User login
Impact of In‐Hospital EVPCR Testing
Non‐polio enteroviruses are the most common cause of aseptic meningitis in children.1 While bacterial meningitis requires parenteral antibiotics, aseptic meningitis requires only supportive care.1 Enteroviral reverse transcription polymerase chain reaction (EVPCR) testing of the cerebrospinal fluid (CSF) allows the virus to be detected with high sensitivity and specificity.2 Because children with a positive EVPCR test are at low risk of bacterial meningitis,3 access to rapid EVPCR results has the potential to impact the clinical management of children with meningitis.4, 5 We studied the impact of implementing an in‐hospital EVPCR testing protocol on the clinical management of children with meningitis in a single‐center retrospective cohort.
MATERIALS AND METHODS
Study Design and Population
We identified children, <19 years of age, with meningitis evaluated at a single tertiary care pediatric center between July 2006 and June 2010. We defined meningitis as a CSF white blood cell (WBC) count 10 cells/mm3 corrected for the presence of CSF red blood cells (RBCs) (1 WBC for every 500 RBCs).6 We excluded children with any of the following: critical illness (defined as hypotension or respiratory failure), purpura, recent neurosurgery, ventricular shunt, immunosuppression, focal bacterial infection requiring parenteral antibiotics, positive CSF Gram stain, or known Lyme disease. The Institutional Review Board approved this study with waiver of informed consent.
Data Collection and Case Definitions
We abstracted historical and physical examination findings, as well as laboratory and microbiologic results, from the medical record. We defined bacterial meningitis as the isolation of pathogenic bacteria from the CSF or blood cultures. Children who had received antibiotics within 72 hours of diagnostic lumbar puncture, with negative cultures, were considered to have pretreated culture‐negative meningitis. Otherwise, children with negative bacterial cultures were classified as having aseptic meningitis.
EVPCR Testing
During the study pre‐period (July 1, 2006 through June 23, 2008), EVPCR tests were flown once daily to a commercial laboratory (ARUP Laboratories, Salt Lake City, UT) where they were run in batches. During the post‐period (June 24, 2008 through June 30, 2010), the study institution replaced the send‐out test with an in‐hospital EVPCR test (Gene Xpert EV Technology; Cepheid, Sunnyvale, CA)7 that allows multiple specimens to be run simultaneously, multiple times daily (between 7:00 AM and 10:00 PM), with results available in as little as 2.5 hours. We defined turnaround time for the test from specimen obtainment to test result.
Outcome Measures
Our 2 primary outcomes were length of stay and duration of parenteral antibiotics. Length of stay was measured as time from emergency department arrival to discharge (emergency department or inpatient discharge). We defined the duration of parenteral antibiotics as time from the first to the last dose of parenteral antibiotics administered, plus the standard antibiotic dosing interval for that antibiotic. For children with Lyme meningitis, the duration of parenteral antibiotic coverage was defined a priori as 48 hours, the standard time to reliably exclude bacterial growth from culture.8
Statistical Methods
Primary outcomes were compared using univariate analyses in 6 patient groups: 1) all patients, and those with 2) a positive EVPCR test, 3) a negative EVPCR test, and a positive test who were 4) 90 days old, 5) >90 days old, and 6) presented during peak enteroviral season (June through October). We utilized MannWhitney tests for continuous variables and 2 tests for proportions. We compared the median turnaround time for EVPCR results and the percentage of tests returning prior to discharge between the pre‐ and post‐periods. We performed interrupted time series spline analyses to assess for trends in our primary outcomes, independent of the change in EVPCR testing protocol. All analyses were conducted using the Statistical Package for the Social Sciences (IBM SPSS Inc, Chicago, IL).9
RESULTS
Of the 593 children with meningitis, 152 (26%) were excluded for the reasons noted above. The 441 patients included in our analyses had the following final diagnoses: bacterial meningitis (1 patient with Streptococcus pneumoniae, 0.2%), pretreated culture‐negative meningitis (42 patients, 10%), and aseptic meningitis (398 patients, 90%).
We compared patient populations and EVPCR testing characteristics between the pre‐ and post‐study periods (Table 1). While CSF glucose differed between study periods, the difference was not felt to be clinically significant. However, during the post‐period, more children presented during enteroviral season. Clinicians were more likely to order an EVPCR test for children with aseptic, than bacterial, meningitis (213/370 [58%] vs 0/1 [0%]).
| Characteristic | Pre‐period (N = 225) | Post‐period (N = 216) | P Value |
|---|---|---|---|
| |||
| Demographics | |||
| Age (months)* | 3 (2106) | 3 (188) | 0.20 |
| Male, n (%) | 135 (60) | 129 (60) | 0.95 |
| Historical features | |||
| Duration of illness (days)* | 2 (14) | 2 (14) | 0.20 |
| Duration of fever (days)* | 1 (12) | 1 (12) | 0.52 |
| Antibiotic pretreatment, n (%) | 29 (13) | 13 (6.0) | 0.015 |
| Temperature at ED presentation* (C) | 37.6 (36.838.4) | 37.8 (37.138.2) | 0.51 |
| Presentation June through October, n (%) | 127 (56) | 143 (66) | 0.040 |
| Laboratory results | |||
| Peripheral WBC (cells/mm3)* | 10.4 (8.213.7) | 10.4 (7.813.6) | 0.67 |
| Peripheral ANC (cells/mm3)* | 5.2 (3.17.4) | 4.9 (2.68.2) | 0.47 |
| CSF WBC (cells/mm3)* | 55 (19176) | 62 (17250) | 0.66 |
| CSF ANC (cells/mm3)* | 8 (045) | 7 (141) | 0.78 |
| CSF glucose (mg/dL)* | 57 (5065) | 54 (4860) | 0.01 |
| CSF protein(mg/dL)* | 50 (3480) | 48 (3470) | 0.73 |
| Traumatic lumbar puncture (CSF RBC 500 cells/mm3), n (%) | 48 (21) | 43 (20) | 0.71 |
| Patient management | |||
| Admission to the hospital, n (%) | 196 (87) | 190 (88) | 0.68 |
| Parenteral antibiotics initiated, n (%) | 206 (92) | 200 (93) | 0.80 |
| Enteroviral PCR Testing | |||
| Testing utilization, n (%) | 62 (28) | 133 (62) | <0.001 |
| 90 days of age, n (%) | 18 (16) | 57/114 (50) | <0.001 |
| >90 days of age, n (%) | 44 (39) | 76/102 (75) | <0.001 |
| Positive test result, n (%) | 33 (53) | 80 (60) | 0.22 |
| Test turnaround time, hours* | 53 (4667) | 12 (617) | <0.001 |
We evaluated the impact of the in‐hospital EVPCR test on the length of stay and duration of parenteral antibiotics for the 6 predefined patient groups (Table 2). Length of stay could be determined for 432 (98%) of study patients, and duration of parenteral antibiotics for 365 (83%). We found a clinically important decrease in both length of stay and duration of parenteral antibiotics for children with a positive EVPCR test in the post‐period. For every hour earlier the EVPCR results returned, length of stay was reduced by 0.3 hours ( = 0.3, 95% confidence interval [CI] 0.10.5), and parenteral antibiotics were reduced by 0.3 hours ( = 0.3, 95% CI 0.10.5). However, even in the post‐period, the median length of time from a positive EVPCR test result to hospital discharge was 14 hours (interquartile range, 533 hours).
| Patient Group | Pre‐Period | Post‐Period | P Value1 |
|---|---|---|---|
| |||
| 1) All study patients | N = 225 | N = 216 | |
| Length of stay* | 49 (2662) | 47 (2662) | 0.09 |
| Duration of parenteral antibiotics* | 48 (2464) | 48 (2460) | 0.23 |
| 2) Children with a positive EVPCR test | N = 32 | N = 80 | |
| Length of stay* | 44 (2854) | 28 (1946) | 0.005 |
| Duration of parenteral antibiotics* | 48 (3072) | 36 (2449) | 0.037 |
| 3) Children with a negative EVPCR test | N = 29 | N = 53 | |
| Length of stay* | 61 (30114) | 59 (45109) | 0.67 |
| Duration of parenteral antibiotics* | 52 (4784) | 54 (4870) | 0.93 |
| 4) Children 90 days of age with positive EVPCR test | N = 9 | N =39 | |
| Length of stay* | 66 (5071) | 37 (2753) | 0.003 |
| Duration of parenteral antibiotics* | 74 (6994) | 48 (3660) | 0.002 |
| 5) Children >90 days of age with positive EVPCR test | N = 23 | N = 41 | |
| Length of stay* | 32 (2750) | 21 (430) | 0.002 |
| Duration of parenteral antibiotics* | 38 (2460) | 24 (2436) | 0.009 |
| 6) Children with a positive EVPCR test who presented during peak enteroviral season | N = 29 | N = 72 | |
| Length of stay* | 43 (2853) | 26 (1738) | 0.002 |
| Duration of parenteral antibiotics* | 46 (2470) | 36 (2448) | 0.05 |
We observed no trend in length of stay in either testing period ( = 0.17, 95% CI 3.9 to 3.6 pre vs = 1.64, 95% CI 6.3 to 3.0 post), with no change following the introduction of the faster EVPCR protocol (P = 0.52). We observed an increase in duration of parenteral antibiotics in the pre‐period ( = 5.4, 95% CI 0.3 to 10.6), with no trend in the post‐period ( = 1.7, 95% CI 5.2 to 1.8), but the difference was not significant (P = 0.08).
DISCUSSION
The in‐hospital EVPCR testing protocol reduced test turnaround time and increased testing. Children with a positive test had a shorter length of stay and duration of parenteral antibiotics. Decreasing the test turnaround time for EVPCR improved the care of children with enteroviral meningitis by reducing the length of unnecessary hospitalizations and parenteral antibiotics, with the potential for reducing the costs associated with these admissions.
Accurate identification of children with enteroviral meningitis, an often self‐limited infection requiring supportive care, can reduce hospitalization and unnecessary antibiotics. Previously, a positive EVPCR test result has been associated with a reduction in length of stay and of parenteral antibiotics,4, 5, 1012 with a direct correlation between test turnaround time and length of stay.12, 13 Moreover, positive EVPCR test results that were available prior to hospital discharge resulted in shorter length of hospital stay and duration of parenteral antibiotics.10
Our study is the largest to investigate the impact of implementing an in‐hospital EVPCR testing protocol, with the goal of making results available in a clinically useful time frame for all patients. Older EVPCR tests were typically performed in batches, or at centralized laboratories.4, 5, 1013 The in‐hospital EVPCR test utilized is a simple testing platform, which can be run multiple times daily. While there were higher charges associated with increased testing in the post‐period, these were more than offset by a reduced length of stay. Using study institution patient charges, we estimate that overall patient charges decreased approximately $80,000 in the post‐period, compared to the pre‐period (an average reduction of $375 per patient).
Many children were not discharged when a positive EVPCR test result became available. Some children with enteroviral meningitis will have persistent symptoms that require inpatient management. In addition, results that returned in the evening or nighttime were less likely to result in immediate hospital discharge. However, children with a positive EVPCR test are at very low risk for bacterial meningitis.3 As clinicians' knowledge of, and comfort with, the EVPCR test increase, this technology has the potential to further decrease the costs of caring for children with enteroviral meningitis.14
Our study had several limitations. First, it was retrospective; however, primary outcomes were objective measures accurately recorded in the medical record for most patients. Second, our study was single‐center, and findings may not be generalizable to other settings. Third, the management of children with meningitis may have been changing over the study period, independent of the in‐hospital EVPCR test. However, among children with a negative test, we observed no change in either of our primary outcomes. Fourth, given the large number of physicians involved with testing and treatment decisions, we could not adjust for clustering at the physician level. Fifth, we corrected CSF WBC in the case of a traumatic lumbar puncture (LP). Although use of this correction might underestimate the true CSF WBC count,6 the percentage of children with traumatic lumbar punctures was the same in both testing periods. Lastly, we evaluated the impact of a diagnostic test immediately after introduction into the clinical setting. As new medical technologies often take time to be adopted into clinical practice,15 we would expect the impact to increase over time.
CONCLUSIONS
In‐hospital EVPCR testing can improve the care of children with meningitis by reducing the length of unnecessary hospitalizations and parenteral antibiotics. Clinicians caring for children with meningitis should have access to in‐hospital EVPCR testing.
Acknowledgements
Disclosure: Nothing to report.
- .Enteroviral infections of the central nervous system.Clin Infect Dis.1995;20(4):971–981.
- ,,, et al.Clinical utility of the polymerase chain reaction for diagnosis of enteroviral meningitis in infancy.J Pediatr.1997;131(3):393–397.
- ,,,.Low risk of bacterial meningitis in children with a positive enteroviral polymerase chain reaction test result.Clin Infect Dis.2010;51(10):1221–1222.
- ,,,,,.Impact of rapid polymerase chain reaction results on management of pediatric patients with enteroviral meningitis.Pediatr Infect Dis J.2002;21(4):283–286.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- ,,,,,.Traumatic lumbar punctures in neonates: test performance of the cerebrospinal fluid white blood cell count.Pediatr Infect Dis J.2008;27(12):1047–1051.
- ,,, et al.Multicenter beta trial of the GeneXpert enterovirus assay.J Clin Microbiol.2007;45(4):1081–1086.
- ,.Most cerebrospinal fluid cultures in children with bacterial meningitis are positive within two days.Pediatr Infect Dis J.1999;18(8):732–733.
- SPSS for Windows [computer program]. Version 19.0.0.Chicago, IL:IBM SPSS Inc;2009.
- ,,,,.Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management.JAMA.2000;283(20):2680–2685.
- ,,,,.The impact of an enteroviral RT‐PCR assay on the diagnosis of aseptic meningitis and patient management.J Clin Virol.2002;25(suppl 1):S19–S26.
- ,,, et al.Impact of rapid enterovirus molecular diagnosis on the management of infants, children, and adults with aseptic meningitis.J Med Virol.2009;81(1):42–48.
- ,,, et al.A one‐step RT‐PCR assay using an enzyme‐linked detection system for the diagnosis of enterovirus meningitis.J Clin Virol.2000;17(3):143–149.
- ,.Cost analysis of enteroviral polymerase chain reaction in infants with fever and cerebrospinal fluid pleocytosis.Arch Pediatr Adolesc Med.2000;154(8):817–821.
- .Adoption of new surgical technology.BMJ.2006;332(7533):112–114.
Non‐polio enteroviruses are the most common cause of aseptic meningitis in children.1 While bacterial meningitis requires parenteral antibiotics, aseptic meningitis requires only supportive care.1 Enteroviral reverse transcription polymerase chain reaction (EVPCR) testing of the cerebrospinal fluid (CSF) allows the virus to be detected with high sensitivity and specificity.2 Because children with a positive EVPCR test are at low risk of bacterial meningitis,3 access to rapid EVPCR results has the potential to impact the clinical management of children with meningitis.4, 5 We studied the impact of implementing an in‐hospital EVPCR testing protocol on the clinical management of children with meningitis in a single‐center retrospective cohort.
MATERIALS AND METHODS
Study Design and Population
We identified children, <19 years of age, with meningitis evaluated at a single tertiary care pediatric center between July 2006 and June 2010. We defined meningitis as a CSF white blood cell (WBC) count 10 cells/mm3 corrected for the presence of CSF red blood cells (RBCs) (1 WBC for every 500 RBCs).6 We excluded children with any of the following: critical illness (defined as hypotension or respiratory failure), purpura, recent neurosurgery, ventricular shunt, immunosuppression, focal bacterial infection requiring parenteral antibiotics, positive CSF Gram stain, or known Lyme disease. The Institutional Review Board approved this study with waiver of informed consent.
Data Collection and Case Definitions
We abstracted historical and physical examination findings, as well as laboratory and microbiologic results, from the medical record. We defined bacterial meningitis as the isolation of pathogenic bacteria from the CSF or blood cultures. Children who had received antibiotics within 72 hours of diagnostic lumbar puncture, with negative cultures, were considered to have pretreated culture‐negative meningitis. Otherwise, children with negative bacterial cultures were classified as having aseptic meningitis.
EVPCR Testing
During the study pre‐period (July 1, 2006 through June 23, 2008), EVPCR tests were flown once daily to a commercial laboratory (ARUP Laboratories, Salt Lake City, UT) where they were run in batches. During the post‐period (June 24, 2008 through June 30, 2010), the study institution replaced the send‐out test with an in‐hospital EVPCR test (Gene Xpert EV Technology; Cepheid, Sunnyvale, CA)7 that allows multiple specimens to be run simultaneously, multiple times daily (between 7:00 AM and 10:00 PM), with results available in as little as 2.5 hours. We defined turnaround time for the test from specimen obtainment to test result.
Outcome Measures
Our 2 primary outcomes were length of stay and duration of parenteral antibiotics. Length of stay was measured as time from emergency department arrival to discharge (emergency department or inpatient discharge). We defined the duration of parenteral antibiotics as time from the first to the last dose of parenteral antibiotics administered, plus the standard antibiotic dosing interval for that antibiotic. For children with Lyme meningitis, the duration of parenteral antibiotic coverage was defined a priori as 48 hours, the standard time to reliably exclude bacterial growth from culture.8
Statistical Methods
Primary outcomes were compared using univariate analyses in 6 patient groups: 1) all patients, and those with 2) a positive EVPCR test, 3) a negative EVPCR test, and a positive test who were 4) 90 days old, 5) >90 days old, and 6) presented during peak enteroviral season (June through October). We utilized MannWhitney tests for continuous variables and 2 tests for proportions. We compared the median turnaround time for EVPCR results and the percentage of tests returning prior to discharge between the pre‐ and post‐periods. We performed interrupted time series spline analyses to assess for trends in our primary outcomes, independent of the change in EVPCR testing protocol. All analyses were conducted using the Statistical Package for the Social Sciences (IBM SPSS Inc, Chicago, IL).9
RESULTS
Of the 593 children with meningitis, 152 (26%) were excluded for the reasons noted above. The 441 patients included in our analyses had the following final diagnoses: bacterial meningitis (1 patient with Streptococcus pneumoniae, 0.2%), pretreated culture‐negative meningitis (42 patients, 10%), and aseptic meningitis (398 patients, 90%).
We compared patient populations and EVPCR testing characteristics between the pre‐ and post‐study periods (Table 1). While CSF glucose differed between study periods, the difference was not felt to be clinically significant. However, during the post‐period, more children presented during enteroviral season. Clinicians were more likely to order an EVPCR test for children with aseptic, than bacterial, meningitis (213/370 [58%] vs 0/1 [0%]).
| Characteristic | Pre‐period (N = 225) | Post‐period (N = 216) | P Value |
|---|---|---|---|
| |||
| Demographics | |||
| Age (months)* | 3 (2106) | 3 (188) | 0.20 |
| Male, n (%) | 135 (60) | 129 (60) | 0.95 |
| Historical features | |||
| Duration of illness (days)* | 2 (14) | 2 (14) | 0.20 |
| Duration of fever (days)* | 1 (12) | 1 (12) | 0.52 |
| Antibiotic pretreatment, n (%) | 29 (13) | 13 (6.0) | 0.015 |
| Temperature at ED presentation* (C) | 37.6 (36.838.4) | 37.8 (37.138.2) | 0.51 |
| Presentation June through October, n (%) | 127 (56) | 143 (66) | 0.040 |
| Laboratory results | |||
| Peripheral WBC (cells/mm3)* | 10.4 (8.213.7) | 10.4 (7.813.6) | 0.67 |
| Peripheral ANC (cells/mm3)* | 5.2 (3.17.4) | 4.9 (2.68.2) | 0.47 |
| CSF WBC (cells/mm3)* | 55 (19176) | 62 (17250) | 0.66 |
| CSF ANC (cells/mm3)* | 8 (045) | 7 (141) | 0.78 |
| CSF glucose (mg/dL)* | 57 (5065) | 54 (4860) | 0.01 |
| CSF protein(mg/dL)* | 50 (3480) | 48 (3470) | 0.73 |
| Traumatic lumbar puncture (CSF RBC 500 cells/mm3), n (%) | 48 (21) | 43 (20) | 0.71 |
| Patient management | |||
| Admission to the hospital, n (%) | 196 (87) | 190 (88) | 0.68 |
| Parenteral antibiotics initiated, n (%) | 206 (92) | 200 (93) | 0.80 |
| Enteroviral PCR Testing | |||
| Testing utilization, n (%) | 62 (28) | 133 (62) | <0.001 |
| 90 days of age, n (%) | 18 (16) | 57/114 (50) | <0.001 |
| >90 days of age, n (%) | 44 (39) | 76/102 (75) | <0.001 |
| Positive test result, n (%) | 33 (53) | 80 (60) | 0.22 |
| Test turnaround time, hours* | 53 (4667) | 12 (617) | <0.001 |
We evaluated the impact of the in‐hospital EVPCR test on the length of stay and duration of parenteral antibiotics for the 6 predefined patient groups (Table 2). Length of stay could be determined for 432 (98%) of study patients, and duration of parenteral antibiotics for 365 (83%). We found a clinically important decrease in both length of stay and duration of parenteral antibiotics for children with a positive EVPCR test in the post‐period. For every hour earlier the EVPCR results returned, length of stay was reduced by 0.3 hours ( = 0.3, 95% confidence interval [CI] 0.10.5), and parenteral antibiotics were reduced by 0.3 hours ( = 0.3, 95% CI 0.10.5). However, even in the post‐period, the median length of time from a positive EVPCR test result to hospital discharge was 14 hours (interquartile range, 533 hours).
| Patient Group | Pre‐Period | Post‐Period | P Value1 |
|---|---|---|---|
| |||
| 1) All study patients | N = 225 | N = 216 | |
| Length of stay* | 49 (2662) | 47 (2662) | 0.09 |
| Duration of parenteral antibiotics* | 48 (2464) | 48 (2460) | 0.23 |
| 2) Children with a positive EVPCR test | N = 32 | N = 80 | |
| Length of stay* | 44 (2854) | 28 (1946) | 0.005 |
| Duration of parenteral antibiotics* | 48 (3072) | 36 (2449) | 0.037 |
| 3) Children with a negative EVPCR test | N = 29 | N = 53 | |
| Length of stay* | 61 (30114) | 59 (45109) | 0.67 |
| Duration of parenteral antibiotics* | 52 (4784) | 54 (4870) | 0.93 |
| 4) Children 90 days of age with positive EVPCR test | N = 9 | N =39 | |
| Length of stay* | 66 (5071) | 37 (2753) | 0.003 |
| Duration of parenteral antibiotics* | 74 (6994) | 48 (3660) | 0.002 |
| 5) Children >90 days of age with positive EVPCR test | N = 23 | N = 41 | |
| Length of stay* | 32 (2750) | 21 (430) | 0.002 |
| Duration of parenteral antibiotics* | 38 (2460) | 24 (2436) | 0.009 |
| 6) Children with a positive EVPCR test who presented during peak enteroviral season | N = 29 | N = 72 | |
| Length of stay* | 43 (2853) | 26 (1738) | 0.002 |
| Duration of parenteral antibiotics* | 46 (2470) | 36 (2448) | 0.05 |
We observed no trend in length of stay in either testing period ( = 0.17, 95% CI 3.9 to 3.6 pre vs = 1.64, 95% CI 6.3 to 3.0 post), with no change following the introduction of the faster EVPCR protocol (P = 0.52). We observed an increase in duration of parenteral antibiotics in the pre‐period ( = 5.4, 95% CI 0.3 to 10.6), with no trend in the post‐period ( = 1.7, 95% CI 5.2 to 1.8), but the difference was not significant (P = 0.08).
DISCUSSION
The in‐hospital EVPCR testing protocol reduced test turnaround time and increased testing. Children with a positive test had a shorter length of stay and duration of parenteral antibiotics. Decreasing the test turnaround time for EVPCR improved the care of children with enteroviral meningitis by reducing the length of unnecessary hospitalizations and parenteral antibiotics, with the potential for reducing the costs associated with these admissions.
Accurate identification of children with enteroviral meningitis, an often self‐limited infection requiring supportive care, can reduce hospitalization and unnecessary antibiotics. Previously, a positive EVPCR test result has been associated with a reduction in length of stay and of parenteral antibiotics,4, 5, 1012 with a direct correlation between test turnaround time and length of stay.12, 13 Moreover, positive EVPCR test results that were available prior to hospital discharge resulted in shorter length of hospital stay and duration of parenteral antibiotics.10
Our study is the largest to investigate the impact of implementing an in‐hospital EVPCR testing protocol, with the goal of making results available in a clinically useful time frame for all patients. Older EVPCR tests were typically performed in batches, or at centralized laboratories.4, 5, 1013 The in‐hospital EVPCR test utilized is a simple testing platform, which can be run multiple times daily. While there were higher charges associated with increased testing in the post‐period, these were more than offset by a reduced length of stay. Using study institution patient charges, we estimate that overall patient charges decreased approximately $80,000 in the post‐period, compared to the pre‐period (an average reduction of $375 per patient).
Many children were not discharged when a positive EVPCR test result became available. Some children with enteroviral meningitis will have persistent symptoms that require inpatient management. In addition, results that returned in the evening or nighttime were less likely to result in immediate hospital discharge. However, children with a positive EVPCR test are at very low risk for bacterial meningitis.3 As clinicians' knowledge of, and comfort with, the EVPCR test increase, this technology has the potential to further decrease the costs of caring for children with enteroviral meningitis.14
Our study had several limitations. First, it was retrospective; however, primary outcomes were objective measures accurately recorded in the medical record for most patients. Second, our study was single‐center, and findings may not be generalizable to other settings. Third, the management of children with meningitis may have been changing over the study period, independent of the in‐hospital EVPCR test. However, among children with a negative test, we observed no change in either of our primary outcomes. Fourth, given the large number of physicians involved with testing and treatment decisions, we could not adjust for clustering at the physician level. Fifth, we corrected CSF WBC in the case of a traumatic lumbar puncture (LP). Although use of this correction might underestimate the true CSF WBC count,6 the percentage of children with traumatic lumbar punctures was the same in both testing periods. Lastly, we evaluated the impact of a diagnostic test immediately after introduction into the clinical setting. As new medical technologies often take time to be adopted into clinical practice,15 we would expect the impact to increase over time.
CONCLUSIONS
In‐hospital EVPCR testing can improve the care of children with meningitis by reducing the length of unnecessary hospitalizations and parenteral antibiotics. Clinicians caring for children with meningitis should have access to in‐hospital EVPCR testing.
Acknowledgements
Disclosure: Nothing to report.
Non‐polio enteroviruses are the most common cause of aseptic meningitis in children.1 While bacterial meningitis requires parenteral antibiotics, aseptic meningitis requires only supportive care.1 Enteroviral reverse transcription polymerase chain reaction (EVPCR) testing of the cerebrospinal fluid (CSF) allows the virus to be detected with high sensitivity and specificity.2 Because children with a positive EVPCR test are at low risk of bacterial meningitis,3 access to rapid EVPCR results has the potential to impact the clinical management of children with meningitis.4, 5 We studied the impact of implementing an in‐hospital EVPCR testing protocol on the clinical management of children with meningitis in a single‐center retrospective cohort.
MATERIALS AND METHODS
Study Design and Population
We identified children, <19 years of age, with meningitis evaluated at a single tertiary care pediatric center between July 2006 and June 2010. We defined meningitis as a CSF white blood cell (WBC) count 10 cells/mm3 corrected for the presence of CSF red blood cells (RBCs) (1 WBC for every 500 RBCs).6 We excluded children with any of the following: critical illness (defined as hypotension or respiratory failure), purpura, recent neurosurgery, ventricular shunt, immunosuppression, focal bacterial infection requiring parenteral antibiotics, positive CSF Gram stain, or known Lyme disease. The Institutional Review Board approved this study with waiver of informed consent.
Data Collection and Case Definitions
We abstracted historical and physical examination findings, as well as laboratory and microbiologic results, from the medical record. We defined bacterial meningitis as the isolation of pathogenic bacteria from the CSF or blood cultures. Children who had received antibiotics within 72 hours of diagnostic lumbar puncture, with negative cultures, were considered to have pretreated culture‐negative meningitis. Otherwise, children with negative bacterial cultures were classified as having aseptic meningitis.
EVPCR Testing
During the study pre‐period (July 1, 2006 through June 23, 2008), EVPCR tests were flown once daily to a commercial laboratory (ARUP Laboratories, Salt Lake City, UT) where they were run in batches. During the post‐period (June 24, 2008 through June 30, 2010), the study institution replaced the send‐out test with an in‐hospital EVPCR test (Gene Xpert EV Technology; Cepheid, Sunnyvale, CA)7 that allows multiple specimens to be run simultaneously, multiple times daily (between 7:00 AM and 10:00 PM), with results available in as little as 2.5 hours. We defined turnaround time for the test from specimen obtainment to test result.
Outcome Measures
Our 2 primary outcomes were length of stay and duration of parenteral antibiotics. Length of stay was measured as time from emergency department arrival to discharge (emergency department or inpatient discharge). We defined the duration of parenteral antibiotics as time from the first to the last dose of parenteral antibiotics administered, plus the standard antibiotic dosing interval for that antibiotic. For children with Lyme meningitis, the duration of parenteral antibiotic coverage was defined a priori as 48 hours, the standard time to reliably exclude bacterial growth from culture.8
Statistical Methods
Primary outcomes were compared using univariate analyses in 6 patient groups: 1) all patients, and those with 2) a positive EVPCR test, 3) a negative EVPCR test, and a positive test who were 4) 90 days old, 5) >90 days old, and 6) presented during peak enteroviral season (June through October). We utilized MannWhitney tests for continuous variables and 2 tests for proportions. We compared the median turnaround time for EVPCR results and the percentage of tests returning prior to discharge between the pre‐ and post‐periods. We performed interrupted time series spline analyses to assess for trends in our primary outcomes, independent of the change in EVPCR testing protocol. All analyses were conducted using the Statistical Package for the Social Sciences (IBM SPSS Inc, Chicago, IL).9
RESULTS
Of the 593 children with meningitis, 152 (26%) were excluded for the reasons noted above. The 441 patients included in our analyses had the following final diagnoses: bacterial meningitis (1 patient with Streptococcus pneumoniae, 0.2%), pretreated culture‐negative meningitis (42 patients, 10%), and aseptic meningitis (398 patients, 90%).
We compared patient populations and EVPCR testing characteristics between the pre‐ and post‐study periods (Table 1). While CSF glucose differed between study periods, the difference was not felt to be clinically significant. However, during the post‐period, more children presented during enteroviral season. Clinicians were more likely to order an EVPCR test for children with aseptic, than bacterial, meningitis (213/370 [58%] vs 0/1 [0%]).
| Characteristic | Pre‐period (N = 225) | Post‐period (N = 216) | P Value |
|---|---|---|---|
| |||
| Demographics | |||
| Age (months)* | 3 (2106) | 3 (188) | 0.20 |
| Male, n (%) | 135 (60) | 129 (60) | 0.95 |
| Historical features | |||
| Duration of illness (days)* | 2 (14) | 2 (14) | 0.20 |
| Duration of fever (days)* | 1 (12) | 1 (12) | 0.52 |
| Antibiotic pretreatment, n (%) | 29 (13) | 13 (6.0) | 0.015 |
| Temperature at ED presentation* (C) | 37.6 (36.838.4) | 37.8 (37.138.2) | 0.51 |
| Presentation June through October, n (%) | 127 (56) | 143 (66) | 0.040 |
| Laboratory results | |||
| Peripheral WBC (cells/mm3)* | 10.4 (8.213.7) | 10.4 (7.813.6) | 0.67 |
| Peripheral ANC (cells/mm3)* | 5.2 (3.17.4) | 4.9 (2.68.2) | 0.47 |
| CSF WBC (cells/mm3)* | 55 (19176) | 62 (17250) | 0.66 |
| CSF ANC (cells/mm3)* | 8 (045) | 7 (141) | 0.78 |
| CSF glucose (mg/dL)* | 57 (5065) | 54 (4860) | 0.01 |
| CSF protein(mg/dL)* | 50 (3480) | 48 (3470) | 0.73 |
| Traumatic lumbar puncture (CSF RBC 500 cells/mm3), n (%) | 48 (21) | 43 (20) | 0.71 |
| Patient management | |||
| Admission to the hospital, n (%) | 196 (87) | 190 (88) | 0.68 |
| Parenteral antibiotics initiated, n (%) | 206 (92) | 200 (93) | 0.80 |
| Enteroviral PCR Testing | |||
| Testing utilization, n (%) | 62 (28) | 133 (62) | <0.001 |
| 90 days of age, n (%) | 18 (16) | 57/114 (50) | <0.001 |
| >90 days of age, n (%) | 44 (39) | 76/102 (75) | <0.001 |
| Positive test result, n (%) | 33 (53) | 80 (60) | 0.22 |
| Test turnaround time, hours* | 53 (4667) | 12 (617) | <0.001 |
We evaluated the impact of the in‐hospital EVPCR test on the length of stay and duration of parenteral antibiotics for the 6 predefined patient groups (Table 2). Length of stay could be determined for 432 (98%) of study patients, and duration of parenteral antibiotics for 365 (83%). We found a clinically important decrease in both length of stay and duration of parenteral antibiotics for children with a positive EVPCR test in the post‐period. For every hour earlier the EVPCR results returned, length of stay was reduced by 0.3 hours ( = 0.3, 95% confidence interval [CI] 0.10.5), and parenteral antibiotics were reduced by 0.3 hours ( = 0.3, 95% CI 0.10.5). However, even in the post‐period, the median length of time from a positive EVPCR test result to hospital discharge was 14 hours (interquartile range, 533 hours).
| Patient Group | Pre‐Period | Post‐Period | P Value1 |
|---|---|---|---|
| |||
| 1) All study patients | N = 225 | N = 216 | |
| Length of stay* | 49 (2662) | 47 (2662) | 0.09 |
| Duration of parenteral antibiotics* | 48 (2464) | 48 (2460) | 0.23 |
| 2) Children with a positive EVPCR test | N = 32 | N = 80 | |
| Length of stay* | 44 (2854) | 28 (1946) | 0.005 |
| Duration of parenteral antibiotics* | 48 (3072) | 36 (2449) | 0.037 |
| 3) Children with a negative EVPCR test | N = 29 | N = 53 | |
| Length of stay* | 61 (30114) | 59 (45109) | 0.67 |
| Duration of parenteral antibiotics* | 52 (4784) | 54 (4870) | 0.93 |
| 4) Children 90 days of age with positive EVPCR test | N = 9 | N =39 | |
| Length of stay* | 66 (5071) | 37 (2753) | 0.003 |
| Duration of parenteral antibiotics* | 74 (6994) | 48 (3660) | 0.002 |
| 5) Children >90 days of age with positive EVPCR test | N = 23 | N = 41 | |
| Length of stay* | 32 (2750) | 21 (430) | 0.002 |
| Duration of parenteral antibiotics* | 38 (2460) | 24 (2436) | 0.009 |
| 6) Children with a positive EVPCR test who presented during peak enteroviral season | N = 29 | N = 72 | |
| Length of stay* | 43 (2853) | 26 (1738) | 0.002 |
| Duration of parenteral antibiotics* | 46 (2470) | 36 (2448) | 0.05 |
We observed no trend in length of stay in either testing period ( = 0.17, 95% CI 3.9 to 3.6 pre vs = 1.64, 95% CI 6.3 to 3.0 post), with no change following the introduction of the faster EVPCR protocol (P = 0.52). We observed an increase in duration of parenteral antibiotics in the pre‐period ( = 5.4, 95% CI 0.3 to 10.6), with no trend in the post‐period ( = 1.7, 95% CI 5.2 to 1.8), but the difference was not significant (P = 0.08).
DISCUSSION
The in‐hospital EVPCR testing protocol reduced test turnaround time and increased testing. Children with a positive test had a shorter length of stay and duration of parenteral antibiotics. Decreasing the test turnaround time for EVPCR improved the care of children with enteroviral meningitis by reducing the length of unnecessary hospitalizations and parenteral antibiotics, with the potential for reducing the costs associated with these admissions.
