User login
Clinical and Radiographic Outcomes of Total Shoulder Arthroplasty With a Hybrid Dual-Radii Glenoid Component
Take-Home Points
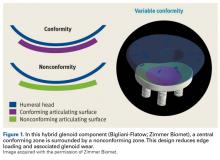
- The authors have developed a total shoulder glenoid prosthesis that conforms with the humeral head in its center and is nonconforming on its peripheral edge.
- All clinical survey and range of motion parameters demonstrated statistically significant improvements at final follow-up.
- Only 3 shoulders (1.7%) required revision surgery.
- Eighty-six (63%) of 136 shoulders demonstrated no radiographic evidence of glenoid loosening.
- This is the first and largest study that evaluates the clinical and radiographic outcomes of this hybrid shoulder prosthesis.
Fixation of the glenoid component is the limiting factor in modern total shoulder arthroplasty (TSA). Glenoid loosening, the most common long-term complication, necessitates revision in up to 12% of patients.1-4 By contrast, humeral component loosening is relatively uncommon, affecting as few as 0.34% of patients.5 Multiple long-term studies have found consistently high rates (45%-93%) of radiolucencies around the glenoid component.3,6,7 Although their clinical significance has been debated, radiolucencies around the glenoid component raise concern about progressive loss of fixation.
Since TSA was introduced in the 1970s, complications with the glenoid component have been addressed with 2 different designs: conforming (congruent) and nonconforming. In a congruent articulation, the radii of curvature of the glenoid and humeral head components are identical, whereas they differ in a nonconforming model. Joint conformity is inversely related to glenohumeral translation.8 Neer’s original TSA was made congruent in order to limit translation and maximize the contact area. However, this design results in edge loading and a so-called rocking-horse phenomenon, which may lead to glenoid loosening.9-13 Surgeons therefore have increasingly turned to nonconforming implants. In the nonconforming design, the radius of curvature of the humeral head is smaller than that of the glenoid. Although this design may reduce edge loading,14 it allows more translation and reduces the relative contact area of the glenohumeral joint. As a result, more contact stress is transmitted to the glenoid component, leading to polyethylene deformation and wear.15,16
Dual radii of curvature are designed to augment joint stability without increasing component wear. Biomechanical data have indicated that edge loading is not increased by having a central conforming region added to a nonconforming model.17 The clinical value of this prosthesis, however, has not been determined. Therefore, we conducted a study to describe the intermediate-term clinical and radiographic outcomes of TSAs that use a novel hybrid glenoid component.
Materials and Methods
This study was approved (protocol AAAD3473) by the Institutional Review Board of Columbia University and was conducted in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations.
Patient Selection
At Columbia University Medical Center, Dr. Bigliani performed 196 TSAs with a hybrid glenoid component (Bigliani-Flatow; Zimmer Biomet) in 169 patients between September 1998 and November 2007. All patients had received a diagnosis of primary glenohumeral arthritis as defined by Neer.18 Patients with previous surgery such as rotator cuff repair or subacromial decompression were included in our review, and patients with a nonprimary form of arthritis, such as rheumatoid, posttraumatic, or post-capsulorrhaphy arthritis, were excluded.
Operative Technique
For all surgeries, Dr. Bigliani performed a subscapularis tenotomy with regional anesthesia and a standard deltopectoral approach. A partial anterior capsulectomy was performed to increase the glenoid’s visibility. The inferior labrum was removed with a needle-tip bovie while the axillary nerve was being protected with a metal finger or narrow Darrach retractor. After reaming and trialing, the final glenoid component was cemented into place. Cement was placed only in the peg or keel holes and pressurized twice before final implantation. Of the 196 glenoid components, 168 (86%) were pegged and 28 (14%) keeled; in addition,190 of these components were all-polyethylene, whereas 6 had trabecular-metal backing. All glenoid components incorporated the hybrid design of dual radii of curvature. After the glenoid was cemented, the final humeral component was placed in 30° of retroversion. Whenever posterior wear was found, retroversion was reduced by 5° to 10°. The humeral prosthesis was cemented in cases (104/196, 53%) of poor bone quality or a large canal.
After surgery, the patient’s sling was fitted with an abduction pillow and a swathe, to be worn the first 24 hours, and the arm was passively ranged. Patients typically were discharged on postoperative day 2. Then, for 2 weeks, they followed an assisted passive range of motion (ROM) protocol, with limited external rotation, for promotion of subscapularis healing.
Clinical Outcomes
Dr. Bigliani assessed preoperative ROM in all planes. During initial evaluation, patients completed a questionnaire that consisted of the 36-Item Short Form Health Survey19,20 (SF-36) and the American Shoulder and Elbow Surgeons21 (ASES) and Simple Shoulder Test22 (SST) surveys. Postoperative clinical data were collected from office follow-up visits, survey questionnaires, or both. Postoperative office data included ROM, subscapularis integrity testing (belly-press or lift-off), and any complications. Patients with <1 year of office follow-up were excluded. In addition, the same survey questionnaire that was used before surgery was mailed to all patients after surgery; then, for anyone who did not respond by mail, we attempted contact by telephone. Neer criteria were based on patients’ subjective assessment of each arm on a 3-point Likert scale (1 = very satisfied, 2 = satisfied, 3 = dissatisfied). Patients were also asked about any specific complications or revision operations since their index procedure.
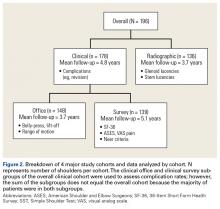
Physical examination and office follow-up data were obtained for 129 patients (148/196 shoulders, 76% follow-up) at a mean of 3.7 years (range 1.0-10.2 years) after surgery. Surveys were completed by 117 patients (139/196 shoulders, 71% follow-up) at a mean of 5.1 years (range, 1.6-11.2 years) after surgery. Only 15 patients had neither 1 year of office follow-up nor a completed questionnaire. The remaining 154 patients (178/196 shoulders, 91% follow-up) had clinical follow-up with office, mail, or telephone questionnaire at a mean of 4.8 years (range, 1.0-11.2 years) after surgery. This cohort of patients was used to determine rates of surgical revisions, subscapularis tears, dislocations, and other complications.
Radiographic Outcomes
Patients were included in the radiographic analysis if they had a shoulder radiograph at least 1 year after surgery. One hundred nineteen patients (136/196 shoulders, 69% follow-up) had radiographic follow-up at a mean of 3.7 years (range, 1.0-9.4 years) after surgery.
Statistical Analysis
Statistical analysis was performed with Stata Version 10.0. Paired t tests were used to compare preoperative and postoperative numerical data, including ROM and survey scores. We calculated 95% confidence intervals (CIs) and set statistical significance at P < .05. For qualitative measures, the Fisher exact test was used. Survivorship analysis was performed according to the Kaplan-Meier method, with right-censored data for no event or missing data.25
Results
Clinical Analysis of Demographics
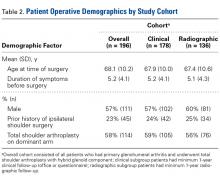
In demographics, the clinical and radiographic patient subgroups were similar to each other and to the overall study population (Table 2). Of 196 patients overall, 16 (8%) had a concomitant rotator cuff repair, and 27 (14%) underwent staged bilateral shoulder arthroplasties.
