User login
FISH and PCR aid in diagnosis of human intestinal spirochetosis
Fluorescence in situ hybridization (FISH) combined with 16S rRNA gene amplification and sequencing allowed researchers to make a definitive diagnosis of human intestinal spirochetosis (HIS) and further identify the causative pathogens.
Pablo Rojas, PhD, of Charité Universitätsmedizin Berlin, and his colleagues evaluated 149 paraffin-embedded or native intestinal biopsies from 91 consecutive patients with histologically diagnosed HIS. The reasons for endoscopy and histological investigation included chronic diarrhea, cancer/adenoma screening, inflammatory bowel disease, and endoscopic detection of polyps or colitis. In all, 12 patients were HIV-positive.
The researchers used a FISH probe to confirm Brachyspira spp. HIS for 77 of the 91 patients. A polymerase chain reaction (PCR) analysis of part of the bacterial 16S rRNA gene confirmed the presence of Brachyspira spp. in 75 patients. The sequencing allowed the researchers to drill down on the pathogen, identifying both the B. aalborgi and B. pilosicoli species lineage among the samples.
There were 14 cases in which researchers could not confirm the diagnosis of HIS with either FISH or RNA sequencing, but they noted that these cases were likely misdiagnosed by histopathology.
“FISH at the interface of histopathology and molecular biology is a valuable diagnostic tool for the diagnosis of HIS. The bright FISH signal in most cases already allowed rapid localization of Brachyspira spp. at 400 magnification. All samples that could be tested via PCR were consistent with the FISH results, whereas 14 cases were diagnosed false positive by histopathology,” the researchers wrote. “On these grounds, we propose to include FISH for the diagnosis and follow-up observation of HIS in routine practice.”
Find the full study in Anaerobe (2017 Oct. doi: 10.1016/j.anaerobe.2017.03.012).
Fluorescence in situ hybridization (FISH) combined with 16S rRNA gene amplification and sequencing allowed researchers to make a definitive diagnosis of human intestinal spirochetosis (HIS) and further identify the causative pathogens.
Pablo Rojas, PhD, of Charité Universitätsmedizin Berlin, and his colleagues evaluated 149 paraffin-embedded or native intestinal biopsies from 91 consecutive patients with histologically diagnosed HIS. The reasons for endoscopy and histological investigation included chronic diarrhea, cancer/adenoma screening, inflammatory bowel disease, and endoscopic detection of polyps or colitis. In all, 12 patients were HIV-positive.
The researchers used a FISH probe to confirm Brachyspira spp. HIS for 77 of the 91 patients. A polymerase chain reaction (PCR) analysis of part of the bacterial 16S rRNA gene confirmed the presence of Brachyspira spp. in 75 patients. The sequencing allowed the researchers to drill down on the pathogen, identifying both the B. aalborgi and B. pilosicoli species lineage among the samples.
There were 14 cases in which researchers could not confirm the diagnosis of HIS with either FISH or RNA sequencing, but they noted that these cases were likely misdiagnosed by histopathology.
“FISH at the interface of histopathology and molecular biology is a valuable diagnostic tool for the diagnosis of HIS. The bright FISH signal in most cases already allowed rapid localization of Brachyspira spp. at 400 magnification. All samples that could be tested via PCR were consistent with the FISH results, whereas 14 cases were diagnosed false positive by histopathology,” the researchers wrote. “On these grounds, we propose to include FISH for the diagnosis and follow-up observation of HIS in routine practice.”
Find the full study in Anaerobe (2017 Oct. doi: 10.1016/j.anaerobe.2017.03.012).
Fluorescence in situ hybridization (FISH) combined with 16S rRNA gene amplification and sequencing allowed researchers to make a definitive diagnosis of human intestinal spirochetosis (HIS) and further identify the causative pathogens.
Pablo Rojas, PhD, of Charité Universitätsmedizin Berlin, and his colleagues evaluated 149 paraffin-embedded or native intestinal biopsies from 91 consecutive patients with histologically diagnosed HIS. The reasons for endoscopy and histological investigation included chronic diarrhea, cancer/adenoma screening, inflammatory bowel disease, and endoscopic detection of polyps or colitis. In all, 12 patients were HIV-positive.
The researchers used a FISH probe to confirm Brachyspira spp. HIS for 77 of the 91 patients. A polymerase chain reaction (PCR) analysis of part of the bacterial 16S rRNA gene confirmed the presence of Brachyspira spp. in 75 patients. The sequencing allowed the researchers to drill down on the pathogen, identifying both the B. aalborgi and B. pilosicoli species lineage among the samples.
There were 14 cases in which researchers could not confirm the diagnosis of HIS with either FISH or RNA sequencing, but they noted that these cases were likely misdiagnosed by histopathology.
“FISH at the interface of histopathology and molecular biology is a valuable diagnostic tool for the diagnosis of HIS. The bright FISH signal in most cases already allowed rapid localization of Brachyspira spp. at 400 magnification. All samples that could be tested via PCR were consistent with the FISH results, whereas 14 cases were diagnosed false positive by histopathology,” the researchers wrote. “On these grounds, we propose to include FISH for the diagnosis and follow-up observation of HIS in routine practice.”
Find the full study in Anaerobe (2017 Oct. doi: 10.1016/j.anaerobe.2017.03.012).
FROM ANAEROBE
U.S. House passes 20-week abortion ban
The U.S. House of Representatives has passed legislation that would ban abortions starting at 20 weeks.
This isn’t the first time in recent years that the Republican-controlled House has passed a 20-week ban. What’s different this time is that it has the support of the White House. Despite the Trump administration’s support for the bill, it’s unlikely to garner the support necessary to come up for a vote in the U.S. Senate.
The American Congress of Obstetricians and Gynecologists (ACOG), which opposes the bill, called it a “cruel ban” that would leave many women without treatment options.
“Many women seek abortion later in pregnancy because restrictive state laws or the lack of abortion providers made it impossible for them to access abortion earlier in their pregnancies,” ACOG said in a statement. “Additionally, many women are delayed in their ability to access abortion care because they need time to raise or save enough money to pay for it.”
Currently, 17 states have enacted their own 20-week abortion bans, according to ACOG.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The U.S. House of Representatives has passed legislation that would ban abortions starting at 20 weeks.
This isn’t the first time in recent years that the Republican-controlled House has passed a 20-week ban. What’s different this time is that it has the support of the White House. Despite the Trump administration’s support for the bill, it’s unlikely to garner the support necessary to come up for a vote in the U.S. Senate.
The American Congress of Obstetricians and Gynecologists (ACOG), which opposes the bill, called it a “cruel ban” that would leave many women without treatment options.
“Many women seek abortion later in pregnancy because restrictive state laws or the lack of abortion providers made it impossible for them to access abortion earlier in their pregnancies,” ACOG said in a statement. “Additionally, many women are delayed in their ability to access abortion care because they need time to raise or save enough money to pay for it.”
Currently, 17 states have enacted their own 20-week abortion bans, according to ACOG.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The U.S. House of Representatives has passed legislation that would ban abortions starting at 20 weeks.
This isn’t the first time in recent years that the Republican-controlled House has passed a 20-week ban. What’s different this time is that it has the support of the White House. Despite the Trump administration’s support for the bill, it’s unlikely to garner the support necessary to come up for a vote in the U.S. Senate.
The American Congress of Obstetricians and Gynecologists (ACOG), which opposes the bill, called it a “cruel ban” that would leave many women without treatment options.
“Many women seek abortion later in pregnancy because restrictive state laws or the lack of abortion providers made it impossible for them to access abortion earlier in their pregnancies,” ACOG said in a statement. “Additionally, many women are delayed in their ability to access abortion care because they need time to raise or save enough money to pay for it.”
Currently, 17 states have enacted their own 20-week abortion bans, according to ACOG.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Zika: CDC begins transition from emergency to long-term response
Officials at the Centers for Disease Control and Prevention are deactivating their emergency operations related to the Zika virus, the agency announced Sept. 29.
The Zika Coordination and Operations Transition Team will instead shift the CDC’s efforts toward long-term activities, including continued support for physicians counseling pregnant women and advice on follow-up care for infants.
Data from the agency show that while there was a steady stream of possible Zika cases reported among pregnant women in the summer of 2017 – about 100 cases every 2 weeks – the numbers were lower than in 2016, when the infection hit its peak. Overall, during 2015-2017, there have been a total of 2,197 cases of possible Zika infection among pregnant women reported in U.S. states and 4,504 in U.S. territories. During that same time period, there have been 98 liveborn infants with birth defects in the U.S. states and 138 in the U.S. territories.
CDC activated the Emergency Operations Center in January 2016 in response to reports on the impact of Zika virus infection in pregnancy, including cases of microcephaly.
“Deactivation does not mean that the threat of Zika has lessened in importance or that people are no longer at risk of infection,” CDC officials said in a statement. “Zika continues to be a public health threat in the United States and internationally. Zika is still a risk for pregnant women, and the continental United States and Hawaii will continue to see some travel-related cases as travelers visit countries and territories with risk of Zika transmission.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Officials at the Centers for Disease Control and Prevention are deactivating their emergency operations related to the Zika virus, the agency announced Sept. 29.
The Zika Coordination and Operations Transition Team will instead shift the CDC’s efforts toward long-term activities, including continued support for physicians counseling pregnant women and advice on follow-up care for infants.
Data from the agency show that while there was a steady stream of possible Zika cases reported among pregnant women in the summer of 2017 – about 100 cases every 2 weeks – the numbers were lower than in 2016, when the infection hit its peak. Overall, during 2015-2017, there have been a total of 2,197 cases of possible Zika infection among pregnant women reported in U.S. states and 4,504 in U.S. territories. During that same time period, there have been 98 liveborn infants with birth defects in the U.S. states and 138 in the U.S. territories.
CDC activated the Emergency Operations Center in January 2016 in response to reports on the impact of Zika virus infection in pregnancy, including cases of microcephaly.
“Deactivation does not mean that the threat of Zika has lessened in importance or that people are no longer at risk of infection,” CDC officials said in a statement. “Zika continues to be a public health threat in the United States and internationally. Zika is still a risk for pregnant women, and the continental United States and Hawaii will continue to see some travel-related cases as travelers visit countries and territories with risk of Zika transmission.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Officials at the Centers for Disease Control and Prevention are deactivating their emergency operations related to the Zika virus, the agency announced Sept. 29.
The Zika Coordination and Operations Transition Team will instead shift the CDC’s efforts toward long-term activities, including continued support for physicians counseling pregnant women and advice on follow-up care for infants.
Data from the agency show that while there was a steady stream of possible Zika cases reported among pregnant women in the summer of 2017 – about 100 cases every 2 weeks – the numbers were lower than in 2016, when the infection hit its peak. Overall, during 2015-2017, there have been a total of 2,197 cases of possible Zika infection among pregnant women reported in U.S. states and 4,504 in U.S. territories. During that same time period, there have been 98 liveborn infants with birth defects in the U.S. states and 138 in the U.S. territories.
CDC activated the Emergency Operations Center in January 2016 in response to reports on the impact of Zika virus infection in pregnancy, including cases of microcephaly.
“Deactivation does not mean that the threat of Zika has lessened in importance or that people are no longer at risk of infection,” CDC officials said in a statement. “Zika continues to be a public health threat in the United States and internationally. Zika is still a risk for pregnant women, and the continental United States and Hawaii will continue to see some travel-related cases as travelers visit countries and territories with risk of Zika transmission.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
FDA approves single-dose, oral bacterial vaginosis treatment
, thanks to its approval by the Food and Drug Administration.
Symbiomix Therapeutics announced Sept. 18 that the FDA had granted approval to secnidazole (Solosec) 2 g oral granules for the treatment of bacterial vaginosis in adult women. Clinical trials of the 5-nitroimidazole antibiotic have shown it to be effective in a single dose, offering the potential for greater patient adherence to treatment over the common regimen of twice-a-day dosing for 7 days.
In a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study of 215 women with bacterial vaginosis, the clinical cure rate was 65.3% for the 2-g secnidazole group, 49.3% for the 1-g secnidazole group, and 19.4% for the placebo group (Obstet Gynecol. 2017 Aug;130[2]:379-86).
Similarly, in a phase 3 double-blind, placebo-controlled study with 189 women, clinical cure rates based on the 2016 FDA guidance were 64.0% for single-dose secnidazole 2 g versus 26.4% for placebo (Am J Obstet Gynecol. 2017 Sep 1. doi: 10.1016/j.ajog.2017.08.017).
The most common adverse events in the trials were vulvovaginal candidiasis (9.6%), headache (3.6%), nausea (3.6%), dysgeusia (3.4%), vomiting (2.5%), diarrhea (2.5%), abdominal pain (2.0%), and vulvovaginal pruritus (2.0%).
The FDA designated the drug as a qualified infectious disease product and granted it fast-track designation, making it eligible for priority review and at least 10 years of market exclusivity.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
, thanks to its approval by the Food and Drug Administration.
Symbiomix Therapeutics announced Sept. 18 that the FDA had granted approval to secnidazole (Solosec) 2 g oral granules for the treatment of bacterial vaginosis in adult women. Clinical trials of the 5-nitroimidazole antibiotic have shown it to be effective in a single dose, offering the potential for greater patient adherence to treatment over the common regimen of twice-a-day dosing for 7 days.
In a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study of 215 women with bacterial vaginosis, the clinical cure rate was 65.3% for the 2-g secnidazole group, 49.3% for the 1-g secnidazole group, and 19.4% for the placebo group (Obstet Gynecol. 2017 Aug;130[2]:379-86).
Similarly, in a phase 3 double-blind, placebo-controlled study with 189 women, clinical cure rates based on the 2016 FDA guidance were 64.0% for single-dose secnidazole 2 g versus 26.4% for placebo (Am J Obstet Gynecol. 2017 Sep 1. doi: 10.1016/j.ajog.2017.08.017).
The most common adverse events in the trials were vulvovaginal candidiasis (9.6%), headache (3.6%), nausea (3.6%), dysgeusia (3.4%), vomiting (2.5%), diarrhea (2.5%), abdominal pain (2.0%), and vulvovaginal pruritus (2.0%).
The FDA designated the drug as a qualified infectious disease product and granted it fast-track designation, making it eligible for priority review and at least 10 years of market exclusivity.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
, thanks to its approval by the Food and Drug Administration.
