User login
Multimodal Treatment of Epidermodysplasia Verruciformis in an HIV-Positive Man
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.
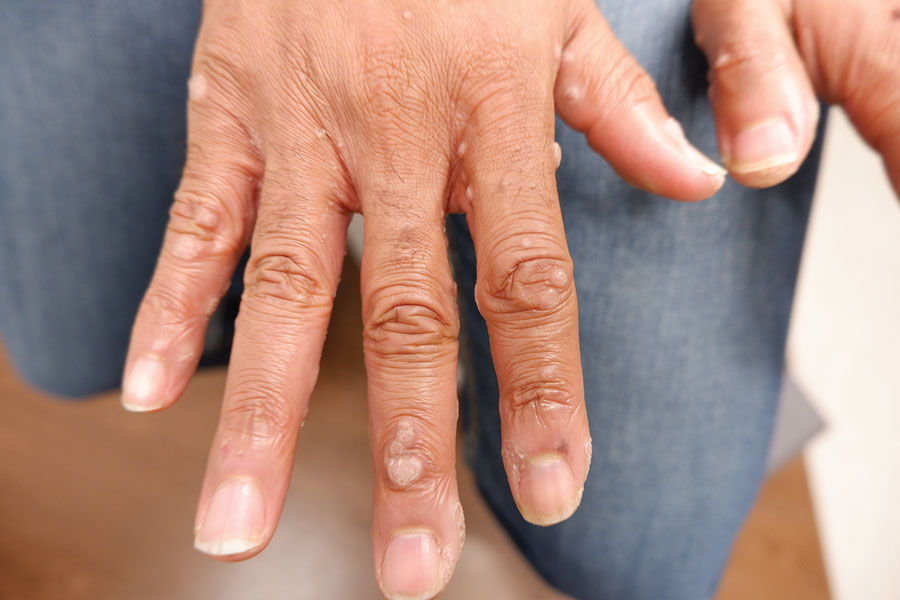
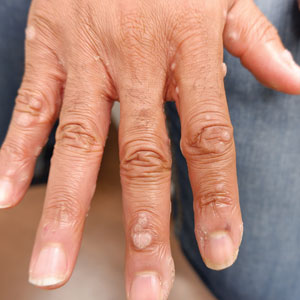
A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.
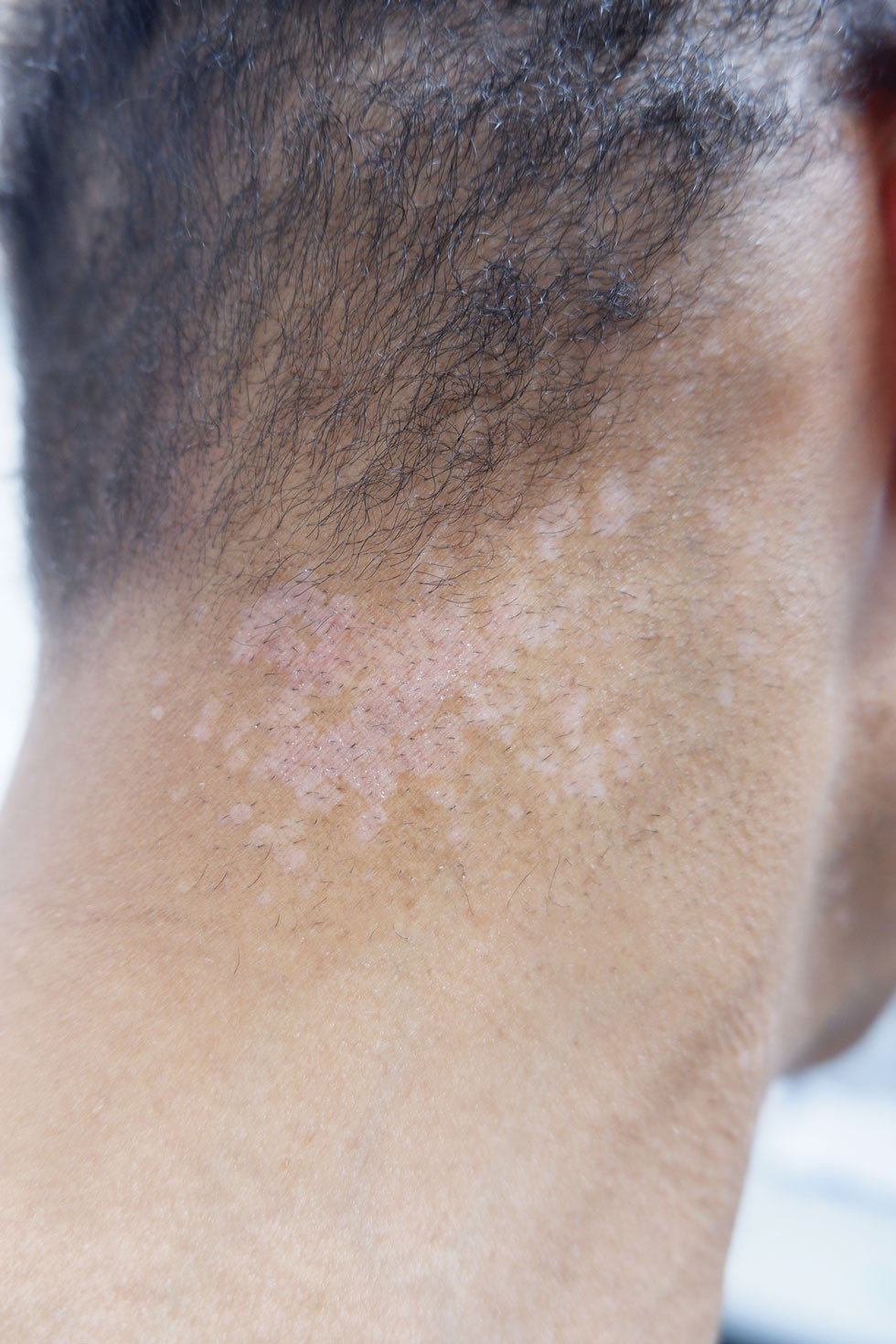
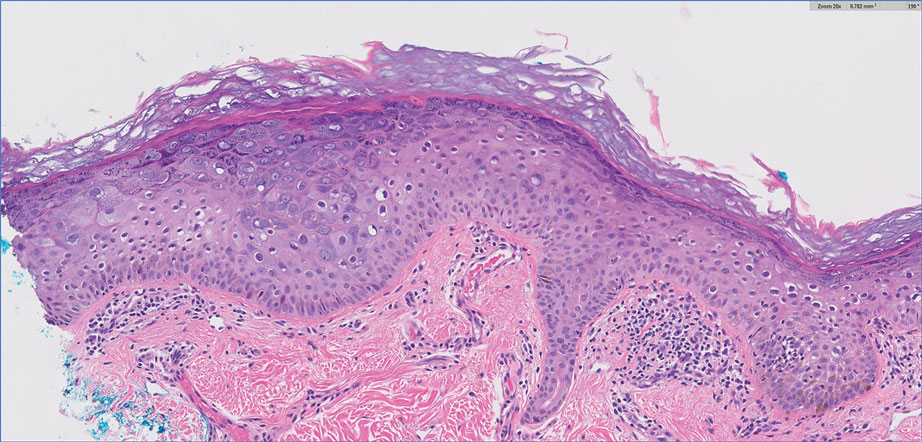
Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.
Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.

A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.

Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.

Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
To the Editor:
Epidermodysplasia verruciformis (EDV) is a rare generalized form of epidermal dysplasia that is linked to certain subtypes of human papillomavirus (HPV) infection and inherited or acquired states of immunodeficiency.1-3 The inherited form most commonly manifests via autosomal-recessive inactivation of the EVER1 and EVER2 genes that encode integral membrane proteins in the endoplasmic reticulum, though cases of autosomal-dominant and X-linked inheritance have been reported.1-3 Acquired cases have been reported in patients lacking immunocompetency, including transplant recipients and patients living with HIV.4-11 We present the case of a patient with HIV-associated EDV who was treated successfully with intralesional Candida albicans antigen, oral acitretin, and cryotherapy.

