User login
New Therapies for Atopic Dermatitis Crowd the Pipeline
Atopic dermatitis, commonly known as eczema, affects at least 10% of adults in the industrialized world. In recent years, research has broadened our understanding of the underlying mechanisms that drive the condition, leading to identification of potential targets and development of new therapies.
Dr Mark Lebwohl, chairman of the Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York City, explains that the March 2017 approval of dupilumab for moderate to severe atopic dermatitis touched off a new era of systemic treatments. Dozens of new drugs are being investigated, many with promising trial results, giving hope to the millions of patients who suffer from the persistent itch that defines the skin condition.
In this ReCAP, Dr Lebwohl summarizes current knowledge of the pathogenesis of atopic dermatitis and highlights the most promising new therapies in the works.
--
Professor and Chairman, Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York, NY.
Mark G. Lebwohl, MD, has disclosed the following relevant financial information:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Allergan; Almirall; Arcutis Biotherapeutics; Avotres Therapeutics; BirchBioMed Inc.; Boehringer Ingelheim; Bristol-Myers Squibb Company; Cara Therapeutics; Castle Biosciences; Corrona; Dermavant Sciences; Evelo Biosciences; Foundation for Research and Education in Dermatology; Inozyme Pharma; Leo Pharma; Meiji Seika Pharma Co.; Menlo Dermatology Medical Group; Mitsubishi Tanabe Pharma America; NeuroDerm; Pfizer Inc.; Promius Pharma LLC; Dr. Reddy's Laboratories; Theravance Biopharma, Inc.; Verrica Pharmaceuticals Inc. ¬
Received research grant from: AbbVie; Amgen Inc.; Arcutis Biotherapeutics; AstraZeneca Pharmaceuticals LP; Boehringer Ingelheim; Celgene Corporation; CLINUVEL; Eli Lilly and Company; Incyte Corporation; Ortho-McNeil-Janssen Pharmaceuticals, Inc.; Kadmon Corporation; Leo Pharma; MedImmune Inc.; Novartis Pharmaceuticals Corporation; Ortho Dermatologics; Pfizer Inc.; SCIderm; UCB, Inc.; Vidac Pharma.
Atopic dermatitis, commonly known as eczema, affects at least 10% of adults in the industrialized world. In recent years, research has broadened our understanding of the underlying mechanisms that drive the condition, leading to identification of potential targets and development of new therapies.
Dr Mark Lebwohl, chairman of the Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York City, explains that the March 2017 approval of dupilumab for moderate to severe atopic dermatitis touched off a new era of systemic treatments. Dozens of new drugs are being investigated, many with promising trial results, giving hope to the millions of patients who suffer from the persistent itch that defines the skin condition.
In this ReCAP, Dr Lebwohl summarizes current knowledge of the pathogenesis of atopic dermatitis and highlights the most promising new therapies in the works.
--
Professor and Chairman, Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York, NY.
Mark G. Lebwohl, MD, has disclosed the following relevant financial information:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Allergan; Almirall; Arcutis Biotherapeutics; Avotres Therapeutics; BirchBioMed Inc.; Boehringer Ingelheim; Bristol-Myers Squibb Company; Cara Therapeutics; Castle Biosciences; Corrona; Dermavant Sciences; Evelo Biosciences; Foundation for Research and Education in Dermatology; Inozyme Pharma; Leo Pharma; Meiji Seika Pharma Co.; Menlo Dermatology Medical Group; Mitsubishi Tanabe Pharma America; NeuroDerm; Pfizer Inc.; Promius Pharma LLC; Dr. Reddy's Laboratories; Theravance Biopharma, Inc.; Verrica Pharmaceuticals Inc. ¬
Received research grant from: AbbVie; Amgen Inc.; Arcutis Biotherapeutics; AstraZeneca Pharmaceuticals LP; Boehringer Ingelheim; Celgene Corporation; CLINUVEL; Eli Lilly and Company; Incyte Corporation; Ortho-McNeil-Janssen Pharmaceuticals, Inc.; Kadmon Corporation; Leo Pharma; MedImmune Inc.; Novartis Pharmaceuticals Corporation; Ortho Dermatologics; Pfizer Inc.; SCIderm; UCB, Inc.; Vidac Pharma.
Atopic dermatitis, commonly known as eczema, affects at least 10% of adults in the industrialized world. In recent years, research has broadened our understanding of the underlying mechanisms that drive the condition, leading to identification of potential targets and development of new therapies.
Dr Mark Lebwohl, chairman of the Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York City, explains that the March 2017 approval of dupilumab for moderate to severe atopic dermatitis touched off a new era of systemic treatments. Dozens of new drugs are being investigated, many with promising trial results, giving hope to the millions of patients who suffer from the persistent itch that defines the skin condition.
In this ReCAP, Dr Lebwohl summarizes current knowledge of the pathogenesis of atopic dermatitis and highlights the most promising new therapies in the works.
--
Professor and Chairman, Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York, NY.
Mark G. Lebwohl, MD, has disclosed the following relevant financial information:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Allergan; Almirall; Arcutis Biotherapeutics; Avotres Therapeutics; BirchBioMed Inc.; Boehringer Ingelheim; Bristol-Myers Squibb Company; Cara Therapeutics; Castle Biosciences; Corrona; Dermavant Sciences; Evelo Biosciences; Foundation for Research and Education in Dermatology; Inozyme Pharma; Leo Pharma; Meiji Seika Pharma Co.; Menlo Dermatology Medical Group; Mitsubishi Tanabe Pharma America; NeuroDerm; Pfizer Inc.; Promius Pharma LLC; Dr. Reddy's Laboratories; Theravance Biopharma, Inc.; Verrica Pharmaceuticals Inc. ¬
Received research grant from: AbbVie; Amgen Inc.; Arcutis Biotherapeutics; AstraZeneca Pharmaceuticals LP; Boehringer Ingelheim; Celgene Corporation; CLINUVEL; Eli Lilly and Company; Incyte Corporation; Ortho-McNeil-Janssen Pharmaceuticals, Inc.; Kadmon Corporation; Leo Pharma; MedImmune Inc.; Novartis Pharmaceuticals Corporation; Ortho Dermatologics; Pfizer Inc.; SCIderm; UCB, Inc.; Vidac Pharma.

Apremilast and Systemic Retinoid Combination Treatment for Moderate to Severe Palmoplantar Psoriasis
To the Editor:
Psoriasis is a chronic inflammatory papulosquamous skin disease affecting 2% to 3% of the population.1 Its pathogenesis is multifactorial, consisting of a disrupted skin barrier and dysregulated immune activation.2
A wide armamentarium of topical and systemic treatments targeting different aspects of the disease pathogenesis have been developed over the years.3,4 Psoriasis was once considered a skin disease exclusively, but accumulating evidence suggests that it is accompanied by a multitude of systemic inflammatory comorbidities.5 This insight supports the concept of systemic treatment for patients with moderate to severe psoriasis. As a chronic disease, psoriasis requires continuous therapy. The treatment approach should focus on achieving efficacy and minimizing side effects. These goals can be achieved by combination, rotational, and sequential treatment approaches.6 Many therapeutic combinations have proven effective, using beneficially different mechanisms of action (MOAs) and toxicity profiles.7 We present a patient with moderate to severe recalcitrant palmoplantar psoriasis who demonstrated improvement with combination therapy.
