User login
Partial Flexor Tendon Laceration Assessment: Interobserver and Intraobserver Reliability
How to manage complete flexor tendon lacerations in the hand is well documented and a subject of relative agreement among authors. However, treatment of partial flexor tendon lacerations is controversial and lacking clear consensus in the literature. Managing these injuries can be challenging, as clinicians must weigh the diminished tensile strength in the injured tendon and the potential for later complications (eg, entrapment, triggering, rupture) against the negative effects of tenorrhaphy.1 Several studies have found impaired tendon gliding on the basis of bulk and inflammatory reaction secondary to suture material within the flexor sheath as well as decreased tendon strength after tenorrhaphy.2-6 This finding led the investigators to recommend nonsurgical management for partial lacerations up to as much as 95% of the cross-sectional area (CSA) of the tendon. According to a survey by McCarthy and colleagues,7 45% of 591 members of the American Society for Surgery of the Hand (ASSH) indicated they would perform tenorrhaphy for a laceration that involved more than 50% of the tendon.
However, accurate assessment of partial-thickness flexor tendon lacerations is difficult owing to the subjectivity of evaluation. In the survey just mentioned,7 the majority of surgeons used the naked eye to make assessments, and only 14% used other means, such as a ruler, a pair of calipers, or loupe magnification. In addition, flexor tendon injuries are often evaluated under less than ideal circumstances—a dirty or bloody field, poor lighting, an uncomfortable patient.
We conducted a study to determine the interobserver and intraobserver reliability of surgeons assessing the percentage of CSA injured in partially lacerated digital flexor tendons. We hypothesized that participants’ accuracy and agreement would be poor.
Materials and Methods
Eight 1-cm transverse, volar skin incisions were made over the midportions of the middle and proximal phalanges of the index, middle, ring, and small fingers of a fresh-frozen human cadaver hand (Figure 1). The tendon sheaths were incised, and the flexor digitorum profundus tendons to each digit were delivered through the wound. With use of a method described previously by Manning and colleagues,8 the tendon was then placed over a flat metal post to be used as a cutting board, and the proposed laceration site was marked with ink. Under loupe magnification, a No. 15 blade was used to create a partial transverse, volar-to-dorsal laceration in each tendon.8 The goal was to create lacerations of about 30%, 50%, and 70% of the total CSA of the tendon. The tendons were then returned to the wound, and visibility of the marked laceration within the wound was ensured. A similar exercise was performed at the level of the proximal palmar crease. Four flexor digitorum superficialis tendons were exposed through 1-cm transverse incisions, and partial lacerations were made in the volar substance of the tendons. The tendons were then returned to the wound, resulting in 12 partially lacerated tendons (8 flexor digitorum profundus, 4 flexor digitorum superficialis).
Six orthopedic surgery residents (2 postgraduate year 1 [PGY-1], 2 PGY-3, 2 PGY-5) and 4 fellowship-trained hand surgeons participated in our study. Each was asked to evaluate the tendons and determine the percentage of total CSA lacerated. Loupe magnification and measuring tools were not permitted, but participants were allowed to handle the tendons. In addition, they were asked if they would perform tenorrhaphy on the injured tendons, given only the amount of injury. The participants repeated this exercise 4 weeks later.
After all measurements were made, a longitudinal incision was made down each of the digits, and the flexor tendons were exposed within the flexor sheath. The transverse incisions in the palm were connected to expose the flexor digitorum superficialis tendons. Under an operating microscope, a pair of digital microcalipers (Kobalt 0.5-ft Metric and SAE Caliper; Figure 2) accurate to 0.01 mm was used to measure the external width (a) and height (b + bˈ) of the tendons just proximal to the lacerations. Measurements were made with the caliper blades just touching the edges of the lacerated tendon, thus minimizing deformation of the tendon. Other measurements made at the laceration site were width of the remaining tendon (c) and height of the remaining tendon (bˈ). CSA of the tendon was calculated assuming a regular ellipsoid shape and using the equation:
Area = 1/2π(b+b')
The area of the tendon injured was determined by calculating the area under a parabola and using the equation:
Area = 2/3c[(b+b')-b']
Last, the percentage of total CSA lacerated was calculated using the equation:
Area (total area)
Statistical analysis was performed to determine accuracy and interobserver and intraobserver reliability. Paired t tests were used in the assessment of accuracy to determine if there were differences between estimated and calibrated measurements.
Results
The 10 participants’ estimates differed significantly (P < .0006) from the calibrated measurements, as did residents’ estimates (P < .0025) and fellowship-trained hand surgeons’ estimates (P < .0002). Estimates were scored 1 to 5 on the basis of proximity to calibrated measurements (Table 1). Thus, more accurate estimates received lower scores. Individual estimates were then scored and stratified into groups for comparison. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. These results are listed in Table 2. Once overall and grouped accuracy was analyzed, κ statistics were calculated to compare interobserver and intraobserver reliability. Overall interobserver agreement was poor for both initial readings (κ = 0.16) and secondary readings (κ = 0.16), indicating poor strength of agreement between individuals both initially and secondarily. Table 3 presents the κ interpretations. There was moderate overall intraobserver agreement (45.83%), indicating participants’ secondary estimates agreed with their primary estimates 46% of the time. Fellowship-trained hand surgeons and first-year residents had the highest intraobserver agreement (50.0%). These results are listed in Table 4.
Discussion
Accurate assessment of partial flexor tendon lacerations is difficult and subjective. There is no standardized method for determining the extent of injury, regardless of whether the evaluation is performed in an emergency department or in the operating room. As McCarthy and colleagues7 noted in their survey of ASSH members, naked eye assessment was by far the most popular means of estimating percentage injured in partial lacerations, and only 10% of the survey respondents used intraoperative measuring devices. Our study showed that participants agreed with one another less than 50% of the time when evaluating injuries without the aid of measuring devices. In addition, interobserver agreement in this study was about 50%, highlighting the difficulty in making an accurate and reproducible assessment.
In a study of canine flexor tendons, McCarthy and colleagues9 found calipers are inaccurate as well and do not provide a reliable means of assessing partial flexor tendon lacerations. They compared caliper measurements with laser micrometer measurements, and the differences averaged 29.3%. They suggested that methods for calculating loss of CSA and for creating precise lacerations must be developed in order to evaluate treatments. One such method is the “tenotome,” devised by Hitchcock and colleagues10: A device with standard scalpel blades is used to make uniform lacerations in tendons by leaving a constant area of the tendon intact, regardless of the size or shape of the original tendon. Measurements made with calipers or rulers assume the tendon has a regular ellipsoid shape, but in reality the shape is a double-ellipse, particularly within the flexor sheath.
Dobyns and colleagues11 observed that changes in CSA size can be related to changes in the size of the bundle pattern of the tendon. They found that, on average, the radial bundle comprised about 60% of the total CSA of the tendon. This finding was clarified by Grewal and colleagues.12 Using histologic sections of tendons plus photomicrographs, they determined that, in zone II of the index and small fingers, the ulnar bundle had an area consistently larger than 50% and the radial bundle less than 50% of the total tendon area. In the ring and middle fingers, the areas of both bundles were almost 50% of the total tendon area. The authors suggested that, using this bundle pattern theory of injury, surgeons could more accurately evaluate the extent of injury with the naked eye.
One of the questions that prompted our study is how reliable is the information a surgeon receives regarding a partial flexor tendon injury evaluated by someone else in another setting. What is done with this information is another question. The scenario can be considered in 2 settings: emergency department and operating room.
Given the poor accuracy and interobserver agreement found in our study, along with the inaccuracy of caliper and ruler measurements, it seems decisions to perform tenorrhaphy based on reported percentages lacerated are unreliable. Our results showed that the ability to accurately assess partial tendon injuries does not improve with surgeon experience, as fellowship-trained hand surgeons were not statistically more accurate or consistent than residents. To this effect, one institution treats all its partial flexor tendon lacerations with wound inspection and irrigation in the emergency department, under digital block and after neurovascular injury has been excluded.8 If the patient is able to actively flex and extend the digit without triggering, then the wound is closed without closing the tendon sheath, a dorsal blocking splint is applied, and motion is begun early, 48 hours later, regardless of laceration severity.
Once the decision has been made to go to the operating room and the injury is being evaluated, what should be done with the information from the measurement, whether made with loupe magnification, calipers, rulers, or the naked eye? Surgeons must weigh the risks for triggering, entrapment, and rupture of untreated partial tendon lacerations1 with the added bulk and potential for adhesions, along with the tensile strength reduction that accompanies tendon repair. Both Reynolds and colleagues13 and Ollinger and colleagues14 found tensile strength significantly diminished in sutured tendons. Ollinger and colleagues14 showed a decrease in tendon gliding after surgical exposure and tenorrhaphy for partial tendon lacerations. Reynolds and colleagues13 concluded that surgical repair leads to poorer results than nonsurgical treatment.
Clinical studies have demonstrated excellent results with nonintervention, and in vivo and in vitro studies have indicated that early motion can be initiated in partial lacerations of up to 95% of total CSA. Wray and Weeks6 treated 26 patients with partial lacerations varying from 25% to 95% of total CSA and noted 1 incidence of trigger finger (which resolved) and no late ruptures. They advocated treatment with early motion and excision or repair of beveled partial lacerations with simple sutures. Stahl and colleagues2 reported comparable outcomes in children with partial lacerations up to 75% of total CSA treated with and without surgery and noted no complications in either group. In a biomechanical study, Hariharan and colleagues4 found lacerations up to 75% can withstand forces associated with active unresisted mobilization.
Conversely, how many patients or surgeons want to return to the operating room to fix a late rupture when it could have been repaired in the primary setting? Schlenker and colleagues,1 reporting on a late flexor pollicus tendon rupture that required tendon grafting, recommended exploration and primary repair of all partial flexor tendon lacerations. Often, it is difficult to determine whether surgical repair is necessary to ensure the best outcome for the patient.
Our study results showed that, in the evaluation of flexor tendon lacerations, both accuracy and interobserver agreement were poor among residents and fellowship-trained hand surgeons, and intraobserver agreement was moderate. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. Our results highlight the difficulty in making accurate assessments of flexor tendon lacerations owing to the subjectivity of evaluation, which appear not to improve with surgeon experience.
1. Schlenker JD, Lister GD, Kleinert HE. Three complications of untreated partial laceration of flexor tendon—entrapment, rupture, and triggering. J Hand Surg Am. 1981;6(4):392-398.
2. Stahl S, Kaufman T, Bialik V. Partial lacerations of flexor tendons in children. Primary repair versus conservative treatment. J Hand Surg Br. 1997;22(3):377-380.
3. Al-Qattan MM. Conservative management of zone II partial flexor tendon lacerations greater than half the width of the tendon. J Hand Surg Am. 2000;25(6):1118-1121.
4. Hariharan JS, Diao E, Soejima O, Lotz JC. Partial lacerations of human digital flexor tendons: a biomechanical analysis. J Hand Surg Am. 1997;22(6):1011-1015.
5. Bishop AT, Cooney WP 3rd, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26(4):301-312.
6. Wray RC Jr, Weeks PM. Treatment of partial tendon lacerations. Hand. 1980;12(2):163-166.
7. McCarthy DM, Boardman ND 3rd, Tramaglini DM, Sotereanos DG, Herndon JH. Clinical management of partially lacerated digital flexor tendons: a survey of hand surgeons. J Hand Surg Am. 1995;20(2):273-275.
8. Manning DW, Spiguel AR, Mass DP. Biomechanical analysis of partial flexor tendon lacerations in zone II of human cadavers. J Hand Surg Am. 2010;35(1):11-18.
9. McCarthy DM, Tramaglini DM, Chan SS, Schmidt CC, Sotereanos DG, Herndon JH. Effect of partial laceration on the structural properties of the canine FDP tendon: an in vitro study. J Hand Surg Am. 1995;20(5):795-800.
10. Hitchcock TF, Candel AG, Light TR, Blevens AD. New technique for producing uniform partial lacerations of tendons. J Orthop Res. 1989;7(3):451-455.
11. Dobyns RC, Cooney WC, Wood MB. Effect of partial lacerations on canine flexor tendons. Minn Med. 1982;65(1):27-32.
12. Grewal R, Sotereanos DG, Rao U, Herndon JH, Woo SL. Bundle pattern of the flexor digitorum profundus tendon in zone II of the hand: a quantitative assessment of the size of a laceration. J Hand Surg Am. 1996;21(6):978-983.
13. Reynolds B, Wray RC Jr, Weeks PM. Should an incompletely severed tendon be sutured? Plast Reconstr Surg. 1976;57(1):36-38.
14. Ollinger H, Wray RC Jr, Weeks PM. Effects of suture on tensile strength gain of partially and completely severed tendons. Surg Forum. 1975;26:63-64.
How to manage complete flexor tendon lacerations in the hand is well documented and a subject of relative agreement among authors. However, treatment of partial flexor tendon lacerations is controversial and lacking clear consensus in the literature. Managing these injuries can be challenging, as clinicians must weigh the diminished tensile strength in the injured tendon and the potential for later complications (eg, entrapment, triggering, rupture) against the negative effects of tenorrhaphy.1 Several studies have found impaired tendon gliding on the basis of bulk and inflammatory reaction secondary to suture material within the flexor sheath as well as decreased tendon strength after tenorrhaphy.2-6 This finding led the investigators to recommend nonsurgical management for partial lacerations up to as much as 95% of the cross-sectional area (CSA) of the tendon. According to a survey by McCarthy and colleagues,7 45% of 591 members of the American Society for Surgery of the Hand (ASSH) indicated they would perform tenorrhaphy for a laceration that involved more than 50% of the tendon.
However, accurate assessment of partial-thickness flexor tendon lacerations is difficult owing to the subjectivity of evaluation. In the survey just mentioned,7 the majority of surgeons used the naked eye to make assessments, and only 14% used other means, such as a ruler, a pair of calipers, or loupe magnification. In addition, flexor tendon injuries are often evaluated under less than ideal circumstances—a dirty or bloody field, poor lighting, an uncomfortable patient.
We conducted a study to determine the interobserver and intraobserver reliability of surgeons assessing the percentage of CSA injured in partially lacerated digital flexor tendons. We hypothesized that participants’ accuracy and agreement would be poor.
Materials and Methods
Eight 1-cm transverse, volar skin incisions were made over the midportions of the middle and proximal phalanges of the index, middle, ring, and small fingers of a fresh-frozen human cadaver hand (Figure 1). The tendon sheaths were incised, and the flexor digitorum profundus tendons to each digit were delivered through the wound. With use of a method described previously by Manning and colleagues,8 the tendon was then placed over a flat metal post to be used as a cutting board, and the proposed laceration site was marked with ink. Under loupe magnification, a No. 15 blade was used to create a partial transverse, volar-to-dorsal laceration in each tendon.8 The goal was to create lacerations of about 30%, 50%, and 70% of the total CSA of the tendon. The tendons were then returned to the wound, and visibility of the marked laceration within the wound was ensured. A similar exercise was performed at the level of the proximal palmar crease. Four flexor digitorum superficialis tendons were exposed through 1-cm transverse incisions, and partial lacerations were made in the volar substance of the tendons. The tendons were then returned to the wound, resulting in 12 partially lacerated tendons (8 flexor digitorum profundus, 4 flexor digitorum superficialis).
Six orthopedic surgery residents (2 postgraduate year 1 [PGY-1], 2 PGY-3, 2 PGY-5) and 4 fellowship-trained hand surgeons participated in our study. Each was asked to evaluate the tendons and determine the percentage of total CSA lacerated. Loupe magnification and measuring tools were not permitted, but participants were allowed to handle the tendons. In addition, they were asked if they would perform tenorrhaphy on the injured tendons, given only the amount of injury. The participants repeated this exercise 4 weeks later.
After all measurements were made, a longitudinal incision was made down each of the digits, and the flexor tendons were exposed within the flexor sheath. The transverse incisions in the palm were connected to expose the flexor digitorum superficialis tendons. Under an operating microscope, a pair of digital microcalipers (Kobalt 0.5-ft Metric and SAE Caliper; Figure 2) accurate to 0.01 mm was used to measure the external width (a) and height (b + bˈ) of the tendons just proximal to the lacerations. Measurements were made with the caliper blades just touching the edges of the lacerated tendon, thus minimizing deformation of the tendon. Other measurements made at the laceration site were width of the remaining tendon (c) and height of the remaining tendon (bˈ). CSA of the tendon was calculated assuming a regular ellipsoid shape and using the equation:
Area = 1/2π(b+b')
The area of the tendon injured was determined by calculating the area under a parabola and using the equation:
Area = 2/3c[(b+b')-b']
Last, the percentage of total CSA lacerated was calculated using the equation:
Area (total area)
Statistical analysis was performed to determine accuracy and interobserver and intraobserver reliability. Paired t tests were used in the assessment of accuracy to determine if there were differences between estimated and calibrated measurements.
Results
The 10 participants’ estimates differed significantly (P < .0006) from the calibrated measurements, as did residents’ estimates (P < .0025) and fellowship-trained hand surgeons’ estimates (P < .0002). Estimates were scored 1 to 5 on the basis of proximity to calibrated measurements (Table 1). Thus, more accurate estimates received lower scores. Individual estimates were then scored and stratified into groups for comparison. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. These results are listed in Table 2. Once overall and grouped accuracy was analyzed, κ statistics were calculated to compare interobserver and intraobserver reliability. Overall interobserver agreement was poor for both initial readings (κ = 0.16) and secondary readings (κ = 0.16), indicating poor strength of agreement between individuals both initially and secondarily. Table 3 presents the κ interpretations. There was moderate overall intraobserver agreement (45.83%), indicating participants’ secondary estimates agreed with their primary estimates 46% of the time. Fellowship-trained hand surgeons and first-year residents had the highest intraobserver agreement (50.0%). These results are listed in Table 4.
Discussion
Accurate assessment of partial flexor tendon lacerations is difficult and subjective. There is no standardized method for determining the extent of injury, regardless of whether the evaluation is performed in an emergency department or in the operating room. As McCarthy and colleagues7 noted in their survey of ASSH members, naked eye assessment was by far the most popular means of estimating percentage injured in partial lacerations, and only 10% of the survey respondents used intraoperative measuring devices. Our study showed that participants agreed with one another less than 50% of the time when evaluating injuries without the aid of measuring devices. In addition, interobserver agreement in this study was about 50%, highlighting the difficulty in making an accurate and reproducible assessment.
In a study of canine flexor tendons, McCarthy and colleagues9 found calipers are inaccurate as well and do not provide a reliable means of assessing partial flexor tendon lacerations. They compared caliper measurements with laser micrometer measurements, and the differences averaged 29.3%. They suggested that methods for calculating loss of CSA and for creating precise lacerations must be developed in order to evaluate treatments. One such method is the “tenotome,” devised by Hitchcock and colleagues10: A device with standard scalpel blades is used to make uniform lacerations in tendons by leaving a constant area of the tendon intact, regardless of the size or shape of the original tendon. Measurements made with calipers or rulers assume the tendon has a regular ellipsoid shape, but in reality the shape is a double-ellipse, particularly within the flexor sheath.
Dobyns and colleagues11 observed that changes in CSA size can be related to changes in the size of the bundle pattern of the tendon. They found that, on average, the radial bundle comprised about 60% of the total CSA of the tendon. This finding was clarified by Grewal and colleagues.12 Using histologic sections of tendons plus photomicrographs, they determined that, in zone II of the index and small fingers, the ulnar bundle had an area consistently larger than 50% and the radial bundle less than 50% of the total tendon area. In the ring and middle fingers, the areas of both bundles were almost 50% of the total tendon area. The authors suggested that, using this bundle pattern theory of injury, surgeons could more accurately evaluate the extent of injury with the naked eye.
One of the questions that prompted our study is how reliable is the information a surgeon receives regarding a partial flexor tendon injury evaluated by someone else in another setting. What is done with this information is another question. The scenario can be considered in 2 settings: emergency department and operating room.
Given the poor accuracy and interobserver agreement found in our study, along with the inaccuracy of caliper and ruler measurements, it seems decisions to perform tenorrhaphy based on reported percentages lacerated are unreliable. Our results showed that the ability to accurately assess partial tendon injuries does not improve with surgeon experience, as fellowship-trained hand surgeons were not statistically more accurate or consistent than residents. To this effect, one institution treats all its partial flexor tendon lacerations with wound inspection and irrigation in the emergency department, under digital block and after neurovascular injury has been excluded.8 If the patient is able to actively flex and extend the digit without triggering, then the wound is closed without closing the tendon sheath, a dorsal blocking splint is applied, and motion is begun early, 48 hours later, regardless of laceration severity.
Once the decision has been made to go to the operating room and the injury is being evaluated, what should be done with the information from the measurement, whether made with loupe magnification, calipers, rulers, or the naked eye? Surgeons must weigh the risks for triggering, entrapment, and rupture of untreated partial tendon lacerations1 with the added bulk and potential for adhesions, along with the tensile strength reduction that accompanies tendon repair. Both Reynolds and colleagues13 and Ollinger and colleagues14 found tensile strength significantly diminished in sutured tendons. Ollinger and colleagues14 showed a decrease in tendon gliding after surgical exposure and tenorrhaphy for partial tendon lacerations. Reynolds and colleagues13 concluded that surgical repair leads to poorer results than nonsurgical treatment.
Clinical studies have demonstrated excellent results with nonintervention, and in vivo and in vitro studies have indicated that early motion can be initiated in partial lacerations of up to 95% of total CSA. Wray and Weeks6 treated 26 patients with partial lacerations varying from 25% to 95% of total CSA and noted 1 incidence of trigger finger (which resolved) and no late ruptures. They advocated treatment with early motion and excision or repair of beveled partial lacerations with simple sutures. Stahl and colleagues2 reported comparable outcomes in children with partial lacerations up to 75% of total CSA treated with and without surgery and noted no complications in either group. In a biomechanical study, Hariharan and colleagues4 found lacerations up to 75% can withstand forces associated with active unresisted mobilization.
Conversely, how many patients or surgeons want to return to the operating room to fix a late rupture when it could have been repaired in the primary setting? Schlenker and colleagues,1 reporting on a late flexor pollicus tendon rupture that required tendon grafting, recommended exploration and primary repair of all partial flexor tendon lacerations. Often, it is difficult to determine whether surgical repair is necessary to ensure the best outcome for the patient.
Our study results showed that, in the evaluation of flexor tendon lacerations, both accuracy and interobserver agreement were poor among residents and fellowship-trained hand surgeons, and intraobserver agreement was moderate. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. Our results highlight the difficulty in making accurate assessments of flexor tendon lacerations owing to the subjectivity of evaluation, which appear not to improve with surgeon experience.
How to manage complete flexor tendon lacerations in the hand is well documented and a subject of relative agreement among authors. However, treatment of partial flexor tendon lacerations is controversial and lacking clear consensus in the literature. Managing these injuries can be challenging, as clinicians must weigh the diminished tensile strength in the injured tendon and the potential for later complications (eg, entrapment, triggering, rupture) against the negative effects of tenorrhaphy.1 Several studies have found impaired tendon gliding on the basis of bulk and inflammatory reaction secondary to suture material within the flexor sheath as well as decreased tendon strength after tenorrhaphy.2-6 This finding led the investigators to recommend nonsurgical management for partial lacerations up to as much as 95% of the cross-sectional area (CSA) of the tendon. According to a survey by McCarthy and colleagues,7 45% of 591 members of the American Society for Surgery of the Hand (ASSH) indicated they would perform tenorrhaphy for a laceration that involved more than 50% of the tendon.