Clinical Analysis of ROM and Survey Scores
Operative shoulder ROM in forward elevation, external rotation at side, external rotation in abduction, and internal rotation all showed statistically significant (P < .001) improvement from before surgery to after surgery. Over 3.7 years, mean (SD) forward elevation improved from 107.3° (34.8°) to 159.0° (29.4°), external rotation at side improved from 20.4° (16.7°) to 49.4° (11.3°), and external rotation in abduction improved from 53.7° (24.3°) to 84.7° (9.1°). Internal rotation improved from a mean (SD) vertebral level of S1 (6.0 levels) to T9 (3.7 levels).
All validated survey scores also showed statistically significant (P < .001) improvement from before surgery to after surgery. Over 5.1 years, mean (SD) SF-36 scores improved from 64.9 (13.4) to 73.6 (17.1), ASES scores improved from 41.1 (22.5) to 82.7 (17.7), SST scores improved from 3.9 (2.8) to 9.7 (2.2), and visual analog scale pain scores improved from 5.6 (3.2) to 1.4 (2.1). Of 139 patients with follow-up, 130 (93.5%) were either satisfied or very satisfied with their TSA, and only 119 (86%) were either satisfied or very satisfied with the nonoperative shoulder.
Clinical Analysis of Postoperative Complications
Of the 178 shoulders evaluated for complications, 3 (1.7%) underwent revision surgery. Mean time to revision was 2.3 years (range, 1.5-3.9 years). Two revisions involved the glenoid component, and the third involved the humerus. In one of the glenoid cases, a 77-year-old woman fell and sustained a fracture at the base of the trabecular metal glenoid pegs; her component was revised to an all-polyethylene component, and she had no further complications. In the other glenoid case, a 73-year-old man’s all-polyethylene component loosened after 2 years and was revised to a trabecular metal implant, which loosened as well and was later converted to a hemiarthroplasty. In the humeral case, a 33-year-old man had his 4-year-old index TSA revised to a cemented stem and had no further complications.
Table 4 compares the clinical and radiographic outcomes of patients who required subscapularis repair, capsular shift, or implant revision with the outcomes of all other study patients, and Figure 3 shows Kaplan-Meier survivorship.
Postoperative Radiographic Analysis
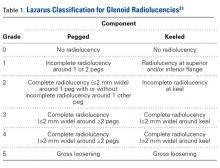
Glenoid Component. At a mean of 3.7 years (minimum, 1 year) after surgery, 86 (63%) of 136 radiographically evaluated shoulders showed no glenoid lucencies; the other 50 (37%) showed ≥1 lucency. Of the 136 shoulders, 33 (24%) had a Lazarus score of 1, 15 (11%) had a score of 2, and only 2 (2%) had a score of 3. None of the shoulders had a score of 4 or 5.
Humeral Component. Of the 136 shoulders, 91 (67%) showed no lucencies in any of the 8 humeral stem zones; the other 45 (33%) showed 1 to 3 lucencies. Thirty (22%) of the 136 shoulders had 1 stem lucency zone, 8 (6%) had 2, and 3 (2%) had 3. None of the shoulders had >3 periprosthetic zones with lucent lines.
Discussion
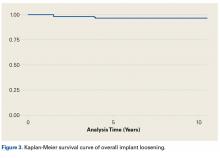
In this article, we describe a hybrid glenoid TSA component with dual radii of curvature. Its central portion is congruent with the humeral head, and its peripheral portion is noncongruent and larger. The most significant finding of our study is the low rate (1.1%) of glenoid component revision 4.8 years after surgery. This rate is the lowest that has been reported in a study of ≥100 patients. Overall implant survival appeared as an almost flat Kaplan-Meir curve. We attribute this low revision rate to improved biomechanics with the hybrid glenoid design.
Symptomatic glenoid component loosening is the most common TSA complication.1,26-28 In a review of 73 Neer TSAs, Cofield7 found glenoid radiolucencies in 71% of patients 3.8 years after surgery. Radiographic evidence of loosening, defined as component migration, or tilt, or a circumferential lucency 1.5 mm thick, was present in another 11% of patients, and 4.1% developed symptomatic loosening that required glenoid revision. In a study with 12.2-year follow-up, Torchia and colleagues3 found rates of 84% for glenoid radiolucencies, 44% for radiographic loosening, and 5.6% for symptomatic loosening that required revision. In a systematic review of studies with follow-up of ≥10 years, Bohsali and colleagues27 found similar lucency and radiographic loosening rates and a 7% glenoid revision rate. These data suggest glenoid radiolucencies may progress to component loosening.
Degree of joint congruence is a key factor in glenoid loosening. Neer’s congruent design increases the contact area with concentric loading and reduces glenohumeral translation, which leads to reduced polyethylene wear and improved joint stability. In extreme arm positions, however, humeral head subluxation results in edge loading and a glenoid rocking-horse effect.9-13,17,29-31 Conversely, nonconforming implants allow increased glenohumeral translation without edge loading,14 though they also reduce the relative glenohumeral contact area and thus transmit more contact stress to the glenoid.16,17 A hybrid glenoid component with central conforming and peripheral nonconforming zones may reduce the rocking-horse effect while maximizing ROM and joint stability. Wang and colleagues32 studied the biomechanical properties of this glenoid design and found that the addition of a central conforming region did not increase edge loading.
Additional results from our study support the efficacy of a hybrid glenoid component. Patients’ clinical outcomes improved significantly. At 5.1 years after surgery, 93.5% of patients were satisfied or very satisfied with their procedure and reported less satisfaction (86%) with the nonoperative shoulder. Also significant was the reduced number of radiolucencies. At 3.7 years after surgery, the overall percentage of shoulders with ≥1 glenoid radiolucency was 37%, considerably lower than the 82% reported by Cofield7 and the rates in more recent studies.3,16,33-36 Of the 178 shoulders in our study, 10 (5.6%) had subscapularis tears, and 6 (3.4%) of 178 had these tears surgically repaired. This 3.4% compares favorably with the 5.9% (of 119 patients) found by Miller and colleagues37 28 months after surgery. Of our 178 shoulders, 27 (15.2%) had clinically significant postoperative complications; 18 (10.1%) of the 178 had these complications surgically treated, and 9 (5.1%) had them managed nonoperatively. Bohsali and colleagues27 systematically reviewed 33 TSA studies and found a slightly higher complication rate (16.3%) 5.3 years after surgery. Furthermore, in our study, the 11 patients who underwent revision, capsular shift, or subscapularis repair had final outcomes comparable to those of the rest of our study population.
Our study had several potential weaknesses. First, its minimum clinical and radiographic follow-up was 1 year, whereas most long-term TSA series set a minimum of 2 years. We used 1 year because this was the first clinical study of the hybrid glenoid component design, and we wanted to maximize its sample size by reporting on intermediate-length outcomes. Even so, 93% (166/178) of our clinical patients and 83% (113/136) of our radiographic patients have had ≥2 years of follow-up, and we continue to follow all study patients for long-term outcomes. Another weakness of the study was its lack of a uniform group of patients with all the office, survey, complications, and radiographic data. Our retrospective study design made it difficult to obtain such a group without significantly reducing the sample size, so we divided patients into 4 data groups. A third potential weakness was the study’s variable method for collecting complications data. Rates of complications in the 178 shoulders were calculated from either office evaluation or patient self-report by mail or telephone. This data collection method is subject to recall bias, but mail and telephone contact was needed so the study would capture the large number of patients who had traveled to our institution for their surgery or had since moved away. Fourth, belly-press and lift-off tests were used in part to assess subscapularis function, but recent literature suggests post-TSA subscapularis assessment can be unreliable.38 These tests may be positive in up to two-thirds of patients after 2 years.39 Fifth, the generalizability of our findings to diagnoses such as rheumatoid and posttraumatic arthritis is limited. We had to restrict the study to patients with primary glenohumeral arthritis in order to minimize confounders.
