User login
In the Literature: Research You Need to Know
Clinical question: What are the changes in the updated Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines?
Background: Chronic obstructive pulmonary disease (COPD) remains a leading cause of death in the U.S. and worldwide. The GOLD guidelines are an international consensus report on COPD diagnosis, management, and prevention, first released in 2001. The 2011 revision to the guidelines was recently published and outlines substantial changes based on updated literature and expert opinion.
Study design: Guidelines based on studies with varying designs.
Setting: Expert panel review of multiple studies from different settings.
Synopsis: While the diagnosis of COPD remains based on a post-bronchodilator fixed ratio of FEV1/FVC <0.70, there is more emphasis on global clinical assessment in the new guidelines. The updated approach describes classifying COPD severity based on risk/symptom frequency using established symptom assessment and the frequency of acute exacerbations of COPD. Instead of five “stages” based on FEV1 measures alone, there are now four "grades" of A through D (A: low risk/fewer symptoms; B: low risk/more symptoms; C: high risk/fewer symptoms; D: high risk/more symptoms) to more easily guide treatment options.
Treatment strategies are also updated, focusing not only on reduction of current symptoms, but also risk of future events. Pharmacologic treatment recommendations include using bronchodilator monotherapy in Group A patients, favoring long-acting over short-acting bronchodilators in Group B patients, prescribing inhaled corticosteroids only in combination with long-acting bronchodilators in Groups C and D patients, and considering newer agents such as phosphodiesterase-4 inhibitors in Group D patients.
Non-pharmacologic interventions include ongoing smoking cessation strategies, exercise promotion, treatment of comorbidities, and even public health strategies in pollution control.
Bottom line: The GOLD guidelines have undergone major revisions that provide a more practical approach to classification of COPD based on symptom severity and risk assessment in order to direct providers in evidence-based treatment that addresses both short-term and long-term impact of the disease.
Citation: Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease website. Accessed Oct. 29, 2012.
For more physician reviews of recent HM-relevant literature, visit our website.
Clinical question: What are the changes in the updated Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines?
Background: Chronic obstructive pulmonary disease (COPD) remains a leading cause of death in the U.S. and worldwide. The GOLD guidelines are an international consensus report on COPD diagnosis, management, and prevention, first released in 2001. The 2011 revision to the guidelines was recently published and outlines substantial changes based on updated literature and expert opinion.
Study design: Guidelines based on studies with varying designs.
Setting: Expert panel review of multiple studies from different settings.
Synopsis: While the diagnosis of COPD remains based on a post-bronchodilator fixed ratio of FEV1/FVC <0.70, there is more emphasis on global clinical assessment in the new guidelines. The updated approach describes classifying COPD severity based on risk/symptom frequency using established symptom assessment and the frequency of acute exacerbations of COPD. Instead of five “stages” based on FEV1 measures alone, there are now four "grades" of A through D (A: low risk/fewer symptoms; B: low risk/more symptoms; C: high risk/fewer symptoms; D: high risk/more symptoms) to more easily guide treatment options.
Treatment strategies are also updated, focusing not only on reduction of current symptoms, but also risk of future events. Pharmacologic treatment recommendations include using bronchodilator monotherapy in Group A patients, favoring long-acting over short-acting bronchodilators in Group B patients, prescribing inhaled corticosteroids only in combination with long-acting bronchodilators in Groups C and D patients, and considering newer agents such as phosphodiesterase-4 inhibitors in Group D patients.
Non-pharmacologic interventions include ongoing smoking cessation strategies, exercise promotion, treatment of comorbidities, and even public health strategies in pollution control.
Bottom line: The GOLD guidelines have undergone major revisions that provide a more practical approach to classification of COPD based on symptom severity and risk assessment in order to direct providers in evidence-based treatment that addresses both short-term and long-term impact of the disease.
Citation: Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease website. Accessed Oct. 29, 2012.
For more physician reviews of recent HM-relevant literature, visit our website.
Clinical question: What are the changes in the updated Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines?
Background: Chronic obstructive pulmonary disease (COPD) remains a leading cause of death in the U.S. and worldwide. The GOLD guidelines are an international consensus report on COPD diagnosis, management, and prevention, first released in 2001. The 2011 revision to the guidelines was recently published and outlines substantial changes based on updated literature and expert opinion.
Study design: Guidelines based on studies with varying designs.
Setting: Expert panel review of multiple studies from different settings.
Synopsis: While the diagnosis of COPD remains based on a post-bronchodilator fixed ratio of FEV1/FVC <0.70, there is more emphasis on global clinical assessment in the new guidelines. The updated approach describes classifying COPD severity based on risk/symptom frequency using established symptom assessment and the frequency of acute exacerbations of COPD. Instead of five “stages” based on FEV1 measures alone, there are now four "grades" of A through D (A: low risk/fewer symptoms; B: low risk/more symptoms; C: high risk/fewer symptoms; D: high risk/more symptoms) to more easily guide treatment options.
Treatment strategies are also updated, focusing not only on reduction of current symptoms, but also risk of future events. Pharmacologic treatment recommendations include using bronchodilator monotherapy in Group A patients, favoring long-acting over short-acting bronchodilators in Group B patients, prescribing inhaled corticosteroids only in combination with long-acting bronchodilators in Groups C and D patients, and considering newer agents such as phosphodiesterase-4 inhibitors in Group D patients.
Non-pharmacologic interventions include ongoing smoking cessation strategies, exercise promotion, treatment of comorbidities, and even public health strategies in pollution control.
Bottom line: The GOLD guidelines have undergone major revisions that provide a more practical approach to classification of COPD based on symptom severity and risk assessment in order to direct providers in evidence-based treatment that addresses both short-term and long-term impact of the disease.
Citation: Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease website. Accessed Oct. 29, 2012.
For more physician reviews of recent HM-relevant literature, visit our website.
ITL: Physician Reviews of HM-Relevant Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- Interventions that improve discharge handovers reviewed
- Duration of in-hospital cardiac resuscitation and survival rates
- Early sepsis intervention strategies to decrease mortality risk
- Hypoglycemia linked to increased mortality in critically ill
- Increased bleeding risk for cardiac patients
- Hospital-run vs. anesthesiologist-run preoperative clinics
- Postoperative delirium and cognitive impairment in cardiac patients
- Benefits of resuming anticoagulants after GI bleeding
- Preoperative hyponatremia and risk of perioperative mortality
Systematic Review Highlights Several Interventions That Improve Discharge Handovers
Clinical question: Do interventions to improve patient handovers at discharge have positive effects on patient care?
Background: The transition from hospital to primary care is often suboptimal and has been associated with unfavorable outcomes, including hospital readmission, increased healthcare utilization, and adverse drug events post-discharge. This review sought to characterize different types of interventions aimed at improving discharge handovers and to evaluate their effects.
Study design: Systematic review of randomized controlled trials.
Setting: Studies published from January 1990 to March 2011.
Synopsis: Review of published databases identified 36 randomized controlled studies on interventions to improve discharge handovers. Studies were blindly evaluated by two reviewers on quality, interventions, and outcomes. There was significant heterogeneity in interventions and outcomes; thus, statistical analysis was not possible. Most studies evaluated multicomponent interventions and had more than one outcome measure.
Of the 36 studies reviewed, 25 reported statistically significant improvements in outcomes, including reduced hospital utilization and improved continuity of care. Effective interventions included medication reconciliation; structured discharge information (facilitated by electronic resources); multidisciplinary discharge planning; shared involvement in arranging care between inpatient and outpatient physicians; and Web-based access to discharge information by the outpatient provider.
The complexity of the interventions and the heterogeneity of reported results did not allow for firm conclusions to be drawn regarding which specific interventions had the strongest effects.
Bottom line: Interventions that target the quality and safety of handovers between hospital and outpatient providers at discharge can significantly reduce hospital utilization and improve continuity of care.
Citation: Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157:417-428.
Longer Duration of In-Hospital Cardiac Resuscitation Associated with Increased Survival
Clinical question: Are prolonged cardiac resuscitation efforts associated with improved outcomes?
Background: There is little evidence or guidelines on how long to maintain resuscitative efforts during in-hospital cardiac arrest, leading to variation in practice. This study characterized patterns of resuscitation duration and relationship to the return of spontaneous circulation and survival to discharge.
Study design: Retrospective observational study.
Setting: Four hundred thirty-five U.S. hospitals reporting data to the American Heart Association’s Get With The Guidelines: Resuscitation registry.
Synopsis: Using duration of resuscitation in nonsurvivors as a surrogate for the tendency of a facility to perform prolonged efforts, hospitals were divided into quartiles. Overall, of 64,339 patients in the registry, 31,198 (48.5%) had return of circulation and 9,912 (15.4%) survived to discharge. Resuscitative efforts in nonsurvivors ranged from a median of 16 to 25 minutes between the lowest and highest quartiles.
There was a stepwise increase in the likelihood for patients to have return of spontaneous circulation and to survive to discharge between each quartile. Specifically, comparing shortest to longest, there was a significant adjusted odds ratio of 1.12 for both the return of circulation (P<0.0001) and survival to discharge (P=0.021). The survival benefit was most apparent for those with pulseless electrical activity or asystole as initial rhythms, as compared to ventricular tachycardia or fibrillation.
Limitations included the study’s observational design, which meant causality could not be determined. Additionally, the study did not account for the quality of resuscitative efforts (e.g. depth of chest compressions, adherence with guidelines), which might have influenced outcomes. Importantly, the study looked at survival to discharge, but it did not evaluate long-term survival or functional status post-discharge, which might better reflect the success of resuscitation.
Bottom line: Prolonged resuscitative efforts were observed to be associated with increased likelihood of return of spontaneous circulation and survival to discharge; this data might provide clinical guidance in determining when to stop resuscitation efforts.
Citation: Goldberger ZD, Chan PS, Berg RA, et al. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: an observational study. Lancet. 2012;380:1473-1481.
Early Sepsis Intervention Strategies Decrease Mortality, Length of Stay, and Cost
Clinical question: For patients with sepsis or septic shock, what is the impact of a real-time comprehensive continuous quality-improvement (QI) initiative on in-hospital mortality, morbidity, and healthcare resource utilization in community and tertiary-care hospitals in the U.S.?
Background: Multiple single-center trials have demonstrated that early sepsis intervention strategies (early goal-directed therapy, resuscitation bundles) improve in-hospital mortality. Little is known about the effectiveness of incorporating these strategies into a real-time continuous QI initiative and implementing interventions across multiple sites simultaneously.
Study design: Pre-post at some sites and concurrent implementation design at other sites.
Setting: Five community and six-tertiary care U.S. hospitals.
Synopsis: The GENeralized Early Sepsis Intervention Strategies (GENESIS) project was a CQI initiative that combined several QI concepts with validated early sepsis interventions known as resuscitation bundles (RB). Continuous QI was implemented on patients with severe sepsis or septic shock in both before and after designs (eight hospitals), and in concurrent designs (three hospitals). The control group was comprised of historical controls treated before GENESIS and patients with incomplete implementation of RB, totaling 1,554 patients. The treatment group included patients treated after GENESIS and those with complete RB compliance, totaling 4,801 patients.
Compared with the control group, patients in the treatment group had a 33% decreased risk of in-hospital mortality (RR 0.67, 95% CI 0.63-0.72), an absolute decrease in hospital length of stay (LOS) by 5.1 days (20.7 days vs. 15.6 days, P<0.001) and a $47,923 reduction in total hospital charges (P<0.001). Limitations included the study design (not a prospective randomized trial), and the possibility of other concurrent unmeasured quality initiatives taking place at the study sites, which might have contributed to improved outcomes.
Bottom line: Early sepsis intervention strategies in the form of a comprehensive continuous QI initiative can decrease mortality, hospital LOS, and cost in both tertiary-care and community hospitals.
Citation: Cannon CM, Holthaus CV, Zubrow MT, et al. The GENESIS project (GENeralized Early Sepsis Intervention Strategies): a multicenter quality improvement collaborative. J Intensive Care Med. 2012; Aug 17. doi:10.1177/0885066612453025.
Hypoglycemia Associated with Increased Mortality in the Critically Ill
Clinical question: Is hypoglycemia associated with mortality in critically ill patients?
Background: Initial studies suggested that intensive glucose control reduces mortality in surgical ICU patients and reduces morbidity in medical ICU patients, but further studies have not supported these findings. Recent literature shows conflicting results on the effects of intense glucose control in critically ill patients.
Study design: Post-hoc analysis of the NICE-SUGAR study database.
Setting: ICUs in 42 hospitals in Australia, New Zealand, and Canada.
Synopsis: The NICE-SUGAR study was a multicenter trial that randomized 6,104 ICU patients to intensive (glucose 80 to 108 mg/dL) or conventional glucose control (≤180 mg/dL). Patients were followed for 90 days or until death, with death being the primary end point. Severe hypoglycemia (<40 mg/dL) was recorded in 6.8% of patients in the intensive glucose control group versus 0.5% in the conventional group. The study showed that intensive glucose control was associated with increased mortality among adult ICU patients.
Using the NICE-SUGAR database, the authors conducted a Cox regression analysis to examine the associations between hypoglycemia and death. A total of 2,714 patients had moderate hypoglycemia (glucose 41 to 70 mg/dL), and 223 patients had severe hypoglycemia. The hazard ratio for mortality was 1.41 (95% CI 1.21-1.62, P<0.001) for patients with moderate hypoglycemia and 2.10 (95% CI 1.59-2.77, P<0.001) for severe hypoglycemia, compared to patients without hypoglycemia.
These findings show a strong association between mortality and hypoglycemia but do not prove causality. Hospitalists caring for ICU patients must be aware that hypoglycemia is associated with mortality and focus on avoiding hypoglycemia. The American Diabetes Association currently recommends a target blood glucose level of 140 to 180 mg/dL for most critically ill patients.
Bottom line: Hypoglycemia (glucose <70 mg/dL) is associated with increased risk of mortality in ICU patients.
Citation: Finfer S, Chittock DR, Su SY, et al. Hypoglycemia and risk of death in critically ill patients. New Engl J Med. 2012;367(12):1108-1118.
Increased Bleeding Risk for Cardiac Patients on Multiple Antithrombotic Drugs
Clinical question: Is there an increased risk of bleeding in atrial fibrillation patients treated with multiple antithrombotic agents following acute myocardial infarction (MI) or percutaneous coronary intervention (PCI)?
Background: Current treatment for atrial fibrillation patients with MI or PCI includes vitamin K antagonist (VKA) therapy to prevent stroke and antiplatelet agents to prevent further coronary events. There are inconsistent findings on the safety and efficacy of combined therapy with VKA, aspirin, and clopidogrel, specifically with regard to bleeding risk.
Study design: Retrospective cohort study.
Setting: Nationwide registry in Denmark.
Synopsis: Using the National Patient Registry in Denmark, 11,480 patients with atrial fibrillation who were admitted for MI or PCI were identified. Patients were grouped by medication regimen, including monotherapy (aspirin, clopidogrel, or VKA), dual therapy (dual antiplatelet or VKA+antiplatelet), or triple therapy (VKA+aspirin+clopidogrel). The primary outcome was nonfatal or fatal bleeding within one year.
