User login
Preoperative Insulin Intensification to Improve Day of Surgery Blood Glucose Control
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
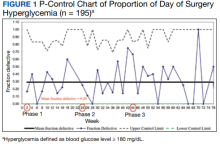
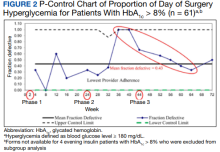
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
In the Lit: Research You Need to Know
Clinical question: Do hospitals caring for a higher volume of patients with congestive heart failure (CHF) provide better, more efficient care for those patients?
Background: For some surgical and cardiovascular procedures, higher procedure volumes have been associated with better outcomes and lower costs. It is unclear whether this association also exists for common medical conditions, such as CHF.
Study design: Retrospective cohort study.
Setting: National sample of Medicare fee-for-service patients 65 years or older.
Synopsis: National Medicare claims data for more than 1 million discharges from 4,095 hospitals were used to examine the relationship between hospital case volume and quality of care, outcomes, and cost for patients with CHF. Quality of care was defined using the Hospital Quality Alliance (HQA) data on four clinical process measures for CHF from 2007. Hospitals were grouped based on their number of CHF discharges during two years: low volume (25-200), medium volume (201-400), and high volume (>400). Risk adjustment was performed.
Hospitals in the low-volume group had lower performance on the process measures (80.2%) than did medium-volume (87.0%) or high-volume (89.1%) hospitals (P<0.001). Thirty-day mortality was highest in low-volume hospitals (10.2%), when compared to medium-volume (9.3%) and high-volume (8.6%) hospitals (P<0.001). Hospital costs were higher at high-volume hospitals ($8,300) than at medium-volume ($7,700) and low-volume ($7,300) hospitals (P<0.001). Readmission rates were not statistically different between hospital groups.
The relationship between volume and outcomes in the study was not linear, and the incremental benefits seen were small beyond the volume of patients seen at medium-volume hospitals.
Bottom line: Hospitals with higher volumes of CHF patients have better CHF process-of-care measures and lower 30-day CHF mortality but also higher CHF costs.
Citation: Joynt KE, Orav EJ, Jha AK. The association between hospital volume and processes, outcomes, and costs of care for congestive heart failure. Ann Intern Med. 2011;154(2):94-102.
For more physician reviews of HM-related research, visit our website.
Clinical question: Do hospitals caring for a higher volume of patients with congestive heart failure (CHF) provide better, more efficient care for those patients?
Background: For some surgical and cardiovascular procedures, higher procedure volumes have been associated with better outcomes and lower costs. It is unclear whether this association also exists for common medical conditions, such as CHF.
Study design: Retrospective cohort study.
Setting: National sample of Medicare fee-for-service patients 65 years or older.
Synopsis: National Medicare claims data for more than 1 million discharges from 4,095 hospitals were used to examine the relationship between hospital case volume and quality of care, outcomes, and cost for patients with CHF. Quality of care was defined using the Hospital Quality Alliance (HQA) data on four clinical process measures for CHF from 2007. Hospitals were grouped based on their number of CHF discharges during two years: low volume (25-200), medium volume (201-400), and high volume (>400). Risk adjustment was performed.
Hospitals in the low-volume group had lower performance on the process measures (80.2%) than did medium-volume (87.0%) or high-volume (89.1%) hospitals (P<0.001). Thirty-day mortality was highest in low-volume hospitals (10.2%), when compared to medium-volume (9.3%) and high-volume (8.6%) hospitals (P<0.001). Hospital costs were higher at high-volume hospitals ($8,300) than at medium-volume ($7,700) and low-volume ($7,300) hospitals (P<0.001). Readmission rates were not statistically different between hospital groups.
The relationship between volume and outcomes in the study was not linear, and the incremental benefits seen were small beyond the volume of patients seen at medium-volume hospitals.
Bottom line: Hospitals with higher volumes of CHF patients have better CHF process-of-care measures and lower 30-day CHF mortality but also higher CHF costs.
Citation: Joynt KE, Orav EJ, Jha AK. The association between hospital volume and processes, outcomes, and costs of care for congestive heart failure. Ann Intern Med. 2011;154(2):94-102.
For more physician reviews of HM-related research, visit our website.
Clinical question: Do hospitals caring for a higher volume of patients with congestive heart failure (CHF) provide better, more efficient care for those patients?
Background: For some surgical and cardiovascular procedures, higher procedure volumes have been associated with better outcomes and lower costs. It is unclear whether this association also exists for common medical conditions, such as CHF.
Study design: Retrospective cohort study.
Setting: National sample of Medicare fee-for-service patients 65 years or older.
Synopsis: National Medicare claims data for more than 1 million discharges from 4,095 hospitals were used to examine the relationship between hospital case volume and quality of care, outcomes, and cost for patients with CHF. Quality of care was defined using the Hospital Quality Alliance (HQA) data on four clinical process measures for CHF from 2007. Hospitals were grouped based on their number of CHF discharges during two years: low volume (25-200), medium volume (201-400), and high volume (>400). Risk adjustment was performed.
Hospitals in the low-volume group had lower performance on the process measures (80.2%) than did medium-volume (87.0%) or high-volume (89.1%) hospitals (P<0.001). Thirty-day mortality was highest in low-volume hospitals (10.2%), when compared to medium-volume (9.3%) and high-volume (8.6%) hospitals (P<0.001). Hospital costs were higher at high-volume hospitals ($8,300) than at medium-volume ($7,700) and low-volume ($7,300) hospitals (P<0.001). Readmission rates were not statistically different between hospital groups.
The relationship between volume and outcomes in the study was not linear, and the incremental benefits seen were small beyond the volume of patients seen at medium-volume hospitals.
Bottom line: Hospitals with higher volumes of CHF patients have better CHF process-of-care measures and lower 30-day CHF mortality but also higher CHF costs.
Citation: Joynt KE, Orav EJ, Jha AK. The association between hospital volume and processes, outcomes, and costs of care for congestive heart failure. Ann Intern Med. 2011;154(2):94-102.
For more physician reviews of HM-related research, visit our website.
In the Literature: HM-Related Research You Need to Know
Literature at a Glance
A guide to this month’s studies
- Rivaroxaban for VTE
- Cost-effectiveness of dabigatran in atrial fibrillation
- Effect of new resident duty-hour limits
- Outcomes of care at acute-stroke centers
- Effect on MIC in patients with MRSA pneumonia
- Optimal hemodialysis frequency
- Effect of BNP testing on hospital length of stay
- Impact of herpes zoster vaccination
- 30-day readmission rates in for-profit hospitals
Oral Rivaroxaban Could Play a Role in VTE Treatment
Clinical question: Is oral rivaroxaban an acceptable treatment option for acute symptomatic deep-vein thrombosis (DVT) and venous thromboembolism (VTE)?
