User login
25-year-old woman • abdominal pain • urticarial rash • recent influenza immunization • Dx?
THE CASE
A 25-year-old woman presented to an infectious diseases (ID) physician with a 4-day history of symptoms following receipt of a quadrivalent influenza vaccine. Two hours after receiving the vaccine, the patient experienced abdominal pain. One hour later, she felt warm and developed diffuse urticaria and rigors. Because of her worsening condition, she presented to the emergency department, where she was given intravenous methylprednisolone 40 mg, ondansetron 8 mg, diphenhydramine 25 mg, and normal saline. Her urticarial rash resolved within 45 minutes, and she was discharged home.
Three days later, she sought additional medical care because of persistent chest tightness, new-onset bronchospasm, pleuritic chest pain, nausea, diarrhea, facial swelling, urticaria, and anorexia. The patient’s vital signs were within normal limits. The oropharynx lacked erythema or obstruction. The lungs were clear to auscultation bilaterally, and heart sounds were regular, with no ectopy or murmurs. Her abdomen was soft, nontender, and nondistended. The patient demonstrated dermatographism on her back.
Historically, the patient had received the influenza vaccine without difficulty. She tolerated latex but had concerns about egg allergy due to vomiting with egg-yolk exposure.
THE DIAGNOSIS
The ID physician, suspecting anaphylaxis and sustained allergic response to the influenza vaccine, arranged for immediate follow-up with an allergist. Multiple tests were done. A negative result on epicutaneous testing to egg was inconsistent with an immunoglobulin (Ig) E-mediated food allergy.
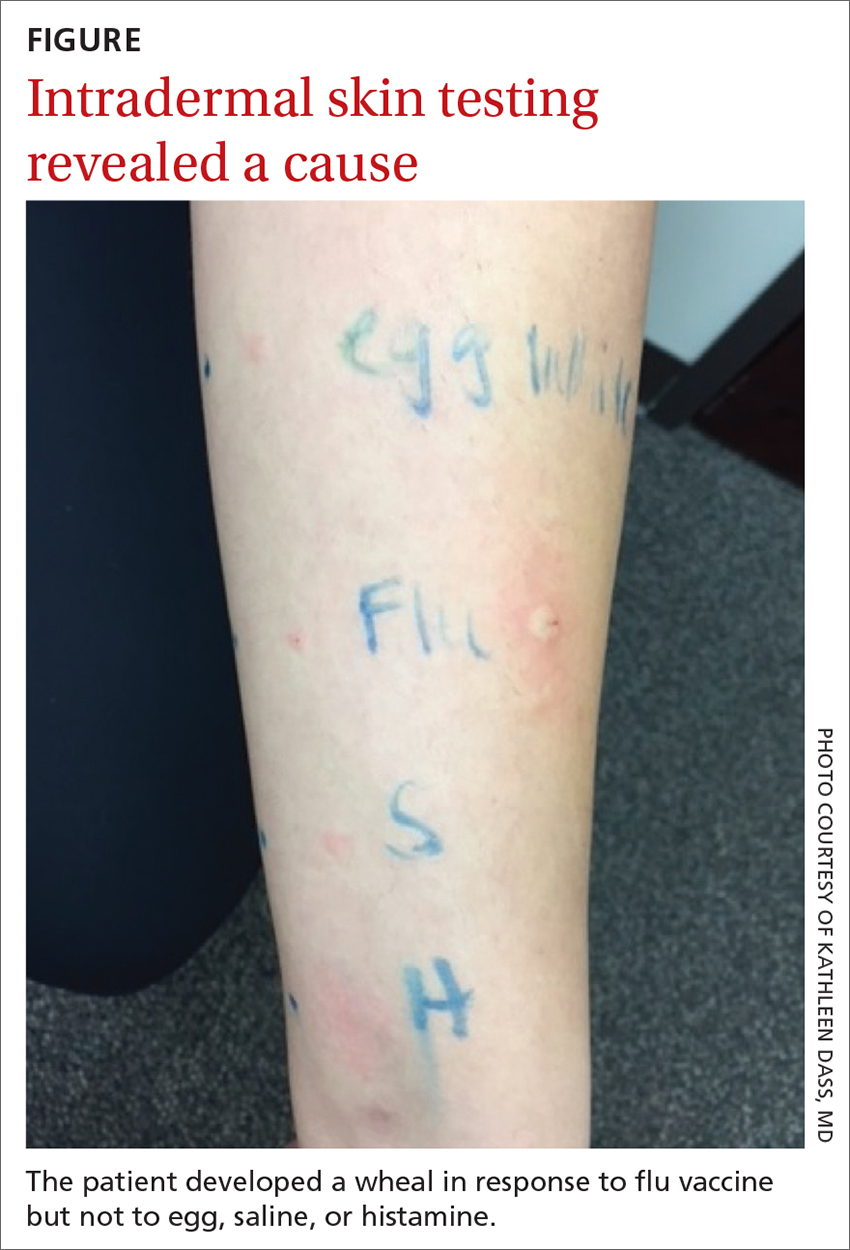
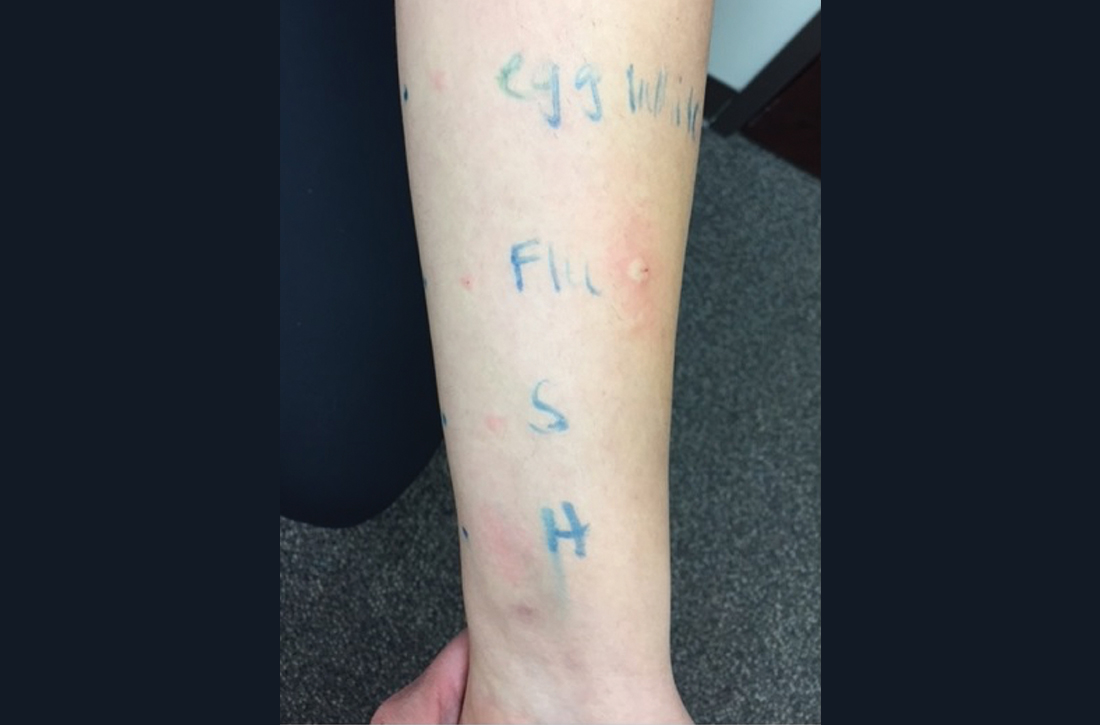
Intradermal testing with the flu vaccine (diluted 1:100) was subsequently performed with appropriate controls. A positive intradermal result is typically a wheal ≥ 5 mm larger than the control. The patient had a 5-mm/15-mm wheal-and-flare response to the flu vaccine, compared to a negative response to saline (FIGURE). (Since the vaccine did not contain gelatin, this was not tested.)
Based on the positive response to flu vaccine and negative response to egg, it was determined that the patient had experienced an anaphylactic reaction to the vaccine itself.
