User login
Asboe-Hansen Sign in Toxic Epidermal Necrolysis
To the Editor:
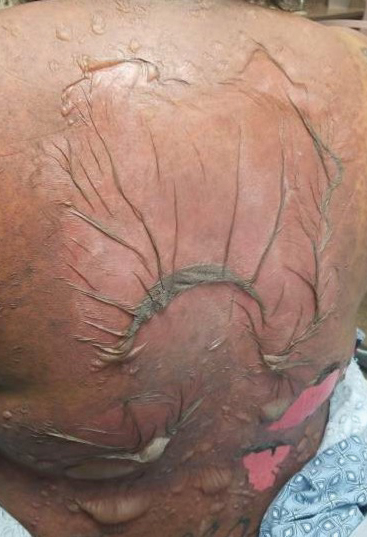
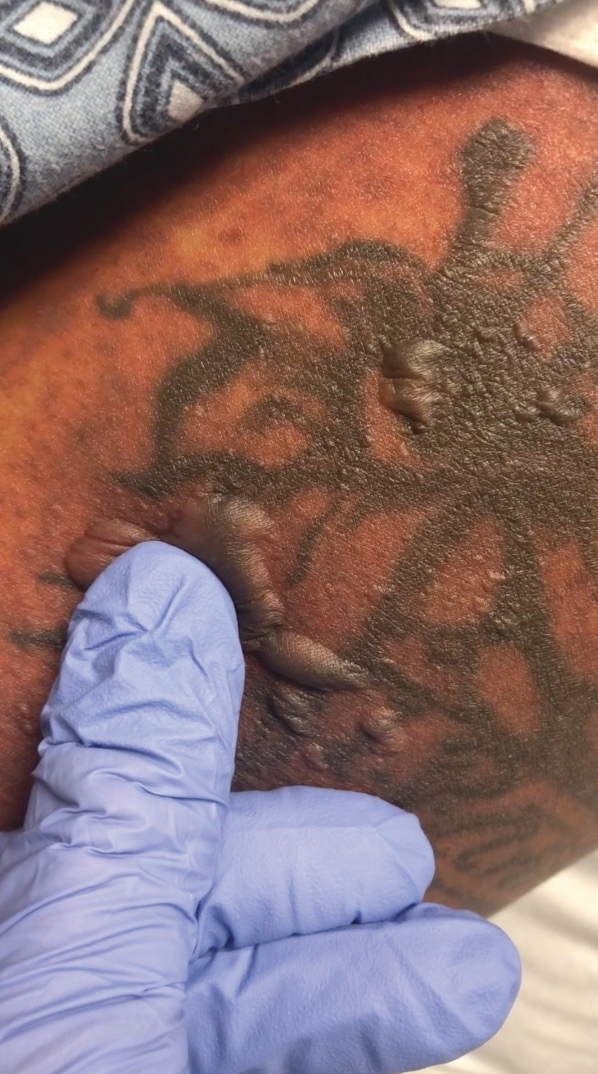
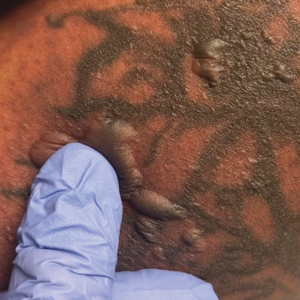
A 25-year-old woman with no notable medical history was admitted to the hospital for suspected Stevens-Johnson syndrome (SJS). The patient was started on amoxicillin 7 days prior to the skin eruption for prophylaxis before removal of an intrauterine device. On the day of admission, she reported ocular discomfort, dysphagia, and dysuria. She developed erythema of the conjunctivae, face, chest, and proximal upper extremities, as well as erosions of the vermilion lips. She presented to the local emergency department and was transferred to our institution for urgent dermatologic consultation. On physical examination by the dermatology service, the patient had erythematous macules coalescing into patches with overlying flaccid bullae, some denuded, involving the face, chest, abdomen, back (Figure 1), bilateral upper extremities, bilateral thighs, and labia majora and minora. Additionally, she had conjunctivitis, superficial erosions of the vermilion lips, and tense bullae of the palms and soles. On palpation of the flaccid bullae, the Asboe-Hansen sign was elicited (Figure 2 and video). A shave biopsy of the newly elicited bullae was performed. Pathology showed a subepidermal bulla with confluent necrosis of the epidermis and minimal inflammatory infiltrate. An additional shave biopsy of perilesional skin was obtained for direct immunofluorescence, which was negative for IgG, C3, IgM, and IgA. Based on the clinical presentation involving more than 30% of the patient’s body surface area (BSA) and the pathology findings, a diagnosis of toxic epidermal necrolysis (TEN) was made. The patient remained in the intensive care unit with a multidisciplinary team consisting of dermatology, ophthalmology, gynecology, gastroenterology, and the general surgery burn group. Following treatment with intravenous immunoglobulin, systemic corticosteroids, and aggressive wound care, the patient made a full recovery.
