User login
Erethism Mercurialis and Reactions to Elemental Mercury
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
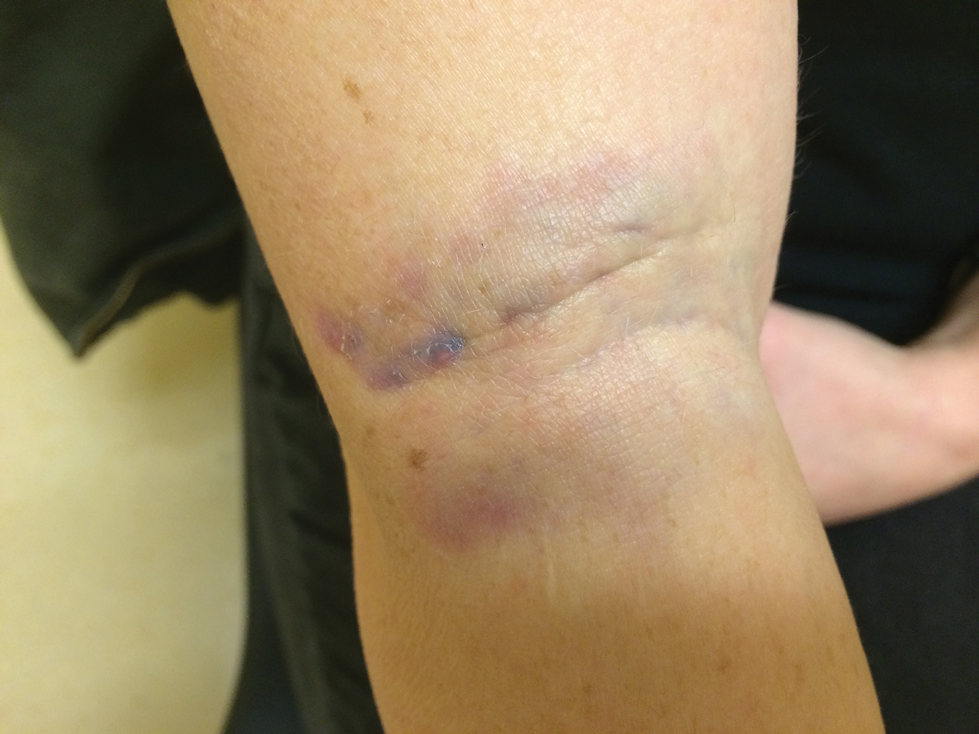
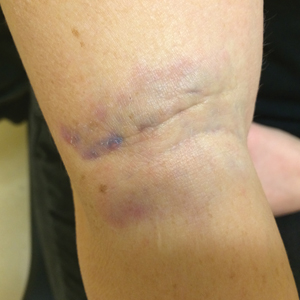
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
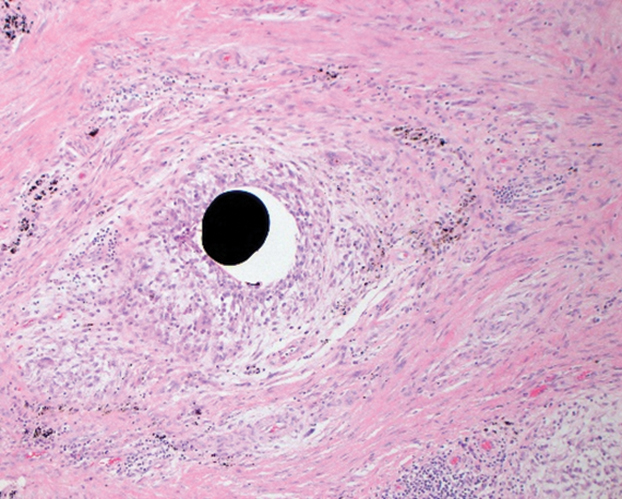
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
Practice Points
- Chronic mercury granulomas can present as firm, erythematous to bluish gray plaques.
- Accidental skin contact to elemental mercury may cause urticaria and dermatitis.
- Blood mercury concentrations below 20 11µg/L are considered within reference range; once blood and urine concentrations exceed 100 11µg/L, clinical signs of acute mercury poisoning typically manifest.
- Mercury is toxic to the central and peripheral nervous systems, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory.
Broadly Distributed Vascular Macules in a Pediatric Patient
The Diagnosis: Capillary Malformation-Arteriovenous Malformation Syndrome
Capillary malformation-arteriovenous malformation (CM-AVM) was suspected, and a sample of the patient's blood was sent for a diagnostic genetic workup. DNA sequencing evaluated the following 5 genes that have been implicated in telangiectasia or AVM disorders: ACVRL1 (activin A receptorlike type 1), ENG (endoglin), GDF2 (growth differentiation factor 2), RASA1 (RAS p21 protein activator 1), and SMAD4 (SMAD family member 4). The patient was found to be heterozygous for a known pathogenic splice-site mutation in the RASA1 gene, consistent with a diagnosis of CM-AVM.