Background: Treatment of acute DVT requires frequent laboratory monitoring, which may be obviated by the use of fixed-dose oral rivaroxaban.
Study designs: Parallel randomized, open-label, event-driven, noninferiority study (the acute DVT study) and randomized, double-blind, placebo-controlled, event-driven superiority trial (continued treatment study).
Setting: Multicenter study.
Synopsis: The acute DVT study randomly assigned 3,449 patients with acute DVT to oral rivaroxaban 15 mg twice daily for three weeks followed by 20 mg daily for three, six, or 12 months or enoxaparin 1 mg/kg subcutaneously twice daily and daily warfarin until a therapeutic INR was achieved, at which time the enoxaparin was discontinued. Rivaroxaban was not inferior in terms of preventing recurrent VTE (2.1% vs. 3.0%; P<0.001). Major or clinically relevant nonmajor bleeding occurred equally in both groups (8.1%).
The continued treatment study randomly assigned 1,196 patients with six to 12 months of prior VTE treatment to rivaroxaban 20 mg daily versus placebo for six or 12 months. Rivaroxaban was superior in preventing recurrent VTE (1.3% vs. 7.1%; P<0.001). A statistically nonsignificant increase in major bleeding was reported with rivaroxaban (0.7% vs. 0.0%). The open-label design and pharmaceutical support create potential for bias.
Bottom line: Oral rivaroxaban might offer a simplified, effective, and safe alternative to enoxaparin and warfarin for short- and long-term VTE treatment.
Citation: The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363 (26):2499-2510.
Dabigatran Might Be a Cost-Effective Alternative to Warfarin in Atrial Fibrillation
Clinical question: Is dabigatran cost-effective compared to warfarin for prevention of stroke in atrial fibrillation?
Background: Dabigatran, a direct thrombin inhibitor, is FDA-approved for the prevention of stroke and systemic embolism in atrial fibrillation. In the 2009 RE-LY trial, dabigatran 150 mg twice daily was associated with fewer embolic strokes than warfarin with similar episodes of major hemorrhage. Dabigatran costs more than warfarin; its cost-effectiveness is unknown.
Study design: Markov decision model.
Setting: Data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY), a multinational randomized trial, and other anticoagulation studies.
Synopsis: This model simulated costs and outcomes for a theoretical cohort of patients >65 with atrial fibrillation and CHADS2 score ≥1 taking either lifelong warfarin or dabigatran. The model included assumptions about costs and quality-of-life effects of INR monitoring, stroke, hemorrhage, and myocardial infarction. Because U.S. pricing for dabigatran was pending, the authors assumed $13 per day.
Both life expectancy in quality-adjusted life years (QALYs) and lifetime costs were higher for dabigatran than for warfarin (10.84 vs. 10.28 QALYs and $168,398 vs. $143,193, respectively). The incremental cost per QALY for dabigatran was $45,372. Limitations include dependence on data from a single-manufacturer-sponsored trial with limited follow-up.
Retail costs for dabigatran are now known to be about $8 per day. When the model is adjusted to that price, an additional QALY would cost $12,000, well below the commonly accepted threshold of $50,000.
Bottom line: Dabigatran is likely a cost-effective alternative to warfarin in nonvalvular atrial fibrillation.
Citation: Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154(1):1-11.
Effects of New ACGME Mandates on Patients and Residents Unclear
Clinical question: How will new intern duty-hour standards impact patient care, residents’ health, and education?
Background: The Accreditation Council for Graduate Medical Education (ACGME) has mandated new duty-hour standards that limit interns’ shifts to 16 hours and night float to six consecutive nights. They also strongly recommend a nighttime nap.
Study design: Systematic review of English-language, original research studies addressing shift length, night float, or protected sleep time, published from 1989 to 2010.
Synopsis: Sixty-four out of 5,345 articles met eligibility criteria, including four randomized controlled trials and five multi-institutional studies. Although 73% of studies examining shift length supported reducing hours, optimal shift duration was not determined. All studies addressing night float examined five to seven consecutive nights of work; data were too heterogeneous for generalization. Data on protected sleep time were too limited to determine effect on residents and patients.
The majority of studies were conducted at single institutions and study designs carried high risk for interpretation bias. Additionally, publication bias might have influenced the results of this review of English-language-only studies.
Bottom line: The available studies that attempt to elucidate the effects of major changes in residency training have significant limitations, and the potential impact of the new standards on patients and residents remains uncertain.
Citation: Reed DA, Fletcher KE, Arora VM. Systematic review: association of shift length, protected sleep time, and night float with patient care, residents’ health, and education. Ann Intern Med. 2010;153:829-842.
Admission to Stroke Centers for Acute Ischemic Stroke Might Improve Mortality
Clinical question: Does admission to a certified stroke center improve survival in patients with acute ischemic stroke?
Background: Since 2003, the Joint Commission has designated fewer than 700 acute-care hospitals as certified stroke centers. However, no large studies have examined whether patients with acute stroke admitted to stroke centers have lower mortality than those admitted to noncertified acute-care hospitals.
Study design: Observational cohort study.
Setting: All acute-care hospitals in New York state.
Synopsis: Data from the New York Statewide Planning and Research Cooperative System identified 30,947 adult patients who were hospitalized with acute stroke over a two-year period. Mean age of patients was 73. Thirty-day all-cause mortality was compared between stroke centers and all other acute-care hospitals. Secondary outcomes were one-day, seven-day, and one-year all-cause mortality. To adjust for unmeasured confounders, the analyses accounted for distance to the nearest stroke center relative to the distance to the nearest acute-care hospital.
Almost half the patients in this study were admitted to stroke centers, where they had an adjusted absolute risk reduction in 30-day mortality of 2.5%. Seven-day mortality was reduced 1.3% and one-year mortality was reduced 3.0%. These findings were statistically significant.
There were no differences in one-day mortality, 30-day readmission rates, or rates of discharge to skilled nursing facilities between hospital designation.
The study was not designed to identify which elements of a certified stroke center contribute to the mortality benefit and did not account for stroke severity. Results may not be generalizable beyond New York state.
Bottom line: Admission to an acute-stroke center is associated with a modest reduction in mortality.
Citation: Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305(4):373-380.
Mortality from MRSA Pneumonia Increases with Higher Vancomycin Minimum Inhibitory Concentration
Clinical question: Does vancomycin minimum inhibitory concentration (MIC) affect mortality due to healthcare-associated pneumonia (HCAP), ventilator-associated pneumonia (VAP), and hospital-acquired pneumonia (HAP) from methicillin-resistant Staphylococcus aureus (MRSA)?