Accurate identification of children with enteroviral meningitis, an often self‐limited infection requiring supportive care, can reduce hospitalization and unnecessary antibiotics. Previously, a positive EVPCR test result has been associated with a reduction in length of stay and of parenteral antibiotics,4, 5, 1012 with a direct correlation between test turnaround time and length of stay.12, 13 Moreover, positive EVPCR test results that were available prior to hospital discharge resulted in shorter length of hospital stay and duration of parenteral antibiotics.10
Our study is the largest to investigate the impact of implementing an in‐hospital EVPCR testing protocol, with the goal of making results available in a clinically useful time frame for all patients. Older EVPCR tests were typically performed in batches, or at centralized laboratories.4, 5, 1013 The in‐hospital EVPCR test utilized is a simple testing platform, which can be run multiple times daily. While there were higher charges associated with increased testing in the post‐period, these were more than offset by a reduced length of stay. Using study institution patient charges, we estimate that overall patient charges decreased approximately $80,000 in the post‐period, compared to the pre‐period (an average reduction of $375 per patient).
Many children were not discharged when a positive EVPCR test result became available. Some children with enteroviral meningitis will have persistent symptoms that require inpatient management. In addition, results that returned in the evening or nighttime were less likely to result in immediate hospital discharge. However, children with a positive EVPCR test are at very low risk for bacterial meningitis.3 As clinicians' knowledge of, and comfort with, the EVPCR test increase, this technology has the potential to further decrease the costs of caring for children with enteroviral meningitis.14
Our study had several limitations. First, it was retrospective; however, primary outcomes were objective measures accurately recorded in the medical record for most patients. Second, our study was single‐center, and findings may not be generalizable to other settings. Third, the management of children with meningitis may have been changing over the study period, independent of the in‐hospital EVPCR test. However, among children with a negative test, we observed no change in either of our primary outcomes. Fourth, given the large number of physicians involved with testing and treatment decisions, we could not adjust for clustering at the physician level. Fifth, we corrected CSF WBC in the case of a traumatic lumbar puncture (LP). Although use of this correction might underestimate the true CSF WBC count,6 the percentage of children with traumatic lumbar punctures was the same in both testing periods. Lastly, we evaluated the impact of a diagnostic test immediately after introduction into the clinical setting. As new medical technologies often take time to be adopted into clinical practice,15 we would expect the impact to increase over time.
CONCLUSIONS
In‐hospital EVPCR testing can improve the care of children with meningitis by reducing the length of unnecessary hospitalizations and parenteral antibiotics. Clinicians caring for children with meningitis should have access to in‐hospital EVPCR testing.
Acknowledgements
Disclosure: Nothing to report.
- .Enteroviral infections of the central nervous system.Clin Infect Dis.1995;20(4):971–981.
- ,,, et al.Clinical utility of the polymerase chain reaction for diagnosis of enteroviral meningitis in infancy.J Pediatr.1997;131(3):393–397.
- ,,,.Low risk of bacterial meningitis in children with a positive enteroviral polymerase chain reaction test result.Clin Infect Dis.2010;51(10):1221–1222.
- ,,,,,.Impact of rapid polymerase chain reaction results on management of pediatric patients with enteroviral meningitis.Pediatr Infect Dis J.2002;21(4):283–286.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- ,,,,,.Traumatic lumbar punctures in neonates: test performance of the cerebrospinal fluid white blood cell count.Pediatr Infect Dis J.2008;27(12):1047–1051.
- ,,, et al.Multicenter beta trial of the GeneXpert enterovirus assay.J Clin Microbiol.2007;45(4):1081–1086.
- ,.Most cerebrospinal fluid cultures in children with bacterial meningitis are positive within two days.Pediatr Infect Dis J.1999;18(8):732–733.
- SPSS for Windows [computer program]. Version 19.0.0.Chicago, IL:IBM SPSS Inc;2009.
- ,,,,.Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management.JAMA.2000;283(20):2680–2685.
- ,,,,.The impact of an enteroviral RT‐PCR assay on the diagnosis of aseptic meningitis and patient management.J Clin Virol.2002;25(suppl 1):S19–S26.
- ,,, et al.Impact of rapid enterovirus molecular diagnosis on the management of infants, children, and adults with aseptic meningitis.J Med Virol.2009;81(1):42–48.
- ,,, et al.A one‐step RT‐PCR assay using an enzyme‐linked detection system for the diagnosis of enterovirus meningitis.J Clin Virol.2000;17(3):143–149.
- ,.Cost analysis of enteroviral polymerase chain reaction in infants with fever and cerebrospinal fluid pleocytosis.Arch Pediatr Adolesc Med.2000;154(8):817–821.
- .Adoption of new surgical technology.BMJ.2006;332(7533):112–114.
- .Enteroviral infections of the central nervous system.Clin Infect Dis.1995;20(4):971–981.
- ,,, et al.Clinical utility of the polymerase chain reaction for diagnosis of enteroviral meningitis in infancy.J Pediatr.1997;131(3):393–397.
- ,,,.Low risk of bacterial meningitis in children with a positive enteroviral polymerase chain reaction test result.Clin Infect Dis.2010;51(10):1221–1222.
- ,,,,,.Impact of rapid polymerase chain reaction results on management of pediatric patients with enteroviral meningitis.Pediatr Infect Dis J.2002;21(4):283–286.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- ,,,,,.Traumatic lumbar punctures in neonates: test performance of the cerebrospinal fluid white blood cell count.Pediatr Infect Dis J.2008;27(12):1047–1051.
- ,,, et al.Multicenter beta trial of the GeneXpert enterovirus assay.J Clin Microbiol.2007;45(4):1081–1086.
- ,.Most cerebrospinal fluid cultures in children with bacterial meningitis are positive within two days.Pediatr Infect Dis J.1999;18(8):732–733.
- SPSS for Windows [computer program]. Version 19.0.0.Chicago, IL:IBM SPSS Inc;2009.
- ,,,,.Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management.JAMA.2000;283(20):2680–2685.
- ,,,,.The impact of an enteroviral RT‐PCR assay on the diagnosis of aseptic meningitis and patient management.J Clin Virol.2002;25(suppl 1):S19–S26.
- ,,, et al.Impact of rapid enterovirus molecular diagnosis on the management of infants, children, and adults with aseptic meningitis.J Med Virol.2009;81(1):42–48.
- ,,, et al.A one‐step RT‐PCR assay using an enzyme‐linked detection system for the diagnosis of enterovirus meningitis.J Clin Virol.2000;17(3):143–149.
- ,.Cost analysis of enteroviral polymerase chain reaction in infants with fever and cerebrospinal fluid pleocytosis.Arch Pediatr Adolesc Med.2000;154(8):817–821.
- .Adoption of new surgical technology.BMJ.2006;332(7533):112–114.
Reliability of CXR for Pneumonia
The chest radiograph (CXR) is the most commonly used diagnostic imaging modality in children, and is considered to be the gold standard for the diagnosis of pneumonia. As such, physicians in developed countries rely on chest radiography to establish the diagnosis of pneumonia.13 However, there are limited data investigating the reliability of this test for the diagnosis of pneumonia in children.2, 46
Prior investigations have noted poor overall agreement by emergency medicine, infectious diseases, and pulmonary medicine physicians, and even radiologists, in their interpretation of chest radiographs for the diagnosis of pneumonia.2, 5, 710 The World Health Organization (WHO) developed criteria to standardize CXR interpretation for the diagnosis of pneumonia in children for use in epidemiologic studies.11 These standardized definitions of pneumonia have been formally evaluated by the WHO6 and utilized in epidemiologic studies of vaccine efficacy,12 but the overall reliability of these radiographic criteria have not been studied outside of these forums.
We conducted this prospective case‐based study to evaluate the reliability of the radiographic diagnosis of pneumonia among children presenting to a pediatric emergency department with clinical suspicion of pneumonia. We were primarily interested in assessing the overall reliability in CXR interpretation for the diagnosis of pneumonia, and identifying which radiographic features of pneumonia were consistently identified by radiologists.
MATERIALS AND METHODS
Study Subjects
We evaluated the reliability of CXR interpretation with respect to the diagnosis of pneumonia among radiologists. Six board‐certified radiologists at 2 academic children's hospitals (Children's Hospital of Philadelphia, Philadelphia, PA [n = 3] and Children's Hospital, Boston, Boston, MA [n = 3]) interpreted the same 110 chest radiographs in a blinded fashion. The radiologists varied with respect to the number of years practicing pediatric radiology (median 8 years, range 3‐36 years). Clinical information such as age, gender, clinical indication for obtaining the radiograph, history, and physical examination findings were not provided. Aside from the study form which stated the WHO classification scheme for radiographic pneumonia, no other information or training was provided to the radiologists as part of this study.
Radiographs were selected among a population of children presenting to the emergency department at Children's Hospital, Boston, who had a radiograph obtained for concern of pneumonia. From this cohort, we selected children who had radiographs which encompassed the spectrum of respiratory disease processes encountered in a pediatric population. The radiographs selected for review included 50 radiographs with a final reading in the medical record without suspicion for pneumonia, and 50 radiographs in which the diagnosis of pneumonia could not be excluded. In the latter group, 25 radiographs had a final reading suggestive of an alveolar infiltrate, and 25 radiographs had a final reading suggestive of an interstitial infiltrate. Ten duplicate radiographs were included to permit assessment of intra‐rater reliability.
Radiograph Interpretation
Radiologists at both sites interpreted the identical 110 radiographs (both anteroposterior [AP] and lateral views for each subject). Digital Imaging and Communications in Medicine (DICOM) images were downloaded from a registry at Children's Hospital, Boston, and were copied to DVDs which were provided to each radiologist. Standardized radiographic imaging software (eFilm Lite [Mississauga, Canada]) was used by each radiologist to view and interpret the radiographs.
Each radiologist completed a study questionnaire for each radiograph interpreted (see Supporting Appendix A in the online version of this article). The questionnaire utilized radiographic descriptors of primary end‐point pneumonia described by the WHO which were procured to standardize the radiographic diagnosis of pneumonia.11, 12 The main outcome of interest was the presence or absence of an infiltrate. Among radiographs in which an infiltrate was identified, radiologists selected whether there was an alveolar infiltrate, interstitial infiltrate, or both. An alveolar infiltrate was defined as a dense or fluffy opacity that occupies a portion or whole of a lobe, or of the entire lung, that may or may not contain air bronchograms.11, 12 An interstitial infiltrate was defined by a lacy pattern involving both lungs, featuring peribronchial thickening and multiple areas of atelectasis.11, 12 It also included minor patchy infiltrates that were not of sufficient magnitude to constitute consolidation, and small areas of atelectasis that in children may be difficult to distinguish from consolidation. Among interstitial infiltrates, radiologists were asked to distinguish infiltrate from atelectasis. A radiograph classified as having either an alveolar infiltrate or interstitial infiltrate (not atelectasis) was considered to have any infiltrate. Additional findings including air bronchograms, hilar adenopathy, pleural effusion, and location of abnormalities were also recorded.
Statistical Analysis
Inter‐rater reliability was assessed using the kappa statistic to determine the overall agreement between the 6 radiologists for each binary outcome (ie, presence or absence of alveolar infiltrate). To calculate 95% confidence intervals (CI) for kappa statistics with more than 2 raters, we employed a bootstrapping method with 1000 replications of samples equal in size to the study sample, using the kapci program as implemented by STATA software (version 10.1, STATA Corp, College Station, TX). Also, intra‐rater reliability was evaluated by examining the agreement within each radiologist upon review of 10 duplicate radiographs that had been randomly inserted into the case‐mix. We used the benchmarks proposed by Landis and Koch to classify the strength of agreement measured by the kappa statistic, as follows: poor (<0.0); slight (0‐0.20); fair (0.21‐0.40); moderate (0.41‐0.60); substantial (0.61‐0.80); almost perfect (0.81‐1.0).13
The study was approved by the institutional review boards at Children's Hospital, Boston and Children's Hospital of Philadelphia.
RESULTS
Patient Sample
The sample of 110 radiographs was obtained from 100 children presenting to the emergency department at Children's Hospital, Boston, with concern of pneumonia. These patients ranged in age from 1 week to 19 years (median, 3.5 years; interquartile range [IQR], 1.6‐6.0 years). Fifty (50%) of these patients were male. As stated above, the sample comprised 50 radiographs with a final reading in the medical record without suspicion for pneumonia, and 50 radiographs in which the diagnosis of pneumonia could not be excluded. The 10 duplicate radiographs encompassed a similar spectrum of findings.
Inter‐Rater Reliability
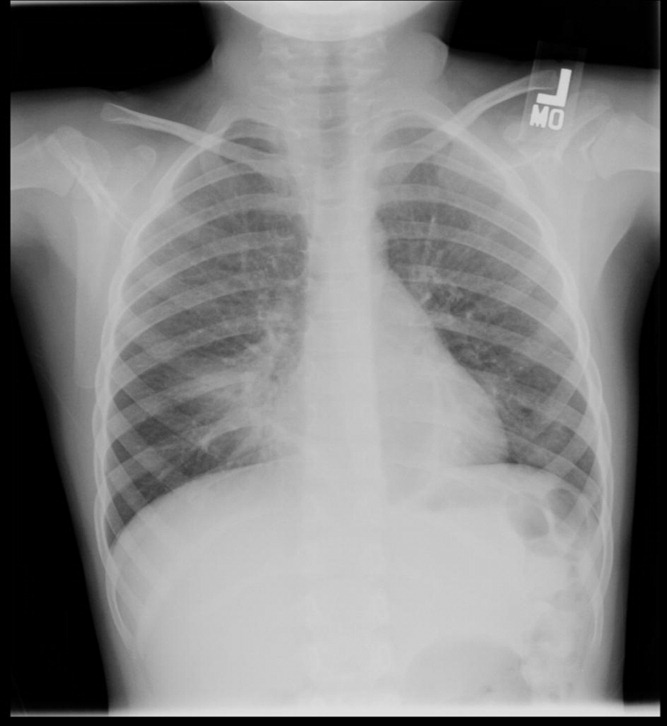
The kappa coefficients of inter‐rater reliability between the radiologists across the 6 clinical measures of interest are displayed in Table 1. As shown, the most reliable measure was that of alveolar infiltrate (Figure 1), which attained a substantial degree of agreement between the radiologists. Two other measures, any infiltrate and pleural effusion, attained moderate reliability, while bronchograms and hilar adenopathy were each classified as having fair reliability. However, interstitial infiltrate (Figure 2) was found to have the lowest kappa estimate, with a slight degree of reliability. When examining inter‐rater reliability among the radiologists separately from each institution, the pattern of results was similar.
| All Radiologists (n = 6) | Kappa | 95% Confidence Interval |
|---|---|---|
| ||
| Any infiltrate | 0.47 | 0.39, 0.56 |
| Alveolar infiltrate | 0.69 | 0.60, 0.78 |
| Interstitial infiltrate | 0.14 | 0.05, 0.23 |
| Air bronchograms | 0.32 | 0.24, 0.42 |
| Hilar adenopathy | 0.21 | 0.08, 0.39 |
| Pleural effusion | 0.45 | 0.29, 0.61 |
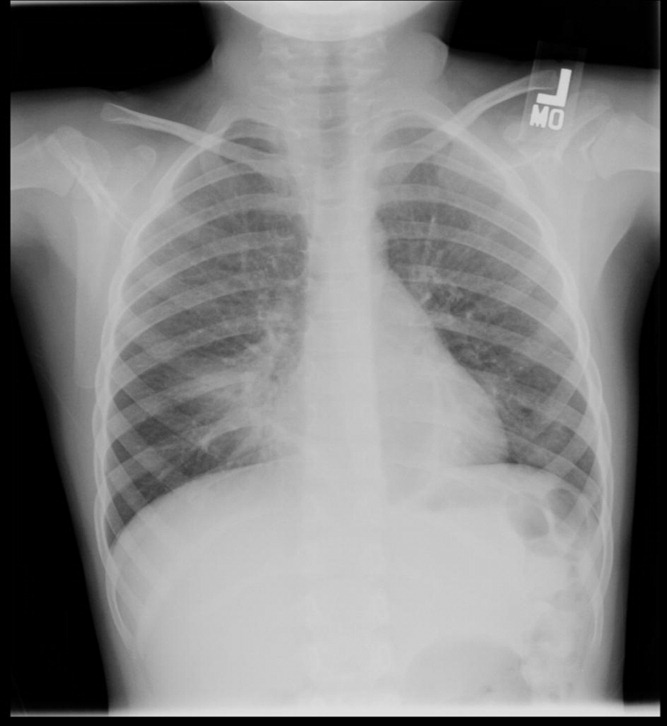
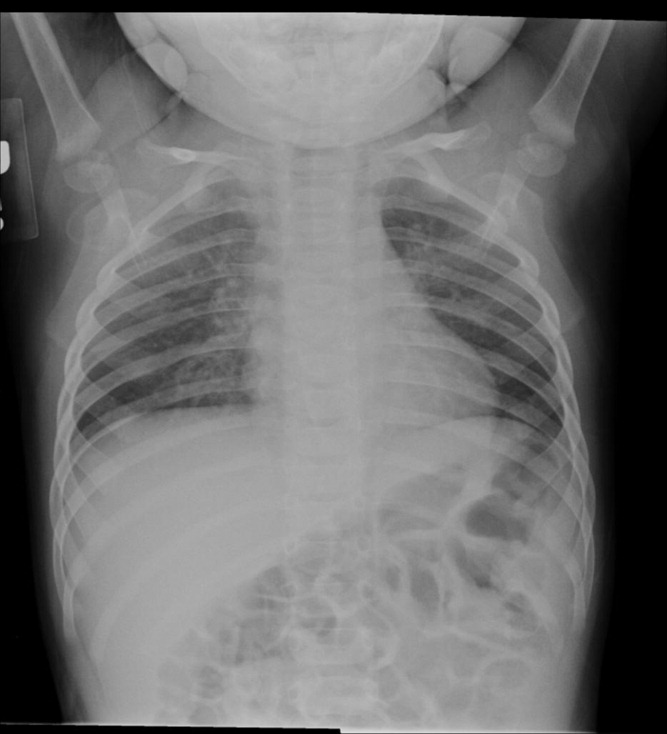
At least 4 of the 6 radiologists agreed on the presence or absence of an alveolar infiltrate for 95 of the 100 unique CXRs; all 6 radiologists agreed regarding the presence or absence of an alveolar infiltrate in 72 of the 100 unique CXRs. At least 4 of the 6 radiologists agreed on the presence or absence of any infiltrate and interstitial infiltrate 96% and 90% of the time, respectively. All 6 of the radiologists agreed on the presence or absence of any infiltrate and interstitial infiltrate 35% and 27% of the time, respectively.
Intra‐Rater Reliability
Estimates of intra‐rater reliability on the primary clinical outcomes (alveolar infiltrate, interstitial infiltrate, and any infiltrate) are found in Table 2. Across the 6 raters, the kappa estimates for alveolar infiltrate were all classified as substantial or almost perfect. The kappa estimates for interstitial infiltrate varied widely, ranging from fair to almost perfect, while for any infiltrate, reliability ranged from moderate to almost perfect.
| Kappa | 95% Confidence Interval | |
|---|---|---|
| ||
| Any infiltrate | ||
| Rater 1 | 1.00 | 1.00, 1.00 |
| Rater 2 | 0.60 | 0.10, 1.00 |
| Rater 3 | 0.80 | 0.44, 1.00 |
| Rater 4 | 1.00 | 1.00, 1.00 |
| Rater 5 | n/a* | |
| Rater 6 | 1.00 | 1.00, 1.00 |
| Alveolar infiltrate | ||
| Rater 1 | 1.00 | 1.00, 1.00 |
| Rater 2 | 1.00 | 1.00, 1.00 |
| Rater 3 | 1.00 | 1.00, 1.00 |
| Rater 4 | 1.00 | 1.00, 1.00 |
| Rater 5 | 0.78 | 0.39, 1.00 |
| Rater 6 | 0.74 | 0.27, 1.00 |
| Interstitial infiltrate | ||
| Rater 1 | 1.00 | 1.00, 1.00 |
| Rater 2 | 0.21 | 0.43, 0.85 |
| Rater 3 | 0.74 | 0.27, 1.00 |
| Rater 4 | n/a | |
| Rater 5 | 0.58 | 0.07, 1.00 |
| Rater 6 | 0.62 | 0.5, 1.00 |
DISCUSSION
The chest radiograph serves as an integral component of the reference standard for the diagnosis of childhood pneumonia. Few prior studies have assessed the reliability of chest radiograph findings in children.3, 5, 12, 14, 15 We found a high degree of agreement among radiologists for radiologic findings consistent with bacterial pneumonia when standardized interpretation criteria were applied. In this study, we identified radiographic features of pneumonia, such as alveolar infiltrate and pleural effusion, that were consistently identified by different radiologists reviewing the same radiograph and by the same radiologist reviewing the same radiograph. These data support the notion that radiographic features most suggestive of bacterial pneumonia are consistently identified by radiologists.16, 17 There was less consistency in the identification of other radiographic findings, such as interstitial infiltrates, air bronchograms, and hilar lymphadenopathy.
Prior studies have found high levels of disagreement among radiologists in the interpretation of chest radiographs.2, 3, 15, 18 Many of these prior studies emphasized variation in detection of radiographic findings that would not typically alter clinical management. We observed high intra‐rater, and inter‐rater reliability among radiologists for the findings of alveolar infiltrate and pleural effusion. These are the radiographic findings most consistent with a bacterial etiologic agent for pneumonia.19 Other studies have also found that the presence of an alveolar infiltrate is a reliable radiographic finding in children18 and adults.7, 9, 10 These findings support the use of the WHO definition of primary endpoint pneumonia for use in epidemiologic studies.4, 6, 11
This study also confirms a previous report by Cherian et al. that findings of many children with asthma, reactive airways disease, bronchiolitis, and viral infections interstitial infiltrates are less reliable.6 This is not surprising considering the fact that these patients often have radiographic findings due to small airway disease and atelectasis.19, 20 The differentiation between atelectasis and interstitial infiltrate is difficult, particularly in young children. A prior study conducted among neonates observed wide variability in the interpretation of chest radiographs, and that the differentiation of pneumonia from atelectasis was difficult for this patient population.5 The decisions around antimicrobial treatment of children with radiographic findings of interstitial infiltrates should be made in the context of the clinical history and physical examination findings, and clinicians should realize that these radiographic features demonstrate poor reliability for the diagnosis of pneumonia.
Overall reliability for the presence of any infiltrate, and its converse, no infiltrate was considered moderate. This is driven by the low reliability and variability around the radiographic diagnosis of interstitial infiltrates. Our findings are similar to those observed in adults with lower respiratory tract infections.9 The low reliability in identification of interstitial infiltrates may explain why prior studies have demonstrated that the CXR results rarely change management in children who have radiographs performed for suspicion of pneumonia.1, 21 Our study highlights the importance of quantifying CXR findings to include specific comments regarding the presence or absence of alveolar infiltrates, rather than the presence or absence of any infiltrate.
The WHO has procured definitions the radiographic diagnosis of pneumonia, and this definition has been utilized to help standardize the interpretation of chest radiographs for the conduct of epidemiological studies.6, 11 Specifically, the definitions utilized not only define the presence or absence of pneumonia, but also attempt to differentiate a primarily bacterial infection (consolidation or pleural effusion), from a viral or atypical presentation (interstitial pattern). Even under the best of circumstances, the differentiation of viral versus bacterial pneumonia is not always possible, and again, is often made by the treating physician by incorporating the clinical setting within which the radiograph was obtained.
This study had several limitations. Firstly, the included radiographs did not reflect the frequency with which certain radiographic findings would be identified in children evaluated for pneumonia in a pediatric emergency department setting. Radiographs were purposefully selected to encompass a broad spectrum of radiologic findings, including less common findings such as hilar lymphadenopathy and pleural effusions. Thus, the prevalence of pneumonia and other abnormal findings in this study was artificially higher than typically observed among a cohort of children for whom pneumonia is considered, a factor that may limit the generalizability of our results. Secondly, the clinical history was not provided to the radiologists to avoid bias by indication. For this study, we notified the radiologists that all radiographs were performed for clinical suspicion of pneumonia without providing details about the subjects' signs and symptoms. The absence of clinical history, however, does not mirror the real world scenario in which the interpretation of the chest radiograph is frequently made in the context of the clinical history. The relevance of this latter issue is unclear, as Tudor et al. found a nonstatistically significant improvement in the overall accuracy in chest radiograph interpretation when radiologists were provided clinical details.10 The radiologists recruited for this study all practice in an academic children's hospital setting, and thus, the generalizability of our findings may be limited to this type of practice setting. Finally, reproducibility does not imply accuracy, and reliability in identifying specific findings does not necessarily lead to improved or different management. Thus, while the reliability of radiographic findings of alveolar infiltrate and pleural effusion is reassuringly high, the validity of these radiographic features for bacterial pneumonia is not known. Ascertainment of validity can only be assessed through the use of invasive testing such as lung biopsy, as the yield from bacterial testing such as blood cultures is low, and the results of other studies such as viral testing of nasopharyngeal washings do not prove an etiologic cause of pneumonia.
CONCLUSIONS
Radiographic findings of alveolar infiltrates and pleural effusions are highly reliable among radiologists. Radiographic interpretation of interstitial infiltrates appears to be less reliable.
- ,,, et al.Usefulness of chest radiographs in children with acute lower respiratory tract disease.J Pediatr.1987;111:187–193.
- ,,, et al.Disagreement in the interpretation of chest radiographs among specialists and clinical outcomes of patients hospitalized with suspected pneumonia.Eur J Intern Med.2006;17:43–47.
- ,,.Problems in the clinical and roentgenographic diagnosis of pneumonia in young children.Clin Pediatr (Phila).1984;23:398–399.
- WHO guidelines on detecting pneumonia in children.Lancet.1991;338:1453–1454.
- ,,, et al.Inter‐ and intra‐observer variability in the assessment of atelectasis and consolidation in neonatal chest radiographs.Pediatr Radiol.1999;29:459–462.
- ,,, et al.Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies.Bull World Health Organ.2005;83:353–359.
- ,,, et al.Interobserver reliability of the chest radiograph in community‐acquired pneumonia. PORT Investigators.Chest.1996;110:343–350.
- ,,, et al.Chest radiographs in the emergency department: is the radiologist really necessary?Postgrad Med J.2003;79:214–217.
- ,,, et al.Inter‐observer variation in the interpretation of chest radiographs for pneumonia in community‐acquired lower respiratory tract infections.Clin Radiol.2004;59:743–752.
- ,,.An assessment of inter‐observer agreement and accuracy when reporting plain radiographs.Clin Radiol.1997;52:235–238.
- Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. In:World Health Organization: Pneumonia Vaccine Trial Investigators' Group.Geneva:Department of Vaccine and Biologics;2001.
- ,,, et al.Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs.Pediatr Infect Dis J.2006;25:779–781.
- ,.The measurement of observer agreement for categorical data.Biometrics.1977;33:159–174.
- ,.Clinical, laboratory, and radiological information in the diagnosis of pneumonia in children.Ann Emerg Med.1988;17:43–46.
- ,.Intraobserver and interobserver agreement of the interpretation of pediatric chest radiographs.Emerg Radiol.17:285–290.
- ,,, et al.Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.Comparison of radiological findings and microbial aetiology of childhood pneumonia.Acta Paediatr.1993;82:360–363.
- Kuhn JP, Slovis TL, Haller JO, eds.Caffey's Pediatric Diagnostic Imaging.10th ed.Philadelphia, PA:Mosby;2004.
- ,,, et al.Clinical predictors of pneumonia among children with wheezing.Pediatrics.2009;124:e29–e36.
- ,,, et al.The clinical impact of the radiology report in wheezing and nonwheezing febrile children: a survey of clinicians.Pediatr Radiol.2009;39:348–353.
The chest radiograph (CXR) is the most commonly used diagnostic imaging modality in children, and is considered to be the gold standard for the diagnosis of pneumonia. As such, physicians in developed countries rely on chest radiography to establish the diagnosis of pneumonia.13 However, there are limited data investigating the reliability of this test for the diagnosis of pneumonia in children.2, 46
Prior investigations have noted poor overall agreement by emergency medicine, infectious diseases, and pulmonary medicine physicians, and even radiologists, in their interpretation of chest radiographs for the diagnosis of pneumonia.2, 5, 710 The World Health Organization (WHO) developed criteria to standardize CXR interpretation for the diagnosis of pneumonia in children for use in epidemiologic studies.11 These standardized definitions of pneumonia have been formally evaluated by the WHO6 and utilized in epidemiologic studies of vaccine efficacy,12 but the overall reliability of these radiographic criteria have not been studied outside of these forums.
We conducted this prospective case‐based study to evaluate the reliability of the radiographic diagnosis of pneumonia among children presenting to a pediatric emergency department with clinical suspicion of pneumonia. We were primarily interested in assessing the overall reliability in CXR interpretation for the diagnosis of pneumonia, and identifying which radiographic features of pneumonia were consistently identified by radiologists.
MATERIALS AND METHODS
Study Subjects
We evaluated the reliability of CXR interpretation with respect to the diagnosis of pneumonia among radiologists. Six board‐certified radiologists at 2 academic children's hospitals (Children's Hospital of Philadelphia, Philadelphia, PA [n = 3] and Children's Hospital, Boston, Boston, MA [n = 3]) interpreted the same 110 chest radiographs in a blinded fashion. The radiologists varied with respect to the number of years practicing pediatric radiology (median 8 years, range 3‐36 years). Clinical information such as age, gender, clinical indication for obtaining the radiograph, history, and physical examination findings were not provided. Aside from the study form which stated the WHO classification scheme for radiographic pneumonia, no other information or training was provided to the radiologists as part of this study.
Radiographs were selected among a population of children presenting to the emergency department at Children's Hospital, Boston, who had a radiograph obtained for concern of pneumonia. From this cohort, we selected children who had radiographs which encompassed the spectrum of respiratory disease processes encountered in a pediatric population. The radiographs selected for review included 50 radiographs with a final reading in the medical record without suspicion for pneumonia, and 50 radiographs in which the diagnosis of pneumonia could not be excluded. In the latter group, 25 radiographs had a final reading suggestive of an alveolar infiltrate, and 25 radiographs had a final reading suggestive of an interstitial infiltrate. Ten duplicate radiographs were included to permit assessment of intra‐rater reliability.
Radiograph Interpretation
Radiologists at both sites interpreted the identical 110 radiographs (both anteroposterior [AP] and lateral views for each subject). Digital Imaging and Communications in Medicine (DICOM) images were downloaded from a registry at Children's Hospital, Boston, and were copied to DVDs which were provided to each radiologist. Standardized radiographic imaging software (eFilm Lite [Mississauga, Canada]) was used by each radiologist to view and interpret the radiographs.
Each radiologist completed a study questionnaire for each radiograph interpreted (see Supporting Appendix A in the online version of this article). The questionnaire utilized radiographic descriptors of primary end‐point pneumonia described by the WHO which were procured to standardize the radiographic diagnosis of pneumonia.11, 12 The main outcome of interest was the presence or absence of an infiltrate. Among radiographs in which an infiltrate was identified, radiologists selected whether there was an alveolar infiltrate, interstitial infiltrate, or both. An alveolar infiltrate was defined as a dense or fluffy opacity that occupies a portion or whole of a lobe, or of the entire lung, that may or may not contain air bronchograms.11, 12 An interstitial infiltrate was defined by a lacy pattern involving both lungs, featuring peribronchial thickening and multiple areas of atelectasis.11, 12 It also included minor patchy infiltrates that were not of sufficient magnitude to constitute consolidation, and small areas of atelectasis that in children may be difficult to distinguish from consolidation. Among interstitial infiltrates, radiologists were asked to distinguish infiltrate from atelectasis. A radiograph classified as having either an alveolar infiltrate or interstitial infiltrate (not atelectasis) was considered to have any infiltrate. Additional findings including air bronchograms, hilar adenopathy, pleural effusion, and location of abnormalities were also recorded.
Statistical Analysis
Inter‐rater reliability was assessed using the kappa statistic to determine the overall agreement between the 6 radiologists for each binary outcome (ie, presence or absence of alveolar infiltrate). To calculate 95% confidence intervals (CI) for kappa statistics with more than 2 raters, we employed a bootstrapping method with 1000 replications of samples equal in size to the study sample, using the kapci program as implemented by STATA software (version 10.1, STATA Corp, College Station, TX). Also, intra‐rater reliability was evaluated by examining the agreement within each radiologist upon review of 10 duplicate radiographs that had been randomly inserted into the case‐mix. We used the benchmarks proposed by Landis and Koch to classify the strength of agreement measured by the kappa statistic, as follows: poor (<0.0); slight (0‐0.20); fair (0.21‐0.40); moderate (0.41‐0.60); substantial (0.61‐0.80); almost perfect (0.81‐1.0).13
The study was approved by the institutional review boards at Children's Hospital, Boston and Children's Hospital of Philadelphia.
RESULTS
Patient Sample
The sample of 110 radiographs was obtained from 100 children presenting to the emergency department at Children's Hospital, Boston, with concern of pneumonia. These patients ranged in age from 1 week to 19 years (median, 3.5 years; interquartile range [IQR], 1.6‐6.0 years). Fifty (50%) of these patients were male. As stated above, the sample comprised 50 radiographs with a final reading in the medical record without suspicion for pneumonia, and 50 radiographs in which the diagnosis of pneumonia could not be excluded. The 10 duplicate radiographs encompassed a similar spectrum of findings.
Inter‐Rater Reliability
The kappa coefficients of inter‐rater reliability between the radiologists across the 6 clinical measures of interest are displayed in Table 1. As shown, the most reliable measure was that of alveolar infiltrate (Figure 1), which attained a substantial degree of agreement between the radiologists. Two other measures, any infiltrate and pleural effusion, attained moderate reliability, while bronchograms and hilar adenopathy were each classified as having fair reliability. However, interstitial infiltrate (Figure 2) was found to have the lowest kappa estimate, with a slight degree of reliability. When examining inter‐rater reliability among the radiologists separately from each institution, the pattern of results was similar.
| All Radiologists (n = 6) | Kappa | 95% Confidence Interval |
|---|---|---|
| ||
| Any infiltrate | 0.47 | 0.39, 0.56 |
| Alveolar infiltrate | 0.69 | 0.60, 0.78 |
| Interstitial infiltrate | 0.14 | 0.05, 0.23 |
| Air bronchograms | 0.32 | 0.24, 0.42 |
| Hilar adenopathy | 0.21 | 0.08, 0.39 |
| Pleural effusion | 0.45 | 0.29, 0.61 |


At least 4 of the 6 radiologists agreed on the presence or absence of an alveolar infiltrate for 95 of the 100 unique CXRs; all 6 radiologists agreed regarding the presence or absence of an alveolar infiltrate in 72 of the 100 unique CXRs. At least 4 of the 6 radiologists agreed on the presence or absence of any infiltrate and interstitial infiltrate 96% and 90% of the time, respectively. All 6 of the radiologists agreed on the presence or absence of any infiltrate and interstitial infiltrate 35% and 27% of the time, respectively.
Intra‐Rater Reliability
Estimates of intra‐rater reliability on the primary clinical outcomes (alveolar infiltrate, interstitial infiltrate, and any infiltrate) are found in Table 2. Across the 6 raters, the kappa estimates for alveolar infiltrate were all classified as substantial or almost perfect. The kappa estimates for interstitial infiltrate varied widely, ranging from fair to almost perfect, while for any infiltrate, reliability ranged from moderate to almost perfect.
| Kappa | 95% Confidence Interval | |
|---|---|---|
| ||
| Any infiltrate | ||
| Rater 1 | 1.00 | 1.00, 1.00 |
| Rater 2 | 0.60 | 0.10, 1.00 |
| Rater 3 | 0.80 | 0.44, 1.00 |
| Rater 4 | 1.00 | 1.00, 1.00 |
| Rater 5 | n/a* | |
| Rater 6 | 1.00 | 1.00, 1.00 |
| Alveolar infiltrate | ||
| Rater 1 | 1.00 | 1.00, 1.00 |
| Rater 2 | 1.00 | 1.00, 1.00 |
| Rater 3 | 1.00 | 1.00, 1.00 |
| Rater 4 | 1.00 | 1.00, 1.00 |
| Rater 5 | 0.78 | 0.39, 1.00 |
| Rater 6 | 0.74 | 0.27, 1.00 |
| Interstitial infiltrate | ||
| Rater 1 | 1.00 | 1.00, 1.00 |
| Rater 2 | 0.21 | 0.43, 0.85 |
| Rater 3 | 0.74 | 0.27, 1.00 |
| Rater 4 | n/a | |
| Rater 5 | 0.58 | 0.07, 1.00 |
| Rater 6 | 0.62 | 0.5, 1.00 |
DISCUSSION
The chest radiograph serves as an integral component of the reference standard for the diagnosis of childhood pneumonia. Few prior studies have assessed the reliability of chest radiograph findings in children.3, 5, 12, 14, 15 We found a high degree of agreement among radiologists for radiologic findings consistent with bacterial pneumonia when standardized interpretation criteria were applied. In this study, we identified radiographic features of pneumonia, such as alveolar infiltrate and pleural effusion, that were consistently identified by different radiologists reviewing the same radiograph and by the same radiologist reviewing the same radiograph. These data support the notion that radiographic features most suggestive of bacterial pneumonia are consistently identified by radiologists.16, 17 There was less consistency in the identification of other radiographic findings, such as interstitial infiltrates, air bronchograms, and hilar lymphadenopathy.
Prior studies have found high levels of disagreement among radiologists in the interpretation of chest radiographs.2, 3, 15, 18 Many of these prior studies emphasized variation in detection of radiographic findings that would not typically alter clinical management. We observed high intra‐rater, and inter‐rater reliability among radiologists for the findings of alveolar infiltrate and pleural effusion. These are the radiographic findings most consistent with a bacterial etiologic agent for pneumonia.19 Other studies have also found that the presence of an alveolar infiltrate is a reliable radiographic finding in children18 and adults.7, 9, 10 These findings support the use of the WHO definition of primary endpoint pneumonia for use in epidemiologic studies.4, 6, 11
This study also confirms a previous report by Cherian et al. that findings of many children with asthma, reactive airways disease, bronchiolitis, and viral infections interstitial infiltrates are less reliable.6 This is not surprising considering the fact that these patients often have radiographic findings due to small airway disease and atelectasis.19, 20 The differentiation between atelectasis and interstitial infiltrate is difficult, particularly in young children. A prior study conducted among neonates observed wide variability in the interpretation of chest radiographs, and that the differentiation of pneumonia from atelectasis was difficult for this patient population.5 The decisions around antimicrobial treatment of children with radiographic findings of interstitial infiltrates should be made in the context of the clinical history and physical examination findings, and clinicians should realize that these radiographic features demonstrate poor reliability for the diagnosis of pneumonia.
Overall reliability for the presence of any infiltrate, and its converse, no infiltrate was considered moderate. This is driven by the low reliability and variability around the radiographic diagnosis of interstitial infiltrates. Our findings are similar to those observed in adults with lower respiratory tract infections.9 The low reliability in identification of interstitial infiltrates may explain why prior studies have demonstrated that the CXR results rarely change management in children who have radiographs performed for suspicion of pneumonia.1, 21 Our study highlights the importance of quantifying CXR findings to include specific comments regarding the presence or absence of alveolar infiltrates, rather than the presence or absence of any infiltrate.
The WHO has procured definitions the radiographic diagnosis of pneumonia, and this definition has been utilized to help standardize the interpretation of chest radiographs for the conduct of epidemiological studies.6, 11 Specifically, the definitions utilized not only define the presence or absence of pneumonia, but also attempt to differentiate a primarily bacterial infection (consolidation or pleural effusion), from a viral or atypical presentation (interstitial pattern). Even under the best of circumstances, the differentiation of viral versus bacterial pneumonia is not always possible, and again, is often made by the treating physician by incorporating the clinical setting within which the radiograph was obtained.
This study had several limitations. Firstly, the included radiographs did not reflect the frequency with which certain radiographic findings would be identified in children evaluated for pneumonia in a pediatric emergency department setting. Radiographs were purposefully selected to encompass a broad spectrum of radiologic findings, including less common findings such as hilar lymphadenopathy and pleural effusions. Thus, the prevalence of pneumonia and other abnormal findings in this study was artificially higher than typically observed among a cohort of children for whom pneumonia is considered, a factor that may limit the generalizability of our results. Secondly, the clinical history was not provided to the radiologists to avoid bias by indication. For this study, we notified the radiologists that all radiographs were performed for clinical suspicion of pneumonia without providing details about the subjects' signs and symptoms. The absence of clinical history, however, does not mirror the real world scenario in which the interpretation of the chest radiograph is frequently made in the context of the clinical history. The relevance of this latter issue is unclear, as Tudor et al. found a nonstatistically significant improvement in the overall accuracy in chest radiograph interpretation when radiologists were provided clinical details.10 The radiologists recruited for this study all practice in an academic children's hospital setting, and thus, the generalizability of our findings may be limited to this type of practice setting. Finally, reproducibility does not imply accuracy, and reliability in identifying specific findings does not necessarily lead to improved or different management. Thus, while the reliability of radiographic findings of alveolar infiltrate and pleural effusion is reassuringly high, the validity of these radiographic features for bacterial pneumonia is not known. Ascertainment of validity can only be assessed through the use of invasive testing such as lung biopsy, as the yield from bacterial testing such as blood cultures is low, and the results of other studies such as viral testing of nasopharyngeal washings do not prove an etiologic cause of pneumonia.
CONCLUSIONS
Radiographic findings of alveolar infiltrates and pleural effusions are highly reliable among radiologists. Radiographic interpretation of interstitial infiltrates appears to be less reliable.
The chest radiograph (CXR) is the most commonly used diagnostic imaging modality in children, and is considered to be the gold standard for the diagnosis of pneumonia. As such, physicians in developed countries rely on chest radiography to establish the diagnosis of pneumonia.13 However, there are limited data investigating the reliability of this test for the diagnosis of pneumonia in children.2, 46
Prior investigations have noted poor overall agreement by emergency medicine, infectious diseases, and pulmonary medicine physicians, and even radiologists, in their interpretation of chest radiographs for the diagnosis of pneumonia.2, 5, 710 The World Health Organization (WHO) developed criteria to standardize CXR interpretation for the diagnosis of pneumonia in children for use in epidemiologic studies.11 These standardized definitions of pneumonia have been formally evaluated by the WHO6 and utilized in epidemiologic studies of vaccine efficacy,12 but the overall reliability of these radiographic criteria have not been studied outside of these forums.
We conducted this prospective case‐based study to evaluate the reliability of the radiographic diagnosis of pneumonia among children presenting to a pediatric emergency department with clinical suspicion of pneumonia. We were primarily interested in assessing the overall reliability in CXR interpretation for the diagnosis of pneumonia, and identifying which radiographic features of pneumonia were consistently identified by radiologists.
MATERIALS AND METHODS
Study Subjects
We evaluated the reliability of CXR interpretation with respect to the diagnosis of pneumonia among radiologists. Six board‐certified radiologists at 2 academic children's hospitals (Children's Hospital of Philadelphia, Philadelphia, PA [n = 3] and Children's Hospital, Boston, Boston, MA [n = 3]) interpreted the same 110 chest radiographs in a blinded fashion. The radiologists varied with respect to the number of years practicing pediatric radiology (median 8 years, range 3‐36 years). Clinical information such as age, gender, clinical indication for obtaining the radiograph, history, and physical examination findings were not provided. Aside from the study form which stated the WHO classification scheme for radiographic pneumonia, no other information or training was provided to the radiologists as part of this study.
Radiographs were selected among a population of children presenting to the emergency department at Children's Hospital, Boston, who had a radiograph obtained for concern of pneumonia. From this cohort, we selected children who had radiographs which encompassed the spectrum of respiratory disease processes encountered in a pediatric population. The radiographs selected for review included 50 radiographs with a final reading in the medical record without suspicion for pneumonia, and 50 radiographs in which the diagnosis of pneumonia could not be excluded. In the latter group, 25 radiographs had a final reading suggestive of an alveolar infiltrate, and 25 radiographs had a final reading suggestive of an interstitial infiltrate. Ten duplicate radiographs were included to permit assessment of intra‐rater reliability.
Radiograph Interpretation
Radiologists at both sites interpreted the identical 110 radiographs (both anteroposterior [AP] and lateral views for each subject). Digital Imaging and Communications in Medicine (DICOM) images were downloaded from a registry at Children's Hospital, Boston, and were copied to DVDs which were provided to each radiologist. Standardized radiographic imaging software (eFilm Lite [Mississauga, Canada]) was used by each radiologist to view and interpret the radiographs.
Each radiologist completed a study questionnaire for each radiograph interpreted (see Supporting Appendix A in the online version of this article). The questionnaire utilized radiographic descriptors of primary end‐point pneumonia described by the WHO which were procured to standardize the radiographic diagnosis of pneumonia.11, 12 The main outcome of interest was the presence or absence of an infiltrate. Among radiographs in which an infiltrate was identified, radiologists selected whether there was an alveolar infiltrate, interstitial infiltrate, or both. An alveolar infiltrate was defined as a dense or fluffy opacity that occupies a portion or whole of a lobe, or of the entire lung, that may or may not contain air bronchograms.11, 12 An interstitial infiltrate was defined by a lacy pattern involving both lungs, featuring peribronchial thickening and multiple areas of atelectasis.11, 12 It also included minor patchy infiltrates that were not of sufficient magnitude to constitute consolidation, and small areas of atelectasis that in children may be difficult to distinguish from consolidation. Among interstitial infiltrates, radiologists were asked to distinguish infiltrate from atelectasis. A radiograph classified as having either an alveolar infiltrate or interstitial infiltrate (not atelectasis) was considered to have any infiltrate. Additional findings including air bronchograms, hilar adenopathy, pleural effusion, and location of abnormalities were also recorded.
Statistical Analysis
Inter‐rater reliability was assessed using the kappa statistic to determine the overall agreement between the 6 radiologists for each binary outcome (ie, presence or absence of alveolar infiltrate). To calculate 95% confidence intervals (CI) for kappa statistics with more than 2 raters, we employed a bootstrapping method with 1000 replications of samples equal in size to the study sample, using the kapci program as implemented by STATA software (version 10.1, STATA Corp, College Station, TX). Also, intra‐rater reliability was evaluated by examining the agreement within each radiologist upon review of 10 duplicate radiographs that had been randomly inserted into the case‐mix. We used the benchmarks proposed by Landis and Koch to classify the strength of agreement measured by the kappa statistic, as follows: poor (<0.0); slight (0‐0.20); fair (0.21‐0.40); moderate (0.41‐0.60); substantial (0.61‐0.80); almost perfect (0.81‐1.0).13
The study was approved by the institutional review boards at Children's Hospital, Boston and Children's Hospital of Philadelphia.
RESULTS
Patient Sample
The sample of 110 radiographs was obtained from 100 children presenting to the emergency department at Children's Hospital, Boston, with concern of pneumonia. These patients ranged in age from 1 week to 19 years (median, 3.5 years; interquartile range [IQR], 1.6‐6.0 years). Fifty (50%) of these patients were male. As stated above, the sample comprised 50 radiographs with a final reading in the medical record without suspicion for pneumonia, and 50 radiographs in which the diagnosis of pneumonia could not be excluded. The 10 duplicate radiographs encompassed a similar spectrum of findings.
Inter‐Rater Reliability
The kappa coefficients of inter‐rater reliability between the radiologists across the 6 clinical measures of interest are displayed in Table 1. As shown, the most reliable measure was that of alveolar infiltrate (Figure 1), which attained a substantial degree of agreement between the radiologists. Two other measures, any infiltrate and pleural effusion, attained moderate reliability, while bronchograms and hilar adenopathy were each classified as having fair reliability. However, interstitial infiltrate (Figure 2) was found to have the lowest kappa estimate, with a slight degree of reliability. When examining inter‐rater reliability among the radiologists separately from each institution, the pattern of results was similar.
| All Radiologists (n = 6) | Kappa | 95% Confidence Interval |
|---|---|---|
| ||
| Any infiltrate | 0.47 | 0.39, 0.56 |
| Alveolar infiltrate | 0.69 | 0.60, 0.78 |
| Interstitial infiltrate | 0.14 | 0.05, 0.23 |
| Air bronchograms | 0.32 | 0.24, 0.42 |
| Hilar adenopathy | 0.21 | 0.08, 0.39 |
| Pleural effusion | 0.45 | 0.29, 0.61 |


At least 4 of the 6 radiologists agreed on the presence or absence of an alveolar infiltrate for 95 of the 100 unique CXRs; all 6 radiologists agreed regarding the presence or absence of an alveolar infiltrate in 72 of the 100 unique CXRs. At least 4 of the 6 radiologists agreed on the presence or absence of any infiltrate and interstitial infiltrate 96% and 90% of the time, respectively. All 6 of the radiologists agreed on the presence or absence of any infiltrate and interstitial infiltrate 35% and 27% of the time, respectively.
Intra‐Rater Reliability
Estimates of intra‐rater reliability on the primary clinical outcomes (alveolar infiltrate, interstitial infiltrate, and any infiltrate) are found in Table 2. Across the 6 raters, the kappa estimates for alveolar infiltrate were all classified as substantial or almost perfect. The kappa estimates for interstitial infiltrate varied widely, ranging from fair to almost perfect, while for any infiltrate, reliability ranged from moderate to almost perfect.
| Kappa | 95% Confidence Interval | |
|---|---|---|
| ||
| Any infiltrate | ||
| Rater 1 | 1.00 | 1.00, 1.00 |
| Rater 2 | 0.60 | 0.10, 1.00 |
| Rater 3 | 0.80 | 0.44, 1.00 |
| Rater 4 | 1.00 | 1.00, 1.00 |
| Rater 5 | n/a* | |
| Rater 6 | 1.00 | 1.00, 1.00 |
| Alveolar infiltrate | ||
| Rater 1 | 1.00 | 1.00, 1.00 |
| Rater 2 | 1.00 | 1.00, 1.00 |
| Rater 3 | 1.00 | 1.00, 1.00 |
| Rater 4 | 1.00 | 1.00, 1.00 |
| Rater 5 | 0.78 | 0.39, 1.00 |
| Rater 6 | 0.74 | 0.27, 1.00 |
| Interstitial infiltrate | ||
| Rater 1 | 1.00 | 1.00, 1.00 |
| Rater 2 | 0.21 | 0.43, 0.85 |
| Rater 3 | 0.74 | 0.27, 1.00 |
| Rater 4 | n/a | |
| Rater 5 | 0.58 | 0.07, 1.00 |
| Rater 6 | 0.62 | 0.5, 1.00 |
DISCUSSION
The chest radiograph serves as an integral component of the reference standard for the diagnosis of childhood pneumonia. Few prior studies have assessed the reliability of chest radiograph findings in children.3, 5, 12, 14, 15 We found a high degree of agreement among radiologists for radiologic findings consistent with bacterial pneumonia when standardized interpretation criteria were applied. In this study, we identified radiographic features of pneumonia, such as alveolar infiltrate and pleural effusion, that were consistently identified by different radiologists reviewing the same radiograph and by the same radiologist reviewing the same radiograph. These data support the notion that radiographic features most suggestive of bacterial pneumonia are consistently identified by radiologists.16, 17 There was less consistency in the identification of other radiographic findings, such as interstitial infiltrates, air bronchograms, and hilar lymphadenopathy.
Prior studies have found high levels of disagreement among radiologists in the interpretation of chest radiographs.2, 3, 15, 18 Many of these prior studies emphasized variation in detection of radiographic findings that would not typically alter clinical management. We observed high intra‐rater, and inter‐rater reliability among radiologists for the findings of alveolar infiltrate and pleural effusion. These are the radiographic findings most consistent with a bacterial etiologic agent for pneumonia.19 Other studies have also found that the presence of an alveolar infiltrate is a reliable radiographic finding in children18 and adults.7, 9, 10 These findings support the use of the WHO definition of primary endpoint pneumonia for use in epidemiologic studies.4, 6, 11
This study also confirms a previous report by Cherian et al. that findings of many children with asthma, reactive airways disease, bronchiolitis, and viral infections interstitial infiltrates are less reliable.6 This is not surprising considering the fact that these patients often have radiographic findings due to small airway disease and atelectasis.19, 20 The differentiation between atelectasis and interstitial infiltrate is difficult, particularly in young children. A prior study conducted among neonates observed wide variability in the interpretation of chest radiographs, and that the differentiation of pneumonia from atelectasis was difficult for this patient population.5 The decisions around antimicrobial treatment of children with radiographic findings of interstitial infiltrates should be made in the context of the clinical history and physical examination findings, and clinicians should realize that these radiographic features demonstrate poor reliability for the diagnosis of pneumonia.
Overall reliability for the presence of any infiltrate, and its converse, no infiltrate was considered moderate. This is driven by the low reliability and variability around the radiographic diagnosis of interstitial infiltrates. Our findings are similar to those observed in adults with lower respiratory tract infections.9 The low reliability in identification of interstitial infiltrates may explain why prior studies have demonstrated that the CXR results rarely change management in children who have radiographs performed for suspicion of pneumonia.1, 21 Our study highlights the importance of quantifying CXR findings to include specific comments regarding the presence or absence of alveolar infiltrates, rather than the presence or absence of any infiltrate.
The WHO has procured definitions the radiographic diagnosis of pneumonia, and this definition has been utilized to help standardize the interpretation of chest radiographs for the conduct of epidemiological studies.6, 11 Specifically, the definitions utilized not only define the presence or absence of pneumonia, but also attempt to differentiate a primarily bacterial infection (consolidation or pleural effusion), from a viral or atypical presentation (interstitial pattern). Even under the best of circumstances, the differentiation of viral versus bacterial pneumonia is not always possible, and again, is often made by the treating physician by incorporating the clinical setting within which the radiograph was obtained.
This study had several limitations. Firstly, the included radiographs did not reflect the frequency with which certain radiographic findings would be identified in children evaluated for pneumonia in a pediatric emergency department setting. Radiographs were purposefully selected to encompass a broad spectrum of radiologic findings, including less common findings such as hilar lymphadenopathy and pleural effusions. Thus, the prevalence of pneumonia and other abnormal findings in this study was artificially higher than typically observed among a cohort of children for whom pneumonia is considered, a factor that may limit the generalizability of our results. Secondly, the clinical history was not provided to the radiologists to avoid bias by indication. For this study, we notified the radiologists that all radiographs were performed for clinical suspicion of pneumonia without providing details about the subjects' signs and symptoms. The absence of clinical history, however, does not mirror the real world scenario in which the interpretation of the chest radiograph is frequently made in the context of the clinical history. The relevance of this latter issue is unclear, as Tudor et al. found a nonstatistically significant improvement in the overall accuracy in chest radiograph interpretation when radiologists were provided clinical details.10 The radiologists recruited for this study all practice in an academic children's hospital setting, and thus, the generalizability of our findings may be limited to this type of practice setting. Finally, reproducibility does not imply accuracy, and reliability in identifying specific findings does not necessarily lead to improved or different management. Thus, while the reliability of radiographic findings of alveolar infiltrate and pleural effusion is reassuringly high, the validity of these radiographic features for bacterial pneumonia is not known. Ascertainment of validity can only be assessed through the use of invasive testing such as lung biopsy, as the yield from bacterial testing such as blood cultures is low, and the results of other studies such as viral testing of nasopharyngeal washings do not prove an etiologic cause of pneumonia.
CONCLUSIONS
Radiographic findings of alveolar infiltrates and pleural effusions are highly reliable among radiologists. Radiographic interpretation of interstitial infiltrates appears to be less reliable.
- ,,, et al.Usefulness of chest radiographs in children with acute lower respiratory tract disease.J Pediatr.1987;111:187–193.
- ,,, et al.Disagreement in the interpretation of chest radiographs among specialists and clinical outcomes of patients hospitalized with suspected pneumonia.Eur J Intern Med.2006;17:43–47.
- ,,.Problems in the clinical and roentgenographic diagnosis of pneumonia in young children.Clin Pediatr (Phila).1984;23:398–399.
- WHO guidelines on detecting pneumonia in children.Lancet.1991;338:1453–1454.
- ,,, et al.Inter‐ and intra‐observer variability in the assessment of atelectasis and consolidation in neonatal chest radiographs.Pediatr Radiol.1999;29:459–462.
- ,,, et al.Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies.Bull World Health Organ.2005;83:353–359.
- ,,, et al.Interobserver reliability of the chest radiograph in community‐acquired pneumonia. PORT Investigators.Chest.1996;110:343–350.
- ,,, et al.Chest radiographs in the emergency department: is the radiologist really necessary?Postgrad Med J.2003;79:214–217.
- ,,, et al.Inter‐observer variation in the interpretation of chest radiographs for pneumonia in community‐acquired lower respiratory tract infections.Clin Radiol.2004;59:743–752.
- ,,.An assessment of inter‐observer agreement and accuracy when reporting plain radiographs.Clin Radiol.1997;52:235–238.
- Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. In:World Health Organization: Pneumonia Vaccine Trial Investigators' Group.Geneva:Department of Vaccine and Biologics;2001.
- ,,, et al.Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs.Pediatr Infect Dis J.2006;25:779–781.
- ,.The measurement of observer agreement for categorical data.Biometrics.1977;33:159–174.
- ,.Clinical, laboratory, and radiological information in the diagnosis of pneumonia in children.Ann Emerg Med.1988;17:43–46.
- ,.Intraobserver and interobserver agreement of the interpretation of pediatric chest radiographs.Emerg Radiol.17:285–290.
- ,,, et al.Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.Comparison of radiological findings and microbial aetiology of childhood pneumonia.Acta Paediatr.1993;82:360–363.
- Kuhn JP, Slovis TL, Haller JO, eds.Caffey's Pediatric Diagnostic Imaging.10th ed.Philadelphia, PA:Mosby;2004.
- ,,, et al.Clinical predictors of pneumonia among children with wheezing.Pediatrics.2009;124:e29–e36.
- ,,, et al.The clinical impact of the radiology report in wheezing and nonwheezing febrile children: a survey of clinicians.Pediatr Radiol.2009;39:348–353.
- ,,, et al.Usefulness of chest radiographs in children with acute lower respiratory tract disease.J Pediatr.1987;111:187–193.
- ,,, et al.Disagreement in the interpretation of chest radiographs among specialists and clinical outcomes of patients hospitalized with suspected pneumonia.Eur J Intern Med.2006;17:43–47.
- ,,.Problems in the clinical and roentgenographic diagnosis of pneumonia in young children.Clin Pediatr (Phila).1984;23:398–399.
- WHO guidelines on detecting pneumonia in children.Lancet.1991;338:1453–1454.
- ,,, et al.Inter‐ and intra‐observer variability in the assessment of atelectasis and consolidation in neonatal chest radiographs.Pediatr Radiol.1999;29:459–462.
- ,,, et al.Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies.Bull World Health Organ.2005;83:353–359.
- ,,, et al.Interobserver reliability of the chest radiograph in community‐acquired pneumonia. PORT Investigators.Chest.1996;110:343–350.
- ,,, et al.Chest radiographs in the emergency department: is the radiologist really necessary?Postgrad Med J.2003;79:214–217.
- ,,, et al.Inter‐observer variation in the interpretation of chest radiographs for pneumonia in community‐acquired lower respiratory tract infections.Clin Radiol.2004;59:743–752.
- ,,.An assessment of inter‐observer agreement and accuracy when reporting plain radiographs.Clin Radiol.1997;52:235–238.
- Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. In:World Health Organization: Pneumonia Vaccine Trial Investigators' Group.Geneva:Department of Vaccine and Biologics;2001.
- ,,, et al.Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs.Pediatr Infect Dis J.2006;25:779–781.
- ,.The measurement of observer agreement for categorical data.Biometrics.1977;33:159–174.
- ,.Clinical, laboratory, and radiological information in the diagnosis of pneumonia in children.Ann Emerg Med.1988;17:43–46.
- ,.Intraobserver and interobserver agreement of the interpretation of pediatric chest radiographs.Emerg Radiol.17:285–290.
- ,,, et al.Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.Comparison of radiological findings and microbial aetiology of childhood pneumonia.Acta Paediatr.1993;82:360–363.
- Kuhn JP, Slovis TL, Haller JO, eds.Caffey's Pediatric Diagnostic Imaging.10th ed.Philadelphia, PA:Mosby;2004.
- ,,, et al.Clinical predictors of pneumonia among children with wheezing.Pediatrics.2009;124:e29–e36.
- ,,, et al.The clinical impact of the radiology report in wheezing and nonwheezing febrile children: a survey of clinicians.Pediatr Radiol.2009;39:348–353.
Copyright © 2011 Society of Hospital Medicine