User login
Osteosarcoma: A Meta-Analysis and Review of the Literature
Osteosarcoma, a primary malignant tumor of the skeleton, is characterized by direct formation of immature bone or osteoid tissue by tumor cells. The World Health Organization histologic classification of bone tumors divides osteosarcoma into central and surface tumors and recognizes a number of subtypes within each group.1 The present review refers only to the classic central high-grade primary osteosarcoma of bone, which represents about 90% of all osteosarcoma cases. Classic osteosarcoma represents about 15% of all biopsy-analyzed primary bone tumors.1 It is the third most common type of neoplasia, preceded by leukemia and lymphoma among older children and adolescents aged 12 to 18 years.2 High-grade primary osteosarcoma is the most common primary skeletal tumor of childhood and adolescence, with an overall annual incidence of 5.6 cases per million children under age 15 years.3-5 Peak incidence is in the second decade of life, and males are affected slightly more often than females.2,6 The period of highest incidence coincides with the growth spurt of the long bones. Osteosarcoma preferentially affects the metaphysis of long bones, the 3 main sites being distal femur, tibia, and proximal humerus.2
Historical Perspective
For most of the 20th century, the 5-year survival rate for classic primary osteosarcoma was under 20%.7 In the 1970s, the first revolution in osteosarcoma treatment arrived with the introduction of adjuvant chemotherapy, which increased survival rates to 50%.8-10 During this expansion of research, several chemotherapeutics (eg, vincristine, bleomycin, dactinomycin) were discarded for poor effectiveness, and others (eg, cisplatin, ifosfamide) were added to doxorubicin and methotrexate, improving 5-year disease-free survival to about 70% in patients with nonmetastatic osteosarcoma. In another significant advance, adjuvant chemotherapy was supplemented with intensive preoperative chemotherapy, resulting in 5-year tumor-free survival that has ranged from 50% to 75% for high-grade osteosarcoma.5,11,12 Adding neoadjuvant chemotherapy and histologic response has allowed for evaluation of surgical margins and early treatment of microscopic disease. Thus, effective limb-sparing procedures can be performed, and the incidence of amputation has decreased from 90% to between 10% and 20%.13,14 However, statistical improvements in survival associated with neoadjuvant treatment may simply delay time of recurrence and metastasis.15 In addition, though chemotherapy has improved survival in osteogenic sarcoma, many have written that this improvement appears to reflect mainly the increase in the intensity of the chemotherapy used, which also leads to a higher propensity for side effects.16
Despite research and advances in chemotherapy regimens, the prognosis of patients with osteosarcoma remains highly variable and often dismal. Mirabello and colleagues17 examined osteosarcoma incidence and survival rates between 1973 and 2004 and found that, with the introduction of neoadjuvant chemotherapy, survival rates improved significantly between 1973 and 1983 and between 1984 and 1993, but there was little improvement between 1993 and 2004.
The long-term outcome for patients with metastatic disease is poor. Investigators have found that 11% to 20% of patients have pulmonary metastasis at initial diagnosis. About half of patients without pulmonary metastases develop them later in the disease course.18 Survival rates for patients with metastasis at initial presentation have ranged from 10% to 40%.19 Recurrent disease still occurs in 30% to 40% of patients, and more than 70% of them die of the tumor.15 The survivors of osteosarcoma are then at increased risk for chronic medical conditions and adverse health status because of the osteosarcoma-related treatments.20
Prognostic Factors
It is important to understand and exploit the influences of different prognostic factors in treating patients with osteosarcoma.7 These factors are important in establishing the best treatment for the individual. Thus, more aggressive treatments can be started in patients with prognostic factors that pose a higher risk of relapse.21 A number of clinical and pathologic features (eg, tumor site, size, subtype; patient sex and age; high alkaline phosphatase or high lactate dehydrogenase [LDH] values; multidrug resistance; genetic variations) have prognostic significance but often with contradictory results because of lack of uniformity in patient analyses and methods.15
Survival for patients with primary osteosarcoma has been analyzed with respect to tumor size and location.7 Studies have found higher survival rates for patients with smaller tumors (<10 cm) and more distal tumor locations.7 These superior survival rates may be the result of earlier detection of tumors and more options for surgical resection of smaller, distal tumors.
Serum LDH levels have helped in risk stratification of patients. High LDH often occurred at time of relapse, and relapse with high LDH correlated with poor prognosis. Meyers and colleagues22 found that 5-year disease-free survival was 72% for patients with normal LDH at presentation and 54% for patients with elevated LDH at presentation.
Several studies have shown that percentage of tumor necrosis on histology is strongly correlated with good prognosis.21 Most groups now define a good histologic response as less than 10% viable tumor cells at time of surgery, and a poor response as more than 10%.23 Results of the Pediatric Oncology Group (POG) protocol for localized osteosarcoma (POG 9351), or Children’s Cancer Group (CCG) 7921, found 45% of patients had favorable responses (>90% necrosis) after preoperative chemotherapy.24 However, several clinicians have recently questioned this finding.
Overall, the prognosis for classic osteosarcoma of the extremity remains highly variable, and there has been little improvement over the past 20 years. The prognosis for younger patients, patients with spinal disease, and patients with metastatic disease remains poor. Although some prognostic factors have been identified and shown to predict a good outcome, it seems few patients have these positive factors. In this article, we describe the literature review and meta-analysis we performed to better define recent survival trends for patients with primary osteosarcoma.
Methods
The MEDLINE, PubMed, and Cochrane databases were searched for eligible studies published in English between 2000 and 2011—a decade of recently reported research. We applied the search strategy [“osteosarcoma” OR “osteogenic sarcoma”] AND [“prognosis” OR “treatment” OR “survival”] and selected reports that specifically addressed factors predicting survival in patients with osteosarcoma—reports that were limited to primary osteosarcoma of the pelvis or extremity and provided 5-year overall survival (OS) data. Abstracts of the selected articles were independently reviewed, and the inclusion and exclusion criteria were applied. We excluded basic science studies and those without pediatric patients, those without primary osteosarcoma, those with periosteal or parosteal osteosarcoma, and those that did not report 5-year OS data.
Statistical Analysis
Number or proportion of patients (whichever was reported) with 5-year OS and number or proportion of patients with 90% necrosis were extracted from each study. For each trial, proportion of patients with 5-year OS and 95% confidence intervals (CIs) and proportion of patients achieving 90% necrosis and 95% CIs were determined. We also calculated proportion of patients with 5-year OS and proportion of patients with 90% necrosis with corresponding 95% CIs of studies that included patients with nonmetastatic disease.
We assessed statistical heterogeneity among trials included in the meta-analysis using the Cochran Q test. Inconsistency was quantified with the I2 statistic, which estimates percentage of total across-studies variation caused by heterogeneity rather than chance.25 We considered I2 higher than 50% as indicating substantial heterogeneity. When substantial heterogeneity was not found, the pooled estimate calculated on the basis of the fixed-effects model was reported using the inverse variance method. When substantial heterogeneity was found, the pooled estimate calculated on the basis of a random-effects model was reported using the DerSimonian and Laird26 method, which takes both within- and between-study variations into account.
Publication bias was assessed through funnel plots and with Begg and Egger tests.27,28 Two-tailed P < .05 was considered statistically significant. All statistical analyses were performed with Stata/SE Version 11.0 (StataCorp).
Results
Our literature search yielded 597 articles. We cross-referenced these articles with the MEDLINE, PubMed, and Cochrane search results using the same keywords and discarded the duplicates. The abstracts of these articles were then reviewed in detail. The 40 articles4,6,11,12,14,15,17-19,21,29-58 that met our study inclusion criteria reported on studies that included patients with metastatic and nonmetastatic osteosarcoma. Because of the significant difference in OS of patients with metastatic disease, we also analyzed articles that included only patients with nonmetastatic disease. Sixteen articles6,14,15,29,32-35,39,47,48,51,53,54,55,57 were included in the analysis of patients with nonmetastatic disease.
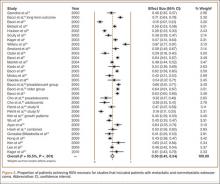
Figure 1 shows 5-year OS for each of the 40 studies. For studies that compared survival of different groups of patients, the survival of each group is shown separately. For example, Bacci and colleagues39 divided patients into adolescent and preadolescent groups and reported 5-year OS for each. In our analysis, we treated each group independently and reported their 5-year OS separately. For each study, 5-year OS, weight of study, and CI are included. Five-year OS ranged from 19% to 94%. Analysis was performed to determine 5-year OS for all studies based on weight given to each study. The random-effects model used for this analysis (heterogeneity test, Q = 656.23; P < .001; I2 = 93.4%) showed 5-year OS of 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma.
Figure 2 shows 5-year OS (range, 53%-94%) for each of the 16 studies that included only patients with nonmetastatic disease. The random-effects model used for this analysis (heterogeneity test, Q = 142.08; P < .001; I2 = 89.4%) showed 5-year OS of 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic disease.
We then examined percentage of patients achieving 90% necrosis on histology in each study. Several studies included in the OS analysis did not report percentage necrosis, leaving 29 studies for the necrosis analysis. Of these 29 studies, all 29 included patients with metastatic and nonmetastatic disease,4,6,11,14,15,18,19,21,29,31-36,37,39,40,43-47,49,50,54-57,59 and 13 included only patients with nonmetastatic disease.6,14,15,29,32-35,40,47,54,55,57 Again, because of the known difference in prognosis between patients with metastatic disease and patients with nonmetastatic disease, we performed separate analyses, one for the combined dataset of all 29 studies (Figure 3) and the other for the 13 nonmetastatic studies (Figure 4). Random-effects models showed 90% necrosis for 50% of patients in both analyses: studies that included patients with metastatic and nonmetastatic disease (95% CI, 45%-54%; heterogeneity test, Q = 692.88; P < .001; I2 = 95.5%) and nonmetastatic studies (95% CI, 41%-59%; heterogeneity test, Q = 385.42; P < .001; I2 = 96.9%).
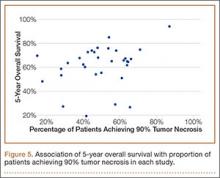
We also performed a meta-regression analysis that included necrosis as a continuous variable for both the overall dataset and the nonmetastatic dataset. Five-year OS was plotted against percentage of patients achieving 90% tumor necrosis for each study. The results are plotted in Figure 5 (combined dataset).
No evidence of publication bias was detected for 5-year OS or percentage necrosis for the analyses of the combined datasets by either Egger test or Begg test. For 5-year OS, Ps were .21 (Egger) and .19 (Begg); for percentage necrosis, Ps were .10 (Egger) and .62 (Begg). In addition, no evidence of publication bias was detected for the analyses of the nonmetastatic studies by either test. For 5-year OS, Ps were .55 (Egger) and .41 (Begg); for percentage necrosis, Ps were .42 (Egger) and .95 (Begg).
Discussion
Five-year OS was 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma and 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic osteosarcoma. These percentages fall within the range found in the literature. Mankin and colleagues37 reviewed 648 cases of patients with osteosarcoma treated at Massachusetts General Hospital in 2004; OS was 68%. In 2011, Sampo and colleagues60 reported 10-year OS of 63% for patients with metastatic and nonmetastatic disease and 73% for patients with local disease at presentation. Five-year OS rates in the literature are consistently about 70%. Ferrari and colleagues61 reported 5-year OS of 73% and 74% for 230 patients treated with 2 different neoadjuvant chemotherapy regimens between 2001 and 2006. The consistency in 5-year OS suggests OS of pediatric patients with osteosarcoma has plateaued, and there has been no significant improvement in survival of patients with osteosarcoma over the past 30 years.
Histologic response to preoperative chemotherapy is strongly associated with survival in pediatric osteosarcoma. Bielack and colleagues31 reported 5-year OS of 75% to 80% for patients who responded well to preoperative chemotherapy (>90% tumor necrosis) and 45% to 55% for patients who responded poorly (<10% necrosis). In our meta-analysis of studies that included patients with nonmetastatic osteosarcoma, 50% achieved necrosis of more than 90%. Percentage of patients achieving necrosis of more than 90% has been about 45%, according to past reports. In 2012, Ferrari and colleagues61 reported that 45% of 230 patients treated with neoadjuvant chemotherapy achieved more than 90% tumor necrosis. Therefore, 5-year OS and percentage of patients achieving 90% necrosis are consistent with previous reports, though this also suggests these numbers have remained constant over the past several decades.
Despite its expansive scale, our study has several important limitations. Data were extracted from published studies, and individual patient data were not available, so we were not able to assess the effects of risk factors (eg, tumor size, location) on 5-year OS. We could not correlate the proportion of patients with 90% necrosis to 5-year OS, as studies did not report OS by necrosis strata. Also, because our numbers were derived from published studies, they may not accurately represent outcomes in the community as a whole. In addition, several successive studies may contain duplicate patient cases. We limited our search to studies published since 2000 to include patients recently diagnosed and treated for osteosarcoma; however, several studies published after 2000 also included patients diagnosed and treated before 2000. Several of these studies are from countries outside the United States and may have a significantly different incidence of osteosarcoma as well as treatment methods and survival rates.
Although this meta-analysis suggests 5-year OS remains about 70% for patients with primary nonmetastatic osteosarcoma, we cannot settle on this conclusion because of the many differences between the studies we included. Therefore, more studies of patients diagnosed and treated within the past 10 years are needed to confirm our beliefs about patient survival.
1. Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667-674.
2. Cho WH, Song WS, Jeon DG, et al. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Ann Surg Oncol. 2010;17(3):702-708.
3. Abate ME, Longhi A, Galletti S, Ferrari S, Bacci G. Non-metastatic osteosarcoma of the extremities in children aged 5 years or younger. Pediatr Blood Cancer. 2010;55(4):652-654.
4. Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011-2018.
5. Pakos EE, Nearchou AD, Grimer RJ, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer. 2009;45(13):2367-2375.
6. Kaste SC, Liu T, Billups CA, Daw NC, Pratt CB, Meyer WH. Tumor size as a predictor of outcome in pediatric non-metastatic osteosarcoma of the extremity. Pediatr Blood Cancer. 2004;43(7):723-728.
7. Brostrom LA, Strander H, Nilsonne U. Survival in osteosarcoma in relation to tumor size and location. Clin Orthop Relat Res. 1982;167:250-254.
8. Harvei S, Solheim O. The prognosis in osteosarcoma: Norwegian national data. Cancer. 1981;48(8):1719-1723.
9. Sutow WW, Sullivan MP, Fernbach DJ, Cangir A, George SL. Adjuvant chemotherapy in primary treatment of osteogenic sarcoma. A Southwest Oncology Group study. Cancer. 1975;36(5):1598-1602.
10. Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5(1):21-26.
11. Hsieh MY, Hung GY, Yen HJ, Chen WM, Chen TH. Osteosarcoma in preadolescent patients: experience in a single institute in Taiwan. J Chin Med Assoc. 2009;72(9):455-461.
12. Longhi A, Pasini E, Bertoni F, Pignotti E, Ferrari C, Bacci G. Twenty-year follow-up of osteosarcoma of the extremity treated with adjuvant chemotherapy. J Chemother. 2004;16(6):582-588.
13. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
14. Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/Osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18(24):4016-4027.
15. Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154-1161.