Symbiomix Therapeutics announced Sept. 18 that the FDA had granted approval to secnidazole (Solosec) 2 g oral granules for the treatment of bacterial vaginosis in adult women. Clinical trials of the 5-nitroimidazole antibiotic have shown it to be effective in a single dose, offering the potential for greater patient adherence to treatment over the common regimen of twice-a-day dosing for 7 days.
In a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study of 215 women with bacterial vaginosis, the clinical cure rate was 65.3% for the 2-g secnidazole group, 49.3% for the 1-g secnidazole group, and 19.4% for the placebo group (Obstet Gynecol. 2017 Aug;130[2]:379-86).
Similarly, in a phase 3 double-blind, placebo-controlled study with 189 women, clinical cure rates based on the 2016 FDA guidance were 64.0% for single-dose secnidazole 2 g versus 26.4% for placebo (Am J Obstet Gynecol. 2017 Sep 1. doi: 10.1016/j.ajog.2017.08.017).
The most common adverse events in the trials were vulvovaginal candidiasis (9.6%), headache (3.6%), nausea (3.6%), dysgeusia (3.4%), vomiting (2.5%), diarrhea (2.5%), abdominal pain (2.0%), and vulvovaginal pruritus (2.0%).
The FDA designated the drug as a qualified infectious disease product and granted it fast-track designation, making it eligible for priority review and at least 10 years of market exclusivity.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
FDA extends Liletta IUD duration of use to 4 years
The Food and Drug Administration has approved a supplemental New Drug Application to extend the duration of use for Liletta (levonorgestrel-releasing intrauterine system) 52 mg, for up to 4 years.
The approval, issued Aug. 3, adds 1 year to the duration of use on the drug label. It is based on additional efficacy and safety data from ACCESS IUS (A Comprehensive Contraceptive Efficacy & Safety Study of an Intrauterine System), an ongoing phase 3 trial with 1,751 U.S. women.
There are three other levonorgestrel-releasing IUDs currently on the market: Mirena and Kyleena, which are both approved for up to 5 years of use; and Skyla, which is approved for up to 3 years of use.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The Food and Drug Administration has approved a supplemental New Drug Application to extend the duration of use for Liletta (levonorgestrel-releasing intrauterine system) 52 mg, for up to 4 years.
The approval, issued Aug. 3, adds 1 year to the duration of use on the drug label. It is based on additional efficacy and safety data from ACCESS IUS (A Comprehensive Contraceptive Efficacy & Safety Study of an Intrauterine System), an ongoing phase 3 trial with 1,751 U.S. women.
There are three other levonorgestrel-releasing IUDs currently on the market: Mirena and Kyleena, which are both approved for up to 5 years of use; and Skyla, which is approved for up to 3 years of use.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The Food and Drug Administration has approved a supplemental New Drug Application to extend the duration of use for Liletta (levonorgestrel-releasing intrauterine system) 52 mg, for up to 4 years.
The approval, issued Aug. 3, adds 1 year to the duration of use on the drug label. It is based on additional efficacy and safety data from ACCESS IUS (A Comprehensive Contraceptive Efficacy & Safety Study of an Intrauterine System), an ongoing phase 3 trial with 1,751 U.S. women.
There are three other levonorgestrel-releasing IUDs currently on the market: Mirena and Kyleena, which are both approved for up to 5 years of use; and Skyla, which is approved for up to 3 years of use.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Ob.gyns. split on cosmetic genital surgery
Ob.gyns. appear divided on whether it is appropriate to perform genital surgery for cosmetic reasons, according to the results of a recent online reader poll.
In a poll conducted by Ob.Gyn. News, 48.7% of respondents said it is appropriate to perform gynecologic procedures, such as labiaplasty, for cosmetic reasons, while 51.3% said it is not appropriate.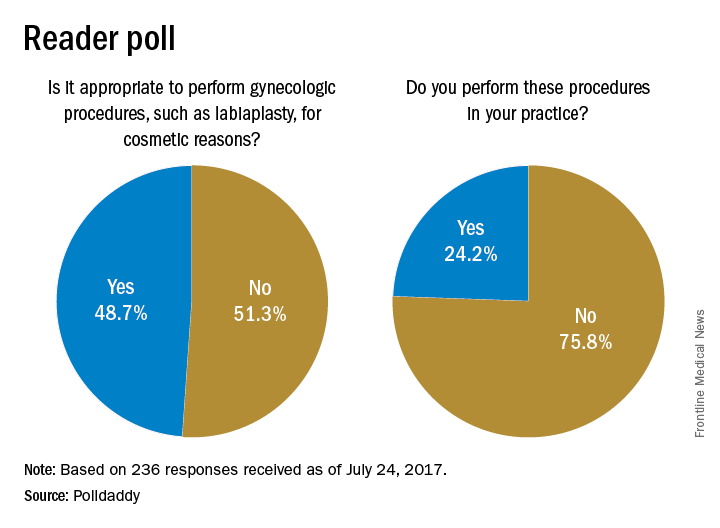
Results are based on 236 reader responses from June 29 to July 24, 2017.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Ob.gyns. appear divided on whether it is appropriate to perform genital surgery for cosmetic reasons, according to the results of a recent online reader poll.
In a poll conducted by Ob.Gyn. News, 48.7% of respondents said it is appropriate to perform gynecologic procedures, such as labiaplasty, for cosmetic reasons, while 51.3% said it is not appropriate.
Results are based on 236 reader responses from June 29 to July 24, 2017.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Ob.gyns. appear divided on whether it is appropriate to perform genital surgery for cosmetic reasons, according to the results of a recent online reader poll.
In a poll conducted by Ob.Gyn. News, 48.7% of respondents said it is appropriate to perform gynecologic procedures, such as labiaplasty, for cosmetic reasons, while 51.3% said it is not appropriate.
Results are based on 236 reader responses from June 29 to July 24, 2017.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Beware hormonal IUD expulsion in obese women
SAN DIEGO – Obese women with a body mass index of 40 or greater are more likely to experience expulsion of levonorgestrel IUDs than women with lower BMI, according to findings from a retrospective cohort study.
Women with class III obesity (a BMI of 40 or greater) had a 3.06-times higher odds of expulsion (95% confidence interval, 1.69-5.57) with a levonorgestrel IUD, compared with a control group of women with a BMI of less than 35, Lynne Saito-Tom, MD, of the University of Hawaii at Manoa, Honolulu, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Dr. Saito-Tom and her colleagues looked at other potential IUD complications, including infection, perforation, and pregnancy. Women with class III obesity also had a higher rate of all complications combined at 24%, compared with 10% among women with class I obesity.
Although complications were higher for more severely obese women, there were no differences between BMI groups in difficulty with insertion (P = .59) or 12-month continuation (P = .69).
The study was unique because it included a diverse ethnic population, with 36% Native Hawaiian/Pacific Islander women and 31% Asian women.
The study wasn’t powered to identify the reasons for greater expulsion in obese women, but some theories include that the IUD could be more difficult to place in larger women without adequate instrumentation or exam tables. Women with class III obesity also tend to have higher rates of heavy menstrual bleeding, which could be driving expulsion, Dr. Saito-Tom said.
However, the results should not deter physicians from placing hormonal IUD in these women, she said. While the expulsion rate was higher than seen generally, it is still an effective method for most obese women. “That’s much more beneficial than discouraging patients,” Dr. Saito-Tom said.
Physicians should continue to educate patients about the benefits of long-acting reversible contraceptives and encourage all patients to utilize them, regardless of their weight, she said.
Dr. Saito-Tom reported having no relevant financial disclosures. One of her colleagues reported grant support from Merck and being a consultant for UpToDate.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
SAN DIEGO – Obese women with a body mass index of 40 or greater are more likely to experience expulsion of levonorgestrel IUDs than women with lower BMI, according to findings from a retrospective cohort study.
Women with class III obesity (a BMI of 40 or greater) had a 3.06-times higher odds of expulsion (95% confidence interval, 1.69-5.57) with a levonorgestrel IUD, compared with a control group of women with a BMI of less than 35, Lynne Saito-Tom, MD, of the University of Hawaii at Manoa, Honolulu, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Dr. Saito-Tom and her colleagues looked at other potential IUD complications, including infection, perforation, and pregnancy. Women with class III obesity also had a higher rate of all complications combined at 24%, compared with 10% among women with class I obesity.
Although complications were higher for more severely obese women, there were no differences between BMI groups in difficulty with insertion (P = .59) or 12-month continuation (P = .69).
The study was unique because it included a diverse ethnic population, with 36% Native Hawaiian/Pacific Islander women and 31% Asian women.
The study wasn’t powered to identify the reasons for greater expulsion in obese women, but some theories include that the IUD could be more difficult to place in larger women without adequate instrumentation or exam tables. Women with class III obesity also tend to have higher rates of heavy menstrual bleeding, which could be driving expulsion, Dr. Saito-Tom said.
However, the results should not deter physicians from placing hormonal IUD in these women, she said. While the expulsion rate was higher than seen generally, it is still an effective method for most obese women. “That’s much more beneficial than discouraging patients,” Dr. Saito-Tom said.
Physicians should continue to educate patients about the benefits of long-acting reversible contraceptives and encourage all patients to utilize them, regardless of their weight, she said.
Dr. Saito-Tom reported having no relevant financial disclosures. One of her colleagues reported grant support from Merck and being a consultant for UpToDate.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
SAN DIEGO – Obese women with a body mass index of 40 or greater are more likely to experience expulsion of levonorgestrel IUDs than women with lower BMI, according to findings from a retrospective cohort study.
Women with class III obesity (a BMI of 40 or greater) had a 3.06-times higher odds of expulsion (95% confidence interval, 1.69-5.57) with a levonorgestrel IUD, compared with a control group of women with a BMI of less than 35, Lynne Saito-Tom, MD, of the University of Hawaii at Manoa, Honolulu, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Dr. Saito-Tom and her colleagues looked at other potential IUD complications, including infection, perforation, and pregnancy. Women with class III obesity also had a higher rate of all complications combined at 24%, compared with 10% among women with class I obesity.
Although complications were higher for more severely obese women, there were no differences between BMI groups in difficulty with insertion (P = .59) or 12-month continuation (P = .69).
The study was unique because it included a diverse ethnic population, with 36% Native Hawaiian/Pacific Islander women and 31% Asian women.
The study wasn’t powered to identify the reasons for greater expulsion in obese women, but some theories include that the IUD could be more difficult to place in larger women without adequate instrumentation or exam tables. Women with class III obesity also tend to have higher rates of heavy menstrual bleeding, which could be driving expulsion, Dr. Saito-Tom said.
However, the results should not deter physicians from placing hormonal IUD in these women, she said. While the expulsion rate was higher than seen generally, it is still an effective method for most obese women. “That’s much more beneficial than discouraging patients,” Dr. Saito-Tom said.
Physicians should continue to educate patients about the benefits of long-acting reversible contraceptives and encourage all patients to utilize them, regardless of their weight, she said.
Dr. Saito-Tom reported having no relevant financial disclosures. One of her colleagues reported grant support from Merck and being a consultant for UpToDate.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Key clinical point:
Major finding: Women with class III obesity had 3.06 higher odds of levonorgestrel IUD expulsion, compared with a control group of women with a BMI of less than 35.
Data source: A retrospective cohort study of 1,071 women who had a levonorgestrel IUD inserted between January 2009 and December 2010.
Disclosures: Dr. Saito-Tom reported having no relevant financial disclosures. One of her colleagues reported grant support from Merck and being a consultant for UpToDate.
Medicaid paperwork adds barrier to postpartum sterilization
SAN DIEGO – Just over half of women who requested immediate postpartum sterilization received it in a prospective study of 334 women, with Medicaid paperwork serving as a barrier for many of the unfulfilled requests.
All of the women in the study delivered a baby and had requested immediate postpartum sterilization at some point before delivery, but just 173 women (52%) received the procedure, Taylor Hahn, MD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. A total of 161 women (48%) did not receive the procedure.
Dr. Hahn, a fourth-year resident at Indiana University, Indianapolis, and her colleagues followed these women for up to 3 months post partum and found that, within the group that didn’t receive immediate postpartum sterilization, just six women – less than 10% – had received sterilization by the end of the follow-up period. The remaining women had chosen an alternative contraceptive method, were still awaiting interval sterilization, or did not receive postpartum care.
This is concerning, Dr. Hahn said, because Medicaid coverage for sterilization typically expires after 60 days post partum.
The consent form was developed in the 1970s to protect women in vulnerable populations from being coerced into sterilization, but Dr. Hahn said that, today, “it really has created such a barrier to these women getting the care that they want and desire.” She contrasted the Medicaid procedure with what is typical in private insurance, which generally covers immediate postpartum sterilization, and the decision can be made the same day.
The researchers reported having no relevant financial disclosures.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
SAN DIEGO – Just over half of women who requested immediate postpartum sterilization received it in a prospective study of 334 women, with Medicaid paperwork serving as a barrier for many of the unfulfilled requests.
All of the women in the study delivered a baby and had requested immediate postpartum sterilization at some point before delivery, but just 173 women (52%) received the procedure, Taylor Hahn, MD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. A total of 161 women (48%) did not receive the procedure.
Dr. Hahn, a fourth-year resident at Indiana University, Indianapolis, and her colleagues followed these women for up to 3 months post partum and found that, within the group that didn’t receive immediate postpartum sterilization, just six women – less than 10% – had received sterilization by the end of the follow-up period. The remaining women had chosen an alternative contraceptive method, were still awaiting interval sterilization, or did not receive postpartum care.
This is concerning, Dr. Hahn said, because Medicaid coverage for sterilization typically expires after 60 days post partum.
The consent form was developed in the 1970s to protect women in vulnerable populations from being coerced into sterilization, but Dr. Hahn said that, today, “it really has created such a barrier to these women getting the care that they want and desire.” She contrasted the Medicaid procedure with what is typical in private insurance, which generally covers immediate postpartum sterilization, and the decision can be made the same day.
The researchers reported having no relevant financial disclosures.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
SAN DIEGO – Just over half of women who requested immediate postpartum sterilization received it in a prospective study of 334 women, with Medicaid paperwork serving as a barrier for many of the unfulfilled requests.