applied to an intact bulla.

Toxic epidermal necrolysis is a rare, acute, life-threatening mucocutaneous disease within a spectrum of adverse cutaneous drug reactions. The estimated worldwide incidence of TEN is 0.4 to 1.9 per million individuals annually.1 Toxic epidermal necrolysis is clinically characterized by diffuse exfoliation of the skin and mucosae with flaccid bullae. These clinical features are a consequence of extensive keratinocyte death, leading to dermoepidermal junction dissociation. Commonly, there is a prodrome of fever, pharyngitis, and painful skin preceding the diffuse erythema and sloughing of skin and mucous membranes. Lesions typically first appear on the trunk and then follow a centrifugal spread, often sparing the distal aspects of the arms and legs.
Toxic epidermal necrolysis is part of a continuous spectrum with SJS. Less than 10% BSA involvement is considered SJS, 10% to 30% BSA involvement is SJS/TEN overlap, and more than 30% BSA detachment is TEN. Stevens-Johnson syndrome can progress to TEN. In TEN, the distribution of cutaneous lesions is more confluent, and mucosal involvement is more severe.2 The differential diagnosis may include staphylococcal scalded skin syndrome, drug-induced linear IgA bullous dermatosis, severe acute graft-vs-host disease, drug reaction with eosinophilia and systemic symptoms, and invasive fungal dermatitis. An accurate diagnosis of TEN is imperative, as the management and morbidity of these diseases are vastly different. Toxic epidermal necrolysis has an estimated mortality rate of 25% to 30%, with sepsis leading to multiorgan failure being the most common cause of death.3
Although the pathophysiology of TEN has yet to be fully elucidated, it is thought to be a T cell–mediated process with CD8+ cells acting as the primary means of keratinocyte death. An estimated 80% to 95% of cases are due to drug reactions.3 The medications that are most commonly associated with TEN include allopurinol, antibiotics, nonsteroidal anti-inflammatory drugs, and anticonvulsants. Symptoms typically begin 7 to 21 days after starting the drug. Less commonly, Mycoplasma pneumoniae, dengue virus, cytomegalovirus, and contrast medium have been reported as inciting factors for TEN.2
The diagnosis of TEN is established by correlating clinical features with a histopathologic examination obtained from a lesional skin biopsy. The classic cutaneous features of TEN begin as erythematous, flesh-colored, dusky to violaceous macules and/or morbilliform or targetoid lesions. These early lesions have the tendency to coalesce. The cutaneous findings will eventually progress into flaccid bullae, diffuse epidermal sloughing, and full-thickness skin necrosis.2,3 The evolution of skin lesions may be rapid or may take several days to develop. On palpation, the Nikolsky (lateral shearing of epidermis with minimal pressure) and Asboe-Hansen sign will be positive in patients with SJS/TEN, demonstrating that the associated blisters are flaccid and may be displaced peripherally.4 For an accurate diagnosis, the biopsy must contain full-thickness epidermis. It is imperative to choose a biopsy site from an acute blister, as old lesions of other diseases, such as erythema multiforme, will eventually become necrotic and mimic the histopathologic appearance of SJS/TEN, potentially leading to an incorrect diagnosis.4 Full-thickness epidermal necrosis has a high sensitivity but low specificity for TEN.3 The histologic features of TEN vary depending on the stage of the disease. Classic histologic findings include satellite necrosis of keratinocytes followed by full-thickness necrosis of keratinocytes and perivascular lymphoid infiltrates. The stratum corneum retains its original structure.4
The Asboe-Hansen sign, also known as the bulla spread sign, was originally described in 1960 as a diagnostic sign for pemphigus vulgaris.5 A positive Asboe-Hansen sign demonstrates the ability to enlarge a bulla in the lateral direction by applying perpendicular mechanical pressure to the roof of an intact bulla. The bulla is extended to adjacent nonblistered skin.6 A positive sign demonstrates decreased adhesion between keratinocytes or between the basal epidermal cells and the dermal connective tissue.5 In addition to pemphigus vulgaris, the Asboe-Hansen sign may be positive in TEN and SJS, as well as other diseases affecting the dermoepidermal junction including pemphigus foliaceus, pemphigus vegetans, and bullous pemphigoid. Asboe-Hansen5 made the argument that a fresh bulla should be biopsied if histopathologic diagnosis is necessary, as older bullae may exhibit epithelial cell regeneration and disturb an accurate diagnosis.
Accurate and early diagnosis of TEN is imperative, as prognosis is strongly correlated with the speed at which the offending drug is discontinued and appropriate medical treatment is initiated. Prompt withdrawal of the offending drug has been reported to reduce the risk for morbidity by 30% per day.7 Although classically associated with the pemphigus group of diseases, the Asboe-Hansen sign is of diagnostic value to the pathologist in diagnosing TEN by reproducing the same microscopic appearance of a fresh spontaneous blister. Due to the notable morbidity and mortality in SJS and TEN, the Asboe-Hansen sign should be attempted for the site of a lesional biopsy, as an accurate diagnosis relies on clinicopathologic correlation.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Frech LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. New York, NY: Elsevier; 2012:332-347.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187.e1–187.e16.
- Elston D, Stratman E, Miller S. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
- Asboe-Hansen G. Blister-spread induced by finger-pressure, a diagnostic sign in pemphigus. J Invest Dermatol. 1960;34:5-9.
- Ganapati S. Eponymous dermatological signs in bullous dermatoses. Indian J Dermatol. 2014;59:21-23.
- Garcia-Doval I, Lecleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323-327.
To the Editor:
A 25-year-old woman with no notable medical history was admitted to the hospital for suspected Stevens-Johnson syndrome (SJS). The patient was started on amoxicillin 7 days prior to the skin eruption for prophylaxis before removal of an intrauterine device. On the day of admission, she reported ocular discomfort, dysphagia, and dysuria. She developed erythema of the conjunctivae, face, chest, and proximal upper extremities, as well as erosions of the vermilion lips. She presented to the local emergency department and was transferred to our institution for urgent dermatologic consultation. On physical examination by the dermatology service, the patient had erythematous macules coalescing into patches with overlying flaccid bullae, some denuded, involving the face, chest, abdomen, back (Figure 1), bilateral upper extremities, bilateral thighs, and labia majora and minora. Additionally, she had conjunctivitis, superficial erosions of the vermilion lips, and tense bullae of the palms and soles. On palpation of the flaccid bullae, the Asboe-Hansen sign was elicited (Figure 2 and video). A shave biopsy of the newly elicited bullae was performed. Pathology showed a subepidermal bulla with confluent necrosis of the epidermis and minimal inflammatory infiltrate. An additional shave biopsy of perilesional skin was obtained for direct immunofluorescence, which was negative for IgG, C3, IgM, and IgA. Based on the clinical presentation involving more than 30% of the patient’s body surface area (BSA) and the pathology findings, a diagnosis of toxic epidermal necrolysis (TEN) was made. The patient remained in the intensive care unit with a multidisciplinary team consisting of dermatology, ophthalmology, gynecology, gastroenterology, and the general surgery burn group. Following treatment with intravenous immunoglobulin, systemic corticosteroids, and aggressive wound care, the patient made a full recovery.
applied to an intact bulla.

