User login
PSA cancer screening: A case for shared decision-making
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
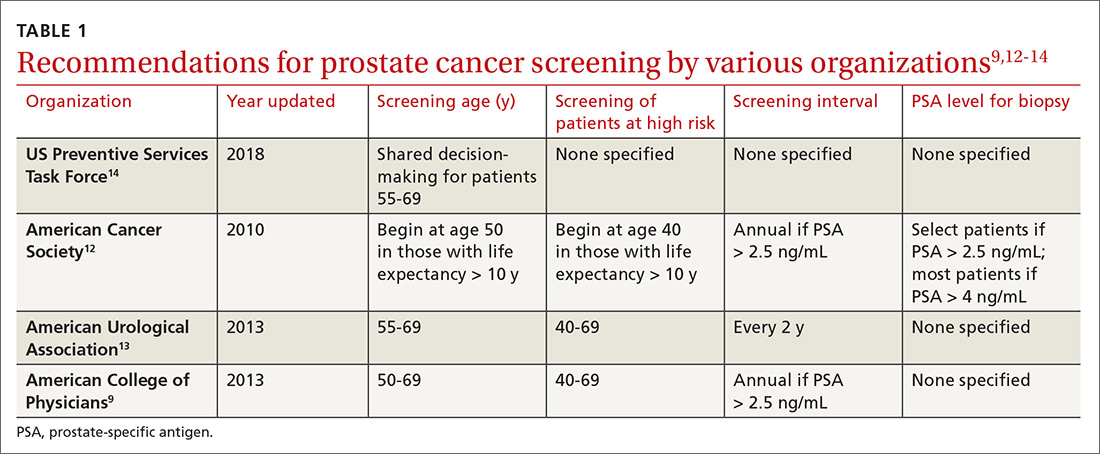
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14
Does PSA-based screening improve patient-centered outcomes?
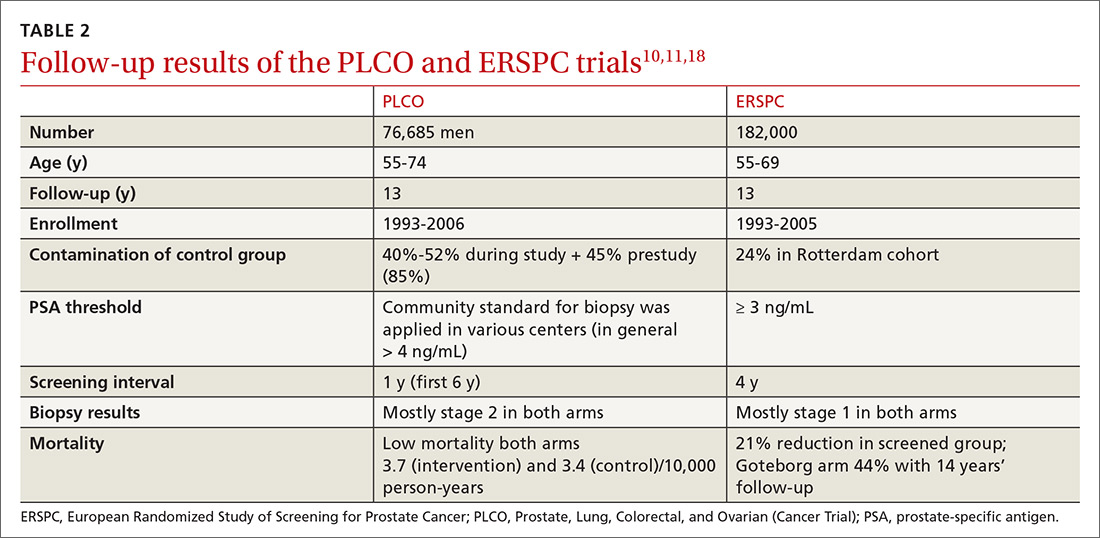
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18
Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
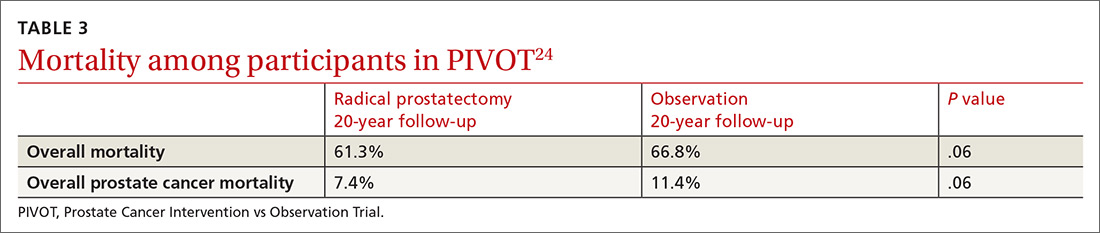
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24
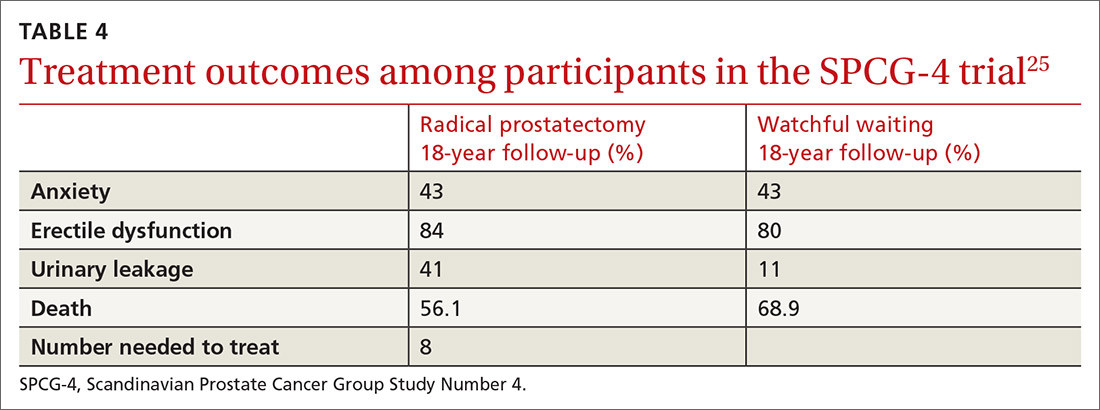
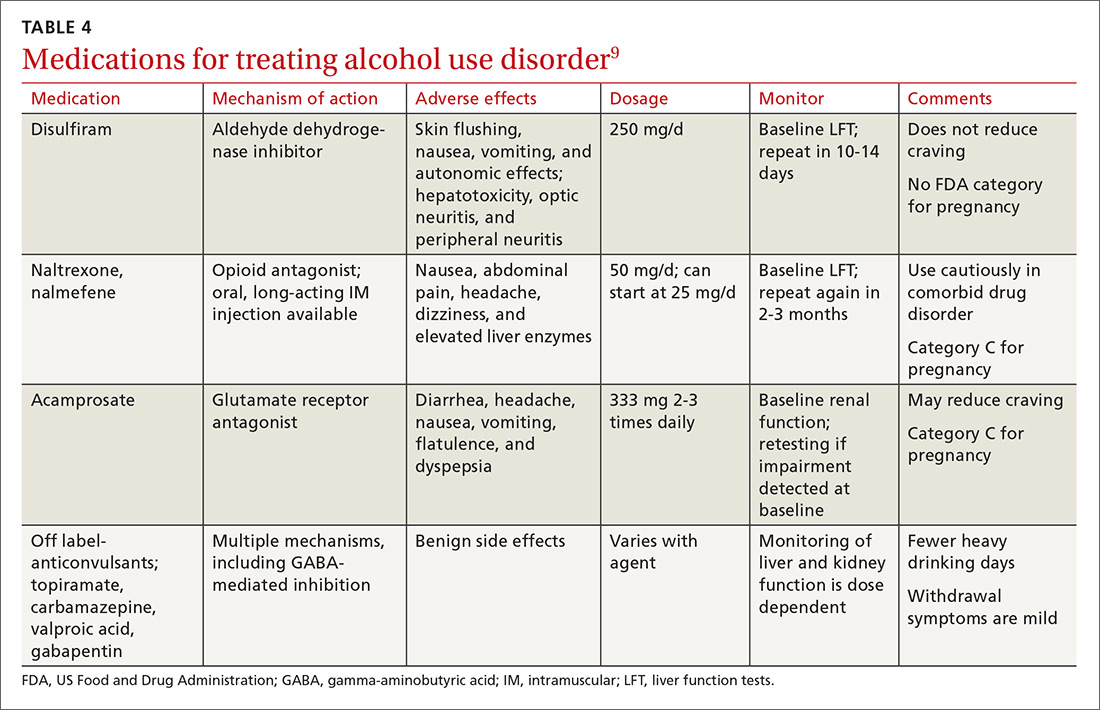
SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.
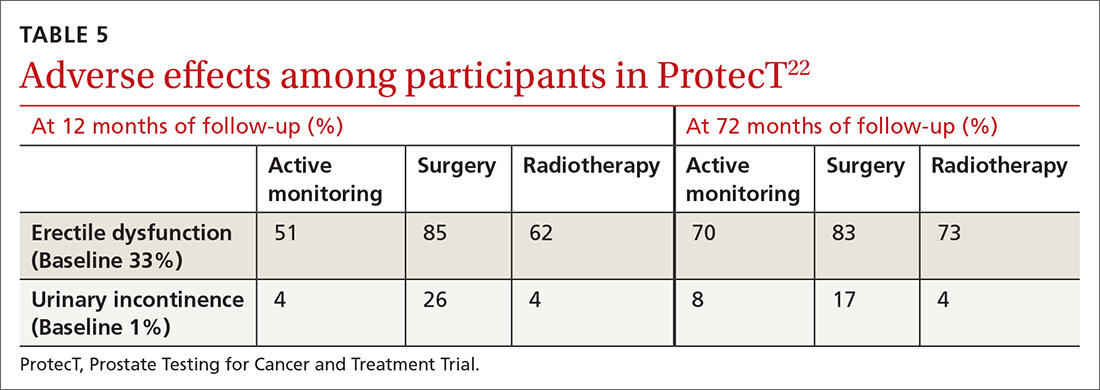
ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22
Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
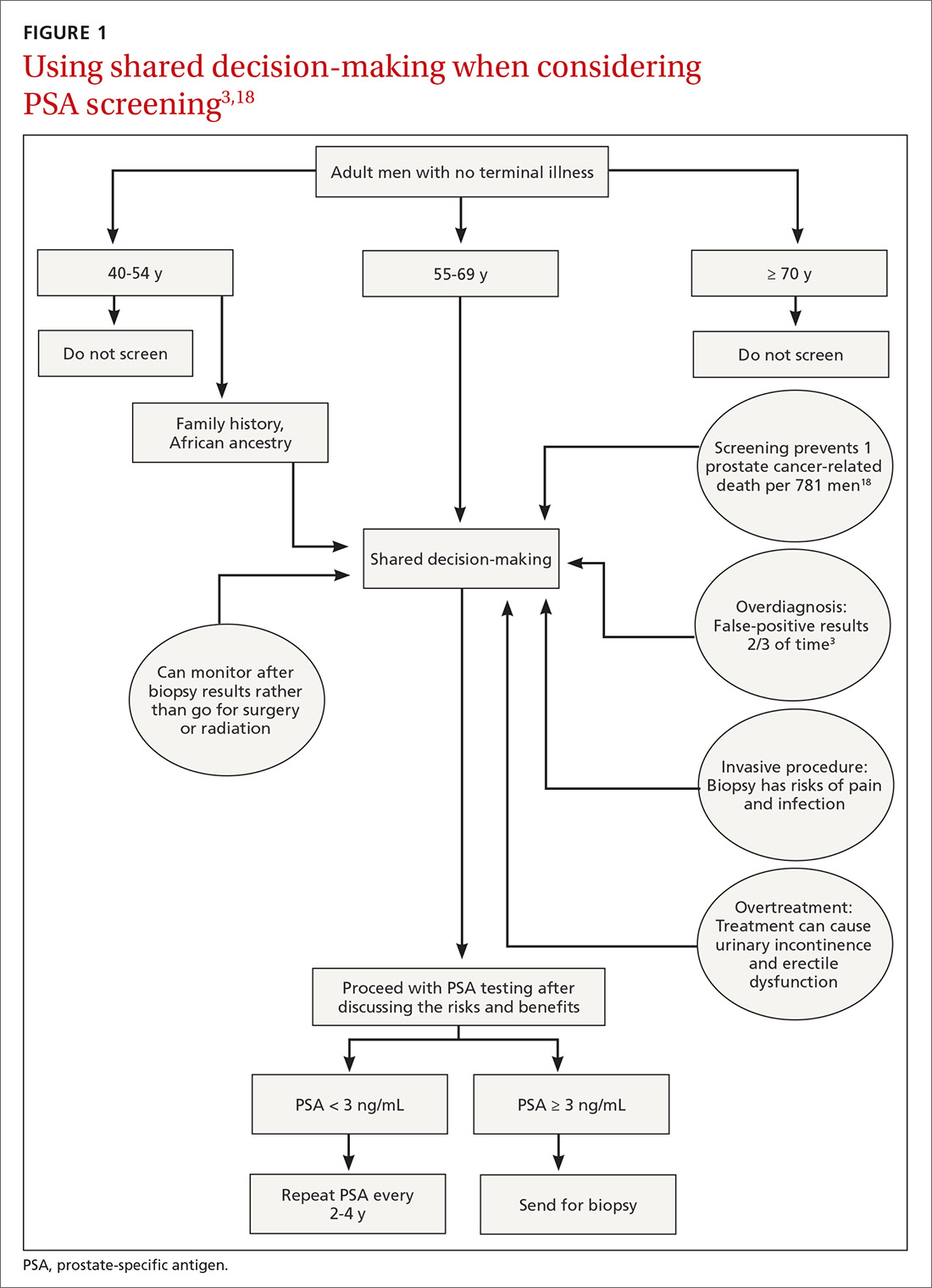
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.
Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; jxd114@case.edu.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14
Does PSA-based screening improve patient-centered outcomes?
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18
Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24
SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.
ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22
Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.
Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; jxd114@case.edu.
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14
Does PSA-based screening improve patient-centered outcomes?
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18
Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24
SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.
ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22
Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.
Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; jxd114@case.edu.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
PRACTICE RECOMMENDATIONS
› Recommend individualized decision-making to men ages 55 to 69 years after discussing the potential benefits and risks of prostate-specific antigen (PSA)-based screening. B
› Do not use a PSA-based screening method for prostate cancer in men ages < 50 years or > 70 years or men with a life expectancy < 10 years. C
› Do not routinely recommend PSA-based screening to men with a family history of prostate cancer or to men who are African American. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hyperextension of the bilateral knees in a 1-day-old neonate • no knee fractures or dislocation on x-ray • Dx?
THE CASE
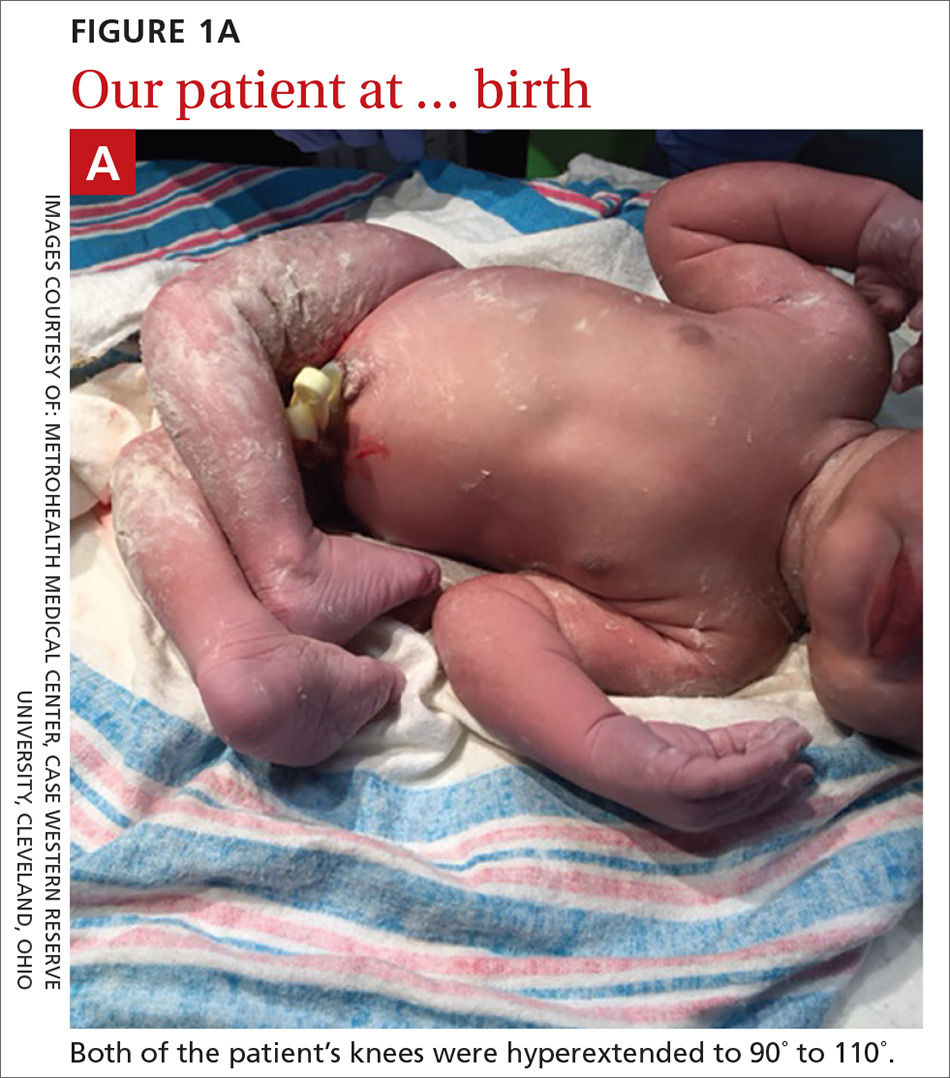
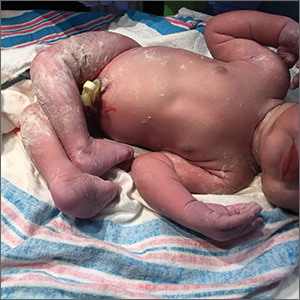
A 29-year-old G7P2315 woman gave birth to a girl at 37 weeks via spontaneous vaginal delivery. APGAR scores were 9 and 9. Birth weight was 2760 g. Cardiovascular and pulmonary examinations were normal (heart rate, 154 beats/min; respiratory rate, 52 breaths/min). Following delivery, the neonate appeared healthy, had a lusty cry, and had no visible craniofacial or cutaneous abnormalities; however, the bilateral knees were hyperextended to 90° to 110° (FIGURE 1A).
The mother had started prenatal care at 7 weeks with 10 total visits to her family physician (JD) throughout the pregnancy. Routine laboratory screening and prenatal ultrasounds (including an anatomy scan) were normal. She had a history of 3 preterm deliveries at 35 weeks, 36 weeks, and 36 weeks, respectively, and had been on progesterone shots once weekly starting at 18 weeks during the current pregnancy. She had no history of infections or recent travel. Her family history was remarkable for a sister who gave birth to a child with
THE DIAGNOSIS
The neonate tolerated passive flexion of the knees to a neutral position. Hip examination demonstrated appropriate range of movement with negative Ortolani and Barlow tests. The infant’s feet aligned correctly, with toes in the front and heels in the back, and an x-ray of the bilateral knees showed no fractures or dislocation.
Based on the clinical examination and x-ray findings, we made a diagnosis of congenital genu recurvatum. A pediatric orthopedics consultation was obtained, and the knees were placed in short leg splints in comfortable flexion to neutral on Day 1 of life. She was discharged the next day.
DISCUSSION
Congenital genu recurvatum, also known as congenital dislocation of the knee, is a rare condition involving abnormal hyperextension of the unilateral or bilateral knees with limited flexion.1 Reports in the literature are limited, but there seems to be a female predominance among known cases of congenital genu recurvatum.2 The clinical presentation varies. Finding may be isolated to the knee(s) but also can present in association with other congenital abnormalities, such as developmental dysplasia of the hip, clubfoot, and hindfoot and forefoot deformities.3,4
Diagnosis is made clinically with radiographic imaging
Diagnosis of congenital genu recurvatum is made clinically and can be confirmed via radiographic imaging of the knees.5 Clinical diagnosis requires assessment of the degree of hyperextension and palpation of the femoral condyles, which become more prominent as the severity of the hyperextension increases.6 X-rays help assess if a true dislocation or subluxation of the tibia on the femur has occurred. Based on the clinical and radiographic findings, congenital genu recurvatum typically is classified according to 3 levels of severity: grade 1 classification only involves hyperextension of the knees without dislocation or subluxation, grade 2 involves the same characteristic hyperextension along with anterior subluxation of the tibia on the femur, and grade 3 includes hyperextension with true dislocation of the tibia on the femur.1 Grades 1 and 2 on this spectrum technically are diagnosed as congenital genu recurvatum while grade 3 is diagnosed as a congenital dislocation of the knee,7 although the 2 terms are used interchangeably in the literature. We classified our case as a grade 1 congenital genu recurvatum based on the clinical and radiographic findings.
Congenital knee hyperextension has intrinsic and extrinsic causes
Hyperextension of the knees at birth may be caused by various intrinsic or extrinsic factors. Intrinsic causes may include breech position, lack of intrauterine space, trauma to the mother, quadriceps contracture or fibrosis, absence of the suprapatellar pouch, deficient or hypoplastic anterior cruciate ligament, pathological tissues, arthrogryposis, or genetic disorders such as Larsen syndrome or achondroplasia.6
Continue to: Extrinsic causes...
Extrinsic causes may include traumatic dislocation during the birthing process3 or intrauterine pressure leading to malposition of the joints. When intrauterine pressure is combined with reduced intrauterine space, this phenomenon is known as packaging disorder.6 Entanglement of the umbilical cord around the legs of the fetus during development may be another potential factor.1
The exact etiology in our patient was unknown, but we determined the cause was extrinsic based on the lack of other genetic abnormalities. We initially considered a possible connection between our patient’s diagnosis and her family history of thrombocytopenia absent radius syndrome, but it was later determined that both were isolated cases and the limb abnormalities were coincidental.
