User login
Abdominal fat linked to lower brain volume in midlife
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AGING AND DISEASES
Do AI chatbots give reliable answers on cancer? Yes and no
two new studies suggest.
AI chatbots, such as ChatGPT (OpenAI), are becoming go-to sources for health information. However, no studies have rigorously evaluated the quality of their medical advice, especially for cancer.
Two new studies published in JAMA Oncology did just that.
One, which looked at common cancer-related Google searches, found that AI chatbots generally provide accurate information to consumers, but the information’s usefulness may be limited by its complexity.
The other, which assessed cancer treatment recommendations, found that AI chatbots overall missed the mark on providing recommendations for breast, prostate, and lung cancers in line with national treatment guidelines.
The medical world is becoming “enamored with our newest potential helper, large language models (LLMs) and in particular chatbots, such as ChatGPT,” Atul Butte, MD, PhD, who heads the Bakar Computational Health Sciences Institute, University of California, San Francisco, wrote in an editorial accompanying the studies. “But maybe our core belief in GPT technology as a clinical partner has not sufficiently been earned yet.”
The first study by Alexander Pan of the State University of New York, Brooklyn, and colleagues analyzed the quality of responses to the top five most searched questions on skin, lung, breast, colorectal, and prostate cancer provided by four AI chatbots: ChatGPT-3.5, Perplexity (Perplexity.AI), Chatsonic (Writesonic), and Bing AI (Microsoft).
Questions included what is skin cancer and what are symptoms of prostate, lung, or breast cancer? The team rated the responses for quality, clarity, actionability, misinformation, and readability.
The researchers found that the four chatbots generated “high-quality” responses about the five cancers and did not appear to spread misinformation. Three of the four chatbots cited reputable sources, such as the American Cancer Society, Mayo Clinic, and Centers for Disease Controls and Prevention, which is “reassuring,” the researchers said.
However, the team also found that the usefulness of the information was “limited” because responses were often written at a college reading level. Another limitation: AI chatbots provided concise answers with no visual aids, which may not be sufficient to explain more complex ideas to consumers.
“These limitations suggest that AI chatbots should be used [supplementally] and not as a primary source for medical information,” the authors said, adding that the chatbots “typically acknowledged their limitations in providing individualized advice and encouraged users to seek medical attention.”
A related study in the journal highlighted the ability of AI chatbots to generate appropriate cancer treatment recommendations.
In this analysis, Shan Chen, MS, with the AI in Medicine Program, Mass General Brigham, Harvard Medical School, Boston, and colleagues benchmarked cancer treatment recommendations made by ChatGPT-3.5 against 2021 National Comprehensive Cancer Network guidelines.
The team created 104 prompts designed to elicit basic treatment strategies for various types of cancer, including breast, prostate, and lung cancer. Questions included “What is the treatment for stage I breast cancer?” Several oncologists then assessed the level of concordance between the chatbot responses and NCCN guidelines.
In 62% of the prompts and answers, all the recommended treatments aligned with the oncologists’ views.
The chatbot provided at least one guideline-concordant treatment for 98% of prompts. However, for 34% of prompts, the chatbot also recommended at least one nonconcordant treatment.
And about 13% of recommended treatments were “hallucinated,” that is, not part of any recommended treatment. Hallucinations were primarily recommendations for localized treatment of advanced disease, targeted therapy, or immunotherapy.
Based on the findings, the team recommended that clinicians advise patients that AI chatbots are not a reliable source of cancer treatment information.
“The chatbot did not perform well at providing accurate cancer treatment recommendations,” the authors said. “The chatbot was most likely to mix in incorrect recommendations among correct ones, an error difficult even for experts to detect.”
In his editorial, Dr. Butte highlighted several caveats, including that the teams evaluated “off the shelf” chatbots, which likely had no specific medical training, and the prompts
designed in both studies were very basic, which may have limited their specificity or actionability. Newer LLMs with specific health care training are being released, he explained.
Despite the mixed study findings, Dr. Butte remains optimistic about the future of AI in medicine.
“Today, the reality is that the highest-quality care is concentrated within a few premier medical systems like the NCI Comprehensive Cancer Centers, accessible only to a small fraction of the global population,” Dr. Butte explained. “However, AI has the potential to change this.”
How can we make this happen?
AI algorithms would need to be trained with “data from the best medical systems globally” and “the latest guidelines from NCCN and elsewhere.” Digital health platforms powered by AI could then be designed to provide resources and advice to patients around the globe, Dr. Butte said.
Although “these algorithms will need to be carefully monitored as they are brought into health systems,” Dr. Butte said, it does not change their potential to “improve care for both the haves and have-nots of health care.”
The study by Mr. Pan and colleagues had no specific funding; one author, Stacy Loeb, MD, MSc, PhD, reported a disclosure; no other disclosures were reported. The study by Shan Chen and colleagues was supported by the Woods Foundation; several authors reported disclosures outside the submitted work. Dr. Butte disclosed relationships with several pharmaceutical companies.
A version of this article first appeared on Medscape.com.
two new studies suggest.
AI chatbots, such as ChatGPT (OpenAI), are becoming go-to sources for health information. However, no studies have rigorously evaluated the quality of their medical advice, especially for cancer.
Two new studies published in JAMA Oncology did just that.
One, which looked at common cancer-related Google searches, found that AI chatbots generally provide accurate information to consumers, but the information’s usefulness may be limited by its complexity.
The other, which assessed cancer treatment recommendations, found that AI chatbots overall missed the mark on providing recommendations for breast, prostate, and lung cancers in line with national treatment guidelines.
The medical world is becoming “enamored with our newest potential helper, large language models (LLMs) and in particular chatbots, such as ChatGPT,” Atul Butte, MD, PhD, who heads the Bakar Computational Health Sciences Institute, University of California, San Francisco, wrote in an editorial accompanying the studies. “But maybe our core belief in GPT technology as a clinical partner has not sufficiently been earned yet.”
The first study by Alexander Pan of the State University of New York, Brooklyn, and colleagues analyzed the quality of responses to the top five most searched questions on skin, lung, breast, colorectal, and prostate cancer provided by four AI chatbots: ChatGPT-3.5, Perplexity (Perplexity.AI), Chatsonic (Writesonic), and Bing AI (Microsoft).
Questions included what is skin cancer and what are symptoms of prostate, lung, or breast cancer? The team rated the responses for quality, clarity, actionability, misinformation, and readability.
The researchers found that the four chatbots generated “high-quality” responses about the five cancers and did not appear to spread misinformation. Three of the four chatbots cited reputable sources, such as the American Cancer Society, Mayo Clinic, and Centers for Disease Controls and Prevention, which is “reassuring,” the researchers said.
However, the team also found that the usefulness of the information was “limited” because responses were often written at a college reading level. Another limitation: AI chatbots provided concise answers with no visual aids, which may not be sufficient to explain more complex ideas to consumers.
“These limitations suggest that AI chatbots should be used [supplementally] and not as a primary source for medical information,” the authors said, adding that the chatbots “typically acknowledged their limitations in providing individualized advice and encouraged users to seek medical attention.”
A related study in the journal highlighted the ability of AI chatbots to generate appropriate cancer treatment recommendations.
In this analysis, Shan Chen, MS, with the AI in Medicine Program, Mass General Brigham, Harvard Medical School, Boston, and colleagues benchmarked cancer treatment recommendations made by ChatGPT-3.5 against 2021 National Comprehensive Cancer Network guidelines.
The team created 104 prompts designed to elicit basic treatment strategies for various types of cancer, including breast, prostate, and lung cancer. Questions included “What is the treatment for stage I breast cancer?” Several oncologists then assessed the level of concordance between the chatbot responses and NCCN guidelines.
In 62% of the prompts and answers, all the recommended treatments aligned with the oncologists’ views.
The chatbot provided at least one guideline-concordant treatment for 98% of prompts. However, for 34% of prompts, the chatbot also recommended at least one nonconcordant treatment.
And about 13% of recommended treatments were “hallucinated,” that is, not part of any recommended treatment. Hallucinations were primarily recommendations for localized treatment of advanced disease, targeted therapy, or immunotherapy.
Based on the findings, the team recommended that clinicians advise patients that AI chatbots are not a reliable source of cancer treatment information.
“The chatbot did not perform well at providing accurate cancer treatment recommendations,” the authors said. “The chatbot was most likely to mix in incorrect recommendations among correct ones, an error difficult even for experts to detect.”
In his editorial, Dr. Butte highlighted several caveats, including that the teams evaluated “off the shelf” chatbots, which likely had no specific medical training, and the prompts
designed in both studies were very basic, which may have limited their specificity or actionability. Newer LLMs with specific health care training are being released, he explained.
Despite the mixed study findings, Dr. Butte remains optimistic about the future of AI in medicine.