Toxic epidermal necrolysis is a rare, acute, life-threatening mucocutaneous disease within a spectrum of adverse cutaneous drug reactions. The estimated worldwide incidence of TEN is 0.4 to 1.9 per million individuals annually.1 Toxic epidermal necrolysis is clinically characterized by diffuse exfoliation of the skin and mucosae with flaccid bullae. These clinical features are a consequence of extensive keratinocyte death, leading to dermoepidermal junction dissociation. Commonly, there is a prodrome of fever, pharyngitis, and painful skin preceding the diffuse erythema and sloughing of skin and mucous membranes. Lesions typically first appear on the trunk and then follow a centrifugal spread, often sparing the distal aspects of the arms and legs.
Toxic epidermal necrolysis is part of a continuous spectrum with SJS. Less than 10% BSA involvement is considered SJS, 10% to 30% BSA involvement is SJS/TEN overlap, and more than 30% BSA detachment is TEN. Stevens-Johnson syndrome can progress to TEN. In TEN, the distribution of cutaneous lesions is more confluent, and mucosal involvement is more severe.2 The differential diagnosis may include staphylococcal scalded skin syndrome, drug-induced linear IgA bullous dermatosis, severe acute graft-vs-host disease, drug reaction with eosinophilia and systemic symptoms, and invasive fungal dermatitis. An accurate diagnosis of TEN is imperative, as the management and morbidity of these diseases are vastly different. Toxic epidermal necrolysis has an estimated mortality rate of 25% to 30%, with sepsis leading to multiorgan failure being the most common cause of death.3
Although the pathophysiology of TEN has yet to be fully elucidated, it is thought to be a T cell–mediated process with CD8+ cells acting as the primary means of keratinocyte death. An estimated 80% to 95% of cases are due to drug reactions.3 The medications that are most commonly associated with TEN include allopurinol, antibiotics, nonsteroidal anti-inflammatory drugs, and anticonvulsants. Symptoms typically begin 7 to 21 days after starting the drug. Less commonly, Mycoplasma pneumoniae, dengue virus, cytomegalovirus, and contrast medium have been reported as inciting factors for TEN.2
The diagnosis of TEN is established by correlating clinical features with a histopathologic examination obtained from a lesional skin biopsy. The classic cutaneous features of TEN begin as erythematous, flesh-colored, dusky to violaceous macules and/or morbilliform or targetoid lesions. These early lesions have the tendency to coalesce. The cutaneous findings will eventually progress into flaccid bullae, diffuse epidermal sloughing, and full-thickness skin necrosis.2,3 The evolution of skin lesions may be rapid or may take several days to develop. On palpation, the Nikolsky (lateral shearing of epidermis with minimal pressure) and Asboe-Hansen sign will be positive in patients with SJS/TEN, demonstrating that the associated blisters are flaccid and may be displaced peripherally.4 For an accurate diagnosis, the biopsy must contain full-thickness epidermis. It is imperative to choose a biopsy site from an acute blister, as old lesions of other diseases, such as erythema multiforme, will eventually become necrotic and mimic the histopathologic appearance of SJS/TEN, potentially leading to an incorrect diagnosis.4 Full-thickness epidermal necrosis has a high sensitivity but low specificity for TEN.3 The histologic features of TEN vary depending on the stage of the disease. Classic histologic findings include satellite necrosis of keratinocytes followed by full-thickness necrosis of keratinocytes and perivascular lymphoid infiltrates. The stratum corneum retains its original structure.4
The Asboe-Hansen sign, also known as the bulla spread sign, was originally described in 1960 as a diagnostic sign for pemphigus vulgaris.5 A positive Asboe-Hansen sign demonstrates the ability to enlarge a bulla in the lateral direction by applying perpendicular mechanical pressure to the roof of an intact bulla. The bulla is extended to adjacent nonblistered skin.6 A positive sign demonstrates decreased adhesion between keratinocytes or between the basal epidermal cells and the dermal connective tissue.5 In addition to pemphigus vulgaris, the Asboe-Hansen sign may be positive in TEN and SJS, as well as other diseases affecting the dermoepidermal junction including pemphigus foliaceus, pemphigus vegetans, and bullous pemphigoid. Asboe-Hansen5 made the argument that a fresh bulla should be biopsied if histopathologic diagnosis is necessary, as older bullae may exhibit epithelial cell regeneration and disturb an accurate diagnosis.
