User login
Breastfeeding by patients with serious mental illness: An ethical approach
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress.Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
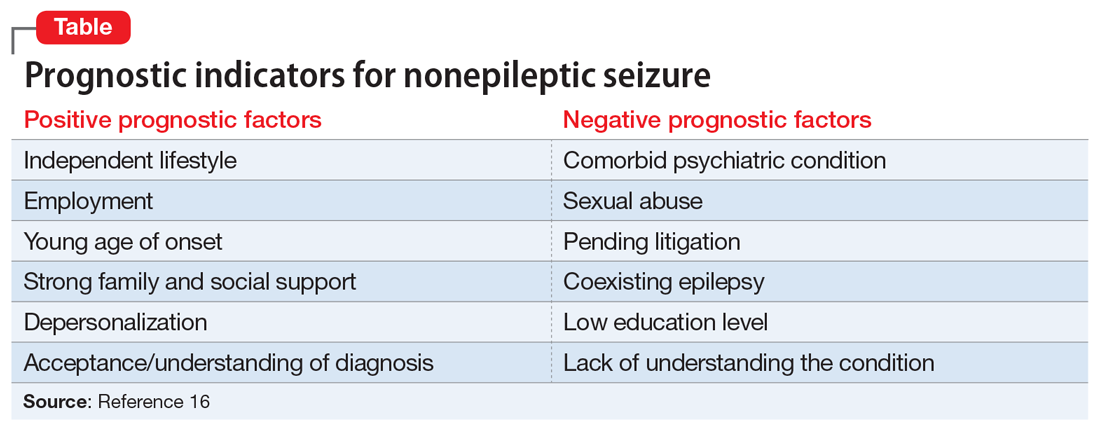
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
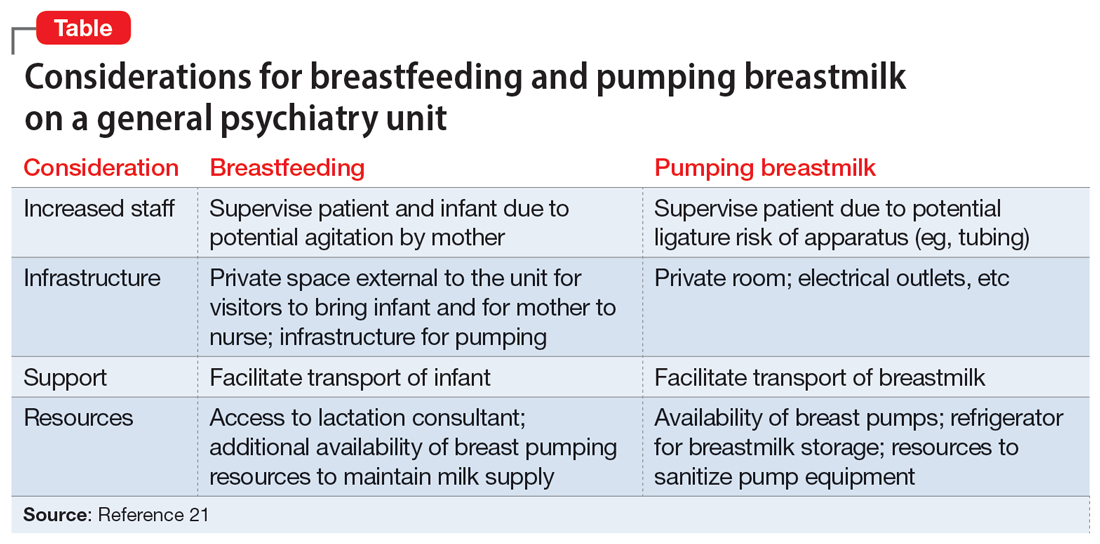
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21
Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
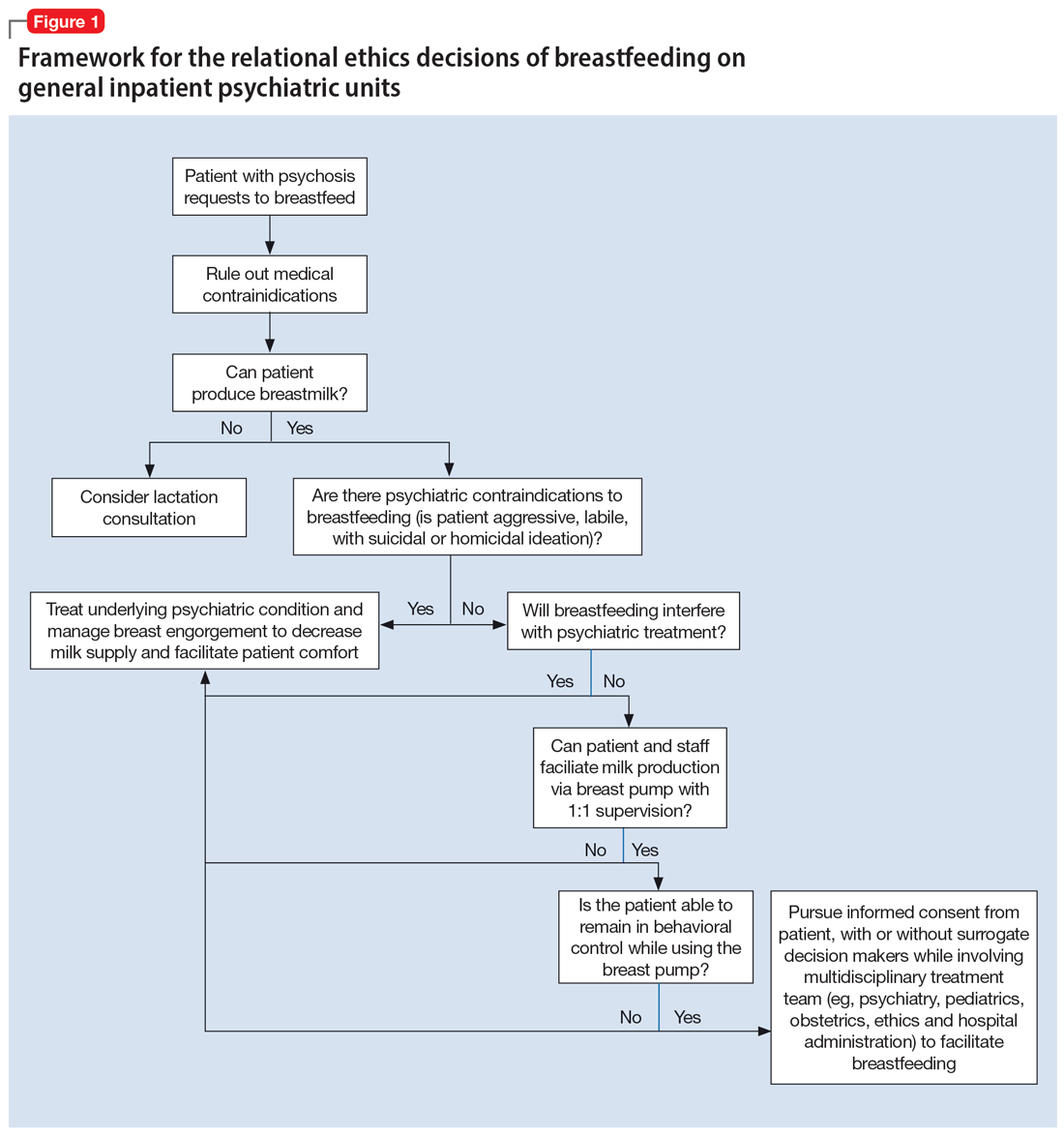
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.
In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
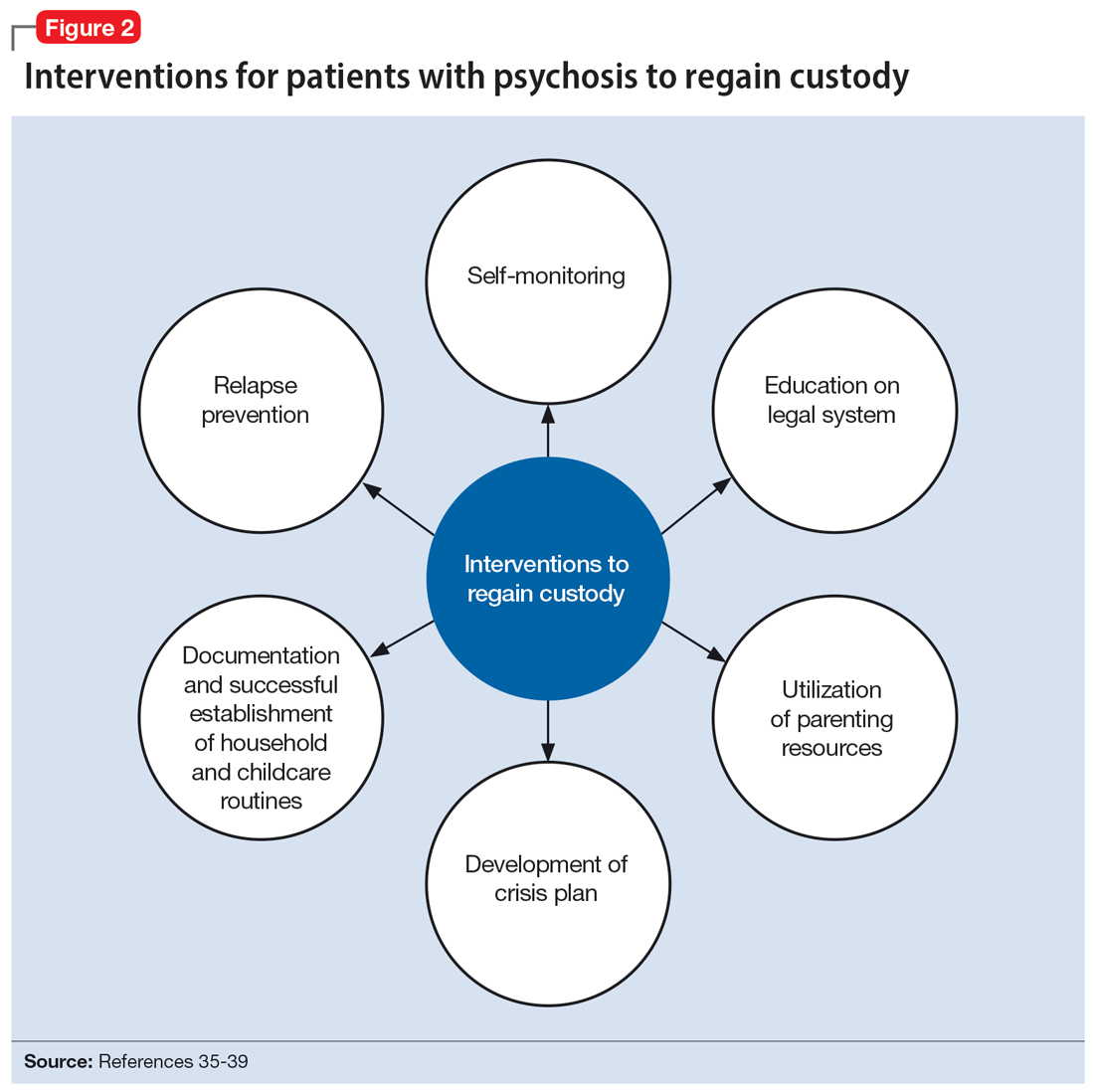
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.
Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress.Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21

Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.

In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.

Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress.Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21

Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.

In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.

Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
Complex trauma in the perinatal period
Complex posttraumatic stress disorder (CPTSD) is a condition characterized by classic trauma-related symptoms in addition to disturbances in self organization (DSO).1-3 DSO symptoms include negative self-concept, emotional dysregulation, and interpersonal problems. CPTSD differs from PTSD in that it includes symptoms of DSO, and differs from borderline personality disorder (BPD) in that it does not include extreme self-injurious behavior, a complete lack of sense of self, and avoidance of rejection or abandonment (Table1,2). The maladaptive traits of CPTSD are often the result of a chronic lack of safety in early childhood, particularly childhood sexual abuse (CSA). CSA may affect up to 20% of women and is defined by the CDC as “any completed or attempted sexual act, sexual contact with, or exploitation of a child by a caregiver.”4,5
Maternal lifetime trauma is more common among women who are in low-income minority groups and can lead to adverse birth outcomes in this vulnerable patient population.6 Recent research has found that trauma can increase cortisol levels during pregnancy, leading to increased placental permeability, inflammatory response, and longstanding alterations in the fetal hypothalamic-pituitary adrenal axis.6 A CPTSD diagnosis is of particular interest during the perinatal period because CPTSD is often a response to interpersonal trauma and attachment adversity, which can be reactivated during the perinatal period.7 CPTSD in survivors of CSA can be exacerbated due to feelings of disempowerment secondary to loss of bodily control throughout pregnancy, childbirth, breastfeeding, and obstetrical exams.5,8 Little is known about perinatal CPTSD, but we can extrapolate from trauma research that it is likely associated with the worsening of other maternal mental health conditions, suicidality, physical complaints, quality of life, maternal-child bonding outcomes, and low birth weight in offspring.5,9,10
Although there are no consensus guidelines on how to diagnose and treat CPTSD during the perinatal period, or how to promote family functioning thereafter, there are many opportunities for intervention. Mental health clinicians are in a particularly important position to care for women in the perinatal period, as collaborative work with obstetricians, pediatricians, and social services can have long-lasting effects.
In this article, we present cases of 3 CSA survivors who experienced worsening of CPTSD symptoms during the perinatal period and received psychiatric care via telehealth during the COVID-19 pandemic. We also identify best practice approaches and highlight areas for future research.
Case descriptions
Case 1
Ms. A, age 33, is married, has 3 children, has asthma, and is vaccinated against COVID-19. Her psychiatric history includes self-reported dissociative identity disorder and bulimia nervosa. At 2 months postpartum following an unplanned yet desired pregnancy, Ms. A presents to the outpatient clinic after a violent episode toward her husband during sexual intercourse. Since the first trimester of her pregnancy, she has expressed increased anxiety and difficulty sleeping, hypervigilance, intimacy avoidance, and negative views of herself and the world, yet she denies persistent depressive, manic, or psychotic symptoms, other maladaptive personality traits, or substance use. She recalls experiencing similar symptoms during her 2 previous peripartum periods, and attributes it to worsening memories of sexual abuse during childhood. Ms. A has a history of psychiatric hospitalizations during adolescence and young adulthood for suicidal ideation. She had been treated with various medications, including chlorpromazine, lamotrigine, carbamazepine, and clonazepam, but self-discontinued these medications in 2016 because she felt they were ineffective. Since becoming a mother, she has consistently denied depressive symptoms or suicidal ideation, and intermittently engaged in interpersonal psychotherapy targeting her conflictual relationship with her husband and parenting struggles.
Ms. A underwent an induced vaginal delivery at 36 weeks gestation due to preeclampsia and had success with breastfeeding. While engaging in sexual activity for the first time postpartum, she dissociated and later learned she had forcefully grabbed her husband’s neck for several seconds but did not cause any longstanding physical damage. Upon learning of this episode, Ms. A’s psychiatrist asks her to complete the International Trauma Questionnaire (ITQ), a brief self-report measure developed for the assessment of the ICD-11 diagnosis of CPTSD (Figure11). Ms. A also completes the PTSD Checklist for DSM-5 (PCL-5), the Dissociative Experiences Scale, and the Edinburgh Postnatal Depression Scale (EPDS) to assist with assessing her symptoms.12-15 The psychiatrist uses ICD-11 criteria to diagnose Ms. A with CPTSD, given her functional impairment associated with both PTSD and DSO symptoms, which have acutely worsened during the perinatal period.
Ms. A initially engages in extensive trauma psychoeducation and supportive psychotherapy for 3 months. She later pursues prolonged exposure psychotherapy targeting intimacy, and after 6 months of treatment, improves her avoidance behaviors and marriage.
Continue to: Case 2
Case 2
Ms. R, age 35, is a partnered mother expecting her third child. She has no relevant medical history and is not vaccinated against COVID-19. Her psychiatric history includes self-reported panic attacks and bipolar affective disorder (BPAD). During the second trimester of a desired, unplanned pregnancy, Ms. R presents to an outpatient psychiatry clinic with symptoms of worsening dysphoria and insomnia. She endorses frequent nightmares and flashbacks of CSA as well as remote intimate partner violence. These symptoms, along with hypervigilance, insomnia, anxiety, dysphoria, negative views of herself and her surroundings, and hallucinations of a shadow that whispers “come” when she is alone, worsened during the first trimester of her pregnancy. She recalls experiencing similar trauma-related symptoms during a previous pregnancy but denies a history of pervasive depressive, manic, or psychotic symptoms. She has no other maladaptive personality traits, denies prior substance use or suicidal behavior, and has never been psychiatrically hospitalized or taken psychotropic medications.
Ms. R completes the PCL-5, ITQ, EPDS, and Mood Disorder Questionnaire (MDQ). The results are notable for significant functional impairment related to PTSD and DSO symptoms with minimal concern for BPAD symptoms. The psychiatrist uses ICD-11 criteria to diagnose Ms. R with CPTSD and discusses treatment options with her and her obstetrician. Ms. R is reluctant to take medication until she delivers her baby. She intermittently attends supportive therapy while pregnant. Her pregnancy is complicated by gestational diabetes, and she often misses appointments with her obstetrician and nutritionist.
Ms. R has an uncomplicated vaginal delivery at 38 weeks gestation and success with breastfeeding, but continues to have CPTSD symptoms. She is prescribed quetiapine 25 mg/d for anxiety, insomnia, mood, and psychotic symptoms, but stops taking the medication after 3 days due to excessive sedation. Ms. R is then prescribed sertraline 50 mg/d, which she finds helpful, but has intermittent adherence. She misses multiple virtual appointments with the psychiatrist and does not want to attend in-person sessions due to fear of contracting COVID-19. The psychiatrist encourages Ms. R to get vaccinated, focuses on organizational skills during sessions to promote attendance, and recommends in-person appointments to increase her motivation for treatment and alliance building. Despite numerous outreach attempts, Ms. R is lost to follow-up at 10 months postpartum.
Case 3
Ms. S, age 29, is a partnered mother expecting her fourth child. Her medical history includes chronic back pain. She is not vaccinated against COVID-19, and her psychiatric history includes BPAD. During the first trimester of an undesired, unplanned pregnancy, Ms. S presents to an outpatient psychiatric clinic following an episode where she held a knife over her gravid abdomen during a fight with her partner. She recounts that she became dysregulated and held a knife to her body to communicate her distress, but she did not cut herself, and adamantly denies wanting to hurt herself or the fetus. Ms. S struggles with affective instability, poor frustration tolerance, and irritability. After 1 month of treatment, she discloses surviving prolonged CSA that led to her current nightmares and flashbacks. She also endorses impaired sleep, intimacy avoidance, hypervigilance, impulsive reckless behaviors (including excessive gambling), and negative views about herself and the world that worsened since she learned she was pregnant. Ms. S reports that these same symptoms were aggravated during prior perinatal periods and recalls 2 episodes of severe dysregulation that led to an interrupted suicide attempt and a violent episode toward a loved one. She denies other self-harm behaviors, substance use, or psychotic symptoms, and denies having a history of psychiatric hospitalizations. Ms. S recalls receiving a brief trial of topiramate for BPAD and migraine when she was last in outpatient psychiatric care 8 years ago.
Her psychiatrist administers the PCL-5, ITQ, MDQ, EPDS, and Borderline Symptoms List 23 (BLS-23). The results are notable for significant PTSD and DSO symptoms.16 The psychiatrist diagnoses Ms. S with CPTSD and bipolar II disorder, exacerbated during the peripartum period. Throughout the remainder of her pregnancy, she endorses mood instability with significant irritability but declines pharmacotherapy. Ms. S intermittently engages in psychotherapy using dialectical behavioral therapy (DBT) focusing on distress tolerance because she is unable to tolerate trauma-focused psychotherapy.
Continue to: Ms. S maintains the pregnancy...
Ms. S maintains the pregnancy without any additional complications and has a vaginal delivery at 39 weeks gestation. She initiates breastfeeding but chooses not to continue after 1 month due to fatigue, insomnia, and worsening mood. Her psychiatrist wants to contact Ms. S’s partner to discuss childcare support at night to promote better sleep conditions for Ms. S, but Ms. S declines. Ms. S intermittently attends virtual appointments, adamantly refuses the COVID-19 vaccine, and is fearful of starting a mood stabilizer despite extensive psychoeducation. At 5 months postpartum, Ms. S reports that she is in a worse mood and does not want to continue the appointment or further treatment, and abruptly ends the telepsychiatry session. Her psychiatrist reaches out the following week to schedule an in-person session if Ms. S agrees to wear personal protective equipment, which she is amenable to. During that appointment, the psychiatrist discusses the risks of bipolar depression and CPTSD on both her and her childrens’ development, against the risk of lamotrigine. Ms. S begins taking lamotrigine, which she tolerates without adverse effects, and quickly notices improvement in her mood as the medication is titrated up slowly to 200 mg/d. Ms. S then engages more consistently in psychotherapy and her CPTSD and bipolar II disorder symptoms much improve at 9 months postpartum.
Ensuring an accurate CPTSD diagnosis
These 3 cases illustrate the diversity and complexity of presentations for perinatal CPTSD following CSA. A CPTSD diagnosis is complicated because the differential is broad for those reporting PTSD and DSO symptoms, and CPTSD is commonly comorbid with other disorders such as anxiety and depression.17 While various scales can facilitate PTSD screening, the ITQ is helpful because it catalogs the symptoms of disturbances in self organization and functional impairment inherent in CPTSD. The ITQ can help clinicians and patients conceptualize symptoms and track progress (Figure11).
