User login
How to Manage Pain in Patients with Renal Insufficiency or End-Stage Renal Disease on Dialysis?
Case
A 70-year-old male with ESRD on hemodialysis presents with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and ankle pain after a fall. An MRI of his ankle is negative, and he is started on acetaminophen and lidocaine patches, which result in adequate pain relief of the ankle. He later develops significant neuropathic pain in both arms, and a CT scan of the cervical spine reveals a cervical abscess and osteomyelitis. The patient desires pain relief but adamantly refuses narcotics, stating: “I don’t want to get addicted.” How can his pain be managed?
Overview
Pain is a common problem in patients with renal insufficiency and end-stage renal disease (ESRD) and can have a significant effect on the patient’s quality of life.1 When assessing a patient’s pain, assess both the severity of the pain (such as on an analogue scale, 0-10) and the characteristics of the pain. Pain is most commonly characterized as nociceptive, neuropathic, or both. Nociceptive pain can be further classified as arising from either somatic or visceral sources, and is often described as dull, throbbing, cramping, and/or pressurelike.1 Neuropathic pain is often described as tingling, numbing, burning, and/or stabbing.
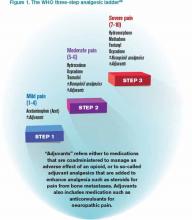
It is a challenge to manage pain in patients with renal insufficiency and dialysis. Renal insufficiency affects the pharmacokinetic properties of most pain medications, including their distribution, clearance, and excretion. The magnitude of the effect of renal insufficiency on drug metabolism varies depending on the agent itself, its metabolite, and the extent of renal failure.3 Multiple factors should be considered when prescribing pain medications for patients on dialysis, including the properties of the parent drug and its metabolites; the physical properties of the dialysis equipment, such as the filter pore size, the flow rate, and the efficiency of the technique used; and the dialysis method (intermittent versus continuous).3 Table 1 provides the recommended dosing of the most commonly prescribed agents, based on the degree of renal impairment. A modified World Health Organization (WHO) ladder has been suggested to treat pain in patients with ESRD, which can lead to effective pain relief in as many as 96% of patients (see Figure 1).2
*Beginning dose: If switching from IR to ER, calculate 24-hour total dose.
**For patients with creatinine clearances (CrCl) of 15 mL/min or less, the daily dosage should be adjusted proportionally (e.g. patients with a CrCl of 7.5 mL/min should receive one-half the dose of a patient with a CrCl of 15 mL/min).
Review of Data
Nonopioid options. Nonopioids, such as acetaminophen and NSAIDs, have no associated tolerance but have a ceiling effect for analgesia, and NSAIDs are associated with dose-dependent acute renal failure, gastrointestinal ulceration and bleeding, and cardiac events. The nonopioids that are considered safe options in patients with renal insufficiency include acetaminophen, ibuprofen, and fenoprofen (Nalfon). However, in the elderly, American Geriatric Society (AGS) guidelines currently recommend avoiding all NSAIDs due to their safety profile in the geriatric population.4 Although all NSAIDs can potentially be used for pain, selected NSAIDs with an FDA indication for acute or chronic pain were included for this review.
Acetaminophen (APAP) is a dialyzable compound that is metabolized in the liver to five inactive metabolites. The terminal elimination half-life of its sulfate and glucuronide metabolites are prolonged in patients with renal failure; therefore, the dosing interval of APAP should be increased to six to eight hours in renally impaired patients.5,6,7 Overall, acetaminophen is considered one of the safest agents to use for the treatment of pain, in renal patients and otherwise, as long as dosing is below the minimal daily dose (see Table 1).
Ibuprofen is metabolized in the liver to inactive compounds. It does not accumulate in renal insufficiency, and two of the inactive compounds are dialyzable.8 It is considered a safe option for the treatment of pain in patients with renal insufficiency or dialysis.9
Fenoprofen is metabolized in the liver to inactive compounds. Renal impairment is likely to cause the accumulation of the inactive metabolites but not the parent compound, so dose reduction is not necessary with the use of this agent in renal insufficiency or dialysis.6
Mefenamic acid (Ponstel) is metabolized in the liver. Mefenamic acid can further deteriorate renal function in patients with underlying renal disease.12 However, the nephrotoxic potential of this agent is of little consideration in ESRD patients on dialysis, and therefore no dosage adjustments are necessary in these patients.6
Ketoprofen is metabolized in the liver, where approximately 80% of the dose is excreted in the urine as a glucuronide metabolite. Dose reduction is recommended in renal insufficiency and dialysis, as it not dialyzable.8
Ketorolac accumulates in renal insufficiency; therefore, it is contraindicated in these patients and in patients at risk for renal failure, including those with volume depletion.10 Ketorolac is unlikely to be removed by dialysis and so should be avoided.10,11
Naproxen is metabolized in the liver to inactive compounds. Use of naproxen is not recommended in patients with moderate to severe renal impairment. If therapy must be initiated, close monitoring of the patient’s renal function is recommended.13
Celecoxib is the only cyclooxygenase-2 (COX-2) inhibitor available in the U.S. It is metabolized extensively by the liver and is unlikely to be removed by dialysis. Therefore, use of COX-2 inhibitors should be avoided in severe renal impairment and in those on dialysis.14,15
Opioid options. The use of opioids in the renally impaired population is challenging, as one must balance opioid-related adverse events with adequate pain control. As such, it is recommended to start with lower-than-recommended doses and slowly titrate up the dose while extending the dosing interval. This will help limit adverse effects, such as respiratory depression and hypotension.3