All of the women in the study delivered a baby and had requested immediate postpartum sterilization at some point before delivery, but just 173 women (52%) received the procedure, Taylor Hahn, MD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. A total of 161 women (48%) did not receive the procedure.
Dr. Hahn, a fourth-year resident at Indiana University, Indianapolis, and her colleagues followed these women for up to 3 months post partum and found that, within the group that didn’t receive immediate postpartum sterilization, just six women – less than 10% – had received sterilization by the end of the follow-up period. The remaining women had chosen an alternative contraceptive method, were still awaiting interval sterilization, or did not receive postpartum care.
This is concerning, Dr. Hahn said, because Medicaid coverage for sterilization typically expires after 60 days post partum.
The consent form was developed in the 1970s to protect women in vulnerable populations from being coerced into sterilization, but Dr. Hahn said that, today, “it really has created such a barrier to these women getting the care that they want and desire.” She contrasted the Medicaid procedure with what is typical in private insurance, which generally covers immediate postpartum sterilization, and the decision can be made the same day.
The researchers reported having no relevant financial disclosures.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Key clinical point:
Major finding: A total of 48% of women who requested immediate postpartum sterilization did not receive it.
Data source: A prospective study of 334 women who delivered and requested immediate postpartum sterilization.
Disclosures: The researchers reported having no relevant financial disclosures.
ACOG vows continued fight against rollback of women’s health protections
SAN DIEGO – Just days after the House passage of the GOP-backed American Health Care Act and signals that the Trump administration will revisit mandated coverage of contraception, leaders of the American Congress of Obstetricians and Gynecologists promised to continue fighting for coverage of women’s health services.
ACOG leaders said they have been working on advocacy in the Senate for months already, recognizing that a bill to repeal the Affordable Care Act could pass the House this year. The House-passed legislation, which still must pass the Senate, could be particularly detrimental to women’s health care, they said, because it includes a provision that would allow states to seek waivers for coverage of the essential health benefits package, which includes coverage of maternity care and contraceptives.
Before the ACA was enacted, only about 12% of insurance companies offered maternity care coverage and only three of four states required it, according to Thomas M. Gellhaus, MD, the outgoing ACOG president and an ob.gyn. in Iowa City. Because of the ACA requirements, all women now have that coverage. Additionally, the contraception coverage is what reduces maternal mortality, he said. “The fight will go on,” Dr. Gellhaus said. “We will not give up on this.”
ACOG has already been coordinating meetings between ob.gyns. and members of Congress and telephone calls before votes to reinforce the concerns with pending legislation and the impact it would have on women’s health care, Dr. Gellhaus said.
But this advocacy effort is not a partisan endeavor, said Mark DeFrancesco, MD, an ACOG past president and an ob.gyn. in Cheshire, Conn. “We’re going to stay out of politics,” he said. “You do that by making the only litmus test, ‘Is this good for women or not?’ ”
He added, “If you get out there with the ‘real facts’ ... it shouldn’t matter what your politics are or what your party is. It’s on us to explain things in such a way that we avoid rhetoric and we avoid the ideology.”
Dr. Brown, who encouraged ob.gyns. and their patients to reach out to their members of Congress in person in their home districts, said the message is simple: “This is what it means for your mother, this what it means for your sister, this is what it means for your wife, this is what it means for your daughters.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
SAN DIEGO – Just days after the House passage of the GOP-backed American Health Care Act and signals that the Trump administration will revisit mandated coverage of contraception, leaders of the American Congress of Obstetricians and Gynecologists promised to continue fighting for coverage of women’s health services.
ACOG leaders said they have been working on advocacy in the Senate for months already, recognizing that a bill to repeal the Affordable Care Act could pass the House this year. The House-passed legislation, which still must pass the Senate, could be particularly detrimental to women’s health care, they said, because it includes a provision that would allow states to seek waivers for coverage of the essential health benefits package, which includes coverage of maternity care and contraceptives.
Before the ACA was enacted, only about 12% of insurance companies offered maternity care coverage and only three of four states required it, according to Thomas M. Gellhaus, MD, the outgoing ACOG president and an ob.gyn. in Iowa City. Because of the ACA requirements, all women now have that coverage. Additionally, the contraception coverage is what reduces maternal mortality, he said. “The fight will go on,” Dr. Gellhaus said. “We will not give up on this.”
ACOG has already been coordinating meetings between ob.gyns. and members of Congress and telephone calls before votes to reinforce the concerns with pending legislation and the impact it would have on women’s health care, Dr. Gellhaus said.
But this advocacy effort is not a partisan endeavor, said Mark DeFrancesco, MD, an ACOG past president and an ob.gyn. in Cheshire, Conn. “We’re going to stay out of politics,” he said. “You do that by making the only litmus test, ‘Is this good for women or not?’ ”
He added, “If you get out there with the ‘real facts’ ... it shouldn’t matter what your politics are or what your party is. It’s on us to explain things in such a way that we avoid rhetoric and we avoid the ideology.”
Dr. Brown, who encouraged ob.gyns. and their patients to reach out to their members of Congress in person in their home districts, said the message is simple: “This is what it means for your mother, this what it means for your sister, this is what it means for your wife, this is what it means for your daughters.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
SAN DIEGO – Just days after the House passage of the GOP-backed American Health Care Act and signals that the Trump administration will revisit mandated coverage of contraception, leaders of the American Congress of Obstetricians and Gynecologists promised to continue fighting for coverage of women’s health services.
ACOG leaders said they have been working on advocacy in the Senate for months already, recognizing that a bill to repeal the Affordable Care Act could pass the House this year. The House-passed legislation, which still must pass the Senate, could be particularly detrimental to women’s health care, they said, because it includes a provision that would allow states to seek waivers for coverage of the essential health benefits package, which includes coverage of maternity care and contraceptives.
Before the ACA was enacted, only about 12% of insurance companies offered maternity care coverage and only three of four states required it, according to Thomas M. Gellhaus, MD, the outgoing ACOG president and an ob.gyn. in Iowa City. Because of the ACA requirements, all women now have that coverage. Additionally, the contraception coverage is what reduces maternal mortality, he said. “The fight will go on,” Dr. Gellhaus said. “We will not give up on this.”
ACOG has already been coordinating meetings between ob.gyns. and members of Congress and telephone calls before votes to reinforce the concerns with pending legislation and the impact it would have on women’s health care, Dr. Gellhaus said.
But this advocacy effort is not a partisan endeavor, said Mark DeFrancesco, MD, an ACOG past president and an ob.gyn. in Cheshire, Conn. “We’re going to stay out of politics,” he said. “You do that by making the only litmus test, ‘Is this good for women or not?’ ”
He added, “If you get out there with the ‘real facts’ ... it shouldn’t matter what your politics are or what your party is. It’s on us to explain things in such a way that we avoid rhetoric and we avoid the ideology.”
Dr. Brown, who encouraged ob.gyns. and their patients to reach out to their members of Congress in person in their home districts, said the message is simple: “This is what it means for your mother, this what it means for your sister, this is what it means for your wife, this is what it means for your daughters.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
AT ACOG 2017
Rural residency offers tool to address ob.gyn. shortages
In 2021, the first obstetrician-gynecologist trained on a separate rural residency track will graduate from the University of Wisconsin.