Once a patient screens positive, a CPTSD diagnosis is best made by the clinician after a full psychiatric interview, similar to other diagnoses. Psychiatrists must use ICD-11 criteria,1 as currently there are no formal DSM-5 criteria for CPTSD.2 Additional scales facilitate CPTSD symptom inventory, such as the PCL-5 to screen and monitor for PTSD symptoms and the BLS-23 to delineate between BPD or DSO symptoms.18 Furthermore, clinicians should screen for other comorbid conditions using additional scales such as the MDQ for BPAD and the EPDS for perinatal mood and anxiety disorders. Sharing a CPTSD diagnosis with a patient is an essential step when initiating treatment. Sensitive psychoeducation on the condition and its application to the perinatal period is key to establishing safety and trust, while also empowering survivors to make their own choices regarding treatment, all essential elements to trauma-informed care.19
A range of treatment options
Once CPTSD is appropriately diagnosed, clinicians must determine whether to use pharmacotherapy, psychotherapy, or both. A meta-analysis by Coventry et al20 sought to determine the best treatment strategies for complex traumatic events such as CSA, Multicomponent interventions were most promising, and psychological interventions were associated with larger effect sizes than pharmacologic interventions for managing PTSD, mood, and sleep. Therapeutic targets include trauma memory processing, self-perception, and dissociation, along with emotion, interpersonal, and somatic regulation.21
Psychotherapy. While there are no standardized guidelines for treating CPTSD, PTSD guidelines suggest using trauma-focused cognitive-behavioral therapy (TF-CBT) as a first-line therapy, though a longer course may be needed to resolve CPTSD symptoms compared to PTSD symptoms.3 DBT for PTSD can be particularly helpful in targeting DSO symptoms.22 Narrative therapy focused on identity, embodiment, and parenting has also shown to be effective for survivors of CSA in the perinatal period, specifically with the goal of meaning-making.5 Therapy can also be effective in a group setting (ie, a “Victim to Survivor” TF-CBT group).23 Sex and couples therapy may be indicated to reestablish trust, especially when it is evident there is sexual inhibition from trauma that influences the relationship, as seen in Case 1.24
Continue to: Pharmacotherapy
Pharmacotherapy. Case 2 and Case 3 both demonstrate that while the peripartum period presents an increased risk for exacerbation of psychiatric symptoms, patients and clinicians may be reluctant to start medications due to concerns for safety during pregnancy or lactation.25 Clinicians must weigh the risks of medication exposure against the risks of exposing the fetus or newborn to untreated psychiatric disease and consult an expert in reproductive psychiatry if questions or concerns arise.26
Adverse effects of psychotropic medications must be considered, especially sedation. Medications that lead to sedation may not be safe or feasible for a mother following delivery, especially if she is breastfeeding. This was exemplified in Case 2, when Ms. R was having troubling hallucinations for which the clinician prescribed quetiapine. The medication resulted in excessive sedation and Ms. R did not feel comfortable performing childcare duties while taking the medication, which greatly influenced future therapy decisions.
Making the decision to prescribe a certain medication for CPTSD is highly influenced by the patient’s most troubling symptoms and their comorbid diagnoses. Selective serotonin reuptake inhibitors (SSRIs) generally are considered safe during pregnancy and breastfeeding, and should be considered as a first-line intervention for PTSD, mood disorders, and anxiety disorders during the perinatal period.27 While prazosin is effective for PTSD symptoms outside of pregnancy, there is limited data regarding its safety during pregnancy and lactation, and it may lead to maternal hypotension and subsequent fetal adverse effects.28
Many patients with a history of CSA experience hallucinations and dissociative symptoms, as demonstrated by Case 1 and Case 2.29 In Case 3, Ms. S displayed features of BPAD with significant hypomanic symptoms and worsening suicidality during prior postpartum periods. The clinician felt comfortable prescribing lamotrigine, a relatively safe medication during the perinatal period compared to other mood stabilizers. Ms. S was amenable to taking lamotrigine, and her clinician avoided the use of an SSRI due to a concern of worsening a bipolar diathesis in this high-risk case.30 Case 2 and Case 3 both highlight the need to closely screen for comorbid conditions such as BPAD and using caution when considering an SSRI in light of the risk of precipitating mania, especially as the patient population is younger and at higher risk for antidepressant-associated mania.31,32
Help patients tap into their sources for strength
Other therapeutic strategies when treating patients with perinatal CPTSD include encouraging survivors to mobilize their support network and sources for strength. Chamberlain et al8 suggest incorporating socioecological and cultural contexts when considering outlets for social support systems and encourage collaborating with families, especially partners, along with community and spiritual networks. As seen in Case 3, clinicians should attempt to speak to family members on behalf of their patients to promote better sleeping conditions, which can greatly alleviate CPTSD and comorbid mood symptoms, and thus reduce suicide risk.33 Sources for strength should be accentuated and clinicians may need to advocate with child protective services to support parenting rights. As demonstrated in Case 1, motherhood can greatly reduce suicide risk, and should be promoted if a child’s safety is not in danger.34
Continue to: Clinicians must recognize...
Clinicians must recognize that patients in the perinatal period face barriers to obtaining health care, especially those with CPTSD, as these patients can be difficult to engage and retain. Each case described in this article challenged the psychiatrist with engagement and alliance-building, stemming from the patient’s CPTSD symptoms of interpersonal difficulties and negative views of surroundings. Case 2 demonstrates how the diagnosis can prevent patients from receiving appropriate prenatal care, while Case 3 shows how clinicians may need more flexible attendance policies and assertive outreach attempts to deliver the mental health care these patients deserve.
These vignettes highlight the psychosocial barriers women face during the perinatal period, such as caring for their child, financial stressors, and COVID-19 pandemic–related factors that can hinder treatment, which can be compounded by trauma. The uncertainty, unpredictability, loss of control, and loss of support structures collectively experienced during the pandemic can be triggering and precipitate worsening CPTSD symptoms.35 Women who experience trauma are less likely to obtain the COVID-19 vaccine for themselves or their children, and this hesitancy is often driven by institutional distrust.36 Policy leaders and clinicians should consider these factors to promote trauma-informed COVID-19 vaccine initiatives and expand mental health access using less orthodox treatment settings, such as telepsychiatry. Telepsychiatry can serve as a bridge to in-person care as patients may feel a higher sense of control when in a familiar home environment. Case 2 and Case 3 exemplify the difficulties of delivering mental health care to perinatal women with CPTSD during the pandemic, especially those who are vaccine-hesitant, and illustrate the importance of adapting a patient’s treatment plan in a personalized and trauma-informed way.
Psychiatrists can help obstetricians and pediatricians by explaining that avoidance patterns and distrust in the clinical setting may be related to trauma and are not grounds for conscious or subconscious punishment or abandonment. Educating other clinicians about trauma-informed care, precautions to use for perinatal patients, and ways to effectively support survivors of CSA can greatly improve health outcomes for perinatal women and their offspring.37
Bottom Line
Complex posttraumatic stress disorder (CPTSD) is characterized by classic PTSD symptoms as well as disturbances in self organization, which can include mood symptoms, psychotic symptoms, and maladaptive personality traits. CPTSD resulting from childhood sexual abuse is of particular concern for women, especially during the perinatal period. Clinicians must know how to recognize the signs and symptoms of CPTSD so they can tailor a trauma-informed treatment plan and promote treatment access in this highly vulnerable patient population.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Keswani M, Nemcek S, Rashid N. Is it bipolar disorder, or a complex form of PTSD? Current Psychiatry. 2021;20(12):42-49. doi:10.12788/cp.0188
Drug Brand Names
Carbamazepine • Carbatrol
Clonazepam • Klonopin
Lamotrigine • Lamictal
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Topiramate • Topamax
1. World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). Complex posttraumatic stress disorder. Accessed November 6, 2021. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/585833559
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Cloitre M, Garvert DW, Brewin CR, et al. Evidence for proposed ICD-11 PTSD and complexPTSD: a latent profile analysis. Eur J Psychotraumatol. 2013;4:10.3402/ejpt.v4i0.20706. doi:10.3402/ejpt.v4i0.20706
4. Leeb RT, Paulozzi LJ, Melanson C, et al. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements, Version 1.0. Centers for Disease Control and Prevention, Department of Health & Human Services; 2008. Accessed August 24, 2022. https://www.cdc.gov/violenceprevention/pdf/cm_surveillance-a.pdf
5. Byrne J, Smart C, Watson G. “I felt like I was being abused all over again”: how survivors of child sexual abuse make sense of the perinatal period through their narratives. J Child Sex Abus. 2017;26(4):465-486. doi:10.1080/10538712.2017.1297880
6. Flom JD, Chiu YM, Hsu HL, et al. Maternal lifetime trauma and birthweight: effect modification by in utero cortisol and child sex. J Pediatr. 2018;203:301-308. doi:10.1016/j.jpeds.2018.07.069
7. Spinazzola J, van der Kolk B, Ford JD. When nowhere is safe: interpersonal trauma and attachment adversity as antecedents of posttraumatic stress disorder and developmental trauma disorder. J Trauma Stress. 2018;31(5):631-642. doi:10.1002/jts.22320
8. Chamberlain C, Gee G, Harfield S, et al. Parenting after a history of childhood maltreatment: a scoping review and map of evidence in the perinatal period. PloS One. 2019;14(3):e0213460. doi:10.1371/journal.pone.0213460
9. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi:10.1016/j.jad.2017.07.045
10. Gavin AR, Morris J. The association between maternal early life forced sexual intercourse and offspring birth weight: the role of socioeconomic status. J Womens Health (Larchmt). 2017;26(5):442-449. doi:10.1089/jwh.2016.5789
11. Cloitre M, Shevlin M, Brewin CR, et al. The international trauma questionnaire: development of a self-report measure of ICD-11 PTSD and complex PTSD. Acta Psychiatr Scand. 2018;138(6):536-546.