DISCUSSION
In adults, the most common adverse reactions to quadrivalent flu vaccine include pain, headache, and fatigue. IgE-mediated reactions to the influenza vaccine, especially anaphylactic reactions, are rare. A Vaccine Safety Datalink study found 10 cases of anaphylaxis after more than 7.4 million doses of inactivated flu vaccine were given, for a rate of 1.35 per 1 million doses.1
Continue to: Don't blame eggs
Don’t blame eggs. It was previously believed that reactions to the flu vaccine were due to egg allergies, because the vaccine may contain a tiny amount of ovalbumin, a protein found in egg. However, multiple studies have supported the safety of injectable influenza vaccine in patients with an egg allergy because the amount of ovalbumin contained in each dose is very low and thus not likely to evoke an allergic response.2,3
How and when to test for allergy. For patients who have a severe allergic reaction or anaphylaxis after immunization, immediate-type allergy skin testing should be performed by an allergist to establish whether the reaction was IgE mediated and to determine the causative agent.
It’s best to wait 4 to 6 weeks after an anaphylactic reaction before doing skin testing, as earlier testing can lead to false-negative results.4 The vaccine should first be tested by using the prick method. If this test is negative, an intradermal test with the vaccine diluted 1:100 should be performed with appropriate controls.5
Should the patient receive future vaccinations?
If skin testing is positive, there are several ways to proceed. A vaccine to which the patient has previously had an allergic reaction and positive skin test can still be administered, with caution.5 With emergency supplies, medication, and equipment immediately available, medical personnel can administer the influenza vaccine in titrated doses. If the full vaccine dose is normally a volume of 0.5 mL, the patient is first given 0.05 mL of a 1:10 dilution and then, at 15-minute intervals, given full-strength vaccine at doses of 0.05, 0.1, 0.15, and finally 0.2 mL, for a cumulative dose of 0.5 mL.5
Alternatively, the patient can forego the vaccination, although this decision has its own risks. In a patient who has previously had an anaphylactic reaction but has negative skin tests—meaning it is unlikely that the patient has IgE antibody to the vaccine—the vaccine can be administered and followed with an observation period of at least 30 minutes.5z Our patient was counseled on both options and decided to forego the vaccine.
THE TAKEAWAY
Anaphylaxis is a life-threatening allergic reaction requiring immediate treatment. Anaphylaxis after vaccine receipt is exceedingly rare.6 Most IgE-mediated allergic reactions post vaccination are attributed to added or residual substances in the vaccine, rather than the immunizing agent itself.6 While common local reactions and fever post vaccination do not contraindicate future vaccination, rare anaphylactic reactions need to be further evaluated, with a referral to an allergist to determine if the patient is, in fact, allergic to additive ingredients within the vaccine vs allergic to the vaccine itself.
CORRESPONDENCE
Kathleen Dass, MD, 24601 Coolidge Highway, Oak Park, MI 48237; kathleen.j.dass@gmail.com
1. Fluarix [package insert]. GlaxoSmithKline Biologicals. Dresden, Germany. 2016. Accessed November 9, 2021. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM220624.pdf
2. Webb L, Petersen M, Boden S, et al. Single-dose influenza vaccination of patients with egg allergy in a multicenter study. J Allergy Clin Immunol. 2011;128:218-219. doi: 10.1016/j.jaci.2011.02.013
3. Howe LE, Conlon ASC, Greenhawt MJ, et al. Safe administration of seasonal influenza vaccine to children with egg allergy of all severities. Ann Allergy Asthma Immunol. 2011;106:446-447. doi: 10.1016/j.anai.2011.01.024
4. Soetens F, Rose M, Fisher M. Timing of skin testing after a suspected anaphylactic reaction during anaesthesia. Acta Anaesthesiol Scand. 2012;56:1042-1046. doi: 10.1111/j.1399-6576.2011.02643.x
5. Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25-43. doi: 10.1016/j.jaci.2012.04.003
6. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878. doi: 10.1016/j.jaci.2015.07.048
THE CASE
A 25-year-old woman presented to an infectious diseases (ID) physician with a 4-day history of symptoms following receipt of a quadrivalent influenza vaccine. Two hours after receiving the vaccine, the patient experienced abdominal pain. One hour later, she felt warm and developed diffuse urticaria and rigors. Because of her worsening condition, she presented to the emergency department, where she was given intravenous methylprednisolone 40 mg, ondansetron 8 mg, diphenhydramine 25 mg, and normal saline. Her urticarial rash resolved within 45 minutes, and she was discharged home.
Three days later, she sought additional medical care because of persistent chest tightness, new-onset bronchospasm, pleuritic chest pain, nausea, diarrhea, facial swelling, urticaria, and anorexia. The patient’s vital signs were within normal limits. The oropharynx lacked erythema or obstruction. The lungs were clear to auscultation bilaterally, and heart sounds were regular, with no ectopy or murmurs. Her abdomen was soft, nontender, and nondistended. The patient demonstrated dermatographism on her back.
Historically, the patient had received the influenza vaccine without difficulty. She tolerated latex but had concerns about egg allergy due to vomiting with egg-yolk exposure.
THE DIAGNOSIS
The ID physician, suspecting anaphylaxis and sustained allergic response to the influenza vaccine, arranged for immediate follow-up with an allergist. Multiple tests were done. A negative result on epicutaneous testing to egg was inconsistent with an immunoglobulin (Ig) E-mediated food allergy.
Intradermal testing with the flu vaccine (diluted 1:100) was subsequently performed with appropriate controls. A positive intradermal result is typically a wheal ≥ 5 mm larger than the control. The patient had a 5-mm/15-mm wheal-and-flare response to the flu vaccine, compared to a negative response to saline (FIGURE). (Since the vaccine did not contain gelatin, this was not tested.)
Based on the positive response to flu vaccine and negative response to egg, it was determined that the patient had experienced an anaphylactic reaction to the vaccine itself.
DISCUSSION
In adults, the most common adverse reactions to quadrivalent flu vaccine include pain, headache, and fatigue. IgE-mediated reactions to the influenza vaccine, especially anaphylactic reactions, are rare. A Vaccine Safety Datalink study found 10 cases of anaphylaxis after more than 7.4 million doses of inactivated flu vaccine were given, for a rate of 1.35 per 1 million doses.1
Continue to: Don't blame eggs
Don’t blame eggs. It was previously believed that reactions to the flu vaccine were due to egg allergies, because the vaccine may contain a tiny amount of ovalbumin, a protein found in egg. However, multiple studies have supported the safety of injectable influenza vaccine in patients with an egg allergy because the amount of ovalbumin contained in each dose is very low and thus not likely to evoke an allergic response.2,3
How and when to test for allergy. For patients who have a severe allergic reaction or anaphylaxis after immunization, immediate-type allergy skin testing should be performed by an allergist to establish whether the reaction was IgE mediated and to determine the causative agent.
It’s best to wait 4 to 6 weeks after an anaphylactic reaction before doing skin testing, as earlier testing can lead to false-negative results.4 The vaccine should first be tested by using the prick method. If this test is negative, an intradermal test with the vaccine diluted 1:100 should be performed with appropriate controls.5
Should the patient receive future vaccinations?
If skin testing is positive, there are several ways to proceed. A vaccine to which the patient has previously had an allergic reaction and positive skin test can still be administered, with caution.5 With emergency supplies, medication, and equipment immediately available, medical personnel can administer the influenza vaccine in titrated doses. If the full vaccine dose is normally a volume of 0.5 mL, the patient is first given 0.05 mL of a 1:10 dilution and then, at 15-minute intervals, given full-strength vaccine at doses of 0.05, 0.1, 0.15, and finally 0.2 mL, for a cumulative dose of 0.5 mL.5
Alternatively, the patient can forego the vaccination, although this decision has its own risks. In a patient who has previously had an anaphylactic reaction but has negative skin tests—meaning it is unlikely that the patient has IgE antibody to the vaccine—the vaccine can be administered and followed with an observation period of at least 30 minutes.5z Our patient was counseled on both options and decided to forego the vaccine.
THE TAKEAWAY
Anaphylaxis is a life-threatening allergic reaction requiring immediate treatment. Anaphylaxis after vaccine receipt is exceedingly rare.6 Most IgE-mediated allergic reactions post vaccination are attributed to added or residual substances in the vaccine, rather than the immunizing agent itself.6 While common local reactions and fever post vaccination do not contraindicate future vaccination, rare anaphylactic reactions need to be further evaluated, with a referral to an allergist to determine if the patient is, in fact, allergic to additive ingredients within the vaccine vs allergic to the vaccine itself.