Accurate and early diagnosis of TEN is imperative, as prognosis is strongly correlated with the speed at which the offending drug is discontinued and appropriate medical treatment is initiated. Prompt withdrawal of the offending drug has been reported to reduce the risk for morbidity by 30% per day.7 Although classically associated with the pemphigus group of diseases, the Asboe-Hansen sign is of diagnostic value to the pathologist in diagnosing TEN by reproducing the same microscopic appearance of a fresh spontaneous blister. Due to the notable morbidity and mortality in SJS and TEN, the Asboe-Hansen sign should be attempted for the site of a lesional biopsy, as an accurate diagnosis relies on clinicopathologic correlation.
To the Editor:
A 25-year-old woman with no notable medical history was admitted to the hospital for suspected Stevens-Johnson syndrome (SJS). The patient was started on amoxicillin 7 days prior to the skin eruption for prophylaxis before removal of an intrauterine device. On the day of admission, she reported ocular discomfort, dysphagia, and dysuria. She developed erythema of the conjunctivae, face, chest, and proximal upper extremities, as well as erosions of the vermilion lips. She presented to the local emergency department and was transferred to our institution for urgent dermatologic consultation. On physical examination by the dermatology service, the patient had erythematous macules coalescing into patches with overlying flaccid bullae, some denuded, involving the face, chest, abdomen, back (Figure 1), bilateral upper extremities, bilateral thighs, and labia majora and minora. Additionally, she had conjunctivitis, superficial erosions of the vermilion lips, and tense bullae of the palms and soles. On palpation of the flaccid bullae, the Asboe-Hansen sign was elicited (Figure 2 and video). A shave biopsy of the newly elicited bullae was performed. Pathology showed a subepidermal bulla with confluent necrosis of the epidermis and minimal inflammatory infiltrate. An additional shave biopsy of perilesional skin was obtained for direct immunofluorescence, which was negative for IgG, C3, IgM, and IgA. Based on the clinical presentation involving more than 30% of the patient’s body surface area (BSA) and the pathology findings, a diagnosis of toxic epidermal necrolysis (TEN) was made. The patient remained in the intensive care unit with a multidisciplinary team consisting of dermatology, ophthalmology, gynecology, gastroenterology, and the general surgery burn group. Following treatment with intravenous immunoglobulin, systemic corticosteroids, and aggressive wound care, the patient made a full recovery.
applied to an intact bulla.

Toxic epidermal necrolysis is a rare, acute, life-threatening mucocutaneous disease within a spectrum of adverse cutaneous drug reactions. The estimated worldwide incidence of TEN is 0.4 to 1.9 per million individuals annually.1 Toxic epidermal necrolysis is clinically characterized by diffuse exfoliation of the skin and mucosae with flaccid bullae. These clinical features are a consequence of extensive keratinocyte death, leading to dermoepidermal junction dissociation. Commonly, there is a prodrome of fever, pharyngitis, and painful skin preceding the diffuse erythema and sloughing of skin and mucous membranes. Lesions typically first appear on the trunk and then follow a centrifugal spread, often sparing the distal aspects of the arms and legs.
Toxic epidermal necrolysis is part of a continuous spectrum with SJS. Less than 10% BSA involvement is considered SJS, 10% to 30% BSA involvement is SJS/TEN overlap, and more than 30% BSA detachment is TEN. Stevens-Johnson syndrome can progress to TEN. In TEN, the distribution of cutaneous lesions is more confluent, and mucosal involvement is more severe.2 The differential diagnosis may include staphylococcal scalded skin syndrome, drug-induced linear IgA bullous dermatosis, severe acute graft-vs-host disease, drug reaction with eosinophilia and systemic symptoms, and invasive fungal dermatitis. An accurate diagnosis of TEN is imperative, as the management and morbidity of these diseases are vastly different. Toxic epidermal necrolysis has an estimated mortality rate of 25% to 30%, with sepsis leading to multiorgan failure being the most common cause of death.3
Although the pathophysiology of TEN has yet to be fully elucidated, it is thought to be a T cell–mediated process with CD8+ cells acting as the primary means of keratinocyte death. An estimated 80% to 95% of cases are due to drug reactions.3 The medications that are most commonly associated with TEN include allopurinol, antibiotics, nonsteroidal anti-inflammatory drugs, and anticonvulsants. Symptoms typically begin 7 to 21 days after starting the drug. Less commonly, Mycoplasma pneumoniae, dengue virus, cytomegalovirus, and contrast medium have been reported as inciting factors for TEN.2
The diagnosis of TEN is established by correlating clinical features with a histopathologic examination obtained from a lesional skin biopsy. The classic cutaneous features of TEN begin as erythematous, flesh-colored, dusky to violaceous macules and/or morbilliform or targetoid lesions. These early lesions have the tendency to coalesce. The cutaneous findings will eventually progress into flaccid bullae, diffuse epidermal sloughing, and full-thickness skin necrosis.2,3 The evolution of skin lesions may be rapid or may take several days to develop. On palpation, the Nikolsky (lateral shearing of epidermis with minimal pressure) and Asboe-Hansen sign will be positive in patients with SJS/TEN, demonstrating that the associated blisters are flaccid and may be displaced peripherally.4 For an accurate diagnosis, the biopsy must contain full-thickness epidermis. It is imperative to choose a biopsy site from an acute blister, as old lesions of other diseases, such as erythema multiforme, will eventually become necrotic and mimic the histopathologic appearance of SJS/TEN, potentially leading to an incorrect diagnosis.4 Full-thickness epidermal necrosis has a high sensitivity but low specificity for TEN.3 The histologic features of TEN vary depending on the stage of the disease. Classic histologic findings include satellite necrosis of keratinocytes followed by full-thickness necrosis of keratinocytes and perivascular lymphoid infiltrates. The stratum corneum retains its original structure.4
The Asboe-Hansen sign, also known as the bulla spread sign, was originally described in 1960 as a diagnostic sign for pemphigus vulgaris.5 A positive Asboe-Hansen sign demonstrates the ability to enlarge a bulla in the lateral direction by applying perpendicular mechanical pressure to the roof of an intact bulla. The bulla is extended to adjacent nonblistered skin.6 A positive sign demonstrates decreased adhesion between keratinocytes or between the basal epidermal cells and the dermal connective tissue.5 In addition to pemphigus vulgaris, the Asboe-Hansen sign may be positive in TEN and SJS, as well as other diseases affecting the dermoepidermal junction including pemphigus foliaceus, pemphigus vegetans, and bullous pemphigoid. Asboe-Hansen5 made the argument that a fresh bulla should be biopsied if histopathologic diagnosis is necessary, as older bullae may exhibit epithelial cell regeneration and disturb an accurate diagnosis.
Accurate and early diagnosis of TEN is imperative, as prognosis is strongly correlated with the speed at which the offending drug is discontinued and appropriate medical treatment is initiated. Prompt withdrawal of the offending drug has been reported to reduce the risk for morbidity by 30% per day.7 Although classically associated with the pemphigus group of diseases, the Asboe-Hansen sign is of diagnostic value to the pathologist in diagnosing TEN by reproducing the same microscopic appearance of a fresh spontaneous blister. Due to the notable morbidity and mortality in SJS and TEN, the Asboe-Hansen sign should be attempted for the site of a lesional biopsy, as an accurate diagnosis relies on clinicopathologic correlation.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Frech LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. New York, NY: Elsevier; 2012:332-347.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187.e1–187.e16.
- Elston D, Stratman E, Miller S. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
- Asboe-Hansen G. Blister-spread induced by finger-pressure, a diagnostic sign in pemphigus. J Invest Dermatol. 1960;34:5-9.
