User login
It’s Time to Use an Age-based Approach to D-dimer
PRACTICE CHANGER
Use an age-adjusted d-dimer cutoff (patient age in years × 10 μg/L) for patients older than 50 when evaluating for venous thromboembolism (VTE); it reduces false-positives without substantially increasing false-negatives.1
STRENGTH OF RECOMMENDATION
A: Based on consistent and good-quality patient-centered evidence from a meta-analysis of cohort studies.1
ILLUSTRATIVE CASE
A 78-year-old woman with no significant medical history or recent immobility comes to your clinic complaining of left lower extremity pain and swelling. Her d-dimer is 650 μg/L. What is your next step?
Although d-dimer is recognized as a reasonable screening tool for VTE, the specificity of d-dimer testing using a conventional cutoff value of 500 μg/L is particularly poor in patients older than 50. In low-risk patients older than 80, the specificity is 14.7%.2-5 As a result, conventional d-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1,000 in younger patients to 8:1,000 in older patients,4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and CT pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
Continued on next page >>
STUDY SUMMARY
Using age-adjusted d-dimer cutoffs significantly reduced false-positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had d-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N = 12,497; 6,969 patients older than 50). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and d-dimer sensitivity and specificity for patients ages ≤ 50, 51 to 60, 61 to 70, 71 to 80, and > 80.
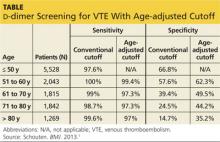
The specificity of the conventional d-dimer cutoff value for VTE decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 μg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (see table). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1,000 patients older than 80, compared with 124/1,000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1,000 patients) is comparable to the false-negative rate in those ages 50 and younger (3 per 1,000). The advantages are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
Continued on next page >>
WHAT’S NEW?
We can now use d-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive d-dimer test was less useful for older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients older than 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted d-dimer cutoff improves the diagnostic accuracy of d-dimer screening in older adults.
CAVEATS
Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of d-dimer results. Not all patients included in this meta-analysis whose d-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of the age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION
You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is its identification of a simple calculation that can directly improve patient care. Clinicians can easily apply an age-adjusted d-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 μg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted d-dimer calculation was provided with the lab results.
REFERENCES
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346: f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:
765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. http://bit.ly/1gPkLoE. Accessed March 3, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Accessed March 3, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(3):155-156, 158.
PRACTICE CHANGER
Use an age-adjusted d-dimer cutoff (patient age in years × 10 μg/L) for patients older than 50 when evaluating for venous thromboembolism (VTE); it reduces false-positives without substantially increasing false-negatives.1
STRENGTH OF RECOMMENDATION
A: Based on consistent and good-quality patient-centered evidence from a meta-analysis of cohort studies.1
ILLUSTRATIVE CASE
A 78-year-old woman with no significant medical history or recent immobility comes to your clinic complaining of left lower extremity pain and swelling. Her d-dimer is 650 μg/L. What is your next step?
Although d-dimer is recognized as a reasonable screening tool for VTE, the specificity of d-dimer testing using a conventional cutoff value of 500 μg/L is particularly poor in patients older than 50. In low-risk patients older than 80, the specificity is 14.7%.2-5 As a result, conventional d-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1,000 in younger patients to 8:1,000 in older patients,4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and CT pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
Continued on next page >>
STUDY SUMMARY
Using age-adjusted d-dimer cutoffs significantly reduced false-positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had d-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N = 12,497; 6,969 patients older than 50). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and d-dimer sensitivity and specificity for patients ages ≤ 50, 51 to 60, 61 to 70, 71 to 80, and > 80.
The specificity of the conventional d-dimer cutoff value for VTE decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 μg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (see table). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1,000 patients older than 80, compared with 124/1,000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1,000 patients) is comparable to the false-negative rate in those ages 50 and younger (3 per 1,000). The advantages are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
Continued on next page >>
WHAT’S NEW?
We can now use d-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive d-dimer test was less useful for older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients older than 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted d-dimer cutoff improves the diagnostic accuracy of d-dimer screening in older adults.
CAVEATS
Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of d-dimer results. Not all patients included in this meta-analysis whose d-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of the age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION
You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is its identification of a simple calculation that can directly improve patient care. Clinicians can easily apply an age-adjusted d-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 μg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted d-dimer calculation was provided with the lab results.
REFERENCES
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346: f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:
765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. http://bit.ly/1gPkLoE. Accessed March 3, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Accessed March 3, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(3):155-156, 158.
PRACTICE CHANGER
Use an age-adjusted d-dimer cutoff (patient age in years × 10 μg/L) for patients older than 50 when evaluating for venous thromboembolism (VTE); it reduces false-positives without substantially increasing false-negatives.1
STRENGTH OF RECOMMENDATION
A: Based on consistent and good-quality patient-centered evidence from a meta-analysis of cohort studies.1
ILLUSTRATIVE CASE
A 78-year-old woman with no significant medical history or recent immobility comes to your clinic complaining of left lower extremity pain and swelling. Her d-dimer is 650 μg/L. What is your next step?
Although d-dimer is recognized as a reasonable screening tool for VTE, the specificity of d-dimer testing using a conventional cutoff value of 500 μg/L is particularly poor in patients older than 50. In low-risk patients older than 80, the specificity is 14.7%.2-5 As a result, conventional d-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1,000 in younger patients to 8:1,000 in older patients,4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and CT pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
Continued on next page >>
STUDY SUMMARY
Using age-adjusted d-dimer cutoffs significantly reduced false-positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had d-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N = 12,497; 6,969 patients older than 50). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and d-dimer sensitivity and specificity for patients ages ≤ 50, 51 to 60, 61 to 70, 71 to 80, and > 80.
The specificity of the conventional d-dimer cutoff value for VTE decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 μg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (see table). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1,000 patients older than 80, compared with 124/1,000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1,000 patients) is comparable to the false-negative rate in those ages 50 and younger (3 per 1,000). The advantages are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
Continued on next page >>
WHAT’S NEW?
We can now use d-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive d-dimer test was less useful for older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients older than 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted d-dimer cutoff improves the diagnostic accuracy of d-dimer screening in older adults.
CAVEATS
Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of d-dimer results. Not all patients included in this meta-analysis whose d-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of the age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION
You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is its identification of a simple calculation that can directly improve patient care. Clinicians can easily apply an age-adjusted d-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 μg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted d-dimer calculation was provided with the lab results.
REFERENCES
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346: f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:
765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. http://bit.ly/1gPkLoE. Accessed March 3, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Accessed March 3, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(3):155-156, 158.
It’s time to use an age-based approach to D-dimer
Use an age-adjusted D-dimer cutoff (patient’s age in years × 10 mcg/L) for patients over age 50 years when evaluating for venous thromboembolism (VTE); it reduces false positives without substantially increasing false negatives.1
Strength of recommendation
A: Based on consistent and good quality patient-centered evidence from a meta-analysis of cohort studies.
Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and metaanalysis. BMJ. 2013;346:f2492.
Illustrative case
A 78-year-old woman with no significant past medical history or recent immobility comes into your clinic complaining of left lower extremity pain and swelling. Her D-dimer is 650 mcg/L. What is your next step?
Although D-dimer is recognized as a reasonable screening tool for VTE, the specificity of D-dimer testing using a conventional cutoff value of 500 mcg/L is particularly poor in patients over 50 years. In low-risk patients over 80 years old, the specificity is 14.7% (95% confidence interval, 11.3%-18.6%).2-5 As a result, conventional D-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1000 in younger patients to 8:1000 in older patients4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and computed tomography pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
STUDY SUMMARY: Using age-adjusted D-dimer cutoffs significantly reduced false positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had D-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies published before June 21, 2012 that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N=12,497; 6969 patients >50 years). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and D-dimer sensitivity and specificity for patients ages ≤50, 51 to 60, 61 to 70, 71 to 80, and >80 years.
The specificity of using the conventional D-dimer cutoff value for VTE (500 mcg/L) decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 mcg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (TABLE). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1000 patients >80 years, compared with 124/1000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than age 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1000 patients) is comparable to the false negative rate in those age, ≤50 (3 per 1000). The advantages of an age-adjusted cutoff are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
WHAT'S NEW?: We can now make use of the D-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive D-dimer test was less useful for our older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients over age 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted D-dimer cutoff improves the diagnostic accuracy of D-dimer screening in older adults.
CAVEATS: Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of D-dimer results. Not all patients included in this meta-analysis whose D-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of using an age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION: You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is it identifies a simple calculation that can directly improve patient care. Physicians can easily apply an age-adjusted D-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 mcg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted D-dimer calculation was provided with the lab results.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013; 346:f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. Available at: http://bit.ly/1vStJtm. Updated January 30, 2014. Accessed February 13, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Updated January 2013. Accessed October 23, 2013.
Use an age-adjusted D-dimer cutoff (patient’s age in years × 10 mcg/L) for patients over age 50 years when evaluating for venous thromboembolism (VTE); it reduces false positives without substantially increasing false negatives.1
Strength of recommendation
A: Based on consistent and good quality patient-centered evidence from a meta-analysis of cohort studies.
Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and metaanalysis. BMJ. 2013;346:f2492.
Illustrative case
A 78-year-old woman with no significant past medical history or recent immobility comes into your clinic complaining of left lower extremity pain and swelling. Her D-dimer is 650 mcg/L. What is your next step?
Although D-dimer is recognized as a reasonable screening tool for VTE, the specificity of D-dimer testing using a conventional cutoff value of 500 mcg/L is particularly poor in patients over 50 years. In low-risk patients over 80 years old, the specificity is 14.7% (95% confidence interval, 11.3%-18.6%).2-5 As a result, conventional D-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1000 in younger patients to 8:1000 in older patients4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and computed tomography pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
STUDY SUMMARY: Using age-adjusted D-dimer cutoffs significantly reduced false positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had D-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies published before June 21, 2012 that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N=12,497; 6969 patients >50 years). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and D-dimer sensitivity and specificity for patients ages ≤50, 51 to 60, 61 to 70, 71 to 80, and >80 years.
The specificity of using the conventional D-dimer cutoff value for VTE (500 mcg/L) decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 mcg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (TABLE). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1000 patients >80 years, compared with 124/1000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than age 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1000 patients) is comparable to the false negative rate in those age, ≤50 (3 per 1000). The advantages of an age-adjusted cutoff are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
WHAT'S NEW?: We can now make use of the D-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive D-dimer test was less useful for our older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients over age 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted D-dimer cutoff improves the diagnostic accuracy of D-dimer screening in older adults.
CAVEATS: Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of D-dimer results. Not all patients included in this meta-analysis whose D-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of using an age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION: You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is it identifies a simple calculation that can directly improve patient care. Physicians can easily apply an age-adjusted D-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 mcg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted D-dimer calculation was provided with the lab results.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Use an age-adjusted D-dimer cutoff (patient’s age in years × 10 mcg/L) for patients over age 50 years when evaluating for venous thromboembolism (VTE); it reduces false positives without substantially increasing false negatives.1
Strength of recommendation
A: Based on consistent and good quality patient-centered evidence from a meta-analysis of cohort studies.
Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and metaanalysis. BMJ. 2013;346:f2492.
Illustrative case
A 78-year-old woman with no significant past medical history or recent immobility comes into your clinic complaining of left lower extremity pain and swelling. Her D-dimer is 650 mcg/L. What is your next step?
Although D-dimer is recognized as a reasonable screening tool for VTE, the specificity of D-dimer testing using a conventional cutoff value of 500 mcg/L is particularly poor in patients over 50 years. In low-risk patients over 80 years old, the specificity is 14.7% (95% confidence interval, 11.3%-18.6%).2-5 As a result, conventional D-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1000 in younger patients to 8:1000 in older patients4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and computed tomography pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
STUDY SUMMARY: Using age-adjusted D-dimer cutoffs significantly reduced false positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had D-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies published before June 21, 2012 that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N=12,497; 6969 patients >50 years). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and D-dimer sensitivity and specificity for patients ages ≤50, 51 to 60, 61 to 70, 71 to 80, and >80 years.
The specificity of using the conventional D-dimer cutoff value for VTE (500 mcg/L) decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 mcg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (TABLE). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1000 patients >80 years, compared with 124/1000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than age 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1000 patients) is comparable to the false negative rate in those age, ≤50 (3 per 1000). The advantages of an age-adjusted cutoff are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
WHAT'S NEW?: We can now make use of the D-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive D-dimer test was less useful for our older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients over age 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted D-dimer cutoff improves the diagnostic accuracy of D-dimer screening in older adults.
CAVEATS: Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of D-dimer results. Not all patients included in this meta-analysis whose D-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of using an age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION: You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is it identifies a simple calculation that can directly improve patient care. Physicians can easily apply an age-adjusted D-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 mcg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted D-dimer calculation was provided with the lab results.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013; 346:f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. Available at: http://bit.ly/1vStJtm. Updated January 30, 2014. Accessed February 13, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Updated January 2013. Accessed October 23, 2013.
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013; 346:f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. Available at: http://bit.ly/1vStJtm. Updated January 30, 2014. Accessed February 13, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Updated January 2013. Accessed October 23, 2013.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
Consider this strategy for upper GI bleeds
Do not order transfusions of red blood cells for patients with acute upper gastrointestinal bleeding unless their hemoglobin level <7 g/dL.
Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.1
A: Based on a single randomized controlled trial (RCT) consistent with other RCTs on recommendations for transfusion.
ILLUSTRATED CASE
An 82-year-old patient presents to the emergency department with several episodes of melena over the past week and one episode of hematemesis this morning. He denies any shortness of breath, dizziness, lightheadedness, or fatigue. He is tachycardic but normotensive. Lab results note a hemoglobin level of 8.3 g/dL. Should you order a transfusion of red blood cells?