12. Cloitre M, Hyland P, Prins A, et al. The international trauma questionnaire (ITQ) measures reliable and clinically significant treatment-related change in PTSD and complex PTSD. Eur J Psychotraumatol. 2021;12(1):1930961. doi:10.1080/20008198.2021.1930961
13. Weathers FW, Litz BT, Keane TM, et al. PTSD Checklist for DSM-5 (PCL-5). US Department of Veterans Affairs. April 11, 2018. Accessed November 25, 2021. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.PDF
14. Dissociative Experiences Scale – II. TraumaDissociation.com. Accessed November 25, 2021. http://traumadissociation.com/des
15. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi:10.1192/bjp.150.6.782
16. Mood Disorder Questionnaire (MDQ). Oregon Health & Science University. Accessed November 7, 2021. https://www.ohsu.edu/sites/default/files/2019-06/cms-quality-bipolar_disorder_mdq_screener.pdf
17. Karatzias T, Hyland P, Bradley A, et al. Risk factors and comorbidity of ICD-11 PTSD and complex PTSD: findings from a trauma-exposed population based sample of adults in the United Kingdom. Depress Anxiety. 2019;36(9):887-894. doi:10.1002/da.22934
18. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
19. Fallot RD, Harris M. A trauma-informed approach to screening and assessment. New Dir Ment Health Serv. 2001;(89):23-31. doi:10.1002/yd.23320018904
20. Coventry PA, Meader N, Melton H, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262. doi:10.1371/journal.pmed.1003262
21. Ford JD. Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Curr Treat Options Psychiatry. 2021;8:1-17. doi:10.1007/s40501-020-00236-6
22. Becker-Sadzio J, Gundel F, Kroczek A, et al. Trauma exposure therapy in a pregnant woman suffering from complex posttraumatic stress disorder after childhood sexual abuse: risk or benefit? Eur J Psychotraumatol. 2020;11(1):1697581. doi:10.1080/20008198.2019.1697581
23. Mendelsohn M, Zachary RS, Harney PA. Group therapy as an ecological bridge to new community for trauma survivors. J Aggress Maltreat Trauma. 2007;14(1-2):227-243. doi:10.1300/J146v14n01_12
24. Macintosh HB, Vaillancourt-Morel MP, Bergeron S. Sex and couple therapy with survivors of childhood trauma. In: Hall KS, Binik YM, eds. Principles and Practice of Sex Therapy. 6th ed. Guilford Press; 2020.
25. Dresner N, Byatt N, Gopalan P, et al. Psychiatric care of peripartum women. Psychiatric Times. 2015;32(12).
26. Zagorski N. How to manage meds before, during, and after pregnancy. Psychiatric News. 2019;54(14):13. https://doi.org/10.1176/APPI.PN.2019.6B36
27. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397-2407. doi:10.1056/NEJMoa1312828
28. Davidson AD, Bhat A, Chu F, et al. A systematic review of the use of prazosin in pregnancy and lactation. Gen Hosp Psychiatry. 2021;71:134-136. doi:10.1016/j.genhosppsych.2021.03.012
29. Shinn AK, Wolff JD, Hwang M, et al. Assessing voice hearing in trauma spectrum disorders: a comparison of two measures and a review of the literature. Front Psychiatry. 2020;10:1011. doi:10.3389/fpsyt.2019.01011
30. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi:10.1016/j.clp.2019.02.004
31. Martin A, Young C, Leckman JF, et al. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adoles Med. 2004;158(8):773-780. doi:10.1001/archpedi.158.8.773
32. Gill N, Bayes A, Parker G. A review of antidepressant-associated hypomania in those diagnosed with unipolar depression-risk factors, conceptual models, and management. Curr Psychiatry Rep. 2020;22(4):20. doi:10.1007/s11920-020-01143-6
33. Harris LM, Huang X, Linthicum KP, et al. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Sci Rep. 2020;10(1):13888. doi:10.1038/s41598-020-70866-6
34. Dehara M, Wells MB, Sjöqvist H, et al. Parenthood is associated with lower suicide risk: a register-based cohort study of 1.5 million Swedes. Acta Psychiatr Scand. 2021;143(3):206-215. doi:10.1111/acps.13240
35. Iyengar U, Jaiprakash B, Haitsuka H, et al. One year into the pandemic: a systematic review of perinatal mental health outcomes during COVID-19. Front Psychiatry. 2021;12:674194. doi:10.3389/fpsyt.2021.674194
36. Milan S, Dáu ALBT. The role of trauma in mothers’ COVID-19 vaccine beliefs and intentions. J Pediatr Psychol. 2021;46(5):526-535. doi:10.1093/jpepsy/jsab043
37. Coles J, Jones K. “Universal precautions”: perinatal touch and examination after childhood sexual abuse. Birth. 2009;36(3):230-236. doi:10.1111/j.1523-536X.2009.00327
Complex posttraumatic stress disorder (CPTSD) is a condition characterized by classic trauma-related symptoms in addition to disturbances in self organization (DSO).1-3 DSO symptoms include negative self-concept, emotional dysregulation, and interpersonal problems. CPTSD differs from PTSD in that it includes symptoms of DSO, and differs from borderline personality disorder (BPD) in that it does not include extreme self-injurious behavior, a complete lack of sense of self, and avoidance of rejection or abandonment (Table1,2). The maladaptive traits of CPTSD are often the result of a chronic lack of safety in early childhood, particularly childhood sexual abuse (CSA). CSA may affect up to 20% of women and is defined by the CDC as “any completed or attempted sexual act, sexual contact with, or exploitation of a child by a caregiver.”4,5

Maternal lifetime trauma is more common among women who are in low-income minority groups and can lead to adverse birth outcomes in this vulnerable patient population.6 Recent research has found that trauma can increase cortisol levels during pregnancy, leading to increased placental permeability, inflammatory response, and longstanding alterations in the fetal hypothalamic-pituitary adrenal axis.6 A CPTSD diagnosis is of particular interest during the perinatal period because CPTSD is often a response to interpersonal trauma and attachment adversity, which can be reactivated during the perinatal period.7 CPTSD in survivors of CSA can be exacerbated due to feelings of disempowerment secondary to loss of bodily control throughout pregnancy, childbirth, breastfeeding, and obstetrical exams.5,8 Little is known about perinatal CPTSD, but we can extrapolate from trauma research that it is likely associated with the worsening of other maternal mental health conditions, suicidality, physical complaints, quality of life, maternal-child bonding outcomes, and low birth weight in offspring.5,9,10
Although there are no consensus guidelines on how to diagnose and treat CPTSD during the perinatal period, or how to promote family functioning thereafter, there are many opportunities for intervention. Mental health clinicians are in a particularly important position to care for women in the perinatal period, as collaborative work with obstetricians, pediatricians, and social services can have long-lasting effects.
In this article, we present cases of 3 CSA survivors who experienced worsening of CPTSD symptoms during the perinatal period and received psychiatric care via telehealth during the COVID-19 pandemic. We also identify best practice approaches and highlight areas for future research.
Case descriptions
Case 1
Ms. A, age 33, is married, has 3 children, has asthma, and is vaccinated against COVID-19. Her psychiatric history includes self-reported dissociative identity disorder and bulimia nervosa. At 2 months postpartum following an unplanned yet desired pregnancy, Ms. A presents to the outpatient clinic after a violent episode toward her husband during sexual intercourse. Since the first trimester of her pregnancy, she has expressed increased anxiety and difficulty sleeping, hypervigilance, intimacy avoidance, and negative views of herself and the world, yet she denies persistent depressive, manic, or psychotic symptoms, other maladaptive personality traits, or substance use. She recalls experiencing similar symptoms during her 2 previous peripartum periods, and attributes it to worsening memories of sexual abuse during childhood. Ms. A has a history of psychiatric hospitalizations during adolescence and young adulthood for suicidal ideation. She had been treated with various medications, including chlorpromazine, lamotrigine, carbamazepine, and clonazepam, but self-discontinued these medications in 2016 because she felt they were ineffective. Since becoming a mother, she has consistently denied depressive symptoms or suicidal ideation, and intermittently engaged in interpersonal psychotherapy targeting her conflictual relationship with her husband and parenting struggles.
Ms. A underwent an induced vaginal delivery at 36 weeks gestation due to preeclampsia and had success with breastfeeding. While engaging in sexual activity for the first time postpartum, she dissociated and later learned she had forcefully grabbed her husband’s neck for several seconds but did not cause any longstanding physical damage. Upon learning of this episode, Ms. A’s psychiatrist asks her to complete the International Trauma Questionnaire (ITQ), a brief self-report measure developed for the assessment of the ICD-11 diagnosis of CPTSD (Figure11). Ms. A also completes the PTSD Checklist for DSM-5 (PCL-5), the Dissociative Experiences Scale, and the Edinburgh Postnatal Depression Scale (EPDS) to assist with assessing her symptoms.12-15 The psychiatrist uses ICD-11 criteria to diagnose Ms. A with CPTSD, given her functional impairment associated with both PTSD and DSO symptoms, which have acutely worsened during the perinatal period.

Ms. A initially engages in extensive trauma psychoeducation and supportive psychotherapy for 3 months. She later pursues prolonged exposure psychotherapy targeting intimacy, and after 6 months of treatment, improves her avoidance behaviors and marriage.
Continue to: Case 2
Case 2
Ms. R, age 35, is a partnered mother expecting her third child. She has no relevant medical history and is not vaccinated against COVID-19. Her psychiatric history includes self-reported panic attacks and bipolar affective disorder (BPAD). During the second trimester of a desired, unplanned pregnancy, Ms. R presents to an outpatient psychiatry clinic with symptoms of worsening dysphoria and insomnia. She endorses frequent nightmares and flashbacks of CSA as well as remote intimate partner violence. These symptoms, along with hypervigilance, insomnia, anxiety, dysphoria, negative views of herself and her surroundings, and hallucinations of a shadow that whispers “come” when she is alone, worsened during the first trimester of her pregnancy. She recalls experiencing similar trauma-related symptoms during a previous pregnancy but denies a history of pervasive depressive, manic, or psychotic symptoms. She has no other maladaptive personality traits, denies prior substance use or suicidal behavior, and has never been psychiatrically hospitalized or taken psychotropic medications.
Ms. R completes the PCL-5, ITQ, EPDS, and Mood Disorder Questionnaire (MDQ). The results are notable for significant functional impairment related to PTSD and DSO symptoms with minimal concern for BPAD symptoms. The psychiatrist uses ICD-11 criteria to diagnose Ms. R with CPTSD and discusses treatment options with her and her obstetrician. Ms. R is reluctant to take medication until she delivers her baby. She intermittently attends supportive therapy while pregnant. Her pregnancy is complicated by gestational diabetes, and she often misses appointments with her obstetrician and nutritionist.
Ms. R has an uncomplicated vaginal delivery at 38 weeks gestation and success with breastfeeding, but continues to have CPTSD symptoms. She is prescribed quetiapine 25 mg/d for anxiety, insomnia, mood, and psychotic symptoms, but stops taking the medication after 3 days due to excessive sedation. Ms. R is then prescribed sertraline 50 mg/d, which she finds helpful, but has intermittent adherence. She misses multiple virtual appointments with the psychiatrist and does not want to attend in-person sessions due to fear of contracting COVID-19. The psychiatrist encourages Ms. R to get vaccinated, focuses on organizational skills during sessions to promote attendance, and recommends in-person appointments to increase her motivation for treatment and alliance building. Despite numerous outreach attempts, Ms. R is lost to follow-up at 10 months postpartum.
