User login
Management of Comorbid Sleep Disorders in Patients With PTSD
[This article originally published online ahead of print April 23, 2015.]
Sleep in the military has traditionally been thought of as a luxury and is sometimes considered at odds with optimal productivity. Every minute that a service member is asleep, he or she is not performing a primary duty, and getting a minimal amount of sleep is often seen as a badge of honor and strength. Research has recently been conducted, underscoring the importance of sleep management as an operational variable that must be accounted for in order to achieve optimal performance and promote resiliency. Both the quality and the duration of sleep must be considered, particularly given the increasingly complicated tasks that every service member must perform during both war and peace.
It has been well established that higher order mental tasks are the most vulnerable to sleep loss, as are those with little mental or physical stimulation, such as guard duty.1,2 Because service members are expected not only to perform in combat, but also to behave and operate ethically in spite of the challenges of war, the importance of adequate sleep must be considered. Many challenges are commonly encountered by service members when attempting to get adequate sleep (Table).3 This review highlights the recent diagnostic and treatment advances with respect to the overlap of sleep disorders and posttraumatic stress disorder (PTSD).
Culture of Sleep Loss
At the United States Military Academy in West Point, New York, a culture of poor sleep is instilled during initial military training; students typically get less than the recommended 7 to 8 hours of sleep per 24 hours.4,5 This sleep restriction continues for most of the time served on active duty: Military members get less sleep on average than does the rest of the U.S. population.6
Studies performed on pilots and during deployment have consistently shown a trend toward inadequate sleep, but only recently has inadequate sleep gained the attention of senior leadership.7,8 The Army Performance Triad, a public health campaign launched in 2013 by the Office of the U.S. Army Surgeon General, equally values sleep, nutrition, and activity. The goal of the Army Performance Triad is to influence behaviors by promoting healthy sleep, activity, and nutrition. Sleep is the apex of the Army Performance Triad.8
Those with chronic sleep restriction may not understand how impaired they are until objective testing is performed.9 In the civilian population, fatal sleep-related traffic accidents have been shown to exceed fatalities due to alcohol and illicit drug use combined.10 When poor sleep is combined with the trauma of war, symptoms exponentially worsen, and treatment becomes more complicated.11 Therefore, even before a formal sleep disorder or psychiatric condition develops, service members put themselves at risk by practicing poor sleep behaviors.11
Once insomnia develops, however, the potential negative health consequences are much more significant. Chronic insomnia, characterized by difficulty initiating or maintaining sleep or by waking too early, is the most common sleep disorder among adults. Thirty percent of adults experience occasional or transient insomnia, and between 9% and 12% of adults have severe chronic insomnia.12,13 This number is likely higher in the military and is much higher in those with PTSD.13
Related: How Effective Is Group Cognitive Behavioral Therapy to Treat PTSD?
The etiology of chronic insomnia is multifactorial and is best conceptualized within a biopsychosocial framework. Physiologic abnormalities, such as increased activity in the central nervous system, hyperarousal of the hypothalamic-pituitary axis, and activation of proinflammatory cytokines, predispose individuals to developing insomnia. In addition, personality traits, such as anxious temperament or an internalizing stress-management style, make it more likely for individuals to respond negatively to stress, the most common precipitating cause of chronic insomnia.
Behavioral factors are also paramount. For example, individuals who experience acute sleep disturbance during deployment might develop maladaptive compensatory behaviors, such as spending excessive time in bed, “trying harder” to sleep, or overusing stimulants. These sleep behaviors can become a chronic condition.14
Comorbidities
Patients with insomnia are at increased risk for medical consequences, such as cardiovascular disease and mortality as well as psychiatric sequelae.15,16 Insomnia is also common among people who have attempted suicide.17 In the military, there was nearly a 20-fold increase in the rate of chronic insomnia among service members between 2000 and 2009, coincident with the dramatic uptick in operations tempo.18
Insomnia is one of the most common reports of returning Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF) veterans and is associated with the development of PTSD.19 Soldiers who reported symptoms of insomnia predeployment were more likely to develop anxiety, depression, and PTSD during deployment than were soldiers who did not report these symptoms.20
Empirically supported and evidence-based treatment options exist. Further, a robust evidence base supports the conclusion that treating insomnia improves not only sleep and quality of life (QOL), but also health-related outcomes in comorbid conditions, including depression, PTSD, chronic pain, and alcohol dependence.21-24 One historical barrier to effective treatment has been poor recognition of the scope of the problem. The army is looking to implement a more robust assessment of sleep in the primary care setting as part of the Army Performance Triad in order to intervene as early as possible. Other government organizations may also follow suit.
Although several FDA-approved medications for insomnia exist, the gold standard treatment for insomnia is cognitive behavioral therapy (CBT).25 Specific behavioral patient preferences that can be used to tailor treatment have been identified within a military population.26 Unfortunately, the most commonly used treatment for chronic insomnia in the military remains sedative- hypnotic medication. Multiple estimates suggest that 15% to 20% of all deployed service members have been prescribed a sedative-hypnotic to aid with sleep initiation, including many off-label antidepressants, antipsychotics, and antihistamines. Indeed, within VA, the use of quetiapine (an antipsychotic used off-label to treat insomnia) increased > 7-fold between 2001 and 2010, making it the second largest single drug expenditure in 2010. Many off-label medications have questionable risk-benefit ratios when used for sleep, and adverse effects can include infection,27 motor vehicle accidents,28 falls,29 and mortality.30 Further, some medications can limit deployability.
There are substantial challenges to incorporating behavioral approaches into the military armamentarium. There is a shortage of behavioral sleep specialists, although training initiatives seem promising.31 Most military facilities now have a medical home model of care with behavioral health providers as intrinsic team members. Their presence makes it easier to refer patients while reducing the stigma associated with behavioral health care. Leveraging technology will also facilitate the provision of quality, physician-directed insomnia treatment to an increasing number of military beneficiaries.
Nightmare Disorder
When patients with PTSD are able to get sleep, nightmares are a frequent occurrence and have been seen in up to 80% of individuals with this disorder.32 Nightmares usually occur during rapid eye movement (REM) sleep and are characterized by distressing dreams that threaten survival or security. They are often well remembered.33 After the nightmare, individuals typically wake up rapidly and report symptoms of distress, which can result in avoiding sleep (thereby perpetuating comorbid insomnia), daytime sleepiness, and fatigue.
Posttraumatic nightmares may have different dream mentation than do other disturbing dreams. The nightmare theme may involve actual events or reliving a prior traumatic experience. Most nightmares, however, have no associated movements or other complex behaviors, because during REM sleep, normal individuals are paralyzed, and thus do not move or act out their dreams.
Trauma-Associated Sleep Disorder
In some cases though, nightmares are accompanied by parasomnia activity.34 Parasomnias are abnormal and unintentional activities that occur during incomplete transitions between sleep stages and are seen more often in military personnel returning from deployment than in the general population. There is limited data regarding parasomnia activity in military personnel and veterans, although a study assessing sleep in 24 OIF/OEF veterans reported that 38% had either non-REM or REM parasomnia.34 Although in some instances these behaviors are simply a combination of genetics and insufficient sleep, in the majority of cases the clinical presentation is more complex.
In the authors’ clinical experience, patients described disruptive nocturnal behaviors (DNBs) which consisted of abnormal vocalizations (screaming, yelling), abnormal movements (tossing, turning, thrashing, sleep walking), or combative behaviors (striking the bed partner). These behaviors were strongly linked to symptoms of autonomic hyperarousal (night sweats, increased heart rate, or fast breathing). The DNBs often mimicked the content of the nightmares. The bed partner or spouse reported many of the cases after they had sustained unintended physical trauma from the combative behaviors.
Initially, REM behavior disorder (RBD) or nightmare disorder were considered potential diagnoses. However, RBD tends to occur in elderly males with neurodegenerative disorders (such as Parkinson disease). Dreams are relatively similar among patients with this disorder.35 Non-REM parasomnias are more common in young children and usually resolve prior to adolescence, although individuals who experienced parasomnias as children may see a reemergence during adulthood as a result of sleep fragmentation, medications, sleep-disordered breathing (SDB), recovery from sleep debt, or recreational drug or alcohol use.36,37
Since these posttraumatic nocturnal behaviors are not formally classified, a condition termed trauma-associated sleep disorder (TSD) was recently proposed.38 Trauma-associated sleep disorder is distinct from other parasomnias, because the onset must relate to a potentially traumatic event. On an overnight polysomnogram, increased muscle activity is seen during REM, and nightmares are almost invariably reported. Trauma-associated sleep disorder seems to involve not only DNB and traumatic dream enactment, but also insomnia and obstructive sleep apnea (OSA).
For patients who present with symptoms of TSD, a sleep study is recommended to evaluate for SDB as well as to characterize whether the patient has abnormal movements in REM sleep (lack of paralysis). There are currently no evidenced-based guidelines for treatment of this newly proposed sleep disorder. Behavioral and environmental modifications are the mainstay of treatment for individuals with any parasomnia. Obtaining an adequate quantity of sleep, avoiding triggers, and promoting a safe sleep environment are critical.
Substances that can lead to sleep fragmentation or impaired cognition, such as drugs and alcohol, should be avoided. Medical conditions that fragment sleep or cause nocturnal awakenings, such as sleep apnea, gastroesophageal reflux disease, and rhinitis should be treated to promote better sleep continuity.
When possible, medications with the potential to cause sleep fragmentation or disruption of normal sleep architecture should be reduced or discontinued. Weapons or objects that could be used as weapons should be removed from the bedroom, and padding should be placed on the sharp corners of furniture. Door and bed alarms, locks, and heavy curtains can minimize the risk of patients leaving the bedroom.
When these interventions are insufficient, medical therapy to suppress these events may be necessary. Some patients respond well to combined treatment with prazosin for nightmares and DNB, CBT for insomnia, and continuous positive airway pressure (CPAP) for OSA.39 Benzodiazepines, particularly clonazepam, may be effective for both slow-wave sleep parasomnias and RBD, but they should be used with caution in those with comorbid PTSD. Melatonin may also be effective, but there is a paucity of high-quality evidence supporting its use.
Obstructive Sleep Apnea
Another common sleep disorder that overlaps with PTSD is SDB. Obstructive sleep apnea is characterized by repetitive oxygen desaturations and arousals from sleep resulting from periodic upper airway collapse. Among middle-aged U.S. adults, about 9% of females and 24% of males have been estimated to have OSA, and rates increase with age and obesity.40 During the past decade, OSA in the military has risen dramatically, from 3,563 to 20,435 cases, with a 4-fold increase among those aged 20 to 24 years.17 Similar to the insomnia data, the increased rate of diagnosis during the recent wars in Southwest Asia coincides with an increase in the prevalence of traumatic brain injury (TBI) and PTSD. Additional reasons for the diagnostic increase may be heightened awareness of the diagnosis, increased availability of sleep disorders centers in the military, and even financial incentives for those undergoing a disability evaluation.
Obstructive sleep apnea is significantly more common in patients with PTSD compared with that in the general population, with rates of OSA ranging from 11.9% to 90%, depending on the study.41-43 Prevalence rates for OSA have been reported in several PTSD populations (violent crime, sexual assault, disasters, and combat). Military studies evaluating recent veterans have found OSA rates between 35% and 67%.44-46 In a recent study looking at SDB in those with PTSD, 53.8% had OSA (67.3% among those with polysomnograms).47 Although the other studies evaluated mixed populations of recent combat veterans, they were enriched for patients with PTSD.
Sleep disorders and PTSD have a “bidirectional” relationship.48 Sleep complaints preceding or temporally related to traumatic events increase the likelihood of subsequent mental health disorders, including PTSD.49-51 Sleep disorders are common in PTSD and are associated with symptoms of depression, relapse of depression, greater reductions in QOL, and suicide.52 Higher rates of OSA among patients who are not physically injured compared with the OSA rates of those with PTSD who also had physical injury (72.9% vs 38%) have also been seen, raising the possibility of different phenotypes of combat-related PTSD and a hypothetical role for premorbid OSA as a risk factor for PTSD.47
The pathophysiology linking SDB and PTSD is based on theories that poor sleep quality limits the ability to manage stress, promotes hyperarousal, confounds environmental stressors (trauma), and hinders the restorative qualities of sleep.49 Rapid eye movement sleep is believed to consolidate emotional memory, which may assist in recovery from traumatic events.53,54 Disrupted sleep architecture from OSA can diminish REM and hinder this process. Sleep fragmentation has been shown to cause upper airway instability and promote SDB.55 In addition, nighttime anxiety may induce hyperventilation with resultant hypocapnia, triggering apneic events.56 Taken together, disrupted sleep architecture, hyperarousal, respiratory instability, and nightmares may exacerbate one another and create a vicious cycle.57
Untreated OSA is associated with worse outcomes in PTSD. Continuous positive airway pressure has been shown to improve symptoms in this group.58-60 A study by Tamanna and colleagues evaluated clinical outcomes related to CPAP use, demonstrating improvements in nightmares, daytime sleepiness, and PTSD symptom severity with increasing adherence.61 Unfortunately, patients with PTSD generally have suboptimal medical adherence, and CPAP adherence decreases in psychiatric disease.62,63 Two recent studies have shown significantly lessened adherence in patients with both PTSD and OSA (compared with OSA alone), in both younger and older veteran populations.64,65 Limited insight and atypical clinical presentations of OSA also limit patient acceptance of treatment. Continuous positive airway pressure usage is decreased by comorbid insomnia, common in PTSD.66 Similarly, nightmares, mask discomfort, air hunger (the feeling of not being able to get a satisfying breath), and claustrophobia have all been associated with poor CPAP adherence in patients with PTSD.