Acute upper gastrointestinal bleeding (UGIB) commonly requires hospital admission, with approximately 61 cases per 100,000 population in the United States in 2009.2 Gastroduodenal peptic ulcer disease accounts for the majority of these cases.3 Although trends indicate an overall decrease in cases requiring hospitalization, UGIB remains a condition associated with a mortality rate of 2.5% and inpatient costs of $2 billion annually.2,3
Studies have been inconclusive—until now
An RCT published in 1999 showed a restrictive transfusion strategy (hemoglobin threshold of 7 g/dL) to be at least as effective as—and possibly superior to—a liberal strategy (threshold of 10 g/dL) in critically ill patients.4 In 2010, an RCT demonstrated that a liberal transfusion strategy (also defined as a transfusion threshold of 10 g/dL) did not reduce the rates of death or in-hospital morbidity in elderly patients after hip surgery.5 A recent Cochrane review of transfusion strategies for UGIB included only 3 small studies (N=93), so its authors could not draw any firm conclusions.6 The results of a new RCT, detailed below, are more conclusive.
STUDY SUMMARY: Restrictive transfusion policy lowers mortality risk
Villanueva et al conducted a nonblinded RCT comparing outcomes in patients admitted to the hospital with moderate-risk acute UGIB transfused on a liberal vs a restrictive strategy.1 The restrictive group used a transfusion hemoglobin threshold of 7 g/dL and a posttransfusion target of 7 to 9 g/dL; the liberal group used a threshold of 9 g/dL, with a posttransfusion target of 9 to 11 g/dL. Patients received one unit of red blood cells at a time until their hemoglobin was above the predetermined threshold.
Patients were excluded if they declined blood transfusion; had massive exsanguinating bleeding, acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, lower GI bleeding, or a transient ischemic attack; had received a transfusion within the previous 90 days; or had a recent history of surgery or trauma. Patients at low risk of rebleeding (as defined by the Rockall risk scoring system) were also excluded. Randomization was stratified by the presence or absence of cirrhosis of the liver.
Participants (N=921) had confirmed hematemesis and/or melena on admission. All underwent emergency gastroscopy within 6 hours of admission, with subsequent interventions based on endoscopic findings. In addition to established hemoglobin levels, patients received a transfusion anytime they developed signs or symptoms related to anemia, massive bleeding, or the need for surgery. Staff monitored hemoglobin levels every 8 hours during the first 48 hours, then daily thereafter.
Both groups had similar baseline characteristics, including hemoglobin on admission and source of bleeding. The authors used intention-to-treat analysis to identify the primary outcome: death from any cause at 45 days. Secondary outcomes were further bleeding and in-hospital complications.
During hospitalization, 49% of patients in the restrictive group and 86% of those in the liberal group received a blood transfusion (P<.001). Thirty-two patients (17 from the restrictive group and 15 from the liberal group) withdrew from the study, leaving 889 patients for overall analysis.
At 45 days, overall mortality from any cause was 5% in the restrictive group and 9% in the liberal group (P=.02; number needed to treat [NNT]=25). Sub-group analysis revealed a lower risk of death in patients with cirrhosis and Child-Pugh class A or B disease assigned to the restrictive transfusion group vs the liberal group. The results showed a trend toward a lower risk of death in patients with bleeding from varices or peptic ulcers for the restrictive group, as well.
In addition, the restrictive transfusion group had a significantly lower rate of adverse events (40% vs 48% for the liberal transfusion group; P=.02, NNT=13), with a significant reduction in transfusion reactions (3% vs 9%; P=.001, NNT=17) and cardiac complications (11% vs 16%; P=.04, NNT=20). The restrictive group had a lower rate of further bleeding (10% vs 16% for the liberal transfusion group; P=.01, NNT=17), as well.
WHAT'S NEW: Many reasons to limit transfusions for acute upper GI bleed
This RCT provides evidence that patients with acute UGIB have improved survival rates and fewer adverse events when a restrictive transfusion strategy is used. In addition to improving patient outcomes, a restrictive strategy will likely reduce costs and overall use of blood products. Thus, the study, along with other recent evaluations, adds evidence to support more restrictive transfusion thresholds.
The AABB (formerly named the American Association of Blood Banks) recently
released guidelines calling for restrictive transfusion thresholds (7-8 g/dL) in stable hospitalized patients.7 In 2012, the American College of Gastroenterology published a practice guideline with a recommended target hemoglobin level of ≥7 g/dL in the management of patients who have ulcer bleeding but no signs of intravascular depletion or comorbidities such as coronary artery disease.8
CAVEATS: Results might differ when endoscopy is delayed
The patients in the study detailed here underwent emergency gastroscopy within 6 hours of admission, and both groups received the same therapies based on endoscopic findings. It remains unclear whether the benefits of a restrictive transfusion strategy would persist in patients who do not undergo endoscopy within that timeframe. And, because the reported baseline characteristics of the patients did not include the prevalence of cardiac disease, caution should be exercised before extrapolating these results to patients with underlying (active or historical) cardiac disease.
CHALLENGES TO IMPLEMENTATION: Changing long-held policies may be difficult
Although RCTs as well as clinical guidelines suggest that restrictive transfusion policies are safe and effective, changing long-held clinical practices is never easy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med.2013;368:11-21.
2. Laine L, Yang H, Chang SC,et al. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 2012; 107:1190-1195.
3. Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928-937.
4. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409-417.
5. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453-2462.
6. Jairath V, Hearnshaw S, Brunskill SJ, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database of Systematic Reviews 2010;CD006613.
7. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49-58.
8. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107:345-360.
Do not order transfusions of red blood cells for patients with acute upper gastrointestinal bleeding unless their hemoglobin level <7 g/dL.
Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.1
A: Based on a single randomized controlled trial (RCT) consistent with other RCTs on recommendations for transfusion.
ILLUSTRATED CASE
An 82-year-old patient presents to the emergency department with several episodes of melena over the past week and one episode of hematemesis this morning. He denies any shortness of breath, dizziness, lightheadedness, or fatigue. He is tachycardic but normotensive. Lab results note a hemoglobin level of 8.3 g/dL. Should you order a transfusion of red blood cells?
Acute upper gastrointestinal bleeding (UGIB) commonly requires hospital admission, with approximately 61 cases per 100,000 population in the United States in 2009.2 Gastroduodenal peptic ulcer disease accounts for the majority of these cases.3 Although trends indicate an overall decrease in cases requiring hospitalization, UGIB remains a condition associated with a mortality rate of 2.5% and inpatient costs of $2 billion annually.2,3
Studies have been inconclusive—until now
An RCT published in 1999 showed a restrictive transfusion strategy (hemoglobin threshold of 7 g/dL) to be at least as effective as—and possibly superior to—a liberal strategy (threshold of 10 g/dL) in critically ill patients.4 In 2010, an RCT demonstrated that a liberal transfusion strategy (also defined as a transfusion threshold of 10 g/dL) did not reduce the rates of death or in-hospital morbidity in elderly patients after hip surgery.5 A recent Cochrane review of transfusion strategies for UGIB included only 3 small studies (N=93), so its authors could not draw any firm conclusions.6 The results of a new RCT, detailed below, are more conclusive.
STUDY SUMMARY: Restrictive transfusion policy lowers mortality risk
Villanueva et al conducted a nonblinded RCT comparing outcomes in patients admitted to the hospital with moderate-risk acute UGIB transfused on a liberal vs a restrictive strategy.1 The restrictive group used a transfusion hemoglobin threshold of 7 g/dL and a posttransfusion target of 7 to 9 g/dL; the liberal group used a threshold of 9 g/dL, with a posttransfusion target of 9 to 11 g/dL. Patients received one unit of red blood cells at a time until their hemoglobin was above the predetermined threshold.
Patients were excluded if they declined blood transfusion; had massive exsanguinating bleeding, acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, lower GI bleeding, or a transient ischemic attack; had received a transfusion within the previous 90 days; or had a recent history of surgery or trauma. Patients at low risk of rebleeding (as defined by the Rockall risk scoring system) were also excluded. Randomization was stratified by the presence or absence of cirrhosis of the liver.
Participants (N=921) had confirmed hematemesis and/or melena on admission. All underwent emergency gastroscopy within 6 hours of admission, with subsequent interventions based on endoscopic findings. In addition to established hemoglobin levels, patients received a transfusion anytime they developed signs or symptoms related to anemia, massive bleeding, or the need for surgery. Staff monitored hemoglobin levels every 8 hours during the first 48 hours, then daily thereafter.
Both groups had similar baseline characteristics, including hemoglobin on admission and source of bleeding. The authors used intention-to-treat analysis to identify the primary outcome: death from any cause at 45 days. Secondary outcomes were further bleeding and in-hospital complications.
During hospitalization, 49% of patients in the restrictive group and 86% of those in the liberal group received a blood transfusion (P<.001). Thirty-two patients (17 from the restrictive group and 15 from the liberal group) withdrew from the study, leaving 889 patients for overall analysis.
At 45 days, overall mortality from any cause was 5% in the restrictive group and 9% in the liberal group (P=.02; number needed to treat [NNT]=25). Sub-group analysis revealed a lower risk of death in patients with cirrhosis and Child-Pugh class A or B disease assigned to the restrictive transfusion group vs the liberal group. The results showed a trend toward a lower risk of death in patients with bleeding from varices or peptic ulcers for the restrictive group, as well.
In addition, the restrictive transfusion group had a significantly lower rate of adverse events (40% vs 48% for the liberal transfusion group; P=.02, NNT=13), with a significant reduction in transfusion reactions (3% vs 9%; P=.001, NNT=17) and cardiac complications (11% vs 16%; P=.04, NNT=20). The restrictive group had a lower rate of further bleeding (10% vs 16% for the liberal transfusion group; P=.01, NNT=17), as well.
WHAT'S NEW: Many reasons to limit transfusions for acute upper GI bleed
This RCT provides evidence that patients with acute UGIB have improved survival rates and fewer adverse events when a restrictive transfusion strategy is used. In addition to improving patient outcomes, a restrictive strategy will likely reduce costs and overall use of blood products. Thus, the study, along with other recent evaluations, adds evidence to support more restrictive transfusion thresholds.
The AABB (formerly named the American Association of Blood Banks) recently
released guidelines calling for restrictive transfusion thresholds (7-8 g/dL) in stable hospitalized patients.7 In 2012, the American College of Gastroenterology published a practice guideline with a recommended target hemoglobin level of ≥7 g/dL in the management of patients who have ulcer bleeding but no signs of intravascular depletion or comorbidities such as coronary artery disease.8
CAVEATS: Results might differ when endoscopy is delayed
The patients in the study detailed here underwent emergency gastroscopy within 6 hours of admission, and both groups received the same therapies based on endoscopic findings. It remains unclear whether the benefits of a restrictive transfusion strategy would persist in patients who do not undergo endoscopy within that timeframe. And, because the reported baseline characteristics of the patients did not include the prevalence of cardiac disease, caution should be exercised before extrapolating these results to patients with underlying (active or historical) cardiac disease.
CHALLENGES TO IMPLEMENTATION: Changing long-held policies may be difficult
Although RCTs as well as clinical guidelines suggest that restrictive transfusion policies are safe and effective, changing long-held clinical practices is never easy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Do not order transfusions of red blood cells for patients with acute upper gastrointestinal bleeding unless their hemoglobin level <7 g/dL.
Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.1
A: Based on a single randomized controlled trial (RCT) consistent with other RCTs on recommendations for transfusion.
ILLUSTRATED CASE
An 82-year-old patient presents to the emergency department with several episodes of melena over the past week and one episode of hematemesis this morning. He denies any shortness of breath, dizziness, lightheadedness, or fatigue. He is tachycardic but normotensive. Lab results note a hemoglobin level of 8.3 g/dL. Should you order a transfusion of red blood cells?
Acute upper gastrointestinal bleeding (UGIB) commonly requires hospital admission, with approximately 61 cases per 100,000 population in the United States in 2009.2 Gastroduodenal peptic ulcer disease accounts for the majority of these cases.3 Although trends indicate an overall decrease in cases requiring hospitalization, UGIB remains a condition associated with a mortality rate of 2.5% and inpatient costs of $2 billion annually.2,3
Studies have been inconclusive—until now
An RCT published in 1999 showed a restrictive transfusion strategy (hemoglobin threshold of 7 g/dL) to be at least as effective as—and possibly superior to—a liberal strategy (threshold of 10 g/dL) in critically ill patients.4 In 2010, an RCT demonstrated that a liberal transfusion strategy (also defined as a transfusion threshold of 10 g/dL) did not reduce the rates of death or in-hospital morbidity in elderly patients after hip surgery.5 A recent Cochrane review of transfusion strategies for UGIB included only 3 small studies (N=93), so its authors could not draw any firm conclusions.6 The results of a new RCT, detailed below, are more conclusive.
STUDY SUMMARY: Restrictive transfusion policy lowers mortality risk
Villanueva et al conducted a nonblinded RCT comparing outcomes in patients admitted to the hospital with moderate-risk acute UGIB transfused on a liberal vs a restrictive strategy.1 The restrictive group used a transfusion hemoglobin threshold of 7 g/dL and a posttransfusion target of 7 to 9 g/dL; the liberal group used a threshold of 9 g/dL, with a posttransfusion target of 9 to 11 g/dL. Patients received one unit of red blood cells at a time until their hemoglobin was above the predetermined threshold.
Patients were excluded if they declined blood transfusion; had massive exsanguinating bleeding, acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, lower GI bleeding, or a transient ischemic attack; had received a transfusion within the previous 90 days; or had a recent history of surgery or trauma. Patients at low risk of rebleeding (as defined by the Rockall risk scoring system) were also excluded. Randomization was stratified by the presence or absence of cirrhosis of the liver.
Participants (N=921) had confirmed hematemesis and/or melena on admission. All underwent emergency gastroscopy within 6 hours of admission, with subsequent interventions based on endoscopic findings. In addition to established hemoglobin levels, patients received a transfusion anytime they developed signs or symptoms related to anemia, massive bleeding, or the need for surgery. Staff monitored hemoglobin levels every 8 hours during the first 48 hours, then daily thereafter.
Both groups had similar baseline characteristics, including hemoglobin on admission and source of bleeding. The authors used intention-to-treat analysis to identify the primary outcome: death from any cause at 45 days. Secondary outcomes were further bleeding and in-hospital complications.
During hospitalization, 49% of patients in the restrictive group and 86% of those in the liberal group received a blood transfusion (P<.001). Thirty-two patients (17 from the restrictive group and 15 from the liberal group) withdrew from the study, leaving 889 patients for overall analysis.
