User login
Women’s health 2015: An update for the internist
Women's health encompasses a broad range of issues unique to the female patient, with a scope that has expanded beyond reproductive health. Providers who care for women must develop cross-disciplinary competencies and understand the complex role of sex and gender on disease expression and treatment outcomes. Staying current with the literature in this rapidly changing field can be challenging for the busy clinician.
This article reviews recent advances in the treatment of depression in pregnancy, nonhormonal therapies for menopausal symptoms, and heart failure therapy in women, highlighting notable studies published in 2014 and early 2015.
TREATMENT OF DEPRESSION IN PREGNANCY
A 32-year-old woman with well-controlled but recurrent depression presents to the clinic for preconception counseling. Her depression has been successfully managed with a selective serotonin reuptake inhibitor (SSRI). She and her husband would like to try to conceive soon, but she is worried that continuing on her current SSRI may harm her baby. How should you advise her?
Concern for teratogenic effects of SSRIs
Depression is common during pregnancy: 11.8% to 13.5% of pregnant women report symptoms of depression,1 and 7.5% of pregnant women take an antidepressant.2
SSRI use during pregnancy has drawn attention because of mixed reports of teratogenic effects on the newborn, such as omphalocele, congenital heart defects, and craniosynostosis.3 Previous observational studies have specifically linked paroxetine to small but significant increases in right ventricular outflow tract obstruction4,5 and have linked sertraline to ventricular septal defects.6
However, reports of associations of congenital malformations and SSRI use in pregnancy in observational studies have been questioned, with concern that these studies had low statistical power, self-reported data leading to recall bias, and limited assessment for confounding factors.3,7
Recent studies refute risk of cardiac malformations
Several newer studies have been published that further examine the association between SSRI use in pregnancy and congenital heart defects, and their findings suggest that once adjusted for confounding variables, SSRI use in pregnancy may not be associated with cardiac malformations.
Huybrechts et al,8 in a large study published in 2014, extracted data on 950,000 pregnant women from the Medicaid database over a 7-year period and examined it for SSRI use during the first 90 days of pregnancy. Though SSRI use was associated with cardiac malformations when unadjusted for confounding variables (unadjusted relative risk 1.25, 95% confidence interval [CI] 1.13–1.38), once the cohort was restricted to women with a diagnosis of only depression and was adjusted based on propensity scoring, the association was no longer statistically significant (adjusted relative risk 1.06, 95% CI 0.93–1.22).
Additionally, there was no association between sertraline and ventricular septal defects (63 cases in 14,040 women exposed to sertraline, adjusted relative risk 1.04, 95% CI 0.76–1.41), or between paroxetine and right ventricular outflow tract obstruction (93 cases in 11,126 women exposed to paroxetine, adjusted relative risk 1.07, 95% CI 0.59–1.93).8
Furu et al7 conducted a sibling-matched case-control comparison published in 2015, in which more than 2 million live births from five Nordic countries were examined in the full cohort study and 2,288 births in the sibling-matched case-control cohort. SSRI or venlafaxine use in the first 90 days of pregnancy was examined. There was a slightly higher rate of cardiac defects in infants born to SSRI or venlafaxine recipients in the cohort study (adjusted odds ratio 1.15, 95% CI 1.05–1.26). However, in the sibling-controlled analyses, neither an SSRI nor venlafaxine was associated with heart defects (adjusted odds ratio 0.92, 95% CI 0.72–1.17), leading the authors to conclude that there might be familial factors or other lifestyle factors that were not taken into consideration and that could have confounded the cohort results.
Bérard et al9 examined antidepressant use in the first trimester of pregnancy in a cohort of women in Canada and concluded that sertraline was associated with congenital atrial and ventricular defects (risk ratio 1.34; 95% CI 1.02–1.76).9 However, this association should be interpreted with caution, as the Canadian cohort was notably smaller than those in other studies we have discussed, with only 18,493 pregnancies in the total cohort, and this conclusion was drawn from 9 cases of ventricular or atrial septal defects in babies of 366 women exposed to sertraline.
Although at first glance SSRIs may appear to be associated with congenital heart defects, these recent studies are reassuring and suggest that the association may actually not be significant. As with any statistical analysis, thoughtful study design, adequate statistical power, and adjustment for confounding factors must be considered before drawing conclusions.
SSRIs, offspring psychiatric outcomes, and miscarriage rates
Clements et al10 studied a cohort extracted from Partners Healthcare consisting of newborns with autism spectrum disorder, newborns with attention-deficit hyperactivity disorder (ADHD), and healthy matched controls and found that SSRI use during pregnancy was not associated with offspring autism spectrum disorder (adjusted odds ratio 1.10, 95% CI 0.7–1.70). However, they did find an increased risk of ADHD with SSRI use during pregnancy (adjusted odds ratio 1.81, 95% CI 1.22–2.70).
Andersen et al11 examined more than 1 million pregnancies in Denmark and found no difference in risk of miscarriage between women who used an SSRI during pregnancy (adjusted hazard ratio 1.27) and women who discontinued their SSRI at least 3 months before pregnancy (adjusted hazard ratio 1.24, P = .47). The authors concluded that because of the similar rate of miscarriage in both groups, there was no association between SSRI use and miscarriage, and that the small increased risk of miscarriage in both groups could have been attributable to a confounding factor that was not measured.
Should our patient continue her SSRI through pregnancy?
Our patient has recurrent depression, and her risk of relapse with antidepressant cessation is high. Though previous, less well-done studies suggested a small risk of congenital heart defects, recent larger high-quality studies provide significant reassurance that SSRI use in pregnancy is not strongly associated with cardiac malformations. Recent studies also show no association with miscarriage or autism spectrum disorder, though there may be risk of offspring ADHD.
She can be counseled that she may continue on her SSRI during pregnancy and can be reassured that the risk to her baby is small compared with her risk of recurrent or postpartum depression.
NONHORMONAL TREATMENT FOR VASOMOTOR SYMPTOMS OF MENOPAUSE
You see a patient who is struggling with symptoms of menopause. She tells you she has terrible hot flashes day and night, and she would like to try drug therapy. She does not want hormone replacement therapy because she is worried about the risk of adverse events. Are there safe and effective nonhormonal pharmacologic treatments for her vasomotor symptoms?
Paroxetine 7.5 mg is approved for vasomotor symptoms of menopause
As many as 75% of menopausal women in the United States experience vasomotor symptoms related to menopause, or hot flashes and night sweats.12 These symptoms can disrupt sleep and negatively affect quality of life. Though previously thought to occur during a short and self-limited time period, a recently published large observational study reported the median duration of vasomotor symptoms was 7.4 years, and in African American women in the cohort the median duration of vasomotor symptoms was 10.1 years—an entire decade of life.13
In 2013, the US Food and Drug Administration (FDA) approved paroxetine 7.5 mg daily for treating moderate to severe hot flashes associated with menopause. It is the only approved nonhormonal treatment for vasomotor symptoms; the only other approved treatments are estrogen therapy for women who have had a hysterectomy and combination estrogen-progesterone therapy for women who have not had a hysterectomy.
Further studies of paroxetine for menopausal symptoms
Since its approval, further studies have been published supporting the use of paroxetine 7.5 mg in treating symptoms of menopause. In addition to reducing hot flashes, this treatment also improves sleep disturbance in women with menopause.14
Pinkerton et al,14 in a pooled analysis of the data from the phase 3 clinical trials of paroxetine 7.5 mg per day, found that participants in groups assigned to paroxetine reported a 62% reduction in nighttime awakenings due to hot flashes compared with a 43% reduction in the placebo group (P < .001). Those who took paroxetine also reported a statistically significantly greater increase in duration of sleep than those who took placebo (37 minutes in the treatment group vs 27 minutes in the placebo group, P = .03).
Some patients are hesitant to take an SSRI because of concerns about adverse effects when used for psychiatric conditions. However, the dose of paroxetine that was studied and approved for vasomotor symptoms is lower than doses used for psychiatric indications and does not appear to be associated with these adverse effects.
Portman et al15 in 2014 examined the effect of paroxetine 7.5 mg vs placebo on weight gain and sexual function in women with vasomotor symptoms of menopause and found no significant increase in weight or decrease in sexual function at 24 weeks of use. Participants were weighed during study visits, and those in the paroxetine group gained on average 0.48% from baseline at 24 weeks, compared with 0.09% in the placebo group (P = .29).
Sexual dysfunction was assessed using the Arizona Sexual Experience Scale, which has been validated in psychiatric patients using antidepressants, and there was no significant difference in symptoms such as sex drive, sexual arousal, vaginal lubrication, or ability to achieve orgasm between the treatment group and placebo group.15
Of note, paroxetine is a potent inhibitor of the cytochrome P-450 CYP2D6 enzyme, and concurrent use of paroxetine with tamoxifen decreases tamoxifen activity.12,16 Since women with a history of breast cancer who cannot use estrogen for hot flashes may be seeking nonhormonal treatment for their vasomotor symptoms, providers should perform careful medication reconciliation and be aware that concomitant use of paroxetine and tamoxifen is not recommended.
Other antidepressants show promise but are not approved for menopausal symptoms
In addition to paroxetine, other nonhormonal drugs have been studied for treating hot flashes, but they have been unable to secure FDA approval for this indication. One of these is the serotonin-norepinephrine reuptake inhibitor venlafaxine, and a 2014 study17 confirmed its efficacy in treating menopausal vasomotor symptoms.
Joffe et al17 performed a three-armed trial comparing venlafaxine 75 mg/day, estradiol 0.5 mg/day, and placebo and found that both of the active treatments were better than placebo at reducing vasomotor symptoms. Compared with each other, estradiol 0.5 mg/day reduced hot flash frequency by an additional 0.6 events per day compared with venlafaxine 75 mg/day (P = .09). Though this difference was statistically significant, the authors pointed out that the clinical significance of such a small absolute difference is questionable. Additionally, providers should be aware that venlafaxine has little or no effect on the metabolism of tamoxifen.16
Shams et al,18 in a meta-analysis published in 2014, concluded that SSRIs as a class are more effective than placebo in treating hot flashes, supporting their widespread off-label use for this purpose. Their analysis examined the results of 11 studies, which included more than 2,000 patients in total, and found that compared with placebo, SSRI use was associated with a significant decrease in hot flashes (mean difference –0.93 events per day, 95% CI –1.49 to –0.37). A mixed treatment comparison analysis was also performed to try to model performance of individual SSRIs based on the pooled data, and the model suggests that escitalopram may be the most efficacious SSRI at reducing hot flash severity.
These studies support the effectiveness of SSRIs18 and venlafaxine17 in reducing hot flashes compared with placebo, though providers should be aware that they are still not FDA-approved for this indication.
Nonhormonal therapy for our patient
We would recommend paroxetine 7.5 mg nightly to this patient, as it is an FDA-approved nonhormonal medication that has been shown to help patients with vasomotor symptoms of menopause as well as sleep disturbance, without sexual side effects or weight gain. If the patient cannot tolerate paroxetine, off-label use of another SSRI or venlafaxine is supported by the recent literature.
HEART DISEASE IN WOMEN: CARDIAC RESYNCHRONIZATION THERAPY
A 68-year-old woman with a history of nonischemic cardiomyopathy presents for routine follow-up in your office. Despite maximal medical therapy on a beta-blocker, an angiotensin II receptor blocker, and a diuretic, she has New York Heart Association (NYHA) class III symptoms. Her most recent studies showed an ejection fraction of 30% by echocardiography and left bundle-branch block on electrocardiography, with a QRS duration of 140 ms. She recently saw her cardiologist, who recommended cardiac resynchronization therapy, and she wants your opinion as to whether or not to proceed with this recommendation. How should you counsel her?
Which patients are candidates for cardiac resynchronization therapy?
Heart disease continues to be the number one cause of death in the United States for both men and women, and almost the same number of women and men die from heart disease every year.19 Though coronary artery disease accounts for most cases of cardiovascular disease in the United States, heart failure is a significant and growing contributor. Approximately 6.6 million adults had heart failure in 2010 in the United States, and an additional 3 million are projected to have heart failure by 2030.20 The burden of disease on our health system is high, with about 1 million hospitalizations and more than 3 million outpatient office visits attributable to heart failure yearly.20
Patients with heart failure may have symptoms of dyspnea, fatigue, orthopnea, and peripheral edema; laboratory and radiologic findings of pulmonary edema, renal insufficiency, and hyponatremia; and electrocardiographic findings of atrial fibrillation or prolonged QRS.21 Intraventricular conduction delay (QRS duration > 120 ms) is associated with dyssynchronous ventricular contraction and impaired pump function and is present in almost one-third of patients who have advanced heart failure.21
Cardiac resynchronization therapy, or biventricular pacing, can improve symptoms and pump function and has been shown to decrease rates of hospitalization and death in these patients.22 According to the joint 2012 guidelines of the American College of Cardiology Foundation, American Heart Association, and Heart Rhythm Society,22 it is indicated for patients with an ejection fraction of 35% or less, left bundle-branch block with QRS duration of 150 ms or more, and NYHA class II to IV symptoms who are in sinus rhythm (class I recommendation, level of evidence A).
Studies of cardiac resynchronization therapy in women
Recently published studies have suggested that women may derive greater benefit than men from cardiac resynchronization therapy.
Zusterzeel et al23 (2014) evaluated sex-specific data from the National Cardiovascular Data Registry, which contains data on all biventricular pacemaker and implantable cardioverter-defibrillator implantations from 80% of US hospitals.23 Of the 21,152 patients who had left bundle-branch block and received cardiac resynchronization therapy, women derived greater benefit in terms of death than men did, with a 21% lower risk of death than men (adjusted hazard ratio 0.79, 95% CI 0.74–0.84, P < .001). This study was also notable in that 36% of the patients were women, whereas in most earlier studies of cardiac resynchronization therapy women accounted for only 22% to 30% of the study population.22
Goldenberg et al24 (2014) performed a follow-up analysis of the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy. Subgroup analysis showed that although both men and women had a lower risk of death if they received cardiac resynchronization therapy compared with an implantable cardioverter-defibrillator only, the magnitude of benefit may be greater for women (hazard ratio 0.48, 95% CI 0.25–0.91, P = .03) than for men (hazard ratio 0.69, 95% CI 0.50–0.95, P = .02).