- Ganapati S. Eponymous dermatological signs in bullous dermatoses. Indian J Dermatol. 2014;59:21-23.
- Garcia-Doval I, Lecleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323-327.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Frech LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. New York, NY: Elsevier; 2012:332-347.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187.e1–187.e16.
- Elston D, Stratman E, Miller S. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
- Asboe-Hansen G. Blister-spread induced by finger-pressure, a diagnostic sign in pemphigus. J Invest Dermatol. 1960;34:5-9.
- Ganapati S. Eponymous dermatological signs in bullous dermatoses. Indian J Dermatol. 2014;59:21-23.
- Garcia-Doval I, Lecleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323-327.
Practice Points
- Asboe-Hansen sign is a useful clinical tool for diagnosing toxic epidermal necrolysis (TEN).
- Asboe-Hansen sign can be employed to generate a fresh bulla for lesional skin biopsy in the evaluation of TEN.
A Case of Leprosy in Central Florida
Case Report
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
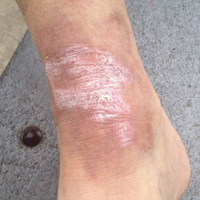
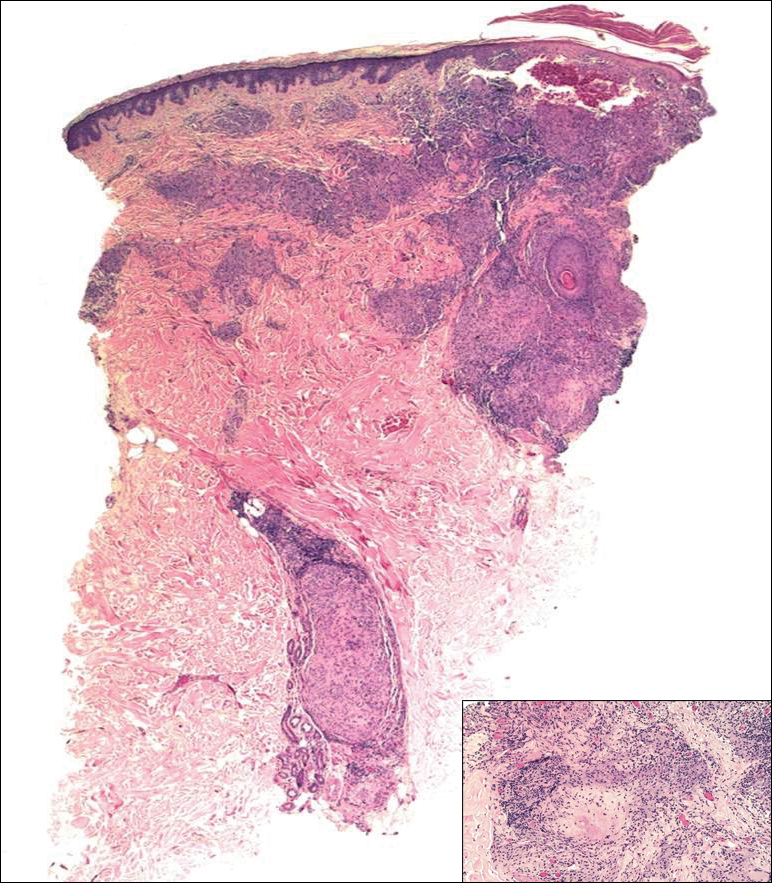
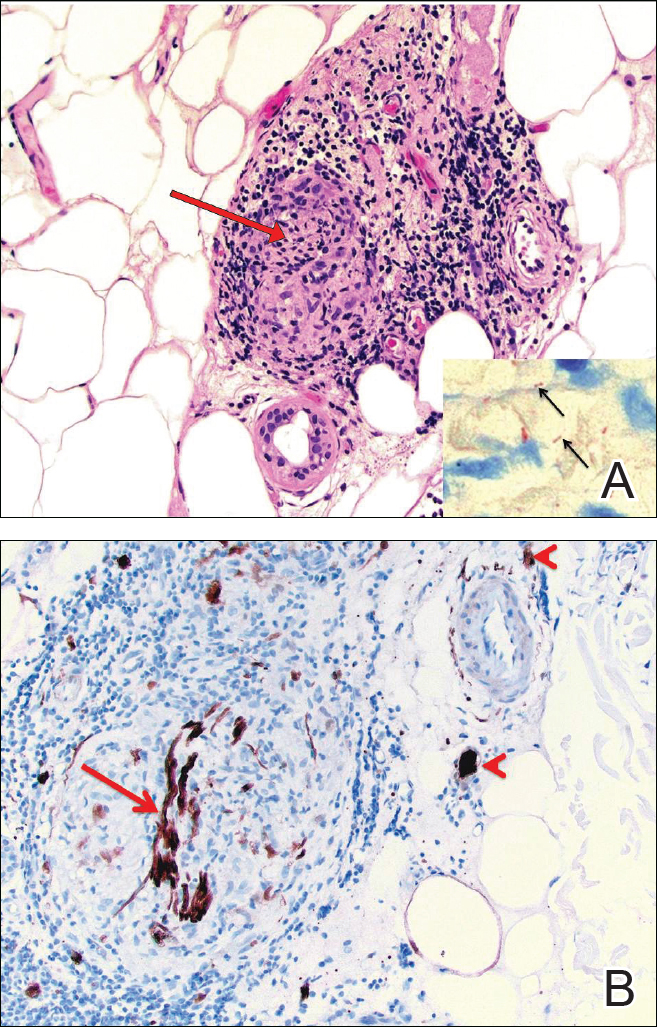
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.
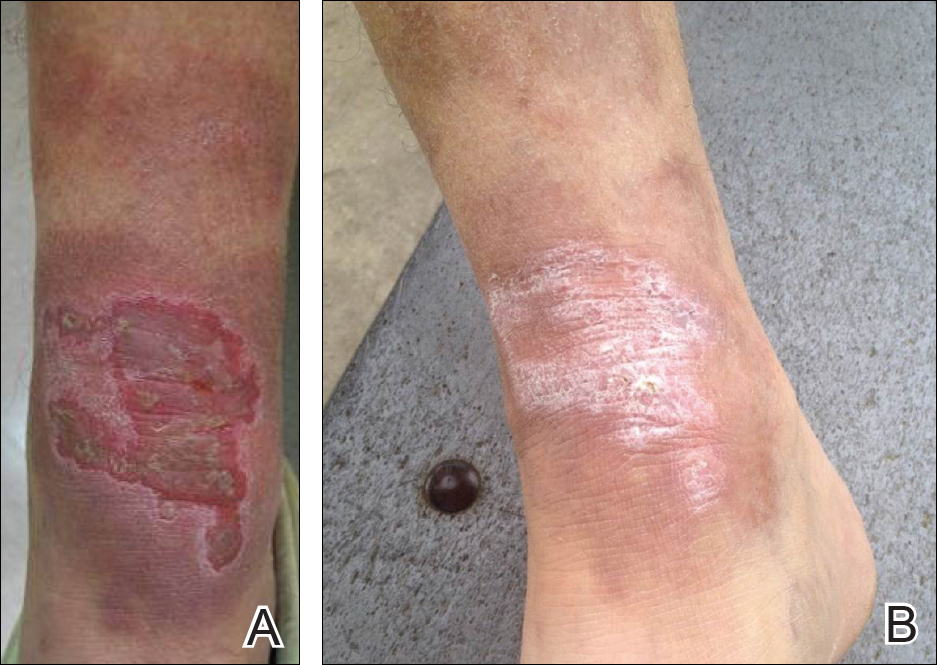
The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
Case Report
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.



The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
Case Report
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.



The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
Practice Points
- A majority of leprosy cases in the United States have been reported in Florida, California, Texas, Louisiana, Hawaii, and New York.
- Leprosy should be included in the differential diagnosis for annular plaques, particularly those not responding to traditional treatment.