User login
WHO Declares "Public Health Emergency" for Microcephaly Linked to Zika Virus
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
WHO declares ‘public health emergency’ for microcephaly linked to Zika virus
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The World Health Organization has declared a “public health emergency of international concern” related to the clusters of microcephaly and other neurological complications reported in Brazil and earlier in French Polynesia.
Though there is a strong association between these cases and the Zika virus, a causal link still has not been scientifically proven, according to the WHO.
The WHO’s emergency declaration clears the way for the international health community to move forward with a coordinated response. Dr. Margaret Chan, WHO Director-General, said her organization plans to take a number of precautionary measures, including improving surveillance and detection of infections, congenital malformations, and neurological complications. They will also work with countries to intensify control of mosquito populations and help expedite the development of diagnostic tests and vaccines to protect at-risk populations.
The recommendations came after a Feb. 1 meeting of the International Health Regulations Emergency Committee, which Dr. Chan convened last week in response to the Zika virus outbreak and the observed increase in neurological disorders and neonatal malformations.
The group of 18 experts advised that the clusters of microcephaly and other complications constitute an “extraordinary event and a public health threat to other parts of the world.” The group did not recommend any restrictions on travel or trade with areas where the Zika virus transmission is ongoing, however.
“At present, the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women,” Dr. Chan said during a press briefing.
Dr. Chan said it’s unclear how long it will take to determine if Zika virus is causing the uptick in microcephaly and other congenital malformations and neurological abnormalities, but health officials are working to set up case-control studies that are scheduled to start in the next 2 weeks.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
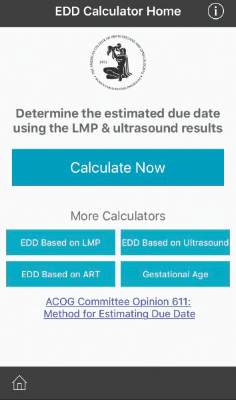
ACOG launches due date calculator app
Is it time to toss out the plastic pregnancy wheel?
The American College of Obstetricians and Gynecologists has launched a new due date calculator application that officials hope will replace the physical wheel and other smartphone apps already on the market.
The Estimated Due Date Calculator (EDD Calculator) incorporates the joint recommendations from ACOG, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine in determining due dates and reconciles the discrepancy in due dates between the first ultrasound and the date of the last menstrual period.
The EDD Calculator – available for free in the Apple and Google Play app stores as part of the ACOG app – also recalculates the due date based on use of assisted reproductive technology and allows users to determine the gestational age at a specific date.
“The EDD Calculator has ACOG guidelines built into the logic, therefore, it is the most accurate tool available for ob.gyns. and their staff,” Dr. Nathaniel DeNicola, ACOG digital and social media expert consultant and senior fellow at the Penn Social Media and Health Innovation Lab, said in a statement.
Although the EDD Calculator is designed for ob.gyns. and other health care providers, it also can be used by patients, according to ACOG.
To get the EDD Calculator, download the ACOG app (www.acog.org/acogapp).
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Is it time to toss out the plastic pregnancy wheel?
The American College of Obstetricians and Gynecologists has launched a new due date calculator application that officials hope will replace the physical wheel and other smartphone apps already on the market.
The Estimated Due Date Calculator (EDD Calculator) incorporates the joint recommendations from ACOG, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine in determining due dates and reconciles the discrepancy in due dates between the first ultrasound and the date of the last menstrual period.
The EDD Calculator – available for free in the Apple and Google Play app stores as part of the ACOG app – also recalculates the due date based on use of assisted reproductive technology and allows users to determine the gestational age at a specific date.
“The EDD Calculator has ACOG guidelines built into the logic, therefore, it is the most accurate tool available for ob.gyns. and their staff,” Dr. Nathaniel DeNicola, ACOG digital and social media expert consultant and senior fellow at the Penn Social Media and Health Innovation Lab, said in a statement.
Although the EDD Calculator is designed for ob.gyns. and other health care providers, it also can be used by patients, according to ACOG.
To get the EDD Calculator, download the ACOG app (www.acog.org/acogapp).
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Is it time to toss out the plastic pregnancy wheel?
The American College of Obstetricians and Gynecologists has launched a new due date calculator application that officials hope will replace the physical wheel and other smartphone apps already on the market.
The Estimated Due Date Calculator (EDD Calculator) incorporates the joint recommendations from ACOG, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine in determining due dates and reconciles the discrepancy in due dates between the first ultrasound and the date of the last menstrual period.
The EDD Calculator – available for free in the Apple and Google Play app stores as part of the ACOG app – also recalculates the due date based on use of assisted reproductive technology and allows users to determine the gestational age at a specific date.
“The EDD Calculator has ACOG guidelines built into the logic, therefore, it is the most accurate tool available for ob.gyns. and their staff,” Dr. Nathaniel DeNicola, ACOG digital and social media expert consultant and senior fellow at the Penn Social Media and Health Innovation Lab, said in a statement.
Although the EDD Calculator is designed for ob.gyns. and other health care providers, it also can be used by patients, according to ACOG.
To get the EDD Calculator, download the ACOG app (www.acog.org/acogapp).
mschneider@frontlinemedcom.com
On Twitter @maryellenny
U.S. Virgin Islands, Dominican Republic added to Zika travel advisory
Officials at the Centers for Disease Control and Prevention have added the U.S. Virgin Islands and the Dominican Republic to the rapidly growing list of areas that are part of the Zika virus travel alert.
The level 2 travel alert cautions travelers to use enhanced precautions to prevent mosquito bites when traveling to areas with ongoing Zika virus transmission. Though the Zika virus is generally mild, with symptoms lasting up to a week, there are more serious risks for pregnant women. Health officials are investigating the connection between microcephaly and other poor outcomes in the babies of pregnant women who were infected with Zika virus.
Until more is known, the CDC is advising pregnant women and those who are trying to become pregnant to postpone travel to these areas. If such travel is necessary, CDC recommends consulting a physician beforehand and strictly following steps to avoid mosquito bites, including wearing long-sleeved shirts and pants, staying indoors, and using insect repellent.
The CDC’s Zika travel alert also includes Puerto Rico (a U.S. territory), Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, and Venezuela.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Officials at the Centers for Disease Control and Prevention have added the U.S. Virgin Islands and the Dominican Republic to the rapidly growing list of areas that are part of the Zika virus travel alert.
The level 2 travel alert cautions travelers to use enhanced precautions to prevent mosquito bites when traveling to areas with ongoing Zika virus transmission. Though the Zika virus is generally mild, with symptoms lasting up to a week, there are more serious risks for pregnant women. Health officials are investigating the connection between microcephaly and other poor outcomes in the babies of pregnant women who were infected with Zika virus.
Until more is known, the CDC is advising pregnant women and those who are trying to become pregnant to postpone travel to these areas. If such travel is necessary, CDC recommends consulting a physician beforehand and strictly following steps to avoid mosquito bites, including wearing long-sleeved shirts and pants, staying indoors, and using insect repellent.
The CDC’s Zika travel alert also includes Puerto Rico (a U.S. territory), Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, and Venezuela.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Officials at the Centers for Disease Control and Prevention have added the U.S. Virgin Islands and the Dominican Republic to the rapidly growing list of areas that are part of the Zika virus travel alert.
The level 2 travel alert cautions travelers to use enhanced precautions to prevent mosquito bites when traveling to areas with ongoing Zika virus transmission. Though the Zika virus is generally mild, with symptoms lasting up to a week, there are more serious risks for pregnant women. Health officials are investigating the connection between microcephaly and other poor outcomes in the babies of pregnant women who were infected with Zika virus.
Until more is known, the CDC is advising pregnant women and those who are trying to become pregnant to postpone travel to these areas. If such travel is necessary, CDC recommends consulting a physician beforehand and strictly following steps to avoid mosquito bites, including wearing long-sleeved shirts and pants, staying indoors, and using insect repellent.
The CDC’s Zika travel alert also includes Puerto Rico (a U.S. territory), Barbados, Bolivia, Brazil, Cape Verde, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Samoa, Suriname, and Venezuela.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Acupuncture no better than sham for relief of hot flashes
Chinese medicine needle acupuncture was about as effective as a sham blunt needle treatment in the relief of hot flashes, although women reported a 40% drop in symptoms with both treatments.
The findings, published online Jan. 18 in Annals of Internal Medicine, add to a growing, but conflicting body of evidence about the benefits of acupuncture in the treatment of menopause symptoms.
Prior to this study, two trials had demonstrated the effectiveness of acupuncture, compared with self care. And a pilot study had shown the effectiveness of acupuncture, compared with a noninsertive sham control. However, a Cochrane review found that acupuncture was more effective, compared with no treatment, and it had a moderate effect size, but was not effective when compared with a sham control (Cochrane Database Syst Rev. 2013 Jul 30;7:CD007410. doi:10.1002/14651858.CD007410.pub2).
The current trial, conducted at multiple sites in Australia, sought to add to the evidence with an adequately powered trial involving a sham control. But Carolyn Ee and her associates at the University of Melbourne noted that their study did not control for the nonspecific effects of acupuncture, such as regular interaction with a therapist.
The researchers randomly assigned 327 women aged older than 40 years who were in late menopause transition or postmenopause and experiencing at least seven moderate daily hot flashes to receive either a standardized Chinese medicine acupuncture treatment or a noninsertive, blunt needle sham acupuncture treatment. Patients received 10 treatments over 8 weeks, and they were assessed at 4 weeks, at the end of treatment, and at 3 and 6 months after treatment (Ann Intern Med. 2016; Jan 18. doi:10.7326/M15-1380.).
Both groups had about a 40% improvement in their hot flashes at the end of treatment, compared with their mean baseline hot flash score. The improvement was sustained at 3 and 6 months after the trial. In the acupuncture group, the mean hot flash scores at the end of treatment were 15.36, compared with 15.04 in the sham group, which was not statistically different. The researchers also found no advantage for acupuncture in quality of life, anxiety, or depression.
“Unless further high-quality evidence emerges, we cannot recommend skin-penetrating acupuncture as an efficacious treatment of this indication; the effects, if any, of acupuncture on these symptoms seem to be unrelated to needling,” the researchers wrote.
Some of the researchers reported receiving grant, scholarship, or fellowship support from the National Health and Medical Research Council of Australia, which funded the study.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Chinese medicine needle acupuncture was about as effective as a sham blunt needle treatment in the relief of hot flashes, although women reported a 40% drop in symptoms with both treatments.
The findings, published online Jan. 18 in Annals of Internal Medicine, add to a growing, but conflicting body of evidence about the benefits of acupuncture in the treatment of menopause symptoms.
Prior to this study, two trials had demonstrated the effectiveness of acupuncture, compared with self care. And a pilot study had shown the effectiveness of acupuncture, compared with a noninsertive sham control. However, a Cochrane review found that acupuncture was more effective, compared with no treatment, and it had a moderate effect size, but was not effective when compared with a sham control (Cochrane Database Syst Rev. 2013 Jul 30;7:CD007410. doi:10.1002/14651858.CD007410.pub2).
The current trial, conducted at multiple sites in Australia, sought to add to the evidence with an adequately powered trial involving a sham control. But Carolyn Ee and her associates at the University of Melbourne noted that their study did not control for the nonspecific effects of acupuncture, such as regular interaction with a therapist.
The researchers randomly assigned 327 women aged older than 40 years who were in late menopause transition or postmenopause and experiencing at least seven moderate daily hot flashes to receive either a standardized Chinese medicine acupuncture treatment or a noninsertive, blunt needle sham acupuncture treatment. Patients received 10 treatments over 8 weeks, and they were assessed at 4 weeks, at the end of treatment, and at 3 and 6 months after treatment (Ann Intern Med. 2016; Jan 18. doi:10.7326/M15-1380.).
Both groups had about a 40% improvement in their hot flashes at the end of treatment, compared with their mean baseline hot flash score. The improvement was sustained at 3 and 6 months after the trial. In the acupuncture group, the mean hot flash scores at the end of treatment were 15.36, compared with 15.04 in the sham group, which was not statistically different. The researchers also found no advantage for acupuncture in quality of life, anxiety, or depression.
“Unless further high-quality evidence emerges, we cannot recommend skin-penetrating acupuncture as an efficacious treatment of this indication; the effects, if any, of acupuncture on these symptoms seem to be unrelated to needling,” the researchers wrote.