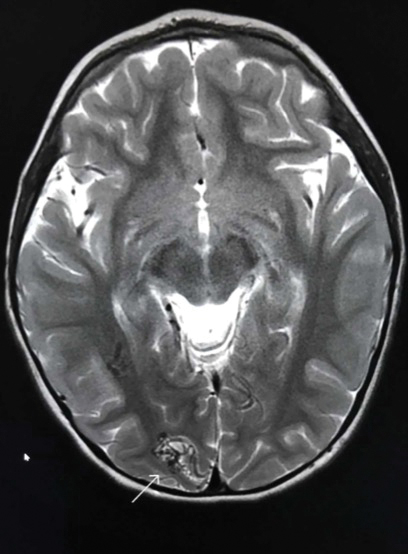
Capillary malformation-arteriovenous malformation presents with multiple small cutaneous CMs and associated arteriovenous fistulas as well as high-flow AVMs located in the soft tissues, bones, or central nervous system (CNS). Occasionally, the cutaneous CMs are surrounded by a blanched halo.1 Because of the potential for CNS involvement in CM-AVM, our patient was further evaluated with spine and brain magnetic resonance imaging (MRI). The brain MRI revealed 2 right occipital pole and fusiform gyral AVMs (Figure). No vascular abnormalities were found in the spine. The patient was referred to interventional neuroradiology to assess the feasibility of ablation to reduce the risk for complications, including intracranial hemorrhage.
Compared to other well-established congenital vascular disorders, CM-AVM has only recently been described in the literature. It was first reported by Eerola and colleagues2 in 2003. They studied several families with CMs and identified heterozygous inactivating RASA1 mutations in 6 families manifesting atypical CMs that were multiple small, round to oval, and pinkish red.2
It has been estimated that RASA1 mutations contribute to 68% of CM-AVM cases. Another gene--EPHB4 (EPH receptor B4)--has been implicated in patients with RASA1-negative disease. Two separate subtypes for patients with CM-AVM have been described: (1) CM-AVM type 1 for patients with RASA1 mutations, and (2) CM-AVM type 2 for those with EPHB4 mutations.3
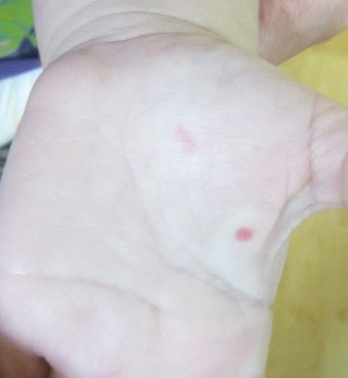
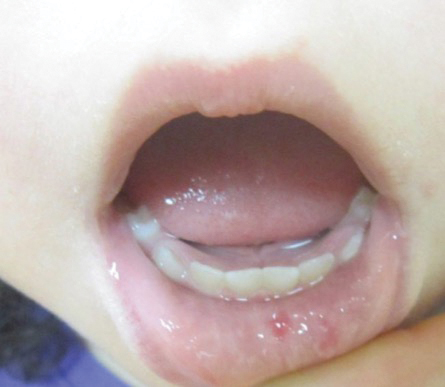
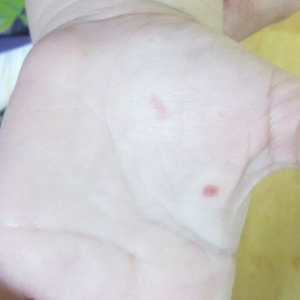
Both CM-AVM types are characterized by small multifocal CMs and an increased risk for CNS fast-flow vascular malformations.4 It has been suggested that there are morphologic differences between the cutaneous manifestations of the 2 types. For example, one group stated Bier spots are more frequently observed in CM-AVM type 2. This same group suggested telangiectases seen primarily on the lips but also in the perioral region and on the upper thorax were seen in CM-AVM type 2 but not in CM-AVM type 1.4 In our patient, it is plausible that the pinpoint red macules on the lips and oral mucosa could be confused for telangiectases (quiz image [bottom]). At this time, we do not feel that there is sufficient evidence to clinically distinguish between CM-AVM types 1 and 2.
Central nervous system involvement seems to be more common in patients with CM-AVM type 1 (10%) than those with CM-AVM type 2 (3%).1,4 Of the 2 CM-AVM type 2 patients found to have intracranial AVMs in one study, both were found to have vein of Galen aneurysmal malformations (VGAMs).4 The study examining CNS involvement in CM-AVM type 1 did not comment on the percentage of VGAMs seen in all patients.1 However, in the retrospective component of the study, the authors reported that in 161 patients with CM-AVM type 1, 24 AVMs were observed, 6 of which were intracranial. Half of these intracranial AVMs were at the vein of Galen, demonstrating that VGAMs are seen in both types of CM-AVM.1 Further study is necessary to better characterize potential phenotypic differences between the 2 forms of CM-AVM.
Overall, the annual risk for hemorrhage associated with brain AVMs is approximately 2% per year.5 Because the morbidity and mortality of undiagnosed CNS malformations is high, it is recommended that patients with both types of CM-AVM undergo spine and brain MRI evaluation. If CNS malformations are identified, patients should be referred to interventional neuroradiology to assess the feasibility of ablation.
It is unclear if patients who initially screen negative for AVMs will go on to develop these fast-flow lesions later. We have noted that new CMs develop over time in our patients. Therefore, it does not seem far-fetched to hypothesize that AVMs of CNS are similarly dynamic. Ultimately, we recommend ongoing screening for brain and spinal AVMs at regular intervals, determined by discussions of risks and benefits between the treating team and patient/family.
It is important to distinguish CM-AVM from hereditary hemorrhagic telangiectasia (HHT), as the distinction affects patient management. Unlike the AVMs found in HHT, AVMs in CM-AVM seldom are found in the lungs or liver.1 Thus, asymptomatic patients with HHT, but not CM-AVM, often are screened for pulmonary AVMs.
The diagnosis of HHT is based on the following 4 findings: spontaneous and recurrent epistaxis; multiple mucocutaneous telangiectasia at characteristic sites, including the lips, oral cavity, fingers, and nose; visceral involvement, such as gastrointestinal, pulmonary, cerebral, or hepatic AVMs; and a first-degree relative with the disorder. Three of the criteria are required for diagnosis.