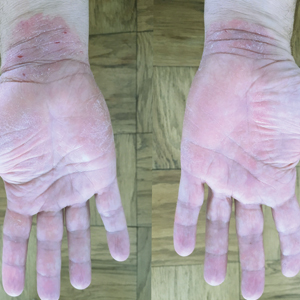
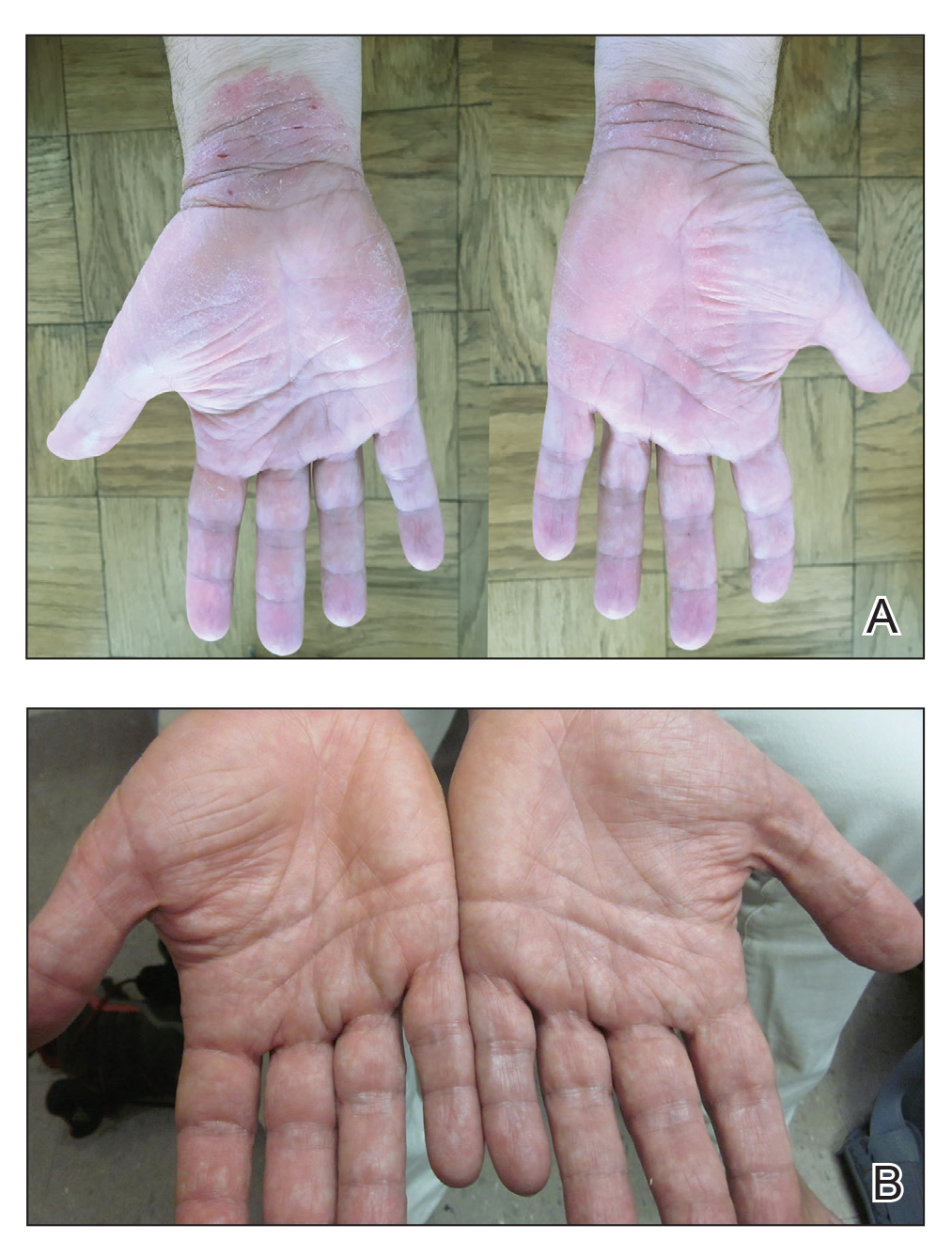
A 50-year-old man presented with palmoplantar psoriasis of 7 years’ duration. His medical history included mild hyperlipidemia treated with atorvastatin. Prior topical treatments including calcipotriene, betamethasone dipropionate, and tacrolimus ointment did not result in improvement. Persistent acral involvement required further intervention, and the excimer laser was added to the therapeutic regimen with a minor additive therapeutic value. Acitretin (25 mg/d) was initiated; however, the disease flared up soon after. Acitretin was discontinued, and the patient was treated with apremilast (30 mg twice daily) for 9 months with a slight improvement. Physical examination revealed erythematous, fissured, scaly plaques involving both the palms and soles. Acitretin (25 mg/d) was reintroduced to the therapeutic regimen, and the acitretin-apremilast combination was used for 2 months. With this regimen, the patient experienced 90% improvement (Figures 1 and 2).

Palmoplantar psoriasis is a debilitating dermatosis that is extremely challenging to treat and is unresponsive to many modalities.8 Increased understanding of psoriasis mechanisms paved the path for the development of highly targeted biologic therapies9 with fewer side effects than drugs such as cyclosporine that indiscriminately neutralize multiple components of the immune system. Although highly specific, these targeted approaches are not without side effects10 and lead to diverse therapeutic outcomes, particularly when prescribed for palmoplantar psoriasis.11,12
The small-molecule inhibitor of phosphodiesterase 4—apremilast—was approved for plaque psoriasis treatment in late 2014. Although not fully elucidated, its MOA involves interfering with intracellular signaling, leading to increased intracellular cyclic adenosine monophosphate levels in inflammatory cells and keratinocytes.13 Proximal interruption of the pathologic cascade leads to the reduction of multiple proinflammatory cytokines with a simultaneous increase in anti-inflammatory mediators.13 Its efficacy and safety in the treatment of psoriasis have been shown in phase 2 and 3 clinical trials.14,15 In contrast to traditional oral therapies for psoriasis (ie, methotrexate, cyclosporine, acitretin), no laboratory test monitoring is needed and the safety profile is notably better.16
Acitretin, the active metabolite of etretinate, modulates epidermal differentiation and has immunomodulating activities.17 It commonly is used for treating palmoplantar psoriasis.8 Until recently, it was the only nonimmunosuppressive systemic treatment for psoriasis, and its combination with other systemic treatments, particularly biologics, has been advocated.18 Prior reports showed remarkable disease improvement when combining acitretin with alefacept, etanercept, infliximab, adalimumab, and ustekinumab.19 The optimal combination should include modalities with different MOAs without overlapping toxicities.19 Apremilast and acitretin have different MOAs and side-effect profiles, but another theoretical advantage is that they both interfere with intracellular signaling on the transcription level rather than affecting extracellular targets.13
Our patient with moderate to severe recalcitrant palmoplantar psoriasis demonstrated approximately 90% improvement following apremilast and acitretin combination therapy. This treatment regimen should be considered in cases of persistent acral disease resistant to other therapeutic efforts.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3-9.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41-44.
- Lebwohl M, Menter A, Koo J, et al. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416-430.
- Cather JC, Menter A. Combining traditional agents and biologics for the treatment of psoriasis. Semin Cutan Med Surg. 2005;24:37-45.
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. J Eur Acad Dermatol Venereol. 2013;27:E384-E389.
- Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis [published online December 29, 2015]. Dermatol Ther (Heidelb). 2015;6:1-12.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Jacobi A, Schuler G, Hertl M. Differential clinical response to alefacept in combination with methotrexate in two patients with refractory palmar psoriasis. Br J Dermatol. 2007;156:178-180.
- Meyer V, Goerge T, Luger TA, et al. Successful treatment of palmoplantar hyperkeratotic psoriasis with a combination of etanercept and alitretinoin. J Clin Aesthet Dermatol. 2011;4:45-46.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Pilkington T, Brogden RN. Acitretin—a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003;49:S118-S124.
- Heinecke GM, Luber AJ, Levitt JO, et al. Combination use of ustekinumab with other systemic therapies: a retrospective study in a tertiary referral center. J Drugs Dermatol. 2013;12:1098-1102.