Program leaders are gambling that the investment in specialized training – including a quarter of the training time spent in smaller, community hospitals – will eventually pay off in terms of more ob.gyns. living and practicing in Wisconsin’s underserved rural settings.
“The key premise that we’re working on and that’s been borne out in the literature on rural training in family medicine is that people who are ultimately going to practice in a rural setting are people who are interested in it, who have a rural background, and people who have experience in a clinical environment in the rural setting,” said Ellen Hartenbach, MD, ob.gyn. residency program director at the University of Wisconsin, Madison.
For now, the program is starting slowly. It matched its first and only rural resident – Laura McDowell of the University of Minnesota – in the 2017 National Resident Match. The new rural-track resident joins the six residents in the school’s main ob.gyn. training program. New residents will be added to the rural track based on the availability of funding. The university relied on a development grant from the state, as well as funding from local community hospitals, to help finance the new position.
Dr. Hartenbach said the selection of the rural-track resident centered around finding someone who had both interest and prior experience in a rural setting, as well as someone who wanted to eventually live in a rural community. The university also had to ensure that applicants weren’t trying to game the system, looking for a way to break into the competitive ob.gyn. residency field, which had a 100% fill rate in 2017. More than 100 medical students applied for the single rural track position.
University of Wisconsin’s program is thought to be the country’s first dedicated, sanctioned rural ob.gyn. residency track. The university has been working for the last 2 years to develop the program and get approval from the Accreditation Council for Graduate Medical Education’s Residency Review Committee.
When the first resident starts on July 1, she will receive 75% of her training in the university hospital in Madison and 25% split among four smaller, community hospitals. The rural resident will receive the same clinical and cognitive skills training as the other residents, with the same number of required procedures. The big difference, Dr. Hartenbach said, comes from the experience gained in working in smaller hospitals.
“There’s a pretty big difference with the ob.gyn. unit in a 50-bed hospital, compared to a 500-bed hospital,” she said. “There’s a difference in terms of the types of patients that come in to smaller medical centers.”
Dr. Hartenbach said she hopes having real-world experience in the rural setting will help to dispel common misperceptions, including that rural physicians are always on call.
The Wisconsin program could be a model for other states by giving residents a chance to understand the dynamics of smaller hospitals, said Thomas Gellhaus, MD, president of the American Congress of Obstetricians and Gynecologists (ACOG). But its success would depend on the presence of community hospitals that have an adequate number of physicians to supervise residents and a case mix that matches up with training requirements. “It can’t just be anywhere,” said Dr. Gellhaus, clinical associate professor at the University of Iowa, Iowa City.
Dr. Hartenbach said the goal behind the new training design is to help bolster the rural OB workforce.
“There’s a lot of rural health disparities in a lot of medical fields, in particular in maternity health services,” she said. “There have been a lot of reports showing that we’re not going to have enough ob.gyns. and a lot of rural hospitals are closing down their maternity services.”
About one out of three Wisconsin counties don’t have an ob.gyn, according to ACOG. In the past, family medicine physicians and nurse midwives have helped to pick up the slack on maternity care in rural settings, but the number of family physicians who offer obstetric services is on the decline. One study showed that the proportion of family physicians providing maternity care dropped from 23% in 2000 to less than 10% in 2010 (J Am Board Fam Med. 2012 May-Jun;25[3]:270-1). That development has contributed to an “acute” crisis in the provision of rural maternity care, Dr. Hartenbach said.
Maldistribution
This trend is national as well. Nearly half of the 3,107 U.S. counties lack ob.gyns. These counties are located in all states but primarily in the Midwest and Mountain West, according to a 2011 workforce report from ACOG.
As of 2010, the national ratio of ob.gyns. per 10,000 women was 2.1, but that ratio decreases from 2.9 in metropolitan counties to 0.7 in rural counties.
William F. Rayburn, MD, distinguished professor and emeritus chair of obstetrics and gynecology at the University of New Mexico, Albuquerque, is working on an update to the workforce report, expected to be released in May 2017 at ACOG’s annual scientific meeting in San Diego.
The maldistribution trend is likely to continue over the next decade for several reasons, said Dr. Rayburn, associate dean for continuing medical education and professional development at the university.
At the top of the list is the stagnant number of residency training slots across the country. While medical schools in the United States and abroad are graduating more students, the number of first-year ob.gyn. residency positions has remained at about 1,200 since 1980. In 2017, there were 1,288 positions offered in the Main Residency Match.
Gender is another factor. Ob.gyn. is now a majority female specialty and by 2025 women will make up about two-thirds of the workforce, Dr. Rayburn said. While women are just as productive as men, they don’t work as many hours and they tend to drop obstetrics from their practices earlier, he added.
In addition, research indicates that women ob.gyns. are more likely to stay in urban areas after training.
“The movement, generally speaking, when people relocate is often to urban areas, from one urban area to another or from a rural area to a more urban area,” Dr. Rayburn said.
There is growing demand for health care from a population of adult women that is increasing at a greater rate than the number of ob.gyn. residents, he said.
The rural residency option being explored in Wisconsin is a great idea, Dr. Rayburn said, provided the trainees receive enough experience in the rural environment to prepare them for the change in practice. “The more you can get people to train in more rural areas, the more likely they are to eventually go there. But that’s far from a guarantee,” he said.
Going forward Dr. Rayburn said he expects to see loan repayment used to draw physicians to underserved areas.
“The problem is that the rural areas tend to have less of a good payer mix,” he said. “In other words, there are more poor people in rural areas. And the health care delivery is more limited in terms of resources, types of surgical equipment, and being able to take care of complicated pregnancies.”
GME funding cap
The Association of American Medical Colleges is focused on easing physician shortages by getting lawmakers to lift the cap on federal funding for graduate medical education (GME) positions that was put in place as part of the Balanced Budget Act of 1997.
“The population is getting larger and aging, which increases the need for more physicians and thus we have to work with Congress to lift the cap,” said Janis Orlowski, MD, Chief Health Care Officer at the Association of American Medical Colleges.
Specifically, the association is calling for funding to train at least 3,000 more physicians each year. In the last Congress, lawmakers introduced bills that would have provided those positions, with one of those bills directing that a portion of those new positions be dedicated to specialties with physician shortages.
Dr. Orlowski said bipartisan support still exists for lifting the cap, though new legislation probably won’t be introduced until after the summer recess when Congress won’t be bogged down with efforts to repeal and replace the Affordable Care Act.
Getting the GME cap lifted is mostly about coming up with the funding, she said. But some lawmakers have expressed concerns about how to ensure that increases go to the specialties with the greatest needs or that physicians trained in these spots will ultimately practice in the areas where care is needed, such as rural America.
“Those are issues that we need to continue to work on and address,” she said.
ACOG supports efforts to lift the GME funding cap and is pushing federal legislation that would establish maternity care health professionals shortage areas, allowing the National Health Service Corps to offer scholarships and loan repayment benefits to providers who work in those areas. Similar programs are already in place for primary care, and dental and mental health. Like the GME funding bill, legislation on this topic was introduced in the last session of Congress but will need to be reintroduced in the current Congress.
“We have to work on the workforce,” Dr. Gellhaus said. “It’s going to be a concern.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
In 2021, the first obstetrician-gynecologist trained on a separate rural residency track will graduate from the University of Wisconsin.
