User login
Human T-Lymphotropic Virus 1 Associated With Adult T-Cell Leukemia/Lymphoma
Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1 (HTLV-1),1-3 which is increasing in incidence in areas of the United States with large immigrant populations.4 Human T-lymphotrophic virus 1 infection is asymptomatic in most patients and has been associated with ATLL as well as tropical spastic paraparesis.5 We present a case of rapid-onset ATLL in an 82-year-old Japanese man who had immigrated to the United States.
Case Report
An 82-year-old Japanese man who had immigrated to the United States presented with papules and nodules on the neck, trunk, and arms of 4 weeks’ duration. Minimal pruritus was associated with the lesions, which were otherwise asymptomatic. The patient reported that he was generally healthy, and a review of systems was negative.
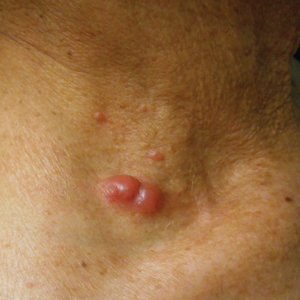
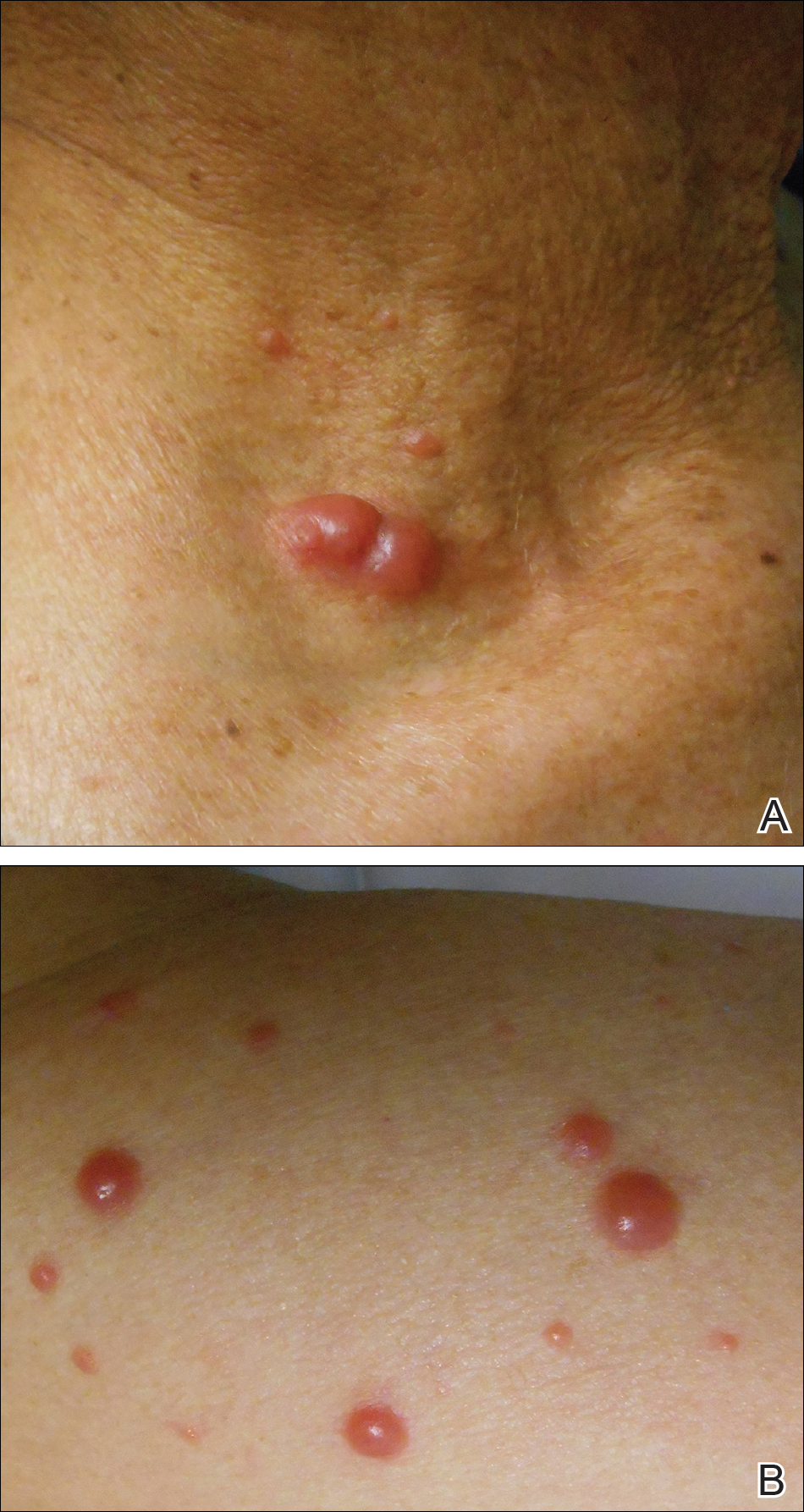
Physical examination revealed numerous erythematous and violaceous papules and nodules on the right side of the neck (Figure 1A), chest, back, abdomen, groin, left arm (Figure 1B), and medial thighs. Bilateral axillary and inguinal lymphadenopathy also was noted.
