User login
Pandemic-era telehealth led to fewer therapy disruptions
TOPLINE:
METHODOLOGY:
- Retrospective study using electronic health records and insurance claims data from three large U.S. health systems.
- Sample included 110,089 patients with mental health conditions who attended at least two psychotherapy visits during the 9 months before and 9 months after the onset of COVID-19, defined in this study as March 14, 2020.
- Outcome was disruption in psychotherapy, defined as a gap of more than 45 days between visits.
TAKEAWAY:
- Before the pandemic, 96.9% of psychotherapy visits were in person and 35.4% were followed by a gap of more than 45 days.
- After the onset of the pandemic, more than half of visits (51.8%) were virtual, and only 17.9% were followed by a gap of more than 45 days.
- Prior to the pandemic, the median time between visits was 27 days, and after the pandemic, it dropped to 14 days, suggesting individuals were more likely to return for additional psychotherapy after the widespread shift to virtual care.
- Over the entire study period, individuals with depressive, anxiety, or bipolar disorders were more likely to maintain consistent psychotherapy visits, whereas those with schizophrenia, ADHD, autism, conduct or disruptive disorders, dementia, or personality disorders were more likely to have a disruption in their visits.
IN PRACTICE:
“These findings support continued use of virtual psychotherapy as an option for care when appropriate infrastructure is in place. In addition, these findings support the continuation of policies that provide access to and coverage for virtual psychotherapy,” the authors write.
SOURCE:
The study, led by Brian K. Ahmedani, PhD, with the Center for Health Policy and Health Services Research, Henry Ford Health, Detroit, was published online in Psychiatric Services.
LIMITATIONS:
The study was conducted in three large health systems with virtual care infrastructure already in place. Researchers did not examine use of virtual care for medication management or for types of care other than psychotherapy, which may present different challenges.
DISCLOSURES:
The study was supported by the National Institute of Mental Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Retrospective study using electronic health records and insurance claims data from three large U.S. health systems.
- Sample included 110,089 patients with mental health conditions who attended at least two psychotherapy visits during the 9 months before and 9 months after the onset of COVID-19, defined in this study as March 14, 2020.
- Outcome was disruption in psychotherapy, defined as a gap of more than 45 days between visits.
TAKEAWAY:
- Before the pandemic, 96.9% of psychotherapy visits were in person and 35.4% were followed by a gap of more than 45 days.
- After the onset of the pandemic, more than half of visits (51.8%) were virtual, and only 17.9% were followed by a gap of more than 45 days.
- Prior to the pandemic, the median time between visits was 27 days, and after the pandemic, it dropped to 14 days, suggesting individuals were more likely to return for additional psychotherapy after the widespread shift to virtual care.
- Over the entire study period, individuals with depressive, anxiety, or bipolar disorders were more likely to maintain consistent psychotherapy visits, whereas those with schizophrenia, ADHD, autism, conduct or disruptive disorders, dementia, or personality disorders were more likely to have a disruption in their visits.
IN PRACTICE:
“These findings support continued use of virtual psychotherapy as an option for care when appropriate infrastructure is in place. In addition, these findings support the continuation of policies that provide access to and coverage for virtual psychotherapy,” the authors write.
SOURCE:
The study, led by Brian K. Ahmedani, PhD, with the Center for Health Policy and Health Services Research, Henry Ford Health, Detroit, was published online in Psychiatric Services.
LIMITATIONS:
The study was conducted in three large health systems with virtual care infrastructure already in place. Researchers did not examine use of virtual care for medication management or for types of care other than psychotherapy, which may present different challenges.
DISCLOSURES:
The study was supported by the National Institute of Mental Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Retrospective study using electronic health records and insurance claims data from three large U.S. health systems.
- Sample included 110,089 patients with mental health conditions who attended at least two psychotherapy visits during the 9 months before and 9 months after the onset of COVID-19, defined in this study as March 14, 2020.
- Outcome was disruption in psychotherapy, defined as a gap of more than 45 days between visits.
TAKEAWAY:
- Before the pandemic, 96.9% of psychotherapy visits were in person and 35.4% were followed by a gap of more than 45 days.
- After the onset of the pandemic, more than half of visits (51.8%) were virtual, and only 17.9% were followed by a gap of more than 45 days.
- Prior to the pandemic, the median time between visits was 27 days, and after the pandemic, it dropped to 14 days, suggesting individuals were more likely to return for additional psychotherapy after the widespread shift to virtual care.
- Over the entire study period, individuals with depressive, anxiety, or bipolar disorders were more likely to maintain consistent psychotherapy visits, whereas those with schizophrenia, ADHD, autism, conduct or disruptive disorders, dementia, or personality disorders were more likely to have a disruption in their visits.
IN PRACTICE:
“These findings support continued use of virtual psychotherapy as an option for care when appropriate infrastructure is in place. In addition, these findings support the continuation of policies that provide access to and coverage for virtual psychotherapy,” the authors write.
SOURCE:
The study, led by Brian K. Ahmedani, PhD, with the Center for Health Policy and Health Services Research, Henry Ford Health, Detroit, was published online in Psychiatric Services.
LIMITATIONS:
The study was conducted in three large health systems with virtual care infrastructure already in place. Researchers did not examine use of virtual care for medication management or for types of care other than psychotherapy, which may present different challenges.
DISCLOSURES:
The study was supported by the National Institute of Mental Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM PSYCHIATRIC SERVICES
The sobering facts about alcohol and cancer
There is an urgent need to raise global awareness about the direct link between alcohol consumption and cancer risk.
That message was delivered by Isabelle Soerjomataram, PhD, with the International Agency for Research on Cancer (IARC), Lyon, France, at a session devoted to alcohol and cancer at the annual meeting of the European Society for Medical Oncology.
Dr. Soerjomataram told the audience. “Health professionals – oncologists, nurses, medical doctors, GPs – have an important role in increasing awareness and bringing this knowledge to people, which may lead to reduced consumption.”
Session chair Gilberto Morgan, MD, medical oncologist, Skåne University Hospital, Lund, Sweden, agreed.
Dr. Morgan noted that healthcare professionals tend to downplay their influence over patients’ drinking habits and often don’t address these behaviors.
But that needs to change.
“We have absolutely no problem asking patients if they take supplements or vitamins or if they’re eating [healthy],” Dr. Morgan said. “So, what is the difference? Why not recommend that they cut down their alcohol intake and leave it up to everybody’s personal choice to do it or not?”
In the session, Dr. Soerjomataram highlighted the global statistics on alcohol use. IARC data show, for instance, that nearly half (46%) of the world’s population consumes alcohol, with rates higher in men (54%) than women (38%).
How much are people drinking?
Globally, on average, the amount comes to about six liters of pure ethanol per year per drinker, or about one wine bottle per week. However, consumption patterns vary widely by country. In France, people consume about 12 liters per year or about two wine bottles per week.
Dr. Soerjomataram stressed the link between alcohol consumption and cancer.
According to IARC data, heavy drinking – defined as more than 60 g/day or about six daily drinks – accounts for 47% of the alcohol-attributable cancers. Risky drinking – between 20 and 60 g/day – accounts for 29%, she explained, while moderate drinking – less than 20 g/day or about two daily drinks – accounts for roughly 14% of cases of alcohol-attributable cancers.
Globally, alcohol intake accounted for 4% of all cancers diagnosed in 2020, according to a 2021 analysis by IARC.
In the United Kingdom alone, “alcohol drinking caused nearly 17,000 cases of cancer in 2020,” Dr. Soerjomataram said, and breast cancer made up almost one in four of those new cases.
In addition to breast cancer, six other cancer types – oral cavity, pharyngeal, laryngeal, esophageal, colorectal, and liver cancer – can be attributed to alcohol consumption, and emerging evidence suggests stomach and pancreatic cancer may be as well.
The good news, said Dr. Soerjomataram, is that long-term trends show declines in alcohol drinking in many countries, including the high wine-producing countries of France and Italy, where large reductions in consumption have been noted since the peak of intake in the 1920s.
“If it’s possible in these countries, I can imagine it’s possible elsewhere,” said Dr. Soerjomataram.
Dr. Soerjomataram and Dr. Morgan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is an urgent need to raise global awareness about the direct link between alcohol consumption and cancer risk.
That message was delivered by Isabelle Soerjomataram, PhD, with the International Agency for Research on Cancer (IARC), Lyon, France, at a session devoted to alcohol and cancer at the annual meeting of the European Society for Medical Oncology.
Dr. Soerjomataram told the audience. “Health professionals – oncologists, nurses, medical doctors, GPs – have an important role in increasing awareness and bringing this knowledge to people, which may lead to reduced consumption.”
Session chair Gilberto Morgan, MD, medical oncologist, Skåne University Hospital, Lund, Sweden, agreed.
Dr. Morgan noted that healthcare professionals tend to downplay their influence over patients’ drinking habits and often don’t address these behaviors.
But that needs to change.
“We have absolutely no problem asking patients if they take supplements or vitamins or if they’re eating [healthy],” Dr. Morgan said. “So, what is the difference? Why not recommend that they cut down their alcohol intake and leave it up to everybody’s personal choice to do it or not?”
In the session, Dr. Soerjomataram highlighted the global statistics on alcohol use. IARC data show, for instance, that nearly half (46%) of the world’s population consumes alcohol, with rates higher in men (54%) than women (38%).