Hydrocodone is metabolized to hydromorphone (Dilaudid), which is then metabolized to its major metabolite hydromorphine-3-glucuronide (H3G) and minor metabolite hydromorphine-6-hydroxy, all of which are excreted renally along with the parent compound. H3G has no analgesic properties, but it can potentially cause neuroexcitation, agitation, confusion, and hallucination. Hydromorphone has been used safely in patients with renal insufficiency and dialysis, as it is expected to be dialyzable. 16,17
Tramadol is metabolized in the liver, producing one active compound. Approximately 30% of the tramadol dose is excreted unchanged in the urine, whereas 60% of the dose is excreted as metabolites. It is recommended to reduce the dose and increase the dosing interval in patients with renal insufficiency, but tramadol is generally well-tolerated in patients with renal insufficiency and dialysis. It is significantly removed by hemodialysis; therefore, redosing after a session may be necessary.18,19
Oxycodone can be used in patients with mild to moderate renal insufficiency but should be used at reduced dosing; it has been associated with significant sedation with usual doses in renal failure patients.16 Its use is generally not recommended in dialysis patients due to lack of data.3
Methadone and its metabolites are excreted in the urine and feces. Methadone has been used safely in patients with renal insufficiency, but it is poorly removed by dialysis and no specific recommendations are available regarding its dosing in dialysis.3,16
Fentanyl is primarily metabolized in the liver to inactive metabolites. Fentanyl clearance is reduced in patients with moderate to severe uremia (BUN >60 mg/dL). It is not expected that fentanyl be dialyzable because of its pharmacokinetic properties (high protein-binding, low water solubility, high molecular weight, and high volume of distribution). Data suggests that fentanyl can be used at usual doses in mild to moderate renal insufficiency and in dialysis patients, although reduced doses may be prudent. Such patients should be monitored for signs of gradual accumulation of the parent drug.3,16
Morphine is metabolized in the liver to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G), all of which are excreted renally, along with the parent compound. Only M6G has analgesic properties, and when it accumulates, it can lead to CNS depression. M3G is associated with behavioral excitation, a side effect that is further magnified in patients with renal insufficiency. Although morphine is dialyzable, it should generally be avoided in patients with any level of renal insufficiency.16,17,20,21
Codeine is metabolized to several active metabolites, all of which are renally excreted. Lower-than-usual doses are recommended in patients with renal insufficiency, and it should be avoided altogether in dialysis patients.3,16
Meperidine is metabolized in the liver to various metabolites, primarily normeperidine, which is toxic and has a long half-life, five to 10 times longer then meperidine. Meperidine should not be used in patients with renal insufficiency or dialysis.3
Adjunctive therapeutic options. Lidocaine patches currently are only FDA-indicated for postherpetic neuralgia but are used for a wide variety of local pain syndromes. Absorption of lidocaine is determined by the duration of application and the surface area over which it is applied. There is no appreciable accumulation of lidocaine or its metabolites in renal insufficiency; therefore, dose adjustments are not required.22,23
Gabapentin is FDA-indicated for partial seizures and postherpetic neuralgia but is also used for a wide variety of neuropathic pain syndromes, including postoperative pain.24 Gabapentin is not metabolized and is excreted in the urine unchanged. Renal clearance of gabapentin is reduced by 40% and the elimination half-life is increased up to 52 hours in renal insufficiency, but it is dialyzable. Therefore, dose adjustments are required with gabapentin in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.25-27
Pregabalin is structurally related to gabapentin and is indicated for a variety of neuropathic pain conditions. Pregabalin is 90% excreted unchanged in the urine, and approximately 50% of drug is removed after four hours of hemodialysis. Dose adjustments are required in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.28
Antidepressant options. Amitriptyline, nortryptiline, and desipramine are the tricyclic antidepressants (TCAs) commonly used for neuropathic pain. TCAs are metabolized in the liver to inactive metabolites, with the exception of amitriptyline, which is metabolized to nortryptiline. Common side effects reported with TCAs include postural hypotension and anticholinergic side effects, such as constipation, urinary retention, blurred vision, dry mouth, delirium, and sedation. It is unlikely that the TCAs can be removed by dialysis. It is suggested that the dosage be reduced in renal insufficiency and that anticholinergic side effects be monitored.29
Back to the Case
The patient’s ankle pain was controlled with acetaminophen and lidocaine patches. For the neuropathic pain in his upper extremities, tramadol was started at 25 mg oral every 12 hours and increased to 50 mg oral every eight hours (below the maximum of 200 mg a day). The tramadol did not result in adequate pain relief, so gabapentin 100 mg at bedtime was initiated, then increased to twice daily over three days with some relief.
A geriatric consult was obtained to help educate him regarding addiction to opioids, as well as to explore goals of care, but he continued to insist on the use of a non-narcotic regimen for his pain.
Bottom Line
Pain management in patients with renal insufficiency and dialysis can be challenging, but there are a number of safe non-narcotic and narcotic pain regimens that can be safely used in this patient population.
Dr. Harisingani is a board-certified hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y., and Drs. Saad and Cassagnol are assistant clinical professors at St. Johns University College of Pharmacy and Health Sciences in Jamaica, N.Y., and clinical pharmacy coordinators at Long Island Jewish Medical Center.