Case 3
Ms. S, age 29, is a partnered mother expecting her fourth child. Her medical history includes chronic back pain. She is not vaccinated against COVID-19, and her psychiatric history includes BPAD. During the first trimester of an undesired, unplanned pregnancy, Ms. S presents to an outpatient psychiatric clinic following an episode where she held a knife over her gravid abdomen during a fight with her partner. She recounts that she became dysregulated and held a knife to her body to communicate her distress, but she did not cut herself, and adamantly denies wanting to hurt herself or the fetus. Ms. S struggles with affective instability, poor frustration tolerance, and irritability. After 1 month of treatment, she discloses surviving prolonged CSA that led to her current nightmares and flashbacks. She also endorses impaired sleep, intimacy avoidance, hypervigilance, impulsive reckless behaviors (including excessive gambling), and negative views about herself and the world that worsened since she learned she was pregnant. Ms. S reports that these same symptoms were aggravated during prior perinatal periods and recalls 2 episodes of severe dysregulation that led to an interrupted suicide attempt and a violent episode toward a loved one. She denies other self-harm behaviors, substance use, or psychotic symptoms, and denies having a history of psychiatric hospitalizations. Ms. S recalls receiving a brief trial of topiramate for BPAD and migraine when she was last in outpatient psychiatric care 8 years ago.
Her psychiatrist administers the PCL-5, ITQ, MDQ, EPDS, and Borderline Symptoms List 23 (BLS-23). The results are notable for significant PTSD and DSO symptoms.16 The psychiatrist diagnoses Ms. S with CPTSD and bipolar II disorder, exacerbated during the peripartum period. Throughout the remainder of her pregnancy, she endorses mood instability with significant irritability but declines pharmacotherapy. Ms. S intermittently engages in psychotherapy using dialectical behavioral therapy (DBT) focusing on distress tolerance because she is unable to tolerate trauma-focused psychotherapy.
Continue to: Ms. S maintains the pregnancy...
Ms. S maintains the pregnancy without any additional complications and has a vaginal delivery at 39 weeks gestation. She initiates breastfeeding but chooses not to continue after 1 month due to fatigue, insomnia, and worsening mood. Her psychiatrist wants to contact Ms. S’s partner to discuss childcare support at night to promote better sleep conditions for Ms. S, but Ms. S declines. Ms. S intermittently attends virtual appointments, adamantly refuses the COVID-19 vaccine, and is fearful of starting a mood stabilizer despite extensive psychoeducation. At 5 months postpartum, Ms. S reports that she is in a worse mood and does not want to continue the appointment or further treatment, and abruptly ends the telepsychiatry session. Her psychiatrist reaches out the following week to schedule an in-person session if Ms. S agrees to wear personal protective equipment, which she is amenable to. During that appointment, the psychiatrist discusses the risks of bipolar depression and CPTSD on both her and her childrens’ development, against the risk of lamotrigine. Ms. S begins taking lamotrigine, which she tolerates without adverse effects, and quickly notices improvement in her mood as the medication is titrated up slowly to 200 mg/d. Ms. S then engages more consistently in psychotherapy and her CPTSD and bipolar II disorder symptoms much improve at 9 months postpartum.
Ensuring an accurate CPTSD diagnosis
These 3 cases illustrate the diversity and complexity of presentations for perinatal CPTSD following CSA. A CPTSD diagnosis is complicated because the differential is broad for those reporting PTSD and DSO symptoms, and CPTSD is commonly comorbid with other disorders such as anxiety and depression.17 While various scales can facilitate PTSD screening, the ITQ is helpful because it catalogs the symptoms of disturbances in self organization and functional impairment inherent in CPTSD. The ITQ can help clinicians and patients conceptualize symptoms and track progress (Figure11).
Once a patient screens positive, a CPTSD diagnosis is best made by the clinician after a full psychiatric interview, similar to other diagnoses. Psychiatrists must use ICD-11 criteria,1 as currently there are no formal DSM-5 criteria for CPTSD.2 Additional scales facilitate CPTSD symptom inventory, such as the PCL-5 to screen and monitor for PTSD symptoms and the BLS-23 to delineate between BPD or DSO symptoms.18 Furthermore, clinicians should screen for other comorbid conditions using additional scales such as the MDQ for BPAD and the EPDS for perinatal mood and anxiety disorders. Sharing a CPTSD diagnosis with a patient is an essential step when initiating treatment. Sensitive psychoeducation on the condition and its application to the perinatal period is key to establishing safety and trust, while also empowering survivors to make their own choices regarding treatment, all essential elements to trauma-informed care.19
A range of treatment options
Once CPTSD is appropriately diagnosed, clinicians must determine whether to use pharmacotherapy, psychotherapy, or both. A meta-analysis by Coventry et al20 sought to determine the best treatment strategies for complex traumatic events such as CSA, Multicomponent interventions were most promising, and psychological interventions were associated with larger effect sizes than pharmacologic interventions for managing PTSD, mood, and sleep. Therapeutic targets include trauma memory processing, self-perception, and dissociation, along with emotion, interpersonal, and somatic regulation.21
Psychotherapy. While there are no standardized guidelines for treating CPTSD, PTSD guidelines suggest using trauma-focused cognitive-behavioral therapy (TF-CBT) as a first-line therapy, though a longer course may be needed to resolve CPTSD symptoms compared to PTSD symptoms.3 DBT for PTSD can be particularly helpful in targeting DSO symptoms.22 Narrative therapy focused on identity, embodiment, and parenting has also shown to be effective for survivors of CSA in the perinatal period, specifically with the goal of meaning-making.5 Therapy can also be effective in a group setting (ie, a “Victim to Survivor” TF-CBT group).23 Sex and couples therapy may be indicated to reestablish trust, especially when it is evident there is sexual inhibition from trauma that influences the relationship, as seen in Case 1.24
Continue to: Pharmacotherapy
Pharmacotherapy. Case 2 and Case 3 both demonstrate that while the peripartum period presents an increased risk for exacerbation of psychiatric symptoms, patients and clinicians may be reluctant to start medications due to concerns for safety during pregnancy or lactation.25 Clinicians must weigh the risks of medication exposure against the risks of exposing the fetus or newborn to untreated psychiatric disease and consult an expert in reproductive psychiatry if questions or concerns arise.26
Adverse effects of psychotropic medications must be considered, especially sedation. Medications that lead to sedation may not be safe or feasible for a mother following delivery, especially if she is breastfeeding. This was exemplified in Case 2, when Ms. R was having troubling hallucinations for which the clinician prescribed quetiapine. The medication resulted in excessive sedation and Ms. R did not feel comfortable performing childcare duties while taking the medication, which greatly influenced future therapy decisions.
Making the decision to prescribe a certain medication for CPTSD is highly influenced by the patient’s most troubling symptoms and their comorbid diagnoses. Selective serotonin reuptake inhibitors (SSRIs) generally are considered safe during pregnancy and breastfeeding, and should be considered as a first-line intervention for PTSD, mood disorders, and anxiety disorders during the perinatal period.27 While prazosin is effective for PTSD symptoms outside of pregnancy, there is limited data regarding its safety during pregnancy and lactation, and it may lead to maternal hypotension and subsequent fetal adverse effects.28
Many patients with a history of CSA experience hallucinations and dissociative symptoms, as demonstrated by Case 1 and Case 2.29 In Case 3, Ms. S displayed features of BPAD with significant hypomanic symptoms and worsening suicidality during prior postpartum periods. The clinician felt comfortable prescribing lamotrigine, a relatively safe medication during the perinatal period compared to other mood stabilizers. Ms. S was amenable to taking lamotrigine, and her clinician avoided the use of an SSRI due to a concern of worsening a bipolar diathesis in this high-risk case.30 Case 2 and Case 3 both highlight the need to closely screen for comorbid conditions such as BPAD and using caution when considering an SSRI in light of the risk of precipitating mania, especially as the patient population is younger and at higher risk for antidepressant-associated mania.31,32
Help patients tap into their sources for strength
Other therapeutic strategies when treating patients with perinatal CPTSD include encouraging survivors to mobilize their support network and sources for strength. Chamberlain et al8 suggest incorporating socioecological and cultural contexts when considering outlets for social support systems and encourage collaborating with families, especially partners, along with community and spiritual networks. As seen in Case 3, clinicians should attempt to speak to family members on behalf of their patients to promote better sleeping conditions, which can greatly alleviate CPTSD and comorbid mood symptoms, and thus reduce suicide risk.33 Sources for strength should be accentuated and clinicians may need to advocate with child protective services to support parenting rights. As demonstrated in Case 1, motherhood can greatly reduce suicide risk, and should be promoted if a child’s safety is not in danger.34
Continue to: Clinicians must recognize...
Clinicians must recognize that patients in the perinatal period face barriers to obtaining health care, especially those with CPTSD, as these patients can be difficult to engage and retain. Each case described in this article challenged the psychiatrist with engagement and alliance-building, stemming from the patient’s CPTSD symptoms of interpersonal difficulties and negative views of surroundings. Case 2 demonstrates how the diagnosis can prevent patients from receiving appropriate prenatal care, while Case 3 shows how clinicians may need more flexible attendance policies and assertive outreach attempts to deliver the mental health care these patients deserve.
These vignettes highlight the psychosocial barriers women face during the perinatal period, such as caring for their child, financial stressors, and COVID-19 pandemic–related factors that can hinder treatment, which can be compounded by trauma. The uncertainty, unpredictability, loss of control, and loss of support structures collectively experienced during the pandemic can be triggering and precipitate worsening CPTSD symptoms.35 Women who experience trauma are less likely to obtain the COVID-19 vaccine for themselves or their children, and this hesitancy is often driven by institutional distrust.36 Policy leaders and clinicians should consider these factors to promote trauma-informed COVID-19 vaccine initiatives and expand mental health access using less orthodox treatment settings, such as telepsychiatry. Telepsychiatry can serve as a bridge to in-person care as patients may feel a higher sense of control when in a familiar home environment. Case 2 and Case 3 exemplify the difficulties of delivering mental health care to perinatal women with CPTSD during the pandemic, especially those who are vaccine-hesitant, and illustrate the importance of adapting a patient’s treatment plan in a personalized and trauma-informed way.
Psychiatrists can help obstetricians and pediatricians by explaining that avoidance patterns and distrust in the clinical setting may be related to trauma and are not grounds for conscious or subconscious punishment or abandonment. Educating other clinicians about trauma-informed care, precautions to use for perinatal patients, and ways to effectively support survivors of CSA can greatly improve health outcomes for perinatal women and their offspring.37
Bottom Line
Complex posttraumatic stress disorder (CPTSD) is characterized by classic PTSD symptoms as well as disturbances in self organization, which can include mood symptoms, psychotic symptoms, and maladaptive personality traits. CPTSD resulting from childhood sexual abuse is of particular concern for women, especially during the perinatal period. Clinicians must know how to recognize the signs and symptoms of CPTSD so they can tailor a trauma-informed treatment plan and promote treatment access in this highly vulnerable patient population.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Keswani M, Nemcek S, Rashid N. Is it bipolar disorder, or a complex form of PTSD? Current Psychiatry. 2021;20(12):42-49. doi:10.12788/cp.0188
Drug Brand Names
Carbamazepine • Carbatrol
Clonazepam • Klonopin
Lamotrigine • Lamictal
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Topiramate • Topamax
Complex posttraumatic stress disorder (CPTSD) is a condition characterized by classic trauma-related symptoms in addition to disturbances in self organization (DSO).1-3 DSO symptoms include negative self-concept, emotional dysregulation, and interpersonal problems. CPTSD differs from PTSD in that it includes symptoms of DSO, and differs from borderline personality disorder (BPD) in that it does not include extreme self-injurious behavior, a complete lack of sense of self, and avoidance of rejection or abandonment (Table1,2). The maladaptive traits of CPTSD are often the result of a chronic lack of safety in early childhood, particularly childhood sexual abuse (CSA). CSA may affect up to 20% of women and is defined by the CDC as “any completed or attempted sexual act, sexual contact with, or exploitation of a child by a caregiver.”4,5

Maternal lifetime trauma is more common among women who are in low-income minority groups and can lead to adverse birth outcomes in this vulnerable patient population.6 Recent research has found that trauma can increase cortisol levels during pregnancy, leading to increased placental permeability, inflammatory response, and longstanding alterations in the fetal hypothalamic-pituitary adrenal axis.6 A CPTSD diagnosis is of particular interest during the perinatal period because CPTSD is often a response to interpersonal trauma and attachment adversity, which can be reactivated during the perinatal period.7 CPTSD in survivors of CSA can be exacerbated due to feelings of disempowerment secondary to loss of bodily control throughout pregnancy, childbirth, breastfeeding, and obstetrical exams.5,8 Little is known about perinatal CPTSD, but we can extrapolate from trauma research that it is likely associated with the worsening of other maternal mental health conditions, suicidality, physical complaints, quality of life, maternal-child bonding outcomes, and low birth weight in offspring.5,9,10
Although there are no consensus guidelines on how to diagnose and treat CPTSD during the perinatal period, or how to promote family functioning thereafter, there are many opportunities for intervention. Mental health clinicians are in a particularly important position to care for women in the perinatal period, as collaborative work with obstetricians, pediatricians, and social services can have long-lasting effects.
In this article, we present cases of 3 CSA survivors who experienced worsening of CPTSD symptoms during the perinatal period and received psychiatric care via telehealth during the COVID-19 pandemic. We also identify best practice approaches and highlight areas for future research.
Case descriptions
Case 1
Ms. A, age 33, is married, has 3 children, has asthma, and is vaccinated against COVID-19. Her psychiatric history includes self-reported dissociative identity disorder and bulimia nervosa. At 2 months postpartum following an unplanned yet desired pregnancy, Ms. A presents to the outpatient clinic after a violent episode toward her husband during sexual intercourse. Since the first trimester of her pregnancy, she has expressed increased anxiety and difficulty sleeping, hypervigilance, intimacy avoidance, and negative views of herself and the world, yet she denies persistent depressive, manic, or psychotic symptoms, other maladaptive personality traits, or substance use. She recalls experiencing similar symptoms during her 2 previous peripartum periods, and attributes it to worsening memories of sexual abuse during childhood. Ms. A has a history of psychiatric hospitalizations during adolescence and young adulthood for suicidal ideation. She had been treated with various medications, including chlorpromazine, lamotrigine, carbamazepine, and clonazepam, but self-discontinued these medications in 2016 because she felt they were ineffective. Since becoming a mother, she has consistently denied depressive symptoms or suicidal ideation, and intermittently engaged in interpersonal psychotherapy targeting her conflictual relationship with her husband and parenting struggles.
Ms. A underwent an induced vaginal delivery at 36 weeks gestation due to preeclampsia and had success with breastfeeding. While engaging in sexual activity for the first time postpartum, she dissociated and later learned she had forcefully grabbed her husband’s neck for several seconds but did not cause any longstanding physical damage. Upon learning of this episode, Ms. A’s psychiatrist asks her to complete the International Trauma Questionnaire (ITQ), a brief self-report measure developed for the assessment of the ICD-11 diagnosis of CPTSD (Figure11). Ms. A also completes the PTSD Checklist for DSM-5 (PCL-5), the Dissociative Experiences Scale, and the Edinburgh Postnatal Depression Scale (EPDS) to assist with assessing her symptoms.12-15 The psychiatrist uses ICD-11 criteria to diagnose Ms. A with CPTSD, given her functional impairment associated with both PTSD and DSO symptoms, which have acutely worsened during the perinatal period.

Ms. A initially engages in extensive trauma psychoeducation and supportive psychotherapy for 3 months. She later pursues prolonged exposure psychotherapy targeting intimacy, and after 6 months of treatment, improves her avoidance behaviors and marriage.
Continue to: Case 2
Case 2
Ms. R, age 35, is a partnered mother expecting her third child. She has no relevant medical history and is not vaccinated against COVID-19. Her psychiatric history includes self-reported panic attacks and bipolar affective disorder (BPAD). During the second trimester of a desired, unplanned pregnancy, Ms. R presents to an outpatient psychiatry clinic with symptoms of worsening dysphoria and insomnia. She endorses frequent nightmares and flashbacks of CSA as well as remote intimate partner violence. These symptoms, along with hypervigilance, insomnia, anxiety, dysphoria, negative views of herself and her surroundings, and hallucinations of a shadow that whispers “come” when she is alone, worsened during the first trimester of her pregnancy. She recalls experiencing similar trauma-related symptoms during a previous pregnancy but denies a history of pervasive depressive, manic, or psychotic symptoms. She has no other maladaptive personality traits, denies prior substance use or suicidal behavior, and has never been psychiatrically hospitalized or taken psychotropic medications.
Ms. R completes the PCL-5, ITQ, EPDS, and Mood Disorder Questionnaire (MDQ). The results are notable for significant functional impairment related to PTSD and DSO symptoms with minimal concern for BPAD symptoms. The psychiatrist uses ICD-11 criteria to diagnose Ms. R with CPTSD and discusses treatment options with her and her obstetrician. Ms. R is reluctant to take medication until she delivers her baby. She intermittently attends supportive therapy while pregnant. Her pregnancy is complicated by gestational diabetes, and she often misses appointments with her obstetrician and nutritionist.
Ms. R has an uncomplicated vaginal delivery at 38 weeks gestation and success with breastfeeding, but continues to have CPTSD symptoms. She is prescribed quetiapine 25 mg/d for anxiety, insomnia, mood, and psychotic symptoms, but stops taking the medication after 3 days due to excessive sedation. Ms. R is then prescribed sertraline 50 mg/d, which she finds helpful, but has intermittent adherence. She misses multiple virtual appointments with the psychiatrist and does not want to attend in-person sessions due to fear of contracting COVID-19. The psychiatrist encourages Ms. R to get vaccinated, focuses on organizational skills during sessions to promote attendance, and recommends in-person appointments to increase her motivation for treatment and alliance building. Despite numerous outreach attempts, Ms. R is lost to follow-up at 10 months postpartum.
Case 3
Ms. S, age 29, is a partnered mother expecting her fourth child. Her medical history includes chronic back pain. She is not vaccinated against COVID-19, and her psychiatric history includes BPAD. During the first trimester of an undesired, unplanned pregnancy, Ms. S presents to an outpatient psychiatric clinic following an episode where she held a knife over her gravid abdomen during a fight with her partner. She recounts that she became dysregulated and held a knife to her body to communicate her distress, but she did not cut herself, and adamantly denies wanting to hurt herself or the fetus. Ms. S struggles with affective instability, poor frustration tolerance, and irritability. After 1 month of treatment, she discloses surviving prolonged CSA that led to her current nightmares and flashbacks. She also endorses impaired sleep, intimacy avoidance, hypervigilance, impulsive reckless behaviors (including excessive gambling), and negative views about herself and the world that worsened since she learned she was pregnant. Ms. S reports that these same symptoms were aggravated during prior perinatal periods and recalls 2 episodes of severe dysregulation that led to an interrupted suicide attempt and a violent episode toward a loved one. She denies other self-harm behaviors, substance use, or psychotic symptoms, and denies having a history of psychiatric hospitalizations. Ms. S recalls receiving a brief trial of topiramate for BPAD and migraine when she was last in outpatient psychiatric care 8 years ago.
Her psychiatrist administers the PCL-5, ITQ, MDQ, EPDS, and Borderline Symptoms List 23 (BLS-23). The results are notable for significant PTSD and DSO symptoms.16 The psychiatrist diagnoses Ms. S with CPTSD and bipolar II disorder, exacerbated during the peripartum period. Throughout the remainder of her pregnancy, she endorses mood instability with significant irritability but declines pharmacotherapy. Ms. S intermittently engages in psychotherapy using dialectical behavioral therapy (DBT) focusing on distress tolerance because she is unable to tolerate trauma-focused psychotherapy.
Continue to: Ms. S maintains the pregnancy...
Ms. S maintains the pregnancy without any additional complications and has a vaginal delivery at 39 weeks gestation. She initiates breastfeeding but chooses not to continue after 1 month due to fatigue, insomnia, and worsening mood. Her psychiatrist wants to contact Ms. S’s partner to discuss childcare support at night to promote better sleep conditions for Ms. S, but Ms. S declines. Ms. S intermittently attends virtual appointments, adamantly refuses the COVID-19 vaccine, and is fearful of starting a mood stabilizer despite extensive psychoeducation. At 5 months postpartum, Ms. S reports that she is in a worse mood and does not want to continue the appointment or further treatment, and abruptly ends the telepsychiatry session. Her psychiatrist reaches out the following week to schedule an in-person session if Ms. S agrees to wear personal protective equipment, which she is amenable to. During that appointment, the psychiatrist discusses the risks of bipolar depression and CPTSD on both her and her childrens’ development, against the risk of lamotrigine. Ms. S begins taking lamotrigine, which she tolerates without adverse effects, and quickly notices improvement in her mood as the medication is titrated up slowly to 200 mg/d. Ms. S then engages more consistently in psychotherapy and her CPTSD and bipolar II disorder symptoms much improve at 9 months postpartum.
Ensuring an accurate CPTSD diagnosis
These 3 cases illustrate the diversity and complexity of presentations for perinatal CPTSD following CSA. A CPTSD diagnosis is complicated because the differential is broad for those reporting PTSD and DSO symptoms, and CPTSD is commonly comorbid with other disorders such as anxiety and depression.17 While various scales can facilitate PTSD screening, the ITQ is helpful because it catalogs the symptoms of disturbances in self organization and functional impairment inherent in CPTSD. The ITQ can help clinicians and patients conceptualize symptoms and track progress (Figure11).
