User login
Clinical chest images power up survival prediction in lung cancer
In patients with stage I lung cancer, adding noncancerous features from CT chest imaging predicts overall survival better than clinical characteristics alone, according to a paper published online in the American Journal of Roentgenology.
Modeling that incorporates noncancerous imaging features captured on chest computed tomography (CT) along with clinical features, when calculated before stereotactic body radiation therapy (SBRT) is administered, improves survival prediction, compared with modeling that relies only on clinical features, the authors report.
“The focus of the study was to look at the environment in which the cancer lives,” said senior author Florian J. Fintelmann, MD, radiologist at Massachusetts General Hospital and associate professor of radiology at Harvard Medical School, both in Boston. “This is looking at parameters like the aortic diameter, body composition – that is, the quantification and characterization of adipose tissue and muscle – coronary artery calcifications, and emphysema quantification.”
CT images are used by radiation oncologists to determine where the radiation should be delivered. “There is more information from these images that we can utilize,” he said.
Survival estimates in patients with state I lung cancer now rely on biological age, ECOG (Eastern Cooperative Oncology Group) score, and the presence of comorbidities, Dr. Fintelmann said.
This retrospective investigation involved 282 patients with a median age of 75 years. There were 168 women and 114 men. All patients had stage I lung cancer and were treated with SBRT between January 2009 and June 2017.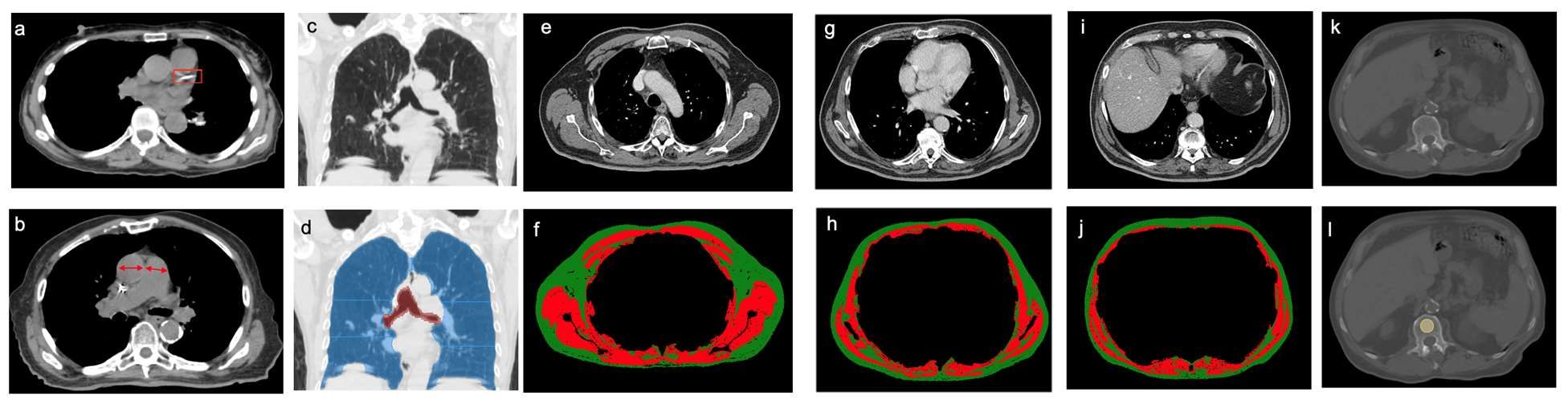
Investigators analyzed pre-treatment chest images with CT. They assessed coronary artery calcium (CAC) score (see above image), pulmonary artery (PA)-to-aorta ratio, emphysema, and several measures of body composition (skeletal muscle and adipose tissue). They developed a statistical model to link clinical and imaging features with overall survival.
An elevated CAC score (11-399: HR, 1.83 [95% confidence interval, 1.15-2.91]; ≥ 400: HR, 1.63 [95% CI, 1.01-2.63]), increased PA-to-aorta ratio (HR, 1.33 [95% CI, 1.16-1.52], per 0.1-unit increase) and decreased thoracic skeletal muscle (HR, 0.88 [95% CI, 0.79-0.98], per 10 cm2/m2 increase) were independently associated with shorter overall survival, investigators observed.
In addition, 5-year overall survival was superior for the model that included clinical and imaging features and inferior for the model restricted to only clinical features. Of all features, the one that emerged the most predictive of overall survival was PA-to-aorta ratio.
In this single-center study of stage I lung cancer patients who were undergoing SBRT, increased CAC score, increased PA-to-aorta ratio, and decreased thoracic skeletal muscle index were independently predictive of poorer overall survival.
“Our modeling shows that these imaging features add so much more [to predicting overall survival],” Dr. Fintelmann said. “The strength of this study is that we show the utility [of the model] and how it exceeds the clinical risk prediction that is currently standard of care. We think this will benefit patients in terms of being able to counsel them and better advise them on their medical decisions.”
This proof-of-concept investigation requires external validation, Dr. Fintelmann stressed. “External data for validation is the next step,” he said, noting he and co-investigators welcome data input from other investigators.
Elsie Nguyen, MD, FRCPC, FNASCI, associate professor of radiology, University of Toronto, responded by email that the study shows that imaging features supplement clinical data in predicting overall survival.
“This study demonstrates the value of extracting non–cancer related computed tomography imaging features to build a model that can better predict overall survival as compared to clinical parameters alone (such as age, performance status and co-morbidities) for stage I lung cancer patients treated with SBRT,” Dr. Nguyen wrote.
“Coronary artery calcium score, pulmonary artery-to-aorta ratio, and sarcopenia independently predicted overall survival,” she wrote. “These results are not surprising, as the prognostic value of each of these imaging features has already been established in the literature.”
Dr. Nguyen pointed out the power in the sum of these imaging features to predict overall survival.
“However, the results of this study demonstrate promising results supportive of the notion that combining clinical and imaging data points can help build a more accurate prediction model for overall survival,” she wrote. “This is analogous to the Brock University (in St. Catharines, Ontario) calculator for solitary pulmonary nodules that calculates malignancy risk based on both clinical and imaging data points. However, external validation of these study results at other centers is first required.”
Dr. Fintelmann and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with stage I lung cancer, adding noncancerous features from CT chest imaging predicts overall survival better than clinical characteristics alone, according to a paper published online in the American Journal of Roentgenology.
Modeling that incorporates noncancerous imaging features captured on chest computed tomography (CT) along with clinical features, when calculated before stereotactic body radiation therapy (SBRT) is administered, improves survival prediction, compared with modeling that relies only on clinical features, the authors report.