“Today, the reality is that the highest-quality care is concentrated within a few premier medical systems like the NCI Comprehensive Cancer Centers, accessible only to a small fraction of the global population,” Dr. Butte explained. “However, AI has the potential to change this.”
How can we make this happen?
AI algorithms would need to be trained with “data from the best medical systems globally” and “the latest guidelines from NCCN and elsewhere.” Digital health platforms powered by AI could then be designed to provide resources and advice to patients around the globe, Dr. Butte said.
Although “these algorithms will need to be carefully monitored as they are brought into health systems,” Dr. Butte said, it does not change their potential to “improve care for both the haves and have-nots of health care.”
The study by Mr. Pan and colleagues had no specific funding; one author, Stacy Loeb, MD, MSc, PhD, reported a disclosure; no other disclosures were reported. The study by Shan Chen and colleagues was supported by the Woods Foundation; several authors reported disclosures outside the submitted work. Dr. Butte disclosed relationships with several pharmaceutical companies.
A version of this article first appeared on Medscape.com.
two new studies suggest.
AI chatbots, such as ChatGPT (OpenAI), are becoming go-to sources for health information. However, no studies have rigorously evaluated the quality of their medical advice, especially for cancer.
Two new studies published in JAMA Oncology did just that.
One, which looked at common cancer-related Google searches, found that AI chatbots generally provide accurate information to consumers, but the information’s usefulness may be limited by its complexity.
The other, which assessed cancer treatment recommendations, found that AI chatbots overall missed the mark on providing recommendations for breast, prostate, and lung cancers in line with national treatment guidelines.
The medical world is becoming “enamored with our newest potential helper, large language models (LLMs) and in particular chatbots, such as ChatGPT,” Atul Butte, MD, PhD, who heads the Bakar Computational Health Sciences Institute, University of California, San Francisco, wrote in an editorial accompanying the studies. “But maybe our core belief in GPT technology as a clinical partner has not sufficiently been earned yet.”
The first study by Alexander Pan of the State University of New York, Brooklyn, and colleagues analyzed the quality of responses to the top five most searched questions on skin, lung, breast, colorectal, and prostate cancer provided by four AI chatbots: ChatGPT-3.5, Perplexity (Perplexity.AI), Chatsonic (Writesonic), and Bing AI (Microsoft).
Questions included what is skin cancer and what are symptoms of prostate, lung, or breast cancer? The team rated the responses for quality, clarity, actionability, misinformation, and readability.
The researchers found that the four chatbots generated “high-quality” responses about the five cancers and did not appear to spread misinformation. Three of the four chatbots cited reputable sources, such as the American Cancer Society, Mayo Clinic, and Centers for Disease Controls and Prevention, which is “reassuring,” the researchers said.
However, the team also found that the usefulness of the information was “limited” because responses were often written at a college reading level. Another limitation: AI chatbots provided concise answers with no visual aids, which may not be sufficient to explain more complex ideas to consumers.
“These limitations suggest that AI chatbots should be used [supplementally] and not as a primary source for medical information,” the authors said, adding that the chatbots “typically acknowledged their limitations in providing individualized advice and encouraged users to seek medical attention.”
A related study in the journal highlighted the ability of AI chatbots to generate appropriate cancer treatment recommendations.
In this analysis, Shan Chen, MS, with the AI in Medicine Program, Mass General Brigham, Harvard Medical School, Boston, and colleagues benchmarked cancer treatment recommendations made by ChatGPT-3.5 against 2021 National Comprehensive Cancer Network guidelines.
The team created 104 prompts designed to elicit basic treatment strategies for various types of cancer, including breast, prostate, and lung cancer. Questions included “What is the treatment for stage I breast cancer?” Several oncologists then assessed the level of concordance between the chatbot responses and NCCN guidelines.
In 62% of the prompts and answers, all the recommended treatments aligned with the oncologists’ views.
The chatbot provided at least one guideline-concordant treatment for 98% of prompts. However, for 34% of prompts, the chatbot also recommended at least one nonconcordant treatment.
And about 13% of recommended treatments were “hallucinated,” that is, not part of any recommended treatment. Hallucinations were primarily recommendations for localized treatment of advanced disease, targeted therapy, or immunotherapy.
Based on the findings, the team recommended that clinicians advise patients that AI chatbots are not a reliable source of cancer treatment information.
“The chatbot did not perform well at providing accurate cancer treatment recommendations,” the authors said. “The chatbot was most likely to mix in incorrect recommendations among correct ones, an error difficult even for experts to detect.”
In his editorial, Dr. Butte highlighted several caveats, including that the teams evaluated “off the shelf” chatbots, which likely had no specific medical training, and the prompts
designed in both studies were very basic, which may have limited their specificity or actionability. Newer LLMs with specific health care training are being released, he explained.
Despite the mixed study findings, Dr. Butte remains optimistic about the future of AI in medicine.
“Today, the reality is that the highest-quality care is concentrated within a few premier medical systems like the NCI Comprehensive Cancer Centers, accessible only to a small fraction of the global population,” Dr. Butte explained. “However, AI has the potential to change this.”
How can we make this happen?
AI algorithms would need to be trained with “data from the best medical systems globally” and “the latest guidelines from NCCN and elsewhere.” Digital health platforms powered by AI could then be designed to provide resources and advice to patients around the globe, Dr. Butte said.
Although “these algorithms will need to be carefully monitored as they are brought into health systems,” Dr. Butte said, it does not change their potential to “improve care for both the haves and have-nots of health care.”
The study by Mr. Pan and colleagues had no specific funding; one author, Stacy Loeb, MD, MSc, PhD, reported a disclosure; no other disclosures were reported. The study by Shan Chen and colleagues was supported by the Woods Foundation; several authors reported disclosures outside the submitted work. Dr. Butte disclosed relationships with several pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
AI tool predicts certain GI cancers years in advance
TOPLINE:
and is more accurate than other tools, a large study suggests.
METHODOLOGY:
- Researchers performed a case-control study using data from the Veterans Affairs Central Cancer Registry.
- They identified 8,430 patients with EAC and 2,965 patients GCA; these patients were compared with more than 10 million control patients.
- K-ECAN uses basic information in the EHR to determine an individual’s future risk of developing EAC or GCA.
TAKEAWAY:
- With an area under the receiver operating characteristic of 0.77, K-ECAN demonstrated better discrimination than previously validated models and published guidelines.
- Using only data from 3 to 5 years prior to diagnosis only slightly diminished its accuracy (AUROC, 0.75).
- K-ECAN remained the most accurate tool when undersampling men to simulate a non-VHA population (AUROC, 0.85).
- Although gastroesophageal reflux disease (GERD) was strongly associated with EAC, it only contributed a small proportion of gain in information for prediction.
IN PRACTICE:
Because K-ECAN does not rely heavily on GERD symptoms to assess risk, it has the “potential to guide providers to increase appropriate uptake of screening. De-emphasizing GERD in decisions to offer screening could paradoxically increase appropriate uptake of screening for EAC and GCA,” the authors wrote.
SOURCE:
The study, with first author Joel H. Rubenstein, MD, with the LTC Charles S. Kettles VA Medical Center, Ann Arbor, Mich., was published online in Gastroenterology.
LIMITATIONS:
K-ECAN was developed and validated among U.S. veterans and needs to be validated in other populations.
DISCLOSURES:
Funding for the study was provided by the Department of Defense. Dr. Rubenstein has received research support from Lucid Diagnostics.
A version of this article first appeared on Medscape.com.
TOPLINE:
and is more accurate than other tools, a large study suggests.
METHODOLOGY:
- Researchers performed a case-control study using data from the Veterans Affairs Central Cancer Registry.
- They identified 8,430 patients with EAC and 2,965 patients GCA; these patients were compared with more than 10 million control patients.
- K-ECAN uses basic information in the EHR to determine an individual’s future risk of developing EAC or GCA.
TAKEAWAY:
- With an area under the receiver operating characteristic of 0.77, K-ECAN demonstrated better discrimination than previously validated models and published guidelines.
- Using only data from 3 to 5 years prior to diagnosis only slightly diminished its accuracy (AUROC, 0.75).
- K-ECAN remained the most accurate tool when undersampling men to simulate a non-VHA population (AUROC, 0.85).
- Although gastroesophageal reflux disease (GERD) was strongly associated with EAC, it only contributed a small proportion of gain in information for prediction.
IN PRACTICE:
Because K-ECAN does not rely heavily on GERD symptoms to assess risk, it has the “potential to guide providers to increase appropriate uptake of screening. De-emphasizing GERD in decisions to offer screening could paradoxically increase appropriate uptake of screening for EAC and GCA,” the authors wrote.