Notably, the lesions seen in HHT and CM-AVM are morphologically different. Our patient did have 1-mm red macules on the lower lip that had clinical features overlapping with telangiectases, but other cutaneous findings including the presence of red macules and small patches, some with blanched halos, were clearly characteristic of CMs, not telangiectases.6 Furthermore, our patient did not have a personal history of epistaxis or a family history of any affected first-degree relatives. Finally, individuals with HHT tend to develop symptoms later in life compared to patients with CM-AVM, starting with epistaxis at 12 years of age.6
Patients with Henoch-Schönlein purpura also present in childhood but typically demonstrate palpable purpura and acute abdominal pain. Patients with Klippel-Trenaunay syndrome present with CM and venous malformation but also typically display limb overgrowth. Most patients with Klippel-Trenaunay syndrome are born with a port-wine stain.
Diffuse neonatal hemangiomatosis is characterized by multiple progressive, rapidly growing cutaneous hemangiomas associated with widespread visceral hemangiomas in the liver, lungs, gastrointestinal tract, brain, and meninges. Our patient's macules were much more slowly progressive.
- Revencu N, Boon LM, Mendola A, et al. RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum Mutat. 2013;34:1632-1641.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Yu J, Streicher JL, Medne L, et al. EPHB4 mutation implicated in capillary malformation-arteriovenous malformation syndrome: a case report. Pediatr Dermatol. 2017;34:227-230.
- Amyere M, Revencu N, Helaers R, et al. Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation. 2017;136:1037-1048.
- Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614-621.
- Edwards LR, Blechman AB, Zlotoff BJ. RASA1 mutation in a family with capillary malformation-arteriovenous malformation syndrome: a discussion of the differential diagnosis. Pediatr Dermatol. 2017;35:e9-e12.
The Diagnosis: Capillary Malformation-Arteriovenous Malformation Syndrome
Capillary malformation-arteriovenous malformation (CM-AVM) was suspected, and a sample of the patient's blood was sent for a diagnostic genetic workup. DNA sequencing evaluated the following 5 genes that have been implicated in telangiectasia or AVM disorders: ACVRL1 (activin A receptorlike type 1), ENG (endoglin), GDF2 (growth differentiation factor 2), RASA1 (RAS p21 protein activator 1), and SMAD4 (SMAD family member 4). The patient was found to be heterozygous for a known pathogenic splice-site mutation in the RASA1 gene, consistent with a diagnosis of CM-AVM.
Capillary malformation-arteriovenous malformation presents with multiple small cutaneous CMs and associated arteriovenous fistulas as well as high-flow AVMs located in the soft tissues, bones, or central nervous system (CNS). Occasionally, the cutaneous CMs are surrounded by a blanched halo.1 Because of the potential for CNS involvement in CM-AVM, our patient was further evaluated with spine and brain magnetic resonance imaging (MRI). The brain MRI revealed 2 right occipital pole and fusiform gyral AVMs (Figure). No vascular abnormalities were found in the spine. The patient was referred to interventional neuroradiology to assess the feasibility of ablation to reduce the risk for complications, including intracranial hemorrhage.
Compared to other well-established congenital vascular disorders, CM-AVM has only recently been described in the literature. It was first reported by Eerola and colleagues2 in 2003. They studied several families with CMs and identified heterozygous inactivating RASA1 mutations in 6 families manifesting atypical CMs that were multiple small, round to oval, and pinkish red.2
It has been estimated that RASA1 mutations contribute to 68% of CM-AVM cases. Another gene--EPHB4 (EPH receptor B4)--has been implicated in patients with RASA1-negative disease. Two separate subtypes for patients with CM-AVM have been described: (1) CM-AVM type 1 for patients with RASA1 mutations, and (2) CM-AVM type 2 for those with EPHB4 mutations.3
Both CM-AVM types are characterized by small multifocal CMs and an increased risk for CNS fast-flow vascular malformations.4 It has been suggested that there are morphologic differences between the cutaneous manifestations of the 2 types. For example, one group stated Bier spots are more frequently observed in CM-AVM type 2. This same group suggested telangiectases seen primarily on the lips but also in the perioral region and on the upper thorax were seen in CM-AVM type 2 but not in CM-AVM type 1.4 In our patient, it is plausible that the pinpoint red macules on the lips and oral mucosa could be confused for telangiectases (quiz image [bottom]). At this time, we do not feel that there is sufficient evidence to clinically distinguish between CM-AVM types 1 and 2.
Central nervous system involvement seems to be more common in patients with CM-AVM type 1 (10%) than those with CM-AVM type 2 (3%).1,4 Of the 2 CM-AVM type 2 patients found to have intracranial AVMs in one study, both were found to have vein of Galen aneurysmal malformations (VGAMs).4 The study examining CNS involvement in CM-AVM type 1 did not comment on the percentage of VGAMs seen in all patients.1 However, in the retrospective component of the study, the authors reported that in 161 patients with CM-AVM type 1, 24 AVMs were observed, 6 of which were intracranial. Half of these intracranial AVMs were at the vein of Galen, demonstrating that VGAMs are seen in both types of CM-AVM.1 Further study is necessary to better characterize potential phenotypic differences between the 2 forms of CM-AVM.
Overall, the annual risk for hemorrhage associated with brain AVMs is approximately 2% per year.5 Because the morbidity and mortality of undiagnosed CNS malformations is high, it is recommended that patients with both types of CM-AVM undergo spine and brain MRI evaluation. If CNS malformations are identified, patients should be referred to interventional neuroradiology to assess the feasibility of ablation.