In addition to deriving greater mortality benefit, women may actually benefit from cardiac resynchronization therapy at shorter QRS durations than what is currently recommended. Women have a shorter baseline QRS than men, and a smaller left ventricular cavity.25 In an FDA meta-analysis published in August 2014, pooled data from more than 4,000 patients in three studies suggested that women with left bundle-branch block benefited from cardiac resynchronization therapy more than men with left bundle-branch block.26 Neither men nor women with left bundle-branch block benefited from it if their QRS duration was less than 130 ms, and both sexes benefited from it if they had left bundle-branch block and a QRS duration longer than 150 ms. However, women who received it who had left bundle-branch block and a QRS duration of 130 to 149 ms had a significant 76% reduction in the primary composite outcome of a heart failure event or death (hazard ratio 0.24, 95% CI 0.11–0.53, P < .001), while men in the same group did not derive significant benefit (hazard ratio 0.85, 95% CI 0.60–1.21, P = .38).
Despite the increasing evidence that there are sex-specific differences in the benefit from cardiac resynchronization therapy, what we know is limited by the low rates of female enrollment in most of the studies of this treatment. In a systematic review published in 2015, Herz et al27 found that 90% of the 183 studies they reviewed enrolled 35% women or less, and half of the studies enrolled less than 23% women. Furthermore, only 20 of the 183 studies reported baseline characteristics by sex.
Recognizing this lack of adequate data, in August 2014 the FDA issued an official guidance statement outlining its expectations regarding sex-specific patient recruitment, data analysis, and data reporting in future medical device studies.28 Hopefully, with this support for sex-specific research by the FDA, future studies will be able to identify therapeutic outcome differences that may exist between male and female patients.
Should our patient receive cardiac resynchronization therapy?
Regarding our patient with heart failure, the above studies suggest she will likely have a lower risk of death if she receives cardiac resynchronization therapy, even though her QRS interval is shorter than 150 ms. Providers who are aware of the emerging data regarding sex differences and treatment response can be powerful advocates for their patients, even in subspecialty areas, as highlighted by this case. We recommend counseling this patient to proceed with cardiac resynchronization therapy.
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ 2001; 323:257–260.
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S; National Birth Defects Prevention Study. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 2011; 205:51.e1–e8.
- Greene MF. Teratogenicity of SSRIs—serious concern or much ado about little? N Engl J Med 2007; 356:2732–2733.
- Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007; 356:2675–2683.
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM; National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007; 356:2684–2692.
- Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ 2009; 339:b3569.
- Furu K, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 2015; 350:h1798.
- Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014; 370:2397–2407.
- Bérard A, Zhao J-P, Sheehy O. Sertraline use during pregnancy and the risk of major malformations. Am J Obstet Gynecol 2015; 212:795.e1–795.e12.
- Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727–734.
- Andersen JT, Andersen NL, Horwitz H, Poulsen HE, Jimenez-Solem E. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol 2014; 124:655–661.
- Orleans RJ, Li L, Kim M-J, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med 2014; 370:1777–1779.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015; 175:531–539.
- Pinkerton JV, Joffe H, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Low-dose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause 2015; 22:50–58.
- Portman DJ, Kaunitz AM, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Effects of low-dose paroxetine 7.5 mg on weight and sexual function during treatment of vasomotor symptoms associated with menopause. Menopause 2014; 21:1082–1090.
- Desmarais JE, Looper KJ. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry 2009; 70:1688–1697.
- Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med 2014; 174:1058–1066.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med 2014; 29:204–213.
- Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H-C. Deaths: final data for 2009. Nat Vital Stat Rep 2012; 60(3):1–117.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—-2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- McMurray JJV. Clinical practice. Systolic heart failure. N Engl J Med 2010; 362:228–238.
- Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013; 61:e6–e75.
- Zusterzeel R, Curtis JP, Canos DA, et al. Sex-specific mortality risk by QRS morphology and duration in patients receiving CRT. J Am Coll Cardiol 2014; 64:887–894.
- Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med 2014; 370:1694–1701.
- Dec GW. Leaning toward a better understanding of CRT in women. J Am Coll Cardiol 2014; 64:895–897.
- Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med 2014; 174:1340–1348.
- Herz ND, Engeda J, Zusterzeel R, et al. Sex differences in device therapy for heart failure: utilization, outcomes, and adverse events. J Women’s Health 2015; 24:261–271.
- U.S. Department of Health and Human Services, Food and Drug Administration. Evaluation of sex-specific data in medical device clinical studies: guidance for industry and Food and Drug Administration staff. 2014; 1–30. www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM283707.pdf. Accessed October 1, 2015.
Women's health encompasses a broad range of issues unique to the female patient, with a scope that has expanded beyond reproductive health. Providers who care for women must develop cross-disciplinary competencies and understand the complex role of sex and gender on disease expression and treatment outcomes. Staying current with the literature in this rapidly changing field can be challenging for the busy clinician.
This article reviews recent advances in the treatment of depression in pregnancy, nonhormonal therapies for menopausal symptoms, and heart failure therapy in women, highlighting notable studies published in 2014 and early 2015.
TREATMENT OF DEPRESSION IN PREGNANCY
A 32-year-old woman with well-controlled but recurrent depression presents to the clinic for preconception counseling. Her depression has been successfully managed with a selective serotonin reuptake inhibitor (SSRI). She and her husband would like to try to conceive soon, but she is worried that continuing on her current SSRI may harm her baby. How should you advise her?
Concern for teratogenic effects of SSRIs
Depression is common during pregnancy: 11.8% to 13.5% of pregnant women report symptoms of depression,1 and 7.5% of pregnant women take an antidepressant.2
SSRI use during pregnancy has drawn attention because of mixed reports of teratogenic effects on the newborn, such as omphalocele, congenital heart defects, and craniosynostosis.3 Previous observational studies have specifically linked paroxetine to small but significant increases in right ventricular outflow tract obstruction4,5 and have linked sertraline to ventricular septal defects.6
However, reports of associations of congenital malformations and SSRI use in pregnancy in observational studies have been questioned, with concern that these studies had low statistical power, self-reported data leading to recall bias, and limited assessment for confounding factors.3,7
Recent studies refute risk of cardiac malformations
Several newer studies have been published that further examine the association between SSRI use in pregnancy and congenital heart defects, and their findings suggest that once adjusted for confounding variables, SSRI use in pregnancy may not be associated with cardiac malformations.
Huybrechts et al,8 in a large study published in 2014, extracted data on 950,000 pregnant women from the Medicaid database over a 7-year period and examined it for SSRI use during the first 90 days of pregnancy. Though SSRI use was associated with cardiac malformations when unadjusted for confounding variables (unadjusted relative risk 1.25, 95% confidence interval [CI] 1.13–1.38), once the cohort was restricted to women with a diagnosis of only depression and was adjusted based on propensity scoring, the association was no longer statistically significant (adjusted relative risk 1.06, 95% CI 0.93–1.22).
Additionally, there was no association between sertraline and ventricular septal defects (63 cases in 14,040 women exposed to sertraline, adjusted relative risk 1.04, 95% CI 0.76–1.41), or between paroxetine and right ventricular outflow tract obstruction (93 cases in 11,126 women exposed to paroxetine, adjusted relative risk 1.07, 95% CI 0.59–1.93).8
Furu et al7 conducted a sibling-matched case-control comparison published in 2015, in which more than 2 million live births from five Nordic countries were examined in the full cohort study and 2,288 births in the sibling-matched case-control cohort. SSRI or venlafaxine use in the first 90 days of pregnancy was examined. There was a slightly higher rate of cardiac defects in infants born to SSRI or venlafaxine recipients in the cohort study (adjusted odds ratio 1.15, 95% CI 1.05–1.26). However, in the sibling-controlled analyses, neither an SSRI nor venlafaxine was associated with heart defects (adjusted odds ratio 0.92, 95% CI 0.72–1.17), leading the authors to conclude that there might be familial factors or other lifestyle factors that were not taken into consideration and that could have confounded the cohort results.
Bérard et al9 examined antidepressant use in the first trimester of pregnancy in a cohort of women in Canada and concluded that sertraline was associated with congenital atrial and ventricular defects (risk ratio 1.34; 95% CI 1.02–1.76).9 However, this association should be interpreted with caution, as the Canadian cohort was notably smaller than those in other studies we have discussed, with only 18,493 pregnancies in the total cohort, and this conclusion was drawn from 9 cases of ventricular or atrial septal defects in babies of 366 women exposed to sertraline.
Although at first glance SSRIs may appear to be associated with congenital heart defects, these recent studies are reassuring and suggest that the association may actually not be significant. As with any statistical analysis, thoughtful study design, adequate statistical power, and adjustment for confounding factors must be considered before drawing conclusions.
SSRIs, offspring psychiatric outcomes, and miscarriage rates
Clements et al10 studied a cohort extracted from Partners Healthcare consisting of newborns with autism spectrum disorder, newborns with attention-deficit hyperactivity disorder (ADHD), and healthy matched controls and found that SSRI use during pregnancy was not associated with offspring autism spectrum disorder (adjusted odds ratio 1.10, 95% CI 0.7–1.70). However, they did find an increased risk of ADHD with SSRI use during pregnancy (adjusted odds ratio 1.81, 95% CI 1.22–2.70).
Andersen et al11 examined more than 1 million pregnancies in Denmark and found no difference in risk of miscarriage between women who used an SSRI during pregnancy (adjusted hazard ratio 1.27) and women who discontinued their SSRI at least 3 months before pregnancy (adjusted hazard ratio 1.24, P = .47). The authors concluded that because of the similar rate of miscarriage in both groups, there was no association between SSRI use and miscarriage, and that the small increased risk of miscarriage in both groups could have been attributable to a confounding factor that was not measured.
Should our patient continue her SSRI through pregnancy?
Our patient has recurrent depression, and her risk of relapse with antidepressant cessation is high. Though previous, less well-done studies suggested a small risk of congenital heart defects, recent larger high-quality studies provide significant reassurance that SSRI use in pregnancy is not strongly associated with cardiac malformations. Recent studies also show no association with miscarriage or autism spectrum disorder, though there may be risk of offspring ADHD.
She can be counseled that she may continue on her SSRI during pregnancy and can be reassured that the risk to her baby is small compared with her risk of recurrent or postpartum depression.
NONHORMONAL TREATMENT FOR VASOMOTOR SYMPTOMS OF MENOPAUSE
You see a patient who is struggling with symptoms of menopause. She tells you she has terrible hot flashes day and night, and she would like to try drug therapy. She does not want hormone replacement therapy because she is worried about the risk of adverse events. Are there safe and effective nonhormonal pharmacologic treatments for her vasomotor symptoms?
Paroxetine 7.5 mg is approved for vasomotor symptoms of menopause
As many as 75% of menopausal women in the United States experience vasomotor symptoms related to menopause, or hot flashes and night sweats.12 These symptoms can disrupt sleep and negatively affect quality of life. Though previously thought to occur during a short and self-limited time period, a recently published large observational study reported the median duration of vasomotor symptoms was 7.4 years, and in African American women in the cohort the median duration of vasomotor symptoms was 10.1 years—an entire decade of life.13
In 2013, the US Food and Drug Administration (FDA) approved paroxetine 7.5 mg daily for treating moderate to severe hot flashes associated with menopause. It is the only approved nonhormonal treatment for vasomotor symptoms; the only other approved treatments are estrogen therapy for women who have had a hysterectomy and combination estrogen-progesterone therapy for women who have not had a hysterectomy.
Further studies of paroxetine for menopausal symptoms
Since its approval, further studies have been published supporting the use of paroxetine 7.5 mg in treating symptoms of menopause. In addition to reducing hot flashes, this treatment also improves sleep disturbance in women with menopause.14
Pinkerton et al,14 in a pooled analysis of the data from the phase 3 clinical trials of paroxetine 7.5 mg per day, found that participants in groups assigned to paroxetine reported a 62% reduction in nighttime awakenings due to hot flashes compared with a 43% reduction in the placebo group (P < .001). Those who took paroxetine also reported a statistically significantly greater increase in duration of sleep than those who took placebo (37 minutes in the treatment group vs 27 minutes in the placebo group, P = .03).
Some patients are hesitant to take an SSRI because of concerns about adverse effects when used for psychiatric conditions. However, the dose of paroxetine that was studied and approved for vasomotor symptoms is lower than doses used for psychiatric indications and does not appear to be associated with these adverse effects.
Portman et al15 in 2014 examined the effect of paroxetine 7.5 mg vs placebo on weight gain and sexual function in women with vasomotor symptoms of menopause and found no significant increase in weight or decrease in sexual function at 24 weeks of use. Participants were weighed during study visits, and those in the paroxetine group gained on average 0.48% from baseline at 24 weeks, compared with 0.09% in the placebo group (P = .29).
Sexual dysfunction was assessed using the Arizona Sexual Experience Scale, which has been validated in psychiatric patients using antidepressants, and there was no significant difference in symptoms such as sex drive, sexual arousal, vaginal lubrication, or ability to achieve orgasm between the treatment group and placebo group.15
Of note, paroxetine is a potent inhibitor of the cytochrome P-450 CYP2D6 enzyme, and concurrent use of paroxetine with tamoxifen decreases tamoxifen activity.12,16 Since women with a history of breast cancer who cannot use estrogen for hot flashes may be seeking nonhormonal treatment for their vasomotor symptoms, providers should perform careful medication reconciliation and be aware that concomitant use of paroxetine and tamoxifen is not recommended.
