User login
When should lipid-lowering therapy be started in the hospitalized patient?
Case
A 52-year-old man with no medical history other than a transient ischemic attack (TIA) three months ago presents to the emergency department (ED) following multiple episodes of substernal (ST) chest pressure. He takes no medication. His electrocardiogram (ECG) revealed lateral ST segment depressions, and his cardiac biomarkers were elevated. He underwent cardiac catheterization, and a single drug-eluting stent was successfully placed to a culprit left circumflex lesion. He is now stable less than 24 hours following his initial presentation, without any evidence of heart failure. His providers prescribe aspirin, clopidogrel, metoprolol, and lisinopril. His fasting LDL level is 92 mg/dL.
What, if any, is the role for lipid-lowering therapy at this time?
Overview
Long-term therapy with HMG CoA reductase inhibitors (statins) has been shown through several large, randomized, controlled trials to reduce the risk for death, myocardial infarction (MI), and stroke in patients with established coronary disease. The most significant effects were evident after approximately two years of treatment.1,2,3,4
Subsequent trials have shown earlier and more significant reductions in the rates of recurrent ischemic cardiovascular events following acute coronary syndromes (ACS) when statins are administered early—within days of the initial event. This is a window of time in which most patients still are hospitalized.4,5,6,7
In addition to this data regarding statin use following ACS, a large, randomized, controlled trial demonstrated similar reductions in the incidence of strokes and cardiovascular events when high-dose atorvastatin was administered within one to six months following TIA or stroke in patients without established coronary disease.8 There is growing data supporting the hypothesis that statins have pleiotropic (non cholesterol-lowering), neuroprotective, properties that may improve patient outcomes following cerebrovascular events.9,10,11 There are ongoing trials investing the role of statins in the acute management of stroke.12,13
Hospitalists frequently manage patients in the stages immediately following ACS and stroke. Based on the large and evolving volume of data regarding the use of statins following these events, when and how should a statin be started in the hospital?
Review of the Data
Following Acute Coronary Syndrome: Death and recurrent ischemic events following ACS are most likely to occur in the early phase of recovery. Based on this observation and evidence supporting the early (in some cases within hours of administration) ‘pleiotropic’ or non-cholesterol lowering effects of statins, including improvement in endothelial function and decreases in platelet aggregation, thrombus deposition, and vascular inflammation, the MIRACL study was designed to answer the question of whether the initiation of treatment with a statin within 24 to 96 hours following ACS would reduce the occurrence of death and recurrent ischemia.4,7,14 Investigators randomized 3,086 patients within 24-96 hours (mean 63 hours) following admission for non-ST segment myocardial infarction (NSTEMI) or unstable angina (UA) to receive either atorvastatin 80 mg/d or placebo.
Investigators monitored patients for the primary end points of ischemic events (death, non-fatal MI, cardiac arrest with resuscitation, symptomatic myocardial ischemia with objective evidence) during a 16-week period. In the treatment arm, the risk of the primary combined end point was significantly reduced—relative risk (RR) 0.84; 95% confidence interval (CI), 0.70-1.00; p=0.048. (See Figure 1, pg. 39)
No significant differences were found between atorvastatin and placebo in the risk of death, non-fatal MI, or cardiac arrest with resuscitation. There was, however, a significantly lower risk of recurrent symptomatic myocardial ischemia with objective evidence requiring emergent re-hospitalization in the treatment arm (RR, 0.74; 95% CI, 0.57-0.95; p=0.02). The mean baseline LDL level in the treatment arm was 124 mg/dL, a value that may represent, in part, suppression of the LDL level in the setting of acute ACS. This is a phenomenon previously described in an analysis of the LUNAR trial.15
Suppression of LDL level after ACS appeared to be minimal, however, and is unlikely to be clinically significant. The benefits of atorvastatin in the MIRACL trial did not appear to depend on baseline LDL level—suggesting the decision to initiate statin therapy after ACS should not be influenced by LDL level at the time of the event.
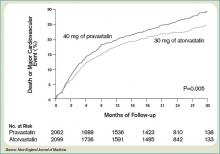
Only one dose of statin was used in the MIRACL trial, and the investigators commented they were unable to determine if a lower dose of atorvastatin or a gradual dose titration to a predetermined LDL target would have achieved similar benefits. The PROVE IT-TIMI 22 trial was designed to compare the reductions in death and major cardiovascular events following ACS between LDL lowering to approximately 100 mg/dL using 40 mg/d of pravastatin, and more intensive LDL lowering to approximately 70 mg/dL using 80 mg/d of atorvastatin.5 Investigators enrolled 4,162 patients for a median of seven days following ACS (STEMI, NSTEMI, or UA) to the two treatment arms. Investigators observed patients for a period of 18 to 36 months for the primary end points of death, MI, UA, revascularization, and stroke. The median LDL level at the time of enrollment was 106 mg/dL in both treatment arms. During follow up, the median LDL levels achieved were 95 mg/dL in the pravastatin group and 62 mg/dL in the atorvastatin group. After two years, a 16% reduction in the hazard ratio for any primary end point was seen favoring 80 mg/d of atorvastatin—p=0.005; 95% CI=5-26%. (See Figure 2, pg. 39) The benefit of high-dose atorvastatin was seen as early as 30 days after randomization and was sustained throughout the trial.
While the PROVE IT-TIMI 22 trial supported a specified dosing strategy for statin use following ACS, Phase Z of the A-to-Z trial was designed to evaluate the early initiation of intensive lipid lowering following ACS, as compared to a delayed and less-intensive strategy.5,6 Investigators randomized 4,497 patients (a mean of 3.7 days following either NSTEMI or STEMI) to receive either placebo for four months followed by simvastatin 20 mg/d or simvastatin 40 mg/d for one month followed by simvastatin 80 mg/d. They followed patients for 24 months for the primary end points of cardiovascular death, MI, readmission for ACS, or stroke. The primary end point occurred in 16.7% of the delayed, lower-intensity treatment group and in 14.4% of the early, higher-intensity treatment group (95% CI 0.76-1.04; p=0.14). Despite the lack of a significant difference in the composite primary end point between the two treatment arms, a significant reduction in the secondary end points of cardiovascular mortality (absolute risk reduction (ARR 1.3%; P=0.05) and congestive heart failure (ARR 1.3%; P=0.04) was evident favoring the early, intensive treatment strategy. These differences were not evident until at least four months after randomization. The A-to-Z trial investigators offered several possible explanations for the delay in evident clinical benefits in their trial when compared against the strong trend toward clinical benefit seen with 30 days following the early initiation of high-dose atorvastatin following ACS in the PROVE IT-TIMI 22 trial. In the PROVE IT trial, patients were enrolled an average of seven days after their index event, and as a result, 69% had undergone revascularization by this time. In the A-to-Z trial, patients were enrolled an average of three to four days earlier, and, therefore, were less likely to have undergone a revascularization procedure by the time of enrollment—and may have continued on with active thrombotic processes relatively less responsive to statin therapy.6 Another notable difference between PROVE IT and A-to-Z subjects was the C-reactive protein (CRP) concentrations in the A-to-Z subjects did not differ between treatment groups within 30 days despite significant differences in their LDL levels.16 This lack of a concurrent, pleiotropic, anti-inflammatory effect in the A-to-Z trial aggressive treatment arm may also have contributed to the delayed treatment effect.
In conclusion, the A-to-Z investigators suggest more intensive statin therapy (than the 40 mg Simvastatin in their intensive treatment arm) may be required to derive the most rapid and maximal clinical benefits during the highest risk period immediately following ACS.
Following stroke: Although there is more robust data supporting the benefits of early, intensive, statin therapy following ACS, there also is established and emerging data supporting similar treatment approaches following stroke.
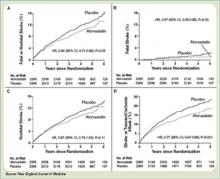
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was designed to determine whether or not atorvastatin 80 mg daily would reduce the risk of stroke in patients without known coronary heart disease who had suffered a TIA or stroke within the preceding six months.8 Patients who experienced a hemorrhagic or ischemic TIA or stroke between one to six months before study entry were randomized to receive either atorvastatin 80 mg/d or placebo.
Investigators followed patients for a mean period of 4.9 years for the primary end point of time to non-fatal or fatal stroke. Secondary composite end points included stroke or TIA, and any coronary or peripheral arterial event, including death from cardiac causes, non-fatal MI, ACS, revascularization (coronary, carotid, peripheral), and death from any cause. No difference in mean baseline LDL levels was witnessed between the treatment and placebo arms (132.7 and 133.7 mg/dL, respectively). Atorvastatin was associated with a 16% relative reduction in the risk of stroke—hazard ratio, 0.84; 95% CI 0.71–0.99; p=0.03. This was found despite an increase in hemorrhagic stroke in the atorvastatin group—a finding that supports an epidemiologic association between low cholesterol levels and brain hemorrhage. The risk of cardiovascular events also was significantly reduced, however, no significant difference in overall mortality was observed between the two groups.
In conclusion, the authors recommend the initiation of high-dose atorvastatin “soon” after stroke or TIA. One can only conclude, based on these data, statin therapy should be initiated within six months of TIA or stroke, in accordance with the study design. There is retrospective data suggesting benefit to statin therapy initiated within four weeks following ischemic stroke, and there are prospective trials in process evaluating the potential benefits of statins initiated within 24 hours following ischemic stroke, however, no large, randomized, controlled trial can demonstrate the effect of statins when used as acute stroke therapy.9,12,13,17
Back to the Case
The patient described in our case has a history of TIA and experienced an acute coronary syndrome (NSTEMI) within the preceding 24 hours. He underwent a revascularization procedure (PCI with stent), and is on appropriate therapy, including dual anti-platelet therapy with aspirin and clopidogrel, a beta-blocker, and an angiotensin-converting enzyme inhibitor. Based on the data and conclusions of the MIRACL, PROVE IT-TIMI 22, and SPARCL trials, high-dose statin therapy with atorvastatin 80 mg/d should be initiated immediately in the patient in order to significantly reduce his risk of recurrent ischemic cardiovascular events and stroke following his acute coronary syndrome and TIA.
Bottom Line
Following ACS, high-dose statin therapy with 80 mg of atorvastatin per day should be initiated when the patient is still in the hospital, irrespective of baseline LDL level. Statin therapy should also strongly be considered for secondary stroke prevention in most patients with a history of stroke or transient ischemic attack. TH
Caleb Hale, MD, is a hospitalist at Beth Israel Deaconess Medical Center in Boston. Joseph Ming Wah Li is director of the hospital medicine program and associate chief, division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston, and assistant professor of Medicine at Harvard Medical School.
References
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
2. Sacks RM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-1009.
3. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-1357.
4. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711-1718.
5. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
6. Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs. a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
7. Waters D, Schwartz GG, Olsson AG. The myocardial ischemia reduction with acute cholesterol lowering (MIRACL) trial: a new frontier for statins? Curr Control Trials Cardiovasc Med. 2001;2;111-114.
8. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
9. Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. 2005;36:1298-1300.
10. Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253-258.
11. Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969-1973.
12. Elkind MS, Sacco RL, MacArthur RB, et al. The neuroprotection with statin therapy for acute recovery trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3:210-218.
13. Montaner J, Chacon P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J of Neurol. 2008;15:82-90.
14. Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20-28.
15. Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. JACC. 2008;51:1440-1445.
16. Wiviott SD, de Lemos JA, Cannon CP, et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials of A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406-1414.
17. Elking MS. Statins as acute-stroke treatment. Int J Stroke. 2006;1:224-225.
Case
A 52-year-old man with no medical history other than a transient ischemic attack (TIA) three months ago presents to the emergency department (ED) following multiple episodes of substernal (ST) chest pressure. He takes no medication. His electrocardiogram (ECG) revealed lateral ST segment depressions, and his cardiac biomarkers were elevated. He underwent cardiac catheterization, and a single drug-eluting stent was successfully placed to a culprit left circumflex lesion. He is now stable less than 24 hours following his initial presentation, without any evidence of heart failure. His providers prescribe aspirin, clopidogrel, metoprolol, and lisinopril. His fasting LDL level is 92 mg/dL.
What, if any, is the role for lipid-lowering therapy at this time?
Overview
Long-term therapy with HMG CoA reductase inhibitors (statins) has been shown through several large, randomized, controlled trials to reduce the risk for death, myocardial infarction (MI), and stroke in patients with established coronary disease. The most significant effects were evident after approximately two years of treatment.1,2,3,4
Subsequent trials have shown earlier and more significant reductions in the rates of recurrent ischemic cardiovascular events following acute coronary syndromes (ACS) when statins are administered early—within days of the initial event. This is a window of time in which most patients still are hospitalized.4,5,6,7
In addition to this data regarding statin use following ACS, a large, randomized, controlled trial demonstrated similar reductions in the incidence of strokes and cardiovascular events when high-dose atorvastatin was administered within one to six months following TIA or stroke in patients without established coronary disease.8 There is growing data supporting the hypothesis that statins have pleiotropic (non cholesterol-lowering), neuroprotective, properties that may improve patient outcomes following cerebrovascular events.9,10,11 There are ongoing trials investing the role of statins in the acute management of stroke.12,13
Hospitalists frequently manage patients in the stages immediately following ACS and stroke. Based on the large and evolving volume of data regarding the use of statins following these events, when and how should a statin be started in the hospital?
Review of the Data
Following Acute Coronary Syndrome: Death and recurrent ischemic events following ACS are most likely to occur in the early phase of recovery. Based on this observation and evidence supporting the early (in some cases within hours of administration) ‘pleiotropic’ or non-cholesterol lowering effects of statins, including improvement in endothelial function and decreases in platelet aggregation, thrombus deposition, and vascular inflammation, the MIRACL study was designed to answer the question of whether the initiation of treatment with a statin within 24 to 96 hours following ACS would reduce the occurrence of death and recurrent ischemia.4,7,14 Investigators randomized 3,086 patients within 24-96 hours (mean 63 hours) following admission for non-ST segment myocardial infarction (NSTEMI) or unstable angina (UA) to receive either atorvastatin 80 mg/d or placebo.
Investigators monitored patients for the primary end points of ischemic events (death, non-fatal MI, cardiac arrest with resuscitation, symptomatic myocardial ischemia with objective evidence) during a 16-week period. In the treatment arm, the risk of the primary combined end point was significantly reduced—relative risk (RR) 0.84; 95% confidence interval (CI), 0.70-1.00; p=0.048. (See Figure 1, pg. 39)
No significant differences were found between atorvastatin and placebo in the risk of death, non-fatal MI, or cardiac arrest with resuscitation. There was, however, a significantly lower risk of recurrent symptomatic myocardial ischemia with objective evidence requiring emergent re-hospitalization in the treatment arm (RR, 0.74; 95% CI, 0.57-0.95; p=0.02). The mean baseline LDL level in the treatment arm was 124 mg/dL, a value that may represent, in part, suppression of the LDL level in the setting of acute ACS. This is a phenomenon previously described in an analysis of the LUNAR trial.15
Suppression of LDL level after ACS appeared to be minimal, however, and is unlikely to be clinically significant. The benefits of atorvastatin in the MIRACL trial did not appear to depend on baseline LDL level—suggesting the decision to initiate statin therapy after ACS should not be influenced by LDL level at the time of the event.
Only one dose of statin was used in the MIRACL trial, and the investigators commented they were unable to determine if a lower dose of atorvastatin or a gradual dose titration to a predetermined LDL target would have achieved similar benefits. The PROVE IT-TIMI 22 trial was designed to compare the reductions in death and major cardiovascular events following ACS between LDL lowering to approximately 100 mg/dL using 40 mg/d of pravastatin, and more intensive LDL lowering to approximately 70 mg/dL using 80 mg/d of atorvastatin.5 Investigators enrolled 4,162 patients for a median of seven days following ACS (STEMI, NSTEMI, or UA) to the two treatment arms. Investigators observed patients for a period of 18 to 36 months for the primary end points of death, MI, UA, revascularization, and stroke. The median LDL level at the time of enrollment was 106 mg/dL in both treatment arms. During follow up, the median LDL levels achieved were 95 mg/dL in the pravastatin group and 62 mg/dL in the atorvastatin group. After two years, a 16% reduction in the hazard ratio for any primary end point was seen favoring 80 mg/d of atorvastatin—p=0.005; 95% CI=5-26%. (See Figure 2, pg. 39) The benefit of high-dose atorvastatin was seen as early as 30 days after randomization and was sustained throughout the trial.
While the PROVE IT-TIMI 22 trial supported a specified dosing strategy for statin use following ACS, Phase Z of the A-to-Z trial was designed to evaluate the early initiation of intensive lipid lowering following ACS, as compared to a delayed and less-intensive strategy.5,6 Investigators randomized 4,497 patients (a mean of 3.7 days following either NSTEMI or STEMI) to receive either placebo for four months followed by simvastatin 20 mg/d or simvastatin 40 mg/d for one month followed by simvastatin 80 mg/d. They followed patients for 24 months for the primary end points of cardiovascular death, MI, readmission for ACS, or stroke. The primary end point occurred in 16.7% of the delayed, lower-intensity treatment group and in 14.4% of the early, higher-intensity treatment group (95% CI 0.76-1.04; p=0.14). Despite the lack of a significant difference in the composite primary end point between the two treatment arms, a significant reduction in the secondary end points of cardiovascular mortality (absolute risk reduction (ARR 1.3%; P=0.05) and congestive heart failure (ARR 1.3%; P=0.04) was evident favoring the early, intensive treatment strategy. These differences were not evident until at least four months after randomization. The A-to-Z trial investigators offered several possible explanations for the delay in evident clinical benefits in their trial when compared against the strong trend toward clinical benefit seen with 30 days following the early initiation of high-dose atorvastatin following ACS in the PROVE IT-TIMI 22 trial. In the PROVE IT trial, patients were enrolled an average of seven days after their index event, and as a result, 69% had undergone revascularization by this time. In the A-to-Z trial, patients were enrolled an average of three to four days earlier, and, therefore, were less likely to have undergone a revascularization procedure by the time of enrollment—and may have continued on with active thrombotic processes relatively less responsive to statin therapy.6 Another notable difference between PROVE IT and A-to-Z subjects was the C-reactive protein (CRP) concentrations in the A-to-Z subjects did not differ between treatment groups within 30 days despite significant differences in their LDL levels.16 This lack of a concurrent, pleiotropic, anti-inflammatory effect in the A-to-Z trial aggressive treatment arm may also have contributed to the delayed treatment effect.
In conclusion, the A-to-Z investigators suggest more intensive statin therapy (than the 40 mg Simvastatin in their intensive treatment arm) may be required to derive the most rapid and maximal clinical benefits during the highest risk period immediately following ACS.
Following stroke: Although there is more robust data supporting the benefits of early, intensive, statin therapy following ACS, there also is established and emerging data supporting similar treatment approaches following stroke.
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was designed to determine whether or not atorvastatin 80 mg daily would reduce the risk of stroke in patients without known coronary heart disease who had suffered a TIA or stroke within the preceding six months.8 Patients who experienced a hemorrhagic or ischemic TIA or stroke between one to six months before study entry were randomized to receive either atorvastatin 80 mg/d or placebo.
Investigators followed patients for a mean period of 4.9 years for the primary end point of time to non-fatal or fatal stroke. Secondary composite end points included stroke or TIA, and any coronary or peripheral arterial event, including death from cardiac causes, non-fatal MI, ACS, revascularization (coronary, carotid, peripheral), and death from any cause. No difference in mean baseline LDL levels was witnessed between the treatment and placebo arms (132.7 and 133.7 mg/dL, respectively). Atorvastatin was associated with a 16% relative reduction in the risk of stroke—hazard ratio, 0.84; 95% CI 0.71–0.99; p=0.03. This was found despite an increase in hemorrhagic stroke in the atorvastatin group—a finding that supports an epidemiologic association between low cholesterol levels and brain hemorrhage. The risk of cardiovascular events also was significantly reduced, however, no significant difference in overall mortality was observed between the two groups.
In conclusion, the authors recommend the initiation of high-dose atorvastatin “soon” after stroke or TIA. One can only conclude, based on these data, statin therapy should be initiated within six months of TIA or stroke, in accordance with the study design. There is retrospective data suggesting benefit to statin therapy initiated within four weeks following ischemic stroke, and there are prospective trials in process evaluating the potential benefits of statins initiated within 24 hours following ischemic stroke, however, no large, randomized, controlled trial can demonstrate the effect of statins when used as acute stroke therapy.9,12,13,17
Back to the Case
The patient described in our case has a history of TIA and experienced an acute coronary syndrome (NSTEMI) within the preceding 24 hours. He underwent a revascularization procedure (PCI with stent), and is on appropriate therapy, including dual anti-platelet therapy with aspirin and clopidogrel, a beta-blocker, and an angiotensin-converting enzyme inhibitor. Based on the data and conclusions of the MIRACL, PROVE IT-TIMI 22, and SPARCL trials, high-dose statin therapy with atorvastatin 80 mg/d should be initiated immediately in the patient in order to significantly reduce his risk of recurrent ischemic cardiovascular events and stroke following his acute coronary syndrome and TIA.
Bottom Line
Following ACS, high-dose statin therapy with 80 mg of atorvastatin per day should be initiated when the patient is still in the hospital, irrespective of baseline LDL level. Statin therapy should also strongly be considered for secondary stroke prevention in most patients with a history of stroke or transient ischemic attack. TH
Caleb Hale, MD, is a hospitalist at Beth Israel Deaconess Medical Center in Boston. Joseph Ming Wah Li is director of the hospital medicine program and associate chief, division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston, and assistant professor of Medicine at Harvard Medical School.
References
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
2. Sacks RM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-1009.
3. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-1357.
4. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711-1718.
5. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
6. Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs. a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
7. Waters D, Schwartz GG, Olsson AG. The myocardial ischemia reduction with acute cholesterol lowering (MIRACL) trial: a new frontier for statins? Curr Control Trials Cardiovasc Med. 2001;2;111-114.
8. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
9. Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. 2005;36:1298-1300.
10. Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253-258.
11. Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969-1973.
12. Elkind MS, Sacco RL, MacArthur RB, et al. The neuroprotection with statin therapy for acute recovery trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3:210-218.
13. Montaner J, Chacon P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J of Neurol. 2008;15:82-90.
14. Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20-28.
15. Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. JACC. 2008;51:1440-1445.
16. Wiviott SD, de Lemos JA, Cannon CP, et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials of A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406-1414.
17. Elking MS. Statins as acute-stroke treatment. Int J Stroke. 2006;1:224-225.
Case
A 52-year-old man with no medical history other than a transient ischemic attack (TIA) three months ago presents to the emergency department (ED) following multiple episodes of substernal (ST) chest pressure. He takes no medication. His electrocardiogram (ECG) revealed lateral ST segment depressions, and his cardiac biomarkers were elevated. He underwent cardiac catheterization, and a single drug-eluting stent was successfully placed to a culprit left circumflex lesion. He is now stable less than 24 hours following his initial presentation, without any evidence of heart failure. His providers prescribe aspirin, clopidogrel, metoprolol, and lisinopril. His fasting LDL level is 92 mg/dL.
What, if any, is the role for lipid-lowering therapy at this time?
Overview
Long-term therapy with HMG CoA reductase inhibitors (statins) has been shown through several large, randomized, controlled trials to reduce the risk for death, myocardial infarction (MI), and stroke in patients with established coronary disease. The most significant effects were evident after approximately two years of treatment.1,2,3,4
Subsequent trials have shown earlier and more significant reductions in the rates of recurrent ischemic cardiovascular events following acute coronary syndromes (ACS) when statins are administered early—within days of the initial event. This is a window of time in which most patients still are hospitalized.4,5,6,7
In addition to this data regarding statin use following ACS, a large, randomized, controlled trial demonstrated similar reductions in the incidence of strokes and cardiovascular events when high-dose atorvastatin was administered within one to six months following TIA or stroke in patients without established coronary disease.8 There is growing data supporting the hypothesis that statins have pleiotropic (non cholesterol-lowering), neuroprotective, properties that may improve patient outcomes following cerebrovascular events.9,10,11 There are ongoing trials investing the role of statins in the acute management of stroke.12,13
Hospitalists frequently manage patients in the stages immediately following ACS and stroke. Based on the large and evolving volume of data regarding the use of statins following these events, when and how should a statin be started in the hospital?
Review of the Data
Following Acute Coronary Syndrome: Death and recurrent ischemic events following ACS are most likely to occur in the early phase of recovery. Based on this observation and evidence supporting the early (in some cases within hours of administration) ‘pleiotropic’ or non-cholesterol lowering effects of statins, including improvement in endothelial function and decreases in platelet aggregation, thrombus deposition, and vascular inflammation, the MIRACL study was designed to answer the question of whether the initiation of treatment with a statin within 24 to 96 hours following ACS would reduce the occurrence of death and recurrent ischemia.4,7,14 Investigators randomized 3,086 patients within 24-96 hours (mean 63 hours) following admission for non-ST segment myocardial infarction (NSTEMI) or unstable angina (UA) to receive either atorvastatin 80 mg/d or placebo.
Investigators monitored patients for the primary end points of ischemic events (death, non-fatal MI, cardiac arrest with resuscitation, symptomatic myocardial ischemia with objective evidence) during a 16-week period. In the treatment arm, the risk of the primary combined end point was significantly reduced—relative risk (RR) 0.84; 95% confidence interval (CI), 0.70-1.00; p=0.048. (See Figure 1, pg. 39)
No significant differences were found between atorvastatin and placebo in the risk of death, non-fatal MI, or cardiac arrest with resuscitation. There was, however, a significantly lower risk of recurrent symptomatic myocardial ischemia with objective evidence requiring emergent re-hospitalization in the treatment arm (RR, 0.74; 95% CI, 0.57-0.95; p=0.02). The mean baseline LDL level in the treatment arm was 124 mg/dL, a value that may represent, in part, suppression of the LDL level in the setting of acute ACS. This is a phenomenon previously described in an analysis of the LUNAR trial.15
Suppression of LDL level after ACS appeared to be minimal, however, and is unlikely to be clinically significant. The benefits of atorvastatin in the MIRACL trial did not appear to depend on baseline LDL level—suggesting the decision to initiate statin therapy after ACS should not be influenced by LDL level at the time of the event.
Only one dose of statin was used in the MIRACL trial, and the investigators commented they were unable to determine if a lower dose of atorvastatin or a gradual dose titration to a predetermined LDL target would have achieved similar benefits. The PROVE IT-TIMI 22 trial was designed to compare the reductions in death and major cardiovascular events following ACS between LDL lowering to approximately 100 mg/dL using 40 mg/d of pravastatin, and more intensive LDL lowering to approximately 70 mg/dL using 80 mg/d of atorvastatin.5 Investigators enrolled 4,162 patients for a median of seven days following ACS (STEMI, NSTEMI, or UA) to the two treatment arms. Investigators observed patients for a period of 18 to 36 months for the primary end points of death, MI, UA, revascularization, and stroke. The median LDL level at the time of enrollment was 106 mg/dL in both treatment arms. During follow up, the median LDL levels achieved were 95 mg/dL in the pravastatin group and 62 mg/dL in the atorvastatin group. After two years, a 16% reduction in the hazard ratio for any primary end point was seen favoring 80 mg/d of atorvastatin—p=0.005; 95% CI=5-26%. (See Figure 2, pg. 39) The benefit of high-dose atorvastatin was seen as early as 30 days after randomization and was sustained throughout the trial.
While the PROVE IT-TIMI 22 trial supported a specified dosing strategy for statin use following ACS, Phase Z of the A-to-Z trial was designed to evaluate the early initiation of intensive lipid lowering following ACS, as compared to a delayed and less-intensive strategy.5,6 Investigators randomized 4,497 patients (a mean of 3.7 days following either NSTEMI or STEMI) to receive either placebo for four months followed by simvastatin 20 mg/d or simvastatin 40 mg/d for one month followed by simvastatin 80 mg/d. They followed patients for 24 months for the primary end points of cardiovascular death, MI, readmission for ACS, or stroke. The primary end point occurred in 16.7% of the delayed, lower-intensity treatment group and in 14.4% of the early, higher-intensity treatment group (95% CI 0.76-1.04; p=0.14). Despite the lack of a significant difference in the composite primary end point between the two treatment arms, a significant reduction in the secondary end points of cardiovascular mortality (absolute risk reduction (ARR 1.3%; P=0.05) and congestive heart failure (ARR 1.3%; P=0.04) was evident favoring the early, intensive treatment strategy. These differences were not evident until at least four months after randomization. The A-to-Z trial investigators offered several possible explanations for the delay in evident clinical benefits in their trial when compared against the strong trend toward clinical benefit seen with 30 days following the early initiation of high-dose atorvastatin following ACS in the PROVE IT-TIMI 22 trial. In the PROVE IT trial, patients were enrolled an average of seven days after their index event, and as a result, 69% had undergone revascularization by this time. In the A-to-Z trial, patients were enrolled an average of three to four days earlier, and, therefore, were less likely to have undergone a revascularization procedure by the time of enrollment—and may have continued on with active thrombotic processes relatively less responsive to statin therapy.6 Another notable difference between PROVE IT and A-to-Z subjects was the C-reactive protein (CRP) concentrations in the A-to-Z subjects did not differ between treatment groups within 30 days despite significant differences in their LDL levels.16 This lack of a concurrent, pleiotropic, anti-inflammatory effect in the A-to-Z trial aggressive treatment arm may also have contributed to the delayed treatment effect.
In conclusion, the A-to-Z investigators suggest more intensive statin therapy (than the 40 mg Simvastatin in their intensive treatment arm) may be required to derive the most rapid and maximal clinical benefits during the highest risk period immediately following ACS.
Following stroke: Although there is more robust data supporting the benefits of early, intensive, statin therapy following ACS, there also is established and emerging data supporting similar treatment approaches following stroke.
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was designed to determine whether or not atorvastatin 80 mg daily would reduce the risk of stroke in patients without known coronary heart disease who had suffered a TIA or stroke within the preceding six months.8 Patients who experienced a hemorrhagic or ischemic TIA or stroke between one to six months before study entry were randomized to receive either atorvastatin 80 mg/d or placebo.
Investigators followed patients for a mean period of 4.9 years for the primary end point of time to non-fatal or fatal stroke. Secondary composite end points included stroke or TIA, and any coronary or peripheral arterial event, including death from cardiac causes, non-fatal MI, ACS, revascularization (coronary, carotid, peripheral), and death from any cause. No difference in mean baseline LDL levels was witnessed between the treatment and placebo arms (132.7 and 133.7 mg/dL, respectively). Atorvastatin was associated with a 16% relative reduction in the risk of stroke—hazard ratio, 0.84; 95% CI 0.71–0.99; p=0.03. This was found despite an increase in hemorrhagic stroke in the atorvastatin group—a finding that supports an epidemiologic association between low cholesterol levels and brain hemorrhage. The risk of cardiovascular events also was significantly reduced, however, no significant difference in overall mortality was observed between the two groups.
In conclusion, the authors recommend the initiation of high-dose atorvastatin “soon” after stroke or TIA. One can only conclude, based on these data, statin therapy should be initiated within six months of TIA or stroke, in accordance with the study design. There is retrospective data suggesting benefit to statin therapy initiated within four weeks following ischemic stroke, and there are prospective trials in process evaluating the potential benefits of statins initiated within 24 hours following ischemic stroke, however, no large, randomized, controlled trial can demonstrate the effect of statins when used as acute stroke therapy.9,12,13,17
Back to the Case
The patient described in our case has a history of TIA and experienced an acute coronary syndrome (NSTEMI) within the preceding 24 hours. He underwent a revascularization procedure (PCI with stent), and is on appropriate therapy, including dual anti-platelet therapy with aspirin and clopidogrel, a beta-blocker, and an angiotensin-converting enzyme inhibitor. Based on the data and conclusions of the MIRACL, PROVE IT-TIMI 22, and SPARCL trials, high-dose statin therapy with atorvastatin 80 mg/d should be initiated immediately in the patient in order to significantly reduce his risk of recurrent ischemic cardiovascular events and stroke following his acute coronary syndrome and TIA.
Bottom Line
Following ACS, high-dose statin therapy with 80 mg of atorvastatin per day should be initiated when the patient is still in the hospital, irrespective of baseline LDL level. Statin therapy should also strongly be considered for secondary stroke prevention in most patients with a history of stroke or transient ischemic attack. TH
Caleb Hale, MD, is a hospitalist at Beth Israel Deaconess Medical Center in Boston. Joseph Ming Wah Li is director of the hospital medicine program and associate chief, division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston, and assistant professor of Medicine at Harvard Medical School.
References
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
2. Sacks RM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-1009.
3. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-1357.
4. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711-1718.
5. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
6. Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs. a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
7. Waters D, Schwartz GG, Olsson AG. The myocardial ischemia reduction with acute cholesterol lowering (MIRACL) trial: a new frontier for statins? Curr Control Trials Cardiovasc Med. 2001;2;111-114.
8. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
9. Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. 2005;36:1298-1300.
10. Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253-258.
11. Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969-1973.
12. Elkind MS, Sacco RL, MacArthur RB, et al. The neuroprotection with statin therapy for acute recovery trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3:210-218.
13. Montaner J, Chacon P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J of Neurol. 2008;15:82-90.
14. Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20-28.
15. Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. JACC. 2008;51:1440-1445.
16. Wiviott SD, de Lemos JA, Cannon CP, et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials of A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406-1414.
17. Elking MS. Statins as acute-stroke treatment. Int J Stroke. 2006;1:224-225.
In the Literature
Semi-Recumbent Position to Prevent Ventilator-Associated Pneumonia: Is It Possible?
By Joseph Ming Wah Li, MD
Van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006 Feb;34(2):396-402.
Ventilator-associated pneumonia (VAP) is a cause of significant morbidity and mortality among mechanically ventilated patients. Studies with radioactive-labeled enteral feeds have demonstrated an increased frequency of endotracheal aspiration of gastric contents in supine patients. The CDC guidelines for prevention of nosocomial pneumonia advise placement of mechanically ventilated patients in a semi-recumbent position as a VAP prevention measure.
Only one previous study, by Drakulovic and colleagues, has assessed this strategy to prevent VAP.1 That study demonstrated a 75% decrease in the incidence of VAP. But van Nieuwenhoven and colleagues raised two important questions about the findings from the previous study: First, the Drakulovic study placed control patients in a horizontal (zero degrees) position, which is not the standard of care in most ICUs. Most patients are placed at 10 degrees, and this position is elevated as patients are weaned. Second, the Drakulovic study measured patients only once daily but did not monitor their body positions in between the daily measurements.
Dr. van Nieuwenhoven and colleagues set out to determine whether it is feasible to keep mechanically ventilated patients in a semi-recumbent position on a continual basis and whether this measure would prevent VAP. This was a prospective multi-centered trial in which mechanically ventilated patients were randomly assigned to the semi-recumbent position with a target backrest elevation of 45 degrees or standard of care (supine position) with a backrest elevation of 10 degrees. They used a transducer with a pendulum, which was placed on the bed frame to measure the backrest elevation every 60 seconds for up to seven days. They calculated a mean degree of elevation for each patient daily. Nurses always respected the patient’s request for positioning, but a dedicated research nurse restored backrest position to the randomized position whenever possible.
Baseline characteristics for both groups were similar. For the supine (control) group, average elevations were 9.8 degrees on day one and 16.1 degrees on day seven. For the semi-recumbent group, average elevations were 28.1 degrees on day one and 22.6 degrees on day seven. There were no significant differences in numbers of patients who developed VAP in either group.
This study suggests that, despite the use of dedicated research nurses to maintain positioning, it may not be possible to keep patients’ backrests elevated to 45 degrees. Keeping patients’ backrests at an elevation of nearly 30 degrees does not appear to prevent VAP more than keeping patients’ backrests at 10 degrees, the present standard of care.
Reference
- Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851-1858.
Bar Codes in Medicine: An Opportunity for Quality Improvement
By Alex Carbo, MD
Poon EG, Cina JL, Churchill W, et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Ann Intern Med. 2006;145:426-434.
Medication errors and adverse drug events (ADEs) have received much attention in the literature; the use of health information technology to mitigate these errors and ADEs has now been proposed in many areas of healthcare. In an effort to decrease medication-dispensing errors, the U.S. Food and Drug Administration (FDA) mandated bar code use for all medications in hospitals, beginning in April 2006. While this technology has been extensively studied in other industries, there is little data describing its effects in the healthcare system.
Poon and colleagues set out to evaluate whether implementation of bar code technology reduced dispensing errors and the ADEs that might be caused by these miscalculations. In a before-and-after evaluation, they studied more than 350,000 dispensed medication doses in an academic medical center between February 2003 and September 2004.
During the bar code conversion process, the hospital pharmacy built a dedicated repackaging center, which was responsible for affixing a bar code to every dose of medication. These medications were then dispensed in three different configurations: two configurations required staff to verify all doses at least once using bar code scanning, and the third configuration—for commonly dispensed medications that could not be accommodated in a standard carousel machine because of their size or need for refrigeration—required scanning only one dose from each batch.
The authors found a 93% to 96% relative reduction in the incidence of target dispensing errors (P<0.001) and an 86% to 97% relative reduction in the incidence of potential ADEs (P<0.001) in the two configurations that required staff to verify all doses by scanning. The greatest reductions were seen in wrong medication errors (56%), wrong strength/dose errors (71%), wrong formulation errors (90%), and expired medication errors (100%).
In the configuration that did not require scanning of every dose, however, there was a 60% relative reduction in the incidence of target dispensing errors (P<0.001), but a 2.4-fold increase in the incidence of target potential ADEs. This included new errors attributable to wrong strength and wrong medication dispensing.
In light of the FDA’s mandate regarding bar codes, it seems that every hospital has the opportunity to improve patient safety and decrease medication error rates with the use of bar code technology. This study suggests that in order to achieve this benefit these systems should be designed to ensure that every medication dose is verified by scanning during the dispensing process.
Evaluation of a Guideline to Guide Resuscitation
By Cindy Lien, MD
Morrison LJ, Visentin LM, Kiss A, et al. Validation of a rule for termination of resuscitation in out-of-hospital cardiac arrest. N Engl J Med. 2006 Aug 3;355(5):478-487.
The survival rate of patients with out-of-hospital cardiac arrest is very low. Thus, guidelines have been developed for termination of resuscitation for those patients who have had no response to advanced cardiac life support provided by emergency medical service (EMS) personnel. Similar guidelines have not yet been developed, however, for situations in which patients receive basic life support from emergency workers trained in the use of an automated external cardiac defibrillator. Patients with little potential for survival are routinely transported to emergency departments, at significant cost to the healthcare system.
Morrison and colleagues present results from the Termination of Resuscitation (TOR) study, a prospective evaluation of a clinical prediction rule for the termination of basic life support by emergency medical personnel trained in the use of automated external defibrillators. The clinical prediction rule, previously developed in a retrospective review of case records from a large urban EMS system, recommends termination of resuscitation if there is no return of spontaneous circulation, no shock administered, and no witness of the arrest by EMS personnel.
In the current study, the authors obtained follow-up data for 1,240 adult patients in Ontario, Canada, who had suffered an arrest of presumed cardiac cause and were subsequently transported to the emergency department after resuscitative efforts. Twenty-four EMS systems participated in the study. The study found that only 0.5% of the patients for whom the clinical prediction rule recommended termination survived (four out of 776 patients). Of the 1,240 total study patients, 41 (3%) survived. The clinical prediction rule recommended continuation of resuscitative efforts for 37 of these 41 patients, resulting in a specificity of 90.2%. The positive predictive value for death was calculated to be 99.5% when termination was recommended.
The TOR trial also determined whether the addition of other criteria to the original prediction rule could further refine the specificity and positive predictive value. They found that the addition to the criteria of a response time greater than eight minutes increased the positive predictive value and specificity to 99.7% and 97.6%, respectively. When the variable “not witnessed by bystander” was added to the clinical prediction rule, both the positive predictive value and specificity increased to 100%. In other words, no patients survived if they had had a completely unwitnessed arrest, no return of spontaneous circulation, and no shocks delivered.
This study identifies a subpopulation of patients with presumed cardiac arrest for whom termination of resuscitative efforts in the field appears reasonable. The authors note that a survival rate of 1% or less has been suggested in past literature as reflective of medical futility. The TOR investigators acknowledge that their study took place before the 2005 AHA Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care were released and that their study protocols were consistent with the 2000 resuscitation guidelines. In light of this information, continued validity testing of the clinical prediction rule under the 2005 AHA protocols is needed.
Nonetheless, it is quite impressive that use of their clinical prediction rule would have resulted in transportation of only 37% of patients (464 of 1,240), rather than 100% of patients, as is currently the practice. If the guidelines described in this article are to be implemented, further studies are necessary to address the training of EMS personnel, who would carry responsibility for terminating resuscitation and notifying families of patients’ deaths.
Prevention of Ventilator-Associated Pneumonia
By Diane Sliwka, MD
Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006 Jun;173(12):1348-1355. Epub 2006 Apr 7.
Ventilator-associated pneumonia (VAP) is an important nosocomial source of morbidity and mortality. The use of prophylactic antimicrobials to decrease VAP raises concern for antimicrobial resistance. This study evaluates the topical antiseptic chlorhexidine (CHX) as an alternative prophylactic intervention for VAP. CHX has previously been shown to decrease VAP in cardiac surgical patients, but has not been studied in higher risk, long-term-ventilated patients. Because CHX works better for gram-positive organisms, the combination of colistin and CHX (COL + CHX) was also studied for improved gram-negative coverage.
This multi-center, randomized, double-blind, placebo-controlled trial enrolled 385 adult patients. Patients who were expected to be intubated for longer than 48 hours were randomized to 3 arms: CHX alone, CHX + COL, and placebo. Exclusion criteria included known preadmission immunocompromised state, pregnancy, and physical limitation to oral application. Pneumonia was defined by clinical decision-making, which was later confirmed by three blinded intensivists’ reviews of the case records and supported by daily clinical pulmonary infection scores.
The primary endpoint of VAP was diagnosed in 52 of 385 patients: 18% placebo, 13% CHX, and 10% CHX + COL. Rate of VAP in the two treatment groups was lower than placebo and reached statistical significance when compared to placebo. The daily hazard ratio for CHX versus placebo was .352 (95% CI .160, .791); for CHX + COL versus placebo, it was .454 (95% CI .224, .925), showing a 65% and 55% reduction in the rate of pneumonia development. Multivariate analysis of variables such as gender, pulmonary admission diagnosis, colonization at time of admission, and antimicrobial use on admission did not affect the data.
The secondary endpoint of endotracheal colonization was evaluated by a twice-weekly endotracheal culture. There was no statistically significant difference in colonization among the three groups in the first (days 1-4) or third (days 9-12) time frames. During the second time frame (days 5-8), there was a statistically significant decrease in colonization for the CHX + COL treatment group when compared to both placebo (16% versus 40% p<.007) and to CHX (16% versus 38%, p<.011); this decrease is thought to be due to gram-negative coverage by COL.
The secondary endpoint of oropharyngeal colonization was evaluated for 87% of all patient days. CHX and CHX + COL were similarly effective for gram-positive bacteria when compared to placebo, with 30% and 27% reduction in rates of colonization, respectively: HR 0.695 for CHX (95% CI, 0.606, 0.796; p < 0.001) and 0.732 (95% CI, 0.640, 0.838; p < 0.001) for CHX + COL. The CHX + COL combination was more effective for gram-negative bacteria: daily HR .534 (95% CI, 0.455, 0.626; p <0.001) alone with a 47% reduction in gram-negative colonization compared to CHX.
No difference was seen in ICU mortality, duration of mechanical ventilation, or duration of ICU stay. One adverse event (tongue swelling) occurred in the CHX + COL group.
Limitations of the study include the following:
- Daily assessments on all patients were not performed;
- The placebo group had more males and more infections on admission than the other two groups, raising the question of randomization error;
- Clinical versus quantitative diagnosis of pneumonia may overestimate VAP in this study;
- It is not known how many patients were not enrolled in the study due to short anticipated ventilator times, but who later had prolonged ventilations; and
- The lack of effect on ventilator time, ICU length of stay, and mortality raises the question of the significance of these findings.
Despite these limitations, the low cost of these treatments, minimal adverse events, low risk of promoting significant antimicrobial resistance, and the finding of decreased VAP and bacterial colonization risk shown in this study support the potential benefit of topical decontamination with CHX and COL in conjunction with other measures of VAP prevention. TH
Reference
- De Riso AJ II, Ladowski JS, Dillon TA, et al. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556-1561.
Semi-Recumbent Position to Prevent Ventilator-Associated Pneumonia: Is It Possible?
By Joseph Ming Wah Li, MD
Van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006 Feb;34(2):396-402.
Ventilator-associated pneumonia (VAP) is a cause of significant morbidity and mortality among mechanically ventilated patients. Studies with radioactive-labeled enteral feeds have demonstrated an increased frequency of endotracheal aspiration of gastric contents in supine patients. The CDC guidelines for prevention of nosocomial pneumonia advise placement of mechanically ventilated patients in a semi-recumbent position as a VAP prevention measure.
Only one previous study, by Drakulovic and colleagues, has assessed this strategy to prevent VAP.1 That study demonstrated a 75% decrease in the incidence of VAP. But van Nieuwenhoven and colleagues raised two important questions about the findings from the previous study: First, the Drakulovic study placed control patients in a horizontal (zero degrees) position, which is not the standard of care in most ICUs. Most patients are placed at 10 degrees, and this position is elevated as patients are weaned. Second, the Drakulovic study measured patients only once daily but did not monitor their body positions in between the daily measurements.
Dr. van Nieuwenhoven and colleagues set out to determine whether it is feasible to keep mechanically ventilated patients in a semi-recumbent position on a continual basis and whether this measure would prevent VAP. This was a prospective multi-centered trial in which mechanically ventilated patients were randomly assigned to the semi-recumbent position with a target backrest elevation of 45 degrees or standard of care (supine position) with a backrest elevation of 10 degrees. They used a transducer with a pendulum, which was placed on the bed frame to measure the backrest elevation every 60 seconds for up to seven days. They calculated a mean degree of elevation for each patient daily. Nurses always respected the patient’s request for positioning, but a dedicated research nurse restored backrest position to the randomized position whenever possible.
Baseline characteristics for both groups were similar. For the supine (control) group, average elevations were 9.8 degrees on day one and 16.1 degrees on day seven. For the semi-recumbent group, average elevations were 28.1 degrees on day one and 22.6 degrees on day seven. There were no significant differences in numbers of patients who developed VAP in either group.
This study suggests that, despite the use of dedicated research nurses to maintain positioning, it may not be possible to keep patients’ backrests elevated to 45 degrees. Keeping patients’ backrests at an elevation of nearly 30 degrees does not appear to prevent VAP more than keeping patients’ backrests at 10 degrees, the present standard of care.
Reference
- Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851-1858.
Bar Codes in Medicine: An Opportunity for Quality Improvement
By Alex Carbo, MD
Poon EG, Cina JL, Churchill W, et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Ann Intern Med. 2006;145:426-434.
Medication errors and adverse drug events (ADEs) have received much attention in the literature; the use of health information technology to mitigate these errors and ADEs has now been proposed in many areas of healthcare. In an effort to decrease medication-dispensing errors, the U.S. Food and Drug Administration (FDA) mandated bar code use for all medications in hospitals, beginning in April 2006. While this technology has been extensively studied in other industries, there is little data describing its effects in the healthcare system.
Poon and colleagues set out to evaluate whether implementation of bar code technology reduced dispensing errors and the ADEs that might be caused by these miscalculations. In a before-and-after evaluation, they studied more than 350,000 dispensed medication doses in an academic medical center between February 2003 and September 2004.
During the bar code conversion process, the hospital pharmacy built a dedicated repackaging center, which was responsible for affixing a bar code to every dose of medication. These medications were then dispensed in three different configurations: two configurations required staff to verify all doses at least once using bar code scanning, and the third configuration—for commonly dispensed medications that could not be accommodated in a standard carousel machine because of their size or need for refrigeration—required scanning only one dose from each batch.
The authors found a 93% to 96% relative reduction in the incidence of target dispensing errors (P<0.001) and an 86% to 97% relative reduction in the incidence of potential ADEs (P<0.001) in the two configurations that required staff to verify all doses by scanning. The greatest reductions were seen in wrong medication errors (56%), wrong strength/dose errors (71%), wrong formulation errors (90%), and expired medication errors (100%).
In the configuration that did not require scanning of every dose, however, there was a 60% relative reduction in the incidence of target dispensing errors (P<0.001), but a 2.4-fold increase in the incidence of target potential ADEs. This included new errors attributable to wrong strength and wrong medication dispensing.
In light of the FDA’s mandate regarding bar codes, it seems that every hospital has the opportunity to improve patient safety and decrease medication error rates with the use of bar code technology. This study suggests that in order to achieve this benefit these systems should be designed to ensure that every medication dose is verified by scanning during the dispensing process.
Evaluation of a Guideline to Guide Resuscitation
By Cindy Lien, MD
Morrison LJ, Visentin LM, Kiss A, et al. Validation of a rule for termination of resuscitation in out-of-hospital cardiac arrest. N Engl J Med. 2006 Aug 3;355(5):478-487.
The survival rate of patients with out-of-hospital cardiac arrest is very low. Thus, guidelines have been developed for termination of resuscitation for those patients who have had no response to advanced cardiac life support provided by emergency medical service (EMS) personnel. Similar guidelines have not yet been developed, however, for situations in which patients receive basic life support from emergency workers trained in the use of an automated external cardiac defibrillator. Patients with little potential for survival are routinely transported to emergency departments, at significant cost to the healthcare system.
Morrison and colleagues present results from the Termination of Resuscitation (TOR) study, a prospective evaluation of a clinical prediction rule for the termination of basic life support by emergency medical personnel trained in the use of automated external defibrillators. The clinical prediction rule, previously developed in a retrospective review of case records from a large urban EMS system, recommends termination of resuscitation if there is no return of spontaneous circulation, no shock administered, and no witness of the arrest by EMS personnel.
In the current study, the authors obtained follow-up data for 1,240 adult patients in Ontario, Canada, who had suffered an arrest of presumed cardiac cause and were subsequently transported to the emergency department after resuscitative efforts. Twenty-four EMS systems participated in the study. The study found that only 0.5% of the patients for whom the clinical prediction rule recommended termination survived (four out of 776 patients). Of the 1,240 total study patients, 41 (3%) survived. The clinical prediction rule recommended continuation of resuscitative efforts for 37 of these 41 patients, resulting in a specificity of 90.2%. The positive predictive value for death was calculated to be 99.5% when termination was recommended.
The TOR trial also determined whether the addition of other criteria to the original prediction rule could further refine the specificity and positive predictive value. They found that the addition to the criteria of a response time greater than eight minutes increased the positive predictive value and specificity to 99.7% and 97.6%, respectively. When the variable “not witnessed by bystander” was added to the clinical prediction rule, both the positive predictive value and specificity increased to 100%. In other words, no patients survived if they had had a completely unwitnessed arrest, no return of spontaneous circulation, and no shocks delivered.
This study identifies a subpopulation of patients with presumed cardiac arrest for whom termination of resuscitative efforts in the field appears reasonable. The authors note that a survival rate of 1% or less has been suggested in past literature as reflective of medical futility. The TOR investigators acknowledge that their study took place before the 2005 AHA Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care were released and that their study protocols were consistent with the 2000 resuscitation guidelines. In light of this information, continued validity testing of the clinical prediction rule under the 2005 AHA protocols is needed.
Nonetheless, it is quite impressive that use of their clinical prediction rule would have resulted in transportation of only 37% of patients (464 of 1,240), rather than 100% of patients, as is currently the practice. If the guidelines described in this article are to be implemented, further studies are necessary to address the training of EMS personnel, who would carry responsibility for terminating resuscitation and notifying families of patients’ deaths.
Prevention of Ventilator-Associated Pneumonia
By Diane Sliwka, MD
Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006 Jun;173(12):1348-1355. Epub 2006 Apr 7.
Ventilator-associated pneumonia (VAP) is an important nosocomial source of morbidity and mortality. The use of prophylactic antimicrobials to decrease VAP raises concern for antimicrobial resistance. This study evaluates the topical antiseptic chlorhexidine (CHX) as an alternative prophylactic intervention for VAP. CHX has previously been shown to decrease VAP in cardiac surgical patients, but has not been studied in higher risk, long-term-ventilated patients. Because CHX works better for gram-positive organisms, the combination of colistin and CHX (COL + CHX) was also studied for improved gram-negative coverage.
This multi-center, randomized, double-blind, placebo-controlled trial enrolled 385 adult patients. Patients who were expected to be intubated for longer than 48 hours were randomized to 3 arms: CHX alone, CHX + COL, and placebo. Exclusion criteria included known preadmission immunocompromised state, pregnancy, and physical limitation to oral application. Pneumonia was defined by clinical decision-making, which was later confirmed by three blinded intensivists’ reviews of the case records and supported by daily clinical pulmonary infection scores.
The primary endpoint of VAP was diagnosed in 52 of 385 patients: 18% placebo, 13% CHX, and 10% CHX + COL. Rate of VAP in the two treatment groups was lower than placebo and reached statistical significance when compared to placebo. The daily hazard ratio for CHX versus placebo was .352 (95% CI .160, .791); for CHX + COL versus placebo, it was .454 (95% CI .224, .925), showing a 65% and 55% reduction in the rate of pneumonia development. Multivariate analysis of variables such as gender, pulmonary admission diagnosis, colonization at time of admission, and antimicrobial use on admission did not affect the data.
The secondary endpoint of endotracheal colonization was evaluated by a twice-weekly endotracheal culture. There was no statistically significant difference in colonization among the three groups in the first (days 1-4) or third (days 9-12) time frames. During the second time frame (days 5-8), there was a statistically significant decrease in colonization for the CHX + COL treatment group when compared to both placebo (16% versus 40% p<.007) and to CHX (16% versus 38%, p<.011); this decrease is thought to be due to gram-negative coverage by COL.
The secondary endpoint of oropharyngeal colonization was evaluated for 87% of all patient days. CHX and CHX + COL were similarly effective for gram-positive bacteria when compared to placebo, with 30% and 27% reduction in rates of colonization, respectively: HR 0.695 for CHX (95% CI, 0.606, 0.796; p < 0.001) and 0.732 (95% CI, 0.640, 0.838; p < 0.001) for CHX + COL. The CHX + COL combination was more effective for gram-negative bacteria: daily HR .534 (95% CI, 0.455, 0.626; p <0.001) alone with a 47% reduction in gram-negative colonization compared to CHX.
No difference was seen in ICU mortality, duration of mechanical ventilation, or duration of ICU stay. One adverse event (tongue swelling) occurred in the CHX + COL group.
Limitations of the study include the following:
- Daily assessments on all patients were not performed;
- The placebo group had more males and more infections on admission than the other two groups, raising the question of randomization error;
- Clinical versus quantitative diagnosis of pneumonia may overestimate VAP in this study;
- It is not known how many patients were not enrolled in the study due to short anticipated ventilator times, but who later had prolonged ventilations; and
- The lack of effect on ventilator time, ICU length of stay, and mortality raises the question of the significance of these findings.
Despite these limitations, the low cost of these treatments, minimal adverse events, low risk of promoting significant antimicrobial resistance, and the finding of decreased VAP and bacterial colonization risk shown in this study support the potential benefit of topical decontamination with CHX and COL in conjunction with other measures of VAP prevention. TH
Reference
- De Riso AJ II, Ladowski JS, Dillon TA, et al. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556-1561.
Semi-Recumbent Position to Prevent Ventilator-Associated Pneumonia: Is It Possible?
By Joseph Ming Wah Li, MD
Van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006 Feb;34(2):396-402.
Ventilator-associated pneumonia (VAP) is a cause of significant morbidity and mortality among mechanically ventilated patients. Studies with radioactive-labeled enteral feeds have demonstrated an increased frequency of endotracheal aspiration of gastric contents in supine patients. The CDC guidelines for prevention of nosocomial pneumonia advise placement of mechanically ventilated patients in a semi-recumbent position as a VAP prevention measure.
Only one previous study, by Drakulovic and colleagues, has assessed this strategy to prevent VAP.1 That study demonstrated a 75% decrease in the incidence of VAP. But van Nieuwenhoven and colleagues raised two important questions about the findings from the previous study: First, the Drakulovic study placed control patients in a horizontal (zero degrees) position, which is not the standard of care in most ICUs. Most patients are placed at 10 degrees, and this position is elevated as patients are weaned. Second, the Drakulovic study measured patients only once daily but did not monitor their body positions in between the daily measurements.
Dr. van Nieuwenhoven and colleagues set out to determine whether it is feasible to keep mechanically ventilated patients in a semi-recumbent position on a continual basis and whether this measure would prevent VAP. This was a prospective multi-centered trial in which mechanically ventilated patients were randomly assigned to the semi-recumbent position with a target backrest elevation of 45 degrees or standard of care (supine position) with a backrest elevation of 10 degrees. They used a transducer with a pendulum, which was placed on the bed frame to measure the backrest elevation every 60 seconds for up to seven days. They calculated a mean degree of elevation for each patient daily. Nurses always respected the patient’s request for positioning, but a dedicated research nurse restored backrest position to the randomized position whenever possible.
Baseline characteristics for both groups were similar. For the supine (control) group, average elevations were 9.8 degrees on day one and 16.1 degrees on day seven. For the semi-recumbent group, average elevations were 28.1 degrees on day one and 22.6 degrees on day seven. There were no significant differences in numbers of patients who developed VAP in either group.
This study suggests that, despite the use of dedicated research nurses to maintain positioning, it may not be possible to keep patients’ backrests elevated to 45 degrees. Keeping patients’ backrests at an elevation of nearly 30 degrees does not appear to prevent VAP more than keeping patients’ backrests at 10 degrees, the present standard of care.
Reference
- Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851-1858.
Bar Codes in Medicine: An Opportunity for Quality Improvement
By Alex Carbo, MD
Poon EG, Cina JL, Churchill W, et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Ann Intern Med. 2006;145:426-434.
Medication errors and adverse drug events (ADEs) have received much attention in the literature; the use of health information technology to mitigate these errors and ADEs has now been proposed in many areas of healthcare. In an effort to decrease medication-dispensing errors, the U.S. Food and Drug Administration (FDA) mandated bar code use for all medications in hospitals, beginning in April 2006. While this technology has been extensively studied in other industries, there is little data describing its effects in the healthcare system.
Poon and colleagues set out to evaluate whether implementation of bar code technology reduced dispensing errors and the ADEs that might be caused by these miscalculations. In a before-and-after evaluation, they studied more than 350,000 dispensed medication doses in an academic medical center between February 2003 and September 2004.
During the bar code conversion process, the hospital pharmacy built a dedicated repackaging center, which was responsible for affixing a bar code to every dose of medication. These medications were then dispensed in three different configurations: two configurations required staff to verify all doses at least once using bar code scanning, and the third configuration—for commonly dispensed medications that could not be accommodated in a standard carousel machine because of their size or need for refrigeration—required scanning only one dose from each batch.
The authors found a 93% to 96% relative reduction in the incidence of target dispensing errors (P<0.001) and an 86% to 97% relative reduction in the incidence of potential ADEs (P<0.001) in the two configurations that required staff to verify all doses by scanning. The greatest reductions were seen in wrong medication errors (56%), wrong strength/dose errors (71%), wrong formulation errors (90%), and expired medication errors (100%).
In the configuration that did not require scanning of every dose, however, there was a 60% relative reduction in the incidence of target dispensing errors (P<0.001), but a 2.4-fold increase in the incidence of target potential ADEs. This included new errors attributable to wrong strength and wrong medication dispensing.
In light of the FDA’s mandate regarding bar codes, it seems that every hospital has the opportunity to improve patient safety and decrease medication error rates with the use of bar code technology. This study suggests that in order to achieve this benefit these systems should be designed to ensure that every medication dose is verified by scanning during the dispensing process.
Evaluation of a Guideline to Guide Resuscitation
By Cindy Lien, MD
Morrison LJ, Visentin LM, Kiss A, et al. Validation of a rule for termination of resuscitation in out-of-hospital cardiac arrest. N Engl J Med. 2006 Aug 3;355(5):478-487.
The survival rate of patients with out-of-hospital cardiac arrest is very low. Thus, guidelines have been developed for termination of resuscitation for those patients who have had no response to advanced cardiac life support provided by emergency medical service (EMS) personnel. Similar guidelines have not yet been developed, however, for situations in which patients receive basic life support from emergency workers trained in the use of an automated external cardiac defibrillator. Patients with little potential for survival are routinely transported to emergency departments, at significant cost to the healthcare system.
Morrison and colleagues present results from the Termination of Resuscitation (TOR) study, a prospective evaluation of a clinical prediction rule for the termination of basic life support by emergency medical personnel trained in the use of automated external defibrillators. The clinical prediction rule, previously developed in a retrospective review of case records from a large urban EMS system, recommends termination of resuscitation if there is no return of spontaneous circulation, no shock administered, and no witness of the arrest by EMS personnel.
In the current study, the authors obtained follow-up data for 1,240 adult patients in Ontario, Canada, who had suffered an arrest of presumed cardiac cause and were subsequently transported to the emergency department after resuscitative efforts. Twenty-four EMS systems participated in the study. The study found that only 0.5% of the patients for whom the clinical prediction rule recommended termination survived (four out of 776 patients). Of the 1,240 total study patients, 41 (3%) survived. The clinical prediction rule recommended continuation of resuscitative efforts for 37 of these 41 patients, resulting in a specificity of 90.2%. The positive predictive value for death was calculated to be 99.5% when termination was recommended.
The TOR trial also determined whether the addition of other criteria to the original prediction rule could further refine the specificity and positive predictive value. They found that the addition to the criteria of a response time greater than eight minutes increased the positive predictive value and specificity to 99.7% and 97.6%, respectively. When the variable “not witnessed by bystander” was added to the clinical prediction rule, both the positive predictive value and specificity increased to 100%. In other words, no patients survived if they had had a completely unwitnessed arrest, no return of spontaneous circulation, and no shocks delivered.
This study identifies a subpopulation of patients with presumed cardiac arrest for whom termination of resuscitative efforts in the field appears reasonable. The authors note that a survival rate of 1% or less has been suggested in past literature as reflective of medical futility. The TOR investigators acknowledge that their study took place before the 2005 AHA Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care were released and that their study protocols were consistent with the 2000 resuscitation guidelines. In light of this information, continued validity testing of the clinical prediction rule under the 2005 AHA protocols is needed.
Nonetheless, it is quite impressive that use of their clinical prediction rule would have resulted in transportation of only 37% of patients (464 of 1,240), rather than 100% of patients, as is currently the practice. If the guidelines described in this article are to be implemented, further studies are necessary to address the training of EMS personnel, who would carry responsibility for terminating resuscitation and notifying families of patients’ deaths.
Prevention of Ventilator-Associated Pneumonia
By Diane Sliwka, MD
Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006 Jun;173(12):1348-1355. Epub 2006 Apr 7.
Ventilator-associated pneumonia (VAP) is an important nosocomial source of morbidity and mortality. The use of prophylactic antimicrobials to decrease VAP raises concern for antimicrobial resistance. This study evaluates the topical antiseptic chlorhexidine (CHX) as an alternative prophylactic intervention for VAP. CHX has previously been shown to decrease VAP in cardiac surgical patients, but has not been studied in higher risk, long-term-ventilated patients. Because CHX works better for gram-positive organisms, the combination of colistin and CHX (COL + CHX) was also studied for improved gram-negative coverage.
This multi-center, randomized, double-blind, placebo-controlled trial enrolled 385 adult patients. Patients who were expected to be intubated for longer than 48 hours were randomized to 3 arms: CHX alone, CHX + COL, and placebo. Exclusion criteria included known preadmission immunocompromised state, pregnancy, and physical limitation to oral application. Pneumonia was defined by clinical decision-making, which was later confirmed by three blinded intensivists’ reviews of the case records and supported by daily clinical pulmonary infection scores.
The primary endpoint of VAP was diagnosed in 52 of 385 patients: 18% placebo, 13% CHX, and 10% CHX + COL. Rate of VAP in the two treatment groups was lower than placebo and reached statistical significance when compared to placebo. The daily hazard ratio for CHX versus placebo was .352 (95% CI .160, .791); for CHX + COL versus placebo, it was .454 (95% CI .224, .925), showing a 65% and 55% reduction in the rate of pneumonia development. Multivariate analysis of variables such as gender, pulmonary admission diagnosis, colonization at time of admission, and antimicrobial use on admission did not affect the data.
The secondary endpoint of endotracheal colonization was evaluated by a twice-weekly endotracheal culture. There was no statistically significant difference in colonization among the three groups in the first (days 1-4) or third (days 9-12) time frames. During the second time frame (days 5-8), there was a statistically significant decrease in colonization for the CHX + COL treatment group when compared to both placebo (16% versus 40% p<.007) and to CHX (16% versus 38%, p<.011); this decrease is thought to be due to gram-negative coverage by COL.
The secondary endpoint of oropharyngeal colonization was evaluated for 87% of all patient days. CHX and CHX + COL were similarly effective for gram-positive bacteria when compared to placebo, with 30% and 27% reduction in rates of colonization, respectively: HR 0.695 for CHX (95% CI, 0.606, 0.796; p < 0.001) and 0.732 (95% CI, 0.640, 0.838; p < 0.001) for CHX + COL. The CHX + COL combination was more effective for gram-negative bacteria: daily HR .534 (95% CI, 0.455, 0.626; p <0.001) alone with a 47% reduction in gram-negative colonization compared to CHX.
No difference was seen in ICU mortality, duration of mechanical ventilation, or duration of ICU stay. One adverse event (tongue swelling) occurred in the CHX + COL group.
Limitations of the study include the following:
- Daily assessments on all patients were not performed;
- The placebo group had more males and more infections on admission than the other two groups, raising the question of randomization error;
- Clinical versus quantitative diagnosis of pneumonia may overestimate VAP in this study;
- It is not known how many patients were not enrolled in the study due to short anticipated ventilator times, but who later had prolonged ventilations; and
- The lack of effect on ventilator time, ICU length of stay, and mortality raises the question of the significance of these findings.
Despite these limitations, the low cost of these treatments, minimal adverse events, low risk of promoting significant antimicrobial resistance, and the finding of decreased VAP and bacterial colonization risk shown in this study support the potential benefit of topical decontamination with CHX and COL in conjunction with other measures of VAP prevention. TH
Reference
- De Riso AJ II, Ladowski JS, Dillon TA, et al. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556-1561.