Background: S. aureus is considered vancomycin-susceptible if the MIC is ≤2 mg/mL. Mortality from MRSA bacteremia increases as vancomycin MIC rises. The effect of higher vancomycin MICs on outcomes in MRSA pneumonia is not known.
Study design: Prospective cohort study.
Setting: Four academic centers in Kentucky, Ohio, Michigan, and Florida.
Synopsis: One hundred fifty-eight patients with HCAP, VAP, or HAP based on American Thoracic Society/Infectious Disease Society of American (ATS/IDSA) definitions and ≥1 MRSA-positive blood or respiratory culture were identified from the prospectively collected Improving Medicine through Pathway Assessment of Critical Therapy in Hospital-Acquired Pneumonia (IMPACT-HAP) database. All were treated with a regimen including vancomycin based on 2005 ATS/IDSA guidelines.
Vancomycin MIC was ≤1 mg/mL in 27% of MRSA isolates; 1.5 mg/mL in 55%; and ≥2mg/mL in 18%. Overall, all-cause 28-day mortality was 32%. After correcting for confounding factors, such as age and comorbid illnesses, all-cause 28-day mortality was higher in patients with higher vancomycin MICs (adjusted odds ratio of death 2.97 per 1 mg/mL increase in vancomycin MIC). Heteroresistance to vancomycin was present in 21% of MRSA isolates but was not associated with an increase in mortality.
Bottom line: Death due to MRSA HCAP, VAP, and HAP increases as the vancomycin MIC increases, even with MICs within the susceptible range.
Citation: Haque NZ, Zuniga LC, Peyrani P, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest. 2010;138(6): 1356-1362.
More Frequent In-Center Hemodialysis Improves Outcomes
Clinical question: Does more frequent hemodialysis reduce mortality, improve cardiovascular outcomes, and improve quality of life in patients undergoing maintenance hemodialysis?
Background: Despite technological improvements over the last 40 years, hemodialysis is still associated with significant morbidity, mortality, and decreased quality of life. The optimal frequency of hemodialysis remains uncertain.
Study design: Randomized clinical trial with blinded analysis.
Setting: Eleven university-based and 54 community-based hemodialysis facilities in North America.
Synopsis: Researchers randomized 245 patients with end-stage renal disease to receive hemodialysis either three times per week or six times per week. Composite of death or one-year increase in left ventricular mass as assessed by cardiac MR was one primary outcome; composite outcome of death or one-year decrease in self-reported physical health was a co-primary outcome.
Frequent hemodialysis was associated with benefits in both composite primary outcomes (hazard ratio [HR] 0.61 for death/increase in left ventricular mass; HR 0.70 for death/decreased physical health). Notably, patients with frequent dialysis were more likely to undergo interventions related to vascular access than with conventional dialysis (HR 1.71). Blood pressure control (P<0.001) and hyperphosphotemia (P=0.002) also were improved with frequent dialysis.
Depression, cognitive performance, albumin, and anemia did not improve. Direct impact on mortality and hospital admission could not be assessed. Results might not be generalizable.
Bottom line: More frequent hemodialysis was associated with a significant reduction in left ventricular mass, improvement in self-reported physical health, and a reduction in mortality using combined composite outcomes. Further cost-benefit and quality-of-life analyses are needed to determine optimal dosing of hemodialysis.
Citation: FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287-2300.
BNP Testing in the Emergency Department Might Decrease Hospital Length Of Stay
Clinical question: Does BNP testing of patients presenting to the ED with acute dyspnea reduce admissions, shorten length of stay (LOS), or improve short-term survival?
Background: B-type natriuretic peptide (BNP) and the N-terminal peptide of its precursor, pro-BNP, are widely used to evaluate patients with acute dyspnea to distinguish cardiac from noncardiac causes. However, clinical outcomes related to this commonly used test are not clearly understood.
Study design: Systematic review and meta-analysis of randomized trials.
Setting: Five randomized controlled trials in EDs in five hospitals (Switzerland, Canada, the Netherlands, United States, and Australia) involving 2,513 patients.
Synopsis: Studies compared BNP testing with routine testing and clinical assessment and described >1 of three clinical outcomes: hospital admission rate, LOS, and mortality. Nonrandomized and retrospective studies and subgroup analyses of larger studies were excluded.
Testing with BNP decreased LOS by a mean of 1.22 days and critical-care-unit stay was modestly reduced (-0.56 days). This change was attributed to improved acute management and more rapid discharge with knowledge of BNP values. There was a nonsignificant trend toward decreased hospital admission from the ED in the BNP group (odds ratio 0.82). The effect of BNP testing on mortality was inconclusive.
Bottom line: BNP testing in the ED is associated with decreased hospital LOS, as well as a trend toward decreased admission rates from the ED. There is no conclusive effect on mortality.
Citation: Lam LL, Cameron PA, Schneider HG, Abramson MJ, Müller C, Krum H. Meta-analysis: effect of B-type natriuretic peptide testing on clinical outcome in patients with acute dyspnea in the emergency setting. Ann Intern Med. 2010;153:728-735.
Vaccination Reduces Incidence of Herpes Zoster in Community-Dwelling Adults Age 60 and Older
Clinical question: What is the impact of herpes zoster vaccination on the incidence of disease in older community-dwelling adults with and without chronic medical conditions?
Background: Live-attenuated vaccination was recently approved in older adults to reduce the incidence of herpes zoster and postherpetic neuralgia. Vaccination practices and efficacy in a clinical setting among patients with varying comorbidities are unknown.
Study design: Retrospective cohort.
Setting: Single health plan in California.
Synopsis: Data were collected from 2007 to 2009 on 75,761 health-plan members who received the vaccine. The data were compared with unvaccinated, age-matched controls. Vaccine recipients were more likely to be white and female, with more outpatient visits and fewer chronic diseases.
A 55% percent reduction in the incidence of herpes zoster was found among recipients. Benefit was seen across all age groups and comorbidities. Incidence of herpes zoster increased as age increased, but the relative rate reduction with vaccination remained nearly constant, including among those older than 80. Patients with chronic diseases also had an increased baseline incidence of herpes zoster but a similar relative reduction with vaccination. The study was not designed to look at post-herpetic neuralgia or to assess severity or duration of symptoms in herpes zoster cases.
Bottom line: Vaccination for herpes zoster is indicated for all adults age 60 and older, including the oldest and most medically complicated, in whom vaccination is not contraindicated.
Citation: Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011; 305(2):160-166.