Other antidepressants show promise but are not approved for menopausal symptoms
In addition to paroxetine, other nonhormonal drugs have been studied for treating hot flashes, but they have been unable to secure FDA approval for this indication. One of these is the serotonin-norepinephrine reuptake inhibitor venlafaxine, and a 2014 study17 confirmed its efficacy in treating menopausal vasomotor symptoms.
Joffe et al17 performed a three-armed trial comparing venlafaxine 75 mg/day, estradiol 0.5 mg/day, and placebo and found that both of the active treatments were better than placebo at reducing vasomotor symptoms. Compared with each other, estradiol 0.5 mg/day reduced hot flash frequency by an additional 0.6 events per day compared with venlafaxine 75 mg/day (P = .09). Though this difference was statistically significant, the authors pointed out that the clinical significance of such a small absolute difference is questionable. Additionally, providers should be aware that venlafaxine has little or no effect on the metabolism of tamoxifen.16
Shams et al,18 in a meta-analysis published in 2014, concluded that SSRIs as a class are more effective than placebo in treating hot flashes, supporting their widespread off-label use for this purpose. Their analysis examined the results of 11 studies, which included more than 2,000 patients in total, and found that compared with placebo, SSRI use was associated with a significant decrease in hot flashes (mean difference –0.93 events per day, 95% CI –1.49 to –0.37). A mixed treatment comparison analysis was also performed to try to model performance of individual SSRIs based on the pooled data, and the model suggests that escitalopram may be the most efficacious SSRI at reducing hot flash severity.
These studies support the effectiveness of SSRIs18 and venlafaxine17 in reducing hot flashes compared with placebo, though providers should be aware that they are still not FDA-approved for this indication.
Nonhormonal therapy for our patient
We would recommend paroxetine 7.5 mg nightly to this patient, as it is an FDA-approved nonhormonal medication that has been shown to help patients with vasomotor symptoms of menopause as well as sleep disturbance, without sexual side effects or weight gain. If the patient cannot tolerate paroxetine, off-label use of another SSRI or venlafaxine is supported by the recent literature.
HEART DISEASE IN WOMEN: CARDIAC RESYNCHRONIZATION THERAPY
A 68-year-old woman with a history of nonischemic cardiomyopathy presents for routine follow-up in your office. Despite maximal medical therapy on a beta-blocker, an angiotensin II receptor blocker, and a diuretic, she has New York Heart Association (NYHA) class III symptoms. Her most recent studies showed an ejection fraction of 30% by echocardiography and left bundle-branch block on electrocardiography, with a QRS duration of 140 ms. She recently saw her cardiologist, who recommended cardiac resynchronization therapy, and she wants your opinion as to whether or not to proceed with this recommendation. How should you counsel her?
Which patients are candidates for cardiac resynchronization therapy?
Heart disease continues to be the number one cause of death in the United States for both men and women, and almost the same number of women and men die from heart disease every year.19 Though coronary artery disease accounts for most cases of cardiovascular disease in the United States, heart failure is a significant and growing contributor. Approximately 6.6 million adults had heart failure in 2010 in the United States, and an additional 3 million are projected to have heart failure by 2030.20 The burden of disease on our health system is high, with about 1 million hospitalizations and more than 3 million outpatient office visits attributable to heart failure yearly.20
Patients with heart failure may have symptoms of dyspnea, fatigue, orthopnea, and peripheral edema; laboratory and radiologic findings of pulmonary edema, renal insufficiency, and hyponatremia; and electrocardiographic findings of atrial fibrillation or prolonged QRS.21 Intraventricular conduction delay (QRS duration > 120 ms) is associated with dyssynchronous ventricular contraction and impaired pump function and is present in almost one-third of patients who have advanced heart failure.21
Cardiac resynchronization therapy, or biventricular pacing, can improve symptoms and pump function and has been shown to decrease rates of hospitalization and death in these patients.22 According to the joint 2012 guidelines of the American College of Cardiology Foundation, American Heart Association, and Heart Rhythm Society,22 it is indicated for patients with an ejection fraction of 35% or less, left bundle-branch block with QRS duration of 150 ms or more, and NYHA class II to IV symptoms who are in sinus rhythm (class I recommendation, level of evidence A).
Studies of cardiac resynchronization therapy in women
Recently published studies have suggested that women may derive greater benefit than men from cardiac resynchronization therapy.
Zusterzeel et al23 (2014) evaluated sex-specific data from the National Cardiovascular Data Registry, which contains data on all biventricular pacemaker and implantable cardioverter-defibrillator implantations from 80% of US hospitals.23 Of the 21,152 patients who had left bundle-branch block and received cardiac resynchronization therapy, women derived greater benefit in terms of death than men did, with a 21% lower risk of death than men (adjusted hazard ratio 0.79, 95% CI 0.74–0.84, P < .001). This study was also notable in that 36% of the patients were women, whereas in most earlier studies of cardiac resynchronization therapy women accounted for only 22% to 30% of the study population.22
Goldenberg et al24 (2014) performed a follow-up analysis of the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy. Subgroup analysis showed that although both men and women had a lower risk of death if they received cardiac resynchronization therapy compared with an implantable cardioverter-defibrillator only, the magnitude of benefit may be greater for women (hazard ratio 0.48, 95% CI 0.25–0.91, P = .03) than for men (hazard ratio 0.69, 95% CI 0.50–0.95, P = .02).
In addition to deriving greater mortality benefit, women may actually benefit from cardiac resynchronization therapy at shorter QRS durations than what is currently recommended. Women have a shorter baseline QRS than men, and a smaller left ventricular cavity.25 In an FDA meta-analysis published in August 2014, pooled data from more than 4,000 patients in three studies suggested that women with left bundle-branch block benefited from cardiac resynchronization therapy more than men with left bundle-branch block.26 Neither men nor women with left bundle-branch block benefited from it if their QRS duration was less than 130 ms, and both sexes benefited from it if they had left bundle-branch block and a QRS duration longer than 150 ms. However, women who received it who had left bundle-branch block and a QRS duration of 130 to 149 ms had a significant 76% reduction in the primary composite outcome of a heart failure event or death (hazard ratio 0.24, 95% CI 0.11–0.53, P < .001), while men in the same group did not derive significant benefit (hazard ratio 0.85, 95% CI 0.60–1.21, P = .38).
Despite the increasing evidence that there are sex-specific differences in the benefit from cardiac resynchronization therapy, what we know is limited by the low rates of female enrollment in most of the studies of this treatment. In a systematic review published in 2015, Herz et al27 found that 90% of the 183 studies they reviewed enrolled 35% women or less, and half of the studies enrolled less than 23% women. Furthermore, only 20 of the 183 studies reported baseline characteristics by sex.
Recognizing this lack of adequate data, in August 2014 the FDA issued an official guidance statement outlining its expectations regarding sex-specific patient recruitment, data analysis, and data reporting in future medical device studies.28 Hopefully, with this support for sex-specific research by the FDA, future studies will be able to identify therapeutic outcome differences that may exist between male and female patients.
Should our patient receive cardiac resynchronization therapy?
Regarding our patient with heart failure, the above studies suggest she will likely have a lower risk of death if she receives cardiac resynchronization therapy, even though her QRS interval is shorter than 150 ms. Providers who are aware of the emerging data regarding sex differences and treatment response can be powerful advocates for their patients, even in subspecialty areas, as highlighted by this case. We recommend counseling this patient to proceed with cardiac resynchronization therapy.
Women's health encompasses a broad range of issues unique to the female patient, with a scope that has expanded beyond reproductive health. Providers who care for women must develop cross-disciplinary competencies and understand the complex role of sex and gender on disease expression and treatment outcomes. Staying current with the literature in this rapidly changing field can be challenging for the busy clinician.
This article reviews recent advances in the treatment of depression in pregnancy, nonhormonal therapies for menopausal symptoms, and heart failure therapy in women, highlighting notable studies published in 2014 and early 2015.
TREATMENT OF DEPRESSION IN PREGNANCY
A 32-year-old woman with well-controlled but recurrent depression presents to the clinic for preconception counseling. Her depression has been successfully managed with a selective serotonin reuptake inhibitor (SSRI). She and her husband would like to try to conceive soon, but she is worried that continuing on her current SSRI may harm her baby. How should you advise her?
Concern for teratogenic effects of SSRIs
Depression is common during pregnancy: 11.8% to 13.5% of pregnant women report symptoms of depression,1 and 7.5% of pregnant women take an antidepressant.2
SSRI use during pregnancy has drawn attention because of mixed reports of teratogenic effects on the newborn, such as omphalocele, congenital heart defects, and craniosynostosis.3 Previous observational studies have specifically linked paroxetine to small but significant increases in right ventricular outflow tract obstruction4,5 and have linked sertraline to ventricular septal defects.6
However, reports of associations of congenital malformations and SSRI use in pregnancy in observational studies have been questioned, with concern that these studies had low statistical power, self-reported data leading to recall bias, and limited assessment for confounding factors.3,7
Recent studies refute risk of cardiac malformations
Several newer studies have been published that further examine the association between SSRI use in pregnancy and congenital heart defects, and their findings suggest that once adjusted for confounding variables, SSRI use in pregnancy may not be associated with cardiac malformations.
Huybrechts et al,8 in a large study published in 2014, extracted data on 950,000 pregnant women from the Medicaid database over a 7-year period and examined it for SSRI use during the first 90 days of pregnancy. Though SSRI use was associated with cardiac malformations when unadjusted for confounding variables (unadjusted relative risk 1.25, 95% confidence interval [CI] 1.13–1.38), once the cohort was restricted to women with a diagnosis of only depression and was adjusted based on propensity scoring, the association was no longer statistically significant (adjusted relative risk 1.06, 95% CI 0.93–1.22).
Additionally, there was no association between sertraline and ventricular septal defects (63 cases in 14,040 women exposed to sertraline, adjusted relative risk 1.04, 95% CI 0.76–1.41), or between paroxetine and right ventricular outflow tract obstruction (93 cases in 11,126 women exposed to paroxetine, adjusted relative risk 1.07, 95% CI 0.59–1.93).8
Furu et al7 conducted a sibling-matched case-control comparison published in 2015, in which more than 2 million live births from five Nordic countries were examined in the full cohort study and 2,288 births in the sibling-matched case-control cohort. SSRI or venlafaxine use in the first 90 days of pregnancy was examined. There was a slightly higher rate of cardiac defects in infants born to SSRI or venlafaxine recipients in the cohort study (adjusted odds ratio 1.15, 95% CI 1.05–1.26). However, in the sibling-controlled analyses, neither an SSRI nor venlafaxine was associated with heart defects (adjusted odds ratio 0.92, 95% CI 0.72–1.17), leading the authors to conclude that there might be familial factors or other lifestyle factors that were not taken into consideration and that could have confounded the cohort results.
Bérard et al9 examined antidepressant use in the first trimester of pregnancy in a cohort of women in Canada and concluded that sertraline was associated with congenital atrial and ventricular defects (risk ratio 1.34; 95% CI 1.02–1.76).9 However, this association should be interpreted with caution, as the Canadian cohort was notably smaller than those in other studies we have discussed, with only 18,493 pregnancies in the total cohort, and this conclusion was drawn from 9 cases of ventricular or atrial septal defects in babies of 366 women exposed to sertraline.
Although at first glance SSRIs may appear to be associated with congenital heart defects, these recent studies are reassuring and suggest that the association may actually not be significant. As with any statistical analysis, thoughtful study design, adequate statistical power, and adjustment for confounding factors must be considered before drawing conclusions.
SSRIs, offspring psychiatric outcomes, and miscarriage rates
Clements et al10 studied a cohort extracted from Partners Healthcare consisting of newborns with autism spectrum disorder, newborns with attention-deficit hyperactivity disorder (ADHD), and healthy matched controls and found that SSRI use during pregnancy was not associated with offspring autism spectrum disorder (adjusted odds ratio 1.10, 95% CI 0.7–1.70). However, they did find an increased risk of ADHD with SSRI use during pregnancy (adjusted odds ratio 1.81, 95% CI 1.22–2.70).
Andersen et al11 examined more than 1 million pregnancies in Denmark and found no difference in risk of miscarriage between women who used an SSRI during pregnancy (adjusted hazard ratio 1.27) and women who discontinued their SSRI at least 3 months before pregnancy (adjusted hazard ratio 1.24, P = .47). The authors concluded that because of the similar rate of miscarriage in both groups, there was no association between SSRI use and miscarriage, and that the small increased risk of miscarriage in both groups could have been attributable to a confounding factor that was not measured.
Should our patient continue her SSRI through pregnancy?
Our patient has recurrent depression, and her risk of relapse with antidepressant cessation is high. Though previous, less well-done studies suggested a small risk of congenital heart defects, recent larger high-quality studies provide significant reassurance that SSRI use in pregnancy is not strongly associated with cardiac malformations. Recent studies also show no association with miscarriage or autism spectrum disorder, though there may be risk of offspring ADHD.
She can be counseled that she may continue on her SSRI during pregnancy and can be reassured that the risk to her baby is small compared with her risk of recurrent or postpartum depression.
NONHORMONAL TREATMENT FOR VASOMOTOR SYMPTOMS OF MENOPAUSE
You see a patient who is struggling with symptoms of menopause. She tells you she has terrible hot flashes day and night, and she would like to try drug therapy. She does not want hormone replacement therapy because she is worried about the risk of adverse events. Are there safe and effective nonhormonal pharmacologic treatments for her vasomotor symptoms?
Paroxetine 7.5 mg is approved for vasomotor symptoms of menopause
As many as 75% of menopausal women in the United States experience vasomotor symptoms related to menopause, or hot flashes and night sweats.12 These symptoms can disrupt sleep and negatively affect quality of life. Though previously thought to occur during a short and self-limited time period, a recently published large observational study reported the median duration of vasomotor symptoms was 7.4 years, and in African American women in the cohort the median duration of vasomotor symptoms was 10.1 years—an entire decade of life.13
In 2013, the US Food and Drug Administration (FDA) approved paroxetine 7.5 mg daily for treating moderate to severe hot flashes associated with menopause. It is the only approved nonhormonal treatment for vasomotor symptoms; the only other approved treatments are estrogen therapy for women who have had a hysterectomy and combination estrogen-progesterone therapy for women who have not had a hysterectomy.
Further studies of paroxetine for menopausal symptoms
Since its approval, further studies have been published supporting the use of paroxetine 7.5 mg in treating symptoms of menopause. In addition to reducing hot flashes, this treatment also improves sleep disturbance in women with menopause.14
Pinkerton et al,14 in a pooled analysis of the data from the phase 3 clinical trials of paroxetine 7.5 mg per day, found that participants in groups assigned to paroxetine reported a 62% reduction in nighttime awakenings due to hot flashes compared with a 43% reduction in the placebo group (P < .001). Those who took paroxetine also reported a statistically significantly greater increase in duration of sleep than those who took placebo (37 minutes in the treatment group vs 27 minutes in the placebo group, P = .03).
Some patients are hesitant to take an SSRI because of concerns about adverse effects when used for psychiatric conditions. However, the dose of paroxetine that was studied and approved for vasomotor symptoms is lower than doses used for psychiatric indications and does not appear to be associated with these adverse effects.
Portman et al15 in 2014 examined the effect of paroxetine 7.5 mg vs placebo on weight gain and sexual function in women with vasomotor symptoms of menopause and found no significant increase in weight or decrease in sexual function at 24 weeks of use. Participants were weighed during study visits, and those in the paroxetine group gained on average 0.48% from baseline at 24 weeks, compared with 0.09% in the placebo group (P = .29).
Sexual dysfunction was assessed using the Arizona Sexual Experience Scale, which has been validated in psychiatric patients using antidepressants, and there was no significant difference in symptoms such as sex drive, sexual arousal, vaginal lubrication, or ability to achieve orgasm between the treatment group and placebo group.15
Of note, paroxetine is a potent inhibitor of the cytochrome P-450 CYP2D6 enzyme, and concurrent use of paroxetine with tamoxifen decreases tamoxifen activity.12,16 Since women with a history of breast cancer who cannot use estrogen for hot flashes may be seeking nonhormonal treatment for their vasomotor symptoms, providers should perform careful medication reconciliation and be aware that concomitant use of paroxetine and tamoxifen is not recommended.
Other antidepressants show promise but are not approved for menopausal symptoms
In addition to paroxetine, other nonhormonal drugs have been studied for treating hot flashes, but they have been unable to secure FDA approval for this indication. One of these is the serotonin-norepinephrine reuptake inhibitor venlafaxine, and a 2014 study17 confirmed its efficacy in treating menopausal vasomotor symptoms.
Joffe et al17 performed a three-armed trial comparing venlafaxine 75 mg/day, estradiol 0.5 mg/day, and placebo and found that both of the active treatments were better than placebo at reducing vasomotor symptoms. Compared with each other, estradiol 0.5 mg/day reduced hot flash frequency by an additional 0.6 events per day compared with venlafaxine 75 mg/day (P = .09). Though this difference was statistically significant, the authors pointed out that the clinical significance of such a small absolute difference is questionable. Additionally, providers should be aware that venlafaxine has little or no effect on the metabolism of tamoxifen.16
Shams et al,18 in a meta-analysis published in 2014, concluded that SSRIs as a class are more effective than placebo in treating hot flashes, supporting their widespread off-label use for this purpose. Their analysis examined the results of 11 studies, which included more than 2,000 patients in total, and found that compared with placebo, SSRI use was associated with a significant decrease in hot flashes (mean difference –0.93 events per day, 95% CI –1.49 to –0.37). A mixed treatment comparison analysis was also performed to try to model performance of individual SSRIs based on the pooled data, and the model suggests that escitalopram may be the most efficacious SSRI at reducing hot flash severity.
These studies support the effectiveness of SSRIs18 and venlafaxine17 in reducing hot flashes compared with placebo, though providers should be aware that they are still not FDA-approved for this indication.
Nonhormonal therapy for our patient
We would recommend paroxetine 7.5 mg nightly to this patient, as it is an FDA-approved nonhormonal medication that has been shown to help patients with vasomotor symptoms of menopause as well as sleep disturbance, without sexual side effects or weight gain. If the patient cannot tolerate paroxetine, off-label use of another SSRI or venlafaxine is supported by the recent literature.
HEART DISEASE IN WOMEN: CARDIAC RESYNCHRONIZATION THERAPY
A 68-year-old woman with a history of nonischemic cardiomyopathy presents for routine follow-up in your office. Despite maximal medical therapy on a beta-blocker, an angiotensin II receptor blocker, and a diuretic, she has New York Heart Association (NYHA) class III symptoms. Her most recent studies showed an ejection fraction of 30% by echocardiography and left bundle-branch block on electrocardiography, with a QRS duration of 140 ms. She recently saw her cardiologist, who recommended cardiac resynchronization therapy, and she wants your opinion as to whether or not to proceed with this recommendation. How should you counsel her?
Which patients are candidates for cardiac resynchronization therapy?
Heart disease continues to be the number one cause of death in the United States for both men and women, and almost the same number of women and men die from heart disease every year.19 Though coronary artery disease accounts for most cases of cardiovascular disease in the United States, heart failure is a significant and growing contributor. Approximately 6.6 million adults had heart failure in 2010 in the United States, and an additional 3 million are projected to have heart failure by 2030.20 The burden of disease on our health system is high, with about 1 million hospitalizations and more than 3 million outpatient office visits attributable to heart failure yearly.20
Patients with heart failure may have symptoms of dyspnea, fatigue, orthopnea, and peripheral edema; laboratory and radiologic findings of pulmonary edema, renal insufficiency, and hyponatremia; and electrocardiographic findings of atrial fibrillation or prolonged QRS.21 Intraventricular conduction delay (QRS duration > 120 ms) is associated with dyssynchronous ventricular contraction and impaired pump function and is present in almost one-third of patients who have advanced heart failure.21
Cardiac resynchronization therapy, or biventricular pacing, can improve symptoms and pump function and has been shown to decrease rates of hospitalization and death in these patients.22 According to the joint 2012 guidelines of the American College of Cardiology Foundation, American Heart Association, and Heart Rhythm Society,22 it is indicated for patients with an ejection fraction of 35% or less, left bundle-branch block with QRS duration of 150 ms or more, and NYHA class II to IV symptoms who are in sinus rhythm (class I recommendation, level of evidence A).
Studies of cardiac resynchronization therapy in women
Recently published studies have suggested that women may derive greater benefit than men from cardiac resynchronization therapy.
Zusterzeel et al23 (2014) evaluated sex-specific data from the National Cardiovascular Data Registry, which contains data on all biventricular pacemaker and implantable cardioverter-defibrillator implantations from 80% of US hospitals.23 Of the 21,152 patients who had left bundle-branch block and received cardiac resynchronization therapy, women derived greater benefit in terms of death than men did, with a 21% lower risk of death than men (adjusted hazard ratio 0.79, 95% CI 0.74–0.84, P < .001). This study was also notable in that 36% of the patients were women, whereas in most earlier studies of cardiac resynchronization therapy women accounted for only 22% to 30% of the study population.22
Goldenberg et al24 (2014) performed a follow-up analysis of the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy. Subgroup analysis showed that although both men and women had a lower risk of death if they received cardiac resynchronization therapy compared with an implantable cardioverter-defibrillator only, the magnitude of benefit may be greater for women (hazard ratio 0.48, 95% CI 0.25–0.91, P = .03) than for men (hazard ratio 0.69, 95% CI 0.50–0.95, P = .02).
In addition to deriving greater mortality benefit, women may actually benefit from cardiac resynchronization therapy at shorter QRS durations than what is currently recommended. Women have a shorter baseline QRS than men, and a smaller left ventricular cavity.25 In an FDA meta-analysis published in August 2014, pooled data from more than 4,000 patients in three studies suggested that women with left bundle-branch block benefited from cardiac resynchronization therapy more than men with left bundle-branch block.26 Neither men nor women with left bundle-branch block benefited from it if their QRS duration was less than 130 ms, and both sexes benefited from it if they had left bundle-branch block and a QRS duration longer than 150 ms. However, women who received it who had left bundle-branch block and a QRS duration of 130 to 149 ms had a significant 76% reduction in the primary composite outcome of a heart failure event or death (hazard ratio 0.24, 95% CI 0.11–0.53, P < .001), while men in the same group did not derive significant benefit (hazard ratio 0.85, 95% CI 0.60–1.21, P = .38).
Despite the increasing evidence that there are sex-specific differences in the benefit from cardiac resynchronization therapy, what we know is limited by the low rates of female enrollment in most of the studies of this treatment. In a systematic review published in 2015, Herz et al27 found that 90% of the 183 studies they reviewed enrolled 35% women or less, and half of the studies enrolled less than 23% women. Furthermore, only 20 of the 183 studies reported baseline characteristics by sex.
Recognizing this lack of adequate data, in August 2014 the FDA issued an official guidance statement outlining its expectations regarding sex-specific patient recruitment, data analysis, and data reporting in future medical device studies.28 Hopefully, with this support for sex-specific research by the FDA, future studies will be able to identify therapeutic outcome differences that may exist between male and female patients.
Should our patient receive cardiac resynchronization therapy?
Regarding our patient with heart failure, the above studies suggest she will likely have a lower risk of death if she receives cardiac resynchronization therapy, even though her QRS interval is shorter than 150 ms. Providers who are aware of the emerging data regarding sex differences and treatment response can be powerful advocates for their patients, even in subspecialty areas, as highlighted by this case. We recommend counseling this patient to proceed with cardiac resynchronization therapy.
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ 2001; 323:257–260.
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S; National Birth Defects Prevention Study. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 2011; 205:51.e1–e8.
- Greene MF. Teratogenicity of SSRIs—serious concern or much ado about little? N Engl J Med 2007; 356:2732–2733.
- Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007; 356:2675–2683.
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM; National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007; 356:2684–2692.
- Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ 2009; 339:b3569.
- Furu K, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 2015; 350:h1798.
- Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014; 370:2397–2407.
- Bérard A, Zhao J-P, Sheehy O. Sertraline use during pregnancy and the risk of major malformations. Am J Obstet Gynecol 2015; 212:795.e1–795.e12.
- Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727–734.
- Andersen JT, Andersen NL, Horwitz H, Poulsen HE, Jimenez-Solem E. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol 2014; 124:655–661.
- Orleans RJ, Li L, Kim M-J, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med 2014; 370:1777–1779.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015; 175:531–539.
- Pinkerton JV, Joffe H, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Low-dose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause 2015; 22:50–58.
- Portman DJ, Kaunitz AM, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Effects of low-dose paroxetine 7.5 mg on weight and sexual function during treatment of vasomotor symptoms associated with menopause. Menopause 2014; 21:1082–1090.
- Desmarais JE, Looper KJ. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry 2009; 70:1688–1697.
- Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med 2014; 174:1058–1066.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med 2014; 29:204–213.
- Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H-C. Deaths: final data for 2009. Nat Vital Stat Rep 2012; 60(3):1–117.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—-2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- McMurray JJV. Clinical practice. Systolic heart failure. N Engl J Med 2010; 362:228–238.
- Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013; 61:e6–e75.
- Zusterzeel R, Curtis JP, Canos DA, et al. Sex-specific mortality risk by QRS morphology and duration in patients receiving CRT. J Am Coll Cardiol 2014; 64:887–894.
- Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med 2014; 370:1694–1701.
- Dec GW. Leaning toward a better understanding of CRT in women. J Am Coll Cardiol 2014; 64:895–897.
- Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med 2014; 174:1340–1348.
- Herz ND, Engeda J, Zusterzeel R, et al. Sex differences in device therapy for heart failure: utilization, outcomes, and adverse events. J Women’s Health 2015; 24:261–271.
- U.S. Department of Health and Human Services, Food and Drug Administration. Evaluation of sex-specific data in medical device clinical studies: guidance for industry and Food and Drug Administration staff. 2014; 1–30. www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM283707.pdf. Accessed October 1, 2015.
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ 2001; 323:257–260.
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S; National Birth Defects Prevention Study. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 2011; 205:51.e1–e8.
- Greene MF. Teratogenicity of SSRIs—serious concern or much ado about little? N Engl J Med 2007; 356:2732–2733.
- Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 2007; 356:2675–2683.
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM; National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 2007; 356:2684–2692.
- Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ 2009; 339:b3569.
- Furu K, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 2015; 350:h1798.
- Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014; 370:2397–2407.
- Bérard A, Zhao J-P, Sheehy O. Sertraline use during pregnancy and the risk of major malformations. Am J Obstet Gynecol 2015; 212:795.e1–795.e12.
- Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727–734.
- Andersen JT, Andersen NL, Horwitz H, Poulsen HE, Jimenez-Solem E. Exposure to selective serotonin reuptake inhibitors in early pregnancy and the risk of miscarriage. Obstet Gynecol 2014; 124:655–661.
- Orleans RJ, Li L, Kim M-J, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med 2014; 370:1777–1779.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015; 175:531–539.
- Pinkerton JV, Joffe H, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Low-dose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause 2015; 22:50–58.
- Portman DJ, Kaunitz AM, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Effects of low-dose paroxetine 7.5 mg on weight and sexual function during treatment of vasomotor symptoms associated with menopause. Menopause 2014; 21:1082–1090.
- Desmarais JE, Looper KJ. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry 2009; 70:1688–1697.
- Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med 2014; 174:1058–1066.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med 2014; 29:204–213.
- Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H-C. Deaths: final data for 2009. Nat Vital Stat Rep 2012; 60(3):1–117.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—-2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- McMurray JJV. Clinical practice. Systolic heart failure. N Engl J Med 2010; 362:228–238.
- Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013; 61:e6–e75.
- Zusterzeel R, Curtis JP, Canos DA, et al. Sex-specific mortality risk by QRS morphology and duration in patients receiving CRT. J Am Coll Cardiol 2014; 64:887–894.
- Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med 2014; 370:1694–1701.
- Dec GW. Leaning toward a better understanding of CRT in women. J Am Coll Cardiol 2014; 64:895–897.
- Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med 2014; 174:1340–1348.
- Herz ND, Engeda J, Zusterzeel R, et al. Sex differences in device therapy for heart failure: utilization, outcomes, and adverse events. J Women’s Health 2015; 24:261–271.
- U.S. Department of Health and Human Services, Food and Drug Administration. Evaluation of sex-specific data in medical device clinical studies: guidance for industry and Food and Drug Administration staff. 2014; 1–30. www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM283707.pdf. Accessed October 1, 2015.
KEY POINTS
- Earlier trials had raised concerns about possible teratogenic effects of selective serotonin reuptake inhibitors, but more recent trials have found no strong association between these drugs and congenital heart defects, and no association with miscarriage or autism spectrum disorder, though there may be a risk of attention deficit hyperactivity disorder in offspring.
- Paroxetine is approved for treating vasomotor symptoms of menopause, but in a lower dose (7.5 mg) than those used for depression and other psychiatric indications. Clinical trials have also shown good results with other antidepressants for treating hot flashes, but the drugs are not yet approved for this indication.
- Women with heart failure and left bundle-branch block can decrease their risk of death with cardiac resynchronization therapy more than men with the same condition. Moreover, women may benefit from this therapy even if their QRS duration is somewhat shorter than the established cutoff, ie, if it is in the range of 130 to 149 ms.
Reducing the risk of breast cancer: A personalized approach
• Use a validated breast cancer risk assessment tool for any woman with a suspicious family history, precancerous breast lesions, or reproductive risk factors. C
• Recommend a semi-annual clinical breast exam and an annual mammogram for women at high risk for invasive breast cancer. C
• Discuss chemoprevention with a selective estrogen-receptor modifier or aromatase inhibitor with women at high risk for breast cancer and low risk for adverse events. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Identifying patients at risk
Among the known risk factors for breast cancer, some are modifiable (use of oral contraceptives and alcohol consumption, for example); others, such as family history and age at which menopause occurs, are not (TABLE 1).4-7 Aging itself confers the greatest risk: The incidence of breast cancer comes close to doubling at each 10-year interval before menopause and continues to climb, but more slowly, thereafter.8,9
TABLE 1
Risk factors for breast cancer4-7
| Nonmodifiable | Age, atypical hyperplasia, chest wall radiation (between the ages of 10-30 y), early menarche, family history, late menopause, race, sex |
| Modifiable | Alcohol consumption, hormone therapy (for menopausal symptoms, oral contraceptives), obesity, parity (first child after age 35, nulliparity) |
Estrogen exposure: The risk is cumulative
A number of studies have linked early onset of menarche (<12 years of age) and late menopause (>55 years) to an increase in breast cancer risk. Nulliparity, or having a first child after age 35, is also associated with greater risk; oophorectomy prior to age 50 may reduce the risk by as much as 40%.4,5,10-13
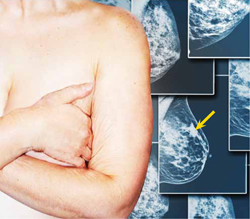
The mammogram shows a malignancy in the superior portion of the breast (arrow). Oral contraceptive use is an additional risk, but the effect slowly diminishes in the 10 years after cessation.4,5 Postmenopausal hormone replacement therapy—specifically, oral conjugated equine estrogen and medroxyprogesterone acetate—was found by the Woman’s Health Initiative to increase breast cancer risk.6
Other nongenetic risk factors include:
Atypical findings on breast biopsy. Evidence of atypical ductal hyperplasia (ADH) or lobular hyperplasia (ALH) is associated with a 4-fold increase in risk.7
Environmental exposure. Radiation, especially to the chest wall (typically as a treatment for Hodgkin’s lymphoma) increases a woman’s risk for breast cancer, particularly if the exposure occurred when she was between the ages of 10 and 30.14
Lifestyle factors. Obesity, particularly in postmenopausal women, and alcohol consumption of more than a drink or two per day are both associated with an increased risk.4
Genetic mutations and breast cancer risk
An estimated 5% to 10% of breast cancers are inherited.5 Genetic susceptibility is generally transmitted as an autosomal dominant trait.
There are 2 known breast cancer genes, BRCA1 and BRCA2, located on the long arm of chromosomes 17 and 13, respectively. The genes themselves encode tumor suppressor proteins. Mutations in these genes impair the DNA repair process, resulting in increased risk.8
The chance of carrying a mutation in either BRCA1 or BRCA2 is estimated at one in 500 to 800 in women of Northern/Western European descent. Among Ashkenazi Jews, however, the frequency is about one in 50.5
A thorough family history that takes into account both the number of affected relatives and their age at diagnosis (TABLE 2)8,15 is helpful in determining whether a patient is at low, high, or very high risk of carrying a genetic mutation. Women who have no first-degree relative with breast cancer—or a relative who was diagnosed with breast cancer after age 50—are at low risk, while those with at least one first-degree relative diagnosed with breast cancer before the age of 50 would be categorized as high risk.
A woman with a family history of early-onset breast or ovarian cancer or a relative who developed both breast and ovarian cancer, bilateral breast cancer, or male breast cancer would be classified as very high risk for a genetic mutation, as would a patient with 2 or more family members affected by breast or ovarian cancer.
Ashkenazi Jewish heritage and a relative who was diagnosed with ovarian or breast cancer indicate an increased likelihood of a BRCA mutation, as well.8 (Other genetic conditions, with mutations that are distinct from the BRCA genes, have also been linked to breast cancer, but occur less frequently.)
BRCA gene testing can confirm very high risk status, prompting the initiation of preventive measures and facilitating early detection. Such testing can also identify—and relieve the anxiety of—noncarriers in high-risk families. Recently published guidelines from the US Preventive Services Task Force (USPSTF) support testing in women with suspicious family histories with a grade B recommendation, indicating that there is at least fair evidence that testing improves important health outcomes and that the benefits of testing outweigh the harms.15
The downside of specific BRCA gene testing for patients who find that they do not have this genetic mutation may include a false sense of security and the failure to identify any other genetic mutations. Patients who learn that they do carry a BRCA gene mutation could face psychosocial or economic harm associated with aggressive surveillance and surgical intervention.5
TABLE 2
Genetic counseling for patients at high risk8,15
Tools can quantify 5-year, 10-year, and lifetime risk
A number of breast cancer risk assessment tools have been developed to help clinicians individualize patient care. None provides the basis for an all-encompassing approach to breast cancer risk or a comprehensive patient discussion of preventive strategies. We have found that, when used in combination, 2 or more predictive models can complement each other and guide the development of a targeted risk reduction approach.
When to use a predictive tool
It is not necessary to use a predictive model for patients at low risk for breast cancer. The tools detailed in TABLE 3 5,14,16-23 are better suited to women who have a suspicious family history, a history of precancerous breast lesions, or known reproductive risks. Although each model has limitations, it is important that you have a working knowledge of circumstances that favor one tool over another. For instance, the Gail model, the most widely used, can help determine if a particular patient is a candidate for chemoprevention.16-20 Others, such as the Tyrer-Cuzick model14,21,22 and the Claus model,14,23 are useful in deciding whether a patient is a candidate for breast magnetic resonance imaging (MRI) as an adjunct to mammography screening. Another useful tool is the BRCAPRO, which is used primarily by genetic counselors to assess the likelihood that a patient carries a BRCA1 or BRCA2 mutation and would benefit from genetic testing.4,5
TABLE 3
Breast cancer risk assessment tools: What you need to know5,14,16-23
| Tool | Intended use | Criteria considered | Results | Limitations | Validation | How to access |
|---|---|---|---|---|---|---|
| Gail model | Assess eligibility for chemoprevention in women >35 years | Reproductive history, history of breast biopsies, first-degree relatives with breast cancer | Estimates 5-year and lifetime risk for invasive breast cancer | Can overestimate risk in patients with previous biopsy and atypical hyperplasia results and family history | Validated in independent projects; widely used to define excess risk; modified model for minorities validated | Available at http://www.cancer.gov/bcrisktool/ |
| Tyrer-Cuzick* model | Assess need for breast MRI | Hormonal and reproductive history, history of breast biopsies, number and age of onset of first- and second-degree relatives with breast cancer | Estimates 10-year and lifetime risk for invasive breast cancer | Potential for significant overestimation of risk in patients with atypical hyperplasia findings on breast biopsy | Not validated | Go to http://www.ems-trials.org/riskevaluator Click on “software downloads” to select the appropriate version |
| Claus model | Assess need for breast MRI | Age of onset of first- and second-degree relatives with history of breast cancer | Estimates incremental 10-year and lifetime risk for invasive breast cancer | Looks only at family history, without considering hormonal or reproductive risk factors | Validation does not extend to minorities | Tables found in Cancer (1994;73:643-651) available at no charge from http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1097-0142/issues |
| BRCAPRO | Determine whether genetic testing is indicated | Family history of breast and ovarian cancer | Estimates likelihood of genetic mutation | Time-consuming; requires highly detailed family history | Validation does not extend to minorities | Not widely available; used primarily by genetic counselors |
| *Also known as the IBIS model. IBIS, International Breast Cancer Intervention Study; MRI, magnetic resonance imaging. | ||||||
Managing patients at all risk levels
Although patients with average, high, or very high risk will all be managed differently, evidence suggests that lifestyle modification as needed, imaging, and chemoprevention, in some cases, can reduce the likelihood of breast cancer for women at all levels of risk.24
For women with an average risk (a 5-year Gail model score ≤1.66% and no significant family history),19 a discussion of the benefits and risks, as well as the limitations, of annual screening mammography beginning at age 40 vs age 50 is in order. Several major organizations, the American College of Obstetricians and Gynecologists25 and American Cancer Society (ACS)16 among them, have guidelines that support annual mammography beginning at 40 years but do not specify at what age to discontinue screening. In contrast, the USPSTF26 recommends biennial mammography between the ages of 50 and 74 years (See “The mammography controvrsy: When should you screen?” J Fam Pract. 2011;60:524-531).
How to proceed? Talk to patients in the 40- to 50-year age range about the benefits and risks of earlier, more frequent screening vs waiting until 50 to start mammography and opting for screening every 2 years. Breast health awareness and the role of clinical breast exams also should be included in a balanced discussion of early detection of breast cancer. A review of the patient’s reproductive status and use of hormone preparations is appropriate, as well.4,5
Patients at high risk (a Gail model score >1.66%; a history of ADH, ALH, or lobular carcinoma in situ; or a family history of breast cancer)3 should be advised to have a clinical breast exam every 6 months and annual mammograms. High-risk patients should also be offered the option of chemoprevention with tamoxifen, raloxifene,27,28 or exemestane29 if the benefits of treatment outweigh the risk of potential adverse effects. The merits of MRI breast surveillance have not been defined for women with this level of risk.14
For very high-risk patients (those with a family history that strongly suggests a genetic predisposition, a confirmed gene mutation, evidence of hereditary breast and ovarian cancer, or a personal history of chest wall irradiation between the ages of 10 and 30 years), a discussion of more aggressive risk-reduction strategies is recommended.4 A clinical breast exam and mammogram should be performed beginning at age 25—or 5 to 10 years before the earliest age at which a first-degree relative was diagnosed.
Starting at age 30, patients at very high risk should undergo annual mammography and breast MRI, either simultaneously or staggered every 6 months, along with a twice-yearly clinical breast exam.14 Breast health awareness and lifestyle modification should be emphasized, and the benefits and risks of chemoprevention should be discussed. Surgical risk-reduction strategies, such as prophylactic mastectomy and oophorectomy, should also be discussed, along with the offer of a referral to a surgeon for consultation.5
What to tell patients about chemoprevention
The USPSTF has issued a grade B recommendation to a discussion of chemoprevention for women who are at high risk for breast cancer and low risk for an adverse event.30 Counseling a patient regarding the risks and benefits of chemoprevention will depend on her age, comorbidities, whether or not she has had a hysterectomy, and her willingness to take the suggested medication.
Selective estrogen receptor modulators (SERMs). The American Society of Clinical Oncology Clinical Practice Guideline Update has reviewed the benefits and potential adverse effects of the SERMs tamoxifen and raloxifene. The Society supports the use of tamoxifen in pre- and postmenopausal women for breast cancer risk reduction; it also supports the use of raloxifene for postmenopausal women, the only patient population for which raloxifene has been approved.27
In a review of 7 placebo-controlled, randomized clinical trials and one head-to-head trial, both drugs reduced the risk for invasive, estrogen receptor–positive breast cancer by about 40% compared with placebo. Breast cancer deaths, however, did not decrease.31
Both tamoxifen and raloxifene were found to increase bone mineral density and reduce fracture risk.31 Thromboembolic events—which occurred less frequently with raloxifene than tamoxifen—was the chief adverse effect, with an incidence of 0.4% to 0.7%. In addition, fewer cases of endometrial cancer were reported with raloxifene compared with tamoxifen, making raloxifene the preferred treatment for postmenopausal women with an intact uterus.31
The National Surgical Adjuvant Breast and Bowel Project STAR study—one of the trials included in the review—initially reported that tamoxifen and raloxifene were equivalent in reducing breast cancer risk in postmenopausal women at increased risk.28 In an updated analysis based on 81 months of use, however, tamoxifen resulted in a 50% reduction in the incidence of breast cancer vs a reduction of 38% for raloxifene.32
The greater reduction in breast cancer risk seen with tamoxifen comes at a potential cost. Tamoxifen was found to have a worse adverse effect profile, leading to a higher risk for endometrial hyperplasia and hysterectomy, as well as thromboembolic events. The difference in all-cause mortality, however, was not statistically significant.32
Aromatase inhibitor therapy. The National Cancer Institute of Canada recently published a major chemoprevention trial, evaluating the effectiveness of aromatase inhibition in breast cancer risk reduction.29 This randomized, double-blind trial of exemestane vs placebo included more than 4500 women with a median follow-up of 3 years, and found that the exemestane reduced the incidence of invasive breast cancer in postmenopausal women at moderate risk by 65% (hazard ratio=0.35; 95% confidence interval, 0.18-0.70; P=.002).29
IBIS-II, a multicenter study in the United Kingdom, randomly assigned 6000 women at increased risk for breast cancer to placebo or anastrozole, an alternative aromatase inhibitor. This trial is ongoing, and breast cancer incidence is the primary endpoint.33 Aromatase inhibitors have not been approved by the US Food and Drug Administration for breast cancer prevention.34
Imaging strategies for those at risk
Although there is evidence that mammography performed on postmenopausal women can reduce breast cancer mortality by 25%, there are known limitations to this detection method.14
One drawback is that in premenopausal women, breast density lowers mammography’s sensitivity. In addition, several studies have found that mammography has a low sensitivity for detecting tumors in patients with a BRCA mutation. This has led to the use of other imaging modalities, especially MRI, for women with a family history that suggests a genetic predisposition.
The first study to demonstrate the superior sensitivity of MRI for detecting invasive breast cancer compared with clinical breast exam and mammography was published in 2004.35 A few years later, the ACS issued guidelines that call for surveillance with MRI as an adjunct to mammography, starting at age 30, for women whose family history, carrier status, or history of chest wall radiation puts them at very high risk (ie, a lifetime risk >20%-25%).14
The ACS found insufficient evidence to recommend for or against breast MRI for women with a lifetime risk of 15% to 20% (or documented high-risk lesions such as lobular carcinoma in situ, ALH, or ADH). Mammographic density, which in itself is a strong risk factor for the development of breast cancer, was not determined to be an indication for MRI screening. In deciding whether MRI is indicated for any high-risk patient, the cost, quality of imaging, and lower specificity must be considered.14
Weighing the benefits of surgery
For women who have a strong family history of breast cancer or are known carriers of a BRCA1 or BRCA gene mutation, the already high risk of developing breast cancer increases as they age. Prophylactic surgery—risk-reduction mastectomy (RRM) and/or bilateral salpingo-oophorectomy (RRSO)—has been found to lower the risk.5,36,37
RRM can reduce the risk of breast cancer by as much as 90% for such patients;38,39 RRSO yields similar results, reducing the risk of ovarian cancer by 80% to 95% and the risk of breast cancer by 40% to 59%, provided the surgery is performed before the patient is 40 years old.36,37
These potential benefits must be weighed against the harm associated with surgically induced menopause, with the attendant risks of cardiovascular disease, osteoporosis, and menopausal symptoms.40 Notably, hormone therapy use after RRSO in women with a gene mutation has not been found to increase the risk of breast cancer. In fact, it may be associated with a decreased risk.5 In general, short-term use of low-dose estrogen—up to the age of 51 or 52 years—is considered to be safe for this population,41,42 but long-term data on breast cancer risk are lacking.
CORRESPONDENCE
Marcia G. Ko, MD, Mayo Clinic, 13737 North 92nd Street, Scottsdale, AZ 85369; ko.marcia@mayo.edu
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2. Smith R. Risk-based screening for breast cancer: is there a practical strategy? Semin Breast Dis. 1999;2:280-291.
3. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279.
4. Pruthi S, Brandt KR, Degnim AC, et al. A multidisciplinary approach to the management of breast cancer, part 1: prevention and diagnosis. Mayo Clin Proc. 2007;82:999-1012.
5. Pruthi S, Gostout BS, Lindor NM. Identification and management of women with BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clin Proc. 2010;85:1111-1120.
6. Rossouw J, Anderson G, Prentice R, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
7. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229-237.
8. Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8:562-594.
9. McPherson K, Steel CM, Dixon JM. ABC of breast diseases. BMJ. 2000;321:624-628.
10. Brinton LA, Schairer C, Hoover RN, et al. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44:783-787.
12. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
13. Narod SA, Brunet JS, Ghadirian P, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet. 2000;356:1876-1881.
14. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
15. U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility. Ann Intern Med. 2005;143:355-361.
16. Gail M, Benichou J. Validation studies on a model for breast cancer risk. J Natl Cancer Inst. 1994;86:573-575.
17. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
18. Pankratz VS, Hartmann LC, Degnim AC, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26:5374-5379.
19. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
20. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
21. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
22. Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28:3591-3596.
23. Claus EB, Risch N, Thompson W. Autosomal dominant inheritance of early-onset breast cancer. Cancer. 1994;73:643-651.
24. Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:384-398.
25. American College of Obstetricians-Gynecologists. Practice bulletin no. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
26. U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
27. Visvanathan K, Chlebowski R, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235-3258.
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
29. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
30. U.S. Preventive Services Task Force. Chemoprevention of breast cancer. Ann Intern Med. 2002;137:56-58.
31. Nelson HD, Fu R, Griffin JC, et al. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151:703-715.
32. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
33. Dunn BK, Ryan A. Phase 3 trials of aromatase inhibitors for breast cancer prevention. Ann N Y Acad Sci. 2009;1155:141-161.
34. National Cancer Institute. Hormone therapy for breast cancer. Reviewed April 11, 2012. Available at: http://www.cancer.gov/cancertopics/factsheet/Therapy/hormone-therapy-breast. Accessed May 18, 2012.
35. Kriege M, Brekelmans CTM, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
36. Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967-975.
37. Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2005;23:7491-7496.
38. Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22:1055-1062.
39. Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77-84.
40. Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111-116.
41. Armstrong K, Schwartz JS, Randall T, et al. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
42. Eisen A, Lubinski J, Gronwald J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100:1361-1367.
;
• Use a validated breast cancer risk assessment tool for any woman with a suspicious family history, precancerous breast lesions, or reproductive risk factors. C
• Recommend a semi-annual clinical breast exam and an annual mammogram for women at high risk for invasive breast cancer. C
• Discuss chemoprevention with a selective estrogen-receptor modifier or aromatase inhibitor with women at high risk for breast cancer and low risk for adverse events. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Identifying patients at risk
Among the known risk factors for breast cancer, some are modifiable (use of oral contraceptives and alcohol consumption, for example); others, such as family history and age at which menopause occurs, are not (TABLE 1).4-7 Aging itself confers the greatest risk: The incidence of breast cancer comes close to doubling at each 10-year interval before menopause and continues to climb, but more slowly, thereafter.8,9
TABLE 1
Risk factors for breast cancer4-7
| Nonmodifiable | Age, atypical hyperplasia, chest wall radiation (between the ages of 10-30 y), early menarche, family history, late menopause, race, sex |
| Modifiable | Alcohol consumption, hormone therapy (for menopausal symptoms, oral contraceptives), obesity, parity (first child after age 35, nulliparity) |
Estrogen exposure: The risk is cumulative
A number of studies have linked early onset of menarche (<12 years of age) and late menopause (>55 years) to an increase in breast cancer risk. Nulliparity, or having a first child after age 35, is also associated with greater risk; oophorectomy prior to age 50 may reduce the risk by as much as 40%.4,5,10-13

The mammogram shows a malignancy in the superior portion of the breast (arrow). Oral contraceptive use is an additional risk, but the effect slowly diminishes in the 10 years after cessation.4,5 Postmenopausal hormone replacement therapy—specifically, oral conjugated equine estrogen and medroxyprogesterone acetate—was found by the Woman’s Health Initiative to increase breast cancer risk.6
Other nongenetic risk factors include:
Atypical findings on breast biopsy. Evidence of atypical ductal hyperplasia (ADH) or lobular hyperplasia (ALH) is associated with a 4-fold increase in risk.7
Environmental exposure. Radiation, especially to the chest wall (typically as a treatment for Hodgkin’s lymphoma) increases a woman’s risk for breast cancer, particularly if the exposure occurred when she was between the ages of 10 and 30.14
Lifestyle factors. Obesity, particularly in postmenopausal women, and alcohol consumption of more than a drink or two per day are both associated with an increased risk.4
Genetic mutations and breast cancer risk
An estimated 5% to 10% of breast cancers are inherited.5 Genetic susceptibility is generally transmitted as an autosomal dominant trait.
There are 2 known breast cancer genes, BRCA1 and BRCA2, located on the long arm of chromosomes 17 and 13, respectively. The genes themselves encode tumor suppressor proteins. Mutations in these genes impair the DNA repair process, resulting in increased risk.8
The chance of carrying a mutation in either BRCA1 or BRCA2 is estimated at one in 500 to 800 in women of Northern/Western European descent. Among Ashkenazi Jews, however, the frequency is about one in 50.5
A thorough family history that takes into account both the number of affected relatives and their age at diagnosis (TABLE 2)8,15 is helpful in determining whether a patient is at low, high, or very high risk of carrying a genetic mutation. Women who have no first-degree relative with breast cancer—or a relative who was diagnosed with breast cancer after age 50—are at low risk, while those with at least one first-degree relative diagnosed with breast cancer before the age of 50 would be categorized as high risk.
A woman with a family history of early-onset breast or ovarian cancer or a relative who developed both breast and ovarian cancer, bilateral breast cancer, or male breast cancer would be classified as very high risk for a genetic mutation, as would a patient with 2 or more family members affected by breast or ovarian cancer.
Ashkenazi Jewish heritage and a relative who was diagnosed with ovarian or breast cancer indicate an increased likelihood of a BRCA mutation, as well.8 (Other genetic conditions, with mutations that are distinct from the BRCA genes, have also been linked to breast cancer, but occur less frequently.)
BRCA gene testing can confirm very high risk status, prompting the initiation of preventive measures and facilitating early detection. Such testing can also identify—and relieve the anxiety of—noncarriers in high-risk families. Recently published guidelines from the US Preventive Services Task Force (USPSTF) support testing in women with suspicious family histories with a grade B recommendation, indicating that there is at least fair evidence that testing improves important health outcomes and that the benefits of testing outweigh the harms.15
The downside of specific BRCA gene testing for patients who find that they do not have this genetic mutation may include a false sense of security and the failure to identify any other genetic mutations. Patients who learn that they do carry a BRCA gene mutation could face psychosocial or economic harm associated with aggressive surveillance and surgical intervention.5
TABLE 2
Genetic counseling for patients at high risk8,15
Tools can quantify 5-year, 10-year, and lifetime risk
A number of breast cancer risk assessment tools have been developed to help clinicians individualize patient care. None provides the basis for an all-encompassing approach to breast cancer risk or a comprehensive patient discussion of preventive strategies. We have found that, when used in combination, 2 or more predictive models can complement each other and guide the development of a targeted risk reduction approach.
When to use a predictive tool
It is not necessary to use a predictive model for patients at low risk for breast cancer. The tools detailed in TABLE 3 5,14,16-23 are better suited to women who have a suspicious family history, a history of precancerous breast lesions, or known reproductive risks. Although each model has limitations, it is important that you have a working knowledge of circumstances that favor one tool over another. For instance, the Gail model, the most widely used, can help determine if a particular patient is a candidate for chemoprevention.16-20 Others, such as the Tyrer-Cuzick model14,21,22 and the Claus model,14,23 are useful in deciding whether a patient is a candidate for breast magnetic resonance imaging (MRI) as an adjunct to mammography screening. Another useful tool is the BRCAPRO, which is used primarily by genetic counselors to assess the likelihood that a patient carries a BRCA1 or BRCA2 mutation and would benefit from genetic testing.4,5
TABLE 3
Breast cancer risk assessment tools: What you need to know5,14,16-23
| Tool | Intended use | Criteria considered | Results | Limitations | Validation | How to access |
|---|---|---|---|---|---|---|
| Gail model | Assess eligibility for chemoprevention in women >35 years | Reproductive history, history of breast biopsies, first-degree relatives with breast cancer | Estimates 5-year and lifetime risk for invasive breast cancer | Can overestimate risk in patients with previous biopsy and atypical hyperplasia results and family history | Validated in independent projects; widely used to define excess risk; modified model for minorities validated | Available at http://www.cancer.gov/bcrisktool/ |
| Tyrer-Cuzick* model | Assess need for breast MRI | Hormonal and reproductive history, history of breast biopsies, number and age of onset of first- and second-degree relatives with breast cancer | Estimates 10-year and lifetime risk for invasive breast cancer | Potential for significant overestimation of risk in patients with atypical hyperplasia findings on breast biopsy | Not validated | Go to http://www.ems-trials.org/riskevaluator Click on “software downloads” to select the appropriate version |
| Claus model | Assess need for breast MRI | Age of onset of first- and second-degree relatives with history of breast cancer | Estimates incremental 10-year and lifetime risk for invasive breast cancer | Looks only at family history, without considering hormonal or reproductive risk factors | Validation does not extend to minorities | Tables found in Cancer (1994;73:643-651) available at no charge from http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1097-0142/issues |
| BRCAPRO | Determine whether genetic testing is indicated | Family history of breast and ovarian cancer | Estimates likelihood of genetic mutation | Time-consuming; requires highly detailed family history | Validation does not extend to minorities | Not widely available; used primarily by genetic counselors |
| *Also known as the IBIS model. IBIS, International Breast Cancer Intervention Study; MRI, magnetic resonance imaging. | ||||||
Managing patients at all risk levels
Although patients with average, high, or very high risk will all be managed differently, evidence suggests that lifestyle modification as needed, imaging, and chemoprevention, in some cases, can reduce the likelihood of breast cancer for women at all levels of risk.24
For women with an average risk (a 5-year Gail model score ≤1.66% and no significant family history),19 a discussion of the benefits and risks, as well as the limitations, of annual screening mammography beginning at age 40 vs age 50 is in order. Several major organizations, the American College of Obstetricians and Gynecologists25 and American Cancer Society (ACS)16 among them, have guidelines that support annual mammography beginning at 40 years but do not specify at what age to discontinue screening. In contrast, the USPSTF26 recommends biennial mammography between the ages of 50 and 74 years (See “The mammography controvrsy: When should you screen?” J Fam Pract. 2011;60:524-531).