Some of the researchers reported receiving grant, scholarship, or fellowship support from the National Health and Medical Research Council of Australia, which funded the study.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Chinese medicine needle acupuncture was about as effective as a sham blunt needle treatment in the relief of hot flashes, although women reported a 40% drop in symptoms with both treatments.
The findings, published online Jan. 18 in Annals of Internal Medicine, add to a growing, but conflicting body of evidence about the benefits of acupuncture in the treatment of menopause symptoms.
Prior to this study, two trials had demonstrated the effectiveness of acupuncture, compared with self care. And a pilot study had shown the effectiveness of acupuncture, compared with a noninsertive sham control. However, a Cochrane review found that acupuncture was more effective, compared with no treatment, and it had a moderate effect size, but was not effective when compared with a sham control (Cochrane Database Syst Rev. 2013 Jul 30;7:CD007410. doi:10.1002/14651858.CD007410.pub2).
The current trial, conducted at multiple sites in Australia, sought to add to the evidence with an adequately powered trial involving a sham control. But Carolyn Ee and her associates at the University of Melbourne noted that their study did not control for the nonspecific effects of acupuncture, such as regular interaction with a therapist.
The researchers randomly assigned 327 women aged older than 40 years who were in late menopause transition or postmenopause and experiencing at least seven moderate daily hot flashes to receive either a standardized Chinese medicine acupuncture treatment or a noninsertive, blunt needle sham acupuncture treatment. Patients received 10 treatments over 8 weeks, and they were assessed at 4 weeks, at the end of treatment, and at 3 and 6 months after treatment (Ann Intern Med. 2016; Jan 18. doi:10.7326/M15-1380.).
Both groups had about a 40% improvement in their hot flashes at the end of treatment, compared with their mean baseline hot flash score. The improvement was sustained at 3 and 6 months after the trial. In the acupuncture group, the mean hot flash scores at the end of treatment were 15.36, compared with 15.04 in the sham group, which was not statistically different. The researchers also found no advantage for acupuncture in quality of life, anxiety, or depression.
“Unless further high-quality evidence emerges, we cannot recommend skin-penetrating acupuncture as an efficacious treatment of this indication; the effects, if any, of acupuncture on these symptoms seem to be unrelated to needling,” the researchers wrote.
Some of the researchers reported receiving grant, scholarship, or fellowship support from the National Health and Medical Research Council of Australia, which funded the study.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Chinese medicine acupuncture was no better than a sham treatment for the relief of hot flashes.
Major finding: After 8 weeks of treatment, mean hot flash scores were 15.36 in the acupuncture group and 15.04 in the sham treatment group, which was not a statistically significant difference.
Data source: A stratified, blind, parallel, randomized, sham-controlled trial of 327 women in late menopause transition or postmenopause.
Disclosures: Some of the researchers reported receiving grant, scholarship, or fellowship support from the National Health and Medical Research Council of Australia, which funded the study.
A look back at 1966
As Ob.Gyn. News celebrates 50 years of publication, we’re taking a look back at our first year – 1966.
Not surprisingly, medicine looked a lot different in the mid-1960s, largely driven by the culture and technology of the time. A review of the 1966 issues of Obstetrics and Gynecology (the Green Journal), offers a snapshot of the state of the science.
With scientists still struggling to develop a rapid test to detect pregnancy, researchers from the Brookdale Hospital Center in Brooklyn, N.Y., detailed the possibility of using elevated breast temperature to get faster results. They compared 50 pregnant and 50 nonpregnant women and found a consistent rise in breast temperature in all pregnant women as early as 1 week after the first missed period. In the March issue, they concluded that the use of temperature difference between the breast and a baseline area on the anterior chest wall could be a rapid, simple, and accurate pregnancy test (Obstet Gynecol. 1966 Mar;27[3]:378-80).
In August, researchers from Australia published promising data on the use of ultrasonic echoscopic examination of the uterus in late pregnancy. They found that the technology was useful in determining fetal position and possible abnormalities and could be repeated as often as necessary to observe changes and growth. The big advantage, they noted, would be the opportunity to avoid excessive fetal exposure to x-rays (Obstet Gynecol. 1966 Aug;28[2]:164-9).
Advertising directed at physicians – in both the Green Journal and in Ob.Gyn. News – provided a glimpse into the practice of medicine at the time. Ob.gyns. saw ads for products such as Eskatrol – a capsule that contained dextroamphetamine sulfate and prochlorperazine – promoted to help women control appetite and “relieve the emotional stress that causes overeating.” And doctors also saw ads for oral contraceptives, first approved by the Food and Drug Administration in 1960.
Ob.gyn. practice was different culturally as well. In a regular column titled “After Office Hours,” published in the Green Journal in January 1966, Dr. Malcolm S. Allan explored a relatively new idea – husband-attended deliveries. Dr. Allan, of Wesson Maternity Hospital in Springfield, Mass., explained that his hospital had conducted a nationwide survey of chiefs of obstetrics after they received a petition seeking to allow husbands into the delivery room, as well as more flexibility for fathers to room in with the mother and baby. The survey, which included responses from 267 hospitals, showed that 81% of hospitals did not allow husbands in the delivery room (Obstet Gynecol. 1966 Jan;27[1]:146-8).
After reviewing the survey results and talking to experts in the area, Dr. Allan and the leadership at Wesson decided not to allow husbands to witness deliveries. He concluded that “some patients in some of these ‘off-beat’ programs are being allowed to assume too much authority for determining the medical management of their pregnancies, while leaving the obstetrician with the responsibility for a healthy outcome.”
But in other ways, not much has changed since 1966. The March edition of “After Office Hours” bemoaned a looming manpower crisis in obstetrics (Obstet Gynecol. 1966 Mar;27[3]:449-52). Dr. Jan Schneider of the University of Michigan, Ann Arbor, wrote that even using conservative estimates of population growth, by 1970 there would be 20,000 obstetricians in the United States delivering on average of 225 babies each, a strain on the workforce. What were some of the factors? An uneven distribution of obstetricians throughout the country and increasing specialization.
In another familiar theme, Dr. Schneider urged physicians to consider team care as one part of the solution, allowing nurse midwives to provide prenatal care and perform normal deliveries under physician supervision.
Some of the clinical debates going on in 1966 are still unresolved. Consider the September 1966 issue of the Green Journal, which features an interim report on contraception with an intrauterine bow inserted immediately postpartum (Obstet Gynecol. 1966 Sep;28[3]:329-31). Five decades later, only about 12 state Medicaid programs cover the cost of insertion of an IUD immediately postpartum. And in the August 1966 issue of the Green Journal, Dr. Carl J. Pauerstein asked, “Once a Section, Always a Trial of Labor?” (Obstet Gynecol. 1966 Aug;28[2]:273-6). A look at the recent Master Class on vaginal birth after cesarean shows that those same questions are still being debated today.
So what will physicians and patients say about obstetrics and gynecology practice 50 years from now?
1966 at a glance
The Surgeon General
In a report to U.S. Surgeon General William H. Stewart titled “Protecting and Improving Health through the Radiological Sciences,” the National Advisory Committee on Radiation warned about emerging problems in the use of ionizing radiation in medicine.
Births
According to data provided by the Centers for Disease Control and Prevention, in 1966, there were 3.6 million births, for a birth rate of 18.4 and a fertility rate of 90.8; 8.4% of births were to unmarried women.
Women get organized
In June, Betty Friedan, Pauli Murray, and several other women launched the National Organization for Women at a conference in Washington, D.C., with Ms. Friedan famously writing N-O-W on a paper napkin.
Medical ethics
Dr. Henry K. Beecher published an article on ethics in the New England Journal of Medicine that is credited with spurring the federal government to set rules on human experimentation and informed consent, including establishment of Institutional Review Boards.
A safety net is born
On July 1, 1966, Medicare coverage began, with more than 19 million beneficiaries.
The AMA
The American Medical Association published the first edition of the Current Procedural Terminology (CPT) code book, creating a system of standardized terms for medical procedures used in documentation. Also in 1966, the AMA encouraged doctors to promote exercise to improve health.
Planned Parenthood
The Planned Parenthood Federation of America awarded its first Margaret Sanger Award. In 1966, four men received the award, including the Rev. Martin Luther King Jr. and President Lyndon B. Johnson.
Pregnancy testing
The first radioimmunoassay for hCG (human chorionic gonadotropin) was described by A.R. Midgley, but the test could not distinguish between hCG and luteinizing hormone. A home pregnancy test was still a decade away.
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
As Ob.Gyn. News celebrates 50 years of publication, we’re taking a look back at our first year – 1966.
Not surprisingly, medicine looked a lot different in the mid-1960s, largely driven by the culture and technology of the time. A review of the 1966 issues of Obstetrics and Gynecology (the Green Journal), offers a snapshot of the state of the science.
With scientists still struggling to develop a rapid test to detect pregnancy, researchers from the Brookdale Hospital Center in Brooklyn, N.Y., detailed the possibility of using elevated breast temperature to get faster results. They compared 50 pregnant and 50 nonpregnant women and found a consistent rise in breast temperature in all pregnant women as early as 1 week after the first missed period. In the March issue, they concluded that the use of temperature difference between the breast and a baseline area on the anterior chest wall could be a rapid, simple, and accurate pregnancy test (Obstet Gynecol. 1966 Mar;27[3]:378-80).
In August, researchers from Australia published promising data on the use of ultrasonic echoscopic examination of the uterus in late pregnancy. They found that the technology was useful in determining fetal position and possible abnormalities and could be repeated as often as necessary to observe changes and growth. The big advantage, they noted, would be the opportunity to avoid excessive fetal exposure to x-rays (Obstet Gynecol. 1966 Aug;28[2]:164-9).
Advertising directed at physicians – in both the Green Journal and in Ob.Gyn. News – provided a glimpse into the practice of medicine at the time. Ob.gyns. saw ads for products such as Eskatrol – a capsule that contained dextroamphetamine sulfate and prochlorperazine – promoted to help women control appetite and “relieve the emotional stress that causes overeating.” And doctors also saw ads for oral contraceptives, first approved by the Food and Drug Administration in 1960.
Ob.gyn. practice was different culturally as well. In a regular column titled “After Office Hours,” published in the Green Journal in January 1966, Dr. Malcolm S. Allan explored a relatively new idea – husband-attended deliveries. Dr. Allan, of Wesson Maternity Hospital in Springfield, Mass., explained that his hospital had conducted a nationwide survey of chiefs of obstetrics after they received a petition seeking to allow husbands into the delivery room, as well as more flexibility for fathers to room in with the mother and baby. The survey, which included responses from 267 hospitals, showed that 81% of hospitals did not allow husbands in the delivery room (Obstet Gynecol. 1966 Jan;27[1]:146-8).
After reviewing the survey results and talking to experts in the area, Dr. Allan and the leadership at Wesson decided not to allow husbands to witness deliveries. He concluded that “some patients in some of these ‘off-beat’ programs are being allowed to assume too much authority for determining the medical management of their pregnancies, while leaving the obstetrician with the responsibility for a healthy outcome.”
But in other ways, not much has changed since 1966. The March edition of “After Office Hours” bemoaned a looming manpower crisis in obstetrics (Obstet Gynecol. 1966 Mar;27[3]:449-52). Dr. Jan Schneider of the University of Michigan, Ann Arbor, wrote that even using conservative estimates of population growth, by 1970 there would be 20,000 obstetricians in the United States delivering on average of 225 babies each, a strain on the workforce. What were some of the factors? An uneven distribution of obstetricians throughout the country and increasing specialization.
In another familiar theme, Dr. Schneider urged physicians to consider team care as one part of the solution, allowing nurse midwives to provide prenatal care and perform normal deliveries under physician supervision.
Some of the clinical debates going on in 1966 are still unresolved. Consider the September 1966 issue of the Green Journal, which features an interim report on contraception with an intrauterine bow inserted immediately postpartum (Obstet Gynecol. 1966 Sep;28[3]:329-31). Five decades later, only about 12 state Medicaid programs cover the cost of insertion of an IUD immediately postpartum. And in the August 1966 issue of the Green Journal, Dr. Carl J. Pauerstein asked, “Once a Section, Always a Trial of Labor?” (Obstet Gynecol. 1966 Aug;28[2]:273-6). A look at the recent Master Class on vaginal birth after cesarean shows that those same questions are still being debated today.
So what will physicians and patients say about obstetrics and gynecology practice 50 years from now?
1966 at a glance
The Surgeon General
In a report to U.S. Surgeon General William H. Stewart titled “Protecting and Improving Health through the Radiological Sciences,” the National Advisory Committee on Radiation warned about emerging problems in the use of ionizing radiation in medicine.
Births
According to data provided by the Centers for Disease Control and Prevention, in 1966, there were 3.6 million births, for a birth rate of 18.4 and a fertility rate of 90.8; 8.4% of births were to unmarried women.
Women get organized
In June, Betty Friedan, Pauli Murray, and several other women launched the National Organization for Women at a conference in Washington, D.C., with Ms. Friedan famously writing N-O-W on a paper napkin.
Medical ethics
Dr. Henry K. Beecher published an article on ethics in the New England Journal of Medicine that is credited with spurring the federal government to set rules on human experimentation and informed consent, including establishment of Institutional Review Boards.
A safety net is born
On July 1, 1966, Medicare coverage began, with more than 19 million beneficiaries.
The AMA
The American Medical Association published the first edition of the Current Procedural Terminology (CPT) code book, creating a system of standardized terms for medical procedures used in documentation. Also in 1966, the AMA encouraged doctors to promote exercise to improve health.
Planned Parenthood
The Planned Parenthood Federation of America awarded its first Margaret Sanger Award. In 1966, four men received the award, including the Rev. Martin Luther King Jr. and President Lyndon B. Johnson.
Pregnancy testing
The first radioimmunoassay for hCG (human chorionic gonadotropin) was described by A.R. Midgley, but the test could not distinguish between hCG and luteinizing hormone. A home pregnancy test was still a decade away.
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
As Ob.Gyn. News celebrates 50 years of publication, we’re taking a look back at our first year – 1966.
Not surprisingly, medicine looked a lot different in the mid-1960s, largely driven by the culture and technology of the time. A review of the 1966 issues of Obstetrics and Gynecology (the Green Journal), offers a snapshot of the state of the science.
With scientists still struggling to develop a rapid test to detect pregnancy, researchers from the Brookdale Hospital Center in Brooklyn, N.Y., detailed the possibility of using elevated breast temperature to get faster results. They compared 50 pregnant and 50 nonpregnant women and found a consistent rise in breast temperature in all pregnant women as early as 1 week after the first missed period. In the March issue, they concluded that the use of temperature difference between the breast and a baseline area on the anterior chest wall could be a rapid, simple, and accurate pregnancy test (Obstet Gynecol. 1966 Mar;27[3]:378-80).
In August, researchers from Australia published promising data on the use of ultrasonic echoscopic examination of the uterus in late pregnancy. They found that the technology was useful in determining fetal position and possible abnormalities and could be repeated as often as necessary to observe changes and growth. The big advantage, they noted, would be the opportunity to avoid excessive fetal exposure to x-rays (Obstet Gynecol. 1966 Aug;28[2]:164-9).
Advertising directed at physicians – in both the Green Journal and in Ob.Gyn. News – provided a glimpse into the practice of medicine at the time. Ob.gyns. saw ads for products such as Eskatrol – a capsule that contained dextroamphetamine sulfate and prochlorperazine – promoted to help women control appetite and “relieve the emotional stress that causes overeating.” And doctors also saw ads for oral contraceptives, first approved by the Food and Drug Administration in 1960.
Ob.gyn. practice was different culturally as well. In a regular column titled “After Office Hours,” published in the Green Journal in January 1966, Dr. Malcolm S. Allan explored a relatively new idea – husband-attended deliveries. Dr. Allan, of Wesson Maternity Hospital in Springfield, Mass., explained that his hospital had conducted a nationwide survey of chiefs of obstetrics after they received a petition seeking to allow husbands into the delivery room, as well as more flexibility for fathers to room in with the mother and baby. The survey, which included responses from 267 hospitals, showed that 81% of hospitals did not allow husbands in the delivery room (Obstet Gynecol. 1966 Jan;27[1]:146-8).
After reviewing the survey results and talking to experts in the area, Dr. Allan and the leadership at Wesson decided not to allow husbands to witness deliveries. He concluded that “some patients in some of these ‘off-beat’ programs are being allowed to assume too much authority for determining the medical management of their pregnancies, while leaving the obstetrician with the responsibility for a healthy outcome.”
But in other ways, not much has changed since 1966. The March edition of “After Office Hours” bemoaned a looming manpower crisis in obstetrics (Obstet Gynecol. 1966 Mar;27[3]:449-52). Dr. Jan Schneider of the University of Michigan, Ann Arbor, wrote that even using conservative estimates of population growth, by 1970 there would be 20,000 obstetricians in the United States delivering on average of 225 babies each, a strain on the workforce. What were some of the factors? An uneven distribution of obstetricians throughout the country and increasing specialization.
In another familiar theme, Dr. Schneider urged physicians to consider team care as one part of the solution, allowing nurse midwives to provide prenatal care and perform normal deliveries under physician supervision.
Some of the clinical debates going on in 1966 are still unresolved. Consider the September 1966 issue of the Green Journal, which features an interim report on contraception with an intrauterine bow inserted immediately postpartum (Obstet Gynecol. 1966 Sep;28[3]:329-31). Five decades later, only about 12 state Medicaid programs cover the cost of insertion of an IUD immediately postpartum. And in the August 1966 issue of the Green Journal, Dr. Carl J. Pauerstein asked, “Once a Section, Always a Trial of Labor?” (Obstet Gynecol. 1966 Aug;28[2]:273-6). A look at the recent Master Class on vaginal birth after cesarean shows that those same questions are still being debated today.
So what will physicians and patients say about obstetrics and gynecology practice 50 years from now?
1966 at a glance
The Surgeon General
In a report to U.S. Surgeon General William H. Stewart titled “Protecting and Improving Health through the Radiological Sciences,” the National Advisory Committee on Radiation warned about emerging problems in the use of ionizing radiation in medicine.
Births
According to data provided by the Centers for Disease Control and Prevention, in 1966, there were 3.6 million births, for a birth rate of 18.4 and a fertility rate of 90.8; 8.4% of births were to unmarried women.
Women get organized
In June, Betty Friedan, Pauli Murray, and several other women launched the National Organization for Women at a conference in Washington, D.C., with Ms. Friedan famously writing N-O-W on a paper napkin.
Medical ethics
Dr. Henry K. Beecher published an article on ethics in the New England Journal of Medicine that is credited with spurring the federal government to set rules on human experimentation and informed consent, including establishment of Institutional Review Boards.
A safety net is born
On July 1, 1966, Medicare coverage began, with more than 19 million beneficiaries.
The AMA
The American Medical Association published the first edition of the Current Procedural Terminology (CPT) code book, creating a system of standardized terms for medical procedures used in documentation. Also in 1966, the AMA encouraged doctors to promote exercise to improve health.
Planned Parenthood
The Planned Parenthood Federation of America awarded its first Margaret Sanger Award. In 1966, four men received the award, including the Rev. Martin Luther King Jr. and President Lyndon B. Johnson.
Pregnancy testing
The first radioimmunoassay for hCG (human chorionic gonadotropin) was described by A.R. Midgley, but the test could not distinguish between hCG and luteinizing hormone. A home pregnancy test was still a decade away.
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
FDA reclassifies transvaginal mesh for pelvic organ prolapse as ‘high risk’
The Food and Drug Administration is tightening its regulation of surgical mesh to repair pelvic organ prolapse transvaginally or through the vagina, citing continued safety risks including severe pelvic pain and organ perforation.
The agency is reclassifying surgical mesh used for transvaginal repair of pelvic organ prolapse from a class II to a class III medical device, moving the devices from the moderate-risk to high-risk category. The FDA is also requiring device manufacturers to submit a premarket approval application that supports the safety and effectiveness of surgical mesh. The premarket approval application requirement only applies to mesh used for transvaginal repair of pelvic organ prolapse, not to mesh used for other indications, such as stress urinary incontinence or abdominal repair of pelvic organ prolapse.
The first mesh device for transvaginal repair of pelvic organ prolapse was cleared for use by the FDA in 2002 and five manufacturers are currently marketing the product, according to the FDA.
For products that are already on the market, manufacturers have 30 months to comply with the premarket approval application requirements.
“These stronger clinical requirements will help to address the significant risks associated with surgical mesh for repair of pelvic organ prolapse,” Dr. William Maisel, deputy director of science and chief scientist for the FDA’s Center for Devices and Radiological Health, said in a statement. “We intend to continue monitoring how women with this device are faring months and years after surgery through continued postmarket surveillance measures.”
This is the latest move by the FDA to signal to physicians and patients that surgical mesh should be used with caution when treating pelvic organ prolapse.
In 2008 and 2011, the FDA issued warnings about increased adverse events reports for surgical mesh used in urogynecological procedures. And in 2012, the agency instructed manufacturers to conduct postmarket surveillance studies on surgical mesh used for tranvaginal repair of pelvic organ prolapse.
The agency first proposed reclassifying surgical mesh to a class III device and requiring premarket approval in May 2014.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The Food and Drug Administration is tightening its regulation of surgical mesh to repair pelvic organ prolapse transvaginally or through the vagina, citing continued safety risks including severe pelvic pain and organ perforation.
The agency is reclassifying surgical mesh used for transvaginal repair of pelvic organ prolapse from a class II to a class III medical device, moving the devices from the moderate-risk to high-risk category. The FDA is also requiring device manufacturers to submit a premarket approval application that supports the safety and effectiveness of surgical mesh. The premarket approval application requirement only applies to mesh used for transvaginal repair of pelvic organ prolapse, not to mesh used for other indications, such as stress urinary incontinence or abdominal repair of pelvic organ prolapse.
The first mesh device for transvaginal repair of pelvic organ prolapse was cleared for use by the FDA in 2002 and five manufacturers are currently marketing the product, according to the FDA.
For products that are already on the market, manufacturers have 30 months to comply with the premarket approval application requirements.
“These stronger clinical requirements will help to address the significant risks associated with surgical mesh for repair of pelvic organ prolapse,” Dr. William Maisel, deputy director of science and chief scientist for the FDA’s Center for Devices and Radiological Health, said in a statement. “We intend to continue monitoring how women with this device are faring months and years after surgery through continued postmarket surveillance measures.”
This is the latest move by the FDA to signal to physicians and patients that surgical mesh should be used with caution when treating pelvic organ prolapse.
In 2008 and 2011, the FDA issued warnings about increased adverse events reports for surgical mesh used in urogynecological procedures. And in 2012, the agency instructed manufacturers to conduct postmarket surveillance studies on surgical mesh used for tranvaginal repair of pelvic organ prolapse.
The agency first proposed reclassifying surgical mesh to a class III device and requiring premarket approval in May 2014.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The Food and Drug Administration is tightening its regulation of surgical mesh to repair pelvic organ prolapse transvaginally or through the vagina, citing continued safety risks including severe pelvic pain and organ perforation.
The agency is reclassifying surgical mesh used for transvaginal repair of pelvic organ prolapse from a class II to a class III medical device, moving the devices from the moderate-risk to high-risk category. The FDA is also requiring device manufacturers to submit a premarket approval application that supports the safety and effectiveness of surgical mesh. The premarket approval application requirement only applies to mesh used for transvaginal repair of pelvic organ prolapse, not to mesh used for other indications, such as stress urinary incontinence or abdominal repair of pelvic organ prolapse.
The first mesh device for transvaginal repair of pelvic organ prolapse was cleared for use by the FDA in 2002 and five manufacturers are currently marketing the product, according to the FDA.
For products that are already on the market, manufacturers have 30 months to comply with the premarket approval application requirements.
“These stronger clinical requirements will help to address the significant risks associated with surgical mesh for repair of pelvic organ prolapse,” Dr. William Maisel, deputy director of science and chief scientist for the FDA’s Center for Devices and Radiological Health, said in a statement. “We intend to continue monitoring how women with this device are faring months and years after surgery through continued postmarket surveillance measures.”
This is the latest move by the FDA to signal to physicians and patients that surgical mesh should be used with caution when treating pelvic organ prolapse.
In 2008 and 2011, the FDA issued warnings about increased adverse events reports for surgical mesh used in urogynecological procedures. And in 2012, the agency instructed manufacturers to conduct postmarket surveillance studies on surgical mesh used for tranvaginal repair of pelvic organ prolapse.
The agency first proposed reclassifying surgical mesh to a class III device and requiring premarket approval in May 2014.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
VIDEO: Could kisspeptin help avoid ovarian hyperstimulation during IVF?
Clinicians may someday be able to cut the risk of ovarian hyperstimulation during in vitro fertilization by using the hormone kisspeptin when maturing and harvesting eggs instead of human chorionic gonadotropin, according to a new study.
Researchers at the Imperial College of London and the Imperial College Healthcare NHS Trust used kisspeptin at varying doses as part of the IVF protocol among 60 women at high risk for ovarian hyperstimulation syndrome (OHSS). The average live birth rate was 45% across all doses of the hormone. No women in the study developed moderate, severe, or critical OHSS during pregnancy.
“Kisspeptin appears to be a promising therapy and further studies are now needed to directly compare kisspeptin with currently available IVF treatments,” said Dr. Ali Abbara, the lead author of the study.
The researchers presented their findings at the Society for Endocrinology’s annual conference in Edinburgh.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Clinicians may someday be able to cut the risk of ovarian hyperstimulation during in vitro fertilization by using the hormone kisspeptin when maturing and harvesting eggs instead of human chorionic gonadotropin, according to a new study.
Researchers at the Imperial College of London and the Imperial College Healthcare NHS Trust used kisspeptin at varying doses as part of the IVF protocol among 60 women at high risk for ovarian hyperstimulation syndrome (OHSS). The average live birth rate was 45% across all doses of the hormone. No women in the study developed moderate, severe, or critical OHSS during pregnancy.
“Kisspeptin appears to be a promising therapy and further studies are now needed to directly compare kisspeptin with currently available IVF treatments,” said Dr. Ali Abbara, the lead author of the study.
The researchers presented their findings at the Society for Endocrinology’s annual conference in Edinburgh.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Clinicians may someday be able to cut the risk of ovarian hyperstimulation during in vitro fertilization by using the hormone kisspeptin when maturing and harvesting eggs instead of human chorionic gonadotropin, according to a new study.
Researchers at the Imperial College of London and the Imperial College Healthcare NHS Trust used kisspeptin at varying doses as part of the IVF protocol among 60 women at high risk for ovarian hyperstimulation syndrome (OHSS). The average live birth rate was 45% across all doses of the hormone. No women in the study developed moderate, severe, or critical OHSS during pregnancy.
“Kisspeptin appears to be a promising therapy and further studies are now needed to directly compare kisspeptin with currently available IVF treatments,” said Dr. Ali Abbara, the lead author of the study.
The researchers presented their findings at the Society for Endocrinology’s annual conference in Edinburgh.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
ACOG, SMFM offer guidance on periviable births
The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine have released a new consensus document recommending best practices in the care of neonates born between 20 and 25 weeks’ gestation.
The periviable birth guidance provides recommendations on the proper level of care at the hospital, the need for counseling on short- and long-term neonatal outcomes, family counseling, and a predelivery plan. The guidance also makes specific recommendations for interventions based on the best estimate of gestational age (Obstet Gynecol. 2015;126:e82-e94).
“Care during the periviable period is incredibly complex, and requires providers to take into account a wide variety of considerations,” Dr. Brian M. Mercer, past president of SMFM and a lead author of the document, said in a statement. “Just as important as trying to predict outcomes is the role of counseling patients in a way that is both accurate and empathetic.”
ACOG and SMFM suggest that clinicians use a prediction tool, rather than relying on gestational age and birth weight estimates alone, when estimating outcomes before a periviable birth. The National Institute of Child Health and Human Development has a tool that can aid in predicting outcomes for extremely preterm births that is based on data from more than 4,400 live births between 22 and 25 weeks’ gestation.
Since both infant and mother will need immediate advanced care after delivery, the groups also recommend that deliveries during the periviable period occur at centers with level III-IV neonatal intensive care units and a level III-IV maternal care designation. These facilities can provide immediate resuscitation, as well as other resources such as respiratory technology and 24-hour newborn imaging. When possible, transfers should be made before delivery, according to the consensus document.
Counseling is another essential element in the management of periviable birth. Family counseling with a team of physicians, including the ob.gyn., maternal-fetal medicine specialist, and neonatologist, should address the likely maternal and neonatal outcomes and the family’s values, including the option for palliative care. ACOG and SMFM recommended making a predelivery plan with the family with the recognition that it would be modified based on the clinical situation.
The document also provides general guidance for performing specific obstetric interventions based on gestational age. For instance, ACOG and SMFM suggest that clinicians “consider” antenatal corticosteriod administration when the estimated gestational age is 23 weeks. They “recommend” antenatal corticosteriods starting at 24 weeks’ gestation through 25 weeks’ gestation.
The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine have released a new consensus document recommending best practices in the care of neonates born between 20 and 25 weeks’ gestation.
The periviable birth guidance provides recommendations on the proper level of care at the hospital, the need for counseling on short- and long-term neonatal outcomes, family counseling, and a predelivery plan. The guidance also makes specific recommendations for interventions based on the best estimate of gestational age (Obstet Gynecol. 2015;126:e82-e94).
“Care during the periviable period is incredibly complex, and requires providers to take into account a wide variety of considerations,” Dr. Brian M. Mercer, past president of SMFM and a lead author of the document, said in a statement. “Just as important as trying to predict outcomes is the role of counseling patients in a way that is both accurate and empathetic.”
ACOG and SMFM suggest that clinicians use a prediction tool, rather than relying on gestational age and birth weight estimates alone, when estimating outcomes before a periviable birth. The National Institute of Child Health and Human Development has a tool that can aid in predicting outcomes for extremely preterm births that is based on data from more than 4,400 live births between 22 and 25 weeks’ gestation.
Since both infant and mother will need immediate advanced care after delivery, the groups also recommend that deliveries during the periviable period occur at centers with level III-IV neonatal intensive care units and a level III-IV maternal care designation. These facilities can provide immediate resuscitation, as well as other resources such as respiratory technology and 24-hour newborn imaging. When possible, transfers should be made before delivery, according to the consensus document.
Counseling is another essential element in the management of periviable birth. Family counseling with a team of physicians, including the ob.gyn., maternal-fetal medicine specialist, and neonatologist, should address the likely maternal and neonatal outcomes and the family’s values, including the option for palliative care. ACOG and SMFM recommended making a predelivery plan with the family with the recognition that it would be modified based on the clinical situation.
The document also provides general guidance for performing specific obstetric interventions based on gestational age. For instance, ACOG and SMFM suggest that clinicians “consider” antenatal corticosteriod administration when the estimated gestational age is 23 weeks. They “recommend” antenatal corticosteriods starting at 24 weeks’ gestation through 25 weeks’ gestation.
The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine have released a new consensus document recommending best practices in the care of neonates born between 20 and 25 weeks’ gestation.
The periviable birth guidance provides recommendations on the proper level of care at the hospital, the need for counseling on short- and long-term neonatal outcomes, family counseling, and a predelivery plan. The guidance also makes specific recommendations for interventions based on the best estimate of gestational age (Obstet Gynecol. 2015;126:e82-e94).
“Care during the periviable period is incredibly complex, and requires providers to take into account a wide variety of considerations,” Dr. Brian M. Mercer, past president of SMFM and a lead author of the document, said in a statement. “Just as important as trying to predict outcomes is the role of counseling patients in a way that is both accurate and empathetic.”
ACOG and SMFM suggest that clinicians use a prediction tool, rather than relying on gestational age and birth weight estimates alone, when estimating outcomes before a periviable birth. The National Institute of Child Health and Human Development has a tool that can aid in predicting outcomes for extremely preterm births that is based on data from more than 4,400 live births between 22 and 25 weeks’ gestation.
Since both infant and mother will need immediate advanced care after delivery, the groups also recommend that deliveries during the periviable period occur at centers with level III-IV neonatal intensive care units and a level III-IV maternal care designation. These facilities can provide immediate resuscitation, as well as other resources such as respiratory technology and 24-hour newborn imaging. When possible, transfers should be made before delivery, according to the consensus document.
Counseling is another essential element in the management of periviable birth. Family counseling with a team of physicians, including the ob.gyn., maternal-fetal medicine specialist, and neonatologist, should address the likely maternal and neonatal outcomes and the family’s values, including the option for palliative care. ACOG and SMFM recommended making a predelivery plan with the family with the recognition that it would be modified based on the clinical situation.
The document also provides general guidance for performing specific obstetric interventions based on gestational age. For instance, ACOG and SMFM suggest that clinicians “consider” antenatal corticosteriod administration when the estimated gestational age is 23 weeks. They “recommend” antenatal corticosteriods starting at 24 weeks’ gestation through 25 weeks’ gestation.
FROM OBSTETRICS AND GYNECOLOGY
NIH expands cancer research program to include breast density
Officials at the National Institutes of Health are taking a closer look at the role that breast density plays in the development of breast cancer.
As part of the agency’s Breast Cancer and the Environment Research Program (BCERP), grant-funded researchers are being tasked with expanding the study of risk factors that precede breast cancer such as breast density. They will also include more racially and ethnically diverse populations in their studies.
The research will be conducted at six centers: Brigham and Women’s Hospital, Boston; City of Hope/Beckman Research Institute, Duarte, Calif.; Columbia University, New York; Georgetown Lombardi Comprehensive Cancer Center, Washington; Michigan State University, Lansing; University of Massachusetts, Amherst. The research will be coordinated by another center at the University of Wisconsin, Madison.
Breast density is a new area of focus for the NIH. The researchers will consider dense breast tissue as a possible intermediate risk factor for breast cancer with the goal of identifying links between environmental exposures and high breast density, which could provide future prevention strategies.
“These priorities reflect our continued commitment to breast cancer prevention,” Caroline H. Dilworth, Ph.D., BCERP program lead at the National Institute of Environmental Health Sciences, said in a statement. “Our goal is to build on the high-quality science we’ve been funding for more than a decade, while also being responsive to the expert recommendations of the Interagency Breast Cancer and Environmental Research Coordinating Committee] report.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Officials at the National Institutes of Health are taking a closer look at the role that breast density plays in the development of breast cancer.
As part of the agency’s Breast Cancer and the Environment Research Program (BCERP), grant-funded researchers are being tasked with expanding the study of risk factors that precede breast cancer such as breast density. They will also include more racially and ethnically diverse populations in their studies.
The research will be conducted at six centers: Brigham and Women’s Hospital, Boston; City of Hope/Beckman Research Institute, Duarte, Calif.; Columbia University, New York; Georgetown Lombardi Comprehensive Cancer Center, Washington; Michigan State University, Lansing; University of Massachusetts, Amherst. The research will be coordinated by another center at the University of Wisconsin, Madison.
Breast density is a new area of focus for the NIH. The researchers will consider dense breast tissue as a possible intermediate risk factor for breast cancer with the goal of identifying links between environmental exposures and high breast density, which could provide future prevention strategies.
“These priorities reflect our continued commitment to breast cancer prevention,” Caroline H. Dilworth, Ph.D., BCERP program lead at the National Institute of Environmental Health Sciences, said in a statement. “Our goal is to build on the high-quality science we’ve been funding for more than a decade, while also being responsive to the expert recommendations of the Interagency Breast Cancer and Environmental Research Coordinating Committee] report.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Officials at the National Institutes of Health are taking a closer look at the role that breast density plays in the development of breast cancer.
As part of the agency’s Breast Cancer and the Environment Research Program (BCERP), grant-funded researchers are being tasked with expanding the study of risk factors that precede breast cancer such as breast density. They will also include more racially and ethnically diverse populations in their studies.
The research will be conducted at six centers: Brigham and Women’s Hospital, Boston; City of Hope/Beckman Research Institute, Duarte, Calif.; Columbia University, New York; Georgetown Lombardi Comprehensive Cancer Center, Washington; Michigan State University, Lansing; University of Massachusetts, Amherst. The research will be coordinated by another center at the University of Wisconsin, Madison.
Breast density is a new area of focus for the NIH. The researchers will consider dense breast tissue as a possible intermediate risk factor for breast cancer with the goal of identifying links between environmental exposures and high breast density, which could provide future prevention strategies.
“These priorities reflect our continued commitment to breast cancer prevention,” Caroline H. Dilworth, Ph.D., BCERP program lead at the National Institute of Environmental Health Sciences, said in a statement. “Our goal is to build on the high-quality science we’ve been funding for more than a decade, while also being responsive to the expert recommendations of the Interagency Breast Cancer and Environmental Research Coordinating Committee] report.”
mschneider@frontlinemedcom.com
On Twitter @maryellenny