User login
Using 3-Dimensional Fluoroscopy to Assess Acute Clavicle Fracture Displacement: A Radiographic Study
Clavicle fractures are common injuries, accounting for 2.6% to 5% of all adult fractures.1,2 Most clavicle fractures (69%-82%) occur in the middle third or midshaft.3,4 Midshaft clavicle fractures are often treated successfully with nonoperative means consisting of shoulder immobilization with either a sling or a figure-of-8 brace. Operative indications historically have been limited to open or impending open injuries and to patients with underlying neurovascular compromise. However, recent clinical studies have found that fractures with particular characteristics may benefit from surgical fixation. Important relative indications for open reduction and internal fixation of midshaft clavicle fractures are complete fracture fragment displacement with no cortical contact, and fractures with axial shortening of more than 20 mm.5,6
Accurately determining the extent of displacement and shortening can therefore be important in guiding treatment recommendations. The standard radiographic view for a clavicle fracture is upright or supine anteroposterior (AP). Typically, an AP radiograph with cephalic tilt of about 20° is obtained as well. On occasion, other supplemental radiographs, such as a 45° angulated view, as originally described by Quesada,7 are obtained. To our knowledge, the literature includes only 2 reports of studies that have compared different radiographic views and their accuracy in measuring fracture shortening8,9; no study has determined the best radiographic view for evaluating fracture displacement.
We conducted a study to determine which radiographic view best captures the most fracture fragment displacement. Acute midshaft clavicle fractures were assessed with simulated angled radiographs created from preoperative upright 3-dimensional (3-D) fluoroscopy scans. Our hypothesis was that a radiographic view with 20° of cephalic tilt would most often detect the most fracture displacement. In addition, we retrospectively reviewed our study patients’ initial AP injury radiographs to determine if obtaining a different view at maximum displacement would have helped identify a larger number of completely displaced midshaft clavicle fractures.
Patients and Methods
Institutional review board approval was obtained. Using our institution’s trauma registry database, we retrospectively identified 10 cases of patients who had undergone preoperative 3-D fluoroscopy for midshaft clavicle fractures. Study inclusion criteria were age 18 years or older, acute midshaft clavicle fracture, and preoperative 3-D fluoroscopy scan of clavicle available. Pediatric patients, nonacute injuries, and clavicle fractures of the lateral or medial third were excluded.
Three-dimensional fluoroscopy was used when the treating surgeon deemed it necessary for preoperative planning. All imaging was performed with a Philips MultiDiagnost Eleva 3-D fluoroscopy imager with patients in the upright standing position. (Informed patient consent was obtained.) Software bundled with the imager was used to create representative radiographs of differing angulation.
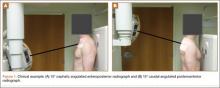
The common practice at most institutions is to obtain 2 radiographic views as part of a standard clavicle series. The additional AP angulated radiograph typically is obtained with 20° to 45° cephalic tilt from the horizontal axis. Therefore, simulated radiographs ranging from 15° to 50° of angulation in 5° increments were created, and the amount of superior displacement of the medial fragment was measured. As the simulated views were constructed from a 3-D composite image, there was none of the magnification error that occurs with AP or posteroanterior (PA) views. The stated degree of angulation mimics a radiograph’s AP cephalic tilt or PA caudal tilt (Figures 1A, 1B). For all radiographic images, displacement between fracture fragments was determined by measuring the distance between the superior cortices at the fracture site of the medial and lateral fragments. Each simulated radiograph was measured by 2 readers using standard computerized radiographic measurement tools. Final displacement was taken as the mean of the 2 measurements.
After determining which radiographic angulation demonstrated the largest number of maximally displaced fractures, we compared the simulated radiographs at that angulation with the injury AP images for all patients. Total number of patients with a completely displaced midshaft clavicle fracture and no cortical contact was recorded for the 2 radiographic views.
The Orthopaedic Trauma Association classification system8 was used to classify the clavicle fractures. Statistical analysis was performed with the Fisher exact test and a regression model, using SPSS Version 19.0 (IBM SPSS Statistics).
Results
Ten patients met the study inclusion criteria. Mean age was 32.9 years (range, 18-65 years). Seven of the 10 patients were male. Six patients had right-side clavicle fractures. Of the 10 patients, 5 had the comminuted wedge fracture pattern (15-B2.3), 2 had the simple spiral pattern (15-B1.1), 2 had the spiral wedge pattern (15-B2.1), and 1 had the oblique pattern (15-B1.2).
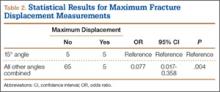
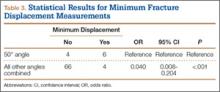
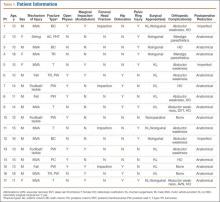
Table 1 summarizes the fracture displacement measurements obtained with the different radiographic views. Of the 10 cases, 5 showed the most displacement with the 15° tilted view (P = .004), and the other 5 showed maximum displacement with different radiographic angulations. In addition, 6 patients showed the least displacement with the 50° angulated view (P < .001). Results of the regression analysis are summarized in Tables 2 and 3.
Initial horizontal AP imaging showed completely displaced midshaft clavicle fractures in 9 of the 10 patients, and 15° simulated radiographs showed completely displaced fractures in all 10 patients (P = .50).
Discussion
Our study results demonstrated that an upright 15° radiographic tilt (AP cephalad or PA caudal) identified the most fracture displacement in the most patients with acute midshaft clavicle fractures. To our knowledge, this is the first study to identify the radiographic angulation that best shows the most clavicle fracture fragment displacement.
Other investigators have studied the accuracy of different radiographic views in the assessment of midshaft clavicle fractures, but they concentrated on fracture shortening. Smekal and colleagues9 used computed tomography (CT) and 3 different radiographic views to evaluate malunited midshaft clavicle fractures. Comparing the horizontal clavicular length measurements obtained with radiographs and CT scans, they determined that PA thoracic radiographs were in highest agreement with the CT scans. The results, however, were not statistically significant. In their study, supine CT was successful because the fractures were healed, and the displacement and shortening amounts were not affected by patient position. Sharr and Mohammed10 studied the accuracy of different views in the assessment of clavicle length in an articulated cadaver specimen. They obtained multiple AP and PA radiographs of different horizontal (medial, lateral) and vertical (cephalad, caudal) angulations. Actual clavicle length was then directly measured and compared with the length measured on the different views. The authors concluded that a PA 15° caudal radiograph was most accurate in assessing clavicular length. Both Smekal and colleagues9 and Sharr and Mohammed10 recommended the PA radiograph because it decreases the degree of magnification on AP radiographs by minimizing the film-to-object distance.
Our findings are important because more accurate determination of fracture displacement in patients with midshaft clavicle fractures may change clinical management. Nowak and colleagues11 investigated various patient and clavicle fracture characteristics that were predictive of a higher rate of long-term sequelae. They found that complete fracture displacement was the strongest radiographic predictor of patients’ beliefs that they were fully recovered from injury at final follow-up. The authors concluded that fractures with no bony contact should receive more “active” management. Robinson and colleagues12 studied a cohort of patients with nonoperatively managed midshaft clavicle fractures and concluded that complete fracture displacement significantly increased risk for nonunion (this risk was 2.3 times higher in patients with displaced fractures than in patients with nondisplaced fractures). Last, McKee and colleagues13 found that shoulder strength and endurance were significantly decreased in nonoperatively treated displaced midshaft clavicle fractures than in the same patients’ uninjured shoulders.
Extending the results of these studies, recent prospective randomized control trials and a meta-analysis have compared the clinical outcomes of nonoperatively and operatively managed displaced midshaft clavicle fractures.14-18 With few exceptions, these studies found improved clinical results with operative fixation. In one such study, the Canadian Orthopaedic Trauma Society14 randomized patients with displaced midshaft clavicle fractures to either operative plate fixation or sling immobilization. The operative group was found to have improved Disability of the Arm, Shoulder, and Hand scores, improved Constant shoulder scores, increased patient satisfaction, faster mean time to bony fracture union, higher satisfaction with shoulder appearance, and lower rates of nonunion and malunion. Given the results of these studies, accurate identification of a displaced midshaft clavicle fracture with no cortical contact is fundamental in deciding whether to recommend operative fixation.
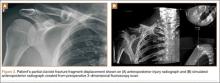
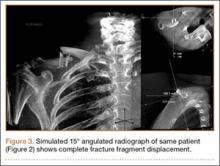
Retrospective review of our cohort’s initial radiographs revealed 1 case in which the patient’s completely displaced midshaft clavicle fracture would not have been diagnosed solely with an AP horizontal image. Cortical contact was seen on a standard AP clavicle radiograph (Figures 2A, 2B), and a 15° tilt radiograph created from 3-D fluoroscopy scan showed complete fracture fragment displacement (Figure 3). A change in fracture classification from partially displaced to fully displaced could alter the type of management used by a treating surgeon.
There were obvious weaknesses to this study. First, its sample size was small (10 patients). Nevertheless, we had sufficient numbers to find a statistically significant angulation. Second, a wider range of radiographic angles could have been studied. Our intent, however, was to investigate the accuracy of the 2 most common supplementary clavicle views (20° and 45° cephalic tilt). Therefore, we selected a range of simulated radiographs that began 5° outside these angulations. Third, we measured only the degree of fracture displacement; we were unable to accurately access fracture shortening, as the 3-D fluoroscopic images were limited to the injured clavicles. A potential solution to this problem is to widen the exposure field in order to include the entire chest and allow clavicular length comparison against the uninjured side. Doing this would have been possible, but at the expense of increasing the patient’s radiation exposure.
This innovative study used 3-D fluoroscopy to capture clavicle fracture images with patients in an upright position. Unlike standard CT, in which patients are supine, this 3-D imaging technology better emulates the patient positioning used for upright radiographs, thereby avoiding potential fracture fragment alignment changes caused by shifts in body position. In addition, 3-D fluoroscopy allows us to create multiple precise simulated radiographic angulations without the magnification error of AP radiographs and, to a lesser extent, PA radiographs. Having a standing PA 15° caudal tilt radiograph obviates the need for CT with 3-D reconstruction. More fine detail may be revealed by CT with 3-D reconstruction than by a standing PA 15° caudal tilt radiograph, but the patient faces less radiation risk and cost with the radiograph.
There is no consensus as to what constitutes the standard radiographic series for clavicle fractures. Radiographic technique can vary with respect to supplemental view angulation, supine or upright patient positioning, and AP or PA radiographic views. Although our study did not address the effect of supine versus upright patient positioning on acute midshaft clavicle fracture displacement, we think that, for all clinical and research purposes, upright 15° caudal PA radiographs should be obtained for patients with acute midshaft clavicle fractures.
Conclusion
Our retrospective study of 10 patients with acute midshaft clavicle fractures and preoperative upright 3-D fluoroscopy scans found that a 15° angulated radiograph most often demonstrated the most fracture fragment displacement. Given these findings, we recommend obtaining an additional PA 15° caudal radiograph in the upright position for patients with midshaft clavicle fractures to best assess the extent of fracture displacement. Accurately identifying the degree of fracture displacement is important, as operative management of completely displaced fractures has been shown to improve clinical outcomes.
1. Postacchini F, Gumina S, De Santis P, Albo F. Epidemiology of clavicle fractures. J Shoulder Elbow Surg. 2002;11(5):452-456.
2. Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clin Orthop Relat Res. 1994;(300):127-132.
3. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
4. Rowe CR. An atlas of anatomy and treatment of midclavicular fractures. Clin Orthop Relat Res. 1968;(58):29-42.
5. Jeray KJ. Acute midshaft clavicular fracture. J Am Acad Orthop Surg. 2007;15(4):239-248.
6. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
7. Quesada F. Technique for the roentgen diagnosis of fractures of the clavicle. Surg Gynecol Obstet. 1926;42:424-428.
8. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
9. Smekal V, Deml C, Irenberger A, et al. Length determination in midshaft clavicle fractures: validation of measurement. J Orthop Trauma. 2008;22(7):458-462.
10. Sharr JR, Mohammed KD. Optimizing the radiographic technique in clavicular fractures. J Shoulder Elbow Surg. 2003;12(2):170-172.
11. Nowak J, Holgersson M, Larsson S. Can we predict long-term sequelae after fractures of the clavicle based on initial findings? A prospective study with nine to ten years of follow-up. J Shoulder Elbow Surg. 2004;13(5):479-486.
12. Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86(7):1359-1365.
13. McKee MD, Pedersen EM, Jones C, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. J Bone Joint Surg Am. 2006;88(1):35-40.
14. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
15. Judd DB, Pallis MP, Smith E, Bottoni CR. Acute operative stabilization versus nonoperative management of clavicle fractures. Am J Orthop. 2009;38(7):341-345.
16. Smekal V, Irenberger A, Struve P, Wambacher M, Krappinger D, Kralinger FS. Elastic stable intramedullary nailing versus nonoperative treatment of displaced midshaft clavicular fractures—a randomized, controlled, clinical trial. J Orthop Trauma. 2009;23(2):106-112.
17. Witzel K. Intramedullary osteosynthesis in fractures of the mid-third of the clavicle in sports traumatology [in German]. Z Orthop Unfall. 2007;145(5):639-642.
18. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
Clavicle fractures are common injuries, accounting for 2.6% to 5% of all adult fractures.1,2 Most clavicle fractures (69%-82%) occur in the middle third or midshaft.3,4 Midshaft clavicle fractures are often treated successfully with nonoperative means consisting of shoulder immobilization with either a sling or a figure-of-8 brace. Operative indications historically have been limited to open or impending open injuries and to patients with underlying neurovascular compromise. However, recent clinical studies have found that fractures with particular characteristics may benefit from surgical fixation. Important relative indications for open reduction and internal fixation of midshaft clavicle fractures are complete fracture fragment displacement with no cortical contact, and fractures with axial shortening of more than 20 mm.5,6
Accurately determining the extent of displacement and shortening can therefore be important in guiding treatment recommendations. The standard radiographic view for a clavicle fracture is upright or supine anteroposterior (AP). Typically, an AP radiograph with cephalic tilt of about 20° is obtained as well. On occasion, other supplemental radiographs, such as a 45° angulated view, as originally described by Quesada,7 are obtained. To our knowledge, the literature includes only 2 reports of studies that have compared different radiographic views and their accuracy in measuring fracture shortening8,9; no study has determined the best radiographic view for evaluating fracture displacement.
We conducted a study to determine which radiographic view best captures the most fracture fragment displacement. Acute midshaft clavicle fractures were assessed with simulated angled radiographs created from preoperative upright 3-dimensional (3-D) fluoroscopy scans. Our hypothesis was that a radiographic view with 20° of cephalic tilt would most often detect the most fracture displacement. In addition, we retrospectively reviewed our study patients’ initial AP injury radiographs to determine if obtaining a different view at maximum displacement would have helped identify a larger number of completely displaced midshaft clavicle fractures.
Patients and Methods
Institutional review board approval was obtained. Using our institution’s trauma registry database, we retrospectively identified 10 cases of patients who had undergone preoperative 3-D fluoroscopy for midshaft clavicle fractures. Study inclusion criteria were age 18 years or older, acute midshaft clavicle fracture, and preoperative 3-D fluoroscopy scan of clavicle available. Pediatric patients, nonacute injuries, and clavicle fractures of the lateral or medial third were excluded.
Three-dimensional fluoroscopy was used when the treating surgeon deemed it necessary for preoperative planning. All imaging was performed with a Philips MultiDiagnost Eleva 3-D fluoroscopy imager with patients in the upright standing position. (Informed patient consent was obtained.) Software bundled with the imager was used to create representative radiographs of differing angulation.
