User login
Thromboprophylaxis: Survey on Barriers
Each year in North America, over 7 million adults are hospitalized with a medical illness.1 Acute illness and decreased mobility in hospital places patients at increased risk for venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and life‐threatening pulmonary embolism (PE).2 Since VTE remains the most preventable cause of death in hospitalized patients, numerous studies have aimed at reducing the incidence of hospital‐acquired DVT. Aside from cost, the impact of VTE to the healthcare system is felt not only by those who diagnose and treat VTE, but also by those responsible for correcting the severe bleeding that can result from inappropriate use of thromboprophylaxis. Approximately 60% of symptomatic VTE occurs in medical patients, and recent hospitalization for medical illness accounts for 25% of all community‐diagnosed VTE. The Agency for Health Research and Quality ranks DVT prevention as the top priority out of 79 patient safety initiatives, and expert consensus groups provide a strong recommendation that DVT prophylaxis with a low‐dose anticoagulant should be administered to at‐risk hospitalized medical patients.2, 3
Despite the availability, efficacy, and safety of DVT prophylaxis,2 it is discouraging that only 21% to 62% of medical patients receive prophylaxis,49 and only 16% to 40% receive appropriate prophylaxis.46, 1012 However, 70% to 90% of patients in other at‐risk groups, such as surgical patients or critically ill patients, receive prophylaxis.1316 The reason why DVT prophylaxis is so underutilized in medical patients is unclear, as explanations for low rates of clinical practice guideline utilization are multifaceted,17 and few studies have investigated the barriers to optimal thromboprophylaxis.1820
To explore possible reasons for this disparity between evidence and practice, we conducted a cross‐sectional survey of 4 clinician groups involved in the care of hospitalized medical patients. Our objective was to identify barriers and potential solutions to the underutilization of DVT prophylaxis in hospitalized medical patients.
METHODS
Instrument Development
The survey focused on 3 domains: perceived importance, effectiveness, and safety of DVT prophylaxis; perceived barriers to implementation; and perceived potential success and feasibility of interventions to optimize DVT prophylaxis. The survey cover letter outlined background information, study design, and a statement on confidentiality. A prior survey of DVT prophylaxis administered to thrombosis experts was used to generate survey questions.21
Only survey respondents who answered yes to the first question, Are you involved in any aspect of the care of hospitalized general medical patients for whom DVT prophylaxis is considered? were asked to complete the remaining sections. Subsequent questions required respondents to check the box on a 7‐point Likert‐type scale that most accurately reflected their perception (Table 1). A successful intervention was defined as one that, if implemented, would yield the anticipated effect and a feasible intervention as one that was easy to implement without major logistical burden. Respondents were also asked which clinician group was best able to provide a daily assessment of patients' need for DVT prophylaxis, ensure DVT prophylaxis is prescribed, and ensure adherence.
|
| Section 1: Perceptions regarding DVT prophylaxis in hospitalized medical patients* |
| 1. How important an issue is the prevention of DVT in hospitalized general medical patients? |
| 2. To your knowledge, how effective are currently used anticoagulant strategies for the prevention of DVT in hospitalized medical patients? |
| 3. How safe are currently used anticoagulant strategies for the prevention of DVT in hospitalized medical patients? |
| 4. Current anticoagulant prophylaxis strategies are: 1 = underutilized, 4 = appropriately utilized, 7 = overutilized. |
| Section 2: Perceptions regarding barriers to the optimal use of DVT prophylaxis |
| 1. Lack of time to consider DVT prophylaxis in every patient |
| 2. Lack of clear indications for DVT prophylaxis (ie, who should get prophylaxis) |
| 3. Lack of clear contraindications for DVT prophylaxis (ie, who should not get prophylaxis) |
| 4. Lack of awareness about effectiveness of DVT prophylaxis |
| 5. Lack of physician agreement with current DVT prophylaxis guidelines |
| 6. Patient discomfort from subcutaneous injections of anticoagulants |
| 7. Clinician concerns about increased bleeding risk from anticoagulant administration |
| Section 3: Perceptions of interventions relating to DVT prophylaxis |
| 1. Yearly multidisciplinary educational meetings: to engage a wide spectrum of healthcare professionals to review DVT prophylaxis in hospitalized medical patients |
| 2. Posters on the wards: to remind healthcare professionals about DVT prophylaxis and patients who are eligible or ineligible for this treatment |
| 3. Laminated pocket cards: to remind healthcare professionals about DVT prophylaxis and patients who are eligible and ineligible for this treatment |
| 4. Preprinted order sheets: to remind healthcare professionals about DVT prophylaxis and patients who are eligible and ineligible for this treatment |
| 5. Periodic audit and feedback to healthcare providers: E‐mails to physicians containing reports on compliance with DVT prevention practice guidelines over recent years |
| 6. Computerized reminders (to the physicians): to prompt the physician to consider DVT prophylaxis upon opening a patient's electronic medical record |
| 7. Nurse reminders (to the physician): to remind the physician about DVT prophylaxis using written or verbal reminders |
| 8. Pharmacist reminders (to the physician): to remind the physician about DVT prophylaxis using written or verbal reminders |
| 9. Physiotherapist reminders (to the physician): to remind the physician about DVT prophylaxis using written or verbal reminders |
| 10. Use of a local opinion leader (within the hospital) to promote evidence‐based use of DVT prophylaxis guidelines: to educate healthcare professionals on best practices for DVT prophylaxis |
Survey Administration
The survey was distributed between April and July 2007 in both paper‐based and web‐based formats using Survey Monkey software. Ontario members of the Canadian Society of Internal Medicine (n = 193) received a direct electronic invitation (from N.S.L., on behalf of J.D.D.) to participate, while members of the Canadian Society of Hospital Pharmacists (CSHP) (n = 1002) received an electronic invitation from an administrator for the CSHP to participate. The CSHP could not ensure that all members receiving the survey were hospital‐based pharmacists, so it was expected that the response rate from this group would be low. Nurse and physiotherapy managers at a convenience sample of 8 hospitals in Ontario, Canada, distributed paper‐based surveys to their staff using stamped, preaddressed envelopes. Nonresponders in all groups were sent reminders at 2 and 4 weeks.22 Data from all completed surveys were entered into an electronic database by a research coordinator (N.S.L.). A research assistant entered paper‐based survey data in duplicate, with discrepancies resolved by consensus and mediation by a third person (J.C.). The study was conducted with Institutional Ethics Review Board approval, and all respondents provided informed consent to participate. All responses were anonymous and confidential.
Statistical Considerations
Given the exploratory nature of this survey, there was no prespecified hypothesis‐driven respondent sample size. Proportions were used to describe response rates. Survey responses scored on the 7‐point Likert‐type scale were expressed as a mean and 95% confidence interval (CI). Important, highly potentially successful, and highly potentially feasible barriers were defined as those with a mean 5 points. Questions without responses, questions with multiple responses, and questions with illegible responses were treated as missing values. All statistical analyses were done using SAS version 9 (Cary, NC).
RESULTS
Survey Responses
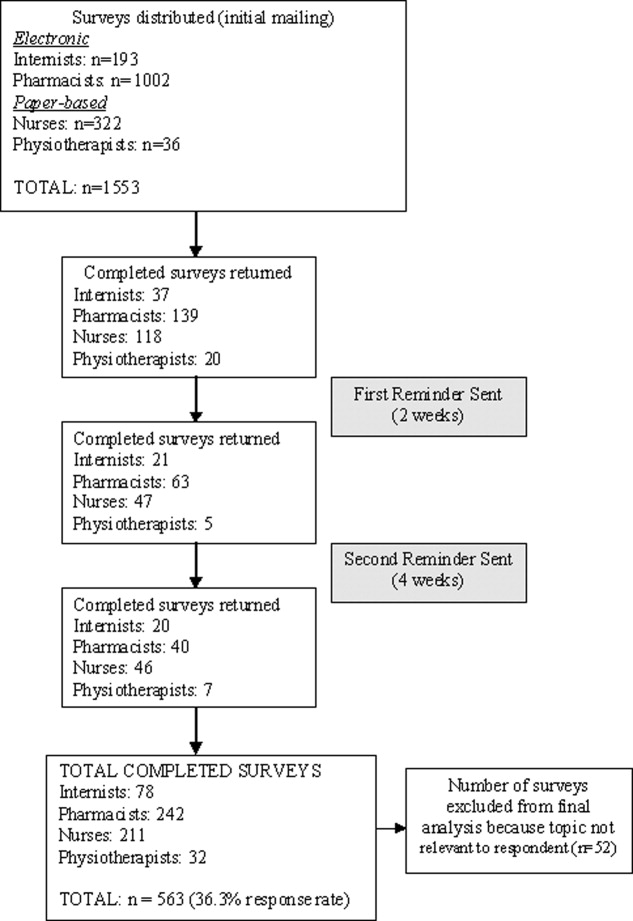
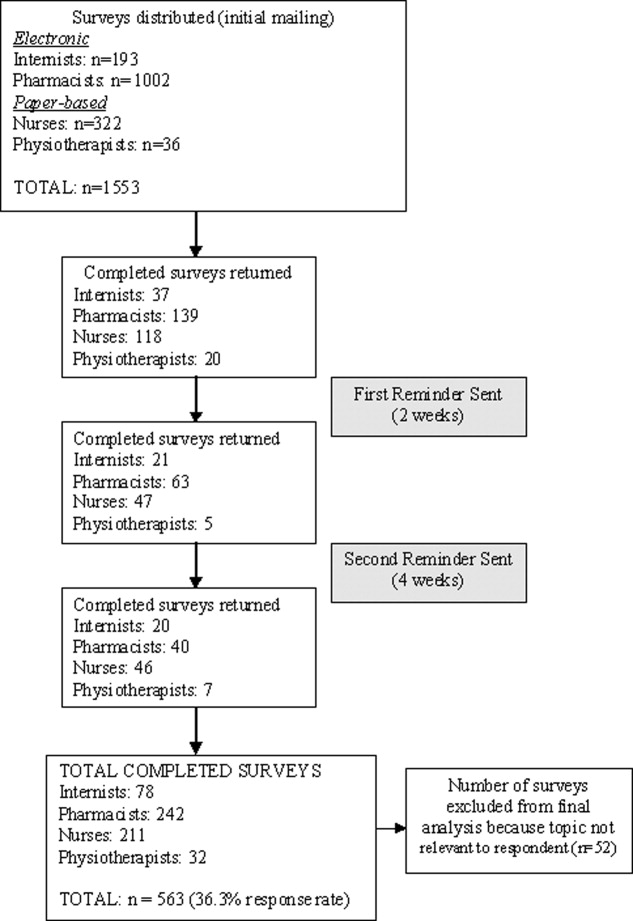
The overall response rate was 36.3% (563/1553), with 65.5% (211/322) of nurses, 40.4% (78/193) of physicians, 24.1% (242/1002) of pharmacists, and 88.8% (32/36) of physiotherapists completing surveys. When pharmacists were removed from the response rate calculation (since it was expected that many of those receiving the survey were not in a primarily hospital‐based practice), the overall response rate rose to 58.3% (321/551). Excluded were 9.2% (52/563) of returned surveys, as respondents indicated the topic was not relevant to their practice. Five hundred eleven surveys were included in the final analysis (Figure 1).
Importance, Effectiveness, Safety, and Appropriateness of DVT Prophylaxis Strategies
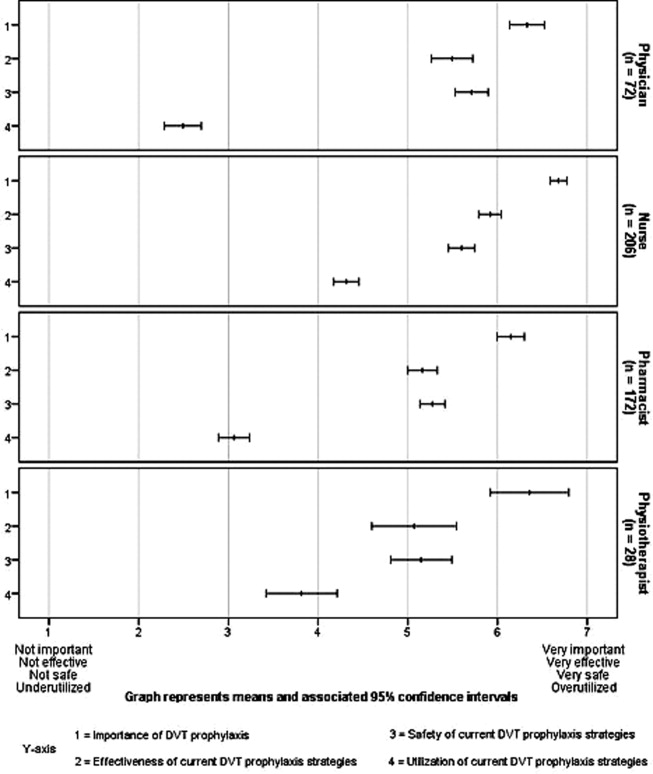
DVT prophylaxis was perceived across clinician groups as important (mean score 6.4; 95% CI 6.3 to 6.5), safe (mean 5.5; 95% CI 5.4 to 5.6), and effective (mean 5.5; 95% CI 5.4 to 6.6) (Figure 2). The mean score for the appropriateness of current DVT prophylaxis practices was 3.5 (95% CI 3.4 to 3.7), suggesting an overall perception of underutilization. However, by respondent groups, DVT prophylaxis was considered to be underutilized by physicians (mean 2.5; 95% CI 2.3 to 2.7) and pharmacists (mean 3.1; 95% CI 2.9 to 3.2), while nurses (mean 4.3; 95% CI 4.2 to 4.5) and physiotherapists (mean 3.8; 95% CI, 3.4 to 4.2) tended to consider current strategies as appropriate.
Potential Barriers to DVT Prophylaxis Utilization
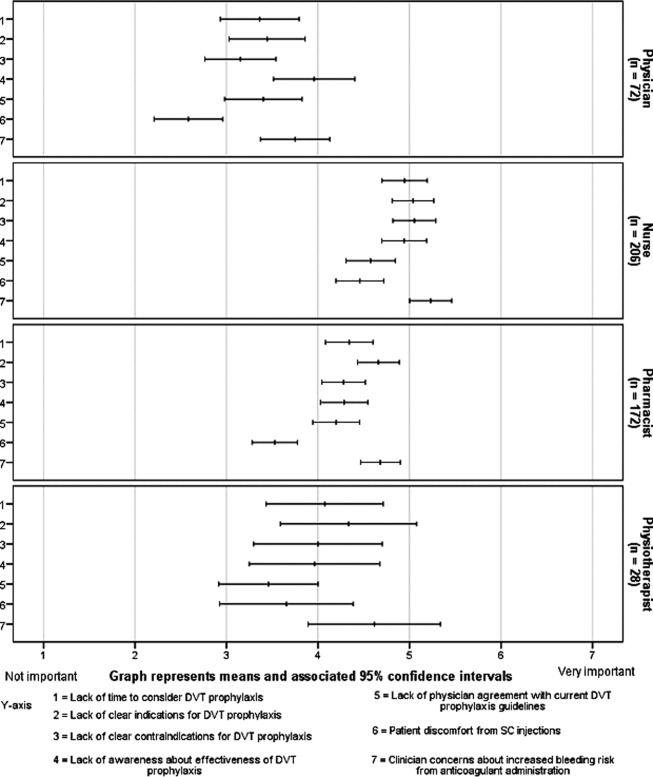
Figure 3 demonstrates that no single barrier to DVT prophylaxis utilization was dominant and no barriers were considered very important. Perceived barriers carrying comparable weight were: concerns about bleeding (mean 4.8; 95% CI 4.6 to 4.9); lack of clear indications (mean 4.6; 95% CI 4.5 to 4.8) and contraindications to DVT prophylaxis (mean 4.4; 95% CI 4.3 to 4.6); lack of awareness about effectiveness of DVT prophylaxis (mean 4.5; 95% CI 4.3 to 4.7); and lack of time to consider DVT prophylaxis in every patient (mean 4.4; 95% CI 4.3 to 4.6). Patient discomfort from subcutaneous injections was perceived as the least important barrier (mean 3.8; 95% CI 3.6 to 4.0). Physicians perceived lack of awareness about the effectiveness of DVT prophylaxis as the most important barrier (mean 4.0; 95% CI 3.5 to 4.4), whereas concern about bleeding was dominant among non‐physicians (nurses' mean 5.2; 95% CI 5.0 to 5.5; pharmacists' mean 4.7; 95% CI 4.5 to 4.9; physiotherapists' mean 4.6; 95% CI 3.9 to 5.3).