This study’s main strength is its description of the clinical and radiographic outcomes of using a single prosthetic system in operations performed by a single surgeon in a large number of patients. This was the first and largest study evaluating the clinical and radiographic outcomes of this hybrid glenoid implant. Excluding patients with nonprimary arthritis allowed us to minimize potential confounding factors that affect patient outcomes. In conclusion, our study results showed the favorable clinical and radiographic outcomes of TSAs that have a hybrid glenoid component with dual radii of curvature. At a mean of 3.7 years after surgery, 63% of patients had no glenoid lucencies, and, at a mean of 4.8 years, only 1.7% of patients required revision. We continue to follow these patients to obtain long-term results of this innovative prosthesis.
1. Rodosky MW, Bigliani LU. Indications for glenoid resurfacing in shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5(3):231-248.
2. Boyd AD Jr, Thomas WH, Scott RD, Sledge CB, Thornhill TS. Total shoulder arthroplasty versus hemiarthroplasty. Indications for glenoid resurfacing. J Arthroplasty. 1990;5(4):329-336.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85(2):251-258.
5. Cofield RH. Degenerative and arthritic problems of the glenohumeral joint. In: Rockwood CA, Matsen FA, eds. The Shoulder. Philadelphia, PA: Saunders; 1990:740-745.
6. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
7. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
8. Karduna AR, Williams GR, Williams JL, Iannotti JP. Kinematics of the glenohumeral joint: influences of muscle forces, ligamentous constraints, and articular geometry. J Orthop Res. 1996;14(6):986-993.
9. Karduna AR, Williams GR, Iannotti JP, Williams JL. Total shoulder arthroplasty biomechanics: a study of the forces and strains at the glenoid component. J Biomech Eng. 1998;120(1):92-99.
10. Karduna AR, Williams GR, Williams JL, Iannotti JP. Glenohumeral joint translations before and after total shoulder arthroplasty. A study in cadavera. J Bone Joint Surg Am. 1997;79(8):1166-1174.
11. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
12. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
13. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
14. Harryman DT, Sidles JA, Harris SL, Lippitt SB, Matsen FA 3rd. The effect of articular conformity and the size of the humeral head component on laxity and motion after glenohumeral arthroplasty. A study in cadavera. J Bone Joint Surg Am. 1995;77(4):555-563.
15. Flatow EL. Prosthetic design considerations in total shoulder arthroplasty. Semin Arthroplasty. 1995;6(4):233-244.
16. Klimkiewicz JJ, Iannotti JP, Rubash HE, Shanbhag AS. Aseptic loosening of the humeral component in total shoulder arthroplasty. J Shoulder Elbow Surg. 1998;7(4):422-426.
17. Wang VM, Krishnan R, Ugwonali OF, Flatow EL, Bigliani LU, Ateshian GA. Biomechanical evaluation of a novel glenoid design in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 suppl S):129S-140S.
18. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
19. Boorman RS, Kopjar B, Fehringer E, Churchill RS, Smith K, Matsen FA 3rd. The effect of total shoulder arthroplasty on self-assessed health status is comparable to that of total hip arthroplasty and coronary artery bypass grafting. J Shoulder Elbow Surg. 2003;12(2):158-163.
20. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
21. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
22. Wright RW, Baumgarten KM. Shoulder outcomes measures. J Am Acad Orthop Surg. 2010;18(7):436-444.
23. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
24. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
25. Dinse GE, Lagakos SW. Nonparametric estimation of lifetime and disease onset distributions from incomplete observations. Biometrics. 1982;38(4):921-932.
26. Baumgarten KM, Lashgari CJ, Yamaguchi K. Glenoid resurfacing in shoulder arthroplasty: indications and contraindications. Instr Course Lect. 2004;53:3-11.
27. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
28. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
29. Poppen NK, Walker PS. Normal and abnormal motion of the shoulder. J Bone Joint Surg Am. 1976;58(2):195-201.
30. Cotton RE, Rideout DF. Tears of the humeral rotator cuff; a radiological and pathological necropsy survey. J Bone Joint Surg Br. 1964;46:314-328.
31. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
32. Wang VM, Sugalski MT, Levine WN, Pawluk RJ, Mow VC, Bigliani LU. Comparison of glenohumeral mechanics following a capsular shift and anterior tightening. J Bone Joint Surg Am. 2005;87(6):1312-1322.
33. Young A, Walch G, Boileau P, et al. A multicentre study of the long-term results of using a flat-back polyethylene glenoid component in shoulder replacement for primary osteoarthritis. J Bone Joint Surg Br. 2011;93(2):210-216.
34. Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600.
35. Walch G, Edwards TB, Boulahia A, Boileau P, Mole D, Adeleine P. The influence of glenohumeral prosthetic mismatch on glenoid radiolucent lines: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2186-2191.
36. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
37. Miller BS, Joseph TA, Noonan TJ, Horan MP, Hawkins RJ. Rupture of the subscapularis tendon after shoulder arthroplasty: diagnosis, treatment, and outcome. J Shoulder Elbow Surg. 2005;14(5):492-496.
38. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
39. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
Take-Home Points
- The authors have developed a total shoulder glenoid prosthesis that conforms with the humeral head in its center and is nonconforming on its peripheral edge.
- All clinical survey and range of motion parameters demonstrated statistically significant improvements at final follow-up.
- Only 3 shoulders (1.7%) required revision surgery.
- Eighty-six (63%) of 136 shoulders demonstrated no radiographic evidence of glenoid loosening.
- This is the first and largest study that evaluates the clinical and radiographic outcomes of this hybrid shoulder prosthesis.
Fixation of the glenoid component is the limiting factor in modern total shoulder arthroplasty (TSA). Glenoid loosening, the most common long-term complication, necessitates revision in up to 12% of patients.1-4 By contrast, humeral component loosening is relatively uncommon, affecting as few as 0.34% of patients.5 Multiple long-term studies have found consistently high rates (45%-93%) of radiolucencies around the glenoid component.3,6,7 Although their clinical significance has been debated, radiolucencies around the glenoid component raise concern about progressive loss of fixation.
Since TSA was introduced in the 1970s, complications with the glenoid component have been addressed with 2 different designs: conforming (congruent) and nonconforming. In a congruent articulation, the radii of curvature of the glenoid and humeral head components are identical, whereas they differ in a nonconforming model. Joint conformity is inversely related to glenohumeral translation.8 Neer’s original TSA was made congruent in order to limit translation and maximize the contact area. However, this design results in edge loading and a so-called rocking-horse phenomenon, which may lead to glenoid loosening.9-13 Surgeons therefore have increasingly turned to nonconforming implants. In the nonconforming design, the radius of curvature of the humeral head is smaller than that of the glenoid. Although this design may reduce edge loading,14 it allows more translation and reduces the relative contact area of the glenohumeral joint. As a result, more contact stress is transmitted to the glenoid component, leading to polyethylene deformation and wear.15,16
Dual radii of curvature are designed to augment joint stability without increasing component wear. Biomechanical data have indicated that edge loading is not increased by having a central conforming region added to a nonconforming model.17 The clinical value of this prosthesis, however, has not been determined. Therefore, we conducted a study to describe the intermediate-term clinical and radiographic outcomes of TSAs that use a novel hybrid glenoid component.
