News
What Is the Clinical Course of Low Back Pain?
- Author:
- Louise Gagnon
Patients with back pain lasting longer than 12 weeks showed less chance of pain reduction.
News

Prehospital COVID therapy effective in rheumatic disease patients
- Author:
- Louise Gagnon
Patients with rheumatic disease and SARS-CoV-2 infection who received outpatient treatment fared better than similar patients who did not receive...
News
Mothers with disabilities less likely to start breastfeeding
- Author:
- Louise Gagnon
The findings suggest the possible need for closer follow-up of a new mother who is breastfeeding and who has an intellectual disability or...
News
Uncombable hair syndrome: One gene, variants responsible for many cases
- Author:
- Louise Gagnon
A deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and...
News
Cannabis industry cribs Big Tobacco’s social responsibility initiatives
- Author:
- Louise Gagnon
“While [corporate social responsibility] activities may have some potential benefits or apparent legitimacy, we have to remember that CSR is also...
News
Poor physician access linked with unplanned return ED visits
- Author:
- Louise Gagnon
Difficulty in accessing primary care was associated with a higher rate of return visits to the ED.
News

Black Americans’ high gout rate stems from social causes
- Author:
- Louise Gagnon
Primary care providers need to address disparities through more comprehensive care and referrals to rheumatologists.
News

Zoster vaccination does not appear to increase flare risk in patients with immune-mediated inflammatory disease
- Author:
- Louise Gagnon
For the study, investigators used medical claims from IBM MarketScan in patients aged 50-64 years, and data from the Centers for Medicare and...
News

Updated pediatric uveitis recommendations advise on expanded treatment options
- Author:
- Louise Gagnon
The recommendations represent an update of 2018 consensus-based recommendations from the SHARE Initiative and the 2019 American College of...
News
Study provides new analysis of isotretinoin and risk for adverse neuropsychiatric outcomes
- Author:
- Louise Gagnon
Although severe neuropsychiatric effects associated with isotretinoin therapy in patients with acne have been reported, “the evidence base ... is...
News

Clinical chest images power up survival prediction in lung cancer
- Author:
- Louise Gagnon
“The results of this study demonstrate promising results supportive of the notion that combining clinical and imaging data points can help build a...
News

Long-term efficacy, safety data for ixekizumab in pediatric psoriasis reported
- Author:
- Louise Gagnon
No new safety findings during weeks 48 to 108 of the trial were reported, including no new cases of inflammatory bowel disease or Candida...
News
Can gram stains guide antibiotics for pneumonia in critical care?
- Author:
- Louise Gagnon
Patients with ventilator-associated pneumonia (VAP) were assessed.
News
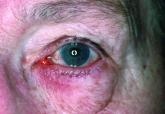
Dryness, conjunctival telangiectasia among ocular symptoms common in rosacea
- Author:
- Louise Gagnon
"The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways."
News
International panel backs energy-based devices as first-line treatment of acne scars
- Author:
- Louise Gagnon
A panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the...