A 56-year-old man presented for evaluation of several cutaneous lesions that had developed over several months on the neck and over many years on the hands and feet. He had a 16-year history of HIV, Castleman disease, and primary effusion lymphoma in remission that was treated with rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride 10 or more years ago. The patient denied pruritus or pain associated with the skin lesions. He was intermittently taking immunosuppressants and antiretrovirals including dolutegravir and emtricitabine-tenofovir for 3 years. Prior treatments of the lesions included cryotherapy and over-the-counter 17% salicylic acid. Physical examination revealed the presence of innumerable, clustered, verrucous, scaly papules on the dorsal and palmoplantar regions of the hands (Figure 1), as well as hypopigmented macules clustered on the neck that morphologically resembled tinea versicolor (Figure 2). The physical examination was otherwise unremarkable.

Complete blood cell counts as well as lipid, liver, and renal function panel results were unremarkable. Laboratory examination also revealed a CD4 cell count of 373/µL (reference range, 320–1900/µL) and an undetectable HIV copy number (<40 copies/mL). A punch biopsy of a hypopigmented macule on the left side of the neck revealed epidermal acanthosis, hypergranulosis, and hyperkeratosis, with blue-gray cytoplasm observed in the keratinocytes (Figure 3). Koilocytes with perinuclear clearing associated with keratinocytes in the upper epidermis were noted. Based on the clinical and histopathologic correlation, acquired EDV was diagnosed.

Given that HIV-associated EDV often is recalcitrant and there is a lack of consistent and effective treatment, the patient initially was prescribed oral acitretin 25 mg/d with intralesional C albicans antigen injected once per month into the lesions along with concurrent cryotherapy. At subsequent monthly follow-ups, the involved areas were notably thinner and flat. The patient reported no remarkable side effects from the systemic retinoid treatment such as abdominal pain, photosensitivity, or headaches, though he did experience mild xerosis. Complete resolution of EDV occurred with multimodal therapy—acitretin, cryotherapy, and intralesional Candida antigen. Palmar verrucae were much improved, and he is currently continuing therapy.
Epidermodysplasia verruciformis is a rare genodermatosis associated with an abnormal susceptibility to cutaneous HPV and can be acquired in immunocompromised patients. Patients with EDV present with a clinically heterogeneous disease that can manifest as hypopigmented, red-brown macules with scaling on the trunk, neck, and extremities, which are morphologically similar to tinea versicolor, or patients can present with flat wartlike papules that are most commonly found on the face, hands, and feet.2,3 Epidermodysplasia verruciformis can be distinguished from EDV-like eruptions and other generalized verrucoses by its characteristic histologic appearance and by the demonstration of HPV within the lesions, typically subtypes HPV-5 and HPV-8.1-3 Classic EDV histopathologic findings include mild to moderate acanthosis and hyperkeratosis with enlarged keratinocytes featuring blue-gray cytoplasm and perinuclear halos.1
The histologic differential diagnosis of EDV is quite broad and includes common verrucae, which may be distinguished by the absence of blue-gray discoloration of the cytoplasm among the individual keratinocytes.1 Verruca plana and condylomata also may mimic EDV, and patients may present with minimal papillomatosis of the surface epidermis.2 Squamous cell carcinoma in situ (SCC-IS) and particularly bowenoid papulosis also may share similar histologic features.2 However, in SCC-IS, there typically is full-thickness dysplasia of the epidermis, which is not present in EDV. Nonetheless, EDV is equivalent to SCC-IS in its clinical behavior. Bowenoid papulosis shares similar findings, but lesions generally are located in the genital areas and linked to HPV-16 and HPV-18.2 Additional histologic features of EDV have been described in the entity of EDV acanthoma, specifically incidental findings present in association with other cutaneous neoplasms including acantholytic acanthomas, condylomas, intradermal nevi, and seborrheic keratoses.12
The pathophysiology of EDV is thought to be specifically associated with patients with immunocompromised conditions. Particular attention has been paid to the association between EDV and HIV. Anselmo et al13 reported a case of HIV-associated acquired EDV with preexisting lesions that were spread along the distribution of the patient’s tattoo, suggesting potential autoinoculation. In individuals living with HIV, the cutaneous features of EDV are not associated with immune status.14
Acquired EDV also may be associated with other conditions including renal transplantation, IgM deficiency, severe combined immunodeficiency, common variable immunodeficiency, systemic lupus erythematosus, and myasthenia gravis.2 Hematologic malignancies such as Hodgkin disease,4 natural killer/T-cell lymphoma,5 cutaneous T-cell lymphoma,6 adult T-cell leukemia,7 intestinal diffuse large B-cell lymphoma,8,9 transformed acute myelogenous lymphoma,10 and chronic myelogenous leukemia11 also may be associated with EDV. In the inherited form, integral membrane proteins of the endoplasmic reticulum encoded by the genes EVER1 and EVER2 on chromosome 17 are thought to act as restriction factors for certain types of HPV.2,3 Inactivating mutations in EVER1 and EVER2 result in defects in cell-mediated immunity, rendering patients susceptible to both benign and oncogenic verrucous infections.2,3 Currently, it is believed that immunosuppressed states may result in defects in cell-mediated immunity that make patients similarly susceptible to these virulent strains of HPV, resulting in an acquired form of EDV.3 Interestingly, the clinical and histologic presentation is identical for acquired EDV and genetic EDV.
Due to the general resistance of EDV to treatment, a variety of options for acquired EDV have been explored including topical and systemic retinoids, cryotherapy, interferon alfa‐2a, zidovudine, ketoconazole, corticosteroids, podophyllotoxin, imiquimod, cidofovir, electrosurgery, 5‐fluorouracil, glycolic acid, temporized diathermy, and methyl aminolevulinate photodynamic therapy.3 Highly active antiretroviral therapy has been proposed as a potential treatment modality for HIV-associated cases; however, acquired EDV has been reported to develop as an immune reconstitution inflammatory syndrome after the initiation of highly active antiretroviral therapy.15
Combination therapy consisting of a systemic retinoid, immunotherapy, and cryotherapy was initiated for our patient. Human papillomavirus infection is marked by epithelial hyperplasia, and retinoids induce antiproliferation through the control of epithelial cell differentiation.16 The specific mechanism of action of retinoids in EDV treatment is unknown; however, the beneficial effects may result from the modification of terminal differentiation, a direct antiviral action, or the enhancement of killer T cells.17 Immunotherapy with C albicans antigen initiates an inflammatory reaction that leads to an immune response directed against the virus, thus reducing the number of warts.2 Cryotherapy aims to destroy the lesion but not the virus.2 The combination of systemic retinoids, immunotherapy, and destruction may target EDV via multiple potentially synergistic mechanisms. Thus, a multimodal approach can be beneficial in patients with recalcitrant acquired EDV.
The occurrence of EDV is rare, and data on treatment are limited in number resulting in general uncertainty about the efficacy of therapies. Elucidation of the specific mechanism of immunosuppression and its effects on T lymphocytes in acquired EDV may shed light on the most effective treatments. We present this novel case of a patient with HIV-associated acquired EDV who responded favorably to a combination treatment of acitretin, intralesional C albicans antigen, and cryotherapy.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
- Nuovo GJ, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Sri JC, Dubina MI, Kao GF, et al. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. J Am Acad Dermatol. 2012;66:292-311.
- Zampetti A, Giurdanella F, Manco S, et al. Acquired epidermodysplasia verruciformis: a comprehensive review and a proposal for treatment. Dermatol Surg. 2013;39:974-980.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Boran P, Tokuc G, Ozberk M, et al. Epidermodysplasia verruciformis associated with natural killer/T cell lymphoma. J Pediatr. 2010;156:340-340.e1.
- Cutlan JE, Rashid RM, Torres-Cabala C, et al. Epidermodysplasia verruciformis after cutaneous T-cell lymphoma: periungual presentation. Dermatol Online J. 2010;16:12.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Slawsky LD, Gilson RT, Hockley AJ, et al. Epidermodysplasia verruciformis associated with severe immunodeficiency, lymphoma, and disseminated molluscum contagiosum. J Am Acad Dermatol. 1992;27:448-450.
- Youssef M, Denguezli M, Ghariani N, et al. Epidermodysplasia verruciformis associated with intestinal lymphoma: a model of viral oncogenicity. Pediatr Dermatol. 2007;24:511-513.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Binkley GW. A case for diagnosis (epidermodysplasia verruciformis?) chronic myeloid leukemia. Arch Derm Syphilol. 1947;55:280-282.
- Ko CJ, Iftner T, Barr RJ, et al. Changes of epidermodysplasia verruciformis in benign skin lesions: the EV acanthoma. J Cutan Pathol. 2007;34:44-48.
- Anselmo F, Ansari U, Gagnier JM, et al. Verrucous lesions in an HIV-positive man. JAAD Case Reports. 2019;5:825-827.
- Huang S, Wu JH, Lewis DJ, et al. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol. 2018;57:1344-1350.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Limmer AL, Wu JH, Doan HQ, et al. Acquired epidermodysplasia verruciformis: a 10-year anniversary update. Br J Dermatol. 2020;182:790-792.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a.J Am Acad Dermatol. 2001;45:296-299.
Practice Points
- Acquired epidermodysplasia verruciformis (EDV) is associated with immunocompromised patients with conditions such as HIV.
- Multimodal treatment of HIV-associated acquired EDV with acitretin, intralesional Candida albicans antigen, and cryotherapy may be efficacious for patients with recalcitrant disease.
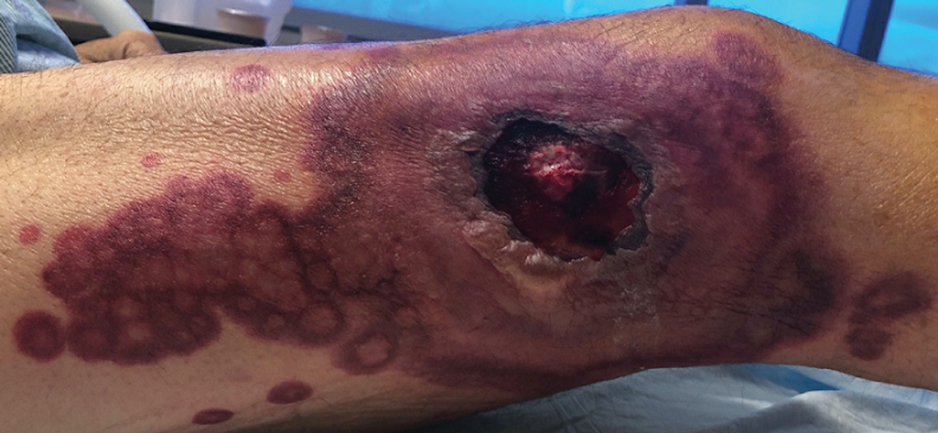
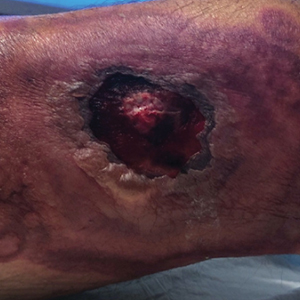
Violaceous-Purpuric Targetoid Macules and Patches With Bullae and Ulceration
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
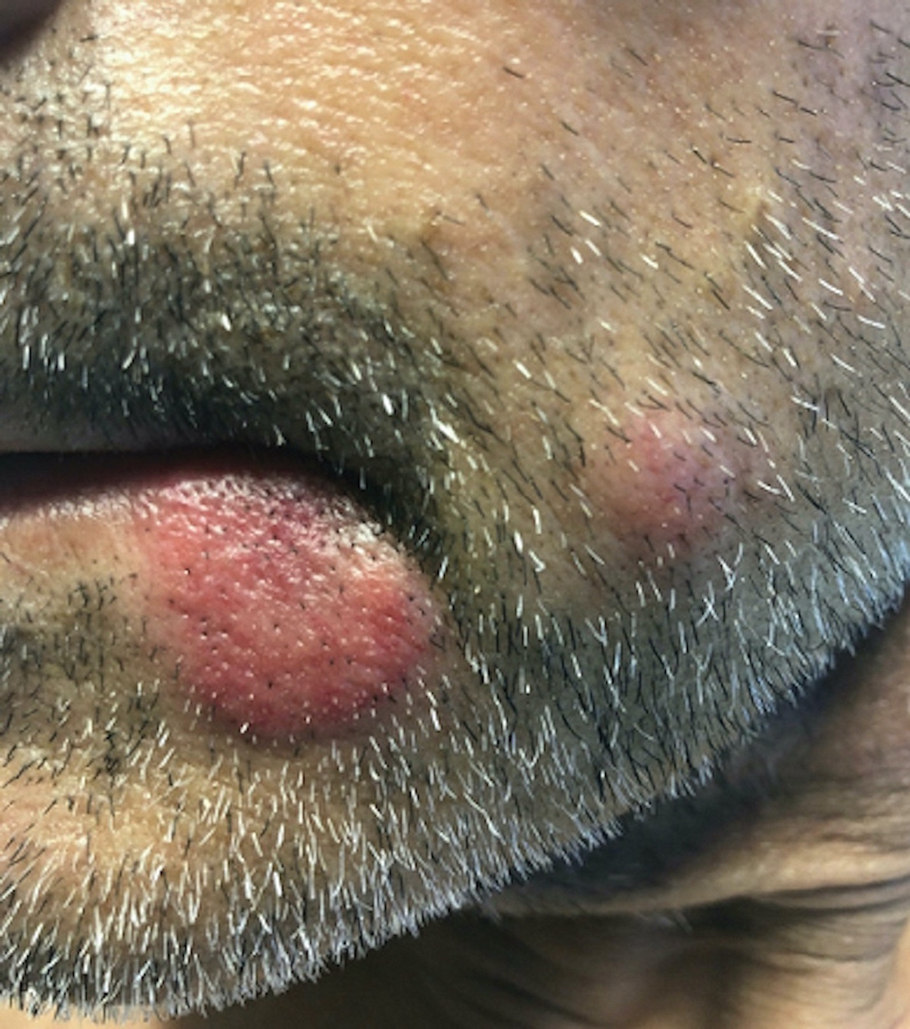
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.
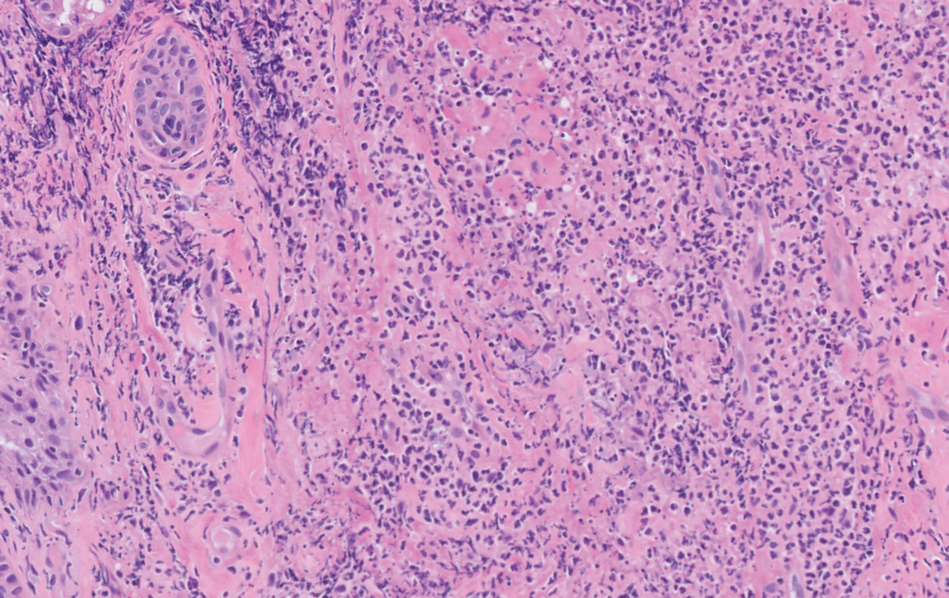
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1
The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.

Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1

The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.

Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1

The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
A 64-year-old man with long-standing myelofibrosis presented with neutropenic fevers as well as progressive painful lesions of 3 days’ duration on the legs. A bone marrow biopsy during this hospitalization demonstrated a recent progression of the patient’s myelofibrosis to acute myeloid leukemia. Physical examination revealed round to oval, violaceous, targetoid plaques. Within a week, new erythematous and nodular lesions appeared on the right arm and left vermilion border. The lesions on the legs enlarged, formed bullae, and ulcerated.