For-Profit Hospital Status Might Increase Risk of 30-Day Readmission to Different Hospitals
Clinical question: Are patients admitted to a for-profit hospital more likely to be readmitted to a different hospital if rehospitalized within 30 days?
Background: Thirty-day readmission occurs in 20% of hospitalized Medicare patients, with at least a quarter of rehospitalized patients admitted to a different hospital. Recent healthcare legislation proposes penalties to reduce readmission rates. This could provide unintended incentives for hospitals to divert patients at high risk for readmission to other hospitals.
Study design: Observational cohort study.
Setting: Hospitalized Medicare patients.
Synopsis: Analysis of a 5% sample of Medicare patients readmitted within 30 days of discharge over a 22-month period identified 74,564 patients who were rehospitalized in a facility different from their initial admission. For-profit status of the initial and subsequent hospital was identified. Twenty-eight percent of patients initially admitted to a for-profit hospital were readmitted to a different hospital within 30 days. By comparison, only 21% of patients initially admitted to a nonprofit hospital were readmitted to a different hospital (P<.001).
The most significant risk factors for readmission to a different hospital were admission to a lower-volume hospital (221% increased risk), disability (21% increased risk), admission to an academic hospital (18% increased risk), and admission to a for-profit hospital (17% increased risk). Thirty-day mortality did not differ between patients readmitted to the same or different hospital, regardless of for-profit status. Admission to a different hospital was associated with increased cost.
This study was not designed to look at why patients were rehospitalized at different hospitals, and findings cannot be generalized beyond Medicare patients.
Bottom line: Discharge from a for-profit hospital is one of several risk factors for 30-day readmission to a different hospital.
Citation: Kind AJ, Bartels C, Mell MW, Mullahy J, Smith M. For-profit hospital status and rehospitalizations at different hospitals: an analysis of Medicare data. Ann Intern Med. 2010;153(11):718-727. TH
Literature at a Glance
A guide to this month’s studies
- Rivaroxaban for VTE
- Cost-effectiveness of dabigatran in atrial fibrillation
- Effect of new resident duty-hour limits
- Outcomes of care at acute-stroke centers
- Effect on MIC in patients with MRSA pneumonia
- Optimal hemodialysis frequency
- Effect of BNP testing on hospital length of stay
- Impact of herpes zoster vaccination
- 30-day readmission rates in for-profit hospitals
Oral Rivaroxaban Could Play a Role in VTE Treatment
Clinical question: Is oral rivaroxaban an acceptable treatment option for acute symptomatic deep-vein thrombosis (DVT) and venous thromboembolism (VTE)?
Background: Treatment of acute DVT requires frequent laboratory monitoring, which may be obviated by the use of fixed-dose oral rivaroxaban.
Study designs: Parallel randomized, open-label, event-driven, noninferiority study (the acute DVT study) and randomized, double-blind, placebo-controlled, event-driven superiority trial (continued treatment study).
Setting: Multicenter study.
Synopsis: The acute DVT study randomly assigned 3,449 patients with acute DVT to oral rivaroxaban 15 mg twice daily for three weeks followed by 20 mg daily for three, six, or 12 months or enoxaparin 1 mg/kg subcutaneously twice daily and daily warfarin until a therapeutic INR was achieved, at which time the enoxaparin was discontinued. Rivaroxaban was not inferior in terms of preventing recurrent VTE (2.1% vs. 3.0%; P<0.001). Major or clinically relevant nonmajor bleeding occurred equally in both groups (8.1%).
The continued treatment study randomly assigned 1,196 patients with six to 12 months of prior VTE treatment to rivaroxaban 20 mg daily versus placebo for six or 12 months. Rivaroxaban was superior in preventing recurrent VTE (1.3% vs. 7.1%; P<0.001). A statistically nonsignificant increase in major bleeding was reported with rivaroxaban (0.7% vs. 0.0%). The open-label design and pharmaceutical support create potential for bias.
Bottom line: Oral rivaroxaban might offer a simplified, effective, and safe alternative to enoxaparin and warfarin for short- and long-term VTE treatment.
Citation: The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363 (26):2499-2510.
Dabigatran Might Be a Cost-Effective Alternative to Warfarin in Atrial Fibrillation
Clinical question: Is dabigatran cost-effective compared to warfarin for prevention of stroke in atrial fibrillation?
Background: Dabigatran, a direct thrombin inhibitor, is FDA-approved for the prevention of stroke and systemic embolism in atrial fibrillation. In the 2009 RE-LY trial, dabigatran 150 mg twice daily was associated with fewer embolic strokes than warfarin with similar episodes of major hemorrhage. Dabigatran costs more than warfarin; its cost-effectiveness is unknown.
Study design: Markov decision model.
Setting: Data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY), a multinational randomized trial, and other anticoagulation studies.
Synopsis: This model simulated costs and outcomes for a theoretical cohort of patients >65 with atrial fibrillation and CHADS2 score ≥1 taking either lifelong warfarin or dabigatran. The model included assumptions about costs and quality-of-life effects of INR monitoring, stroke, hemorrhage, and myocardial infarction. Because U.S. pricing for dabigatran was pending, the authors assumed $13 per day.
Both life expectancy in quality-adjusted life years (QALYs) and lifetime costs were higher for dabigatran than for warfarin (10.84 vs. 10.28 QALYs and $168,398 vs. $143,193, respectively). The incremental cost per QALY for dabigatran was $45,372. Limitations include dependence on data from a single-manufacturer-sponsored trial with limited follow-up.
Retail costs for dabigatran are now known to be about $8 per day. When the model is adjusted to that price, an additional QALY would cost $12,000, well below the commonly accepted threshold of $50,000.
Bottom line: Dabigatran is likely a cost-effective alternative to warfarin in nonvalvular atrial fibrillation.
Citation: Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154(1):1-11.
Effects of New ACGME Mandates on Patients and Residents Unclear
Clinical question: How will new intern duty-hour standards impact patient care, residents’ health, and education?
Background: The Accreditation Council for Graduate Medical Education (ACGME) has mandated new duty-hour standards that limit interns’ shifts to 16 hours and night float to six consecutive nights. They also strongly recommend a nighttime nap.
Study design: Systematic review of English-language, original research studies addressing shift length, night float, or protected sleep time, published from 1989 to 2010.
Synopsis: Sixty-four out of 5,345 articles met eligibility criteria, including four randomized controlled trials and five multi-institutional studies. Although 73% of studies examining shift length supported reducing hours, optimal shift duration was not determined. All studies addressing night float examined five to seven consecutive nights of work; data were too heterogeneous for generalization. Data on protected sleep time were too limited to determine effect on residents and patients.