“The focus of the study was to look at the environment in which the cancer lives,” said senior author Florian J. Fintelmann, MD, radiologist at Massachusetts General Hospital and associate professor of radiology at Harvard Medical School, both in Boston. “This is looking at parameters like the aortic diameter, body composition – that is, the quantification and characterization of adipose tissue and muscle – coronary artery calcifications, and emphysema quantification.”
CT images are used by radiation oncologists to determine where the radiation should be delivered. “There is more information from these images that we can utilize,” he said.
Survival estimates in patients with state I lung cancer now rely on biological age, ECOG (Eastern Cooperative Oncology Group) score, and the presence of comorbidities, Dr. Fintelmann said.
This retrospective investigation involved 282 patients with a median age of 75 years. There were 168 women and 114 men. All patients had stage I lung cancer and were treated with SBRT between January 2009 and June 2017.
Investigators analyzed pre-treatment chest images with CT. They assessed coronary artery calcium (CAC) score (see above image), pulmonary artery (PA)-to-aorta ratio, emphysema, and several measures of body composition (skeletal muscle and adipose tissue). They developed a statistical model to link clinical and imaging features with overall survival.
An elevated CAC score (11-399: HR, 1.83 [95% confidence interval, 1.15-2.91]; ≥ 400: HR, 1.63 [95% CI, 1.01-2.63]), increased PA-to-aorta ratio (HR, 1.33 [95% CI, 1.16-1.52], per 0.1-unit increase) and decreased thoracic skeletal muscle (HR, 0.88 [95% CI, 0.79-0.98], per 10 cm2/m2 increase) were independently associated with shorter overall survival, investigators observed.
In addition, 5-year overall survival was superior for the model that included clinical and imaging features and inferior for the model restricted to only clinical features. Of all features, the one that emerged the most predictive of overall survival was PA-to-aorta ratio.
In this single-center study of stage I lung cancer patients who were undergoing SBRT, increased CAC score, increased PA-to-aorta ratio, and decreased thoracic skeletal muscle index were independently predictive of poorer overall survival.
“Our modeling shows that these imaging features add so much more [to predicting overall survival],” Dr. Fintelmann said. “The strength of this study is that we show the utility [of the model] and how it exceeds the clinical risk prediction that is currently standard of care. We think this will benefit patients in terms of being able to counsel them and better advise them on their medical decisions.”
This proof-of-concept investigation requires external validation, Dr. Fintelmann stressed. “External data for validation is the next step,” he said, noting he and co-investigators welcome data input from other investigators.
Elsie Nguyen, MD, FRCPC, FNASCI, associate professor of radiology, University of Toronto, responded by email that the study shows that imaging features supplement clinical data in predicting overall survival.
“This study demonstrates the value of extracting non–cancer related computed tomography imaging features to build a model that can better predict overall survival as compared to clinical parameters alone (such as age, performance status and co-morbidities) for stage I lung cancer patients treated with SBRT,” Dr. Nguyen wrote.
“Coronary artery calcium score, pulmonary artery-to-aorta ratio, and sarcopenia independently predicted overall survival,” she wrote. “These results are not surprising, as the prognostic value of each of these imaging features has already been established in the literature.”
Dr. Nguyen pointed out the power in the sum of these imaging features to predict overall survival.
“However, the results of this study demonstrate promising results supportive of the notion that combining clinical and imaging data points can help build a more accurate prediction model for overall survival,” she wrote. “This is analogous to the Brock University (in St. Catharines, Ontario) calculator for solitary pulmonary nodules that calculates malignancy risk based on both clinical and imaging data points. However, external validation of these study results at other centers is first required.”
Dr. Fintelmann and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with stage I lung cancer, adding noncancerous features from CT chest imaging predicts overall survival better than clinical characteristics alone, according to a paper published online in the American Journal of Roentgenology.
Modeling that incorporates noncancerous imaging features captured on chest computed tomography (CT) along with clinical features, when calculated before stereotactic body radiation therapy (SBRT) is administered, improves survival prediction, compared with modeling that relies only on clinical features, the authors report.
“The focus of the study was to look at the environment in which the cancer lives,” said senior author Florian J. Fintelmann, MD, radiologist at Massachusetts General Hospital and associate professor of radiology at Harvard Medical School, both in Boston. “This is looking at parameters like the aortic diameter, body composition – that is, the quantification and characterization of adipose tissue and muscle – coronary artery calcifications, and emphysema quantification.”
CT images are used by radiation oncologists to determine where the radiation should be delivered. “There is more information from these images that we can utilize,” he said.
Survival estimates in patients with state I lung cancer now rely on biological age, ECOG (Eastern Cooperative Oncology Group) score, and the presence of comorbidities, Dr. Fintelmann said.
This retrospective investigation involved 282 patients with a median age of 75 years. There were 168 women and 114 men. All patients had stage I lung cancer and were treated with SBRT between January 2009 and June 2017.
Investigators analyzed pre-treatment chest images with CT. They assessed coronary artery calcium (CAC) score (see above image), pulmonary artery (PA)-to-aorta ratio, emphysema, and several measures of body composition (skeletal muscle and adipose tissue). They developed a statistical model to link clinical and imaging features with overall survival.
An elevated CAC score (11-399: HR, 1.83 [95% confidence interval, 1.15-2.91]; ≥ 400: HR, 1.63 [95% CI, 1.01-2.63]), increased PA-to-aorta ratio (HR, 1.33 [95% CI, 1.16-1.52], per 0.1-unit increase) and decreased thoracic skeletal muscle (HR, 0.88 [95% CI, 0.79-0.98], per 10 cm2/m2 increase) were independently associated with shorter overall survival, investigators observed.
In addition, 5-year overall survival was superior for the model that included clinical and imaging features and inferior for the model restricted to only clinical features. Of all features, the one that emerged the most predictive of overall survival was PA-to-aorta ratio.
In this single-center study of stage I lung cancer patients who were undergoing SBRT, increased CAC score, increased PA-to-aorta ratio, and decreased thoracic skeletal muscle index were independently predictive of poorer overall survival.
“Our modeling shows that these imaging features add so much more [to predicting overall survival],” Dr. Fintelmann said. “The strength of this study is that we show the utility [of the model] and how it exceeds the clinical risk prediction that is currently standard of care. We think this will benefit patients in terms of being able to counsel them and better advise them on their medical decisions.”
This proof-of-concept investigation requires external validation, Dr. Fintelmann stressed. “External data for validation is the next step,” he said, noting he and co-investigators welcome data input from other investigators.
