User login
Combo treatment under review for Waldenstrom macroglobulinemia
by the Food and Drug Administration.
Ibrutinib, a Bruton’s tyrosine kinase inhibitor, is already approved as a single agent for WM. The addition of rituximab to the indication is based on positive results from the phase 3 INNOVATE study. In particular, the trial showed a superior progression-free survival rate at 30 months for the ibrutinib-rituximab combination at 82%, compared with placebo plus rituximab at 28% (N Engl J Med. 2018;378:2399-410).
The study’s lead investigator, Meletios A. Dimopoulos, MD, called the combination a “new standard of care” for WM at the recent annual meeting of the American Society of Clinical Oncology.
Ibrutinib, marketed as Imbruvica, is jointly developed and commercialized by Pharmacyclics and Janssen Biotech.
by the Food and Drug Administration.
Ibrutinib, a Bruton’s tyrosine kinase inhibitor, is already approved as a single agent for WM. The addition of rituximab to the indication is based on positive results from the phase 3 INNOVATE study. In particular, the trial showed a superior progression-free survival rate at 30 months for the ibrutinib-rituximab combination at 82%, compared with placebo plus rituximab at 28% (N Engl J Med. 2018;378:2399-410).
The study’s lead investigator, Meletios A. Dimopoulos, MD, called the combination a “new standard of care” for WM at the recent annual meeting of the American Society of Clinical Oncology.
Ibrutinib, marketed as Imbruvica, is jointly developed and commercialized by Pharmacyclics and Janssen Biotech.
by the Food and Drug Administration.
Ibrutinib, a Bruton’s tyrosine kinase inhibitor, is already approved as a single agent for WM. The addition of rituximab to the indication is based on positive results from the phase 3 INNOVATE study. In particular, the trial showed a superior progression-free survival rate at 30 months for the ibrutinib-rituximab combination at 82%, compared with placebo plus rituximab at 28% (N Engl J Med. 2018;378:2399-410).
The study’s lead investigator, Meletios A. Dimopoulos, MD, called the combination a “new standard of care” for WM at the recent annual meeting of the American Society of Clinical Oncology.
Ibrutinib, marketed as Imbruvica, is jointly developed and commercialized by Pharmacyclics and Janssen Biotech.
Hemophilia adherence tied to perception of disease
More than half (56%) of adult patients with hemophilia are adherent to a prescribed prophylaxis regimen, but compliance appears less likely among patients who are having difficulty coping with pain or have a high conviction of disease.
Ana Torres-Ortuño, PhD, of the University of Murcia (Spain) and her colleagues performed a multicenter, cross-sectional descriptive study of 23 adult patients with severe hemophilia A or hemophilia B using various validated questionnaires that measured quality of life, disease perception, coping strategies, and treatment adherence.
The researchers found that Patients who experienced haemarthrosis with greater frequency had significantly greater adherence in terms of dosing (P less than .05), planning (P less than .05), and skipping (P less than .01). Similarly, patients with HIV infection were more adherent in terms of frequency of infusion than patients without infection.
The researchers also found significant correlations among all the psychosocial variables measured and adherence to prophylaxis. For instance, patients who had poorer quality of life related to managing their physical health, pain, and emotions showed poorer planning of their treatment. Patients who had difficulty remembering treatment had poorer quality of life related to pain and vitality, but they also had greater conviction of disease and hypochondriasis.
“Intervention programmes should be aimed more at changing barriers that patients and caregivers encounter when accepting diagnosis and how they can adapt their resources and skills to better take advantage of the progress made in treatments,” the researchers wrote.
The study was supported by a grant from Pfizer. The researchers reported having no financial disclosures.
SOURCE: Torres-Ortuño A et al. Vox Sang. 2018 May 24. doi: 10.1111/vox.12669.
More than half (56%) of adult patients with hemophilia are adherent to a prescribed prophylaxis regimen, but compliance appears less likely among patients who are having difficulty coping with pain or have a high conviction of disease.
Ana Torres-Ortuño, PhD, of the University of Murcia (Spain) and her colleagues performed a multicenter, cross-sectional descriptive study of 23 adult patients with severe hemophilia A or hemophilia B using various validated questionnaires that measured quality of life, disease perception, coping strategies, and treatment adherence.
The researchers found that Patients who experienced haemarthrosis with greater frequency had significantly greater adherence in terms of dosing (P less than .05), planning (P less than .05), and skipping (P less than .01). Similarly, patients with HIV infection were more adherent in terms of frequency of infusion than patients without infection.
The researchers also found significant correlations among all the psychosocial variables measured and adherence to prophylaxis. For instance, patients who had poorer quality of life related to managing their physical health, pain, and emotions showed poorer planning of their treatment. Patients who had difficulty remembering treatment had poorer quality of life related to pain and vitality, but they also had greater conviction of disease and hypochondriasis.
“Intervention programmes should be aimed more at changing barriers that patients and caregivers encounter when accepting diagnosis and how they can adapt their resources and skills to better take advantage of the progress made in treatments,” the researchers wrote.
The study was supported by a grant from Pfizer. The researchers reported having no financial disclosures.