To the Editor:
Psoriasis is a chronic inflammatory papulosquamous skin disease affecting 2% to 3% of the population.1 Its pathogenesis is multifactorial, consisting of a disrupted skin barrier and dysregulated immune activation.2
A wide armamentarium of topical and systemic treatments targeting different aspects of the disease pathogenesis have been developed over the years.3,4 Psoriasis was once considered a skin disease exclusively, but accumulating evidence suggests that it is accompanied by a multitude of systemic inflammatory comorbidities.5 This insight supports the concept of systemic treatment for patients with moderate to severe psoriasis. As a chronic disease, psoriasis requires continuous therapy. The treatment approach should focus on achieving efficacy and minimizing side effects. These goals can be achieved by combination, rotational, and sequential treatment approaches.6 Many therapeutic combinations have proven effective, using beneficially different mechanisms of action (MOAs) and toxicity profiles.7 We present a patient with moderate to severe recalcitrant palmoplantar psoriasis who demonstrated improvement with combination therapy.
A 50-year-old man presented with palmoplantar psoriasis of 7 years’ duration. His medical history included mild hyperlipidemia treated with atorvastatin. Prior topical treatments including calcipotriene, betamethasone dipropionate, and tacrolimus ointment did not result in improvement. Persistent acral involvement required further intervention, and the excimer laser was added to the therapeutic regimen with a minor additive therapeutic value. Acitretin (25 mg/d) was initiated; however, the disease flared up soon after. Acitretin was discontinued, and the patient was treated with apremilast (30 mg twice daily) for 9 months with a slight improvement. Physical examination revealed erythematous, fissured, scaly plaques involving both the palms and soles. Acitretin (25 mg/d) was reintroduced to the therapeutic regimen, and the acitretin-apremilast combination was used for 2 months. With this regimen, the patient experienced 90% improvement (Figures 1 and 2).


Palmoplantar psoriasis is a debilitating dermatosis that is extremely challenging to treat and is unresponsive to many modalities.8 Increased understanding of psoriasis mechanisms paved the path for the development of highly targeted biologic therapies9 with fewer side effects than drugs such as cyclosporine that indiscriminately neutralize multiple components of the immune system. Although highly specific, these targeted approaches are not without side effects10 and lead to diverse therapeutic outcomes, particularly when prescribed for palmoplantar psoriasis.11,12
The small-molecule inhibitor of phosphodiesterase 4—apremilast—was approved for plaque psoriasis treatment in late 2014. Although not fully elucidated, its MOA involves interfering with intracellular signaling, leading to increased intracellular cyclic adenosine monophosphate levels in inflammatory cells and keratinocytes.13 Proximal interruption of the pathologic cascade leads to the reduction of multiple proinflammatory cytokines with a simultaneous increase in anti-inflammatory mediators.13 Its efficacy and safety in the treatment of psoriasis have been shown in phase 2 and 3 clinical trials.14,15 In contrast to traditional oral therapies for psoriasis (ie, methotrexate, cyclosporine, acitretin), no laboratory test monitoring is needed and the safety profile is notably better.16
Acitretin, the active metabolite of etretinate, modulates epidermal differentiation and has immunomodulating activities.17 It commonly is used for treating palmoplantar psoriasis.8 Until recently, it was the only nonimmunosuppressive systemic treatment for psoriasis, and its combination with other systemic treatments, particularly biologics, has been advocated.18 Prior reports showed remarkable disease improvement when combining acitretin with alefacept, etanercept, infliximab, adalimumab, and ustekinumab.19 The optimal combination should include modalities with different MOAs without overlapping toxicities.19 Apremilast and acitretin have different MOAs and side-effect profiles, but another theoretical advantage is that they both interfere with intracellular signaling on the transcription level rather than affecting extracellular targets.13
Our patient with moderate to severe recalcitrant palmoplantar psoriasis demonstrated approximately 90% improvement following apremilast and acitretin combination therapy. This treatment regimen should be considered in cases of persistent acral disease resistant to other therapeutic efforts.
To the Editor:
Psoriasis is a chronic inflammatory papulosquamous skin disease affecting 2% to 3% of the population.1 Its pathogenesis is multifactorial, consisting of a disrupted skin barrier and dysregulated immune activation.2
A wide armamentarium of topical and systemic treatments targeting different aspects of the disease pathogenesis have been developed over the years.3,4 Psoriasis was once considered a skin disease exclusively, but accumulating evidence suggests that it is accompanied by a multitude of systemic inflammatory comorbidities.5 This insight supports the concept of systemic treatment for patients with moderate to severe psoriasis. As a chronic disease, psoriasis requires continuous therapy. The treatment approach should focus on achieving efficacy and minimizing side effects. These goals can be achieved by combination, rotational, and sequential treatment approaches.6 Many therapeutic combinations have proven effective, using beneficially different mechanisms of action (MOAs) and toxicity profiles.7 We present a patient with moderate to severe recalcitrant palmoplantar psoriasis who demonstrated improvement with combination therapy.
A 50-year-old man presented with palmoplantar psoriasis of 7 years’ duration. His medical history included mild hyperlipidemia treated with atorvastatin. Prior topical treatments including calcipotriene, betamethasone dipropionate, and tacrolimus ointment did not result in improvement. Persistent acral involvement required further intervention, and the excimer laser was added to the therapeutic regimen with a minor additive therapeutic value. Acitretin (25 mg/d) was initiated; however, the disease flared up soon after. Acitretin was discontinued, and the patient was treated with apremilast (30 mg twice daily) for 9 months with a slight improvement. Physical examination revealed erythematous, fissured, scaly plaques involving both the palms and soles. Acitretin (25 mg/d) was reintroduced to the therapeutic regimen, and the acitretin-apremilast combination was used for 2 months. With this regimen, the patient experienced 90% improvement (Figures 1 and 2).


Palmoplantar psoriasis is a debilitating dermatosis that is extremely challenging to treat and is unresponsive to many modalities.8 Increased understanding of psoriasis mechanisms paved the path for the development of highly targeted biologic therapies9 with fewer side effects than drugs such as cyclosporine that indiscriminately neutralize multiple components of the immune system. Although highly specific, these targeted approaches are not without side effects10 and lead to diverse therapeutic outcomes, particularly when prescribed for palmoplantar psoriasis.11,12
The small-molecule inhibitor of phosphodiesterase 4—apremilast—was approved for plaque psoriasis treatment in late 2014. Although not fully elucidated, its MOA involves interfering with intracellular signaling, leading to increased intracellular cyclic adenosine monophosphate levels in inflammatory cells and keratinocytes.13 Proximal interruption of the pathologic cascade leads to the reduction of multiple proinflammatory cytokines with a simultaneous increase in anti-inflammatory mediators.13 Its efficacy and safety in the treatment of psoriasis have been shown in phase 2 and 3 clinical trials.14,15 In contrast to traditional oral therapies for psoriasis (ie, methotrexate, cyclosporine, acitretin), no laboratory test monitoring is needed and the safety profile is notably better.16
Acitretin, the active metabolite of etretinate, modulates epidermal differentiation and has immunomodulating activities.17 It commonly is used for treating palmoplantar psoriasis.8 Until recently, it was the only nonimmunosuppressive systemic treatment for psoriasis, and its combination with other systemic treatments, particularly biologics, has been advocated.18 Prior reports showed remarkable disease improvement when combining acitretin with alefacept, etanercept, infliximab, adalimumab, and ustekinumab.19 The optimal combination should include modalities with different MOAs without overlapping toxicities.19 Apremilast and acitretin have different MOAs and side-effect profiles, but another theoretical advantage is that they both interfere with intracellular signaling on the transcription level rather than affecting extracellular targets.13
Our patient with moderate to severe recalcitrant palmoplantar psoriasis demonstrated approximately 90% improvement following apremilast and acitretin combination therapy. This treatment regimen should be considered in cases of persistent acral disease resistant to other therapeutic efforts.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3-9.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41-44.