SOURCE:
The study, with first author Joel H. Rubenstein, MD, with the LTC Charles S. Kettles VA Medical Center, Ann Arbor, Mich., was published online in Gastroenterology.
LIMITATIONS:
K-ECAN was developed and validated among U.S. veterans and needs to be validated in other populations.
DISCLOSURES:
Funding for the study was provided by the Department of Defense. Dr. Rubenstein has received research support from Lucid Diagnostics.
A version of this article first appeared on Medscape.com.
TOPLINE:
and is more accurate than other tools, a large study suggests.
METHODOLOGY:
- Researchers performed a case-control study using data from the Veterans Affairs Central Cancer Registry.
- They identified 8,430 patients with EAC and 2,965 patients GCA; these patients were compared with more than 10 million control patients.
- K-ECAN uses basic information in the EHR to determine an individual’s future risk of developing EAC or GCA.
TAKEAWAY:
- With an area under the receiver operating characteristic of 0.77, K-ECAN demonstrated better discrimination than previously validated models and published guidelines.
- Using only data from 3 to 5 years prior to diagnosis only slightly diminished its accuracy (AUROC, 0.75).
- K-ECAN remained the most accurate tool when undersampling men to simulate a non-VHA population (AUROC, 0.85).
- Although gastroesophageal reflux disease (GERD) was strongly associated with EAC, it only contributed a small proportion of gain in information for prediction.
IN PRACTICE:
Because K-ECAN does not rely heavily on GERD symptoms to assess risk, it has the “potential to guide providers to increase appropriate uptake of screening. De-emphasizing GERD in decisions to offer screening could paradoxically increase appropriate uptake of screening for EAC and GCA,” the authors wrote.
SOURCE:
The study, with first author Joel H. Rubenstein, MD, with the LTC Charles S. Kettles VA Medical Center, Ann Arbor, Mich., was published online in Gastroenterology.
LIMITATIONS:
K-ECAN was developed and validated among U.S. veterans and needs to be validated in other populations.
DISCLOSURES:
Funding for the study was provided by the Department of Defense. Dr. Rubenstein has received research support from Lucid Diagnostics.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
‘Game changer’ data for vitamin D in digestive tract cancers
In the p53-immunoreactive subgroup, daily vitamin D supplementation reduced the risk of relapse or death by 73%. Overall, the 5-year relapse-free survival (RFS) among those receiving vitamin D was 81% vs. almost 31% in the placebo group.
Vitamin D supplementation, however, had no effect on survival outcomes in the non–p53-immunoreactive subgroup.
These findings represent a “game changer” for vitamin D and cancer, Michael Holick, PhD, MD, with Boston University, said in an editorial accompanying the study, published online in JAMA Network Open. The AMATERASU trial “provides an additional variable in our understanding of whether improving vitamin D status has any benefit for reducing risk of developing cancer as well as improving relapse-free and mortality outcomes.”
A growing body of research suggests that vitamin D supplementation may reduce the risk of cancer mortality, but the evidence remains mixed and efficacy may hinge on a patient’s tumor biology, specifically the p53 protein, the authors of the current analysis explained.
A 2019 randomized controlled trial from the research team found vitamin D supplements of 2000 IU/day did not improve RFS at 5 years in patients with digestive tract cancers. However, a post hoc analysis of the AMATERASU trial published in 2020 suggested that vitamin D supplementation improved RFS in a subgroup of patients with p53-positive digestive tract cancers, as seen using immunohistochemistry (IHC) staining (79% vs. 57% in the placebo group; hazard ratio, 0.52; P = .02).
In the current post hoc analysis of the AMATERASU trial, the research team explored whether vitamin D supplementation reduced the risk of relapse or death in the subgroup of patients who were p53 immunoreactive, defined as positivity for both nuclear accumulation of the p53 protein in more than 99% of cancer cells, as seen on IHC staining, as well as anti-p53 antibodies in serum.
In the trial, patients with stage I-III luminal gastrointestinal cancer who had undergone complete tumor resection were randomly assigned to receive placebo or oral vitamin D supplements of 2,000 IU/day from their first postoperative visit through the end of the trial, up to 8 years.
The current post hoc analysis by p53-immunoreactive status included 392 patients, of whom 47% had colorectal cancer, 43% had gastric cancer, 9% had esophageal cancer, and 0.5% had small-bowel cancer.
The post hoc analysis found that, among the p53-immunoreactive subgroup of 80 patients, relapse or death occurred in 9 of 54 patients (17%) in the vitamin D group and 14 of 26 patients (54%) in the placebo group. The 5-year RFS was significantly higher in the vitamin D group than the placebo group (81% vs. 31%; HR, 0.27; P = .002).
This was not the case in the 272 patients in the non–p53-immunoreactive subgroup. In this group, vitamin D supplementation had no apparent effect on 5-year RFS, compared with placebo (22% vs. 21%; HR, 1.09; 95% confidence interval, 0.65-1.84).
The main findings of this study were that daily supplementation of 2000 IU of vitamin D reduced the risk of relapse or death, compared with placebo, in the p53-immunoreactive subgroup, and they “suggest the importance of developing cancer immunotherapy targeting mutated p53 proteins,” study investigator Mitsuyoshi Urashima, MD, PhD, MPH, with Jikei University, Tokyo, and colleagues concluded.
Support for the study was provided by the Japan-Supported Program for the Strategic Research Foundation at Private Universities and a grant from the Japan Society for the Promotion of Science of the Ministry of Education, Culture, Sports, Science, and Technology. The authors report no relevant financial relationships. Dr. Holick reported grants from Carbogen Amcis and Solius; personal fees from Biogena, Sanofi, Faes Farma, Eric Anthony Nepute, and others; nonfinancial support from Ontometrics outside the submitted work; and had a patent for Novel Use of 25 hydroxy vitamin D pending for Carbogen Amcis BV and Aamanya AG.
A version of this article appeared on Medscape.com.
In the p53-immunoreactive subgroup, daily vitamin D supplementation reduced the risk of relapse or death by 73%. Overall, the 5-year relapse-free survival (RFS) among those receiving vitamin D was 81% vs. almost 31% in the placebo group.
Vitamin D supplementation, however, had no effect on survival outcomes in the non–p53-immunoreactive subgroup.
These findings represent a “game changer” for vitamin D and cancer, Michael Holick, PhD, MD, with Boston University, said in an editorial accompanying the study, published online in JAMA Network Open. The AMATERASU trial “provides an additional variable in our understanding of whether improving vitamin D status has any benefit for reducing risk of developing cancer as well as improving relapse-free and mortality outcomes.”
A growing body of research suggests that vitamin D supplementation may reduce the risk of cancer mortality, but the evidence remains mixed and efficacy may hinge on a patient’s tumor biology, specifically the p53 protein, the authors of the current analysis explained.
A 2019 randomized controlled trial from the research team found vitamin D supplements of 2000 IU/day did not improve RFS at 5 years in patients with digestive tract cancers. However, a post hoc analysis of the AMATERASU trial published in 2020 suggested that vitamin D supplementation improved RFS in a subgroup of patients with p53-positive digestive tract cancers, as seen using immunohistochemistry (IHC) staining (79% vs. 57% in the placebo group; hazard ratio, 0.52; P = .02).
In the current post hoc analysis of the AMATERASU trial, the research team explored whether vitamin D supplementation reduced the risk of relapse or death in the subgroup of patients who were p53 immunoreactive, defined as positivity for both nuclear accumulation of the p53 protein in more than 99% of cancer cells, as seen on IHC staining, as well as anti-p53 antibodies in serum.
In the trial, patients with stage I-III luminal gastrointestinal cancer who had undergone complete tumor resection were randomly assigned to receive placebo or oral vitamin D supplements of 2,000 IU/day from their first postoperative visit through the end of the trial, up to 8 years.
The current post hoc analysis by p53-immunoreactive status included 392 patients, of whom 47% had colorectal cancer, 43% had gastric cancer, 9% had esophageal cancer, and 0.5% had small-bowel cancer.
The post hoc analysis found that, among the p53-immunoreactive subgroup of 80 patients, relapse or death occurred in 9 of 54 patients (17%) in the vitamin D group and 14 of 26 patients (54%) in the placebo group. The 5-year RFS was significantly higher in the vitamin D group than the placebo group (81% vs. 31%; HR, 0.27; P = .002).
This was not the case in the 272 patients in the non–p53-immunoreactive subgroup. In this group, vitamin D supplementation had no apparent effect on 5-year RFS, compared with placebo (22% vs. 21%; HR, 1.09; 95% confidence interval, 0.65-1.84).
The main findings of this study were that daily supplementation of 2000 IU of vitamin D reduced the risk of relapse or death, compared with placebo, in the p53-immunoreactive subgroup, and they “suggest the importance of developing cancer immunotherapy targeting mutated p53 proteins,” study investigator Mitsuyoshi Urashima, MD, PhD, MPH, with Jikei University, Tokyo, and colleagues concluded.