References
- Mid-Atlantic Renal Coalition and the Kidney End-of-Life Coalition. Clinical algorithm & preferred medications to treat pain in dialysis patients. Coalition for Supportive Care of Kidney Patients website. Available at: http://www.kidneysupportivecare.org/Physicians-Clinicians/Pain—Symptom-Management.aspx. Accessed Nov. 18, 2012.
- Barakzoy AS, Moss AH. Efficacy of the World Health Organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198-3203.
- Johnson SJ. Opioid safety in patients with renal or hepatic dysfunction. Pain Treatment Topics website. Available at: http://pain-topics.org/pdf/Opioids-Renal-Hepatic-Dysfunction.pdf. Accessed Nov. 28, 2012.
- Ferrell B, Argoff CE, Epplin J, et al. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ. Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(3):291-297.
- Launay-Vacher V, Karie S, Fau JB, Izzedine H, Deray G. Treatment of pain in patients with renal insufficiency: the World Health Organization three-step ladder adapted. J Pain. 2006;6(3):137-148.
- Berg KJ, Djøseland O, Gjellan A, et al. Acute effects of paracetamol on prostaglandin synthesis and renal function in normal man and in patients with renal failure. Clin Nephrol. 1990;34:255-262.
- Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 R.P.) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976;1976(0):45-52.
- Shen CH, Hung CJ, Wu CC, Huang HW, Ho WM. Rhabdomyolysis-induced acute renal failure after morphine overdose—a case report. Acta Anaesthesiol Sin. 1999;37(3):159-162.
- Ketorolac tromethamine oral tablets [package insert]. St. Louis: Ethex Corp.: 2008.
- Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23:415-427. Erratum in: Clin Pharmacokinet. 1999;24(3):270.
- Ponstel [package insert]. Alpharetta, GA: First Horizon Pharmaceutical Corp.; 2006.
- Naprosyn [package insert]. Nutley, NJ: Roche Laboratories Inc.; 2008.
- Celebrex [package insert]. New York: G.D. Searle LLC; 2011.
- Catella-Lawson F, McAdam B, Morrison BW, et al. Effects of specific inhibition of cyclooygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735-741.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497-504.
- Lee MA, Leng ME, Tiernan EJ. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med. 2001;15(1):26-34.
- Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCI. Am J. Med. 1996;101(1A):47S-53S.
- Izzedine H, Launay-Vacher V, Abbara C, Aymard G, Bassilios N, Deray G. Pharmacokinetics of tramadol in a hemodialysis patient. Nephron. 2002;92(3):755-756.
- Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24(4):344-354.
- Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25(1):74-91.
- Lidoderm [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010.
- Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12(2):447-459.
- Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;15:126(1-3):91-101.
- Neurontin [package insert]. New York: Parke-Davis; 2010.
- Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51(4):358-363.
- Srivastava U, Kumar A, Saxena S, et al: Effect of preoperative gabapentin on postoperative pain and tramadol consumption after minilap open cholecystectomy: a randomized double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2010;27(N4):331-335.
- Lyrica [package insert]. New York: Pfizer Inc.; 2012.
- Broadbent A, Khor K, Heaney A. Palliation and chronic renal failure: opioid and other palliative medications—dosage guidelines. Progress in Palliative Care. 2003;11(4):183-190(8).
- Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease: a review of analgesics in renal failure. J Nephrol. 2011;24(1):35-40.
- Wolters Kluwer Health. Facts & comparisons. Wolters Kluwer Health website. Available at: http://www.factsandcomparisons.com. Accessed Jan. 14, 2013.
- Lexicomp. Lexicomp Online. Lexicomp website. Available at: http://www.lexi.com/institutions/products/online/.
Case
A 70-year-old male with ESRD on hemodialysis presents with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and ankle pain after a fall. An MRI of his ankle is negative, and he is started on acetaminophen and lidocaine patches, which result in adequate pain relief of the ankle. He later develops significant neuropathic pain in both arms, and a CT scan of the cervical spine reveals a cervical abscess and osteomyelitis. The patient desires pain relief but adamantly refuses narcotics, stating: “I don’t want to get addicted.” How can his pain be managed?
Overview
Pain is a common problem in patients with renal insufficiency and end-stage renal disease (ESRD) and can have a significant effect on the patient’s quality of life.1 When assessing a patient’s pain, assess both the severity of the pain (such as on an analogue scale, 0-10) and the characteristics of the pain. Pain is most commonly characterized as nociceptive, neuropathic, or both. Nociceptive pain can be further classified as arising from either somatic or visceral sources, and is often described as dull, throbbing, cramping, and/or pressurelike.1 Neuropathic pain is often described as tingling, numbing, burning, and/or stabbing.
It is a challenge to manage pain in patients with renal insufficiency and dialysis. Renal insufficiency affects the pharmacokinetic properties of most pain medications, including their distribution, clearance, and excretion. The magnitude of the effect of renal insufficiency on drug metabolism varies depending on the agent itself, its metabolite, and the extent of renal failure.3 Multiple factors should be considered when prescribing pain medications for patients on dialysis, including the properties of the parent drug and its metabolites; the physical properties of the dialysis equipment, such as the filter pore size, the flow rate, and the efficiency of the technique used; and the dialysis method (intermittent versus continuous).3 Table 1 provides the recommended dosing of the most commonly prescribed agents, based on the degree of renal impairment. A modified World Health Organization (WHO) ladder has been suggested to treat pain in patients with ESRD, which can lead to effective pain relief in as many as 96% of patients (see Figure 1).2
*Beginning dose: If switching from IR to ER, calculate 24-hour total dose.