Program leaders are gambling that the investment in specialized training – including a quarter of the training time spent in smaller, community hospitals – will eventually pay off in terms of more ob.gyns. living and practicing in Wisconsin’s underserved rural settings.
“The key premise that we’re working on and that’s been borne out in the literature on rural training in family medicine is that people who are ultimately going to practice in a rural setting are people who are interested in it, who have a rural background, and people who have experience in a clinical environment in the rural setting,” said Ellen Hartenbach, MD, ob.gyn. residency program director at the University of Wisconsin, Madison.
For now, the program is starting slowly. It matched its first and only rural resident – Laura McDowell of the University of Minnesota – in the 2017 National Resident Match. The new rural-track resident joins the six residents in the school’s main ob.gyn. training program. New residents will be added to the rural track based on the availability of funding. The university relied on a development grant from the state, as well as funding from local community hospitals, to help finance the new position.
Dr. Hartenbach said the selection of the rural-track resident centered around finding someone who had both interest and prior experience in a rural setting, as well as someone who wanted to eventually live in a rural community. The university also had to ensure that applicants weren’t trying to game the system, looking for a way to break into the competitive ob.gyn. residency field, which had a 100% fill rate in 2017. More than 100 medical students applied for the single rural track position.
University of Wisconsin’s program is thought to be the country’s first dedicated, sanctioned rural ob.gyn. residency track. The university has been working for the last 2 years to develop the program and get approval from the Accreditation Council for Graduate Medical Education’s Residency Review Committee.
When the first resident starts on July 1, she will receive 75% of her training in the university hospital in Madison and 25% split among four smaller, community hospitals. The rural resident will receive the same clinical and cognitive skills training as the other residents, with the same number of required procedures. The big difference, Dr. Hartenbach said, comes from the experience gained in working in smaller hospitals.
“There’s a pretty big difference with the ob.gyn. unit in a 50-bed hospital, compared to a 500-bed hospital,” she said. “There’s a difference in terms of the types of patients that come in to smaller medical centers.”
Dr. Hartenbach said she hopes having real-world experience in the rural setting will help to dispel common misperceptions, including that rural physicians are always on call.
The Wisconsin program could be a model for other states by giving residents a chance to understand the dynamics of smaller hospitals, said Thomas Gellhaus, MD, president of the American Congress of Obstetricians and Gynecologists (ACOG). But its success would depend on the presence of community hospitals that have an adequate number of physicians to supervise residents and a case mix that matches up with training requirements. “It can’t just be anywhere,” said Dr. Gellhaus, clinical associate professor at the University of Iowa, Iowa City.
Dr. Hartenbach said the goal behind the new training design is to help bolster the rural OB workforce.
“There’s a lot of rural health disparities in a lot of medical fields, in particular in maternity health services,” she said. “There have been a lot of reports showing that we’re not going to have enough ob.gyns. and a lot of rural hospitals are closing down their maternity services.”
About one out of three Wisconsin counties don’t have an ob.gyn, according to ACOG. In the past, family medicine physicians and nurse midwives have helped to pick up the slack on maternity care in rural settings, but the number of family physicians who offer obstetric services is on the decline. One study showed that the proportion of family physicians providing maternity care dropped from 23% in 2000 to less than 10% in 2010 (J Am Board Fam Med. 2012 May-Jun;25[3]:270-1). That development has contributed to an “acute” crisis in the provision of rural maternity care, Dr. Hartenbach said.
Maldistribution
This trend is national as well. Nearly half of the 3,107 U.S. counties lack ob.gyns. These counties are located in all states but primarily in the Midwest and Mountain West, according to a 2011 workforce report from ACOG.
As of 2010, the national ratio of ob.gyns. per 10,000 women was 2.1, but that ratio decreases from 2.9 in metropolitan counties to 0.7 in rural counties.
William F. Rayburn, MD, distinguished professor and emeritus chair of obstetrics and gynecology at the University of New Mexico, Albuquerque, is working on an update to the workforce report, expected to be released in May 2017 at ACOG’s annual scientific meeting in San Diego.
The maldistribution trend is likely to continue over the next decade for several reasons, said Dr. Rayburn, associate dean for continuing medical education and professional development at the university.
At the top of the list is the stagnant number of residency training slots across the country. While medical schools in the United States and abroad are graduating more students, the number of first-year ob.gyn. residency positions has remained at about 1,200 since 1980. In 2017, there were 1,288 positions offered in the Main Residency Match.
Gender is another factor. Ob.gyn. is now a majority female specialty and by 2025 women will make up about two-thirds of the workforce, Dr. Rayburn said. While women are just as productive as men, they don’t work as many hours and they tend to drop obstetrics from their practices earlier, he added.
In addition, research indicates that women ob.gyns. are more likely to stay in urban areas after training.
“The movement, generally speaking, when people relocate is often to urban areas, from one urban area to another or from a rural area to a more urban area,” Dr. Rayburn said.
There is growing demand for health care from a population of adult women that is increasing at a greater rate than the number of ob.gyn. residents, he said.
The rural residency option being explored in Wisconsin is a great idea, Dr. Rayburn said, provided the trainees receive enough experience in the rural environment to prepare them for the change in practice. “The more you can get people to train in more rural areas, the more likely they are to eventually go there. But that’s far from a guarantee,” he said.
Going forward Dr. Rayburn said he expects to see loan repayment used to draw physicians to underserved areas.
“The problem is that the rural areas tend to have less of a good payer mix,” he said. “In other words, there are more poor people in rural areas. And the health care delivery is more limited in terms of resources, types of surgical equipment, and being able to take care of complicated pregnancies.”
GME funding cap
The Association of American Medical Colleges is focused on easing physician shortages by getting lawmakers to lift the cap on federal funding for graduate medical education (GME) positions that was put in place as part of the Balanced Budget Act of 1997.
“The population is getting larger and aging, which increases the need for more physicians and thus we have to work with Congress to lift the cap,” said Janis Orlowski, MD, Chief Health Care Officer at the Association of American Medical Colleges.
Specifically, the association is calling for funding to train at least 3,000 more physicians each year. In the last Congress, lawmakers introduced bills that would have provided those positions, with one of those bills directing that a portion of those new positions be dedicated to specialties with physician shortages.
Dr. Orlowski said bipartisan support still exists for lifting the cap, though new legislation probably won’t be introduced until after the summer recess when Congress won’t be bogged down with efforts to repeal and replace the Affordable Care Act.
Getting the GME cap lifted is mostly about coming up with the funding, she said. But some lawmakers have expressed concerns about how to ensure that increases go to the specialties with the greatest needs or that physicians trained in these spots will ultimately practice in the areas where care is needed, such as rural America.
“Those are issues that we need to continue to work on and address,” she said.
ACOG supports efforts to lift the GME funding cap and is pushing federal legislation that would establish maternity care health professionals shortage areas, allowing the National Health Service Corps to offer scholarships and loan repayment benefits to providers who work in those areas. Similar programs are already in place for primary care, and dental and mental health. Like the GME funding bill, legislation on this topic was introduced in the last session of Congress but will need to be reintroduced in the current Congress.
“We have to work on the workforce,” Dr. Gellhaus said. “It’s going to be a concern.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
In 2021, the first obstetrician-gynecologist trained on a separate rural residency track will graduate from the University of Wisconsin.