16. Cohen IJ, Kaplinsky C, Katz K, et al. Improved results in osteogenic sarcoma 1973–79 vs. 1980–86: analysis of results from a single center. Isr J Med Sci. 1993;29(1):27-29.
17. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results program. Cancer. 2009;115(7):1531-1543.
18. Kager L, Zoubek A, Dominkus M, et al. Osteosarcoma in very young children: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2010;116(22):5316-5324.
19. Szendroi M, Papai Z, Koos R, Illes T. Limb-saving surgery, survival, and prognostic factors for osteosarcoma: the Hungarian experience. J Surg Oncol. 2000;73(2):87-94.
20. Nagarajan R, Kamruzzaman A, Ness KK, et al. Twenty years of follow-up of survivors of childhood osteosarcoma: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117(3):625-634.
21. Bacci G, Longhi A, Ferrari S, et al. Prognostic significance of serum lactate dehydrogenase in osteosarcoma of the extremity: experience at Rizzoli on 1421 patients treated over the last 30 years. Tumori. 2004;90(5):478-484.
22. Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5-15.
23. Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422-441.
24. Hendershot E, Pappo A, Malkin D, Sung L. Tumor necrosis in pediatric osteosarcoma: impact of modern therapies. J Pediatr Oncol Nurs. 2006;23(4):176-181.
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101.
28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634.
29. Bacci G, Ferrari S, Longhi A, Mellano D, Giacomini S, Forni C. Delay in diagnosis of high-grade osteosarcoma of the extremities. Has it any effect on the stage of disease? Tumori. 2000;86(3):204-206.
30. Bacci G, Ferrari S, Longhi A, et al. Neoadjuvant chemotherapy for high grade osteosarcoma of the extremities: long-term results for patients treated according to the Rizzoli IOR/OS-3b protocol. J Chemother. 2001;13(1):93-99.
31. Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776-790.
32. Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38(9):1218-1225.
33. Scully SP, Ghert MA, Zurakowski D, Thompson RC, Gebhardt MC. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J Bone Joint Surg Am. 2002;84(1):49-57.
34. Wilkins RM, Cullen JW, Odom L, et al. Superior survival in treatment of primary nonmetastatic pediatric osteosarcoma of the extremity. Ann Surg Oncol. 2003;10(5):498-507.
35. Smeland S, Muller C, Alvegard TA, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488-494.
36. Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21(2):334-341.
37. Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;429:286-291.
38. Donati D, Giacomini S, Gozzi E, et al. Osteosarcoma of the pelvis. Eur J Surg Oncol. 2004;30(3):332-340.
39. Bacci G, Longhi A, Bertoni F, et al. Primary high-grade osteosarcoma: comparison between preadolescent and older patients. J Pediatr Hematol Oncol. 2005;27(3):129-134.
40. Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41(18):2836-2845.
41. Matsuo T, Sugita T, Sato K, et al. Clinical outcomes of 54 pelvic osteosarcomas registered by Japanese musculoskeletal oncology group. Oncology. 2005;68(4-6):375-381.
42. Kuhelj D, Jereb B. Pediatric osteosarcoma: a 35-year experience in Slovenia. Pediatr Hematol Oncol. 2005;22(4):335-343.
43. Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer. 2005;104(5):1100-1109.
44. Daecke W, Bielack S, Martini AK, et al. Osteosarcoma of the hand and forearm: experience of the Cooperative Osteosarcoma Study Group. Ann Surg Oncol. 2005;12(4):322-331.
45. Cho WH, Lee SY, Song WS, Park JH. Osteosarcoma in pre-adolescent patients. J Int Med Res. 2006;34(6):676-681.
46. Petrilli AS, de Camargo B, Filho VO, et al. Results of the Brazilian Osteosarcoma Treatment Group studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161-1168.
47. Kim MS, Lee SY, Cho WH, et al. Growth patterns of osteosarcoma predict patient survival. Arch Orthop Trauma Surg. 2009;129(9):1189-1196.
48. Lee JA, Kim MS, Kim DH, et al. Osteosarcoma developed in the period of maximal growth rate have inferior prognosis. J Pediatr Hematol Oncol. 2008;30(6):419-424.
49. Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis: clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol. 2009;39(8):514-522.
50. Ayan I, Kebudi R, Ozger H. Childhood osteosarcoma: multimodal therapy in a single-institution Turkish series. Cancer Treat Res. 2009;152:319-338.
51. Bruland OS, Bauer H, Alvegaard T, Smeland S. Treatment of osteosarcoma. The Scandinavian Sarcoma Group experience. Cancer Treat Res. 2009;152:309-318.
52. Bielack S, Jurgens H, Jundt G, et al. Osteosarcoma: the COSS experience. Cancer Treat Res. 2009;152:289-308.
53. Bispo Júnior RZ, Camargo OP. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clinics (Sao Paulo). 2009;64(12):1177-1186.
54. Gonzalez-Billalabeitia E, Hitt R, Fernandez J, et al. Pre-treatment serum lactate dehydrogenase level is an important prognostic factor in high-grade extremity osteosarcoma. Clin Transl Oncol. 2009;11(7):479-483.
55. Kong CB, Kim MS, Lee SY, et al. Prognostic effect of diaphyseal location in osteosarcoma: a cohort case–control study at a single institute. Ann Surg Oncol. 2009;16(11):3094-3100.
56. Kim MS, Lee SY, Cho WH, et al. Prognostic effects of doctor-associated diagnostic delays in osteosarcoma. Arch Orthop Trauma Surg. 2009;129(10):1421-1425.
57. Lee JA, Kim MS, Kim DH, et al. Risk stratification based on the clinical factors at diagnosis is closely related to the survival of localized osteosarcoma. Pediatr Blood Cancer. 2009;52(3):340-345.
58. Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Osteosarcoma in children 5 years of age or younger at initial diagnosis. Pediatr Blood Cancer. 2010;55(2):285-289.
59. Munajat I, Zulmi W, Norazman MZ, Wan Faisham WI. Tumour volume and lung metastasis in patients with osteosarcoma. J Orthop Surg (Hong Kong). 2008;16(2):182-185.
60. Sampo M, Koivikko M, Taskinen M, et al. Incidence, epidemiology and treatment results of osteosarcoma in Finland - a nationwide population-based study. Acta Oncol. 2011;50(8):1206-1214.
61. Ferrari S, Ruggieri P, Cefalo G, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian Sarcoma Group trial ISG/OS-1. J Clin Oncol. 2012;30(17):2112-2118.
Osteosarcoma, a primary malignant tumor of the skeleton, is characterized by direct formation of immature bone or osteoid tissue by tumor cells. The World Health Organization histologic classification of bone tumors divides osteosarcoma into central and surface tumors and recognizes a number of subtypes within each group.1 The present review refers only to the classic central high-grade primary osteosarcoma of bone, which represents about 90% of all osteosarcoma cases. Classic osteosarcoma represents about 15% of all biopsy-analyzed primary bone tumors.1 It is the third most common type of neoplasia, preceded by leukemia and lymphoma among older children and adolescents aged 12 to 18 years.2 High-grade primary osteosarcoma is the most common primary skeletal tumor of childhood and adolescence, with an overall annual incidence of 5.6 cases per million children under age 15 years.3-5 Peak incidence is in the second decade of life, and males are affected slightly more often than females.2,6 The period of highest incidence coincides with the growth spurt of the long bones. Osteosarcoma preferentially affects the metaphysis of long bones, the 3 main sites being distal femur, tibia, and proximal humerus.2
Historical Perspective
For most of the 20th century, the 5-year survival rate for classic primary osteosarcoma was under 20%.7 In the 1970s, the first revolution in osteosarcoma treatment arrived with the introduction of adjuvant chemotherapy, which increased survival rates to 50%.8-10 During this expansion of research, several chemotherapeutics (eg, vincristine, bleomycin, dactinomycin) were discarded for poor effectiveness, and others (eg, cisplatin, ifosfamide) were added to doxorubicin and methotrexate, improving 5-year disease-free survival to about 70% in patients with nonmetastatic osteosarcoma. In another significant advance, adjuvant chemotherapy was supplemented with intensive preoperative chemotherapy, resulting in 5-year tumor-free survival that has ranged from 50% to 75% for high-grade osteosarcoma.5,11,12 Adding neoadjuvant chemotherapy and histologic response has allowed for evaluation of surgical margins and early treatment of microscopic disease. Thus, effective limb-sparing procedures can be performed, and the incidence of amputation has decreased from 90% to between 10% and 20%.13,14 However, statistical improvements in survival associated with neoadjuvant treatment may simply delay time of recurrence and metastasis.15 In addition, though chemotherapy has improved survival in osteogenic sarcoma, many have written that this improvement appears to reflect mainly the increase in the intensity of the chemotherapy used, which also leads to a higher propensity for side effects.16
Despite research and advances in chemotherapy regimens, the prognosis of patients with osteosarcoma remains highly variable and often dismal. Mirabello and colleagues17 examined osteosarcoma incidence and survival rates between 1973 and 2004 and found that, with the introduction of neoadjuvant chemotherapy, survival rates improved significantly between 1973 and 1983 and between 1984 and 1993, but there was little improvement between 1993 and 2004.
The long-term outcome for patients with metastatic disease is poor. Investigators have found that 11% to 20% of patients have pulmonary metastasis at initial diagnosis. About half of patients without pulmonary metastases develop them later in the disease course.18 Survival rates for patients with metastasis at initial presentation have ranged from 10% to 40%.19 Recurrent disease still occurs in 30% to 40% of patients, and more than 70% of them die of the tumor.15 The survivors of osteosarcoma are then at increased risk for chronic medical conditions and adverse health status because of the osteosarcoma-related treatments.20
Prognostic Factors
It is important to understand and exploit the influences of different prognostic factors in treating patients with osteosarcoma.7 These factors are important in establishing the best treatment for the individual. Thus, more aggressive treatments can be started in patients with prognostic factors that pose a higher risk of relapse.21 A number of clinical and pathologic features (eg, tumor site, size, subtype; patient sex and age; high alkaline phosphatase or high lactate dehydrogenase [LDH] values; multidrug resistance; genetic variations) have prognostic significance but often with contradictory results because of lack of uniformity in patient analyses and methods.15
Survival for patients with primary osteosarcoma has been analyzed with respect to tumor size and location.7 Studies have found higher survival rates for patients with smaller tumors (<10 cm) and more distal tumor locations.7 These superior survival rates may be the result of earlier detection of tumors and more options for surgical resection of smaller, distal tumors.
Serum LDH levels have helped in risk stratification of patients. High LDH often occurred at time of relapse, and relapse with high LDH correlated with poor prognosis. Meyers and colleagues22 found that 5-year disease-free survival was 72% for patients with normal LDH at presentation and 54% for patients with elevated LDH at presentation.
Several studies have shown that percentage of tumor necrosis on histology is strongly correlated with good prognosis.21 Most groups now define a good histologic response as less than 10% viable tumor cells at time of surgery, and a poor response as more than 10%.23 Results of the Pediatric Oncology Group (POG) protocol for localized osteosarcoma (POG 9351), or Children’s Cancer Group (CCG) 7921, found 45% of patients had favorable responses (>90% necrosis) after preoperative chemotherapy.24 However, several clinicians have recently questioned this finding.
Overall, the prognosis for classic osteosarcoma of the extremity remains highly variable, and there has been little improvement over the past 20 years. The prognosis for younger patients, patients with spinal disease, and patients with metastatic disease remains poor. Although some prognostic factors have been identified and shown to predict a good outcome, it seems few patients have these positive factors. In this article, we describe the literature review and meta-analysis we performed to better define recent survival trends for patients with primary osteosarcoma.
Methods
The MEDLINE, PubMed, and Cochrane databases were searched for eligible studies published in English between 2000 and 2011—a decade of recently reported research. We applied the search strategy [“osteosarcoma” OR “osteogenic sarcoma”] AND [“prognosis” OR “treatment” OR “survival”] and selected reports that specifically addressed factors predicting survival in patients with osteosarcoma—reports that were limited to primary osteosarcoma of the pelvis or extremity and provided 5-year overall survival (OS) data. Abstracts of the selected articles were independently reviewed, and the inclusion and exclusion criteria were applied. We excluded basic science studies and those without pediatric patients, those without primary osteosarcoma, those with periosteal or parosteal osteosarcoma, and those that did not report 5-year OS data.
Statistical Analysis
Number or proportion of patients (whichever was reported) with 5-year OS and number or proportion of patients with 90% necrosis were extracted from each study. For each trial, proportion of patients with 5-year OS and 95% confidence intervals (CIs) and proportion of patients achieving 90% necrosis and 95% CIs were determined. We also calculated proportion of patients with 5-year OS and proportion of patients with 90% necrosis with corresponding 95% CIs of studies that included patients with nonmetastatic disease.
We assessed statistical heterogeneity among trials included in the meta-analysis using the Cochran Q test. Inconsistency was quantified with the I2 statistic, which estimates percentage of total across-studies variation caused by heterogeneity rather than chance.25 We considered I2 higher than 50% as indicating substantial heterogeneity. When substantial heterogeneity was not found, the pooled estimate calculated on the basis of the fixed-effects model was reported using the inverse variance method. When substantial heterogeneity was found, the pooled estimate calculated on the basis of a random-effects model was reported using the DerSimonian and Laird26 method, which takes both within- and between-study variations into account.
Publication bias was assessed through funnel plots and with Begg and Egger tests.27,28 Two-tailed P < .05 was considered statistically significant. All statistical analyses were performed with Stata/SE Version 11.0 (StataCorp).
Results
Our literature search yielded 597 articles. We cross-referenced these articles with the MEDLINE, PubMed, and Cochrane search results using the same keywords and discarded the duplicates. The abstracts of these articles were then reviewed in detail. The 40 articles4,6,11,12,14,15,17-19,21,29-58 that met our study inclusion criteria reported on studies that included patients with metastatic and nonmetastatic osteosarcoma. Because of the significant difference in OS of patients with metastatic disease, we also analyzed articles that included only patients with nonmetastatic disease. Sixteen articles6,14,15,29,32-35,39,47,48,51,53,54,55,57 were included in the analysis of patients with nonmetastatic disease.
Figure 1 shows 5-year OS for each of the 40 studies. For studies that compared survival of different groups of patients, the survival of each group is shown separately. For example, Bacci and colleagues39 divided patients into adolescent and preadolescent groups and reported 5-year OS for each. In our analysis, we treated each group independently and reported their 5-year OS separately. For each study, 5-year OS, weight of study, and CI are included. Five-year OS ranged from 19% to 94%. Analysis was performed to determine 5-year OS for all studies based on weight given to each study. The random-effects model used for this analysis (heterogeneity test, Q = 656.23; P < .001; I2 = 93.4%) showed 5-year OS of 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma.
Figure 2 shows 5-year OS (range, 53%-94%) for each of the 16 studies that included only patients with nonmetastatic disease. The random-effects model used for this analysis (heterogeneity test, Q = 142.08; P < .001; I2 = 89.4%) showed 5-year OS of 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic disease.
We then examined percentage of patients achieving 90% necrosis on histology in each study. Several studies included in the OS analysis did not report percentage necrosis, leaving 29 studies for the necrosis analysis. Of these 29 studies, all 29 included patients with metastatic and nonmetastatic disease,4,6,11,14,15,18,19,21,29,31-36,37,39,40,43-47,49,50,54-57,59 and 13 included only patients with nonmetastatic disease.6,14,15,29,32-35,40,47,54,55,57 Again, because of the known difference in prognosis between patients with metastatic disease and patients with nonmetastatic disease, we performed separate analyses, one for the combined dataset of all 29 studies (Figure 3) and the other for the 13 nonmetastatic studies (Figure 4). Random-effects models showed 90% necrosis for 50% of patients in both analyses: studies that included patients with metastatic and nonmetastatic disease (95% CI, 45%-54%; heterogeneity test, Q = 692.88; P < .001; I2 = 95.5%) and nonmetastatic studies (95% CI, 41%-59%; heterogeneity test, Q = 385.42; P < .001; I2 = 96.9%).
We also performed a meta-regression analysis that included necrosis as a continuous variable for both the overall dataset and the nonmetastatic dataset. Five-year OS was plotted against percentage of patients achieving 90% tumor necrosis for each study. The results are plotted in Figure 5 (combined dataset).
No evidence of publication bias was detected for 5-year OS or percentage necrosis for the analyses of the combined datasets by either Egger test or Begg test. For 5-year OS, Ps were .21 (Egger) and .19 (Begg); for percentage necrosis, Ps were .10 (Egger) and .62 (Begg). In addition, no evidence of publication bias was detected for the analyses of the nonmetastatic studies by either test. For 5-year OS, Ps were .55 (Egger) and .41 (Begg); for percentage necrosis, Ps were .42 (Egger) and .95 (Begg).
Discussion
Five-year OS was 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma and 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic osteosarcoma. These percentages fall within the range found in the literature. Mankin and colleagues37 reviewed 648 cases of patients with osteosarcoma treated at Massachusetts General Hospital in 2004; OS was 68%. In 2011, Sampo and colleagues60 reported 10-year OS of 63% for patients with metastatic and nonmetastatic disease and 73% for patients with local disease at presentation. Five-year OS rates in the literature are consistently about 70%. Ferrari and colleagues61 reported 5-year OS of 73% and 74% for 230 patients treated with 2 different neoadjuvant chemotherapy regimens between 2001 and 2006. The consistency in 5-year OS suggests OS of pediatric patients with osteosarcoma has plateaued, and there has been no significant improvement in survival of patients with osteosarcoma over the past 30 years.
Histologic response to preoperative chemotherapy is strongly associated with survival in pediatric osteosarcoma. Bielack and colleagues31 reported 5-year OS of 75% to 80% for patients who responded well to preoperative chemotherapy (>90% tumor necrosis) and 45% to 55% for patients who responded poorly (<10% necrosis). In our meta-analysis of studies that included patients with nonmetastatic osteosarcoma, 50% achieved necrosis of more than 90%. Percentage of patients achieving necrosis of more than 90% has been about 45%, according to past reports. In 2012, Ferrari and colleagues61 reported that 45% of 230 patients treated with neoadjuvant chemotherapy achieved more than 90% tumor necrosis. Therefore, 5-year OS and percentage of patients achieving 90% necrosis are consistent with previous reports, though this also suggests these numbers have remained constant over the past several decades.
Despite its expansive scale, our study has several important limitations. Data were extracted from published studies, and individual patient data were not available, so we were not able to assess the effects of risk factors (eg, tumor size, location) on 5-year OS. We could not correlate the proportion of patients with 90% necrosis to 5-year OS, as studies did not report OS by necrosis strata. Also, because our numbers were derived from published studies, they may not accurately represent outcomes in the community as a whole. In addition, several successive studies may contain duplicate patient cases. We limited our search to studies published since 2000 to include patients recently diagnosed and treated for osteosarcoma; however, several studies published after 2000 also included patients diagnosed and treated before 2000. Several of these studies are from countries outside the United States and may have a significantly different incidence of osteosarcoma as well as treatment methods and survival rates.
Although this meta-analysis suggests 5-year OS remains about 70% for patients with primary nonmetastatic osteosarcoma, we cannot settle on this conclusion because of the many differences between the studies we included. Therefore, more studies of patients diagnosed and treated within the past 10 years are needed to confirm our beliefs about patient survival.
Osteosarcoma, a primary malignant tumor of the skeleton, is characterized by direct formation of immature bone or osteoid tissue by tumor cells. The World Health Organization histologic classification of bone tumors divides osteosarcoma into central and surface tumors and recognizes a number of subtypes within each group.1 The present review refers only to the classic central high-grade primary osteosarcoma of bone, which represents about 90% of all osteosarcoma cases. Classic osteosarcoma represents about 15% of all biopsy-analyzed primary bone tumors.1 It is the third most common type of neoplasia, preceded by leukemia and lymphoma among older children and adolescents aged 12 to 18 years.2 High-grade primary osteosarcoma is the most common primary skeletal tumor of childhood and adolescence, with an overall annual incidence of 5.6 cases per million children under age 15 years.3-5 Peak incidence is in the second decade of life, and males are affected slightly more often than females.2,6 The period of highest incidence coincides with the growth spurt of the long bones. Osteosarcoma preferentially affects the metaphysis of long bones, the 3 main sites being distal femur, tibia, and proximal humerus.2
Historical Perspective
For most of the 20th century, the 5-year survival rate for classic primary osteosarcoma was under 20%.7 In the 1970s, the first revolution in osteosarcoma treatment arrived with the introduction of adjuvant chemotherapy, which increased survival rates to 50%.8-10 During this expansion of research, several chemotherapeutics (eg, vincristine, bleomycin, dactinomycin) were discarded for poor effectiveness, and others (eg, cisplatin, ifosfamide) were added to doxorubicin and methotrexate, improving 5-year disease-free survival to about 70% in patients with nonmetastatic osteosarcoma. In another significant advance, adjuvant chemotherapy was supplemented with intensive preoperative chemotherapy, resulting in 5-year tumor-free survival that has ranged from 50% to 75% for high-grade osteosarcoma.5,11,12 Adding neoadjuvant chemotherapy and histologic response has allowed for evaluation of surgical margins and early treatment of microscopic disease. Thus, effective limb-sparing procedures can be performed, and the incidence of amputation has decreased from 90% to between 10% and 20%.13,14 However, statistical improvements in survival associated with neoadjuvant treatment may simply delay time of recurrence and metastasis.15 In addition, though chemotherapy has improved survival in osteogenic sarcoma, many have written that this improvement appears to reflect mainly the increase in the intensity of the chemotherapy used, which also leads to a higher propensity for side effects.16
Despite research and advances in chemotherapy regimens, the prognosis of patients with osteosarcoma remains highly variable and often dismal. Mirabello and colleagues17 examined osteosarcoma incidence and survival rates between 1973 and 2004 and found that, with the introduction of neoadjuvant chemotherapy, survival rates improved significantly between 1973 and 1983 and between 1984 and 1993, but there was little improvement between 1993 and 2004.
The long-term outcome for patients with metastatic disease is poor. Investigators have found that 11% to 20% of patients have pulmonary metastasis at initial diagnosis. About half of patients without pulmonary metastases develop them later in the disease course.18 Survival rates for patients with metastasis at initial presentation have ranged from 10% to 40%.19 Recurrent disease still occurs in 30% to 40% of patients, and more than 70% of them die of the tumor.15 The survivors of osteosarcoma are then at increased risk for chronic medical conditions and adverse health status because of the osteosarcoma-related treatments.20
Prognostic Factors
It is important to understand and exploit the influences of different prognostic factors in treating patients with osteosarcoma.7 These factors are important in establishing the best treatment for the individual. Thus, more aggressive treatments can be started in patients with prognostic factors that pose a higher risk of relapse.21 A number of clinical and pathologic features (eg, tumor site, size, subtype; patient sex and age; high alkaline phosphatase or high lactate dehydrogenase [LDH] values; multidrug resistance; genetic variations) have prognostic significance but often with contradictory results because of lack of uniformity in patient analyses and methods.15
Survival for patients with primary osteosarcoma has been analyzed with respect to tumor size and location.7 Studies have found higher survival rates for patients with smaller tumors (<10 cm) and more distal tumor locations.7 These superior survival rates may be the result of earlier detection of tumors and more options for surgical resection of smaller, distal tumors.
Serum LDH levels have helped in risk stratification of patients. High LDH often occurred at time of relapse, and relapse with high LDH correlated with poor prognosis. Meyers and colleagues22 found that 5-year disease-free survival was 72% for patients with normal LDH at presentation and 54% for patients with elevated LDH at presentation.
Several studies have shown that percentage of tumor necrosis on histology is strongly correlated with good prognosis.21 Most groups now define a good histologic response as less than 10% viable tumor cells at time of surgery, and a poor response as more than 10%.23 Results of the Pediatric Oncology Group (POG) protocol for localized osteosarcoma (POG 9351), or Children’s Cancer Group (CCG) 7921, found 45% of patients had favorable responses (>90% necrosis) after preoperative chemotherapy.24 However, several clinicians have recently questioned this finding.
Overall, the prognosis for classic osteosarcoma of the extremity remains highly variable, and there has been little improvement over the past 20 years. The prognosis for younger patients, patients with spinal disease, and patients with metastatic disease remains poor. Although some prognostic factors have been identified and shown to predict a good outcome, it seems few patients have these positive factors. In this article, we describe the literature review and meta-analysis we performed to better define recent survival trends for patients with primary osteosarcoma.
Methods
The MEDLINE, PubMed, and Cochrane databases were searched for eligible studies published in English between 2000 and 2011—a decade of recently reported research. We applied the search strategy [“osteosarcoma” OR “osteogenic sarcoma”] AND [“prognosis” OR “treatment” OR “survival”] and selected reports that specifically addressed factors predicting survival in patients with osteosarcoma—reports that were limited to primary osteosarcoma of the pelvis or extremity and provided 5-year overall survival (OS) data. Abstracts of the selected articles were independently reviewed, and the inclusion and exclusion criteria were applied. We excluded basic science studies and those without pediatric patients, those without primary osteosarcoma, those with periosteal or parosteal osteosarcoma, and those that did not report 5-year OS data.
Statistical Analysis
Number or proportion of patients (whichever was reported) with 5-year OS and number or proportion of patients with 90% necrosis were extracted from each study. For each trial, proportion of patients with 5-year OS and 95% confidence intervals (CIs) and proportion of patients achieving 90% necrosis and 95% CIs were determined. We also calculated proportion of patients with 5-year OS and proportion of patients with 90% necrosis with corresponding 95% CIs of studies that included patients with nonmetastatic disease.
We assessed statistical heterogeneity among trials included in the meta-analysis using the Cochran Q test. Inconsistency was quantified with the I2 statistic, which estimates percentage of total across-studies variation caused by heterogeneity rather than chance.25 We considered I2 higher than 50% as indicating substantial heterogeneity. When substantial heterogeneity was not found, the pooled estimate calculated on the basis of the fixed-effects model was reported using the inverse variance method. When substantial heterogeneity was found, the pooled estimate calculated on the basis of a random-effects model was reported using the DerSimonian and Laird26 method, which takes both within- and between-study variations into account.
Publication bias was assessed through funnel plots and with Begg and Egger tests.27,28 Two-tailed P < .05 was considered statistically significant. All statistical analyses were performed with Stata/SE Version 11.0 (StataCorp).
Results
Our literature search yielded 597 articles. We cross-referenced these articles with the MEDLINE, PubMed, and Cochrane search results using the same keywords and discarded the duplicates. The abstracts of these articles were then reviewed in detail. The 40 articles4,6,11,12,14,15,17-19,21,29-58 that met our study inclusion criteria reported on studies that included patients with metastatic and nonmetastatic osteosarcoma. Because of the significant difference in OS of patients with metastatic disease, we also analyzed articles that included only patients with nonmetastatic disease. Sixteen articles6,14,15,29,32-35,39,47,48,51,53,54,55,57 were included in the analysis of patients with nonmetastatic disease.
Figure 1 shows 5-year OS for each of the 40 studies. For studies that compared survival of different groups of patients, the survival of each group is shown separately. For example, Bacci and colleagues39 divided patients into adolescent and preadolescent groups and reported 5-year OS for each. In our analysis, we treated each group independently and reported their 5-year OS separately. For each study, 5-year OS, weight of study, and CI are included. Five-year OS ranged from 19% to 94%. Analysis was performed to determine 5-year OS for all studies based on weight given to each study. The random-effects model used for this analysis (heterogeneity test, Q = 656.23; P < .001; I2 = 93.4%) showed 5-year OS of 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma.
Figure 2 shows 5-year OS (range, 53%-94%) for each of the 16 studies that included only patients with nonmetastatic disease. The random-effects model used for this analysis (heterogeneity test, Q = 142.08; P < .001; I2 = 89.4%) showed 5-year OS of 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic disease.
We then examined percentage of patients achieving 90% necrosis on histology in each study. Several studies included in the OS analysis did not report percentage necrosis, leaving 29 studies for the necrosis analysis. Of these 29 studies, all 29 included patients with metastatic and nonmetastatic disease,4,6,11,14,15,18,19,21,29,31-36,37,39,40,43-47,49,50,54-57,59 and 13 included only patients with nonmetastatic disease.6,14,15,29,32-35,40,47,54,55,57 Again, because of the known difference in prognosis between patients with metastatic disease and patients with nonmetastatic disease, we performed separate analyses, one for the combined dataset of all 29 studies (Figure 3) and the other for the 13 nonmetastatic studies (Figure 4). Random-effects models showed 90% necrosis for 50% of patients in both analyses: studies that included patients with metastatic and nonmetastatic disease (95% CI, 45%-54%; heterogeneity test, Q = 692.88; P < .001; I2 = 95.5%) and nonmetastatic studies (95% CI, 41%-59%; heterogeneity test, Q = 385.42; P < .001; I2 = 96.9%).
We also performed a meta-regression analysis that included necrosis as a continuous variable for both the overall dataset and the nonmetastatic dataset. Five-year OS was plotted against percentage of patients achieving 90% tumor necrosis for each study. The results are plotted in Figure 5 (combined dataset).
No evidence of publication bias was detected for 5-year OS or percentage necrosis for the analyses of the combined datasets by either Egger test or Begg test. For 5-year OS, Ps were .21 (Egger) and .19 (Begg); for percentage necrosis, Ps were .10 (Egger) and .62 (Begg). In addition, no evidence of publication bias was detected for the analyses of the nonmetastatic studies by either test. For 5-year OS, Ps were .55 (Egger) and .41 (Begg); for percentage necrosis, Ps were .42 (Egger) and .95 (Begg).
Discussion
Five-year OS was 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma and 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic osteosarcoma. These percentages fall within the range found in the literature. Mankin and colleagues37 reviewed 648 cases of patients with osteosarcoma treated at Massachusetts General Hospital in 2004; OS was 68%. In 2011, Sampo and colleagues60 reported 10-year OS of 63% for patients with metastatic and nonmetastatic disease and 73% for patients with local disease at presentation. Five-year OS rates in the literature are consistently about 70%. Ferrari and colleagues61 reported 5-year OS of 73% and 74% for 230 patients treated with 2 different neoadjuvant chemotherapy regimens between 2001 and 2006. The consistency in 5-year OS suggests OS of pediatric patients with osteosarcoma has plateaued, and there has been no significant improvement in survival of patients with osteosarcoma over the past 30 years.
Histologic response to preoperative chemotherapy is strongly associated with survival in pediatric osteosarcoma. Bielack and colleagues31 reported 5-year OS of 75% to 80% for patients who responded well to preoperative chemotherapy (>90% tumor necrosis) and 45% to 55% for patients who responded poorly (<10% necrosis). In our meta-analysis of studies that included patients with nonmetastatic osteosarcoma, 50% achieved necrosis of more than 90%. Percentage of patients achieving necrosis of more than 90% has been about 45%, according to past reports. In 2012, Ferrari and colleagues61 reported that 45% of 230 patients treated with neoadjuvant chemotherapy achieved more than 90% tumor necrosis. Therefore, 5-year OS and percentage of patients achieving 90% necrosis are consistent with previous reports, though this also suggests these numbers have remained constant over the past several decades.
Despite its expansive scale, our study has several important limitations. Data were extracted from published studies, and individual patient data were not available, so we were not able to assess the effects of risk factors (eg, tumor size, location) on 5-year OS. We could not correlate the proportion of patients with 90% necrosis to 5-year OS, as studies did not report OS by necrosis strata. Also, because our numbers were derived from published studies, they may not accurately represent outcomes in the community as a whole. In addition, several successive studies may contain duplicate patient cases. We limited our search to studies published since 2000 to include patients recently diagnosed and treated for osteosarcoma; however, several studies published after 2000 also included patients diagnosed and treated before 2000. Several of these studies are from countries outside the United States and may have a significantly different incidence of osteosarcoma as well as treatment methods and survival rates.
Although this meta-analysis suggests 5-year OS remains about 70% for patients with primary nonmetastatic osteosarcoma, we cannot settle on this conclusion because of the many differences between the studies we included. Therefore, more studies of patients diagnosed and treated within the past 10 years are needed to confirm our beliefs about patient survival.
1. Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667-674.
2. Cho WH, Song WS, Jeon DG, et al. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Ann Surg Oncol. 2010;17(3):702-708.
3. Abate ME, Longhi A, Galletti S, Ferrari S, Bacci G. Non-metastatic osteosarcoma of the extremities in children aged 5 years or younger. Pediatr Blood Cancer. 2010;55(4):652-654.
4. Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011-2018.
5. Pakos EE, Nearchou AD, Grimer RJ, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer. 2009;45(13):2367-2375.
6. Kaste SC, Liu T, Billups CA, Daw NC, Pratt CB, Meyer WH. Tumor size as a predictor of outcome in pediatric non-metastatic osteosarcoma of the extremity. Pediatr Blood Cancer. 2004;43(7):723-728.
7. Brostrom LA, Strander H, Nilsonne U. Survival in osteosarcoma in relation to tumor size and location. Clin Orthop Relat Res. 1982;167:250-254.
8. Harvei S, Solheim O. The prognosis in osteosarcoma: Norwegian national data. Cancer. 1981;48(8):1719-1723.
9. Sutow WW, Sullivan MP, Fernbach DJ, Cangir A, George SL. Adjuvant chemotherapy in primary treatment of osteogenic sarcoma. A Southwest Oncology Group study. Cancer. 1975;36(5):1598-1602.
10. Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5(1):21-26.
11. Hsieh MY, Hung GY, Yen HJ, Chen WM, Chen TH. Osteosarcoma in preadolescent patients: experience in a single institute in Taiwan. J Chin Med Assoc. 2009;72(9):455-461.
12. Longhi A, Pasini E, Bertoni F, Pignotti E, Ferrari C, Bacci G. Twenty-year follow-up of osteosarcoma of the extremity treated with adjuvant chemotherapy. J Chemother. 2004;16(6):582-588.
13. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
14. Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/Osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18(24):4016-4027.
15. Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154-1161.
16. Cohen IJ, Kaplinsky C, Katz K, et al. Improved results in osteogenic sarcoma 1973–79 vs. 1980–86: analysis of results from a single center. Isr J Med Sci. 1993;29(1):27-29.
17. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results program. Cancer. 2009;115(7):1531-1543.
18. Kager L, Zoubek A, Dominkus M, et al. Osteosarcoma in very young children: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2010;116(22):5316-5324.
19. Szendroi M, Papai Z, Koos R, Illes T. Limb-saving surgery, survival, and prognostic factors for osteosarcoma: the Hungarian experience. J Surg Oncol. 2000;73(2):87-94.
20. Nagarajan R, Kamruzzaman A, Ness KK, et al. Twenty years of follow-up of survivors of childhood osteosarcoma: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117(3):625-634.
21. Bacci G, Longhi A, Ferrari S, et al. Prognostic significance of serum lactate dehydrogenase in osteosarcoma of the extremity: experience at Rizzoli on 1421 patients treated over the last 30 years. Tumori. 2004;90(5):478-484.
22. Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5-15.
23. Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422-441.
24. Hendershot E, Pappo A, Malkin D, Sung L. Tumor necrosis in pediatric osteosarcoma: impact of modern therapies. J Pediatr Oncol Nurs. 2006;23(4):176-181.
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101.
28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634.
29. Bacci G, Ferrari S, Longhi A, Mellano D, Giacomini S, Forni C. Delay in diagnosis of high-grade osteosarcoma of the extremities. Has it any effect on the stage of disease? Tumori. 2000;86(3):204-206.
30. Bacci G, Ferrari S, Longhi A, et al. Neoadjuvant chemotherapy for high grade osteosarcoma of the extremities: long-term results for patients treated according to the Rizzoli IOR/OS-3b protocol. J Chemother. 2001;13(1):93-99.
31. Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776-790.
32. Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38(9):1218-1225.
33. Scully SP, Ghert MA, Zurakowski D, Thompson RC, Gebhardt MC. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J Bone Joint Surg Am. 2002;84(1):49-57.
34. Wilkins RM, Cullen JW, Odom L, et al. Superior survival in treatment of primary nonmetastatic pediatric osteosarcoma of the extremity. Ann Surg Oncol. 2003;10(5):498-507.
35. Smeland S, Muller C, Alvegard TA, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488-494.
36. Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21(2):334-341.
37. Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;429:286-291.
38. Donati D, Giacomini S, Gozzi E, et al. Osteosarcoma of the pelvis. Eur J Surg Oncol. 2004;30(3):332-340.
39. Bacci G, Longhi A, Bertoni F, et al. Primary high-grade osteosarcoma: comparison between preadolescent and older patients. J Pediatr Hematol Oncol. 2005;27(3):129-134.
40. Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41(18):2836-2845.
41. Matsuo T, Sugita T, Sato K, et al. Clinical outcomes of 54 pelvic osteosarcomas registered by Japanese musculoskeletal oncology group. Oncology. 2005;68(4-6):375-381.
42. Kuhelj D, Jereb B. Pediatric osteosarcoma: a 35-year experience in Slovenia. Pediatr Hematol Oncol. 2005;22(4):335-343.
43. Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer. 2005;104(5):1100-1109.
44. Daecke W, Bielack S, Martini AK, et al. Osteosarcoma of the hand and forearm: experience of the Cooperative Osteosarcoma Study Group. Ann Surg Oncol. 2005;12(4):322-331.
45. Cho WH, Lee SY, Song WS, Park JH. Osteosarcoma in pre-adolescent patients. J Int Med Res. 2006;34(6):676-681.
46. Petrilli AS, de Camargo B, Filho VO, et al. Results of the Brazilian Osteosarcoma Treatment Group studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161-1168.
47. Kim MS, Lee SY, Cho WH, et al. Growth patterns of osteosarcoma predict patient survival. Arch Orthop Trauma Surg. 2009;129(9):1189-1196.
48. Lee JA, Kim MS, Kim DH, et al. Osteosarcoma developed in the period of maximal growth rate have inferior prognosis. J Pediatr Hematol Oncol. 2008;30(6):419-424.
49. Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis: clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol. 2009;39(8):514-522.
50. Ayan I, Kebudi R, Ozger H. Childhood osteosarcoma: multimodal therapy in a single-institution Turkish series. Cancer Treat Res. 2009;152:319-338.
51. Bruland OS, Bauer H, Alvegaard T, Smeland S. Treatment of osteosarcoma. The Scandinavian Sarcoma Group experience. Cancer Treat Res. 2009;152:309-318.
52. Bielack S, Jurgens H, Jundt G, et al. Osteosarcoma: the COSS experience. Cancer Treat Res. 2009;152:289-308.
53. Bispo Júnior RZ, Camargo OP. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clinics (Sao Paulo). 2009;64(12):1177-1186.
54. Gonzalez-Billalabeitia E, Hitt R, Fernandez J, et al. Pre-treatment serum lactate dehydrogenase level is an important prognostic factor in high-grade extremity osteosarcoma. Clin Transl Oncol. 2009;11(7):479-483.
55. Kong CB, Kim MS, Lee SY, et al. Prognostic effect of diaphyseal location in osteosarcoma: a cohort case–control study at a single institute. Ann Surg Oncol. 2009;16(11):3094-3100.
56. Kim MS, Lee SY, Cho WH, et al. Prognostic effects of doctor-associated diagnostic delays in osteosarcoma. Arch Orthop Trauma Surg. 2009;129(10):1421-1425.
57. Lee JA, Kim MS, Kim DH, et al. Risk stratification based on the clinical factors at diagnosis is closely related to the survival of localized osteosarcoma. Pediatr Blood Cancer. 2009;52(3):340-345.
58. Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Osteosarcoma in children 5 years of age or younger at initial diagnosis. Pediatr Blood Cancer. 2010;55(2):285-289.
59. Munajat I, Zulmi W, Norazman MZ, Wan Faisham WI. Tumour volume and lung metastasis in patients with osteosarcoma. J Orthop Surg (Hong Kong). 2008;16(2):182-185.
60. Sampo M, Koivikko M, Taskinen M, et al. Incidence, epidemiology and treatment results of osteosarcoma in Finland - a nationwide population-based study. Acta Oncol. 2011;50(8):1206-1214.
61. Ferrari S, Ruggieri P, Cefalo G, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian Sarcoma Group trial ISG/OS-1. J Clin Oncol. 2012;30(17):2112-2118.
1. Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667-674.
2. Cho WH, Song WS, Jeon DG, et al. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Ann Surg Oncol. 2010;17(3):702-708.
3. Abate ME, Longhi A, Galletti S, Ferrari S, Bacci G. Non-metastatic osteosarcoma of the extremities in children aged 5 years or younger. Pediatr Blood Cancer. 2010;55(4):652-654.
4. Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011-2018.
5. Pakos EE, Nearchou AD, Grimer RJ, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer. 2009;45(13):2367-2375.
6. Kaste SC, Liu T, Billups CA, Daw NC, Pratt CB, Meyer WH. Tumor size as a predictor of outcome in pediatric non-metastatic osteosarcoma of the extremity. Pediatr Blood Cancer. 2004;43(7):723-728.
7. Brostrom LA, Strander H, Nilsonne U. Survival in osteosarcoma in relation to tumor size and location. Clin Orthop Relat Res. 1982;167:250-254.
8. Harvei S, Solheim O. The prognosis in osteosarcoma: Norwegian national data. Cancer. 1981;48(8):1719-1723.
9. Sutow WW, Sullivan MP, Fernbach DJ, Cangir A, George SL. Adjuvant chemotherapy in primary treatment of osteogenic sarcoma. A Southwest Oncology Group study. Cancer. 1975;36(5):1598-1602.
10. Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5(1):21-26.
11. Hsieh MY, Hung GY, Yen HJ, Chen WM, Chen TH. Osteosarcoma in preadolescent patients: experience in a single institute in Taiwan. J Chin Med Assoc. 2009;72(9):455-461.
12. Longhi A, Pasini E, Bertoni F, Pignotti E, Ferrari C, Bacci G. Twenty-year follow-up of osteosarcoma of the extremity treated with adjuvant chemotherapy. J Chemother. 2004;16(6):582-588.
13. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
14. Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/Osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18(24):4016-4027.
15. Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154-1161.
16. Cohen IJ, Kaplinsky C, Katz K, et al. Improved results in osteogenic sarcoma 1973–79 vs. 1980–86: analysis of results from a single center. Isr J Med Sci. 1993;29(1):27-29.
17. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results program. Cancer. 2009;115(7):1531-1543.
18. Kager L, Zoubek A, Dominkus M, et al. Osteosarcoma in very young children: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2010;116(22):5316-5324.
19. Szendroi M, Papai Z, Koos R, Illes T. Limb-saving surgery, survival, and prognostic factors for osteosarcoma: the Hungarian experience. J Surg Oncol. 2000;73(2):87-94.
20. Nagarajan R, Kamruzzaman A, Ness KK, et al. Twenty years of follow-up of survivors of childhood osteosarcoma: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117(3):625-634.
21. Bacci G, Longhi A, Ferrari S, et al. Prognostic significance of serum lactate dehydrogenase in osteosarcoma of the extremity: experience at Rizzoli on 1421 patients treated over the last 30 years. Tumori. 2004;90(5):478-484.
22. Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5-15.
23. Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422-441.
24. Hendershot E, Pappo A, Malkin D, Sung L. Tumor necrosis in pediatric osteosarcoma: impact of modern therapies. J Pediatr Oncol Nurs. 2006;23(4):176-181.
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101.
28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634.
29. Bacci G, Ferrari S, Longhi A, Mellano D, Giacomini S, Forni C. Delay in diagnosis of high-grade osteosarcoma of the extremities. Has it any effect on the stage of disease? Tumori. 2000;86(3):204-206.
30. Bacci G, Ferrari S, Longhi A, et al. Neoadjuvant chemotherapy for high grade osteosarcoma of the extremities: long-term results for patients treated according to the Rizzoli IOR/OS-3b protocol. J Chemother. 2001;13(1):93-99.
31. Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776-790.
32. Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38(9):1218-1225.
33. Scully SP, Ghert MA, Zurakowski D, Thompson RC, Gebhardt MC. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J Bone Joint Surg Am. 2002;84(1):49-57.
34. Wilkins RM, Cullen JW, Odom L, et al. Superior survival in treatment of primary nonmetastatic pediatric osteosarcoma of the extremity. Ann Surg Oncol. 2003;10(5):498-507.
35. Smeland S, Muller C, Alvegard TA, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488-494.
36. Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21(2):334-341.
37. Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;429:286-291.
38. Donati D, Giacomini S, Gozzi E, et al. Osteosarcoma of the pelvis. Eur J Surg Oncol. 2004;30(3):332-340.
39. Bacci G, Longhi A, Bertoni F, et al. Primary high-grade osteosarcoma: comparison between preadolescent and older patients. J Pediatr Hematol Oncol. 2005;27(3):129-134.
40. Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41(18):2836-2845.
41. Matsuo T, Sugita T, Sato K, et al. Clinical outcomes of 54 pelvic osteosarcomas registered by Japanese musculoskeletal oncology group. Oncology. 2005;68(4-6):375-381.
42. Kuhelj D, Jereb B. Pediatric osteosarcoma: a 35-year experience in Slovenia. Pediatr Hematol Oncol. 2005;22(4):335-343.
43. Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer. 2005;104(5):1100-1109.
44. Daecke W, Bielack S, Martini AK, et al. Osteosarcoma of the hand and forearm: experience of the Cooperative Osteosarcoma Study Group. Ann Surg Oncol. 2005;12(4):322-331.
