitalization and then disinfecting for reuse. For safety reasons, MDIs of COVID-19 patients should be reused only for other patients with COVID-19.8
- Do not use posteroanterior and lateral chest X-ray as initial imaging. Use a portable chest X-ray instead.
The CDC does not currently recommend diagnosing COVID-19 by chest X-ray (CXR).7 When used appropriately, CXR can provide information to support a COVID-19 diagnosis and rule out other etiologies that cause respiratory symptoms.9 Posteroanterior (PA) and lateral CXR are more sensitive than portable CXR for detecting pleural effusions, and lateral CXR is needed to examine structures along the axis of the body. Portable CXR also may cause the heart to appear magnified and the mediastinum widened, the diaphragm to appear higher, and vascular shadows to be obscured.10 The improved ability to detect these subtle differences should be weighed against the increased risk to HCWs required to perform PA and lateral CXR. A portable CXR exposes a relatively smaller number of staff who come to the bedside versus the larger number of people exposed in transporting the patient out of the room and into the hallway, elevator, and the radiology suite for a PA and lateral CXR.
- Avoid in-person evaluations in favor of virtual communication unless necessary.
To minimize HCW exposure to COVID-19 and optimize infection control, the CDC recommends the use of telemedicine when possible.7 Telemedicine refers to the use of technology to support clinical care across some distance, which includes video visits and remote clinical monitoring. At the time of writing, the Centers for Medicare & Medicaid Services had waived the rural site of care requirement for Medicare beneficiaries, granted 49 Medicaid waivers to states to enhance flexibility, and (at least temporarily) added inpatient care to the list of reimbursed telemedicine services.11 Funding for expanded coverage under Medicare is included in the recent Coronavirus Preparedness and Response Supplemental Appropriations Act.12 These federal changes open the door for commercial payers and state Medicaid programs to further boost telemedicine through reimbursement parity to in-person visits and other coverage policies. Hospitalists can ride this momentum and learn from ambulatory colleagues to harness the power of telemedicine and minimize unnecessary face-to-face interactions with patients who are suspected or confirmed to have COVID-19.13 Even if providers have to enter the patient’s room, telemedicine may still allow for large virtual family meetings despite strict visitor restrictions and physical distance with loved ones. If in-person visits are necessary, only one designated person should enter the patient’s room instead of the entire team.
- Do not delay goals of care conversations for hospitalized patients who are unlikely to benefit from life-sustaining treatments.
The COVID-19 pandemic amplifies the need for early goals of care discussions. Mortality rates range higher with acute respiratory distress syndrome from COVID-19, compared with other etiologies, and is associated with extended intensive care unit stays.14 The harms extend beyond the patient and families to our HCWs through psychological distress and heightened exposure from aerosolization during resuscitation. Advance care planning should center on the values and preferences of the patient. Rather than asking if the patient or family would want certain treatments, it is crucial for clinicians to be direct in making do-not-resuscitate recommendations if deemed futile care.15 This practice is well within legal confines and is distinct from withdrawal or withholding of life-sustaining resources.15
CONCLUSION
HCWs providing inpatient care during this pandemic remain among the highest risk for contracting the infection. As of April 9, 2020, nearly 9,300 HCWs in the United States have contracted COVID-19.16 One thing remains clear: If we want to protect our patients, we must start by protecting our HCWs. We must think critically to evaluate the potential harms to our extended healthcare teams and strive further to eliminate overuse from our care.
Acknowledgment
The authors represent members of the High Value Practice Academic Alliance. The High Value Practice Academic Alliance is a consortium of academic medical centers in the United States and Canada working to advance high-value healthcare through collaborative quality improvement, research, and education. Additional information is available at http://www.hvpaa.org.