Continuous positive airway pressure adherence is determined early (days to weeks), and initial use predicts long-term adherence.67-70 Patients are most likely to abandon therapy or fail to initiate therapy during this period. Given the potential adverse outcomes of comorbid mental illness and sleep disorders, including suicide, interventions should begin early.71 Continuous positive airway pressure devices with heated humidification, group education, peer success stories, and telephonic follow-up are all methods that improve adherence.72 There is conflicting evidence regarding the efficacy of nonbenzodiazepine sedative- hypnotics for improving diagnostic accuracy and CPAP adherence.73-76
Related: Using Light to Manage Sleep-Wake Issues in Patients With Dementia
Given this population’s high rate of comorbid insomnia, polypharmacy, and potentially pharmacotherapy refractory insomnia, the approach should be used cautiously in patients with PTSD OSA.77 Emerging efforts incorporate a biopsychosocial approach with an individualized focus on a patient’s unique barriers to adherence. Incorporating approaches such as motivational enhancement (for those ambivalent about change), educational approaches, and CBT may all be useful adjuncts.78-80
Ongoing VA trials have been designed to evaluate the impact of CPAP therapy on symptoms of PTSD and to compare CPAP and mandibular advancement devices with regards to efficacy in reducing the apneas and/or hypopneas per hour of sleep and improving symptoms.81,82
Discussion
Service members, like most adults, need about 8 hours of quality sleep per night to function at optimal levels and maximize operational readiness. The medical community is increasingly recognizing that sleep disturbances are inextricably linked to psychiatric disorders, particularly PTSD, depression, and anxiety.83,84 Balancing occupational performance and the demand of military missions with service member health remains a difficult leadership challenge.
Recent evidence suggests that disordered sleep may precede other PTSD symptom clusters.43,85 Sleep architecture in PTSD is disrupted, and abnormalities in both REM and non-REM sleep have been described.86,87 Insomnia not only is a component of depressive and anxiety disorders, but also impacts the course of disease severity.88 Sleep deprivation has been shown to be a risk factor for major depression in adolescents.89 In those with comorbid sleep problems, PTSD, and TBI, each disorder worsens QOL in an additive fashion.90
Severe mental illness impacts the military through a service member’s lost workdays, decreased productivity, impaired social relationships, and even suicide. Given that sleep quality is related to outcomes for patients with mental illnesses, access to medical professionals with specific training in sleep disorders becomes an integral part of a multidisciplinary approach to military health care. Encouragingly, treatment of insomnia and nightmares has been shown to improve PTSD symptom severity as well as headaches in veterans with mild TBI, even if neurologic deficits remain static.91 Similarly, treatment of insomnia is known to improve depressive symptoms in those with comorbid conditions.
Conclusion
The importance of sleep as a combat multiplier is increasingly recognized. The U.S. Army Surgeon General has acknowledged the interplay between inadequate sleep and impairments in other functional areas and placed specific emphasis on sleep as part of the Army Performance Triad. A core tenant of the Army Surgeon General’s message is that army medicine is on a mission to transform from a health care system to a system for health. The Army Wellness Centers, Army Medical Homes, Soldier-Centered Medical Homes, and embedded behavioral health are supporting the health of the force in these capacities. These functional areas treat behavioral health and sleep-related concerns across the continuum of disease from prevention, timely initial intervention once a condition has been identified, long-term treatment programs, and rehabilitative services.
Getting the proper quantity and quality of sleep, in addition to healthy activity and nutrition, increases readiness so that when called on to perform, soldiers are ready. A recent article by Wesensten and Balkin from the Walter Reed Army Institute of Research summarizes some guidelines for sleep from the Army Performance Triad Working Group to include sleep hygiene tips and judicious use of naps and caffeine.92 Efforts to improve soldier resiliency by improving sleep-related disorders have yet to be studied in a meaningful way, so additional research is needed to determine best practices and evidence-based guidelines.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
2. U.S. Department of the Army. Combat and Operational Stress Control Manual for Leaders and Soldiers. Washington, DC: Government Printing Office; 2009. FM 6-22.5.
3. Williams SG, Collen J, Wickwire E, Lettieri CJ, Mysliwiec V. The impact of sleep on soldier performance. Curr Psychiatry Rep. 2014;16(8):459.
4. Miller NL, Shattuck LG, Tvaryanas AP, Matsangas P. Effects of Sleep on Training Effectiveness in Soldiers at Fort Leonard Wood, Missouri. Monterey, CA: Naval Post Graduate School; 2010.
5. Miller NL, Shattuck LG, Matsangas P. Longitudinal study of sleep patterns of United States Military Academy cadets. Sleep. 2010;33(12):1623-1631.
6. Barlas FM, Higgins WB, Pflieger JC, Diecker K. 2011 Health Related Behaviors Survey of Active Duty Military Personnel. Fairfax, VA: Department of Defense; 2013.
7. Caldwell JL, Gilreath SR. Work and sleep hours of U.S. Army aviation personnel working reverse cycle. Mil Med. 2001;166(2):159-166.
8. Mental Health Advisory Team 9. Mental Health Advisory Team 9 (MHAT 9), Operation Enduring Freedom (OEF) 2013 Afghanistan. Army Medicine Website. http://armymedicine.mil/Documents /MHAT_9_OEF_Report.pdf. Published October 10, 2013. Accessed March 3, 2015.
9. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117-126.
10. Czeisler CA. The Gordon Wilson lecture: work hours, sleep and patient safety in residency training. Trans Am Clin Climatol Assoc. 2006;117:159-188.
11. Krakow B, Melendrez D, Pedersen B, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49(11): 948-953.
12. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;229(suppl 2):S347-S353.
13. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479-1484.
14. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541-553.
15. Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4): 227-247.
16. Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213-218.
17. Sjöström N, Waern M, Hetta J. Nightmares and sleep disturbances in relation to suicidality in suicide attempters. Sleep. 2007;30(1):91-95.
18. Armed Forces Health Surveillance Center. Insomnia, Active Component, U.S. Armed Forces, January 2000-December 2009 Medical Surveillance Monthly Report. 2010;17(5):12-15.
19. McLay RN, Klam WP, Volkert SL. Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in U.S. service members returning from military deployments. Mil Med. 2010;175(10):759-762.
20. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
21. Manber R, Edinger JD, Gress JL, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489-495.
22. Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327-341.
23. Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61(6):947-956.
24. Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. 2011;49(4):227-233.
25. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
26. Epstein DR, Babcock-Parziale JL, Haynes PL, Herb CA. Insomnia treatment acceptability and p of male Iraq and Afghanistan combat veterans and their healthcare providers. J Rehabil Res Dev. 2012;49(6):867-878.
27. Joya FL, Kripke DF, Loving RT, Dawson A, Kline LE. Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. J Clin Sleep Med. 2009;5(4):377-383.
28. Farkas RH, Unger EF, Temple R. Zolpidem and driving impairment—identifying persons at risk. N Engl J Med. 2013;369(8):689-691.
29. Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1-6.
30. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: A matched cohort study. BMJ Open. 2012;2:e000850.
31. Karlin BE, Trockel M, Taylor CB, Gimeno J, Manber R. National dissemination of cognitive behavioral therapy for insomnia in veterans: therapist- and patient-level outcomes. J Consult Clin Psychol. 2013;81(5):912-917.
32. Aurora RN, Zak RS, Auerbach SH, et al; Standards of Practice Committee, American Academy of Sleep Medicine. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
33. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
34. Wallace DM, Shafazand S, Ramos AR, et al. Insomnia characteristics and clinical correlates in Operation Enduring Freedom/Operation Iraqi Freedom veterans with post-traumatic stress disorder and mild traumatic brain injury: an exploratory study. Sleep Med. 2011;12(9):850-859.
35. Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. CCJM. 1990;57(suppl):S9-S23.
36. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(12):3494-3509.
37. Provini F, Piazzi G, Tinuper P, et al. Nocturnal frontal lobe epilepsy: a clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122(6):1017-1031.
38. Mysliwiec V, O’Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares and rem without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143-1148.
39. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
40. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-1235.
41. Krakow B, Haynes PL, Warner TD, et al. Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress. 2004;17(3):257-268.
42. Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405-1411.
43. Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169-184.
44. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
45. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US Military Personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
46. Mysliwiec V, McGraw L, Pierce R, et al. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
47. Williams SG, Collen J, Orr N, Holley AB, Lettieri CJ. Sleep disorders in combat-related PTSD. Sleep Breath. 2015;19(1):175-182.
48. Krakow B, Melendrez D, Warner TD, et al. To breathe, perchance to sleep: sleep-disordered breathing and chronic insomnia among trauma survivors. Sleep Breath. 2002;6(4):189-202.
49. Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69-74.
50. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
51. Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, Moore D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol. 2011;67(12):1240-1258.
52. Pittman JO, Goldsmith AA, Lemmer JA, Kilmer MT, Baker DG. Post-traumatic stress disorder, depression, and health-related quality of life in OEF/OIF veterans. Quality of Life Res. 2012;21(1):99-103.
53. Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696-1701.
54. Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8(2):112-119.
55. Sériès F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485.
56. Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825-1832.
57. van Liempt S. Sleep disturbances and PTSD: a perpetual circle? Eur J Psychotraumatology. 2012;3.
58. Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry. 2007;68(8):1257-1270.
59. Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291-298.
60. Sateia MJ. Update on sleep and psychiatric disorders. Chest. 2009;135(5):1370-1379.
61. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
62. Lockwood A, Steinke DT, Botts SR. Medication adherence and its effect on relapse among patients discharged from a Veterans Affairs posttraumatic stress disorder treatment program. Ann Pharmacother. 2009;43(7):1227-1232.
63. Means MK, Ulmer CS, Edinger JD. Ethnic differences in continuous positive airway pressure (CPAP) adherence in veterans with and without psychiatric disorders. Behav Sleep Med. 2010;8(4):260-273.
64. El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495-1500.
65. Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667-672.
66. Wickwire EM, Smith MT, Birnbaum S, Collop NA. Sleep maintenance insomnia complaints predict poor CPAP adherence: a clinical case series. Sleep Med. 2010;11(8):772-776.
67. Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5(3):229-240.
68. Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320-324.
69. Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27(1):134-138.
70. Pépin JL, Krieger J, Rodenstein D, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999;160(4):1124-1129.
71. Ribeiro JD, Pease JL, Gutierrez PM, et al. Sleep problems outperform depression and hopelessness as cross-sectional and longitudinal predictors of suicidal ideation and behavior in young adults in the military. J Affect Disord. 2012;136(3):743-750.
72. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343-356.
73. Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest. 2006;130(5):1369-1376.
74. Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136(5):1263-1268.
75. Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep. 2008;31(9):1310-1316.
76. Lettieri CJ, Shah AA, Holley AB, et al. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151(10):696-702.
77. Mysliwiec V, Roth B. Pharmacotherapy refractory insomnia in soldiers with traumatic brain injury. Chest. 2013;143(2):582-583.
78. Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: A randomized controlled trial. Sleep. 2013;36(11):1655-1662.
79. Bartlett D, Wong K, Richards D, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep. 2013;36(11):1647-1654.
80. Dickerson SS, Obeidat R, Dean G, et al. Development and usability testing of a self-management intervention to support individuals with obstructive sleep apnea in accommodating to CPAP treatment. Heart Lung. 2013;42(5):346-352.
81. Effects of CPAP therapy on PTSD symptoms. ClinicalTrials.gov Identifier NCT02019914. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT02019914. Updated September 3, 2014. Accessed March 4, 2015.
82. A randomized cross over trial of two treatments for obstructive sleep apnea in veterans with post-traumatic stress disorder. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT01535586. Updated February 16, 2012. Accessed March 4, 2015.
83. Babson KA, Boden MT, Woodward S, Alvarez J, Bonn-Miller M. Anxiety sensitivity and sleep quality: independent and interactive predictors of posttraumatic stress disorder symptoms. J Nerv Ment Dis. 2013;201(1):48-51.
84. Belleville G, Guay S, Marchand A. Impact of sleep disturbances on PTSD symptoms and perceived health. J Nerv Ment Dis. 2009;197(2):126-132.
85. van Liempt S, van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety. 2013;30(5):469-474.
86. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
87. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382.
88. Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9-15.
89. Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37(2):239-244.
90. Macera CA, Aralis HJ, Rauh MJ, MacGregor AJ. Do sleep problems mediate the relationship between traumatic brain injury and development of mental health symptoms after deployment? Sleep. 2013;36(1):83-90.
91. Ruff RL, Riechers RG, Wang XF et al. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. 2012;49(9):1305-1320.
92. Wesensten NJ, Balkin TJ. The challenge of sleep management in military operations. US Army Med Dep J. 2013;109-118.
[This article originally published online ahead of print April 23, 2015.]
Sleep in the military has traditionally been thought of as a luxury and is sometimes considered at odds with optimal productivity. Every minute that a service member is asleep, he or she is not performing a primary duty, and getting a minimal amount of sleep is often seen as a badge of honor and strength. Research has recently been conducted, underscoring the importance of sleep management as an operational variable that must be accounted for in order to achieve optimal performance and promote resiliency. Both the quality and the duration of sleep must be considered, particularly given the increasingly complicated tasks that every service member must perform during both war and peace.
It has been well established that higher order mental tasks are the most vulnerable to sleep loss, as are those with little mental or physical stimulation, such as guard duty.1,2 Because service members are expected not only to perform in combat, but also to behave and operate ethically in spite of the challenges of war, the importance of adequate sleep must be considered. Many challenges are commonly encountered by service members when attempting to get adequate sleep (Table).3 This review highlights the recent diagnostic and treatment advances with respect to the overlap of sleep disorders and posttraumatic stress disorder (PTSD).
Culture of Sleep Loss
At the United States Military Academy in West Point, New York, a culture of poor sleep is instilled during initial military training; students typically get less than the recommended 7 to 8 hours of sleep per 24 hours.4,5 This sleep restriction continues for most of the time served on active duty: Military members get less sleep on average than does the rest of the U.S. population.6
Studies performed on pilots and during deployment have consistently shown a trend toward inadequate sleep, but only recently has inadequate sleep gained the attention of senior leadership.7,8 The Army Performance Triad, a public health campaign launched in 2013 by the Office of the U.S. Army Surgeon General, equally values sleep, nutrition, and activity. The goal of the Army Performance Triad is to influence behaviors by promoting healthy sleep, activity, and nutrition. Sleep is the apex of the Army Performance Triad.8
Those with chronic sleep restriction may not understand how impaired they are until objective testing is performed.9 In the civilian population, fatal sleep-related traffic accidents have been shown to exceed fatalities due to alcohol and illicit drug use combined.10 When poor sleep is combined with the trauma of war, symptoms exponentially worsen, and treatment becomes more complicated.11 Therefore, even before a formal sleep disorder or psychiatric condition develops, service members put themselves at risk by practicing poor sleep behaviors.11
Once insomnia develops, however, the potential negative health consequences are much more significant. Chronic insomnia, characterized by difficulty initiating or maintaining sleep or by waking too early, is the most common sleep disorder among adults. Thirty percent of adults experience occasional or transient insomnia, and between 9% and 12% of adults have severe chronic insomnia.12,13 This number is likely higher in the military and is much higher in those with PTSD.13
Related: How Effective Is Group Cognitive Behavioral Therapy to Treat PTSD?