CORRESPONDENCE
Kathleen Dass, MD, 24601 Coolidge Highway, Oak Park, MI 48237; kathleen.j.dass@gmail.com
THE CASE
A 25-year-old woman presented to an infectious diseases (ID) physician with a 4-day history of symptoms following receipt of a quadrivalent influenza vaccine. Two hours after receiving the vaccine, the patient experienced abdominal pain. One hour later, she felt warm and developed diffuse urticaria and rigors. Because of her worsening condition, she presented to the emergency department, where she was given intravenous methylprednisolone 40 mg, ondansetron 8 mg, diphenhydramine 25 mg, and normal saline. Her urticarial rash resolved within 45 minutes, and she was discharged home.
Three days later, she sought additional medical care because of persistent chest tightness, new-onset bronchospasm, pleuritic chest pain, nausea, diarrhea, facial swelling, urticaria, and anorexia. The patient’s vital signs were within normal limits. The oropharynx lacked erythema or obstruction. The lungs were clear to auscultation bilaterally, and heart sounds were regular, with no ectopy or murmurs. Her abdomen was soft, nontender, and nondistended. The patient demonstrated dermatographism on her back.
Historically, the patient had received the influenza vaccine without difficulty. She tolerated latex but had concerns about egg allergy due to vomiting with egg-yolk exposure.
THE DIAGNOSIS
The ID physician, suspecting anaphylaxis and sustained allergic response to the influenza vaccine, arranged for immediate follow-up with an allergist. Multiple tests were done. A negative result on epicutaneous testing to egg was inconsistent with an immunoglobulin (Ig) E-mediated food allergy.
Intradermal testing with the flu vaccine (diluted 1:100) was subsequently performed with appropriate controls. A positive intradermal result is typically a wheal ≥ 5 mm larger than the control. The patient had a 5-mm/15-mm wheal-and-flare response to the flu vaccine, compared to a negative response to saline (FIGURE). (Since the vaccine did not contain gelatin, this was not tested.)
Based on the positive response to flu vaccine and negative response to egg, it was determined that the patient had experienced an anaphylactic reaction to the vaccine itself.
DISCUSSION
In adults, the most common adverse reactions to quadrivalent flu vaccine include pain, headache, and fatigue. IgE-mediated reactions to the influenza vaccine, especially anaphylactic reactions, are rare. A Vaccine Safety Datalink study found 10 cases of anaphylaxis after more than 7.4 million doses of inactivated flu vaccine were given, for a rate of 1.35 per 1 million doses.1
Continue to: Don't blame eggs
Don’t blame eggs. It was previously believed that reactions to the flu vaccine were due to egg allergies, because the vaccine may contain a tiny amount of ovalbumin, a protein found in egg. However, multiple studies have supported the safety of injectable influenza vaccine in patients with an egg allergy because the amount of ovalbumin contained in each dose is very low and thus not likely to evoke an allergic response.2,3
How and when to test for allergy. For patients who have a severe allergic reaction or anaphylaxis after immunization, immediate-type allergy skin testing should be performed by an allergist to establish whether the reaction was IgE mediated and to determine the causative agent.
It’s best to wait 4 to 6 weeks after an anaphylactic reaction before doing skin testing, as earlier testing can lead to false-negative results.4 The vaccine should first be tested by using the prick method. If this test is negative, an intradermal test with the vaccine diluted 1:100 should be performed with appropriate controls.5
Should the patient receive future vaccinations?
If skin testing is positive, there are several ways to proceed. A vaccine to which the patient has previously had an allergic reaction and positive skin test can still be administered, with caution.5 With emergency supplies, medication, and equipment immediately available, medical personnel can administer the influenza vaccine in titrated doses. If the full vaccine dose is normally a volume of 0.5 mL, the patient is first given 0.05 mL of a 1:10 dilution and then, at 15-minute intervals, given full-strength vaccine at doses of 0.05, 0.1, 0.15, and finally 0.2 mL, for a cumulative dose of 0.5 mL.5
Alternatively, the patient can forego the vaccination, although this decision has its own risks. In a patient who has previously had an anaphylactic reaction but has negative skin tests—meaning it is unlikely that the patient has IgE antibody to the vaccine—the vaccine can be administered and followed with an observation period of at least 30 minutes.5z Our patient was counseled on both options and decided to forego the vaccine.
THE TAKEAWAY
Anaphylaxis is a life-threatening allergic reaction requiring immediate treatment. Anaphylaxis after vaccine receipt is exceedingly rare.6 Most IgE-mediated allergic reactions post vaccination are attributed to added or residual substances in the vaccine, rather than the immunizing agent itself.6 While common local reactions and fever post vaccination do not contraindicate future vaccination, rare anaphylactic reactions need to be further evaluated, with a referral to an allergist to determine if the patient is, in fact, allergic to additive ingredients within the vaccine vs allergic to the vaccine itself.
CORRESPONDENCE
Kathleen Dass, MD, 24601 Coolidge Highway, Oak Park, MI 48237; kathleen.j.dass@gmail.com
1. Fluarix [package insert]. GlaxoSmithKline Biologicals. Dresden, Germany. 2016. Accessed November 9, 2021. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM220624.pdf
2. Webb L, Petersen M, Boden S, et al. Single-dose influenza vaccination of patients with egg allergy in a multicenter study. J Allergy Clin Immunol. 2011;128:218-219. doi: 10.1016/j.jaci.2011.02.013
3. Howe LE, Conlon ASC, Greenhawt MJ, et al. Safe administration of seasonal influenza vaccine to children with egg allergy of all severities. Ann Allergy Asthma Immunol. 2011;106:446-447. doi: 10.1016/j.anai.2011.01.024
4. Soetens F, Rose M, Fisher M. Timing of skin testing after a suspected anaphylactic reaction during anaesthesia. Acta Anaesthesiol Scand. 2012;56:1042-1046. doi: 10.1111/j.1399-6576.2011.02643.x
5. Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25-43. doi: 10.1016/j.jaci.2012.04.003
6. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878. doi: 10.1016/j.jaci.2015.07.048
1. Fluarix [package insert]. GlaxoSmithKline Biologicals. Dresden, Germany. 2016. Accessed November 9, 2021. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM220624.pdf
2. Webb L, Petersen M, Boden S, et al. Single-dose influenza vaccination of patients with egg allergy in a multicenter study. J Allergy Clin Immunol. 2011;128:218-219. doi: 10.1016/j.jaci.2011.02.013
3. Howe LE, Conlon ASC, Greenhawt MJ, et al. Safe administration of seasonal influenza vaccine to children with egg allergy of all severities. Ann Allergy Asthma Immunol. 2011;106:446-447. doi: 10.1016/j.anai.2011.01.024
4. Soetens F, Rose M, Fisher M. Timing of skin testing after a suspected anaphylactic reaction during anaesthesia. Acta Anaesthesiol Scand. 2012;56:1042-1046. doi: 10.1111/j.1399-6576.2011.02643.x
5. Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25-43. doi: 10.1016/j.jaci.2012.04.003
6. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878. doi: 10.1016/j.jaci.2015.07.048
Acute Severe Urticaria From Minocycline
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
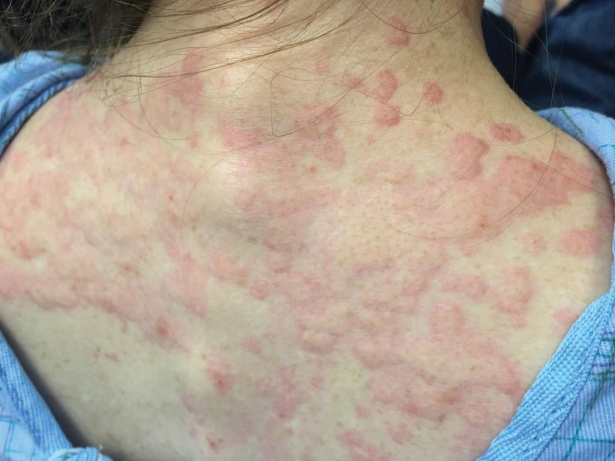
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
Practice Points
- Minocycline is a commonly prescribed long-term treatment for acne vulgaris.
- Minocycline-induced acute urticaria and anaphylaxis are rare adverse events.