45. Cho WH, Lee SY, Song WS, Park JH. Osteosarcoma in pre-adolescent patients. J Int Med Res. 2006;34(6):676-681.
46. Petrilli AS, de Camargo B, Filho VO, et al. Results of the Brazilian Osteosarcoma Treatment Group studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161-1168.
47. Kim MS, Lee SY, Cho WH, et al. Growth patterns of osteosarcoma predict patient survival. Arch Orthop Trauma Surg. 2009;129(9):1189-1196.
48. Lee JA, Kim MS, Kim DH, et al. Osteosarcoma developed in the period of maximal growth rate have inferior prognosis. J Pediatr Hematol Oncol. 2008;30(6):419-424.
49. Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis: clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol. 2009;39(8):514-522.
50. Ayan I, Kebudi R, Ozger H. Childhood osteosarcoma: multimodal therapy in a single-institution Turkish series. Cancer Treat Res. 2009;152:319-338.
51. Bruland OS, Bauer H, Alvegaard T, Smeland S. Treatment of osteosarcoma. The Scandinavian Sarcoma Group experience. Cancer Treat Res. 2009;152:309-318.
52. Bielack S, Jurgens H, Jundt G, et al. Osteosarcoma: the COSS experience. Cancer Treat Res. 2009;152:289-308.
53. Bispo Júnior RZ, Camargo OP. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clinics (Sao Paulo). 2009;64(12):1177-1186.
54. Gonzalez-Billalabeitia E, Hitt R, Fernandez J, et al. Pre-treatment serum lactate dehydrogenase level is an important prognostic factor in high-grade extremity osteosarcoma. Clin Transl Oncol. 2009;11(7):479-483.
55. Kong CB, Kim MS, Lee SY, et al. Prognostic effect of diaphyseal location in osteosarcoma: a cohort case–control study at a single institute. Ann Surg Oncol. 2009;16(11):3094-3100.
56. Kim MS, Lee SY, Cho WH, et al. Prognostic effects of doctor-associated diagnostic delays in osteosarcoma. Arch Orthop Trauma Surg. 2009;129(10):1421-1425.
57. Lee JA, Kim MS, Kim DH, et al. Risk stratification based on the clinical factors at diagnosis is closely related to the survival of localized osteosarcoma. Pediatr Blood Cancer. 2009;52(3):340-345.
58. Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Osteosarcoma in children 5 years of age or younger at initial diagnosis. Pediatr Blood Cancer. 2010;55(2):285-289.
59. Munajat I, Zulmi W, Norazman MZ, Wan Faisham WI. Tumour volume and lung metastasis in patients with osteosarcoma. J Orthop Surg (Hong Kong). 2008;16(2):182-185.
60. Sampo M, Koivikko M, Taskinen M, et al. Incidence, epidemiology and treatment results of osteosarcoma in Finland - a nationwide population-based study. Acta Oncol. 2011;50(8):1206-1214.
61. Ferrari S, Ruggieri P, Cefalo G, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian Sarcoma Group trial ISG/OS-1. J Clin Oncol. 2012;30(17):2112-2118.
Harrington Rod Revision After Failed Total Hip Arthroplasty Due to Missed Acetabular Metastasis
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
Spontaneous, Chronic Expanding Posterior Thigh Hematoma Mimicking Soft-Tissue Sarcoma in a Morbidly Obese Pregnant Woman
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
Pilot Study for an Orthopedic Surgical Training Laboratory for Basic Motor Skills
For the resident, the surgical residency is physically, emotionally, and intellectually demanding, requiring longitudinally concentrated effort. Although education of orthopedic surgeons necessarily occurs within the context of the health care delivery system, vital lessons also are taught in laboratories, skill stations, and surgical simulators. Before practice-based learning can take place, residents must gain experience and demonstrate growth in surgical skills, including decision-making and technical skills. These skill sets are difficult to systematically teach and objectively analyze.
The most effective way to teach and assess a resident’s knowledge of musculoskeletal medicine remains unclear at this point. Much of the current literature addresses the issue at the medical student level.1-7 Some studies have shown the effectiveness of surgical training programs, both cadaveric and computer-based simulators, in teaching various surgical skill sets.8-14 The orthopedic literature has seen a boom in surgical simulators aimed at the upper-level resident. Many of the topics involve use of arthroscopic simulators.15-19 Evidence suggests that simulators can discriminate between novice and expert users, but discrimination between novice and intermediate trainees in surgical education should be paramount.20
The American Board of Orthopaedic Surgery (ABOS) and the orthopedic Residency Review Committee (RRC) recommended new requirements for structured motor skills training in basic orthopedic surgery education,21 which were approved by the Accreditation Council for Graduate Medical Education (ACGME) board of directors and went into effect on July 1, 2013. In response to the new ACGME guidelines, our institution created a skills laboratory devoted to surgical simulation. Our focus in implementing this surgical skills simulation was junior-level, specifically postgraduate year 1 to 3 (PGY-1 to PGY-3), orthopedic residents. Our first goal was to set up a series of surgical training stations to educate junior-level residents in 4 core areas: handling and comfort with basic power equipment, casting/splinting, suturing, and surgical instrument identification. A secondary goal was to objectively evaluate the residents through written examinations (presession–postsession) and a novel ankle fracture model (pre–post).
Materials and Methods
Institutional review board approval was obtained before beginning the investigation.
Written Examination
We created a multiple-choice 25-question written examination (Appendix) and administered it to 11 junior residents before and after they participated in the training. This examination assessed their knowledge base of basic orthopedic tenets, including basic bone healing, basic fracture repair (Arbeitsgemeinschaft für Osteosynthesefragen [AO] principles22), suturing, surgical instrument identification, casting/splinting, and elementary implant-design rationale.
Evaluator Scorecard
We created an evaluation scorecard (Figure 1) and had 2 faculty members and 2 senior-level residents complete it independently. Junior residents were evaluated on a sawbones lateral malleolar ankle fracture model at 2 time points. As with the written examinations, the junior residents completed the fracture model both before and immediately after the multiple skill sessions. Each of the 15 data points was scored from 1 to 4, for a total of 60 points.
Facility for Surgical Training Session
Our Clinical Skills Education and Assessment Center houses small-group interactive laboratories for administration, debriefing, and assessment of simulations with the latest in audiovisual equipment. Five stations were created: hands-on introduction to surgical power equipment using sawbones, wood, and polyvinylchloride (PVC) pipe; hands-on introduction to casting and splinting; hands-on introduction to suturing; hands-on interaction with surgical scrub technician assisting with instrument identification; and didactic PowerPoint (Microsoft, Redmond, Washington) presentation focusing on core trauma competencies, basic orthopedic design rationale, and basic bone biology.
Development of Surgical Skills Training Session
Multiple faculty members and senior-level residents collaborated to create the skill stations (Figure 2), which were designed based on ACGME recommendations and on weaknesses our program had seen in junior-level residents. We devoted an afternoon to this session, excusing our program’s junior residents from clinical responsibilities. Four PGY-1, 5 PGY-2, and 2 PGY-3 residents participated. (Four of our 15 junior residents were unable to attend because of clinical responsibilities.) The afternoon started by dividing the 11 junior residents into 2 groups. Before the session, while one group performed the ankle fracture model and was being evaluated, the other took the written examination. This closely timed portion was allotted only 20 minutes. Then residents were divided into 5 groups of 2 or 3 and were rotated through all 5 stations. Forty minutes were allotted for each station. Residents were not evaluated during this portion. The stations were intended solely for education, and each station was staffed by a faculty member and/or senior-level resident.
Cordless reciprocating saws and drills were purchased to introduce and refine junior residents’ motor skills. Sawbones, 2×4-in sections of wood, and PVC pipe were used in the training. Emphasis was placed on tactile feel and feedback with both sawing and drilling. For the casting and splinting session, we used 4-in fiberglass, 4-in plaster rolls, and cotton soft roll to demonstrate a multitude of common casts and splints (Figure 3). Casts included short- and long-arm casts and short-leg casts. Splinting included coaptation, sugar tong, and ulnar gutter splints for the upper extremity and a short-leg posterior splint for the lower extremity.
The didactic PowerPoint presentation drew largely from content in chapters of the book AO Principles of Fracture Management.22 Content included condensed, to-the-point high-yield summaries of AO tenets, basic bone healing and biology, and orthopedic implant-design rationale focused on these elementary principles:
◾ Basic screw design, including cortical, cancellous, and locking screw designs.
◾ Evolution of plate osteosynthesis to currently used locking compression plate.
◾ Locking plate principles.
◾ Lag technique.
◾ Plate use: compression mode, neutralization, bridging, buttress, anti-glide.
The suturing portion was performed with thawed ham hocks (Figure 4). This model replicates live tissue layers and allows a layered closure technique as a training tool. Both 0 and 2-0 absorbable suture were available for a layered, deep fascial closure; also available was 2-0 nonabsorbable nylon for the skin. Staple guns were available, as were basic surgical instruments, including quality needle drivers, Adson forceps, and suture scissors. The knots demonstrated included simple, horizontal mattress, vertical mattress, and tension-relieving. One- and 2-hand tying and instrument tying were reinforced.
The final session consisted of surgical instrument identification. A certified orthopedic scrub technician participated. On site were multiple trays, including a basic bone set, a hand-and-foot set, small and large fragment sets, and a hip set. A detailed review of each set was led by the surgical technician. This review was followed by a question-and-answer session with the junior residents. After the session, we ended with the written examination and the ankle fracture model.
Statistical Methods
We report presession and postsession means, modes, and medians as measures of score-central tendencies. Our small sample size makes the assumption of Gaussian distribution tenuous and more susceptible to outliers. Therefore, in addition to reporting means, we include medians and modes to more accurately account for outliers. Moreover, the κ statistic is a robust measure of interrater agreement for 2 or more groups. We report κ statistics to determine the interrater reliability of 4 independent observers.
Results
Written Examination
Eleven residents (PGY-1 to PGY-3) completed the examination (Table 1). For the entire group, mean (SD) presession percentile was 87.3 (10.4), median was 88, and mode was 96; mean (SD) was 80 (12.6) for PGY-1, 89.6 (6.7) for PGY-2, and 96 (5.7) for PGY-3. For the entire group, mean (SD) postsession percentile was 92 (8.4), median was 96, and mode was 96; mean (SD) was 85 (10.5) for PGY-1, 96 (4) for PGY-2, and 96 (0) for PGY-3 (Table 2).
There was a significant presession–postsession difference in scores among all test takers, regardless of training level (P = .019). The PGY-1 level did not reach statistical significance in improvement from presession to postsession (P = .080); the PGY-2 level also did not reach statistical significance in improvement (P = .099); the PGY-3 level did not have enough participants to calculate a P value based on a paired Student t test.
Ankle Fracture Model
Actual percentile scores are listed in Table 3. For the entire group, mean (SD) overall presession percentile was 68.6 (13.9), median was 67, and mode was 67; mean (SD) was 58.8 (9.8) for PGY-1, 76.1 (13.6) for PGY-2, and 69.5 (9.8) for PGY-3. For the entire group, mean (SD) postsession percentile was 95.2 (5.2), median was 97, and mode was 97; mean (SD) was 91.8 (6.3) for PGY-1, 97.1 (3.5) for PGY-2, and 97.3 (2.4) for PGY-3.
There was a large and significant presession–postsession difference in scores among all test takers, regardless of training level (P = .03). Each group reached statistical significance in improvement from presession to postsession: PGY-1 (P = .04), PGY-2 (P = .01), and PGY-3 (P = .03).
For κ calculations, we adjusted all scores to ordinal data and thus used a standard grading system:
Score Grade
90–100 A
80–89 B
70–79 C
60–69 D
0–59 F
For the presession fracture model, the κ among the 4 independent observational scorers was 0.1148 (Table 4), which is poor based on κ scoring criteria and which we attribute to the particularly harsh grading by 1 observational scorer (faculty 1) relative to the other scorers’. Examination of the κ scores of faculty 1 and faculty 2 indicated only 9.09% agreement. Conversely, the κ among resident scorers agreed 54.55% of the time. Removing faculty 1 as an outlier raised the κ score dramatically, to 0.3125 (fair interobserver agreement).
For the postsession fracture model, the κ among the 4 independent observational scorers improved only marginally, to 0.1156 (still poor), again attributed to a difference in severity of grading: faculty 1 (harsh) versus faculty 2 (relatively kind). Examination of the κ scores of faculty 1 and faculty 2 revealed 72.73% agreement; residents agreed 81.82% of the time.
Discussion
The importance of surgical skill development in resident education is emphasized in the ACGME Core Competencies.23 The ACGME instructed all programs to require residents to gain competency in 6 areas: patient care, interpersonal and communication skills, medical knowledge, professionalism, practice-based learning and systems-based practice. Although many surgeon educators and residents are focused on these 6 Core Competencies, current standards do not require surgical skills laboratory training and simply require residents to log cases into the ACGME website. Minimal case number recommendations are in place for graduating senior residents, but these numbers are based on averages with no strong scientific basis.
Although sweeping changes in orthopedic residency training went into effect July 1, 2013, this system remains untested and may offer room for improvement. One change is the restructuring of the PGY-1 internship. A basic surgical skills curriculum must include goals, objectives, and assessment metrics; skills used in the initial management of injured patients, including splinting, casting, application of traction devices, and other types of immobilization; and basic operative skills, including soft-tissue management, suturing, bone management, arthroscopy, fluoroscopy, and use of basic orthopedic equipment.21
Orthopedic program directors and residents were recently surveyed regarding the current state of orthopedic motor skills training.24 Three key findings deserve emphasis: There is a lack of objective criteria for evaluating resident performance in the skills laboratory; most program directors who have a laboratory do not understand the associated costs; and the most significant issue for program directors is the financial challenge of operating a motor skills laboratory. The survey findings strongly suggest that proposed changes in skills training should be accompanied by careful cost analysis before widespread implementation.
Although various online demonstrations of entire surgeries are available, as are textbooks describing a generalized approach to musculoskeletal surgery, we assume that, as laid out in the Core Competencies, residents are fine-tuning their surgical skills by actively participating in operating rooms under direct observation of attending physicians. To our knowledge, however, there are no data regarding how often this happens in the operative setting, where volume and efficiency are becoming increasingly scrutinized. There has been much concern over how hour restrictions will affect residents’ total operative experience.25,26 Finally, we have no means to objectively evaluate residents’ surgical skills on graduation.
Other programs have implemented surgical skill simulators, but an orthopedics-specific surgical skills laboratory, to our knowledge, has been discussed in only 1 study.21 Results from randomized controlled trials reported in the general surgery literature have proved simulation-based training leads to detectable benefits for learners in clinical settings.27-29 Over the past decade, some alternative surgical skills training methods have been adopted in orthopedic surgery as well. These methods include hands-on training in specifically designed surgical skills laboratories using cadaver models or synthetic bones; software tools; and computerized simulators. In recent years, numerous studies reported in the orthopedic literature have examined arthroscopic simulators in residency training.18-20,30-34 However, these studies are arguably more specific to sports subspecialties and thus more pertinent to upper-level trainees.