At 45 days, overall mortality from any cause was 5% in the restrictive group and 9% in the liberal group (P=.02; number needed to treat [NNT]=25). Sub-group analysis revealed a lower risk of death in patients with cirrhosis and Child-Pugh class A or B disease assigned to the restrictive transfusion group vs the liberal group. The results showed a trend toward a lower risk of death in patients with bleeding from varices or peptic ulcers for the restrictive group, as well.
In addition, the restrictive transfusion group had a significantly lower rate of adverse events (40% vs 48% for the liberal transfusion group; P=.02, NNT=13), with a significant reduction in transfusion reactions (3% vs 9%; P=.001, NNT=17) and cardiac complications (11% vs 16%; P=.04, NNT=20). The restrictive group had a lower rate of further bleeding (10% vs 16% for the liberal transfusion group; P=.01, NNT=17), as well.
WHAT'S NEW: Many reasons to limit transfusions for acute upper GI bleed
This RCT provides evidence that patients with acute UGIB have improved survival rates and fewer adverse events when a restrictive transfusion strategy is used. In addition to improving patient outcomes, a restrictive strategy will likely reduce costs and overall use of blood products. Thus, the study, along with other recent evaluations, adds evidence to support more restrictive transfusion thresholds.
The AABB (formerly named the American Association of Blood Banks) recently
released guidelines calling for restrictive transfusion thresholds (7-8 g/dL) in stable hospitalized patients.7 In 2012, the American College of Gastroenterology published a practice guideline with a recommended target hemoglobin level of ≥7 g/dL in the management of patients who have ulcer bleeding but no signs of intravascular depletion or comorbidities such as coronary artery disease.8
CAVEATS: Results might differ when endoscopy is delayed
The patients in the study detailed here underwent emergency gastroscopy within 6 hours of admission, and both groups received the same therapies based on endoscopic findings. It remains unclear whether the benefits of a restrictive transfusion strategy would persist in patients who do not undergo endoscopy within that timeframe. And, because the reported baseline characteristics of the patients did not include the prevalence of cardiac disease, caution should be exercised before extrapolating these results to patients with underlying (active or historical) cardiac disease.
CHALLENGES TO IMPLEMENTATION: Changing long-held policies may be difficult
Although RCTs as well as clinical guidelines suggest that restrictive transfusion policies are safe and effective, changing long-held clinical practices is never easy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med.2013;368:11-21.
2. Laine L, Yang H, Chang SC,et al. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 2012; 107:1190-1195.
3. Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928-937.
4. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409-417.
5. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453-2462.
6. Jairath V, Hearnshaw S, Brunskill SJ, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database of Systematic Reviews 2010;CD006613.
7. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49-58.
8. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107:345-360.
1. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med.2013;368:11-21.
2. Laine L, Yang H, Chang SC,et al. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol 2012; 107:1190-1195.
3. Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med 2008; 359:928-937.
4. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409-417.
5. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011; 365:2453-2462.
6. Jairath V, Hearnshaw S, Brunskill SJ, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database of Systematic Reviews 2010;CD006613.
7. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49-58.
8. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107:345-360.
Copyright 2013. The Family Physicians Inquiries Network. All rights reserved.
Prescribing an Antibiotic? Pair It With Probiotics
PRACTICE CHANGER
Recommend that patients taking antibiotics also take probiotics, which have been found to be effective both for the prevention and treatment of antibiotic-associated diarrhea (AAD).1
STRENGTH OF
RECOMMENDATION
A: Based on a systematic review and meta-analysis of randomized controlled trials (RCTs).
ILLUSTRATIVE CASE
When you prescribe an antibiotic for a 45-year-old patient with Helicobacter pylori, he worries that the medication will cause diarrhea. Should you recommend that he take probiotics?
More than a third of patients taking antibiotics develop AAD,2 and in 17% of cases, AAD is fatal.3,4 Although the diarrhea may be the result of increased gastrointestinal (GI) motility in some cases, a disruption of the GI flora that normally acts as a barrier to infection and aids in the digestion of carbohydrates is a far more common cause.
Morbidity and mortality are high
AAD is associated with several pathogens, including Clostridium difficile, Clostridium perfringens, Klebsiella oxytoca, and Staphylococcus aureus,2 and varies widely in severity. Pseudomembranous colitis secondary to C difficile is the main cause of AAD-related mortality, which more than doubled from 2002 to 2009.3,4 C difficile infections cost the US health care system up to $1.3 billion annually.5 With such high rates of morbidity and mortality and high health care costs associated with AAD, even a small reduction in the number of cases would have a big impact.
Probiotics replenish the natural GI flora with nonpathogenic organisms. A 2006 meta-analysis of 31 RCTs assessing the efficacy of probiotics for both the prevention of AAD and treatment of C difficile found a pooled relative risk (RR) of 0.43 for AAD in the patients taking probiotics.6 However, many of the studies included in that meta-analysis were small. As a result, in 2010, the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) recommended against the use of probiotics for the prevention of primary C difficile infection, citing a lack of high-quality evidence.7
Nonetheless, that same year, 98% of gastroenterologists surveyed expressed a belief that probiotics had a role in the treatment of GI illness.8 And in 2011, the Third Yale Working Group on Probiotic Use published recommendations for probiotic use based on expert opinion.9 The meta-analysis detailed in this PURL, which included more than 30 trials published since the 2006 meta-analysis, addressed the efficacy of probiotics for prevention and treatment of AAD.
STUDY SUMMARY
Probiotics significantly reduce AAD
Hempel et al reviewed 82 studies and pooled data from 63 RCTs (N = 11,811) to identify the RR of AAD among patients who received probiotics during antibiotic treatment, compared with those who received no probiotics or were given a placebo.1 The studies encompassed a variety of antibiotics, taken alone or in combination, and several probiotics, including Lactobacillus, Bifidobacterium, Saccharomyces, and some combinations.
The outcome: The pooled RR for AAD in the probiotics groups was 0.58, with a number needed to treat of 13. Although the authors reported that the overall quality of the included trials was poor, a sensitivity analysis of the higher-quality studies yielded similar results.
Subgroup analyses by type of probiotic and duration of antibiotic treatment were also consistent with the overall pooled RR. In subgroup analysis by age, a similar decrease in AAD was found among the youngest patients (ages 0 to 17) and those between the ages of 17 and 65. Among patients older than 65—for whom there were just three studies—a nonsignificant decrease in risk was found. Twenty-three of the studies assessed adverse outcomes, of which none were reported.
WHAT'S NEW
A reason to pair antibiotics and probiotics
This meta-analysis reached a similar conclusion to the 2006 meta-analysis: Probiotics appear to be effective in preventing and treating AAD in children and adults receiving a wide variety of antibiotics for a number of conditions. The results were also consistent with those of a new meta-analysis that looked specifically at one pathogen—and found a reduction of 66% in C difficile–associated diarrhea in patients taking probiotics with their antibiotics.10
CAVEATS
Limited data on the safety
of probiotics exist
There was some heterogeneity among the studies in the meta-analysis by Hempel et al,1 and some of the studies were of poor quality. Because of this, the authors used subgroup and sensitivity analysis, which supported their initial conclusion.
Probiotics have generally been considered safe; however, there have been rare reports of sepsis and fungemia associated with probiotic use, especially in immunosuppressed patients.1 Fifty-nine of the included studies did not assess adverse events, which limited the ability of this meta-analysis to assess safety.1 Patients taking probiotics should be monitored for adverse effects.
CHALLENGES TO IMPLEMENTATION
Lack of guidance on dosing and duration
Since probiotics are considered food supplements, health insurance will not cover the cost (which will likely be more than $20 per month). No single probiotic strain has high-quality evidence; however, most of the RCTs included in the meta-analysis used combinations of Lactobacillus species, which are usually found in OTC antidiarrheal probiotic supplements. No standard dose exists, but dose ranges in RCTs are 107 to 1010 colony-forming units per capsule (taken one to three times daily);1 however, product labels have variable accuracy.11 The duration of treatment ranges from one to three weeks—or as long as the patient continues to take antibiotics. .
REFERENCES
1. Hempel S, Newberry S, Maher A, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA. 2012; 307:1959-1969.
2. McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563-578.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile–associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037-1042.
4. Perry A, Dellon E, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 Update. Gastroenterology. 2012;143: 1179-1187.
5. Dubberke E, Wertheimer A. A review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30:57-66.
6. McFarland L. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:
812-822.
7. Cohen S, Gerding D, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America. Infect Control Hosp Epidemiol. 2010;31: 431-455.
8. Williams M, Ha C, Ciorba M. Probiotics as therapy in gastroenterology. J Clin Gastroenterol. 2010;44:631-636.
9. Floch M, Walker A, Madsne K, et al. Recommendations for probiotic use—2011 update. J Clin Gastroenterol. 2011;45(suppl):S168-S171.
10. Johnston BC, Ma SS, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
11. Hamilton-Miller J, Shah S. Deficiencies in microbiological quality and labeling of probiotic supplements. Int J Food Microbiol. 2002; 72:175-176.
Acknowledgement
The PURLs Surveillance System was developed with support from Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2012. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2012;61:673-674.
PRACTICE CHANGER
Recommend that patients taking antibiotics also take probiotics, which have been found to be effective both for the prevention and treatment of antibiotic-associated diarrhea (AAD).1
STRENGTH OF
RECOMMENDATION
A: Based on a systematic review and meta-analysis of randomized controlled trials (RCTs).
ILLUSTRATIVE CASE
When you prescribe an antibiotic for a 45-year-old patient with Helicobacter pylori, he worries that the medication will cause diarrhea. Should you recommend that he take probiotics?
More than a third of patients taking antibiotics develop AAD,2 and in 17% of cases, AAD is fatal.3,4 Although the diarrhea may be the result of increased gastrointestinal (GI) motility in some cases, a disruption of the GI flora that normally acts as a barrier to infection and aids in the digestion of carbohydrates is a far more common cause.
Morbidity and mortality are high
AAD is associated with several pathogens, including Clostridium difficile, Clostridium perfringens, Klebsiella oxytoca, and Staphylococcus aureus,2 and varies widely in severity. Pseudomembranous colitis secondary to C difficile is the main cause of AAD-related mortality, which more than doubled from 2002 to 2009.3,4 C difficile infections cost the US health care system up to $1.3 billion annually.5 With such high rates of morbidity and mortality and high health care costs associated with AAD, even a small reduction in the number of cases would have a big impact.
Probiotics replenish the natural GI flora with nonpathogenic organisms. A 2006 meta-analysis of 31 RCTs assessing the efficacy of probiotics for both the prevention of AAD and treatment of C difficile found a pooled relative risk (RR) of 0.43 for AAD in the patients taking probiotics.6 However, many of the studies included in that meta-analysis were small. As a result, in 2010, the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) recommended against the use of probiotics for the prevention of primary C difficile infection, citing a lack of high-quality evidence.7
Nonetheless, that same year, 98% of gastroenterologists surveyed expressed a belief that probiotics had a role in the treatment of GI illness.8 And in 2011, the Third Yale Working Group on Probiotic Use published recommendations for probiotic use based on expert opinion.9 The meta-analysis detailed in this PURL, which included more than 30 trials published since the 2006 meta-analysis, addressed the efficacy of probiotics for prevention and treatment of AAD.
STUDY SUMMARY
Probiotics significantly reduce AAD
Hempel et al reviewed 82 studies and pooled data from 63 RCTs (N = 11,811) to identify the RR of AAD among patients who received probiotics during antibiotic treatment, compared with those who received no probiotics or were given a placebo.1 The studies encompassed a variety of antibiotics, taken alone or in combination, and several probiotics, including Lactobacillus, Bifidobacterium, Saccharomyces, and some combinations.
The outcome: The pooled RR for AAD in the probiotics groups was 0.58, with a number needed to treat of 13. Although the authors reported that the overall quality of the included trials was poor, a sensitivity analysis of the higher-quality studies yielded similar results.
Subgroup analyses by type of probiotic and duration of antibiotic treatment were also consistent with the overall pooled RR. In subgroup analysis by age, a similar decrease in AAD was found among the youngest patients (ages 0 to 17) and those between the ages of 17 and 65. Among patients older than 65—for whom there were just three studies—a nonsignificant decrease in risk was found. Twenty-three of the studies assessed adverse outcomes, of which none were reported.
WHAT'S NEW
A reason to pair antibiotics and probiotics
This meta-analysis reached a similar conclusion to the 2006 meta-analysis: Probiotics appear to be effective in preventing and treating AAD in children and adults receiving a wide variety of antibiotics for a number of conditions. The results were also consistent with those of a new meta-analysis that looked specifically at one pathogen—and found a reduction of 66% in C difficile–associated diarrhea in patients taking probiotics with their antibiotics.10
CAVEATS
Limited data on the safety
of probiotics exist
There was some heterogeneity among the studies in the meta-analysis by Hempel et al,1 and some of the studies were of poor quality. Because of this, the authors used subgroup and sensitivity analysis, which supported their initial conclusion.
Probiotics have generally been considered safe; however, there have been rare reports of sepsis and fungemia associated with probiotic use, especially in immunosuppressed patients.1 Fifty-nine of the included studies did not assess adverse events, which limited the ability of this meta-analysis to assess safety.1 Patients taking probiotics should be monitored for adverse effects.
CHALLENGES TO IMPLEMENTATION
Lack of guidance on dosing and duration
Since probiotics are considered food supplements, health insurance will not cover the cost (which will likely be more than $20 per month). No single probiotic strain has high-quality evidence; however, most of the RCTs included in the meta-analysis used combinations of Lactobacillus species, which are usually found in OTC antidiarrheal probiotic supplements. No standard dose exists, but dose ranges in RCTs are 107 to 1010 colony-forming units per capsule (taken one to three times daily);1 however, product labels have variable accuracy.11 The duration of treatment ranges from one to three weeks—or as long as the patient continues to take antibiotics. .
REFERENCES
1. Hempel S, Newberry S, Maher A, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA. 2012; 307:1959-1969.
2. McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563-578.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile–associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037-1042.
4. Perry A, Dellon E, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 Update. Gastroenterology. 2012;143: 1179-1187.
5. Dubberke E, Wertheimer A. A review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30:57-66.
6. McFarland L. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:
812-822.
7. Cohen S, Gerding D, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America. Infect Control Hosp Epidemiol. 2010;31: 431-455.
8. Williams M, Ha C, Ciorba M. Probiotics as therapy in gastroenterology. J Clin Gastroenterol. 2010;44:631-636.
9. Floch M, Walker A, Madsne K, et al. Recommendations for probiotic use—2011 update. J Clin Gastroenterol. 2011;45(suppl):S168-S171.
10. Johnston BC, Ma SS, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
11. Hamilton-Miller J, Shah S. Deficiencies in microbiological quality and labeling of probiotic supplements. Int J Food Microbiol. 2002; 72:175-176.
Acknowledgement
The PURLs Surveillance System was developed with support from Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2012. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2012;61:673-674.
PRACTICE CHANGER
Recommend that patients taking antibiotics also take probiotics, which have been found to be effective both for the prevention and treatment of antibiotic-associated diarrhea (AAD).1
STRENGTH OF
RECOMMENDATION
A: Based on a systematic review and meta-analysis of randomized controlled trials (RCTs).
ILLUSTRATIVE CASE
When you prescribe an antibiotic for a 45-year-old patient with Helicobacter pylori, he worries that the medication will cause diarrhea. Should you recommend that he take probiotics?
More than a third of patients taking antibiotics develop AAD,2 and in 17% of cases, AAD is fatal.3,4 Although the diarrhea may be the result of increased gastrointestinal (GI) motility in some cases, a disruption of the GI flora that normally acts as a barrier to infection and aids in the digestion of carbohydrates is a far more common cause.
Morbidity and mortality are high
AAD is associated with several pathogens, including Clostridium difficile, Clostridium perfringens, Klebsiella oxytoca, and Staphylococcus aureus,2 and varies widely in severity. Pseudomembranous colitis secondary to C difficile is the main cause of AAD-related mortality, which more than doubled from 2002 to 2009.3,4 C difficile infections cost the US health care system up to $1.3 billion annually.5 With such high rates of morbidity and mortality and high health care costs associated with AAD, even a small reduction in the number of cases would have a big impact.
Probiotics replenish the natural GI flora with nonpathogenic organisms. A 2006 meta-analysis of 31 RCTs assessing the efficacy of probiotics for both the prevention of AAD and treatment of C difficile found a pooled relative risk (RR) of 0.43 for AAD in the patients taking probiotics.6 However, many of the studies included in that meta-analysis were small. As a result, in 2010, the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) recommended against the use of probiotics for the prevention of primary C difficile infection, citing a lack of high-quality evidence.7
Nonetheless, that same year, 98% of gastroenterologists surveyed expressed a belief that probiotics had a role in the treatment of GI illness.8 And in 2011, the Third Yale Working Group on Probiotic Use published recommendations for probiotic use based on expert opinion.9 The meta-analysis detailed in this PURL, which included more than 30 trials published since the 2006 meta-analysis, addressed the efficacy of probiotics for prevention and treatment of AAD.
STUDY SUMMARY
Probiotics significantly reduce AAD
Hempel et al reviewed 82 studies and pooled data from 63 RCTs (N = 11,811) to identify the RR of AAD among patients who received probiotics during antibiotic treatment, compared with those who received no probiotics or were given a placebo.1 The studies encompassed a variety of antibiotics, taken alone or in combination, and several probiotics, including Lactobacillus, Bifidobacterium, Saccharomyces, and some combinations.
The outcome: The pooled RR for AAD in the probiotics groups was 0.58, with a number needed to treat of 13. Although the authors reported that the overall quality of the included trials was poor, a sensitivity analysis of the higher-quality studies yielded similar results.
Subgroup analyses by type of probiotic and duration of antibiotic treatment were also consistent with the overall pooled RR. In subgroup analysis by age, a similar decrease in AAD was found among the youngest patients (ages 0 to 17) and those between the ages of 17 and 65. Among patients older than 65—for whom there were just three studies—a nonsignificant decrease in risk was found. Twenty-three of the studies assessed adverse outcomes, of which none were reported.
WHAT'S NEW
A reason to pair antibiotics and probiotics
This meta-analysis reached a similar conclusion to the 2006 meta-analysis: Probiotics appear to be effective in preventing and treating AAD in children and adults receiving a wide variety of antibiotics for a number of conditions. The results were also consistent with those of a new meta-analysis that looked specifically at one pathogen—and found a reduction of 66% in C difficile–associated diarrhea in patients taking probiotics with their antibiotics.10
CAVEATS
Limited data on the safety
of probiotics exist
There was some heterogeneity among the studies in the meta-analysis by Hempel et al,1 and some of the studies were of poor quality. Because of this, the authors used subgroup and sensitivity analysis, which supported their initial conclusion.
Probiotics have generally been considered safe; however, there have been rare reports of sepsis and fungemia associated with probiotic use, especially in immunosuppressed patients.1 Fifty-nine of the included studies did not assess adverse events, which limited the ability of this meta-analysis to assess safety.1 Patients taking probiotics should be monitored for adverse effects.
CHALLENGES TO IMPLEMENTATION
Lack of guidance on dosing and duration
Since probiotics are considered food supplements, health insurance will not cover the cost (which will likely be more than $20 per month). No single probiotic strain has high-quality evidence; however, most of the RCTs included in the meta-analysis used combinations of Lactobacillus species, which are usually found in OTC antidiarrheal probiotic supplements. No standard dose exists, but dose ranges in RCTs are 107 to 1010 colony-forming units per capsule (taken one to three times daily);1 however, product labels have variable accuracy.11 The duration of treatment ranges from one to three weeks—or as long as the patient continues to take antibiotics. .
REFERENCES
1. Hempel S, Newberry S, Maher A, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA. 2012; 307:1959-1969.
2. McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563-578.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile–associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037-1042.
4. Perry A, Dellon E, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 Update. Gastroenterology. 2012;143: 1179-1187.
5. Dubberke E, Wertheimer A. A review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30:57-66.
6. McFarland L. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:
812-822.
7. Cohen S, Gerding D, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America. Infect Control Hosp Epidemiol. 2010;31: 431-455.
8. Williams M, Ha C, Ciorba M. Probiotics as therapy in gastroenterology. J Clin Gastroenterol. 2010;44:631-636.
9. Floch M, Walker A, Madsne K, et al. Recommendations for probiotic use—2011 update. J Clin Gastroenterol. 2011;45(suppl):S168-S171.
10. Johnston BC, Ma SS, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
11. Hamilton-Miller J, Shah S. Deficiencies in microbiological quality and labeling of probiotic supplements. Int J Food Microbiol. 2002; 72:175-176.
Acknowledgement
The PURLs Surveillance System was developed with support from Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2012. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2012;61:673-674.
Prescribing an antibiotic? Pair it with probiotics
Recommend that patients taking antibiotics also take probiotics, which have been found to be effective both for the prevention and treatment of antibiotic-associated diarrhea (AAD).1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of randomized controlled trials.
Hempel S, Newberry S, Maher A, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA. 2012;307: 1959-1969.
ILLUSTRATIVE CASE
When you prescribe an antibiotic for a 45-year-old patient with Helicobacter pylori, he worries that the medication will cause diarrhea. Should you recommend that he take probiotics?
More than a third of patients taking antibiotics develop AAD,2 and in 17% of cases, AAD is fatal.3,4 Although the diarrhea may be the result of increased gastrointestinal (GI) motility in some cases, a disruption of the GI flora that normally acts as a barrier to infection and aids in the digestion of carbohydrates is a far more common cause.
Morbidity and mortality are high
AAD is associated with several pathogens, including Clostridium difficile, Clostridium perfringens, Klebsiella oxytoca, and Staphylococcus aureus,2 and varies widely in severity. Pseudomembranous colitis secondary to C difficile is the main cause of AAD-related mortality, which more than doubled from 2002 to 2009.3,4 C difficile infections cost the US health care system up to $1.3 billion annually.5 With such high rates of morbidity and mortality and high health care costs associated with AAD, even a small reduction in the number of cases would have a big impact.
Probiotics replenish the natural GI flora with nonpathogenic organisms. A 2006 meta-analysis of 31 randomized controlled trials (RCTs) assessing the efficacy of probiotics for both the prevention of AAD and treatment of C difficile found a pooled relative risk of 0.43 for AAD in the patients taking probiotics.6 However, many of the studies included in that meta-analysis were small. As a result, in 2010, the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) recommended against the use of probiotics for the prevention of primary C difficile infection, citing a lack of high-quality evidence.7
Nonetheless, that same year, 98% of gastroenterologists surveyed expressed a belief that probiotics had a role in the treatment of GI illness.8 And in 2011, the 3rd Yale Working Group on Probiotic Use published recommendations for probiotic use based on expert opinion.9 The meta-analysis detailed in this PURL, which included more than 30 trials published since the 2006 meta-analysis, addressed the efficacy of probiotics for prevention and treatment of AAD.
STUDY SUMMARY: Probiotics significantly reduce AAD
Hempel et al reviewed 82 studies and pooled data from 63 RCTs (N=11,811) to identify the relative risk (RR) of AAD among patients who received probiotics during antibiotic treatment compared with those who received no probiotics or were given a placebo.1 The studies encompassed a variety of antibiotics, taken alone or in combination, and several probiotics, including Lactobacillus, Bifidobacterium, Saccharomyces, and some combinations.
The outcome: The pooled RR for AAD in the probiotics groups was 0.58 (95% confidence interval, 0.50-0.68; P<.001), with a number needed to treat of 13. Although the authors reported that the overall quality of the included trials was poor, a sensitivity analysis of the higher quality studies yielded similar results.
Subgroup analyses by type of probiotic and duration of antibiotic treatment were also consistent with the overall pooled RR. In subgroup analysis by age, a similar decrease in AAD was found among the youngest patients (0-17 years) and those between the ages of 17 and 65 years. Among patients older than 65 years—for whom there were just 3 studies—a non-significant decrease in risk was found. Twenty-three of the studies assessed adverse outcomes, and none was found.
WHAT’S NEW: A reason to pair antibiotics and probiotics
This meta-analysis reached a similar conclusion as the 2006 meta-analysis: Probiotics appear to be effective in preventing and treating AAD in children and adults receiving a wide variety of antibiotics for a number of conditions. The results were also consistent with those of a new meta-analysis that looked specifically at one pathogen—and found a reduction of 66% in C difficile-associated diarrhea in patients taking probiotics with their antibiotics.10
CAVEATS: Limited data on the safety of probiotics exist
There was some heterogeneity among the studies in the meta-analysis by Hempel et al, and some of the studies were of poor quality. Because of this, the authors used subgroup and sensitivity analysis, which supported their initial conclusion.
Probiotics have generally been considered safe; however, there have been rare reports of sepsis and fungemia associated with probiotic use, especially in immunosuppressed patients.1 Fifty-nine of the included studies did not assess adverse events, which limited the ability of this meta-analysis to assess safety.1 Patients taking probiotics should be monitored for adverse effects.
CHALLENGES TO IMPLEMENTATION: Lack of guidance on dosing and duration
Since probiotics are considered food supplements, health insurance will not cover the cost (which will likely be more than $20 per month; www.walgreens.com). No single probiotic strain has high-quality evidence; however, most of the RCTs included in the meta-analysis used combinations of Lactobacillus species, which are usually found in over-the-counter antidiarrheal probiotic supplements. No standard dose exists, but dose ranges in RCTs are 107 to 1010 colony-forming units per capsule (taken one to 3 times daily);1 however, product labels have variable accuracy.11 The duration of treatment ranges from one to 3 weeks—or as long as the patient continues to take antibiotics.
Acknowledgement
The PURLs Surveillance System was developed with support from Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Hempel S, Newberry S, Maher A, et al. probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA. 2012;307:1959-1969.
2. McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563-578.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037-1042.
4. Perry A, Dellon E, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 Update. Gastroenterology. 2012;143:1179-1187.
5. Dubberke E, Wertheimer A. review of current literature on the economic burden of Clostridium difficile Infection. Infect Control Hosp Epidemiol. 2009;30:57-66.
6. McFarland L. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812-822.
7. Cohen S, Gerding D, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America. Infect Control Hosp Epidemiol. 2010;31:431-455.
8. Williams M, Ha C, Ciorba M. Probiotics as therapy in gastroenterology. J Clin Gastroenterol. 2010;44:631-636.
9. Floch M, Walker A, Madsne K, et al. Recommendations for probiotic use—2011 update. J Clin Gastroenterol. 2011;45(suppl):S168-S171.
10. Johnston BC, Ma SS, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
11. Hamilton-Miller J, Shah S. Deficiencies in microbiological quality and labeling of probiotic supplements. Int J Food Microbiol. 2002;72:175-176.
Recommend that patients taking antibiotics also take probiotics, which have been found to be effective both for the prevention and treatment of antibiotic-associated diarrhea (AAD).1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of randomized controlled trials.
Hempel S, Newberry S, Maher A, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA. 2012;307: 1959-1969.
ILLUSTRATIVE CASE
When you prescribe an antibiotic for a 45-year-old patient with Helicobacter pylori, he worries that the medication will cause diarrhea. Should you recommend that he take probiotics?
More than a third of patients taking antibiotics develop AAD,2 and in 17% of cases, AAD is fatal.3,4 Although the diarrhea may be the result of increased gastrointestinal (GI) motility in some cases, a disruption of the GI flora that normally acts as a barrier to infection and aids in the digestion of carbohydrates is a far more common cause.
Morbidity and mortality are high
AAD is associated with several pathogens, including Clostridium difficile, Clostridium perfringens, Klebsiella oxytoca, and Staphylococcus aureus,2 and varies widely in severity. Pseudomembranous colitis secondary to C difficile is the main cause of AAD-related mortality, which more than doubled from 2002 to 2009.3,4 C difficile infections cost the US health care system up to $1.3 billion annually.5 With such high rates of morbidity and mortality and high health care costs associated with AAD, even a small reduction in the number of cases would have a big impact.
Probiotics replenish the natural GI flora with nonpathogenic organisms. A 2006 meta-analysis of 31 randomized controlled trials (RCTs) assessing the efficacy of probiotics for both the prevention of AAD and treatment of C difficile found a pooled relative risk of 0.43 for AAD in the patients taking probiotics.6 However, many of the studies included in that meta-analysis were small. As a result, in 2010, the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) recommended against the use of probiotics for the prevention of primary C difficile infection, citing a lack of high-quality evidence.7
Nonetheless, that same year, 98% of gastroenterologists surveyed expressed a belief that probiotics had a role in the treatment of GI illness.8 And in 2011, the 3rd Yale Working Group on Probiotic Use published recommendations for probiotic use based on expert opinion.9 The meta-analysis detailed in this PURL, which included more than 30 trials published since the 2006 meta-analysis, addressed the efficacy of probiotics for prevention and treatment of AAD.