Potential Success and Feasibility of Interventions to Optimize DVT Prophylaxis Utilization
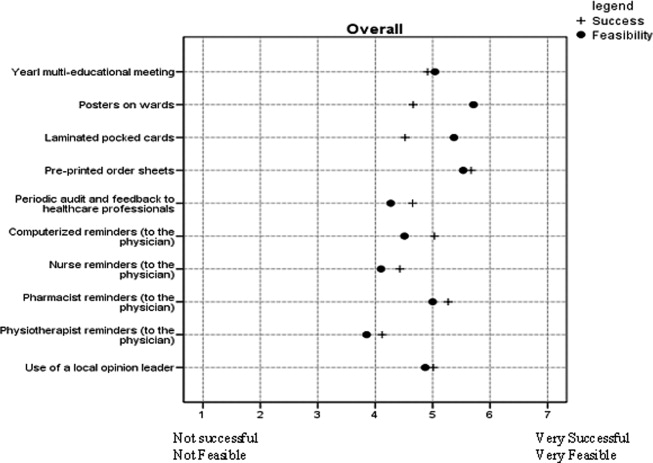
Interventions considered across clinician groups as highly potentially successful were: preprinted order sheets (5.7; 95% CI 5.6 to 5.8); pharmacist reminders to physicians (mean 5.3; 95% CI 5.1 to 5.4); computerized reminders to physicians (mean 5.0; 95% CI 4.9 to 5.2); and use of a local opinion leader (mean 5.0; 95% CI 4.9 to 5.2). Interventions considered highly potentially feasible were: posters (mean 5.7; CI 5.6 to 5.8); preprinted order sheets (mean 5.5; 95% CI 5.4 to 5.7); laminated pocket cards (mean 5.4; 95% CI 5.2 to 5.5); multidisciplinary educational meetings (mean 5.0; 95% CI 4.9 to 5.2); and pharmacist reminders to physicians (mean 5.0; 95% CI 4.9 to 5.1). Preprinted orders and pharmacist reminders were perceived by all clinician groups as having both high potential success and feasibility (Figure 4).
Perceptions on Which Clinician Group Is Best Able to Assess and Implement DVT Prophylaxis
Respondents were divided between considering the attending physician and the bedside nurse as best able to perform a daily assessment of patients' need for DVT prophylaxis (43.4% [204/470] vs 44.0% [207/470], respectively). Respondents from these groups each predominantly thought this responsibility was theirs, with 68.1% (49/72) of physicians and 61.5% (123/200) of nurses perceiving this as their responsibility (Figure 5).
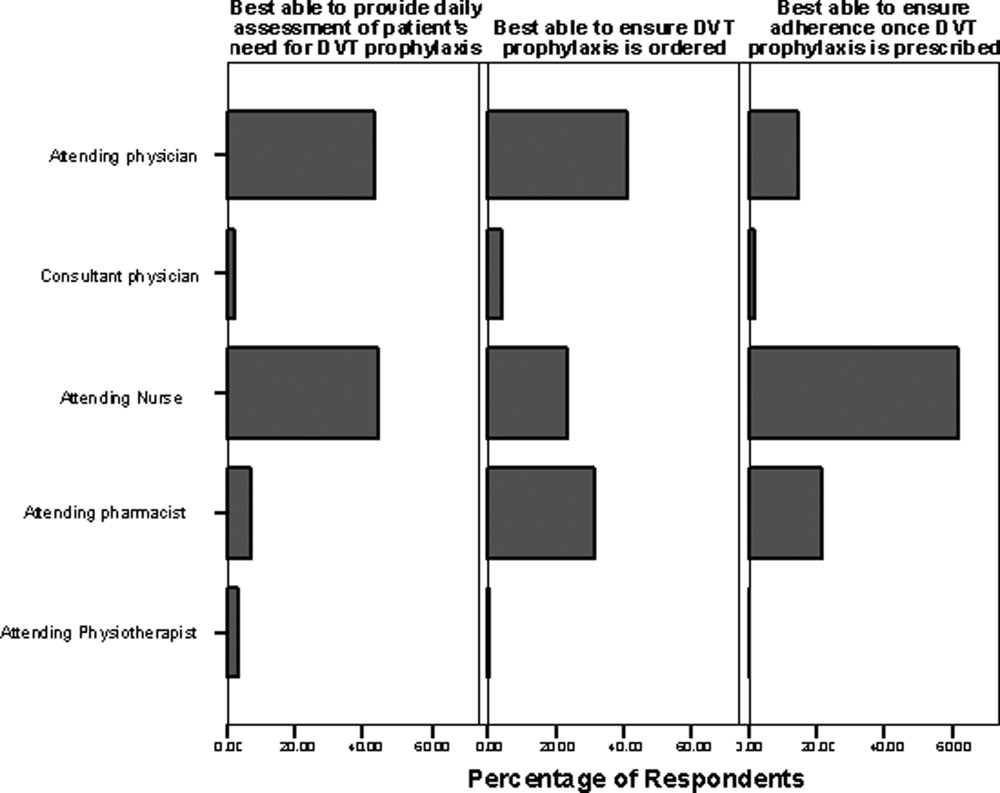
Forty‐one percent (193/471) of respondents perceived the attending physician as best able to ensure that DVT prophylaxis is ordered, while 31.2% (147/471) identified the pharmacist and 23.3% (110/471) identified the bedside nurse as best suited to this role. Among pharmacists, 66.3% (114/172) perceived that the attending pharmacist is best able to perform this task. Among respondents, 61.9% (296/478) felt the bedside nurse is best able to ensure adherence to DVT prophylaxis, with good agreement among all clinician groups.
DISCUSSION
Our survey identified several perceived barriers to optimizing DVT prophylaxis, consistent with those reported in the White Paper sponsored by the American Public Health Association.23 While no single barrier outlined in our survey was dominant, 2 novel barriers were identified: misperception of DVT prophylaxis underutilization, and confusion about roles and responsibilities in the area of DVT prophylaxis. Attention to these barriers may be helpful in developing an intervention aimed at bridging the gap between evidence and practice.
While our survey demonstrates agreement across clinician groups on the importance, efficacy, and safety of DVT prophylaxis, the discordant perceptions that exist about whether DVT prophylaxis is utilized appropriately is an important concern. Physician and pharmacist‐respondents demonstrated awareness that thromboprophylaxis is underutilized in medical patients. However, despite overwhelming published evidence to the contrary, nurses responding to our survey did not tend to recognize the problem of DVT prophylaxis underutilization in hospitalized medical patients. This knowledge deficit may be a significant barrier particularly since the pooled group of respondents indicated that nurses are among those caregivers best able to conduct a daily assessment of patients' need for DVT prophylaxis. A possible explanation for the finding that nurses and physiotherapists demonstrated a relative lack of awareness of the problem of DVT prophylaxis underutilization is ward‐specific healthcare priorities. Nursing and physiotherapy care on surgical wards is aimed at preventing postoperative complications, including DVT. However, its primary focus on medical wards is the management of acute medical problems. Prevention of hospital‐related complications, such as DVT, is often a secondary focus. Therefore, ensuring that all clinician groups are educated about the problem of DVT prophylaxis underutilization is necessary to drive quality improvement. A physician‐based survey on antithrombotic therapies demonstrated a similar need for education on guideline recommendations.20
A second important barrier identified in our survey is that both attending nurses and physicians feel that daily assessment of a patient's need for DVT prophylaxis is their responsibility. Confusion about roles and responsibilities in this area of patient care was reported by Cook et al., who identified that multidisciplinary care was perceived as a barrier to effective VTE prevention.18 Uncertainty as to which group should take ownership of DVT prophylaxis can lead to a diffusion of responsibility, a lack of accountability, and a gap in care. A resolution to whether DVT risk assessment is a nursing or a physician role could be reached through increased interdisciplinary communication and provision of clear definitions of roles to hospital staff.
Survey respondents felt that preprinted orders and pharmacist reminders to physicians were potentially successful and feasible strategies to optimize DVT prophylaxis. These components could be part of a simple tool to initiate prophylaxis. While electronic alerts have been shown to increase prophylaxis rates,24 we suspect that many respondents did not view these as highly important because of limited use of computerized order entry at their facilities. Interestingly, survey respondents did not perceive audit‐and‐feedback systems or local opinion leaders as potentially successful, though previous studies have demonstrated that they can change clinician behavior.25, 26 This may be because respondents may not be aware of the strength of technology‐based interventions (eg, electronic orders) and the role of opinion leaders, and the evidence in support of such interventions.24, 26 A systematic review of studies to improve DVT prophylaxis in hospitals reported that a combination of multiple active strategies is most effective, particularly those that link physician reminders with audit‐and‐feedback.27 For example, in the define study, a multicomponent intervention consisting of interactive educational sessions, verbal and computerized prompts, and individual performance feedback significantly improved adherence to DVT prophylaxis guidelines in critically ill patients.28 Whether a similar intervention could improve adherence to DVT prophylaxis guidelines in hospitalized medical patients merits further study. Any intervention must be paired with better education about which patients should, and should not, receive prophylaxis, as this may address many reported barriers in our survey (including concerns about bleeding). Respondents' uncertainty about these issues is not surprising, as studies of DVT prophylaxis in medical patients are not plentiful.2 However, recent guidelines do identify subgroups of medically ill patients in whom DVT prophylaxis is indicated.2 A clear and simple DVT risk assessment algorithm that identifies medical patients in whom DVT prophylaxis should (or should not) be administered may help to overcome respondents' concerns.
A limitation of our survey is the overall response rate of 36.3%, largely driven by the considerable number of nonresponding pharmacists (n = 760, reflecting 49% of the entire sample). However, the majority of the pharmacists were likely not hospital‐based, were thus not a target of this study, and their low response rate is not surprising. After excluding pharmacists, the response rate was 58.3% (321/551), which is consistent with response rates of other large‐sample surveys.29 The lower response rate for physicians and pharmacists may also reflect web‐based survey dissemination which, despite its feasibility, has lower response rates than paper‐based dissemination.3032 While the sample of physicians was relatively small compared to the other respondent groups surveyed, we aimed to identify barriers to actually implementing VTE prophylaxis, not just ordering prophylaxis, which is a multidisciplinary concern.
Although this survey was based on Canadian healthcare providers' perspectives, we believe the results are generalizable since both US and Canadian‐based studies have found that VTE prophylaxis is underutilized among hospitalized medical patients.4, 6 Furthermore, the American College of Chest Physicians (ACCP) guidelines on VTE prophylaxis, which are well‐recognized in both the United States and Canada, were developed with input from Canadian and American content experts.2 And while the US and Canadian healthcare systems are organized differently, at the patient‐care level, the roles of healthcare professionals are very similar. The generalizability of our findings is, however, limited by the institutional characteristics of respondents. We do not purport that the responses of any of the 4 clinician groups are generalizable to those groups as a whole. Although we surveyed clinicians in teaching and nonteaching, urban and rural practices, perceptions about DVT prophylaxis may be influenced by other factors, including the availability of local preprinted orders, electronic medical records, and quality improvement programs. Another potential limitation is that we did not assess all possible strategies to improve DVT prophylaxis, such as nurse practitioners and computerized decision support systems. These were purposely excluded, as they are not financially feasible in all centers, and thus not generalizable. Finally, like all self‐administered surveys, our findings reflect respondents' perceptions rather than objective observations about practice.
In conclusion, we identified novel and important barriers to optimal DVT prophylaxis utilization and potential interventions to address this important safety concern in hospitalized medical patients. To overcome some of these barriers, we propose an educational intervention prior to delivery of a top‐down, evidence‐based intervention to first increase healthcare providers' knowledge of the safety of DVT prophylaxis, system and team‐based approaches, and which interventions are most likely to be successful so as to encourage greater compliance with the intervention. A top‐down, system‐wide approach, involving the entire healthcare team and hospital administrators, can help drive this communication. As DVT prophylaxis becomes an increasingly important component in hospital accreditation, such solutions become appealing to facilitate change in practices. Results of this survey may inform future knowledge translation interventions by eliminating perceived barriers to DVT prophylaxis and by incorporating strategies that are perceived by healthcare professionals to be successful, feasible, and supported by evidence.
- ,.National hospital discharge survey: annual summary, 1996.Vital Health Stat.1999;13:1–46.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133:381S–443S.
- ,,, et al. Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment: No. 43. AHRQ Publication No. 01‐E058, July 2001. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.ahrq.gov/clinic/ptsafety/. Accessed October 9,2007.
- ,,, et al.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119:145–155.
- ,,,.Hospitals' compliance with prophylaxis guidelines for venous thromboembolism.Am J Health Syst Pharm.2007;64:69–76.
- ,,,.Thromboprophylaxis rates in US medical centers: success or failure?J Thromb Haemost.2007;5:1610–1616.
- ,,.A retrospective evaluation of adherence to guidelines for prevention of thromboembolic events in general medical inpatients.Can J Hosp Pharm.2006;59:258–263.
- ,,, et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132:936–945.
- ,,,.Venous thromboembolism prophylaxis in medical inpatients: a retrospective chart review.Thromb Res.2003;111:215–219.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of DVT prophylaxis guidelines.Chest2001;120:1964–1971.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns.Haematologica.2002;87:746–750.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371:387–394.
- ,,,,.The use of low molecular weight heparins for the prevention of postoperative venous thromboembolism in general surgery. A survey of practice in the United States.Int Angiol.2002;1:78–85.
- ,,, et al.Venous thromboembolic disease management patterns in total hip arthroplasty and total knee arthroplasty patients: a survey of the AAHKS membership.J Arthroplasty.2001;6:679–688.
- ,,.Thromboprophylaxis in medical‐surgical intensive care unit patients.J Crit Care.2005;20:320–323.
- ,.Utilization of venous thromboembolism prophylaxis in a medical‐surgical ICU.Chest.1998;113:162–164.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,,, et al.Thromboprophylaxis for hospitalized medical patients: a multicenter qualitative study.J Hosp Med.2009;4;269–275.
- ,,, et al.Definition of immobility in studies of thromboprophylaxis in hospitalized medical patients: a systematic review.J Vasc Nurs.2010;28:54–66.
- ,,, et al.The use of antithrombotic therapies in the prevention and treatment of arterial and venous thrombosis: a survey of current knowledge and practice supporting the need for clinical education.Crit Pathw Cardiol.2010;9:41–48.
- ,,, et al.Antithrombotic and thrombolytic therapy: from evidence to application: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:688S–696S.
- .Mail and internet surveys: the tailored design method.New York, NY:John Wiley 2000.
- Deep‐vein thrombosis: advancing awareness to protect patient lives. Public Health Leadership Conference on Deep‐Vein Thrombosis. American Public Health Association. Available at: http://www.apha.org/NR/rdonlyres/A209F84A‐7C0E‐4761–9ECF‐61D22E1E11F7/0/DVT_White_Paper.pdf. Accessed May 28,2008.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,, et al.Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43:23–25.
- ,,,.Local opinion leaders: effects on professional practice and health care outcomes.Cochrane Database Syst Rev.2007;24(1):CD000125.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- ,,, et al.Minimizing errors of omission: behavioural reinforcement of heparin to avert venous emboli: the BEHAVE study.Crit Care Med.2006;34:694–699.
- ,,,.Using the Internet to conduct surveys of health professionals: a valid alternative?Fam Pract.2003;20:545–551.
- ,,,,,.Use of new technology in endourology and laparoscopy by American urologists: Internet and postal survey.Urology.2000;56:760–765.
- ,,,,.E‐mail versus conventional postal mail survey of geriatric chiefs.Gerontologist.2001;41:799–804.
- ,,, et al.Internet versus mailed questionnaires: a randomized comparison.J Med Internet Res.2004;6:e30.
Each year in North America, over 7 million adults are hospitalized with a medical illness.1 Acute illness and decreased mobility in hospital places patients at increased risk for venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and life‐threatening pulmonary embolism (PE).2 Since VTE remains the most preventable cause of death in hospitalized patients, numerous studies have aimed at reducing the incidence of hospital‐acquired DVT. Aside from cost, the impact of VTE to the healthcare system is felt not only by those who diagnose and treat VTE, but also by those responsible for correcting the severe bleeding that can result from inappropriate use of thromboprophylaxis. Approximately 60% of symptomatic VTE occurs in medical patients, and recent hospitalization for medical illness accounts for 25% of all community‐diagnosed VTE. The Agency for Health Research and Quality ranks DVT prevention as the top priority out of 79 patient safety initiatives, and expert consensus groups provide a strong recommendation that DVT prophylaxis with a low‐dose anticoagulant should be administered to at‐risk hospitalized medical patients.2, 3
Despite the availability, efficacy, and safety of DVT prophylaxis,2 it is discouraging that only 21% to 62% of medical patients receive prophylaxis,49 and only 16% to 40% receive appropriate prophylaxis.46, 1012 However, 70% to 90% of patients in other at‐risk groups, such as surgical patients or critically ill patients, receive prophylaxis.1316 The reason why DVT prophylaxis is so underutilized in medical patients is unclear, as explanations for low rates of clinical practice guideline utilization are multifaceted,17 and few studies have investigated the barriers to optimal thromboprophylaxis.1820
To explore possible reasons for this disparity between evidence and practice, we conducted a cross‐sectional survey of 4 clinician groups involved in the care of hospitalized medical patients. Our objective was to identify barriers and potential solutions to the underutilization of DVT prophylaxis in hospitalized medical patients.