Treatment options and outcomes for extrinsic and intrinsic etiologies depend on the severity of the hyperextension and any associated abnormalities, as well as the time in which therapy is initiated.1 Reduction of the hyperextension within 24 hours of birth has been associated with excellent outcomes.8 Regardless of the cause, all cases of congenital genu recurvatum should first be treated conservatively. Evidence has suggested that conservative therapy involving early gentle manipulation of the knee combined with serial splinting and casting should be the first line of treatment.6 If initial treatment attempts fail or in cases occurring later in life, surgical interventions (eg, quadriceps release procedures such as percutaneous quadriceps recession or V-Y quadricepsplasty, proximal tibial closing-wedge, anterior displacement osteotomy) likely is warranted.6,9
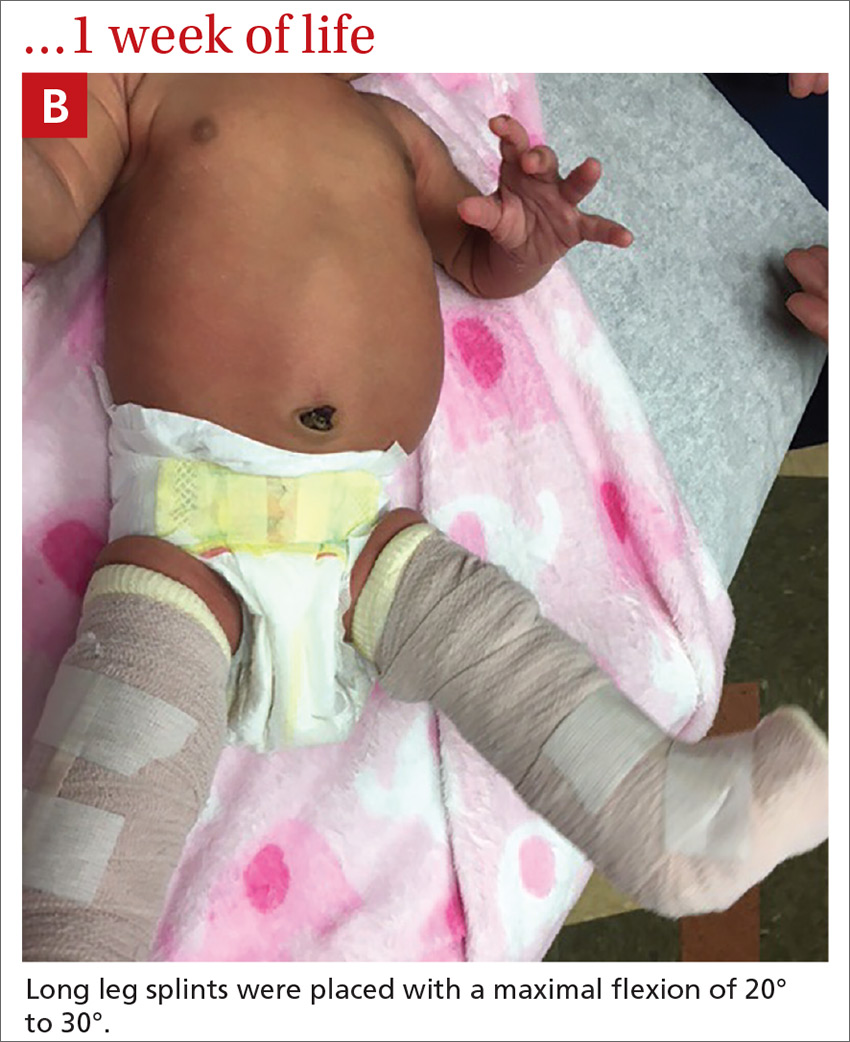
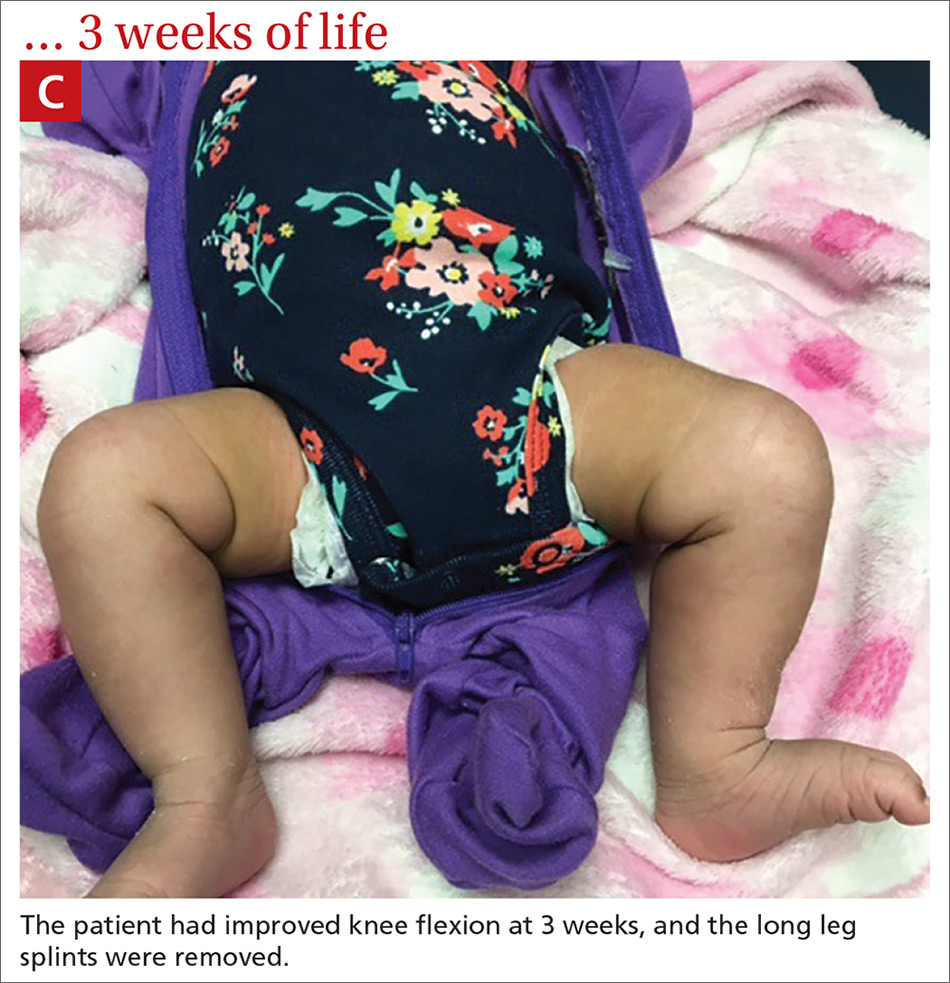
Our patient. At 1 week of life, our patient’s short leg splints were replaced with long leg splints with a maximal flexion of 20° to 30° (FIGURE 1B). Weekly follow-ups with serial casting were initiated in the pediatric orthopedics clinic. At 3 weeks of life, the patient’s knee flexion had improved and the splints were removed (FIGURE 1C). Upon clinical examination, the bilateral knees were extended to a neutral position, and both could be actively and passively flexed to 90°. The patient was referred to Physical Therapy to perform range of movement exercises on the knees.
At 8 weeks of life, the bilateral legs were in full extension, and knee flexion was up to 130°. Physical therapy for knee range of movement exercise was continued on a weekly basis until 6 months of life, then twice monthly until the patient was 1 year old. Ultimately, the hyperextension was corrected, and the patient started walking at around 16 months of age. Her prognosis is good, and she will be able to participate in low-impact sports, after consulting with her orthopedist.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Congenital genu recurvatum is a rare condition that presents with abnormal hyperextension of the knee(s) with limited flexion. Early diagnosis and assessment of the severity of the hyperextension is crucial in determining the type of intervention to pursue. Conservative management entails serial casting and splinting to increase knee flexion. If conservative management fails or if the diagnosis is made later in life, surgical options often are pursued.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 MetroHealth Medical Drive, Cleveland, OH 44109; jxd114@case.edu
1. Donaire AR, Sethuram S, Kitsos E, et al. Congenital bilateral knee hyperextension in a well-newborn infant. Res J Clin Pediatr. 2017;1. https://www.scitechnol.com/peer-review/congenital-bilateral-knee-hyperextension-in-a-wellnewborn-infant-V63Y.php?article_id=5940. Accessed April 2, 2019.
2. Osakwe GO, Asuquo EJ, Abang EI, et al. Congenital knee dislocation: challenges in management in a low resource center. Journal of dental and medical sciences. 2016;15:78-82.
3. Katz MP, Grogono BJ, Soper KC. The etiology and treatment of congenital dislocation of the knee. J Bone Joint Surg Br. 1967;49:112-20.
4. Elmada M, Ceylan H, Erdil M, et al. Congenital dislocation of knee. Eur J Med. 2013;10:164-166.
5. Abdelaziz TH, Samir S. Congenital dislocation of the knee: a protocol for management based on degree of knee flexion. J Child Orthop. 2011;5:143-149.
6. Tiwari M, Sharma N. Unilateral congenital knee and hip dislocation with bilateral clubfoot—a rare packaging disorder. J Orthop Case Rep. 2013;3:21-24.
7. Ahmadi B, Shahriaree H, Silver CM. Severe congenital genu recurvatum. case report. J Bone Joint Surg Am. 1979;61:622-623.
8. Cheng CC, Ko JY. Early reduction for congenital dislocation of the knee within twenty-four hours of birth. Chang Gung Med J. 2010;33:266-273.
9. Youssef AO. Limited open quadriceps release for treatment of congenital dislocation of the knee. J Pediatric Orthop. 2017;37:192-198.
THE CASE
A 29-year-old G7P2315 woman gave birth to a girl at 37 weeks via spontaneous vaginal delivery. APGAR scores were 9 and 9. Birth weight was 2760 g. Cardiovascular and pulmonary examinations were normal (heart rate, 154 beats/min; respiratory rate, 52 breaths/min). Following delivery, the neonate appeared healthy, had a lusty cry, and had no visible craniofacial or cutaneous abnormalities; however, the bilateral knees were hyperextended to 90° to 110° (FIGURE 1A).
The mother had started prenatal care at 7 weeks with 10 total visits to her family physician (JD) throughout the pregnancy. Routine laboratory screening and prenatal ultrasounds (including an anatomy scan) were normal. She had a history of 3 preterm deliveries at 35 weeks, 36 weeks, and 36 weeks, respectively, and had been on progesterone shots once weekly starting at 18 weeks during the current pregnancy. She had no history of infections or recent travel. Her family history was remarkable for a sister who gave birth to a child with
THE DIAGNOSIS
The neonate tolerated passive flexion of the knees to a neutral position. Hip examination demonstrated appropriate range of movement with negative Ortolani and Barlow tests. The infant’s feet aligned correctly, with toes in the front and heels in the back, and an x-ray of the bilateral knees showed no fractures or dislocation.
Based on the clinical examination and x-ray findings, we made a diagnosis of congenital genu recurvatum. A pediatric orthopedics consultation was obtained, and the knees were placed in short leg splints in comfortable flexion to neutral on Day 1 of life. She was discharged the next day.
DISCUSSION
Congenital genu recurvatum, also known as congenital dislocation of the knee, is a rare condition involving abnormal hyperextension of the unilateral or bilateral knees with limited flexion.1 Reports in the literature are limited, but there seems to be a female predominance among known cases of congenital genu recurvatum.2 The clinical presentation varies. Finding may be isolated to the knee(s) but also can present in association with other congenital abnormalities, such as developmental dysplasia of the hip, clubfoot, and hindfoot and forefoot deformities.3,4
Diagnosis is made clinically with radiographic imaging
Diagnosis of congenital genu recurvatum is made clinically and can be confirmed via radiographic imaging of the knees.5 Clinical diagnosis requires assessment of the degree of hyperextension and palpation of the femoral condyles, which become more prominent as the severity of the hyperextension increases.6 X-rays help assess if a true dislocation or subluxation of the tibia on the femur has occurred. Based on the clinical and radiographic findings, congenital genu recurvatum typically is classified according to 3 levels of severity: grade 1 classification only involves hyperextension of the knees without dislocation or subluxation, grade 2 involves the same characteristic hyperextension along with anterior subluxation of the tibia on the femur, and grade 3 includes hyperextension with true dislocation of the tibia on the femur.1 Grades 1 and 2 on this spectrum technically are diagnosed as congenital genu recurvatum while grade 3 is diagnosed as a congenital dislocation of the knee,7 although the 2 terms are used interchangeably in the literature. We classified our case as a grade 1 congenital genu recurvatum based on the clinical and radiographic findings.
Congenital knee hyperextension has intrinsic and extrinsic causes
Hyperextension of the knees at birth may be caused by various intrinsic or extrinsic factors. Intrinsic causes may include breech position, lack of intrauterine space, trauma to the mother, quadriceps contracture or fibrosis, absence of the suprapatellar pouch, deficient or hypoplastic anterior cruciate ligament, pathological tissues, arthrogryposis, or genetic disorders such as Larsen syndrome or achondroplasia.6
Continue to: Extrinsic causes...
Extrinsic causes may include traumatic dislocation during the birthing process3 or intrauterine pressure leading to malposition of the joints. When intrauterine pressure is combined with reduced intrauterine space, this phenomenon is known as packaging disorder.6 Entanglement of the umbilical cord around the legs of the fetus during development may be another potential factor.1
The exact etiology in our patient was unknown, but we determined the cause was extrinsic based on the lack of other genetic abnormalities. We initially considered a possible connection between our patient’s diagnosis and her family history of thrombocytopenia absent radius syndrome, but it was later determined that both were isolated cases and the limb abnormalities were coincidental.
Treatment options and outcomes for extrinsic and intrinsic etiologies depend on the severity of the hyperextension and any associated abnormalities, as well as the time in which therapy is initiated.1 Reduction of the hyperextension within 24 hours of birth has been associated with excellent outcomes.8 Regardless of the cause, all cases of congenital genu recurvatum should first be treated conservatively. Evidence has suggested that conservative therapy involving early gentle manipulation of the knee combined with serial splinting and casting should be the first line of treatment.6 If initial treatment attempts fail or in cases occurring later in life, surgical interventions (eg, quadriceps release procedures such as percutaneous quadriceps recession or V-Y quadricepsplasty, proximal tibial closing-wedge, anterior displacement osteotomy) likely is warranted.6,9
Our patient. At 1 week of life, our patient’s short leg splints were replaced with long leg splints with a maximal flexion of 20° to 30° (FIGURE 1B). Weekly follow-ups with serial casting were initiated in the pediatric orthopedics clinic. At 3 weeks of life, the patient’s knee flexion had improved and the splints were removed (FIGURE 1C). Upon clinical examination, the bilateral knees were extended to a neutral position, and both could be actively and passively flexed to 90°. The patient was referred to Physical Therapy to perform range of movement exercises on the knees.