Our study results showed that surgical skills laboratory training should be a required aspect of our residents’ training. Although less of a dramatic improvement was noted in the written examination component of the laboratory, the overall knowledge base improved (Table 3). This was especially evident at the PGY-1 level, where written examination scores increased from a presession median of 80% to a postsession median of 85%. A larger degree of improvement was found with the ankle fracture model, and there was statistical improvement at all training levels, from PGY-1 to PGY-3. Previous work has shown that intensive laboratory-based training can be effective, particularly for first-year residents. Sonnadara and colleagues35 demonstrated that a 30-day intensive surgical skills course effectively helped first-year orthopedic residents develop targeted basic surgical skills. In a follow-up study, Sonnadara and colleagues36 demonstrated that a surgical skills course completed at the beginning of a residency was effective in teaching targeted technical skills, and that skills taught in this manner can have excellent retention rates.
There are limitations inherent in our skills course. The κ agreement in the ankle fracture model was low before and after administration, which we attribute to 1 observer outlier. This could be amended by removing outliers and further objectifying and simplifying the scoring system (A–F). Right now, we do not have enough data to determine whether the scores actually improve significantly through the training years or whether they will correlate with operating room experience. Our study had no control. For future investigations, we are considering having general orthopedic surgeons from the community perform the same scenarios and be graded with the same checklists as a control. Implementation, however, may be a challenge. Both our written examination and our ankle fracture model checklist have not been validated—this is one of our next steps. The point system used to score the ankle fracture model was subjectively developed and would benefit from further standardization before drawing conclusions about true validity.
Conclusion
Orthopedic residency programs, like programs in other surgical specialties, are increasingly focused on teaching and documenting the learning of core competencies, even as work-hour restrictions and demands for clinical efficiency limit the amount of time residents spend in the operating room. We have demonstrated the potential value of an intensive laboratory in improving junior-level residents’ basic surgical skills and knowledge. We will continue to refine our methods, with a goal being to create reproducible models that could be adapted by other orthopedic residency programs and by other surgical educators.
1. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
2. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
3. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
4. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
5. Corbett EC Jr, Elnicki DM, Conaway MR. When should students learn essential physical examination skills? Views of internal medicine clerkship directors in North America. Acad Med. 2008;83(1):96-99.
6. Coady DA, Walker DJ, Kay LJ. Teaching medical students musculoskeletal examination skills: identifying barriers to learning and ways of overcoming them. Scand J Rheumatol. 2004;33(1):47-51.
7. Saleh K, Messner R, Axtell S, Harris I, Mahowald ML. Development and evaluation of an integrated musculoskeletal disease course for medical students. J Bone Joint Surg Am. 2004;86(8):1653-1658.
8. van Empel PJ, Verdam MG, Huirne JA, Bonjer HJ, Meijerink WJ, Scheele F. Open knot-tying skills: resident skills assessed. J Obstet Gynaecol Res. 2013;39(5):1030-1036.
9. Barrier BF, Thompson AB, McCullough MW, Occhino JA. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7(6):374-379.
10. Liss MA, McDougall EM. Robotic surgical simulation. Cancer J. 2013;19(2):124-129.
11. Stegemann AP, Ahmed K, Syed JR, et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81(4):767-774.
12. Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg. 2013;58(2):524-528.
13. Kuhls DA, Risucci DA, Bowyer MW, Luchette FA. Advanced surgical skills for exposure in trauma: a new surgical skills cadaver course for surgery residents and fellows. J Trauma Acute Care Surg. 2013;74(2):664-670.
14. Sanfey HA, Dunnington GL. Basic surgical skills testing for junior residents: current views of general surgery program directors. J Am Coll Surg. 2011;212(3):406-412.
15. Alvand A, Khan T, Al-Ali S, Jackson WF, Price AJ, Rees JL. Simple visual parameters for objective assessment of arthroscopic skill. J Bone Joint Surg Am. 2012;94(13):e97.
16. Jackson WF, Khan T, Alvand A, et al. Learning and retaining simulated arthroscopic meniscal repair skills. J Bone Joint Surg Am. 2012;94(17):e132.
17. Pernar LI, Smink DS, Hicks G, Peyre SE. Residents can successfully teach basic surgical skills in the simulation center. J Surg Educ. 2012;69(5):617-622.
18. Tuijthof GJ, Visser P, Sierevelt IN, Van Dijk CN, Kerkhoffs GM. Does perception of usefulness of arthroscopic simulators differ with levels of experience? Clin Orthop. 2011;469(6):1701-1708.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Slade Shantz JA, Leiter JR, Gottschalk T, MacDonald PB. The internal validity of arthroscopic simulators and their effectiveness in arthroscopic education. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):33-40.
21. Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11(5):747-757.
22. Ruedi TP, Buckley RE, Moran CG. AO Principles of Fracture Management. Stuttgart, Germany: Thieme; 2007.
23. Chen CL, Chen WC, Chiang JH, Ho CF. Interscapular hibernoma: case report and literature review. Kaohsiung J Med Sci. 2011;27(8):348-352.
24. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
25. Baskies MA, Ruchelsman DE, Capeci CM, Zuckerman JD, Egol KA. Operative experience in an orthopaedic surgery residency program: the effect of work-hour restrictions. J Bone Joint Surg Am. 2008;90(4):924-927.
26. Pappas AJ, Teague DC. The impact of the Accreditation Council for Graduate Medical Education work-hour regulations on the surgical experience of orthopaedic surgery residents. J Bone Joint Surg Am. 2007;89(4):904-909.
27. Palter VN, Grantcharov T, Harvey A, Macrae HM. Ex vivo technical skills training transfers to the operating room and enhances cognitive learning: a randomized controlled trial. Ann Surg. 2011;253(5):886-889.
28. Franzeck FM, Rosenthal R, Muller MK, et al. Prospective randomized controlled trial of simulator-based versus traditional in-surgery laparoscopic camera navigation training. Surg Endosc. 2012;26(1):235-241.
29. Zendejas B, Cook DA, Bingener J, et al. Simulation-based mastery learning improves patient outcomes in laparoscopic inguinal hernia repair: a randomized controlled trial. Ann Surg. 2011;254(3):502-509.
30. Hui Y, Safir O, Dubrowski A, Carnahan H. What skills should simulation training in arthroscopy teach residents? A focus on resident input. Int J Comput Assist Radiol Surg. 2013;8(6):945-953.
31. Butler A, Olson T, Koehler R, Nicandri G. Do the skills acquired by novice surgeons using anatomic dry models transfer effectively to the task of diagnostic knee arthroscopy performed on cadaveric specimens? J Bone Joint Surg Am. 2013;95(3):e15(1-8).
32. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
33. Elliott MJ, Caprise PA, Henning AE, Kurtz CA, Sekiya JK. Diagnostic knee arthroscopy: a pilot study to evaluate surgical skills. Arthroscopy. 2012;28(2):218-224.
34. Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop. 2011;82(1):90-95.
35. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
36. Sonnadara RR, Garbedian S, Safir O, et al. Orthopaedic boot camp II: examining the retention rates of an intensive surgical skills course. Surgery. 2012;151(6):803-807.
For the resident, the surgical residency is physically, emotionally, and intellectually demanding, requiring longitudinally concentrated effort. Although education of orthopedic surgeons necessarily occurs within the context of the health care delivery system, vital lessons also are taught in laboratories, skill stations, and surgical simulators. Before practice-based learning can take place, residents must gain experience and demonstrate growth in surgical skills, including decision-making and technical skills. These skill sets are difficult to systematically teach and objectively analyze.
The most effective way to teach and assess a resident’s knowledge of musculoskeletal medicine remains unclear at this point. Much of the current literature addresses the issue at the medical student level.1-7 Some studies have shown the effectiveness of surgical training programs, both cadaveric and computer-based simulators, in teaching various surgical skill sets.8-14 The orthopedic literature has seen a boom in surgical simulators aimed at the upper-level resident. Many of the topics involve use of arthroscopic simulators.15-19 Evidence suggests that simulators can discriminate between novice and expert users, but discrimination between novice and intermediate trainees in surgical education should be paramount.20
The American Board of Orthopaedic Surgery (ABOS) and the orthopedic Residency Review Committee (RRC) recommended new requirements for structured motor skills training in basic orthopedic surgery education,21 which were approved by the Accreditation Council for Graduate Medical Education (ACGME) board of directors and went into effect on July 1, 2013. In response to the new ACGME guidelines, our institution created a skills laboratory devoted to surgical simulation. Our focus in implementing this surgical skills simulation was junior-level, specifically postgraduate year 1 to 3 (PGY-1 to PGY-3), orthopedic residents. Our first goal was to set up a series of surgical training stations to educate junior-level residents in 4 core areas: handling and comfort with basic power equipment, casting/splinting, suturing, and surgical instrument identification. A secondary goal was to objectively evaluate the residents through written examinations (presession–postsession) and a novel ankle fracture model (pre–post).
Materials and Methods
Institutional review board approval was obtained before beginning the investigation.
Written Examination
We created a multiple-choice 25-question written examination (Appendix) and administered it to 11 junior residents before and after they participated in the training. This examination assessed their knowledge base of basic orthopedic tenets, including basic bone healing, basic fracture repair (Arbeitsgemeinschaft für Osteosynthesefragen [AO] principles22), suturing, surgical instrument identification, casting/splinting, and elementary implant-design rationale.
Evaluator Scorecard
We created an evaluation scorecard (Figure 1) and had 2 faculty members and 2 senior-level residents complete it independently. Junior residents were evaluated on a sawbones lateral malleolar ankle fracture model at 2 time points. As with the written examinations, the junior residents completed the fracture model both before and immediately after the multiple skill sessions. Each of the 15 data points was scored from 1 to 4, for a total of 60 points.
Facility for Surgical Training Session
Our Clinical Skills Education and Assessment Center houses small-group interactive laboratories for administration, debriefing, and assessment of simulations with the latest in audiovisual equipment. Five stations were created: hands-on introduction to surgical power equipment using sawbones, wood, and polyvinylchloride (PVC) pipe; hands-on introduction to casting and splinting; hands-on introduction to suturing; hands-on interaction with surgical scrub technician assisting with instrument identification; and didactic PowerPoint (Microsoft, Redmond, Washington) presentation focusing on core trauma competencies, basic orthopedic design rationale, and basic bone biology.
Development of Surgical Skills Training Session
Multiple faculty members and senior-level residents collaborated to create the skill stations (Figure 2), which were designed based on ACGME recommendations and on weaknesses our program had seen in junior-level residents. We devoted an afternoon to this session, excusing our program’s junior residents from clinical responsibilities. Four PGY-1, 5 PGY-2, and 2 PGY-3 residents participated. (Four of our 15 junior residents were unable to attend because of clinical responsibilities.) The afternoon started by dividing the 11 junior residents into 2 groups. Before the session, while one group performed the ankle fracture model and was being evaluated, the other took the written examination. This closely timed portion was allotted only 20 minutes. Then residents were divided into 5 groups of 2 or 3 and were rotated through all 5 stations. Forty minutes were allotted for each station. Residents were not evaluated during this portion. The stations were intended solely for education, and each station was staffed by a faculty member and/or senior-level resident.
Cordless reciprocating saws and drills were purchased to introduce and refine junior residents’ motor skills. Sawbones, 2×4-in sections of wood, and PVC pipe were used in the training. Emphasis was placed on tactile feel and feedback with both sawing and drilling. For the casting and splinting session, we used 4-in fiberglass, 4-in plaster rolls, and cotton soft roll to demonstrate a multitude of common casts and splints (Figure 3). Casts included short- and long-arm casts and short-leg casts. Splinting included coaptation, sugar tong, and ulnar gutter splints for the upper extremity and a short-leg posterior splint for the lower extremity.
The didactic PowerPoint presentation drew largely from content in chapters of the book AO Principles of Fracture Management.22 Content included condensed, to-the-point high-yield summaries of AO tenets, basic bone healing and biology, and orthopedic implant-design rationale focused on these elementary principles:
◾ Basic screw design, including cortical, cancellous, and locking screw designs.
◾ Evolution of plate osteosynthesis to currently used locking compression plate.
◾ Locking plate principles.
◾ Lag technique.
◾ Plate use: compression mode, neutralization, bridging, buttress, anti-glide.
The suturing portion was performed with thawed ham hocks (Figure 4). This model replicates live tissue layers and allows a layered closure technique as a training tool. Both 0 and 2-0 absorbable suture were available for a layered, deep fascial closure; also available was 2-0 nonabsorbable nylon for the skin. Staple guns were available, as were basic surgical instruments, including quality needle drivers, Adson forceps, and suture scissors. The knots demonstrated included simple, horizontal mattress, vertical mattress, and tension-relieving. One- and 2-hand tying and instrument tying were reinforced.
The final session consisted of surgical instrument identification. A certified orthopedic scrub technician participated. On site were multiple trays, including a basic bone set, a hand-and-foot set, small and large fragment sets, and a hip set. A detailed review of each set was led by the surgical technician. This review was followed by a question-and-answer session with the junior residents. After the session, we ended with the written examination and the ankle fracture model.
Statistical Methods
We report presession and postsession means, modes, and medians as measures of score-central tendencies. Our small sample size makes the assumption of Gaussian distribution tenuous and more susceptible to outliers. Therefore, in addition to reporting means, we include medians and modes to more accurately account for outliers. Moreover, the κ statistic is a robust measure of interrater agreement for 2 or more groups. We report κ statistics to determine the interrater reliability of 4 independent observers.
Results
Written Examination
Eleven residents (PGY-1 to PGY-3) completed the examination (Table 1). For the entire group, mean (SD) presession percentile was 87.3 (10.4), median was 88, and mode was 96; mean (SD) was 80 (12.6) for PGY-1, 89.6 (6.7) for PGY-2, and 96 (5.7) for PGY-3. For the entire group, mean (SD) postsession percentile was 92 (8.4), median was 96, and mode was 96; mean (SD) was 85 (10.5) for PGY-1, 96 (4) for PGY-2, and 96 (0) for PGY-3 (Table 2).
There was a significant presession–postsession difference in scores among all test takers, regardless of training level (P = .019). The PGY-1 level did not reach statistical significance in improvement from presession to postsession (P = .080); the PGY-2 level also did not reach statistical significance in improvement (P = .099); the PGY-3 level did not have enough participants to calculate a P value based on a paired Student t test.
Ankle Fracture Model
Actual percentile scores are listed in Table 3. For the entire group, mean (SD) overall presession percentile was 68.6 (13.9), median was 67, and mode was 67; mean (SD) was 58.8 (9.8) for PGY-1, 76.1 (13.6) for PGY-2, and 69.5 (9.8) for PGY-3. For the entire group, mean (SD) postsession percentile was 95.2 (5.2), median was 97, and mode was 97; mean (SD) was 91.8 (6.3) for PGY-1, 97.1 (3.5) for PGY-2, and 97.3 (2.4) for PGY-3.
There was a large and significant presession–postsession difference in scores among all test takers, regardless of training level (P = .03). Each group reached statistical significance in improvement from presession to postsession: PGY-1 (P = .04), PGY-2 (P = .01), and PGY-3 (P = .03).
