User login
Opportunities for Stewardship in the Transition From Intravenous to Enteral Antibiotics in Hospitalized Pediatric Patients
Bacterial infections are a common reason for pediatric hospital admissions in the United States.1 Antibiotics are the mainstay of treatment, and whether to administer them intravenously (IV) or enterally is an important and, at times, challenging decision. Not all hospitalized patients with infections require IV antibiotics, and safe, effective early transitions to enteral therapy have been described for numerous infections.2-7 However, guidelines describing the ideal initial route of antibiotic administration and when to transition to oral therapy are lacking.5,7,8 This lack of high-quality evidence-based guidance may contribute to overuse of IV antibiotics for many hospitalized pediatric patients, even when safe and effective enteral options exist.9
Significant costs and harms are associated with the use of IV antibiotics. In particular, studies have demonstrated longer length of stay (LOS), increased costs, and worsened pain or anxiety related to complications (eg, phlebitis, extravasation injury, thrombosis, catheter-associated bloodstream infections) associated with IV antibiotics.3,4,10-13 Earlier transition to enteral therapy, however, can mitigate these increased risks and costs.
The Centers for Disease Control and Prevention lists the transition from IV to oral antibiotics as a key stewardship intervention for improving antibiotic use.14 The Infectious Diseases Society of America (IDSA) antibiotic stewardship program guidelines strongly recommend the timely conversion from IV to oral antibiotics, stating that efforts focusing on this transition should be integrated into routine practice.15 There are a few metrics in the literature to measure this intervention, but none is universally used, and a modified delphi process could not reach consensus on IV-to-oral transition metrics.16
Few studies describe the opportunity to transition to enteral antibiotics in hospitalized patients with common bacterial infections or explore variation across hospitals. It is critical to understand current practice of antibiotic administration in order to identify opportunities to optimize patient outcomes and promote high-value care. Furthermore, few studies have evaluated the feasibility of IV-to-oral transition metrics using an administrative database. Thus, the aims of this study were to (1) determine opportunities to transition from IV to enteral antibiotics for pediatric patients hospitalized with common bacterial infections based on their ability to tolerate other enteral medications, (2) describe variation in transition practices among children’s hospitals, and (3) evaluate the feasibility of novel IV-to-oral transition metrics using an administrative database to inform stewardship efforts.
METHODS
Study Design and Setting
This multicenter, retrospective cohort study used data from the Pediatric Health Information System (PHIS), an administrative and billing database containing encounter-level data from 52 tertiary care pediatric hospitals across the United States affiliated with the Children’s Hospital Association (Lenexa, Kansas). Hospitals submit encounter-level data, including demographics, medications, and diagnoses based on International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Data were de-identified at the time of submission, and data quality and reliability were assured by joint efforts between the Children’s Hospital Association and participating hospitals.
Study Population
This study included pediatric patients aged 60 days to 18 years who were hospitalized (inpatient or observation status) at one of the participating hospitals between January 1, 2017, and December 31, 2018, for one of the following seven common bacterial infections: community-acquired pneumonia (CAP), neck infection (superficial and deep), periorbital/orbital infection, urinary tract infection (UTI), osteomyelitis, septic arthritis, or skin and soft tissue infection (SSTI). The diagnosis cohorts were defined based on ICD-10-CM discharge diagnoses adapted from previous studies (Appendix Table 1).3,17-23 To define a cohort of generally healthy pediatric patients with an acute infection, we excluded patients hospitalized in the intensive care unit, patients with nonhome discharges, and patients with complex chronic conditions.24 We also excluded hospitals with incomplete data during the study period (n=1). The Institutional Review Board at Cincinnati Children’s Hospital Medical Center determined this study to be non–human-subjects research.
Outcomes
The primary outcomes were the number of opportunity days and the percent of days with opportunity to transition from IV to enteral therapy. Opportunity days, or days in which there was a potential opportunity to transition from IV to enteral antibiotics, were defined as days patients received only IV antibiotic doses and at least one enteral nonantibiotic medication, suggesting an ability to take enteral medications.13 We excluded days patients received IV antibiotics for which there was no enteral alternative (eg, vancomycin, Appendix Table 2). When measuring opportunity, to be conservative (ie, to underestimate rather than overestimate opportunity), we did not count as an opportunity day any day in which patients received both IV and enteral antibiotics. Percent opportunity, or the percent of days patients received antibiotics in which there was potential opportunity to transition from IV to enteral antibiotics, was defined as the number of opportunity days divided by number of inpatient days patients received enteral antibiotics or IV antibiotics with at least one enteral nonantibiotic medication (antibiotic days). Similar to opportunity days, antibiotic days excluded days patients were on IV antibiotics for which there was no enteral alternative. Based on our definition, a lower percent opportunity indicates that a hospital is using enteral antibiotics earlier during the hospitalization (earlier transition), while a higher percent opportunity represents later enteral antibiotic use (later transition).
Statistical Analysis
Demographic and clinical characteristics were summarized by diagnosis with descriptive statistics, including frequency with percentage, mean with standard deviation, and median with interquartile range (IQR). For each diagnosis, we evaluated aggregate opportunity days (sum of opportunity days among all hospitals), opportunity days per encounter, and aggregate percent opportunity using frequencies, mean with standard deviation, and percentages, respectively. We also calculated aggregate opportunity days for diagnosis-antibiotic combinations. To visually show variation in the percent opportunity across hospitals, we displayed the percent opportunity on a heat map, and evaluated percent opportunity across hospitals using chi-square tests. To compare the variability in the percent opportunity across and within hospitals, we used a generalized linear model with two fixed effects (hospital and diagnosis), and parsed the variability using the sum of squares. We performed a sensitivity analysis and excluded days that patients received antiemetic medications (eg, ondansetron, granisetron, prochlorperazine, promethazine), as these suggest potential intolerance of enteral medications. All statistical analyses were performed using SAS v.9.4 (SAS Institute Inc, Cary, North Carolina) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, California), and P values < .05 were considered statistically significant.
RESULTS
During the 2-year study period, 100,103 hospitalizations met our inclusion criteria across 51 hospitals and seven diagnosis categories (Table 1). Diagnosis cohorts ranged in size from 1,462 encounters for septic arthritis to 35,665 encounters for neck infections. Overall, we identified 88,522 aggregate opportunity days on which there was an opportunity to switch from IV to enteral treatment in the majority of participants (percent opportunity, 57%).
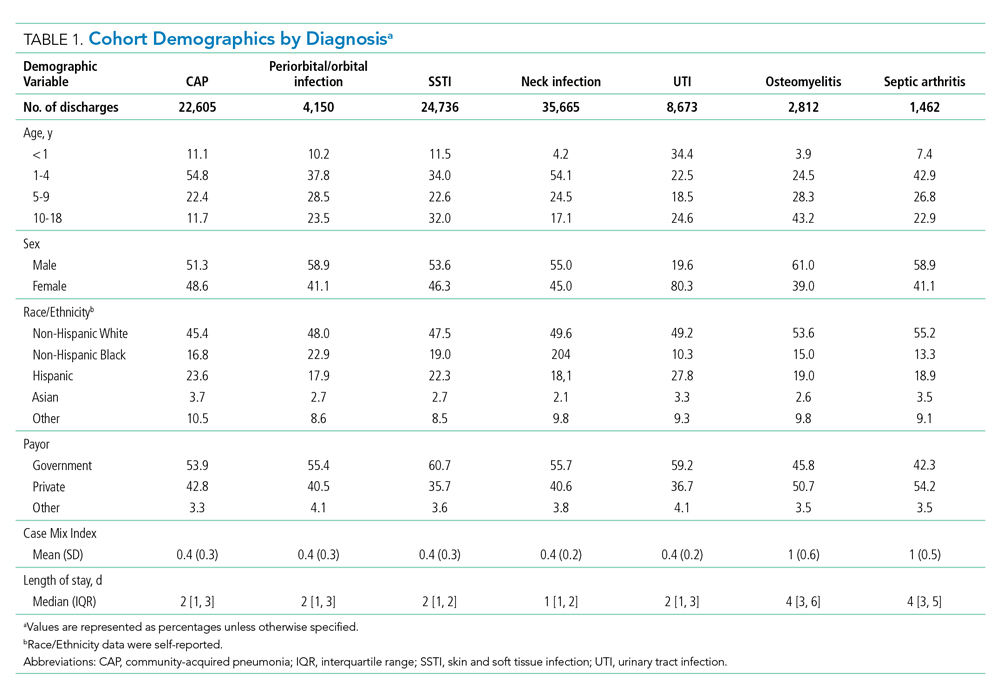
Opportunity by Diagnosis
The number of opportunity days (aggregate and mean per encounter) and percent opportunity varied by diagnosis (Table 2). The aggregate number of opportunity days ranged from 3,693 in patients with septic arthritis to 25,359 in patients with SSTI, and mean opportunity days per encounter ranged from 0.9 in CAP to 2.8 in septic arthritis. Percent opportunity was highest for septic arthritis at 72.7% and lowest for CAP at 39.7%.
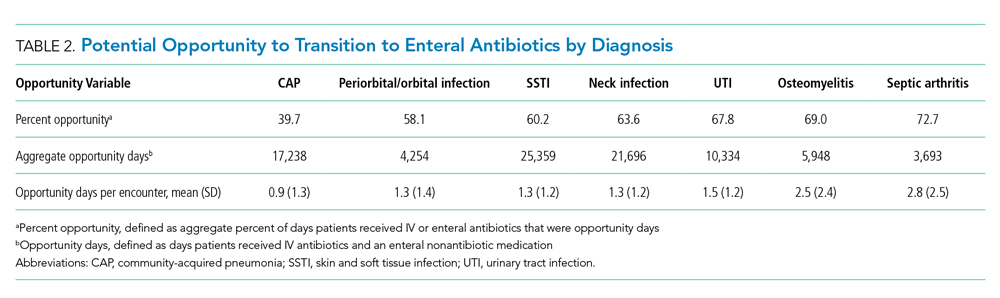
Variation in Opportunity Among Hospitals
The variation in the percent opportunity across hospitals was statistically significant for all diagnoses (Figure). Within hospitals, we observed similar practice patterns across diagnoses. For example, hospitals with a higher percent opportunity for one diagnosis tended to have higher percent opportunity for the other diagnoses (as noted in the top portion of the Figure), and those with lower percent opportunity for one diagnosis tended to also have lower percent opportunity for the other diagnoses studied (as noted in the bottom portion of the Figure). When evaluating variability in the percent opportunity, 45% of the variability was attributable to the hospital-effect and 35% to the diagnosis; the remainder was unexplained variability. Sensitivity analysis excluding days when patients received an antiemetic medication yielded no differences in our results.

Opportunity by Antibiotic
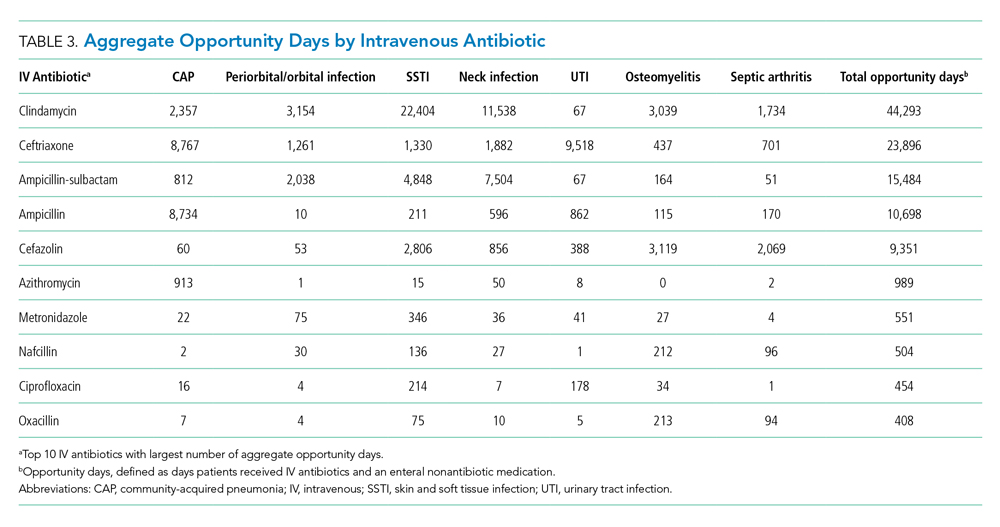
The aggregate number of opportunity days varied by antibiotic (Table 3). Intravenous antibiotics with the largest number of opportunity days included clindamycin (44,293), ceftriaxone (23,896), and ampicillin-sulbactam (15,484). Antibiotic-diagnosis combinations with the largest number of opportunity days for each diagnosis included ceftriaxone and ampicillin in CAP; clindamycin in cellulitis, SSTI, and neck infections; ceftriaxone in UTI; and cefazolin in osteomyelitis and septic arthritis.
DISCUSSION
In this multicenter study of pediatric patients hospitalized with common bacterial infections, there was the potential to transition from IV to enteral treatment in over half of the antibiotic days. The degree of opportunity varied by infection, antibiotic, and hospital. Antibiotics with a large aggregate number of opportunity days for enteral transition included clindamycin, which has excellent bioavailability; and ampicillin and ampicillin-sulbactam, which can achieve pharmacodynamic targets with oral equivalents.25-29 The across-hospital variation for a given diagnosis suggests that certain hospitals have strategies in place which permit an earlier transition to enteral antibiotics compared to other institutions in which there were likely missed opportunities to do so. This variability is likely due to limited evidence, emphasizing the need for robust studies to better understand the optimal initial antibiotic route and transition time. Our findings highlight the need for, and large potential impact of, stewardship efforts to promote earlier transition for specific drug targets. This study also demonstrates the feasibility of obtaining two metrics—percent opportunity and opportunity days—from administrative databases to inform stewardship efforts within and across hospitals.
Opportunity days and percent opportunity varied among diagnoses. The variation in aggregate opportunity days was largely a reflection of the number of encounters: Diagnoses such as SSTI, neck infections, and CAP had a large number of both aggregate opportunity days and encounters. The range of opportunity days per encounter (0.9-2.5) suggests potential missed opportunities to transition to enteral antibiotics across all diagnoses (Table 2). The higher opportunity days per encounter in osteomyelitis and septic arthritis may be related to longer LOS and higher percent opportunity. Percent opportunity likely varied among diagnoses due to differences in admission and discharge readiness criteria, diagnostic evaluation, frequency of antibiotic administration, and evidence on the optimal route of initial antibiotics and when to transition to oral formulations. For example, we hypothesize that certain diagnoses, such as osteomyelitis and septic arthritis, have admission and discharge readiness criteria directly tied to the perceived need for IV antibiotics, which may limit in-hospital days on enteral antibiotics and explain the high percent opportunity that we observed. The high percent opportunity seen in musculoskeletal infections also may be due to delays in initiating targeted treatment until culture results were available. Encounters for CAP had the lowest percent opportunity; we hypothesize that this is because admission and discharge readiness may be determined by factors other than the need for IV antibiotics (eg, need for supplemental oxygen), which may increase days on enteral antibiotics and lead to a lower percent opportunity.30
Urinary tract infection encounters had a high percent opportunity. As with musculoskeletal infection, this may be related to delays in initiating targeted treatment until culture results became available. Another reason for the high percent opportunity in UTI could be the common use of ceftriaxone, which, dosed every 24 hours, likely reduced the opportunity to transition to enteral antibiotics. There is strong evidence demonstrating no difference in outcomes based on antibiotic routes for UTI, and we would expect this to result in a low percent opportunity.2,31 While the observed high opportunity in UTI may relate to an initial unknown diagnosis or concern for systemic infection, this highlights potential opportunities for quality improvement initiatives to promote empiric oral antibiotics in clinically stable patients hospitalized with suspected UTI.
There was substantial variation in percent opportunity across hospitals for a given diagnosis, with less variation across diagnoses for a given hospital. Variation across hospitals but consistency within individual hospitals suggests that some hospitals may promote earlier transition from IV to enteral antibiotics as standard practice for all diagnoses, while other hospitals continue IV antibiotics for the entire hospitalization, highlighting potential missed opportunities at some institutions. While emerging data suggest that traditional long durations of IV antibiotics are not necessary for many infections, the limited evidence on the optimal time to switch to oral antibiotics may have influenced this variation.2-7 Many guidelines recommend initial IV antibiotics for hospitalized pediatric patients, but there are few studies comparing IV and enteral therapy.2,5,9 Limited evidence leaves significant room for hospital culture, antibiotic stewardship efforts, reimbursement considerations, and/or hospital workflow to influence transition timing and overall opportunity at individual hospitals.7,8,32-34 These findings emphasize the importance of research to identify optimal transition time and comparative effectiveness studies to evaluate whether initial IV antibiotics are truly needed for mild—and even severe—disease presentations. Since many patients are admitted for the perceived need for IV antibiotics, earlier use of enteral antibiotics could reduce rates of hospitalizations, LOS, healthcare costs, and resource utilization.
Antibiotics with a high number of opportunity days included clindamycin, ceftriaxone, ampicillin-sublactam, and ampicillin. Our findings are consistent with another study which found that most bioavailable drugs, including clindamycin, were administered via the IV route and accounted for a large number of antibiotic days.35 The Infectious Diseases Society of America recommends that hospitals promote earlier transition to oral formulations for highly bioavailable drugs.7 Given the high bioavailability of clindamycin, its common use in high-frequency encounters such as SSTI and neck infections, and the fact that it accounted for a large number of opportunity days, quality improvement initiatives promoting earlier transition to oral clindamycin could have a large impact across health systems.25,26 Additionally, although beta-lactam antibiotics such as amoxicillin and amoxicillin-sulbactam are not highly bioavailable, oral dosing can achieve sufficient serum concentrations to reach pharmacodynamic targets for common clinical indications; this could be an important quality improvement initiative.27-29 Several single-site studies have successfully implemented quality improvement initiatives to promote earlier IV-to-enteral transition, with resulting reductions in costs and no adverse events noted, highlighting the feasibility and impact of such efforts.13,36-38
This study also demonstrates the feasibility of collecting two metrics (percent opportunity and opportunity days) from administrative databases to inform IV-to-oral transition benchmarking and stewardship efforts. While there are several metrics in the literature for evaluating antibiotic transition (eg, days of IV or oral therapy, percentage of antibiotics given via the oral route, time to switch from IV to oral, and acceptance rate of suggested changes to antibiotic route), none are universally used or agreed upon.15,16,39 The opportunity metrics used in this study have several strengths, including the feasibility of obtaining them from existing databases and the ability to account for intake of other enteral medications; the latter is not evaluated in other metrics. These opportunity metrics can be used together to identify the percent of time in which there is opportunity to transition and total number of days to understand the full extent of potential opportunity for future interventions. As demonstrated in this study, these metrics can be measured by diagnosis, antibiotic, or diagnosis-antibiotic combination, and they can be used to evaluate stewardship efforts at a single institution over time or compare efforts across hospitals.
These findings should be interpreted in the context of important limitations. First, we attempted to characterize potential opportunity to transition to enteral medications based on a patient’s ability to tolerate nonenteral medications. However, there are other factors that could limit the opportunity to transition that we could not account for with an administrative dataset, including the use of antibiotics prior to admission, disease progression, severity of illness, and malabsorptive concerns. Thus, though we may have overestimated the true opportunity to transition to enteral antibiotics, it is unlikely that this would account for all of the variation in transition times that we observed across hospitals. Second, while our study required patients to have one of seven types of infection, we did not exclude any additional infectious diagnoses (eg, concurrent bacteremia, Clostridioides difficile, otitis media) that could have driven the choice of antibiotic type and modality. Although emerging evidence is supporting earlier transitions to oral therapy, bacteremia is typically treated with IV antibiotics; this may have led to an overestimation of true opportunity.40 “Clostridioides” difficile and otitis media are typically treated with enteral therapy; concurrent infections such as these may have led to an underestimation of opportunity given the fact that, based on our definition, the days on which patients received both IV and enteral antibiotics were not counted as opportunity days. Third, because PHIS uses billing days to capture medication use, we were unable to distinguish transitions that occurred early in the day vs those that took place later in the day. This could have led to an underestimation of percent opportunity, particularly for diagnoses with a short LOS; it also likely led to an underestimation of the variability observed across hospitals. Fourth, because we used an administrative dataset, we are unable to understand reasoning behind transitioning time from IV to oral antibiotics, as well as provider, patient, and institutional level factors that influenced these decisions.
CONCLUSION
Children hospitalized with bacterial infections often receive IV antibiotics, and the timing of transition from IV to enteral antibiotics varies significantly across hospitals. Further research is needed to compare the effectiveness of IV and enteral antibiotics and better define criteria for transition to enteral therapy. We identified ample opportunities for quality improvement initiatives to promote earlier transition, which have the potential to reduce healthcare utilization and promote optimal patient-directed high-value care.
1. Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X
3. Keren R, Shah SS, Srivastava R, et al; for the Pediatric Research Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e201692. https://doi.org/10.1542/peds.2016-1692
5. Li HK, Agweyu A, English M, Bejon P. An unsupported preference for intravenous antibiotics. PLoS Med. 2015;12(5):e1001825. https://dx.doi.org/10.1371%2Fjournal.pmed.1001825
6. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393
7. Bradley JS, Byington CL, Shah SS, et al; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Management of community-acquired pneumonia (CAP) in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1542/peds.2011-2385
8. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8-S14. https://doi.org/10.1093/cid/cir363
9. Schroeder AR, Ralston SL. Intravenous antibiotic durations for common bacterial infections in children: when is enough? J Hosp Med. 2014;9(9):604-609. https://doi.org/10.1002/jhm.2239
10. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatric Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003
11. van Zanten AR, Engelfriet PM, van Dillen K, van Veen M, Nuijten MJ, Polderman KH. Importance of nondrug costs of intravenous antibiotic therapy. Crit Care. 2003;7(6):R184-R190. https://doi.org/10.1186/cc2388
12. Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117(4):1210-1215. https://doi.org/10.1542/peds.2005-1465
13. Girdwood SCT, Sellas MN, Courter JD, et al. Improving the transition of intravenous to enteral antibiotics in pediatric patients with pneumonia or skin and soft tissue infections. J Hosp Med. 2020;15(1):10-15. https://doi.org/10.12788/jhm.3253
14. Core Elements of Hospital Antibiotic Stewardship Programs. Centers for Disease Control and Prevention. Published 2019. Accessed May 30, 2020. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
15. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. https://doi.org/10.1093/cid/ciw118
16. Science M, Timberlake K, Morris A, Read S, Le Saux N; Groupe Antibiothérapie en Pédiatrie Canada Alliance for Stewardship of Antimicrobials in Pediatrics (GAP Can ASAP). Quality metrics for antimicrobial stewardship programs. Pediatrics. 2019;143(4):e20182372. https://doi.org/10.1542/peds.2018-2372
17. Tchou MJ, Hall M, Shah SS, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Patterns of electrolyte testing at children’s hospitals for common inpatient diagnoses. Pediatrics. 2019;144(1):e20181644. https://doi.org/10.1542/peds.2018-1644
18. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
19. Desai S, Shah SS, Hall M, Richardson TE, Thomson JE; Pediatric Research in Inpatient Settings (PRIS) Network. Imaging strategies and outcomes in children hospitalized with cervical lymphadenitis. J Hosp Med. 2020;15(4):197-203. https://doi.org/10.12788/jhm.3333
20. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
21. Tieder JS, Hall M, Auger KA, et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128(2):323-330. https://doi.org/10.1542/peds.2010-2064
22. Singh JA, Yu S. The burden of septic arthritis on the U.S. inpatient care: a national study. PLoS One. 2017;12(8):e0182577. https://doi.org/10.1371/journal.pone.0182577
23. Foradori DM, Lopez MA, Hall M, et al. Invasive bacterial infections in infants younger than 60 days with skin and soft tissue infections. Pediatr Emerg Care. 2018. https://doi.org/10.1097/pec.0000000000001584
24. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
25. Arancibia A, Icarte A, González C, Morasso I. Dose-dependent bioavailability of amoxycillin. Int J Clin Pharmacol Ther Toxicol. 1988;26(6):300-303.
26. Grayson ML, Cosgrove S, Crowe S, et al. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. 7th ed. CRC Press; 2018.
27. Downes KJ, Hahn A, Wiles J, Courter JD, Inks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in pediatrics’. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006
28. Gras-Le Guen C, Boscher C, Godon N, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol. 2007;63(7):657-662. https://doi.org/10.1007/s00228-007-0307-3
29. Sanchez Navarro A. New formulations of amoxicillin/clavulanic acid: a pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2005;44(11):1097-1115. https://doi.org/10.2165/00003088-200544110-00001
30. Fine MJ, Hough LJ, Medsger AR, et al. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):36-44. https://doi.org/10.1001/archinte.1997.00440220040006
31. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007(4):CD003237. https://doi.org/10.1002/14651858.cd003237.pub2
32. Nageswaran S, Woods CR, Benjamin DK Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25(8):695-699. https://doi.org/10.1097/01.inf.0000227820.36036.f1
33. Al-Nammari S, Roberton B, Ferguson C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Should a child with preseptal periorbital cellulitis be treated with intravenous or oral antibiotics? Emerg Med J. 2007;24(2):128-129. https://doi.org/10.1136/emj.2006.045245
34. Vieira F, Allen SM, Stocks RMS, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483, vii. https://doi.org/10.1016/j.otc.2008.01.002
35. Smith M, Shah S, Kronman M, Patel S, Thurm C, Hersh AL. Route of administration for highly orally bioavailable antibiotics. Open Forum Infect Dis. 2017;4(Suppl 1):S498-S499. https://doi.org/10.1093/ofid/ofx163.1291
36. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
37. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3
38. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585
39. Public Health Ontario. Antimicrobial stewardship programs metric examples. Published 2017. Accessed June 1, 2020. https://www.publichealthontario.ca/-/media/documents/A/2017/asp-metrics-examples.pdf?la=en
40. Desai S, Aronson PL, Shabanova V, et al; Febrile Young Infant Research Collaborative. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
Bacterial infections are a common reason for pediatric hospital admissions in the United States.1 Antibiotics are the mainstay of treatment, and whether to administer them intravenously (IV) or enterally is an important and, at times, challenging decision. Not all hospitalized patients with infections require IV antibiotics, and safe, effective early transitions to enteral therapy have been described for numerous infections.2-7 However, guidelines describing the ideal initial route of antibiotic administration and when to transition to oral therapy are lacking.5,7,8 This lack of high-quality evidence-based guidance may contribute to overuse of IV antibiotics for many hospitalized pediatric patients, even when safe and effective enteral options exist.9
Significant costs and harms are associated with the use of IV antibiotics. In particular, studies have demonstrated longer length of stay (LOS), increased costs, and worsened pain or anxiety related to complications (eg, phlebitis, extravasation injury, thrombosis, catheter-associated bloodstream infections) associated with IV antibiotics.3,4,10-13 Earlier transition to enteral therapy, however, can mitigate these increased risks and costs.
The Centers for Disease Control and Prevention lists the transition from IV to oral antibiotics as a key stewardship intervention for improving antibiotic use.14 The Infectious Diseases Society of America (IDSA) antibiotic stewardship program guidelines strongly recommend the timely conversion from IV to oral antibiotics, stating that efforts focusing on this transition should be integrated into routine practice.15 There are a few metrics in the literature to measure this intervention, but none is universally used, and a modified delphi process could not reach consensus on IV-to-oral transition metrics.16
Few studies describe the opportunity to transition to enteral antibiotics in hospitalized patients with common bacterial infections or explore variation across hospitals. It is critical to understand current practice of antibiotic administration in order to identify opportunities to optimize patient outcomes and promote high-value care. Furthermore, few studies have evaluated the feasibility of IV-to-oral transition metrics using an administrative database. Thus, the aims of this study were to (1) determine opportunities to transition from IV to enteral antibiotics for pediatric patients hospitalized with common bacterial infections based on their ability to tolerate other enteral medications, (2) describe variation in transition practices among children’s hospitals, and (3) evaluate the feasibility of novel IV-to-oral transition metrics using an administrative database to inform stewardship efforts.
METHODS
Study Design and Setting
This multicenter, retrospective cohort study used data from the Pediatric Health Information System (PHIS), an administrative and billing database containing encounter-level data from 52 tertiary care pediatric hospitals across the United States affiliated with the Children’s Hospital Association (Lenexa, Kansas). Hospitals submit encounter-level data, including demographics, medications, and diagnoses based on International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Data were de-identified at the time of submission, and data quality and reliability were assured by joint efforts between the Children’s Hospital Association and participating hospitals.
Study Population
This study included pediatric patients aged 60 days to 18 years who were hospitalized (inpatient or observation status) at one of the participating hospitals between January 1, 2017, and December 31, 2018, for one of the following seven common bacterial infections: community-acquired pneumonia (CAP), neck infection (superficial and deep), periorbital/orbital infection, urinary tract infection (UTI), osteomyelitis, septic arthritis, or skin and soft tissue infection (SSTI). The diagnosis cohorts were defined based on ICD-10-CM discharge diagnoses adapted from previous studies (Appendix Table 1).3,17-23 To define a cohort of generally healthy pediatric patients with an acute infection, we excluded patients hospitalized in the intensive care unit, patients with nonhome discharges, and patients with complex chronic conditions.24 We also excluded hospitals with incomplete data during the study period (n=1). The Institutional Review Board at Cincinnati Children’s Hospital Medical Center determined this study to be non–human-subjects research.
Outcomes
The primary outcomes were the number of opportunity days and the percent of days with opportunity to transition from IV to enteral therapy. Opportunity days, or days in which there was a potential opportunity to transition from IV to enteral antibiotics, were defined as days patients received only IV antibiotic doses and at least one enteral nonantibiotic medication, suggesting an ability to take enteral medications.13 We excluded days patients received IV antibiotics for which there was no enteral alternative (eg, vancomycin, Appendix Table 2). When measuring opportunity, to be conservative (ie, to underestimate rather than overestimate opportunity), we did not count as an opportunity day any day in which patients received both IV and enteral antibiotics. Percent opportunity, or the percent of days patients received antibiotics in which there was potential opportunity to transition from IV to enteral antibiotics, was defined as the number of opportunity days divided by number of inpatient days patients received enteral antibiotics or IV antibiotics with at least one enteral nonantibiotic medication (antibiotic days). Similar to opportunity days, antibiotic days excluded days patients were on IV antibiotics for which there was no enteral alternative. Based on our definition, a lower percent opportunity indicates that a hospital is using enteral antibiotics earlier during the hospitalization (earlier transition), while a higher percent opportunity represents later enteral antibiotic use (later transition).
Statistical Analysis
Demographic and clinical characteristics were summarized by diagnosis with descriptive statistics, including frequency with percentage, mean with standard deviation, and median with interquartile range (IQR). For each diagnosis, we evaluated aggregate opportunity days (sum of opportunity days among all hospitals), opportunity days per encounter, and aggregate percent opportunity using frequencies, mean with standard deviation, and percentages, respectively. We also calculated aggregate opportunity days for diagnosis-antibiotic combinations. To visually show variation in the percent opportunity across hospitals, we displayed the percent opportunity on a heat map, and evaluated percent opportunity across hospitals using chi-square tests. To compare the variability in the percent opportunity across and within hospitals, we used a generalized linear model with two fixed effects (hospital and diagnosis), and parsed the variability using the sum of squares. We performed a sensitivity analysis and excluded days that patients received antiemetic medications (eg, ondansetron, granisetron, prochlorperazine, promethazine), as these suggest potential intolerance of enteral medications. All statistical analyses were performed using SAS v.9.4 (SAS Institute Inc, Cary, North Carolina) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, California), and P values < .05 were considered statistically significant.
RESULTS
During the 2-year study period, 100,103 hospitalizations met our inclusion criteria across 51 hospitals and seven diagnosis categories (Table 1). Diagnosis cohorts ranged in size from 1,462 encounters for septic arthritis to 35,665 encounters for neck infections. Overall, we identified 88,522 aggregate opportunity days on which there was an opportunity to switch from IV to enteral treatment in the majority of participants (percent opportunity, 57%).

Opportunity by Diagnosis
The number of opportunity days (aggregate and mean per encounter) and percent opportunity varied by diagnosis (Table 2). The aggregate number of opportunity days ranged from 3,693 in patients with septic arthritis to 25,359 in patients with SSTI, and mean opportunity days per encounter ranged from 0.9 in CAP to 2.8 in septic arthritis. Percent opportunity was highest for septic arthritis at 72.7% and lowest for CAP at 39.7%.

Variation in Opportunity Among Hospitals
The variation in the percent opportunity across hospitals was statistically significant for all diagnoses (Figure). Within hospitals, we observed similar practice patterns across diagnoses. For example, hospitals with a higher percent opportunity for one diagnosis tended to have higher percent opportunity for the other diagnoses (as noted in the top portion of the Figure), and those with lower percent opportunity for one diagnosis tended to also have lower percent opportunity for the other diagnoses studied (as noted in the bottom portion of the Figure). When evaluating variability in the percent opportunity, 45% of the variability was attributable to the hospital-effect and 35% to the diagnosis; the remainder was unexplained variability. Sensitivity analysis excluding days when patients received an antiemetic medication yielded no differences in our results.

Opportunity by Antibiotic
The aggregate number of opportunity days varied by antibiotic (Table 3). Intravenous antibiotics with the largest number of opportunity days included clindamycin (44,293), ceftriaxone (23,896), and ampicillin-sulbactam (15,484). Antibiotic-diagnosis combinations with the largest number of opportunity days for each diagnosis included ceftriaxone and ampicillin in CAP; clindamycin in cellulitis, SSTI, and neck infections; ceftriaxone in UTI; and cefazolin in osteomyelitis and septic arthritis.

DISCUSSION
In this multicenter study of pediatric patients hospitalized with common bacterial infections, there was the potential to transition from IV to enteral treatment in over half of the antibiotic days. The degree of opportunity varied by infection, antibiotic, and hospital. Antibiotics with a large aggregate number of opportunity days for enteral transition included clindamycin, which has excellent bioavailability; and ampicillin and ampicillin-sulbactam, which can achieve pharmacodynamic targets with oral equivalents.25-29 The across-hospital variation for a given diagnosis suggests that certain hospitals have strategies in place which permit an earlier transition to enteral antibiotics compared to other institutions in which there were likely missed opportunities to do so. This variability is likely due to limited evidence, emphasizing the need for robust studies to better understand the optimal initial antibiotic route and transition time. Our findings highlight the need for, and large potential impact of, stewardship efforts to promote earlier transition for specific drug targets. This study also demonstrates the feasibility of obtaining two metrics—percent opportunity and opportunity days—from administrative databases to inform stewardship efforts within and across hospitals.
Opportunity days and percent opportunity varied among diagnoses. The variation in aggregate opportunity days was largely a reflection of the number of encounters: Diagnoses such as SSTI, neck infections, and CAP had a large number of both aggregate opportunity days and encounters. The range of opportunity days per encounter (0.9-2.5) suggests potential missed opportunities to transition to enteral antibiotics across all diagnoses (Table 2). The higher opportunity days per encounter in osteomyelitis and septic arthritis may be related to longer LOS and higher percent opportunity. Percent opportunity likely varied among diagnoses due to differences in admission and discharge readiness criteria, diagnostic evaluation, frequency of antibiotic administration, and evidence on the optimal route of initial antibiotics and when to transition to oral formulations. For example, we hypothesize that certain diagnoses, such as osteomyelitis and septic arthritis, have admission and discharge readiness criteria directly tied to the perceived need for IV antibiotics, which may limit in-hospital days on enteral antibiotics and explain the high percent opportunity that we observed. The high percent opportunity seen in musculoskeletal infections also may be due to delays in initiating targeted treatment until culture results were available. Encounters for CAP had the lowest percent opportunity; we hypothesize that this is because admission and discharge readiness may be determined by factors other than the need for IV antibiotics (eg, need for supplemental oxygen), which may increase days on enteral antibiotics and lead to a lower percent opportunity.30
Urinary tract infection encounters had a high percent opportunity. As with musculoskeletal infection, this may be related to delays in initiating targeted treatment until culture results became available. Another reason for the high percent opportunity in UTI could be the common use of ceftriaxone, which, dosed every 24 hours, likely reduced the opportunity to transition to enteral antibiotics. There is strong evidence demonstrating no difference in outcomes based on antibiotic routes for UTI, and we would expect this to result in a low percent opportunity.2,31 While the observed high opportunity in UTI may relate to an initial unknown diagnosis or concern for systemic infection, this highlights potential opportunities for quality improvement initiatives to promote empiric oral antibiotics in clinically stable patients hospitalized with suspected UTI.
There was substantial variation in percent opportunity across hospitals for a given diagnosis, with less variation across diagnoses for a given hospital. Variation across hospitals but consistency within individual hospitals suggests that some hospitals may promote earlier transition from IV to enteral antibiotics as standard practice for all diagnoses, while other hospitals continue IV antibiotics for the entire hospitalization, highlighting potential missed opportunities at some institutions. While emerging data suggest that traditional long durations of IV antibiotics are not necessary for many infections, the limited evidence on the optimal time to switch to oral antibiotics may have influenced this variation.2-7 Many guidelines recommend initial IV antibiotics for hospitalized pediatric patients, but there are few studies comparing IV and enteral therapy.2,5,9 Limited evidence leaves significant room for hospital culture, antibiotic stewardship efforts, reimbursement considerations, and/or hospital workflow to influence transition timing and overall opportunity at individual hospitals.7,8,32-34 These findings emphasize the importance of research to identify optimal transition time and comparative effectiveness studies to evaluate whether initial IV antibiotics are truly needed for mild—and even severe—disease presentations. Since many patients are admitted for the perceived need for IV antibiotics, earlier use of enteral antibiotics could reduce rates of hospitalizations, LOS, healthcare costs, and resource utilization.
Antibiotics with a high number of opportunity days included clindamycin, ceftriaxone, ampicillin-sublactam, and ampicillin. Our findings are consistent with another study which found that most bioavailable drugs, including clindamycin, were administered via the IV route and accounted for a large number of antibiotic days.35 The Infectious Diseases Society of America recommends that hospitals promote earlier transition to oral formulations for highly bioavailable drugs.7 Given the high bioavailability of clindamycin, its common use in high-frequency encounters such as SSTI and neck infections, and the fact that it accounted for a large number of opportunity days, quality improvement initiatives promoting earlier transition to oral clindamycin could have a large impact across health systems.25,26 Additionally, although beta-lactam antibiotics such as amoxicillin and amoxicillin-sulbactam are not highly bioavailable, oral dosing can achieve sufficient serum concentrations to reach pharmacodynamic targets for common clinical indications; this could be an important quality improvement initiative.27-29 Several single-site studies have successfully implemented quality improvement initiatives to promote earlier IV-to-enteral transition, with resulting reductions in costs and no adverse events noted, highlighting the feasibility and impact of such efforts.13,36-38
This study also demonstrates the feasibility of collecting two metrics (percent opportunity and opportunity days) from administrative databases to inform IV-to-oral transition benchmarking and stewardship efforts. While there are several metrics in the literature for evaluating antibiotic transition (eg, days of IV or oral therapy, percentage of antibiotics given via the oral route, time to switch from IV to oral, and acceptance rate of suggested changes to antibiotic route), none are universally used or agreed upon.15,16,39 The opportunity metrics used in this study have several strengths, including the feasibility of obtaining them from existing databases and the ability to account for intake of other enteral medications; the latter is not evaluated in other metrics. These opportunity metrics can be used together to identify the percent of time in which there is opportunity to transition and total number of days to understand the full extent of potential opportunity for future interventions. As demonstrated in this study, these metrics can be measured by diagnosis, antibiotic, or diagnosis-antibiotic combination, and they can be used to evaluate stewardship efforts at a single institution over time or compare efforts across hospitals.
These findings should be interpreted in the context of important limitations. First, we attempted to characterize potential opportunity to transition to enteral medications based on a patient’s ability to tolerate nonenteral medications. However, there are other factors that could limit the opportunity to transition that we could not account for with an administrative dataset, including the use of antibiotics prior to admission, disease progression, severity of illness, and malabsorptive concerns. Thus, though we may have overestimated the true opportunity to transition to enteral antibiotics, it is unlikely that this would account for all of the variation in transition times that we observed across hospitals. Second, while our study required patients to have one of seven types of infection, we did not exclude any additional infectious diagnoses (eg, concurrent bacteremia, Clostridioides difficile, otitis media) that could have driven the choice of antibiotic type and modality. Although emerging evidence is supporting earlier transitions to oral therapy, bacteremia is typically treated with IV antibiotics; this may have led to an overestimation of true opportunity.40 “Clostridioides” difficile and otitis media are typically treated with enteral therapy; concurrent infections such as these may have led to an underestimation of opportunity given the fact that, based on our definition, the days on which patients received both IV and enteral antibiotics were not counted as opportunity days. Third, because PHIS uses billing days to capture medication use, we were unable to distinguish transitions that occurred early in the day vs those that took place later in the day. This could have led to an underestimation of percent opportunity, particularly for diagnoses with a short LOS; it also likely led to an underestimation of the variability observed across hospitals. Fourth, because we used an administrative dataset, we are unable to understand reasoning behind transitioning time from IV to oral antibiotics, as well as provider, patient, and institutional level factors that influenced these decisions.
CONCLUSION
Children hospitalized with bacterial infections often receive IV antibiotics, and the timing of transition from IV to enteral antibiotics varies significantly across hospitals. Further research is needed to compare the effectiveness of IV and enteral antibiotics and better define criteria for transition to enteral therapy. We identified ample opportunities for quality improvement initiatives to promote earlier transition, which have the potential to reduce healthcare utilization and promote optimal patient-directed high-value care.
Bacterial infections are a common reason for pediatric hospital admissions in the United States.1 Antibiotics are the mainstay of treatment, and whether to administer them intravenously (IV) or enterally is an important and, at times, challenging decision. Not all hospitalized patients with infections require IV antibiotics, and safe, effective early transitions to enteral therapy have been described for numerous infections.2-7 However, guidelines describing the ideal initial route of antibiotic administration and when to transition to oral therapy are lacking.5,7,8 This lack of high-quality evidence-based guidance may contribute to overuse of IV antibiotics for many hospitalized pediatric patients, even when safe and effective enteral options exist.9
Significant costs and harms are associated with the use of IV antibiotics. In particular, studies have demonstrated longer length of stay (LOS), increased costs, and worsened pain or anxiety related to complications (eg, phlebitis, extravasation injury, thrombosis, catheter-associated bloodstream infections) associated with IV antibiotics.3,4,10-13 Earlier transition to enteral therapy, however, can mitigate these increased risks and costs.
The Centers for Disease Control and Prevention lists the transition from IV to oral antibiotics as a key stewardship intervention for improving antibiotic use.14 The Infectious Diseases Society of America (IDSA) antibiotic stewardship program guidelines strongly recommend the timely conversion from IV to oral antibiotics, stating that efforts focusing on this transition should be integrated into routine practice.15 There are a few metrics in the literature to measure this intervention, but none is universally used, and a modified delphi process could not reach consensus on IV-to-oral transition metrics.16
Few studies describe the opportunity to transition to enteral antibiotics in hospitalized patients with common bacterial infections or explore variation across hospitals. It is critical to understand current practice of antibiotic administration in order to identify opportunities to optimize patient outcomes and promote high-value care. Furthermore, few studies have evaluated the feasibility of IV-to-oral transition metrics using an administrative database. Thus, the aims of this study were to (1) determine opportunities to transition from IV to enteral antibiotics for pediatric patients hospitalized with common bacterial infections based on their ability to tolerate other enteral medications, (2) describe variation in transition practices among children’s hospitals, and (3) evaluate the feasibility of novel IV-to-oral transition metrics using an administrative database to inform stewardship efforts.
METHODS
Study Design and Setting
This multicenter, retrospective cohort study used data from the Pediatric Health Information System (PHIS), an administrative and billing database containing encounter-level data from 52 tertiary care pediatric hospitals across the United States affiliated with the Children’s Hospital Association (Lenexa, Kansas). Hospitals submit encounter-level data, including demographics, medications, and diagnoses based on International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Data were de-identified at the time of submission, and data quality and reliability were assured by joint efforts between the Children’s Hospital Association and participating hospitals.
Study Population
This study included pediatric patients aged 60 days to 18 years who were hospitalized (inpatient or observation status) at one of the participating hospitals between January 1, 2017, and December 31, 2018, for one of the following seven common bacterial infections: community-acquired pneumonia (CAP), neck infection (superficial and deep), periorbital/orbital infection, urinary tract infection (UTI), osteomyelitis, septic arthritis, or skin and soft tissue infection (SSTI). The diagnosis cohorts were defined based on ICD-10-CM discharge diagnoses adapted from previous studies (Appendix Table 1).3,17-23 To define a cohort of generally healthy pediatric patients with an acute infection, we excluded patients hospitalized in the intensive care unit, patients with nonhome discharges, and patients with complex chronic conditions.24 We also excluded hospitals with incomplete data during the study period (n=1). The Institutional Review Board at Cincinnati Children’s Hospital Medical Center determined this study to be non–human-subjects research.
Outcomes
The primary outcomes were the number of opportunity days and the percent of days with opportunity to transition from IV to enteral therapy. Opportunity days, or days in which there was a potential opportunity to transition from IV to enteral antibiotics, were defined as days patients received only IV antibiotic doses and at least one enteral nonantibiotic medication, suggesting an ability to take enteral medications.13 We excluded days patients received IV antibiotics for which there was no enteral alternative (eg, vancomycin, Appendix Table 2). When measuring opportunity, to be conservative (ie, to underestimate rather than overestimate opportunity), we did not count as an opportunity day any day in which patients received both IV and enteral antibiotics. Percent opportunity, or the percent of days patients received antibiotics in which there was potential opportunity to transition from IV to enteral antibiotics, was defined as the number of opportunity days divided by number of inpatient days patients received enteral antibiotics or IV antibiotics with at least one enteral nonantibiotic medication (antibiotic days). Similar to opportunity days, antibiotic days excluded days patients were on IV antibiotics for which there was no enteral alternative. Based on our definition, a lower percent opportunity indicates that a hospital is using enteral antibiotics earlier during the hospitalization (earlier transition), while a higher percent opportunity represents later enteral antibiotic use (later transition).
Statistical Analysis
Demographic and clinical characteristics were summarized by diagnosis with descriptive statistics, including frequency with percentage, mean with standard deviation, and median with interquartile range (IQR). For each diagnosis, we evaluated aggregate opportunity days (sum of opportunity days among all hospitals), opportunity days per encounter, and aggregate percent opportunity using frequencies, mean with standard deviation, and percentages, respectively. We also calculated aggregate opportunity days for diagnosis-antibiotic combinations. To visually show variation in the percent opportunity across hospitals, we displayed the percent opportunity on a heat map, and evaluated percent opportunity across hospitals using chi-square tests. To compare the variability in the percent opportunity across and within hospitals, we used a generalized linear model with two fixed effects (hospital and diagnosis), and parsed the variability using the sum of squares. We performed a sensitivity analysis and excluded days that patients received antiemetic medications (eg, ondansetron, granisetron, prochlorperazine, promethazine), as these suggest potential intolerance of enteral medications. All statistical analyses were performed using SAS v.9.4 (SAS Institute Inc, Cary, North Carolina) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, California), and P values < .05 were considered statistically significant.
RESULTS
During the 2-year study period, 100,103 hospitalizations met our inclusion criteria across 51 hospitals and seven diagnosis categories (Table 1). Diagnosis cohorts ranged in size from 1,462 encounters for septic arthritis to 35,665 encounters for neck infections. Overall, we identified 88,522 aggregate opportunity days on which there was an opportunity to switch from IV to enteral treatment in the majority of participants (percent opportunity, 57%).

Opportunity by Diagnosis
The number of opportunity days (aggregate and mean per encounter) and percent opportunity varied by diagnosis (Table 2). The aggregate number of opportunity days ranged from 3,693 in patients with septic arthritis to 25,359 in patients with SSTI, and mean opportunity days per encounter ranged from 0.9 in CAP to 2.8 in septic arthritis. Percent opportunity was highest for septic arthritis at 72.7% and lowest for CAP at 39.7%.

Variation in Opportunity Among Hospitals
The variation in the percent opportunity across hospitals was statistically significant for all diagnoses (Figure). Within hospitals, we observed similar practice patterns across diagnoses. For example, hospitals with a higher percent opportunity for one diagnosis tended to have higher percent opportunity for the other diagnoses (as noted in the top portion of the Figure), and those with lower percent opportunity for one diagnosis tended to also have lower percent opportunity for the other diagnoses studied (as noted in the bottom portion of the Figure). When evaluating variability in the percent opportunity, 45% of the variability was attributable to the hospital-effect and 35% to the diagnosis; the remainder was unexplained variability. Sensitivity analysis excluding days when patients received an antiemetic medication yielded no differences in our results.

Opportunity by Antibiotic
The aggregate number of opportunity days varied by antibiotic (Table 3). Intravenous antibiotics with the largest number of opportunity days included clindamycin (44,293), ceftriaxone (23,896), and ampicillin-sulbactam (15,484). Antibiotic-diagnosis combinations with the largest number of opportunity days for each diagnosis included ceftriaxone and ampicillin in CAP; clindamycin in cellulitis, SSTI, and neck infections; ceftriaxone in UTI; and cefazolin in osteomyelitis and septic arthritis.

DISCUSSION
In this multicenter study of pediatric patients hospitalized with common bacterial infections, there was the potential to transition from IV to enteral treatment in over half of the antibiotic days. The degree of opportunity varied by infection, antibiotic, and hospital. Antibiotics with a large aggregate number of opportunity days for enteral transition included clindamycin, which has excellent bioavailability; and ampicillin and ampicillin-sulbactam, which can achieve pharmacodynamic targets with oral equivalents.25-29 The across-hospital variation for a given diagnosis suggests that certain hospitals have strategies in place which permit an earlier transition to enteral antibiotics compared to other institutions in which there were likely missed opportunities to do so. This variability is likely due to limited evidence, emphasizing the need for robust studies to better understand the optimal initial antibiotic route and transition time. Our findings highlight the need for, and large potential impact of, stewardship efforts to promote earlier transition for specific drug targets. This study also demonstrates the feasibility of obtaining two metrics—percent opportunity and opportunity days—from administrative databases to inform stewardship efforts within and across hospitals.
Opportunity days and percent opportunity varied among diagnoses. The variation in aggregate opportunity days was largely a reflection of the number of encounters: Diagnoses such as SSTI, neck infections, and CAP had a large number of both aggregate opportunity days and encounters. The range of opportunity days per encounter (0.9-2.5) suggests potential missed opportunities to transition to enteral antibiotics across all diagnoses (Table 2). The higher opportunity days per encounter in osteomyelitis and septic arthritis may be related to longer LOS and higher percent opportunity. Percent opportunity likely varied among diagnoses due to differences in admission and discharge readiness criteria, diagnostic evaluation, frequency of antibiotic administration, and evidence on the optimal route of initial antibiotics and when to transition to oral formulations. For example, we hypothesize that certain diagnoses, such as osteomyelitis and septic arthritis, have admission and discharge readiness criteria directly tied to the perceived need for IV antibiotics, which may limit in-hospital days on enteral antibiotics and explain the high percent opportunity that we observed. The high percent opportunity seen in musculoskeletal infections also may be due to delays in initiating targeted treatment until culture results were available. Encounters for CAP had the lowest percent opportunity; we hypothesize that this is because admission and discharge readiness may be determined by factors other than the need for IV antibiotics (eg, need for supplemental oxygen), which may increase days on enteral antibiotics and lead to a lower percent opportunity.30
Urinary tract infection encounters had a high percent opportunity. As with musculoskeletal infection, this may be related to delays in initiating targeted treatment until culture results became available. Another reason for the high percent opportunity in UTI could be the common use of ceftriaxone, which, dosed every 24 hours, likely reduced the opportunity to transition to enteral antibiotics. There is strong evidence demonstrating no difference in outcomes based on antibiotic routes for UTI, and we would expect this to result in a low percent opportunity.2,31 While the observed high opportunity in UTI may relate to an initial unknown diagnosis or concern for systemic infection, this highlights potential opportunities for quality improvement initiatives to promote empiric oral antibiotics in clinically stable patients hospitalized with suspected UTI.
There was substantial variation in percent opportunity across hospitals for a given diagnosis, with less variation across diagnoses for a given hospital. Variation across hospitals but consistency within individual hospitals suggests that some hospitals may promote earlier transition from IV to enteral antibiotics as standard practice for all diagnoses, while other hospitals continue IV antibiotics for the entire hospitalization, highlighting potential missed opportunities at some institutions. While emerging data suggest that traditional long durations of IV antibiotics are not necessary for many infections, the limited evidence on the optimal time to switch to oral antibiotics may have influenced this variation.2-7 Many guidelines recommend initial IV antibiotics for hospitalized pediatric patients, but there are few studies comparing IV and enteral therapy.2,5,9 Limited evidence leaves significant room for hospital culture, antibiotic stewardship efforts, reimbursement considerations, and/or hospital workflow to influence transition timing and overall opportunity at individual hospitals.7,8,32-34 These findings emphasize the importance of research to identify optimal transition time and comparative effectiveness studies to evaluate whether initial IV antibiotics are truly needed for mild—and even severe—disease presentations. Since many patients are admitted for the perceived need for IV antibiotics, earlier use of enteral antibiotics could reduce rates of hospitalizations, LOS, healthcare costs, and resource utilization.
Antibiotics with a high number of opportunity days included clindamycin, ceftriaxone, ampicillin-sublactam, and ampicillin. Our findings are consistent with another study which found that most bioavailable drugs, including clindamycin, were administered via the IV route and accounted for a large number of antibiotic days.35 The Infectious Diseases Society of America recommends that hospitals promote earlier transition to oral formulations for highly bioavailable drugs.7 Given the high bioavailability of clindamycin, its common use in high-frequency encounters such as SSTI and neck infections, and the fact that it accounted for a large number of opportunity days, quality improvement initiatives promoting earlier transition to oral clindamycin could have a large impact across health systems.25,26 Additionally, although beta-lactam antibiotics such as amoxicillin and amoxicillin-sulbactam are not highly bioavailable, oral dosing can achieve sufficient serum concentrations to reach pharmacodynamic targets for common clinical indications; this could be an important quality improvement initiative.27-29 Several single-site studies have successfully implemented quality improvement initiatives to promote earlier IV-to-enteral transition, with resulting reductions in costs and no adverse events noted, highlighting the feasibility and impact of such efforts.13,36-38
This study also demonstrates the feasibility of collecting two metrics (percent opportunity and opportunity days) from administrative databases to inform IV-to-oral transition benchmarking and stewardship efforts. While there are several metrics in the literature for evaluating antibiotic transition (eg, days of IV or oral therapy, percentage of antibiotics given via the oral route, time to switch from IV to oral, and acceptance rate of suggested changes to antibiotic route), none are universally used or agreed upon.15,16,39 The opportunity metrics used in this study have several strengths, including the feasibility of obtaining them from existing databases and the ability to account for intake of other enteral medications; the latter is not evaluated in other metrics. These opportunity metrics can be used together to identify the percent of time in which there is opportunity to transition and total number of days to understand the full extent of potential opportunity for future interventions. As demonstrated in this study, these metrics can be measured by diagnosis, antibiotic, or diagnosis-antibiotic combination, and they can be used to evaluate stewardship efforts at a single institution over time or compare efforts across hospitals.
These findings should be interpreted in the context of important limitations. First, we attempted to characterize potential opportunity to transition to enteral medications based on a patient’s ability to tolerate nonenteral medications. However, there are other factors that could limit the opportunity to transition that we could not account for with an administrative dataset, including the use of antibiotics prior to admission, disease progression, severity of illness, and malabsorptive concerns. Thus, though we may have overestimated the true opportunity to transition to enteral antibiotics, it is unlikely that this would account for all of the variation in transition times that we observed across hospitals. Second, while our study required patients to have one of seven types of infection, we did not exclude any additional infectious diagnoses (eg, concurrent bacteremia, Clostridioides difficile, otitis media) that could have driven the choice of antibiotic type and modality. Although emerging evidence is supporting earlier transitions to oral therapy, bacteremia is typically treated with IV antibiotics; this may have led to an overestimation of true opportunity.40 “Clostridioides” difficile and otitis media are typically treated with enteral therapy; concurrent infections such as these may have led to an underestimation of opportunity given the fact that, based on our definition, the days on which patients received both IV and enteral antibiotics were not counted as opportunity days. Third, because PHIS uses billing days to capture medication use, we were unable to distinguish transitions that occurred early in the day vs those that took place later in the day. This could have led to an underestimation of percent opportunity, particularly for diagnoses with a short LOS; it also likely led to an underestimation of the variability observed across hospitals. Fourth, because we used an administrative dataset, we are unable to understand reasoning behind transitioning time from IV to oral antibiotics, as well as provider, patient, and institutional level factors that influenced these decisions.
CONCLUSION
Children hospitalized with bacterial infections often receive IV antibiotics, and the timing of transition from IV to enteral antibiotics varies significantly across hospitals. Further research is needed to compare the effectiveness of IV and enteral antibiotics and better define criteria for transition to enteral therapy. We identified ample opportunities for quality improvement initiatives to promote earlier transition, which have the potential to reduce healthcare utilization and promote optimal patient-directed high-value care.
1. Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X
3. Keren R, Shah SS, Srivastava R, et al; for the Pediatric Research Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e201692. https://doi.org/10.1542/peds.2016-1692
5. Li HK, Agweyu A, English M, Bejon P. An unsupported preference for intravenous antibiotics. PLoS Med. 2015;12(5):e1001825. https://dx.doi.org/10.1371%2Fjournal.pmed.1001825
6. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393
7. Bradley JS, Byington CL, Shah SS, et al; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Management of community-acquired pneumonia (CAP) in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1542/peds.2011-2385
8. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8-S14. https://doi.org/10.1093/cid/cir363
9. Schroeder AR, Ralston SL. Intravenous antibiotic durations for common bacterial infections in children: when is enough? J Hosp Med. 2014;9(9):604-609. https://doi.org/10.1002/jhm.2239
10. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatric Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003
11. van Zanten AR, Engelfriet PM, van Dillen K, van Veen M, Nuijten MJ, Polderman KH. Importance of nondrug costs of intravenous antibiotic therapy. Crit Care. 2003;7(6):R184-R190. https://doi.org/10.1186/cc2388
12. Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117(4):1210-1215. https://doi.org/10.1542/peds.2005-1465
13. Girdwood SCT, Sellas MN, Courter JD, et al. Improving the transition of intravenous to enteral antibiotics in pediatric patients with pneumonia or skin and soft tissue infections. J Hosp Med. 2020;15(1):10-15. https://doi.org/10.12788/jhm.3253
14. Core Elements of Hospital Antibiotic Stewardship Programs. Centers for Disease Control and Prevention. Published 2019. Accessed May 30, 2020. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
15. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. https://doi.org/10.1093/cid/ciw118
16. Science M, Timberlake K, Morris A, Read S, Le Saux N; Groupe Antibiothérapie en Pédiatrie Canada Alliance for Stewardship of Antimicrobials in Pediatrics (GAP Can ASAP). Quality metrics for antimicrobial stewardship programs. Pediatrics. 2019;143(4):e20182372. https://doi.org/10.1542/peds.2018-2372
17. Tchou MJ, Hall M, Shah SS, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Patterns of electrolyte testing at children’s hospitals for common inpatient diagnoses. Pediatrics. 2019;144(1):e20181644. https://doi.org/10.1542/peds.2018-1644
18. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
19. Desai S, Shah SS, Hall M, Richardson TE, Thomson JE; Pediatric Research in Inpatient Settings (PRIS) Network. Imaging strategies and outcomes in children hospitalized with cervical lymphadenitis. J Hosp Med. 2020;15(4):197-203. https://doi.org/10.12788/jhm.3333
20. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
21. Tieder JS, Hall M, Auger KA, et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128(2):323-330. https://doi.org/10.1542/peds.2010-2064
22. Singh JA, Yu S. The burden of septic arthritis on the U.S. inpatient care: a national study. PLoS One. 2017;12(8):e0182577. https://doi.org/10.1371/journal.pone.0182577
23. Foradori DM, Lopez MA, Hall M, et al. Invasive bacterial infections in infants younger than 60 days with skin and soft tissue infections. Pediatr Emerg Care. 2018. https://doi.org/10.1097/pec.0000000000001584
24. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
25. Arancibia A, Icarte A, González C, Morasso I. Dose-dependent bioavailability of amoxycillin. Int J Clin Pharmacol Ther Toxicol. 1988;26(6):300-303.
26. Grayson ML, Cosgrove S, Crowe S, et al. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. 7th ed. CRC Press; 2018.
27. Downes KJ, Hahn A, Wiles J, Courter JD, Inks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in pediatrics’. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006
28. Gras-Le Guen C, Boscher C, Godon N, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol. 2007;63(7):657-662. https://doi.org/10.1007/s00228-007-0307-3
29. Sanchez Navarro A. New formulations of amoxicillin/clavulanic acid: a pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2005;44(11):1097-1115. https://doi.org/10.2165/00003088-200544110-00001
30. Fine MJ, Hough LJ, Medsger AR, et al. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):36-44. https://doi.org/10.1001/archinte.1997.00440220040006
31. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007(4):CD003237. https://doi.org/10.1002/14651858.cd003237.pub2
32. Nageswaran S, Woods CR, Benjamin DK Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25(8):695-699. https://doi.org/10.1097/01.inf.0000227820.36036.f1
33. Al-Nammari S, Roberton B, Ferguson C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Should a child with preseptal periorbital cellulitis be treated with intravenous or oral antibiotics? Emerg Med J. 2007;24(2):128-129. https://doi.org/10.1136/emj.2006.045245
34. Vieira F, Allen SM, Stocks RMS, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483, vii. https://doi.org/10.1016/j.otc.2008.01.002
35. Smith M, Shah S, Kronman M, Patel S, Thurm C, Hersh AL. Route of administration for highly orally bioavailable antibiotics. Open Forum Infect Dis. 2017;4(Suppl 1):S498-S499. https://doi.org/10.1093/ofid/ofx163.1291
36. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
37. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3
38. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585
39. Public Health Ontario. Antimicrobial stewardship programs metric examples. Published 2017. Accessed June 1, 2020. https://www.publichealthontario.ca/-/media/documents/A/2017/asp-metrics-examples.pdf?la=en
40. Desai S, Aronson PL, Shabanova V, et al; Febrile Young Infant Research Collaborative. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
1. Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X
3. Keren R, Shah SS, Srivastava R, et al; for the Pediatric Research Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e201692. https://doi.org/10.1542/peds.2016-1692
5. Li HK, Agweyu A, English M, Bejon P. An unsupported preference for intravenous antibiotics. PLoS Med. 2015;12(5):e1001825. https://dx.doi.org/10.1371%2Fjournal.pmed.1001825
6. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393
7. Bradley JS, Byington CL, Shah SS, et al; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Management of community-acquired pneumonia (CAP) in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1542/peds.2011-2385
8. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8-S14. https://doi.org/10.1093/cid/cir363
9. Schroeder AR, Ralston SL. Intravenous antibiotic durations for common bacterial infections in children: when is enough? J Hosp Med. 2014;9(9):604-609. https://doi.org/10.1002/jhm.2239
10. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatric Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003
11. van Zanten AR, Engelfriet PM, van Dillen K, van Veen M, Nuijten MJ, Polderman KH. Importance of nondrug costs of intravenous antibiotic therapy. Crit Care. 2003;7(6):R184-R190. https://doi.org/10.1186/cc2388
12. Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117(4):1210-1215. https://doi.org/10.1542/peds.2005-1465
13. Girdwood SCT, Sellas MN, Courter JD, et al. Improving the transition of intravenous to enteral antibiotics in pediatric patients with pneumonia or skin and soft tissue infections. J Hosp Med. 2020;15(1):10-15. https://doi.org/10.12788/jhm.3253
14. Core Elements of Hospital Antibiotic Stewardship Programs. Centers for Disease Control and Prevention. Published 2019. Accessed May 30, 2020. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
15. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. https://doi.org/10.1093/cid/ciw118
16. Science M, Timberlake K, Morris A, Read S, Le Saux N; Groupe Antibiothérapie en Pédiatrie Canada Alliance for Stewardship of Antimicrobials in Pediatrics (GAP Can ASAP). Quality metrics for antimicrobial stewardship programs. Pediatrics. 2019;143(4):e20182372. https://doi.org/10.1542/peds.2018-2372
17. Tchou MJ, Hall M, Shah SS, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Patterns of electrolyte testing at children’s hospitals for common inpatient diagnoses. Pediatrics. 2019;144(1):e20181644. https://doi.org/10.1542/peds.2018-1644
18. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
19. Desai S, Shah SS, Hall M, Richardson TE, Thomson JE; Pediatric Research in Inpatient Settings (PRIS) Network. Imaging strategies and outcomes in children hospitalized with cervical lymphadenitis. J Hosp Med. 2020;15(4):197-203. https://doi.org/10.12788/jhm.3333
20. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
21. Tieder JS, Hall M, Auger KA, et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128(2):323-330. https://doi.org/10.1542/peds.2010-2064
22. Singh JA, Yu S. The burden of septic arthritis on the U.S. inpatient care: a national study. PLoS One. 2017;12(8):e0182577. https://doi.org/10.1371/journal.pone.0182577
23. Foradori DM, Lopez MA, Hall M, et al. Invasive bacterial infections in infants younger than 60 days with skin and soft tissue infections. Pediatr Emerg Care. 2018. https://doi.org/10.1097/pec.0000000000001584
24. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
25. Arancibia A, Icarte A, González C, Morasso I. Dose-dependent bioavailability of amoxycillin. Int J Clin Pharmacol Ther Toxicol. 1988;26(6):300-303.
26. Grayson ML, Cosgrove S, Crowe S, et al. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. 7th ed. CRC Press; 2018.
27. Downes KJ, Hahn A, Wiles J, Courter JD, Inks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in pediatrics’. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006
28. Gras-Le Guen C, Boscher C, Godon N, et al. Therapeutic amoxicillin levels achieved with oral administration in term neonates. Eur J Clin Pharmacol. 2007;63(7):657-662. https://doi.org/10.1007/s00228-007-0307-3
29. Sanchez Navarro A. New formulations of amoxicillin/clavulanic acid: a pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2005;44(11):1097-1115. https://doi.org/10.2165/00003088-200544110-00001
30. Fine MJ, Hough LJ, Medsger AR, et al. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157(1):36-44. https://doi.org/10.1001/archinte.1997.00440220040006
31. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007(4):CD003237. https://doi.org/10.1002/14651858.cd003237.pub2
32. Nageswaran S, Woods CR, Benjamin DK Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25(8):695-699. https://doi.org/10.1097/01.inf.0000227820.36036.f1
33. Al-Nammari S, Roberton B, Ferguson C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Should a child with preseptal periorbital cellulitis be treated with intravenous or oral antibiotics? Emerg Med J. 2007;24(2):128-129. https://doi.org/10.1136/emj.2006.045245
34. Vieira F, Allen SM, Stocks RMS, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459-483, vii. https://doi.org/10.1016/j.otc.2008.01.002
35. Smith M, Shah S, Kronman M, Patel S, Thurm C, Hersh AL. Route of administration for highly orally bioavailable antibiotics. Open Forum Infect Dis. 2017;4(Suppl 1):S498-S499. https://doi.org/10.1093/ofid/ofx163.1291
36. Brady PW, Brinkman WB, Simmons JM, et al. Oral antibiotics at discharge for children with acute osteomyelitis: a rapid cycle improvement project. BMJ Qual Saf. 2014;23(6):499-507. https://doi.org/10.1136/bmjqs-2013-002179
37. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3
38. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585
39. Public Health Ontario. Antimicrobial stewardship programs metric examples. Published 2017. Accessed June 1, 2020. https://www.publichealthontario.ca/-/media/documents/A/2017/asp-metrics-examples.pdf?la=en
40. Desai S, Aronson PL, Shabanova V, et al; Febrile Young Infant Research Collaborative. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
© 2021 Society of Hospital Medicine
Things We Do for No Reason: Neuroimaging for Hospitalized Patients with Delirium
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 67-year-old woman with a history of hypertension and osteoarthritis was hospitalized for fever, flank pain, and dysuria with pyuria on urinalysis. She was diagnosed with acute pyelonephritis and started ceftriaxone, ondansetron for nausea, and oxycodone for pain. On hospital day two, she developed acute confusion that waxed and waned in severity throughout the day. On examination, she appeared mildly agitated, inattentive, and was noted to pick at her linens and garment. She was oriented to person only and had a nonfocal neurologic examination. Her nurse reported no recent falls or trauma. As part of the patient’s evaluation, her attending physician ordered a head computed tomography (CT) scan.
BACKGROUND
Delirium is commonly diagnosed in hospitalized patients. It has a prevalence of 29%-64% and is associated with longer lengths of stay, higher mortality, and costs of over $164 billion per year in the United States.1 While a number of practice guidelines have been created to help guide delirium diagnosis and management, there is not a clear consensus on when neuroimaging should be performed during the evaluation.2-4 It should also be noted that numerous guidelines for delirium management exist, with variable quality and a heavy reliance on expert opinion.5 Perhaps due to this lack of consensus, neuroimaging is performed in 33% to 67% of hospitalized patients with delirium.6,7
WHY YOU MAY THINK NEUROIMAGING IS HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
Delirium is known to be associated with intracranial processes. For example, delirium occurs in 13% to 48% of patients with acute stroke8 and conversely 7% of patients with new confusion evaluated in emergency departments or inpatient settings were found to have an acute stroke.9 The inclusion of neuroimaging as part of a delirium evaluation is supported in certain circumstances, such as in patients with recent falls, focal neurologic signs (including papilledema), systemic anticoagulation,2 or increased risk of intracranial processes such as metastatic malignancy.4
WHY NEUROIMAGING IS NOT HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
A number of studies have evaluated the diagnostic yield of neuroimaging in hospitalized patients with delirium (Table).6,7,10,11 Two studies included patients with delirium that developed after hospitalization10,11 and two included patients with delirium at admission.6,7
Theisen-Toupal et al. conducted a retrospective study of 220 hospitalized general medical patients who underwent head CT scans for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence or unresponsiveness.10 Patients were excluded if they had a history of falls, head trauma, or new neurologic deficits in the preceding two weeks or if the admitting diagnosis was stroke or cerebral hemorrhage. Additionally, the authors limited patients to those who developed delirium 24 hours or more after admission. There were 6/220 (2.7%) patients identified with an acute intracranial process. Of these six patients, three were receiving anticoagulation. An additional 4/220 (1.8%) head CT scans were identified as equivocal, prompting further neuroimaging, which ultimately showed chronic findings.
Vijayakrishnan et al. performed a retrospective review of 400 hospitalized patients who underwent inpatient CT scans, then limited to those with new delirium.11 They identified 36 patients, of which four (11%) had acute findings on CT: one case each of acute hemorrhage, subdural hematoma, brain metastases, and septic emboli. The authors state “all the four patients had preimaging clinical symptoms and signs, which warranted imaging as per guidelines suggested by the British Geriatrics Society and the Australian and New Zealand Society for Geriatric Medicine,” though they do not provide further details. The strength of this paper is that it isolated patients who developed delirium while hospitalized; however, conclusions were limited by the small sample size.
Lai et al.’s case-control study evaluated 300 consecutive patients admitted to a delirium unit over 18 months.6 Of these 300 patients, 200 (67%) had CT performed; 29/200 (14.5%) had intracranial findings on CT that explained their delirium, including 13 ischemic strokes, seven subdural hemorrhages, nine intracerebral hemorrhages, and three additional ischemic strokes that evolved on follow-up imaging but were not present on the initial scans. The authors performed univariate and multivariate analyses to identify risk factors for an intracranial cause of delirium. Only 3/29 patients with a positive scan did not have one of three main risk factors the authors identified: a fall in the preceding two weeks, new neurologic findings, or sudden deterioration of consciousness. It should be noted that authors did not define “deterioration of consciousness” and that all patients had confusion on admission to the unit, rather than developing during hospitalization.
Hijazi et al. conducted a retrospective cohort study over a 20-month period of 1,653 patients with delirium at the time of admission or during their hospitalization. Patients with delirium due to drug or medication withdrawal or “psychiatric reasons” were excluded. Overall, 538 (32.5%) patients underwent CT, MRI or both, and 78 (14.5%) patients had a positive finding on neuroimaging. This study’s 14.5 % overall yield matches that of Lai et al. Unfortunately, the study included all patients with delirium and did not report the rates of fall, neurologic deficits, and/or use of anticoagulation among those with positive neuroimaging. This limits the generalizability of the findings to a cohort of patients without intracranial pathology risk factors.
The reported yield of neuroimaging for hospitalized patients with delirium ranged from 2.7% to 14.5% across studies. However, in studies taking into account specific patient risk factors; the reported yields in patients without focal neurologic findings, new decline in mental status, systemic anticoagulation, or recent falls were 0%,11 1.4%,10 and 1.5%.6 While a rate of 1.5% may appear high for a serious outcome such as stroke or intracranial bleeding, it is comparable to rates reported for missed major cardiac events in clinical algorithms for evaluating chest pain.12 It should also be noted that neuroimaging is imperfect for acute stroke, and thus the positive or negative predictive value may be poor in the setting of low prevalence. For example, for detection of any acute stroke, the sensitivity/specificity of MRI and CT are 83%/97% and 26%/98% respectively.13
Neuroimaging is expensive and has risks. The average charge for a head CT is approximately $1,400 at academic institutions.14 Moreover, computed tomography exposes patients to significant radiation and up to 2% of malignancies in the United States may be attributable to prior tomography exposure.15 Additionally, there are non-negligible rates of incidental findings during neuroimaging, 1% for CT16 and 2.7%-13.7% for MRI,17,18 which may result in further evaluation or treatment that causes significant patient anxiety. Obtaining neuroimaging on delirious patients can be time consuming and labor intensive, which could delay care to other patients. Additionally, sedating medications are often administered to agitated patients prior to imaging, which risk worsening delirium. Ordering neuroimaging for all patients with acute delirium, therefore, exposes the large majority to unnecessary costs and potential harms.
WHEN NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS COULD BE REASONABLE
The diagnostic yield of head CT in the evaluation of delirium is significantly higher in patients with specific risk factors. Lai et al. found adjusted odds ratios for abnormal CT of 18.2 in patients with new focal deficits, 5.6 with a fall in the preceding two weeks and 4.6 in patients with deterioration in consciousness. Patients with systemic anticoagulation had higher unadjusted, (OR 2.4) though not adjusted odds of having an abnormal CT.6 Thiesen-Toupal et al. excluded patients with recent falls or neurologic deficits but reported that three out of six delirious patients with abnormal neuroimaging were anticoagulated.10 Vijayakrishnan et al. found that all four delirious patients with intracranial findings met guideline criteria for neuroimaging.11 Thus, current recommendations for neuroimaging in delirious patients with falls, focal neurologic deficits, or systemic anticoagulation are appropriate. In situations when a provider lacks an accurate history and is unable to determine if risk factors are present (for example a confused patient found sitting on the floor next to the bed), it may also be reasonable to consider neuroimaging.
Data are limited, but some authors advocate for neuroimaging in cases of delirium that do not improve with treatment.6 Additionally, it may be reasonable to consider neuroimaging in delirium patients with predispositions to embolic or metastatic intracranial processes such as endovascular infections and certain malignancies.4
WHAT YOU SHOULD DO INSTEAD OF NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS
Hospitalized patients with acute confusion should be assessed for delirium with a validated instrument such as the Confusion Assessment Method (CAM).19,20 The original CAM included several components: acute change in mental status with a fluctuating course and inattention, plus either disorganized thinking and/or altered level of consciousness. Multiple delirium assessment tools have been created and validated, all of which include inattention as a required feature. A recent hospital-based study using a two item bedside test asking the patient to name the day of the week and list the months of the year backwards detected delirium with a sensitivity of 93% and specificity of 64%.21 Once the diagnosis of delirium is established, evaluation should begin with a careful history and physical examination focused on the identification of risk factors such as physical restraints, indwelling urinary catheters, and drugs known to precipitate delirium, particularly those with withdrawal potential, anticholinergic properties, and sedative-hypnotic agents.22-24 Delirium may be the first harbinger of serious medical illness and specific testing should be guided by clinical suspicion. In general, a thorough physical examination should look for focal neurologic deficits, hypoxia, signs of infection, and other inflammatory or painful processes that could precipitate delirium.25 Targeted laboratory evaluation may include a basic metabolic panel to identify electrolyte (including calcium) and metabolic derangements, complete blood count, and urinalysis if infection is suspected.
RECOMMENDATIONS
- Use a validated instrument such as CAM to evaluate hospitalized patients who develop altered mental status.
- Delirious patients should undergo a thorough history including a review of medications, physical exam, and targeted laboratory testing aimed at identifying common risk factors and precipitants of delirium that should be addressed.
- Perform neuroimaging if there is a history of fall or head trauma in the preceding two weeks, any new focal abnormalities on neurologic exam or if the patient is receiving systemic anticoagulation.
- It may be reasonable to consider neuroimaging for patients with an atypical course of delirium, such as a sudden decline in the level of consciousness, persistence despite addressing identified factors, or if there is a high degree of suspicion for embolic or metastatic processes.
CONCLUSIONS
Performing neuroimaging in undifferentiated patients who develop delirium while hospitalized has a low diagnostic yield, is costly, and is potentially harmful. Neuroimaging should be reserved for those with identified risk factors for intracranial pathology. For the patient described in the initial vignette with no risk factors for intracranial cause, neuroimaging would be unlikely to contribute to her care. To change provider beliefs and behaviors regarding neuroimaging, prospective studies evaluating guideline implementation are needed. However, based on the current evidence, neuroimaging should be reserved for those with identified risk factors.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1. PubMed
2. Clinical practice guidelines for the management of delirium in older people. 2006; https://www2.health.vic.gov.au/Api/downloadmedia/%7BDAA8404B-FEE7-4BDA-8A1E-A32494783B7F%7D. Accessed June 4, 2018.
3. Delirium: prevention, diagnosis and management. NICE Guidance 2010; https://www.nice.org.uk/guidance/cg103. Accessed June 4, 2018.
4. Michaud L, Bula C, Berney A, et al. Delirium: guidelines for general hospitals. J Psychosom Res. 2007;62(3):371-383. doi: 10.1016/j.jpsychores.2006.10.004. PubMed
5. Bush SH, Marchington KL, Agar M, Davis DH, Sikora L, Tsang TW. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7(3):e013809. doi: 10.1136/bmjopen-2016-013809. PubMed
6. Lai MM, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012;42(4):422-427. doi: 10.1111/j.1445-5994.2010.02400.x. PubMed
7. Hijazi Z, Lange P, Watson R, Maier AB. The use of cerebral imaging for investigating delirium aetiology. Eur J Intern Med. 2018;52:35-39. doi: 10.1016/j.ejim.2018.01.024. PubMed
8. Oldenbeuving AW, de Kort PL, Jansen BP, Roks G, Kappelle LJ. Delirium in acute stroke: a review. Int J Stroke. 2007;2(4):270-275. doi: 10.1111/j.1747-4949.2007.00163.x. PubMed
9. Benbadis SR, Sila CA, Cristea RL. Mental status changes and stroke. J Stroke Cerebrovasc Dis. 1994;4(4):216-219. doi: 10.1016/S1052-3057(10)80093-X. PubMed
10. Theisen-Toupal J, Breu AC, Mattison ML, Arnaout R. Diagnostic yield of head computed tomography for the hospitalized medical patient with delirium. J Hosp Med. 2014;9(8):497-501. doi: 10.1002/jhm.2198. PubMed
11. Vijayakrishnan R, Ramasubramanian A, Dhand S. Utility of head CT scan for acute inpatient delirium. Hosp Top. 2015;93(1):9-12. doi: 10.1080/00185868.2015.1012928. PubMed
12. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255. PubMed
13. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298. doi: 10.1016/S0140-6736(07)60151-2. PubMed
14. Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12(5):453-457. doi: 10.1016/j.jacr.2014.12.016. PubMed
15. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149. PubMed
16. Eskandary H, Sabbagh M, Khajehpour F, Eskandari M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63(6):550-553; discussion 553. doi: 10.1016/j.surneu.2004.07.049. PubMed
17. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828. doi: 10.1056/NEJMoa070972. PubMed
18. Morris Z, Whiteley WN, Longstreth WT, Jr., et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. PubMed
19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941. PubMed
20. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830. doi: 10.1111/j.1532-5415.2008.01674.x. PubMed
21. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. doi: 10.1002/jhm.2418. PubMed
22. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing the risk of delirium. Am J Psychiatry. 1992;149(10):1393-1394. doi: 10.1176/ajp.149.10.1393. PubMed
23. Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care. Am J Med. 1999;106(5):565-573. doi: 0.1016/S0002-9343(99)00070-4. PubMed
24. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. doi: 10.1056/NEJMc1714932. PubMed
25. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857. doi: 10.1001/jama.1996.03530350034031. PubMed
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 67-year-old woman with a history of hypertension and osteoarthritis was hospitalized for fever, flank pain, and dysuria with pyuria on urinalysis. She was diagnosed with acute pyelonephritis and started ceftriaxone, ondansetron for nausea, and oxycodone for pain. On hospital day two, she developed acute confusion that waxed and waned in severity throughout the day. On examination, she appeared mildly agitated, inattentive, and was noted to pick at her linens and garment. She was oriented to person only and had a nonfocal neurologic examination. Her nurse reported no recent falls or trauma. As part of the patient’s evaluation, her attending physician ordered a head computed tomography (CT) scan.
BACKGROUND
Delirium is commonly diagnosed in hospitalized patients. It has a prevalence of 29%-64% and is associated with longer lengths of stay, higher mortality, and costs of over $164 billion per year in the United States.1 While a number of practice guidelines have been created to help guide delirium diagnosis and management, there is not a clear consensus on when neuroimaging should be performed during the evaluation.2-4 It should also be noted that numerous guidelines for delirium management exist, with variable quality and a heavy reliance on expert opinion.5 Perhaps due to this lack of consensus, neuroimaging is performed in 33% to 67% of hospitalized patients with delirium.6,7
WHY YOU MAY THINK NEUROIMAGING IS HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
Delirium is known to be associated with intracranial processes. For example, delirium occurs in 13% to 48% of patients with acute stroke8 and conversely 7% of patients with new confusion evaluated in emergency departments or inpatient settings were found to have an acute stroke.9 The inclusion of neuroimaging as part of a delirium evaluation is supported in certain circumstances, such as in patients with recent falls, focal neurologic signs (including papilledema), systemic anticoagulation,2 or increased risk of intracranial processes such as metastatic malignancy.4
WHY NEUROIMAGING IS NOT HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
A number of studies have evaluated the diagnostic yield of neuroimaging in hospitalized patients with delirium (Table).6,7,10,11 Two studies included patients with delirium that developed after hospitalization10,11 and two included patients with delirium at admission.6,7
Theisen-Toupal et al. conducted a retrospective study of 220 hospitalized general medical patients who underwent head CT scans for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence or unresponsiveness.10 Patients were excluded if they had a history of falls, head trauma, or new neurologic deficits in the preceding two weeks or if the admitting diagnosis was stroke or cerebral hemorrhage. Additionally, the authors limited patients to those who developed delirium 24 hours or more after admission. There were 6/220 (2.7%) patients identified with an acute intracranial process. Of these six patients, three were receiving anticoagulation. An additional 4/220 (1.8%) head CT scans were identified as equivocal, prompting further neuroimaging, which ultimately showed chronic findings.
Vijayakrishnan et al. performed a retrospective review of 400 hospitalized patients who underwent inpatient CT scans, then limited to those with new delirium.11 They identified 36 patients, of which four (11%) had acute findings on CT: one case each of acute hemorrhage, subdural hematoma, brain metastases, and septic emboli. The authors state “all the four patients had preimaging clinical symptoms and signs, which warranted imaging as per guidelines suggested by the British Geriatrics Society and the Australian and New Zealand Society for Geriatric Medicine,” though they do not provide further details. The strength of this paper is that it isolated patients who developed delirium while hospitalized; however, conclusions were limited by the small sample size.
Lai et al.’s case-control study evaluated 300 consecutive patients admitted to a delirium unit over 18 months.6 Of these 300 patients, 200 (67%) had CT performed; 29/200 (14.5%) had intracranial findings on CT that explained their delirium, including 13 ischemic strokes, seven subdural hemorrhages, nine intracerebral hemorrhages, and three additional ischemic strokes that evolved on follow-up imaging but were not present on the initial scans. The authors performed univariate and multivariate analyses to identify risk factors for an intracranial cause of delirium. Only 3/29 patients with a positive scan did not have one of three main risk factors the authors identified: a fall in the preceding two weeks, new neurologic findings, or sudden deterioration of consciousness. It should be noted that authors did not define “deterioration of consciousness” and that all patients had confusion on admission to the unit, rather than developing during hospitalization.
Hijazi et al. conducted a retrospective cohort study over a 20-month period of 1,653 patients with delirium at the time of admission or during their hospitalization. Patients with delirium due to drug or medication withdrawal or “psychiatric reasons” were excluded. Overall, 538 (32.5%) patients underwent CT, MRI or both, and 78 (14.5%) patients had a positive finding on neuroimaging. This study’s 14.5 % overall yield matches that of Lai et al. Unfortunately, the study included all patients with delirium and did not report the rates of fall, neurologic deficits, and/or use of anticoagulation among those with positive neuroimaging. This limits the generalizability of the findings to a cohort of patients without intracranial pathology risk factors.
The reported yield of neuroimaging for hospitalized patients with delirium ranged from 2.7% to 14.5% across studies. However, in studies taking into account specific patient risk factors; the reported yields in patients without focal neurologic findings, new decline in mental status, systemic anticoagulation, or recent falls were 0%,11 1.4%,10 and 1.5%.6 While a rate of 1.5% may appear high for a serious outcome such as stroke or intracranial bleeding, it is comparable to rates reported for missed major cardiac events in clinical algorithms for evaluating chest pain.12 It should also be noted that neuroimaging is imperfect for acute stroke, and thus the positive or negative predictive value may be poor in the setting of low prevalence. For example, for detection of any acute stroke, the sensitivity/specificity of MRI and CT are 83%/97% and 26%/98% respectively.13
Neuroimaging is expensive and has risks. The average charge for a head CT is approximately $1,400 at academic institutions.14 Moreover, computed tomography exposes patients to significant radiation and up to 2% of malignancies in the United States may be attributable to prior tomography exposure.15 Additionally, there are non-negligible rates of incidental findings during neuroimaging, 1% for CT16 and 2.7%-13.7% for MRI,17,18 which may result in further evaluation or treatment that causes significant patient anxiety. Obtaining neuroimaging on delirious patients can be time consuming and labor intensive, which could delay care to other patients. Additionally, sedating medications are often administered to agitated patients prior to imaging, which risk worsening delirium. Ordering neuroimaging for all patients with acute delirium, therefore, exposes the large majority to unnecessary costs and potential harms.
WHEN NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS COULD BE REASONABLE
The diagnostic yield of head CT in the evaluation of delirium is significantly higher in patients with specific risk factors. Lai et al. found adjusted odds ratios for abnormal CT of 18.2 in patients with new focal deficits, 5.6 with a fall in the preceding two weeks and 4.6 in patients with deterioration in consciousness. Patients with systemic anticoagulation had higher unadjusted, (OR 2.4) though not adjusted odds of having an abnormal CT.6 Thiesen-Toupal et al. excluded patients with recent falls or neurologic deficits but reported that three out of six delirious patients with abnormal neuroimaging were anticoagulated.10 Vijayakrishnan et al. found that all four delirious patients with intracranial findings met guideline criteria for neuroimaging.11 Thus, current recommendations for neuroimaging in delirious patients with falls, focal neurologic deficits, or systemic anticoagulation are appropriate. In situations when a provider lacks an accurate history and is unable to determine if risk factors are present (for example a confused patient found sitting on the floor next to the bed), it may also be reasonable to consider neuroimaging.
Data are limited, but some authors advocate for neuroimaging in cases of delirium that do not improve with treatment.6 Additionally, it may be reasonable to consider neuroimaging in delirium patients with predispositions to embolic or metastatic intracranial processes such as endovascular infections and certain malignancies.4
WHAT YOU SHOULD DO INSTEAD OF NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS
Hospitalized patients with acute confusion should be assessed for delirium with a validated instrument such as the Confusion Assessment Method (CAM).19,20 The original CAM included several components: acute change in mental status with a fluctuating course and inattention, plus either disorganized thinking and/or altered level of consciousness. Multiple delirium assessment tools have been created and validated, all of which include inattention as a required feature. A recent hospital-based study using a two item bedside test asking the patient to name the day of the week and list the months of the year backwards detected delirium with a sensitivity of 93% and specificity of 64%.21 Once the diagnosis of delirium is established, evaluation should begin with a careful history and physical examination focused on the identification of risk factors such as physical restraints, indwelling urinary catheters, and drugs known to precipitate delirium, particularly those with withdrawal potential, anticholinergic properties, and sedative-hypnotic agents.22-24 Delirium may be the first harbinger of serious medical illness and specific testing should be guided by clinical suspicion. In general, a thorough physical examination should look for focal neurologic deficits, hypoxia, signs of infection, and other inflammatory or painful processes that could precipitate delirium.25 Targeted laboratory evaluation may include a basic metabolic panel to identify electrolyte (including calcium) and metabolic derangements, complete blood count, and urinalysis if infection is suspected.
RECOMMENDATIONS
- Use a validated instrument such as CAM to evaluate hospitalized patients who develop altered mental status.
- Delirious patients should undergo a thorough history including a review of medications, physical exam, and targeted laboratory testing aimed at identifying common risk factors and precipitants of delirium that should be addressed.
- Perform neuroimaging if there is a history of fall or head trauma in the preceding two weeks, any new focal abnormalities on neurologic exam or if the patient is receiving systemic anticoagulation.
- It may be reasonable to consider neuroimaging for patients with an atypical course of delirium, such as a sudden decline in the level of consciousness, persistence despite addressing identified factors, or if there is a high degree of suspicion for embolic or metastatic processes.
CONCLUSIONS
Performing neuroimaging in undifferentiated patients who develop delirium while hospitalized has a low diagnostic yield, is costly, and is potentially harmful. Neuroimaging should be reserved for those with identified risk factors for intracranial pathology. For the patient described in the initial vignette with no risk factors for intracranial cause, neuroimaging would be unlikely to contribute to her care. To change provider beliefs and behaviors regarding neuroimaging, prospective studies evaluating guideline implementation are needed. However, based on the current evidence, neuroimaging should be reserved for those with identified risk factors.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 67-year-old woman with a history of hypertension and osteoarthritis was hospitalized for fever, flank pain, and dysuria with pyuria on urinalysis. She was diagnosed with acute pyelonephritis and started ceftriaxone, ondansetron for nausea, and oxycodone for pain. On hospital day two, she developed acute confusion that waxed and waned in severity throughout the day. On examination, she appeared mildly agitated, inattentive, and was noted to pick at her linens and garment. She was oriented to person only and had a nonfocal neurologic examination. Her nurse reported no recent falls or trauma. As part of the patient’s evaluation, her attending physician ordered a head computed tomography (CT) scan.
BACKGROUND
Delirium is commonly diagnosed in hospitalized patients. It has a prevalence of 29%-64% and is associated with longer lengths of stay, higher mortality, and costs of over $164 billion per year in the United States.1 While a number of practice guidelines have been created to help guide delirium diagnosis and management, there is not a clear consensus on when neuroimaging should be performed during the evaluation.2-4 It should also be noted that numerous guidelines for delirium management exist, with variable quality and a heavy reliance on expert opinion.5 Perhaps due to this lack of consensus, neuroimaging is performed in 33% to 67% of hospitalized patients with delirium.6,7
WHY YOU MAY THINK NEUROIMAGING IS HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
Delirium is known to be associated with intracranial processes. For example, delirium occurs in 13% to 48% of patients with acute stroke8 and conversely 7% of patients with new confusion evaluated in emergency departments or inpatient settings were found to have an acute stroke.9 The inclusion of neuroimaging as part of a delirium evaluation is supported in certain circumstances, such as in patients with recent falls, focal neurologic signs (including papilledema), systemic anticoagulation,2 or increased risk of intracranial processes such as metastatic malignancy.4
WHY NEUROIMAGING IS NOT HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
A number of studies have evaluated the diagnostic yield of neuroimaging in hospitalized patients with delirium (Table).6,7,10,11 Two studies included patients with delirium that developed after hospitalization10,11 and two included patients with delirium at admission.6,7
Theisen-Toupal et al. conducted a retrospective study of 220 hospitalized general medical patients who underwent head CT scans for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence or unresponsiveness.10 Patients were excluded if they had a history of falls, head trauma, or new neurologic deficits in the preceding two weeks or if the admitting diagnosis was stroke or cerebral hemorrhage. Additionally, the authors limited patients to those who developed delirium 24 hours or more after admission. There were 6/220 (2.7%) patients identified with an acute intracranial process. Of these six patients, three were receiving anticoagulation. An additional 4/220 (1.8%) head CT scans were identified as equivocal, prompting further neuroimaging, which ultimately showed chronic findings.
Vijayakrishnan et al. performed a retrospective review of 400 hospitalized patients who underwent inpatient CT scans, then limited to those with new delirium.11 They identified 36 patients, of which four (11%) had acute findings on CT: one case each of acute hemorrhage, subdural hematoma, brain metastases, and septic emboli. The authors state “all the four patients had preimaging clinical symptoms and signs, which warranted imaging as per guidelines suggested by the British Geriatrics Society and the Australian and New Zealand Society for Geriatric Medicine,” though they do not provide further details. The strength of this paper is that it isolated patients who developed delirium while hospitalized; however, conclusions were limited by the small sample size.
Lai et al.’s case-control study evaluated 300 consecutive patients admitted to a delirium unit over 18 months.6 Of these 300 patients, 200 (67%) had CT performed; 29/200 (14.5%) had intracranial findings on CT that explained their delirium, including 13 ischemic strokes, seven subdural hemorrhages, nine intracerebral hemorrhages, and three additional ischemic strokes that evolved on follow-up imaging but were not present on the initial scans. The authors performed univariate and multivariate analyses to identify risk factors for an intracranial cause of delirium. Only 3/29 patients with a positive scan did not have one of three main risk factors the authors identified: a fall in the preceding two weeks, new neurologic findings, or sudden deterioration of consciousness. It should be noted that authors did not define “deterioration of consciousness” and that all patients had confusion on admission to the unit, rather than developing during hospitalization.
Hijazi et al. conducted a retrospective cohort study over a 20-month period of 1,653 patients with delirium at the time of admission or during their hospitalization. Patients with delirium due to drug or medication withdrawal or “psychiatric reasons” were excluded. Overall, 538 (32.5%) patients underwent CT, MRI or both, and 78 (14.5%) patients had a positive finding on neuroimaging. This study’s 14.5 % overall yield matches that of Lai et al. Unfortunately, the study included all patients with delirium and did not report the rates of fall, neurologic deficits, and/or use of anticoagulation among those with positive neuroimaging. This limits the generalizability of the findings to a cohort of patients without intracranial pathology risk factors.
The reported yield of neuroimaging for hospitalized patients with delirium ranged from 2.7% to 14.5% across studies. However, in studies taking into account specific patient risk factors; the reported yields in patients without focal neurologic findings, new decline in mental status, systemic anticoagulation, or recent falls were 0%,11 1.4%,10 and 1.5%.6 While a rate of 1.5% may appear high for a serious outcome such as stroke or intracranial bleeding, it is comparable to rates reported for missed major cardiac events in clinical algorithms for evaluating chest pain.12 It should also be noted that neuroimaging is imperfect for acute stroke, and thus the positive or negative predictive value may be poor in the setting of low prevalence. For example, for detection of any acute stroke, the sensitivity/specificity of MRI and CT are 83%/97% and 26%/98% respectively.13
Neuroimaging is expensive and has risks. The average charge for a head CT is approximately $1,400 at academic institutions.14 Moreover, computed tomography exposes patients to significant radiation and up to 2% of malignancies in the United States may be attributable to prior tomography exposure.15 Additionally, there are non-negligible rates of incidental findings during neuroimaging, 1% for CT16 and 2.7%-13.7% for MRI,17,18 which may result in further evaluation or treatment that causes significant patient anxiety. Obtaining neuroimaging on delirious patients can be time consuming and labor intensive, which could delay care to other patients. Additionally, sedating medications are often administered to agitated patients prior to imaging, which risk worsening delirium. Ordering neuroimaging for all patients with acute delirium, therefore, exposes the large majority to unnecessary costs and potential harms.
WHEN NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS COULD BE REASONABLE
The diagnostic yield of head CT in the evaluation of delirium is significantly higher in patients with specific risk factors. Lai et al. found adjusted odds ratios for abnormal CT of 18.2 in patients with new focal deficits, 5.6 with a fall in the preceding two weeks and 4.6 in patients with deterioration in consciousness. Patients with systemic anticoagulation had higher unadjusted, (OR 2.4) though not adjusted odds of having an abnormal CT.6 Thiesen-Toupal et al. excluded patients with recent falls or neurologic deficits but reported that three out of six delirious patients with abnormal neuroimaging were anticoagulated.10 Vijayakrishnan et al. found that all four delirious patients with intracranial findings met guideline criteria for neuroimaging.11 Thus, current recommendations for neuroimaging in delirious patients with falls, focal neurologic deficits, or systemic anticoagulation are appropriate. In situations when a provider lacks an accurate history and is unable to determine if risk factors are present (for example a confused patient found sitting on the floor next to the bed), it may also be reasonable to consider neuroimaging.
Data are limited, but some authors advocate for neuroimaging in cases of delirium that do not improve with treatment.6 Additionally, it may be reasonable to consider neuroimaging in delirium patients with predispositions to embolic or metastatic intracranial processes such as endovascular infections and certain malignancies.4
WHAT YOU SHOULD DO INSTEAD OF NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS
Hospitalized patients with acute confusion should be assessed for delirium with a validated instrument such as the Confusion Assessment Method (CAM).19,20 The original CAM included several components: acute change in mental status with a fluctuating course and inattention, plus either disorganized thinking and/or altered level of consciousness. Multiple delirium assessment tools have been created and validated, all of which include inattention as a required feature. A recent hospital-based study using a two item bedside test asking the patient to name the day of the week and list the months of the year backwards detected delirium with a sensitivity of 93% and specificity of 64%.21 Once the diagnosis of delirium is established, evaluation should begin with a careful history and physical examination focused on the identification of risk factors such as physical restraints, indwelling urinary catheters, and drugs known to precipitate delirium, particularly those with withdrawal potential, anticholinergic properties, and sedative-hypnotic agents.22-24 Delirium may be the first harbinger of serious medical illness and specific testing should be guided by clinical suspicion. In general, a thorough physical examination should look for focal neurologic deficits, hypoxia, signs of infection, and other inflammatory or painful processes that could precipitate delirium.25 Targeted laboratory evaluation may include a basic metabolic panel to identify electrolyte (including calcium) and metabolic derangements, complete blood count, and urinalysis if infection is suspected.
RECOMMENDATIONS
- Use a validated instrument such as CAM to evaluate hospitalized patients who develop altered mental status.
- Delirious patients should undergo a thorough history including a review of medications, physical exam, and targeted laboratory testing aimed at identifying common risk factors and precipitants of delirium that should be addressed.
- Perform neuroimaging if there is a history of fall or head trauma in the preceding two weeks, any new focal abnormalities on neurologic exam or if the patient is receiving systemic anticoagulation.
- It may be reasonable to consider neuroimaging for patients with an atypical course of delirium, such as a sudden decline in the level of consciousness, persistence despite addressing identified factors, or if there is a high degree of suspicion for embolic or metastatic processes.
CONCLUSIONS
Performing neuroimaging in undifferentiated patients who develop delirium while hospitalized has a low diagnostic yield, is costly, and is potentially harmful. Neuroimaging should be reserved for those with identified risk factors for intracranial pathology. For the patient described in the initial vignette with no risk factors for intracranial cause, neuroimaging would be unlikely to contribute to her care. To change provider beliefs and behaviors regarding neuroimaging, prospective studies evaluating guideline implementation are needed. However, based on the current evidence, neuroimaging should be reserved for those with identified risk factors.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1. PubMed
2. Clinical practice guidelines for the management of delirium in older people. 2006; https://www2.health.vic.gov.au/Api/downloadmedia/%7BDAA8404B-FEE7-4BDA-8A1E-A32494783B7F%7D. Accessed June 4, 2018.
3. Delirium: prevention, diagnosis and management. NICE Guidance 2010; https://www.nice.org.uk/guidance/cg103. Accessed June 4, 2018.
4. Michaud L, Bula C, Berney A, et al. Delirium: guidelines for general hospitals. J Psychosom Res. 2007;62(3):371-383. doi: 10.1016/j.jpsychores.2006.10.004. PubMed
5. Bush SH, Marchington KL, Agar M, Davis DH, Sikora L, Tsang TW. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7(3):e013809. doi: 10.1136/bmjopen-2016-013809. PubMed
6. Lai MM, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012;42(4):422-427. doi: 10.1111/j.1445-5994.2010.02400.x. PubMed
7. Hijazi Z, Lange P, Watson R, Maier AB. The use of cerebral imaging for investigating delirium aetiology. Eur J Intern Med. 2018;52:35-39. doi: 10.1016/j.ejim.2018.01.024. PubMed
8. Oldenbeuving AW, de Kort PL, Jansen BP, Roks G, Kappelle LJ. Delirium in acute stroke: a review. Int J Stroke. 2007;2(4):270-275. doi: 10.1111/j.1747-4949.2007.00163.x. PubMed
9. Benbadis SR, Sila CA, Cristea RL. Mental status changes and stroke. J Stroke Cerebrovasc Dis. 1994;4(4):216-219. doi: 10.1016/S1052-3057(10)80093-X. PubMed
10. Theisen-Toupal J, Breu AC, Mattison ML, Arnaout R. Diagnostic yield of head computed tomography for the hospitalized medical patient with delirium. J Hosp Med. 2014;9(8):497-501. doi: 10.1002/jhm.2198. PubMed
11. Vijayakrishnan R, Ramasubramanian A, Dhand S. Utility of head CT scan for acute inpatient delirium. Hosp Top. 2015;93(1):9-12. doi: 10.1080/00185868.2015.1012928. PubMed
12. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255. PubMed
13. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298. doi: 10.1016/S0140-6736(07)60151-2. PubMed
14. Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12(5):453-457. doi: 10.1016/j.jacr.2014.12.016. PubMed
15. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149. PubMed
16. Eskandary H, Sabbagh M, Khajehpour F, Eskandari M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63(6):550-553; discussion 553. doi: 10.1016/j.surneu.2004.07.049. PubMed
17. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828. doi: 10.1056/NEJMoa070972. PubMed
18. Morris Z, Whiteley WN, Longstreth WT, Jr., et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. PubMed
19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941. PubMed
20. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830. doi: 10.1111/j.1532-5415.2008.01674.x. PubMed
21. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. doi: 10.1002/jhm.2418. PubMed
22. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing the risk of delirium. Am J Psychiatry. 1992;149(10):1393-1394. doi: 10.1176/ajp.149.10.1393. PubMed
23. Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care. Am J Med. 1999;106(5):565-573. doi: 0.1016/S0002-9343(99)00070-4. PubMed
24. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. doi: 10.1056/NEJMc1714932. PubMed
25. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857. doi: 10.1001/jama.1996.03530350034031. PubMed
1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1. PubMed
2. Clinical practice guidelines for the management of delirium in older people. 2006; https://www2.health.vic.gov.au/Api/downloadmedia/%7BDAA8404B-FEE7-4BDA-8A1E-A32494783B7F%7D. Accessed June 4, 2018.
3. Delirium: prevention, diagnosis and management. NICE Guidance 2010; https://www.nice.org.uk/guidance/cg103. Accessed June 4, 2018.
4. Michaud L, Bula C, Berney A, et al. Delirium: guidelines for general hospitals. J Psychosom Res. 2007;62(3):371-383. doi: 10.1016/j.jpsychores.2006.10.004. PubMed
5. Bush SH, Marchington KL, Agar M, Davis DH, Sikora L, Tsang TW. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7(3):e013809. doi: 10.1136/bmjopen-2016-013809. PubMed
6. Lai MM, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012;42(4):422-427. doi: 10.1111/j.1445-5994.2010.02400.x. PubMed
7. Hijazi Z, Lange P, Watson R, Maier AB. The use of cerebral imaging for investigating delirium aetiology. Eur J Intern Med. 2018;52:35-39. doi: 10.1016/j.ejim.2018.01.024. PubMed
8. Oldenbeuving AW, de Kort PL, Jansen BP, Roks G, Kappelle LJ. Delirium in acute stroke: a review. Int J Stroke. 2007;2(4):270-275. doi: 10.1111/j.1747-4949.2007.00163.x. PubMed
9. Benbadis SR, Sila CA, Cristea RL. Mental status changes and stroke. J Stroke Cerebrovasc Dis. 1994;4(4):216-219. doi: 10.1016/S1052-3057(10)80093-X. PubMed
10. Theisen-Toupal J, Breu AC, Mattison ML, Arnaout R. Diagnostic yield of head computed tomography for the hospitalized medical patient with delirium. J Hosp Med. 2014;9(8):497-501. doi: 10.1002/jhm.2198. PubMed
11. Vijayakrishnan R, Ramasubramanian A, Dhand S. Utility of head CT scan for acute inpatient delirium. Hosp Top. 2015;93(1):9-12. doi: 10.1080/00185868.2015.1012928. PubMed
12. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255. PubMed
13. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298. doi: 10.1016/S0140-6736(07)60151-2. PubMed
14. Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12(5):453-457. doi: 10.1016/j.jacr.2014.12.016. PubMed
15. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149. PubMed
16. Eskandary H, Sabbagh M, Khajehpour F, Eskandari M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63(6):550-553; discussion 553. doi: 10.1016/j.surneu.2004.07.049. PubMed
17. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828. doi: 10.1056/NEJMoa070972. PubMed
18. Morris Z, Whiteley WN, Longstreth WT, Jr., et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. PubMed
19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941. PubMed
20. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830. doi: 10.1111/j.1532-5415.2008.01674.x. PubMed
21. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. doi: 10.1002/jhm.2418. PubMed
22. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing the risk of delirium. Am J Psychiatry. 1992;149(10):1393-1394. doi: 10.1176/ajp.149.10.1393. PubMed
23. Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care. Am J Med. 1999;106(5):565-573. doi: 0.1016/S0002-9343(99)00070-4. PubMed
24. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. doi: 10.1056/NEJMc1714932. PubMed
25. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857. doi: 10.1001/jama.1996.03530350034031. PubMed
© 2019 Society of Hospital Medicine
The Association of Discharge Before Noon and Length of Stay in Hospitalized Pediatric Patients
Many hospitals and emergency departments (EDs) face challenges posed by overcrowding and hospital throughput. Slow ED throughput has been associated with worse patient outcomes.1 One strategy increasingly employed to improve hospital throughput is to increase the rate of inpatient discharges earlier in the day, which is often defined as discharges before noon (DCBNs). The hypothesis behind DCBN is that earlier hospital discharges will allow for earlier ED admissions and thus mitigate ED overcrowding while optimizing inpatient hospital flow. Previous quality improvement efforts to increase the percentage of DCBNs have been successfully implemented. For example, Wertheimer et al. implemented a process for earlier discharges and reported a 27-percentage point (11% to 38%) increase in DCBN on general medicine units.2 In a recent survey among leaders in hospital medicine programs, a majority reported early discharge as an important institutional goal.3
Studies of the effectiveness of DCBN initiatives on improving throughput and shortening length of stay (LOS) in adult patients have had mixed results. Computer modeling has supported the idea that earlier inpatient discharges would shorten ED patient boarding time.4
A question of interest for hospitals is if DCBN is a good indicator of shorter LOS, or is DCBN an arbitrary indicator, as morning discharges might just be the result of a delayed discharge of a patient ready for discharge the prior afternoon/evening. Our study objectives were: (1) to determine whether DCBN is associated with a shorter LOS in a pediatric population at an academic medical center, and (2) to examine separately this association in medical and surgical patients given the different provider workflow and patient clinical characteristics in those groups.
PATIENTS AND METHODS
Patients and Settings
We included patients 21 years or younger with an inpatient admission to any of the following pediatric medical or surgical services: cardiac surgery, cardiology, endocrinology, gastroenterology, general services, hematology/oncology, nephrology, orthopedics, otolaryngology, plastic surgery, pulmonology, and urology. Patients whose stay did not extend beyond one midnight were excluded because discharge time of day for these short stays was strongly related to the time of admission. We also excluded patients whose stay extended beyond two standard deviations of the average LOS for the discharge service under the assumption that these patients represented atypical circumstances. Finally, we excluded patients who died or left against medical advice. A consortium diagram of all exclusion criteria can be found in Supplemental Figure 1. Discharge data were extracted from the Carolina Database Warehouse, a data repository of the University of North Carolina Health System. The University of North Carolina Institutional Review Board reviewed and approved this study (IRB 17-0500).
Measures
The outcome of interest was LOS, defined as discharge date and time minus admission date and time, and thus a continuous measure of time in the hospital rather than a number of midnights. Rajkomar et al. used the same definition of LOS.6 The independent variable of interest was whether the discharge occurred before noon. Because discharges between midnight and 8:00
All model covariates were collected at the patient level (Table 1)
Statistical Analysis
Student t tests and χ2 statistics were used to compare baseline characteristics of hospitalizations of patients DCBN and after noon. We used ordinary least squares (OLS) regression models to assess the association between DCBN and LOS. Because DCBN may be correlated with patient characteristics, we used propensity score weighted models. Propensity scores were estimated using a logistic regression predicting DCBN using the variables given in Table 1 (excluding the outcome variable LOS). To estimate the average treatment effect on the entire sample for each model, we weighted each observation by the inverse-probability of treatment as per recent propensity score methods detailed by Garrido et al.9 In the inverse-probability weighted models, we clustered on attending physician to adjust for the autocorrelation caused by unobservable similarities of discharges by the same attending. We tested for multicollinearity using the variance inflation factor (VIF). To test our secondary hypothesis that there was a difference in the relationship between DCBN and LOS based on service type (medical versus surgical), we tested if the service type moderated any of the coefficients using a joint Wald test on the 10 coefficients interacted with the service type.
For our sensitivity analysis, we reran all surgical and medical discharges models changing the LOS outlier exclusion criteria to greater than three and then four standard deviations. Statistical modeling and analysis were completed using Stata version 14 (StataCorp, College Station, Texas).
RESULTS
Our study sample comprised 8,226 pediatric hospitalizations with a LOS mean of 5.10 and a median of 3.91 days respectively (range, 1.25-32.83 days). There were 1,531 (18.6%) DCBNs. Compared to those discharged after noon, patients with DCBN had a higher probability of being surgical patients, having commercial insurance, discharge home with self-care, discharge on the weekend, and discharge from a nonquality improvement unit (Table 1). Patients with DCBN were also more likely to be white, non-Hispanic, and male.
Our propensity score weighted ordinary least score (OLS) LOS regression results are presented in Table 2. In the bivariate analysis, DCBN was associated with an average 0.40 day, or roughly 10 hours, shorter LOS (P < .001). In the multivariate model of all discharges, we found that DCBN was associated with a mean of 0.27 day (P = .010) shorter LOS when compared to discharge in the afternoon when controlling for age, race, ethnicity, weekend discharge, discharge from quality improvement unit, discharge service type, CMI, insurance type, and discharge disposition.
There was no evidence of multicollinearity (mean VIF of 1.14). The Wald test returned an F statistic of 27.50 (P < .001) indicating there was a structural difference in the relationship between LOS and DCBN dependent on discharge service type; thus, we ran separate surgical and medical discharge models to interpret model coefficients for both service types. When we analyzed surgical and medical discharges in separate models, the effect of DCBN on LOS in the medical discharges model was significantly associated with a 0.30 day (P = .017) shorter LOS (Table 2). The association was not significant in the surgical discharges model.
To further test the analysis, we increased the LOS outlier exclusion criteria to three and four standard deviations.
DISCUSSION
The differential effect of DCBN on LOS in surgical and medical discharges suggests that the relationship between DCBN and LOS may be related to provider team workflow. For example, surgical teams may tend to round one time per day early in the morning before spending the entire day in the operating room, and thus completing more early morning discharge orders compared to medical teams. However, if a patient on a surgical service is not ready for discharge first thing in the morning, the patient may be more likely to wait until the following morning for a discharge order. On medical services, physician schedules may allow for more flexibility for rounding and responding with a discharge order when a patient becomes ready; however, medical services may round later in the day compared to surgeons and for a longer period of time, delaying discharges beyond noon that could have been made earlier. Another possibility, given UNC pediatric services are loosely regionalized with surgical patients concentrated more in one unit, is that unit-level differences in how staff processed discharges could have contributed to the difference observed between medical and surgical patients, particularly as there was a unit-level quality improvement effort for decreasing discharge time on one of two medical floors. However, we analyzed for differences based on the discharging unit and found no association. The influence of outliers on the association between DCBN and LOS increases also suggests that this group of children who have extremely long hospital stays might need further exploration.
Our study has some similar and some contrasting results with prior studies in adult patients. Our findings support the modeling literature that suggests DCBN may improve discharge efficiency by shortening patient LOS for some discharges.4 These findings contrast with Rajkomar et al., who reported that DCBN was associated with a longer LOS in adult patients.6 The contrasting findings could be due to differences in pediatric versus adult patients.
Our study has several limitations. While we controlled for observable characteristics using covariates and propensity score weighted analyses, there are likely unobservable characteristics that confound our analysis. We did not measure other factors that may affect discharge time of day such as high occupancy, staffing levels, patient transportation availability, and patient and family preferences. Given these limitations, we caution against interpreting a causal relationship between independent variables and the outcome. Finally, this analysis was conducted at a single tertiary care, academic medical center. The majority of pediatric admissions at this institution are either transferred from other hospitals or scheduled admissions for medical or surgical care. A smaller proportion of discharges are acute, unplanned admissions through our emergency department in children with or without underlying medical complexity. These factors plus the exclusion of observation, extended recovery, and all the less than two-day stays in this study contribute to a relatively higher average LOS. These factors potentially limit generalizability to other care settings. Additionally, the majority of the care teams involve care by resident physicians, and they are often the primary caregivers and write the majority of orders in patient charts such as discharge orders. While we were not able to control for within resident physician similarities between patients, we did control for autocorrelation at the attending level.
CONCLUSION
The results of our study suggest that DCBN is associated with a decreased LOS for medical but not surgical pediatric patients. DCBN may not be an appropriate measure for all services. Further research should be done to identify other feasible but more valid indicators for shorter LOS.
Disclosures
The authors have no financial relationships relative to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
Funding
There were no external sources of funding for this work.
1. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. doi:10.1111/j.1553-2712.2008.00295.x. PubMed
2. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
3. Patel H, Fang MC, Mourad M, et al. Hospitalist and internal medicine leaders’ perspectives of early discharge challenges at academic medical centers. J Hosp Med. 2017;13(6):388-391. doi: 10.12788/jhm.2885. PubMed
4. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi:10.1016/j.jemermed.2010.06.028. PubMed
5. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi:10.1002/jhm.2412. PubMed
6. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. doi:10.1002/jhm.2529. PubMed
7. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. doi:10.1016/j.amjmed.2014.12.011. PubMed
8. Sauer B, Brookhart MA, Roy JA, VanderWeele TJ. Covariate selection. In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, eds. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. PubMed
9. Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49(5):1701-1720. doi:10.1111/1475-6773.12182. PubMed
10. Maguire P. Do discharge-before-noon Intiatives work? 2016. https://www.todayshospitalist.com/do-discharge-before-noon-initiatives-work/. Accessed April, 2018.
Many hospitals and emergency departments (EDs) face challenges posed by overcrowding and hospital throughput. Slow ED throughput has been associated with worse patient outcomes.1 One strategy increasingly employed to improve hospital throughput is to increase the rate of inpatient discharges earlier in the day, which is often defined as discharges before noon (DCBNs). The hypothesis behind DCBN is that earlier hospital discharges will allow for earlier ED admissions and thus mitigate ED overcrowding while optimizing inpatient hospital flow. Previous quality improvement efforts to increase the percentage of DCBNs have been successfully implemented. For example, Wertheimer et al. implemented a process for earlier discharges and reported a 27-percentage point (11% to 38%) increase in DCBN on general medicine units.2 In a recent survey among leaders in hospital medicine programs, a majority reported early discharge as an important institutional goal.3
Studies of the effectiveness of DCBN initiatives on improving throughput and shortening length of stay (LOS) in adult patients have had mixed results. Computer modeling has supported the idea that earlier inpatient discharges would shorten ED patient boarding time.4
A question of interest for hospitals is if DCBN is a good indicator of shorter LOS, or is DCBN an arbitrary indicator, as morning discharges might just be the result of a delayed discharge of a patient ready for discharge the prior afternoon/evening. Our study objectives were: (1) to determine whether DCBN is associated with a shorter LOS in a pediatric population at an academic medical center, and (2) to examine separately this association in medical and surgical patients given the different provider workflow and patient clinical characteristics in those groups.
PATIENTS AND METHODS
Patients and Settings
We included patients 21 years or younger with an inpatient admission to any of the following pediatric medical or surgical services: cardiac surgery, cardiology, endocrinology, gastroenterology, general services, hematology/oncology, nephrology, orthopedics, otolaryngology, plastic surgery, pulmonology, and urology. Patients whose stay did not extend beyond one midnight were excluded because discharge time of day for these short stays was strongly related to the time of admission. We also excluded patients whose stay extended beyond two standard deviations of the average LOS for the discharge service under the assumption that these patients represented atypical circumstances. Finally, we excluded patients who died or left against medical advice. A consortium diagram of all exclusion criteria can be found in Supplemental Figure 1. Discharge data were extracted from the Carolina Database Warehouse, a data repository of the University of North Carolina Health System. The University of North Carolina Institutional Review Board reviewed and approved this study (IRB 17-0500).
Measures
The outcome of interest was LOS, defined as discharge date and time minus admission date and time, and thus a continuous measure of time in the hospital rather than a number of midnights. Rajkomar et al. used the same definition of LOS.6 The independent variable of interest was whether the discharge occurred before noon. Because discharges between midnight and 8:00
All model covariates were collected at the patient level (Table 1)
Statistical Analysis
Student t tests and χ2 statistics were used to compare baseline characteristics of hospitalizations of patients DCBN and after noon. We used ordinary least squares (OLS) regression models to assess the association between DCBN and LOS. Because DCBN may be correlated with patient characteristics, we used propensity score weighted models. Propensity scores were estimated using a logistic regression predicting DCBN using the variables given in Table 1 (excluding the outcome variable LOS). To estimate the average treatment effect on the entire sample for each model, we weighted each observation by the inverse-probability of treatment as per recent propensity score methods detailed by Garrido et al.9 In the inverse-probability weighted models, we clustered on attending physician to adjust for the autocorrelation caused by unobservable similarities of discharges by the same attending. We tested for multicollinearity using the variance inflation factor (VIF). To test our secondary hypothesis that there was a difference in the relationship between DCBN and LOS based on service type (medical versus surgical), we tested if the service type moderated any of the coefficients using a joint Wald test on the 10 coefficients interacted with the service type.
For our sensitivity analysis, we reran all surgical and medical discharges models changing the LOS outlier exclusion criteria to greater than three and then four standard deviations. Statistical modeling and analysis were completed using Stata version 14 (StataCorp, College Station, Texas).
RESULTS
Our study sample comprised 8,226 pediatric hospitalizations with a LOS mean of 5.10 and a median of 3.91 days respectively (range, 1.25-32.83 days). There were 1,531 (18.6%) DCBNs. Compared to those discharged after noon, patients with DCBN had a higher probability of being surgical patients, having commercial insurance, discharge home with self-care, discharge on the weekend, and discharge from a nonquality improvement unit (Table 1). Patients with DCBN were also more likely to be white, non-Hispanic, and male.
Our propensity score weighted ordinary least score (OLS) LOS regression results are presented in Table 2. In the bivariate analysis, DCBN was associated with an average 0.40 day, or roughly 10 hours, shorter LOS (P < .001). In the multivariate model of all discharges, we found that DCBN was associated with a mean of 0.27 day (P = .010) shorter LOS when compared to discharge in the afternoon when controlling for age, race, ethnicity, weekend discharge, discharge from quality improvement unit, discharge service type, CMI, insurance type, and discharge disposition.
There was no evidence of multicollinearity (mean VIF of 1.14). The Wald test returned an F statistic of 27.50 (P < .001) indicating there was a structural difference in the relationship between LOS and DCBN dependent on discharge service type; thus, we ran separate surgical and medical discharge models to interpret model coefficients for both service types. When we analyzed surgical and medical discharges in separate models, the effect of DCBN on LOS in the medical discharges model was significantly associated with a 0.30 day (P = .017) shorter LOS (Table 2). The association was not significant in the surgical discharges model.
To further test the analysis, we increased the LOS outlier exclusion criteria to three and four standard deviations.
DISCUSSION
The differential effect of DCBN on LOS in surgical and medical discharges suggests that the relationship between DCBN and LOS may be related to provider team workflow. For example, surgical teams may tend to round one time per day early in the morning before spending the entire day in the operating room, and thus completing more early morning discharge orders compared to medical teams. However, if a patient on a surgical service is not ready for discharge first thing in the morning, the patient may be more likely to wait until the following morning for a discharge order. On medical services, physician schedules may allow for more flexibility for rounding and responding with a discharge order when a patient becomes ready; however, medical services may round later in the day compared to surgeons and for a longer period of time, delaying discharges beyond noon that could have been made earlier. Another possibility, given UNC pediatric services are loosely regionalized with surgical patients concentrated more in one unit, is that unit-level differences in how staff processed discharges could have contributed to the difference observed between medical and surgical patients, particularly as there was a unit-level quality improvement effort for decreasing discharge time on one of two medical floors. However, we analyzed for differences based on the discharging unit and found no association. The influence of outliers on the association between DCBN and LOS increases also suggests that this group of children who have extremely long hospital stays might need further exploration.
Our study has some similar and some contrasting results with prior studies in adult patients. Our findings support the modeling literature that suggests DCBN may improve discharge efficiency by shortening patient LOS for some discharges.4 These findings contrast with Rajkomar et al., who reported that DCBN was associated with a longer LOS in adult patients.6 The contrasting findings could be due to differences in pediatric versus adult patients.
Our study has several limitations. While we controlled for observable characteristics using covariates and propensity score weighted analyses, there are likely unobservable characteristics that confound our analysis. We did not measure other factors that may affect discharge time of day such as high occupancy, staffing levels, patient transportation availability, and patient and family preferences. Given these limitations, we caution against interpreting a causal relationship between independent variables and the outcome. Finally, this analysis was conducted at a single tertiary care, academic medical center. The majority of pediatric admissions at this institution are either transferred from other hospitals or scheduled admissions for medical or surgical care. A smaller proportion of discharges are acute, unplanned admissions through our emergency department in children with or without underlying medical complexity. These factors plus the exclusion of observation, extended recovery, and all the less than two-day stays in this study contribute to a relatively higher average LOS. These factors potentially limit generalizability to other care settings. Additionally, the majority of the care teams involve care by resident physicians, and they are often the primary caregivers and write the majority of orders in patient charts such as discharge orders. While we were not able to control for within resident physician similarities between patients, we did control for autocorrelation at the attending level.
CONCLUSION
The results of our study suggest that DCBN is associated with a decreased LOS for medical but not surgical pediatric patients. DCBN may not be an appropriate measure for all services. Further research should be done to identify other feasible but more valid indicators for shorter LOS.
Disclosures
The authors have no financial relationships relative to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
Funding
There were no external sources of funding for this work.
Many hospitals and emergency departments (EDs) face challenges posed by overcrowding and hospital throughput. Slow ED throughput has been associated with worse patient outcomes.1 One strategy increasingly employed to improve hospital throughput is to increase the rate of inpatient discharges earlier in the day, which is often defined as discharges before noon (DCBNs). The hypothesis behind DCBN is that earlier hospital discharges will allow for earlier ED admissions and thus mitigate ED overcrowding while optimizing inpatient hospital flow. Previous quality improvement efforts to increase the percentage of DCBNs have been successfully implemented. For example, Wertheimer et al. implemented a process for earlier discharges and reported a 27-percentage point (11% to 38%) increase in DCBN on general medicine units.2 In a recent survey among leaders in hospital medicine programs, a majority reported early discharge as an important institutional goal.3
Studies of the effectiveness of DCBN initiatives on improving throughput and shortening length of stay (LOS) in adult patients have had mixed results. Computer modeling has supported the idea that earlier inpatient discharges would shorten ED patient boarding time.4
A question of interest for hospitals is if DCBN is a good indicator of shorter LOS, or is DCBN an arbitrary indicator, as morning discharges might just be the result of a delayed discharge of a patient ready for discharge the prior afternoon/evening. Our study objectives were: (1) to determine whether DCBN is associated with a shorter LOS in a pediatric population at an academic medical center, and (2) to examine separately this association in medical and surgical patients given the different provider workflow and patient clinical characteristics in those groups.
PATIENTS AND METHODS
Patients and Settings
We included patients 21 years or younger with an inpatient admission to any of the following pediatric medical or surgical services: cardiac surgery, cardiology, endocrinology, gastroenterology, general services, hematology/oncology, nephrology, orthopedics, otolaryngology, plastic surgery, pulmonology, and urology. Patients whose stay did not extend beyond one midnight were excluded because discharge time of day for these short stays was strongly related to the time of admission. We also excluded patients whose stay extended beyond two standard deviations of the average LOS for the discharge service under the assumption that these patients represented atypical circumstances. Finally, we excluded patients who died or left against medical advice. A consortium diagram of all exclusion criteria can be found in Supplemental Figure 1. Discharge data were extracted from the Carolina Database Warehouse, a data repository of the University of North Carolina Health System. The University of North Carolina Institutional Review Board reviewed and approved this study (IRB 17-0500).
Measures
The outcome of interest was LOS, defined as discharge date and time minus admission date and time, and thus a continuous measure of time in the hospital rather than a number of midnights. Rajkomar et al. used the same definition of LOS.6 The independent variable of interest was whether the discharge occurred before noon. Because discharges between midnight and 8:00
All model covariates were collected at the patient level (Table 1)
Statistical Analysis
Student t tests and χ2 statistics were used to compare baseline characteristics of hospitalizations of patients DCBN and after noon. We used ordinary least squares (OLS) regression models to assess the association between DCBN and LOS. Because DCBN may be correlated with patient characteristics, we used propensity score weighted models. Propensity scores were estimated using a logistic regression predicting DCBN using the variables given in Table 1 (excluding the outcome variable LOS). To estimate the average treatment effect on the entire sample for each model, we weighted each observation by the inverse-probability of treatment as per recent propensity score methods detailed by Garrido et al.9 In the inverse-probability weighted models, we clustered on attending physician to adjust for the autocorrelation caused by unobservable similarities of discharges by the same attending. We tested for multicollinearity using the variance inflation factor (VIF). To test our secondary hypothesis that there was a difference in the relationship between DCBN and LOS based on service type (medical versus surgical), we tested if the service type moderated any of the coefficients using a joint Wald test on the 10 coefficients interacted with the service type.
For our sensitivity analysis, we reran all surgical and medical discharges models changing the LOS outlier exclusion criteria to greater than three and then four standard deviations. Statistical modeling and analysis were completed using Stata version 14 (StataCorp, College Station, Texas).
RESULTS
Our study sample comprised 8,226 pediatric hospitalizations with a LOS mean of 5.10 and a median of 3.91 days respectively (range, 1.25-32.83 days). There were 1,531 (18.6%) DCBNs. Compared to those discharged after noon, patients with DCBN had a higher probability of being surgical patients, having commercial insurance, discharge home with self-care, discharge on the weekend, and discharge from a nonquality improvement unit (Table 1). Patients with DCBN were also more likely to be white, non-Hispanic, and male.
Our propensity score weighted ordinary least score (OLS) LOS regression results are presented in Table 2. In the bivariate analysis, DCBN was associated with an average 0.40 day, or roughly 10 hours, shorter LOS (P < .001). In the multivariate model of all discharges, we found that DCBN was associated with a mean of 0.27 day (P = .010) shorter LOS when compared to discharge in the afternoon when controlling for age, race, ethnicity, weekend discharge, discharge from quality improvement unit, discharge service type, CMI, insurance type, and discharge disposition.
There was no evidence of multicollinearity (mean VIF of 1.14). The Wald test returned an F statistic of 27.50 (P < .001) indicating there was a structural difference in the relationship between LOS and DCBN dependent on discharge service type; thus, we ran separate surgical and medical discharge models to interpret model coefficients for both service types. When we analyzed surgical and medical discharges in separate models, the effect of DCBN on LOS in the medical discharges model was significantly associated with a 0.30 day (P = .017) shorter LOS (Table 2). The association was not significant in the surgical discharges model.
To further test the analysis, we increased the LOS outlier exclusion criteria to three and four standard deviations.
DISCUSSION
The differential effect of DCBN on LOS in surgical and medical discharges suggests that the relationship between DCBN and LOS may be related to provider team workflow. For example, surgical teams may tend to round one time per day early in the morning before spending the entire day in the operating room, and thus completing more early morning discharge orders compared to medical teams. However, if a patient on a surgical service is not ready for discharge first thing in the morning, the patient may be more likely to wait until the following morning for a discharge order. On medical services, physician schedules may allow for more flexibility for rounding and responding with a discharge order when a patient becomes ready; however, medical services may round later in the day compared to surgeons and for a longer period of time, delaying discharges beyond noon that could have been made earlier. Another possibility, given UNC pediatric services are loosely regionalized with surgical patients concentrated more in one unit, is that unit-level differences in how staff processed discharges could have contributed to the difference observed between medical and surgical patients, particularly as there was a unit-level quality improvement effort for decreasing discharge time on one of two medical floors. However, we analyzed for differences based on the discharging unit and found no association. The influence of outliers on the association between DCBN and LOS increases also suggests that this group of children who have extremely long hospital stays might need further exploration.
Our study has some similar and some contrasting results with prior studies in adult patients. Our findings support the modeling literature that suggests DCBN may improve discharge efficiency by shortening patient LOS for some discharges.4 These findings contrast with Rajkomar et al., who reported that DCBN was associated with a longer LOS in adult patients.6 The contrasting findings could be due to differences in pediatric versus adult patients.
Our study has several limitations. While we controlled for observable characteristics using covariates and propensity score weighted analyses, there are likely unobservable characteristics that confound our analysis. We did not measure other factors that may affect discharge time of day such as high occupancy, staffing levels, patient transportation availability, and patient and family preferences. Given these limitations, we caution against interpreting a causal relationship between independent variables and the outcome. Finally, this analysis was conducted at a single tertiary care, academic medical center. The majority of pediatric admissions at this institution are either transferred from other hospitals or scheduled admissions for medical or surgical care. A smaller proportion of discharges are acute, unplanned admissions through our emergency department in children with or without underlying medical complexity. These factors plus the exclusion of observation, extended recovery, and all the less than two-day stays in this study contribute to a relatively higher average LOS. These factors potentially limit generalizability to other care settings. Additionally, the majority of the care teams involve care by resident physicians, and they are often the primary caregivers and write the majority of orders in patient charts such as discharge orders. While we were not able to control for within resident physician similarities between patients, we did control for autocorrelation at the attending level.
CONCLUSION
The results of our study suggest that DCBN is associated with a decreased LOS for medical but not surgical pediatric patients. DCBN may not be an appropriate measure for all services. Further research should be done to identify other feasible but more valid indicators for shorter LOS.
Disclosures
The authors have no financial relationships relative to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
Funding
There were no external sources of funding for this work.
1. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. doi:10.1111/j.1553-2712.2008.00295.x. PubMed
2. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
3. Patel H, Fang MC, Mourad M, et al. Hospitalist and internal medicine leaders’ perspectives of early discharge challenges at academic medical centers. J Hosp Med. 2017;13(6):388-391. doi: 10.12788/jhm.2885. PubMed
4. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi:10.1016/j.jemermed.2010.06.028. PubMed
5. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi:10.1002/jhm.2412. PubMed
6. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. doi:10.1002/jhm.2529. PubMed
7. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. doi:10.1016/j.amjmed.2014.12.011. PubMed
8. Sauer B, Brookhart MA, Roy JA, VanderWeele TJ. Covariate selection. In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, eds. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. PubMed
9. Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49(5):1701-1720. doi:10.1111/1475-6773.12182. PubMed
10. Maguire P. Do discharge-before-noon Intiatives work? 2016. https://www.todayshospitalist.com/do-discharge-before-noon-initiatives-work/. Accessed April, 2018.
1. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. doi:10.1111/j.1553-2712.2008.00295.x. PubMed
2. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
3. Patel H, Fang MC, Mourad M, et al. Hospitalist and internal medicine leaders’ perspectives of early discharge challenges at academic medical centers. J Hosp Med. 2017;13(6):388-391. doi: 10.12788/jhm.2885. PubMed
4. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi:10.1016/j.jemermed.2010.06.028. PubMed
5. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi:10.1002/jhm.2412. PubMed
6. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. doi:10.1002/jhm.2529. PubMed
7. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. doi:10.1016/j.amjmed.2014.12.011. PubMed
8. Sauer B, Brookhart MA, Roy JA, VanderWeele TJ. Covariate selection. In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, eds. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. PubMed
9. Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49(5):1701-1720. doi:10.1111/1475-6773.12182. PubMed
10. Maguire P. Do discharge-before-noon Intiatives work? 2016. https://www.todayshospitalist.com/do-discharge-before-noon-initiatives-work/. Accessed April, 2018.
© 2019 Society of Hospital Medicine