The majority of studies were conducted at single institutions and study designs carried high risk for interpretation bias. Additionally, publication bias might have influenced the results of this review of English-language-only studies.
Bottom line: The available studies that attempt to elucidate the effects of major changes in residency training have significant limitations, and the potential impact of the new standards on patients and residents remains uncertain.
Citation: Reed DA, Fletcher KE, Arora VM. Systematic review: association of shift length, protected sleep time, and night float with patient care, residents’ health, and education. Ann Intern Med. 2010;153:829-842.
Admission to Stroke Centers for Acute Ischemic Stroke Might Improve Mortality
Clinical question: Does admission to a certified stroke center improve survival in patients with acute ischemic stroke?
Background: Since 2003, the Joint Commission has designated fewer than 700 acute-care hospitals as certified stroke centers. However, no large studies have examined whether patients with acute stroke admitted to stroke centers have lower mortality than those admitted to noncertified acute-care hospitals.
Study design: Observational cohort study.
Setting: All acute-care hospitals in New York state.
Synopsis: Data from the New York Statewide Planning and Research Cooperative System identified 30,947 adult patients who were hospitalized with acute stroke over a two-year period. Mean age of patients was 73. Thirty-day all-cause mortality was compared between stroke centers and all other acute-care hospitals. Secondary outcomes were one-day, seven-day, and one-year all-cause mortality. To adjust for unmeasured confounders, the analyses accounted for distance to the nearest stroke center relative to the distance to the nearest acute-care hospital.
Almost half the patients in this study were admitted to stroke centers, where they had an adjusted absolute risk reduction in 30-day mortality of 2.5%. Seven-day mortality was reduced 1.3% and one-year mortality was reduced 3.0%. These findings were statistically significant.
There were no differences in one-day mortality, 30-day readmission rates, or rates of discharge to skilled nursing facilities between hospital designation.
The study was not designed to identify which elements of a certified stroke center contribute to the mortality benefit and did not account for stroke severity. Results may not be generalizable beyond New York state.
Bottom line: Admission to an acute-stroke center is associated with a modest reduction in mortality.
Citation: Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305(4):373-380.
Mortality from MRSA Pneumonia Increases with Higher Vancomycin Minimum Inhibitory Concentration
Clinical question: Does vancomycin minimum inhibitory concentration (MIC) affect mortality due to healthcare-associated pneumonia (HCAP), ventilator-associated pneumonia (VAP), and hospital-acquired pneumonia (HAP) from methicillin-resistant Staphylococcus aureus (MRSA)?
Background: S. aureus is considered vancomycin-susceptible if the MIC is ≤2 mg/mL. Mortality from MRSA bacteremia increases as vancomycin MIC rises. The effect of higher vancomycin MICs on outcomes in MRSA pneumonia is not known.
Study design: Prospective cohort study.
Setting: Four academic centers in Kentucky, Ohio, Michigan, and Florida.
Synopsis: One hundred fifty-eight patients with HCAP, VAP, or HAP based on American Thoracic Society/Infectious Disease Society of American (ATS/IDSA) definitions and ≥1 MRSA-positive blood or respiratory culture were identified from the prospectively collected Improving Medicine through Pathway Assessment of Critical Therapy in Hospital-Acquired Pneumonia (IMPACT-HAP) database. All were treated with a regimen including vancomycin based on 2005 ATS/IDSA guidelines.
Vancomycin MIC was ≤1 mg/mL in 27% of MRSA isolates; 1.5 mg/mL in 55%; and ≥2mg/mL in 18%. Overall, all-cause 28-day mortality was 32%. After correcting for confounding factors, such as age and comorbid illnesses, all-cause 28-day mortality was higher in patients with higher vancomycin MICs (adjusted odds ratio of death 2.97 per 1 mg/mL increase in vancomycin MIC). Heteroresistance to vancomycin was present in 21% of MRSA isolates but was not associated with an increase in mortality.
Bottom line: Death due to MRSA HCAP, VAP, and HAP increases as the vancomycin MIC increases, even with MICs within the susceptible range.
Citation: Haque NZ, Zuniga LC, Peyrani P, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest. 2010;138(6): 1356-1362.
More Frequent In-Center Hemodialysis Improves Outcomes
Clinical question: Does more frequent hemodialysis reduce mortality, improve cardiovascular outcomes, and improve quality of life in patients undergoing maintenance hemodialysis?
Background: Despite technological improvements over the last 40 years, hemodialysis is still associated with significant morbidity, mortality, and decreased quality of life. The optimal frequency of hemodialysis remains uncertain.
Study design: Randomized clinical trial with blinded analysis.
Setting: Eleven university-based and 54 community-based hemodialysis facilities in North America.
Synopsis: Researchers randomized 245 patients with end-stage renal disease to receive hemodialysis either three times per week or six times per week. Composite of death or one-year increase in left ventricular mass as assessed by cardiac MR was one primary outcome; composite outcome of death or one-year decrease in self-reported physical health was a co-primary outcome.
Frequent hemodialysis was associated with benefits in both composite primary outcomes (hazard ratio [HR] 0.61 for death/increase in left ventricular mass; HR 0.70 for death/decreased physical health). Notably, patients with frequent dialysis were more likely to undergo interventions related to vascular access than with conventional dialysis (HR 1.71). Blood pressure control (P<0.001) and hyperphosphotemia (P=0.002) also were improved with frequent dialysis.
Depression, cognitive performance, albumin, and anemia did not improve. Direct impact on mortality and hospital admission could not be assessed. Results might not be generalizable.
Bottom line: More frequent hemodialysis was associated with a significant reduction in left ventricular mass, improvement in self-reported physical health, and a reduction in mortality using combined composite outcomes. Further cost-benefit and quality-of-life analyses are needed to determine optimal dosing of hemodialysis.
Citation: FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287-2300.
BNP Testing in the Emergency Department Might Decrease Hospital Length Of Stay
Clinical question: Does BNP testing of patients presenting to the ED with acute dyspnea reduce admissions, shorten length of stay (LOS), or improve short-term survival?
Background: B-type natriuretic peptide (BNP) and the N-terminal peptide of its precursor, pro-BNP, are widely used to evaluate patients with acute dyspnea to distinguish cardiac from noncardiac causes. However, clinical outcomes related to this commonly used test are not clearly understood.
Study design: Systematic review and meta-analysis of randomized trials.
Setting: Five randomized controlled trials in EDs in five hospitals (Switzerland, Canada, the Netherlands, United States, and Australia) involving 2,513 patients.
