Article
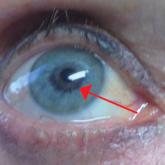
Bimatoprost-Induced Iris Hyperpigmentation: Beauty in the Darkened Eye of the Beholder
- Author:
- Michael B. Lipp, DO
- Leela Athalye, DO
- Navid Nami, DO
Iris hyperpigmentation can occur when bimatoprost eye drops are applied to the eyes for treatment of ocular hypertension and glaucoma, but reports...
Article
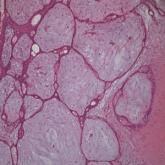
Concomitant Fibrofolliculoma and Trichodiscoma on the Abdomen
- Author:
- Jessica Riley, DO
- Leela Athalye, DO
- Donna Tran, DO
- Stephanie Fogelson, MD
- Paul Shitabata, MD
Fibrofolliculoma and trichodiscoma are adnexal tumors that arise from or around hair follicles and are two of the many characteristic features of...
Article
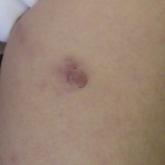
A Rare Case of Primary Cutaneous Diffuse Large B-Cell Lymphoma, Leg Type
- Author:
- Leela Athalye, DO
- Navid Nami, DO
- Paul Shitabata, MD
Primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) is a rare, intermediately aggressive form of primary cutaneous B-cell lymphoma...
Article

Collagenous and Elastotic Marginal Plaques of the Hands
- Author:
- Mayha Patel, DO
- Leela Athalye, DO
- Paul Shitabata, MD
- Mark F. Maida, MD
- Navid Nami, DO