Materials and Methods
This study was approved (protocol AAAD3473) by the Institutional Review Board of Columbia University and was conducted in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations.
Patient Selection
At Columbia University Medical Center, Dr. Bigliani performed 196 TSAs with a hybrid glenoid component (Bigliani-Flatow; Zimmer Biomet) in 169 patients between September 1998 and November 2007. All patients had received a diagnosis of primary glenohumeral arthritis as defined by Neer.18 Patients with previous surgery such as rotator cuff repair or subacromial decompression were included in our review, and patients with a nonprimary form of arthritis, such as rheumatoid, posttraumatic, or post-capsulorrhaphy arthritis, were excluded.
Operative Technique
For all surgeries, Dr. Bigliani performed a subscapularis tenotomy with regional anesthesia and a standard deltopectoral approach. A partial anterior capsulectomy was performed to increase the glenoid’s visibility. The inferior labrum was removed with a needle-tip bovie while the axillary nerve was being protected with a metal finger or narrow Darrach retractor. After reaming and trialing, the final glenoid component was cemented into place. Cement was placed only in the peg or keel holes and pressurized twice before final implantation. Of the 196 glenoid components, 168 (86%) were pegged and 28 (14%) keeled; in addition,190 of these components were all-polyethylene, whereas 6 had trabecular-metal backing. All glenoid components incorporated the hybrid design of dual radii of curvature. After the glenoid was cemented, the final humeral component was placed in 30° of retroversion. Whenever posterior wear was found, retroversion was reduced by 5° to 10°. The humeral prosthesis was cemented in cases (104/196, 53%) of poor bone quality or a large canal.
After surgery, the patient’s sling was fitted with an abduction pillow and a swathe, to be worn the first 24 hours, and the arm was passively ranged. Patients typically were discharged on postoperative day 2. Then, for 2 weeks, they followed an assisted passive range of motion (ROM) protocol, with limited external rotation, for promotion of subscapularis healing.
Clinical Outcomes
Dr. Bigliani assessed preoperative ROM in all planes. During initial evaluation, patients completed a questionnaire that consisted of the 36-Item Short Form Health Survey19,20 (SF-36) and the American Shoulder and Elbow Surgeons21 (ASES) and Simple Shoulder Test22 (SST) surveys. Postoperative clinical data were collected from office follow-up visits, survey questionnaires, or both. Postoperative office data included ROM, subscapularis integrity testing (belly-press or lift-off), and any complications. Patients with <1 year of office follow-up were excluded. In addition, the same survey questionnaire that was used before surgery was mailed to all patients after surgery; then, for anyone who did not respond by mail, we attempted contact by telephone. Neer criteria were based on patients’ subjective assessment of each arm on a 3-point Likert scale (1 = very satisfied, 2 = satisfied, 3 = dissatisfied). Patients were also asked about any specific complications or revision operations since their index procedure.
Physical examination and office follow-up data were obtained for 129 patients (148/196 shoulders, 76% follow-up) at a mean of 3.7 years (range 1.0-10.2 years) after surgery. Surveys were completed by 117 patients (139/196 shoulders, 71% follow-up) at a mean of 5.1 years (range, 1.6-11.2 years) after surgery. Only 15 patients had neither 1 year of office follow-up nor a completed questionnaire. The remaining 154 patients (178/196 shoulders, 91% follow-up) had clinical follow-up with office, mail, or telephone questionnaire at a mean of 4.8 years (range, 1.0-11.2 years) after surgery. This cohort of patients was used to determine rates of surgical revisions, subscapularis tears, dislocations, and other complications.
Radiographic Outcomes
Patients were included in the radiographic analysis if they had a shoulder radiograph at least 1 year after surgery. One hundred nineteen patients (136/196 shoulders, 69% follow-up) had radiographic follow-up at a mean of 3.7 years (range, 1.0-9.4 years) after surgery.
Statistical Analysis
Statistical analysis was performed with Stata Version 10.0. Paired t tests were used to compare preoperative and postoperative numerical data, including ROM and survey scores. We calculated 95% confidence intervals (CIs) and set statistical significance at P < .05. For qualitative measures, the Fisher exact test was used. Survivorship analysis was performed according to the Kaplan-Meier method, with right-censored data for no event or missing data.25
Results
Clinical Analysis of Demographics
In demographics, the clinical and radiographic patient subgroups were similar to each other and to the overall study population (Table 2). Of 196 patients overall, 16 (8%) had a concomitant rotator cuff repair, and 27 (14%) underwent staged bilateral shoulder arthroplasties.
Clinical Analysis of ROM and Survey Scores
Operative shoulder ROM in forward elevation, external rotation at side, external rotation in abduction, and internal rotation all showed statistically significant (P < .001) improvement from before surgery to after surgery. Over 3.7 years, mean (SD) forward elevation improved from 107.3° (34.8°) to 159.0° (29.4°), external rotation at side improved from 20.4° (16.7°) to 49.4° (11.3°), and external rotation in abduction improved from 53.7° (24.3°) to 84.7° (9.1°). Internal rotation improved from a mean (SD) vertebral level of S1 (6.0 levels) to T9 (3.7 levels).
All validated survey scores also showed statistically significant (P < .001) improvement from before surgery to after surgery. Over 5.1 years, mean (SD) SF-36 scores improved from 64.9 (13.4) to 73.6 (17.1), ASES scores improved from 41.1 (22.5) to 82.7 (17.7), SST scores improved from 3.9 (2.8) to 9.7 (2.2), and visual analog scale pain scores improved from 5.6 (3.2) to 1.4 (2.1). Of 139 patients with follow-up, 130 (93.5%) were either satisfied or very satisfied with their TSA, and only 119 (86%) were either satisfied or very satisfied with the nonoperative shoulder.
Clinical Analysis of Postoperative Complications
Of the 178 shoulders evaluated for complications, 3 (1.7%) underwent revision surgery. Mean time to revision was 2.3 years (range, 1.5-3.9 years). Two revisions involved the glenoid component, and the third involved the humerus. In one of the glenoid cases, a 77-year-old woman fell and sustained a fracture at the base of the trabecular metal glenoid pegs; her component was revised to an all-polyethylene component, and she had no further complications. In the other glenoid case, a 73-year-old man’s all-polyethylene component loosened after 2 years and was revised to a trabecular metal implant, which loosened as well and was later converted to a hemiarthroplasty. In the humeral case, a 33-year-old man had his 4-year-old index TSA revised to a cemented stem and had no further complications.
Table 4 compares the clinical and radiographic outcomes of patients who required subscapularis repair, capsular shift, or implant revision with the outcomes of all other study patients, and Figure 3 shows Kaplan-Meier survivorship.
Postoperative Radiographic Analysis
Glenoid Component. At a mean of 3.7 years (minimum, 1 year) after surgery, 86 (63%) of 136 radiographically evaluated shoulders showed no glenoid lucencies; the other 50 (37%) showed ≥1 lucency. Of the 136 shoulders, 33 (24%) had a Lazarus score of 1, 15 (11%) had a score of 2, and only 2 (2%) had a score of 3. None of the shoulders had a score of 4 or 5.
Humeral Component. Of the 136 shoulders, 91 (67%) showed no lucencies in any of the 8 humeral stem zones; the other 45 (33%) showed 1 to 3 lucencies. Thirty (22%) of the 136 shoulders had 1 stem lucency zone, 8 (6%) had 2, and 3 (2%) had 3. None of the shoulders had >3 periprosthetic zones with lucent lines.