Synopsis: Studies compared BNP testing with routine testing and clinical assessment and described >1 of three clinical outcomes: hospital admission rate, LOS, and mortality. Nonrandomized and retrospective studies and subgroup analyses of larger studies were excluded.
Testing with BNP decreased LOS by a mean of 1.22 days and critical-care-unit stay was modestly reduced (-0.56 days). This change was attributed to improved acute management and more rapid discharge with knowledge of BNP values. There was a nonsignificant trend toward decreased hospital admission from the ED in the BNP group (odds ratio 0.82). The effect of BNP testing on mortality was inconclusive.
Bottom line: BNP testing in the ED is associated with decreased hospital LOS, as well as a trend toward decreased admission rates from the ED. There is no conclusive effect on mortality.
Citation: Lam LL, Cameron PA, Schneider HG, Abramson MJ, Müller C, Krum H. Meta-analysis: effect of B-type natriuretic peptide testing on clinical outcome in patients with acute dyspnea in the emergency setting. Ann Intern Med. 2010;153:728-735.
Vaccination Reduces Incidence of Herpes Zoster in Community-Dwelling Adults Age 60 and Older
Clinical question: What is the impact of herpes zoster vaccination on the incidence of disease in older community-dwelling adults with and without chronic medical conditions?
Background: Live-attenuated vaccination was recently approved in older adults to reduce the incidence of herpes zoster and postherpetic neuralgia. Vaccination practices and efficacy in a clinical setting among patients with varying comorbidities are unknown.
Study design: Retrospective cohort.
Setting: Single health plan in California.
Synopsis: Data were collected from 2007 to 2009 on 75,761 health-plan members who received the vaccine. The data were compared with unvaccinated, age-matched controls. Vaccine recipients were more likely to be white and female, with more outpatient visits and fewer chronic diseases.
A 55% percent reduction in the incidence of herpes zoster was found among recipients. Benefit was seen across all age groups and comorbidities. Incidence of herpes zoster increased as age increased, but the relative rate reduction with vaccination remained nearly constant, including among those older than 80. Patients with chronic diseases also had an increased baseline incidence of herpes zoster but a similar relative reduction with vaccination. The study was not designed to look at post-herpetic neuralgia or to assess severity or duration of symptoms in herpes zoster cases.
Bottom line: Vaccination for herpes zoster is indicated for all adults age 60 and older, including the oldest and most medically complicated, in whom vaccination is not contraindicated.
Citation: Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011; 305(2):160-166.
For-Profit Hospital Status Might Increase Risk of 30-Day Readmission to Different Hospitals
Clinical question: Are patients admitted to a for-profit hospital more likely to be readmitted to a different hospital if rehospitalized within 30 days?
Background: Thirty-day readmission occurs in 20% of hospitalized Medicare patients, with at least a quarter of rehospitalized patients admitted to a different hospital. Recent healthcare legislation proposes penalties to reduce readmission rates. This could provide unintended incentives for hospitals to divert patients at high risk for readmission to other hospitals.
Study design: Observational cohort study.
Setting: Hospitalized Medicare patients.
Synopsis: Analysis of a 5% sample of Medicare patients readmitted within 30 days of discharge over a 22-month period identified 74,564 patients who were rehospitalized in a facility different from their initial admission. For-profit status of the initial and subsequent hospital was identified. Twenty-eight percent of patients initially admitted to a for-profit hospital were readmitted to a different hospital within 30 days. By comparison, only 21% of patients initially admitted to a nonprofit hospital were readmitted to a different hospital (P<.001).
The most significant risk factors for readmission to a different hospital were admission to a lower-volume hospital (221% increased risk), disability (21% increased risk), admission to an academic hospital (18% increased risk), and admission to a for-profit hospital (17% increased risk). Thirty-day mortality did not differ between patients readmitted to the same or different hospital, regardless of for-profit status. Admission to a different hospital was associated with increased cost.
This study was not designed to look at why patients were rehospitalized at different hospitals, and findings cannot be generalized beyond Medicare patients.
Bottom line: Discharge from a for-profit hospital is one of several risk factors for 30-day readmission to a different hospital.
Citation: Kind AJ, Bartels C, Mell MW, Mullahy J, Smith M. For-profit hospital status and rehospitalizations at different hospitals: an analysis of Medicare data. Ann Intern Med. 2010;153(11):718-727. TH
Literature at a Glance
A guide to this month’s studies
- Rivaroxaban for VTE
- Cost-effectiveness of dabigatran in atrial fibrillation
- Effect of new resident duty-hour limits
- Outcomes of care at acute-stroke centers
- Effect on MIC in patients with MRSA pneumonia
- Optimal hemodialysis frequency
- Effect of BNP testing on hospital length of stay
- Impact of herpes zoster vaccination
- 30-day readmission rates in for-profit hospitals
Oral Rivaroxaban Could Play a Role in VTE Treatment
Clinical question: Is oral rivaroxaban an acceptable treatment option for acute symptomatic deep-vein thrombosis (DVT) and venous thromboembolism (VTE)?
Background: Treatment of acute DVT requires frequent laboratory monitoring, which may be obviated by the use of fixed-dose oral rivaroxaban.
Study designs: Parallel randomized, open-label, event-driven, noninferiority study (the acute DVT study) and randomized, double-blind, placebo-controlled, event-driven superiority trial (continued treatment study).
Setting: Multicenter study.
Synopsis: The acute DVT study randomly assigned 3,449 patients with acute DVT to oral rivaroxaban 15 mg twice daily for three weeks followed by 20 mg daily for three, six, or 12 months or enoxaparin 1 mg/kg subcutaneously twice daily and daily warfarin until a therapeutic INR was achieved, at which time the enoxaparin was discontinued. Rivaroxaban was not inferior in terms of preventing recurrent VTE (2.1% vs. 3.0%; P<0.001). Major or clinically relevant nonmajor bleeding occurred equally in both groups (8.1%).
The continued treatment study randomly assigned 1,196 patients with six to 12 months of prior VTE treatment to rivaroxaban 20 mg daily versus placebo for six or 12 months. Rivaroxaban was superior in preventing recurrent VTE (1.3% vs. 7.1%; P<0.001). A statistically nonsignificant increase in major bleeding was reported with rivaroxaban (0.7% vs. 0.0%). The open-label design and pharmaceutical support create potential for bias.
Bottom line: Oral rivaroxaban might offer a simplified, effective, and safe alternative to enoxaparin and warfarin for short- and long-term VTE treatment.
Citation: The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363 (26):2499-2510.
Dabigatran Might Be a Cost-Effective Alternative to Warfarin in Atrial Fibrillation
Clinical question: Is dabigatran cost-effective compared to warfarin for prevention of stroke in atrial fibrillation?
Background: Dabigatran, a direct thrombin inhibitor, is FDA-approved for the prevention of stroke and systemic embolism in atrial fibrillation. In the 2009 RE-LY trial, dabigatran 150 mg twice daily was associated with fewer embolic strokes than warfarin with similar episodes of major hemorrhage. Dabigatran costs more than warfarin; its cost-effectiveness is unknown.
Study design: Markov decision model.
Setting: Data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY), a multinational randomized trial, and other anticoagulation studies.
Synopsis: This model simulated costs and outcomes for a theoretical cohort of patients >65 with atrial fibrillation and CHADS2 score ≥1 taking either lifelong warfarin or dabigatran. The model included assumptions about costs and quality-of-life effects of INR monitoring, stroke, hemorrhage, and myocardial infarction. Because U.S. pricing for dabigatran was pending, the authors assumed $13 per day.
Both life expectancy in quality-adjusted life years (QALYs) and lifetime costs were higher for dabigatran than for warfarin (10.84 vs. 10.28 QALYs and $168,398 vs. $143,193, respectively). The incremental cost per QALY for dabigatran was $45,372. Limitations include dependence on data from a single-manufacturer-sponsored trial with limited follow-up.
Retail costs for dabigatran are now known to be about $8 per day. When the model is adjusted to that price, an additional QALY would cost $12,000, well below the commonly accepted threshold of $50,000.
Bottom line: Dabigatran is likely a cost-effective alternative to warfarin in nonvalvular atrial fibrillation.
Citation: Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154(1):1-11.
Effects of New ACGME Mandates on Patients and Residents Unclear
Clinical question: How will new intern duty-hour standards impact patient care, residents’ health, and education?
Background: The Accreditation Council for Graduate Medical Education (ACGME) has mandated new duty-hour standards that limit interns’ shifts to 16 hours and night float to six consecutive nights. They also strongly recommend a nighttime nap.
Study design: Systematic review of English-language, original research studies addressing shift length, night float, or protected sleep time, published from 1989 to 2010.
Synopsis: Sixty-four out of 5,345 articles met eligibility criteria, including four randomized controlled trials and five multi-institutional studies. Although 73% of studies examining shift length supported reducing hours, optimal shift duration was not determined. All studies addressing night float examined five to seven consecutive nights of work; data were too heterogeneous for generalization. Data on protected sleep time were too limited to determine effect on residents and patients.
The majority of studies were conducted at single institutions and study designs carried high risk for interpretation bias. Additionally, publication bias might have influenced the results of this review of English-language-only studies.
Bottom line: The available studies that attempt to elucidate the effects of major changes in residency training have significant limitations, and the potential impact of the new standards on patients and residents remains uncertain.
Citation: Reed DA, Fletcher KE, Arora VM. Systematic review: association of shift length, protected sleep time, and night float with patient care, residents’ health, and education. Ann Intern Med. 2010;153:829-842.
Admission to Stroke Centers for Acute Ischemic Stroke Might Improve Mortality
Clinical question: Does admission to a certified stroke center improve survival in patients with acute ischemic stroke?
Background: Since 2003, the Joint Commission has designated fewer than 700 acute-care hospitals as certified stroke centers. However, no large studies have examined whether patients with acute stroke admitted to stroke centers have lower mortality than those admitted to noncertified acute-care hospitals.
Study design: Observational cohort study.
Setting: All acute-care hospitals in New York state.
Synopsis: Data from the New York Statewide Planning and Research Cooperative System identified 30,947 adult patients who were hospitalized with acute stroke over a two-year period. Mean age of patients was 73. Thirty-day all-cause mortality was compared between stroke centers and all other acute-care hospitals. Secondary outcomes were one-day, seven-day, and one-year all-cause mortality. To adjust for unmeasured confounders, the analyses accounted for distance to the nearest stroke center relative to the distance to the nearest acute-care hospital.
Almost half the patients in this study were admitted to stroke centers, where they had an adjusted absolute risk reduction in 30-day mortality of 2.5%. Seven-day mortality was reduced 1.3% and one-year mortality was reduced 3.0%. These findings were statistically significant.
There were no differences in one-day mortality, 30-day readmission rates, or rates of discharge to skilled nursing facilities between hospital designation.
The study was not designed to identify which elements of a certified stroke center contribute to the mortality benefit and did not account for stroke severity. Results may not be generalizable beyond New York state.
Bottom line: Admission to an acute-stroke center is associated with a modest reduction in mortality.
Citation: Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305(4):373-380.
Mortality from MRSA Pneumonia Increases with Higher Vancomycin Minimum Inhibitory Concentration
Clinical question: Does vancomycin minimum inhibitory concentration (MIC) affect mortality due to healthcare-associated pneumonia (HCAP), ventilator-associated pneumonia (VAP), and hospital-acquired pneumonia (HAP) from methicillin-resistant Staphylococcus aureus (MRSA)?
Background: S. aureus is considered vancomycin-susceptible if the MIC is ≤2 mg/mL. Mortality from MRSA bacteremia increases as vancomycin MIC rises. The effect of higher vancomycin MICs on outcomes in MRSA pneumonia is not known.
Study design: Prospective cohort study.
Setting: Four academic centers in Kentucky, Ohio, Michigan, and Florida.
Synopsis: One hundred fifty-eight patients with HCAP, VAP, or HAP based on American Thoracic Society/Infectious Disease Society of American (ATS/IDSA) definitions and ≥1 MRSA-positive blood or respiratory culture were identified from the prospectively collected Improving Medicine through Pathway Assessment of Critical Therapy in Hospital-Acquired Pneumonia (IMPACT-HAP) database. All were treated with a regimen including vancomycin based on 2005 ATS/IDSA guidelines.
Vancomycin MIC was ≤1 mg/mL in 27% of MRSA isolates; 1.5 mg/mL in 55%; and ≥2mg/mL in 18%. Overall, all-cause 28-day mortality was 32%. After correcting for confounding factors, such as age and comorbid illnesses, all-cause 28-day mortality was higher in patients with higher vancomycin MICs (adjusted odds ratio of death 2.97 per 1 mg/mL increase in vancomycin MIC). Heteroresistance to vancomycin was present in 21% of MRSA isolates but was not associated with an increase in mortality.
Bottom line: Death due to MRSA HCAP, VAP, and HAP increases as the vancomycin MIC increases, even with MICs within the susceptible range.
Citation: Haque NZ, Zuniga LC, Peyrani P, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest. 2010;138(6): 1356-1362.