STUDY SUMMARY: Probiotics significantly reduce AAD
Hempel et al reviewed 82 studies and pooled data from 63 RCTs (N=11,811) to identify the relative risk (RR) of AAD among patients who received probiotics during antibiotic treatment compared with those who received no probiotics or were given a placebo.1 The studies encompassed a variety of antibiotics, taken alone or in combination, and several probiotics, including Lactobacillus, Bifidobacterium, Saccharomyces, and some combinations.
The outcome: The pooled RR for AAD in the probiotics groups was 0.58 (95% confidence interval, 0.50-0.68; P<.001), with a number needed to treat of 13. Although the authors reported that the overall quality of the included trials was poor, a sensitivity analysis of the higher quality studies yielded similar results.
Subgroup analyses by type of probiotic and duration of antibiotic treatment were also consistent with the overall pooled RR. In subgroup analysis by age, a similar decrease in AAD was found among the youngest patients (0-17 years) and those between the ages of 17 and 65 years. Among patients older than 65 years—for whom there were just 3 studies—a non-significant decrease in risk was found. Twenty-three of the studies assessed adverse outcomes, and none was found.
WHAT’S NEW: A reason to pair antibiotics and probiotics
This meta-analysis reached a similar conclusion as the 2006 meta-analysis: Probiotics appear to be effective in preventing and treating AAD in children and adults receiving a wide variety of antibiotics for a number of conditions. The results were also consistent with those of a new meta-analysis that looked specifically at one pathogen—and found a reduction of 66% in C difficile-associated diarrhea in patients taking probiotics with their antibiotics.10
CAVEATS: Limited data on the safety of probiotics exist
There was some heterogeneity among the studies in the meta-analysis by Hempel et al, and some of the studies were of poor quality. Because of this, the authors used subgroup and sensitivity analysis, which supported their initial conclusion.
Probiotics have generally been considered safe; however, there have been rare reports of sepsis and fungemia associated with probiotic use, especially in immunosuppressed patients.1 Fifty-nine of the included studies did not assess adverse events, which limited the ability of this meta-analysis to assess safety.1 Patients taking probiotics should be monitored for adverse effects.
CHALLENGES TO IMPLEMENTATION: Lack of guidance on dosing and duration
Since probiotics are considered food supplements, health insurance will not cover the cost (which will likely be more than $20 per month; www.walgreens.com). No single probiotic strain has high-quality evidence; however, most of the RCTs included in the meta-analysis used combinations of Lactobacillus species, which are usually found in over-the-counter antidiarrheal probiotic supplements. No standard dose exists, but dose ranges in RCTs are 107 to 1010 colony-forming units per capsule (taken one to 3 times daily);1 however, product labels have variable accuracy.11 The duration of treatment ranges from one to 3 weeks—or as long as the patient continues to take antibiotics.
Acknowledgement
The PURLs Surveillance System was developed with support from Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Recommend that patients taking antibiotics also take probiotics, which have been found to be effective both for the prevention and treatment of antibiotic-associated diarrhea (AAD).1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of randomized controlled trials.
Hempel S, Newberry S, Maher A, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA. 2012;307: 1959-1969.
ILLUSTRATIVE CASE
When you prescribe an antibiotic for a 45-year-old patient with Helicobacter pylori, he worries that the medication will cause diarrhea. Should you recommend that he take probiotics?
More than a third of patients taking antibiotics develop AAD,2 and in 17% of cases, AAD is fatal.3,4 Although the diarrhea may be the result of increased gastrointestinal (GI) motility in some cases, a disruption of the GI flora that normally acts as a barrier to infection and aids in the digestion of carbohydrates is a far more common cause.
Morbidity and mortality are high
AAD is associated with several pathogens, including Clostridium difficile, Clostridium perfringens, Klebsiella oxytoca, and Staphylococcus aureus,2 and varies widely in severity. Pseudomembranous colitis secondary to C difficile is the main cause of AAD-related mortality, which more than doubled from 2002 to 2009.3,4 C difficile infections cost the US health care system up to $1.3 billion annually.5 With such high rates of morbidity and mortality and high health care costs associated with AAD, even a small reduction in the number of cases would have a big impact.
Probiotics replenish the natural GI flora with nonpathogenic organisms. A 2006 meta-analysis of 31 randomized controlled trials (RCTs) assessing the efficacy of probiotics for both the prevention of AAD and treatment of C difficile found a pooled relative risk of 0.43 for AAD in the patients taking probiotics.6 However, many of the studies included in that meta-analysis were small. As a result, in 2010, the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) recommended against the use of probiotics for the prevention of primary C difficile infection, citing a lack of high-quality evidence.7
Nonetheless, that same year, 98% of gastroenterologists surveyed expressed a belief that probiotics had a role in the treatment of GI illness.8 And in 2011, the 3rd Yale Working Group on Probiotic Use published recommendations for probiotic use based on expert opinion.9 The meta-analysis detailed in this PURL, which included more than 30 trials published since the 2006 meta-analysis, addressed the efficacy of probiotics for prevention and treatment of AAD.
STUDY SUMMARY: Probiotics significantly reduce AAD
Hempel et al reviewed 82 studies and pooled data from 63 RCTs (N=11,811) to identify the relative risk (RR) of AAD among patients who received probiotics during antibiotic treatment compared with those who received no probiotics or were given a placebo.1 The studies encompassed a variety of antibiotics, taken alone or in combination, and several probiotics, including Lactobacillus, Bifidobacterium, Saccharomyces, and some combinations.
The outcome: The pooled RR for AAD in the probiotics groups was 0.58 (95% confidence interval, 0.50-0.68; P<.001), with a number needed to treat of 13. Although the authors reported that the overall quality of the included trials was poor, a sensitivity analysis of the higher quality studies yielded similar results.
Subgroup analyses by type of probiotic and duration of antibiotic treatment were also consistent with the overall pooled RR. In subgroup analysis by age, a similar decrease in AAD was found among the youngest patients (0-17 years) and those between the ages of 17 and 65 years. Among patients older than 65 years—for whom there were just 3 studies—a non-significant decrease in risk was found. Twenty-three of the studies assessed adverse outcomes, and none was found.
WHAT’S NEW: A reason to pair antibiotics and probiotics
This meta-analysis reached a similar conclusion as the 2006 meta-analysis: Probiotics appear to be effective in preventing and treating AAD in children and adults receiving a wide variety of antibiotics for a number of conditions. The results were also consistent with those of a new meta-analysis that looked specifically at one pathogen—and found a reduction of 66% in C difficile-associated diarrhea in patients taking probiotics with their antibiotics.10
CAVEATS: Limited data on the safety of probiotics exist
There was some heterogeneity among the studies in the meta-analysis by Hempel et al, and some of the studies were of poor quality. Because of this, the authors used subgroup and sensitivity analysis, which supported their initial conclusion.
Probiotics have generally been considered safe; however, there have been rare reports of sepsis and fungemia associated with probiotic use, especially in immunosuppressed patients.1 Fifty-nine of the included studies did not assess adverse events, which limited the ability of this meta-analysis to assess safety.1 Patients taking probiotics should be monitored for adverse effects.
CHALLENGES TO IMPLEMENTATION: Lack of guidance on dosing and duration
Since probiotics are considered food supplements, health insurance will not cover the cost (which will likely be more than $20 per month; www.walgreens.com). No single probiotic strain has high-quality evidence; however, most of the RCTs included in the meta-analysis used combinations of Lactobacillus species, which are usually found in over-the-counter antidiarrheal probiotic supplements. No standard dose exists, but dose ranges in RCTs are 107 to 1010 colony-forming units per capsule (taken one to 3 times daily);1 however, product labels have variable accuracy.11 The duration of treatment ranges from one to 3 weeks—or as long as the patient continues to take antibiotics.
Acknowledgement
The PURLs Surveillance System was developed with support from Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Hempel S, Newberry S, Maher A, et al. probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA. 2012;307:1959-1969.
2. McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563-578.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037-1042.
4. Perry A, Dellon E, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 Update. Gastroenterology. 2012;143:1179-1187.
5. Dubberke E, Wertheimer A. review of current literature on the economic burden of Clostridium difficile Infection. Infect Control Hosp Epidemiol. 2009;30:57-66.
6. McFarland L. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812-822.
7. Cohen S, Gerding D, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America. Infect Control Hosp Epidemiol. 2010;31:431-455.
8. Williams M, Ha C, Ciorba M. Probiotics as therapy in gastroenterology. J Clin Gastroenterol. 2010;44:631-636.
9. Floch M, Walker A, Madsne K, et al. Recommendations for probiotic use—2011 update. J Clin Gastroenterol. 2011;45(suppl):S168-S171.
10. Johnston BC, Ma SS, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
11. Hamilton-Miller J, Shah S. Deficiencies in microbiological quality and labeling of probiotic supplements. Int J Food Microbiol. 2002;72:175-176.
1. Hempel S, Newberry S, Maher A, et al. probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA. 2012;307:1959-1969.
2. McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563-578.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037-1042.
4. Perry A, Dellon E, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 Update. Gastroenterology. 2012;143:1179-1187.
5. Dubberke E, Wertheimer A. review of current literature on the economic burden of Clostridium difficile Infection. Infect Control Hosp Epidemiol. 2009;30:57-66.
6. McFarland L. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812-822.
7. Cohen S, Gerding D, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America. Infect Control Hosp Epidemiol. 2010;31:431-455.
8. Williams M, Ha C, Ciorba M. Probiotics as therapy in gastroenterology. J Clin Gastroenterol. 2010;44:631-636.
9. Floch M, Walker A, Madsne K, et al. Recommendations for probiotic use—2011 update. J Clin Gastroenterol. 2011;45(suppl):S168-S171.
10. Johnston BC, Ma SS, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
11. Hamilton-Miller J, Shah S. Deficiencies in microbiological quality and labeling of probiotic supplements. Int J Food Microbiol. 2002;72:175-176.
Copyright © 2013 The Family Physicians Inquiries Network. All rights reserved.
BP meds: This simple change improves outcomes
Advise patients with uncontrolled hypertension to take at least one of their blood pressure (BP) medications at bedtime instead of in the morning. Nighttime dosing leads to better control and lowers the risk of major cardiovascular events.1,2
STRENGTH OF RECOMMENDATION
B: Based on a well-done randomized clinical trial (RCT) and a subgroup analysis.
Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
ILLUSTRATIVE CASES
- A 60-year-old man has struggled to get his BP under control despite the use of 3 anti-hypertensives. Is there anything you can recommend to improve his BP control and lower his cardiovascular risk?
- You prescribe hydrochlorothiazide for a 55-year-old woman with newly diagnosed hypertension. What can you tell her about how to take the medication to maximize its beneficial effects?
Management of hypertension often centers around BP measurements taken in a doctor’s office during the day, although both BP and metabolism fluctuate with circadian rhythms. Most people experience an increase in pressure during the day, with peaks in the morning and evening, followed by a decline in BP while they sleep at night.3
The focus belongs on nighttime BP
Sleeping BP is getting considerable attention, particularly the phenomenon of nondipping. Commonly defined as a <10% decline in systolic pressure during sleep, nondipping is associated with an increased risk of cardiovascular events, such as heart attack and stroke.4 What’s more, mean BP during the night is a better predictor of cardiovascular disease (CVD) risk than BP while the patient is awake.5,6
Evidence suggests that taking an anti-hypertensive medication at night increases its therapeutic effect,7 yet most patients take it in the morning.8 The study detailed in this PURL was designed to investigate whether bedtime dosing significantly affects BP control and CVD risk.
STUDY SUMMARY: Bedtime dosing benefits patients, and there’s no downside
The MAPEC study was an open-label RCT conducted at a single center in Spain.1 Patients were enrolled if they had a diagnosis of either untreated hypertension (based on ambulatory BP monitoring [ABPM] criteria) or resistant hypertension (uncontrolled on ≥3 optimally dosed antihypertensive medications). Exclusion criteria included pregnancy, a history of drug/alcohol abuse, night shift work, acquired immune deficiency syndrome, type 1 diabetes, secondary hypertension, and a previous CVD diagnosis.
Patients were randomly assigned to one of 2 time-of-day dosing groups: morning dosing of all their BP medications (n=1109) or dosing of ≥1 BP medications at bedtime (n=1092). ABPM—in which patients wore a monitor that recorded their BP every 20 minutes during the day and every 30 minutes at night for 48 hours—was conducted once a year, or more frequently when medication adjustments occurred. The use of a specific drug was not required, but physicians were instructed to adjust medications according to a study-specific ABPM protocol.
Patients were followed for a mean of 5.6 years for the endpoints of CVD events and mortality. These endpoints were assessed by researchers blinded to patients’ treatment assignment.
At baseline, the 2 groups were similar in age (mean of 55 years), percentage of men (48%), presence of comorbidities, and baseline clinic and ambulatory BP. Throughout the study, patients in the bedtime dosing group had lower mean asleep systolic and diastolic BP, a lower prevalence of a non-dipping pattern, and a higher prevalence of controlled ambulatory BP. The bedtime group also had a lower risk of total CVD events (relative risk [RR]=0.39; 95% confidence interval [CI], 0.29-0.51; P<.001) and major CVD events (RR=0.33; 95% CI, 0.19-0.55; P<.001), and fewer overall deaths (4.16/1000 vs 2.11/1000 patient-years; P=.008) (TABLE). To prevent one CVD event, 63 patients would need to take their BP medication at bedtime instead of in the morning for one year. To prevent one death, 488 patient would need to adhere to the nighttime schedule for one year.
A subgroup analysis of patients with type 2 diabetes (n=448)2 had similar results: For this population, too, bedtime dosing led to lower asleep BP, a lower prevalence of a non-dipping pattern, and a higher prevalence of controlled ambulatory BP, as well as a lower risk of total CVD events, major CVD events, and CVD-related death. The differences persisted after correction for the use of statins and aspirin. Among those in this subgroup analysis, 29 patients would need to take their BP medications at bedtime for one year to prevent one CVD event, and 263 patients would need to be treated for one year to prevent one death.
TABLE
Dosing of BP meds: A look at outcomes
| Events/1000 patient-years | Morning dosing | Bedtime dosing | P between groups |
|---|---|---|---|
| overall (n=2201)1 | |||
| Total events* | 27.80 | 11.95 | <.001 |
| CVd death | 2.08 | 0.53 | .006 |
| Cardiovascular events | 11.00 | 5.27 | <.001 |
| Cerebrovascular events | 3.57 | 1.23 | .001 |
| Diabetes subgroup (n=448)2 | |||
| Total events* | 54.24 | 19.80 | <.001 |
| CVd death | 4.79 | 0.86 | .038 |
| Cardiovascular events | 15.95 | 6.89 | .008 |
| Cerebrovascular events | 6.38 | 0.86 | .010 |
| *Includes death from all causes and cardiovascular and cerebrovascular events. BP, blood pressure; CVD, cardiovascular disease. | |||
WHAT’S NEW: Advantages of preventing nondipping are clearly established
We’ve known that a nondipping pattern is associated with higher cardiovascular risks and that taking antihypertensives at bedtime decreases the prevalence of nondipping patterns. The MAPEC study, however, is the first prospective trial to show that bedtime dosing of BP medications lowers the risk of CVD events and death.
CAVEATS: Methodology, non-US guidelines raise questions about applicability here
MAPEC was an open-label study, meaning that the physicians adjusting BP medications were aware of the treatment groups to which their patients were allocated. Physicians were given guidelines for the titration of medications, but it is unclear whether they treated patients in both treatment groups identically. Patients were also aware of their treatment group, which creates the potential for bias if one group adhered to their medications more closely than the other.
The study was a single-center trial conducted in Spain, which may limit its generalizability to the United States. Notably, Spain’s medication guidelines differ from ours, with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and calcium channel blockers as first-line medications and hydrochlorothiazide as a second-line option.
While ABPM appears to be a better indicator of CVD risk compared with clinic BP monitoring, most US physicians still rely on readings taken in their office for diagnosing and managing hypertension. How ambulatory BP translates to clinic BP is somewhat unclear.
CHALLENGES TO IMPLEMENTATION: Some patients and providers may resist the switch
We see few challenges to implementing bedtime dosing of BP medications for patients with uncontrolled hypertension. It is possible, however, that patients who have a long-standing routine of taking their medications in the morning may be resistant to change. Also, pharmacists and nurses, as well as some physicians, may continue recommending morning dosing, which could be confusing for patients.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
2. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
3. Hermida RC, Ayala DE, Portaluppi F. Circadian variation of blood pressure: the basis for the chronotherapy of hypertension. Adv Drug Deliv Rev. 2007;59:904-922.
4. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793-801.
5. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin study outcome. Hypertension. 2005;46:156-161.
6. Hermida RC, Ayala DE, Mojón A, et al. Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 2011;58:1165-1173.
7. De la Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466-472.
8. Hermida RC, Ayala DE, Calvo C, et al. Chronotherapy of hypertension: administration-time-dependent effects of treatment on the circadian pattern of blood pressure. Adv Drug Deliv Rev. 2007;59:923-939.
Advise patients with uncontrolled hypertension to take at least one of their blood pressure (BP) medications at bedtime instead of in the morning. Nighttime dosing leads to better control and lowers the risk of major cardiovascular events.1,2
STRENGTH OF RECOMMENDATION
B: Based on a well-done randomized clinical trial (RCT) and a subgroup analysis.
Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
ILLUSTRATIVE CASES
- A 60-year-old man has struggled to get his BP under control despite the use of 3 anti-hypertensives. Is there anything you can recommend to improve his BP control and lower his cardiovascular risk?
- You prescribe hydrochlorothiazide for a 55-year-old woman with newly diagnosed hypertension. What can you tell her about how to take the medication to maximize its beneficial effects?
Management of hypertension often centers around BP measurements taken in a doctor’s office during the day, although both BP and metabolism fluctuate with circadian rhythms. Most people experience an increase in pressure during the day, with peaks in the morning and evening, followed by a decline in BP while they sleep at night.3
The focus belongs on nighttime BP
Sleeping BP is getting considerable attention, particularly the phenomenon of nondipping. Commonly defined as a <10% decline in systolic pressure during sleep, nondipping is associated with an increased risk of cardiovascular events, such as heart attack and stroke.4 What’s more, mean BP during the night is a better predictor of cardiovascular disease (CVD) risk than BP while the patient is awake.5,6
Evidence suggests that taking an anti-hypertensive medication at night increases its therapeutic effect,7 yet most patients take it in the morning.8 The study detailed in this PURL was designed to investigate whether bedtime dosing significantly affects BP control and CVD risk.
STUDY SUMMARY: Bedtime dosing benefits patients, and there’s no downside
The MAPEC study was an open-label RCT conducted at a single center in Spain.1 Patients were enrolled if they had a diagnosis of either untreated hypertension (based on ambulatory BP monitoring [ABPM] criteria) or resistant hypertension (uncontrolled on ≥3 optimally dosed antihypertensive medications). Exclusion criteria included pregnancy, a history of drug/alcohol abuse, night shift work, acquired immune deficiency syndrome, type 1 diabetes, secondary hypertension, and a previous CVD diagnosis.
Patients were randomly assigned to one of 2 time-of-day dosing groups: morning dosing of all their BP medications (n=1109) or dosing of ≥1 BP medications at bedtime (n=1092). ABPM—in which patients wore a monitor that recorded their BP every 20 minutes during the day and every 30 minutes at night for 48 hours—was conducted once a year, or more frequently when medication adjustments occurred. The use of a specific drug was not required, but physicians were instructed to adjust medications according to a study-specific ABPM protocol.
Patients were followed for a mean of 5.6 years for the endpoints of CVD events and mortality. These endpoints were assessed by researchers blinded to patients’ treatment assignment.
At baseline, the 2 groups were similar in age (mean of 55 years), percentage of men (48%), presence of comorbidities, and baseline clinic and ambulatory BP. Throughout the study, patients in the bedtime dosing group had lower mean asleep systolic and diastolic BP, a lower prevalence of a non-dipping pattern, and a higher prevalence of controlled ambulatory BP. The bedtime group also had a lower risk of total CVD events (relative risk [RR]=0.39; 95% confidence interval [CI], 0.29-0.51; P<.001) and major CVD events (RR=0.33; 95% CI, 0.19-0.55; P<.001), and fewer overall deaths (4.16/1000 vs 2.11/1000 patient-years; P=.008) (TABLE). To prevent one CVD event, 63 patients would need to take their BP medication at bedtime instead of in the morning for one year. To prevent one death, 488 patient would need to adhere to the nighttime schedule for one year.
A subgroup analysis of patients with type 2 diabetes (n=448)2 had similar results: For this population, too, bedtime dosing led to lower asleep BP, a lower prevalence of a non-dipping pattern, and a higher prevalence of controlled ambulatory BP, as well as a lower risk of total CVD events, major CVD events, and CVD-related death. The differences persisted after correction for the use of statins and aspirin. Among those in this subgroup analysis, 29 patients would need to take their BP medications at bedtime for one year to prevent one CVD event, and 263 patients would need to be treated for one year to prevent one death.
TABLE
Dosing of BP meds: A look at outcomes
| Events/1000 patient-years | Morning dosing | Bedtime dosing | P between groups |
|---|---|---|---|
| overall (n=2201)1 | |||
| Total events* | 27.80 | 11.95 | <.001 |
| CVd death | 2.08 | 0.53 | .006 |
| Cardiovascular events | 11.00 | 5.27 | <.001 |
| Cerebrovascular events | 3.57 | 1.23 | .001 |
| Diabetes subgroup (n=448)2 | |||
| Total events* | 54.24 | 19.80 | <.001 |
| CVd death | 4.79 | 0.86 | .038 |
| Cardiovascular events | 15.95 | 6.89 | .008 |
| Cerebrovascular events | 6.38 | 0.86 | .010 |
| *Includes death from all causes and cardiovascular and cerebrovascular events. BP, blood pressure; CVD, cardiovascular disease. | |||
WHAT’S NEW: Advantages of preventing nondipping are clearly established
We’ve known that a nondipping pattern is associated with higher cardiovascular risks and that taking antihypertensives at bedtime decreases the prevalence of nondipping patterns. The MAPEC study, however, is the first prospective trial to show that bedtime dosing of BP medications lowers the risk of CVD events and death.
CAVEATS: Methodology, non-US guidelines raise questions about applicability here
MAPEC was an open-label study, meaning that the physicians adjusting BP medications were aware of the treatment groups to which their patients were allocated. Physicians were given guidelines for the titration of medications, but it is unclear whether they treated patients in both treatment groups identically. Patients were also aware of their treatment group, which creates the potential for bias if one group adhered to their medications more closely than the other.
The study was a single-center trial conducted in Spain, which may limit its generalizability to the United States. Notably, Spain’s medication guidelines differ from ours, with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and calcium channel blockers as first-line medications and hydrochlorothiazide as a second-line option.
While ABPM appears to be a better indicator of CVD risk compared with clinic BP monitoring, most US physicians still rely on readings taken in their office for diagnosing and managing hypertension. How ambulatory BP translates to clinic BP is somewhat unclear.
CHALLENGES TO IMPLEMENTATION: Some patients and providers may resist the switch
We see few challenges to implementing bedtime dosing of BP medications for patients with uncontrolled hypertension. It is possible, however, that patients who have a long-standing routine of taking their medications in the morning may be resistant to change. Also, pharmacists and nurses, as well as some physicians, may continue recommending morning dosing, which could be confusing for patients.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Advise patients with uncontrolled hypertension to take at least one of their blood pressure (BP) medications at bedtime instead of in the morning. Nighttime dosing leads to better control and lowers the risk of major cardiovascular events.1,2
STRENGTH OF RECOMMENDATION
B: Based on a well-done randomized clinical trial (RCT) and a subgroup analysis.
Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
ILLUSTRATIVE CASES
- A 60-year-old man has struggled to get his BP under control despite the use of 3 anti-hypertensives. Is there anything you can recommend to improve his BP control and lower his cardiovascular risk?
- You prescribe hydrochlorothiazide for a 55-year-old woman with newly diagnosed hypertension. What can you tell her about how to take the medication to maximize its beneficial effects?
Management of hypertension often centers around BP measurements taken in a doctor’s office during the day, although both BP and metabolism fluctuate with circadian rhythms. Most people experience an increase in pressure during the day, with peaks in the morning and evening, followed by a decline in BP while they sleep at night.3
The focus belongs on nighttime BP
Sleeping BP is getting considerable attention, particularly the phenomenon of nondipping. Commonly defined as a <10% decline in systolic pressure during sleep, nondipping is associated with an increased risk of cardiovascular events, such as heart attack and stroke.4 What’s more, mean BP during the night is a better predictor of cardiovascular disease (CVD) risk than BP while the patient is awake.5,6
Evidence suggests that taking an anti-hypertensive medication at night increases its therapeutic effect,7 yet most patients take it in the morning.8 The study detailed in this PURL was designed to investigate whether bedtime dosing significantly affects BP control and CVD risk.
STUDY SUMMARY: Bedtime dosing benefits patients, and there’s no downside
The MAPEC study was an open-label RCT conducted at a single center in Spain.1 Patients were enrolled if they had a diagnosis of either untreated hypertension (based on ambulatory BP monitoring [ABPM] criteria) or resistant hypertension (uncontrolled on ≥3 optimally dosed antihypertensive medications). Exclusion criteria included pregnancy, a history of drug/alcohol abuse, night shift work, acquired immune deficiency syndrome, type 1 diabetes, secondary hypertension, and a previous CVD diagnosis.
Patients were randomly assigned to one of 2 time-of-day dosing groups: morning dosing of all their BP medications (n=1109) or dosing of ≥1 BP medications at bedtime (n=1092). ABPM—in which patients wore a monitor that recorded their BP every 20 minutes during the day and every 30 minutes at night for 48 hours—was conducted once a year, or more frequently when medication adjustments occurred. The use of a specific drug was not required, but physicians were instructed to adjust medications according to a study-specific ABPM protocol.
Patients were followed for a mean of 5.6 years for the endpoints of CVD events and mortality. These endpoints were assessed by researchers blinded to patients’ treatment assignment.
At baseline, the 2 groups were similar in age (mean of 55 years), percentage of men (48%), presence of comorbidities, and baseline clinic and ambulatory BP. Throughout the study, patients in the bedtime dosing group had lower mean asleep systolic and diastolic BP, a lower prevalence of a non-dipping pattern, and a higher prevalence of controlled ambulatory BP. The bedtime group also had a lower risk of total CVD events (relative risk [RR]=0.39; 95% confidence interval [CI], 0.29-0.51; P<.001) and major CVD events (RR=0.33; 95% CI, 0.19-0.55; P<.001), and fewer overall deaths (4.16/1000 vs 2.11/1000 patient-years; P=.008) (TABLE). To prevent one CVD event, 63 patients would need to take their BP medication at bedtime instead of in the morning for one year. To prevent one death, 488 patient would need to adhere to the nighttime schedule for one year.
A subgroup analysis of patients with type 2 diabetes (n=448)2 had similar results: For this population, too, bedtime dosing led to lower asleep BP, a lower prevalence of a non-dipping pattern, and a higher prevalence of controlled ambulatory BP, as well as a lower risk of total CVD events, major CVD events, and CVD-related death. The differences persisted after correction for the use of statins and aspirin. Among those in this subgroup analysis, 29 patients would need to take their BP medications at bedtime for one year to prevent one CVD event, and 263 patients would need to be treated for one year to prevent one death.
TABLE
Dosing of BP meds: A look at outcomes
| Events/1000 patient-years | Morning dosing | Bedtime dosing | P between groups |
|---|---|---|---|
| overall (n=2201)1 | |||
| Total events* | 27.80 | 11.95 | <.001 |
| CVd death | 2.08 | 0.53 | .006 |
| Cardiovascular events | 11.00 | 5.27 | <.001 |
| Cerebrovascular events | 3.57 | 1.23 | .001 |
| Diabetes subgroup (n=448)2 | |||
| Total events* | 54.24 | 19.80 | <.001 |
| CVd death | 4.79 | 0.86 | .038 |
| Cardiovascular events | 15.95 | 6.89 | .008 |
| Cerebrovascular events | 6.38 | 0.86 | .010 |
| *Includes death from all causes and cardiovascular and cerebrovascular events. BP, blood pressure; CVD, cardiovascular disease. | |||
WHAT’S NEW: Advantages of preventing nondipping are clearly established
We’ve known that a nondipping pattern is associated with higher cardiovascular risks and that taking antihypertensives at bedtime decreases the prevalence of nondipping patterns. The MAPEC study, however, is the first prospective trial to show that bedtime dosing of BP medications lowers the risk of CVD events and death.
