User login
When Should Hypopituitarism Be Suspected?
Case
A 53-year-old woman with a history of a suprasellar meningioma resected nine years ago with recurrence of a 4.5x2 cm mass one year ago and recent ventriculoperitoneal (VP) shunt placement for hydrocephalus presented with altered mental status (AMS) and hallucinations. She was admitted for radiation therapy to the mass. The patient had little improvement in her mental status four weeks into a six-week, 4860 cGy course of photon therapy.
The internal medicine service was consulted for new onset tachycardia (103), hypotension (83/55), and fever (38.6 C). Laboratory data revealed a white blood cell count 4.8 x 109 cells/L, sodium 137 mmol/L, potassium 4.1 mmol/L, chloride 110 mmol/L, bicarbonate 28 mmol/L, blood urea nitrogen 3 mg/dl, creatinine 0.6 mg/dl, and glucose 91 mg/dl. Thyroid-stimulating hormone (TSH) was low at 0.38 mIU/mL. Urine specific gravity was 1.006. Workups for infectious and thromboembolic diseases were unremarkable.
Discussion
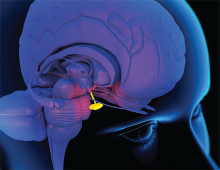
Hypopituitarism is a disorder of impaired hormone production from the anterior and, less commonly, posterior pituitary gland. The condition can originate from several broad categories of diseases affecting the hypothalamus, pituitary stalk, or pituitary gland. In adults, the etiology is often from the mass effect of tumors or from treatment with surgery or radiotherapy. Other causes include vascular, infectious, infiltrative, inflammatory, and idiopathic. Well-substantiated data on the incidence and prevalence of hypopituitarism is sparse. It has an estimated prevalence of 45.5 cases per 100,000 and incidence of 4.2 cases per 100,000 per year.1
Clinical manifestations of hypopituitarism depend on the type and severity of hormone deficiency. The consequences of adrenal insufficiency (AI) range from smoldering and nonspecific findings (e.g. fatigue, lethargy, indistinct gastrointestinal symptoms, eosinophilia, fever) to full-fledged crisis (e.g. AMS, severe electrolyte abnormalities, hemodynamic compromise, shock). The presentation of central AI (i.e., arising from hypothalamic or pituitary pathology) is often more subtle than primary AI. In central AI, only glucocorticoid (GC) function is disrupted, leaving the renin-angiotensin-aldosterone system and mineralocorticoid (MC) function intact. This is in stark contrast to primary AI resulting from direct adrenal gland injury, which nearly always disrupts both GC and MC function, leading to more profound circulatory collapse and electrolyte disturbance.2
Aside from orthostatic blood pressure or possible low-grade fever, few physical exam features are associated with central AI. Hyperpigmentation is not seen due to the lack of anterior pituitary-derived melanocortins that stimulate melanocytes and induce pigmentation. As for laboratory findings, hyperkalemia is a feature of primary AI (due to hypoaldosteronism) but is not seen in central AI. Hyponatremia occurs in both types of AI and is vasopressin-mediated. Hyponatremia is more common in primary AI, resulting from appropriate vasopressin release that occurs due to hypotension. Hyponatremia also occurs in secondary AI because of increased vasopressin secretion mediated directly by hypocortisolemia.3,4
In summary, hyperpigmentation and the electrolyte pattern of hyponatremia and hyperkalemia are distinguishing clinical characteristics of primary AI, occurring in up to 90% of cases, but these features would not be expected with central AI.5
In the hospitalized patient with multiple active acute illnesses and infectious risk factors, it can be difficult to recognize the diagnosis of AI or hypopituitarism. Not only do signs and symptoms frequently overlap, but concomitant acute illness is usually a triggering event. Crisis should be suspected in the setting of unexplained fever, dehydration, or shock out of proportion to severity of current illness.5
Not surprisingly, high rates of partial or complete hypopituitarism are seen in patients following surgical removal of pituitary tumors or nearby neoplasms (e.g. craniopharyngiomas). Both surgery and radiotherapy for non-pituitary brain tumors are also major risk factors for development of hypopituitarism, occurring in up to 38% and 41% of patients, respectively.6 The strongest predictors of hormone failure are higher radiation doses, proximity to the pituitary-hypothalamus, and longer time interval after completion of radiotherapy. Within 10 years after a median dose of 5000 rad (50Gy) directed at the skull base, nasopharynx, or cranium, up to three-fourths of patients will develop some degree of pituitary insufficiency. Later onset of hormone failure usually reflects hypothalamic injury, whereas higher irradiation doses can lead to earlier onset pituitary damage.5
Not all hormone-secreting cells of the hypothalamus or pituitary are equally susceptible to injury; there is a characteristic sequence of hormonal failure. The typical order of hormone deficiency from pituitary compression or destruction is as follows: growth hormone (GH) > follicle-stimulating hormone (FSH) > luteinizing hormone (LH) > TSH > adrenocorticotropic hormone (ACTH) > vasopressin. A similar pattern is seen following brain irradiation: GH > FSH and LH > ACTH > TSH. A recent systematic review of 18 studies with 813 patients receiving cranial radiotherapy for non-pituitary tumors found pituitary dysfunction was 45% for GH deficiency, compared to 22% for ACTH deficiency.7
Biochemical diagnosis of hypopituitarism consists of measuring the various pituitary and target hormone levels as well as provocation testing. When interpreting these tests, whether to identify excess or deficient states, it is important to remember the individual values are part of the broader hypothalamic-pituitary axis feedback loops. Thus, it can be more useful designating if a high or low test value is appropriately or inappropriately high or low. In the presented case, low TSH level could be misinterpreted as excess thyroid hormone supplementation. An appropriately elevated free T4 level would confirm this, but an inappropriately low free T4 would raise suspicion of central hypothalamic-pituitary dysfunction.
With high enough clinical suspicion of hypopituitarism, empiric treatment with thyroid supplementation and corticosteroids should be started before confirmation of the diagnosis, to prevent secondary organ dysfunction and improve morbidity and mortality.2 Rapid administration with intravenous levothyroxine can be given in severe hypothyroidism or myxedema.
“Stress-dose” steroids are generally recommended for patients who are also administered levothyroxine, as the desired increased in metabolic rate can deplete existing pituitary-adrenocortical hormone reserves, precipitating adrenal crisis.5 Stress-dose corticosteroids also ensure recruitment of a mineralocorticoid response. Cortisol has both GC and MC stimulating effects but is rapidly metabolized to cortisone, which lacks MC stimulating effects. Thus, high doses overwhelm this conversion step and allow remaining cortisol to stimulate MC receptors.2 These high doses may not be necessary in secondary AI (i.e., preserved MC function) but would be reasonable in an unstable patient or until confirmation is made with an inappropriately low ACTH.
Back to the Case
Morning cortisol returned undetectable, and ACTH was 14 pg/mL (6-58). Past records revealed a down-trending TSH from 1.12 to 0.38 mIU/mL, which had inappropriately prompted a levothyroxine dose reduction from 50 mcg to 25 mcg. A free thyroxine (T4) was low at 0.67 ng/dL (0.89-1.76). Estradiol, FSH, and LH were undetectable. Prolactin was 23 ng/mL (3-27). She was started on prednisone, 5 mg daily, and her levothyroxine was adjusted to a weight-based dose. Her fever resolved with the initiation of prednisone, and all cultures remained negative. Over two weeks, she improved back to her baseline, was discharged to a rehabilitation center, and eventually returned home.
Dr. Inman is a hospitalist at St. Mary’s Hospital and Regional Medical Center in Grand Junction, Colo. Dr. Bridenstine is an endocrinologist at the University of Colorado Denver. Dr. Cumbler is a hospitalist at the University of Colorado Denver.
References
- Regal M, Pàramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol. 2001;55(6):735-740.
- Bouillon R. Acute adrenal insufficiency. Endocrinol Metab Clin North Am. 2006;35(4):767-75, ix.
- Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol. 1987;252(4 Pt 2):R635-644.
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066-2073.
- Melmed S, Polonski KS, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia, Pa.: Saunders/Elsevier; 2012.
- Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369(9571):1461-1470.
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metabol. 2011;96(8):2330-2340.
Case
A 53-year-old woman with a history of a suprasellar meningioma resected nine years ago with recurrence of a 4.5x2 cm mass one year ago and recent ventriculoperitoneal (VP) shunt placement for hydrocephalus presented with altered mental status (AMS) and hallucinations. She was admitted for radiation therapy to the mass. The patient had little improvement in her mental status four weeks into a six-week, 4860 cGy course of photon therapy.
The internal medicine service was consulted for new onset tachycardia (103), hypotension (83/55), and fever (38.6 C). Laboratory data revealed a white blood cell count 4.8 x 109 cells/L, sodium 137 mmol/L, potassium 4.1 mmol/L, chloride 110 mmol/L, bicarbonate 28 mmol/L, blood urea nitrogen 3 mg/dl, creatinine 0.6 mg/dl, and glucose 91 mg/dl. Thyroid-stimulating hormone (TSH) was low at 0.38 mIU/mL. Urine specific gravity was 1.006. Workups for infectious and thromboembolic diseases were unremarkable.
Discussion
Hypopituitarism is a disorder of impaired hormone production from the anterior and, less commonly, posterior pituitary gland. The condition can originate from several broad categories of diseases affecting the hypothalamus, pituitary stalk, or pituitary gland. In adults, the etiology is often from the mass effect of tumors or from treatment with surgery or radiotherapy. Other causes include vascular, infectious, infiltrative, inflammatory, and idiopathic. Well-substantiated data on the incidence and prevalence of hypopituitarism is sparse. It has an estimated prevalence of 45.5 cases per 100,000 and incidence of 4.2 cases per 100,000 per year.1
Clinical manifestations of hypopituitarism depend on the type and severity of hormone deficiency. The consequences of adrenal insufficiency (AI) range from smoldering and nonspecific findings (e.g. fatigue, lethargy, indistinct gastrointestinal symptoms, eosinophilia, fever) to full-fledged crisis (e.g. AMS, severe electrolyte abnormalities, hemodynamic compromise, shock). The presentation of central AI (i.e., arising from hypothalamic or pituitary pathology) is often more subtle than primary AI. In central AI, only glucocorticoid (GC) function is disrupted, leaving the renin-angiotensin-aldosterone system and mineralocorticoid (MC) function intact. This is in stark contrast to primary AI resulting from direct adrenal gland injury, which nearly always disrupts both GC and MC function, leading to more profound circulatory collapse and electrolyte disturbance.2
Aside from orthostatic blood pressure or possible low-grade fever, few physical exam features are associated with central AI. Hyperpigmentation is not seen due to the lack of anterior pituitary-derived melanocortins that stimulate melanocytes and induce pigmentation. As for laboratory findings, hyperkalemia is a feature of primary AI (due to hypoaldosteronism) but is not seen in central AI. Hyponatremia occurs in both types of AI and is vasopressin-mediated. Hyponatremia is more common in primary AI, resulting from appropriate vasopressin release that occurs due to hypotension. Hyponatremia also occurs in secondary AI because of increased vasopressin secretion mediated directly by hypocortisolemia.3,4
In summary, hyperpigmentation and the electrolyte pattern of hyponatremia and hyperkalemia are distinguishing clinical characteristics of primary AI, occurring in up to 90% of cases, but these features would not be expected with central AI.5
In the hospitalized patient with multiple active acute illnesses and infectious risk factors, it can be difficult to recognize the diagnosis of AI or hypopituitarism. Not only do signs and symptoms frequently overlap, but concomitant acute illness is usually a triggering event. Crisis should be suspected in the setting of unexplained fever, dehydration, or shock out of proportion to severity of current illness.5
Not surprisingly, high rates of partial or complete hypopituitarism are seen in patients following surgical removal of pituitary tumors or nearby neoplasms (e.g. craniopharyngiomas). Both surgery and radiotherapy for non-pituitary brain tumors are also major risk factors for development of hypopituitarism, occurring in up to 38% and 41% of patients, respectively.6 The strongest predictors of hormone failure are higher radiation doses, proximity to the pituitary-hypothalamus, and longer time interval after completion of radiotherapy. Within 10 years after a median dose of 5000 rad (50Gy) directed at the skull base, nasopharynx, or cranium, up to three-fourths of patients will develop some degree of pituitary insufficiency. Later onset of hormone failure usually reflects hypothalamic injury, whereas higher irradiation doses can lead to earlier onset pituitary damage.5
Not all hormone-secreting cells of the hypothalamus or pituitary are equally susceptible to injury; there is a characteristic sequence of hormonal failure. The typical order of hormone deficiency from pituitary compression or destruction is as follows: growth hormone (GH) > follicle-stimulating hormone (FSH) > luteinizing hormone (LH) > TSH > adrenocorticotropic hormone (ACTH) > vasopressin. A similar pattern is seen following brain irradiation: GH > FSH and LH > ACTH > TSH. A recent systematic review of 18 studies with 813 patients receiving cranial radiotherapy for non-pituitary tumors found pituitary dysfunction was 45% for GH deficiency, compared to 22% for ACTH deficiency.7
Biochemical diagnosis of hypopituitarism consists of measuring the various pituitary and target hormone levels as well as provocation testing. When interpreting these tests, whether to identify excess or deficient states, it is important to remember the individual values are part of the broader hypothalamic-pituitary axis feedback loops. Thus, it can be more useful designating if a high or low test value is appropriately or inappropriately high or low. In the presented case, low TSH level could be misinterpreted as excess thyroid hormone supplementation. An appropriately elevated free T4 level would confirm this, but an inappropriately low free T4 would raise suspicion of central hypothalamic-pituitary dysfunction.
With high enough clinical suspicion of hypopituitarism, empiric treatment with thyroid supplementation and corticosteroids should be started before confirmation of the diagnosis, to prevent secondary organ dysfunction and improve morbidity and mortality.2 Rapid administration with intravenous levothyroxine can be given in severe hypothyroidism or myxedema.
“Stress-dose” steroids are generally recommended for patients who are also administered levothyroxine, as the desired increased in metabolic rate can deplete existing pituitary-adrenocortical hormone reserves, precipitating adrenal crisis.5 Stress-dose corticosteroids also ensure recruitment of a mineralocorticoid response. Cortisol has both GC and MC stimulating effects but is rapidly metabolized to cortisone, which lacks MC stimulating effects. Thus, high doses overwhelm this conversion step and allow remaining cortisol to stimulate MC receptors.2 These high doses may not be necessary in secondary AI (i.e., preserved MC function) but would be reasonable in an unstable patient or until confirmation is made with an inappropriately low ACTH.
Back to the Case
Morning cortisol returned undetectable, and ACTH was 14 pg/mL (6-58). Past records revealed a down-trending TSH from 1.12 to 0.38 mIU/mL, which had inappropriately prompted a levothyroxine dose reduction from 50 mcg to 25 mcg. A free thyroxine (T4) was low at 0.67 ng/dL (0.89-1.76). Estradiol, FSH, and LH were undetectable. Prolactin was 23 ng/mL (3-27). She was started on prednisone, 5 mg daily, and her levothyroxine was adjusted to a weight-based dose. Her fever resolved with the initiation of prednisone, and all cultures remained negative. Over two weeks, she improved back to her baseline, was discharged to a rehabilitation center, and eventually returned home.
Dr. Inman is a hospitalist at St. Mary’s Hospital and Regional Medical Center in Grand Junction, Colo. Dr. Bridenstine is an endocrinologist at the University of Colorado Denver. Dr. Cumbler is a hospitalist at the University of Colorado Denver.
References
- Regal M, Pàramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol. 2001;55(6):735-740.
- Bouillon R. Acute adrenal insufficiency. Endocrinol Metab Clin North Am. 2006;35(4):767-75, ix.
- Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol. 1987;252(4 Pt 2):R635-644.
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066-2073.
- Melmed S, Polonski KS, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia, Pa.: Saunders/Elsevier; 2012.
- Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369(9571):1461-1470.
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metabol. 2011;96(8):2330-2340.
Case
A 53-year-old woman with a history of a suprasellar meningioma resected nine years ago with recurrence of a 4.5x2 cm mass one year ago and recent ventriculoperitoneal (VP) shunt placement for hydrocephalus presented with altered mental status (AMS) and hallucinations. She was admitted for radiation therapy to the mass. The patient had little improvement in her mental status four weeks into a six-week, 4860 cGy course of photon therapy.
The internal medicine service was consulted for new onset tachycardia (103), hypotension (83/55), and fever (38.6 C). Laboratory data revealed a white blood cell count 4.8 x 109 cells/L, sodium 137 mmol/L, potassium 4.1 mmol/L, chloride 110 mmol/L, bicarbonate 28 mmol/L, blood urea nitrogen 3 mg/dl, creatinine 0.6 mg/dl, and glucose 91 mg/dl. Thyroid-stimulating hormone (TSH) was low at 0.38 mIU/mL. Urine specific gravity was 1.006. Workups for infectious and thromboembolic diseases were unremarkable.
Discussion
Hypopituitarism is a disorder of impaired hormone production from the anterior and, less commonly, posterior pituitary gland. The condition can originate from several broad categories of diseases affecting the hypothalamus, pituitary stalk, or pituitary gland. In adults, the etiology is often from the mass effect of tumors or from treatment with surgery or radiotherapy. Other causes include vascular, infectious, infiltrative, inflammatory, and idiopathic. Well-substantiated data on the incidence and prevalence of hypopituitarism is sparse. It has an estimated prevalence of 45.5 cases per 100,000 and incidence of 4.2 cases per 100,000 per year.1
Clinical manifestations of hypopituitarism depend on the type and severity of hormone deficiency. The consequences of adrenal insufficiency (AI) range from smoldering and nonspecific findings (e.g. fatigue, lethargy, indistinct gastrointestinal symptoms, eosinophilia, fever) to full-fledged crisis (e.g. AMS, severe electrolyte abnormalities, hemodynamic compromise, shock). The presentation of central AI (i.e., arising from hypothalamic or pituitary pathology) is often more subtle than primary AI. In central AI, only glucocorticoid (GC) function is disrupted, leaving the renin-angiotensin-aldosterone system and mineralocorticoid (MC) function intact. This is in stark contrast to primary AI resulting from direct adrenal gland injury, which nearly always disrupts both GC and MC function, leading to more profound circulatory collapse and electrolyte disturbance.2
Aside from orthostatic blood pressure or possible low-grade fever, few physical exam features are associated with central AI. Hyperpigmentation is not seen due to the lack of anterior pituitary-derived melanocortins that stimulate melanocytes and induce pigmentation. As for laboratory findings, hyperkalemia is a feature of primary AI (due to hypoaldosteronism) but is not seen in central AI. Hyponatremia occurs in both types of AI and is vasopressin-mediated. Hyponatremia is more common in primary AI, resulting from appropriate vasopressin release that occurs due to hypotension. Hyponatremia also occurs in secondary AI because of increased vasopressin secretion mediated directly by hypocortisolemia.3,4
In summary, hyperpigmentation and the electrolyte pattern of hyponatremia and hyperkalemia are distinguishing clinical characteristics of primary AI, occurring in up to 90% of cases, but these features would not be expected with central AI.5
In the hospitalized patient with multiple active acute illnesses and infectious risk factors, it can be difficult to recognize the diagnosis of AI or hypopituitarism. Not only do signs and symptoms frequently overlap, but concomitant acute illness is usually a triggering event. Crisis should be suspected in the setting of unexplained fever, dehydration, or shock out of proportion to severity of current illness.5
Not surprisingly, high rates of partial or complete hypopituitarism are seen in patients following surgical removal of pituitary tumors or nearby neoplasms (e.g. craniopharyngiomas). Both surgery and radiotherapy for non-pituitary brain tumors are also major risk factors for development of hypopituitarism, occurring in up to 38% and 41% of patients, respectively.6 The strongest predictors of hormone failure are higher radiation doses, proximity to the pituitary-hypothalamus, and longer time interval after completion of radiotherapy. Within 10 years after a median dose of 5000 rad (50Gy) directed at the skull base, nasopharynx, or cranium, up to three-fourths of patients will develop some degree of pituitary insufficiency. Later onset of hormone failure usually reflects hypothalamic injury, whereas higher irradiation doses can lead to earlier onset pituitary damage.5
Not all hormone-secreting cells of the hypothalamus or pituitary are equally susceptible to injury; there is a characteristic sequence of hormonal failure. The typical order of hormone deficiency from pituitary compression or destruction is as follows: growth hormone (GH) > follicle-stimulating hormone (FSH) > luteinizing hormone (LH) > TSH > adrenocorticotropic hormone (ACTH) > vasopressin. A similar pattern is seen following brain irradiation: GH > FSH and LH > ACTH > TSH. A recent systematic review of 18 studies with 813 patients receiving cranial radiotherapy for non-pituitary tumors found pituitary dysfunction was 45% for GH deficiency, compared to 22% for ACTH deficiency.7
Biochemical diagnosis of hypopituitarism consists of measuring the various pituitary and target hormone levels as well as provocation testing. When interpreting these tests, whether to identify excess or deficient states, it is important to remember the individual values are part of the broader hypothalamic-pituitary axis feedback loops. Thus, it can be more useful designating if a high or low test value is appropriately or inappropriately high or low. In the presented case, low TSH level could be misinterpreted as excess thyroid hormone supplementation. An appropriately elevated free T4 level would confirm this, but an inappropriately low free T4 would raise suspicion of central hypothalamic-pituitary dysfunction.
With high enough clinical suspicion of hypopituitarism, empiric treatment with thyroid supplementation and corticosteroids should be started before confirmation of the diagnosis, to prevent secondary organ dysfunction and improve morbidity and mortality.2 Rapid administration with intravenous levothyroxine can be given in severe hypothyroidism or myxedema.
“Stress-dose” steroids are generally recommended for patients who are also administered levothyroxine, as the desired increased in metabolic rate can deplete existing pituitary-adrenocortical hormone reserves, precipitating adrenal crisis.5 Stress-dose corticosteroids also ensure recruitment of a mineralocorticoid response. Cortisol has both GC and MC stimulating effects but is rapidly metabolized to cortisone, which lacks MC stimulating effects. Thus, high doses overwhelm this conversion step and allow remaining cortisol to stimulate MC receptors.2 These high doses may not be necessary in secondary AI (i.e., preserved MC function) but would be reasonable in an unstable patient or until confirmation is made with an inappropriately low ACTH.
Back to the Case
Morning cortisol returned undetectable, and ACTH was 14 pg/mL (6-58). Past records revealed a down-trending TSH from 1.12 to 0.38 mIU/mL, which had inappropriately prompted a levothyroxine dose reduction from 50 mcg to 25 mcg. A free thyroxine (T4) was low at 0.67 ng/dL (0.89-1.76). Estradiol, FSH, and LH were undetectable. Prolactin was 23 ng/mL (3-27). She was started on prednisone, 5 mg daily, and her levothyroxine was adjusted to a weight-based dose. Her fever resolved with the initiation of prednisone, and all cultures remained negative. Over two weeks, she improved back to her baseline, was discharged to a rehabilitation center, and eventually returned home.
Dr. Inman is a hospitalist at St. Mary’s Hospital and Regional Medical Center in Grand Junction, Colo. Dr. Bridenstine is an endocrinologist at the University of Colorado Denver. Dr. Cumbler is a hospitalist at the University of Colorado Denver.
References
- Regal M, Pàramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol. 2001;55(6):735-740.
- Bouillon R. Acute adrenal insufficiency. Endocrinol Metab Clin North Am. 2006;35(4):767-75, ix.
- Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol. 1987;252(4 Pt 2):R635-644.
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066-2073.
- Melmed S, Polonski KS, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia, Pa.: Saunders/Elsevier; 2012.
- Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369(9571):1461-1470.
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metabol. 2011;96(8):2330-2340.