At 8 weeks of life, the bilateral legs were in full extension, and knee flexion was up to 130°. Physical therapy for knee range of movement exercise was continued on a weekly basis until 6 months of life, then twice monthly until the patient was 1 year old. Ultimately, the hyperextension was corrected, and the patient started walking at around 16 months of age. Her prognosis is good, and she will be able to participate in low-impact sports, after consulting with her orthopedist.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Congenital genu recurvatum is a rare condition that presents with abnormal hyperextension of the knee(s) with limited flexion. Early diagnosis and assessment of the severity of the hyperextension is crucial in determining the type of intervention to pursue. Conservative management entails serial casting and splinting to increase knee flexion. If conservative management fails or if the diagnosis is made later in life, surgical options often are pursued.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 MetroHealth Medical Drive, Cleveland, OH 44109; jxd114@case.edu
THE CASE
A 29-year-old G7P2315 woman gave birth to a girl at 37 weeks via spontaneous vaginal delivery. APGAR scores were 9 and 9. Birth weight was 2760 g. Cardiovascular and pulmonary examinations were normal (heart rate, 154 beats/min; respiratory rate, 52 breaths/min). Following delivery, the neonate appeared healthy, had a lusty cry, and had no visible craniofacial or cutaneous abnormalities; however, the bilateral knees were hyperextended to 90° to 110° (FIGURE 1A).
The mother had started prenatal care at 7 weeks with 10 total visits to her family physician (JD) throughout the pregnancy. Routine laboratory screening and prenatal ultrasounds (including an anatomy scan) were normal. She had a history of 3 preterm deliveries at 35 weeks, 36 weeks, and 36 weeks, respectively, and had been on progesterone shots once weekly starting at 18 weeks during the current pregnancy. She had no history of infections or recent travel. Her family history was remarkable for a sister who gave birth to a child with
THE DIAGNOSIS
The neonate tolerated passive flexion of the knees to a neutral position. Hip examination demonstrated appropriate range of movement with negative Ortolani and Barlow tests. The infant’s feet aligned correctly, with toes in the front and heels in the back, and an x-ray of the bilateral knees showed no fractures or dislocation.
Based on the clinical examination and x-ray findings, we made a diagnosis of congenital genu recurvatum. A pediatric orthopedics consultation was obtained, and the knees were placed in short leg splints in comfortable flexion to neutral on Day 1 of life. She was discharged the next day.
DISCUSSION
Congenital genu recurvatum, also known as congenital dislocation of the knee, is a rare condition involving abnormal hyperextension of the unilateral or bilateral knees with limited flexion.1 Reports in the literature are limited, but there seems to be a female predominance among known cases of congenital genu recurvatum.2 The clinical presentation varies. Finding may be isolated to the knee(s) but also can present in association with other congenital abnormalities, such as developmental dysplasia of the hip, clubfoot, and hindfoot and forefoot deformities.3,4
Diagnosis is made clinically with radiographic imaging
Diagnosis of congenital genu recurvatum is made clinically and can be confirmed via radiographic imaging of the knees.5 Clinical diagnosis requires assessment of the degree of hyperextension and palpation of the femoral condyles, which become more prominent as the severity of the hyperextension increases.6 X-rays help assess if a true dislocation or subluxation of the tibia on the femur has occurred. Based on the clinical and radiographic findings, congenital genu recurvatum typically is classified according to 3 levels of severity: grade 1 classification only involves hyperextension of the knees without dislocation or subluxation, grade 2 involves the same characteristic hyperextension along with anterior subluxation of the tibia on the femur, and grade 3 includes hyperextension with true dislocation of the tibia on the femur.1 Grades 1 and 2 on this spectrum technically are diagnosed as congenital genu recurvatum while grade 3 is diagnosed as a congenital dislocation of the knee,7 although the 2 terms are used interchangeably in the literature. We classified our case as a grade 1 congenital genu recurvatum based on the clinical and radiographic findings.
Congenital knee hyperextension has intrinsic and extrinsic causes
Hyperextension of the knees at birth may be caused by various intrinsic or extrinsic factors. Intrinsic causes may include breech position, lack of intrauterine space, trauma to the mother, quadriceps contracture or fibrosis, absence of the suprapatellar pouch, deficient or hypoplastic anterior cruciate ligament, pathological tissues, arthrogryposis, or genetic disorders such as Larsen syndrome or achondroplasia.6
Continue to: Extrinsic causes...
Extrinsic causes may include traumatic dislocation during the birthing process3 or intrauterine pressure leading to malposition of the joints. When intrauterine pressure is combined with reduced intrauterine space, this phenomenon is known as packaging disorder.6 Entanglement of the umbilical cord around the legs of the fetus during development may be another potential factor.1
The exact etiology in our patient was unknown, but we determined the cause was extrinsic based on the lack of other genetic abnormalities. We initially considered a possible connection between our patient’s diagnosis and her family history of thrombocytopenia absent radius syndrome, but it was later determined that both were isolated cases and the limb abnormalities were coincidental.
Treatment options and outcomes for extrinsic and intrinsic etiologies depend on the severity of the hyperextension and any associated abnormalities, as well as the time in which therapy is initiated.1 Reduction of the hyperextension within 24 hours of birth has been associated with excellent outcomes.8 Regardless of the cause, all cases of congenital genu recurvatum should first be treated conservatively. Evidence has suggested that conservative therapy involving early gentle manipulation of the knee combined with serial splinting and casting should be the first line of treatment.6 If initial treatment attempts fail or in cases occurring later in life, surgical interventions (eg, quadriceps release procedures such as percutaneous quadriceps recession or V-Y quadricepsplasty, proximal tibial closing-wedge, anterior displacement osteotomy) likely is warranted.6,9
Our patient. At 1 week of life, our patient’s short leg splints were replaced with long leg splints with a maximal flexion of 20° to 30° (FIGURE 1B). Weekly follow-ups with serial casting were initiated in the pediatric orthopedics clinic. At 3 weeks of life, the patient’s knee flexion had improved and the splints were removed (FIGURE 1C). Upon clinical examination, the bilateral knees were extended to a neutral position, and both could be actively and passively flexed to 90°. The patient was referred to Physical Therapy to perform range of movement exercises on the knees.
At 8 weeks of life, the bilateral legs were in full extension, and knee flexion was up to 130°. Physical therapy for knee range of movement exercise was continued on a weekly basis until 6 months of life, then twice monthly until the patient was 1 year old. Ultimately, the hyperextension was corrected, and the patient started walking at around 16 months of age. Her prognosis is good, and she will be able to participate in low-impact sports, after consulting with her orthopedist.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Congenital genu recurvatum is a rare condition that presents with abnormal hyperextension of the knee(s) with limited flexion. Early diagnosis and assessment of the severity of the hyperextension is crucial in determining the type of intervention to pursue. Conservative management entails serial casting and splinting to increase knee flexion. If conservative management fails or if the diagnosis is made later in life, surgical options often are pursued.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 MetroHealth Medical Drive, Cleveland, OH 44109; jxd114@case.edu
1. Donaire AR, Sethuram S, Kitsos E, et al. Congenital bilateral knee hyperextension in a well-newborn infant. Res J Clin Pediatr. 2017;1. https://www.scitechnol.com/peer-review/congenital-bilateral-knee-hyperextension-in-a-wellnewborn-infant-V63Y.php?article_id=5940. Accessed April 2, 2019.
2. Osakwe GO, Asuquo EJ, Abang EI, et al. Congenital knee dislocation: challenges in management in a low resource center. Journal of dental and medical sciences. 2016;15:78-82.
3. Katz MP, Grogono BJ, Soper KC. The etiology and treatment of congenital dislocation of the knee. J Bone Joint Surg Br. 1967;49:112-20.
4. Elmada M, Ceylan H, Erdil M, et al. Congenital dislocation of knee. Eur J Med. 2013;10:164-166.
5. Abdelaziz TH, Samir S. Congenital dislocation of the knee: a protocol for management based on degree of knee flexion. J Child Orthop. 2011;5:143-149.
6. Tiwari M, Sharma N. Unilateral congenital knee and hip dislocation with bilateral clubfoot—a rare packaging disorder. J Orthop Case Rep. 2013;3:21-24.
7. Ahmadi B, Shahriaree H, Silver CM. Severe congenital genu recurvatum. case report. J Bone Joint Surg Am. 1979;61:622-623.
8. Cheng CC, Ko JY. Early reduction for congenital dislocation of the knee within twenty-four hours of birth. Chang Gung Med J. 2010;33:266-273.
9. Youssef AO. Limited open quadriceps release for treatment of congenital dislocation of the knee. J Pediatric Orthop. 2017;37:192-198.
1. Donaire AR, Sethuram S, Kitsos E, et al. Congenital bilateral knee hyperextension in a well-newborn infant. Res J Clin Pediatr. 2017;1. https://www.scitechnol.com/peer-review/congenital-bilateral-knee-hyperextension-in-a-wellnewborn-infant-V63Y.php?article_id=5940. Accessed April 2, 2019.
2. Osakwe GO, Asuquo EJ, Abang EI, et al. Congenital knee dislocation: challenges in management in a low resource center. Journal of dental and medical sciences. 2016;15:78-82.
3. Katz MP, Grogono BJ, Soper KC. The etiology and treatment of congenital dislocation of the knee. J Bone Joint Surg Br. 1967;49:112-20.
4. Elmada M, Ceylan H, Erdil M, et al. Congenital dislocation of knee. Eur J Med. 2013;10:164-166.
5. Abdelaziz TH, Samir S. Congenital dislocation of the knee: a protocol for management based on degree of knee flexion. J Child Orthop. 2011;5:143-149.
6. Tiwari M, Sharma N. Unilateral congenital knee and hip dislocation with bilateral clubfoot—a rare packaging disorder. J Orthop Case Rep. 2013;3:21-24.
7. Ahmadi B, Shahriaree H, Silver CM. Severe congenital genu recurvatum. case report. J Bone Joint Surg Am. 1979;61:622-623.
8. Cheng CC, Ko JY. Early reduction for congenital dislocation of the knee within twenty-four hours of birth. Chang Gung Med J. 2010;33:266-273.
9. Youssef AO. Limited open quadriceps release for treatment of congenital dislocation of the knee. J Pediatric Orthop. 2017;37:192-198.
Alcohol use disorder: How best to screen and intervene
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12
Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12
Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12
Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
Non-alcoholic fatty liver disease: What’s in our arsenal?
› Screen patients with non-alcoholic fatty liver disease (NAFLD) for type 2 diabetes mellitus. A
› Treat components of the metabolic syndrome to improve the clinical outcome in patients with NAFLD. A
› Consider liver-directed pharmacotherapy, such as antioxidants (eg, vitamin E), insulin sensitizers, bile acid sequestrants, and pentoxifylline, to treat severe NAFLD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 39-year-old Hispanic man with a body mass index (BMI) of 35 kg/m2, type 2 diabetes mellitus (T2DM), and hypertension is referred for evaluation of abnormal liver function tests (LFTs) and fatty liver on ultrasound. He is taking metformin and lisinopril, and a patient alcohol screening survey is negative. LFT results reveal the following: alanine aminotransferase (ALT) 27 IU/dL; aspartate aminotransferase (AST) 43 IU/dL; albumin 4.2 g/dL; gamma glutamyl transferase 22 u/L; alkaline phosphatase 51 IU/L; and total bilirubin 0.3 mg/dL. Lactate dehydrogenase and prothrombin time are normal.
Results of his liver screen are as follows: hepatitis B surface antigen, hepatitis C antibody, antimitochondrial antibody, and anti-smooth muscle antibody are negative, and iron, transferrin saturation, and ceruloplasmin are in normal range. Antinuclear antibody (1:20 dilution) is weakly positive, and alpha-1 antitrypsin (264 mg/dL) and serum ferritin (300 ng/mL) are mildly increased.
The patient undergoes a liver biopsy that shows grade 2 steatosis, grade 1 lobular inflammation, few ballooned hepatocytes, and stage 1 fibrosis. Based on these clinical findings, he is given a diagnosis of non-alcoholic fatty liver disease (NAFLD).