CAVEATS: Methodology, non-US guidelines raise questions about applicability here
MAPEC was an open-label study, meaning that the physicians adjusting BP medications were aware of the treatment groups to which their patients were allocated. Physicians were given guidelines for the titration of medications, but it is unclear whether they treated patients in both treatment groups identically. Patients were also aware of their treatment group, which creates the potential for bias if one group adhered to their medications more closely than the other.
The study was a single-center trial conducted in Spain, which may limit its generalizability to the United States. Notably, Spain’s medication guidelines differ from ours, with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and calcium channel blockers as first-line medications and hydrochlorothiazide as a second-line option.
While ABPM appears to be a better indicator of CVD risk compared with clinic BP monitoring, most US physicians still rely on readings taken in their office for diagnosing and managing hypertension. How ambulatory BP translates to clinic BP is somewhat unclear.
CHALLENGES TO IMPLEMENTATION: Some patients and providers may resist the switch
We see few challenges to implementing bedtime dosing of BP medications for patients with uncontrolled hypertension. It is possible, however, that patients who have a long-standing routine of taking their medications in the morning may be resistant to change. Also, pharmacists and nurses, as well as some physicians, may continue recommending morning dosing, which could be confusing for patients.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
2. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
3. Hermida RC, Ayala DE, Portaluppi F. Circadian variation of blood pressure: the basis for the chronotherapy of hypertension. Adv Drug Deliv Rev. 2007;59:904-922.
4. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793-801.
5. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin study outcome. Hypertension. 2005;46:156-161.
6. Hermida RC, Ayala DE, Mojón A, et al. Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 2011;58:1165-1173.
7. De la Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466-472.
8. Hermida RC, Ayala DE, Calvo C, et al. Chronotherapy of hypertension: administration-time-dependent effects of treatment on the circadian pattern of blood pressure. Adv Drug Deliv Rev. 2007;59:923-939.
1. Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651.
2. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
3. Hermida RC, Ayala DE, Portaluppi F. Circadian variation of blood pressure: the basis for the chronotherapy of hypertension. Adv Drug Deliv Rev. 2007;59:904-922.
4. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793-801.
5. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin study outcome. Hypertension. 2005;46:156-161.
6. Hermida RC, Ayala DE, Mojón A, et al. Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 2011;58:1165-1173.
7. De la Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466-472.
8. Hermida RC, Ayala DE, Calvo C, et al. Chronotherapy of hypertension: administration-time-dependent effects of treatment on the circadian pattern of blood pressure. Adv Drug Deliv Rev. 2007;59:923-939.
Copyright © 2012 The Family Physicians Inquiries Network.
All rights reserved.
Time to try this warfarin alternative?
Consider dabigatran, an oral anticoagulant that does not require monitoring, for the prevention of stroke and thromboembolism in patients with atrial fibrillation.1
STRENGTH OF RECOMMENDATION
B: Based on a single well-done randomized controlled trial (RCT).
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
ILLUSTRATIVE CASE
A 75-year-old man with persistent atrial fibrillation and diabetes comes to your office for a check of his international normalized ratio (INR). It has been hard to keep his INR within the normal range of 2 to 3 in recent months, and today is no different: The patient’s INR is 1.7, although he insists he has been compliant with his warfarin regimen and has had no change in diet or other medications. What other anticoagulation options can you offer him?
Patients with atrial fibrillation have a 3% to 8% annual risk of stroke.2 Both adjusted-dose warfarin and antiplatelet agents such as aspirin have been shown to be effective at reducing this risk, although warfarin is significantly more effective.3
Those who have atrial fibrillation and a previous history of thromboembolism or rheumatic mitral stenosis or more than one moderate risk factor (age ≥75 years, hypertension, heart failure, impaired left ventricular systolic function, or diabetes) have the highest stroke risk. The American College of Cardiology/American Heart Association Task Force/ European Society of Cardiology (ACC/AHA/ ESC) 2006 guidelines for the management of atrial fibrillation recommend chronic anticoagulation with an oral vitamin K antagonist, such as warfarin, for these high-risk patients.4
Warfarin therapy is challenging
We have all experienced the frustrations of maintaining our patients on warfarin at a therapeutic INR; the average patient is within this range only about 67% of the time, although this varies dramatically from patient to patient.5
Many of our patients have experienced the inconvenience and cost of repeated monitoring, as well as the morbidity associated with both major and minor bleeding related to warfarin use. And there are many potential interactions between warfarin and foods or other drugs.
Is the new oral anticoagulant a better bet?
There are anticoagulants that do not require monitoring (eg, enoxaparin), but few patients are willing to undergo daily subcutaneous injections, and the cost is often prohibitive. Now there is another alternative.
Dabigatran (Pradaxa), an oral direct thrombin inhibitor, was approved by the US Food and Drug Administration in October 2010 for the prevention of stroke and systemic embolism in patients with atrial fibrillation.6 Dabigatran is administered twice daily in a fixed dose. Because it has a relatively short half-life (12-17 hours), it does not require INR monitoring. Dabigatran has no known interactions with foods and minimal interactions with other medications. Its value as a warfarin alternative for patients with atrial fibrillation was addressed in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study detailed below.
STUDY SUMMARY: At higher dose, dabigatran prevents more strokes than warfarin
RE-LY included 18,113 patients from 951 facilities in 44 countries. To be eligible for the study, patients had to have atrial fibrillation documented on an electrocardiogram and at least one additional risk factor for stroke.
Participants were randomized into one of 3 groups: dabigatran 110 mg twice daily, dabigatran 150 mg twice daily (both administered in a blinded fashion), or warfarin (administered in an unblinded fashion and dosed to maintain an INR between 2 and 3). Baseline characteristics, such as age, sex, and CHADS2 (congestive heart failure, hypertension, age, diabetes, prior stroke) score, were similar across all 3 groups. The median duration of follow-up was 2 years, and complete follow-up occurred in 99.9% of participants.
The primary outcome of the study was stroke or systemic embolism. The primary safety outcome was major hemorrhage, defined as a reduction in hemoglobin of ≥2 g/dL, transfusion of ≥2 units of blood, or symptomatic bleeding in a critical area/organ. Other outcomes were death, myocardial infarction (MI), pulmonary embolism, transient ischemic attack, and hospitalization.
For the primary outcome of prevention of stroke or systemic embolism, the 150-mg dose of dabigatran was superior to warfarin (1.11% vs 1.69% per year, relative risk [RR], 0.66; 95% confidence interval [CI], 0.53-0.82; P<.001 for superiority). The major bleeding rates were similar for dabigatran 150 mg and warfarin, although major gastrointestinal bleeding rates were significantly higher with this dose of dabigatran compared with warfarin (TABLE). Minor bleeding was more common in the warfarin group (16.37% vs 14.84%; RR, 0.91; 95% CI, 0.85-0.97; P=.005).
The 110-mg dose of dabigatran (which is not available in the United States) was neither inferior nor superior to warfarin for the prevention of stroke or systemic embolism. This dose of dabigatran had a lower risk of major bleeding compared with warfarin.
TABLE
Dabigatran vs warfarin: A look at the evidence1
| Event | Incidence (%/y) | NNT/NNH with dabigatran instead of warfarin | Relative risk (95% CI) | P value | |
|---|---|---|---|---|---|
| Dabigatran (150 mg) | Warfarin | ||||
| Stroke or systemic embolism | 1.11 | 1.69 | NNT: 173 | 0.66 (0.53-0.82) | <.001* <.001 |
| Hemorrhagic stroke | 0.10 | 0.38 | NNT: 477 | 0.26 (0.14-0.49) | <.001 |
| MI | 0.74 | 0.53 | NNH: 477 | 1.38 (1.00-1.91) | .048 |
| Death from any cause | 3.64 | 4.13 | NS | 0.88 (0.77-1.00) | .051 |
| Major bleeding | 3.11 | 3.36 | NS | 0.93 (0.81-1.07) | .31 |
| Intracranial bleeding | 0.30 | 0.74 | NNT: 228 | 0.40 (0.27-0.60) | <.001 |
| GI bleeding | 1.51 | 1.02 | NNH: 205 | 1.50 (1.19-1.89) | <.001 |
| Life-threatening bleeding | 1.45 | 1.80 | NNT: 286 | 0.81 (0.66-0.99) | .04 |
| CI, confidence interval; GI, gastrointestinal; MI, myocardial infarction; NNH, number needed to harm; NNT, number needed to treat; NS, no significant difference. *P value for noninferiority; all other P values are for superiority. | |||||
Mortality rates are similar
Rates of death from any cause were similar among the 3 treatment groups. The rates of hemorrhagic stroke were lower in both dabigatran groups compared with the warfarin group, while rates of MI were lower in the warfarin group than in either of the dabigatran groups.
Dyspepsia was the only other adverse effect that was significantly more common among dabigatran users vs warfarin users. Rates of hepatotoxicity, which was a problem wiThearlier oral direct thrombin inhibitors, were similar for both drugs. Multiple subgroup analyses revealed no significant interaction between the treatment effect of dabigatran and variables such as sex, body mass index, creatinine clearance, CHADS2 score, aspirin use, or previous long-term use of a vitamin K antagonist.
WHAT’S NEW: This easier-to-use oral anticoagulant is a viable option
Dabigatran gives physicians and patients with atrial fibrillation an option that is more convenient than warfarin for stroke prevention. Its 150-mg dose is more effective in preventing stroke compared with warfarin, and comparable in terms of bleeding risk.
CAVEATS: Unknown long-term effects, potential for bias
The median follow-up in the RE-LY study was 2 years. Longer-term efficacy and safety data may differ from the initial results.
The trial was funded by Boehringer Ingelheim, the manufacturer of dabigatran (Pradaxa). However, study coordination, data management, and analysis were performed independently by the Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
Patients taking dabigatran received the medication in a blinded fashion, but the warfarin group could not be blinded because of the need for INR monitoring and dosage adjustments. To decrease potential bias, the outcome events were assessed by 2 independent investigators who were blinded to the treatment assignments.
CHALLENGES TO IMPLEMENTATION: Cost of dabigatran may be a barrier
The wholesale price of dabigatran, as quoted by Boehringer Ingelheim, is $6.75 per day; the retail price for a 30-day supply is approximately $235, according to drugstore.com, Walgreens, and Walmart). In comparison, a one-month supply of warfarin is about $15. Out-of-pocket costs for many patients will likely be high until dabigatran is added to insurers’ formularies. When costs for monitoring and hospitalizations or treatment for complications associated with warfarin are factored in, however, dabigatran is cost effective, a recent study indicates.7
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Connolly SJ, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Wolf PA, et al. Atrial fibrillation as an independent risk factor for stroke; the Framingham study. Stroke. 1991;22:983-988.
3. Hart RG, et al. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
4. Fuster V, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation–executive summary. J Am Coll Cardiol. 2006;48:854-906.
5. Rose AJ, et al. Warfarin for atrial fibrillation in community-based practice. J Thromb Haemost. 2008;6:1647-1654.
6. US Food and Drug Administration. FDA approves Pradaxa in people with atrial fibrillation. October 19, 2010.
7. Freeman JV, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1-11.
Consider dabigatran, an oral anticoagulant that does not require monitoring, for the prevention of stroke and thromboembolism in patients with atrial fibrillation.1
STRENGTH OF RECOMMENDATION
B: Based on a single well-done randomized controlled trial (RCT).
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
ILLUSTRATIVE CASE
A 75-year-old man with persistent atrial fibrillation and diabetes comes to your office for a check of his international normalized ratio (INR). It has been hard to keep his INR within the normal range of 2 to 3 in recent months, and today is no different: The patient’s INR is 1.7, although he insists he has been compliant with his warfarin regimen and has had no change in diet or other medications. What other anticoagulation options can you offer him?
Patients with atrial fibrillation have a 3% to 8% annual risk of stroke.2 Both adjusted-dose warfarin and antiplatelet agents such as aspirin have been shown to be effective at reducing this risk, although warfarin is significantly more effective.3
Those who have atrial fibrillation and a previous history of thromboembolism or rheumatic mitral stenosis or more than one moderate risk factor (age ≥75 years, hypertension, heart failure, impaired left ventricular systolic function, or diabetes) have the highest stroke risk. The American College of Cardiology/American Heart Association Task Force/ European Society of Cardiology (ACC/AHA/ ESC) 2006 guidelines for the management of atrial fibrillation recommend chronic anticoagulation with an oral vitamin K antagonist, such as warfarin, for these high-risk patients.4
Warfarin therapy is challenging
We have all experienced the frustrations of maintaining our patients on warfarin at a therapeutic INR; the average patient is within this range only about 67% of the time, although this varies dramatically from patient to patient.5
Many of our patients have experienced the inconvenience and cost of repeated monitoring, as well as the morbidity associated with both major and minor bleeding related to warfarin use. And there are many potential interactions between warfarin and foods or other drugs.
Is the new oral anticoagulant a better bet?
There are anticoagulants that do not require monitoring (eg, enoxaparin), but few patients are willing to undergo daily subcutaneous injections, and the cost is often prohibitive. Now there is another alternative.
Dabigatran (Pradaxa), an oral direct thrombin inhibitor, was approved by the US Food and Drug Administration in October 2010 for the prevention of stroke and systemic embolism in patients with atrial fibrillation.6 Dabigatran is administered twice daily in a fixed dose. Because it has a relatively short half-life (12-17 hours), it does not require INR monitoring. Dabigatran has no known interactions with foods and minimal interactions with other medications. Its value as a warfarin alternative for patients with atrial fibrillation was addressed in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study detailed below.
STUDY SUMMARY: At higher dose, dabigatran prevents more strokes than warfarin
RE-LY included 18,113 patients from 951 facilities in 44 countries. To be eligible for the study, patients had to have atrial fibrillation documented on an electrocardiogram and at least one additional risk factor for stroke.