With more than 3 million people diagnosed and more than 200,000 deaths worldwide at the time this article was written, coronavirus disease of 2019 (COVID-19) poses an unprecedented challenge to the public and to our healthcare system.1 The United States has surpassed every other country in the total number of COVID-19 cases. Hospitals in hotspots are operating beyond capacity, while others prepare for a predicted surge of patients suffering from COVID-19. Now more than ever, clinicians need to prioritize limited time and resources wisely in this rapidly changing environment. Our most precious limited resource, healthcare workers (HCWs), bravely care for patients while trying to avoid acquiring the infection. With each test and treatment, clinicians must carefully consider harms and benefits, including exposing themselves and other HCWs to SARS-CoV-2, the virus causing this disease.
Delivering any healthcare service in which the potential harm exceeds benefit represents one form of overuse. In the era of COVID-19, the harmful consequences of overuse go beyond the patient to the healthcare team. For example, unnecessary chest computed tomography (CT) to help diagnose COVID-19 comes with the usual risks to the patient including radiation, but it may also reveal a suspicious nodule. That incidental finding can lead to downstream consequences, such as more imaging, blood work, and biopsy. In the current pandemic, however, that CT comes with more than just the usual risk. The initial unnecessary chest CT can risk exposing the transporter, the staff in the hallways and elevator en route, the radiology staff operating the CT scanner, and the maintenance staff who must clean the room and scanner afterward. Potential downstream harms to staff include exposure of the pulmonary and interventional radiology consultants, as well as the staff who perform repeat imaging after the biopsy. Evaluation of the nodule potentially prolongs the patient’s stay and exposes more staff. Clinicians must weigh the benefits and harms of each test and treatment carefully with consideration of both the patient and the staff involved. Moreover, it may turn out that the patient and staff without symptoms of COVID-19 may pose the most risk to one another.

RECOMMENDATIONS
Choosing Wisely® partnered with patients and clinician societies to develop a Top 5 recommendations list for eliminating unnecessary testing and treatment. Our multi-institutional group from the High Value Practice Academic Alliance proposed this Top 5 list of overuse practices in hospital medicine that can lead to harm of both patients and HCWs in the COVID-19 era (Table). The following recommendations apply to all patients with unsuspected, suspected, or confirmed SARS-CoV-2 infection in the hospital setting.
- Do not obtain nonurgent labs in separate blood draws if they can be batched together.
This recommendation expands on the original Society of Hospital Medicine Choosing Wisely recommendation: Don’t perform repetitive complete blood count and chemistry testing in the face of clinical and lab stability.2 Aside from patient harms such as pain and hospital-acquired anemia, the risk of exposure to HCWs who perform phlebotomy (phlebotomists, nurses, and other clinicians), as well as staff who transport, handle, and process the bloodwork in the lab, must be minimized. Most prior interventions to eliminate unnecessary bloodwork focused on the number of lab tests,3 but some also aimed to batch nonurgent labs together to effectively reduce unnecessary needlesticks (“think twice, stick once”).4 This concept can be brought into this pandemic to provide safe and appropriate care for both patients and HCWs.
- Do not use bronchodilators unless there is active obstructive airway disease, and if needed, use metered dose inhalers instead of nebulizers.
We do not recommend using bronchodilators to treat COVID-19 symptoms unless patients develop acute bronchospastic symptoms of their underlying obstructive airway disease.5 When needed, use metered dose inhalers (MDIs),6 if available, instead of nebulizers because the latter potentiates aerosolization that could lead to higher risk of spreading the infection. The risk extends to respiratory technicians and nurses who administer the nebulizer, as well as other HCWs who enter the room during or after administration. The Centers for Disease Control and Prevention (CDC) considers nebulized bronchodilator therapy a “high-risk” exposure for HCWs not wearing the proper personal protectvie equipment.7 Moreover, MDI therapy produces equivalent outcomes to nebulized treatments for patients who are not critically ill.6 Unfortunately, the supply of MDIs during this crisis has not kept up with the increased demand.8
There are no clear guidelines for reuse of MDIs in COVID-19; however, options include labeling patients’ MDIs to use for hospitalization and discharge or labeling an MDI for use during hospitalization and then disinfecting for reuse. For safety reasons, MDIs of COVID-19 patients should be reused only for other patients with COVID-19.8
- Do not use posteroanterior and lateral chest X-ray as initial imaging. Use a portable chest X-ray instead.