- Lebwohl M, Menter A, Koo J, et al. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416-430.
- Cather JC, Menter A. Combining traditional agents and biologics for the treatment of psoriasis. Semin Cutan Med Surg. 2005;24:37-45.
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. J Eur Acad Dermatol Venereol. 2013;27:E384-E389.
- Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis [published online December 29, 2015]. Dermatol Ther (Heidelb). 2015;6:1-12.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Jacobi A, Schuler G, Hertl M. Differential clinical response to alefacept in combination with methotrexate in two patients with refractory palmar psoriasis. Br J Dermatol. 2007;156:178-180.
- Meyer V, Goerge T, Luger TA, et al. Successful treatment of palmoplantar hyperkeratotic psoriasis with a combination of etanercept and alitretinoin. J Clin Aesthet Dermatol. 2011;4:45-46.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Pilkington T, Brogden RN. Acitretin—a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003;49:S118-S124.
- Heinecke GM, Luber AJ, Levitt JO, et al. Combination use of ustekinumab with other systemic therapies: a retrospective study in a tertiary referral center. J Drugs Dermatol. 2013;12:1098-1102.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3-9.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41-44.
- Lebwohl M, Menter A, Koo J, et al. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416-430.
- Cather JC, Menter A. Combining traditional agents and biologics for the treatment of psoriasis. Semin Cutan Med Surg. 2005;24:37-45.
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. J Eur Acad Dermatol Venereol. 2013;27:E384-E389.
- Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis [published online December 29, 2015]. Dermatol Ther (Heidelb). 2015;6:1-12.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Jacobi A, Schuler G, Hertl M. Differential clinical response to alefacept in combination with methotrexate in two patients with refractory palmar psoriasis. Br J Dermatol. 2007;156:178-180.
- Meyer V, Goerge T, Luger TA, et al. Successful treatment of palmoplantar hyperkeratotic psoriasis with a combination of etanercept and alitretinoin. J Clin Aesthet Dermatol. 2011;4:45-46.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Pilkington T, Brogden RN. Acitretin—a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003;49:S118-S124.
- Heinecke GM, Luber AJ, Levitt JO, et al. Combination use of ustekinumab with other systemic therapies: a retrospective study in a tertiary referral center. J Drugs Dermatol. 2013;12:1098-1102.
Practice Points
- Palmoplantar psoriasis is challenging to treat and is unresponsive to many modalities.
- Combination, rotational, and sequential treatment approaches may minimize side effects and loss of efficacy as well as enhance treatment responses.
- Apremilast and acitretin combination therapy led to 90% skin improvement in a case of severe recalcitrant palmoplantar psoriasis.
Melkersson-Rosenthal Syndrome Successfully Treated With Adalimumab
Melkersson-Rosenthal syndrome (MRS) is a rare condition comprised of unilateral peripheral facial nerve palsy, episodic or progressive facial edema, and lingua plicata (also known as fissured tongue). Melkersson-Rosenthal syndrome is a subtype of orofacial granulomatosis and often is mistaken for angioedema or pseudoangioedema due to the swelling of the lips and eyelids. We present a case of MRS that cleared in response to adalimumab therapy.
Case Report
A 69-year-old woman presented to our dermatology clinic with facial edema and a fissured tongue of 4 years’ duration. These symptoms had failed to improve with doxycycline, tacrolimus ointment 0.1%, and cortisone injections of the upper lip, as well as a balsam-free diet, fragrance-free skin products, and flavor-free toothpaste prescribed by multiple physicians over 4 years. Two weeks prior to the current presentation the patient developed left facial nerve palsy that was diagnosed by an outside physician as Bell palsy, and the patient completed a 7-day course of prednisone 1 day prior to presentation. The patient’s medical history was remarkable for type 2 diabetes mellitus controlled with metformin, hyperlipidemia controlled with ezetimibe-simvastatin, and psoriasis. She reported no family history of autoimmune or dermatologic disorders and denied any fever, unintentional weight loss, nausea, vomiting, or diarrhea.
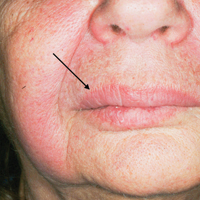
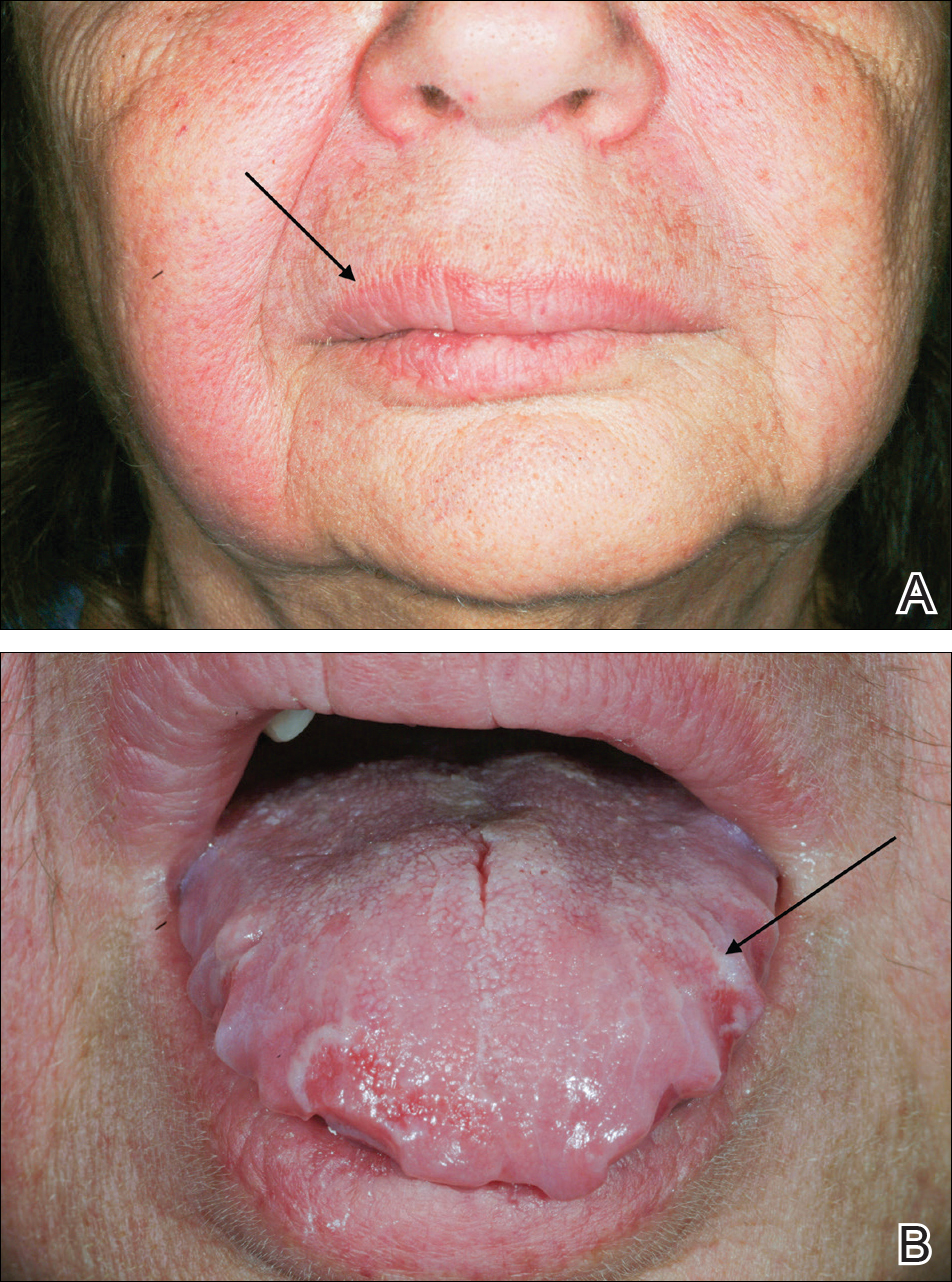