NAFLD is the most frequent cause of chronic liver disease both in the United States and globally.1 In fact, a number of long-term epidemiologic studies report that nearly one-third of the US population has the disease.2 The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH) to cirrhosis. Of patients with NAFLD, 10% to 30% have the more severe form—NASH—and about 10% of those with NASH progress to cirrhosis and other liver-related complications.3
People with NAFLD consume no alcohol, or only a modest amount (ie, weekly intake <140 g in women and <210 g in men). Typically, they are asymptomatic with normal or mildly abnormal LFTs discovered as part of a preventive health screening. In patients with simple hepatic steatosis alone, serum ALT levels are higher than serum AST levels. (In contrast, patients with alcoholic liver injury and NASH with progressive fibrosis have higher serum AST than ALT levels.) A serum hepatitis panel and liver screen are negative for other explanations of chronic liver disease.
NAFLD is strongly associated with obesity, insulin resistance/T2DM, and hyperlipidemia, all of which are components of metabolic syndrome. Obesity, particularly central obesity, is highly predictive of hepatic steatosis and disease progression.4 T2DM occurs 5 to 9 times more frequently in people with NAFLD than in the general population,5 and, conversely, nearly 66% of patients with T2DM have NAFLD.6,7 Furthermore, nearly 70% of patients with T2DM develop fatty liver and its consequences, including NASH, fibrosis, cirrhosis, and hepatocellular carcinoma.5,7
4 therapeutic strategies. Based on our current understanding of the pathogenesis of NAFLD, there are 4 main therapeutic avenues: lifestyle modification, liver-directed pharmacotherapy, management of metabolic syndrome, and surveillance of the complications of cirrhosis. The review that follows explores the evidence to date for each.
Take steps to reduce weight and increase physical activity
The primary objective with NAFLD is to right the imbalance between calorie intake and utilization so as to reverse the obesity and insulin resistance underlying the disease.
Target carbohydrates. Current data clearly suggest that energy intake is significantly higher in patients with NAFLD than in those without the disease.8 Thus, reducing dietary carbohydrate and overall energy intake is beneficial to preventing and halting the progression of liver damage. Increased intake of high fructose corn syrup may be at least partially to blame; research has linked the substance to the occurrence of obesity, metabolic syndrome, and NAFLD.9
The optimal diet to treat NAFLD is not known because of the difficulties inherent to performing well-designed dietary intervention trials and ensuring long-term compliance. At least one study reported that a Mediterranean diet helped reduce hepatic steatosis and improve insulin sensitivity in nondiabetic individuals.10 Generally, patients should avoid saturated fats, simple carbohydrates, and sweetened drinks, and they should be instructed to restrict calories to cause weight loss of about .5 kg to 1 kg per week until the target weight is achieved.11
Current observational studies indicate that prudent calorie restriction combined with increased physical activity is the best strategy for achieving and sustaining optimum body weight; severe calorie restriction is likely to cause skeletal muscle loss that can aggravate NAFLD.
Encourage exercise. Aerobic exercise improves skeletal muscle insulin sensitivity—the primary underlying mechanism that causes NAFLD.12 Although the optimum duration and intensity of exercise is not known, several randomized controlled trials (RCTs) found that moderately intense training, high-intensity training, and/or resistance training improved hepatic steatosis and insulin resistance, but an effect on ALT was inconsistent.13 (None of these studies included histology as an outcome measure.)
Given the multitude of benefits of aerobic exercise, there is no question that patients with NAFLD should try to increase their physical activity and incorporate exercise into their daily routine.
Hold off on pharmacologic weight loss. Orlistat, an enteric lipase inhibitor, causes malabsorption of dietary fat, which leads to weight loss. Although one study demonstrated that orlistat improves ALT and steatosis in patients with NAFLD, a subsequent RCT concluded that orlistat with caloric restriction and vitamin E (800 IU/d) did not enhance weight loss over caloric restriction and vitamin E alone.14 Additionally, in patients with weight loss >9% of body weight, histologic improvement occurred independent of orlistat.14 Therefore, orlistat is not currently recommended for weight loss in patients with NAFLD.
Keep bariatric surgery on your radar. Bariatric-metabolic surgery provides the most reliable method for achieving sustained weight loss in morbidly obese individuals with NAFLD. Commonly used surgical procedures are associated with reduced steatosis and lobular inflammatory changes, but reports are conflicting regarding fibrosis.15
The majority of published data indicate that bariatric surgery improves the histologic and metabolic changes associated with NAFLD and has potential as a treatment option for patients with morbid obesity and NAFLD. However, the timing and type of surgery that is most effective, and whether bariatric surgery can cure the disease, remain unanswered questions. Long-term follow-up and RCTs are needed to address these issues. As a result, no definitive recommendations regarding bariatric surgery as a treatment for NAFLD can be made at this time.15
Liver-directed pharmacotherapy: Evidence is lacking for many agents
Lifestyle modification remains the mainstay of therapy for NAFLD because of its efficacy and lack of adverse effects. But low compliance rates often make pharmacotherapy necessary to reduce the health burden related to NAFLD. Despite the success rate of pharmacologic agents that focus on insulin resistance and lipid metabolism and that have antioxidant properties, the long-term safety and efficacy of many of these agents is largely unknown. Furthermore, the FDA has not approved any pharmacologic agents specifically for the treatment of NAFLD. Here’s what we know:
Vitamin E. Five RCTs have evaluated the antioxidant vitamin E in patients with NASH. The best study published to date found that 96 weeks of therapy with 800 IU/d vitamin E was associated with a 42% improvement in hepatic histology, compared with 19% improvement in the placebo group.16 Vitamin E was also associated with improved serum ALT.
Although vitamin E seems to be a promising agent for the treatment of NASH, concerns exist about its long-term safety because of an increased risk of all-cause mortality and hemorrhagic stroke.17 In addition, because the optimal dose and duration of treatment is unknown and because studies have not evaluated the supplement in patients who have diabetes and NASH, vitamin E is not currently considered to be a standard therapy for NASH.
Insulin sensitizers. Because insulin resistance is believed to be the underlying mechanism for the development and progression of NAFLD, a compelling rationale exists for the use of insulin sensitizers in the management of the disease. Metformin, an activator of adenosine monophosphate-activated protein kinase, and the thiazolidinediones (pioglitazone and rosiglitazone) are the most extensively studied agents in clinical trials. A number of studies looking at the effects of metformin on NAFLD found that liver function, steatosis, and insulin sensitivity improved;18 however, a recent meta-analysis found that metformin failed to improve liver histology.19
Similarly, although clinical trials have shown that thiazolidinediones improve liver enzymes, inflammatory markers, and hepatic steatosis, questions surround their long-term safety.20 The largest placebo-controlled trial on this issue to date—PIVENS (pioglitazone vs vitamin E vs placebo)—found that pioglitazone was beneficial in improving hepatic histology.16 However, the well-recognized adverse effects of pioglitazone (eg, upper respiratory tract infection, edema, and hypoglycemia) may temper its utility.
Clinical trials involving newer antidiabetic agents, such as dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP1) analogues, indicate that such agents improve insulin resistance, steatosis, and inflammation.21 However, these drugs are not considered to be routine therapy because of limited data and the lack of long-term benefits.
Bile acid regulatory agents. Ursodeoxycholic acid (UDCA), a bile acid with antiapoptotic and cytoprotective properties, is used as a hepatoprotectant in NAFLD. Although early studies showed no significant differences in LFT results between UDCA-treated and untreated groups, recent RCTs indicate that UDCA improves ALT and serum fibrosis.22,23 The FLINT trial, a recent multicenter RCT involving obeticholic acid, found that UDCA was associated with improvement in histologic outcomes, although long-term benefits and safety—especially with regard to worsening hyperlipidemia—are questionable.24
Pentoxifylline. Researchers have evaluated pentoxifylline, a hepatoprotectant with anti-tumor necrosis factor effect, in the treatment of NAFLD.25 In fact, pooled results from 5 well-designed studies indicate that pentoxifylline significantly reduces ALT and AST and improves steatosis, lobular inflammation, and fibrosis.26 Although these data suggest that pentoxifylline holds promise as a therapeutic option, the lack of large multicenter studies and FDA approval temper its utility in the management of NASH at this time.
Cholesterol-lowering agents. Statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase in the liver and have anti-inflammatory and anti-fibrogenic properties. They have been used in patients with NAFLD, primarily because of their cardiovascular benefit. Two RCTs with high risk of bias and a small number of participants found statin therapy to be associated with improved serum transaminases and ultrasound findings; however, liver biopsies were not performed in either of these studies.27
Lowering cholesterol using an absorption inhibitor, such as ezetimibe, was associated with improvement in liver histology in a single RCT.28 Even though statins are not considered to be a treatment for NAFLD, they can be used to safely lower plasma cholesterol in patients with the disease.
Renin-angiotensin system (RAS) inhibitors. Research in animals indicates that activation of the renin-angiotensin system contributes to the pathogenesis of NAFLD, but data on the benefits of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in patients with NAFLD are limited, conflicting, and derived largely from retrospective29 and pilot prospective studies.
Based on currently published literature, RAS inhibitors are not considered an NAFLD treatment. However, because cardiovascular disease is a major cause of death in patients with NAFLD, the renal and cardiovascular protection offered by these agents likely lowers mortality in patients with the disease.
Probiotics. The use of probiotics in the treatment of NAFLD is based on the premise that alterations in intestinal microbes and the inflammatory response may improve the disease. Three RCTs involving different formulations of probiotics, synbiotics, or placebo, showed improvement in serum liver markers and insulin resistance, but did not include histologic outcome measures.30 Furthermore, the long-term consequences of altered gut flora are presently unknown. As such, the available evidence does not support the use of probiotics for the treatment of NAFLD.
Polyunsaturated fatty acids (PUFA). Clearly, omega-3 fatty acids have beneficial effects on cardiometabolic risk factors and positively impact lipid metabolism and insulin sensitivity. In addition, a few studies have reported improvement in non-histologic outcome measures of NAFLD, but 2 high-quality RCTs found no benefit of fish oil-based PUFA on histology.31,32 Thus, current evidence does not support recommending PUFA supplementation for the treatment of NAFLD.
Chinese herbal medicines. At least 56 trials have looked at 75 different Chinese herbal medicines in varying formulations, dosages, routes of administration, and durations of treatment, using various controlled interventions.33 No trial reported primary outcomes, such as hepatic-related mortality, morbidity, or health care quality of life. Although a large number of the trials reported some positive effects on various biochemical or radiologic measures, the high risk of bias and the limited number of trials testing individual herbal medicines leave efficacy and safety open to question. As such, no Chinese herbal medicines are regarded as treatment for NAFLD at this time.
Target components of metabolic syndrome
Management of the components of metabolic syndrome remains one of the safest and most effective ways to manage NAFLD. Therefore, screening for and treating T2DM, hypertension, and dyslipidemia are priorities. Although obstructive sleep apnea (OSA) is not part of metabolic syndrome, the condition frequently coexists with metabolic syndrome because both entities have obesity as a risk factor.
T2DM. Screen all patients with NAFLD for T2DM and vice-versa because, as noted earlier, patients with diabetes have more severe and progressive NAFLD, and a high proportion of patients with NAFLD have T2DM.5,6 Although research has not shown metformin to improve histology in NASH, metformin is recommended as a first-line agent for the treatment of T2DM because it aids in weight loss and lowers diabetes-related mortality.34
Pioglitazone is considered a second-line agent. Despite its beneficial effects on insulin sensitivity and hepatic histology, there are concerns about the adverse effects of thiazolidinediones. GLP1 analogues, which improve liver enzymes and reduce hepatic steatosis, are considered third-line agents.
Hypertension. Because approximately 70% of patients with NAFLD have hypertension,35 it is imperative to screen patients for the condition. If blood pressure is >140/90 mm Hg, patients should be managed according to hypertension guidelines. ACE inhibitors or ARBs are recommended as first-line therapy, since blocking the renin-angiotensin system potentially reduces hepatic fibrosis,36 and ARBs may lower transaminases and improve insulin sensitivity in NAFLD.
Dyslipidemia. Treatment of dyslipidemia is essential to lowering cardiovascular mortality in patients with NAFLD. Even though elevated transaminases occur with NAFLD, this should not preclude starting therapy to lower triglycerides to <150 mg/dL and total cholesterol to <200 mg/dL.