METHODS
Instrument Development
The survey focused on 3 domains: perceived importance, effectiveness, and safety of DVT prophylaxis; perceived barriers to implementation; and perceived potential success and feasibility of interventions to optimize DVT prophylaxis. The survey cover letter outlined background information, study design, and a statement on confidentiality. A prior survey of DVT prophylaxis administered to thrombosis experts was used to generate survey questions.21
Only survey respondents who answered yes to the first question, Are you involved in any aspect of the care of hospitalized general medical patients for whom DVT prophylaxis is considered? were asked to complete the remaining sections. Subsequent questions required respondents to check the box on a 7‐point Likert‐type scale that most accurately reflected their perception (Table 1). A successful intervention was defined as one that, if implemented, would yield the anticipated effect and a feasible intervention as one that was easy to implement without major logistical burden. Respondents were also asked which clinician group was best able to provide a daily assessment of patients' need for DVT prophylaxis, ensure DVT prophylaxis is prescribed, and ensure adherence.
|
| Section 1: Perceptions regarding DVT prophylaxis in hospitalized medical patients* |
| 1. How important an issue is the prevention of DVT in hospitalized general medical patients? |
| 2. To your knowledge, how effective are currently used anticoagulant strategies for the prevention of DVT in hospitalized medical patients? |
| 3. How safe are currently used anticoagulant strategies for the prevention of DVT in hospitalized medical patients? |
| 4. Current anticoagulant prophylaxis strategies are: 1 = underutilized, 4 = appropriately utilized, 7 = overutilized. |
| Section 2: Perceptions regarding barriers to the optimal use of DVT prophylaxis |
| 1. Lack of time to consider DVT prophylaxis in every patient |
| 2. Lack of clear indications for DVT prophylaxis (ie, who should get prophylaxis) |
| 3. Lack of clear contraindications for DVT prophylaxis (ie, who should not get prophylaxis) |
| 4. Lack of awareness about effectiveness of DVT prophylaxis |
| 5. Lack of physician agreement with current DVT prophylaxis guidelines |
| 6. Patient discomfort from subcutaneous injections of anticoagulants |
| 7. Clinician concerns about increased bleeding risk from anticoagulant administration |
| Section 3: Perceptions of interventions relating to DVT prophylaxis |
| 1. Yearly multidisciplinary educational meetings: to engage a wide spectrum of healthcare professionals to review DVT prophylaxis in hospitalized medical patients |
| 2. Posters on the wards: to remind healthcare professionals about DVT prophylaxis and patients who are eligible or ineligible for this treatment |
| 3. Laminated pocket cards: to remind healthcare professionals about DVT prophylaxis and patients who are eligible and ineligible for this treatment |
| 4. Preprinted order sheets: to remind healthcare professionals about DVT prophylaxis and patients who are eligible and ineligible for this treatment |
| 5. Periodic audit and feedback to healthcare providers: E‐mails to physicians containing reports on compliance with DVT prevention practice guidelines over recent years |
| 6. Computerized reminders (to the physicians): to prompt the physician to consider DVT prophylaxis upon opening a patient's electronic medical record |
| 7. Nurse reminders (to the physician): to remind the physician about DVT prophylaxis using written or verbal reminders |
| 8. Pharmacist reminders (to the physician): to remind the physician about DVT prophylaxis using written or verbal reminders |
| 9. Physiotherapist reminders (to the physician): to remind the physician about DVT prophylaxis using written or verbal reminders |
| 10. Use of a local opinion leader (within the hospital) to promote evidence‐based use of DVT prophylaxis guidelines: to educate healthcare professionals on best practices for DVT prophylaxis |
Survey Administration
The survey was distributed between April and July 2007 in both paper‐based and web‐based formats using Survey Monkey software. Ontario members of the Canadian Society of Internal Medicine (n = 193) received a direct electronic invitation (from N.S.L., on behalf of J.D.D.) to participate, while members of the Canadian Society of Hospital Pharmacists (CSHP) (n = 1002) received an electronic invitation from an administrator for the CSHP to participate. The CSHP could not ensure that all members receiving the survey were hospital‐based pharmacists, so it was expected that the response rate from this group would be low. Nurse and physiotherapy managers at a convenience sample of 8 hospitals in Ontario, Canada, distributed paper‐based surveys to their staff using stamped, preaddressed envelopes. Nonresponders in all groups were sent reminders at 2 and 4 weeks.22 Data from all completed surveys were entered into an electronic database by a research coordinator (N.S.L.). A research assistant entered paper‐based survey data in duplicate, with discrepancies resolved by consensus and mediation by a third person (J.C.). The study was conducted with Institutional Ethics Review Board approval, and all respondents provided informed consent to participate. All responses were anonymous and confidential.
Statistical Considerations
Given the exploratory nature of this survey, there was no prespecified hypothesis‐driven respondent sample size. Proportions were used to describe response rates. Survey responses scored on the 7‐point Likert‐type scale were expressed as a mean and 95% confidence interval (CI). Important, highly potentially successful, and highly potentially feasible barriers were defined as those with a mean 5 points. Questions without responses, questions with multiple responses, and questions with illegible responses were treated as missing values. All statistical analyses were done using SAS version 9 (Cary, NC).
RESULTS
Survey Responses
The overall response rate was 36.3% (563/1553), with 65.5% (211/322) of nurses, 40.4% (78/193) of physicians, 24.1% (242/1002) of pharmacists, and 88.8% (32/36) of physiotherapists completing surveys. When pharmacists were removed from the response rate calculation (since it was expected that many of those receiving the survey were not in a primarily hospital‐based practice), the overall response rate rose to 58.3% (321/551). Excluded were 9.2% (52/563) of returned surveys, as respondents indicated the topic was not relevant to their practice. Five hundred eleven surveys were included in the final analysis (Figure 1).

Importance, Effectiveness, Safety, and Appropriateness of DVT Prophylaxis Strategies
DVT prophylaxis was perceived across clinician groups as important (mean score 6.4; 95% CI 6.3 to 6.5), safe (mean 5.5; 95% CI 5.4 to 5.6), and effective (mean 5.5; 95% CI 5.4 to 6.6) (Figure 2). The mean score for the appropriateness of current DVT prophylaxis practices was 3.5 (95% CI 3.4 to 3.7), suggesting an overall perception of underutilization. However, by respondent groups, DVT prophylaxis was considered to be underutilized by physicians (mean 2.5; 95% CI 2.3 to 2.7) and pharmacists (mean 3.1; 95% CI 2.9 to 3.2), while nurses (mean 4.3; 95% CI 4.2 to 4.5) and physiotherapists (mean 3.8; 95% CI, 3.4 to 4.2) tended to consider current strategies as appropriate.

Potential Barriers to DVT Prophylaxis Utilization
Figure 3 demonstrates that no single barrier to DVT prophylaxis utilization was dominant and no barriers were considered very important. Perceived barriers carrying comparable weight were: concerns about bleeding (mean 4.8; 95% CI 4.6 to 4.9); lack of clear indications (mean 4.6; 95% CI 4.5 to 4.8) and contraindications to DVT prophylaxis (mean 4.4; 95% CI 4.3 to 4.6); lack of awareness about effectiveness of DVT prophylaxis (mean 4.5; 95% CI 4.3 to 4.7); and lack of time to consider DVT prophylaxis in every patient (mean 4.4; 95% CI 4.3 to 4.6). Patient discomfort from subcutaneous injections was perceived as the least important barrier (mean 3.8; 95% CI 3.6 to 4.0). Physicians perceived lack of awareness about the effectiveness of DVT prophylaxis as the most important barrier (mean 4.0; 95% CI 3.5 to 4.4), whereas concern about bleeding was dominant among non‐physicians (nurses' mean 5.2; 95% CI 5.0 to 5.5; pharmacists' mean 4.7; 95% CI 4.5 to 4.9; physiotherapists' mean 4.6; 95% CI 3.9 to 5.3).

Potential Success and Feasibility of Interventions to Optimize DVT Prophylaxis Utilization
Interventions considered across clinician groups as highly potentially successful were: preprinted order sheets (5.7; 95% CI 5.6 to 5.8); pharmacist reminders to physicians (mean 5.3; 95% CI 5.1 to 5.4); computerized reminders to physicians (mean 5.0; 95% CI 4.9 to 5.2); and use of a local opinion leader (mean 5.0; 95% CI 4.9 to 5.2). Interventions considered highly potentially feasible were: posters (mean 5.7; CI 5.6 to 5.8); preprinted order sheets (mean 5.5; 95% CI 5.4 to 5.7); laminated pocket cards (mean 5.4; 95% CI 5.2 to 5.5); multidisciplinary educational meetings (mean 5.0; 95% CI 4.9 to 5.2); and pharmacist reminders to physicians (mean 5.0; 95% CI 4.9 to 5.1). Preprinted orders and pharmacist reminders were perceived by all clinician groups as having both high potential success and feasibility (Figure 4).

Perceptions on Which Clinician Group Is Best Able to Assess and Implement DVT Prophylaxis
Respondents were divided between considering the attending physician and the bedside nurse as best able to perform a daily assessment of patients' need for DVT prophylaxis (43.4% [204/470] vs 44.0% [207/470], respectively). Respondents from these groups each predominantly thought this responsibility was theirs, with 68.1% (49/72) of physicians and 61.5% (123/200) of nurses perceiving this as their responsibility (Figure 5).

Forty‐one percent (193/471) of respondents perceived the attending physician as best able to ensure that DVT prophylaxis is ordered, while 31.2% (147/471) identified the pharmacist and 23.3% (110/471) identified the bedside nurse as best suited to this role. Among pharmacists, 66.3% (114/172) perceived that the attending pharmacist is best able to perform this task. Among respondents, 61.9% (296/478) felt the bedside nurse is best able to ensure adherence to DVT prophylaxis, with good agreement among all clinician groups.
DISCUSSION
Our survey identified several perceived barriers to optimizing DVT prophylaxis, consistent with those reported in the White Paper sponsored by the American Public Health Association.23 While no single barrier outlined in our survey was dominant, 2 novel barriers were identified: misperception of DVT prophylaxis underutilization, and confusion about roles and responsibilities in the area of DVT prophylaxis. Attention to these barriers may be helpful in developing an intervention aimed at bridging the gap between evidence and practice.
While our survey demonstrates agreement across clinician groups on the importance, efficacy, and safety of DVT prophylaxis, the discordant perceptions that exist about whether DVT prophylaxis is utilized appropriately is an important concern. Physician and pharmacist‐respondents demonstrated awareness that thromboprophylaxis is underutilized in medical patients. However, despite overwhelming published evidence to the contrary, nurses responding to our survey did not tend to recognize the problem of DVT prophylaxis underutilization in hospitalized medical patients. This knowledge deficit may be a significant barrier particularly since the pooled group of respondents indicated that nurses are among those caregivers best able to conduct a daily assessment of patients' need for DVT prophylaxis. A possible explanation for the finding that nurses and physiotherapists demonstrated a relative lack of awareness of the problem of DVT prophylaxis underutilization is ward‐specific healthcare priorities. Nursing and physiotherapy care on surgical wards is aimed at preventing postoperative complications, including DVT. However, its primary focus on medical wards is the management of acute medical problems. Prevention of hospital‐related complications, such as DVT, is often a secondary focus. Therefore, ensuring that all clinician groups are educated about the problem of DVT prophylaxis underutilization is necessary to drive quality improvement. A physician‐based survey on antithrombotic therapies demonstrated a similar need for education on guideline recommendations.20
A second important barrier identified in our survey is that both attending nurses and physicians feel that daily assessment of a patient's need for DVT prophylaxis is their responsibility. Confusion about roles and responsibilities in this area of patient care was reported by Cook et al., who identified that multidisciplinary care was perceived as a barrier to effective VTE prevention.18 Uncertainty as to which group should take ownership of DVT prophylaxis can lead to a diffusion of responsibility, a lack of accountability, and a gap in care. A resolution to whether DVT risk assessment is a nursing or a physician role could be reached through increased interdisciplinary communication and provision of clear definitions of roles to hospital staff.
Survey respondents felt that preprinted orders and pharmacist reminders to physicians were potentially successful and feasible strategies to optimize DVT prophylaxis. These components could be part of a simple tool to initiate prophylaxis. While electronic alerts have been shown to increase prophylaxis rates,24 we suspect that many respondents did not view these as highly important because of limited use of computerized order entry at their facilities. Interestingly, survey respondents did not perceive audit‐and‐feedback systems or local opinion leaders as potentially successful, though previous studies have demonstrated that they can change clinician behavior.25, 26 This may be because respondents may not be aware of the strength of technology‐based interventions (eg, electronic orders) and the role of opinion leaders, and the evidence in support of such interventions.24, 26 A systematic review of studies to improve DVT prophylaxis in hospitals reported that a combination of multiple active strategies is most effective, particularly those that link physician reminders with audit‐and‐feedback.27 For example, in the define study, a multicomponent intervention consisting of interactive educational sessions, verbal and computerized prompts, and individual performance feedback significantly improved adherence to DVT prophylaxis guidelines in critically ill patients.28 Whether a similar intervention could improve adherence to DVT prophylaxis guidelines in hospitalized medical patients merits further study. Any intervention must be paired with better education about which patients should, and should not, receive prophylaxis, as this may address many reported barriers in our survey (including concerns about bleeding). Respondents' uncertainty about these issues is not surprising, as studies of DVT prophylaxis in medical patients are not plentiful.2 However, recent guidelines do identify subgroups of medically ill patients in whom DVT prophylaxis is indicated.2 A clear and simple DVT risk assessment algorithm that identifies medical patients in whom DVT prophylaxis should (or should not) be administered may help to overcome respondents' concerns.
A limitation of our survey is the overall response rate of 36.3%, largely driven by the considerable number of nonresponding pharmacists (n = 760, reflecting 49% of the entire sample). However, the majority of the pharmacists were likely not hospital‐based, were thus not a target of this study, and their low response rate is not surprising. After excluding pharmacists, the response rate was 58.3% (321/551), which is consistent with response rates of other large‐sample surveys.29 The lower response rate for physicians and pharmacists may also reflect web‐based survey dissemination which, despite its feasibility, has lower response rates than paper‐based dissemination.3032 While the sample of physicians was relatively small compared to the other respondent groups surveyed, we aimed to identify barriers to actually implementing VTE prophylaxis, not just ordering prophylaxis, which is a multidisciplinary concern.
Although this survey was based on Canadian healthcare providers' perspectives, we believe the results are generalizable since both US and Canadian‐based studies have found that VTE prophylaxis is underutilized among hospitalized medical patients.4, 6 Furthermore, the American College of Chest Physicians (ACCP) guidelines on VTE prophylaxis, which are well‐recognized in both the United States and Canada, were developed with input from Canadian and American content experts.2 And while the US and Canadian healthcare systems are organized differently, at the patient‐care level, the roles of healthcare professionals are very similar. The generalizability of our findings is, however, limited by the institutional characteristics of respondents. We do not purport that the responses of any of the 4 clinician groups are generalizable to those groups as a whole. Although we surveyed clinicians in teaching and nonteaching, urban and rural practices, perceptions about DVT prophylaxis may be influenced by other factors, including the availability of local preprinted orders, electronic medical records, and quality improvement programs. Another potential limitation is that we did not assess all possible strategies to improve DVT prophylaxis, such as nurse practitioners and computerized decision support systems. These were purposely excluded, as they are not financially feasible in all centers, and thus not generalizable. Finally, like all self‐administered surveys, our findings reflect respondents' perceptions rather than objective observations about practice.
In conclusion, we identified novel and important barriers to optimal DVT prophylaxis utilization and potential interventions to address this important safety concern in hospitalized medical patients. To overcome some of these barriers, we propose an educational intervention prior to delivery of a top‐down, evidence‐based intervention to first increase healthcare providers' knowledge of the safety of DVT prophylaxis, system and team‐based approaches, and which interventions are most likely to be successful so as to encourage greater compliance with the intervention. A top‐down, system‐wide approach, involving the entire healthcare team and hospital administrators, can help drive this communication. As DVT prophylaxis becomes an increasingly important component in hospital accreditation, such solutions become appealing to facilitate change in practices. Results of this survey may inform future knowledge translation interventions by eliminating perceived barriers to DVT prophylaxis and by incorporating strategies that are perceived by healthcare professionals to be successful, feasible, and supported by evidence.
Each year in North America, over 7 million adults are hospitalized with a medical illness.1 Acute illness and decreased mobility in hospital places patients at increased risk for venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and life‐threatening pulmonary embolism (PE).2 Since VTE remains the most preventable cause of death in hospitalized patients, numerous studies have aimed at reducing the incidence of hospital‐acquired DVT. Aside from cost, the impact of VTE to the healthcare system is felt not only by those who diagnose and treat VTE, but also by those responsible for correcting the severe bleeding that can result from inappropriate use of thromboprophylaxis. Approximately 60% of symptomatic VTE occurs in medical patients, and recent hospitalization for medical illness accounts for 25% of all community‐diagnosed VTE. The Agency for Health Research and Quality ranks DVT prevention as the top priority out of 79 patient safety initiatives, and expert consensus groups provide a strong recommendation that DVT prophylaxis with a low‐dose anticoagulant should be administered to at‐risk hospitalized medical patients.2, 3
Despite the availability, efficacy, and safety of DVT prophylaxis,2 it is discouraging that only 21% to 62% of medical patients receive prophylaxis,49 and only 16% to 40% receive appropriate prophylaxis.46, 1012 However, 70% to 90% of patients in other at‐risk groups, such as surgical patients or critically ill patients, receive prophylaxis.1316 The reason why DVT prophylaxis is so underutilized in medical patients is unclear, as explanations for low rates of clinical practice guideline utilization are multifaceted,17 and few studies have investigated the barriers to optimal thromboprophylaxis.1820
To explore possible reasons for this disparity between evidence and practice, we conducted a cross‐sectional survey of 4 clinician groups involved in the care of hospitalized medical patients. Our objective was to identify barriers and potential solutions to the underutilization of DVT prophylaxis in hospitalized medical patients.
METHODS
Instrument Development
The survey focused on 3 domains: perceived importance, effectiveness, and safety of DVT prophylaxis; perceived barriers to implementation; and perceived potential success and feasibility of interventions to optimize DVT prophylaxis. The survey cover letter outlined background information, study design, and a statement on confidentiality. A prior survey of DVT prophylaxis administered to thrombosis experts was used to generate survey questions.21
Only survey respondents who answered yes to the first question, Are you involved in any aspect of the care of hospitalized general medical patients for whom DVT prophylaxis is considered? were asked to complete the remaining sections. Subsequent questions required respondents to check the box on a 7‐point Likert‐type scale that most accurately reflected their perception (Table 1). A successful intervention was defined as one that, if implemented, would yield the anticipated effect and a feasible intervention as one that was easy to implement without major logistical burden. Respondents were also asked which clinician group was best able to provide a daily assessment of patients' need for DVT prophylaxis, ensure DVT prophylaxis is prescribed, and ensure adherence.
|
| Section 1: Perceptions regarding DVT prophylaxis in hospitalized medical patients* |
| 1. How important an issue is the prevention of DVT in hospitalized general medical patients? |
| 2. To your knowledge, how effective are currently used anticoagulant strategies for the prevention of DVT in hospitalized medical patients? |
| 3. How safe are currently used anticoagulant strategies for the prevention of DVT in hospitalized medical patients? |
| 4. Current anticoagulant prophylaxis strategies are: 1 = underutilized, 4 = appropriately utilized, 7 = overutilized. |
| Section 2: Perceptions regarding barriers to the optimal use of DVT prophylaxis |
| 1. Lack of time to consider DVT prophylaxis in every patient |
| 2. Lack of clear indications for DVT prophylaxis (ie, who should get prophylaxis) |
| 3. Lack of clear contraindications for DVT prophylaxis (ie, who should not get prophylaxis) |
| 4. Lack of awareness about effectiveness of DVT prophylaxis |
| 5. Lack of physician agreement with current DVT prophylaxis guidelines |
| 6. Patient discomfort from subcutaneous injections of anticoagulants |
| 7. Clinician concerns about increased bleeding risk from anticoagulant administration |
| Section 3: Perceptions of interventions relating to DVT prophylaxis |
| 1. Yearly multidisciplinary educational meetings: to engage a wide spectrum of healthcare professionals to review DVT prophylaxis in hospitalized medical patients |
| 2. Posters on the wards: to remind healthcare professionals about DVT prophylaxis and patients who are eligible or ineligible for this treatment |
| 3. Laminated pocket cards: to remind healthcare professionals about DVT prophylaxis and patients who are eligible and ineligible for this treatment |
| 4. Preprinted order sheets: to remind healthcare professionals about DVT prophylaxis and patients who are eligible and ineligible for this treatment |
| 5. Periodic audit and feedback to healthcare providers: E‐mails to physicians containing reports on compliance with DVT prevention practice guidelines over recent years |
| 6. Computerized reminders (to the physicians): to prompt the physician to consider DVT prophylaxis upon opening a patient's electronic medical record |
| 7. Nurse reminders (to the physician): to remind the physician about DVT prophylaxis using written or verbal reminders |
| 8. Pharmacist reminders (to the physician): to remind the physician about DVT prophylaxis using written or verbal reminders |
| 9. Physiotherapist reminders (to the physician): to remind the physician about DVT prophylaxis using written or verbal reminders |
| 10. Use of a local opinion leader (within the hospital) to promote evidence‐based use of DVT prophylaxis guidelines: to educate healthcare professionals on best practices for DVT prophylaxis |
Survey Administration
The survey was distributed between April and July 2007 in both paper‐based and web‐based formats using Survey Monkey software. Ontario members of the Canadian Society of Internal Medicine (n = 193) received a direct electronic invitation (from N.S.L., on behalf of J.D.D.) to participate, while members of the Canadian Society of Hospital Pharmacists (CSHP) (n = 1002) received an electronic invitation from an administrator for the CSHP to participate. The CSHP could not ensure that all members receiving the survey were hospital‐based pharmacists, so it was expected that the response rate from this group would be low. Nurse and physiotherapy managers at a convenience sample of 8 hospitals in Ontario, Canada, distributed paper‐based surveys to their staff using stamped, preaddressed envelopes. Nonresponders in all groups were sent reminders at 2 and 4 weeks.22 Data from all completed surveys were entered into an electronic database by a research coordinator (N.S.L.). A research assistant entered paper‐based survey data in duplicate, with discrepancies resolved by consensus and mediation by a third person (J.C.). The study was conducted with Institutional Ethics Review Board approval, and all respondents provided informed consent to participate. All responses were anonymous and confidential.
Statistical Considerations
Given the exploratory nature of this survey, there was no prespecified hypothesis‐driven respondent sample size. Proportions were used to describe response rates. Survey responses scored on the 7‐point Likert‐type scale were expressed as a mean and 95% confidence interval (CI). Important, highly potentially successful, and highly potentially feasible barriers were defined as those with a mean 5 points. Questions without responses, questions with multiple responses, and questions with illegible responses were treated as missing values. All statistical analyses were done using SAS version 9 (Cary, NC).
RESULTS
Survey Responses
The overall response rate was 36.3% (563/1553), with 65.5% (211/322) of nurses, 40.4% (78/193) of physicians, 24.1% (242/1002) of pharmacists, and 88.8% (32/36) of physiotherapists completing surveys. When pharmacists were removed from the response rate calculation (since it was expected that many of those receiving the survey were not in a primarily hospital‐based practice), the overall response rate rose to 58.3% (321/551). Excluded were 9.2% (52/563) of returned surveys, as respondents indicated the topic was not relevant to their practice. Five hundred eleven surveys were included in the final analysis (Figure 1).

Importance, Effectiveness, Safety, and Appropriateness of DVT Prophylaxis Strategies
DVT prophylaxis was perceived across clinician groups as important (mean score 6.4; 95% CI 6.3 to 6.5), safe (mean 5.5; 95% CI 5.4 to 5.6), and effective (mean 5.5; 95% CI 5.4 to 6.6) (Figure 2). The mean score for the appropriateness of current DVT prophylaxis practices was 3.5 (95% CI 3.4 to 3.7), suggesting an overall perception of underutilization. However, by respondent groups, DVT prophylaxis was considered to be underutilized by physicians (mean 2.5; 95% CI 2.3 to 2.7) and pharmacists (mean 3.1; 95% CI 2.9 to 3.2), while nurses (mean 4.3; 95% CI 4.2 to 4.5) and physiotherapists (mean 3.8; 95% CI, 3.4 to 4.2) tended to consider current strategies as appropriate.

Potential Barriers to DVT Prophylaxis Utilization
Figure 3 demonstrates that no single barrier to DVT prophylaxis utilization was dominant and no barriers were considered very important. Perceived barriers carrying comparable weight were: concerns about bleeding (mean 4.8; 95% CI 4.6 to 4.9); lack of clear indications (mean 4.6; 95% CI 4.5 to 4.8) and contraindications to DVT prophylaxis (mean 4.4; 95% CI 4.3 to 4.6); lack of awareness about effectiveness of DVT prophylaxis (mean 4.5; 95% CI 4.3 to 4.7); and lack of time to consider DVT prophylaxis in every patient (mean 4.4; 95% CI 4.3 to 4.6). Patient discomfort from subcutaneous injections was perceived as the least important barrier (mean 3.8; 95% CI 3.6 to 4.0). Physicians perceived lack of awareness about the effectiveness of DVT prophylaxis as the most important barrier (mean 4.0; 95% CI 3.5 to 4.4), whereas concern about bleeding was dominant among non‐physicians (nurses' mean 5.2; 95% CI 5.0 to 5.5; pharmacists' mean 4.7; 95% CI 4.5 to 4.9; physiotherapists' mean 4.6; 95% CI 3.9 to 5.3).

Potential Success and Feasibility of Interventions to Optimize DVT Prophylaxis Utilization
Interventions considered across clinician groups as highly potentially successful were: preprinted order sheets (5.7; 95% CI 5.6 to 5.8); pharmacist reminders to physicians (mean 5.3; 95% CI 5.1 to 5.4); computerized reminders to physicians (mean 5.0; 95% CI 4.9 to 5.2); and use of a local opinion leader (mean 5.0; 95% CI 4.9 to 5.2). Interventions considered highly potentially feasible were: posters (mean 5.7; CI 5.6 to 5.8); preprinted order sheets (mean 5.5; 95% CI 5.4 to 5.7); laminated pocket cards (mean 5.4; 95% CI 5.2 to 5.5); multidisciplinary educational meetings (mean 5.0; 95% CI 4.9 to 5.2); and pharmacist reminders to physicians (mean 5.0; 95% CI 4.9 to 5.1). Preprinted orders and pharmacist reminders were perceived by all clinician groups as having both high potential success and feasibility (Figure 4).

Perceptions on Which Clinician Group Is Best Able to Assess and Implement DVT Prophylaxis
Respondents were divided between considering the attending physician and the bedside nurse as best able to perform a daily assessment of patients' need for DVT prophylaxis (43.4% [204/470] vs 44.0% [207/470], respectively). Respondents from these groups each predominantly thought this responsibility was theirs, with 68.1% (49/72) of physicians and 61.5% (123/200) of nurses perceiving this as their responsibility (Figure 5).

Forty‐one percent (193/471) of respondents perceived the attending physician as best able to ensure that DVT prophylaxis is ordered, while 31.2% (147/471) identified the pharmacist and 23.3% (110/471) identified the bedside nurse as best suited to this role. Among pharmacists, 66.3% (114/172) perceived that the attending pharmacist is best able to perform this task. Among respondents, 61.9% (296/478) felt the bedside nurse is best able to ensure adherence to DVT prophylaxis, with good agreement among all clinician groups.
DISCUSSION
Our survey identified several perceived barriers to optimizing DVT prophylaxis, consistent with those reported in the White Paper sponsored by the American Public Health Association.23 While no single barrier outlined in our survey was dominant, 2 novel barriers were identified: misperception of DVT prophylaxis underutilization, and confusion about roles and responsibilities in the area of DVT prophylaxis. Attention to these barriers may be helpful in developing an intervention aimed at bridging the gap between evidence and practice.
While our survey demonstrates agreement across clinician groups on the importance, efficacy, and safety of DVT prophylaxis, the discordant perceptions that exist about whether DVT prophylaxis is utilized appropriately is an important concern. Physician and pharmacist‐respondents demonstrated awareness that thromboprophylaxis is underutilized in medical patients. However, despite overwhelming published evidence to the contrary, nurses responding to our survey did not tend to recognize the problem of DVT prophylaxis underutilization in hospitalized medical patients. This knowledge deficit may be a significant barrier particularly since the pooled group of respondents indicated that nurses are among those caregivers best able to conduct a daily assessment of patients' need for DVT prophylaxis. A possible explanation for the finding that nurses and physiotherapists demonstrated a relative lack of awareness of the problem of DVT prophylaxis underutilization is ward‐specific healthcare priorities. Nursing and physiotherapy care on surgical wards is aimed at preventing postoperative complications, including DVT. However, its primary focus on medical wards is the management of acute medical problems. Prevention of hospital‐related complications, such as DVT, is often a secondary focus. Therefore, ensuring that all clinician groups are educated about the problem of DVT prophylaxis underutilization is necessary to drive quality improvement. A physician‐based survey on antithrombotic therapies demonstrated a similar need for education on guideline recommendations.20
A second important barrier identified in our survey is that both attending nurses and physicians feel that daily assessment of a patient's need for DVT prophylaxis is their responsibility. Confusion about roles and responsibilities in this area of patient care was reported by Cook et al., who identified that multidisciplinary care was perceived as a barrier to effective VTE prevention.18 Uncertainty as to which group should take ownership of DVT prophylaxis can lead to a diffusion of responsibility, a lack of accountability, and a gap in care. A resolution to whether DVT risk assessment is a nursing or a physician role could be reached through increased interdisciplinary communication and provision of clear definitions of roles to hospital staff.
Survey respondents felt that preprinted orders and pharmacist reminders to physicians were potentially successful and feasible strategies to optimize DVT prophylaxis. These components could be part of a simple tool to initiate prophylaxis. While electronic alerts have been shown to increase prophylaxis rates,24 we suspect that many respondents did not view these as highly important because of limited use of computerized order entry at their facilities. Interestingly, survey respondents did not perceive audit‐and‐feedback systems or local opinion leaders as potentially successful, though previous studies have demonstrated that they can change clinician behavior.25, 26 This may be because respondents may not be aware of the strength of technology‐based interventions (eg, electronic orders) and the role of opinion leaders, and the evidence in support of such interventions.24, 26 A systematic review of studies to improve DVT prophylaxis in hospitals reported that a combination of multiple active strategies is most effective, particularly those that link physician reminders with audit‐and‐feedback.27 For example, in the define study, a multicomponent intervention consisting of interactive educational sessions, verbal and computerized prompts, and individual performance feedback significantly improved adherence to DVT prophylaxis guidelines in critically ill patients.28 Whether a similar intervention could improve adherence to DVT prophylaxis guidelines in hospitalized medical patients merits further study. Any intervention must be paired with better education about which patients should, and should not, receive prophylaxis, as this may address many reported barriers in our survey (including concerns about bleeding). Respondents' uncertainty about these issues is not surprising, as studies of DVT prophylaxis in medical patients are not plentiful.2 However, recent guidelines do identify subgroups of medically ill patients in whom DVT prophylaxis is indicated.2 A clear and simple DVT risk assessment algorithm that identifies medical patients in whom DVT prophylaxis should (or should not) be administered may help to overcome respondents' concerns.
A limitation of our survey is the overall response rate of 36.3%, largely driven by the considerable number of nonresponding pharmacists (n = 760, reflecting 49% of the entire sample). However, the majority of the pharmacists were likely not hospital‐based, were thus not a target of this study, and their low response rate is not surprising. After excluding pharmacists, the response rate was 58.3% (321/551), which is consistent with response rates of other large‐sample surveys.29 The lower response rate for physicians and pharmacists may also reflect web‐based survey dissemination which, despite its feasibility, has lower response rates than paper‐based dissemination.3032 While the sample of physicians was relatively small compared to the other respondent groups surveyed, we aimed to identify barriers to actually implementing VTE prophylaxis, not just ordering prophylaxis, which is a multidisciplinary concern.
Although this survey was based on Canadian healthcare providers' perspectives, we believe the results are generalizable since both US and Canadian‐based studies have found that VTE prophylaxis is underutilized among hospitalized medical patients.4, 6 Furthermore, the American College of Chest Physicians (ACCP) guidelines on VTE prophylaxis, which are well‐recognized in both the United States and Canada, were developed with input from Canadian and American content experts.2 And while the US and Canadian healthcare systems are organized differently, at the patient‐care level, the roles of healthcare professionals are very similar. The generalizability of our findings is, however, limited by the institutional characteristics of respondents. We do not purport that the responses of any of the 4 clinician groups are generalizable to those groups as a whole. Although we surveyed clinicians in teaching and nonteaching, urban and rural practices, perceptions about DVT prophylaxis may be influenced by other factors, including the availability of local preprinted orders, electronic medical records, and quality improvement programs. Another potential limitation is that we did not assess all possible strategies to improve DVT prophylaxis, such as nurse practitioners and computerized decision support systems. These were purposely excluded, as they are not financially feasible in all centers, and thus not generalizable. Finally, like all self‐administered surveys, our findings reflect respondents' perceptions rather than objective observations about practice.
In conclusion, we identified novel and important barriers to optimal DVT prophylaxis utilization and potential interventions to address this important safety concern in hospitalized medical patients. To overcome some of these barriers, we propose an educational intervention prior to delivery of a top‐down, evidence‐based intervention to first increase healthcare providers' knowledge of the safety of DVT prophylaxis, system and team‐based approaches, and which interventions are most likely to be successful so as to encourage greater compliance with the intervention. A top‐down, system‐wide approach, involving the entire healthcare team and hospital administrators, can help drive this communication. As DVT prophylaxis becomes an increasingly important component in hospital accreditation, such solutions become appealing to facilitate change in practices. Results of this survey may inform future knowledge translation interventions by eliminating perceived barriers to DVT prophylaxis and by incorporating strategies that are perceived by healthcare professionals to be successful, feasible, and supported by evidence.
- ,.National hospital discharge survey: annual summary, 1996.Vital Health Stat.1999;13:1–46.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133:381S–443S.
- ,,, et al. Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment: No. 43. AHRQ Publication No. 01‐E058, July 2001. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.ahrq.gov/clinic/ptsafety/. Accessed October 9,2007.
- ,,, et al.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119:145–155.
- ,,,.Hospitals' compliance with prophylaxis guidelines for venous thromboembolism.Am J Health Syst Pharm.2007;64:69–76.
- ,,,.Thromboprophylaxis rates in US medical centers: success or failure?J Thromb Haemost.2007;5:1610–1616.
- ,,.A retrospective evaluation of adherence to guidelines for prevention of thromboembolic events in general medical inpatients.Can J Hosp Pharm.2006;59:258–263.
- ,,, et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132:936–945.
- ,,,.Venous thromboembolism prophylaxis in medical inpatients: a retrospective chart review.Thromb Res.2003;111:215–219.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of DVT prophylaxis guidelines.Chest2001;120:1964–1971.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns.Haematologica.2002;87:746–750.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371:387–394.
- ,,,,.The use of low molecular weight heparins for the prevention of postoperative venous thromboembolism in general surgery. A survey of practice in the United States.Int Angiol.2002;1:78–85.
- ,,, et al.Venous thromboembolic disease management patterns in total hip arthroplasty and total knee arthroplasty patients: a survey of the AAHKS membership.J Arthroplasty.2001;6:679–688.
- ,,.Thromboprophylaxis in medical‐surgical intensive care unit patients.J Crit Care.2005;20:320–323.
- ,.Utilization of venous thromboembolism prophylaxis in a medical‐surgical ICU.Chest.1998;113:162–164.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,,, et al.Thromboprophylaxis for hospitalized medical patients: a multicenter qualitative study.J Hosp Med.2009;4;269–275.
- ,,, et al.Definition of immobility in studies of thromboprophylaxis in hospitalized medical patients: a systematic review.J Vasc Nurs.2010;28:54–66.
- ,,, et al.The use of antithrombotic therapies in the prevention and treatment of arterial and venous thrombosis: a survey of current knowledge and practice supporting the need for clinical education.Crit Pathw Cardiol.2010;9:41–48.
- ,,, et al.Antithrombotic and thrombolytic therapy: from evidence to application: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:688S–696S.
- .Mail and internet surveys: the tailored design method.New York, NY:John Wiley 2000.
- Deep‐vein thrombosis: advancing awareness to protect patient lives. Public Health Leadership Conference on Deep‐Vein Thrombosis. American Public Health Association. Available at: http://www.apha.org/NR/rdonlyres/A209F84A‐7C0E‐4761–9ECF‐61D22E1E11F7/0/DVT_White_Paper.pdf. Accessed May 28,2008.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,, et al.Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43:23–25.
- ,,,.Local opinion leaders: effects on professional practice and health care outcomes.Cochrane Database Syst Rev.2007;24(1):CD000125.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- ,,, et al.Minimizing errors of omission: behavioural reinforcement of heparin to avert venous emboli: the BEHAVE study.Crit Care Med.2006;34:694–699.
- ,,,.Using the Internet to conduct surveys of health professionals: a valid alternative?Fam Pract.2003;20:545–551.
- ,,,,,.Use of new technology in endourology and laparoscopy by American urologists: Internet and postal survey.Urology.2000;56:760–765.
- ,,,,.E‐mail versus conventional postal mail survey of geriatric chiefs.Gerontologist.2001;41:799–804.
- ,,, et al.Internet versus mailed questionnaires: a randomized comparison.J Med Internet Res.2004;6:e30.
- ,.National hospital discharge survey: annual summary, 1996.Vital Health Stat.1999;13:1–46.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133:381S–443S.
- ,,, et al. Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment: No. 43. AHRQ Publication No. 01‐E058, July 2001. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.ahrq.gov/clinic/ptsafety/. Accessed October 9,2007.
- ,,, et al.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119:145–155.
- ,,,.Hospitals' compliance with prophylaxis guidelines for venous thromboembolism.Am J Health Syst Pharm.2007;64:69–76.
- ,,,.Thromboprophylaxis rates in US medical centers: success or failure?J Thromb Haemost.2007;5:1610–1616.
- ,,.A retrospective evaluation of adherence to guidelines for prevention of thromboembolic events in general medical inpatients.Can J Hosp Pharm.2006;59:258–263.
- ,,, et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132:936–945.
- ,,,.Venous thromboembolism prophylaxis in medical inpatients: a retrospective chart review.Thromb Res.2003;111:215–219.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of DVT prophylaxis guidelines.Chest2001;120:1964–1971.
- ,,, et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns.Haematologica.2002;87:746–750.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371:387–394.
- ,,,,.The use of low molecular weight heparins for the prevention of postoperative venous thromboembolism in general surgery. A survey of practice in the United States.Int Angiol.2002;1:78–85.
- ,,, et al.Venous thromboembolic disease management patterns in total hip arthroplasty and total knee arthroplasty patients: a survey of the AAHKS membership.J Arthroplasty.2001;6:679–688.
- ,,.Thromboprophylaxis in medical‐surgical intensive care unit patients.J Crit Care.2005;20:320–323.
- ,.Utilization of venous thromboembolism prophylaxis in a medical‐surgical ICU.Chest.1998;113:162–164.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,,, et al.Thromboprophylaxis for hospitalized medical patients: a multicenter qualitative study.J Hosp Med.2009;4;269–275.
- ,,, et al.Definition of immobility in studies of thromboprophylaxis in hospitalized medical patients: a systematic review.J Vasc Nurs.2010;28:54–66.
- ,,, et al.The use of antithrombotic therapies in the prevention and treatment of arterial and venous thrombosis: a survey of current knowledge and practice supporting the need for clinical education.Crit Pathw Cardiol.2010;9:41–48.
- ,,, et al.Antithrombotic and thrombolytic therapy: from evidence to application: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:688S–696S.
- .Mail and internet surveys: the tailored design method.New York, NY:John Wiley 2000.
- Deep‐vein thrombosis: advancing awareness to protect patient lives. Public Health Leadership Conference on Deep‐Vein Thrombosis. American Public Health Association. Available at: http://www.apha.org/NR/rdonlyres/A209F84A‐7C0E‐4761–9ECF‐61D22E1E11F7/0/DVT_White_Paper.pdf. Accessed May 28,2008.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,, et al.Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43:23–25.
- ,,,.Local opinion leaders: effects on professional practice and health care outcomes.Cochrane Database Syst Rev.2007;24(1):CD000125.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- ,,, et al.Minimizing errors of omission: behavioural reinforcement of heparin to avert venous emboli: the BEHAVE study.Crit Care Med.2006;34:694–699.
- ,,,.Using the Internet to conduct surveys of health professionals: a valid alternative?Fam Pract.2003;20:545–551.
- ,,,,,.Use of new technology in endourology and laparoscopy by American urologists: Internet and postal survey.Urology.2000;56:760–765.
- ,,,,.E‐mail versus conventional postal mail survey of geriatric chiefs.Gerontologist.2001;41:799–804.
- ,,, et al.Internet versus mailed questionnaires: a randomized comparison.J Med Internet Res.2004;6:e30.
Copyright © 2011 Society of Hospital Medicine
Preventing venous thromboembolism in long-term care residents: Cautious advice based on limited data
Randomized trials that included more than 20,000 medical patients have shown that anticoagulant therapy is safe and effective in preventing venous thromboembolism (VTE), ie, deep vein thrombosis and pulmonary embolism.
However, these trials were done in hospitalized patients, who typically had an acute medical illness and who, if eligible, received a short (7- to 10-day) course of anticoagulant prophylaxis.
Little attention has been given to VTE prophylaxis in residents of long-term care facilities. These patients have risk profiles similar to those of hospitalized medical patients. Some of them may have been transferred from an acute care hospital. In addition, most are elderly, and many have reduced mobility and are at risk for illnesses such as stroke and cardiorespiratory insufficiency, which increase the risk of VTE.
VTE in residents of long-term care facilities is a growing concern. By some estimates, by the year 2030 more than 20% of the US population (70.2 million people) will be over 65 years of age.1 Of those who reached age 65 in 1990, an estimated 43% will enter a nursing home at least once before they die—32% for 3 months, 24% for at least a year, and 9% for at least 5 years.2
Against this background, the objectives of this review are to consider:
- The scope of the problem of VTE in long-term care residents
- Why VTE prophylaxis is often overlooked in medical patients
- Evidence—or lack of evidence—for the safety and efficacy of VTE prophylaxis in long-term care residents and other medical patients
- Available options for VTE prophylaxis
- Which long-term care residents should or should not be considered for prophylaxis.
THE TRUE SCOPE OF THE PROBLEM IS UNKNOWN
The incidence of acute VTE among nursing home residents is reported to be 1.3 events per 100 person-years.3 About 8% of cases of pulmonary embolism and 10% of cases of deep venous thrombosis in the elderly are in nursing home residents.4
However, only 20% of patients with VTE have typical symptoms such as leg pain and swelling or acute dyspnea and chest pain, while 80% have no symptoms.5
Furthermore, deep venous thrombosis is more likely to be clinically silent in patients whose mobility is impaired, such as nursing home residents, as the symptoms arising from obstruction of venous flow are more pronounced with walking.
Pulmonary embolism is also underdiagnosed in this group. An autopsy study of 234 nursing home residents found undiagnosed pulmonary embolism to be the cause of death in 8%, and 40% of cases of pulmonary embolism were not suspected before the patient died.6 Yet pulmonary embolism has a higher case-fatality rate in the elderly than in younger patients, particularly when elderly patients have comorbidities.7
A reason why the diagnosis is so often missed is that pulmonary embolism can present atypically in the elderly, with syncope being more common and tachycardia being less common than in younger patients.8
Since so many cases of VTE are clinically silent and most long-term care residents who die do not undergo autopsy, the true scope of VTE as a clinical problem in these patients is unknown. Consequently, the best way to diagnose, prevent, and treat VTE is also unclear.
WHY IS VTE PREVENTION SO OFTEN OVERLOOKED IN MEDICAL PATIENTS?
In general, nonsurgical patients receive suboptimal thromboprophylaxis. National and international chart audits and cross-sectional studies show that only 16% to 33% of hospitalized medical patients at risk for VTE receive appropriate anticoagulant prophylaxis.9 Though no audits in long-term care facilities have been published, the rate of appropriate prophylaxis is likely comparable to or possibly less than that in medical patients in the hospital. In contrast, in surgical patients the rate is much higher—up to 90%.10,11
Why is VTE prophylaxis so underused in medical patients?
One reason is that we do not really know the baseline risk of VTE in medical patients, particularly in those with chronic illness who require long-term care.12 This is relevant because, in the absence of data about patients’ baseline risk, anticoagulant prophylaxis should be ordered selectively, as it poses known risks of bleeding. The risk is greater in elderly people with comorbidities, as are the associated costs.
In addition, relatively few studies have assessed thromboprophylaxis in medical patients, especially in residents of long-term care facilities.
Another reason is that we lack practice guidelines for patients who need long-term care. The well-accepted guidelines from the American College of Chest Physicians (ACCP) cite advanced age and immobility as risk factors for VTE and strongly recommend prophylaxis in acutely ill medical patients who have limited mobility and an additional risk factor such as infection or cancer.13 Though elderly residents of long-term care facilities may share some of these risk factors, the ACCP guidelines make no specific recommendations for this group.
The attitudes of health care professionals may also pose a barrier. Lloyd et al (unpublished data, 2009) surveyed 1,601 health care professionals in Ontario, Canada, in 2007, to assess potential barriers to anticoagulant prophylaxis in hospitalized medical patients. Respondents cited concerns about the risk of bleeding from anticoagulants, lack of clear indications and contraindications for anticoagulant prophylaxis, and lack of time to consider VTE prophylaxis in every patient. (They did not, however, cite disagreement with guidelines or patient discomfort from subcutaneous anticoagulant injections as barriers.) It is reasonable to assume that these attitudes may also pose a problem in long-term care residents.
Finally, no randomized trials have evaluated the efficacy and safety of anticoagulant drugs or mechanical methods of prophylaxis in long-term care residents. Studies have shown that a short course (7–10 days) of an anticoagulant drug effectively prevents VTE in acutely ill patients, but the efficacy of an extended course in patients with chronic illness who require long-term care is not clear. Therefore, recommendations about thromboprophylaxis in long-term care residents should be made with the caveat that they are based on indirect evidence from other patient groups. This is a considerable limitation.
OPTIONS FOR THROMBOPROPHYLAXIS IN LONG-TERM CARE RESIDENTS
Options for thromboprophylaxis fall into two broad categories: anticoagulant drugs and mechanical devices.
Anticoagulant prophylactic drugs
These agents have been assessed in randomized trials in surgical or acutely ill medical patients, although fondaparinux and tinzaparin are not approved for use in medical patients. Furthermore, none of them has been evaluated in residents of long-term care facilities.
The choice of anticoagulant for prophylaxis is determined largely by clinical factors.
Low-molecular-weight heparins are popular both in and out of the hospital because they have predictable pharmacokinetic properties, they come in convenient prefilled syringes, and they can be given once daily. However, some of them may bioaccumulate in patients with impaired renal function, as they are cleared primarily by the kidney.
Unfractionated heparin is likely to be safer in patients with severe renal insufficiency (creatinine clearance < 30 mL/min), as it is cleared via nonrenal mechanisms.
However, a recent single-arm trial of dalteparin 5,000 IU once daily in critically ill patients with severe renal insufficiency found no evidence of an excessive anticoagulant effect or of drug bioaccumulation.15 Dalteparin may thus be an alternative to unfractionated heparin in medical patients with impaired renal function.
Fondaparinux, a newer anticoagulant, is also given once daily. It is the anticoagulant of choice in patients who have had heparin-induced thrombocytopenia because it is not derived from heparin and likely does not cross-react with heparin-induced thrombocytopenia antibodies.16,17
Limited data on benefit of prophylactic anticoagulant drugs
As mentioned, the trials that confirmed the efficacy and safety of anticoagulant prophylaxis were in surgical patients and hospitalized medical patients, not elderly long-term care residents. The poor evidence for anticoagulant prophylaxis in these patients may be strengthened if extended-duration, out-of-hospital prophylaxis were shown to be effective in medical patients. Long-term care residents could more reasonably be compared with medical patients discharged home with a chronic or resolving illness than with those who are hospitalized.
There is some evidence, although with caveats, that extended anticoagulant prophylaxis, started after an acute illness has resolved, confers a benefit. A recent randomized trial compared extended-duration and short-duration prophylaxis (5 weeks vs 10 days) with enoxaparin 40 mg once daily in 4,726 medical patients with impaired mobility.18 The risk of any VTE event was 44% lower with extended-duration prophylaxis (2.8% vs 4.9%; P = .001) and the risk of symptomatic VTE was 73% lower (0.3% vs 1.1%; P = .004), and this benefit persisted 2 months after treatment was stopped (3.0% vs 5.2%; P = .0015). However, extended treatment conferred a fourfold higher risk of major bleeding (0.6% vs 0.15%; P = .019).
These findings should also be considered in terms of absolute benefit and harm. Treating 1,000 patients for 5 weeks instead of 10 days would prevent eight episodes of symptomatic VTE (absolute risk reduction = 0.8%, number needed to treat = 125) at the cost of four to five episodes of major bleeding (absolute risk increase = 0.45%, number needed to harm = 222). This is a modest net therapeutic benefit.
The therapeutic benefit would be greater if we consider all episodes of VTE, both symptomatic and asymptomatic. Treating 1,000 patients for 5 weeks would prevent 20 episodes of symptomatic or asymptomatic VTE (absolute risk reduction = 2.1%, number needed to treat = 48). However, the clinical importance of asymptomatic VTE is questionable.
Given these considerations, if extended-duration anticoagulant prophylaxis is considered, it should be for patients at highest risk to optimize both its net therapeutic benefits and its cost-effectiveness.
Mechanical prophylaxis
Mechanical thromboprophylactic devices—graduated or elastic compression stockings and intermittent pneumatic compression devices—are effective when used by themselves in surgical patients.13 However, in a randomized controlled trial in patients with ischemic stroke, the rate of VTE was 10.0% with graduated compression stockings in addition to “usual care VTE prophylaxis” vs 10.5% with usual care alone, and patients in the stocking group had a fourfold higher risk of developing skin breaks, ulcers, blisters, or necrosis (5% vs 1%; odds ratio 4.18; 95% CI 2.4–7.3).19 Furthermore, improperly fitted stockings, especially those that are thigh-length, can be uncomfortable to wear and difficult to apply.
Overall, the role of mechanical thromboprophylaxis in long-term care facilities is not clear. If it is considered, there should be a compelling reason to use it—for example, for patients at high risk in whom anticoagulants are contraindicated because of ongoing bleeding or a higher risk of bleeding (eg, recent gastrointestinal bleeding, hemorrhagic stroke, coagulopathy, or thrombocytopenia). Furthermore, if stockings are used, they should be properly fitted and routinely monitored for adverse effects, since elderly patients are likely to be most susceptible to skin breakdown.
WHICH LONG-TERM CARE RESIDENTS SHOULD RECEIVE VTE PROPHYLAXIS?
Old age and immobility are not the only risk factors
The current ACCP guidelines suggest considering thromboprophylaxis for hospitalized medical patients over age 75 who cannot walk without assistance.13 However, we lack evidence to suggest a similar strategy in long-term care residents.
The ACCP guidelines are based on data on risk. Nearly 25% of elderly patients with confirmed pulmonary embolism had been immobile prior to their diagnosis.8 In addition, prolonged bed rest (> 14 days) has been reported to be the strongest independent risk factor for symptomatic deep venous thrombosis, increasing the risk more than fivefold.20 Advanced age is also considered a risk factor for VTE, as risk starts to increase at age 40 and doubles each decade of life thereafter.18
No study has assessed the impact of these factors on the risk of VTE in long-term care residents. Since most of such patients are elderly and have impaired mobility, we believe a more selective approach should be used in assigning VTE risk status, one that does not use advanced age and immobility as the only criteria for starting thromboprophylaxis.
Residents of long-term care facilities may be immobile because of underlying illness or disability, such as cognitive impairment, sensory impairment (eg, poor access to corrective lenses and hearing aids), or poor access to assist devices (eg, walkers, canes). In addition, iatrogenic factors that decrease mobility such as indwelling bladder catheters and physical restraints are also common in such patients.
Efforts to improve mobility should be encouraged. However, we recommend that thromboprophylaxis be considered only in patients who have both impaired mobility and an intercurrent acute medical illness such as an acute infection or acute inflammatory disease.13
A related issue is the difference between long-term care residents with a chronic but stable disease and those with acute disease. Patients with acute exacerbations of congestive heart failure or chronic obstructive lung disease may be considered for thromboprophylaxis, as they become more comparable to acutely ill medical patients in whom clinical trials have shown the effectiveness of anticoagulant prophylaxis. On the other hand, patients with these diseases who remain stable may not need prophylaxis.
This approach avoids giving long-term anticoagulant prophylaxis to patients who have irreversible diseases and limits the use of these drugs and devices to higher-risk periods.
Consider thromboprophylaxis if…
- An acute exacerbation of congestive heart failure or chronic obstructive pulmonary disease
- Acute infection (eg, urosepsis, pneumonia, cellulitis, infectious diarrhea)
- An acute exacerbation of an inflammatory disease (eg, rheumatoid arthritis)
- Active cancer (eg, patient receiving radiation therapy or chemotherapy)
- Immobility and prior VTE.
Do not routinely consider prophylaxis if…
We also believe patients should not be routinely considered for anticoagulant VTE prophylaxis if they have:
- Chronic but stable cardiorespiratory disease
- Chronic but stable infectious or inflammatory disease
- Terminal cancer with very limited life expectancy
- Any contraindication to anticoagulants (eg, active bleeding, recent bleeding, coagulopathy, thrombocytopenia).
ANTICOAGULANT PROPHYLAXIS POSES RISKS IN LONG-TERM CARE RESIDENTS
Bleeding is the principal risk
The incidence of clinically important bleeding associated with anticoagulant prophylaxis is 0.2% to 5.6%, and the risk of fatal bleeding is 0.02% to 0.5%.21–24
As no randomized trial has examined anticoagulant prophylaxis in elderly long-term care residents, their bleeding risk with this therapy is unclear. However, older patients are likely to be at higher risk than younger patients because they have more comorbidities, take more drugs that could interact with heparin and potentiate bleeding, and have fragile skin, predisposing to injury from subcutaneous injections.
Also, renal function tends to decline with age. In a retrospective study of 854 outpatients over age 65, 29% had moderate renal insufficiency (creatinine clearance 30–50 mL/min), and 6% had severe renal insufficiency (creatinine clearance < 30 mL/min).25 Recent evidence suggests that some low-molecular-weight heparins (dalteparin and tinzaparin) do not bioaccumulate in patients with impaired renal function. However, enoxaparin and fondaparinux should be used with caution in patients with moderate to severe renal impairment.
Though much attention has recently been paid to increasing anticoagulant doses if the patient is obese, residents of long-term care facilities are more likely to be underweight. Dose adjustment should be considered when a low-molecular-weight heparin or fondaparinux is given to patients weighing less than 50 kg.
Heparin-induced thrombocytopenia
The other major risk of anticoagulant prophylaxis is heparin-induced thrombocytopenia, an infrequent but life-threatening complication caused by the formation of antibodies to the heparin-derived anticoagulant and a platelet surface antigen. It is associated with moderate thrombocytopenia and an incidence of venous or arterial thrombosis that is over 50%.26
No study has assessed the incidence of heparin-induced thrombocytopenia in long-term care residents. A meta-analysis reported that the risk with anticoagulant prophylaxis was 1.6% with unfractionated heparin (95% confidence interval [CI] 1.2%–2.1%) and 0.6% with low-molecular-weight heparin (95% CI 0.4%–0.9%), and that this risk increased with the duration of prophylaxis.27 If anticoagulant prophylaxis were given to all long-term care residents for extended durations (eg, for the duration of reduced mobility), the incidence and prevalence of heparin-induced thrombocytopenia would likely become a major concern.
Whenever anticoagulant prophylaxis is considered, the risks of both thrombosis and bleeding should be considered. Patients who are receiving anticoagulant prophylaxis should also be monitored for bleeding and heparin-induced thrombocytopenia. This is particularly true in long-term care residents, in whom the risks and benefits of anticoagulant prophylaxis are extrapolated from data from other populations.
MORE RESEARCH IS NEEDED
To date, we lack audits of thromboprophylaxis, clinical practice guidelines, and clear indications and contraindications for anticoagulant prophylaxis in long-term care residents. In the absence of such data, extrapolating the efficacy and safety of thromboprophylaxis from hospitalized patients to long-term care residents is difficult.
Clearly, additional research is needed to identify which long-term care residents would benefit most from thromboprophylaxis. In the meantime, a selective approach to identifying patients who should be considered for thromboprophylaxis should be adopted.
- Cornman JM. Questions for societies with “third age” populations. The Extension-of-Life Working Group, The Gerontological Society of America. Acad Med 1997; 72:856–862.
- Kemper P, Murtaugh CM. Lifetime use of nursing home care. N Engl J Med 1991; 324:595–600.
- Gomes JP, Shaheen WH, Truong SV, Brown EF, Beasley BW, Gajewski BJ. Incidence of venous thromboembolic events among nursing home residents. J Gen Intern Med 2003; 18:934–936.
- Kniffin WD, Baron JA, Barrett J, Birkmeyer JD, Anderson FA. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med 1994; 154:861–866.
- Bounameaux H. Integrating pharmacologic and mechanical prophylaxis of venous thromboembolism. Thromb Haemost 1999; 82:931–937.
- Gross JS, Neufeld RR, Libow LS, Gerber I, Rodstein M. Autopsy study of the elderly institutionalized patient. Review of 234 autopsies. Arch Intern Med 1988; 148:173–176.
- Spyropoulos AC, Merli G. Management of venous thromboembolism in the elderly. Drugs Aging 2006; 23:651–671.
- Punukollu H, Khan IA, Punukollu G, Gowda RM, Mendoza C, Sacchi TJ. Acute pulmonary embolism in elderly: clinical characteristics and outcome. Int J Cardiol 2005; 99:213–216.
- Douketis JD. Prevention of venous thromboembolism in hospitalized medical patients: addressing some practical questions. Curr Opin Pulm Med 2008; 14:381–388.
- Cohen AT, Tapson VF, Bergmann JF, et al; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Haas S, Spyropoulos AC. Primary prevention of venous thromboembolism in long-term care: identifying and managing the risk. Clin Appl Thromb Hemost 2008; 14:149–158.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133( suppl 6):381S–453S.
- Francis CW. Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med 2007; 356:1438–1444.
- Douketis J, Cook D, Meade M, et al; Canadian Critical Care Trials Group. Prophylaxis against deep vein thrombosis in critically ill patients with severe renal insufficiency with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the DIRECT study. Arch Intern Med 2008; 168:1805–1812.
- Lobo B, Finch C, Howard A, Minhas S. Fondaparinux for the treatment of patients with acute heparin-induced thrombocytopenia. Thromb Haemost 2008; 99:208–214.
- Spinler SA. New concepts in heparin-induced thrombocytopenia: diagnosis and management. J Thromb Thrombolysis 2006; 21:17–21.
- Hull RD, Schellong SM, Tapson VF, et al. Extended-duration thromboprophylaxis in acutely ill medical patients with recent reduced mobility: methodology for the EXCLAIM study. J Thromb Thrombolysis 2006; 22:31–38.
- Dennis M, Sandercock PA, Reid J, et al; CLOTS Trials Collaboration Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009; 373:1958–1965.
- Weill-Engerer S, Meaume S, Lahlou A, et al. Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case-control multicenter study. J Am Geriatr Soc 2004; 52:1299–1304.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med 2006; 166:853–859.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139:893–900.
- Lloyd NS, Douketis JD, Moinuddin I, Lim W, Crowther MA. Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta-analysis. J Thromb Haemost 2008; 6:405–414.
- Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 2003; 163:356–360.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Stein PD, Hull RD, Matta F, Yaekoub AY, Liang J. Incidence of thrombocytopenia in hospitalized patients with venous thromboembolism. Am J Med 2009; 122:919–930.
Randomized trials that included more than 20,000 medical patients have shown that anticoagulant therapy is safe and effective in preventing venous thromboembolism (VTE), ie, deep vein thrombosis and pulmonary embolism.
However, these trials were done in hospitalized patients, who typically had an acute medical illness and who, if eligible, received a short (7- to 10-day) course of anticoagulant prophylaxis.
Little attention has been given to VTE prophylaxis in residents of long-term care facilities. These patients have risk profiles similar to those of hospitalized medical patients. Some of them may have been transferred from an acute care hospital. In addition, most are elderly, and many have reduced mobility and are at risk for illnesses such as stroke and cardiorespiratory insufficiency, which increase the risk of VTE.
VTE in residents of long-term care facilities is a growing concern. By some estimates, by the year 2030 more than 20% of the US population (70.2 million people) will be over 65 years of age.1 Of those who reached age 65 in 1990, an estimated 43% will enter a nursing home at least once before they die—32% for 3 months, 24% for at least a year, and 9% for at least 5 years.2
Against this background, the objectives of this review are to consider:
- The scope of the problem of VTE in long-term care residents
- Why VTE prophylaxis is often overlooked in medical patients
- Evidence—or lack of evidence—for the safety and efficacy of VTE prophylaxis in long-term care residents and other medical patients
- Available options for VTE prophylaxis
- Which long-term care residents should or should not be considered for prophylaxis.
THE TRUE SCOPE OF THE PROBLEM IS UNKNOWN
The incidence of acute VTE among nursing home residents is reported to be 1.3 events per 100 person-years.3 About 8% of cases of pulmonary embolism and 10% of cases of deep venous thrombosis in the elderly are in nursing home residents.4
However, only 20% of patients with VTE have typical symptoms such as leg pain and swelling or acute dyspnea and chest pain, while 80% have no symptoms.5
Furthermore, deep venous thrombosis is more likely to be clinically silent in patients whose mobility is impaired, such as nursing home residents, as the symptoms arising from obstruction of venous flow are more pronounced with walking.
Pulmonary embolism is also underdiagnosed in this group. An autopsy study of 234 nursing home residents found undiagnosed pulmonary embolism to be the cause of death in 8%, and 40% of cases of pulmonary embolism were not suspected before the patient died.6 Yet pulmonary embolism has a higher case-fatality rate in the elderly than in younger patients, particularly when elderly patients have comorbidities.7
A reason why the diagnosis is so often missed is that pulmonary embolism can present atypically in the elderly, with syncope being more common and tachycardia being less common than in younger patients.8
Since so many cases of VTE are clinically silent and most long-term care residents who die do not undergo autopsy, the true scope of VTE as a clinical problem in these patients is unknown. Consequently, the best way to diagnose, prevent, and treat VTE is also unclear.
WHY IS VTE PREVENTION SO OFTEN OVERLOOKED IN MEDICAL PATIENTS?
In general, nonsurgical patients receive suboptimal thromboprophylaxis. National and international chart audits and cross-sectional studies show that only 16% to 33% of hospitalized medical patients at risk for VTE receive appropriate anticoagulant prophylaxis.9 Though no audits in long-term care facilities have been published, the rate of appropriate prophylaxis is likely comparable to or possibly less than that in medical patients in the hospital. In contrast, in surgical patients the rate is much higher—up to 90%.10,11
Why is VTE prophylaxis so underused in medical patients?
One reason is that we do not really know the baseline risk of VTE in medical patients, particularly in those with chronic illness who require long-term care.12 This is relevant because, in the absence of data about patients’ baseline risk, anticoagulant prophylaxis should be ordered selectively, as it poses known risks of bleeding. The risk is greater in elderly people with comorbidities, as are the associated costs.
In addition, relatively few studies have assessed thromboprophylaxis in medical patients, especially in residents of long-term care facilities.
Another reason is that we lack practice guidelines for patients who need long-term care. The well-accepted guidelines from the American College of Chest Physicians (ACCP) cite advanced age and immobility as risk factors for VTE and strongly recommend prophylaxis in acutely ill medical patients who have limited mobility and an additional risk factor such as infection or cancer.13 Though elderly residents of long-term care facilities may share some of these risk factors, the ACCP guidelines make no specific recommendations for this group.
The attitudes of health care professionals may also pose a barrier. Lloyd et al (unpublished data, 2009) surveyed 1,601 health care professionals in Ontario, Canada, in 2007, to assess potential barriers to anticoagulant prophylaxis in hospitalized medical patients. Respondents cited concerns about the risk of bleeding from anticoagulants, lack of clear indications and contraindications for anticoagulant prophylaxis, and lack of time to consider VTE prophylaxis in every patient. (They did not, however, cite disagreement with guidelines or patient discomfort from subcutaneous anticoagulant injections as barriers.) It is reasonable to assume that these attitudes may also pose a problem in long-term care residents.
Finally, no randomized trials have evaluated the efficacy and safety of anticoagulant drugs or mechanical methods of prophylaxis in long-term care residents. Studies have shown that a short course (7–10 days) of an anticoagulant drug effectively prevents VTE in acutely ill patients, but the efficacy of an extended course in patients with chronic illness who require long-term care is not clear. Therefore, recommendations about thromboprophylaxis in long-term care residents should be made with the caveat that they are based on indirect evidence from other patient groups. This is a considerable limitation.
OPTIONS FOR THROMBOPROPHYLAXIS IN LONG-TERM CARE RESIDENTS
Options for thromboprophylaxis fall into two broad categories: anticoagulant drugs and mechanical devices.