**For patients with creatinine clearances (CrCl) of 15 mL/min or less, the daily dosage should be adjusted proportionally (e.g. patients with a CrCl of 7.5 mL/min should receive one-half the dose of a patient with a CrCl of 15 mL/min).
Review of Data
Nonopioid options. Nonopioids, such as acetaminophen and NSAIDs, have no associated tolerance but have a ceiling effect for analgesia, and NSAIDs are associated with dose-dependent acute renal failure, gastrointestinal ulceration and bleeding, and cardiac events. The nonopioids that are considered safe options in patients with renal insufficiency include acetaminophen, ibuprofen, and fenoprofen (Nalfon). However, in the elderly, American Geriatric Society (AGS) guidelines currently recommend avoiding all NSAIDs due to their safety profile in the geriatric population.4 Although all NSAIDs can potentially be used for pain, selected NSAIDs with an FDA indication for acute or chronic pain were included for this review.
Acetaminophen (APAP) is a dialyzable compound that is metabolized in the liver to five inactive metabolites. The terminal elimination half-life of its sulfate and glucuronide metabolites are prolonged in patients with renal failure; therefore, the dosing interval of APAP should be increased to six to eight hours in renally impaired patients.5,6,7 Overall, acetaminophen is considered one of the safest agents to use for the treatment of pain, in renal patients and otherwise, as long as dosing is below the minimal daily dose (see Table 1).
Ibuprofen is metabolized in the liver to inactive compounds. It does not accumulate in renal insufficiency, and two of the inactive compounds are dialyzable.8 It is considered a safe option for the treatment of pain in patients with renal insufficiency or dialysis.9
Fenoprofen is metabolized in the liver to inactive compounds. Renal impairment is likely to cause the accumulation of the inactive metabolites but not the parent compound, so dose reduction is not necessary with the use of this agent in renal insufficiency or dialysis.6
Mefenamic acid (Ponstel) is metabolized in the liver. Mefenamic acid can further deteriorate renal function in patients with underlying renal disease.12 However, the nephrotoxic potential of this agent is of little consideration in ESRD patients on dialysis, and therefore no dosage adjustments are necessary in these patients.6
Ketoprofen is metabolized in the liver, where approximately 80% of the dose is excreted in the urine as a glucuronide metabolite. Dose reduction is recommended in renal insufficiency and dialysis, as it not dialyzable.8
Ketorolac accumulates in renal insufficiency; therefore, it is contraindicated in these patients and in patients at risk for renal failure, including those with volume depletion.10 Ketorolac is unlikely to be removed by dialysis and so should be avoided.10,11
Naproxen is metabolized in the liver to inactive compounds. Use of naproxen is not recommended in patients with moderate to severe renal impairment. If therapy must be initiated, close monitoring of the patient’s renal function is recommended.13
Celecoxib is the only cyclooxygenase-2 (COX-2) inhibitor available in the U.S. It is metabolized extensively by the liver and is unlikely to be removed by dialysis. Therefore, use of COX-2 inhibitors should be avoided in severe renal impairment and in those on dialysis.14,15
Opioid options. The use of opioids in the renally impaired population is challenging, as one must balance opioid-related adverse events with adequate pain control. As such, it is recommended to start with lower-than-recommended doses and slowly titrate up the dose while extending the dosing interval. This will help limit adverse effects, such as respiratory depression and hypotension.3
Hydrocodone is metabolized to hydromorphone (Dilaudid), which is then metabolized to its major metabolite hydromorphine-3-glucuronide (H3G) and minor metabolite hydromorphine-6-hydroxy, all of which are excreted renally along with the parent compound. H3G has no analgesic properties, but it can potentially cause neuroexcitation, agitation, confusion, and hallucination. Hydromorphone has been used safely in patients with renal insufficiency and dialysis, as it is expected to be dialyzable. 16,17
Tramadol is metabolized in the liver, producing one active compound. Approximately 30% of the tramadol dose is excreted unchanged in the urine, whereas 60% of the dose is excreted as metabolites. It is recommended to reduce the dose and increase the dosing interval in patients with renal insufficiency, but tramadol is generally well-tolerated in patients with renal insufficiency and dialysis. It is significantly removed by hemodialysis; therefore, redosing after a session may be necessary.18,19
Oxycodone can be used in patients with mild to moderate renal insufficiency but should be used at reduced dosing; it has been associated with significant sedation with usual doses in renal failure patients.16 Its use is generally not recommended in dialysis patients due to lack of data.3
Methadone and its metabolites are excreted in the urine and feces. Methadone has been used safely in patients with renal insufficiency, but it is poorly removed by dialysis and no specific recommendations are available regarding its dosing in dialysis.3,16
Fentanyl is primarily metabolized in the liver to inactive metabolites. Fentanyl clearance is reduced in patients with moderate to severe uremia (BUN >60 mg/dL). It is not expected that fentanyl be dialyzable because of its pharmacokinetic properties (high protein-binding, low water solubility, high molecular weight, and high volume of distribution). Data suggests that fentanyl can be used at usual doses in mild to moderate renal insufficiency and in dialysis patients, although reduced doses may be prudent. Such patients should be monitored for signs of gradual accumulation of the parent drug.3,16