Support for the study was provided by the Japan-Supported Program for the Strategic Research Foundation at Private Universities and a grant from the Japan Society for the Promotion of Science of the Ministry of Education, Culture, Sports, Science, and Technology. The authors report no relevant financial relationships. Dr. Holick reported grants from Carbogen Amcis and Solius; personal fees from Biogena, Sanofi, Faes Farma, Eric Anthony Nepute, and others; nonfinancial support from Ontometrics outside the submitted work; and had a patent for Novel Use of 25 hydroxy vitamin D pending for Carbogen Amcis BV and Aamanya AG.
A version of this article appeared on Medscape.com.
In the p53-immunoreactive subgroup, daily vitamin D supplementation reduced the risk of relapse or death by 73%. Overall, the 5-year relapse-free survival (RFS) among those receiving vitamin D was 81% vs. almost 31% in the placebo group.
Vitamin D supplementation, however, had no effect on survival outcomes in the non–p53-immunoreactive subgroup.
These findings represent a “game changer” for vitamin D and cancer, Michael Holick, PhD, MD, with Boston University, said in an editorial accompanying the study, published online in JAMA Network Open. The AMATERASU trial “provides an additional variable in our understanding of whether improving vitamin D status has any benefit for reducing risk of developing cancer as well as improving relapse-free and mortality outcomes.”
A growing body of research suggests that vitamin D supplementation may reduce the risk of cancer mortality, but the evidence remains mixed and efficacy may hinge on a patient’s tumor biology, specifically the p53 protein, the authors of the current analysis explained.
A 2019 randomized controlled trial from the research team found vitamin D supplements of 2000 IU/day did not improve RFS at 5 years in patients with digestive tract cancers. However, a post hoc analysis of the AMATERASU trial published in 2020 suggested that vitamin D supplementation improved RFS in a subgroup of patients with p53-positive digestive tract cancers, as seen using immunohistochemistry (IHC) staining (79% vs. 57% in the placebo group; hazard ratio, 0.52; P = .02).
In the current post hoc analysis of the AMATERASU trial, the research team explored whether vitamin D supplementation reduced the risk of relapse or death in the subgroup of patients who were p53 immunoreactive, defined as positivity for both nuclear accumulation of the p53 protein in more than 99% of cancer cells, as seen on IHC staining, as well as anti-p53 antibodies in serum.
In the trial, patients with stage I-III luminal gastrointestinal cancer who had undergone complete tumor resection were randomly assigned to receive placebo or oral vitamin D supplements of 2,000 IU/day from their first postoperative visit through the end of the trial, up to 8 years.
The current post hoc analysis by p53-immunoreactive status included 392 patients, of whom 47% had colorectal cancer, 43% had gastric cancer, 9% had esophageal cancer, and 0.5% had small-bowel cancer.
The post hoc analysis found that, among the p53-immunoreactive subgroup of 80 patients, relapse or death occurred in 9 of 54 patients (17%) in the vitamin D group and 14 of 26 patients (54%) in the placebo group. The 5-year RFS was significantly higher in the vitamin D group than the placebo group (81% vs. 31%; HR, 0.27; P = .002).
This was not the case in the 272 patients in the non–p53-immunoreactive subgroup. In this group, vitamin D supplementation had no apparent effect on 5-year RFS, compared with placebo (22% vs. 21%; HR, 1.09; 95% confidence interval, 0.65-1.84).
The main findings of this study were that daily supplementation of 2000 IU of vitamin D reduced the risk of relapse or death, compared with placebo, in the p53-immunoreactive subgroup, and they “suggest the importance of developing cancer immunotherapy targeting mutated p53 proteins,” study investigator Mitsuyoshi Urashima, MD, PhD, MPH, with Jikei University, Tokyo, and colleagues concluded.
Support for the study was provided by the Japan-Supported Program for the Strategic Research Foundation at Private Universities and a grant from the Japan Society for the Promotion of Science of the Ministry of Education, Culture, Sports, Science, and Technology. The authors report no relevant financial relationships. Dr. Holick reported grants from Carbogen Amcis and Solius; personal fees from Biogena, Sanofi, Faes Farma, Eric Anthony Nepute, and others; nonfinancial support from Ontometrics outside the submitted work; and had a patent for Novel Use of 25 hydroxy vitamin D pending for Carbogen Amcis BV and Aamanya AG.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
FDA okays first biosimilar for multiple sclerosis
including clinically isolated syndrome, relapsing remitting MS, and active secondary progressive disease.
“Biosimilar medications offer additional effective treatment options that have the potential to increase access for people living with relapsing forms of multiple sclerosis. [This] approval could have a meaningful impact for patients managing their disease,” Paul R. Lee, MD, PhD, director of the division of neurology II, FDA Center for Drug Evaluation and Research, said in a statement.
The natalizumab biosimilar is given using the same dosing and administration schedule. Like the reference product, it is indicated for adults with moderately to severely active Crohn’s disease unresponsive to other medications.
The approval of the natalizumab biosimilar is based on results of the phase 3 Antelope trial, which showed no clinically meaningful differences between it and the reference product.
The trial included 264 adults (mean age, 36 years; 61% women) with relapsing remitting MS from 48 centers in seven Eastern European countries.
All were randomly assigned to receive intravenous infusions every 4 weeks of 300 mg of the natalizumab biosimilar or the reference product for a total of 12 infusions.
At 24 and 48 weeks, there were no between-group differences in annualized relapse rates or Expanded Disability Status Scale scores, which were similar between treatment groups at baseline. There were also no significant differences in safety, tolerability, or immunogenicity.
The prescribing information for both natalizumab products includes a boxed warning about the increased risk of progressive multifocal leukoencephalopathy (PML), a viral infection of the brain that usually leads to death or severe disability.
Risk factors for the development of PML include the presence of antibodies to the JC virus, longer duration of therapy, and prior use of immunosuppressants.
“These factors should be considered in the context of expected benefit when initiating and continuing treatment with natalizumab products, and health care providers should monitor patients and withhold treatment immediately at the first sign or symptom suggestive of PML,” the FDA advises.
Because of the risks of PML, natalizumab products are available only through a restricted drug distribution program under a risk evaluation and mitigation strategy.
In a statement, Sandoz said it’s committed to having the product available in the United States “as soon as possible.”
A version of this article appeared on Medscape.com.
including clinically isolated syndrome, relapsing remitting MS, and active secondary progressive disease.
“Biosimilar medications offer additional effective treatment options that have the potential to increase access for people living with relapsing forms of multiple sclerosis. [This] approval could have a meaningful impact for patients managing their disease,” Paul R. Lee, MD, PhD, director of the division of neurology II, FDA Center for Drug Evaluation and Research, said in a statement.
The natalizumab biosimilar is given using the same dosing and administration schedule. Like the reference product, it is indicated for adults with moderately to severely active Crohn’s disease unresponsive to other medications.
The approval of the natalizumab biosimilar is based on results of the phase 3 Antelope trial, which showed no clinically meaningful differences between it and the reference product.
The trial included 264 adults (mean age, 36 years; 61% women) with relapsing remitting MS from 48 centers in seven Eastern European countries.
All were randomly assigned to receive intravenous infusions every 4 weeks of 300 mg of the natalizumab biosimilar or the reference product for a total of 12 infusions.
At 24 and 48 weeks, there were no between-group differences in annualized relapse rates or Expanded Disability Status Scale scores, which were similar between treatment groups at baseline. There were also no significant differences in safety, tolerability, or immunogenicity.
The prescribing information for both natalizumab products includes a boxed warning about the increased risk of progressive multifocal leukoencephalopathy (PML), a viral infection of the brain that usually leads to death or severe disability.
Risk factors for the development of PML include the presence of antibodies to the JC virus, longer duration of therapy, and prior use of immunosuppressants.
“These factors should be considered in the context of expected benefit when initiating and continuing treatment with natalizumab products, and health care providers should monitor patients and withhold treatment immediately at the first sign or symptom suggestive of PML,” the FDA advises.
Because of the risks of PML, natalizumab products are available only through a restricted drug distribution program under a risk evaluation and mitigation strategy.
In a statement, Sandoz said it’s committed to having the product available in the United States “as soon as possible.”
A version of this article appeared on Medscape.com.
including clinically isolated syndrome, relapsing remitting MS, and active secondary progressive disease.
“Biosimilar medications offer additional effective treatment options that have the potential to increase access for people living with relapsing forms of multiple sclerosis. [This] approval could have a meaningful impact for patients managing their disease,” Paul R. Lee, MD, PhD, director of the division of neurology II, FDA Center for Drug Evaluation and Research, said in a statement.
The natalizumab biosimilar is given using the same dosing and administration schedule. Like the reference product, it is indicated for adults with moderately to severely active Crohn’s disease unresponsive to other medications.
The approval of the natalizumab biosimilar is based on results of the phase 3 Antelope trial, which showed no clinically meaningful differences between it and the reference product.
The trial included 264 adults (mean age, 36 years; 61% women) with relapsing remitting MS from 48 centers in seven Eastern European countries.
All were randomly assigned to receive intravenous infusions every 4 weeks of 300 mg of the natalizumab biosimilar or the reference product for a total of 12 infusions.
At 24 and 48 weeks, there were no between-group differences in annualized relapse rates or Expanded Disability Status Scale scores, which were similar between treatment groups at baseline. There were also no significant differences in safety, tolerability, or immunogenicity.
The prescribing information for both natalizumab products includes a boxed warning about the increased risk of progressive multifocal leukoencephalopathy (PML), a viral infection of the brain that usually leads to death or severe disability.
Risk factors for the development of PML include the presence of antibodies to the JC virus, longer duration of therapy, and prior use of immunosuppressants.
“These factors should be considered in the context of expected benefit when initiating and continuing treatment with natalizumab products, and health care providers should monitor patients and withhold treatment immediately at the first sign or symptom suggestive of PML,” the FDA advises.
Because of the risks of PML, natalizumab products are available only through a restricted drug distribution program under a risk evaluation and mitigation strategy.
In a statement, Sandoz said it’s committed to having the product available in the United States “as soon as possible.”
A version of this article appeared on Medscape.com.
Risky drinking common in cancer survivors
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
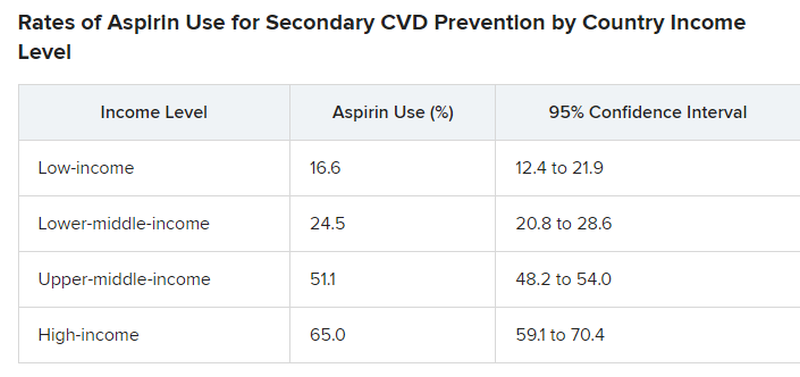
Aspirin for secondary CVD prevention underused worldwide
Nationally representative survey data from 51 countries showed that fewer than half of eligible people overall, including less than one-quarter in low-income and lower-middle–income countries, were taking aspirin for secondary CVD prevention.
“Our findings were not surprising but rather disappointing,” first author Sang Gune Yoo, MD, fellow in cardiovascular disease at Washington University in St Louis, said in an interview.
“We had hoped that the rates of aspirin use for secondary prevention would have increased after decades of effort to promote cardiovascular health worldwide,” Dr. Yoo said.
In high-income countries, such as the United States, rates of aspirin use for secondary CVD prevention were higher – at around 65% – but that’s also “really low and not particularly good or anything to be proud of,” Deepak Bhatt, MD, MPH, director of Mount Sinai Heart, New York, who wasn’t involved in the study, said in an interview.
The study was published online in JAMA. It provides the most extensive and up-to-date estimates of the worldwide use of aspirin for secondary prevention of CVD.
The researchers did a cross-sectional analysis using pooled, individual participant data from nationally representative health surveys conducted from 2013 to 2020 in 51 low-, middle-, and high-income countries.
The overall pooled sample included 124,505 nonpregnant adults (mean age, 52 years; 51% women). A total of 10,589 (8.1%) had a self-reported history of CVD and about 40% of these individuals were taking aspirin.
However, rates differed markedly by country, with use rates lowest in low-income and lower-middle–income countries and highest in upper-middle–income and high-income countries.
Primary vs. secondary prevention
The study did not explore the factors or reasons behind suboptimal aspirin use for secondary CVD prevention.
For example, it did not investigate whether data demonstrating that aspirin is not helpful in primary prevention is having a negative effect on use rates for secondary prevention. However, “rates of aspirin use for secondary prevention were low previously and remain suboptimal,” Dr. Yoo said.
Dr. Bhatt said that the “suboptimal” use of aspirin for secondary prevention is “a bit perplexing because this is a medicine that’s familiar, the data in secondary prevention are broadly known to physicians and it’s a cheap medicine so we can’t, in this case, blame high cost.”
Dr. Bhatt said it’s possible that coverage in the lay media of “negative” aspirin trials that may not distinguish between a primary and secondary prevention trial may contribute to confusion about aspirin. “In some cases, the doctor may think the patient is taking aspirin, but self discontinues it based on something they read or saw on the Internet.”
Dr. Yoo and colleagues said that, to meet the goal of reducing premature mortality from noncommunicable diseases, including CVD, “national health policies and health systems must develop, implement and evaluate strategies to promote evidence-based use of aspirin.”
“Strategies to boost appropriate aspirin use must be contextualized to the country and its health system,” Dr. Yoo added.
The study had no commercial funding. Dr. Yoo has disclosed no relevant financial relationships. Dr. Bhatt disclosed receiving grants and/or personal fees from many companies, publications, and organizations.
A version of this article appeared on Medscape.com.
Nationally representative survey data from 51 countries showed that fewer than half of eligible people overall, including less than one-quarter in low-income and lower-middle–income countries, were taking aspirin for secondary CVD prevention.
“Our findings were not surprising but rather disappointing,” first author Sang Gune Yoo, MD, fellow in cardiovascular disease at Washington University in St Louis, said in an interview.
“We had hoped that the rates of aspirin use for secondary prevention would have increased after decades of effort to promote cardiovascular health worldwide,” Dr. Yoo said.
In high-income countries, such as the United States, rates of aspirin use for secondary CVD prevention were higher – at around 65% – but that’s also “really low and not particularly good or anything to be proud of,” Deepak Bhatt, MD, MPH, director of Mount Sinai Heart, New York, who wasn’t involved in the study, said in an interview.
The study was published online in JAMA. It provides the most extensive and up-to-date estimates of the worldwide use of aspirin for secondary prevention of CVD.
The researchers did a cross-sectional analysis using pooled, individual participant data from nationally representative health surveys conducted from 2013 to 2020 in 51 low-, middle-, and high-income countries.
The overall pooled sample included 124,505 nonpregnant adults (mean age, 52 years; 51% women). A total of 10,589 (8.1%) had a self-reported history of CVD and about 40% of these individuals were taking aspirin.
However, rates differed markedly by country, with use rates lowest in low-income and lower-middle–income countries and highest in upper-middle–income and high-income countries.
Primary vs. secondary prevention
The study did not explore the factors or reasons behind suboptimal aspirin use for secondary CVD prevention.
For example, it did not investigate whether data demonstrating that aspirin is not helpful in primary prevention is having a negative effect on use rates for secondary prevention. However, “rates of aspirin use for secondary prevention were low previously and remain suboptimal,” Dr. Yoo said.
Dr. Bhatt said that the “suboptimal” use of aspirin for secondary prevention is “a bit perplexing because this is a medicine that’s familiar, the data in secondary prevention are broadly known to physicians and it’s a cheap medicine so we can’t, in this case, blame high cost.”
Dr. Bhatt said it’s possible that coverage in the lay media of “negative” aspirin trials that may not distinguish between a primary and secondary prevention trial may contribute to confusion about aspirin. “In some cases, the doctor may think the patient is taking aspirin, but self discontinues it based on something they read or saw on the Internet.”
Dr. Yoo and colleagues said that, to meet the goal of reducing premature mortality from noncommunicable diseases, including CVD, “national health policies and health systems must develop, implement and evaluate strategies to promote evidence-based use of aspirin.”
“Strategies to boost appropriate aspirin use must be contextualized to the country and its health system,” Dr. Yoo added.
The study had no commercial funding. Dr. Yoo has disclosed no relevant financial relationships. Dr. Bhatt disclosed receiving grants and/or personal fees from many companies, publications, and organizations.
A version of this article appeared on Medscape.com.
Nationally representative survey data from 51 countries showed that fewer than half of eligible people overall, including less than one-quarter in low-income and lower-middle–income countries, were taking aspirin for secondary CVD prevention.
“Our findings were not surprising but rather disappointing,” first author Sang Gune Yoo, MD, fellow in cardiovascular disease at Washington University in St Louis, said in an interview.
“We had hoped that the rates of aspirin use for secondary prevention would have increased after decades of effort to promote cardiovascular health worldwide,” Dr. Yoo said.
In high-income countries, such as the United States, rates of aspirin use for secondary CVD prevention were higher – at around 65% – but that’s also “really low and not particularly good or anything to be proud of,” Deepak Bhatt, MD, MPH, director of Mount Sinai Heart, New York, who wasn’t involved in the study, said in an interview.
The study was published online in JAMA. It provides the most extensive and up-to-date estimates of the worldwide use of aspirin for secondary prevention of CVD.
The researchers did a cross-sectional analysis using pooled, individual participant data from nationally representative health surveys conducted from 2013 to 2020 in 51 low-, middle-, and high-income countries.
The overall pooled sample included 124,505 nonpregnant adults (mean age, 52 years; 51% women). A total of 10,589 (8.1%) had a self-reported history of CVD and about 40% of these individuals were taking aspirin.
However, rates differed markedly by country, with use rates lowest in low-income and lower-middle–income countries and highest in upper-middle–income and high-income countries.
Primary vs. secondary prevention
The study did not explore the factors or reasons behind suboptimal aspirin use for secondary CVD prevention.
For example, it did not investigate whether data demonstrating that aspirin is not helpful in primary prevention is having a negative effect on use rates for secondary prevention. However, “rates of aspirin use for secondary prevention were low previously and remain suboptimal,” Dr. Yoo said.
Dr. Bhatt said that the “suboptimal” use of aspirin for secondary prevention is “a bit perplexing because this is a medicine that’s familiar, the data in secondary prevention are broadly known to physicians and it’s a cheap medicine so we can’t, in this case, blame high cost.”
Dr. Bhatt said it’s possible that coverage in the lay media of “negative” aspirin trials that may not distinguish between a primary and secondary prevention trial may contribute to confusion about aspirin. “In some cases, the doctor may think the patient is taking aspirin, but self discontinues it based on something they read or saw on the Internet.”
Dr. Yoo and colleagues said that, to meet the goal of reducing premature mortality from noncommunicable diseases, including CVD, “national health policies and health systems must develop, implement and evaluate strategies to promote evidence-based use of aspirin.”
“Strategies to boost appropriate aspirin use must be contextualized to the country and its health system,” Dr. Yoo added.
The study had no commercial funding. Dr. Yoo has disclosed no relevant financial relationships. Dr. Bhatt disclosed receiving grants and/or personal fees from many companies, publications, and organizations.
A version of this article appeared on Medscape.com.
FROM JAMA
Big geographic access gaps for oncofertility services in U.S.
TOPLINE:
, a study shows.
METHODOLOGY:
- In this cross-sectional analysis, researchers identified 370 fertility centers in the United States (361 in the continental U.S.) that provide oncofertility services and that met criteria for an oncofertility center.
- Clinics were considered oncofertility centers if they offered oocyte cryopreservation, had performed at least one fertility preservation cycle in 2018, reported serving people without partners, and had an embryology laboratory that was accredited per Centers for Disease Control and Prevention guidelines.
- Researchers then quantified the number of young women potentially eligible for oncofertility services who lived farther than 2 hours from an oncofertility center.
- In a secondary analysis, the team assessed the association between geographic access and state fertility preservation insurance mandates.
TAKEAWAY:
- Overall, 3.63 million (5.7%) young women of reproductive age in the United States (aged 15-4) live more than 2 hours from an oncofertility center.
- The greatest gaps in access are in the Mountain West, West North Central, and Southwest regions; for instance, in Montana, North Dakota, and Wyoming fewer than half of the at-risk population has geographic access to an oncofertility center.
- Among the 11 states with fertility preservation insurance mandates, 98.5% of at-risk women have geographic access to an oncofertility center; in the 17 states without fertility preservation legislation, 79.6% of at-risk women have access to an oncofertility center.
IN PRACTICE:
Just over 3.6 million “reproductive-age female individuals lack geographic access to oncofertility services, especially in the Mountain West and West North Central regions,” the authors concluded. “Significant geographic disparities in access to fertility preservation in the U.S. require strategic expansion of care, especially given the growing demand for oncofertility services.”
SOURCE:
The study, led by Benjamin Peipert, MD, with Duke University, Durham, N.C., was published online in JAMA Oncology.
LIMITATIONS:
The authors relied on clinic data reported to the CDC, made assumptions about reasonable travel time, and may have underestimated access in some areas.
DISCLOSURES:
The study had no commercial funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a study shows.
METHODOLOGY:
- In this cross-sectional analysis, researchers identified 370 fertility centers in the United States (361 in the continental U.S.) that provide oncofertility services and that met criteria for an oncofertility center.
- Clinics were considered oncofertility centers if they offered oocyte cryopreservation, had performed at least one fertility preservation cycle in 2018, reported serving people without partners, and had an embryology laboratory that was accredited per Centers for Disease Control and Prevention guidelines.
- Researchers then quantified the number of young women potentially eligible for oncofertility services who lived farther than 2 hours from an oncofertility center.
- In a secondary analysis, the team assessed the association between geographic access and state fertility preservation insurance mandates.
TAKEAWAY:
- Overall, 3.63 million (5.7%) young women of reproductive age in the United States (aged 15-4) live more than 2 hours from an oncofertility center.
- The greatest gaps in access are in the Mountain West, West North Central, and Southwest regions; for instance, in Montana, North Dakota, and Wyoming fewer than half of the at-risk population has geographic access to an oncofertility center.
- Among the 11 states with fertility preservation insurance mandates, 98.5% of at-risk women have geographic access to an oncofertility center; in the 17 states without fertility preservation legislation, 79.6% of at-risk women have access to an oncofertility center.
IN PRACTICE:
Just over 3.6 million “reproductive-age female individuals lack geographic access to oncofertility services, especially in the Mountain West and West North Central regions,” the authors concluded. “Significant geographic disparities in access to fertility preservation in the U.S. require strategic expansion of care, especially given the growing demand for oncofertility services.”
SOURCE:
The study, led by Benjamin Peipert, MD, with Duke University, Durham, N.C., was published online in JAMA Oncology.
LIMITATIONS:
The authors relied on clinic data reported to the CDC, made assumptions about reasonable travel time, and may have underestimated access in some areas.
DISCLOSURES:
The study had no commercial funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a study shows.
METHODOLOGY:
- In this cross-sectional analysis, researchers identified 370 fertility centers in the United States (361 in the continental U.S.) that provide oncofertility services and that met criteria for an oncofertility center.
- Clinics were considered oncofertility centers if they offered oocyte cryopreservation, had performed at least one fertility preservation cycle in 2018, reported serving people without partners, and had an embryology laboratory that was accredited per Centers for Disease Control and Prevention guidelines.
- Researchers then quantified the number of young women potentially eligible for oncofertility services who lived farther than 2 hours from an oncofertility center.
- In a secondary analysis, the team assessed the association between geographic access and state fertility preservation insurance mandates.
TAKEAWAY:
- Overall, 3.63 million (5.7%) young women of reproductive age in the United States (aged 15-4) live more than 2 hours from an oncofertility center.
- The greatest gaps in access are in the Mountain West, West North Central, and Southwest regions; for instance, in Montana, North Dakota, and Wyoming fewer than half of the at-risk population has geographic access to an oncofertility center.
- Among the 11 states with fertility preservation insurance mandates, 98.5% of at-risk women have geographic access to an oncofertility center; in the 17 states without fertility preservation legislation, 79.6% of at-risk women have access to an oncofertility center.
IN PRACTICE:
Just over 3.6 million “reproductive-age female individuals lack geographic access to oncofertility services, especially in the Mountain West and West North Central regions,” the authors concluded. “Significant geographic disparities in access to fertility preservation in the U.S. require strategic expansion of care, especially given the growing demand for oncofertility services.”
SOURCE:
The study, led by Benjamin Peipert, MD, with Duke University, Durham, N.C., was published online in JAMA Oncology.
LIMITATIONS:
The authors relied on clinic data reported to the CDC, made assumptions about reasonable travel time, and may have underestimated access in some areas.
DISCLOSURES:
The study had no commercial funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Most with early AD not eligible for new antiamyloid drugs
Only a small fraction of older adults in the early stages of Alzheimer’s disease (AD) meet eligibility criteria to receive treatment with newly approved antiamyloid drugs, largely because of the presence of medical conditions or neuroimaging findings, new research shows.
Applying the clinical trial criteria, only about 8%-17% of amyloid-positive individuals with early AD would be eligible for lecanemab (Leqembi), and even fewer, 5%-9%, would be eligible for aducanumab (Aduhelm), the researchers found.
This study highlights the “limited suitability” of most adults with mild cognitive impairment (MCI) or mild dementia with elevated brain amyloid for treatment with these anti–beta amyloid monoclonal antibodies, write Maria Vassilaki, MD, PhD, and colleagues with Mayo Clinic, Rochester, Minn.
The study was published online in Neurology
The authors of an accompanying editorial write that this study “provides an important estimate of treatment eligibility for amyloid-lowering monoclonal antibodies for early AD to help health systems make realistic plans for providing these treatments.”
More real-world data needed
Dr. Vassilaki and colleagues applied eligibility criteria for lecanemab and aducanumab to 237 older adults with MCI or mild dementia and increased brain amyloid burden from the Mayo Clinic Study of Aging (MCSA). Their mean age was 80.9 years, 55% were men, and most were White.
After applying lecanemab’s inclusion criteria, less than half of the study population was eligible to receive treatment (112 of 237, or 47%).
A total of 21 people were excluded because of a body mass index less than 17 or greater than or equal to 35; 48 due to a Clinical Dementia Rating (CDR) global score other than 0.5 or 1.0; 46 because they did not meet WMS-R Logical Memory II scores for age; 8 because of a Mini Mental State Examination (MMSE) score outside the bounds of 22-30; and two because of a CDR memory score less than 0.5.
Applying lecanemab’s exclusion criteria further narrowed the number of eligible participants from 112 to 19 (8% of 237).
Notable exclusions included cardiopulmonary contraindications, central nervous system–related exclusions such as brain cancer, Parkinson’s disease, epilepsy or brain injury, imaging findings, and history of cancer.
The results were similar for aducanumab, with 104 of the 237 participants (44%) meeting the trial’s inclusion criteria. Applying aducanumab’s exclusion criteria further reduced the number of eligible participants to 12 (5% of 237).
A sensitivity analysis including participants with MCI, without CDR global, MMSE, or WMS-R Logical Memory II score restrictions, resulted in a somewhat higher percentage of eligible participants (17.4% for lecanemab and 8.9% for aducanumab).
Shared decision-making
“Clinicians and health systems should be aware that by applying the clinical trial criteria, a smaller percentage might be eligible for these treatments than originally anticipated,” Dr. Vassilaki told this news organization. To help clinicians, there are published recommendations for the appropriate use of these treatments, she noted.
Given that clinical trial participants are typically healthier than the general population, Dr. Vassilaki said that research is needed to examine the safety and efficacy of antiamyloid therapies in larger, more diverse populations as well as in less healthy populations, before these therapies may be more widely available to people with AD.
“We can take advantage of the postmarketing surveillance of side effects, and also enrollment of patients receiving these treatments to registries could provide us with data useful for any necessary adjustment to drug use,” Dr. Vassilaki told this news organization.
‘Sharp focus’
This study “brings the issue of eligibility for amyloid-lowering antibody treatment into sharp focus,” Matthew Howe, MD, PhD, with Butler Hospital Memory & Aging Program, Providence, R.I., and colleagues note in their editorial.
“The results underscore the importance of careful patient selection to help identify patients most likely to benefit from treatment and exclude those at risk for serious outcomes,” they write.
They also write that appropriate use recommendations for lecanemab and aducanumab “will be revisited as more real-world data emerge, especially about safety.”
For now, clinicians “must exercise clinical judgment in selecting patients for treatment with shared decision-making with patients and families,” they add.
The study was supported by the National Institutes of Health, the National Institute on Aging, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic, the Mayo Foundation for Medical Education and Research, the Liston Award, the GHR Foundation and the Schuler Foundation. Dr. Vassilaki has consulted for F. Hoffmann-La Roche and has equity ownership in Abbott Laboratories, Johnson & Johnson, Medtronic, Merck, and Amgen. Dr. Howe has no conflicts of interest.
A version of this article first appeared on Medscape.com.
Only a small fraction of older adults in the early stages of Alzheimer’s disease (AD) meet eligibility criteria to receive treatment with newly approved antiamyloid drugs, largely because of the presence of medical conditions or neuroimaging findings, new research shows.
Applying the clinical trial criteria, only about 8%-17% of amyloid-positive individuals with early AD would be eligible for lecanemab (Leqembi), and even fewer, 5%-9%, would be eligible for aducanumab (Aduhelm), the researchers found.
This study highlights the “limited suitability” of most adults with mild cognitive impairment (MCI) or mild dementia with elevated brain amyloid for treatment with these anti–beta amyloid monoclonal antibodies, write Maria Vassilaki, MD, PhD, and colleagues with Mayo Clinic, Rochester, Minn.
The study was published online in Neurology
The authors of an accompanying editorial write that this study “provides an important estimate of treatment eligibility for amyloid-lowering monoclonal antibodies for early AD to help health systems make realistic plans for providing these treatments.”
More real-world data needed
Dr. Vassilaki and colleagues applied eligibility criteria for lecanemab and aducanumab to 237 older adults with MCI or mild dementia and increased brain amyloid burden from the Mayo Clinic Study of Aging (MCSA). Their mean age was 80.9 years, 55% were men, and most were White.
After applying lecanemab’s inclusion criteria, less than half of the study population was eligible to receive treatment (112 of 237, or 47%).
A total of 21 people were excluded because of a body mass index less than 17 or greater than or equal to 35; 48 due to a Clinical Dementia Rating (CDR) global score other than 0.5 or 1.0; 46 because they did not meet WMS-R Logical Memory II scores for age; 8 because of a Mini Mental State Examination (MMSE) score outside the bounds of 22-30; and two because of a CDR memory score less than 0.5.
Applying lecanemab’s exclusion criteria further narrowed the number of eligible participants from 112 to 19 (8% of 237).
Notable exclusions included cardiopulmonary contraindications, central nervous system–related exclusions such as brain cancer, Parkinson’s disease, epilepsy or brain injury, imaging findings, and history of cancer.
The results were similar for aducanumab, with 104 of the 237 participants (44%) meeting the trial’s inclusion criteria. Applying aducanumab’s exclusion criteria further reduced the number of eligible participants to 12 (5% of 237).
A sensitivity analysis including participants with MCI, without CDR global, MMSE, or WMS-R Logical Memory II score restrictions, resulted in a somewhat higher percentage of eligible participants (17.4% for lecanemab and 8.9% for aducanumab).
Shared decision-making
“Clinicians and health systems should be aware that by applying the clinical trial criteria, a smaller percentage might be eligible for these treatments than originally anticipated,” Dr. Vassilaki told this news organization. To help clinicians, there are published recommendations for the appropriate use of these treatments, she noted.
Given that clinical trial participants are typically healthier than the general population, Dr. Vassilaki said that research is needed to examine the safety and efficacy of antiamyloid therapies in larger, more diverse populations as well as in less healthy populations, before these therapies may be more widely available to people with AD.
“We can take advantage of the postmarketing surveillance of side effects, and also enrollment of patients receiving these treatments to registries could provide us with data useful for any necessary adjustment to drug use,” Dr. Vassilaki told this news organization.
‘Sharp focus’
This study “brings the issue of eligibility for amyloid-lowering antibody treatment into sharp focus,” Matthew Howe, MD, PhD, with Butler Hospital Memory & Aging Program, Providence, R.I., and colleagues note in their editorial.
“The results underscore the importance of careful patient selection to help identify patients most likely to benefit from treatment and exclude those at risk for serious outcomes,” they write.
They also write that appropriate use recommendations for lecanemab and aducanumab “will be revisited as more real-world data emerge, especially about safety.”
For now, clinicians “must exercise clinical judgment in selecting patients for treatment with shared decision-making with patients and families,” they add.
The study was supported by the National Institutes of Health, the National Institute on Aging, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic, the Mayo Foundation for Medical Education and Research, the Liston Award, the GHR Foundation and the Schuler Foundation. Dr. Vassilaki has consulted for F. Hoffmann-La Roche and has equity ownership in Abbott Laboratories, Johnson & Johnson, Medtronic, Merck, and Amgen. Dr. Howe has no conflicts of interest.
A version of this article first appeared on Medscape.com.
Only a small fraction of older adults in the early stages of Alzheimer’s disease (AD) meet eligibility criteria to receive treatment with newly approved antiamyloid drugs, largely because of the presence of medical conditions or neuroimaging findings, new research shows.
Applying the clinical trial criteria, only about 8%-17% of amyloid-positive individuals with early AD would be eligible for lecanemab (Leqembi), and even fewer, 5%-9%, would be eligible for aducanumab (Aduhelm), the researchers found.
This study highlights the “limited suitability” of most adults with mild cognitive impairment (MCI) or mild dementia with elevated brain amyloid for treatment with these anti–beta amyloid monoclonal antibodies, write Maria Vassilaki, MD, PhD, and colleagues with Mayo Clinic, Rochester, Minn.