Elsie Nguyen, MD, FRCPC, FNASCI, associate professor of radiology, University of Toronto, responded by email that the study shows that imaging features supplement clinical data in predicting overall survival.
“This study demonstrates the value of extracting non–cancer related computed tomography imaging features to build a model that can better predict overall survival as compared to clinical parameters alone (such as age, performance status and co-morbidities) for stage I lung cancer patients treated with SBRT,” Dr. Nguyen wrote.
“Coronary artery calcium score, pulmonary artery-to-aorta ratio, and sarcopenia independently predicted overall survival,” she wrote. “These results are not surprising, as the prognostic value of each of these imaging features has already been established in the literature.”
Dr. Nguyen pointed out the power in the sum of these imaging features to predict overall survival.
“However, the results of this study demonstrate promising results supportive of the notion that combining clinical and imaging data points can help build a more accurate prediction model for overall survival,” she wrote. “This is analogous to the Brock University (in St. Catharines, Ontario) calculator for solitary pulmonary nodules that calculates malignancy risk based on both clinical and imaging data points. However, external validation of these study results at other centers is first required.”
Dr. Fintelmann and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term efficacy, safety data for ixekizumab in pediatric psoriasis reported
with the interleukin (IL)-17 inhibitor, investigators reported.
In addition, findings of a substudy, which evaluated randomized withdrawal of treatment after 60 weeks, suggest patients were able to regain benefit after not being treated for a period.
Ixekizumab (Taltz) was approved by the U.S. Food and Drug Administration for treating pediatric psoriasis in March 2020 for patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
The trial (IXORA-PEDS) involved 171 patients aged 6-17 years (mean age, 13.5 years; 99 females and 72 males), who were randomly assigned to receive ixekizumab via subcutaneous administration every 4 weeks (115) or placebo for 12 weeks (56). Thereafter, 166 patients continued in an open-label maintenance period in which they were treated every 4 weeks for 12-60 weeks. This was followed by an extension period of up to 108 weeks, which was completed by 139 patients (83.7%). At baseline, the patients’ Psoriasis Area and Severity Index (PASI) score was 12 or higher, the static Physician’s Global Assessment (sPGA) score was 3 or higher, and 10% or more of body surface area was affected.
In the study, at 12 weeks, treatment with ixekizumab was superior to placebo, with sustained responses through 48 weeks. In the follow-up phase, primary and secondary endpoints were sustained through week 108, with patients achieving or maintaining PASI 75 (91.7%), PASI 90 (79%), PASI 100 (55.1%), sPGA 0 or 1 (78.3%), and sPGA 0 (52.4%). Significant improvements in itch were seen at 12 weeks and were sustained with “meaningful improvements in itch for 78.5% of these patients at week 108,” the investigators report.
Among the patients who received ixekizumab, clearance rates in areas that are difficult to treat increased from week 12 to week 108 among those affected. During this time, clearance of nail psoriasis increased from 22.8% to 68.1%, clearance of palmoplantar psoriasis increased from 46.2% to 90%, clearance of scalp psoriasis increased from 70.7% to 76.2%, and clearance of genital psoriasis increased from 83.3% to 87.5%.
No new safety findings during weeks 48-108 of the trial were reported, including no new cases of inflammatory bowel disease (IBD) or Candida infections. The results were reported in JAMA Dermatology.
“Safety is really what we think of most when we are talking about pediatric patients, especially since they may be on these for decades and ... since they most commonly start these therapies in adolescence,” said Amy Paller, MD, the study’s lead author, in an interview. “To be able to take this out 108 weeks, 2 years, is starting to get to a point where we are getting more comfortable with safety. Clearly, no new signals arose.” Dr. Paller is chair of the department of dermatology and professor of dermatology and pediatrics, Northwestern University, Chicago.
One of the biggest concerns with using IL-17 inhibitors such as ixekizumab to manage psoriasis is the development of IBD, said Dr. Paller. She noted that four cases of IBD were reported before the extension phase of the trial but that no new IBD cases were reported after week 48.
“We would not start this as a treatment of choice in someone with Crohn’s disease, or perhaps we would think twice about using it in someone with a strong family history [of Crohn’s disease],” said Dr. Paller, who is also the director of the Skin Biology and Diseases Resource-Based Center at Northwestern. “Otherwise, it does not make me concerned about its use.”
Commenting on the study, Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, said that the trial’s results provide additional evidence regarding the optimal management of pediatric psoriasis.
“The landscape has shifted toward involving more pediatric patients in clinical trials, thereby providing dermatologists with data to select safe and effective therapies to manage children with psoriasis,” Dr. Cordoro said in an interview. “We have data showing that children with psoriasis have been undertreated, likely because of concerns about safety. The more evidence available from trials such as this, the more likely children are to receive necessary treatment.”
The efficacy data from the study on difficult-to-treat areas of psoriasis, in addition to improvements in BSA and PASI measures, are significant for clinicians deciding on a therapy for patients with psoriasis concentrated in specific body sites. “It was very valuable that the efficacy data was provided by site, such as scalp, palmoplantar, nails, and genital psoriasis, as these are low-BSA but high-impact areas for patients,” said Dr. Cordoro.
The trial data on Crohn’s disease buttress her decision to continue to refrain from initiating ixekizumab in a child with IBD or who is at high risk for IBD. “I was happy to see that there was not a signal for Candida infection,” she added.
Interestingly, in the substudy in the European population, in which there was a double-blind, randomized withdrawal period, fewer patients who were reassigned to receive ixekizumab experienced relapse, compared with those who were reassigned to receive placebo. A total of 90.9% of patients who received placebo experienced relapse, compared with 17.6% of patients treated with ixekizumab. The median time to relapse in the placebo group was 149 days.
“There are data in the adult population that suggest intermittent treatment does allow for recapture of clinical response,” said Dr. Cordoro. “While it is not a large enough dataset to know definitively, this substudy of patients suggests the possibility of intermittent treatment and the ability to regain control [of psoriasis] after a period off drug.”
The study was funded by Eli Lilly. Dr. Paller is an investigator and consultant for Eli Lilly. Several other authors have received grants, personal fees, and/or were a consultant to Eli Lilly, and two authors are Eli Lilly employees. Dr. Cordoro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
with the interleukin (IL)-17 inhibitor, investigators reported.
In addition, findings of a substudy, which evaluated randomized withdrawal of treatment after 60 weeks, suggest patients were able to regain benefit after not being treated for a period.