It is unclear if patients who initially screen negative for AVMs will go on to develop these fast-flow lesions later. We have noted that new CMs develop over time in our patients. Therefore, it does not seem far-fetched to hypothesize that AVMs of CNS are similarly dynamic. Ultimately, we recommend ongoing screening for brain and spinal AVMs at regular intervals, determined by discussions of risks and benefits between the treating team and patient/family.
It is important to distinguish CM-AVM from hereditary hemorrhagic telangiectasia (HHT), as the distinction affects patient management. Unlike the AVMs found in HHT, AVMs in CM-AVM seldom are found in the lungs or liver.1 Thus, asymptomatic patients with HHT, but not CM-AVM, often are screened for pulmonary AVMs.
The diagnosis of HHT is based on the following 4 findings: spontaneous and recurrent epistaxis; multiple mucocutaneous telangiectasia at characteristic sites, including the lips, oral cavity, fingers, and nose; visceral involvement, such as gastrointestinal, pulmonary, cerebral, or hepatic AVMs; and a first-degree relative with the disorder. Three of the criteria are required for diagnosis.
Notably, the lesions seen in HHT and CM-AVM are morphologically different. Our patient did have 1-mm red macules on the lower lip that had clinical features overlapping with telangiectases, but other cutaneous findings including the presence of red macules and small patches, some with blanched halos, were clearly characteristic of CMs, not telangiectases.6 Furthermore, our patient did not have a personal history of epistaxis or a family history of any affected first-degree relatives. Finally, individuals with HHT tend to develop symptoms later in life compared to patients with CM-AVM, starting with epistaxis at 12 years of age.6
Patients with Henoch-Schönlein purpura also present in childhood but typically demonstrate palpable purpura and acute abdominal pain. Patients with Klippel-Trenaunay syndrome present with CM and venous malformation but also typically display limb overgrowth. Most patients with Klippel-Trenaunay syndrome are born with a port-wine stain.
Diffuse neonatal hemangiomatosis is characterized by multiple progressive, rapidly growing cutaneous hemangiomas associated with widespread visceral hemangiomas in the liver, lungs, gastrointestinal tract, brain, and meninges. Our patient's macules were much more slowly progressive.
The Diagnosis: Capillary Malformation-Arteriovenous Malformation Syndrome
Capillary malformation-arteriovenous malformation (CM-AVM) was suspected, and a sample of the patient's blood was sent for a diagnostic genetic workup. DNA sequencing evaluated the following 5 genes that have been implicated in telangiectasia or AVM disorders: ACVRL1 (activin A receptorlike type 1), ENG (endoglin), GDF2 (growth differentiation factor 2), RASA1 (RAS p21 protein activator 1), and SMAD4 (SMAD family member 4). The patient was found to be heterozygous for a known pathogenic splice-site mutation in the RASA1 gene, consistent with a diagnosis of CM-AVM.
Capillary malformation-arteriovenous malformation presents with multiple small cutaneous CMs and associated arteriovenous fistulas as well as high-flow AVMs located in the soft tissues, bones, or central nervous system (CNS). Occasionally, the cutaneous CMs are surrounded by a blanched halo.1 Because of the potential for CNS involvement in CM-AVM, our patient was further evaluated with spine and brain magnetic resonance imaging (MRI). The brain MRI revealed 2 right occipital pole and fusiform gyral AVMs (Figure). No vascular abnormalities were found in the spine. The patient was referred to interventional neuroradiology to assess the feasibility of ablation to reduce the risk for complications, including intracranial hemorrhage.
Compared to other well-established congenital vascular disorders, CM-AVM has only recently been described in the literature. It was first reported by Eerola and colleagues2 in 2003. They studied several families with CMs and identified heterozygous inactivating RASA1 mutations in 6 families manifesting atypical CMs that were multiple small, round to oval, and pinkish red.2
It has been estimated that RASA1 mutations contribute to 68% of CM-AVM cases. Another gene--EPHB4 (EPH receptor B4)--has been implicated in patients with RASA1-negative disease. Two separate subtypes for patients with CM-AVM have been described: (1) CM-AVM type 1 for patients with RASA1 mutations, and (2) CM-AVM type 2 for those with EPHB4 mutations.3
Both CM-AVM types are characterized by small multifocal CMs and an increased risk for CNS fast-flow vascular malformations.4 It has been suggested that there are morphologic differences between the cutaneous manifestations of the 2 types. For example, one group stated Bier spots are more frequently observed in CM-AVM type 2. This same group suggested telangiectases seen primarily on the lips but also in the perioral region and on the upper thorax were seen in CM-AVM type 2 but not in CM-AVM type 1.4 In our patient, it is plausible that the pinpoint red macules on the lips and oral mucosa could be confused for telangiectases (quiz image [bottom]). At this time, we do not feel that there is sufficient evidence to clinically distinguish between CM-AVM types 1 and 2.
Central nervous system involvement seems to be more common in patients with CM-AVM type 1 (10%) than those with CM-AVM type 2 (3%).1,4 Of the 2 CM-AVM type 2 patients found to have intracranial AVMs in one study, both were found to have vein of Galen aneurysmal malformations (VGAMs).4 The study examining CNS involvement in CM-AVM type 1 did not comment on the percentage of VGAMs seen in all patients.1 However, in the retrospective component of the study, the authors reported that in 161 patients with CM-AVM type 1, 24 AVMs were observed, 6 of which were intracranial. Half of these intracranial AVMs were at the vein of Galen, demonstrating that VGAMs are seen in both types of CM-AVM.1 Further study is necessary to better characterize potential phenotypic differences between the 2 forms of CM-AVM.
Overall, the annual risk for hemorrhage associated with brain AVMs is approximately 2% per year.5 Because the morbidity and mortality of undiagnosed CNS malformations is high, it is recommended that patients with both types of CM-AVM undergo spine and brain MRI evaluation. If CNS malformations are identified, patients should be referred to interventional neuroradiology to assess the feasibility of ablation.
It is unclear if patients who initially screen negative for AVMs will go on to develop these fast-flow lesions later. We have noted that new CMs develop over time in our patients. Therefore, it does not seem far-fetched to hypothesize that AVMs of CNS are similarly dynamic. Ultimately, we recommend ongoing screening for brain and spinal AVMs at regular intervals, determined by discussions of risks and benefits between the treating team and patient/family.
It is important to distinguish CM-AVM from hereditary hemorrhagic telangiectasia (HHT), as the distinction affects patient management. Unlike the AVMs found in HHT, AVMs in CM-AVM seldom are found in the lungs or liver.1 Thus, asymptomatic patients with HHT, but not CM-AVM, often are screened for pulmonary AVMs.
The diagnosis of HHT is based on the following 4 findings: spontaneous and recurrent epistaxis; multiple mucocutaneous telangiectasia at characteristic sites, including the lips, oral cavity, fingers, and nose; visceral involvement, such as gastrointestinal, pulmonary, cerebral, or hepatic AVMs; and a first-degree relative with the disorder. Three of the criteria are required for diagnosis.
Notably, the lesions seen in HHT and CM-AVM are morphologically different. Our patient did have 1-mm red macules on the lower lip that had clinical features overlapping with telangiectases, but other cutaneous findings including the presence of red macules and small patches, some with blanched halos, were clearly characteristic of CMs, not telangiectases.6 Furthermore, our patient did not have a personal history of epistaxis or a family history of any affected first-degree relatives. Finally, individuals with HHT tend to develop symptoms later in life compared to patients with CM-AVM, starting with epistaxis at 12 years of age.6
Patients with Henoch-Schönlein purpura also present in childhood but typically demonstrate palpable purpura and acute abdominal pain. Patients with Klippel-Trenaunay syndrome present with CM and venous malformation but also typically display limb overgrowth. Most patients with Klippel-Trenaunay syndrome are born with a port-wine stain.
Diffuse neonatal hemangiomatosis is characterized by multiple progressive, rapidly growing cutaneous hemangiomas associated with widespread visceral hemangiomas in the liver, lungs, gastrointestinal tract, brain, and meninges. Our patient's macules were much more slowly progressive.
- Revencu N, Boon LM, Mendola A, et al. RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum Mutat. 2013;34:1632-1641.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Yu J, Streicher JL, Medne L, et al. EPHB4 mutation implicated in capillary malformation-arteriovenous malformation syndrome: a case report. Pediatr Dermatol. 2017;34:227-230.
- Amyere M, Revencu N, Helaers R, et al. Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation. 2017;136:1037-1048.
- Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614-621.
- Edwards LR, Blechman AB, Zlotoff BJ. RASA1 mutation in a family with capillary malformation-arteriovenous malformation syndrome: a discussion of the differential diagnosis. Pediatr Dermatol. 2017;35:e9-e12.
- Revencu N, Boon LM, Mendola A, et al. RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum Mutat. 2013;34:1632-1641.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Yu J, Streicher JL, Medne L, et al. EPHB4 mutation implicated in capillary malformation-arteriovenous malformation syndrome: a case report. Pediatr Dermatol. 2017;34:227-230.
- Amyere M, Revencu N, Helaers R, et al. Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation. 2017;136:1037-1048.
- Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614-621.
- Edwards LR, Blechman AB, Zlotoff BJ. RASA1 mutation in a family with capillary malformation-arteriovenous malformation syndrome: a discussion of the differential diagnosis. Pediatr Dermatol. 2017;35:e9-e12.
A 2-year-old girl presented with an erythematous macule on the left nasal sidewall that had been present since birth as well as other similar-appearing macules that had slowly evolved over the last 2 years. The patient was born via normal spontaneous vaginal delivery to healthy parents. She had 2 healthy siblings. Her parents reported that she was otherwise growing and developing normally. The patient had no history of epistaxis, and there was no family history of vascular anomalies. Physical examination revealed 2- to 6-mm vascular macules that blanched with pressure and filled quickly thereafter on the left nasal sidewall, upper (top) and lower extremities, and trunk. Some macules were surrounded by blanched halos. Several 1-mm red macules also were noted on the exterior and interior of the mucosal lower lip (bottom).