SOURCE: Torres-Ortuño A et al. Vox Sang. 2018 May 24. doi: 10.1111/vox.12669.
More than half (56%) of adult patients with hemophilia are adherent to a prescribed prophylaxis regimen, but compliance appears less likely among patients who are having difficulty coping with pain or have a high conviction of disease.
Ana Torres-Ortuño, PhD, of the University of Murcia (Spain) and her colleagues performed a multicenter, cross-sectional descriptive study of 23 adult patients with severe hemophilia A or hemophilia B using various validated questionnaires that measured quality of life, disease perception, coping strategies, and treatment adherence.
The researchers found that Patients who experienced haemarthrosis with greater frequency had significantly greater adherence in terms of dosing (P less than .05), planning (P less than .05), and skipping (P less than .01). Similarly, patients with HIV infection were more adherent in terms of frequency of infusion than patients without infection.
The researchers also found significant correlations among all the psychosocial variables measured and adherence to prophylaxis. For instance, patients who had poorer quality of life related to managing their physical health, pain, and emotions showed poorer planning of their treatment. Patients who had difficulty remembering treatment had poorer quality of life related to pain and vitality, but they also had greater conviction of disease and hypochondriasis.
“Intervention programmes should be aimed more at changing barriers that patients and caregivers encounter when accepting diagnosis and how they can adapt their resources and skills to better take advantage of the progress made in treatments,” the researchers wrote.
The study was supported by a grant from Pfizer. The researchers reported having no financial disclosures.
SOURCE: Torres-Ortuño A et al. Vox Sang. 2018 May 24. doi: 10.1111/vox.12669.
FROM VOX SANGUINIS
British good practice paper offers MCL diagnosis pearls
Immunohistochemical panels used in the diagnosis of mantle cell lymphoma should include cyclin D1 and SOX11 immunostaining, according to a good practice paper from the British Society of Haematology.
Pamela McKay, MD, of the Beatson West of Scotland Cancer Centre, Glasgow, and her colleagues provided based on a review of literature from 1980 to 2017. The good practice paper aims to offer best practice advice based on consensus where the evidence is limited. Specifically, the paper incorporates new information on molecular pathology and the use of positron emission tomography/computed tomography (PET/CT) scanning in staging of disease.
The top recommendations related to MCL diagnosis include performing lymph node excision or adequate core biopsy for diagnosis of nodal MCL. For non-nodal presentation, a tissue biopsy or peripheral blood can be used. Additionally, immunohistochemical panels should include cyclin D1 and SOX11 immunostaining.
In cases of atypical morphology, aberrant immunophenotype, equivocal cyclin D1 positivity, or unusual clinical presentation, the authors recommended fluorescence in situ hybridization (FISH) to demonstrate the presence of the t(11;14) translocation. They also recommended recording the Ki67 Proliferation Index at baseline, with an index of greater than 30% being indicative of a poorer outcome.
In terms of staging disease, Dr. McKay and her associates recommended that patients undergo staging with CT of the neck, chest, abdomen, and pelvis. They recommended against routine use of fluorodeoxyglucose PET for MCL staging, but said it could be considered if radical radiotherapy is being proposed for early-stage disease.
For cases with suspicion of central nervous system involvement, lumbar puncture with cytospin and immunophenotyping is recommended.
They recommended that all MCL patients have either their simplified or combined MCL international prognostic index score recorded at baseline.
All the authors made a declaration of interest to the British Society of Haematology and task force chairs, which may be viewed on request.
SOURCE: McKay P et al. Br J Haematol. 2018 Jun 8. doi: 10.1111/bjh.15281.
Immunohistochemical panels used in the diagnosis of mantle cell lymphoma should include cyclin D1 and SOX11 immunostaining, according to a good practice paper from the British Society of Haematology.
Pamela McKay, MD, of the Beatson West of Scotland Cancer Centre, Glasgow, and her colleagues provided based on a review of literature from 1980 to 2017. The good practice paper aims to offer best practice advice based on consensus where the evidence is limited. Specifically, the paper incorporates new information on molecular pathology and the use of positron emission tomography/computed tomography (PET/CT) scanning in staging of disease.
The top recommendations related to MCL diagnosis include performing lymph node excision or adequate core biopsy for diagnosis of nodal MCL. For non-nodal presentation, a tissue biopsy or peripheral blood can be used. Additionally, immunohistochemical panels should include cyclin D1 and SOX11 immunostaining.
In cases of atypical morphology, aberrant immunophenotype, equivocal cyclin D1 positivity, or unusual clinical presentation, the authors recommended fluorescence in situ hybridization (FISH) to demonstrate the presence of the t(11;14) translocation. They also recommended recording the Ki67 Proliferation Index at baseline, with an index of greater than 30% being indicative of a poorer outcome.
In terms of staging disease, Dr. McKay and her associates recommended that patients undergo staging with CT of the neck, chest, abdomen, and pelvis. They recommended against routine use of fluorodeoxyglucose PET for MCL staging, but said it could be considered if radical radiotherapy is being proposed for early-stage disease.
For cases with suspicion of central nervous system involvement, lumbar puncture with cytospin and immunophenotyping is recommended.