Ixekizumab (Taltz) was approved by the U.S. Food and Drug Administration for treating pediatric psoriasis in March 2020 for patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
The trial (IXORA-PEDS) involved 171 patients aged 6-17 years (mean age, 13.5 years; 99 females and 72 males), who were randomly assigned to receive ixekizumab via subcutaneous administration every 4 weeks (115) or placebo for 12 weeks (56). Thereafter, 166 patients continued in an open-label maintenance period in which they were treated every 4 weeks for 12-60 weeks. This was followed by an extension period of up to 108 weeks, which was completed by 139 patients (83.7%). At baseline, the patients’ Psoriasis Area and Severity Index (PASI) score was 12 or higher, the static Physician’s Global Assessment (sPGA) score was 3 or higher, and 10% or more of body surface area was affected.
In the study, at 12 weeks, treatment with ixekizumab was superior to placebo, with sustained responses through 48 weeks. In the follow-up phase, primary and secondary endpoints were sustained through week 108, with patients achieving or maintaining PASI 75 (91.7%), PASI 90 (79%), PASI 100 (55.1%), sPGA 0 or 1 (78.3%), and sPGA 0 (52.4%). Significant improvements in itch were seen at 12 weeks and were sustained with “meaningful improvements in itch for 78.5% of these patients at week 108,” the investigators report.
Among the patients who received ixekizumab, clearance rates in areas that are difficult to treat increased from week 12 to week 108 among those affected. During this time, clearance of nail psoriasis increased from 22.8% to 68.1%, clearance of palmoplantar psoriasis increased from 46.2% to 90%, clearance of scalp psoriasis increased from 70.7% to 76.2%, and clearance of genital psoriasis increased from 83.3% to 87.5%.
No new safety findings during weeks 48-108 of the trial were reported, including no new cases of inflammatory bowel disease (IBD) or Candida infections. The results were reported in JAMA Dermatology.
“Safety is really what we think of most when we are talking about pediatric patients, especially since they may be on these for decades and ... since they most commonly start these therapies in adolescence,” said Amy Paller, MD, the study’s lead author, in an interview. “To be able to take this out 108 weeks, 2 years, is starting to get to a point where we are getting more comfortable with safety. Clearly, no new signals arose.” Dr. Paller is chair of the department of dermatology and professor of dermatology and pediatrics, Northwestern University, Chicago.
One of the biggest concerns with using IL-17 inhibitors such as ixekizumab to manage psoriasis is the development of IBD, said Dr. Paller. She noted that four cases of IBD were reported before the extension phase of the trial but that no new IBD cases were reported after week 48.
“We would not start this as a treatment of choice in someone with Crohn’s disease, or perhaps we would think twice about using it in someone with a strong family history [of Crohn’s disease],” said Dr. Paller, who is also the director of the Skin Biology and Diseases Resource-Based Center at Northwestern. “Otherwise, it does not make me concerned about its use.”
Commenting on the study, Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, said that the trial’s results provide additional evidence regarding the optimal management of pediatric psoriasis.
“The landscape has shifted toward involving more pediatric patients in clinical trials, thereby providing dermatologists with data to select safe and effective therapies to manage children with psoriasis,” Dr. Cordoro said in an interview. “We have data showing that children with psoriasis have been undertreated, likely because of concerns about safety. The more evidence available from trials such as this, the more likely children are to receive necessary treatment.”
The efficacy data from the study on difficult-to-treat areas of psoriasis, in addition to improvements in BSA and PASI measures, are significant for clinicians deciding on a therapy for patients with psoriasis concentrated in specific body sites. “It was very valuable that the efficacy data was provided by site, such as scalp, palmoplantar, nails, and genital psoriasis, as these are low-BSA but high-impact areas for patients,” said Dr. Cordoro.
The trial data on Crohn’s disease buttress her decision to continue to refrain from initiating ixekizumab in a child with IBD or who is at high risk for IBD. “I was happy to see that there was not a signal for Candida infection,” she added.
Interestingly, in the substudy in the European population, in which there was a double-blind, randomized withdrawal period, fewer patients who were reassigned to receive ixekizumab experienced relapse, compared with those who were reassigned to receive placebo. A total of 90.9% of patients who received placebo experienced relapse, compared with 17.6% of patients treated with ixekizumab. The median time to relapse in the placebo group was 149 days.
“There are data in the adult population that suggest intermittent treatment does allow for recapture of clinical response,” said Dr. Cordoro. “While it is not a large enough dataset to know definitively, this substudy of patients suggests the possibility of intermittent treatment and the ability to regain control [of psoriasis] after a period off drug.”
The study was funded by Eli Lilly. Dr. Paller is an investigator and consultant for Eli Lilly. Several other authors have received grants, personal fees, and/or were a consultant to Eli Lilly, and two authors are Eli Lilly employees. Dr. Cordoro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
with the interleukin (IL)-17 inhibitor, investigators reported.
In addition, findings of a substudy, which evaluated randomized withdrawal of treatment after 60 weeks, suggest patients were able to regain benefit after not being treated for a period.
Ixekizumab (Taltz) was approved by the U.S. Food and Drug Administration for treating pediatric psoriasis in March 2020 for patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
The trial (IXORA-PEDS) involved 171 patients aged 6-17 years (mean age, 13.5 years; 99 females and 72 males), who were randomly assigned to receive ixekizumab via subcutaneous administration every 4 weeks (115) or placebo for 12 weeks (56). Thereafter, 166 patients continued in an open-label maintenance period in which they were treated every 4 weeks for 12-60 weeks. This was followed by an extension period of up to 108 weeks, which was completed by 139 patients (83.7%). At baseline, the patients’ Psoriasis Area and Severity Index (PASI) score was 12 or higher, the static Physician’s Global Assessment (sPGA) score was 3 or higher, and 10% or more of body surface area was affected.
In the study, at 12 weeks, treatment with ixekizumab was superior to placebo, with sustained responses through 48 weeks. In the follow-up phase, primary and secondary endpoints were sustained through week 108, with patients achieving or maintaining PASI 75 (91.7%), PASI 90 (79%), PASI 100 (55.1%), sPGA 0 or 1 (78.3%), and sPGA 0 (52.4%). Significant improvements in itch were seen at 12 weeks and were sustained with “meaningful improvements in itch for 78.5% of these patients at week 108,” the investigators report.
Among the patients who received ixekizumab, clearance rates in areas that are difficult to treat increased from week 12 to week 108 among those affected. During this time, clearance of nail psoriasis increased from 22.8% to 68.1%, clearance of palmoplantar psoriasis increased from 46.2% to 90%, clearance of scalp psoriasis increased from 70.7% to 76.2%, and clearance of genital psoriasis increased from 83.3% to 87.5%.
No new safety findings during weeks 48-108 of the trial were reported, including no new cases of inflammatory bowel disease (IBD) or Candida infections. The results were reported in JAMA Dermatology.
“Safety is really what we think of most when we are talking about pediatric patients, especially since they may be on these for decades and ... since they most commonly start these therapies in adolescence,” said Amy Paller, MD, the study’s lead author, in an interview. “To be able to take this out 108 weeks, 2 years, is starting to get to a point where we are getting more comfortable with safety. Clearly, no new signals arose.” Dr. Paller is chair of the department of dermatology and professor of dermatology and pediatrics, Northwestern University, Chicago.
One of the biggest concerns with using IL-17 inhibitors such as ixekizumab to manage psoriasis is the development of IBD, said Dr. Paller. She noted that four cases of IBD were reported before the extension phase of the trial but that no new IBD cases were reported after week 48.
“We would not start this as a treatment of choice in someone with Crohn’s disease, or perhaps we would think twice about using it in someone with a strong family history [of Crohn’s disease],” said Dr. Paller, who is also the director of the Skin Biology and Diseases Resource-Based Center at Northwestern. “Otherwise, it does not make me concerned about its use.”
Commenting on the study, Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, said that the trial’s results provide additional evidence regarding the optimal management of pediatric psoriasis.
“The landscape has shifted toward involving more pediatric patients in clinical trials, thereby providing dermatologists with data to select safe and effective therapies to manage children with psoriasis,” Dr. Cordoro said in an interview. “We have data showing that children with psoriasis have been undertreated, likely because of concerns about safety. The more evidence available from trials such as this, the more likely children are to receive necessary treatment.”
The efficacy data from the study on difficult-to-treat areas of psoriasis, in addition to improvements in BSA and PASI measures, are significant for clinicians deciding on a therapy for patients with psoriasis concentrated in specific body sites. “It was very valuable that the efficacy data was provided by site, such as scalp, palmoplantar, nails, and genital psoriasis, as these are low-BSA but high-impact areas for patients,” said Dr. Cordoro.
The trial data on Crohn’s disease buttress her decision to continue to refrain from initiating ixekizumab in a child with IBD or who is at high risk for IBD. “I was happy to see that there was not a signal for Candida infection,” she added.
Interestingly, in the substudy in the European population, in which there was a double-blind, randomized withdrawal period, fewer patients who were reassigned to receive ixekizumab experienced relapse, compared with those who were reassigned to receive placebo. A total of 90.9% of patients who received placebo experienced relapse, compared with 17.6% of patients treated with ixekizumab. The median time to relapse in the placebo group was 149 days.
“There are data in the adult population that suggest intermittent treatment does allow for recapture of clinical response,” said Dr. Cordoro. “While it is not a large enough dataset to know definitively, this substudy of patients suggests the possibility of intermittent treatment and the ability to regain control [of psoriasis] after a period off drug.”
The study was funded by Eli Lilly. Dr. Paller is an investigator and consultant for Eli Lilly. Several other authors have received grants, personal fees, and/or were a consultant to Eli Lilly, and two authors are Eli Lilly employees. Dr. Cordoro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Can gram stains guide antibiotics for pneumonia in critical care?
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
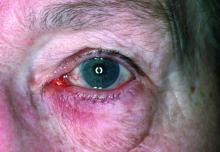
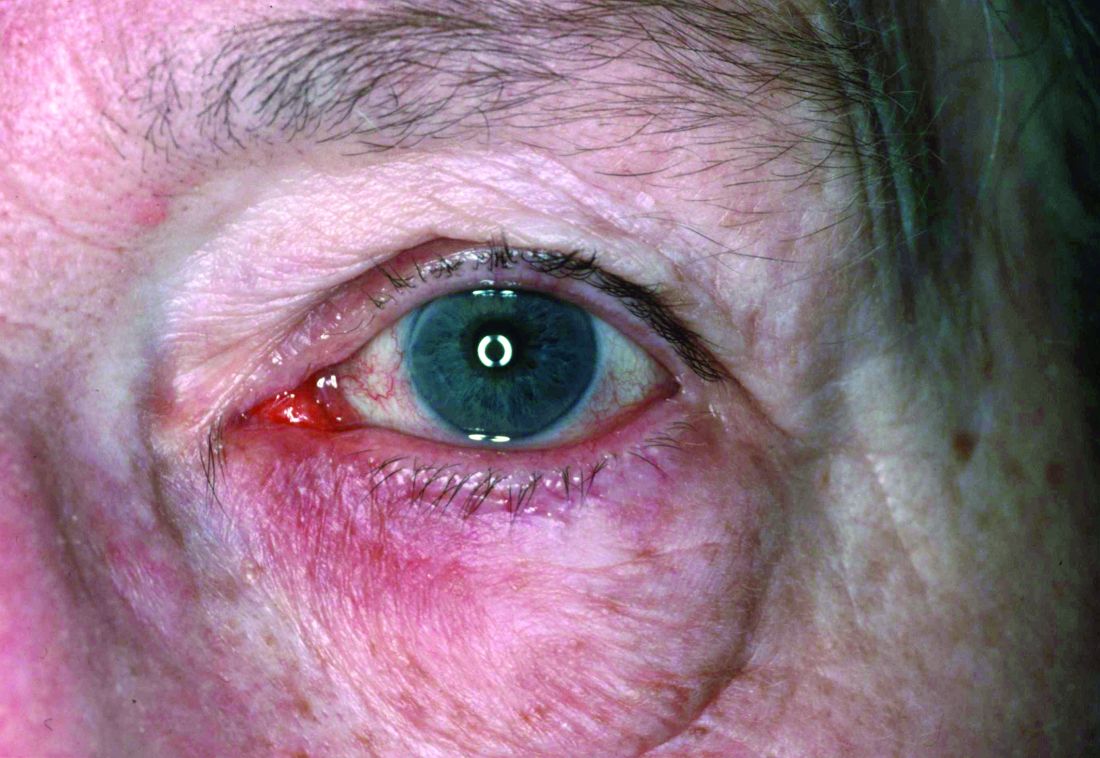
Dryness, conjunctival telangiectasia among ocular symptoms common in rosacea
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNATIONAL OPTHALMOLOGY
International panel backs energy-based devices as first-line treatment of acne scars
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New x-ray technique shows COVID-19 lung in unprecedented detail
A recent article published in Nature Methods highlights how hierarchical phase-contrast tomography (HiP-CT), an x-ray phase propagation technique that uses spatial coherence to conduct three-dimensional scans of organs ex vivo, may offer clinicians greater insights into disease processes.
“It is not a clinical technique as such,” said Claire Walsh PhD, a biophysicist and senior research fellow at the Center for Advanced Biomedical Imaging, University College London, and one of the authors of the article. She stressed that HiP-CT is used ex vivo.
“This technology uses x-rays from a fourth-generation x-ray source, the European Synchrotron Radiation Facility’s Extremely Brilliant Source. It is an incredibly bright x-ray source,” said Dr. Walsh in an interview. She said synchrotron x-ray tomography provides a much enhanced view of the lungs of persons who had had COVID-19. “We are looking at a different property of the x-ray waves. We are looking at a phase shift. [HiP-CT] is much, much more sensitive to small changes in the tissue than x-ray or CT. Another massive advantage of HiP-CT is the resolution it offers. The resolution goes down to single cells inside an intact human organ,” she said.
The resolution permits researchers to view blood vessels 5 μm in diameter in an intact lung. In comparison, clinical CT images show blood vessels of around 1 mm in diameter – 200 times larger.
“This technique will help us understand the structure of organs at a more fundamental level,” said Dr. Walsh. She noted that the technology has been valuable in allowing greater understanding of COVID-19 disease process. “This is about building an understanding of what the disease is doing in our bodies. If we don’t understand what the disease is changing structurally, it is very hard to understand how to go about developing treatments,” she said.
There are few synchrotron radiation facilities, so this technology is not widely available. Because of the very high radiation dose, the technique will be used ex vivo for the foreseeable future, Dr. Walsh said.
“The x-ray dose is incredibly high; 2-kg normal CT scans are approximately 100 mG [milligauss]. This is 20,000 times more than a medical CT scan,” explained Dr. Walsh. “We don’t really have plans for this to become an in vivo human technique. We are aiming that we will be able to register clinical scans to HiP-CT in a few cases, and so HiP-CT will become a calibration for analyzing clinical techniques.”
Elsie T. Nguyen, MD, FRCPC, vice-president of the Canadian Society of Thoracic Radiology and associate professor of radiology, University of Toronto, noted that the technology will be valuable in pathology and radiology.
“HiP-CT appears to be an exciting new development that can help physicians, including radiologists, understand pathology that was once beyond the spatial resolution of computed tomography scans,” said Dr. Nguyen in an interview. “The fact that vascular abnormalities particularly relating to severe COVID-19 pneumonia can be visualized to the micron level is very novel and exciting. This will help us understand better from a mechanistic point of view what is happening to the blood vessels that contributes to worse outcomes, like shunting of blood or blood clots, and may have applications for prognostication to predict which patients are likely to survive severe COVID-19 pneumonia.”
Dr. Nguyen noted that HiP-CT could help thoracic radiologists better visualize honeycomb cysts associated with fibrotic interstitial lung disease (ILD). It could help to classify the type of fibrotic ILD and inform patient prognosis.
“Currently, we struggle to differentiate early honeycomb cysts, which are a sign of more advanced lung destruction, from traction bronchiolectasis, that is, dilated airways due to surrounding fibrotic lung, on high-resolution computed tomography of the lungs,” said Dr. Nguyen. She said HiP-CT was very promising and had many applications in addition to visualizing the lungs.
The research was funded by the Chan Zuckerberg Initiative, the ESRF, the UK-MRC, and the Royal Academy of Engineering. Dr. Walsh and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A recent article published in Nature Methods highlights how hierarchical phase-contrast tomography (HiP-CT), an x-ray phase propagation technique that uses spatial coherence to conduct three-dimensional scans of organs ex vivo, may offer clinicians greater insights into disease processes.
“It is not a clinical technique as such,” said Claire Walsh PhD, a biophysicist and senior research fellow at the Center for Advanced Biomedical Imaging, University College London, and one of the authors of the article. She stressed that HiP-CT is used ex vivo.
“This technology uses x-rays from a fourth-generation x-ray source, the European Synchrotron Radiation Facility’s Extremely Brilliant Source. It is an incredibly bright x-ray source,” said Dr. Walsh in an interview. She said synchrotron x-ray tomography provides a much enhanced view of the lungs of persons who had had COVID-19. “We are looking at a different property of the x-ray waves. We are looking at a phase shift. [HiP-CT] is much, much more sensitive to small changes in the tissue than x-ray or CT. Another massive advantage of HiP-CT is the resolution it offers. The resolution goes down to single cells inside an intact human organ,” she said.
The resolution permits researchers to view blood vessels 5 μm in diameter in an intact lung. In comparison, clinical CT images show blood vessels of around 1 mm in diameter – 200 times larger.
“This technique will help us understand the structure of organs at a more fundamental level,” said Dr. Walsh. She noted that the technology has been valuable in allowing greater understanding of COVID-19 disease process. “This is about building an understanding of what the disease is doing in our bodies. If we don’t understand what the disease is changing structurally, it is very hard to understand how to go about developing treatments,” she said.
There are few synchrotron radiation facilities, so this technology is not widely available. Because of the very high radiation dose, the technique will be used ex vivo for the foreseeable future, Dr. Walsh said.
“The x-ray dose is incredibly high; 2-kg normal CT scans are approximately 100 mG [milligauss]. This is 20,000 times more than a medical CT scan,” explained Dr. Walsh. “We don’t really have plans for this to become an in vivo human technique. We are aiming that we will be able to register clinical scans to HiP-CT in a few cases, and so HiP-CT will become a calibration for analyzing clinical techniques.”
Elsie T. Nguyen, MD, FRCPC, vice-president of the Canadian Society of Thoracic Radiology and associate professor of radiology, University of Toronto, noted that the technology will be valuable in pathology and radiology.
“HiP-CT appears to be an exciting new development that can help physicians, including radiologists, understand pathology that was once beyond the spatial resolution of computed tomography scans,” said Dr. Nguyen in an interview. “The fact that vascular abnormalities particularly relating to severe COVID-19 pneumonia can be visualized to the micron level is very novel and exciting. This will help us understand better from a mechanistic point of view what is happening to the blood vessels that contributes to worse outcomes, like shunting of blood or blood clots, and may have applications for prognostication to predict which patients are likely to survive severe COVID-19 pneumonia.”
Dr. Nguyen noted that HiP-CT could help thoracic radiologists better visualize honeycomb cysts associated with fibrotic interstitial lung disease (ILD). It could help to classify the type of fibrotic ILD and inform patient prognosis.
“Currently, we struggle to differentiate early honeycomb cysts, which are a sign of more advanced lung destruction, from traction bronchiolectasis, that is, dilated airways due to surrounding fibrotic lung, on high-resolution computed tomography of the lungs,” said Dr. Nguyen. She said HiP-CT was very promising and had many applications in addition to visualizing the lungs.
The research was funded by the Chan Zuckerberg Initiative, the ESRF, the UK-MRC, and the Royal Academy of Engineering. Dr. Walsh and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A recent article published in Nature Methods highlights how hierarchical phase-contrast tomography (HiP-CT), an x-ray phase propagation technique that uses spatial coherence to conduct three-dimensional scans of organs ex vivo, may offer clinicians greater insights into disease processes.
“It is not a clinical technique as such,” said Claire Walsh PhD, a biophysicist and senior research fellow at the Center for Advanced Biomedical Imaging, University College London, and one of the authors of the article. She stressed that HiP-CT is used ex vivo.
“This technology uses x-rays from a fourth-generation x-ray source, the European Synchrotron Radiation Facility’s Extremely Brilliant Source. It is an incredibly bright x-ray source,” said Dr. Walsh in an interview. She said synchrotron x-ray tomography provides a much enhanced view of the lungs of persons who had had COVID-19. “We are looking at a different property of the x-ray waves. We are looking at a phase shift. [HiP-CT] is much, much more sensitive to small changes in the tissue than x-ray or CT. Another massive advantage of HiP-CT is the resolution it offers. The resolution goes down to single cells inside an intact human organ,” she said.
The resolution permits researchers to view blood vessels 5 μm in diameter in an intact lung. In comparison, clinical CT images show blood vessels of around 1 mm in diameter – 200 times larger.
“This technique will help us understand the structure of organs at a more fundamental level,” said Dr. Walsh. She noted that the technology has been valuable in allowing greater understanding of COVID-19 disease process. “This is about building an understanding of what the disease is doing in our bodies. If we don’t understand what the disease is changing structurally, it is very hard to understand how to go about developing treatments,” she said.
There are few synchrotron radiation facilities, so this technology is not widely available. Because of the very high radiation dose, the technique will be used ex vivo for the foreseeable future, Dr. Walsh said.
“The x-ray dose is incredibly high; 2-kg normal CT scans are approximately 100 mG [milligauss]. This is 20,000 times more than a medical CT scan,” explained Dr. Walsh. “We don’t really have plans for this to become an in vivo human technique. We are aiming that we will be able to register clinical scans to HiP-CT in a few cases, and so HiP-CT will become a calibration for analyzing clinical techniques.”
Elsie T. Nguyen, MD, FRCPC, vice-president of the Canadian Society of Thoracic Radiology and associate professor of radiology, University of Toronto, noted that the technology will be valuable in pathology and radiology.
“HiP-CT appears to be an exciting new development that can help physicians, including radiologists, understand pathology that was once beyond the spatial resolution of computed tomography scans,” said Dr. Nguyen in an interview. “The fact that vascular abnormalities particularly relating to severe COVID-19 pneumonia can be visualized to the micron level is very novel and exciting. This will help us understand better from a mechanistic point of view what is happening to the blood vessels that contributes to worse outcomes, like shunting of blood or blood clots, and may have applications for prognostication to predict which patients are likely to survive severe COVID-19 pneumonia.”
Dr. Nguyen noted that HiP-CT could help thoracic radiologists better visualize honeycomb cysts associated with fibrotic interstitial lung disease (ILD). It could help to classify the type of fibrotic ILD and inform patient prognosis.
“Currently, we struggle to differentiate early honeycomb cysts, which are a sign of more advanced lung destruction, from traction bronchiolectasis, that is, dilated airways due to surrounding fibrotic lung, on high-resolution computed tomography of the lungs,” said Dr. Nguyen. She said HiP-CT was very promising and had many applications in addition to visualizing the lungs.
The research was funded by the Chan Zuckerberg Initiative, the ESRF, the UK-MRC, and the Royal Academy of Engineering. Dr. Walsh and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Handheld device highly sensitive in detecting amblyopia; can be used in children as young as 2 years of age
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data sharing to improve AI used in breast-imaging research
The curated dataset, which consists of 22,032 DBT volumes associated with 5,610 studies from 5,060 patients, was published online in JAMA Network Open. The studies were divided into types: normal studies (91.4%), actionable studies that required additional imaging but no biopsy (5.0%), benign biopsied studies (2.0%), and studies that detected cancer (1.6%).
To develop and evaluate their deep-learning model for the detection of architectural distortions and masses, the researchers used a test set of 460 studies from 418 patients with cancer. Their algorithm reached a breast-based sensitivity of two false positives per DBT volume, or 65%.
“The main focus of this publication is on the dataset, rather than on a specific hypothesis,” said principal researcher Maciej A. Mazurowski, PhD, scientific director of the Duke Center for Artificial Intelligence in Radiology in Durham, N.C.
“We have publicly shared a large dataset of digital breast tomosynthesis images, which are sometimes referred to as 3D mammograms, for more than 5,000 patients. There are two purposes for sharing data like these. One is to improve research and development of machine-learning algorithms. You can train models with these data. The other reason, maybe even more important, is to provide a benchmark to test algorithms,” he said in an interview.
The large-scale sharing of data is a key step toward transparency in science, said Dr. Mazurowski. “It is about making sure results can be easily reproduced and setting benchmarks.”
The dataset includes masses and architectural distortions that were annotated by two experienced radiologists, but does not include annotations for calcifications and/or microcalcifications.
This lack of calcifications is a limitation of the study, said Jean Seely, MD, professor of radiology at the University of Ottawa, who is president of the Canadian Society of Breast Imaging and regional lead for the Ontario Breast Screening Program.
“About 45% of invasive breast cancers are diagnosed based on calcifications,” she explained.
Still, although the sensitivity of the AI algorithm was not high (65%) – the average sensitivity of 2D mammography is 85% – the researchers should be commended for releasing such a large dataset, said Dr. Seely.
“The fact that they have made it publicly available is very, very useful,” she said, adding that the dataset can be leveraged in future breast-imaging research.
Although DBT is much better at identifying breast cancers than mammography, DBT exams take about 30% more time to read.
“There’s a lot of work being done in artificial intelligence in breast imaging to not only improve the workflow for breast radiologists, but also to help with the diagnosis and detection,” she noted. “Anything that helps improve the confidence and the accuracy of the radiologist is really what we’re aiming for right now.”
The size and the content of this dataset will contribute to breast-imaging research, said Jaron Chong, MD, of the department of medical imaging at Western University in London, Ontario, who is chair of the AI Standing Committee at the Canadian Association of Radiologists.
“The contribution could be valuable in the long term because DBT is a rare dataset in comparison to conventional 2D mammography,” said Dr. Chong. “Most existing datasets have focused on two-dimensional imaging. We might see more research papers reference this dataset in the future, iterating and improving upon this article’s algorithm performance.”
Dr. Mazurowski reports serving as an adviser to Gradient Health. Dr. Seely is an unpaid principal investigator for the Ottawa site of the Tomosynthesis Mammographic Imaging Screening Trial (TMIST). Dr. Chong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The curated dataset, which consists of 22,032 DBT volumes associated with 5,610 studies from 5,060 patients, was published online in JAMA Network Open. The studies were divided into types: normal studies (91.4%), actionable studies that required additional imaging but no biopsy (5.0%), benign biopsied studies (2.0%), and studies that detected cancer (1.6%).
To develop and evaluate their deep-learning model for the detection of architectural distortions and masses, the researchers used a test set of 460 studies from 418 patients with cancer. Their algorithm reached a breast-based sensitivity of two false positives per DBT volume, or 65%.
“The main focus of this publication is on the dataset, rather than on a specific hypothesis,” said principal researcher Maciej A. Mazurowski, PhD, scientific director of the Duke Center for Artificial Intelligence in Radiology in Durham, N.C.
“We have publicly shared a large dataset of digital breast tomosynthesis images, which are sometimes referred to as 3D mammograms, for more than 5,000 patients. There are two purposes for sharing data like these. One is to improve research and development of machine-learning algorithms. You can train models with these data. The other reason, maybe even more important, is to provide a benchmark to test algorithms,” he said in an interview.
The large-scale sharing of data is a key step toward transparency in science, said Dr. Mazurowski. “It is about making sure results can be easily reproduced and setting benchmarks.”
The dataset includes masses and architectural distortions that were annotated by two experienced radiologists, but does not include annotations for calcifications and/or microcalcifications.
This lack of calcifications is a limitation of the study, said Jean Seely, MD, professor of radiology at the University of Ottawa, who is president of the Canadian Society of Breast Imaging and regional lead for the Ontario Breast Screening Program.
“About 45% of invasive breast cancers are diagnosed based on calcifications,” she explained.
Still, although the sensitivity of the AI algorithm was not high (65%) – the average sensitivity of 2D mammography is 85% – the researchers should be commended for releasing such a large dataset, said Dr. Seely.
“The fact that they have made it publicly available is very, very useful,” she said, adding that the dataset can be leveraged in future breast-imaging research.
Although DBT is much better at identifying breast cancers than mammography, DBT exams take about 30% more time to read.
“There’s a lot of work being done in artificial intelligence in breast imaging to not only improve the workflow for breast radiologists, but also to help with the diagnosis and detection,” she noted. “Anything that helps improve the confidence and the accuracy of the radiologist is really what we’re aiming for right now.”
The size and the content of this dataset will contribute to breast-imaging research, said Jaron Chong, MD, of the department of medical imaging at Western University in London, Ontario, who is chair of the AI Standing Committee at the Canadian Association of Radiologists.
“The contribution could be valuable in the long term because DBT is a rare dataset in comparison to conventional 2D mammography,” said Dr. Chong. “Most existing datasets have focused on two-dimensional imaging. We might see more research papers reference this dataset in the future, iterating and improving upon this article’s algorithm performance.”
Dr. Mazurowski reports serving as an adviser to Gradient Health. Dr. Seely is an unpaid principal investigator for the Ottawa site of the Tomosynthesis Mammographic Imaging Screening Trial (TMIST). Dr. Chong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The curated dataset, which consists of 22,032 DBT volumes associated with 5,610 studies from 5,060 patients, was published online in JAMA Network Open. The studies were divided into types: normal studies (91.4%), actionable studies that required additional imaging but no biopsy (5.0%), benign biopsied studies (2.0%), and studies that detected cancer (1.6%).
To develop and evaluate their deep-learning model for the detection of architectural distortions and masses, the researchers used a test set of 460 studies from 418 patients with cancer. Their algorithm reached a breast-based sensitivity of two false positives per DBT volume, or 65%.
“The main focus of this publication is on the dataset, rather than on a specific hypothesis,” said principal researcher Maciej A. Mazurowski, PhD, scientific director of the Duke Center for Artificial Intelligence in Radiology in Durham, N.C.
“We have publicly shared a large dataset of digital breast tomosynthesis images, which are sometimes referred to as 3D mammograms, for more than 5,000 patients. There are two purposes for sharing data like these. One is to improve research and development of machine-learning algorithms. You can train models with these data. The other reason, maybe even more important, is to provide a benchmark to test algorithms,” he said in an interview.
The large-scale sharing of data is a key step toward transparency in science, said Dr. Mazurowski. “It is about making sure results can be easily reproduced and setting benchmarks.”
The dataset includes masses and architectural distortions that were annotated by two experienced radiologists, but does not include annotations for calcifications and/or microcalcifications.
This lack of calcifications is a limitation of the study, said Jean Seely, MD, professor of radiology at the University of Ottawa, who is president of the Canadian Society of Breast Imaging and regional lead for the Ontario Breast Screening Program.
“About 45% of invasive breast cancers are diagnosed based on calcifications,” she explained.
Still, although the sensitivity of the AI algorithm was not high (65%) – the average sensitivity of 2D mammography is 85% – the researchers should be commended for releasing such a large dataset, said Dr. Seely.
“The fact that they have made it publicly available is very, very useful,” she said, adding that the dataset can be leveraged in future breast-imaging research.
Although DBT is much better at identifying breast cancers than mammography, DBT exams take about 30% more time to read.
“There’s a lot of work being done in artificial intelligence in breast imaging to not only improve the workflow for breast radiologists, but also to help with the diagnosis and detection,” she noted. “Anything that helps improve the confidence and the accuracy of the radiologist is really what we’re aiming for right now.”
The size and the content of this dataset will contribute to breast-imaging research, said Jaron Chong, MD, of the department of medical imaging at Western University in London, Ontario, who is chair of the AI Standing Committee at the Canadian Association of Radiologists.
“The contribution could be valuable in the long term because DBT is a rare dataset in comparison to conventional 2D mammography,” said Dr. Chong. “Most existing datasets have focused on two-dimensional imaging. We might see more research papers reference this dataset in the future, iterating and improving upon this article’s algorithm performance.”
Dr. Mazurowski reports serving as an adviser to Gradient Health. Dr. Seely is an unpaid principal investigator for the Ottawa site of the Tomosynthesis Mammographic Imaging Screening Trial (TMIST). Dr. Chong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.