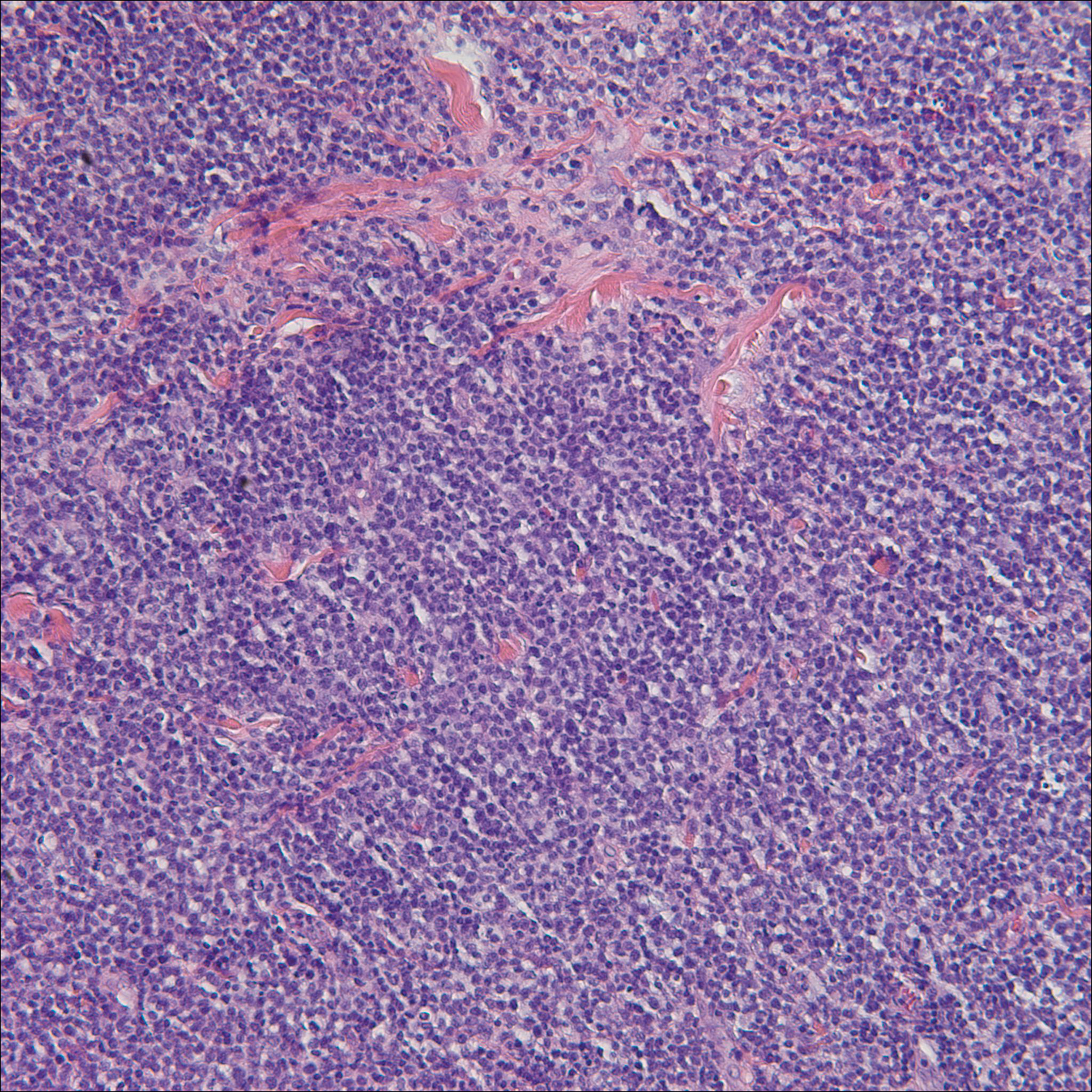
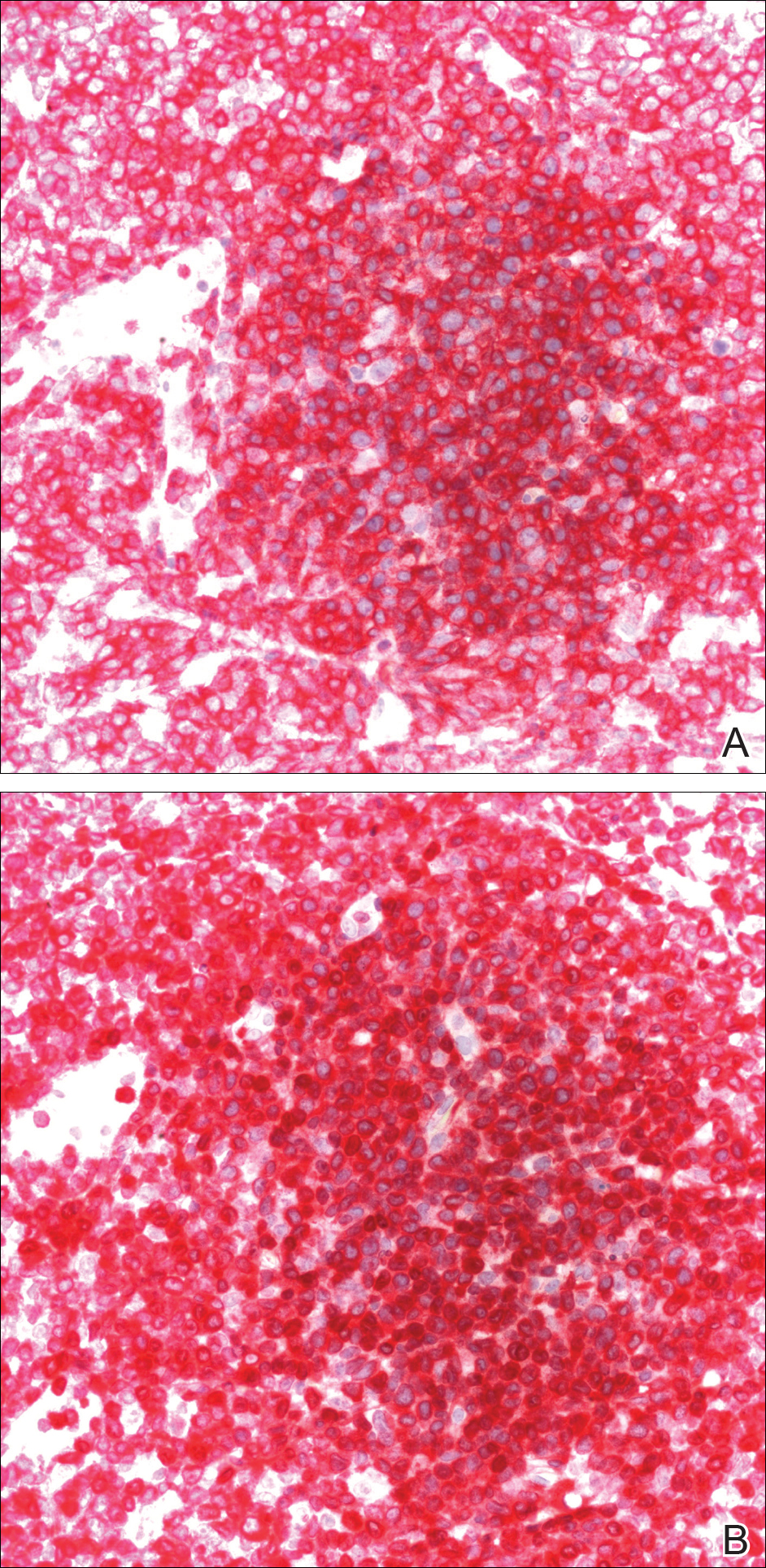
A biopsy from the abdomen revealed a dense, atypical, pandermal lymphoid infiltrate comprised of medium-sized lymphocytes with oval nuclei, fine chromatin, and pale cytoplasm (Figure 2). Mitotic figures and apoptotic cells also were observed. Immunostaining was strongly and diffusely positive for CD4 (Figure 3A), B-cell lymphoma 2 (Bcl-2)(Figure 3B), CD3, and programmed death 1, and was negative for CD8, CD10, CD20, CD30, and myeloperoxidase.
A bone marrow biopsy revealed an atypical T-cell population on flow cytometry. Western blot analysis for HTLV-1 antibodies was positive. Complete blood cell count and complete metabolic panel were within reference range.
Clinical and histopathologic findings fit the diagnosis of ATLL. The patient was referred to hematology/oncology, but the rapid progression of lesions continued, and the patient died within 4 months of initial presentation.
Comment
Etiology
First described in 1977, ATLL is an uncommon neoplasm of mature T cells.6 The etiology is associated with infection by the retrovirus HTLV-1, which is endemic in Southern Japan, the Caribbean, Central and West Africa, and Central and South America, with increasing incidence in areas of the United States with large immigrant populations.7 The incidence of ATLL among all registered lymphoma cases from 2003 to 2008 in Japan was 8.3% compared to 0.2% in the United States.7
Transmission of HTLV-1
Human T-lymphotropic virus 1 is a retrovirus most commonly found in CD4+T cells and can be transmitted through breast milk, sexual intercourse, and blood exposure (eg, blood transfusion), with breastfeeding and blood exposure being the most common.8-10 Human T-lymphotrophic virus 1 has been described as the causative agent for 3 entities: (1) ATLL, (2) a nervous system degenerative disorder known as HTLV-1–associated myelopathy or tropical spastic paraparesis, and (3) HTLV-1 uveitis.5,11 It is thought that 10 to 20 million individuals worldwide are infected with HTLV-1.12
The evolution from infection with HTLV-1 to ATLL is thought to involve multiple steps.13,14 Those who contract the virus later in life rarely, if ever, develop ATLL, suggesting that this progression requires considerable time to evolve to carcinogenesis. More than 90% of those infected with HTLV-1 remain asymptomatic, while only 2% to 3% of women and 6% to 7% of men develop ATLL with a median incubation period greater than 15 to 20 years.7
Subtypes
Adult T-cell leukemia/lymphoma has been divided into 4 clinical subtypes based on clinical presentation and prognosis.15 The acute type is more aggressive and has a poorer prognosis, while the chronic and smoldering types have a more indolent course. The smoldering variant largely has only cutaneous involvement with less than 1% of the peripheral leukocytes being atypical lymphocytes.16 A cutaneous subtype in which few to no leukemic cells are present also has been described and may overlap with the smoldering variant.The cutaneous variant has been further classified into 2 subtypes, tumoral and erythematopapular, with the tumoral subtype carrying a worse prognosis.17,18 Clinically, 39% to 57% of ATLL cases have skin involvement, with nearly one-third reporting skin manifestations as the first symptom.19,20 The cutaneous manifestations vary greatly and may include papules, plaques, nodules, tumors, erythematous patches, or erythroderma.4,21 In addition to skin manifestations, most patients with acute ATLL demonstrate leukemia, lymphadenopathy, organomegaly, and hypercalcemia.22
Histopathology
Histologically, both the smoldering and chronic forms of tumoral or erythematopapular ATLL demonstrate a cutaneous, dermal, or subcutaneous infiltrate of small- to medium-sized CD4+ T cells with histiocytes and admixed granulomas.4 Epidermotropism and Pautrier microabscesses often are limited or absent but can be seen.
Differential Diagnosis
The differential diagnosis includes other small- or medium-sized T-cell lymphomas. The chronic and smoldering types can be difficult to distinguish from mycosis fungoides.
Treatment
Treatment decisions should be made based on the subclassification and prognostic factors at the time of diagnosis. High doses of interferon alfa and zidovudine may show some benefit, but many cases require multiagent chemotherapy.22 The only possible curative treatment is allogeneic stem cell transplant. Mogamulizumab, an antichemokine receptor 4 monoclonal antibody, has demonstrated some ATLL antitumor activity.24
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481-492.
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retro-virus particles form fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415-7419.
- Hinuma Y, Gotoh Y, Sugamura K, et al. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gan. 1982;73:341-344.
- Marchetti MA, Pulitzer MP, Myskowski PL, et al. Cutaneous manifestations of human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a single-center, retrospective study. J Am Acad Dermatol. 2015;72:293-301.
- Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407-410.
- Takatsuki K, Uchiyama T, Sagawa K, et al. Adult T cell leukemia in Japan. In: Seno S, Takasu F, Irino S, eds. Topics in Hematology. Amsterdam, Netherlands: Excerpta Medica; 1977:73-77.
- Yoshida N, Chihara D. Incidence of adult T-cell leukemia/lymphoma in nonendemic areas. Curr Treat Options Oncol. 2015;16:7.
- Tajima K, Tominaga S, Suchi T, et al. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan. 1982;73:893-901.
- Kajiyama W, Kashiwagi S, Ikematsu H, et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986;154:851-857.
- Ichimaru M, Ikeda S, Kinoshita K, et al. Mother-to-child transmission of HTLV-1. Cancer Detect Prev. 1991;15:177-181.
- Lyra-da-Silva JO, de Mello Gonzaga YB, de Melo Espíndola O, et al. Adult t-cell leukemia/lymphoma: a case report of primary cutaneous tumoral type. Dermatol Pract Concept. 2012;2:202a03.
- Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J Emerg Med. 2000;18:109-119.
- Magalhaes M, Oliveira PD, Bittencourt AL, et al. Microsatellite alterations are also present in the less aggressive types of adult T-cell leukemia-lymphoma. PLoS Negl Trop Dis. 2015;9:e0003403.
- Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191-195.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428-437.
- Takahashi K, Tanaka T, Fujita M, et al. Cutaneous-type adult T-cell leukemia lymphoma. a unique clinical feature with monoclonal T-cell proliferation detected by Southern blot analysis Arch Dermatol. 1988;124:399-404.
- Amano M, Kurokawa M, Ogata K, et al. New entity, definition and diagnostic criteria of cutaneous adult T-cell leukemia/lymphoma: human T-lymphotropic virus type 1 proviral DNA load can distinguish between cutaneous and smoldering types. J Dermatol. 2008;35:270-275.
- Johno M, Ohishi M, Kojo Y, et al. Cutaneous manifestations of adult T-cell leukemia lymphoma. Gann Monogr Cancer Res. 1992;39:33-42.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: a report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991;79:428-437.
- Levine PH, Manns A, Jaffe ES, et al. The effect of ethnic differences on the pattern of HTLV-I-associated T-cell leukemia/lymphoma (HATL) in the United States. Int J Cancer. 1994;56:177-181.
- Pezeshkpoor F, Yazdanpanah MJ, Shirdel A. Specific cutaneous manifestations in adult T-cell leukemia/lymphoma. Int J Dermatol. 2008;47:359-362.
- Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453-459.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-4130.
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837-842.
Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1 (HTLV-1),1-3 which is increasing in incidence in areas of the United States with large immigrant populations.4 Human T-lymphotrophic virus 1 infection is asymptomatic in most patients and has been associated with ATLL as well as tropical spastic paraparesis.5 We present a case of rapid-onset ATLL in an 82-year-old Japanese man who had immigrated to the United States.
Case Report
An 82-year-old Japanese man who had immigrated to the United States presented with papules and nodules on the neck, trunk, and arms of 4 weeks’ duration. Minimal pruritus was associated with the lesions, which were otherwise asymptomatic. The patient reported that he was generally healthy, and a review of systems was negative.
Physical examination revealed numerous erythematous and violaceous papules and nodules on the right side of the neck (Figure 1A), chest, back, abdomen, groin, left arm (Figure 1B), and medial thighs. Bilateral axillary and inguinal lymphadenopathy also was noted.

A biopsy from the abdomen revealed a dense, atypical, pandermal lymphoid infiltrate comprised of medium-sized lymphocytes with oval nuclei, fine chromatin, and pale cytoplasm (Figure 2). Mitotic figures and apoptotic cells also were observed. Immunostaining was strongly and diffusely positive for CD4 (Figure 3A), B-cell lymphoma 2 (Bcl-2)(Figure 3B), CD3, and programmed death 1, and was negative for CD8, CD10, CD20, CD30, and myeloperoxidase.


A bone marrow biopsy revealed an atypical T-cell population on flow cytometry. Western blot analysis for HTLV-1 antibodies was positive. Complete blood cell count and complete metabolic panel were within reference range.
Clinical and histopathologic findings fit the diagnosis of ATLL. The patient was referred to hematology/oncology, but the rapid progression of lesions continued, and the patient died within 4 months of initial presentation.
Comment
Etiology
First described in 1977, ATLL is an uncommon neoplasm of mature T cells.6 The etiology is associated with infection by the retrovirus HTLV-1, which is endemic in Southern Japan, the Caribbean, Central and West Africa, and Central and South America, with increasing incidence in areas of the United States with large immigrant populations.7 The incidence of ATLL among all registered lymphoma cases from 2003 to 2008 in Japan was 8.3% compared to 0.2% in the United States.7
Transmission of HTLV-1
Human T-lymphotropic virus 1 is a retrovirus most commonly found in CD4+T cells and can be transmitted through breast milk, sexual intercourse, and blood exposure (eg, blood transfusion), with breastfeeding and blood exposure being the most common.8-10 Human T-lymphotrophic virus 1 has been described as the causative agent for 3 entities: (1) ATLL, (2) a nervous system degenerative disorder known as HTLV-1–associated myelopathy or tropical spastic paraparesis, and (3) HTLV-1 uveitis.5,11 It is thought that 10 to 20 million individuals worldwide are infected with HTLV-1.12
The evolution from infection with HTLV-1 to ATLL is thought to involve multiple steps.13,14 Those who contract the virus later in life rarely, if ever, develop ATLL, suggesting that this progression requires considerable time to evolve to carcinogenesis. More than 90% of those infected with HTLV-1 remain asymptomatic, while only 2% to 3% of women and 6% to 7% of men develop ATLL with a median incubation period greater than 15 to 20 years.7
Subtypes
Adult T-cell leukemia/lymphoma has been divided into 4 clinical subtypes based on clinical presentation and prognosis.15 The acute type is more aggressive and has a poorer prognosis, while the chronic and smoldering types have a more indolent course. The smoldering variant largely has only cutaneous involvement with less than 1% of the peripheral leukocytes being atypical lymphocytes.16 A cutaneous subtype in which few to no leukemic cells are present also has been described and may overlap with the smoldering variant.The cutaneous variant has been further classified into 2 subtypes, tumoral and erythematopapular, with the tumoral subtype carrying a worse prognosis.17,18 Clinically, 39% to 57% of ATLL cases have skin involvement, with nearly one-third reporting skin manifestations as the first symptom.19,20 The cutaneous manifestations vary greatly and may include papules, plaques, nodules, tumors, erythematous patches, or erythroderma.4,21 In addition to skin manifestations, most patients with acute ATLL demonstrate leukemia, lymphadenopathy, organomegaly, and hypercalcemia.22
Histopathology
Histologically, both the smoldering and chronic forms of tumoral or erythematopapular ATLL demonstrate a cutaneous, dermal, or subcutaneous infiltrate of small- to medium-sized CD4+ T cells with histiocytes and admixed granulomas.4 Epidermotropism and Pautrier microabscesses often are limited or absent but can be seen.
Differential Diagnosis
The differential diagnosis includes other small- or medium-sized T-cell lymphomas. The chronic and smoldering types can be difficult to distinguish from mycosis fungoides.
Treatment
Treatment decisions should be made based on the subclassification and prognostic factors at the time of diagnosis. High doses of interferon alfa and zidovudine may show some benefit, but many cases require multiagent chemotherapy.22 The only possible curative treatment is allogeneic stem cell transplant. Mogamulizumab, an antichemokine receptor 4 monoclonal antibody, has demonstrated some ATLL antitumor activity.24
Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1 (HTLV-1),1-3 which is increasing in incidence in areas of the United States with large immigrant populations.4 Human T-lymphotrophic virus 1 infection is asymptomatic in most patients and has been associated with ATLL as well as tropical spastic paraparesis.5 We present a case of rapid-onset ATLL in an 82-year-old Japanese man who had immigrated to the United States.
Case Report
An 82-year-old Japanese man who had immigrated to the United States presented with papules and nodules on the neck, trunk, and arms of 4 weeks’ duration. Minimal pruritus was associated with the lesions, which were otherwise asymptomatic. The patient reported that he was generally healthy, and a review of systems was negative.
Physical examination revealed numerous erythematous and violaceous papules and nodules on the right side of the neck (Figure 1A), chest, back, abdomen, groin, left arm (Figure 1B), and medial thighs. Bilateral axillary and inguinal lymphadenopathy also was noted.

A biopsy from the abdomen revealed a dense, atypical, pandermal lymphoid infiltrate comprised of medium-sized lymphocytes with oval nuclei, fine chromatin, and pale cytoplasm (Figure 2). Mitotic figures and apoptotic cells also were observed. Immunostaining was strongly and diffusely positive for CD4 (Figure 3A), B-cell lymphoma 2 (Bcl-2)(Figure 3B), CD3, and programmed death 1, and was negative for CD8, CD10, CD20, CD30, and myeloperoxidase.


A bone marrow biopsy revealed an atypical T-cell population on flow cytometry. Western blot analysis for HTLV-1 antibodies was positive. Complete blood cell count and complete metabolic panel were within reference range.
Clinical and histopathologic findings fit the diagnosis of ATLL. The patient was referred to hematology/oncology, but the rapid progression of lesions continued, and the patient died within 4 months of initial presentation.
Comment
Etiology
First described in 1977, ATLL is an uncommon neoplasm of mature T cells.6 The etiology is associated with infection by the retrovirus HTLV-1, which is endemic in Southern Japan, the Caribbean, Central and West Africa, and Central and South America, with increasing incidence in areas of the United States with large immigrant populations.7 The incidence of ATLL among all registered lymphoma cases from 2003 to 2008 in Japan was 8.3% compared to 0.2% in the United States.7
Transmission of HTLV-1
Human T-lymphotropic virus 1 is a retrovirus most commonly found in CD4+T cells and can be transmitted through breast milk, sexual intercourse, and blood exposure (eg, blood transfusion), with breastfeeding and blood exposure being the most common.8-10 Human T-lymphotrophic virus 1 has been described as the causative agent for 3 entities: (1) ATLL, (2) a nervous system degenerative disorder known as HTLV-1–associated myelopathy or tropical spastic paraparesis, and (3) HTLV-1 uveitis.5,11 It is thought that 10 to 20 million individuals worldwide are infected with HTLV-1.12
The evolution from infection with HTLV-1 to ATLL is thought to involve multiple steps.13,14 Those who contract the virus later in life rarely, if ever, develop ATLL, suggesting that this progression requires considerable time to evolve to carcinogenesis. More than 90% of those infected with HTLV-1 remain asymptomatic, while only 2% to 3% of women and 6% to 7% of men develop ATLL with a median incubation period greater than 15 to 20 years.7
Subtypes
Adult T-cell leukemia/lymphoma has been divided into 4 clinical subtypes based on clinical presentation and prognosis.15 The acute type is more aggressive and has a poorer prognosis, while the chronic and smoldering types have a more indolent course. The smoldering variant largely has only cutaneous involvement with less than 1% of the peripheral leukocytes being atypical lymphocytes.16 A cutaneous subtype in which few to no leukemic cells are present also has been described and may overlap with the smoldering variant.The cutaneous variant has been further classified into 2 subtypes, tumoral and erythematopapular, with the tumoral subtype carrying a worse prognosis.17,18 Clinically, 39% to 57% of ATLL cases have skin involvement, with nearly one-third reporting skin manifestations as the first symptom.19,20 The cutaneous manifestations vary greatly and may include papules, plaques, nodules, tumors, erythematous patches, or erythroderma.4,21 In addition to skin manifestations, most patients with acute ATLL demonstrate leukemia, lymphadenopathy, organomegaly, and hypercalcemia.22
Histopathology
Histologically, both the smoldering and chronic forms of tumoral or erythematopapular ATLL demonstrate a cutaneous, dermal, or subcutaneous infiltrate of small- to medium-sized CD4+ T cells with histiocytes and admixed granulomas.4 Epidermotropism and Pautrier microabscesses often are limited or absent but can be seen.
Differential Diagnosis
The differential diagnosis includes other small- or medium-sized T-cell lymphomas. The chronic and smoldering types can be difficult to distinguish from mycosis fungoides.
Treatment
Treatment decisions should be made based on the subclassification and prognostic factors at the time of diagnosis. High doses of interferon alfa and zidovudine may show some benefit, but many cases require multiagent chemotherapy.22 The only possible curative treatment is allogeneic stem cell transplant. Mogamulizumab, an antichemokine receptor 4 monoclonal antibody, has demonstrated some ATLL antitumor activity.24
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481-492.
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retro-virus particles form fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415-7419.
- Hinuma Y, Gotoh Y, Sugamura K, et al. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gan. 1982;73:341-344.
- Marchetti MA, Pulitzer MP, Myskowski PL, et al. Cutaneous manifestations of human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a single-center, retrospective study. J Am Acad Dermatol. 2015;72:293-301.
- Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407-410.
- Takatsuki K, Uchiyama T, Sagawa K, et al. Adult T cell leukemia in Japan. In: Seno S, Takasu F, Irino S, eds. Topics in Hematology. Amsterdam, Netherlands: Excerpta Medica; 1977:73-77.
- Yoshida N, Chihara D. Incidence of adult T-cell leukemia/lymphoma in nonendemic areas. Curr Treat Options Oncol. 2015;16:7.
- Tajima K, Tominaga S, Suchi T, et al. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan. 1982;73:893-901.
- Kajiyama W, Kashiwagi S, Ikematsu H, et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986;154:851-857.
- Ichimaru M, Ikeda S, Kinoshita K, et al. Mother-to-child transmission of HTLV-1. Cancer Detect Prev. 1991;15:177-181.
- Lyra-da-Silva JO, de Mello Gonzaga YB, de Melo Espíndola O, et al. Adult t-cell leukemia/lymphoma: a case report of primary cutaneous tumoral type. Dermatol Pract Concept. 2012;2:202a03.
- Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J Emerg Med. 2000;18:109-119.
- Magalhaes M, Oliveira PD, Bittencourt AL, et al. Microsatellite alterations are also present in the less aggressive types of adult T-cell leukemia-lymphoma. PLoS Negl Trop Dis. 2015;9:e0003403.
- Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191-195.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428-437.
- Takahashi K, Tanaka T, Fujita M, et al. Cutaneous-type adult T-cell leukemia lymphoma. a unique clinical feature with monoclonal T-cell proliferation detected by Southern blot analysis Arch Dermatol. 1988;124:399-404.
- Amano M, Kurokawa M, Ogata K, et al. New entity, definition and diagnostic criteria of cutaneous adult T-cell leukemia/lymphoma: human T-lymphotropic virus type 1 proviral DNA load can distinguish between cutaneous and smoldering types. J Dermatol. 2008;35:270-275.
- Johno M, Ohishi M, Kojo Y, et al. Cutaneous manifestations of adult T-cell leukemia lymphoma. Gann Monogr Cancer Res. 1992;39:33-42.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: a report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991;79:428-437.
- Levine PH, Manns A, Jaffe ES, et al. The effect of ethnic differences on the pattern of HTLV-I-associated T-cell leukemia/lymphoma (HATL) in the United States. Int J Cancer. 1994;56:177-181.
- Pezeshkpoor F, Yazdanpanah MJ, Shirdel A. Specific cutaneous manifestations in adult T-cell leukemia/lymphoma. Int J Dermatol. 2008;47:359-362.
- Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453-459.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-4130.
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837-842.
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481-492.
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retro-virus particles form fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415-7419.
- Hinuma Y, Gotoh Y, Sugamura K, et al. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gan. 1982;73:341-344.
- Marchetti MA, Pulitzer MP, Myskowski PL, et al. Cutaneous manifestations of human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a single-center, retrospective study. J Am Acad Dermatol. 2015;72:293-301.
- Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407-410.
- Takatsuki K, Uchiyama T, Sagawa K, et al. Adult T cell leukemia in Japan. In: Seno S, Takasu F, Irino S, eds. Topics in Hematology. Amsterdam, Netherlands: Excerpta Medica; 1977:73-77.
- Yoshida N, Chihara D. Incidence of adult T-cell leukemia/lymphoma in nonendemic areas. Curr Treat Options Oncol. 2015;16:7.
- Tajima K, Tominaga S, Suchi T, et al. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan. 1982;73:893-901.
- Kajiyama W, Kashiwagi S, Ikematsu H, et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986;154:851-857.
- Ichimaru M, Ikeda S, Kinoshita K, et al. Mother-to-child transmission of HTLV-1. Cancer Detect Prev. 1991;15:177-181.
- Lyra-da-Silva JO, de Mello Gonzaga YB, de Melo Espíndola O, et al. Adult t-cell leukemia/lymphoma: a case report of primary cutaneous tumoral type. Dermatol Pract Concept. 2012;2:202a03.
- Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J Emerg Med. 2000;18:109-119.
- Magalhaes M, Oliveira PD, Bittencourt AL, et al. Microsatellite alterations are also present in the less aggressive types of adult T-cell leukemia-lymphoma. PLoS Negl Trop Dis. 2015;9:e0003403.
- Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191-195.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428-437.
- Takahashi K, Tanaka T, Fujita M, et al. Cutaneous-type adult T-cell leukemia lymphoma. a unique clinical feature with monoclonal T-cell proliferation detected by Southern blot analysis Arch Dermatol. 1988;124:399-404.
- Amano M, Kurokawa M, Ogata K, et al. New entity, definition and diagnostic criteria of cutaneous adult T-cell leukemia/lymphoma: human T-lymphotropic virus type 1 proviral DNA load can distinguish between cutaneous and smoldering types. J Dermatol. 2008;35:270-275.
- Johno M, Ohishi M, Kojo Y, et al. Cutaneous manifestations of adult T-cell leukemia lymphoma. Gann Monogr Cancer Res. 1992;39:33-42.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: a report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991;79:428-437.
- Levine PH, Manns A, Jaffe ES, et al. The effect of ethnic differences on the pattern of HTLV-I-associated T-cell leukemia/lymphoma (HATL) in the United States. Int J Cancer. 1994;56:177-181.
- Pezeshkpoor F, Yazdanpanah MJ, Shirdel A. Specific cutaneous manifestations in adult T-cell leukemia/lymphoma. Int J Dermatol. 2008;47:359-362.
- Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453-459.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-4130.
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837-842.
Practice Points
- Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1.
- In the United States, ATLL is increasing in incidence in areas with large immigrant populations.
- High suspicion and clinical features must be present to make the diagnosis of ATLL due to considerable histologic overlap with other cutaneous T-cell lymphomas.
Reticulated erythematous patch on teenager’s foot
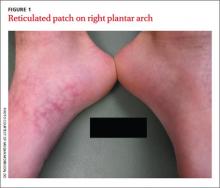
An 18-year-old Caucasian male sought care for an ill-defined reticulated patch on his right plantar arch (FIGURE 1). The patient said that the lesion had gradually appeared 2 years earlier, had grown slowly, and was occasionally itchy. Physical exam revealed a lacy violaceous, hyperpigmented, reticulated patch that was blanchable and nontender to palpation.
Our patient denied having a history of trauma to the area or a coagulation or connective tissue disorder. The lesion didn’t vary with temperature or season, and there were no known triggers. The patient’s left plantar arch was unchanged.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema ab igne
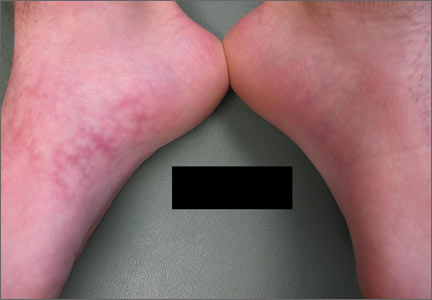
Upon further questioning, the patient acknowledged that he occasionally rested his bare feet around a portable heater under his desk while using his computer for a few hours each day (FIGURE 2). He often kept his right foot on the heater while he let his left foot rest on the ground. A punch biopsy was performed; the findings, when combined with the patient’s report of having exposed his foot to heat, supported the diagnosis of erythema ab igne (EAI).
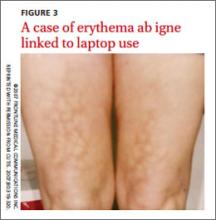
EAI commonly presents as an asymptomatic reticulated erythematous to violaceous patch in an area of the body that has been in contact with heat.1 It originally was described on the bilateral anterior lower extremities after prolonged exposure to burning stoves or open fires.1 With the advent of central heating, these presentations have decreased, but there has been a resurgence of EAI with atypical distributions as a result of evolving technology and new heating sources. Reported causes of EAI include heating pads,1,2 laptop computers3 (FIGURE 3), car seat heaters,4 hot water bottles, popcorn bags, cell phones,5 and space heaters that have resulted in patches on the breast, thighs, arms, and, in our patient, foot.1-5
Blood work, biopsy can help narrow the differential
The differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectasia. Livedo reticularis can be associated with autoimmune conditions and coagulopathies. Livedo racemosa is a typical sign of Sneddon’s syndrome and can be seen in up to 70% of patients with antiphospholipid-antibody syndrome and systemic lupus erythematosus. Diagnosis of these conditions is confirmed by elevated coagulation factors, presence of autoimmune antibodies, or history of cerebrovascular accident.6 These tests would be normal in EAI.
Histopathologic changes observed in EAI include an atrophic epidermis with an interface dermatitis, vasodilation, and dermal pigmentation. Necrotic keratinocytes and focal hyperkeratosis can be noted, along with squamous atypia. Although these changes are nonspecific, they can be used to confirm an EAI diagnosis in patients for whom the affected area has been exposed to a heat source.
Histologically, EAI is similar to actinic keratosis, with epidermal changes showing squamous atypia.2 Due to the similarities, these lesions are sometimes referred to as “thermal keratosis.” Some researchers have suggested that the thermal heat may induce epithelial changes in the same way that ultraviolet light produces epithelial changes.7
Rarely, EAI can turn into cancer. There have been a few reported cases of EAI transforming into squamous cell carcinoma or Merkel cell carcinoma; squamous cell carcinoma is more common, and tends to occur after a long latent period (up to 30 years).7-9 EAI lesions often begin as a chronic ulcer and tend not to heal. If the lesion continues to evolve (ie, ulcerate), a biopsy may be warranted to rule out a malignant transformation.
Eliminate heat exposure, consider a topical treatment
Treatment of acute EAI involves eliminating the offending heat source. The hyperpigmentation will slowly resolve over months to years.4 Persistent exposure to heat sources can lead to chronic EAI, which is more difficult to eliminate.
Because hyperpigmentation can be visually unappealing and emotionally distressing, some patients prefer active treatment. EAI has been effectively treated with 4% hydroquinone topical cream twice a day and tretinoin topical cream at night.2,10,11 Lesions that have epithelial atypia have improved with 5-fluorouracil topical cream.7
EAI also has been successfully treated with laser therapy with the 1064-nm Q-switched Nd:YAG laser with low fluence at 2-week intervals.9
Our patient declined topical therapy. He improved after a few months of avoiding the heater under his desk.
CORRESPONDENCE
Megan Morrison, DO, 5333 McAuley Drive Suite R-5003, Ypsilanti, MI 48197; memorrison10@gmail.com
1. Huynh N, Sarma D, Huerter C. Erythema ab igne: a case report and review of the literature. Cutis. 2011;88:290-292.
2. Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
3. Fu LW, Vender R. Erythema ab igne caused by laptop computer gaming—a case report. Int J Dermatol. 2012;51:716-717.
4. Brodell D, Mostow EN. Automobile seat heater-induced erythema ab igne. Arch Dermtol. 2012;148:264-265.
5. Dela Rosa K, Satter EK. Erythematous patches on the chest. Arch Dermatol. 2012;148:113-118.
6. Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
7. Bilic M, Adams B. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974.
8. Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1998;124:110-113.
9. Cho S, Jung JY, Lee JH. Erythema ab igne successfully treated using 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet laser with low fluence. Dermatol Surg. 2011;37:551-553.
10. Cardona LFC, Parsons AC, Sangueza OP. Erythematous lesions on the back of a man: challenge. Erythema ab igne. Am J Dermatopathol. 2011;33:185,199.
11. Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
An 18-year-old Caucasian male sought care for an ill-defined reticulated patch on his right plantar arch (FIGURE 1). The patient said that the lesion had gradually appeared 2 years earlier, had grown slowly, and was occasionally itchy. Physical exam revealed a lacy violaceous, hyperpigmented, reticulated patch that was blanchable and nontender to palpation.
Our patient denied having a history of trauma to the area or a coagulation or connective tissue disorder. The lesion didn’t vary with temperature or season, and there were no known triggers. The patient’s left plantar arch was unchanged.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema ab igne
Upon further questioning, the patient acknowledged that he occasionally rested his bare feet around a portable heater under his desk while using his computer for a few hours each day (FIGURE 2). He often kept his right foot on the heater while he let his left foot rest on the ground. A punch biopsy was performed; the findings, when combined with the patient’s report of having exposed his foot to heat, supported the diagnosis of erythema ab igne (EAI).
EAI commonly presents as an asymptomatic reticulated erythematous to violaceous patch in an area of the body that has been in contact with heat.1 It originally was described on the bilateral anterior lower extremities after prolonged exposure to burning stoves or open fires.1 With the advent of central heating, these presentations have decreased, but there has been a resurgence of EAI with atypical distributions as a result of evolving technology and new heating sources. Reported causes of EAI include heating pads,1,2 laptop computers3 (FIGURE 3), car seat heaters,4 hot water bottles, popcorn bags, cell phones,5 and space heaters that have resulted in patches on the breast, thighs, arms, and, in our patient, foot.1-5
Blood work, biopsy can help narrow the differential
The differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectasia. Livedo reticularis can be associated with autoimmune conditions and coagulopathies. Livedo racemosa is a typical sign of Sneddon’s syndrome and can be seen in up to 70% of patients with antiphospholipid-antibody syndrome and systemic lupus erythematosus. Diagnosis of these conditions is confirmed by elevated coagulation factors, presence of autoimmune antibodies, or history of cerebrovascular accident.6 These tests would be normal in EAI.
Histopathologic changes observed in EAI include an atrophic epidermis with an interface dermatitis, vasodilation, and dermal pigmentation. Necrotic keratinocytes and focal hyperkeratosis can be noted, along with squamous atypia. Although these changes are nonspecific, they can be used to confirm an EAI diagnosis in patients for whom the affected area has been exposed to a heat source.
Histologically, EAI is similar to actinic keratosis, with epidermal changes showing squamous atypia.2 Due to the similarities, these lesions are sometimes referred to as “thermal keratosis.” Some researchers have suggested that the thermal heat may induce epithelial changes in the same way that ultraviolet light produces epithelial changes.7
Rarely, EAI can turn into cancer. There have been a few reported cases of EAI transforming into squamous cell carcinoma or Merkel cell carcinoma; squamous cell carcinoma is more common, and tends to occur after a long latent period (up to 30 years).7-9 EAI lesions often begin as a chronic ulcer and tend not to heal. If the lesion continues to evolve (ie, ulcerate), a biopsy may be warranted to rule out a malignant transformation.
Eliminate heat exposure, consider a topical treatment
Treatment of acute EAI involves eliminating the offending heat source. The hyperpigmentation will slowly resolve over months to years.4 Persistent exposure to heat sources can lead to chronic EAI, which is more difficult to eliminate.
Because hyperpigmentation can be visually unappealing and emotionally distressing, some patients prefer active treatment. EAI has been effectively treated with 4% hydroquinone topical cream twice a day and tretinoin topical cream at night.2,10,11 Lesions that have epithelial atypia have improved with 5-fluorouracil topical cream.7
EAI also has been successfully treated with laser therapy with the 1064-nm Q-switched Nd:YAG laser with low fluence at 2-week intervals.9
Our patient declined topical therapy. He improved after a few months of avoiding the heater under his desk.
CORRESPONDENCE
Megan Morrison, DO, 5333 McAuley Drive Suite R-5003, Ypsilanti, MI 48197; memorrison10@gmail.com
An 18-year-old Caucasian male sought care for an ill-defined reticulated patch on his right plantar arch (FIGURE 1). The patient said that the lesion had gradually appeared 2 years earlier, had grown slowly, and was occasionally itchy. Physical exam revealed a lacy violaceous, hyperpigmented, reticulated patch that was blanchable and nontender to palpation.
Our patient denied having a history of trauma to the area or a coagulation or connective tissue disorder. The lesion didn’t vary with temperature or season, and there were no known triggers. The patient’s left plantar arch was unchanged.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema ab igne
Upon further questioning, the patient acknowledged that he occasionally rested his bare feet around a portable heater under his desk while using his computer for a few hours each day (FIGURE 2). He often kept his right foot on the heater while he let his left foot rest on the ground. A punch biopsy was performed; the findings, when combined with the patient’s report of having exposed his foot to heat, supported the diagnosis of erythema ab igne (EAI).
EAI commonly presents as an asymptomatic reticulated erythematous to violaceous patch in an area of the body that has been in contact with heat.1 It originally was described on the bilateral anterior lower extremities after prolonged exposure to burning stoves or open fires.1 With the advent of central heating, these presentations have decreased, but there has been a resurgence of EAI with atypical distributions as a result of evolving technology and new heating sources. Reported causes of EAI include heating pads,1,2 laptop computers3 (FIGURE 3), car seat heaters,4 hot water bottles, popcorn bags, cell phones,5 and space heaters that have resulted in patches on the breast, thighs, arms, and, in our patient, foot.1-5
Blood work, biopsy can help narrow the differential
The differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectasia. Livedo reticularis can be associated with autoimmune conditions and coagulopathies. Livedo racemosa is a typical sign of Sneddon’s syndrome and can be seen in up to 70% of patients with antiphospholipid-antibody syndrome and systemic lupus erythematosus. Diagnosis of these conditions is confirmed by elevated coagulation factors, presence of autoimmune antibodies, or history of cerebrovascular accident.6 These tests would be normal in EAI.
Histopathologic changes observed in EAI include an atrophic epidermis with an interface dermatitis, vasodilation, and dermal pigmentation. Necrotic keratinocytes and focal hyperkeratosis can be noted, along with squamous atypia. Although these changes are nonspecific, they can be used to confirm an EAI diagnosis in patients for whom the affected area has been exposed to a heat source.
Histologically, EAI is similar to actinic keratosis, with epidermal changes showing squamous atypia.2 Due to the similarities, these lesions are sometimes referred to as “thermal keratosis.” Some researchers have suggested that the thermal heat may induce epithelial changes in the same way that ultraviolet light produces epithelial changes.7
Rarely, EAI can turn into cancer. There have been a few reported cases of EAI transforming into squamous cell carcinoma or Merkel cell carcinoma; squamous cell carcinoma is more common, and tends to occur after a long latent period (up to 30 years).7-9 EAI lesions often begin as a chronic ulcer and tend not to heal. If the lesion continues to evolve (ie, ulcerate), a biopsy may be warranted to rule out a malignant transformation.
Eliminate heat exposure, consider a topical treatment
Treatment of acute EAI involves eliminating the offending heat source. The hyperpigmentation will slowly resolve over months to years.4 Persistent exposure to heat sources can lead to chronic EAI, which is more difficult to eliminate.
Because hyperpigmentation can be visually unappealing and emotionally distressing, some patients prefer active treatment. EAI has been effectively treated with 4% hydroquinone topical cream twice a day and tretinoin topical cream at night.2,10,11 Lesions that have epithelial atypia have improved with 5-fluorouracil topical cream.7
EAI also has been successfully treated with laser therapy with the 1064-nm Q-switched Nd:YAG laser with low fluence at 2-week intervals.9
Our patient declined topical therapy. He improved after a few months of avoiding the heater under his desk.
CORRESPONDENCE
Megan Morrison, DO, 5333 McAuley Drive Suite R-5003, Ypsilanti, MI 48197; memorrison10@gmail.com
1. Huynh N, Sarma D, Huerter C. Erythema ab igne: a case report and review of the literature. Cutis. 2011;88:290-292.
2. Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
3. Fu LW, Vender R. Erythema ab igne caused by laptop computer gaming—a case report. Int J Dermatol. 2012;51:716-717.
4. Brodell D, Mostow EN. Automobile seat heater-induced erythema ab igne. Arch Dermtol. 2012;148:264-265.
5. Dela Rosa K, Satter EK. Erythematous patches on the chest. Arch Dermatol. 2012;148:113-118.
6. Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
7. Bilic M, Adams B. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974.
8. Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1998;124:110-113.
9. Cho S, Jung JY, Lee JH. Erythema ab igne successfully treated using 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet laser with low fluence. Dermatol Surg. 2011;37:551-553.
10. Cardona LFC, Parsons AC, Sangueza OP. Erythematous lesions on the back of a man: challenge. Erythema ab igne. Am J Dermatopathol. 2011;33:185,199.
11. Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.
1. Huynh N, Sarma D, Huerter C. Erythema ab igne: a case report and review of the literature. Cutis. 2011;88:290-292.
2. Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
3. Fu LW, Vender R. Erythema ab igne caused by laptop computer gaming—a case report. Int J Dermatol. 2012;51:716-717.
4. Brodell D, Mostow EN. Automobile seat heater-induced erythema ab igne. Arch Dermtol. 2012;148:264-265.
5. Dela Rosa K, Satter EK. Erythematous patches on the chest. Arch Dermatol. 2012;148:113-118.
6. Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
7. Bilic M, Adams B. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974.
8. Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1998;124:110-113.
9. Cho S, Jung JY, Lee JH. Erythema ab igne successfully treated using 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet laser with low fluence. Dermatol Surg. 2011;37:551-553.
10. Cardona LFC, Parsons AC, Sangueza OP. Erythematous lesions on the back of a man: challenge. Erythema ab igne. Am J Dermatopathol. 2011;33:185,199.
11. Sahl WJ, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol. 1992;27:109-110.