User login
Impact of Ketogenic and Low-Glycemic Diets on Inflammatory Skin Conditions
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17
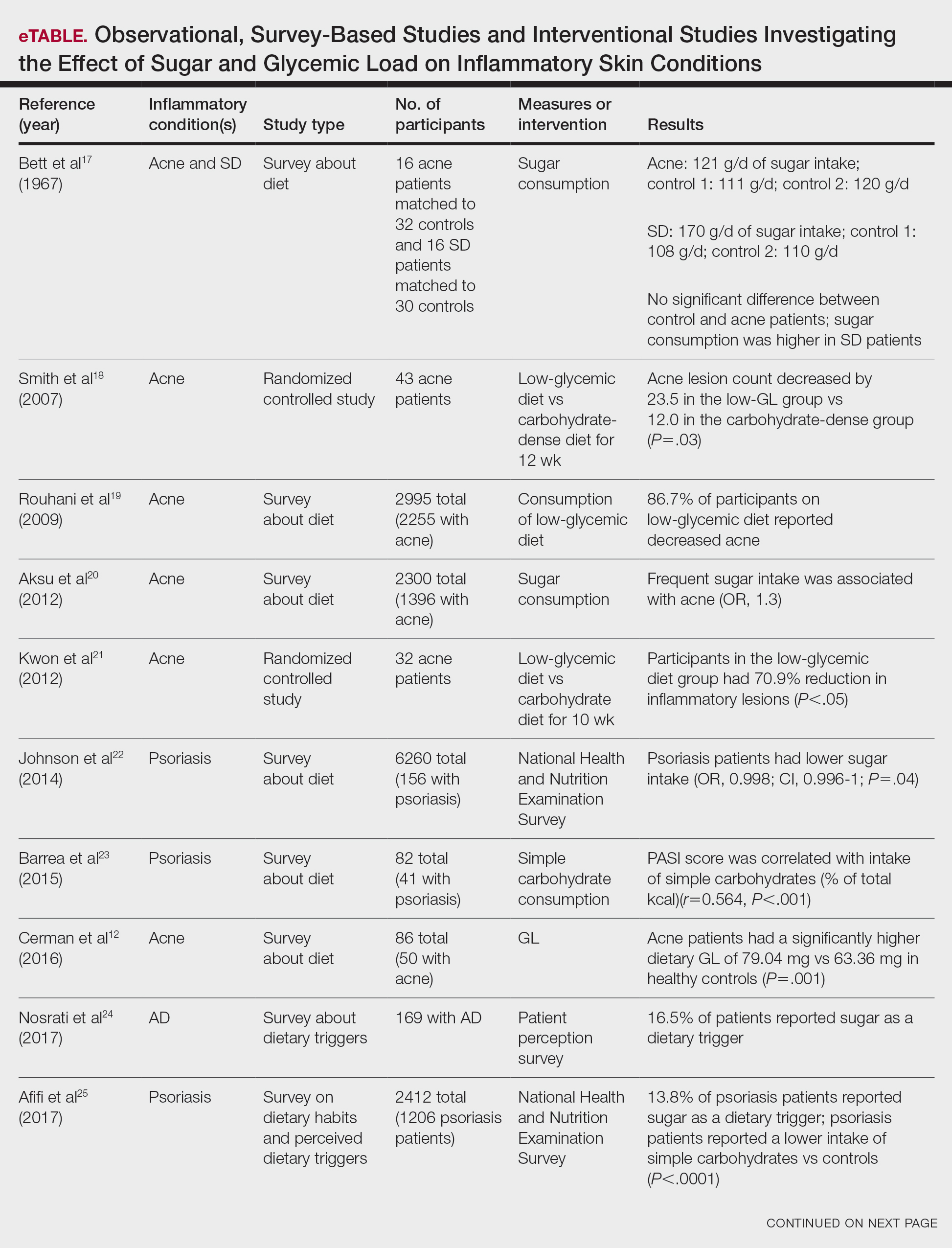

Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Practice Points
- As the ketogenic diet gains in popularity, dermatologists may inform patients that there is emerging evidence supporting the idea that low-glycemic diets may contribute to improvement in inflammatory skin conditions.
- Dermatologists may educate patients about the potential benefits of a low-glycemic diet as a supplementary treatment for acne based on existing evidence.
- Current evidence is insufficient to endorse a ketogenic diet as superior to other dietary approaches in treating inflammatory skin conditions.