Morphine is metabolized in the liver to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G), all of which are excreted renally, along with the parent compound. Only M6G has analgesic properties, and when it accumulates, it can lead to CNS depression. M3G is associated with behavioral excitation, a side effect that is further magnified in patients with renal insufficiency. Although morphine is dialyzable, it should generally be avoided in patients with any level of renal insufficiency.16,17,20,21
Codeine is metabolized to several active metabolites, all of which are renally excreted. Lower-than-usual doses are recommended in patients with renal insufficiency, and it should be avoided altogether in dialysis patients.3,16
Meperidine is metabolized in the liver to various metabolites, primarily normeperidine, which is toxic and has a long half-life, five to 10 times longer then meperidine. Meperidine should not be used in patients with renal insufficiency or dialysis.3
Adjunctive therapeutic options. Lidocaine patches currently are only FDA-indicated for postherpetic neuralgia but are used for a wide variety of local pain syndromes. Absorption of lidocaine is determined by the duration of application and the surface area over which it is applied. There is no appreciable accumulation of lidocaine or its metabolites in renal insufficiency; therefore, dose adjustments are not required.22,23
Gabapentin is FDA-indicated for partial seizures and postherpetic neuralgia but is also used for a wide variety of neuropathic pain syndromes, including postoperative pain.24 Gabapentin is not metabolized and is excreted in the urine unchanged. Renal clearance of gabapentin is reduced by 40% and the elimination half-life is increased up to 52 hours in renal insufficiency, but it is dialyzable. Therefore, dose adjustments are required with gabapentin in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.25-27
Pregabalin is structurally related to gabapentin and is indicated for a variety of neuropathic pain conditions. Pregabalin is 90% excreted unchanged in the urine, and approximately 50% of drug is removed after four hours of hemodialysis. Dose adjustments are required in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.28
Antidepressant options. Amitriptyline, nortryptiline, and desipramine are the tricyclic antidepressants (TCAs) commonly used for neuropathic pain. TCAs are metabolized in the liver to inactive metabolites, with the exception of amitriptyline, which is metabolized to nortryptiline. Common side effects reported with TCAs include postural hypotension and anticholinergic side effects, such as constipation, urinary retention, blurred vision, dry mouth, delirium, and sedation. It is unlikely that the TCAs can be removed by dialysis. It is suggested that the dosage be reduced in renal insufficiency and that anticholinergic side effects be monitored.29
Back to the Case
The patient’s ankle pain was controlled with acetaminophen and lidocaine patches. For the neuropathic pain in his upper extremities, tramadol was started at 25 mg oral every 12 hours and increased to 50 mg oral every eight hours (below the maximum of 200 mg a day). The tramadol did not result in adequate pain relief, so gabapentin 100 mg at bedtime was initiated, then increased to twice daily over three days with some relief.
A geriatric consult was obtained to help educate him regarding addiction to opioids, as well as to explore goals of care, but he continued to insist on the use of a non-narcotic regimen for his pain.
Bottom Line
Pain management in patients with renal insufficiency and dialysis can be challenging, but there are a number of safe non-narcotic and narcotic pain regimens that can be safely used in this patient population.
Dr. Harisingani is a board-certified hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y., and Drs. Saad and Cassagnol are assistant clinical professors at St. Johns University College of Pharmacy and Health Sciences in Jamaica, N.Y., and clinical pharmacy coordinators at Long Island Jewish Medical Center.
References
- Mid-Atlantic Renal Coalition and the Kidney End-of-Life Coalition. Clinical algorithm & preferred medications to treat pain in dialysis patients. Coalition for Supportive Care of Kidney Patients website. Available at: http://www.kidneysupportivecare.org/Physicians-Clinicians/Pain—Symptom-Management.aspx. Accessed Nov. 18, 2012.
- Barakzoy AS, Moss AH. Efficacy of the World Health Organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198-3203.
- Johnson SJ. Opioid safety in patients with renal or hepatic dysfunction. Pain Treatment Topics website. Available at: http://pain-topics.org/pdf/Opioids-Renal-Hepatic-Dysfunction.pdf. Accessed Nov. 28, 2012.
- Ferrell B, Argoff CE, Epplin J, et al. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ. Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(3):291-297.
- Launay-Vacher V, Karie S, Fau JB, Izzedine H, Deray G. Treatment of pain in patients with renal insufficiency: the World Health Organization three-step ladder adapted. J Pain. 2006;6(3):137-148.
- Berg KJ, Djøseland O, Gjellan A, et al. Acute effects of paracetamol on prostaglandin synthesis and renal function in normal man and in patients with renal failure. Clin Nephrol. 1990;34:255-262.
- Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 R.P.) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976;1976(0):45-52.
- Shen CH, Hung CJ, Wu CC, Huang HW, Ho WM. Rhabdomyolysis-induced acute renal failure after morphine overdose—a case report. Acta Anaesthesiol Sin. 1999;37(3):159-162.
- Ketorolac tromethamine oral tablets [package insert]. St. Louis: Ethex Corp.: 2008.
- Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23:415-427. Erratum in: Clin Pharmacokinet. 1999;24(3):270.
- Ponstel [package insert]. Alpharetta, GA: First Horizon Pharmaceutical Corp.; 2006.
- Naprosyn [package insert]. Nutley, NJ: Roche Laboratories Inc.; 2008.
- Celebrex [package insert]. New York: G.D. Searle LLC; 2011.
- Catella-Lawson F, McAdam B, Morrison BW, et al. Effects of specific inhibition of cyclooygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735-741.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497-504.
- Lee MA, Leng ME, Tiernan EJ. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med. 2001;15(1):26-34.
- Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCI. Am J. Med. 1996;101(1A):47S-53S.
- Izzedine H, Launay-Vacher V, Abbara C, Aymard G, Bassilios N, Deray G. Pharmacokinetics of tramadol in a hemodialysis patient. Nephron. 2002;92(3):755-756.
- Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24(4):344-354.
- Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25(1):74-91.
- Lidoderm [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010.
- Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12(2):447-459.
- Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;15:126(1-3):91-101.
- Neurontin [package insert]. New York: Parke-Davis; 2010.
- Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51(4):358-363.
- Srivastava U, Kumar A, Saxena S, et al: Effect of preoperative gabapentin on postoperative pain and tramadol consumption after minilap open cholecystectomy: a randomized double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2010;27(N4):331-335.
- Lyrica [package insert]. New York: Pfizer Inc.; 2012.
- Broadbent A, Khor K, Heaney A. Palliation and chronic renal failure: opioid and other palliative medications—dosage guidelines. Progress in Palliative Care. 2003;11(4):183-190(8).
- Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease: a review of analgesics in renal failure. J Nephrol. 2011;24(1):35-40.
- Wolters Kluwer Health. Facts & comparisons. Wolters Kluwer Health website. Available at: http://www.factsandcomparisons.com. Accessed Jan. 14, 2013.
- Lexicomp. Lexicomp Online. Lexicomp website. Available at: http://www.lexi.com/institutions/products/online/.
Case
A 70-year-old male with ESRD on hemodialysis presents with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and ankle pain after a fall. An MRI of his ankle is negative, and he is started on acetaminophen and lidocaine patches, which result in adequate pain relief of the ankle. He later develops significant neuropathic pain in both arms, and a CT scan of the cervical spine reveals a cervical abscess and osteomyelitis. The patient desires pain relief but adamantly refuses narcotics, stating: “I don’t want to get addicted.” How can his pain be managed?
Overview
Pain is a common problem in patients with renal insufficiency and end-stage renal disease (ESRD) and can have a significant effect on the patient’s quality of life.1 When assessing a patient’s pain, assess both the severity of the pain (such as on an analogue scale, 0-10) and the characteristics of the pain. Pain is most commonly characterized as nociceptive, neuropathic, or both. Nociceptive pain can be further classified as arising from either somatic or visceral sources, and is often described as dull, throbbing, cramping, and/or pressurelike.1 Neuropathic pain is often described as tingling, numbing, burning, and/or stabbing.
It is a challenge to manage pain in patients with renal insufficiency and dialysis. Renal insufficiency affects the pharmacokinetic properties of most pain medications, including their distribution, clearance, and excretion. The magnitude of the effect of renal insufficiency on drug metabolism varies depending on the agent itself, its metabolite, and the extent of renal failure.3 Multiple factors should be considered when prescribing pain medications for patients on dialysis, including the properties of the parent drug and its metabolites; the physical properties of the dialysis equipment, such as the filter pore size, the flow rate, and the efficiency of the technique used; and the dialysis method (intermittent versus continuous).3 Table 1 provides the recommended dosing of the most commonly prescribed agents, based on the degree of renal impairment. A modified World Health Organization (WHO) ladder has been suggested to treat pain in patients with ESRD, which can lead to effective pain relief in as many as 96% of patients (see Figure 1).2
*Beginning dose: If switching from IR to ER, calculate 24-hour total dose.
**For patients with creatinine clearances (CrCl) of 15 mL/min or less, the daily dosage should be adjusted proportionally (e.g. patients with a CrCl of 7.5 mL/min should receive one-half the dose of a patient with a CrCl of 15 mL/min).
Review of Data
Nonopioid options. Nonopioids, such as acetaminophen and NSAIDs, have no associated tolerance but have a ceiling effect for analgesia, and NSAIDs are associated with dose-dependent acute renal failure, gastrointestinal ulceration and bleeding, and cardiac events. The nonopioids that are considered safe options in patients with renal insufficiency include acetaminophen, ibuprofen, and fenoprofen (Nalfon). However, in the elderly, American Geriatric Society (AGS) guidelines currently recommend avoiding all NSAIDs due to their safety profile in the geriatric population.4 Although all NSAIDs can potentially be used for pain, selected NSAIDs with an FDA indication for acute or chronic pain were included for this review.
Acetaminophen (APAP) is a dialyzable compound that is metabolized in the liver to five inactive metabolites. The terminal elimination half-life of its sulfate and glucuronide metabolites are prolonged in patients with renal failure; therefore, the dosing interval of APAP should be increased to six to eight hours in renally impaired patients.5,6,7 Overall, acetaminophen is considered one of the safest agents to use for the treatment of pain, in renal patients and otherwise, as long as dosing is below the minimal daily dose (see Table 1).
Ibuprofen is metabolized in the liver to inactive compounds. It does not accumulate in renal insufficiency, and two of the inactive compounds are dialyzable.8 It is considered a safe option for the treatment of pain in patients with renal insufficiency or dialysis.9
Fenoprofen is metabolized in the liver to inactive compounds. Renal impairment is likely to cause the accumulation of the inactive metabolites but not the parent compound, so dose reduction is not necessary with the use of this agent in renal insufficiency or dialysis.6
Mefenamic acid (Ponstel) is metabolized in the liver. Mefenamic acid can further deteriorate renal function in patients with underlying renal disease.12 However, the nephrotoxic potential of this agent is of little consideration in ESRD patients on dialysis, and therefore no dosage adjustments are necessary in these patients.6
Ketoprofen is metabolized in the liver, where approximately 80% of the dose is excreted in the urine as a glucuronide metabolite. Dose reduction is recommended in renal insufficiency and dialysis, as it not dialyzable.8
Ketorolac accumulates in renal insufficiency; therefore, it is contraindicated in these patients and in patients at risk for renal failure, including those with volume depletion.10 Ketorolac is unlikely to be removed by dialysis and so should be avoided.10,11
Naproxen is metabolized in the liver to inactive compounds. Use of naproxen is not recommended in patients with moderate to severe renal impairment. If therapy must be initiated, close monitoring of the patient’s renal function is recommended.13
Celecoxib is the only cyclooxygenase-2 (COX-2) inhibitor available in the U.S. It is metabolized extensively by the liver and is unlikely to be removed by dialysis. Therefore, use of COX-2 inhibitors should be avoided in severe renal impairment and in those on dialysis.14,15
Opioid options. The use of opioids in the renally impaired population is challenging, as one must balance opioid-related adverse events with adequate pain control. As such, it is recommended to start with lower-than-recommended doses and slowly titrate up the dose while extending the dosing interval. This will help limit adverse effects, such as respiratory depression and hypotension.3
Hydrocodone is metabolized to hydromorphone (Dilaudid), which is then metabolized to its major metabolite hydromorphine-3-glucuronide (H3G) and minor metabolite hydromorphine-6-hydroxy, all of which are excreted renally along with the parent compound. H3G has no analgesic properties, but it can potentially cause neuroexcitation, agitation, confusion, and hallucination. Hydromorphone has been used safely in patients with renal insufficiency and dialysis, as it is expected to be dialyzable. 16,17
Tramadol is metabolized in the liver, producing one active compound. Approximately 30% of the tramadol dose is excreted unchanged in the urine, whereas 60% of the dose is excreted as metabolites. It is recommended to reduce the dose and increase the dosing interval in patients with renal insufficiency, but tramadol is generally well-tolerated in patients with renal insufficiency and dialysis. It is significantly removed by hemodialysis; therefore, redosing after a session may be necessary.18,19
Oxycodone can be used in patients with mild to moderate renal insufficiency but should be used at reduced dosing; it has been associated with significant sedation with usual doses in renal failure patients.16 Its use is generally not recommended in dialysis patients due to lack of data.3
Methadone and its metabolites are excreted in the urine and feces. Methadone has been used safely in patients with renal insufficiency, but it is poorly removed by dialysis and no specific recommendations are available regarding its dosing in dialysis.3,16
Fentanyl is primarily metabolized in the liver to inactive metabolites. Fentanyl clearance is reduced in patients with moderate to severe uremia (BUN >60 mg/dL). It is not expected that fentanyl be dialyzable because of its pharmacokinetic properties (high protein-binding, low water solubility, high molecular weight, and high volume of distribution). Data suggests that fentanyl can be used at usual doses in mild to moderate renal insufficiency and in dialysis patients, although reduced doses may be prudent. Such patients should be monitored for signs of gradual accumulation of the parent drug.3,16
Morphine is metabolized in the liver to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G), all of which are excreted renally, along with the parent compound. Only M6G has analgesic properties, and when it accumulates, it can lead to CNS depression. M3G is associated with behavioral excitation, a side effect that is further magnified in patients with renal insufficiency. Although morphine is dialyzable, it should generally be avoided in patients with any level of renal insufficiency.16,17,20,21
Codeine is metabolized to several active metabolites, all of which are renally excreted. Lower-than-usual doses are recommended in patients with renal insufficiency, and it should be avoided altogether in dialysis patients.3,16
Meperidine is metabolized in the liver to various metabolites, primarily normeperidine, which is toxic and has a long half-life, five to 10 times longer then meperidine. Meperidine should not be used in patients with renal insufficiency or dialysis.3
Adjunctive therapeutic options. Lidocaine patches currently are only FDA-indicated for postherpetic neuralgia but are used for a wide variety of local pain syndromes. Absorption of lidocaine is determined by the duration of application and the surface area over which it is applied. There is no appreciable accumulation of lidocaine or its metabolites in renal insufficiency; therefore, dose adjustments are not required.22,23
Gabapentin is FDA-indicated for partial seizures and postherpetic neuralgia but is also used for a wide variety of neuropathic pain syndromes, including postoperative pain.24 Gabapentin is not metabolized and is excreted in the urine unchanged. Renal clearance of gabapentin is reduced by 40% and the elimination half-life is increased up to 52 hours in renal insufficiency, but it is dialyzable. Therefore, dose adjustments are required with gabapentin in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.25-27
Pregabalin is structurally related to gabapentin and is indicated for a variety of neuropathic pain conditions. Pregabalin is 90% excreted unchanged in the urine, and approximately 50% of drug is removed after four hours of hemodialysis. Dose adjustments are required in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.28
Antidepressant options. Amitriptyline, nortryptiline, and desipramine are the tricyclic antidepressants (TCAs) commonly used for neuropathic pain. TCAs are metabolized in the liver to inactive metabolites, with the exception of amitriptyline, which is metabolized to nortryptiline. Common side effects reported with TCAs include postural hypotension and anticholinergic side effects, such as constipation, urinary retention, blurred vision, dry mouth, delirium, and sedation. It is unlikely that the TCAs can be removed by dialysis. It is suggested that the dosage be reduced in renal insufficiency and that anticholinergic side effects be monitored.29
Back to the Case
The patient’s ankle pain was controlled with acetaminophen and lidocaine patches. For the neuropathic pain in his upper extremities, tramadol was started at 25 mg oral every 12 hours and increased to 50 mg oral every eight hours (below the maximum of 200 mg a day). The tramadol did not result in adequate pain relief, so gabapentin 100 mg at bedtime was initiated, then increased to twice daily over three days with some relief.
A geriatric consult was obtained to help educate him regarding addiction to opioids, as well as to explore goals of care, but he continued to insist on the use of a non-narcotic regimen for his pain.
Bottom Line
Pain management in patients with renal insufficiency and dialysis can be challenging, but there are a number of safe non-narcotic and narcotic pain regimens that can be safely used in this patient population.
Dr. Harisingani is a board-certified hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y., and Drs. Saad and Cassagnol are assistant clinical professors at St. Johns University College of Pharmacy and Health Sciences in Jamaica, N.Y., and clinical pharmacy coordinators at Long Island Jewish Medical Center.
References
- Mid-Atlantic Renal Coalition and the Kidney End-of-Life Coalition. Clinical algorithm & preferred medications to treat pain in dialysis patients. Coalition for Supportive Care of Kidney Patients website. Available at: http://www.kidneysupportivecare.org/Physicians-Clinicians/Pain—Symptom-Management.aspx. Accessed Nov. 18, 2012.
- Barakzoy AS, Moss AH. Efficacy of the World Health Organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198-3203.
- Johnson SJ. Opioid safety in patients with renal or hepatic dysfunction. Pain Treatment Topics website. Available at: http://pain-topics.org/pdf/Opioids-Renal-Hepatic-Dysfunction.pdf. Accessed Nov. 28, 2012.
- Ferrell B, Argoff CE, Epplin J, et al. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ. Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(3):291-297.
- Launay-Vacher V, Karie S, Fau JB, Izzedine H, Deray G. Treatment of pain in patients with renal insufficiency: the World Health Organization three-step ladder adapted. J Pain. 2006;6(3):137-148.
- Berg KJ, Djøseland O, Gjellan A, et al. Acute effects of paracetamol on prostaglandin synthesis and renal function in normal man and in patients with renal failure. Clin Nephrol. 1990;34:255-262.
- Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 R.P.) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976;1976(0):45-52.
- Shen CH, Hung CJ, Wu CC, Huang HW, Ho WM. Rhabdomyolysis-induced acute renal failure after morphine overdose—a case report. Acta Anaesthesiol Sin. 1999;37(3):159-162.
- Ketorolac tromethamine oral tablets [package insert]. St. Louis: Ethex Corp.: 2008.
- Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23:415-427. Erratum in: Clin Pharmacokinet. 1999;24(3):270.
- Ponstel [package insert]. Alpharetta, GA: First Horizon Pharmaceutical Corp.; 2006.
- Naprosyn [package insert]. Nutley, NJ: Roche Laboratories Inc.; 2008.
- Celebrex [package insert]. New York: G.D. Searle LLC; 2011.
- Catella-Lawson F, McAdam B, Morrison BW, et al. Effects of specific inhibition of cyclooygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735-741.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497-504.
- Lee MA, Leng ME, Tiernan EJ. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med. 2001;15(1):26-34.
- Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCI. Am J. Med. 1996;101(1A):47S-53S.
- Izzedine H, Launay-Vacher V, Abbara C, Aymard G, Bassilios N, Deray G. Pharmacokinetics of tramadol in a hemodialysis patient. Nephron. 2002;92(3):755-756.
- Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24(4):344-354.
- Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25(1):74-91.
- Lidoderm [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010.
- Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12(2):447-459.
- Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;15:126(1-3):91-101.
- Neurontin [package insert]. New York: Parke-Davis; 2010.
- Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51(4):358-363.
- Srivastava U, Kumar A, Saxena S, et al: Effect of preoperative gabapentin on postoperative pain and tramadol consumption after minilap open cholecystectomy: a randomized double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2010;27(N4):331-335.
- Lyrica [package insert]. New York: Pfizer Inc.; 2012.
- Broadbent A, Khor K, Heaney A. Palliation and chronic renal failure: opioid and other palliative medications—dosage guidelines. Progress in Palliative Care. 2003;11(4):183-190(8).
- Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease: a review of analgesics in renal failure. J Nephrol. 2011;24(1):35-40.
- Wolters Kluwer Health. Facts & comparisons. Wolters Kluwer Health website. Available at: http://www.factsandcomparisons.com. Accessed Jan. 14, 2013.
- Lexicomp. Lexicomp Online. Lexicomp website. Available at: http://www.lexi.com/institutions/products/online/.