The etiology of chronic insomnia is multifactorial and is best conceptualized within a biopsychosocial framework. Physiologic abnormalities, such as increased activity in the central nervous system, hyperarousal of the hypothalamic-pituitary axis, and activation of proinflammatory cytokines, predispose individuals to developing insomnia. In addition, personality traits, such as anxious temperament or an internalizing stress-management style, make it more likely for individuals to respond negatively to stress, the most common precipitating cause of chronic insomnia.
Behavioral factors are also paramount. For example, individuals who experience acute sleep disturbance during deployment might develop maladaptive compensatory behaviors, such as spending excessive time in bed, “trying harder” to sleep, or overusing stimulants. These sleep behaviors can become a chronic condition.14
Comorbidities
Patients with insomnia are at increased risk for medical consequences, such as cardiovascular disease and mortality as well as psychiatric sequelae.15,16 Insomnia is also common among people who have attempted suicide.17 In the military, there was nearly a 20-fold increase in the rate of chronic insomnia among service members between 2000 and 2009, coincident with the dramatic uptick in operations tempo.18
Insomnia is one of the most common reports of returning Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF) veterans and is associated with the development of PTSD.19 Soldiers who reported symptoms of insomnia predeployment were more likely to develop anxiety, depression, and PTSD during deployment than were soldiers who did not report these symptoms.20
Empirically supported and evidence-based treatment options exist. Further, a robust evidence base supports the conclusion that treating insomnia improves not only sleep and quality of life (QOL), but also health-related outcomes in comorbid conditions, including depression, PTSD, chronic pain, and alcohol dependence.21-24 One historical barrier to effective treatment has been poor recognition of the scope of the problem. The army is looking to implement a more robust assessment of sleep in the primary care setting as part of the Army Performance Triad in order to intervene as early as possible. Other government organizations may also follow suit.
Although several FDA-approved medications for insomnia exist, the gold standard treatment for insomnia is cognitive behavioral therapy (CBT).25 Specific behavioral patient preferences that can be used to tailor treatment have been identified within a military population.26 Unfortunately, the most commonly used treatment for chronic insomnia in the military remains sedative- hypnotic medication. Multiple estimates suggest that 15% to 20% of all deployed service members have been prescribed a sedative-hypnotic to aid with sleep initiation, including many off-label antidepressants, antipsychotics, and antihistamines. Indeed, within VA, the use of quetiapine (an antipsychotic used off-label to treat insomnia) increased > 7-fold between 2001 and 2010, making it the second largest single drug expenditure in 2010. Many off-label medications have questionable risk-benefit ratios when used for sleep, and adverse effects can include infection,27 motor vehicle accidents,28 falls,29 and mortality.30 Further, some medications can limit deployability.
There are substantial challenges to incorporating behavioral approaches into the military armamentarium. There is a shortage of behavioral sleep specialists, although training initiatives seem promising.31 Most military facilities now have a medical home model of care with behavioral health providers as intrinsic team members. Their presence makes it easier to refer patients while reducing the stigma associated with behavioral health care. Leveraging technology will also facilitate the provision of quality, physician-directed insomnia treatment to an increasing number of military beneficiaries.
Nightmare Disorder
When patients with PTSD are able to get sleep, nightmares are a frequent occurrence and have been seen in up to 80% of individuals with this disorder.32 Nightmares usually occur during rapid eye movement (REM) sleep and are characterized by distressing dreams that threaten survival or security. They are often well remembered.33 After the nightmare, individuals typically wake up rapidly and report symptoms of distress, which can result in avoiding sleep (thereby perpetuating comorbid insomnia), daytime sleepiness, and fatigue.
Posttraumatic nightmares may have different dream mentation than do other disturbing dreams. The nightmare theme may involve actual events or reliving a prior traumatic experience. Most nightmares, however, have no associated movements or other complex behaviors, because during REM sleep, normal individuals are paralyzed, and thus do not move or act out their dreams.
Trauma-Associated Sleep Disorder
In some cases though, nightmares are accompanied by parasomnia activity.34 Parasomnias are abnormal and unintentional activities that occur during incomplete transitions between sleep stages and are seen more often in military personnel returning from deployment than in the general population. There is limited data regarding parasomnia activity in military personnel and veterans, although a study assessing sleep in 24 OIF/OEF veterans reported that 38% had either non-REM or REM parasomnia.34 Although in some instances these behaviors are simply a combination of genetics and insufficient sleep, in the majority of cases the clinical presentation is more complex.
In the authors’ clinical experience, patients described disruptive nocturnal behaviors (DNBs) which consisted of abnormal vocalizations (screaming, yelling), abnormal movements (tossing, turning, thrashing, sleep walking), or combative behaviors (striking the bed partner). These behaviors were strongly linked to symptoms of autonomic hyperarousal (night sweats, increased heart rate, or fast breathing). The DNBs often mimicked the content of the nightmares. The bed partner or spouse reported many of the cases after they had sustained unintended physical trauma from the combative behaviors.
Initially, REM behavior disorder (RBD) or nightmare disorder were considered potential diagnoses. However, RBD tends to occur in elderly males with neurodegenerative disorders (such as Parkinson disease). Dreams are relatively similar among patients with this disorder.35 Non-REM parasomnias are more common in young children and usually resolve prior to adolescence, although individuals who experienced parasomnias as children may see a reemergence during adulthood as a result of sleep fragmentation, medications, sleep-disordered breathing (SDB), recovery from sleep debt, or recreational drug or alcohol use.36,37
Since these posttraumatic nocturnal behaviors are not formally classified, a condition termed trauma-associated sleep disorder (TSD) was recently proposed.38 Trauma-associated sleep disorder is distinct from other parasomnias, because the onset must relate to a potentially traumatic event. On an overnight polysomnogram, increased muscle activity is seen during REM, and nightmares are almost invariably reported. Trauma-associated sleep disorder seems to involve not only DNB and traumatic dream enactment, but also insomnia and obstructive sleep apnea (OSA).
For patients who present with symptoms of TSD, a sleep study is recommended to evaluate for SDB as well as to characterize whether the patient has abnormal movements in REM sleep (lack of paralysis). There are currently no evidenced-based guidelines for treatment of this newly proposed sleep disorder. Behavioral and environmental modifications are the mainstay of treatment for individuals with any parasomnia. Obtaining an adequate quantity of sleep, avoiding triggers, and promoting a safe sleep environment are critical.
Substances that can lead to sleep fragmentation or impaired cognition, such as drugs and alcohol, should be avoided. Medical conditions that fragment sleep or cause nocturnal awakenings, such as sleep apnea, gastroesophageal reflux disease, and rhinitis should be treated to promote better sleep continuity.
When possible, medications with the potential to cause sleep fragmentation or disruption of normal sleep architecture should be reduced or discontinued. Weapons or objects that could be used as weapons should be removed from the bedroom, and padding should be placed on the sharp corners of furniture. Door and bed alarms, locks, and heavy curtains can minimize the risk of patients leaving the bedroom.
When these interventions are insufficient, medical therapy to suppress these events may be necessary. Some patients respond well to combined treatment with prazosin for nightmares and DNB, CBT for insomnia, and continuous positive airway pressure (CPAP) for OSA.39 Benzodiazepines, particularly clonazepam, may be effective for both slow-wave sleep parasomnias and RBD, but they should be used with caution in those with comorbid PTSD. Melatonin may also be effective, but there is a paucity of high-quality evidence supporting its use.
Obstructive Sleep Apnea
Another common sleep disorder that overlaps with PTSD is SDB. Obstructive sleep apnea is characterized by repetitive oxygen desaturations and arousals from sleep resulting from periodic upper airway collapse. Among middle-aged U.S. adults, about 9% of females and 24% of males have been estimated to have OSA, and rates increase with age and obesity.40 During the past decade, OSA in the military has risen dramatically, from 3,563 to 20,435 cases, with a 4-fold increase among those aged 20 to 24 years.17 Similar to the insomnia data, the increased rate of diagnosis during the recent wars in Southwest Asia coincides with an increase in the prevalence of traumatic brain injury (TBI) and PTSD. Additional reasons for the diagnostic increase may be heightened awareness of the diagnosis, increased availability of sleep disorders centers in the military, and even financial incentives for those undergoing a disability evaluation.
Obstructive sleep apnea is significantly more common in patients with PTSD compared with that in the general population, with rates of OSA ranging from 11.9% to 90%, depending on the study.41-43 Prevalence rates for OSA have been reported in several PTSD populations (violent crime, sexual assault, disasters, and combat). Military studies evaluating recent veterans have found OSA rates between 35% and 67%.44-46 In a recent study looking at SDB in those with PTSD, 53.8% had OSA (67.3% among those with polysomnograms).47 Although the other studies evaluated mixed populations of recent combat veterans, they were enriched for patients with PTSD.
Sleep disorders and PTSD have a “bidirectional” relationship.48 Sleep complaints preceding or temporally related to traumatic events increase the likelihood of subsequent mental health disorders, including PTSD.49-51 Sleep disorders are common in PTSD and are associated with symptoms of depression, relapse of depression, greater reductions in QOL, and suicide.52 Higher rates of OSA among patients who are not physically injured compared with the OSA rates of those with PTSD who also had physical injury (72.9% vs 38%) have also been seen, raising the possibility of different phenotypes of combat-related PTSD and a hypothetical role for premorbid OSA as a risk factor for PTSD.47
The pathophysiology linking SDB and PTSD is based on theories that poor sleep quality limits the ability to manage stress, promotes hyperarousal, confounds environmental stressors (trauma), and hinders the restorative qualities of sleep.49 Rapid eye movement sleep is believed to consolidate emotional memory, which may assist in recovery from traumatic events.53,54 Disrupted sleep architecture from OSA can diminish REM and hinder this process. Sleep fragmentation has been shown to cause upper airway instability and promote SDB.55 In addition, nighttime anxiety may induce hyperventilation with resultant hypocapnia, triggering apneic events.56 Taken together, disrupted sleep architecture, hyperarousal, respiratory instability, and nightmares may exacerbate one another and create a vicious cycle.57
Untreated OSA is associated with worse outcomes in PTSD. Continuous positive airway pressure has been shown to improve symptoms in this group.58-60 A study by Tamanna and colleagues evaluated clinical outcomes related to CPAP use, demonstrating improvements in nightmares, daytime sleepiness, and PTSD symptom severity with increasing adherence.61 Unfortunately, patients with PTSD generally have suboptimal medical adherence, and CPAP adherence decreases in psychiatric disease.62,63 Two recent studies have shown significantly lessened adherence in patients with both PTSD and OSA (compared with OSA alone), in both younger and older veteran populations.64,65 Limited insight and atypical clinical presentations of OSA also limit patient acceptance of treatment. Continuous positive airway pressure usage is decreased by comorbid insomnia, common in PTSD.66 Similarly, nightmares, mask discomfort, air hunger (the feeling of not being able to get a satisfying breath), and claustrophobia have all been associated with poor CPAP adherence in patients with PTSD.
Continuous positive airway pressure adherence is determined early (days to weeks), and initial use predicts long-term adherence.67-70 Patients are most likely to abandon therapy or fail to initiate therapy during this period. Given the potential adverse outcomes of comorbid mental illness and sleep disorders, including suicide, interventions should begin early.71 Continuous positive airway pressure devices with heated humidification, group education, peer success stories, and telephonic follow-up are all methods that improve adherence.72 There is conflicting evidence regarding the efficacy of nonbenzodiazepine sedative- hypnotics for improving diagnostic accuracy and CPAP adherence.73-76
Related: Using Light to Manage Sleep-Wake Issues in Patients With Dementia
Given this population’s high rate of comorbid insomnia, polypharmacy, and potentially pharmacotherapy refractory insomnia, the approach should be used cautiously in patients with PTSD OSA.77 Emerging efforts incorporate a biopsychosocial approach with an individualized focus on a patient’s unique barriers to adherence. Incorporating approaches such as motivational enhancement (for those ambivalent about change), educational approaches, and CBT may all be useful adjuncts.78-80
Ongoing VA trials have been designed to evaluate the impact of CPAP therapy on symptoms of PTSD and to compare CPAP and mandibular advancement devices with regards to efficacy in reducing the apneas and/or hypopneas per hour of sleep and improving symptoms.81,82
Discussion
Service members, like most adults, need about 8 hours of quality sleep per night to function at optimal levels and maximize operational readiness. The medical community is increasingly recognizing that sleep disturbances are inextricably linked to psychiatric disorders, particularly PTSD, depression, and anxiety.83,84 Balancing occupational performance and the demand of military missions with service member health remains a difficult leadership challenge.
Recent evidence suggests that disordered sleep may precede other PTSD symptom clusters.43,85 Sleep architecture in PTSD is disrupted, and abnormalities in both REM and non-REM sleep have been described.86,87 Insomnia not only is a component of depressive and anxiety disorders, but also impacts the course of disease severity.88 Sleep deprivation has been shown to be a risk factor for major depression in adolescents.89 In those with comorbid sleep problems, PTSD, and TBI, each disorder worsens QOL in an additive fashion.90
Severe mental illness impacts the military through a service member’s lost workdays, decreased productivity, impaired social relationships, and even suicide. Given that sleep quality is related to outcomes for patients with mental illnesses, access to medical professionals with specific training in sleep disorders becomes an integral part of a multidisciplinary approach to military health care. Encouragingly, treatment of insomnia and nightmares has been shown to improve PTSD symptom severity as well as headaches in veterans with mild TBI, even if neurologic deficits remain static.91 Similarly, treatment of insomnia is known to improve depressive symptoms in those with comorbid conditions.