Anticoagulant prophylactic drugs
These agents have been assessed in randomized trials in surgical or acutely ill medical patients, although fondaparinux and tinzaparin are not approved for use in medical patients. Furthermore, none of them has been evaluated in residents of long-term care facilities.
The choice of anticoagulant for prophylaxis is determined largely by clinical factors.
Low-molecular-weight heparins are popular both in and out of the hospital because they have predictable pharmacokinetic properties, they come in convenient prefilled syringes, and they can be given once daily. However, some of them may bioaccumulate in patients with impaired renal function, as they are cleared primarily by the kidney.
Unfractionated heparin is likely to be safer in patients with severe renal insufficiency (creatinine clearance < 30 mL/min), as it is cleared via nonrenal mechanisms.
However, a recent single-arm trial of dalteparin 5,000 IU once daily in critically ill patients with severe renal insufficiency found no evidence of an excessive anticoagulant effect or of drug bioaccumulation.15 Dalteparin may thus be an alternative to unfractionated heparin in medical patients with impaired renal function.
Fondaparinux, a newer anticoagulant, is also given once daily. It is the anticoagulant of choice in patients who have had heparin-induced thrombocytopenia because it is not derived from heparin and likely does not cross-react with heparin-induced thrombocytopenia antibodies.16,17
Limited data on benefit of prophylactic anticoagulant drugs
As mentioned, the trials that confirmed the efficacy and safety of anticoagulant prophylaxis were in surgical patients and hospitalized medical patients, not elderly long-term care residents. The poor evidence for anticoagulant prophylaxis in these patients may be strengthened if extended-duration, out-of-hospital prophylaxis were shown to be effective in medical patients. Long-term care residents could more reasonably be compared with medical patients discharged home with a chronic or resolving illness than with those who are hospitalized.
There is some evidence, although with caveats, that extended anticoagulant prophylaxis, started after an acute illness has resolved, confers a benefit. A recent randomized trial compared extended-duration and short-duration prophylaxis (5 weeks vs 10 days) with enoxaparin 40 mg once daily in 4,726 medical patients with impaired mobility.18 The risk of any VTE event was 44% lower with extended-duration prophylaxis (2.8% vs 4.9%; P = .001) and the risk of symptomatic VTE was 73% lower (0.3% vs 1.1%; P = .004), and this benefit persisted 2 months after treatment was stopped (3.0% vs 5.2%; P = .0015). However, extended treatment conferred a fourfold higher risk of major bleeding (0.6% vs 0.15%; P = .019).
These findings should also be considered in terms of absolute benefit and harm. Treating 1,000 patients for 5 weeks instead of 10 days would prevent eight episodes of symptomatic VTE (absolute risk reduction = 0.8%, number needed to treat = 125) at the cost of four to five episodes of major bleeding (absolute risk increase = 0.45%, number needed to harm = 222). This is a modest net therapeutic benefit.
The therapeutic benefit would be greater if we consider all episodes of VTE, both symptomatic and asymptomatic. Treating 1,000 patients for 5 weeks would prevent 20 episodes of symptomatic or asymptomatic VTE (absolute risk reduction = 2.1%, number needed to treat = 48). However, the clinical importance of asymptomatic VTE is questionable.
Given these considerations, if extended-duration anticoagulant prophylaxis is considered, it should be for patients at highest risk to optimize both its net therapeutic benefits and its cost-effectiveness.
Mechanical prophylaxis
Mechanical thromboprophylactic devices—graduated or elastic compression stockings and intermittent pneumatic compression devices—are effective when used by themselves in surgical patients.13 However, in a randomized controlled trial in patients with ischemic stroke, the rate of VTE was 10.0% with graduated compression stockings in addition to “usual care VTE prophylaxis” vs 10.5% with usual care alone, and patients in the stocking group had a fourfold higher risk of developing skin breaks, ulcers, blisters, or necrosis (5% vs 1%; odds ratio 4.18; 95% CI 2.4–7.3).19 Furthermore, improperly fitted stockings, especially those that are thigh-length, can be uncomfortable to wear and difficult to apply.
Overall, the role of mechanical thromboprophylaxis in long-term care facilities is not clear. If it is considered, there should be a compelling reason to use it—for example, for patients at high risk in whom anticoagulants are contraindicated because of ongoing bleeding or a higher risk of bleeding (eg, recent gastrointestinal bleeding, hemorrhagic stroke, coagulopathy, or thrombocytopenia). Furthermore, if stockings are used, they should be properly fitted and routinely monitored for adverse effects, since elderly patients are likely to be most susceptible to skin breakdown.
WHICH LONG-TERM CARE RESIDENTS SHOULD RECEIVE VTE PROPHYLAXIS?
Old age and immobility are not the only risk factors
The current ACCP guidelines suggest considering thromboprophylaxis for hospitalized medical patients over age 75 who cannot walk without assistance.13 However, we lack evidence to suggest a similar strategy in long-term care residents.
The ACCP guidelines are based on data on risk. Nearly 25% of elderly patients with confirmed pulmonary embolism had been immobile prior to their diagnosis.8 In addition, prolonged bed rest (> 14 days) has been reported to be the strongest independent risk factor for symptomatic deep venous thrombosis, increasing the risk more than fivefold.20 Advanced age is also considered a risk factor for VTE, as risk starts to increase at age 40 and doubles each decade of life thereafter.18
No study has assessed the impact of these factors on the risk of VTE in long-term care residents. Since most of such patients are elderly and have impaired mobility, we believe a more selective approach should be used in assigning VTE risk status, one that does not use advanced age and immobility as the only criteria for starting thromboprophylaxis.
Residents of long-term care facilities may be immobile because of underlying illness or disability, such as cognitive impairment, sensory impairment (eg, poor access to corrective lenses and hearing aids), or poor access to assist devices (eg, walkers, canes). In addition, iatrogenic factors that decrease mobility such as indwelling bladder catheters and physical restraints are also common in such patients.
Efforts to improve mobility should be encouraged. However, we recommend that thromboprophylaxis be considered only in patients who have both impaired mobility and an intercurrent acute medical illness such as an acute infection or acute inflammatory disease.13
A related issue is the difference between long-term care residents with a chronic but stable disease and those with acute disease. Patients with acute exacerbations of congestive heart failure or chronic obstructive lung disease may be considered for thromboprophylaxis, as they become more comparable to acutely ill medical patients in whom clinical trials have shown the effectiveness of anticoagulant prophylaxis. On the other hand, patients with these diseases who remain stable may not need prophylaxis.
This approach avoids giving long-term anticoagulant prophylaxis to patients who have irreversible diseases and limits the use of these drugs and devices to higher-risk periods.
Consider thromboprophylaxis if…
- An acute exacerbation of congestive heart failure or chronic obstructive pulmonary disease
- Acute infection (eg, urosepsis, pneumonia, cellulitis, infectious diarrhea)
- An acute exacerbation of an inflammatory disease (eg, rheumatoid arthritis)
- Active cancer (eg, patient receiving radiation therapy or chemotherapy)
- Immobility and prior VTE.
Do not routinely consider prophylaxis if…
We also believe patients should not be routinely considered for anticoagulant VTE prophylaxis if they have:
- Chronic but stable cardiorespiratory disease
- Chronic but stable infectious or inflammatory disease
- Terminal cancer with very limited life expectancy
- Any contraindication to anticoagulants (eg, active bleeding, recent bleeding, coagulopathy, thrombocytopenia).
ANTICOAGULANT PROPHYLAXIS POSES RISKS IN LONG-TERM CARE RESIDENTS
Bleeding is the principal risk
The incidence of clinically important bleeding associated with anticoagulant prophylaxis is 0.2% to 5.6%, and the risk of fatal bleeding is 0.02% to 0.5%.21–24
As no randomized trial has examined anticoagulant prophylaxis in elderly long-term care residents, their bleeding risk with this therapy is unclear. However, older patients are likely to be at higher risk than younger patients because they have more comorbidities, take more drugs that could interact with heparin and potentiate bleeding, and have fragile skin, predisposing to injury from subcutaneous injections.
Also, renal function tends to decline with age. In a retrospective study of 854 outpatients over age 65, 29% had moderate renal insufficiency (creatinine clearance 30–50 mL/min), and 6% had severe renal insufficiency (creatinine clearance < 30 mL/min).25 Recent evidence suggests that some low-molecular-weight heparins (dalteparin and tinzaparin) do not bioaccumulate in patients with impaired renal function. However, enoxaparin and fondaparinux should be used with caution in patients with moderate to severe renal impairment.
Though much attention has recently been paid to increasing anticoagulant doses if the patient is obese, residents of long-term care facilities are more likely to be underweight. Dose adjustment should be considered when a low-molecular-weight heparin or fondaparinux is given to patients weighing less than 50 kg.
Heparin-induced thrombocytopenia
The other major risk of anticoagulant prophylaxis is heparin-induced thrombocytopenia, an infrequent but life-threatening complication caused by the formation of antibodies to the heparin-derived anticoagulant and a platelet surface antigen. It is associated with moderate thrombocytopenia and an incidence of venous or arterial thrombosis that is over 50%.26
No study has assessed the incidence of heparin-induced thrombocytopenia in long-term care residents. A meta-analysis reported that the risk with anticoagulant prophylaxis was 1.6% with unfractionated heparin (95% confidence interval [CI] 1.2%–2.1%) and 0.6% with low-molecular-weight heparin (95% CI 0.4%–0.9%), and that this risk increased with the duration of prophylaxis.27 If anticoagulant prophylaxis were given to all long-term care residents for extended durations (eg, for the duration of reduced mobility), the incidence and prevalence of heparin-induced thrombocytopenia would likely become a major concern.
Whenever anticoagulant prophylaxis is considered, the risks of both thrombosis and bleeding should be considered. Patients who are receiving anticoagulant prophylaxis should also be monitored for bleeding and heparin-induced thrombocytopenia. This is particularly true in long-term care residents, in whom the risks and benefits of anticoagulant prophylaxis are extrapolated from data from other populations.
MORE RESEARCH IS NEEDED
To date, we lack audits of thromboprophylaxis, clinical practice guidelines, and clear indications and contraindications for anticoagulant prophylaxis in long-term care residents. In the absence of such data, extrapolating the efficacy and safety of thromboprophylaxis from hospitalized patients to long-term care residents is difficult.
Clearly, additional research is needed to identify which long-term care residents would benefit most from thromboprophylaxis. In the meantime, a selective approach to identifying patients who should be considered for thromboprophylaxis should be adopted.
Randomized trials that included more than 20,000 medical patients have shown that anticoagulant therapy is safe and effective in preventing venous thromboembolism (VTE), ie, deep vein thrombosis and pulmonary embolism.
However, these trials were done in hospitalized patients, who typically had an acute medical illness and who, if eligible, received a short (7- to 10-day) course of anticoagulant prophylaxis.
Little attention has been given to VTE prophylaxis in residents of long-term care facilities. These patients have risk profiles similar to those of hospitalized medical patients. Some of them may have been transferred from an acute care hospital. In addition, most are elderly, and many have reduced mobility and are at risk for illnesses such as stroke and cardiorespiratory insufficiency, which increase the risk of VTE.
VTE in residents of long-term care facilities is a growing concern. By some estimates, by the year 2030 more than 20% of the US population (70.2 million people) will be over 65 years of age.1 Of those who reached age 65 in 1990, an estimated 43% will enter a nursing home at least once before they die—32% for 3 months, 24% for at least a year, and 9% for at least 5 years.2
Against this background, the objectives of this review are to consider:
- The scope of the problem of VTE in long-term care residents
- Why VTE prophylaxis is often overlooked in medical patients
- Evidence—or lack of evidence—for the safety and efficacy of VTE prophylaxis in long-term care residents and other medical patients
- Available options for VTE prophylaxis
- Which long-term care residents should or should not be considered for prophylaxis.
THE TRUE SCOPE OF THE PROBLEM IS UNKNOWN
The incidence of acute VTE among nursing home residents is reported to be 1.3 events per 100 person-years.3 About 8% of cases of pulmonary embolism and 10% of cases of deep venous thrombosis in the elderly are in nursing home residents.4
However, only 20% of patients with VTE have typical symptoms such as leg pain and swelling or acute dyspnea and chest pain, while 80% have no symptoms.5
Furthermore, deep venous thrombosis is more likely to be clinically silent in patients whose mobility is impaired, such as nursing home residents, as the symptoms arising from obstruction of venous flow are more pronounced with walking.
Pulmonary embolism is also underdiagnosed in this group. An autopsy study of 234 nursing home residents found undiagnosed pulmonary embolism to be the cause of death in 8%, and 40% of cases of pulmonary embolism were not suspected before the patient died.6 Yet pulmonary embolism has a higher case-fatality rate in the elderly than in younger patients, particularly when elderly patients have comorbidities.7
A reason why the diagnosis is so often missed is that pulmonary embolism can present atypically in the elderly, with syncope being more common and tachycardia being less common than in younger patients.8
Since so many cases of VTE are clinically silent and most long-term care residents who die do not undergo autopsy, the true scope of VTE as a clinical problem in these patients is unknown. Consequently, the best way to diagnose, prevent, and treat VTE is also unclear.
WHY IS VTE PREVENTION SO OFTEN OVERLOOKED IN MEDICAL PATIENTS?
In general, nonsurgical patients receive suboptimal thromboprophylaxis. National and international chart audits and cross-sectional studies show that only 16% to 33% of hospitalized medical patients at risk for VTE receive appropriate anticoagulant prophylaxis.9 Though no audits in long-term care facilities have been published, the rate of appropriate prophylaxis is likely comparable to or possibly less than that in medical patients in the hospital. In contrast, in surgical patients the rate is much higher—up to 90%.10,11
Why is VTE prophylaxis so underused in medical patients?
One reason is that we do not really know the baseline risk of VTE in medical patients, particularly in those with chronic illness who require long-term care.12 This is relevant because, in the absence of data about patients’ baseline risk, anticoagulant prophylaxis should be ordered selectively, as it poses known risks of bleeding. The risk is greater in elderly people with comorbidities, as are the associated costs.
In addition, relatively few studies have assessed thromboprophylaxis in medical patients, especially in residents of long-term care facilities.
Another reason is that we lack practice guidelines for patients who need long-term care. The well-accepted guidelines from the American College of Chest Physicians (ACCP) cite advanced age and immobility as risk factors for VTE and strongly recommend prophylaxis in acutely ill medical patients who have limited mobility and an additional risk factor such as infection or cancer.13 Though elderly residents of long-term care facilities may share some of these risk factors, the ACCP guidelines make no specific recommendations for this group.
The attitudes of health care professionals may also pose a barrier. Lloyd et al (unpublished data, 2009) surveyed 1,601 health care professionals in Ontario, Canada, in 2007, to assess potential barriers to anticoagulant prophylaxis in hospitalized medical patients. Respondents cited concerns about the risk of bleeding from anticoagulants, lack of clear indications and contraindications for anticoagulant prophylaxis, and lack of time to consider VTE prophylaxis in every patient. (They did not, however, cite disagreement with guidelines or patient discomfort from subcutaneous anticoagulant injections as barriers.) It is reasonable to assume that these attitudes may also pose a problem in long-term care residents.
Finally, no randomized trials have evaluated the efficacy and safety of anticoagulant drugs or mechanical methods of prophylaxis in long-term care residents. Studies have shown that a short course (7–10 days) of an anticoagulant drug effectively prevents VTE in acutely ill patients, but the efficacy of an extended course in patients with chronic illness who require long-term care is not clear. Therefore, recommendations about thromboprophylaxis in long-term care residents should be made with the caveat that they are based on indirect evidence from other patient groups. This is a considerable limitation.
OPTIONS FOR THROMBOPROPHYLAXIS IN LONG-TERM CARE RESIDENTS
Options for thromboprophylaxis fall into two broad categories: anticoagulant drugs and mechanical devices.
Anticoagulant prophylactic drugs
These agents have been assessed in randomized trials in surgical or acutely ill medical patients, although fondaparinux and tinzaparin are not approved for use in medical patients. Furthermore, none of them has been evaluated in residents of long-term care facilities.
The choice of anticoagulant for prophylaxis is determined largely by clinical factors.
Low-molecular-weight heparins are popular both in and out of the hospital because they have predictable pharmacokinetic properties, they come in convenient prefilled syringes, and they can be given once daily. However, some of them may bioaccumulate in patients with impaired renal function, as they are cleared primarily by the kidney.
Unfractionated heparin is likely to be safer in patients with severe renal insufficiency (creatinine clearance < 30 mL/min), as it is cleared via nonrenal mechanisms.
However, a recent single-arm trial of dalteparin 5,000 IU once daily in critically ill patients with severe renal insufficiency found no evidence of an excessive anticoagulant effect or of drug bioaccumulation.15 Dalteparin may thus be an alternative to unfractionated heparin in medical patients with impaired renal function.
Fondaparinux, a newer anticoagulant, is also given once daily. It is the anticoagulant of choice in patients who have had heparin-induced thrombocytopenia because it is not derived from heparin and likely does not cross-react with heparin-induced thrombocytopenia antibodies.16,17
Limited data on benefit of prophylactic anticoagulant drugs
As mentioned, the trials that confirmed the efficacy and safety of anticoagulant prophylaxis were in surgical patients and hospitalized medical patients, not elderly long-term care residents. The poor evidence for anticoagulant prophylaxis in these patients may be strengthened if extended-duration, out-of-hospital prophylaxis were shown to be effective in medical patients. Long-term care residents could more reasonably be compared with medical patients discharged home with a chronic or resolving illness than with those who are hospitalized.
There is some evidence, although with caveats, that extended anticoagulant prophylaxis, started after an acute illness has resolved, confers a benefit. A recent randomized trial compared extended-duration and short-duration prophylaxis (5 weeks vs 10 days) with enoxaparin 40 mg once daily in 4,726 medical patients with impaired mobility.18 The risk of any VTE event was 44% lower with extended-duration prophylaxis (2.8% vs 4.9%; P = .001) and the risk of symptomatic VTE was 73% lower (0.3% vs 1.1%; P = .004), and this benefit persisted 2 months after treatment was stopped (3.0% vs 5.2%; P = .0015). However, extended treatment conferred a fourfold higher risk of major bleeding (0.6% vs 0.15%; P = .019).
These findings should also be considered in terms of absolute benefit and harm. Treating 1,000 patients for 5 weeks instead of 10 days would prevent eight episodes of symptomatic VTE (absolute risk reduction = 0.8%, number needed to treat = 125) at the cost of four to five episodes of major bleeding (absolute risk increase = 0.45%, number needed to harm = 222). This is a modest net therapeutic benefit.
The therapeutic benefit would be greater if we consider all episodes of VTE, both symptomatic and asymptomatic. Treating 1,000 patients for 5 weeks would prevent 20 episodes of symptomatic or asymptomatic VTE (absolute risk reduction = 2.1%, number needed to treat = 48). However, the clinical importance of asymptomatic VTE is questionable.
Given these considerations, if extended-duration anticoagulant prophylaxis is considered, it should be for patients at highest risk to optimize both its net therapeutic benefits and its cost-effectiveness.
Mechanical prophylaxis
Mechanical thromboprophylactic devices—graduated or elastic compression stockings and intermittent pneumatic compression devices—are effective when used by themselves in surgical patients.13 However, in a randomized controlled trial in patients with ischemic stroke, the rate of VTE was 10.0% with graduated compression stockings in addition to “usual care VTE prophylaxis” vs 10.5% with usual care alone, and patients in the stocking group had a fourfold higher risk of developing skin breaks, ulcers, blisters, or necrosis (5% vs 1%; odds ratio 4.18; 95% CI 2.4–7.3).19 Furthermore, improperly fitted stockings, especially those that are thigh-length, can be uncomfortable to wear and difficult to apply.
Overall, the role of mechanical thromboprophylaxis in long-term care facilities is not clear. If it is considered, there should be a compelling reason to use it—for example, for patients at high risk in whom anticoagulants are contraindicated because of ongoing bleeding or a higher risk of bleeding (eg, recent gastrointestinal bleeding, hemorrhagic stroke, coagulopathy, or thrombocytopenia). Furthermore, if stockings are used, they should be properly fitted and routinely monitored for adverse effects, since elderly patients are likely to be most susceptible to skin breakdown.
WHICH LONG-TERM CARE RESIDENTS SHOULD RECEIVE VTE PROPHYLAXIS?
Old age and immobility are not the only risk factors
The current ACCP guidelines suggest considering thromboprophylaxis for hospitalized medical patients over age 75 who cannot walk without assistance.13 However, we lack evidence to suggest a similar strategy in long-term care residents.
The ACCP guidelines are based on data on risk. Nearly 25% of elderly patients with confirmed pulmonary embolism had been immobile prior to their diagnosis.8 In addition, prolonged bed rest (> 14 days) has been reported to be the strongest independent risk factor for symptomatic deep venous thrombosis, increasing the risk more than fivefold.20 Advanced age is also considered a risk factor for VTE, as risk starts to increase at age 40 and doubles each decade of life thereafter.18
No study has assessed the impact of these factors on the risk of VTE in long-term care residents. Since most of such patients are elderly and have impaired mobility, we believe a more selective approach should be used in assigning VTE risk status, one that does not use advanced age and immobility as the only criteria for starting thromboprophylaxis.
Residents of long-term care facilities may be immobile because of underlying illness or disability, such as cognitive impairment, sensory impairment (eg, poor access to corrective lenses and hearing aids), or poor access to assist devices (eg, walkers, canes). In addition, iatrogenic factors that decrease mobility such as indwelling bladder catheters and physical restraints are also common in such patients.
Efforts to improve mobility should be encouraged. However, we recommend that thromboprophylaxis be considered only in patients who have both impaired mobility and an intercurrent acute medical illness such as an acute infection or acute inflammatory disease.13
A related issue is the difference between long-term care residents with a chronic but stable disease and those with acute disease. Patients with acute exacerbations of congestive heart failure or chronic obstructive lung disease may be considered for thromboprophylaxis, as they become more comparable to acutely ill medical patients in whom clinical trials have shown the effectiveness of anticoagulant prophylaxis. On the other hand, patients with these diseases who remain stable may not need prophylaxis.
This approach avoids giving long-term anticoagulant prophylaxis to patients who have irreversible diseases and limits the use of these drugs and devices to higher-risk periods.
Consider thromboprophylaxis if…
- An acute exacerbation of congestive heart failure or chronic obstructive pulmonary disease
- Acute infection (eg, urosepsis, pneumonia, cellulitis, infectious diarrhea)
- An acute exacerbation of an inflammatory disease (eg, rheumatoid arthritis)
- Active cancer (eg, patient receiving radiation therapy or chemotherapy)
- Immobility and prior VTE.
Do not routinely consider prophylaxis if…
We also believe patients should not be routinely considered for anticoagulant VTE prophylaxis if they have:
- Chronic but stable cardiorespiratory disease
- Chronic but stable infectious or inflammatory disease
- Terminal cancer with very limited life expectancy
- Any contraindication to anticoagulants (eg, active bleeding, recent bleeding, coagulopathy, thrombocytopenia).
ANTICOAGULANT PROPHYLAXIS POSES RISKS IN LONG-TERM CARE RESIDENTS
Bleeding is the principal risk
The incidence of clinically important bleeding associated with anticoagulant prophylaxis is 0.2% to 5.6%, and the risk of fatal bleeding is 0.02% to 0.5%.21–24
As no randomized trial has examined anticoagulant prophylaxis in elderly long-term care residents, their bleeding risk with this therapy is unclear. However, older patients are likely to be at higher risk than younger patients because they have more comorbidities, take more drugs that could interact with heparin and potentiate bleeding, and have fragile skin, predisposing to injury from subcutaneous injections.
Also, renal function tends to decline with age. In a retrospective study of 854 outpatients over age 65, 29% had moderate renal insufficiency (creatinine clearance 30–50 mL/min), and 6% had severe renal insufficiency (creatinine clearance < 30 mL/min).25 Recent evidence suggests that some low-molecular-weight heparins (dalteparin and tinzaparin) do not bioaccumulate in patients with impaired renal function. However, enoxaparin and fondaparinux should be used with caution in patients with moderate to severe renal impairment.
Though much attention has recently been paid to increasing anticoagulant doses if the patient is obese, residents of long-term care facilities are more likely to be underweight. Dose adjustment should be considered when a low-molecular-weight heparin or fondaparinux is given to patients weighing less than 50 kg.
Heparin-induced thrombocytopenia
The other major risk of anticoagulant prophylaxis is heparin-induced thrombocytopenia, an infrequent but life-threatening complication caused by the formation of antibodies to the heparin-derived anticoagulant and a platelet surface antigen. It is associated with moderate thrombocytopenia and an incidence of venous or arterial thrombosis that is over 50%.26
No study has assessed the incidence of heparin-induced thrombocytopenia in long-term care residents. A meta-analysis reported that the risk with anticoagulant prophylaxis was 1.6% with unfractionated heparin (95% confidence interval [CI] 1.2%–2.1%) and 0.6% with low-molecular-weight heparin (95% CI 0.4%–0.9%), and that this risk increased with the duration of prophylaxis.27 If anticoagulant prophylaxis were given to all long-term care residents for extended durations (eg, for the duration of reduced mobility), the incidence and prevalence of heparin-induced thrombocytopenia would likely become a major concern.
Whenever anticoagulant prophylaxis is considered, the risks of both thrombosis and bleeding should be considered. Patients who are receiving anticoagulant prophylaxis should also be monitored for bleeding and heparin-induced thrombocytopenia. This is particularly true in long-term care residents, in whom the risks and benefits of anticoagulant prophylaxis are extrapolated from data from other populations.
MORE RESEARCH IS NEEDED
To date, we lack audits of thromboprophylaxis, clinical practice guidelines, and clear indications and contraindications for anticoagulant prophylaxis in long-term care residents. In the absence of such data, extrapolating the efficacy and safety of thromboprophylaxis from hospitalized patients to long-term care residents is difficult.
Clearly, additional research is needed to identify which long-term care residents would benefit most from thromboprophylaxis. In the meantime, a selective approach to identifying patients who should be considered for thromboprophylaxis should be adopted.
- Cornman JM. Questions for societies with “third age” populations. The Extension-of-Life Working Group, The Gerontological Society of America. Acad Med 1997; 72:856–862.
- Kemper P, Murtaugh CM. Lifetime use of nursing home care. N Engl J Med 1991; 324:595–600.
- Gomes JP, Shaheen WH, Truong SV, Brown EF, Beasley BW, Gajewski BJ. Incidence of venous thromboembolic events among nursing home residents. J Gen Intern Med 2003; 18:934–936.
- Kniffin WD, Baron JA, Barrett J, Birkmeyer JD, Anderson FA. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med 1994; 154:861–866.
- Bounameaux H. Integrating pharmacologic and mechanical prophylaxis of venous thromboembolism. Thromb Haemost 1999; 82:931–937.
- Gross JS, Neufeld RR, Libow LS, Gerber I, Rodstein M. Autopsy study of the elderly institutionalized patient. Review of 234 autopsies. Arch Intern Med 1988; 148:173–176.
- Spyropoulos AC, Merli G. Management of venous thromboembolism in the elderly. Drugs Aging 2006; 23:651–671.
- Punukollu H, Khan IA, Punukollu G, Gowda RM, Mendoza C, Sacchi TJ. Acute pulmonary embolism in elderly: clinical characteristics and outcome. Int J Cardiol 2005; 99:213–216.
- Douketis JD. Prevention of venous thromboembolism in hospitalized medical patients: addressing some practical questions. Curr Opin Pulm Med 2008; 14:381–388.
- Cohen AT, Tapson VF, Bergmann JF, et al; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Haas S, Spyropoulos AC. Primary prevention of venous thromboembolism in long-term care: identifying and managing the risk. Clin Appl Thromb Hemost 2008; 14:149–158.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133( suppl 6):381S–453S.
- Francis CW. Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med 2007; 356:1438–1444.
- Douketis J, Cook D, Meade M, et al; Canadian Critical Care Trials Group. Prophylaxis against deep vein thrombosis in critically ill patients with severe renal insufficiency with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the DIRECT study. Arch Intern Med 2008; 168:1805–1812.
- Lobo B, Finch C, Howard A, Minhas S. Fondaparinux for the treatment of patients with acute heparin-induced thrombocytopenia. Thromb Haemost 2008; 99:208–214.
- Spinler SA. New concepts in heparin-induced thrombocytopenia: diagnosis and management. J Thromb Thrombolysis 2006; 21:17–21.
- Hull RD, Schellong SM, Tapson VF, et al. Extended-duration thromboprophylaxis in acutely ill medical patients with recent reduced mobility: methodology for the EXCLAIM study. J Thromb Thrombolysis 2006; 22:31–38.
- Dennis M, Sandercock PA, Reid J, et al; CLOTS Trials Collaboration Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009; 373:1958–1965.
- Weill-Engerer S, Meaume S, Lahlou A, et al. Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case-control multicenter study. J Am Geriatr Soc 2004; 52:1299–1304.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med 2006; 166:853–859.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139:893–900.
- Lloyd NS, Douketis JD, Moinuddin I, Lim W, Crowther MA. Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta-analysis. J Thromb Haemost 2008; 6:405–414.
- Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 2003; 163:356–360.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Stein PD, Hull RD, Matta F, Yaekoub AY, Liang J. Incidence of thrombocytopenia in hospitalized patients with venous thromboembolism. Am J Med 2009; 122:919–930.
- Cornman JM. Questions for societies with “third age” populations. The Extension-of-Life Working Group, The Gerontological Society of America. Acad Med 1997; 72:856–862.
- Kemper P, Murtaugh CM. Lifetime use of nursing home care. N Engl J Med 1991; 324:595–600.
- Gomes JP, Shaheen WH, Truong SV, Brown EF, Beasley BW, Gajewski BJ. Incidence of venous thromboembolic events among nursing home residents. J Gen Intern Med 2003; 18:934–936.
- Kniffin WD, Baron JA, Barrett J, Birkmeyer JD, Anderson FA. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med 1994; 154:861–866.
- Bounameaux H. Integrating pharmacologic and mechanical prophylaxis of venous thromboembolism. Thromb Haemost 1999; 82:931–937.
- Gross JS, Neufeld RR, Libow LS, Gerber I, Rodstein M. Autopsy study of the elderly institutionalized patient. Review of 234 autopsies. Arch Intern Med 1988; 148:173–176.
- Spyropoulos AC, Merli G. Management of venous thromboembolism in the elderly. Drugs Aging 2006; 23:651–671.
- Punukollu H, Khan IA, Punukollu G, Gowda RM, Mendoza C, Sacchi TJ. Acute pulmonary embolism in elderly: clinical characteristics and outcome. Int J Cardiol 2005; 99:213–216.
- Douketis JD. Prevention of venous thromboembolism in hospitalized medical patients: addressing some practical questions. Curr Opin Pulm Med 2008; 14:381–388.
- Cohen AT, Tapson VF, Bergmann JF, et al; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Haas S, Spyropoulos AC. Primary prevention of venous thromboembolism in long-term care: identifying and managing the risk. Clin Appl Thromb Hemost 2008; 14:149–158.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133( suppl 6):381S–453S.
- Francis CW. Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med 2007; 356:1438–1444.
- Douketis J, Cook D, Meade M, et al; Canadian Critical Care Trials Group. Prophylaxis against deep vein thrombosis in critically ill patients with severe renal insufficiency with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the DIRECT study. Arch Intern Med 2008; 168:1805–1812.
- Lobo B, Finch C, Howard A, Minhas S. Fondaparinux for the treatment of patients with acute heparin-induced thrombocytopenia. Thromb Haemost 2008; 99:208–214.
- Spinler SA. New concepts in heparin-induced thrombocytopenia: diagnosis and management. J Thromb Thrombolysis 2006; 21:17–21.
- Hull RD, Schellong SM, Tapson VF, et al. Extended-duration thromboprophylaxis in acutely ill medical patients with recent reduced mobility: methodology for the EXCLAIM study. J Thromb Thrombolysis 2006; 22:31–38.
- Dennis M, Sandercock PA, Reid J, et al; CLOTS Trials Collaboration Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009; 373:1958–1965.
- Weill-Engerer S, Meaume S, Lahlou A, et al. Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case-control multicenter study. J Am Geriatr Soc 2004; 52:1299–1304.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med 2006; 166:853–859.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139:893–900.
- Lloyd NS, Douketis JD, Moinuddin I, Lim W, Crowther MA. Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta-analysis. J Thromb Haemost 2008; 6:405–414.
- Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 2003; 163:356–360.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Stein PD, Hull RD, Matta F, Yaekoub AY, Liang J. Incidence of thrombocytopenia in hospitalized patients with venous thromboembolism. Am J Med 2009; 122:919–930.
KEY POINTS
- Assessment of VTE risk and consideration of need for anticoagulant prophylaxis in long-term care residents are based on indirect data, derived primarily from studies of acutely ill hospitalized medical patients.
- Drugs and devices for thromboprophylaxis have been studied in medical and surgical populations, but issues of efficacy and safety are likely to also pertain to long-term care residents.
- Thromboprophylaxis should be considered for long-term care residents if they are definitely at increased risk of VTE—ie, if they have an acute exacerbation of congestive heart failure or chronic obstructive pulmonary disease; acute inflammatory disease; acute infection; active cancer; or immobility and prior VTE.