How much are people drinking?
Globally, on average, the amount comes to about six liters of pure ethanol per year per drinker, or about one wine bottle per week. However, consumption patterns vary widely by country. In France, people consume about 12 liters per year or about two wine bottles per week.
Dr. Soerjomataram stressed the link between alcohol consumption and cancer.
According to IARC data, heavy drinking – defined as more than 60 g/day or about six daily drinks – accounts for 47% of the alcohol-attributable cancers. Risky drinking – between 20 and 60 g/day – accounts for 29%, she explained, while moderate drinking – less than 20 g/day or about two daily drinks – accounts for roughly 14% of cases of alcohol-attributable cancers.
Globally, alcohol intake accounted for 4% of all cancers diagnosed in 2020, according to a 2021 analysis by IARC.
In the United Kingdom alone, “alcohol drinking caused nearly 17,000 cases of cancer in 2020,” Dr. Soerjomataram said, and breast cancer made up almost one in four of those new cases.
In addition to breast cancer, six other cancer types – oral cavity, pharyngeal, laryngeal, esophageal, colorectal, and liver cancer – can be attributed to alcohol consumption, and emerging evidence suggests stomach and pancreatic cancer may be as well.
The good news, said Dr. Soerjomataram, is that long-term trends show declines in alcohol drinking in many countries, including the high wine-producing countries of France and Italy, where large reductions in consumption have been noted since the peak of intake in the 1920s.
“If it’s possible in these countries, I can imagine it’s possible elsewhere,” said Dr. Soerjomataram.
Dr. Soerjomataram and Dr. Morgan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is an urgent need to raise global awareness about the direct link between alcohol consumption and cancer risk.
That message was delivered by Isabelle Soerjomataram, PhD, with the International Agency for Research on Cancer (IARC), Lyon, France, at a session devoted to alcohol and cancer at the annual meeting of the European Society for Medical Oncology.
Dr. Soerjomataram told the audience. “Health professionals – oncologists, nurses, medical doctors, GPs – have an important role in increasing awareness and bringing this knowledge to people, which may lead to reduced consumption.”
Session chair Gilberto Morgan, MD, medical oncologist, Skåne University Hospital, Lund, Sweden, agreed.
Dr. Morgan noted that healthcare professionals tend to downplay their influence over patients’ drinking habits and often don’t address these behaviors.
But that needs to change.
“We have absolutely no problem asking patients if they take supplements or vitamins or if they’re eating [healthy],” Dr. Morgan said. “So, what is the difference? Why not recommend that they cut down their alcohol intake and leave it up to everybody’s personal choice to do it or not?”
In the session, Dr. Soerjomataram highlighted the global statistics on alcohol use. IARC data show, for instance, that nearly half (46%) of the world’s population consumes alcohol, with rates higher in men (54%) than women (38%).
How much are people drinking?
Globally, on average, the amount comes to about six liters of pure ethanol per year per drinker, or about one wine bottle per week. However, consumption patterns vary widely by country. In France, people consume about 12 liters per year or about two wine bottles per week.
Dr. Soerjomataram stressed the link between alcohol consumption and cancer.
According to IARC data, heavy drinking – defined as more than 60 g/day or about six daily drinks – accounts for 47% of the alcohol-attributable cancers. Risky drinking – between 20 and 60 g/day – accounts for 29%, she explained, while moderate drinking – less than 20 g/day or about two daily drinks – accounts for roughly 14% of cases of alcohol-attributable cancers.
Globally, alcohol intake accounted for 4% of all cancers diagnosed in 2020, according to a 2021 analysis by IARC.
In the United Kingdom alone, “alcohol drinking caused nearly 17,000 cases of cancer in 2020,” Dr. Soerjomataram said, and breast cancer made up almost one in four of those new cases.
In addition to breast cancer, six other cancer types – oral cavity, pharyngeal, laryngeal, esophageal, colorectal, and liver cancer – can be attributed to alcohol consumption, and emerging evidence suggests stomach and pancreatic cancer may be as well.
The good news, said Dr. Soerjomataram, is that long-term trends show declines in alcohol drinking in many countries, including the high wine-producing countries of France and Italy, where large reductions in consumption have been noted since the peak of intake in the 1920s.
“If it’s possible in these countries, I can imagine it’s possible elsewhere,” said Dr. Soerjomataram.
Dr. Soerjomataram and Dr. Morgan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESMO 2023
Hitting the snooze button may provide cognitive benefit
TOPLINE:
Challenging conventional wisdom,
METHODOLOGY:
- Researchers did two studies to determine why intermittent morning alarms are used and how they affect sleep, cognition, cortisol, and mood.
- Study 1 was a survey of 1,732 healthy adults (mean age 34 years; 66% women) designed to elucidate the characteristics of people who snooze and why they choose to delay their waking in this way.
- Study 2 was a within-subject polysomnography study of 31 healthy habitual snoozers (mean age 27 years; 18 women) designed to explore the acute effects of snoozing on sleep architecture, sleepiness, cognitive ability, mood, and cortisol awakening response.
TAKEAWAY:
- Overall, 69% reported using the snooze button or setting multiple alarms at least sometimes, most often on workdays (71%), with an average snooze time per morning of 22 minutes.
- Sleep quality did not differ between snoozers and nonsnoozers, but snoozers were more likely to feel mentally drowsy on waking (odds ratio, 3.0; P < .001) and had slightly shorter sleep time on workdays (13 minutes).
- In the polysomnography study, compared with waking up abruptly, 30 minutes of snoozing in the morning improved or did not affect performance on standard cognitive tests completed directly on final awakening.
- Snoozing resulted in about 6 minutes of lost sleep, but it prevented awakening from slow-wave sleep and had no clear effects on the cortisol awakening response, morning sleepiness, mood, or overnight sleep architecture.
IN PRACTICE:
“The findings indicate that there is no reason to stop snoozing in the morning if you enjoy it, at least not for snooze times around 30 minutes. In fact, it may even help those with morning drowsiness to be slightly more awake once they get up,” corresponding author Tina Sundelin, PhD, of Stockholm University, said in a statement.
SOURCE:
The study was published online in the Journal of Sleep Research.
LIMITATIONS:
Study 1 focused on waking preferences in a convenience sample of adults. Study 2 included only habitual snoozers making it difficult to generalize the findings to people who don’t usually snooze. The study investigated only the effect of 30 minutes of snoozing on the studied parameters. It’s possible that shorter or longer snooze times have different cognitive effects.
DISCLOSURES:
Support for the study was provided by the Stress Research Institute, Stockholm University, and a grant from Vetenskapsrådet. The authors disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Challenging conventional wisdom,
METHODOLOGY:
- Researchers did two studies to determine why intermittent morning alarms are used and how they affect sleep, cognition, cortisol, and mood.
- Study 1 was a survey of 1,732 healthy adults (mean age 34 years; 66% women) designed to elucidate the characteristics of people who snooze and why they choose to delay their waking in this way.
- Study 2 was a within-subject polysomnography study of 31 healthy habitual snoozers (mean age 27 years; 18 women) designed to explore the acute effects of snoozing on sleep architecture, sleepiness, cognitive ability, mood, and cortisol awakening response.
TAKEAWAY:
- Overall, 69% reported using the snooze button or setting multiple alarms at least sometimes, most often on workdays (71%), with an average snooze time per morning of 22 minutes.
- Sleep quality did not differ between snoozers and nonsnoozers, but snoozers were more likely to feel mentally drowsy on waking (odds ratio, 3.0; P < .001) and had slightly shorter sleep time on workdays (13 minutes).
- In the polysomnography study, compared with waking up abruptly, 30 minutes of snoozing in the morning improved or did not affect performance on standard cognitive tests completed directly on final awakening.
- Snoozing resulted in about 6 minutes of lost sleep, but it prevented awakening from slow-wave sleep and had no clear effects on the cortisol awakening response, morning sleepiness, mood, or overnight sleep architecture.
IN PRACTICE:
“The findings indicate that there is no reason to stop snoozing in the morning if you enjoy it, at least not for snooze times around 30 minutes. In fact, it may even help those with morning drowsiness to be slightly more awake once they get up,” corresponding author Tina Sundelin, PhD, of Stockholm University, said in a statement.
SOURCE:
The study was published online in the Journal of Sleep Research.
LIMITATIONS:
Study 1 focused on waking preferences in a convenience sample of adults. Study 2 included only habitual snoozers making it difficult to generalize the findings to people who don’t usually snooze. The study investigated only the effect of 30 minutes of snoozing on the studied parameters. It’s possible that shorter or longer snooze times have different cognitive effects.
DISCLOSURES:
Support for the study was provided by the Stress Research Institute, Stockholm University, and a grant from Vetenskapsrådet. The authors disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Challenging conventional wisdom,
METHODOLOGY:
- Researchers did two studies to determine why intermittent morning alarms are used and how they affect sleep, cognition, cortisol, and mood.
- Study 1 was a survey of 1,732 healthy adults (mean age 34 years; 66% women) designed to elucidate the characteristics of people who snooze and why they choose to delay their waking in this way.
- Study 2 was a within-subject polysomnography study of 31 healthy habitual snoozers (mean age 27 years; 18 women) designed to explore the acute effects of snoozing on sleep architecture, sleepiness, cognitive ability, mood, and cortisol awakening response.
TAKEAWAY:
- Overall, 69% reported using the snooze button or setting multiple alarms at least sometimes, most often on workdays (71%), with an average snooze time per morning of 22 minutes.
- Sleep quality did not differ between snoozers and nonsnoozers, but snoozers were more likely to feel mentally drowsy on waking (odds ratio, 3.0; P < .001) and had slightly shorter sleep time on workdays (13 minutes).
- In the polysomnography study, compared with waking up abruptly, 30 minutes of snoozing in the morning improved or did not affect performance on standard cognitive tests completed directly on final awakening.
- Snoozing resulted in about 6 minutes of lost sleep, but it prevented awakening from slow-wave sleep and had no clear effects on the cortisol awakening response, morning sleepiness, mood, or overnight sleep architecture.
IN PRACTICE:
“The findings indicate that there is no reason to stop snoozing in the morning if you enjoy it, at least not for snooze times around 30 minutes. In fact, it may even help those with morning drowsiness to be slightly more awake once they get up,” corresponding author Tina Sundelin, PhD, of Stockholm University, said in a statement.
SOURCE:
The study was published online in the Journal of Sleep Research.
LIMITATIONS:
Study 1 focused on waking preferences in a convenience sample of adults. Study 2 included only habitual snoozers making it difficult to generalize the findings to people who don’t usually snooze. The study investigated only the effect of 30 minutes of snoozing on the studied parameters. It’s possible that shorter or longer snooze times have different cognitive effects.
DISCLOSURES:
Support for the study was provided by the Stress Research Institute, Stockholm University, and a grant from Vetenskapsrådet. The authors disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
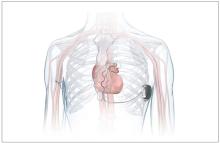
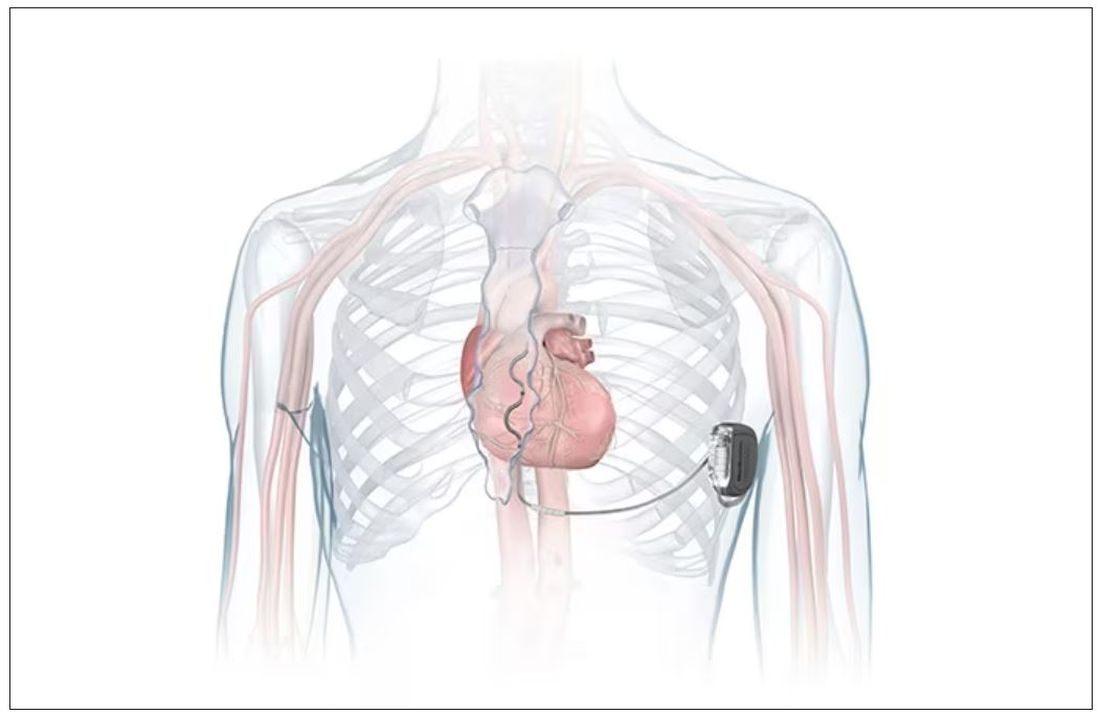
FDA okays first extravascular ICD system
which uses a single lead implanted substernally to allow antitachycardia pacing and low-energy defibrillation while avoiding the vascular space for lead placement.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, said in a company news release.
“Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology,” said Dr. Knight, who was involved in the pivotal trial that led to U.S. approval.
The approval, which includes the system’s proprietary procedure implant tools, was supported by results from a global pivotal study that demonstrated the safety and effectiveness of the system.
Results of the study were presented at the annual meeting of the European Society of Cardiology in 2022.
The study enrolled 356 patients who were at risk of sudden cardiac death and who had a class I or IIa indication for ICD. Participants were enrolled at 46 sites in 17 countries.
The device’s effectiveness in delivering defibrillation therapy at implant (primary efficacy endpoint) was 98.7%, compared with a prespecified target of 88%.
There were no major intraprocedural complications, nor were any unique complications observed that were related to the EV ICD procedure or system, compared with transvenous and subcutaneous ICDs.
Additionally, 33 defibrillation shocks were avoided by having antitachycardia pacing programmed “on.”
At 6 months, 92.6% of patients (Kaplan-Meier estimate) were free from major system- and/or procedure-related major complications, such as hospitalization, system revision, or death.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, who have not previously undergone sternotomy, and who do not need long-term bradycardia pacing.
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional transvenous ICDs.
Medtronic said the Aurora EV-ICD system will be commercially available on a limited basis in the United States in the coming weeks.
A version of this article first appeared on Medscape.com.
which uses a single lead implanted substernally to allow antitachycardia pacing and low-energy defibrillation while avoiding the vascular space for lead placement.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, said in a company news release.
“Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology,” said Dr. Knight, who was involved in the pivotal trial that led to U.S. approval.
The approval, which includes the system’s proprietary procedure implant tools, was supported by results from a global pivotal study that demonstrated the safety and effectiveness of the system.
Results of the study were presented at the annual meeting of the European Society of Cardiology in 2022.
The study enrolled 356 patients who were at risk of sudden cardiac death and who had a class I or IIa indication for ICD. Participants were enrolled at 46 sites in 17 countries.
The device’s effectiveness in delivering defibrillation therapy at implant (primary efficacy endpoint) was 98.7%, compared with a prespecified target of 88%.
There were no major intraprocedural complications, nor were any unique complications observed that were related to the EV ICD procedure or system, compared with transvenous and subcutaneous ICDs.
Additionally, 33 defibrillation shocks were avoided by having antitachycardia pacing programmed “on.”
At 6 months, 92.6% of patients (Kaplan-Meier estimate) were free from major system- and/or procedure-related major complications, such as hospitalization, system revision, or death.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, who have not previously undergone sternotomy, and who do not need long-term bradycardia pacing.
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional transvenous ICDs.
Medtronic said the Aurora EV-ICD system will be commercially available on a limited basis in the United States in the coming weeks.
A version of this article first appeared on Medscape.com.
which uses a single lead implanted substernally to allow antitachycardia pacing and low-energy defibrillation while avoiding the vascular space for lead placement.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, said in a company news release.
“Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology,” said Dr. Knight, who was involved in the pivotal trial that led to U.S. approval.
The approval, which includes the system’s proprietary procedure implant tools, was supported by results from a global pivotal study that demonstrated the safety and effectiveness of the system.
Results of the study were presented at the annual meeting of the European Society of Cardiology in 2022.
The study enrolled 356 patients who were at risk of sudden cardiac death and who had a class I or IIa indication for ICD. Participants were enrolled at 46 sites in 17 countries.
The device’s effectiveness in delivering defibrillation therapy at implant (primary efficacy endpoint) was 98.7%, compared with a prespecified target of 88%.
There were no major intraprocedural complications, nor were any unique complications observed that were related to the EV ICD procedure or system, compared with transvenous and subcutaneous ICDs.
Additionally, 33 defibrillation shocks were avoided by having antitachycardia pacing programmed “on.”
At 6 months, 92.6% of patients (Kaplan-Meier estimate) were free from major system- and/or procedure-related major complications, such as hospitalization, system revision, or death.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, who have not previously undergone sternotomy, and who do not need long-term bradycardia pacing.
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional transvenous ICDs.
Medtronic said the Aurora EV-ICD system will be commercially available on a limited basis in the United States in the coming weeks.
A version of this article first appeared on Medscape.com.
Study reveals potentially unnecessary CRC screening in older adults
TOPLINE:
Older adults with limited life expectancy are just as likely to undergo colorectal cancer (CRC) screening as those with longer life expectancy, a new study shows.
METHODOLOGY:
- Researchers used national survey data to estimate the prevalence and factors associated with CRC screening in 25,888 community-dwelling adults aged 65-84 according to their predicted 10-year mortality risk.
- They estimated 10-year mortality risk using a validated index. From the lowest to highest quintiles, mortality risk was 12%, 24%, 39%, 58%, and 79%, respectively.
- Investigators determined the proportion of screening performed in adults with life expectancy less than 10 years, defined as 10-year mortality risk ≥ 50% (that is, quintiles 4 and 5).
TAKEAWAY:
- In this cohort of older adults previously not up to date with CRC screening, the overall prevalence of past-year screening was 38.5%.
- The prevalence of past-year CRC screening decreased with advancing age but did not differ significantly by 10-year mortality risk. From lowest to highest quintile, prevalence was 39.5%, 40.6%, 38.7%, 36.4%, and 35.4%, respectively.
- The likelihood of CRC screening did not differ between adults in the lowest vs. highest quintile of 10-year mortality risk (adjusted odds ratio, 1.05).
- More than one-quarter (27.9%) of past-year screening occurred in adults with life expectancy less than 10 years, and 50.7% of adults aged 75-84 years had life expectancy less than 10 years at the time of screening.
- Paradoxically, the prevalence of invasive screening increased with lowered life expectancy among adults aged 70-79 years.
IN PRACTICE:
“Our results suggest that health status and life expectancy may be overlooked in current CRC screening programs, and personalized screening incorporating individual life expectancy may improve the value of screening,” the authors write.
SOURCE:
The study, with first author Po-Hong Liu, MD, MPH, division of digestive and liver diseases, University of Texas Southwestern Medical Center, Dallas, was published online in the American Journal of Gastroenterology.
LIMITATIONS:
The survey data were self-reported and were not validated by medical records. The data did not include information to identify individuals who were at higher risk for CRC or to trace prior CRC screening history. It was not possible to reliably classify test indication (screening vs. surveillance vs. diagnostic).
DISCLOSURES:
The study was supported by the National Institutes of Health. One author disclosed serving as a consultant or on advisory boards for Exact Sciences, Universal Dx, Roche, and Freenome. Another disclosed consulting for Freenome.
A version of this article first appeared on Medscape.com.
TOPLINE:
Older adults with limited life expectancy are just as likely to undergo colorectal cancer (CRC) screening as those with longer life expectancy, a new study shows.
METHODOLOGY:
- Researchers used national survey data to estimate the prevalence and factors associated with CRC screening in 25,888 community-dwelling adults aged 65-84 according to their predicted 10-year mortality risk.
- They estimated 10-year mortality risk using a validated index. From the lowest to highest quintiles, mortality risk was 12%, 24%, 39%, 58%, and 79%, respectively.
- Investigators determined the proportion of screening performed in adults with life expectancy less than 10 years, defined as 10-year mortality risk ≥ 50% (that is, quintiles 4 and 5).
TAKEAWAY:
- In this cohort of older adults previously not up to date with CRC screening, the overall prevalence of past-year screening was 38.5%.
- The prevalence of past-year CRC screening decreased with advancing age but did not differ significantly by 10-year mortality risk. From lowest to highest quintile, prevalence was 39.5%, 40.6%, 38.7%, 36.4%, and 35.4%, respectively.
- The likelihood of CRC screening did not differ between adults in the lowest vs. highest quintile of 10-year mortality risk (adjusted odds ratio, 1.05).
- More than one-quarter (27.9%) of past-year screening occurred in adults with life expectancy less than 10 years, and 50.7% of adults aged 75-84 years had life expectancy less than 10 years at the time of screening.
- Paradoxically, the prevalence of invasive screening increased with lowered life expectancy among adults aged 70-79 years.
IN PRACTICE:
“Our results suggest that health status and life expectancy may be overlooked in current CRC screening programs, and personalized screening incorporating individual life expectancy may improve the value of screening,” the authors write.
SOURCE:
The study, with first author Po-Hong Liu, MD, MPH, division of digestive and liver diseases, University of Texas Southwestern Medical Center, Dallas, was published online in the American Journal of Gastroenterology.
LIMITATIONS:
The survey data were self-reported and were not validated by medical records. The data did not include information to identify individuals who were at higher risk for CRC or to trace prior CRC screening history. It was not possible to reliably classify test indication (screening vs. surveillance vs. diagnostic).
DISCLOSURES:
The study was supported by the National Institutes of Health. One author disclosed serving as a consultant or on advisory boards for Exact Sciences, Universal Dx, Roche, and Freenome. Another disclosed consulting for Freenome.
A version of this article first appeared on Medscape.com.
TOPLINE:
Older adults with limited life expectancy are just as likely to undergo colorectal cancer (CRC) screening as those with longer life expectancy, a new study shows.
METHODOLOGY:
- Researchers used national survey data to estimate the prevalence and factors associated with CRC screening in 25,888 community-dwelling adults aged 65-84 according to their predicted 10-year mortality risk.
- They estimated 10-year mortality risk using a validated index. From the lowest to highest quintiles, mortality risk was 12%, 24%, 39%, 58%, and 79%, respectively.
- Investigators determined the proportion of screening performed in adults with life expectancy less than 10 years, defined as 10-year mortality risk ≥ 50% (that is, quintiles 4 and 5).
TAKEAWAY:
- In this cohort of older adults previously not up to date with CRC screening, the overall prevalence of past-year screening was 38.5%.
- The prevalence of past-year CRC screening decreased with advancing age but did not differ significantly by 10-year mortality risk. From lowest to highest quintile, prevalence was 39.5%, 40.6%, 38.7%, 36.4%, and 35.4%, respectively.
- The likelihood of CRC screening did not differ between adults in the lowest vs. highest quintile of 10-year mortality risk (adjusted odds ratio, 1.05).
- More than one-quarter (27.9%) of past-year screening occurred in adults with life expectancy less than 10 years, and 50.7% of adults aged 75-84 years had life expectancy less than 10 years at the time of screening.
- Paradoxically, the prevalence of invasive screening increased with lowered life expectancy among adults aged 70-79 years.
IN PRACTICE:
“Our results suggest that health status and life expectancy may be overlooked in current CRC screening programs, and personalized screening incorporating individual life expectancy may improve the value of screening,” the authors write.
SOURCE:
The study, with first author Po-Hong Liu, MD, MPH, division of digestive and liver diseases, University of Texas Southwestern Medical Center, Dallas, was published online in the American Journal of Gastroenterology.
LIMITATIONS:
The survey data were self-reported and were not validated by medical records. The data did not include information to identify individuals who were at higher risk for CRC or to trace prior CRC screening history. It was not possible to reliably classify test indication (screening vs. surveillance vs. diagnostic).
DISCLOSURES:
The study was supported by the National Institutes of Health. One author disclosed serving as a consultant or on advisory boards for Exact Sciences, Universal Dx, Roche, and Freenome. Another disclosed consulting for Freenome.
A version of this article first appeared on Medscape.com.
CMS ‘million hearts’ CVD risk reduction model works
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Durable LVAD for advanced HF still underutilized
The prognosis for patients with advanced heart failure (HF) who fail guideline-directed medical therapy is poor, but
Those are the key takeaways from a scientific statement on durable mechanical circulatory support, published online in the Journal of the American College of Cardiology.
“I think it is important to highlight this issue because of the sheer impact that heart failure has on American citizens,” corresponding author Jennifer Cowger, MD, MS, advanced heart failure specialist, Henry Ford Health, Detroit, said in an interview.
“End-stage heart failure has no medication that has shown a gain in survival, and most are dead by 1 year,” she said.
This scientific statement highlights the “amazing evolution of LVAD support and associated improvement in outcomes,” Dr. Cowger said.
Yet because LVADs are only implanted at roughly 170 U.S. centers, “many cardiologists are not aware of the amazing survival improvement with modern LVAD technology, and patients are under-referred,” Dr. Cowger noted.
Contemporary outcomes on par with heart transplant
The authors note that survival with durable LVAD (dLVAD) has markedly improved over the years. Current survival is approximately 87% at 1 year for patients supported with a contemporary LVAD.
Average patient survival is now similar to that of heart transplantation at 2 years, with 5-year dLVAD survival now approaching 60%, they point out.
Contemporary dLVAD yields significant and sustained improvements in functional capacity. Data show that roughly 80% of patients improve to NYHA functional class I and II, with significant improvements in 6-minute walk distances and health-related quality of life, the authors note.
In addition, innovations in dLVAD technology have reduced the risk of several adverse events, including pump thrombosis, stroke, and bleeding.
“Novel devices are on the horizon of clinical investigation, offering smaller size, permitting less invasive surgical implantation, and eliminating the percutaneous lead for power supply,” the authors note.
“Unfortunately, greater adoption of dLVAD therapy has not been realized due to delayed referral of patients to advanced HF centers, insufficient clinician knowledge of contemporary dLVAD outcomes (including gains in quality of life), and deprioritization of patients with dLVAD support waiting for heart transplantation,” they write.
In addition to highlighting contemporary outcomes with dLVAD support, the 18-page statement also includes sections on:
- Current indications and timing of referral
- Surgical considerations (device selection, surgical techniques and approach to concomitant valvular disease, and management of acute right ventricular dysfunction)
- Unique patient populations (women, children, and adult congenital heart disease)
- Summary, gaps, and future directions
A recent workshop held by the National Heart, Lung, and Blood Institute (NHLBI) identified critical gaps in the field of advanced HF.
One of the major gaps identified was the need to improve mechanical circulatory support use as a “complement or alternative” therapy to heart transplantation. The workshop also emphasized the need to “synergize” LVAD and heart transplant in the same patient to maximize health-related quality of life and survival benefit.