How to proceed? Talk to patients in the 40- to 50-year age range about the benefits and risks of earlier, more frequent screening vs waiting until 50 to start mammography and opting for screening every 2 years. Breast health awareness and the role of clinical breast exams also should be included in a balanced discussion of early detection of breast cancer. A review of the patient’s reproductive status and use of hormone preparations is appropriate, as well.4,5
Patients at high risk (a Gail model score >1.66%; a history of ADH, ALH, or lobular carcinoma in situ; or a family history of breast cancer)3 should be advised to have a clinical breast exam every 6 months and annual mammograms. High-risk patients should also be offered the option of chemoprevention with tamoxifen, raloxifene,27,28 or exemestane29 if the benefits of treatment outweigh the risk of potential adverse effects. The merits of MRI breast surveillance have not been defined for women with this level of risk.14
For very high-risk patients (those with a family history that strongly suggests a genetic predisposition, a confirmed gene mutation, evidence of hereditary breast and ovarian cancer, or a personal history of chest wall irradiation between the ages of 10 and 30 years), a discussion of more aggressive risk-reduction strategies is recommended.4 A clinical breast exam and mammogram should be performed beginning at age 25—or 5 to 10 years before the earliest age at which a first-degree relative was diagnosed.
Starting at age 30, patients at very high risk should undergo annual mammography and breast MRI, either simultaneously or staggered every 6 months, along with a twice-yearly clinical breast exam.14 Breast health awareness and lifestyle modification should be emphasized, and the benefits and risks of chemoprevention should be discussed. Surgical risk-reduction strategies, such as prophylactic mastectomy and oophorectomy, should also be discussed, along with the offer of a referral to a surgeon for consultation.5
What to tell patients about chemoprevention
The USPSTF has issued a grade B recommendation to a discussion of chemoprevention for women who are at high risk for breast cancer and low risk for an adverse event.30 Counseling a patient regarding the risks and benefits of chemoprevention will depend on her age, comorbidities, whether or not she has had a hysterectomy, and her willingness to take the suggested medication.
Selective estrogen receptor modulators (SERMs). The American Society of Clinical Oncology Clinical Practice Guideline Update has reviewed the benefits and potential adverse effects of the SERMs tamoxifen and raloxifene. The Society supports the use of tamoxifen in pre- and postmenopausal women for breast cancer risk reduction; it also supports the use of raloxifene for postmenopausal women, the only patient population for which raloxifene has been approved.27
In a review of 7 placebo-controlled, randomized clinical trials and one head-to-head trial, both drugs reduced the risk for invasive, estrogen receptor–positive breast cancer by about 40% compared with placebo. Breast cancer deaths, however, did not decrease.31
Both tamoxifen and raloxifene were found to increase bone mineral density and reduce fracture risk.31 Thromboembolic events—which occurred less frequently with raloxifene than tamoxifen—was the chief adverse effect, with an incidence of 0.4% to 0.7%. In addition, fewer cases of endometrial cancer were reported with raloxifene compared with tamoxifen, making raloxifene the preferred treatment for postmenopausal women with an intact uterus.31
The National Surgical Adjuvant Breast and Bowel Project STAR study—one of the trials included in the review—initially reported that tamoxifen and raloxifene were equivalent in reducing breast cancer risk in postmenopausal women at increased risk.28 In an updated analysis based on 81 months of use, however, tamoxifen resulted in a 50% reduction in the incidence of breast cancer vs a reduction of 38% for raloxifene.32
The greater reduction in breast cancer risk seen with tamoxifen comes at a potential cost. Tamoxifen was found to have a worse adverse effect profile, leading to a higher risk for endometrial hyperplasia and hysterectomy, as well as thromboembolic events. The difference in all-cause mortality, however, was not statistically significant.32
Aromatase inhibitor therapy. The National Cancer Institute of Canada recently published a major chemoprevention trial, evaluating the effectiveness of aromatase inhibition in breast cancer risk reduction.29 This randomized, double-blind trial of exemestane vs placebo included more than 4500 women with a median follow-up of 3 years, and found that the exemestane reduced the incidence of invasive breast cancer in postmenopausal women at moderate risk by 65% (hazard ratio=0.35; 95% confidence interval, 0.18-0.70; P=.002).29
IBIS-II, a multicenter study in the United Kingdom, randomly assigned 6000 women at increased risk for breast cancer to placebo or anastrozole, an alternative aromatase inhibitor. This trial is ongoing, and breast cancer incidence is the primary endpoint.33 Aromatase inhibitors have not been approved by the US Food and Drug Administration for breast cancer prevention.34
Imaging strategies for those at risk
Although there is evidence that mammography performed on postmenopausal women can reduce breast cancer mortality by 25%, there are known limitations to this detection method.14
One drawback is that in premenopausal women, breast density lowers mammography’s sensitivity. In addition, several studies have found that mammography has a low sensitivity for detecting tumors in patients with a BRCA mutation. This has led to the use of other imaging modalities, especially MRI, for women with a family history that suggests a genetic predisposition.
The first study to demonstrate the superior sensitivity of MRI for detecting invasive breast cancer compared with clinical breast exam and mammography was published in 2004.35 A few years later, the ACS issued guidelines that call for surveillance with MRI as an adjunct to mammography, starting at age 30, for women whose family history, carrier status, or history of chest wall radiation puts them at very high risk (ie, a lifetime risk >20%-25%).14
The ACS found insufficient evidence to recommend for or against breast MRI for women with a lifetime risk of 15% to 20% (or documented high-risk lesions such as lobular carcinoma in situ, ALH, or ADH). Mammographic density, which in itself is a strong risk factor for the development of breast cancer, was not determined to be an indication for MRI screening. In deciding whether MRI is indicated for any high-risk patient, the cost, quality of imaging, and lower specificity must be considered.14
Weighing the benefits of surgery
For women who have a strong family history of breast cancer or are known carriers of a BRCA1 or BRCA gene mutation, the already high risk of developing breast cancer increases as they age. Prophylactic surgery—risk-reduction mastectomy (RRM) and/or bilateral salpingo-oophorectomy (RRSO)—has been found to lower the risk.5,36,37
RRM can reduce the risk of breast cancer by as much as 90% for such patients;38,39 RRSO yields similar results, reducing the risk of ovarian cancer by 80% to 95% and the risk of breast cancer by 40% to 59%, provided the surgery is performed before the patient is 40 years old.36,37
These potential benefits must be weighed against the harm associated with surgically induced menopause, with the attendant risks of cardiovascular disease, osteoporosis, and menopausal symptoms.40 Notably, hormone therapy use after RRSO in women with a gene mutation has not been found to increase the risk of breast cancer. In fact, it may be associated with a decreased risk.5 In general, short-term use of low-dose estrogen—up to the age of 51 or 52 years—is considered to be safe for this population,41,42 but long-term data on breast cancer risk are lacking.
CORRESPONDENCE
Marcia G. Ko, MD, Mayo Clinic, 13737 North 92nd Street, Scottsdale, AZ 85369; ko.marcia@mayo.edu
• Use a validated breast cancer risk assessment tool for any woman with a suspicious family history, precancerous breast lesions, or reproductive risk factors. C
• Recommend a semi-annual clinical breast exam and an annual mammogram for women at high risk for invasive breast cancer. C
• Discuss chemoprevention with a selective estrogen-receptor modifier or aromatase inhibitor with women at high risk for breast cancer and low risk for adverse events. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Identifying patients at risk
Among the known risk factors for breast cancer, some are modifiable (use of oral contraceptives and alcohol consumption, for example); others, such as family history and age at which menopause occurs, are not (TABLE 1).4-7 Aging itself confers the greatest risk: The incidence of breast cancer comes close to doubling at each 10-year interval before menopause and continues to climb, but more slowly, thereafter.8,9
TABLE 1
Risk factors for breast cancer4-7
| Nonmodifiable | Age, atypical hyperplasia, chest wall radiation (between the ages of 10-30 y), early menarche, family history, late menopause, race, sex |
| Modifiable | Alcohol consumption, hormone therapy (for menopausal symptoms, oral contraceptives), obesity, parity (first child after age 35, nulliparity) |
Estrogen exposure: The risk is cumulative
A number of studies have linked early onset of menarche (<12 years of age) and late menopause (>55 years) to an increase in breast cancer risk. Nulliparity, or having a first child after age 35, is also associated with greater risk; oophorectomy prior to age 50 may reduce the risk by as much as 40%.4,5,10-13

The mammogram shows a malignancy in the superior portion of the breast (arrow). Oral contraceptive use is an additional risk, but the effect slowly diminishes in the 10 years after cessation.4,5 Postmenopausal hormone replacement therapy—specifically, oral conjugated equine estrogen and medroxyprogesterone acetate—was found by the Woman’s Health Initiative to increase breast cancer risk.6
Other nongenetic risk factors include:
Atypical findings on breast biopsy. Evidence of atypical ductal hyperplasia (ADH) or lobular hyperplasia (ALH) is associated with a 4-fold increase in risk.7
Environmental exposure. Radiation, especially to the chest wall (typically as a treatment for Hodgkin’s lymphoma) increases a woman’s risk for breast cancer, particularly if the exposure occurred when she was between the ages of 10 and 30.14
Lifestyle factors. Obesity, particularly in postmenopausal women, and alcohol consumption of more than a drink or two per day are both associated with an increased risk.4
Genetic mutations and breast cancer risk
An estimated 5% to 10% of breast cancers are inherited.5 Genetic susceptibility is generally transmitted as an autosomal dominant trait.
There are 2 known breast cancer genes, BRCA1 and BRCA2, located on the long arm of chromosomes 17 and 13, respectively. The genes themselves encode tumor suppressor proteins. Mutations in these genes impair the DNA repair process, resulting in increased risk.8
The chance of carrying a mutation in either BRCA1 or BRCA2 is estimated at one in 500 to 800 in women of Northern/Western European descent. Among Ashkenazi Jews, however, the frequency is about one in 50.5
A thorough family history that takes into account both the number of affected relatives and their age at diagnosis (TABLE 2)8,15 is helpful in determining whether a patient is at low, high, or very high risk of carrying a genetic mutation. Women who have no first-degree relative with breast cancer—or a relative who was diagnosed with breast cancer after age 50—are at low risk, while those with at least one first-degree relative diagnosed with breast cancer before the age of 50 would be categorized as high risk.
A woman with a family history of early-onset breast or ovarian cancer or a relative who developed both breast and ovarian cancer, bilateral breast cancer, or male breast cancer would be classified as very high risk for a genetic mutation, as would a patient with 2 or more family members affected by breast or ovarian cancer.
Ashkenazi Jewish heritage and a relative who was diagnosed with ovarian or breast cancer indicate an increased likelihood of a BRCA mutation, as well.8 (Other genetic conditions, with mutations that are distinct from the BRCA genes, have also been linked to breast cancer, but occur less frequently.)
BRCA gene testing can confirm very high risk status, prompting the initiation of preventive measures and facilitating early detection. Such testing can also identify—and relieve the anxiety of—noncarriers in high-risk families. Recently published guidelines from the US Preventive Services Task Force (USPSTF) support testing in women with suspicious family histories with a grade B recommendation, indicating that there is at least fair evidence that testing improves important health outcomes and that the benefits of testing outweigh the harms.15
The downside of specific BRCA gene testing for patients who find that they do not have this genetic mutation may include a false sense of security and the failure to identify any other genetic mutations. Patients who learn that they do carry a BRCA gene mutation could face psychosocial or economic harm associated with aggressive surveillance and surgical intervention.5
TABLE 2
Genetic counseling for patients at high risk8,15
Tools can quantify 5-year, 10-year, and lifetime risk
A number of breast cancer risk assessment tools have been developed to help clinicians individualize patient care. None provides the basis for an all-encompassing approach to breast cancer risk or a comprehensive patient discussion of preventive strategies. We have found that, when used in combination, 2 or more predictive models can complement each other and guide the development of a targeted risk reduction approach.
When to use a predictive tool
It is not necessary to use a predictive model for patients at low risk for breast cancer. The tools detailed in TABLE 3 5,14,16-23 are better suited to women who have a suspicious family history, a history of precancerous breast lesions, or known reproductive risks. Although each model has limitations, it is important that you have a working knowledge of circumstances that favor one tool over another. For instance, the Gail model, the most widely used, can help determine if a particular patient is a candidate for chemoprevention.16-20 Others, such as the Tyrer-Cuzick model14,21,22 and the Claus model,14,23 are useful in deciding whether a patient is a candidate for breast magnetic resonance imaging (MRI) as an adjunct to mammography screening. Another useful tool is the BRCAPRO, which is used primarily by genetic counselors to assess the likelihood that a patient carries a BRCA1 or BRCA2 mutation and would benefit from genetic testing.4,5
TABLE 3
Breast cancer risk assessment tools: What you need to know5,14,16-23
| Tool | Intended use | Criteria considered | Results | Limitations | Validation | How to access |
|---|---|---|---|---|---|---|
| Gail model | Assess eligibility for chemoprevention in women >35 years | Reproductive history, history of breast biopsies, first-degree relatives with breast cancer | Estimates 5-year and lifetime risk for invasive breast cancer | Can overestimate risk in patients with previous biopsy and atypical hyperplasia results and family history | Validated in independent projects; widely used to define excess risk; modified model for minorities validated | Available at http://www.cancer.gov/bcrisktool/ |
| Tyrer-Cuzick* model | Assess need for breast MRI | Hormonal and reproductive history, history of breast biopsies, number and age of onset of first- and second-degree relatives with breast cancer | Estimates 10-year and lifetime risk for invasive breast cancer | Potential for significant overestimation of risk in patients with atypical hyperplasia findings on breast biopsy | Not validated | Go to http://www.ems-trials.org/riskevaluator Click on “software downloads” to select the appropriate version |
| Claus model | Assess need for breast MRI | Age of onset of first- and second-degree relatives with history of breast cancer | Estimates incremental 10-year and lifetime risk for invasive breast cancer | Looks only at family history, without considering hormonal or reproductive risk factors | Validation does not extend to minorities | Tables found in Cancer (1994;73:643-651) available at no charge from http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1097-0142/issues |
| BRCAPRO | Determine whether genetic testing is indicated | Family history of breast and ovarian cancer | Estimates likelihood of genetic mutation | Time-consuming; requires highly detailed family history | Validation does not extend to minorities | Not widely available; used primarily by genetic counselors |
| *Also known as the IBIS model. IBIS, International Breast Cancer Intervention Study; MRI, magnetic resonance imaging. | ||||||
Managing patients at all risk levels
Although patients with average, high, or very high risk will all be managed differently, evidence suggests that lifestyle modification as needed, imaging, and chemoprevention, in some cases, can reduce the likelihood of breast cancer for women at all levels of risk.24
For women with an average risk (a 5-year Gail model score ≤1.66% and no significant family history),19 a discussion of the benefits and risks, as well as the limitations, of annual screening mammography beginning at age 40 vs age 50 is in order. Several major organizations, the American College of Obstetricians and Gynecologists25 and American Cancer Society (ACS)16 among them, have guidelines that support annual mammography beginning at 40 years but do not specify at what age to discontinue screening. In contrast, the USPSTF26 recommends biennial mammography between the ages of 50 and 74 years (See “The mammography controvrsy: When should you screen?” J Fam Pract. 2011;60:524-531).
How to proceed? Talk to patients in the 40- to 50-year age range about the benefits and risks of earlier, more frequent screening vs waiting until 50 to start mammography and opting for screening every 2 years. Breast health awareness and the role of clinical breast exams also should be included in a balanced discussion of early detection of breast cancer. A review of the patient’s reproductive status and use of hormone preparations is appropriate, as well.4,5
Patients at high risk (a Gail model score >1.66%; a history of ADH, ALH, or lobular carcinoma in situ; or a family history of breast cancer)3 should be advised to have a clinical breast exam every 6 months and annual mammograms. High-risk patients should also be offered the option of chemoprevention with tamoxifen, raloxifene,27,28 or exemestane29 if the benefits of treatment outweigh the risk of potential adverse effects. The merits of MRI breast surveillance have not been defined for women with this level of risk.14
For very high-risk patients (those with a family history that strongly suggests a genetic predisposition, a confirmed gene mutation, evidence of hereditary breast and ovarian cancer, or a personal history of chest wall irradiation between the ages of 10 and 30 years), a discussion of more aggressive risk-reduction strategies is recommended.4 A clinical breast exam and mammogram should be performed beginning at age 25—or 5 to 10 years before the earliest age at which a first-degree relative was diagnosed.
Starting at age 30, patients at very high risk should undergo annual mammography and breast MRI, either simultaneously or staggered every 6 months, along with a twice-yearly clinical breast exam.14 Breast health awareness and lifestyle modification should be emphasized, and the benefits and risks of chemoprevention should be discussed. Surgical risk-reduction strategies, such as prophylactic mastectomy and oophorectomy, should also be discussed, along with the offer of a referral to a surgeon for consultation.5
What to tell patients about chemoprevention
The USPSTF has issued a grade B recommendation to a discussion of chemoprevention for women who are at high risk for breast cancer and low risk for an adverse event.30 Counseling a patient regarding the risks and benefits of chemoprevention will depend on her age, comorbidities, whether or not she has had a hysterectomy, and her willingness to take the suggested medication.
Selective estrogen receptor modulators (SERMs). The American Society of Clinical Oncology Clinical Practice Guideline Update has reviewed the benefits and potential adverse effects of the SERMs tamoxifen and raloxifene. The Society supports the use of tamoxifen in pre- and postmenopausal women for breast cancer risk reduction; it also supports the use of raloxifene for postmenopausal women, the only patient population for which raloxifene has been approved.27
In a review of 7 placebo-controlled, randomized clinical trials and one head-to-head trial, both drugs reduced the risk for invasive, estrogen receptor–positive breast cancer by about 40% compared with placebo. Breast cancer deaths, however, did not decrease.31
Both tamoxifen and raloxifene were found to increase bone mineral density and reduce fracture risk.31 Thromboembolic events—which occurred less frequently with raloxifene than tamoxifen—was the chief adverse effect, with an incidence of 0.4% to 0.7%. In addition, fewer cases of endometrial cancer were reported with raloxifene compared with tamoxifen, making raloxifene the preferred treatment for postmenopausal women with an intact uterus.31
The National Surgical Adjuvant Breast and Bowel Project STAR study—one of the trials included in the review—initially reported that tamoxifen and raloxifene were equivalent in reducing breast cancer risk in postmenopausal women at increased risk.28 In an updated analysis based on 81 months of use, however, tamoxifen resulted in a 50% reduction in the incidence of breast cancer vs a reduction of 38% for raloxifene.32
The greater reduction in breast cancer risk seen with tamoxifen comes at a potential cost. Tamoxifen was found to have a worse adverse effect profile, leading to a higher risk for endometrial hyperplasia and hysterectomy, as well as thromboembolic events. The difference in all-cause mortality, however, was not statistically significant.32
Aromatase inhibitor therapy. The National Cancer Institute of Canada recently published a major chemoprevention trial, evaluating the effectiveness of aromatase inhibition in breast cancer risk reduction.29 This randomized, double-blind trial of exemestane vs placebo included more than 4500 women with a median follow-up of 3 years, and found that the exemestane reduced the incidence of invasive breast cancer in postmenopausal women at moderate risk by 65% (hazard ratio=0.35; 95% confidence interval, 0.18-0.70; P=.002).29
IBIS-II, a multicenter study in the United Kingdom, randomly assigned 6000 women at increased risk for breast cancer to placebo or anastrozole, an alternative aromatase inhibitor. This trial is ongoing, and breast cancer incidence is the primary endpoint.33 Aromatase inhibitors have not been approved by the US Food and Drug Administration for breast cancer prevention.34
Imaging strategies for those at risk
Although there is evidence that mammography performed on postmenopausal women can reduce breast cancer mortality by 25%, there are known limitations to this detection method.14
One drawback is that in premenopausal women, breast density lowers mammography’s sensitivity. In addition, several studies have found that mammography has a low sensitivity for detecting tumors in patients with a BRCA mutation. This has led to the use of other imaging modalities, especially MRI, for women with a family history that suggests a genetic predisposition.
The first study to demonstrate the superior sensitivity of MRI for detecting invasive breast cancer compared with clinical breast exam and mammography was published in 2004.35 A few years later, the ACS issued guidelines that call for surveillance with MRI as an adjunct to mammography, starting at age 30, for women whose family history, carrier status, or history of chest wall radiation puts them at very high risk (ie, a lifetime risk >20%-25%).14
The ACS found insufficient evidence to recommend for or against breast MRI for women with a lifetime risk of 15% to 20% (or documented high-risk lesions such as lobular carcinoma in situ, ALH, or ADH). Mammographic density, which in itself is a strong risk factor for the development of breast cancer, was not determined to be an indication for MRI screening. In deciding whether MRI is indicated for any high-risk patient, the cost, quality of imaging, and lower specificity must be considered.14
Weighing the benefits of surgery
For women who have a strong family history of breast cancer or are known carriers of a BRCA1 or BRCA gene mutation, the already high risk of developing breast cancer increases as they age. Prophylactic surgery—risk-reduction mastectomy (RRM) and/or bilateral salpingo-oophorectomy (RRSO)—has been found to lower the risk.5,36,37
RRM can reduce the risk of breast cancer by as much as 90% for such patients;38,39 RRSO yields similar results, reducing the risk of ovarian cancer by 80% to 95% and the risk of breast cancer by 40% to 59%, provided the surgery is performed before the patient is 40 years old.36,37
These potential benefits must be weighed against the harm associated with surgically induced menopause, with the attendant risks of cardiovascular disease, osteoporosis, and menopausal symptoms.40 Notably, hormone therapy use after RRSO in women with a gene mutation has not been found to increase the risk of breast cancer. In fact, it may be associated with a decreased risk.5 In general, short-term use of low-dose estrogen—up to the age of 51 or 52 years—is considered to be safe for this population,41,42 but long-term data on breast cancer risk are lacking.
CORRESPONDENCE
Marcia G. Ko, MD, Mayo Clinic, 13737 North 92nd Street, Scottsdale, AZ 85369; ko.marcia@mayo.edu
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2. Smith R. Risk-based screening for breast cancer: is there a practical strategy? Semin Breast Dis. 1999;2:280-291.
3. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279.
4. Pruthi S, Brandt KR, Degnim AC, et al. A multidisciplinary approach to the management of breast cancer, part 1: prevention and diagnosis. Mayo Clin Proc. 2007;82:999-1012.
5. Pruthi S, Gostout BS, Lindor NM. Identification and management of women with BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clin Proc. 2010;85:1111-1120.
6. Rossouw J, Anderson G, Prentice R, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
7. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229-237.
8. Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8:562-594.
9. McPherson K, Steel CM, Dixon JM. ABC of breast diseases. BMJ. 2000;321:624-628.
10. Brinton LA, Schairer C, Hoover RN, et al. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44:783-787.
12. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
13. Narod SA, Brunet JS, Ghadirian P, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet. 2000;356:1876-1881.
14. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
15. U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility. Ann Intern Med. 2005;143:355-361.
16. Gail M, Benichou J. Validation studies on a model for breast cancer risk. J Natl Cancer Inst. 1994;86:573-575.
17. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
18. Pankratz VS, Hartmann LC, Degnim AC, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26:5374-5379.
19. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
20. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
21. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
22. Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28:3591-3596.
23. Claus EB, Risch N, Thompson W. Autosomal dominant inheritance of early-onset breast cancer. Cancer. 1994;73:643-651.
24. Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:384-398.
25. American College of Obstetricians-Gynecologists. Practice bulletin no. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
26. U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
27. Visvanathan K, Chlebowski R, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235-3258.
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
29. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
30. U.S. Preventive Services Task Force. Chemoprevention of breast cancer. Ann Intern Med. 2002;137:56-58.
31. Nelson HD, Fu R, Griffin JC, et al. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151:703-715.
32. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
33. Dunn BK, Ryan A. Phase 3 trials of aromatase inhibitors for breast cancer prevention. Ann N Y Acad Sci. 2009;1155:141-161.
34. National Cancer Institute. Hormone therapy for breast cancer. Reviewed April 11, 2012. Available at: http://www.cancer.gov/cancertopics/factsheet/Therapy/hormone-therapy-breast. Accessed May 18, 2012.
35. Kriege M, Brekelmans CTM, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
36. Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967-975.
37. Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2005;23:7491-7496.
38. Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22:1055-1062.
39. Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77-84.
40. Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111-116.
41. Armstrong K, Schwartz JS, Randall T, et al. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
42. Eisen A, Lubinski J, Gronwald J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100:1361-1367.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2. Smith R. Risk-based screening for breast cancer: is there a practical strategy? Semin Breast Dis. 1999;2:280-291.
3. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279.
4. Pruthi S, Brandt KR, Degnim AC, et al. A multidisciplinary approach to the management of breast cancer, part 1: prevention and diagnosis. Mayo Clin Proc. 2007;82:999-1012.
5. Pruthi S, Gostout BS, Lindor NM. Identification and management of women with BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clin Proc. 2010;85:1111-1120.
6. Rossouw J, Anderson G, Prentice R, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
7. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229-237.
8. Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8:562-594.
9. McPherson K, Steel CM, Dixon JM. ABC of breast diseases. BMJ. 2000;321:624-628.
10. Brinton LA, Schairer C, Hoover RN, et al. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44:783-787.
12. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
13. Narod SA, Brunet JS, Ghadirian P, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet. 2000;356:1876-1881.
14. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
15. U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility. Ann Intern Med. 2005;143:355-361.
16. Gail M, Benichou J. Validation studies on a model for breast cancer risk. J Natl Cancer Inst. 1994;86:573-575.
17. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
18. Pankratz VS, Hartmann LC, Degnim AC, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26:5374-5379.
19. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
20. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
21. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
22. Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28:3591-3596.
23. Claus EB, Risch N, Thompson W. Autosomal dominant inheritance of early-onset breast cancer. Cancer. 1994;73:643-651.
24. Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:384-398.
25. American College of Obstetricians-Gynecologists. Practice bulletin no. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
26. U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
27. Visvanathan K, Chlebowski R, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235-3258.
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
29. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
30. U.S. Preventive Services Task Force. Chemoprevention of breast cancer. Ann Intern Med. 2002;137:56-58.
31. Nelson HD, Fu R, Griffin JC, et al. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151:703-715.
32. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
33. Dunn BK, Ryan A. Phase 3 trials of aromatase inhibitors for breast cancer prevention. Ann N Y Acad Sci. 2009;1155:141-161.
34. National Cancer Institute. Hormone therapy for breast cancer. Reviewed April 11, 2012. Available at: http://www.cancer.gov/cancertopics/factsheet/Therapy/hormone-therapy-breast. Accessed May 18, 2012.
35. Kriege M, Brekelmans CTM, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
36. Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967-975.
37. Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2005;23:7491-7496.
38. Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22:1055-1062.
39. Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77-84.
40. Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111-116.
41. Armstrong K, Schwartz JS, Randall T, et al. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
42. Eisen A, Lubinski J, Gronwald J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100:1361-1367.
;
;