Program leaders are gambling that the investment in specialized training – including a quarter of the training time spent in smaller, community hospitals – will eventually pay off in terms of more ob.gyns. living and practicing in Wisconsin’s underserved rural settings.
“The key premise that we’re working on and that’s been borne out in the literature on rural training in family medicine is that people who are ultimately going to practice in a rural setting are people who are interested in it, who have a rural background, and people who have experience in a clinical environment in the rural setting,” said Ellen Hartenbach, MD, ob.gyn. residency program director at the University of Wisconsin, Madison.
For now, the program is starting slowly. It matched its first and only rural resident – Laura McDowell of the University of Minnesota – in the 2017 National Resident Match. The new rural-track resident joins the six residents in the school’s main ob.gyn. training program. New residents will be added to the rural track based on the availability of funding. The university relied on a development grant from the state, as well as funding from local community hospitals, to help finance the new position.
Dr. Hartenbach said the selection of the rural-track resident centered around finding someone who had both interest and prior experience in a rural setting, as well as someone who wanted to eventually live in a rural community. The university also had to ensure that applicants weren’t trying to game the system, looking for a way to break into the competitive ob.gyn. residency field, which had a 100% fill rate in 2017. More than 100 medical students applied for the single rural track position.
University of Wisconsin’s program is thought to be the country’s first dedicated, sanctioned rural ob.gyn. residency track. The university has been working for the last 2 years to develop the program and get approval from the Accreditation Council for Graduate Medical Education’s Residency Review Committee.
When the first resident starts on July 1, she will receive 75% of her training in the university hospital in Madison and 25% split among four smaller, community hospitals. The rural resident will receive the same clinical and cognitive skills training as the other residents, with the same number of required procedures. The big difference, Dr. Hartenbach said, comes from the experience gained in working in smaller hospitals.
“There’s a pretty big difference with the ob.gyn. unit in a 50-bed hospital, compared to a 500-bed hospital,” she said. “There’s a difference in terms of the types of patients that come in to smaller medical centers.”
Dr. Hartenbach said she hopes having real-world experience in the rural setting will help to dispel common misperceptions, including that rural physicians are always on call.
The Wisconsin program could be a model for other states by giving residents a chance to understand the dynamics of smaller hospitals, said Thomas Gellhaus, MD, president of the American Congress of Obstetricians and Gynecologists (ACOG). But its success would depend on the presence of community hospitals that have an adequate number of physicians to supervise residents and a case mix that matches up with training requirements. “It can’t just be anywhere,” said Dr. Gellhaus, clinical associate professor at the University of Iowa, Iowa City.
Dr. Hartenbach said the goal behind the new training design is to help bolster the rural OB workforce.
“There’s a lot of rural health disparities in a lot of medical fields, in particular in maternity health services,” she said. “There have been a lot of reports showing that we’re not going to have enough ob.gyns. and a lot of rural hospitals are closing down their maternity services.”
About one out of three Wisconsin counties don’t have an ob.gyn, according to ACOG. In the past, family medicine physicians and nurse midwives have helped to pick up the slack on maternity care in rural settings, but the number of family physicians who offer obstetric services is on the decline. One study showed that the proportion of family physicians providing maternity care dropped from 23% in 2000 to less than 10% in 2010 (J Am Board Fam Med. 2012 May-Jun;25[3]:270-1). That development has contributed to an “acute” crisis in the provision of rural maternity care, Dr. Hartenbach said.
Maldistribution
This trend is national as well. Nearly half of the 3,107 U.S. counties lack ob.gyns. These counties are located in all states but primarily in the Midwest and Mountain West, according to a 2011 workforce report from ACOG.
As of 2010, the national ratio of ob.gyns. per 10,000 women was 2.1, but that ratio decreases from 2.9 in metropolitan counties to 0.7 in rural counties.
William F. Rayburn, MD, distinguished professor and emeritus chair of obstetrics and gynecology at the University of New Mexico, Albuquerque, is working on an update to the workforce report, expected to be released in May 2017 at ACOG’s annual scientific meeting in San Diego.
The maldistribution trend is likely to continue over the next decade for several reasons, said Dr. Rayburn, associate dean for continuing medical education and professional development at the university.
At the top of the list is the stagnant number of residency training slots across the country. While medical schools in the United States and abroad are graduating more students, the number of first-year ob.gyn. residency positions has remained at about 1,200 since 1980. In 2017, there were 1,288 positions offered in the Main Residency Match.
Gender is another factor. Ob.gyn. is now a majority female specialty and by 2025 women will make up about two-thirds of the workforce, Dr. Rayburn said. While women are just as productive as men, they don’t work as many hours and they tend to drop obstetrics from their practices earlier, he added.
In addition, research indicates that women ob.gyns. are more likely to stay in urban areas after training.
“The movement, generally speaking, when people relocate is often to urban areas, from one urban area to another or from a rural area to a more urban area,” Dr. Rayburn said.
There is growing demand for health care from a population of adult women that is increasing at a greater rate than the number of ob.gyn. residents, he said.
The rural residency option being explored in Wisconsin is a great idea, Dr. Rayburn said, provided the trainees receive enough experience in the rural environment to prepare them for the change in practice. “The more you can get people to train in more rural areas, the more likely they are to eventually go there. But that’s far from a guarantee,” he said.
Going forward Dr. Rayburn said he expects to see loan repayment used to draw physicians to underserved areas.
“The problem is that the rural areas tend to have less of a good payer mix,” he said. “In other words, there are more poor people in rural areas. And the health care delivery is more limited in terms of resources, types of surgical equipment, and being able to take care of complicated pregnancies.”
GME funding cap
The Association of American Medical Colleges is focused on easing physician shortages by getting lawmakers to lift the cap on federal funding for graduate medical education (GME) positions that was put in place as part of the Balanced Budget Act of 1997.
“The population is getting larger and aging, which increases the need for more physicians and thus we have to work with Congress to lift the cap,” said Janis Orlowski, MD, Chief Health Care Officer at the Association of American Medical Colleges.
Specifically, the association is calling for funding to train at least 3,000 more physicians each year. In the last Congress, lawmakers introduced bills that would have provided those positions, with one of those bills directing that a portion of those new positions be dedicated to specialties with physician shortages.
Dr. Orlowski said bipartisan support still exists for lifting the cap, though new legislation probably won’t be introduced until after the summer recess when Congress won’t be bogged down with efforts to repeal and replace the Affordable Care Act.
Getting the GME cap lifted is mostly about coming up with the funding, she said. But some lawmakers have expressed concerns about how to ensure that increases go to the specialties with the greatest needs or that physicians trained in these spots will ultimately practice in the areas where care is needed, such as rural America.
“Those are issues that we need to continue to work on and address,” she said.
ACOG supports efforts to lift the GME funding cap and is pushing federal legislation that would establish maternity care health professionals shortage areas, allowing the National Health Service Corps to offer scholarships and loan repayment benefits to providers who work in those areas. Similar programs are already in place for primary care, and dental and mental health. Like the GME funding bill, legislation on this topic was introduced in the last session of Congress but will need to be reintroduced in the current Congress.
“We have to work on the workforce,” Dr. Gellhaus said. “It’s going to be a concern.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny