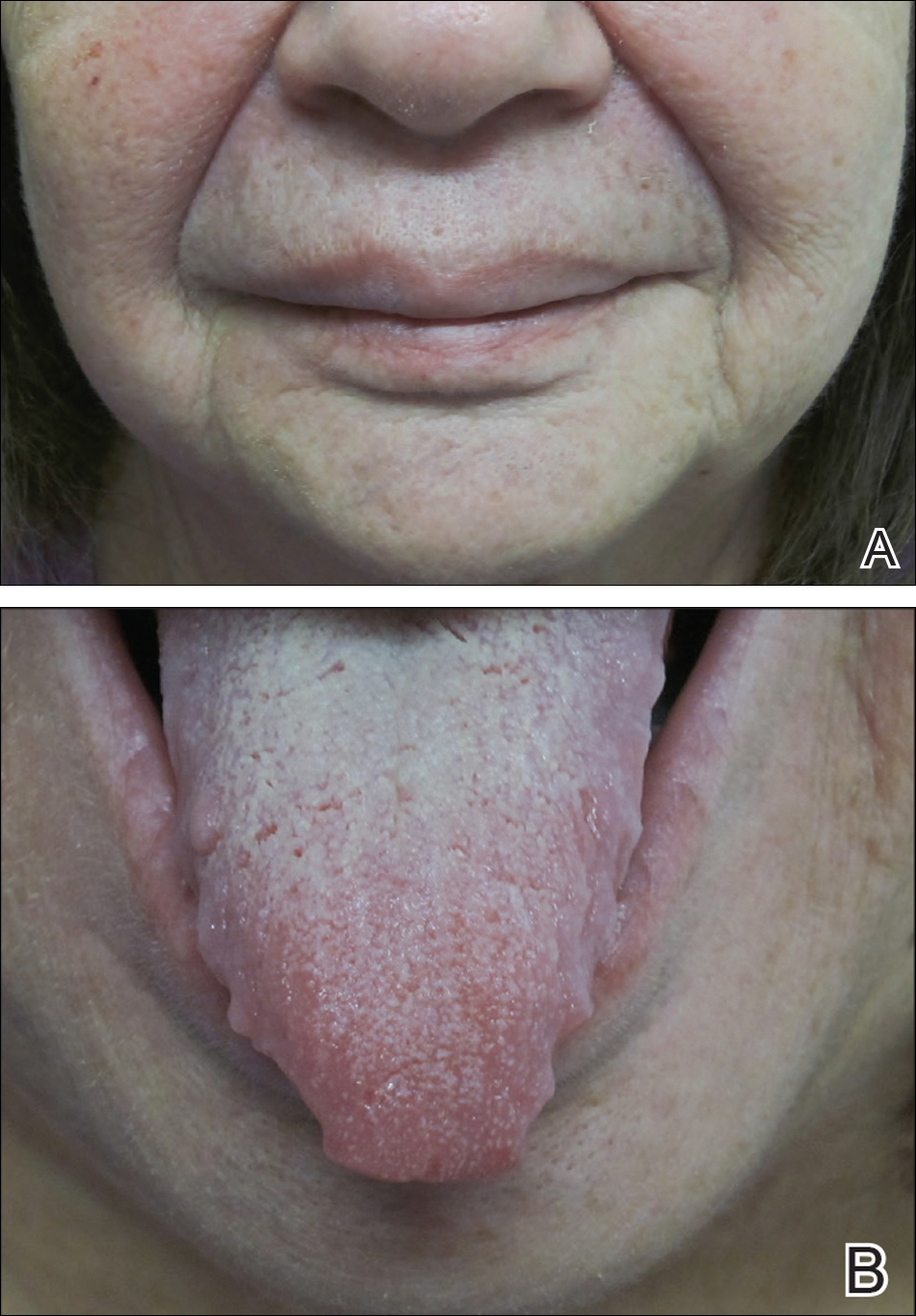
On physical examination, the patient had considerable perioral edema and erythema without warmth or tenderness (Figure 1A). The tongue was fissured with notable scalloping at the lateral margins (Figure 1B). There were no aphthous ulcers or lymphadenopathy, and the remainder of the neurologic examination was normal. The patient had erythematous plaques with scaling on the bilateral elbows. Cardiopulmonary, musculoskeletal, and abdominal examinations were otherwise normal.
Laboratory data revealed an elevated white blood cell count of 17,500/µL (reference range, 4500–11,000/µL), an elevated absolute neutrophil count of 14,018/µL (reference range, 0–700/µL), and an absolute eosinophil count of 0/µL (reference range, 0–450/µL), with the rest of the complete blood cell count within reference range. A basic metabolic panel showed an elevated glucose level of 326 mg/dL (reference range, 70–110 mg/dL), consistent with diabetes and most likely exacerbated by the recent steroid course. A lipid panel was consistent with diagnosed hyperlipidemia (total cholesterol, 236 mg/dL [reference range, <200 mg/dL]; low-density lipoprotein, 134 mg/dL [reference range, 10–30 mg/dL]; triglycerides, 188 mg/dL [reference range, <160 mg/dL]). Hepatitis B and C tests were negative. A punch biopsy of the buccal and labial mucosa was taken, revealing a parakeratinized stratified squamous epithelium with an unusual pattern of surface keratinization with foci of intracellular and extracellular edema in the spinous layer. The underlying fibrous connective tissue was edematous with infiltrates of lymphocytes, mast cells, macrophages, and a few plasma cells. The pathology report listed the diagnosis as nonspecific “chronic mucositis,” with a list of differential diagnoses that included angioedema, hypersensitivity reaction, or other possible autoimmune disorders.
On consideration of these differential diagnoses, it was felt most likely to be MRS, which remains a primarily clinical diagnosis characterized by the triad of symptoms seen in this patient. Treatment of this condition emphasizes inflammation, and steroid therapy often is utilized, as it was in our patient. After the diagnosis of MRS was made, the patient received adalimumab 80 mg subcutaneously on day 1 and 40 mg on day 8 as a loading dose; she subsequently began a course of subcutaneous injections of adalimumab 40 mg once every other week for treatment of psoriasis with the goal of simultaneously treating the MRS. The symptoms did not completely resolve at this dose, so it was increased to 40 mg once weekly. The patient reported that the facial edema, lingua plicata, and facial nerve palsy resolved concomitantly over approximately 3 months with greater improvement at 5 months (Figure 2). The patient has had no relapses as of the last follow-up at 11 months.
Comment
Melkersson-Rosenthal syndrome usually presents sporadically, though there are reports of familial association,1-3 and only 8% to 25% of patients worldwide present with the complete triad of symptoms.4 The pathogenesis of the syndrome is controversial. Granulomatous changes have been found in patients experiencing chronic edema. However, according to Zimmer et al5 in a study of 42 MRS patients, only 46% (19/42) had granulomatous changes; 36% (15/42) had nonspecific inflammation, 11% (5/42) had incidental findings, and 7% (3/42) showed no histopathologic abnormalities. Granulomatous cheilitis is a subtype of orofacial granulomatosis, an idiopathic process that causes swelling of the face and lips as well as intraoral swelling and ulceration. Orofacial granulomatosis is referred to as granulomatous cheilitis when the lip is involved. Melkersson-Rosenthal syndrome is another subtype of orofacial granulomatosis that includes facial palsy and fissured tongue.6,7
In a clinical study of 7 patients with MRS, Liu and Yu1 found 3 (42%) patients to have dysarthria, dysphagia, and tongue muscle atrophy; 1 patient to have migrainelike headaches; 1 patient to have decreased vision and an ocular movement disorder; 1 patient to have ipsilateral hearing loss; and 1 patient to lack any other symptoms. Halevy et al8 suggested a possible association of MRS with psoriasis. In their review of 12 patients, 1 (8%) had psoriatic arthritis, 2 (17%) had skin biopsy–proven psoriasis, and 3 (25%) had a family history of psoriasis.8 Because the disease is quite rare, it is difficult to determine other symptoms that may be associated with the disease.
Tumor necrosis factor α (TNF-α) is needed for granuloma formation, and TNF-α antagonists have been used to treat a number of granulomatous conditions including Crohn disease and sarcoidosis.9-11 Two case reports indicate that infliximab, a mouse/human chimeric monoclonal antibody to TNF-α, has been used successfully to clear MRS.12,13 One report cited the use of adalimumab for maintenance therapy of MRS,12 and more recently, adalimumab has been reported for refractory MRS.14 However, there currently are no known reports regarding the efficacy of adalimumab as a first-line treatment of MRS.
Adalimumab is a fully human monoclonal antibody to TNF-α, which is administered via subcutaneous injections. Infliximab must be administered at an infusion center, making treatment logistically more difficult for patients, and can be associated with the development of infusion reactions, though the exact data on infusion reactions are difficult to estimate due to variations in reporting.15,16
In 2014, Stein et al
Conclusion
We present a case of a 69-year-old woman who presented with facial nerve palsy, facial edema, and a fissured tongue, which is the classic triad of MRS, and all 3 symptoms improved with adalimumab.
- Liu R, Yu S. Melk
ersson-Rosenthal syndrome: a review of seven patients [published online May 7, 2013]. J Clin Neurosci. 2013;20:993-995. - Sun B, Zhou C, Han Z. Facial palsy in Melkersson-Rosenthal syndrome and Bell’s palsy: familial history and recurrence tendency [published online August 13, 2014]. Ann Otol Rhinol Laryngol. 2015;124:107-109.
- Meisel-Stosiek M, Hornstein OP, Stosiek N. Family study on Melkersson-Rosenthal syndrome. some hereditary aspects of the disease and review of literature. Acta Derm Venereol. 1990;70:221-226.
- Sciubba JJ, Said-Al-Naief N. Orofacial granulomatosis: presentation, pathology and management of 13 cases. J Oral Pathol Med. 2003;32:576-585.
- Zimmer WM, Rogers
RS 3rd, Reeve CM, et al. Orofacial manifestations of Melkersson-Rosenthal syndrome. a study of 42 patients and review of 220 cases from the literature. Oral Surg Oral Med Oral Pathol. 1992;74:610-619. - Critchlow WA, Cha
ng D. Cheilitis granulomatosa: a review [published online September 22, 2013]. Head Neck Pathol. 2014;8:209-213. - Allen CM, Camisa
C. Oral disease. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012:1157-1160. - Halevy S, Shalom
G, Trattner A, et al. Melkersson-Rosenthal syndrome: a possible association with psoriasis. J Am Acad Dermatol. 2012;67:795-796. - Algood HM, Lin P
L, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(suppl 3):S189-S193. - Yee AM, Pochapin
MB. Treatment of complicated sarcoidosis with infliximab anti-tumor necrosis factor-alpha therapy. Ann Intern Med. 2001;135:27-31. - Targan SR, Hanau
er SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. - Kakimoto C, Spar
ks C, White AA. Melkersson-Rosenthal syndrome: a form of pseudoangioedema. Ann Allergy Asthma Immunol. 2007;99:185-189. - Wickramasinghe N
, Gunasekara CN, Fernando WS, et al. Vulvitis granulomatosa, Melkersson-Rosenthal syndrome, and Crohn’s disease: dramatic response to infliximab therapy. Int J Dermatol. 2012;51:966-968. - Stein J, Paulke
A, Schacher B, et al. An extraordinary form of the Melkersson-Rosenthal syndrome successfully treated with the tumour necrosis factor-α blocker adalimumab [published online May 14, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204674. - Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315-1324.
- Choquette D, Faraawi R, Chow A, et al. Incidence and management of infusion reactions to infliximab in a prospective real-world community registry. J Rheumatol. 2015;42:1105-1111.
- Ruiz Villaverde R, Sánchez Cano D. Successful treatment of granulomatous cheilitis with adalimumab. Int J Dermatol. 2012;51:118-120.
Melkersson-Rosenthal syndrome (MRS) is a rare condition comprised of unilateral peripheral facial nerve palsy, episodic or progressive facial edema, and lingua plicata (also known as fissured tongue). Melkersson-Rosenthal syndrome is a subtype of orofacial granulomatosis and often is mistaken for angioedema or pseudoangioedema due to the swelling of the lips and eyelids. We present a case of MRS that cleared in response to adalimumab therapy.
Case Report
A 69-year-old woman presented to our dermatology clinic with facial edema and a fissured tongue of 4 years’ duration. These symptoms had failed to improve with doxycycline, tacrolimus ointment 0.1%, and cortisone injections of the upper lip, as well as a balsam-free diet, fragrance-free skin products, and flavor-free toothpaste prescribed by multiple physicians over 4 years. Two weeks prior to the current presentation the patient developed left facial nerve palsy that was diagnosed by an outside physician as Bell palsy, and the patient completed a 7-day course of prednisone 1 day prior to presentation. The patient’s medical history was remarkable for type 2 diabetes mellitus controlled with metformin, hyperlipidemia controlled with ezetimibe-simvastatin, and psoriasis. She reported no family history of autoimmune or dermatologic disorders and denied any fever, unintentional weight loss, nausea, vomiting, or diarrhea.
On physical examination, the patient had considerable perioral edema and erythema without warmth or tenderness (Figure 1A). The tongue was fissured with notable scalloping at the lateral margins (Figure 1B). There were no aphthous ulcers or lymphadenopathy, and the remainder of the neurologic examination was normal. The patient had erythematous plaques with scaling on the bilateral elbows. Cardiopulmonary, musculoskeletal, and abdominal examinations were otherwise normal.

Laboratory data revealed an elevated white blood cell count of 17,500/µL (reference range, 4500–11,000/µL), an elevated absolute neutrophil count of 14,018/µL (reference range, 0–700/µL), and an absolute eosinophil count of 0/µL (reference range, 0–450/µL), with the rest of the complete blood cell count within reference range. A basic metabolic panel showed an elevated glucose level of 326 mg/dL (reference range, 70–110 mg/dL), consistent with diabetes and most likely exacerbated by the recent steroid course. A lipid panel was consistent with diagnosed hyperlipidemia (total cholesterol, 236 mg/dL [reference range, <200 mg/dL]; low-density lipoprotein, 134 mg/dL [reference range, 10–30 mg/dL]; triglycerides, 188 mg/dL [reference range, <160 mg/dL]). Hepatitis B and C tests were negative. A punch biopsy of the buccal and labial mucosa was taken, revealing a parakeratinized stratified squamous epithelium with an unusual pattern of surface keratinization with foci of intracellular and extracellular edema in the spinous layer. The underlying fibrous connective tissue was edematous with infiltrates of lymphocytes, mast cells, macrophages, and a few plasma cells. The pathology report listed the diagnosis as nonspecific “chronic mucositis,” with a list of differential diagnoses that included angioedema, hypersensitivity reaction, or other possible autoimmune disorders.
On consideration of these differential diagnoses, it was felt most likely to be MRS, which remains a primarily clinical diagnosis characterized by the triad of symptoms seen in this patient. Treatment of this condition emphasizes inflammation, and steroid therapy often is utilized, as it was in our patient. After the diagnosis of MRS was made, the patient received adalimumab 80 mg subcutaneously on day 1 and 40 mg on day 8 as a loading dose; she subsequently began a course of subcutaneous injections of adalimumab 40 mg once every other week for treatment of psoriasis with the goal of simultaneously treating the MRS. The symptoms did not completely resolve at this dose, so it was increased to 40 mg once weekly. The patient reported that the facial edema, lingua plicata, and facial nerve palsy resolved concomitantly over approximately 3 months with greater improvement at 5 months (Figure 2). The patient has had no relapses as of the last follow-up at 11 months.

Comment
Melkersson-Rosenthal syndrome usually presents sporadically, though there are reports of familial association,1-3 and only 8% to 25% of patients worldwide present with the complete triad of symptoms.4 The pathogenesis of the syndrome is controversial. Granulomatous changes have been found in patients experiencing chronic edema. However, according to Zimmer et al5 in a study of 42 MRS patients, only 46% (19/42) had granulomatous changes; 36% (15/42) had nonspecific inflammation, 11% (5/42) had incidental findings, and 7% (3/42) showed no histopathologic abnormalities. Granulomatous cheilitis is a subtype of orofacial granulomatosis, an idiopathic process that causes swelling of the face and lips as well as intraoral swelling and ulceration. Orofacial granulomatosis is referred to as granulomatous cheilitis when the lip is involved. Melkersson-Rosenthal syndrome is another subtype of orofacial granulomatosis that includes facial palsy and fissured tongue.6,7
In a clinical study of 7 patients with MRS, Liu and Yu1 found 3 (42%) patients to have dysarthria, dysphagia, and tongue muscle atrophy; 1 patient to have migrainelike headaches; 1 patient to have decreased vision and an ocular movement disorder; 1 patient to have ipsilateral hearing loss; and 1 patient to lack any other symptoms. Halevy et al8 suggested a possible association of MRS with psoriasis. In their review of 12 patients, 1 (8%) had psoriatic arthritis, 2 (17%) had skin biopsy–proven psoriasis, and 3 (25%) had a family history of psoriasis.8 Because the disease is quite rare, it is difficult to determine other symptoms that may be associated with the disease.
Tumor necrosis factor α (TNF-α) is needed for granuloma formation, and TNF-α antagonists have been used to treat a number of granulomatous conditions including Crohn disease and sarcoidosis.9-11 Two case reports indicate that infliximab, a mouse/human chimeric monoclonal antibody to TNF-α, has been used successfully to clear MRS.12,13 One report cited the use of adalimumab for maintenance therapy of MRS,12 and more recently, adalimumab has been reported for refractory MRS.14 However, there currently are no known reports regarding the efficacy of adalimumab as a first-line treatment of MRS.
Adalimumab is a fully human monoclonal antibody to TNF-α, which is administered via subcutaneous injections. Infliximab must be administered at an infusion center, making treatment logistically more difficult for patients, and can be associated with the development of infusion reactions, though the exact data on infusion reactions are difficult to estimate due to variations in reporting.15,16
In 2014, Stein et al
Conclusion
We present a case of a 69-year-old woman who presented with facial nerve palsy, facial edema, and a fissured tongue, which is the classic triad of MRS, and all 3 symptoms improved with adalimumab.
Melkersson-Rosenthal syndrome (MRS) is a rare condition comprised of unilateral peripheral facial nerve palsy, episodic or progressive facial edema, and lingua plicata (also known as fissured tongue). Melkersson-Rosenthal syndrome is a subtype of orofacial granulomatosis and often is mistaken for angioedema or pseudoangioedema due to the swelling of the lips and eyelids. We present a case of MRS that cleared in response to adalimumab therapy.
Case Report
A 69-year-old woman presented to our dermatology clinic with facial edema and a fissured tongue of 4 years’ duration. These symptoms had failed to improve with doxycycline, tacrolimus ointment 0.1%, and cortisone injections of the upper lip, as well as a balsam-free diet, fragrance-free skin products, and flavor-free toothpaste prescribed by multiple physicians over 4 years. Two weeks prior to the current presentation the patient developed left facial nerve palsy that was diagnosed by an outside physician as Bell palsy, and the patient completed a 7-day course of prednisone 1 day prior to presentation. The patient’s medical history was remarkable for type 2 diabetes mellitus controlled with metformin, hyperlipidemia controlled with ezetimibe-simvastatin, and psoriasis. She reported no family history of autoimmune or dermatologic disorders and denied any fever, unintentional weight loss, nausea, vomiting, or diarrhea.
On physical examination, the patient had considerable perioral edema and erythema without warmth or tenderness (Figure 1A). The tongue was fissured with notable scalloping at the lateral margins (Figure 1B). There were no aphthous ulcers or lymphadenopathy, and the remainder of the neurologic examination was normal. The patient had erythematous plaques with scaling on the bilateral elbows. Cardiopulmonary, musculoskeletal, and abdominal examinations were otherwise normal.

Laboratory data revealed an elevated white blood cell count of 17,500/µL (reference range, 4500–11,000/µL), an elevated absolute neutrophil count of 14,018/µL (reference range, 0–700/µL), and an absolute eosinophil count of 0/µL (reference range, 0–450/µL), with the rest of the complete blood cell count within reference range. A basic metabolic panel showed an elevated glucose level of 326 mg/dL (reference range, 70–110 mg/dL), consistent with diabetes and most likely exacerbated by the recent steroid course. A lipid panel was consistent with diagnosed hyperlipidemia (total cholesterol, 236 mg/dL [reference range, <200 mg/dL]; low-density lipoprotein, 134 mg/dL [reference range, 10–30 mg/dL]; triglycerides, 188 mg/dL [reference range, <160 mg/dL]). Hepatitis B and C tests were negative. A punch biopsy of the buccal and labial mucosa was taken, revealing a parakeratinized stratified squamous epithelium with an unusual pattern of surface keratinization with foci of intracellular and extracellular edema in the spinous layer. The underlying fibrous connective tissue was edematous with infiltrates of lymphocytes, mast cells, macrophages, and a few plasma cells. The pathology report listed the diagnosis as nonspecific “chronic mucositis,” with a list of differential diagnoses that included angioedema, hypersensitivity reaction, or other possible autoimmune disorders.
On consideration of these differential diagnoses, it was felt most likely to be MRS, which remains a primarily clinical diagnosis characterized by the triad of symptoms seen in this patient. Treatment of this condition emphasizes inflammation, and steroid therapy often is utilized, as it was in our patient. After the diagnosis of MRS was made, the patient received adalimumab 80 mg subcutaneously on day 1 and 40 mg on day 8 as a loading dose; she subsequently began a course of subcutaneous injections of adalimumab 40 mg once every other week for treatment of psoriasis with the goal of simultaneously treating the MRS. The symptoms did not completely resolve at this dose, so it was increased to 40 mg once weekly. The patient reported that the facial edema, lingua plicata, and facial nerve palsy resolved concomitantly over approximately 3 months with greater improvement at 5 months (Figure 2). The patient has had no relapses as of the last follow-up at 11 months.

Comment
Melkersson-Rosenthal syndrome usually presents sporadically, though there are reports of familial association,1-3 and only 8% to 25% of patients worldwide present with the complete triad of symptoms.4 The pathogenesis of the syndrome is controversial. Granulomatous changes have been found in patients experiencing chronic edema. However, according to Zimmer et al5 in a study of 42 MRS patients, only 46% (19/42) had granulomatous changes; 36% (15/42) had nonspecific inflammation, 11% (5/42) had incidental findings, and 7% (3/42) showed no histopathologic abnormalities. Granulomatous cheilitis is a subtype of orofacial granulomatosis, an idiopathic process that causes swelling of the face and lips as well as intraoral swelling and ulceration. Orofacial granulomatosis is referred to as granulomatous cheilitis when the lip is involved. Melkersson-Rosenthal syndrome is another subtype of orofacial granulomatosis that includes facial palsy and fissured tongue.6,7
In a clinical study of 7 patients with MRS, Liu and Yu1 found 3 (42%) patients to have dysarthria, dysphagia, and tongue muscle atrophy; 1 patient to have migrainelike headaches; 1 patient to have decreased vision and an ocular movement disorder; 1 patient to have ipsilateral hearing loss; and 1 patient to lack any other symptoms. Halevy et al8 suggested a possible association of MRS with psoriasis. In their review of 12 patients, 1 (8%) had psoriatic arthritis, 2 (17%) had skin biopsy–proven psoriasis, and 3 (25%) had a family history of psoriasis.8 Because the disease is quite rare, it is difficult to determine other symptoms that may be associated with the disease.
Tumor necrosis factor α (TNF-α) is needed for granuloma formation, and TNF-α antagonists have been used to treat a number of granulomatous conditions including Crohn disease and sarcoidosis.9-11 Two case reports indicate that infliximab, a mouse/human chimeric monoclonal antibody to TNF-α, has been used successfully to clear MRS.12,13 One report cited the use of adalimumab for maintenance therapy of MRS,12 and more recently, adalimumab has been reported for refractory MRS.14 However, there currently are no known reports regarding the efficacy of adalimumab as a first-line treatment of MRS.
Adalimumab is a fully human monoclonal antibody to TNF-α, which is administered via subcutaneous injections. Infliximab must be administered at an infusion center, making treatment logistically more difficult for patients, and can be associated with the development of infusion reactions, though the exact data on infusion reactions are difficult to estimate due to variations in reporting.15,16
In 2014, Stein et al
Conclusion
We present a case of a 69-year-old woman who presented with facial nerve palsy, facial edema, and a fissured tongue, which is the classic triad of MRS, and all 3 symptoms improved with adalimumab.
- Liu R, Yu S. Melk
ersson-Rosenthal syndrome: a review of seven patients [published online May 7, 2013]. J Clin Neurosci. 2013;20:993-995. - Sun B, Zhou C, Han Z. Facial palsy in Melkersson-Rosenthal syndrome and Bell’s palsy: familial history and recurrence tendency [published online August 13, 2014]. Ann Otol Rhinol Laryngol. 2015;124:107-109.
- Meisel-Stosiek M, Hornstein OP, Stosiek N. Family study on Melkersson-Rosenthal syndrome. some hereditary aspects of the disease and review of literature. Acta Derm Venereol. 1990;70:221-226.
- Sciubba JJ, Said-Al-Naief N. Orofacial granulomatosis: presentation, pathology and management of 13 cases. J Oral Pathol Med. 2003;32:576-585.
- Zimmer WM, Rogers
RS 3rd, Reeve CM, et al. Orofacial manifestations of Melkersson-Rosenthal syndrome. a study of 42 patients and review of 220 cases from the literature. Oral Surg Oral Med Oral Pathol. 1992;74:610-619. - Critchlow WA, Cha
ng D. Cheilitis granulomatosa: a review [published online September 22, 2013]. Head Neck Pathol. 2014;8:209-213. - Allen CM, Camisa
C. Oral disease. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012:1157-1160. - Halevy S, Shalom
G, Trattner A, et al. Melkersson-Rosenthal syndrome: a possible association with psoriasis. J Am Acad Dermatol. 2012;67:795-796. - Algood HM, Lin P
L, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(suppl 3):S189-S193. - Yee AM, Pochapin
MB. Treatment of complicated sarcoidosis with infliximab anti-tumor necrosis factor-alpha therapy. Ann Intern Med. 2001;135:27-31. - Targan SR, Hanau
er SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. - Kakimoto C, Spar
ks C, White AA. Melkersson-Rosenthal syndrome: a form of pseudoangioedema. Ann Allergy Asthma Immunol. 2007;99:185-189. - Wickramasinghe N
, Gunasekara CN, Fernando WS, et al. Vulvitis granulomatosa, Melkersson-Rosenthal syndrome, and Crohn’s disease: dramatic response to infliximab therapy. Int J Dermatol. 2012;51:966-968. - Stein J, Paulke
A, Schacher B, et al. An extraordinary form of the Melkersson-Rosenthal syndrome successfully treated with the tumour necrosis factor-α blocker adalimumab [published online May 14, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204674. - Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315-1324.
- Choquette D, Faraawi R, Chow A, et al. Incidence and management of infusion reactions to infliximab in a prospective real-world community registry. J Rheumatol. 2015;42:1105-1111.
- Ruiz Villaverde R, Sánchez Cano D. Successful treatment of granulomatous cheilitis with adalimumab. Int J Dermatol. 2012;51:118-120.
- Liu R, Yu S. Melk
ersson-Rosenthal syndrome: a review of seven patients [published online May 7, 2013]. J Clin Neurosci. 2013;20:993-995. - Sun B, Zhou C, Han Z. Facial palsy in Melkersson-Rosenthal syndrome and Bell’s palsy: familial history and recurrence tendency [published online August 13, 2014]. Ann Otol Rhinol Laryngol. 2015;124:107-109.
- Meisel-Stosiek M, Hornstein OP, Stosiek N. Family study on Melkersson-Rosenthal syndrome. some hereditary aspects of the disease and review of literature. Acta Derm Venereol. 1990;70:221-226.
- Sciubba JJ, Said-Al-Naief N. Orofacial granulomatosis: presentation, pathology and management of 13 cases. J Oral Pathol Med. 2003;32:576-585.
- Zimmer WM, Rogers
RS 3rd, Reeve CM, et al. Orofacial manifestations of Melkersson-Rosenthal syndrome. a study of 42 patients and review of 220 cases from the literature. Oral Surg Oral Med Oral Pathol. 1992;74:610-619. - Critchlow WA, Cha
ng D. Cheilitis granulomatosa: a review [published online September 22, 2013]. Head Neck Pathol. 2014;8:209-213. - Allen CM, Camisa
C. Oral disease. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012:1157-1160. - Halevy S, Shalom
G, Trattner A, et al. Melkersson-Rosenthal syndrome: a possible association with psoriasis. J Am Acad Dermatol. 2012;67:795-796. - Algood HM, Lin P
L, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(suppl 3):S189-S193. - Yee AM, Pochapin
MB. Treatment of complicated sarcoidosis with infliximab anti-tumor necrosis factor-alpha therapy. Ann Intern Med. 2001;135:27-31. - Targan SR, Hanau
er SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. - Kakimoto C, Spar
ks C, White AA. Melkersson-Rosenthal syndrome: a form of pseudoangioedema. Ann Allergy Asthma Immunol. 2007;99:185-189. - Wickramasinghe N
, Gunasekara CN, Fernando WS, et al. Vulvitis granulomatosa, Melkersson-Rosenthal syndrome, and Crohn’s disease: dramatic response to infliximab therapy. Int J Dermatol. 2012;51:966-968. - Stein J, Paulke
A, Schacher B, et al. An extraordinary form of the Melkersson-Rosenthal syndrome successfully treated with the tumour necrosis factor-α blocker adalimumab [published online May 14, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204674. - Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315-1324.
- Choquette D, Faraawi R, Chow A, et al. Incidence and management of infusion reactions to infliximab in a prospective real-world community registry. J Rheumatol. 2015;42:1105-1111.
- Ruiz Villaverde R, Sánchez Cano D. Successful treatment of granulomatous cheilitis with adalimumab. Int J Dermatol. 2012;51:118-120.
Practice Points
- The classical triad of Melkersson-Rosenthal syndrome (MRS), which includes facial nerve palsy, facial edema, and lingua plicata, can present gradually over time and should therefore be kept in the differential of cheilitis.
- Tumor necrosis factor α therapy may play a crucial role in rare granulomatous diseases, including MRS.