OSA. Because of the high prevalence of OSA in patients with NAFLD, physicians should have a high index of suspicion and screen this population for sleep disorders. OSA is associated with an increased risk of NAFLD and advanced fibrosis in NASH.37 Treatment of OSA improves quality of life and controls blood pressure in patients with NAFLD, but it’s currently unclear whether targeting sleep disorders can slow the progression of fibrosis in NAFLD.
Concentrate on the complications of cirrhosis
Patients with NASH cirrhosis, like those with cirrhosis of other etiologies, are at risk for complications, including hepatic encephalopathy, ascites, hepatorenal syndrome, and esophageal variceal hemorrhage. Surveillance to detect these include an annual liver ultrasound, an alpha-fetoprotein test every 6 months, esophagogastroduodenoscopy for varices, and an assessment for liver transplantation. For more on these complications, see, “Cirrhosis complications: Keeping them under control,” J Fam Pract. 2015;64:338-342. NAFLD-associated cirrhosis is the third most frequent indication for liver transplantation in the United States and may become the most frequent indication in the next decade.38
CASE › Because the patient’s liver biopsy showed early NASH, we recommended that he aggressively pursue lifestyle modification, including regular physical activity and dietary changes. Additionally, we discussed optimization of glycemic control and continued use of lisinopril for control of hypertension. On follow-up 6 months later, he had lost weight and his BMI was 32 kg/m2. In addition, his transaminase levels had improved, but they had not normalized.
We recommended that he continue the same measures, with follow-up every 6 months to ensure compliance with lifestyle modifications and with diabetes and hypertension control.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Metro Health Medical Center, 2500 Metro Health Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
1. Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52.
2. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of non-alcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45.
3. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131.
4. Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974.
5. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344.
6 Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121.
7. Stefan N, Häring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011-2017.
8. Capristo E, Miele L, Forgione A, et al. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2005;9:265-268.
9. Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721-729.
10. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143.
11. Centre for Public Health Excellence at NICE. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Clinical Excellence; 2006.
12. Kirwan JP, Solomon TP, Wojta DM, et al. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156.
13. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166.
14. Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86.
15. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340.
16. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685.
17. Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702.
18. Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig. 2014;34:1-7.
19. Li Y, Liu L, Wang B, et al. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64.
20. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307.
21. Olaywi M, Bhatia T, Anand S, et al. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int. 2013;12:584-588.
22. Troisi G, Crisciotti F, Gianturco V, et al. The treatment with ursodeoxycholic acid in elderly patients affected by NAFLD and metabolic syndrome: a case-control study. Clin Ter. 2013;164:203-207.
23. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodeoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019.
24. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:946.
25. Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619.
26. Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577.
27. Eslami L, Merat S, Malekzadeh R, et al. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623.
28. akeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890.
29. Goh GB, Pagadala MR, Dasarathy J, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979-985.
30. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918.
31. Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137-144.
32. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.
33. Liu ZL, Xie LZ, Zhu J, et al. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;8:CD009059.
34. National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians; 2008.
35. Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141-145.
36. Georgescu EF, Ionescu R, Niculescu M. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954.
37. Musso G, Cassader M, Olivetti C, et al. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431.
38. Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253.
› Screen patients with non-alcoholic fatty liver disease (NAFLD) for type 2 diabetes mellitus. A
› Treat components of the metabolic syndrome to improve the clinical outcome in patients with NAFLD. A
› Consider liver-directed pharmacotherapy, such as antioxidants (eg, vitamin E), insulin sensitizers, bile acid sequestrants, and pentoxifylline, to treat severe NAFLD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 39-year-old Hispanic man with a body mass index (BMI) of 35 kg/m2, type 2 diabetes mellitus (T2DM), and hypertension is referred for evaluation of abnormal liver function tests (LFTs) and fatty liver on ultrasound. He is taking metformin and lisinopril, and a patient alcohol screening survey is negative. LFT results reveal the following: alanine aminotransferase (ALT) 27 IU/dL; aspartate aminotransferase (AST) 43 IU/dL; albumin 4.2 g/dL; gamma glutamyl transferase 22 u/L; alkaline phosphatase 51 IU/L; and total bilirubin 0.3 mg/dL. Lactate dehydrogenase and prothrombin time are normal.
Results of his liver screen are as follows: hepatitis B surface antigen, hepatitis C antibody, antimitochondrial antibody, and anti-smooth muscle antibody are negative, and iron, transferrin saturation, and ceruloplasmin are in normal range. Antinuclear antibody (1:20 dilution) is weakly positive, and alpha-1 antitrypsin (264 mg/dL) and serum ferritin (300 ng/mL) are mildly increased.
The patient undergoes a liver biopsy that shows grade 2 steatosis, grade 1 lobular inflammation, few ballooned hepatocytes, and stage 1 fibrosis. Based on these clinical findings, he is given a diagnosis of non-alcoholic fatty liver disease (NAFLD).
NAFLD is the most frequent cause of chronic liver disease both in the United States and globally.1 In fact, a number of long-term epidemiologic studies report that nearly one-third of the US population has the disease.2 The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH) to cirrhosis. Of patients with NAFLD, 10% to 30% have the more severe form—NASH—and about 10% of those with NASH progress to cirrhosis and other liver-related complications.3
People with NAFLD consume no alcohol, or only a modest amount (ie, weekly intake <140 g in women and <210 g in men). Typically, they are asymptomatic with normal or mildly abnormal LFTs discovered as part of a preventive health screening. In patients with simple hepatic steatosis alone, serum ALT levels are higher than serum AST levels. (In contrast, patients with alcoholic liver injury and NASH with progressive fibrosis have higher serum AST than ALT levels.) A serum hepatitis panel and liver screen are negative for other explanations of chronic liver disease.
NAFLD is strongly associated with obesity, insulin resistance/T2DM, and hyperlipidemia, all of which are components of metabolic syndrome. Obesity, particularly central obesity, is highly predictive of hepatic steatosis and disease progression.4 T2DM occurs 5 to 9 times more frequently in people with NAFLD than in the general population,5 and, conversely, nearly 66% of patients with T2DM have NAFLD.6,7 Furthermore, nearly 70% of patients with T2DM develop fatty liver and its consequences, including NASH, fibrosis, cirrhosis, and hepatocellular carcinoma.5,7
4 therapeutic strategies. Based on our current understanding of the pathogenesis of NAFLD, there are 4 main therapeutic avenues: lifestyle modification, liver-directed pharmacotherapy, management of metabolic syndrome, and surveillance of the complications of cirrhosis. The review that follows explores the evidence to date for each.
Take steps to reduce weight and increase physical activity
The primary objective with NAFLD is to right the imbalance between calorie intake and utilization so as to reverse the obesity and insulin resistance underlying the disease.
Target carbohydrates. Current data clearly suggest that energy intake is significantly higher in patients with NAFLD than in those without the disease.8 Thus, reducing dietary carbohydrate and overall energy intake is beneficial to preventing and halting the progression of liver damage. Increased intake of high fructose corn syrup may be at least partially to blame; research has linked the substance to the occurrence of obesity, metabolic syndrome, and NAFLD.9
The optimal diet to treat NAFLD is not known because of the difficulties inherent to performing well-designed dietary intervention trials and ensuring long-term compliance. At least one study reported that a Mediterranean diet helped reduce hepatic steatosis and improve insulin sensitivity in nondiabetic individuals.10 Generally, patients should avoid saturated fats, simple carbohydrates, and sweetened drinks, and they should be instructed to restrict calories to cause weight loss of about .5 kg to 1 kg per week until the target weight is achieved.11
Current observational studies indicate that prudent calorie restriction combined with increased physical activity is the best strategy for achieving and sustaining optimum body weight; severe calorie restriction is likely to cause skeletal muscle loss that can aggravate NAFLD.
Encourage exercise. Aerobic exercise improves skeletal muscle insulin sensitivity—the primary underlying mechanism that causes NAFLD.12 Although the optimum duration and intensity of exercise is not known, several randomized controlled trials (RCTs) found that moderately intense training, high-intensity training, and/or resistance training improved hepatic steatosis and insulin resistance, but an effect on ALT was inconsistent.13 (None of these studies included histology as an outcome measure.)
Given the multitude of benefits of aerobic exercise, there is no question that patients with NAFLD should try to increase their physical activity and incorporate exercise into their daily routine.
Hold off on pharmacologic weight loss. Orlistat, an enteric lipase inhibitor, causes malabsorption of dietary fat, which leads to weight loss. Although one study demonstrated that orlistat improves ALT and steatosis in patients with NAFLD, a subsequent RCT concluded that orlistat with caloric restriction and vitamin E (800 IU/d) did not enhance weight loss over caloric restriction and vitamin E alone.14 Additionally, in patients with weight loss >9% of body weight, histologic improvement occurred independent of orlistat.14 Therefore, orlistat is not currently recommended for weight loss in patients with NAFLD.
Keep bariatric surgery on your radar. Bariatric-metabolic surgery provides the most reliable method for achieving sustained weight loss in morbidly obese individuals with NAFLD. Commonly used surgical procedures are associated with reduced steatosis and lobular inflammatory changes, but reports are conflicting regarding fibrosis.15
The majority of published data indicate that bariatric surgery improves the histologic and metabolic changes associated with NAFLD and has potential as a treatment option for patients with morbid obesity and NAFLD. However, the timing and type of surgery that is most effective, and whether bariatric surgery can cure the disease, remain unanswered questions. Long-term follow-up and RCTs are needed to address these issues. As a result, no definitive recommendations regarding bariatric surgery as a treatment for NAFLD can be made at this time.15
Liver-directed pharmacotherapy: Evidence is lacking for many agents
Lifestyle modification remains the mainstay of therapy for NAFLD because of its efficacy and lack of adverse effects. But low compliance rates often make pharmacotherapy necessary to reduce the health burden related to NAFLD. Despite the success rate of pharmacologic agents that focus on insulin resistance and lipid metabolism and that have antioxidant properties, the long-term safety and efficacy of many of these agents is largely unknown. Furthermore, the FDA has not approved any pharmacologic agents specifically for the treatment of NAFLD. Here’s what we know:
Vitamin E. Five RCTs have evaluated the antioxidant vitamin E in patients with NASH. The best study published to date found that 96 weeks of therapy with 800 IU/d vitamin E was associated with a 42% improvement in hepatic histology, compared with 19% improvement in the placebo group.16 Vitamin E was also associated with improved serum ALT.
Although vitamin E seems to be a promising agent for the treatment of NASH, concerns exist about its long-term safety because of an increased risk of all-cause mortality and hemorrhagic stroke.17 In addition, because the optimal dose and duration of treatment is unknown and because studies have not evaluated the supplement in patients who have diabetes and NASH, vitamin E is not currently considered to be a standard therapy for NASH.
Insulin sensitizers. Because insulin resistance is believed to be the underlying mechanism for the development and progression of NAFLD, a compelling rationale exists for the use of insulin sensitizers in the management of the disease. Metformin, an activator of adenosine monophosphate-activated protein kinase, and the thiazolidinediones (pioglitazone and rosiglitazone) are the most extensively studied agents in clinical trials. A number of studies looking at the effects of metformin on NAFLD found that liver function, steatosis, and insulin sensitivity improved;18 however, a recent meta-analysis found that metformin failed to improve liver histology.19
Similarly, although clinical trials have shown that thiazolidinediones improve liver enzymes, inflammatory markers, and hepatic steatosis, questions surround their long-term safety.20 The largest placebo-controlled trial on this issue to date—PIVENS (pioglitazone vs vitamin E vs placebo)—found that pioglitazone was beneficial in improving hepatic histology.16 However, the well-recognized adverse effects of pioglitazone (eg, upper respiratory tract infection, edema, and hypoglycemia) may temper its utility.
Clinical trials involving newer antidiabetic agents, such as dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP1) analogues, indicate that such agents improve insulin resistance, steatosis, and inflammation.21 However, these drugs are not considered to be routine therapy because of limited data and the lack of long-term benefits.
Bile acid regulatory agents. Ursodeoxycholic acid (UDCA), a bile acid with antiapoptotic and cytoprotective properties, is used as a hepatoprotectant in NAFLD. Although early studies showed no significant differences in LFT results between UDCA-treated and untreated groups, recent RCTs indicate that UDCA improves ALT and serum fibrosis.22,23 The FLINT trial, a recent multicenter RCT involving obeticholic acid, found that UDCA was associated with improvement in histologic outcomes, although long-term benefits and safety—especially with regard to worsening hyperlipidemia—are questionable.24
Pentoxifylline. Researchers have evaluated pentoxifylline, a hepatoprotectant with anti-tumor necrosis factor effect, in the treatment of NAFLD.25 In fact, pooled results from 5 well-designed studies indicate that pentoxifylline significantly reduces ALT and AST and improves steatosis, lobular inflammation, and fibrosis.26 Although these data suggest that pentoxifylline holds promise as a therapeutic option, the lack of large multicenter studies and FDA approval temper its utility in the management of NASH at this time.