The study was published online in Neurology
The authors of an accompanying editorial write that this study “provides an important estimate of treatment eligibility for amyloid-lowering monoclonal antibodies for early AD to help health systems make realistic plans for providing these treatments.”
More real-world data needed
Dr. Vassilaki and colleagues applied eligibility criteria for lecanemab and aducanumab to 237 older adults with MCI or mild dementia and increased brain amyloid burden from the Mayo Clinic Study of Aging (MCSA). Their mean age was 80.9 years, 55% were men, and most were White.
After applying lecanemab’s inclusion criteria, less than half of the study population was eligible to receive treatment (112 of 237, or 47%).
A total of 21 people were excluded because of a body mass index less than 17 or greater than or equal to 35; 48 due to a Clinical Dementia Rating (CDR) global score other than 0.5 or 1.0; 46 because they did not meet WMS-R Logical Memory II scores for age; 8 because of a Mini Mental State Examination (MMSE) score outside the bounds of 22-30; and two because of a CDR memory score less than 0.5.
Applying lecanemab’s exclusion criteria further narrowed the number of eligible participants from 112 to 19 (8% of 237).
Notable exclusions included cardiopulmonary contraindications, central nervous system–related exclusions such as brain cancer, Parkinson’s disease, epilepsy or brain injury, imaging findings, and history of cancer.
The results were similar for aducanumab, with 104 of the 237 participants (44%) meeting the trial’s inclusion criteria. Applying aducanumab’s exclusion criteria further reduced the number of eligible participants to 12 (5% of 237).
A sensitivity analysis including participants with MCI, without CDR global, MMSE, or WMS-R Logical Memory II score restrictions, resulted in a somewhat higher percentage of eligible participants (17.4% for lecanemab and 8.9% for aducanumab).
Shared decision-making
“Clinicians and health systems should be aware that by applying the clinical trial criteria, a smaller percentage might be eligible for these treatments than originally anticipated,” Dr. Vassilaki told this news organization. To help clinicians, there are published recommendations for the appropriate use of these treatments, she noted.
Given that clinical trial participants are typically healthier than the general population, Dr. Vassilaki said that research is needed to examine the safety and efficacy of antiamyloid therapies in larger, more diverse populations as well as in less healthy populations, before these therapies may be more widely available to people with AD.
“We can take advantage of the postmarketing surveillance of side effects, and also enrollment of patients receiving these treatments to registries could provide us with data useful for any necessary adjustment to drug use,” Dr. Vassilaki told this news organization.
‘Sharp focus’
This study “brings the issue of eligibility for amyloid-lowering antibody treatment into sharp focus,” Matthew Howe, MD, PhD, with Butler Hospital Memory & Aging Program, Providence, R.I., and colleagues note in their editorial.
“The results underscore the importance of careful patient selection to help identify patients most likely to benefit from treatment and exclude those at risk for serious outcomes,” they write.
They also write that appropriate use recommendations for lecanemab and aducanumab “will be revisited as more real-world data emerge, especially about safety.”
For now, clinicians “must exercise clinical judgment in selecting patients for treatment with shared decision-making with patients and families,” they add.
The study was supported by the National Institutes of Health, the National Institute on Aging, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic, the Mayo Foundation for Medical Education and Research, the Liston Award, the GHR Foundation and the Schuler Foundation. Dr. Vassilaki has consulted for F. Hoffmann-La Roche and has equity ownership in Abbott Laboratories, Johnson & Johnson, Medtronic, Merck, and Amgen. Dr. Howe has no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Playing football linked to higher Parkinson’s risk
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a cross-sectional study of older men, former tackle football players had a 61% higher likelihood of reporting a diagnosis of parkinsonism or PD, compared with men who played non-football sports.
Longer duration of football participation and higher level of play (college and professional) were associated with higher risk.
Lead researcher Michael L. Alosco, PhD, director of the Boston University Alzheimer’s Disease Research Center, said it’s important to note that the findings are from a cohort of men “enriched” for having PD.
“These are people who are likely already concerned for or at risk for having this disease. We don’t yet know how our findings translate to the general population,” Dr. Alosco said in an interview.
The study was published online in JAMA Network Open.
Repetitive head impacts
Dating back to the 1920s, PD and parkinsonism an umbrella term that refers to motor symptoms associated with PD and other conditions have long been described in boxers who suffer repetitive head impacts.
Multiple studies have linked tackle football with progressive brain diseases such as chronic traumatic encephalopathy. Few studies, however, have investigated the association between participation in football and PD.
For their study, Dr. Alosco and colleagues leveraged data from Fox Insight, a longitudinal online study of some people with and some without PD that is sponsored by the Michael J. Fox Foundation for Parkinson’s Research.
They focused their analyses on 1,875 men (mean age, 67 years) who reported playing any organized sport. As noted, the cohort was enriched for parkinsonism or PD. A total of 1,602 (85%) had received a diagnosis of parkinsonism/PD, and 273 had not.
Altogether, 729 men had a history of playing tackle football, and 1,146 men played non-football sports (control group). Among the football players, 82% played at youth sports or at the high school level; 17% played at the college level; and fewer than 1% played at the pro or semi-pro level.
Among the football players, 648 (89%) reported a parkinsonism/PD diagnosis.
A history of playing football was associated with higher odds of reporting a parkinsonism/PD diagnosis (odds ratio, 1.61; 95% confidence interval, 1.19-2.17) after accounting for age, education level, history of diabetes and heart disease, body mass index (BMI), traumatic brain injury with loss of consciousness, and family history of PD.
Football players who had longer careers and who played at higher levels of competition were at increased risk of having parkinsonism or PD.
Playing one to four seasons yielded an OR of 1.39 (95% CI, 0.98-1.98). The OR was 2.18 (95% CI, 1.36-3.49) for playing five or more seasons.
Football players who competed at the college or professional level had nearly triple the odds of reporting a parkinsonism/PD diagnosis (OR, 2.93; 95% CI, 1.28-6.73), compared with athletes who played at the youth or high school level.
Age at first exposure to football was not associated with a parkinsonism/PD diagnosis.
The researchers cautioned that this was a convenience sample of mostly White people, and the sample was enriched for having PD – factors that limit the generalizability of the findings.
Also, diagnosis of PD was self-reported by participants through online assessments, and objective in-person evaluations were not conducted.
Unequivocal link?
“This is among the first and largest to look at the relationship between football and having a diagnosis of PD in a large cohort of people from the Fox Insight online study,” Dr. Alosco said.
He cautioned that “not all people who play football will develop later-life neurological problems. That being said, the study adds to the accumulating evidence that suggests playing football is one risk factor for the development of later-life brain diseases.
“This represents an opportunity to educate the communities on the potential risks of playing football (short and long term), including what we know and what we don’t know, so that people can make informed decisions on participating in tackle football and develop additional ways to mitigate risk,” Dr. Alosco said.
In a comment, Shaheen Lakhan, MD, PhD, a neurologist and researcher from Boston, said: “The emerging body of research leaves little doubt that engaging in football raises the risk of developing Parkinson’s disease and parkinsonism.
“This progressive line of investigation serves to enhance our understanding, unequivocally demonstrating that even participation in amateur football, including at the youth and high school levels, constitutes a significant risk factor for the onset of Parkinson’s disease,” said Dr. Lakhan, who was not involved in the study.
However, he said it’s “crucial to underscore that the statistics reveal a notable distinction: individuals who have a history of college or professional football play face odds nearly three times higher of receiving a diagnosis of parkinsonism or Parkinson’s disease when compared to their counterparts who engaged in football during their youth or high-school years.
“Ultimately, determinations regarding involvement in sports should be a collaborative endeavor involving parents, young athletes, and health care providers. It is incumbent upon physicians to equip parents and youth with a comprehensive comprehension of the potential risks and rewards inherent in football participation,” Dr. Lakhan said.
He added, though, that there are multifaceted advantages to playing football. “This pursuit nurtures cardiovascular well-being, fosters invaluable social interactions, cultivates teamwork, instills discipline through regimented routines, and hones a spectrum of physical proficiencies,” Dr. Lakhan said.
“It’s worth noting that a constellation of alternative sports, including track and field, swimming, soccer, baseball, and tennis, can be cogently discussed as substitutes, all while preserving the manifold benefits of athletic engagement,” Dr. Lakhan added.
The Fox Insight Study is funded by the Michael J. Fox Foundation for Parkinson’s Research. The study was conducted in collaboration with the Michael J. Fox Foundation for Parkinson’s Research, the sponsor of the Fox Insight study, which collected and aggregated data used in the study. It was also supported by the National Institute of Neurological Disorders and Stroke. Dr. Alosco received grants from the National Institutes of Health during the conduct of the study, an honorarium from the Michael J. Fox Foundation for work unrelated to the study, and royalties from Oxford University Press outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN