User login
Acute Kidney Injury in the ICU: Medication Dosing
Q: As a hospitalist, I often see patients in our ICU develop AKI. Our pharmacist helps us with medication dosing, but sometimes I feel as if we're pulling a dose out of the air. Are there any studies or guidelines we can refer to?
Standard medication dosing adjustments for patients with impaired renal function are generally based on estimated glomerular filtration rate (eGFR). Because SCr is a lagging indicator of AKI, all methods of deriving eGFR from SCr are valid only when the patient is in a steady state.10
SCr has yet to be replaced by a real-time biomarker for AKI; this has left clinicians in the ICU setting with no simple or concise method for real-time assessment of renal function. In response to this common clinical conundrum, the RIFLE criteria,8 mentioned above, incorporates urinary output and relative increase in SCr as assessment criteria (see table for definitions).
This revised classification system may help the clinician define the severity of AKI in the acute setting. However, no medication dosing guidelines currently correspond with RIFLE staging. To further complicate the picture, there is evidence to suggest that AKI may affect drug metabolism through nonrenal pathways, such as hepatic clearance and transport functions.11 Add to this the potential for impaired drug absorption, distribution, and/or clearance due to variance in intravascular volume status, hepatic hypoperfusion, hypoxia, decreased protein synthesis, and competitive inhibition from concomitant medications—in short, the variables become too complex for calculating therapeutic drug dosing to be possible.
In the absence of definitive guidelines, the clinician plays a critical role in medication dosing adjustment for the ICU patient with AKI. The clinician must use astute clinical judgment to assess and prioritize the unique constellation of factors in any given case. Some of the factors that should be carefully considered when estimating medication dose adjustments in this context include RIFLE staging, trend in SCr, baseline SCr, nephrotoxicity of the medication to be administered, the drug's volume of distribution, the metabolic pathways of drug excretion, and the patient's weight.
A serum drug level, when available, is generally the best guide for dosing adjustment.10 The RIFLE staging does offer some clinical pearls that may be helpful. Though not evidence-based recommendations, these guides are commonly used in the clinical environment.
When patients are in the Failure stage, for example (see specifics in the table), they are generally considered to have an eGFR of less than 15 mL/min for purposes of drug dose adjustment (personal communication, Gideon Kayanan, PharmD, February 2013). However, patients in this category are much more likely than others to be undergoing dialysis, in which case the pharmacokinetics and pharmacodynamics are further complicated. In some cases, it may be appropriate to order creatinine clearance studies with a 6- or 12-hour urine collection and extrapolate a 24-hour creatinine clearance from this value.

The dearth of literature addressing this topic (despite the prevalence of AKI in the acute care setting) is a clear indication of the complexity of creating guidelines to address such a dynamic, multivariate pharmacokinetic process. Review of the literature clearly demonstrates that medical science in this area is not yet sufficiently developed to produce a standardized, data-driven guideline for dose adjustment calculation in patients with AKI.10 Until biomarkers are detected that offer real-time assessment of renal function and that can be used in the clinical setting, there will continue to be a component of estimation, analysis of trends, and reliance on clinical judgment in adjusting medication doses for inpatients with AKI. —AC
References
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Q: As a hospitalist, I often see patients in our ICU develop AKI. Our pharmacist helps us with medication dosing, but sometimes I feel as if we're pulling a dose out of the air. Are there any studies or guidelines we can refer to?
Standard medication dosing adjustments for patients with impaired renal function are generally based on estimated glomerular filtration rate (eGFR). Because SCr is a lagging indicator of AKI, all methods of deriving eGFR from SCr are valid only when the patient is in a steady state.10
SCr has yet to be replaced by a real-time biomarker for AKI; this has left clinicians in the ICU setting with no simple or concise method for real-time assessment of renal function. In response to this common clinical conundrum, the RIFLE criteria,8 mentioned above, incorporates urinary output and relative increase in SCr as assessment criteria (see table for definitions).
This revised classification system may help the clinician define the severity of AKI in the acute setting. However, no medication dosing guidelines currently correspond with RIFLE staging. To further complicate the picture, there is evidence to suggest that AKI may affect drug metabolism through nonrenal pathways, such as hepatic clearance and transport functions.11 Add to this the potential for impaired drug absorption, distribution, and/or clearance due to variance in intravascular volume status, hepatic hypoperfusion, hypoxia, decreased protein synthesis, and competitive inhibition from concomitant medications—in short, the variables become too complex for calculating therapeutic drug dosing to be possible.
In the absence of definitive guidelines, the clinician plays a critical role in medication dosing adjustment for the ICU patient with AKI. The clinician must use astute clinical judgment to assess and prioritize the unique constellation of factors in any given case. Some of the factors that should be carefully considered when estimating medication dose adjustments in this context include RIFLE staging, trend in SCr, baseline SCr, nephrotoxicity of the medication to be administered, the drug's volume of distribution, the metabolic pathways of drug excretion, and the patient's weight.
A serum drug level, when available, is generally the best guide for dosing adjustment.10 The RIFLE staging does offer some clinical pearls that may be helpful. Though not evidence-based recommendations, these guides are commonly used in the clinical environment.
When patients are in the Failure stage, for example (see specifics in the table), they are generally considered to have an eGFR of less than 15 mL/min for purposes of drug dose adjustment (personal communication, Gideon Kayanan, PharmD, February 2013). However, patients in this category are much more likely than others to be undergoing dialysis, in which case the pharmacokinetics and pharmacodynamics are further complicated. In some cases, it may be appropriate to order creatinine clearance studies with a 6- or 12-hour urine collection and extrapolate a 24-hour creatinine clearance from this value.

The dearth of literature addressing this topic (despite the prevalence of AKI in the acute care setting) is a clear indication of the complexity of creating guidelines to address such a dynamic, multivariate pharmacokinetic process. Review of the literature clearly demonstrates that medical science in this area is not yet sufficiently developed to produce a standardized, data-driven guideline for dose adjustment calculation in patients with AKI.10 Until biomarkers are detected that offer real-time assessment of renal function and that can be used in the clinical setting, there will continue to be a component of estimation, analysis of trends, and reliance on clinical judgment in adjusting medication doses for inpatients with AKI. —AC
References
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Q: As a hospitalist, I often see patients in our ICU develop AKI. Our pharmacist helps us with medication dosing, but sometimes I feel as if we're pulling a dose out of the air. Are there any studies or guidelines we can refer to?
Standard medication dosing adjustments for patients with impaired renal function are generally based on estimated glomerular filtration rate (eGFR). Because SCr is a lagging indicator of AKI, all methods of deriving eGFR from SCr are valid only when the patient is in a steady state.10
SCr has yet to be replaced by a real-time biomarker for AKI; this has left clinicians in the ICU setting with no simple or concise method for real-time assessment of renal function. In response to this common clinical conundrum, the RIFLE criteria,8 mentioned above, incorporates urinary output and relative increase in SCr as assessment criteria (see table for definitions).
This revised classification system may help the clinician define the severity of AKI in the acute setting. However, no medication dosing guidelines currently correspond with RIFLE staging. To further complicate the picture, there is evidence to suggest that AKI may affect drug metabolism through nonrenal pathways, such as hepatic clearance and transport functions.11 Add to this the potential for impaired drug absorption, distribution, and/or clearance due to variance in intravascular volume status, hepatic hypoperfusion, hypoxia, decreased protein synthesis, and competitive inhibition from concomitant medications—in short, the variables become too complex for calculating therapeutic drug dosing to be possible.
In the absence of definitive guidelines, the clinician plays a critical role in medication dosing adjustment for the ICU patient with AKI. The clinician must use astute clinical judgment to assess and prioritize the unique constellation of factors in any given case. Some of the factors that should be carefully considered when estimating medication dose adjustments in this context include RIFLE staging, trend in SCr, baseline SCr, nephrotoxicity of the medication to be administered, the drug's volume of distribution, the metabolic pathways of drug excretion, and the patient's weight.
A serum drug level, when available, is generally the best guide for dosing adjustment.10 The RIFLE staging does offer some clinical pearls that may be helpful. Though not evidence-based recommendations, these guides are commonly used in the clinical environment.
When patients are in the Failure stage, for example (see specifics in the table), they are generally considered to have an eGFR of less than 15 mL/min for purposes of drug dose adjustment (personal communication, Gideon Kayanan, PharmD, February 2013). However, patients in this category are much more likely than others to be undergoing dialysis, in which case the pharmacokinetics and pharmacodynamics are further complicated. In some cases, it may be appropriate to order creatinine clearance studies with a 6- or 12-hour urine collection and extrapolate a 24-hour creatinine clearance from this value.

The dearth of literature addressing this topic (despite the prevalence of AKI in the acute care setting) is a clear indication of the complexity of creating guidelines to address such a dynamic, multivariate pharmacokinetic process. Review of the literature clearly demonstrates that medical science in this area is not yet sufficiently developed to produce a standardized, data-driven guideline for dose adjustment calculation in patients with AKI.10 Until biomarkers are detected that offer real-time assessment of renal function and that can be used in the clinical setting, there will continue to be a component of estimation, analysis of trends, and reliance on clinical judgment in adjusting medication doses for inpatients with AKI. —AC
References
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Acute Kidney Injury in the ICU: Increasing Prevalence
Q: In 10 years as a hospitalist advanced practitioner, I've been seeing more and more AKI in our ICU. Is this true everywhere, or are we doing something wrong?
AKI is on the rise nationwide (see hospitalization data in figure), and it carries grim implications for patient outcomes.1-3 AKI with a rise in serum creatinine (SCr) as modest as 0.3 mg/dL is associated with a 70% increase in mortality risk. A rise in SCr exceeding 0.5 mg/dL has been associated with a 6.5-fold rise in the risk for death, even when adjusted for age and gender.4 This is higher than the mortality rate for inpatients admitted with cardiovascular disease or cancer, and just slightly more favorable than the mortality risk associated with sepsis (odds ratios, 6.6 and 7.5, respectively). AKI management in the non-ICU setting incurs the third highest median direct hospital cost, after acute MI and stroke.3
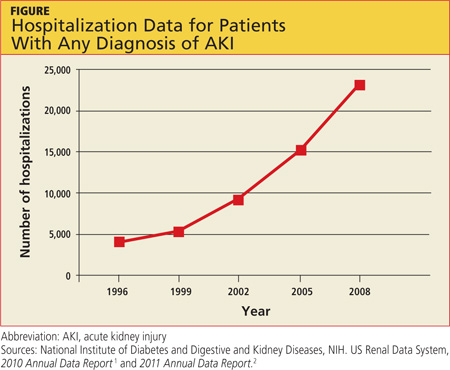
A recent retrospective analysis of hospital admissions nationwide from 2000 to 2009 shows a 10% annual increase in the incidence of AKI requiring dialysis, with at least doubling of the incidence and the number of deaths during that 10-year time period.5 Analyzing the incidence of AKI not requiring dialysis over time is more challenging because the criteria to define AKI have not been static; however, the rise in AKI requiring dialysis has mirrored the rise in AKI not requiring dialysis—suggesting that there is in fact an increased incidence of AKI, independent of variability in the defining criteria.3
Researchers reported in 2012 that during the previous year, the incidence of AKI among all hospitalized patients was 1 in 5.6 In the ICU, incidence of AKI has been reported at 39%, with a mortality rate of 25%.7 Based on the RIFLE criteria (a recently revised classification system whose name refers to Risk, Injury, Failure; Loss and End-stage kidney disease),8 as many as two-thirds of patients admitted to the ICU meet criteria for a diagnosis of AKI.

Predictors for AKI include advancing age, baseline SCr below 1.2 mg/dL, the presence of diabetes, use of IV contrast, acute coronary syndromes, sepsis, liver or heart failure, and use of nephrotoxic medications.3
It is important for clinicians to recognize the implications of AKI, even when it manifests as a relatively minor rise in SCr. In addition to its association with poor outcomes in hospitalized patients, AKI increases the risk for chronic kidney disease and for readmissions within six months after hospital discharge.9 Unfortunately, our increased awareness of the implications of AKI in the inpatient setting has yet to translate into significant improvement in outcomes.
The evolution and availability of epidemiologic and outcome data, we can only hope, will serve to direct more resources and further study toward this issue. Clinicians' efforts to prevent and treat AKI can have profound implications for many of our nation's most chronically and critically ill patients. —AC
REFERENCES
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Q: In 10 years as a hospitalist advanced practitioner, I've been seeing more and more AKI in our ICU. Is this true everywhere, or are we doing something wrong?
AKI is on the rise nationwide (see hospitalization data in figure), and it carries grim implications for patient outcomes.1-3 AKI with a rise in serum creatinine (SCr) as modest as 0.3 mg/dL is associated with a 70% increase in mortality risk. A rise in SCr exceeding 0.5 mg/dL has been associated with a 6.5-fold rise in the risk for death, even when adjusted for age and gender.4 This is higher than the mortality rate for inpatients admitted with cardiovascular disease or cancer, and just slightly more favorable than the mortality risk associated with sepsis (odds ratios, 6.6 and 7.5, respectively). AKI management in the non-ICU setting incurs the third highest median direct hospital cost, after acute MI and stroke.3

A recent retrospective analysis of hospital admissions nationwide from 2000 to 2009 shows a 10% annual increase in the incidence of AKI requiring dialysis, with at least doubling of the incidence and the number of deaths during that 10-year time period.5 Analyzing the incidence of AKI not requiring dialysis over time is more challenging because the criteria to define AKI have not been static; however, the rise in AKI requiring dialysis has mirrored the rise in AKI not requiring dialysis—suggesting that there is in fact an increased incidence of AKI, independent of variability in the defining criteria.3
Researchers reported in 2012 that during the previous year, the incidence of AKI among all hospitalized patients was 1 in 5.6 In the ICU, incidence of AKI has been reported at 39%, with a mortality rate of 25%.7 Based on the RIFLE criteria (a recently revised classification system whose name refers to Risk, Injury, Failure; Loss and End-stage kidney disease),8 as many as two-thirds of patients admitted to the ICU meet criteria for a diagnosis of AKI.

Predictors for AKI include advancing age, baseline SCr below 1.2 mg/dL, the presence of diabetes, use of IV contrast, acute coronary syndromes, sepsis, liver or heart failure, and use of nephrotoxic medications.3
It is important for clinicians to recognize the implications of AKI, even when it manifests as a relatively minor rise in SCr. In addition to its association with poor outcomes in hospitalized patients, AKI increases the risk for chronic kidney disease and for readmissions within six months after hospital discharge.9 Unfortunately, our increased awareness of the implications of AKI in the inpatient setting has yet to translate into significant improvement in outcomes.
The evolution and availability of epidemiologic and outcome data, we can only hope, will serve to direct more resources and further study toward this issue. Clinicians' efforts to prevent and treat AKI can have profound implications for many of our nation's most chronically and critically ill patients. —AC
REFERENCES
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Q: In 10 years as a hospitalist advanced practitioner, I've been seeing more and more AKI in our ICU. Is this true everywhere, or are we doing something wrong?
AKI is on the rise nationwide (see hospitalization data in figure), and it carries grim implications for patient outcomes.1-3 AKI with a rise in serum creatinine (SCr) as modest as 0.3 mg/dL is associated with a 70% increase in mortality risk. A rise in SCr exceeding 0.5 mg/dL has been associated with a 6.5-fold rise in the risk for death, even when adjusted for age and gender.4 This is higher than the mortality rate for inpatients admitted with cardiovascular disease or cancer, and just slightly more favorable than the mortality risk associated with sepsis (odds ratios, 6.6 and 7.5, respectively). AKI management in the non-ICU setting incurs the third highest median direct hospital cost, after acute MI and stroke.3

A recent retrospective analysis of hospital admissions nationwide from 2000 to 2009 shows a 10% annual increase in the incidence of AKI requiring dialysis, with at least doubling of the incidence and the number of deaths during that 10-year time period.5 Analyzing the incidence of AKI not requiring dialysis over time is more challenging because the criteria to define AKI have not been static; however, the rise in AKI requiring dialysis has mirrored the rise in AKI not requiring dialysis—suggesting that there is in fact an increased incidence of AKI, independent of variability in the defining criteria.3
Researchers reported in 2012 that during the previous year, the incidence of AKI among all hospitalized patients was 1 in 5.6 In the ICU, incidence of AKI has been reported at 39%, with a mortality rate of 25%.7 Based on the RIFLE criteria (a recently revised classification system whose name refers to Risk, Injury, Failure; Loss and End-stage kidney disease),8 as many as two-thirds of patients admitted to the ICU meet criteria for a diagnosis of AKI.

Predictors for AKI include advancing age, baseline SCr below 1.2 mg/dL, the presence of diabetes, use of IV contrast, acute coronary syndromes, sepsis, liver or heart failure, and use of nephrotoxic medications.3
It is important for clinicians to recognize the implications of AKI, even when it manifests as a relatively minor rise in SCr. In addition to its association with poor outcomes in hospitalized patients, AKI increases the risk for chronic kidney disease and for readmissions within six months after hospital discharge.9 Unfortunately, our increased awareness of the implications of AKI in the inpatient setting has yet to translate into significant improvement in outcomes.
The evolution and availability of epidemiologic and outcome data, we can only hope, will serve to direct more resources and further study toward this issue. Clinicians' efforts to prevent and treat AKI can have profound implications for many of our nation's most chronically and critically ill patients. —AC
REFERENCES
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Man, 48, With Excruciating Leg Pain
A 48-year-old black man, on hemodialysis since August 2002, presented to his primary care provider (PCP) in July 2006 with excruciating leg pain. According to the patient, the leg pain had worsened during the previous six months and was so severe that he was barely able to walk without pain. He was a full-time night security guard and reported walking three to five miles each night.
The man was undergoing hemodialysis three times per week, necessitated by nephritic range proteinuria. He had a questionable history of diabetes but a known diagnosis of hypertension. Definitive diagnosis through kidney biopsy was not obtained because of the associated risk, the patient's obesity, and his aversion to the procedure.
The patient had recently been hospitalized with shortness of breath and fluid overload. Intensive dialysis allowed a significant drop in his dialysis target weight. He was readmitted a few days later with chills, fever, cough, and shortness of breath. He was diagnosed with bilateral pulmonary emboli. The patient said his hypercoagulation work-up was negative, but he was started on warfarin before discharge.
On current presentation, he had swollen, tender legs and multiple excoriations over the calves, explained by the patient's admitted scratching. His skin was shiny and tight. He was still taking warfarin, with an international normalized ratio of 2.1. The patient denied shortness of breath, pruritus (any more than expected with renal disease), or increased fluid.
In addition to warfarin, he was taking esomeprazole 40 mg/d, extended-release metoprolol 25 mg bid, cinacalcet 90 mg/d, sevelamer 4,000 mg and lanthanum 5,000 mg before every meal, mometasone furoate as needed, hydroxyzine 25 mg every four hours as needed, miconazole powder applied to the feet as needed, and a daily prescription multivitamin complex.
Laboratory tests included normal findings (for a dialysis patient) on the complete blood count; blood urea nitrogen, 101 mg/dL (reference range, 7 to 20 mg/dL); serum creatinine, 16.6 mg/dL (0.8 to 1.4 mg/dL); Kt/V (a measure of adequacy of dialysis), 1.37 (acceptable); calcium, 9.6 mg/dL (8.2 to 10.2 mg/dL); serum phosphorus, 5.6 mg/dL (2.4 to 4.1 mg/dL); intact parathyroid hormone, 359 ng/L (10 to 65 ng/L).
The patient's PCP prescribed oxycodone for the pain and referred him to the vascular clinic for evaluation of his legs. A lower leg duplex scan with ankle/brachial indices performed on July 18 showed significant bilateral peripheral vascular disease. Subsequent magnetic resonance angiography (MRA) showed a questionable adrenal gland mass. Abdominal CT with and without contrast yielded negative results for the adrenal mass but showed a cyst in the right kidney. Although cysts are commonly found in dialysis patients, the vascular surgeon elected to evaluate the cyst with an MRI with gadolinium; the mass was found to be hemorrhagic.
Further vascular work-up continued, including MRI with gadolinium on September 26, 2006, which revealed two-vessel runoff in the right foot and three-vessel runoff in the left foot. According to the vascular consult, there was no area to bypass. The patient was sent back to his PCP. At this point, he was taking oxycodone four times per day and continuing to work full-time as a night security guard.
The patient was then sent to neurology for evaluation. By this time, the severity of his leg pain had increased 90%, with worsening swelling and persistent shininess (see figure). The neurologist was unable to obtain electromyograms due to the severity of the patient's pain and lower extremity swelling. No definitive diagnosis could be made.
About one year later, the man's attending nephrology group received copies of the work-up that the PCP sent to the dialysis center. It was apparent that neither the patient's PCP nor the vascular, radiology, or neurology consultants had seen the FDA warning released in June 20061 regarding the use of gadolinium in patients with renal disease. What had started out as a peripheral neuropathy (either renal or diabetic in etiology) was now a full-blown case of nephrogenic systemic fibrosis (NSF).
Open biopsy performed on October 29, 2007, confirmed the presence of gadolinium in the patient's epidermis. He became the first documented case of NSF in the Washington, DC area.
Discussion
In the late 1990s, several reports of an unknown sclerosing dermopathy in patients with chronic kidney disease began to emerge. In 2000, the new entity was named nephrogenic systemic fibrosis, with a disease course demonstrating systemic involvement that affected multiple organ systems and often resulted in severe joint limitations. A Web-based reporting system for this newly described disease, created by Shawn Cowper, MD, of Yale University,2 made it possible to investigate associated epidemiologic factors.
Neither gender, race, nor age appeared relevant. However, all patients had renal disease—acute, chronic, or transient—and more than 90% of patients were dialysis dependent. Factors since recognized to confirm a diagnosis of NSF are severe renal impairment (ie, glomerular filtration rate [GFR] < 30 mL/min/1.73 m2),3 CD34+ dendritic cells found on deep biopsy,4 and the following clinical manifestations:
• Skin. Burning or itching, reddened or darkened patches; possible skin swelling, hardening, and/or tightening.
• Eyes. Yellow raised spots in the whites of the eyes.
• Bones, joints, muscles. Joint stiffness; limited range of motion in the arms, hands, legs, or feet; pain deep in the hip bone or ribs; and/or muscle weakness.3
Theories abounded on the cause of NSF. While the presence of renal disease is a requirement, dialysis did not seem to be.5 Ten percent of NSF cases are patients who have never been dialyzed, and thousands of dialysis patients never develop NSF. Neither was any temporal correlation to dialysis found: While some patients developed NSF soon after starting dialysis, many had been on dialysis for years before NSF occurred. No association was found between NSF and the type of dialysis (inpatient, outpatient, hemodialysis, or peritoneal dialysis), the filter, manufacturer, dialysate, technique, or dialysis unit.2
Authors of a retrospective study involving two large tissue repositories looked for cases of NSF before 1997, but none were found.6 If dialysis was not causing NSF, and the disease did not appear to have existed before 1997, what renal toxin had been introduced in the 1990s to explain it?
One early suspicion involved erythropoietin (EPO), used to treat anemia in patients with kidney disease. Skin changes had been reported in some patients after initiation of treatment with EPO, and the NSF patients received a significantly higher mean dose of EPO than controls received.7
Ninety percent of patients with NSF had fistula reconstruction or dialysis catheter placement, but these are common in renal disease patients.8 Forty-eight percent of patients had had liver or kidney transplants, and 12% had hypercoagulable states. Most patients with NSF had never received ACE inhibitors. Were the protective antifibrogenic properties of these agents missing?
Mystery Solved
In a triumph for the Internet and its capacity to disseminate information around the world, a breakthrough came in 2006 from a small town in Austria. Grobner9 described nine patients who had received gadodiamide (Omniscan™)–enhanced MRA, five of whom developed NSF. Upon release of this report, researchers reexamined the original cases and detected a clear correlation between gadolinium and NSF. Because the contrast dose given for MRA can be as much as three times that required for routine MRI, the absence of NSF cases before 1997 suddenly made sense.
In May 2006, researchers for the Danish Medicines Agency reported 13 cases of NSF in patients injected with gadodiamide.10 Within months, 28 biopsy-proven cases were reported in St. Louis, six in Texas, and 13 at the University of Wisconsin—all involving patients exposed to gadolinium.11-13 It was apparent that NSF was iatrogenic and could be controlled.
What We Have Learned Since
In subsequent research, it has been found that more than 90% of reported cases of NSF occurred following exposure to gadodiamide—although gadodiamide accounts for only 15% of all gadolinium injections worldwide,14 and this number is decreasing as more cases are reported. The correlation between gadodiamide and NSF is so strong that its manufacturer, GE Healthcare, sent practitioners a letter in June 2006 warning of NSF as an adverse effect of gadolinium exposure.15 Two days later, the FDA issued an advisory on gadolinium-enhanced imaging procedures, recommending prompt hemodialysis after gadolinium exposure and reminding radiologists and nephrologists that gadolinium is not FDA approved for MRA.1
Although the 44% incidence rate of NSF reported by Grobner9 has never been replicated, a retrospective review of all known NSF cases affirmed that more than 90% of patients had been exposed to gadolinium.14 Two 2007 reports published in the Journal of the American Academy of Dermatology demonstrated that gadolinium was detectable in the tissues of patients with NSF.16,17
In Europe, in response to the May 2006 report from the Danish Medicines Agency,10 the European Society of Urogenital Radiology revised its guidelines with a directive that gadodiamide not be administered in any patients who had reduced kidney function or were undergoing dialysis.18 Shortly thereafter, the European Committee for Medicinal Products for Human Use issued a contraindication for gadodiamide use in patients with severe renal impairment and advised that these patients not be given gadolinium unless there was no other choice.19 A contraindication was also issued for gadodiamide use in patients with previous or anticipated liver transplantation.
The American College of Radiology guidelines published in 200720 stated that patients with any level of renal disease should not receive gadodiamide.
In March 2007, GE Healthcare published a paper on NSF, reiterating the safety of gadodiamide while acknowledging that 120 more cases had been reported to them ("usually associated with exposure at high doses").21 The FDA upholds an alert regarding use of all gadolinium-based contrast agents for patients with acute or chronic severe renal insufficiency,3 while stopping short of a ban on gadodiamide in such patients.
How Common Is NSF?
In a 2007 study conducted at the University of Wisconsin, Sadowski et al13 reported 13 cases of gadolinium-induced NSF, 11 involving patients with a GFR below 30 mL/min/1.73 m2 but two with a GFR between 30 and 60 mL/min/1.73 m2 (ie, with renal insufficiency, although the authors noted that renal insufficiency was acute in these two patients). The incidence of NSF was 4.6% among hospitalized patients with a GFR be-low 60 mL/min/1.73 m2 who underwent gadolinium-enhanced MRI at the university hospital's radiology department. A reexamination of the charts of the patients with a GFR between 30 and 60 mL/min/1.73 m2 revealed that these patients had levels below 30 mL/min/1.73 m2 when their gadolinium exposure took place.
In an outpatient population–based calculation performed by Deo et al,22 a 2.4% chance of NSF was determined for each gadolinium exposure. Incidence of NSF was calculated at 4.3 cases per 1,000 patient-years in this population, making NSF as common as contrast-induced nephropathy. Nearly 5% of patients with NSF have an exceedingly rapid and fulminant disease course that may result in death. NSF, of itself, is not a cause of death but may contribute to death by restricting effective ventilation or by restricting mobility to the point of causing an accidental fall that may be further exacerbated by fractures and clotting complications. NSF survivors may experience disabling systemic symptoms. Full recovery occurs only in patients who recover renal function, either naturally or by kidney transplantation.4
Why Is NSF More Common With Gadodiamide?
As of June 2008, five gadolinium-based contrast agents were FDA approved for use with MRI (none with MRA)3: gadobenate (MultiHance®), gadodiamide (Omniscan), gadopentetate (Magnevist®), gadoteridol (ProHance®), and gadoversetamide (Opti-MARK®). More than 90% of NSF cases are associated with gadodiamide. Because this agent is the least stable thermodynamically, it may be more likely than the others to transmetallate.14 All gadolinium chelates are excreted by the kidney, and the decreased renal clearances associated with renal impairment may expose patients to prolonged gadolinium transmetallation, allowing the agent to accumulate in bone and other tissue.
Gadoterate (Dotarem®), a cyclic gadolinium-based agent that is available in Europe but not the US, is considered more stable than other agents. It has been suggested that such agents may be safer choices for patients with decreased renal function.14,19
Strategies to Prevent NSF
In the US and Europe, only a physician who has consulted with a radiologist can write an order for gadolinium use in a patient with a GFR below 30 mL/min/1.73 m2.18,20 European guidelines do not allow use of gadodiamide in such patients.
Although the actual population-based occurrence of NSF is low, the nature of the disease calls for an effort to limit vulnerable patients' exposure to gadolinium (see box). Outside of withholding imaging procedures, the only currently known strategies to reduce the incidence of NSF are to use a more stable, nonchelating gadolinium14 and to remove the gadolinium as soon as possible.3,24
It has been recommended that patients with renal disease who are presently undergoing dialysis be dialyzed within two to three hours of gadolinium exposure, then again within 24 and 48 hours, provided it is clinically safe.20,24 This has been shown to remove 99% of the gadolinium.23
Since peritoneal dialysis clears gadolinium poorly, hemodialysis is recommended for peritoneal dialysis patients after gadolinium exposure, following the regimen outlined above.20
No consensus has been reached regarding the patient with a GFR between 30 and 60 mL/min/1.73 m2, nor for the patient with a lower GFR and no access for dialysis to be administered. Placement of a catheter for two days' dialysis incurs both surgical and renal risks for these patients.8
Patient Outcome
The only known cure for NSF is kidney transplantation, which is associated with a complete cure rate of 40%.4,25 Nevertheless, while this manuscript was in preparation, the patient presented in this case study underwent kidney transplantation. On day 8 postsurgery, he was no longer taking oxycodone, his skin condition was clearing up, and he was feeling considerably better. His health care providers hope for further regression from his disease.
Conclusion
NSF is just one example of iatrogenic conditions that can occur in any hospital, office, or clinic. Health care providers cannot be too vigilant in keeping abreast of warnings from the FDA and other agencies. In this case, several clinicians overlooked a recent, urgent public health advisory, with significant consequences.
1. US Food and Drug Administration. Public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance. www.fda.gov/cder/drug/advisory/gadolinium_agents.htm. Accessed July 24, 2008.
2. Cowper SE, Su L, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23(5):383-393.
3. US Food and Drug Administration. Information for healthcare professionals: gadolinium-based contrast agents for magnetic resonance imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). Last updated June 4, 2008. www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm. Accessed July 24, 2008.
4. International Center for Nephrogenic Fibrosing Dermopathy Research. www.icnfdr.org. Accessed July 24, 2008.
5. DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19(3):191-194.
6. Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18(6):614-617.
7. Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med. 2006;145(3):234-235.
8. Miskulin D, Gul A, Rudnick MR, Cowper SE. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in advanced renal failure. www.uptodate.com/patients/content/topic.do?topicKey=dialysis/48700. Accessed July 24, 2008.
9. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
10. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
11. Centers for Disease Control and Prevention. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002-2006. MMWR Morb Mortal Wkly Rep. 2007;56(7):137-141.
12. Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol. 2007;42(2):139-145.
13. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148-157.
14. Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80(950):73-76.
15. GE Healthcare. Omniscan safety update. http://md.gehealthcare.com/omniscan/safety/index.html. Accessed July 24, 2008.
16. Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56(1):27-30.
17. High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56(1):21-26.
18. Thomsen H; European Society of Urogenital Radiology. European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol. 2007;17(1):70-76.
19. Bongartz G. Imaging in the time of NFD/NSF: do we have to change our routines concerning renal insufficiency? MAGMA. 2007;20(2):57-62.
20. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
21. GE Healthcare Paper on Nephrogenic Systemic Fibrosis (March 2007). http://md.gehealthcare.com/omniscan/GE% 20Healthcare%20Paper%20On%20Nephrogenic%20 Systemic%20Fibrosis.pdf. Accessed July 24, 2008.
22. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267.
23. Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol. 2001;42(3):339-341.
24. Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647-649.
25. Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis. 2005;46(4):763-765.
A 48-year-old black man, on hemodialysis since August 2002, presented to his primary care provider (PCP) in July 2006 with excruciating leg pain. According to the patient, the leg pain had worsened during the previous six months and was so severe that he was barely able to walk without pain. He was a full-time night security guard and reported walking three to five miles each night.
The man was undergoing hemodialysis three times per week, necessitated by nephritic range proteinuria. He had a questionable history of diabetes but a known diagnosis of hypertension. Definitive diagnosis through kidney biopsy was not obtained because of the associated risk, the patient's obesity, and his aversion to the procedure.
The patient had recently been hospitalized with shortness of breath and fluid overload. Intensive dialysis allowed a significant drop in his dialysis target weight. He was readmitted a few days later with chills, fever, cough, and shortness of breath. He was diagnosed with bilateral pulmonary emboli. The patient said his hypercoagulation work-up was negative, but he was started on warfarin before discharge.
On current presentation, he had swollen, tender legs and multiple excoriations over the calves, explained by the patient's admitted scratching. His skin was shiny and tight. He was still taking warfarin, with an international normalized ratio of 2.1. The patient denied shortness of breath, pruritus (any more than expected with renal disease), or increased fluid.
In addition to warfarin, he was taking esomeprazole 40 mg/d, extended-release metoprolol 25 mg bid, cinacalcet 90 mg/d, sevelamer 4,000 mg and lanthanum 5,000 mg before every meal, mometasone furoate as needed, hydroxyzine 25 mg every four hours as needed, miconazole powder applied to the feet as needed, and a daily prescription multivitamin complex.
Laboratory tests included normal findings (for a dialysis patient) on the complete blood count; blood urea nitrogen, 101 mg/dL (reference range, 7 to 20 mg/dL); serum creatinine, 16.6 mg/dL (0.8 to 1.4 mg/dL); Kt/V (a measure of adequacy of dialysis), 1.37 (acceptable); calcium, 9.6 mg/dL (8.2 to 10.2 mg/dL); serum phosphorus, 5.6 mg/dL (2.4 to 4.1 mg/dL); intact parathyroid hormone, 359 ng/L (10 to 65 ng/L).
The patient's PCP prescribed oxycodone for the pain and referred him to the vascular clinic for evaluation of his legs. A lower leg duplex scan with ankle/brachial indices performed on July 18 showed significant bilateral peripheral vascular disease. Subsequent magnetic resonance angiography (MRA) showed a questionable adrenal gland mass. Abdominal CT with and without contrast yielded negative results for the adrenal mass but showed a cyst in the right kidney. Although cysts are commonly found in dialysis patients, the vascular surgeon elected to evaluate the cyst with an MRI with gadolinium; the mass was found to be hemorrhagic.
Further vascular work-up continued, including MRI with gadolinium on September 26, 2006, which revealed two-vessel runoff in the right foot and three-vessel runoff in the left foot. According to the vascular consult, there was no area to bypass. The patient was sent back to his PCP. At this point, he was taking oxycodone four times per day and continuing to work full-time as a night security guard.
The patient was then sent to neurology for evaluation. By this time, the severity of his leg pain had increased 90%, with worsening swelling and persistent shininess (see figure). The neurologist was unable to obtain electromyograms due to the severity of the patient's pain and lower extremity swelling. No definitive diagnosis could be made.
About one year later, the man's attending nephrology group received copies of the work-up that the PCP sent to the dialysis center. It was apparent that neither the patient's PCP nor the vascular, radiology, or neurology consultants had seen the FDA warning released in June 20061 regarding the use of gadolinium in patients with renal disease. What had started out as a peripheral neuropathy (either renal or diabetic in etiology) was now a full-blown case of nephrogenic systemic fibrosis (NSF).
Open biopsy performed on October 29, 2007, confirmed the presence of gadolinium in the patient's epidermis. He became the first documented case of NSF in the Washington, DC area.
Discussion
In the late 1990s, several reports of an unknown sclerosing dermopathy in patients with chronic kidney disease began to emerge. In 2000, the new entity was named nephrogenic systemic fibrosis, with a disease course demonstrating systemic involvement that affected multiple organ systems and often resulted in severe joint limitations. A Web-based reporting system for this newly described disease, created by Shawn Cowper, MD, of Yale University,2 made it possible to investigate associated epidemiologic factors.
Neither gender, race, nor age appeared relevant. However, all patients had renal disease—acute, chronic, or transient—and more than 90% of patients were dialysis dependent. Factors since recognized to confirm a diagnosis of NSF are severe renal impairment (ie, glomerular filtration rate [GFR] < 30 mL/min/1.73 m2),3 CD34+ dendritic cells found on deep biopsy,4 and the following clinical manifestations:
• Skin. Burning or itching, reddened or darkened patches; possible skin swelling, hardening, and/or tightening.
• Eyes. Yellow raised spots in the whites of the eyes.
• Bones, joints, muscles. Joint stiffness; limited range of motion in the arms, hands, legs, or feet; pain deep in the hip bone or ribs; and/or muscle weakness.3
Theories abounded on the cause of NSF. While the presence of renal disease is a requirement, dialysis did not seem to be.5 Ten percent of NSF cases are patients who have never been dialyzed, and thousands of dialysis patients never develop NSF. Neither was any temporal correlation to dialysis found: While some patients developed NSF soon after starting dialysis, many had been on dialysis for years before NSF occurred. No association was found between NSF and the type of dialysis (inpatient, outpatient, hemodialysis, or peritoneal dialysis), the filter, manufacturer, dialysate, technique, or dialysis unit.2
Authors of a retrospective study involving two large tissue repositories looked for cases of NSF before 1997, but none were found.6 If dialysis was not causing NSF, and the disease did not appear to have existed before 1997, what renal toxin had been introduced in the 1990s to explain it?
One early suspicion involved erythropoietin (EPO), used to treat anemia in patients with kidney disease. Skin changes had been reported in some patients after initiation of treatment with EPO, and the NSF patients received a significantly higher mean dose of EPO than controls received.7
Ninety percent of patients with NSF had fistula reconstruction or dialysis catheter placement, but these are common in renal disease patients.8 Forty-eight percent of patients had had liver or kidney transplants, and 12% had hypercoagulable states. Most patients with NSF had never received ACE inhibitors. Were the protective antifibrogenic properties of these agents missing?
Mystery Solved
In a triumph for the Internet and its capacity to disseminate information around the world, a breakthrough came in 2006 from a small town in Austria. Grobner9 described nine patients who had received gadodiamide (Omniscan™)–enhanced MRA, five of whom developed NSF. Upon release of this report, researchers reexamined the original cases and detected a clear correlation between gadolinium and NSF. Because the contrast dose given for MRA can be as much as three times that required for routine MRI, the absence of NSF cases before 1997 suddenly made sense.
In May 2006, researchers for the Danish Medicines Agency reported 13 cases of NSF in patients injected with gadodiamide.10 Within months, 28 biopsy-proven cases were reported in St. Louis, six in Texas, and 13 at the University of Wisconsin—all involving patients exposed to gadolinium.11-13 It was apparent that NSF was iatrogenic and could be controlled.
What We Have Learned Since
In subsequent research, it has been found that more than 90% of reported cases of NSF occurred following exposure to gadodiamide—although gadodiamide accounts for only 15% of all gadolinium injections worldwide,14 and this number is decreasing as more cases are reported. The correlation between gadodiamide and NSF is so strong that its manufacturer, GE Healthcare, sent practitioners a letter in June 2006 warning of NSF as an adverse effect of gadolinium exposure.15 Two days later, the FDA issued an advisory on gadolinium-enhanced imaging procedures, recommending prompt hemodialysis after gadolinium exposure and reminding radiologists and nephrologists that gadolinium is not FDA approved for MRA.1
Although the 44% incidence rate of NSF reported by Grobner9 has never been replicated, a retrospective review of all known NSF cases affirmed that more than 90% of patients had been exposed to gadolinium.14 Two 2007 reports published in the Journal of the American Academy of Dermatology demonstrated that gadolinium was detectable in the tissues of patients with NSF.16,17
In Europe, in response to the May 2006 report from the Danish Medicines Agency,10 the European Society of Urogenital Radiology revised its guidelines with a directive that gadodiamide not be administered in any patients who had reduced kidney function or were undergoing dialysis.18 Shortly thereafter, the European Committee for Medicinal Products for Human Use issued a contraindication for gadodiamide use in patients with severe renal impairment and advised that these patients not be given gadolinium unless there was no other choice.19 A contraindication was also issued for gadodiamide use in patients with previous or anticipated liver transplantation.
The American College of Radiology guidelines published in 200720 stated that patients with any level of renal disease should not receive gadodiamide.
In March 2007, GE Healthcare published a paper on NSF, reiterating the safety of gadodiamide while acknowledging that 120 more cases had been reported to them ("usually associated with exposure at high doses").21 The FDA upholds an alert regarding use of all gadolinium-based contrast agents for patients with acute or chronic severe renal insufficiency,3 while stopping short of a ban on gadodiamide in such patients.
How Common Is NSF?
In a 2007 study conducted at the University of Wisconsin, Sadowski et al13 reported 13 cases of gadolinium-induced NSF, 11 involving patients with a GFR below 30 mL/min/1.73 m2 but two with a GFR between 30 and 60 mL/min/1.73 m2 (ie, with renal insufficiency, although the authors noted that renal insufficiency was acute in these two patients). The incidence of NSF was 4.6% among hospitalized patients with a GFR be-low 60 mL/min/1.73 m2 who underwent gadolinium-enhanced MRI at the university hospital's radiology department. A reexamination of the charts of the patients with a GFR between 30 and 60 mL/min/1.73 m2 revealed that these patients had levels below 30 mL/min/1.73 m2 when their gadolinium exposure took place.
In an outpatient population–based calculation performed by Deo et al,22 a 2.4% chance of NSF was determined for each gadolinium exposure. Incidence of NSF was calculated at 4.3 cases per 1,000 patient-years in this population, making NSF as common as contrast-induced nephropathy. Nearly 5% of patients with NSF have an exceedingly rapid and fulminant disease course that may result in death. NSF, of itself, is not a cause of death but may contribute to death by restricting effective ventilation or by restricting mobility to the point of causing an accidental fall that may be further exacerbated by fractures and clotting complications. NSF survivors may experience disabling systemic symptoms. Full recovery occurs only in patients who recover renal function, either naturally or by kidney transplantation.4
Why Is NSF More Common With Gadodiamide?
As of June 2008, five gadolinium-based contrast agents were FDA approved for use with MRI (none with MRA)3: gadobenate (MultiHance®), gadodiamide (Omniscan), gadopentetate (Magnevist®), gadoteridol (ProHance®), and gadoversetamide (Opti-MARK®). More than 90% of NSF cases are associated with gadodiamide. Because this agent is the least stable thermodynamically, it may be more likely than the others to transmetallate.14 All gadolinium chelates are excreted by the kidney, and the decreased renal clearances associated with renal impairment may expose patients to prolonged gadolinium transmetallation, allowing the agent to accumulate in bone and other tissue.
Gadoterate (Dotarem®), a cyclic gadolinium-based agent that is available in Europe but not the US, is considered more stable than other agents. It has been suggested that such agents may be safer choices for patients with decreased renal function.14,19
Strategies to Prevent NSF
In the US and Europe, only a physician who has consulted with a radiologist can write an order for gadolinium use in a patient with a GFR below 30 mL/min/1.73 m2.18,20 European guidelines do not allow use of gadodiamide in such patients.
Although the actual population-based occurrence of NSF is low, the nature of the disease calls for an effort to limit vulnerable patients' exposure to gadolinium (see box). Outside of withholding imaging procedures, the only currently known strategies to reduce the incidence of NSF are to use a more stable, nonchelating gadolinium14 and to remove the gadolinium as soon as possible.3,24
It has been recommended that patients with renal disease who are presently undergoing dialysis be dialyzed within two to three hours of gadolinium exposure, then again within 24 and 48 hours, provided it is clinically safe.20,24 This has been shown to remove 99% of the gadolinium.23
Since peritoneal dialysis clears gadolinium poorly, hemodialysis is recommended for peritoneal dialysis patients after gadolinium exposure, following the regimen outlined above.20
No consensus has been reached regarding the patient with a GFR between 30 and 60 mL/min/1.73 m2, nor for the patient with a lower GFR and no access for dialysis to be administered. Placement of a catheter for two days' dialysis incurs both surgical and renal risks for these patients.8
Patient Outcome
The only known cure for NSF is kidney transplantation, which is associated with a complete cure rate of 40%.4,25 Nevertheless, while this manuscript was in preparation, the patient presented in this case study underwent kidney transplantation. On day 8 postsurgery, he was no longer taking oxycodone, his skin condition was clearing up, and he was feeling considerably better. His health care providers hope for further regression from his disease.
Conclusion
NSF is just one example of iatrogenic conditions that can occur in any hospital, office, or clinic. Health care providers cannot be too vigilant in keeping abreast of warnings from the FDA and other agencies. In this case, several clinicians overlooked a recent, urgent public health advisory, with significant consequences.
A 48-year-old black man, on hemodialysis since August 2002, presented to his primary care provider (PCP) in July 2006 with excruciating leg pain. According to the patient, the leg pain had worsened during the previous six months and was so severe that he was barely able to walk without pain. He was a full-time night security guard and reported walking three to five miles each night.
The man was undergoing hemodialysis three times per week, necessitated by nephritic range proteinuria. He had a questionable history of diabetes but a known diagnosis of hypertension. Definitive diagnosis through kidney biopsy was not obtained because of the associated risk, the patient's obesity, and his aversion to the procedure.
The patient had recently been hospitalized with shortness of breath and fluid overload. Intensive dialysis allowed a significant drop in his dialysis target weight. He was readmitted a few days later with chills, fever, cough, and shortness of breath. He was diagnosed with bilateral pulmonary emboli. The patient said his hypercoagulation work-up was negative, but he was started on warfarin before discharge.
On current presentation, he had swollen, tender legs and multiple excoriations over the calves, explained by the patient's admitted scratching. His skin was shiny and tight. He was still taking warfarin, with an international normalized ratio of 2.1. The patient denied shortness of breath, pruritus (any more than expected with renal disease), or increased fluid.
In addition to warfarin, he was taking esomeprazole 40 mg/d, extended-release metoprolol 25 mg bid, cinacalcet 90 mg/d, sevelamer 4,000 mg and lanthanum 5,000 mg before every meal, mometasone furoate as needed, hydroxyzine 25 mg every four hours as needed, miconazole powder applied to the feet as needed, and a daily prescription multivitamin complex.
Laboratory tests included normal findings (for a dialysis patient) on the complete blood count; blood urea nitrogen, 101 mg/dL (reference range, 7 to 20 mg/dL); serum creatinine, 16.6 mg/dL (0.8 to 1.4 mg/dL); Kt/V (a measure of adequacy of dialysis), 1.37 (acceptable); calcium, 9.6 mg/dL (8.2 to 10.2 mg/dL); serum phosphorus, 5.6 mg/dL (2.4 to 4.1 mg/dL); intact parathyroid hormone, 359 ng/L (10 to 65 ng/L).
The patient's PCP prescribed oxycodone for the pain and referred him to the vascular clinic for evaluation of his legs. A lower leg duplex scan with ankle/brachial indices performed on July 18 showed significant bilateral peripheral vascular disease. Subsequent magnetic resonance angiography (MRA) showed a questionable adrenal gland mass. Abdominal CT with and without contrast yielded negative results for the adrenal mass but showed a cyst in the right kidney. Although cysts are commonly found in dialysis patients, the vascular surgeon elected to evaluate the cyst with an MRI with gadolinium; the mass was found to be hemorrhagic.
Further vascular work-up continued, including MRI with gadolinium on September 26, 2006, which revealed two-vessel runoff in the right foot and three-vessel runoff in the left foot. According to the vascular consult, there was no area to bypass. The patient was sent back to his PCP. At this point, he was taking oxycodone four times per day and continuing to work full-time as a night security guard.
The patient was then sent to neurology for evaluation. By this time, the severity of his leg pain had increased 90%, with worsening swelling and persistent shininess (see figure). The neurologist was unable to obtain electromyograms due to the severity of the patient's pain and lower extremity swelling. No definitive diagnosis could be made.
About one year later, the man's attending nephrology group received copies of the work-up that the PCP sent to the dialysis center. It was apparent that neither the patient's PCP nor the vascular, radiology, or neurology consultants had seen the FDA warning released in June 20061 regarding the use of gadolinium in patients with renal disease. What had started out as a peripheral neuropathy (either renal or diabetic in etiology) was now a full-blown case of nephrogenic systemic fibrosis (NSF).
Open biopsy performed on October 29, 2007, confirmed the presence of gadolinium in the patient's epidermis. He became the first documented case of NSF in the Washington, DC area.
Discussion
In the late 1990s, several reports of an unknown sclerosing dermopathy in patients with chronic kidney disease began to emerge. In 2000, the new entity was named nephrogenic systemic fibrosis, with a disease course demonstrating systemic involvement that affected multiple organ systems and often resulted in severe joint limitations. A Web-based reporting system for this newly described disease, created by Shawn Cowper, MD, of Yale University,2 made it possible to investigate associated epidemiologic factors.
Neither gender, race, nor age appeared relevant. However, all patients had renal disease—acute, chronic, or transient—and more than 90% of patients were dialysis dependent. Factors since recognized to confirm a diagnosis of NSF are severe renal impairment (ie, glomerular filtration rate [GFR] < 30 mL/min/1.73 m2),3 CD34+ dendritic cells found on deep biopsy,4 and the following clinical manifestations:
• Skin. Burning or itching, reddened or darkened patches; possible skin swelling, hardening, and/or tightening.
• Eyes. Yellow raised spots in the whites of the eyes.
• Bones, joints, muscles. Joint stiffness; limited range of motion in the arms, hands, legs, or feet; pain deep in the hip bone or ribs; and/or muscle weakness.3
Theories abounded on the cause of NSF. While the presence of renal disease is a requirement, dialysis did not seem to be.5 Ten percent of NSF cases are patients who have never been dialyzed, and thousands of dialysis patients never develop NSF. Neither was any temporal correlation to dialysis found: While some patients developed NSF soon after starting dialysis, many had been on dialysis for years before NSF occurred. No association was found between NSF and the type of dialysis (inpatient, outpatient, hemodialysis, or peritoneal dialysis), the filter, manufacturer, dialysate, technique, or dialysis unit.2
Authors of a retrospective study involving two large tissue repositories looked for cases of NSF before 1997, but none were found.6 If dialysis was not causing NSF, and the disease did not appear to have existed before 1997, what renal toxin had been introduced in the 1990s to explain it?
One early suspicion involved erythropoietin (EPO), used to treat anemia in patients with kidney disease. Skin changes had been reported in some patients after initiation of treatment with EPO, and the NSF patients received a significantly higher mean dose of EPO than controls received.7
Ninety percent of patients with NSF had fistula reconstruction or dialysis catheter placement, but these are common in renal disease patients.8 Forty-eight percent of patients had had liver or kidney transplants, and 12% had hypercoagulable states. Most patients with NSF had never received ACE inhibitors. Were the protective antifibrogenic properties of these agents missing?
Mystery Solved
In a triumph for the Internet and its capacity to disseminate information around the world, a breakthrough came in 2006 from a small town in Austria. Grobner9 described nine patients who had received gadodiamide (Omniscan™)–enhanced MRA, five of whom developed NSF. Upon release of this report, researchers reexamined the original cases and detected a clear correlation between gadolinium and NSF. Because the contrast dose given for MRA can be as much as three times that required for routine MRI, the absence of NSF cases before 1997 suddenly made sense.
In May 2006, researchers for the Danish Medicines Agency reported 13 cases of NSF in patients injected with gadodiamide.10 Within months, 28 biopsy-proven cases were reported in St. Louis, six in Texas, and 13 at the University of Wisconsin—all involving patients exposed to gadolinium.11-13 It was apparent that NSF was iatrogenic and could be controlled.
What We Have Learned Since
In subsequent research, it has been found that more than 90% of reported cases of NSF occurred following exposure to gadodiamide—although gadodiamide accounts for only 15% of all gadolinium injections worldwide,14 and this number is decreasing as more cases are reported. The correlation between gadodiamide and NSF is so strong that its manufacturer, GE Healthcare, sent practitioners a letter in June 2006 warning of NSF as an adverse effect of gadolinium exposure.15 Two days later, the FDA issued an advisory on gadolinium-enhanced imaging procedures, recommending prompt hemodialysis after gadolinium exposure and reminding radiologists and nephrologists that gadolinium is not FDA approved for MRA.1
Although the 44% incidence rate of NSF reported by Grobner9 has never been replicated, a retrospective review of all known NSF cases affirmed that more than 90% of patients had been exposed to gadolinium.14 Two 2007 reports published in the Journal of the American Academy of Dermatology demonstrated that gadolinium was detectable in the tissues of patients with NSF.16,17
In Europe, in response to the May 2006 report from the Danish Medicines Agency,10 the European Society of Urogenital Radiology revised its guidelines with a directive that gadodiamide not be administered in any patients who had reduced kidney function or were undergoing dialysis.18 Shortly thereafter, the European Committee for Medicinal Products for Human Use issued a contraindication for gadodiamide use in patients with severe renal impairment and advised that these patients not be given gadolinium unless there was no other choice.19 A contraindication was also issued for gadodiamide use in patients with previous or anticipated liver transplantation.
The American College of Radiology guidelines published in 200720 stated that patients with any level of renal disease should not receive gadodiamide.
In March 2007, GE Healthcare published a paper on NSF, reiterating the safety of gadodiamide while acknowledging that 120 more cases had been reported to them ("usually associated with exposure at high doses").21 The FDA upholds an alert regarding use of all gadolinium-based contrast agents for patients with acute or chronic severe renal insufficiency,3 while stopping short of a ban on gadodiamide in such patients.
How Common Is NSF?
In a 2007 study conducted at the University of Wisconsin, Sadowski et al13 reported 13 cases of gadolinium-induced NSF, 11 involving patients with a GFR below 30 mL/min/1.73 m2 but two with a GFR between 30 and 60 mL/min/1.73 m2 (ie, with renal insufficiency, although the authors noted that renal insufficiency was acute in these two patients). The incidence of NSF was 4.6% among hospitalized patients with a GFR be-low 60 mL/min/1.73 m2 who underwent gadolinium-enhanced MRI at the university hospital's radiology department. A reexamination of the charts of the patients with a GFR between 30 and 60 mL/min/1.73 m2 revealed that these patients had levels below 30 mL/min/1.73 m2 when their gadolinium exposure took place.
In an outpatient population–based calculation performed by Deo et al,22 a 2.4% chance of NSF was determined for each gadolinium exposure. Incidence of NSF was calculated at 4.3 cases per 1,000 patient-years in this population, making NSF as common as contrast-induced nephropathy. Nearly 5% of patients with NSF have an exceedingly rapid and fulminant disease course that may result in death. NSF, of itself, is not a cause of death but may contribute to death by restricting effective ventilation or by restricting mobility to the point of causing an accidental fall that may be further exacerbated by fractures and clotting complications. NSF survivors may experience disabling systemic symptoms. Full recovery occurs only in patients who recover renal function, either naturally or by kidney transplantation.4
Why Is NSF More Common With Gadodiamide?
As of June 2008, five gadolinium-based contrast agents were FDA approved for use with MRI (none with MRA)3: gadobenate (MultiHance®), gadodiamide (Omniscan), gadopentetate (Magnevist®), gadoteridol (ProHance®), and gadoversetamide (Opti-MARK®). More than 90% of NSF cases are associated with gadodiamide. Because this agent is the least stable thermodynamically, it may be more likely than the others to transmetallate.14 All gadolinium chelates are excreted by the kidney, and the decreased renal clearances associated with renal impairment may expose patients to prolonged gadolinium transmetallation, allowing the agent to accumulate in bone and other tissue.
Gadoterate (Dotarem®), a cyclic gadolinium-based agent that is available in Europe but not the US, is considered more stable than other agents. It has been suggested that such agents may be safer choices for patients with decreased renal function.14,19
Strategies to Prevent NSF
In the US and Europe, only a physician who has consulted with a radiologist can write an order for gadolinium use in a patient with a GFR below 30 mL/min/1.73 m2.18,20 European guidelines do not allow use of gadodiamide in such patients.
Although the actual population-based occurrence of NSF is low, the nature of the disease calls for an effort to limit vulnerable patients' exposure to gadolinium (see box). Outside of withholding imaging procedures, the only currently known strategies to reduce the incidence of NSF are to use a more stable, nonchelating gadolinium14 and to remove the gadolinium as soon as possible.3,24
It has been recommended that patients with renal disease who are presently undergoing dialysis be dialyzed within two to three hours of gadolinium exposure, then again within 24 and 48 hours, provided it is clinically safe.20,24 This has been shown to remove 99% of the gadolinium.23
Since peritoneal dialysis clears gadolinium poorly, hemodialysis is recommended for peritoneal dialysis patients after gadolinium exposure, following the regimen outlined above.20
No consensus has been reached regarding the patient with a GFR between 30 and 60 mL/min/1.73 m2, nor for the patient with a lower GFR and no access for dialysis to be administered. Placement of a catheter for two days' dialysis incurs both surgical and renal risks for these patients.8
Patient Outcome
The only known cure for NSF is kidney transplantation, which is associated with a complete cure rate of 40%.4,25 Nevertheless, while this manuscript was in preparation, the patient presented in this case study underwent kidney transplantation. On day 8 postsurgery, he was no longer taking oxycodone, his skin condition was clearing up, and he was feeling considerably better. His health care providers hope for further regression from his disease.
Conclusion
NSF is just one example of iatrogenic conditions that can occur in any hospital, office, or clinic. Health care providers cannot be too vigilant in keeping abreast of warnings from the FDA and other agencies. In this case, several clinicians overlooked a recent, urgent public health advisory, with significant consequences.
1. US Food and Drug Administration. Public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance. www.fda.gov/cder/drug/advisory/gadolinium_agents.htm. Accessed July 24, 2008.
2. Cowper SE, Su L, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23(5):383-393.
3. US Food and Drug Administration. Information for healthcare professionals: gadolinium-based contrast agents for magnetic resonance imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). Last updated June 4, 2008. www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm. Accessed July 24, 2008.
4. International Center for Nephrogenic Fibrosing Dermopathy Research. www.icnfdr.org. Accessed July 24, 2008.
5. DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19(3):191-194.
6. Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18(6):614-617.
7. Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med. 2006;145(3):234-235.
8. Miskulin D, Gul A, Rudnick MR, Cowper SE. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in advanced renal failure. www.uptodate.com/patients/content/topic.do?topicKey=dialysis/48700. Accessed July 24, 2008.
9. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
10. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
11. Centers for Disease Control and Prevention. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002-2006. MMWR Morb Mortal Wkly Rep. 2007;56(7):137-141.
12. Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol. 2007;42(2):139-145.
13. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148-157.
14. Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80(950):73-76.
15. GE Healthcare. Omniscan safety update. http://md.gehealthcare.com/omniscan/safety/index.html. Accessed July 24, 2008.
16. Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56(1):27-30.
17. High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56(1):21-26.
18. Thomsen H; European Society of Urogenital Radiology. European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol. 2007;17(1):70-76.
19. Bongartz G. Imaging in the time of NFD/NSF: do we have to change our routines concerning renal insufficiency? MAGMA. 2007;20(2):57-62.
20. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
21. GE Healthcare Paper on Nephrogenic Systemic Fibrosis (March 2007). http://md.gehealthcare.com/omniscan/GE% 20Healthcare%20Paper%20On%20Nephrogenic%20 Systemic%20Fibrosis.pdf. Accessed July 24, 2008.
22. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267.
23. Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol. 2001;42(3):339-341.
24. Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647-649.
25. Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis. 2005;46(4):763-765.
1. US Food and Drug Administration. Public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance. www.fda.gov/cder/drug/advisory/gadolinium_agents.htm. Accessed July 24, 2008.
2. Cowper SE, Su L, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23(5):383-393.
3. US Food and Drug Administration. Information for healthcare professionals: gadolinium-based contrast agents for magnetic resonance imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). Last updated June 4, 2008. www.fda.gov/cder/drug/InfoSheets/HCP/gcca_200705.htm. Accessed July 24, 2008.
4. International Center for Nephrogenic Fibrosing Dermopathy Research. www.icnfdr.org. Accessed July 24, 2008.
5. DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin Dial. 2006;19(3):191-194.
6. Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18(6):614-617.
7. Swaminathan S, Ahmed I, McCarthy JT, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med. 2006;145(3):234-235.
8. Miskulin D, Gul A, Rudnick MR, Cowper SE. Nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in advanced renal failure. www.uptodate.com/patients/content/topic.do?topicKey=dialysis/48700. Accessed July 24, 2008.
9. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
10. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
11. Centers for Disease Control and Prevention. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents—St. Louis, Missouri, 2002-2006. MMWR Morb Mortal Wkly Rep. 2007;56(7):137-141.
12. Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol. 2007;42(2):139-145.
13. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148-157.
14. Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br J Radiol. 2007;80(950):73-76.
15. GE Healthcare. Omniscan safety update. http://md.gehealthcare.com/omniscan/safety/index.html. Accessed July 24, 2008.
16. Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol. 2007;56(1):27-30.
17. High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56(1):21-26.
18. Thomsen H; European Society of Urogenital Radiology. European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol. 2007;17(1):70-76.
19. Bongartz G. Imaging in the time of NFD/NSF: do we have to change our routines concerning renal insufficiency? MAGMA. 2007;20(2):57-62.
20. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
21. GE Healthcare Paper on Nephrogenic Systemic Fibrosis (March 2007). http://md.gehealthcare.com/omniscan/GE% 20Healthcare%20Paper%20On%20Nephrogenic%20 Systemic%20Fibrosis.pdf. Accessed July 24, 2008.
22. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267.
23. Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol. 2001;42(3):339-341.
24. Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647-649.
25. Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis. 2005;46(4):763-765.