Cholesterol-lowering agents. Statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase in the liver and have anti-inflammatory and anti-fibrogenic properties. They have been used in patients with NAFLD, primarily because of their cardiovascular benefit. Two RCTs with high risk of bias and a small number of participants found statin therapy to be associated with improved serum transaminases and ultrasound findings; however, liver biopsies were not performed in either of these studies.27
Lowering cholesterol using an absorption inhibitor, such as ezetimibe, was associated with improvement in liver histology in a single RCT.28 Even though statins are not considered to be a treatment for NAFLD, they can be used to safely lower plasma cholesterol in patients with the disease.
Renin-angiotensin system (RAS) inhibitors. Research in animals indicates that activation of the renin-angiotensin system contributes to the pathogenesis of NAFLD, but data on the benefits of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in patients with NAFLD are limited, conflicting, and derived largely from retrospective29 and pilot prospective studies.
Based on currently published literature, RAS inhibitors are not considered an NAFLD treatment. However, because cardiovascular disease is a major cause of death in patients with NAFLD, the renal and cardiovascular protection offered by these agents likely lowers mortality in patients with the disease.
Probiotics. The use of probiotics in the treatment of NAFLD is based on the premise that alterations in intestinal microbes and the inflammatory response may improve the disease. Three RCTs involving different formulations of probiotics, synbiotics, or placebo, showed improvement in serum liver markers and insulin resistance, but did not include histologic outcome measures.30 Furthermore, the long-term consequences of altered gut flora are presently unknown. As such, the available evidence does not support the use of probiotics for the treatment of NAFLD.
Polyunsaturated fatty acids (PUFA). Clearly, omega-3 fatty acids have beneficial effects on cardiometabolic risk factors and positively impact lipid metabolism and insulin sensitivity. In addition, a few studies have reported improvement in non-histologic outcome measures of NAFLD, but 2 high-quality RCTs found no benefit of fish oil-based PUFA on histology.31,32 Thus, current evidence does not support recommending PUFA supplementation for the treatment of NAFLD.
Chinese herbal medicines. At least 56 trials have looked at 75 different Chinese herbal medicines in varying formulations, dosages, routes of administration, and durations of treatment, using various controlled interventions.33 No trial reported primary outcomes, such as hepatic-related mortality, morbidity, or health care quality of life. Although a large number of the trials reported some positive effects on various biochemical or radiologic measures, the high risk of bias and the limited number of trials testing individual herbal medicines leave efficacy and safety open to question. As such, no Chinese herbal medicines are regarded as treatment for NAFLD at this time.
Target components of metabolic syndrome
Management of the components of metabolic syndrome remains one of the safest and most effective ways to manage NAFLD. Therefore, screening for and treating T2DM, hypertension, and dyslipidemia are priorities. Although obstructive sleep apnea (OSA) is not part of metabolic syndrome, the condition frequently coexists with metabolic syndrome because both entities have obesity as a risk factor.
T2DM. Screen all patients with NAFLD for T2DM and vice-versa because, as noted earlier, patients with diabetes have more severe and progressive NAFLD, and a high proportion of patients with NAFLD have T2DM.5,6 Although research has not shown metformin to improve histology in NASH, metformin is recommended as a first-line agent for the treatment of T2DM because it aids in weight loss and lowers diabetes-related mortality.34
Pioglitazone is considered a second-line agent. Despite its beneficial effects on insulin sensitivity and hepatic histology, there are concerns about the adverse effects of thiazolidinediones. GLP1 analogues, which improve liver enzymes and reduce hepatic steatosis, are considered third-line agents.
Hypertension. Because approximately 70% of patients with NAFLD have hypertension,35 it is imperative to screen patients for the condition. If blood pressure is >140/90 mm Hg, patients should be managed according to hypertension guidelines. ACE inhibitors or ARBs are recommended as first-line therapy, since blocking the renin-angiotensin system potentially reduces hepatic fibrosis,36 and ARBs may lower transaminases and improve insulin sensitivity in NAFLD.
Dyslipidemia. Treatment of dyslipidemia is essential to lowering cardiovascular mortality in patients with NAFLD. Even though elevated transaminases occur with NAFLD, this should not preclude starting therapy to lower triglycerides to <150 mg/dL and total cholesterol to <200 mg/dL.
OSA. Because of the high prevalence of OSA in patients with NAFLD, physicians should have a high index of suspicion and screen this population for sleep disorders. OSA is associated with an increased risk of NAFLD and advanced fibrosis in NASH.37 Treatment of OSA improves quality of life and controls blood pressure in patients with NAFLD, but it’s currently unclear whether targeting sleep disorders can slow the progression of fibrosis in NAFLD.
Concentrate on the complications of cirrhosis
Patients with NASH cirrhosis, like those with cirrhosis of other etiologies, are at risk for complications, including hepatic encephalopathy, ascites, hepatorenal syndrome, and esophageal variceal hemorrhage. Surveillance to detect these include an annual liver ultrasound, an alpha-fetoprotein test every 6 months, esophagogastroduodenoscopy for varices, and an assessment for liver transplantation. For more on these complications, see, “Cirrhosis complications: Keeping them under control,” J Fam Pract. 2015;64:338-342. NAFLD-associated cirrhosis is the third most frequent indication for liver transplantation in the United States and may become the most frequent indication in the next decade.38
CASE › Because the patient’s liver biopsy showed early NASH, we recommended that he aggressively pursue lifestyle modification, including regular physical activity and dietary changes. Additionally, we discussed optimization of glycemic control and continued use of lisinopril for control of hypertension. On follow-up 6 months later, he had lost weight and his BMI was 32 kg/m2. In addition, his transaminase levels had improved, but they had not normalized.
We recommended that he continue the same measures, with follow-up every 6 months to ensure compliance with lifestyle modifications and with diabetes and hypertension control.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Metro Health Medical Center, 2500 Metro Health Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
› Screen patients with non-alcoholic fatty liver disease (NAFLD) for type 2 diabetes mellitus. A
› Treat components of the metabolic syndrome to improve the clinical outcome in patients with NAFLD. A
› Consider liver-directed pharmacotherapy, such as antioxidants (eg, vitamin E), insulin sensitizers, bile acid sequestrants, and pentoxifylline, to treat severe NAFLD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 39-year-old Hispanic man with a body mass index (BMI) of 35 kg/m2, type 2 diabetes mellitus (T2DM), and hypertension is referred for evaluation of abnormal liver function tests (LFTs) and fatty liver on ultrasound. He is taking metformin and lisinopril, and a patient alcohol screening survey is negative. LFT results reveal the following: alanine aminotransferase (ALT) 27 IU/dL; aspartate aminotransferase (AST) 43 IU/dL; albumin 4.2 g/dL; gamma glutamyl transferase 22 u/L; alkaline phosphatase 51 IU/L; and total bilirubin 0.3 mg/dL. Lactate dehydrogenase and prothrombin time are normal.
Results of his liver screen are as follows: hepatitis B surface antigen, hepatitis C antibody, antimitochondrial antibody, and anti-smooth muscle antibody are negative, and iron, transferrin saturation, and ceruloplasmin are in normal range. Antinuclear antibody (1:20 dilution) is weakly positive, and alpha-1 antitrypsin (264 mg/dL) and serum ferritin (300 ng/mL) are mildly increased.
The patient undergoes a liver biopsy that shows grade 2 steatosis, grade 1 lobular inflammation, few ballooned hepatocytes, and stage 1 fibrosis. Based on these clinical findings, he is given a diagnosis of non-alcoholic fatty liver disease (NAFLD).
NAFLD is the most frequent cause of chronic liver disease both in the United States and globally.1 In fact, a number of long-term epidemiologic studies report that nearly one-third of the US population has the disease.2 The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH) to cirrhosis. Of patients with NAFLD, 10% to 30% have the more severe form—NASH—and about 10% of those with NASH progress to cirrhosis and other liver-related complications.3
People with NAFLD consume no alcohol, or only a modest amount (ie, weekly intake <140 g in women and <210 g in men). Typically, they are asymptomatic with normal or mildly abnormal LFTs discovered as part of a preventive health screening. In patients with simple hepatic steatosis alone, serum ALT levels are higher than serum AST levels. (In contrast, patients with alcoholic liver injury and NASH with progressive fibrosis have higher serum AST than ALT levels.) A serum hepatitis panel and liver screen are negative for other explanations of chronic liver disease.
NAFLD is strongly associated with obesity, insulin resistance/T2DM, and hyperlipidemia, all of which are components of metabolic syndrome. Obesity, particularly central obesity, is highly predictive of hepatic steatosis and disease progression.4 T2DM occurs 5 to 9 times more frequently in people with NAFLD than in the general population,5 and, conversely, nearly 66% of patients with T2DM have NAFLD.6,7 Furthermore, nearly 70% of patients with T2DM develop fatty liver and its consequences, including NASH, fibrosis, cirrhosis, and hepatocellular carcinoma.5,7
4 therapeutic strategies. Based on our current understanding of the pathogenesis of NAFLD, there are 4 main therapeutic avenues: lifestyle modification, liver-directed pharmacotherapy, management of metabolic syndrome, and surveillance of the complications of cirrhosis. The review that follows explores the evidence to date for each.
Take steps to reduce weight and increase physical activity
The primary objective with NAFLD is to right the imbalance between calorie intake and utilization so as to reverse the obesity and insulin resistance underlying the disease.
Target carbohydrates. Current data clearly suggest that energy intake is significantly higher in patients with NAFLD than in those without the disease.8 Thus, reducing dietary carbohydrate and overall energy intake is beneficial to preventing and halting the progression of liver damage. Increased intake of high fructose corn syrup may be at least partially to blame; research has linked the substance to the occurrence of obesity, metabolic syndrome, and NAFLD.9
The optimal diet to treat NAFLD is not known because of the difficulties inherent to performing well-designed dietary intervention trials and ensuring long-term compliance. At least one study reported that a Mediterranean diet helped reduce hepatic steatosis and improve insulin sensitivity in nondiabetic individuals.10 Generally, patients should avoid saturated fats, simple carbohydrates, and sweetened drinks, and they should be instructed to restrict calories to cause weight loss of about .5 kg to 1 kg per week until the target weight is achieved.11
Current observational studies indicate that prudent calorie restriction combined with increased physical activity is the best strategy for achieving and sustaining optimum body weight; severe calorie restriction is likely to cause skeletal muscle loss that can aggravate NAFLD.
Encourage exercise. Aerobic exercise improves skeletal muscle insulin sensitivity—the primary underlying mechanism that causes NAFLD.12 Although the optimum duration and intensity of exercise is not known, several randomized controlled trials (RCTs) found that moderately intense training, high-intensity training, and/or resistance training improved hepatic steatosis and insulin resistance, but an effect on ALT was inconsistent.13 (None of these studies included histology as an outcome measure.)
Given the multitude of benefits of aerobic exercise, there is no question that patients with NAFLD should try to increase their physical activity and incorporate exercise into their daily routine.
Hold off on pharmacologic weight loss. Orlistat, an enteric lipase inhibitor, causes malabsorption of dietary fat, which leads to weight loss. Although one study demonstrated that orlistat improves ALT and steatosis in patients with NAFLD, a subsequent RCT concluded that orlistat with caloric restriction and vitamin E (800 IU/d) did not enhance weight loss over caloric restriction and vitamin E alone.14 Additionally, in patients with weight loss >9% of body weight, histologic improvement occurred independent of orlistat.14 Therefore, orlistat is not currently recommended for weight loss in patients with NAFLD.
Keep bariatric surgery on your radar. Bariatric-metabolic surgery provides the most reliable method for achieving sustained weight loss in morbidly obese individuals with NAFLD. Commonly used surgical procedures are associated with reduced steatosis and lobular inflammatory changes, but reports are conflicting regarding fibrosis.15
The majority of published data indicate that bariatric surgery improves the histologic and metabolic changes associated with NAFLD and has potential as a treatment option for patients with morbid obesity and NAFLD. However, the timing and type of surgery that is most effective, and whether bariatric surgery can cure the disease, remain unanswered questions. Long-term follow-up and RCTs are needed to address these issues. As a result, no definitive recommendations regarding bariatric surgery as a treatment for NAFLD can be made at this time.15
Liver-directed pharmacotherapy: Evidence is lacking for many agents
Lifestyle modification remains the mainstay of therapy for NAFLD because of its efficacy and lack of adverse effects. But low compliance rates often make pharmacotherapy necessary to reduce the health burden related to NAFLD. Despite the success rate of pharmacologic agents that focus on insulin resistance and lipid metabolism and that have antioxidant properties, the long-term safety and efficacy of many of these agents is largely unknown. Furthermore, the FDA has not approved any pharmacologic agents specifically for the treatment of NAFLD. Here’s what we know:
Vitamin E. Five RCTs have evaluated the antioxidant vitamin E in patients with NASH. The best study published to date found that 96 weeks of therapy with 800 IU/d vitamin E was associated with a 42% improvement in hepatic histology, compared with 19% improvement in the placebo group.16 Vitamin E was also associated with improved serum ALT.
Although vitamin E seems to be a promising agent for the treatment of NASH, concerns exist about its long-term safety because of an increased risk of all-cause mortality and hemorrhagic stroke.17 In addition, because the optimal dose and duration of treatment is unknown and because studies have not evaluated the supplement in patients who have diabetes and NASH, vitamin E is not currently considered to be a standard therapy for NASH.
Insulin sensitizers. Because insulin resistance is believed to be the underlying mechanism for the development and progression of NAFLD, a compelling rationale exists for the use of insulin sensitizers in the management of the disease. Metformin, an activator of adenosine monophosphate-activated protein kinase, and the thiazolidinediones (pioglitazone and rosiglitazone) are the most extensively studied agents in clinical trials. A number of studies looking at the effects of metformin on NAFLD found that liver function, steatosis, and insulin sensitivity improved;18 however, a recent meta-analysis found that metformin failed to improve liver histology.19
Similarly, although clinical trials have shown that thiazolidinediones improve liver enzymes, inflammatory markers, and hepatic steatosis, questions surround their long-term safety.20 The largest placebo-controlled trial on this issue to date—PIVENS (pioglitazone vs vitamin E vs placebo)—found that pioglitazone was beneficial in improving hepatic histology.16 However, the well-recognized adverse effects of pioglitazone (eg, upper respiratory tract infection, edema, and hypoglycemia) may temper its utility.
Clinical trials involving newer antidiabetic agents, such as dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP1) analogues, indicate that such agents improve insulin resistance, steatosis, and inflammation.21 However, these drugs are not considered to be routine therapy because of limited data and the lack of long-term benefits.
Bile acid regulatory agents. Ursodeoxycholic acid (UDCA), a bile acid with antiapoptotic and cytoprotective properties, is used as a hepatoprotectant in NAFLD. Although early studies showed no significant differences in LFT results between UDCA-treated and untreated groups, recent RCTs indicate that UDCA improves ALT and serum fibrosis.22,23 The FLINT trial, a recent multicenter RCT involving obeticholic acid, found that UDCA was associated with improvement in histologic outcomes, although long-term benefits and safety—especially with regard to worsening hyperlipidemia—are questionable.24
Pentoxifylline. Researchers have evaluated pentoxifylline, a hepatoprotectant with anti-tumor necrosis factor effect, in the treatment of NAFLD.25 In fact, pooled results from 5 well-designed studies indicate that pentoxifylline significantly reduces ALT and AST and improves steatosis, lobular inflammation, and fibrosis.26 Although these data suggest that pentoxifylline holds promise as a therapeutic option, the lack of large multicenter studies and FDA approval temper its utility in the management of NASH at this time.
Cholesterol-lowering agents. Statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase in the liver and have anti-inflammatory and anti-fibrogenic properties. They have been used in patients with NAFLD, primarily because of their cardiovascular benefit. Two RCTs with high risk of bias and a small number of participants found statin therapy to be associated with improved serum transaminases and ultrasound findings; however, liver biopsies were not performed in either of these studies.27
Lowering cholesterol using an absorption inhibitor, such as ezetimibe, was associated with improvement in liver histology in a single RCT.28 Even though statins are not considered to be a treatment for NAFLD, they can be used to safely lower plasma cholesterol in patients with the disease.
Renin-angiotensin system (RAS) inhibitors. Research in animals indicates that activation of the renin-angiotensin system contributes to the pathogenesis of NAFLD, but data on the benefits of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in patients with NAFLD are limited, conflicting, and derived largely from retrospective29 and pilot prospective studies.
Based on currently published literature, RAS inhibitors are not considered an NAFLD treatment. However, because cardiovascular disease is a major cause of death in patients with NAFLD, the renal and cardiovascular protection offered by these agents likely lowers mortality in patients with the disease.
Probiotics. The use of probiotics in the treatment of NAFLD is based on the premise that alterations in intestinal microbes and the inflammatory response may improve the disease. Three RCTs involving different formulations of probiotics, synbiotics, or placebo, showed improvement in serum liver markers and insulin resistance, but did not include histologic outcome measures.30 Furthermore, the long-term consequences of altered gut flora are presently unknown. As such, the available evidence does not support the use of probiotics for the treatment of NAFLD.
Polyunsaturated fatty acids (PUFA). Clearly, omega-3 fatty acids have beneficial effects on cardiometabolic risk factors and positively impact lipid metabolism and insulin sensitivity. In addition, a few studies have reported improvement in non-histologic outcome measures of NAFLD, but 2 high-quality RCTs found no benefit of fish oil-based PUFA on histology.31,32 Thus, current evidence does not support recommending PUFA supplementation for the treatment of NAFLD.
Chinese herbal medicines. At least 56 trials have looked at 75 different Chinese herbal medicines in varying formulations, dosages, routes of administration, and durations of treatment, using various controlled interventions.33 No trial reported primary outcomes, such as hepatic-related mortality, morbidity, or health care quality of life. Although a large number of the trials reported some positive effects on various biochemical or radiologic measures, the high risk of bias and the limited number of trials testing individual herbal medicines leave efficacy and safety open to question. As such, no Chinese herbal medicines are regarded as treatment for NAFLD at this time.
Target components of metabolic syndrome
Management of the components of metabolic syndrome remains one of the safest and most effective ways to manage NAFLD. Therefore, screening for and treating T2DM, hypertension, and dyslipidemia are priorities. Although obstructive sleep apnea (OSA) is not part of metabolic syndrome, the condition frequently coexists with metabolic syndrome because both entities have obesity as a risk factor.
T2DM. Screen all patients with NAFLD for T2DM and vice-versa because, as noted earlier, patients with diabetes have more severe and progressive NAFLD, and a high proportion of patients with NAFLD have T2DM.5,6 Although research has not shown metformin to improve histology in NASH, metformin is recommended as a first-line agent for the treatment of T2DM because it aids in weight loss and lowers diabetes-related mortality.34
Pioglitazone is considered a second-line agent. Despite its beneficial effects on insulin sensitivity and hepatic histology, there are concerns about the adverse effects of thiazolidinediones. GLP1 analogues, which improve liver enzymes and reduce hepatic steatosis, are considered third-line agents.
Hypertension. Because approximately 70% of patients with NAFLD have hypertension,35 it is imperative to screen patients for the condition. If blood pressure is >140/90 mm Hg, patients should be managed according to hypertension guidelines. ACE inhibitors or ARBs are recommended as first-line therapy, since blocking the renin-angiotensin system potentially reduces hepatic fibrosis,36 and ARBs may lower transaminases and improve insulin sensitivity in NAFLD.
Dyslipidemia. Treatment of dyslipidemia is essential to lowering cardiovascular mortality in patients with NAFLD. Even though elevated transaminases occur with NAFLD, this should not preclude starting therapy to lower triglycerides to <150 mg/dL and total cholesterol to <200 mg/dL.
OSA. Because of the high prevalence of OSA in patients with NAFLD, physicians should have a high index of suspicion and screen this population for sleep disorders. OSA is associated with an increased risk of NAFLD and advanced fibrosis in NASH.37 Treatment of OSA improves quality of life and controls blood pressure in patients with NAFLD, but it’s currently unclear whether targeting sleep disorders can slow the progression of fibrosis in NAFLD.
Concentrate on the complications of cirrhosis
Patients with NASH cirrhosis, like those with cirrhosis of other etiologies, are at risk for complications, including hepatic encephalopathy, ascites, hepatorenal syndrome, and esophageal variceal hemorrhage. Surveillance to detect these include an annual liver ultrasound, an alpha-fetoprotein test every 6 months, esophagogastroduodenoscopy for varices, and an assessment for liver transplantation. For more on these complications, see, “Cirrhosis complications: Keeping them under control,” J Fam Pract. 2015;64:338-342. NAFLD-associated cirrhosis is the third most frequent indication for liver transplantation in the United States and may become the most frequent indication in the next decade.38
CASE › Because the patient’s liver biopsy showed early NASH, we recommended that he aggressively pursue lifestyle modification, including regular physical activity and dietary changes. Additionally, we discussed optimization of glycemic control and continued use of lisinopril for control of hypertension. On follow-up 6 months later, he had lost weight and his BMI was 32 kg/m2. In addition, his transaminase levels had improved, but they had not normalized.
We recommended that he continue the same measures, with follow-up every 6 months to ensure compliance with lifestyle modifications and with diabetes and hypertension control.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Metro Health Medical Center, 2500 Metro Health Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
1. Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52.
2. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of non-alcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45.
3. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131.
4. Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974.
5. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344.
6 Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121.
7. Stefan N, Häring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011-2017.
8. Capristo E, Miele L, Forgione A, et al. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2005;9:265-268.
9. Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721-729.
10. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143.
11. Centre for Public Health Excellence at NICE. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Clinical Excellence; 2006.
12. Kirwan JP, Solomon TP, Wojta DM, et al. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156.
13. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166.
14. Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86.
15. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340.
16. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685.
17. Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702.
18. Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig. 2014;34:1-7.
19. Li Y, Liu L, Wang B, et al. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64.
20. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307.
21. Olaywi M, Bhatia T, Anand S, et al. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int. 2013;12:584-588.
22. Troisi G, Crisciotti F, Gianturco V, et al. The treatment with ursodeoxycholic acid in elderly patients affected by NAFLD and metabolic syndrome: a case-control study. Clin Ter. 2013;164:203-207.
23. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodeoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019.
24. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:946.
25. Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619.
26. Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577.
27. Eslami L, Merat S, Malekzadeh R, et al. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623.
28. akeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890.
29. Goh GB, Pagadala MR, Dasarathy J, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979-985.
30. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918.
31. Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137-144.
32. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.
33. Liu ZL, Xie LZ, Zhu J, et al. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;8:CD009059.
34. National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians; 2008.
35. Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141-145.
36. Georgescu EF, Ionescu R, Niculescu M. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954.
37. Musso G, Cassader M, Olivetti C, et al. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431.
38. Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253.
1. Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52.
2. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of non-alcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45.
3. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131.
4. Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974.
5. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344.
6 Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121.
7. Stefan N, Häring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011-2017.
8. Capristo E, Miele L, Forgione A, et al. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2005;9:265-268.
9. Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721-729.
10. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143.
11. Centre for Public Health Excellence at NICE. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Clinical Excellence; 2006.
12. Kirwan JP, Solomon TP, Wojta DM, et al. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156.
13. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166.
14. Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86.
15. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340.
16. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685.
17. Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702.
18. Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig. 2014;34:1-7.
19. Li Y, Liu L, Wang B, et al. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64.
20. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307.
21. Olaywi M, Bhatia T, Anand S, et al. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int. 2013;12:584-588.
22. Troisi G, Crisciotti F, Gianturco V, et al. The treatment with ursodeoxycholic acid in elderly patients affected by NAFLD and metabolic syndrome: a case-control study. Clin Ter. 2013;164:203-207.
23. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodeoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019.
24. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:946.
25. Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619.
26. Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577.
27. Eslami L, Merat S, Malekzadeh R, et al. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623.
28. akeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890.
29. Goh GB, Pagadala MR, Dasarathy J, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979-985.
30. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918.
31. Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137-144.
32. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.
33. Liu ZL, Xie LZ, Zhu J, et al. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;8:CD009059.
34. National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians; 2008.
35. Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141-145.
36. Georgescu EF, Ionescu R, Niculescu M. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954.
37. Musso G, Cassader M, Olivetti C, et al. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431.
38. Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253.