Patients receiving triple therapy had the highest rate of bleeding, with a crude incidence of 14.2 events per 100 person-years. Patients treated with VKA+aspirin+clopidogel were significantly more likely than those on VKA+antiplatelet (HR 1.47, 95% CI 1.04-2.08) or dual antiplatelet (HR 2.20, 95% CI 1.58-3.08) treatment to have a bleeding event within 90 days, and similar trends were seen at 90 to 360 days. There was no significant difference in thromboembolic events among patients on VKA+aspirin+clopidogrel versus VKA+antiplatelet therapy.
Only bleeding events that required hospitalization were recorded, which might underestimate the bleeding risks of these regimens. Additionally, INR levels were not determined, which could impact both bleeding and thromboembolic outcomes.
However, this study does suggest that there are significant bleeding risks among patients treated with triple therapy. Hospitalists should weigh the risks of thromboembolic events with bleeding risks in patients with atrial fibrillation and MI/PCI, and only prescribe VKA+aspirin+clopidogrel with these risks in mind.
Bottom line: Immediate and continued bleeding risk is increased in patients with atrial fibrillation admitted with PCI or MI who are placed on triple antithrombotic therapy with VKA+aspirin+clopidogrel.
Citation: Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention. Circulation. 2012;126:1185-1193.
Hospitalist-Run Preoperative Clinic Improves Outcomes in Complex Surgical Patients
Clinical question: Do hospitalist-run preoperative clinics improve outcomes for medically complex patients undergoing noncardiac surgery compared to traditional anesthesiologist-run preoperative clinics?
Background: Studies of perioperative medical consultation have shown inconsistent effects on quality of care, but preoperative medical consultation has only been evaluated in the immediate preoperative period (one day prior to surgery or less). Little is known about the impact of involving hospitalists earlier in the preoperative period.
Study design: Retrospective, pre-post study.
Setting: Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS).
Synopsis: In July 2004, the VAGLAHS Preoperative Clinic transitioned from being anesthesiologist-run to hospitalist-run. Mid-level providers were trained on preoperative medical assessment, and patients were only evaluated by anesthesia staff on the day of surgery, after they had been deemed medically acceptable for surgery. All patients seen in the clinic from July 2003 to July 2005 were included in the study. Period A included patients evaluated when anesthesia staff supervised the clinic. Period B included patients evaluated during the first year of the hospitalist-run system.
There were 1,101 patients with inpatient surgeries in Period A, and 1,126 patients in Period B. Mean length of stay (LOS) decreased to 5.28 days during the hospitalist-run model from 9.87 days during the anesthesiologist-run model. LOS reductions were most notable in patients with ASA scores of 3 or higher; LOS reductions were not seen in an internal control of surgical patients who were not evaluated in the preoperative clinic. Inpatient mortality was also reduced in Period B compared with Period A, to 4 cases (0.36%) from 14 cases (1.27%) (P=0.0158). Specific processes that led to improved outcomes for patients in Period B could not be identified. The VA study setting might limit the generalizability of the results.
Bottom line: A hospitalist-run preoperative clinic was associated with decreased LOS and inpatient mortality compared with a traditional anesthesiologist-run clinic.
Citation: Vazirani S, Lankarani-Fard A, Lian LJ, Stelzner M, Asch SM. Preoperative processes and outcomes after implementation of a hospitalist-run preoperative clinic. J Hosp Med. 2012 Sep 7. doi:10.1002/jhm.1968.
Delirium after Cardiac Surgery Associated with Prolonged Cognitive Impairment
Clinical question: Is postoperative delirium associated with decreased cognitive function in the first year after cardiac surgery?
Background: In general populations, delirium has been associated with long-term decline in cognitive ability. Delirium and cognitive dysfunction are both common following cardiac surgery, but the effects of postoperative delirium on the trajectory of cognitive function over time is unclear.
Study design: Prospective cohort study.
Setting: Two academic medical centers and a Veterans Administration hospital.
Synopsis: Two hundred twenty-five patients aged 60 or older who were scheduled to undergo coronary artery bypass grafting or valve replacement surgery were included. Patients underwent preoperative assessment cognitive function with the use of the Mini-Mental State Examination (MMSE). Starting on postoperative Day 2, patients underwent daily assessment for delirium. After discharge, cognitive function was reassessed at months one, six, and 12.
Postoperative delirium occurred in 103 patients (46%). Patients with delirium were older, had higher comorbidity scores, and had lower MMSE scores at baseline. Among the overall study population, adjusted MMSE scores dropped 4.6 points from baseline to postoperative Day 2, then were observed to increase by approximately one point per day during postoperative days 3 to 5 with minimal change thereafter. Patients with delirium had greater decrease in cognitive function in the immediate postoperative period compared to patients without delirium (7.7 points vs. 2.1, P<0.001).
Patients without delirium returned to their baseline cognitive ability by one month postoperatively, while patients who had delirium were still making gains up to six months post-operatively, never returning to baseline level of function by one year. Unmeasured confounders and uncertain sensitivity of the MMSE to detect mild cognitive impairment might limit these findings.
Bottom line: Cognitive function decreases in the immediate postoperative period following cardiac surgery. Compared to patients without delirium, patients with delirium experience more dramatic and prolonged cognitive impairment postoperatively, without returning to their preoperative level of cognitive function at one year.
Citation: Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. New Engl J Med. 2012;367:30-39.
Benefits of Resuming Anticoagulation after GI Bleed Outweigh Risks for Most Patients
Clinical question: In warfarin-treated patients who have experienced gastrointestinal (GI) bleeding, what are the patterns of restarting warfarin therapy and the incidence of thrombosis, recurrent GI bleed, and death in the 90 days following index bleed?
Background: In warfarin-treated patients who experience GI bleeding, warfarin is often temporarily held or permanently discontinued, placing patients at increased risk for developing thromboembolism. Little is known about the risks, benefits, and timing of restarting warfarin in this patient population.
Study design: Retrospective cohort study.
Setting: Kaiser Permanente Colorado.
Synopsis: Using clinical and administrative databases, 442 patients who presented with GI bleeding while receiving warfarin therapy were identified. Patients were grouped by whether they resumed warfarin (n=260, including 41 patients in whom anticoagulation was never interrupted), or did not resume warfarin therapy (n=182) in the 90 days following index GI bleed. Patients with prosthetic heart valves or GI bleeding localized to the rectum/anus were more commonly restarted on warfarin, whereas older patients and those in whom the source of bleeding was not identified were less likely to be restarted on warfarin therapy.
Restarting warfarin therapy after index GI bleed was associated with lower risk of thrombosis (HR 0.05, 95% CI 0.01-0.58) and death from any cause (HR 0.31, 95% CI 0.15-0.62), and it was not associated with a significant increase in risk for recurrent GI bleed (HR 1.32, 95% CI 0.50-3.57).
The authors concluded that for many patients who experience a warfarin-associated GI bleed, the benefits of restarting warfarin therapy outweigh the risks. No conclusions were made regarding the optimal timing of resuming therapy. Limitations included the use of administrative data and inability to determine the potential influence of aspirin use on outcomes.
Bottom line: Resuming warfarin in the 90 days following a warfarin-associated GI bleed is associated with decreased risk of thrombosis and death without increased risk for recurrent GI bleed.
Citation: Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012 Sep 12. doi:10.1001/archinternmed.2012.4261.
Preoperative Hyponatremia Associated with Increased Risk for Perioperative Complications and Mortality
Clinical question: Is preoperative hyponatremia an indicator of perioperative morbidity and mortality?
Background: Hyponatremia is a common diagnosis in the hospital setting and is associated with adverse outcomes, even in mild cases. However, it is unclear if this association exists in surgical patients when detected preoperatively.
Study design: Retrospective cohort study.
Setting: Academic and community hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program (NSQIP).