However, accurate assessment of partial-thickness flexor tendon lacerations is difficult owing to the subjectivity of evaluation. In the survey just mentioned,7 the majority of surgeons used the naked eye to make assessments, and only 14% used other means, such as a ruler, a pair of calipers, or loupe magnification. In addition, flexor tendon injuries are often evaluated under less than ideal circumstances—a dirty or bloody field, poor lighting, an uncomfortable patient.
We conducted a study to determine the interobserver and intraobserver reliability of surgeons assessing the percentage of CSA injured in partially lacerated digital flexor tendons. We hypothesized that participants’ accuracy and agreement would be poor.
Materials and Methods
Eight 1-cm transverse, volar skin incisions were made over the midportions of the middle and proximal phalanges of the index, middle, ring, and small fingers of a fresh-frozen human cadaver hand (Figure 1). The tendon sheaths were incised, and the flexor digitorum profundus tendons to each digit were delivered through the wound. With use of a method described previously by Manning and colleagues,8 the tendon was then placed over a flat metal post to be used as a cutting board, and the proposed laceration site was marked with ink. Under loupe magnification, a No. 15 blade was used to create a partial transverse, volar-to-dorsal laceration in each tendon.8 The goal was to create lacerations of about 30%, 50%, and 70% of the total CSA of the tendon. The tendons were then returned to the wound, and visibility of the marked laceration within the wound was ensured. A similar exercise was performed at the level of the proximal palmar crease. Four flexor digitorum superficialis tendons were exposed through 1-cm transverse incisions, and partial lacerations were made in the volar substance of the tendons. The tendons were then returned to the wound, resulting in 12 partially lacerated tendons (8 flexor digitorum profundus, 4 flexor digitorum superficialis).
Six orthopedic surgery residents (2 postgraduate year 1 [PGY-1], 2 PGY-3, 2 PGY-5) and 4 fellowship-trained hand surgeons participated in our study. Each was asked to evaluate the tendons and determine the percentage of total CSA lacerated. Loupe magnification and measuring tools were not permitted, but participants were allowed to handle the tendons. In addition, they were asked if they would perform tenorrhaphy on the injured tendons, given only the amount of injury. The participants repeated this exercise 4 weeks later.
After all measurements were made, a longitudinal incision was made down each of the digits, and the flexor tendons were exposed within the flexor sheath. The transverse incisions in the palm were connected to expose the flexor digitorum superficialis tendons. Under an operating microscope, a pair of digital microcalipers (Kobalt 0.5-ft Metric and SAE Caliper; Figure 2) accurate to 0.01 mm was used to measure the external width (a) and height (b + bˈ) of the tendons just proximal to the lacerations. Measurements were made with the caliper blades just touching the edges of the lacerated tendon, thus minimizing deformation of the tendon. Other measurements made at the laceration site were width of the remaining tendon (c) and height of the remaining tendon (bˈ). CSA of the tendon was calculated assuming a regular ellipsoid shape and using the equation:
Area = 1/2π(b+b')
The area of the tendon injured was determined by calculating the area under a parabola and using the equation:
Area = 2/3c[(b+b')-b']
Last, the percentage of total CSA lacerated was calculated using the equation:
Area (total area)
Statistical analysis was performed to determine accuracy and interobserver and intraobserver reliability. Paired t tests were used in the assessment of accuracy to determine if there were differences between estimated and calibrated measurements.
Results
The 10 participants’ estimates differed significantly (P < .0006) from the calibrated measurements, as did residents’ estimates (P < .0025) and fellowship-trained hand surgeons’ estimates (P < .0002). Estimates were scored 1 to 5 on the basis of proximity to calibrated measurements (Table 1). Thus, more accurate estimates received lower scores. Individual estimates were then scored and stratified into groups for comparison. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. These results are listed in Table 2. Once overall and grouped accuracy was analyzed, κ statistics were calculated to compare interobserver and intraobserver reliability. Overall interobserver agreement was poor for both initial readings (κ = 0.16) and secondary readings (κ = 0.16), indicating poor strength of agreement between individuals both initially and secondarily. Table 3 presents the κ interpretations. There was moderate overall intraobserver agreement (45.83%), indicating participants’ secondary estimates agreed with their primary estimates 46% of the time. Fellowship-trained hand surgeons and first-year residents had the highest intraobserver agreement (50.0%). These results are listed in Table 4.
Discussion
Accurate assessment of partial flexor tendon lacerations is difficult and subjective. There is no standardized method for determining the extent of injury, regardless of whether the evaluation is performed in an emergency department or in the operating room. As McCarthy and colleagues7 noted in their survey of ASSH members, naked eye assessment was by far the most popular means of estimating percentage injured in partial lacerations, and only 10% of the survey respondents used intraoperative measuring devices. Our study showed that participants agreed with one another less than 50% of the time when evaluating injuries without the aid of measuring devices. In addition, interobserver agreement in this study was about 50%, highlighting the difficulty in making an accurate and reproducible assessment.
In a study of canine flexor tendons, McCarthy and colleagues9 found calipers are inaccurate as well and do not provide a reliable means of assessing partial flexor tendon lacerations. They compared caliper measurements with laser micrometer measurements, and the differences averaged 29.3%. They suggested that methods for calculating loss of CSA and for creating precise lacerations must be developed in order to evaluate treatments. One such method is the “tenotome,” devised by Hitchcock and colleagues10: A device with standard scalpel blades is used to make uniform lacerations in tendons by leaving a constant area of the tendon intact, regardless of the size or shape of the original tendon. Measurements made with calipers or rulers assume the tendon has a regular ellipsoid shape, but in reality the shape is a double-ellipse, particularly within the flexor sheath.
Dobyns and colleagues11 observed that changes in CSA size can be related to changes in the size of the bundle pattern of the tendon. They found that, on average, the radial bundle comprised about 60% of the total CSA of the tendon. This finding was clarified by Grewal and colleagues.12 Using histologic sections of tendons plus photomicrographs, they determined that, in zone II of the index and small fingers, the ulnar bundle had an area consistently larger than 50% and the radial bundle less than 50% of the total tendon area. In the ring and middle fingers, the areas of both bundles were almost 50% of the total tendon area. The authors suggested that, using this bundle pattern theory of injury, surgeons could more accurately evaluate the extent of injury with the naked eye.
One of the questions that prompted our study is how reliable is the information a surgeon receives regarding a partial flexor tendon injury evaluated by someone else in another setting. What is done with this information is another question. The scenario can be considered in 2 settings: emergency department and operating room.
Given the poor accuracy and interobserver agreement found in our study, along with the inaccuracy of caliper and ruler measurements, it seems decisions to perform tenorrhaphy based on reported percentages lacerated are unreliable. Our results showed that the ability to accurately assess partial tendon injuries does not improve with surgeon experience, as fellowship-trained hand surgeons were not statistically more accurate or consistent than residents. To this effect, one institution treats all its partial flexor tendon lacerations with wound inspection and irrigation in the emergency department, under digital block and after neurovascular injury has been excluded.8 If the patient is able to actively flex and extend the digit without triggering, then the wound is closed without closing the tendon sheath, a dorsal blocking splint is applied, and motion is begun early, 48 hours later, regardless of laceration severity.
Once the decision has been made to go to the operating room and the injury is being evaluated, what should be done with the information from the measurement, whether made with loupe magnification, calipers, rulers, or the naked eye? Surgeons must weigh the risks for triggering, entrapment, and rupture of untreated partial tendon lacerations1 with the added bulk and potential for adhesions, along with the tensile strength reduction that accompanies tendon repair. Both Reynolds and colleagues13 and Ollinger and colleagues14 found tensile strength significantly diminished in sutured tendons. Ollinger and colleagues14 showed a decrease in tendon gliding after surgical exposure and tenorrhaphy for partial tendon lacerations. Reynolds and colleagues13 concluded that surgical repair leads to poorer results than nonsurgical treatment.
Clinical studies have demonstrated excellent results with nonintervention, and in vivo and in vitro studies have indicated that early motion can be initiated in partial lacerations of up to 95% of total CSA. Wray and Weeks6 treated 26 patients with partial lacerations varying from 25% to 95% of total CSA and noted 1 incidence of trigger finger (which resolved) and no late ruptures. They advocated treatment with early motion and excision or repair of beveled partial lacerations with simple sutures. Stahl and colleagues2 reported comparable outcomes in children with partial lacerations up to 75% of total CSA treated with and without surgery and noted no complications in either group. In a biomechanical study, Hariharan and colleagues4 found lacerations up to 75% can withstand forces associated with active unresisted mobilization.
Conversely, how many patients or surgeons want to return to the operating room to fix a late rupture when it could have been repaired in the primary setting? Schlenker and colleagues,1 reporting on a late flexor pollicus tendon rupture that required tendon grafting, recommended exploration and primary repair of all partial flexor tendon lacerations. Often, it is difficult to determine whether surgical repair is necessary to ensure the best outcome for the patient.
Our study results showed that, in the evaluation of flexor tendon lacerations, both accuracy and interobserver agreement were poor among residents and fellowship-trained hand surgeons, and intraobserver agreement was moderate. Third-year residents were the most accurate residents, and there was no difference in accuracy between residents and fellowship-trained hand surgeons. Our results highlight the difficulty in making accurate assessments of flexor tendon lacerations owing to the subjectivity of evaluation, which appear not to improve with surgeon experience.
1. Schlenker JD, Lister GD, Kleinert HE. Three complications of untreated partial laceration of flexor tendon—entrapment, rupture, and triggering. J Hand Surg Am. 1981;6(4):392-398.
2. Stahl S, Kaufman T, Bialik V. Partial lacerations of flexor tendons in children. Primary repair versus conservative treatment. J Hand Surg Br. 1997;22(3):377-380.
3. Al-Qattan MM. Conservative management of zone II partial flexor tendon lacerations greater than half the width of the tendon. J Hand Surg Am. 2000;25(6):1118-1121.
4. Hariharan JS, Diao E, Soejima O, Lotz JC. Partial lacerations of human digital flexor tendons: a biomechanical analysis. J Hand Surg Am. 1997;22(6):1011-1015.
5. Bishop AT, Cooney WP 3rd, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26(4):301-312.
6. Wray RC Jr, Weeks PM. Treatment of partial tendon lacerations. Hand. 1980;12(2):163-166.
7. McCarthy DM, Boardman ND 3rd, Tramaglini DM, Sotereanos DG, Herndon JH. Clinical management of partially lacerated digital flexor tendons: a survey of hand surgeons. J Hand Surg Am. 1995;20(2):273-275.
8. Manning DW, Spiguel AR, Mass DP. Biomechanical analysis of partial flexor tendon lacerations in zone II of human cadavers. J Hand Surg Am. 2010;35(1):11-18.
9. McCarthy DM, Tramaglini DM, Chan SS, Schmidt CC, Sotereanos DG, Herndon JH. Effect of partial laceration on the structural properties of the canine FDP tendon: an in vitro study. J Hand Surg Am. 1995;20(5):795-800.
10. Hitchcock TF, Candel AG, Light TR, Blevens AD. New technique for producing uniform partial lacerations of tendons. J Orthop Res. 1989;7(3):451-455.
11. Dobyns RC, Cooney WC, Wood MB. Effect of partial lacerations on canine flexor tendons. Minn Med. 1982;65(1):27-32.
12. Grewal R, Sotereanos DG, Rao U, Herndon JH, Woo SL. Bundle pattern of the flexor digitorum profundus tendon in zone II of the hand: a quantitative assessment of the size of a laceration. J Hand Surg Am. 1996;21(6):978-983.
13. Reynolds B, Wray RC Jr, Weeks PM. Should an incompletely severed tendon be sutured? Plast Reconstr Surg. 1976;57(1):36-38.
14. Ollinger H, Wray RC Jr, Weeks PM. Effects of suture on tensile strength gain of partially and completely severed tendons. Surg Forum. 1975;26:63-64.
1. Schlenker JD, Lister GD, Kleinert HE. Three complications of untreated partial laceration of flexor tendon—entrapment, rupture, and triggering. J Hand Surg Am. 1981;6(4):392-398.
2. Stahl S, Kaufman T, Bialik V. Partial lacerations of flexor tendons in children. Primary repair versus conservative treatment. J Hand Surg Br. 1997;22(3):377-380.
3. Al-Qattan MM. Conservative management of zone II partial flexor tendon lacerations greater than half the width of the tendon. J Hand Surg Am. 2000;25(6):1118-1121.
4. Hariharan JS, Diao E, Soejima O, Lotz JC. Partial lacerations of human digital flexor tendons: a biomechanical analysis. J Hand Surg Am. 1997;22(6):1011-1015.
5. Bishop AT, Cooney WP 3rd, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26(4):301-312.
6. Wray RC Jr, Weeks PM. Treatment of partial tendon lacerations. Hand. 1980;12(2):163-166.
7. McCarthy DM, Boardman ND 3rd, Tramaglini DM, Sotereanos DG, Herndon JH. Clinical management of partially lacerated digital flexor tendons: a survey of hand surgeons. J Hand Surg Am. 1995;20(2):273-275.
8. Manning DW, Spiguel AR, Mass DP. Biomechanical analysis of partial flexor tendon lacerations in zone II of human cadavers. J Hand Surg Am. 2010;35(1):11-18.
9. McCarthy DM, Tramaglini DM, Chan SS, Schmidt CC, Sotereanos DG, Herndon JH. Effect of partial laceration on the structural properties of the canine FDP tendon: an in vitro study. J Hand Surg Am. 1995;20(5):795-800.
10. Hitchcock TF, Candel AG, Light TR, Blevens AD. New technique for producing uniform partial lacerations of tendons. J Orthop Res. 1989;7(3):451-455.
11. Dobyns RC, Cooney WC, Wood MB. Effect of partial lacerations on canine flexor tendons. Minn Med. 1982;65(1):27-32.
12. Grewal R, Sotereanos DG, Rao U, Herndon JH, Woo SL. Bundle pattern of the flexor digitorum profundus tendon in zone II of the hand: a quantitative assessment of the size of a laceration. J Hand Surg Am. 1996;21(6):978-983.
13. Reynolds B, Wray RC Jr, Weeks PM. Should an incompletely severed tendon be sutured? Plast Reconstr Surg. 1976;57(1):36-38.
14. Ollinger H, Wray RC Jr, Weeks PM. Effects of suture on tensile strength gain of partially and completely severed tendons. Surg Forum. 1975;26:63-64.
Harrington Rod Revision After Failed Total Hip Arthroplasty Due to Missed Acetabular Metastasis
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
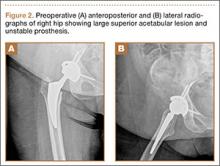
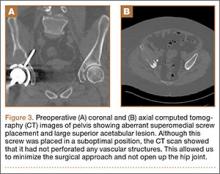
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
Pilot Study for an Orthopedic Surgical Training Laboratory for Basic Motor Skills
For the resident, the surgical residency is physically, emotionally, and intellectually demanding, requiring longitudinally concentrated effort. Although education of orthopedic surgeons necessarily occurs within the context of the health care delivery system, vital lessons also are taught in laboratories, skill stations, and surgical simulators. Before practice-based learning can take place, residents must gain experience and demonstrate growth in surgical skills, including decision-making and technical skills. These skill sets are difficult to systematically teach and objectively analyze.
The most effective way to teach and assess a resident’s knowledge of musculoskeletal medicine remains unclear at this point. Much of the current literature addresses the issue at the medical student level.1-7 Some studies have shown the effectiveness of surgical training programs, both cadaveric and computer-based simulators, in teaching various surgical skill sets.8-14 The orthopedic literature has seen a boom in surgical simulators aimed at the upper-level resident. Many of the topics involve use of arthroscopic simulators.15-19 Evidence suggests that simulators can discriminate between novice and expert users, but discrimination between novice and intermediate trainees in surgical education should be paramount.20
The American Board of Orthopaedic Surgery (ABOS) and the orthopedic Residency Review Committee (RRC) recommended new requirements for structured motor skills training in basic orthopedic surgery education,21 which were approved by the Accreditation Council for Graduate Medical Education (ACGME) board of directors and went into effect on July 1, 2013. In response to the new ACGME guidelines, our institution created a skills laboratory devoted to surgical simulation. Our focus in implementing this surgical skills simulation was junior-level, specifically postgraduate year 1 to 3 (PGY-1 to PGY-3), orthopedic residents. Our first goal was to set up a series of surgical training stations to educate junior-level residents in 4 core areas: handling and comfort with basic power equipment, casting/splinting, suturing, and surgical instrument identification. A secondary goal was to objectively evaluate the residents through written examinations (presession–postsession) and a novel ankle fracture model (pre–post).
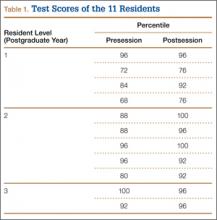
Materials and Methods
Institutional review board approval was obtained before beginning the investigation.
Written Examination
We created a multiple-choice 25-question written examination (Appendix) and administered it to 11 junior residents before and after they participated in the training. This examination assessed their knowledge base of basic orthopedic tenets, including basic bone healing, basic fracture repair (Arbeitsgemeinschaft für Osteosynthesefragen [AO] principles22), suturing, surgical instrument identification, casting/splinting, and elementary implant-design rationale.
Evaluator Scorecard
We created an evaluation scorecard (Figure 1) and had 2 faculty members and 2 senior-level residents complete it independently. Junior residents were evaluated on a sawbones lateral malleolar ankle fracture model at 2 time points. As with the written examinations, the junior residents completed the fracture model both before and immediately after the multiple skill sessions. Each of the 15 data points was scored from 1 to 4, for a total of 60 points.
Facility for Surgical Training Session
Our Clinical Skills Education and Assessment Center houses small-group interactive laboratories for administration, debriefing, and assessment of simulations with the latest in audiovisual equipment. Five stations were created: hands-on introduction to surgical power equipment using sawbones, wood, and polyvinylchloride (PVC) pipe; hands-on introduction to casting and splinting; hands-on introduction to suturing; hands-on interaction with surgical scrub technician assisting with instrument identification; and didactic PowerPoint (Microsoft, Redmond, Washington) presentation focusing on core trauma competencies, basic orthopedic design rationale, and basic bone biology.
Development of Surgical Skills Training Session
Multiple faculty members and senior-level residents collaborated to create the skill stations (Figure 2), which were designed based on ACGME recommendations and on weaknesses our program had seen in junior-level residents. We devoted an afternoon to this session, excusing our program’s junior residents from clinical responsibilities. Four PGY-1, 5 PGY-2, and 2 PGY-3 residents participated. (Four of our 15 junior residents were unable to attend because of clinical responsibilities.) The afternoon started by dividing the 11 junior residents into 2 groups. Before the session, while one group performed the ankle fracture model and was being evaluated, the other took the written examination. This closely timed portion was allotted only 20 minutes. Then residents were divided into 5 groups of 2 or 3 and were rotated through all 5 stations. Forty minutes were allotted for each station. Residents were not evaluated during this portion. The stations were intended solely for education, and each station was staffed by a faculty member and/or senior-level resident.
Cordless reciprocating saws and drills were purchased to introduce and refine junior residents’ motor skills. Sawbones, 2×4-in sections of wood, and PVC pipe were used in the training. Emphasis was placed on tactile feel and feedback with both sawing and drilling. For the casting and splinting session, we used 4-in fiberglass, 4-in plaster rolls, and cotton soft roll to demonstrate a multitude of common casts and splints (Figure 3). Casts included short- and long-arm casts and short-leg casts. Splinting included coaptation, sugar tong, and ulnar gutter splints for the upper extremity and a short-leg posterior splint for the lower extremity.
The didactic PowerPoint presentation drew largely from content in chapters of the book AO Principles of Fracture Management.22 Content included condensed, to-the-point high-yield summaries of AO tenets, basic bone healing and biology, and orthopedic implant-design rationale focused on these elementary principles:
◾ Basic screw design, including cortical, cancellous, and locking screw designs.
◾ Evolution of plate osteosynthesis to currently used locking compression plate.
◾ Locking plate principles.
◾ Lag technique.
◾ Plate use: compression mode, neutralization, bridging, buttress, anti-glide.
The suturing portion was performed with thawed ham hocks (Figure 4). This model replicates live tissue layers and allows a layered closure technique as a training tool. Both 0 and 2-0 absorbable suture were available for a layered, deep fascial closure; also available was 2-0 nonabsorbable nylon for the skin. Staple guns were available, as were basic surgical instruments, including quality needle drivers, Adson forceps, and suture scissors. The knots demonstrated included simple, horizontal mattress, vertical mattress, and tension-relieving. One- and 2-hand tying and instrument tying were reinforced.
The final session consisted of surgical instrument identification. A certified orthopedic scrub technician participated. On site were multiple trays, including a basic bone set, a hand-and-foot set, small and large fragment sets, and a hip set. A detailed review of each set was led by the surgical technician. This review was followed by a question-and-answer session with the junior residents. After the session, we ended with the written examination and the ankle fracture model.
Statistical Methods
We report presession and postsession means, modes, and medians as measures of score-central tendencies. Our small sample size makes the assumption of Gaussian distribution tenuous and more susceptible to outliers. Therefore, in addition to reporting means, we include medians and modes to more accurately account for outliers. Moreover, the κ statistic is a robust measure of interrater agreement for 2 or more groups. We report κ statistics to determine the interrater reliability of 4 independent observers.
Results
Written Examination
Eleven residents (PGY-1 to PGY-3) completed the examination (Table 1). For the entire group, mean (SD) presession percentile was 87.3 (10.4), median was 88, and mode was 96; mean (SD) was 80 (12.6) for PGY-1, 89.6 (6.7) for PGY-2, and 96 (5.7) for PGY-3. For the entire group, mean (SD) postsession percentile was 92 (8.4), median was 96, and mode was 96; mean (SD) was 85 (10.5) for PGY-1, 96 (4) for PGY-2, and 96 (0) for PGY-3 (Table 2).
There was a significant presession–postsession difference in scores among all test takers, regardless of training level (P = .019). The PGY-1 level did not reach statistical significance in improvement from presession to postsession (P = .080); the PGY-2 level also did not reach statistical significance in improvement (P = .099); the PGY-3 level did not have enough participants to calculate a P value based on a paired Student t test.
Ankle Fracture Model
Actual percentile scores are listed in Table 3. For the entire group, mean (SD) overall presession percentile was 68.6 (13.9), median was 67, and mode was 67; mean (SD) was 58.8 (9.8) for PGY-1, 76.1 (13.6) for PGY-2, and 69.5 (9.8) for PGY-3. For the entire group, mean (SD) postsession percentile was 95.2 (5.2), median was 97, and mode was 97; mean (SD) was 91.8 (6.3) for PGY-1, 97.1 (3.5) for PGY-2, and 97.3 (2.4) for PGY-3.
There was a large and significant presession–postsession difference in scores among all test takers, regardless of training level (P = .03). Each group reached statistical significance in improvement from presession to postsession: PGY-1 (P = .04), PGY-2 (P = .01), and PGY-3 (P = .03).
For κ calculations, we adjusted all scores to ordinal data and thus used a standard grading system:
Score Grade
90–100 A
80–89 B
70–79 C
60–69 D
0–59 F
For the presession fracture model, the κ among the 4 independent observational scorers was 0.1148 (Table 4), which is poor based on κ scoring criteria and which we attribute to the particularly harsh grading by 1 observational scorer (faculty 1) relative to the other scorers’. Examination of the κ scores of faculty 1 and faculty 2 indicated only 9.09% agreement. Conversely, the κ among resident scorers agreed 54.55% of the time. Removing faculty 1 as an outlier raised the κ score dramatically, to 0.3125 (fair interobserver agreement).
For the postsession fracture model, the κ among the 4 independent observational scorers improved only marginally, to 0.1156 (still poor), again attributed to a difference in severity of grading: faculty 1 (harsh) versus faculty 2 (relatively kind). Examination of the κ scores of faculty 1 and faculty 2 revealed 72.73% agreement; residents agreed 81.82% of the time.
Discussion
The importance of surgical skill development in resident education is emphasized in the ACGME Core Competencies.23 The ACGME instructed all programs to require residents to gain competency in 6 areas: patient care, interpersonal and communication skills, medical knowledge, professionalism, practice-based learning and systems-based practice. Although many surgeon educators and residents are focused on these 6 Core Competencies, current standards do not require surgical skills laboratory training and simply require residents to log cases into the ACGME website. Minimal case number recommendations are in place for graduating senior residents, but these numbers are based on averages with no strong scientific basis.
Although sweeping changes in orthopedic residency training went into effect July 1, 2013, this system remains untested and may offer room for improvement. One change is the restructuring of the PGY-1 internship. A basic surgical skills curriculum must include goals, objectives, and assessment metrics; skills used in the initial management of injured patients, including splinting, casting, application of traction devices, and other types of immobilization; and basic operative skills, including soft-tissue management, suturing, bone management, arthroscopy, fluoroscopy, and use of basic orthopedic equipment.21
Orthopedic program directors and residents were recently surveyed regarding the current state of orthopedic motor skills training.24 Three key findings deserve emphasis: There is a lack of objective criteria for evaluating resident performance in the skills laboratory; most program directors who have a laboratory do not understand the associated costs; and the most significant issue for program directors is the financial challenge of operating a motor skills laboratory. The survey findings strongly suggest that proposed changes in skills training should be accompanied by careful cost analysis before widespread implementation.
Although various online demonstrations of entire surgeries are available, as are textbooks describing a generalized approach to musculoskeletal surgery, we assume that, as laid out in the Core Competencies, residents are fine-tuning their surgical skills by actively participating in operating rooms under direct observation of attending physicians. To our knowledge, however, there are no data regarding how often this happens in the operative setting, where volume and efficiency are becoming increasingly scrutinized. There has been much concern over how hour restrictions will affect residents’ total operative experience.25,26 Finally, we have no means to objectively evaluate residents’ surgical skills on graduation.
Other programs have implemented surgical skill simulators, but an orthopedics-specific surgical skills laboratory, to our knowledge, has been discussed in only 1 study.21 Results from randomized controlled trials reported in the general surgery literature have proved simulation-based training leads to detectable benefits for learners in clinical settings.27-29 Over the past decade, some alternative surgical skills training methods have been adopted in orthopedic surgery as well. These methods include hands-on training in specifically designed surgical skills laboratories using cadaver models or synthetic bones; software tools; and computerized simulators. In recent years, numerous studies reported in the orthopedic literature have examined arthroscopic simulators in residency training.18-20,30-34 However, these studies are arguably more specific to sports subspecialties and thus more pertinent to upper-level trainees.
Our study results showed that surgical skills laboratory training should be a required aspect of our residents’ training. Although less of a dramatic improvement was noted in the written examination component of the laboratory, the overall knowledge base improved (Table 3). This was especially evident at the PGY-1 level, where written examination scores increased from a presession median of 80% to a postsession median of 85%. A larger degree of improvement was found with the ankle fracture model, and there was statistical improvement at all training levels, from PGY-1 to PGY-3. Previous work has shown that intensive laboratory-based training can be effective, particularly for first-year residents. Sonnadara and colleagues35 demonstrated that a 30-day intensive surgical skills course effectively helped first-year orthopedic residents develop targeted basic surgical skills. In a follow-up study, Sonnadara and colleagues36 demonstrated that a surgical skills course completed at the beginning of a residency was effective in teaching targeted technical skills, and that skills taught in this manner can have excellent retention rates.
There are limitations inherent in our skills course. The κ agreement in the ankle fracture model was low before and after administration, which we attribute to 1 observer outlier. This could be amended by removing outliers and further objectifying and simplifying the scoring system (A–F). Right now, we do not have enough data to determine whether the scores actually improve significantly through the training years or whether they will correlate with operating room experience. Our study had no control. For future investigations, we are considering having general orthopedic surgeons from the community perform the same scenarios and be graded with the same checklists as a control. Implementation, however, may be a challenge. Both our written examination and our ankle fracture model checklist have not been validated—this is one of our next steps. The point system used to score the ankle fracture model was subjectively developed and would benefit from further standardization before drawing conclusions about true validity.
Conclusion
Orthopedic residency programs, like programs in other surgical specialties, are increasingly focused on teaching and documenting the learning of core competencies, even as work-hour restrictions and demands for clinical efficiency limit the amount of time residents spend in the operating room. We have demonstrated the potential value of an intensive laboratory in improving junior-level residents’ basic surgical skills and knowledge. We will continue to refine our methods, with a goal being to create reproducible models that could be adapted by other orthopedic residency programs and by other surgical educators.
1. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
2. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
3. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
4. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
5. Corbett EC Jr, Elnicki DM, Conaway MR. When should students learn essential physical examination skills? Views of internal medicine clerkship directors in North America. Acad Med. 2008;83(1):96-99.
6. Coady DA, Walker DJ, Kay LJ. Teaching medical students musculoskeletal examination skills: identifying barriers to learning and ways of overcoming them. Scand J Rheumatol. 2004;33(1):47-51.
7. Saleh K, Messner R, Axtell S, Harris I, Mahowald ML. Development and evaluation of an integrated musculoskeletal disease course for medical students. J Bone Joint Surg Am. 2004;86(8):1653-1658.
8. van Empel PJ, Verdam MG, Huirne JA, Bonjer HJ, Meijerink WJ, Scheele F. Open knot-tying skills: resident skills assessed. J Obstet Gynaecol Res. 2013;39(5):1030-1036.
9. Barrier BF, Thompson AB, McCullough MW, Occhino JA. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7(6):374-379.
10. Liss MA, McDougall EM. Robotic surgical simulation. Cancer J. 2013;19(2):124-129.
11. Stegemann AP, Ahmed K, Syed JR, et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81(4):767-774.
12. Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg. 2013;58(2):524-528.
13. Kuhls DA, Risucci DA, Bowyer MW, Luchette FA. Advanced surgical skills for exposure in trauma: a new surgical skills cadaver course for surgery residents and fellows. J Trauma Acute Care Surg. 2013;74(2):664-670.
14. Sanfey HA, Dunnington GL. Basic surgical skills testing for junior residents: current views of general surgery program directors. J Am Coll Surg. 2011;212(3):406-412.
15. Alvand A, Khan T, Al-Ali S, Jackson WF, Price AJ, Rees JL. Simple visual parameters for objective assessment of arthroscopic skill. J Bone Joint Surg Am. 2012;94(13):e97.
16. Jackson WF, Khan T, Alvand A, et al. Learning and retaining simulated arthroscopic meniscal repair skills. J Bone Joint Surg Am. 2012;94(17):e132.
17. Pernar LI, Smink DS, Hicks G, Peyre SE. Residents can successfully teach basic surgical skills in the simulation center. J Surg Educ. 2012;69(5):617-622.
18. Tuijthof GJ, Visser P, Sierevelt IN, Van Dijk CN, Kerkhoffs GM. Does perception of usefulness of arthroscopic simulators differ with levels of experience? Clin Orthop. 2011;469(6):1701-1708.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Slade Shantz JA, Leiter JR, Gottschalk T, MacDonald PB. The internal validity of arthroscopic simulators and their effectiveness in arthroscopic education. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):33-40.
21. Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11(5):747-757.
22. Ruedi TP, Buckley RE, Moran CG. AO Principles of Fracture Management. Stuttgart, Germany: Thieme; 2007.
23. Chen CL, Chen WC, Chiang JH, Ho CF. Interscapular hibernoma: case report and literature review. Kaohsiung J Med Sci. 2011;27(8):348-352.
24. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
25. Baskies MA, Ruchelsman DE, Capeci CM, Zuckerman JD, Egol KA. Operative experience in an orthopaedic surgery residency program: the effect of work-hour restrictions. J Bone Joint Surg Am. 2008;90(4):924-927.
26. Pappas AJ, Teague DC. The impact of the Accreditation Council for Graduate Medical Education work-hour regulations on the surgical experience of orthopaedic surgery residents. J Bone Joint Surg Am. 2007;89(4):904-909.
27. Palter VN, Grantcharov T, Harvey A, Macrae HM. Ex vivo technical skills training transfers to the operating room and enhances cognitive learning: a randomized controlled trial. Ann Surg. 2011;253(5):886-889.
28. Franzeck FM, Rosenthal R, Muller MK, et al. Prospective randomized controlled trial of simulator-based versus traditional in-surgery laparoscopic camera navigation training. Surg Endosc. 2012;26(1):235-241.
29. Zendejas B, Cook DA, Bingener J, et al. Simulation-based mastery learning improves patient outcomes in laparoscopic inguinal hernia repair: a randomized controlled trial. Ann Surg. 2011;254(3):502-509.
30. Hui Y, Safir O, Dubrowski A, Carnahan H. What skills should simulation training in arthroscopy teach residents? A focus on resident input. Int J Comput Assist Radiol Surg. 2013;8(6):945-953.
31. Butler A, Olson T, Koehler R, Nicandri G. Do the skills acquired by novice surgeons using anatomic dry models transfer effectively to the task of diagnostic knee arthroscopy performed on cadaveric specimens? J Bone Joint Surg Am. 2013;95(3):e15(1-8).
32. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
33. Elliott MJ, Caprise PA, Henning AE, Kurtz CA, Sekiya JK. Diagnostic knee arthroscopy: a pilot study to evaluate surgical skills. Arthroscopy. 2012;28(2):218-224.
34. Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop. 2011;82(1):90-95.
35. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
36. Sonnadara RR, Garbedian S, Safir O, et al. Orthopaedic boot camp II: examining the retention rates of an intensive surgical skills course. Surgery. 2012;151(6):803-807.
For the resident, the surgical residency is physically, emotionally, and intellectually demanding, requiring longitudinally concentrated effort. Although education of orthopedic surgeons necessarily occurs within the context of the health care delivery system, vital lessons also are taught in laboratories, skill stations, and surgical simulators. Before practice-based learning can take place, residents must gain experience and demonstrate growth in surgical skills, including decision-making and technical skills. These skill sets are difficult to systematically teach and objectively analyze.
The most effective way to teach and assess a resident’s knowledge of musculoskeletal medicine remains unclear at this point. Much of the current literature addresses the issue at the medical student level.1-7 Some studies have shown the effectiveness of surgical training programs, both cadaveric and computer-based simulators, in teaching various surgical skill sets.8-14 The orthopedic literature has seen a boom in surgical simulators aimed at the upper-level resident. Many of the topics involve use of arthroscopic simulators.15-19 Evidence suggests that simulators can discriminate between novice and expert users, but discrimination between novice and intermediate trainees in surgical education should be paramount.20
The American Board of Orthopaedic Surgery (ABOS) and the orthopedic Residency Review Committee (RRC) recommended new requirements for structured motor skills training in basic orthopedic surgery education,21 which were approved by the Accreditation Council for Graduate Medical Education (ACGME) board of directors and went into effect on July 1, 2013. In response to the new ACGME guidelines, our institution created a skills laboratory devoted to surgical simulation. Our focus in implementing this surgical skills simulation was junior-level, specifically postgraduate year 1 to 3 (PGY-1 to PGY-3), orthopedic residents. Our first goal was to set up a series of surgical training stations to educate junior-level residents in 4 core areas: handling and comfort with basic power equipment, casting/splinting, suturing, and surgical instrument identification. A secondary goal was to objectively evaluate the residents through written examinations (presession–postsession) and a novel ankle fracture model (pre–post).
Materials and Methods
Institutional review board approval was obtained before beginning the investigation.
Written Examination
We created a multiple-choice 25-question written examination (Appendix) and administered it to 11 junior residents before and after they participated in the training. This examination assessed their knowledge base of basic orthopedic tenets, including basic bone healing, basic fracture repair (Arbeitsgemeinschaft für Osteosynthesefragen [AO] principles22), suturing, surgical instrument identification, casting/splinting, and elementary implant-design rationale.
Evaluator Scorecard
We created an evaluation scorecard (Figure 1) and had 2 faculty members and 2 senior-level residents complete it independently. Junior residents were evaluated on a sawbones lateral malleolar ankle fracture model at 2 time points. As with the written examinations, the junior residents completed the fracture model both before and immediately after the multiple skill sessions. Each of the 15 data points was scored from 1 to 4, for a total of 60 points.
Facility for Surgical Training Session
Our Clinical Skills Education and Assessment Center houses small-group interactive laboratories for administration, debriefing, and assessment of simulations with the latest in audiovisual equipment. Five stations were created: hands-on introduction to surgical power equipment using sawbones, wood, and polyvinylchloride (PVC) pipe; hands-on introduction to casting and splinting; hands-on introduction to suturing; hands-on interaction with surgical scrub technician assisting with instrument identification; and didactic PowerPoint (Microsoft, Redmond, Washington) presentation focusing on core trauma competencies, basic orthopedic design rationale, and basic bone biology.
Development of Surgical Skills Training Session
Multiple faculty members and senior-level residents collaborated to create the skill stations (Figure 2), which were designed based on ACGME recommendations and on weaknesses our program had seen in junior-level residents. We devoted an afternoon to this session, excusing our program’s junior residents from clinical responsibilities. Four PGY-1, 5 PGY-2, and 2 PGY-3 residents participated. (Four of our 15 junior residents were unable to attend because of clinical responsibilities.) The afternoon started by dividing the 11 junior residents into 2 groups. Before the session, while one group performed the ankle fracture model and was being evaluated, the other took the written examination. This closely timed portion was allotted only 20 minutes. Then residents were divided into 5 groups of 2 or 3 and were rotated through all 5 stations. Forty minutes were allotted for each station. Residents were not evaluated during this portion. The stations were intended solely for education, and each station was staffed by a faculty member and/or senior-level resident.
Cordless reciprocating saws and drills were purchased to introduce and refine junior residents’ motor skills. Sawbones, 2×4-in sections of wood, and PVC pipe were used in the training. Emphasis was placed on tactile feel and feedback with both sawing and drilling. For the casting and splinting session, we used 4-in fiberglass, 4-in plaster rolls, and cotton soft roll to demonstrate a multitude of common casts and splints (Figure 3). Casts included short- and long-arm casts and short-leg casts. Splinting included coaptation, sugar tong, and ulnar gutter splints for the upper extremity and a short-leg posterior splint for the lower extremity.
The didactic PowerPoint presentation drew largely from content in chapters of the book AO Principles of Fracture Management.22 Content included condensed, to-the-point high-yield summaries of AO tenets, basic bone healing and biology, and orthopedic implant-design rationale focused on these elementary principles:
◾ Basic screw design, including cortical, cancellous, and locking screw designs.
◾ Evolution of plate osteosynthesis to currently used locking compression plate.
◾ Locking plate principles.
◾ Lag technique.
◾ Plate use: compression mode, neutralization, bridging, buttress, anti-glide.
The suturing portion was performed with thawed ham hocks (Figure 4). This model replicates live tissue layers and allows a layered closure technique as a training tool. Both 0 and 2-0 absorbable suture were available for a layered, deep fascial closure; also available was 2-0 nonabsorbable nylon for the skin. Staple guns were available, as were basic surgical instruments, including quality needle drivers, Adson forceps, and suture scissors. The knots demonstrated included simple, horizontal mattress, vertical mattress, and tension-relieving. One- and 2-hand tying and instrument tying were reinforced.
The final session consisted of surgical instrument identification. A certified orthopedic scrub technician participated. On site were multiple trays, including a basic bone set, a hand-and-foot set, small and large fragment sets, and a hip set. A detailed review of each set was led by the surgical technician. This review was followed by a question-and-answer session with the junior residents. After the session, we ended with the written examination and the ankle fracture model.
Statistical Methods
We report presession and postsession means, modes, and medians as measures of score-central tendencies. Our small sample size makes the assumption of Gaussian distribution tenuous and more susceptible to outliers. Therefore, in addition to reporting means, we include medians and modes to more accurately account for outliers. Moreover, the κ statistic is a robust measure of interrater agreement for 2 or more groups. We report κ statistics to determine the interrater reliability of 4 independent observers.
Results
Written Examination
Eleven residents (PGY-1 to PGY-3) completed the examination (Table 1). For the entire group, mean (SD) presession percentile was 87.3 (10.4), median was 88, and mode was 96; mean (SD) was 80 (12.6) for PGY-1, 89.6 (6.7) for PGY-2, and 96 (5.7) for PGY-3. For the entire group, mean (SD) postsession percentile was 92 (8.4), median was 96, and mode was 96; mean (SD) was 85 (10.5) for PGY-1, 96 (4) for PGY-2, and 96 (0) for PGY-3 (Table 2).
There was a significant presession–postsession difference in scores among all test takers, regardless of training level (P = .019). The PGY-1 level did not reach statistical significance in improvement from presession to postsession (P = .080); the PGY-2 level also did not reach statistical significance in improvement (P = .099); the PGY-3 level did not have enough participants to calculate a P value based on a paired Student t test.
Ankle Fracture Model
Actual percentile scores are listed in Table 3. For the entire group, mean (SD) overall presession percentile was 68.6 (13.9), median was 67, and mode was 67; mean (SD) was 58.8 (9.8) for PGY-1, 76.1 (13.6) for PGY-2, and 69.5 (9.8) for PGY-3. For the entire group, mean (SD) postsession percentile was 95.2 (5.2), median was 97, and mode was 97; mean (SD) was 91.8 (6.3) for PGY-1, 97.1 (3.5) for PGY-2, and 97.3 (2.4) for PGY-3.
There was a large and significant presession–postsession difference in scores among all test takers, regardless of training level (P = .03). Each group reached statistical significance in improvement from presession to postsession: PGY-1 (P = .04), PGY-2 (P = .01), and PGY-3 (P = .03).
For κ calculations, we adjusted all scores to ordinal data and thus used a standard grading system:
Score Grade
90–100 A
80–89 B
70–79 C
60–69 D
0–59 F
For the presession fracture model, the κ among the 4 independent observational scorers was 0.1148 (Table 4), which is poor based on κ scoring criteria and which we attribute to the particularly harsh grading by 1 observational scorer (faculty 1) relative to the other scorers’. Examination of the κ scores of faculty 1 and faculty 2 indicated only 9.09% agreement. Conversely, the κ among resident scorers agreed 54.55% of the time. Removing faculty 1 as an outlier raised the κ score dramatically, to 0.3125 (fair interobserver agreement).
For the postsession fracture model, the κ among the 4 independent observational scorers improved only marginally, to 0.1156 (still poor), again attributed to a difference in severity of grading: faculty 1 (harsh) versus faculty 2 (relatively kind). Examination of the κ scores of faculty 1 and faculty 2 revealed 72.73% agreement; residents agreed 81.82% of the time.
Discussion
The importance of surgical skill development in resident education is emphasized in the ACGME Core Competencies.23 The ACGME instructed all programs to require residents to gain competency in 6 areas: patient care, interpersonal and communication skills, medical knowledge, professionalism, practice-based learning and systems-based practice. Although many surgeon educators and residents are focused on these 6 Core Competencies, current standards do not require surgical skills laboratory training and simply require residents to log cases into the ACGME website. Minimal case number recommendations are in place for graduating senior residents, but these numbers are based on averages with no strong scientific basis.
Although sweeping changes in orthopedic residency training went into effect July 1, 2013, this system remains untested and may offer room for improvement. One change is the restructuring of the PGY-1 internship. A basic surgical skills curriculum must include goals, objectives, and assessment metrics; skills used in the initial management of injured patients, including splinting, casting, application of traction devices, and other types of immobilization; and basic operative skills, including soft-tissue management, suturing, bone management, arthroscopy, fluoroscopy, and use of basic orthopedic equipment.21
Orthopedic program directors and residents were recently surveyed regarding the current state of orthopedic motor skills training.24 Three key findings deserve emphasis: There is a lack of objective criteria for evaluating resident performance in the skills laboratory; most program directors who have a laboratory do not understand the associated costs; and the most significant issue for program directors is the financial challenge of operating a motor skills laboratory. The survey findings strongly suggest that proposed changes in skills training should be accompanied by careful cost analysis before widespread implementation.
Although various online demonstrations of entire surgeries are available, as are textbooks describing a generalized approach to musculoskeletal surgery, we assume that, as laid out in the Core Competencies, residents are fine-tuning their surgical skills by actively participating in operating rooms under direct observation of attending physicians. To our knowledge, however, there are no data regarding how often this happens in the operative setting, where volume and efficiency are becoming increasingly scrutinized. There has been much concern over how hour restrictions will affect residents’ total operative experience.25,26 Finally, we have no means to objectively evaluate residents’ surgical skills on graduation.
Other programs have implemented surgical skill simulators, but an orthopedics-specific surgical skills laboratory, to our knowledge, has been discussed in only 1 study.21 Results from randomized controlled trials reported in the general surgery literature have proved simulation-based training leads to detectable benefits for learners in clinical settings.27-29 Over the past decade, some alternative surgical skills training methods have been adopted in orthopedic surgery as well. These methods include hands-on training in specifically designed surgical skills laboratories using cadaver models or synthetic bones; software tools; and computerized simulators. In recent years, numerous studies reported in the orthopedic literature have examined arthroscopic simulators in residency training.18-20,30-34 However, these studies are arguably more specific to sports subspecialties and thus more pertinent to upper-level trainees.
Our study results showed that surgical skills laboratory training should be a required aspect of our residents’ training. Although less of a dramatic improvement was noted in the written examination component of the laboratory, the overall knowledge base improved (Table 3). This was especially evident at the PGY-1 level, where written examination scores increased from a presession median of 80% to a postsession median of 85%. A larger degree of improvement was found with the ankle fracture model, and there was statistical improvement at all training levels, from PGY-1 to PGY-3. Previous work has shown that intensive laboratory-based training can be effective, particularly for first-year residents. Sonnadara and colleagues35 demonstrated that a 30-day intensive surgical skills course effectively helped first-year orthopedic residents develop targeted basic surgical skills. In a follow-up study, Sonnadara and colleagues36 demonstrated that a surgical skills course completed at the beginning of a residency was effective in teaching targeted technical skills, and that skills taught in this manner can have excellent retention rates.
There are limitations inherent in our skills course. The κ agreement in the ankle fracture model was low before and after administration, which we attribute to 1 observer outlier. This could be amended by removing outliers and further objectifying and simplifying the scoring system (A–F). Right now, we do not have enough data to determine whether the scores actually improve significantly through the training years or whether they will correlate with operating room experience. Our study had no control. For future investigations, we are considering having general orthopedic surgeons from the community perform the same scenarios and be graded with the same checklists as a control. Implementation, however, may be a challenge. Both our written examination and our ankle fracture model checklist have not been validated—this is one of our next steps. The point system used to score the ankle fracture model was subjectively developed and would benefit from further standardization before drawing conclusions about true validity.
Conclusion
Orthopedic residency programs, like programs in other surgical specialties, are increasingly focused on teaching and documenting the learning of core competencies, even as work-hour restrictions and demands for clinical efficiency limit the amount of time residents spend in the operating room. We have demonstrated the potential value of an intensive laboratory in improving junior-level residents’ basic surgical skills and knowledge. We will continue to refine our methods, with a goal being to create reproducible models that could be adapted by other orthopedic residency programs and by other surgical educators.
For the resident, the surgical residency is physically, emotionally, and intellectually demanding, requiring longitudinally concentrated effort. Although education of orthopedic surgeons necessarily occurs within the context of the health care delivery system, vital lessons also are taught in laboratories, skill stations, and surgical simulators. Before practice-based learning can take place, residents must gain experience and demonstrate growth in surgical skills, including decision-making and technical skills. These skill sets are difficult to systematically teach and objectively analyze.
The most effective way to teach and assess a resident’s knowledge of musculoskeletal medicine remains unclear at this point. Much of the current literature addresses the issue at the medical student level.1-7 Some studies have shown the effectiveness of surgical training programs, both cadaveric and computer-based simulators, in teaching various surgical skill sets.8-14 The orthopedic literature has seen a boom in surgical simulators aimed at the upper-level resident. Many of the topics involve use of arthroscopic simulators.15-19 Evidence suggests that simulators can discriminate between novice and expert users, but discrimination between novice and intermediate trainees in surgical education should be paramount.20
The American Board of Orthopaedic Surgery (ABOS) and the orthopedic Residency Review Committee (RRC) recommended new requirements for structured motor skills training in basic orthopedic surgery education,21 which were approved by the Accreditation Council for Graduate Medical Education (ACGME) board of directors and went into effect on July 1, 2013. In response to the new ACGME guidelines, our institution created a skills laboratory devoted to surgical simulation. Our focus in implementing this surgical skills simulation was junior-level, specifically postgraduate year 1 to 3 (PGY-1 to PGY-3), orthopedic residents. Our first goal was to set up a series of surgical training stations to educate junior-level residents in 4 core areas: handling and comfort with basic power equipment, casting/splinting, suturing, and surgical instrument identification. A secondary goal was to objectively evaluate the residents through written examinations (presession–postsession) and a novel ankle fracture model (pre–post).
Materials and Methods
Institutional review board approval was obtained before beginning the investigation.
Written Examination
We created a multiple-choice 25-question written examination (Appendix) and administered it to 11 junior residents before and after they participated in the training. This examination assessed their knowledge base of basic orthopedic tenets, including basic bone healing, basic fracture repair (Arbeitsgemeinschaft für Osteosynthesefragen [AO] principles22), suturing, surgical instrument identification, casting/splinting, and elementary implant-design rationale.
Evaluator Scorecard
We created an evaluation scorecard (Figure 1) and had 2 faculty members and 2 senior-level residents complete it independently. Junior residents were evaluated on a sawbones lateral malleolar ankle fracture model at 2 time points. As with the written examinations, the junior residents completed the fracture model both before and immediately after the multiple skill sessions. Each of the 15 data points was scored from 1 to 4, for a total of 60 points.
Facility for Surgical Training Session
Our Clinical Skills Education and Assessment Center houses small-group interactive laboratories for administration, debriefing, and assessment of simulations with the latest in audiovisual equipment. Five stations were created: hands-on introduction to surgical power equipment using sawbones, wood, and polyvinylchloride (PVC) pipe; hands-on introduction to casting and splinting; hands-on introduction to suturing; hands-on interaction with surgical scrub technician assisting with instrument identification; and didactic PowerPoint (Microsoft, Redmond, Washington) presentation focusing on core trauma competencies, basic orthopedic design rationale, and basic bone biology.
Development of Surgical Skills Training Session
Multiple faculty members and senior-level residents collaborated to create the skill stations (Figure 2), which were designed based on ACGME recommendations and on weaknesses our program had seen in junior-level residents. We devoted an afternoon to this session, excusing our program’s junior residents from clinical responsibilities. Four PGY-1, 5 PGY-2, and 2 PGY-3 residents participated. (Four of our 15 junior residents were unable to attend because of clinical responsibilities.) The afternoon started by dividing the 11 junior residents into 2 groups. Before the session, while one group performed the ankle fracture model and was being evaluated, the other took the written examination. This closely timed portion was allotted only 20 minutes. Then residents were divided into 5 groups of 2 or 3 and were rotated through all 5 stations. Forty minutes were allotted for each station. Residents were not evaluated during this portion. The stations were intended solely for education, and each station was staffed by a faculty member and/or senior-level resident.
Cordless reciprocating saws and drills were purchased to introduce and refine junior residents’ motor skills. Sawbones, 2×4-in sections of wood, and PVC pipe were used in the training. Emphasis was placed on tactile feel and feedback with both sawing and drilling. For the casting and splinting session, we used 4-in fiberglass, 4-in plaster rolls, and cotton soft roll to demonstrate a multitude of common casts and splints (Figure 3). Casts included short- and long-arm casts and short-leg casts. Splinting included coaptation, sugar tong, and ulnar gutter splints for the upper extremity and a short-leg posterior splint for the lower extremity.
The didactic PowerPoint presentation drew largely from content in chapters of the book AO Principles of Fracture Management.22 Content included condensed, to-the-point high-yield summaries of AO tenets, basic bone healing and biology, and orthopedic implant-design rationale focused on these elementary principles:
◾ Basic screw design, including cortical, cancellous, and locking screw designs.
◾ Evolution of plate osteosynthesis to currently used locking compression plate.
◾ Locking plate principles.
◾ Lag technique.
◾ Plate use: compression mode, neutralization, bridging, buttress, anti-glide.
The suturing portion was performed with thawed ham hocks (Figure 4). This model replicates live tissue layers and allows a layered closure technique as a training tool. Both 0 and 2-0 absorbable suture were available for a layered, deep fascial closure; also available was 2-0 nonabsorbable nylon for the skin. Staple guns were available, as were basic surgical instruments, including quality needle drivers, Adson forceps, and suture scissors. The knots demonstrated included simple, horizontal mattress, vertical mattress, and tension-relieving. One- and 2-hand tying and instrument tying were reinforced.
The final session consisted of surgical instrument identification. A certified orthopedic scrub technician participated. On site were multiple trays, including a basic bone set, a hand-and-foot set, small and large fragment sets, and a hip set. A detailed review of each set was led by the surgical technician. This review was followed by a question-and-answer session with the junior residents. After the session, we ended with the written examination and the ankle fracture model.
Statistical Methods
We report presession and postsession means, modes, and medians as measures of score-central tendencies. Our small sample size makes the assumption of Gaussian distribution tenuous and more susceptible to outliers. Therefore, in addition to reporting means, we include medians and modes to more accurately account for outliers. Moreover, the κ statistic is a robust measure of interrater agreement for 2 or more groups. We report κ statistics to determine the interrater reliability of 4 independent observers.
Results
Written Examination
Eleven residents (PGY-1 to PGY-3) completed the examination (Table 1). For the entire group, mean (SD) presession percentile was 87.3 (10.4), median was 88, and mode was 96; mean (SD) was 80 (12.6) for PGY-1, 89.6 (6.7) for PGY-2, and 96 (5.7) for PGY-3. For the entire group, mean (SD) postsession percentile was 92 (8.4), median was 96, and mode was 96; mean (SD) was 85 (10.5) for PGY-1, 96 (4) for PGY-2, and 96 (0) for PGY-3 (Table 2).
There was a significant presession–postsession difference in scores among all test takers, regardless of training level (P = .019). The PGY-1 level did not reach statistical significance in improvement from presession to postsession (P = .080); the PGY-2 level also did not reach statistical significance in improvement (P = .099); the PGY-3 level did not have enough participants to calculate a P value based on a paired Student t test.
Ankle Fracture Model
Actual percentile scores are listed in Table 3. For the entire group, mean (SD) overall presession percentile was 68.6 (13.9), median was 67, and mode was 67; mean (SD) was 58.8 (9.8) for PGY-1, 76.1 (13.6) for PGY-2, and 69.5 (9.8) for PGY-3. For the entire group, mean (SD) postsession percentile was 95.2 (5.2), median was 97, and mode was 97; mean (SD) was 91.8 (6.3) for PGY-1, 97.1 (3.5) for PGY-2, and 97.3 (2.4) for PGY-3.
There was a large and significant presession–postsession difference in scores among all test takers, regardless of training level (P = .03). Each group reached statistical significance in improvement from presession to postsession: PGY-1 (P = .04), PGY-2 (P = .01), and PGY-3 (P = .03).
For κ calculations, we adjusted all scores to ordinal data and thus used a standard grading system:
Score Grade
90–100 A
80–89 B
70–79 C
60–69 D
0–59 F
For the presession fracture model, the κ among the 4 independent observational scorers was 0.1148 (Table 4), which is poor based on κ scoring criteria and which we attribute to the particularly harsh grading by 1 observational scorer (faculty 1) relative to the other scorers’. Examination of the κ scores of faculty 1 and faculty 2 indicated only 9.09% agreement. Conversely, the κ among resident scorers agreed 54.55% of the time. Removing faculty 1 as an outlier raised the κ score dramatically, to 0.3125 (fair interobserver agreement).
For the postsession fracture model, the κ among the 4 independent observational scorers improved only marginally, to 0.1156 (still poor), again attributed to a difference in severity of grading: faculty 1 (harsh) versus faculty 2 (relatively kind). Examination of the κ scores of faculty 1 and faculty 2 revealed 72.73% agreement; residents agreed 81.82% of the time.
Discussion
The importance of surgical skill development in resident education is emphasized in the ACGME Core Competencies.23 The ACGME instructed all programs to require residents to gain competency in 6 areas: patient care, interpersonal and communication skills, medical knowledge, professionalism, practice-based learning and systems-based practice. Although many surgeon educators and residents are focused on these 6 Core Competencies, current standards do not require surgical skills laboratory training and simply require residents to log cases into the ACGME website. Minimal case number recommendations are in place for graduating senior residents, but these numbers are based on averages with no strong scientific basis.
Although sweeping changes in orthopedic residency training went into effect July 1, 2013, this system remains untested and may offer room for improvement. One change is the restructuring of the PGY-1 internship. A basic surgical skills curriculum must include goals, objectives, and assessment metrics; skills used in the initial management of injured patients, including splinting, casting, application of traction devices, and other types of immobilization; and basic operative skills, including soft-tissue management, suturing, bone management, arthroscopy, fluoroscopy, and use of basic orthopedic equipment.21
Orthopedic program directors and residents were recently surveyed regarding the current state of orthopedic motor skills training.24 Three key findings deserve emphasis: There is a lack of objective criteria for evaluating resident performance in the skills laboratory; most program directors who have a laboratory do not understand the associated costs; and the most significant issue for program directors is the financial challenge of operating a motor skills laboratory. The survey findings strongly suggest that proposed changes in skills training should be accompanied by careful cost analysis before widespread implementation.
Although various online demonstrations of entire surgeries are available, as are textbooks describing a generalized approach to musculoskeletal surgery, we assume that, as laid out in the Core Competencies, residents are fine-tuning their surgical skills by actively participating in operating rooms under direct observation of attending physicians. To our knowledge, however, there are no data regarding how often this happens in the operative setting, where volume and efficiency are becoming increasingly scrutinized. There has been much concern over how hour restrictions will affect residents’ total operative experience.25,26 Finally, we have no means to objectively evaluate residents’ surgical skills on graduation.
Other programs have implemented surgical skill simulators, but an orthopedics-specific surgical skills laboratory, to our knowledge, has been discussed in only 1 study.21 Results from randomized controlled trials reported in the general surgery literature have proved simulation-based training leads to detectable benefits for learners in clinical settings.27-29 Over the past decade, some alternative surgical skills training methods have been adopted in orthopedic surgery as well. These methods include hands-on training in specifically designed surgical skills laboratories using cadaver models or synthetic bones; software tools; and computerized simulators. In recent years, numerous studies reported in the orthopedic literature have examined arthroscopic simulators in residency training.18-20,30-34 However, these studies are arguably more specific to sports subspecialties and thus more pertinent to upper-level trainees.
Our study results showed that surgical skills laboratory training should be a required aspect of our residents’ training. Although less of a dramatic improvement was noted in the written examination component of the laboratory, the overall knowledge base improved (Table 3). This was especially evident at the PGY-1 level, where written examination scores increased from a presession median of 80% to a postsession median of 85%. A larger degree of improvement was found with the ankle fracture model, and there was statistical improvement at all training levels, from PGY-1 to PGY-3. Previous work has shown that intensive laboratory-based training can be effective, particularly for first-year residents. Sonnadara and colleagues35 demonstrated that a 30-day intensive surgical skills course effectively helped first-year orthopedic residents develop targeted basic surgical skills. In a follow-up study, Sonnadara and colleagues36 demonstrated that a surgical skills course completed at the beginning of a residency was effective in teaching targeted technical skills, and that skills taught in this manner can have excellent retention rates.
There are limitations inherent in our skills course. The κ agreement in the ankle fracture model was low before and after administration, which we attribute to 1 observer outlier. This could be amended by removing outliers and further objectifying and simplifying the scoring system (A–F). Right now, we do not have enough data to determine whether the scores actually improve significantly through the training years or whether they will correlate with operating room experience. Our study had no control. For future investigations, we are considering having general orthopedic surgeons from the community perform the same scenarios and be graded with the same checklists as a control. Implementation, however, may be a challenge. Both our written examination and our ankle fracture model checklist have not been validated—this is one of our next steps. The point system used to score the ankle fracture model was subjectively developed and would benefit from further standardization before drawing conclusions about true validity.
Conclusion
Orthopedic residency programs, like programs in other surgical specialties, are increasingly focused on teaching and documenting the learning of core competencies, even as work-hour restrictions and demands for clinical efficiency limit the amount of time residents spend in the operating room. We have demonstrated the potential value of an intensive laboratory in improving junior-level residents’ basic surgical skills and knowledge. We will continue to refine our methods, with a goal being to create reproducible models that could be adapted by other orthopedic residency programs and by other surgical educators.
1. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
2. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
3. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
4. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
5. Corbett EC Jr, Elnicki DM, Conaway MR. When should students learn essential physical examination skills? Views of internal medicine clerkship directors in North America. Acad Med. 2008;83(1):96-99.
6. Coady DA, Walker DJ, Kay LJ. Teaching medical students musculoskeletal examination skills: identifying barriers to learning and ways of overcoming them. Scand J Rheumatol. 2004;33(1):47-51.
7. Saleh K, Messner R, Axtell S, Harris I, Mahowald ML. Development and evaluation of an integrated musculoskeletal disease course for medical students. J Bone Joint Surg Am. 2004;86(8):1653-1658.
8. van Empel PJ, Verdam MG, Huirne JA, Bonjer HJ, Meijerink WJ, Scheele F. Open knot-tying skills: resident skills assessed. J Obstet Gynaecol Res. 2013;39(5):1030-1036.
9. Barrier BF, Thompson AB, McCullough MW, Occhino JA. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7(6):374-379.
10. Liss MA, McDougall EM. Robotic surgical simulation. Cancer J. 2013;19(2):124-129.
11. Stegemann AP, Ahmed K, Syed JR, et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81(4):767-774.
12. Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg. 2013;58(2):524-528.
13. Kuhls DA, Risucci DA, Bowyer MW, Luchette FA. Advanced surgical skills for exposure in trauma: a new surgical skills cadaver course for surgery residents and fellows. J Trauma Acute Care Surg. 2013;74(2):664-670.
14. Sanfey HA, Dunnington GL. Basic surgical skills testing for junior residents: current views of general surgery program directors. J Am Coll Surg. 2011;212(3):406-412.
15. Alvand A, Khan T, Al-Ali S, Jackson WF, Price AJ, Rees JL. Simple visual parameters for objective assessment of arthroscopic skill. J Bone Joint Surg Am. 2012;94(13):e97.
16. Jackson WF, Khan T, Alvand A, et al. Learning and retaining simulated arthroscopic meniscal repair skills. J Bone Joint Surg Am. 2012;94(17):e132.
17. Pernar LI, Smink DS, Hicks G, Peyre SE. Residents can successfully teach basic surgical skills in the simulation center. J Surg Educ. 2012;69(5):617-622.
18. Tuijthof GJ, Visser P, Sierevelt IN, Van Dijk CN, Kerkhoffs GM. Does perception of usefulness of arthroscopic simulators differ with levels of experience? Clin Orthop. 2011;469(6):1701-1708.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Slade Shantz JA, Leiter JR, Gottschalk T, MacDonald PB. The internal validity of arthroscopic simulators and their effectiveness in arthroscopic education. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):33-40.
21. Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11(5):747-757.
22. Ruedi TP, Buckley RE, Moran CG. AO Principles of Fracture Management. Stuttgart, Germany: Thieme; 2007.
23. Chen CL, Chen WC, Chiang JH, Ho CF. Interscapular hibernoma: case report and literature review. Kaohsiung J Med Sci. 2011;27(8):348-352.
24. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
25. Baskies MA, Ruchelsman DE, Capeci CM, Zuckerman JD, Egol KA. Operative experience in an orthopaedic surgery residency program: the effect of work-hour restrictions. J Bone Joint Surg Am. 2008;90(4):924-927.
26. Pappas AJ, Teague DC. The impact of the Accreditation Council for Graduate Medical Education work-hour regulations on the surgical experience of orthopaedic surgery residents. J Bone Joint Surg Am. 2007;89(4):904-909.
27. Palter VN, Grantcharov T, Harvey A, Macrae HM. Ex vivo technical skills training transfers to the operating room and enhances cognitive learning: a randomized controlled trial. Ann Surg. 2011;253(5):886-889.
28. Franzeck FM, Rosenthal R, Muller MK, et al. Prospective randomized controlled trial of simulator-based versus traditional in-surgery laparoscopic camera navigation training. Surg Endosc. 2012;26(1):235-241.
29. Zendejas B, Cook DA, Bingener J, et al. Simulation-based mastery learning improves patient outcomes in laparoscopic inguinal hernia repair: a randomized controlled trial. Ann Surg. 2011;254(3):502-509.
30. Hui Y, Safir O, Dubrowski A, Carnahan H. What skills should simulation training in arthroscopy teach residents? A focus on resident input. Int J Comput Assist Radiol Surg. 2013;8(6):945-953.
31. Butler A, Olson T, Koehler R, Nicandri G. Do the skills acquired by novice surgeons using anatomic dry models transfer effectively to the task of diagnostic knee arthroscopy performed on cadaveric specimens? J Bone Joint Surg Am. 2013;95(3):e15(1-8).
32. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
33. Elliott MJ, Caprise PA, Henning AE, Kurtz CA, Sekiya JK. Diagnostic knee arthroscopy: a pilot study to evaluate surgical skills. Arthroscopy. 2012;28(2):218-224.
34. Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop. 2011;82(1):90-95.
35. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
36. Sonnadara RR, Garbedian S, Safir O, et al. Orthopaedic boot camp II: examining the retention rates of an intensive surgical skills course. Surgery. 2012;151(6):803-807.
1. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
2. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
3. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
4. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
5. Corbett EC Jr, Elnicki DM, Conaway MR. When should students learn essential physical examination skills? Views of internal medicine clerkship directors in North America. Acad Med. 2008;83(1):96-99.
6. Coady DA, Walker DJ, Kay LJ. Teaching medical students musculoskeletal examination skills: identifying barriers to learning and ways of overcoming them. Scand J Rheumatol. 2004;33(1):47-51.
7. Saleh K, Messner R, Axtell S, Harris I, Mahowald ML. Development and evaluation of an integrated musculoskeletal disease course for medical students. J Bone Joint Surg Am. 2004;86(8):1653-1658.
8. van Empel PJ, Verdam MG, Huirne JA, Bonjer HJ, Meijerink WJ, Scheele F. Open knot-tying skills: resident skills assessed. J Obstet Gynaecol Res. 2013;39(5):1030-1036.
9. Barrier BF, Thompson AB, McCullough MW, Occhino JA. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7(6):374-379.
10. Liss MA, McDougall EM. Robotic surgical simulation. Cancer J. 2013;19(2):124-129.
11. Stegemann AP, Ahmed K, Syed JR, et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81(4):767-774.
12. Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg. 2013;58(2):524-528.
13. Kuhls DA, Risucci DA, Bowyer MW, Luchette FA. Advanced surgical skills for exposure in trauma: a new surgical skills cadaver course for surgery residents and fellows. J Trauma Acute Care Surg. 2013;74(2):664-670.
14. Sanfey HA, Dunnington GL. Basic surgical skills testing for junior residents: current views of general surgery program directors. J Am Coll Surg. 2011;212(3):406-412.
15. Alvand A, Khan T, Al-Ali S, Jackson WF, Price AJ, Rees JL. Simple visual parameters for objective assessment of arthroscopic skill. J Bone Joint Surg Am. 2012;94(13):e97.
16. Jackson WF, Khan T, Alvand A, et al. Learning and retaining simulated arthroscopic meniscal repair skills. J Bone Joint Surg Am. 2012;94(17):e132.
17. Pernar LI, Smink DS, Hicks G, Peyre SE. Residents can successfully teach basic surgical skills in the simulation center. J Surg Educ. 2012;69(5):617-622.
18. Tuijthof GJ, Visser P, Sierevelt IN, Van Dijk CN, Kerkhoffs GM. Does perception of usefulness of arthroscopic simulators differ with levels of experience? Clin Orthop. 2011;469(6):1701-1708.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Slade Shantz JA, Leiter JR, Gottschalk T, MacDonald PB. The internal validity of arthroscopic simulators and their effectiveness in arthroscopic education. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):33-40.
21. Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11(5):747-757.
22. Ruedi TP, Buckley RE, Moran CG. AO Principles of Fracture Management. Stuttgart, Germany: Thieme; 2007.
23. Chen CL, Chen WC, Chiang JH, Ho CF. Interscapular hibernoma: case report and literature review. Kaohsiung J Med Sci. 2011;27(8):348-352.
24. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
25. Baskies MA, Ruchelsman DE, Capeci CM, Zuckerman JD, Egol KA. Operative experience in an orthopaedic surgery residency program: the effect of work-hour restrictions. J Bone Joint Surg Am. 2008;90(4):924-927.
26. Pappas AJ, Teague DC. The impact of the Accreditation Council for Graduate Medical Education work-hour regulations on the surgical experience of orthopaedic surgery residents. J Bone Joint Surg Am. 2007;89(4):904-909.
27. Palter VN, Grantcharov T, Harvey A, Macrae HM. Ex vivo technical skills training transfers to the operating room and enhances cognitive learning: a randomized controlled trial. Ann Surg. 2011;253(5):886-889.
28. Franzeck FM, Rosenthal R, Muller MK, et al. Prospective randomized controlled trial of simulator-based versus traditional in-surgery laparoscopic camera navigation training. Surg Endosc. 2012;26(1):235-241.
29. Zendejas B, Cook DA, Bingener J, et al. Simulation-based mastery learning improves patient outcomes in laparoscopic inguinal hernia repair: a randomized controlled trial. Ann Surg. 2011;254(3):502-509.
30. Hui Y, Safir O, Dubrowski A, Carnahan H. What skills should simulation training in arthroscopy teach residents? A focus on resident input. Int J Comput Assist Radiol Surg. 2013;8(6):945-953.
31. Butler A, Olson T, Koehler R, Nicandri G. Do the skills acquired by novice surgeons using anatomic dry models transfer effectively to the task of diagnostic knee arthroscopy performed on cadaveric specimens? J Bone Joint Surg Am. 2013;95(3):e15(1-8).
32. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
33. Elliott MJ, Caprise PA, Henning AE, Kurtz CA, Sekiya JK. Diagnostic knee arthroscopy: a pilot study to evaluate surgical skills. Arthroscopy. 2012;28(2):218-224.
34. Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop. 2011;82(1):90-95.
35. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
36. Sonnadara RR, Garbedian S, Safir O, et al. Orthopaedic boot camp II: examining the retention rates of an intensive surgical skills course. Surgery. 2012;151(6):803-807.