Conclusion
The importance of sleep as a combat multiplier is increasingly recognized. The U.S. Army Surgeon General has acknowledged the interplay between inadequate sleep and impairments in other functional areas and placed specific emphasis on sleep as part of the Army Performance Triad. A core tenant of the Army Surgeon General’s message is that army medicine is on a mission to transform from a health care system to a system for health. The Army Wellness Centers, Army Medical Homes, Soldier-Centered Medical Homes, and embedded behavioral health are supporting the health of the force in these capacities. These functional areas treat behavioral health and sleep-related concerns across the continuum of disease from prevention, timely initial intervention once a condition has been identified, long-term treatment programs, and rehabilitative services.
Getting the proper quantity and quality of sleep, in addition to healthy activity and nutrition, increases readiness so that when called on to perform, soldiers are ready. A recent article by Wesensten and Balkin from the Walter Reed Army Institute of Research summarizes some guidelines for sleep from the Army Performance Triad Working Group to include sleep hygiene tips and judicious use of naps and caffeine.92 Efforts to improve soldier resiliency by improving sleep-related disorders have yet to be studied in a meaningful way, so additional research is needed to determine best practices and evidence-based guidelines.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[This article originally published online ahead of print April 23, 2015.]
Sleep in the military has traditionally been thought of as a luxury and is sometimes considered at odds with optimal productivity. Every minute that a service member is asleep, he or she is not performing a primary duty, and getting a minimal amount of sleep is often seen as a badge of honor and strength. Research has recently been conducted, underscoring the importance of sleep management as an operational variable that must be accounted for in order to achieve optimal performance and promote resiliency. Both the quality and the duration of sleep must be considered, particularly given the increasingly complicated tasks that every service member must perform during both war and peace.
It has been well established that higher order mental tasks are the most vulnerable to sleep loss, as are those with little mental or physical stimulation, such as guard duty.1,2 Because service members are expected not only to perform in combat, but also to behave and operate ethically in spite of the challenges of war, the importance of adequate sleep must be considered. Many challenges are commonly encountered by service members when attempting to get adequate sleep (Table).3 This review highlights the recent diagnostic and treatment advances with respect to the overlap of sleep disorders and posttraumatic stress disorder (PTSD).
Culture of Sleep Loss
At the United States Military Academy in West Point, New York, a culture of poor sleep is instilled during initial military training; students typically get less than the recommended 7 to 8 hours of sleep per 24 hours.4,5 This sleep restriction continues for most of the time served on active duty: Military members get less sleep on average than does the rest of the U.S. population.6
Studies performed on pilots and during deployment have consistently shown a trend toward inadequate sleep, but only recently has inadequate sleep gained the attention of senior leadership.7,8 The Army Performance Triad, a public health campaign launched in 2013 by the Office of the U.S. Army Surgeon General, equally values sleep, nutrition, and activity. The goal of the Army Performance Triad is to influence behaviors by promoting healthy sleep, activity, and nutrition. Sleep is the apex of the Army Performance Triad.8
Those with chronic sleep restriction may not understand how impaired they are until objective testing is performed.9 In the civilian population, fatal sleep-related traffic accidents have been shown to exceed fatalities due to alcohol and illicit drug use combined.10 When poor sleep is combined with the trauma of war, symptoms exponentially worsen, and treatment becomes more complicated.11 Therefore, even before a formal sleep disorder or psychiatric condition develops, service members put themselves at risk by practicing poor sleep behaviors.11
Once insomnia develops, however, the potential negative health consequences are much more significant. Chronic insomnia, characterized by difficulty initiating or maintaining sleep or by waking too early, is the most common sleep disorder among adults. Thirty percent of adults experience occasional or transient insomnia, and between 9% and 12% of adults have severe chronic insomnia.12,13 This number is likely higher in the military and is much higher in those with PTSD.13
Related: How Effective Is Group Cognitive Behavioral Therapy to Treat PTSD?
The etiology of chronic insomnia is multifactorial and is best conceptualized within a biopsychosocial framework. Physiologic abnormalities, such as increased activity in the central nervous system, hyperarousal of the hypothalamic-pituitary axis, and activation of proinflammatory cytokines, predispose individuals to developing insomnia. In addition, personality traits, such as anxious temperament or an internalizing stress-management style, make it more likely for individuals to respond negatively to stress, the most common precipitating cause of chronic insomnia.
Behavioral factors are also paramount. For example, individuals who experience acute sleep disturbance during deployment might develop maladaptive compensatory behaviors, such as spending excessive time in bed, “trying harder” to sleep, or overusing stimulants. These sleep behaviors can become a chronic condition.14
Comorbidities
Patients with insomnia are at increased risk for medical consequences, such as cardiovascular disease and mortality as well as psychiatric sequelae.15,16 Insomnia is also common among people who have attempted suicide.17 In the military, there was nearly a 20-fold increase in the rate of chronic insomnia among service members between 2000 and 2009, coincident with the dramatic uptick in operations tempo.18
Insomnia is one of the most common reports of returning Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF) veterans and is associated with the development of PTSD.19 Soldiers who reported symptoms of insomnia predeployment were more likely to develop anxiety, depression, and PTSD during deployment than were soldiers who did not report these symptoms.20
Empirically supported and evidence-based treatment options exist. Further, a robust evidence base supports the conclusion that treating insomnia improves not only sleep and quality of life (QOL), but also health-related outcomes in comorbid conditions, including depression, PTSD, chronic pain, and alcohol dependence.21-24 One historical barrier to effective treatment has been poor recognition of the scope of the problem. The army is looking to implement a more robust assessment of sleep in the primary care setting as part of the Army Performance Triad in order to intervene as early as possible. Other government organizations may also follow suit.
Although several FDA-approved medications for insomnia exist, the gold standard treatment for insomnia is cognitive behavioral therapy (CBT).25 Specific behavioral patient preferences that can be used to tailor treatment have been identified within a military population.26 Unfortunately, the most commonly used treatment for chronic insomnia in the military remains sedative- hypnotic medication. Multiple estimates suggest that 15% to 20% of all deployed service members have been prescribed a sedative-hypnotic to aid with sleep initiation, including many off-label antidepressants, antipsychotics, and antihistamines. Indeed, within VA, the use of quetiapine (an antipsychotic used off-label to treat insomnia) increased > 7-fold between 2001 and 2010, making it the second largest single drug expenditure in 2010. Many off-label medications have questionable risk-benefit ratios when used for sleep, and adverse effects can include infection,27 motor vehicle accidents,28 falls,29 and mortality.30 Further, some medications can limit deployability.
There are substantial challenges to incorporating behavioral approaches into the military armamentarium. There is a shortage of behavioral sleep specialists, although training initiatives seem promising.31 Most military facilities now have a medical home model of care with behavioral health providers as intrinsic team members. Their presence makes it easier to refer patients while reducing the stigma associated with behavioral health care. Leveraging technology will also facilitate the provision of quality, physician-directed insomnia treatment to an increasing number of military beneficiaries.
Nightmare Disorder
When patients with PTSD are able to get sleep, nightmares are a frequent occurrence and have been seen in up to 80% of individuals with this disorder.32 Nightmares usually occur during rapid eye movement (REM) sleep and are characterized by distressing dreams that threaten survival or security. They are often well remembered.33 After the nightmare, individuals typically wake up rapidly and report symptoms of distress, which can result in avoiding sleep (thereby perpetuating comorbid insomnia), daytime sleepiness, and fatigue.
Posttraumatic nightmares may have different dream mentation than do other disturbing dreams. The nightmare theme may involve actual events or reliving a prior traumatic experience. Most nightmares, however, have no associated movements or other complex behaviors, because during REM sleep, normal individuals are paralyzed, and thus do not move or act out their dreams.
Trauma-Associated Sleep Disorder
In some cases though, nightmares are accompanied by parasomnia activity.34 Parasomnias are abnormal and unintentional activities that occur during incomplete transitions between sleep stages and are seen more often in military personnel returning from deployment than in the general population. There is limited data regarding parasomnia activity in military personnel and veterans, although a study assessing sleep in 24 OIF/OEF veterans reported that 38% had either non-REM or REM parasomnia.34 Although in some instances these behaviors are simply a combination of genetics and insufficient sleep, in the majority of cases the clinical presentation is more complex.
In the authors’ clinical experience, patients described disruptive nocturnal behaviors (DNBs) which consisted of abnormal vocalizations (screaming, yelling), abnormal movements (tossing, turning, thrashing, sleep walking), or combative behaviors (striking the bed partner). These behaviors were strongly linked to symptoms of autonomic hyperarousal (night sweats, increased heart rate, or fast breathing). The DNBs often mimicked the content of the nightmares. The bed partner or spouse reported many of the cases after they had sustained unintended physical trauma from the combative behaviors.
Initially, REM behavior disorder (RBD) or nightmare disorder were considered potential diagnoses. However, RBD tends to occur in elderly males with neurodegenerative disorders (such as Parkinson disease). Dreams are relatively similar among patients with this disorder.35 Non-REM parasomnias are more common in young children and usually resolve prior to adolescence, although individuals who experienced parasomnias as children may see a reemergence during adulthood as a result of sleep fragmentation, medications, sleep-disordered breathing (SDB), recovery from sleep debt, or recreational drug or alcohol use.36,37
Since these posttraumatic nocturnal behaviors are not formally classified, a condition termed trauma-associated sleep disorder (TSD) was recently proposed.38 Trauma-associated sleep disorder is distinct from other parasomnias, because the onset must relate to a potentially traumatic event. On an overnight polysomnogram, increased muscle activity is seen during REM, and nightmares are almost invariably reported. Trauma-associated sleep disorder seems to involve not only DNB and traumatic dream enactment, but also insomnia and obstructive sleep apnea (OSA).
For patients who present with symptoms of TSD, a sleep study is recommended to evaluate for SDB as well as to characterize whether the patient has abnormal movements in REM sleep (lack of paralysis). There are currently no evidenced-based guidelines for treatment of this newly proposed sleep disorder. Behavioral and environmental modifications are the mainstay of treatment for individuals with any parasomnia. Obtaining an adequate quantity of sleep, avoiding triggers, and promoting a safe sleep environment are critical.
Substances that can lead to sleep fragmentation or impaired cognition, such as drugs and alcohol, should be avoided. Medical conditions that fragment sleep or cause nocturnal awakenings, such as sleep apnea, gastroesophageal reflux disease, and rhinitis should be treated to promote better sleep continuity.
When possible, medications with the potential to cause sleep fragmentation or disruption of normal sleep architecture should be reduced or discontinued. Weapons or objects that could be used as weapons should be removed from the bedroom, and padding should be placed on the sharp corners of furniture. Door and bed alarms, locks, and heavy curtains can minimize the risk of patients leaving the bedroom.
When these interventions are insufficient, medical therapy to suppress these events may be necessary. Some patients respond well to combined treatment with prazosin for nightmares and DNB, CBT for insomnia, and continuous positive airway pressure (CPAP) for OSA.39 Benzodiazepines, particularly clonazepam, may be effective for both slow-wave sleep parasomnias and RBD, but they should be used with caution in those with comorbid PTSD. Melatonin may also be effective, but there is a paucity of high-quality evidence supporting its use.
Obstructive Sleep Apnea
Another common sleep disorder that overlaps with PTSD is SDB. Obstructive sleep apnea is characterized by repetitive oxygen desaturations and arousals from sleep resulting from periodic upper airway collapse. Among middle-aged U.S. adults, about 9% of females and 24% of males have been estimated to have OSA, and rates increase with age and obesity.40 During the past decade, OSA in the military has risen dramatically, from 3,563 to 20,435 cases, with a 4-fold increase among those aged 20 to 24 years.17 Similar to the insomnia data, the increased rate of diagnosis during the recent wars in Southwest Asia coincides with an increase in the prevalence of traumatic brain injury (TBI) and PTSD. Additional reasons for the diagnostic increase may be heightened awareness of the diagnosis, increased availability of sleep disorders centers in the military, and even financial incentives for those undergoing a disability evaluation.
Obstructive sleep apnea is significantly more common in patients with PTSD compared with that in the general population, with rates of OSA ranging from 11.9% to 90%, depending on the study.41-43 Prevalence rates for OSA have been reported in several PTSD populations (violent crime, sexual assault, disasters, and combat). Military studies evaluating recent veterans have found OSA rates between 35% and 67%.44-46 In a recent study looking at SDB in those with PTSD, 53.8% had OSA (67.3% among those with polysomnograms).47 Although the other studies evaluated mixed populations of recent combat veterans, they were enriched for patients with PTSD.
Sleep disorders and PTSD have a “bidirectional” relationship.48 Sleep complaints preceding or temporally related to traumatic events increase the likelihood of subsequent mental health disorders, including PTSD.49-51 Sleep disorders are common in PTSD and are associated with symptoms of depression, relapse of depression, greater reductions in QOL, and suicide.52 Higher rates of OSA among patients who are not physically injured compared with the OSA rates of those with PTSD who also had physical injury (72.9% vs 38%) have also been seen, raising the possibility of different phenotypes of combat-related PTSD and a hypothetical role for premorbid OSA as a risk factor for PTSD.47
The pathophysiology linking SDB and PTSD is based on theories that poor sleep quality limits the ability to manage stress, promotes hyperarousal, confounds environmental stressors (trauma), and hinders the restorative qualities of sleep.49 Rapid eye movement sleep is believed to consolidate emotional memory, which may assist in recovery from traumatic events.53,54 Disrupted sleep architecture from OSA can diminish REM and hinder this process. Sleep fragmentation has been shown to cause upper airway instability and promote SDB.55 In addition, nighttime anxiety may induce hyperventilation with resultant hypocapnia, triggering apneic events.56 Taken together, disrupted sleep architecture, hyperarousal, respiratory instability, and nightmares may exacerbate one another and create a vicious cycle.57
Untreated OSA is associated with worse outcomes in PTSD. Continuous positive airway pressure has been shown to improve symptoms in this group.58-60 A study by Tamanna and colleagues evaluated clinical outcomes related to CPAP use, demonstrating improvements in nightmares, daytime sleepiness, and PTSD symptom severity with increasing adherence.61 Unfortunately, patients with PTSD generally have suboptimal medical adherence, and CPAP adherence decreases in psychiatric disease.62,63 Two recent studies have shown significantly lessened adherence in patients with both PTSD and OSA (compared with OSA alone), in both younger and older veteran populations.64,65 Limited insight and atypical clinical presentations of OSA also limit patient acceptance of treatment. Continuous positive airway pressure usage is decreased by comorbid insomnia, common in PTSD.66 Similarly, nightmares, mask discomfort, air hunger (the feeling of not being able to get a satisfying breath), and claustrophobia have all been associated with poor CPAP adherence in patients with PTSD.
Continuous positive airway pressure adherence is determined early (days to weeks), and initial use predicts long-term adherence.67-70 Patients are most likely to abandon therapy or fail to initiate therapy during this period. Given the potential adverse outcomes of comorbid mental illness and sleep disorders, including suicide, interventions should begin early.71 Continuous positive airway pressure devices with heated humidification, group education, peer success stories, and telephonic follow-up are all methods that improve adherence.72 There is conflicting evidence regarding the efficacy of nonbenzodiazepine sedative- hypnotics for improving diagnostic accuracy and CPAP adherence.73-76
Related: Using Light to Manage Sleep-Wake Issues in Patients With Dementia
Given this population’s high rate of comorbid insomnia, polypharmacy, and potentially pharmacotherapy refractory insomnia, the approach should be used cautiously in patients with PTSD OSA.77 Emerging efforts incorporate a biopsychosocial approach with an individualized focus on a patient’s unique barriers to adherence. Incorporating approaches such as motivational enhancement (for those ambivalent about change), educational approaches, and CBT may all be useful adjuncts.78-80
Ongoing VA trials have been designed to evaluate the impact of CPAP therapy on symptoms of PTSD and to compare CPAP and mandibular advancement devices with regards to efficacy in reducing the apneas and/or hypopneas per hour of sleep and improving symptoms.81,82
Discussion
Service members, like most adults, need about 8 hours of quality sleep per night to function at optimal levels and maximize operational readiness. The medical community is increasingly recognizing that sleep disturbances are inextricably linked to psychiatric disorders, particularly PTSD, depression, and anxiety.83,84 Balancing occupational performance and the demand of military missions with service member health remains a difficult leadership challenge.
Recent evidence suggests that disordered sleep may precede other PTSD symptom clusters.43,85 Sleep architecture in PTSD is disrupted, and abnormalities in both REM and non-REM sleep have been described.86,87 Insomnia not only is a component of depressive and anxiety disorders, but also impacts the course of disease severity.88 Sleep deprivation has been shown to be a risk factor for major depression in adolescents.89 In those with comorbid sleep problems, PTSD, and TBI, each disorder worsens QOL in an additive fashion.90
Severe mental illness impacts the military through a service member’s lost workdays, decreased productivity, impaired social relationships, and even suicide. Given that sleep quality is related to outcomes for patients with mental illnesses, access to medical professionals with specific training in sleep disorders becomes an integral part of a multidisciplinary approach to military health care. Encouragingly, treatment of insomnia and nightmares has been shown to improve PTSD symptom severity as well as headaches in veterans with mild TBI, even if neurologic deficits remain static.91 Similarly, treatment of insomnia is known to improve depressive symptoms in those with comorbid conditions.
Conclusion
The importance of sleep as a combat multiplier is increasingly recognized. The U.S. Army Surgeon General has acknowledged the interplay between inadequate sleep and impairments in other functional areas and placed specific emphasis on sleep as part of the Army Performance Triad. A core tenant of the Army Surgeon General’s message is that army medicine is on a mission to transform from a health care system to a system for health. The Army Wellness Centers, Army Medical Homes, Soldier-Centered Medical Homes, and embedded behavioral health are supporting the health of the force in these capacities. These functional areas treat behavioral health and sleep-related concerns across the continuum of disease from prevention, timely initial intervention once a condition has been identified, long-term treatment programs, and rehabilitative services.
Getting the proper quantity and quality of sleep, in addition to healthy activity and nutrition, increases readiness so that when called on to perform, soldiers are ready. A recent article by Wesensten and Balkin from the Walter Reed Army Institute of Research summarizes some guidelines for sleep from the Army Performance Triad Working Group to include sleep hygiene tips and judicious use of naps and caffeine.92 Efforts to improve soldier resiliency by improving sleep-related disorders have yet to be studied in a meaningful way, so additional research is needed to determine best practices and evidence-based guidelines.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
2. U.S. Department of the Army. Combat and Operational Stress Control Manual for Leaders and Soldiers. Washington, DC: Government Printing Office; 2009. FM 6-22.5.
3. Williams SG, Collen J, Wickwire E, Lettieri CJ, Mysliwiec V. The impact of sleep on soldier performance. Curr Psychiatry Rep. 2014;16(8):459.
4. Miller NL, Shattuck LG, Tvaryanas AP, Matsangas P. Effects of Sleep on Training Effectiveness in Soldiers at Fort Leonard Wood, Missouri. Monterey, CA: Naval Post Graduate School; 2010.
5. Miller NL, Shattuck LG, Matsangas P. Longitudinal study of sleep patterns of United States Military Academy cadets. Sleep. 2010;33(12):1623-1631.
6. Barlas FM, Higgins WB, Pflieger JC, Diecker K. 2011 Health Related Behaviors Survey of Active Duty Military Personnel. Fairfax, VA: Department of Defense; 2013.
7. Caldwell JL, Gilreath SR. Work and sleep hours of U.S. Army aviation personnel working reverse cycle. Mil Med. 2001;166(2):159-166.
8. Mental Health Advisory Team 9. Mental Health Advisory Team 9 (MHAT 9), Operation Enduring Freedom (OEF) 2013 Afghanistan. Army Medicine Website. http://armymedicine.mil/Documents /MHAT_9_OEF_Report.pdf. Published October 10, 2013. Accessed March 3, 2015.
9. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117-126.
10. Czeisler CA. The Gordon Wilson lecture: work hours, sleep and patient safety in residency training. Trans Am Clin Climatol Assoc. 2006;117:159-188.
11. Krakow B, Melendrez D, Pedersen B, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49(11): 948-953.
12. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;229(suppl 2):S347-S353.
13. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479-1484.
14. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541-553.
15. Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4): 227-247.
16. Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213-218.
17. Sjöström N, Waern M, Hetta J. Nightmares and sleep disturbances in relation to suicidality in suicide attempters. Sleep. 2007;30(1):91-95.
18. Armed Forces Health Surveillance Center. Insomnia, Active Component, U.S. Armed Forces, January 2000-December 2009 Medical Surveillance Monthly Report. 2010;17(5):12-15.
19. McLay RN, Klam WP, Volkert SL. Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in U.S. service members returning from military deployments. Mil Med. 2010;175(10):759-762.
20. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
21. Manber R, Edinger JD, Gress JL, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489-495.
22. Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327-341.
23. Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61(6):947-956.
24. Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. 2011;49(4):227-233.
25. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
26. Epstein DR, Babcock-Parziale JL, Haynes PL, Herb CA. Insomnia treatment acceptability and p of male Iraq and Afghanistan combat veterans and their healthcare providers. J Rehabil Res Dev. 2012;49(6):867-878.
27. Joya FL, Kripke DF, Loving RT, Dawson A, Kline LE. Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. J Clin Sleep Med. 2009;5(4):377-383.
28. Farkas RH, Unger EF, Temple R. Zolpidem and driving impairment—identifying persons at risk. N Engl J Med. 2013;369(8):689-691.
29. Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1-6.
30. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: A matched cohort study. BMJ Open. 2012;2:e000850.
31. Karlin BE, Trockel M, Taylor CB, Gimeno J, Manber R. National dissemination of cognitive behavioral therapy for insomnia in veterans: therapist- and patient-level outcomes. J Consult Clin Psychol. 2013;81(5):912-917.
32. Aurora RN, Zak RS, Auerbach SH, et al; Standards of Practice Committee, American Academy of Sleep Medicine. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
33. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
34. Wallace DM, Shafazand S, Ramos AR, et al. Insomnia characteristics and clinical correlates in Operation Enduring Freedom/Operation Iraqi Freedom veterans with post-traumatic stress disorder and mild traumatic brain injury: an exploratory study. Sleep Med. 2011;12(9):850-859.
35. Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. CCJM. 1990;57(suppl):S9-S23.
36. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(12):3494-3509.
37. Provini F, Piazzi G, Tinuper P, et al. Nocturnal frontal lobe epilepsy: a clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122(6):1017-1031.
38. Mysliwiec V, O’Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares and rem without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143-1148.
39. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
40. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-1235.
41. Krakow B, Haynes PL, Warner TD, et al. Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress. 2004;17(3):257-268.
42. Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405-1411.
43. Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169-184.
44. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
45. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US Military Personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
46. Mysliwiec V, McGraw L, Pierce R, et al. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
47. Williams SG, Collen J, Orr N, Holley AB, Lettieri CJ. Sleep disorders in combat-related PTSD. Sleep Breath. 2015;19(1):175-182.
48. Krakow B, Melendrez D, Warner TD, et al. To breathe, perchance to sleep: sleep-disordered breathing and chronic insomnia among trauma survivors. Sleep Breath. 2002;6(4):189-202.
49. Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69-74.
50. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
51. Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, Moore D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol. 2011;67(12):1240-1258.
52. Pittman JO, Goldsmith AA, Lemmer JA, Kilmer MT, Baker DG. Post-traumatic stress disorder, depression, and health-related quality of life in OEF/OIF veterans. Quality of Life Res. 2012;21(1):99-103.
53. Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696-1701.
54. Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8(2):112-119.
55. Sériès F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485.
56. Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825-1832.
57. van Liempt S. Sleep disturbances and PTSD: a perpetual circle? Eur J Psychotraumatology. 2012;3.
58. Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry. 2007;68(8):1257-1270.
59. Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291-298.
60. Sateia MJ. Update on sleep and psychiatric disorders. Chest. 2009;135(5):1370-1379.
61. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
62. Lockwood A, Steinke DT, Botts SR. Medication adherence and its effect on relapse among patients discharged from a Veterans Affairs posttraumatic stress disorder treatment program. Ann Pharmacother. 2009;43(7):1227-1232.
63. Means MK, Ulmer CS, Edinger JD. Ethnic differences in continuous positive airway pressure (CPAP) adherence in veterans with and without psychiatric disorders. Behav Sleep Med. 2010;8(4):260-273.
64. El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495-1500.
65. Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667-672.
66. Wickwire EM, Smith MT, Birnbaum S, Collop NA. Sleep maintenance insomnia complaints predict poor CPAP adherence: a clinical case series. Sleep Med. 2010;11(8):772-776.
67. Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5(3):229-240.
68. Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320-324.
69. Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27(1):134-138.
70. Pépin JL, Krieger J, Rodenstein D, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999;160(4):1124-1129.
71. Ribeiro JD, Pease JL, Gutierrez PM, et al. Sleep problems outperform depression and hopelessness as cross-sectional and longitudinal predictors of suicidal ideation and behavior in young adults in the military. J Affect Disord. 2012;136(3):743-750.
72. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343-356.
73. Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest. 2006;130(5):1369-1376.
74. Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136(5):1263-1268.
75. Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep. 2008;31(9):1310-1316.
76. Lettieri CJ, Shah AA, Holley AB, et al. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151(10):696-702.
77. Mysliwiec V, Roth B. Pharmacotherapy refractory insomnia in soldiers with traumatic brain injury. Chest. 2013;143(2):582-583.
78. Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: A randomized controlled trial. Sleep. 2013;36(11):1655-1662.
79. Bartlett D, Wong K, Richards D, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep. 2013;36(11):1647-1654.
80. Dickerson SS, Obeidat R, Dean G, et al. Development and usability testing of a self-management intervention to support individuals with obstructive sleep apnea in accommodating to CPAP treatment. Heart Lung. 2013;42(5):346-352.
81. Effects of CPAP therapy on PTSD symptoms. ClinicalTrials.gov Identifier NCT02019914. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT02019914. Updated September 3, 2014. Accessed March 4, 2015.
82. A randomized cross over trial of two treatments for obstructive sleep apnea in veterans with post-traumatic stress disorder. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT01535586. Updated February 16, 2012. Accessed March 4, 2015.
83. Babson KA, Boden MT, Woodward S, Alvarez J, Bonn-Miller M. Anxiety sensitivity and sleep quality: independent and interactive predictors of posttraumatic stress disorder symptoms. J Nerv Ment Dis. 2013;201(1):48-51.
84. Belleville G, Guay S, Marchand A. Impact of sleep disturbances on PTSD symptoms and perceived health. J Nerv Ment Dis. 2009;197(2):126-132.
85. van Liempt S, van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety. 2013;30(5):469-474.
86. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
87. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382.
88. Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9-15.
89. Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37(2):239-244.
90. Macera CA, Aralis HJ, Rauh MJ, MacGregor AJ. Do sleep problems mediate the relationship between traumatic brain injury and development of mental health symptoms after deployment? Sleep. 2013;36(1):83-90.
91. Ruff RL, Riechers RG, Wang XF et al. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. 2012;49(9):1305-1320.
92. Wesensten NJ, Balkin TJ. The challenge of sleep management in military operations. US Army Med Dep J. 2013;109-118.
1. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335-352.
2. U.S. Department of the Army. Combat and Operational Stress Control Manual for Leaders and Soldiers. Washington, DC: Government Printing Office; 2009. FM 6-22.5.
3. Williams SG, Collen J, Wickwire E, Lettieri CJ, Mysliwiec V. The impact of sleep on soldier performance. Curr Psychiatry Rep. 2014;16(8):459.
4. Miller NL, Shattuck LG, Tvaryanas AP, Matsangas P. Effects of Sleep on Training Effectiveness in Soldiers at Fort Leonard Wood, Missouri. Monterey, CA: Naval Post Graduate School; 2010.