Discussion
In this article, we describe a hybrid glenoid TSA component with dual radii of curvature. Its central portion is congruent with the humeral head, and its peripheral portion is noncongruent and larger. The most significant finding of our study is the low rate (1.1%) of glenoid component revision 4.8 years after surgery. This rate is the lowest that has been reported in a study of ≥100 patients. Overall implant survival appeared as an almost flat Kaplan-Meir curve. We attribute this low revision rate to improved biomechanics with the hybrid glenoid design.
Symptomatic glenoid component loosening is the most common TSA complication.1,26-28 In a review of 73 Neer TSAs, Cofield7 found glenoid radiolucencies in 71% of patients 3.8 years after surgery. Radiographic evidence of loosening, defined as component migration, or tilt, or a circumferential lucency 1.5 mm thick, was present in another 11% of patients, and 4.1% developed symptomatic loosening that required glenoid revision. In a study with 12.2-year follow-up, Torchia and colleagues3 found rates of 84% for glenoid radiolucencies, 44% for radiographic loosening, and 5.6% for symptomatic loosening that required revision. In a systematic review of studies with follow-up of ≥10 years, Bohsali and colleagues27 found similar lucency and radiographic loosening rates and a 7% glenoid revision rate. These data suggest glenoid radiolucencies may progress to component loosening.
Degree of joint congruence is a key factor in glenoid loosening. Neer’s congruent design increases the contact area with concentric loading and reduces glenohumeral translation, which leads to reduced polyethylene wear and improved joint stability. In extreme arm positions, however, humeral head subluxation results in edge loading and a glenoid rocking-horse effect.9-13,17,29-31 Conversely, nonconforming implants allow increased glenohumeral translation without edge loading,14 though they also reduce the relative glenohumeral contact area and thus transmit more contact stress to the glenoid.16,17 A hybrid glenoid component with central conforming and peripheral nonconforming zones may reduce the rocking-horse effect while maximizing ROM and joint stability. Wang and colleagues32 studied the biomechanical properties of this glenoid design and found that the addition of a central conforming region did not increase edge loading.
Additional results from our study support the efficacy of a hybrid glenoid component. Patients’ clinical outcomes improved significantly. At 5.1 years after surgery, 93.5% of patients were satisfied or very satisfied with their procedure and reported less satisfaction (86%) with the nonoperative shoulder. Also significant was the reduced number of radiolucencies. At 3.7 years after surgery, the overall percentage of shoulders with ≥1 glenoid radiolucency was 37%, considerably lower than the 82% reported by Cofield7 and the rates in more recent studies.3,16,33-36 Of the 178 shoulders in our study, 10 (5.6%) had subscapularis tears, and 6 (3.4%) of 178 had these tears surgically repaired. This 3.4% compares favorably with the 5.9% (of 119 patients) found by Miller and colleagues37 28 months after surgery. Of our 178 shoulders, 27 (15.2%) had clinically significant postoperative complications; 18 (10.1%) of the 178 had these complications surgically treated, and 9 (5.1%) had them managed nonoperatively. Bohsali and colleagues27 systematically reviewed 33 TSA studies and found a slightly higher complication rate (16.3%) 5.3 years after surgery. Furthermore, in our study, the 11 patients who underwent revision, capsular shift, or subscapularis repair had final outcomes comparable to those of the rest of our study population.
Our study had several potential weaknesses. First, its minimum clinical and radiographic follow-up was 1 year, whereas most long-term TSA series set a minimum of 2 years. We used 1 year because this was the first clinical study of the hybrid glenoid component design, and we wanted to maximize its sample size by reporting on intermediate-length outcomes. Even so, 93% (166/178) of our clinical patients and 83% (113/136) of our radiographic patients have had ≥2 years of follow-up, and we continue to follow all study patients for long-term outcomes. Another weakness of the study was its lack of a uniform group of patients with all the office, survey, complications, and radiographic data. Our retrospective study design made it difficult to obtain such a group without significantly reducing the sample size, so we divided patients into 4 data groups. A third potential weakness was the study’s variable method for collecting complications data. Rates of complications in the 178 shoulders were calculated from either office evaluation or patient self-report by mail or telephone. This data collection method is subject to recall bias, but mail and telephone contact was needed so the study would capture the large number of patients who had traveled to our institution for their surgery or had since moved away. Fourth, belly-press and lift-off tests were used in part to assess subscapularis function, but recent literature suggests post-TSA subscapularis assessment can be unreliable.38 These tests may be positive in up to two-thirds of patients after 2 years.39 Fifth, the generalizability of our findings to diagnoses such as rheumatoid and posttraumatic arthritis is limited. We had to restrict the study to patients with primary glenohumeral arthritis in order to minimize confounders.
This study’s main strength is its description of the clinical and radiographic outcomes of using a single prosthetic system in operations performed by a single surgeon in a large number of patients. This was the first and largest study evaluating the clinical and radiographic outcomes of this hybrid glenoid implant. Excluding patients with nonprimary arthritis allowed us to minimize potential confounding factors that affect patient outcomes. In conclusion, our study results showed the favorable clinical and radiographic outcomes of TSAs that have a hybrid glenoid component with dual radii of curvature. At a mean of 3.7 years after surgery, 63% of patients had no glenoid lucencies, and, at a mean of 4.8 years, only 1.7% of patients required revision. We continue to follow these patients to obtain long-term results of this innovative prosthesis.
Take-Home Points
- The authors have developed a total shoulder glenoid prosthesis that conforms with the humeral head in its center and is nonconforming on its peripheral edge.
- All clinical survey and range of motion parameters demonstrated statistically significant improvements at final follow-up.
- Only 3 shoulders (1.7%) required revision surgery.
- Eighty-six (63%) of 136 shoulders demonstrated no radiographic evidence of glenoid loosening.
- This is the first and largest study that evaluates the clinical and radiographic outcomes of this hybrid shoulder prosthesis.
Fixation of the glenoid component is the limiting factor in modern total shoulder arthroplasty (TSA). Glenoid loosening, the most common long-term complication, necessitates revision in up to 12% of patients.1-4 By contrast, humeral component loosening is relatively uncommon, affecting as few as 0.34% of patients.5 Multiple long-term studies have found consistently high rates (45%-93%) of radiolucencies around the glenoid component.3,6,7 Although their clinical significance has been debated, radiolucencies around the glenoid component raise concern about progressive loss of fixation.
Since TSA was introduced in the 1970s, complications with the glenoid component have been addressed with 2 different designs: conforming (congruent) and nonconforming. In a congruent articulation, the radii of curvature of the glenoid and humeral head components are identical, whereas they differ in a nonconforming model. Joint conformity is inversely related to glenohumeral translation.8 Neer’s original TSA was made congruent in order to limit translation and maximize the contact area. However, this design results in edge loading and a so-called rocking-horse phenomenon, which may lead to glenoid loosening.9-13 Surgeons therefore have increasingly turned to nonconforming implants. In the nonconforming design, the radius of curvature of the humeral head is smaller than that of the glenoid. Although this design may reduce edge loading,14 it allows more translation and reduces the relative contact area of the glenohumeral joint. As a result, more contact stress is transmitted to the glenoid component, leading to polyethylene deformation and wear.15,16
Dual radii of curvature are designed to augment joint stability without increasing component wear. Biomechanical data have indicated that edge loading is not increased by having a central conforming region added to a nonconforming model.17 The clinical value of this prosthesis, however, has not been determined. Therefore, we conducted a study to describe the intermediate-term clinical and radiographic outcomes of TSAs that use a novel hybrid glenoid component.
Materials and Methods
This study was approved (protocol AAAD3473) by the Institutional Review Board of Columbia University and was conducted in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations.
Patient Selection
At Columbia University Medical Center, Dr. Bigliani performed 196 TSAs with a hybrid glenoid component (Bigliani-Flatow; Zimmer Biomet) in 169 patients between September 1998 and November 2007. All patients had received a diagnosis of primary glenohumeral arthritis as defined by Neer.18 Patients with previous surgery such as rotator cuff repair or subacromial decompression were included in our review, and patients with a nonprimary form of arthritis, such as rheumatoid, posttraumatic, or post-capsulorrhaphy arthritis, were excluded.
Operative Technique
For all surgeries, Dr. Bigliani performed a subscapularis tenotomy with regional anesthesia and a standard deltopectoral approach. A partial anterior capsulectomy was performed to increase the glenoid’s visibility. The inferior labrum was removed with a needle-tip bovie while the axillary nerve was being protected with a metal finger or narrow Darrach retractor. After reaming and trialing, the final glenoid component was cemented into place. Cement was placed only in the peg or keel holes and pressurized twice before final implantation. Of the 196 glenoid components, 168 (86%) were pegged and 28 (14%) keeled; in addition,190 of these components were all-polyethylene, whereas 6 had trabecular-metal backing. All glenoid components incorporated the hybrid design of dual radii of curvature. After the glenoid was cemented, the final humeral component was placed in 30° of retroversion. Whenever posterior wear was found, retroversion was reduced by 5° to 10°. The humeral prosthesis was cemented in cases (104/196, 53%) of poor bone quality or a large canal.
After surgery, the patient’s sling was fitted with an abduction pillow and a swathe, to be worn the first 24 hours, and the arm was passively ranged. Patients typically were discharged on postoperative day 2. Then, for 2 weeks, they followed an assisted passive range of motion (ROM) protocol, with limited external rotation, for promotion of subscapularis healing.
Clinical Outcomes
Dr. Bigliani assessed preoperative ROM in all planes. During initial evaluation, patients completed a questionnaire that consisted of the 36-Item Short Form Health Survey19,20 (SF-36) and the American Shoulder and Elbow Surgeons21 (ASES) and Simple Shoulder Test22 (SST) surveys. Postoperative clinical data were collected from office follow-up visits, survey questionnaires, or both. Postoperative office data included ROM, subscapularis integrity testing (belly-press or lift-off), and any complications. Patients with <1 year of office follow-up were excluded. In addition, the same survey questionnaire that was used before surgery was mailed to all patients after surgery; then, for anyone who did not respond by mail, we attempted contact by telephone. Neer criteria were based on patients’ subjective assessment of each arm on a 3-point Likert scale (1 = very satisfied, 2 = satisfied, 3 = dissatisfied). Patients were also asked about any specific complications or revision operations since their index procedure.
Physical examination and office follow-up data were obtained for 129 patients (148/196 shoulders, 76% follow-up) at a mean of 3.7 years (range 1.0-10.2 years) after surgery. Surveys were completed by 117 patients (139/196 shoulders, 71% follow-up) at a mean of 5.1 years (range, 1.6-11.2 years) after surgery. Only 15 patients had neither 1 year of office follow-up nor a completed questionnaire. The remaining 154 patients (178/196 shoulders, 91% follow-up) had clinical follow-up with office, mail, or telephone questionnaire at a mean of 4.8 years (range, 1.0-11.2 years) after surgery. This cohort of patients was used to determine rates of surgical revisions, subscapularis tears, dislocations, and other complications.
Radiographic Outcomes
Patients were included in the radiographic analysis if they had a shoulder radiograph at least 1 year after surgery. One hundred nineteen patients (136/196 shoulders, 69% follow-up) had radiographic follow-up at a mean of 3.7 years (range, 1.0-9.4 years) after surgery.
Statistical Analysis
Statistical analysis was performed with Stata Version 10.0. Paired t tests were used to compare preoperative and postoperative numerical data, including ROM and survey scores. We calculated 95% confidence intervals (CIs) and set statistical significance at P < .05. For qualitative measures, the Fisher exact test was used. Survivorship analysis was performed according to the Kaplan-Meier method, with right-censored data for no event or missing data.25
Results
Clinical Analysis of Demographics
In demographics, the clinical and radiographic patient subgroups were similar to each other and to the overall study population (Table 2). Of 196 patients overall, 16 (8%) had a concomitant rotator cuff repair, and 27 (14%) underwent staged bilateral shoulder arthroplasties.
Clinical Analysis of ROM and Survey Scores
Operative shoulder ROM in forward elevation, external rotation at side, external rotation in abduction, and internal rotation all showed statistically significant (P < .001) improvement from before surgery to after surgery. Over 3.7 years, mean (SD) forward elevation improved from 107.3° (34.8°) to 159.0° (29.4°), external rotation at side improved from 20.4° (16.7°) to 49.4° (11.3°), and external rotation in abduction improved from 53.7° (24.3°) to 84.7° (9.1°). Internal rotation improved from a mean (SD) vertebral level of S1 (6.0 levels) to T9 (3.7 levels).
All validated survey scores also showed statistically significant (P < .001) improvement from before surgery to after surgery. Over 5.1 years, mean (SD) SF-36 scores improved from 64.9 (13.4) to 73.6 (17.1), ASES scores improved from 41.1 (22.5) to 82.7 (17.7), SST scores improved from 3.9 (2.8) to 9.7 (2.2), and visual analog scale pain scores improved from 5.6 (3.2) to 1.4 (2.1). Of 139 patients with follow-up, 130 (93.5%) were either satisfied or very satisfied with their TSA, and only 119 (86%) were either satisfied or very satisfied with the nonoperative shoulder.
Clinical Analysis of Postoperative Complications
Of the 178 shoulders evaluated for complications, 3 (1.7%) underwent revision surgery. Mean time to revision was 2.3 years (range, 1.5-3.9 years). Two revisions involved the glenoid component, and the third involved the humerus. In one of the glenoid cases, a 77-year-old woman fell and sustained a fracture at the base of the trabecular metal glenoid pegs; her component was revised to an all-polyethylene component, and she had no further complications. In the other glenoid case, a 73-year-old man’s all-polyethylene component loosened after 2 years and was revised to a trabecular metal implant, which loosened as well and was later converted to a hemiarthroplasty. In the humeral case, a 33-year-old man had his 4-year-old index TSA revised to a cemented stem and had no further complications.
Table 4 compares the clinical and radiographic outcomes of patients who required subscapularis repair, capsular shift, or implant revision with the outcomes of all other study patients, and Figure 3 shows Kaplan-Meier survivorship.
Postoperative Radiographic Analysis
Glenoid Component. At a mean of 3.7 years (minimum, 1 year) after surgery, 86 (63%) of 136 radiographically evaluated shoulders showed no glenoid lucencies; the other 50 (37%) showed ≥1 lucency. Of the 136 shoulders, 33 (24%) had a Lazarus score of 1, 15 (11%) had a score of 2, and only 2 (2%) had a score of 3. None of the shoulders had a score of 4 or 5.
