User login
Referral for CT Pulmonary Angiography
Approximately 10 million patients present to emergency departments each year with symptoms raising concern of thromboembolism (VTE).1 The current gold standard for diagnosis of VTE is pulmonary angiography.2 As this study is invasive, alternative imaging protocols have been sought. CTPA, when combined with measures of pretest probability, equals or surpasses the ability of pulmonary angiography to detect VTE and can improve the ability of clinicians to rule out VTE.38 In a study of 930 patients, application of clinical rules in addition to D‐dimer testing decreased the number of CTPAs ordered by 50%.9 One of the most common clinical rule sets is the Wells Score, which relies on historical features related to the risk of DVT/VTE and physical examination findings.10, 11
Other institutions have demonstrated an increase in the number of CTPAs ordered and VTE diagnoses since the study became widely available.13 Based on the observation of an increasing number of CTPAs ordered at our institution without an increase in the number of VTEs diagnosed, we aimed to ascertain the physician ordering practices for CTPA. We hypothesized that CTPAs were ordered at a greater frequency in a low‐risk population because an institutional clinical algorithm was lacking.
METHODS
Charts of all patients aged 18‐100 with CTPA ordered to rule out acute VTE were retrospectively examined. A Simplified Wells Score was applied using only the information available to the ordering physician at the time the CTPA was performed. Patients were stratified by their Simplified Wells Score to low (0‐1 points), intermediate (2‐6 points), or high (>6 points) pretest clinical probability. A D‐dimer value, if ordered, was used to further stratify patients based on a positive or negative result. The official radiologic report of the CTPA was used to determine the rate of VTE diagnosis for the study population.
RESULTS
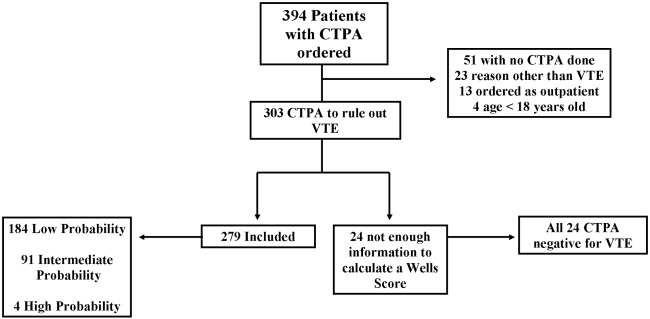
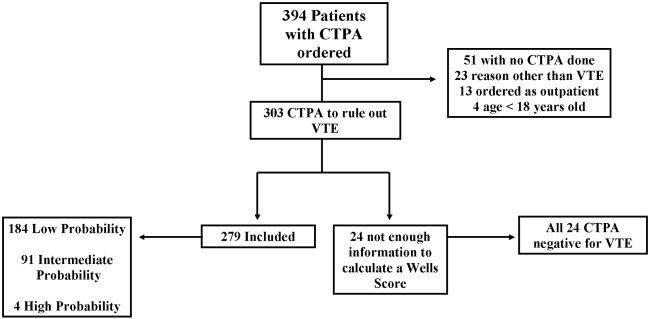
Three hundred and ninety‐four patients were referred for CTPA (Fig. 1). Two hundred and seventy‐nine had adequate clinical data to calculate a Simplified Wells Score and were included in the study. Of the 279 studies included, 75% were ordered through the emergency department and 25% from inpatient services (Table 1). The study patients were stratified according to the Simplified Wells criteria: 184 patients (66%) had low clinical probability, 91 (33%) had intermediate clinical probability, and 4 (1%) had high clinical probability. Nineteen (7%) patients had a history of DVT or VTE, and 28 (10%) had a history of active cancer at the time of their CTPA. One hundred and twenty‐five of the 279 patients had a D‐dimer performed (Fig. 2). One hundred and eight were positive, and 17 were negative. Of the 17 patients who had a negative D‐dimer and underwent CTPA testing, none were diagnosed with VTE. Eighty‐three low‐clinical‐probability patients underwent CTPA without D‐dimer testing, 4 of whom were diagnosed with VTE.
| Low (n = 184) | Intermediate (n = 91) | High (n = 4) | Total (n = 279) | |
|---|---|---|---|---|
| Age (years), mean | 52 | 59 | 62 | 58 |
| Male, n (%) | 82 (45) | 44 (48) | 1 (25) | 127 (46) |
| Female, n (%) | 102 (55) | 47 (52) | 3 (75) | 152 (54) |
| Emergency department, n (%) | 150 (82) | 47 (52) | 4 (100) | 225 (75) |
| Medical, n (%) | 22 (12) | 18 (20) | 0 | 40 (13) |
| Surgical, n (%) | 9 (5) | 14 (15) | 0 | 23 (7) |
| ICU, n (%) | 3 (1) | 12 (13) | 0 | 15 (5) |
| Wells Score, mean | 0.72 | 3.4 | 7.8 | 1.6 |
| D‐dimer performed, n (%) | 101 (55) | 21 (23) | 3 (75) | 125 (45) |
| D‐dimer positive, n (%) | 89 (88) | 16 (76) | 3 (100) | 108 (86) |
| D‐dimer negative, n (%) | 12 (12) | 5 (24) | 0 | 17 (14) |
| CTPA positive, n (%) | 8 (4) | 11 (12) | 1 (25) | 20 (7) |
| CPTA negative, n (%) | 176 (96) | 80 (88) | 3 (75) | 259 (93) |
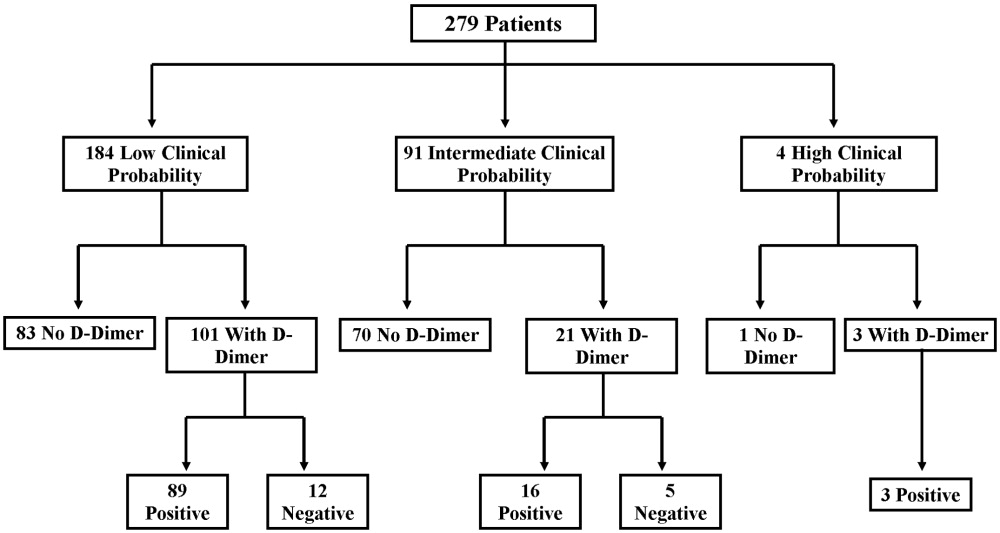
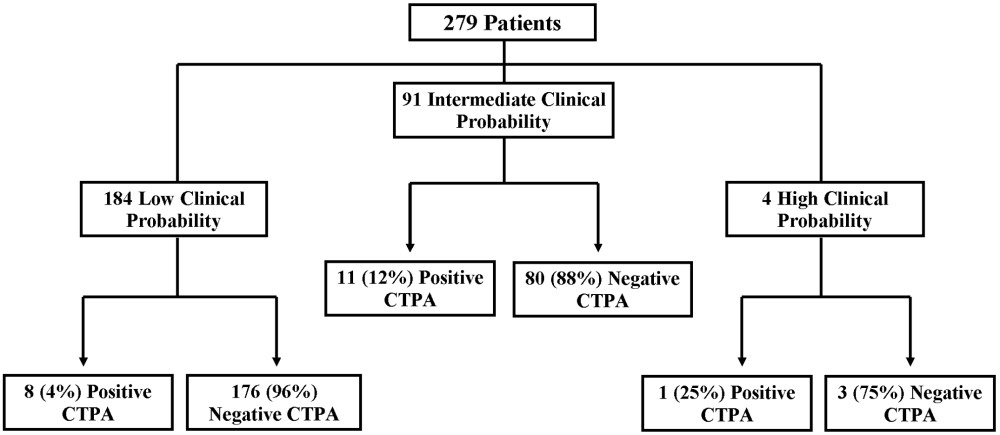
There were 20 positive CTPAs in the study group (Fig. 3). Review of the records for 3 months after the study of patients whose CTPA was negative disclosed no diagnoses of VTE by other modalities. VTE was diagnosed in 4% of patients in the low‐clinical‐probability group, 12% in the intermediate‐clinical‐probability group, and 25% in the high‐clinical‐probability group. The overall positive CTPA rate was 7.2%.