Once a patient screens positive, a CPTSD diagnosis is best made by the clinician after a full psychiatric interview, similar to other diagnoses. Psychiatrists must use ICD-11 criteria,1 as currently there are no formal DSM-5 criteria for CPTSD.2 Additional scales facilitate CPTSD symptom inventory, such as the PCL-5 to screen and monitor for PTSD symptoms and the BLS-23 to delineate between BPD or DSO symptoms.18 Furthermore, clinicians should screen for other comorbid conditions using additional scales such as the MDQ for BPAD and the EPDS for perinatal mood and anxiety disorders. Sharing a CPTSD diagnosis with a patient is an essential step when initiating treatment. Sensitive psychoeducation on the condition and its application to the perinatal period is key to establishing safety and trust, while also empowering survivors to make their own choices regarding treatment, all essential elements to trauma-informed care.19
A range of treatment options
Once CPTSD is appropriately diagnosed, clinicians must determine whether to use pharmacotherapy, psychotherapy, or both. A meta-analysis by Coventry et al20 sought to determine the best treatment strategies for complex traumatic events such as CSA, Multicomponent interventions were most promising, and psychological interventions were associated with larger effect sizes than pharmacologic interventions for managing PTSD, mood, and sleep. Therapeutic targets include trauma memory processing, self-perception, and dissociation, along with emotion, interpersonal, and somatic regulation.21
Psychotherapy. While there are no standardized guidelines for treating CPTSD, PTSD guidelines suggest using trauma-focused cognitive-behavioral therapy (TF-CBT) as a first-line therapy, though a longer course may be needed to resolve CPTSD symptoms compared to PTSD symptoms.3 DBT for PTSD can be particularly helpful in targeting DSO symptoms.22 Narrative therapy focused on identity, embodiment, and parenting has also shown to be effective for survivors of CSA in the perinatal period, specifically with the goal of meaning-making.5 Therapy can also be effective in a group setting (ie, a “Victim to Survivor” TF-CBT group).23 Sex and couples therapy may be indicated to reestablish trust, especially when it is evident there is sexual inhibition from trauma that influences the relationship, as seen in Case 1.24
Continue to: Pharmacotherapy
Pharmacotherapy. Case 2 and Case 3 both demonstrate that while the peripartum period presents an increased risk for exacerbation of psychiatric symptoms, patients and clinicians may be reluctant to start medications due to concerns for safety during pregnancy or lactation.25 Clinicians must weigh the risks of medication exposure against the risks of exposing the fetus or newborn to untreated psychiatric disease and consult an expert in reproductive psychiatry if questions or concerns arise.26
Adverse effects of psychotropic medications must be considered, especially sedation. Medications that lead to sedation may not be safe or feasible for a mother following delivery, especially if she is breastfeeding. This was exemplified in Case 2, when Ms. R was having troubling hallucinations for which the clinician prescribed quetiapine. The medication resulted in excessive sedation and Ms. R did not feel comfortable performing childcare duties while taking the medication, which greatly influenced future therapy decisions.
Making the decision to prescribe a certain medication for CPTSD is highly influenced by the patient’s most troubling symptoms and their comorbid diagnoses. Selective serotonin reuptake inhibitors (SSRIs) generally are considered safe during pregnancy and breastfeeding, and should be considered as a first-line intervention for PTSD, mood disorders, and anxiety disorders during the perinatal period.27 While prazosin is effective for PTSD symptoms outside of pregnancy, there is limited data regarding its safety during pregnancy and lactation, and it may lead to maternal hypotension and subsequent fetal adverse effects.28
Many patients with a history of CSA experience hallucinations and dissociative symptoms, as demonstrated by Case 1 and Case 2.29 In Case 3, Ms. S displayed features of BPAD with significant hypomanic symptoms and worsening suicidality during prior postpartum periods. The clinician felt comfortable prescribing lamotrigine, a relatively safe medication during the perinatal period compared to other mood stabilizers. Ms. S was amenable to taking lamotrigine, and her clinician avoided the use of an SSRI due to a concern of worsening a bipolar diathesis in this high-risk case.30 Case 2 and Case 3 both highlight the need to closely screen for comorbid conditions such as BPAD and using caution when considering an SSRI in light of the risk of precipitating mania, especially as the patient population is younger and at higher risk for antidepressant-associated mania.31,32
Help patients tap into their sources for strength
Other therapeutic strategies when treating patients with perinatal CPTSD include encouraging survivors to mobilize their support network and sources for strength. Chamberlain et al8 suggest incorporating socioecological and cultural contexts when considering outlets for social support systems and encourage collaborating with families, especially partners, along with community and spiritual networks. As seen in Case 3, clinicians should attempt to speak to family members on behalf of their patients to promote better sleeping conditions, which can greatly alleviate CPTSD and comorbid mood symptoms, and thus reduce suicide risk.33 Sources for strength should be accentuated and clinicians may need to advocate with child protective services to support parenting rights. As demonstrated in Case 1, motherhood can greatly reduce suicide risk, and should be promoted if a child’s safety is not in danger.34
Continue to: Clinicians must recognize...
Clinicians must recognize that patients in the perinatal period face barriers to obtaining health care, especially those with CPTSD, as these patients can be difficult to engage and retain. Each case described in this article challenged the psychiatrist with engagement and alliance-building, stemming from the patient’s CPTSD symptoms of interpersonal difficulties and negative views of surroundings. Case 2 demonstrates how the diagnosis can prevent patients from receiving appropriate prenatal care, while Case 3 shows how clinicians may need more flexible attendance policies and assertive outreach attempts to deliver the mental health care these patients deserve.
These vignettes highlight the psychosocial barriers women face during the perinatal period, such as caring for their child, financial stressors, and COVID-19 pandemic–related factors that can hinder treatment, which can be compounded by trauma. The uncertainty, unpredictability, loss of control, and loss of support structures collectively experienced during the pandemic can be triggering and precipitate worsening CPTSD symptoms.35 Women who experience trauma are less likely to obtain the COVID-19 vaccine for themselves or their children, and this hesitancy is often driven by institutional distrust.36 Policy leaders and clinicians should consider these factors to promote trauma-informed COVID-19 vaccine initiatives and expand mental health access using less orthodox treatment settings, such as telepsychiatry. Telepsychiatry can serve as a bridge to in-person care as patients may feel a higher sense of control when in a familiar home environment. Case 2 and Case 3 exemplify the difficulties of delivering mental health care to perinatal women with CPTSD during the pandemic, especially those who are vaccine-hesitant, and illustrate the importance of adapting a patient’s treatment plan in a personalized and trauma-informed way.
Psychiatrists can help obstetricians and pediatricians by explaining that avoidance patterns and distrust in the clinical setting may be related to trauma and are not grounds for conscious or subconscious punishment or abandonment. Educating other clinicians about trauma-informed care, precautions to use for perinatal patients, and ways to effectively support survivors of CSA can greatly improve health outcomes for perinatal women and their offspring.37
Bottom Line
Complex posttraumatic stress disorder (CPTSD) is characterized by classic PTSD symptoms as well as disturbances in self organization, which can include mood symptoms, psychotic symptoms, and maladaptive personality traits. CPTSD resulting from childhood sexual abuse is of particular concern for women, especially during the perinatal period. Clinicians must know how to recognize the signs and symptoms of CPTSD so they can tailor a trauma-informed treatment plan and promote treatment access in this highly vulnerable patient population.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Keswani M, Nemcek S, Rashid N. Is it bipolar disorder, or a complex form of PTSD? Current Psychiatry. 2021;20(12):42-49. doi:10.12788/cp.0188
Drug Brand Names
Carbamazepine • Carbatrol
Clonazepam • Klonopin
Lamotrigine • Lamictal
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Topiramate • Topamax
1. World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). Complex posttraumatic stress disorder. Accessed November 6, 2021. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/585833559
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Cloitre M, Garvert DW, Brewin CR, et al. Evidence for proposed ICD-11 PTSD and complexPTSD: a latent profile analysis. Eur J Psychotraumatol. 2013;4:10.3402/ejpt.v4i0.20706. doi:10.3402/ejpt.v4i0.20706
4. Leeb RT, Paulozzi LJ, Melanson C, et al. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements, Version 1.0. Centers for Disease Control and Prevention, Department of Health & Human Services; 2008. Accessed August 24, 2022. https://www.cdc.gov/violenceprevention/pdf/cm_surveillance-a.pdf
5. Byrne J, Smart C, Watson G. “I felt like I was being abused all over again”: how survivors of child sexual abuse make sense of the perinatal period through their narratives. J Child Sex Abus. 2017;26(4):465-486. doi:10.1080/10538712.2017.1297880
6. Flom JD, Chiu YM, Hsu HL, et al. Maternal lifetime trauma and birthweight: effect modification by in utero cortisol and child sex. J Pediatr. 2018;203:301-308. doi:10.1016/j.jpeds.2018.07.069
7. Spinazzola J, van der Kolk B, Ford JD. When nowhere is safe: interpersonal trauma and attachment adversity as antecedents of posttraumatic stress disorder and developmental trauma disorder. J Trauma Stress. 2018;31(5):631-642. doi:10.1002/jts.22320
8. Chamberlain C, Gee G, Harfield S, et al. Parenting after a history of childhood maltreatment: a scoping review and map of evidence in the perinatal period. PloS One. 2019;14(3):e0213460. doi:10.1371/journal.pone.0213460
9. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi:10.1016/j.jad.2017.07.045
10. Gavin AR, Morris J. The association between maternal early life forced sexual intercourse and offspring birth weight: the role of socioeconomic status. J Womens Health (Larchmt). 2017;26(5):442-449. doi:10.1089/jwh.2016.5789
11. Cloitre M, Shevlin M, Brewin CR, et al. The international trauma questionnaire: development of a self-report measure of ICD-11 PTSD and complex PTSD. Acta Psychiatr Scand. 2018;138(6):536-546.