The NHLBI workshop also highlighted the need to model how different patient subset characteristics may affect mechanical circulatory support outcomes to inform bridge-to-transplantation or bridge-to-decision/candidacy opportunities more appropriately.
This research had no commercial funding. A number of study authors disclosed relationships with industry. The full list is available with the original article.
A version of this article first appeared on Medscape.com.
The prognosis for patients with advanced heart failure (HF) who fail guideline-directed medical therapy is poor, but
Those are the key takeaways from a scientific statement on durable mechanical circulatory support, published online in the Journal of the American College of Cardiology.
“I think it is important to highlight this issue because of the sheer impact that heart failure has on American citizens,” corresponding author Jennifer Cowger, MD, MS, advanced heart failure specialist, Henry Ford Health, Detroit, said in an interview.
“End-stage heart failure has no medication that has shown a gain in survival, and most are dead by 1 year,” she said.
This scientific statement highlights the “amazing evolution of LVAD support and associated improvement in outcomes,” Dr. Cowger said.
Yet because LVADs are only implanted at roughly 170 U.S. centers, “many cardiologists are not aware of the amazing survival improvement with modern LVAD technology, and patients are under-referred,” Dr. Cowger noted.
Contemporary outcomes on par with heart transplant
The authors note that survival with durable LVAD (dLVAD) has markedly improved over the years. Current survival is approximately 87% at 1 year for patients supported with a contemporary LVAD.
Average patient survival is now similar to that of heart transplantation at 2 years, with 5-year dLVAD survival now approaching 60%, they point out.
Contemporary dLVAD yields significant and sustained improvements in functional capacity. Data show that roughly 80% of patients improve to NYHA functional class I and II, with significant improvements in 6-minute walk distances and health-related quality of life, the authors note.
In addition, innovations in dLVAD technology have reduced the risk of several adverse events, including pump thrombosis, stroke, and bleeding.
“Novel devices are on the horizon of clinical investigation, offering smaller size, permitting less invasive surgical implantation, and eliminating the percutaneous lead for power supply,” the authors note.
“Unfortunately, greater adoption of dLVAD therapy has not been realized due to delayed referral of patients to advanced HF centers, insufficient clinician knowledge of contemporary dLVAD outcomes (including gains in quality of life), and deprioritization of patients with dLVAD support waiting for heart transplantation,” they write.
In addition to highlighting contemporary outcomes with dLVAD support, the 18-page statement also includes sections on:
- Current indications and timing of referral
- Surgical considerations (device selection, surgical techniques and approach to concomitant valvular disease, and management of acute right ventricular dysfunction)
- Unique patient populations (women, children, and adult congenital heart disease)
- Summary, gaps, and future directions
A recent workshop held by the National Heart, Lung, and Blood Institute (NHLBI) identified critical gaps in the field of advanced HF.
One of the major gaps identified was the need to improve mechanical circulatory support use as a “complement or alternative” therapy to heart transplantation. The workshop also emphasized the need to “synergize” LVAD and heart transplant in the same patient to maximize health-related quality of life and survival benefit.
The NHLBI workshop also highlighted the need to model how different patient subset characteristics may affect mechanical circulatory support outcomes to inform bridge-to-transplantation or bridge-to-decision/candidacy opportunities more appropriately.
This research had no commercial funding. A number of study authors disclosed relationships with industry. The full list is available with the original article.
A version of this article first appeared on Medscape.com.
The prognosis for patients with advanced heart failure (HF) who fail guideline-directed medical therapy is poor, but
Those are the key takeaways from a scientific statement on durable mechanical circulatory support, published online in the Journal of the American College of Cardiology.
“I think it is important to highlight this issue because of the sheer impact that heart failure has on American citizens,” corresponding author Jennifer Cowger, MD, MS, advanced heart failure specialist, Henry Ford Health, Detroit, said in an interview.
“End-stage heart failure has no medication that has shown a gain in survival, and most are dead by 1 year,” she said.
This scientific statement highlights the “amazing evolution of LVAD support and associated improvement in outcomes,” Dr. Cowger said.
Yet because LVADs are only implanted at roughly 170 U.S. centers, “many cardiologists are not aware of the amazing survival improvement with modern LVAD technology, and patients are under-referred,” Dr. Cowger noted.
Contemporary outcomes on par with heart transplant
The authors note that survival with durable LVAD (dLVAD) has markedly improved over the years. Current survival is approximately 87% at 1 year for patients supported with a contemporary LVAD.
Average patient survival is now similar to that of heart transplantation at 2 years, with 5-year dLVAD survival now approaching 60%, they point out.
Contemporary dLVAD yields significant and sustained improvements in functional capacity. Data show that roughly 80% of patients improve to NYHA functional class I and II, with significant improvements in 6-minute walk distances and health-related quality of life, the authors note.
In addition, innovations in dLVAD technology have reduced the risk of several adverse events, including pump thrombosis, stroke, and bleeding.
“Novel devices are on the horizon of clinical investigation, offering smaller size, permitting less invasive surgical implantation, and eliminating the percutaneous lead for power supply,” the authors note.
“Unfortunately, greater adoption of dLVAD therapy has not been realized due to delayed referral of patients to advanced HF centers, insufficient clinician knowledge of contemporary dLVAD outcomes (including gains in quality of life), and deprioritization of patients with dLVAD support waiting for heart transplantation,” they write.
In addition to highlighting contemporary outcomes with dLVAD support, the 18-page statement also includes sections on:
- Current indications and timing of referral
- Surgical considerations (device selection, surgical techniques and approach to concomitant valvular disease, and management of acute right ventricular dysfunction)
- Unique patient populations (women, children, and adult congenital heart disease)
- Summary, gaps, and future directions
A recent workshop held by the National Heart, Lung, and Blood Institute (NHLBI) identified critical gaps in the field of advanced HF.
One of the major gaps identified was the need to improve mechanical circulatory support use as a “complement or alternative” therapy to heart transplantation. The workshop also emphasized the need to “synergize” LVAD and heart transplant in the same patient to maximize health-related quality of life and survival benefit.
The NHLBI workshop also highlighted the need to model how different patient subset characteristics may affect mechanical circulatory support outcomes to inform bridge-to-transplantation or bridge-to-decision/candidacy opportunities more appropriately.
This research had no commercial funding. A number of study authors disclosed relationships with industry. The full list is available with the original article.
A version of this article first appeared on Medscape.com.
FROM JACC
Active surveillance preferred in low-risk prostate cancer
TOPLINE:
When provided detailed information on options, most men with low-risk prostate cancer chose active surveillance over treatment, and there was no difference in outcomes, new research from Italy shows.
METHODOLOGY:
- Active surveillance for patients with low-risk prostate cancer has been recommended for years, but its adoption often varies within and between countries.
- The current study, based in Italy, aimed to promote the adoption of active surveillance in two regions in Northern Italy and to understand patient acceptance and outcomes in comparison with active treatment.
- Men newly diagnosed with low-risk prostate cancer between June 2015 and December 2021 were eligible. All were informed of treatment options and were offered active surveillance.
- Multilevel models identified factors associated with choosing active surveillance over active treatment, which consisted of either radical prostatectomy or radiation therapy.
TAKEAWAY:
- Overall, 83% (706 of 852) men chose active surveillance over immediate treatment. There was an upward trend over time, from 78% in 2015-2017 to 90% in 2020-2021.
- Patients who chose active surveillance over any radical treatment were more likely to be aged 75 years or older (odds ratio, 4.27), to have a Charlson Comorbidity Index ≥ 2 (OR, 1.98), to have undergone independent revision of the first biopsy (OR, 2.35), and to have undergone multidisciplinary assessment (OR, 2.65).
- Worse prostate cancer prognostic factors, such as stage T2a (OR, 0.54) and Gleason Score 3+4 (OR, 0.20), were associated with lower odds of choosing active surveillance than any radical treatment.
- In an adjusted intention-to-treat analysis, among patients who initially chose active surveillance, overall survival was not worse in comparison with those who chose any radical treatment (hazard ratio, 0.86; 95% confidence interval, 0.41-1.79) or in comparison with those who chose radical prostatectomy (HR, 0.90; 95% CI, 0.37-2.20).
IN PRACTICE:
“The main remarkable finding of [the trial] is represented by the widespread adoption of active surveillance in our [Regional Oncology Network] since the beginning of the study, and the increasing trend over time, reaching approximately 90% of eligible patients in 2020 to 2021,” the authors wrote.
SOURCE:
The study, with first author Giovannino Ciccone, MD, PhD, AOU City of Health and Science of Turin, Italy, was published online in JAMA Network Open.
LIMITATIONS:
Key limitations include the relatively short follow-up (median, 57 months), variability between centers in terms of enrolling patients and discussing their choices, and the high rate of patients who abandoned active surveillance by year 2 of follow-up. Overall, about 281 patients (~40%) abandoned active surveillance by year 2, most commonly because of biochemical progression.
DISCLOSURES:
The START project was funded by the Fondazione Compagnia di San Paolo and partially by Rete Oncologica del Piemonte e Valle d’Aosta, Turin, Italy. Dr. Ciccone has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
When provided detailed information on options, most men with low-risk prostate cancer chose active surveillance over treatment, and there was no difference in outcomes, new research from Italy shows.