5. Miller NL, Shattuck LG, Matsangas P. Longitudinal study of sleep patterns of United States Military Academy cadets. Sleep. 2010;33(12):1623-1631.
6. Barlas FM, Higgins WB, Pflieger JC, Diecker K. 2011 Health Related Behaviors Survey of Active Duty Military Personnel. Fairfax, VA: Department of Defense; 2013.
7. Caldwell JL, Gilreath SR. Work and sleep hours of U.S. Army aviation personnel working reverse cycle. Mil Med. 2001;166(2):159-166.
8. Mental Health Advisory Team 9. Mental Health Advisory Team 9 (MHAT 9), Operation Enduring Freedom (OEF) 2013 Afghanistan. Army Medicine Website. http://armymedicine.mil/Documents /MHAT_9_OEF_Report.pdf. Published October 10, 2013. Accessed March 3, 2015.
9. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117-126.
10. Czeisler CA. The Gordon Wilson lecture: work hours, sleep and patient safety in residency training. Trans Am Clin Climatol Assoc. 2006;117:159-188.
11. Krakow B, Melendrez D, Pedersen B, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49(11): 948-953.
12. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;229(suppl 2):S347-S353.
13. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479-1484.
14. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541-553.
15. Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4): 227-247.
16. Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213-218.
17. Sjöström N, Waern M, Hetta J. Nightmares and sleep disturbances in relation to suicidality in suicide attempters. Sleep. 2007;30(1):91-95.
18. Armed Forces Health Surveillance Center. Insomnia, Active Component, U.S. Armed Forces, January 2000-December 2009 Medical Surveillance Monthly Report. 2010;17(5):12-15.
19. McLay RN, Klam WP, Volkert SL. Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in U.S. service members returning from military deployments. Mil Med. 2010;175(10):759-762.
20. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
21. Manber R, Edinger JD, Gress JL, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489-495.
22. Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327-341.
23. Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61(6):947-956.
24. Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. 2011;49(4):227-233.
25. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
26. Epstein DR, Babcock-Parziale JL, Haynes PL, Herb CA. Insomnia treatment acceptability and p of male Iraq and Afghanistan combat veterans and their healthcare providers. J Rehabil Res Dev. 2012;49(6):867-878.
27. Joya FL, Kripke DF, Loving RT, Dawson A, Kline LE. Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. J Clin Sleep Med. 2009;5(4):377-383.
28. Farkas RH, Unger EF, Temple R. Zolpidem and driving impairment—identifying persons at risk. N Engl J Med. 2013;369(8):689-691.
29. Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1-6.
30. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: A matched cohort study. BMJ Open. 2012;2:e000850.
31. Karlin BE, Trockel M, Taylor CB, Gimeno J, Manber R. National dissemination of cognitive behavioral therapy for insomnia in veterans: therapist- and patient-level outcomes. J Consult Clin Psychol. 2013;81(5):912-917.
32. Aurora RN, Zak RS, Auerbach SH, et al; Standards of Practice Committee, American Academy of Sleep Medicine. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
33. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
34. Wallace DM, Shafazand S, Ramos AR, et al. Insomnia characteristics and clinical correlates in Operation Enduring Freedom/Operation Iraqi Freedom veterans with post-traumatic stress disorder and mild traumatic brain injury: an exploratory study. Sleep Med. 2011;12(9):850-859.
35. Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. CCJM. 1990;57(suppl):S9-S23.
36. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(12):3494-3509.
37. Provini F, Piazzi G, Tinuper P, et al. Nocturnal frontal lobe epilepsy: a clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122(6):1017-1031.
38. Mysliwiec V, O’Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares and rem without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143-1148.
39. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
40. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-1235.
41. Krakow B, Haynes PL, Warner TD, et al. Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress. 2004;17(3):257-268.
42. Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405-1411.
43. Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169-184.
44. Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142(3):622-630.
45. Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US Military Personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549-557.
46. Mysliwiec V, McGraw L, Pierce R, et al. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174.
47. Williams SG, Collen J, Orr N, Holley AB, Lettieri CJ. Sleep disorders in combat-related PTSD. Sleep Breath. 2015;19(1):175-182.
48. Krakow B, Melendrez D, Warner TD, et al. To breathe, perchance to sleep: sleep-disordered breathing and chronic insomnia among trauma survivors. Sleep Breath. 2002;6(4):189-202.
49. Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69-74.
50. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009-1018.
51. Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, Moore D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol. 2011;67(12):1240-1258.
52. Pittman JO, Goldsmith AA, Lemmer JA, Kilmer MT, Baker DG. Post-traumatic stress disorder, depression, and health-related quality of life in OEF/OIF veterans. Quality of Life Res. 2012;21(1):99-103.
53. Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696-1701.
54. Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8(2):112-119.
55. Sériès F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485.
56. Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825-1832.
57. van Liempt S. Sleep disturbances and PTSD: a perpetual circle? Eur J Psychotraumatology. 2012;3.
58. Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry. 2007;68(8):1257-1270.
59. Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291-298.
60. Sateia MJ. Update on sleep and psychiatric disorders. Chest. 2009;135(5):1370-1379.
61. Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA). J Clin Sleep Med. 2014;10(6):631-636.
62. Lockwood A, Steinke DT, Botts SR. Medication adherence and its effect on relapse among patients discharged from a Veterans Affairs posttraumatic stress disorder treatment program. Ann Pharmacother. 2009;43(7):1227-1232.
63. Means MK, Ulmer CS, Edinger JD. Ethnic differences in continuous positive airway pressure (CPAP) adherence in veterans with and without psychiatric disorders. Behav Sleep Med. 2010;8(4):260-273.
64. El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495-1500.
65. Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667-672.
66. Wickwire EM, Smith MT, Birnbaum S, Collop NA. Sleep maintenance insomnia complaints predict poor CPAP adherence: a clinical case series. Sleep Med. 2010;11(8):772-776.
67. Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5(3):229-240.
68. Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320-324.
69. Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27(1):134-138.
70. Pépin JL, Krieger J, Rodenstein D, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999;160(4):1124-1129.
71. Ribeiro JD, Pease JL, Gutierrez PM, et al. Sleep problems outperform depression and hopelessness as cross-sectional and longitudinal predictors of suicidal ideation and behavior in young adults in the military. J Affect Disord. 2012;136(3):743-750.
72. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343-356.
73. Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest. 2006;130(5):1369-1376.
74. Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136(5):1263-1268.
75. Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep. 2008;31(9):1310-1316.
76. Lettieri CJ, Shah AA, Holley AB, et al. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151(10):696-702.
77. Mysliwiec V, Roth B. Pharmacotherapy refractory insomnia in soldiers with traumatic brain injury. Chest. 2013;143(2):582-583.
78. Aloia MS, Arnedt JT, Strand M, Millman RP, Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: A randomized controlled trial. Sleep. 2013;36(11):1655-1662.
79. Bartlett D, Wong K, Richards D, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep. 2013;36(11):1647-1654.
80. Dickerson SS, Obeidat R, Dean G, et al. Development and usability testing of a self-management intervention to support individuals with obstructive sleep apnea in accommodating to CPAP treatment. Heart Lung. 2013;42(5):346-352.
81. Effects of CPAP therapy on PTSD symptoms. ClinicalTrials.gov Identifier NCT02019914. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT02019914. Updated September 3, 2014. Accessed March 4, 2015.
82. A randomized cross over trial of two treatments for obstructive sleep apnea in veterans with post-traumatic stress disorder. ClinicalTrials.gov Website. https://clinicaltrials.gov/ct2/show/NCT01535586. Updated February 16, 2012. Accessed March 4, 2015.
83. Babson KA, Boden MT, Woodward S, Alvarez J, Bonn-Miller M. Anxiety sensitivity and sleep quality: independent and interactive predictors of posttraumatic stress disorder symptoms. J Nerv Ment Dis. 2013;201(1):48-51.
84. Belleville G, Guay S, Marchand A. Impact of sleep disturbances on PTSD symptoms and perceived health. J Nerv Ment Dis. 2009;197(2):126-132.
85. van Liempt S, van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety. 2013;30(5):469-474.
86. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
87. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372-382.
88. Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9-15.
89. Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37(2):239-244.
90. Macera CA, Aralis HJ, Rauh MJ, MacGregor AJ. Do sleep problems mediate the relationship between traumatic brain injury and development of mental health symptoms after deployment? Sleep. 2013;36(1):83-90.
91. Ruff RL, Riechers RG, Wang XF et al. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. 2012;49(9):1305-1320.
92. Wesensten NJ, Balkin TJ. The challenge of sleep management in military operations. US Army Med Dep J. 2013;109-118.
Referral for CT Pulmonary Angiography
Approximately 10 million patients present to emergency departments each year with symptoms raising concern of thromboembolism (VTE).1 The current gold standard for diagnosis of VTE is pulmonary angiography.2 As this study is invasive, alternative imaging protocols have been sought. CTPA, when combined with measures of pretest probability, equals or surpasses the ability of pulmonary angiography to detect VTE and can improve the ability of clinicians to rule out VTE.38 In a study of 930 patients, application of clinical rules in addition to D‐dimer testing decreased the number of CTPAs ordered by 50%.9 One of the most common clinical rule sets is the Wells Score, which relies on historical features related to the risk of DVT/VTE and physical examination findings.10, 11
Other institutions have demonstrated an increase in the number of CTPAs ordered and VTE diagnoses since the study became widely available.13 Based on the observation of an increasing number of CTPAs ordered at our institution without an increase in the number of VTEs diagnosed, we aimed to ascertain the physician ordering practices for CTPA. We hypothesized that CTPAs were ordered at a greater frequency in a low‐risk population because an institutional clinical algorithm was lacking.
METHODS
Charts of all patients aged 18‐100 with CTPA ordered to rule out acute VTE were retrospectively examined. A Simplified Wells Score was applied using only the information available to the ordering physician at the time the CTPA was performed. Patients were stratified by their Simplified Wells Score to low (0‐1 points), intermediate (2‐6 points), or high (>6 points) pretest clinical probability. A D‐dimer value, if ordered, was used to further stratify patients based on a positive or negative result. The official radiologic report of the CTPA was used to determine the rate of VTE diagnosis for the study population.
RESULTS
Three hundred and ninety‐four patients were referred for CTPA (Fig. 1). Two hundred and seventy‐nine had adequate clinical data to calculate a Simplified Wells Score and were included in the study. Of the 279 studies included, 75% were ordered through the emergency department and 25% from inpatient services (Table 1). The study patients were stratified according to the Simplified Wells criteria: 184 patients (66%) had low clinical probability, 91 (33%) had intermediate clinical probability, and 4 (1%) had high clinical probability. Nineteen (7%) patients had a history of DVT or VTE, and 28 (10%) had a history of active cancer at the time of their CTPA. One hundred and twenty‐five of the 279 patients had a D‐dimer performed (Fig. 2). One hundred and eight were positive, and 17 were negative. Of the 17 patients who had a negative D‐dimer and underwent CTPA testing, none were diagnosed with VTE. Eighty‐three low‐clinical‐probability patients underwent CTPA without D‐dimer testing, 4 of whom were diagnosed with VTE.
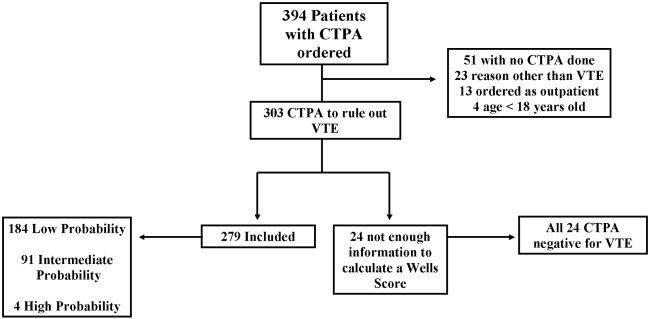
| Low (n = 184) | Intermediate (n = 91) | High (n = 4) | Total (n = 279) | |
|---|---|---|---|---|
| Age (years), mean | 52 | 59 | 62 | 58 |
| Male, n (%) | 82 (45) | 44 (48) | 1 (25) | 127 (46) |
| Female, n (%) | 102 (55) | 47 (52) | 3 (75) | 152 (54) |
| Emergency department, n (%) | 150 (82) | 47 (52) | 4 (100) | 225 (75) |
| Medical, n (%) | 22 (12) | 18 (20) | 0 | 40 (13) |
| Surgical, n (%) | 9 (5) | 14 (15) | 0 | 23 (7) |
| ICU, n (%) | 3 (1) | 12 (13) | 0 | 15 (5) |
| Wells Score, mean | 0.72 | 3.4 | 7.8 | 1.6 |
| D‐dimer performed, n (%) | 101 (55) | 21 (23) | 3 (75) | 125 (45) |
| D‐dimer positive, n (%) | 89 (88) | 16 (76) | 3 (100) | 108 (86) |
| D‐dimer negative, n (%) | 12 (12) | 5 (24) | 0 | 17 (14) |
| CTPA positive, n (%) | 8 (4) | 11 (12) | 1 (25) | 20 (7) |
| CPTA negative, n (%) | 176 (96) | 80 (88) | 3 (75) | 259 (93) |
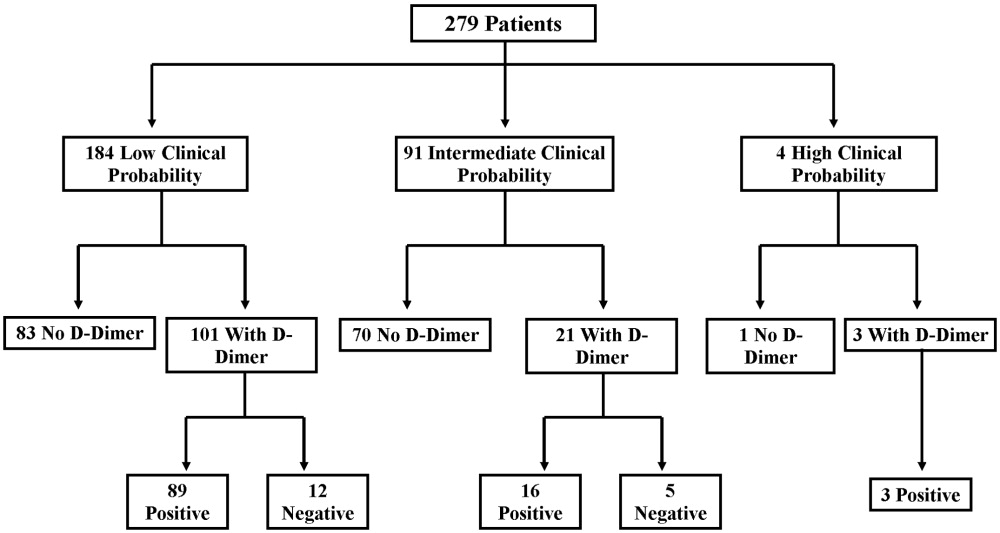
There were 20 positive CTPAs in the study group (Fig. 3). Review of the records for 3 months after the study of patients whose CTPA was negative disclosed no diagnoses of VTE by other modalities. VTE was diagnosed in 4% of patients in the low‐clinical‐probability group, 12% in the intermediate‐clinical‐probability group, and 25% in the high‐clinical‐probability group. The overall positive CTPA rate was 7.2%.
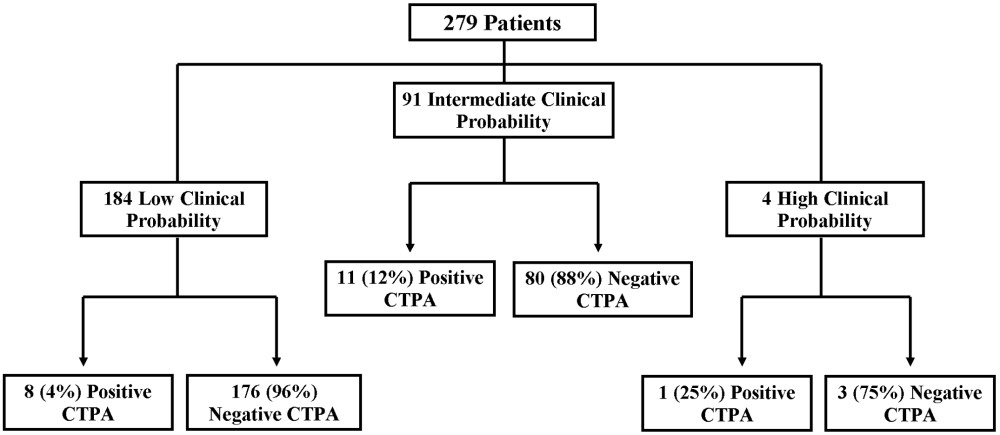
DISCUSSION
Many studies have examined the application of clinical rule sets in addition to D‐dimer testing and CTPA to exclude acute VTE.39 Most of these studies have shown that the use of an algorithm is safe and frequently reduces referral for CTPA in low‐clinical‐probability patients. However, others have noted that some physicians do not routinely use validated algorithms when making decisions related to patient evaluation.13 Our rate of positive CTPA was low compared with rates reported in the literature.3, 14 We believe the most likely explanation is the large number of low‐clinical‐probability patients who underwent CTPA, possibly because providers do not routinely use a validated clinical algorithm.
When our patient population was risk stratified by Simplified Wells criteria and compared with similar data from published studies, we had a much higher proportion of patients classified as low clinical probability.7, 8, 15 The low‐clinical‐probability group's mean Simplified Wells Score was 0.71; one‐third had a Simplified Wells Score of 0. This reflects a low‐risk population for VTE, supported by the low prevalence of prior DVT/VTE and active cancer in our population.4, 10 The rationale for referring patients with so few risk factors for CTPA is unclear. It is possible that providers used CTPA to evaluate symptoms not clearly explained and obtained the study to look for other diagnoses in addition to VTE. By not applying a clinical algorithm, very‐low‐risk patients underwent CTPA, increasing the number of negative studies and decreasing the overall positive rate.
Not using a clinical algorithm also resulted in indiscriminate D‐dimer testing. There were 83 patients risk‐stratified as low clinical probability who did not have a D‐dimer prior to undergoing CTPA. Some of these patients may well have had a negative D‐dimer, requiring no further workup to rule out VTE. Seventeen patients had a negative D‐dimer and still underwent CTPA; all these patients were negative for VTE. These aberrations likely occurred from unfamiliarity with use of the D‐dimer test or doubts about its ability to reliably exclude VTE. Appropriate application of D‐dimer testing could have decreased the number of CTPAs ordered and increased our overall rate of positive VTE diagnosis.
Perrier et al., Brown et al., and Kelly and Wells all describe different methods of introducing clinical algorithms to aid the diagnosis of VTE.46, 9 All agree that patients should be risk stratified by pretest clinical probability, and low‐probability patients should undergo intermediate testing with D‐dimer prior to CTPA. Implementation of a similar clinical algorithm at our facility would likely decrease the number of CTPAs ordered. If all patients presenting at our facility with signs and symptoms raising concern for VTE were first risk‐stratified by pretest clinical probability, and all low‐probability patients underwent highly‐sensitive D‐dimer testing as an initial step, fewer CTPAs would be performed on low‐probability patients. The largest group of patients in our study were low probability; therefore, decreasing CTPA in this group could have a significant effect on our institution.
The retrospective nature of our study resulted in the following limitations. It is impossible to determine how the ordering provider viewed the patient's pretest probability. In most of the medical records, a pretest clinical probability was not documented. We attempted to validate the ordering provider's decision by being as generous as possible in applying points to the Wells Score. For example, if a patient had a remote history of cancer and the ordering provider documented this as a risk factor for VTE, the point value for cancer was given even though the Wells Score has a much narrower definition of this category.10 This practice favors assigning patients a potentially higher clinical probability and may have increased the number of patients designated as intermediate and high clinical probability in our study.
Our hospital primarily relies on CTPA with lower extremity venogram as the diagnostic test for VTE. Indeterminate tests may have occurred and thus falsely lowered the number of VTEs diagnosed. However, no patient with a negative CTPA was diagnosed with VTE by any modality in the 3 months after their initial study at our institution; a diagnosis of VTE could have been made at another hospital. The Simplified Wells Score uses both objective and subjective components to arrive at a point total. Our results might be different if newer algorithms, such as the Revised Geneva Score,16 which relies only on objective measurements, had been used.
CONCLUSIONS
The reliance on CTPA alone to exclude a potentially life‐threatening illness without additional risk stratification or clinical information leads to overuse of this test in patients with very low to no clinical risk for VTE and a low rate of diagnosed VTE. Implementation of a clinical algorithm for the diagnosis of suspected VTE may eliminate the need for many CTPAs, improving the yield of this test without compromising patient safety, especially at institutions with a low prevalence of PE.
Acknowledgements
The authors thank Dr. John Rinard, DO, for assistance with initial editing of the abstract and Troy Patience for his assistance with statistical analysis.
- ,,, et al.Impact of a rapid‐rule out protocol for pulmonary embolism on the rate of screening, missed cases and pulmonary vascular imaging in an urban US emergency department.Ann Emerg Med.2004;44:490–502.
- ,,, et al.ATS 1999 Clinical practice guideline for the diagnostic approach to acute venous thromboembolism.Am J Respir Crit Care Med.1999;160:1043–1066.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism.JAMA.2005;293:2012–2017.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,.An emergency department guideline for the diagnosis of pulmonary embolism: an outcome study.Acad Emerg Med.2005;12:20–25.
- ,.A clinical probability assessment and D‐dimer measurement should be the initial step in the investigation of suspected venous thromboembolism.Chest.2003;124:1116–1119.
- ,,, et al.Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44:503–510.
- ,.External validation and comparison of recently described prediction rules for suspected pulmonary embolism.Curr Opin Pulm Med.2004;10:345–349.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D‐dimer.Ann Intern Med.2001;135:98–107.
- ,,, et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- ,,, et al.Assessing the clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161:92–97.
- ,,, et al.The effect of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- Simplifying the evaluation of pulmonary embolism.Chest.2006:129:1400–1401.
- ,,, et al.Meta‐Analysis: Outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,,, et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354:2317–2327.
- ,,, et al.Prediction of pulmonary embolism in the emergency department: the revised Geneva score.Ann Intern Med.2006;144(3):165–171.
Approximately 10 million patients present to emergency departments each year with symptoms raising concern of thromboembolism (VTE).1 The current gold standard for diagnosis of VTE is pulmonary angiography.2 As this study is invasive, alternative imaging protocols have been sought. CTPA, when combined with measures of pretest probability, equals or surpasses the ability of pulmonary angiography to detect VTE and can improve the ability of clinicians to rule out VTE.38 In a study of 930 patients, application of clinical rules in addition to D‐dimer testing decreased the number of CTPAs ordered by 50%.9 One of the most common clinical rule sets is the Wells Score, which relies on historical features related to the risk of DVT/VTE and physical examination findings.10, 11
Other institutions have demonstrated an increase in the number of CTPAs ordered and VTE diagnoses since the study became widely available.13 Based on the observation of an increasing number of CTPAs ordered at our institution without an increase in the number of VTEs diagnosed, we aimed to ascertain the physician ordering practices for CTPA. We hypothesized that CTPAs were ordered at a greater frequency in a low‐risk population because an institutional clinical algorithm was lacking.
METHODS
Charts of all patients aged 18‐100 with CTPA ordered to rule out acute VTE were retrospectively examined. A Simplified Wells Score was applied using only the information available to the ordering physician at the time the CTPA was performed. Patients were stratified by their Simplified Wells Score to low (0‐1 points), intermediate (2‐6 points), or high (>6 points) pretest clinical probability. A D‐dimer value, if ordered, was used to further stratify patients based on a positive or negative result. The official radiologic report of the CTPA was used to determine the rate of VTE diagnosis for the study population.
RESULTS
Three hundred and ninety‐four patients were referred for CTPA (Fig. 1). Two hundred and seventy‐nine had adequate clinical data to calculate a Simplified Wells Score and were included in the study. Of the 279 studies included, 75% were ordered through the emergency department and 25% from inpatient services (Table 1). The study patients were stratified according to the Simplified Wells criteria: 184 patients (66%) had low clinical probability, 91 (33%) had intermediate clinical probability, and 4 (1%) had high clinical probability. Nineteen (7%) patients had a history of DVT or VTE, and 28 (10%) had a history of active cancer at the time of their CTPA. One hundred and twenty‐five of the 279 patients had a D‐dimer performed (Fig. 2). One hundred and eight were positive, and 17 were negative. Of the 17 patients who had a negative D‐dimer and underwent CTPA testing, none were diagnosed with VTE. Eighty‐three low‐clinical‐probability patients underwent CTPA without D‐dimer testing, 4 of whom were diagnosed with VTE.

| Low (n = 184) | Intermediate (n = 91) | High (n = 4) | Total (n = 279) | |
|---|---|---|---|---|
| Age (years), mean | 52 | 59 | 62 | 58 |
| Male, n (%) | 82 (45) | 44 (48) | 1 (25) | 127 (46) |
| Female, n (%) | 102 (55) | 47 (52) | 3 (75) | 152 (54) |
| Emergency department, n (%) | 150 (82) | 47 (52) | 4 (100) | 225 (75) |
| Medical, n (%) | 22 (12) | 18 (20) | 0 | 40 (13) |
| Surgical, n (%) | 9 (5) | 14 (15) | 0 | 23 (7) |
| ICU, n (%) | 3 (1) | 12 (13) | 0 | 15 (5) |
| Wells Score, mean | 0.72 | 3.4 | 7.8 | 1.6 |
| D‐dimer performed, n (%) | 101 (55) | 21 (23) | 3 (75) | 125 (45) |
| D‐dimer positive, n (%) | 89 (88) | 16 (76) | 3 (100) | 108 (86) |
| D‐dimer negative, n (%) | 12 (12) | 5 (24) | 0 | 17 (14) |
| CTPA positive, n (%) | 8 (4) | 11 (12) | 1 (25) | 20 (7) |
| CPTA negative, n (%) | 176 (96) | 80 (88) | 3 (75) | 259 (93) |

There were 20 positive CTPAs in the study group (Fig. 3). Review of the records for 3 months after the study of patients whose CTPA was negative disclosed no diagnoses of VTE by other modalities. VTE was diagnosed in 4% of patients in the low‐clinical‐probability group, 12% in the intermediate‐clinical‐probability group, and 25% in the high‐clinical‐probability group. The overall positive CTPA rate was 7.2%.

DISCUSSION
Many studies have examined the application of clinical rule sets in addition to D‐dimer testing and CTPA to exclude acute VTE.39 Most of these studies have shown that the use of an algorithm is safe and frequently reduces referral for CTPA in low‐clinical‐probability patients. However, others have noted that some physicians do not routinely use validated algorithms when making decisions related to patient evaluation.13 Our rate of positive CTPA was low compared with rates reported in the literature.3, 14 We believe the most likely explanation is the large number of low‐clinical‐probability patients who underwent CTPA, possibly because providers do not routinely use a validated clinical algorithm.
When our patient population was risk stratified by Simplified Wells criteria and compared with similar data from published studies, we had a much higher proportion of patients classified as low clinical probability.7, 8, 15 The low‐clinical‐probability group's mean Simplified Wells Score was 0.71; one‐third had a Simplified Wells Score of 0. This reflects a low‐risk population for VTE, supported by the low prevalence of prior DVT/VTE and active cancer in our population.4, 10 The rationale for referring patients with so few risk factors for CTPA is unclear. It is possible that providers used CTPA to evaluate symptoms not clearly explained and obtained the study to look for other diagnoses in addition to VTE. By not applying a clinical algorithm, very‐low‐risk patients underwent CTPA, increasing the number of negative studies and decreasing the overall positive rate.
Not using a clinical algorithm also resulted in indiscriminate D‐dimer testing. There were 83 patients risk‐stratified as low clinical probability who did not have a D‐dimer prior to undergoing CTPA. Some of these patients may well have had a negative D‐dimer, requiring no further workup to rule out VTE. Seventeen patients had a negative D‐dimer and still underwent CTPA; all these patients were negative for VTE. These aberrations likely occurred from unfamiliarity with use of the D‐dimer test or doubts about its ability to reliably exclude VTE. Appropriate application of D‐dimer testing could have decreased the number of CTPAs ordered and increased our overall rate of positive VTE diagnosis.
Perrier et al., Brown et al., and Kelly and Wells all describe different methods of introducing clinical algorithms to aid the diagnosis of VTE.46, 9 All agree that patients should be risk stratified by pretest clinical probability, and low‐probability patients should undergo intermediate testing with D‐dimer prior to CTPA. Implementation of a similar clinical algorithm at our facility would likely decrease the number of CTPAs ordered. If all patients presenting at our facility with signs and symptoms raising concern for VTE were first risk‐stratified by pretest clinical probability, and all low‐probability patients underwent highly‐sensitive D‐dimer testing as an initial step, fewer CTPAs would be performed on low‐probability patients. The largest group of patients in our study were low probability; therefore, decreasing CTPA in this group could have a significant effect on our institution.
The retrospective nature of our study resulted in the following limitations. It is impossible to determine how the ordering provider viewed the patient's pretest probability. In most of the medical records, a pretest clinical probability was not documented. We attempted to validate the ordering provider's decision by being as generous as possible in applying points to the Wells Score. For example, if a patient had a remote history of cancer and the ordering provider documented this as a risk factor for VTE, the point value for cancer was given even though the Wells Score has a much narrower definition of this category.10 This practice favors assigning patients a potentially higher clinical probability and may have increased the number of patients designated as intermediate and high clinical probability in our study.
Our hospital primarily relies on CTPA with lower extremity venogram as the diagnostic test for VTE. Indeterminate tests may have occurred and thus falsely lowered the number of VTEs diagnosed. However, no patient with a negative CTPA was diagnosed with VTE by any modality in the 3 months after their initial study at our institution; a diagnosis of VTE could have been made at another hospital. The Simplified Wells Score uses both objective and subjective components to arrive at a point total. Our results might be different if newer algorithms, such as the Revised Geneva Score,16 which relies only on objective measurements, had been used.
CONCLUSIONS
The reliance on CTPA alone to exclude a potentially life‐threatening illness without additional risk stratification or clinical information leads to overuse of this test in patients with very low to no clinical risk for VTE and a low rate of diagnosed VTE. Implementation of a clinical algorithm for the diagnosis of suspected VTE may eliminate the need for many CTPAs, improving the yield of this test without compromising patient safety, especially at institutions with a low prevalence of PE.
Acknowledgements
The authors thank Dr. John Rinard, DO, for assistance with initial editing of the abstract and Troy Patience for his assistance with statistical analysis.
Approximately 10 million patients present to emergency departments each year with symptoms raising concern of thromboembolism (VTE).1 The current gold standard for diagnosis of VTE is pulmonary angiography.2 As this study is invasive, alternative imaging protocols have been sought. CTPA, when combined with measures of pretest probability, equals or surpasses the ability of pulmonary angiography to detect VTE and can improve the ability of clinicians to rule out VTE.38 In a study of 930 patients, application of clinical rules in addition to D‐dimer testing decreased the number of CTPAs ordered by 50%.9 One of the most common clinical rule sets is the Wells Score, which relies on historical features related to the risk of DVT/VTE and physical examination findings.10, 11
Other institutions have demonstrated an increase in the number of CTPAs ordered and VTE diagnoses since the study became widely available.13 Based on the observation of an increasing number of CTPAs ordered at our institution without an increase in the number of VTEs diagnosed, we aimed to ascertain the physician ordering practices for CTPA. We hypothesized that CTPAs were ordered at a greater frequency in a low‐risk population because an institutional clinical algorithm was lacking.
METHODS
Charts of all patients aged 18‐100 with CTPA ordered to rule out acute VTE were retrospectively examined. A Simplified Wells Score was applied using only the information available to the ordering physician at the time the CTPA was performed. Patients were stratified by their Simplified Wells Score to low (0‐1 points), intermediate (2‐6 points), or high (>6 points) pretest clinical probability. A D‐dimer value, if ordered, was used to further stratify patients based on a positive or negative result. The official radiologic report of the CTPA was used to determine the rate of VTE diagnosis for the study population.
RESULTS
Three hundred and ninety‐four patients were referred for CTPA (Fig. 1). Two hundred and seventy‐nine had adequate clinical data to calculate a Simplified Wells Score and were included in the study. Of the 279 studies included, 75% were ordered through the emergency department and 25% from inpatient services (Table 1). The study patients were stratified according to the Simplified Wells criteria: 184 patients (66%) had low clinical probability, 91 (33%) had intermediate clinical probability, and 4 (1%) had high clinical probability. Nineteen (7%) patients had a history of DVT or VTE, and 28 (10%) had a history of active cancer at the time of their CTPA. One hundred and twenty‐five of the 279 patients had a D‐dimer performed (Fig. 2). One hundred and eight were positive, and 17 were negative. Of the 17 patients who had a negative D‐dimer and underwent CTPA testing, none were diagnosed with VTE. Eighty‐three low‐clinical‐probability patients underwent CTPA without D‐dimer testing, 4 of whom were diagnosed with VTE.

| Low (n = 184) | Intermediate (n = 91) | High (n = 4) | Total (n = 279) | |
|---|---|---|---|---|
| Age (years), mean | 52 | 59 | 62 | 58 |
| Male, n (%) | 82 (45) | 44 (48) | 1 (25) | 127 (46) |
| Female, n (%) | 102 (55) | 47 (52) | 3 (75) | 152 (54) |
| Emergency department, n (%) | 150 (82) | 47 (52) | 4 (100) | 225 (75) |
| Medical, n (%) | 22 (12) | 18 (20) | 0 | 40 (13) |
| Surgical, n (%) | 9 (5) | 14 (15) | 0 | 23 (7) |
| ICU, n (%) | 3 (1) | 12 (13) | 0 | 15 (5) |
| Wells Score, mean | 0.72 | 3.4 | 7.8 | 1.6 |
| D‐dimer performed, n (%) | 101 (55) | 21 (23) | 3 (75) | 125 (45) |
| D‐dimer positive, n (%) | 89 (88) | 16 (76) | 3 (100) | 108 (86) |
| D‐dimer negative, n (%) | 12 (12) | 5 (24) | 0 | 17 (14) |
| CTPA positive, n (%) | 8 (4) | 11 (12) | 1 (25) | 20 (7) |
| CPTA negative, n (%) | 176 (96) | 80 (88) | 3 (75) | 259 (93) |

There were 20 positive CTPAs in the study group (Fig. 3). Review of the records for 3 months after the study of patients whose CTPA was negative disclosed no diagnoses of VTE by other modalities. VTE was diagnosed in 4% of patients in the low‐clinical‐probability group, 12% in the intermediate‐clinical‐probability group, and 25% in the high‐clinical‐probability group. The overall positive CTPA rate was 7.2%.

DISCUSSION
Many studies have examined the application of clinical rule sets in addition to D‐dimer testing and CTPA to exclude acute VTE.39 Most of these studies have shown that the use of an algorithm is safe and frequently reduces referral for CTPA in low‐clinical‐probability patients. However, others have noted that some physicians do not routinely use validated algorithms when making decisions related to patient evaluation.13 Our rate of positive CTPA was low compared with rates reported in the literature.3, 14 We believe the most likely explanation is the large number of low‐clinical‐probability patients who underwent CTPA, possibly because providers do not routinely use a validated clinical algorithm.
When our patient population was risk stratified by Simplified Wells criteria and compared with similar data from published studies, we had a much higher proportion of patients classified as low clinical probability.7, 8, 15 The low‐clinical‐probability group's mean Simplified Wells Score was 0.71; one‐third had a Simplified Wells Score of 0. This reflects a low‐risk population for VTE, supported by the low prevalence of prior DVT/VTE and active cancer in our population.4, 10 The rationale for referring patients with so few risk factors for CTPA is unclear. It is possible that providers used CTPA to evaluate symptoms not clearly explained and obtained the study to look for other diagnoses in addition to VTE. By not applying a clinical algorithm, very‐low‐risk patients underwent CTPA, increasing the number of negative studies and decreasing the overall positive rate.
Not using a clinical algorithm also resulted in indiscriminate D‐dimer testing. There were 83 patients risk‐stratified as low clinical probability who did not have a D‐dimer prior to undergoing CTPA. Some of these patients may well have had a negative D‐dimer, requiring no further workup to rule out VTE. Seventeen patients had a negative D‐dimer and still underwent CTPA; all these patients were negative for VTE. These aberrations likely occurred from unfamiliarity with use of the D‐dimer test or doubts about its ability to reliably exclude VTE. Appropriate application of D‐dimer testing could have decreased the number of CTPAs ordered and increased our overall rate of positive VTE diagnosis.
Perrier et al., Brown et al., and Kelly and Wells all describe different methods of introducing clinical algorithms to aid the diagnosis of VTE.46, 9 All agree that patients should be risk stratified by pretest clinical probability, and low‐probability patients should undergo intermediate testing with D‐dimer prior to CTPA. Implementation of a similar clinical algorithm at our facility would likely decrease the number of CTPAs ordered. If all patients presenting at our facility with signs and symptoms raising concern for VTE were first risk‐stratified by pretest clinical probability, and all low‐probability patients underwent highly‐sensitive D‐dimer testing as an initial step, fewer CTPAs would be performed on low‐probability patients. The largest group of patients in our study were low probability; therefore, decreasing CTPA in this group could have a significant effect on our institution.
The retrospective nature of our study resulted in the following limitations. It is impossible to determine how the ordering provider viewed the patient's pretest probability. In most of the medical records, a pretest clinical probability was not documented. We attempted to validate the ordering provider's decision by being as generous as possible in applying points to the Wells Score. For example, if a patient had a remote history of cancer and the ordering provider documented this as a risk factor for VTE, the point value for cancer was given even though the Wells Score has a much narrower definition of this category.10 This practice favors assigning patients a potentially higher clinical probability and may have increased the number of patients designated as intermediate and high clinical probability in our study.
Our hospital primarily relies on CTPA with lower extremity venogram as the diagnostic test for VTE. Indeterminate tests may have occurred and thus falsely lowered the number of VTEs diagnosed. However, no patient with a negative CTPA was diagnosed with VTE by any modality in the 3 months after their initial study at our institution; a diagnosis of VTE could have been made at another hospital. The Simplified Wells Score uses both objective and subjective components to arrive at a point total. Our results might be different if newer algorithms, such as the Revised Geneva Score,16 which relies only on objective measurements, had been used.
CONCLUSIONS
The reliance on CTPA alone to exclude a potentially life‐threatening illness without additional risk stratification or clinical information leads to overuse of this test in patients with very low to no clinical risk for VTE and a low rate of diagnosed VTE. Implementation of a clinical algorithm for the diagnosis of suspected VTE may eliminate the need for many CTPAs, improving the yield of this test without compromising patient safety, especially at institutions with a low prevalence of PE.
Acknowledgements
The authors thank Dr. John Rinard, DO, for assistance with initial editing of the abstract and Troy Patience for his assistance with statistical analysis.
- ,,, et al.Impact of a rapid‐rule out protocol for pulmonary embolism on the rate of screening, missed cases and pulmonary vascular imaging in an urban US emergency department.Ann Emerg Med.2004;44:490–502.
- ,,, et al.ATS 1999 Clinical practice guideline for the diagnostic approach to acute venous thromboembolism.Am J Respir Crit Care Med.1999;160:1043–1066.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism.JAMA.2005;293:2012–2017.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,.An emergency department guideline for the diagnosis of pulmonary embolism: an outcome study.Acad Emerg Med.2005;12:20–25.
- ,.A clinical probability assessment and D‐dimer measurement should be the initial step in the investigation of suspected venous thromboembolism.Chest.2003;124:1116–1119.
- ,,, et al.Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44:503–510.
- ,.External validation and comparison of recently described prediction rules for suspected pulmonary embolism.Curr Opin Pulm Med.2004;10:345–349.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D‐dimer.Ann Intern Med.2001;135:98–107.
- ,,, et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- ,,, et al.Assessing the clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161:92–97.
- ,,, et al.The effect of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- Simplifying the evaluation of pulmonary embolism.Chest.2006:129:1400–1401.
- ,,, et al.Meta‐Analysis: Outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,,, et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354:2317–2327.
- ,,, et al.Prediction of pulmonary embolism in the emergency department: the revised Geneva score.Ann Intern Med.2006;144(3):165–171.
- ,,, et al.Impact of a rapid‐rule out protocol for pulmonary embolism on the rate of screening, missed cases and pulmonary vascular imaging in an urban US emergency department.Ann Emerg Med.2004;44:490–502.
- ,,, et al.ATS 1999 Clinical practice guideline for the diagnostic approach to acute venous thromboembolism.Am J Respir Crit Care Med.1999;160:1043–1066.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism.JAMA.2005;293:2012–2017.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,.An emergency department guideline for the diagnosis of pulmonary embolism: an outcome study.Acad Emerg Med.2005;12:20–25.
- ,.A clinical probability assessment and D‐dimer measurement should be the initial step in the investigation of suspected venous thromboembolism.Chest.2003;124:1116–1119.
- ,,, et al.Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism.Ann Emerg Med.2004;44:503–510.
- ,.External validation and comparison of recently described prediction rules for suspected pulmonary embolism.Curr Opin Pulm Med.2004;10:345–349.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D‐dimer.Ann Intern Med.2001;135:98–107.
- ,,, et al.Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis.N Engl J Med.2003;349:1227–1235.
- ,,, et al.Assessing the clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161:92–97.
- ,,, et al.The effect of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- Simplifying the evaluation of pulmonary embolism.Chest.2006:129:1400–1401.
- ,,, et al.Meta‐Analysis: Outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,,, et al.Multidetector computed tomography for acute pulmonary embolism.N Engl J Med.2006;354:2317–2327.
- ,,, et al.Prediction of pulmonary embolism in the emergency department: the revised Geneva score.Ann Intern Med.2006;144(3):165–171.