For κ calculations, we adjusted all scores to ordinal data and thus used a standard grading system:
Score Grade
90–100 A
80–89 B
70–79 C
60–69 D
0–59 F
For the presession fracture model, the κ among the 4 independent observational scorers was 0.1148 (Table 4), which is poor based on κ scoring criteria and which we attribute to the particularly harsh grading by 1 observational scorer (faculty 1) relative to the other scorers’. Examination of the κ scores of faculty 1 and faculty 2 indicated only 9.09% agreement. Conversely, the κ among resident scorers agreed 54.55% of the time. Removing faculty 1 as an outlier raised the κ score dramatically, to 0.3125 (fair interobserver agreement).
For the postsession fracture model, the κ among the 4 independent observational scorers improved only marginally, to 0.1156 (still poor), again attributed to a difference in severity of grading: faculty 1 (harsh) versus faculty 2 (relatively kind). Examination of the κ scores of faculty 1 and faculty 2 revealed 72.73% agreement; residents agreed 81.82% of the time.
Discussion
The importance of surgical skill development in resident education is emphasized in the ACGME Core Competencies.23 The ACGME instructed all programs to require residents to gain competency in 6 areas: patient care, interpersonal and communication skills, medical knowledge, professionalism, practice-based learning and systems-based practice. Although many surgeon educators and residents are focused on these 6 Core Competencies, current standards do not require surgical skills laboratory training and simply require residents to log cases into the ACGME website. Minimal case number recommendations are in place for graduating senior residents, but these numbers are based on averages with no strong scientific basis.
Although sweeping changes in orthopedic residency training went into effect July 1, 2013, this system remains untested and may offer room for improvement. One change is the restructuring of the PGY-1 internship. A basic surgical skills curriculum must include goals, objectives, and assessment metrics; skills used in the initial management of injured patients, including splinting, casting, application of traction devices, and other types of immobilization; and basic operative skills, including soft-tissue management, suturing, bone management, arthroscopy, fluoroscopy, and use of basic orthopedic equipment.21
Orthopedic program directors and residents were recently surveyed regarding the current state of orthopedic motor skills training.24 Three key findings deserve emphasis: There is a lack of objective criteria for evaluating resident performance in the skills laboratory; most program directors who have a laboratory do not understand the associated costs; and the most significant issue for program directors is the financial challenge of operating a motor skills laboratory. The survey findings strongly suggest that proposed changes in skills training should be accompanied by careful cost analysis before widespread implementation.
Although various online demonstrations of entire surgeries are available, as are textbooks describing a generalized approach to musculoskeletal surgery, we assume that, as laid out in the Core Competencies, residents are fine-tuning their surgical skills by actively participating in operating rooms under direct observation of attending physicians. To our knowledge, however, there are no data regarding how often this happens in the operative setting, where volume and efficiency are becoming increasingly scrutinized. There has been much concern over how hour restrictions will affect residents’ total operative experience.25,26 Finally, we have no means to objectively evaluate residents’ surgical skills on graduation.
Other programs have implemented surgical skill simulators, but an orthopedics-specific surgical skills laboratory, to our knowledge, has been discussed in only 1 study.21 Results from randomized controlled trials reported in the general surgery literature have proved simulation-based training leads to detectable benefits for learners in clinical settings.27-29 Over the past decade, some alternative surgical skills training methods have been adopted in orthopedic surgery as well. These methods include hands-on training in specifically designed surgical skills laboratories using cadaver models or synthetic bones; software tools; and computerized simulators. In recent years, numerous studies reported in the orthopedic literature have examined arthroscopic simulators in residency training.18-20,30-34 However, these studies are arguably more specific to sports subspecialties and thus more pertinent to upper-level trainees.
Our study results showed that surgical skills laboratory training should be a required aspect of our residents’ training. Although less of a dramatic improvement was noted in the written examination component of the laboratory, the overall knowledge base improved (Table 3). This was especially evident at the PGY-1 level, where written examination scores increased from a presession median of 80% to a postsession median of 85%. A larger degree of improvement was found with the ankle fracture model, and there was statistical improvement at all training levels, from PGY-1 to PGY-3. Previous work has shown that intensive laboratory-based training can be effective, particularly for first-year residents. Sonnadara and colleagues35 demonstrated that a 30-day intensive surgical skills course effectively helped first-year orthopedic residents develop targeted basic surgical skills. In a follow-up study, Sonnadara and colleagues36 demonstrated that a surgical skills course completed at the beginning of a residency was effective in teaching targeted technical skills, and that skills taught in this manner can have excellent retention rates.
There are limitations inherent in our skills course. The κ agreement in the ankle fracture model was low before and after administration, which we attribute to 1 observer outlier. This could be amended by removing outliers and further objectifying and simplifying the scoring system (A–F). Right now, we do not have enough data to determine whether the scores actually improve significantly through the training years or whether they will correlate with operating room experience. Our study had no control. For future investigations, we are considering having general orthopedic surgeons from the community perform the same scenarios and be graded with the same checklists as a control. Implementation, however, may be a challenge. Both our written examination and our ankle fracture model checklist have not been validated—this is one of our next steps. The point system used to score the ankle fracture model was subjectively developed and would benefit from further standardization before drawing conclusions about true validity.
Conclusion
Orthopedic residency programs, like programs in other surgical specialties, are increasingly focused on teaching and documenting the learning of core competencies, even as work-hour restrictions and demands for clinical efficiency limit the amount of time residents spend in the operating room. We have demonstrated the potential value of an intensive laboratory in improving junior-level residents’ basic surgical skills and knowledge. We will continue to refine our methods, with a goal being to create reproducible models that could be adapted by other orthopedic residency programs and by other surgical educators.
For the resident, the surgical residency is physically, emotionally, and intellectually demanding, requiring longitudinally concentrated effort. Although education of orthopedic surgeons necessarily occurs within the context of the health care delivery system, vital lessons also are taught in laboratories, skill stations, and surgical simulators. Before practice-based learning can take place, residents must gain experience and demonstrate growth in surgical skills, including decision-making and technical skills. These skill sets are difficult to systematically teach and objectively analyze.
The most effective way to teach and assess a resident’s knowledge of musculoskeletal medicine remains unclear at this point. Much of the current literature addresses the issue at the medical student level.1-7 Some studies have shown the effectiveness of surgical training programs, both cadaveric and computer-based simulators, in teaching various surgical skill sets.8-14 The orthopedic literature has seen a boom in surgical simulators aimed at the upper-level resident. Many of the topics involve use of arthroscopic simulators.15-19 Evidence suggests that simulators can discriminate between novice and expert users, but discrimination between novice and intermediate trainees in surgical education should be paramount.20
The American Board of Orthopaedic Surgery (ABOS) and the orthopedic Residency Review Committee (RRC) recommended new requirements for structured motor skills training in basic orthopedic surgery education,21 which were approved by the Accreditation Council for Graduate Medical Education (ACGME) board of directors and went into effect on July 1, 2013. In response to the new ACGME guidelines, our institution created a skills laboratory devoted to surgical simulation. Our focus in implementing this surgical skills simulation was junior-level, specifically postgraduate year 1 to 3 (PGY-1 to PGY-3), orthopedic residents. Our first goal was to set up a series of surgical training stations to educate junior-level residents in 4 core areas: handling and comfort with basic power equipment, casting/splinting, suturing, and surgical instrument identification. A secondary goal was to objectively evaluate the residents through written examinations (presession–postsession) and a novel ankle fracture model (pre–post).
Materials and Methods
Institutional review board approval was obtained before beginning the investigation.
Written Examination
We created a multiple-choice 25-question written examination (Appendix) and administered it to 11 junior residents before and after they participated in the training. This examination assessed their knowledge base of basic orthopedic tenets, including basic bone healing, basic fracture repair (Arbeitsgemeinschaft für Osteosynthesefragen [AO] principles22), suturing, surgical instrument identification, casting/splinting, and elementary implant-design rationale.
Evaluator Scorecard
We created an evaluation scorecard (Figure 1) and had 2 faculty members and 2 senior-level residents complete it independently. Junior residents were evaluated on a sawbones lateral malleolar ankle fracture model at 2 time points. As with the written examinations, the junior residents completed the fracture model both before and immediately after the multiple skill sessions. Each of the 15 data points was scored from 1 to 4, for a total of 60 points.
Facility for Surgical Training Session
Our Clinical Skills Education and Assessment Center houses small-group interactive laboratories for administration, debriefing, and assessment of simulations with the latest in audiovisual equipment. Five stations were created: hands-on introduction to surgical power equipment using sawbones, wood, and polyvinylchloride (PVC) pipe; hands-on introduction to casting and splinting; hands-on introduction to suturing; hands-on interaction with surgical scrub technician assisting with instrument identification; and didactic PowerPoint (Microsoft, Redmond, Washington) presentation focusing on core trauma competencies, basic orthopedic design rationale, and basic bone biology.
Development of Surgical Skills Training Session
Multiple faculty members and senior-level residents collaborated to create the skill stations (Figure 2), which were designed based on ACGME recommendations and on weaknesses our program had seen in junior-level residents. We devoted an afternoon to this session, excusing our program’s junior residents from clinical responsibilities. Four PGY-1, 5 PGY-2, and 2 PGY-3 residents participated. (Four of our 15 junior residents were unable to attend because of clinical responsibilities.) The afternoon started by dividing the 11 junior residents into 2 groups. Before the session, while one group performed the ankle fracture model and was being evaluated, the other took the written examination. This closely timed portion was allotted only 20 minutes. Then residents were divided into 5 groups of 2 or 3 and were rotated through all 5 stations. Forty minutes were allotted for each station. Residents were not evaluated during this portion. The stations were intended solely for education, and each station was staffed by a faculty member and/or senior-level resident.
Cordless reciprocating saws and drills were purchased to introduce and refine junior residents’ motor skills. Sawbones, 2×4-in sections of wood, and PVC pipe were used in the training. Emphasis was placed on tactile feel and feedback with both sawing and drilling. For the casting and splinting session, we used 4-in fiberglass, 4-in plaster rolls, and cotton soft roll to demonstrate a multitude of common casts and splints (Figure 3). Casts included short- and long-arm casts and short-leg casts. Splinting included coaptation, sugar tong, and ulnar gutter splints for the upper extremity and a short-leg posterior splint for the lower extremity.
The didactic PowerPoint presentation drew largely from content in chapters of the book AO Principles of Fracture Management.22 Content included condensed, to-the-point high-yield summaries of AO tenets, basic bone healing and biology, and orthopedic implant-design rationale focused on these elementary principles:
◾ Basic screw design, including cortical, cancellous, and locking screw designs.
◾ Evolution of plate osteosynthesis to currently used locking compression plate.
◾ Locking plate principles.
◾ Lag technique.
◾ Plate use: compression mode, neutralization, bridging, buttress, anti-glide.
The suturing portion was performed with thawed ham hocks (Figure 4). This model replicates live tissue layers and allows a layered closure technique as a training tool. Both 0 and 2-0 absorbable suture were available for a layered, deep fascial closure; also available was 2-0 nonabsorbable nylon for the skin. Staple guns were available, as were basic surgical instruments, including quality needle drivers, Adson forceps, and suture scissors. The knots demonstrated included simple, horizontal mattress, vertical mattress, and tension-relieving. One- and 2-hand tying and instrument tying were reinforced.
The final session consisted of surgical instrument identification. A certified orthopedic scrub technician participated. On site were multiple trays, including a basic bone set, a hand-and-foot set, small and large fragment sets, and a hip set. A detailed review of each set was led by the surgical technician. This review was followed by a question-and-answer session with the junior residents. After the session, we ended with the written examination and the ankle fracture model.
Statistical Methods
We report presession and postsession means, modes, and medians as measures of score-central tendencies. Our small sample size makes the assumption of Gaussian distribution tenuous and more susceptible to outliers. Therefore, in addition to reporting means, we include medians and modes to more accurately account for outliers. Moreover, the κ statistic is a robust measure of interrater agreement for 2 or more groups. We report κ statistics to determine the interrater reliability of 4 independent observers.
Results
Written Examination
Eleven residents (PGY-1 to PGY-3) completed the examination (Table 1). For the entire group, mean (SD) presession percentile was 87.3 (10.4), median was 88, and mode was 96; mean (SD) was 80 (12.6) for PGY-1, 89.6 (6.7) for PGY-2, and 96 (5.7) for PGY-3. For the entire group, mean (SD) postsession percentile was 92 (8.4), median was 96, and mode was 96; mean (SD) was 85 (10.5) for PGY-1, 96 (4) for PGY-2, and 96 (0) for PGY-3 (Table 2).
There was a significant presession–postsession difference in scores among all test takers, regardless of training level (P = .019). The PGY-1 level did not reach statistical significance in improvement from presession to postsession (P = .080); the PGY-2 level also did not reach statistical significance in improvement (P = .099); the PGY-3 level did not have enough participants to calculate a P value based on a paired Student t test.
Ankle Fracture Model
Actual percentile scores are listed in Table 3. For the entire group, mean (SD) overall presession percentile was 68.6 (13.9), median was 67, and mode was 67; mean (SD) was 58.8 (9.8) for PGY-1, 76.1 (13.6) for PGY-2, and 69.5 (9.8) for PGY-3. For the entire group, mean (SD) postsession percentile was 95.2 (5.2), median was 97, and mode was 97; mean (SD) was 91.8 (6.3) for PGY-1, 97.1 (3.5) for PGY-2, and 97.3 (2.4) for PGY-3.
There was a large and significant presession–postsession difference in scores among all test takers, regardless of training level (P = .03). Each group reached statistical significance in improvement from presession to postsession: PGY-1 (P = .04), PGY-2 (P = .01), and PGY-3 (P = .03).
For κ calculations, we adjusted all scores to ordinal data and thus used a standard grading system:
Score Grade
90–100 A
80–89 B
70–79 C
60–69 D
0–59 F
For the presession fracture model, the κ among the 4 independent observational scorers was 0.1148 (Table 4), which is poor based on κ scoring criteria and which we attribute to the particularly harsh grading by 1 observational scorer (faculty 1) relative to the other scorers’. Examination of the κ scores of faculty 1 and faculty 2 indicated only 9.09% agreement. Conversely, the κ among resident scorers agreed 54.55% of the time. Removing faculty 1 as an outlier raised the κ score dramatically, to 0.3125 (fair interobserver agreement).
For the postsession fracture model, the κ among the 4 independent observational scorers improved only marginally, to 0.1156 (still poor), again attributed to a difference in severity of grading: faculty 1 (harsh) versus faculty 2 (relatively kind). Examination of the κ scores of faculty 1 and faculty 2 revealed 72.73% agreement; residents agreed 81.82% of the time.
Discussion
The importance of surgical skill development in resident education is emphasized in the ACGME Core Competencies.23 The ACGME instructed all programs to require residents to gain competency in 6 areas: patient care, interpersonal and communication skills, medical knowledge, professionalism, practice-based learning and systems-based practice. Although many surgeon educators and residents are focused on these 6 Core Competencies, current standards do not require surgical skills laboratory training and simply require residents to log cases into the ACGME website. Minimal case number recommendations are in place for graduating senior residents, but these numbers are based on averages with no strong scientific basis.
Although sweeping changes in orthopedic residency training went into effect July 1, 2013, this system remains untested and may offer room for improvement. One change is the restructuring of the PGY-1 internship. A basic surgical skills curriculum must include goals, objectives, and assessment metrics; skills used in the initial management of injured patients, including splinting, casting, application of traction devices, and other types of immobilization; and basic operative skills, including soft-tissue management, suturing, bone management, arthroscopy, fluoroscopy, and use of basic orthopedic equipment.21
Orthopedic program directors and residents were recently surveyed regarding the current state of orthopedic motor skills training.24 Three key findings deserve emphasis: There is a lack of objective criteria for evaluating resident performance in the skills laboratory; most program directors who have a laboratory do not understand the associated costs; and the most significant issue for program directors is the financial challenge of operating a motor skills laboratory. The survey findings strongly suggest that proposed changes in skills training should be accompanied by careful cost analysis before widespread implementation.
Although various online demonstrations of entire surgeries are available, as are textbooks describing a generalized approach to musculoskeletal surgery, we assume that, as laid out in the Core Competencies, residents are fine-tuning their surgical skills by actively participating in operating rooms under direct observation of attending physicians. To our knowledge, however, there are no data regarding how often this happens in the operative setting, where volume and efficiency are becoming increasingly scrutinized. There has been much concern over how hour restrictions will affect residents’ total operative experience.25,26 Finally, we have no means to objectively evaluate residents’ surgical skills on graduation.
Other programs have implemented surgical skill simulators, but an orthopedics-specific surgical skills laboratory, to our knowledge, has been discussed in only 1 study.21 Results from randomized controlled trials reported in the general surgery literature have proved simulation-based training leads to detectable benefits for learners in clinical settings.27-29 Over the past decade, some alternative surgical skills training methods have been adopted in orthopedic surgery as well. These methods include hands-on training in specifically designed surgical skills laboratories using cadaver models or synthetic bones; software tools; and computerized simulators. In recent years, numerous studies reported in the orthopedic literature have examined arthroscopic simulators in residency training.18-20,30-34 However, these studies are arguably more specific to sports subspecialties and thus more pertinent to upper-level trainees.
Our study results showed that surgical skills laboratory training should be a required aspect of our residents’ training. Although less of a dramatic improvement was noted in the written examination component of the laboratory, the overall knowledge base improved (Table 3). This was especially evident at the PGY-1 level, where written examination scores increased from a presession median of 80% to a postsession median of 85%. A larger degree of improvement was found with the ankle fracture model, and there was statistical improvement at all training levels, from PGY-1 to PGY-3. Previous work has shown that intensive laboratory-based training can be effective, particularly for first-year residents. Sonnadara and colleagues35 demonstrated that a 30-day intensive surgical skills course effectively helped first-year orthopedic residents develop targeted basic surgical skills. In a follow-up study, Sonnadara and colleagues36 demonstrated that a surgical skills course completed at the beginning of a residency was effective in teaching targeted technical skills, and that skills taught in this manner can have excellent retention rates.
There are limitations inherent in our skills course. The κ agreement in the ankle fracture model was low before and after administration, which we attribute to 1 observer outlier. This could be amended by removing outliers and further objectifying and simplifying the scoring system (A–F). Right now, we do not have enough data to determine whether the scores actually improve significantly through the training years or whether they will correlate with operating room experience. Our study had no control. For future investigations, we are considering having general orthopedic surgeons from the community perform the same scenarios and be graded with the same checklists as a control. Implementation, however, may be a challenge. Both our written examination and our ankle fracture model checklist have not been validated—this is one of our next steps. The point system used to score the ankle fracture model was subjectively developed and would benefit from further standardization before drawing conclusions about true validity.
Conclusion
Orthopedic residency programs, like programs in other surgical specialties, are increasingly focused on teaching and documenting the learning of core competencies, even as work-hour restrictions and demands for clinical efficiency limit the amount of time residents spend in the operating room. We have demonstrated the potential value of an intensive laboratory in improving junior-level residents’ basic surgical skills and knowledge. We will continue to refine our methods, with a goal being to create reproducible models that could be adapted by other orthopedic residency programs and by other surgical educators.
1. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
2. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
3. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
4. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
5. Corbett EC Jr, Elnicki DM, Conaway MR. When should students learn essential physical examination skills? Views of internal medicine clerkship directors in North America. Acad Med. 2008;83(1):96-99.
6. Coady DA, Walker DJ, Kay LJ. Teaching medical students musculoskeletal examination skills: identifying barriers to learning and ways of overcoming them. Scand J Rheumatol. 2004;33(1):47-51.
7. Saleh K, Messner R, Axtell S, Harris I, Mahowald ML. Development and evaluation of an integrated musculoskeletal disease course for medical students. J Bone Joint Surg Am. 2004;86(8):1653-1658.
8. van Empel PJ, Verdam MG, Huirne JA, Bonjer HJ, Meijerink WJ, Scheele F. Open knot-tying skills: resident skills assessed. J Obstet Gynaecol Res. 2013;39(5):1030-1036.
9. Barrier BF, Thompson AB, McCullough MW, Occhino JA. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7(6):374-379.
10. Liss MA, McDougall EM. Robotic surgical simulation. Cancer J. 2013;19(2):124-129.
11. Stegemann AP, Ahmed K, Syed JR, et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81(4):767-774.
12. Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg. 2013;58(2):524-528.
13. Kuhls DA, Risucci DA, Bowyer MW, Luchette FA. Advanced surgical skills for exposure in trauma: a new surgical skills cadaver course for surgery residents and fellows. J Trauma Acute Care Surg. 2013;74(2):664-670.
14. Sanfey HA, Dunnington GL. Basic surgical skills testing for junior residents: current views of general surgery program directors. J Am Coll Surg. 2011;212(3):406-412.
15. Alvand A, Khan T, Al-Ali S, Jackson WF, Price AJ, Rees JL. Simple visual parameters for objective assessment of arthroscopic skill. J Bone Joint Surg Am. 2012;94(13):e97.
16. Jackson WF, Khan T, Alvand A, et al. Learning and retaining simulated arthroscopic meniscal repair skills. J Bone Joint Surg Am. 2012;94(17):e132.
17. Pernar LI, Smink DS, Hicks G, Peyre SE. Residents can successfully teach basic surgical skills in the simulation center. J Surg Educ. 2012;69(5):617-622.
18. Tuijthof GJ, Visser P, Sierevelt IN, Van Dijk CN, Kerkhoffs GM. Does perception of usefulness of arthroscopic simulators differ with levels of experience? Clin Orthop. 2011;469(6):1701-1708.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Slade Shantz JA, Leiter JR, Gottschalk T, MacDonald PB. The internal validity of arthroscopic simulators and their effectiveness in arthroscopic education. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):33-40.
21. Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11(5):747-757.
22. Ruedi TP, Buckley RE, Moran CG. AO Principles of Fracture Management. Stuttgart, Germany: Thieme; 2007.
23. Chen CL, Chen WC, Chiang JH, Ho CF. Interscapular hibernoma: case report and literature review. Kaohsiung J Med Sci. 2011;27(8):348-352.
24. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
25. Baskies MA, Ruchelsman DE, Capeci CM, Zuckerman JD, Egol KA. Operative experience in an orthopaedic surgery residency program: the effect of work-hour restrictions. J Bone Joint Surg Am. 2008;90(4):924-927.
26. Pappas AJ, Teague DC. The impact of the Accreditation Council for Graduate Medical Education work-hour regulations on the surgical experience of orthopaedic surgery residents. J Bone Joint Surg Am. 2007;89(4):904-909.
27. Palter VN, Grantcharov T, Harvey A, Macrae HM. Ex vivo technical skills training transfers to the operating room and enhances cognitive learning: a randomized controlled trial. Ann Surg. 2011;253(5):886-889.
28. Franzeck FM, Rosenthal R, Muller MK, et al. Prospective randomized controlled trial of simulator-based versus traditional in-surgery laparoscopic camera navigation training. Surg Endosc. 2012;26(1):235-241.
29. Zendejas B, Cook DA, Bingener J, et al. Simulation-based mastery learning improves patient outcomes in laparoscopic inguinal hernia repair: a randomized controlled trial. Ann Surg. 2011;254(3):502-509.
30. Hui Y, Safir O, Dubrowski A, Carnahan H. What skills should simulation training in arthroscopy teach residents? A focus on resident input. Int J Comput Assist Radiol Surg. 2013;8(6):945-953.
31. Butler A, Olson T, Koehler R, Nicandri G. Do the skills acquired by novice surgeons using anatomic dry models transfer effectively to the task of diagnostic knee arthroscopy performed on cadaveric specimens? J Bone Joint Surg Am. 2013;95(3):e15(1-8).
32. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
33. Elliott MJ, Caprise PA, Henning AE, Kurtz CA, Sekiya JK. Diagnostic knee arthroscopy: a pilot study to evaluate surgical skills. Arthroscopy. 2012;28(2):218-224.
34. Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop. 2011;82(1):90-95.
35. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
36. Sonnadara RR, Garbedian S, Safir O, et al. Orthopaedic boot camp II: examining the retention rates of an intensive surgical skills course. Surgery. 2012;151(6):803-807.
1. Schmale GA. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop. 2005;(437):251-259.
2. Day CS, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
3. Bilderback K, Eggerstedt J, Sadasivan KK, et al. Design and implementation of a system-based course in musculoskeletal medicine for medical students. J Bone Joint Surg Am. 2008;90(10):2292-2300.
4. Freedman KB, Bernstein J. Educational deficiencies in musculoskeletal medicine. J Bone Joint Surg Am. 2002;84(4):604-608.
5. Corbett EC Jr, Elnicki DM, Conaway MR. When should students learn essential physical examination skills? Views of internal medicine clerkship directors in North America. Acad Med. 2008;83(1):96-99.
6. Coady DA, Walker DJ, Kay LJ. Teaching medical students musculoskeletal examination skills: identifying barriers to learning and ways of overcoming them. Scand J Rheumatol. 2004;33(1):47-51.
7. Saleh K, Messner R, Axtell S, Harris I, Mahowald ML. Development and evaluation of an integrated musculoskeletal disease course for medical students. J Bone Joint Surg Am. 2004;86(8):1653-1658.
8. van Empel PJ, Verdam MG, Huirne JA, Bonjer HJ, Meijerink WJ, Scheele F. Open knot-tying skills: resident skills assessed. J Obstet Gynaecol Res. 2013;39(5):1030-1036.
9. Barrier BF, Thompson AB, McCullough MW, Occhino JA. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7(6):374-379.
10. Liss MA, McDougall EM. Robotic surgical simulation. Cancer J. 2013;19(2):124-129.
11. Stegemann AP, Ahmed K, Syed JR, et al. Fundamental skills of robotic surgery: a multi-institutional randomized controlled trial for validation of a simulation-based curriculum. Urology. 2013;81(4):767-774.
12. Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg. 2013;58(2):524-528.
13. Kuhls DA, Risucci DA, Bowyer MW, Luchette FA. Advanced surgical skills for exposure in trauma: a new surgical skills cadaver course for surgery residents and fellows. J Trauma Acute Care Surg. 2013;74(2):664-670.
14. Sanfey HA, Dunnington GL. Basic surgical skills testing for junior residents: current views of general surgery program directors. J Am Coll Surg. 2011;212(3):406-412.
15. Alvand A, Khan T, Al-Ali S, Jackson WF, Price AJ, Rees JL. Simple visual parameters for objective assessment of arthroscopic skill. J Bone Joint Surg Am. 2012;94(13):e97.
16. Jackson WF, Khan T, Alvand A, et al. Learning and retaining simulated arthroscopic meniscal repair skills. J Bone Joint Surg Am. 2012;94(17):e132.
17. Pernar LI, Smink DS, Hicks G, Peyre SE. Residents can successfully teach basic surgical skills in the simulation center. J Surg Educ. 2012;69(5):617-622.
18. Tuijthof GJ, Visser P, Sierevelt IN, Van Dijk CN, Kerkhoffs GM. Does perception of usefulness of arthroscopic simulators differ with levels of experience? Clin Orthop. 2011;469(6):1701-1708.
19. Martin KD, Cameron K, Belmont PJ, Schoenfeld A, Owens BD. Shoulder arthroscopy simulator performance correlates with resident and shoulder arthroscopy experience. J Bone Joint Surg Am. 2012;94(21):e160.
20. Slade Shantz JA, Leiter JR, Gottschalk T, MacDonald PB. The internal validity of arthroscopic simulators and their effectiveness in arthroscopic education. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):33-40.
21. Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11(5):747-757.
22. Ruedi TP, Buckley RE, Moran CG. AO Principles of Fracture Management. Stuttgart, Germany: Thieme; 2007.
23. Chen CL, Chen WC, Chiang JH, Ho CF. Interscapular hibernoma: case report and literature review. Kaohsiung J Med Sci. 2011;27(8):348-352.
24. Karam MD, Pedowitz RA, Natividad H, Murray J, Marsh JL. Current and future use of surgical skills training laboratories in orthopaedic resident education: a national survey. J Bone Joint Surg Am. 2013;95(1):e4.
25. Baskies MA, Ruchelsman DE, Capeci CM, Zuckerman JD, Egol KA. Operative experience in an orthopaedic surgery residency program: the effect of work-hour restrictions. J Bone Joint Surg Am. 2008;90(4):924-927.
26. Pappas AJ, Teague DC. The impact of the Accreditation Council for Graduate Medical Education work-hour regulations on the surgical experience of orthopaedic surgery residents. J Bone Joint Surg Am. 2007;89(4):904-909.
27. Palter VN, Grantcharov T, Harvey A, Macrae HM. Ex vivo technical skills training transfers to the operating room and enhances cognitive learning: a randomized controlled trial. Ann Surg. 2011;253(5):886-889.
28. Franzeck FM, Rosenthal R, Muller MK, et al. Prospective randomized controlled trial of simulator-based versus traditional in-surgery laparoscopic camera navigation training. Surg Endosc. 2012;26(1):235-241.
29. Zendejas B, Cook DA, Bingener J, et al. Simulation-based mastery learning improves patient outcomes in laparoscopic inguinal hernia repair: a randomized controlled trial. Ann Surg. 2011;254(3):502-509.
30. Hui Y, Safir O, Dubrowski A, Carnahan H. What skills should simulation training in arthroscopy teach residents? A focus on resident input. Int J Comput Assist Radiol Surg. 2013;8(6):945-953.
31. Butler A, Olson T, Koehler R, Nicandri G. Do the skills acquired by novice surgeons using anatomic dry models transfer effectively to the task of diagnostic knee arthroscopy performed on cadaveric specimens? J Bone Joint Surg Am. 2013;95(3):e15(1-8).
32. Martin KD, Belmont PJ, Schoenfeld AJ, Todd M, Cameron KL, Owens BD. Arthroscopic basic task performance in shoulder simulator model correlates with similar task performance in cadavers. J Bone Joint Surg Am. 2011;93(21):e1271-e1275.
33. Elliott MJ, Caprise PA, Henning AE, Kurtz CA, Sekiya JK. Diagnostic knee arthroscopy: a pilot study to evaluate surgical skills. Arthroscopy. 2012;28(2):218-224.
34. Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop. 2011;82(1):90-95.
35. Sonnadara RR, Van Vliet A, Safir O, et al. Orthopedic boot camp: examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149(6):745-749.
36. Sonnadara RR, Garbedian S, Safir O, et al. Orthopaedic boot camp II: examining the retention rates of an intensive surgical skills course. Surgery. 2012;151(6):803-807.