METHODOLOGY:
- Active surveillance for patients with low-risk prostate cancer has been recommended for years, but its adoption often varies within and between countries.
- The current study, based in Italy, aimed to promote the adoption of active surveillance in two regions in Northern Italy and to understand patient acceptance and outcomes in comparison with active treatment.
- Men newly diagnosed with low-risk prostate cancer between June 2015 and December 2021 were eligible. All were informed of treatment options and were offered active surveillance.
- Multilevel models identified factors associated with choosing active surveillance over active treatment, which consisted of either radical prostatectomy or radiation therapy.
TAKEAWAY:
- Overall, 83% (706 of 852) men chose active surveillance over immediate treatment. There was an upward trend over time, from 78% in 2015-2017 to 90% in 2020-2021.
- Patients who chose active surveillance over any radical treatment were more likely to be aged 75 years or older (odds ratio, 4.27), to have a Charlson Comorbidity Index ≥ 2 (OR, 1.98), to have undergone independent revision of the first biopsy (OR, 2.35), and to have undergone multidisciplinary assessment (OR, 2.65).
- Worse prostate cancer prognostic factors, such as stage T2a (OR, 0.54) and Gleason Score 3+4 (OR, 0.20), were associated with lower odds of choosing active surveillance than any radical treatment.
- In an adjusted intention-to-treat analysis, among patients who initially chose active surveillance, overall survival was not worse in comparison with those who chose any radical treatment (hazard ratio, 0.86; 95% confidence interval, 0.41-1.79) or in comparison with those who chose radical prostatectomy (HR, 0.90; 95% CI, 0.37-2.20).
IN PRACTICE:
“The main remarkable finding of [the trial] is represented by the widespread adoption of active surveillance in our [Regional Oncology Network] since the beginning of the study, and the increasing trend over time, reaching approximately 90% of eligible patients in 2020 to 2021,” the authors wrote.
SOURCE:
The study, with first author Giovannino Ciccone, MD, PhD, AOU City of Health and Science of Turin, Italy, was published online in JAMA Network Open.
LIMITATIONS:
Key limitations include the relatively short follow-up (median, 57 months), variability between centers in terms of enrolling patients and discussing their choices, and the high rate of patients who abandoned active surveillance by year 2 of follow-up. Overall, about 281 patients (~40%) abandoned active surveillance by year 2, most commonly because of biochemical progression.
DISCLOSURES:
The START project was funded by the Fondazione Compagnia di San Paolo and partially by Rete Oncologica del Piemonte e Valle d’Aosta, Turin, Italy. Dr. Ciccone has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
When provided detailed information on options, most men with low-risk prostate cancer chose active surveillance over treatment, and there was no difference in outcomes, new research from Italy shows.
METHODOLOGY:
- Active surveillance for patients with low-risk prostate cancer has been recommended for years, but its adoption often varies within and between countries.
- The current study, based in Italy, aimed to promote the adoption of active surveillance in two regions in Northern Italy and to understand patient acceptance and outcomes in comparison with active treatment.
- Men newly diagnosed with low-risk prostate cancer between June 2015 and December 2021 were eligible. All were informed of treatment options and were offered active surveillance.
- Multilevel models identified factors associated with choosing active surveillance over active treatment, which consisted of either radical prostatectomy or radiation therapy.
TAKEAWAY:
- Overall, 83% (706 of 852) men chose active surveillance over immediate treatment. There was an upward trend over time, from 78% in 2015-2017 to 90% in 2020-2021.
- Patients who chose active surveillance over any radical treatment were more likely to be aged 75 years or older (odds ratio, 4.27), to have a Charlson Comorbidity Index ≥ 2 (OR, 1.98), to have undergone independent revision of the first biopsy (OR, 2.35), and to have undergone multidisciplinary assessment (OR, 2.65).
- Worse prostate cancer prognostic factors, such as stage T2a (OR, 0.54) and Gleason Score 3+4 (OR, 0.20), were associated with lower odds of choosing active surveillance than any radical treatment.
- In an adjusted intention-to-treat analysis, among patients who initially chose active surveillance, overall survival was not worse in comparison with those who chose any radical treatment (hazard ratio, 0.86; 95% confidence interval, 0.41-1.79) or in comparison with those who chose radical prostatectomy (HR, 0.90; 95% CI, 0.37-2.20).
IN PRACTICE:
“The main remarkable finding of [the trial] is represented by the widespread adoption of active surveillance in our [Regional Oncology Network] since the beginning of the study, and the increasing trend over time, reaching approximately 90% of eligible patients in 2020 to 2021,” the authors wrote.
SOURCE:
The study, with first author Giovannino Ciccone, MD, PhD, AOU City of Health and Science of Turin, Italy, was published online in JAMA Network Open.
LIMITATIONS:
Key limitations include the relatively short follow-up (median, 57 months), variability between centers in terms of enrolling patients and discussing their choices, and the high rate of patients who abandoned active surveillance by year 2 of follow-up. Overall, about 281 patients (~40%) abandoned active surveillance by year 2, most commonly because of biochemical progression.
DISCLOSURES:
The START project was funded by the Fondazione Compagnia di San Paolo and partially by Rete Oncologica del Piemonte e Valle d’Aosta, Turin, Italy. Dr. Ciccone has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ESG yields significant, sustained weight loss across obesity classes
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective analysis of 1,506 adults (85% female, 70% White) with severe obesity (501 class I, 546 class II, and 459 class III) who underwent ESG at seven academic and private U.S. centers from 2013 to 2022.
- Average percent total body weight loss (%TBWL) was evaluated at 6, 12, 18, and 24 months after the procedure.
- Weight loss and safety outcomes were evaluated according to obesity class.
TAKEAWAY:
- At 12 months, 83.2% of patients achieved ≥10% TBWL and 60.9% achieved ≥15% TBWL across all obesity classes.
- There was a significant difference in TBWL by baseline obesity class, with average weight loss significantly greater in class III than classes I and II at all time points. At 24 months, class III patients had mean TBWL of 20.4%, compared with 13.3% for class I and 13.6% for class II patients.
- As early as 6 months post-ESG, patients in all BMI classes were able to drop to the next lower BMI class and remained there through 2 years. However, ongoing improvement in BMI until the end of follow-up was seen only in class III patients. Notably, class III patients were significantly younger and taller than class I and class II patients.
- There were no differences in adverse events between obesity classes. Only 2.6% of patients had an adverse event requiring hospitalization. Most of these events (86%) were for symptom management and/or fluid replacement.
IN PRACTICE:
“Traditionally, ESG has been proposed as a treatment choice for patients with class I and II obesity because of its modest weight loss outcomes. However, our data show a %TBWL crossing 20% in patients with class III disease, which may push the envelope of perceived utility of ESG,” the authors write.
SOURCE:
The study, with first author Khushboo Gala, MBBS, division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., was published online in Clinical and Translational Gastroenterology.
LIMITATIONS:
Limitations include the retrospective design, with outcomes only out to 2 years, and loss of follow-up, with only 339 of the 1506 patients evaluated at 2 years.
DISCLOSURES:
The study had no financial support. Several study authors reported ties to industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective analysis of 1,506 adults (85% female, 70% White) with severe obesity (501 class I, 546 class II, and 459 class III) who underwent ESG at seven academic and private U.S. centers from 2013 to 2022.
- Average percent total body weight loss (%TBWL) was evaluated at 6, 12, 18, and 24 months after the procedure.
- Weight loss and safety outcomes were evaluated according to obesity class.
TAKEAWAY:
- At 12 months, 83.2% of patients achieved ≥10% TBWL and 60.9% achieved ≥15% TBWL across all obesity classes.
- There was a significant difference in TBWL by baseline obesity class, with average weight loss significantly greater in class III than classes I and II at all time points. At 24 months, class III patients had mean TBWL of 20.4%, compared with 13.3% for class I and 13.6% for class II patients.
- As early as 6 months post-ESG, patients in all BMI classes were able to drop to the next lower BMI class and remained there through 2 years. However, ongoing improvement in BMI until the end of follow-up was seen only in class III patients. Notably, class III patients were significantly younger and taller than class I and class II patients.
- There were no differences in adverse events between obesity classes. Only 2.6% of patients had an adverse event requiring hospitalization. Most of these events (86%) were for symptom management and/or fluid replacement.
IN PRACTICE:
“Traditionally, ESG has been proposed as a treatment choice for patients with class I and II obesity because of its modest weight loss outcomes. However, our data show a %TBWL crossing 20% in patients with class III disease, which may push the envelope of perceived utility of ESG,” the authors write.
SOURCE:
The study, with first author Khushboo Gala, MBBS, division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., was published online in Clinical and Translational Gastroenterology.
LIMITATIONS:
Limitations include the retrospective design, with outcomes only out to 2 years, and loss of follow-up, with only 339 of the 1506 patients evaluated at 2 years.
DISCLOSURES:
The study had no financial support. Several study authors reported ties to industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective analysis of 1,506 adults (85% female, 70% White) with severe obesity (501 class I, 546 class II, and 459 class III) who underwent ESG at seven academic and private U.S. centers from 2013 to 2022.
- Average percent total body weight loss (%TBWL) was evaluated at 6, 12, 18, and 24 months after the procedure.
- Weight loss and safety outcomes were evaluated according to obesity class.
TAKEAWAY:
- At 12 months, 83.2% of patients achieved ≥10% TBWL and 60.9% achieved ≥15% TBWL across all obesity classes.
- There was a significant difference in TBWL by baseline obesity class, with average weight loss significantly greater in class III than classes I and II at all time points. At 24 months, class III patients had mean TBWL of 20.4%, compared with 13.3% for class I and 13.6% for class II patients.
- As early as 6 months post-ESG, patients in all BMI classes were able to drop to the next lower BMI class and remained there through 2 years. However, ongoing improvement in BMI until the end of follow-up was seen only in class III patients. Notably, class III patients were significantly younger and taller than class I and class II patients.
- There were no differences in adverse events between obesity classes. Only 2.6% of patients had an adverse event requiring hospitalization. Most of these events (86%) were for symptom management and/or fluid replacement.
IN PRACTICE:
“Traditionally, ESG has been proposed as a treatment choice for patients with class I and II obesity because of its modest weight loss outcomes. However, our data show a %TBWL crossing 20% in patients with class III disease, which may push the envelope of perceived utility of ESG,” the authors write.
SOURCE:
The study, with first author Khushboo Gala, MBBS, division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., was published online in Clinical and Translational Gastroenterology.
LIMITATIONS:
Limitations include the retrospective design, with outcomes only out to 2 years, and loss of follow-up, with only 339 of the 1506 patients evaluated at 2 years.
DISCLOSURES:
The study had no financial support. Several study authors reported ties to industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Remote symptom monitoring in advanced cancer improves quality of life
During treatment for metastatic cancer, remote monitoring of symptoms using electronic patient-reported outcomes (ePROs) reduced health care visits and improved patients’ physical function and quality of life, but did not impact overall survival, according to findings from the PRO-TECT trial.
said Ethan Basch, MD, University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, who presented the findings at the annual meeting of the European Society for Medical Oncology.
Jiyoung Ahn, PhD, professor of population health at NYU Langone Health and associate director of population science, NYU Langone Perlmutter Cancer Center, both in New York, said this study “provides exciting scientific evidence” supporting real-time, remote monitoring of PROs. Dr. Ahn was not involved with the PRO-TECT trial.
Symptoms among patients with advanced cancer receiving treatment are “exceedingly common,” Dr. Basch explained, but “unfortunately, evidence demonstrates that we as clinicians miss up to 50% of our patients’ symptoms with potential serious downstream consequences.”
Remote monitoring with ePROs can help clinicians detect patients’ symptoms early so they can intervene early.
In the PRO-TECT cluster-randomized trial, 52 oncology practices in the United States were randomly assigned (1:1) to remote monitoring with ePRO surveys or usual care. The cohort included 1,191 patients with metastatic cancer – with 593 patients at PRO practices and 598 patients at control practices. Participating practices could enroll up to 50 patients with any type of metastatic cancer, except for indolent lymphoma or acute leukemia, who were receiving systemic treatment.
Patients in the ePRO practices completed weekly surveys either online or using an automated telephone system for up to 1 year. The survey included questions related to nine common symptoms, performance status, and falls.
For symptoms that are severe or worsening, a real-time alert goes to the care team through the electronic health record or by an email, Dr. Basch explained. Similarly, reports highlighting the longitudinal trajectory of symptoms can be generated at patient visits and reviewed by clinicians, which can bring “the patient and the care team closer together by elevating those issues that are particularly salient to the patient’s experience,” he noted.
Patients completed over 91% of the electronic symptom surveys. After 24 months, the team observed no significant difference in the primary outcome of overall survival – 42.0 months with ePRO vs. 43.5 months with usual care (hazard ratio, 0.99; P = .86).
Dr. Basch and colleagues did, however, observe a 6% reduction in emergency or hospital admissions in the ePRO group, compared with usual care. The ePRO group also had a significantly longer time to first emergency admission (HR, 0.84; P = .03) and a decreased average number of admissions per patient over 1 year (1.48 vs. 1.81; P = .006).
At multiple time points, the team also observed “clinically meaningful and statistically significant” benefits in physical functioning, symptom control, and health-related quality of life, Dr. Basch reported. More patients in the ePRO than the usual-care group experienced benefits in fatigue (odds ratio, 1.77; P < .001), anorexia (OR, 1.32; P = .03), nausea/vomiting (OR, 1.40; P = .01), and sleep (OR, 1.73; P < .001).
Patients’ impressions of the ePRO symptom monitoring system were also “overwhelmingly” positive, Dr. Basch said. Most found the questions relevant and easy to understand and felt that their care team used the information, which made patients feel more in control of their care.
Nurses generally had a favorable impression of the system, with the majority stating that the information was helpful for electronic health record documentation and that it improved discussions with their patients and improved their efficiency.
However, about one-quarter of the nurses expressed reluctance about continuing to use the system, citing the “added work of the ePROs, particularly alerts that were triggered that prompted them to call their patients, particularly during the pandemic when nurses in the United States were pulled in many directions,” Dr. Basch said.
He noted that future ePRO implementations should aim to integrate ePROs into care processes and adjust nurse responsibilities to allow time for ePRO work.
It will also be important to offer a variety of ePRO platforms that are easily accessible for different patient groups. “Notably,” said Dr. Basch, about one-third of the patients selected the automated telephone option. These were largely patients living in rural areas of the United States with lower socioeconomic status and lower health literacy, “suggesting that we need to think about our technologies to meet patients where they are,” he said.
Despite the positive outcomes, there are “challenges to widespread adoption,” agreed NYU’s Dr. Ahn.
These challenges include the need for physician adaptation to new technologies, data security, and ensuring patient engagement and compliance with remote monitoring systems.
“Successfully addressing these challenges is crucial for optimizing the integration of ePROs into cancer care,” Dr. Ahn said.
ESMO’s invited discussant, Anne Letsch, MD, noted that “cancer therapies are getting more complex, and it’s important that patients are well informed and empowered to get together with the treatment teams throughout therapy.”
The high completion rate with ePRO symptom surveys was “quite remarkable,” said Dr. Letsch, head of the Cancer Center at the University Hospital Schleswig Holstein, Kiel, Germany.
But, Dr. Letsch said, it’s “a pity” that there was no overall survival benefit among patients in the ePRO group. Perhaps overall survival is not what matters most in this context, she said. Instead, she asked, “are other outcomes, like health-related quality of life, symptom control and treatment safety, much more important?”
Dr. Basch also questioned whether the survival differences between the two groups may have been blunted because a substantial portion of the trial was conducted during the COVID-19 pandemic, when medical resources and treatments were delayed and diverted.
Dr. Basch pointed to a 2017 study he and colleagues conducted at a single tertiary care medical center, in which patients monitored with ePROs did demonstrate an overall survival benefit, compared with usual care.
Overall, though, the study demonstrated that “symptom monitoring with ePROs is feasible during routine treatment for advanced cancers across diverse practices in the U.S.” and improved patients’ quality of life, Dr. Basch said.
Funding for the study was provided by a grant from the Patient-Centered Outcomes Research Institute. Dr. Basch has disclosed relationships with Resilience Health, Sivan Health, Navigating Cancer, and AstraZeneca. Dr. Letsch and Dr. Ahn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
During treatment for metastatic cancer, remote monitoring of symptoms using electronic patient-reported outcomes (ePROs) reduced health care visits and improved patients’ physical function and quality of life, but did not impact overall survival, according to findings from the PRO-TECT trial.
said Ethan Basch, MD, University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, who presented the findings at the annual meeting of the European Society for Medical Oncology.
Jiyoung Ahn, PhD, professor of population health at NYU Langone Health and associate director of population science, NYU Langone Perlmutter Cancer Center, both in New York, said this study “provides exciting scientific evidence” supporting real-time, remote monitoring of PROs. Dr. Ahn was not involved with the PRO-TECT trial.
Symptoms among patients with advanced cancer receiving treatment are “exceedingly common,” Dr. Basch explained, but “unfortunately, evidence demonstrates that we as clinicians miss up to 50% of our patients’ symptoms with potential serious downstream consequences.”
Remote monitoring with ePROs can help clinicians detect patients’ symptoms early so they can intervene early.
In the PRO-TECT cluster-randomized trial, 52 oncology practices in the United States were randomly assigned (1:1) to remote monitoring with ePRO surveys or usual care. The cohort included 1,191 patients with metastatic cancer – with 593 patients at PRO practices and 598 patients at control practices. Participating practices could enroll up to 50 patients with any type of metastatic cancer, except for indolent lymphoma or acute leukemia, who were receiving systemic treatment.
Patients in the ePRO practices completed weekly surveys either online or using an automated telephone system for up to 1 year. The survey included questions related to nine common symptoms, performance status, and falls.
For symptoms that are severe or worsening, a real-time alert goes to the care team through the electronic health record or by an email, Dr. Basch explained. Similarly, reports highlighting the longitudinal trajectory of symptoms can be generated at patient visits and reviewed by clinicians, which can bring “the patient and the care team closer together by elevating those issues that are particularly salient to the patient’s experience,” he noted.
Patients completed over 91% of the electronic symptom surveys. After 24 months, the team observed no significant difference in the primary outcome of overall survival – 42.0 months with ePRO vs. 43.5 months with usual care (hazard ratio, 0.99; P = .86).
Dr. Basch and colleagues did, however, observe a 6% reduction in emergency or hospital admissions in the ePRO group, compared with usual care. The ePRO group also had a significantly longer time to first emergency admission (HR, 0.84; P = .03) and a decreased average number of admissions per patient over 1 year (1.48 vs. 1.81; P = .006).
At multiple time points, the team also observed “clinically meaningful and statistically significant” benefits in physical functioning, symptom control, and health-related quality of life, Dr. Basch reported. More patients in the ePRO than the usual-care group experienced benefits in fatigue (odds ratio, 1.77; P < .001), anorexia (OR, 1.32; P = .03), nausea/vomiting (OR, 1.40; P = .01), and sleep (OR, 1.73; P < .001).
Patients’ impressions of the ePRO symptom monitoring system were also “overwhelmingly” positive, Dr. Basch said. Most found the questions relevant and easy to understand and felt that their care team used the information, which made patients feel more in control of their care.
Nurses generally had a favorable impression of the system, with the majority stating that the information was helpful for electronic health record documentation and that it improved discussions with their patients and improved their efficiency.
However, about one-quarter of the nurses expressed reluctance about continuing to use the system, citing the “added work of the ePROs, particularly alerts that were triggered that prompted them to call their patients, particularly during the pandemic when nurses in the United States were pulled in many directions,” Dr. Basch said.
He noted that future ePRO implementations should aim to integrate ePROs into care processes and adjust nurse responsibilities to allow time for ePRO work.
It will also be important to offer a variety of ePRO platforms that are easily accessible for different patient groups. “Notably,” said Dr. Basch, about one-third of the patients selected the automated telephone option. These were largely patients living in rural areas of the United States with lower socioeconomic status and lower health literacy, “suggesting that we need to think about our technologies to meet patients where they are,” he said.
Despite the positive outcomes, there are “challenges to widespread adoption,” agreed NYU’s Dr. Ahn.
These challenges include the need for physician adaptation to new technologies, data security, and ensuring patient engagement and compliance with remote monitoring systems.
“Successfully addressing these challenges is crucial for optimizing the integration of ePROs into cancer care,” Dr. Ahn said.
ESMO’s invited discussant, Anne Letsch, MD, noted that “cancer therapies are getting more complex, and it’s important that patients are well informed and empowered to get together with the treatment teams throughout therapy.”
The high completion rate with ePRO symptom surveys was “quite remarkable,” said Dr. Letsch, head of the Cancer Center at the University Hospital Schleswig Holstein, Kiel, Germany.
But, Dr. Letsch said, it’s “a pity” that there was no overall survival benefit among patients in the ePRO group. Perhaps overall survival is not what matters most in this context, she said. Instead, she asked, “are other outcomes, like health-related quality of life, symptom control and treatment safety, much more important?”
Dr. Basch also questioned whether the survival differences between the two groups may have been blunted because a substantial portion of the trial was conducted during the COVID-19 pandemic, when medical resources and treatments were delayed and diverted.
Dr. Basch pointed to a 2017 study he and colleagues conducted at a single tertiary care medical center, in which patients monitored with ePROs did demonstrate an overall survival benefit, compared with usual care.
Overall, though, the study demonstrated that “symptom monitoring with ePROs is feasible during routine treatment for advanced cancers across diverse practices in the U.S.” and improved patients’ quality of life, Dr. Basch said.
Funding for the study was provided by a grant from the Patient-Centered Outcomes Research Institute. Dr. Basch has disclosed relationships with Resilience Health, Sivan Health, Navigating Cancer, and AstraZeneca. Dr. Letsch and Dr. Ahn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
During treatment for metastatic cancer, remote monitoring of symptoms using electronic patient-reported outcomes (ePROs) reduced health care visits and improved patients’ physical function and quality of life, but did not impact overall survival, according to findings from the PRO-TECT trial.
said Ethan Basch, MD, University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, who presented the findings at the annual meeting of the European Society for Medical Oncology.
Jiyoung Ahn, PhD, professor of population health at NYU Langone Health and associate director of population science, NYU Langone Perlmutter Cancer Center, both in New York, said this study “provides exciting scientific evidence” supporting real-time, remote monitoring of PROs. Dr. Ahn was not involved with the PRO-TECT trial.
Symptoms among patients with advanced cancer receiving treatment are “exceedingly common,” Dr. Basch explained, but “unfortunately, evidence demonstrates that we as clinicians miss up to 50% of our patients’ symptoms with potential serious downstream consequences.”
Remote monitoring with ePROs can help clinicians detect patients’ symptoms early so they can intervene early.
In the PRO-TECT cluster-randomized trial, 52 oncology practices in the United States were randomly assigned (1:1) to remote monitoring with ePRO surveys or usual care. The cohort included 1,191 patients with metastatic cancer – with 593 patients at PRO practices and 598 patients at control practices. Participating practices could enroll up to 50 patients with any type of metastatic cancer, except for indolent lymphoma or acute leukemia, who were receiving systemic treatment.
Patients in the ePRO practices completed weekly surveys either online or using an automated telephone system for up to 1 year. The survey included questions related to nine common symptoms, performance status, and falls.
For symptoms that are severe or worsening, a real-time alert goes to the care team through the electronic health record or by an email, Dr. Basch explained. Similarly, reports highlighting the longitudinal trajectory of symptoms can be generated at patient visits and reviewed by clinicians, which can bring “the patient and the care team closer together by elevating those issues that are particularly salient to the patient’s experience,” he noted.
Patients completed over 91% of the electronic symptom surveys. After 24 months, the team observed no significant difference in the primary outcome of overall survival – 42.0 months with ePRO vs. 43.5 months with usual care (hazard ratio, 0.99; P = .86).
Dr. Basch and colleagues did, however, observe a 6% reduction in emergency or hospital admissions in the ePRO group, compared with usual care. The ePRO group also had a significantly longer time to first emergency admission (HR, 0.84; P = .03) and a decreased average number of admissions per patient over 1 year (1.48 vs. 1.81; P = .006).
At multiple time points, the team also observed “clinically meaningful and statistically significant” benefits in physical functioning, symptom control, and health-related quality of life, Dr. Basch reported. More patients in the ePRO than the usual-care group experienced benefits in fatigue (odds ratio, 1.77; P < .001), anorexia (OR, 1.32; P = .03), nausea/vomiting (OR, 1.40; P = .01), and sleep (OR, 1.73; P < .001).
Patients’ impressions of the ePRO symptom monitoring system were also “overwhelmingly” positive, Dr. Basch said. Most found the questions relevant and easy to understand and felt that their care team used the information, which made patients feel more in control of their care.
Nurses generally had a favorable impression of the system, with the majority stating that the information was helpful for electronic health record documentation and that it improved discussions with their patients and improved their efficiency.
However, about one-quarter of the nurses expressed reluctance about continuing to use the system, citing the “added work of the ePROs, particularly alerts that were triggered that prompted them to call their patients, particularly during the pandemic when nurses in the United States were pulled in many directions,” Dr. Basch said.
He noted that future ePRO implementations should aim to integrate ePROs into care processes and adjust nurse responsibilities to allow time for ePRO work.
It will also be important to offer a variety of ePRO platforms that are easily accessible for different patient groups. “Notably,” said Dr. Basch, about one-third of the patients selected the automated telephone option. These were largely patients living in rural areas of the United States with lower socioeconomic status and lower health literacy, “suggesting that we need to think about our technologies to meet patients where they are,” he said.
Despite the positive outcomes, there are “challenges to widespread adoption,” agreed NYU’s Dr. Ahn.
These challenges include the need for physician adaptation to new technologies, data security, and ensuring patient engagement and compliance with remote monitoring systems.
“Successfully addressing these challenges is crucial for optimizing the integration of ePROs into cancer care,” Dr. Ahn said.
ESMO’s invited discussant, Anne Letsch, MD, noted that “cancer therapies are getting more complex, and it’s important that patients are well informed and empowered to get together with the treatment teams throughout therapy.”
The high completion rate with ePRO symptom surveys was “quite remarkable,” said Dr. Letsch, head of the Cancer Center at the University Hospital Schleswig Holstein, Kiel, Germany.
But, Dr. Letsch said, it’s “a pity” that there was no overall survival benefit among patients in the ePRO group. Perhaps overall survival is not what matters most in this context, she said. Instead, she asked, “are other outcomes, like health-related quality of life, symptom control and treatment safety, much more important?”
Dr. Basch also questioned whether the survival differences between the two groups may have been blunted because a substantial portion of the trial was conducted during the COVID-19 pandemic, when medical resources and treatments were delayed and diverted.
Dr. Basch pointed to a 2017 study he and colleagues conducted at a single tertiary care medical center, in which patients monitored with ePROs did demonstrate an overall survival benefit, compared with usual care.
Overall, though, the study demonstrated that “symptom monitoring with ePROs is feasible during routine treatment for advanced cancers across diverse practices in the U.S.” and improved patients’ quality of life, Dr. Basch said.
Funding for the study was provided by a grant from the Patient-Centered Outcomes Research Institute. Dr. Basch has disclosed relationships with Resilience Health, Sivan Health, Navigating Cancer, and AstraZeneca. Dr. Letsch and Dr. Ahn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESMO CONGRESS 2023