Synopsis: A total of 75,423 adult patients with hyponatremia (sodium <135 mEq/L) who were undergoing major surgery were compared to 888,840 patients with normal preoperative sodium levels over a six-year period. The primary outcome was 30-day mortality. Secondary outcomes included postoperative major coronary events, stroke, wound infection, pneumonia, and length of stay (LOS).
Compared to patients with normal sodium levels, those with preoperative hyponatremia had higher rates of perioperative mortality (5.2% vs. 1.3%; adjusted odds ratio 1.44, 95% CI 1.38-1.50), with increased risk that correlated with increasing severity of hyponatremia. Association with postoperative mortality was particularly strong among hyponatremic patients with ASA scores of 1 or 2 and those undergoing nonemergency surgery.
Patients with preoperative hyponatremia were also found to have increased risk for all postoperative complications evaluated, with the exception of stroke. Limitations included the potential for unmeasured confounders and not being able to account for the role of medications used perioperatively. Research is needed to determine whether correcting preoperative hyponatremia lessens the risk of mortality and other postoperative complications.
Bottom line: Among patients undergoing major surgery, preoperative hyponatremia is a predictor of postoperative 30-day mortality and morbidity.
Citation: Leung AA, McAlister FA, Rogers SO, et al. Preoperative hyponatremia and perioperative complications. Arch Intern Med. 2012;172:1-8.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Interventions that improve discharge handovers reviewed
- Duration of in-hospital cardiac resuscitation and survival rates
- Early sepsis intervention strategies to decrease mortality risk
- Hypoglycemia linked to increased mortality in critically ill
- Increased bleeding risk for cardiac patients
- Hospital-run vs. anesthesiologist-run preoperative clinics
- Postoperative delirium and cognitive impairment in cardiac patients
- Benefits of resuming anticoagulants after GI bleeding
- Preoperative hyponatremia and risk of perioperative mortality
Systematic Review Highlights Several Interventions That Improve Discharge Handovers
Clinical question: Do interventions to improve patient handovers at discharge have positive effects on patient care?
Background: The transition from hospital to primary care is often suboptimal and has been associated with unfavorable outcomes, including hospital readmission, increased healthcare utilization, and adverse drug events post-discharge. This review sought to characterize different types of interventions aimed at improving discharge handovers and to evaluate their effects.
Study design: Systematic review of randomized controlled trials.
Setting: Studies published from January 1990 to March 2011.
Synopsis: Review of published databases identified 36 randomized controlled studies on interventions to improve discharge handovers. Studies were blindly evaluated by two reviewers on quality, interventions, and outcomes. There was significant heterogeneity in interventions and outcomes; thus, statistical analysis was not possible. Most studies evaluated multicomponent interventions and had more than one outcome measure.
Of the 36 studies reviewed, 25 reported statistically significant improvements in outcomes, including reduced hospital utilization and improved continuity of care. Effective interventions included medication reconciliation; structured discharge information (facilitated by electronic resources); multidisciplinary discharge planning; shared involvement in arranging care between inpatient and outpatient physicians; and Web-based access to discharge information by the outpatient provider.
The complexity of the interventions and the heterogeneity of reported results did not allow for firm conclusions to be drawn regarding which specific interventions had the strongest effects.
Bottom line: Interventions that target the quality and safety of handovers between hospital and outpatient providers at discharge can significantly reduce hospital utilization and improve continuity of care.
Citation: Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157:417-428.
Longer Duration of In-Hospital Cardiac Resuscitation Associated with Increased Survival
Clinical question: Are prolonged cardiac resuscitation efforts associated with improved outcomes?
Background: There is little evidence or guidelines on how long to maintain resuscitative efforts during in-hospital cardiac arrest, leading to variation in practice. This study characterized patterns of resuscitation duration and relationship to the return of spontaneous circulation and survival to discharge.
Study design: Retrospective observational study.
Setting: Four hundred thirty-five U.S. hospitals reporting data to the American Heart Association’s Get With The Guidelines: Resuscitation registry.
Synopsis: Using duration of resuscitation in nonsurvivors as a surrogate for the tendency of a facility to perform prolonged efforts, hospitals were divided into quartiles. Overall, of 64,339 patients in the registry, 31,198 (48.5%) had return of circulation and 9,912 (15.4%) survived to discharge. Resuscitative efforts in nonsurvivors ranged from a median of 16 to 25 minutes between the lowest and highest quartiles.
There was a stepwise increase in the likelihood for patients to have return of spontaneous circulation and to survive to discharge between each quartile. Specifically, comparing shortest to longest, there was a significant adjusted odds ratio of 1.12 for both the return of circulation (P<0.0001) and survival to discharge (P=0.021). The survival benefit was most apparent for those with pulseless electrical activity or asystole as initial rhythms, as compared to ventricular tachycardia or fibrillation.
Limitations included the study’s observational design, which meant causality could not be determined. Additionally, the study did not account for the quality of resuscitative efforts (e.g. depth of chest compressions, adherence with guidelines), which might have influenced outcomes. Importantly, the study looked at survival to discharge, but it did not evaluate long-term survival or functional status post-discharge, which might better reflect the success of resuscitation.
Bottom line: Prolonged resuscitative efforts were observed to be associated with increased likelihood of return of spontaneous circulation and survival to discharge; this data might provide clinical guidance in determining when to stop resuscitation efforts.
Citation: Goldberger ZD, Chan PS, Berg RA, et al. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: an observational study. Lancet. 2012;380:1473-1481.
Early Sepsis Intervention Strategies Decrease Mortality, Length of Stay, and Cost
Clinical question: For patients with sepsis or septic shock, what is the impact of a real-time comprehensive continuous quality-improvement (QI) initiative on in-hospital mortality, morbidity, and healthcare resource utilization in community and tertiary-care hospitals in the U.S.?
Background: Multiple single-center trials have demonstrated that early sepsis intervention strategies (early goal-directed therapy, resuscitation bundles) improve in-hospital mortality. Little is known about the effectiveness of incorporating these strategies into a real-time continuous QI initiative and implementing interventions across multiple sites simultaneously.
Study design: Pre-post at some sites and concurrent implementation design at other sites.
Setting: Five community and six-tertiary care U.S. hospitals.
Synopsis: The GENeralized Early Sepsis Intervention Strategies (GENESIS) project was a CQI initiative that combined several QI concepts with validated early sepsis interventions known as resuscitation bundles (RB). Continuous QI was implemented on patients with severe sepsis or septic shock in both before and after designs (eight hospitals), and in concurrent designs (three hospitals). The control group was comprised of historical controls treated before GENESIS and patients with incomplete implementation of RB, totaling 1,554 patients. The treatment group included patients treated after GENESIS and those with complete RB compliance, totaling 4,801 patients.
Compared with the control group, patients in the treatment group had a 33% decreased risk of in-hospital mortality (RR 0.67, 95% CI 0.63-0.72), an absolute decrease in hospital length of stay (LOS) by 5.1 days (20.7 days vs. 15.6 days, P<0.001) and a $47,923 reduction in total hospital charges (P<0.001). Limitations included the study design (not a prospective randomized trial), and the possibility of other concurrent unmeasured quality initiatives taking place at the study sites, which might have contributed to improved outcomes.
Bottom line: Early sepsis intervention strategies in the form of a comprehensive continuous QI initiative can decrease mortality, hospital LOS, and cost in both tertiary-care and community hospitals.
Citation: Cannon CM, Holthaus CV, Zubrow MT, et al. The GENESIS project (GENeralized Early Sepsis Intervention Strategies): a multicenter quality improvement collaborative. J Intensive Care Med. 2012; Aug 17. doi:10.1177/0885066612453025.
Hypoglycemia Associated with Increased Mortality in the Critically Ill
Clinical question: Is hypoglycemia associated with mortality in critically ill patients?
Background: Initial studies suggested that intensive glucose control reduces mortality in surgical ICU patients and reduces morbidity in medical ICU patients, but further studies have not supported these findings. Recent literature shows conflicting results on the effects of intense glucose control in critically ill patients.
Study design: Post-hoc analysis of the NICE-SUGAR study database.
Setting: ICUs in 42 hospitals in Australia, New Zealand, and Canada.
Synopsis: The NICE-SUGAR study was a multicenter trial that randomized 6,104 ICU patients to intensive (glucose 80 to 108 mg/dL) or conventional glucose control (≤180 mg/dL). Patients were followed for 90 days or until death, with death being the primary end point. Severe hypoglycemia (<40 mg/dL) was recorded in 6.8% of patients in the intensive glucose control group versus 0.5% in the conventional group. The study showed that intensive glucose control was associated with increased mortality among adult ICU patients.
Using the NICE-SUGAR database, the authors conducted a Cox regression analysis to examine the associations between hypoglycemia and death. A total of 2,714 patients had moderate hypoglycemia (glucose 41 to 70 mg/dL), and 223 patients had severe hypoglycemia. The hazard ratio for mortality was 1.41 (95% CI 1.21-1.62, P<0.001) for patients with moderate hypoglycemia and 2.10 (95% CI 1.59-2.77, P<0.001) for severe hypoglycemia, compared to patients without hypoglycemia.
These findings show a strong association between mortality and hypoglycemia but do not prove causality. Hospitalists caring for ICU patients must be aware that hypoglycemia is associated with mortality and focus on avoiding hypoglycemia. The American Diabetes Association currently recommends a target blood glucose level of 140 to 180 mg/dL for most critically ill patients.
Bottom line: Hypoglycemia (glucose <70 mg/dL) is associated with increased risk of mortality in ICU patients.
Citation: Finfer S, Chittock DR, Su SY, et al. Hypoglycemia and risk of death in critically ill patients. New Engl J Med. 2012;367(12):1108-1118.
Increased Bleeding Risk for Cardiac Patients on Multiple Antithrombotic Drugs
Clinical question: Is there an increased risk of bleeding in atrial fibrillation patients treated with multiple antithrombotic agents following acute myocardial infarction (MI) or percutaneous coronary intervention (PCI)?
Background: Current treatment for atrial fibrillation patients with MI or PCI includes vitamin K antagonist (VKA) therapy to prevent stroke and antiplatelet agents to prevent further coronary events. There are inconsistent findings on the safety and efficacy of combined therapy with VKA, aspirin, and clopidogrel, specifically with regard to bleeding risk.
Study design: Retrospective cohort study.
Setting: Nationwide registry in Denmark.
Synopsis: Using the National Patient Registry in Denmark, 11,480 patients with atrial fibrillation who were admitted for MI or PCI were identified. Patients were grouped by medication regimen, including monotherapy (aspirin, clopidogrel, or VKA), dual therapy (dual antiplatelet or VKA+antiplatelet), or triple therapy (VKA+aspirin+clopidogrel). The primary outcome was nonfatal or fatal bleeding within one year.
Patients receiving triple therapy had the highest rate of bleeding, with a crude incidence of 14.2 events per 100 person-years. Patients treated with VKA+aspirin+clopidogel were significantly more likely than those on VKA+antiplatelet (HR 1.47, 95% CI 1.04-2.08) or dual antiplatelet (HR 2.20, 95% CI 1.58-3.08) treatment to have a bleeding event within 90 days, and similar trends were seen at 90 to 360 days. There was no significant difference in thromboembolic events among patients on VKA+aspirin+clopidogrel versus VKA+antiplatelet therapy.
Only bleeding events that required hospitalization were recorded, which might underestimate the bleeding risks of these regimens. Additionally, INR levels were not determined, which could impact both bleeding and thromboembolic outcomes.
However, this study does suggest that there are significant bleeding risks among patients treated with triple therapy. Hospitalists should weigh the risks of thromboembolic events with bleeding risks in patients with atrial fibrillation and MI/PCI, and only prescribe VKA+aspirin+clopidogrel with these risks in mind.
Bottom line: Immediate and continued bleeding risk is increased in patients with atrial fibrillation admitted with PCI or MI who are placed on triple antithrombotic therapy with VKA+aspirin+clopidogrel.
Citation: Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention. Circulation. 2012;126:1185-1193.
Hospitalist-Run Preoperative Clinic Improves Outcomes in Complex Surgical Patients
Clinical question: Do hospitalist-run preoperative clinics improve outcomes for medically complex patients undergoing noncardiac surgery compared to traditional anesthesiologist-run preoperative clinics?
Background: Studies of perioperative medical consultation have shown inconsistent effects on quality of care, but preoperative medical consultation has only been evaluated in the immediate preoperative period (one day prior to surgery or less). Little is known about the impact of involving hospitalists earlier in the preoperative period.
Study design: Retrospective, pre-post study.
Setting: Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS).
Synopsis: In July 2004, the VAGLAHS Preoperative Clinic transitioned from being anesthesiologist-run to hospitalist-run. Mid-level providers were trained on preoperative medical assessment, and patients were only evaluated by anesthesia staff on the day of surgery, after they had been deemed medically acceptable for surgery. All patients seen in the clinic from July 2003 to July 2005 were included in the study. Period A included patients evaluated when anesthesia staff supervised the clinic. Period B included patients evaluated during the first year of the hospitalist-run system.
There were 1,101 patients with inpatient surgeries in Period A, and 1,126 patients in Period B. Mean length of stay (LOS) decreased to 5.28 days during the hospitalist-run model from 9.87 days during the anesthesiologist-run model. LOS reductions were most notable in patients with ASA scores of 3 or higher; LOS reductions were not seen in an internal control of surgical patients who were not evaluated in the preoperative clinic. Inpatient mortality was also reduced in Period B compared with Period A, to 4 cases (0.36%) from 14 cases (1.27%) (P=0.0158). Specific processes that led to improved outcomes for patients in Period B could not be identified. The VA study setting might limit the generalizability of the results.
Bottom line: A hospitalist-run preoperative clinic was associated with decreased LOS and inpatient mortality compared with a traditional anesthesiologist-run clinic.
Citation: Vazirani S, Lankarani-Fard A, Lian LJ, Stelzner M, Asch SM. Preoperative processes and outcomes after implementation of a hospitalist-run preoperative clinic. J Hosp Med. 2012 Sep 7. doi:10.1002/jhm.1968.
Delirium after Cardiac Surgery Associated with Prolonged Cognitive Impairment
Clinical question: Is postoperative delirium associated with decreased cognitive function in the first year after cardiac surgery?
Background: In general populations, delirium has been associated with long-term decline in cognitive ability. Delirium and cognitive dysfunction are both common following cardiac surgery, but the effects of postoperative delirium on the trajectory of cognitive function over time is unclear.
Study design: Prospective cohort study.
Setting: Two academic medical centers and a Veterans Administration hospital.
Synopsis: Two hundred twenty-five patients aged 60 or older who were scheduled to undergo coronary artery bypass grafting or valve replacement surgery were included. Patients underwent preoperative assessment cognitive function with the use of the Mini-Mental State Examination (MMSE). Starting on postoperative Day 2, patients underwent daily assessment for delirium. After discharge, cognitive function was reassessed at months one, six, and 12.
Postoperative delirium occurred in 103 patients (46%). Patients with delirium were older, had higher comorbidity scores, and had lower MMSE scores at baseline. Among the overall study population, adjusted MMSE scores dropped 4.6 points from baseline to postoperative Day 2, then were observed to increase by approximately one point per day during postoperative days 3 to 5 with minimal change thereafter. Patients with delirium had greater decrease in cognitive function in the immediate postoperative period compared to patients without delirium (7.7 points vs. 2.1, P<0.001).
Patients without delirium returned to their baseline cognitive ability by one month postoperatively, while patients who had delirium were still making gains up to six months post-operatively, never returning to baseline level of function by one year. Unmeasured confounders and uncertain sensitivity of the MMSE to detect mild cognitive impairment might limit these findings.
Bottom line: Cognitive function decreases in the immediate postoperative period following cardiac surgery. Compared to patients without delirium, patients with delirium experience more dramatic and prolonged cognitive impairment postoperatively, without returning to their preoperative level of cognitive function at one year.
Citation: Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. New Engl J Med. 2012;367:30-39.
Benefits of Resuming Anticoagulation after GI Bleed Outweigh Risks for Most Patients
Clinical question: In warfarin-treated patients who have experienced gastrointestinal (GI) bleeding, what are the patterns of restarting warfarin therapy and the incidence of thrombosis, recurrent GI bleed, and death in the 90 days following index bleed?
Background: In warfarin-treated patients who experience GI bleeding, warfarin is often temporarily held or permanently discontinued, placing patients at increased risk for developing thromboembolism. Little is known about the risks, benefits, and timing of restarting warfarin in this patient population.
Study design: Retrospective cohort study.
Setting: Kaiser Permanente Colorado.
Synopsis: Using clinical and administrative databases, 442 patients who presented with GI bleeding while receiving warfarin therapy were identified. Patients were grouped by whether they resumed warfarin (n=260, including 41 patients in whom anticoagulation was never interrupted), or did not resume warfarin therapy (n=182) in the 90 days following index GI bleed. Patients with prosthetic heart valves or GI bleeding localized to the rectum/anus were more commonly restarted on warfarin, whereas older patients and those in whom the source of bleeding was not identified were less likely to be restarted on warfarin therapy.
Restarting warfarin therapy after index GI bleed was associated with lower risk of thrombosis (HR 0.05, 95% CI 0.01-0.58) and death from any cause (HR 0.31, 95% CI 0.15-0.62), and it was not associated with a significant increase in risk for recurrent GI bleed (HR 1.32, 95% CI 0.50-3.57).
The authors concluded that for many patients who experience a warfarin-associated GI bleed, the benefits of restarting warfarin therapy outweigh the risks. No conclusions were made regarding the optimal timing of resuming therapy. Limitations included the use of administrative data and inability to determine the potential influence of aspirin use on outcomes.
Bottom line: Resuming warfarin in the 90 days following a warfarin-associated GI bleed is associated with decreased risk of thrombosis and death without increased risk for recurrent GI bleed.
Citation: Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012 Sep 12. doi:10.1001/archinternmed.2012.4261.
Preoperative Hyponatremia Associated with Increased Risk for Perioperative Complications and Mortality
Clinical question: Is preoperative hyponatremia an indicator of perioperative morbidity and mortality?
Background: Hyponatremia is a common diagnosis in the hospital setting and is associated with adverse outcomes, even in mild cases. However, it is unclear if this association exists in surgical patients when detected preoperatively.
Study design: Retrospective cohort study.
Setting: Academic and community hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program (NSQIP).
Synopsis: A total of 75,423 adult patients with hyponatremia (sodium <135 mEq/L) who were undergoing major surgery were compared to 888,840 patients with normal preoperative sodium levels over a six-year period. The primary outcome was 30-day mortality. Secondary outcomes included postoperative major coronary events, stroke, wound infection, pneumonia, and length of stay (LOS).
Compared to patients with normal sodium levels, those with preoperative hyponatremia had higher rates of perioperative mortality (5.2% vs. 1.3%; adjusted odds ratio 1.44, 95% CI 1.38-1.50), with increased risk that correlated with increasing severity of hyponatremia. Association with postoperative mortality was particularly strong among hyponatremic patients with ASA scores of 1 or 2 and those undergoing nonemergency surgery.
Patients with preoperative hyponatremia were also found to have increased risk for all postoperative complications evaluated, with the exception of stroke. Limitations included the potential for unmeasured confounders and not being able to account for the role of medications used perioperatively. Research is needed to determine whether correcting preoperative hyponatremia lessens the risk of mortality and other postoperative complications.
Bottom line: Among patients undergoing major surgery, preoperative hyponatremia is a predictor of postoperative 30-day mortality and morbidity.
Citation: Leung AA, McAlister FA, Rogers SO, et al. Preoperative hyponatremia and perioperative complications. Arch Intern Med. 2012;172:1-8.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Interventions that improve discharge handovers reviewed
- Duration of in-hospital cardiac resuscitation and survival rates
- Early sepsis intervention strategies to decrease mortality risk
- Hypoglycemia linked to increased mortality in critically ill
- Increased bleeding risk for cardiac patients
- Hospital-run vs. anesthesiologist-run preoperative clinics
- Postoperative delirium and cognitive impairment in cardiac patients
- Benefits of resuming anticoagulants after GI bleeding
- Preoperative hyponatremia and risk of perioperative mortality
Systematic Review Highlights Several Interventions That Improve Discharge Handovers
Clinical question: Do interventions to improve patient handovers at discharge have positive effects on patient care?
Background: The transition from hospital to primary care is often suboptimal and has been associated with unfavorable outcomes, including hospital readmission, increased healthcare utilization, and adverse drug events post-discharge. This review sought to characterize different types of interventions aimed at improving discharge handovers and to evaluate their effects.
Study design: Systematic review of randomized controlled trials.
Setting: Studies published from January 1990 to March 2011.
Synopsis: Review of published databases identified 36 randomized controlled studies on interventions to improve discharge handovers. Studies were blindly evaluated by two reviewers on quality, interventions, and outcomes. There was significant heterogeneity in interventions and outcomes; thus, statistical analysis was not possible. Most studies evaluated multicomponent interventions and had more than one outcome measure.
Of the 36 studies reviewed, 25 reported statistically significant improvements in outcomes, including reduced hospital utilization and improved continuity of care. Effective interventions included medication reconciliation; structured discharge information (facilitated by electronic resources); multidisciplinary discharge planning; shared involvement in arranging care between inpatient and outpatient physicians; and Web-based access to discharge information by the outpatient provider.
The complexity of the interventions and the heterogeneity of reported results did not allow for firm conclusions to be drawn regarding which specific interventions had the strongest effects.
Bottom line: Interventions that target the quality and safety of handovers between hospital and outpatient providers at discharge can significantly reduce hospital utilization and improve continuity of care.
Citation: Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157:417-428.
Longer Duration of In-Hospital Cardiac Resuscitation Associated with Increased Survival
Clinical question: Are prolonged cardiac resuscitation efforts associated with improved outcomes?
Background: There is little evidence or guidelines on how long to maintain resuscitative efforts during in-hospital cardiac arrest, leading to variation in practice. This study characterized patterns of resuscitation duration and relationship to the return of spontaneous circulation and survival to discharge.
Study design: Retrospective observational study.
Setting: Four hundred thirty-five U.S. hospitals reporting data to the American Heart Association’s Get With The Guidelines: Resuscitation registry.
Synopsis: Using duration of resuscitation in nonsurvivors as a surrogate for the tendency of a facility to perform prolonged efforts, hospitals were divided into quartiles. Overall, of 64,339 patients in the registry, 31,198 (48.5%) had return of circulation and 9,912 (15.4%) survived to discharge. Resuscitative efforts in nonsurvivors ranged from a median of 16 to 25 minutes between the lowest and highest quartiles.
There was a stepwise increase in the likelihood for patients to have return of spontaneous circulation and to survive to discharge between each quartile. Specifically, comparing shortest to longest, there was a significant adjusted odds ratio of 1.12 for both the return of circulation (P<0.0001) and survival to discharge (P=0.021). The survival benefit was most apparent for those with pulseless electrical activity or asystole as initial rhythms, as compared to ventricular tachycardia or fibrillation.
Limitations included the study’s observational design, which meant causality could not be determined. Additionally, the study did not account for the quality of resuscitative efforts (e.g. depth of chest compressions, adherence with guidelines), which might have influenced outcomes. Importantly, the study looked at survival to discharge, but it did not evaluate long-term survival or functional status post-discharge, which might better reflect the success of resuscitation.
Bottom line: Prolonged resuscitative efforts were observed to be associated with increased likelihood of return of spontaneous circulation and survival to discharge; this data might provide clinical guidance in determining when to stop resuscitation efforts.
Citation: Goldberger ZD, Chan PS, Berg RA, et al. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: an observational study. Lancet. 2012;380:1473-1481.
Early Sepsis Intervention Strategies Decrease Mortality, Length of Stay, and Cost
Clinical question: For patients with sepsis or septic shock, what is the impact of a real-time comprehensive continuous quality-improvement (QI) initiative on in-hospital mortality, morbidity, and healthcare resource utilization in community and tertiary-care hospitals in the U.S.?
Background: Multiple single-center trials have demonstrated that early sepsis intervention strategies (early goal-directed therapy, resuscitation bundles) improve in-hospital mortality. Little is known about the effectiveness of incorporating these strategies into a real-time continuous QI initiative and implementing interventions across multiple sites simultaneously.
Study design: Pre-post at some sites and concurrent implementation design at other sites.
Setting: Five community and six-tertiary care U.S. hospitals.
Synopsis: The GENeralized Early Sepsis Intervention Strategies (GENESIS) project was a CQI initiative that combined several QI concepts with validated early sepsis interventions known as resuscitation bundles (RB). Continuous QI was implemented on patients with severe sepsis or septic shock in both before and after designs (eight hospitals), and in concurrent designs (three hospitals). The control group was comprised of historical controls treated before GENESIS and patients with incomplete implementation of RB, totaling 1,554 patients. The treatment group included patients treated after GENESIS and those with complete RB compliance, totaling 4,801 patients.
Compared with the control group, patients in the treatment group had a 33% decreased risk of in-hospital mortality (RR 0.67, 95% CI 0.63-0.72), an absolute decrease in hospital length of stay (LOS) by 5.1 days (20.7 days vs. 15.6 days, P<0.001) and a $47,923 reduction in total hospital charges (P<0.001). Limitations included the study design (not a prospective randomized trial), and the possibility of other concurrent unmeasured quality initiatives taking place at the study sites, which might have contributed to improved outcomes.
Bottom line: Early sepsis intervention strategies in the form of a comprehensive continuous QI initiative can decrease mortality, hospital LOS, and cost in both tertiary-care and community hospitals.
Citation: Cannon CM, Holthaus CV, Zubrow MT, et al. The GENESIS project (GENeralized Early Sepsis Intervention Strategies): a multicenter quality improvement collaborative. J Intensive Care Med. 2012; Aug 17. doi:10.1177/0885066612453025.
Hypoglycemia Associated with Increased Mortality in the Critically Ill
Clinical question: Is hypoglycemia associated with mortality in critically ill patients?
Background: Initial studies suggested that intensive glucose control reduces mortality in surgical ICU patients and reduces morbidity in medical ICU patients, but further studies have not supported these findings. Recent literature shows conflicting results on the effects of intense glucose control in critically ill patients.
Study design: Post-hoc analysis of the NICE-SUGAR study database.
Setting: ICUs in 42 hospitals in Australia, New Zealand, and Canada.
Synopsis: The NICE-SUGAR study was a multicenter trial that randomized 6,104 ICU patients to intensive (glucose 80 to 108 mg/dL) or conventional glucose control (≤180 mg/dL). Patients were followed for 90 days or until death, with death being the primary end point. Severe hypoglycemia (<40 mg/dL) was recorded in 6.8% of patients in the intensive glucose control group versus 0.5% in the conventional group. The study showed that intensive glucose control was associated with increased mortality among adult ICU patients.
Using the NICE-SUGAR database, the authors conducted a Cox regression analysis to examine the associations between hypoglycemia and death. A total of 2,714 patients had moderate hypoglycemia (glucose 41 to 70 mg/dL), and 223 patients had severe hypoglycemia. The hazard ratio for mortality was 1.41 (95% CI 1.21-1.62, P<0.001) for patients with moderate hypoglycemia and 2.10 (95% CI 1.59-2.77, P<0.001) for severe hypoglycemia, compared to patients without hypoglycemia.
These findings show a strong association between mortality and hypoglycemia but do not prove causality. Hospitalists caring for ICU patients must be aware that hypoglycemia is associated with mortality and focus on avoiding hypoglycemia. The American Diabetes Association currently recommends a target blood glucose level of 140 to 180 mg/dL for most critically ill patients.
Bottom line: Hypoglycemia (glucose <70 mg/dL) is associated with increased risk of mortality in ICU patients.
Citation: Finfer S, Chittock DR, Su SY, et al. Hypoglycemia and risk of death in critically ill patients. New Engl J Med. 2012;367(12):1108-1118.
Increased Bleeding Risk for Cardiac Patients on Multiple Antithrombotic Drugs
Clinical question: Is there an increased risk of bleeding in atrial fibrillation patients treated with multiple antithrombotic agents following acute myocardial infarction (MI) or percutaneous coronary intervention (PCI)?
Background: Current treatment for atrial fibrillation patients with MI or PCI includes vitamin K antagonist (VKA) therapy to prevent stroke and antiplatelet agents to prevent further coronary events. There are inconsistent findings on the safety and efficacy of combined therapy with VKA, aspirin, and clopidogrel, specifically with regard to bleeding risk.
Study design: Retrospective cohort study.
Setting: Nationwide registry in Denmark.
Synopsis: Using the National Patient Registry in Denmark, 11,480 patients with atrial fibrillation who were admitted for MI or PCI were identified. Patients were grouped by medication regimen, including monotherapy (aspirin, clopidogrel, or VKA), dual therapy (dual antiplatelet or VKA+antiplatelet), or triple therapy (VKA+aspirin+clopidogrel). The primary outcome was nonfatal or fatal bleeding within one year.
Patients receiving triple therapy had the highest rate of bleeding, with a crude incidence of 14.2 events per 100 person-years. Patients treated with VKA+aspirin+clopidogel were significantly more likely than those on VKA+antiplatelet (HR 1.47, 95% CI 1.04-2.08) or dual antiplatelet (HR 2.20, 95% CI 1.58-3.08) treatment to have a bleeding event within 90 days, and similar trends were seen at 90 to 360 days. There was no significant difference in thromboembolic events among patients on VKA+aspirin+clopidogrel versus VKA+antiplatelet therapy.
Only bleeding events that required hospitalization were recorded, which might underestimate the bleeding risks of these regimens. Additionally, INR levels were not determined, which could impact both bleeding and thromboembolic outcomes.
However, this study does suggest that there are significant bleeding risks among patients treated with triple therapy. Hospitalists should weigh the risks of thromboembolic events with bleeding risks in patients with atrial fibrillation and MI/PCI, and only prescribe VKA+aspirin+clopidogrel with these risks in mind.
Bottom line: Immediate and continued bleeding risk is increased in patients with atrial fibrillation admitted with PCI or MI who are placed on triple antithrombotic therapy with VKA+aspirin+clopidogrel.
Citation: Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention. Circulation. 2012;126:1185-1193.
Hospitalist-Run Preoperative Clinic Improves Outcomes in Complex Surgical Patients
Clinical question: Do hospitalist-run preoperative clinics improve outcomes for medically complex patients undergoing noncardiac surgery compared to traditional anesthesiologist-run preoperative clinics?
Background: Studies of perioperative medical consultation have shown inconsistent effects on quality of care, but preoperative medical consultation has only been evaluated in the immediate preoperative period (one day prior to surgery or less). Little is known about the impact of involving hospitalists earlier in the preoperative period.
Study design: Retrospective, pre-post study.
Setting: Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS).
Synopsis: In July 2004, the VAGLAHS Preoperative Clinic transitioned from being anesthesiologist-run to hospitalist-run. Mid-level providers were trained on preoperative medical assessment, and patients were only evaluated by anesthesia staff on the day of surgery, after they had been deemed medically acceptable for surgery. All patients seen in the clinic from July 2003 to July 2005 were included in the study. Period A included patients evaluated when anesthesia staff supervised the clinic. Period B included patients evaluated during the first year of the hospitalist-run system.
There were 1,101 patients with inpatient surgeries in Period A, and 1,126 patients in Period B. Mean length of stay (LOS) decreased to 5.28 days during the hospitalist-run model from 9.87 days during the anesthesiologist-run model. LOS reductions were most notable in patients with ASA scores of 3 or higher; LOS reductions were not seen in an internal control of surgical patients who were not evaluated in the preoperative clinic. Inpatient mortality was also reduced in Period B compared with Period A, to 4 cases (0.36%) from 14 cases (1.27%) (P=0.0158). Specific processes that led to improved outcomes for patients in Period B could not be identified. The VA study setting might limit the generalizability of the results.
Bottom line: A hospitalist-run preoperative clinic was associated with decreased LOS and inpatient mortality compared with a traditional anesthesiologist-run clinic.
Citation: Vazirani S, Lankarani-Fard A, Lian LJ, Stelzner M, Asch SM. Preoperative processes and outcomes after implementation of a hospitalist-run preoperative clinic. J Hosp Med. 2012 Sep 7. doi:10.1002/jhm.1968.
Delirium after Cardiac Surgery Associated with Prolonged Cognitive Impairment
Clinical question: Is postoperative delirium associated with decreased cognitive function in the first year after cardiac surgery?
Background: In general populations, delirium has been associated with long-term decline in cognitive ability. Delirium and cognitive dysfunction are both common following cardiac surgery, but the effects of postoperative delirium on the trajectory of cognitive function over time is unclear.
Study design: Prospective cohort study.
Setting: Two academic medical centers and a Veterans Administration hospital.
Synopsis: Two hundred twenty-five patients aged 60 or older who were scheduled to undergo coronary artery bypass grafting or valve replacement surgery were included. Patients underwent preoperative assessment cognitive function with the use of the Mini-Mental State Examination (MMSE). Starting on postoperative Day 2, patients underwent daily assessment for delirium. After discharge, cognitive function was reassessed at months one, six, and 12.
Postoperative delirium occurred in 103 patients (46%). Patients with delirium were older, had higher comorbidity scores, and had lower MMSE scores at baseline. Among the overall study population, adjusted MMSE scores dropped 4.6 points from baseline to postoperative Day 2, then were observed to increase by approximately one point per day during postoperative days 3 to 5 with minimal change thereafter. Patients with delirium had greater decrease in cognitive function in the immediate postoperative period compared to patients without delirium (7.7 points vs. 2.1, P<0.001).
Patients without delirium returned to their baseline cognitive ability by one month postoperatively, while patients who had delirium were still making gains up to six months post-operatively, never returning to baseline level of function by one year. Unmeasured confounders and uncertain sensitivity of the MMSE to detect mild cognitive impairment might limit these findings.
Bottom line: Cognitive function decreases in the immediate postoperative period following cardiac surgery. Compared to patients without delirium, patients with delirium experience more dramatic and prolonged cognitive impairment postoperatively, without returning to their preoperative level of cognitive function at one year.
Citation: Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. New Engl J Med. 2012;367:30-39.
Benefits of Resuming Anticoagulation after GI Bleed Outweigh Risks for Most Patients
Clinical question: In warfarin-treated patients who have experienced gastrointestinal (GI) bleeding, what are the patterns of restarting warfarin therapy and the incidence of thrombosis, recurrent GI bleed, and death in the 90 days following index bleed?
Background: In warfarin-treated patients who experience GI bleeding, warfarin is often temporarily held or permanently discontinued, placing patients at increased risk for developing thromboembolism. Little is known about the risks, benefits, and timing of restarting warfarin in this patient population.
Study design: Retrospective cohort study.
Setting: Kaiser Permanente Colorado.
Synopsis: Using clinical and administrative databases, 442 patients who presented with GI bleeding while receiving warfarin therapy were identified. Patients were grouped by whether they resumed warfarin (n=260, including 41 patients in whom anticoagulation was never interrupted), or did not resume warfarin therapy (n=182) in the 90 days following index GI bleed. Patients with prosthetic heart valves or GI bleeding localized to the rectum/anus were more commonly restarted on warfarin, whereas older patients and those in whom the source of bleeding was not identified were less likely to be restarted on warfarin therapy.
Restarting warfarin therapy after index GI bleed was associated with lower risk of thrombosis (HR 0.05, 95% CI 0.01-0.58) and death from any cause (HR 0.31, 95% CI 0.15-0.62), and it was not associated with a significant increase in risk for recurrent GI bleed (HR 1.32, 95% CI 0.50-3.57).
The authors concluded that for many patients who experience a warfarin-associated GI bleed, the benefits of restarting warfarin therapy outweigh the risks. No conclusions were made regarding the optimal timing of resuming therapy. Limitations included the use of administrative data and inability to determine the potential influence of aspirin use on outcomes.
Bottom line: Resuming warfarin in the 90 days following a warfarin-associated GI bleed is associated with decreased risk of thrombosis and death without increased risk for recurrent GI bleed.
Citation: Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012 Sep 12. doi:10.1001/archinternmed.2012.4261.
Preoperative Hyponatremia Associated with Increased Risk for Perioperative Complications and Mortality
Clinical question: Is preoperative hyponatremia an indicator of perioperative morbidity and mortality?
Background: Hyponatremia is a common diagnosis in the hospital setting and is associated with adverse outcomes, even in mild cases. However, it is unclear if this association exists in surgical patients when detected preoperatively.
Study design: Retrospective cohort study.
Setting: Academic and community hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program (NSQIP).
Synopsis: A total of 75,423 adult patients with hyponatremia (sodium <135 mEq/L) who were undergoing major surgery were compared to 888,840 patients with normal preoperative sodium levels over a six-year period. The primary outcome was 30-day mortality. Secondary outcomes included postoperative major coronary events, stroke, wound infection, pneumonia, and length of stay (LOS).
Compared to patients with normal sodium levels, those with preoperative hyponatremia had higher rates of perioperative mortality (5.2% vs. 1.3%; adjusted odds ratio 1.44, 95% CI 1.38-1.50), with increased risk that correlated with increasing severity of hyponatremia. Association with postoperative mortality was particularly strong among hyponatremic patients with ASA scores of 1 or 2 and those undergoing nonemergency surgery.
Patients with preoperative hyponatremia were also found to have increased risk for all postoperative complications evaluated, with the exception of stroke. Limitations included the potential for unmeasured confounders and not being able to account for the role of medications used perioperatively. Research is needed to determine whether correcting preoperative hyponatremia lessens the risk of mortality and other postoperative complications.
Bottom line: Among patients undergoing major surgery, preoperative hyponatremia is a predictor of postoperative 30-day mortality and morbidity.
Citation: Leung AA, McAlister FA, Rogers SO, et al. Preoperative hyponatremia and perioperative complications. Arch Intern Med. 2012;172:1-8.
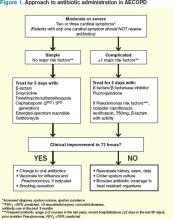
What Is the Appropriate Use of Antibiotics In Acute Exacerbations of COPD?
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.