User login
Opioid Management in Older Adults: Lessons Learned From a Geriatric Patient-Centered Medical Home
The United States continues to confront an opioid crisis that also affects older adults. According to the Substance Abuse and Mental Health Services Administration from 1999 to 2010, there has been a 4-fold increase in opioid overdose deaths.1 Between 2010 and 2015, the rate of opioid-related inpatient stays and emergency department (ED) visits for people aged ≥ 65 years increased by 34% and 74%, respectively, and opioid-related overdose deaths continue to increase among older patients.1,2
Background
Chronic pain is estimated to affect 50 million US adults.3 Individuals receiving long-term opioid therapy may not have experienced relief with other medications or cannot take them for medical safety reasons. Losing access to opioid prescriptions can contribute to misuse of illicit opioids. Implementing best practices for prescription opioid management in older adults is challenging. Older adults have a high prevalence of chronic pain, which is linked to disability and loss of function, reduced mobility, falls, depression, anxiety, sleep disorders, social isolation, and suicide or suicidal ideation.4 Until recently, chronic pain in older adults was often treated primarily with long-term opioid prescriptions, despite little evidence for the effectiveness of that treatment for chronic conditions. The prevalence of long-term opioid use in adults has increased from 1.8% (1999-2000) to 5.4% (2013-2014), and 25% of adult long-term opioid users are aged ≥ 65 years.5
Older adults are especially vulnerable to developing adverse events (AEs) from opioid use, including constipation, confusion, nausea, falls, and overdose. These factors make safe prescribing more challenging even when opioids are an appropriate therapeutic choice. Older adults often have multiple chronic conditions and take multiple medications that increase risk of AEs due to drug-disease and drug-drug interactions. Finding appropriate alternatives for pain management can be challenging in the presence of dementia if other pharmacologic options are contraindicated or mobility issues limit access to other therapeutic options.
Pain treatment plans should be based on realistic functional goals using a shared decision-making approach accounting for patient and provider expectations. All reasonable nondrug and nonopioid treatments should be considered before opioids are initiated. A comprehensive, person-centered, approach to pain management in older adults that includes opioids, other medications, and complementary and integrative care could improve both pain control and function,and reduce the harms of unnecessary opioid exposure.6 A validated risk review should be performed and documented on all patients starting opioids except patients enrolled in hospice care.
In 2018, the US Department of Veterans Affairs (VA) required all facilities to complete case reviews for veterans identified in the Stratification Tool for Opioid Risk Mitigation (STORM) dashboard as being at particularly high risk for AEs among patients prescribed opioids.7 We present our experience with a 1-year management of 48 high-risk older patients receiving chronic prescription opioid therapy. These patients obtained all their care at the VA with complete record documentation.
Methods
The Tennessee Valley Healthcare System (TVHS) is an integrated VA health care system with > 100,000 veteran patients in middle Tennessee with 2 medical centers 40 miles apart, and 12 community-based outpatient clinics. In 2011, TVHS developed a geriatric patient-centered medical home model for geriatric primary care—the geriatric patient aligned care team (GeriPACT).8 GeriPACT consists of a GeriPACT primary care provider (geriatrician or geriatric nurse practitioner with a panel of about 800 outpatients), social worker, clinical pharmacist, registered nurse care manager, licensed vocational nurse, and clerical staff. GeriPACT is a special population PACT within primary care for complex geriatric and other high-risk vulnerable veterans providing integrated, interdisciplinary assessment and longitudinal management, and coordination of both VA and non-VA-funded (eg, Medicare and Medicaid) services for patients and caregivers. GeriPACT at the Nashville TVHS campus has an enrollment of 745 patients of whom 48 receive chronic prescription opioid therapy. The practice is supported by the VA Computerized Patients Record System (CPRS), including the electronic patient portal, My healtheVet, with telemedicine capabilities. Data were collected by chart review with operations data extracted from the Veterans Health Information System and Technology Architecture.
Best practices for prescription opioids for chronic pain follow the US Department of Health and Human Services Interagency Task Force pain management recommendations.4 These include: (1) Effective pain evaluation and management, including diagnostic evaluation and indicated referrals; (2) appropriately prescribed opioids when indicated; and (3) active management of opioid users to prevent AEs and misuse. Strategies used in GeriPACT were adopted from the pain management task force and designed to address needs and challenges associated with responsible chronic opioid prescribing (Table 1).
All 48 patients who were prescribed chronic opioid therapy received routine primary care at GeriPACT. A data tracking sheet was maintained from July 1, 2019 to June 30, 2020. Patients were presented for interdisciplinary collaboration and management at weekly GeriPACT where applicable continuous improvement processes were incorporated. Patients were seen every 3 to 6 months and offered dose reduction and alternative therapies at those times. All patients initiated monthly calls for medication refills and were monitored with an initial opioid contract and quarterly unannounced urine drug screens (UDSs) as well as a quarterly review of the prescription drug monitoring database (PDMD). Additionally, all patients received an Opioid Risk Tool assessment (scale 0-26; high risk ≥ 8) and a Risk Index for Overdose or Serious Opioid-Induced Respiratory Depression (RIOSORD) Score (scale 0-115).9,10 Patients with RIOSORD scores ≥ 25 (14% risk of opioid induced respiratory depression) were issued naloxone kits.
All VA patients additionally receive a risk stratification, the clinical assessment of need (CAN) score, which is a clinical predictor of hospitalization and death developed for VA populations.11 This methodology extracts predictors from 6 categories: social demographics, medical conditions, vital signs, prior year use of health services, medications, and laboratory tests and constructs logistic regression models to predict outcomes. CAN scores are on a 99-point scale, with higher scores corresponding to an increased probability of future health care events.
Our overall study was designed to meet standards for quality improvement reporting excellence (SQUIRE) criteria, and this report meets the quality improvement minimum quality criteria set (QI-MQCS) domains for reporting quality improvement work.12,13 The TVHS Institutional Review Board determined this study to be a quality improvement initiative.
Results
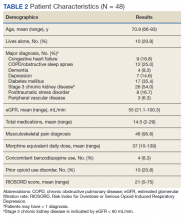
Chronic opioid patients comprised 6.4% of the GeriPACT population. These patients had many comorbidities, including diabetes mellitus (35%), pulmonary disease (25%), congestive heart failure (18.8%), and dementia (8%). There were 54% with estimated glomerular filtration rates (eGFR) < 60 mL/min, indicating at least stage 3 chronic kidney disease (Table 2). Patients had an average RIOSORD Score of 21 and a 14% risk of opioid induced respiratory depression, and 20% received mental health services.
The mean CAN score was 83.1, suggesting a 1-year risk of 20% for a major AE and 5% mortality risk. Many patients with chronic opioid use were transferred to GeriPACT from primary care due to presence of complex medical issues in addition to need for chronic pain management. In this population, 8% were coprescribed benzodiazepines and opioids. Consults were obtained from interventional pain for 37.5% of patients and palliative care for 27% of patients, the majority for goals of care related to chronic illness and advance directive discussions, and in 1 patient for pain and symptom management. The majority of patients (81%) had advance care planning documents or discussions documented in the electronic health record, and 87.5% of patients received home and community-based support in addition to GeriPACT care.
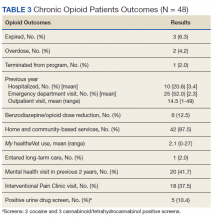
My healtheVet patient portal secure messaging was used a mean 2.1 times per patient (range 0-27) to maintain contact with GeriPACT providers and patients had a mean 14.5 outpatient visits yearly (range, 1-49) in addition to monthly clinic contact for opioid prescription refills (Table 3). One patient entered long-term care. Three patients expired due to congestive heart failure, sepsis, and complications following a hip fracture. Of the patients who expired, all had advance directives or hospice care involvement. The VA STORM risk tool identifies the highest risk patients: suicide risk, current opioid or substance use disorder, suicide attempt or overdose during the past year, and potential for opioid-related respiratory depression on the RIOSORD scale. None of the panel patients met high-risk criteria on the Opioid Risk Tool assessment or were identified on the facility’s highest risk category by the STORM risk tool.
Medication Reduction
Pharmacists routinely counseled patients regarding the appropriate timing of refills and made monthly calls to request refills of controlled drugs. Three patients agreed to opioid dose reduction due to improved clinical status. Two patients had 25% and 30% dose reductions, respectively, and 1 patient was able to be discontinue opioids. This was achieved through reduction of therapy and or substitution of alternative nonopioid pain medications. One patient was initiated on a slow benzodiazepine taper schedule after decades of benzodiazepine use in addition to engagement with a whole health coach and primary care mental health integration provider. Another patient was disenrolled from the clinic because of repeated nonadherence and positive UDSs for polysubstance use disorder.
Accidental Overdoses
There were 2 patients with accidental overdoses who survived, both on high morphine equivalent daily doses (MEDDs). One patient was admitted to the intensive care unit for increasing confusion after taking more than the prescribed opioids (120 mg MEDD) due to uncontrolled pain for 2 months following surgery. The second patient was taking 66 mg MEDD with multiple risk factors for respiratory depression (severe chronic obstructive pulmonary disease requiring oxygen, obstructive sleep apnea, and concomitant benzodiazepine use) who repeatedly refused tapering of opioids and benzodiazepines. He was found unresponsive in respiratory depression by home health staff. Both patients had naloxone kits in their home that were not administered.
Urine Drug Screening
There was an occasional negative opioid UDS attributed to patients on a low-dose opioid administered more than 24 hours before. Five patients (10.4%) had positive UDSs. Two patients were positive for cocaine, and because of chronic persistent pain and complex medical problems cared for in the clinic, counseled and continued on therapy with no repeat infractions. Two patients were positive for cannabinoids attributed to CBD oil products, which are legal in Tennessee. One patient had repeated positive UDSs for polysubstance abuse and was terminated from the clinic, preferring to use cannabinoids and other substances illegally. Meperidine, fentanyl, tramadol, and other synthetic opioids are not detected on a routine UDS.
Discussion
Primary care is critical in optimizing the prescribing and use of opioids in older adults. The patient-centered medical home can give health care providers the tools and support to provide evidence—based pain management for their older adult patients and to facilitate prescription and monitoring of appropriate opioid use to minimizing AEs and OUD risk. This includes a reliable health information technology monitoring system as part of a collaborative, person-centered care practice capable of managing frail older patients with multiple chronic conditions as well as social risk factors. GeriPACT was able to implement guideline—based evaluation and treatment of chronic pain patients through optimal management of opioids, risk reduction, and monitoring and management of AEs, misuse, and dose tapering using shared decision-making strategies when appropriate.
Complex older patients on chronic opioid treatment can do well and are best managed by an interdisciplinary team. Our panel had a high prevalence of chronic opioid patients and a high expected mortality based on the severity of comorbidities. Patients had frequent access to care with utilization of many support services. Patients received care for many chronic illnesses at the same time they received opioid therapy and generally were satisfied and adherent to their treatment plan. Published reports of the prevalence of coprescribing of benzodiazepines and opioids of 1.1 to 2.7% in the general population, may be lower than our veteran population.14 Despite the fact that nearly 20% of the population had a history of opioid misuse, only 1 patient was terminated from the clinic because of repeated episodes of polysubstance use disorder.
GeriPACT has the capability to be responsive to the changing needs of older chronic pain patients as a learning health system using continuous process improvement with frequent team meetings and interdisciplinary care.15 Our experience with chronic pain management demonstrates the feasibility and quality of guideline-based management and enhances our understanding of the intersection of care, chronic pain management, and opioid use disorder in older adults.
Limitations
Our experience with this population of older veterans may not be applicable to other geriatric populations. While all patients received their primary care at VA and patients were seen regularly, our data may not account for possible use of some community services, including hospitalization and long-term care.
Conclusions
Guideline-based patient-centered medical home management of patients with chronic pain treated with opioids can be an effective model to maintain and improve measures of health and well-being in older patients. Primary care management is critical in optimizing the prescribing and use of opioids in older adults. These complex older patients are best managed by an interdisciplinary team.
Acknowledgments
This work was supported in part by the Geriatric Workforce Enhancement Program, HRSA Grant: 1-U1Q-HP 033085-01-00.
1. Weiss AJ, Heslin KC, Barrett ML, Izar R, Bierman AS. Opioid-related inpatient stays and emergency department visits among patients aged 65 years and older, 2010 and 2015: Statistical Brief #244. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); September 18, 2018.
2. Centers for Disease Control and Prevention. New data show significant changes in drug overdose deaths. Published March 18, 2020. Accessed March 11, 2021. https://www.cdc.gov/media/releases/2020/p0318-data-show-changes-overdose-deaths.html
3. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. Published 2018 Sep 14. doi:10.15585/mmwr.mm6736a2
4. National Institutes of Health, Interagency Pain Research Coordinating Committee. National pain strategy overview. Updated March 11, 2021. Accessed March 11, 2021. https://www.iprcc.nih.gov/national-pain-strategy-overview
5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018;27(5):526-534. doi:10.1002/pds.4278
6. US Department of Health and Human Services. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. Published May 9, 2019. Accessed March 17, 2021.https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
7. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
8. US Department of Veterans Affairs, Veterans Health Administration. Geriatric patient aligned care team (Geri-PACT). Published June 15, 2015. Accessed March 11, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3115
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432-442. doi:10.1111/j.1526-4637.2005.00072.x
10. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
11. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1097/MLR.0b013e31827da95a
12. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32. doi:10.1136/qshc.2008.029058
13. Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ Qual Saf. 2015;24(12):796-804. doi:10.1136/bmjqs-2014-003151
14. Rhee TG. Coprescribing of Benzodiazepines and Opioids in Older Adults: Rates, Correlates, and National Trends. J Gerontol A Biol Sci Med Sci. 2019;74(12):1910-1915. doi:10.1093/gerona/gly283
15. National Academy of Medicine. The Learning Healthcare System: Workshop Summary. The National Academies Press; 2007. doi:10.17226/11903.
The United States continues to confront an opioid crisis that also affects older adults. According to the Substance Abuse and Mental Health Services Administration from 1999 to 2010, there has been a 4-fold increase in opioid overdose deaths.1 Between 2010 and 2015, the rate of opioid-related inpatient stays and emergency department (ED) visits for people aged ≥ 65 years increased by 34% and 74%, respectively, and opioid-related overdose deaths continue to increase among older patients.1,2
Background
Chronic pain is estimated to affect 50 million US adults.3 Individuals receiving long-term opioid therapy may not have experienced relief with other medications or cannot take them for medical safety reasons. Losing access to opioid prescriptions can contribute to misuse of illicit opioids. Implementing best practices for prescription opioid management in older adults is challenging. Older adults have a high prevalence of chronic pain, which is linked to disability and loss of function, reduced mobility, falls, depression, anxiety, sleep disorders, social isolation, and suicide or suicidal ideation.4 Until recently, chronic pain in older adults was often treated primarily with long-term opioid prescriptions, despite little evidence for the effectiveness of that treatment for chronic conditions. The prevalence of long-term opioid use in adults has increased from 1.8% (1999-2000) to 5.4% (2013-2014), and 25% of adult long-term opioid users are aged ≥ 65 years.5
Older adults are especially vulnerable to developing adverse events (AEs) from opioid use, including constipation, confusion, nausea, falls, and overdose. These factors make safe prescribing more challenging even when opioids are an appropriate therapeutic choice. Older adults often have multiple chronic conditions and take multiple medications that increase risk of AEs due to drug-disease and drug-drug interactions. Finding appropriate alternatives for pain management can be challenging in the presence of dementia if other pharmacologic options are contraindicated or mobility issues limit access to other therapeutic options.
Pain treatment plans should be based on realistic functional goals using a shared decision-making approach accounting for patient and provider expectations. All reasonable nondrug and nonopioid treatments should be considered before opioids are initiated. A comprehensive, person-centered, approach to pain management in older adults that includes opioids, other medications, and complementary and integrative care could improve both pain control and function,and reduce the harms of unnecessary opioid exposure.6 A validated risk review should be performed and documented on all patients starting opioids except patients enrolled in hospice care.
In 2018, the US Department of Veterans Affairs (VA) required all facilities to complete case reviews for veterans identified in the Stratification Tool for Opioid Risk Mitigation (STORM) dashboard as being at particularly high risk for AEs among patients prescribed opioids.7 We present our experience with a 1-year management of 48 high-risk older patients receiving chronic prescription opioid therapy. These patients obtained all their care at the VA with complete record documentation.
Methods
The Tennessee Valley Healthcare System (TVHS) is an integrated VA health care system with > 100,000 veteran patients in middle Tennessee with 2 medical centers 40 miles apart, and 12 community-based outpatient clinics. In 2011, TVHS developed a geriatric patient-centered medical home model for geriatric primary care—the geriatric patient aligned care team (GeriPACT).8 GeriPACT consists of a GeriPACT primary care provider (geriatrician or geriatric nurse practitioner with a panel of about 800 outpatients), social worker, clinical pharmacist, registered nurse care manager, licensed vocational nurse, and clerical staff. GeriPACT is a special population PACT within primary care for complex geriatric and other high-risk vulnerable veterans providing integrated, interdisciplinary assessment and longitudinal management, and coordination of both VA and non-VA-funded (eg, Medicare and Medicaid) services for patients and caregivers. GeriPACT at the Nashville TVHS campus has an enrollment of 745 patients of whom 48 receive chronic prescription opioid therapy. The practice is supported by the VA Computerized Patients Record System (CPRS), including the electronic patient portal, My healtheVet, with telemedicine capabilities. Data were collected by chart review with operations data extracted from the Veterans Health Information System and Technology Architecture.
Best practices for prescription opioids for chronic pain follow the US Department of Health and Human Services Interagency Task Force pain management recommendations.4 These include: (1) Effective pain evaluation and management, including diagnostic evaluation and indicated referrals; (2) appropriately prescribed opioids when indicated; and (3) active management of opioid users to prevent AEs and misuse. Strategies used in GeriPACT were adopted from the pain management task force and designed to address needs and challenges associated with responsible chronic opioid prescribing (Table 1).
All 48 patients who were prescribed chronic opioid therapy received routine primary care at GeriPACT. A data tracking sheet was maintained from July 1, 2019 to June 30, 2020. Patients were presented for interdisciplinary collaboration and management at weekly GeriPACT where applicable continuous improvement processes were incorporated. Patients were seen every 3 to 6 months and offered dose reduction and alternative therapies at those times. All patients initiated monthly calls for medication refills and were monitored with an initial opioid contract and quarterly unannounced urine drug screens (UDSs) as well as a quarterly review of the prescription drug monitoring database (PDMD). Additionally, all patients received an Opioid Risk Tool assessment (scale 0-26; high risk ≥ 8) and a Risk Index for Overdose or Serious Opioid-Induced Respiratory Depression (RIOSORD) Score (scale 0-115).9,10 Patients with RIOSORD scores ≥ 25 (14% risk of opioid induced respiratory depression) were issued naloxone kits.
All VA patients additionally receive a risk stratification, the clinical assessment of need (CAN) score, which is a clinical predictor of hospitalization and death developed for VA populations.11 This methodology extracts predictors from 6 categories: social demographics, medical conditions, vital signs, prior year use of health services, medications, and laboratory tests and constructs logistic regression models to predict outcomes. CAN scores are on a 99-point scale, with higher scores corresponding to an increased probability of future health care events.
Our overall study was designed to meet standards for quality improvement reporting excellence (SQUIRE) criteria, and this report meets the quality improvement minimum quality criteria set (QI-MQCS) domains for reporting quality improvement work.12,13 The TVHS Institutional Review Board determined this study to be a quality improvement initiative.
Results
Chronic opioid patients comprised 6.4% of the GeriPACT population. These patients had many comorbidities, including diabetes mellitus (35%), pulmonary disease (25%), congestive heart failure (18.8%), and dementia (8%). There were 54% with estimated glomerular filtration rates (eGFR) < 60 mL/min, indicating at least stage 3 chronic kidney disease (Table 2). Patients had an average RIOSORD Score of 21 and a 14% risk of opioid induced respiratory depression, and 20% received mental health services.
The mean CAN score was 83.1, suggesting a 1-year risk of 20% for a major AE and 5% mortality risk. Many patients with chronic opioid use were transferred to GeriPACT from primary care due to presence of complex medical issues in addition to need for chronic pain management. In this population, 8% were coprescribed benzodiazepines and opioids. Consults were obtained from interventional pain for 37.5% of patients and palliative care for 27% of patients, the majority for goals of care related to chronic illness and advance directive discussions, and in 1 patient for pain and symptom management. The majority of patients (81%) had advance care planning documents or discussions documented in the electronic health record, and 87.5% of patients received home and community-based support in addition to GeriPACT care.
My healtheVet patient portal secure messaging was used a mean 2.1 times per patient (range 0-27) to maintain contact with GeriPACT providers and patients had a mean 14.5 outpatient visits yearly (range, 1-49) in addition to monthly clinic contact for opioid prescription refills (Table 3). One patient entered long-term care. Three patients expired due to congestive heart failure, sepsis, and complications following a hip fracture. Of the patients who expired, all had advance directives or hospice care involvement. The VA STORM risk tool identifies the highest risk patients: suicide risk, current opioid or substance use disorder, suicide attempt or overdose during the past year, and potential for opioid-related respiratory depression on the RIOSORD scale. None of the panel patients met high-risk criteria on the Opioid Risk Tool assessment or were identified on the facility’s highest risk category by the STORM risk tool.
Medication Reduction
Pharmacists routinely counseled patients regarding the appropriate timing of refills and made monthly calls to request refills of controlled drugs. Three patients agreed to opioid dose reduction due to improved clinical status. Two patients had 25% and 30% dose reductions, respectively, and 1 patient was able to be discontinue opioids. This was achieved through reduction of therapy and or substitution of alternative nonopioid pain medications. One patient was initiated on a slow benzodiazepine taper schedule after decades of benzodiazepine use in addition to engagement with a whole health coach and primary care mental health integration provider. Another patient was disenrolled from the clinic because of repeated nonadherence and positive UDSs for polysubstance use disorder.
Accidental Overdoses
There were 2 patients with accidental overdoses who survived, both on high morphine equivalent daily doses (MEDDs). One patient was admitted to the intensive care unit for increasing confusion after taking more than the prescribed opioids (120 mg MEDD) due to uncontrolled pain for 2 months following surgery. The second patient was taking 66 mg MEDD with multiple risk factors for respiratory depression (severe chronic obstructive pulmonary disease requiring oxygen, obstructive sleep apnea, and concomitant benzodiazepine use) who repeatedly refused tapering of opioids and benzodiazepines. He was found unresponsive in respiratory depression by home health staff. Both patients had naloxone kits in their home that were not administered.
Urine Drug Screening
There was an occasional negative opioid UDS attributed to patients on a low-dose opioid administered more than 24 hours before. Five patients (10.4%) had positive UDSs. Two patients were positive for cocaine, and because of chronic persistent pain and complex medical problems cared for in the clinic, counseled and continued on therapy with no repeat infractions. Two patients were positive for cannabinoids attributed to CBD oil products, which are legal in Tennessee. One patient had repeated positive UDSs for polysubstance abuse and was terminated from the clinic, preferring to use cannabinoids and other substances illegally. Meperidine, fentanyl, tramadol, and other synthetic opioids are not detected on a routine UDS.
Discussion
Primary care is critical in optimizing the prescribing and use of opioids in older adults. The patient-centered medical home can give health care providers the tools and support to provide evidence—based pain management for their older adult patients and to facilitate prescription and monitoring of appropriate opioid use to minimizing AEs and OUD risk. This includes a reliable health information technology monitoring system as part of a collaborative, person-centered care practice capable of managing frail older patients with multiple chronic conditions as well as social risk factors. GeriPACT was able to implement guideline—based evaluation and treatment of chronic pain patients through optimal management of opioids, risk reduction, and monitoring and management of AEs, misuse, and dose tapering using shared decision-making strategies when appropriate.
Complex older patients on chronic opioid treatment can do well and are best managed by an interdisciplinary team. Our panel had a high prevalence of chronic opioid patients and a high expected mortality based on the severity of comorbidities. Patients had frequent access to care with utilization of many support services. Patients received care for many chronic illnesses at the same time they received opioid therapy and generally were satisfied and adherent to their treatment plan. Published reports of the prevalence of coprescribing of benzodiazepines and opioids of 1.1 to 2.7% in the general population, may be lower than our veteran population.14 Despite the fact that nearly 20% of the population had a history of opioid misuse, only 1 patient was terminated from the clinic because of repeated episodes of polysubstance use disorder.
GeriPACT has the capability to be responsive to the changing needs of older chronic pain patients as a learning health system using continuous process improvement with frequent team meetings and interdisciplinary care.15 Our experience with chronic pain management demonstrates the feasibility and quality of guideline-based management and enhances our understanding of the intersection of care, chronic pain management, and opioid use disorder in older adults.
Limitations
Our experience with this population of older veterans may not be applicable to other geriatric populations. While all patients received their primary care at VA and patients were seen regularly, our data may not account for possible use of some community services, including hospitalization and long-term care.
Conclusions
Guideline-based patient-centered medical home management of patients with chronic pain treated with opioids can be an effective model to maintain and improve measures of health and well-being in older patients. Primary care management is critical in optimizing the prescribing and use of opioids in older adults. These complex older patients are best managed by an interdisciplinary team.
Acknowledgments
This work was supported in part by the Geriatric Workforce Enhancement Program, HRSA Grant: 1-U1Q-HP 033085-01-00.
The United States continues to confront an opioid crisis that also affects older adults. According to the Substance Abuse and Mental Health Services Administration from 1999 to 2010, there has been a 4-fold increase in opioid overdose deaths.1 Between 2010 and 2015, the rate of opioid-related inpatient stays and emergency department (ED) visits for people aged ≥ 65 years increased by 34% and 74%, respectively, and opioid-related overdose deaths continue to increase among older patients.1,2
Background
Chronic pain is estimated to affect 50 million US adults.3 Individuals receiving long-term opioid therapy may not have experienced relief with other medications or cannot take them for medical safety reasons. Losing access to opioid prescriptions can contribute to misuse of illicit opioids. Implementing best practices for prescription opioid management in older adults is challenging. Older adults have a high prevalence of chronic pain, which is linked to disability and loss of function, reduced mobility, falls, depression, anxiety, sleep disorders, social isolation, and suicide or suicidal ideation.4 Until recently, chronic pain in older adults was often treated primarily with long-term opioid prescriptions, despite little evidence for the effectiveness of that treatment for chronic conditions. The prevalence of long-term opioid use in adults has increased from 1.8% (1999-2000) to 5.4% (2013-2014), and 25% of adult long-term opioid users are aged ≥ 65 years.5
Older adults are especially vulnerable to developing adverse events (AEs) from opioid use, including constipation, confusion, nausea, falls, and overdose. These factors make safe prescribing more challenging even when opioids are an appropriate therapeutic choice. Older adults often have multiple chronic conditions and take multiple medications that increase risk of AEs due to drug-disease and drug-drug interactions. Finding appropriate alternatives for pain management can be challenging in the presence of dementia if other pharmacologic options are contraindicated or mobility issues limit access to other therapeutic options.
Pain treatment plans should be based on realistic functional goals using a shared decision-making approach accounting for patient and provider expectations. All reasonable nondrug and nonopioid treatments should be considered before opioids are initiated. A comprehensive, person-centered, approach to pain management in older adults that includes opioids, other medications, and complementary and integrative care could improve both pain control and function,and reduce the harms of unnecessary opioid exposure.6 A validated risk review should be performed and documented on all patients starting opioids except patients enrolled in hospice care.
In 2018, the US Department of Veterans Affairs (VA) required all facilities to complete case reviews for veterans identified in the Stratification Tool for Opioid Risk Mitigation (STORM) dashboard as being at particularly high risk for AEs among patients prescribed opioids.7 We present our experience with a 1-year management of 48 high-risk older patients receiving chronic prescription opioid therapy. These patients obtained all their care at the VA with complete record documentation.
Methods
The Tennessee Valley Healthcare System (TVHS) is an integrated VA health care system with > 100,000 veteran patients in middle Tennessee with 2 medical centers 40 miles apart, and 12 community-based outpatient clinics. In 2011, TVHS developed a geriatric patient-centered medical home model for geriatric primary care—the geriatric patient aligned care team (GeriPACT).8 GeriPACT consists of a GeriPACT primary care provider (geriatrician or geriatric nurse practitioner with a panel of about 800 outpatients), social worker, clinical pharmacist, registered nurse care manager, licensed vocational nurse, and clerical staff. GeriPACT is a special population PACT within primary care for complex geriatric and other high-risk vulnerable veterans providing integrated, interdisciplinary assessment and longitudinal management, and coordination of both VA and non-VA-funded (eg, Medicare and Medicaid) services for patients and caregivers. GeriPACT at the Nashville TVHS campus has an enrollment of 745 patients of whom 48 receive chronic prescription opioid therapy. The practice is supported by the VA Computerized Patients Record System (CPRS), including the electronic patient portal, My healtheVet, with telemedicine capabilities. Data were collected by chart review with operations data extracted from the Veterans Health Information System and Technology Architecture.
Best practices for prescription opioids for chronic pain follow the US Department of Health and Human Services Interagency Task Force pain management recommendations.4 These include: (1) Effective pain evaluation and management, including diagnostic evaluation and indicated referrals; (2) appropriately prescribed opioids when indicated; and (3) active management of opioid users to prevent AEs and misuse. Strategies used in GeriPACT were adopted from the pain management task force and designed to address needs and challenges associated with responsible chronic opioid prescribing (Table 1).
All 48 patients who were prescribed chronic opioid therapy received routine primary care at GeriPACT. A data tracking sheet was maintained from July 1, 2019 to June 30, 2020. Patients were presented for interdisciplinary collaboration and management at weekly GeriPACT where applicable continuous improvement processes were incorporated. Patients were seen every 3 to 6 months and offered dose reduction and alternative therapies at those times. All patients initiated monthly calls for medication refills and were monitored with an initial opioid contract and quarterly unannounced urine drug screens (UDSs) as well as a quarterly review of the prescription drug monitoring database (PDMD). Additionally, all patients received an Opioid Risk Tool assessment (scale 0-26; high risk ≥ 8) and a Risk Index for Overdose or Serious Opioid-Induced Respiratory Depression (RIOSORD) Score (scale 0-115).9,10 Patients with RIOSORD scores ≥ 25 (14% risk of opioid induced respiratory depression) were issued naloxone kits.
All VA patients additionally receive a risk stratification, the clinical assessment of need (CAN) score, which is a clinical predictor of hospitalization and death developed for VA populations.11 This methodology extracts predictors from 6 categories: social demographics, medical conditions, vital signs, prior year use of health services, medications, and laboratory tests and constructs logistic regression models to predict outcomes. CAN scores are on a 99-point scale, with higher scores corresponding to an increased probability of future health care events.
Our overall study was designed to meet standards for quality improvement reporting excellence (SQUIRE) criteria, and this report meets the quality improvement minimum quality criteria set (QI-MQCS) domains for reporting quality improvement work.12,13 The TVHS Institutional Review Board determined this study to be a quality improvement initiative.
Results
Chronic opioid patients comprised 6.4% of the GeriPACT population. These patients had many comorbidities, including diabetes mellitus (35%), pulmonary disease (25%), congestive heart failure (18.8%), and dementia (8%). There were 54% with estimated glomerular filtration rates (eGFR) < 60 mL/min, indicating at least stage 3 chronic kidney disease (Table 2). Patients had an average RIOSORD Score of 21 and a 14% risk of opioid induced respiratory depression, and 20% received mental health services.
The mean CAN score was 83.1, suggesting a 1-year risk of 20% for a major AE and 5% mortality risk. Many patients with chronic opioid use were transferred to GeriPACT from primary care due to presence of complex medical issues in addition to need for chronic pain management. In this population, 8% were coprescribed benzodiazepines and opioids. Consults were obtained from interventional pain for 37.5% of patients and palliative care for 27% of patients, the majority for goals of care related to chronic illness and advance directive discussions, and in 1 patient for pain and symptom management. The majority of patients (81%) had advance care planning documents or discussions documented in the electronic health record, and 87.5% of patients received home and community-based support in addition to GeriPACT care.
My healtheVet patient portal secure messaging was used a mean 2.1 times per patient (range 0-27) to maintain contact with GeriPACT providers and patients had a mean 14.5 outpatient visits yearly (range, 1-49) in addition to monthly clinic contact for opioid prescription refills (Table 3). One patient entered long-term care. Three patients expired due to congestive heart failure, sepsis, and complications following a hip fracture. Of the patients who expired, all had advance directives or hospice care involvement. The VA STORM risk tool identifies the highest risk patients: suicide risk, current opioid or substance use disorder, suicide attempt or overdose during the past year, and potential for opioid-related respiratory depression on the RIOSORD scale. None of the panel patients met high-risk criteria on the Opioid Risk Tool assessment or were identified on the facility’s highest risk category by the STORM risk tool.
Medication Reduction
Pharmacists routinely counseled patients regarding the appropriate timing of refills and made monthly calls to request refills of controlled drugs. Three patients agreed to opioid dose reduction due to improved clinical status. Two patients had 25% and 30% dose reductions, respectively, and 1 patient was able to be discontinue opioids. This was achieved through reduction of therapy and or substitution of alternative nonopioid pain medications. One patient was initiated on a slow benzodiazepine taper schedule after decades of benzodiazepine use in addition to engagement with a whole health coach and primary care mental health integration provider. Another patient was disenrolled from the clinic because of repeated nonadherence and positive UDSs for polysubstance use disorder.
Accidental Overdoses
There were 2 patients with accidental overdoses who survived, both on high morphine equivalent daily doses (MEDDs). One patient was admitted to the intensive care unit for increasing confusion after taking more than the prescribed opioids (120 mg MEDD) due to uncontrolled pain for 2 months following surgery. The second patient was taking 66 mg MEDD with multiple risk factors for respiratory depression (severe chronic obstructive pulmonary disease requiring oxygen, obstructive sleep apnea, and concomitant benzodiazepine use) who repeatedly refused tapering of opioids and benzodiazepines. He was found unresponsive in respiratory depression by home health staff. Both patients had naloxone kits in their home that were not administered.
Urine Drug Screening
There was an occasional negative opioid UDS attributed to patients on a low-dose opioid administered more than 24 hours before. Five patients (10.4%) had positive UDSs. Two patients were positive for cocaine, and because of chronic persistent pain and complex medical problems cared for in the clinic, counseled and continued on therapy with no repeat infractions. Two patients were positive for cannabinoids attributed to CBD oil products, which are legal in Tennessee. One patient had repeated positive UDSs for polysubstance abuse and was terminated from the clinic, preferring to use cannabinoids and other substances illegally. Meperidine, fentanyl, tramadol, and other synthetic opioids are not detected on a routine UDS.
Discussion
Primary care is critical in optimizing the prescribing and use of opioids in older adults. The patient-centered medical home can give health care providers the tools and support to provide evidence—based pain management for their older adult patients and to facilitate prescription and monitoring of appropriate opioid use to minimizing AEs and OUD risk. This includes a reliable health information technology monitoring system as part of a collaborative, person-centered care practice capable of managing frail older patients with multiple chronic conditions as well as social risk factors. GeriPACT was able to implement guideline—based evaluation and treatment of chronic pain patients through optimal management of opioids, risk reduction, and monitoring and management of AEs, misuse, and dose tapering using shared decision-making strategies when appropriate.
Complex older patients on chronic opioid treatment can do well and are best managed by an interdisciplinary team. Our panel had a high prevalence of chronic opioid patients and a high expected mortality based on the severity of comorbidities. Patients had frequent access to care with utilization of many support services. Patients received care for many chronic illnesses at the same time they received opioid therapy and generally were satisfied and adherent to their treatment plan. Published reports of the prevalence of coprescribing of benzodiazepines and opioids of 1.1 to 2.7% in the general population, may be lower than our veteran population.14 Despite the fact that nearly 20% of the population had a history of opioid misuse, only 1 patient was terminated from the clinic because of repeated episodes of polysubstance use disorder.
GeriPACT has the capability to be responsive to the changing needs of older chronic pain patients as a learning health system using continuous process improvement with frequent team meetings and interdisciplinary care.15 Our experience with chronic pain management demonstrates the feasibility and quality of guideline-based management and enhances our understanding of the intersection of care, chronic pain management, and opioid use disorder in older adults.
Limitations
Our experience with this population of older veterans may not be applicable to other geriatric populations. While all patients received their primary care at VA and patients were seen regularly, our data may not account for possible use of some community services, including hospitalization and long-term care.
Conclusions
Guideline-based patient-centered medical home management of patients with chronic pain treated with opioids can be an effective model to maintain and improve measures of health and well-being in older patients. Primary care management is critical in optimizing the prescribing and use of opioids in older adults. These complex older patients are best managed by an interdisciplinary team.
Acknowledgments
This work was supported in part by the Geriatric Workforce Enhancement Program, HRSA Grant: 1-U1Q-HP 033085-01-00.
1. Weiss AJ, Heslin KC, Barrett ML, Izar R, Bierman AS. Opioid-related inpatient stays and emergency department visits among patients aged 65 years and older, 2010 and 2015: Statistical Brief #244. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); September 18, 2018.
2. Centers for Disease Control and Prevention. New data show significant changes in drug overdose deaths. Published March 18, 2020. Accessed March 11, 2021. https://www.cdc.gov/media/releases/2020/p0318-data-show-changes-overdose-deaths.html
3. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. Published 2018 Sep 14. doi:10.15585/mmwr.mm6736a2
4. National Institutes of Health, Interagency Pain Research Coordinating Committee. National pain strategy overview. Updated March 11, 2021. Accessed March 11, 2021. https://www.iprcc.nih.gov/national-pain-strategy-overview
5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018;27(5):526-534. doi:10.1002/pds.4278
6. US Department of Health and Human Services. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. Published May 9, 2019. Accessed March 17, 2021.https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
7. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
8. US Department of Veterans Affairs, Veterans Health Administration. Geriatric patient aligned care team (Geri-PACT). Published June 15, 2015. Accessed March 11, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3115
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432-442. doi:10.1111/j.1526-4637.2005.00072.x
10. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
11. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1097/MLR.0b013e31827da95a
12. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32. doi:10.1136/qshc.2008.029058
13. Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ Qual Saf. 2015;24(12):796-804. doi:10.1136/bmjqs-2014-003151
14. Rhee TG. Coprescribing of Benzodiazepines and Opioids in Older Adults: Rates, Correlates, and National Trends. J Gerontol A Biol Sci Med Sci. 2019;74(12):1910-1915. doi:10.1093/gerona/gly283
15. National Academy of Medicine. The Learning Healthcare System: Workshop Summary. The National Academies Press; 2007. doi:10.17226/11903.
1. Weiss AJ, Heslin KC, Barrett ML, Izar R, Bierman AS. Opioid-related inpatient stays and emergency department visits among patients aged 65 years and older, 2010 and 2015: Statistical Brief #244. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); September 18, 2018.
2. Centers for Disease Control and Prevention. New data show significant changes in drug overdose deaths. Published March 18, 2020. Accessed March 11, 2021. https://www.cdc.gov/media/releases/2020/p0318-data-show-changes-overdose-deaths.html
3. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. Published 2018 Sep 14. doi:10.15585/mmwr.mm6736a2
4. National Institutes of Health, Interagency Pain Research Coordinating Committee. National pain strategy overview. Updated March 11, 2021. Accessed March 11, 2021. https://www.iprcc.nih.gov/national-pain-strategy-overview
5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018;27(5):526-534. doi:10.1002/pds.4278
6. US Department of Health and Human Services. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. Published May 9, 2019. Accessed March 17, 2021.https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
7. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
8. US Department of Veterans Affairs, Veterans Health Administration. Geriatric patient aligned care team (Geri-PACT). Published June 15, 2015. Accessed March 11, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3115
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432-442. doi:10.1111/j.1526-4637.2005.00072.x
10. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
11. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1097/MLR.0b013e31827da95a
12. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32. doi:10.1136/qshc.2008.029058
13. Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ Qual Saf. 2015;24(12):796-804. doi:10.1136/bmjqs-2014-003151
14. Rhee TG. Coprescribing of Benzodiazepines and Opioids in Older Adults: Rates, Correlates, and National Trends. J Gerontol A Biol Sci Med Sci. 2019;74(12):1910-1915. doi:10.1093/gerona/gly283
15. National Academy of Medicine. The Learning Healthcare System: Workshop Summary. The National Academies Press; 2007. doi:10.17226/11903.
Impaired Arousal and Mortality
Arousal is defined as the patient's overall level of responsiveness to the environment. Its assessment is standard of care in most intensive care units (ICUs) to monitor depth of sedation and underlying brain dysfunction. There has been recent interest in expanding the role of arousal assessment beyond the ICU. Specifically, the Veterans Affairs Delirium Working Group proposed that simple arousal assessment be a vital sign to quantify underlying brain dysfunction.[1] The rationale is that impaired arousal is closely linked with delirium,[2] and is an integral component of multiple delirium assessments.[3, 4, 5] Chester et al. observed that the presence of impaired arousal was 64% sensitive and 93% specific for delirium diagnosed by a psychiatrist.[2] Delirium is an under‐recognized public health problem that affects up to 25% of older hospitalized patients,[6, 7] is associated with a multitude of adverse outcomes such as death and accelerated cognitive decline,[8] and costs the US healthcare system an excess of $152 billion dollars.[9]
Most delirium assessments require the patient to undergo additional cognitive testing. The assessment of arousal, however, requires the rater to merely observe the patient during routine clinical care and can be easily integrated into the clinical workflow.[10] Because of its simplicity and brevity, assessing arousal alone using validated scales such as the Richmond Agitation‐Sedation Scale (RASS) may be a more appealing alternative to traditional, more complex delirium screening in the acute care setting. Its clinical utility would be further strengthened if impaired arousal was also associated with mortality, and conferred risk even in the absence of delirium. As a result, we sought to determine if impaired arousal at initial presentation in older acutely ill patients predicted 6‐month mortality and whether this relationship was present in the absence of delirium.
METHODS
Design Overview
We performed a planned secondary analysis of 2 prospective cohorts that enrolled patients from May 2007 to August 2008 between 8 am and 10 pm during the weekdays, and July 2009 to February 2012 between 8 am and 4 pm during the weekdays. The first cohort was designed to evaluate the relationship between delirium and patient outcomes.[11, 12] The second cohort was used to validate brief delirium assessments using a psychiatrist's assessment as the reference standard.[5, 13] The local institutional review board approved these studies.
Setting and Participants
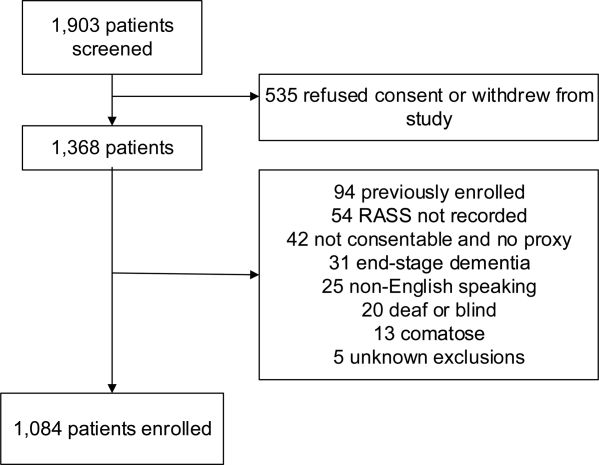
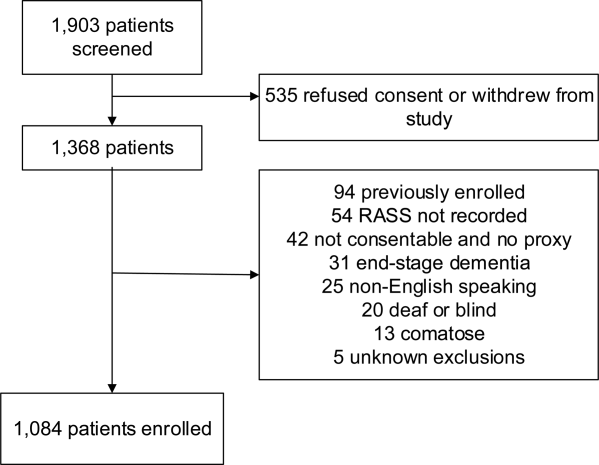
These studies were conducted in an urban emergency department located within an academic, tertiary care hospital with over 57,000 visits annually. Patients were included if they were 65 years or older and in the emergency department for <12 hours at the time of enrollment. The 12‐hour cutoff was used to include patients who presented to the emergency department in the evening and early morning hours. Patients were excluded if they were previously enrolled, non‐English speaking, comatose, or were nonverbal and unable to follow simple commands prior to the acute illness. Because the July 2009 to February 2012 cohort was designed to validate delirium assessments with auditory and visual components, patients were also excluded if they were deaf or blind.
Measurement of Arousal
RASS is an arousal scale commonly used in ICUs to assess depth of sedation and ranges from 5 (unarousable) to +4 (combative); 0 represents normal arousal.[10, 14] The RASS simply requires the rater to observe the patient during their routine interactions and does not require any additional cognitive testing. The RASS terms sedation was modified to drowsy (Table 1), because we wanted to capture impaired arousal regardless of sedation administration. We did not use the modified RASS (mRASS) proposed by the Veteran's Affairs Delirium Working Group, because it was published after data collection began.[1] The mRASS is very similar to the RASS, except it also incorporates a very informal inattention assessment. The RASS was ascertained by research assistants who were college students and graduates, and emergency medical technician basics and paramedics. The principal investigator gave them a 5‐minute didactic lecture about the RASS and observed them perform the RASS in at least 5 patients prior to the start of the study. Inter‐rater reliability between trained research assistants and a physician was assessed for 456 (42.0%) patients of the study sample. The weighted kappa of the RASS was 0.61, indicating very good inter‐rater reliability. Because the 81.7% of patients with impaired arousal had a RASS of 1, the RASS dichotomized as normal (RASS=0) or impaired (RASS other than 0).
| Score | Term | Description |
|---|---|---|
| ||
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent nonpurposeful movement |
| +1 | Restless | Anxious but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| 1 | Slight drowsy | Not fully alert, but has sustained awakening (eye opening/eye contact) to voice (>10 seconds) |
| 2 | Moderately drowsy | Briefly awakens with eye contact to voice (<10 seconds) |
| 3 | Very drowsy | Movement or eye opening to voice (but no eye contact) |
| 4 | Awakens to pain only | No response to voice, but movement or eye opening to physical stimulation |
| 5 | Unarousable | No response to voice or physical stimulation |
Death Ascertainment
Death within 6 months was ascertained using the following algorithm: (1) The electronic medical record was searched to determine the patient's death status. (2) Patients who had a documented emergency department visit, outpatient clinic visit, or hospitalization after 6 months were considered to be alive at 6 months. (3) For the remaining patients, date of death was searched in the Social Security Death Index (SSDI). (4) Patients without a death recorded in the SSDI 1 year after the index visit was considered to be alive at 6 months. Nine hundred thirty‐one (85.9%) out of 1084 patients had a recorded death in the medical record or SSDI, or had an emergency department or hospital visit documented in their record 6 months after the index visit.
Additional Variables Collected
Patients were considered to have dementia if they had: (1) documented dementia in the medical record, (2) a short form Informant Questionnaire on Cognitive Decline in the Elderly score (IQCODE) greater than 3.38,[15] or (3) prescribed cholinesterase inhibitors prior to admission. The short form IQCODE is an informant questionnaire with 16 items; a cutoff of 3.38 out of 5.00 is 79% sensitive and 82% specific for dementia.[16] Premorbid functional status was determined by the Katz Activities of Daily Living (Katz ADL) and ranges from 0 (completely dependent) to 6 (completely independent).[17] Patients with a score <5 were considered to be functionally dependent. Both the IQCODE and Katz ADL were prospectively collected in the emergency department at the time of enrollment.
The Charlson Comorbidity Index was used to measure comorbid burden.[18] The Acute Physiology Score (APS) of the Acute Physiology and Chronic Health Evaluation II score was used to quantify severity of illness.[19] The Glasgow Coma Scale was not included in the APS because it was not collected. Intravenous, intramuscular, and oral benzodiazepine and opioids given in the prehospital and emergency department were also recorded. The Charlson Comorbidity Index, APS, and benzodiazepine and opioid administration were collected after patient enrollment using the electronic medical record.
Within 3 hours of the RASS, a subset of 406 patients was evaluated by a consultation‐liaison psychiatrist who determined the patient's delirium status using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) criteria.[20] Details of their comprehensive assessments have been described in a previous report.[5]
Statistical Analysis
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. For simple comparisons, Wilcoxon rank sum tests were performed for continuous data, and 2 analyses or Fisher exact test were performed for categorical data. To evaluate the predictive validity of impaired arousal on 6‐month mortality, the cumulative probability of survival was estimated within 6 months from the study enrollment date using the Kaplan‐Meier method. Cox proportional hazards regression was performed to assess if impaired arousal was independently associated with 6‐month mortality after adjusting for age, gender, nonwhite race, comorbidity burden (Charlson Comorbidity Index), severity of illness (APS), dementia, functional dependence (Katz ADL <5), nursing home residence, admission status, and benzodiazepine or opioid medication administration. Patients were censored at the end of 6 months. The selection of covariates was based upon expert opinion and literature review. The number of covariates used for the model was limited by the number of events to minimize overfitting; 1 df was allowed for every 10 to 15 events.[21] Because severity of illness, psychoactive medication administration, and admission status might modify the relationship between 6‐month mortality and impaired arousal, 2‐way interaction terms were incorporated. To maintain parsimony and minimize overfitting and collinearity, nonsignificant interaction terms (P>0.20) were removed in the final model.[22] Hazard ratios (HR) with their 95% confidence interval (95% CI) were reported.
To determine if arousal was associated with 6‐month mortality in the absence of delirium, we performed another Cox proportional hazard regression in a subset of 406 patients who received a psychiatrist assessment. Six‐month mortality was the dependent variable, and the independent variable was a 3‐level categorical variable of different arousal/delirium combinations: (1) impaired arousal/delirium positive, (2) impaired arousal/delirium negative, and (3) normal arousal (with or without delirium). Because there were only 8 patients who had normal arousal with delirium, this group was collapsed into the normal arousal without delirium group. Because there were 55 deaths, the number of covariates that could be entered into the Cox proportional hazard regression model was limited. We used the inverse weighted propensity score method to help minimize residual confounding.[23] Traditional propensity score adjustment could not be performed because there were 3 arousal/delirium categories. Similar to propensity score adjustment, inverse weighted propensity score method was used to help balance the distribution of patient characteristics among the exposure groups and also allow adjustment for multiple confounders while minimizing the degrees of freedom expended. A propensity score was the probability of having a particular arousal/delirium category based upon baseline patient characteristics. Multinomial logistic regression was performed to calculate the propensity score, and the baseline covariates used were age, gender, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, and nursing home residence. For the Cox proportional hazard regression model, each observation was weighted by the inverse of the propensity score for their given arousal/delirium category; propensity scores exceeding the 95th percentile were trimmed to avoid overly influential weighting. Benzodiazepine and opioid medications given in the emergency department and admission status were adjusted as covariates in the weighted Cox proportional hazard regression model.
Nineteen patients (1.8%) had missing Katz ADL; these missing values were imputed using multiple imputation. The reliability of the final regression models were internally validated using the bootstrap method.[21] Two thousand sets of bootstrap samples were generated by resampling the original data, and the optimism was estimated to determine the degree of overfitting.[21] An optimism value >0.85 indicated no evidence of substantial overfitting.[21] Variance inflation factors were used to check multicollinearity. Schoenfeld residuals were also analyzed to determine goodness‐of‐fit and assess for outliers. P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and open source R statistical software version 3.0.1 (
RESULTS
A total of 1903 patients were screened, and 1084 patients met enrollment criteria (Figure 1). Of these, 1051 (97.0%) were non‐ICU patients. Patient characteristics of this cohort can be seen in Table 2. Enrolled patients and potentially eligible patients who presented to the emergency department during the enrollment window were similar in age, gender, and severity of illness, but enrolled patients were slightly more likely to have a chief complaint of chest pain and syncope (unpublished data).
| Variables | Normal Arousal, n=835 | Impaired Arousal, n=249 | P Value |
|---|---|---|---|
| |||
| Median age, y (IQR) | 74 (6980) | 75 (7083) | 0.005 |
| Female gender | 459 (55.0%) | 132 (53.0%) | 0.586 |
| Nonwhite race | 122 (14.6%) | 51 (20.5%) | 0.027 |
| Residence | <0.001 | ||
| Home | 752 (90.1%) | 204 (81.9%) | |
| Assisted living | 29 (3.5%) | 13 (5.2%) | |
| Rehabilitation | 8 (1.0%) | 5 (2.0%) | |
| Nursing home | 42 (5.0%) | 27 (10.8%) | |
| Dementia* | 175 (21.0%) | 119 (47.8%) | <0.001 |
| Dependent | 120 (14.4%) | 99 (39.8%) | <0.001 |
| Median Charlson (IQR) | 2 (1, 4) | 3 (2, 5) | <0.001 |
| Median APS (IQR) | 2 (1, 4) | 2 (1, 5) | <0.001 |
| Primary complaint | <0.001 | ||
| Abdominal pain | 45 (5.4%) | 13 (5.2%) | |
| Altered mental status | 12 (1.4%) | 36 (14.5%) | |
| Chest pain | 128 (15.3%) | 31 (12.5%) | |
| Disturbances of sensation | 17 (2.0%) | 2 (0.8%) | |
| Dizziness | 16 (1.9%) | 2 (0.8%) | |
| Fever | 11 (1.3%) | 7 (2.8%) | |
| General illness, malaise | 26 (3.1%) | 5 (2.0%) | |
| General weakness | 68 (8.1%) | 29 (11.7%) | |
| Nausea/vomiting | 29 (3.5%) | 4 (1.6%) | |
| Shortness of breath | 85 (10.2%) | 21 (8.4%) | |
| Syncope | 46 (5.5%) | 10 (4.0%) | |
| Trauma, multiple organs | 19 (2.3%) | 8 (3.2%) | |
| Other | 333 (39.9%) | 81 (32.5%) | |
| Benzodiazepines or opioid medications administration | 188 (22.5%) | 67 (26.9%) | 0.152 |
| Admitted to the hospital | 478 (57.3%) | 191 (76.7%) | 0.002 |
| Internal medicine | 411 (86.0%) | 153 (80.1%) | |
| Surgery | 38 (8.0%) | 21 (11.0%) | |
| Neurology | 19 (4.0%) | 13 (6.8%) | |
| Psychiatry | 1 (0.2%) | 2 (1.1%) | |
| Unknown/missing | 9 (1.9%) | 2 (1.1%) | |
| Death within 6 months | 81 (9.7%) | 59 (23.7%) | <0.001 |
Of those enrolled, 249 (23.0%) had an abnormal RASS at initial presentation, and their distribution can be seen in Figure 2. Within 6 months, patients with an abnormal RASS were more likely to die compared with patients with a RASS of 0 (23.7% vs 9.7%, P<0.001). The Kaplan‐Meier survival curves for all enrolled patients with impaired and normal RASS can be seen in Figure 3; the survival curve declined more slowly in patients with a normal RASS compared with those with an abnormal RASS.
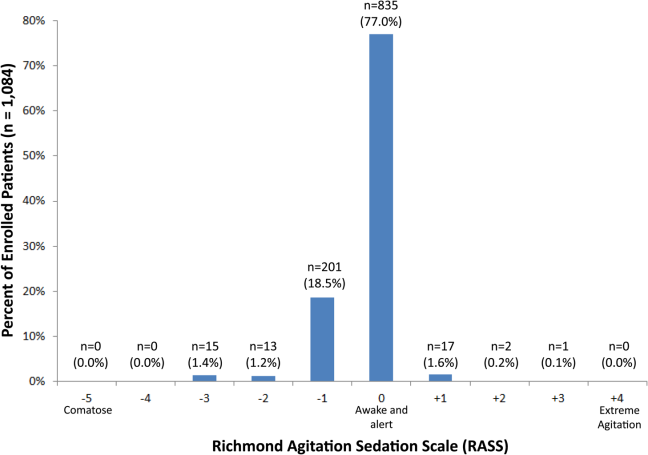
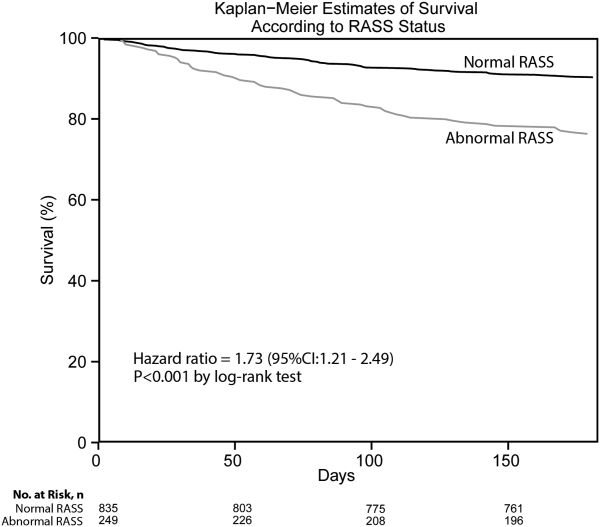
Using Cox proportional hazards regression, the relationship between an abnormal RASS at initial presentation and 6‐month mortality persisted (HR: 1.73, 95% CI: 1.21‐2.49) after adjusting for age, sex, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, nursing home residence, psychoactive medications given, and admission status. The interaction between an abnormal RASS and APS (severity of illness) had a P value of 0.52. The interaction between an abnormal RASS and benzodiazepine or opioid medication administration had a P value of 0.38. The interaction between an abnormal RASS and admission status had a P value of 0.57. This indicated that severity of illness, psychoactive medication administration, and admission status did not modify the relationship between an abnormal RASS and 6‐month mortality.
We analyzed a subset of 406 patients who received a psychiatrist's assessment to determine if an abnormal RASS was associated with 6‐month mortality regardless of delirium status using Cox proportional hazard regression weighted by the inverse of the propensity score. Patients with an abnormal RASS and no delirium were significantly associated with higher mortality compared to those with a normal RASS (HR: 2.20, 95% CI: 1.10‐4.41). Patients with an abnormal RASS with delirium also had an increased risk for 6‐month mortality (HR: 2.86, 95% CI: 1.29‐6.34).
All regression models were internally validated. There was no evidence of substantial overfitting or collinearity. The Schoenfeld residuals for each model were examined graphically and there was good model fit overall, and no significant outliers were observed.
DISCUSSION
Vital sign measurements are a fundamental component of patient care, and abnormalities can serve as an early warning signal of the patient's clinical deterioration. However, traditional vital signs do not include an assessment of the patient's brain function. Our chief finding is that impaired arousal at initial presentation, as determined by the nonphysician research staff, increased the risk of 6‐month mortality by 73% after adjusting for confounders in a diverse group of acutely ill older patients. This relationship existed regardless of severity of illness, administration of psychoactive medications, and admission status. Though impaired arousal is closely linked with delirium,[2, 24] which is another well‐known predictor of mortality,[11, 25, 26] the prognostic significance of impaired arousal appeared to extend beyond delirium. We observed that the relationship between 6‐month mortality and impaired arousal in the absence of delirium was remarkably similar to that observed with impaired arousal with delirium. Arousal can be assessed for by simply observing the patient during routine clinical care and can be performed by nonphysician and physician healthcare providers. Its assessment should be performed and communicated in conjunction with traditional vital sign measurements in the emergency department and inpatient settings.[1]
Most of the data linking impaired arousal to death have been collected in the ICU. Coma, which represents the most severe form of depressed arousal, has been shown to increase the likelihood of death regardless of underlying etiology.[27, 28, 29, 30, 31] This includes patients who have impaired arousal because they received sedative medications during mechanical ventilation.[32] Few studies have investigated the effect of impaired arousal in a non‐ICU patient population. Zuliani et al. observed that impaired arousal was associated with 30‐day mortality, but their study was conducted in 469 older stroke patients, limiting the study's external validity to a more general patient population.[33] Our data advance the current stage of knowledge; we observed a similar relationship between impaired arousal and 6‐month mortality in a much broader clinical population who were predominantly not critically ill regardless of delirium status. Additionally, most of our impaired arousal cohort had a RASS of 1, indicating that even subtle abnormalities portended adverse outcomes.
In addition to long‐term prognosis, the presence of impaired arousal has immediate clinical implications. Using arousal scales like the RASS can serve as a way for healthcare providers to succinctly communicate the patient's mental status in a standardized manner during transitions of care (eg, emergency physician to inpatient team). Regardless of which clinical setting they are in, older acutely ill patients with an impaired arousal may also require close monitoring, especially if the impairment is acute. Because of its close relationship with delirium, these patients likely have an underlying acute medical illness that precipitated their impaired arousal.
Understanding the true clinical significance of impaired arousal in the absence of delirium requires further study. Because of the fluctuating nature of delirium, it is possible that these patients may have initially been delirious and then became nondelirious during the psychiatrist's evaluation. Conversely, it is also possible that these patients may have eventually transitioned into delirium at later point in time; the presence of impaired arousal alone may be a precursor to delirium. Last, these patients may have had subsyndromal delirium, which is defined as having 1 or more delirium symptoms without ever meeting full DSM‐IV‐TR criteria for delirium.[34] Patients with subsyndromal delirium have poorer outcomes, such as prolonged hospitalizations, and higher mortality than patients without delirium symptoms.[34]
Additional studies are also needed to further clarify the impact of impaired arousal on nonmortality outcomes such as functional and cognitive decline. The prognostic significance of serial arousal measurements also requires further study. It is possible that patients whose impaired arousal rapidly resolves after an intervention may have better prognoses than those who have persistent impairment. The measurement of arousal may have additional clinical applications in disease prognosis models. The presence of altered mental status is incorporated in various disease‐specific risk scores such as the CURB‐65 or Pneumonia Severity Index for pneumonia,[35, 36] and the Pulmonary Embolism Severity Index for pulmonary embolism.[37] However, the definition of altered mental status is highly variable; it ranges from subjective impressions that can be unreliable to formal cognitive testing, which can be time consuming. Arousal scales such as the RASS may allow for more feasible, reliable, and standardized assessment of mental status. Future studies should investigate if incorporating the RASS would improve the discrimination of these disease‐severity indices.
This study has several notable limitations. We excluded patients with a RASS of 4 and 5, which represented comatose patients. This exclusion, however, likely biased our findings toward the null. We enrolled a convenience sample that may have introduced selection bias. However, our enrolled cohort was similar to all potentially eligible patients who presented to the emergency department during the study period. We also attempted to mitigate this selection bias by using multivariable regression and adjusting for factors that may have confounded the relationship between RASS and 6‐month mortality. This study was performed at a single, urban, academic hospital and enrolled patients who were aged 65 years and older. Our findings may not be generalizable to other settings and to those who are under 65 years of age. Because 406 patients received a psychiatric evaluation, this limited the number of covariates that could be incorporated into the multivariable model to evaluate if impaired arousal in the absence of delirium is associated with 6‐month mortality. To minimize residual confounding, we used the inverse weighted propensity score, but we acknowledge that this bias may still exist. Larger studies are needed to clarify the relationships between arousal, delirium, and mortality.
CONCLUSION
In conclusion, impaired arousal at initial presentation is an independent predictor for 6‐month mortality in a diverse group of acutely ill older patients, and this risk appears to be present even in the absence of delirium. Because of its ease of use and prognostic significance, it may be a useful vital sign for underlying brain dysfunction. Routine standardized assessment and communication of arousal during routine clinical care may be warranted.
Disclosures: Research reported in this publication was supported by the Vanderbilt Physician Scientist Development Award, Emergency Medicine Foundation, and National Institute on Aging of the National Institutes of Health under award number K23AG032355. This study was also supported by the National Center for Research Resources, grant UL1 RR024975‐01, and is now at the National Center for Advancing Translational Sciences, grant 2 UL1 TR000445‐06. Dr. Vasilevskis was supported in part by the National Institute on Aging of the National Institutes of Health under award number K23AG040157. Dr. Powers was supported by Health Resources and Services Administration Geriatric Education Centers, grant 1D31HP08823‐01‐00. Dr. Storrow was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K12HL1090 and the National Center for Advancing Translational Sciences under award number UL1TR000445. Dr. Ely was supported in part by the National Institute on Aging of the National Institutes of Health under award numbers R01AG027472 and R01AG035117, and a Veteran Affairs MERIT award. Drs. Vasilevskis, Schnelle, Dittus, Powers, and Ely were supported by the Veteran Affairs Geriatric Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vanderbilt University, Emergency Medicine Foundation, National Institutes of Health, and Veterans Affairs. The funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
J.H.H., E.W.E., J.F.S., A.B.S., and R.D.S. conceived the trial. J.H.H., E.W.E., A.B.S., J.F.S., R.D.S., A.S., and A.W. participated in the study design. J.H.H. and A.W. recruited patients and collected the data. J.H.H., A.J.G., and A.S. analyzed the data. All authors participated in the interpretation of results. J.H.H. drafted the manuscript, and all authors contributed to the critical review and revision of the manuscript.
The authors report no conflicts of interest.
- , , , et al. The development of a mental status vital sign for use across the spectrum of care. J Am Med Dir Assoc. 2009;10:379–380.
- , , , . Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen And The Brief Confusion Assessment Method. Ann Emerg Med. 2013;62:457–465.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , . Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19:158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304:443–451.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32.
- , , , et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
- , , , et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56:244–252.
- , , , et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med. 2011;18:451–457.
- , , , et al. Validation of the Confusion Assessment Method For The Intensive Care Unit in older emergency department patients. Acad Emerg Med. 2014;21:180–187.
- , , , et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
- , , , . Does this patient have dementia? JAMA. 2007;297:2391–2404.
- . A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychol Med. 1994;24:145–153.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727.
- , , , , . Charlson Index is associated with one‐year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- American Psychiatric Association. Task Force on DSM‐IV. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
- . Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4.
- . An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65:207–214.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
- , , . Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med. 1982;10:86–95.
- , . Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484.
- , , , , , . Predicting outcome from hypoxic‐ischemic coma. JAMA. 1985;253:1420–1426.
- , , , et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263.
- , , , . Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879.
- , , , et al. Early intensive care sedation predicts long‐term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731.
- , , , , , . Risk factors for short‐term mortality in older subjects with acute ischemic stroke. Gerontology. 2006;52:231–236.
- , , , . The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046.
Arousal is defined as the patient's overall level of responsiveness to the environment. Its assessment is standard of care in most intensive care units (ICUs) to monitor depth of sedation and underlying brain dysfunction. There has been recent interest in expanding the role of arousal assessment beyond the ICU. Specifically, the Veterans Affairs Delirium Working Group proposed that simple arousal assessment be a vital sign to quantify underlying brain dysfunction.[1] The rationale is that impaired arousal is closely linked with delirium,[2] and is an integral component of multiple delirium assessments.[3, 4, 5] Chester et al. observed that the presence of impaired arousal was 64% sensitive and 93% specific for delirium diagnosed by a psychiatrist.[2] Delirium is an under‐recognized public health problem that affects up to 25% of older hospitalized patients,[6, 7] is associated with a multitude of adverse outcomes such as death and accelerated cognitive decline,[8] and costs the US healthcare system an excess of $152 billion dollars.[9]
Most delirium assessments require the patient to undergo additional cognitive testing. The assessment of arousal, however, requires the rater to merely observe the patient during routine clinical care and can be easily integrated into the clinical workflow.[10] Because of its simplicity and brevity, assessing arousal alone using validated scales such as the Richmond Agitation‐Sedation Scale (RASS) may be a more appealing alternative to traditional, more complex delirium screening in the acute care setting. Its clinical utility would be further strengthened if impaired arousal was also associated with mortality, and conferred risk even in the absence of delirium. As a result, we sought to determine if impaired arousal at initial presentation in older acutely ill patients predicted 6‐month mortality and whether this relationship was present in the absence of delirium.
METHODS
Design Overview
We performed a planned secondary analysis of 2 prospective cohorts that enrolled patients from May 2007 to August 2008 between 8 am and 10 pm during the weekdays, and July 2009 to February 2012 between 8 am and 4 pm during the weekdays. The first cohort was designed to evaluate the relationship between delirium and patient outcomes.[11, 12] The second cohort was used to validate brief delirium assessments using a psychiatrist's assessment as the reference standard.[5, 13] The local institutional review board approved these studies.
Setting and Participants
These studies were conducted in an urban emergency department located within an academic, tertiary care hospital with over 57,000 visits annually. Patients were included if they were 65 years or older and in the emergency department for <12 hours at the time of enrollment. The 12‐hour cutoff was used to include patients who presented to the emergency department in the evening and early morning hours. Patients were excluded if they were previously enrolled, non‐English speaking, comatose, or were nonverbal and unable to follow simple commands prior to the acute illness. Because the July 2009 to February 2012 cohort was designed to validate delirium assessments with auditory and visual components, patients were also excluded if they were deaf or blind.
Measurement of Arousal
RASS is an arousal scale commonly used in ICUs to assess depth of sedation and ranges from 5 (unarousable) to +4 (combative); 0 represents normal arousal.[10, 14] The RASS simply requires the rater to observe the patient during their routine interactions and does not require any additional cognitive testing. The RASS terms sedation was modified to drowsy (Table 1), because we wanted to capture impaired arousal regardless of sedation administration. We did not use the modified RASS (mRASS) proposed by the Veteran's Affairs Delirium Working Group, because it was published after data collection began.[1] The mRASS is very similar to the RASS, except it also incorporates a very informal inattention assessment. The RASS was ascertained by research assistants who were college students and graduates, and emergency medical technician basics and paramedics. The principal investigator gave them a 5‐minute didactic lecture about the RASS and observed them perform the RASS in at least 5 patients prior to the start of the study. Inter‐rater reliability between trained research assistants and a physician was assessed for 456 (42.0%) patients of the study sample. The weighted kappa of the RASS was 0.61, indicating very good inter‐rater reliability. Because the 81.7% of patients with impaired arousal had a RASS of 1, the RASS dichotomized as normal (RASS=0) or impaired (RASS other than 0).
| Score | Term | Description |
|---|---|---|
| ||
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent nonpurposeful movement |
| +1 | Restless | Anxious but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| 1 | Slight drowsy | Not fully alert, but has sustained awakening (eye opening/eye contact) to voice (>10 seconds) |
| 2 | Moderately drowsy | Briefly awakens with eye contact to voice (<10 seconds) |
| 3 | Very drowsy | Movement or eye opening to voice (but no eye contact) |
| 4 | Awakens to pain only | No response to voice, but movement or eye opening to physical stimulation |
| 5 | Unarousable | No response to voice or physical stimulation |
Death Ascertainment
Death within 6 months was ascertained using the following algorithm: (1) The electronic medical record was searched to determine the patient's death status. (2) Patients who had a documented emergency department visit, outpatient clinic visit, or hospitalization after 6 months were considered to be alive at 6 months. (3) For the remaining patients, date of death was searched in the Social Security Death Index (SSDI). (4) Patients without a death recorded in the SSDI 1 year after the index visit was considered to be alive at 6 months. Nine hundred thirty‐one (85.9%) out of 1084 patients had a recorded death in the medical record or SSDI, or had an emergency department or hospital visit documented in their record 6 months after the index visit.
Additional Variables Collected
Patients were considered to have dementia if they had: (1) documented dementia in the medical record, (2) a short form Informant Questionnaire on Cognitive Decline in the Elderly score (IQCODE) greater than 3.38,[15] or (3) prescribed cholinesterase inhibitors prior to admission. The short form IQCODE is an informant questionnaire with 16 items; a cutoff of 3.38 out of 5.00 is 79% sensitive and 82% specific for dementia.[16] Premorbid functional status was determined by the Katz Activities of Daily Living (Katz ADL) and ranges from 0 (completely dependent) to 6 (completely independent).[17] Patients with a score <5 were considered to be functionally dependent. Both the IQCODE and Katz ADL were prospectively collected in the emergency department at the time of enrollment.
The Charlson Comorbidity Index was used to measure comorbid burden.[18] The Acute Physiology Score (APS) of the Acute Physiology and Chronic Health Evaluation II score was used to quantify severity of illness.[19] The Glasgow Coma Scale was not included in the APS because it was not collected. Intravenous, intramuscular, and oral benzodiazepine and opioids given in the prehospital and emergency department were also recorded. The Charlson Comorbidity Index, APS, and benzodiazepine and opioid administration were collected after patient enrollment using the electronic medical record.
Within 3 hours of the RASS, a subset of 406 patients was evaluated by a consultation‐liaison psychiatrist who determined the patient's delirium status using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) criteria.[20] Details of their comprehensive assessments have been described in a previous report.[5]
Statistical Analysis
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. For simple comparisons, Wilcoxon rank sum tests were performed for continuous data, and 2 analyses or Fisher exact test were performed for categorical data. To evaluate the predictive validity of impaired arousal on 6‐month mortality, the cumulative probability of survival was estimated within 6 months from the study enrollment date using the Kaplan‐Meier method. Cox proportional hazards regression was performed to assess if impaired arousal was independently associated with 6‐month mortality after adjusting for age, gender, nonwhite race, comorbidity burden (Charlson Comorbidity Index), severity of illness (APS), dementia, functional dependence (Katz ADL <5), nursing home residence, admission status, and benzodiazepine or opioid medication administration. Patients were censored at the end of 6 months. The selection of covariates was based upon expert opinion and literature review. The number of covariates used for the model was limited by the number of events to minimize overfitting; 1 df was allowed for every 10 to 15 events.[21] Because severity of illness, psychoactive medication administration, and admission status might modify the relationship between 6‐month mortality and impaired arousal, 2‐way interaction terms were incorporated. To maintain parsimony and minimize overfitting and collinearity, nonsignificant interaction terms (P>0.20) were removed in the final model.[22] Hazard ratios (HR) with their 95% confidence interval (95% CI) were reported.
To determine if arousal was associated with 6‐month mortality in the absence of delirium, we performed another Cox proportional hazard regression in a subset of 406 patients who received a psychiatrist assessment. Six‐month mortality was the dependent variable, and the independent variable was a 3‐level categorical variable of different arousal/delirium combinations: (1) impaired arousal/delirium positive, (2) impaired arousal/delirium negative, and (3) normal arousal (with or without delirium). Because there were only 8 patients who had normal arousal with delirium, this group was collapsed into the normal arousal without delirium group. Because there were 55 deaths, the number of covariates that could be entered into the Cox proportional hazard regression model was limited. We used the inverse weighted propensity score method to help minimize residual confounding.[23] Traditional propensity score adjustment could not be performed because there were 3 arousal/delirium categories. Similar to propensity score adjustment, inverse weighted propensity score method was used to help balance the distribution of patient characteristics among the exposure groups and also allow adjustment for multiple confounders while minimizing the degrees of freedom expended. A propensity score was the probability of having a particular arousal/delirium category based upon baseline patient characteristics. Multinomial logistic regression was performed to calculate the propensity score, and the baseline covariates used were age, gender, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, and nursing home residence. For the Cox proportional hazard regression model, each observation was weighted by the inverse of the propensity score for their given arousal/delirium category; propensity scores exceeding the 95th percentile were trimmed to avoid overly influential weighting. Benzodiazepine and opioid medications given in the emergency department and admission status were adjusted as covariates in the weighted Cox proportional hazard regression model.
Nineteen patients (1.8%) had missing Katz ADL; these missing values were imputed using multiple imputation. The reliability of the final regression models were internally validated using the bootstrap method.[21] Two thousand sets of bootstrap samples were generated by resampling the original data, and the optimism was estimated to determine the degree of overfitting.[21] An optimism value >0.85 indicated no evidence of substantial overfitting.[21] Variance inflation factors were used to check multicollinearity. Schoenfeld residuals were also analyzed to determine goodness‐of‐fit and assess for outliers. P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and open source R statistical software version 3.0.1 (
RESULTS
A total of 1903 patients were screened, and 1084 patients met enrollment criteria (Figure 1). Of these, 1051 (97.0%) were non‐ICU patients. Patient characteristics of this cohort can be seen in Table 2. Enrolled patients and potentially eligible patients who presented to the emergency department during the enrollment window were similar in age, gender, and severity of illness, but enrolled patients were slightly more likely to have a chief complaint of chest pain and syncope (unpublished data).

| Variables | Normal Arousal, n=835 | Impaired Arousal, n=249 | P Value |
|---|---|---|---|
| |||
| Median age, y (IQR) | 74 (6980) | 75 (7083) | 0.005 |
| Female gender | 459 (55.0%) | 132 (53.0%) | 0.586 |
| Nonwhite race | 122 (14.6%) | 51 (20.5%) | 0.027 |
| Residence | <0.001 | ||
| Home | 752 (90.1%) | 204 (81.9%) | |
| Assisted living | 29 (3.5%) | 13 (5.2%) | |
| Rehabilitation | 8 (1.0%) | 5 (2.0%) | |
| Nursing home | 42 (5.0%) | 27 (10.8%) | |
| Dementia* | 175 (21.0%) | 119 (47.8%) | <0.001 |
| Dependent | 120 (14.4%) | 99 (39.8%) | <0.001 |
| Median Charlson (IQR) | 2 (1, 4) | 3 (2, 5) | <0.001 |
| Median APS (IQR) | 2 (1, 4) | 2 (1, 5) | <0.001 |
| Primary complaint | <0.001 | ||
| Abdominal pain | 45 (5.4%) | 13 (5.2%) | |
| Altered mental status | 12 (1.4%) | 36 (14.5%) | |
| Chest pain | 128 (15.3%) | 31 (12.5%) | |
| Disturbances of sensation | 17 (2.0%) | 2 (0.8%) | |
| Dizziness | 16 (1.9%) | 2 (0.8%) | |
| Fever | 11 (1.3%) | 7 (2.8%) | |
| General illness, malaise | 26 (3.1%) | 5 (2.0%) | |
| General weakness | 68 (8.1%) | 29 (11.7%) | |
| Nausea/vomiting | 29 (3.5%) | 4 (1.6%) | |
| Shortness of breath | 85 (10.2%) | 21 (8.4%) | |
| Syncope | 46 (5.5%) | 10 (4.0%) | |
| Trauma, multiple organs | 19 (2.3%) | 8 (3.2%) | |
| Other | 333 (39.9%) | 81 (32.5%) | |
| Benzodiazepines or opioid medications administration | 188 (22.5%) | 67 (26.9%) | 0.152 |
| Admitted to the hospital | 478 (57.3%) | 191 (76.7%) | 0.002 |
| Internal medicine | 411 (86.0%) | 153 (80.1%) | |
| Surgery | 38 (8.0%) | 21 (11.0%) | |
| Neurology | 19 (4.0%) | 13 (6.8%) | |
| Psychiatry | 1 (0.2%) | 2 (1.1%) | |
| Unknown/missing | 9 (1.9%) | 2 (1.1%) | |
| Death within 6 months | 81 (9.7%) | 59 (23.7%) | <0.001 |
Of those enrolled, 249 (23.0%) had an abnormal RASS at initial presentation, and their distribution can be seen in Figure 2. Within 6 months, patients with an abnormal RASS were more likely to die compared with patients with a RASS of 0 (23.7% vs 9.7%, P<0.001). The Kaplan‐Meier survival curves for all enrolled patients with impaired and normal RASS can be seen in Figure 3; the survival curve declined more slowly in patients with a normal RASS compared with those with an abnormal RASS.


Using Cox proportional hazards regression, the relationship between an abnormal RASS at initial presentation and 6‐month mortality persisted (HR: 1.73, 95% CI: 1.21‐2.49) after adjusting for age, sex, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, nursing home residence, psychoactive medications given, and admission status. The interaction between an abnormal RASS and APS (severity of illness) had a P value of 0.52. The interaction between an abnormal RASS and benzodiazepine or opioid medication administration had a P value of 0.38. The interaction between an abnormal RASS and admission status had a P value of 0.57. This indicated that severity of illness, psychoactive medication administration, and admission status did not modify the relationship between an abnormal RASS and 6‐month mortality.
We analyzed a subset of 406 patients who received a psychiatrist's assessment to determine if an abnormal RASS was associated with 6‐month mortality regardless of delirium status using Cox proportional hazard regression weighted by the inverse of the propensity score. Patients with an abnormal RASS and no delirium were significantly associated with higher mortality compared to those with a normal RASS (HR: 2.20, 95% CI: 1.10‐4.41). Patients with an abnormal RASS with delirium also had an increased risk for 6‐month mortality (HR: 2.86, 95% CI: 1.29‐6.34).
All regression models were internally validated. There was no evidence of substantial overfitting or collinearity. The Schoenfeld residuals for each model were examined graphically and there was good model fit overall, and no significant outliers were observed.
DISCUSSION
Vital sign measurements are a fundamental component of patient care, and abnormalities can serve as an early warning signal of the patient's clinical deterioration. However, traditional vital signs do not include an assessment of the patient's brain function. Our chief finding is that impaired arousal at initial presentation, as determined by the nonphysician research staff, increased the risk of 6‐month mortality by 73% after adjusting for confounders in a diverse group of acutely ill older patients. This relationship existed regardless of severity of illness, administration of psychoactive medications, and admission status. Though impaired arousal is closely linked with delirium,[2, 24] which is another well‐known predictor of mortality,[11, 25, 26] the prognostic significance of impaired arousal appeared to extend beyond delirium. We observed that the relationship between 6‐month mortality and impaired arousal in the absence of delirium was remarkably similar to that observed with impaired arousal with delirium. Arousal can be assessed for by simply observing the patient during routine clinical care and can be performed by nonphysician and physician healthcare providers. Its assessment should be performed and communicated in conjunction with traditional vital sign measurements in the emergency department and inpatient settings.[1]
Most of the data linking impaired arousal to death have been collected in the ICU. Coma, which represents the most severe form of depressed arousal, has been shown to increase the likelihood of death regardless of underlying etiology.[27, 28, 29, 30, 31] This includes patients who have impaired arousal because they received sedative medications during mechanical ventilation.[32] Few studies have investigated the effect of impaired arousal in a non‐ICU patient population. Zuliani et al. observed that impaired arousal was associated with 30‐day mortality, but their study was conducted in 469 older stroke patients, limiting the study's external validity to a more general patient population.[33] Our data advance the current stage of knowledge; we observed a similar relationship between impaired arousal and 6‐month mortality in a much broader clinical population who were predominantly not critically ill regardless of delirium status. Additionally, most of our impaired arousal cohort had a RASS of 1, indicating that even subtle abnormalities portended adverse outcomes.
In addition to long‐term prognosis, the presence of impaired arousal has immediate clinical implications. Using arousal scales like the RASS can serve as a way for healthcare providers to succinctly communicate the patient's mental status in a standardized manner during transitions of care (eg, emergency physician to inpatient team). Regardless of which clinical setting they are in, older acutely ill patients with an impaired arousal may also require close monitoring, especially if the impairment is acute. Because of its close relationship with delirium, these patients likely have an underlying acute medical illness that precipitated their impaired arousal.
Understanding the true clinical significance of impaired arousal in the absence of delirium requires further study. Because of the fluctuating nature of delirium, it is possible that these patients may have initially been delirious and then became nondelirious during the psychiatrist's evaluation. Conversely, it is also possible that these patients may have eventually transitioned into delirium at later point in time; the presence of impaired arousal alone may be a precursor to delirium. Last, these patients may have had subsyndromal delirium, which is defined as having 1 or more delirium symptoms without ever meeting full DSM‐IV‐TR criteria for delirium.[34] Patients with subsyndromal delirium have poorer outcomes, such as prolonged hospitalizations, and higher mortality than patients without delirium symptoms.[34]
Additional studies are also needed to further clarify the impact of impaired arousal on nonmortality outcomes such as functional and cognitive decline. The prognostic significance of serial arousal measurements also requires further study. It is possible that patients whose impaired arousal rapidly resolves after an intervention may have better prognoses than those who have persistent impairment. The measurement of arousal may have additional clinical applications in disease prognosis models. The presence of altered mental status is incorporated in various disease‐specific risk scores such as the CURB‐65 or Pneumonia Severity Index for pneumonia,[35, 36] and the Pulmonary Embolism Severity Index for pulmonary embolism.[37] However, the definition of altered mental status is highly variable; it ranges from subjective impressions that can be unreliable to formal cognitive testing, which can be time consuming. Arousal scales such as the RASS may allow for more feasible, reliable, and standardized assessment of mental status. Future studies should investigate if incorporating the RASS would improve the discrimination of these disease‐severity indices.
This study has several notable limitations. We excluded patients with a RASS of 4 and 5, which represented comatose patients. This exclusion, however, likely biased our findings toward the null. We enrolled a convenience sample that may have introduced selection bias. However, our enrolled cohort was similar to all potentially eligible patients who presented to the emergency department during the study period. We also attempted to mitigate this selection bias by using multivariable regression and adjusting for factors that may have confounded the relationship between RASS and 6‐month mortality. This study was performed at a single, urban, academic hospital and enrolled patients who were aged 65 years and older. Our findings may not be generalizable to other settings and to those who are under 65 years of age. Because 406 patients received a psychiatric evaluation, this limited the number of covariates that could be incorporated into the multivariable model to evaluate if impaired arousal in the absence of delirium is associated with 6‐month mortality. To minimize residual confounding, we used the inverse weighted propensity score, but we acknowledge that this bias may still exist. Larger studies are needed to clarify the relationships between arousal, delirium, and mortality.
CONCLUSION
In conclusion, impaired arousal at initial presentation is an independent predictor for 6‐month mortality in a diverse group of acutely ill older patients, and this risk appears to be present even in the absence of delirium. Because of its ease of use and prognostic significance, it may be a useful vital sign for underlying brain dysfunction. Routine standardized assessment and communication of arousal during routine clinical care may be warranted.
Disclosures: Research reported in this publication was supported by the Vanderbilt Physician Scientist Development Award, Emergency Medicine Foundation, and National Institute on Aging of the National Institutes of Health under award number K23AG032355. This study was also supported by the National Center for Research Resources, grant UL1 RR024975‐01, and is now at the National Center for Advancing Translational Sciences, grant 2 UL1 TR000445‐06. Dr. Vasilevskis was supported in part by the National Institute on Aging of the National Institutes of Health under award number K23AG040157. Dr. Powers was supported by Health Resources and Services Administration Geriatric Education Centers, grant 1D31HP08823‐01‐00. Dr. Storrow was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K12HL1090 and the National Center for Advancing Translational Sciences under award number UL1TR000445. Dr. Ely was supported in part by the National Institute on Aging of the National Institutes of Health under award numbers R01AG027472 and R01AG035117, and a Veteran Affairs MERIT award. Drs. Vasilevskis, Schnelle, Dittus, Powers, and Ely were supported by the Veteran Affairs Geriatric Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vanderbilt University, Emergency Medicine Foundation, National Institutes of Health, and Veterans Affairs. The funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
J.H.H., E.W.E., J.F.S., A.B.S., and R.D.S. conceived the trial. J.H.H., E.W.E., A.B.S., J.F.S., R.D.S., A.S., and A.W. participated in the study design. J.H.H. and A.W. recruited patients and collected the data. J.H.H., A.J.G., and A.S. analyzed the data. All authors participated in the interpretation of results. J.H.H. drafted the manuscript, and all authors contributed to the critical review and revision of the manuscript.
The authors report no conflicts of interest.
Arousal is defined as the patient's overall level of responsiveness to the environment. Its assessment is standard of care in most intensive care units (ICUs) to monitor depth of sedation and underlying brain dysfunction. There has been recent interest in expanding the role of arousal assessment beyond the ICU. Specifically, the Veterans Affairs Delirium Working Group proposed that simple arousal assessment be a vital sign to quantify underlying brain dysfunction.[1] The rationale is that impaired arousal is closely linked with delirium,[2] and is an integral component of multiple delirium assessments.[3, 4, 5] Chester et al. observed that the presence of impaired arousal was 64% sensitive and 93% specific for delirium diagnosed by a psychiatrist.[2] Delirium is an under‐recognized public health problem that affects up to 25% of older hospitalized patients,[6, 7] is associated with a multitude of adverse outcomes such as death and accelerated cognitive decline,[8] and costs the US healthcare system an excess of $152 billion dollars.[9]
Most delirium assessments require the patient to undergo additional cognitive testing. The assessment of arousal, however, requires the rater to merely observe the patient during routine clinical care and can be easily integrated into the clinical workflow.[10] Because of its simplicity and brevity, assessing arousal alone using validated scales such as the Richmond Agitation‐Sedation Scale (RASS) may be a more appealing alternative to traditional, more complex delirium screening in the acute care setting. Its clinical utility would be further strengthened if impaired arousal was also associated with mortality, and conferred risk even in the absence of delirium. As a result, we sought to determine if impaired arousal at initial presentation in older acutely ill patients predicted 6‐month mortality and whether this relationship was present in the absence of delirium.
METHODS
Design Overview
We performed a planned secondary analysis of 2 prospective cohorts that enrolled patients from May 2007 to August 2008 between 8 am and 10 pm during the weekdays, and July 2009 to February 2012 between 8 am and 4 pm during the weekdays. The first cohort was designed to evaluate the relationship between delirium and patient outcomes.[11, 12] The second cohort was used to validate brief delirium assessments using a psychiatrist's assessment as the reference standard.[5, 13] The local institutional review board approved these studies.
Setting and Participants
These studies were conducted in an urban emergency department located within an academic, tertiary care hospital with over 57,000 visits annually. Patients were included if they were 65 years or older and in the emergency department for <12 hours at the time of enrollment. The 12‐hour cutoff was used to include patients who presented to the emergency department in the evening and early morning hours. Patients were excluded if they were previously enrolled, non‐English speaking, comatose, or were nonverbal and unable to follow simple commands prior to the acute illness. Because the July 2009 to February 2012 cohort was designed to validate delirium assessments with auditory and visual components, patients were also excluded if they were deaf or blind.
Measurement of Arousal
RASS is an arousal scale commonly used in ICUs to assess depth of sedation and ranges from 5 (unarousable) to +4 (combative); 0 represents normal arousal.[10, 14] The RASS simply requires the rater to observe the patient during their routine interactions and does not require any additional cognitive testing. The RASS terms sedation was modified to drowsy (Table 1), because we wanted to capture impaired arousal regardless of sedation administration. We did not use the modified RASS (mRASS) proposed by the Veteran's Affairs Delirium Working Group, because it was published after data collection began.[1] The mRASS is very similar to the RASS, except it also incorporates a very informal inattention assessment. The RASS was ascertained by research assistants who were college students and graduates, and emergency medical technician basics and paramedics. The principal investigator gave them a 5‐minute didactic lecture about the RASS and observed them perform the RASS in at least 5 patients prior to the start of the study. Inter‐rater reliability between trained research assistants and a physician was assessed for 456 (42.0%) patients of the study sample. The weighted kappa of the RASS was 0.61, indicating very good inter‐rater reliability. Because the 81.7% of patients with impaired arousal had a RASS of 1, the RASS dichotomized as normal (RASS=0) or impaired (RASS other than 0).
| Score | Term | Description |
|---|---|---|
| ||
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s), aggressive |
| +2 | Agitated | Frequent nonpurposeful movement |
| +1 | Restless | Anxious but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| 1 | Slight drowsy | Not fully alert, but has sustained awakening (eye opening/eye contact) to voice (>10 seconds) |
| 2 | Moderately drowsy | Briefly awakens with eye contact to voice (<10 seconds) |
| 3 | Very drowsy | Movement or eye opening to voice (but no eye contact) |
| 4 | Awakens to pain only | No response to voice, but movement or eye opening to physical stimulation |
| 5 | Unarousable | No response to voice or physical stimulation |
Death Ascertainment
Death within 6 months was ascertained using the following algorithm: (1) The electronic medical record was searched to determine the patient's death status. (2) Patients who had a documented emergency department visit, outpatient clinic visit, or hospitalization after 6 months were considered to be alive at 6 months. (3) For the remaining patients, date of death was searched in the Social Security Death Index (SSDI). (4) Patients without a death recorded in the SSDI 1 year after the index visit was considered to be alive at 6 months. Nine hundred thirty‐one (85.9%) out of 1084 patients had a recorded death in the medical record or SSDI, or had an emergency department or hospital visit documented in their record 6 months after the index visit.
Additional Variables Collected
Patients were considered to have dementia if they had: (1) documented dementia in the medical record, (2) a short form Informant Questionnaire on Cognitive Decline in the Elderly score (IQCODE) greater than 3.38,[15] or (3) prescribed cholinesterase inhibitors prior to admission. The short form IQCODE is an informant questionnaire with 16 items; a cutoff of 3.38 out of 5.00 is 79% sensitive and 82% specific for dementia.[16] Premorbid functional status was determined by the Katz Activities of Daily Living (Katz ADL) and ranges from 0 (completely dependent) to 6 (completely independent).[17] Patients with a score <5 were considered to be functionally dependent. Both the IQCODE and Katz ADL were prospectively collected in the emergency department at the time of enrollment.
The Charlson Comorbidity Index was used to measure comorbid burden.[18] The Acute Physiology Score (APS) of the Acute Physiology and Chronic Health Evaluation II score was used to quantify severity of illness.[19] The Glasgow Coma Scale was not included in the APS because it was not collected. Intravenous, intramuscular, and oral benzodiazepine and opioids given in the prehospital and emergency department were also recorded. The Charlson Comorbidity Index, APS, and benzodiazepine and opioid administration were collected after patient enrollment using the electronic medical record.
Within 3 hours of the RASS, a subset of 406 patients was evaluated by a consultation‐liaison psychiatrist who determined the patient's delirium status using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) criteria.[20] Details of their comprehensive assessments have been described in a previous report.[5]
Statistical Analysis
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. For simple comparisons, Wilcoxon rank sum tests were performed for continuous data, and 2 analyses or Fisher exact test were performed for categorical data. To evaluate the predictive validity of impaired arousal on 6‐month mortality, the cumulative probability of survival was estimated within 6 months from the study enrollment date using the Kaplan‐Meier method. Cox proportional hazards regression was performed to assess if impaired arousal was independently associated with 6‐month mortality after adjusting for age, gender, nonwhite race, comorbidity burden (Charlson Comorbidity Index), severity of illness (APS), dementia, functional dependence (Katz ADL <5), nursing home residence, admission status, and benzodiazepine or opioid medication administration. Patients were censored at the end of 6 months. The selection of covariates was based upon expert opinion and literature review. The number of covariates used for the model was limited by the number of events to minimize overfitting; 1 df was allowed for every 10 to 15 events.[21] Because severity of illness, psychoactive medication administration, and admission status might modify the relationship between 6‐month mortality and impaired arousal, 2‐way interaction terms were incorporated. To maintain parsimony and minimize overfitting and collinearity, nonsignificant interaction terms (P>0.20) were removed in the final model.[22] Hazard ratios (HR) with their 95% confidence interval (95% CI) were reported.
To determine if arousal was associated with 6‐month mortality in the absence of delirium, we performed another Cox proportional hazard regression in a subset of 406 patients who received a psychiatrist assessment. Six‐month mortality was the dependent variable, and the independent variable was a 3‐level categorical variable of different arousal/delirium combinations: (1) impaired arousal/delirium positive, (2) impaired arousal/delirium negative, and (3) normal arousal (with or without delirium). Because there were only 8 patients who had normal arousal with delirium, this group was collapsed into the normal arousal without delirium group. Because there were 55 deaths, the number of covariates that could be entered into the Cox proportional hazard regression model was limited. We used the inverse weighted propensity score method to help minimize residual confounding.[23] Traditional propensity score adjustment could not be performed because there were 3 arousal/delirium categories. Similar to propensity score adjustment, inverse weighted propensity score method was used to help balance the distribution of patient characteristics among the exposure groups and also allow adjustment for multiple confounders while minimizing the degrees of freedom expended. A propensity score was the probability of having a particular arousal/delirium category based upon baseline patient characteristics. Multinomial logistic regression was performed to calculate the propensity score, and the baseline covariates used were age, gender, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, and nursing home residence. For the Cox proportional hazard regression model, each observation was weighted by the inverse of the propensity score for their given arousal/delirium category; propensity scores exceeding the 95th percentile were trimmed to avoid overly influential weighting. Benzodiazepine and opioid medications given in the emergency department and admission status were adjusted as covariates in the weighted Cox proportional hazard regression model.
Nineteen patients (1.8%) had missing Katz ADL; these missing values were imputed using multiple imputation. The reliability of the final regression models were internally validated using the bootstrap method.[21] Two thousand sets of bootstrap samples were generated by resampling the original data, and the optimism was estimated to determine the degree of overfitting.[21] An optimism value >0.85 indicated no evidence of substantial overfitting.[21] Variance inflation factors were used to check multicollinearity. Schoenfeld residuals were also analyzed to determine goodness‐of‐fit and assess for outliers. P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and open source R statistical software version 3.0.1 (
RESULTS
A total of 1903 patients were screened, and 1084 patients met enrollment criteria (Figure 1). Of these, 1051 (97.0%) were non‐ICU patients. Patient characteristics of this cohort can be seen in Table 2. Enrolled patients and potentially eligible patients who presented to the emergency department during the enrollment window were similar in age, gender, and severity of illness, but enrolled patients were slightly more likely to have a chief complaint of chest pain and syncope (unpublished data).

| Variables | Normal Arousal, n=835 | Impaired Arousal, n=249 | P Value |
|---|---|---|---|
| |||
| Median age, y (IQR) | 74 (6980) | 75 (7083) | 0.005 |
| Female gender | 459 (55.0%) | 132 (53.0%) | 0.586 |
| Nonwhite race | 122 (14.6%) | 51 (20.5%) | 0.027 |
| Residence | <0.001 | ||
| Home | 752 (90.1%) | 204 (81.9%) | |
| Assisted living | 29 (3.5%) | 13 (5.2%) | |
| Rehabilitation | 8 (1.0%) | 5 (2.0%) | |
| Nursing home | 42 (5.0%) | 27 (10.8%) | |
| Dementia* | 175 (21.0%) | 119 (47.8%) | <0.001 |
| Dependent | 120 (14.4%) | 99 (39.8%) | <0.001 |
| Median Charlson (IQR) | 2 (1, 4) | 3 (2, 5) | <0.001 |
| Median APS (IQR) | 2 (1, 4) | 2 (1, 5) | <0.001 |
| Primary complaint | <0.001 | ||
| Abdominal pain | 45 (5.4%) | 13 (5.2%) | |
| Altered mental status | 12 (1.4%) | 36 (14.5%) | |
| Chest pain | 128 (15.3%) | 31 (12.5%) | |
| Disturbances of sensation | 17 (2.0%) | 2 (0.8%) | |
| Dizziness | 16 (1.9%) | 2 (0.8%) | |
| Fever | 11 (1.3%) | 7 (2.8%) | |
| General illness, malaise | 26 (3.1%) | 5 (2.0%) | |
| General weakness | 68 (8.1%) | 29 (11.7%) | |
| Nausea/vomiting | 29 (3.5%) | 4 (1.6%) | |
| Shortness of breath | 85 (10.2%) | 21 (8.4%) | |
| Syncope | 46 (5.5%) | 10 (4.0%) | |
| Trauma, multiple organs | 19 (2.3%) | 8 (3.2%) | |
| Other | 333 (39.9%) | 81 (32.5%) | |
| Benzodiazepines or opioid medications administration | 188 (22.5%) | 67 (26.9%) | 0.152 |
| Admitted to the hospital | 478 (57.3%) | 191 (76.7%) | 0.002 |
| Internal medicine | 411 (86.0%) | 153 (80.1%) | |
| Surgery | 38 (8.0%) | 21 (11.0%) | |
| Neurology | 19 (4.0%) | 13 (6.8%) | |
| Psychiatry | 1 (0.2%) | 2 (1.1%) | |
| Unknown/missing | 9 (1.9%) | 2 (1.1%) | |
| Death within 6 months | 81 (9.7%) | 59 (23.7%) | <0.001 |
Of those enrolled, 249 (23.0%) had an abnormal RASS at initial presentation, and their distribution can be seen in Figure 2. Within 6 months, patients with an abnormal RASS were more likely to die compared with patients with a RASS of 0 (23.7% vs 9.7%, P<0.001). The Kaplan‐Meier survival curves for all enrolled patients with impaired and normal RASS can be seen in Figure 3; the survival curve declined more slowly in patients with a normal RASS compared with those with an abnormal RASS.


Using Cox proportional hazards regression, the relationship between an abnormal RASS at initial presentation and 6‐month mortality persisted (HR: 1.73, 95% CI: 1.21‐2.49) after adjusting for age, sex, nonwhite race, comorbidity burden, severity of illness, dementia, functional dependence, nursing home residence, psychoactive medications given, and admission status. The interaction between an abnormal RASS and APS (severity of illness) had a P value of 0.52. The interaction between an abnormal RASS and benzodiazepine or opioid medication administration had a P value of 0.38. The interaction between an abnormal RASS and admission status had a P value of 0.57. This indicated that severity of illness, psychoactive medication administration, and admission status did not modify the relationship between an abnormal RASS and 6‐month mortality.
We analyzed a subset of 406 patients who received a psychiatrist's assessment to determine if an abnormal RASS was associated with 6‐month mortality regardless of delirium status using Cox proportional hazard regression weighted by the inverse of the propensity score. Patients with an abnormal RASS and no delirium were significantly associated with higher mortality compared to those with a normal RASS (HR: 2.20, 95% CI: 1.10‐4.41). Patients with an abnormal RASS with delirium also had an increased risk for 6‐month mortality (HR: 2.86, 95% CI: 1.29‐6.34).
All regression models were internally validated. There was no evidence of substantial overfitting or collinearity. The Schoenfeld residuals for each model were examined graphically and there was good model fit overall, and no significant outliers were observed.
DISCUSSION
Vital sign measurements are a fundamental component of patient care, and abnormalities can serve as an early warning signal of the patient's clinical deterioration. However, traditional vital signs do not include an assessment of the patient's brain function. Our chief finding is that impaired arousal at initial presentation, as determined by the nonphysician research staff, increased the risk of 6‐month mortality by 73% after adjusting for confounders in a diverse group of acutely ill older patients. This relationship existed regardless of severity of illness, administration of psychoactive medications, and admission status. Though impaired arousal is closely linked with delirium,[2, 24] which is another well‐known predictor of mortality,[11, 25, 26] the prognostic significance of impaired arousal appeared to extend beyond delirium. We observed that the relationship between 6‐month mortality and impaired arousal in the absence of delirium was remarkably similar to that observed with impaired arousal with delirium. Arousal can be assessed for by simply observing the patient during routine clinical care and can be performed by nonphysician and physician healthcare providers. Its assessment should be performed and communicated in conjunction with traditional vital sign measurements in the emergency department and inpatient settings.[1]
Most of the data linking impaired arousal to death have been collected in the ICU. Coma, which represents the most severe form of depressed arousal, has been shown to increase the likelihood of death regardless of underlying etiology.[27, 28, 29, 30, 31] This includes patients who have impaired arousal because they received sedative medications during mechanical ventilation.[32] Few studies have investigated the effect of impaired arousal in a non‐ICU patient population. Zuliani et al. observed that impaired arousal was associated with 30‐day mortality, but their study was conducted in 469 older stroke patients, limiting the study's external validity to a more general patient population.[33] Our data advance the current stage of knowledge; we observed a similar relationship between impaired arousal and 6‐month mortality in a much broader clinical population who were predominantly not critically ill regardless of delirium status. Additionally, most of our impaired arousal cohort had a RASS of 1, indicating that even subtle abnormalities portended adverse outcomes.
In addition to long‐term prognosis, the presence of impaired arousal has immediate clinical implications. Using arousal scales like the RASS can serve as a way for healthcare providers to succinctly communicate the patient's mental status in a standardized manner during transitions of care (eg, emergency physician to inpatient team). Regardless of which clinical setting they are in, older acutely ill patients with an impaired arousal may also require close monitoring, especially if the impairment is acute. Because of its close relationship with delirium, these patients likely have an underlying acute medical illness that precipitated their impaired arousal.
Understanding the true clinical significance of impaired arousal in the absence of delirium requires further study. Because of the fluctuating nature of delirium, it is possible that these patients may have initially been delirious and then became nondelirious during the psychiatrist's evaluation. Conversely, it is also possible that these patients may have eventually transitioned into delirium at later point in time; the presence of impaired arousal alone may be a precursor to delirium. Last, these patients may have had subsyndromal delirium, which is defined as having 1 or more delirium symptoms without ever meeting full DSM‐IV‐TR criteria for delirium.[34] Patients with subsyndromal delirium have poorer outcomes, such as prolonged hospitalizations, and higher mortality than patients without delirium symptoms.[34]
Additional studies are also needed to further clarify the impact of impaired arousal on nonmortality outcomes such as functional and cognitive decline. The prognostic significance of serial arousal measurements also requires further study. It is possible that patients whose impaired arousal rapidly resolves after an intervention may have better prognoses than those who have persistent impairment. The measurement of arousal may have additional clinical applications in disease prognosis models. The presence of altered mental status is incorporated in various disease‐specific risk scores such as the CURB‐65 or Pneumonia Severity Index for pneumonia,[35, 36] and the Pulmonary Embolism Severity Index for pulmonary embolism.[37] However, the definition of altered mental status is highly variable; it ranges from subjective impressions that can be unreliable to formal cognitive testing, which can be time consuming. Arousal scales such as the RASS may allow for more feasible, reliable, and standardized assessment of mental status. Future studies should investigate if incorporating the RASS would improve the discrimination of these disease‐severity indices.
This study has several notable limitations. We excluded patients with a RASS of 4 and 5, which represented comatose patients. This exclusion, however, likely biased our findings toward the null. We enrolled a convenience sample that may have introduced selection bias. However, our enrolled cohort was similar to all potentially eligible patients who presented to the emergency department during the study period. We also attempted to mitigate this selection bias by using multivariable regression and adjusting for factors that may have confounded the relationship between RASS and 6‐month mortality. This study was performed at a single, urban, academic hospital and enrolled patients who were aged 65 years and older. Our findings may not be generalizable to other settings and to those who are under 65 years of age. Because 406 patients received a psychiatric evaluation, this limited the number of covariates that could be incorporated into the multivariable model to evaluate if impaired arousal in the absence of delirium is associated with 6‐month mortality. To minimize residual confounding, we used the inverse weighted propensity score, but we acknowledge that this bias may still exist. Larger studies are needed to clarify the relationships between arousal, delirium, and mortality.
CONCLUSION
In conclusion, impaired arousal at initial presentation is an independent predictor for 6‐month mortality in a diverse group of acutely ill older patients, and this risk appears to be present even in the absence of delirium. Because of its ease of use and prognostic significance, it may be a useful vital sign for underlying brain dysfunction. Routine standardized assessment and communication of arousal during routine clinical care may be warranted.
Disclosures: Research reported in this publication was supported by the Vanderbilt Physician Scientist Development Award, Emergency Medicine Foundation, and National Institute on Aging of the National Institutes of Health under award number K23AG032355. This study was also supported by the National Center for Research Resources, grant UL1 RR024975‐01, and is now at the National Center for Advancing Translational Sciences, grant 2 UL1 TR000445‐06. Dr. Vasilevskis was supported in part by the National Institute on Aging of the National Institutes of Health under award number K23AG040157. Dr. Powers was supported by Health Resources and Services Administration Geriatric Education Centers, grant 1D31HP08823‐01‐00. Dr. Storrow was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K12HL1090 and the National Center for Advancing Translational Sciences under award number UL1TR000445. Dr. Ely was supported in part by the National Institute on Aging of the National Institutes of Health under award numbers R01AG027472 and R01AG035117, and a Veteran Affairs MERIT award. Drs. Vasilevskis, Schnelle, Dittus, Powers, and Ely were supported by the Veteran Affairs Geriatric Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of Vanderbilt University, Emergency Medicine Foundation, National Institutes of Health, and Veterans Affairs. The funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
J.H.H., E.W.E., J.F.S., A.B.S., and R.D.S. conceived the trial. J.H.H., E.W.E., A.B.S., J.F.S., R.D.S., A.S., and A.W. participated in the study design. J.H.H. and A.W. recruited patients and collected the data. J.H.H., A.J.G., and A.S. analyzed the data. All authors participated in the interpretation of results. J.H.H. drafted the manuscript, and all authors contributed to the critical review and revision of the manuscript.
The authors report no conflicts of interest.
- , , , et al. The development of a mental status vital sign for use across the spectrum of care. J Am Med Dir Assoc. 2009;10:379–380.
- , , , . Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen And The Brief Confusion Assessment Method. Ann Emerg Med. 2013;62:457–465.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , . Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19:158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304:443–451.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32.
- , , , et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
- , , , et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56:244–252.
- , , , et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med. 2011;18:451–457.
- , , , et al. Validation of the Confusion Assessment Method For The Intensive Care Unit in older emergency department patients. Acad Emerg Med. 2014;21:180–187.
- , , , et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
- , , , . Does this patient have dementia? JAMA. 2007;297:2391–2404.
- . A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychol Med. 1994;24:145–153.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727.
- , , , , . Charlson Index is associated with one‐year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- American Psychiatric Association. Task Force on DSM‐IV. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
- . Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4.
- . An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65:207–214.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
- , , . Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med. 1982;10:86–95.
- , . Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484.
- , , , , , . Predicting outcome from hypoxic‐ischemic coma. JAMA. 1985;253:1420–1426.
- , , , et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263.
- , , , . Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879.
- , , , et al. Early intensive care sedation predicts long‐term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731.
- , , , , , . Risk factors for short‐term mortality in older subjects with acute ischemic stroke. Gerontology. 2006;52:231–236.
- , , , . The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046.
- , , , et al. The development of a mental status vital sign for use across the spectrum of care. J Am Med Dir Assoc. 2009;10:379–380.
- , , , . Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453.
- , , , , , . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , et al. Diagnosing delirium in older emergency department patients: validity and reliability of the Delirium Triage Screen And The Brief Confusion Assessment Method. Ann Emerg Med. 2013;62:457–465.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , . Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19:158–163.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304:443–451.
- , , , , . One‐year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32.
- , , , et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344.
- , , , et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56:244–252.
- , , , et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med. 2011;18:451–457.
- , , , et al. Validation of the Confusion Assessment Method For The Intensive Care Unit in older emergency department patients. Acad Emerg Med. 2014;21:180–187.
- , , , et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
- , , , . Does this patient have dementia? JAMA. 2007;297:2391–2404.
- . A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychol Med. 1994;24:145–153.
- . Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727.
- , , , , . Charlson Index is associated with one‐year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13:530–536.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- American Psychiatric Association. Task Force on DSM‐IV. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
- . Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4.
- . An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res. 2008;65:207–214.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762.
- , , . Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med. 1982;10:86–95.
- , . Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484.
- , , , , , . Predicting outcome from hypoxic‐ischemic coma. JAMA. 1985;253:1420–1426.
- , , , et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263.
- , , , . Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291:870–879.
- , , , et al. Early intensive care sedation predicts long‐term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731.
- , , , , , . Risk factors for short‐term mortality in older subjects with acute ischemic stroke. Gerontology. 2006;52:231–236.
- , , , . The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336:243–250.
- , , , et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046.
© 2014 Society of Hospital Medicine