Humeral Component. Of the 136 shoulders, 91 (67%) showed no lucencies in any of the 8 humeral stem zones; the other 45 (33%) showed 1 to 3 lucencies. Thirty (22%) of the 136 shoulders had 1 stem lucency zone, 8 (6%) had 2, and 3 (2%) had 3. None of the shoulders had >3 periprosthetic zones with lucent lines.
Discussion
In this article, we describe a hybrid glenoid TSA component with dual radii of curvature. Its central portion is congruent with the humeral head, and its peripheral portion is noncongruent and larger. The most significant finding of our study is the low rate (1.1%) of glenoid component revision 4.8 years after surgery. This rate is the lowest that has been reported in a study of ≥100 patients. Overall implant survival appeared as an almost flat Kaplan-Meir curve. We attribute this low revision rate to improved biomechanics with the hybrid glenoid design.
Symptomatic glenoid component loosening is the most common TSA complication.1,26-28 In a review of 73 Neer TSAs, Cofield7 found glenoid radiolucencies in 71% of patients 3.8 years after surgery. Radiographic evidence of loosening, defined as component migration, or tilt, or a circumferential lucency 1.5 mm thick, was present in another 11% of patients, and 4.1% developed symptomatic loosening that required glenoid revision. In a study with 12.2-year follow-up, Torchia and colleagues3 found rates of 84% for glenoid radiolucencies, 44% for radiographic loosening, and 5.6% for symptomatic loosening that required revision. In a systematic review of studies with follow-up of ≥10 years, Bohsali and colleagues27 found similar lucency and radiographic loosening rates and a 7% glenoid revision rate. These data suggest glenoid radiolucencies may progress to component loosening.
Degree of joint congruence is a key factor in glenoid loosening. Neer’s congruent design increases the contact area with concentric loading and reduces glenohumeral translation, which leads to reduced polyethylene wear and improved joint stability. In extreme arm positions, however, humeral head subluxation results in edge loading and a glenoid rocking-horse effect.9-13,17,29-31 Conversely, nonconforming implants allow increased glenohumeral translation without edge loading,14 though they also reduce the relative glenohumeral contact area and thus transmit more contact stress to the glenoid.16,17 A hybrid glenoid component with central conforming and peripheral nonconforming zones may reduce the rocking-horse effect while maximizing ROM and joint stability. Wang and colleagues32 studied the biomechanical properties of this glenoid design and found that the addition of a central conforming region did not increase edge loading.
Additional results from our study support the efficacy of a hybrid glenoid component. Patients’ clinical outcomes improved significantly. At 5.1 years after surgery, 93.5% of patients were satisfied or very satisfied with their procedure and reported less satisfaction (86%) with the nonoperative shoulder. Also significant was the reduced number of radiolucencies. At 3.7 years after surgery, the overall percentage of shoulders with ≥1 glenoid radiolucency was 37%, considerably lower than the 82% reported by Cofield7 and the rates in more recent studies.3,16,33-36 Of the 178 shoulders in our study, 10 (5.6%) had subscapularis tears, and 6 (3.4%) of 178 had these tears surgically repaired. This 3.4% compares favorably with the 5.9% (of 119 patients) found by Miller and colleagues37 28 months after surgery. Of our 178 shoulders, 27 (15.2%) had clinically significant postoperative complications; 18 (10.1%) of the 178 had these complications surgically treated, and 9 (5.1%) had them managed nonoperatively. Bohsali and colleagues27 systematically reviewed 33 TSA studies and found a slightly higher complication rate (16.3%) 5.3 years after surgery. Furthermore, in our study, the 11 patients who underwent revision, capsular shift, or subscapularis repair had final outcomes comparable to those of the rest of our study population.
Our study had several potential weaknesses. First, its minimum clinical and radiographic follow-up was 1 year, whereas most long-term TSA series set a minimum of 2 years. We used 1 year because this was the first clinical study of the hybrid glenoid component design, and we wanted to maximize its sample size by reporting on intermediate-length outcomes. Even so, 93% (166/178) of our clinical patients and 83% (113/136) of our radiographic patients have had ≥2 years of follow-up, and we continue to follow all study patients for long-term outcomes. Another weakness of the study was its lack of a uniform group of patients with all the office, survey, complications, and radiographic data. Our retrospective study design made it difficult to obtain such a group without significantly reducing the sample size, so we divided patients into 4 data groups. A third potential weakness was the study’s variable method for collecting complications data. Rates of complications in the 178 shoulders were calculated from either office evaluation or patient self-report by mail or telephone. This data collection method is subject to recall bias, but mail and telephone contact was needed so the study would capture the large number of patients who had traveled to our institution for their surgery or had since moved away. Fourth, belly-press and lift-off tests were used in part to assess subscapularis function, but recent literature suggests post-TSA subscapularis assessment can be unreliable.38 These tests may be positive in up to two-thirds of patients after 2 years.39 Fifth, the generalizability of our findings to diagnoses such as rheumatoid and posttraumatic arthritis is limited. We had to restrict the study to patients with primary glenohumeral arthritis in order to minimize confounders.
This study’s main strength is its description of the clinical and radiographic outcomes of using a single prosthetic system in operations performed by a single surgeon in a large number of patients. This was the first and largest study evaluating the clinical and radiographic outcomes of this hybrid glenoid implant. Excluding patients with nonprimary arthritis allowed us to minimize potential confounding factors that affect patient outcomes. In conclusion, our study results showed the favorable clinical and radiographic outcomes of TSAs that have a hybrid glenoid component with dual radii of curvature. At a mean of 3.7 years after surgery, 63% of patients had no glenoid lucencies, and, at a mean of 4.8 years, only 1.7% of patients required revision. We continue to follow these patients to obtain long-term results of this innovative prosthesis.
1. Rodosky MW, Bigliani LU. Indications for glenoid resurfacing in shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5(3):231-248.
2. Boyd AD Jr, Thomas WH, Scott RD, Sledge CB, Thornhill TS. Total shoulder arthroplasty versus hemiarthroplasty. Indications for glenoid resurfacing. J Arthroplasty. 1990;5(4):329-336.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85(2):251-258.
5. Cofield RH. Degenerative and arthritic problems of the glenohumeral joint. In: Rockwood CA, Matsen FA, eds. The Shoulder. Philadelphia, PA: Saunders; 1990:740-745.
6. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
7. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
8. Karduna AR, Williams GR, Williams JL, Iannotti JP. Kinematics of the glenohumeral joint: influences of muscle forces, ligamentous constraints, and articular geometry. J Orthop Res. 1996;14(6):986-993.
9. Karduna AR, Williams GR, Iannotti JP, Williams JL. Total shoulder arthroplasty biomechanics: a study of the forces and strains at the glenoid component. J Biomech Eng. 1998;120(1):92-99.
10. Karduna AR, Williams GR, Williams JL, Iannotti JP. Glenohumeral joint translations before and after total shoulder arthroplasty. A study in cadavera. J Bone Joint Surg Am. 1997;79(8):1166-1174.
11. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
12. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
13. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
14. Harryman DT, Sidles JA, Harris SL, Lippitt SB, Matsen FA 3rd. The effect of articular conformity and the size of the humeral head component on laxity and motion after glenohumeral arthroplasty. A study in cadavera. J Bone Joint Surg Am. 1995;77(4):555-563.
15. Flatow EL. Prosthetic design considerations in total shoulder arthroplasty. Semin Arthroplasty. 1995;6(4):233-244.
16. Klimkiewicz JJ, Iannotti JP, Rubash HE, Shanbhag AS. Aseptic loosening of the humeral component in total shoulder arthroplasty. J Shoulder Elbow Surg. 1998;7(4):422-426.
17. Wang VM, Krishnan R, Ugwonali OF, Flatow EL, Bigliani LU, Ateshian GA. Biomechanical evaluation of a novel glenoid design in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 suppl S):129S-140S.
18. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
19. Boorman RS, Kopjar B, Fehringer E, Churchill RS, Smith K, Matsen FA 3rd. The effect of total shoulder arthroplasty on self-assessed health status is comparable to that of total hip arthroplasty and coronary artery bypass grafting. J Shoulder Elbow Surg. 2003;12(2):158-163.
20. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
21. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
22. Wright RW, Baumgarten KM. Shoulder outcomes measures. J Am Acad Orthop Surg. 2010;18(7):436-444.
23. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
24. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
25. Dinse GE, Lagakos SW. Nonparametric estimation of lifetime and disease onset distributions from incomplete observations. Biometrics. 1982;38(4):921-932.
26. Baumgarten KM, Lashgari CJ, Yamaguchi K. Glenoid resurfacing in shoulder arthroplasty: indications and contraindications. Instr Course Lect. 2004;53:3-11.
27. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
28. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
29. Poppen NK, Walker PS. Normal and abnormal motion of the shoulder. J Bone Joint Surg Am. 1976;58(2):195-201.
30. Cotton RE, Rideout DF. Tears of the humeral rotator cuff; a radiological and pathological necropsy survey. J Bone Joint Surg Br. 1964;46:314-328.
31. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
32. Wang VM, Sugalski MT, Levine WN, Pawluk RJ, Mow VC, Bigliani LU. Comparison of glenohumeral mechanics following a capsular shift and anterior tightening. J Bone Joint Surg Am. 2005;87(6):1312-1322.
33. Young A, Walch G, Boileau P, et al. A multicentre study of the long-term results of using a flat-back polyethylene glenoid component in shoulder replacement for primary osteoarthritis. J Bone Joint Surg Br. 2011;93(2):210-216.
34. Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600.
35. Walch G, Edwards TB, Boulahia A, Boileau P, Mole D, Adeleine P. The influence of glenohumeral prosthetic mismatch on glenoid radiolucent lines: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2186-2191.
36. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
37. Miller BS, Joseph TA, Noonan TJ, Horan MP, Hawkins RJ. Rupture of the subscapularis tendon after shoulder arthroplasty: diagnosis, treatment, and outcome. J Shoulder Elbow Surg. 2005;14(5):492-496.
38. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
39. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
1. Rodosky MW, Bigliani LU. Indications for glenoid resurfacing in shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5(3):231-248.
2. Boyd AD Jr, Thomas WH, Scott RD, Sledge CB, Thornhill TS. Total shoulder arthroplasty versus hemiarthroplasty. Indications for glenoid resurfacing. J Arthroplasty. 1990;5(4):329-336.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85(2):251-258.
5. Cofield RH. Degenerative and arthritic problems of the glenohumeral joint. In: Rockwood CA, Matsen FA, eds. The Shoulder. Philadelphia, PA: Saunders; 1990:740-745.
6. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
7. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
8. Karduna AR, Williams GR, Williams JL, Iannotti JP. Kinematics of the glenohumeral joint: influences of muscle forces, ligamentous constraints, and articular geometry. J Orthop Res. 1996;14(6):986-993.
9. Karduna AR, Williams GR, Iannotti JP, Williams JL. Total shoulder arthroplasty biomechanics: a study of the forces and strains at the glenoid component. J Biomech Eng. 1998;120(1):92-99.
10. Karduna AR, Williams GR, Williams JL, Iannotti JP. Glenohumeral joint translations before and after total shoulder arthroplasty. A study in cadavera. J Bone Joint Surg Am. 1997;79(8):1166-1174.
11. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
12. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
13. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
14. Harryman DT, Sidles JA, Harris SL, Lippitt SB, Matsen FA 3rd. The effect of articular conformity and the size of the humeral head component on laxity and motion after glenohumeral arthroplasty. A study in cadavera. J Bone Joint Surg Am. 1995;77(4):555-563.
15. Flatow EL. Prosthetic design considerations in total shoulder arthroplasty. Semin Arthroplasty. 1995;6(4):233-244.
16. Klimkiewicz JJ, Iannotti JP, Rubash HE, Shanbhag AS. Aseptic loosening of the humeral component in total shoulder arthroplasty. J Shoulder Elbow Surg. 1998;7(4):422-426.
17. Wang VM, Krishnan R, Ugwonali OF, Flatow EL, Bigliani LU, Ateshian GA. Biomechanical evaluation of a novel glenoid design in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 suppl S):129S-140S.
18. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
19. Boorman RS, Kopjar B, Fehringer E, Churchill RS, Smith K, Matsen FA 3rd. The effect of total shoulder arthroplasty on self-assessed health status is comparable to that of total hip arthroplasty and coronary artery bypass grafting. J Shoulder Elbow Surg. 2003;12(2):158-163.
20. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
21. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
22. Wright RW, Baumgarten KM. Shoulder outcomes measures. J Am Acad Orthop Surg. 2010;18(7):436-444.
23. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
24. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
25. Dinse GE, Lagakos SW. Nonparametric estimation of lifetime and disease onset distributions from incomplete observations. Biometrics. 1982;38(4):921-932.
26. Baumgarten KM, Lashgari CJ, Yamaguchi K. Glenoid resurfacing in shoulder arthroplasty: indications and contraindications. Instr Course Lect. 2004;53:3-11.
27. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
28. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
29. Poppen NK, Walker PS. Normal and abnormal motion of the shoulder. J Bone Joint Surg Am. 1976;58(2):195-201.
30. Cotton RE, Rideout DF. Tears of the humeral rotator cuff; a radiological and pathological necropsy survey. J Bone Joint Surg Br. 1964;46:314-328.
31. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
32. Wang VM, Sugalski MT, Levine WN, Pawluk RJ, Mow VC, Bigliani LU. Comparison of glenohumeral mechanics following a capsular shift and anterior tightening. J Bone Joint Surg Am. 2005;87(6):1312-1322.
33. Young A, Walch G, Boileau P, et al. A multicentre study of the long-term results of using a flat-back polyethylene glenoid component in shoulder replacement for primary osteoarthritis. J Bone Joint Surg Br. 2011;93(2):210-216.
34. Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600.
35. Walch G, Edwards TB, Boulahia A, Boileau P, Mole D, Adeleine P. The influence of glenohumeral prosthetic mismatch on glenoid radiolucent lines: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2186-2191.
36. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
37. Miller BS, Joseph TA, Noonan TJ, Horan MP, Hawkins RJ. Rupture of the subscapularis tendon after shoulder arthroplasty: diagnosis, treatment, and outcome. J Shoulder Elbow Surg. 2005;14(5):492-496.
38. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
39. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
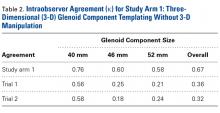
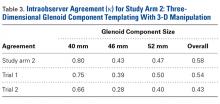
Reliability of 3-Dimensional Glenoid Component Templating and Correlation to Intraoperative Component Selection
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
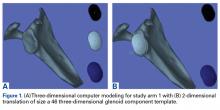
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
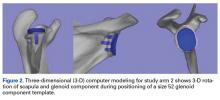
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.