Participants were randomized into one of 3 groups: dabigatran 110 mg twice daily, dabigatran 150 mg twice daily (both administered in a blinded fashion), or warfarin (administered in an unblinded fashion and dosed to maintain an INR between 2 and 3). Baseline characteristics, such as age, sex, and CHADS2 (congestive heart failure, hypertension, age, diabetes, prior stroke) score, were similar across all 3 groups. The median duration of follow-up was 2 years, and complete follow-up occurred in 99.9% of participants.
The primary outcome of the study was stroke or systemic embolism. The primary safety outcome was major hemorrhage, defined as a reduction in hemoglobin of ≥2 g/dL, transfusion of ≥2 units of blood, or symptomatic bleeding in a critical area/organ. Other outcomes were death, myocardial infarction (MI), pulmonary embolism, transient ischemic attack, and hospitalization.
For the primary outcome of prevention of stroke or systemic embolism, the 150-mg dose of dabigatran was superior to warfarin (1.11% vs 1.69% per year, relative risk [RR], 0.66; 95% confidence interval [CI], 0.53-0.82; P<.001 for superiority). The major bleeding rates were similar for dabigatran 150 mg and warfarin, although major gastrointestinal bleeding rates were significantly higher with this dose of dabigatran compared with warfarin (TABLE). Minor bleeding was more common in the warfarin group (16.37% vs 14.84%; RR, 0.91; 95% CI, 0.85-0.97; P=.005).
The 110-mg dose of dabigatran (which is not available in the United States) was neither inferior nor superior to warfarin for the prevention of stroke or systemic embolism. This dose of dabigatran had a lower risk of major bleeding compared with warfarin.
TABLE
Dabigatran vs warfarin: A look at the evidence1
| Event | Incidence (%/y) | NNT/NNH with dabigatran instead of warfarin | Relative risk (95% CI) | P value | |
|---|---|---|---|---|---|
| Dabigatran (150 mg) | Warfarin | ||||
| Stroke or systemic embolism | 1.11 | 1.69 | NNT: 173 | 0.66 (0.53-0.82) | <.001* <.001 |
| Hemorrhagic stroke | 0.10 | 0.38 | NNT: 477 | 0.26 (0.14-0.49) | <.001 |
| MI | 0.74 | 0.53 | NNH: 477 | 1.38 (1.00-1.91) | .048 |
| Death from any cause | 3.64 | 4.13 | NS | 0.88 (0.77-1.00) | .051 |
| Major bleeding | 3.11 | 3.36 | NS | 0.93 (0.81-1.07) | .31 |
| Intracranial bleeding | 0.30 | 0.74 | NNT: 228 | 0.40 (0.27-0.60) | <.001 |
| GI bleeding | 1.51 | 1.02 | NNH: 205 | 1.50 (1.19-1.89) | <.001 |
| Life-threatening bleeding | 1.45 | 1.80 | NNT: 286 | 0.81 (0.66-0.99) | .04 |
| CI, confidence interval; GI, gastrointestinal; MI, myocardial infarction; NNH, number needed to harm; NNT, number needed to treat; NS, no significant difference. *P value for noninferiority; all other P values are for superiority. | |||||
Mortality rates are similar
Rates of death from any cause were similar among the 3 treatment groups. The rates of hemorrhagic stroke were lower in both dabigatran groups compared with the warfarin group, while rates of MI were lower in the warfarin group than in either of the dabigatran groups.
Dyspepsia was the only other adverse effect that was significantly more common among dabigatran users vs warfarin users. Rates of hepatotoxicity, which was a problem wiThearlier oral direct thrombin inhibitors, were similar for both drugs. Multiple subgroup analyses revealed no significant interaction between the treatment effect of dabigatran and variables such as sex, body mass index, creatinine clearance, CHADS2 score, aspirin use, or previous long-term use of a vitamin K antagonist.
WHAT’S NEW: This easier-to-use oral anticoagulant is a viable option
Dabigatran gives physicians and patients with atrial fibrillation an option that is more convenient than warfarin for stroke prevention. Its 150-mg dose is more effective in preventing stroke compared with warfarin, and comparable in terms of bleeding risk.
CAVEATS: Unknown long-term effects, potential for bias
The median follow-up in the RE-LY study was 2 years. Longer-term efficacy and safety data may differ from the initial results.
The trial was funded by Boehringer Ingelheim, the manufacturer of dabigatran (Pradaxa). However, study coordination, data management, and analysis were performed independently by the Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
Patients taking dabigatran received the medication in a blinded fashion, but the warfarin group could not be blinded because of the need for INR monitoring and dosage adjustments. To decrease potential bias, the outcome events were assessed by 2 independent investigators who were blinded to the treatment assignments.
CHALLENGES TO IMPLEMENTATION: Cost of dabigatran may be a barrier
The wholesale price of dabigatran, as quoted by Boehringer Ingelheim, is $6.75 per day; the retail price for a 30-day supply is approximately $235, according to drugstore.com, Walgreens, and Walmart). In comparison, a one-month supply of warfarin is about $15. Out-of-pocket costs for many patients will likely be high until dabigatran is added to insurers’ formularies. When costs for monitoring and hospitalizations or treatment for complications associated with warfarin are factored in, however, dabigatran is cost effective, a recent study indicates.7
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
Consider dabigatran, an oral anticoagulant that does not require monitoring, for the prevention of stroke and thromboembolism in patients with atrial fibrillation.1
STRENGTH OF RECOMMENDATION
B: Based on a single well-done randomized controlled trial (RCT).
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
ILLUSTRATIVE CASE
A 75-year-old man with persistent atrial fibrillation and diabetes comes to your office for a check of his international normalized ratio (INR). It has been hard to keep his INR within the normal range of 2 to 3 in recent months, and today is no different: The patient’s INR is 1.7, although he insists he has been compliant with his warfarin regimen and has had no change in diet or other medications. What other anticoagulation options can you offer him?
Patients with atrial fibrillation have a 3% to 8% annual risk of stroke.2 Both adjusted-dose warfarin and antiplatelet agents such as aspirin have been shown to be effective at reducing this risk, although warfarin is significantly more effective.3
Those who have atrial fibrillation and a previous history of thromboembolism or rheumatic mitral stenosis or more than one moderate risk factor (age ≥75 years, hypertension, heart failure, impaired left ventricular systolic function, or diabetes) have the highest stroke risk. The American College of Cardiology/American Heart Association Task Force/ European Society of Cardiology (ACC/AHA/ ESC) 2006 guidelines for the management of atrial fibrillation recommend chronic anticoagulation with an oral vitamin K antagonist, such as warfarin, for these high-risk patients.4
Warfarin therapy is challenging
We have all experienced the frustrations of maintaining our patients on warfarin at a therapeutic INR; the average patient is within this range only about 67% of the time, although this varies dramatically from patient to patient.5
Many of our patients have experienced the inconvenience and cost of repeated monitoring, as well as the morbidity associated with both major and minor bleeding related to warfarin use. And there are many potential interactions between warfarin and foods or other drugs.
Is the new oral anticoagulant a better bet?
There are anticoagulants that do not require monitoring (eg, enoxaparin), but few patients are willing to undergo daily subcutaneous injections, and the cost is often prohibitive. Now there is another alternative.
Dabigatran (Pradaxa), an oral direct thrombin inhibitor, was approved by the US Food and Drug Administration in October 2010 for the prevention of stroke and systemic embolism in patients with atrial fibrillation.6 Dabigatran is administered twice daily in a fixed dose. Because it has a relatively short half-life (12-17 hours), it does not require INR monitoring. Dabigatran has no known interactions with foods and minimal interactions with other medications. Its value as a warfarin alternative for patients with atrial fibrillation was addressed in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study detailed below.
STUDY SUMMARY: At higher dose, dabigatran prevents more strokes than warfarin
RE-LY included 18,113 patients from 951 facilities in 44 countries. To be eligible for the study, patients had to have atrial fibrillation documented on an electrocardiogram and at least one additional risk factor for stroke.
Participants were randomized into one of 3 groups: dabigatran 110 mg twice daily, dabigatran 150 mg twice daily (both administered in a blinded fashion), or warfarin (administered in an unblinded fashion and dosed to maintain an INR between 2 and 3). Baseline characteristics, such as age, sex, and CHADS2 (congestive heart failure, hypertension, age, diabetes, prior stroke) score, were similar across all 3 groups. The median duration of follow-up was 2 years, and complete follow-up occurred in 99.9% of participants.
The primary outcome of the study was stroke or systemic embolism. The primary safety outcome was major hemorrhage, defined as a reduction in hemoglobin of ≥2 g/dL, transfusion of ≥2 units of blood, or symptomatic bleeding in a critical area/organ. Other outcomes were death, myocardial infarction (MI), pulmonary embolism, transient ischemic attack, and hospitalization.
For the primary outcome of prevention of stroke or systemic embolism, the 150-mg dose of dabigatran was superior to warfarin (1.11% vs 1.69% per year, relative risk [RR], 0.66; 95% confidence interval [CI], 0.53-0.82; P<.001 for superiority). The major bleeding rates were similar for dabigatran 150 mg and warfarin, although major gastrointestinal bleeding rates were significantly higher with this dose of dabigatran compared with warfarin (TABLE). Minor bleeding was more common in the warfarin group (16.37% vs 14.84%; RR, 0.91; 95% CI, 0.85-0.97; P=.005).
The 110-mg dose of dabigatran (which is not available in the United States) was neither inferior nor superior to warfarin for the prevention of stroke or systemic embolism. This dose of dabigatran had a lower risk of major bleeding compared with warfarin.
TABLE
Dabigatran vs warfarin: A look at the evidence1
| Event | Incidence (%/y) | NNT/NNH with dabigatran instead of warfarin | Relative risk (95% CI) | P value | |
|---|---|---|---|---|---|
| Dabigatran (150 mg) | Warfarin | ||||
| Stroke or systemic embolism | 1.11 | 1.69 | NNT: 173 | 0.66 (0.53-0.82) | <.001* <.001 |
| Hemorrhagic stroke | 0.10 | 0.38 | NNT: 477 | 0.26 (0.14-0.49) | <.001 |
| MI | 0.74 | 0.53 | NNH: 477 | 1.38 (1.00-1.91) | .048 |
| Death from any cause | 3.64 | 4.13 | NS | 0.88 (0.77-1.00) | .051 |
| Major bleeding | 3.11 | 3.36 | NS | 0.93 (0.81-1.07) | .31 |
| Intracranial bleeding | 0.30 | 0.74 | NNT: 228 | 0.40 (0.27-0.60) | <.001 |
| GI bleeding | 1.51 | 1.02 | NNH: 205 | 1.50 (1.19-1.89) | <.001 |
| Life-threatening bleeding | 1.45 | 1.80 | NNT: 286 | 0.81 (0.66-0.99) | .04 |
| CI, confidence interval; GI, gastrointestinal; MI, myocardial infarction; NNH, number needed to harm; NNT, number needed to treat; NS, no significant difference. *P value for noninferiority; all other P values are for superiority. | |||||
Mortality rates are similar
Rates of death from any cause were similar among the 3 treatment groups. The rates of hemorrhagic stroke were lower in both dabigatran groups compared with the warfarin group, while rates of MI were lower in the warfarin group than in either of the dabigatran groups.
Dyspepsia was the only other adverse effect that was significantly more common among dabigatran users vs warfarin users. Rates of hepatotoxicity, which was a problem wiThearlier oral direct thrombin inhibitors, were similar for both drugs. Multiple subgroup analyses revealed no significant interaction between the treatment effect of dabigatran and variables such as sex, body mass index, creatinine clearance, CHADS2 score, aspirin use, or previous long-term use of a vitamin K antagonist.
WHAT’S NEW: This easier-to-use oral anticoagulant is a viable option
Dabigatran gives physicians and patients with atrial fibrillation an option that is more convenient than warfarin for stroke prevention. Its 150-mg dose is more effective in preventing stroke compared with warfarin, and comparable in terms of bleeding risk.
CAVEATS: Unknown long-term effects, potential for bias
The median follow-up in the RE-LY study was 2 years. Longer-term efficacy and safety data may differ from the initial results.
The trial was funded by Boehringer Ingelheim, the manufacturer of dabigatran (Pradaxa). However, study coordination, data management, and analysis were performed independently by the Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
Patients taking dabigatran received the medication in a blinded fashion, but the warfarin group could not be blinded because of the need for INR monitoring and dosage adjustments. To decrease potential bias, the outcome events were assessed by 2 independent investigators who were blinded to the treatment assignments.
CHALLENGES TO IMPLEMENTATION: Cost of dabigatran may be a barrier
The wholesale price of dabigatran, as quoted by Boehringer Ingelheim, is $6.75 per day; the retail price for a 30-day supply is approximately $235, according to drugstore.com, Walgreens, and Walmart). In comparison, a one-month supply of warfarin is about $15. Out-of-pocket costs for many patients will likely be high until dabigatran is added to insurers’ formularies. When costs for monitoring and hospitalizations or treatment for complications associated with warfarin are factored in, however, dabigatran is cost effective, a recent study indicates.7
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Connolly SJ, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Wolf PA, et al. Atrial fibrillation as an independent risk factor for stroke; the Framingham study. Stroke. 1991;22:983-988.
3. Hart RG, et al. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
4. Fuster V, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation–executive summary. J Am Coll Cardiol. 2006;48:854-906.
5. Rose AJ, et al. Warfarin for atrial fibrillation in community-based practice. J Thromb Haemost. 2008;6:1647-1654.
6. US Food and Drug Administration. FDA approves Pradaxa in people with atrial fibrillation. October 19, 2010.
7. Freeman JV, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1-11.
1. Connolly SJ, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Wolf PA, et al. Atrial fibrillation as an independent risk factor for stroke; the Framingham study. Stroke. 1991;22:983-988.
3. Hart RG, et al. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
4. Fuster V, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation–executive summary. J Am Coll Cardiol. 2006;48:854-906.
5. Rose AJ, et al. Warfarin for atrial fibrillation in community-based practice. J Thromb Haemost. 2008;6:1647-1654.
6. US Food and Drug Administration. FDA approves Pradaxa in people with atrial fibrillation. October 19, 2010.
7. Freeman JV, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1-11.
Copyright © 2011 The Family Physicians Inquiries Network.
All rights reserved.