They recommended that all MCL patients have either their simplified or combined MCL international prognostic index score recorded at baseline.
All the authors made a declaration of interest to the British Society of Haematology and task force chairs, which may be viewed on request.
SOURCE: McKay P et al. Br J Haematol. 2018 Jun 8. doi: 10.1111/bjh.15281.
Immunohistochemical panels used in the diagnosis of mantle cell lymphoma should include cyclin D1 and SOX11 immunostaining, according to a good practice paper from the British Society of Haematology.
Pamela McKay, MD, of the Beatson West of Scotland Cancer Centre, Glasgow, and her colleagues provided based on a review of literature from 1980 to 2017. The good practice paper aims to offer best practice advice based on consensus where the evidence is limited. Specifically, the paper incorporates new information on molecular pathology and the use of positron emission tomography/computed tomography (PET/CT) scanning in staging of disease.
The top recommendations related to MCL diagnosis include performing lymph node excision or adequate core biopsy for diagnosis of nodal MCL. For non-nodal presentation, a tissue biopsy or peripheral blood can be used. Additionally, immunohistochemical panels should include cyclin D1 and SOX11 immunostaining.
In cases of atypical morphology, aberrant immunophenotype, equivocal cyclin D1 positivity, or unusual clinical presentation, the authors recommended fluorescence in situ hybridization (FISH) to demonstrate the presence of the t(11;14) translocation. They also recommended recording the Ki67 Proliferation Index at baseline, with an index of greater than 30% being indicative of a poorer outcome.
In terms of staging disease, Dr. McKay and her associates recommended that patients undergo staging with CT of the neck, chest, abdomen, and pelvis. They recommended against routine use of fluorodeoxyglucose PET for MCL staging, but said it could be considered if radical radiotherapy is being proposed for early-stage disease.
For cases with suspicion of central nervous system involvement, lumbar puncture with cytospin and immunophenotyping is recommended.
They recommended that all MCL patients have either their simplified or combined MCL international prognostic index score recorded at baseline.
All the authors made a declaration of interest to the British Society of Haematology and task force chairs, which may be viewed on request.
SOURCE: McKay P et al. Br J Haematol. 2018 Jun 8. doi: 10.1111/bjh.15281.
FROM THE BRITISH JOURNAL OF HAEMATOLOGY
Emicizumab gets priority review for hemophilia A without inhibitors
The Food and Drug Administration has granted priority review to Roche’s emicizumab-kxwh (Hemlibra) for the treatment of adults and children with hemophilia A without factor VIII inhibitors.
The agency is scheduled to make a decision on approval in October 2018.
Among patients aged 12 years and older without factor VIII inhibitors, emicizumab-kxwh prophylaxis every week reduced treated bleeds by 96% (P less than .0001) and treated bleeds were reduced by 97% (P less than .0001) in patients who were treated every 2 weeks, according to Roche. The drug-treated group was compared with patients who received no prophylaxis. Another arm of the study examined patients who had previously received factor VIII prophylaxis and then switched to emicizumab-kxwh prophylaxis. In an intrapatient comparison, emicizumab-kxwh showed a 68% reduction in treated bleeds, which was statistically significant and demonstrated superior efficacy to factor VIII prophylaxis.
Emicizumab-kxwh was approved by FDA in November 2017 for routine prophylaxis for adults and children with hemophilia A with factor VIII inhibitors. That approval was based on results from the HAVEN 1 and HAVEN 2 studies.
The Food and Drug Administration has granted priority review to Roche’s emicizumab-kxwh (Hemlibra) for the treatment of adults and children with hemophilia A without factor VIII inhibitors.
The agency is scheduled to make a decision on approval in October 2018.
Among patients aged 12 years and older without factor VIII inhibitors, emicizumab-kxwh prophylaxis every week reduced treated bleeds by 96% (P less than .0001) and treated bleeds were reduced by 97% (P less than .0001) in patients who were treated every 2 weeks, according to Roche. The drug-treated group was compared with patients who received no prophylaxis. Another arm of the study examined patients who had previously received factor VIII prophylaxis and then switched to emicizumab-kxwh prophylaxis. In an intrapatient comparison, emicizumab-kxwh showed a 68% reduction in treated bleeds, which was statistically significant and demonstrated superior efficacy to factor VIII prophylaxis.
Emicizumab-kxwh was approved by FDA in November 2017 for routine prophylaxis for adults and children with hemophilia A with factor VIII inhibitors. That approval was based on results from the HAVEN 1 and HAVEN 2 studies.
The Food and Drug Administration has granted priority review to Roche’s emicizumab-kxwh (Hemlibra) for the treatment of adults and children with hemophilia A without factor VIII inhibitors.
The agency is scheduled to make a decision on approval in October 2018.
Among patients aged 12 years and older without factor VIII inhibitors, emicizumab-kxwh prophylaxis every week reduced treated bleeds by 96% (P less than .0001) and treated bleeds were reduced by 97% (P less than .0001) in patients who were treated every 2 weeks, according to Roche. The drug-treated group was compared with patients who received no prophylaxis. Another arm of the study examined patients who had previously received factor VIII prophylaxis and then switched to emicizumab-kxwh prophylaxis. In an intrapatient comparison, emicizumab-kxwh showed a 68% reduction in treated bleeds, which was statistically significant and demonstrated superior efficacy to factor VIII prophylaxis.
Emicizumab-kxwh was approved by FDA in November 2017 for routine prophylaxis for adults and children with hemophilia A with factor VIII inhibitors. That approval was based on results from the HAVEN 1 and HAVEN 2 studies.
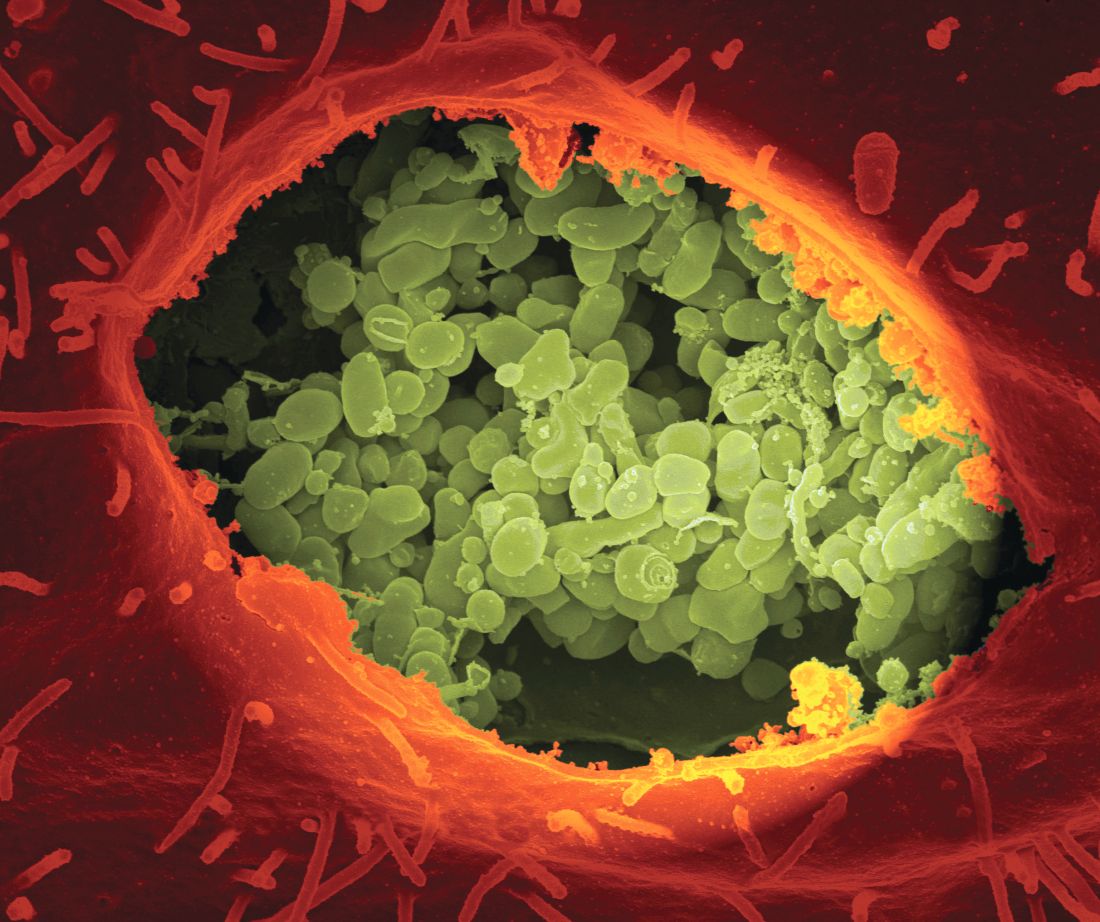
FDA grants priority review to first-line SAA treatment
The Food and Drug Administration has granted priority review to Novartis for their severe aplastic anemia drug.
in combination with standard immunosuppressive therapy (IST). The drug is already approved in the United States for treatment of refractory SAA patients. It is also approved for treatment of chronic immune thrombocytopenia in adults and children who are refractory to other treatments or patients with chronic hepatitis C virus. [[{"fid":"","view_mode":"","fields":{"format":"","field_file_image_alt_text[und][0][value]":"","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html"},"type":"media","attributes":{"class":"media-element file-"},"field_deltas":{"1":{"field_file_image_caption[und][0][format]":"filtered_html"}}}]]
The priority review status was granted based on preliminary findings showing that eltrombopag plus IST outperformed IST alone in treatment-naïve patients. The study showed that 52% of newly diagnosed patients achieved a complete response at 6 months with eltrombopag plus IST, which was 35% higher than patients treated with IST alone. The overall response rate was 85% at 6 months in the eltrombopag group, according to Novartis.
The drugmaker received a breakthrough therapy designation from the FDA for eltrombopag for first-line use in SAA in January 2018.
The Food and Drug Administration has granted priority review to Novartis for their severe aplastic anemia drug.
in combination with standard immunosuppressive therapy (IST). The drug is already approved in the United States for treatment of refractory SAA patients. It is also approved for treatment of chronic immune thrombocytopenia in adults and children who are refractory to other treatments or patients with chronic hepatitis C virus. [[{"fid":"","view_mode":"","fields":{"format":"","field_file_image_alt_text[und][0][value]":"","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html"},"type":"media","attributes":{"class":"media-element file-"},"field_deltas":{"1":{"field_file_image_caption[und][0][format]":"filtered_html"}}}]]
The priority review status was granted based on preliminary findings showing that eltrombopag plus IST outperformed IST alone in treatment-naïve patients. The study showed that 52% of newly diagnosed patients achieved a complete response at 6 months with eltrombopag plus IST, which was 35% higher than patients treated with IST alone. The overall response rate was 85% at 6 months in the eltrombopag group, according to Novartis.
The drugmaker received a breakthrough therapy designation from the FDA for eltrombopag for first-line use in SAA in January 2018.
The Food and Drug Administration has granted priority review to Novartis for their severe aplastic anemia drug.
in combination with standard immunosuppressive therapy (IST). The drug is already approved in the United States for treatment of refractory SAA patients. It is also approved for treatment of chronic immune thrombocytopenia in adults and children who are refractory to other treatments or patients with chronic hepatitis C virus. [[{"fid":"","view_mode":"","fields":{"format":"","field_file_image_alt_text[und][0][value]":"","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][value]":"","field_file_image_caption[und][0][format]":"filtered_html"},"type":"media","attributes":{"class":"media-element file-"},"field_deltas":{"1":{"field_file_image_caption[und][0][format]":"filtered_html"}}}]]
The priority review status was granted based on preliminary findings showing that eltrombopag plus IST outperformed IST alone in treatment-naïve patients. The study showed that 52% of newly diagnosed patients achieved a complete response at 6 months with eltrombopag plus IST, which was 35% higher than patients treated with IST alone. The overall response rate was 85% at 6 months in the eltrombopag group, according to Novartis.
The drugmaker received a breakthrough therapy designation from the FDA for eltrombopag for first-line use in SAA in January 2018.
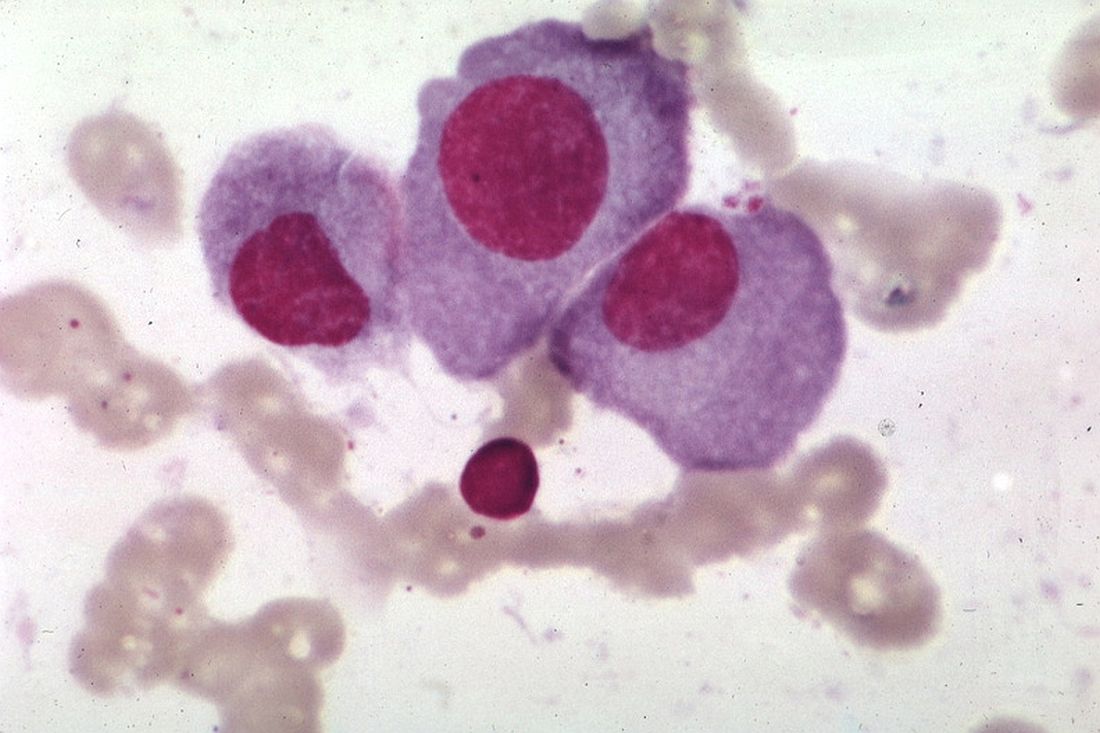
Overcoming TP53 mutation proves difficult in MCL
, new findings suggest.
“TP53 mutated MCL remains a major challenge, and our results underline the importance of molecular profiling, including TP53 status, in future trials exploring novel agents,” wrote Christian Winther Eskelund, MD, of Rigshospitalet in Copenhagen, and his colleagues. The findings were published in Haematologica.
The researchers noted that the results will need validation in a larger cohort of patients.
They performed an analysis of 50 MCL patients who enrolled in the Nordic MCL4 trial between 2009 and 2013. Patients were either over age 65 years or were younger but unfit for autologous stem cell transplantation. Despite the addition of lenalidomide to the chemoimmunotherapy regimen, patients with TP53 mutations had worse overall and progression-free survival and were significantly quicker to experience relapse.
After a median follow up of 47 months, median overall survival was 25 months for patients with TP53 mutations, compared with 69 months for those without (P less than .0001). Similarly, median progression-free survival was 10 months in patients with the mutation, compared with 42 months in patients without it (P = .001). Time to relapse was a median of 10 months in these mutated patients, compared with 58 months for unmutated MCL patients (P less than .0001).
TP53 mutations were identified in six patients (14%), one of whom withdrew consent at day 28 of the study. Of the remaining patients with mutations, all of them either progressed or relapsed during the study and none were alive at the most recent follow-up. During the study, patients received an induction phase of six cycles of lenalidomide plus bendamustine-rituximab (weeks 1-24), followed by a maintenance phase of lenalidomide (weeks 25-56).
SOURCE: Eskelund CW et al. Haematologica. 2018 May 24. doi: 10.3324/haematol.2018.194399.
, new findings suggest.
“TP53 mutated MCL remains a major challenge, and our results underline the importance of molecular profiling, including TP53 status, in future trials exploring novel agents,” wrote Christian Winther Eskelund, MD, of Rigshospitalet in Copenhagen, and his colleagues. The findings were published in Haematologica.
The researchers noted that the results will need validation in a larger cohort of patients.
They performed an analysis of 50 MCL patients who enrolled in the Nordic MCL4 trial between 2009 and 2013. Patients were either over age 65 years or were younger but unfit for autologous stem cell transplantation. Despite the addition of lenalidomide to the chemoimmunotherapy regimen, patients with TP53 mutations had worse overall and progression-free survival and were significantly quicker to experience relapse.
After a median follow up of 47 months, median overall survival was 25 months for patients with TP53 mutations, compared with 69 months for those without (P less than .0001). Similarly, median progression-free survival was 10 months in patients with the mutation, compared with 42 months in patients without it (P = .001). Time to relapse was a median of 10 months in these mutated patients, compared with 58 months for unmutated MCL patients (P less than .0001).
TP53 mutations were identified in six patients (14%), one of whom withdrew consent at day 28 of the study. Of the remaining patients with mutations, all of them either progressed or relapsed during the study and none were alive at the most recent follow-up. During the study, patients received an induction phase of six cycles of lenalidomide plus bendamustine-rituximab (weeks 1-24), followed by a maintenance phase of lenalidomide (weeks 25-56).
SOURCE: Eskelund CW et al. Haematologica. 2018 May 24. doi: 10.3324/haematol.2018.194399.
, new findings suggest.
“TP53 mutated MCL remains a major challenge, and our results underline the importance of molecular profiling, including TP53 status, in future trials exploring novel agents,” wrote Christian Winther Eskelund, MD, of Rigshospitalet in Copenhagen, and his colleagues. The findings were published in Haematologica.
The researchers noted that the results will need validation in a larger cohort of patients.
They performed an analysis of 50 MCL patients who enrolled in the Nordic MCL4 trial between 2009 and 2013. Patients were either over age 65 years or were younger but unfit for autologous stem cell transplantation. Despite the addition of lenalidomide to the chemoimmunotherapy regimen, patients with TP53 mutations had worse overall and progression-free survival and were significantly quicker to experience relapse.
After a median follow up of 47 months, median overall survival was 25 months for patients with TP53 mutations, compared with 69 months for those without (P less than .0001). Similarly, median progression-free survival was 10 months in patients with the mutation, compared with 42 months in patients without it (P = .001). Time to relapse was a median of 10 months in these mutated patients, compared with 58 months for unmutated MCL patients (P less than .0001).
TP53 mutations were identified in six patients (14%), one of whom withdrew consent at day 28 of the study. Of the remaining patients with mutations, all of them either progressed or relapsed during the study and none were alive at the most recent follow-up. During the study, patients received an induction phase of six cycles of lenalidomide plus bendamustine-rituximab (weeks 1-24), followed by a maintenance phase of lenalidomide (weeks 25-56).
SOURCE: Eskelund CW et al. Haematologica. 2018 May 24. doi: 10.3324/haematol.2018.194399.
FROM HAEMATOLOGICA
CAR T therapy to enter early testing in multiple myeloma
Janssen Biotech is launching a phase 1b/2 trial of an .
The trial, which was cleared by the Food and Drug Administration to begin in the second half of 2018, will evaluate the safety and efficacy of LCAR-B38M (JNJ-68284528). The CAR T therapy targets B-cell Maturation Antigen and expresses a CAR protein that is identical to a product that was developed by Legend Biotech and evaluated in a first-in-human clinical study in China.
The drug is being developed as part of a collaboration between Legend Biotech and Janssen Biotech.
Janssen Biotech is launching a phase 1b/2 trial of an .
The trial, which was cleared by the Food and Drug Administration to begin in the second half of 2018, will evaluate the safety and efficacy of LCAR-B38M (JNJ-68284528). The CAR T therapy targets B-cell Maturation Antigen and expresses a CAR protein that is identical to a product that was developed by Legend Biotech and evaluated in a first-in-human clinical study in China.
The drug is being developed as part of a collaboration between Legend Biotech and Janssen Biotech.
Janssen Biotech is launching a phase 1b/2 trial of an .
The trial, which was cleared by the Food and Drug Administration to begin in the second half of 2018, will evaluate the safety and efficacy of LCAR-B38M (JNJ-68284528). The CAR T therapy targets B-cell Maturation Antigen and expresses a CAR protein that is identical to a product that was developed by Legend Biotech and evaluated in a first-in-human clinical study in China.
The drug is being developed as part of a collaboration between Legend Biotech and Janssen Biotech.
FDA to review FLT3 agent for refractory AML
The Food and Drug Administration has granted priority review to an FMS-like tyrosine kinase 3 (FLT3)–targeting agent for the treatment of adults with relapsed or refractory acute myeloid leukemia (AML).
If approved, it would be the first FLT3 inhibitor available for this indication.
The application is based on the ongoing ADMIRAL trial, a phase 3, open-label, randomized study of gilteritinib versus salvage chemotherapy. The trial is designed to enroll 369 patients with FLT3 mutations present in bone marrow or whole blood who are refractory or have relapsed on first-line therapy. The primary endpoints are overall survival and rates of complete remission and complete remission with partial hematologic recovery.
The FDA has set Nov. 29 as a target date for reaching a decision on approval of the drug.
The Food and Drug Administration has granted priority review to an FMS-like tyrosine kinase 3 (FLT3)–targeting agent for the treatment of adults with relapsed or refractory acute myeloid leukemia (AML).
If approved, it would be the first FLT3 inhibitor available for this indication.
The application is based on the ongoing ADMIRAL trial, a phase 3, open-label, randomized study of gilteritinib versus salvage chemotherapy. The trial is designed to enroll 369 patients with FLT3 mutations present in bone marrow or whole blood who are refractory or have relapsed on first-line therapy. The primary endpoints are overall survival and rates of complete remission and complete remission with partial hematologic recovery.
The FDA has set Nov. 29 as a target date for reaching a decision on approval of the drug.
The Food and Drug Administration has granted priority review to an FMS-like tyrosine kinase 3 (FLT3)–targeting agent for the treatment of adults with relapsed or refractory acute myeloid leukemia (AML).
If approved, it would be the first FLT3 inhibitor available for this indication.
The application is based on the ongoing ADMIRAL trial, a phase 3, open-label, randomized study of gilteritinib versus salvage chemotherapy. The trial is designed to enroll 369 patients with FLT3 mutations present in bone marrow or whole blood who are refractory or have relapsed on first-line therapy. The primary endpoints are overall survival and rates of complete remission and complete remission with partial hematologic recovery.
The FDA has set Nov. 29 as a target date for reaching a decision on approval of the drug.
FDA approves epoetin alfa biosimilar to treat anemia
, a treatment for anemia brought on by chronic kidney disease, chemotherapy, or use of zidovudine.
The biosimilar product is also approved to reduce the chance of red blood cell transfusion before and after surgery.
FDA’s approval, issued on May 15, is based on review of structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other safety and effectiveness information showing that the epoetin alfa-epbx is biosimilar to the reference product epoetin alfa. By approving epoetin alfa-epbx as a biosimilar, the FDA is saying that there are “no clinically meaningful differences in safety, purity, and potency” from epoetin alfa.
The agency’s approval comes almost a year after the Oncologic Drugs Advisory Committee voted 14-1 to support approval of the biosimilar. The FDA had rejected the application in 2017, citing manufacturing issues at a facility in Kansas, before ultimately approving the product in 2018.
The biosimilar product must be dispensed with a patient Medication Guide with information about uses and risks and carries a boxed warning about an increased risk of death, heart problems, stroke, and tumor growth or recurrence.
The biosimilar product is marketed by Hospira Inc., a Pfizer company.
, a treatment for anemia brought on by chronic kidney disease, chemotherapy, or use of zidovudine.
The biosimilar product is also approved to reduce the chance of red blood cell transfusion before and after surgery.
FDA’s approval, issued on May 15, is based on review of structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other safety and effectiveness information showing that the epoetin alfa-epbx is biosimilar to the reference product epoetin alfa. By approving epoetin alfa-epbx as a biosimilar, the FDA is saying that there are “no clinically meaningful differences in safety, purity, and potency” from epoetin alfa.
The agency’s approval comes almost a year after the Oncologic Drugs Advisory Committee voted 14-1 to support approval of the biosimilar. The FDA had rejected the application in 2017, citing manufacturing issues at a facility in Kansas, before ultimately approving the product in 2018.
The biosimilar product must be dispensed with a patient Medication Guide with information about uses and risks and carries a boxed warning about an increased risk of death, heart problems, stroke, and tumor growth or recurrence.
The biosimilar product is marketed by Hospira Inc., a Pfizer company.
, a treatment for anemia brought on by chronic kidney disease, chemotherapy, or use of zidovudine.
The biosimilar product is also approved to reduce the chance of red blood cell transfusion before and after surgery.
FDA’s approval, issued on May 15, is based on review of structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other safety and effectiveness information showing that the epoetin alfa-epbx is biosimilar to the reference product epoetin alfa. By approving epoetin alfa-epbx as a biosimilar, the FDA is saying that there are “no clinically meaningful differences in safety, purity, and potency” from epoetin alfa.
The agency’s approval comes almost a year after the Oncologic Drugs Advisory Committee voted 14-1 to support approval of the biosimilar. The FDA had rejected the application in 2017, citing manufacturing issues at a facility in Kansas, before ultimately approving the product in 2018.
The biosimilar product must be dispensed with a patient Medication Guide with information about uses and risks and carries a boxed warning about an increased risk of death, heart problems, stroke, and tumor growth or recurrence.
The biosimilar product is marketed by Hospira Inc., a Pfizer company.
Study: No link between non-Hodgkin lymphoma and Q fever
Sonja E. van Roeden, MD, and colleagues from Utrecht University, the Netherlands, performed a retrospective, population-based analysis of the entire general population of the Netherlands from 2002 to 2013, encompassing the 3-year period of a large Q fever epidemic in the country.
In total, there were 48,760 cases of NHL diagnosed between Jan. 1, 2002 and Dec. 31, 2013, with the annual incidence ranging from 21.4 cases per 100,000 population in 2002 to 26.7 in 2010. While researchers found a significant association between NHL incidence and areas of high endemicity of Q fever in 2009 (relative risk 1.16; P = .029), there were no other associations.
Among the 439 people with chronic Q fever, 5 went on to develop NHL, resulting in a relative risk of 4.99 (P = .0003), compared with the general population of the Netherlands.
“The absence of an exposure-response relation between the intensity of exposure and risk of non-Hodgkin lymphoma, based on reported incidence of acute Q fever, does not imply a causal relation,” the researchers wrote. “However, one could consider exposure to C. burnetii in people with chronic Q fever as more intense than in acute Q fever because of the ongoing character and duration of exposure, and assume an exposure-response relation on the basis of the higher risk for non-Hodgkin lymphoma in patients with chronic Q fever.”
The researchers reported having no financial disclosures.
SOURCE: van Roeden SE et al. Lancet Haematol. 2018 May;5:e211-9.
Sonja E. van Roeden, MD, and colleagues from Utrecht University, the Netherlands, performed a retrospective, population-based analysis of the entire general population of the Netherlands from 2002 to 2013, encompassing the 3-year period of a large Q fever epidemic in the country.
In total, there were 48,760 cases of NHL diagnosed between Jan. 1, 2002 and Dec. 31, 2013, with the annual incidence ranging from 21.4 cases per 100,000 population in 2002 to 26.7 in 2010. While researchers found a significant association between NHL incidence and areas of high endemicity of Q fever in 2009 (relative risk 1.16; P = .029), there were no other associations.
Among the 439 people with chronic Q fever, 5 went on to develop NHL, resulting in a relative risk of 4.99 (P = .0003), compared with the general population of the Netherlands.
“The absence of an exposure-response relation between the intensity of exposure and risk of non-Hodgkin lymphoma, based on reported incidence of acute Q fever, does not imply a causal relation,” the researchers wrote. “However, one could consider exposure to C. burnetii in people with chronic Q fever as more intense than in acute Q fever because of the ongoing character and duration of exposure, and assume an exposure-response relation on the basis of the higher risk for non-Hodgkin lymphoma in patients with chronic Q fever.”
The researchers reported having no financial disclosures.
SOURCE: van Roeden SE et al. Lancet Haematol. 2018 May;5:e211-9.
Sonja E. van Roeden, MD, and colleagues from Utrecht University, the Netherlands, performed a retrospective, population-based analysis of the entire general population of the Netherlands from 2002 to 2013, encompassing the 3-year period of a large Q fever epidemic in the country.
In total, there were 48,760 cases of NHL diagnosed between Jan. 1, 2002 and Dec. 31, 2013, with the annual incidence ranging from 21.4 cases per 100,000 population in 2002 to 26.7 in 2010. While researchers found a significant association between NHL incidence and areas of high endemicity of Q fever in 2009 (relative risk 1.16; P = .029), there were no other associations.
Among the 439 people with chronic Q fever, 5 went on to develop NHL, resulting in a relative risk of 4.99 (P = .0003), compared with the general population of the Netherlands.
“The absence of an exposure-response relation between the intensity of exposure and risk of non-Hodgkin lymphoma, based on reported incidence of acute Q fever, does not imply a causal relation,” the researchers wrote. “However, one could consider exposure to C. burnetii in people with chronic Q fever as more intense than in acute Q fever because of the ongoing character and duration of exposure, and assume an exposure-response relation on the basis of the higher risk for non-Hodgkin lymphoma in patients with chronic Q fever.”
The researchers reported having no financial disclosures.
SOURCE: van Roeden SE et al. Lancet Haematol. 2018 May;5:e211-9.
FROM LANCET HAEMATOLOGY