The common practice at most institutions is to obtain 2 radiographic views as part of a standard clavicle series. The additional AP angulated radiograph typically is obtained with 20° to 45° cephalic tilt from the horizontal axis. Therefore, simulated radiographs ranging from 15° to 50° of angulation in 5° increments were created, and the amount of superior displacement of the medial fragment was measured. As the simulated views were constructed from a 3-D composite image, there was none of the magnification error that occurs with AP or posteroanterior (PA) views. The stated degree of angulation mimics a radiograph’s AP cephalic tilt or PA caudal tilt (Figures 1A, 1B). For all radiographic images, displacement between fracture fragments was determined by measuring the distance between the superior cortices at the fracture site of the medial and lateral fragments. Each simulated radiograph was measured by 2 readers using standard computerized radiographic measurement tools. Final displacement was taken as the mean of the 2 measurements.
After determining which radiographic angulation demonstrated the largest number of maximally displaced fractures, we compared the simulated radiographs at that angulation with the injury AP images for all patients. Total number of patients with a completely displaced midshaft clavicle fracture and no cortical contact was recorded for the 2 radiographic views.
The Orthopaedic Trauma Association classification system8 was used to classify the clavicle fractures. Statistical analysis was performed with the Fisher exact test and a regression model, using SPSS Version 19.0 (IBM SPSS Statistics).
Results
Ten patients met the study inclusion criteria. Mean age was 32.9 years (range, 18-65 years). Seven of the 10 patients were male. Six patients had right-side clavicle fractures. Of the 10 patients, 5 had the comminuted wedge fracture pattern (15-B2.3), 2 had the simple spiral pattern (15-B1.1), 2 had the spiral wedge pattern (15-B2.1), and 1 had the oblique pattern (15-B1.2).
Table 1 summarizes the fracture displacement measurements obtained with the different radiographic views. Of the 10 cases, 5 showed the most displacement with the 15° tilted view (P = .004), and the other 5 showed maximum displacement with different radiographic angulations. In addition, 6 patients showed the least displacement with the 50° angulated view (P < .001). Results of the regression analysis are summarized in Tables 2 and 3.
Initial horizontal AP imaging showed completely displaced midshaft clavicle fractures in 9 of the 10 patients, and 15° simulated radiographs showed completely displaced fractures in all 10 patients (P = .50).
Discussion
Our study results demonstrated that an upright 15° radiographic tilt (AP cephalad or PA caudal) identified the most fracture displacement in the most patients with acute midshaft clavicle fractures. To our knowledge, this is the first study to identify the radiographic angulation that best shows the most clavicle fracture fragment displacement.
Other investigators have studied the accuracy of different radiographic views in the assessment of midshaft clavicle fractures, but they concentrated on fracture shortening. Smekal and colleagues9 used computed tomography (CT) and 3 different radiographic views to evaluate malunited midshaft clavicle fractures. Comparing the horizontal clavicular length measurements obtained with radiographs and CT scans, they determined that PA thoracic radiographs were in highest agreement with the CT scans. The results, however, were not statistically significant. In their study, supine CT was successful because the fractures were healed, and the displacement and shortening amounts were not affected by patient position. Sharr and Mohammed10 studied the accuracy of different views in the assessment of clavicle length in an articulated cadaver specimen. They obtained multiple AP and PA radiographs of different horizontal (medial, lateral) and vertical (cephalad, caudal) angulations. Actual clavicle length was then directly measured and compared with the length measured on the different views. The authors concluded that a PA 15° caudal radiograph was most accurate in assessing clavicular length. Both Smekal and colleagues9 and Sharr and Mohammed10 recommended the PA radiograph because it decreases the degree of magnification on AP radiographs by minimizing the film-to-object distance.
Our findings are important because more accurate determination of fracture displacement in patients with midshaft clavicle fractures may change clinical management. Nowak and colleagues11 investigated various patient and clavicle fracture characteristics that were predictive of a higher rate of long-term sequelae. They found that complete fracture displacement was the strongest radiographic predictor of patients’ beliefs that they were fully recovered from injury at final follow-up. The authors concluded that fractures with no bony contact should receive more “active” management. Robinson and colleagues12 studied a cohort of patients with nonoperatively managed midshaft clavicle fractures and concluded that complete fracture displacement significantly increased risk for nonunion (this risk was 2.3 times higher in patients with displaced fractures than in patients with nondisplaced fractures). Last, McKee and colleagues13 found that shoulder strength and endurance were significantly decreased in nonoperatively treated displaced midshaft clavicle fractures than in the same patients’ uninjured shoulders.
Extending the results of these studies, recent prospective randomized control trials and a meta-analysis have compared the clinical outcomes of nonoperatively and operatively managed displaced midshaft clavicle fractures.14-18 With few exceptions, these studies found improved clinical results with operative fixation. In one such study, the Canadian Orthopaedic Trauma Society14 randomized patients with displaced midshaft clavicle fractures to either operative plate fixation or sling immobilization. The operative group was found to have improved Disability of the Arm, Shoulder, and Hand scores, improved Constant shoulder scores, increased patient satisfaction, faster mean time to bony fracture union, higher satisfaction with shoulder appearance, and lower rates of nonunion and malunion. Given the results of these studies, accurate identification of a displaced midshaft clavicle fracture with no cortical contact is fundamental in deciding whether to recommend operative fixation.
Retrospective review of our cohort’s initial radiographs revealed 1 case in which the patient’s completely displaced midshaft clavicle fracture would not have been diagnosed solely with an AP horizontal image. Cortical contact was seen on a standard AP clavicle radiograph (Figures 2A, 2B), and a 15° tilt radiograph created from 3-D fluoroscopy scan showed complete fracture fragment displacement (Figure 3). A change in fracture classification from partially displaced to fully displaced could alter the type of management used by a treating surgeon.
There were obvious weaknesses to this study. First, its sample size was small (10 patients). Nevertheless, we had sufficient numbers to find a statistically significant angulation. Second, a wider range of radiographic angles could have been studied. Our intent, however, was to investigate the accuracy of the 2 most common supplementary clavicle views (20° and 45° cephalic tilt). Therefore, we selected a range of simulated radiographs that began 5° outside these angulations. Third, we measured only the degree of fracture displacement; we were unable to accurately access fracture shortening, as the 3-D fluoroscopic images were limited to the injured clavicles. A potential solution to this problem is to widen the exposure field in order to include the entire chest and allow clavicular length comparison against the uninjured side. Doing this would have been possible, but at the expense of increasing the patient’s radiation exposure.
This innovative study used 3-D fluoroscopy to capture clavicle fracture images with patients in an upright position. Unlike standard CT, in which patients are supine, this 3-D imaging technology better emulates the patient positioning used for upright radiographs, thereby avoiding potential fracture fragment alignment changes caused by shifts in body position. In addition, 3-D fluoroscopy allows us to create multiple precise simulated radiographic angulations without the magnification error of AP radiographs and, to a lesser extent, PA radiographs. Having a standing PA 15° caudal tilt radiograph obviates the need for CT with 3-D reconstruction. More fine detail may be revealed by CT with 3-D reconstruction than by a standing PA 15° caudal tilt radiograph, but the patient faces less radiation risk and cost with the radiograph.
There is no consensus as to what constitutes the standard radiographic series for clavicle fractures. Radiographic technique can vary with respect to supplemental view angulation, supine or upright patient positioning, and AP or PA radiographic views. Although our study did not address the effect of supine versus upright patient positioning on acute midshaft clavicle fracture displacement, we think that, for all clinical and research purposes, upright 15° caudal PA radiographs should be obtained for patients with acute midshaft clavicle fractures.
Conclusion
Our retrospective study of 10 patients with acute midshaft clavicle fractures and preoperative upright 3-D fluoroscopy scans found that a 15° angulated radiograph most often demonstrated the most fracture fragment displacement. Given these findings, we recommend obtaining an additional PA 15° caudal radiograph in the upright position for patients with midshaft clavicle fractures to best assess the extent of fracture displacement. Accurately identifying the degree of fracture displacement is important, as operative management of completely displaced fractures has been shown to improve clinical outcomes.
Clavicle fractures are common injuries, accounting for 2.6% to 5% of all adult fractures.1,2 Most clavicle fractures (69%-82%) occur in the middle third or midshaft.3,4 Midshaft clavicle fractures are often treated successfully with nonoperative means consisting of shoulder immobilization with either a sling or a figure-of-8 brace. Operative indications historically have been limited to open or impending open injuries and to patients with underlying neurovascular compromise. However, recent clinical studies have found that fractures with particular characteristics may benefit from surgical fixation. Important relative indications for open reduction and internal fixation of midshaft clavicle fractures are complete fracture fragment displacement with no cortical contact, and fractures with axial shortening of more than 20 mm.5,6
Accurately determining the extent of displacement and shortening can therefore be important in guiding treatment recommendations. The standard radiographic view for a clavicle fracture is upright or supine anteroposterior (AP). Typically, an AP radiograph with cephalic tilt of about 20° is obtained as well. On occasion, other supplemental radiographs, such as a 45° angulated view, as originally described by Quesada,7 are obtained. To our knowledge, the literature includes only 2 reports of studies that have compared different radiographic views and their accuracy in measuring fracture shortening8,9; no study has determined the best radiographic view for evaluating fracture displacement.
We conducted a study to determine which radiographic view best captures the most fracture fragment displacement. Acute midshaft clavicle fractures were assessed with simulated angled radiographs created from preoperative upright 3-dimensional (3-D) fluoroscopy scans. Our hypothesis was that a radiographic view with 20° of cephalic tilt would most often detect the most fracture displacement. In addition, we retrospectively reviewed our study patients’ initial AP injury radiographs to determine if obtaining a different view at maximum displacement would have helped identify a larger number of completely displaced midshaft clavicle fractures.
Patients and Methods
Institutional review board approval was obtained. Using our institution’s trauma registry database, we retrospectively identified 10 cases of patients who had undergone preoperative 3-D fluoroscopy for midshaft clavicle fractures. Study inclusion criteria were age 18 years or older, acute midshaft clavicle fracture, and preoperative 3-D fluoroscopy scan of clavicle available. Pediatric patients, nonacute injuries, and clavicle fractures of the lateral or medial third were excluded.
Three-dimensional fluoroscopy was used when the treating surgeon deemed it necessary for preoperative planning. All imaging was performed with a Philips MultiDiagnost Eleva 3-D fluoroscopy imager with patients in the upright standing position. (Informed patient consent was obtained.) Software bundled with the imager was used to create representative radiographs of differing angulation.
The common practice at most institutions is to obtain 2 radiographic views as part of a standard clavicle series. The additional AP angulated radiograph typically is obtained with 20° to 45° cephalic tilt from the horizontal axis. Therefore, simulated radiographs ranging from 15° to 50° of angulation in 5° increments were created, and the amount of superior displacement of the medial fragment was measured. As the simulated views were constructed from a 3-D composite image, there was none of the magnification error that occurs with AP or posteroanterior (PA) views. The stated degree of angulation mimics a radiograph’s AP cephalic tilt or PA caudal tilt (Figures 1A, 1B). For all radiographic images, displacement between fracture fragments was determined by measuring the distance between the superior cortices at the fracture site of the medial and lateral fragments. Each simulated radiograph was measured by 2 readers using standard computerized radiographic measurement tools. Final displacement was taken as the mean of the 2 measurements.
After determining which radiographic angulation demonstrated the largest number of maximally displaced fractures, we compared the simulated radiographs at that angulation with the injury AP images for all patients. Total number of patients with a completely displaced midshaft clavicle fracture and no cortical contact was recorded for the 2 radiographic views.
The Orthopaedic Trauma Association classification system8 was used to classify the clavicle fractures. Statistical analysis was performed with the Fisher exact test and a regression model, using SPSS Version 19.0 (IBM SPSS Statistics).
Results
Ten patients met the study inclusion criteria. Mean age was 32.9 years (range, 18-65 years). Seven of the 10 patients were male. Six patients had right-side clavicle fractures. Of the 10 patients, 5 had the comminuted wedge fracture pattern (15-B2.3), 2 had the simple spiral pattern (15-B1.1), 2 had the spiral wedge pattern (15-B2.1), and 1 had the oblique pattern (15-B1.2).
Table 1 summarizes the fracture displacement measurements obtained with the different radiographic views. Of the 10 cases, 5 showed the most displacement with the 15° tilted view (P = .004), and the other 5 showed maximum displacement with different radiographic angulations. In addition, 6 patients showed the least displacement with the 50° angulated view (P < .001). Results of the regression analysis are summarized in Tables 2 and 3.
Initial horizontal AP imaging showed completely displaced midshaft clavicle fractures in 9 of the 10 patients, and 15° simulated radiographs showed completely displaced fractures in all 10 patients (P = .50).
Discussion
Our study results demonstrated that an upright 15° radiographic tilt (AP cephalad or PA caudal) identified the most fracture displacement in the most patients with acute midshaft clavicle fractures. To our knowledge, this is the first study to identify the radiographic angulation that best shows the most clavicle fracture fragment displacement.
Other investigators have studied the accuracy of different radiographic views in the assessment of midshaft clavicle fractures, but they concentrated on fracture shortening. Smekal and colleagues9 used computed tomography (CT) and 3 different radiographic views to evaluate malunited midshaft clavicle fractures. Comparing the horizontal clavicular length measurements obtained with radiographs and CT scans, they determined that PA thoracic radiographs were in highest agreement with the CT scans. The results, however, were not statistically significant. In their study, supine CT was successful because the fractures were healed, and the displacement and shortening amounts were not affected by patient position. Sharr and Mohammed10 studied the accuracy of different views in the assessment of clavicle length in an articulated cadaver specimen. They obtained multiple AP and PA radiographs of different horizontal (medial, lateral) and vertical (cephalad, caudal) angulations. Actual clavicle length was then directly measured and compared with the length measured on the different views. The authors concluded that a PA 15° caudal radiograph was most accurate in assessing clavicular length. Both Smekal and colleagues9 and Sharr and Mohammed10 recommended the PA radiograph because it decreases the degree of magnification on AP radiographs by minimizing the film-to-object distance.
Our findings are important because more accurate determination of fracture displacement in patients with midshaft clavicle fractures may change clinical management. Nowak and colleagues11 investigated various patient and clavicle fracture characteristics that were predictive of a higher rate of long-term sequelae. They found that complete fracture displacement was the strongest radiographic predictor of patients’ beliefs that they were fully recovered from injury at final follow-up. The authors concluded that fractures with no bony contact should receive more “active” management. Robinson and colleagues12 studied a cohort of patients with nonoperatively managed midshaft clavicle fractures and concluded that complete fracture displacement significantly increased risk for nonunion (this risk was 2.3 times higher in patients with displaced fractures than in patients with nondisplaced fractures). Last, McKee and colleagues13 found that shoulder strength and endurance were significantly decreased in nonoperatively treated displaced midshaft clavicle fractures than in the same patients’ uninjured shoulders.
Extending the results of these studies, recent prospective randomized control trials and a meta-analysis have compared the clinical outcomes of nonoperatively and operatively managed displaced midshaft clavicle fractures.14-18 With few exceptions, these studies found improved clinical results with operative fixation. In one such study, the Canadian Orthopaedic Trauma Society14 randomized patients with displaced midshaft clavicle fractures to either operative plate fixation or sling immobilization. The operative group was found to have improved Disability of the Arm, Shoulder, and Hand scores, improved Constant shoulder scores, increased patient satisfaction, faster mean time to bony fracture union, higher satisfaction with shoulder appearance, and lower rates of nonunion and malunion. Given the results of these studies, accurate identification of a displaced midshaft clavicle fracture with no cortical contact is fundamental in deciding whether to recommend operative fixation.