12. Cloitre M, Hyland P, Prins A, et al. The international trauma questionnaire (ITQ) measures reliable and clinically significant treatment-related change in PTSD and complex PTSD. Eur J Psychotraumatol. 2021;12(1):1930961. doi:10.1080/20008198.2021.1930961
13. Weathers FW, Litz BT, Keane TM, et al. PTSD Checklist for DSM-5 (PCL-5). US Department of Veterans Affairs. April 11, 2018. Accessed November 25, 2021. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.PDF
14. Dissociative Experiences Scale – II. TraumaDissociation.com. Accessed November 25, 2021. http://traumadissociation.com/des
15. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi:10.1192/bjp.150.6.782
16. Mood Disorder Questionnaire (MDQ). Oregon Health & Science University. Accessed November 7, 2021. https://www.ohsu.edu/sites/default/files/2019-06/cms-quality-bipolar_disorder_mdq_screener.pdf
17. Karatzias T, Hyland P, Bradley A, et al. Risk factors and comorbidity of ICD-11 PTSD and complex PTSD: findings from a trauma-exposed population based sample of adults in the United Kingdom. Depress Anxiety. 2019;36(9):887-894. doi:10.1002/da.22934
18. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
19. Fallot RD, Harris M. A trauma-informed approach to screening and assessment. New Dir Ment Health Serv. 2001;(89):23-31. doi:10.1002/yd.23320018904
20. Coventry PA, Meader N, Melton H, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262. doi:10.1371/journal.pmed.1003262
21. Ford JD. Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Curr Treat Options Psychiatry. 2021;8:1-17. doi:10.1007/s40501-020-00236-6
22. Becker-Sadzio J, Gundel F, Kroczek A, et al. Trauma exposure therapy in a pregnant woman suffering from complex posttraumatic stress disorder after childhood sexual abuse: risk or benefit? Eur J Psychotraumatol. 2020;11(1):1697581. doi:10.1080/20008198.2019.1697581
23. Mendelsohn M, Zachary RS, Harney PA. Group therapy as an ecological bridge to new community for trauma survivors. J Aggress Maltreat Trauma. 2007;14(1-2):227-243. doi:10.1300/J146v14n01_12
24. Macintosh HB, Vaillancourt-Morel MP, Bergeron S. Sex and couple therapy with survivors of childhood trauma. In: Hall KS, Binik YM, eds. Principles and Practice of Sex Therapy. 6th ed. Guilford Press; 2020.
25. Dresner N, Byatt N, Gopalan P, et al. Psychiatric care of peripartum women. Psychiatric Times. 2015;32(12).
26. Zagorski N. How to manage meds before, during, and after pregnancy. Psychiatric News. 2019;54(14):13. https://doi.org/10.1176/APPI.PN.2019.6B36
27. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397-2407. doi:10.1056/NEJMoa1312828
28. Davidson AD, Bhat A, Chu F, et al. A systematic review of the use of prazosin in pregnancy and lactation. Gen Hosp Psychiatry. 2021;71:134-136. doi:10.1016/j.genhosppsych.2021.03.012
29. Shinn AK, Wolff JD, Hwang M, et al. Assessing voice hearing in trauma spectrum disorders: a comparison of two measures and a review of the literature. Front Psychiatry. 2020;10:1011. doi:10.3389/fpsyt.2019.01011
30. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi:10.1016/j.clp.2019.02.004
31. Martin A, Young C, Leckman JF, et al. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adoles Med. 2004;158(8):773-780. doi:10.1001/archpedi.158.8.773
32. Gill N, Bayes A, Parker G. A review of antidepressant-associated hypomania in those diagnosed with unipolar depression-risk factors, conceptual models, and management. Curr Psychiatry Rep. 2020;22(4):20. doi:10.1007/s11920-020-01143-6
33. Harris LM, Huang X, Linthicum KP, et al. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Sci Rep. 2020;10(1):13888. doi:10.1038/s41598-020-70866-6
34. Dehara M, Wells MB, Sjöqvist H, et al. Parenthood is associated with lower suicide risk: a register-based cohort study of 1.5 million Swedes. Acta Psychiatr Scand. 2021;143(3):206-215. doi:10.1111/acps.13240
35. Iyengar U, Jaiprakash B, Haitsuka H, et al. One year into the pandemic: a systematic review of perinatal mental health outcomes during COVID-19. Front Psychiatry. 2021;12:674194. doi:10.3389/fpsyt.2021.674194
36. Milan S, Dáu ALBT. The role of trauma in mothers’ COVID-19 vaccine beliefs and intentions. J Pediatr Psychol. 2021;46(5):526-535. doi:10.1093/jpepsy/jsab043
37. Coles J, Jones K. “Universal precautions”: perinatal touch and examination after childhood sexual abuse. Birth. 2009;36(3):230-236. doi:10.1111/j.1523-536X.2009.00327
1. World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). Complex posttraumatic stress disorder. Accessed November 6, 2021. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/585833559
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Cloitre M, Garvert DW, Brewin CR, et al. Evidence for proposed ICD-11 PTSD and complexPTSD: a latent profile analysis. Eur J Psychotraumatol. 2013;4:10.3402/ejpt.v4i0.20706. doi:10.3402/ejpt.v4i0.20706
4. Leeb RT, Paulozzi LJ, Melanson C, et al. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements, Version 1.0. Centers for Disease Control and Prevention, Department of Health & Human Services; 2008. Accessed August 24, 2022. https://www.cdc.gov/violenceprevention/pdf/cm_surveillance-a.pdf
5. Byrne J, Smart C, Watson G. “I felt like I was being abused all over again”: how survivors of child sexual abuse make sense of the perinatal period through their narratives. J Child Sex Abus. 2017;26(4):465-486. doi:10.1080/10538712.2017.1297880
6. Flom JD, Chiu YM, Hsu HL, et al. Maternal lifetime trauma and birthweight: effect modification by in utero cortisol and child sex. J Pediatr. 2018;203:301-308. doi:10.1016/j.jpeds.2018.07.069
7. Spinazzola J, van der Kolk B, Ford JD. When nowhere is safe: interpersonal trauma and attachment adversity as antecedents of posttraumatic stress disorder and developmental trauma disorder. J Trauma Stress. 2018;31(5):631-642. doi:10.1002/jts.22320
8. Chamberlain C, Gee G, Harfield S, et al. Parenting after a history of childhood maltreatment: a scoping review and map of evidence in the perinatal period. PloS One. 2019;14(3):e0213460. doi:10.1371/journal.pone.0213460
9. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi:10.1016/j.jad.2017.07.045
10. Gavin AR, Morris J. The association between maternal early life forced sexual intercourse and offspring birth weight: the role of socioeconomic status. J Womens Health (Larchmt). 2017;26(5):442-449. doi:10.1089/jwh.2016.5789
11. Cloitre M, Shevlin M, Brewin CR, et al. The international trauma questionnaire: development of a self-report measure of ICD-11 PTSD and complex PTSD. Acta Psychiatr Scand. 2018;138(6):536-546.
12. Cloitre M, Hyland P, Prins A, et al. The international trauma questionnaire (ITQ) measures reliable and clinically significant treatment-related change in PTSD and complex PTSD. Eur J Psychotraumatol. 2021;12(1):1930961. doi:10.1080/20008198.2021.1930961
13. Weathers FW, Litz BT, Keane TM, et al. PTSD Checklist for DSM-5 (PCL-5). US Department of Veterans Affairs. April 11, 2018. Accessed November 25, 2021. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.PDF
14. Dissociative Experiences Scale – II. TraumaDissociation.com. Accessed November 25, 2021. http://traumadissociation.com/des
15. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi:10.1192/bjp.150.6.782
16. Mood Disorder Questionnaire (MDQ). Oregon Health & Science University. Accessed November 7, 2021. https://www.ohsu.edu/sites/default/files/2019-06/cms-quality-bipolar_disorder_mdq_screener.pdf
17. Karatzias T, Hyland P, Bradley A, et al. Risk factors and comorbidity of ICD-11 PTSD and complex PTSD: findings from a trauma-exposed population based sample of adults in the United Kingdom. Depress Anxiety. 2019;36(9):887-894. doi:10.1002/da.22934
18. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
19. Fallot RD, Harris M. A trauma-informed approach to screening and assessment. New Dir Ment Health Serv. 2001;(89):23-31. doi:10.1002/yd.23320018904
20. Coventry PA, Meader N, Melton H, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262. doi:10.1371/journal.pmed.1003262
21. Ford JD. Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Curr Treat Options Psychiatry. 2021;8:1-17. doi:10.1007/s40501-020-00236-6
22. Becker-Sadzio J, Gundel F, Kroczek A, et al. Trauma exposure therapy in a pregnant woman suffering from complex posttraumatic stress disorder after childhood sexual abuse: risk or benefit? Eur J Psychotraumatol. 2020;11(1):1697581. doi:10.1080/20008198.2019.1697581
23. Mendelsohn M, Zachary RS, Harney PA. Group therapy as an ecological bridge to new community for trauma survivors. J Aggress Maltreat Trauma. 2007;14(1-2):227-243. doi:10.1300/J146v14n01_12
24. Macintosh HB, Vaillancourt-Morel MP, Bergeron S. Sex and couple therapy with survivors of childhood trauma. In: Hall KS, Binik YM, eds. Principles and Practice of Sex Therapy. 6th ed. Guilford Press; 2020.
25. Dresner N, Byatt N, Gopalan P, et al. Psychiatric care of peripartum women. Psychiatric Times. 2015;32(12).
26. Zagorski N. How to manage meds before, during, and after pregnancy. Psychiatric News. 2019;54(14):13. https://doi.org/10.1176/APPI.PN.2019.6B36
27. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397-2407. doi:10.1056/NEJMoa1312828
28. Davidson AD, Bhat A, Chu F, et al. A systematic review of the use of prazosin in pregnancy and lactation. Gen Hosp Psychiatry. 2021;71:134-136. doi:10.1016/j.genhosppsych.2021.03.012
29. Shinn AK, Wolff JD, Hwang M, et al. Assessing voice hearing in trauma spectrum disorders: a comparison of two measures and a review of the literature. Front Psychiatry. 2020;10:1011. doi:10.3389/fpsyt.2019.01011
30. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi:10.1016/j.clp.2019.02.004
31. Martin A, Young C, Leckman JF, et al. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adoles Med. 2004;158(8):773-780. doi:10.1001/archpedi.158.8.773
32. Gill N, Bayes A, Parker G. A review of antidepressant-associated hypomania in those diagnosed with unipolar depression-risk factors, conceptual models, and management. Curr Psychiatry Rep. 2020;22(4):20. doi:10.1007/s11920-020-01143-6
33. Harris LM, Huang X, Linthicum KP, et al. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Sci Rep. 2020;10(1):13888. doi:10.1038/s41598-020-70866-6
34. Dehara M, Wells MB, Sjöqvist H, et al. Parenthood is associated with lower suicide risk: a register-based cohort study of 1.5 million Swedes. Acta Psychiatr Scand. 2021;143(3):206-215. doi:10.1111/acps.13240
35. Iyengar U, Jaiprakash B, Haitsuka H, et al. One year into the pandemic: a systematic review of perinatal mental health outcomes during COVID-19. Front Psychiatry. 2021;12:674194. doi:10.3389/fpsyt.2021.674194
36. Milan S, Dáu ALBT. The role of trauma in mothers’ COVID-19 vaccine beliefs and intentions. J Pediatr Psychol. 2021;46(5):526-535. doi:10.1093/jpepsy/jsab043
37. Coles J, Jones K. “Universal precautions”: perinatal touch and examination after childhood sexual abuse. Birth. 2009;36(3):230-236. doi:10.1111/j.1523-536X.2009.00327
Seizure-like episodes, but is it really epilepsy?
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.
Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.
A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.
Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.
A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.
Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.
A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.