More Frequent In-Center Hemodialysis Improves Outcomes
Clinical question: Does more frequent hemodialysis reduce mortality, improve cardiovascular outcomes, and improve quality of life in patients undergoing maintenance hemodialysis?
Background: Despite technological improvements over the last 40 years, hemodialysis is still associated with significant morbidity, mortality, and decreased quality of life. The optimal frequency of hemodialysis remains uncertain.
Study design: Randomized clinical trial with blinded analysis.
Setting: Eleven university-based and 54 community-based hemodialysis facilities in North America.
Synopsis: Researchers randomized 245 patients with end-stage renal disease to receive hemodialysis either three times per week or six times per week. Composite of death or one-year increase in left ventricular mass as assessed by cardiac MR was one primary outcome; composite outcome of death or one-year decrease in self-reported physical health was a co-primary outcome.
Frequent hemodialysis was associated with benefits in both composite primary outcomes (hazard ratio [HR] 0.61 for death/increase in left ventricular mass; HR 0.70 for death/decreased physical health). Notably, patients with frequent dialysis were more likely to undergo interventions related to vascular access than with conventional dialysis (HR 1.71). Blood pressure control (P<0.001) and hyperphosphotemia (P=0.002) also were improved with frequent dialysis.
Depression, cognitive performance, albumin, and anemia did not improve. Direct impact on mortality and hospital admission could not be assessed. Results might not be generalizable.
Bottom line: More frequent hemodialysis was associated with a significant reduction in left ventricular mass, improvement in self-reported physical health, and a reduction in mortality using combined composite outcomes. Further cost-benefit and quality-of-life analyses are needed to determine optimal dosing of hemodialysis.
Citation: FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287-2300.
BNP Testing in the Emergency Department Might Decrease Hospital Length Of Stay
Clinical question: Does BNP testing of patients presenting to the ED with acute dyspnea reduce admissions, shorten length of stay (LOS), or improve short-term survival?
Background: B-type natriuretic peptide (BNP) and the N-terminal peptide of its precursor, pro-BNP, are widely used to evaluate patients with acute dyspnea to distinguish cardiac from noncardiac causes. However, clinical outcomes related to this commonly used test are not clearly understood.
Study design: Systematic review and meta-analysis of randomized trials.
Setting: Five randomized controlled trials in EDs in five hospitals (Switzerland, Canada, the Netherlands, United States, and Australia) involving 2,513 patients.
Synopsis: Studies compared BNP testing with routine testing and clinical assessment and described >1 of three clinical outcomes: hospital admission rate, LOS, and mortality. Nonrandomized and retrospective studies and subgroup analyses of larger studies were excluded.
Testing with BNP decreased LOS by a mean of 1.22 days and critical-care-unit stay was modestly reduced (-0.56 days). This change was attributed to improved acute management and more rapid discharge with knowledge of BNP values. There was a nonsignificant trend toward decreased hospital admission from the ED in the BNP group (odds ratio 0.82). The effect of BNP testing on mortality was inconclusive.
Bottom line: BNP testing in the ED is associated with decreased hospital LOS, as well as a trend toward decreased admission rates from the ED. There is no conclusive effect on mortality.
Citation: Lam LL, Cameron PA, Schneider HG, Abramson MJ, Müller C, Krum H. Meta-analysis: effect of B-type natriuretic peptide testing on clinical outcome in patients with acute dyspnea in the emergency setting. Ann Intern Med. 2010;153:728-735.
Vaccination Reduces Incidence of Herpes Zoster in Community-Dwelling Adults Age 60 and Older
Clinical question: What is the impact of herpes zoster vaccination on the incidence of disease in older community-dwelling adults with and without chronic medical conditions?
Background: Live-attenuated vaccination was recently approved in older adults to reduce the incidence of herpes zoster and postherpetic neuralgia. Vaccination practices and efficacy in a clinical setting among patients with varying comorbidities are unknown.
Study design: Retrospective cohort.
Setting: Single health plan in California.
Synopsis: Data were collected from 2007 to 2009 on 75,761 health-plan members who received the vaccine. The data were compared with unvaccinated, age-matched controls. Vaccine recipients were more likely to be white and female, with more outpatient visits and fewer chronic diseases.
A 55% percent reduction in the incidence of herpes zoster was found among recipients. Benefit was seen across all age groups and comorbidities. Incidence of herpes zoster increased as age increased, but the relative rate reduction with vaccination remained nearly constant, including among those older than 80. Patients with chronic diseases also had an increased baseline incidence of herpes zoster but a similar relative reduction with vaccination. The study was not designed to look at post-herpetic neuralgia or to assess severity or duration of symptoms in herpes zoster cases.
Bottom line: Vaccination for herpes zoster is indicated for all adults age 60 and older, including the oldest and most medically complicated, in whom vaccination is not contraindicated.
Citation: Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011; 305(2):160-166.
For-Profit Hospital Status Might Increase Risk of 30-Day Readmission to Different Hospitals
Clinical question: Are patients admitted to a for-profit hospital more likely to be readmitted to a different hospital if rehospitalized within 30 days?
Background: Thirty-day readmission occurs in 20% of hospitalized Medicare patients, with at least a quarter of rehospitalized patients admitted to a different hospital. Recent healthcare legislation proposes penalties to reduce readmission rates. This could provide unintended incentives for hospitals to divert patients at high risk for readmission to other hospitals.
Study design: Observational cohort study.
Setting: Hospitalized Medicare patients.
Synopsis: Analysis of a 5% sample of Medicare patients readmitted within 30 days of discharge over a 22-month period identified 74,564 patients who were rehospitalized in a facility different from their initial admission. For-profit status of the initial and subsequent hospital was identified. Twenty-eight percent of patients initially admitted to a for-profit hospital were readmitted to a different hospital within 30 days. By comparison, only 21% of patients initially admitted to a nonprofit hospital were readmitted to a different hospital (P<.001).
The most significant risk factors for readmission to a different hospital were admission to a lower-volume hospital (221% increased risk), disability (21% increased risk), admission to an academic hospital (18% increased risk), and admission to a for-profit hospital (17% increased risk). Thirty-day mortality did not differ between patients readmitted to the same or different hospital, regardless of for-profit status. Admission to a different hospital was associated with increased cost.
This study was not designed to look at why patients were rehospitalized at different hospitals, and findings cannot be generalized beyond Medicare patients.
Bottom line: Discharge from a for-profit hospital is one of several risk factors for 30-day readmission to a different hospital.
Citation: Kind AJ, Bartels C, Mell MW, Mullahy J, Smith M. For-profit hospital status and rehospitalizations at different hospitals: an analysis of Medicare data. Ann Intern Med. 2010;153(11):718-727. TH