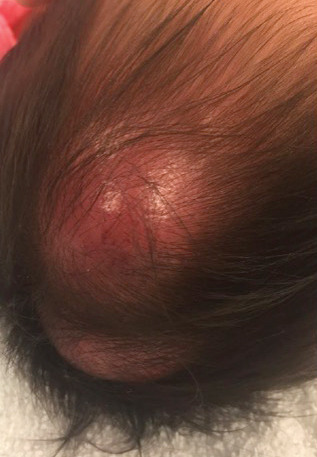
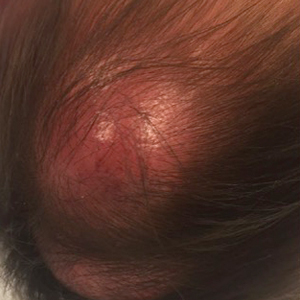
Rapidly Growing Cutaneous Nodules on the Scalp
The Diagnosis: B-Cell Acute Lymphoblastic Leukemia
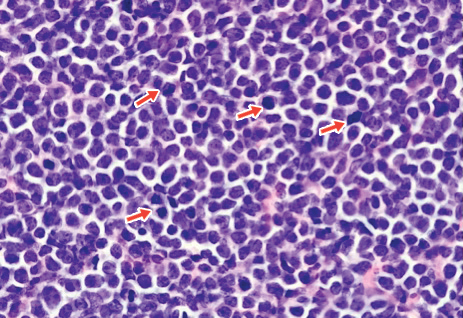
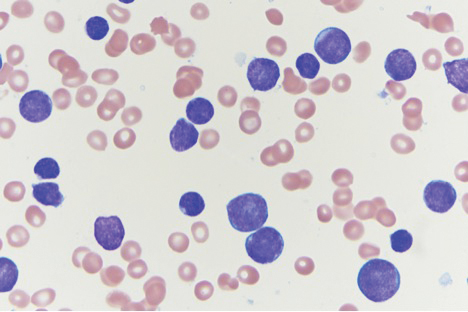
A 4-mm punch biopsy of one of the scalp lesions showed a diffuse infiltrate of intermediately sized cells with variably mature chromatin and irregular nuclear contours, consistent with a neoplastic process. Numerous mitotic figures were present, indicating a high proliferation rate (Figure 1). At that time there was no evidence of systemic involvement. A repeat biopsy with concurrent bone marrow biopsy was scheduled 10 days after the patient's initial presentation for further classification. Laboratory studies at that time revealed leukocytosis with elevated neutrophils and lymphocytes as well as a high absolute blast count.
On immunohistochemical staining, the neoplastic cells were positive for CD45, which indicated the neoplasm was hematopoietic, as well as CD10 and the B-cell antigens PAX-5 and CD79a. The cells were negative for CD20, which also is a B-cell marker, but this marker is only expressed in approximately half of pediatric acute lymphoblastic leukemia (ALL) cases with B-cell precursor origin.1 Markers that typically are expressed in B-cell acute lymphoblastic leukemia (B-ALL)--CD34 and terminal deoxynucleotidyl transferase--were both negative. These results were somewhat contradictory, and the differential remained open to both B-ALL and mature B-cell lymphoma. A bone marrow biopsy showed approximately 65% blasts or leukemic cells (Figure 2). Flow cytometry showed the cells were positive for CD10, CD19, weak CD79a, and variable lambda surface antigen expression. The cells were negative for expression of CD20, CD34, terminal deoxynucleotidyl transferase, myeloid antigens, and CD3. Ultimately, the morphology and immunophenotype were most consistent with a diagnosis of B-ALL. Fluorescence in situ hybridization revealed mixed lineage leukemia, MLL, gene rearrangements.
In general, when considering the differential diagnosis of superficial nodules, 5 elements are helpful to consider: the number of nodules (single vs multiple); the location; and the presence or absence of tenderness, pigmentation or erythema, and firmness.2 Our patient had multiple nodules on the scalp, which were erythematous to slightly purple and firm. The differential diagnosis can be categorized into malignant; infectious; and benign inflammatory, vascular, and fibrous tumors.
Potential oncologic processes include leukemia cutis, lymphoblastic leukemia/lymphoma, Langerhans cell histiocytosis, and rhabdomyosarcoma. Initial laboratory test results were reassuring. Infectious processes in the differential include deep fungal infections such as coccidioidomycosis and nontuberculous mycobacterial infections. Coccidioidomycosis was the most likely to cause skin lesions or masses in our patient; however, it was considered less likely because the patient's family had not traveled or been exposed to an endemic area.3
Benign tumors in the differential include deep hemangioma, which was deemed less likely in our patient because most hemangiomas reach 80% of their maximum size by 5 months of age.4 Another possible benign tumor is infantile myofibromatosis, which is rare but is the most common fibrous tumor of infancy.5
Early-onset childhood sarcoidosis also has been shown to produce multiple nontender firm nodules.2 This process was considered unlikely in our patient because not only is the disease relatively rare in the pediatric population, but most reported childhood cases have occurred in patients aged 13 to 15 years.6 Additionally, no uveitis or arthritis was observed in this case.
Ultimately, histopathology and bone marrow biopsy were necessary to determine the diagnosis of B-ALL. Although uncommon, cutaneous involvement can be an early sign of ALL in children.7 Thus, neoplastic etiologies should be considered in the workup of cutaneous nodules in children, especially when these nodules are hard, rapidly growing, ulcerated, fixed, and/or vascular.8 Once the diagnosis is established, initial workup of ALL in children should include complete blood cell count with manual differential, prothrombin time, partial thromboplastin time, electrolytes, uric acid, and renal and liver function tests. Often, baseline viral titers such as cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, hepatitis B virus, and varicella-zoster virus also are included. Patients are risk stratified to the appropriate level of treatment based on tumor immunophenotype, cytogenetic findings, patient age, white blood cell count at the time of diagnosis, and response to initial therapy. Treatment typically is comprised of a multidrug regimen divided into several phases--induction, consolidation, and maintenance--as well as therapy directed to the central nervous system. Treatment protocols usually take 2 to 3 years to complete.
Our patient was treated with 1 dose of intrathecal methotrexate before starting the Interfant-06 protocol with a 7-day methylprednisolone prophase. The patient's nodules shrank over time and were no longer present after 14 days of treatment.
- Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982-3988.
- Whelan JP, Zembowicz A. Case records of the Massachusetts General Hospital. case 19-2006. a 22-month-old boy with the rapid growth of subcutaneous nodules. N Engl J Med. 2006;354:2697-2704.
- Malo J, Luraschi-Monjagatta C, Wolk DM, et al. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11:243-253.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Schurr P, Moulsdale W. Infantile myofibroma. Adv Neonatal Care. 2008;8:13-20.
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16.
- Millot F, Robert A, Bertrand Y, et al. Cutaneous involvement in children with acute lymphoblastic leukemia or lymphoblastic lymphoma. The Children's Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics. 1997;100:60-64.
- Fogelson S, Dohil M. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20:180-191.
The Diagnosis: B-Cell Acute Lymphoblastic Leukemia
A 4-mm punch biopsy of one of the scalp lesions showed a diffuse infiltrate of intermediately sized cells with variably mature chromatin and irregular nuclear contours, consistent with a neoplastic process. Numerous mitotic figures were present, indicating a high proliferation rate (Figure 1). At that time there was no evidence of systemic involvement. A repeat biopsy with concurrent bone marrow biopsy was scheduled 10 days after the patient's initial presentation for further classification. Laboratory studies at that time revealed leukocytosis with elevated neutrophils and lymphocytes as well as a high absolute blast count.
On immunohistochemical staining, the neoplastic cells were positive for CD45, which indicated the neoplasm was hematopoietic, as well as CD10 and the B-cell antigens PAX-5 and CD79a. The cells were negative for CD20, which also is a B-cell marker, but this marker is only expressed in approximately half of pediatric acute lymphoblastic leukemia (ALL) cases with B-cell precursor origin.1 Markers that typically are expressed in B-cell acute lymphoblastic leukemia (B-ALL)--CD34 and terminal deoxynucleotidyl transferase--were both negative. These results were somewhat contradictory, and the differential remained open to both B-ALL and mature B-cell lymphoma. A bone marrow biopsy showed approximately 65% blasts or leukemic cells (Figure 2). Flow cytometry showed the cells were positive for CD10, CD19, weak CD79a, and variable lambda surface antigen expression. The cells were negative for expression of CD20, CD34, terminal deoxynucleotidyl transferase, myeloid antigens, and CD3. Ultimately, the morphology and immunophenotype were most consistent with a diagnosis of B-ALL. Fluorescence in situ hybridization revealed mixed lineage leukemia, MLL, gene rearrangements.
In general, when considering the differential diagnosis of superficial nodules, 5 elements are helpful to consider: the number of nodules (single vs multiple); the location; and the presence or absence of tenderness, pigmentation or erythema, and firmness.2 Our patient had multiple nodules on the scalp, which were erythematous to slightly purple and firm. The differential diagnosis can be categorized into malignant; infectious; and benign inflammatory, vascular, and fibrous tumors.
Potential oncologic processes include leukemia cutis, lymphoblastic leukemia/lymphoma, Langerhans cell histiocytosis, and rhabdomyosarcoma. Initial laboratory test results were reassuring. Infectious processes in the differential include deep fungal infections such as coccidioidomycosis and nontuberculous mycobacterial infections. Coccidioidomycosis was the most likely to cause skin lesions or masses in our patient; however, it was considered less likely because the patient's family had not traveled or been exposed to an endemic area.3
Benign tumors in the differential include deep hemangioma, which was deemed less likely in our patient because most hemangiomas reach 80% of their maximum size by 5 months of age.4 Another possible benign tumor is infantile myofibromatosis, which is rare but is the most common fibrous tumor of infancy.5
Early-onset childhood sarcoidosis also has been shown to produce multiple nontender firm nodules.2 This process was considered unlikely in our patient because not only is the disease relatively rare in the pediatric population, but most reported childhood cases have occurred in patients aged 13 to 15 years.6 Additionally, no uveitis or arthritis was observed in this case.
Ultimately, histopathology and bone marrow biopsy were necessary to determine the diagnosis of B-ALL. Although uncommon, cutaneous involvement can be an early sign of ALL in children.7 Thus, neoplastic etiologies should be considered in the workup of cutaneous nodules in children, especially when these nodules are hard, rapidly growing, ulcerated, fixed, and/or vascular.8 Once the diagnosis is established, initial workup of ALL in children should include complete blood cell count with manual differential, prothrombin time, partial thromboplastin time, electrolytes, uric acid, and renal and liver function tests. Often, baseline viral titers such as cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, hepatitis B virus, and varicella-zoster virus also are included. Patients are risk stratified to the appropriate level of treatment based on tumor immunophenotype, cytogenetic findings, patient age, white blood cell count at the time of diagnosis, and response to initial therapy. Treatment typically is comprised of a multidrug regimen divided into several phases--induction, consolidation, and maintenance--as well as therapy directed to the central nervous system. Treatment protocols usually take 2 to 3 years to complete.
Our patient was treated with 1 dose of intrathecal methotrexate before starting the Interfant-06 protocol with a 7-day methylprednisolone prophase. The patient's nodules shrank over time and were no longer present after 14 days of treatment.
The Diagnosis: B-Cell Acute Lymphoblastic Leukemia
A 4-mm punch biopsy of one of the scalp lesions showed a diffuse infiltrate of intermediately sized cells with variably mature chromatin and irregular nuclear contours, consistent with a neoplastic process. Numerous mitotic figures were present, indicating a high proliferation rate (Figure 1). At that time there was no evidence of systemic involvement. A repeat biopsy with concurrent bone marrow biopsy was scheduled 10 days after the patient's initial presentation for further classification. Laboratory studies at that time revealed leukocytosis with elevated neutrophils and lymphocytes as well as a high absolute blast count.