Retrospective review of our cohort’s initial radiographs revealed 1 case in which the patient’s completely displaced midshaft clavicle fracture would not have been diagnosed solely with an AP horizontal image. Cortical contact was seen on a standard AP clavicle radiograph (Figures 2A, 2B), and a 15° tilt radiograph created from 3-D fluoroscopy scan showed complete fracture fragment displacement (Figure 3). A change in fracture classification from partially displaced to fully displaced could alter the type of management used by a treating surgeon.
There were obvious weaknesses to this study. First, its sample size was small (10 patients). Nevertheless, we had sufficient numbers to find a statistically significant angulation. Second, a wider range of radiographic angles could have been studied. Our intent, however, was to investigate the accuracy of the 2 most common supplementary clavicle views (20° and 45° cephalic tilt). Therefore, we selected a range of simulated radiographs that began 5° outside these angulations. Third, we measured only the degree of fracture displacement; we were unable to accurately access fracture shortening, as the 3-D fluoroscopic images were limited to the injured clavicles. A potential solution to this problem is to widen the exposure field in order to include the entire chest and allow clavicular length comparison against the uninjured side. Doing this would have been possible, but at the expense of increasing the patient’s radiation exposure.
This innovative study used 3-D fluoroscopy to capture clavicle fracture images with patients in an upright position. Unlike standard CT, in which patients are supine, this 3-D imaging technology better emulates the patient positioning used for upright radiographs, thereby avoiding potential fracture fragment alignment changes caused by shifts in body position. In addition, 3-D fluoroscopy allows us to create multiple precise simulated radiographic angulations without the magnification error of AP radiographs and, to a lesser extent, PA radiographs. Having a standing PA 15° caudal tilt radiograph obviates the need for CT with 3-D reconstruction. More fine detail may be revealed by CT with 3-D reconstruction than by a standing PA 15° caudal tilt radiograph, but the patient faces less radiation risk and cost with the radiograph.
There is no consensus as to what constitutes the standard radiographic series for clavicle fractures. Radiographic technique can vary with respect to supplemental view angulation, supine or upright patient positioning, and AP or PA radiographic views. Although our study did not address the effect of supine versus upright patient positioning on acute midshaft clavicle fracture displacement, we think that, for all clinical and research purposes, upright 15° caudal PA radiographs should be obtained for patients with acute midshaft clavicle fractures.
Conclusion
Our retrospective study of 10 patients with acute midshaft clavicle fractures and preoperative upright 3-D fluoroscopy scans found that a 15° angulated radiograph most often demonstrated the most fracture fragment displacement. Given these findings, we recommend obtaining an additional PA 15° caudal radiograph in the upright position for patients with midshaft clavicle fractures to best assess the extent of fracture displacement. Accurately identifying the degree of fracture displacement is important, as operative management of completely displaced fractures has been shown to improve clinical outcomes.
1. Postacchini F, Gumina S, De Santis P, Albo F. Epidemiology of clavicle fractures. J Shoulder Elbow Surg. 2002;11(5):452-456.
2. Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clin Orthop Relat Res. 1994;(300):127-132.
3. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
4. Rowe CR. An atlas of anatomy and treatment of midclavicular fractures. Clin Orthop Relat Res. 1968;(58):29-42.
5. Jeray KJ. Acute midshaft clavicular fracture. J Am Acad Orthop Surg. 2007;15(4):239-248.
6. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
7. Quesada F. Technique for the roentgen diagnosis of fractures of the clavicle. Surg Gynecol Obstet. 1926;42:424-428.
8. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
9. Smekal V, Deml C, Irenberger A, et al. Length determination in midshaft clavicle fractures: validation of measurement. J Orthop Trauma. 2008;22(7):458-462.
10. Sharr JR, Mohammed KD. Optimizing the radiographic technique in clavicular fractures. J Shoulder Elbow Surg. 2003;12(2):170-172.
11. Nowak J, Holgersson M, Larsson S. Can we predict long-term sequelae after fractures of the clavicle based on initial findings? A prospective study with nine to ten years of follow-up. J Shoulder Elbow Surg. 2004;13(5):479-486.
12. Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86(7):1359-1365.
13. McKee MD, Pedersen EM, Jones C, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. J Bone Joint Surg Am. 2006;88(1):35-40.
14. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
15. Judd DB, Pallis MP, Smith E, Bottoni CR. Acute operative stabilization versus nonoperative management of clavicle fractures. Am J Orthop. 2009;38(7):341-345.
16. Smekal V, Irenberger A, Struve P, Wambacher M, Krappinger D, Kralinger FS. Elastic stable intramedullary nailing versus nonoperative treatment of displaced midshaft clavicular fractures—a randomized, controlled, clinical trial. J Orthop Trauma. 2009;23(2):106-112.
17. Witzel K. Intramedullary osteosynthesis in fractures of the mid-third of the clavicle in sports traumatology [in German]. Z Orthop Unfall. 2007;145(5):639-642.
18. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
1. Postacchini F, Gumina S, De Santis P, Albo F. Epidemiology of clavicle fractures. J Shoulder Elbow Surg. 2002;11(5):452-456.
2. Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clin Orthop Relat Res. 1994;(300):127-132.
3. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
4. Rowe CR. An atlas of anatomy and treatment of midclavicular fractures. Clin Orthop Relat Res. 1968;(58):29-42.
5. Jeray KJ. Acute midshaft clavicular fracture. J Am Acad Orthop Surg. 2007;15(4):239-248.
6. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
7. Quesada F. Technique for the roentgen diagnosis of fractures of the clavicle. Surg Gynecol Obstet. 1926;42:424-428.
8. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
9. Smekal V, Deml C, Irenberger A, et al. Length determination in midshaft clavicle fractures: validation of measurement. J Orthop Trauma. 2008;22(7):458-462.
10. Sharr JR, Mohammed KD. Optimizing the radiographic technique in clavicular fractures. J Shoulder Elbow Surg. 2003;12(2):170-172.
11. Nowak J, Holgersson M, Larsson S. Can we predict long-term sequelae after fractures of the clavicle based on initial findings? A prospective study with nine to ten years of follow-up. J Shoulder Elbow Surg. 2004;13(5):479-486.
12. Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86(7):1359-1365.
13. McKee MD, Pedersen EM, Jones C, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. J Bone Joint Surg Am. 2006;88(1):35-40.
14. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
15. Judd DB, Pallis MP, Smith E, Bottoni CR. Acute operative stabilization versus nonoperative management of clavicle fractures. Am J Orthop. 2009;38(7):341-345.
16. Smekal V, Irenberger A, Struve P, Wambacher M, Krappinger D, Kralinger FS. Elastic stable intramedullary nailing versus nonoperative treatment of displaced midshaft clavicular fractures—a randomized, controlled, clinical trial. J Orthop Trauma. 2009;23(2):106-112.
17. Witzel K. Intramedullary osteosynthesis in fractures of the mid-third of the clavicle in sports traumatology [in German]. Z Orthop Unfall. 2007;145(5):639-642.
18. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
Treatment of Acetabular Fractures in Adolescents
In children, pelvic fractures are uncommon, with an incidence ranging from 1% to 4.6% of all pediatric fractures,1-4 and acetabular fractures make up only 0.8% to 15% of pelvic fractures.1,3,5,6 Acetabular fractures are so uncommon in children partly because of the cartilaginous nature of the immature acetabulum. The increased cartilage volume relative to adults provides greater capacity for energy absorption, resulting in greater elastic and plastic deformation before fracture occurrence. More force is therefore required to cause a fracture, and associated visceral injuries, head injuries, and long-bone fractures are common.3,7,8
The impact of acetabular fractures on adolescents warrants special attention because any resulting disability will affect them during their most productive years. Both avascular necrosis (AVN) and degenerative arthritis are particularly devastating complications in this age group. Complications such as premature physeal closure9-15 are unique to adolescents, and there is little information available on how injury in older children affects growth in this area.
There have been very few studies of the outcomes of these injuries in children. Mostly, there have been case reports and small series primarily dealing with nonoperative management of acetabular fractures in adolescents.3,10,11,16-20 By contrast, operative treatment of acetabular fractures in adults has been well described, and outcomes widely reported. As a result, much of our knowledge about managing these injuries is extrapolated from the adult literature. Although treatment of acetabular fractures in adults has evolved substantially, treatment of these injuries in adolescents remains primarily nonoperative. We conducted a study to evaluate outcomes of treatment of adolescent acetabular fractures.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the cases of all adolescent patients admitted with a diagnosis of acetabular fracture to 2 academic institutions between 1991 and 2003. Thirty-eight patients (28 males, 10 females) were identified. Mean age at time of injury was 15 years (range, 11-18 years). Mean follow-up was 3.2 years (range, 5-180 months).
Data on fracture types, treatment methods, associated injuries, complications, union rates, pain, and return to normal activities were collected. Acetabular fractures were classified according to the system of Letournel and Judet.21 There were 20 elementary and 18 associated fractures.
Of the 38 patients, 30 sustained high-energy trauma in motor vehicle accidents (25) or in falls from significant heights (5). The other 8 patients injured themselves playing sports (4 had severe traumatic brain injury, 2 had labial wounds, and 2 had injuries involving the abdominal viscera). Twelve patients had associated pelvic ring injuries, 18 had femoral head dislocations, 2 had femoral head fractures, and 13 had evidence of impaction injury to the femoral head articular cartilage. Twelve patients had marginal impaction of the acetabular wall. Fifteen patients had open triradiate physes at time of injury (Table 1).
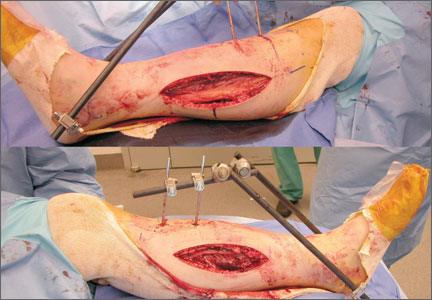
Thirty-seven of the 38 patients were treated with open reduction and internal fixation (ORIF) by an experienced orthopedic trauma surgeon; 1 patient with a stable posterior wall fracture was treated nonoperatively. Surgical indications were articular displacement of more than 1 mm, hip joint instability, irreducible hip dislocation, and intra-articular fracture fragments. In the 37 surgically treated cases, the approaches used were Kocher-Langenbeck (22), ilioinguinal (8), combined Kocher-Langenbeck/ilioinguinal (5), and triradiate (2).
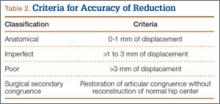
Immediate postoperative radiographs were evaluated by 3 orthopedic surgeons blinded to the patients’ clinical outcomes. Displacement was evaluated on anteroposterior (AP) and Judet views of the pelvis, as described by Matta,22 and reductions were classified as anatomical (0-1 mm of displacement), imperfect (>1 to 3 mm), poor (>3 mm), or surgical secondary congruence (Table 2).
Results
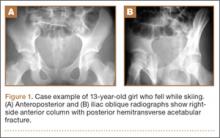
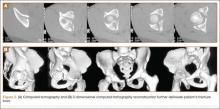
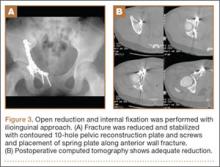
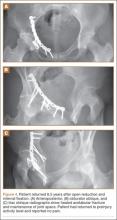
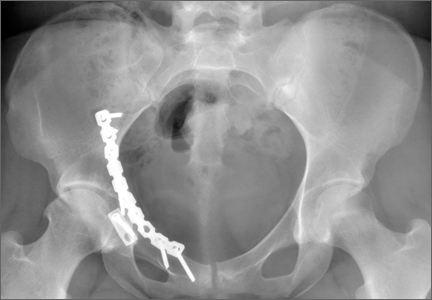
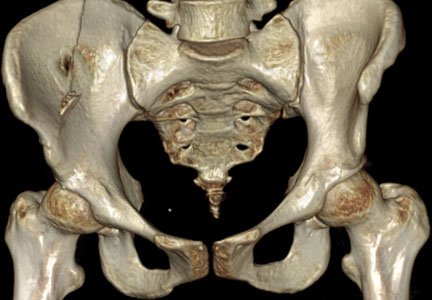
Thirty-seven patients underwent acetabular fracture ORIF. Immediate postoperative radiographs showed 30 anatomical reductions and 7 imperfect reductions. One patient had surgical secondary congruence and developed AVN of the hip. We could not identify an association between the quality of the reduction and the outcome with respect to pain or return to activity. However, no patient had a poor reduction. An illustrative case is presented in Figures 1 to 4.
All acetabular fractures united within 4.5 months (range, 3.0-8.0 months) after the index procedure. Early postoperative complications included 3 cases of meralgia paresthetica and 13 cases of abductor weakness. Meralgia paresthetica resolved spontaneously in all 3 patients. Of the 13 patients with abductor weakness, 11 improved with physical therapy, 1 was limited by the head injury, and 1 subsequently underwent hip fusion. One patient had a deep vein thrombosis (DVT) that was identified before surgery and managed with warfarin.
Other complications included 1 case of deep infection of the surgical wound. This infection presented 4 months after surgery and was treated with débridement, hardware removal, and a 3-month course of antibiotics. Two patients who sustained hip dislocations at time of injury developed AVN of the femoral head. Both developed osteoarthritis, and 1 underwent hip fusion. Eight patients developed heterotopic ossification on the side of the acetabular fracture; 4 of them underwent surgical excision. Four patients required a separate operation for hardware removal. Four patients with triradiate cartilage involvement went on to premature closure. No patient had any leg-length discrepancy or dysplasia at time of follow-up.
Thirty-four of the 38 patients returned to their regular activities. For these patients, mean time to return to full activity was 7.0 months (range, 3-30 months); there was no difference in mean time to return to full activity between skeletally mature and skeletally immature patients (6.6 vs 7.4 months; P = .57). Of the other 4 patients, 1 had permanent cognitive and physical disability with an ataxic gait as a result of a traumatic brain injury, 2 were limited by AVN (1 underwent hip fusion), and 1 was limited by an ipsilateral knee injury.
Of the 38 patients, 29 were pain-free; 6 had occasional, intermittent mild pain that did not limit their activities; and 3 had severe, activity-limiting pain. Of the 6 patients with mild pain, 2 had femoral impaction injuries, and 4 had marginal impaction injuries. Of the 3 patients with severe pain, 2 developed femoral head AVN, and 1 had multiple ipsilateral extremity injuries involving the femur, knee, and tibia.
Discussion
The traditional treatment for acetabular fractures in children has been nonoperative,8,10 and there are few specific treatment guidelines.13 Recent recommendations are nonoperative treatment for minimally displaced fractures (<1 mm) and acetabular fracture ORIF for fractures displaced more than 2 mm.11 No clear consensus exists on management for fractures displaced 1 to 2 mm. Few studies have investigated the outcomes of operative management of these fractures in the pediatric or adolescent population.
In our series of adolescent acetabular fractures, we examined unions, complications, and return to activity. Of 38 patients with acetabular fractures, 37 were treated with ORIF. Anatomical reduction was achieved in the majority of patients. Posterior wall fractures were by far the most common fracture type, which is consistent with previous reports.10,11 All acetabular fractures united, and most patients were pain-free at latest follow-up. There was a low incidence of major complications in our patient population. One major complication was a DVT in a 14-year-old boy who was in a motor vehicle accident and sustained a T-type fracture of the right acetabulum with contralateral femoral shaft and ankle fractures. The DVT was in the right internal iliac and common femoral veins and was diagnosed on magnetic resonance venography. The patient was treated with warfarin for 3 months without incident.
Two patients developed AVN of the femoral head. One of these patients was an 11-year-old girl who was in a motor vehicle accident and sustained a T-type fracture with marginal impaction of the posterior wall, posterior hip dislocation, and a pelvic ring injury. She was treated with ORIF through combined Kocher-Langenbeck/ilioinguinal approaches. By 4 months after surgery, the acetabular fracture was united. Nine months after surgery, she still had pain (activity-limiting) and a 35° flexion contracture of the hip, and she was ambulating with a cane. The diagnosis was AVN of the hip. The patient underwent hip fusion 1 year after surgery.
The second patient with femoral head AVN was a 12-year-old boy who fell while skiing and sustained a fracture of the posterior wall and a hip dislocation with impaction of the femoral head. Initial treatment at an outside institution consisted of open reduction of the hip and excision of a “loose body” from the joint. Eight weeks after surgery, the patient continued to have pain and was referred to our institution. A second operation was performed. Findings included a defect involving 40% of the posterior wall, and signs that the posterior wall had been excised during the initial operation. The patient eventually developed AVN of the hip. This patient was also diagnosed with a deep wound infection 4 months after surgery. He presented with pain and a fluid collection around the hip. The infection was not confirmed through fluid culture, and, as he eventually developed AVN of the hip, his symptoms may have been the result of chondrolysis or AVN rather than infection.
There were no cases of nonunion or malunion, leg-length discrepancy, or permanent sciatic nerve palsy. Although there were a few cases of premature closure of the triradiate cartilage, no acetabular dysplasia was seen at latest follow-up, likely because of the relative maturity of our pediatric group (age range, 11-18 years). Age at time of injury is thought to be the most important factor influencing growth and development of the acetabulum.9,13 In addition, previous studies have demonstrated a tendency toward acetabular fractures in patients with mature triradiate cartilage—versus pelvic ring injuries in patients with immature triradiate cartilage.8,11 This may also account for the older age of our study group.
Minor complications (eg, meralgia paresthetica) resolved spontaneously. The most common complications were abductor weakness and heterotopic ossification. In only 4 cases was a secondary procedure for excision of the heterotopic bone required. Abductor weakness, more commonly associated with a Kocher-Langenbeck approach to the hip, resolved with therapy in almost all cases. Only 4 of our patients required removal of hardware from the acetabulum.
Although the majority of acetabular fractures resulted from high-energy trauma, 8 cases were sports-related. Six of these involved posterior wall fractures, suggesting the injury resulted from a fall on flexed knee and hip. This was not known to be a common mechanism of injury in this age group.3,7 An additional concern was how to size the posterior wall fragment when not ossified. At one center, preoperative magnetic resonance imaging (MRI) was effectively used to size the osteochondral posterior wall fragment as standard protocol for patients with posterior wall fractures in this age group—resulting in better decisions regarding the need for ORIF. At the other institution, preoperative MRI was not performed routinely for this subset of patients.
Thirty-four of our 38 patients returned to their normal activities. Of the other 4 patients, 1 was permanently disabled secondary to traumatic brain injury, 1 had other ipsilateral extremity injuries that limited his mobility, and 2 developed AVN of the femoral head. Both patients with AVN had hip dislocations. Four of the 6 patients who were symptomatic during activity sustained impaction injuries of the femoral head or posterior wall. This suggests that poorer outcomes may be associated with dislocation or with articular injuries—similar to what has been reported in the adult literature.
This study had several limitations. First, it was a retrospective case series, so there was no control group for comparison. Second, the relatively short follow-up did not allow evaluation of the incidence of degenerative arthritis secondary to articular injury, the symptoms of which may develop 1 to 2 decades after injury.13 This phenomenon was well described by Letournel and Judet21 in the adult population, and there is no reason to presume the adolescent population is any different. Third, our sample was small and unlikely to represent a uniform sampling of the general pediatric population. Fourth, it was not possible to draw detailed conclusions about the outcome of ORIF for a particular type of acetabular fracture. Fifth, we did not see as many of the associated visceral injuries that are so prevalent in the literature. This may reflect improvement in safety specifications for automobiles, or our group may not have had the most severe or high-energy injuries. Here our population sample may have skewed our results, leading to better than expected outcomes.
One last study limitation, a major one, was the age of our population, 11 to 18 years, which makes it difficult to extrapolate results to the entire pediatric population. On one hand, a more immature skeleton has a higher chance of remodeling and is more forgiving of deformities and small amounts of displacement. On the other hand, injury and premature triradiate cartilage fusion in a younger patient can lead to significant deformity and acetabular dysplasia.9 Whether ORIF of these fractures would alter the outcome of an injury to the triradiate cartilage is yet to be determined.
Conclusion
In agreement with earlier studies,10,11,15,18 the good outcomes in our series correlated with congruence of reduction. Outcome predictors such as dislocation, femoral head injury, and marginal impaction are similar to those described in the adult literature. Although our study did not have a nonoperative group for comparison, the favorable outcomes of ORIF of acetabular fractures suggest that a more aggressive approach to treatment should be considered. Given the added benefits of early, pain-free mobilization, we think that only stable, undisplaced fractures (<1 mm) should be managed nonoperatively. In the adolescent population, we recommend ORIF for optimal management of unstable acetabular fractures, fractures with any hip subluxation, and fractures displaced more than 1 mm.
1. Canale ST, Beaty JH. Fractures of the pelvis. In: Beaty JH, Kassler JR, eds. Rockwood and Wilkin’s Fractures in Children. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:883-991.
2. Demetriades D, Karaiskakis M, Velmahos GC, Alo K, Murray J, Chan L. Pelvic fractures in pediatric and adult trauma patients: are they different injuries? J Trauma. 2003;54(6):1146-1151.
3. Grisoni N, Connor S, Marsh E, Thompson GH, Cooperman DR, Blakemore LC. Pelvic fractures in a pediatric level I trauma center. J Orthop Trauma. 2002;16(7):458-463.
4. Ismail N, Bellemare JF, Mollitt DL, Di Scala C, Koeppel B, Tepas JJ. Death from pelvic fracture: children are different. J Pediatr Surg. 1996;31(1):82-85.
5. Schlickwei W, Keck T. Pelvic and acetabular fractures in childhood. Injury. 2005;36(suppl 1):A57-A63.
6. Swiontkowski MF. Fractures and dislocations about the hip and pelvis. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia, PA: Saunders; 2003:371-406.
7. Silber JS, Flynn JM, Koffler KM, Dormans JP, Drummond DS. Analysis of the cause, classification, and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop. 2001;21(4):446-450.
8. Silber JS, Flynn JM. Changing patterns of pediatric pelvic fractures with skeletal maturation: implications for classification and management. J Pediatr Orthop. 2002;22(1):22-26.
9. Bucholz RW, Ezaki M, Ogden JA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64(4):600-609.
10. Heeg M, Klasen HJ, Visser JD. Acetabular fractures in children and adolescents. J Bone Joint Surg Br. 1989;71(3):418-421.
11. Heeg M, de Ridder VA, Tornetta P, de Lange S, Klasen HJ. Acetabular fractures in children and adolescents. Clin Orthop Relat Res. 2000;(376):80-86.
12. Heeg M, Visser JD, Oostvogel HJ. Injuries of the acetabular triradiate cartilage and sacroiliac joint. J Bone Joint Surg Br. 1988;70(1):34-37.
13. Liporace FA, Ong B, Mohaideen A, Ong A, Koval KJ. Development and injury of the triradiate cartilage with its effects on acetabular development: review of the literature. J Trauma. 2003;54(6):1245-1249.
14. Rodrigues KF. Injury of the acetabular epiphysis. Injury. 1973;4(3):258-260.
15. Trousdale RT, Ganz R. Posttraumatic acetabular dysplasia. Clin Orthop Relat Res. 1994;(305):124-132.
16. Brooks E, Rosman M. Central fracture-dislocation of the hip in a child. J Trauma. 1988;28(11):1590-1592.
17. Habacker TA, Heinrich SD, Dehne R. Fracture of the superior pelvic quadrant in a child. J Pediatr Orthop. 1995;15(1):69-72.
18. Karunakar MA, Goulet JA, Mueller KL, Bedi A, Le TT. Operative treatment of unstable pediatric pelvis and acetabular fractures. J Pediatr Orthop. 2005;25(1):34-38.
19. Rieger H, Brug E. Fractures of the pelvis in children. Clin Orthop Relat Res. 1997;(336);226-239.
20. Torode I, Zieg D. Pelvic fractures in children. J Pediatr Orthop. 1985;5(1):76-84.
21. Letournel E, Judet R. Fractures of the Acetabulum. 2nd ed. New York, NY: Springer-Verlag; 1993.
22. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks of the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645.
In children, pelvic fractures are uncommon, with an incidence ranging from 1% to 4.6% of all pediatric fractures,1-4 and acetabular fractures make up only 0.8% to 15% of pelvic fractures.1,3,5,6 Acetabular fractures are so uncommon in children partly because of the cartilaginous nature of the immature acetabulum. The increased cartilage volume relative to adults provides greater capacity for energy absorption, resulting in greater elastic and plastic deformation before fracture occurrence. More force is therefore required to cause a fracture, and associated visceral injuries, head injuries, and long-bone fractures are common.3,7,8
The impact of acetabular fractures on adolescents warrants special attention because any resulting disability will affect them during their most productive years. Both avascular necrosis (AVN) and degenerative arthritis are particularly devastating complications in this age group. Complications such as premature physeal closure9-15 are unique to adolescents, and there is little information available on how injury in older children affects growth in this area.
There have been very few studies of the outcomes of these injuries in children. Mostly, there have been case reports and small series primarily dealing with nonoperative management of acetabular fractures in adolescents.3,10,11,16-20 By contrast, operative treatment of acetabular fractures in adults has been well described, and outcomes widely reported. As a result, much of our knowledge about managing these injuries is extrapolated from the adult literature. Although treatment of acetabular fractures in adults has evolved substantially, treatment of these injuries in adolescents remains primarily nonoperative. We conducted a study to evaluate outcomes of treatment of adolescent acetabular fractures.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the cases of all adolescent patients admitted with a diagnosis of acetabular fracture to 2 academic institutions between 1991 and 2003. Thirty-eight patients (28 males, 10 females) were identified. Mean age at time of injury was 15 years (range, 11-18 years). Mean follow-up was 3.2 years (range, 5-180 months).
Data on fracture types, treatment methods, associated injuries, complications, union rates, pain, and return to normal activities were collected. Acetabular fractures were classified according to the system of Letournel and Judet.21 There were 20 elementary and 18 associated fractures.
Of the 38 patients, 30 sustained high-energy trauma in motor vehicle accidents (25) or in falls from significant heights (5). The other 8 patients injured themselves playing sports (4 had severe traumatic brain injury, 2 had labial wounds, and 2 had injuries involving the abdominal viscera). Twelve patients had associated pelvic ring injuries, 18 had femoral head dislocations, 2 had femoral head fractures, and 13 had evidence of impaction injury to the femoral head articular cartilage. Twelve patients had marginal impaction of the acetabular wall. Fifteen patients had open triradiate physes at time of injury (Table 1).
Thirty-seven of the 38 patients were treated with open reduction and internal fixation (ORIF) by an experienced orthopedic trauma surgeon; 1 patient with a stable posterior wall fracture was treated nonoperatively. Surgical indications were articular displacement of more than 1 mm, hip joint instability, irreducible hip dislocation, and intra-articular fracture fragments. In the 37 surgically treated cases, the approaches used were Kocher-Langenbeck (22), ilioinguinal (8), combined Kocher-Langenbeck/ilioinguinal (5), and triradiate (2).
Immediate postoperative radiographs were evaluated by 3 orthopedic surgeons blinded to the patients’ clinical outcomes. Displacement was evaluated on anteroposterior (AP) and Judet views of the pelvis, as described by Matta,22 and reductions were classified as anatomical (0-1 mm of displacement), imperfect (>1 to 3 mm), poor (>3 mm), or surgical secondary congruence (Table 2).
Results
Thirty-seven patients underwent acetabular fracture ORIF. Immediate postoperative radiographs showed 30 anatomical reductions and 7 imperfect reductions. One patient had surgical secondary congruence and developed AVN of the hip. We could not identify an association between the quality of the reduction and the outcome with respect to pain or return to activity. However, no patient had a poor reduction. An illustrative case is presented in Figures 1 to 4.
All acetabular fractures united within 4.5 months (range, 3.0-8.0 months) after the index procedure. Early postoperative complications included 3 cases of meralgia paresthetica and 13 cases of abductor weakness. Meralgia paresthetica resolved spontaneously in all 3 patients. Of the 13 patients with abductor weakness, 11 improved with physical therapy, 1 was limited by the head injury, and 1 subsequently underwent hip fusion. One patient had a deep vein thrombosis (DVT) that was identified before surgery and managed with warfarin.
Other complications included 1 case of deep infection of the surgical wound. This infection presented 4 months after surgery and was treated with débridement, hardware removal, and a 3-month course of antibiotics. Two patients who sustained hip dislocations at time of injury developed AVN of the femoral head. Both developed osteoarthritis, and 1 underwent hip fusion. Eight patients developed heterotopic ossification on the side of the acetabular fracture; 4 of them underwent surgical excision. Four patients required a separate operation for hardware removal. Four patients with triradiate cartilage involvement went on to premature closure. No patient had any leg-length discrepancy or dysplasia at time of follow-up.
Thirty-four of the 38 patients returned to their regular activities. For these patients, mean time to return to full activity was 7.0 months (range, 3-30 months); there was no difference in mean time to return to full activity between skeletally mature and skeletally immature patients (6.6 vs 7.4 months; P = .57). Of the other 4 patients, 1 had permanent cognitive and physical disability with an ataxic gait as a result of a traumatic brain injury, 2 were limited by AVN (1 underwent hip fusion), and 1 was limited by an ipsilateral knee injury.
Of the 38 patients, 29 were pain-free; 6 had occasional, intermittent mild pain that did not limit their activities; and 3 had severe, activity-limiting pain. Of the 6 patients with mild pain, 2 had femoral impaction injuries, and 4 had marginal impaction injuries. Of the 3 patients with severe pain, 2 developed femoral head AVN, and 1 had multiple ipsilateral extremity injuries involving the femur, knee, and tibia.
Discussion
The traditional treatment for acetabular fractures in children has been nonoperative,8,10 and there are few specific treatment guidelines.13 Recent recommendations are nonoperative treatment for minimally displaced fractures (<1 mm) and acetabular fracture ORIF for fractures displaced more than 2 mm.11 No clear consensus exists on management for fractures displaced 1 to 2 mm. Few studies have investigated the outcomes of operative management of these fractures in the pediatric or adolescent population.
In our series of adolescent acetabular fractures, we examined unions, complications, and return to activity. Of 38 patients with acetabular fractures, 37 were treated with ORIF. Anatomical reduction was achieved in the majority of patients. Posterior wall fractures were by far the most common fracture type, which is consistent with previous reports.10,11 All acetabular fractures united, and most patients were pain-free at latest follow-up. There was a low incidence of major complications in our patient population. One major complication was a DVT in a 14-year-old boy who was in a motor vehicle accident and sustained a T-type fracture of the right acetabulum with contralateral femoral shaft and ankle fractures. The DVT was in the right internal iliac and common femoral veins and was diagnosed on magnetic resonance venography. The patient was treated with warfarin for 3 months without incident.
Two patients developed AVN of the femoral head. One of these patients was an 11-year-old girl who was in a motor vehicle accident and sustained a T-type fracture with marginal impaction of the posterior wall, posterior hip dislocation, and a pelvic ring injury. She was treated with ORIF through combined Kocher-Langenbeck/ilioinguinal approaches. By 4 months after surgery, the acetabular fracture was united. Nine months after surgery, she still had pain (activity-limiting) and a 35° flexion contracture of the hip, and she was ambulating with a cane. The diagnosis was AVN of the hip. The patient underwent hip fusion 1 year after surgery.
The second patient with femoral head AVN was a 12-year-old boy who fell while skiing and sustained a fracture of the posterior wall and a hip dislocation with impaction of the femoral head. Initial treatment at an outside institution consisted of open reduction of the hip and excision of a “loose body” from the joint. Eight weeks after surgery, the patient continued to have pain and was referred to our institution. A second operation was performed. Findings included a defect involving 40% of the posterior wall, and signs that the posterior wall had been excised during the initial operation. The patient eventually developed AVN of the hip. This patient was also diagnosed with a deep wound infection 4 months after surgery. He presented with pain and a fluid collection around the hip. The infection was not confirmed through fluid culture, and, as he eventually developed AVN of the hip, his symptoms may have been the result of chondrolysis or AVN rather than infection.
There were no cases of nonunion or malunion, leg-length discrepancy, or permanent sciatic nerve palsy. Although there were a few cases of premature closure of the triradiate cartilage, no acetabular dysplasia was seen at latest follow-up, likely because of the relative maturity of our pediatric group (age range, 11-18 years). Age at time of injury is thought to be the most important factor influencing growth and development of the acetabulum.9,13 In addition, previous studies have demonstrated a tendency toward acetabular fractures in patients with mature triradiate cartilage—versus pelvic ring injuries in patients with immature triradiate cartilage.8,11 This may also account for the older age of our study group.
Minor complications (eg, meralgia paresthetica) resolved spontaneously. The most common complications were abductor weakness and heterotopic ossification. In only 4 cases was a secondary procedure for excision of the heterotopic bone required. Abductor weakness, more commonly associated with a Kocher-Langenbeck approach to the hip, resolved with therapy in almost all cases. Only 4 of our patients required removal of hardware from the acetabulum.
Although the majority of acetabular fractures resulted from high-energy trauma, 8 cases were sports-related. Six of these involved posterior wall fractures, suggesting the injury resulted from a fall on flexed knee and hip. This was not known to be a common mechanism of injury in this age group.3,7 An additional concern was how to size the posterior wall fragment when not ossified. At one center, preoperative magnetic resonance imaging (MRI) was effectively used to size the osteochondral posterior wall fragment as standard protocol for patients with posterior wall fractures in this age group—resulting in better decisions regarding the need for ORIF. At the other institution, preoperative MRI was not performed routinely for this subset of patients.
Thirty-four of our 38 patients returned to their normal activities. Of the other 4 patients, 1 was permanently disabled secondary to traumatic brain injury, 1 had other ipsilateral extremity injuries that limited his mobility, and 2 developed AVN of the femoral head. Both patients with AVN had hip dislocations. Four of the 6 patients who were symptomatic during activity sustained impaction injuries of the femoral head or posterior wall. This suggests that poorer outcomes may be associated with dislocation or with articular injuries—similar to what has been reported in the adult literature.
This study had several limitations. First, it was a retrospective case series, so there was no control group for comparison. Second, the relatively short follow-up did not allow evaluation of the incidence of degenerative arthritis secondary to articular injury, the symptoms of which may develop 1 to 2 decades after injury.13 This phenomenon was well described by Letournel and Judet21 in the adult population, and there is no reason to presume the adolescent population is any different. Third, our sample was small and unlikely to represent a uniform sampling of the general pediatric population. Fourth, it was not possible to draw detailed conclusions about the outcome of ORIF for a particular type of acetabular fracture. Fifth, we did not see as many of the associated visceral injuries that are so prevalent in the literature. This may reflect improvement in safety specifications for automobiles, or our group may not have had the most severe or high-energy injuries. Here our population sample may have skewed our results, leading to better than expected outcomes.
One last study limitation, a major one, was the age of our population, 11 to 18 years, which makes it difficult to extrapolate results to the entire pediatric population. On one hand, a more immature skeleton has a higher chance of remodeling and is more forgiving of deformities and small amounts of displacement. On the other hand, injury and premature triradiate cartilage fusion in a younger patient can lead to significant deformity and acetabular dysplasia.9 Whether ORIF of these fractures would alter the outcome of an injury to the triradiate cartilage is yet to be determined.
Conclusion
In agreement with earlier studies,10,11,15,18 the good outcomes in our series correlated with congruence of reduction. Outcome predictors such as dislocation, femoral head injury, and marginal impaction are similar to those described in the adult literature. Although our study did not have a nonoperative group for comparison, the favorable outcomes of ORIF of acetabular fractures suggest that a more aggressive approach to treatment should be considered. Given the added benefits of early, pain-free mobilization, we think that only stable, undisplaced fractures (<1 mm) should be managed nonoperatively. In the adolescent population, we recommend ORIF for optimal management of unstable acetabular fractures, fractures with any hip subluxation, and fractures displaced more than 1 mm.
In children, pelvic fractures are uncommon, with an incidence ranging from 1% to 4.6% of all pediatric fractures,1-4 and acetabular fractures make up only 0.8% to 15% of pelvic fractures.1,3,5,6 Acetabular fractures are so uncommon in children partly because of the cartilaginous nature of the immature acetabulum. The increased cartilage volume relative to adults provides greater capacity for energy absorption, resulting in greater elastic and plastic deformation before fracture occurrence. More force is therefore required to cause a fracture, and associated visceral injuries, head injuries, and long-bone fractures are common.3,7,8
The impact of acetabular fractures on adolescents warrants special attention because any resulting disability will affect them during their most productive years. Both avascular necrosis (AVN) and degenerative arthritis are particularly devastating complications in this age group. Complications such as premature physeal closure9-15 are unique to adolescents, and there is little information available on how injury in older children affects growth in this area.
There have been very few studies of the outcomes of these injuries in children. Mostly, there have been case reports and small series primarily dealing with nonoperative management of acetabular fractures in adolescents.3,10,11,16-20 By contrast, operative treatment of acetabular fractures in adults has been well described, and outcomes widely reported. As a result, much of our knowledge about managing these injuries is extrapolated from the adult literature. Although treatment of acetabular fractures in adults has evolved substantially, treatment of these injuries in adolescents remains primarily nonoperative. We conducted a study to evaluate outcomes of treatment of adolescent acetabular fractures.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the cases of all adolescent patients admitted with a diagnosis of acetabular fracture to 2 academic institutions between 1991 and 2003. Thirty-eight patients (28 males, 10 females) were identified. Mean age at time of injury was 15 years (range, 11-18 years). Mean follow-up was 3.2 years (range, 5-180 months).
Data on fracture types, treatment methods, associated injuries, complications, union rates, pain, and return to normal activities were collected. Acetabular fractures were classified according to the system of Letournel and Judet.21 There were 20 elementary and 18 associated fractures.
Of the 38 patients, 30 sustained high-energy trauma in motor vehicle accidents (25) or in falls from significant heights (5). The other 8 patients injured themselves playing sports (4 had severe traumatic brain injury, 2 had labial wounds, and 2 had injuries involving the abdominal viscera). Twelve patients had associated pelvic ring injuries, 18 had femoral head dislocations, 2 had femoral head fractures, and 13 had evidence of impaction injury to the femoral head articular cartilage. Twelve patients had marginal impaction of the acetabular wall. Fifteen patients had open triradiate physes at time of injury (Table 1).
Thirty-seven of the 38 patients were treated with open reduction and internal fixation (ORIF) by an experienced orthopedic trauma surgeon; 1 patient with a stable posterior wall fracture was treated nonoperatively. Surgical indications were articular displacement of more than 1 mm, hip joint instability, irreducible hip dislocation, and intra-articular fracture fragments. In the 37 surgically treated cases, the approaches used were Kocher-Langenbeck (22), ilioinguinal (8), combined Kocher-Langenbeck/ilioinguinal (5), and triradiate (2).
Immediate postoperative radiographs were evaluated by 3 orthopedic surgeons blinded to the patients’ clinical outcomes. Displacement was evaluated on anteroposterior (AP) and Judet views of the pelvis, as described by Matta,22 and reductions were classified as anatomical (0-1 mm of displacement), imperfect (>1 to 3 mm), poor (>3 mm), or surgical secondary congruence (Table 2).
Results
Thirty-seven patients underwent acetabular fracture ORIF. Immediate postoperative radiographs showed 30 anatomical reductions and 7 imperfect reductions. One patient had surgical secondary congruence and developed AVN of the hip. We could not identify an association between the quality of the reduction and the outcome with respect to pain or return to activity. However, no patient had a poor reduction. An illustrative case is presented in Figures 1 to 4.
All acetabular fractures united within 4.5 months (range, 3.0-8.0 months) after the index procedure. Early postoperative complications included 3 cases of meralgia paresthetica and 13 cases of abductor weakness. Meralgia paresthetica resolved spontaneously in all 3 patients. Of the 13 patients with abductor weakness, 11 improved with physical therapy, 1 was limited by the head injury, and 1 subsequently underwent hip fusion. One patient had a deep vein thrombosis (DVT) that was identified before surgery and managed with warfarin.
Other complications included 1 case of deep infection of the surgical wound. This infection presented 4 months after surgery and was treated with débridement, hardware removal, and a 3-month course of antibiotics. Two patients who sustained hip dislocations at time of injury developed AVN of the femoral head. Both developed osteoarthritis, and 1 underwent hip fusion. Eight patients developed heterotopic ossification on the side of the acetabular fracture; 4 of them underwent surgical excision. Four patients required a separate operation for hardware removal. Four patients with triradiate cartilage involvement went on to premature closure. No patient had any leg-length discrepancy or dysplasia at time of follow-up.
Thirty-four of the 38 patients returned to their regular activities. For these patients, mean time to return to full activity was 7.0 months (range, 3-30 months); there was no difference in mean time to return to full activity between skeletally mature and skeletally immature patients (6.6 vs 7.4 months; P = .57). Of the other 4 patients, 1 had permanent cognitive and physical disability with an ataxic gait as a result of a traumatic brain injury, 2 were limited by AVN (1 underwent hip fusion), and 1 was limited by an ipsilateral knee injury.
Of the 38 patients, 29 were pain-free; 6 had occasional, intermittent mild pain that did not limit their activities; and 3 had severe, activity-limiting pain. Of the 6 patients with mild pain, 2 had femoral impaction injuries, and 4 had marginal impaction injuries. Of the 3 patients with severe pain, 2 developed femoral head AVN, and 1 had multiple ipsilateral extremity injuries involving the femur, knee, and tibia.
Discussion
The traditional treatment for acetabular fractures in children has been nonoperative,8,10 and there are few specific treatment guidelines.13 Recent recommendations are nonoperative treatment for minimally displaced fractures (<1 mm) and acetabular fracture ORIF for fractures displaced more than 2 mm.11 No clear consensus exists on management for fractures displaced 1 to 2 mm. Few studies have investigated the outcomes of operative management of these fractures in the pediatric or adolescent population.
In our series of adolescent acetabular fractures, we examined unions, complications, and return to activity. Of 38 patients with acetabular fractures, 37 were treated with ORIF. Anatomical reduction was achieved in the majority of patients. Posterior wall fractures were by far the most common fracture type, which is consistent with previous reports.10,11 All acetabular fractures united, and most patients were pain-free at latest follow-up. There was a low incidence of major complications in our patient population. One major complication was a DVT in a 14-year-old boy who was in a motor vehicle accident and sustained a T-type fracture of the right acetabulum with contralateral femoral shaft and ankle fractures. The DVT was in the right internal iliac and common femoral veins and was diagnosed on magnetic resonance venography. The patient was treated with warfarin for 3 months without incident.
Two patients developed AVN of the femoral head. One of these patients was an 11-year-old girl who was in a motor vehicle accident and sustained a T-type fracture with marginal impaction of the posterior wall, posterior hip dislocation, and a pelvic ring injury. She was treated with ORIF through combined Kocher-Langenbeck/ilioinguinal approaches. By 4 months after surgery, the acetabular fracture was united. Nine months after surgery, she still had pain (activity-limiting) and a 35° flexion contracture of the hip, and she was ambulating with a cane. The diagnosis was AVN of the hip. The patient underwent hip fusion 1 year after surgery.
The second patient with femoral head AVN was a 12-year-old boy who fell while skiing and sustained a fracture of the posterior wall and a hip dislocation with impaction of the femoral head. Initial treatment at an outside institution consisted of open reduction of the hip and excision of a “loose body” from the joint. Eight weeks after surgery, the patient continued to have pain and was referred to our institution. A second operation was performed. Findings included a defect involving 40% of the posterior wall, and signs that the posterior wall had been excised during the initial operation. The patient eventually developed AVN of the hip. This patient was also diagnosed with a deep wound infection 4 months after surgery. He presented with pain and a fluid collection around the hip. The infection was not confirmed through fluid culture, and, as he eventually developed AVN of the hip, his symptoms may have been the result of chondrolysis or AVN rather than infection.
There were no cases of nonunion or malunion, leg-length discrepancy, or permanent sciatic nerve palsy. Although there were a few cases of premature closure of the triradiate cartilage, no acetabular dysplasia was seen at latest follow-up, likely because of the relative maturity of our pediatric group (age range, 11-18 years). Age at time of injury is thought to be the most important factor influencing growth and development of the acetabulum.9,13 In addition, previous studies have demonstrated a tendency toward acetabular fractures in patients with mature triradiate cartilage—versus pelvic ring injuries in patients with immature triradiate cartilage.8,11 This may also account for the older age of our study group.
Minor complications (eg, meralgia paresthetica) resolved spontaneously. The most common complications were abductor weakness and heterotopic ossification. In only 4 cases was a secondary procedure for excision of the heterotopic bone required. Abductor weakness, more commonly associated with a Kocher-Langenbeck approach to the hip, resolved with therapy in almost all cases. Only 4 of our patients required removal of hardware from the acetabulum.
Although the majority of acetabular fractures resulted from high-energy trauma, 8 cases were sports-related. Six of these involved posterior wall fractures, suggesting the injury resulted from a fall on flexed knee and hip. This was not known to be a common mechanism of injury in this age group.3,7 An additional concern was how to size the posterior wall fragment when not ossified. At one center, preoperative magnetic resonance imaging (MRI) was effectively used to size the osteochondral posterior wall fragment as standard protocol for patients with posterior wall fractures in this age group—resulting in better decisions regarding the need for ORIF. At the other institution, preoperative MRI was not performed routinely for this subset of patients.
Thirty-four of our 38 patients returned to their normal activities. Of the other 4 patients, 1 was permanently disabled secondary to traumatic brain injury, 1 had other ipsilateral extremity injuries that limited his mobility, and 2 developed AVN of the femoral head. Both patients with AVN had hip dislocations. Four of the 6 patients who were symptomatic during activity sustained impaction injuries of the femoral head or posterior wall. This suggests that poorer outcomes may be associated with dislocation or with articular injuries—similar to what has been reported in the adult literature.
This study had several limitations. First, it was a retrospective case series, so there was no control group for comparison. Second, the relatively short follow-up did not allow evaluation of the incidence of degenerative arthritis secondary to articular injury, the symptoms of which may develop 1 to 2 decades after injury.13 This phenomenon was well described by Letournel and Judet21 in the adult population, and there is no reason to presume the adolescent population is any different. Third, our sample was small and unlikely to represent a uniform sampling of the general pediatric population. Fourth, it was not possible to draw detailed conclusions about the outcome of ORIF for a particular type of acetabular fracture. Fifth, we did not see as many of the associated visceral injuries that are so prevalent in the literature. This may reflect improvement in safety specifications for automobiles, or our group may not have had the most severe or high-energy injuries. Here our population sample may have skewed our results, leading to better than expected outcomes.
One last study limitation, a major one, was the age of our population, 11 to 18 years, which makes it difficult to extrapolate results to the entire pediatric population. On one hand, a more immature skeleton has a higher chance of remodeling and is more forgiving of deformities and small amounts of displacement. On the other hand, injury and premature triradiate cartilage fusion in a younger patient can lead to significant deformity and acetabular dysplasia.9 Whether ORIF of these fractures would alter the outcome of an injury to the triradiate cartilage is yet to be determined.
Conclusion
In agreement with earlier studies,10,11,15,18 the good outcomes in our series correlated with congruence of reduction. Outcome predictors such as dislocation, femoral head injury, and marginal impaction are similar to those described in the adult literature. Although our study did not have a nonoperative group for comparison, the favorable outcomes of ORIF of acetabular fractures suggest that a more aggressive approach to treatment should be considered. Given the added benefits of early, pain-free mobilization, we think that only stable, undisplaced fractures (<1 mm) should be managed nonoperatively. In the adolescent population, we recommend ORIF for optimal management of unstable acetabular fractures, fractures with any hip subluxation, and fractures displaced more than 1 mm.
1. Canale ST, Beaty JH. Fractures of the pelvis. In: Beaty JH, Kassler JR, eds. Rockwood and Wilkin’s Fractures in Children. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:883-991.
2. Demetriades D, Karaiskakis M, Velmahos GC, Alo K, Murray J, Chan L. Pelvic fractures in pediatric and adult trauma patients: are they different injuries? J Trauma. 2003;54(6):1146-1151.
3. Grisoni N, Connor S, Marsh E, Thompson GH, Cooperman DR, Blakemore LC. Pelvic fractures in a pediatric level I trauma center. J Orthop Trauma. 2002;16(7):458-463.
4. Ismail N, Bellemare JF, Mollitt DL, Di Scala C, Koeppel B, Tepas JJ. Death from pelvic fracture: children are different. J Pediatr Surg. 1996;31(1):82-85.
5. Schlickwei W, Keck T. Pelvic and acetabular fractures in childhood. Injury. 2005;36(suppl 1):A57-A63.
6. Swiontkowski MF. Fractures and dislocations about the hip and pelvis. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia, PA: Saunders; 2003:371-406.
7. Silber JS, Flynn JM, Koffler KM, Dormans JP, Drummond DS. Analysis of the cause, classification, and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop. 2001;21(4):446-450.
8. Silber JS, Flynn JM. Changing patterns of pediatric pelvic fractures with skeletal maturation: implications for classification and management. J Pediatr Orthop. 2002;22(1):22-26.
9. Bucholz RW, Ezaki M, Ogden JA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64(4):600-609.
10. Heeg M, Klasen HJ, Visser JD. Acetabular fractures in children and adolescents. J Bone Joint Surg Br. 1989;71(3):418-421.
11. Heeg M, de Ridder VA, Tornetta P, de Lange S, Klasen HJ. Acetabular fractures in children and adolescents. Clin Orthop Relat Res. 2000;(376):80-86.
12. Heeg M, Visser JD, Oostvogel HJ. Injuries of the acetabular triradiate cartilage and sacroiliac joint. J Bone Joint Surg Br. 1988;70(1):34-37.
13. Liporace FA, Ong B, Mohaideen A, Ong A, Koval KJ. Development and injury of the triradiate cartilage with its effects on acetabular development: review of the literature. J Trauma. 2003;54(6):1245-1249.
14. Rodrigues KF. Injury of the acetabular epiphysis. Injury. 1973;4(3):258-260.
15. Trousdale RT, Ganz R. Posttraumatic acetabular dysplasia. Clin Orthop Relat Res. 1994;(305):124-132.
16. Brooks E, Rosman M. Central fracture-dislocation of the hip in a child. J Trauma. 1988;28(11):1590-1592.
17. Habacker TA, Heinrich SD, Dehne R. Fracture of the superior pelvic quadrant in a child. J Pediatr Orthop. 1995;15(1):69-72.
18. Karunakar MA, Goulet JA, Mueller KL, Bedi A, Le TT. Operative treatment of unstable pediatric pelvis and acetabular fractures. J Pediatr Orthop. 2005;25(1):34-38.
19. Rieger H, Brug E. Fractures of the pelvis in children. Clin Orthop Relat Res. 1997;(336);226-239.
20. Torode I, Zieg D. Pelvic fractures in children. J Pediatr Orthop. 1985;5(1):76-84.
21. Letournel E, Judet R. Fractures of the Acetabulum. 2nd ed. New York, NY: Springer-Verlag; 1993.
22. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks of the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645.
1. Canale ST, Beaty JH. Fractures of the pelvis. In: Beaty JH, Kassler JR, eds. Rockwood and Wilkin’s Fractures in Children. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:883-991.
2. Demetriades D, Karaiskakis M, Velmahos GC, Alo K, Murray J, Chan L. Pelvic fractures in pediatric and adult trauma patients: are they different injuries? J Trauma. 2003;54(6):1146-1151.
3. Grisoni N, Connor S, Marsh E, Thompson GH, Cooperman DR, Blakemore LC. Pelvic fractures in a pediatric level I trauma center. J Orthop Trauma. 2002;16(7):458-463.
4. Ismail N, Bellemare JF, Mollitt DL, Di Scala C, Koeppel B, Tepas JJ. Death from pelvic fracture: children are different. J Pediatr Surg. 1996;31(1):82-85.
5. Schlickwei W, Keck T. Pelvic and acetabular fractures in childhood. Injury. 2005;36(suppl 1):A57-A63.
6. Swiontkowski MF. Fractures and dislocations about the hip and pelvis. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. Philadelphia, PA: Saunders; 2003:371-406.
7. Silber JS, Flynn JM, Koffler KM, Dormans JP, Drummond DS. Analysis of the cause, classification, and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop. 2001;21(4):446-450.
8. Silber JS, Flynn JM. Changing patterns of pediatric pelvic fractures with skeletal maturation: implications for classification and management. J Pediatr Orthop. 2002;22(1):22-26.
9. Bucholz RW, Ezaki M, Ogden JA. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg Am. 1982;64(4):600-609.
10. Heeg M, Klasen HJ, Visser JD. Acetabular fractures in children and adolescents. J Bone Joint Surg Br. 1989;71(3):418-421.
11. Heeg M, de Ridder VA, Tornetta P, de Lange S, Klasen HJ. Acetabular fractures in children and adolescents. Clin Orthop Relat Res. 2000;(376):80-86.
12. Heeg M, Visser JD, Oostvogel HJ. Injuries of the acetabular triradiate cartilage and sacroiliac joint. J Bone Joint Surg Br. 1988;70(1):34-37.
13. Liporace FA, Ong B, Mohaideen A, Ong A, Koval KJ. Development and injury of the triradiate cartilage with its effects on acetabular development: review of the literature. J Trauma. 2003;54(6):1245-1249.
14. Rodrigues KF. Injury of the acetabular epiphysis. Injury. 1973;4(3):258-260.
15. Trousdale RT, Ganz R. Posttraumatic acetabular dysplasia. Clin Orthop Relat Res. 1994;(305):124-132.
16. Brooks E, Rosman M. Central fracture-dislocation of the hip in a child. J Trauma. 1988;28(11):1590-1592.
17. Habacker TA, Heinrich SD, Dehne R. Fracture of the superior pelvic quadrant in a child. J Pediatr Orthop. 1995;15(1):69-72.
18. Karunakar MA, Goulet JA, Mueller KL, Bedi A, Le TT. Operative treatment of unstable pediatric pelvis and acetabular fractures. J Pediatr Orthop. 2005;25(1):34-38.
19. Rieger H, Brug E. Fractures of the pelvis in children. Clin Orthop Relat Res. 1997;(336);226-239.
20. Torode I, Zieg D. Pelvic fractures in children. J Pediatr Orthop. 1985;5(1):76-84.
21. Letournel E, Judet R. Fractures of the Acetabulum. 2nd ed. New York, NY: Springer-Verlag; 1993.
22. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks of the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645.
Treatment of Proximal Humerus Fractures: Comparison of Shoulder and Trauma Surgeons
Proximal humerus fractures (PHFs), AO/OTA (Ar beitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 11,1 are common, representing 4% to 5% of all fractures in adults.2 However, there is no consensus as to optimal management of these injuries, with some reports supporting and others rejecting the various fixation methods,3 and there are no evidence-based practice guidelines informing treatment decisions.4 Not surprisingly, orthopedic surgeons do not agree on ideal treatment for PHFs5,6 and differ by region in their rates of surgical management.2 In addition, analyses of national databases have found variation in choice of surgical treatment for PHFs between surgeons and between hospitals of different patient volumes.4 Few studies have assessed surgeon agreement on treatment decisions. Findings from these limited investigations indicate there is little agreement on treatment choices, but training may have some impact.5-7 In 3 studies,5-7 shoulder and trauma fellowship–trained surgeons differed in their management of PHFs both in terms of rates of operative treatment5,7 and specific operative management choices.5,6 No study has assessed surgeon agreement on radiographic outcomes.
We conducted a study to compare expert shoulder and trauma surgeons’ treatment decision-making and agreement on final radiographic outcomes of surgically treated PHFs. We hypothesized there would be poor agreement on treatment decisions and better agreement on radiographic outcomes, with a difference between shoulder and trauma fellowship–trained surgeons.
Materials and Methods
After receiving institutional review board approval for this study, we collected data on 100 consecutive PHFs (AO/OTA type 111) surgically treated at 2 affiliated level I trauma centers between January 2004 and July 2008. None of the cases in the series was managed by any of the surgeons participating in this study.
We created a PowerPoint (Microsoft, Redmond, Washington) survey that included radiographs (preoperative, immediate postoperative, final postoperative) and, if available, a computed tomography image. This survey was sent to 4 orthopedic surgeons: Drs. Gardner, Gerber, Lorich, and Walch. Two of these authors are fellowship-trained in shoulder surgery, the other 2 in orthopedic traumatology with specialization in treating PHFs. All are internationally renowned in PHF management. Using the survey images and a 4-point Likert scale ranging from disagree strongly to agree strongly, the examiners rated their agreement with treatment decisions (arthroplasty vs fixation). They also rated (very poor to very good) immediate postoperative reduction or arthroplasty placement, immediate postoperative fixation methods for fractures treated with open reduction and internal fixation (ORIF), and final radiographic outcomes.
Interobserver agreement was calculated using the intraclass correlation coefficient (ICC),8,9 with scores of <0.2 (poor), 0.21 to 0.4 (fair), 0.41 to 0.6 (moderate), 0.61 to 0.8 (good), and >0.8 (excellent) used to indicate agreement among observers. ICC scores were determined by treating the 4 examiners as independent entities. Subgroup analyses were also performed to determine ICC scores comparing the 2 shoulder surgeons, comparing the 2 trauma surgeons, and comparing the shoulder surgeons and trauma surgeons as 2 separate groups. ICC scores were used instead of κ coefficients to assess agreement because ICC scores treat ratings as continuous variables, allow for comparison of 2 or more raters, and allow for assessment of correlation among raters, whereas κ coefficients treat data as categorical variables and assume the ratings have no natural ordering. ICC scores were generated by SAS 9.1.3 software (SAS Institute, Cary, North Carolina).
Results
The 4 surgeons’ overall ICC scores for agreement with the rating of immediate reduction or arthroplasty placement and the rating of final radiographic outcome indicated moderate levels of agreement (Table 1). Regarding treatment decision-making and ratings of fixation, the surgeons demonstrated poor and fair levels of agreement, respectively.
The ICC scores comparing the shoulder and trauma surgeons revealed similar levels of agreement (Table 2): moderate levels of agreement for ratings of both immediate postoperative reduction or arthroplasty placement and final radiographic outcomes, but poor and fair levels of agreement regarding treatment decision-making and the rating of immediate postoperative fixation methods for fractures treated with ORIF, respectively.
Subgroup analysis revealed that the 2 shoulder surgeons had poor and fair levels of agreement for treatment decisions and rating of immediate postoperative fixation, respectively, though they moderately agreed on rating of immediate postoperative reduction or arthroplasty placement and rating of final radiographic outcome (Table 3). When the 2 trauma surgeons were compared with each other, ICC scores revealed higher levels of agreement overall (Table 4). In other words, the 2 trauma surgeons agreed with each other more than the 2 shoulder surgeons agreed with each other.
Discussion
This study had 3 major findings: (1) Surgeons do not agree on treatment decisions, including fixation methods, regarding PHFs; (2) regardless of their opinions on ideal treatment, they moderately agree on reductions and final radiographic outcomes; (3) expert trauma surgeons may agree more on treatment decisions than expert shoulder surgeons do. In other words, surgeons do not agree on the best treatment, but they radiographically recognize when a procedure has been performed technically well or poorly. These results support our hypothesis and the limited current literature.
An analysis of Medicare databases showed marked regional variation in rates of operative treatment of PHFs.2 Similarly, a Nationwide Inpatient Sample analysis revealed nationwide variation in operative management of PHFs.4 Both findings are consistent with our results of poor agreement about treatment decisions and ratings of postoperative fixation of PHFs. In 2010, Petit and colleagues6 reported that surgeons do not agree on PHF management. In 2011, Foroohar and colleagues10 similarly reported low interobserver agreement for treatment recommendations made by 4 upper extremity orthopedic specialists, 4 general orthopedic surgeons, 4 senior residents, and 4 junior residents, for a series of 16 PHFs—also consistent with our findings.
The lack of agreement about PHF treatment may reflect a difference in training, particularly in light of the recent expansion of shoulder and elbow fellowships.2 Three separate studies performed at 2 affiliated level I trauma centers demonstrated significant differences in treatment decision-making between shoulder and trauma fellowship–trained surgeons.5-7 Our results are consistent with the hypothesis that training affects treatment decision-making, as we found poor agreement between shoulder and trauma fellowship–trained surgeons regarding treatment decision for PHFs. Subanalyses revealed that expert trauma surgeons agreed with each other on treatment decisions more than expert shoulder surgeons agreed with each other, further suggesting that training may affect how surgeons manage PHFs. Differences in fellowship training even within the same specialty may account for the observed lesser levels of agreement between the shoulder surgeons, even among experts in the field.
The evidence for optimal treatment historically has been poor,4,6 with few high-quality prospective, randomized controlled studies on the topic up until the past few years. The most recent Cochrane Review on optimal PHF treatment concluded that there is insufficient evidence to make an evidence-based recommendation and that the long-term benefit of surgery is unclear.11 However, at least 5 controlled trials on the topic have been published within the past 5 years.12-16 The evidence is striking and generally supports nonoperative treatment for most PHFs, including some displaced fractures—contrary to general orthopedic practice in many parts of the United States,2 which hitherto had been based mainly on individual surgeon experience and the limited literature. Without strong evidence to support one treatment option over another, surgeons are left with no objective, scientific way of coming to agreement.
Related to the poor status quo of evidence for PHF treatments is new technology (eg, locking plates, reverse total shoulder arthroplasty) that has expanded surgical indications.2,17 Although such developments have the potential to improve surgical treatments, they may also exacerbate the disagreement between surgeons regarding optimal operative treatment of PHFs. This potential consequence of new technology may be reflected in our finding of disagreement among surgeons on immediate postoperative fixation methods. Precisely because they are new, such technological innovations have limited evidence supporting their use. This leaves surgeons with little to nothing to inform their decisions to use these devices, other than familiarity with and impressions of the new technology.
Our study had several limitations. First is the small sample size, of surgeons who are leaders in the field. Our sample therefore may not be generalizable to the general population of shoulder and trauma surgeons. Second, we did not calculate intraobserver variability. Third, inherent to studies of interobserver agreement is the uncertainty of their clinical relevance. In the clinical setting, a surgeon has much more information at hand (eg, patient history, physical examination findings, colleague consultations), thus raising the possibility of underestimations of interobserver agreements.18 Fourth, our comparison of surgeons’ ratings of outcomes was purely radiographic, which may or may not represent or be indicative of clinical outcomes (eg, pain relief, function, range of motion, patient satisfaction). The conclusions we may draw are accordingly limited, as we did not directly evaluate clinical outcome parameters.
Our study had several strengths as well. First, to our knowledge this is the first study to assess interobserver variability in surgeons’ ratings of radiographic outcomes. Its findings may provide further insight into the reasons for poor agreement among orthopedic surgeons on both classification and treatment of PHFs. Second, our surveying of internationally renowned expert surgeons from 4 different institutions may have helped reduce single-institution bias, and it presents the highest level of expertise in the treatment of PHFs.
Although the surgeons in our study moderately agreed on final radiographic outcomes of PHFs, such levels of agreement may still be clinically unacceptable.19 The overall disagreement on treatment decisions highlights the need for better evidence for optimal treatment of PHFs in order to improve consensus, particularly with anticipated increases in age and comorbidities in the population in coming years.4 Subgroup analysis suggested trauma fellowships may contribute to better treatment agreement, though this idea requires further study, perhaps by surveying shoulder and trauma fellowship directors and their curricula for variability in teaching treatment decision-making. The surgeons in our study agreed more on what they consider acceptable final radiographic outcomes, which is encouraging. However, treatment consensus is the primary goal. The recent publication of prospective, randomized studies is helping with this issue, but more studies are needed. It is encouraging that several are planned or under way.20-22
Conclusion
The surgeons surveyed in this study did not agree on ideal treatment for PHFs but moderately agreed on quality of radiographic outcomes. These differences may reflect a difference in training. We conducted this study to compare experienced shoulder and trauma fellowship–trained surgeons’ treatment decision-making and ratings of radiographic outcomes of PHFs when presented with the same group of patients managed at 2 level I trauma centers. We hypothesized there would be little agreement on treatment decisions, better agreement on final radiographic outcome, and a difference between decision-making and ratings of radiographic outcomes between expert shoulder and trauma surgeons. Our results showed that surgeons do not agree on the best treatment for PHFs but radiographically recognize when an operative treatment has been performed well or poorly. Regarding treatment decisions, our results also showed that expert trauma surgeons may agree more with each other than shoulder surgeons agree with each other. These results support our hypothesis and the limited current literature. The overall disagreement among the surgeons in our study and an aging population that grows sicker each year highlight the need for better evidence for the optimal treatment of PHFs in order to improve consensus.
1. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium – 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
2. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
3. McLaurin TM. Proximal humerus fractures in the elderly are we operating on too many? Bull Hosp Jt Dis. 2004;62(1-2):24-32.
4. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop. 2012;471(2):655-664.
5. Boykin RE, Jawa A, O’Brien T, Higgins LD, Warner JJP. Variability in operative management of proximal humerus fractures. Shoulder Elbow. 2011;3(4):197-201.
6. Petit CJ, Millett PJ, Endres NK, Diller D, Harris MB, Warner JJP. Management of proximal humeral fractures: surgeons don’t agree. J Shoulder Elbow Surg. 2010;19(3):446-451.
7. Okike K, Lee OC, Makanji H, Harris MB, Vrahas MS. Factors associated with the decision for operative versus non-operative treatment of displaced proximal humerus fractures in the elderly. Injury. 2013;44(4):448-455.
8. Kodali P, Jones MH, Polster J, Miniaci A, Fening SD. Accuracy of measurement of Hill-Sachs lesions with computed tomography. J Shoulder Elbow Surg. 2011;20(8):1328-1334.
9. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428.
10. Foroohar A, Tosti R, Richmond JM, Gaughan JP, Ilyas AM. Classification and treatment of proximal humerus fractures: inter-observer reliability and agreement across imaging modalities and experience. J Orthop Surg Res. 2011;6:38.
11. Handoll HH, Ollivere BJ. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2010;(12):CD000434.
12. Boons HW, Goosen JH, van Grinsven S, van Susante JL, van Loon CJ. Hemiarthroplasty for humeral four-part fractures for patients 65 years and older: a randomized controlled trial. Clin Orthop. 2012;470(12):3483-3491.
13. Fjalestad T, Hole MØ, Hovden IAH, Blücher J, Strømsøe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
14. Fjalestad T, Hole MØ, Jørgensen JJ, Strømsøe K, Kristiansen IS. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury. 2010;41(6):599-605.
15. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
16. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
17. Agudelo J, Schürmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
18. Brorson S, Hróbjartsson A. Training improves agreement among doctors using the Neer system for proximal humeral fractures in a systematic review. J Clin Epidemiol. 2008;61(1):7-16.
19. Brorson S, Olsen BS, Frich LH, et al. Surgeons agree more on treatment recommendations than on classification of proximal humeral fractures. BMC Musculoskelet Disord. 2012;13:114.
20. Handoll H, Brealey S, Rangan A, et al. Protocol for the ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial: a pragmatic multi-centre randomised controlled trial of surgical versus non-surgical treatment for proximal fracture of the humerus in adults. BMC Musculoskelet Disord. 2009;10:140.
21. Den Hartog D, Van Lieshout EMM, Tuinebreijer WE, et al. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon): a multicenter randomized controlled trial. BMC Musculoskelet Disord. 2010;11:97.
22. Verbeek PA, van den Akker-Scheek I, Wendt KW, Diercks RL. Hemiarthroplasty versus angle-stable locking compression plate osteosynthesis in the treatment of three- and four-part fractures of the proximal humerus in the elderly: design of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:16.
Proximal humerus fractures (PHFs), AO/OTA (Ar beitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 11,1 are common, representing 4% to 5% of all fractures in adults.2 However, there is no consensus as to optimal management of these injuries, with some reports supporting and others rejecting the various fixation methods,3 and there are no evidence-based practice guidelines informing treatment decisions.4 Not surprisingly, orthopedic surgeons do not agree on ideal treatment for PHFs5,6 and differ by region in their rates of surgical management.2 In addition, analyses of national databases have found variation in choice of surgical treatment for PHFs between surgeons and between hospitals of different patient volumes.4 Few studies have assessed surgeon agreement on treatment decisions. Findings from these limited investigations indicate there is little agreement on treatment choices, but training may have some impact.5-7 In 3 studies,5-7 shoulder and trauma fellowship–trained surgeons differed in their management of PHFs both in terms of rates of operative treatment5,7 and specific operative management choices.5,6 No study has assessed surgeon agreement on radiographic outcomes.
We conducted a study to compare expert shoulder and trauma surgeons’ treatment decision-making and agreement on final radiographic outcomes of surgically treated PHFs. We hypothesized there would be poor agreement on treatment decisions and better agreement on radiographic outcomes, with a difference between shoulder and trauma fellowship–trained surgeons.
Materials and Methods
After receiving institutional review board approval for this study, we collected data on 100 consecutive PHFs (AO/OTA type 111) surgically treated at 2 affiliated level I trauma centers between January 2004 and July 2008. None of the cases in the series was managed by any of the surgeons participating in this study.
We created a PowerPoint (Microsoft, Redmond, Washington) survey that included radiographs (preoperative, immediate postoperative, final postoperative) and, if available, a computed tomography image. This survey was sent to 4 orthopedic surgeons: Drs. Gardner, Gerber, Lorich, and Walch. Two of these authors are fellowship-trained in shoulder surgery, the other 2 in orthopedic traumatology with specialization in treating PHFs. All are internationally renowned in PHF management. Using the survey images and a 4-point Likert scale ranging from disagree strongly to agree strongly, the examiners rated their agreement with treatment decisions (arthroplasty vs fixation). They also rated (very poor to very good) immediate postoperative reduction or arthroplasty placement, immediate postoperative fixation methods for fractures treated with open reduction and internal fixation (ORIF), and final radiographic outcomes.
Interobserver agreement was calculated using the intraclass correlation coefficient (ICC),8,9 with scores of <0.2 (poor), 0.21 to 0.4 (fair), 0.41 to 0.6 (moderate), 0.61 to 0.8 (good), and >0.8 (excellent) used to indicate agreement among observers. ICC scores were determined by treating the 4 examiners as independent entities. Subgroup analyses were also performed to determine ICC scores comparing the 2 shoulder surgeons, comparing the 2 trauma surgeons, and comparing the shoulder surgeons and trauma surgeons as 2 separate groups. ICC scores were used instead of κ coefficients to assess agreement because ICC scores treat ratings as continuous variables, allow for comparison of 2 or more raters, and allow for assessment of correlation among raters, whereas κ coefficients treat data as categorical variables and assume the ratings have no natural ordering. ICC scores were generated by SAS 9.1.3 software (SAS Institute, Cary, North Carolina).
Results
The 4 surgeons’ overall ICC scores for agreement with the rating of immediate reduction or arthroplasty placement and the rating of final radiographic outcome indicated moderate levels of agreement (Table 1). Regarding treatment decision-making and ratings of fixation, the surgeons demonstrated poor and fair levels of agreement, respectively.
The ICC scores comparing the shoulder and trauma surgeons revealed similar levels of agreement (Table 2): moderate levels of agreement for ratings of both immediate postoperative reduction or arthroplasty placement and final radiographic outcomes, but poor and fair levels of agreement regarding treatment decision-making and the rating of immediate postoperative fixation methods for fractures treated with ORIF, respectively.
Subgroup analysis revealed that the 2 shoulder surgeons had poor and fair levels of agreement for treatment decisions and rating of immediate postoperative fixation, respectively, though they moderately agreed on rating of immediate postoperative reduction or arthroplasty placement and rating of final radiographic outcome (Table 3). When the 2 trauma surgeons were compared with each other, ICC scores revealed higher levels of agreement overall (Table 4). In other words, the 2 trauma surgeons agreed with each other more than the 2 shoulder surgeons agreed with each other.
Discussion
This study had 3 major findings: (1) Surgeons do not agree on treatment decisions, including fixation methods, regarding PHFs; (2) regardless of their opinions on ideal treatment, they moderately agree on reductions and final radiographic outcomes; (3) expert trauma surgeons may agree more on treatment decisions than expert shoulder surgeons do. In other words, surgeons do not agree on the best treatment, but they radiographically recognize when a procedure has been performed technically well or poorly. These results support our hypothesis and the limited current literature.
An analysis of Medicare databases showed marked regional variation in rates of operative treatment of PHFs.2 Similarly, a Nationwide Inpatient Sample analysis revealed nationwide variation in operative management of PHFs.4 Both findings are consistent with our results of poor agreement about treatment decisions and ratings of postoperative fixation of PHFs. In 2010, Petit and colleagues6 reported that surgeons do not agree on PHF management. In 2011, Foroohar and colleagues10 similarly reported low interobserver agreement for treatment recommendations made by 4 upper extremity orthopedic specialists, 4 general orthopedic surgeons, 4 senior residents, and 4 junior residents, for a series of 16 PHFs—also consistent with our findings.
The lack of agreement about PHF treatment may reflect a difference in training, particularly in light of the recent expansion of shoulder and elbow fellowships.2 Three separate studies performed at 2 affiliated level I trauma centers demonstrated significant differences in treatment decision-making between shoulder and trauma fellowship–trained surgeons.5-7 Our results are consistent with the hypothesis that training affects treatment decision-making, as we found poor agreement between shoulder and trauma fellowship–trained surgeons regarding treatment decision for PHFs. Subanalyses revealed that expert trauma surgeons agreed with each other on treatment decisions more than expert shoulder surgeons agreed with each other, further suggesting that training may affect how surgeons manage PHFs. Differences in fellowship training even within the same specialty may account for the observed lesser levels of agreement between the shoulder surgeons, even among experts in the field.
The evidence for optimal treatment historically has been poor,4,6 with few high-quality prospective, randomized controlled studies on the topic up until the past few years. The most recent Cochrane Review on optimal PHF treatment concluded that there is insufficient evidence to make an evidence-based recommendation and that the long-term benefit of surgery is unclear.11 However, at least 5 controlled trials on the topic have been published within the past 5 years.12-16 The evidence is striking and generally supports nonoperative treatment for most PHFs, including some displaced fractures—contrary to general orthopedic practice in many parts of the United States,2 which hitherto had been based mainly on individual surgeon experience and the limited literature. Without strong evidence to support one treatment option over another, surgeons are left with no objective, scientific way of coming to agreement.
Related to the poor status quo of evidence for PHF treatments is new technology (eg, locking plates, reverse total shoulder arthroplasty) that has expanded surgical indications.2,17 Although such developments have the potential to improve surgical treatments, they may also exacerbate the disagreement between surgeons regarding optimal operative treatment of PHFs. This potential consequence of new technology may be reflected in our finding of disagreement among surgeons on immediate postoperative fixation methods. Precisely because they are new, such technological innovations have limited evidence supporting their use. This leaves surgeons with little to nothing to inform their decisions to use these devices, other than familiarity with and impressions of the new technology.
Our study had several limitations. First is the small sample size, of surgeons who are leaders in the field. Our sample therefore may not be generalizable to the general population of shoulder and trauma surgeons. Second, we did not calculate intraobserver variability. Third, inherent to studies of interobserver agreement is the uncertainty of their clinical relevance. In the clinical setting, a surgeon has much more information at hand (eg, patient history, physical examination findings, colleague consultations), thus raising the possibility of underestimations of interobserver agreements.18 Fourth, our comparison of surgeons’ ratings of outcomes was purely radiographic, which may or may not represent or be indicative of clinical outcomes (eg, pain relief, function, range of motion, patient satisfaction). The conclusions we may draw are accordingly limited, as we did not directly evaluate clinical outcome parameters.
Our study had several strengths as well. First, to our knowledge this is the first study to assess interobserver variability in surgeons’ ratings of radiographic outcomes. Its findings may provide further insight into the reasons for poor agreement among orthopedic surgeons on both classification and treatment of PHFs. Second, our surveying of internationally renowned expert surgeons from 4 different institutions may have helped reduce single-institution bias, and it presents the highest level of expertise in the treatment of PHFs.
Although the surgeons in our study moderately agreed on final radiographic outcomes of PHFs, such levels of agreement may still be clinically unacceptable.19 The overall disagreement on treatment decisions highlights the need for better evidence for optimal treatment of PHFs in order to improve consensus, particularly with anticipated increases in age and comorbidities in the population in coming years.4 Subgroup analysis suggested trauma fellowships may contribute to better treatment agreement, though this idea requires further study, perhaps by surveying shoulder and trauma fellowship directors and their curricula for variability in teaching treatment decision-making. The surgeons in our study agreed more on what they consider acceptable final radiographic outcomes, which is encouraging. However, treatment consensus is the primary goal. The recent publication of prospective, randomized studies is helping with this issue, but more studies are needed. It is encouraging that several are planned or under way.20-22
Conclusion
The surgeons surveyed in this study did not agree on ideal treatment for PHFs but moderately agreed on quality of radiographic outcomes. These differences may reflect a difference in training. We conducted this study to compare experienced shoulder and trauma fellowship–trained surgeons’ treatment decision-making and ratings of radiographic outcomes of PHFs when presented with the same group of patients managed at 2 level I trauma centers. We hypothesized there would be little agreement on treatment decisions, better agreement on final radiographic outcome, and a difference between decision-making and ratings of radiographic outcomes between expert shoulder and trauma surgeons. Our results showed that surgeons do not agree on the best treatment for PHFs but radiographically recognize when an operative treatment has been performed well or poorly. Regarding treatment decisions, our results also showed that expert trauma surgeons may agree more with each other than shoulder surgeons agree with each other. These results support our hypothesis and the limited current literature. The overall disagreement among the surgeons in our study and an aging population that grows sicker each year highlight the need for better evidence for the optimal treatment of PHFs in order to improve consensus.
Proximal humerus fractures (PHFs), AO/OTA (Ar beitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 11,1 are common, representing 4% to 5% of all fractures in adults.2 However, there is no consensus as to optimal management of these injuries, with some reports supporting and others rejecting the various fixation methods,3 and there are no evidence-based practice guidelines informing treatment decisions.4 Not surprisingly, orthopedic surgeons do not agree on ideal treatment for PHFs5,6 and differ by region in their rates of surgical management.2 In addition, analyses of national databases have found variation in choice of surgical treatment for PHFs between surgeons and between hospitals of different patient volumes.4 Few studies have assessed surgeon agreement on treatment decisions. Findings from these limited investigations indicate there is little agreement on treatment choices, but training may have some impact.5-7 In 3 studies,5-7 shoulder and trauma fellowship–trained surgeons differed in their management of PHFs both in terms of rates of operative treatment5,7 and specific operative management choices.5,6 No study has assessed surgeon agreement on radiographic outcomes.
We conducted a study to compare expert shoulder and trauma surgeons’ treatment decision-making and agreement on final radiographic outcomes of surgically treated PHFs. We hypothesized there would be poor agreement on treatment decisions and better agreement on radiographic outcomes, with a difference between shoulder and trauma fellowship–trained surgeons.
Materials and Methods
After receiving institutional review board approval for this study, we collected data on 100 consecutive PHFs (AO/OTA type 111) surgically treated at 2 affiliated level I trauma centers between January 2004 and July 2008. None of the cases in the series was managed by any of the surgeons participating in this study.
We created a PowerPoint (Microsoft, Redmond, Washington) survey that included radiographs (preoperative, immediate postoperative, final postoperative) and, if available, a computed tomography image. This survey was sent to 4 orthopedic surgeons: Drs. Gardner, Gerber, Lorich, and Walch. Two of these authors are fellowship-trained in shoulder surgery, the other 2 in orthopedic traumatology with specialization in treating PHFs. All are internationally renowned in PHF management. Using the survey images and a 4-point Likert scale ranging from disagree strongly to agree strongly, the examiners rated their agreement with treatment decisions (arthroplasty vs fixation). They also rated (very poor to very good) immediate postoperative reduction or arthroplasty placement, immediate postoperative fixation methods for fractures treated with open reduction and internal fixation (ORIF), and final radiographic outcomes.
Interobserver agreement was calculated using the intraclass correlation coefficient (ICC),8,9 with scores of <0.2 (poor), 0.21 to 0.4 (fair), 0.41 to 0.6 (moderate), 0.61 to 0.8 (good), and >0.8 (excellent) used to indicate agreement among observers. ICC scores were determined by treating the 4 examiners as independent entities. Subgroup analyses were also performed to determine ICC scores comparing the 2 shoulder surgeons, comparing the 2 trauma surgeons, and comparing the shoulder surgeons and trauma surgeons as 2 separate groups. ICC scores were used instead of κ coefficients to assess agreement because ICC scores treat ratings as continuous variables, allow for comparison of 2 or more raters, and allow for assessment of correlation among raters, whereas κ coefficients treat data as categorical variables and assume the ratings have no natural ordering. ICC scores were generated by SAS 9.1.3 software (SAS Institute, Cary, North Carolina).
Results
The 4 surgeons’ overall ICC scores for agreement with the rating of immediate reduction or arthroplasty placement and the rating of final radiographic outcome indicated moderate levels of agreement (Table 1). Regarding treatment decision-making and ratings of fixation, the surgeons demonstrated poor and fair levels of agreement, respectively.
The ICC scores comparing the shoulder and trauma surgeons revealed similar levels of agreement (Table 2): moderate levels of agreement for ratings of both immediate postoperative reduction or arthroplasty placement and final radiographic outcomes, but poor and fair levels of agreement regarding treatment decision-making and the rating of immediate postoperative fixation methods for fractures treated with ORIF, respectively.
Subgroup analysis revealed that the 2 shoulder surgeons had poor and fair levels of agreement for treatment decisions and rating of immediate postoperative fixation, respectively, though they moderately agreed on rating of immediate postoperative reduction or arthroplasty placement and rating of final radiographic outcome (Table 3). When the 2 trauma surgeons were compared with each other, ICC scores revealed higher levels of agreement overall (Table 4). In other words, the 2 trauma surgeons agreed with each other more than the 2 shoulder surgeons agreed with each other.
Discussion
This study had 3 major findings: (1) Surgeons do not agree on treatment decisions, including fixation methods, regarding PHFs; (2) regardless of their opinions on ideal treatment, they moderately agree on reductions and final radiographic outcomes; (3) expert trauma surgeons may agree more on treatment decisions than expert shoulder surgeons do. In other words, surgeons do not agree on the best treatment, but they radiographically recognize when a procedure has been performed technically well or poorly. These results support our hypothesis and the limited current literature.
An analysis of Medicare databases showed marked regional variation in rates of operative treatment of PHFs.2 Similarly, a Nationwide Inpatient Sample analysis revealed nationwide variation in operative management of PHFs.4 Both findings are consistent with our results of poor agreement about treatment decisions and ratings of postoperative fixation of PHFs. In 2010, Petit and colleagues6 reported that surgeons do not agree on PHF management. In 2011, Foroohar and colleagues10 similarly reported low interobserver agreement for treatment recommendations made by 4 upper extremity orthopedic specialists, 4 general orthopedic surgeons, 4 senior residents, and 4 junior residents, for a series of 16 PHFs—also consistent with our findings.
The lack of agreement about PHF treatment may reflect a difference in training, particularly in light of the recent expansion of shoulder and elbow fellowships.2 Three separate studies performed at 2 affiliated level I trauma centers demonstrated significant differences in treatment decision-making between shoulder and trauma fellowship–trained surgeons.5-7 Our results are consistent with the hypothesis that training affects treatment decision-making, as we found poor agreement between shoulder and trauma fellowship–trained surgeons regarding treatment decision for PHFs. Subanalyses revealed that expert trauma surgeons agreed with each other on treatment decisions more than expert shoulder surgeons agreed with each other, further suggesting that training may affect how surgeons manage PHFs. Differences in fellowship training even within the same specialty may account for the observed lesser levels of agreement between the shoulder surgeons, even among experts in the field.
The evidence for optimal treatment historically has been poor,4,6 with few high-quality prospective, randomized controlled studies on the topic up until the past few years. The most recent Cochrane Review on optimal PHF treatment concluded that there is insufficient evidence to make an evidence-based recommendation and that the long-term benefit of surgery is unclear.11 However, at least 5 controlled trials on the topic have been published within the past 5 years.12-16 The evidence is striking and generally supports nonoperative treatment for most PHFs, including some displaced fractures—contrary to general orthopedic practice in many parts of the United States,2 which hitherto had been based mainly on individual surgeon experience and the limited literature. Without strong evidence to support one treatment option over another, surgeons are left with no objective, scientific way of coming to agreement.
Related to the poor status quo of evidence for PHF treatments is new technology (eg, locking plates, reverse total shoulder arthroplasty) that has expanded surgical indications.2,17 Although such developments have the potential to improve surgical treatments, they may also exacerbate the disagreement between surgeons regarding optimal operative treatment of PHFs. This potential consequence of new technology may be reflected in our finding of disagreement among surgeons on immediate postoperative fixation methods. Precisely because they are new, such technological innovations have limited evidence supporting their use. This leaves surgeons with little to nothing to inform their decisions to use these devices, other than familiarity with and impressions of the new technology.
Our study had several limitations. First is the small sample size, of surgeons who are leaders in the field. Our sample therefore may not be generalizable to the general population of shoulder and trauma surgeons. Second, we did not calculate intraobserver variability. Third, inherent to studies of interobserver agreement is the uncertainty of their clinical relevance. In the clinical setting, a surgeon has much more information at hand (eg, patient history, physical examination findings, colleague consultations), thus raising the possibility of underestimations of interobserver agreements.18 Fourth, our comparison of surgeons’ ratings of outcomes was purely radiographic, which may or may not represent or be indicative of clinical outcomes (eg, pain relief, function, range of motion, patient satisfaction). The conclusions we may draw are accordingly limited, as we did not directly evaluate clinical outcome parameters.
Our study had several strengths as well. First, to our knowledge this is the first study to assess interobserver variability in surgeons’ ratings of radiographic outcomes. Its findings may provide further insight into the reasons for poor agreement among orthopedic surgeons on both classification and treatment of PHFs. Second, our surveying of internationally renowned expert surgeons from 4 different institutions may have helped reduce single-institution bias, and it presents the highest level of expertise in the treatment of PHFs.
Although the surgeons in our study moderately agreed on final radiographic outcomes of PHFs, such levels of agreement may still be clinically unacceptable.19 The overall disagreement on treatment decisions highlights the need for better evidence for optimal treatment of PHFs in order to improve consensus, particularly with anticipated increases in age and comorbidities in the population in coming years.4 Subgroup analysis suggested trauma fellowships may contribute to better treatment agreement, though this idea requires further study, perhaps by surveying shoulder and trauma fellowship directors and their curricula for variability in teaching treatment decision-making. The surgeons in our study agreed more on what they consider acceptable final radiographic outcomes, which is encouraging. However, treatment consensus is the primary goal. The recent publication of prospective, randomized studies is helping with this issue, but more studies are needed. It is encouraging that several are planned or under way.20-22
Conclusion
The surgeons surveyed in this study did not agree on ideal treatment for PHFs but moderately agreed on quality of radiographic outcomes. These differences may reflect a difference in training. We conducted this study to compare experienced shoulder and trauma fellowship–trained surgeons’ treatment decision-making and ratings of radiographic outcomes of PHFs when presented with the same group of patients managed at 2 level I trauma centers. We hypothesized there would be little agreement on treatment decisions, better agreement on final radiographic outcome, and a difference between decision-making and ratings of radiographic outcomes between expert shoulder and trauma surgeons. Our results showed that surgeons do not agree on the best treatment for PHFs but radiographically recognize when an operative treatment has been performed well or poorly. Regarding treatment decisions, our results also showed that expert trauma surgeons may agree more with each other than shoulder surgeons agree with each other. These results support our hypothesis and the limited current literature. The overall disagreement among the surgeons in our study and an aging population that grows sicker each year highlight the need for better evidence for the optimal treatment of PHFs in order to improve consensus.
1. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium – 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
2. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
3. McLaurin TM. Proximal humerus fractures in the elderly are we operating on too many? Bull Hosp Jt Dis. 2004;62(1-2):24-32.
4. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop. 2012;471(2):655-664.
5. Boykin RE, Jawa A, O’Brien T, Higgins LD, Warner JJP. Variability in operative management of proximal humerus fractures. Shoulder Elbow. 2011;3(4):197-201.
6. Petit CJ, Millett PJ, Endres NK, Diller D, Harris MB, Warner JJP. Management of proximal humeral fractures: surgeons don’t agree. J Shoulder Elbow Surg. 2010;19(3):446-451.
7. Okike K, Lee OC, Makanji H, Harris MB, Vrahas MS. Factors associated with the decision for operative versus non-operative treatment of displaced proximal humerus fractures in the elderly. Injury. 2013;44(4):448-455.
8. Kodali P, Jones MH, Polster J, Miniaci A, Fening SD. Accuracy of measurement of Hill-Sachs lesions with computed tomography. J Shoulder Elbow Surg. 2011;20(8):1328-1334.
9. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428.
10. Foroohar A, Tosti R, Richmond JM, Gaughan JP, Ilyas AM. Classification and treatment of proximal humerus fractures: inter-observer reliability and agreement across imaging modalities and experience. J Orthop Surg Res. 2011;6:38.
11. Handoll HH, Ollivere BJ. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2010;(12):CD000434.
12. Boons HW, Goosen JH, van Grinsven S, van Susante JL, van Loon CJ. Hemiarthroplasty for humeral four-part fractures for patients 65 years and older: a randomized controlled trial. Clin Orthop. 2012;470(12):3483-3491.
13. Fjalestad T, Hole MØ, Hovden IAH, Blücher J, Strømsøe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
14. Fjalestad T, Hole MØ, Jørgensen JJ, Strømsøe K, Kristiansen IS. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury. 2010;41(6):599-605.
15. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
16. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
17. Agudelo J, Schürmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
18. Brorson S, Hróbjartsson A. Training improves agreement among doctors using the Neer system for proximal humeral fractures in a systematic review. J Clin Epidemiol. 2008;61(1):7-16.
19. Brorson S, Olsen BS, Frich LH, et al. Surgeons agree more on treatment recommendations than on classification of proximal humeral fractures. BMC Musculoskelet Disord. 2012;13:114.
20. Handoll H, Brealey S, Rangan A, et al. Protocol for the ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial: a pragmatic multi-centre randomised controlled trial of surgical versus non-surgical treatment for proximal fracture of the humerus in adults. BMC Musculoskelet Disord. 2009;10:140.
21. Den Hartog D, Van Lieshout EMM, Tuinebreijer WE, et al. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon): a multicenter randomized controlled trial. BMC Musculoskelet Disord. 2010;11:97.
22. Verbeek PA, van den Akker-Scheek I, Wendt KW, Diercks RL. Hemiarthroplasty versus angle-stable locking compression plate osteosynthesis in the treatment of three- and four-part fractures of the proximal humerus in the elderly: design of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:16.
1. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium – 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
2. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
3. McLaurin TM. Proximal humerus fractures in the elderly are we operating on too many? Bull Hosp Jt Dis. 2004;62(1-2):24-32.
4. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop. 2012;471(2):655-664.
5. Boykin RE, Jawa A, O’Brien T, Higgins LD, Warner JJP. Variability in operative management of proximal humerus fractures. Shoulder Elbow. 2011;3(4):197-201.
6. Petit CJ, Millett PJ, Endres NK, Diller D, Harris MB, Warner JJP. Management of proximal humeral fractures: surgeons don’t agree. J Shoulder Elbow Surg. 2010;19(3):446-451.
7. Okike K, Lee OC, Makanji H, Harris MB, Vrahas MS. Factors associated with the decision for operative versus non-operative treatment of displaced proximal humerus fractures in the elderly. Injury. 2013;44(4):448-455.
8. Kodali P, Jones MH, Polster J, Miniaci A, Fening SD. Accuracy of measurement of Hill-Sachs lesions with computed tomography. J Shoulder Elbow Surg. 2011;20(8):1328-1334.
9. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428.
10. Foroohar A, Tosti R, Richmond JM, Gaughan JP, Ilyas AM. Classification and treatment of proximal humerus fractures: inter-observer reliability and agreement across imaging modalities and experience. J Orthop Surg Res. 2011;6:38.
11. Handoll HH, Ollivere BJ. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2010;(12):CD000434.
12. Boons HW, Goosen JH, van Grinsven S, van Susante JL, van Loon CJ. Hemiarthroplasty for humeral four-part fractures for patients 65 years and older: a randomized controlled trial. Clin Orthop. 2012;470(12):3483-3491.
13. Fjalestad T, Hole MØ, Hovden IAH, Blücher J, Strømsøe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
14. Fjalestad T, Hole MØ, Jørgensen JJ, Strømsøe K, Kristiansen IS. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury. 2010;41(6):599-605.
15. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
16. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
17. Agudelo J, Schürmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
18. Brorson S, Hróbjartsson A. Training improves agreement among doctors using the Neer system for proximal humeral fractures in a systematic review. J Clin Epidemiol. 2008;61(1):7-16.
19. Brorson S, Olsen BS, Frich LH, et al. Surgeons agree more on treatment recommendations than on classification of proximal humeral fractures. BMC Musculoskelet Disord. 2012;13:114.
20. Handoll H, Brealey S, Rangan A, et al. Protocol for the ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial: a pragmatic multi-centre randomised controlled trial of surgical versus non-surgical treatment for proximal fracture of the humerus in adults. BMC Musculoskelet Disord. 2009;10:140.
21. Den Hartog D, Van Lieshout EMM, Tuinebreijer WE, et al. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon): a multicenter randomized controlled trial. BMC Musculoskelet Disord. 2010;11:97.
22. Verbeek PA, van den Akker-Scheek I, Wendt KW, Diercks RL. Hemiarthroplasty versus angle-stable locking compression plate osteosynthesis in the treatment of three- and four-part fractures of the proximal humerus in the elderly: design of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:16.