The CDC does not currently recommend diagnosing COVID-19 by chest X-ray (CXR).7 When used appropriately, CXR can provide information to support a COVID-19 diagnosis and rule out other etiologies that cause respiratory symptoms.9 Posteroanterior (PA) and lateral CXR are more sensitive than portable CXR for detecting pleural effusions, and lateral CXR is needed to examine structures along the axis of the body. Portable CXR also may cause the heart to appear magnified and the mediastinum widened, the diaphragm to appear higher, and vascular shadows to be obscured.10 The improved ability to detect these subtle differences should be weighed against the increased risk to HCWs required to perform PA and lateral CXR. A portable CXR exposes a relatively smaller number of staff who come to the bedside versus the larger number of people exposed in transporting the patient out of the room and into the hallway, elevator, and the radiology suite for a PA and lateral CXR.
- Avoid in-person evaluations in favor of virtual communication unless necessary.
To minimize HCW exposure to COVID-19 and optimize infection control, the CDC recommends the use of telemedicine when possible.7 Telemedicine refers to the use of technology to support clinical care across some distance, which includes video visits and remote clinical monitoring. At the time of writing, the Centers for Medicare & Medicaid Services had waived the rural site of care requirement for Medicare beneficiaries, granted 49 Medicaid waivers to states to enhance flexibility, and (at least temporarily) added inpatient care to the list of reimbursed telemedicine services.11 Funding for expanded coverage under Medicare is included in the recent Coronavirus Preparedness and Response Supplemental Appropriations Act.12 These federal changes open the door for commercial payers and state Medicaid programs to further boost telemedicine through reimbursement parity to in-person visits and other coverage policies. Hospitalists can ride this momentum and learn from ambulatory colleagues to harness the power of telemedicine and minimize unnecessary face-to-face interactions with patients who are suspected or confirmed to have COVID-19.13 Even if providers have to enter the patient’s room, telemedicine may still allow for large virtual family meetings despite strict visitor restrictions and physical distance with loved ones. If in-person visits are necessary, only one designated person should enter the patient’s room instead of the entire team.
- Do not delay goals of care conversations for hospitalized patients who are unlikely to benefit from life-sustaining treatments.
The COVID-19 pandemic amplifies the need for early goals of care discussions. Mortality rates range higher with acute respiratory distress syndrome from COVID-19, compared with other etiologies, and is associated with extended intensive care unit stays.14 The harms extend beyond the patient and families to our HCWs through psychological distress and heightened exposure from aerosolization during resuscitation. Advance care planning should center on the values and preferences of the patient. Rather than asking if the patient or family would want certain treatments, it is crucial for clinicians to be direct in making do-not-resuscitate recommendations if deemed futile care.15 This practice is well within legal confines and is distinct from withdrawal or withholding of life-sustaining resources.15
CONCLUSION
HCWs providing inpatient care during this pandemic remain among the highest risk for contracting the infection. As of April 9, 2020, nearly 9,300 HCWs in the United States have contracted COVID-19.16 One thing remains clear: If we want to protect our patients, we must start by protecting our HCWs. We must think critically to evaluate the potential harms to our extended healthcare teams and strive further to eliminate overuse from our care.
Acknowledgment
The authors represent members of the High Value Practice Academic Alliance. The High Value Practice Academic Alliance is a consortium of academic medical centers in the United States and Canada working to advance high-value healthcare through collaborative quality improvement, research, and education. Additional information is available at http://www.hvpaa.org.
1. World Health Organization. Coronavirus disease (COVID-19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed May 3, 2020.
2. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063.
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. https://doi.org/10.1001/jamainternmed.2017.5152.
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549.
5. Respiratory care committee of Chinese Thoracic Society. [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;17(0):E020. https://doi.org/10.3760/cma.j.issn.1001-0939.2020.0020.
6. Moriates C, Feldman L. Nebulized bronchodilators instead of metered-dose inhalers for obstructive pulmonary symptoms. J Hosp Med. 2015;10(10):691-693. https://doi.org/10.1002/jhm.2386.
7. Centers for Disease Control and Prevention. Interim US Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19). April 15, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed May 3, 2020.
8. Institute for Safe Medication Practices. Revisiting the Need for MDI Common Canister Protocols During the COVID-19 Pandemic. March 26, 2020. https://ismp.org/resources/revisiting-need-mdi-common-canister-protocols-during-covid-19-pandemic. Accessed May 3, 2020.
9. American College of Radiology. ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. March 11, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed May 3, 2020.
10. Bell DJ, Jones J, et al. https://radiopaedia.org/articles/chest-radiograph?lang=us. Accessed April 4, 2020.
11. Centers for Medicare & Medicaid Services. List of Telehealth Services. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes. Accessed April 17, 2020.
12. Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020, HR 6074, 116th Cong (2020). Accessed May 3, 2020. https://congress.gov/bill/116th-congress/house-bill/6074/.
13. Doshi A, Platt Y, Dressen JR, Mathews Benji, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(5):302-304. https://doi.org/10.12788/jhm.3419.
14. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. https://doi.org/10.1001/jama.2020.5394.
15. Curtis JR, Kross EK, Stapleton RD. The importance of addressing advance care planning and decisions about do-not-resuscitate orders during novel coronavirus 2019 (COVID-19) [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.4894.
16. CDC COVID-19 Response Team. Characteristics of health care personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-481.
1. World Health Organization. Coronavirus disease (COVID-19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed May 3, 2020.
2. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063.
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. https://doi.org/10.1001/jamainternmed.2017.5152.
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549.
5. Respiratory care committee of Chinese Thoracic Society. [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;17(0):E020. https://doi.org/10.3760/cma.j.issn.1001-0939.2020.0020.
6. Moriates C, Feldman L. Nebulized bronchodilators instead of metered-dose inhalers for obstructive pulmonary symptoms. J Hosp Med. 2015;10(10):691-693. https://doi.org/10.1002/jhm.2386.
7. Centers for Disease Control and Prevention. Interim US Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease 2019 (COVID-19). April 15, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed May 3, 2020.
8. Institute for Safe Medication Practices. Revisiting the Need for MDI Common Canister Protocols During the COVID-19 Pandemic. March 26, 2020. https://ismp.org/resources/revisiting-need-mdi-common-canister-protocols-during-covid-19-pandemic. Accessed May 3, 2020.
9. American College of Radiology. ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. March 11, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed May 3, 2020.
10. Bell DJ, Jones J, et al. https://radiopaedia.org/articles/chest-radiograph?lang=us. Accessed April 4, 2020.
11. Centers for Medicare & Medicaid Services. List of Telehealth Services. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes. Accessed April 17, 2020.
12. Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020, HR 6074, 116th Cong (2020). Accessed May 3, 2020. https://congress.gov/bill/116th-congress/house-bill/6074/.
13. Doshi A, Platt Y, Dressen JR, Mathews Benji, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(5):302-304. https://doi.org/10.12788/jhm.3419.
14. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. https://doi.org/10.1001/jama.2020.5394.
15. Curtis JR, Kross EK, Stapleton RD. The importance of addressing advance care planning and decisions about do-not-resuscitate orders during novel coronavirus 2019 (COVID-19) [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.4894.
16. CDC COVID-19 Response Team. Characteristics of health care personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-481.
© 2020 Society of Hospital Medicine