On immunohistochemical staining, the neoplastic cells were positive for CD45, which indicated the neoplasm was hematopoietic, as well as CD10 and the B-cell antigens PAX-5 and CD79a. The cells were negative for CD20, which also is a B-cell marker, but this marker is only expressed in approximately half of pediatric acute lymphoblastic leukemia (ALL) cases with B-cell precursor origin.1 Markers that typically are expressed in B-cell acute lymphoblastic leukemia (B-ALL)--CD34 and terminal deoxynucleotidyl transferase--were both negative. These results were somewhat contradictory, and the differential remained open to both B-ALL and mature B-cell lymphoma. A bone marrow biopsy showed approximately 65% blasts or leukemic cells (Figure 2). Flow cytometry showed the cells were positive for CD10, CD19, weak CD79a, and variable lambda surface antigen expression. The cells were negative for expression of CD20, CD34, terminal deoxynucleotidyl transferase, myeloid antigens, and CD3. Ultimately, the morphology and immunophenotype were most consistent with a diagnosis of B-ALL. Fluorescence in situ hybridization revealed mixed lineage leukemia, MLL, gene rearrangements.
In general, when considering the differential diagnosis of superficial nodules, 5 elements are helpful to consider: the number of nodules (single vs multiple); the location; and the presence or absence of tenderness, pigmentation or erythema, and firmness.2 Our patient had multiple nodules on the scalp, which were erythematous to slightly purple and firm. The differential diagnosis can be categorized into malignant; infectious; and benign inflammatory, vascular, and fibrous tumors.
Potential oncologic processes include leukemia cutis, lymphoblastic leukemia/lymphoma, Langerhans cell histiocytosis, and rhabdomyosarcoma. Initial laboratory test results were reassuring. Infectious processes in the differential include deep fungal infections such as coccidioidomycosis and nontuberculous mycobacterial infections. Coccidioidomycosis was the most likely to cause skin lesions or masses in our patient; however, it was considered less likely because the patient's family had not traveled or been exposed to an endemic area.3
Benign tumors in the differential include deep hemangioma, which was deemed less likely in our patient because most hemangiomas reach 80% of their maximum size by 5 months of age.4 Another possible benign tumor is infantile myofibromatosis, which is rare but is the most common fibrous tumor of infancy.5
Early-onset childhood sarcoidosis also has been shown to produce multiple nontender firm nodules.2 This process was considered unlikely in our patient because not only is the disease relatively rare in the pediatric population, but most reported childhood cases have occurred in patients aged 13 to 15 years.6 Additionally, no uveitis or arthritis was observed in this case.
Ultimately, histopathology and bone marrow biopsy were necessary to determine the diagnosis of B-ALL. Although uncommon, cutaneous involvement can be an early sign of ALL in children.7 Thus, neoplastic etiologies should be considered in the workup of cutaneous nodules in children, especially when these nodules are hard, rapidly growing, ulcerated, fixed, and/or vascular.8 Once the diagnosis is established, initial workup of ALL in children should include complete blood cell count with manual differential, prothrombin time, partial thromboplastin time, electrolytes, uric acid, and renal and liver function tests. Often, baseline viral titers such as cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, hepatitis B virus, and varicella-zoster virus also are included. Patients are risk stratified to the appropriate level of treatment based on tumor immunophenotype, cytogenetic findings, patient age, white blood cell count at the time of diagnosis, and response to initial therapy. Treatment typically is comprised of a multidrug regimen divided into several phases--induction, consolidation, and maintenance--as well as therapy directed to the central nervous system. Treatment protocols usually take 2 to 3 years to complete.
Our patient was treated with 1 dose of intrathecal methotrexate before starting the Interfant-06 protocol with a 7-day methylprednisolone prophase. The patient's nodules shrank over time and were no longer present after 14 days of treatment.
- Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982-3988.
- Whelan JP, Zembowicz A. Case records of the Massachusetts General Hospital. case 19-2006. a 22-month-old boy with the rapid growth of subcutaneous nodules. N Engl J Med. 2006;354:2697-2704.
- Malo J, Luraschi-Monjagatta C, Wolk DM, et al. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11:243-253.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Schurr P, Moulsdale W. Infantile myofibroma. Adv Neonatal Care. 2008;8:13-20.
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16.
- Millot F, Robert A, Bertrand Y, et al. Cutaneous involvement in children with acute lymphoblastic leukemia or lymphoblastic lymphoma. The Children's Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics. 1997;100:60-64.
- Fogelson S, Dohil M. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20:180-191.
- Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982-3988.
- Whelan JP, Zembowicz A. Case records of the Massachusetts General Hospital. case 19-2006. a 22-month-old boy with the rapid growth of subcutaneous nodules. N Engl J Med. 2006;354:2697-2704.
- Malo J, Luraschi-Monjagatta C, Wolk DM, et al. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11:243-253.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Schurr P, Moulsdale W. Infantile myofibroma. Adv Neonatal Care. 2008;8:13-20.
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16.
- Millot F, Robert A, Bertrand Y, et al. Cutaneous involvement in children with acute lymphoblastic leukemia or lymphoblastic lymphoma. The Children's Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics. 1997;100:60-64.
- Fogelson S, Dohil M. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20:180-191.
An 8-month-old infant girl presented with rapidly growing cutaneous nodules on the scalp of 1 month's duration. Her parents reported that she disliked lying flat but was otherwise growing and developing normally. Nondiagnostic ultrasonography of the head and brain had been performed as well as a skull radiograph, which found no evidence of lytic lesions. On physical examination, 3 erythematous to violaceous, subcutaneous, firm, fixed nodules were observed on the scalp. Notable cervical lymphadenopathy with several distinct, fixed, firm, subcutaneous nodules in the postauricular lymph chains also were noted. The patient had no pertinent medical history and was born via normal spontaneous vaginal delivery to healthy parents. The remainder of the physical examination and review of systems was negative.