DISCUSSION
Many studies have examined the application of clinical rule sets in addition to D‐dimer testing and CTPA to exclude acute VTE.39 Most of these studies have shown that the use of an algorithm is safe and frequently reduces referral for CTPA in low‐clinical‐probability patients. However, others have noted that some physicians do not routinely use validated algorithms when making decisions related to patient evaluation.13 Our rate of positive CTPA was low compared with rates reported in the literature.3, 14 We believe the most likely explanation is the large number of low‐clinical‐probability patients who underwent CTPA, possibly because providers do not routinely use a validated clinical algorithm.
When our patient population was risk stratified by Simplified Wells criteria and compared with similar data from published studies, we had a much higher proportion of patients classified as low clinical probability.7, 8, 15 The low‐clinical‐probability group's mean Simplified Wells Score was 0.71; one‐third had a Simplified Wells Score of 0. This reflects a low‐risk population for VTE, supported by the low prevalence of prior DVT/VTE and active cancer in our population.4, 10 The rationale for referring patients with so few risk factors for CTPA is unclear. It is possible that providers used CTPA to evaluate symptoms not clearly explained and obtained the study to look for other diagnoses in addition to VTE. By not applying a clinical algorithm, very‐low‐risk patients underwent CTPA, increasing the number of negative studies and decreasing the overall positive rate.
Not using a clinical algorithm also resulted in indiscriminate D‐dimer testing. There were 83 patients risk‐stratified as low clinical probability who did not have a D‐dimer prior to undergoing CTPA. Some of these patients may well have had a negative D‐dimer, requiring no further workup to rule out VTE. Seventeen patients had a negative D‐dimer and still underwent CTPA; all these patients were negative for VTE. These aberrations likely occurred from unfamiliarity with use of the D‐dimer test or doubts about its ability to reliably exclude VTE. Appropriate application of D‐dimer testing could have decreased the number of CTPAs ordered and increased our overall rate of positive VTE diagnosis.
Perrier et al., Brown et al., and Kelly and Wells all describe different methods of introducing clinical algorithms to aid the diagnosis of VTE.46, 9 All agree that patients should be risk stratified by pretest clinical probability, and low‐probability patients should undergo intermediate testing with D‐dimer prior to CTPA. Implementation of a similar clinical algorithm at our facility would likely decrease the number of CTPAs ordered. If all patients presenting at our facility with signs and symptoms raising concern for VTE were first risk‐stratified by pretest clinical probability, and all low‐probability patients underwent highly‐sensitive D‐dimer testing as an initial step, fewer CTPAs would be performed on low‐probability patients. The largest group of patients in our study were low probability; therefore, decreasing CTPA in this group could have a significant effect on our institution.
The retrospective nature of our study resulted in the following limitations. It is impossible to determine how the ordering provider viewed the patient's pretest probability. In most of the medical records, a pretest clinical probability was not documented. We attempted to validate the ordering provider's decision by being as generous as possible in applying points to the Wells Score. For example, if a patient had a remote history of cancer and the ordering provider documented this as a risk factor for VTE, the point value for cancer was given even though the Wells Score has a much narrower definition of this category.10 This practice favors assigning patients a potentially higher clinical probability and may have increased the number of patients designated as intermediate and high clinical probability in our study.
Our hospital primarily relies on CTPA with lower extremity venogram as the diagnostic test for VTE. Indeterminate tests may have occurred and thus falsely lowered the number of VTEs diagnosed. However, no patient with a negative CTPA was diagnosed with VTE by any modality in the 3 months after their initial study at our institution; a diagnosis of VTE could have been made at another hospital. The Simplified Wells Score uses both objective and subjective components to arrive at a point total. Our results might be different if newer algorithms, such as the Revised Geneva Score,16 which relies only on objective measurements, had been used.
CONCLUSIONS
The reliance on CTPA alone to exclude a potentially life‐threatening illness without additional risk stratification or clinical information leads to overuse of this test in patients with very low to no clinical risk for VTE and a low rate of diagnosed VTE. Implementation of a clinical algorithm for the diagnosis of suspected VTE may eliminate the need for many CTPAs, improving the yield of this test without compromising patient safety, especially at institutions with a low prevalence of PE.
Acknowledgements
The authors thank Dr. John Rinard, DO, for assistance with initial editing of the abstract and Troy Patience for his assistance with statistical analysis.
- ,,, et al.Impact of a rapid‐rule out protocol for pulmonary embolism on the rate of screening, missed cases and pulmonary vascular imaging in an urban US emergency department.Ann Emerg Med.2004;44:490–502.
- ,,, et al.ATS 1999 Clinical practice guideline for the diagnostic approach to acute venous thromboembolism.Am J Respir Crit Care Med.1999;160:1043–1066.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism.JAMA.2005;293:2012–2017.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,.An emergency department guideline for the diagnosis of pulmonary embolism: an outcome study.Acad Emerg Med.2005;12:20–25.
- ,.A clinical probability assessment and D‐dimer measurement should be the initial step in the investigation of suspected venous thromboembolism.Chest.2003;124:1116–1119.
- ,,, et al.Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44:503–510.
- ,.External validation and comparison of recently described prediction rules for suspected pulmonary embolism.Curr Opin Pulm Med.2004;10:345–349.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D‐dimer.Ann Intern Med.2001;135:98–107.
- ,,, et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- ,,, et al.Assessing the clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161:92–97.
- ,,, et al.The effect of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- Simplifying the evaluation of pulmonary embolism.Chest.2006:129:1400–1401.
- ,,, et al.Meta‐Analysis: Outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,,, et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354:2317–2327.
- ,,, et al.Prediction of pulmonary embolism in the emergency department: the revised Geneva score.Ann Intern Med.2006;144(3):165–171.
Approximately 10 million patients present to emergency departments each year with symptoms raising concern of thromboembolism (VTE).1 The current gold standard for diagnosis of VTE is pulmonary angiography.2 As this study is invasive, alternative imaging protocols have been sought. CTPA, when combined with measures of pretest probability, equals or surpasses the ability of pulmonary angiography to detect VTE and can improve the ability of clinicians to rule out VTE.38 In a study of 930 patients, application of clinical rules in addition to D‐dimer testing decreased the number of CTPAs ordered by 50%.9 One of the most common clinical rule sets is the Wells Score, which relies on historical features related to the risk of DVT/VTE and physical examination findings.10, 11
Other institutions have demonstrated an increase in the number of CTPAs ordered and VTE diagnoses since the study became widely available.13 Based on the observation of an increasing number of CTPAs ordered at our institution without an increase in the number of VTEs diagnosed, we aimed to ascertain the physician ordering practices for CTPA. We hypothesized that CTPAs were ordered at a greater frequency in a low‐risk population because an institutional clinical algorithm was lacking.
METHODS
Charts of all patients aged 18‐100 with CTPA ordered to rule out acute VTE were retrospectively examined. A Simplified Wells Score was applied using only the information available to the ordering physician at the time the CTPA was performed. Patients were stratified by their Simplified Wells Score to low (0‐1 points), intermediate (2‐6 points), or high (>6 points) pretest clinical probability. A D‐dimer value, if ordered, was used to further stratify patients based on a positive or negative result. The official radiologic report of the CTPA was used to determine the rate of VTE diagnosis for the study population.
RESULTS
Three hundred and ninety‐four patients were referred for CTPA (Fig. 1). Two hundred and seventy‐nine had adequate clinical data to calculate a Simplified Wells Score and were included in the study. Of the 279 studies included, 75% were ordered through the emergency department and 25% from inpatient services (Table 1). The study patients were stratified according to the Simplified Wells criteria: 184 patients (66%) had low clinical probability, 91 (33%) had intermediate clinical probability, and 4 (1%) had high clinical probability. Nineteen (7%) patients had a history of DVT or VTE, and 28 (10%) had a history of active cancer at the time of their CTPA. One hundred and twenty‐five of the 279 patients had a D‐dimer performed (Fig. 2). One hundred and eight were positive, and 17 were negative. Of the 17 patients who had a negative D‐dimer and underwent CTPA testing, none were diagnosed with VTE. Eighty‐three low‐clinical‐probability patients underwent CTPA without D‐dimer testing, 4 of whom were diagnosed with VTE.

| Low (n = 184) | Intermediate (n = 91) | High (n = 4) | Total (n = 279) | |
|---|---|---|---|---|
| Age (years), mean | 52 | 59 | 62 | 58 |
| Male, n (%) | 82 (45) | 44 (48) | 1 (25) | 127 (46) |
| Female, n (%) | 102 (55) | 47 (52) | 3 (75) | 152 (54) |
| Emergency department, n (%) | 150 (82) | 47 (52) | 4 (100) | 225 (75) |
| Medical, n (%) | 22 (12) | 18 (20) | 0 | 40 (13) |
| Surgical, n (%) | 9 (5) | 14 (15) | 0 | 23 (7) |
| ICU, n (%) | 3 (1) | 12 (13) | 0 | 15 (5) |
| Wells Score, mean | 0.72 | 3.4 | 7.8 | 1.6 |
| D‐dimer performed, n (%) | 101 (55) | 21 (23) | 3 (75) | 125 (45) |
| D‐dimer positive, n (%) | 89 (88) | 16 (76) | 3 (100) | 108 (86) |
| D‐dimer negative, n (%) | 12 (12) | 5 (24) | 0 | 17 (14) |
| CTPA positive, n (%) | 8 (4) | 11 (12) | 1 (25) | 20 (7) |
| CPTA negative, n (%) | 176 (96) | 80 (88) | 3 (75) | 259 (93) |

There were 20 positive CTPAs in the study group (Fig. 3). Review of the records for 3 months after the study of patients whose CTPA was negative disclosed no diagnoses of VTE by other modalities. VTE was diagnosed in 4% of patients in the low‐clinical‐probability group, 12% in the intermediate‐clinical‐probability group, and 25% in the high‐clinical‐probability group. The overall positive CTPA rate was 7.2%.

DISCUSSION
Many studies have examined the application of clinical rule sets in addition to D‐dimer testing and CTPA to exclude acute VTE.39 Most of these studies have shown that the use of an algorithm is safe and frequently reduces referral for CTPA in low‐clinical‐probability patients. However, others have noted that some physicians do not routinely use validated algorithms when making decisions related to patient evaluation.13 Our rate of positive CTPA was low compared with rates reported in the literature.3, 14 We believe the most likely explanation is the large number of low‐clinical‐probability patients who underwent CTPA, possibly because providers do not routinely use a validated clinical algorithm.
When our patient population was risk stratified by Simplified Wells criteria and compared with similar data from published studies, we had a much higher proportion of patients classified as low clinical probability.7, 8, 15 The low‐clinical‐probability group's mean Simplified Wells Score was 0.71; one‐third had a Simplified Wells Score of 0. This reflects a low‐risk population for VTE, supported by the low prevalence of prior DVT/VTE and active cancer in our population.4, 10 The rationale for referring patients with so few risk factors for CTPA is unclear. It is possible that providers used CTPA to evaluate symptoms not clearly explained and obtained the study to look for other diagnoses in addition to VTE. By not applying a clinical algorithm, very‐low‐risk patients underwent CTPA, increasing the number of negative studies and decreasing the overall positive rate.
Not using a clinical algorithm also resulted in indiscriminate D‐dimer testing. There were 83 patients risk‐stratified as low clinical probability who did not have a D‐dimer prior to undergoing CTPA. Some of these patients may well have had a negative D‐dimer, requiring no further workup to rule out VTE. Seventeen patients had a negative D‐dimer and still underwent CTPA; all these patients were negative for VTE. These aberrations likely occurred from unfamiliarity with use of the D‐dimer test or doubts about its ability to reliably exclude VTE. Appropriate application of D‐dimer testing could have decreased the number of CTPAs ordered and increased our overall rate of positive VTE diagnosis.
Perrier et al., Brown et al., and Kelly and Wells all describe different methods of introducing clinical algorithms to aid the diagnosis of VTE.46, 9 All agree that patients should be risk stratified by pretest clinical probability, and low‐probability patients should undergo intermediate testing with D‐dimer prior to CTPA. Implementation of a similar clinical algorithm at our facility would likely decrease the number of CTPAs ordered. If all patients presenting at our facility with signs and symptoms raising concern for VTE were first risk‐stratified by pretest clinical probability, and all low‐probability patients underwent highly‐sensitive D‐dimer testing as an initial step, fewer CTPAs would be performed on low‐probability patients. The largest group of patients in our study were low probability; therefore, decreasing CTPA in this group could have a significant effect on our institution.
The retrospective nature of our study resulted in the following limitations. It is impossible to determine how the ordering provider viewed the patient's pretest probability. In most of the medical records, a pretest clinical probability was not documented. We attempted to validate the ordering provider's decision by being as generous as possible in applying points to the Wells Score. For example, if a patient had a remote history of cancer and the ordering provider documented this as a risk factor for VTE, the point value for cancer was given even though the Wells Score has a much narrower definition of this category.10 This practice favors assigning patients a potentially higher clinical probability and may have increased the number of patients designated as intermediate and high clinical probability in our study.
Our hospital primarily relies on CTPA with lower extremity venogram as the diagnostic test for VTE. Indeterminate tests may have occurred and thus falsely lowered the number of VTEs diagnosed. However, no patient with a negative CTPA was diagnosed with VTE by any modality in the 3 months after their initial study at our institution; a diagnosis of VTE could have been made at another hospital. The Simplified Wells Score uses both objective and subjective components to arrive at a point total. Our results might be different if newer algorithms, such as the Revised Geneva Score,16 which relies only on objective measurements, had been used.
CONCLUSIONS
The reliance on CTPA alone to exclude a potentially life‐threatening illness without additional risk stratification or clinical information leads to overuse of this test in patients with very low to no clinical risk for VTE and a low rate of diagnosed VTE. Implementation of a clinical algorithm for the diagnosis of suspected VTE may eliminate the need for many CTPAs, improving the yield of this test without compromising patient safety, especially at institutions with a low prevalence of PE.
Acknowledgements
The authors thank Dr. John Rinard, DO, for assistance with initial editing of the abstract and Troy Patience for his assistance with statistical analysis.
Approximately 10 million patients present to emergency departments each year with symptoms raising concern of thromboembolism (VTE).1 The current gold standard for diagnosis of VTE is pulmonary angiography.2 As this study is invasive, alternative imaging protocols have been sought. CTPA, when combined with measures of pretest probability, equals or surpasses the ability of pulmonary angiography to detect VTE and can improve the ability of clinicians to rule out VTE.38 In a study of 930 patients, application of clinical rules in addition to D‐dimer testing decreased the number of CTPAs ordered by 50%.9 One of the most common clinical rule sets is the Wells Score, which relies on historical features related to the risk of DVT/VTE and physical examination findings.10, 11
Other institutions have demonstrated an increase in the number of CTPAs ordered and VTE diagnoses since the study became widely available.13 Based on the observation of an increasing number of CTPAs ordered at our institution without an increase in the number of VTEs diagnosed, we aimed to ascertain the physician ordering practices for CTPA. We hypothesized that CTPAs were ordered at a greater frequency in a low‐risk population because an institutional clinical algorithm was lacking.
METHODS
Charts of all patients aged 18‐100 with CTPA ordered to rule out acute VTE were retrospectively examined. A Simplified Wells Score was applied using only the information available to the ordering physician at the time the CTPA was performed. Patients were stratified by their Simplified Wells Score to low (0‐1 points), intermediate (2‐6 points), or high (>6 points) pretest clinical probability. A D‐dimer value, if ordered, was used to further stratify patients based on a positive or negative result. The official radiologic report of the CTPA was used to determine the rate of VTE diagnosis for the study population.
RESULTS
Three hundred and ninety‐four patients were referred for CTPA (Fig. 1). Two hundred and seventy‐nine had adequate clinical data to calculate a Simplified Wells Score and were included in the study. Of the 279 studies included, 75% were ordered through the emergency department and 25% from inpatient services (Table 1). The study patients were stratified according to the Simplified Wells criteria: 184 patients (66%) had low clinical probability, 91 (33%) had intermediate clinical probability, and 4 (1%) had high clinical probability. Nineteen (7%) patients had a history of DVT or VTE, and 28 (10%) had a history of active cancer at the time of their CTPA. One hundred and twenty‐five of the 279 patients had a D‐dimer performed (Fig. 2). One hundred and eight were positive, and 17 were negative. Of the 17 patients who had a negative D‐dimer and underwent CTPA testing, none were diagnosed with VTE. Eighty‐three low‐clinical‐probability patients underwent CTPA without D‐dimer testing, 4 of whom were diagnosed with VTE.

| Low (n = 184) | Intermediate (n = 91) | High (n = 4) | Total (n = 279) | |
|---|---|---|---|---|
| Age (years), mean | 52 | 59 | 62 | 58 |
| Male, n (%) | 82 (45) | 44 (48) | 1 (25) | 127 (46) |
| Female, n (%) | 102 (55) | 47 (52) | 3 (75) | 152 (54) |
| Emergency department, n (%) | 150 (82) | 47 (52) | 4 (100) | 225 (75) |
| Medical, n (%) | 22 (12) | 18 (20) | 0 | 40 (13) |
| Surgical, n (%) | 9 (5) | 14 (15) | 0 | 23 (7) |
| ICU, n (%) | 3 (1) | 12 (13) | 0 | 15 (5) |
| Wells Score, mean | 0.72 | 3.4 | 7.8 | 1.6 |
| D‐dimer performed, n (%) | 101 (55) | 21 (23) | 3 (75) | 125 (45) |
| D‐dimer positive, n (%) | 89 (88) | 16 (76) | 3 (100) | 108 (86) |
| D‐dimer negative, n (%) | 12 (12) | 5 (24) | 0 | 17 (14) |
| CTPA positive, n (%) | 8 (4) | 11 (12) | 1 (25) | 20 (7) |
| CPTA negative, n (%) | 176 (96) | 80 (88) | 3 (75) | 259 (93) |

There were 20 positive CTPAs in the study group (Fig. 3). Review of the records for 3 months after the study of patients whose CTPA was negative disclosed no diagnoses of VTE by other modalities. VTE was diagnosed in 4% of patients in the low‐clinical‐probability group, 12% in the intermediate‐clinical‐probability group, and 25% in the high‐clinical‐probability group. The overall positive CTPA rate was 7.2%.

DISCUSSION
Many studies have examined the application of clinical rule sets in addition to D‐dimer testing and CTPA to exclude acute VTE.39 Most of these studies have shown that the use of an algorithm is safe and frequently reduces referral for CTPA in low‐clinical‐probability patients. However, others have noted that some physicians do not routinely use validated algorithms when making decisions related to patient evaluation.13 Our rate of positive CTPA was low compared with rates reported in the literature.3, 14 We believe the most likely explanation is the large number of low‐clinical‐probability patients who underwent CTPA, possibly because providers do not routinely use a validated clinical algorithm.
When our patient population was risk stratified by Simplified Wells criteria and compared with similar data from published studies, we had a much higher proportion of patients classified as low clinical probability.7, 8, 15 The low‐clinical‐probability group's mean Simplified Wells Score was 0.71; one‐third had a Simplified Wells Score of 0. This reflects a low‐risk population for VTE, supported by the low prevalence of prior DVT/VTE and active cancer in our population.4, 10 The rationale for referring patients with so few risk factors for CTPA is unclear. It is possible that providers used CTPA to evaluate symptoms not clearly explained and obtained the study to look for other diagnoses in addition to VTE. By not applying a clinical algorithm, very‐low‐risk patients underwent CTPA, increasing the number of negative studies and decreasing the overall positive rate.
Not using a clinical algorithm also resulted in indiscriminate D‐dimer testing. There were 83 patients risk‐stratified as low clinical probability who did not have a D‐dimer prior to undergoing CTPA. Some of these patients may well have had a negative D‐dimer, requiring no further workup to rule out VTE. Seventeen patients had a negative D‐dimer and still underwent CTPA; all these patients were negative for VTE. These aberrations likely occurred from unfamiliarity with use of the D‐dimer test or doubts about its ability to reliably exclude VTE. Appropriate application of D‐dimer testing could have decreased the number of CTPAs ordered and increased our overall rate of positive VTE diagnosis.
Perrier et al., Brown et al., and Kelly and Wells all describe different methods of introducing clinical algorithms to aid the diagnosis of VTE.46, 9 All agree that patients should be risk stratified by pretest clinical probability, and low‐probability patients should undergo intermediate testing with D‐dimer prior to CTPA. Implementation of a similar clinical algorithm at our facility would likely decrease the number of CTPAs ordered. If all patients presenting at our facility with signs and symptoms raising concern for VTE were first risk‐stratified by pretest clinical probability, and all low‐probability patients underwent highly‐sensitive D‐dimer testing as an initial step, fewer CTPAs would be performed on low‐probability patients. The largest group of patients in our study were low probability; therefore, decreasing CTPA in this group could have a significant effect on our institution.
The retrospective nature of our study resulted in the following limitations. It is impossible to determine how the ordering provider viewed the patient's pretest probability. In most of the medical records, a pretest clinical probability was not documented. We attempted to validate the ordering provider's decision by being as generous as possible in applying points to the Wells Score. For example, if a patient had a remote history of cancer and the ordering provider documented this as a risk factor for VTE, the point value for cancer was given even though the Wells Score has a much narrower definition of this category.10 This practice favors assigning patients a potentially higher clinical probability and may have increased the number of patients designated as intermediate and high clinical probability in our study.
Our hospital primarily relies on CTPA with lower extremity venogram as the diagnostic test for VTE. Indeterminate tests may have occurred and thus falsely lowered the number of VTEs diagnosed. However, no patient with a negative CTPA was diagnosed with VTE by any modality in the 3 months after their initial study at our institution; a diagnosis of VTE could have been made at another hospital. The Simplified Wells Score uses both objective and subjective components to arrive at a point total. Our results might be different if newer algorithms, such as the Revised Geneva Score,16 which relies only on objective measurements, had been used.
CONCLUSIONS
The reliance on CTPA alone to exclude a potentially life‐threatening illness without additional risk stratification or clinical information leads to overuse of this test in patients with very low to no clinical risk for VTE and a low rate of diagnosed VTE. Implementation of a clinical algorithm for the diagnosis of suspected VTE may eliminate the need for many CTPAs, improving the yield of this test without compromising patient safety, especially at institutions with a low prevalence of PE.
Acknowledgements
The authors thank Dr. John Rinard, DO, for assistance with initial editing of the abstract and Troy Patience for his assistance with statistical analysis.
- ,,, et al.Impact of a rapid‐rule out protocol for pulmonary embolism on the rate of screening, missed cases and pulmonary vascular imaging in an urban US emergency department.Ann Emerg Med.2004;44:490–502.
- ,,, et al.ATS 1999 Clinical practice guideline for the diagnostic approach to acute venous thromboembolism.Am J Respir Crit Care Med.1999;160:1043–1066.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism.JAMA.2005;293:2012–2017.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,.An emergency department guideline for the diagnosis of pulmonary embolism: an outcome study.Acad Emerg Med.2005;12:20–25.
- ,.A clinical probability assessment and D‐dimer measurement should be the initial step in the investigation of suspected venous thromboembolism.Chest.2003;124:1116–1119.
- ,,, et al.Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44:503–510.
- ,.External validation and comparison of recently described prediction rules for suspected pulmonary embolism.Curr Opin Pulm Med.2004;10:345–349.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D‐dimer.Ann Intern Med.2001;135:98–107.
- ,,, et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- ,,, et al.Assessing the clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161:92–97.
- ,,, et al.The effect of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- Simplifying the evaluation of pulmonary embolism.Chest.2006:129:1400–1401.
- ,,, et al.Meta‐Analysis: Outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,,, et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354:2317–2327.
- ,,, et al.Prediction of pulmonary embolism in the emergency department: the revised Geneva score.Ann Intern Med.2006;144(3):165–171.
- ,,, et al.Impact of a rapid‐rule out protocol for pulmonary embolism on the rate of screening, missed cases and pulmonary vascular imaging in an urban US emergency department.Ann Emerg Med.2004;44:490–502.
- ,,, et al.ATS 1999 Clinical practice guideline for the diagnostic approach to acute venous thromboembolism.Am J Respir Crit Care Med.1999;160:1043–1066.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism.JAMA.2005;293:2012–2017.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,.An emergency department guideline for the diagnosis of pulmonary embolism: an outcome study.Acad Emerg Med.2005;12:20–25.
- ,.A clinical probability assessment and D‐dimer measurement should be the initial step in the investigation of suspected venous thromboembolism.Chest.2003;124:1116–1119.
- ,,, et al.Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44:503–510.
- ,.External validation and comparison of recently described prediction rules for suspected pulmonary embolism.Curr Opin Pulm Med.2004;10:345–349.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D‐dimer.Ann Intern Med.2001;135:98–107.
- ,,, et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- ,,, et al.Assessing the clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161:92–97.
- ,,, et al.The effect of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- Simplifying the evaluation of pulmonary embolism.Chest.2006:129:1400–1401.
- ,,, et al.Meta‐Analysis: Outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,,, et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354:2317–2327.
- ,,, et al.Prediction of pulmonary embolism in the emergency department: the revised Geneva score.Ann Intern Med.2006;144(3):165–171.